-
作为一种具有高毒性、生物累积和生物放大效应的重金属,汞(Hg)主要以气态形式被排放到大气中,随后可通过大气环流进行全球传输并沉降到陆地和海洋生态系统. 化石燃料燃烧、金属冶炼和垃圾焚烧等人为活动每年产生2000—3000 t的汞排放,而地质活动等自然源的每年汞排放量可达5500 t[1]. 目前,在无明显人为源的偏远地区也普遍检出汞[2],如青藏高原的海螺沟冰川融水中汞含量为6.96—10.78 ng·L-1[3],北极苔原也有显著大气汞沉降[4]. 为降低汞污染对生态系统与人体健康的危害,2013年世界上128个国家和地区签署了《关于汞的水俣公约》以减少汞的使用与人为排放[5],2018年全球人为导致的汞排放较2013年已出现下降[6]. 然而,由于历史上累积汞在表生环境中的再释放和循环,汞污染问题仍将长期存在[7]. 此外,全球变暖可能导致冰川与冻土中汞的释放[8],而升温引起的生态系统中初级生产力提高、生物习性改变以及食物网结构的变化也可能加剧生物体中汞的累积[9].
引起水俣病等公害事件的甲基汞(MeHg)是毒性最高的汞形态之一,其主要来源于硫酸盐还原菌、铁还原菌和产甲烷菌等含有hgcAB基因簇微生物的甲基化[10]. 甲基汞通过与含巯基的蛋白质结合,对生物体造成以神经系统为主的全身性损伤,并可跨过胎盘屏障导致先天性疾病[11-13]. 甲基汞的健康风险与甲基汞暴露直接相关,如食用鱼肉等水产品是甲基汞的重要暴露途径[12,14]. 尽管水中甲基汞在总汞中的占比通常较低,但鱼体中甲基汞可达总汞的95%左右[15],该现象主要是因为甲基汞的生物累积和生物放大效应. 作为海洋中的初级生产者,藻类具有极强的汞富集能力,部分藻中汞浓度可达水环境的103—106倍[16]. 藻类富集的甲基汞可通过食物链传递并在其他生物体内累积,甲基汞的营养级放大斜率可达0.15 —0.35,表现出显著的生物放大效应[17-20]. 同时,日趋严重的全球变暖、水体酸化和富营养化等问题带来了环境因子、藻类丰度、汞浓度与可利用性的新变化.
开展甲基汞在藻类中的富集过程研究以及这一过程对水体甲基汞生物累积与放大的关键影响研究对于揭示汞的生物富集、传递特性以及预测其风险至关重要. 因此,本文对藻类富集甲基汞的特征、机理及影响因素进行详细讨论,总结甲基汞在藻类中的分布、转化与后续营养传递特征,并对相关研究的发展方向进行展望.
-
藻类对甲基汞的富集涉及吸收、吸附、转运、解吸和外排等多个过程(图1),这些过程对甲基汞在藻类中的累积和食物链传递有重要影响. 首先,藻类可通过吸附、吸收、解吸和外排影响进入食物链的甲基汞的量;此外,藻类及其伴生菌与胞外分泌物可能通过去甲基化或甲基化改变食物链中甲基汞浓度;再者,甲基汞在藻体的不同亚细胞结构中的分布会影响甲基汞在食物链中的传递行为.
-
藻类可显著累积水体甲基汞,基于野外数据建立的模型显示浮游植物对甲基汞的生物累积因子(bioaccumulation factor,BAF)达102.4—105.9[21]. 由于实际环境中浮游生物是由多种藻类组成,难以确定不同藻类对甲基汞富集的具体贡献,所以藻类对甲基汞的富集研究通常选择环境中的硅藻与绿藻等优势藻种在实验室条件下开展工作[22]. 该部分论述了藻对甲基汞的累积规律,以及相关的吸附与吸收机制.
-
藻类累积甲基汞研究的主要指标包括藻类的体积富集因子(volume concentration factor,VCF)、富集率以及累积速率(具体计算方法见表1注). 表1比较了不同研究结果,其中,甲基汞的形态和暴露时间是影响藻类富集甲基汞的重要因素. 首先,不同形态甲基汞在藻类中的富集存在差异,相较于氯化甲基汞,半胱氨酸与蛋氨酸与甲基汞的络合会减弱藻类对甲基汞的富集[23-24]. 此外,藻类对甲基汞的富集具有一定的时间变化规律,通常在初始5 min内非常迅速,在24 h左右达到平衡[25],因此暴露24 h可测定平衡状态下藻类的富集率等指标,暴露1 h内可反映短期的甲基汞富集动力学变化.
藻类富集甲基汞主要通过吸收和吸附两种途径[26]. 一些研究采用乙二胺四乙酸(EDTA)或半胱氨酸等络合剂清洗藻类表面吸附的甲基汞,用以区分藻类通过吸附与吸收富集的甲基汞. 其中,0.8 mmol·L−1半胱氨酸清洗5 min或8 mmol·L−1半胱氨酸清洗1 min,藻类吸附甲基汞的解吸率可达90%以上[27]. 研究发现,藻类富集甲基汞的差异可能来源于藻类对甲基汞吸附与吸收的比例不同. 如5 ng·L−1 的甲基汞暴露下,斜生栅藻(Scenedesmus obliquus)平衡后的吸附率(藻吸收的甲基汞量/水体甲基汞量)为16.3%,低于蛋白核小球藻(Chlorella pyrenoidosa)(28.2%),但二者的吸附率(藻吸附的甲基汞量/水体甲基汞量)接近,且随甲基汞暴露浓度增加,斜生栅藻吸收率的增加幅度显著高于蛋白核小球藻[28]. 另外,暴露时间由24 h增加至168 h,蛋白核小球藻的吸附率由21.2%下降至11.3%,吸收率则由38.3%上升至46.9%[28]. 由于吸附速率大于吸收速率,推测藻类富集甲基汞前期以胞外快速吸附为主,平衡后通过胞内缓慢吸收进行富集[29].
-
藻类累积甲基汞的反应动力学研究发现水华束丝藻(Aphanizomenon flosaquae)与铜绿微囊藻(Microcystis aeruginosa)对甲基汞的吸附均较好地符合准二级动力学模型(R2 > 0.99),提示这一过程可能涉及物理吸附与化学吸附,化学吸附可能涉及藻类表面基团与甲基汞间的电子转移或共用;这两种藻类对甲基汞的吸附也可较好地由Freundlich等温吸附模型(R2 = 0.9248与0.8614)拟合[25],提示在藻细胞表面可能存在多个吸附位点同时起作用,且吸附位点间表现出不同的吸附自由能与剩余度.
通过对椭圆小球藻(Chlorella ellipticus)、鱼腥藻(Anabaena)、水华束丝藻和铜绿微囊藻的红外光谱研究,藻类通过细胞壁或胞外多聚物(extracellular polymeric substances,EPS)上广泛存在的氨基、巯基、羧基和羟基等官能团与甲基汞结合[35-36]. 不同官能团与甲基汞的结合机理不同,羟基和羧基等官能团通过孤对电子与甲基汞形成配位键[37],磺酸基等官能团则通过失去电子形成负电荷后的静电作用吸附甲基汞[38]. 与藻体内含量较低的巯基等含硫基团相比,羟基与羧基对藻类吸附甲基汞的贡献可能较大也更易体现[36,39]. 羟基与羧基的吸附过程为环境中的甲基汞-配体复合物(CH3Hg-L)与细胞表面的官能团(R)经配体交换形成新的络合物(CH3Hg-R),CH3Hg-L与CH3Hg-R的相对热力学稳定常数决定了络合速率,此外L与R的空间位阻也可能影响吸附的速率,如谷胱甘肽、N-乙酰-L-半胱氨酸和N-乙酰-青霉胺等较大尺寸或支链较多的硫醇配体结合甲基汞的程度小于半胱氨酸和巯基乙酸等小分子配体[23].
-
研究发现三磷酸腺苷(adenosine triphosphate,ATP)和转运蛋白参与了藻类对甲基汞的吸收,表明藻类可以通过主动运输富集甲基汞. 光系统Ⅰ抑制剂(甲基紫精)、光系统Ⅱ抑制剂(二氯酚二甲基脲)、避光与γ-辐射等减少ATP的措施可抑制藻类对甲基汞的吸收,证明藻类存在需要ATP的甲基汞吸收过程[40-41]. 尽管藻类的甲基汞转运蛋白尚未确定,但在仓鼠卵巢细胞(ATCC CCL-61)中,LAT1转运蛋白承担了甲基汞-半胱氨酸复合物(MeHg-L-cysteine)的运输功能[41]. 研究猜测MeHg-L-cysteine的结构类似于LAT1转运蛋白的底物蛋氨酸,从而导致甲基汞被错误运输[42]. LAT1蛋白过表达的CHO-k1细胞中MeHg-L-cysteine与蛋氨酸的竞争吸收现象进一步支持了上述猜测的合理性[41]. 而参与藻类主动吸收甲基汞途径的转运蛋白仍需进一步研究.
另外,藻类也可通过被动扩散吸收水体中的甲基汞. 如不同pH和氯离子浓度下,甲基汞的辛醇-水分配系数与威氏海链藻对甲基汞的吸收速率呈正相关关系(r = 0.75)[43],而且温度升高10 ℃对甲基汞吸收速率的影响(Q10)值仅为0.7,显著低于温度对主动运输吸收速率的影响(普遍为2—3)[40,44],表明甲基汞可通过被动扩散进入藻细胞. 但有研究发现经热灭活破坏主动运输途径后,梅尼小环藻(Cyclotella meneghiniana)的甲基汞吸收量显著下降,甲基汞在细胞质中占比由64%降至4%,证明被动扩散在甲基汞吸收中的贡献可能较低[45].
-
藻类可能存在解吸与外排降低藻类中甲基汞的含量(图1). 在暴露168 h后,蛋白核小球藻的甲基汞吸附量相较最高吸附量(24 h)下降约40%,单个斜生栅藻的甲基汞吸收量相较最高吸收量(24 h)下降约74%[28]. 以上藻类对甲基汞的吸附和吸收的降低与藻类生长稀释以及增殖稀释有关[21],但也可能是由于甲基汞发生解吸和外排.
由于藻类对甲基汞的吸附、吸收、解吸和外排等过程可能同时发生,藻类对甲基汞的吸附和外排过程难以观察与定量. 藻类中甲基汞的解吸过程未见报道,但有研究表明震荡可使椭圆小球藻、水华束丝藻和铜绿微囊藻中69%、42%和34%的二价汞发生解吸[46]. 对于藻类中甲基汞的外排,过去研究认为甲基汞胁迫下藻类细胞膜通透性改变是原因之一[47]. 另外,甲基汞胁迫会导致莱茵衣藻(Chlamydomonas reinhardtii)中的铁转运蛋白、锌转运蛋白、ATP结合转运蛋白与阳离子扩散蛋白等金属转运相关蛋白质表达上调,因此,甲基汞外排可能需要转运蛋白的参与[26]. 但甲基汞胁迫下,一些蛋白质的高表达并非参与甲基汞的外排,如ATP结合蛋白的基因表达上调增加了胞内MRP2蛋白的含量,此蛋白可将甲基汞转移到液泡中而非排出体外[48]. 因此,后续需对甲基汞外排相关转运蛋白的具体作用机制进行研究.
-
目前,没有证据表明藻类可以直接产生甲基汞,但有研究认为藻类逆境中产生的代谢物二甲基硫分解为甲磺酸的过程可能导致甲基汞的生成[49]. 以往研究发现北极环境雪与地表水样品中的甲磺酸量与甲基汞量呈正相关[50],且二甲基硫转化过程中具有与硫还原菌相似的四氢叶酸途径[51],表明该途径可能存在,但暂未得到证实.
已有研究证据表明藻类可通过影响产甲基汞菌活性间接改变甲基汞浓度. 如向巴西采集的含微生物环境样品中加入蓝藻后,甲基汞净产率由6.8%增加至24.6%,而仅存在蓝藻的对照组水样中无甲基汞的产生[52]. 产甲基汞菌活性的提高可能是因为蓝藻增加了细菌群落所需的氢气[53],同时藻源性的叶绿素、蛋白质、细胞壁和脂质等物质也可增加产甲基汞细菌活性[54]. 另外,不同环境样品中蓝藻生物量与甲基汞含量呈正相关趋势[55]. 然而,藻类也可能抑制甲基汞产生,如中肋骨条藻(Skeletonema costatum)通过吸附二价汞(47%)降低了铁还原菌的甲基汞产量[56]. 此外,藻类也可能通过改变水体透光率以及溶解氧含量等影响汞的甲基化[57].
除光致去甲基化[58]与化学去甲基化[59]外,最近研究发现浮游生物群落也可在24 h内降解水样中12%的甲基汞[60]. 双富集同位素技术证明三角褐指藻(Phaeodactylum tricornutum)与旋链角毛藻(Chaetoceros curvisetus)等6种藻类可以引起甲基汞去甲基化,其中三角褐指藻等藻类可通过胞外分泌物实现甲基汞的光致去甲基化,东海原甲藻(Prorocentrum donghaiense)的去甲基化能力源于其伴生细菌,旋链角刺藻的去甲基化作用则来自于伴生细菌与胞外分泌物的共同作用[61]. 上述不同途径的去甲基化速率存在差异,胞外分泌物介导的光致去甲基化速率(0.01—0.39 d−1)显著高于伴生细菌的生物去甲基化(0.03—0.14 d−1)[61],该降解速率差异是由于反应机理不同[62-63]. 胞外分泌物被认为通过硫醇介导光致去甲基化[61],其可作为软碱与甲基汞的结合促进甲基汞的光降解[64];伴生细菌则可能通过甲基汞裂解酶(MerB)等途径降解甲基汞[65]. 但针对藻类及其伴生菌去甲基化过程的研究较少.
藻体内还存在其他改变环境中汞迁移性和生物可利用性的转化途径. 藻类可将二价汞还原为毒性低而挥发性强的溶解性零价汞[66],但此途径效率较低(< 5%),且机理尚不明确[67]. 通过冷原子荧光检测改进的酸碱还原差值法分析,硫化汞(β-HgS)也可能存在于藻类细胞中[68]. 有观点认为β-HgS是藻类中主要的无机汞形态(20%—90%),并推断其主要来源于液泡中二价汞的转化,但此前检测方法可能无法有效区分二价汞与硫化汞[69]. 未来可借助扩展X射线吸收精细结构谱等手段对硫化汞的生成过程进行详细研究[70].
-
通过差速离心、热处理与化学处理可将藻类分为细胞碎屑(包括细胞壁与细胞膜等)、细胞器、热稳定蛋白(如植物螯合肽等)、热变性蛋白(如酶等)与富金属矿体5部分[71-72]. 通常将富金属矿体、细胞碎屑与热稳定蛋白统称为金属解毒部分即生物非活性部分,将细胞器与热变性蛋白划分为金属敏感组分. 热稳定蛋白是甲基汞在藻类中的主要结合部分,其中甲基汞的累积量可占细胞总累积量的44%(假微型海链藻)、68%(小球藻)和80%(等鞭金藻)[31]. 在热稳定蛋白中,甲基汞主要与含巯基的半胱氨酸、谷胱甘肽和植物螯合肽等组分络合[33,73]. 与二价汞类似,甲基汞在热稳定蛋白中达到饱和后,可能会进一步累积到其他结构中,因此细胞碎屑与细胞器组分中也存在甲基汞,但均未超过总累积量的30%[31].
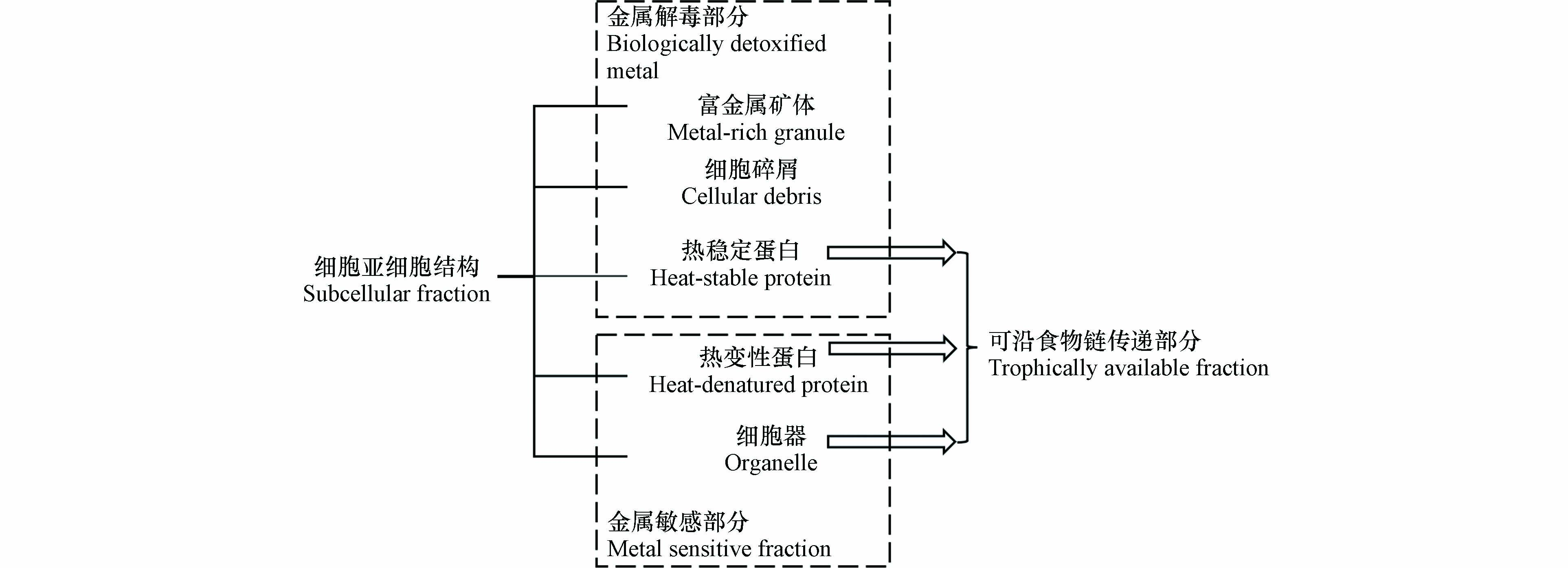
藻类中甲基汞的亚细胞分布对其生物可利用性可能有重要影响[74]. 图2总结了不同累积位点重金属的生物可利用性差异,细胞器与热变性蛋白等金属敏感部分中镉与银等重金属可沿食物链传递,而细胞碎屑与富金属颗粒等解毒组分中的重金属则不会沿食物链传递[75-77]. 以往研究通过明胶包裹含甲基汞的贻贝不同亚细胞组分喂食鱼类发现,热稳定蛋白、热变性蛋白和细胞器等组分中甲基汞被同化的比例高于不溶物等其他组分[78]. 而分布于不同亚细胞结构的重金属在食物链传递中存在差异[79]. 目前尚未开展藻类富集甲基汞的相关研究,藻类不同亚细胞结构在甲基汞传递中的作用还需进一步研究.
-
藻类富集甲基汞受生物因素和环境因素影响. 藻类的相对表面积、结构以及活性均是影响富集效果的重要生物因素,环境因素则包括pH、温度、溶解性有机质(dissolved organic matter,DOM)、氯离子和硒等.
-
藻类的相对表面积可影响藻类富集甲基汞的能力. 齿状藻等藻类甲基汞的VCF与相对表面积的相关系数可达0.97[34]. 因此,相对表面积更大的原核藻类蓝藻的甲基汞富集能力高于常见的硅藻与隐藻等真核藻类[47].
藻类结构也影响甲基汞的富集. 如没有叶绿体的原核藻类裂须藻的甲基汞富集率显著低于被内质网包裹叶绿体的真核藻类海链藻[80],且这两种藻类的甲基汞富集量均小于含有完整的脂质双分子层叶绿体的鼓藻与月牙藻(Selenastrum)[40],说明了叶绿体膜在转运和累积甲基汞可能发挥重要功能. 此外,藻类结构差异可能导致甲基汞的后续传递差异,如浮游动物与聚球藻以及原绿球藻的甲基汞浓度之比仅为0.7左右,显著低于硅藻等真核藻类,这可能因为原核细胞的细胞器较少,胞内膜结构不发达使甲基汞更趋于在藻类细胞碎屑等金属解毒部分累积,进而降低甲基汞在食物链中的传递效率(图2)[81].
藻类的细胞活性也是影响其富集甲基汞的重要因素. 稳定生长阶段藻类用于主动运输的能量较多[40],因此鼓藻在稳定生长阶段甲基汞吸收量(759 amol·cell−1)显著高于其在指数生长阶段的吸收量(38.1 amol·cell−1).
-
(1)pH:藻类对重金属的吸收受pH调控. 以往研究认为pH下降时,甲基汞与H+竞争细胞表面的阳离子吸附位点引起藻类的甲基汞吸附量的降低[82],但目前证据显示pH下降增加藻类对甲基汞的富集. 研究发现全球范围内pH较低水体中藻类甲基汞含量普遍高于高pH水体[83]. 进一步的研究发现酸化增加藻类对甲基汞的富集,如当pH由6.5降低至5.5后,莱茵衣藻对氯化甲基汞的富集程度增加了1.6倍到2倍[84]. 这种现象可能与水体中甲基汞的赋存形态以及浓度改变有关:首先,酸化导致水体中与有机质结合的甲基汞释放,增加了具有生物可利用性的甲基汞量[85];其次,pH较低时,甲基汞的主要形态为更易进入细胞的氯化甲基汞,其生物可利用高于其他汞形态[43];另外,酸化条件下,细胞膜通透性会随藻类脂质与叶绿体改变而变化,促进甲基汞的扩散[86].
(2)温度:气候变暖已经成为全球面临的重要问题. 2010年全球海水表层平均温度较100年前升高了0.6 ℃[87]. 温度升高不仅引起海洋的酸化[88],也可促进藻类的生长繁殖,提高藻类生产力,进而影响藻类对甲基汞的富集[89]. 实验室条件下模拟实验显示温度由20 ℃增长至40 ℃时,羊角月牙藻的甲基汞吸收速率上升70%[40]. 但温度改变对单位生物量藻中甲基汞含量影响较小,提示升温可能通过增加藻类生物量促进其累积甲基汞[90]. 因此,虽然藻类生长繁殖受温度改变影响较大,藻类富集甲基汞的能力对温度变化并不敏感[21].
(3)溶解性有机质:多数情况下,DOM通过巯基结合甲基汞进而降低藻类对甲基汞的富集[91]. 高浓度DOM(20 mg·L−1)的添加可使梅尼小环藻甲基汞的VCF下降90%,低浓度的DOM(1.5 mg·L−1)也使其VCF下降一半[92]. 此外,疏水DOM对藻类富集甲基汞的抑制效果比亲水DOM更显著,这可能是由于疏水DOM中与甲基汞结合的双齿芳香基团的含量更高,比亲水DOM更易络合甲基汞[92].
但是,也有研究发现梅尼小环藻、莱茵衣藻和隐鞭藻在高DOM环境水体(旧金山湾三角洲)中甲基汞VCF是其在低DOM环境水体(科苏姆内斯河)中的2倍以上[45]. 该现象可能与DOM的异质性有关. 不同来源的DOM对藻类累积甲基汞的影响存在差异. 通过大肠杆菌生物传感器揭示DOM对甲基汞细胞富集的影响,结果为来自原绿藻和易变裸藻的EPS严重抑制了甲基汞的富集,而来自纤细裸藻的EPS的抑制效果并不显著[93]. 此外,蓝藻的EPS也在一定程度上降低铜绿假单胞菌(Microcystis aeruginosa)对甲基汞的富集[94],上述结果提示了不同来源的EPS可能对藻类富集甲基汞的影响效果存在差异. 通过分离和分析EPS中不同分子量的组分发现氨基酸与多胺的量与甲基汞富集量呈正相关,而羧基及其衍生物与甲基汞富集量呈负相关[93]. 对小分子DOM模型研究发现,不同DOM的形态与配位作用差异也影响了谷胱甘肽、半胱氨酸和巯基乙酸等含巯基化合物对藻类富集甲基汞的抑制效果[24].
(4)其他因素:研究发现水体中氯离子和硒也影响藻类累积甲基汞. 氯离子可与甲基汞络合,当氯离子浓度由0.47 mmol·L−1增加至470 mmol·L−1时,水体中氯化甲基汞的占比由13%增至99%,布氏双尾藻对甲基汞的VCF由4.0×104增至1.1×105[34],这可能是由于氯化甲基汞的膜渗透率高于氢氧化甲基汞[43]. 硒代甲硫氨酸可使海链藻对二价汞的4 h富集率由30%增长至70%,使甲基汞的4 h富集率从75%下降至44%[95]. 这可能是由于二价汞、甲基汞与硒代甲硫氨酸形成的复合物的跨膜特性不同[96],而胞内汞硒复合物的生成也可能影响藻类对甲基汞的吸收、外排及营养级传递[95].
-
浮游动物可通过摄食藻类进而累积汞,其对甲基汞与二价汞的同化效率存在显著差异[20,97]. 如汤氏纺锤水溞(Acartia tonsa)对藻类饵料中甲基汞的同化效率为58%—79%,对二价汞的同化效率仅为25%—31%,导致甲基汞的营养级放大因子(> 1)显著高于二价汞(0.2)[98].
甲基汞营养级传递受藻类和浮游动物等生物因素影响. 研究表明藻类细胞质中甲基汞含量与浮游动物的同化率有密切关系(r = 0.95),提示细胞质中甲基汞更易被浮游动物同化[98]. 另外,藻类种类也影响甲基汞在浮游动物中的同化作用,如汤氏纺锤水溞对小型假微型海链藻甲基汞的同化率(71%)低于同属大体积的威氏海链藻的同化率(88%)[99]. 因此,不同藻类对食物链甲基汞传递的贡献不同,模型结果表明硅藻与聚球藻分别贡献了沿食物链转移甲基汞的35%与25%,而其余藻类对甲基汞的贡献为40%[81]. 此外,浮游动物的甲基汞浓度随其体积增加而增加[100],甲基汞同化率随着肠道通过时间的增加而增加[101].
环境变化也是影响甲基汞食物链传递的重要因素[102]. 升温可在一定程度上增加产甲基汞菌的甲基化活性[103]以及藻类对甲基汞的累积[100],也可通过改变藻类消费者的生命活动(如排泄与生长速率等)影响甲基汞的食物链传递[90]. 当从14 ℃增至24 ℃时,大型溞对藻类的甲基汞同化率无显著变化,但显著影响排泄和生殖过程对大型溞体内甲基汞排出量的贡献,其中排泄的贡献率由52%升至85%,生殖的贡献率由43%降至11%,总外排量降低使甲基汞累积增加[104]. 因此,温度变化可通过改变浮游动物生命活动来影响甲基汞食物链传递.
目前广泛发生的富营养化也影响甲基汞的食物链传递,但是具体效果存在争议. 一种观点认为富营养化可以增加藻类生物量,降低单个藻类甲基汞的累积,如富营养化使藻类生物量增加3倍时,浮游植物甲基汞含量降低,进而导致水溞的甲基汞含量下降70%[105]. 另一种观点认为富营养化可提高水体中甲基汞的浓度. 富营养化引起的藻源性有机质的增加可刺激微生物汞甲基化[106],藻类的大规模凋亡阶段也会释放大量甲基汞[107]. 模型结果表明富营养化可使波罗的海水体内甲基汞总量增加4倍,间接增加藻类对甲基汞的富集[106]. 在巢湖、东湖与滇池水样品中加入藻类可使甲基汞产量提升了24.3%—15918%[108]. 但是,富营养化对甲基汞食物链传递的综合影响尚需深入探究.
-
藻类可通过吸附、主动运输和被动扩散富集水中的甲基汞,并通过转化、解吸、外排和食物链传递等过程影响甲基汞的环境归趋. 生物与环境因素是影响甲基汞藻类累积及其食物链传递的重要因素. 由于藻类种类繁多且生理状态多变,不同区域pH、温度和DOM等环境条件差异巨大,因此藻类富集和传递甲基汞过程非常复杂. 目前,藻类富集甲基汞的机制与影响因素尚待厘清. 如藻类主动吸收甲基汞的转运蛋白与机制仍不清楚,藻类对甲基汞的主动外排和转化途径(如甲基化与硫化)仍有待证实. 此外,pH和DOM等环境因素对藻类富集甲基汞与后续食物链传递的影响存在争议,需深入研究.
目前,藻类富集甲基汞及其食物链传递的研究大部分为实验室模拟实验,未来可将实验室模拟实验与长期的现场研究相结合,以明确实际环境中甲基汞的藻类累积和食物链传递行为. 多同位素示踪、全细胞生物传感器和同步辐射等技术可望在阐明酸化、富营养化和全球变暖等背景下藻类对甲基汞富集与后续营养传递中扮演重要角色.
藻类对甲基汞的富集与传递研究进展
Research progress on the bioconcentration of methylmercury in algae and its trophic transfer
-
摘要: 甲基汞是一种高毒性的污染物,其易累积在水生生物体内,从而对水生生态系统和人体健康产生危害. 作为水生生态系统的初级生产者,藻类控制着进入食物链的甲基汞浓度和总量. 藻类对甲基汞的显著富集作用及水生食物链传递过程导致其在高营养级生物中显著累积. 因此,厘清藻类在甲基汞的富集与食物链传递过程中的作用对于揭示甲基汞的生物累积和预测甲基汞的环境风险具有重要意义. 本文概述了藻类对甲基汞的富集与食物链传递特征与机制,总结了影响富集与食物链传递的生物与环境因素,讨论了全球变暖和富营养化等环境变化对藻类富集与传递甲基汞的影响,并展望了藻类富集甲基汞研究的发展方向.Abstract: Methylmercury (MeHg), a highly toxic pollutant, can be bioaccumulated by aquatic organisms and cause risks to both the aquatic ecosystem and human health. As the primary producer in the aquatic ecosystem, algae determine the level of MeHg introduced into the food web. The high bioconcentration of MeHg in algae from water and its further trophic transfer lead to its biomagnification in organisms at higher trophic level, which is of great significance to reveal bioaccumulation of MeHg and predict its risk. In this review, we outline the characteristics and mechanisms of MeHg bioconcentration in algae and further trophic transfer. The biological and environmental factors affecting bioconcentration and trophic transfer are discussed. Future research directions on MeHg bioconcentration in algae are also proposed.
-
Key words:
- algae /
- methylmercury /
- bioconcentration /
- trophic transfer /
- adsorption /
- absorption.
-
作为一种具有高毒性、生物累积和生物放大效应的重金属,汞(Hg)主要以气态形式被排放到大气中,随后可通过大气环流进行全球传输并沉降到陆地和海洋生态系统. 化石燃料燃烧、金属冶炼和垃圾焚烧等人为活动每年产生2000—3000 t的汞排放,而地质活动等自然源的每年汞排放量可达5500 t[1]. 目前,在无明显人为源的偏远地区也普遍检出汞[2],如青藏高原的海螺沟冰川融水中汞含量为6.96—10.78 ng·L-1[3],北极苔原也有显著大气汞沉降[4]. 为降低汞污染对生态系统与人体健康的危害,2013年世界上128个国家和地区签署了《关于汞的水俣公约》以减少汞的使用与人为排放[5],2018年全球人为导致的汞排放较2013年已出现下降[6]. 然而,由于历史上累积汞在表生环境中的再释放和循环,汞污染问题仍将长期存在[7]. 此外,全球变暖可能导致冰川与冻土中汞的释放[8],而升温引起的生态系统中初级生产力提高、生物习性改变以及食物网结构的变化也可能加剧生物体中汞的累积[9].
引起水俣病等公害事件的甲基汞(MeHg)是毒性最高的汞形态之一,其主要来源于硫酸盐还原菌、铁还原菌和产甲烷菌等含有hgcAB基因簇微生物的甲基化[10]. 甲基汞通过与含巯基的蛋白质结合,对生物体造成以神经系统为主的全身性损伤,并可跨过胎盘屏障导致先天性疾病[11-13]. 甲基汞的健康风险与甲基汞暴露直接相关,如食用鱼肉等水产品是甲基汞的重要暴露途径[12,14]. 尽管水中甲基汞在总汞中的占比通常较低,但鱼体中甲基汞可达总汞的95%左右[15],该现象主要是因为甲基汞的生物累积和生物放大效应. 作为海洋中的初级生产者,藻类具有极强的汞富集能力,部分藻中汞浓度可达水环境的103—106倍[16]. 藻类富集的甲基汞可通过食物链传递并在其他生物体内累积,甲基汞的营养级放大斜率可达0.15 —0.35,表现出显著的生物放大效应[17-20]. 同时,日趋严重的全球变暖、水体酸化和富营养化等问题带来了环境因子、藻类丰度、汞浓度与可利用性的新变化.
开展甲基汞在藻类中的富集过程研究以及这一过程对水体甲基汞生物累积与放大的关键影响研究对于揭示汞的生物富集、传递特性以及预测其风险至关重要. 因此,本文对藻类富集甲基汞的特征、机理及影响因素进行详细讨论,总结甲基汞在藻类中的分布、转化与后续营养传递特征,并对相关研究的发展方向进行展望.
1. 藻类富集甲基汞的特征、机制和影响因素(The patterns, mechanisms, and influencing factors of methylmercury bioconcentration in algae)
藻类对甲基汞的富集涉及吸收、吸附、转运、解吸和外排等多个过程(图1),这些过程对甲基汞在藻类中的累积和食物链传递有重要影响. 首先,藻类可通过吸附、吸收、解吸和外排影响进入食物链的甲基汞的量;此外,藻类及其伴生菌与胞外分泌物可能通过去甲基化或甲基化改变食物链中甲基汞浓度;再者,甲基汞在藻体的不同亚细胞结构中的分布会影响甲基汞在食物链中的传递行为.
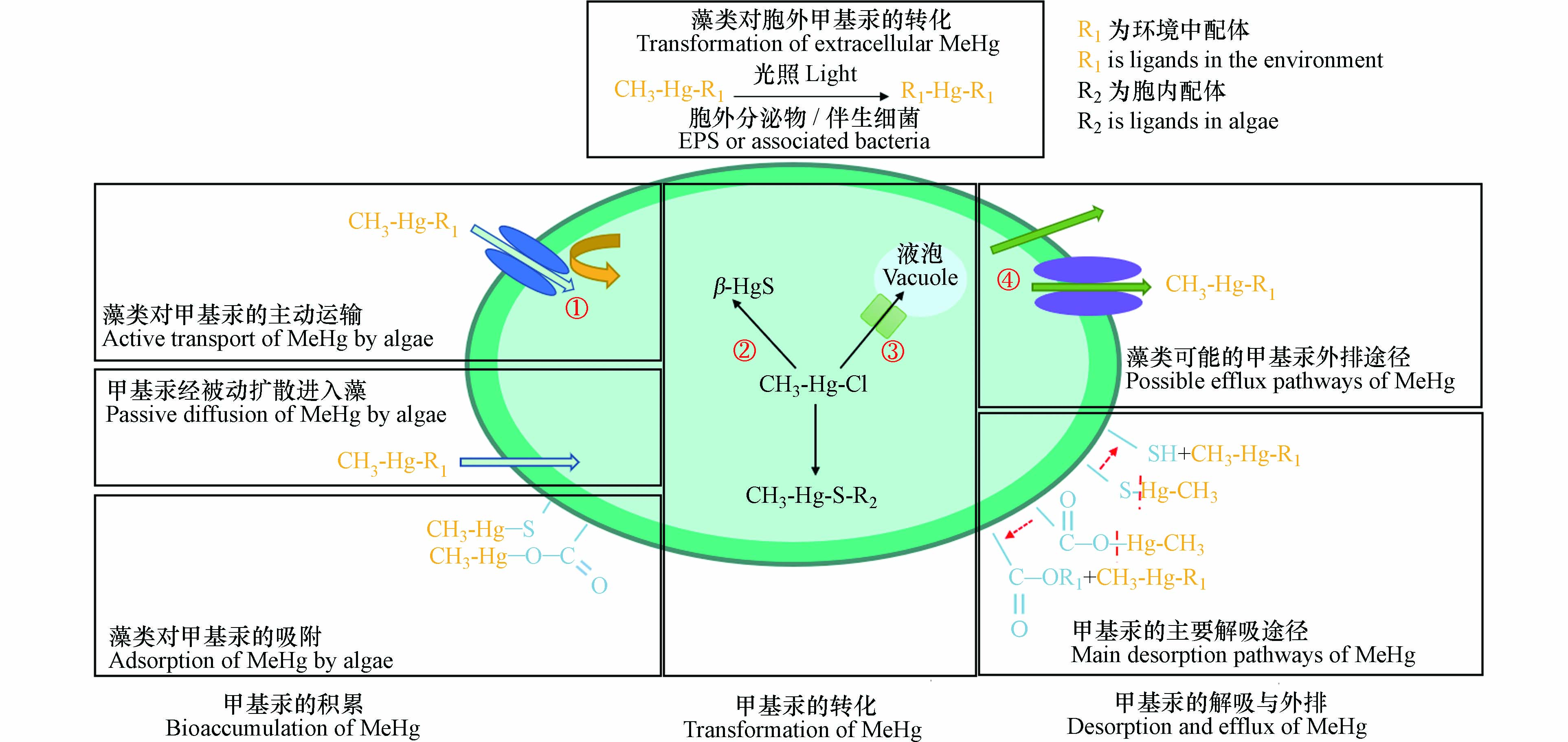 图 1 藻类吸附、吸收、转化、解吸与外排甲基汞的相关机制Figure 1. The proposed mechanisms of adsorption, absorption, transformation, desorption and efflux of MeHg by algae(① 未确定甲基汞主动运输的转运蛋白;② 未确定甲基汞是否可以转化为硫化汞颗粒;③ 未确定甲基汞进入液泡的途径;④ 未确定甲基汞的解吸与外排)(① The transporter of MeHg via active transport has not been identified; ② It is unknown whether MeHg can be converted to β-HgS; ③ The pathway of MeHg entering the vacuole has not been determined; ④ The desorption and efflux of MeHg are not determined)
图 1 藻类吸附、吸收、转化、解吸与外排甲基汞的相关机制Figure 1. The proposed mechanisms of adsorption, absorption, transformation, desorption and efflux of MeHg by algae(① 未确定甲基汞主动运输的转运蛋白;② 未确定甲基汞是否可以转化为硫化汞颗粒;③ 未确定甲基汞进入液泡的途径;④ 未确定甲基汞的解吸与外排)(① The transporter of MeHg via active transport has not been identified; ② It is unknown whether MeHg can be converted to β-HgS; ③ The pathway of MeHg entering the vacuole has not been determined; ④ The desorption and efflux of MeHg are not determined)1.1 甲基汞在藻类中的累积规律与机制
藻类可显著累积水体甲基汞,基于野外数据建立的模型显示浮游植物对甲基汞的生物累积因子(bioaccumulation factor,BAF)达102.4—105.9[21]. 由于实际环境中浮游生物是由多种藻类组成,难以确定不同藻类对甲基汞富集的具体贡献,所以藻类对甲基汞的富集研究通常选择环境中的硅藻与绿藻等优势藻种在实验室条件下开展工作[22]. 该部分论述了藻对甲基汞的累积规律,以及相关的吸附与吸收机制.
1.1.1 甲基汞在藻类中的累积规律
藻类累积甲基汞研究的主要指标包括藻类的体积富集因子(volume concentration factor,VCF)、富集率以及累积速率(具体计算方法见表1注). 表1比较了不同研究结果,其中,甲基汞的形态和暴露时间是影响藻类富集甲基汞的重要因素. 首先,不同形态甲基汞在藻类中的富集存在差异,相较于氯化甲基汞,半胱氨酸与蛋氨酸与甲基汞的络合会减弱藻类对甲基汞的富集[23-24]. 此外,藻类对甲基汞的富集具有一定的时间变化规律,通常在初始5 min内非常迅速,在24 h左右达到平衡[25],因此暴露24 h可测定平衡状态下藻类的富集率等指标,暴露1 h内可反映短期的甲基汞富集动力学变化.
表 1 实验室条件下常见藻类对甲基汞的富集效果Table 1. Bioconcentration of MeHg in common algae under laboratory conditions所属纲Class 受试藻类Subject algae 甲基汞形态MeHg species 甲基汞浓度/(nmol·L−1)Concentration 暴露时间/hExposure time 是否清洗Whether cleaned 吸收速率/(cell−1 h−1·nmol·L−1)aAbsorption rate lgVCFb/生物累积因子lgVCF/BCF 富集率cEnrichment ratio 文献References 绿藻纲 葡萄鼓藻Cosmarium botrytis CH3HgCl 0.02 24e 否 —f 5.94 ± 0.69b —f [30] 绿藻纲 钙化裂须藻Schizothrix calcicola CH3HgCl 0.02 24d 否 —f 5.60 ± 0.21b —f [30] 绿藻纲 小球藻Chlorella autotrophica CH3HgCl 3.29 72g 8 mmol·L−1半胱氨酸pH = 8.2,1 min —f 113.8h —f [31] 绿藻纲 羊角月牙藻Selenastrum capricornutum CH3HgCl 1.5 1 否 1.3×10−4 —f 0.97 [23] 绿藻纲 羊角月牙藻Selenastrum capricornutum CH3Hg-GSH 1.5i 1 否 1.03×10−4 —f 0.93 [23] 绿藻纲 羊角月牙藻Selenastrum capricornutum CH3HgCl 0.02 24e 否 —f 6.67 ± 0.13b —f [30] 绿藻纲 羊角月牙藻Selenastrum capricornutum CH3Hg-Cys 1.5i 1 否 1.11×10−4 —f 0.92 [23] 绿藻纲 蛋白核小球藻Chlorella pyrenoidsa CH3HgCl 0.4 24e 0.8 mmol·L−1半胱氨酸pH = 7.1,5 min 0.08 —f 0.46 [28] 绿藻纲 杜氏藻Dunaliella tertiolecta CH3HgCl 0.29—0.42 4d 否 0.63 ± 0.03 4.46b —f [32] 绿藻纲 斜生栅藻Scenedesmus obliquus CH3HgCl 0.4 24e 0.8 mmol·L−1半胱氨酸pH = 7.1,5 min 0.02 —f 0.86 [28] 蓝藻纲 聚球藻Synechococcus bacillaris CH3HgCl 0.29—0.42 4d 否 0.97±0.03 6.34b —f [32] 硅藻纲 海链藻Thalassiosira sp. CH3HgCl 0.02 24e 否 —f 5.37 ± 0.04b —f [30] 硅藻纲 假微型海链藻Thalassiosira pseudonana CH3Hg-Cys 4×10−3 1d 否 11—14×10−3 5.47b —f [32] 硅藻纲 假微型海链藻Thalassiosira pseudonana CH3HgCl 0.29—0.42 4d 否 15.00 ± 3.00 5.44b —f [32] 硅藻纲 假微型海链藻Thalassiosira pseudonana CH3Hg-Met 0.28—0.48 l 4 否 16.9 ± 0.2 6.22b —f [24] 硅藻纲 假微型海链藻Thalassiosira pseudonana CH3Hg-HA 0.28—0.48l 4 否 11.9 ± 1.5 6.07b —f [24] 硅藻纲 假微型海链藻Thalassiosira pseudonana CH3HgCl 7.32 72g 8 mmol·L−1半胱氨酸pH = 8.2,1 min —f 14.7h —f [31] 硅藻纲 威氏海链藻Thalassiosira weissflogii CH3HgCl 1.85 1d 8 mmol·L−1半胱氨酸pH = 8.2,1 min 4.6 9.8×106 j —f [33] 硅藻纲 威氏海链藻Thalassiosira weissflogii CH3HgCl 1.85 1k 8 mmol·L−1半胱氨酸pH = 8.2,1 min 3.01 7.4×106 j —f [33] 硅藻纲 布氏双尾藻Ditylum brightwellii CH3HgCl 0.1 1d 否 130 5.49b —f [34] 硅藻纲 旋链角毛藻Chaetoceros curvisetus CH3HgCl 2.5 5d 否 1.8 6.04b —f [34] 鞭藻纲 等鞭金藻Isochrysis galbana CH3HgCl 4.59 72g 8 mmol·L−1 半胱氨酸pH = 8.2,1 min —f 79.23h —f [31] 注:GSH: Glutathione; Cys: Cysteine; Met: Methionine; HA: Humic acid. (a. 单位为cell−1 h−1 nmol·L−1;b.体积富集因子(Volume concentration factor,VCF)=平衡后藻类中的甲基汞浓度(mol·µm−3)/水中的甲基汞浓度(mol·µm−3);c.富集率 = 平衡后藻类中的甲基汞含量(µg)/水中的甲基汞含量(µg);d.培养过程为全程光照条件;e.培养中明暗时间比为12 : 12;f.文章内未给出具体数据,用“—”表示;g.培养中明暗时间比为14 : 10;h.生物富集因子(Bioaccumulation factor,BAF)= 平衡后藻类中的甲基汞浓度(µg·g−1 湿重)/水中的甲基汞浓度(µg·g−1);i.藻类暴露甲基汞浓度为1.5 nmol·L−1,表中谷胱甘肽或半胱氨酸的浓度为90 nmol·L−1;j.生物富集因子 = 平衡后藻类中的甲基汞浓度(µg·mol−1 以生物炭计)/水中的甲基汞浓度(µg·L−1);k.藻在暴露前在2 µg·L−1的甲基汞溶液中驯化18 d(明暗时间比为14 : 10),大约为第18代藻类;l.暴露的蛋氨酸浓度为1 nmol·L−1,胡敏酸浓度为1 mg·L−1 C) (a. cell−1 h−1 nmol−1; b. VCF = MeHg concentration in algae (mol·µm−3) / MeHg concentration in water (mol·µm−3) after equilibrium; c. The enrichment rate = MeHg concentration in algae (µg) / MeHg concentration in water (µg) after equilibrium; d. The cultivation process was under full light; e. The ratio of light and dark time in culture was 12 : 12; f. No specific data is given in the reference, which is indicated by "-"; g. The ratio of light and dark time in culture was 14 : 10; h. BAF = MeHg concentration in algae (µg·g−1 wet weight) / MeHg concentration in water (µg·g−1) after equilibrium; i. The concentration of MeHg exposed to algae is 1.5 nmol·L−1, and the concentration of glutathione or cysteine in the table is 90 nmol·L−1; j. Bioconcentration factor = MeHg concentration in algae (µg·mol−1 in biochar) / MeHg concentration in water (µg·L−1) after equilibrium; k. Algae were acclimated in 2 µg·L−1 MeHg solution for 18 days before exposure (the ratio of light and dark time was 14:10), which was about the 18th generation of algae; l. The exposed methionine concentration was 1 nmol·L−1 and the humic acid concentration was 1 mg·L−1 C.) 藻类富集甲基汞主要通过吸收和吸附两种途径[26]. 一些研究采用乙二胺四乙酸(EDTA)或半胱氨酸等络合剂清洗藻类表面吸附的甲基汞,用以区分藻类通过吸附与吸收富集的甲基汞. 其中,0.8 mmol·L−1半胱氨酸清洗5 min或8 mmol·L−1半胱氨酸清洗1 min,藻类吸附甲基汞的解吸率可达90%以上[27]. 研究发现,藻类富集甲基汞的差异可能来源于藻类对甲基汞吸附与吸收的比例不同. 如5 ng·L−1 的甲基汞暴露下,斜生栅藻(Scenedesmus obliquus)平衡后的吸附率(藻吸收的甲基汞量/水体甲基汞量)为16.3%,低于蛋白核小球藻(Chlorella pyrenoidosa)(28.2%),但二者的吸附率(藻吸附的甲基汞量/水体甲基汞量)接近,且随甲基汞暴露浓度增加,斜生栅藻吸收率的增加幅度显著高于蛋白核小球藻[28]. 另外,暴露时间由24 h增加至168 h,蛋白核小球藻的吸附率由21.2%下降至11.3%,吸收率则由38.3%上升至46.9%[28]. 由于吸附速率大于吸收速率,推测藻类富集甲基汞前期以胞外快速吸附为主,平衡后通过胞内缓慢吸收进行富集[29].
1.1.2 藻类对甲基汞的吸附机制
藻类累积甲基汞的反应动力学研究发现水华束丝藻(Aphanizomenon flosaquae)与铜绿微囊藻(Microcystis aeruginosa)对甲基汞的吸附均较好地符合准二级动力学模型(R2 > 0.99),提示这一过程可能涉及物理吸附与化学吸附,化学吸附可能涉及藻类表面基团与甲基汞间的电子转移或共用;这两种藻类对甲基汞的吸附也可较好地由Freundlich等温吸附模型(R2 = 0.9248与0.8614)拟合[25],提示在藻细胞表面可能存在多个吸附位点同时起作用,且吸附位点间表现出不同的吸附自由能与剩余度.
通过对椭圆小球藻(Chlorella ellipticus)、鱼腥藻(Anabaena)、水华束丝藻和铜绿微囊藻的红外光谱研究,藻类通过细胞壁或胞外多聚物(extracellular polymeric substances,EPS)上广泛存在的氨基、巯基、羧基和羟基等官能团与甲基汞结合[35-36]. 不同官能团与甲基汞的结合机理不同,羟基和羧基等官能团通过孤对电子与甲基汞形成配位键[37],磺酸基等官能团则通过失去电子形成负电荷后的静电作用吸附甲基汞[38]. 与藻体内含量较低的巯基等含硫基团相比,羟基与羧基对藻类吸附甲基汞的贡献可能较大也更易体现[36,39]. 羟基与羧基的吸附过程为环境中的甲基汞-配体复合物(CH3Hg-L)与细胞表面的官能团(R)经配体交换形成新的络合物(CH3Hg-R),CH3Hg-L与CH3Hg-R的相对热力学稳定常数决定了络合速率,此外L与R的空间位阻也可能影响吸附的速率,如谷胱甘肽、N-乙酰-L-半胱氨酸和N-乙酰-青霉胺等较大尺寸或支链较多的硫醇配体结合甲基汞的程度小于半胱氨酸和巯基乙酸等小分子配体[23].
1.1.3 藻类对甲基汞的吸收机制
研究发现三磷酸腺苷(adenosine triphosphate,ATP)和转运蛋白参与了藻类对甲基汞的吸收,表明藻类可以通过主动运输富集甲基汞. 光系统Ⅰ抑制剂(甲基紫精)、光系统Ⅱ抑制剂(二氯酚二甲基脲)、避光与γ-辐射等减少ATP的措施可抑制藻类对甲基汞的吸收,证明藻类存在需要ATP的甲基汞吸收过程[40-41]. 尽管藻类的甲基汞转运蛋白尚未确定,但在仓鼠卵巢细胞(ATCC CCL-61)中,LAT1转运蛋白承担了甲基汞-半胱氨酸复合物(MeHg-L-cysteine)的运输功能[41]. 研究猜测MeHg-L-cysteine的结构类似于LAT1转运蛋白的底物蛋氨酸,从而导致甲基汞被错误运输[42]. LAT1蛋白过表达的CHO-k1细胞中MeHg-L-cysteine与蛋氨酸的竞争吸收现象进一步支持了上述猜测的合理性[41]. 而参与藻类主动吸收甲基汞途径的转运蛋白仍需进一步研究.
另外,藻类也可通过被动扩散吸收水体中的甲基汞. 如不同pH和氯离子浓度下,甲基汞的辛醇-水分配系数与威氏海链藻对甲基汞的吸收速率呈正相关关系(r = 0.75)[43],而且温度升高10 ℃对甲基汞吸收速率的影响(Q10)值仅为0.7,显著低于温度对主动运输吸收速率的影响(普遍为2—3)[40,44],表明甲基汞可通过被动扩散进入藻细胞. 但有研究发现经热灭活破坏主动运输途径后,梅尼小环藻(Cyclotella meneghiniana)的甲基汞吸收量显著下降,甲基汞在细胞质中占比由64%降至4%,证明被动扩散在甲基汞吸收中的贡献可能较低[45].
1.2 藻类对甲基汞的解吸与外排
藻类可能存在解吸与外排降低藻类中甲基汞的含量(图1). 在暴露168 h后,蛋白核小球藻的甲基汞吸附量相较最高吸附量(24 h)下降约40%,单个斜生栅藻的甲基汞吸收量相较最高吸收量(24 h)下降约74%[28]. 以上藻类对甲基汞的吸附和吸收的降低与藻类生长稀释以及增殖稀释有关[21],但也可能是由于甲基汞发生解吸和外排.
由于藻类对甲基汞的吸附、吸收、解吸和外排等过程可能同时发生,藻类对甲基汞的吸附和外排过程难以观察与定量. 藻类中甲基汞的解吸过程未见报道,但有研究表明震荡可使椭圆小球藻、水华束丝藻和铜绿微囊藻中69%、42%和34%的二价汞发生解吸[46]. 对于藻类中甲基汞的外排,过去研究认为甲基汞胁迫下藻类细胞膜通透性改变是原因之一[47]. 另外,甲基汞胁迫会导致莱茵衣藻(Chlamydomonas reinhardtii)中的铁转运蛋白、锌转运蛋白、ATP结合转运蛋白与阳离子扩散蛋白等金属转运相关蛋白质表达上调,因此,甲基汞外排可能需要转运蛋白的参与[26]. 但甲基汞胁迫下,一些蛋白质的高表达并非参与甲基汞的外排,如ATP结合蛋白的基因表达上调增加了胞内MRP2蛋白的含量,此蛋白可将甲基汞转移到液泡中而非排出体外[48]. 因此,后续需对甲基汞外排相关转运蛋白的具体作用机制进行研究.
1.3 藻类对汞甲基化和去甲基化的作用
目前,没有证据表明藻类可以直接产生甲基汞,但有研究认为藻类逆境中产生的代谢物二甲基硫分解为甲磺酸的过程可能导致甲基汞的生成[49]. 以往研究发现北极环境雪与地表水样品中的甲磺酸量与甲基汞量呈正相关[50],且二甲基硫转化过程中具有与硫还原菌相似的四氢叶酸途径[51],表明该途径可能存在,但暂未得到证实.
已有研究证据表明藻类可通过影响产甲基汞菌活性间接改变甲基汞浓度. 如向巴西采集的含微生物环境样品中加入蓝藻后,甲基汞净产率由6.8%增加至24.6%,而仅存在蓝藻的对照组水样中无甲基汞的产生[52]. 产甲基汞菌活性的提高可能是因为蓝藻增加了细菌群落所需的氢气[53],同时藻源性的叶绿素、蛋白质、细胞壁和脂质等物质也可增加产甲基汞细菌活性[54]. 另外,不同环境样品中蓝藻生物量与甲基汞含量呈正相关趋势[55]. 然而,藻类也可能抑制甲基汞产生,如中肋骨条藻(Skeletonema costatum)通过吸附二价汞(47%)降低了铁还原菌的甲基汞产量[56]. 此外,藻类也可能通过改变水体透光率以及溶解氧含量等影响汞的甲基化[57].
除光致去甲基化[58]与化学去甲基化[59]外,最近研究发现浮游生物群落也可在24 h内降解水样中12%的甲基汞[60]. 双富集同位素技术证明三角褐指藻(Phaeodactylum tricornutum)与旋链角毛藻(Chaetoceros curvisetus)等6种藻类可以引起甲基汞去甲基化,其中三角褐指藻等藻类可通过胞外分泌物实现甲基汞的光致去甲基化,东海原甲藻(Prorocentrum donghaiense)的去甲基化能力源于其伴生细菌,旋链角刺藻的去甲基化作用则来自于伴生细菌与胞外分泌物的共同作用[61]. 上述不同途径的去甲基化速率存在差异,胞外分泌物介导的光致去甲基化速率(0.01—0.39 d−1)显著高于伴生细菌的生物去甲基化(0.03—0.14 d−1)[61],该降解速率差异是由于反应机理不同[62-63]. 胞外分泌物被认为通过硫醇介导光致去甲基化[61],其可作为软碱与甲基汞的结合促进甲基汞的光降解[64];伴生细菌则可能通过甲基汞裂解酶(MerB)等途径降解甲基汞[65]. 但针对藻类及其伴生菌去甲基化过程的研究较少.
藻体内还存在其他改变环境中汞迁移性和生物可利用性的转化途径. 藻类可将二价汞还原为毒性低而挥发性强的溶解性零价汞[66],但此途径效率较低(< 5%),且机理尚不明确[67]. 通过冷原子荧光检测改进的酸碱还原差值法分析,硫化汞(β-HgS)也可能存在于藻类细胞中[68]. 有观点认为β-HgS是藻类中主要的无机汞形态(20%—90%),并推断其主要来源于液泡中二价汞的转化,但此前检测方法可能无法有效区分二价汞与硫化汞[69]. 未来可借助扩展X射线吸收精细结构谱等手段对硫化汞的生成过程进行详细研究[70].
1.4 藻类中甲基汞的亚细胞分布
通过差速离心、热处理与化学处理可将藻类分为细胞碎屑(包括细胞壁与细胞膜等)、细胞器、热稳定蛋白(如植物螯合肽等)、热变性蛋白(如酶等)与富金属矿体5部分[71-72]. 通常将富金属矿体、细胞碎屑与热稳定蛋白统称为金属解毒部分即生物非活性部分,将细胞器与热变性蛋白划分为金属敏感组分. 热稳定蛋白是甲基汞在藻类中的主要结合部分,其中甲基汞的累积量可占细胞总累积量的44%(假微型海链藻)、68%(小球藻)和80%(等鞭金藻)[31]. 在热稳定蛋白中,甲基汞主要与含巯基的半胱氨酸、谷胱甘肽和植物螯合肽等组分络合[33,73]. 与二价汞类似,甲基汞在热稳定蛋白中达到饱和后,可能会进一步累积到其他结构中,因此细胞碎屑与细胞器组分中也存在甲基汞,但均未超过总累积量的30%[31].
藻类中甲基汞的亚细胞分布对其生物可利用性可能有重要影响[74]. 图2总结了不同累积位点重金属的生物可利用性差异,细胞器与热变性蛋白等金属敏感部分中镉与银等重金属可沿食物链传递,而细胞碎屑与富金属颗粒等解毒组分中的重金属则不会沿食物链传递[75-77]. 以往研究通过明胶包裹含甲基汞的贻贝不同亚细胞组分喂食鱼类发现,热稳定蛋白、热变性蛋白和细胞器等组分中甲基汞被同化的比例高于不溶物等其他组分[78]. 而分布于不同亚细胞结构的重金属在食物链传递中存在差异[79]. 目前尚未开展藻类富集甲基汞的相关研究,藻类不同亚细胞结构在甲基汞传递中的作用还需进一步研究.
1.5 影响藻类富集甲基汞的因素
藻类富集甲基汞受生物因素和环境因素影响. 藻类的相对表面积、结构以及活性均是影响富集效果的重要生物因素,环境因素则包括pH、温度、溶解性有机质(dissolved organic matter,DOM)、氯离子和硒等.
1.5.1 生物因素
藻类的相对表面积可影响藻类富集甲基汞的能力. 齿状藻等藻类甲基汞的VCF与相对表面积的相关系数可达0.97[34]. 因此,相对表面积更大的原核藻类蓝藻的甲基汞富集能力高于常见的硅藻与隐藻等真核藻类[47].
藻类结构也影响甲基汞的富集. 如没有叶绿体的原核藻类裂须藻的甲基汞富集率显著低于被内质网包裹叶绿体的真核藻类海链藻[80],且这两种藻类的甲基汞富集量均小于含有完整的脂质双分子层叶绿体的鼓藻与月牙藻(Selenastrum)[40],说明了叶绿体膜在转运和累积甲基汞可能发挥重要功能. 此外,藻类结构差异可能导致甲基汞的后续传递差异,如浮游动物与聚球藻以及原绿球藻的甲基汞浓度之比仅为0.7左右,显著低于硅藻等真核藻类,这可能因为原核细胞的细胞器较少,胞内膜结构不发达使甲基汞更趋于在藻类细胞碎屑等金属解毒部分累积,进而降低甲基汞在食物链中的传递效率(图2)[81].
藻类的细胞活性也是影响其富集甲基汞的重要因素. 稳定生长阶段藻类用于主动运输的能量较多[40],因此鼓藻在稳定生长阶段甲基汞吸收量(759 amol·cell−1)显著高于其在指数生长阶段的吸收量(38.1 amol·cell−1).
1.5.2 环境因素
(1)pH:藻类对重金属的吸收受pH调控. 以往研究认为pH下降时,甲基汞与H+竞争细胞表面的阳离子吸附位点引起藻类的甲基汞吸附量的降低[82],但目前证据显示pH下降增加藻类对甲基汞的富集. 研究发现全球范围内pH较低水体中藻类甲基汞含量普遍高于高pH水体[83]. 进一步的研究发现酸化增加藻类对甲基汞的富集,如当pH由6.5降低至5.5后,莱茵衣藻对氯化甲基汞的富集程度增加了1.6倍到2倍[84]. 这种现象可能与水体中甲基汞的赋存形态以及浓度改变有关:首先,酸化导致水体中与有机质结合的甲基汞释放,增加了具有生物可利用性的甲基汞量[85];其次,pH较低时,甲基汞的主要形态为更易进入细胞的氯化甲基汞,其生物可利用高于其他汞形态[43];另外,酸化条件下,细胞膜通透性会随藻类脂质与叶绿体改变而变化,促进甲基汞的扩散[86].
(2)温度:气候变暖已经成为全球面临的重要问题. 2010年全球海水表层平均温度较100年前升高了0.6 ℃[87]. 温度升高不仅引起海洋的酸化[88],也可促进藻类的生长繁殖,提高藻类生产力,进而影响藻类对甲基汞的富集[89]. 实验室条件下模拟实验显示温度由20 ℃增长至40 ℃时,羊角月牙藻的甲基汞吸收速率上升70%[40]. 但温度改变对单位生物量藻中甲基汞含量影响较小,提示升温可能通过增加藻类生物量促进其累积甲基汞[90]. 因此,虽然藻类生长繁殖受温度改变影响较大,藻类富集甲基汞的能力对温度变化并不敏感[21].
(3)溶解性有机质:多数情况下,DOM通过巯基结合甲基汞进而降低藻类对甲基汞的富集[91]. 高浓度DOM(20 mg·L−1)的添加可使梅尼小环藻甲基汞的VCF下降90%,低浓度的DOM(1.5 mg·L−1)也使其VCF下降一半[92]. 此外,疏水DOM对藻类富集甲基汞的抑制效果比亲水DOM更显著,这可能是由于疏水DOM中与甲基汞结合的双齿芳香基团的含量更高,比亲水DOM更易络合甲基汞[92].
但是,也有研究发现梅尼小环藻、莱茵衣藻和隐鞭藻在高DOM环境水体(旧金山湾三角洲)中甲基汞VCF是其在低DOM环境水体(科苏姆内斯河)中的2倍以上[45]. 该现象可能与DOM的异质性有关. 不同来源的DOM对藻类累积甲基汞的影响存在差异. 通过大肠杆菌生物传感器揭示DOM对甲基汞细胞富集的影响,结果为来自原绿藻和易变裸藻的EPS严重抑制了甲基汞的富集,而来自纤细裸藻的EPS的抑制效果并不显著[93]. 此外,蓝藻的EPS也在一定程度上降低铜绿假单胞菌(Microcystis aeruginosa)对甲基汞的富集[94],上述结果提示了不同来源的EPS可能对藻类富集甲基汞的影响效果存在差异. 通过分离和分析EPS中不同分子量的组分发现氨基酸与多胺的量与甲基汞富集量呈正相关,而羧基及其衍生物与甲基汞富集量呈负相关[93]. 对小分子DOM模型研究发现,不同DOM的形态与配位作用差异也影响了谷胱甘肽、半胱氨酸和巯基乙酸等含巯基化合物对藻类富集甲基汞的抑制效果[24].
(4)其他因素:研究发现水体中氯离子和硒也影响藻类累积甲基汞. 氯离子可与甲基汞络合,当氯离子浓度由0.47 mmol·L−1增加至470 mmol·L−1时,水体中氯化甲基汞的占比由13%增至99%,布氏双尾藻对甲基汞的VCF由4.0×104增至1.1×105[34],这可能是由于氯化甲基汞的膜渗透率高于氢氧化甲基汞[43]. 硒代甲硫氨酸可使海链藻对二价汞的4 h富集率由30%增长至70%,使甲基汞的4 h富集率从75%下降至44%[95]. 这可能是由于二价汞、甲基汞与硒代甲硫氨酸形成的复合物的跨膜特性不同[96],而胞内汞硒复合物的生成也可能影响藻类对甲基汞的吸收、外排及营养级传递[95].
2. 藻类与浮游动物间甲基汞传递特征(Trophic transfer characteristics of methylmercury bioaccumulated from algae to zooplankton)
浮游动物可通过摄食藻类进而累积汞,其对甲基汞与二价汞的同化效率存在显著差异[20,97]. 如汤氏纺锤水溞(Acartia tonsa)对藻类饵料中甲基汞的同化效率为58%—79%,对二价汞的同化效率仅为25%—31%,导致甲基汞的营养级放大因子(> 1)显著高于二价汞(0.2)[98].
甲基汞营养级传递受藻类和浮游动物等生物因素影响. 研究表明藻类细胞质中甲基汞含量与浮游动物的同化率有密切关系(r = 0.95),提示细胞质中甲基汞更易被浮游动物同化[98]. 另外,藻类种类也影响甲基汞在浮游动物中的同化作用,如汤氏纺锤水溞对小型假微型海链藻甲基汞的同化率(71%)低于同属大体积的威氏海链藻的同化率(88%)[99]. 因此,不同藻类对食物链甲基汞传递的贡献不同,模型结果表明硅藻与聚球藻分别贡献了沿食物链转移甲基汞的35%与25%,而其余藻类对甲基汞的贡献为40%[81]. 此外,浮游动物的甲基汞浓度随其体积增加而增加[100],甲基汞同化率随着肠道通过时间的增加而增加[101].
环境变化也是影响甲基汞食物链传递的重要因素[102]. 升温可在一定程度上增加产甲基汞菌的甲基化活性[103]以及藻类对甲基汞的累积[100],也可通过改变藻类消费者的生命活动(如排泄与生长速率等)影响甲基汞的食物链传递[90]. 当从14 ℃增至24 ℃时,大型溞对藻类的甲基汞同化率无显著变化,但显著影响排泄和生殖过程对大型溞体内甲基汞排出量的贡献,其中排泄的贡献率由52%升至85%,生殖的贡献率由43%降至11%,总外排量降低使甲基汞累积增加[104]. 因此,温度变化可通过改变浮游动物生命活动来影响甲基汞食物链传递.
目前广泛发生的富营养化也影响甲基汞的食物链传递,但是具体效果存在争议. 一种观点认为富营养化可以增加藻类生物量,降低单个藻类甲基汞的累积,如富营养化使藻类生物量增加3倍时,浮游植物甲基汞含量降低,进而导致水溞的甲基汞含量下降70%[105]. 另一种观点认为富营养化可提高水体中甲基汞的浓度. 富营养化引起的藻源性有机质的增加可刺激微生物汞甲基化[106],藻类的大规模凋亡阶段也会释放大量甲基汞[107]. 模型结果表明富营养化可使波罗的海水体内甲基汞总量增加4倍,间接增加藻类对甲基汞的富集[106]. 在巢湖、东湖与滇池水样品中加入藻类可使甲基汞产量提升了24.3%—15918%[108]. 但是,富营养化对甲基汞食物链传递的综合影响尚需深入探究.
3. 总结与展望(Conclusions and perspectives)
藻类可通过吸附、主动运输和被动扩散富集水中的甲基汞,并通过转化、解吸、外排和食物链传递等过程影响甲基汞的环境归趋. 生物与环境因素是影响甲基汞藻类累积及其食物链传递的重要因素. 由于藻类种类繁多且生理状态多变,不同区域pH、温度和DOM等环境条件差异巨大,因此藻类富集和传递甲基汞过程非常复杂. 目前,藻类富集甲基汞的机制与影响因素尚待厘清. 如藻类主动吸收甲基汞的转运蛋白与机制仍不清楚,藻类对甲基汞的主动外排和转化途径(如甲基化与硫化)仍有待证实. 此外,pH和DOM等环境因素对藻类富集甲基汞与后续食物链传递的影响存在争议,需深入研究.
目前,藻类富集甲基汞及其食物链传递的研究大部分为实验室模拟实验,未来可将实验室模拟实验与长期的现场研究相结合,以明确实际环境中甲基汞的藻类累积和食物链传递行为. 多同位素示踪、全细胞生物传感器和同步辐射等技术可望在阐明酸化、富营养化和全球变暖等背景下藻类对甲基汞富集与后续营养传递中扮演重要角色.
-
表 1 实验室条件下常见藻类对甲基汞的富集效果
Table 1. Bioconcentration of MeHg in common algae under laboratory conditions
所属纲Class 受试藻类Subject algae 甲基汞形态MeHg species 甲基汞浓度/(nmol·L−1)Concentration 暴露时间/hExposure time 是否清洗Whether cleaned 吸收速率/(cell−1 h−1·nmol·L−1)aAbsorption rate lgVCFb/生物累积因子lgVCF/BCF 富集率cEnrichment ratio 文献References 绿藻纲 葡萄鼓藻Cosmarium botrytis CH3HgCl 0.02 24e 否 —f 5.94 ± 0.69b —f [30] 绿藻纲 钙化裂须藻Schizothrix calcicola CH3HgCl 0.02 24d 否 —f 5.60 ± 0.21b —f [30] 绿藻纲 小球藻Chlorella autotrophica CH3HgCl 3.29 72g 8 mmol·L−1半胱氨酸pH = 8.2,1 min —f 113.8h —f [31] 绿藻纲 羊角月牙藻Selenastrum capricornutum CH3HgCl 1.5 1 否 1.3×10−4 —f 0.97 [23] 绿藻纲 羊角月牙藻Selenastrum capricornutum CH3Hg-GSH 1.5i 1 否 1.03×10−4 —f 0.93 [23] 绿藻纲 羊角月牙藻Selenastrum capricornutum CH3HgCl 0.02 24e 否 —f 6.67 ± 0.13b —f [30] 绿藻纲 羊角月牙藻Selenastrum capricornutum CH3Hg-Cys 1.5i 1 否 1.11×10−4 —f 0.92 [23] 绿藻纲 蛋白核小球藻Chlorella pyrenoidsa CH3HgCl 0.4 24e 0.8 mmol·L−1半胱氨酸pH = 7.1,5 min 0.08 —f 0.46 [28] 绿藻纲 杜氏藻Dunaliella tertiolecta CH3HgCl 0.29—0.42 4d 否 0.63 ± 0.03 4.46b —f [32] 绿藻纲 斜生栅藻Scenedesmus obliquus CH3HgCl 0.4 24e 0.8 mmol·L−1半胱氨酸pH = 7.1,5 min 0.02 —f 0.86 [28] 蓝藻纲 聚球藻Synechococcus bacillaris CH3HgCl 0.29—0.42 4d 否 0.97±0.03 6.34b —f [32] 硅藻纲 海链藻Thalassiosira sp. CH3HgCl 0.02 24e 否 —f 5.37 ± 0.04b —f [30] 硅藻纲 假微型海链藻Thalassiosira pseudonana CH3Hg-Cys 4×10−3 1d 否 11—14×10−3 5.47b —f [32] 硅藻纲 假微型海链藻Thalassiosira pseudonana CH3HgCl 0.29—0.42 4d 否 15.00 ± 3.00 5.44b —f [32] 硅藻纲 假微型海链藻Thalassiosira pseudonana CH3Hg-Met 0.28—0.48 l 4 否 16.9 ± 0.2 6.22b —f [24] 硅藻纲 假微型海链藻Thalassiosira pseudonana CH3Hg-HA 0.28—0.48l 4 否 11.9 ± 1.5 6.07b —f [24] 硅藻纲 假微型海链藻Thalassiosira pseudonana CH3HgCl 7.32 72g 8 mmol·L−1半胱氨酸pH = 8.2,1 min —f 14.7h —f [31] 硅藻纲 威氏海链藻Thalassiosira weissflogii CH3HgCl 1.85 1d 8 mmol·L−1半胱氨酸pH = 8.2,1 min 4.6 9.8×106 j —f [33] 硅藻纲 威氏海链藻Thalassiosira weissflogii CH3HgCl 1.85 1k 8 mmol·L−1半胱氨酸pH = 8.2,1 min 3.01 7.4×106 j —f [33] 硅藻纲 布氏双尾藻Ditylum brightwellii CH3HgCl 0.1 1d 否 130 5.49b —f [34] 硅藻纲 旋链角毛藻Chaetoceros curvisetus CH3HgCl 2.5 5d 否 1.8 6.04b —f [34] 鞭藻纲 等鞭金藻Isochrysis galbana CH3HgCl 4.59 72g 8 mmol·L−1 半胱氨酸pH = 8.2,1 min —f 79.23h —f [31] 注:GSH: Glutathione; Cys: Cysteine; Met: Methionine; HA: Humic acid. (a. 单位为cell−1 h−1 nmol·L−1;b.体积富集因子(Volume concentration factor,VCF)=平衡后藻类中的甲基汞浓度(mol·µm−3)/水中的甲基汞浓度(mol·µm−3);c.富集率 = 平衡后藻类中的甲基汞含量(µg)/水中的甲基汞含量(µg);d.培养过程为全程光照条件;e.培养中明暗时间比为12 : 12;f.文章内未给出具体数据,用“—”表示;g.培养中明暗时间比为14 : 10;h.生物富集因子(Bioaccumulation factor,BAF)= 平衡后藻类中的甲基汞浓度(µg·g−1 湿重)/水中的甲基汞浓度(µg·g−1);i.藻类暴露甲基汞浓度为1.5 nmol·L−1,表中谷胱甘肽或半胱氨酸的浓度为90 nmol·L−1;j.生物富集因子 = 平衡后藻类中的甲基汞浓度(µg·mol−1 以生物炭计)/水中的甲基汞浓度(µg·L−1);k.藻在暴露前在2 µg·L−1的甲基汞溶液中驯化18 d(明暗时间比为14 : 10),大约为第18代藻类;l.暴露的蛋氨酸浓度为1 nmol·L−1,胡敏酸浓度为1 mg·L−1 C) (a. cell−1 h−1 nmol−1; b. VCF = MeHg concentration in algae (mol·µm−3) / MeHg concentration in water (mol·µm−3) after equilibrium; c. The enrichment rate = MeHg concentration in algae (µg) / MeHg concentration in water (µg) after equilibrium; d. The cultivation process was under full light; e. The ratio of light and dark time in culture was 12 : 12; f. No specific data is given in the reference, which is indicated by "-"; g. The ratio of light and dark time in culture was 14 : 10; h. BAF = MeHg concentration in algae (µg·g−1 wet weight) / MeHg concentration in water (µg·g−1) after equilibrium; i. The concentration of MeHg exposed to algae is 1.5 nmol·L−1, and the concentration of glutathione or cysteine in the table is 90 nmol·L−1; j. Bioconcentration factor = MeHg concentration in algae (µg·mol−1 in biochar) / MeHg concentration in water (µg·L−1) after equilibrium; k. Algae were acclimated in 2 µg·L−1 MeHg solution for 18 days before exposure (the ratio of light and dark time was 14:10), which was about the 18th generation of algae; l. The exposed methionine concentration was 1 nmol·L−1 and the humic acid concentration was 1 mg·L−1 C.) -
[1] FUTSAETER G, WILSON S. The UNEP global mercury assessment: Sources, emissions and transport[C]. Proceedings of the 16th International Conference on Heavy Metals in the Environment (ICHMET), Rome, ITALY, 2012. [2] 吴飞, 王训, 罗辑, 等. 青藏高原林线森林汞的空间分布格局及对大气环境汞污染的指示 [J]. 环境化学, 2019, 38(7): 1619-1627. doi: 10.7524/j.issn.0254-6108.2018092302 WU F, WANG X, LUO J, et al. Spatial distribution of total mercury in timberline forest of Tibetan Plateau regions and its implications of atmospheric mercury pollution [J]. Environmental Chemistry, 2019, 38(7): 1619-1627(in Chinese). doi: 10.7524/j.issn.0254-6108.2018092302
[3] 王洁, 孙学军, 李明月, 等. 海螺沟冰川融水径流中汞与悬浮颗粒物的季节变化特征及其关系研究 [J]. 地球与环境, 2022, 50(3): 320-327. WANG J, SUN X J, LI M Y, et al. Seasonal variation and relationship between mercury and suspended particulate matter in Hailuogou glacier meltwater runoff [J]. Earth and Environment, 2022, 50(3): 320-327(in Chinese).
[4] OBRIST D, AGNAN Y, JISKRA M, et al. Tundra uptake of atmospheric elemental mercury drives Arctic mercury pollution [J]. Nature, 2017, 547(7662): 201-204. doi: 10.1038/nature22997 [5] 冯新斌, 史建波, 李平, 等. 我国汞污染研究与履约进展 [J]. 中国科学院院刊, 2020, 35(11): 1344-1350. doi: 10.16418/j.issn.1000-3045.20201015002 FENG X B, SHI J B, LI P, et al. Progress of mercury pollution research and implementation of minamata convention in China [J]. Bulletin of Chinese Academy of Sciences, 2020, 35(11): 1344-1350(in Chinese). doi: 10.16418/j.issn.1000-3045.20201015002
[6] OUTRIDGE P M, MASON R P, WANG F, et al. Updated global and oceanic mercury budgets for the united nations global mercury assessment 2018 [J]. Environmental Science & Technology, 2018, 52(20): 11466-11477. [7] WANG F Y, OUTRIDGE P M, FENG X B, et al. How closely do mercury trends in fish and other aquatic wildlife track those in the atmosphere?- Implications for evaluating the effectiveness of the Minamata Convention [J]. Science of the Total Environment, 2019, 674: 58-70. doi: 10.1016/j.scitotenv.2019.04.101 [8] SUN X J, ZHANG Q G, ZHANG G S, et al. Melting Himalayas and mercury export: Results of continuous observations from the Rongbuk Glacier on Mt. Everest and future insights [J]. Water Research, 2022, 218: 118474. doi: 10.1016/j.watres.2022.118474 [9] JANSEN W. Mercury concentrations in commercial fish species from Lake Winnipeg, 1971-2019 [J]. Journal of Great Lakes Research, 2021, 47(3): 648-662. doi: 10.1016/j.jglr.2021.02.001 [10] ZHAO L D, CHEN H M, LU X, et al. Contrasting effects of dissolved organic matter on mercury methylation by Geobacter sulfurreducens PCA and Desulfovibrio desulfuricans ND132 [J]. Environmental Science & Technology, 2017, 51(18): 10468-10475. [11] LU F C. Mercury as a food contaminant [J]. WHO Chronicle, 1974, 28(1): 8-11. [12] MERGLER D, ANDERSON H A, CHAN L H M, et al. Methylmercury exposure and health effects in humans: A worldwide concern [J]. Ambio, 2007, 36(1): 3-11. doi: 10.1579/0044-7447(2007)36[3:MEAHEI]2.0.CO;2 [13] TRASANDE L, LANDRIGAN P J, SCHECHTER C. Public health and economic consequences of methyl mercury toxicity to the developing brain [J]. Environmental Health Perspectives, 2005, 113(5): 590-596. doi: 10.1289/ehp.7743 [14] BATTUELLO M, SARTOR R M, BRIZIO P, et al. The influence of feeding strategies on trace element bioaccumulation in copepods (Calanoida) [J]. Ecological Indicators, 2017, 74: 311-320. doi: 10.1016/j.ecolind.2016.11.041 [15] WATRAS C J, BLOOM N S. Mercury and methylmercury, in individual zooplankton: Implications for bioaccumulation [J]. Limnology and Oceanography, 1992, 37(6): 1313-1318. doi: 10.4319/lo.1992.37.6.1313 [16] 毋赟, 王文雄. 汞在海洋浮游植物中的生物累积和毒性效应 [J]. 生态毒理学报, 2014, 9(5): 810-818. WU Y, WANG W X. Bioaccumulation and toxicity of mercury in marine phytoplankton [J]. Asian Journal of Ecotoxicology, 2014, 9(5): 810-818(in Chinese).
[17] CÓRDOBA-TOVAR L, MARRUGO-NEGRETE J, BARÓN P R, et al. Drivers of biomagnification of Hg, As and Se in aquatic food webs: A review[J]. Environmental Research, 2022, 204(Pt C): 112226. [18] LEHNHERR I. Methylmercury biogeochemistry: A review with special reference to Arctic aquatic ecosystems [J]. Environmental Reviews, 2014, 22(3): 229-243. doi: 10.1139/er-2013-0059 [19] DRANGUET P, le FAUCHEUR S, SLAVEYKOVA V I. Mercury bioavailability, transformations, and effects on freshwater biofilms [J]. Environmental Toxicology and Chemistry, 2017, 36(12): 3194-3205. doi: 10.1002/etc.3934 [20] CHEN C L, AMIRBAHMAN A, FISHER N, et al. Methylmercury in marine ecosystems: Spatial patterns and processes of production, bioaccumulation, and biomagnification [J]. EcoHealth, 2008, 5(4): 399-408. doi: 10.1007/s10393-008-0201-1 [21] SCHARTUP A T, QURESHI A, DASSUNCAO C, et al. A model for methylmercury uptake and trophic transfer by marine plankton [J]. Environmental Science & Technology, 2018, 52(2): 654-662. [22] HARDING G, DALZIEL J, VASS P. Bioaccumulation of methylmercury within the marine food web of the outer Bay of Fundy, Gulf of Maine [J]. PLoS One, 2018, 13(7): e0197220. doi: 10.1371/journal.pone.0197220 [23] SKROBONJA A, GOJKOVIC Z, SOERENSEN A L, et al. Uptake kinetics of methylmercury in a freshwater alga exposed to methylmercury complexes with environmentally relevant thiols [J]. Environmental Science & Technology, 2019, 53(23): 13757-13766. [24] LEE C S, FISHER N S. Bioaccumulation of methylmercury in a marine diatom and the influence of dissolved organic matter [J]. Marine Chemistry, 2017, 197: 70-79. doi: 10.1016/j.marchem.2017.09.005 [25] 金林, 孙荣国, 莫雅斐, 等. 椭圆小球藻对汞的吸附-解吸探究 [J]. 地球与环境, 2018, 46(6): 599-605. doi: 10.14050/j.cnki.1672-9250.2018.46.117 JIN L, SUN R G, MO Y F, et al. Study on adsorption-desorption of mercury by Chlorella ellipsoidea [J]. Earth and Environment, 2018, 46(6): 599-605(in Chinese). doi: 10.14050/j.cnki.1672-9250.2018.46.117
[26] le FAUCHEUR S, CAMPBELL P G C, FORTIN C, et al. Interactions between mercury and phytoplankton: Speciation, bioavailability, and internal handling [J]. Environmental Toxicology and Chemistry, 2014, 33(6): 1211-1224. doi: 10.1002/etc.2424 [27] FUJITA M, HASHIZUME K. Status of uptake of mercury by the fresh water diatom, Synedra ulna [J]. Water Research, 1975, 9(10): 889-894. doi: 10.1016/0043-1354(75)90038-X [28] 刘军晖, 麻冰涓, 毛宇翔, 等. 微藻对无机汞和甲基汞的吸附和吸收特性 [J]. 环境化学, 2017, 36(7): 1602-1613. doi: 10.7524/j.issn.0254-6108.2017.07.2016110701 LIU J H, MA B J, MAO Y X, et al. Adsorption and absorption characteristics of inorganic mercury and methylmercury by microalgae [J]. Environmental Chemistry, 2017, 36(7): 1602-1613(in Chinese). doi: 10.7524/j.issn.0254-6108.2017.07.2016110701
[29] 刘瑞霞, 汤鸿霄, 劳伟雄. 重金属的生物吸附机理及吸附平衡模式研究 [J]. 化学进展, 2002, 14(2): 87-92. doi: 10.3321/j.issn:1005-281X.2002.02.002 LIU R X, TANG H X, LAO W X. Advances in biosorption mechanism and equilibrium modeling for heavy metals on biomaterials [J]. Progress in Chemistry, 2002, 14(2): 87-92(in Chinese). doi: 10.3321/j.issn:1005-281X.2002.02.002
[30] MILES C J, MOYE H A, PHLIPS E J, et al. Partitioning of monomethylmercury between freshwater algae and water [J]. Environmental Science & Technology, 2001, 35(21): 4277-4282. [31] WU Y, WANG W X. Accumulation, subcellular distribution and toxicity of inorganic mercury and methylmercury in marine phytoplankton [J]. Environmental Pollution, 2011, 159(10): 3097-3105. doi: 10.1016/j.envpol.2011.04.012 [32] LEE C S, FISHER N S. Methylmercury uptake by diverse marine phytoplankton [J]. Limnology and Oceanography, 2016, 61(5): 1626-1639. doi: 10.1002/lno.10318 [33] WU Y, WANG W X. Differential acclimation of a marine diatom to inorganic mercury and methylmercury exposure [J]. Aquatic Toxicology, 2013, 138/139: 52-59. doi: 10.1016/j.aquatox.2013.04.012 [34] KIM H, van DUONG H, KIM E, et al. Effects of phytoplankton cell size and chloride concentration on the bioaccumulation of methylmercury in marine phytoplankton [J]. Environmental Toxicology, 2014, 29(8): 936-941. doi: 10.1002/tox.21821 [35] DENG G F, ZHANG T W, YANG L M, et al. Studies of biouptake and transformation of mercury by a typical unicellular diatom Phaeodactylum tricornutum [J]. Chinese Science Bulletin, 2013, 58(2): 256-265. doi: 10.1007/s11434-012-5514-3 [36] 刘朝淑, 莫雅斐, 孙荣国, 等. 水华束丝藻和铜绿微囊藻对水中甲基汞的吸附特征及动力学研究 [J]. 地球与环境, 2020, 48(4): 518-524. doi: 10.14050/j.cnki.1672-9250.2020.48.066 LIU C S, MO Y F, SUN R G, et al. Study on adsorption characteristics and kinetics of methylmercury in water by Aphanizomenon flosaquae and Microcystis aeruginosa [J]. Earth and Environment, 2020, 48(4): 518-524(in Chinese). doi: 10.14050/j.cnki.1672-9250.2020.48.066
[37] DAVIS T A, VOLESKY B, MUCCI A. A review of the biochemistry of heavy metal biosorption by brown algae [J]. Water Research, 2003, 37(18): 4311-4330. doi: 10.1016/S0043-1354(03)00293-8 [38] LI X G, FENG H, HUANG M R. Strong adsorbability of mercury ions on aniline/sulfoanisidine copolymer nanosorbents [J]. Chemistry - A European Journal, 2009, 15(18): 4573-4581. doi: 10.1002/chem.200802431 [39] 王倩雅, 张莹, 李爱芬, 等. 硫素营养水平对产油尖状栅藻光合生理及生化组成的影响 [J]. 水生生物学报, 2017, 41(4): 904-913. doi: 10.7541/2017.113 WANG Q Y, ZHANG Y, LI A F, et al. Effects of sulfur concentration on the photosynthetic physiology and biochemical composition of Scenedesmus acuminatus [J]. Acta Hydrobiologica Sinica, 2017, 41(4): 904-913(in Chinese). doi: 10.7541/2017.113
[40] MOYE H A, MILES C J, PHLIPS E J, et al. Kinetics and uptake mechanisms for monomethylmercury between freshwater algae and water [J]. Environmental Science & Technology, 2002, 36(16): 3550-3555. [41] YIN Z B, JIANG H Y, SYVERSEN T, et al. The methylmercury-l-cysteine conjugate is a substrate for the L-type large neutral amino acid transporter [J]. Journal of Neurochemistry, 2008, 107(4): 1083-1090. [42] ASCHNER M, CLARKSON T W. Methyl mercury uptake across bovine brain capillary endothelial cells in vitro: The role of amino acids [J]. Pharmacology & Toxicology, 1989, 64(3): 293-297. [43] MASON R P, REINFELDER J R, MOREL F M M. Uptake, toxicity, and trophic transfer of mercury in a coastal diatom [J]. Environmental Science & Technology, 1996, 30(6): 1835-1845. [44] DHAKA A, VISWANATH V, PATAPOUTIAN A. Trp ion channels and temperature sensation [J]. Annual Review of Neuroscience, 2006, 29: 135-161. doi: 10.1146/annurev.neuro.29.051605.112958 [45] PICKHARDT P C, FISHER N S. Accumulation of inorganic and methylmercury by freshwater phytoplankton in two contrasting water bodies [J]. Environmental Science & Technology, 2007, 41(1): 125-131. [46] 金林. 贵州喀斯特水库中几种典型藻对汞吸附—解吸及光还原的影响[D]. 贵阳: 贵州师范大学, 2018. JIN L. Effects of several typical algae on adsorption-desorption and photoreduction of mercury in the Karst reservoir in Guizhou[D]. Guiyang: Guizhou Normal University, 2018(in Chinese).
[47] VALLEE B L, ULMER D D. Biochemical effects of mercury, cadmium, and lead [J]. Annual Review of Biochemistry, 1972, 41(10): 91-128. [48] SIMON D F, DESCOMBES P, ZERGES W, et al. Global expression profiling of Chlamydomonas reinhardtii exposed to trace levels of free cadmium [J]. Environmental Toxicology and Chemistry, 2008, 27(8): 1668-1675. doi: 10.1897/07-649.1 [49] 张晓华, 刘骥, 柳敬丽, 等. DMSP的生物合成与裂解及其在硫循环中的作用 [J]. 中国科学基金, 2018, 32(5): 471-478. doi: 10.16262/j.cnki.1000-8217.2018.05.005 ZHANG X H, LIU J, LIU J L, et al. Biosynthesis and cleavage of DMSP and their roles in global sulfur cycle [J]. Bulletin of National Natural Science Foundation of China, 2018, 32(5): 471-478(in Chinese). doi: 10.16262/j.cnki.1000-8217.2018.05.005
[50] LAROSE C, DOMMERGUE A, de ANGELIS M, et al. Springtime changes in snow chemistry lead to new insights into mercury methylation in the Arctic [J]. Geochimica et Cosmochimica Acta, 2010, 74(22): 6263-6275. doi: 10.1016/j.gca.2010.08.043 [51] REISCH C R, MORAN M A, WHITMAN W B. Dimethylsulfoniopropionate-dependent demethylase (DmdA) from Pelagibacter ubique and Silicibacter pomeroyi [J]. Journal of Bacteriology, 2008, 190(24): 8018-8024. doi: 10.1128/JB.00770-08 [52] LÁZARO W L, DÍEZ S, BRAVO A G, et al. Cyanobacteria as regulators of methylmercury production in periphyton [J]. Science of the Total Environment, 2019, 668: 723-729. doi: 10.1016/j.scitotenv.2019.02.233 [53] BAUMGARTNER L K, REID R P, DUPRAZ C, et al. Sulfate reducing bacteria in microbial mats: Changing paradigms, new discoveries [J]. Sedimentary Geology, 2006, 185(3/4): 131-145. [54] BRAVO A G, BOUCHET S, TOLU J, et al. Molecular composition of organic matter controls methylmercury formation in boreal lakes [J]. Nature Communications, 2017, 8: 14255. doi: 10.1038/ncomms14255 [55] LÁZARO W L, GUIMARÃES J R D, IGNÁCIO A R A, et al. Cyanobacteria enhance methylmercury production: A hypothesis tested in the periphyton of two lakes in the Pantanal floodplain, Brazil [J]. Science of the Total Environment, 2013, 456/457: 231-238. doi: 10.1016/j.scitotenv.2013.03.022 [56] DING L Y, HE N N, YANG S, et al. Inhibitory effects of Skeletonema costatum on mercury methylation by Geobacter sulfurreducens PCA [J]. Chemosphere, 2019, 216: 179-185. doi: 10.1016/j.chemosphere.2018.10.121 [57] SEELOS M, BEUTEL M, AUSTIN C M, et al. Effects of hypolimnetic oxygenation on fish tissue mercury in reservoirs near the new Almaden Mining District, California, USA[J]. Environmental Pollution, 2021, 268(Pt A): 115759. [58] LUO H W, CHENG Q Q, PAN X L. Photochemical behaviors of mercury (Hg) species in aquatic systems: A systematic review on reaction process, mechanism, and influencing factor [J]. Science of the Total Environment, 2020, 720: 137540. doi: 10.1016/j.scitotenv.2020.137540 [59] MORENO F N, ANDERSON C W N, STEWART R B, et al. Phytofiltration of mercury-contaminated water: Volatilisation and plant-accumulation aspects [J]. Environmental and Experimental Botany, 2008, 62(1): 78-85. doi: 10.1016/j.envexpbot.2007.07.007 [60] COSSART T, GARCIA-CALLEJA J, WORMS I A M, et al. Species-specific isotope tracking of mercury uptake and transformations by pico-nanoplankton in an eutrophic lake [J]. Environmental Pollution, 2021, 288: 117771. doi: 10.1016/j.envpol.2021.117771 [61] LI Y, LI D, SONG B B, et al. The potential of mercury methylation and demethylation by 15 species of marine microalgae [J]. Water Research, 2022, 215: 118266. doi: 10.1016/j.watres.2022.118266 [62] DIMENTO B P, MASON R P. Factors controlling the photochemical degradation of methylmercury in coastal and oceanic waters [J]. Marine Chemistry, 2017, 196: 116-125. doi: 10.1016/j.marchem.2017.08.006 [63] MONPERRUS M, TESSIER E, AMOUROUX D, et al. Mercury methylation, demethylation and reduction rates in coastal and marine surface waters of the Mediterranean Sea [J]. Marine Chemistry, 2007, 107(1): 49-63. doi: 10.1016/j.marchem.2007.01.018 [64] TOSSELL J A. Theoretical study of the photodecomposition of methyl Hg complexes [J]. The Journal of Physical Chemistry A, 1998, 102(20): 3587-3591. doi: 10.1021/jp980244u [65] TREVORS J T. The role of microbial metal resistance and detoxification mechanisms in environmental bioassay research [J]. Hydrobiologia, 1989, 188/189(1): 143-147. doi: 10.1007/BF00027779 [66] MASON R P, MOREL F M M, HEMOND H F. The role of microorganisms in elemental mercury formation in natural waters [J]. Water, Air, and Soil Pollution, 1995, 80(1/2/3/4): 775-787. [67] MORELLI E, FERRARA R, BELLINI B, et al. Changes in the non-protein thiol pool and production of dissolved gaseous mercury in the marine diatom Thalassiosira weissflogii under mercury exposure [J]. The Science of the Total Environment, 2009, 408(2): 286-293. doi: 10.1016/j.scitotenv.2009.09.047 [68] KELLY D, BUDD K, LEFEBVRE D D. Mercury analysis of acid- and alkaline-reduced biological samples: Identification of meta-cinnabar as the major biotransformed compound in algae [J]. Applied and Environmental Microbiology, 2006, 72(1): 361-367. doi: 10.1128/AEM.72.1.361-367.2006 [69] WU Y, WANG W X. Intracellular speciation and transformation of inorganic mercury in marine phytoplankton [J]. Aquatic Toxicology, 2014, 148: 122-129. doi: 10.1016/j.aquatox.2014.01.005 [70] CARRASCO-GIL S, SIEBNER H, LEDUC D L, et al. Mercury localization and speciation in plants grown hydroponically or in a natural environment [J]. Environmental Science & Technology, 2013, 47(7): 3082-3090. [71] LAVOIE M, BERNIER J, FORTIN C, et al. Cell homogenization and subcellular fractionation in two phytoplanktonic algae: Implications for the assessment of metal subcellular distributions [J]. Limnology and Oceanography:Methods, 2009, 7(4): 277-286. doi: 10.4319/lom.2009.7.277 [72] WALLACE W G, LUOMA S N. Subcellular compartmentalization of Cd and Zn in two bivalves. II. Significance of trophically available metal (TAM) [J]. Marine Ecology Progress Series, 2003, 257: 125-137. doi: 10.3354/meps257125 [73] SATOH M, HIRACHI Y, YOSHIOKA A, et al. Determination of cellular levels of nonprotein thiols in phytoplankton and their correlations with susceptibility to mercury [J]. Journal of Phycology, 2002, 38(5): 983-990. doi: 10.1046/j.1529-8817.2002.t01-1-01223.x [74] RAINBOW P S. Trace metal concentrations in aquatic invertebrates: Why and so what? [J]. Environmental Pollution, 2002, 120(3): 497-507. doi: 10.1016/S0269-7491(02)00238-5 [75] REINFELDER J R, FISHER N S. The assimilation of elements ingested by marine planktonic bivalve larvae [J]. Limnology and Oceanography, 1994, 39(1): 12-20. doi: 10.4319/lo.1994.39.1.0012 [76] LAVOIE M, le FAUCHEUR S, FORTIN C, et al. Cadmium detoxification strategies in two phytoplankton species: Metal binding by newly synthesized thiolated peptides and metal sequestration in granules [J]. Aquatic Toxicology, 2009, 92(2): 65-75. doi: 10.1016/j.aquatox.2008.12.007 [77] RAINBOW P S, AMIARD J C, AMIARD-TRIQUET C, et al. Trophic transfer of trace metals: Subcellular compartmentalization in bivalve prey, assimilation by a gastropod predator and in vitro digestion simulations [J]. Marine Ecology Progress Series, 2007, 348: 125-138. doi: 10.3354/meps07086 [78] DANG F, WANG W X. Subcellular controls of mercury trophic transfer to a marine fish [J]. Aquatic Toxicology, 2010, 99(4): 500-506. doi: 10.1016/j.aquatox.2010.06.010 [79] NG T Y T, WANG W X. Dynamics of metal subcellular distribution and its relationship with metal uptake in marine mussels [J]. Environmental Toxicology and Chemistry, 2005, 24(9): 2365-2372. doi: 10.1897/04-637R.1 [80] LEE R E. Phycology [M]. Cambridge: Cambridge University Press, 1999. [81] ZHANG Y X, SOERENSEN A L, SCHARTUP A T, et al. A global model for methylmercury formation and uptake at the base of marine food webs [J]. Global Biogeochemical Cycles, 2020, 34(2): e2019GB006348. [82] 邓龙. 浮游植物对汞和甲基汞的富集特征研究[D]. 贵阳: 贵州师范大学, 2016. DENG L. Enrichment features of mercury and methylmercury in phytoplankton[D]. Guiyang: Guizhou Normal University, 2016(in Chinese).
[83] BEUTEL M, FUHRMANN B, HERBON G, et al. Cycling of methylmercury and other redox-sensitive compounds in the profundal zone of a hypereutrophic water supply reservoir [J]. Hydrobiologia, 2020, 847(21): 4425-4446. doi: 10.1007/s10750-020-04192-3 [84] le FAUCHEUR S, TREMBLAY Y, FORTIN C, et al. Acidification increases mercury uptake by a freshwater alga, Chlamydomonas reinhardtii [J]. Environmental Chemistry, 2011, 8(6): 612-622. doi: 10.1071/EN11006 [85] HINTELMANN H, WELBOURN P M, EVANS R D. Binding of methylmercury compounds by humic and fulvic acids [J]. Water, Air, and Soil Pollution, 1995, 80(1/2/3/4): 1031-1034. [86] RAZAVI N R, QU M Z, CHEN D M, et al. Effect of eutrophication on mercury (Hg) dynamics in subtropical reservoirs from a high Hg deposition ecoregion [J]. Limnology and Oceanography, 2015, 60(2): 386-401. doi: 10.1002/lno.10036 [87] 叶幼亭, 史大林. 全球变化对海洋生态系统初级生产关键过程的影响 [J]. 植物生态学报, 2020, 44(5): 575-582. doi: 10.17521/cjpe.2019.0313 YE Y T, SHI D L. Effects of global change on key processes of primary production in marine ecosystems [J]. Chinese Journal of Plant Ecology, 2020, 44(5): 575-582(in Chinese). doi: 10.17521/cjpe.2019.0313
[88] WANG X M, CHENG H, CHE H Z, et al. Modern dust aerosol availability in northwestern China [J]. Scientific Reports, 2017, 7: 8741. doi: 10.1038/s41598-017-09458-w [89] JIANG S, LIU X D, CHEN Q Q. Distribution of total mercury and methylmercury in lake sediments in Arctic Ny-Ålesund [J]. Chemosphere, 2011, 83(8): 1108-1116. doi: 10.1016/j.chemosphere.2011.01.031 [90] WU P P, KAINZ M J, VALDÉS F, et al. Elevated temperature and browning increase dietary methylmercury, but decrease essential fatty acids at the base of lake food webs [J]. Scientific Reports, 2021, 11: 16859. doi: 10.1038/s41598-021-95742-9 [91] AIKEN G, HAITZER M, RYAN J N, et al. Interactions between dissolved organic matter and mercury in the Florida Everglades [J]. Journal De Physique Ⅳ, 2003, 107: 29-32. [92] LUENGEN A C, FISHER N S, BERGAMASCHI B A. Dissolved organic matter reduces algal accumulation of methylmercury [J]. Environmental Toxicology and Chemistry, 2012, 31(8): 1712-1719. doi: 10.1002/etc.1885 [93] MANGAL V, STENZLER B R, POULAIN A J, et al. Aerobic and anaerobic bacterial mercury uptake is driven by algal organic matter composition and molecular weight [J]. Environmental Science & Technology, 2019, 53(1): 157-165. [94] CHEN H W, WU Y Y, LI Y X, et al. Methylmercury accumulation and toxicity to cyanobacteria: Implications of extracellural polymeric substances and growth properties [J]. Water Environment Research, 2014, 86(7): 626-634. doi: 10.2175/106143014X13975035525465 [95] WANG W X, WONG R S K, WANG J F, et al. Influences of different selenium species on the uptake and assimilation of Hg(II) and methylmercury by diatoms and green mussels [J]. Aquatic Toxicology, 2004, 68(1): 39-50. doi: 10.1016/j.aquatox.2004.02.003 [96] AMIARD-TRIQUET C, AMIARD J C. Influence of ecological factors on accumulation of metal mixtures [J]. Metal Metabolism in Aquatic Environments, 1998: 316-386. [97] GOSNELL K J, BALCOM P H, TOBIAS C R, et al. Spatial and temporal trophic transfer dynamics of mercury and methylmercury into zooplankton and phytoplankton of Long Island Sound [J]. Limnology and Oceanography, 2017, 62(3): 1122-1138. doi: 10.1002/lno.10490 [98] LEE C S, FISHER N S. Bioaccumulation of methylmercury in a marine copepod [J]. Environmental Toxicology and Chemistry, 2017, 36(5): 1287-1293. doi: 10.1002/etc.3660 [99] GOSNELL K J, DAM H G, MASON R P. Mercury and methylmercury uptake and trophic transfer from marine diatoms to copepods and field collected zooplankton [J]. Marine Environmental Research, 2021, 170: 105446. doi: 10.1016/j.marenvres.2021.105446 [100] BEŁDOWSKA M, KOBOS J. Mercury concentration in phytoplankton in response to warming of an autumn-winter season [J]. Environmental Pollution, 2016, 215: 38-47. doi: 10.1016/j.envpol.2016.05.002 [101] 王文雄, 潘进芬. 重金属在海洋食物链中的传递 [J]. 生态学报, 2004, 24(3): 599-604. doi: 10.3321/j.issn:1000-0933.2004.03.030 WANG W X, PAN J F. The transfer of metals in marine food chains: A review [J]. Acta Ecologica Sinica, 2004, 24(3): 599-604(in Chinese). doi: 10.3321/j.issn:1000-0933.2004.03.030
[102] LONG S X, HAMILTON P B, YANG Y, et al. Differential bioaccumulation of mercury by zooplankton taxa in a mercury-contaminated reservoir Guizhou China [J]. Environmental Pollution, 2018, 239: 147-160. doi: 10.1016/j.envpol.2018.04.008 [103] DIJKSTRA J A, BUCKMAN K L, WARD D, et al. Experimental and natural warming elevates mercury concentrations in estuarine fish [J]. PLoS one, 2013, 8(3): e0058401. [104] TSUI M T K, WANG W X. Temperature influences on the accumulation and elimination of mercury in a freshwater cladoceran, Daphnia magna [J]. Aquatic Toxicology (Amsterdam, Netherlands), 2004, 70(3): 245-256. doi: 10.1016/j.aquatox.2004.09.006 [105] PICKHARDT P C, FOLT C L, CHEN C Y, et al. Algal blooms reduce the uptake of toxic methylmercury in freshwater food webs [J]. Proceedings of the National Academy of Sciences of the United States of America, 2002, 99(7): 4419-4423. doi: 10.1073/pnas.072531099 [106] SOERENSEN A L, SCHARTUP A T, GUSTAFSSON E, et al. Eutrophication increases phytoplankton methylmercury concentrations in a coastal sea-A Baltic sea case study [J]. Environmental Science & Technology, 2016, 50(21): 11787-11796. [107] TADA Y Y, MARUMOTO K. Uptake of methylmercury by marine microalgae and its bioaccumulation in them [J]. Journal of Oceanography, 2020, 76(1): 63-70. doi: 10.1007/s10872-019-00525-6 [108] LEI P, ZHANG J, ZHU J J, et al. Algal organic matter drives methanogen-mediated methylmercury production in water from eutrophic shallow lakes [J]. Environmental Science & Technology, 2021, 55(15): 10811-10820. -




 下载:
下载: