-
全氟和多氟烷基化合物(per- and polyfluoroalkyl substances,PFASs)是一类人工合成的化合物,具有优良的表面活性、化学稳定性和疏油疏水等特性。全氟辛酸(perfluorooctanoic acid,PFOA)和全氟辛磺酸(perfluorooctane sulfonate,PFOS)作为典型的PFASs,已广泛应用于消费品生产和工业生产过程中[1-2]。由于具有环境持久性、生物累积性和潜在的生物毒性,PFOS和PFOA及其盐类先后被列入《关于持久性有机污染物的斯德哥尔摩公约》的受限制名单[3]。随着PFOA和PFOS在各行业的消减与淘汰,大量工业替代品被开发并投入使用[4],例如,氯代多氟醚基磺酸(chlorinated polyfluorinated ether sulfonates,Cl-PFESAs)[5]、多氟调聚磺酸/羧酸(fluorotelomer sulfonic/carboxylic acids,FTSs/FTCAs)[6]、六氟环氧丙烷多聚酸(hexafluoropropylene oxide dimer/trimer acid,HFPO-DA/TA)[7]和全氟壬烯氧基苯磺酸钠(sodium p-perfluorous nonenoxybenzene sulfonate,OBS)[8]等。然而,这些新型PFASs在分子结构和化学性质上与PFOA和PFOS相似,大量使用可能导致同样的环境问题和健康风险。目前已在水体、沉积物和土壤等环境介质中检测到上述新型PFASs [9-12]。
昆虫作为环境污染物指示性物种,利用其监测这些典型和新型PFASs已经受到越来越多的关注。例如,水生昆虫(如蜻蜓幼虫)被用于监测加拿大和坦桑尼亚河流中PFASs的污染水平[13-14],而蜻蜓成虫也用于监测南非地区典型PFASs的时空分布[15]。Koch 等[16-17]研究证明羽化的水生昆虫(如蚊、蜻蜓等)作为重要载体将PFASs从水生生态系统传输到陆地生态系统。Lan等[18]在蝗虫体内检测到多种新型PFASs(如6:2 FTS、6:2 和8:2 Cl-PFESAs),认为蝗虫可作为评估农田生态系统中PFASs的暴露风险的指示性生物。也有研究发现蜱虫可作为“哨兵”监测陆生生态系统中PFASs的污染特征[19]。然而,对于昆虫体内PFASs的检测方法并不一致。Velesia等[15]采用先碱消解后离子对萃取再通过Oasis HLB和Oasis MCX固相萃取小柱(SPE)进行净化的方法处理昆虫样品。Koch 等[20]采用氢氧化钠/甲醇(NaOH/MeOH)超声萃取和Oasis WAX 柱净化的方法。而De Solla等[13]使用一种分散固相萃取吸附剂(dSPE,Envi-Carb)对昆虫样品进行净化。另外,Lan等[18]将PFASs分为中长链(C≥4)和超短链(C2—C3),中长链PFASs采用离子对萃取和GCB-Carbon柱净化的方法,超短链 PFASs则是采用NaOH/MeOH超声萃取和poly-sery PWAX柱净化的方法。
相比于上述使用的SPE净化方法,QuEChERS样品净化方法具有成本低、操作便捷、目标物损失少和回收率高等优点。目前已广泛运用于生物组织(鱼肉、肝脏等)[21-23]和食品(牛奶、鸡蛋等)[24-26]中PFASs的快速检测,但还未见采用QuEChERS前处理方法检测昆虫体内PFASs的报道。与其他动物组织样本不同,昆虫样品在提取和净化过程中的主要干扰物质是蛋白质、不饱和脂肪酸和色素。另外,文献报道的目标化合物多以传统的PFOA和PFOS为主,缺乏对新型PFASs的关注。因此,本研究优化了多种新型PFASs的UPLC-MS/MS仪器参数,同时比较了不同萃取剂对昆虫样品中PFASs提取效率的影响,以及不同dSPE的使用对昆虫样品净化效果的影响,最终确定了一套昆虫样品中35种传统和新型PFASs的检测方法。
-
超高效液相色谱-串联质谱仪(Agilent Technologies 1290 Infinity-6460 Triple Quad,美国Agilent公司),高速冷冻离心机(H1850R,湖南湘仪),数控超声波提取仪(KQ-600DE,昆山舒美),干式氮吹仪(EFAA-AC-02,上海安谱),多管涡旋混合仪(EOAA-HM-01,上海安谱),恒温培养振荡器(ZWYR-240,上海智城),真空冷冻干燥仪(FD-1C-50,北京博医康),微量分析天平(AUY220,日本岛津)。
全氟丁酸(PFBA)、全氟戊酸(PFPeA)、全氟己酸(PFHxA)、全氟庚酸(PFHpA)、PFOA、全氟壬酸(PFNA)、全氟葵酸(PFDA)、全氟十一烷酸(PFUnDA)、全氟十二烷酸(PFDoDA)、全氟丁烷磺酸(PFBS)、全氟戊烷磺酸(PFPeS)、全氟己烷磺酸(PFHxS)、全氟庚烷磺酸(PFHpS)、PFOS、全氟辛基磺酰胺(FOSA-I)、全氟-4-(五氟乙基)环己烷磺酸(PFECHS)、全氟-3-氧杂戊烷磺酸(PFEESA)、全氟-3,6-二氧杂庚酸(3,6-OPFHpA)、全氟-3-甲氧基丙酸(PF4OPeA)、全氟-4-甲氧基丁酸(PF5OHxA)、3H-全氟-3-(3-甲氧基丙氧基)丙酸(ADONA)、HFPO-DA、6:2和8:2 Cl-PFESAs、6:2、8:2和10:2 FTCAs、4:2、6:2和8:2 FTSs、多氟调聚磷酸二酯(6:2、6:2/8:2和8:2 diPAPs)和同位素标记的PFASs(包括13C4-PFBA、13C2-PFHxA、13C4-PFOA、13C5-PFNA、13C2-PFDA、13C2-PFUnDA、13C2-PFDoDA、13C3-PFBS、18O2-PFHxS、13C4-PFOS、13C3-HFPO-DA、13C2-6:2 FTCA、13C2-8:2 FTCA、13C2-10:2 FTCA、13C2-4:2 FTS、13C2-6:2 FTS、13C2-8:2 FTS、13C4-6:2 diPAP、13C4-8:2 diPAP)均购自加拿大Wellington Laboratories(纯度大于98%); HFPO-TA购自加拿大Toronto Research Chemicals(≥ 95%);OBS购自广东滃江化学试剂有限公司(≥ 98%); MeOH(色谱级),乙腈(ACN,色谱级)和甲基叔丁基醚(MTBE,色谱级)均购自德国Merck公司;四丁基硫酸氢氨(TBAH,≥ 98%),乙酸铵(NH4Ac,优级纯),无水碳酸钠(Na2CO3,优级纯),碳酸氢钠(NaHCO3,优级纯)和NaOH(优级纯)均购自上海阿拉丁生化科技有限公司。浓盐酸(HCL,分析纯)购自中国医药集团。两种分散固相萃取吸附剂:Supelclean Envi-Carb(120-400目,100 m2·g−1)和Discovery DSC-18(C18,50 μm,480 m2·g−1)购自美国Supelco公司。
-
2021年7月采集到3个目的5种昆虫,样品采集后立即进行分类鉴定,按生境可分为水生昆虫(蜻蜓成虫和幼虫)和陆生昆虫(蝗虫、蝼蛄、蟋蟀和蝴蝶)。其中,蜻蜓幼虫、蝼蛄、蟋蟀和部分蝗虫样品购买自山东某昆虫市场,蜻蜓、蝴蝶和部分蝗虫样品采集自江西某电镀工业园区下游水库及周边农田。
-
所有昆虫样品用超纯水清洗干净后,再冷冻干燥48 h后研磨成粉末。
TBAH/MTBE离子对萃取法:称取0.1 g干重(dw)昆虫粉末于15 mL聚丙烯(PP)管中,加入2 mL 0.25 mol·L−1碳酸钠缓冲溶液(pH = 10)和1 mL 0.5 mol·L−1 TBAH,再加入3 ng 内标(IS),混匀后加入5 mL MTBE,振荡(300 r·min−1)提取20 min。在4 ℃下12000 r·min−1,离心5 min,取上清液于干净的15 mL PP管中。原PP管中加入10 mL MTBE,进行多次萃取,将上清液氮吹至4 mL,再加入0.1 g Envi-Carb和0.05 g C18,涡旋1 min以12000 r·min−1,离心7 min后取上清。上清液氮吹至净干后,用70%的MeOH/水定容至300 μL,以15000 r·min−1离心15 min,取上清液到进样瓶中待测。
有机溶剂萃取法:MeOH萃取时,样品加入5 mL MeOH和IS后震荡提取过夜(避光),离心取上清液后,再加入5 mL MeOH重复萃取两次; 10 mmol·L−1 NaOH/MeOH萃取时,样品加入5 mL萃取液和IS后震荡提取过夜(避光),离心取上清液加入 0.05 mL的2 mol·L−1 HCL调节pH,再加入5 mL MeOH重复萃取两次;0.1% HCL/ACN萃取时,样品加入5 mL萃取液和IS后超声萃取20 min,再震荡提取过夜(避光),上清液取出后再加入5 mL ACN重复萃取两次。有机溶剂萃取法后续的净化、氮吹、定容等步骤与TBAH/MTBE离子对萃取法相同。
-
样品中35种PFASs的定性和定量分析采用超高效液相色谱-质谱联用仪(UPLC-MS/MS)。
色谱条件:色谱柱为Agilent Eclipse Plus C18(3.0 mm × 50 mm,1.8 µm);柱温为40 ℃;进样体积为5 µL;流速为0.3 mL·min−1;流动相A为2 mmol·L−1 NH4Ac,流动相B为ACN;洗脱梯度为0—7 min,70%—30% A;7—9 min,30%—10% A;9—10 min,10%—70% A。进样时间为10 min,后置保留时间为2 min。
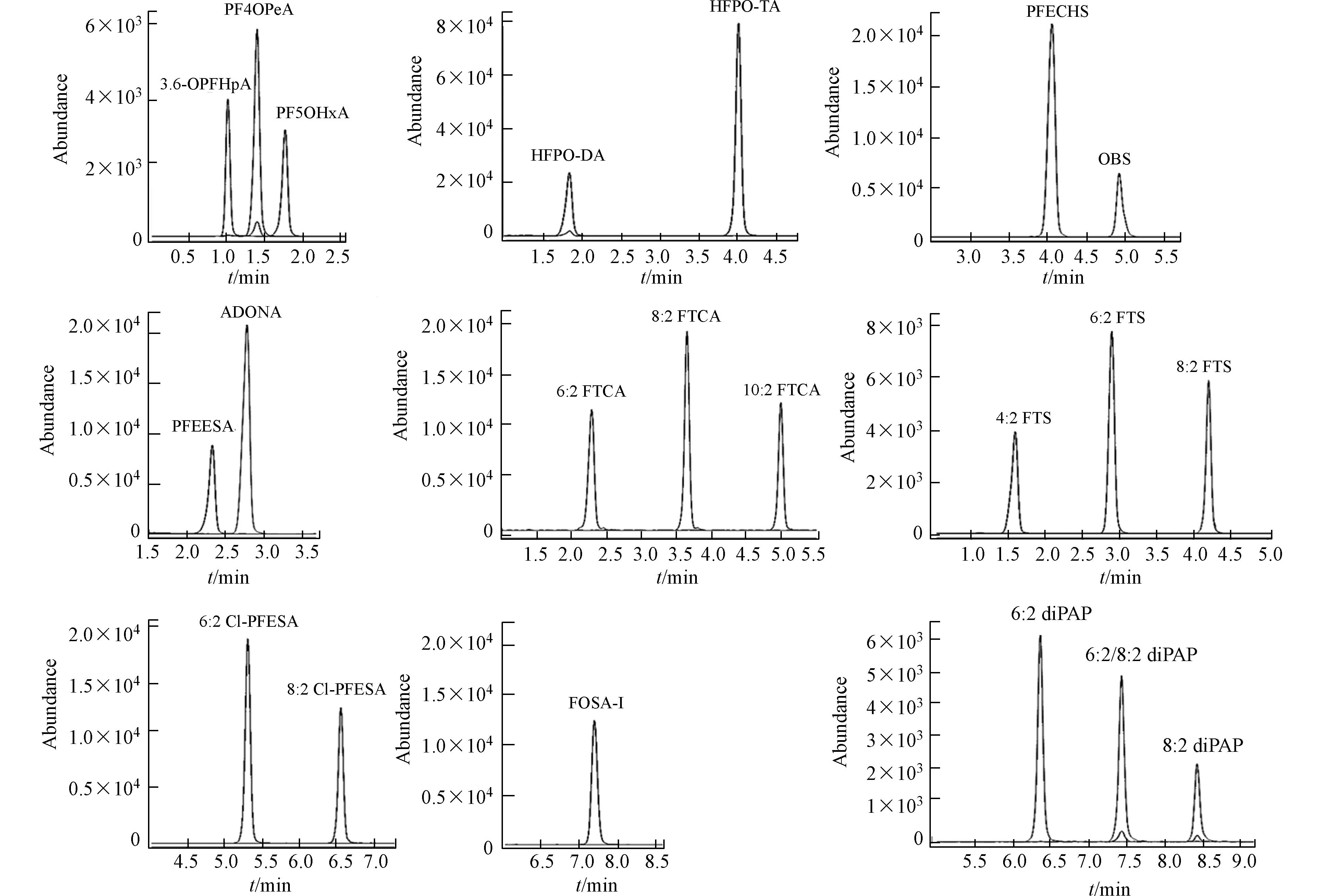
质谱条件:采用电喷雾负离子源(ESI−)和多反应检测(MRM)模式;离子源温度:300 ℃;毛细管电压:3500 V;加电压(EMV):400 V;干燥气流速:6.0 mL·min−1,干燥气温度:325 ℃,雾化气电压:30 psi。各单体PFASs的质谱参数见表1,21种新型PFASs的MRM图见图1。
-
采用美国Agilent Technologies公司MassHunter B.07软件进行定量分析,Origin 8.5软件进行作图和数据处理。
-
采用 70% MeOH/水溶液配制35种PFASs和19种IS的混合标准液,浓度范围为0.20、0.50、1、2、5、10、20、50、100 ng·mL−1。通过内标法定量,IS浓度为10 ng·mL−1。根据优化的UPLC-MS/MS质谱分析方法得到标准曲线,详细信息如表2所示。结果表明,35 种PFASs均在0.20—100 ng·mL−1范围内线性良好,相关系数(R2)均在0.999以上。仪器检出限(LOD)和方法定量限(LOQ)分别通过3倍和10倍的信噪比(S/N)计算得到,目标化合物的LOD和LOQ值范围分别为0.0105—0.0329 ng·mL−1和0.0314—0.0988 ng·g−1 dw ,与前人报道的方法相当[23, 25]。
-
通过加标实验来优化昆虫样品的前处理方法,即在空白样品和昆虫样品中添加10 ng·mL−1水平的目标PFASs标准品,每种方法重复实验3 次,计算不同方法的加标回收率。
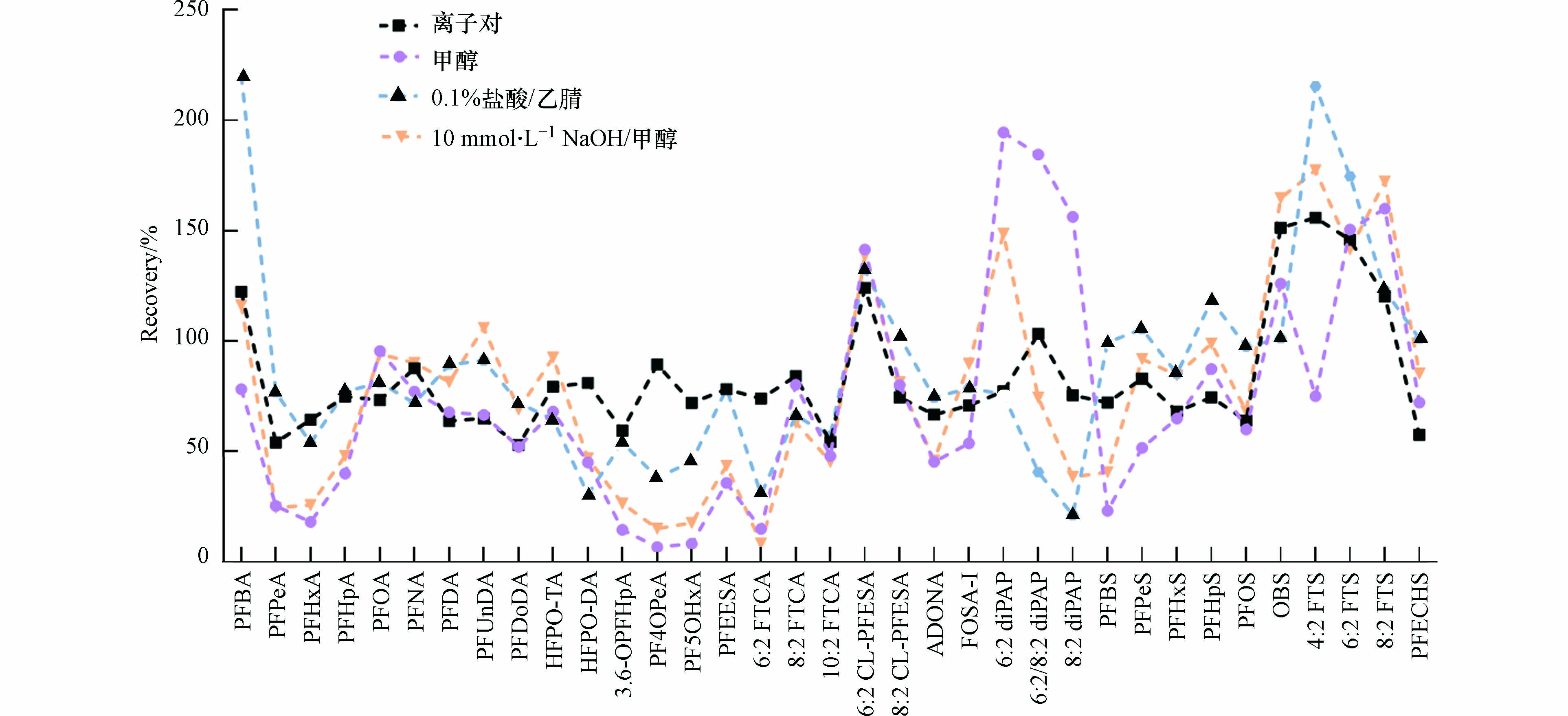
首先对PFASs的萃取方法进行优化,本实验比较了4种方法(MeOH、10 mmol·L−1 NaOH/MeOH、0.1% HCL/ACN和TBAH/MTBE离子对萃取)对目标化合物回收率的影响,如图2所示。结果表明,MeOH、10 mmol·L−1 NaOH/MeOH和0.1% HCL/ACN萃取方法中PFASs回收率的波动较大,其中MeOH为7%—195%,10 mmol·L−1 NaOH/MeOH为8%—177%,0.1% HCL/ACN为21%—220%。MeOH和10 mmol·L−1 NaOH/MeOH萃取的PFPeA、PFHxA、3.6-OPFHpA、PF4OPeA、PF5OHxA等短链PFASs回收率过低(7%—26%),而6:2 diPAP、6:2/8:2 diPAP、8:2FTS和OBS等长链PFASs回收率过高(160%—195%)。0.1% HCL/ACN萃取方法中,PFBA和4:2 FTS的回收率分别高达220%和215%,而HFPO-DA、6:2 FTCA和8:2 diPAP的回收率仅为31%、30%和21%。TBAH/MTBE离子对萃取方法对PFASs回收率的影响相对较小(53%—155%),仅4:2 FTS和OBS的回收率略高于150%,这可能是由于基质效应。对比不同方法可以看出,TBAH/MTBE离子对萃取对PFASs回收率的影响整体较小,因此选择该方法提取昆虫样品中的PFASs。TBAH/MTBE离子对萃取的方法也常用于肝脏等生物组织样品中PFASs的提取[27-28]。另外,从图2可以看出,4种萃取方法分析的6:2 Cl-PFESA、OBS和FTSs等目标化合物的回收率偏高,说明存在基质干扰,需进一步优化昆虫样品的QuEChERS净化方法。
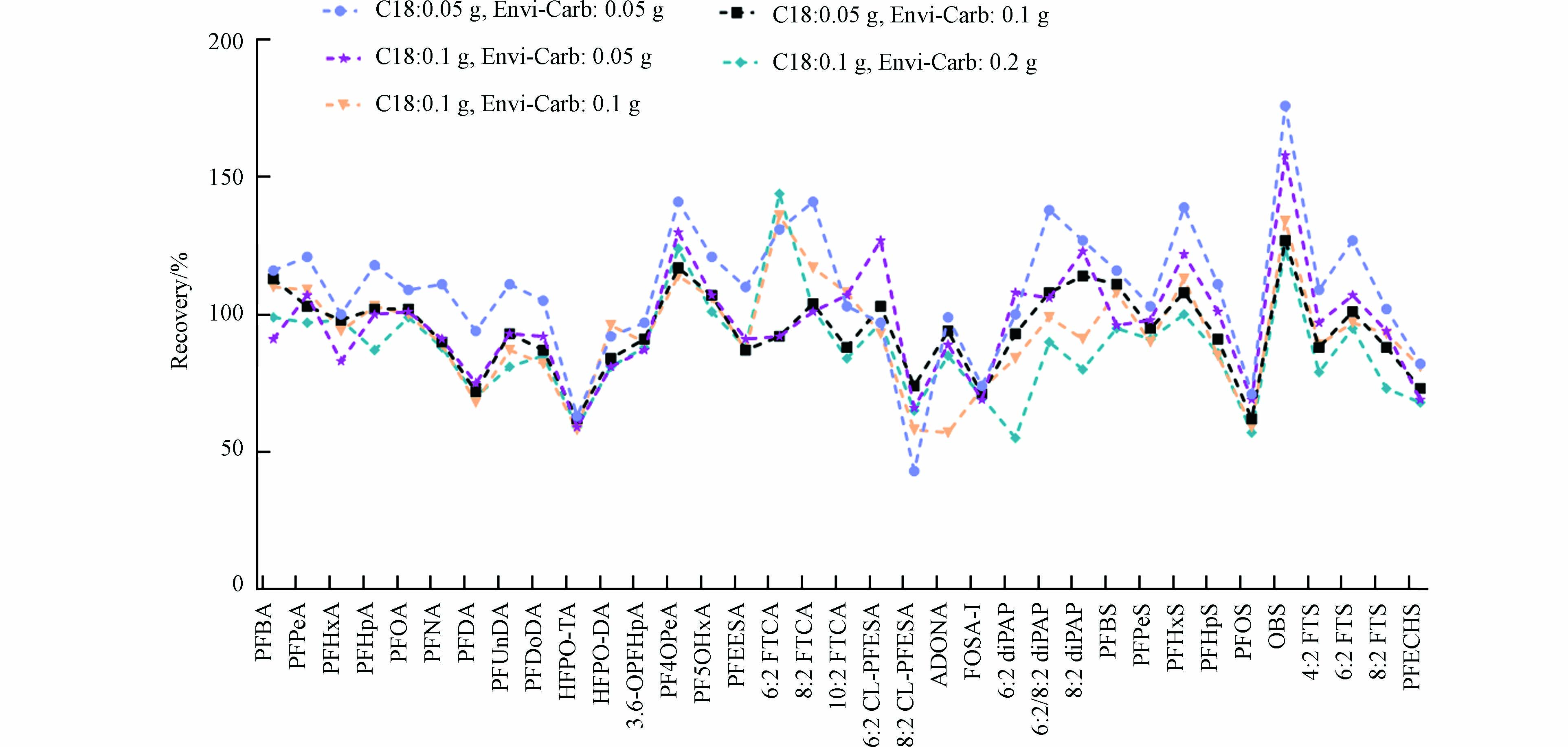
本研究主要采用的dSPE吸附剂为Envi-Carb和C18,其中Envi-Carb常用的去除极性共萃物,如叶绿素和固醇类[29];C18则主要吸附非极性共萃物,如三酰甘油和其他亲脂性化合物[30]。N-丙基乙二胺(PSA)也常用于QuEChERS共萃物的净化,但是PSA作为弱阴离子交换吸附剂对酸性的PFASs具有一定的吸附作用[25]。已有研究也发现PSA的用量超过0.1 g时会吸附部分PFASs[23],因此在本研究中未使用PSA作吸附剂。对于昆虫样品来说,萃取液中含有大量蛋白质、脂肪和色素等共萃物,单独使用C18或Envi-Carb效果并不好,因此同时添加C18和Envi-Carb来净化样品。本实验比较了0.05 g C18 + 0.05 g Envi-Carb、0.1 g C18 + 0.05 g Envi-Carb、0.05 g C18 + 0.1 g Envi-Carb、0.1 g C18 + 0.1 g Envi-Carb和0.1 g C18 + 0.2 g Envi-Carb对PFASs回收率的影响。图3中,当C18为0.05 g时,Envi-Carb质量过少导致PFASs回收率偏高(大部分目标化合物回收率超过100%);当Envi-Carb为0.1 g时,C18的增加导致一些新型PFASs回收率降低。总的来说,0.05 g C18+0.1 g Envi-Carb的组合对PFASs的影响最小,回收率范围在62%—128%,因此选择该组合对昆虫样品萃取液进行净化。优化后的QuEChERS净化方法不仅能保证回收率还能有效去除溶液中的蛋白质、脂肪和色素等干扰物质,使昆虫样品达到仪器检测要求。
-
通过不同浓度的加标实验来保证监测实际昆虫样品中PFASs的适用性,即称取0.1 g dw水生昆虫(蜻蜓幼虫)和陆生昆虫(蝗虫)样品,分别加入低、中、高三个浓度水平(定容浓度为 1、10、00 ng·mL−1)的混合PFASs标准品以及IS(10 ng·mL−1)。每个加标浓度重复做3个平行样品,分别计算水生昆虫和陆生昆虫中PFASs的回收率和相对标准偏差(relative standard deviation,RSD)。结果如表3所示,水生昆虫和陆生昆虫中35种PFASs的回收率分别为70%—126%和64%—115%,RSD分别为1.1%—20%和0.15%—24%,说明该方法能满足对实际昆虫样品的定量分析。
-
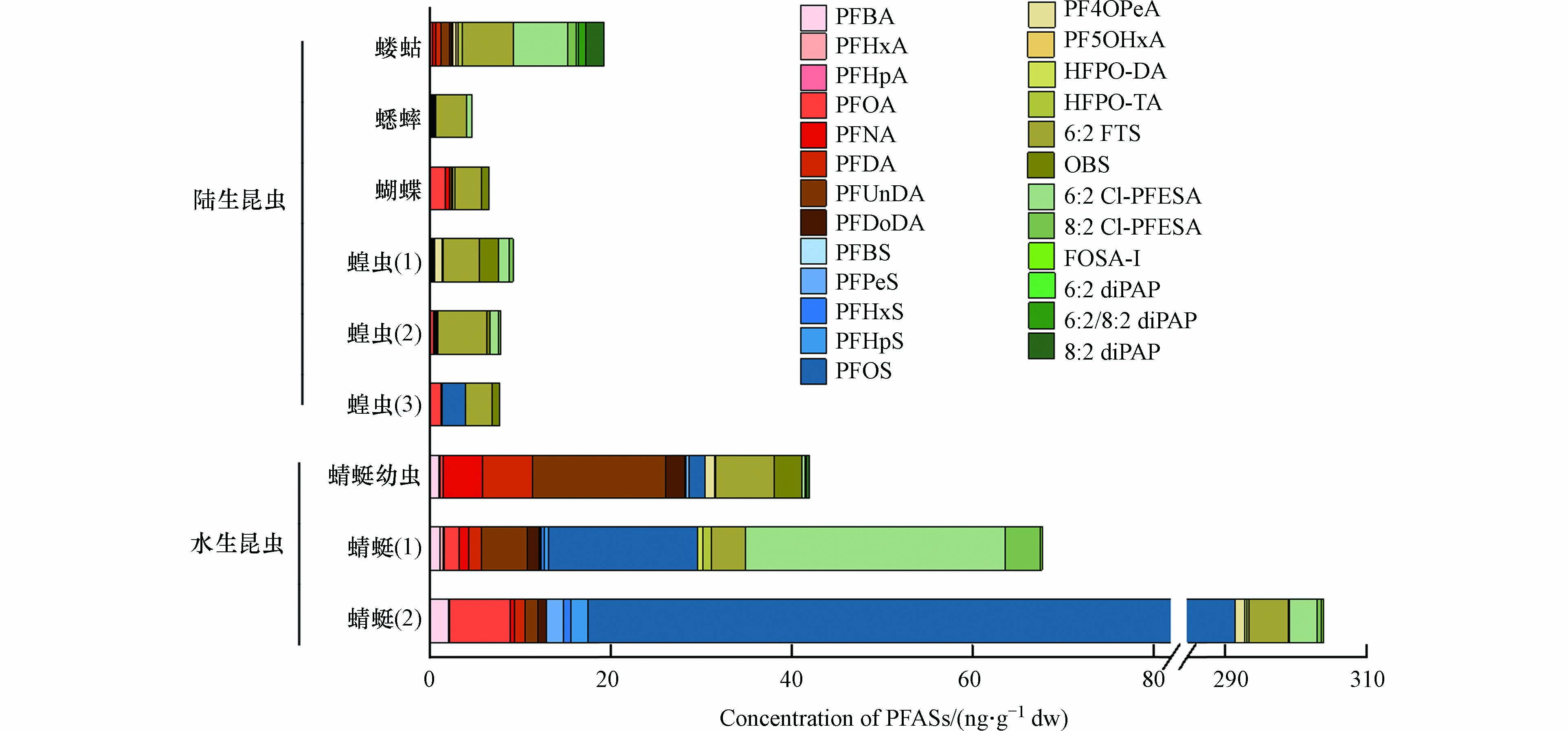
本研究的昆虫包括3个目的5种昆虫,分别为蜻蜓目(蜻蜓幼虫和成虫),直翅目(蝗虫,蝼蛄,蟋蟀)和鳞翅目(蝴蝶),按生境可分为水生昆虫和陆生昆虫。如图4所示,水生昆虫和陆生昆虫体内PFASs的浓度和组成差异明显。水生昆虫中ƩPFASs浓度(42—304 ng·g−1 dw)明显高于陆生昆虫(4.7—19 ng·g−1 dw),这与Koch等[16] 的报道结果一致,主要是由于PFASs通常随污水排放到水环境中[20]。从组成上看,水生昆虫以传统的PFCAs(如PFOA、PFNA、PFDA 和PFUnDA)和PFOS为主,而陆生昆虫主要是以新型PFASs(如6:2 FTS、OBS、6:2和8:2 Cl-PFESAs)为主。值得注意的是,在蜻蜓(1)和蝼蛄中浓度最高的PFASs是6:2 Cl-PFESA,说明6:2 Cl-PFESA作为PFOS替代品在我国电镀行业中被广泛使用。另外,6:2 FTS在陆生昆虫体内的浓度均高于PFOS,而OBS和HFPO-DA/TA也在大部分昆虫样品(除了蟋蟀)中检出,这说明近年来这些新型替代品已经在我国广泛生产和应用。而6:2、6:2/8:2 和8:2 diPAPs仅在蜻蜓幼虫和蝼蛄体内检出,说明其使用量在我国还较小。
在工业园区采集的蜻蜓(1)和蜻蜓(2)中ƩPFASs浓度分别为68 ng·g−1 dw和304 ng·g−1 dw,与加拿大某国际机场下游采集的蜻蜓体内浓度相当(180 ng·g−1 ww)[13],远高于南非未受污染区域的蜻蜓中7种典型PFASs的浓度(0.17—21 ng·g−1 ww)[15],但是低于瑞典某军用机场下游采集的蜻蜓中PFASs的浓度(120—3500 ng·g−1 dw)[16]。而在山东昆虫市场购买的蜻蜓幼虫中ƩPFASs浓度(42 ng·g−1 dw)与坦桑尼亚采集的蜻蜓幼虫中污染水平相当(6.1—24 ng·g−1 ww)[14]。对于陆生昆虫,蝼蛄中ƩPFASs的浓度最高为19 ng·g−1 dw,其次为蝗虫((8.3 ± 0.65) ng·g−1 dw)和蝴蝶(6.6 ng·g−1 dw),蟋蟀浓度最低(4.7 ng·g−1 dw),这可能与其食性相关,蝼蛄为杂食性昆虫,而其他3种陆生昆虫为植食性昆虫。另外,蝗虫体内PFASs的浓度略高于环北京-天津-河北地区农田中采集的蝗虫中PFASs的浓度(3.7 ± 1.8) ng·g−1 dw)[18]。对于其他陆生昆虫,目前并未见到相关PFASs的报道。
-
本研究建立了一套QuEChERS结合超高效液相色谱-串联质谱法测定昆虫体内的35种典型和新型PFASs,具有快速、高效、便捷、经济、准确等优点。采用该方法对实际昆虫样品进行分析,结果表明水生昆虫中PFASs浓度(42—304 ng·g−1 dw)明显高于陆生昆虫(4.7—19 ng·g−1 dw)。陆生昆虫PFASs的组成以新型PFASs为主,主要是6:2 FTS、6:2 Cl-PFESA 和OBS;而水生昆虫则以传统PFASs为主,如PFOA、PFNA、PFDA 、PFUnDA和PFOS。
QuEChERS-超高效液相色谱-串联质谱法测定昆虫体内35种全氟和多氟烷基化合物
Determination of 35 per- and polyfluoroalkyl substances (PFASs) in insect by QuEChERS pretreatment combined with ultra-performance liquid chromatography-tandem mass spectrometry
-
摘要: 昆虫作为常见的指示生物,已被用于监测环境中全氟和多氟烷基化合物(PFASs)的污染特征。本文基于QuEChERS前处理方法结合超高效液相色谱-串联质谱(UPLC-MS/MS),建立了昆虫中35种PFASs的检测方法,包括9种全氟羧酸(PFCAs)、5 种全氟磺酸(PFSAs)、3种多氟调聚羧酸(FTCAs)、3种多氟调聚磺酸(FTSs)、3种多氟调聚磷酸二酯(diPAPs)、2种氯代多氟醚基磺酸(Cl-PFESAs)、7种全氟聚醚羧酸(PFECAs)以及全氟辛基磺酰胺(FOSA)、全氟-4-(五氟乙基)环己烷磺酸(PFECHS)和全氟壬烯氧基苯磺酸钠(OBS)。昆虫样品采用离子对萃取,0.1 g Envi-Carb和0.05 g DSC-18为吸附剂的前处理方法,在多反应负离子监测模式下进行PFASs检测,内标法定量。结果表明,35种PFASs的回收率在62%—128%,方法定量限为0.0314—0.0876 ng·g−1 dw,标准曲线线性关系良好(R2 > 0.999)。利用本方法对3个目的5种昆虫进行测定,结果表明水生昆虫中ƩPFASs浓度(42—304 ng·g−1 dw)明显高于陆生昆虫(4.7—19 ng·g−1 dw)。水生昆虫中污染物以长链PFCAs(PFOA、PFNA、PFDA和PFUnDA)和PFOS为主,而陆生昆虫以新型PFASs(6:2 FTS、OBS和Cl-PFESAs)为主。本研究建立的方法具有经济、便捷、高效、准确、灵敏等优点,可同时监测不同种类昆虫体内多种典型和新型PFASs。
-
关键词:
- 全氟和多氟烷基化合物 /
- 昆虫 /
- QuEChERS /
- 超高效液相色谱-串联质谱 /
- 工业园
Abstract: Insects as common indicator species have been used to monitor the occurrence of per- and polyfluoroalkyl substances (PFASs) in the environment. In this study, a method for the detection of 35 PFASs in insects was established based on QuEChERS pretreatment method combined with ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). These PFASs include 9 perfluoroalkyl carboxylic acids (PFCAs), 5 perfluoroalkyl sulfonic acids (PFSAs), 3 fluorotelomer carboxylic acids (FTCAs), 3 fluorotelomer sulfonates (FTSs), 3 polyfluoroalkyl phosphoric acid diester (diPAPs), 2 chlorinated polyfluorinated ether sulfonic acids (Cl-PFESAs), 7 perfluoroalkyl ether carboxylic acids (PFECAs), perfluorooctane sulfonamide (FOSA), perfluoro-4-ethylcyclohexane sulfonate (PFECHS) and sodium p-perfluorous nonenoxybenzene sulfonate (OBS). The insect samples were pretreated by ion-pair liquid-liquid extraction, and purified with 0.1g ENVI-Carb and 0.05 g DSC-18 as adsorbent. The target PFASs were detected by UPLC-MS/MS in the negative electrospray ionization mode with multiple reaction monitoring, and quantified by internal standard. The results showed that the recoveries of PFASs ranged from 62% to 128%, and the method limits of quantitation were 0.0314—0.0876 ng·g−1 dw. The standard curves of individual PFASs have good linear correlation (R2 > 0.999). The levels of PFASs in 5 species of insects from 3 orders were determined by the optimized methods. The concentrations of ƩPFASs in aquatic insects (42—304 ng·g−1 dw) were obviously higher than that in terrestrial insects (4.7—19 ng·g−1 dw). Long-chain PFCAs (PFOA, PFNA, PFDA and PFUnDA) and PFOS were predominated PFASs in aquatic insects, while emerging PFASs (6:2 FTS, OBS and Cl-PFESAs) were dominant in terrestrial insects. The method established in this study has the advantages of economy, convenience, efficiency, accuracy and sensitivity, and can be used to monitor legacy and emerging PFASs in different species of insects.-
Key words:
- PFASs /
- insect /
- QuEChERS /
- UPLC-MS/MS /
- industrial park
-
全氟和多氟烷基化合物(per- and polyfluoroalkyl substances,PFASs)是一类人工合成的化合物,具有优良的表面活性、化学稳定性和疏油疏水等特性。全氟辛酸(perfluorooctanoic acid,PFOA)和全氟辛磺酸(perfluorooctane sulfonate,PFOS)作为典型的PFASs,已广泛应用于消费品生产和工业生产过程中[1-2]。由于具有环境持久性、生物累积性和潜在的生物毒性,PFOS和PFOA及其盐类先后被列入《关于持久性有机污染物的斯德哥尔摩公约》的受限制名单[3]。随着PFOA和PFOS在各行业的消减与淘汰,大量工业替代品被开发并投入使用[4],例如,氯代多氟醚基磺酸(chlorinated polyfluorinated ether sulfonates,Cl-PFESAs)[5]、多氟调聚磺酸/羧酸(fluorotelomer sulfonic/carboxylic acids,FTSs/FTCAs)[6]、六氟环氧丙烷多聚酸(hexafluoropropylene oxide dimer/trimer acid,HFPO-DA/TA)[7]和全氟壬烯氧基苯磺酸钠(sodium p-perfluorous nonenoxybenzene sulfonate,OBS)[8]等。然而,这些新型PFASs在分子结构和化学性质上与PFOA和PFOS相似,大量使用可能导致同样的环境问题和健康风险。目前已在水体、沉积物和土壤等环境介质中检测到上述新型PFASs [9-12]。
昆虫作为环境污染物指示性物种,利用其监测这些典型和新型PFASs已经受到越来越多的关注。例如,水生昆虫(如蜻蜓幼虫)被用于监测加拿大和坦桑尼亚河流中PFASs的污染水平[13-14],而蜻蜓成虫也用于监测南非地区典型PFASs的时空分布[15]。Koch 等[16-17]研究证明羽化的水生昆虫(如蚊、蜻蜓等)作为重要载体将PFASs从水生生态系统传输到陆地生态系统。Lan等[18]在蝗虫体内检测到多种新型PFASs(如6:2 FTS、6:2 和8:2 Cl-PFESAs),认为蝗虫可作为评估农田生态系统中PFASs的暴露风险的指示性生物。也有研究发现蜱虫可作为“哨兵”监测陆生生态系统中PFASs的污染特征[19]。然而,对于昆虫体内PFASs的检测方法并不一致。Velesia等[15]采用先碱消解后离子对萃取再通过Oasis HLB和Oasis MCX固相萃取小柱(SPE)进行净化的方法处理昆虫样品。Koch 等[20]采用氢氧化钠/甲醇(NaOH/MeOH)超声萃取和Oasis WAX 柱净化的方法。而De Solla等[13]使用一种分散固相萃取吸附剂(dSPE,Envi-Carb)对昆虫样品进行净化。另外,Lan等[18]将PFASs分为中长链(C≥4)和超短链(C2—C3),中长链PFASs采用离子对萃取和GCB-Carbon柱净化的方法,超短链 PFASs则是采用NaOH/MeOH超声萃取和poly-sery PWAX柱净化的方法。
相比于上述使用的SPE净化方法,QuEChERS样品净化方法具有成本低、操作便捷、目标物损失少和回收率高等优点。目前已广泛运用于生物组织(鱼肉、肝脏等)[21-23]和食品(牛奶、鸡蛋等)[24-26]中PFASs的快速检测,但还未见采用QuEChERS前处理方法检测昆虫体内PFASs的报道。与其他动物组织样本不同,昆虫样品在提取和净化过程中的主要干扰物质是蛋白质、不饱和脂肪酸和色素。另外,文献报道的目标化合物多以传统的PFOA和PFOS为主,缺乏对新型PFASs的关注。因此,本研究优化了多种新型PFASs的UPLC-MS/MS仪器参数,同时比较了不同萃取剂对昆虫样品中PFASs提取效率的影响,以及不同dSPE的使用对昆虫样品净化效果的影响,最终确定了一套昆虫样品中35种传统和新型PFASs的检测方法。
1. 实验部分(Experimental section)
1.1 仪器与试剂
超高效液相色谱-串联质谱仪(Agilent Technologies 1290 Infinity-6460 Triple Quad,美国Agilent公司),高速冷冻离心机(H1850R,湖南湘仪),数控超声波提取仪(KQ-600DE,昆山舒美),干式氮吹仪(EFAA-AC-02,上海安谱),多管涡旋混合仪(EOAA-HM-01,上海安谱),恒温培养振荡器(ZWYR-240,上海智城),真空冷冻干燥仪(FD-1C-50,北京博医康),微量分析天平(AUY220,日本岛津)。
全氟丁酸(PFBA)、全氟戊酸(PFPeA)、全氟己酸(PFHxA)、全氟庚酸(PFHpA)、PFOA、全氟壬酸(PFNA)、全氟葵酸(PFDA)、全氟十一烷酸(PFUnDA)、全氟十二烷酸(PFDoDA)、全氟丁烷磺酸(PFBS)、全氟戊烷磺酸(PFPeS)、全氟己烷磺酸(PFHxS)、全氟庚烷磺酸(PFHpS)、PFOS、全氟辛基磺酰胺(FOSA-I)、全氟-4-(五氟乙基)环己烷磺酸(PFECHS)、全氟-3-氧杂戊烷磺酸(PFEESA)、全氟-3,6-二氧杂庚酸(3,6-OPFHpA)、全氟-3-甲氧基丙酸(PF4OPeA)、全氟-4-甲氧基丁酸(PF5OHxA)、3H-全氟-3-(3-甲氧基丙氧基)丙酸(ADONA)、HFPO-DA、6:2和8:2 Cl-PFESAs、6:2、8:2和10:2 FTCAs、4:2、6:2和8:2 FTSs、多氟调聚磷酸二酯(6:2、6:2/8:2和8:2 diPAPs)和同位素标记的PFASs(包括13C4-PFBA、13C2-PFHxA、13C4-PFOA、13C5-PFNA、13C2-PFDA、13C2-PFUnDA、13C2-PFDoDA、13C3-PFBS、18O2-PFHxS、13C4-PFOS、13C3-HFPO-DA、13C2-6:2 FTCA、13C2-8:2 FTCA、13C2-10:2 FTCA、13C2-4:2 FTS、13C2-6:2 FTS、13C2-8:2 FTS、13C4-6:2 diPAP、13C4-8:2 diPAP)均购自加拿大Wellington Laboratories(纯度大于98%); HFPO-TA购自加拿大Toronto Research Chemicals(≥ 95%);OBS购自广东滃江化学试剂有限公司(≥ 98%); MeOH(色谱级),乙腈(ACN,色谱级)和甲基叔丁基醚(MTBE,色谱级)均购自德国Merck公司;四丁基硫酸氢氨(TBAH,≥ 98%),乙酸铵(NH4Ac,优级纯),无水碳酸钠(Na2CO3,优级纯),碳酸氢钠(NaHCO3,优级纯)和NaOH(优级纯)均购自上海阿拉丁生化科技有限公司。浓盐酸(HCL,分析纯)购自中国医药集团。两种分散固相萃取吸附剂:Supelclean Envi-Carb(120-400目,100 m2·g−1)和Discovery DSC-18(C18,50 μm,480 m2·g−1)购自美国Supelco公司。
1.2 实验方法
1.2.1 昆虫样品采集
2021年7月采集到3个目的5种昆虫,样品采集后立即进行分类鉴定,按生境可分为水生昆虫(蜻蜓成虫和幼虫)和陆生昆虫(蝗虫、蝼蛄、蟋蟀和蝴蝶)。其中,蜻蜓幼虫、蝼蛄、蟋蟀和部分蝗虫样品购买自山东某昆虫市场,蜻蜓、蝴蝶和部分蝗虫样品采集自江西某电镀工业园区下游水库及周边农田。
1.2.2 样品前处理
所有昆虫样品用超纯水清洗干净后,再冷冻干燥48 h后研磨成粉末。
TBAH/MTBE离子对萃取法:称取0.1 g干重(dw)昆虫粉末于15 mL聚丙烯(PP)管中,加入2 mL 0.25 mol·L−1碳酸钠缓冲溶液(pH = 10)和1 mL 0.5 mol·L−1 TBAH,再加入3 ng 内标(IS),混匀后加入5 mL MTBE,振荡(300 r·min−1)提取20 min。在4 ℃下12000 r·min−1,离心5 min,取上清液于干净的15 mL PP管中。原PP管中加入10 mL MTBE,进行多次萃取,将上清液氮吹至4 mL,再加入0.1 g Envi-Carb和0.05 g C18,涡旋1 min以12000 r·min−1,离心7 min后取上清。上清液氮吹至净干后,用70%的MeOH/水定容至300 μL,以15000 r·min−1离心15 min,取上清液到进样瓶中待测。
有机溶剂萃取法:MeOH萃取时,样品加入5 mL MeOH和IS后震荡提取过夜(避光),离心取上清液后,再加入5 mL MeOH重复萃取两次; 10 mmol·L−1 NaOH/MeOH萃取时,样品加入5 mL萃取液和IS后震荡提取过夜(避光),离心取上清液加入 0.05 mL的2 mol·L−1 HCL调节pH,再加入5 mL MeOH重复萃取两次;0.1% HCL/ACN萃取时,样品加入5 mL萃取液和IS后超声萃取20 min,再震荡提取过夜(避光),上清液取出后再加入5 mL ACN重复萃取两次。有机溶剂萃取法后续的净化、氮吹、定容等步骤与TBAH/MTBE离子对萃取法相同。
1.3 仪器分析
样品中35种PFASs的定性和定量分析采用超高效液相色谱-质谱联用仪(UPLC-MS/MS)。
色谱条件:色谱柱为Agilent Eclipse Plus C18(3.0 mm × 50 mm,1.8 µm);柱温为40 ℃;进样体积为5 µL;流速为0.3 mL·min−1;流动相A为2 mmol·L−1 NH4Ac,流动相B为ACN;洗脱梯度为0—7 min,70%—30% A;7—9 min,30%—10% A;9—10 min,10%—70% A。进样时间为10 min,后置保留时间为2 min。
质谱条件:采用电喷雾负离子源(ESI−)和多反应检测(MRM)模式;离子源温度:300 ℃;毛细管电压:3500 V;加电压(EMV):400 V;干燥气流速:6.0 mL·min−1,干燥气温度:325 ℃,雾化气电压:30 psi。各单体PFASs的质谱参数见表1,21种新型PFASs的MRM图见图1。
表 1 35种PFASs以及19种内标的质谱参数Table 1. Detailed mass spectrometry parameters of 35 individual PFASs and 19 isotopic internal standards简称Acronym 化合物全称Compound name 母离子Precursor Ion (m/z) 子离子Product Ion (m/z) 碎裂电压/VFragmentor 碰撞能/eVCollision Energy PFBA Perfluorobutanoic acid 212.8 168.8 60 6 PFPeA Perfluoropentanoic acid 262.9 218.8 80 5 PFHxA Perfluorohexanoic acid 312.9 268.9 80 5 PFHpA Perfluoroheptanoic acid 362.8 318.9 80 5 PFOA Perfluorooctanoic acid 412.9 368.9 90 5 PFNA Perfluorononanoic acid 462.8 418.9 90 5 PFDA Perfluorodecanoic acid 512.8 468.9 100 5 PFUnDA Perfluoroundecanoic acid 562.9 518.9 80 5 PFDoDA Perfluorododecanoic acid 612.9 568.9 100 10 PFBS Potassium perfluorobutane sulfonate 298.8 79.9 150 45 PFPeS Sodium perfluoropentane sulfonate 348.9 79.9 150 50 PFHxS Sodium perfluorohexane sulfonate 398.8 79.9 150 60 PFHpS Sodium perfluoroheptane sulfonate 448.9 79.9 150 60 PFOS Sodium perfluorooctane sulfonate 498.8 79.9 150 60 FOSA-I Perfluoro-1-octanesulfonamide 497.8 77.9 150 50 3.6-OPFHpA Perfluoro-3,6-dioxaheptanoic acid 201 84.9 80 10 PF4OPeA Perfluoro-4-oxapentanoic acid 228.9 84.9 50 10 PF5OHxA Perfluoro-5-oxahexanoic acid 278.9 84.9 50 12 ADONA 3H-perfluoro-3-(3-methoxy-propoxy)-propanoate acid 376.9 250.6 80 10 PFEESA Perfluoro (2-ethoxyethane) sulfonic acid 314.9 134.7 100 25 HFPO-DA Hexafluoropropylene oxide dimer acid 284.9 168.8 60 5 HFPO-TA Hexafluoropropylene oxide trimer acid 184.7 118.7 80 15 6:2 FTCA 6:2 fluorotelomer carboxylic acid 376.9 292.9 80 10 8:2 FTCA 8:2 fluorotelomer carboxylic acid 476.6 392.9 80 12 10:2 FTCA 10:2 fluorotelomer carboxylic acid 576.6 492.9 90 10 4:2 FTS 4:2 fluorotelomer sulfonate acid 326.9 306.7 150 20 6:2 FTS 6:2 fluorotelomer sulfonate acid 426.9 406.7 180 25 8:2 FTS 8:2 fluorotelomer sulfonate acid 526.9 506.7 200 30 6:2 Cl-PFESA 6:2 chlorinated polyfluorinated ether sulfonates 530.9 350.8 150 30 8:2 Cl-PFESA 8:2 chlorinated polyfluorinated ether sulfonates 630.9 450.8 150 35 OBS Sodium p-perfluorous nonenoxybenzene sulfonate 602.8 171.6 200 50 PFECHS Potassium perfluoro-4-ethylcyclohexanesulfonate 460.8 380.8 150 30 6:2 diPAP Sodium bis(1H,1H,2H,2H-perfluorooctyl) phosphate 788.7 96.7 195 40 6:2/8:2 diPAP Sodium bis(1H,1H,2H,2H-perfluorooctyl-1H,1H,2H,2H- perfluorodecyl) phosphate 888.7 96.7 200 50 8:2 diPAP Sodium bis(1H,1H,2H,2H-perfluorodecyl) phosphate 988.7 96.7 200 50 13C4-PFBA Perfluoro-(13C4)-butanoic acid 216.9 171.9 60 6 13C2-PFHxA Perfluoro-(13C2)-hexanoic acid 314.9 269.9 80 5 13C4-PFOA Perfluoro-(13C4)-octanoic acid 416.8 371.9 90 5 13C5-PFNA Perfluoro-(13C5)-nonanoic acid 467.9 422.9 90 5 13C2-PFDA Perfluoro-(13C2)-decanoic acid 514.9 469.9 100 5 13C2-PFUnDA Perfluoro-(13C2)-undecanoic acid 564.9 519.9 80 5 13C2-PFDoDA Perfluoro-(13C2)-dodecanoic acid 614.9 569.9 100 10 13C3-PFBS Perfluoro-(13C3)- butane sulfonate 301.8 79.9 150 45 18O2-PFHxS Perfluoro-(18O2)-hexane sulfonic acid 402.9 79.9 150 60 13C4-PFOS Perfluoro-(13C4)-octane sulfonic acid 502.8 79.9 150 60 13C3-HFPO-DA Hexafluoropropylene-(13C3)-dimer acid 286.9 168.8 60 5 13C2-6:2 FTCA 2-Perfluorohexyl-(13C2)-ethanoic acid 378.9 293.6 80 10 13C2-8:2 FTCA 2-Perfluorooctyl-(13C2)-ethanoic acid 478.6 393.9 80 12 13C2-10:2 FTCA 2-Perfluorodecyl-(13C2)-ethanoic acid 578.6 493.9 90 10 13C2-4:2 FTS 4:2 fluorotelomer-(13C2)-sulfonate 328.9 80.9 150 20 13C2-6:2 FTS 6:2 fluorotelomer-(13C2)-sulfonate 428.9 80.9 180 25 13C2-8:2 FTS 8:2 fluorotelomer-(13C2)-sulfonate 528.9 80.9 200 30 13C4-6:2 diPAP Sodium bis(1H,1H,2H,2H-[1,2-13C2]perfluorooctyl) phosphate 792.7 445.8 200 40 13C4-8:2 diPAP Sodium bis(1H,1H,2H,2H-[1,2-13C2]perfluorodecyl) phosphate 992.7 545.8 200 50 1.4 数据处理
采用美国Agilent Technologies公司MassHunter B.07软件进行定量分析,Origin 8.5软件进行作图和数据处理。
2. 结果与讨论(Results and discussion)
2.1 仪器检出限和方法定量限
采用 70% MeOH/水溶液配制35种PFASs和19种IS的混合标准液,浓度范围为0.20、0.50、1、2、5、10、20、50、100 ng·mL−1。通过内标法定量,IS浓度为10 ng·mL−1。根据优化的UPLC-MS/MS质谱分析方法得到标准曲线,详细信息如表2所示。结果表明,35 种PFASs均在0.20—100 ng·mL−1范围内线性良好,相关系数(R2)均在0.999以上。仪器检出限(LOD)和方法定量限(LOQ)分别通过3倍和10倍的信噪比(S/N)计算得到,目标化合物的LOD和LOQ值范围分别为0.0105—0.0329 ng·mL−1和0.0314—0.0988 ng·g−1 dw ,与前人报道的方法相当[23, 25]。
表 2 35种PFASs的线性方程、相关系数(R2)、仪器检出限和方法定量限Table 2. Linear equation, correlation coefficient (R2), limit of detection (LOD), and limit of quantitation (LOQ,) for 35 target PFASs目标化合物Target compound 内标Internal standard 线性方程Linear equation 相关系数(R2)Correlation coefficient 检出限/(ng·mL−1)LOD 定量限/(ng·g−1 dw)LOQ PFBA 13C4-PFBA y=0.026676x−0.017817 0.99951 0.0135 0.0405 PFPeA 13C4-PFBA y=0.015960x−0.004434 0.99979 0.0222 0.0665 PFHxA 13C2-PFHxA y=0.031886x−0.009241 0.99986 0.0199 0.0596 PFHpA 13C2-PFHxA y=0.034467x−0.009820 0.99989 0.0187 0.0561 PFOA 13C4-PFOA y=0.034039x−0.016462 0.99972 0.0198 0.0595 PFNA 13C5-PFNA y=0.030688x−0.007462 0.99992 0.0198 0.0595 PFDA 13C2-PFDA y=0.030758x−0.014403 0.99983 0.0176 0.0527 PFUnDA 13C2-PFUnDA y=0.035521x−0.035578 0.99992 0.0184 0.0553 PFDoDA 13C2-PFDoDA y=0.029815x−0.011676 0.99988 0.0166 0.0498 PFBS 13C3-PFBS y=0.043224x−0.001482 0.99980 0.0181 0.0542 PFPeS 13C3-PFBS y=0.047911x+0.001343 1.00000 0.0189 0.0567 PFHxS 18O2-PFHxS y=0.058056x−0.006899 0.99989 0.0204 0.0612 PFHpS 18O2-PFHxS y=0.035339x−0.011198 0.99986 0.0105 0.0314 PFOS 13C4-PFOS y=0.041710x+0.042938 0.99981 0.0204 0.0613 FOSA-I 13C4-PFOS y=0.016368x−0.001861 0.99987 0.0292 0.0876 3.6-OPFHpA 13C4-PFBA y=0.017046x−0.013055 0.99929 0.0202 0.0606 PF4OPeA 13C4-PFBA y=0.016543x−0.011943 0.99939 0.0188 0.0563 PF5OHxA 13C2-PFHxA y=0.014955x−0.010679 0.99941 0.0192 0.0577 ADONA 13C4-PFOA y=0.058757x−0.041735 0.99942 0.0190 0.0571 PFEESA 13C4-PFOA y=0.047871x−0.042587 0.99901 0.0186 0.0558 HFPO-DA 13C3-HFPO-DA y=0.007638x−0.006091 0.99913 0.0182 0.0547 HFPO-TA 13C3-HFPO-DA y=0.024974x−0.008596 0.99951 0.0279 0.0836 6:2 FTCA 13C2-6:2 FTCA y=0.001298x−0.000705 0.99968 0.0310 0.0930 8:2 FTCA 13C2-8:2 FTCA y=0.001433x−0.000481 0.99988 0.0192 0.0576 10:2 FTCA 13C2-10:2 FTCA y=0.001310x−0.000431 0.99971 0.0177 0.0531 4:2 FTS 13C2-4:2 FTS y=0.028611x−0.004785 0.99981 0.0176 0.0528 6:2 FTS 13C2-6:2 FTS y=0.026094x+0.008380 0.99949 0.0191 0.0573 8:2 FTS 13C2-8:2 FTS y=0.017183x+0.003359 0.99921 0.0152 0.0457 6:2 CL-PFESA 13C4-PFOS y=0.175038x−0.086573 0.99973 0.0173 0.0520 8:2 CL-PFESA 13C4-PFOS y=0.140150x−0.055419 0.99988 0.0163 0.0488 OBS 13C4-PFOS y=0.018987x+0.002797 0.99928 0.0129 0.0387 PFECHS 13C4-PFOS y=0.156627x−0.016528 0.99986 0.0196 0.0588 6:2 diPAP 13C4-6:2 diPAP y=0.007340x−0.003177 0.99975 0.0240 0.0719 6:2/8:2 diPAP 13C4-6:2 diPAP y=0.012541x−0.000963 0.99912 0.0209 0.0626 8:2 diPAP 13C4-8:2 diPAP y=0.004813x−0.000242 0.99927 0.0329 0.0988 2.2 样品前处理方法的优化
通过加标实验来优化昆虫样品的前处理方法,即在空白样品和昆虫样品中添加10 ng·mL−1水平的目标PFASs标准品,每种方法重复实验3 次,计算不同方法的加标回收率。
首先对PFASs的萃取方法进行优化,本实验比较了4种方法(MeOH、10 mmol·L−1 NaOH/MeOH、0.1% HCL/ACN和TBAH/MTBE离子对萃取)对目标化合物回收率的影响,如图2所示。结果表明,MeOH、10 mmol·L−1 NaOH/MeOH和0.1% HCL/ACN萃取方法中PFASs回收率的波动较大,其中MeOH为7%—195%,10 mmol·L−1 NaOH/MeOH为8%—177%,0.1% HCL/ACN为21%—220%。MeOH和10 mmol·L−1 NaOH/MeOH萃取的PFPeA、PFHxA、3.6-OPFHpA、PF4OPeA、PF5OHxA等短链PFASs回收率过低(7%—26%),而6:2 diPAP、6:2/8:2 diPAP、8:2FTS和OBS等长链PFASs回收率过高(160%—195%)。0.1% HCL/ACN萃取方法中,PFBA和4:2 FTS的回收率分别高达220%和215%,而HFPO-DA、6:2 FTCA和8:2 diPAP的回收率仅为31%、30%和21%。TBAH/MTBE离子对萃取方法对PFASs回收率的影响相对较小(53%—155%),仅4:2 FTS和OBS的回收率略高于150%,这可能是由于基质效应。对比不同方法可以看出,TBAH/MTBE离子对萃取对PFASs回收率的影响整体较小,因此选择该方法提取昆虫样品中的PFASs。TBAH/MTBE离子对萃取的方法也常用于肝脏等生物组织样品中PFASs的提取[27-28]。另外,从图2可以看出,4种萃取方法分析的6:2 Cl-PFESA、OBS和FTSs等目标化合物的回收率偏高,说明存在基质干扰,需进一步优化昆虫样品的QuEChERS净化方法。
本研究主要采用的dSPE吸附剂为Envi-Carb和C18,其中Envi-Carb常用的去除极性共萃物,如叶绿素和固醇类[29];C18则主要吸附非极性共萃物,如三酰甘油和其他亲脂性化合物[30]。N-丙基乙二胺(PSA)也常用于QuEChERS共萃物的净化,但是PSA作为弱阴离子交换吸附剂对酸性的PFASs具有一定的吸附作用[25]。已有研究也发现PSA的用量超过0.1 g时会吸附部分PFASs[23],因此在本研究中未使用PSA作吸附剂。对于昆虫样品来说,萃取液中含有大量蛋白质、脂肪和色素等共萃物,单独使用C18或Envi-Carb效果并不好,因此同时添加C18和Envi-Carb来净化样品。本实验比较了0.05 g C18 + 0.05 g Envi-Carb、0.1 g C18 + 0.05 g Envi-Carb、0.05 g C18 + 0.1 g Envi-Carb、0.1 g C18 + 0.1 g Envi-Carb和0.1 g C18 + 0.2 g Envi-Carb对PFASs回收率的影响。图3中,当C18为0.05 g时,Envi-Carb质量过少导致PFASs回收率偏高(大部分目标化合物回收率超过100%);当Envi-Carb为0.1 g时,C18的增加导致一些新型PFASs回收率降低。总的来说,0.05 g C18+0.1 g Envi-Carb的组合对PFASs的影响最小,回收率范围在62%—128%,因此选择该组合对昆虫样品萃取液进行净化。优化后的QuEChERS净化方法不仅能保证回收率还能有效去除溶液中的蛋白质、脂肪和色素等干扰物质,使昆虫样品达到仪器检测要求。
2.3 不同种类昆虫中PFASs的加标回收率
通过不同浓度的加标实验来保证监测实际昆虫样品中PFASs的适用性,即称取0.1 g dw水生昆虫(蜻蜓幼虫)和陆生昆虫(蝗虫)样品,分别加入低、中、高三个浓度水平(定容浓度为 1、10、00 ng·mL−1)的混合PFASs标准品以及IS(10 ng·mL−1)。每个加标浓度重复做3个平行样品,分别计算水生昆虫和陆生昆虫中PFASs的回收率和相对标准偏差(relative standard deviation,RSD)。结果如表3所示,水生昆虫和陆生昆虫中35种PFASs的回收率分别为70%—126%和64%—115%,RSD分别为1.1%—20%和0.15%—24%,说明该方法能满足对实际昆虫样品的定量分析。
表 3 水生和陆生昆虫中35种PFASs的加标回收率和标准偏差(%)Table 3. The recoveries and relative standard deviation (%) for 35 target PFASs in aquatic and terrestrial insects目标化合物Target compound 水生昆虫Aquatic insect 陆生昆虫Terrestrial insect 1 ng·mL−1 10 ng·mL−1 100 ng·mL−1 1 ng·mL−1 10 ng·mL−1 100 ng·mL−1 PFBA 118.9 ± 2.3 ª 99.3 ± 2.9 98.5 ± 4.2 101.7 ± 8.7 87.2 ± 5.0 81.5 ± 0.72 PFPeA 94.5 ± 1.8 99.6 ± 5.4 99.0 ± 6.4 87.8 ± 2.7 73.5 ± 4.3 70.3 ± 3.0 PFHxA 94.0 ± 8.5 95.8 ± 4.5 96.1 ± 5.1 97.6 ± 3.4 81.1 ± 1.5 87.1 ± 3.6 PFHpA 89.5 ± 5.4 94.3 ± 9.8 101.5 ± 6.5 76.2 ± 7.9 76.4 ± 0.50 95.1 ± 4.0 PFOA 89.3 ± 5.8 93.1 ± 11 99.4 ± 6.6 77.4 ± 8.5 75.2 ± 3.6 99.5 ± 6.2 PFNA 82.2 ± 3.8 104.8 ± 3.0 102.9 ± 6.5 79.1 ± 7.8 78.9 ± 3.7 101.0 ± 6.0 PFDA 82.7 ± 4.8 117.0 ± 8.7 106.2 ± 6.2 73.8 ± 5.8 78.2 ± 2.7 98.5 ± 6.1 PFUnDA 85.0 ± 5.1 91.3 ± 2.8 108.8 ± 4.4 67.8 ± 1.4 70.4 ± 1.3 85.2 ± 5.6 PFDoDA 82.8 ± 3.4 93.5 ± 7.7 104.8 ± 5.9 81.9 ± 8.8 95.0 ± 1.2 95.7 ± 4.3 PFBS 101.4 ± 11 81.4 ± 8.1 101.5 ± 7.6 107.2 ± 10 79.5 ± 1.2 106.1 ± 4.3 PFPeS 108.6 ± 3.8 99.8 ± 8.3 107.6 ± 7.8 96.4 ± 16 80.9 ± 2.1 106.8 ± 6.1 PFHxS 84.8 ± 7.0 90.0 ± 7.9 86.6 ± 7.0 83.0 ± 11 72.8 ± 1.6 80.5 ± 4.9 PFHpS 110.1 ± 5.8 103.7 ± 4.8 119.8 ± 6.0 97.7 ± 11 94.7 ± 7.3 108.6 ± 5.1 PFOS 124.6 ± 8.5 94.8 ± 5.0 86.9 ± 6.0 102.8 ± 24 98.1 ± 6.1 79.8 ± 5.0 FOSA-I 78.1 ± 5.5 80.2 ± 8.3 76.7 ± 6.1 89.6 ± 6.8 98.6 ± 1.0 86.5 ± 2.5 3.6-OPFHpA 81.4 ± 8.1 86.3 ± 6.3 95.0 ± 4.2 78.2 ± 3.7 76.8 ± 5.1 79.4 ± 0.66 PF4OPeA 118.4 ± 5.7 104.8 ± 4.9 106.1 ± 2.3 96.3 ± 6.3 80.0 ± 4.4 81.0 ± 2.8 PF5OHxA 93.1 ± 4.9 105.2 ± 2.6 111.5 ± 4.0 75.5 ± 4.8 71.3 ± 4.9 71.4 ± 2.5 ADONA 70.0 ± 2.0 81.4 ± 6.3 93.0 ± 5.5 67.8 ± 1.4 65.2 ± 2.4 79.8 ± 1.5 PFEESA 73.7 ± 8.1 78.4 ± 6.4 95.0 ± 5.8 69.9 ± 1.1 67.7 ± 2.3 81.7 ± 2.0 HFPO-DA 94.6 ± 10 90.1 ± 4.0 96.0 ± 4.9 103.5 ± 5.2 76.8 ± 2.6 76.7 ± 1.6 HFPO-TA 104.8 ± 4.7 72.8 ± 6.2 85.7 ± 8.1 95.2 ± 5.5 104.1 ± 4.7 63.7 ± 1.1 6:2 FTCA 115.8 ± 3.7 89.9 ± 9.4 114.2 ± 4.2 101.5 ± 9.3 86.7 ± 5.4 102.6 ± 4.1 8:2 FTCA 100.3 ± 2.4 107.3 ± 12 109.0 ± 4.8 93.3 ± 7.7 115.0 ± 2.9 103.1 ± 5.6 10:2 FTCA 126.0 ± 4.2 79.6 ± 3.7 91.9 ± 2.7 101.3 ± 9.9 72.7 ± 7.8 78.0 ± 1.3 4:2 FTS 110.1 ± 20 102.0 ± 7.0 84.5 ± 5.4 98.4 ± 9.5 100.7 ± 6.1 75.4 ± 2.4 6:2 FTS 97.7 ± 17 99.8 ± 9.8 75.5 ± 5.3 106.3 ± 11 93.2 ± 7.0 73.5 ± 0.15 8:2 FTS 69.9 ± 3.8 104.5 ± 14 96.2 ± 5.5 67.3 ± 3.8 101.4 ± 8.9 107.1 ± 0.19 6:2 Cl-PFESA 117.4 ± 8.1 83.8 ± 3.3 103.9 ± 4.2 93.1 ± 2.6 92.9 ± 5.2 96.7 ± 2.2 8:2 Cl-PFESA 82.5 ± 8.6 101.2 ± 2.1 111.1 ± 4.3 100.8 ± 2.1 98.7 ± 4.9 99.4 ± 2.6 OBS 88.0 ± 13 89.1 ± 4.6 81.0 ± 6.7 99.6 ± 9.4 97.7 ± 6.6 79.0 ± 1.0 PFECHS 87.9 ± 6.2 79.2 ± 2.1 112.1 ± 6.6 71.5 ± 6.1 73.5 ± 3.9 96.9 ± 2.1 6:2 diPAP 109.0 ± 2.5 91.0 ± 16 113.9 ± 16 112.4 ± 5.1 92.4 ± 7.1 115.1 ± 9.9 6:2/8:2 diPAP 101.0 ± 20 94.0 ± 3.8 116.0 ± 3.4 92.4 ± 2.4 80.3 ± 11 89.9 ± 1.1 8:2 diPAP 121.5 ± 5.4 79.8 ± 1.1 91.4 ± 11 111.3 ± 6.4 109.5 ± 11 92.8 ± 3.6 ª 均值±相对标准偏差。ª Mean ± RSD. 2.4 实际昆虫样品中PFASs分析
本研究的昆虫包括3个目的5种昆虫,分别为蜻蜓目(蜻蜓幼虫和成虫),直翅目(蝗虫,蝼蛄,蟋蟀)和鳞翅目(蝴蝶),按生境可分为水生昆虫和陆生昆虫。如图4所示,水生昆虫和陆生昆虫体内PFASs的浓度和组成差异明显。水生昆虫中ƩPFASs浓度(42—304 ng·g−1 dw)明显高于陆生昆虫(4.7—19 ng·g−1 dw),这与Koch等[16] 的报道结果一致,主要是由于PFASs通常随污水排放到水环境中[20]。从组成上看,水生昆虫以传统的PFCAs(如PFOA、PFNA、PFDA 和PFUnDA)和PFOS为主,而陆生昆虫主要是以新型PFASs(如6:2 FTS、OBS、6:2和8:2 Cl-PFESAs)为主。值得注意的是,在蜻蜓(1)和蝼蛄中浓度最高的PFASs是6:2 Cl-PFESA,说明6:2 Cl-PFESA作为PFOS替代品在我国电镀行业中被广泛使用。另外,6:2 FTS在陆生昆虫体内的浓度均高于PFOS,而OBS和HFPO-DA/TA也在大部分昆虫样品(除了蟋蟀)中检出,这说明近年来这些新型替代品已经在我国广泛生产和应用。而6:2、6:2/8:2 和8:2 diPAPs仅在蜻蜓幼虫和蝼蛄体内检出,说明其使用量在我国还较小。
在工业园区采集的蜻蜓(1)和蜻蜓(2)中ƩPFASs浓度分别为68 ng·g−1 dw和304 ng·g−1 dw,与加拿大某国际机场下游采集的蜻蜓体内浓度相当(180 ng·g−1 ww)[13],远高于南非未受污染区域的蜻蜓中7种典型PFASs的浓度(0.17—21 ng·g−1 ww)[15],但是低于瑞典某军用机场下游采集的蜻蜓中PFASs的浓度(120—3500 ng·g−1 dw)[16]。而在山东昆虫市场购买的蜻蜓幼虫中ƩPFASs浓度(42 ng·g−1 dw)与坦桑尼亚采集的蜻蜓幼虫中污染水平相当(6.1—24 ng·g−1 ww)[14]。对于陆生昆虫,蝼蛄中ƩPFASs的浓度最高为19 ng·g−1 dw,其次为蝗虫((8.3 ± 0.65) ng·g−1 dw)和蝴蝶(6.6 ng·g−1 dw),蟋蟀浓度最低(4.7 ng·g−1 dw),这可能与其食性相关,蝼蛄为杂食性昆虫,而其他3种陆生昆虫为植食性昆虫。另外,蝗虫体内PFASs的浓度略高于环北京-天津-河北地区农田中采集的蝗虫中PFASs的浓度(3.7 ± 1.8) ng·g−1 dw)[18]。对于其他陆生昆虫,目前并未见到相关PFASs的报道。
3. 结论(Conclusion)
本研究建立了一套QuEChERS结合超高效液相色谱-串联质谱法测定昆虫体内的35种典型和新型PFASs,具有快速、高效、便捷、经济、准确等优点。采用该方法对实际昆虫样品进行分析,结果表明水生昆虫中PFASs浓度(42—304 ng·g−1 dw)明显高于陆生昆虫(4.7—19 ng·g−1 dw)。陆生昆虫PFASs的组成以新型PFASs为主,主要是6:2 FTS、6:2 Cl-PFESA 和OBS;而水生昆虫则以传统PFASs为主,如PFOA、PFNA、PFDA 、PFUnDA和PFOS。
-
表 1 35种PFASs以及19种内标的质谱参数
Table 1. Detailed mass spectrometry parameters of 35 individual PFASs and 19 isotopic internal standards
简称Acronym 化合物全称Compound name 母离子Precursor Ion (m/z) 子离子Product Ion (m/z) 碎裂电压/VFragmentor 碰撞能/eVCollision Energy PFBA Perfluorobutanoic acid 212.8 168.8 60 6 PFPeA Perfluoropentanoic acid 262.9 218.8 80 5 PFHxA Perfluorohexanoic acid 312.9 268.9 80 5 PFHpA Perfluoroheptanoic acid 362.8 318.9 80 5 PFOA Perfluorooctanoic acid 412.9 368.9 90 5 PFNA Perfluorononanoic acid 462.8 418.9 90 5 PFDA Perfluorodecanoic acid 512.8 468.9 100 5 PFUnDA Perfluoroundecanoic acid 562.9 518.9 80 5 PFDoDA Perfluorododecanoic acid 612.9 568.9 100 10 PFBS Potassium perfluorobutane sulfonate 298.8 79.9 150 45 PFPeS Sodium perfluoropentane sulfonate 348.9 79.9 150 50 PFHxS Sodium perfluorohexane sulfonate 398.8 79.9 150 60 PFHpS Sodium perfluoroheptane sulfonate 448.9 79.9 150 60 PFOS Sodium perfluorooctane sulfonate 498.8 79.9 150 60 FOSA-I Perfluoro-1-octanesulfonamide 497.8 77.9 150 50 3.6-OPFHpA Perfluoro-3,6-dioxaheptanoic acid 201 84.9 80 10 PF4OPeA Perfluoro-4-oxapentanoic acid 228.9 84.9 50 10 PF5OHxA Perfluoro-5-oxahexanoic acid 278.9 84.9 50 12 ADONA 3H-perfluoro-3-(3-methoxy-propoxy)-propanoate acid 376.9 250.6 80 10 PFEESA Perfluoro (2-ethoxyethane) sulfonic acid 314.9 134.7 100 25 HFPO-DA Hexafluoropropylene oxide dimer acid 284.9 168.8 60 5 HFPO-TA Hexafluoropropylene oxide trimer acid 184.7 118.7 80 15 6:2 FTCA 6:2 fluorotelomer carboxylic acid 376.9 292.9 80 10 8:2 FTCA 8:2 fluorotelomer carboxylic acid 476.6 392.9 80 12 10:2 FTCA 10:2 fluorotelomer carboxylic acid 576.6 492.9 90 10 4:2 FTS 4:2 fluorotelomer sulfonate acid 326.9 306.7 150 20 6:2 FTS 6:2 fluorotelomer sulfonate acid 426.9 406.7 180 25 8:2 FTS 8:2 fluorotelomer sulfonate acid 526.9 506.7 200 30 6:2 Cl-PFESA 6:2 chlorinated polyfluorinated ether sulfonates 530.9 350.8 150 30 8:2 Cl-PFESA 8:2 chlorinated polyfluorinated ether sulfonates 630.9 450.8 150 35 OBS Sodium p-perfluorous nonenoxybenzene sulfonate 602.8 171.6 200 50 PFECHS Potassium perfluoro-4-ethylcyclohexanesulfonate 460.8 380.8 150 30 6:2 diPAP Sodium bis(1H,1H,2H,2H-perfluorooctyl) phosphate 788.7 96.7 195 40 6:2/8:2 diPAP Sodium bis(1H,1H,2H,2H-perfluorooctyl-1H,1H,2H,2H- perfluorodecyl) phosphate 888.7 96.7 200 50 8:2 diPAP Sodium bis(1H,1H,2H,2H-perfluorodecyl) phosphate 988.7 96.7 200 50 13C4-PFBA Perfluoro-(13C4)-butanoic acid 216.9 171.9 60 6 13C2-PFHxA Perfluoro-(13C2)-hexanoic acid 314.9 269.9 80 5 13C4-PFOA Perfluoro-(13C4)-octanoic acid 416.8 371.9 90 5 13C5-PFNA Perfluoro-(13C5)-nonanoic acid 467.9 422.9 90 5 13C2-PFDA Perfluoro-(13C2)-decanoic acid 514.9 469.9 100 5 13C2-PFUnDA Perfluoro-(13C2)-undecanoic acid 564.9 519.9 80 5 13C2-PFDoDA Perfluoro-(13C2)-dodecanoic acid 614.9 569.9 100 10 13C3-PFBS Perfluoro-(13C3)- butane sulfonate 301.8 79.9 150 45 18O2-PFHxS Perfluoro-(18O2)-hexane sulfonic acid 402.9 79.9 150 60 13C4-PFOS Perfluoro-(13C4)-octane sulfonic acid 502.8 79.9 150 60 13C3-HFPO-DA Hexafluoropropylene-(13C3)-dimer acid 286.9 168.8 60 5 13C2-6:2 FTCA 2-Perfluorohexyl-(13C2)-ethanoic acid 378.9 293.6 80 10 13C2-8:2 FTCA 2-Perfluorooctyl-(13C2)-ethanoic acid 478.6 393.9 80 12 13C2-10:2 FTCA 2-Perfluorodecyl-(13C2)-ethanoic acid 578.6 493.9 90 10 13C2-4:2 FTS 4:2 fluorotelomer-(13C2)-sulfonate 328.9 80.9 150 20 13C2-6:2 FTS 6:2 fluorotelomer-(13C2)-sulfonate 428.9 80.9 180 25 13C2-8:2 FTS 8:2 fluorotelomer-(13C2)-sulfonate 528.9 80.9 200 30 13C4-6:2 diPAP Sodium bis(1H,1H,2H,2H-[1,2-13C2]perfluorooctyl) phosphate 792.7 445.8 200 40 13C4-8:2 diPAP Sodium bis(1H,1H,2H,2H-[1,2-13C2]perfluorodecyl) phosphate 992.7 545.8 200 50 表 2 35种PFASs的线性方程、相关系数(R2)、仪器检出限和方法定量限
Table 2. Linear equation, correlation coefficient (R2), limit of detection (LOD), and limit of quantitation (LOQ,) for 35 target PFASs
目标化合物Target compound 内标Internal standard 线性方程Linear equation 相关系数(R2)Correlation coefficient 检出限/(ng·mL−1)LOD 定量限/(ng·g−1 dw)LOQ PFBA 13C4-PFBA y=0.026676x−0.017817 0.99951 0.0135 0.0405 PFPeA 13C4-PFBA y=0.015960x−0.004434 0.99979 0.0222 0.0665 PFHxA 13C2-PFHxA y=0.031886x−0.009241 0.99986 0.0199 0.0596 PFHpA 13C2-PFHxA y=0.034467x−0.009820 0.99989 0.0187 0.0561 PFOA 13C4-PFOA y=0.034039x−0.016462 0.99972 0.0198 0.0595 PFNA 13C5-PFNA y=0.030688x−0.007462 0.99992 0.0198 0.0595 PFDA 13C2-PFDA y=0.030758x−0.014403 0.99983 0.0176 0.0527 PFUnDA 13C2-PFUnDA y=0.035521x−0.035578 0.99992 0.0184 0.0553 PFDoDA 13C2-PFDoDA y=0.029815x−0.011676 0.99988 0.0166 0.0498 PFBS 13C3-PFBS y=0.043224x−0.001482 0.99980 0.0181 0.0542 PFPeS 13C3-PFBS y=0.047911x+0.001343 1.00000 0.0189 0.0567 PFHxS 18O2-PFHxS y=0.058056x−0.006899 0.99989 0.0204 0.0612 PFHpS 18O2-PFHxS y=0.035339x−0.011198 0.99986 0.0105 0.0314 PFOS 13C4-PFOS y=0.041710x+0.042938 0.99981 0.0204 0.0613 FOSA-I 13C4-PFOS y=0.016368x−0.001861 0.99987 0.0292 0.0876 3.6-OPFHpA 13C4-PFBA y=0.017046x−0.013055 0.99929 0.0202 0.0606 PF4OPeA 13C4-PFBA y=0.016543x−0.011943 0.99939 0.0188 0.0563 PF5OHxA 13C2-PFHxA y=0.014955x−0.010679 0.99941 0.0192 0.0577 ADONA 13C4-PFOA y=0.058757x−0.041735 0.99942 0.0190 0.0571 PFEESA 13C4-PFOA y=0.047871x−0.042587 0.99901 0.0186 0.0558 HFPO-DA 13C3-HFPO-DA y=0.007638x−0.006091 0.99913 0.0182 0.0547 HFPO-TA 13C3-HFPO-DA y=0.024974x−0.008596 0.99951 0.0279 0.0836 6:2 FTCA 13C2-6:2 FTCA y=0.001298x−0.000705 0.99968 0.0310 0.0930 8:2 FTCA 13C2-8:2 FTCA y=0.001433x−0.000481 0.99988 0.0192 0.0576 10:2 FTCA 13C2-10:2 FTCA y=0.001310x−0.000431 0.99971 0.0177 0.0531 4:2 FTS 13C2-4:2 FTS y=0.028611x−0.004785 0.99981 0.0176 0.0528 6:2 FTS 13C2-6:2 FTS y=0.026094x+0.008380 0.99949 0.0191 0.0573 8:2 FTS 13C2-8:2 FTS y=0.017183x+0.003359 0.99921 0.0152 0.0457 6:2 CL-PFESA 13C4-PFOS y=0.175038x−0.086573 0.99973 0.0173 0.0520 8:2 CL-PFESA 13C4-PFOS y=0.140150x−0.055419 0.99988 0.0163 0.0488 OBS 13C4-PFOS y=0.018987x+0.002797 0.99928 0.0129 0.0387 PFECHS 13C4-PFOS y=0.156627x−0.016528 0.99986 0.0196 0.0588 6:2 diPAP 13C4-6:2 diPAP y=0.007340x−0.003177 0.99975 0.0240 0.0719 6:2/8:2 diPAP 13C4-6:2 diPAP y=0.012541x−0.000963 0.99912 0.0209 0.0626 8:2 diPAP 13C4-8:2 diPAP y=0.004813x−0.000242 0.99927 0.0329 0.0988 表 3 水生和陆生昆虫中35种PFASs的加标回收率和标准偏差(%)
Table 3. The recoveries and relative standard deviation (%) for 35 target PFASs in aquatic and terrestrial insects
目标化合物Target compound 水生昆虫Aquatic insect 陆生昆虫Terrestrial insect 1 ng·mL−1 10 ng·mL−1 100 ng·mL−1 1 ng·mL−1 10 ng·mL−1 100 ng·mL−1 PFBA 118.9 ± 2.3 ª 99.3 ± 2.9 98.5 ± 4.2 101.7 ± 8.7 87.2 ± 5.0 81.5 ± 0.72 PFPeA 94.5 ± 1.8 99.6 ± 5.4 99.0 ± 6.4 87.8 ± 2.7 73.5 ± 4.3 70.3 ± 3.0 PFHxA 94.0 ± 8.5 95.8 ± 4.5 96.1 ± 5.1 97.6 ± 3.4 81.1 ± 1.5 87.1 ± 3.6 PFHpA 89.5 ± 5.4 94.3 ± 9.8 101.5 ± 6.5 76.2 ± 7.9 76.4 ± 0.50 95.1 ± 4.0 PFOA 89.3 ± 5.8 93.1 ± 11 99.4 ± 6.6 77.4 ± 8.5 75.2 ± 3.6 99.5 ± 6.2 PFNA 82.2 ± 3.8 104.8 ± 3.0 102.9 ± 6.5 79.1 ± 7.8 78.9 ± 3.7 101.0 ± 6.0 PFDA 82.7 ± 4.8 117.0 ± 8.7 106.2 ± 6.2 73.8 ± 5.8 78.2 ± 2.7 98.5 ± 6.1 PFUnDA 85.0 ± 5.1 91.3 ± 2.8 108.8 ± 4.4 67.8 ± 1.4 70.4 ± 1.3 85.2 ± 5.6 PFDoDA 82.8 ± 3.4 93.5 ± 7.7 104.8 ± 5.9 81.9 ± 8.8 95.0 ± 1.2 95.7 ± 4.3 PFBS 101.4 ± 11 81.4 ± 8.1 101.5 ± 7.6 107.2 ± 10 79.5 ± 1.2 106.1 ± 4.3 PFPeS 108.6 ± 3.8 99.8 ± 8.3 107.6 ± 7.8 96.4 ± 16 80.9 ± 2.1 106.8 ± 6.1 PFHxS 84.8 ± 7.0 90.0 ± 7.9 86.6 ± 7.0 83.0 ± 11 72.8 ± 1.6 80.5 ± 4.9 PFHpS 110.1 ± 5.8 103.7 ± 4.8 119.8 ± 6.0 97.7 ± 11 94.7 ± 7.3 108.6 ± 5.1 PFOS 124.6 ± 8.5 94.8 ± 5.0 86.9 ± 6.0 102.8 ± 24 98.1 ± 6.1 79.8 ± 5.0 FOSA-I 78.1 ± 5.5 80.2 ± 8.3 76.7 ± 6.1 89.6 ± 6.8 98.6 ± 1.0 86.5 ± 2.5 3.6-OPFHpA 81.4 ± 8.1 86.3 ± 6.3 95.0 ± 4.2 78.2 ± 3.7 76.8 ± 5.1 79.4 ± 0.66 PF4OPeA 118.4 ± 5.7 104.8 ± 4.9 106.1 ± 2.3 96.3 ± 6.3 80.0 ± 4.4 81.0 ± 2.8 PF5OHxA 93.1 ± 4.9 105.2 ± 2.6 111.5 ± 4.0 75.5 ± 4.8 71.3 ± 4.9 71.4 ± 2.5 ADONA 70.0 ± 2.0 81.4 ± 6.3 93.0 ± 5.5 67.8 ± 1.4 65.2 ± 2.4 79.8 ± 1.5 PFEESA 73.7 ± 8.1 78.4 ± 6.4 95.0 ± 5.8 69.9 ± 1.1 67.7 ± 2.3 81.7 ± 2.0 HFPO-DA 94.6 ± 10 90.1 ± 4.0 96.0 ± 4.9 103.5 ± 5.2 76.8 ± 2.6 76.7 ± 1.6 HFPO-TA 104.8 ± 4.7 72.8 ± 6.2 85.7 ± 8.1 95.2 ± 5.5 104.1 ± 4.7 63.7 ± 1.1 6:2 FTCA 115.8 ± 3.7 89.9 ± 9.4 114.2 ± 4.2 101.5 ± 9.3 86.7 ± 5.4 102.6 ± 4.1 8:2 FTCA 100.3 ± 2.4 107.3 ± 12 109.0 ± 4.8 93.3 ± 7.7 115.0 ± 2.9 103.1 ± 5.6 10:2 FTCA 126.0 ± 4.2 79.6 ± 3.7 91.9 ± 2.7 101.3 ± 9.9 72.7 ± 7.8 78.0 ± 1.3 4:2 FTS 110.1 ± 20 102.0 ± 7.0 84.5 ± 5.4 98.4 ± 9.5 100.7 ± 6.1 75.4 ± 2.4 6:2 FTS 97.7 ± 17 99.8 ± 9.8 75.5 ± 5.3 106.3 ± 11 93.2 ± 7.0 73.5 ± 0.15 8:2 FTS 69.9 ± 3.8 104.5 ± 14 96.2 ± 5.5 67.3 ± 3.8 101.4 ± 8.9 107.1 ± 0.19 6:2 Cl-PFESA 117.4 ± 8.1 83.8 ± 3.3 103.9 ± 4.2 93.1 ± 2.6 92.9 ± 5.2 96.7 ± 2.2 8:2 Cl-PFESA 82.5 ± 8.6 101.2 ± 2.1 111.1 ± 4.3 100.8 ± 2.1 98.7 ± 4.9 99.4 ± 2.6 OBS 88.0 ± 13 89.1 ± 4.6 81.0 ± 6.7 99.6 ± 9.4 97.7 ± 6.6 79.0 ± 1.0 PFECHS 87.9 ± 6.2 79.2 ± 2.1 112.1 ± 6.6 71.5 ± 6.1 73.5 ± 3.9 96.9 ± 2.1 6:2 diPAP 109.0 ± 2.5 91.0 ± 16 113.9 ± 16 112.4 ± 5.1 92.4 ± 7.1 115.1 ± 9.9 6:2/8:2 diPAP 101.0 ± 20 94.0 ± 3.8 116.0 ± 3.4 92.4 ± 2.4 80.3 ± 11 89.9 ± 1.1 8:2 diPAP 121.5 ± 5.4 79.8 ± 1.1 91.4 ± 11 111.3 ± 6.4 109.5 ± 11 92.8 ± 3.6 ª 均值±相对标准偏差。ª Mean ± RSD. -
[1] WANG Z Y, DEWITT J C, HIGGINS C P, et al. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? [J]. Environmental Science & Technology, 2017, 51(5): 2508-2518. [2] 金航标, 祝凌燕, 单国强. 高效液相色谱-串联质谱法同时测定人血清中的全氟辛烷磺酸及其前体物 [J]. 环境化学, 2016, 35(6): 1180-1188. doi: 10.7524/j.issn.0254-6108.2016.06.2015111705 JIN H B, ZHU L Y, SHAN G Q. Determination of perfluorooctane sulfonate and its precursors in human serum samples by high performance liquid chromatography electrospray tandem mass spectrometry [J]. Environmental Chemistry, 2016, 35(6): 1180-1188(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.06.2015111705
[3] ZHANG B, HE Y, YANG G, et al. Legacy and emerging poly- and perfluoroalkyl substances in finless porpoises from East China Sea: Temporal trends and tissue-specific accumulation [J]. Environmental Science & Technology, 2022,56(10): 6113-6122. [4] 林泳峰, 阮挺, 江桂斌. 新型全氟和多氟烷基化合物的分析、行为与效应研究进展 [J]. 科学通报, 2017, 62(24): 2724-2738. doi: 10.1360/N972017-00223 LIN Y F, RUAN T, JIANG G B. Progress on analytical methods and environmental behavior of emerging per-and polyfluoroalkyl substances [J]. Chinese Science Bulletin, 2017, 62(24): 2724-2738(in Chinese). doi: 10.1360/N972017-00223
[5] WANG S W, HUANG J, YANG Y, et al. First report of a Chinese PFOS alternative overlooked for 30 years: Its toxicity, persistence, and presence in the environment [J]. Environmental Science & Technology, 2013, 47(18): 10163-10170. [6] NAKAYAMA S F, YOSHIKANE M, ONODA Y, et al. Worldwide trends in tracing poly- and perfluoroalkyl substances (PFAS) in the environment [J]. TrAC Trends in Analytical Chemistry, 2019, 121: 115410. doi: 10.1016/j.trac.2019.02.011 [7] YAO J Z, PAN Y T, SHENG N, et al. Novel perfluoroalkyl ether carboxylic acids (PFECAs) and sulfonic acids (PFESAs): Occurrence and association with serum biochemical parameters in residents living near a fluorochemical plant in China [J]. Environmental Science & Technology, 2020, 54(21): 13389-13398. [8] XU L, SHI Y L, LI C X, et al. Discovery of a novel polyfluoroalkyl benzenesulfonic acid around oilfields in Northern China [J]. Environmental Science & Technology, 2017, 51(24): 14173-14181. [9] HU H M, ZHANG Y Y, ZHAO N, et al. Legacy and emerging poly- and perfluorochemicals in seawater and sediment from East China Sea [J]. Science of the Total Environment, 2021, 797: 149052. doi: 10.1016/j.scitotenv.2021.149052 [10] MUNOZ G, LIU J X, VO DUY S, et al. Analysis of F-53B, Gen-X, ADONA, and emerging fluoroalkylether substances in environmental and biomonitoring samples: A review [J]. Trends in Environmental Analytical Chemistry, 2019, 23: e00066. doi: 10.1016/j.teac.2019.e00066 [11] GALLOWAY J E, MORENO A V P, LINDSTROM A B, et al. Evidence of air dispersion: HFPO-DA and PFOA in Ohio and west Virginia surface water and soil near a fluoropolymer production facility [J]. Environmental Science & Technology, 2020, 54(12): 7175-7184. [12] MENG L Y, SONG B Y, ZHONG H F, et al. Legacy and emerging per- and polyfluoroalkyl substances (PFAS) in the Bohai Sea and its inflow rivers [J]. Environment International, 2021, 156: 106735. doi: 10.1016/j.envint.2021.106735 [13] de SOLLA S R, de SILVA A O, LETCHER R J. Highly elevated levels of perfluorooctane sulfonate and other perfluorinated acids found in biota and surface water downstream of an international airport, Hamilton, Ontario, Canada [J]. Environment International, 2012, 39(1): 19-26. doi: 10.1016/j.envint.2011.09.011 [14] GROFFEN T, RIJNDERS J, van DOORN L, et al. Preliminary study on the distribution of metals and persistent organic pollutants (POPs), including perfluoroalkylated acids (PFAS), in the aquatic environment near Morogoro, Tanzania, and the potential health risks for humans [J]. Environmental Research, 2021, 192: 110299. doi: 10.1016/j.envres.2020.110299 [15] LESCH V, BOUWMAN H, KINOSHITA A, et al. First report of perfluoroalkyl substances in South African Odonata [J]. Chemosphere, 2017, 175: 153-160. doi: 10.1016/j.chemosphere.2017.02.020 [16] KOCH A, JONSSON M, YEUNG L W Y, et al. Per- and polyfluoroalkyl-contaminated freshwater impacts adjacent riparian food webs [J]. Environmental Science & Technology, 2020, 54(19): 11951-11960. [17] KOCH A, JONSSON M, YEUNG L W Y, et al. Quantification of biodriven transfer of per- and polyfluoroalkyl substances from the aquatic to the terrestrial environment via emergent insects [J]. Environmental Science & Technology, 2021, 55(12): 7900-7909. [18] LAN Z H, YAO Y M, XU J Y, et al. Novel and legacy per- and polyfluoroalkyl substances (PFASs) in a farmland environment: Soil distribution and biomonitoring with plant leaves and locusts [J]. Environmental Pollution, 2020, 263: 114487. doi: 10.1016/j.envpol.2020.114487 [19] ARISTIZABAL-HENAO J J, BROWN H J, GRIFFIN E K, et al. Ticks as novel sentinels to monitor environmental levels of per- and polyfluoroalkyl substances (PFAS) [J]. Environmental Science. Processes & Impacts, 2021, 23(9): 1301-1307. [20] KOCH A, KÄRRMAN A, YEUNG L W Y, et al. Point source characterization of per- and polyfluoroalkyl substances (PFASs) and extractable organofluorine (EOF) in freshwater and aquatic invertebrates [J]. Environmental Science. Processes & Impacts, 2019, 21(11): 1887-1898. [21] GAO Y, ZHANG Q H, LI X M, et al. Simultaneous determination of legacy and emerging per- and polyfluoroalkyl substances in fish by QuEChERS coupled with ultrahigh performance liquid chromatography tandem mass spectrometry [J]. Analytical Methods, 2018, 10(47): 5715-5722. doi: 10.1039/C8AY01478G [22] KANNAN K, TAO L, SINCLAIR E, et al. Perfluorinated compounds in aquatic organisms at various trophic levels in a Great Lakes food chain [J]. Archives of Environmental Contamination and Toxicology, 2005, 48(4): 559-566. doi: 10.1007/s00244-004-0133-x [23] 朱萍萍, 岳振峰, 郑宗坤, 等. 分散固相萃取结合高效液相色谱-串联质谱法测定羊肝中19种全氟烷基酸 [J]. 色谱, 2015, 33(5): 494-500. doi: 10.3724/SP.J.1123.2014.12034 ZHU P P, YUE Z F, ZHENG Z K, et al. Determination of perfluoroalkyl acids in lamb liver by high performance liquid chromatographytandem mass spectrometry combined with dispersive solid phase extraction [J]. Chinese Journal of Chromatography, 2015, 33(5): 494-500(in Chinese). doi: 10.3724/SP.J.1123.2014.12034
[24] GENUALDI S, YOUNG W, DEJAGER L, et al. Method development and validation of per- and polyfluoroalkyl substances in foods from FDA's total diet study program [J]. Journal of Agricultural and Food Chemistry, 2021, 69(20): 5599-5606. doi: 10.1021/acs.jafc.1c01777 [25] LACINA O, HRADKOVA P, PULKRABOVA J, et al. Simple, high throughput ultra-high performance liquid chromatography/tandem mass spectrometry trace analysis of perfluorinated alkylated substances in food of animal origin: Milk and fish [J]. Journal of Chromatography A, 2011, 1218(28): 4312-4321. doi: 10.1016/j.chroma.2011.04.061 [26] 李帅, 陈辉, 金铃和, 等. 高效液相色谱-串联质谱法测定蜂蜜中20种全氟烷基化合物 [J]. 色谱, 2017, 35(5): 495-501. doi: 10.3724/SP.J.1123.2016.12016 LI S, CHEN H, JIN L H, et al. Determination of 20 perfluorinated alkyl substances in honey by high performance liquid chromatography-tandem mass spectrometry [J]. Chinese Journal of Chromatography, 2017, 35(5): 495-501(in Chinese). doi: 10.3724/SP.J.1123.2016.12016
[27] GURUGE K S, MANAGE P M, YAMANAKA N, et al. Species-specific concentrations of perfluoroalkyl contaminants in farm and pet animals in Japan[J]. Chemosphere, 2008, 73(1 Suppl): S210-S215. [28] ZABALETA I, BIZKARGUENAGA E, PRIETO A, et al. Simultaneous determination of perfluorinated compounds and their potential precursors in mussel tissue and fish muscle tissue and liver samples by liquid chromatography-electrospray-tandem mass spectrometry [J]. Journal of Chromatography A, 2015, 1387: 13-23. doi: 10.1016/j.chroma.2015.01.089 [29] LEHOTAY S J, MAŠTOVSKÁ K, LIGHTFIELD A R. Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruits and vegetables [J]. Journal of AOAC INTERNATIONAL, 2019, 88(2): 615-629. [30] LANKOVA D, LACINA O, PULKRABOVA J, et al. The determination of perfluoroalkyl substances, brominated flame retardants and their metabolites in human breast milk and infant formula [J]. Talanta, 2013, 117: 318-325. doi: 10.1016/j.talanta.2013.08.040 -




 DownLoad:
DownLoad: