-
β-N-甲氨基-L-丙氨酸(β-N-methylamino-L-alanine, BMAA)是一种可由多种蓝藻产生[1]、具有慢性神经毒性的毒素. 毒性研究显示,BMAA可能与肌萎缩性侧索硬化症、阿尔茨海默氏病、帕金森综合症等神经系统退行性疾病有关[2]. 人体可以通过各种各样的形式暴露于BMAA,如食用被污染的水或食物、在受污染的水体中游泳、吸入受BMAA污染的气溶胶等. 随着全球日趋严重的水体富营养化现状,人们对藻毒素的研究逐渐增多,但大部分研究关注重点在微囊藻毒素,由于BMAA检测难度较高(包括多种同分异构体难分离、分子量较小易受各类基质影响、环境中多种赋存形态等)、环境浓度较低[3-4],目前对BMAA的研究非常有限. 本文拟通过对BMAA的检测方法、水环境及各类水产食物的检测水平等进行总结概述,以了解当前人群对BMAA的暴露风险.
-
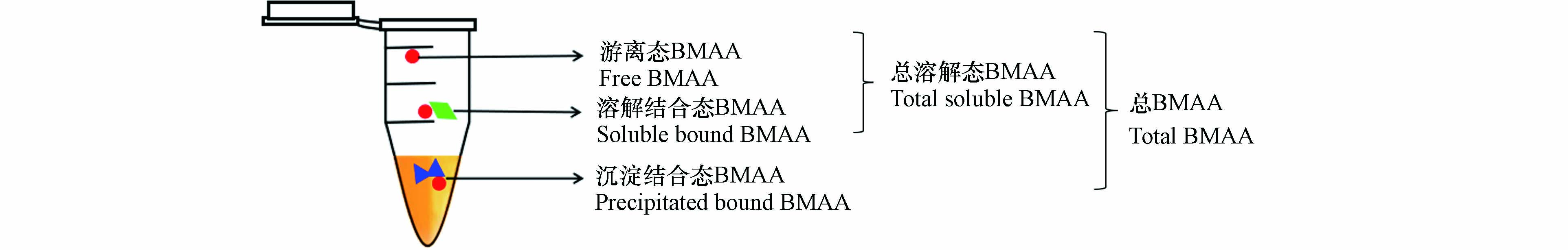
BMAA是一种非蛋白质氨基酸,其分子中含有羧基、一级胺和二级胺基团(图1),结构与人体必需的赖氨酸相似,分子式C4H10N2O2,相对分子质量为118.13 g·mol−1. BMAA极易溶于水,在环境中有明显的生物累积和生物学放大现象[5]. 目前,已在自然界中发现了多种BMAA的同分异构体,常见的有 2,4-二氨基丁酸(2,4-diaminobutyric acid, DAB)、N-2-氨乙基甘氨酸[N-(2-aminoethyl)glycine, AEG]、β-氨基-N-甲基丙氨酸(β-amino-N-methylalanine, BAMA)(图1). 其中,DAB是一种存在于许多原核和真核生物体内具有肝毒性和神经毒性的毒素,在许多生物样品中均与BMAA共存[6],其熔点、沸点及酸度系数均低于BMAA(表1);AEG是BMAA同分异构体中毒性最强的[7],可被肝脏大量吸收[8],具有肝毒性,其沸点和酸度与BMAA相近,但熔点更低;而BAMA的研究数据有限,仅有证据表明其同样具有神经毒性. BMAA在环境中以游离或结合形式存在,具体分为以下3种形态(图2):(1)以游离分子形式存在的游离态BMAA;(2)与短肽链结合存在的溶解结合态BMAA;(3)错误插入蛋白质中的沉淀结合态BMAA(也称为蛋白结合态BMAA).
其中,溶解结合态与游离态又称为总溶解态BMAA[9]. 由于不同形态的BMAA致神经毒性的作用通路存在差异(如游离态BMAA与重碳酸盐结合后是谷氨酸受体激动剂,可介导神经元变性[10-11];而结合态BMAA可能引起蛋白质的错误折叠从而造成蛋白质功能障碍,引起神经系统损害[12]),区分不同形态的BMAA的污染特征对其生态风险或人群健康风险评价都具有重要意义.
-
目前已报道的BMAA检测方法有高效液相色谱法[13](high performance liquid chromatography, HPLC)、气相色谱-质谱联用法[14](gas chromatograph-mass spectrometer, GC-MS)、液相色谱-串联质谱法[15](liquid chromatography tandem mass spectrometer, LC-MS/MS)等. GC-MS法是早期BMAA的检测方法,由于其前处理较为复杂,目前应用较少. HPLC分析法常与荧光检测器(Fluorescence detector, FLD)联用. 由于BMAA本身无荧光活性且是一种极性小分子化合物,因此在测定前需对BMAA进行衍生化处理,最常用的衍生剂是6-氨基喹啉基-N-羟基琥珀酰亚氨基甲酸酯(6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, AQC)[16]. 然而,荧光检测的低特异性及与其他衍生物的共洗脱会造成BMAA的误鉴,可能导致定量结果的假阳性或浓度被高估[17]. 自2012年后,已少有研究采用HPLC法和GC-MS法分析样本中的BMAA,多采用LC-MS/MS法. LC-MS/MS法通过保留时间、前体离子质荷比、产物离子质荷比和产物离子丰度比[17]的4个指标来定性,是目前分析各类样品中BMAA毒素及其同分异构体最常用的方法. 如表2所示,LC-MS/MS法根据是否使用衍生剂可进一步分为AQC衍生法和直接分析法. 其中,AQC衍生法因其能够检测复杂样品基质中的低浓度BMAA及其同分异构体,同时具有较高选择性而应用更为广泛. 基于LC-MS/MS的AQC衍生法的方法检出限一般比直接进样法低3—5倍,比HPLC-FLD等方法低10倍以上[18]. 经AQC衍生的LC-MS/MS法是美国分析化学家协会(Association of Official Analytical Chemists, AOAC)认可的唯一经过验证的方法. BMAA同分异构体与BMAA结构非常类似,因而可以检测BMAA的方法均可同时测定BMAA及其同分异构体. 近年的研究多同时分析BMAA和DAB、AEG、BAMA的3种同分异构体[19-27](表2). 研究发现色谱柱的选择和洗脱梯度的设定对BMAA及其同分异构体的分离度起决定作用:BAMA常与BMAA共洗脱,二者分离难度较高;DAB与AEG能与BMAA实现较好的色谱分离[28],但易产生相互干扰. 值的注意的是,有些早期文献仅检测了BMAA[29-31],不排除存在高估BMAA浓度的可能性.
基于LC-MS/MS仪器方法,目前已有文献报道了水体、藻类、海产品等样品中BMAA的前处理方法[21、32-34]. 在这些文献中,通常采用固相萃取法进行水样的富集或基质的净化. 其中,最常用的固相萃取小柱是Oasis MCX小柱,该小柱对BMAA的回收率接近100%[15];也有采用HLB和PCX小柱的报道,但应用很少[35]. 如前文所述,区分BMAA的不同赋存形态对于了解其污染特征及风险评价具有重要意义,因此,许多研究在相关方面进行了探索. BMAA三种赋存形态的分离主要借助三氯乙酸(Trichloroacetic acid, TCA)提取、盐酸水解实现,主要报道的形态(见图2)包括:(1)游离态BMAA:TCA提取后的上清液,直接进行BMAA测定;(2)总溶解态BMAA:TCA提取液经盐酸水解后的上清液,进行后续测定;(3)沉淀结合态BMAA:TCA提取后的沉淀经盐酸水解进行后续处理;(4)总BMAA:样品直接经盐酸水解后,进行BMAA测定. 经过以上形态分离的样品,后续可采用MCX小柱等固相萃取方法进行进一步净化,并根据仪器方法要求,可直接上机分析或进行AQC衍生后再进行上机分析[36]. 其中,单独测定总溶解态BMAA的研究相对较少[37]. BMAA同分异构体各形态分析的前处理方法与BMAA基本一致.
-
在国外水体中BMAA的报道屡见不鲜:Lage等[29]在瑞典芬妮湖水中检出了2—6 μg·L−1的总BMAA;Combes等[38]在法国塞纳河水样中检测到2—13.5 μg·L−1的游离态BMAA;Al-Sammak等[39]在美国内布拉斯加州的7个水库水中检测到了BMAA和DAB,其中,柯克曼湾水样中的BMAA浓度(游离态和沉淀结合态BMAA)高达25.3 μg·L−1,而柳溪镇水样中DAB浓度高达21.1 μg·L−1. 即使在远离海洋和湖泊的沙漠地区的水样中,BMAA也有检出. Craighead等[40]在蒙古国内戈壁沙漠的饮用水和浅水水洼中检出了BMAA和DAB,其中,DAB(100%)的检出率显著高于BMAA(55.6%). 作为BMAA毒素的主要“生产者”,藻类中BMAA的报道更加广泛. 波罗的海的藻类中游离态和沉淀结合态BMAA的浓度分别为0.0097—0.7025 μg·g−1和0.008—2.58 μg·g−1[30];葡萄牙河口的藻类样本中游离态和沉淀结合态BMAA浓度分别为0.04—8 μg·g−1和2—63 μg·g−1,这是首次在河口处报道BMAA毒素的存在[31]. Faassen等[41]在荷兰21个城市采集的藻类浮渣中,有9个地区的样本中检出了游离态BMAA,浓度为4—42 μg·g−1;2个地区的样品中检出了1 μg·g−1和4 μg·g−1的DAB. 英国1990—2004年间采集的12种藻类样品中BMAA(游离态和沉淀结合态)的检出浓度为8—287 μg·g−1[42];值得注意的是,其中5个检出了BMAA毒素的水体发生了动物接触后死亡事件,虽未证明BMAA是其直接死因,但仍不能忽视BMAA可能带来的健康风险. 此外,Roy-Lachapelle等[43]采集了加拿大5个湖泊中的藻类样品,分别在4个湖泊中检出了BMAA(10—300 μg·g−1),4个湖泊中检出了DAB(8—40 μg·g−1),以及在3个湖泊中检出了AEG(9—80 μg·g−1). 可见,BMAA及其同分异构体在水环境中的污染状况在不同国家和地区不尽相同,但都存在一定程度上的污染.
目前关于我国水环境中BMAA检出情况的报道如表3所示,已有的调查表明我国水环境中BMAA的污染情况并不乐观. 闫博引等[35]在全国14个地区调查了不同水体(海水、湖泊水、水库水)中BMAA的污染情况,其结果显示:水库水样中均未发现BMAA的存在;青岛海域、厦门海域水样中BMAA疑似存在,但无法准确定量(<0.01 μg·g−1);而在观赏性湖泊水样中,BMAA的检出率高达70%,表明我国水环境中存在BMAA污染的风险,且水质较差的景观湖污染风险最大. Cao等[32]在太湖水样中检出了0.23 μg·L−1的BMAA和0.002 μg·L−1左右的DAB. 顾笑笑等[44]在湖州市淡水混和养殖池塘的水体和底泥中分别检测到3.081—3.203 μg·L−1和0.681—0.711 μg·g−1的BMAA,表明在淡水养殖环境中也存在BMAA的污染. Wang等[45]在青岛胶州湾浮游植物中检测到0.03—1.00 μg·g−1的BMAA和0.01—0.48 μg·g−1的DAB,同时发现浮游植物中BMAA的浓度存在季节性变化. 此外,作为藻华无公害处理的产物—蓝藻有机肥中也被检出数量可观的BMAA毒素(游离态BMAA:1.8—16.3 μg·g−1、结合态BMAA:3.43—13.67 μg·g−1)[46].
-
由于水环境中存在广泛的BMAA污染,水产品也无法避免地受到污染. 相较于水体和藻类,水产品中BMAA主要检测游离态和沉淀结合态,检出品种包括滤食性软体动物、甲壳动物和鱼类等. 斯德哥尔摩市售的贻贝、生蚝和虾样品中总BMAA的检出浓度为0.08—0.90 μg·g−1[47];法国肖泻湖的贻贝样品中BMAA(游离态和总BMAA)、AEG、DAB的检出率分别为47%、91.2%、100%[48];波罗的海的贻贝中总BMAA的浓度范围为0.63—1.6 μg·g−1[49];南非水库的鱼类中BMAA(游离态和沉淀结合态BMAA)的检出浓度为1.63—3.055 μg·g−1[50];Christensen[51]在美国东南部地区的螃蟹样品中检出4.7—14.1 μg·g−1的总BMAA. 此外,Field等[52]在调查美国马里兰州安纳波利斯的3名肌萎缩侧索硬化症(Amyotrophic Lateral Sclerosis, ALS)患者的发病原因时,发现3人均食用了当地切萨皮克湾的青蟹,而在切萨皮克湾青蟹的蟹钳样品中均检测到了浓度高达115.2 μg·g−1的总BMAA和72.7 μg·g−1的DAB,提示BMAA毒素及其同分异构体可能是ALS发病的危险因素. 我国的水产品中也有BMAA检出的相关报道(表4):陈咏梅等[53]在武汉官桥湖水华爆发期间检出了鱼体内的BMAA残留,平均含量为(0.32±0.317) μg·g−1;Wu等[54]调查了太湖流域6种淡水养殖产品可食用部分BMAA的浓度范围为0.28—5.47 μg·g−1;顾笑笑等[44]在研究湖州市水华严重的淡水养殖池塘中水产的污染状况时发现软体动物、甲壳动物、鱼类中均可检出BMAA,其含量分别为0.528—1.065 μg·g−1、0.456—0.555 μg·g−1、0.358—0.494 μg·g−1;Li等[55]在中国东海、黄海海域的扁玉螺中检测到0.86—3.97 μg·g−1的BMAA和0.21—0.40 μg·g−1的DAB.
更值得关注的是,BMAA具有生物富集和生物放大的特性,营养级别高的水产品一旦被污染,将大大增加人群对BMAA的食用风险. 王超等[56]在黄海海域比较了不同营养级生物—浮游植物、浮游动物、软体动物和节肢动物体内BMAA的含量,发现BMAA浓度沿食物链:浮游植物(0.1 μg·g−1)—浮游动物(1 μg·g−1)—软体动物(3.5 μg·g−1)—节肢动物(12 μg·g−1)逐级升高,生物富集和生物放大效应明显. Jiao等[5]通过监测太湖贡湖湾不同营养级生物中的BMAA浓度,发现软体动物、甲壳类动物和鱼类的BMAA平均水平分别达到3.21、3.76、6.05 μg·g−1;而含量最高的是营养层次较高的翘嘴红鮊. 多项关于鲨鱼产品的调查结果显示,鲨鱼中BMAA残留极高:太平洋和大西洋鲨鱼鱼鳍和肌肉样本中,总BMAA浓度范围为34—2011 μg·g−1[57];美国南佛罗里达州的7种市售鱼翅中总BMAA检出浓度为144—1836 μg·g−1[58],市售的94%的鲨鱼软骨粉(15/16)中检出了75—352 μg·g−1的总BMAA,16种软骨粉中均检出DAB(69—1483 μg·g−1)和AEG(1298—1729 μg·g−1)[59]. 由此可见,营养级别越高的水产品中BMAA的食用风险越高,应引起人们的高度关注.
-
近年来,藻类相关产品因其广泛的药用价值和丰富的营养价值受到人们的青睐,螺旋藻衍生的一系列保健品已在70多个国家和地区进行销售[61]. 但据研究显示,相关产品的藻类来源可能被BMAA污染:加拿大的4种市售螺旋藻蛋白粉样品中BMAA、DAB和AEG均可检出,浓度分别为0.13—0.74 μg·g−1、9.32—107.06 μg·g−1和0.14—6.48 μg·g−1[26];美国18种市售藻类膳食补充剂中有2种检出0.04 μg·g−1和0.55 μg·g−1的总BMAA[62];德国33种藻类膳食补充剂中有1种检出了0.08 μg·g−1的DAB[63]. 藻类保健品的健康效应数据来源多是体外实验或实验动物,缺乏人体临床试验,不具有普遍性;且藻类保健品生产行业大多是自我管理,存在低估藻类保健品带来健康风险的可能性. 樊华等[64]分析了我国市面上11个较流通品牌的螺旋藻制剂,结果显示均含有0.004—0.02 μg·g−1游离态的DAB,但还未见有关于藻类保健品中BMAA毒素检出的报道,在该方面存在较大的研究空白.
-
相比于微囊藻毒素和贝类毒素,目前关于BMAA的基础研究相对较少,因此,目前仍无官方的健康指导值或限量要求. 世界卫生组织(World Health Organization, WHO)目前仅对蓝藻毒素中的微囊藻毒素等个别毒素提出了限量标准,如MC-LR的每日容许摄入量为0.04 μg·kg−1[65],食用贝类中石房蛤毒素的允许浓度为80 μg·100 g−1新鲜组织[66]. 已有学者试图建立水产品中BMAA的安全摄入标准限值(Guideline Values, GVs). Wu等[54]依据美国环保署(Environmental Protection Agency, EPA)提出的健康风险评价方法,以大鼠幼崽纹状体神经肽系统发生变化作为毒性终点[67],并结合我国藻类污染现状提出了水产品中BMAA的建议GVs限值为7.2 μg·g−1干重(成人)和1.8 μg·g−1干重(儿童). 顾笑笑等[44]则参照美国国家科学院(National Academy of Sciences, NAS)提出的健康风险评价方法,采用猕猴出现皮质神经功能障碍、帕金森特征及行为异常作为毒性终点[2],提出水产品中BMAA的GVs值为12 μg·g−1干重(成人)和3 μg·g−1干重(儿童). 值得注意的是,这两项研究中均采用100 g·d−1干重作为人群对水产品的平均消费量. 通过查阅资料[68]发现,2013—2020年我国居民人均水产品消费量从28.5 g·d−1湿重增加到38.1 g·d−1湿重,平均每年以0.47的速度增长. 考虑水产品中含水量一般为70%—80%,取25%作为干湿重的比值[69],我国居民人均水产品消费量仅为7.1—9.5 g·d−1干重,远低于两篇文献中的取值. 基于此消费数据重新估算水产品中BMAA的GVs值,分别为:成人为75.6—101.1 μg·g−1干重,儿童为18.9—25.3 μg·g−1干重(采用Wu计算方法);成人为126—168.5 μg·g−1干重,儿童为42—56.2 μg·g−1干重(采用顾笑笑计算方法). 考虑到我国不同地区人群对水产品消费的巨大差异,我们进一步采用我国水产品消费量最大的海南省的消费数据进行估算. 2015—2020年海南省人均水产消费量[68]为17.5—21.6 g·d−1干重,则两种方法估算的BMAA的GVs值分别为:成人33.3 μg·g−1干重和55.4 μg·g−1干重,儿童8.3 μg·g−1干重和18.5 μg·g−1干重. 鉴于我国近年来人均水产消费量逐年递增的现象,应根据实际情况,及时修正BMAA的健康指导值.
-
综上所述,作为一种具有慢性神经毒性的蓝藻毒素,BMAA在国内外水环境、水产品以及藻类保健品中被广泛检出;尤其是在营养级别较高的水生生物体内的残留高达mg·g−1级别,严重威胁人群的身体健康. 作为一个湖泊众多、水产业兴旺、且水体富营养化严重的国家,我国目前对于相关环境及产品中BMAA的调查研究还非常有限;在分析检测方法上还有许多的提升空间;对这类物质的毒性异构体的关注不够等等.
在未来,应对BMAA毒素及其同分异构体展开以下两方面的研究,以期全面了解我国BMAA毒素的污染现状:(1)开发更加可靠、具有选择性的分析方法来检测我国环境中BMAA毒素的暴露水平和污染程度,以评估与BMAA暴露相关的公共健康风险,减少对人类健康的潜在影响. (2)全面对BMAA赋存状态进行分析研究,得到所检测物质中准确的BMAA毒素含量,为生态风险评估工作提供基础理论依据.
水环境和水产品中β-N-甲氨基-L-丙氨酸(BMAA)检测方法和检出情况的研究进展
Research progress on detection methods and detection levels of β-N-methylamino-L-alanine (BMAA) in water environment and aquatic samples
-
摘要: 本文对蓝藻毒素β-N-甲氨基-L-丙氨酸(β-N-methylamino-L-alanine, BMAA)的结构形态、检测方法、环境和食物中的检出情况等方面进行系统综述. 研究结果表明,BMAA主要存在3种赋存形态(游离态、溶解结合态、沉淀结合态);环境和食物中的BMAA类目前主要采用经过AQC衍生的LC-MS/MS法检测,样品需经形态分离后、采用MCX固相萃取小柱处理. 水环境、水产品和藻类保健品中BMAA类的污染已被大量报道. 亚洲、北美洲、欧洲的相关环境和食物中均有一定的检出(检出浓度:水样<0.01—25.3 μg·L-1;藻类<0.01—300 μg·g-1;非鲨鱼水产品中0.08—115.3 μg·g-1;藻类保健品中0.04—0.73 μg·g-1). BMAA存在生物积累和生物放大的现象,水产品中营养级别越高的生物检出浓度就越高,如鲨鱼类产品中检出浓度高达34—2011 μg·g-1,需要引起重视. 我国目前对BMAA的相关研究还很有限,未来应加强检测方法、现场调查、风险评估等相关方面的研究,以全面了解我国人群对BMAA类物质的暴露风险.
-
关键词:
- β-N-甲氨基-L-丙氨酸(BMAA) /
- LC-MS/MS /
- 水环境 /
- 水产品 /
- 藻类产品.
Abstract: This paper systematically reviewed the structure, morphology, detection methods, environment and the level of β-N-methylamino-L-alanine (BMAA) in environment and food samples. The results show that BMAA mainly exists in three forms, including free BMAA, soluble bound BMAA, and precipitated bound BMAA. Currently, BMAAs in the environment and food are mainly detected by the AQC-derived LC-MS/MS method. The samples need to be morphologically separated and processed with MCX solid phase extraction cartridges. The contamination of BMAA has been reported in the water environment, aquatic products and algae health products in Asia, North America and Europe, with the detectable concentrations of < 0.01—25.3 μg·L-1 in water samples , < 0.01—300 μg·g-1 in algae samples, 0.08—115.3 μg·g-1 in non-shark aquatic products and 0.04—0.73 μg·g-1 in algal health products. Due to the bioaccumulation and biomagnification of BMAA, the aquatic products with higher nutrient levels showed higher concentrations. For example, the BMAA levels reported in the shark products were up to 34—2011 μg·g-1. Therefore, more attentions should be paid to these animals. At present, the relevant researches on BMAA in our country are very limited. More efforts should be made in analytical method development, on-site investigation and risk assessments to comprehensively assess the exposure risk of Chinese population to BMAAs. -
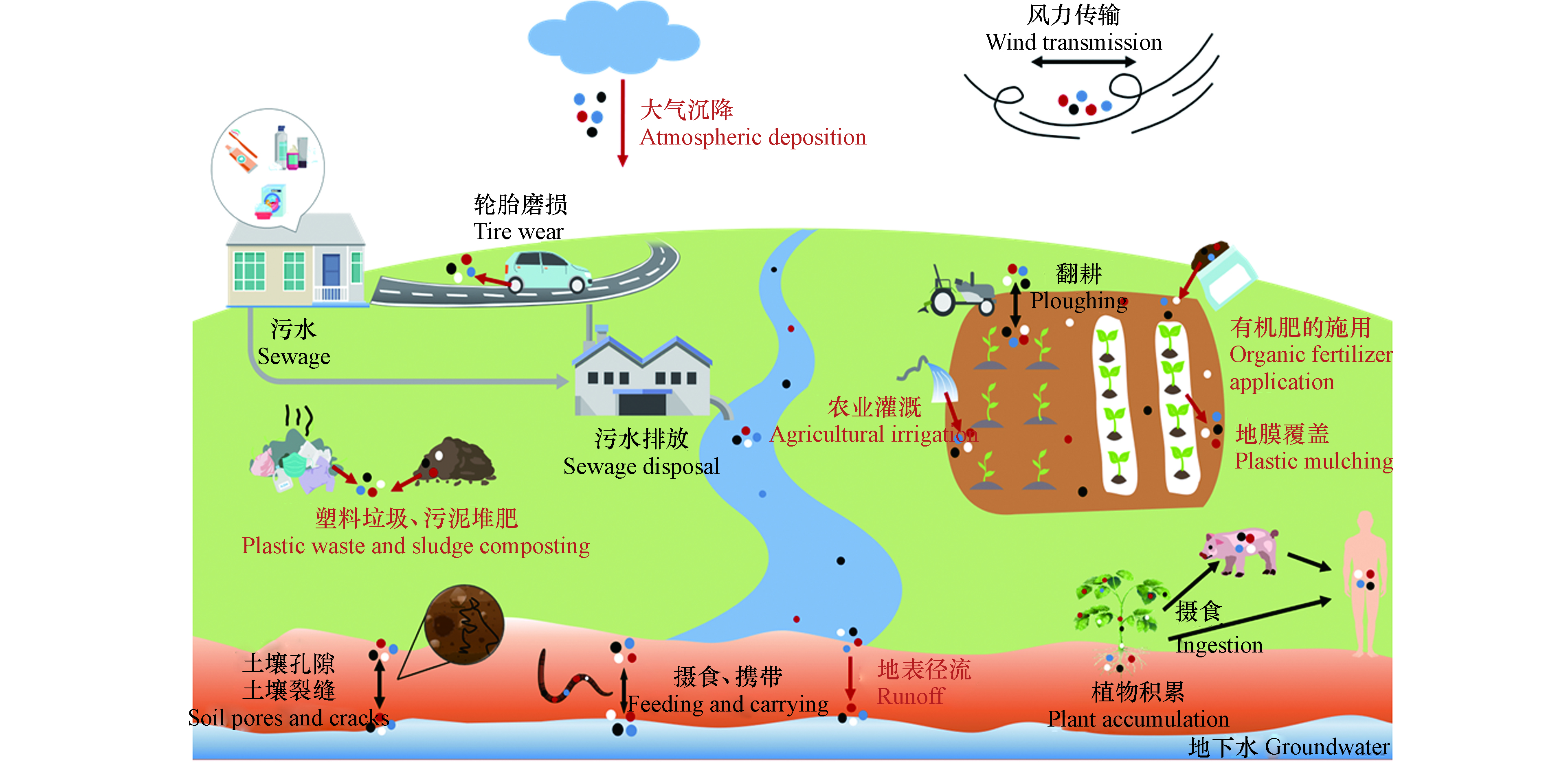
塑料的商业生产始于20世纪50年代[1],现广泛应用于包装、医疗、农业等行业,仅2019年全球塑料产量就高达3.68亿吨[2]。塑料在光照辐射、机械磨损、风化侵蚀、动物和微生物的作用下,可逐渐分解成粒径更小的塑料颗粒[3]。微塑料(microplastics, MPs)的概念最早出现在2004年Science发表的一篇文章[4],定义为粒径小于5 mm的塑料颗粒[5],粒径小于100 nm的被称为“纳米塑料”(nanoplastics, NPs)[6]。MPs通过大气、洋流等作用在全球范围内长距离运输[7],并在环境中持续存在和积累。水体[8]、沉积物[9]、土壤[10]、大气[11]甚至深海和极地都能检测到MPs[7]。尽管多项研究回顾了MPs在水环境中的发生、分布、生态风险及水体MPs与其他污染物的环境地球化学行为[8, 12-13],但关于陆地MPs的综述论文却很少[14-15]。陆地MPs是海洋MPs的主要来源,其MPs污染程度可能是海洋的4—23倍[16]。土壤作为陆地系统中MPs的汇[17],对MPs的储存和转移起着至关重要的作用[18]。因此,充分认识MPs在土壤环境中的丰度、来源、迁移和生态毒性对于科学评估和源头控制土壤MPs污染十分关键。
在Web of Science核心数据库中以“microplastics”和“soil”为关键词进行了搜索(截至2021年8月21日),产生了608篇文献。通过共现网络分析(图1),发现土壤环境MPs的研究始于2016年,相关研究主要包括:1)土壤类型,全球学者普遍注重农田土壤MPs的研究;2)MPs的来源,包括未合理处置的塑料垃圾、污泥堆肥、有机肥料的施用、污水灌溉和地膜覆盖等;3)MPs的分析方法,包括采样、分离(筛分、密度分离、消解等)、鉴定(目检法、光谱法、热解质谱分析法等);4)土壤MPs的丰度、类型(如聚丙烯(PP)、聚乙烯(PE)、聚苯乙烯(PS))、形状(如纤维、薄膜、碎片、颗粒等);5)MPs的生物效应,包括对植物、动物和微生物的影响。由此可见,MPs的来源、种类、分布、检测方法及生态健康风险是当前土壤MPs污染研究的热点方向。已发表的文献中,Praveena等[19]、陈雅兰等[20]较为全面的综述了土壤中MPs的提取与鉴定方法,郝爱红等[14]、Zhao等[15]从土壤中MPs的来源、迁移、分析方法、污染特征和生态风险等方面入手,揭示了土壤MPs的归宿和生态风险,但有关土壤MPs与多种有害污染物共同暴露的生物毒性、土壤中老化或降解MPs的生态风险鲜有报道。有学者对全球土壤MPs污染做了简单的总结[17, 21],但所收集的数据不够全面。因此,本文在总结最新国内外研究进展的基础上,从土壤环境中MPs的来源、丰度、迁移及其生态健康风险方面进行了综述,并提出了相关领域未来的研究重点。相比先前的研究,本文更加全面的总结了土壤中MPs的丰度,通过绘制分布图以更加直观的形式展现了全球土壤MPs污染,并将土壤老化/降解MPs的生态风险以及MPs的复合污染毒性和潜在生态风险展开了系统地回顾和展望,填补该领域综述论文的空白。本文将为评估土壤MPs潜在的生态健康风险提供有价值的参考。
 图 1 已发表论文中以“微塑料”、“土壤”为关键词的共现网络分析图[22]。Figure 1. Co-occurrence network analysis of published research papers with “microplastics” and “soil” as keywords每个节点(关键词)大小与其出现频次成正比,连线颜色表示论文发表年份,数据截至2021年8月21日
图 1 已发表论文中以“微塑料”、“土壤”为关键词的共现网络分析图[22]。Figure 1. Co-occurrence network analysis of published research papers with “microplastics” and “soil” as keywords每个节点(关键词)大小与其出现频次成正比,连线颜色表示论文发表年份,数据截至2021年8月21日1. 土壤MPs的来源、丰度和迁移 (Source, abundance and migration of soil MPs)
1.1 土壤MPs的来源
土壤中MPs的来源十分广泛(图2),人们日常生活(如未合理处置的塑料垃圾)和农业活动(如污泥堆肥、有机肥施用、地膜覆盖及农田灌溉等)产生的MPs会直接进入土壤[23-26],或通过地表径流[27]和大气沉降[28]间接输送到土壤环境。
1.1.1 未合理处置的塑料垃圾
土壤中存在着与水环境类似、种类繁多的MPs碎片[29],它们与塑料污染密不可分。根据目前的塑料废弃物管理趋势预测,2050年全球产生的塑料垃圾中将有120万吨进入垃圾填埋场或自然环境[30],必然会对生态环境造成影响。日常生活使用的一次性塑料袋/瓶、口罩/手套、衣服等均含有塑料,如使用后被随意丢弃在路边或非法倾倒地点[31],会造成附近土壤塑料污染。作为塑料垃圾的重要组成部分,塑料袋全球每年的消费量约为5000—10000亿个,其中900多亿个塑料袋不可回收[32],可在环境中老化降解生成MPs。自2020年新冠疫情爆发以来,大量一次性口罩排放到环境中。据估计,2020年全球生产的一次性口罩约520亿个[33]。每片新口罩中可释放(183.0±78.4)个MPs,而使用过的口罩因附着了空气中的MPs会释放更多的MPs(每片(1246.6±403.5) 个) [34]。由此,未合理处置的一次性口罩引起的土壤塑料和MPs污染不容忽视。
1.1.2 污泥堆肥的长期积累
污泥堆肥可能导致土壤MPs的增加[24]。生活废水经污水处理厂,可大大减少MPs(去除率约99%)向水环境直接排放[24],但未被处理的MPs通常积聚在污泥中[35],由于污泥含有丰富的N、P、K等营养元素[36],许多地区将污泥用作农田肥料[24],MPs便由此进入土壤。不同国家污泥中MPs的含量与经济发展水平、人口密度和废物处置等因素有关[37]。对于经济发达、人口密度高的国家,因使用药品、个人护理品(PPCPs)及洗衣产生的污水量大[38],污泥中MPs的含量相应较高。在欧洲和北美地区,每年通过污泥堆肥进入农田的MPs分别有约6.3×104—4.3×105和4.4×104—3.0×105吨[39]。土壤MPs的丰度随污泥施用量的增加而增加[24]。研究发现,在农田中仅施用一次污泥,15年后该区域土壤中仍可检测出塑料纤维[40],表明MPs在土壤中难以降解,会产生持久性污染。
1.1.3 有机肥料的施用
有机肥料的重复施用除了会引起重金属和抗生素等污染残留[41],还会导致土壤MPs污染,而后者常常被人们忽视[42]。研究发现,有机肥中普遍含有的MPs可能来自运输饲料的塑料管道、储存消毒剂或抗生素的塑料瓶[43]。江西鹰潭,猪粪中MPs的平均年丰度约为(1250±640)个·kg−1(干重),施用了猪粪的农田中MPs的年均累积量约为(1.25±0.61)个·kg−1[42];施用猪粪22年后的农田中MPs丰度((43.8±16.2)个·kg−1)明显高于未施用猪粪的农田((16.4±2.7)个·kg−1)[42]。德国是全球对肥料质量要求最严格的国家之一,但每年通过施用有机肥进入农田的MPs高达3.5×1010—2.2×1012个[26]。我国作为有机肥生产和使用大国,据估计,我国每年通过有机肥进入农田土壤中的MPs可达52.4—26400吨[3]。但该数据仅仅基于德国波恩、斯洛文尼亚等地区关于有机肥中塑料污染的报道[23, 26, 44],并结合我国有机肥每年实际施用量(2200万吨左右)来进行估算的,该估算忽略了粒径小于0.5 mm的MPs,且缺乏我国有机肥中关于MPs丰度的报道,因此,未来的研究中还应多关注我国有机肥中MPs的污染情况,以便全面评估我国通过有机肥进入土壤的MPs量。
1.1.4 农业灌溉和地表径流
农业灌溉是MPs进入土壤的又一重要途径。据统计,全球每年生活污水产生量超过356 km3,处理后的出水中有23.8 km3主要用于农业灌溉[45]。生活污水中含有大量源于PPCPs和衣物的MPs。虽然常规的处理工艺可有效去除污水中绝大部分MPs,但出水中仍有残留的MPs通过农业灌溉进入土壤环境[15]。在部分水资源匮乏的国家,未经处理的污水也会被用于灌溉农田[23]。据报道,全球约有3.6×105 km2的农田是使用未处理或者部分处理的生活污水进行灌溉的[46],必然会向土壤中输入更多的MPs。此外,天然水体中也存在MPs,例如:我国长江水中MPs高达6.6×103个·m−3[47],珠江水中MPs的丰度介于397—7924个·m−3之间[48],即使在偏远的内陆湖泊沿岸也有大量MPs存在,如青藏高原湖泊中MPs丰度可达(625±411)个·m−3[49]。这些水环境中的MPs也可通过灌溉或随地表径流进入土壤环境中。随着研究的深入,人们开始对生态环境敏感区(如青藏高原[49]、沙漠[50]、黄土高原[51])MPs污染进行研究,作为东南亚多条河流重要发源地的青藏高原,无处不在的MPs可能使其污染范围不断扩大到其他水系,或通过地表径流进入土壤环境,而该地区生态环境脆弱,存在调查难度大、恢复年限长等问题,未来的研究应该更加注重生态环境敏感区MPs污染及其健康风险评价。
1.1.5 地膜的广泛应用
地膜是农田土壤MPs污染的重要来源[23, 25]。2016年全球农用塑料薄膜市场交易量为400万吨,预计到2030年将以每年5.6%的速度增长[25]。全球约有1.29×105 km2的农田覆盖有地膜[52],我国地膜使用量最大,占全世界地膜覆盖面积的90%[17]。从田地中去除地膜费时费力,大量被残留的地膜在阳光辐射等作用下逐步破碎裂解,形成MPs[29]。农田土壤中MPs的含量随覆盖时间的延长逐渐增加[17]。在我国石河子市,随着地膜连续覆盖时间从5年增加至30年,MPs丰度从10.10 mg·kg−1增加到了61.05 mg·kg−1[53]。目前,大力研制与推广的环保型可降解地膜是解决塑料污染最有效的途径,但研究表明,MPs对污染物(如抗生素、农药等)的吸附能力大小排序为:老化可降解MPs>可降解MPs>非可降解MPs,且老化程度越高对污染物的吸附量越大[54-55],在这种情况下可降解地膜的使用,特别是地膜在环境中不可避免的老化行为,可能会给环境带来更大的生态危害,在未来的农业发展中应该重视这一问题。
1.1.6 大气沉降输入
土壤MPs也有部分来自大气中悬浮的塑料颗粒。多项研究表明,大气中存在MPs,如南海西北部大气中MPs的丰度为(0.035±0.015)n·m−3[56]。大气中的MPs主要来源于建筑材料、纺织品磨损、灰尘、道路油漆、轮胎和制动器磨损[57]。轮胎磨损产生的MPs主要来自各种车辆,全球车辆轮胎磨损的MPs排放量为人均0.81 kg·a−1[58],飞机轮胎磨损释放的MPs相对较少,约占荷兰轮胎磨损MPs排放总量的2%[58]。空气中密度小的大塑料颗粒和MPs可通过大气沉降和风力传输沉积在城市或乡村陆地表面[59],还可传输到偏远、人烟稀少的地区[28]。据报道,我国烟台市大气MPs沉降通量达1.5×105个·(m2 a)−1[60];法国巴黎大气MPs沉降通量达2—355个·(m2 d)−1,且该地区每年有3—10吨的纤维被大气沉降物沉积[59]。由此可见,大气沉降是MPs沉积到陆地的重要途径。值得思考的是,粒径小于50 μm的MPs可以重新悬浮到大气中[61],增加人体吸入MPs的风险,而多数国家并没有将大气中的MPs作为空气污染的一部分进行监测,为了明晰MPs对人类健康构成的潜在风险,将MPs纳入空气污染的监测范围迫在眉睫,尤其是在MPs污染严重的大城市。
总体来看,国内外大量关于土壤中MPs的来源研究仅停留在对来源的简单陈述,只有少部分做了MPs的溯源追踪方法。目前,环境中MPs的溯源方法主要集中于水体和沉积物,通过非仪器分析法(目视分析法、密度分析法、灼烧分析法等)从MPs的颜色、形状、密度等特性初步判识MPs的外观及用途[62],或通过仪器检测(光谱分析法、显微分析法、色谱质谱分析法等)判识MPs的化学成分及结构[63],两者相结合可追溯环境中MPs的来源。从已有研究成果来看,土壤MPs的溯源依旧没有可靠且简单易行的检测方法。值得注意的是,进入到环境的塑料碎片和MPs,由于各种物理化学作用,最终会破碎形成NPs,更小的粒径以及颜色、形状等特性不够显著增加了对MPs来源追溯的难度,因此亟需建立适合更小粒径的NPs的检测方法和理化指标。
1.2 土壤MPs的迁移行为
MPs在土壤中可发生水平和垂直迁移[64],其迁移行为受土壤和MPs理化性质的影响[21, 65]。土壤的理化性质(包括孔隙度、土壤质地、矿物和腐殖质含量等)对MPs的迁移有重要影响。土壤的孔隙大小由其质地决定,可直接影响MPs的迁移[30],砂土表面的MPs在渗透作用下可垂直迁移至距地表1.5—7.5 cm的土壤中[66]。由于土壤裂缝,干燥气候可能会加速MPs向下移动[66]。土壤矿物和腐殖酸共存时会增加MPs的垂直传输距离(9—10 cm)[67]。Wu等[68]发现, PS微球的迁移能力随土壤矿物(Fe/Al氧化物)含量的增高而降低,这是由于带负电的MPs与带正电的Fe/Al氧化物发生静电吸引所致。此外,MPs的特性(包括粒径、形状、电荷和表面化学等)也会影响其在土壤中的迁移。当MPs的粒径小于土壤孔隙尺寸时,MPs能通过土壤孔隙和裂缝向下移动,粒径小的MPs也容易被土壤动物摄食而转移到更深层的土壤中[69-70]。由于MPs与土壤团聚体的相互作用不同,不同形状的MPs可能对土壤中MPs的迁移产生阻塞作用影响其迁移行为[65]。如:塑料微球和微粒比微纤维更易下移到土壤深层,因为微纤维与土壤颗粒缠结形成土块后无法迁移[71]。高密度的MPs(如PET(聚对苯二甲酸乙二醇酯))可能会因重力作用而促进其在土壤中的迁移[72]。表面含有羧基、磺酸基、低密度氨基官能团的PS微球,比含有高密度氨基官能团的PS微球更易在海沙中迁移,这是由于带正电的高密度氨基MPs与带负电的沙粒之间存在静电吸引,从而阻碍MPs的迁移行为[73]。
除了在土壤内部迁移外,土壤中的MPs也会在风力、气流、地表径流等作用下迁移到空气和水等环境介质中[64, 66]。土壤表面的MPs尤其是微纤维等轻质塑料颗粒,可以被风和气流抬升到空气中,最终长距离传播到其他陆地或地表水中[59]。此外,地表径流可促使MPs进入深层土壤甚至含水层。据报道,澳大利亚维多利亚州地下水中MPs的平均丰度为38个·L−1[74],向地下水迁移的MPs可能带来新的环境问题,但目前仍缺乏对地下水MPs污染的环境风险预测、评估和防控研究。
1.3 全球土壤环境中MPs的丰度
我们收集了全球不同地区土壤环境中检出的MPs的理化性质和丰度,绘制了图3。目前,虽然只有少量研究报道了土壤环境中MPs的丰度情况,但可看出MPs广泛存在于多种土壤中(如农业土壤、公园土壤、湿地土壤、沙漠土壤等),其丰度从几个·kg−1到数万个·kg−1不等,多数地区土壤MPs丰度在0—5×103个·kg−1之间,粒径大多小于1 mm[75-77];MPs形状有纤维、薄膜、碎片、颗粒等,PP、PE、PS是土壤中最主要的聚合物类型。土壤环境中MPs的丰度普遍高于水和沉积物中的[8],说明土壤环境是MPs重要的汇。在全球范围内,亚洲、欧洲、北美、大洋洲的土壤环境中都发现了MPs,且不同地区丰度差异较大。从图3中可看出,智利梅利皮利亚县田地因长期施用污泥导致土壤MPs丰度高达18000—41000个·kg−1,明显高于其他地区[24];西班牙东南部穆尔西亚蔬菜农田土壤和墨西哥坎佩切家庭花园土壤中也检测到了数量较高的MPs,丰度分别为(2116±1024)个·kg−1和(870±1900)个·kg−1[78-79];但德国石勒苏益格-荷尔斯泰因州农田表层土壤中MPs仅有(5.8±8)个·kg−1[80],且该国弗兰科尼亚中部农田中MPs的丰度最低,仅为(0.34±0.36)个·kg−1[81]。
作为最大的塑料生产国和消费国[82],我国土壤MPs污染引起了越来越多的关注。在我国大多数受人为活动影响较少的土壤中MPs含量较低,如山东东营黄河三角洲湿地无植物覆盖的土壤和长江沿岸休耕的土壤中MPs丰度仅为60个·kg−1[83]和(28.4±22.0)个·kg−1[84];但农业土壤中MPs的含量通常较高,如:云南滇池柴河流域土壤MPs丰度为7100—42960个·kg−1[85];湖北武汉、山东寿光的农田土壤中也含有较高丰度的MPs(4.3×104—6.2×105、275—4165个·kg−1)[76-77],这可能是塑料地膜老化降解、污泥施用和污水灌溉所致。而少数地区如黄土高原[51]、上海菜地[75]等农田土壤中MPs丰度较小。在工业活动频繁的地区,也可能会引入较高丰度的MPs,广东贵屿电子废物拆解区土壤中MPs的丰度达34100个·kg−1[86]。沿海地区可通过海水养殖、旅游和港口建设等活动引入大量MPs[87]。一些偏远地区也存在少量MPs,可能是通过游客活动、卡车轮胎磨损和农用地膜引入的[88],或与大气传输有关。
土壤中MPs的垂直分布没有明显的规律[76]。例如我国上海郊区[75]、山东寿光[76]和德国石勒苏益格-荷尔斯泰因州[80]农田中表层土壤MPs丰度高于深层土壤MPs丰度,黄土高原[51]、山东胶州湾菜地和果园土壤[89]、毛里求斯农业土壤[90]中深层土壤含有更多的MPs,而我国云南滇池柴河流域农田[85]和墨西哥家庭花园[79]的表层和深层土壤MPs含量无显著差异。不同地区土壤MPs垂直分布可能会受到土壤翻耕、地表径流等因素的影响[51],动物的摄食和排泄行为也可能影响MPs在表层和深层土壤之间的垂直转移[58, 64]。此外,少数研究还报道了土壤质地、植被覆盖、栽培时间、恢复年限等与MPs丰度的关系[50, 76, 85]。例如: 我国山东寿光的农业土壤和砂质壤土中MPs丰度显著高于粉质壤土[76],毛乌素沙漠土壤MPs丰度高于草地和林地[50];设施栽培时间>25与<10年的农田土壤中MPs丰度差异不显著,表明早期的设施栽培措施导致土壤中MPs的累积数量不高[85]。由此可见,土壤中MPs无处不在,不同地区土壤MPs污染水平之间的差异是人类农业活动、工业生产等因素共同作用的结果。值得注意的是,已有研究采用的分离、计数MPs的方法不一,在单位上也有区别,可能会低估或高估了土壤中MPs的真实污染水平。因此,未来的研究亟需建立土壤MPs分离和检测标准。在深层土壤中,MPs受阳光辐照的影响减小,且可降解塑料的微生物种群较少[91],这意味着土壤深处MPs的老化降解可能减慢,其持久性可能会更长。那么,除了表层土壤,检测深层土壤中MPs的含量才能全面评估土壤中MPs的污染状况。
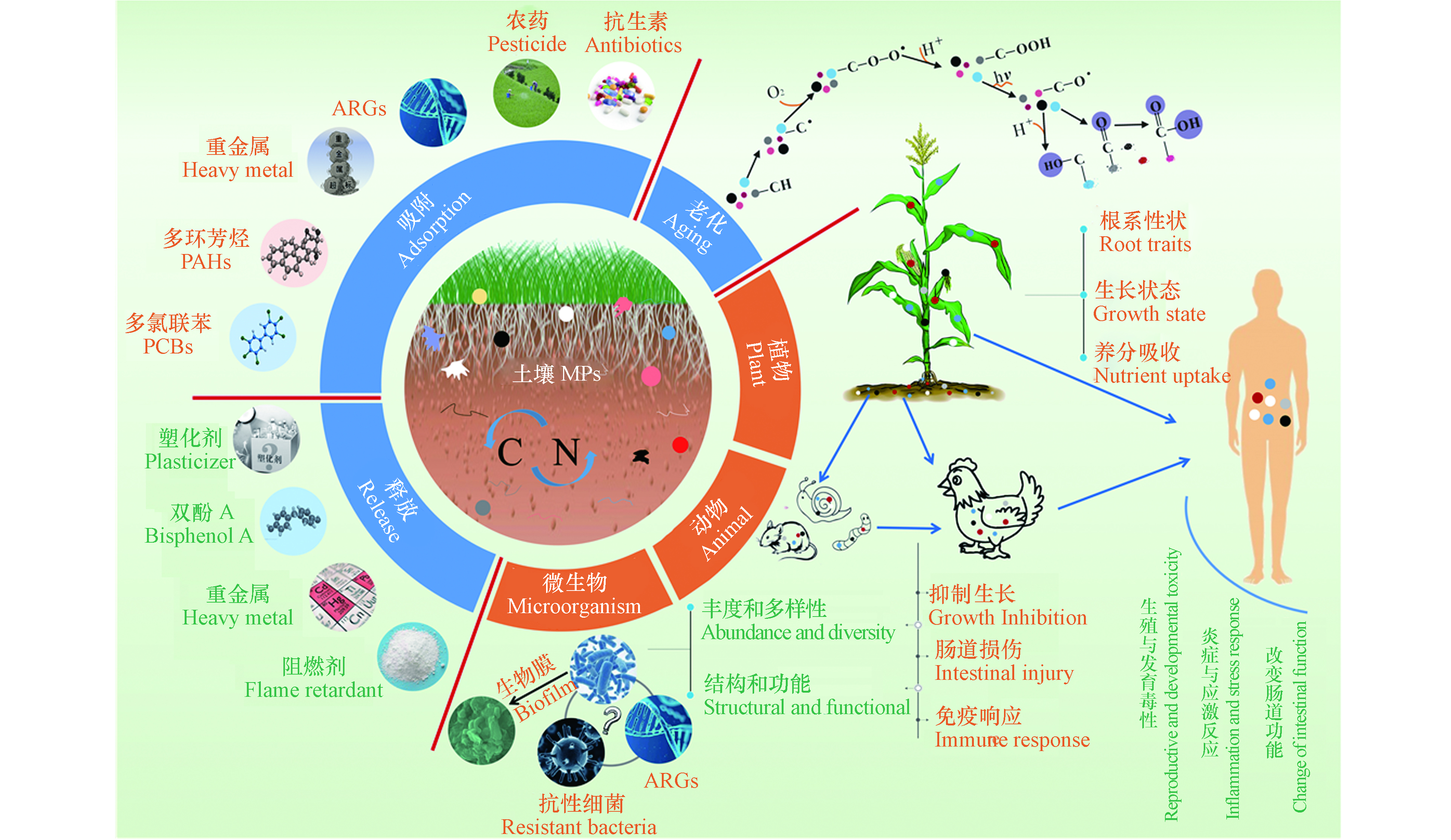
2. 土壤MPs污染的生态健康风险(Ecological health risks of soil MPs pollution)
土壤MPs可通过多种途径对生态系统构成潜在威胁(图4)。MPs的存在可直接影响土壤动植物、微生物的生长[92-94],后经食物链的积累和传递可能对人体健康构成潜在威胁[79]。土壤MPs在土壤环境中能够吸附多种污染物质(如重金属、抗生素、农药等)[58, 95],或与自身释放的添加剂(如增塑剂、抗氧化剂、阻燃剂等)形成复合污染[96],这会给土壤动植物的生长带来极大的危害,而土壤环境中的MPs大多处于老化/降解状态,较原生MPs对污染物表现为更高的吸附能力[97],可能会对土壤生态系统构成更大的威胁。
2.1 MPs对陆生植物的影响
MPs进入农业土壤会对植物产生暴露,阻塞种子孔隙、限制根吸收水和养分[92],影响植物的芽高、生物量和发芽率等[98-100]。Bosker 等[101]发现,绿色荧光塑料颗粒(50、500、4800 nm, 107个·mL−1)因堵塞种子的荚膜孔道会限制水芹种子发芽。而含PP、高密度聚乙烯(HDPE)、低密度聚乙烯(LDPE)和PET的土壤MPs能促进番茄植株的生长,但会延迟结果和降低果实产量[102]。MPs还可通过改变土壤结构、容重、持水能力和营养成分[103-104],间接影响植物根系性状、生长状态和养分吸收[99, 105]。de Souza Machado等[100]发现,MPs污染使得土壤容重降低,通气增加,有助于植物根系渗透到土壤中。然而,MPs(如微纤维)也会缠住幼根,阻碍幼苗的生长[92]。
MPs对植物生长的影响与其类型、暴露浓度、粒径等因素有关。de Souza Machado等[105]发现,PA、PE、HDPE、PP(均为2.0%)均会改变大葱的生物量、元素组成和根系性状,其影响程度因聚合物类型而异。Boots等[98]对比研究了生物降解的聚乳酸(PLA, 65.6 μm, 0.1% W/W)和难降解合成纤维((丙烯酸(AA)和尼龙混合物), 0.001% W/W)对黑麦草发芽的影响,发现两种MPs均会降低发芽率,PLA还会降低芽高。Qi等[99]也报道了类似的结果,即1%的淀粉基生物降解塑料和PE均抑制了小麦生长,且前者比后者的抑制作用更强。由此,生物降解材料来源的MPs对植物可能产生更强的毒性效应,值得进一步研究。一些研究表明粒径大小不同的MPs对植物的影响也不同,与5 μm PS(10、50、100 mg·L−1)相比,100 nm PS对蚕豆的生长抑制作用、遗传毒性和氧化损伤更强[106]。但目前,对于MPs在植物中的积累和转运以及对植物的毒性作用和机制等的认识仍不清楚。
2.2 MPs对陆生动物的影响
MPs被动物摄入后会影响其摄食行为、生长和繁殖[107]。与水生动物相比,MPs对陆生动物影响的生态毒理学研究非常有限,且主要集中在无脊椎动物(如蚯蚓)[93]。已有研究证实MPs暴露对蚯蚓的毒性作用主要包括抑制生长、体重减轻、肠道损伤、免疫响应、肠道微生物群落的改变,以及死亡率增加[70, 108-109]。少数研究报道了土壤MPs也会影响蜗牛[110]、土壤线虫[111]、小鼠[112]等的健康。MPs对动物的影响存在剂量-效应关系。Huerta Lwanga等[107]发现,0.2%的PE(<150 μm)对蚯蚓(Lumbricidae)的生长和存活没有影响,但较高的添加量(1.2%)有抑制作用。Cao等[108]同样发现,低剂量(≤0.5%)的PS(58 μm)对蚯蚓生长的影响不明显,但高剂量(1%、2%)的MPs显著抑制了蚯蚓的生长,死亡率达40%。PS(0.05—0.1 μm)在高暴露量(10%)下可观察到蚯蚓肠道微生物群的明显变化[113]。虽然低浓度MPs暴露不会明显影响动物的生长和引起动物死亡,但会诱使动物组织病理损伤和免疫响应[70]。在评估MPs对动物健康的影响时,粒径是除暴露剂量之外的重要影响因素,Lei等[111]研究了不同粒径的PS(0.1、0.5、1.0、2.0、5.0 μm)对土壤线虫(Caenorhabditis elegans)的影响,发现相同质量浓度(1 mg·L−1)下1.0 μm PS暴露后土壤线虫的存活率最低。然而,对于MPs对陆生动物的潜在影响,如MPs在动物组织中的积累和运输、MPs对动物的毒性作用和机制等方面的认识仍存在空白。
2.3 MPs对土壤微生物的影响
MPs内含或吸附的有机物可为微生物提供碳源[21],微生物在MPs表面定殖后形成生物膜[114],继而构成具有特殊微生物群落组成和功能的“塑料圈”[115]。研究发现,电子拆解厂区域的MPs(如PP、聚碳酸酯(PC)和ABS)及其周围环境的细菌群落存在显著差异,这可能是因为MPs为微生物提供了新的生态位[116],或通过改变土壤理化性质(如破坏土壤结构、降低土壤密度、改变土壤持水能力等)影响了微生物的群落结构和功能[65, 117]。添加MPs后土壤微生物群落多样性的影响研究还处于起步阶段,Huang、Judy等[118-119]认为,HDPE(<2 mm, 0.1%、0.25%、0.5%、1% W/W)、PVC(<2 mm, 0.01%、0.1%、0.25%、0.5%、1% W/W)、PET(<2 mm, 0.1%、0.25%、0.5%、1% W/W)和LDPE(2 mm×2 mm, 0.076 g·kg−1)的存在并没有显著改变土壤微生物群落的丰度和多样性。但也有研究发现土壤中添加低或高浓度(1%、5%)的LDPE(678 μm)和高浓度(5%)的PVC(18 μm)均显著增加了β变形杆菌目(Betaproteobacteriales)和假单胞菌目(Pseudomonadales)的相对丰度,而高浓度的PVC(18 μm, 5%)显著降低了鞘脂单胞菌科(Sphingomonadaceae)的丰度[120]。这些研究结果之间的差异可能与MPs的类型、浓度、以及土壤的理化性质有关。不同类型的MPs对微生物活性影响不同,PP颗粒(<180 μm, 7%、28%)对土壤微生物活性有积极影响[103],然而,Lozano等[94]发现PP碎片(<5 mm, 20%)会降低土壤微生物活性,PS颗粒(32.6 nm±11.9 nm, 1000 ng·g−1)、LDPE(643 μm, 17%)也对土壤微生物活性显示出负面影响[65, 121],de Souza Machado等[105]的研究也报道了类似的结果,但在这些研究中,MPs粒径、形状、大小和浓度各不相同,因此很难得出MPs对微生物毒性的一般性结论。
此外,MPs作为致病菌和耐药菌的载体[122],可能影响土壤中ARGs的分布和迁移。MPs与ARGs在环境中广泛共存,由于ARGs对人类健康的潜在不利影响,其传播越来越受到关注。水生环境中,多项研究表明MPs(如PVC、聚乙烯醇(PVA))可影响ARGs的分布和传播[123]。在土壤中,PS(0.08—0.10 mm, 0.1%)的存在已被证实会增加抗生素和ARGs的保留时间[124],Lu等[125]也得出了类似的结果,MPs可促进土壤中ARGs丰度和数量,但还需要更多的证据来证实MPs污染是否促进ARGs在土壤环境中传播的结论。此外,Zhu等[126]发现土壤温度和湿度的升高均显著提高了MPs上ARGs的丰度,因此,在全球气候变化的情况下,土壤MPs对ARGs影响需引起更多的关注。
2.4 MPs对人类健康的潜在影响
MPs可通过改变土壤理化性质、降低土壤肥力,影响土壤的生态功能和粮食生产[127],对人类的生存和发展产生潜在影响。MPs也可经陆生食物链传递进入人体。MPs及其吸附的污染物可在动植物体内积累[79],食用植物可以从土壤中吸收和积累微型(0.2 μm)荧光PS珠[128],100 nm PS可以在蚕豆、生菜根中积累,然后运输到茎叶[106]。一些重要的家禽(如鸡)也可食用MPs[79],而当人们食用被污染的家禽或蔬菜时,MPs可能在人体内大量积累。据估计,在墨西哥每人每年通过食用鸡肉就可摄入840个塑料颗粒[79],MPs一旦进入人体,可能引起炎症与应激反应、产生生殖与发育毒性,或改变肠道微生物的组成和功能[129]。MPs(<150 μm)可能会从肠腔转移到淋巴和循环系统,进而导致全身暴露[129]。Schirinzi等[130]证明了MPs(PS, 10 μm)和NPs(PS, 40、250 nm)可诱导人体细胞发生氧化应激,并在细胞水平上引起细胞毒性。MPs和NPs与免疫系统作用还可能会导致免疫毒性,进而引发不良反应(即免疫抑制、免疫激活和异常炎症反应)[131]。Prata[132]还发现,由于摄入MPs引起的慢性炎症和刺激可能会因DNA损伤而导致癌症。此外,常见的塑料添加剂,如邻苯二甲酸盐、阻燃剂、双酚A等,与生殖和发育障碍有关,可能引发乳腺癌、血液感染、青春期过早和生殖器缺陷[133]。目前开展的土壤MPs由食物链传递被吸食进入人体的研究还比较少,但已经在人类食物[129]和粪便[134]中检测到了MPs,甚至在人类胎盘、婴儿粪便、婴儿内脏中也发现了MPs的存在[135],虽然没有证据表明这些MPs是来源于土壤环境,但该结果应该足以引起人们对土壤MPs的重视。此外,大气MPs或许能通过反射阳光辐射对气候有冷却效果[136],而土壤中的MPs通过扬尘进入大气环境是否也有同样的效应,进而引起一系列的生态健康问题,如气候变化、水文调节及粮食安全等[137]。
2.5 MPs与其他污染物的复合污染毒性及生态风险
MPs因疏水性强、比表面积大[138],可以吸附多种有机和无机污染物,如多环芳烃(PAHs)、多氯联苯(PCBs)、重金属等[58, 95],或与自身释放的添加剂(如增塑剂、抗氧化剂、阻燃剂等)形成复合污染[96],从而影响土壤动植物的生长。对植物来说,Gao等[96]发现当加入邻苯二甲酸二丁酯(DBP)时,PS (100—1000 nm、>10000 nm)加重了DBP诱导的植物毒性,增强了对生菜(Lactuca sativa L. var . ramosa Hort)的负面影响,且小粒径PS(100—1000 nm)对生菜的不利影响略大。Liu等[139]发现土壤中PE(200—250 μm,0.5%、1%、2%、5%、8% W/W)和菲(100 mg·kg−1)共同污染比单一处理对小麦幼苗(Triticum aestivum L. cv. NAU 9918)的毒性更强,PE的单一污染破坏了小麦叶片的光合系统,而PE和菲复合污染则加剧了这种破坏。MPs与土壤中重金属等无机污染物的复合污染也引起了人们的关注。Dong等[140]研究发现,在As(Ⅲ)存在下,大尺寸的PS(5 µm)可以迁移到胡萝卜的叶和根部,这是由于As(Ⅲ)增加了PS表面的负电荷,同时As(Ⅲ)也会导致细胞壁扭曲和变形,并导致更多的MPs进入胡萝卜,降低其质量。另一项研究表明,PET(<2 mm)还可以作为载体将重金属运输到小麦根际区域[141]。而Zong等[142]的研究表明,与单一重金属处理相比,PS(0.5 µm, 100 mg·L−1)与Cu2+、Cd2+的结合增加了小麦中叶绿素含量,增强了光合作用,减少了活性氧(ROS)的积累,表明PS(0.5 µm, 100 mg·L−1)对Cu2+、Cd2+的生物利用度和毒性具有缓解作用。对动物来说,Zhou等[143]发现PP(<150 μm, 0.03%、0.3%、0.6%、0.9%)与重金属(Cd, 8 mg·kg−1)二者联合暴露会对蚯蚓(Eisenia foetida)产生更强的负面影响,降低蚯蚓的生长速度并增加其死亡率。而另一项研究却发现,PVC可能通过吸附/结合As(Ⅴ),降低As(Ⅴ)的生物利用度来缓解As(Ⅴ)对肠道菌群的影响,从而防止As(Ⅴ)的减少和总砷在肠道中的积累,降低对蚯蚓(Metaphire californica)的毒性[144]。然而,Sun等[145]发现,MPs(40—50 μm, 10 mg·kg−1、300 mg·kg−1)可显著增加毒氟磷杀虫剂在蚯蚓体(Eisenia fetida)内的生物蓄积性,加重对蚯蚓的氧化损伤和干扰代谢。Boughattas等[146]将MPs(100 µg·kg−1)和除草剂2,4-二氯苯氧乙酸(2-4-D)(7 mg·kg−1)共同暴露于土壤中,结果表明,MPs增加了蚯蚓中的2,4-D生物积累,破坏了溶酶体膜的稳定性和氧化状态,并增加了抗氧化基因的表达。
目前,不管是对MPs的单一毒性研究还是与其他污染物的复合毒性研究,都存在受试动植物类别有限、土壤类型单一、研究周期短等问题,且MPs的种类、大小和浓度与实际土壤环境有一定的差异,如实验室研究中所用MPs浓度往往会高于实际土壤环境中MPs的最大浓度(6.7%)[147],未来的研究应在环境相关浓度条件下评估生态效应。更重要的是,没有充分考虑自然环境因素,真实土壤环境中MPs更多是处于老化或被生物膜定殖的状态,这无疑增加了MPs上的吸附位点,可能使得MPs上吸附的污染物更多,对陆地生态系统构成更严重的威胁。此外,粒径较小的MPs,特别是NPs,可能对陆地生态系统的健康风险更大[21],应作为重点评估的对象。
2.6 MPs的老化/降解及其潜在生态风险
MPs在土壤中的长期积累可以进一步老化或降解[21]。除光照辐射、机械磨损、风化侵蚀外,土壤环境中动物群和微生物(如细菌和真菌)也可以降解MPs[21, 107, 148]。从土壤中分离得到的假单胞菌属细菌AKS2对LDPE的降解率在45 d内达到4%—6%[149],在地膜中分离得到的红球菌C208对PE塑料薄膜的降解率在30 d内达8%[150]。但目前从土壤中分离出能降解MPs的菌株种类较少,因此探究用于降解土壤MPs的微生物可能是进一步研究的方向之一。而生物体可以通过咬、咀嚼或消化碎片来物理降解MPs[151-152]。蜡螟(Waxworms)、印度谷螟(Indian Mealmoths)已被证实能吞食PE并在其肠道微生物的帮助下降解塑料聚合物[153]。此外,大麦虫(Zophobas Morio)、黄粉虫(Tenebrio molitor)、蚯蚓等均具有降解MPs的能力[154-156]。老化/降解会改变MPs的表面结构、疏水性、结晶度和比表面积,并增加MPs表面C—O、C=O、—OH等含氧官能团的数量[8, 97],导致老化或降解MPs具有更高的吸附能力,使其可以吸附其他污染物质,对土壤生态系统构成更大的威胁。
目前,关于土壤MPs的老化或降解对陆地生态系统的危害研究并不多,主要是以下几个方面。首先,长期风化会使MPs分解成为NPs,许多研究已证明粒径较小的NPs可能较MPs具有更大的环境流动性和毒性[111]。Muhammad等[157]发现家蚕(Bombyx mori)暴露于PS MPs(5—5.9 μm, 10 μg·mL−1)的个体在感染后存活得更好,而暴露于PS NPs(50—100 nm, 10 μg·mL−1)的个体则表现出更高的死亡率。Liu等[158]也得出了类似的结果,相较于100 nm PS NPs,20 nm PS NPs(0.1—100 μg·L−1)对线虫(Caenorhabditis elegans)表现出更强的毒性。其次,老化MPs对污染物表现为更强的吸附能力,且老化的可降解MPs更强[51, 159]。Zhang等[159]研究发现,搁浅的PS泡沫对土霉素的吸附能力高于原始PS泡沫的吸附能力,Fan等[55]的研究也发现通过紫外线的老化过程,PLA、PVC对四环素、环丙沙星的吸附能力增加,且可降解PLA表现出更好的吸附能力,这些研究表明更多的有机污染物可以吸附并浓缩到老化的MPs上,形成的复合污染可能对生物体造成更严重的危害。最后,一些研究还探究了在超纯水和模拟肠液中,抗生素在原生/老化MPs上的解吸行为,发现与原生MPs相比,抗生素在老化MPs上解吸量更大,且模拟肠液中的抗生素解吸量比超纯水中大,这可能会对生物体造成更严重的危害[55]。除了老化MPs对生物体的危害外,也可能会带来其他的环境问题,如老化后形成的NPs由于粒径太小,如何从土壤环境中检测丰度及去除也是一大难题。综上,老化MPs的生态毒性问题及其带来的环境污染问题值得高度关注。
3. 结论与展望(Conclusion and perspective)
(1)土壤MPs的来源途径很多,包括未合理处置的塑料垃圾、污泥堆肥、有机肥的施用、农业灌溉、地膜覆盖等,但当前的研究仅停留在对土壤MPs来源的描述上,很少聚焦MPs的溯源研究,现有的技术条件无法将MPs从环境中根除,因此从源头管控就显得尤为重要。但如今土壤MPs溯源几乎处于空白状态,建议加强这方面的研究,为土壤中MPs的源头控制提供关键支撑。
(2)MPs污染在全球土壤环境中普遍存在,应加大力度调查土壤MPs丰度。不同地点、土地类型、不同深度土壤中MPs污染水平和特征存在较大差异,频繁的农业活动导致农田土壤MPs污染较为严重,PE、PP、PS是土壤中最常见的MPs类型。通过大气传输、植物积累、动物摄食、翻耕等多种途径,MPs最终可迁移到深层土壤甚至含水层,因此检测深层土壤中MPs的含量才能全面评估土壤MPs的污染状况。迁移到地下水中的MPs可能带来新的环境问题,但相关的环境风险预测、评估和防控仍缺乏。
(3)土壤MPs的存在会对动植物的生长产生不同影响,关于这方面的研究存在暴露时间短、受试动植物类别有限、土壤类型单一以及MPs种类、粒径大小和浓度与实际土壤环境有一定差异等问题,未来应结合实际土壤环境状况加强这方面的研究。土壤MPs经陆生食物链的传递和积累,可能对人类健康构成严重威胁,但关于环境相关浓度土壤MPs对不同类型动植物的阈值毒性水平及其在食物链中转移的研究还不足,这些问题在后续研究中需重点考虑,以全面揭示陆地生态系统中MPs带来的生态风险。
(4)MPs因疏水性强、比表面积大,可以吸附多种有机和无机污染物,从而影响土壤生物的生长,MPs还可与自身释放的添加剂等形成复合污染,使得MPs的环境行为更加复杂。但目前关于土壤MPs与其携带的污染物结合和释放的机理尚不清楚,与多种有害污染物共同暴露对陆生生物的毒性效应和人体健康的风险亟待研究。未来的研究重点应关注MPs进入到土壤中如何参与其他元素(如重金属)和污染物的环境地球化学行为及生物效应。
(5)土壤MPs的存在可改变微生物的群落结构和功能,反过来,在微生物、土壤动物、光照辐射等作用下MPs可进一步老化或降解,可能对土壤生态系统构成更大的威胁。但MPs影响土壤微生物的机制和途径暂不明晰,未来探究MPs对微生物群落结构、微生物活性的影响,MPs对全球生态系统和生物地球化学循环及对ARGs的影响是研究的重点方向之一。此外,还应寻找绿色、高效且环保的控制措施,以减少生物体对MPs的吸收,并降低其在土壤生态系统中的迁移。
-
表 1 BMAA及其同分异构体的理化性质
Table 1. Physicochemical properties of BMAA and its isomers
物质名称Substance 熔点Melting point 沸点Boiling point 酸度系数pKa 水溶性Water-solubility 稳定性Stability BMAA 177℃ 284.2℃ 2.1 易溶于水 稳定,不易分解 DAB 144℃ 220.7℃ 1.8 AEG 140-143℃ 290℃ 2.2 表 2 2018-2022年LC-MS/MS法在不同介质中BMAA及其同分异构体的检出限
Table 2. Detection limits of BMAA and its isomers in different matrixes from 2018 to 2022 by LC-MS/MS
样本类型Type of matrix 是否衍生Derivatization 检测物质Type of toxin 检出限/(μg·L−1)Detection of limit 参考文献References BMAA BAMA DAB AEG 水 水库水 直接进样 总BMAA、总DAB、总AEG 0.015 NR 0.0091 0.0061 [19] 地表水 FMOCa衍生 游离态BMAA、游离态BAMA、游离态DAB、游离态AEG 0.005 0.005 0.003 0.002 [20] 藻类 淡水及海洋藻类 AQC衍生 总BMAA、总DAB、总AEG 0.01 NR 0.01 0.01 [21] 水华藻类 EZ:faast衍生b 游离态BMAA、游离态DAB 0.02 NR 0.04 NR [22] 生物结皮c AQC衍生 总BMAA、总BAMA、总DAB、总AEG 0.01 0.037 0.01 0.01 [23] 生物基质 藻类、贝类、血浆、脑脊液 AQC衍生 总BMAA、总DAB、总AEG 0.01 NR 0.01 0.01 [24] 贝类 AQC衍生 总溶解态BMAA、总溶解态DAB 0.31d NR 0.013d NR [25] 藻类相关产品 螺旋藻天然保健品 AQC衍生 总BMAA、总DAB、总AEG 0.187 NR 0.187 0.187 [26] 螺旋藻粉 AQC衍生 总BMAA、总DAB、总AEG 0.01 NR 0.02 0.01 [27] 注:NR:Not Report, 未报道;aFMOC(9-fluorenylmethyl chlorofor mate):9-芴基甲基氯甲酸酯;b一种游离氨基酸衍生试剂盒;c一种沙漠地表覆被类型;d检出限单位为μg·g−1. 表 3 BMAA在我国部分水环境中的检出情况
Table 3. Detection of BMAA in some water environments in China
样品类别Species 检测地点Location 检出浓度Concentration 存在形态Existence 前处理Pretreatment 参考文献References 水体(湖泊水+海水+水库水) 太湖无锡流域 0.129/0.105 游离态/沉淀结合态BMAA 直接分析法 [35] 南京玄武湖 0.628/0.072 豫园景观湖 0.697/0.116 南昌孔目湖 1.648/0.19 南山景观湖 0.486/0.215 云南滇池 1.493/0.359 安徽巢湖 0.108/0.099 武汉东湖 <0.01 洞庭湖 <0.01 杭州西湖 <0.01 大连海域 <0.01 滨海流域 <0.01 青岛流域 <0.01 厦门海域 <0.01 密云水库 <0.01 深圳水库 <0.01 太湖 0.23a 总BMAA 直接分析法 [32] 蓝藻有机肥 太湖 1.8—16.3/3.43—13.67 游离态/沉淀结合态BMAA AQC-衍生法 [46] 水体 湖州市淡水养殖池塘 3.081—3.203a 总BMAA AQC-衍生法 [44] 底泥 0.681—0.711 浮游植物 青岛胶州湾 0.03—1.00 总溶解态BMAA 直接分析法 [45] 注:a检出浓度单位为μg·L−1,未带上标的检出浓度单位为μg·g−1. 表 4 BMAA在我国水产品中的检出情况
Table 4. Detection of BMAA in Chinese aquatic products
样品类别Species 样品名称Samples 检测地点Location 检出浓度/(μg·g−1)Concentration 存在形态Existence 前处理Pretreatment 参考文献References 软体动物 扁玉螺 黄海海域 3.54/20.92 游离态/总溶解态BMAA 直接分析法 [9] 脉红螺 0.64 栉江珧 2.57 菲律宾蛤仔 0.86 扁玉螺 1.76/4.07 AQC-衍生法 脉红螺 0.40 栉江珧 1.17 菲律宾蛤仔 0.51 背角无齿蚌 太湖贡湖湾 0.147—0.173/3.26—3.78 游离态/沉淀结合态BMAA AQC-衍生法 [60] 铜锈环棱螺 0.09—0.141/3.154—3.786 厚壳贻贝 舟山市 0.45 游离态BMAA 直接分析法 [55] 长竹蛏 荣成市 0.66 软体动物 扁玉螺 荣成市 2.15 游离态BMAA 直接分析法 [55] 大连市 3.97 连云港市 0.99 莱州市 0.86 青岛市 1.43 河蚬 湖州市淡水养殖池塘 0.528—0.540 总BMAA AQC-衍生法 [44] 铜锈环棱螺 1.015—1.065 贻贝 太湖贡湖湾 2.437—4.663 游离态BMAA+沉淀结合态BMAA AQC-衍生法 [5] 淡水蜗牛 0.63—3.85 亚洲蛤 0.80—6.72 河蚬 太湖 1.27—4.01 游离态BMAA+沉淀结合态BMAA AQC-衍生法 [54] 无齿蚌 0.28—4.92 甲壳动物 日本沼虾 湖州市淡水养殖池塘 0.456—0.468 总BMAA AQC-衍生法 [44] 中华绒螯蟹 0.543—0.555 日本沼虾 太湖贡湖湾 0.044—0.076/0.888—1.132 游离态/沉淀结合态BMAA AQC-衍生法 [60] 太湖秀丽白虾 ND/0.078—0.162 淡水蟹 太湖贡湖湾 5.727—11.793 游离态BMAA+沉淀结合态BMAA AQC-衍生法 [5] 螃蟹 6.479—6.481 淡水虾 0.177—1.903 西伯利亚对虾 0.104—0.136 对虾 3.141—7.099 日本沼虾 太湖 0.65—3.45 游离态BMAA+沉淀结合态BMAA AQC-衍生法 [54] 中华绒螯蟹 1.82—4.16 鱼类 草鱼 太湖 1.78—3.10 游离态BMAA+沉淀结合态BMAA AQC-衍生法 [54] 青鱼 2.95—5.47 青鱼 湖州市淡水养殖池塘 0.358—0.370 总BMAA AQC-衍生法 [44] 鲫 0.482—0.494 麦穗鱼 太湖贡湖湾 0.038—0.102/0.07—0.13 游离态/沉淀结合态BMAA AQC-衍生法 [60] 梅鲚鱼 ND/6.96—7.72 鲢鱼 0.121—0.159/10.27—11.31 黄颡鱼 0.52—0.60/8.67—9.45 鳑鲏 武汉官桥湖 0.038—0.448/ND 游离态/沉淀结合态BMAA AQC-衍生法 [53] 鲫 0.086—0.166/ND 鲤 0.1—0.5/0.233—0.393 鲢 0.01—0.046/ND 鳙 0.018—0.06/ND 鳊 0.631—1.121/0.001—0.003 鱼类 太湖贡湖湾 0.049—49.31 游离态BMAA+沉淀结合态BMAA AQC-衍生法 [5] 注:ND:Not Detected, 未检测到. -
[1] COX P A, BANACK S A, MURCH S J, et al. Diverse taxa of cyanobacteria produce β-N-methylamino-L-alanine, a neurotoxic amino acid [J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(14): 5074-5078. doi: 10.1073/pnas.0501526102 [2] SPENCER P S, NUNN P B, HUGON J, et al. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin [J]. Science, 1987, 237(4814): 517-522. doi: 10.1126/science.3603037 [3] CHERNOFF N, HILL D J, DIGGS D L, et al. A critical review of the postulated role of the non-essential amino acid, β-N-methylamino-L-alanine, in neurodegenerative disease in humans [J]. Journal of Toxicology and Environmental Health. Part B, Critical Reviews, 2017, 20(4): 1-47. [4] MANOLIDI K, TRIANTIS T M, KALOUDIS T, et al. Neurotoxin BMAA and its isomeric amino acids in cyanobacteria and cyanobacteria-based food supplements [J]. Journal of Hazardous Materials, 2019, 365: 346-365. doi: 10.1016/j.jhazmat.2018.10.084 [5] JIAO Y Y, CHEN Q K, CHEN X, et al. Occurrence and transfer of a cyanobacterial neurotoxin β-methylamino-L-alanine within the aquatic food webs of Gonghu Bay (Lake Taihu, China) to evaluate the potential human health risk [J]. Science of the Total Environment, 2014, 468/469: 457-463. doi: 10.1016/j.scitotenv.2013.08.064 [6] RÉVEILLON D, SÉCHET V, HESS P, et al. Systematic detection of BMAA (β-N-methylamino-L-alanine) and DAB (2,4-diaminobutyric acid) in mollusks collected in shellfish production areas along the French coasts [J]. Toxicon, 2016, 110: 35-46. doi: 10.1016/j.toxicon.2015.11.011 [7] SCHNEIDER T, SIMPSON C, DESAI P, et al. Neurotoxicity of isomers of the environmental toxin L-BMAA [J]. Toxicon, 2020, 184: 175-179. doi: 10.1016/j.toxicon.2020.06.014 [8] GALLO-TORRES H E, HEIMER E, SCHEIDL F, et al. The gastrointestinal absorption, tissue distribution, urinary excretion and metabolism of N-(2-aminoethyl)-Glycine (AEG) in the rat [J]. Life Sciences, 1980, 27(24): 2347-2357. doi: 10.1016/0024-3205(80)90504-4 [9] 王超, 邱江兵, 宋甲亮, 等. 不同液相色谱-质谱联用法分析贝类样品中神经毒素β-N-甲氨基-L-丙氨酸的比较 [J]. 中国渔业质量与标准, 2021, 11(1): 34-45. WANG C, QIU J B, SONG J L, et al. A comparative study on the analytical performance of different liquid chromatography coupled with tandem mass spectrometry for neurotoxin β-N-methylamino-L-alanine in mollusks [J]. Chinese Fishery Quality and Standards, 2021, 11(1): 34-45(in Chinese).
[10] HOEFFER C A, KLANN E. mTOR signaling: At the crossroads of plasticity, memory and disease [J]. Trends in Neurosciences, 2010, 33(2): 67-75. doi: 10.1016/j.tins.2009.11.003 [11] CHONG Z Z, SHANG Y C, WANG S H, et al. A critical kinase cascade in neurological disorders: PI 3-K, Akt, and mTOR [J]. Future Neurology, 2012, 7(6): 733-748. doi: 10.2217/fnl.12.72 [12] PAPAPETROPOULOS S. Is there a role for naturally occurring cyanobacterial toxins in neurodegeneration? The beta-N-methylamino-L-alanine (BMAA) paradigm [J]. Neurochemistry International, 2007, 50(7/8): 998-1003. [13] EMMONS R V, KARAJ E, CUDJOE E, et al. Leveraging multi-mode microextraction and liquid chromatography stationary phases for quantitative analysis of neurotoxin β-N-methylamino-L-alanine and other non-proteinogenic amino acids [J]. Journal Of Chromatography A, 2022, 1685: 463636. doi: 10.1016/j.chroma.2022.463636 [14] PAGLIARA P, DE BENEDETTO G E, FRANCAVILLA M, et al. Bioactive potential of two marine picocyanobacteria belonging to cyanobium and synechococcus genera[J]. Microorganisms, 2021, 9(10):2048. [15] ZHAO P, QIU J B, LI A F, et al. Matrix effect of diverse biological samples extracted with different extraction ratios on the detection of β-N-methylamino-L-alanine by two common LC-MS/MS analysis methods [J]. Toxins, 2022, 14(6): 387. doi: 10.3390/toxins14060387 [16] ROSÉN J, WESTERBERG E, HELLENÄS K E, et al. A new method for analysis of underivatized free β-methylamino-alanine: Validation and method comparison [J]. Toxicon, 2016, 121: 105-108. doi: 10.1016/j.toxicon.2016.08.021 [17] FAASSEN E J, GILLISSEN F, LÜRLING M. A comparative study on three analytical methods for the determination of the neurotoxin BMAA in cyanobacteria [J]. PLoS One, 2012, 7(5): e36667. doi: 10.1371/journal.pone.0036667 [18] BISHOP S L, MURCH S J. A systematic review of analytical methods for the detection and quantification of β-N-methylamino-L-alanine (BMAA) [J]. Analyst, 2019, 145(1): 13-28. [19] APARICIO-MURIANA M M, CARMONA-MOLERO R, LARA F J, et al. Multiclass cyanotoxin analysis in reservoir waters: Tandem solid-phase extraction followed by zwitterionic hydrophilic interaction liquid chromatography-mass spectrometry [J]. Talanta, 2022, 237: 122929. doi: 10.1016/j.talanta.2021.122929 [20] VO DUY S, MUNOZ G, DINH Q T, et al. Analysis of the neurotoxin β-N-methylamino-L-alanine (BMAA) and isomers in surface water by FMOC derivatization liquid chromatography high resolution mass spectrometry [J]. PLoS One, 2019, 14(8): e0220698. doi: 10.1371/journal.pone.0220698 [21] METCALF J S, BANACK S A, WESSEL R A, et al. Toxin analysis of freshwater cyanobacterial and marine harmful algal blooms on the west coast of Florida and implications for estuarine environments [J]. Neurotoxicity Research, 2021, 39(1): 27-35. doi: 10.1007/s12640-020-00248-3 [22] MAIN B J, BOWLING L C, PADULA M P, et al. Detection of the suspected neurotoxin β-methylamino-L-alanine (BMAA) in cyanobacterial blooms from multiple water bodies in Eastern Australia [J]. Harmful Algae, 2018, 74: 10-18. doi: 10.1016/j.hal.2018.03.004 [23] CHATZIEFTHIMIOU A D, BANACK S A, COX P A. Biocrust-produced cyanotoxins are found vertically in the desert soil profile [J]. Neurotoxicity Research, 2021, 39(1): 42-48. doi: 10.1007/s12640-020-00224-x [24] BANACK S A. Second laboratory validation of β-N-methylamino-L-alanine, N-(2-aminoethyl)glycine, and 2, 4-diaminobuytric acid by ultra-performance liquid chromatography and tandem mass spectrometry [J]. Neurotoxicity Research, 2021, 39(1): 107-116. doi: 10.1007/s12640-020-00208-x [25] LI A, HU Y, SONG J, et al. Ubiquity of the neurotoxin β-N-methylamino-L-alanine and its isomers confirmed by two different mass spectrometric methods in diverse marine mollusks [J]. Toxicon, 2018, 151: 129-136. doi: 10.1016/j.toxicon.2018.07.004 [26] GLOVER W B, BAKER T C, MURCH S J, et al. Determination of β-N-methylamino-L-alanine, N-(2-aminoethyl)glycine, and 2,4-diaminobutyric acid in food products containing cyanobacteria by ultra-performance liquid chromatography and tandem mass spectrometry: Single-laboratory validation [J]. Journal of AOAC International, 2015, 98(6): 1559-1565. doi: 10.5740/jaoacint.15-084 [27] BAKER T C, TYMM F J M, MURCH S J. Assessing environmental exposure to β-N-methylamino-L-alanine (BMAA) in complex sample matrices: A comparison of the three most popular LC-MS/MS methods [J]. Neurotoxicity Research, 2018, 33(1): 43-54. doi: 10.1007/s12640-017-9764-3 [28] TYMM F J M, BISHOP S L, MURCH S J. A single laboratory validation for the analysis of underivatized β-N-methylamino-L-alanine (BMAA) [J]. Neurotoxicity Research, 2021, 39(1): 49-71. doi: 10.1007/s12640-019-00137-4 [29] LAGE S, ANNADOTTER H, RASMUSSEN U, et al. Biotransfer of β-N-methylamino-L-alanine (BMAA) in a eutrophicated freshwater lake [J]. Marine Drugs, 2015, 13(3): 1185-1201. doi: 10.3390/md13031185 [30] LAGE S, BURIAN A, RASMUSSEN U, et al. BMAA extraction of cyanobacteria samples: Which method to choose? [J]. Environmental Science and Pollution Research International, 2016, 23(1): 338-350. doi: 10.1007/s11356-015-5266-0 [31] CERVANTES CIANCA R C, BAPTISTA M S, LOPES V R, et al. The non-protein amino acid β-N-methylamino-L-alanine in portuguese cyanobacterial isolates [J]. Amino Acids, 2012, 42(6): 2473-2479. doi: 10.1007/s00726-011-1057-1 [32] CAO Y, HU S Y, GONG T T, et al. Decomposition of β-N-methylamino-L-alanine (BMAA) and 2,4-diaminobutyric acid (DAB) during chlorination and consequent disinfection byproducts formation [J]. Water Research, 2019, 159: 365-374. doi: 10.1016/j.watres.2019.05.007 [33] VIOLI J P, MITROVIC S M, COLVILLE A, et al. Prevalence of β-methylamino-L-alanine (BMAA) and its isomers in freshwater cyanobacteria isolated from eastern Australia [J]. Ecotoxicology and Environmental Safety, 2019, 172: 72-81. doi: 10.1016/j.ecoenv.2019.01.046 [34] CHATZIEFTHIMIOU A D, DEITCH E J, GLOVER W B, et al. Analysis of neurotoxic amino acids from marine waters, microbial mats, and seafood destined for human consumption in the Arabian Gulf [J]. Neurotoxicity Research, 2018, 33(1): 143-152. doi: 10.1007/s12640-017-9772-3 [35] 闫博引. 蓝藻神经毒素BMAA在水中赋存状态及氧化降解机制[D]. 哈尔滨: 哈尔滨工业大学, 2020. YAN B Y. Occurrence state and oxidative degragation mechanism of cyanobacterial neurotoxin BMAA in water[D]. Harbin: Harbin Institute of Technology, 2020(in Chinese).
[36] FAASSEN E J, ANTONIOU M G, BEEKMAN-LUKASSEN W, et al. A collaborative evaluation of LC-MS/MS based methods for BMAA analysis: Soluble bound BMAA found to be an important fraction [J]. Marine Drugs, 2016, 14(3): 45. doi: 10.3390/md14030045 [37] LANCE E, ARNICH N, MAIGNIEN T, et al. Occurrence of β-N-methylamino-L-alanine (BMAA) and isomers in aquatic environments and aquatic food sources for humans [J]. Toxins, 2018, 10(2): 83. doi: 10.3390/toxins10020083 [38] COMBES A, EL ABDELLAOUI S, SARAZIN C, et al. Validation of the analytical procedure for the determination of the neurotoxin β-N-methylamino-L-alanine in complex environmental samples [J]. Analytica Chimica Acta, 2013, 771: 42-49. doi: 10.1016/j.aca.2013.02.016 [39] AL-SAMMAK M A, HOAGLAND K D, CASSADA D, et al. Co-occurrence of the cyanotoxins BMAA, DABA and Anatoxin-a in Nebraska reservoirs, fish, and aquatic plants [J]. Toxins, 2014, 6(2): 488-508. doi: 10.3390/toxins6020488 [40] CRAIGHEAD D, METCALF J S, BANACK S A, et al. Presence of the neurotoxic amino acids β-N-methylamino-L-alanine (BMAA) and 2, 4-diaminobutyric acid (DAB) in shallow springs from the Gobi Desert [J]. Amyotrophic Lateral Sclerosis, 2009, 10(sup2): 96-100. doi: 10.3109/17482960903278469 [41] FAASSEN E J, GILLISSEN F, ZWEERS H A J, et al. Determination of the neurotoxins BMAA (β-N-methylamino-L-alanine) and DAB (α-, γ-diaminobutyric acid) by LC-MSMS in Dutch urban waters with cyanobacterial blooms [J]. Amyotrophic Lateral Sclerosis, 2009, 10(sup2): 79-84. doi: 10.3109/17482960903272967 [42] METCALF J S, BANACK S A, LINDSAY J, et al. Co-occurrence of β-N-methylamino-L-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990-2004 [J]. Environmental Microbiology, 2008, 10(3): 702-708. doi: 10.1111/j.1462-2920.2007.01492.x [43] ROY-LACHAPELLE A, SOLLIEC M, SAUVÉ S. Determination of BMAA and three alkaloid cyanotoxins in lake water using dansyl chloride derivatization and high-resolution mass spectrometry [J]. Analytical and Bioanalytical Chemistry, 2015, 407(18): 5487-5501. doi: 10.1007/s00216-015-8722-2 [44] 顾笑笑, 吴湘, 张爱, 等. 神经毒素BMAA在淡水池塘水体中的健康风险及调控技术 [J]. 水生生物学报, 2022, 46(2): 176-183. doi: 10.7541/2021.2021.012 GU X X, WU X, ZHANG A, et al. Health risk of neurotoxin β-N-methylamino-L-alanine(BMAA) in freshwater aquaculture ponds and its control technology [J]. Acta Hydrobiologica Sinica, 2022, 46(2): 176-183(in Chinese). doi: 10.7541/2021.2021.012
[45] WANG C, YAN C, QIU J B, et al. Food web biomagnification of the neurotoxin β-N-methylamino-L-alanine in a diatom-dominated marine ecosystem in China [J]. Journal of Hazardous Materials, 2021, 404: 124217. doi: 10.1016/j.jhazmat.2020.124217 [46] 李博. 藻毒素BMAA在土壤与作物间的迁移累积及其对秀丽隐杆线虫的影响[D]. 南京: 南京农业大学, 2019. LI B. Transfer and bioaccumulation of A cyanobacterial neurotoxin BMAA between soil and crop and its effects on Caenorhabditis elegans[D]. Nanjing: Nanjing Agricultural University, 2019(in Chinese).
[47] JIANG L Y, KISELOVA N, ROSÉN J, et al. Quantification of neurotoxin BMAA (β-N-methylamino-L-alanine) in seafood from Swedish markets [J]. Scientific Reports, 2014, 4: 6931. doi: 10.1038/srep06931 [48] RÉVEILLON D, ABADIE E, SÉCHET V, et al. β-N-methylamino-L-alanine (BMAA) and isomers: Distribution in different food web compartments of Thau lagoon, French Mediterranean Sea [J]. Marine Environmental Research, 2015, 110: 8-18. doi: 10.1016/j.marenvres.2015.07.015 [49] LAMPINEN SALOMONSSON M, HANSSON A, BONDESSON U. Development and in-house validation of a method for quantification of BMAA in mussels using dansyl chloride derivatization and ultra performance liquid chromatography tandem mass spectrometry [J]. Analytical Methods, 2013, 5(18): 4865. doi: 10.1039/c3ay40657a [50] SCOTT L L, DOWNING S, DOWNING T. Potential for dietary exposure to β-N-methylamino-L-alanine and microcystin from a freshwater system [J]. Toxicon, 2018, 150: 261-266. doi: 10.1016/j.toxicon.2018.06.076 [51] CHRISTENSEN S J, HEMSCHEIDT T K, TRAPIDO-ROSENTHAL H, et al. Detection and quantification of β-methylamino-L-alanine in aquatic invertebrates [J]. Limnology and Oceanography:Methods, 2012, 10(11): 891-898. doi: 10.4319/lom.2012.10.891 [52] FIELD N C, METCALF J S, CALLER T A, et al. Linking β-methylamino-L-alanine exposure to sporadic amyotrophic lateral sclerosis in Annapolis, MD [J]. Toxicon, 2013, 70: 179-183. doi: 10.1016/j.toxicon.2013.04.010 [53] 陈咏梅, 赵以军, 陈默, 等. 武汉官桥湖蓝藻毒素BMAA的生物累积与健康风险评估 [J]. 水生态学杂志, 2019, 40(4): 22-29. doi: 10.15928/j.1674-3075.2019.04.004 CHEN Y M, ZHAO Y J, CHEN M, et al. Bioaccumulation and health risk assessment of the cyanobacterial neurotoxin BMAA in Guanqiao Lake, Wuhan [J]. Journal of Hydroecology, 2019, 40(4): 22-29(in Chinese). doi: 10.15928/j.1674-3075.2019.04.004
[54] WU X, WU H, GU X X, et al. Biomagnification characteristics and health risk assessment of the neurotoxin BMAA in freshwater aquaculture products of Taihu Lake Basin, China [J]. Chemosphere, 2019, 229: 332-340. doi: 10.1016/j.chemosphere.2019.04.210 [55] LI A F, SONG J L, HU Y, et al. New typical vector of neurotoxin β-N-methylamino-L-alanine (BMAA) in the marine benthic ecosystem [J]. Marine Drugs, 2016, 14(11): 202. doi: 10.3390/md14110202 [56] 王超, 邱江兵, 柳超, 等. 神经毒素BMAA沿海洋食物链的迁移转化行为研究[C]. 第十四届生物毒素毒理学术大会暨第一届生物毒素——从生存适应到转化医学专题学术会议会刊, 2019. WANG C, QIU J B, LIU C, et al. Migration and transformation behavior of neurotoxin BMAA along the Marine food chain[C]. Proceedings of the 14th Biotoxin Toxicology Conference and the 1st Biotoxin: From Survival Adaptation to Translational Medicine Symposium, 2019(in Chinese).
[57] HAMMERSCHLAG N, DAVIS D A, MONDO K, et al. Cyanobacterial neurotoxin BMAA and mercury in sharks [J]. Toxins, 2016, 8(8): 238. doi: 10.3390/toxins8080238 [58] MONDO K, HAMMERSCHLAG N, BASILE M, et al. Cyanobacterial neurotoxin β-N-methylamino-L-alanine (BMAA) in shark fins [J]. Marine Drugs, 2012, 10(2): 509-520. [59] MONDO K, BROC GLOVER W, MURCH S J, et al. Environmental neurotoxins β-N-methylamino-L-alanine (BMAA) and mercury in shark cartilage dietary supplements [J]. Food Chem Toxicol, 2014, 70: 26-32. doi: 10.1016/j.fct.2014.04.015 [60] 焦一滢. 蓝藻神经毒素β-N-甲氨基-L-丙氨酸在太湖食物链中赋存与环境行为研究[D]. 南京: 南京大学, 2014. JIAO Y Y. Occurrence of the cyanobacterial neurotoxin β-N-methylamino-L-alanine in foodchains of lake Tai and the study of environmental fates[D]. Nanjing: Nanjing University, 2014(in Chinese).
[61] GANTAR M, SVIRČEV Z. Microalgae and cyanobacteria: Food for thought(1) [J]. Journal of Phycology, 2008, 44(2): 260-268. doi: 10.1111/j.1529-8817.2008.00469.x [62] ROY-LACHAPELLE A, SOLLIEC M, BOUCHARD M F, et al. Detection of cyanotoxins in algae dietary supplements [J]. Toxins, 2017, 9(3): 76. doi: 10.3390/toxins9030076 [63] KRÜGER T, MÖNCH B, OPPENHÄUSER S, et al. LC-MS/MS determination of the isomeric neurotoxins BMAA (β-N-methylamino-L-alanine) and DAB (2, 4-diaminobutyric acid) in cyanobacteria and seeds of Cycas revoluta and Lathyrus latifolius [J]. Toxicon, 2010, 55(2/3): 547-557. [64] 樊华. 淡水蓝藻产生神经毒素BMAA和DAB的潜力及其环境影响因子研究[D]. 青岛: 中国海洋大学, 2013. FAN H. Potential production of neurotoxins BMAA and DAB in freshwater cyanobacteria and effects environmental factors[D]. Qingdao: Ocean University of China, 2013(in Chinese).
[65] DROBAC D, TOKODI N, SIMEUNOVIĆ J, et al. Human exposure to cyanotoxins and their effects on health [J]. Arhiv Za Higijenu Rada i Toksikologiju, 2013, 64(2): 119-130. [66] FALCONER I R, HUMPAGE A R. Health risk assessment of cyanobacterial (blue-green algal) toxins in drinking water [J]. International Journal of Environmental Research and Public Health, 2005, 2(1): 43-50. doi: 10.3390/ijerph2005010043 [67] KARLSSON O, KULTIMA K, WADENSTEN H, et al. Neurotoxin-induced neuropeptide perturbations in striatum of neonatal rats [J]. Journal of Proteome Research, 2013, 12(4): 1678-1690. doi: 10.1021/pr3010265 [68] 全国居民主要食品消费量[EB/OL]. 国家统计局, 2013-2020. National consumption of major food products[EB/OL]. National Bureau of Statistics, 2013-2020.
[69] 闫云君, 梁彦龄. 水生大型无脊椎动物的干湿重比的研究 [J]. 华中理工大学学报, 1999, 27(9): 61-63. YAN Y J, LIANG Y L. A study of dry to wet weight ratio of aquatic macroinvertebrates [J]. Journal of Huazhong University of Science and Technology, 1999, 27(9): 61-63(in Chinese).
-




 下载:
下载: