-
羰基化合物是大气中一类重要的含氧挥发性有机化合物(OVOCs),在对流层光化学作用中起重要作用,是大气中O3、过氧乙酰硝酸酯(PANs)、有机酸等二次污染物形成的中间产物,影响对流层大气的氧化潜势[1]. 羰基化合物的光解被认为是大气中许多自由基生成的主要途径,如ROx等,这些自由基通过影响大气中NO向NO2转化的化学速率,从而进一步影响O3的二次生成[2-3]. 羰基化合物对气溶胶中有机组分的贡献也十分显著,是大气中二次有机气溶胶(SOA)生成的重要中间产物[4-5]. 城市大气中羰基化合物的来源主要包括一次来源和二次生成,一次来源可分为机动车尾气、餐饮业烟气、燃烧(家用燃料和烟草)和工业废气等一次人为排放源以及少量的生物排放,二次生成主要发生在光化学反应过程中[1, 6-10].
浙江省地处长江三角洲地区南翼,地势自西南向东北呈阶梯状倾,受地理位置和地形条件的影响,与周边地区大气对流充分,季风特点显著. 杭州是浙江省的省会,是长三角地区第二大城市,汽车保有量从39万辆(2000年)增加到282万辆(2020年),2020年常住人口1196万. 绍兴市是长三角城市群重要城市,以纺织印染工业为主导产业,2020年常住人口529万,汽车保有量162万辆. 当前长三角大部分城市进入臭氧污染和PM2.5污染并重的大气控制新阶段,因此作为二者共同前体物的VOCs成为关注焦点,杭州[11-12]和绍兴[13] 城区以及工业源排放的VOCs均有报道,但目前针对含氧有机物(OVOC)特别是羰基化合物的研究仍较少.
在本研究中,于2020年11月在杭州采样,2021年1—11月在绍兴市采样,实地测量了两地环境空气中羰基化合物浓度. 研究目的是:(1)认识杭州和绍兴大气中羰基化合物的浓度及其日变化特征;(2)研究大气中羰基化合物对大气氧化性的贡献.
-
本文以杭州湾的两大城市,杭州市和绍兴市作为研究区域. 杭州湾地区属于典型的亚热带季风气候,冬季主要风向为西北风,温度较低;夏季主要风向为东南风,天气湿热. 杭州市地处长江三角洲南翼和钱塘江流域,西部属浙西丘陵区,东部为平原地区,地势低平,河网、湖泊密布. 绍兴市北邻钱塘江,南部为丘陵地区,印染纺织工厂和石化工厂多分布在北部. 绍兴北部海拔约10 m,其他方向分布着许多丘陵(海拔约50—500 m)[14].
-
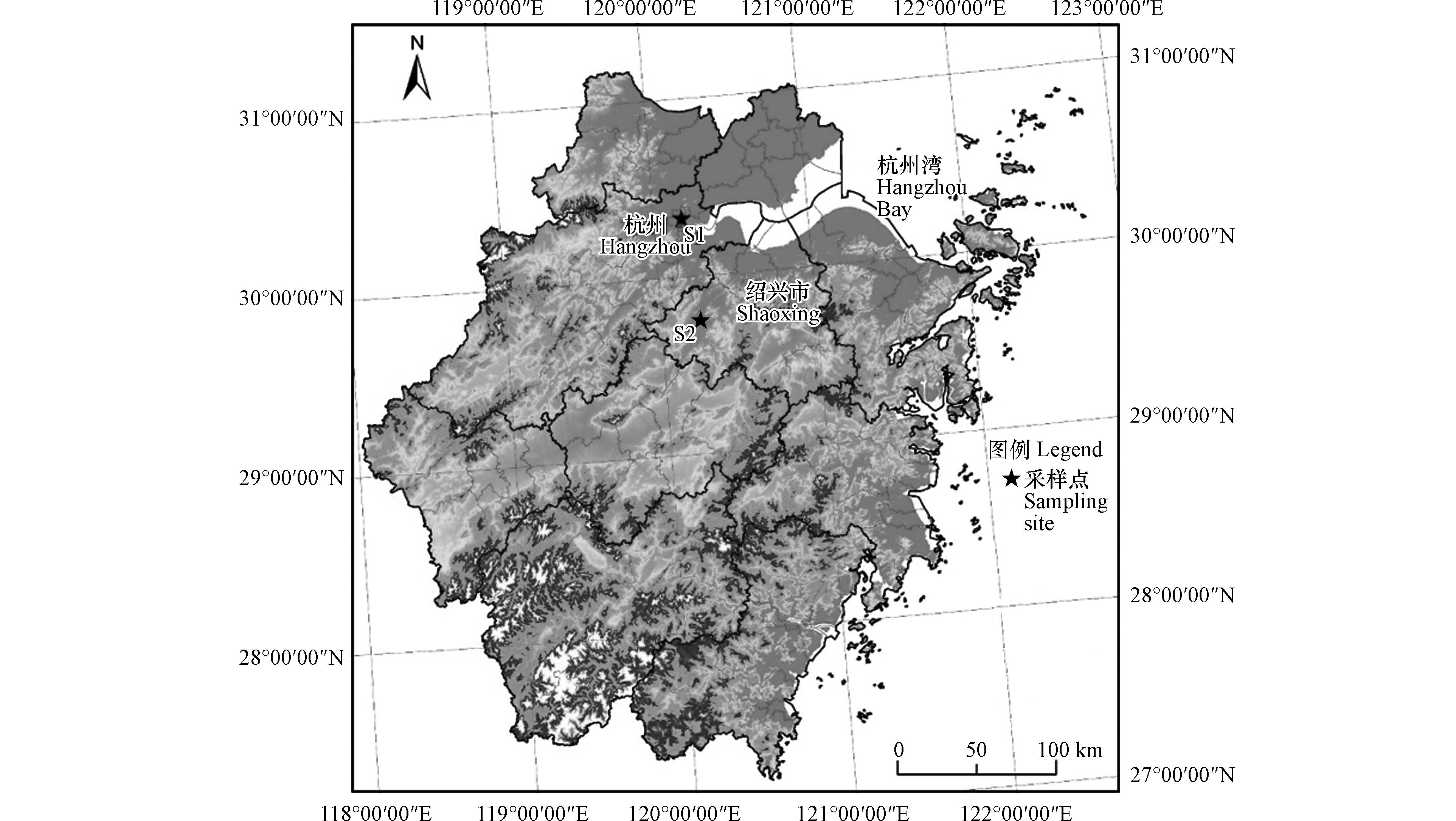
采样点位设在中国长江三角洲地区,包括杭州(S1)和绍兴(S2)两个城市站点(图1),两个采样点相隔60 km. 杭州采样点(33°17′35′′N,120°9′42′′E)位于城市中心区,周围是商业区和居住区. 采样装置放置在浙江工业大学朝晖校区子良楼楼顶(距地面约20 m),距离潮王路约200 m. 绍兴采样点(30°3′26′′N,120°37′53′′E)位于绍兴市洋泾湖科创园2号楼楼顶(距地面约25 m),采样点西北方向为公园(距采样点约600 m),西南为建筑工地(距采样点约400 m),东南为村庄和农业用地. 整个采样期间杭州和绍兴的气象条件见表1,颗粒物(PM2.5和PM10)、NO2、CO、SO2、O3、温湿度数据均取自真气网(https://www.zq12369.com/),风速数据取自wunderground网站(https://www.wunderground.com/).
-
本实验采样及分析方法依据美国环保署(EPA)标准方法TO-11A(USA EPA,1999)[15],采用2,4-二硝基苯肼(DNPH)/HPLC法测定大气羰基化合物含量. 使用的溶剂为高效液相色谱纯(HPLC级),实验用水为超纯水. 乙腈购于美国Supelco公司,标准样品为美国O2si公司(Organic Standards Solutions International LLC)生产的包含15种醛酮类化合物苯腙标准样品的TO11混合标准品. 采样管为DNPH(阿拉丁)涂敷过的Sep-pek硅胶柱(Waters),DNPH涂渍液在实验室内配置,采样时将硅胶小柱连接在自主研发的多通道气体自动采样器上,该采样器由微型电脑控制,具备温压校准系统、流量校准系统、定时采样和循环采样功能,通过多通道旋转气路切换阀控制气路在指定时间段启动以实现连续采样,可连续采集12个气体样品. 气体流速通过流量校准系统精确控制为1 L·min−1,在硅胶小柱前端连接碘化钾小柱以消除环境空气中O3对样品采集的影响. 采样结束后立即密封好样品柱,并置于冰箱中冷藏保存,采样柱在采样后48 h 内用2 mL乙腈洗脱[16],并使用HPLC分析样品溶液. 使用的高效液相色谱仪(HPLC)为Agilent1260Ⅱ,色谱柱为Agilent ZORBAX Eclipse Plus C18柱(5 μm,250 mm×4.6 mm). 在360 nm波长下检测,进样量为5 μL,洗脱梯度在之前的工作中有详细介绍[17]. 用浓度范围0.15—0.45 μg·mL−1的混合标样在相同的色谱条件下分离,以保留时间定性,峰面积外标法定量. 标准曲线的线性回归系数R2均大于0.999.
采样流速均为1 L·min−1,在杭州采样点设置采样时间为1 h,一天采集24个样品,秋季(2020年11月)采样天数为9 d,共采集209个样品. 在绍兴采样点,冬季(2021年1月6号,12—16号)采样时间为1 h,每天采集24个样品,采样天数为6 d,共采集142个样品;春季(2021年4月28号—5月5号)每天采集19个样品(9:00—17:00时间段内1 h采集1个样品,18:00—次日7:30时间段内1.5 h采集1个样品),采样天数为8 d,共采集148个样品;夏季(2021年9月3—7号)每天采集19个样品(采样时间同春季),采样天数为5 d,共采集95个样品;秋季(2021年11月10—16号)每天采集19个样品(采样时间同春季),采样天数为6 d,共采集114个样品.
-
每10个样品后分别做1个实验室空白和1个现场空白,空白柱结果符合EPA空白标准. 将目标化合物浓度为0.15 μg·mL−1的标准溶液分析至少7次,以计算最低检测限(MDL),根据公式:
MDL=t(n−1,1−α=99%)×S . 其中,n为重复分析次数,t(n-1,1-α=99%)表示自由度为n-1时的t分布,S表示标准偏差. 当采样体积为60 L,定容体积为2 mL时,MDL值范围为0. 05—0.1 μg∙m−3. -
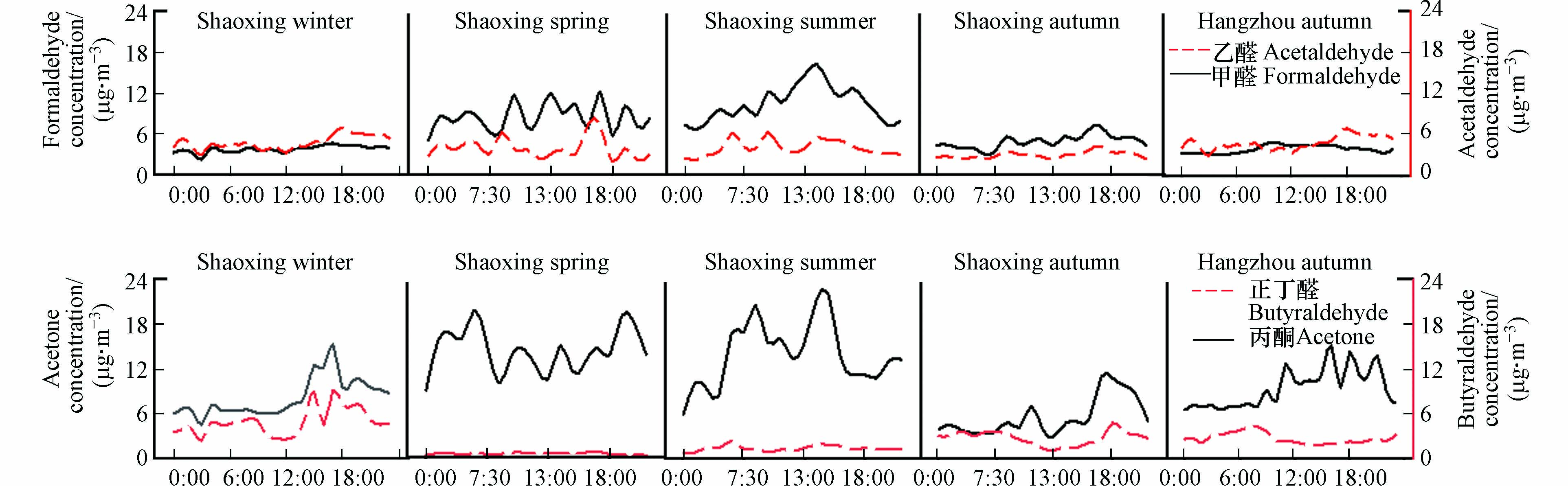
在杭州和绍兴观测到的羰基化合物浓度如表2所示,定量了15种羰基物,其中甲醛、乙醛、丙酮和正丁醛的浓度最大,4种羰基化合物的总浓度为16.09—29.54 μg∙m−3. 丙酮是绍兴和杭州城市空气中丰度最大的羰基化合物,与国内外一些城市的观测结果相似[6, 8, 18-20]. 羰基化合物平均总浓度(36.12 μg∙m−3)在夏季最高,甲醛在夏季/冬季和秋季/冬季的质量浓度比分别为2.79和1.32,夏季绍兴市平均气温在25—35 ℃,日照强度相对较高,环境空气中的O3浓度显著升高,光化学反应加快,导致碳氢化合物迅速光氧化,这可能是绍兴市夏季羰基化合物的主要形成途径[21-22]. 机动车尾气是环境空气中羰基化合物的主要来源[23],2020年绍兴市机动车保有量约为175万辆,其中汽车保有量约为162万辆.
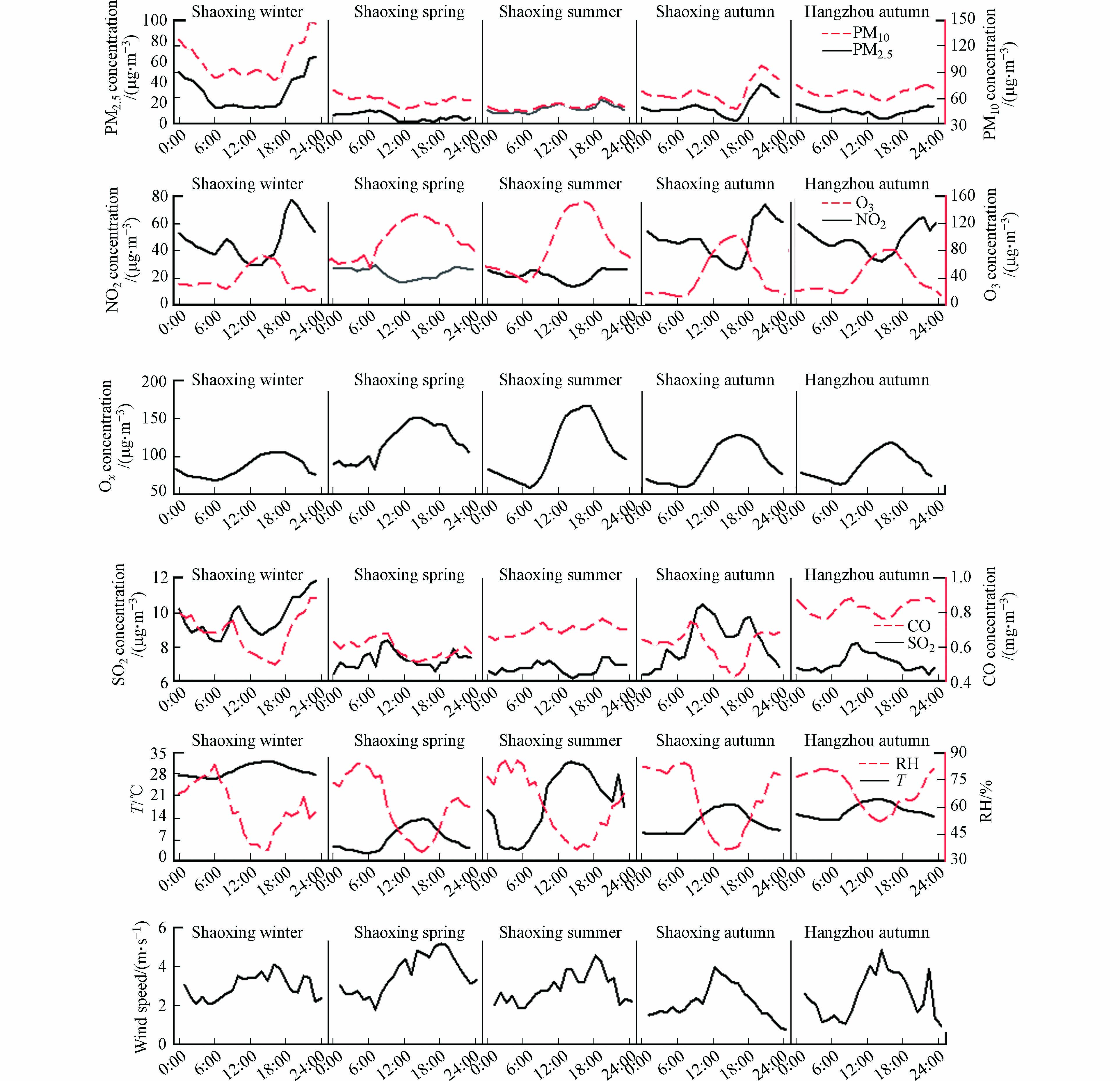
图2和图3显示了观测期间主要痕量气体(包括测量到的4种主要羰基化合物)、大气氧化剂Ox(NO2和O3)、颗粒物(PM2.5和PM10)和气象参数的平均日变化. 这些日变化反映了大气边界层、初级排放和大气光化学的演变. 颗粒物受风速影响较大,在白天风速超过2 m·s−1时段内出现谷值,在夜间微风或静风条件下出现峰值. O3呈单峰分布,在残留层中富含O3的空气夹带的影响下[24-25],O3浓度在日出后迅速升高,14:00—15:00时段达到峰值,然后持续下降至次日早晨,变化趋势与Ox一致. NO2、SO2和CO均在早高峰时段(7:00—9:00)和晚高峰时段(18:00—19:00)出现峰值,甲醛和乙醛的日变化与这些无机痕量气体的日变化相似,除夏季外,分别在9:00和19:00出现两个峰值,这些日变化趋势表明,机动车尾气可能会对杭州和绍兴的人为污染物排放产生重大影响.
利用Ox评价大气氧化能力[1]. Ox从7:00左右开始持续上升,并在12:00—18:00保持较高的水平. 甲醛和乙醛在早晨出现小峰值,在18:00左右出现谷值,结合O3与Ox的日变化特征推测,甲醛和乙醛在夜间积累,随着日出后光化学反应开始进行,大气氧化能力升高,这两种羰基化合物不断被消耗,在日落后其质量浓度开始回升,此时环境空气中大部分臭氧被NO滴定,甲醛和乙醛的消耗速率较低[26-27]. 凌晨出现低值则可能是由于夜间人类活动强度低,人为排放量减少所致.
杭州市环境空气中甲醛和乙醛的日变化趋势为中午和傍晚高、早晨和下午低. 在绍兴,除冬季外,环境空气中甲醛和乙醛浓度有明显变化,甲醛的平均浓度在午后(13:00—16:00)较高,与O3浓度变化趋势一致性良好,乙醛浓度在中午和晚上较低,与南宁市的观测结果相似[22],这可能是由乙醛在白天通过光解和OH自由基迅速反应的光化学损失导致的[28]. 在杭州市和绍兴市均观察到丙酮的日变化很大,浓度范围分别为3.55—88.84 μg∙m−3和0.24—67.48 μg∙m−3,且丙酮在下午和晚上出现峰值,这可能是由于丙酮长期存在于大气中[21],说明杭州市和绍兴市环境空气中丙酮来源是复杂的. 甲醛、乙醛和丙酮全天都保持在较高水平,这三种羰基化合物的日变化可能是一次排放和二次生成的综合作用. 在绍兴市的整个采样周期中,正丁醛在冬季浓度跨度最大,范围为0.70—20.40 μg∙m−3,其它季节浓度相对较低且无明显日变化特征,这是因为对流层中正丁醛与OH自由基的反应活性较高,其反应速率常数为23.7×10−12 cm3 molecule−1·s−1,发生反应后正丁醛在大气中的生命周期仅为4.7 h[29],日出后光化学反应增强,丁醛与大气中的OH自由基迅速发生反应,这可能是导致正丁醛浓度在中午下降而在早晨浓度升高的原因. 总体而言,这些羰基化合物的日变化表明初级和次级来源都可能对杭州市和绍兴市检测到的羰基化合物有贡献[30, 23],特别是甲醛、乙醛和丙酮.
-
甲醛、乙醛、丙酮是环境空气中最丰富的3种羰基化合物,在杭州市和绍兴市,这3种羰基化合物之和分别占总羰基化合物的76.2%和80.5%,丰度与国内外城市地区以前的研究结果一致[21, 17, 22, 9]. 为对比杭州市和绍兴市的羰基化合物水平,表3中列出了国内外城市地区的甲醛、乙醛和丙酮的浓度水平及甲醛/乙醛浓度比值(C1/C2). 杭州市秋季环境空气中甲醛(3.81 μg∙m−3)和乙醛(4.71 μg∙m−3)的平均浓度明显低于中国典型特大城市,绍兴市环境空气中甲醛(5.85 μg∙m−3)和乙醛(3.88 μg∙m−3)与中国香港[31]、日本大阪[18]、意大利罗马[8]等地区相当,而两处观测点的丙酮浓度(杭州10.34 μg∙m−3,绍兴8.30 μg∙m−3)则与北京[6]、上海[32]等高污染典型城市相当,明显高于贵阳[21]、奥尔良[33]等城市. 城市地区汽车尾气是大气羰基化合物的重要排放源[19],而丙酮的主要来源可能与溶剂和涂料挥发等不规则排放有关[11]. 绍兴采样点位于绍兴市东北部,Li等对绍兴市环境空气中VOCs源解析的结果表明,绍兴市溶剂使用量主要集中在东部地区,近年来工业发展和人口增长导致了严重的空气污染[13].
环境空气中甲醛/乙醛浓度比值(C1/C2)和乙醛/丙醛浓度比值(C2/C3)可用来大致判断大气羰基化合物的主要来源[34],在人类活动的影响下,城市大气中C1/C2比值一般为1—2,由于自然排放的碳氢化合物光氧化生成甲醛的产率高于乙醛,因此,森林及偏远郊区大气中C1/C2比值为10左右[19-20, 22, 35]. 本研究中(见表3),杭州秋季和绍兴冬季C1/C2的平均值分别为0.81、0.86,与广州[35](0.84)相似. 绍兴除冬季外,其它三个季节的C1/C2平均值均 > 2,与长沙[20](2.10)、西安[9](2.14)和奥尔良[33](2.18)相似,其中夏季最高(2.73),春季次之(2.18),秋季第三(2.06). 研究表明[21, 1],羰基化合物的光解及其与OH自由基和O3的化学反应是大气中羰基化合物的主要去除途径,此途径可通过改变大气中甲醛、乙醛的分布来改变C1/C2比值. 与OH自由基发生化学反应的去除率取决于各羰基化合物的反应性,Ji等[36]的研究表明,乙醛与OH自由基反应速率仅8.8 h,大于甲醛(1.2 d),因此在更强烈的光化学条件下,C1/C2比值可能会更高. 绍兴冬季观测到的C1/C2比值最低,说明在其它季节中,强烈的光化学反应导致大气中羰基化合物的形成.
-
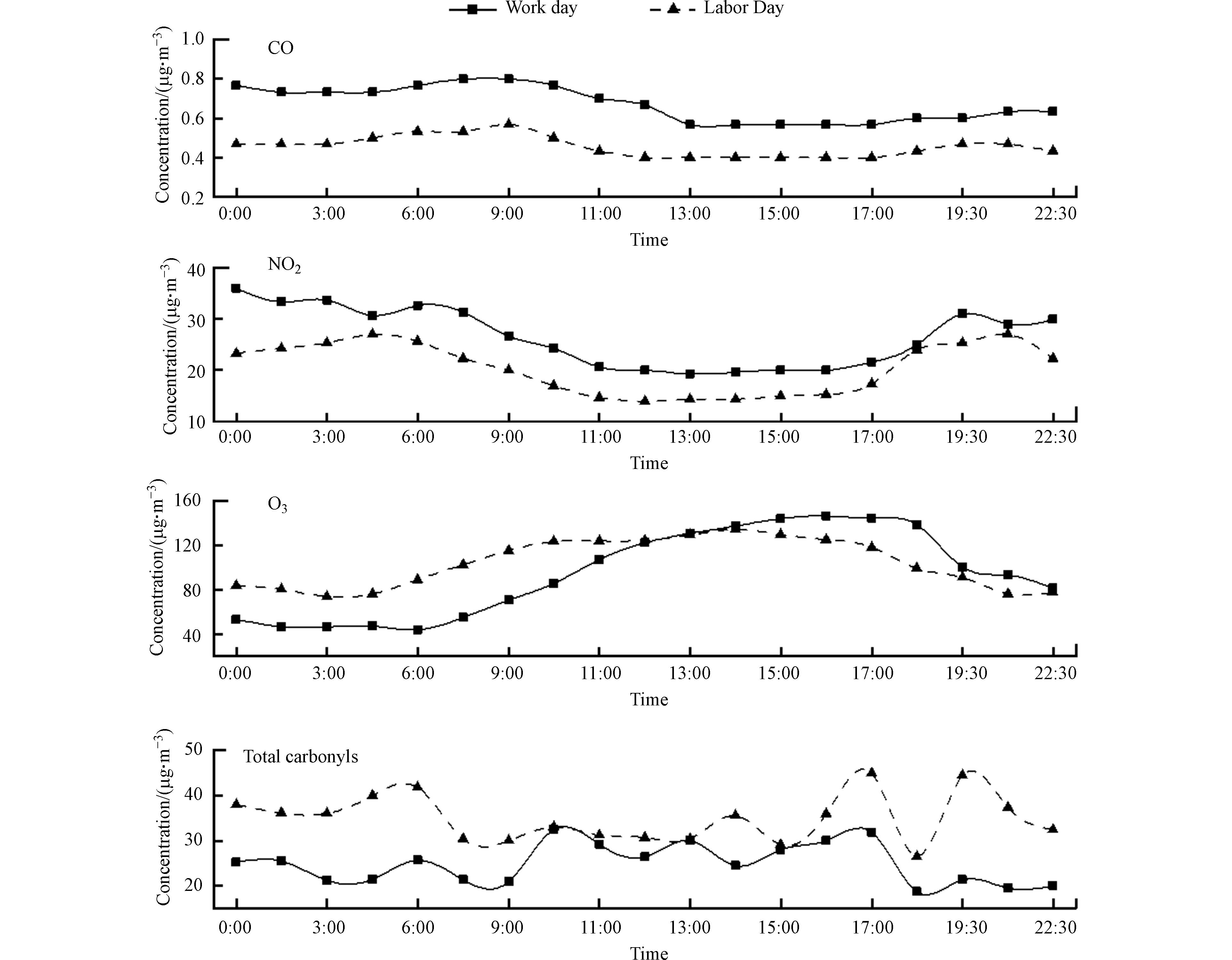
对工作日(2021年4月28—30日)和节假日(2021年5月1—5日)绍兴市大气羰基化合物质量浓度的日变化进行了分析. 其中工作日早晨6:00—8:00期间有雾出现,早晨和夜间微风,中午和午后风速较大(> 4 m·s−1),而节假日天气晴朗,夜间微风或静风,白天风速较大(> 4 m·s−1). 节假日期间羰基化合物浓度高于工作日(图4),这与其他研究报道的不同[37]. 工作日期间大气中CO和NO2浓度均高于节假日,说明节假日期间的高浓度羰基化合物与机动车尾气排放的关系不大. 结合采样期间环境O3浓度可知(表4),节假日期间存在较严重的臭氧污染,臭氧浓度最大达190 μg∙m−3,超过GB3095-2012《环境空气质量标准》[38]中规定的二级标准限值(160 μg∙m−3),由此可知,节假日期间的臭氧污染可能与羰基化合物的高浓度有关.
如图4所示,工作日白天羰基化合物浓度高于夜间,在早晨9:00上升,10:00时达到日最大值,午后太阳光辐射最强、气温最高,机动车尾气排放增加,在一次排放和光化学氧化二次生成的共同影响下[39, 1],午后羰基化合物浓度升高. 羰基化合物浓度在17:00—18:00时段出现最大值,这是因为该时段正处于下班高峰期,车流量显著增加导致机动车尾气排放迅速增加. 傍晚以后随着光化学反应强度的降低,羰基化合物浓度迅速降低,并在夜间浓度较低. 工作日的日变化趋势说明羰基化合物受光化学反应和人为源一次排放的双重影响.
而在发生臭氧污染的节假日期间,夜间羰基化合物浓度明显高于白天(图4),与O3日变化趋势相反,这与其他研究报道的结果正好相反[17]. 这是因为污染天白天大气氧化性较强,光化学反应速率快,羰基化合物被迅速消耗,从节假日期间白天(7:30—18:00)羰基化合物的变化趋势来看,羰基化合物浓度在7:30—10:00有轻微的上升趋势,在中午浓度平稳(11:00—13:00),14:00时出现一个小峰值,午后(15:00—17:00)受光化学反应影响,羰基化合物浓度上升,并在17:00时达到日最大值,而后迅速降低,在18:00时降至日最低值,傍晚以后随着光化学反应的中止、大气氧化能力减弱、边界层迅速降低导致扩散条件不利等因素的共同影响下,羰基化合物浓度继续升高,在19:30—21:00时段内出现峰值,并在夜间保持较高浓度水平,但由于此时环境空气中仍存在未被滴定的较高浓度的臭氧,活性较强的羰基化合物仍以较快速率被氧化消耗[27].
-
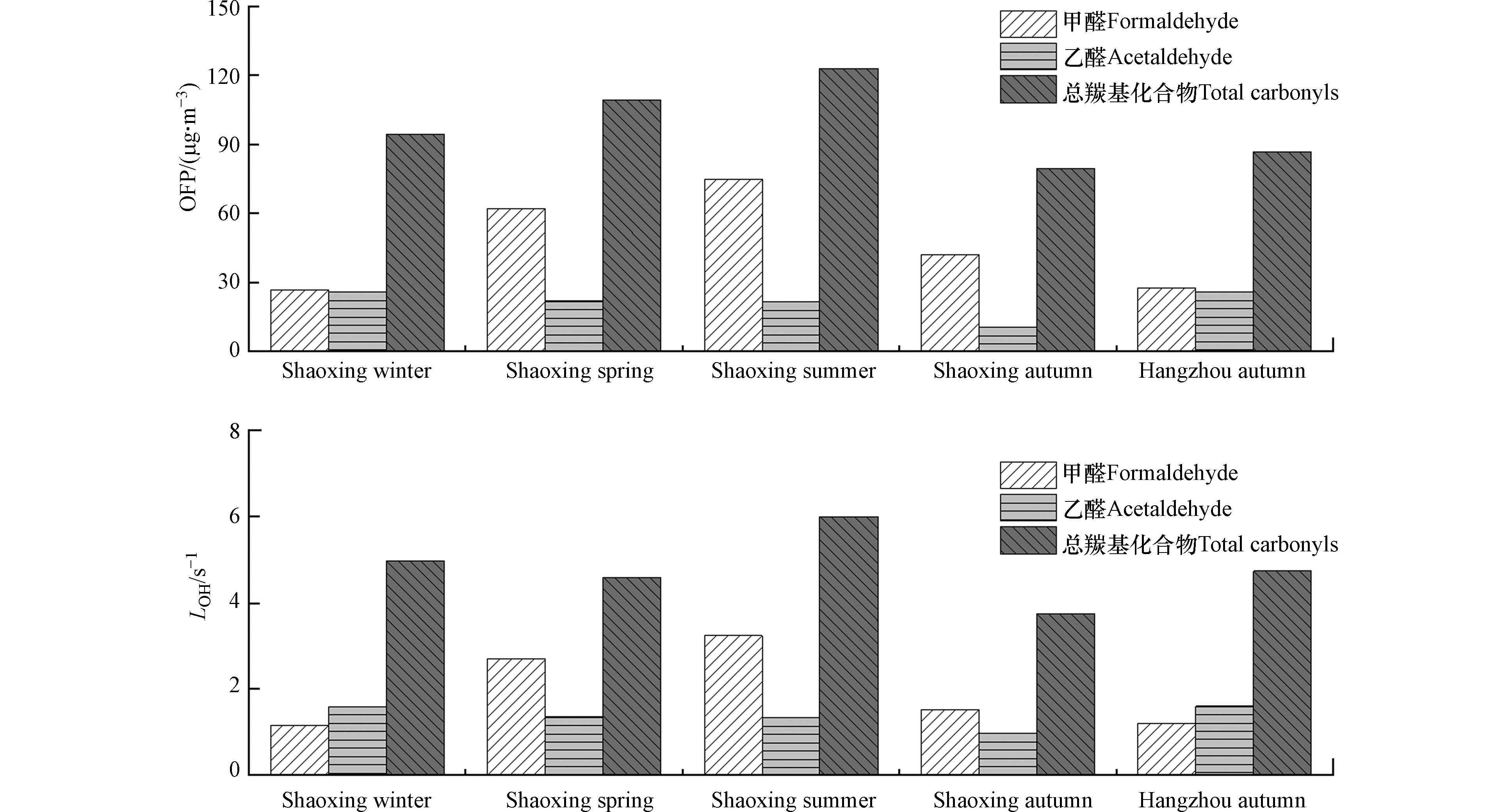
在本次研究中,使用臭氧生成潜势(OFP)和OH自由基消耗速率(LOH)评估大气中羰基化合物对臭氧的二次生成的贡献及其光化学反应活性. 用下列公示计算,MIR分别来源于Carter[40]、Duan[7]的研究.
其中,
MIRi 为挥发性有机化合物i的最大反应性增量(g O3/g VOCs),KOHi(cm3⋅(molecule⋅s)−1) 为298 K时[VOC]i 与OH自由基反应的速率常数.研究表明,长江三角洲城市地区的臭氧生成在臭氧高峰期处于挥发性有机化合物(VOC)受限状态,而不是NOx的限制状态[3]. Geng[41]等以上海市为例,发现醛、酮和乙醇对光化学臭氧形成的贡献率达10%. 如图5所示,绍兴市由羰基化合物贡献的OFP为80—123 μg∙m−3,总羰基化合物的LOH为3.7—6.0(s−1),均表现为夏高秋低,秋季杭州市由羰基化合物贡献的OFP和LOH均高于绍兴秋季,且低于冬季绍兴市,这是因为秋季杭州市的总羰基化合物浓度高于绍兴市. 在羰基化合物中,甲醛和乙醛丰度最高,是LOH的主要贡献者,说明甲醛和乙醛是光化学活性最大的两个羰基物种. 在绍兴市,这两种化合物对OFP的贡献为55.8%—78.2%,特别是在春季和夏季,秋季杭州市和绍兴市的占比相当(61%和66%). 图5显示,甲醛总是OFP的第一贡献者,其次是乙醛,说明甲醛和乙醛对大气中臭氧的二次生成有显著贡献,均是杭州市和绍兴市大气臭氧的主要前体物.
-
杭州市和绍兴市大气中羰基化合物测定表明,两个城市存在较高浓度的羰基化合物污染,甲醛、乙醛和丙酮是3种含量最丰富的羰基化合物,分别占杭州和绍兴羰基化合物总量的76.2%和80.5%. 绍兴市夏季大气中羰基化合物浓度显著高于其他季节. 季节变化和日变化表明,光氧化和人为来源(如机动车尾气排放和工业排放)是绍兴市大气羰基化合物的重要来源. 结合C1/C2比值,表明人为来源是杭州市秋季大气羰基化合物的主要来源. 在发生臭氧污染的节假日期间,羰基化合物浓度明显高于工作日,对比其日变化趋势,发现臭氧污染期间羰基化合物浓度夜间较高. 对杭州市和绍兴市OFP贡献较大的羰基化合物是甲醛和乙醛,它们在杭州市秋季大气中对OFP的贡献达53.4%,在绍兴市全年大气中对OFP的贡献为55.8%—78.2%,以夏季最高. 总的来说,在杭州和绍兴观测区,甲醛和乙醛是大气臭氧的主要前体物,对大气光氧化污染有重要贡献,建议生态环保部门重点针对甲醛和乙醛进行管控.
杭州及绍兴大气中羰基化合物的污染特征、来源及影响
The pollution levels, sources and impact of carbonyls in Hangzhou and Shaoxing atmosphere
-
摘要: 长江三角洲地区是我国“三区十群”大气污染防治重点区域之一,城市大气光化学污染严重,但对其形成机制研究较少. 本论文采用2,4-二硝基苯肼固相吸附采样,高效液相色谱法(HPLC)分离检测技术,测定了杭州和绍兴两个长三角典型城市大气羰基化合物,研究了羰基化合物的时空分布特征及其对大气光氧化的贡献,并利用比值法初步分析了羰基化合物的来源. 结果表明,杭州市和绍兴市大气中主要羰基化合物都是甲醛、乙醛和丙酮,其在杭州市的质量浓度分别是(3.81±1.15)μg·m−3、(4.71±2.18)μg·m−3、(10.34±10.20)μg·m−3(n=209),在绍兴市的质量浓度分别为(5.85±3.93)μg·m−3、(3.88±1.97)μg·m−3、(8.30±6.87)μg·m−3(n=499),这3种化合物占杭州市和绍兴市羰基化合物总量分别是76.2%、80.5%. 绍兴市夏季羰基化合物的平均总浓度明显高于其他季节,大气光化学污染可能是绍兴大气羰基化合物的主要来源. 2021年4月28日—5月5日,在绍兴的一次臭氧轻度污染过程期间,臭氧污染的节假日期间羰基化合物总量高于工作日,且夜间高于白天. 对杭州和绍兴臭氧生成潜势(OFP)贡献较大的羰基化合物是甲醛和乙醛,它们对杭州市秋季大气中OFP的贡献达53.4%,对绍兴市全年大气中总OFP的贡献值为55.8%—78.2%. 本研究结果有助于认识大气羰基化合物对杭州市和绍兴市臭氧的贡献.Abstract: The Yangtze River Delta region (YRD) is one of China’s “Three Regions and Ten Groups” air pollution prevention and control areas. Although the urban atmospheric photochemical pollution is quite serious, there are few studies on its formation mechanism. To investigate the pollution characteristics of carbonyls in urban ambient air in the YRD region, Hangzhou City and Shaoxing City were selected as typical cities. The levels of carbonyl compounds in Hangzhou and Shaoxing were measured by using a high-performance liquid chromatography (HPLC). The spatiotemporal variations and the contributions to atmospheric photo-oxidation of carbonyl compounds were studied. The preliminary sources analysis of carbonyls was carried out by a ratio method. Formaldehyde, acetaldehyde, and acetone were the three most abundant carbonyls of both cities with the mean concentrations of (3.81±1.15) μg∙m−3, (4.71±2.18) μg∙m−3 and (10.34±10.20) μg∙m−3 (n=209), respectively, in Hangzhou city. The mean concentrations of formaldehyde, acetaldehyde and acetone in Shaoxing city were (5.85±3.93) μg∙m−3, (3.88±1.97) μg∙m−3, and (8.30±6.87) μg∙m−3 (n=499), respectively. These three compounds accounted for above 76.2% and 80.5% of total carbonyls in Hangzhou and Shaoxing. The total carbonyl concentrations showed significant higher levels in summer compared to other seasons at Shaoxing. The photo-oxidation of hydrocarbons might be the important sources to carbonyls in Shaoxing. A slight ozone pollution process occurred in Shaoxing from April 28 to May 5, 2021, the total carbonyl concentrations during Labor Day period of ozone pollution was higher than that of working days, and concentrations during night were higher than those during daytime. The most important species of carbonyls which contributed to the ozone formation potentials (OFP) were formaldehyde and acetaldehyde both in Hangzhou and Shaoxing. These two species contributed 53.4% to the OFP in autumn of Hangzhou, and 55.8%—78.2% to the OFP in Shaoxing for the whole year. This study helps to understand the contribution of atmospheric carbonyls to ozone in Hangzhou and Shaoxing, may be useful to control ozone pollution.
-
Key words:
- carbonyl compounds /
- spatiotemporal variations /
- photo-oxidation /
- the Yangtze River Delta /
- Hangzhou /
- Shaoxing
-
因产业布局、结构、管理等原因,我国现阶段突发环境风险形势严峻,突发环境事件总量仍处高位,呈现事件诱因复杂、危害影响大的特点。近年来,我国多次发生由于尾矿库泄漏引起的突发性重金属污染事件,如2015年甘陕川锑污染、2016年新疆阿勒泰地区克兰河污染、2017年河南栾川钼污染、2017年嘉陵江铊污染、2020年黑龙江伊春鹿鸣矿业尾矿库泄漏等。对突发水环境重金属污染事件开展污染溯源分析,可有力支撑事件应急处置、灾害生态风险评估和责任追究等。目前,国内外采用的污染溯源方法主要基于水质模型法、地学统计法、遗传算法、贝叶斯法、反向位置概率密度函数法等,其中以贝叶斯法及其衍生法为主[1-6]。这些方法均需要大量的基础数据支撑,而在突发环境事件发生的短期内基本不能满足要求,由此会因得出的结果存在较大误差而导致溯源不准;而且,因这些方法在演算过程中存在的不确定性误差,亦会导致溯源偏差。在污染源识别方面,现阶段国内外主要依靠构建污染源区域监测网络来对污染源进行监控。然而,我国很大一部分突发水污染事件的肇事企业是非法生产企业(未登记在册)或者企业瞒报,从而使得即使突发水污染事件被发现,却不能判定特征污染物的来源和排放特征。这个问题导致我国突发流域性环境事件时,往往错过事故的最佳断源时机,同时也为后续事件追责造成一定影响。
本文系统总结了突发水环境重金属污染溯源方法,并以2017年嘉陵江铊污染事件为例,介绍了溯源过程,还原了污染事件全过程,以期为类似污染事件溯源提供参考。
1. 溯源方法
本文所述溯源方法包含污染物特征指纹分析、污染物总量分析、水体中污染物浓度梯度变化分析、污染物迁移时间与路径分析等4部分的综合运用。同时,结合区域现场踏勘、产业结果与分布、人员访谈等情况进行综合分析也非常重要。
1.1 污染物特征指纹分析
分析所有环境应急监测数据,厘清造成本次突发水污染事件的主要影响因子,即主要超标指标;随后围绕上游区域产业行业结构特征,分析其工艺特征,研究其可能产生的特征污染物,并与主要超标指标进行比对,预判造成本次事件的可能肇事企业类型与区域。
1.2 污染物总量分析
计算有记录以来环境应急监测中特征污染物浓度的平均值,结合污染事件发生水体的流量(或水量)、流速等水文条件,估算出此次事件特征污染物泄漏进入河流的总量;随后在疑似企业中根据工艺及物料平衡估算企业排入水体中的污染物总量;将企业排出和水体中检出的量进行比较,进一步确定肇事企业。
1.3 水体中污染物浓度梯度变化分析
根据疑似肇事企业污染物排放浓度与应急监测得到的污染物浓度做对比,评估其浓度在水体中的变化趋势是否符合污染物在水体中的混合扩散模型。
1.4 污染物迁移时间与路径分析
综合水体流量、流速等水文条件、气象条件,从监测到的污染物前锋反推污染物可能的排放源头和排放时间;随后结合上游区域疑似企业排查,进一步锁定肇事企业。
2. 案例概况
2.1 污染事件概况
2017年5月5日,四川省某市环保局监测发现,其市级集中式饮用水水源地铊浓度超过《地表水环境质量标准》(GB 3838-2002)中标准限值4.6倍,定性为饮用水水源地水质污染,具体原因不明。据此,该市人民政府认定嘉陵江该流域发生了铊污染事件,并于5月5日22时启动突发环境事件应急II级响应。5月8日,受生态环境部应急办及川陕两省委托,笔者团队运用本文提出的溯源方法在嘉陵江沿线开展污染源排查,当日初步锁定嫌疑企业;5月9日,开展核查并锁定肇事企业;5月10日20:00,嘉陵江受污染河段各应急监测断面铊浓度均达标;5月11日,该市终止此次事件的应急响应。
2.2 事件原因分析
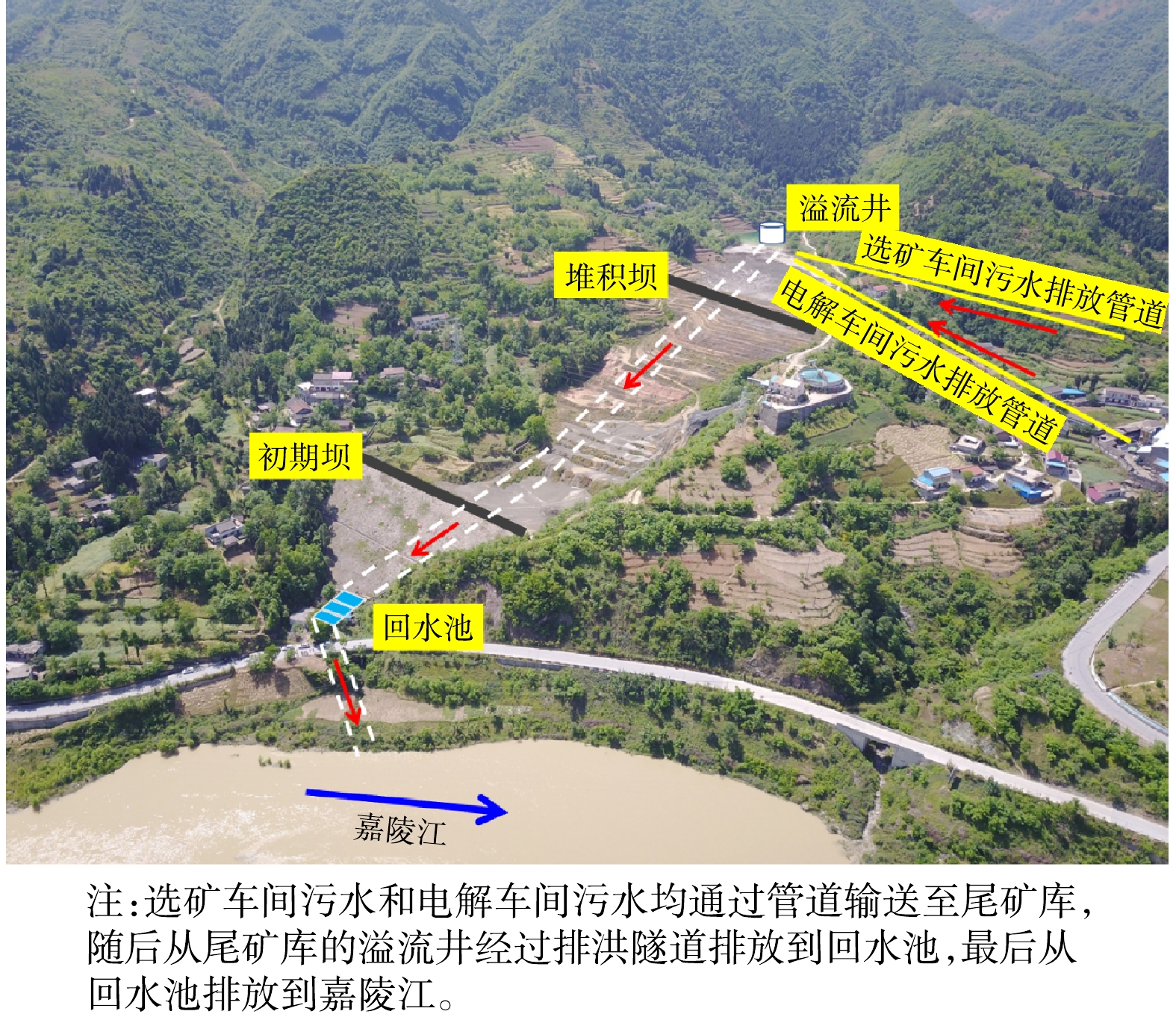
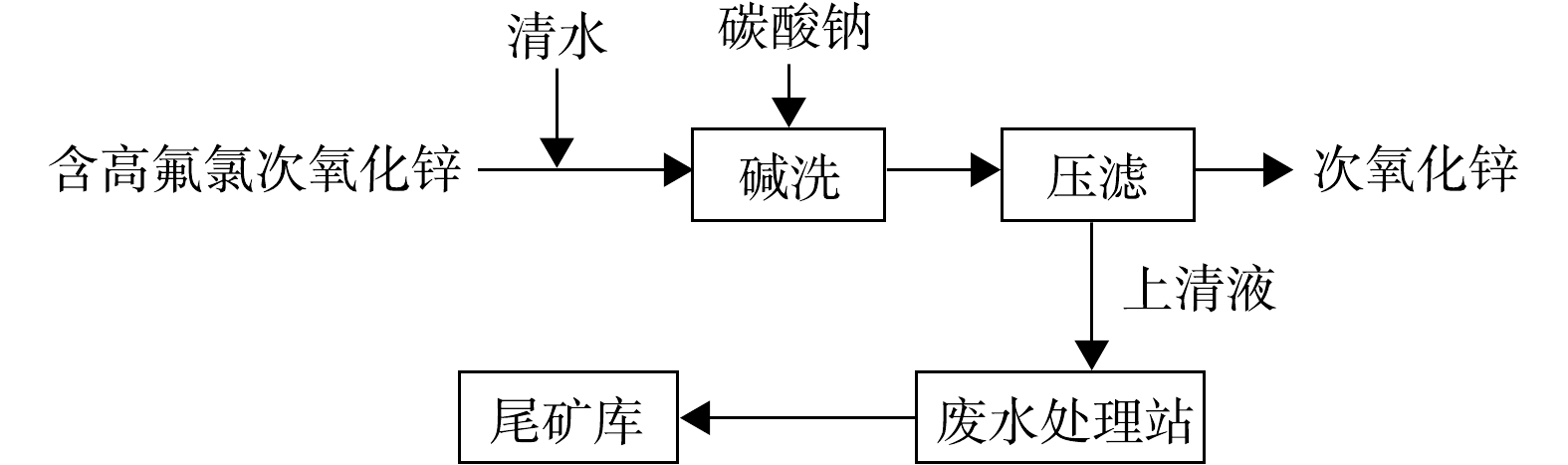
经调查,事件背景为:1) 2017年3月以来,陕西省某企业以来料加工方式碱洗除氟氯处理后铊含量高的多膛炉次氧化锌烟灰原料(碱洗除氟氯工艺流程见图1),产生的未经有效处理的高浓度含铊废水被排入尾矿库;2) 5月2—3日,某企业所在县出现强降水天气,导致该企业尾矿库高浓度含铊雨污混合水经溢流井集中后外排,在嘉陵江形成污染水团,造成本次事件。
2.3 污染源概况
该企业尾矿库下游设拦渣坝,上游设拦洪坝,库区左岸设钢筋砼方涵泄洪。尾矿库设计库容为1.98×106 m3,属三等尾矿库。库区排洪系统采用溢流井-隧洞泄洪的方式,当尾矿库存水超过溢流水位时,会通过溢流井经回水池流入嘉陵江干流。
3. 案例的溯源分析
3.1 特征污染物定性与控制标准
1)确定特征污染物种类。2017年5月4日上午10时50分左右,该市环境监测站会同第三方监测机构于川陕交界界面(八庙沟断面)进行例行饮用水水质监测,取河道左岸和右岸水样分别进行饮用水全指标分析。5月5日,水样监测指标出现铊浓度超标(其他指标未见超标)。5月5日11时和18时,对该市水源地水源水进行了取样检测,结果显示铊浓度超标。据此认定该流域发生突发铊污染事件。
5月9日,环境保护部工作组会同两地公安、环保等部门对嫌疑企业进行了现场检查。经查实,该企业选矿车间原料中铊质量浓度约为2 500 mg·kg−1,现场残存待排入尾矿库的废水中铊浓度约为9.16 mg·L−1,与本次嘉陵江流域水体污染事件中特征超标因子铊相吻合。另外,2016年以来,该企业从澳大利亚、土耳其、伊朗等国家采购锌精矿,部分锌精矿中铊质量浓度为100~200 mg·kg−1。锌精矿中的铊经冶炼后富集到多膛炉次氧化锌烟灰中,现场监测表明,多膛炉次氧化锌烟灰中铊质量浓度约2 500 mg·kg −1。
2)特征污染物性质与控制标准。本次事件特征污染物铊是一种剧毒物质,易溶于硝酸和硫酸,不溶于水。铊有2种氧化态形式,即一价铊和三价铊。水体中铊常以一价存在,更易形成稳定化合物,并随水体迁移进入其他环境介质或生物体内。铊对哺乳动物的毒害作用远超过Hg、As、Cd、Pd、Sb等重金属[7]。铊主要为伴生,常通过含铊矿山的采选冶炼等途径进入水环境[8]。铊可通过饮水以及食物链等进入人体,并在人体中的骨髓、肾脏等器官累积,损害人体肌肉、中枢神经系统等。我国《地表水环境质量标准》(GB 3838-2002)中“表3 集中式生活饮用水地表水源地特定项目”以及《生活饮用水卫生标准》(GB 5749-2006)中“表3 水质非常规指标及限值”规定了铊的允许质量浓度为0.000 1 mg·L−1。
鉴于受此事件影响的有集中式生活饮用水取水口,故将应急处置工作目标确定为地表水体中铊浓度达到集中式生活饮用水地表水源地特定项目标准限值的0.000 1 mg·L−1。
3.2 特征污染物总量核算
1)企业排入尾矿库的铊总量。该企业选矿车间于2017年4月18日—5月2日使用高含铊多膛炉次氧化锌烟灰作为原料,进行碱洗以除氟氯。排入尾矿库废水量约120 m3·d−1。铊质量浓度以厂区残存废水浓度9.16 mg·L−1计,日均铊排量约为1.10 kg。在15 d生产期内铊排放总量为16.49 kg。
2)尾矿库排入嘉陵江铊总量。当尾矿库存水超过溢流水位时,会通过溢流井溢流排入嘉陵江。经现场测算,该尾矿库库区可存水量约1 300 m3,4月18日至5月2日选矿车间排入尾矿库废水总量约1 800 m3,超过尾矿库可存水量。同时,尾矿库存水经下渗、自然蒸发等损耗有限。因此,可推断在5月2日降水前尾矿库溢流井应处于溢流或接近溢流的状态。
5月2—3日,企业所在区域降水量共28.6 mm,此次降水导致尾矿库中污水集中溢流排放。经现场测算,该场降水后尾矿库库区汇入雨水量约1 500 m3。在尾矿库处于溢流或接近溢流的状态下,不考虑中间损耗,排入嘉陵江雨污混合水水量以1 500 m3计,铊质量浓度以尾矿库现存雨污混合水浓度5.31 mg·L−1计,则5月2日至3日尾矿库通过溢流井集中排入嘉陵江铊总量约为7.97 kg。
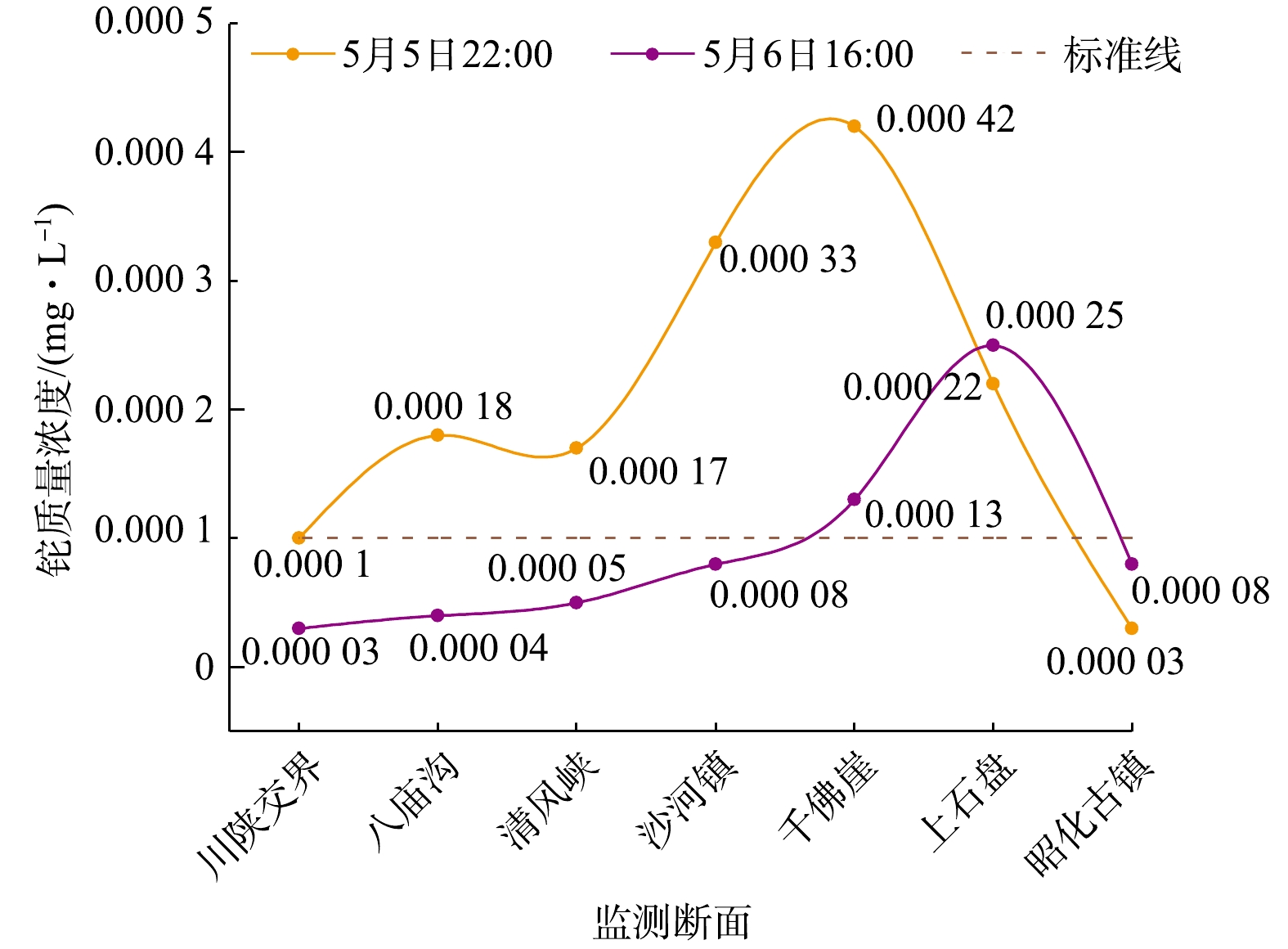
3)嘉陵江流域受污染水体中铊总量。根据2017年5月5日22时市监测站提供的水质监测数据和水文站提供的水文数据,对排入嘉陵江流域的铊总量进行了核算,主要计算数据见表1。5月5日22时污染水团前锋已达上石盘断面附近,尾峰在川陕交界断面附近,污染水团集中在2个断面之间约83 km的河段内。根据区间污染物分布情况,采用积分法计算出污染水团中铊的总量约6.61 kg。
表 1 5月5日22时各监测断面水文水质数据表Table 1. Hydrological and water quality data sheets for each monitoring section at 22:00 on the 5th应急监测断面 铊质量浓度/(mg·L−1) 断面流量/(m3·s−1) 断面流速/(m·s−1) 断面截面积/m2 相邻断面距离/km 川陕交界 0.000 10 107 0.72 148.61 12 八庙沟 0.000 18 107 0.72 148.61 22 清风峡 0.000 17 107 0.72 148.61 13 沙河镇 0.000 33 139 0.52 267.3 14 千佛崖 0.000 42 171 0.31 551.61 22 上石盘 0.000 22 171 0.31 551.61 7 昭化古镇 0.000 03 — — — — 根据以上数据来分析,本次污染事件中嘉陵江污染水团中铊总量约6.61 kg,比前述尾矿库排入嘉陵江铊总量7.97 kg略低。考虑到铊进入水体后,污染物会被泥沙等吸附沉降转移至沉积相,故两者的量基本吻合。
3.3 特征污染物浓度及其校核
该企业选矿车间残存的待排入尾矿库废水中铊质量浓度为9.16 mg·L−1、尾矿库存水铊浓度为5.31 mg·L−1,分别超标约9.16×104倍和5.31×104倍;据5月4日事发后监测数据,嘉陵江铊最大超标倍数约10倍。上述情况与高浓度污染物集中外排,进入水体后因掺混稀释作用的浓度梯度变化特征相符。
3.4 特征污染物浓度变化趋势校核
根据应急监测数据分析,各应急监测断面铊质量浓度呈较完整的正态分布(见图2)。从图2中各监测断面的过程峰形可以看出,此次污染事件的排放特征为污染物短时间大量排放,与强降水时尾矿库存水通过溢流井集中溢流特征吻合。
3.5 特征污染物迁移与水文数据校核
根据实测水文数据反演,尾矿库自5月2日12时开始集中溢流。这与5月2日10时开始降水后,尾矿水存水集中溢流时间基本吻合。污染团前锋迁移时间估算见表2。
表 2 污染团前锋迁移时间估算Table 2. Estimation of the migration time of the front of the contaminated mass应急监测断面 距尾矿库距离/km 流速/(m·s−1) 相邻断面传播时长/h 污染团前锋到达时间 尾矿库 0 0.72 0 5月2日12:00 川陕交界 16 0.72 6 5月2日18:00 八庙沟 28 0.72 5 5月2日23:00 清风峡 50 0.72 8 5月3日07:00 沙河镇 63 0.52 7 5月3日14:00 千佛崖 77 0.31 13 5月4日02:00 上石盘 99 0.14 44 5月5日22:00 3.6 污染物排放路径判定
经现场查勘、监测分析及企业人员确认,选矿车间多膛炉烟灰碱洗除氟氯产生的废水、电解车间洗铜废水中含铊废水,在未经有效处置的情况下,由泵经专管排入尾矿库。当尾矿库存水超过溢流井溢流水位时,含铊污水经溢流井-排洪隧洞排入回水池后进入100 m外的嘉陵江干流。污染物排放路径如图3所示。
综上所述,通过特征污染物、总量核算、质量浓度梯度、污染过程峰型、时间序列等综合分析,可判定本次嘉陵江流域铊污染事件为5月2—3日该企业矿库含铊污水在降水后集中溢流外排所致。
4. 结语
本研究提出的溯源方法是一种利用有限数据并结合现场踏勘,在短时间内快速准确锁定污染源的方法。该溯源方法还成功应用于2012年龙江河镉污染、2016年仙女湖镉污染等突发水环境重金属污染等未知源事件的溯源工作。如在2012年龙江河突发环境事件中,成功锁定肇事企业是一家未登记的非法炼铟企业,其非法将大量生产废液注人地下溶洞,在河流水位突降时引发突发水污染事件。实践证明,该溯源方法及其改进方法可有效适用于一次性及连续性排污的未知污染源溯源工作,可支撑突发环境事件的应急处置。
-
表 1 杭州和绍兴采样期间气象条件信息
Table 1. Meteorological parameters in the sampling periods at the Hangzhou site (S1) and Shaoxing site (S2)
温度/℃Temperature 相对湿度/%Relative humidity 风速/(m·s−1)Wind speed 天气Weather 杭州 秋季 9—24 24—95 0—5.812 晴、多云、小雨 冬季 -2—20 15—82 0.898—7.184 晴、阴、多云 绍兴 春季 18—23 57—100 0.898—4.041 晴、多云、小雨 夏季 25—35 49—91 0.447—5.812 晴、多云 秋季 6—21 24—91 0—8.047 晴、多云 表 2 杭州市秋季、绍兴市四季羰基化合物的浓度(μg∙m−3)
Table 2. Average concentration of the carbonyls in each sampling periods at Hangzhou site and Shaoxing site (μg∙m−3)
甲醛Formaldehyde 乙醛Acetaldehyde 丙酮Acetone 正丁醛Butyraldehyde 其它9种羰基化合物Other 9 carbonyls 合计Total 杭州秋季 范围 1.79—7.53 2.02—17.26 3.55—88.84 1.86—3.15 0.43—18.71 4.33—141.02 平均浓度 3.81±1.15 4.71±2.18 10.34±10.20 2.61±1.57 3.29±3.22 24.76±18.33 绍兴冬季 范围 1.13—8.90 1.13—13.00 3.03—38.20 0.70—20.40 0.80—9.93 6.83—81.53 平均浓度 3.72±1.41 4.69±2.29 8.08±6.31 4.49±3.43 3.81±3.81 25.61±19.05 绍兴春季 范围 1.20—40.33 0.12—23.02 2.53—67.48 0.08—1.40 0.14—10.56 1.69—101.46 平均浓度 8.63±5.62 3.97±3.82 14.71±10.97 0.56±0.31 2.32±3.42 31.01±25.29 绍兴夏季 范围 6.62—25.30 2.23—6.14 5.82—21.82 0.66—2.24 1.22—17.69 16.55—64.03 平均浓度 10.38±2.61 3.92±1.09 13.97±4.25 1.27±0.37 6.58±5.72 36.12±14.04 绍兴秋季 范围 0.85—9.97 0.61—6.51 0.24—41.63 0.03—8.54 0.07—15.80 1.62—60.97 平均浓度 4.90±2.03 2.90±1.14 5.62±5.65 2.66±2.01 3.07±6.04 18.88±18.36 表 3 不同城市羰基化合物质量浓度对比(μg∙m−3)
Table 3. A comparison of atmospheric formaldehyde, acetaldehyde, acetone and C1/C2 value in different urban cities (μg∙m−3)
地区Location 采样时间Period 甲醛Formaldehyde 乙醛Acetaldehyde 丙酮Acetone C1/C2 杭州 2020.11 3.81 4.71 10.34 0.81 绍兴 2021.01 3.72 4.69 8.08 0.86 绍兴 2021.05 8.63 3.97 14.71 2.18 绍兴 2021.09 10.38 3.92 13.97 2.73 绍兴 2021.11 4.90 2.90 5.62 2.06 杭州[11] 2006.03—04 22.2 6.37 18.4 — 长沙[20] 2014.12—2015.01 5.88 4.84 7.84 1.33 长沙[20] 2015.06—07 14.09 8.28 9.02 2.10 上海[32] 2007.01—10 19.4 15.9 11.9 — 武汉[19] 2017—2018 6.57 4.63 7.46 1.42 香港[31] 2013全年 5.2 2.8 — — 北京[6] 2008冬 4.8 6.0 11.6 — 广州[35] 2005.11 6.02 7.37 8.33 0.84 南宁[22] 2012.10—2012.07 6.79 15.81 5.43 0.43 日本,大阪[18] 2003.07—10 3.0—6.9 1.3—4.6 3.9—14 — 西安[9] 2009夏 7.92 3.7 6.41 2.14 西安[9] 2010冬 5.57 12.0 9.33 0.47 贵阳[21] 2008.12—2009.08 4.80 5.70 5.10 — 法国,奥尔良*[33] 2011.01 1.79 1.21 2.51 2.18 意大利,罗马[8] 2005.07—09 5.11 1.73 9.30 — “—”表示数据在相关引用中不可用;“*”表示原文中单位是ppbv,进行换算: 1μg⋅m−3=Mg/24.45ppbv 表 4 “五一”节假日前后天气条件和其它污染物浓度范围
Table 4. The concentration range of weather conditions and other pollutants before and after Labor Day
工作日(2021.04.28—30)Weekday 节假日(2021.05.01—05)Holiday 天气 晴、多云、雾 晴、多云 CO 0.4—1.2 mg∙m−3 0.4—0.9 mg∙m−3 NO2 14—55 μg∙m−3 10—82 μg∙m−3 O3 21—161 μg∙m−3 33—190 μg∙m−3 风速 0—7.152 m∙s−1 0.894—8.046 m∙s−1 -
[1] ZHANG Y N, XUE L K, DONG C, et al. Gaseous carbonyls in China's atmosphere: Tempo-spatial distributions, sources, photochemical formation, and impact on air quality [J]. Atmospheric Environment, 2019, 214: 116863. doi: 10.1016/j.atmosenv.2019.116863 [2] WANG T, XUE L K, BRIMBLECOMBE P, et al. Ozone pollution in China: A review of concentrations, meteorological influences, chemical precursors, and effects [J]. Science of the Total Environment, 2017, 575: 1582-1596. doi: 10.1016/j.scitotenv.2016.10.081 [3] LI L, CHEN C H, HUANG C, et al. Process analysis of regional ozone formation over the Yangtze River Delta, China using the Community Multi-scale Air Quality modeling system [J]. Atmospheric Chemistry and Physics, 2012, 12(22): 10971-10987. doi: 10.5194/acp-12-10971-2012 [4] FU T M, JACOB D J, WITTROCK F, et al. Global budgets of atmospheric glyoxal and methylglyoxal, and implications for formation of secondary organic aerosols [J]. Journal of Geophysical Research:Atmospheres, 2008, 113(D15): D15303. doi: 10.1029/2007JD009505 [5] LI J Y, MAO J Q, MIN K E, et al. Observational constraints on glyoxal production from isoprene oxidation and its contribution to organic aerosol over the Southeast United States [J]. Journal of Geophysical Research:Atmospheres, 2016, 121(16): 9849-9861. doi: 10.1002/2016JD025331 [6] ZHANG Y J, MU Y J, LIU J F, et al. Levels, sources and health risks of carbonyls and BTEX in the ambient air of Beijing, China [J]. Journal of Environmental Sciences, 2012, 24(1): 124-130. doi: 10.1016/S1001-0742(11)60735-3 [7] DUAN J C, TAN J H, YANG L, et al. Concentration, sources and ozone formation potential of volatile organic compounds (VOCs) during ozone episode in Beijing [J]. Atmospheric Research, 2008, 88(1): 25-35. doi: 10.1016/j.atmosres.2007.09.004 [8] POSSANZINI M, TAGLIACOZZO G, CECINATO A. Ambient levels and sources of lower carbonyls at montelibretti, Rome (Italy) [J]. Water, Air, and Soil Pollution, 2007, 183(1/2/3/4): 447-454. [9] DAI W T, HO S S H, HO K F, et al. Seasonal and diurnal variations of mono- and di-carbonyls in Xi'an, China [J]. Atmospheric Research, 2012, 113: 102-112. doi: 10.1016/j.atmosres.2012.05.001 [10] 李元昭, 李少华, 张成龙, 等. 北京市大兴区夏季大气中醛酮类化合物的污染水平、来源及影响 [J]. 环境化学, 2021, 40(7): 1999-2015. doi: 10.7524/j.issn.0254-6108.2020030103 LI Y Z, LI S H, ZHANG C L, et al. The pollution levels, sources and impact of atmospheric carbonyls in summer of Daxing District, Beijing [J]. Environmental Chemistry, 2021, 40(7): 1999-2015(in Chinese). doi: 10.7524/j.issn.0254-6108.2020030103
[11] WENG M L, ZHU L Z, YANG K, et al. Levels and health risks of carbonyl compounds in selected public places in Hangzhou, China [J]. Journal of Hazardous Materials, 2009, 164(2/3): 700-706. [12] LI K W, CHEN L H, YING F, et al. Meteorological and chemical impacts on ozone formation: A case study in Hangzhou, China [J]. Atmospheric Research, 2017, 196: 40-52. doi: 10.1016/j.atmosres.2017.06.003 [13] LI B W, HO S S H, QU L L, et al. Temporal and spatial discrepancies of VOCs in an industrial-dominant city in China during summertime [J]. Chemosphere, 2021, 264: 128536. doi: 10.1016/j.chemosphere.2020.128536 [14] SUN H S, LI J H, ZHANG Y Q, et al. Early Paleozoic tectonic reactivation of the Shaoxing-Jiangshan fault zone: Structural and geochronological constraints from the Chencai domain, South China [J]. Journal of Structural Geology, 2018, 110: 116-130. doi: 10.1016/j.jsg.2018.03.003 [15] EPA U. Compendium of methods for the determination of toxic organic compounds in ambient air[S]. 1999. [16] HO K F, SAI HANG HO S, CHENG Y, et al. Real-world emission factors of fifteen carbonyl compounds measured in a Hong Kong tunnel [J]. Atmospheric Environment, 2007, 41(8): 1747-1758. doi: 10.1016/j.atmosenv.2006.10.027 [17] PANG X B, MU Y J. Seasonal and diurnal variations of carbonyl compounds in Beijing ambient air [J]. Atmospheric Environment, 2006, 40(33): 6313-6320. doi: 10.1016/j.atmosenv.2006.05.044 [18] UEBORI M, IMAMURA K. Analysis of aliphatic and aromatic carbonyl compounds in ambient air by LC/MS/MS [J]. Analytical Sciences, 2004, 20(10): 1459-1462. doi: 10.2116/analsci.20.1459 [19] YANG Z, CHENG H R, WANG Z W, et al. Chemical characteristics of atmospheric carbonyl compounds and source identification of formaldehyde in Wuhan, Central China [J]. Atmospheric Research, 2019, 228: 95-106. doi: 10.1016/j.atmosres.2019.05.020 [20] JIANG Z H, ZHENG X, ZHAI H Q, et al. Seasonal and diurnal characteristics of carbonyls in the urban atmosphere of Changsha, a mountainous city in south-central China [J]. Environmental Pollution, 2019, 253: 259-267. doi: 10.1016/j.envpol.2019.06.127 [21] PANG X B, LEE X Q. Temporal variations of atmospheric carbonyls in urban ambient air and street canyons of a Mountainous City in Southwest China [J]. Atmospheric Environment, 2010, 44(17): 2098-2106. doi: 10.1016/j.atmosenv.2010.03.006 [22] GUO S J, CHEN M, TAN J H. Seasonal and diurnal characteristics of atmospheric carbonyls in Nanning, China [J]. Atmospheric Research, 2016, 169: 46-53. doi: 10.1016/j.atmosres.2015.09.028 [23] PANG X B, MU Y J, LEE X Q, et al. Influences of characteristic meteorological conditions on atmospheric carbonyls in Beijing, China [J]. Atmospheric Research, 2009, 93(4): 913-919. doi: 10.1016/j.atmosres.2009.05.001 [24] GALBALLY I E, ROY C R. Destruction of ozone at the earth's surface [J]. Quarterly Journal of the Royal Meteorological Society, 1980, 106(449): 599-620. doi: 10.1002/qj.49710644915 [25] KARLSSON P E, KLINGBERG J, ENGARDT M, et al. Past, present and future concentrations of ground-level ozone and potential impacts on ecosystems and human health in northern Europe [J]. Science of the Total Environment, 2017, 576: 22-35. doi: 10.1016/j.scitotenv.2016.10.061 [26] VAIRAVAMURTHY A, ROBERTS J M, NEWMAN L. Methods for determination of low molecular weight carbonyl compounds in the atmosphere: A review [J]. Atmospheric Environment. Part A. General Topics, 1992, 26(11): 1965-1993. doi: 10.1016/0960-1686(92)90083-W [27] 王楚涵, 张鑫, 吴鸣, 等. 沈阳市郊区环境空气中醛酮类化合物的污染特征与来源分析 [J]. 环境科学研究, 2020, 33(12): 2771-2784. doi: 10.13198/j.issn.1001-6929.2020.03.39 WANG C H, ZHANG X, WU M, et al. Pollution characterization and source analyses of carbonyls in the ambient air in a suburban area of Shenyang [J]. Research of Environmental Sciences, 2020, 33(12): 2771-2784(in Chinese). doi: 10.13198/j.issn.1001-6929.2020.03.39
[28] ATKINSON R. Atmospheric chemistry of VOCs and NOx [J]. Atmospheric Environment, 2000, 34(12/13/14): 2063-2101. [29] MELLOUKI A, WALLINGTON T J, CHEN J. Atmospheric chemistry of oxygenated volatile organic compounds: Impacts on air quality and climate [J]. Chemical Reviews, 2015, 115(10): 3984-4014. doi: 10.1021/cr500549n [30] SHEN H Q, LIU Y H, ZHAO M, et al. Significance of carbonyl compounds to photochemical ozone formation in a coastal city (Shantou) in Eastern China [J]. Science of the Total Environment, 2021, 764: 144031. doi: 10.1016/j.scitotenv.2020.144031 [31] LUI K H, HO S S H, LOUIE P K K, et al. Seasonal behavior of carbonyls and source characterization of formaldehyde (HCHO) in ambient air [J]. Atmospheric Environment, 2017, 152: 51-60. doi: 10.1016/j.atmosenv.2016.12.004 [32] HUANG J, FENG Y L, LI J, et al. Characteristics of carbonyl compounds in ambient air of Shanghai, China [J]. Journal of Atmospheric Chemistry, 2008, 61(1): 1-20. doi: 10.1007/s10874-009-9121-x [33] JIANG Z H, GROSSELIN B, DAËLE V, et al. Seasonal, diurnal and nocturnal variations of carbonyl compounds in the semi-urban environment of Orléans, France [J]. Journal of Environmental Sciences, 2016, 40: 84-91. doi: 10.1016/j.jes.2015.11.016 [34] SHEPSON P B, HASTIE D R, SCHIFF H I, et al. Atmospheric concentrations and temporal variations of C1–C3 carbonyl compounds at two rural sites in central Ontario [J]. Atmospheric Environment. Part A. General Topics, 1991, 25(9): 2001-2015. doi: 10.1016/0960-1686(91)90280-K [35] LÜ H, CAI Q Y, WEN S, et al. Carbonyl compounds in the ambient air of hazy days and clear days in Guangzhou, China [J]. Atmospheric Research, 2009, 94(3): 363-372. doi: 10.1016/j.atmosres.2009.06.014 [36] JI Y M, GAO Y P, LI G Y, et al. Theoretical study of the reaction mechanism and kinetics of low-molecular-weight atmospheric aldehydes (C1-C4) with NO2 [J]. Atmospheric Environment, 2012, 54: 288-295. doi: 10.1016/j.atmosenv.2012.02.040 [37] 史建武, 庞小兵, 白志鹏, 等. 天津及渤海大气中醛酮化合物的检测 [J]. 天津大学学报, 2011, 44(3): 233-241. doi: 10.3969/j.issn.0493-2137.2011.03.008 SHI J W, PANG X B, BAI Z P, et al. Measurement of carbonyl compounds in ambient air of Tianjin City and Bohai Sea [J]. Journal of Tianjin University, 2011, 44(3): 233-241(in Chinese). doi: 10.3969/j.issn.0493-2137.2011.03.008
[38] 国家质量监督检验检疫总局, 中国国家标准化管理委员会. 环境空气质量标准: GB 3095—2012[S]. 北京: 中国环境科学出版社, 2016. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of the People's Republic of China. Ambient air quality standard: GB 3095—2012[S]. Beijing: China Environment Science Press, 2016(in Chinese).
[39] ATKINSON R, AREY J. Atmospheric degradation of volatile organic compounds[J]. ChemInform, 2003, 103(12): 4605–4638. [40] CARTER W P L. Development of ozone reactivity scales for volatile organic compounds [J]. Air & Waste, 1994, 44(7): 881-899. [41] GENG F H, ZHAO C S, TANG X, et al. Analysis of ozone and VOCs measured in Shanghai: A case study [J]. Atmospheric Environment, 2007, 41(5): 989-1001. doi: 10.1016/j.atmosenv.2006.09.023 期刊类型引用(4)
1. 龚世飞,肖能武,丁武汉,郭元平,叶青松,王巍,李虎. 丹江口水库核心水源区化肥施用分布特征及其环境风险评价. 长江流域资源与环境. 2022(10): 2259-2271 .  百度学术
百度学术
2. 龚世飞,丁武汉,居学海,肖能武,叶青松,黄进,李虎. 典型农业小流域面源污染源解析与控制策略——以丹江口水源涵养区为例. 中国农业科学. 2021(18): 3919-3931 .  百度学术
百度学术
3. 王超,张洪,雷俊山,贾海燕,雷沛,尹炜. 南水北调中线水源地陡坡型库岸生态屏障构建. 环境工程学报. 2020(12): 3243-3250 .  本站查看
本站查看
4. 龚世飞,丁武汉,肖能武,郭元平,叶青松,王巍,李虎. 丹江口水库核心水源区典型流域农业面源污染特征. 农业环境科学学报. 2019(12): 2816-2825 .  百度学术
百度学术
其他类型引用(1)
-






 下载:
下载: