-
羟基多氯联苯 (hydroxylated PCBs, OH-PCBs) 的母体化合物多氯联苯 (polychlorinated biphenyls, PCBs) 是斯德哥尔摩公约限制的首批持久性有机污染物 (persistent organic pollutants, POPs) 之一,曾被广泛应用于电力设备、化工、印刷等领域[1-2]. 由于PCBs具有POPs属性(持久性、生物蓄积性、半挥发性以及生物毒性),自20世纪80年代被大批禁用后,至今在环境中仍难以被彻底消除[3-4]. 环境中的PCBs经过一系列化学/生理生化反应能够产生羟基多氯联苯 (hydroxylated PCBs, OH-PCBs)、甲磺基-PCBs、PCBs硫酸盐等多种二代持久性污染物[5-7]. OH-PCBs是环境样品中最常见的PCBs的代谢/反应产物之一,酚羟基基团的加入使得分子的辛醇/水分配系数减小,从而在生物体内存在的PCBs向OH-PCBs的转化过程普遍被认为是一种有效的生物解毒途径[8-9].
OH-PCBs既能通过生物代谢转化而来,又能在自然环境条件下通过自发进行的化学反应形成[10-11]. 因此,环境中OH-PCBs的来源主要可分为生物代谢途径和非生物过程转化途径. 细胞色素P450单加氧化酶 (CYP450) 是一个庞大的酶系家族,主要存在于肝脏[12],该酶系主要对药物及进入体内的其它外源性化合物进行催化氧化,是药物代谢的重要催化酶[13]. PCBs通过食物摄入、呼吸作用、皮肤接触等过程进入生物体后,在CYP450的作用下,经氧化芳烃介导或在苯环上直接嵌入—OH基团而转化为OH-PCBs[8, 14-15]. 近年来,越来越多的研究显示,PCBs通过大气进行远距离迁移过程中,部分低氯化PCBs以蒸汽形态存在,并能与羟基自由基 (OH·) 发生反应形成OH-PCBs[11]. 两种来源的OH-PCBs经雨水冲刷、大气沉降、地表径流等途径汇聚于江、河、湖、海,从而使水生环境成为OH-PCBs等持久性污染物的主要蓄积库之一.
水生环境蓄积库中的OH-PCBs通过各级生物的不断富集,最终流向包括人类在内位于食物链顶端的生物. 通过对全球各地区研究人员调查结果的统计发现,在水产品摄入量较高地区的人群体内以及以水生生物为主要捕食对象的掠食类动物体内均发现有不同程度OH-PCBs的蓄积[16-22]. 现有的研究结果表明,蓄积在动物体内的OH-PCBs会引发一系列毒性效应,例如内分泌干扰效应[2, 23-24]、生殖毒性[25]及神经行为毒性[26]等. 作为公认的环境内分泌干扰物 (environmental disrupt compounds, EDCs),内分泌干扰效应是OH-PCBs最典型的毒性效应. 研究发现,很多OH-PCBs与生物内源性分泌物 (如:雌二醇、甲状腺素 (thyroxine, T4)、三碘甲状腺原氨酸 (triiodothyronine, T3)) 具有相似的结构特征或酶结合位点,推测该性质与OH-PCBs在血液中的持久残留性有关[27-28]. 目前已经有很多学者针对OH-PCBs的内分泌干扰效应开展调查研究,并从不同角度剖析OH-PCBs的内分泌干扰活性[27, 29-30],但关于其发挥内分泌干扰作用的机理研究仍缺乏直接有力的证据. 随着相关研究的不断深入,越来越多证据表明残留在动物体内的OH-PCBs或具有比母体化合物更强的毒性,而关于OH-PCBs通过食物链进入生物体后的二次代谢过程及其生理危害缺乏系统性研究. 本文对水生环境蓄积库中OH-PCBs的污染现状、生物体内的蓄积传递特征及其内分泌干扰效应机制研究进展进行综述,为进一步研究OH-PCBs的生物有效性、在生物体内的二次代谢及其毒理学评价提供参考.
-
在219种PCBs的同系物中,根据—OH基团在分子上位置的差异,可能共存在837种OH-PCBs[5]. 具有半挥发性的PCBs以大气为介质进行远距离传输过程中,能够通过与大气中的OH· 反应产生OH-PCBs[11];同时,环境中的PCBs经各种生理活动(如呼吸、摄食、直接接触等)进入到动/植物体内,在CYP450系酶的催化下代谢产生OH-PCBs[31]. 水生环境是众多持久性污染物的主要蓄积库之一[32-33],本文系统性地分析了水生环境蓄积库中OH-PCBs的主要来源途径及污染现状.
-
水体、沉积物及水生生物是水生环境生态系统的重要组成部分,水生环境的污染对人类及其他高等动物的生命健康造成威胁[3, 34-35]. 相比于PCBs,OH-PCBs由于—OH的加入增加了分子的极性[8],同时这也增加了OH-PCBs在水生环境中赋存的可能性. 然而,根据对现有文献的统计分析,关于水体OH-PCBs污染情况的可参考数据非常有限. 一方面,OH-PCBs分子的性质决定了其难以以游离态长期存在于水体;另一方面,目前常用的仪器分析方法的灵敏度不足以支持水体中存在的极其微量OH-PCBs的定量分析. 即便是在电子拆解典型污染水域,在7个不同采样点所采集的水样中,其中只有2个采样点的水样中检测到OH-PCBs(∑OH-PCBs, 4.12 ng·g−1)[36]. Ueno等[37]采用气相色谱串联高分辨率质谱法对加拿大安大略省的雨水、雪水和地表水样进行了分析,采集的样品中PCBs和OH-PCBs均有检出,但含量非常低微,其中地表水样中OH-PCBs的平均检出浓度为22 pg·L−1. 目前关于国内外环境水体中OH-PCBs的污染状况的监测,需要引起相关部门的更多关注.
水生环境蓄积库中的OH-PCBs可能会长期吸附在浮游生物等有机体或其他载体上,被低等生物捕食或经水体沉淀作用赋存于沉积物中;与此同时,蓄积在水生生物体内的部分OH-PCBs最终随着生物残骸进入水底沉积物. Ibeto等[38]调查了尼日尔河水生环境中PCBs的含量,发现相比于河上游,河下游的鱼体及河底沉积物中的PCBs的浓度均较高,这说明PCBs等污染物在河底沉积物中的含量可能与河床地势等因素有关. Marek等[39]对芝加哥的印第安纳港附近水域的表层沉积物中的OH-PCBs(0.2—26.0 ng·g−1)浓度进行了测量,其结果表明任何曾受Aroclors污染的地区都可能存在OH-PCBs的污染. 国内学者对太湖水域的底泥分析发现,有多种OH-PCBs(e.g. 3-OH-PCB101)被检出(0.46—12.2 ng·g−1)[40]. 王帅等[36]调查了浙江省台州市PCBs典型污染区底泥污染状况,在4个采样点所采集的底泥中均有OH-PCBs检出(ND—14.4 ng·g−1). 最新的一项调查研究表明,在特定区域内,进入环境的PCBs可能在特定条件影响下向某种OH-PCBs的同系物定向转化[41],该现象可能会影响OH-PCBs在水域附近人群/动物体内的蓄积浓度.
水生生物是水生环境蓄积库中OH-PCBs等污染物随食物链传递的主要载体,水生环境介质中的OH-PCBs通过水生生物的生理过程主动运输或被动扩散进入其体内,并通过食物链在不同营养层级的生物体内传递和蓄积. 本课题组前期在对上海地区淡水水产品及东海海域的野生生物样品中PCB101及其羟基化等代谢物进行调查时发现,不同生活习性和栖息环境的生物体所受污染程度存在较大差异(结果见表1)[42]. 与该调查结果类似,对取自中国浙江省污染水域的水生生物样本进行分析过程中同样发现,底栖鱼类(e.g. 鲫鱼,3.38—29.43 ng·g−1)以及多栖息于腐殖质淤泥中的鱼类(e.g. 泥鳅,16.98—49.93 ng·g−1)体内蓄积浓度较高[36]. 根据学者对美国底特律河中的不同种类的鱼的检测发现,OH-PCBs的蓄积浓度(0.47—171.6 ng·g−1 ww)普遍高于国内的检测水平,但底栖鱼类与食肉/远洋鱼的污染水平未显示出显著差异[43]. 相比于食物链底层水生生物,高等水生掠食动物体内OH-PCBs的蓄积量更高,例如在2009年关于北大西洋的调查结果显示在灰海豹体内检出33种OH-PCBs,累计浓度高达1838.38 ng·g−1 ww[44]. 国内外关于OH-PCBs在水生生物体内的污染情况统计结果见表1.
以上各调查研究结果表明,OH-PCBs的污染已经渗入各级水生环境蓄积库中,其分布不仅与水生环境区域位置、介质种类有关,而且与水生生物栖息环境及其所处食物链/网的营养层级均等存在相关性. 水生环境中赋存的OH-PCBs通过食物链对高级动物包括人类生命健康产生威胁,引起全球学者的关注. 对水生环境中OH-PCBs的来源进行系统性分析,有助于从根源上对局部地域污染情况的预防和治理.
-
自然环境中的OH-PCBs主要源于PCBs的转化,该过程主要分为生物代谢转化和非生物转化. 一方面,PCBs可能通过生物体内的CYP450酶系的催化氧化转化为OH-PCBs;另一方面,大气等环境介质中存在的PCBs能够通过与OH· 发生反应进而转化为OH-PCBs.
-
关于环境中OH-PCBs的非生物转化途径,目前领域内主要集中在关于PCBs与环境介质中OH· 反应的研究. 作为一种半挥发性有机化合物,PCBs在大气以较高浓度存在,该性质对其在环境中的迁移与转化具有深远影响. 大气中的OH· 主要来源于有水蒸气存在的条件下臭氧的光解,因而在对流层中有大量OH· 存在[50]. OH· 具有高活性,几乎能与所有有机化合物发生化学反应. 据相关学者估计,每年通过与OH· 发生反应而从大气中去除的PCBs的量可能高达8300吨[51]. Totten等[52]报告了美国多个地区白天对流层气相PCBs的消耗情况,结合对OH· 日消耗率的观测结果,证明了PCBs的去除是通过与大气OH· 反应实现的. Mandalakis等[53]通过研究提供了亚热带地区(克里特岛和希腊)气相PCBs与大气OH· 反应的直接证据. 研究发现,随着氯化程度的增加,PCBs的还原性减少,导致PCBs与OH· 的反应性下降. 最近,有国内学者使用量子化学和过渡态理论,计算了PCBs与OH· 反应的速率系数,其结果表明所选定的PCBs的半衰期均超过24 h[11]. 值得注意的是,Brubaker等在实验室条件下研究了PCBs和大气中的OH· 之间的反应过程,证实了该反应能够产生OH-PCBs[54].
-
P450酶系是普遍存在于动、植物及微生物的含血红素蛋白家族,参与体内类固醇、脂肪酸、部分维生素以及进入体内的大部分外源化学物(包括医药、污染物、天然化合物和其它化学品)的代谢[12]. P450酶亚型在动物体的肝、肠、肺等多个器官均有表达,肮脏为外源化学物在动物体内进行生物转化的主要器官[55]. 目前的研究普遍认为,PCBs在动物体内的代谢过程主要分为3个阶段:a. 分子的活化;b. 进一步反应并与生物体的内源性分子结合;c. 蓄积在组织中或被排出体外[6, 56]. 产生OH-PCBs的第一阶段主要发生在肝脏,由P450酶催化直接或间接(经氧化芳烃介导转化)产生OH-PCBs,这两种氧化途径在早期已被证实[57]. 因此,动物体内产生的OH-PCBs可能主要来源于PCBs在动物体内发生的第一阶段反应.
研究发现,PCB在生物体内的转化率和转化效率主要取决于分子中Cl—的数量和位置[58]. 一般来说,联苯上Cl—的数量越少,代谢速率越快;与此同时,分子上的非氯取代位置也直接影响了P450酶催化第一阶段反应的发生. 分析对人体血清进行检测的数据发现,在4'- 位置的羟基化PCBs是在血清中发现的主要OH-PCB同系物[59-60]. 此外,近年来关于PCBs在植物和细菌、酵母等低等生物体内通过P450酶催化氧化转化为OH-PCBs得代谢过程也已经被证实[61-63].
-
蓄积在生物体内的OH-PCBs已被证明可能具有比其母体化合物PCBs更高的毒理学效应[64]. 不同来源的OH-PCBs汇聚于水生环境,又通过摄食、呼吸、浸透渗入等途径进入水生生物体内,最终沿着食物链被生物累积和放大,对高等动物的正常生命活动产生影响. 了解OH-PCBs重新进入生物体内后的迁移分布特征及其“再代谢”过程的发生,有助于进一步探索OH-PCBs在生物体内产生的毒理效应机制,预防和治理OH-PCBs引起的潜在污染危害.
-
根据当前对水生环境中OH-PCBs污染现状的调查结果,水生生物体内蓄积PCBs和OH-PCBs的量不仅与附近水域的污染程度有关,同时与其所在食物链、食物网的营养层级具有强相关性[65-66]. 水生环境中OH-PCBs向生物体迁移的过程可分为3个层次. 首先,水生环境中的贝类等处于食物链底层的生物,通过身体的直接接触及在摄食过程接触到OH-PCBs等污染物;其次,富集在浮游生物及其他有机载体上的OH-PCBs,被虾蟹等消费者吸食后,蓄积在其生物体内;第三,鱼类及水生环境中的其他高级捕食者通过捕食、呼吸、身体接触等过程,暴露于水生环境中的OH-PCBs. 多项调查结果表明,OH-PCBs已经通过食物链向哺乳动物及人类体内蓄积[60].
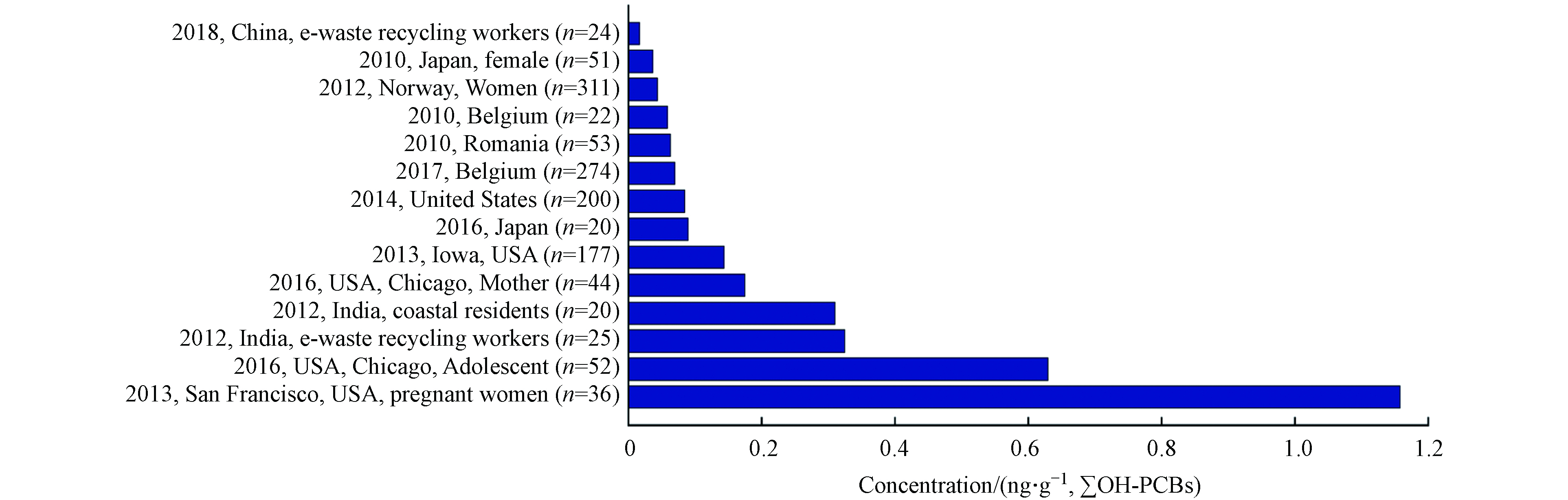
Quinete等[67]对全球各地区不同人群中OH-PCBs的污染状况(1993—2009)进行了统计,发现各地区的污染状况随着时间的推移普遍有明显改善,检出浓度较高者多为长期居住在沿海地区且其膳食结构中海产品占比较大的人群或各国电子垃圾回收工作人员. 本文对近几年 (2010—2018) 更新的研究调查数据进行了统计,见图1. 研究人员对挪威的311名女性血液样本进行分析检测,并根据该部分人群日常摄食海产品的量进行分组,结果表明各组间的OH-PCBs的含量没有显著差异[68]. 然而有更多研究表明,居住在沿海地区的人群通过食品摄入是其暴露于OH-PCBs的重要途径. 日本学者调查了居民体内POPs的蓄积水平,结合人群血液样本和市售海产食品样本检测结果,证明了人群体内存在的OH-PBDE、MeO-PBDE主要来源于食用海藻和鱼类[66]. Dufour等通过对目标人群 (n=274) 开展问卷调查并收集血液样本,建立了涉及年龄、BMI和海鱼消费量的多变量模型,并指出海鱼消费量是影响预测结果的重要变量[69].
自然界中的顶级捕食者,尤其是以鱼类等为食的动物是自然环境中OH-PCBs的主要蓄积载体[19, 46, 76-77]. Gebbink等[78]曾在格陵兰岛东部地区的北极熊血液样本中检测到33种OH-PCBs,总浓度高达1020 ng·g−1. 研究人员通过对比分析,10年前后斯瓦尔巴群岛地区北极熊血液样本中的OH-PCBs浓度几乎减少了一半 ((49 ± 21) ng·g−1),但仍普遍高于其他高等哺乳动物[79]. 在其他高等捕食者身上也发生着类似的变化[19, 80],该现象说明随着PCBs在全球范围禁用时间的推移,环境中赋存的PCBs及OH-PCBs等污染物虽然在减少,但仍然存在由水生环境中的OH-PCBs向外输出的动态趋势. 因此,膳食结构中鱼、虾、蟹等食品占比高,甚至有食用海洋哺乳动物习惯的局部地区人群暴露OH-CBs的风险及其引起的潜在隐患,需要进一步开展相关调查研究.
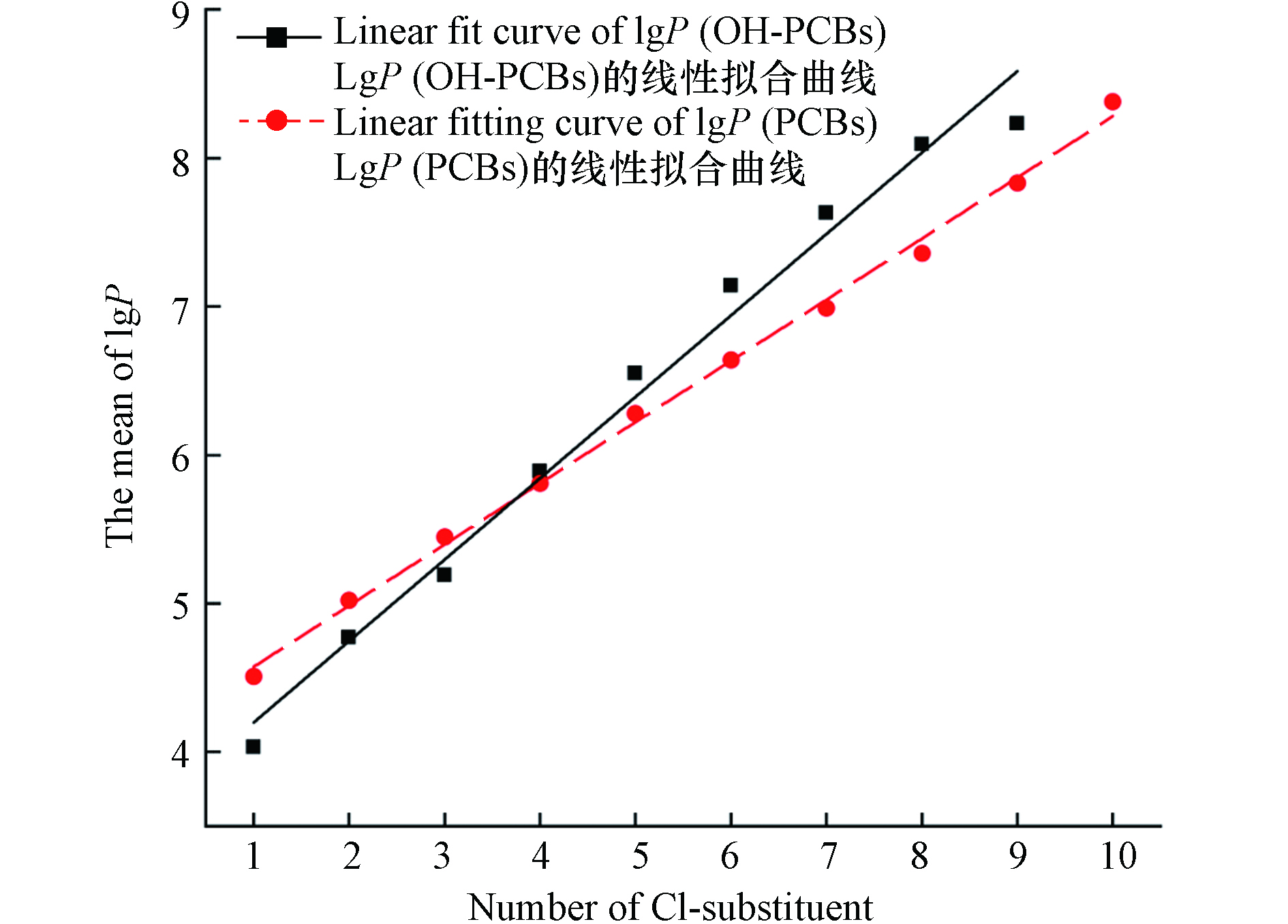
与母体化合物PCBs的性质类似,OH-PCBs的油-水分配系数 (lg P) 较大,且联苯中—Cl取代基团的增加而增大(两者的R2均大于0.95,见图2). 在生物体内经过第一阶段代谢转化产生的OH-PCBs在排出体外之前,似乎优先持留于血液中并在其它组织中产生蓄积[6]. OH-PCBs在生物体内的第二阶段反应包括葡萄糖醛酸化、磺化反应以及与大分子物质的结合[5]. 随着鱼类等食物源性暴露对人类及其他动物体内OH-PCBs贡献率的揭示,部分研究人员将目光聚焦于从外部摄入体内的OH-PCBs在生物体内发生的一些系列生理生化变化.
-
OH-PCBs在生物体内经过第一阶段反应产生之后,在第二阶段转化中趋于和其他官能团形成共轭体系,进而产生更易于排出体外的极性化合物. 诸如OH-PCBs等带有酚羟基的化合物,由于其结构的特异性,能够作为生物体UDP葡萄糖醛酸转移酶 (UDP-glucuronosyltransferases, UGTs) 和硫酸基转移酶 (sulfotransferases, SULTs) 的底物发生催化反应[81-83]. 因此,研究已经证明OH-PCBs在生物体内发生的共轭反应多为葡萄糖醛酸化和硫酸化. 葡萄糖醛酸代谢物是在UGTs催化的酶促反应中,葡萄糖醛酸基从尿苷-5'-二磷酸-α-D-葡萄糖醛酸转移至苯酚形成的. 类似地,哺乳动物胞浆SULT通过转移3'-磷酸腺苷-5'-磷酸硫酸盐中的硫酰基来催化硫酸盐代谢产物的形成.
据报道,体内的OH-PCBs在作为UGTs和SULTs的底物进一步转化过程中,可能同时抑制了这些酶对其他物质的催化. Jia等[84]研究了OH-PCBs对4种人体SULT亚型的潜在抑制能力,在测试过程中发现OH-PCBs对所选择的4种SULT亚型的活性均具有显著抑制效果,且OH-PCBs对SULTs的抑制作用具有浓度依赖性. Liu等[83]利用不同OH-PCBs测试其对人体SULT2A1活性的影响,结果表明其中两种OH-PCBs作为SULT2A1的底物,能够对其他底物造成竞争性抑制;另一种OH-PCBs非SULT2A1底物,但仍然表现出对SULT2A1的抑制作用. Li等[85]报道了OH-PCBs对UGT的抑制作用,推测OH-PCBs对UGTs亚型的抑制作用存在复杂的构效关系,不同Cl—数量的OH-PCBs会对UGT的不同亚型产生抑制效果.
有证据显示OH-PCBs能够被多次氧化,生成双(多)羟基化代谢产物[86-87]. 双羟基化PCBs在体内可进一步转化产生儿茶酚类和其他对苯二酚类物质,该类物质出现在生物机体能引起氧化应激并造成其他一系列损害作用[88].
-
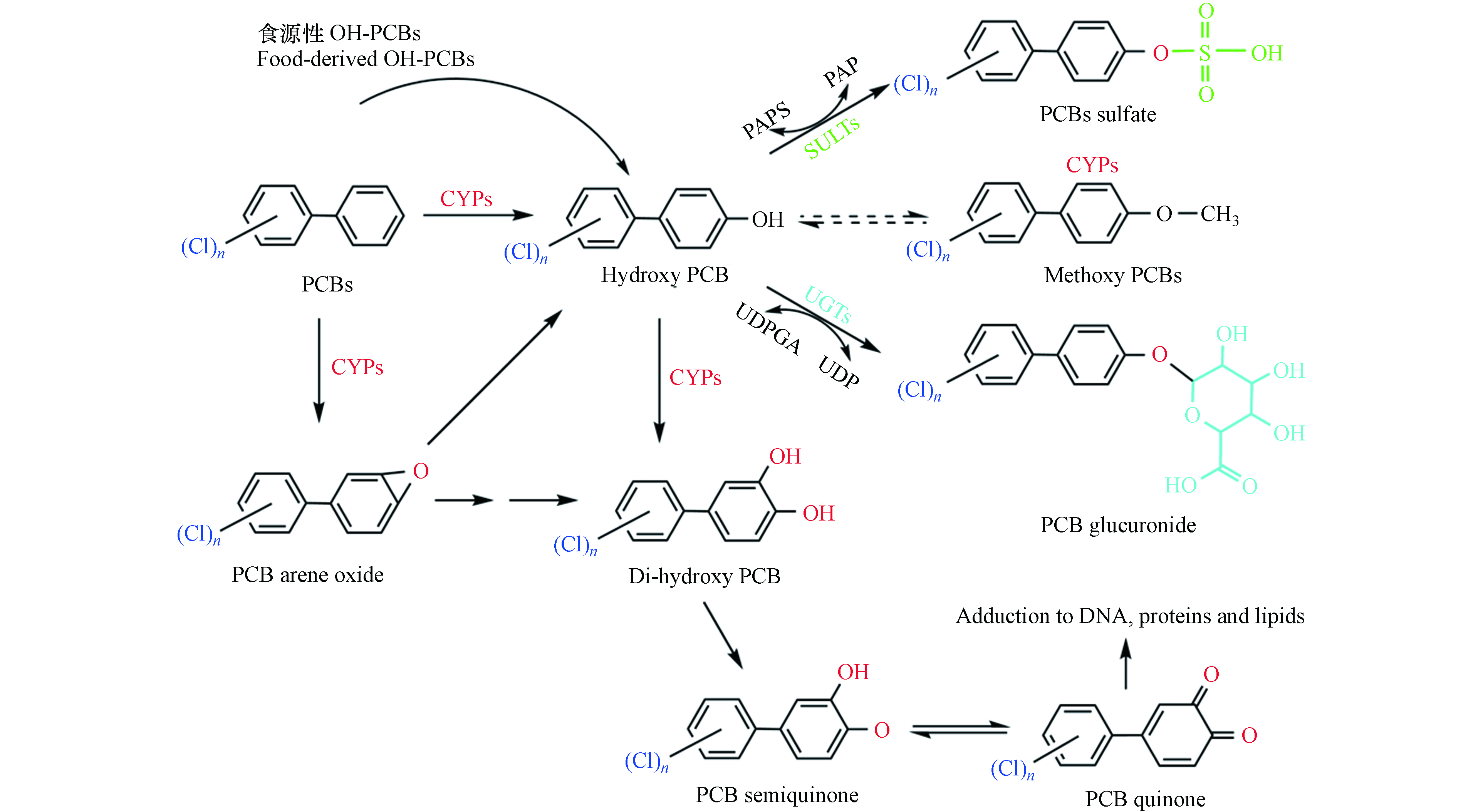
通过各类食品接触OH-PCBs仍然是一条不可忽视的暴露途径. 现有的研究多集中在对典型地区食源性接触OH-PCBs的人群进行污染状况调查,而OH-PCBs通过食物进入动物/人类体内发生的一系列变化有待进一步探索. OH-PCBs进入生物体后,经消化系统吸收进入血液循环,部分OH-PCBs发生与内源性代谢产生的OH-PCBs相同的生物转化(图3),通过第二阶段的反应从而排出体外;其余部分被持留在血液的可能性较大. 留存在血液中的OH-PCBs,关于其在各组织器官中的蓄积分布情况及其影响方面的调查是比较缺乏.
最近的一项研究弥补了这一领域研究的空白,Wang等[89]以鲫鱼为食用水产品代表,向鲫鱼的可食肌肉组织中添加代表性OH-PCBs制备暴露饲料对小鼠进行饲喂暴露实验,结果表明OH-PCBs经摄食暴露后,可能存在向母体化合物PCBs逆向转化的代谢过程,并初步揭示了OH-PCBs在模式生物体内的蓄积分布及迁移特征,相关的代谢路径需要进一步的研究阐明.
近几年的一些研究表明,OH-PCBs在生物体内能够转化为MeO-PCBs,且反应是可逆的. Sun等[90]在对中国境内的污水处理厂附近的污泥样品分析过程中率先发现了这一转化过程,通过实验验证,确定该可逆转化过程为微生物的代谢作用,且MeO-PCBs的去甲基化过程优于OH-PCBs的甲基化. 随后该团队以枯草芽孢杆菌为实验对象,系统性研究了微生物对PCBs的代谢,并确定出与OH-PCBs、MeO-PCBs相互转化相关的甲基转移酶的基因[91]. 研究人员在植物体内观察到类似的可逆转化,向水培杨树植株分别暴露4'-MeO-PCB3和4'-PCB3硫酸盐后均有OH-PCBs产生,并确定了三者之间的相互转化关系[92]. 有实验证明,相比于OH-PCBs,MeO-PCBs在生物体产生的生长抑制和氧化损伤更强,该现象与两者之间的相互转化率是否相关并未得到证实[93]. 关于动物体内是否会发生OH-PCBs向MeO-PCBs的可逆转化问题,有必要开展实验进一步探索.
-
OH-PCBs被归为EDCs已有多年[94-95],经过多项研究证明,OH-PCBs在生物体引发的生殖毒性[25, 96]、生长发育障碍[97]、智力低下[98-99]等多种毒理学效应似乎多与其产生的内分泌干扰效应有关. EDCs产生内分泌干扰效应的机制可分为3种,包括对调节激素水平相关酶的抑制、影响激素类物质的运输以及对与激素水平相关信号的干扰. 根据对相关研究的统计结果,关于OH-PCBs的内分泌干扰效应的研究主要围绕对雌激素和甲状腺激素的干扰,同时这也是目前该领域研究的热点.
-
雌激素在雌性动物的生命周期扮演极其重要的角色,对雌性动物的生长、发育、代谢及生殖等生命活动起着调节作用. 雌激素的分泌和调节反馈机制复杂,同时也意味着体内雌激素水平可能被外源化学物从各个方面造成影响.
雌激素SULTs是一种在内分泌下游调节雌激素水平的酶,有多项研究证明,生物体内低浓度的OH-PCBs便能对雌激素SULTs产生抑制作用[100]. Parker等[101]调查了5种低氯化OH-PCBs对SULTs的影响,发现几种低氯化OH-PCBs是SULT1E1和SULT2A1的强效抑制剂,并有可能对细胞内雌激素相关的底物浓度的调节造成破坏. 研究人员在开展OH-PCBs对人体SULT亚型抑制能力的研究中发现,OH-PCBs对SULTs的抑制作用具有显著的浓度依赖性[84]. Carlson等[102]分别向虹鳟鱼的饲料中加入氯化程度不同的OH-PCBs(4-OH-PCB30、4-OH-PCB61)和天然雌激素(17β-雌二醇和雌酮),证明几种化合物均以剂量依赖的方式诱导血浆卵黄蛋白原 (vitellogenin, VTG),且4-OH-PCB30的诱导效果比4-OH-PCB61更强.
OH-PCBs对雌激素分泌产生干扰过程中,除了暴露剂量外,OH-PCBs的氯化程度和—OH和—Cl基团的位置也决定了化合物的干扰能力. Nomiyama等[103]通过体外原代培养的成年雄性爪蟾肝细胞研究了21种OH-PCBs的VTG诱导活性,且诱导活性较强的均为低氯化OH-PCBs,其中对羟基的活性最高,间氯取代酚环的OH-PCBs在VTG检测中未显示雌激素活性. Takeuchi等[104]筛查了100种OH-PCBs对雌激素等类固醇类激素受体的活化/拮抗作用,结果显示分别有45种、6种OH-PCBs充当雌激素受体的激动剂和拮抗剂,且这些OH-PCBs多具有对羟基和邻位羟基结构. 与低氯化OH-PCBs的性质类似,学者在研究多溴联苯醚 (polybrominated diphenyl ethers, PBDEs) 羟基化代谢物的雌激素干扰效应过程中,低溴化的OH-PBDEs对雌激素水平的干扰力更强[105-106].
研究显示,由于酚羟基的存在,OH-PCBs能够与一些激素类化合物的受体竞争性结合,进而影响激素水平[29, 107]. 研究人员通过体外细胞实验证实了几种OH-PCBs对雄性激素 (androgen receptor, AR) 和雌性激素 (estrogen receptor, ER) 基因活性的影响(表现出抗雄激素和雌激素活性),具有雌激素活性的OH-PCBs对维生素代谢和钙的转运也造成干扰,同时各种迹象表明,OH-PCBs通过直接改变雄激素或雌激素介导的信号传导途径对雌/雄激素水平造成干扰[108]. Cao等[109]对比分析了PCBs、OH-PCBs及PCBs硫酸盐与ERγ结合的亲和力,显然OH-PCBs的亲和力有关,并推测可能与OH-PCBs能够和氨基酸G1u316和Arg247形成氢键有关. 随着研究的不断深入,已有学者比较分子场分析和比较相似性指数分析构建出OH-PCBs与ER的三维定量构效关系模型[110],为进一步揭示OH-PCBs与ER的相互作用机制提供便利.
-
甲状腺激素对动物生理活动的调节是无时无刻的,几乎遍布于机体的组织和细胞. 甲状腺激素的分泌主要受下丘脑-垂体-甲状腺(HPG)轴的调控,有效成分主要为T4、T3和降钙素,其中T4在血浆中含量最多. 分析化合物的结构发现,多种OH-PCBs具有与T4、T3极其相似的活性结合位点,主要通过竞争性与甲状腺素转运蛋白 (transthyretin, TTR) 等运载蛋白的特异性结合而造成TH分泌的紊乱[111].
不同OH-PCBs同系物对TH的干扰效力取决于其结构特性. Lans等[94]通过体外实验研究了OH-PCBs与T4竞争性结合TTR的特性,观察到测试的所有—OH在间位或对位的OH-PCBs的相对结合效力均高于T4. 该团队同时测试了OH-PCBs与TTR、甲状腺素结合球蛋白的结合效力,结果表明OH-PCBs能够有效结合TTR而竞争性抑制T4的结合[95]. OH-PCBs通过竞争性与TTR结合而延长其在血液中的半衰期,不仅影响甲状腺激素水平,并且能够以结合体的形式透过血-脑、胎盘屏障进而造成更多毒理学效应[112-113]. Otake等[114]通过实验证明,产前宫内暴露OH-PCBs的浓度与新生儿体内T4的水平显著相关. 日本学者测量了被诊断为乳腺癌的女性 (n=51) 和健康孕妇 (n=129) 血清中OH-PCBs和TH水平,然而其分析结果表明OH-PCBs水平与患者体内的T4和T3的量没有显著相关性[71, 115]. 与此不同的是,Hisada等[24]在日本调查了79名孕妇妊娠早期体内OH-PCBs的浓度与TH、促甲状腺激素 (thyroid stimulating hormone, TSH) 水平之间的相关性,通过多元回归分析结果表明,一些OH-PCBs的浓度与新生儿体内TSH的浓度之间存在显著的正相关. 学者在对越南部分地区女性血液样本进行检测时发现,OH-PCBs的浓度与T4、T3呈正相关,而与TSH浓度呈负相关,这也说明不同OH-PCBs在影响机体TH水平过程中存在本质的区别[116].
研究发现OH-PCBs不仅能抑制TH诱导的TR的转录激活[117],同时也可能会抑制TR介导的天然启动子的转录[118],并通过诱导脱碘酶或硫酸酯酶加快TH的排泄或代谢[119],说明OH-PCBs对TH的干扰是多途径的. 有学者从OH-PCBs在第二阶段反应产物 (e.g. PCBs硫酸盐) 的角度分析OH-PCBs对TH的干扰,通过实验证明,PCBs硫酸盐是人体TTR的高亲和力配体,并首次表明PCBs硫酸盐在PCBs诱导的TH水平紊乱中具有潜在的相关性[120-121]. 最近的一项研究发现OH-PCBs进入动物大脑中,可能对尿素循环、脯氨酸相关化合物、嘌呤、嘧啶、谷胱甘肽和氨基酸的代谢有关[34],推测OH-PCBs对TH的干扰可能发生在HPG轴及其相关物质的合成、代谢通路等任意环节.
目前在OH-PCBs的内分泌干扰机制方面的研究中,关于其浓度对激素水平及相关转运蛋白的影响已经开展了较多的研究,且研究方法学较为成熟. 关于OH-PCBs等物质对激素分泌信号通路的影响效应需要进一步结合代谢组学和分子生物学方法开展深入研究,从而能够实现对OH-PCBs的内分泌干扰机制有较为全面的认识. 与此同时,最新的研究结果表明,生物体接触OH-PCBs后可能同时导致其体内存在母体化合物PCBs负荷[89],而关于两者的联合内分泌干扰效应是否对OH-PCBs发挥干扰效应产生影响,需要开展实验来说明.
-
根据全球各地区的污染调查结果,水生环境蓄积库中的OH-PCBs通过食物链等途径向人类社会不断输出,OH-PCBs仍然是生态环境中不可忽视的危害因子. 我国作为临海大国和水产品消费大国,OH-PCBs对国民生命健康的影响应受到进一步关注. 在开展此篇综述查阅文献过程中,作者认为以下几个问题应当给予关注:
(1)与一些发达国家相比,国内对于水生环境及人群体内OH-PCBs的污染状况监测力度相对缺乏,可供参考的数据较少,借此倡导相关部门推进该领域的工作安排,并且应结合我国淡水水产品巨大消费量的特点,同时推进对淡/海水水生环境及相关人群的调查工作.
(2)越来越多的研究表明,食源性OH-PCBs是动物/人体内OH-PCBs的重要来源,但关于通过食物进入动物/人体后的蓄积、分布、迁移等药代动力学基本参数缺乏,且OH-PCBs进入机体后的“再代谢”路径有待进一步探索.
(3)目前关于OH-PCBs的内分泌干扰效应的研究较多,但还未能从本质上解释其发挥干扰效应的毒理机制,尤其是关于OH-PCBs对TH影响的研究多停留在剂量-效应关系层面,有待深入探索,并且根据目前的调查结果,OH-PCBs多与母体化合物PCBs同时出现在生物体,两者在产生内分泌干扰效应过程中的关系需要被进一步研究.
水生环境中OH-PCBs的来源、污染现状及其内分泌干扰机制研究进展
Research progress on the source and pollution status of OH-PCBs in aquatic environment and their endocrine disruption mechanism
-
摘要: 羟基多氯联苯 (hydroxylated polychlorinated biphenyls,OH-PCBs) 是典型持久性污染物多氯联苯 (polychlorinated biphenyls,PCBs) 在环境中经多种机制氧化产生的主要产物之一,属于二代环境持久性污染物,在水生环境中被广泛检出(水体 <4.12 ng·g−1,沉积物 <26.0 ng·g−1,水生生物<1838.38 ng·g−1). 水生环境中OH-PCBs的来源主要包括两方面,一方面,PCBs在生物体内由细胞色素P450 (CYP450) 单加氧化酶系直接氧化或经氧化芳烃介导而产生OH-PCBs;另一方面,PCBs能够与大气中的羟基自由基发生反应转化为OH-PCBs,最终环境中的OH-PCBs经过雨水冲刷、大气沉降等作用进入水生环境中. 水生环境介质中痕量的OH-PCBs经食物链的传递和放大作用,不仅对水生生物的生理活动造成影响,而且能够通过水产品等食物途径进入到人体. 由于部分OH-PCBs的结构与天然雌激素、甲状腺激素等内分泌物质类似,在动物体内会导致一系列内分泌干扰效应,但其干扰机制仍未阐明;并且蓄积在生物体内的OH-PCBs能够进一步发生代谢转化,产生的多种代谢产物及其毒理效应有待进一步研究. 本文对目前OH-PCBs在水生环境中的污染现状、生物体内的蓄积及“再代谢”特征及其内分泌干扰效应机制研究进展进行综述,进而为OH-PCBs的暴露风险评价、蓄积代谢规律以及毒理学效应研究提供参考.Abstract: Hydroxylated polychlorinated biphenyls (OH-PCBs) are one of the main products of typical persistent pollutants, polychlorinated biphenyls (PCBs), produced by oxidation in the environment through various mechanisms, and are the second generation environmental persistent pollutants. This class of substances is currently widely detected in the aquatic environment (water < 4.12 ng·g−1, sediment < 26.0 ng·g−1, aquatic organisms < 1838.38 ng·g−1). The sources of OH-PCBs in the aquatic environment mainly include two aspects. On the one hand, PCBs are directly oxidized by cytochrome P450 (CYP450) mono-additive oxidase system or mediated by oxidized aromatic hydrocarbons to produce OH-PCBs in living organisms; on the other hand, PCBs can react with atmospheric hydroxyl radicals to convert into OH-PCBs. Finally, the OH-PCBs in the environment are accumulated in the aquatic environment through the effects of rain erosion and atmospheric deposition. Since the structure of some OH-PCBs is similar to natural estrogen, thyroid hormone and other endocrine substances, it will cause a series of endocrine disrupting effects in animals. However, these interference mechanisms remain unclear. Moreover, the OH-PCBs accumulated in the organism can further undergo metabolic transformation, and the various metabolites produced and their toxicological effects need further study. This paper reviews the current pollution status of OH-PCBs in aquatic environment, the characteristics of accumulation and "re-metabolism" in organisms, and the research progress on the mechanism of endocrine disrupting effect. The purpose is to provide a reference for the exposure risk assessment, accumulation metabolism law and toxicological effect research of OH-PCBs.
-
表 1 OH-PCBs在国内外各地区水生生物样本的污染现状
Table 1. Pollution status of OH-PCBs in aquatic biological samples in various regions at home and abroad
地区 Area 样品 Sample 浓度/(ng·g−1, ww) Concentration 文献 References 中国上海 河虾 3.48—6.42 [42] 乌鳢 0—4.12 鳜鱼 2.75—6.41 中国东海 带鱼 0.004—0.09 章鱼 0.03—0.60 虎头鲨 0.27—1.04 贻贝 < LOQ 缢蛏 < LOQ 中国浙江省台州市 河虾 7.49—12.9 [36] 黄颡鱼 6.02—12.95 螺蛳 1.52—17.35 鲫鱼 3.38—29.43 泥鳅 16.98—49.93 五大湖地区 湖鳟 <0.105 [45] 美国底特律河 食肉/远洋鱼 1.40—171.60 [43] 底栖鱼类 0.47—117.84 美国阿拉斯加州 弓头鲸(血液) 1.26—1.74 [46] 北大西洋西部 灰海豹(脑脊液) 1838.38 [44] 美国旧金山湾 海豹(肝脏) 20—690 [19] 俄罗斯贝加尔湖 海豹(血液) 0.71—4.6 [47] 加拿大安大略省 云斑鮰(血浆) 3.6 [48] 日本沿海 扇形双髻鲨 29—141 [49] 日本琥珀鱼 230—650 注:LOQ: limit of quantification. -
[1] ROSS P S, VOS J G, BIRNBAUM L S, et al. PCBs are a health risk for humans and wildlife [J]. Science, 2000, 289(5486): 1878-1879. [2] 杨方星, 徐盈. 多氯联苯的羟基化代谢产物及其内分泌干扰机制 [J]. 化学进展, 2005, 17(4): 740-748. doi: 10.3321/j.issn:1005-281X.2005.04.022 YANG F X, XU Y. Hydroxylated metabolites of polychlorinated biphenyls and their endocrine disrupting mechanism [J]. Progress in Chemistry, 2005, 17(4): 740-748(in Chinese). doi: 10.3321/j.issn:1005-281X.2005.04.022
[3] DESFORGES J P, HALL A, MCCONNELL B, et al. Predicting global killer whale population collapse from PCB pollution [J]. Science, 2018, 361(6409): 1373-1376. doi: 10.1126/science.aat1953 [4] SIMMONS D B D, MCMASTER M E, REINER E J, et al. Wild fish from the Bay of Quinte Area of Concern contain elevated tissue concentrations of PCBs and exhibit evidence of endocrine-related health effects [J]. Environment International, 2014, 66: 124-137. doi: 10.1016/j.envint.2014.01.009 [5] GRIMM F A, HU D F, KANIA-KORWEL I, et al. Metabolism and metabolites of polychlorinated biphenyls [J]. Critical Reviews in Toxicology, 2015, 45(3): 245-272. doi: 10.3109/10408444.2014.999365 [6] LETCHER R J, KLASSON-WEHLER E, BERGMAN A. Methyl Sulfone and Hydroxylated Metabolites of Polychlorinated Biphenyls Biphenyls [M]. Berlin, Heidelberg: Springer Berlin Heidelberg, 2000. [7] 王帅, 乔艺飘, 黄宣运, 等. 2, 2’, 4, 5, 5’-五氯联苯在小鼠体内代谢产物的鉴定 [J]. 分析化学, 2019, 47(12): 1951-1959. WANG S, QIAO Y P, HUANG X Y, et al. Identification of metabolites of 2, 2', 4, 5, 5'-pentachlorodiphenyl in mice [J]. Chinese Journal of Analytical Chemistry, 2019, 47(12): 1951-1959(in Chinese).
[8] DHAKAL K, GADUPUDI G S, LEHMLER H J, et al. Sources and toxicities of phenolic polychlorinated biphenyls (OH-PCBs) [J]. Environmental Science and Pollution Research International, 2018, 25(17): 16277-16290. doi: 10.1007/s11356-017-9694-x [9] TEHRANI R, van AKEN B. Hydroxylated polychlorinated biphenyls in the environment: Sources, fate, and toxicities [J]. Environmental Science and Pollution Research International, 2014, 21(10): 6334-6345. doi: 10.1007/s11356-013-1742-6 [10] HARAGUCHI K, KATO Y, KOGA N, et al. Metabolism of polychlorinated biphenyls by Gunn rats: Identification and serum retention of catechol metabolites [J]. Chemical Research in Toxicology, 2004, 17(12): 1684-1691. doi: 10.1021/tx0498096 [11] LIAO Z H, ZENG M, WANG L M. Atmospheric oxidation mechansim of polychlorinated biphenyls (PCBs) initiated by OH radicals [J]. Chemosphere, 2020, 240: 124756. doi: 10.1016/j.chemosphere.2019.124756 [12] FURGE L L, GUENGERICH F P. Cytochrome P450 enzymes in drug metabolism and chemical toxicology: An introduction [J]. Biochemistry and Molecular Biology Education, 2006, 34(2): 66-74. doi: 10.1002/bmb.2006.49403402066 [13] GUENGERICH F P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity [J]. Chemical Research in Toxicology, 2001, 14(6): 611-650. doi: 10.1021/tx0002583 [14] WU X N, ZHAI G S, SCHNOOR J L, et al. Atropselective disposition of 2, 2', 3, 4', 6-pentachlorobiphenyl (PCB 91) and identification of its metabolites in mice with liver-specific deletion of cytochrome P450 reductase [J]. Chemical Research in Toxicology, 2020, 33(6): 1328-1338. doi: 10.1021/acs.chemrestox.9b00255 [15] UWIMANA E, RUIZ P, LI X S, et al. Human CYP2A6, CYP2B6, AND CYP2E1 atropselectively metabolize polychlorinated biphenyls to hydroxylated metabolites [J]. Environmental Science & Technology, 2019, 53(4): 2114-2123. [16] SOECHITRAM S D, ATHANASIADOU M, HOVANDER L, et al. Fetal exposure to PCBs and their hydroxylated metabolites in a Dutch cohort [J]. Environmental Health Perspectives, 2004, 112(11): 1208-1212. doi: 10.1289/ehp.6424 [17] HOVANDER L, LINDERHOLM L, ATHANASIADOU M, et al. Levels of PCBs and their metabolites in the serum of residents of a highly contaminated area in eastern Slovakia [J]. Environmental Science & Technology, 2006, 40(12): 3696-3703. [18] VERREAULT J, SHAHMIRI S, GABRIELSEN G W, et al. Organohalogen and metabolically-derived contaminants and associations with whole body constituents in Norwegian Arctic glaucous gulls [J]. Environment International, 2007, 33(6): 823-830. doi: 10.1016/j.envint.2007.03.013 [19] PARK J S, KALANTZI O I, KOPEC D, et al. Polychlorinated biphenyls (PCBs) and their hydroxylated metabolites (OH-PCBs) in livers of harbor seals (Phoca vitulina) from San francisco bay, California and gulf of Maine [J]. Marine Environmental Research, 2009, 67(3): 129-135. doi: 10.1016/j.marenvres.2008.12.003 [20] GUSTAVSON L, CIESIELSKI T M, BYTINGSVIK J, et al. Hydroxylated polychlorinated biphenyls decrease circulating steroids in female polar bears (Ursus maritimus) [J]. Environmental Research, 2015, 138: 191-201. doi: 10.1016/j.envres.2015.02.011 [21] ZHENG J, YU L H, CHEN S J, et al. Polychlorinated biphenyls (PCBs) in human hair and serum from E-waste recycling workers in Southern China: Concentrations, chiral signatures, correlations, and source identification [J]. Environmental Science & Technology, 2016, 50(3): 1579-1586. [22] MA S T, REN G F, ZENG X Y, et al. Polychlorinated biphenyls and their hydroxylated metabolites in the serum of e-waste dismantling workers from Eastern China [J]. Environmental Geochemistry and Health, 2018, 40(5): 1931-1940. doi: 10.1007/s10653-017-9958-x [23] PARKER V S, SQUIREWELL E, JOACHIM-LEHMLER H, et al. Hydroxylated metabolites of common airborne polychlorinated biphenyls and their potential for disrupting estrogen homeostasis and adipogenesis [J]. The FASEB Journal, 2018, 32: 605.8. [24] HISADA A, SHIMODAIRA K, OKAI T, et al. Associations between levels of hydroxylated PCBs and PCBs in serum of pregnant women and blood thyroid hormone levels and body size of neonates [J]. International Journal of Hygiene and Environmental Health, 2014, 217(4/5): 546-553. [25] NOMIYAMA K, TSUJISAWA Y, ASHIDA E, et al. Mother to fetus transfer of hydroxylated polychlorinated biphenyl congeners (OH-PCBs) in the Japanese macaque (Macaca fuscata): Extrapolation of exposure scenarios to humans [J]. Environmental Science & Technology, 2020, 54(18): 11386-11395. [26] KIMURA-KURODA J, NAGATA I, KURODA Y. Disrupting effects of hydroxy-polychlorinated biphenyl (PCB) congeners on neuronal development of cerebellar Purkinje cells: A possible causal factor for developmental brain disorders? [J]. Chemosphere, 2007, 67(9): S412-S420. doi: 10.1016/j.chemosphere.2006.05.137 [27] KIMURA-KURODA J, NAGATA I, KURODA Y. Hydroxylated metabolites of polychlorinated biphenyls inhibit thyroid-hormone-dependent extension of cerebellar Purkinje cell dendrites [J]. Developmental Brain Research, 2005, 154(2): 259-263. doi: 10.1016/j.devbrainres.2004.11.004 [28] UCÁN-MARIN F, ARUKWE A, MORTENSEN A S, et al. Recombinant albumin and transthyretin transport proteins from two gull species and human: Chlorinated and brominated contaminant binding and thyroid hormones [J]. Environmental Science & Technology, 2010, 44(1): 497-504. [29] WANG T, COOK I, LEYH T S. The molecular basis of OH-PCB estrogen receptor activation [J]. Journal of Biological Chemistry, 2021, 296: 100353. doi: 10.1016/j.jbc.2021.100353 [30] 史熊杰, 刘春生, 余珂, 等. 环境内分泌干扰物毒理学研究[J]. 化学进展, 2009, 21(S1): 340-349. SHI X J, LIU C S, YU K, et al. Toxicological research on environmental endocrine disruptors[J]. Progress in Chemistry, 2009, 21(Sup 1): 340-349(in Chinese).
[31] SANDAU C D, AYOTTE P, DEWAILLY E, et al. Analysis of hydroxylated metabolites of PCBs (OH-PCBs) and other chlorinated phenolic compounds in whole blood from Canadian Inuit [J]. Environmental Health Perspectives, 2000, 108(7): 611-616. doi: 10.1289/ehp.00108611 [32] LI Y Y, LIN T, CHEN Y J, et al. Polybrominated diphenyl ethers (PBDEs) in sediments of the coastal East China Sea: Occurrence, distribution and mass inventory [J]. Environmental Pollution, 2012, 171: 155-161. doi: 10.1016/j.envpol.2012.07.039 [33] BEYER A, BIZIUK M. Environmental fate and global distribution of polychlorinated biphenyls [J]. Reviews of Environmental Contamination and Toxicology, 2009, 201: 137-158. [34] NOMIYAMA K, EGUCHI A, TAKAGUCHI K, et al. Targeted metabolome analysis of the dog brain exposed to PCBs suggests inhibition of oxidative phosphorylation by hydroxylated PCBs [J]. Toxicology and Applied Pharmacology, 2019, 377: 114620. doi: 10.1016/j.taap.2019.114620 [35] RUEL M V M, BOS A F, SOECHITRAM S D, et al. Prenatal exposure to organohalogen compounds and children's mental and motor development at 18 and 30 months of age [J]. NeuroToxicology, 2019, 72: 6-14. doi: 10.1016/j.neuro.2019.01.003 [36] 王帅, 史永富. 电子废物拆解区羟基多氯联苯的分布及传递特征研究 [J]. 安徽农业科学, 2019, 47(16): 68-72,77. doi: 10.3969/j.issn.0517-6611.2019.16.021 WANG S, SHI Y F. Study on distribution and transmitting characteristics of hydroxyl polychlorinated biphenyls in E-waste dismantling area [J]. Journal of Anhui Agricultural Sciences, 2019, 47(16): 68-72,77(in Chinese). doi: 10.3969/j.issn.0517-6611.2019.16.021
[37] UENO D, DARLING C, ALAEE M, et al. Detection of hydroxylated polychlorinated biphenyls (OH-PCBs) in the abiotic environment: Surface water and precipitation from Ontario, Canada [J]. Environmental Science & Technology, 2007, 41(6): 1841-1848. [38] IBETO C N, NKECHI W C, EKERE N R. Health risks of polychlorinated biphenyls (PCBs) levels in fish and sediment from river Niger (onitsha axis) [J]. Journal of Aquatic Food Product Technology, 2019, 28(2): 138-149. doi: 10.1080/10498850.2019.1568332 [39] MAREK R F, MARTINEZ A, HORNBUCKLE K C. Discovery of hydroxylated polychlorinated biphenyls (OH-PCBs) in sediment from a lake Michigan waterway and original commercial aroclors [J]. Environmental Science & Technology, 2013, 47(15): 8204-8210. [40] 王帅, 黄宣运, 袁瑞, 等. 气相色谱法检测水产养殖底泥中10种羟基多氯联苯 [J]. 环境化学, 2018, 37(7): 1575-1582. doi: 10.7524/j.issn.0254-6108.2017110706 WANG S, HUANG X Y, YUAN R, et al. Determination of 10 hydroxylated polychlorinated biphenyls in aquaculture sediment by gas chromatography [J]. Environmental Chemistry, 2018, 37(7): 1575-1582(in Chinese). doi: 10.7524/j.issn.0254-6108.2017110706
[41] SAKTRAKULKLA P, LI X S, MARTINEZ A, et al. Hydroxylated polychlorinated biphenyls are emerging legacy pollutants in contaminated sediments [J]. Environmental Science & Technology, 2022, 56(4): 2269-2278. [42] WANG S, HUANG X Y, WANG M Y, et al. Simultaneous determination of polychlorinated biphenyl 101 (PCB101) and its hydroxylated, methoxylated and methyl sulfonated metabolites in aquatic organisms by solid-phase extraction (SPE) and gas chromatography-microelectron capture detection (GC-μECD) [J]. Analytical Letters, 2022, 55(5): 784-795. doi: 10.1080/00032719.2021.1967369 [43] LI H X, DROUILLARD K G, BENNETT E, et al. Plasma-associated halogenated phenolic contaminants in benthic and pelagic fish species from the Detroit River [J]. Environmental Science & Technology, 2003, 37(5): 832-839. [44] MONTIE E W, REDDY C M, GEBBINK W A, et al. Organohalogen contaminants and metabolites in cerebrospinal fluid and cerebellum gray matter in short-beaked common dolphins and Atlantic white-sided dolphins from the western North Atlantic [J]. Environmental Pollution, 2009, 157(8/9): 2345-2358. [45] CAMPBELL L M, MUIR D C, WHITTLE D M, et al. Hydroxylated PCBs and other chlorinated phenolic compounds in lake trout (Salvelinus namaycush) blood plasma from the Great Lakes region [J]. Environmental Science & Technology, 2003, 37(9): 1720-1725. [46] HOEKSTRA P F, LETCHER R J, O'HARA T M, et al. Hydroxylated and methylsulfone-containing metabolites of polychlorinated biphenyls in the plasma and blubber of bowhead whales (Balaena mysticetus) [J]. Environmental Toxicology and Chemistry, 2003, 22(11): 2650-2658. doi: 10.1897/02-641 [47] IMAEDA D, NOMIYAMA K, KUNISUE T, et al. Blood levels of polychlorinated biphenyls and their hydroxylated metabolites in Baikal seals (Pusa sibirica): Emphasis on inter species comparison, gender difference and association with blood thyroid hormone levels [J]. Chemosphere, 2014, 114: 1-8. doi: 10.1016/j.chemosphere.2014.03.089 [48] GILROY È A M, MUIR D G C, MCMASTER M E, et al. Polychlorinated biphenyls and their hydroxylated metabolites in wild fish from Wheatley Harbour Area of Concern, Ontario, Canada [J]. Environmental Toxicology and Chemistry, 2012, 31(12): 2788-2797. doi: 10.1002/etc.2023 [49] NOMIYAMA K, UCHIYAMA Y, HORIUCHI S, et al. Organohalogen compounds and their metabolites in the blood of Japanese amberjack (Seriola quinqueradiata) and scalloped hammerhead shark (Sphyrna lewini) from Japanese coastal waters [J]. Chemosphere, 2011, 85(3): 315-321. doi: 10.1016/j.chemosphere.2011.06.092 [50] ATKINSON R. Atmospheric reactions of alkoxy and β-hydroxyalkoxy radicals [J]. International Journal of Chemical Kinetics, 1997, 29(2): 99-111. doi: 10.1002/(SICI)1097-4601(1997)29:2<99::AID-KIN3>3.0.CO;2-F [51] ANDERSON P N, HITES R A. OH radical reactions: The major removal pathway for polychlorinated biphenyls from the atmosphere [J]. Environmental Science & Technology, 1996, 30(5): 1756-1763. [52] TOTTEN L A, EISENREICH S J, BRUNCIAK P A. Evidence for destruction of PCBs by the OH radical in urban atmospheres [J]. Chemosphere, 2002, 47(7): 735-746. doi: 10.1016/S0045-6535(01)00326-5 [53] MANDALAKIS M, BERRESHEIM H, STEPHANOU E G. Direct evidence for destruction of polychlorobiphenyls by OH radicals in the subtropical troposphere [J]. Environmental Science & Technology, 2003, 37(3): 542-547. [54] BRUBAKER W W, HITES R A. Gas-phase oxidation products of biphenyl and polychlorinated biphenyls [J]. Environmental Science & Technology, 1998, 32(24): 3913-3918. [55] STAMOU M, WU X N, KANIA-KORWEL I, et al. Cytochrome p450 mRNA expression in the rodent brain: Species-, sex-, and region-dependent differences [J]. Drug Metabolism and Disposition:the Biological Fate of Chemicals, 2014, 42(2): 239-244. doi: 10.1124/dmd.113.054239 [56] 王帅, 田良良, 孔聪, 等. 水产品中多氯联苯代谢产物的研究进展 [J]. 环境化学, 2019, 38(1): 114-130. doi: 10.7524/j.issn.0254-6108.2018080202 WANG S, TIAN L L, KONG C, et al. Research progress of polychlorinated biphenyl metabolites aquatic products [J]. Environmental Chemistry, 2019, 38(1): 114-130(in Chinese). doi: 10.7524/j.issn.0254-6108.2018080202
[57] WYNDHAM C, SAFE S. In vitro metabolism of 4-chlorobiphenyl by control and induced rat liver microsomes [J]. Biochemistry, 1978, 17(2): 208-215. doi: 10.1021/bi00595a002 [58] MILLS R A, MILLIS C D, DANNAN G A, et al. Studies on the structure-activity relationships for the metabolism of polybrominated biphenyls by rat liver microsomes [J]. Toxicology and Applied Pharmacology, 1985, 78(1): 96-104. doi: 10.1016/0041-008X(85)90309-6 [59] KOH W X, HORNBUCKLE K C, THORNE P S. Human serum from urban and rural adolescents and their mothers shows exposure to polychlorinated biphenyls not found in commercial mixtures [J]. Environmental Science & Technology, 2015, 49(13): 8105-8112. [60] MAREK R F, THORNE P S, DEWALL J, et al. Variability in PCB and OH-PCB serum levels in children and their mothers in urban and rural US communities [J]. Environmental Science & Technology, 2014, 48(22): 13459-13467. [61] KAMATA R, SHIRAISHI F, KAGEYAMA S, et al. Detection and measurement of the agonistic activities of PCBs and mono-hydroxylated PCBs to the constitutive androstane receptor using a recombinant yeast assay [J]. Toxicology in Vitro, 2015, 29(7): 1859-1867. doi: 10.1016/j.tiv.2015.07.021 [62] MA C X, ZHAI G S, WU H M, et al. Identification of a novel hydroxylated metabolite of 2, 2', 3, 5', 6-pentachlorobiphenyl formed in whole poplar plants [J]. Environmental Science and Pollution Research International, 2016, 23(3): 2089-2098. doi: 10.1007/s11356-015-5939-8 [63] BORJA J, TALEON D M, AURESENIA J, et al. Polychlorinated biphenyls and their biodegradation [J]. Process Biochemistry, 2005, 40(6): 1999-2013. doi: 10.1016/j.procbio.2004.08.006 [64] MONTAÑO M, GUTLEB A C, MURK A J. Persistent toxic burdens of halogenated phenolic compounds in humans and wildlife [J]. Environmental Science & Technology, 2013, 47(12): 6071-6081. [65] BORGÅ K, WOLKERS H, SKAARE J U, et al. Bioaccumulation of PCBs in Arctic seabirds: Influence of dietary exposure and congener biotransformation [J]. Environmental Pollution, 2005, 134(3): 397-409. doi: 10.1016/j.envpol.2004.09.016 [66] HARAGUCHI K, ITO Y, TAKAGI M, et al. Levels, profiles and dietary sources of hydroxylated PCBs and hydroxylated and methoxylated PBDEs in Japanese women serum samples [J]. Environment International, 2016, 97: 155-162. doi: 10.1016/j.envint.2016.08.022 [67] QUINETE N, SCHETTGEN T, BERTRAM J, et al. Occurrence and distribution of PCB metabolites in blood and their potential health effects in humans: A review [J]. Environmental Science and Pollution Research International, 2014, 21(20): 11951-11972. doi: 10.1007/s11356-014-3136-9 [68] RYLANDER C, LUND E, FRØYLAND L, et al. Predictors of PCP, OH-PCBs, PCBs and chlorinated pesticides in a general female Norwegian population [J]. Environment International, 2012, 43: 13-20. doi: 10.1016/j.envint.2012.02.008 [69] DUFOUR P, PIRARD C, CHARLIER C. Determination of phenolic organohalogens in human serum from a Belgian population and assessment of parameters affecting the human contamination [J]. Science of the Total Environment, 2017, 599/600: 1856-1866. doi: 10.1016/j.scitotenv.2017.05.157 [70] DIRTU A C, JASPERS V L B, CERNAT R, et al. Distribution of PCBs, their hydroxylated metabolites, and other phenolic contaminants in human serum from two European countries [J]. Environmental Science & Technology, 2010, 44(8): 2876-2883. [71] NOMIYAMA K, YONEHARA T, YONEMURA S, et al. Determination and characterization of hydroxylated polychlorinated biphenyls (OH-PCBs) in serum and adipose tissue of Japanese women diagnosed with breast cancer [J]. Environmental Science & Technology, 2010, 44(8): 2890-2896. [72] EGUCHI A, NOMIYAMA K, DEVANATHAN G, et al. Different profiles of anthropogenic and naturally produced organohalogen compounds in serum from residents living near a coastal area and e-waste recycling workers in India [J]. Environment International, 2012, 47: 8-16. doi: 10.1016/j.envint.2012.05.003 [73] MAREK R F, THORNE P S, WANG K, et al. PCBs and OH-PCBs in serum from children and mothers in urban and rural US communities [J]. Environmental Science & Technology, 2013, 47(7): 3353-3361. [74] ZOTA A R, LINDERHOLM L, PARK J S, et al. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California [J]. Environmental Science & Technology, 2013, 47(20): 11776-11784. [75] KOH W X, HORNBUCKLE K C, MAREK R F, et al. Hydroxylated polychlorinated biphenyls in human sera from adolescents and their mothers living in two US Midwestern communities [J]. Chemosphere, 2016, 147: 389-395. doi: 10.1016/j.chemosphere.2015.12.113 [76] FÄNGSTRÖM B, ATHANASIADOU M, ATHANASSIADIS I, et al. Hydroxylated PCB metabolites in nonhatched fulmar eggs from the Faroe Islands [J]. Ambio, 2005, 34(3): 184-187. doi: 10.1579/0044-7447-34.3.184 [77] NOMIYAMA K, MURATA S, KUNISUE T, et al. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBs) in the blood of toothed and baleen whales stranded along Japanese coastal waters [J]. Environmental Science & Technology, 2010, 44(10): 3732-3738. [78] GEBBINK W A, SONNE C, DIETZ R, et al. Tissue-specific congener composition of organohalogen and metabolite contaminants in East Greenland polar bears (Ursus maritimus) [J]. Environmental Pollution, 2008, 152(3): 621-629. doi: 10.1016/j.envpol.2007.07.001 [79] BYTINGSVIK J, LIE E, AARS J, et al. PCBs and OH-PCBs in polar bear mother-cub pairs: A comparative study based on plasma levels in 1998 and 2008 [J]. Science of the Total Environment, 2012, 417/418: 117-128. doi: 10.1016/j.scitotenv.2011.12.033 [80] GABRIELSEN K M, VILLANGER G D, LIE E, et al. Levels and patterns of hydroxylated polychlorinated biphenyls (OH-PCBs) and their associations with thyroid hormones in hooded seal (Cystophora cristata) mother-pup pairs [J]. Aquatic Toxicology, 2011, 105(3/4): 482-491. [81] EKUASE E J, LIU Y G, LEHMLER H J, et al. Structure-activity relationships for hydroxylated polychlorinated biphenyls as inhibitors of the sulfation of dehydroepiandrosterone catalyzed by human hydroxysteroid sulfotransferase SULT2A1 [J]. Chemical Research in Toxicology, 2011, 24(10): 1720-1728. doi: 10.1021/tx200260h [82] TAMPAL N, LEHMLER H J, ESPANDIARI P, et al. Glucuronidation of hydroxylated polychlorinated biphenyls (PCBs) [J]. Chemical Research in Toxicology, 2002, 15(10): 1259-1266. doi: 10.1021/tx0200212 [83] LIU Y G, APAK T I, LEHMLER H J, et al. Hydroxylated polychlorinated biphenyls are substrates and inhibitors of human hydroxysteroid sulfotransferase SULT2A1 [J]. Chemical Research in Toxicology, 2006, 19(11): 1420-1425. doi: 10.1021/tx060160+ [84] JIA R Y, ZHANG Z P, QIN G Q, et al. Inhibition of hydroxylated polychlorinated biphenyls (OH-PCBs) on sulfotransferases (SULTs) [J]. Environmental Pollution, 2021, 291: 118214. doi: 10.1016/j.envpol.2021.118214 [85] LI S N, CAO Y F, SUN X Y, et al. Hydroxy metabolites of polychlorinated biphenyls (OH-PCBs) exhibit inhibitory effects on UDP-glucuronosyltransferases (UGTs) [J]. Chemosphere, 2018, 212: 513-522. doi: 10.1016/j.chemosphere.2018.08.040 [86] LI X S, LIU Y N, MARTIN J W, et al. Nontarget analysis reveals gut microbiome-dependent differences in the fecal PCB metabolite profiles of germ-free and conventional mice [J]. Environmental Pollution, 2021, 268: 115726. doi: 10.1016/j.envpol.2020.115726 [87] ZHANG C Y, FLOR S, RUIZ P, et al. 3, 3'-dichlorobiphenyl is metabolized to a complex mixture of oxidative metabolites, including novel methoxylated metabolites, by HepG2 cells [J]. Environmental Science & Technology, 2020, 54(19): 12345-12357. [88] SONG Y, WAGNER B A, LEHMLER H J, et al. Semiquinone radicals from oxygenated polychlorinated biphenyls: Electron paramagnetic resonance studies [J]. Chemical Research in Toxicology, 2008, 21(7): 1359-1367. doi: 10.1021/tx8000175 [89] WANG M Y, ZHANG L F, WU D, et al. Simulation experiment on OH-PCB being ingested through daily diet: Accumulation, transformation and distribution of hydroxylated-2, 2', 4, 5, 5'-pentachlorobiphenyl (OH-PCB101) in mice [J]. Science of the Total Environment, 2022, 802: 149891. doi: 10.1016/j.scitotenv.2021.149891 [90] SUN J T, ZHU L Z, PAN L L, et al. Detection of methoxylated and hydroxylated polychlorinated biphenyls in sewage sludge in China with evidence for their microbial transformation [J]. Scientific Reports, 2016, 6: 29782. doi: 10.1038/srep29782 [91] SUN J T, PAN L L, ZHU L Z. Formation of hydroxylated and methoxylated polychlorinated biphenyls by Bacillus subtilis: New insights into microbial metabolism [J]. Science of the Total Environment, 2018, 613/614: 54-61. doi: 10.1016/j.scitotenv.2017.09.063 [92] LI Y L, BAKO C M, SAKTRAKULKLA P, et al. Interconversion between methoxylated, hydroxylated and sulfated metabolites of PCB 3 in whole poplar plants [J]. Science of the Total Environment, 2021, 785: 147341. doi: 10.1016/j.scitotenv.2021.147341 [93] LIN F J, SUN J T, LIU N, et al. Phytotoxicity and metabolic responses induced by tetrachlorobiphenyl and its hydroxylated and methoxylated derivatives in rice (Oryza sative L. ) [J]. Environment International, 2020, 139: 105695. doi: 10.1016/j.envint.2020.105695 [94] LANS M C, KLASSON-WEHLER E, WILLEMSEN M, et al. Structure-dependent, competitive interaction of hydroxy-polychlorobiphenyls, -dibenzo-p-dioxins and-dibenzofurans with human transthyretin [J]. Chemico-Biological Interactions, 1993, 88(1): 7-21. doi: 10.1016/0009-2797(93)90081-9 [95] LANS M C, SPIERTZ C, BROUWER A, et al. Different competition of thyroxine binding to transthyretin and thyroxine-binding globulin by hydroxy-PCBs, PCDDs and PCDFs [J]. European Journal of Pharmacology, 1994, 270(2/3): 129-136. [96] PARK J S, BERGMAN Å, LINDERHOLM L, et al. Placental transfer of polychlorinated biphenyls, their hydroxylated metabolites and pentachlorophenol in pregnant women from eastern Slovakia [J]. Chemosphere, 2008, 70(9): 1676-1684. doi: 10.1016/j.chemosphere.2007.07.049 [97] BERGHUIS S A, SOECHITRAM S D, HITZERT M M, et al. Prenatal exposure to polychlorinated biphenyls and their hydroxylated metabolites is associated with motor development of three-month-old infants [J]. NeuroToxicology, 2013, 38: 124-130. doi: 10.1016/j.neuro.2013.07.003 [98] BERGHUIS S A, van BRAECKEL K N J A, SAUER P J J, et al. Prenatal exposure to persistent organic pollutants and cognition and motor performance in adolescence [J]. Environment International, 2018, 121: 13-22. doi: 10.1016/j.envint.2018.08.030 [99] HAIJIMA A, LESMANA R, SHIMOKAWA N, et al. Differential neurotoxic effects of in utero and lactational exposure to hydroxylated polychlorinated biphenyl (OH-PCB 106) on spontaneous locomotor activity and motor coordination in young adult male mice [J]. The Journal of Toxicological Sciences, 2017, 42(4): 407-416. doi: 10.2131/jts.42.407 [100] SHEVTSOV S, PETROTCHENKO E V, PEDERSEN L C, et al. Crystallographic analysis of a hydroxylated polychlorinated biphenyl (OH-PCB) bound to the catalytic estrogen binding site of human estrogen sulfotransferase [J]. Environmental Health Perspectives, 2003, 111(7): 884-888. doi: 10.1289/ehp.6056 [101] PARKER V S, SQUIREWELL E J, LEHMLER H J, et al. Hydroxylated and sulfated metabolites of commonly occurring airborne polychlorinated biphenyls inhibit human steroid sulfotransferases SULT1E1 and SULT2A1 [J]. Environmental Toxicology and Pharmacology, 2018, 58: 196-201. doi: 10.1016/j.etap.2018.01.010 [102] CARLSON D B, WILLIAMS D E. 4-hydroxy-2', 4', 6'-trichlorobiphenyl and 4-hydroxy-2', 3', 4', 5'-tetrachlorobiphenyl are estrogenic in rainbow trout [J]. Environmental Toxicology and Chemistry, 2001, 20(2): 351-358. doi: 10.1002/etc.5620200216 [103] NOMIYAMA K, NOMURA Y, TAKAHASHI T, et al. Hydroxylated polychlorinated biphenyls (OH-PCBs) induce vitellogenin through estrogenic activity in primary-cultured hepatocytes of the Xenopus laevis [J]. Chemosphere, 2010, 78(7): 800-806. doi: 10.1016/j.chemosphere.2009.12.018 [104] TAKEUCHI S, SHIRAISHI F, KITAMURA S, et al. Characterization of steroid hormone receptor activities in 100 hydroxylated polychlorinated biphenyls, including congeners identified in humans [J]. Toxicology, 2011, 289(2/3): 112-121. [105] LI X X, GAO Y, GUO L H, et al. Structure-dependent activities of hydroxylated polybrominated diphenyl ethers on human estrogen receptor [J]. Toxicology, 2013, 309: 15-22. doi: 10.1016/j.tox.2013.04.001 [106] PĚNČÍKOVÁ K, SVRŽKOVÁ L, STRAPÁČOVÁ S, et al. In vitro profiling of toxic effects of prominent environmental lower-chlorinated PCB congeners linked with endocrine disruption and tumor promotion [J]. Environmental Pollution, 2018, 237: 473-486. doi: 10.1016/j.envpol.2018.02.067 [107] RUIZ P, INGALE K, WHEELER J S, et al. 3D QSAR studies of hydroxylated polychlorinated biphenyls as potential xenoestrogens [J]. Chemosphere, 2016, 144: 2238-2246. doi: 10.1016/j.chemosphere.2015.11.004 [108] TAM N, LAI K P, KONG R Y C. Comparative transcriptomic analysis reveals reproductive impairments caused by PCBs and OH-PCBs through the dysregulation of ER and AR signaling [J]. Science of the Total Environment, 2022, 802: 149913. doi: 10.1016/j.scitotenv.2021.149913 [109] CAO L Y, REN X M, GUO L H. Estrogen-related receptor γ is a novel target for Lower-Chlorinated Polychlorinated Biphenyls and their hydroxylated and sulfated metabolites [J]. Environmental Pollution, 2019, 254: 113088. doi: 10.1016/j.envpol.2019.113088 [110] WANG F F, QIU Y C, ZHOU B. In silico exploration of hydroxylated polychlorinated biphenyls as estrogen receptor β ligands by 3D-QSAR, molecular docking and molecular dynamics simulations[J]. Journal of Biomolecular Structure and Dynamics, 2022, 40(15): 6798-6809. [111] MONTAÑO M, COCCO E, GUIGNARD C, et al. New approaches to assess the transthyretin binding capacity of bioactivated thyroid hormone disruptors [J]. Toxicological Sciences, 2012, 130(1): 94-105. doi: 10.1093/toxsci/kfs228 [112] MEERTS I A T M, ASSINK Y, CENIJN P H, et al. Placental transfer of a hydroxylated polychlorinated biphenyl and effects on fetal and maternal thyroid hormone homeostasis in the rat [J]. Toxicological Sciences, 2002, 68(2): 361-371. doi: 10.1093/toxsci/68.2.361 [113] MALMBERG T, HOOGSTRAATE J, BERGMAN A, et al. Pharmacokinetics of two major hydroxylated polychlorinated biphenyl metabolites with specific retention in rat blood [J]. Xenobiotica, 2004, 34(6): 581-589. doi: 10.1080/00498250410001713078 [114] OTAKE T, YOSHINAGA J, ENOMOTO T, et al. Thyroid hormone status of newborns in relation to in utero exposure to PCBs and hydroxylated PCB metabolites [J]. Environmental Research, 2007, 105(2): 240-246. doi: 10.1016/j.envres.2007.03.010 [115] HISADA A, SHIMODAIRA K, OKAI T, et al. Serum levels of hydroxylated PCBs, PCBs and thyroid hormone measures of Japanese pregnant women [J]. Environmental Health and Preventive Medicine, 2013, 18(3): 205-214. doi: 10.1007/s12199-012-0306-6 [116] EGUCHI A, NOMIYAMA K, MINH TUE N, et al. Residue profiles of organohalogen compounds in human serum from e-waste recycling sites in North Vietnam: Association with thyroid hormone levels [J]. Environmental Research, 2015, 137: 440-449. doi: 10.1016/j.envres.2015.01.007 [117] MIYAZAKI W, IWASAKI T, TAKESHITA A, et al. Polychlorinated biphenyls suppress thyroid hormone receptor-mediated transcription through a novel mechanism [J]. Journal of Biological Chemistry, 2004, 279(18): 18195-18202. doi: 10.1074/jbc.M310531200 [118] AMANO I, MIYAZAKI W, IWASAKI T, et al. The effect of hydroxylated polychlorinated biphenyl (OH-PCB) on thyroid hormone receptor (TR)-mediated transcription through native-thyroid hormone response element (TRE) [J]. Industrial Health, 2010, 48(1): 115-118. doi: 10.2486/indhealth.48.115 [119] SOECHITRAM S D, BERGHUIS S A, VISSER T J, et al. Polychlorinated biphenyl exposure and deiodinase activity in young infants [J]. Science of the Total Environment, 2017, 574: 1117-1124. doi: 10.1016/j.scitotenv.2016.09.098 [120] GRIMM F A, LEHMLER H J, HE X R, et al. Sulfated metabolites of polychlorinated biphenyls are high-affinity ligands for the thyroid hormone transport protein transthyretin [J]. Environmental Health Perspectives, 2013, 121(6): 657-662. doi: 10.1289/ehp.1206198 [121] REN X M, LI C H, ZHANG J Q, et al. Binding and activity of sulfated metabolites of lower-chlorinated polychlorinated biphenyls towards thyroid hormone receptor alpha [J]. Ecotoxicology and Environmental Safety, 2019, 180: 686-692. doi: 10.1016/j.ecoenv.2019.05.056 -




 下载:
下载: