-
我国重金属的生产量与消费量与日俱增,这带来了一系列环境污染问题[1-3]. 铬(Cr)具有高毒性、普遍性和持久性,被美国环保署(EPA)列为首要污染物之一[4]. Cr一般以两种形式存在于环境中:Cr(Ⅵ)和Cr(Ⅲ). Cr(Ⅲ)毒性较小且不溶,而Cr(Ⅵ)毒性是Cr(Ⅲ)的100倍,且具有高度的溶解性和流动性[5],对人体有严重危害[6]. 全球大约80%的Cr被开采后用于冶金行业[7],这些Cr废弃物的自然浸出会使得Cr(Ⅵ)在环境中迁移,造成污染[8]. 我国每年产出大量的Cr废弃物[9],土壤中Cr含量平均值已达78.94 mg·kg-1[10],高于规定要求,需要对Cr污染土壤进行有效治理.
零价铁(ZVI)具有比表面积大、反应活性高、还原能力强等优点,被广泛应用于Cr(Ⅵ)污染土壤的修复[11-12]. 黄铁矿(FeS2)常被用于吸附有机污染物和重金属,其成分为Fe2+和S22−还原基团,可以有效地促进Cr(Ⅵ)的还原与固定[13]. 但ZVI容易表面聚集,会降低其还原能力,且在施用过程中存在过度释放Fe的问题[14],导致土壤孔隙度降低并引起骨料胶结,影响土壤结构[15];天然黄铁矿表面钝化严重[16],导致其与Cr(Ⅵ)反应较慢,这些问题限制了二者的应用. 生物炭(Biochar)是由富含碳的生物质在缺氧条件下热解产生的[17],其原料来源广且价格低[18],是一种环境友好型材料[19]. 生物炭表面官能团丰富,其中羟基、氧羧基和酚类官能团可与土壤中的污染物结合[20],羧酸(COOH)、C=O等可与重金属结合[21]. 生物炭的多孔结构和大比表面积为重金属提供了可观的吸附位点[22],可降低其在土壤中的迁移性[23],已被广泛应用于土壤修复方面[24-25]. 此外,生物炭可作为ZVI等金属材料的载体[26],起到分散作用,减缓钝化现象,有利于重金属污染的治理. 水热炭(Hydrochar)是指一定湿度的生物质在较低温度和一定压力下进行炭化得到的生物炭[27]. 相比热解炭,水热炭无需预处理,耗能低,产率高,孔隙结构发达,有机质含量更高[28-29],对污染土壤具有良好的修复潜力. Teng等[30]利用Fe改性水热炭降低了土壤中Pb和Sb的生物有效性. Xia等[31]制备氨基改性水热炭,施用后土壤中Cu、Pb和Cd的生物有效性、淋溶毒性及在水稻中的富集量均不同程度下降. 然而相比于热解生物炭的广泛应用,水热炭针对特定土壤环境的改性应用研究较少,需要进一步进行实验探究.
机械球磨法[32]可将材料尺寸粉碎至纳米级,并使元素分布均匀,经济高效且操作简单. 本实验采用机械球磨法将ZVI、黄铁矿分别负载在玉米秸秆水热炭上,制备成两种铁改性水热炭,主要目的如下:(1)通过土壤提取实验,研究ZVI、黄铁矿、水热炭及改性炭对土壤中Cr的固定作用,并测定土壤中有效铁的含量,验证两种改性水热炭是否有助于解决过度释放Fe的问题;(2)通过土柱淋溶实验进一步探索改性水热炭对土壤中Cr的固化效能,分析土壤中Cr的纵向迁移规律,同时对实验材料进行表征分析,初步探究水热炭对Cr污染土壤的机制,得出最佳改性水热炭.
-
Cr污染土壤取自山东省某化工厂,土壤风干后,去除石子等杂质,研磨后过40目筛备用,同时取普通未污染土壤进行相同处理. 对两种土壤基本理化性质进行测定,结果见表1. 主要实验仪器见表2,实验所用零价铁(ZVI)平均粒径为48 μm;黄铁矿(FeS2)平均粒径为45 μm.
-
将玉米秸秆置于马弗炉中,在500 ℃下反应3 h,制得热解生物炭(BC);将玉米秸秆与水以1:20的质量比混合后置于反应釜中,在300 ℃下反应1 h,制得水热生物炭(SBC);将SBC分别与ZVI、黄铁矿以不同质量比置于球磨机中,以550 r·min−1运行3 h,制得不同炭铁质量比的ZVI改性水热生物炭(ZBC)和黄铁矿改性水热生物炭(HBC),所有制得的生物炭均过100目筛使其均质. 对改性水热炭的表面形貌、官能团以及施加到土壤前后的元素及价态的变化进行表征.
-
(1)热解炭与水热炭对土壤中Cr赋存形态的影响
以8 g·kg−1 的投加量向20 g污染土壤分别投加BC、SBC,混匀后置于50 mL离心管,调土壤含水率为30%,静置15 d后采用Tessier法[33]对土壤的Cr进行提取分析,每个处理重复3次并作对照实验.
(2)改性水热炭对土壤中Cr固化效能的影响
以10 g·kg−1 的投加量向10 g污染土壤分别投加不同改性水热炭,混匀后置于锥形瓶中,再加入100 mL水后放置于摇床中,设置速度为120 r·min−1振荡48 h,分时间取上清液过0.45 μm滤膜后测定总铬浓度,每个处理重复3次并作对照实验.
(3)不同材料对土壤中Cr固化效能的影响
以不同投加量向20 g污染土壤分别投加不同材料,混匀后置于50 mL离心管,调土壤含水率为30%,静置15 d后测定土壤浸出液总铬浓度,并对投加ZVI、黄铁矿、ZBC和HBC的土壤有效铁含量进行测定,每个处理重复3次并作对照实验.
-
向Cr污染土壤中分别加入5 g·kg−1的ZBC和HBC,保持土壤含水率为30%,在恒温培养箱中培养20 d备用,并作未施加炭的对照实验(CK). 土柱装置为高20 cm、内径4 cm的圆型有机玻璃柱,底部开口连接橡胶管,用于收集浸出液. 向土柱下层填充10 cm的未污染土壤,上层分别填充8 cm不同处理的污染土壤,每个处理重复3次. 从底部注水使土壤饱和后静置24 h,随后从顶部进行淋洗,淋洗液总体积为900 mL,采用间歇浸出法. 得到的浸出液过0.45 μm滤膜后测定总铬浓度. 实验结束后将土柱上下层分段取出,将上层土壤风干后过100目筛得到施加到土壤中的炭,并进行表征;测定下层土壤总铬与Cr(Ⅵ)浓度,观察Cr的纵向迁移性.
-
土壤总铬浓度的测定参照HJ 491—2019,Cr(Ⅵ)浓度的测定参照HJ 1082—2019,土壤浸出液中总铬浓度的测定参照HJ 749—2015;土壤有效铁含量的测定采用火焰原子吸收分光光度法测定,测定前使用二乙基三氨五乙酸法浸提.
-
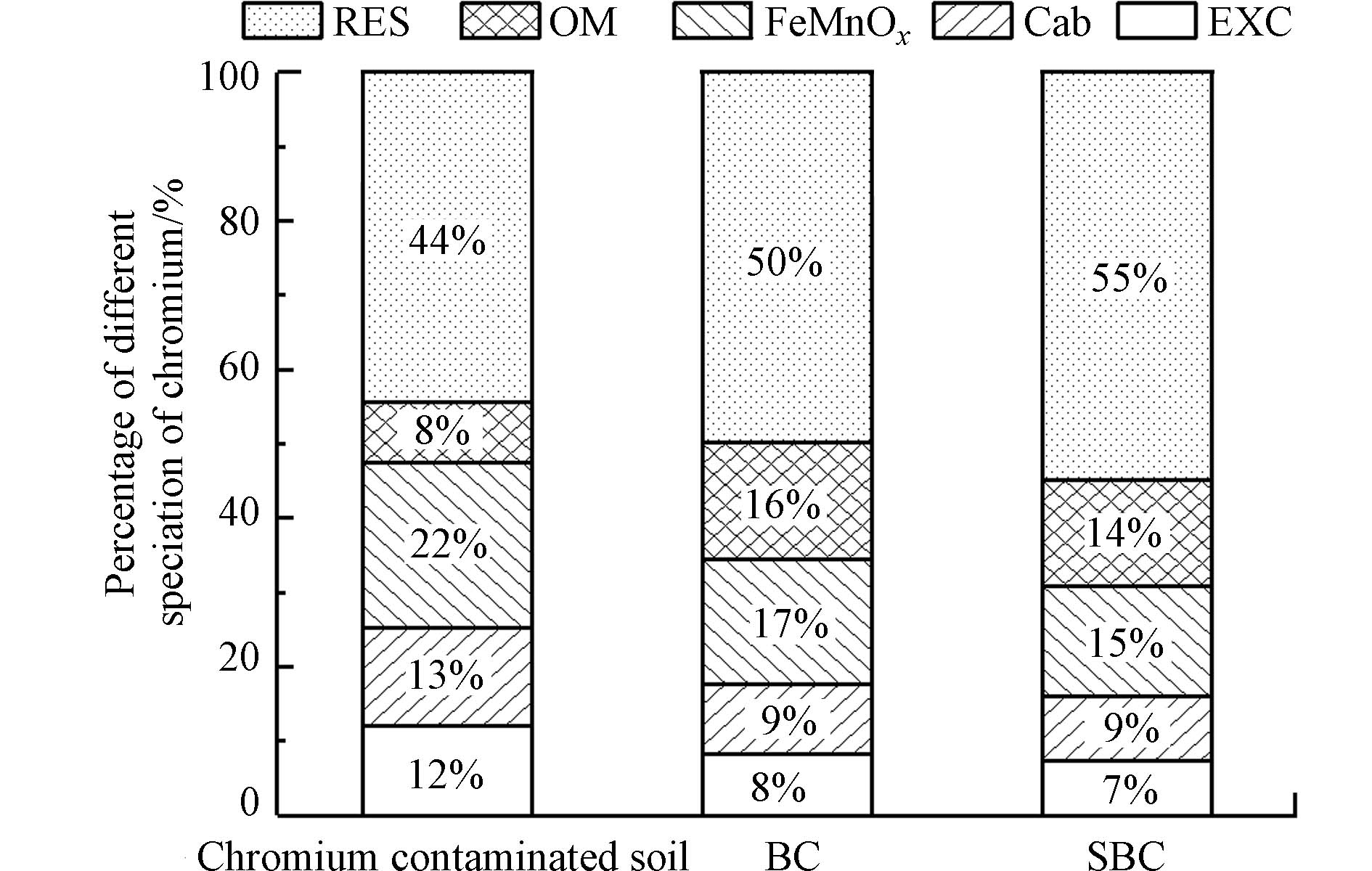
Tessier五步提取法可以把土壤中的Cr分为5种形态,这些形态按照生物利用度和其毒性大小由低到高依次为残渣晶格结合态(RES)、有机质及硫化物结合态(OM)、铁锰氧化物结合态(FeMnOx)、碳酸盐结合态(Cab)、金属可交换态(EXC). 结果如图1所示,与对照土壤相比,施用生物炭处理促进了EXC、Cab和FeMnOx向OM和RES转化,土壤中Cr的稳定性提高,毒性下降. EXC、Cab和FeMnOx组分主要由可溶性高、交换性较强的重金属离子及其碳酸盐态组成[34],这些组分的减少说明生物炭施用后土壤中Cr的稳定性提高. 除生物炭的吸附作用外[35],生物炭与Cr(Ⅵ)的静电吸引以及与Cr(Ⅲ)的络合反应[36]也可能是Cr稳定性提升的原因. 而与BC处理相比,SBC处理后(OM+RES)组分占比增加更为显著,这表明水热炭对土壤中Cr的固定效果更好,可能是由于水热炭拥有更丰富的表面官能团,通过配位键等作用将重金属由活性状态转化为惰性状态[37].
-
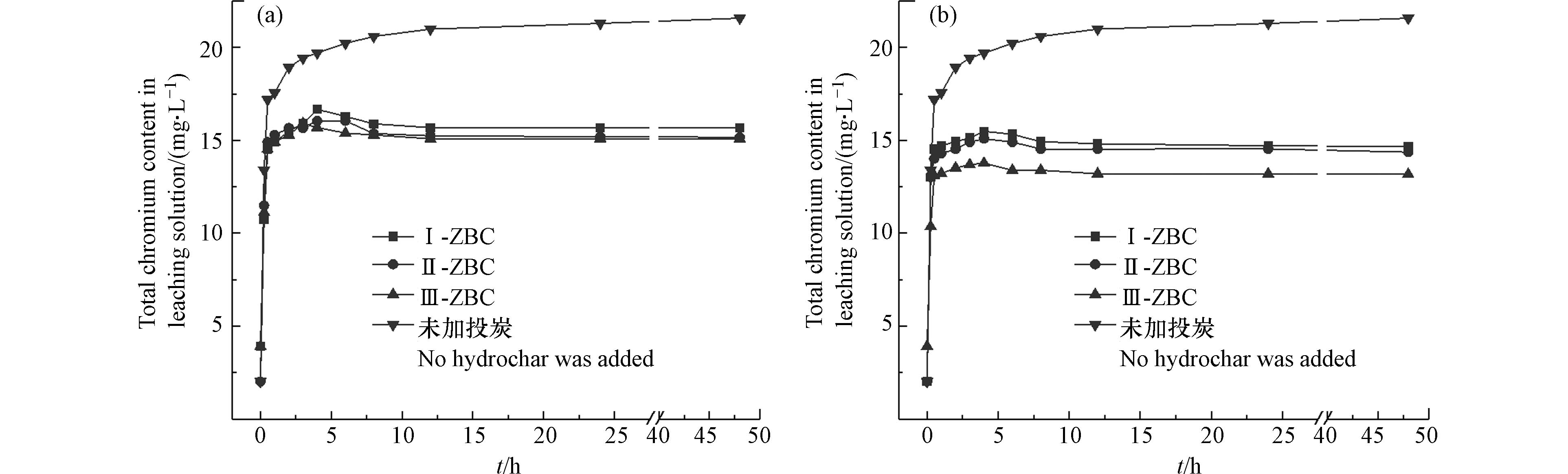
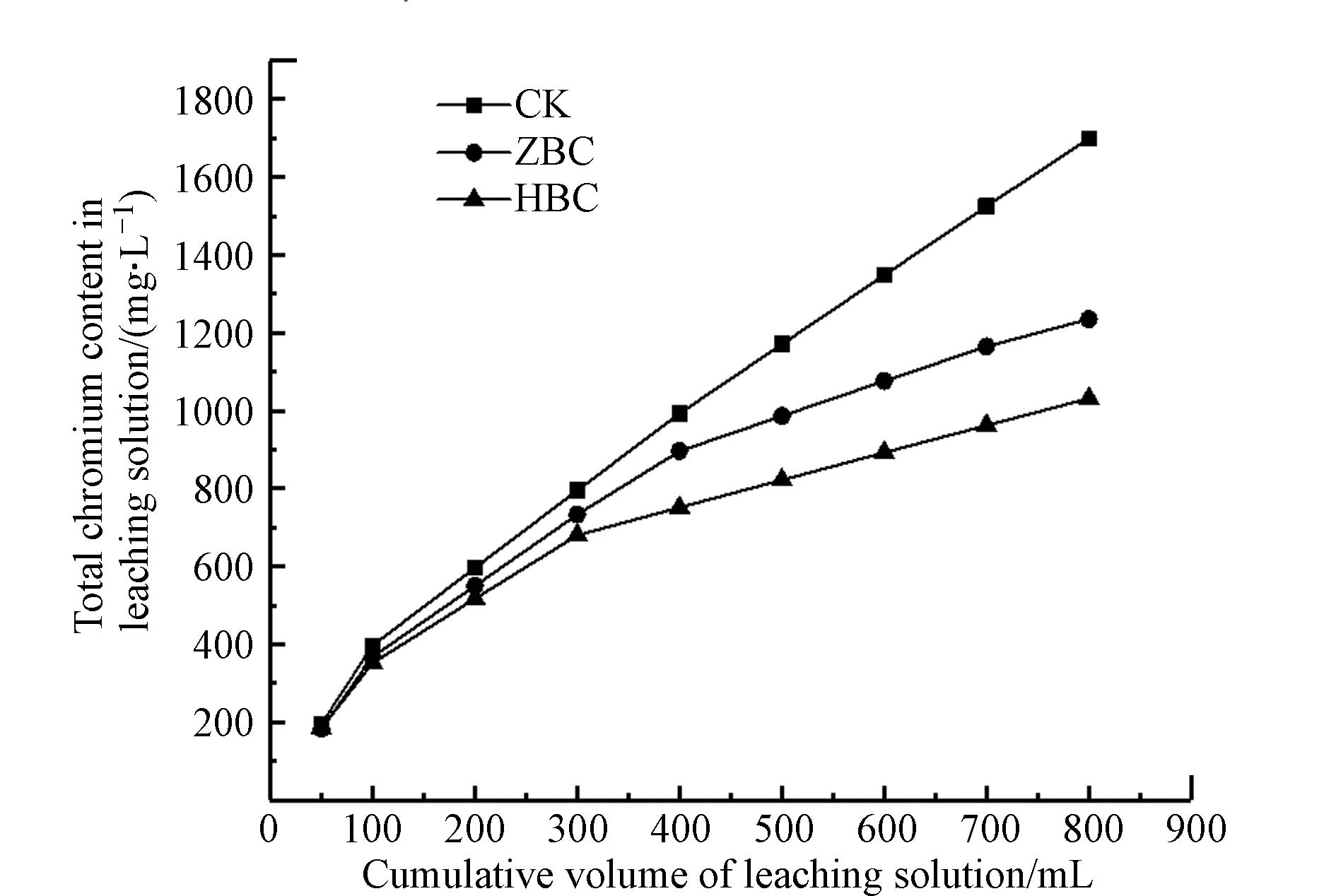
按炭铁质量比2:1、1:1、1:2,将ZVI改性水热炭依次记作Ⅰ-ZBC、Ⅱ-ZBC、Ⅲ-ZBC,将黄铁矿改性生物炭依次记作Ⅰ-HBC、Ⅱ-HBC、Ⅲ-HBC. 由图2可知,施用改性水热炭均降低浸出液总铬含量. 其中施用Ⅰ-ZBC、Ⅱ-ZBC、Ⅲ-ZBC浸出液总铬浓度分别下降27.4%、29.7%、30.0%,彼此无显著差异;施用Ⅰ-HBC、Ⅱ-HBC、Ⅲ-HBC浸出液总铬浓度分别下降32.0%、33.4%、38.9%,整体处理效果优于ZBC,Ⅲ-HBC处理效果突出.
从图2中看出,Cr的释放过程分为两个阶段[38]. 第一阶段土壤表面吸附的Cr和土壤中迁移性较强的Cr(Ⅵ)迅速释放到溶液中,浸出液总铬浓度快速升高. 与对照实验(CK)相比,添加ZBC、HBC后总铬含量显著降低,且增长速率减缓. 在反应进行1 h后进入第二阶段,此时Cr的释放由土壤颗粒表面转为内部,释放速度降低. 施加ZBC、HBC的土壤浸出液总铬浓度在4 h达到最大值后呈下降趋势. 而未投加炭的对照组总铬浓度在6 h后变化趋于平稳,但仍呈上升状态. 因此,施加ZBC、HBC对土壤中的Cr有固定作用. 根据实验结果,选择处理效果较好的Ⅲ-ZBC和Ⅲ-HBC进行后续实验研究,后续提到的ZBC、HBC均为Ⅲ-ZBC、Ⅲ-HBC.
-
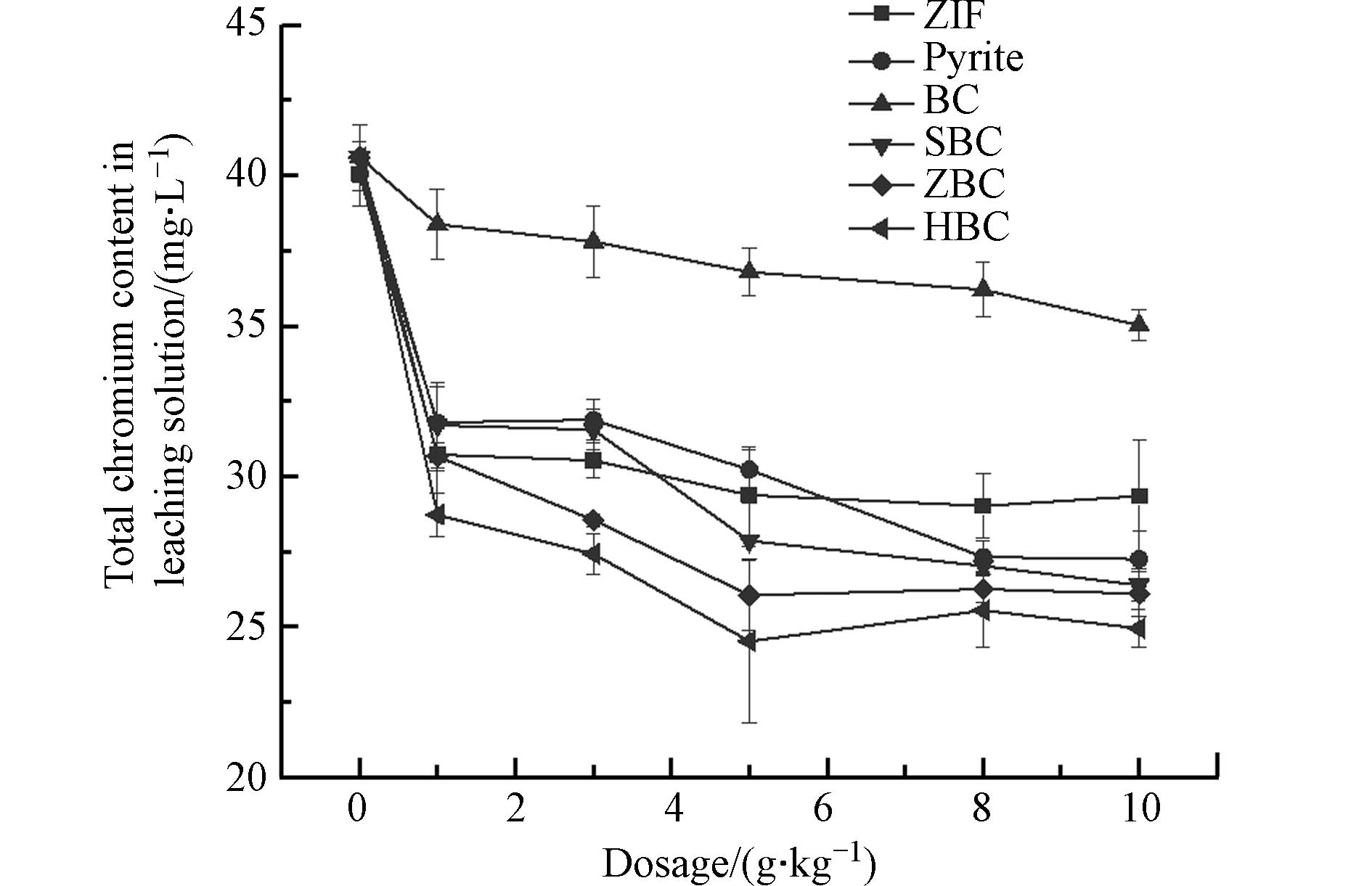
从图3可知,随材料投加量的增加,土壤浸出液中总铬含量整体均呈下降趋势. ZVI在投加量为8 g·kg−1时达到最佳处理效果,此时总铬含量为29.012 mg·L−1,与对照组相比降低27.5%. 增大投加量到10 g·kg−1时,总铬浓度反而上升,可能是高投加量下ZVI会因其磁性造成颗粒团聚,导致炭表面活性位点减少[14]. 且ZVI将Cr(Ⅵ)还原成Cr(Ⅲ)会在其表面形成氧化膜,阻碍活性位点与重金属接触[39]. 相比ZVI,黄铁矿处理效果更好,在投加量为8 g·kg−1时浸出液总铬含量降低到27.3 mg·L−1,与对照组相比降低31.8%. 在所有材料中,BC处理效果最差,投加量为10 g·kg−1时浸出液总铬含量仅降低13.4%,而SBC处理效果较好. 随SBC投加量的增加,其处理效果显著增强. 当投加量为10 g·kg−1时,浸出液总铬含量降低到26.4 mg·L−1,与对照组相比降低35.0%. 这可能是由于水热炭拥有更丰富的表面含氧官能团(如羧基、羟基等),对Cr有更强的吸附能力[40]. 两种改性水热炭处理效果更为优秀,且达到最佳处理效果所需投加量较低,节省材料使用量. 这可能是因为球磨改性后炭颗粒粒径变小,比表面积增大[41],ZVI、黄铁矿较好地负载到炭骨架上,且铁颗粒的团聚现象减弱,增强改性炭对土壤中Cr的吸附和还原能力[26]. 在5 g·kg−1最佳投加量下,ZBC处理后浸出液总铬含量降低到26.0 mg·L−1,比对照组降低35.8%;而HBC处理效果最好,其浸出液总铬含量降低到24.5 mg·L−1,比对照组降低39.6%.
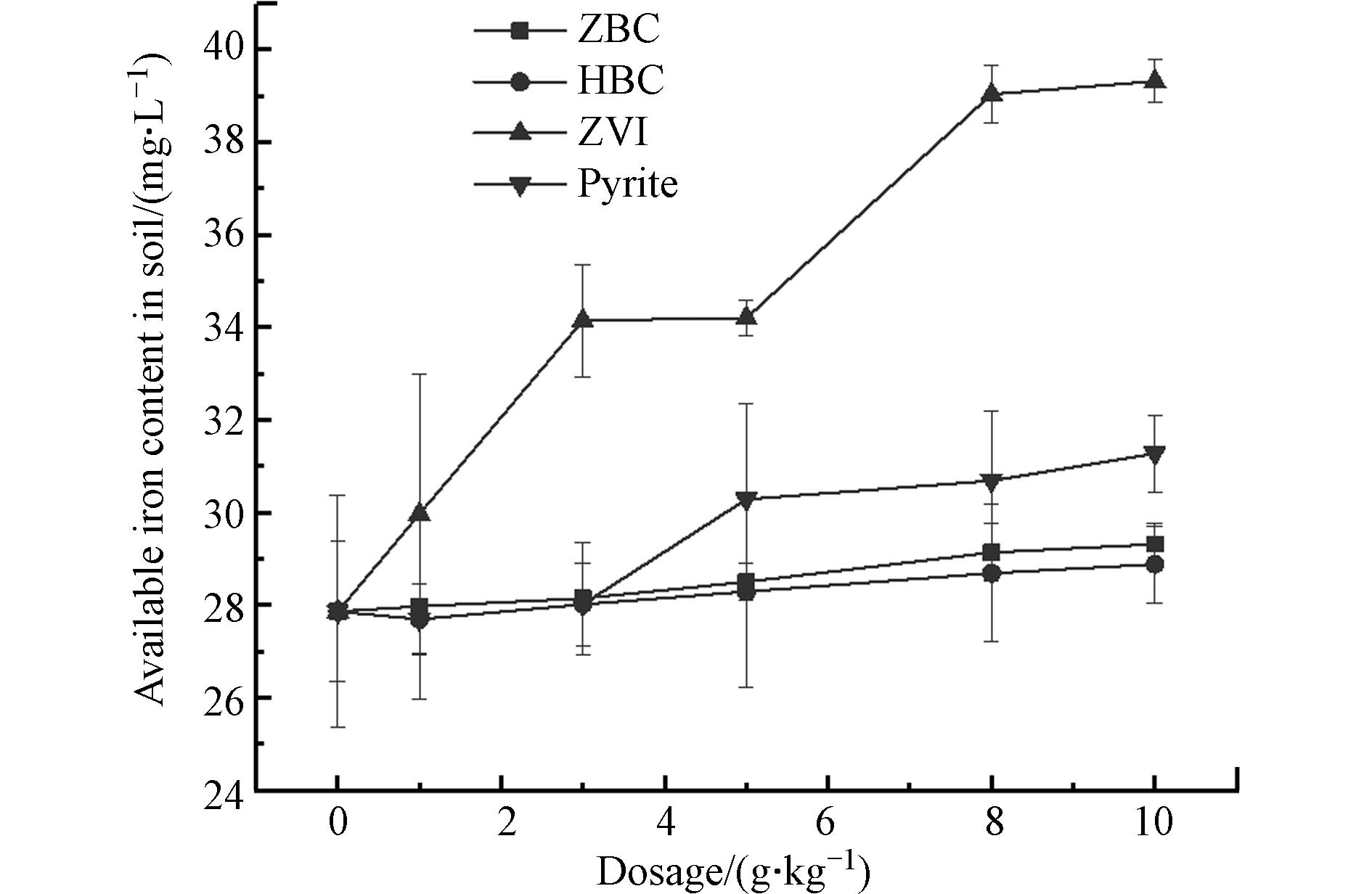
施用ZVI、黄铁矿等进行土壤修复时存在Fe释放过度的问题[14],因此对投加ZVI、黄铁矿、ZBC和HBC的土壤中的有效铁含量进行测定,结果如图4所示.
随ZVI、黄铁矿投加量的增加,土壤中有效铁含量增加. 在最佳投加量8 g·kg−1的条件下,ZVI处理使土壤中有效铁含量增加40.1%,黄铁矿处理使土壤中有效铁含量增加10.1%. 而ZBC、HBC投加量的增加对土壤中有效铁含量影响较小,在投加量为5 g·kg−1的条件下,土壤中有效铁含量的分别增长0.6 mg·L−1和0.4 mg·L−1,涨幅均小于0.1%,有效解决Fe释放过度的问题.
-
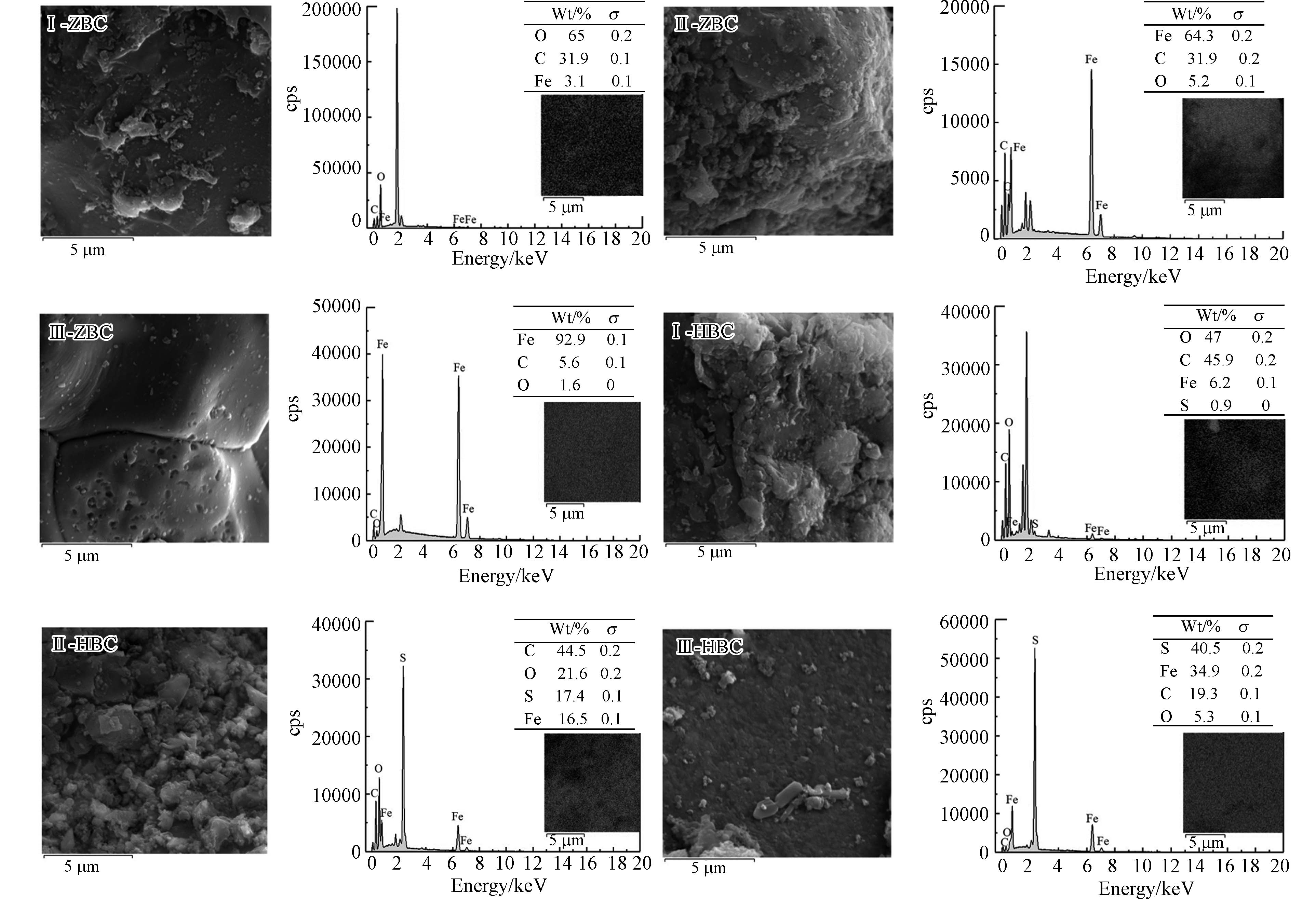
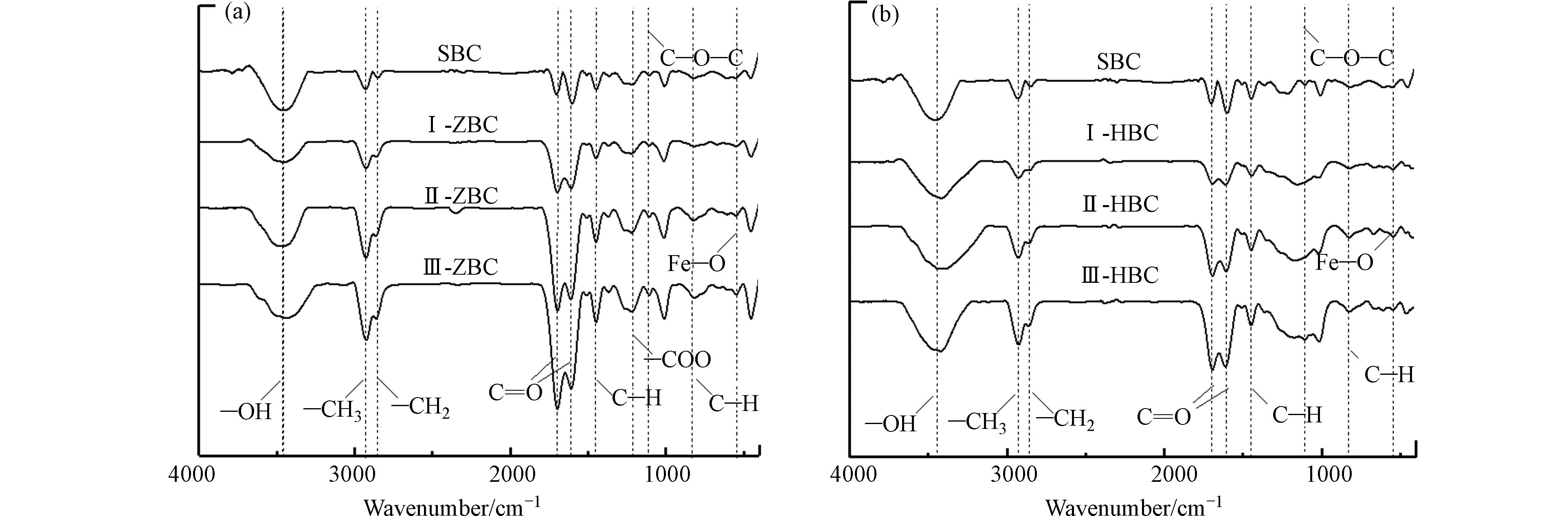
6种改性水热炭的表面形貌及对应的mapping测试结果见图5,可以看到经过球磨后,ZVI、黄铁矿在炭表面分散,炭的表面粗糙,结构不规则. 低铁炭掺杂比的炭存在着Fe元素分布较少或颗粒团聚的现象[14],这可能会导致炭有效孔隙和活性位点减少,降低炭的吸附能力. 随着铁炭掺杂比增大,Fe元素重量百分比上升,分布愈发均匀. 这可能是由于较多ZVI、黄铁矿可与水热炭在球磨过程中更充分地相互摩擦和碰撞,通过球磨介质的作用,使其在生物炭断裂、变形过程中分布到生物炭的表面及孔隙结构中[26],增加炭的活性位点,增强对Cr的吸附能力[42].
-
为进一步探究水热炭改性后对土壤中Cr的固定机理,对SBC和6种改性水热炭的红外特征峰进行分析(图6). O—H等氧化还原活性官能团被认为是生物炭氧化还原能力的驱动力[17],可与重金属阳离子交换[43]. 1730—1734 cm−1处羧基、醛、酮和酯类基团上的C=O峰和1612—1615 cm−1处C=C、C=O峰的强度随着铁炭掺杂比的增加而增大,说明改性后炭含氧官能团增加. C=O等含氧官能团可以为重金属提供大量结合位点,增加炭吸附能力,形成络合物[21]. 543 cm−1处为Fe—O的弱峰[44],证明Fe与含氧基团结合,成功地负载在水热炭表面.
-
对两种改性水热炭施加到土壤前后的样品进行XPS测定,结果如图7所示. 观察全谱图可得,二者全谱图中均存在Fe峰,说明Fe成功负载到炭骨架上,其中HBC表面还存在S元素. 炭在施加到土壤后全谱图中均出现Cr峰,且O峰的强度增加,说明改性水热炭可能将Cr吸附在表面并形成铁铬氧化物. 对比施加前后的Fe2p谱图,代表Fe(Ⅱ)的峰强度均下降,Fe(Ⅲ)峰强度相对增强,表明ZBC、HBC中的Fe对土中的Cr(Ⅵ)具有还原能力. 在HBC的Fe2p谱图中,代表FeS2[45-46]的峰强度前后变化明显,这表明FeS2参与了对Cr(Ⅵ)的还原. Cr2p谱图中在577—579 eV处存在代表Cr(Ⅲ)的多重轨道分裂峰[47],表明炭表面存在Cr的氧化物和氢氧化物;在580 eV附近存在代表Cr(VI)的弱峰且拟合较差,进一步说明ZBC、HBC将污染土中的Cr(Ⅵ)还原成Cr(Ⅲ),炭表面不存在或存在极少量的Cr(Ⅵ).
-
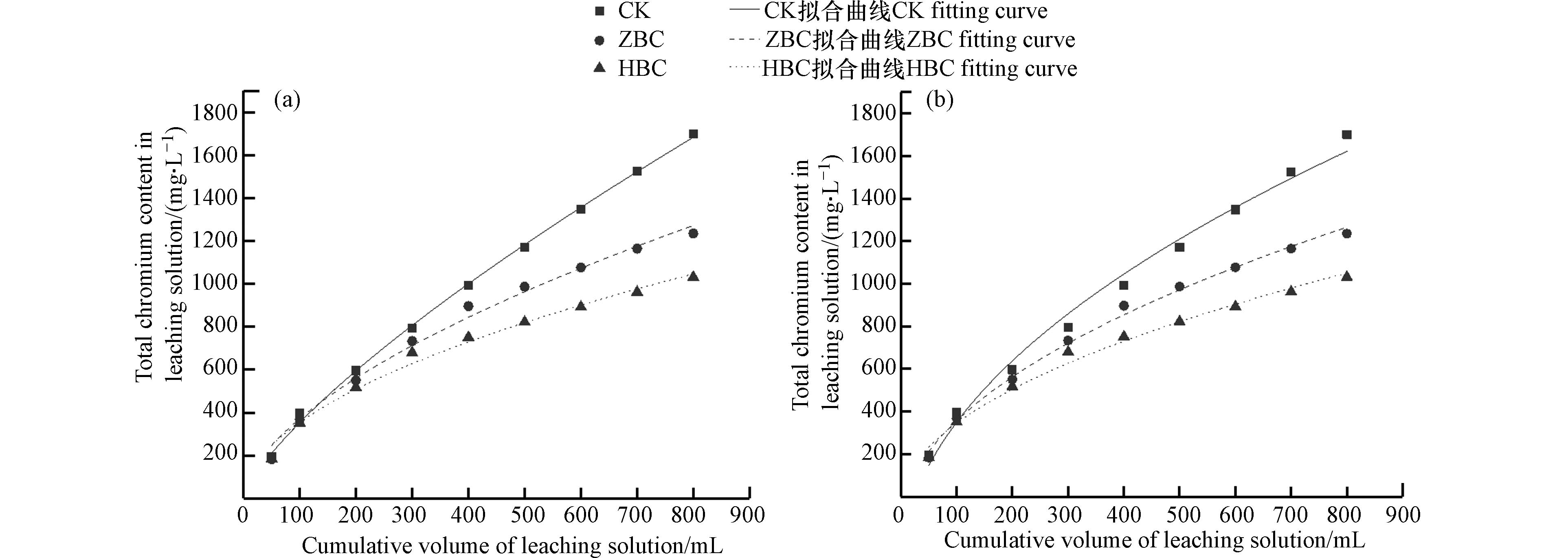
各土柱淋溶液中总铬累积含量变化如图8所示. 在淋溶初期,可溶态重金属快速释放到浸出液中[48],淋溶液中的重金属累积量均快速增加,当淋溶液体积达到300 mL时,ZBC、HBC凭借表面官能团和优秀的吸附性能,将土壤颗粒内部释放的Cr(Ⅵ)还原为较稳定的Cr(Ⅲ)并吸附在炭表面,使得淋溶液中总铬含量的增长速率减缓,而CK淋溶液中的总铬含量一直呈快速上升状态. 实验结束时CK、ZBC、HBC淋溶液总铬含量分别为1700.22、1235.22、1031.49 mg·L−1. 与CK相比,ZBC、HBC总铬含量分别下降27.3%、39.3%,均表现出良好的Cr固定效果,降低了Cr的迁移性.
土壤中重金属迁移、释放和转化的影响因素复杂,采用动力学模型拟合重金属的累积释放有助于了解过程,阐述机理. 双常数速率方程、抛物线扩散方程常用于描述土壤化学过程,表达式如下:
式中,y表示重金属释放量;x表示淋溶体积;a、b为常数.
双常数速率方程是一种经验方程,可用于反映重金属与土壤表面吸附亲和力的差异[49]. 而抛物线扩散方程常用于描述土壤内部物质的扩散,反映多个扩散机制共同控制的动力学过程[50]. 采用这两种方程对土壤中Cr的累积释放过程进行拟合,得到结果如图9、表3所示. 总的来说两种动力学模型均能较好地描述各土柱淋溶时释放Cr的动力学过程,这说明Cr在炭土环境中的释放机制复杂. 其中双常数速率方程对CK的拟合效果更为优秀,其模拟结果R2值为0.9985. 这说明未投加改性水热炭时,土壤表面吸附点位对Cr亲和力的差异较大,不能有效固定Cr. 抛物线扩散方程对ZBC和HBC的拟合效果更为优秀,其模拟结果R2值分别为0.9954、0.9887. 这说明投加改性水热炭后,土壤表面的Cr被有效吸附,淋溶液中的Cr主要来自于土壤颗粒内部的扩散作用.
-
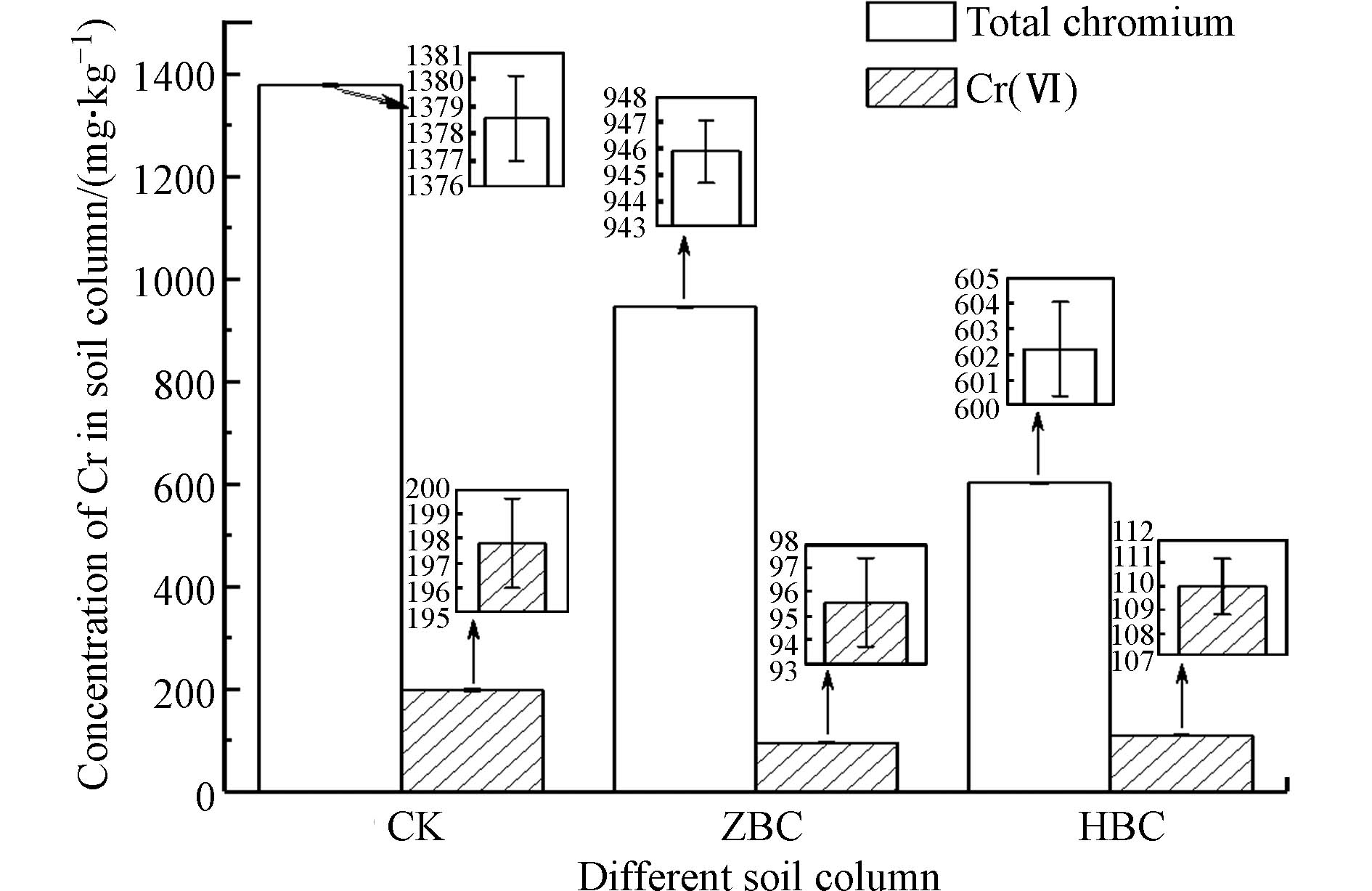
由图10可知,CK土柱中Cr表现出较强的纵向迁移性,在水的淋洗与重力沉降等作用下由上部污染土壤向下部未污染土壤迁移,最终CK土柱下部土壤中总铬浓度为1378.550 mg·kg−1,Cr(Ⅵ)浓度为197.802 mg·kg−1,污染严重. 与CK相比,施加ZBC和HBC后土柱下部土壤的总铬和Cr(Ⅵ)浓度明显下降,其中投加ZBC的土柱下部土壤中总铬含量相比CK降低31.4%,Cr(Ⅵ)浓度相比CK降低51.7%;投加HBC的土柱下部土壤中总铬含量相比CK降低56.3%,Cr(Ⅵ)浓度相比CK降低44.4%.
Cr(Ⅵ)在土壤中的离子态主要为HCrO4−和CrO4−,由于污染土壤pH为8.49,偏碱性,HCrO4−更多地转变为CrO4−. 负载ZVI的ZBC会与CrO4−在土壤中发生以下反应[51]:
通过Fe0、Fe2+的还原能力,最终将Cr(Ⅵ)还原成Cr(Ⅲ),并形成CrxFe1-x(OH)3,固定在土壤中. 从反应式可以看出,酸性条件更利于反应进行,而本实验污染土壤偏碱性,这可能是导致ZBC施加后总铬浓度降低效果较HBC差的一个原因. 而负载黄铁矿(FeS2)的HBC则与CrO4−在土壤中发生以下反应[52]:
且最终Fe3+与Cr3+会发生式(5)反应. 其中S0及其水解产物(S2O32−、S22−)可长期保持土壤对Cr(Ⅵ)的还原能力[52]. 结合XPS表征结果,可知HBC中的FeS2参加反应,而在整个氧化过程中,FeS2可向Fe3+和SO42−提供15个电子,更有利于对Cr(Ⅵ)的还原,故施加HBC后土柱总铬浓度下降明显,更多的Cr(Ⅵ)被还原成Cr(Ⅲ)固定在土壤中. 但ZBC施加后Cr(Ⅵ)浓度降低效果优于HBC,结合浸出液总铬浓度进行分析,可能是由于土壤偏碱性,ZBC与土壤中的Cr(Ⅵ)反应较缓,未完全反应的Cr(Ⅵ)随淋洗液的冲洗快速下沉,使得浸出液中总铬浓度明显升高,而土柱中Cr(Ⅵ)浓度下降. 总的来看,施加HBC后土柱及其淋洗液中的总铬浓度下降程度更大,说明其对污染土壤中的Cr具有更好的固定效果.
-
(1)施加水热炭(SBC)使土壤中稳定性高、毒性低的有机质及硫化物结合态(OM)和残渣晶格结合态(RES)的Cr增加17%,处理效果优于热解炭(BC).
(2)土壤提取实验表明,较大铁炭掺杂比(2:1)制备的改性水热炭ZBC、HBC在低投加量(5 g·kg−1)下对土壤中的Cr表现出更好的固定效果,与对照组相比其土壤浸出液中总铬浓度分别降低了35.8%、39.6%,既节省了材料用量,且不存在向土壤中过度释放铁的现象.
(3)土柱淋溶实验表明,Cr在炭土环境中的释放机制复杂,双常数速率方程对CK土柱拟合较好,表明未施加炭时,土壤表面吸附点位对Cr亲和力的差异较大,不能有效固定Cr;抛物线扩散方程对ZBC和HBC土柱拟合较好,表明投加炭后土壤表面的Cr被有效吸附,淋溶液中的Cr主要来自土壤颗粒内部的扩散作用.
(4)土柱实验结束后,ZBC土柱下部未污染土壤中总铬含量相比CK降低了31.4%,Cr(Ⅵ)浓度相比CK降低了51.7%;HBC土柱下部未污染土壤中总铬含量相比CK降低了56.3%,Cr(Ⅵ)浓度相比CK降低了44.4%. 结合表征结果可得,ZBC、HBC可吸附对土中的Cr,水热炭负载的Fe对土中的Cr(Ⅵ)具有还原能力,可将其还原成Cr(Ⅲ)固定在土壤中. HBC中存在FeS2,有效参与对Cr(Ⅵ)的还原,对污染土壤中Cr的固化效果更好. 此材料可为水热炭修复重金属污染土壤的应用提供思路与探索.
两种铁改性水热炭对土壤中铬固化效能及其形态影响
Effect of two Fe-modified hydrochar carbons on the curing efficiency and morphology of chromium in soil
-
摘要: 本实验采用机械球磨法分别制备零价铁(ZVI)、黄铁矿负载水热炭,通过土壤提取实验和土柱淋溶实验,对比添加不同材料后土壤中铬(Cr)含量的变化,分析淋溶液中Cr累积释放规律及Cr在土柱中的纵向迁移行为. 实验结果表明,与其他材料相比,施加ZVI改性水热炭(ZBC)和黄铁矿改性水热炭(HBC)对土壤中Cr均有优秀的固化效果. 在低投加量(5 g·kg−1)下ZBC、HBC使土壤浸出液中总铬浓度分别降低35.8%、39.6%,而土壤中有效铁含量涨幅均小于0.1%,解决了使用ZVI和黄铁矿引起的土壤铁含量过度增加的问题. 在土柱淋溶实验中,施加ZBC、HBC后淋溶液总铬含量较CK(未加改性水热炭的对照组)分别下降27.3%、39.3%;土柱下部未污染土壤总铬含量分别降低31.4%、56.3%,Cr(Ⅵ)浓度分别降低51.7%、44.4%. 结合表征可得,土壤中的Cr可被ZBC、HBC吸附,水热炭负载的Fe可将Cr(Ⅵ)还原成Cr(Ⅲ)并附着在土壤中,HBC中的FeS2有效参与了对Cr(Ⅵ)的还原. 总的来看,HBC对土壤中Cr的固化效果更好,可为水热炭修复重金属污染土壤的应用提供思路与探索.Abstract: In this work, zero-valent iron (ZVI) or pyrite loaded hydrochar was prepared by mechanical ball-milling method, respectively. Through soil extraction experiment and soil column leaching experiment, the changes of chromium (Cr) content in soil after adding different materials were compared. The cumulative release of Cr in leaching solution and the longitudinal migration behavior of Cr in soil column were analyzed. The results showed that compared with other materials, both ZVI modified hydrochar (ZBC) and pyrite modified hydrochar (HBC) had excellent effect on the immobilization of Cr in soil. Under low dosage (5 g·kg−1), ZBC and HBC reduced the total Cr concentration in soil leaching solution by 35.8% and 39.6%, respectively. While the increase of available iron (Fe) content in soil was less than 0.1%, which solved the problem of excessive increase of Fe content in soil caused by ZVI and pyrite. In soil column leaching experiments, compared with CK (control group without modified hydrochar), the total Cr content in ZBC and HBC leaching solution decreased by 27.3% and 39.3%, respectively. The total Cr content in the unpolluted soil at the bottom of the soil column decreased by 31.4% and 56.3%, respectively, and the concentration of Cr(Ⅵ) decreased by 51.7% and 44.4%, respectively. The characterization result proves that Cr in soil can be adsorbed by ZBC and HBC. Cr(Ⅵ) can be reduced to Cr(Ⅲ) and fixed in soil by Fe load on hydrochar. The presence of FeS2 in HBC effectively participates in the reduction of Cr(Ⅵ). In general, HBC has better effect on the immobilization of Cr in soil, which can provide ideas and exploration for the application of hydrochar in remediation of heavy metal contaminated soil.
-
Key words:
- chromium /
- remediation of contaminated soil /
- hydrochar /
- zero-valent iron /
- pyrite.
-
城市具有环境界面多样、污染物迁移归趋过程复杂等特征,正面对着全球变暖和区域环境恶化的严峻挑战. 城市土地覆被正快速被人工不透水面(建筑屋顶、路面等)所取代,形成了“城市第二自然地理格局”[1]. 不透水面改变了城市水文、能量分布和非点源污染负载,是城市环境系统最重要的环境介质之一[2-3]. 多环芳烃(polycyclic aromatic hydrocarbons, PAHs)广泛分布于城市环境系统中,其具有的“致癌、致畸、致突变”效应和持续输入特征,对城市生态和居民健康构成了极大的威胁. 目前,关于城市环境系统中PAHs的研究多聚焦在大气气溶胶[4]、城市植被[5]、道路灰尘[6]、水体[7]和降水[8]等环境介质,并已检测出较高质量浓度水平的PAHs富集.
Gustafson等发现城市大气中半挥发性有机污染物质量浓度随温度的升高呈指数增加,认为污染物可能来源于被污染的路面[9]. Diamond等发现了典型不透水面(玻璃)表面有机膜存在的证据,认为玻璃可以吸附大气中半挥发性有机污染物[10]. 随后,Diamond等利用多介质逸度模型对城市环境系统中半挥发性有机污染物的迁移归趋过程进行了模拟,发现不透水面的存在增加了污染物在环境中的停留时间[11],认为不透水面是PAHs主要的“汇”[12],而汽车尾气和本地短距离传输的污染物是不透水面PAHs的主要来源[13]. 此外,不透水面的存在增强了PAHs在城市环境系统中的可迁移性,通过大气-不透水面-水体系统增加了水体中PAHs的含量,造成地表水的污染[14]. 玻璃表面已检测出较高质量浓度的有机污染物,如美国“911”恐怖袭击现场玻璃表面PAHs质量浓度高达154 μg·m-2[15],上海市金山工业区玻璃表面PAHs质量浓度达87.8 μg·m-2[16]. 当环境条件改变时,玻璃表面富集的PAHs又会释放到室内空气中,居民日常生活和工作与玻璃直接接触也较频繁,这样不可避免的暴露于PAHs风险之中. 基于此,本研究选择上海市公园绿地玻璃为研究介质,了解玻璃表面PAHs富集水平和组分特征,进一步对污染源进行分析,以期为城市多介质PAHs研究提供理论和数据支撑.
1. 材料与方法(Materials and methods)
1.1 样品采集
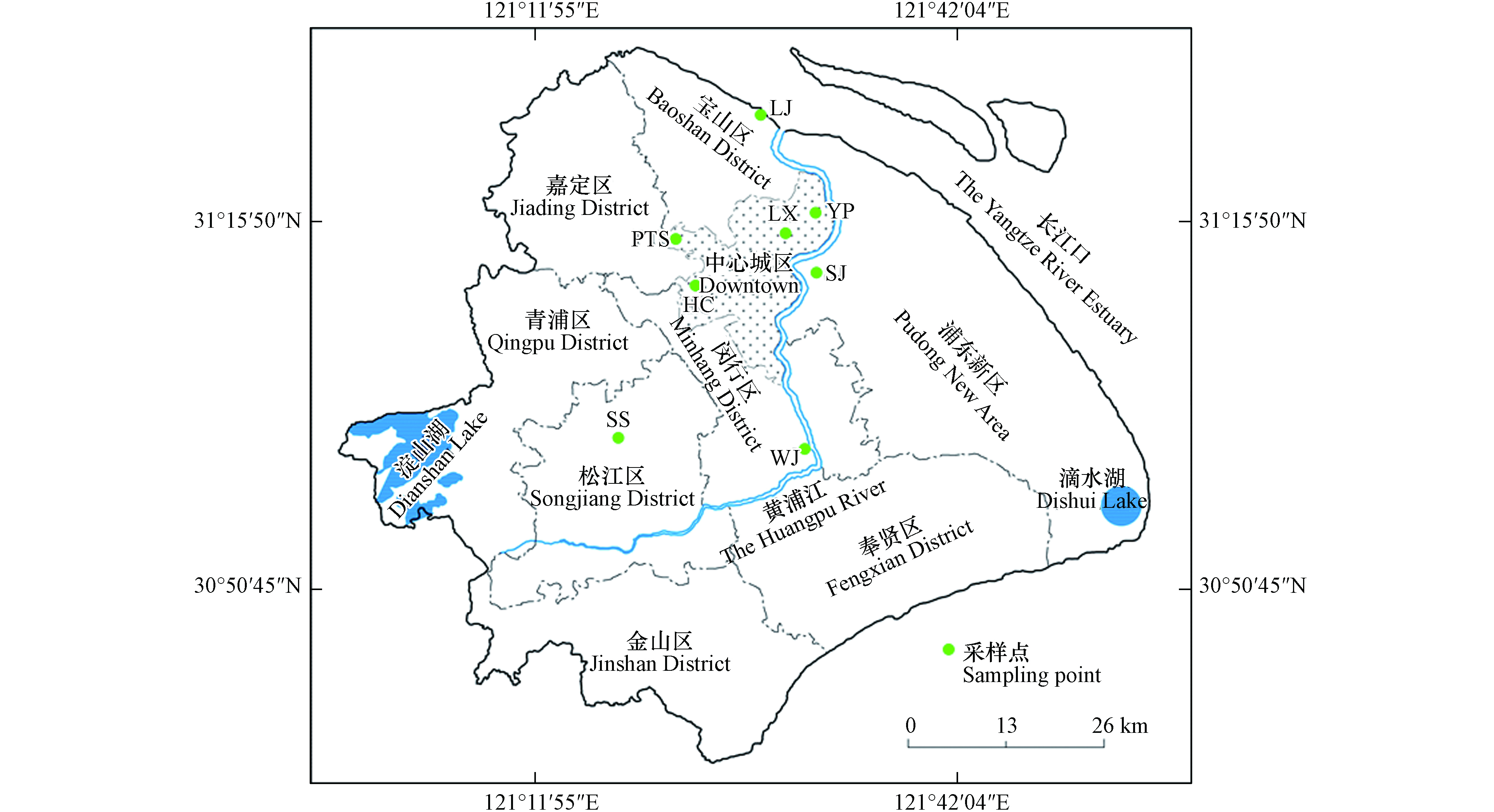
本研究共选择了8个代表性公园绿地,其中杨浦公园(YP)、鲁迅公园(LX)、华漕公园(HC)和普陀体育公园(PTS)位于上海市中心城区,主要满足于附近居民日常休闲需要;世纪公园(SJ)靠近陆家嘴商务区,为4A级旅游景区,汽车流量大;临江公园(LJ)和吴泾公园(WJ)分别位于宝山工业区和吴泾工业区附近,为附近居民日常休闲活动场所;佘山森林公园(SS)位于上海市松江区,为远郊区旅游景区,工业排放和汽车尾气对其影响较小(图1). 考虑降雨时采样点附近草本植物叶片喷溅对玻璃表面PAHs累积的影响和采样的方便性,选择不受降雨冲刷影响且高于地面约2 m左右的玻璃作为采样对象. 根据玻璃大小确定采样面积,保证采样区域距玻璃边框10 cm以上,以免玻璃边框密封材料污染样品. 由于采样玻璃表面PAHs的累积时间不确定,用低尘擦拭纸沾取二氯甲烷将玻璃擦拭干净,然后每隔3个月擦拭玻璃表面采集样品1次,共采集4次,分别代表春季、夏季、秋季和冬季. 采样时,用已净化的低尘擦拭纸(Kimwipes,购于美国kimberly clark公司)沾取二氯甲烷定面积擦拭玻璃表面,采集好的样品用铝箔包好装于自封袋中. 每个采样点采集2个样品,共计64个. 为检验大气中气相PAHs对采样过程的影响,同时采集野外空白样品,用镊子夹取低尘擦拭纸沾取二氯甲烷后在空气中挥动至风干后用铝箔包好,所有样品带回实验室-20℃保存待分析.
1.2 样品处理与分析
样品冷冻风干后称重,加适量铜粉和无水硫酸钠一并填装于滤纸槽中,加入氘代回收率内标物(购于德国Dr. Ehrenstorfer公司),用丙酮和二氯甲烷混合溶剂(120 mL,体积比1:1)在索式抽提器中以4次·h−1的速率连续回流抽提20 h,将抽提液旋转蒸发至约1 mL,用25 mL正己烷进行溶剂置换后再蒸发至1 mL,过硅胶-氧化铝层析柱去除杂质(体积比2∶1,湿法填装),用15 mL正己烷和70 mL混合溶剂(二氯甲烷和正己烷体积比为3∶7)分别淋洗出烷烃和芳烃组分,含芳烃组分浓缩定容至1mL上机分析. 利用气相色谱-质谱联用仪(Agilent, 7890A/5975C, 美国Agilent公司)分析16种优控PAHs(萘(Na)、苊(Acy)、二氢苊(Ace)、芴(Fluo)、菲(Phe)、蒽(An)、荧蒽(Fl)、芘(Pyr)、苯并[a]蒽(BaA)、䓛(Chry)、苯并[b]荧蒽(BbF)、苯并[k]荧蒽(BkF)、苯并[a]芘(BaP)、茚并[1,2,3-cd]芘(InP)、二苯并[a,h]蒽(DahA)、苯并[ghi]苝(BghiP)). 色谱柱为DB-5聚硅氧烷聚合物色谱柱(30 m×0.25 mm×0.25 μm). 升温程序为色谱柱在55 ℃维持2 min,然后以20 ℃·min−1速率升温到280 ℃,以10 ℃·min−1 速率升温到310 ℃,维持5 min. 载气为高纯He,流速1 mL·min−1. 扫描模式:SIM模式.
1.3 质量保证与质量控制
部分空白样品检出Na和Phe(含量低于样品实际含量的3%),方法空白实验无PAHs被检出,16种PAHs加标空白回收率为70%—110%. 氘代内标物回收率为:萘-d8:79.8%—95.1%,二氢苊-d10:79.85%—95.73%,菲-d10:74.83%—98.89%,䓛-d12:77.8%—106.2%,苝-d12:80.1%—101.65%.
1.4 数据处理与制图
玻璃表面PAHs质量浓度有两种表达方式:面积归一化和质量归一化. 计算公式如下:(1)面积归一化质量浓度(ANMC)=C/A(单位为ng·m−2,C为每个采样点2个样品的平均质量浓度,A为采样面积,采样时用刻度尺标出采样范围,一般为30 cm×30 cm);(2)质量归一化质量浓度(MNMC)=C/M(单位为ng·g−1,C为每个采样点2个样品的平均质量浓度,M为采样前后低尘擦拭纸质量差)[15]. 应用毒性当量因子(TEFs)计算PAHs的毒性当量(TEQ),计算公式如下:TEQ=(Ci×TEFs),Ci为单体PAH的浓度,TEFs为毒性当量因子[17]. 运用Freehand,Origin 7.5软件进行绘图.
2. 结果与讨论(Results and discussion)
2.1 PAHs质量浓度水平与组成特征
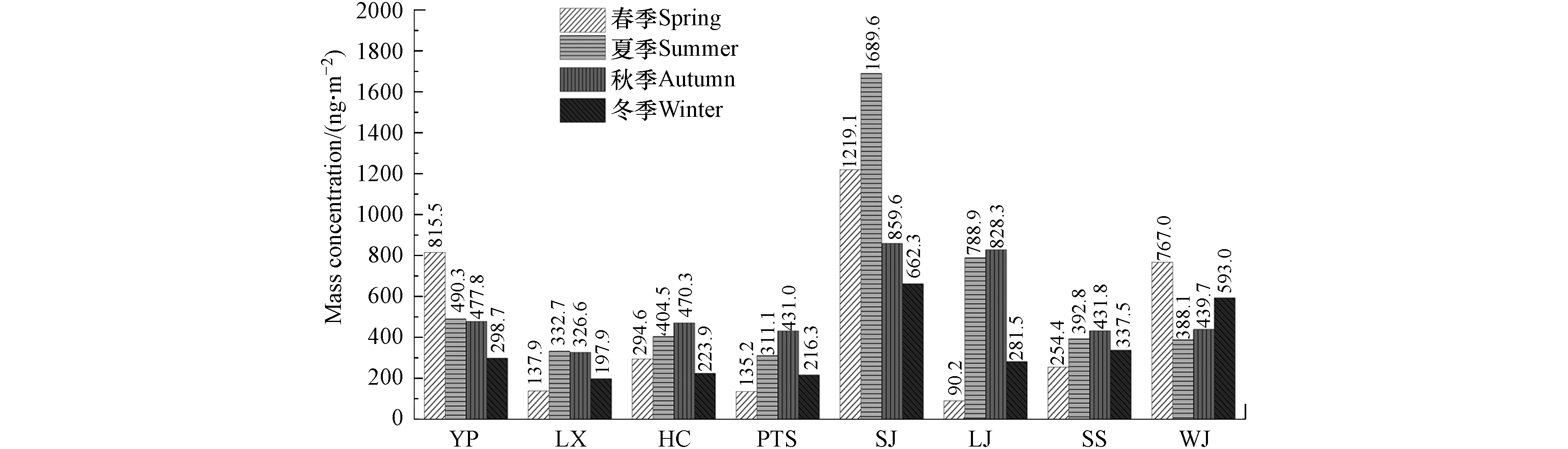
公园绿地玻璃表面PAHs面积归一化质量浓度如表1所示,16种PAHs在春季和夏季的检出率为100%;YP采样点InP、DahA和BghiP,SJ和LJ采样点Acy在秋季低于检出限,而YP、LX、HC、LJ和WJ采样点Acy在冬季低于检出限. 从季节平均质量浓度看,玻璃表面Phe最高(66.9—111.6 ng·m−2),不同季节质量浓度占比在14%—22%之间,其次为Fl(质量浓度在51.3—78.8 ng·m−2之间,占比为9%—17%)和Pyr(质量浓度在32.4—60.7 ng·m−2之间,占比为5%—14%),而DahA最低(质量浓度在2.8—16.4 ng·m−2之间,占比为1%—3%),说明玻璃表面更易吸附大气中的中低环数PAHs,使得高环PAHs的质量浓度较低. 这一结果与上海市大气干湿沉降样品中PAHs富集特征相似,Phe、Fl和Pyr的占比最高[4].
表 1 公园绿地玻璃表面PAHs质量浓度水平(ng·m−2)Table 1. Mass concentration level of PAHs on the glass surface of the park green space(ng·m−2)PAHs YP LX HC PTS SJ LJ SS WJ Na 20.4—113.0 9.1—50.6 31.5—80.8 11.0—40.3 51.6—147.4 14.9—75.5 12.4—71.0 25.3—92.0 Acy 0.0—23.7 0.0—25.2 0.0—25.3 2.3—23.1 0.0—48.6 0.0—67.0 1.5—21.0 0.0—23.4 Ace 2.0—38.2 2.8—20.6 1.9—43.9 2.8—46.0 2.7—42.7 1.5—72.7 1.8—40.5 4.4—52.6 Fluo 20.4—35.1 6.3—26.1 9.8—29.1 5.3—28.0 23.4—49.9 5.2—63.6 5.3—19.2 11.9—28.8 Phe 71.3—121.9 56.3—78.9 56.7—116.8 27.2—118.2 106.0—152.9 11.6—176.5 31.6—74.1 107.7—128.9 An 2.5—12.5 1.4—7.9 2.5—10.8 2.5—10.1 11.3—22.0 1.5—29.9 2.5—7.9 8.8—12.9 Fl 48.1—147.7 18.4—35.9 28.2—43.8 23.2—37.2 93.4—199.4 13.3—98.7 41.7—55.4 25.0—141.5 Pyr 36.0—111.9 12.1—24.1 31.0—36.9 17.7—39.1 61.3—163.7 10.7—74.4 7.7—46.7 31.4—103.3 BaA 9.1—44.6 2.4—17.5 5.8—20.0 5.0—13.3 27.2—82.5 3.4—49.5 11.7—20.7 7.6—32.8 Chry 36.0—98.8 10.0—26.5 20.6—31.9 16.1—28.5 54.2—109.8 6.5—84.7 33.7—43.0 24.3—80.5 B[b+k]F 17.1—63.3 3.7—25.3 9.0—26.6 8.7—20.3 46.0—133.5 7.5—202.1 22.3—33.9 10.3—76.3 BaP 4.8—47.9 2.0—10.0 3.2—11.3 3.6—7.4 22.2—136.0 3.0—88.3 12.6—19.0 4.8—42.8 InP 0.0—32.8 2.1—13.3 3.9—9.8 3.6—10.0 25.3—63.8 2.7—266.1 11.4—16.9 6.6—38.1 DahA 0.0—9.9 1.2—5.7 1.2—5.0 1.2—5.8 5.2—18.1 0.6—91.1 2.9—5.6 4.3—12.2 BghiP 0.0—41.4 2.8—15.2 3.3—10.9 5.0—11.5 32.2—70.5 3.4—402.5 9.7—27.2 8.6—54.2 T-PAHs 298.7—731.5 131.9—332.7 223.9—470.3 125.4—431.0 662.3—1066.7 83.6—1689.6 221.1—431.8 388.1—662.4 公园绿地玻璃表面PAHs的质量浓度范围在83.6—1689.6 ng·m−2之间(表1). 玻璃表面PAHs质量浓度在夏季最高(599.7 ng·m−2),其次秋季(533.1 ng·m−2)和春季(464.2 ng·m−2),冬季最低(351.4 ng·m−2). 在夏季,高温使得中低环数PAHs从被污染的土壤、植物叶片和路面等介质挥发到大气中,促进了玻璃表面有机膜的冷凝过程[9]. 与其它研究相比,远低于广州城区(1290 ng·m−2)[18]、上海市交通区(4007.9 ng·m−2)和商业区(3655.6 ng·m−2)[12]、巴尔的摩城区(5408 ng·m−2)[19]、多伦多城区(6100 ng·m−2)[15]和911恐怖袭击后的纽约世贸中心废墟附近玻璃表面PAHs质量浓度(38744 ng·m−2)[20],但高于多伦多农村(210 ng·m−2)[15]. 具体来看(图2),紧邻上海陆家嘴金融区的SJ玻璃表面PAHs季节平均质量浓度最高(1107.6 ng·m−2),SJ采样点附近交通流量大,PAHs更多来源于交通排放[21];其次为WJ(546.9 ng·m−2),该采样点位于黄浦江边,北面为吴泾火电厂,往来船舶尾气和火电厂燃煤是PAHs的主要来源.
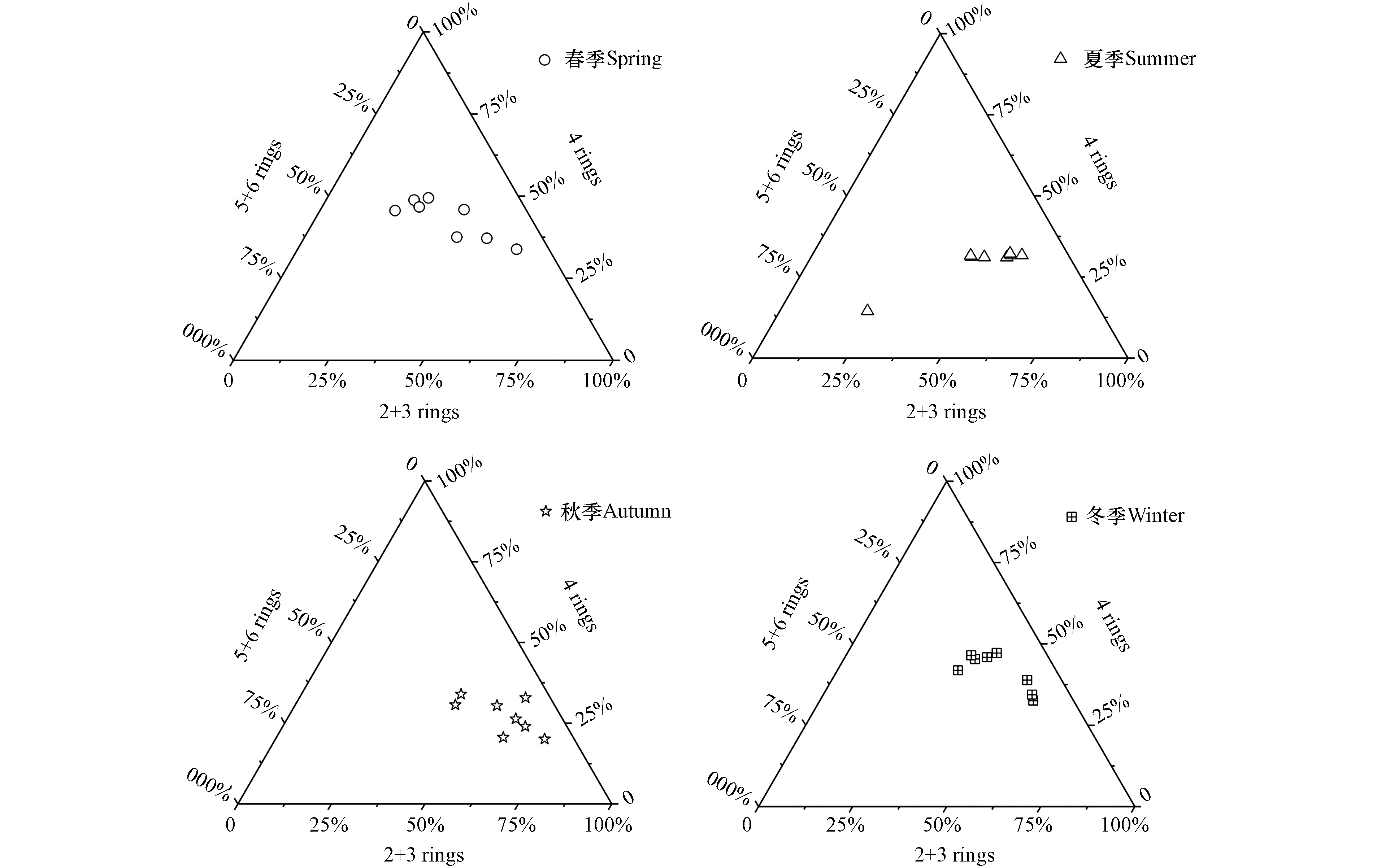
由于气-固分配系数的差异,大气中低环数PAHs以气相形式存在,而高环数PAHs以颗粒相形式存在[22]. 由图3可知,公园绿地玻璃表面PAHs组成特征季节差异明显,4环PAHs的质量浓度平均占比在春季(43%)和冬季(42%)最高;随着季节转换,2+3环PAHs质量浓度平均占比在秋季(57%)和夏季(46%)增加明显,说明大气中PAHs存在形式季节差异明显,进一步影响玻璃表面PAHs吸附过程,这种富集模式表征了城市大气颗粒物中PAHs的老化[15];而5+6环PAHs质量浓度平均占比在夏季(24%)>春季(22%)>秋季(15%)=冬季(15%). 总体上,玻璃表面以2+3环和4环PAHs为主,这与Toronto、香港和广州玻璃表面PAHs组成相似[15,18]. 从单体PAH来看,春季和冬季以Phe、Fl和Pyr为主,夏季以Phe和BghiP为主,而秋季以Phe、Na和Fl为主.
2.2 PAHs溯源分析
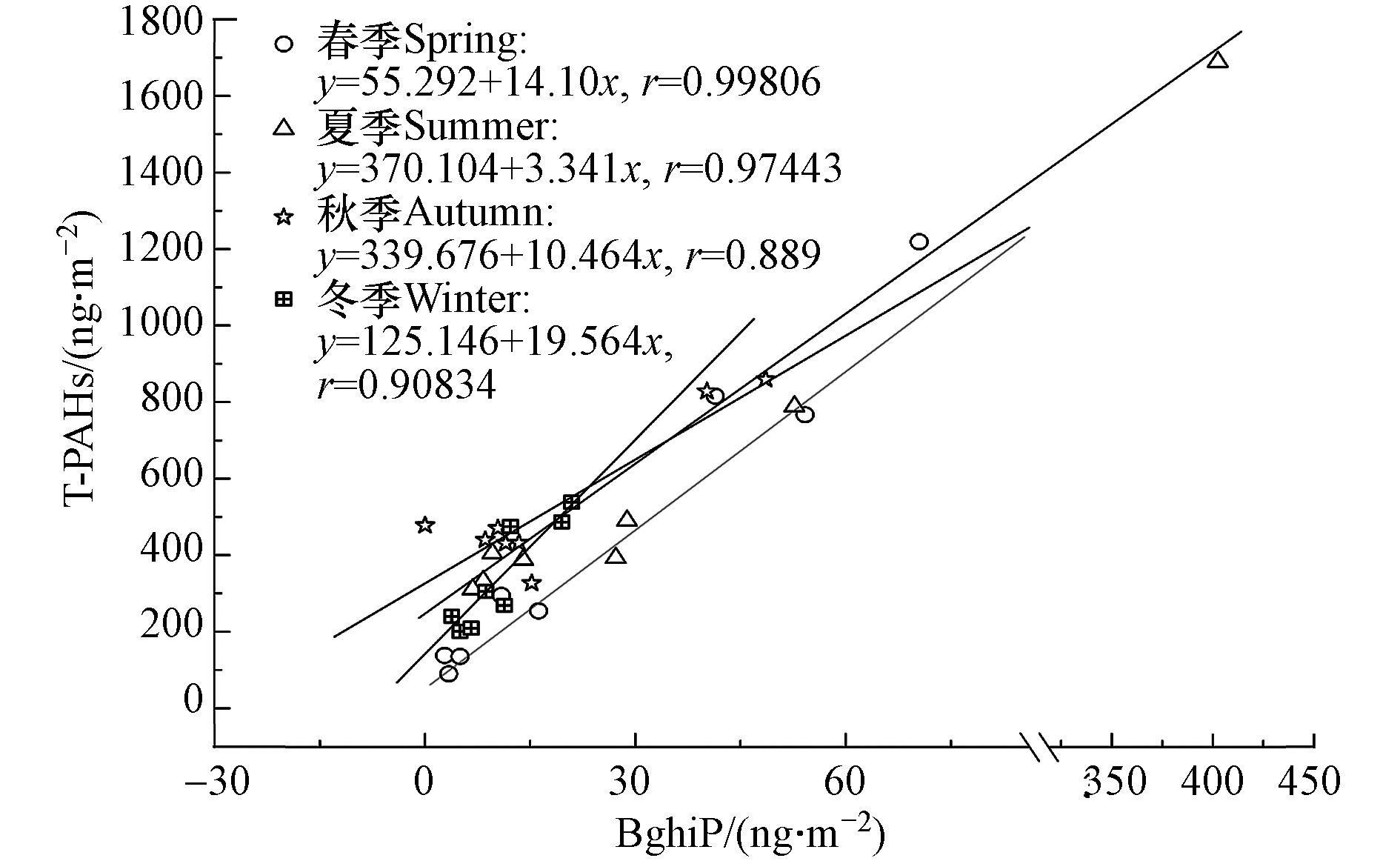
汽车尾气、工业排放和居民生活排放是城市环境系统中PAHs的主要来源[23~25]. 上海市路网稠密、汽车保有量大,汽车尾气对环境中PAHs的贡献不容忽视,而汽车尾气中气相有机物又是玻璃表面有机膜形成的主要物质[13]. 上海地区源成分谱研究表明,柴油车(卡车和客车)尾气中Na、Phe和Fl占比最高,而汽油车尾气中BaP、BghiP和Pyr占比最高[26]. 朱利中等研究认为,BghiP是汽油引擎或柴油内燃机的主要排放物[23],因此,BghiP与T-PAHs之间相关性常被作为城市PAHs的判源指标. 如图4所示,不同季节玻璃表面BghiP与T-PAHs之间均存在很强的正相关关系,春季的相关系数(r=0.99806)>夏季(r=0.97443)>冬季(r=0.90834)>秋季(r=0.889),说明汽车尾气是公园绿地玻璃表面PAHs的主要贡献源.
PAHs同分异构体的热稳定性不同,可利用同分异构体比值判断PAHs来源类型[27]. An/(Phe+An)和Fl/(Fl+Pyr)、InP/(InP+BghiP)和BaA/(BaA+Chry)异构体比值常被用作环境介质中PAHs的判源指标[28]. 不同研究区域环境参数差异较大,使得PAHs在迁移归趋过程中的降解转化不同,所以利用其它研究区异构体比值得出的判源结果可能存在偏差. 本研究利用上海市PAHs污染源成分谱数据对玻璃表面PAHs进行源解析研究[26]. 如图5所示,从An/(Phe+An)和Fl/(Fl+Pyr)异构体比值来看,春季PAHs异构体比值与柴油车,降尘、裸露表土和油烟中PAHs成分谱相近,夏季PAHs异构体比值与柴油车、裸露表土和烧烤PAHs成分谱相似,而秋季和冬季PAHs异构体比值主要与裸露表土和降尘PAHs成分谱相似,可以看出不同季节玻璃表面PAHs污染源存在差异,但主要污染源相对稳定,即为汽车尾气和扬尘源(降尘和裸露表土),而夏季户外烧烤对PAHs有一定的贡献. 从InP/(InP+BghiP)和BaA/(BaA+Chry)异构体比值来看,春季PAHs异构体比值与秸秆燃烧物、草木灰、裸露表土和工业区路面尘、降尘相似,夏季主要与油烟、汽车修理厂表土和草木灰相似,秋季和冬季与秸秆燃烧物和裸露表土相似,说明玻璃表面PAHs在春季、秋季和冬季可能更多来源于扬尘源(裸露表土)和秸秆焚烧,油烟和修理厂表土挥发出的气相PAHs在夏季的贡献较为明显. 相较于其它环境介质(土壤、水体和大气颗粒物等),吸附在玻璃表面的PAHs在光照和气温发生变化时更易发生光降解[12],所以玻璃表面PAHs的溯源还需结合其它判源方法进一步研究.
 图 5 不同季节玻璃表面PAHs特征比值Figure 5. Characteristic ratio of PAHs on glass surface in different seasons1. 柴油车(客车)Diesel vehicle(bus),2. 油烟Lampblack,3. 裸露表土1 Exposed topsoil 1,4. 柴油车(卡车)Diesel vehicle(truck),5. 烧烤Barbecue,6. 宝山区降尘Dust fall(Baoshan),7. 秸秆燃烧物Straw incineration,8. 工地开挖土方1 Site earthwork 1,9. 汽车修理厂表土Topsoil of garage,10. 草木灰Plant ash,11. 宝山区路面尘Road dust(Baoshan),12. 工地开挖土方2 Site earthwork 2,13. 金山区路面尘Road dust(Jinshan),14. 裸露表土2 Exposed topsoil 2
图 5 不同季节玻璃表面PAHs特征比值Figure 5. Characteristic ratio of PAHs on glass surface in different seasons1. 柴油车(客车)Diesel vehicle(bus),2. 油烟Lampblack,3. 裸露表土1 Exposed topsoil 1,4. 柴油车(卡车)Diesel vehicle(truck),5. 烧烤Barbecue,6. 宝山区降尘Dust fall(Baoshan),7. 秸秆燃烧物Straw incineration,8. 工地开挖土方1 Site earthwork 1,9. 汽车修理厂表土Topsoil of garage,10. 草木灰Plant ash,11. 宝山区路面尘Road dust(Baoshan),12. 工地开挖土方2 Site earthwork 2,13. 金山区路面尘Road dust(Jinshan),14. 裸露表土2 Exposed topsoil 22.3 PAHs毒性当量
在城市环境中,PAHs以气相或颗粒相吸附在玻璃表面,居民日常生活不可避免的暴露于PAHs风险之中,所以开展玻璃表面PAHs的毒性当量评估非常必要. BaP毒性当量浓度(toxic equivalent quantity, TEQ)已被广泛地应用于评估PAHs的潜在风险,通过毒性当量因子(toxic equivalence factors, TEFs)将不同毒性的PAHs质量浓度转化为生物毒理学数据,进而了解PAHs的潜在风险[17]. 有关玻璃表面PAHs研究开展较晚且资料较少,对玻璃表面PAHs进行质量归一化处理后的7种致癌性PAHs(BaA、Chry、B[b]F、B[k]F、BaP、InP和DahA)的质量浓度范围为58.3—1311.8 ng·g−1. 计算后的TEQ浓度为夏季(466.6 ng·g−1)>春季(361.0 ng·g−1)>秋季(262.9 ng·g−1)>冬季(214.6 ng·g−1),与上海市文教区(490 ng·g−1)和商业区(261 ng·g−1)玻璃表面TEQ浓度相当[12];但远低于上海市PM2.5中PAHs的TEQ浓度[4];也低于上海市路边土壤中TEQ浓度(892 ng·g−1),与绿化带(401 ng·g−1)、商业区(341 ng·g−1)和公园(324 ng·g−1)土壤中TEQ浓度相当[29];高于上海市不同功能区香樟树叶片中PAHs的TEQ浓度值[30]. BaP、DahA和B[b+k]F是玻璃表面主要的致癌单体PAH,共计占TEQ浓度的80%—91%,其中BaP的TEQ浓度占比高达50%—62%.
3. 结论(Conclusion)
(1)玻璃表面PAHs质量浓度在83.6—1689.6 ng·m−2之间. 在夏季最高(599.7 ng·m−2),冬季最低(351.4 ng·m−2). SJ玻璃表面PAHs平均质量浓度最高(1107.6 ng·m−2),可能受控于附近繁忙的交通排放. 单体PAH中Phe的质量浓度最高(66.9—111.6 ng·m−2),其次为Fl(51.3—78.8 ng·m−2),DahA的质量浓度最低(2.8—16.4 ng·m−2). 玻璃表面以2+3环和4环PAHs为主,4环PAHs在春季和冬季占比最高;2+3环PAHs在秋季和夏季占比最高.
(2)BghiP与T-PAHs之间相关系数呈春季(r=0.99806)>夏季(r=0.97443)>冬季(r=0.90834)>秋季(r=0.889). An/(Phe+An)和Fl/(Fl+Pyr)异构体比值表明主要污染源相对稳定,即为汽车尾气和扬尘源(降尘和裸露表土);而InP/(InP+BghiP)和BaA/(BaA+Chry)异构体比值说明玻璃表面PAHs在春季、秋季和冬季可能更多来源于扬尘源(裸露表土)和秸秆焚烧,油烟和汽车修理厂表土挥发的气相PAHs对夏季PAHs的贡献较明显.
(3)质量归一化处理后,TEQ浓度呈现出夏季(466.6 ng·g−1)>春季(361.0 ng·g−1)>秋季(262.9 ng·g−1)>冬季(214.6 ng·g−1). BaP、DahA和B[b+k]F是玻璃表面主要的致癌单体PAH,共计占TEQ浓度的80%—91%,其中BaP的TEQ浓度占比高达50%—62%.
-
表 1 土壤理化性质
Table 1. Soil physicochemical properties
土壤Soil pH 有机质/(g·kg−1)Organic matter 阳离子交换容量/(cmol·kg−1)Cation exchange capacity 总铬/(mg·kg−1)Total chromium Cr(Ⅵ)/(mg·kg−1) 有效铁/(mg·kg−1)Available iron 污染土壤 8.49 ± 0.05 40.64 ± 0.05 21.84 ± 1.05 9540.51 ± 7.5 1059.51 ± 5 27.86 ± 2.51 未污染土壤 7.64 ± 0.05 20.51 ± 1.04 12.44 ± 0.75 ND ND 4.86 ± 1.05 注:ND未检出. ND, no detected. 表 2 主要实验仪器
Table 2. Main experimental instruments
名称Instrument name 型号Product model 厂家Manufacturer 火焰原子吸收分光光度计 ICE 3500 赛默飞世尔科技公司 行星式球磨仪 QXQM-80 长沙天创粉末技术有限公司 马弗炉 SX2-8-10Z 上海博迅实业有限公司医疗设备厂 反应釜 SLM100 北京世纪森朗实验仪器有限公司 恒温振荡摇床 SHA-CA 常州恒睿仪器设备制造有限公司 扫描电子显微镜 FEI Quanta 400 FEG 美国FEI公司 傅里叶变换红外光谱仪 TENSOR Ⅱ 德国布鲁克光谱仪器公司 X射线光电子能谱仪 K-Alpha 赛默飞世尔科技公司 表 3 总铬累积释放的动力学拟合结果
Table 3. Kinetic fitting results of cumulative release of total chromium
土柱Soil column 双常数速率方程Two-constant rate equation 抛物线扩散方程Parabolic diffusion equation a b R2 a b R2 CK 11.1507 0.7506 0.9985 −346.6836 69.6050 0.9882 ZBC 24.3499 0.5919 0.9900 −140.8598 49.7365 0.9954 HBC 32.3184 0.5203 0.9865 −41.4815 38.5962 0.9887 -
[1] LIU X Y, BAI Z K, SHI H D, et al. Heavy metal pollution of soils from coal mines in China [J]. Natural Hazards, 2019, 99(2): 1163-1177. doi: 10.1007/s11069-019-03771-5 [2] YANG Q Q, LI Z Y, LU X N, et al. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment [J]. Science of the Total Environment, 2018, 642: 690-700. doi: 10.1016/j.scitotenv.2018.06.068 [3] MA Y, WANG L N, CAO Y Z, et al. Stabilization and remediation of heavy metal-contaminated soils in China: insights from a decade-long national survey [J]. Environmental Science and Pollution Research, 2022, 29(26): 39077-39087. doi: 10.1007/s11356-021-18346-w [4] KEITH L, TELLIARD W. ES&T special report: priority pollutants: Ia perspective view [J]. Environmental Science & Technology, 1979, 13(4): 416-423. [5] SAHA R, NANDI R, SAHA B. Sources and toxicity of hexavalent chromium [J]. Journal of Coordination Chemistry, 2011, 64(10): 1782-1806. doi: 10.1080/00958972.2011.583646 [6] ALVAREZ C C, GÓMEZ M E B, ZAVALA A H. Hexavalent chromium: Regulation and health effects [J]. Journal of Trace Elements in Medicine and Biology, 2021, 65: 126729. doi: 10.1016/j.jtemb.2021.126729 [7] COETZEE J J, BANSAL N, CHIRWA E M N. Chromium in environment, its toxic effect from chromite-mining and ferrochrome industries, and its possible bioremediation [J]. Exposure and Health, 2020, 12(1): 51-62. doi: 10.1007/s12403-018-0284-z [8] DHAL B, THATOI H N, DAS N N, et al. Chemical and microbial remediation of hexavalent chromium from contaminated soil and mining/metallurgical solid waste: A review [J]. Journal of Hazardous Materials, 2013, 250: 272-291. [9] HE B, YUN Z J, SHI J B, et al. Research progress of heavy metal pollution in China: Sources, analytical methods, status, and toxicity [J]. Chinese Science Bulletin, 2013, 58(2): 134-140. doi: 10.1007/s11434-012-5541-0 [10] ZHANG X Y, ZHONG T Y, LIU L, et al. Chromium occurrences in arable soil and its influence on food production in China [J]. Environmental Earth Sciences, 2016, 75(3): 1-8. [11] ZHU F, LIU T, ZHANG Z C, et al. Remediation of hexavalent chromium in column by green synthesized nanoscale zero-valent iron/nickel: Factors, migration model and numerical simulation [J]. Ecotoxicology and Environmental Safety, 2021, 207: 111572. doi: 10.1016/j.ecoenv.2020.111572 [12] CHEN X L, LI X M, XU D D, et al. Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review [J]. Nanotechnology Reviews, 2020, 9(1): 736-750. doi: 10.1515/ntrev-2020-0059 [13] WANG T, QIAN T W, HUO L J, et al. Immobilization of hexavalent chromium in soil and groundwater using synthetic pyrite particles [J]. Environmental Pollution, 2019, 255: 112992. doi: 10.1016/j.envpol.2019.112992 [14] SU H J, FANG Z Q, TSANG P E, et al. Stabilisation of nanoscale zero-valent iron with biochar for enhanced transport and in situ remediation of hexavalent chromium in soil [J]. Environmental Pollution, 2016, 214: 94-100. doi: 10.1016/j.envpol.2016.03.072 [15] SNEATH H E, HUTCHINGS T R, DELEIJ F A A M. Assessment of biochar and iron filing amendments for the remediation of a metal, arsenic and phenanthrene co-contaminated spoil [J]. Environmental Pollution, 2013, 178: 361-366. doi: 10.1016/j.envpol.2013.03.009 [16] YE Y X, SHAN C, ZHANG X L, et al. Water decontamination from Cr(Ⅲ)-organic complexes based on pyrite/H2O2: Performance, mechanism, and validation [J]. Environmental Science & Technology, 2018, 52(18): 10657-10664. [17] ZHENG C J, YANG Z H, SI M Y, et al. Application of biochars in the remediation of chromium contamination: Fabrication, mechanisms, and interfering species [J]. Journal of Hazardous Materials, 2021, 407: 124376. doi: 10.1016/j.jhazmat.2020.124376 [18] HASSAN M, LIU Y J, NAIDU R, et al. Influences of feedstock sources and pyrolysis temperature on the properties of biochar and functionality as adsorbents: A meta-analysis [J]. Science of the Total Environment, 2020, 744: 140714. doi: 10.1016/j.scitotenv.2020.140714 [19] CHEN W F, MENG J, HAN X R, et al. Past, present, and future of biochar [J]. Biochar, 2019, 1(1): 75-87. doi: 10.1007/s42773-019-00008-3 [20] BAO Z J, FENG H Y, TU W Y, et al. Method and mechanism of chromium removal from soil: A systematic review [J]. Environmental Science and Pollution Research, 2022, 29(24): 35501-35517. doi: 10.1007/s11356-022-19452-z [21] ABDIN Y, USMAN A, OK Y S, et al. Competitive sorption and availability of coexisting heavy metals in mining-contaminated soil: Contrasting effects of mesquite and fishbone biochars [J]. Environmental Research, 2020, 181: 108846. doi: 10.1016/j.envres.2019.108846 [22] ANTÓN-HERRERO R, GARCÍA-DELGADO C, ALONSO-IZQUIERDO M, et al. Comparative adsorption of tetracyclines on biochars and stevensite: Looking for the most effective adsorbent [J]. Applied Clay Science, 2018, 160: 162-172. doi: 10.1016/j.clay.2017.12.023 [23] CHEN H B, QIN P, YANG X, et al. Sorption of diethyl phthalate and cadmium by pig carcass and green waste-derived biochars under single and binary systems [J]. Environmental Research, 2021, 193: 110594. doi: 10.1016/j.envres.2020.110594 [24] ZHU S H, WANG S, YANG X, et al. Green sustainable and highly efficient hematite nanoparticles modified biochar-clay granular composite for Cr(VI) removal and related mechanism [J]. Journal of Cleaner Production, 2020, 276: 123009. doi: 10.1016/j.jclepro.2020.123009 [25] LI Y Y, ZHANG T T, NING Z, et al. Characteristics and applications of sewage sludge biochar modified by ferrous sulfate for remediating Cr(Ⅵ)-contaminated soils [J]. Advances in Civil Engineering, 2020, 2020: 6521638. [26] WANG S S, ZHAO M Y, ZHOU M, et al. Biochar-supported nZVI (nZVI/BC) for contaminant removal from soil and water: A critical review [J]. Journal of Hazardous Materials, 2019, 373: 820-834. doi: 10.1016/j.jhazmat.2019.03.080 [27] NZEDIEGWU C, NAETH M A, CHANG S X. Carbonization temperature and feedstock type interactively affect chemical, fuel, and surface properties of hydrochars [J]. Bioresource Technology, 2021, 330: 124976. doi: 10.1016/j.biortech.2021.124976 [28] KHAN N, MOHAN S, DINESHA P. Regimes of hydrochar yield from hydrothermal degradation of various lignocellulosic biomass: A review [J]. Journal of Cleaner Production, 2021, 288: 125629. doi: 10.1016/j.jclepro.2020.125629 [29] WANG T F, ZHAI Y B, ZHU Y, et al. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties [J]. Renewable and Sustainable Energy Reviews, 2018, 90: 223-247. doi: 10.1016/j.rser.2018.03.071 [30] TENG F Y, ZHANG Y X, WANG D Q, et al. Iron-modified rice husk hydrochar and its immobilization effect for Pb and Sb in contaminated soil [J]. Journal of Hazardous Materials, 2020, 398: 122977. doi: 10.1016/j.jhazmat.2020.122977 [31] XIA Y, LUO H N, LI D, et al. Efficient immobilization of toxic heavy metals in multi-contaminated agricultural soils by amino-functionalized hydrochar: Performance, plant responses and immobilization mechanisms [J]. Environmental Pollution, 2020, 261: 114217. doi: 10.1016/j.envpol.2020.114217 [32] ZHANG Q R, WANG J M, LYU H H, et al. Ball-milled biochar for galaxolide removal: Sorption performance and governing mechanisms [J]. Science of the Total Environment, 2019, 659: 1537-1545. doi: 10.1016/j.scitotenv.2019.01.005 [33] TESSIER A, CAMPBELL P G C, BISSON M. Sequential extraction procedure for the speciation of particulate trace metals [J]. Analytical Chemistry, 1979, 51(7): 844-851. doi: 10.1021/ac50043a017 [34] FUENTES A, LLORÉNS M, SÁEZ J, et al. Comparative study of six different sludges by sequential speciation of heavy metals [J]. Bioresource Technology, 2008, 99(3): 517-525. doi: 10.1016/j.biortech.2007.01.025 [35] ZHANG S Q, ZHANG H Q, LIU F, et al. Effective removal of Cr(Ⅵ) from aqueous solution by biochar supported manganese sulfide [J]. RSC Advances, 2019, 9(54): 31333-31342. doi: 10.1039/C9RA06028F [36] DONG X L, MA L Q, LI Y C. Characteristics and mechanisms of hexavalent chromium removal by biochar from sugar beet tailing [J]. Journal of Hazardous Materials, 2011, 190(1/2/3): 909-915. [37] REN J, WANG F H, ZHAI Y B, et al. Effect of sewage sludge hydrochar on soil properties and Cd immobilization in a contaminated soil [J]. Chemosphere, 2017, 189: 627-633. doi: 10.1016/j.chemosphere.2017.09.102 [38] FONSECA B, MAIO H, QUINTELAS C, et al. Retention of Cr(Ⅵ) and Pb(Ⅱ) on a loamy sand soil [J]. Chemical Engineering Journal, 2009, 152(1): 212-219. doi: 10.1016/j.cej.2009.04.045 [39] FU R B, ZHANG X, XU Z, et al. Fast and highly efficient removal of chromium (Ⅵ) using humus-supported nanoscale zero-valent iron: Influencing factors, kinetics and mechanism [J]. Separation and Purification Technology, 2017, 174: 362-371. doi: 10.1016/j.seppur.2016.10.058 [40] REGMI P, GARCIA MOSCOSO J L, KUMAR S, et al. Removal of copper and cadmium from aqueous solution using switchgrass biochar produced via hydrothermal carbonization process [J]. Journal of Environmental Management, 2012, 109: 61-69. [41] ZHANG P, BING X, JIAO L, et al. Amelioration effects of coastal saline-alkali soil by ball-milled red phosphorus-loaded biochar [J]. Chemical Engineering Journal, 2022, 431: 133904. doi: 10.1016/j.cej.2021.133904 [42] SHAN D N, DENG S B, ZHAO T N, et al. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling [J]. Journal of Hazardous Materials, 2016, 305: 156-163. doi: 10.1016/j.jhazmat.2015.11.047 [43] INYANG M, GAO B, YAO Y, et al. Removal of heavy metals from aqueous solution by biochars derived from anaerobically digested biomass [J]. Bioresource Technology, 2012, 110: 50-56. doi: 10.1016/j.biortech.2012.01.072 [44] ZHANG Y T, JIAO X Q, LIU N, et al. Enhanced removal of aqueous Cr(Ⅵ) by a green synthesized nanoscale zero-valent iron supported on oak wood biochar [J]. Chemosphere, 2020, 245: 125542. doi: 10.1016/j.chemosphere.2019.125542 [45] LAAJALEHTO K, KARTIO I, NOWAK P. XPS study of clean metal sulfide surfaces [J]. Applied Surface Science, 1994, 81(1): 11-15. doi: 10.1016/0169-4332(94)90080-9 [46] VAN DER HEIDE H, HEMMEL R, VAN BRUGGEN C F, et al. X-ray photoelectron spectra of 3d transition metal pyrites [J]. Journal of Solid State Chemistry, 1980, 33(1): 17-25. doi: 10.1016/0022-4596(80)90543-5 [47] YOLSHINA V A, YOLSHINA L A, PRYAKHINA V I. SEM and XPS study of Cr6+ removal from wastewater via reduction and adsorption by hierarchically structured carbon composite in neutral media [J]. Journal of Inorganic and Organometallic Polymers and Materials, 2021, 31(8): 3624-3635. doi: 10.1007/s10904-021-02003-3 [48] DAI Z J, WANG L L, TANG H, et al. Speciation analysis and leaching behaviors of selected trace elements in spent SCR catalyst [J]. Chemosphere, 2018, 207: 440-448. doi: 10.1016/j.chemosphere.2018.05.119 [49] YU J Z, KLARUP D. Extraction kinetics of copper, zinc, iron, and manganese from contaminated sediment using disodium ethylenediaminetetraacetate [J]. Water, Air, and Soil Pollution, 1994, 75(3/4): 205-225. [50] MOTAGHIAN H R, HOSSEINPUR A R. Copper desorption kinetics in wheat (Triticum aestivum L. ) rhizosphere in some sewage sludge amended soils [J]. Environmental Earth Sciences, 2013, 70(4): 1571-1580. doi: 10.1007/s12665-013-2242-1 [51] JI B, SHU Y R, LI Y X, et al. Chromium (Ⅵ) removal from water using starch coated nanoscale zerovalent iron particles supported on activated carbon [J]. Chemical Engineering Communications, 2019, 206(6): 708-715. doi: 10.1080/00986445.2018.1521390 [52] LI Y Y, LIANG J L, HE X, et al. Kinetics and mechanisms of amorphous FeS2 induced Cr(Ⅵ) reduction [J]. Journal of Hazardous Materials, 2016, 320: 216-225. doi: 10.1016/j.jhazmat.2016.08.010 -




 下载:
下载: