-
饮用水消毒技术的广泛应用,使得霍乱和伤寒等介水传染病的发生率大大降低。但是,消毒剂与水中天然有机物和溴/碘离子等反应,生成有毒的消毒副产物 (DBPs),如三卤甲烷、卤乙酸和溴酸盐等[1-5]。然而,水中仍有大量DBPs因为检测技术的限制而无法被鉴定识别[2, 6-11]。
cis-2-丁烯-1,4-二醛 (BDA)是近年报道的一种新型DBPs[12-15]。BDA是一种不饱和的脂肪族醛类化合物,具有强亲核性,易与氨基酸、多肽、蛋白质和DNA等生物分子快速反应[16-19]。BDA会导致氨基酸之间的交联,造成生物体中毒[18-20]。同时,BDA是DNA直接活性诱变剂,可通过与DNA反应形成诱变加合物,例如:BDA可以与肝脏核苷酸和肾脏核苷酸形成加合物,产生量为 (33 ± 21) 个加合物/108个肝脏核苷酸和 (13 ± 5) 加合物/108个肾脏核苷酸,导致DNA单链断裂和DNA交联,增加致癌风险。另有报道,BDA会刺激肝细胞增殖,导致肿瘤的形成,例如,BDA与肝脏蛋白质共价结合,增加小鼠的肿瘤发病率[18, 20-21]。
在水体消毒过程中,酚类和呋喃类化合物有较大的BDA生成潜能,例如,实际水体中酚类物质的最高浓度可达到10 μg·L−1,经过氯消毒后BDA的产率高达20%[12, 22- 23]。同时,大气中芳香类化合物的氧化过程[24, 25]和食品中的呋喃在生物体的代谢过程也可以产生BDA [20]。鉴于BDA的生物毒性强,筛查鉴定消毒后生成的BDA非常重要。
目前有关水体中BDA的分析检测还未能引起人们广泛的关注。Churchwell等[20]利用生物标记物2-脱氧胞苷,采用四极杆飞行时间质谱 (Quadrupole-Time-of-Flight mass spectrometer) 技术分析了肝细胞中产生的BDA,衍生生物样品中BDA用到缓冲盐、水解酶和核酸外切酶等多种试剂,操作繁琐且价格昂贵,不适用于水体中BDA的检测。Prasse等[15]采用高效液相色谱-串联质谱 (HPLC-MS/MS) 技术,用标准加入法定量了紫外/过氧化氢 (UV/H2O2) 及氯消毒过程中产生的BDA,但是该方法操作繁琐,不适用于大批量样品的测试,并且其检测范围在1—10 μmol·L−1之间,定量限较高。本文拟建立适用于大批量样品测试的外标定量分析方法。在众多分析方法中,HPLC-MS/MS由于其灵敏度高和使用方便等优点,广泛应用于未知有机化合物的筛查和已知有机化合物的定量分析等[26-33]。
鉴于BDA是反应性亲电试剂,与蛋白质中的亲核部分,包括半胱氨酸、赖氨酸和组氨酸以及DNA中的伯胺或硫醇基团,发生特异性反应[12-15]。例如,1当量的BDA与1当量的氨基或硫醇反应 (当有两种氨基酸为衍生剂时,1分子BDA中的两个羰基均会参与亲核反应,形成2分子氨基酸衍生物),产生吡咯环结构加合物[15]。BDA与N-乙酰基赖氨酸 (NAL) 形成的吡咯结构的衍生物较稳定,且在HPLC-MS/MS具有较高的响应[12, 15]。
针对近年引起关注的饮用水消毒副产物BDA分析方法不完善的问题,本文选择NAL为目标生物分子,对BDA进行衍生化,再进一步选用HPLC-MS/MS在正离子条件下,应用多反应监测 (MRM) 模式进行定量分析,建立了BDA的HPLC-MS/MS分析方法,并进行了方法学验证。
-
本文所用试剂除标准品外,其余均为分析纯。2,5-二甲氧基-2,5-二氢呋喃 (纯度 > 97%,日本TCI);甲醇、乙腈 (HPLC级,德国Merck公司);甲酸 (LC-MS级,Sigma-Aldrich);硫代硫酸钠 (分析纯,日本TCI);次氯酸钠 (4.00%—4.99%,Sigma-Aldrich);硼砂;硼酸 (国药集团);水系PTFE微孔滤膜 (0.22 μm,上海安谱);Poly-Sery HLB Pro固相萃取柱 (1 g/6 mL,CNW);实验用水均为Milli-Q Reference纯水仪 (Millipore)制备的高纯水。
-
本研究主要分析仪器为HPLC-MS/MS分析仪:高效液相色谱 (ExionLC,美国AB Sciex公司)和三重四极杆质谱仪 (Triple Quad 5500+,美国AB Sciex公司)。
-
由于BDA没有商业化标准品,本实验根据文献中的方法进行BDA的制备合成[15, 18, 23]。制备过程如下:将2.6030 g的2,5-二甲氧基-2,5-二氢呋喃溶解于20.0 mL的超纯水中,配制成1 mol·L−1的溶液,将此无色透明溶液转移至密封瓶中,放置到水浴锅中以55 ℃条件恒温反应18 h。反应结束后液体为黄色。对溶液进行提纯,在90 ℃条件下,对该黄色溶液进行减压蒸馏除去溶液中的水,得到橙黄色油状液体,将此液体作为BDA的标准品使用。制备所得BDA的纯度通过以下方法鉴定:对BDA及其原料2,5-二甲氧基-2,5-二氢呋喃进行薄层色谱分析,在产物点区域未发现原料点;同时HPLC-紫外检测器和MS谱图中,均未发现杂峰,说明BDA的原料完全转化为BDA且无其他物质生成,制备所得的BDA的纯度满足分析要求。
因为BDA在质谱中无可电离的官能团,无法直接测定,所以需要对BDA进行衍生化前处理。文献中常用的衍生化试剂有:NAL、谷胱甘肽 (GSH) 和N-乙酰基半胱氨酸 (NAC) 等[15, 18]。从BDA的衍生物的稳定性和信号强度等方面考虑,本文选用NAL为衍生化试剂。取样5 mL后,添加氢氧化钠或盐酸调节溶液pH为中性,加入50 μL 50 mmol·L−1的NAL,在室温条件下反应24 h后,进行HPLC-MS/MS分析。衍生反应如图1所示(本文测定的BDA均为与NAL的衍生物 (m/z 255.1) 的碎片离子m/z 209.1和m/z 167.1)。
-
色谱柱:Phenomenex Kinetex® C18 100 Å LC Column (100 mm × 2.1 mm,2.6 μm);流动相:0.05%甲酸水溶液 (A) 和甲醇 (B),梯度洗脱:0—2 min 5% B,2—5 min 5%—95% B,5—6 min 95% B,6—7 min 95% —5% B,7—8.5 min 5% B,流速为0.5 mL·min−1;柱温为35 ℃;进样量为20 μL。
-
AB SCIEX质谱仪配备电喷雾离子源 (ESI),采用正离子模式扫描,多反应监测 (MRM) 模式;质谱参数:离子化电压 (IS):4500 V;离子源温度 (TEM):550 ℃;气帘气 (CUR):20 psi;喷雾器 (GS1):15 psi;辅助加热器 (GS2):0 psi。
-
鉴于含盐系统应该使用在线 (或离线) 固相萃取 (SPE) -HPLC-MS/MS测试,但前期使用过程中发现经在线固相萃取后,BDA的峰型较差,重现性不佳。采用直接HPLC-MS/MS测试方法,使用在线切换阀将2 min前切入废液,避免盐分进入质谱,可以得到更好的峰型,且重现性更佳,因此本文均采用直接HPLC-MS/MS方法对BDA进行检测。
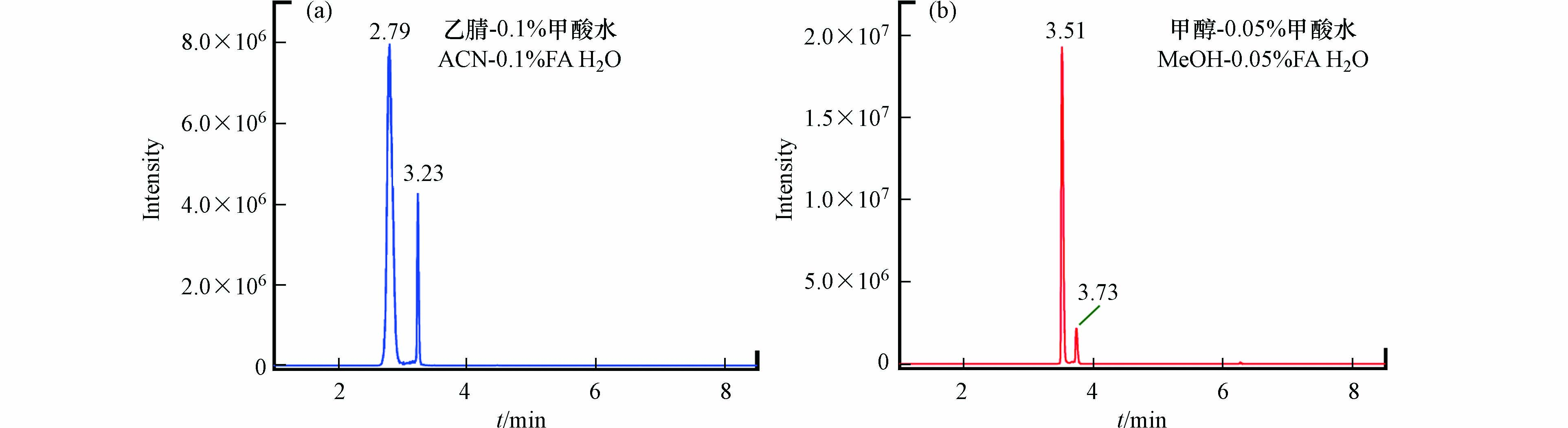
采用Phenomenex C18色谱柱分析,比较纯水、0.05%或0.1%甲酸水溶液为流动相A,甲醇、乙腈或0.1%甲酸乙腈为流动相B的效果 (注意图2仅给出最优的两种流动相所得色谱图)。因测定的是BDA与衍生剂NAL的加合物,在流动相中适量加入甲酸可以抑制[M+Na]+峰而促进[M+H]+峰的形成,能够在一定程度上改变色谱峰的峰形并提高响应值,从而提高检测的灵敏度,因此流动相A选择含甲酸的纯水。
BDA的加合物有两个碎片离子m/z 167.1和m/z 209.1,其中m/z 167.1的响应相比m/z 209.1的高,因此本文选择碎片离子m/z 167.1为定量离子。根据图2可以看出,在使用乙腈-0.1%甲酸水和甲醇-0.05%甲酸水为流动相时均有较好的峰型,但是在甲醇-0.05%甲酸水体系中峰宽较窄,峰高较高,具有更好的灵敏度,因此选择甲醇-0.05%甲酸水为测定的流动相。
采用优化的最佳色谱条件 (1.3.1) 对消毒后水中的BDA进行测定,灵敏度高,重现性好。
-
在正离子模式下,采用子离子扫描模式优化条件得到了母离子m/z 255.1的两个碎片离子m/z 167.1和m/z 209.1 (质谱碎裂过程见图1),并通过MRM模式优化了两对离子对的去簇电压和最佳碰撞能,优化的质谱条件如表1所示。
-
根据《HJ/T 168—2010》中空白实验未检出目标物质 (或检出目标物质浓度可以忽略不计) 的检出限测定方法,选择0.1 μmol·L−1作为检出限的测定浓度,配制7份BDA浓度为0.1 μmol·L−1的水样,经0.22 μm滤膜过滤后进行HPLC-MS/MS分析,7次测定结果的标准偏差 (S) 为0.012。计算得出方法的检出限 (limit of detection, LOD) 为:LOD = t0.99 × S = 3.143 × 0.012 = 0.035 μmol·L−1,方法的定量限 (limit of quantification, LOQ) 为:LOQ = 3 × LOD = 3 × 0.035 μmol·L−1 = 0.105 μmol·L−1。
-
水消毒过程后,往往有水质背景与待测物质共存,这些基质成分会造成基质效应,即基质成分改变目标化合物离子化效率,导致其测定时分析信号增强或减弱[32-33]。因此在实际消毒水体中BDA的测试,要通过基质匹配的标准曲线进行定量,以消除基质效应导致的影响。
本文分别测定了纯水和基质匹配标准溶液的标准曲线。此处的基质为模拟消毒过程中的溶液,即在50 mmol·L−1硼酸盐缓冲液中加入0.1 mmol·L−1苯酚 (BDA的前体化合物),磁力搅拌下加入3 mmol·L−1的次氯酸钠,反应1 min,以保证苯酚完全反应且BDA的生成量可以忽略不计,立即加入过量硫代硫酸钠淬灭余氯终止反应,继续搅拌15 min以上。用该溶液为基质溶液,按纯水标准曲线溶液的配制方法配制基质匹配的标准曲线溶液。由基质匹配水样的MRM色谱图和纯水标准溶液的MRM色谱图可知,BDA的峰型均较尖锐,但BDA加合物在基质匹配水样中的离子峰强度仅为在纯水中的离子峰强度的20%左右,说明基质效应抑制了BDA加合物的电离,因此对于实际样品的测定,需要选用基质匹配的标准曲线进行定量,以补偿基质效应带来的影响。
考察建立的BDA的测试方法的线性范围,BDA在浓度:0、0.05、0.1、0.2、0.5、1、2、5、10 μmol·L−1和20 μmol·L−1范围内,得到线性方程:y = 5.31×106 x,线性相关性系数R2 = 0.9945,线性相关性较好 (图3a)。由于BDA加合物的测定受到基质抑制作用,考察了基质匹配的标准曲线的线性范围,在同样的浓度范围内,所得线性方程:y = 1.38×106 x,线性相关性系数R2 = 0.9973,同样具有较好的线性相关性 (图3b)。由于没有合适的内标物质,外标法所测结果易受其他因素影响,在实际测样时,每批次均会重新测定并绘制标准曲线。
-
选择BDA浓度为0.2 μmol·L−1的样品,进行日内精密度的测定。日内精密度为一天分4个时段进行测定,得到的浓度分别为:0.177、0.194、0.180、0.180 μmol·L−1,RSD为6.24%。RSD < 10%,由此说明建立的方法对BDA的测定精密度良好。
-
为了考察方法的定量准确性,采用建立的HPLC-MS/MS方法对实际消毒水样进行了低、中、高的3个加标浓度水平的回收率测定。由表2可知,通过对空白样品和加标样品的对比分析,回收率在83.6%—112%,可以得到良好的定量结果,说明该方法用于消毒水样中BDA的测定具有较好的准确度。
-
根据文献[12],测试苯酚的在氯化过程中产生BDA的情况,向0.1 mmol·L−1的苯酚溶液中加入3 mmol·L−1的次氯酸钠,氯化30 min时可以得到18 μmol·L−1的BDA。在相同的实验条件下,本文的方法测定的BDA生成量为17 μmol·L−1,与文献结果接近。说明该方法具有较好的准确度和重现性。
水体中的有机污染物,尤其是酚类物质,在实际消毒过程中有生成BDA的潜在风险。例如:相同含量的2,4,6-三氯酚、2,6-二氯酚等的BDA产率与苯酚生成BDA的产率基本一致,达到20%左右。此结果与文献值基本一致,说明方法可以满足实验室检测需求。
在实际废水中,酚类物质的浓度可能高达10 μg·L−1,能产生约2 μg·L−1的BDA,为了能用本方法定量,可以对样品进行固相萃取 (SPE) 处理。即对100 mL (若浓度较低可以取更大体积) 实际废水,加入适量的NaOH或HCl将溶液调至中性,再加入100 μL 50 mmol·L−1的NAL储备液,室温下衍生化24 h。鉴于BDA与NAL加合物是弱极性化合物,选用HLB固相萃取小柱富集,甲醇为洗脱液。洗脱液使用高纯氮气吹干后用超纯水定容至1 mL,充分混匀溶解,用0.22 μm滤膜过滤,待测HPLC-MS/MS。本文选取0.1、0.01、0.001 μmol·L−1的3个浓度的基质匹配溶液进行SPE前处理和准确度验证,回收率为80%—108% (见表3),说明该方法经过SPE处理同样可以保障结果的准确度。
-
为了分析水消毒后新型消毒副产物BDA的生成,本文建立了以基质加标为定量方式的HPLC-MS/MS方法。方法学验证结果表明,该方法线性范围宽:0.1—20 μmol·L−1 (文献中为1—10 μmol·L−1),检出限和定量限低。低、中、高的3个浓度梯度的加标回收率较高,RSD < 10%,说明该方法具有较高的准确度和精密度。本方法测定苯酚氯化后BDA的生成量与文献值基本一致,进一步说明该方法检测结果准确,重现性好。实际消毒水体中BDA含量较低,经过SPE前处理后依然可以满足定量要求。本研究为消毒后水中不饱和醛类化合物的检测提供了可靠依据。
新型消毒副产物cis-2-丁烯-1,4-二醛的检测方法建立与评估
Establishment and evaluation of the determination method of an emerging disinfection by-product of cis-2-butene-1,4-diformaldehyde
-
摘要: 饮用水消毒对病原微生物的预防起到至关重要的作用,但是有毒有害消毒副产物的产生,对人类健康造成潜在威胁。cis-2-丁烯-1,4-二醛 (BDA) 是近年检出的新型消毒副产物,生物毒性强,具有严重的潜在危害。本文建立了BDA的高效液相色谱-串联质谱的分析方法,旨在定量筛查出水体中的BDA。结果表明:BDA的纯溶剂标准曲线和基质匹配标准曲线的线性较好,相关系数 (R2) 均大于0.99。方法检出限为0.035 μmol·L−1,定量限值为0.105 μmol·L−1。回收率在83.6% ‒ 112%之间,相对标准偏差小于10% (n = 7)。该方法能够快速高效地完成水体中BDA的分析。
-
关键词:
- 消毒 /
- 消毒副产物 /
- cis-2-丁烯-1,4-二醛 /
- 高效液相色谱-串联质谱 (HPLC-MS/MS) /
- N-乙酰基赖氨酸衍生
Abstract: Disinfection plays a crucial role in prevention of pathogenic microorganisms in drinking water. However, the formation of toxic disinfection by-products posed a potential threat to human health. An emerging disinfection by-product, cis-2-butene-1,4-dialdehyde (BDA), possesses strong biological toxicity and serious potential hazards. This study established an analytical method for BDA by using high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS), which aimed to screen and quantify BDA in water. The results showed that the linearity of standard curves in both pure water and matrix-match solution were good, with the correlation coefficients (R2) higher than 0.99. The detection limit of the method was 0.035 μmol·L−1, and the quantification limit was 0.105 μmol·L−1. The recovery rate ranged from 83.6% to 112%, and the relative standard deviation was less than 10% (n = 7). This method is able to accomplish quick and efficient analysis of BDA in water. -
表 1 MRM模式下BDA的质谱参数
Table 1. Mass spectrometry parameters of BDA in MRM mode
母离子/DaQ1 Mass/Da 子离子/DaQ3 Mass/Da 停留时间/msDwell time/ms 去簇电压/VDeclustering potential/V 碰撞能/VCollision energy/V 255.1 167.1 10 100 24 255.1 209.1 10 147 18 表 2 实际消毒水样结果验证总结
Table 2. Summary of result verificationof real disinfection water samples
加标梯度Spiking level 峰面积Area 加标浓度/(μmol·L−1)Spiking concentrition 实测浓度/(μmol·L−1)Experimental concentration 标准偏差Standard deviation 平均值Mean value RSD/% 回收率/% Recovery 空白Blank 1.09×106 0 0.15 0 0.15 3.05 — 1.09×106 0 0.16 — 1.02×106 0 0.14 — 低浓度加标Low level spiking 1.27×106 0.2 0.18 0.01 0.18 5.56 83.6 低浓度加标Low level spiking 1.24×106 0.2 0.18 0.01 0.18 5.56 97.1 1.26×106 0.2 0.18 83.6 中浓度加标Medium level spiking 7.62×106 1 1.09 0.01 1.11 1.16 93.6 7.78×106 1 1.11 96 7.85×106 1 1.12 97 高浓度加标 High level spiking 3.81×107 5 5.45 0.13 5.64 2.33 106 4.03×107 5 5.76 112 4.01×107 5 5.73 112 表 3 SPE对低浓度BDA测定准确度的影响
Table 3. The influence of SPE on the accuracy of low-concentration BDA analysis
SPE前加标浓度/ (μmol·L−1)Spiking concentration before SPE SPE后理论浓度/ (μmol·L−1)Theory concentration after SPE 实测值/ (μmol·L−1)Experimental concentration 加标回收率/ %Spiking recovery 0.1 10.0 9.25 92.5 0.01 1.0 1.08 108 0.001 0.1 0.08 80 -
[1] GHANBARI F, MORADI M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review [J]. Chemical Engineering Journal, 2017, 310: 41-62. doi: 10.1016/j.cej.2016.10.064 [2] BOND T, HUANG J, TEMPLETON M R, et al. Occurrence and control of nitrogenous disinfection by-products in drinking water - A review [J]. Water Research, 2011, 45(15): 4341-4354. doi: 10.1016/j.watres.2011.05.034 [3] RICHARDSON S D, PLEWA M J, WAGNER E D, et al. Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: A review and roadmap for research [J]. Mutation Research/Reviews in Mutation Research, 2007, 636(1/2/3): 178-242. [4] BOND T, TEMPLETON M R, GRAHAM N. Precursors of nitrogenous disinfection by-products in drinking water: A critical review and analysis [J]. Journal of Hazardous Materials, 2012, 235/236: 1-16. doi: 10.1016/j.jhazmat.2012.07.017 [5] DEBORDE M, von GUNTEN U. Reactions of chlorine with inorganic and organic compounds during water treatment—Kinetics and mechanisms: A critical review [J]. Water Research, 2008, 42(1/2): 13-51. [6] ESCHER B I, ALLINSON M, ALTENBURGER R, et al. Benchmarking organic micropollutants in wastewater, recycled water and drinking water with in vitro bioassays [J]. Environmental Science & Technology, 2014, 48(3): 1940-1956. [7] LIU X K, LIU R R, ZHU B, et al. Characterization of carbonyl disinfection by-products during ozonation, chlorination, and chloramination of dissolved organic matters [J]. Environmental Science & Technology, 2020, 54(4): 2218-2227. [8] LIU X K, LIN Y F, RUAN T, et al. Identification of N-nitrosamines and nitrogenous heterocyclic byproducts during chloramination of aromatic secondary amine precursors [J]. Environmental Science & Technology, 2020, 54(20): 12949-12958. [9] XIANG Y Y, GONSIOR M, SCHMITT-KOPPLIN P, et al. Influence of the UV/H2O2 advanced oxidation process on dissolved organic matter and the connection between elemental composition and disinfection byproduct formation [J]. Environmental Science & Technology, 2020, 54(23): 14964-14973. [10] KRASNER S W, WEINBERG H S, RICHARDSON S D, et al. Occurrence of a new generation of disinfection byproducts [J]. Environmental Science & Technology, 2006, 40(23): 7175-7185. [11] RICHARDSON S D, TERNES T A. Water analysis: Emerging contaminants and current issues [J]. Analytical Chemistry, 2018, 90(1): 398-428. doi: 10.1021/acs.analchem.7b04577 [12] PRASSE C, von GUNTEN U, SEDLAK D L. Chlorination of phenols revisited: Unexpected formation of α, β-unsaturated C4-dicarbonyl ring cleavage products [J]. Environmental Science & Technology, 2020, 54(2): 826-834. [13] MARRON E L, van BUREN J, CUTHBERTSON A A, et al. Reactions of α, β-unsaturated carbonyls with free chlorine, free bromine, and combined chlorine [J]. Environmental Science & Technology, 2021, 55(5): 3305-3312. [14] van BUREN J, PRASSE C, MARRON E L, et al. Ring-cleavage products produced during the initial phase of oxidative treatment of alkyl-substituted aromatic compounds [J]. Environmental Science & Technology, 2020, 54(13): 8352-8361. [15] PRASSE C, FORD B, NOMURA D K, et al. Unexpected transformation of dissolved phenols to toxic dicarbonyls by hydroxyl radicals and UV light [J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(10): 2311-2316. doi: 10.1073/pnas.1715821115 [16] KELLERT M, WAGNER S, LUTZ U, et al. Biomarkers of furan exposure by metabolic profiling of rat urine with liquid chromatography-tandem mass spectrometry and principal component analysis [J]. Chemical Research in Toxicology, 2008, 21(3): 761-768. doi: 10.1021/tx7004212 [17] CHEN L J, HECHT S S, PETERSON L A. Characterization of amino acid and glutathione adducts of cis-2-butene-1,4-dial, a reactive metabolite of furan [J]. Chemical Research in Toxicology, 1997, 10(8): 866-874. [18] PETERSON L A, CUMMINGS M E, CHAN J Y, et al. Identification of a cis-2-butene-1, 4-dial-derived glutathione conjugate in the urine of furan-treated rats [J]. Chemical Research in Toxicology, 2006, 19(9): 1138-1141. doi: 10.1021/tx060111x [19] GATES L A, LU D, PETERSON L A. Trapping of cis-2-butene-1, 4-dial to measure furan metabolism in human liver microsomes by cytochrome P450 enzymes [J]. Drug Metabolism and Disposition:the Biological Fate of Chemicals, 2012, 40(3): 596-601. doi: 10.1124/dmd.111.043679 [20] CHURCHWELL M I, SCHERI R C, von TUNGELN L S, et al. Evaluation of serum and liver toxicokinetics for furan and liver DNA adduct formation in male Fischer 344 rats [J]. Food and Chemical Toxicology, 2015, 86: 1-8. doi: 10.1016/j.fct.2015.08.029 [21] CHEN L J, HECHT S S, PETERSON L A. Identification of cis-2-butene-1, 4-dial as a microsomal metabolite of furan [J]. Chemical Research in Toxicology, 1995, 8(7): 903-906. doi: 10.1021/tx00049a001 [22] ACERO J L, PIRIOU P, von GUNTEN U. Kinetics and mechanisms of formation of bromophenols during drinking water chlorination: Assessment of taste and odor development [J]. Water Research, 2005, 39(13): 2979-2993. doi: 10.1016/j.watres.2005.04.055 [23] PIRIOU P, SOULET C, ACERO J L, et al. Understanding medicinal taste and odour formation in drinking waters [J]. Water Science and Technology, 2007, 55(5): 85-94. doi: 10.2166/wst.2007.166 [24] WANG L M, WU R R, XU C. Atmospheric oxidation mechanism of benzene. Fates of alkoxy radical intermediates and revised mechanism [J]. The Journal of Physical Chemistry. A, 2013, 117(51): 14163-14168. doi: 10.1021/jp4101762 [25] WU R R, PAN S S, LI Y, et al. Atmospheric oxidation mechanism of toluene [J]. The Journal of Physical Chemistry. A, 2014, 118(25): 4533-4547. doi: 10.1021/jp500077f [26] 黄晓梅, 吴杨, 崔君涛, 等. 高分辨质谱在氯化石蜡分析方法中的应用 [J]. 分析化学, 2019, 47(3): 323-334. doi: 10.1016/S1872-2040(19)61144-8 HUANG X M, WU Y, CUI J T, et al. Applications of high-resolution mass spectrometry in determination of chlorinated paraffins [J]. Chinese Journal of Analytical Chemistry, 2019, 47(3): 323-334(in Chinese). doi: 10.1016/S1872-2040(19)61144-8
[27] 黄林艳, 鲁炳闻, 赵彦辉, 等. 高效液相色谱分析四溴双酚A标准样品方法优化及应用 [J]. 环境化学, 2020, 39(12): 3524-3530. HUANG L Y, LU B W, ZHAO Y H, et al. Optimization and application of HPLC method for tetrabromobisphenol A reference material analysis [J]. Environmental Chemistry, 2020, 39(12): 3524-3530(in Chinese).
[28] 孙腾飞, 向垒, 陈雷, 等. 环境水样及固相样品中全氟化合物分析方法研究进展 [J]. 分析化学, 2017, 45(4): 601-610. doi: 10.11895/j.issn.0253-3820.160817 SUN T F, XIANG L, CHEN L, et al. Research progresses of determination of perfluorinated compounds in environmental water and solid samples [J]. Chinese Journal of Analytical Chemistry, 2017, 45(4): 601-610(in Chinese). doi: 10.11895/j.issn.0253-3820.160817
[29] 康春莉, 王英, 杜尧国, 等. 水体中挥发酚测定方法的改进 [J]. 分析化学, 2000, 28(7): 872-875. doi: 10.3321/j.issn:0253-3820.2000.07.019 KANG C L, WANG Y, DU Y G, et al. Improvement on the determination of volatile phenols in water [J]. Chinese Journal of Analytieal Chemistry, 2000, 28(7): 872-875(in Chinese). doi: 10.3321/j.issn:0253-3820.2000.07.019
[30] 高婷婷, 杜鹏, 徐泽琼, 等. 污水中常见违禁药物分析方法优化及验证 [J]. 环境科学, 2017, 38(1): 201-211. GAO T T, DU P, XU Z Q, et al. Optimization and validation of the analytical method to detect common illicit drugs in wastewater [J]. Environmental Science, 2017, 38(1): 201-211(in Chinese).
[31] 寇弘儒, 刘士峰, 孙艳超, 等. 基于两种前处理对苹果叶片中虫酰肼残留的液相分析方法 [J]. 环境化学, 2020, 39(1): 179-187. doi: 10.7524/j.issn.0254-6108.2019061201 KOU H R, LIU S F, SUN Y C, et al. Liquid phase analysis of tebufenozide residues in apple leaves based on two pretreatments [J]. Environmental Chemistry, 2020, 39(1): 179-187(in Chinese). doi: 10.7524/j.issn.0254-6108.2019061201
[32] ZHANG Z P, ZHANG R J, XIAO H, et al. Development of a standardized food model for studying the impact of food matrix effects on the gastrointestinal fate and toxicity of ingested nanomaterials [J]. NanoImpact, 2019, 13: 13-25. doi: 10.1016/j.impact.2018.11.002 [33] CAPPIELLO A, FAMIGLINI G, PALMA P, et al. Overcoming matrix effects in liquid chromatography-mass spectrometry [J]. Analytical Chemistry, 2008, 80(23): 9343-9348. doi: 10.1021/ac8018312 -






 下载:
下载: