-
磁性纳米铁具有特殊的物理化学性质,随着纳米科学技术的快速发展,磁性纳米铁已成功应用于催化、生物技术、生物医药、磁共振成像、数据存储、生物传感器、环境污染物去除等广泛领域[1],但与此同时,此种材料所带来的潜在环境危害也有所增加[2]. 磁性纳米铁除了被用于土壤污染物去除(例如去除土壤中的重金属离子、有机污染物、无机污染物)时进入土壤,还会在生产与废弃过程中通过各种途径以“三废”的形式在土壤中积累[3].
土壤为陆生生物提供生活所必需的矿质元素和水分,是生态系统中物质与能量交换的重要场所;同时也是生态系统中生物部分和无机环境部分相互作用的产物[4]. 磁性纳米铁在土壤中的积累除了会对土壤理化性质、酶活性和温室气体排出造成影响,还会影响到土壤微生物群落的结构、功能和代谢,从而影响到整个土壤生态系统[5]. 本研究以此为出发点,总结了磁性纳米铁(以四氧化三铁磁性纳米颗粒(Fe3O4-NPs)、三氧化二铁磁性纳米颗粒(Fe2O3-NPs)和纳米零价铁(nZVI-NPs)为主)在土壤中积累所带来影响的相关研究,以期为合理利用磁性纳米铁修复土壤污染的同时尽可能减少对土壤以及微生物的危害提供理论依据.
-
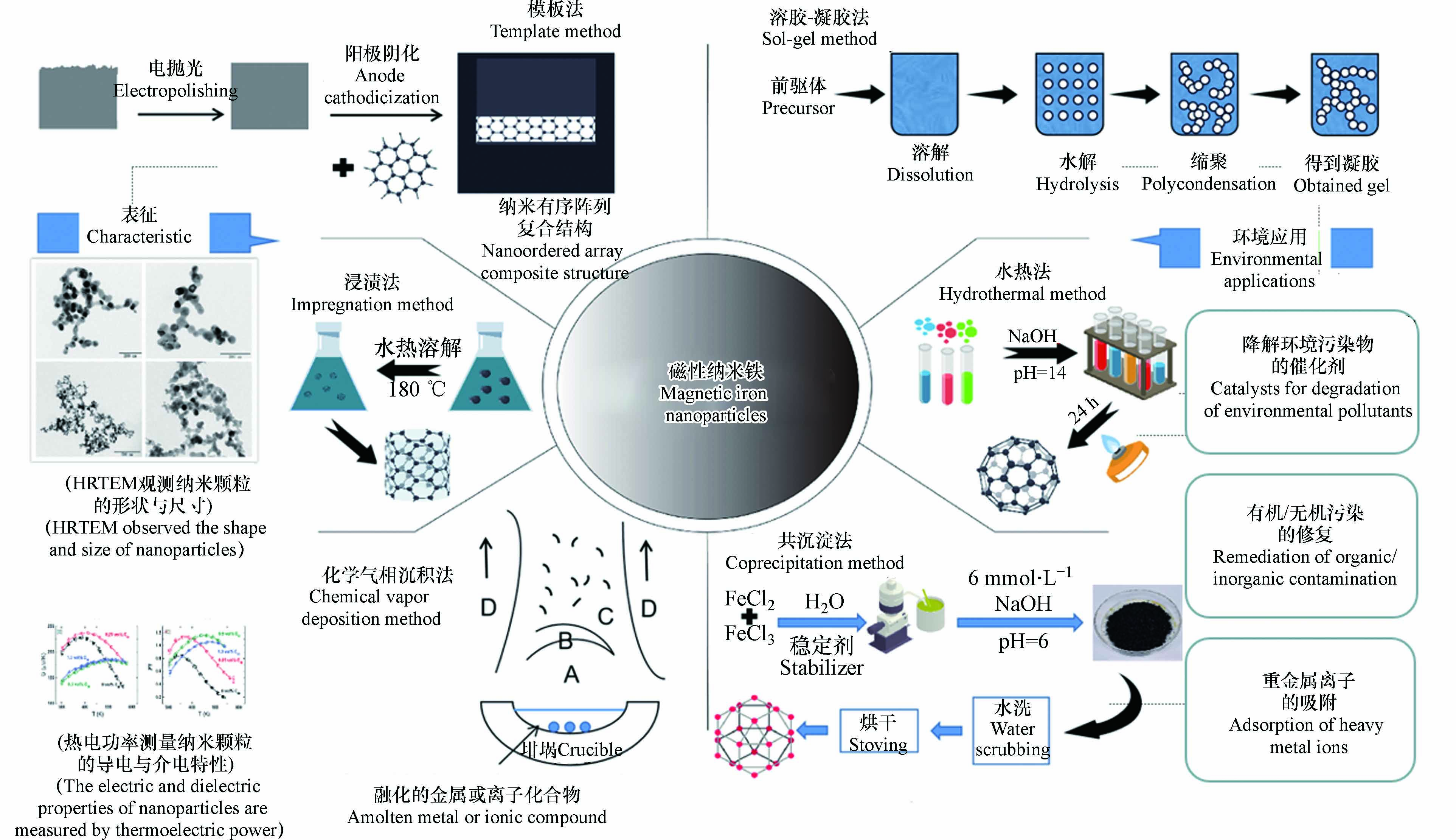
磁性纳米铁可以通过原子组装或块体材料断裂来制备,常用的制备方法有溶胶-凝胶法、水热法、共沉淀法、浸渍法、模板法、化学气相沉积法(图1)[6].
溶胶-凝胶法制备Fe3O4-NPs以亚硝酸铁为前驱体经过溶解、水解、缩聚、老化等步骤制得[7];采用水热法制备Fe2O3-NPs,样品形成的起始点是由氢氧化钠、去离子水、乙醇和油酸混合制备的溶液与七水硫酸铁水溶液混合后电磁搅拌6 h,将混合溶液在160 ℃加热24 h后,在去离子水和乙醇中洗涤6次,在75 ℃温度下干燥12 h后得到[8];采用化学共沉淀法合成Fe3O4-NPs的材料有: 六水合氯化铁(FeCl3·6H2O)、四水合氯化铁(FeCl2·4H2O)、水溶液混合后得到混合溶液,向混合溶液中添加NaOH将混合溶液pH调整至6后共沉淀生成Fe3O4-NPs;用浸渍法制备Fe2O3-NPs时,首先是将制备好的水凝胶与铁盐浸渍,然后在180 ℃温度下煅烧,合成分散在硅干凝胶中不同含量的Fe2O3-NPs样品[9];在绿色合成战略中,对环境无害的溶剂、无毒化学品和可再生材料的利用是要考虑的一些关键问题,例如利用纤维素薄膜作为辅助材料,通过电抛光、阳极阴化等步骤合成了单分散磁性Fe2O3-NPs[10];化学气相沉积是一种用于生产高质量、高性能固体材料的化学过程. 在利用化学气相沉积法合成Fe2O3-NPs、Fe3O4-NPs的过程中,水(基材)暴露在一种或多种挥发性前驱体中,这些前驱体在基材表面发生反应和分解,以产生所需的沉积[11].
nZVI-NPs的合成可以通过铣削、蚀刻和机加工等机械和化学过程将大尺寸材料转化为nZVI-NPs;或是通过化学合成、自组装、位置组装等方法逐个将原子或分子组装为nZVI-NPs,具体可分为氯化铁合成法、硫酸亚铁合成法以及自上而下法合成nZVI-NPs[12].
-
为了研究不同合成技术制备的磁性纳米铁的性能,目前已有多种技术用于磁性纳米铁的表征,例如采用X射线衍射(XRD)、场发射散射电子显微镜(FESEM)、高分辨透射电子显微镜(HRTEM)、原子力显微镜(AFM)、光子相关光谱(PCS)、动态光散射(DLS)、凝聚粒子计数器(CPC)等设备获得所制备样品的尺寸和形状,通过扫描电子显微镜、透射电子显微镜等设备观察纳米粒子的表面形貌以及长度和直径[13 − 14].
介电损耗切线、电阻率、电导率等是研究者通常观察到的磁性纳米铁的性质. 热电功率(TEP)测量和红外光谱证实,激光辐照纳米颗粒后,随着温度的升高,纳米颗粒的电导率增加,电阻率降低[15]. 磁性纳米铁的光电导性质,无论正负,都可以通过对光电导研究来证实,同时在对磁性纳米铁光学性质的表征上,目前已有一系列模型来确定纳米颗粒的光学性质,但其中最流行的模型是Tauc模型[16]. Tauc模型目前已经被用来观察铁酸镁(MgFe2O4)纳米材料的能量带隙等光学性质[17].
-
磁性纳米铁作为一种新型的纳米材料,因其独特的性质,如纳米效应、磁性、热/pH敏感性、生物相容性和稳定性等而备受关注[18]. 因此,磁性纳米铁应用越来越广泛,特别是在环境保护领域,例如作为环境污染物的催化剂、有机/无机污染物的环境修复治理等. 本节讨论了铁基磁性纳米材料用于环境应用的最新进展[19].
-
磁性纳米铁可以作为催化剂将污染物分子分解或转化为毒性较小的代谢物. 浸渍氧化-沉淀法合成的Fe3O4-NPs是降解泮托拉唑的非均相催化剂,在最佳条件下降解效率达到98.0%[20]. 另一种采用先进的反向共沉淀法制备的Fe3O4-NPs在超声辐照和活化H2O2作用下,60 min内可以降解约90%的罗丹明B[21]. 此外,Fe3O4-NPs还被用作非均相催化剂载体,以获得易于分散在普通溶剂中、高比表面积和可重复利用性的生物制品[22]. 例如,制备氨基功能化的Fe3O4-NPs并将其与戊二醛偶联(粒径范围为10—100 nm),可催化多种生物转化反应,包括乙酸乙酯合成和植物油酯交换制备生物柴油[23].
-
近年来,nZVI-NPs因其卓越的环境修复能力而受到极大关注. 它们的修复机制取决于nZVI-NPs的吸附和还原性能以及污染物的性质[24]. 实验研究表明,nZVI-NPs可以有效转化各种环境有机污染物,包括有机污染物,如卤代烃(如三氯乙烯)和有机染料(如甲基橙、紫红),采用nZVI-NPs对三氯乙烯进行脱氯,三氯乙烯去除率大于99%[25]. nZVI-NPs还可以有效转化多种环境无机污染物,包括重金属离子(如镍、汞、砷、铬)、放射性元素(如铀)和类金属(如硝酸盐或磷酸盐)[26].
-
利用Fe3O4-NPs修饰酵母细胞制成的纳米复合材料是去除铬的良好生物吸附剂[27]. Fe3O4-NPs修饰后的酵母细胞的吸附能力比未修饰的酵母细胞提高了3倍. 改性吸附剂对1000 mg·g−1铬溶液在最佳条件下的吸附量为186.32 mg·g−1,而未改性吸附剂的吸附量为137.31 mg·g−1[28]. 此外,表1归纳了部分通过各种方法合成的磁性纳米铁去除重金属应用的相关研究.
-
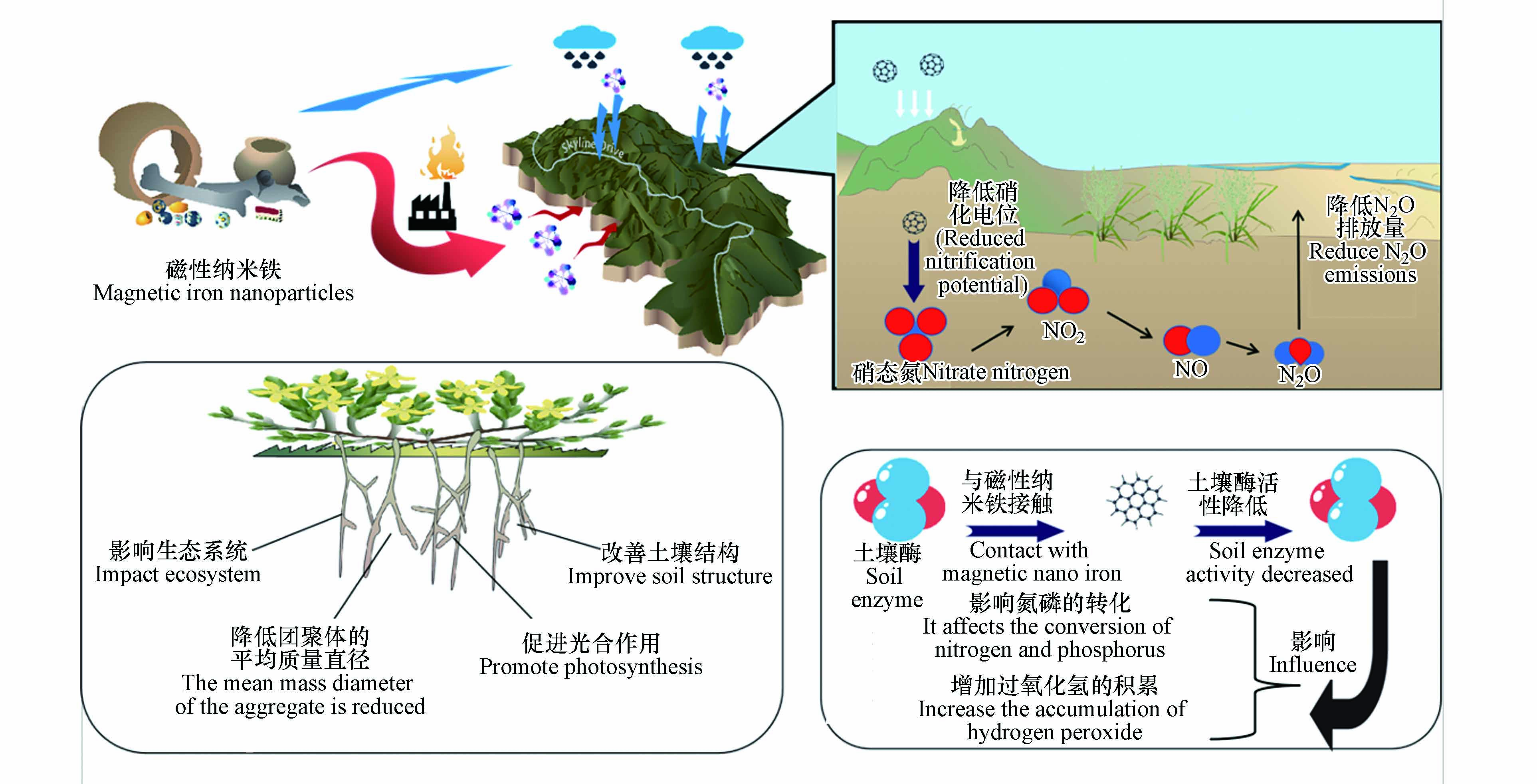
一些向环境释放大量磁性纳米铁的产品包括涂料、颜料、油漆、电子仪器,在生产阶段,大约有0.1%—2%的磁性纳米铁会到达环境中[35]. 其中进入土壤的磁性纳米铁占进入环境总量的10%—25%[36],磁性纳米铁不仅通过农业中的直接土地应用进入土壤(例如以磁性纳米铁为原料制成的具有成本效益、生物相容性和生物降解性的功能纳米材料被人为释放到土壤中以增加作物产量和有针对性地输送农药和营养物质,增强植物对各种胁迫因素的抵抗力,并作为纳米传感器检测各种污染物、植物疾病和植物营养不足)[37],还可通过垃圾焚烧进入大气沉积、降水和农业灌溉从大气和水环境中进入土壤[38],之后对土壤生态系统、团聚体的平均质量直径、光合作用和土壤结构等方面产生影响[39].
-
磁性纳米铁通过废物处理、空中沉积或污水处理厂等直接或间接处理方法进入土壤,因此需要了解磁性纳米铁与土壤之间的相互作用以及对土壤理化性质的影响[40],天然纳米颗粒如铁氧化物、黏土、有机质和其他矿物,是陆地生态系统进行生化过程的重要组成部分[41 − 42]. 磁性纳米铁的进入可能通过改变天然纳米颗粒的分布而影响土壤的发育或行为,从而影响生态系统[43]. 此外,磁性纳米铁的存在也会改变土壤性质,Sun等将Fe3O4-NPs应用于低钙寒农田,结果表明,Fe3O4-NPs降低了团聚体的平均质量直径,并且增加了土壤容重和土壤体积含水量. 此外,增加Fe3O4-NPs含量也会增加土壤的渗透阻力,从而使土壤的渗透阻力增大,减少根系生长,同时降低土壤饱和导水率,影响植物吸水[44 − 45]. 此外Fe3O4-NPs的积累会增加土壤中三价铁的浓度,从而使土壤pH值升高,pH的升高同样会影响植物生长. 另一方面,当土壤的水饱和时,三价铁可以转化为亚铁,亚铁参与其中叶绿素的合成,在光合作用中起着重要的作用[46].
此外,根据Yan等的研究,向土壤中释放Fe3O4-NPs会提高玉米的抗氧化能力,促进玉米的生长,其原因是Fe3O4-NPs造成土壤中叶绿素、糖、蛋白质和营养元素含量的增加以及丙二醛含量的降低,对土壤中的有机质含量产生了影响[47].
-
土壤酶是土壤中最活跃的有机成分之一[48],磁性纳米铁与土壤酶接触后,会降低部分土壤酶的活性,并最终会影响氮磷的转化、增加过氧化氢的积累、造成土壤肥力降低和对微生物产生不良影响. 此部分总结了磁性纳米铁对转化酶、脲酶、磷酸酶和过氧化氢酶等酶活性的影响,以评估磁性纳米铁对土壤酶活性的影响[49]. You等[50]用3种纳米材料(纳米氧化锌、纳米氧化铈、纳米氧化钛)与Fe3O4-NPs对土壤酶活性的影响进行比较,结果表明两种土壤微生物群落在纳米氧化锌处理后均表现出过氧化氢酶活性降低、脲酶活性升高的趋势. 此外,与对照相比,黑土中含纳米氧化锌的土壤转化酶活性显著提高,盐碱地土壤转化酶活性呈下降趋势,而在在黑土中,只有纳米氧化锌处理后显著降低了磷酸酶活性[50]. 因此,在上述几类磁性纳米铁中,纳米氧化锌对4种酶活性的影响均强于其他材料. 与含纳米氧化锌的土壤相比,含纳米氧化铈的土壤过氧化氢酶和磷酸酶活性与对照差异不显著,含纳米氧化铈的土壤的脲酶活性显著低于对照组. 纳米氧化钛和Fe3O4-NPs处理的过氧化氢酶和转化酶活性在盐碱地显著降低,而在黑土中不显著. 在不同浓度的磁性纳米铁处理下,黑土和盐碱土土壤酶活性变化一致[51].
此外,Fe2O3-NPs和Fe3O4-NPs增加土壤中转化酶和脲酶的数量,促进土壤中碳、氮的周转[52]. 然而,只有某些特定的细菌受益于这种变化,而不是整个土壤微生物种群,这是由于土壤微生物的脱氢酶没有得到增强[53].
-
通过各种途径释放到土壤中的磁性纳米铁不仅影响土壤养分循环,还影响CO2、CH4和N2O等温室气体的排放[54],并影响全球气候变化,N2O是全球变暖的重要温室气体之一[55],在100年时间尺度上其全球变暖趋势是CO2的298倍,生态系统过程在N2O排放中起着重要作用,农业系统每年排放N2O约为2.7亿t[56]. 不同类型的纳米颗粒直接影响土壤硝化和反硝化过程,并影响N2O的排放,例如磁性氧化铁纳米颗粒可显著影响土壤的硝化电位,从而降低土壤中N2O的排放量,硝化菌和反硝化菌对纳米颗粒的响应随纳米颗粒类型和土壤类型的不同而不同[57](如图2所示).
Fe2O3-NPs由于其所具有的磁性从而对土壤呼吸产生影响,在Fe2O3-NPs磁性的辐射作用下,土壤微生物增强对土壤有机碳的分解能力,增强了土壤的呼吸作用,最终导致土壤排放量增加[58],并且会通过影响土壤中的电子迁移过程来抑制CH4的排放,此外,Fe2O3-NPs的磁性会对土壤微生物的活性有激活作用,并且对土壤呼吸产生影响,最终导致土壤中CO2的排放量增加[59].
YANG等[60]的研究发现,Fe3O4-NPs会促进土壤中一种高度富集的丁酸盐氧化联合体的富集转移过程,在Fe3O4-NPs的作用下会持续增强丁酸盐氧化和CH4的生成,添加碳纳米管替代Fe3O4-NPs也产生了类似的刺激效果,而Fe3O4-NPs表面的二氧化硅涂层则完全消除了这种刺激作用,说明Fe3O4-NPs的导电性在促进共氧化过程释放CH4中起着关键作用[60].
-
微生物在地球化学循环中发挥着重要作用,对土壤功能,特别是有机质分解和养分循环至关重要;因此,它们在调节植物生产力、群落动态和土壤形成中都很重要[61]. 微生物通过其代谢活性参与土壤无机组分的转化、移动和固定化,一个表面为1 Ha、度为30 cm的土壤可能总共含有10 mg的细菌和10 mg的真菌[62],这种微生物群落在维持土壤功能和陆地生态系统方面发挥着重要作用[63]. 它们也是污染物降解过程中的主要参与者,如有机质矿化,促进生态系统中的养分循环,从而促进土壤肥力[64]. 微生物之间以及微生物与高等生物之间也保持着关键的共生和致病关系[65],由于其关键而广泛的作用,土壤微生物的代谢活动已被用作评估土壤中人为活动释放的污染物(如金属和农药)的影响的测量端点. 这些测量值通常被用于生态风险评估[66].
-
Fe2O3-NPs和Fe3O4-NPs对细菌群落的影响可能归因于纳米颗粒的两种特性及其对微生物代谢的促进作用[67],由于其微小的尺寸和稳定性,Fe2O3-NPs和Fe3O4-NPs极容易运输到土壤中[68],提高了土壤表面积与体积之比,因此相对于块状材料,纳米颗粒更容易部分分解和释放离子[69]. 此外,纳米颗粒具有最活跃的表面位点(主要是Fe2O3-NPs上的Fe-OH位点,能够与天然有机化合物结合)[70]. 例如,在土壤中的有机化合物,如腐殖酸和黄腐酸的辅助下,磁性纳米铁的添加可以增强铁对土壤细菌的生物有效性. 腐植酸是动植物残基在理化和微生物降解过程中形成的,在自然系统中含量丰富,它具有由烷基和芳香族单元组成的骨架,与羧酸、酚羟基和醌官能团结合,可与磁性纳米铁表面产生很强的亲和力[71]. 腐殖酸对磁性纳米铁的吸收通常通过空间和静电效应的结合提高其稳定性. 此外,由于腐殖酸与氧化铁表面位之间的配体交换反应,溶解的三价铁离子从磁性纳米铁表面进入水相. 因此,土壤中的生物有效铁离子增加,进而刺激土壤中某些微生物的生长[72].
并且Fe2O3-NPs对土壤细菌群落的影响更有利,Zeng等的研究指出,大部分磁性纳米铁由于产生活性氧(ROS)而具有毒性,而化学稳定的Fe2O3-NPs没有明显的细胞毒性,含有Fe2+的纳米颗粒会导致大肠杆菌存活率的剂量依赖性下降,主要是由于氧化应激,纳米颗粒由完全氧化的晶体组成,因此在环境中高度稳定,产生氧化应激的能力较低[73]. 相比之下,Fe3O4-NPs由于其结构内电子迁移率高和Fe2+的扩散而不稳定,因此,部分磁性纳米铁的不良影响可以通过Fe3O4-NPs释放Fe2+来抵消,导致细菌群落丰富度的增强较弱,细菌群落组成的变化较小[74].
-
nZVI-NPs可以潜在地刺激细菌生长,从而可能发生土壤细菌群落结构的一些变化[75 − 76]. 采用荧光原位杂交(FISH)、变性梯度凝胶电泳(DGGE)和磷脂脂肪酸分析(PLFA)研究nZVI-NPs对微生物多样性的影响[77]. 施用nZVI-NPs 72 h后,Fajardo等观察到土壤微生物群落结构和系统发育组成发生了显著变化[78]. 荧光原位杂交分析表明,当nZVI-NPs对微生物群落施加选择性压力时,微生物多样性发生了变化,促进了某些微生物类群(古菌、α-变形菌和低G + C革兰氏阳性菌)的优势,而其他微生物类群(β-变形菌和γ-变形菌及其亚纲)的减少,添加10 g·kg−1 nZVI-NPs 28 d后的性梯度凝胶电泳图谱也表明细菌群落组成发生了显著变化. Pawlett等人的报告提到,nZVI-NPs导致所有测试的土壤质地中的磷脂脂肪酸分析剖面发生改变,但这些影响取决于土壤中的有机质含量[79]. 这些研究表明,nZVI-NPs可在短期内(4个月)显著改变土壤微生物群落结构,影响细菌、古菌和真菌的种群数量[80].
Liu等将不同浓度nZVI-NPs暴露后,观测土壤生态系统的响应,结果表明长期暴露nZVI-NPs对土壤微生物群落特征无显著影响,但可以促进蚯蚓的生长,从而进一步提高了土壤营养元素的生物有效性,为nZVI-NPs应用于污染修复与治理的环境安全性评估提供了科学依据[81].
Kasem等[82]的研究结果表明,注射羧甲基纤维素稳定的nZVI-NPs通过促进厌氧菌和脱氯菌的生长,对污染修复产生了积极的影响. 此外,这种影响是非常持久的,可能是由于羧甲基纤维素的缓慢发酵和生物质的缓慢腐烂[82]. 根据Kocur等观察到的含氯挥发性有机物降解产物,在使用注射羧甲基纤维素稳定的nZVI-NPs后,呼吸有机卤化物的微生物会得到增强[83],在两年的监测期间,含氯挥发性有机污染物在处理区域持续降解. Ahmad等的实验结果表明,nZVI-NPs会降低抑瘤性化合物和氯仿等生物可降解化合物的浓度,从而降低对微生物活性的抑制作用,由此产生的条件促进了有机卤化物呼吸微生物的丰度的增加[84]. 随着纳米技术产业的不断发展,以及其对环境所具有的潜在污染性质,纳米颗粒对自然系统的影响正成为一个日益活跃的研究领域,实验结果表明施用注射羧甲基纤维素稳定的nZVI-NPs后,微生物种群数量显著增加,其中包括负责含氯挥发性有机物降解的脱卤菌. 由于观察到这种显著的含氯挥发性有机物降解是由于两种电子供体(nZVI-NPs和羧甲基纤维素)的结合,因此需要做更多的工作来进一步评估纳米颗粒和纳米技术对环境的影响[85].
-
单一碳源利用(SCSU)系统基于对土壤微生物群落代谢特性的广泛调查,检测土壤微生物种群的功能能力,所得数据可以使用多元技术进行分析,以比较群落的代谢能力[86]. 然而,由于土壤微生物群落是由快速生长和缓慢生长的有机体组成的,因此生长缓慢的有机体可能不包括在本分析中[87]. SCSU的优势包括其区分微生物群落的能力强、操作简便、可重复性和大量描述群落代谢特征数据的生成[88].
微量热法是通过微量热仪对代谢过程的热效应进行实时、高频、精确的监测、绘制微量热曲线,展示土壤微生物稳定期及对数生长期的热散逸[89],再通过模型拟合,可分析获得微生物生物量,微生物群落的潜在最大代谢活性、响应时间、比生长速率、周转速率,活性微生物比例等参数,用于表征微生物群落的代谢特征[90 − 91].
-
磁性纳米铁会直接作用或通过废水排放等间接方式作用于土壤微生物群落,许多磁性纳米铁对微生物具有杀伤特性,从而进一步抑制土壤微生物群落的代谢活动,如固氮、氨化、反硝化、溶磷和其他促进微生物生长的活动[92]. 但是某些磁性纳米铁在低浓度下会促进某些微生物代谢过程,从而促进微生物生长,对土壤微生物群落的数量及丰富度产生影响[93]. 例如,Rajput等过研究对纳米银和Fe2O3-NPs对土壤微生物硝化作用的影响,发现Fe2O3-NPs能促进土壤微生物的硝化作用,而纳米银则抑制微生物硝化作用,且存在剂量效应[94 − 95]. 当浓度增大时,氨氮不易转化,氨氧化较弱,而后通过实时定量PCR发现,纳米银降低了对土壤中微生物数量的影响,而Fe2O3-NPs的影响不显著,然后通过分析土壤中氨氧化细菌和古菌,发现随着纳米银浓度的增加,土壤中氨氧化细菌和古菌数量显著减少[96 − 97],Fe2O3-NPs增加了土壤中氨氧化细菌的数量[98]. 通过微热仪表征土壤微生物的代谢特征,发现纳米氧化银对土壤微生物的代谢活动有负作用,且表现为剂量效应,即随着纳米银浓度的增加,负作用越大. 相反,Fe2O3-NPs对微生物代谢有正向作用,且随着浓度的增加而增加[99],此外土壤中有机卤化物呼吸微生物在Fe2O3-NPs的刺激下,在提高对含氯挥发性有机污染物降解的同时,微生物丰富度也会发生变化(如图3).
-
环境中的各种因素(如天然有机物(NOM)、酸碱度(pH)、离子(IONS)和背景离子)可以改变磁性纳米铁和测试生物的表面性质,从而改变磁性纳米铁对土壤微生物代谢的影响. 溶液pH和IS在磁性纳米铁和藻类细胞的异质团聚中发挥重要作用,这有助于稀释纳米颗粒的细胞毒性[100]. Ranmadugala等报道了Ca2+在影响nZVI-NPs对大肠杆菌的毒性方面的双重作用. 一方面,Ca2+的存在可以通过增强nZVI-NPs的聚集和沉淀来抑制nZVI-NPs的杀菌作用;另一方面,Ca2+可能作为桥梁促进nZVI-NPs对细菌的粘附,从而造成更严重的影响[101]. 相对高浓度的Ca2+(40 mg·L−1)甚至可以将带负电荷的nZVI-NPs转化为带正电荷的nZVI-NPs,使颗粒更有利于吸附带负电荷的大肠杆菌. 每种生物都有特定的适宜温度,光的进入对某些生物,特别是藻类和植物的生长至关重要. 环境中营养物质的可获得性也是一个决定性因素,一般来说,充足的营养物质的供应可以提高生物体对磁性纳米铁的耐受性. 基质类型(水、土壤或沉积物)是控制纳米颗粒毒性的重要因素. 组分尤其是有机质和黏土会强烈影响磁性纳米铁的流动性和有效性,导致磁性纳米铁在土壤或沉积物中的毒性低于水体. 在现实环境中,生物接触到的是转化后的磁性纳米铁,因此,在环境相关的测试条件下,磁性纳米铁对微生物代谢影响的研究备受关注,这使我们能够更好地了解其生态危害,尽可能减轻其对土壤微生物群落的不良影响[102].
-
随着磁性纳米铁应用的日益广泛,人们对其在环境中发展潜力和潜在影响进行了研究,磁性纳米铁通过多种方式释放到土壤中后,与土壤中的生物化学成分及微生物相互作用,最终对土壤理化性质以及微生物群落自身及代谢产生影响. 磁性纳米铁进入土壤后,会对土壤容重、土壤体积含水量、渗透阻力以及有机物质含量等产生影响,且在不同类型的土壤中,磁性纳米铁对土壤酶活的影响不同. 此外,磁性纳米铁通过改变土壤的硝化电位、微生物对有机碳的分解能力及电子迁移来改变土壤温室气体排放量. 磁性纳米铁对微生物群落的影响则体现在刺激土壤微生物的生长、改变微生物群落的结构与数量以及对微生物代谢的影响. 未来的研究方向可从以下几个方面开展:
(1)磁性纳米铁在环境领域和农业上的应用使得它通过主动或被动的方式进入土壤环境,在最大限度地减少或消除废物的产生和减少污染的同时,自身在土壤中的积累也会对土壤理化性质以及微生物群落的结构、功能以及代谢产生负面效应;因此今后除了加强农业及环境领域应用的同时,还需要考虑自身积聚所带来的负面生态效应,寻求用最适合的用量来达到最优的效果.
(2)重金属等有害有毒污染物(如铅、镉、汞等)和有机化合物(如苯、二甲苯、甲苯溶剂和农药)农药是环境中的整治目标,通过共价键、马来酰亚胺、配体交换,在磁性纳米铁表面设计并引入功能和活性位点,可提高磁性纳米铁的反应性和与重金属等污染物的反应程度,应用前景广阔,因为通过这些途径功能化的磁性纳米铁吸附剂用于污染物去除的研究十分匮乏.
(3)在研究磁性纳米铁对土壤组成结构及微生物群落代谢特征的影响的同时,以微生物为基础合成磁性纳米铁的研究取得了重大进展,然而,磁性纳米铁生物合成过程的改进和应用还需要进行大量的研究. 与物理和化学方法相比,微生物合成纳米颗粒的过程相对较慢,在合成中不建议使用病原微生物,因为它可能限制所产生的磁性纳米铁在涉及人类或动物接触的应用中使用,制备的磁性纳米铁应对生物和环境无毒,因此对利用微生物合成磁性纳米铁进行毒性研究至关重要,对能够产生稳定磁性纳米铁的微生物的研究有助于开发可靠的生产工艺,促进磁性纳米铁的发展.
磁性纳米铁对土壤理化性质及微生物群落和代谢的影响研究进展
Effects of magnetic nano - iron on soil physicochemical properties and microbial community and metabolism
-
摘要: 近年来,磁性纳米铁因其独特性能在生物医学、农业、工业、生命科学及环境保护领域广泛应用. 随着磁性纳米铁的大范围应用,其对环境和人体健康将带来的潜在影响,已经引起科学界的广泛关注. 本文系统总结了近年来磁性纳米铁的相关文献资料,探讨了磁性纳米铁的合成方法、表征及环境应用;我们重点强调了磁性纳米铁对土壤理化性质及微生物群落和代谢的潜在影响. 最后展望了磁性纳米铁在未来的环境应用、发展及纳米毒理学亟待研究的重要问题. 本文旨在更加全面地揭示磁性纳米铁的环境可持续性,为其安全使用和环境功能化应用提供一定的参考.Abstract: In recent years, magnetic nano-iron has been widely used in biomedicine, agriculture, industry, life science and environmental protection due to its unique properties. With the wide application of magnetic nano-iron, their potential impact on the environment and human health has attracted wide attention from the scientific community. In this paper, we systematically summarized the relevant literature of magnetic nano- iron in recent years, and also discussed the synthesis method, characterization and environmental application of magnetic iron nanoparticles. We highlight the potential effects of magnetic nano-iron on the soil physicochemical properties, microbial communities and metabolism. Finally, we prospect the important problems about the environmental application, development and nanotoxicology of magnetic nano-iron in the future . This paper aims to comprehensively reveal the environmental sustainability of magnetic nano-iron, and provide some references for its safe use and environmental functional applications.
-
Key words:
- smagnetic-nano iron /
- soil /
- environmental implication /
- microbiological population /
- metabolism.
-
全氟和多氟化合物(per- and polyfluoroalkyl substances,PFASs)是一类氢原子完全或部分被氟取代的人工合成有机化合物[1]. 由于其独特的物理化学性质,PFASs在工业和消费品中具有多种用途,例如纺织品、食品包装、化妆品、和消防泡沫等[2]. 然而,接触PFASs可能会造成激素分泌、生殖、免疫、代谢以及胎儿生长发育等方面的毒理作用[3]. 根据全球许多研究,PFASs已在地表水、土壤、沉积物、动物、空气、灰尘等基质中被发现[4],它们在各环境介质中的普遍存在引起了越来越多的关注. PFASs的环境持久性和生物累积性导致其最终在动物、植物和人体内蓄积. 太湖作为长三角地区的重要水源地,其污染状况一直备受关注. 太湖流域包括江苏、浙江等几个人口密集、工业发达的地区,PFASs生产及纺织处理、金属电镀、消防、半导体等相关产业分布密集[5-6],PFASs生产使用量及其环境排放潜力较大. 目前已经有少量研究报道了太湖地表水和沉积物中PFASs的污染特征[7-10],但其关注的目标PFASs化合物种类较少,尤其是关于一些新型PFASs还鲜有报道. 目前,国内外持久性有机污染物(POPs)履约行动力度逐渐加强,对一些库存PFASs例如全氟辛基磺酸(PFOS)等使用、生产的限制管控和替代不断强化,世界范围内的许多区域出现了PFOS等管控目标污染水平的逐年降低,也伴随着各种新型短链以及杂原子取代型PFASs环境污染水平的逐渐升高. 因此在环境监测中将更多的新型PFASs纳入分析目标更有利于对PFASs污染的全面准确认识.
梅梁湾是太湖污染最严重的地区[9-10]. 因此,本研究选取靠近无锡市的太湖梅梁湾为研究区域,设置5个湖体采样点和3个河流入湖口采样点,采集地表水和沉积物样品,对其中的包括多种新型PFASs在内的32种PFASs目标物进行分析测定. 在此基础上研究PFASs组成特征和PFASs在地表水和沉积物两相之间的分配,并从生态和人体健康两方面对PFASs进行了风险评估,以期全面了解该区域PFASs的污染状况和风险,为污染预防管控和治理提供基础数据.
1. 实验部分(Experimental section)
1.1 试剂和材料
甲醇(色谱纯)购自德国Merck公司;乙酸铵(色谱纯)购自美国Thermo Fisher Scientific公司;氨水(色谱纯,~50% V/V)和冰醋酸(色谱纯,>99.8%)均购自美国Alfa Aesar公司;超纯水(电阻率>18.2 MΩ·cm)经Milli-Q(美国,Milipore公司)系统制得. 弱阴离子交换吸附剂填料的Oasis WAX固相萃取小柱(150 mg,6 cm3)购自美国Waters公司.
包含30种PFASs的PFAC30PAR标准品和10:2氯代多氟醚磺酸(10:2 Cl-PFESA)标准品以及质量标记的内标液MPFAC-MXA(包括13C4-PFBA、13C4-PFOA、13C2-PFDA、13C2-PFDoDA、18O2-PFHxS和13C4-PFOS)均购自惠灵顿实验室(Guelph,ON,Canada). 全氟壬烯氧基苯磺酸(OBS)标准品是从凯尔氟新材料有限公司(Kaierfu New Material Co.,Ltd.)的商业产品(95%,工业级)中纯化而来的. 32种PFASs混标储备液(100 ng·mL−1)采用甲醇配制,置于4 ℃的冰箱中保存.
1.2 样品采集和前处理
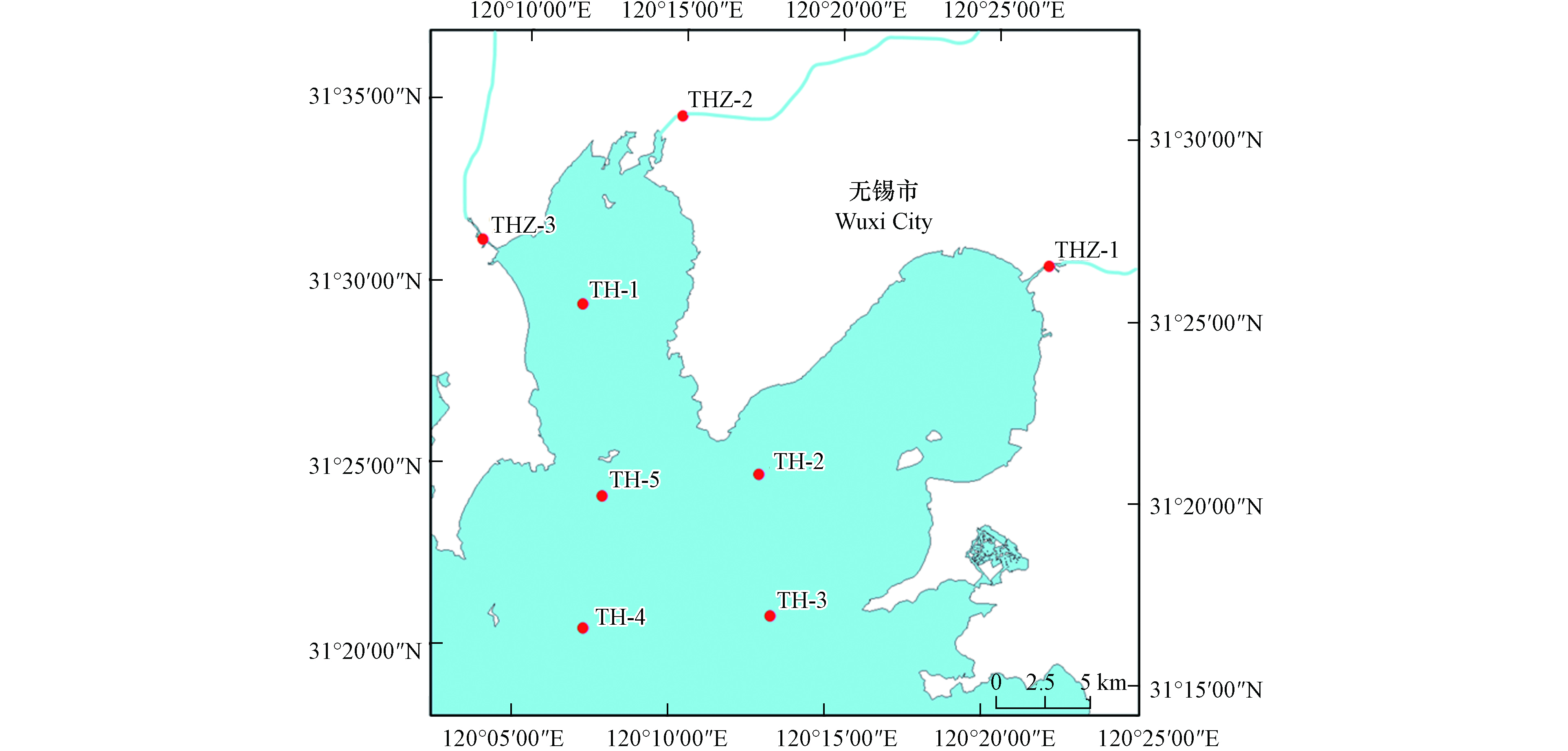
2021年6月,在江苏省无锡市太湖梅梁湾布设了5个湖体采样点(TH1—5),以及3个无锡市主要河流入湖口采样点(THZ1—3,望虞河、梁溪河、直湖港河)(如图1所示),采集了地表水和沉积物样品. 水样使用不锈钢水桶采集,沉积物则采用不锈钢抓泥斗. 所有样品均收集在聚丙烯(PP)采样瓶中,寄到实验室前均暂存于冰箱,两天内运送到实验室,水样置于4 ℃的冰箱中冷藏保存,沉积物样品在−20 ℃下冷冻保存直至处理.
水样经0.7 μm玻璃纤维滤膜抽滤后,准确量取400 mL,加入2 ng的MPFAC-MXA,然后经固相萃取净化处理. 固相萃取小柱Oasis WAX依次用4 mL 1%的氨水-甲醇溶液、4 mL甲醇和8 mL超纯水进行活化. 之后上样,该过程流速控制在3 mL·min−1. 上样完成后依次加入4 mL 2.5 mmol·L−1的醋酸铵-冰醋酸缓冲液和8 mL超纯水清洗WAX小柱. 待清洗液流干后,WAX小柱真空泵抽干1.5 h,最后用4 mL甲醇和4 mL 0.1%的氨水-甲醇溶液洗脱待测物,将合并后的洗脱液氮吹定容至1 mL,以4500 r·min−1的转速离心10 min后,取上清液转移至进样小瓶待测.
沉积物经真空冷冻干燥后,研磨过筛. 准确称取0.5 g沉积物置于15 mL聚丙烯离心管,加入2 ng 的MPFAC-MXA,充分涡旋振荡混匀后老化30 min. 加入10 mL甲醇,50 ℃水浴超声提取30 min,混匀后用摇床以250 r·min−1速度振荡提取16 h,离心(4500 r·min−1)后收集上清液;残留部分加入5 mL甲醇进行二次提取,摇床30 min(250 r·min−1),离心10 min(4500 r·min−1),合并上清液. 氮吹至约1 mL,离心(4500 r·min−1)10 min,取上清液加超纯水稀释至50 mL,用活化好的WAX小柱富集净化(净化过程与水样一致),洗脱液氮吹定容至1 mL,离心(4500 r·min−1)后取上清液进样分析.
1.3 仪器分析
样品分析采用配有二元梯度泵、自动进样器和柱温箱的高效液相色谱HPLC(Ultimate 3000,Thermo Fisher Scientific)与配有ESI源的三重四极杆质谱(API 3200,Applied Biosystems/MDS SCIEX)串联仪器进行. 使用Acclaim 120 C18色谱柱(5 μm,4.6 mm×150 mm,Thermo Fisher Scientific,USA)分离PFASs,柱温25 ℃. 流动相A为甲醇,B为50 mmol·L−1乙酸铵,梯度洗脱程序为:0 min流动相A比例为72%,4 min内增加到95%,在此状态下保持3 min,最后在0.1 min内恢复到72%,并维持2.9 min,分析总时长为10 min. 流速为1 mL·min−1,进样量为10 μL. 质谱在ESI负离子模式下使用多反应监测(MRM)模式运行,气帘气压20 psi,碰撞气压6 psi,离子源喷雾电压−1000 V,温度350 ℃,雾化气60 psi,辅助雾化气40 psi,PFASs详细的质谱参数和离子对见表1.
表 1 PFASs的离子对和质谱参数Table 1. Mass transitions and parameters of mass spectrometry化合物Compound 名称 Name 母离子Q1 子离子Q3 解簇电压/ VDP 入口电压/ VEP 碰撞入口电压/ VCEP 碰撞能量/ eVCE 碰撞出口电压/ VCXP PFCAs 全氟烷基羧酸 PFBA 全氟丁酸 212.8 168.8 −15.5 −10.0 −15.0 −16.2 −4.7 PFPeA 全氟戊酸 262.8 218.9 −21.5 −10.0 −19.2 −15.5 −5.8 PFHxA 全氟己酸 312.8 269.0 −18.7 −10.0 −19.5 −15.6 −7.8 PFHpA 全氟庚酸 362.8 319.0 −23.0 −10.0 −20.0 −13.0 −9.0 PFOA 全氟辛酸 412.8 369.0* −16.5 −10.0 −21.7 −15.2 −10.4 412.8 168.8 −20.6 −10.0 −40.0 −24.5 −4.4 PFNA 全氟壬酸 462.8 419.1 −31.4 −10.0 −43.2 −19.1 −12.1 PFDA 全氟癸酸 512.8 469.1 −24.4 −10.0 −37.0 −24.0 −7.4 PFUnDA 全氟十一酸 562.8 519.1 −23.4 −10.0 −50.2 −30.0 −8.5 PFDoDA 全氟十二酸 612.8 569.0 −24.0 −10.0 −40.3 −25.6 −9.2 PFTrDA 全氟十三酸 662.8 619.0 −33.4 −10.0 −40.7 −24.0 −10.3 PFTeDA 全氟十四酸 712.8 669.0 −52.9 −10.0 −38.1 −21.1 −11.2 PFSAs 全氟烷基磺酸 PFBS 全氟丁基磺酸 298.8 79.9* −71.2 −10.0 −29.8 −51.2 −3.5 298.8 99.0 −70.2 −10.0 −31.0 −40.0 −5.1 PFPeS 全氟戊基磺酸 348.8 79.9* −72.0 −10.0 −26.4 −63.0 −3.4 348.8 99.0 −72.8 −10.0 −93.3 −42.5 −5.0 PFHxS 全氟己基磺酸 398.8 79.9* −75.3 −10.0 −44.2 −66.0 −3.1 398.8 99.0 −72.6 −10.0 −30.2 −51.4 −2.0 PFHpS 全氟庚基磺酸 448.8 79.9* −81.0 −10.0 −46.0 −75.0 −3.6 448.8 99.0 −85.0 −10.0 −40.0 −56.1 −2.0 PFOS 全氟辛基磺酸 498.8 79.9* −89.1 −10.0 −34.0 −86.1 −3.4 498.8 99.0 −82.0 −10.0 −56.1 −62.7 −2.0 PFNS 全氟壬基磺酸 548.8 79.9* −90.6 −10.0 −34.0 −92.1 −3.3 548.8 99.0 −94.6 −10.0 −31.5 −75.8 −2.0 PFDS 全氟癸基磺酸 599.3 79.9* −97.0 −10.0 −21.3 −76.0 −3.6 599.3 99.0 −98.2 −10.0 −36.1 −81.0 −2.0 Cl-PFESAs 氯代多氟醚磺酸 6:2 Cl-PFESA 6:2氯代多氟醚磺酸 530.6 351.0* −66.0 −10.0 −40.0 −41.6 −9.8 530.6 83.0 −56.0 −10.0 −38.0 −50.6 −1.6 8:2 Cl-PFESA 8:2氯代多氟醚磺酸 631.0 451.2* −31.0 −10.0 −35.5 −35.0 −7.0 631.0 83.2 −75.0 −10.0 −52.9 −53.0 −4.3 10:2 Cl-PFESA 10:2氯代多氟醚磺酸 731.1 551.1 −90.1 −10.0 −32.5 −33.6 −8.6 731.1 83.1 −91.5 −10.0 −64.6 −70.7 −1.5 FTSs 氟调聚磺酸 4:2 FTS 4:2氟调聚磺酸 326.8 81.0 −50.1 −10.0 −31.8 −46.4 −4.0 326.8 307.0 −51.5 −10.0 −22.5 −32.7 −8.9 6:2 FTS 6:2氟调聚磺酸 426.8 81.0 −54.4 −10.0 −35.2 −61.0 −4.0 426.8 407.0 −55.2 −10.0 −28.2 −39.0 −6.1 8:2 FTS 8:2氟调聚磺酸 526.9 81.0 −65.9 −10.0 −37.9 −73.2 −4.0 526.9 506.9 −71.0 −10.0 −33.9 −43.2 −15.0 FSAs 全氟磺酰胺 FBSA 全氟丁基磺酰胺 297.8 77.9 −46.7 −10.0 −23.9 −39.8 −3.8 FHxSA 全氟己基磺酰胺 397.8 77.9 −60.0 −10.0 −31.8 −47.2 −4.3 FOSA 全氟辛基磺酰胺 497.8 77.9 −74.1 −10.0 −33.9 −60.0 −4.5 N-MeFOSAA N-甲基全氟-1-辛烷磺酰胺基乙酸 569.7 419.2 −50.5 −10.0 −31.2 −31.9 −6.4 569.7 511.8 −42.1 −10.0 −29.1 −32.0 −7.9 N-EtFOSAA N-乙基全氟-1-辛烷磺酰胺基乙酸 583.8 419.2 −47.2 −10.0 −34.0 −33.0 −5.3 583.8 525.9 −44.0 −10.0 −31.2 −32.2 −8.3 NaDONA 4,8-二氧杂-3-氢-全氟壬酸 377.0 85.0* −25.0 −10.0 −27.0 −48.9 −4.9 377.0 251.0 −21.0 −10.0 −26.0 −18.8 −7.0 HFPO-DA 六氟环氧丙烷二聚体 329.0 285.0 −11.0 −10.0 −18.5 −11.8 −7.9 OBS 全氟壬烯氧基苯磺酸 603.1 172.0* −87.1 −10.0 −49.5 −52.6 −4.2 602.5 108.0 −82.2 −10.0 −41.5 −85.9 −2.5 602.5 465.0 −101.9 −10.0 −32.2 −49.0 −6.3 IS 内标 13C4−PFBA 13C标记全氟丁酸 216.9 171.9 −24.0 −10.0 −12.6 −13.5 −4.8 13C4−PFOA 13C标记全氟辛酸 416.8 372.1 −25.4 −10.0 −20.7 −13.5 −5.5 13C2−PFDA 13C标记全氟癸酸 515.0 470.0 −22.3 −7.0 −26.2 −18.2 −13.2 13C2−PFDoDA 13C标记全氟十二酸 614.8 570.0 −25.6 −10.0 −33.1 −22.0 −9.4 18O2−PFHxS 18O标记全氟己基磺酸 403.0 102.9 −81.2 −9.0 −40.9 −48.0 −2.5 13C4−PFOS 13C标记全氟辛基磺酸 502.8 79.9 −91.8 −10.0 −44.4 −78.3 −3.6 *定量离子quantification ion. 1.4 质量保证与控制
在整个实验过程中,完全避免使用含PFASs材料制成的容器和试剂. 在实验前,对所有使用的实验用品和试剂进行分析,确保其浓度始终低于根据信噪比(S/N)为3确定的仪器检出限,方法检出限(LOD)按照400 mL水,0.5 g沉积物计算. 另外,在每10个样品中加入程序空白,检查背景污染;并在测定20个样品后测定标准质控,检查信号漂移. 使用内标法对目标物进行定量,标准曲线系列浓度为0.05、0.1、0.2、0.5、1.0、2.0、5.0、10、20、50 ng·mL−1. 使用1/x2加权回归,所有PFASs目标物的相关系数均>0.99. 分析过程中如果标准品测定值与其真实值的偏差超过±20%,须重新构建标准曲线. 进行加标回收实验以评估整个方法的准确性和精密度,将PFASs标准品加入到地表水和沉积物样品中并采用与实际样品相同的提取和净化步骤进行处理. 大部分PFASs的平均基质加标回收率(n=3)在80%至120%之间(表2).
表 2 PFASs的加标回收率和检出限(LODs)Table 2. The spike recoveries and limits of detection (LODs) of PFASs化合物Compound 地表水Water 沉积物Sediment 回收率/%Recovery 相对标准偏差/%RSD 方法检出限/(ng·L−1)LOD 回收率/%Recovery 相对标准偏差/%RSD 方法检出限/ (ng·g−1)LOD PFBA 92.9±6.9 7.4 0.23 98.3±4.4 4.5 0.09 PFPeA 101.0±8.5 8.4 0.14 92.9±7.8 8.3 0.06 PFHxA 101.7±9.5 9.3 0.09 101.3±9.3 9.1 0.03 PFHpA 110.5±8.5 7.6 0.12 98.3±8.1 8.2 0.05 PFOA 104.7±10.5 9.9 0.36 92.0±7.3 7.0 0.14 PFNA 103.4±7.7 7.4 0.18 100.1±10 10.0 0.07 PFDA 102.9±9.5 9.3 0.17 109.9±6.6 6.0 0.07 PFUnDA 92.2±7.2 7.6 0.18 91.5±7.4 8.1 0.07 PFDoDA 95.2±12.3 12.8 0.20 92.3±4.1 4.4 0.08 PFTrDA 97.5±9.3 9.4 0.28 96.5±10.9 11.3 0.11 PFTeDA 102.7±10.9 9.5 0.68 102.9±12.1 11.8 0.27 PFBS 105.1±12.3 11.6 0.07 102.6±4.6 4.4 0.03 PFPeS 109.6±9.1 8.3 0.09 98.3±3.0 3.0 0.04 PFHxS 99.0±9.6 9.7 0.08 93.7±4.5 4.8 0.03 PFHpS 99.1±8.7 8.7 0.06 95.1±3.9 4.1 0.03 PFOS 99.2±8.7 7.3 0.07 99.6±7.1 6.3 0.03 PFNS 89.1±7.7 8.4 0.07 98.4±4.8 4.9 0.03 PFDS 80.7±4.1 5.1 1.36 94.2±5.5 5.7 0.55 6:2 Cl-PFESA 86.9±6.1 7.0 0.20 96.8±4.5 4.5 0.08 8:2 Cl-PFESA 75.4±6.1 8.0 0.12 84.1±8.8 10.5 0.05 10:2 Cl-PFESA 78.3±7.5 9.5 0.27 77.3±4.6 5.6 0.11 4:2 FTS 154.3±11 6.9 0.79 94.1±8.5 7.3 0.32 6:2 FTS 124.7±15 11.7 0.50 119.9±8.5 6.9 0.20 8:2 FTS 115.3±3.4 2.9 0.28 112.6±6.0 5.1 0.11 FBSA 90.8±6.9 7.6 0.05 82.2±3.4 4.2 0.02 FHxSA 78.4±2.1 2.7 0.03 72.6±5.1 7.1 0.01 FOSA 97.0±8.5 8.8 0.03 78.7±3.1 3.9 0.01 NaDONA 104.6±7.5 7.2 0.19 96.0±4.3 4.5 0.08 HFPO-DA 107.9±7.8 7.3 1.58 70.9±3.7 4.4 0.63 N-MeFOSAA 92.0±9.0 9.8 0.68 108.8±7 6.4 0.27 N-EtFOSAA 100.3±10.6 10.5 0.71 104.8±2.0 1.9 0.29 OBS 100.9±4.5 4.4 0.36 131.0±4.9 3.7 0.14 1.5 数据处理
沉积物-水分配系数(Kd,单位L·kg−1)常用于评估污染物在沉积物中的吸附能力[11],计算公式如下:
Kd=Csediment/Cwater×1000 其中,Csediment代表沉积物中目标物浓度(ng·g−1干重),Cwater代表水样目标物浓度(ng·L−1).
进一步地,沉积物有机碳归一化分配系数(Koc,单位L·kg−1)计算如下[11]:
Koc=Kd/foc 其中,foc为沉积物有机质含量(%),采用配备有固体附件SSM-5000A的TOC分析仪(Shimadzu,Japan)测定.
文献中常用用风险商(RQs)来评价化合物对水生生物的潜在影响[12-13],计算公式如下所示:
RQ=MEC/PNEC 这里MEC是地表水中所测得的化合物的最大浓度(ng·L−1);PNEC是预测的化合物的无效应浓度(ng·L−1),其数值按下面公式进行计算:
PNEC=(EC50orLC50)/f 其中,最低中位数有效浓度EC50或LC50(mg·L−1)基于模型操作(ECOSAR™软件,EPA)得出[13];f是安全系数,根据欧洲水框架指令, f一般取1000. RQ小于0.1表示化合物对水生生物风险较低,在0.1到1之间属于中等程度风险,大于1表示风险较高.
太湖作为重要的饮用水水源地,且采样点TH-2为无锡市自来水厂沙渚取水口,饮用水摄入是附近居民暴露PFASs的可能途径,参照先前报道[10, 12],以湖水PFASs浓度代替当地居民饮用水浓度,评估人群通过饮用水对PFASs的单位体重日摄入量(EDI)(以体重计,ng·(kg·d)−1),计算公式如下[12]:
EDI=(C×IR)/BW 其中,C为目标PFASs的平均浓度(ng·L−1),IR为每天饮用水摄入量(L·d−1),BW为平均体重(kg). IR和BW的成人和儿童数据引自中国人群暴露参数手册[14].
采用IBM PASW统计18.0(SPSS Inc.,1993—2007)进行统计分析,统计显著性阈值为P<0.05. 低于LOD的值被指定为LOD/2. 沉积物的浓度均为干重(dw)浓度.
2. 结果与讨论 (Results and discussion)
2.1 地表水中PFASs的污染特征
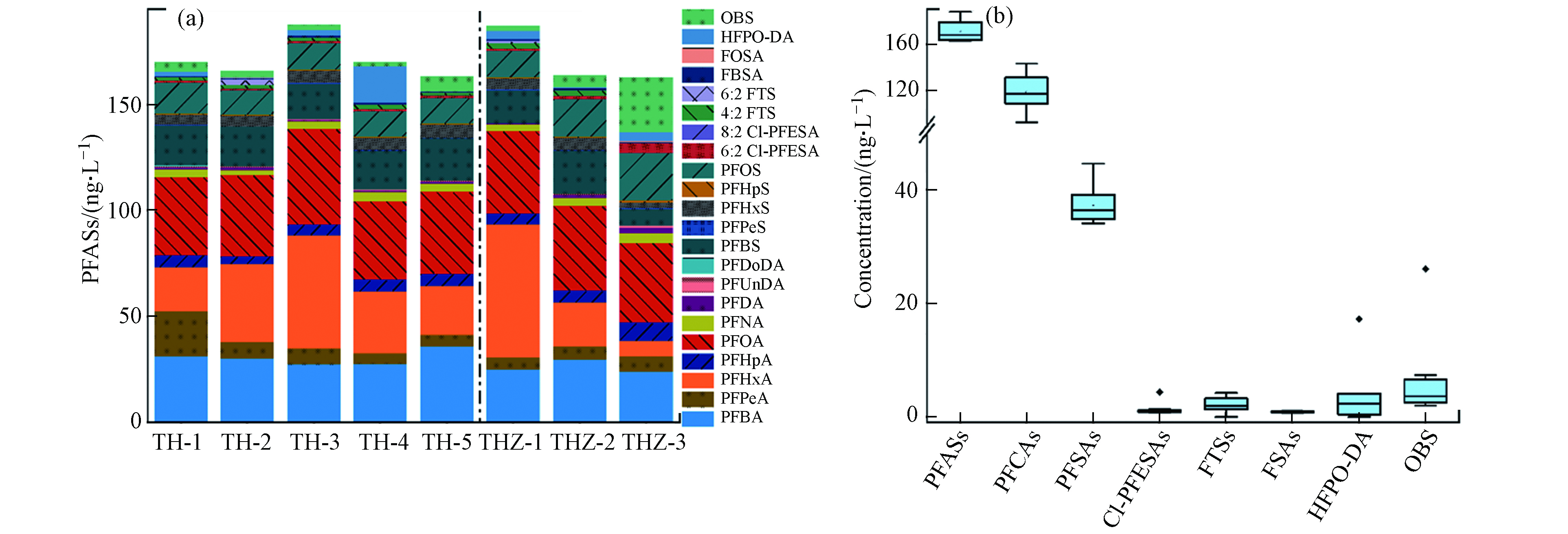
太湖梅梁湾地表水中共检出22种PFASs,其中15种PFASs的检出率为100%,主要包括C4—10 PFCAs、C4—8 PFSAs、6:2 Cl-PFESA、FBSA和OBS(图2a),说明PFASs应用的广泛性和污染的普遍性,尤其是新型PFASs的广泛检出值得进一步关注. 根据文献报道,全氟丁基磺酰胺(FBSA)是全氟丁基磺酸(PFBS)的前体物,它可能来自新的全氟丁基类替代品或其他短链全氟烷基类产品[15]. 然而,FBSA在环境中并不常见,此次在太湖梅梁湾地表水中检出浓度范围为0.65—1.05 ng·L−1(平均值0.82),可能与当地的食品包装、纺织、农药等[6]工业生产有关. 另外,OBS和Cl-PFESA是两种被忽视40余年的新型PFASs,在PFOS被公约限制后被认为是其主要替代品. 其中OBS在中国作为消防泡沫助剂和石油工业采油助剂被大量使用,其已经被证明具有与PFOS相似的急性毒性[16]和生物蓄积性[17]. 因此,这种新型PFAS在太湖梅梁湾中的普遍检出(2.01—26.06 ng·L−1,平均值6.67)需引起重视. 6:2 Cl-PFESA主要应用于金属电镀领域,虽然其检出浓度(范围0.73—4.25 ng·L−1,平均值1.38)并不高,但由于具有很强的生物蓄积性[18],所以其对水生生物和当地人群的影响不可忽略.
水样中∑PFASs范围为162.82—187.62 ng·L−1(平均值171.34,中位值167.82),此浓度与之前测得的∑PFASs:96.2—330 ng·L−1(平均值147)相当[7, 9]. 与其他湖泊相比,太湖梅梁湾∑PFASs要高于同位于华东地区的淡水湖—巢湖(平均值14.46 ng·L−1)[19],以及北京城区地表水(∑PFASs范围2.88—309.23 ng·L−1,平均值46.14)[13],同样高于北美五大湖[20],但远低于位于武汉氟化学工业生产基地附近的汤逊湖(∑PFASs范围4570—11890 ng·L−1,平均值9850)[21].
通过浓度组成分布(图2a)可以发现,地表水中以传统PFASs为主,主要是中短链PFCAs和PFSAs,其具有较高的水溶性和解离度,更容易赋存在水中,所以在地表水中占比大. 所有目标PFASs中,以平均值计算,太湖梅梁湾地表水中主要化合物浓度和贡献分别为:PFOA(39.13 ng·L−1,23%)、PFHxA(31.71 ng·L−1,19%)、PFBA(28.80 ng·L−1,17%)、PFBS(16.93 ng·L−1,10%)和PFOS(14.49 ng·L−1,8%). ∑PFCAs和∑PFSAs两者之和占比约为90%,而且∑PFCAs始终高于∑PFSAs浓度(图2b),说明PFCAs相比于PFSAs亲水性更强. 地表水中均以中短链PFASs为主,且C4-8 PFCAs是最主要的PFASs,这与前人研究一致[22]. 在测得的10种PFCAs同系物中,除PFHxA和PFOA外,其浓度大致随着碳链的增加而降低. 其中,PFBA、PFHxA、PFOA是最重要的PFCAs,这在一定程度上可能反映了短链PFASs对长链PFASs的替代结果.
由图1和图2a可知,太湖梅梁湾湖体和河流入湖口水样中检出的主要PFASs种类总体一致,说明这些物质可随水流迁移;其中TH-1点位PFASs组成特征与入湖口THZ-2和THZ-3相似,而TH-2和TH-3与入湖口THZ-1的组成特征相似,说明入湖河流对太湖梅梁湾湖体中PFASs污染有一定影响. 另外,采用Kruskal-Waillis检验对不同采样点之间的∑PFASs浓度差异进行统计学分析,显示入湖河流和湖体间没有显著性差异,这说明两者具有相似的污染来源;河流经过城市,纳污量大,这在一定程度上预示着人类活动对水产养殖可能存在影响. 该区域属于长三角地区,经济发达,工业密集,多种PFASs的检出与其周边完备的生产体系有很大联系. 根据以前的报道,太湖梅梁湾地区大多数PFASs直接来自于人类活动,如工业生产和家庭使用,而不是通过间接或远距离运输[23]. 另外,太湖梅梁湾本身较为封闭,所以PFASs浓度相对较高.
2.2 沉积物PFASs的污染特征
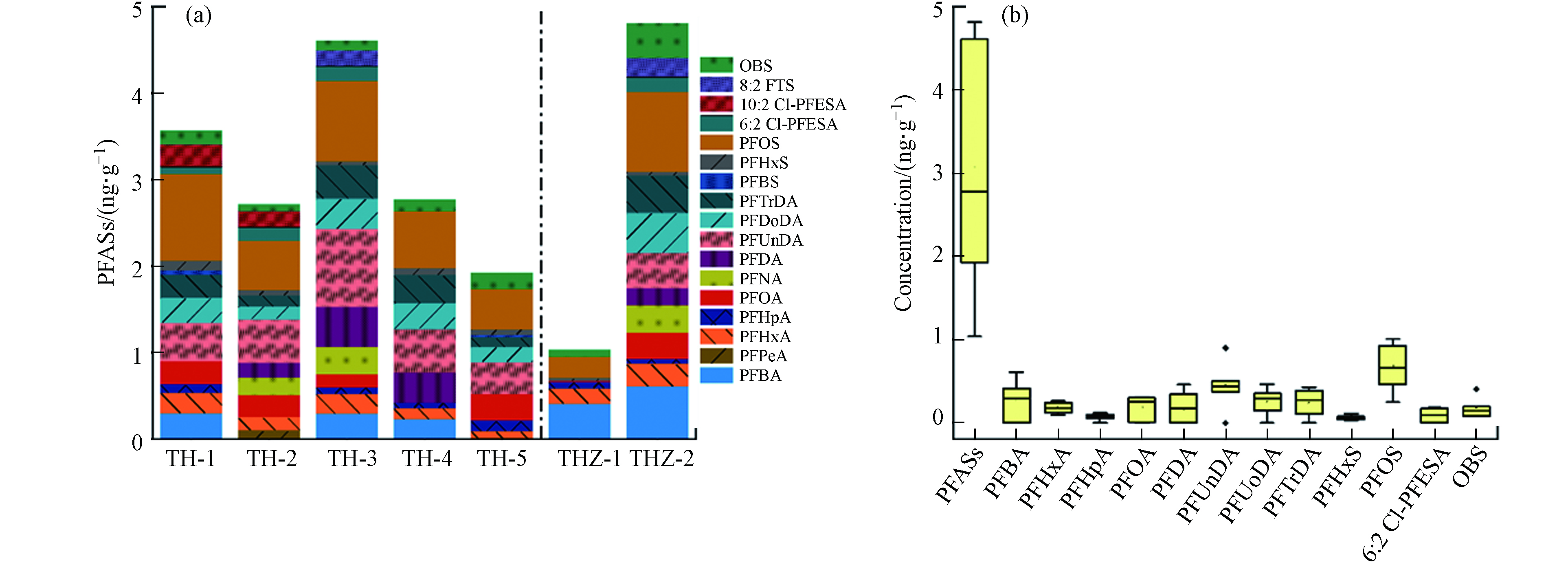
太湖梅梁湾地表水中的PFASs通过分配作用吸附在沉积物中,入湖河流与湖体沉积物中PFASs浓度水平与组成如图3a所示. 检出的17种PFASs中,只有PFHxA、PFHxS、PFOS和OBS的检出率在90%以上. 以平均值计算,太湖梅梁湾沉积物中主要化合物对∑PFASs贡献由大到小为:PFOS(21%),PFUnDA(14%),PFBA(9%),PFDoDA(8%),PFTrDA(7%),PFOA(6%),可以看出除PFBA外,其余均为中长链PFAAs,体现出其更高的亲脂性,更易于在沉积物中累积. 特别是PFDoDA和PFTrDA在地表水中未检出或浓度很低,说明相比于地表水来说,其更易吸附在沉积物中. 如图3b所示,PFOS作为沉积物中浓度最高的PFASs,其浓度范围在0.25—1.01 ng·g−1 dw(平均值0.69),与文献报道浓度相近[7]. 在太湖梅梁湾地表水中普遍检出的两种新型PFASs,OBS和6:2 Cl-PFESA在沉积物中的平均浓度分别为0.17 ng·g−1 dw和0.10 ng·g−1 dw.
总体来看,太湖梅梁湾沉积物中PFASs浓度处于较低水平(<5 ng·g−1 dw),∑PFASs浓度范围为1.04—4.81 ng·g−1 dw(平均值3.06),与之前测得太湖沉积物浓度相似:0.62—5.55 ng·g−1 dw(平均值2.15)[7],1.11—8.21 ng·g−1 dw(平均值2.42)[8]. 太湖梅梁湾∑PFASs浓度与渤海周边河流沉积物∑PFASs浓度(平均值3.34 ng·g−1 dw)持平,但高于渤海中海底沉积物∑PFASs浓度(范围0.37—4.18 ng·g−1 dw,平均值0.98 ng·g−1 dw)[11].
2.3 PFASs在沉积物-水中的分配
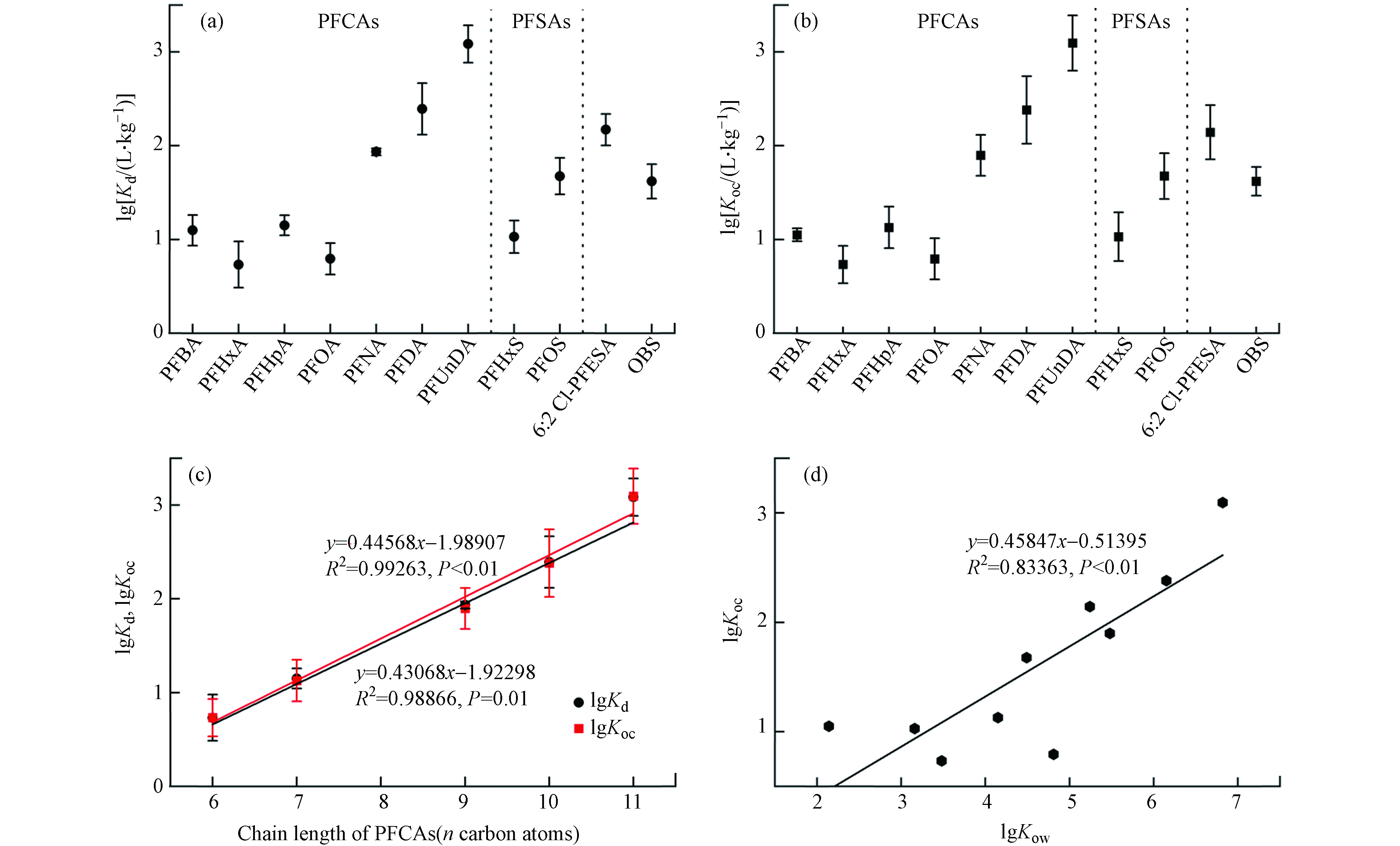
水和沉积物中PFASs的浓度低于LOD值的指定为LOD/2,计算了太湖梅梁湾两种介质中检出率高于40%的PFASs的沉积物-水分配系数(Kd)和沉积物有机碳归一化分配系数(Koc). 如图4a和b所示,太湖梅梁湾中PFOS的lg Kd(0.74—3.77)始终高于具有相同碳链长的PFOA(0.17—3.19),同样PFOS的lg Koc值也高于PFOA. 该结果与多数先前报道结果相似,即PFSAs的lg Kd值比具有相同碳数的PFCAs高0.71—0.76个对数单位[24]. 太湖梅梁湾中4种磺酸类化合物的lg Kd值顺序为:PFHxS(0.82—1.34,平均值:1.08)<OBS(1.42—1.85,1.66)<PFOS(1.29—1.86,1.70)<6:2 Cl-PFESA(1.56—2.32,2.05).
众所周知,疏水相互作用是PFASs分配到沉积物中的关键因素. 6:2 Cl-PFESA的醚键和更大的分子体积可以增加其疏水性[25],这是其Kd值高于PFOS的主要原因. 另外,OBS的Kd值与PFOS的Kd和Koc值非常接近,表明两种化合物在水和沉积物之间的分配特征相似. 相关研究表明,全氟烷基链长是影响PFASs沉积物吸附的主要因素,其lg Kd值通常随全氟碳链长度的增加而增加[26],这与图4c呈现的结果基本吻合. PFASs的Kd值在不同采样点之间的差异可能是因为Kd值受PFASs的理化性质以及多种环境因素(例如沉积物的有机碳含量、pH、水温和盐度)的影响[26]. 本研究中,PFASs的辛醇-水分配系数(Kow)来源于EPA EPIWEB 4.1,图4d中lg Koc与lg Kow呈正相关(R2 = 0.83363,P < 0.01),表明疏水性更高的PFASs能够更有效地从水相分配到沉积物中.
2.4 风险评估
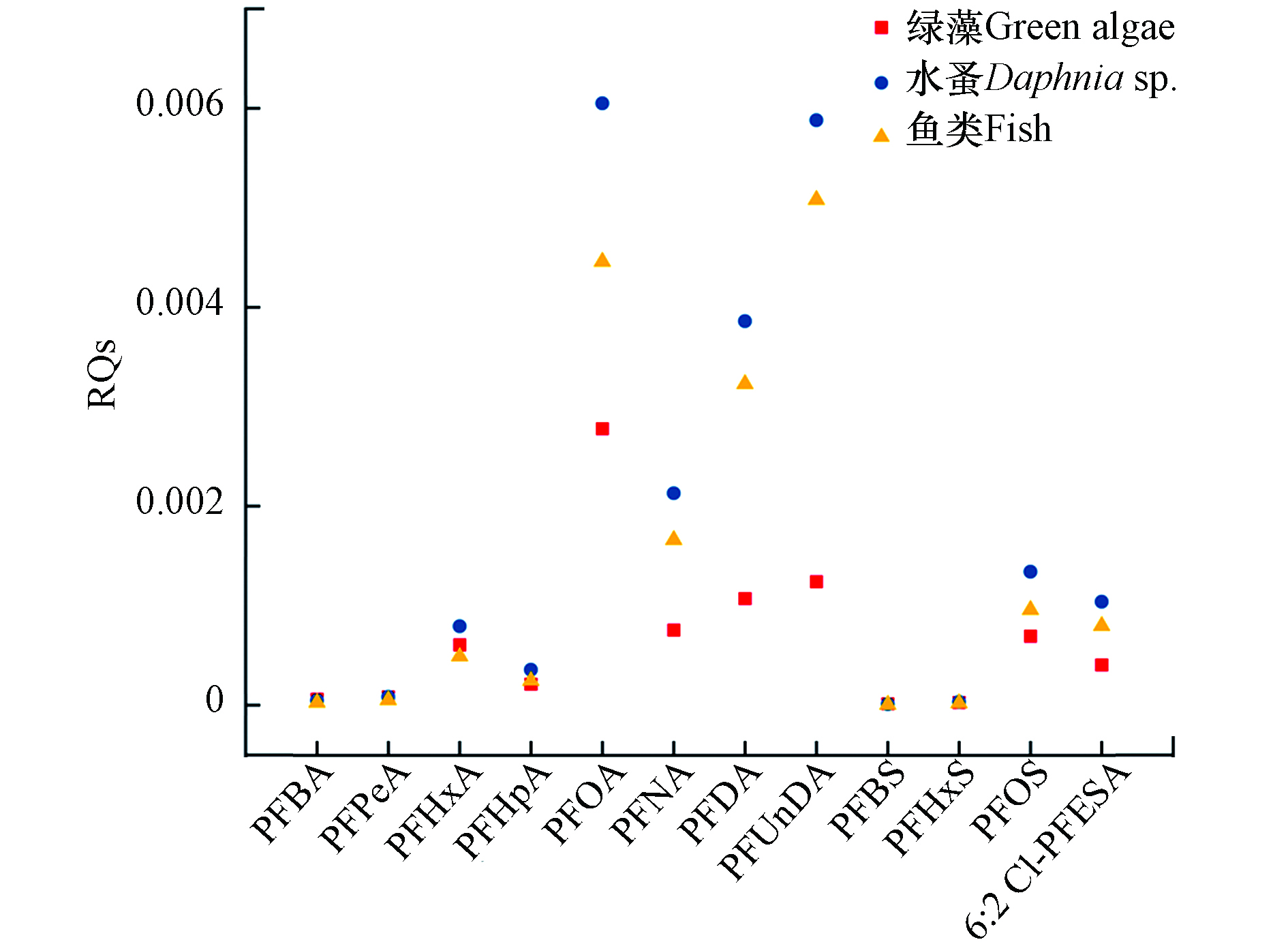
近年来大量毒理学研究报道了PFASs可对水生生物造成不良影响,本研究利用目前可获得的数据,对该区域水生环境中PFASs环境风险进行评估. 有研究提出了中国水环境中PFOA和PFOS的最大基准浓度(CMC)和持续基准浓度(CCC)[27]:CMC分别为45.54 mg·L−1和3.78 mg·L−1;CCC分别为3.52 mg·L−1和0.25 mg·L−1. 本研究中PFOA(36.80—45.05 ng·L−1)和PFOS(11.36—22.67 ng·L−1)的浓度均远低于上述值,说明太湖梅梁湾中PFOS和PFOA对水生生物风险较低. 另外,本文采用风险商(RQs)进行PFASs的生态风险评价. 选取地表水中检出率比较高的PFASs,计算了它们对于绿藻、水蚤和鱼类的RQs,发现其均低于0.1(图5),说明这些化合物对当地的水生生物风险较低. 但部分PFASs,如PFOA、PFUnDA等的RQs较高,其对水生生物的生物蓄积性和毒性仍需长期监控.
有文献[28]根据相对来源贡献推测出我国PFOA和PFOS的饮用水健康指导值分别为85 ng·L−1和47 ng·L−1;即将实施的《生活饮用水卫生标准(GB 5749—2022)》[29]规定了生活饮用水中PFOA和PFOS的限值分别为80 ng·L−1和40 ng·L−1,太湖梅梁湾水环境中PFOA和PFOS均低于上述健康指导值和国家标准. 然而,目前我国尚缺乏对许多新型PFASs的阈值和标准,因此进一步的系统研究很有必要.
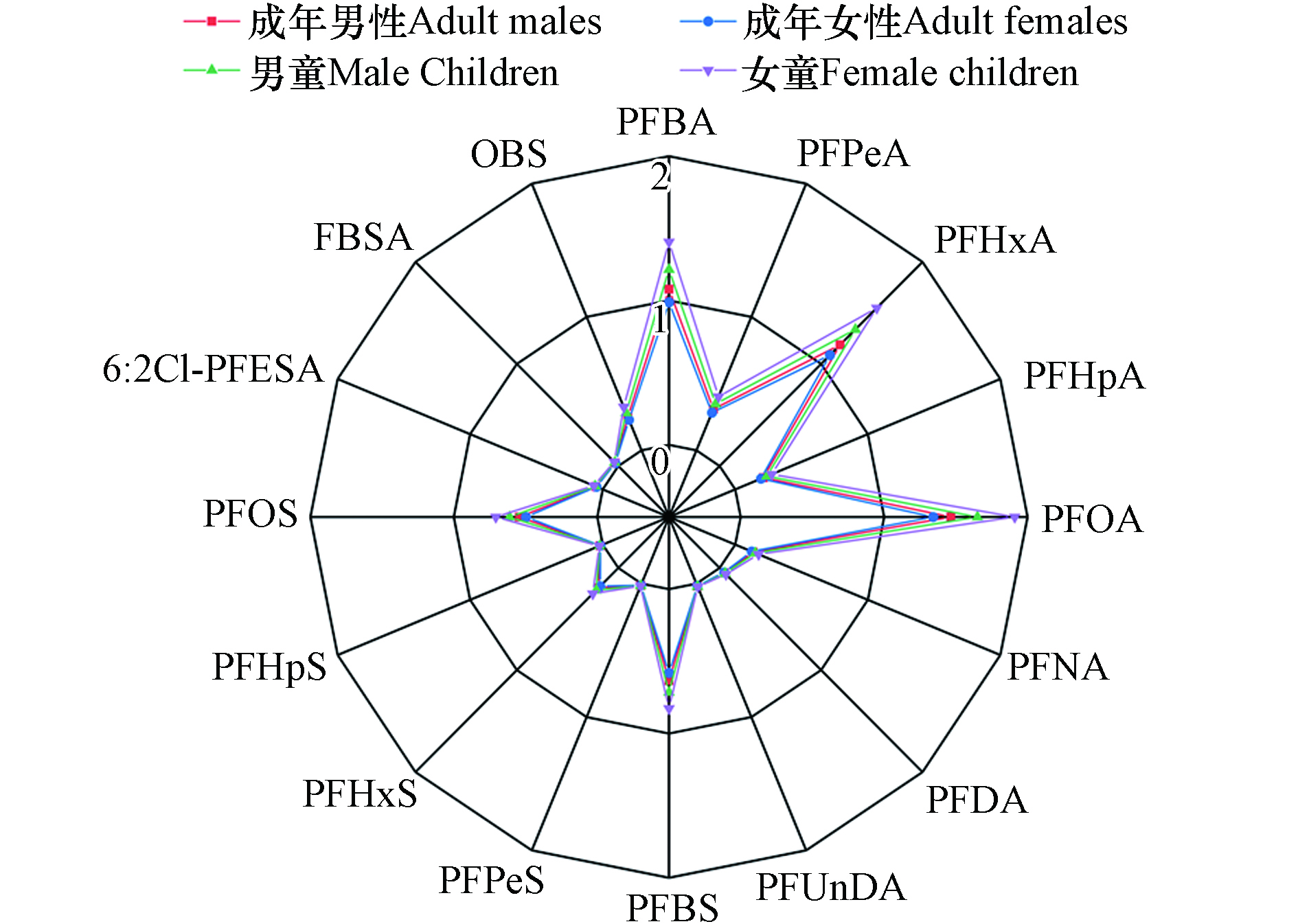
图6显示了通过饮用水估计的成年和儿童PFASs的每日摄入量(EDI). 成年男性、成年女性和男童、女童∑PFASs的EDIs分别为6.42、5.87 ng·(kg·d)−1和7.22、8.36 ng·(kg·d)−1,单个PFAS在不同人群中的EDI均小于2 ng·(kg·d)−1.
在成人群体中,男性的EDI值略高于女性;而女童的EDI高于男童,说明具有更高的暴露风险. 儿童的EDI高于成年人,主要是因为他们的单位体重耗水量较高,这表明摄入PFASs可能会对儿童造成更严重的危害[30]. PFOA的EDI对∑PFASs的EDI贡献最大,其次是PFBA、PFHxA、PFBA、PFBS和PFOS等. 该地区PFOA的EDI(1.34—1.91 ng·(kg·d)−1)和PFOS的EDI(0.50—0.71 ng·(kg·d)−1)高于其它水源地[30-31]. 成年人通过饮用水摄入PFOS的量低于通过太湖鲫鱼估计的EDI(16.4 ng·(kg·d)−1),但通过饮用水摄入PFOA的量高于通过太湖鲫鱼估计的EDI值(0.531 ng·(kg·d)−1)[32],可能会导致人类暴露PFOA的潜在健康风险. 本研究中PFOA和PFOS的EDI比欧洲食品安全局规定的每日可耐受摄入量(1500 ng·(kg·d)−1和150 ng·(kg·d)−1)[33]低很多,这意味着饮用水中的PFOA和PFOS对居民几乎无害. 然而,其他暴露途径(如食物或空气)未被考虑在内,由于PFASs的持久性、生物积累和长期健康影响,当地居民通过与水接触的累积不应被忽视. 因此,持续监测是必要的,以避免因接触PFASs而导致的不良健康效应.
3. 结论(Conclusion)
PFASs在太湖梅梁湾地表水和沉积物样品中的普遍检出,表明了PFASs在该地区的广泛应用,一些新型PFASs在地表水和沉积物中的检出尤其应该引起重视. 地表水中∑PFASs为162.82—187.62 ng·L−1(平均值171.34),主要以C4—8 PFCAs、PFBS等中短链化合物为主;沉积物中∑PFASs为1.04—4.81 ng·g−1干重(平均值3.06),以中长链化合物为主,PFOS贡献升高. PFASs在地表水和沉积物之间的分配与其疏水性及碳链长度有很大关系,并可能受到环境因素的影响. 虽然生态风险和人体健康风险评估的结果表明PFASs具有较低风险,但作为当地饮用水源,长期暴露的危害值得进一步关注.
-
表 1 绿色合成的磁性纳米铁去除重金属的应用
Table 1. Application of green synthetic magnetic iron nanoparticles for removal of heavy metals
磁性纳米铁种类Magnetic nano iron species 去除金属种类Removing metal type 去除效率Removal efficiency 参考文献References 纳米零价铁 六价铬 90 min 99.45% Huang et al. (2017)[29] 四氧化三铁纳米颗粒 钙和镉 120 min 钙55%, 镉40% Sebastian et al.(2018)[30] 铁纳米颗粒 六价铬 90 min 99.29% Wei et al. (2017)[31] 纳米零价铁、 氧化铁纳米颗粒 六价铬 35 min 98.9% Jin et al. (2017)[32] 氧化铁纳米颗粒 镉 90 min 90.0% Ehrampoush et al.(2015)[33] 纳米零价铁 六价铬 90 min 89.9% Qiu et al. (2017)[34] -
[1] AJINKYA N, YU X F, KAITHAL P, et al. Magnetic iron oxide nanoparticle (IONP) synthesis to applications: Present andfuture[J]. Materials (Basel, Switzerland), 2020, 13(20): 4644. doi: 10.3390/ma13204644 [2] ANDRADE R G D, VELOSO S R S, CASTANHEIRA E M S. Shape anisotropic iron oxide-based magnetic nanoparticles: Synthesis and biomedical applications[J]. International Journal of Molecular Sciences, 2020, 21(7): 2455. doi: 10.3390/ijms21072455 [3] FATIMA H, LEE D W, YUN H J, et al. Shape-controlled synthesis of magnetic Fe3O4 nanoparticles with different iron precursors and capping agents[J]. RSC Advances, 2018, 8(41): 22917-22923. doi: 10.1039/C8RA02909A [4] JACINTO M J, SILVA V C, VALLADÃO D S, et al. Biosynthesis of magnetic iron oxide nanoparticles: A review[J]. Biotechnology Letters, 2021, 43(1): 1-12. doi: 10.1007/s10529-020-03047-0 [5] DOLORES MÁRQUEZ-MEDINA M, RODRÍGUEZ-PADRÓN D, BALU A M, et al. Mechanochemically synthesized supported magnetic Fe-nanoparticles as catalysts for efficient vanillin production[J]. Catalysts, 2019, 9(3): 290. doi: 10.3390/catal9030290 [6] NAYEEM J, ALIM AL-BARI M A, MAHIUDDIN M, et al. Silica coating of iron oxide magnetic nanoparticles by reverse microemulsion method and their functionalization with cationic polymer P(NIPAm-co-AMPTMA) for antibacterial vancomycin immobilization[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 611: 125857. doi: 10.1016/j.colsurfa.2020.125857 [7] TAKAI Z I, MUSTAFA M, ASMAN S, et al. Preparation and characterization of magnetite (Fe3O4) nanoparticles by sol-gel method[J]. Int J Nanoelectron Mater, 2019, 12: 37-46. [8] TADIC M, PANJAN M, DAMNJANOVIC V, et al. Magnetic properties of hematite (α-Fe2O3) nanoparticles prepared by hydrothermal synthesis method[J]. Applied Surface Science, 2014, 320: 183-187. doi: 10.1016/j.apsusc.2014.08.193 [9] MAHARJAN A, DIKSHIT P K, GUPTA A, et al. Catalytic activity of magnetic iron oxide nanoparticles for hydrogen peroxide decomposition: Optimization and characterization[J]. Journal of Chemical Technology & Biotechnology, 2020, 95(9): 2495-2508. [10] LIU S L, TAO D D, ZHANG L N. Cellulose scaffold: A green template for the controlling synthesis of magnetic inorganic nanoparticles[J]. Powder Technology, 2012, 217: 502-509. doi: 10.1016/j.powtec.2011.11.010 [11] RANE A V, KANNY K, ABITHA V K, et al. Methods for synthesis of nanoparticles and fabrication of nanocomposites[M]//Synthesis of Inorganic Nanomaterials. Amsterdam: Elsevier, 2018: 121-139. [12] MUKHERJEE R, KUMAR R, SINHA A, et al. A review on synthesis, characterization, and applications of nano zero valent iron (nZVI) for environmental remediation[J]. Critical Reviews in Environmental Science and Technology, 2016, 46(5): 443-466. doi: 10.1080/10643389.2015.1103832 [13] SAMROT A V, SAHITHYA C S, SELVARANI A J, et al. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles[J]. Current Research in Green and Sustainable Chemistry, 2021, 4: 100042. doi: 10.1016/j.crgsc.2020.100042 [14] SARWAR A, WANG J, KHAN M S, et al. Iron oxide (Fe3O4)-supported SiO2 magnetic nanocomposites for efficient adsorption of fluoride from drinking water: Synthesis, characterization, and adsorption isotherm analysis[J]. Water, 2021, 13(11): 1514. doi: 10.3390/w13111514 [15] SHUKLA S, KHAN R, DAVEREY A. Synthesis and characterization of magnetic nanoparticles, and their applications in wastewater treatment: A review[J]. Environmental Technology & Innovation, 2021, 24: 101924. [16] SMALLMAN R E, NGAN A H W. Characterization and analysis[M]//Modern Physical Metallurgy. Amsterdam: Elsevier, 2014: 159-250. [17] YOSHIDA T, NAKAMURA T, HIGASHI O, et al. Magnetic fractionation and characterization of magnetic nanoparticles for magnetic particle imaging[J]. Japanese Journal of Applied Physics, 2018, 57(8): 080302. doi: 10.7567/JJAP.57.080302 [18] BHALERAO T S. Magnetic nanostructures: environmental and agricultural applications[M]//Nanotechnology in the Life Sciences. Cham: Springer International Publishing, 2019: 213-224. [19] BHATERIA R, SINGH R. A review on nanotechnological application of magnetic iron oxides for heavy metal removal[J]. Journal of Water Process Engineering, 2019, 31: 100845. doi: 10.1016/j.jwpe.2019.100845 [20] HE Y Z, WANG Z W, WANG H, et al. Metal-organic framework-derived nanomaterials in environment related fields: Fundamentals, properties and applications[J]. Coordination Chemistry Reviews, 2021, 429: 213618. doi: 10.1016/j.ccr.2020.213618 [21] SAHARAN P, CHAUDHARY G R, MEHTA S K, et al. Removal of water contaminants by iron oxide nanomaterials[J]. Journal of Nanoscience and Nanotechnology, 2014, 14(1): 627-643. doi: 10.1166/jnn.2014.9053 [22] LI W L, FORTNER J D. (Super)paramagnetic nanoparticles as platform materials for environmental applications: From synthesis to demonstration[J]. Frontiers of Environmental Science & Engineering, 2020, 14(5): 1-9. [23] KOLLURU S S, AGARWAL S, SIREESHA S, et al. Heavy metal removal from wastewater using nanomaterials-process and engineering aspects[J]. Process Safety and Environmental Protection, 2021, 150: 323-355. doi: 10.1016/j.psep.2021.04.025 [24] MOHAMMED L, GOMAA H G, RAGAB D, et al. Magnetic nanoparticles for environmental and biomedical applications: A review[J]. Particuology, 2017, 30: 1-14. doi: 10.1016/j.partic.2016.06.001 [25] NGUYEN M D, TRAN H V, XU S J, et al. Fe3O4 nanoparticles: Structures, synthesis, magnetic properties, surface functionalization, and emerging applications[J]. Applied Sciences (Basel, Switzerland), 2021, 11(23): 11301. [26] REHMAN A U, NAZIR S, IRSHAD R, et al. Toxicity of heavy metals in plants and animals and their uptake by magnetic iron oxide nanoparticles[J]. Journal of Molecular Liquids, 2021, 321: 114455. doi: 10.1016/j.molliq.2020.114455 [27] SOHAIL M I, WARIS A A, AYUB M A, et al. Environmental application of nanomaterials: A promise to sustainable future[M]//Engineered Nanomaterials and Phytonanotechnology: Challenges for Plant Sustainability. Amsterdam: Elsevier, 2019: 1-54. [28] ZHOU Q X, LI J, WANG M Y, et al. Iron-based magnetic nanomaterials and their environmental applications[J]. Critical Reviews in Environmental Science and Technology, 2016, 46(8): 783-826. doi: 10.1080/10643389.2016.1160815 [29] HUANG X Y, WANG W, LING L, et al. Heavy metal-nZVI reactions: The core-shell structure and applications for heavy metal treatment[J]. Acta Chimica Sinica, 2017, 75(6): 529. doi: 10.6023/A17020051 [30] SEBASTIAN A, NANGIA A, PRASAD M N V. A green synthetic route to phenolics fabricated magnetite nanoparticles from coconut husk extract: Implications to treat metal contaminated water and heavy metal stress in Oryza sativa L[J]. Journal of Cleaner Production, 2018, 174: 355-366. doi: 10.1016/j.jclepro.2017.10.343 [31] WEI Y F, FANG Z Q, ZHENG L C, et al. Biosynthesized iron nanoparticles in aqueous extracts of Eichhornia crassipes and its mechanism in the hexavalent chromium removal[J]. Applied Surface Science, 2017, 399: 322-329. doi: 10.1016/j.apsusc.2016.12.090 [32] JIN S Y, PARK B C, HAM W S, et al. Effect of the magnetic core size of amino-functionalized Fe3O4-mesoporous SiO2 core-shell nanoparticles on the removal of heavy metal ions[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2017, 531: 133-140. [33] EHRAMPOUSH M H, MIRIA M, SALMANI M H, et al. Cadmium removal from aqueous solution by green synthesis iron oxide nanoparticles with tangerine peel extract[J]. Journal of Environmental Health Science and Engineering, 2015, 13(1): 84. doi: 10.1186/s40201-015-0237-4 [34] QIU Y, ZHANG Q, GAO B, et al. Removal mechanisms of Cr(VI) and Cr(III) by biochar supported nanosized zero-valent iron: Synergy of adsorption, reduction and transformation[J]. Environmental Pollution, 2020, 265: 115018. doi: 10.1016/j.envpol.2020.115018 [35] HAN X, WANG F, Zhao Y, et al. Recycling of iron ore tailings into magnetic nanoparticles and nanoporous materials for the remediation of water, air and soil: a review[J]. Environmental Chemistry Letters, 2022: 1-24. [36] CHUANG P Y, CHIA Y, LIOU Y H, et al. Characterization of preferential flow paths between boreholes in fractured rock using a nanoscale zero-valent iron tracer test[J]. Hydrogeology Journal, 2016, 24(7): 1651-1662. doi: 10.1007/s10040-016-1426-7 [37] BANIJAMALI S, FEIZIAN M, BIDABADI A A, et al. Evaluation uptake and translocation of iron oxide nanoparticles and its effect on photosynthetic pigmentation of Chrysanthemum (Chrysanthemum morifolium) ‘Salvador’[J]. Journal of Ornamental Plants, 2019, 9(4): 245-258. [38] AHMED B, RIZVI A, ALI K, et al. Nanoparticles in the soil–plant system: a review[J]. Environmental Chemistry Letters, 2021, 19(2): 1545-1609. doi: 10.1007/s10311-020-01138-y [39] MEDINA-PÉREZ G, FERNÁNDEZ-LUQUEÑO F, VAZQUEZ-NUÑEZ E, et al. Remediating polluted soils using nanotechnologies: Environmental benefits and risks[J]. Polish Journal of Environmental Studies, 2019, 28(3): 1013-1030. doi: 10.15244/pjoes/87099 [40] RAFFI M M, HUSEN A. Impact of fabricated nanoparticles on the rhizospheric microorganisms and soil environment[M]//Nanomaterials and Plant Potential. Cham: Springer International Publishing, 2019: 529-552. [41] RAWAT S, PULLAGURALA V L R, ADISA I O, et al. Factors affecting fate and transport of engineered nanomaterials in terrestrial environments[J]. Current Opinion in Environmental Science & Health, 2018, 6: 47-53. [42] XU Z X, LONG X, JIA Y, et al. Occurrence, transport, and toxicity of nanomaterials in soil ecosystems: A review[J]. Environmental Chemistry Letters, 2022, 20(6): 3943-3969. doi: 10.1007/s10311-022-01507-9 [43] 张旭升. 不同植被修复模式下土壤真菌的研究及纳米材料对土壤理化性质和酶活性的影响[D]. 太原: 山西大学, 2021. ZHANG X S. Study on soil fungi under different vegetation restoration patterns and the effect of nanomaterials on soil physical and chemical properties and enzyme activities[D]. Taiyuan: Shanxi University, 2021 (in Chinese).
[44] SUN P, SUN Y Y, LUO Y H, et al. The application progress of nano materials for remediation in contaminated soil[J]. IOP Conference Series: Earth and Environmental Science, 2021, 692(3): 032035. doi: 10.1088/1755-1315/692/3/032035 [45] XIN X P, ZHAO F L, ZHAO H M, et al. Comparative assessment of polymeric and other nanoparticles impacts on soil microbial and biochemical properties[J]. Geoderma, 2020, 367: 114278. doi: 10.1016/j.geoderma.2020.114278 [46] JAIN A, SINGH N, KHAN S. Nanomaterials for soil reclamation[M]//Advances in Environmental Engineering and Green Technologies. IGI Global, 2021: 530-541. [47] YAN L, LI P Y, ZHAO X P, et al. Physiological and metabolic responses of maize (Zea mays) plants to Fe3O4 nanoparticles[J]. Science of the Total Environment, 2020, 718: 137400. doi: 10.1016/j.scitotenv.2020.137400 [48] GALAKTIONOVA L, GAVRISH I, LEBEDEV S. Bioeffects of Zn and Cu nanoparticles in soil systems[J]. Toxicology and Environmental Health Sciences, 2019, 11(4): 259-270. doi: 10.1007/s13530-019-0413-5 [49] ASADISHAD B, CHAHAL S, AKBARI A, et al. Amendment of agricultural soil with metal nanoparticles: Effects on soil enzyme activity and microbial community composition[J]. Environmental Science & Technology, 2018, 52(4): 1908-1918. [50] YOU T T, LIU D D, CHEN J, et al. Effects of metal oxide nanoparticles on soil enzyme activities and bacterial communities in two different soil types[J]. Journal of Soils and Sediments, 2018, 18(1): 211-221. doi: 10.1007/s11368-017-1716-2 [51] SUN W, DOU F, Li C, et al. Impacts of metallic nanoparticles and transformed products on soil health[J]. Critical Reviews in Environmental Science and Technology, 2021, 51(10): 973-1002. YANG H Y, ZHANG X, DANG D, et al. Effects of iron oxide nanoparticles on CH4 and N2O emissions and microbial communities in two typical paddy soils[J]. Chinese Journal of Applied and Environmental Biology, 2021, 27(3): 725-733 (in Chinese). [52] ELHAMBAKHSH A, GHANAATIAN A, KESHAVARZ P. Glutamine functionalized iron oxide nanoparticles for high-performance carbon dioxide absorption[J]. Journal of Natural Gas Science and Engineering, 2021, 94: 104081. doi: 10.1016/j.jngse.2021.104081 [53] DIMKPA C O. Can nanotechnology deliver the promised benefits without negatively impacting soil microbial life?[J]. Journal of Basic Microbiology, 2014, 54(9): 889-904. doi: 10.1002/jobm.201400298 [54] PEREA VELEZ Y S, CARRILLO-GONZALEZ R, GONZÁLEZ-CHÁVEZ M. Interaction of metal nanoparticles–plants–microorganisms in agriculture and soil remediation[J]. Journal of Nanoparticle Research, 2021, 23(9): 1-48. [55] HU L F, FENG Z Y, YU Y X, et al. Effects of metal oxide nanoparticles on nitrous oxide emissions in agriculture soil[J]. Agriculture, 2022, 12(6): 770. doi: 10.3390/agriculture12060770 [56] RAJA M A, HUSEN A. Role of nanomaterials in soil and water quality management[M]//Nanomaterials for Agriculture and Forestry Applications. Amsterdam: Elsevier, 2020: 491-503. [57] PUSPITASARI P, YAZIRIN C, BACHTIAR L A, et al. Application of nanocatalyst iron oxide (Fe2O3) to reduce exhaust emissions (CO and HC)[J]. IOP Conference Series: Materials Science and Engineering, 2018, 432: 012004. doi: 10.1088/1757-899X/432/1/012004 [58] 吴江利, 罗学刚, 李宝强, 等. 微生物菌肥作用下荒漠土壤微生物群落结构和功能研究[J]. 中国农学通报, 2015, 31(9) 216-223. WU J L, LUO X G, LI B Q, et al. Researches on microbial community structure and function in desert soil under microbial fertilizer[J]. Chinese Agricultural Science Bulletin, 2015, 31(9): 216-223 (in Chinese).
[59] FU L, SONG T Z, ZHANG W, et al. Stimulatory effect of magnetite nanoparticles on a highly enriched butyrate-oxidizing consortium[J]. Frontiers in Microbiology, 2018, 9: 1480. doi: 10.3389/fmicb.2018.01480 [60] 杨浩宇, 张潇, 党迪, 等. 纳米氧化铁对水稻土CH4和N2O排放及微生物的影响[J]. 应用与环境生物学报, 2021, 27(3): 725-733. YANG H Y, ZHANG X, DANG D, et al. Effects of iron oxide nanoparticles on CH4 and N2O emissions and microbial communities in two typical paddy soils[J]. Chinese Journal of Applied and Environmental Biology, 2021, 27(3): 725-733 (in Chinese).
[61] CHEN Q L, DING J, ZHU Y G, et al. Soil bacterial taxonomic diversity is critical to maintaining the plant productivity[J]. Environment International, 2020, 140: 105766. doi: 10.1016/j.envint.2020.105766 [62] JIA Y, WHALEN J K. A new perspective on functional redundancy and phylogenetic niche conservatism in soil microbial communities[J]. Pedosphere, 2020, 30(1): 18-24. doi: 10.1016/S1002-0160(19)60826-X [63] ZHONG Y, YAN W M, WANG R W, et al. Decreased occurrence of carbon cycle functions in microbial communities along with long-term secondary succession[J]. Soil Biology and Biochemistry, 2018, 123: 207-217. doi: 10.1016/j.soilbio.2018.05.017 [64] van der BOM F, NUNES I, RAYMOND N S, et al. Long-term fertilisation form, level and duration affect the diversity, structure and functioning of soil microbial communities in the field[J]. Soil Biology and Biochemistry, 2018, 122: 91-103. doi: 10.1016/j.soilbio.2018.04.003 [65] ZHENG Q, HU Y T, ZHANG S S, et al. Soil multifunctionality is affected by the soil environment and by microbial community composition and diversity[J]. Soil Biology and Biochemistry, 2019, 136: 107521. doi: 10.1016/j.soilbio.2019.107521 [66] 尹雪梅, 王晓凤. 纳米Fe3O4对玉米叶面积和根际微生物群落功能多样性的影响[C]//中国土壤学会土壤环境专业委员会第十九次会议暨“农田土壤污染与修复研讨会”第二届山东省土壤污染防控与修复技术研讨会摘要集. 济南, 2017: 139. YIN X M, WANG X F. Effects of nano Fe3O4 on leaf area and rhizosphere microbial community functional diversity in maize[C]//. The 19th Conference of Soil Environment Committee of Soil Society of China and the 2nd Workshop on Soil Pollution Control and Remediation in Shandong Province Abstract Collection. Jinan, 2017: 139(in Chinese).
[67] HE S Y, FENG Y Z, REN H X, et al. The impact of iron oxide magnetic nanoparticles on the soil bacterial community[J]. Journal of Soils and Sediments, 2011, 11(8): 1408-1417. doi: 10.1007/s11368-011-0415-7 [68] KUMAR P, BURMAN U, KAUL R K. Ecological risks of nanoparticles[M]//Nanomaterials in Plants, Algae, and Microorganisms. Amsterdam: Elsevier, 2018: 429-452. [69] REN X M, GUO L, CHEN Y, et al. Effect of magnet powder (Fe3O4) on aerobic granular sludge (AGS) formation and microbial community structure characteristics[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(8): 9707-9715. [70] SAIF S, TAHIR A, CHEN Y S. Green synthesis of iron nanoparticles and their environmental applications and implications[J]. Nanomaterials (Basel, Switzerland), 2016, 6(11): 209. doi: 10.3390/nano6110209 [71] SHEN Y X, JIANG B, XING Y. Recent advances in the application of magnetic Fe3O4 nanomaterials for the removal of emerging contaminants[J]. Environmental Science and Pollution Research, 2021, 28(7): 7599-7620. doi: 10.1007/s11356-020-11877-8 [72] MAI T, HILT J Z. Magnetic nanoparticles: reactive oxygen species generation and potential therapeutic applications[J]. Journal of Nanoparticle Research, 2017, 19(7): 1-10. [73] ZENG Q Z, XU J, HOU Y, et al. Effect of Fe3O4 nanoparticles exposure on the treatment efficiency of phenol wastewater and community shifts in SBR system[J]. Journal of Hazardous Materials, 2021, 407: 124828. doi: 10.1016/j.jhazmat.2020.124828 [74] LEFEVRE E, BOSSA N, WIESNER M R, et al. A review of the environmental implications of in situ remediation by nanoscale zero valent iron (nZVI): Behavior, transport and impacts on microbial communities[J]. Science of the Total Environment, 2016, 565: 889-901. doi: 10.1016/j.scitotenv.2016.02.003 [75] FAJARDO C, GARCÍA-CANTALEJO J, BOTÍAS P, et al. New insights into the impact of nZVI on soil microbial biodiversity and functionality[J]. Journal of Environmental Science and Health, Part A, 2019, 54(3): 157-167. doi: 10.1080/10934529.2018.1535159 [76] ANZA M, SALAZAR O, EPELDE L, et al. The application of nanoscale zero-valent iron promotes soil remediation while negatively affecting soil microbial biomass and activity[J]. Frontiers in Environmental Science, 2019, 7: 19. doi: 10.3389/fenvs.2019.00019 [77] FAJARDO C, ORTÍZ L T, RODRÍGUEZ-MEMBIBRE M L, et al. Assessing the impact of zero-valent iron (ZVI) nanotechnology on soil microbial structure and functionality: A molecular approach[J]. Chemosphere, 2012, 86(8): 802-808. doi: 10.1016/j.chemosphere.2011.11.041 [78] PAWLETT M, RITZ K, DOREY R A, et al. The impact of zero-valent iron nanoparticles upon soil microbial communities is context dependent[J]. Environmental Science and Pollution Research, 2013, 20(2): 1041-1049. doi: 10.1007/s11356-012-1196-2 [79] YE W F, LU J, YE J F, et al. The effects and mechanisms of zero-valent iron on anaerobic digestion of solid waste: A mini-review[J]. Journal of Cleaner Production, 2021, 278: 123567. doi: 10.1016/j.jclepro.2020.123567 [80] KOCUR C M D, LOMHEIM L, MOLENDA O, et al. Long-term field study of microbial community and dechlorinating activity following carboxymethyl cellulose-stabilized nanoscale zero-valent iron injection[J]. Environmental Science & Technology, 2016, 50(14): 7658-7670. [81] LIU C E, YUE M H, TAN H L, et al. Effects of nano-zero-valent iron(nZVI) on earthworm-bacteria-soil systems[J]. Chinese Journal of Eco-Agriculture, 2021, 29(10): 1722-1732. [82] KASEM K K, MOSTAFA M, ABD-ELSALAM K A. Iron-based nanomaterials: Effect on soil microbes and soil health[M]//Nanotechnology in the Life Sciences. Cham: Springer International Publishing, 2019: 261-285. [83] AHMAD S, LIU X M, TANG J C, et al. Biochar-supported nanosized zero-valent iron (nZVI/BC) composites for removal of nitro and chlorinated contaminants[J]. Chemical Engineering Journal, 2022, 431: 133187. doi: 10.1016/j.cej.2021.133187 [84] PENG D H, WU B, TAN H, et al. Effect of multiple iron-based nanoparticles on availability of lead and iron, and micro-ecology in lead contaminated soil[J]. Chemosphere, 2019, 228: 44-53. doi: 10.1016/j.chemosphere.2019.04.106 [85] ADHIKARI K, HARTEMINK A E. Linking soils to ecosystem services—a global review[J]. Geoderma, 2016, 262: 101-111. doi: 10.1016/j.geoderma.2015.08.009 [86] LI L, XU M G, EYAKUB ALI M, et al. Factors affecting soil microbial biomass and functional diversity with the application of organic amendments in three contrasting cropland soils during a field experiment[J]. PLoS One, 2018, 13(9): e0203812. doi: 10.1371/journal.pone.0203812 [87] MOSCATELLI M C, SECONDI L, MARABOTTINI R, et al. Assessment of soil microbial functional diversity: Land use and soil properties affect CLPP-MicroResp and enzymes responses[J]. Pedobiologia, 2018, 66: 36-42. doi: 10.1016/j.pedobi.2018.01.001 [88] NKONGOLO K K, NARENDRULA-KOTHA R. Advances in monitoring soil microbial community dynamic and function[J]. Journal of Applied Genetics, 2020, 61(2): 249-263. doi: 10.1007/s13353-020-00549-5 [89] GHOSH S, JOSHI K, WEBSTER T J. Removal of heavy metals by microbial communities[M]//Wastewater Treatment Reactors. Amsterdam: Elsevier, 2021: 537-566. [90] FAKRUDDIN M, BIN MANNAN K S. Methods for analyzing diversity of microbial communities in natural environments[J]. Ceylon Journal of Science (Biological Sciences), 2013, 42(1): 19. doi: 10.4038/cjsbs.v42i1.5896 [91] KHAN S T. Interaction of engineered nanomaterials with soil microbiome and plants: Their impact on plant and soil health[M]//Sustainable Agriculture Reviews 41. Cham: Springer International Publishing, 2020: 181-199. [92] QIAN H F, KE M J, QU Q, et al. Ecological effects of single-walled carbon nanotubes on soil microbial communities and soil fertility[J]. Bulletin of Environmental Contamination and Toxicology, 2018, 101(4): 536-542. doi: 10.1007/s00128-018-2437-y [93] 曹鑫磊, 姜浩, 杨宝山, 等. 纳米银对小麦秸秆还田土壤中酶活性及微生物群落功能多样性的影响[J]. 山东科学, 2021, 34(3): 80-89. CAO X L, JIANG H, YANG B S, et al. Effects of nano-silver on enzyme activity and microbial community functional diversity in wheat straw returning soil[J]. Shandong Science, 2021, 34(3): 80-89.(in Chinese).
[94] RAJPUT V D, MINKINA T M, BEHAL A, et al. Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms A review[J]. Environmental Nanotechnology, Monitoring & Management, 2018, 9: 76-84. [95] SHI X D, WEI W, WU L, et al. Zero-valent iron mediated biological wastewater and sludge treatment[J]. Chemical Engineering Journal, 2021, 426: 130821. doi: 10.1016/j.cej.2021.130821 [96] TIAN L Y, SHEN J P, SUN G X, et al. Foliar application of SiO2 nanoparticles alters soil metabolite profiles and microbial community composition in the pakchoi (Brassica chinensis L. ) rhizosphere grown in contaminated mine soil[J]. Environmental Science & Technology, 2020, 54(20): 13137-13146. [97] VANZETTO G V, THOMÉ A. Bibliometric study of the toxicology of nanoescale zero valent iron used in soil remediation[J]. Environmental Pollution, 2019, 252: 74-83. doi: 10.1016/j.envpol.2019.05.092 [98] ZHU X W, BLANCO E, BHATTI M, et al. Impact of metallic nanoparticles on anaerobic digestion: A systematic review[J]. Science of the Total Environment, 2021, 757: 143747. doi: 10.1016/j.scitotenv.2020.143747 [99] LEI C, SUN Y Q, TSANG D C W, et al. Environmental transformations and ecological effects of iron-based nanoparticles[J]. Environmental Pollution, 2018, 232: 10-30. doi: 10.1016/j.envpol.2017.09.052 [100] MAHANTY B, JESUDAS S, PADMAPRABHA A. Toxicity of surface functionalized iron oxide nanoparticles toward pure suspension culture and soil microcosm[J]. Environmental Nanotechnology, Monitoring & Management, 2019, 12: 100235. [101] RANMADUGALA D, EBRAHIMINEZHAD A, MANLEY-HARRIS M, et al. Magnetic immobilization of bacteria using iron oxide nanoparticles[J]. Biotechnology Letters, 2018, 40(2): 237-248. doi: 10.1007/s10529-017-2477-0 [102] SEIFAN M, EBRAHIMINEZHAD A, GHASEMI Y, et al. The role of magnetic iron oxide nanoparticles in the bacterially induced calcium carbonate precipitation[J]. Applied Microbiology and Biotechnology, 2018, 102(8): 3595-3606. doi: 10.1007/s00253-018-8860-5 -






 下载:
下载: