-
近年来,国内外大力推广新能源汽车。磷酸铁锂作为理想的动力电池正极材料,被广泛应用于新能源汽车动力电池的制造 [1-5]。磷酸铁锂电池业已成为新能源汽车行业动力电池的主流品种。动力电池使用年限一般为3—5年,随着新能源汽车销量的稳步增长和磷酸铁锂电池用量的不断上升,磷酸铁锂电池的报废量将逐年增加[6-8],开发废旧磷酸铁锂电池资源化、高值化利用技术尤为迫切。目前,废旧磷酸铁锂电池资源化的工艺主要侧重于锂的回收提取,缺乏对剩余磷酸铁高值化利用的有效途径[9].
我国是纺织品生产和出口第一大国,印染行业的发展导致了印染废水大量排放等问题。在印染行业使用的众多有机染料中,偶氮染料的使用量占80%左右。偶氮染料具有色度高、难降解的特点,其废水处理技术一直是研究的热点[10-11]。多相光助-芬顿技术是一种高效降解水中有机污染物的方法,其优点是能够避免铁离子的二次污染、拓宽适用的pH值范围,在染料等难降解废水处理技术开发中倍受关注[12-14]。该技术开发的核心是高效、稳定多相催化剂的研制。国内外众多学者对铁氧化物、铁硫化物和铁负载型等铁基催化剂多相光助-芬顿催化过程的讨论最为深入[15-16]。研究表明[17],在铁基催化剂光助-芬顿催化过程中,多相催化剂不仅是引发均相芬顿反应铁离子的源,而且是铁离子以固相形式回收的汇。通过调控多相催化剂上铁离子的释放过程,能够明显促进体系对难降解有机污染物的矿化效率[18]。目前,促进多相催化剂上铁离子释放的方法主要有络合法[15]和还原法[19]等两大类。络合法促进多相催化剂释放的是络合态的铁离子,而还原法能够使多相催化剂直接释放亚铁离子,因而对芬顿反应增效能力更为显著[19]。通过添加强还原能力的试剂,可以使催化剂表面的FeⅢ直接化学还原为FeⅡ,进而促进催化剂亚铁离子的释放 [20-22]。相比较而言,利用苯醌等光敏性物质光照产生水合电子的还原法,不仅能促进催化剂亚铁离子的转化和释放,而且可以为体系中·OH的生成提供新的途径,体系对有机污染物的降解能力更强[23]。
磷酸铁和磷酸亚铁的溶度积常数分别为1.3×10−22和1×10−36 [24],利用可见光下苯醌类化合物产生的光生水合电子 [25-26],可以将磷酸铁还原成磷酸亚铁,有望促进亚铁离子的释放并引发均相芬顿反应降解有机污染物;利用磷酸铁难溶性的特点,可以将均相芬顿反应转化的铁离子以固体形式再次回收并循环利用。将磷酸铁作为芬顿反应铁源的开发,是对其高值化利用的一种有效途径,但迄今为止,其芬顿反应过程中催化剂铁离子源汇机制的研究鲜见报道。
本文探讨了可见光下苯醌类化合物对磷酸铁释放亚铁离子的影响,以偶氮染料橙Ⅱ作为探针分子,考察了可见光下苯醌类化合物诱导磷酸铁芬顿反应降解橙Ⅱ的效率,分析了橙Ⅱ降解过程中铁离子和亚铁离子之间的转化以及羟基自由基浓度的变化,探明了可见光下苯醌诱导磷酸铁芬顿反应铁离子的源汇机制。通过这些研究,开发了磷酸铁用于有机废水处理高值化利用的途径,为废旧磷酸铁锂电池的资源化利用提供理论依据。
-
QE65Pro光谱仪(美国OceanOptics);Optima2100DVICP发射光谱仪(美国PerkinElmer公司); UV-2300紫外分光光度计(中国上海天美科学仪器);79-1磁力加热搅拌器(金坛市大地自动化仪器厂);Multi N/C2100总有机碳分析仪(德国耶拿分析仪器股份有限公司);PCX50C Discover多通道光催化反应系统(北京泊菲莱科技有限公司)。
-
以下试剂无特殊说明均为分析纯。橙Ⅱ(生物染色剂)、30% H2O2、盐酸、硝酸、硫酸,邻菲罗啉、乙酸、乙酸铵、磷酸二氢铵购于西陇科学有限公司;99% CTAB购于上海思域化工科技有限公司;九水硝酸铁、氨水、磷酸、乙醇、硫酸钛(化学纯)、磷酸氢二钠、磷酸二氢钠均购于国药集团化学试剂有限公司;苯醌(99%)购于麦克林生化科技有限公司;香豆素、对二甲基醌购于阿拉丁生化科技股份有限公司;2-氯-1,4-苯醌(95%)购于上海吉至生化科技有限公司。试剂溶液均用去离子水配置。
-
磷酸铁用沉淀法制备,具体步骤如下:将一定量的去离子水和铁源质量1.5%的表面活性剂CTAB加入三口烧瓶中,置于设定温度的水浴锅中。称取相同摩尔量的硝酸铁和磷酸二氢铵,分别配成等体积的溶液(铁离子与磷酸根物质的量之比为1:1);将二者同时滴加到上述三口烧瓶中,持续搅拌至溶液滴加完毕,停止加热,冷却至室温;抽滤、醇洗、水洗、干燥得到FePO4·xH2O。以上反应过程中用磷酸和氨水控制反应溶液pH值在2.0—2.5,控制反应温度70 ℃,在此情况下磷酸铁结晶水含量x=2.0—2.2。产物研磨过200目筛装瓶,避光保存。
-
实验在PCX50C Discover多通道光催化反应系统中进行,主机有9个光催化反应位,每个光反应位配有一个50 mL平底石英反应瓶,底部设有磁力搅拌装置,转速设定为200 r·min−1;反应通道底部光源为白光可见光(PCX50 LED灯珠白光);体系由循环恒温水控制反应温度为30 ℃。
橙Ⅱ降解实验:橙Ⅱ初始浓度为0.2 mmol·L−1,加入盐酸调节初始pH值为3.0,磷酸铁用量为1 g·L−1,过氧化氢用量为10 mmol·L−1。实验先于无光情况下搅拌30 min达到吸附平衡,而后加入过氧化氢,打开光源,开始反应计时。
橙Ⅱ降解循环实验:在催化剂对橙Ⅱ进行暗吸附30 min和3 h脱色后,对其进行过滤、洗涤、干燥、研磨、回收。利用回收的催化剂分别进行橙Ⅱ第二次循环脱色实验,并监测总铁离子浓度的变化。第三次循环实验的操作与第二次循环实验相同。
总铁离子和亚铁离子的测定预先用0.22 μm水系滤头过滤,其他测定项目预先用0.45 μm水系滤头过滤。
-
橙Ⅱ浓度采用分光光度法测定:在设定的时刻取样、过滤,于484 nm处测得吸光度值,用标准曲线计算得到橙Ⅱ浓度。亚铁离子浓度采用邻菲罗啉分光光度法测定;总铁离子浓度采用原子吸收法测定;TOC利用有机碳分析仪进行测定;过氧化氢浓度采用磷酸钛分光光度法测定;羟基自由基浓度利用香豆素荧光光度法测定[15]。实验过程中每组数据重复3次,取平均值。
-
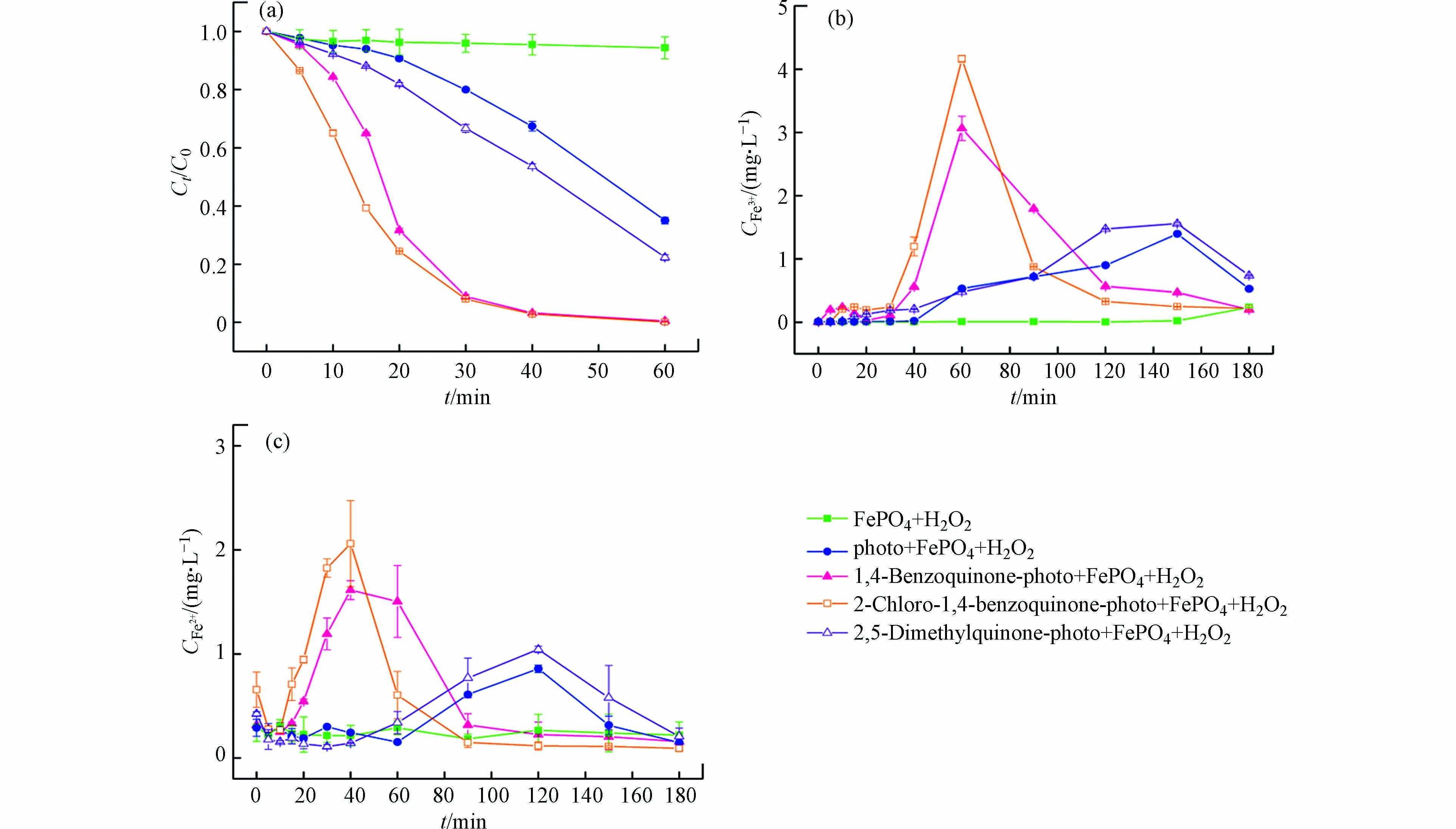
图1(a)是橙Ⅱ在不同反应体系中脱色情况的对比,图1(b)和1(c)是橙Ⅱ脱色过程中不同体系铁离子和亚铁离子浓度随时间的变化。
由图1(a)可见,磷酸铁-过氧化氢体系对橙Ⅱ几乎没有降解能力,橙Ⅱ在60 min内基本没有脱色。磷酸铁是难溶化合物,即使在pH 3.0时的10 mmol·L−1的H2O2溶液中仍然很稳定,溶液中铁离子和亚铁离子的生成可以忽略不计(见图1b和1c)。该结果说明,磷酸铁上固态的铁离子(FeⅢ)无法有效催化过氧化氢产生羟基自由基。
可见光-磷酸铁-过氧化氢体系中,橙Ⅱ在反应20 min后脱色明显,反应60 min时脱色率可达65%(见图1a)。研究表明[27],偶氮染料能够吸收可见光转化成激发态,后者能够与固体催化剂表面的FeⅢ发生电子转移从而将FeⅢ还原成FeⅡ。对于磷酸铁固体,当其转化成磷酸亚铁时,亚铁离子的溶解度急剧增加。根据两者浓度积可知,溶液中铁离子的理论平衡浓度可以由1.14×10−11 mol·L−1(Fe3+)增加到6.31×10−8 mol·L−1(Fe2+)。磷酸亚铁释放的亚铁离子可以与过氧化氢发生芬顿反应产生羟基自由基,进而将橙Ⅱ降解脱色。可见光无法有效光解过氧化氢产生羟基自由基[28],因此橙Ⅱ在反应后期脱色的原因主要是均相芬顿反应所致,具体的反应过程如式(1)—(4)所示。由于溶液中Fe2+能通过芬顿反应快速转化成Fe3+,因此该体系在60 min内只能检测到Fe3+浓度的增加(见图1b)。
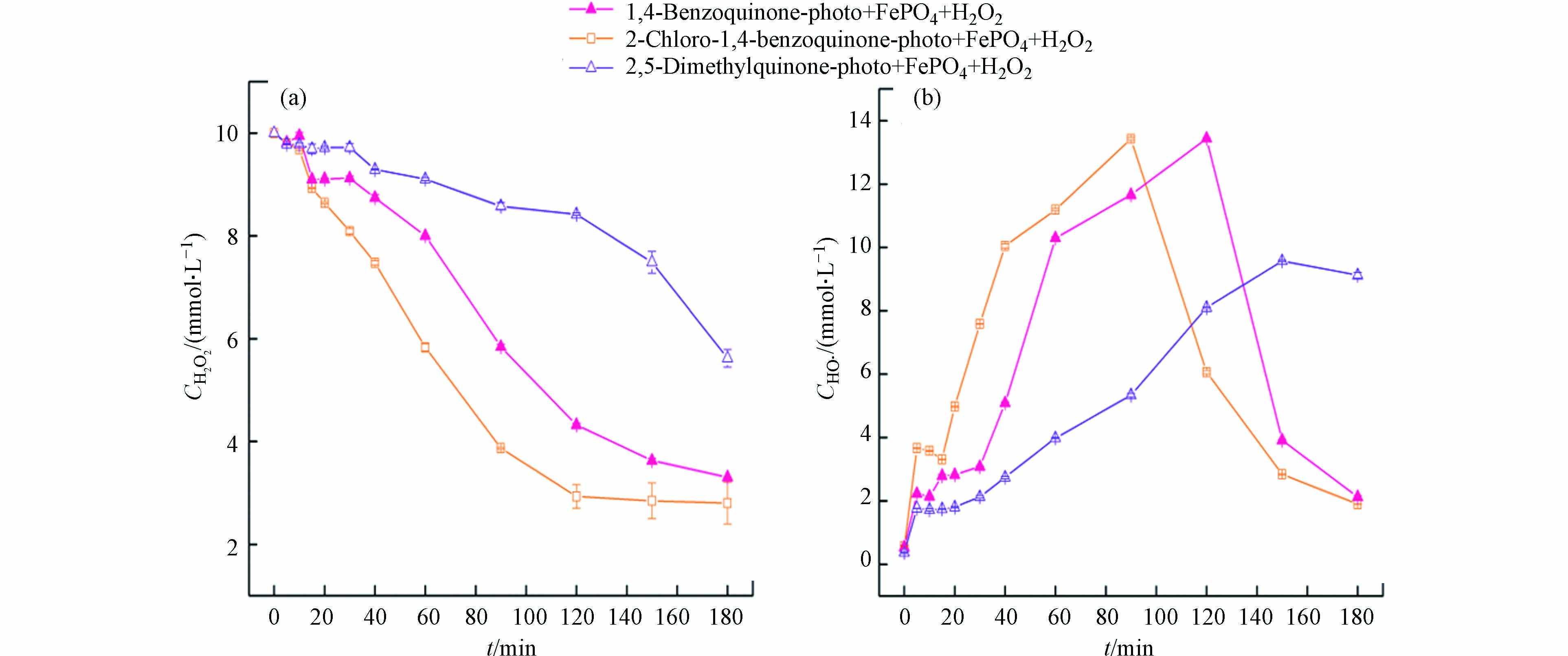
将苯醌类化合物加入到可见光-磷酸铁-过氧化氢体系时,由于苯醌类化合物光敏性极高,在紫外光[18]和可见光下[29]均能释放出大量的水合电子eaq−(式5—7)。水合电子能高效地使磷酸铁转化成磷酸亚铁(式8),并进行如式(3)和(4)的反应。需要指出的是,相对于激发态橙Ⅱ电子转移还原磷酸铁的能力,苯醌类化合物光生水合电子的还原能力更为高效,其铁离子和亚铁离子浓度明显增加(图1b、1c)。继而,溶液均相芬顿反应激发并导致橙Ⅱ的脱色速率明显增加。由图1(a)可见,添加苯醌、2-氯-1,4-苯醌和对二甲基醌的体系,反应20 min时橙Ⅱ的脱色率分别为68.3%、75.5%和18.1%;反应60 min时,橙Ⅱ的脱色率分别增加到99.5%、98.1%和77.7%。由图1(c)可见,添加2-氯-1,4-苯醌、苯醌和对二甲基醌的体系,溶液中亚铁离子浓度峰值(对应时间)从基础实验的0.86 mg·L−1 (120 min)分别提高到2.06 mg·L−1 (40 min)、1.61 mg·L−1 (40 min)和1.04 mg·L−1 (120 min)。
添加的苯醌类化合物中,存在不同的吸电子基团和给电子基团,其对苯环的作用是吸电子诱导和给电子共轭两种效应的综合结果。当和苯环直接相连的是—Cl时,其吸电子诱导效应大于给电子共轭效应,钝化苯环;当和苯环直接相连的是—CH3基团时,基团给电子共轭效应大于吸电子诱导效应,具有活化苯环的作用[30]。相对于2-氯-1,4-苯醌和苯醌,对二甲基苯醌更易被活性物质·OH降解,无法持续产生光生水合电子,中断了亚铁离子的持续转化,减弱了体系对橙Ⅱ的降解能力。



苯醌类化合物-可见光-磷酸铁-过氧化氢体系中,橙Ⅱ的降解主要通过均相芬顿反应完成,磷酸铁参与的催化过程可以分为3个阶段:反应初期,磷酸铁还原成磷酸亚铁并将亚铁离子释放到溶液中;反应中期,均相芬顿反应产生羟基自由基降解橙Ⅱ;反应后期,沉淀反应使铁离子以磷酸铁形式回收。其中,磷酸亚铁的生成和亚铁离子的释放是橙Ⅱ降解的决定步骤。由图1(c)可见,反应初期,亚铁离子释放速度(式3)会超过均相芬顿反应速度(式4),导致溶液中亚铁离子浓度会高于铁离子浓度;反应中期,随着苯醌类化合物的降解,亚铁离子释放受阻,芬顿反应将亚铁离子快速转化为铁离子,使得后者浓度增加;反应后期,当沉淀反应(式9)占优时,溶液中铁离子浓度会重新下降并以磷酸铁沉淀的形式再次回收。因此,同一苯醌类化合物-可见光-磷酸铁-过氧化氢体系,亚铁离子浓度的峰值出现总先于铁离子的峰值,且反应180 min时溶液中铁离子浓度会大幅降低至0.2 mg·L−1左右(图1b)。
研究表明[18],苯醌类化合物在光的照射下会生成苯酚,苯酚会进一步生成苯酚正离子自由基和光生水合电子。本质上,在苯醌类化合物-可见光-磷酸铁-过氧化氢体系中,磷酸铁所起的作用是间接的铁源和直接的铁汇,而苯醌类化合物的光生水合电子则是铁源释放的激发物质。由图1(c)可见,苯醌类化合物光生水合电子的还原能力明显强于橙Ⅱ激发态,因此亚铁离子能够更为有效地释放,其相应体系中亚铁离子浓度的峰值增加、对应的时间提前。当不同的苯醌类化合物加入到可见光-磷酸铁-过氧化氢体系中时,橙Ⅱ脱色效率由高到低的顺序为:2-氯-1,4-苯醌 > 苯醌 > 对二甲基醌(图1a),该结果与亚铁离子浓度的变化趋势相一致(图1c)。
-
苯醌类化合物产生光生水合电子的效率决定了亚铁离子的释放,进而影响体系H2O2分解和·OH生成。需要指出的是,在该体系中,羟基自由基除主要由芬顿反应外,还可以通过光生水合电子与H2O2直接反应产生(式10)[31-32],因此氧化性物种的生成途径更为丰富。苯醌类化合物-可见光-磷酸铁-过氧化氢体系中,羟基自由基和过氧化氢浓度变化如图2所示。
由图2可见,在催化反应初期,溶液中铁离子和亚铁离子较低,导致均相芬顿反应不足、过氧化氢分解缓慢、羟基自由基浓度增加不明显。在催化反应中期,大量释放于溶液中的亚铁离子会快速催化分解过氧化氢并产生羟基自由基,因此后者浓度急剧增加。2-氯-1,4-苯醌、苯醌和对二甲基醌均能明显促进可见光-磷酸铁-过氧化氢体系羟基自由基的生成,羟基自由基最大浓度及其对应的时间分别为13.43 μmol·L−1 (90 min)、13.44 μmol·L−1(120 min)和9.57 μmol·L−1(150 min)。此外,对比图1(b)与图2可知,体系羟基自由基的最大浓度与Fe2+浓度呈正相关,但羟基自由基浓度的峰值要滞后于Fe2+浓度的峰值。该结果进一步证明Fe2+的生成和释放是氧化性物种羟基自由基生成的决定因素。在催化反应后期,铁离子和过氧化氢浓度下降、羟基自由基生成不足,同时体系中有机物对羟基自由基消耗以及羟基自由基的相互淬灭(式11),这些作用导致体系中羟基自由基浓度下降明显。羟基自由基相互淬灭会再次生成过氧化氢,使得后者在催化反应后期能维持一定浓度(见图2a)。
氧化性物种羟基自由基的形成,能够高效降解体系中的有机物。在可见光-磷酸铁-过氧化氢体系加入苯醌类化合物可以促进羟基自由基的生成;同时,苯醌类化合物自身也是有机化合物,必然会与橙Ⅱ竞争羟基自由基。测定体系TOC的变化情况,可以比较各体系对有机物矿化的效果,结果如图3所示。3种苯醌类化合物和橙Ⅱ被羟基自由基完全降解的理论化学反应式如式(12) —(15) 所示:
反应体系中,橙Ⅱ和3种苯醌类化合物初始浓度均为0.2 mmol·L−1,但反应式中羟基自由基的系数不同,因此随体系TOC的减低消耗羟基自由基的主要有机物是橙Ⅱ。比较图2和图3可见,反应体系有机物的矿化与羟基自由基浓度相对应:羟基自由基浓度低时,体系有机物矿化不显著;羟基自由基浓度急剧增加时,体系有机物矿化度明显增加。当分别外加2-氯-1,4-苯醌、苯醌和对二甲基醌到可见光-磷酸铁-过氧化氢体系时,体系有机物矿化率差异明显:反应60 min时,分别为33.5%、22.7% 和5.53%;反应180 min时,分别为78.8%、77.6%和52.4%。一方面,由3种苯醌类化合物矿化的化学反应式可知,尽管对二甲基醌易于降解但相同浓度下对二甲基醌完全矿化所需的羟基自由浓度最高;另一方面,对二甲基醌还原磷酸铁释放亚铁离子的能力最弱。这些因素导致3种苯醌类化合物中,对二甲基醌促进橙Ⅱ脱色(图1a)和矿化(图3)的能力最差。
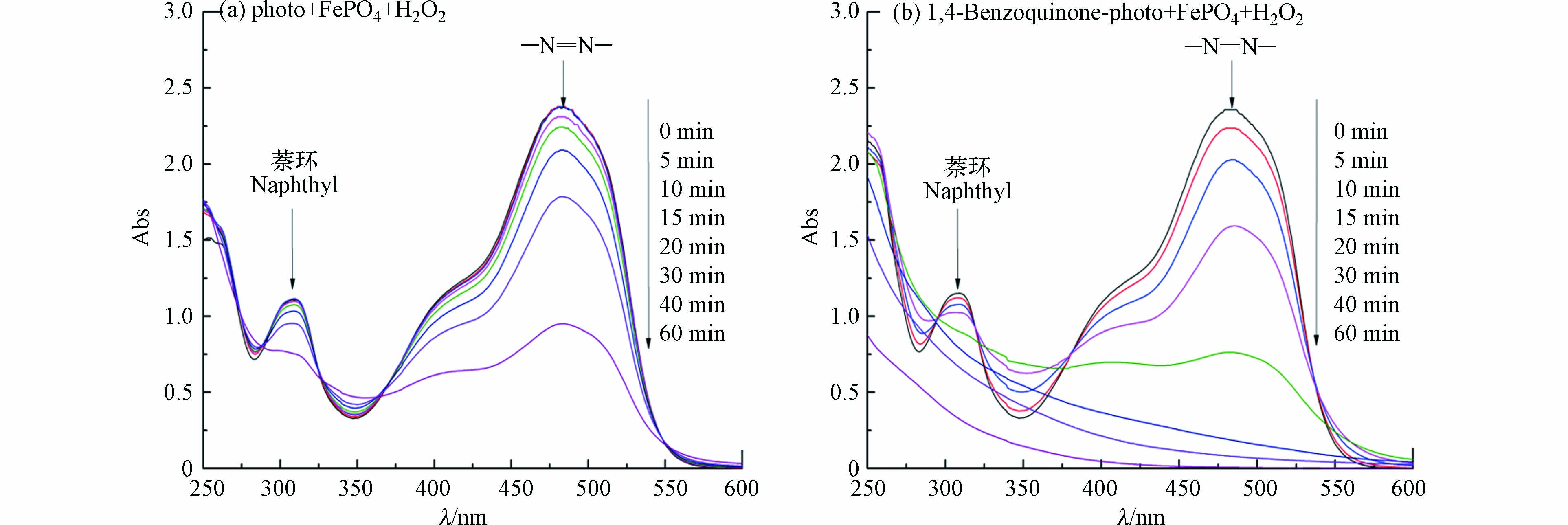
对降解体系不同时刻进行全波长扫描,可以直观地分析橙Ⅱ的降解过程。以苯醌-可见光-磷酸铁-过氧化氢体系为例,其不同反应时刻体系全波长扫描结果如图4所示。可见,相对于可见光-磷酸铁-过氧化氢体系,苯醌的加入可以明显促进体系中橙Ⅱ结构中萘环(310 nm)和偶氮键(484 nm)的破坏,促进橙Ⅱ的脱色和矿化。橙Ⅱ的脱色只需要破坏偶氮键即可,而其矿化则需要将C、H和N等元素彻底氧化(式15),因此橙Ⅱ的脱色总是先于矿化完成,该规律与课题组前期的研究结果相一致[18-19]。
-
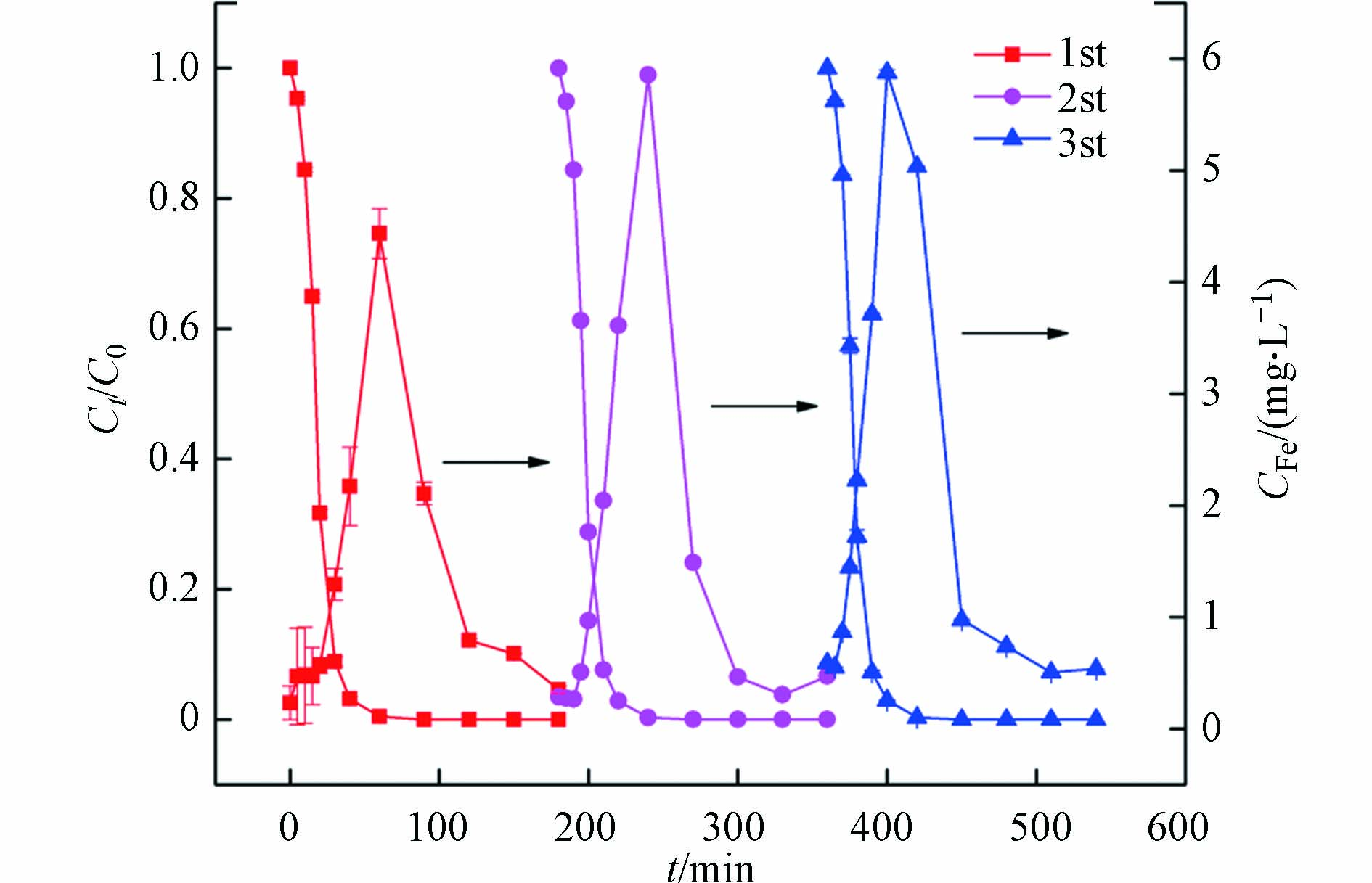
在苯醌类化合物-可见光-磷酸铁-过氧化氢体系中,磷酸铁主要的作用是亚铁离子释放的间接源和三价铁离子沉淀的直接汇。为了讨论磷酸铁对亚铁离子释放和三价铁离子回收的重现性,分析了磷酸铁循环使用过程中橙Ⅱ脱色和总铁离子浓度变化曲线,结果如图5所示。
由图5可见,磷酸铁循环使用过程中,溶液中的总铁离子呈现规律性的释放和回收现象。该结果说明,在苯醌-可见光-磷酸铁-过氧化氢体系中,磷酸铁能够作为铁离子稳定的源和汇。在循环反应的起始时刻,溶液中总铁离子在0.2—0.6 mg·L−1之间,反映了磷酸铁高度的化学稳定性;在循环反应前期,磷酸铁能够被苯醌光生水合电子还原并释放亚铁离子,使得溶液中铁离子浓度增加并引发均相芬顿反应产生羟基自由基,从而导致橙Ⅱ快速脱色和苯醌降解;在循环反应后期,苯醌光生水合电子不足限制了磷酸铁的持续还原,由此三价铁离子的沉淀反应超过亚铁离子的释放,溶液中铁离子浓度会急剧降低;在循环反应结束时,沉淀作用使得总铁离子浓度重新降至0.2—0.4 mg·L−1左右,可以有效避免铁离子的二次污染。需要指出的是,在橙Ⅱ的脱色过程中,铁离子浓度的峰值会随循环次数的增加而略有增大,说明再沉淀的磷酸铁对铁离子的释放能力有所增加,这也是橙Ⅱ的脱色速率随循环次数增加而略有提高的原因。
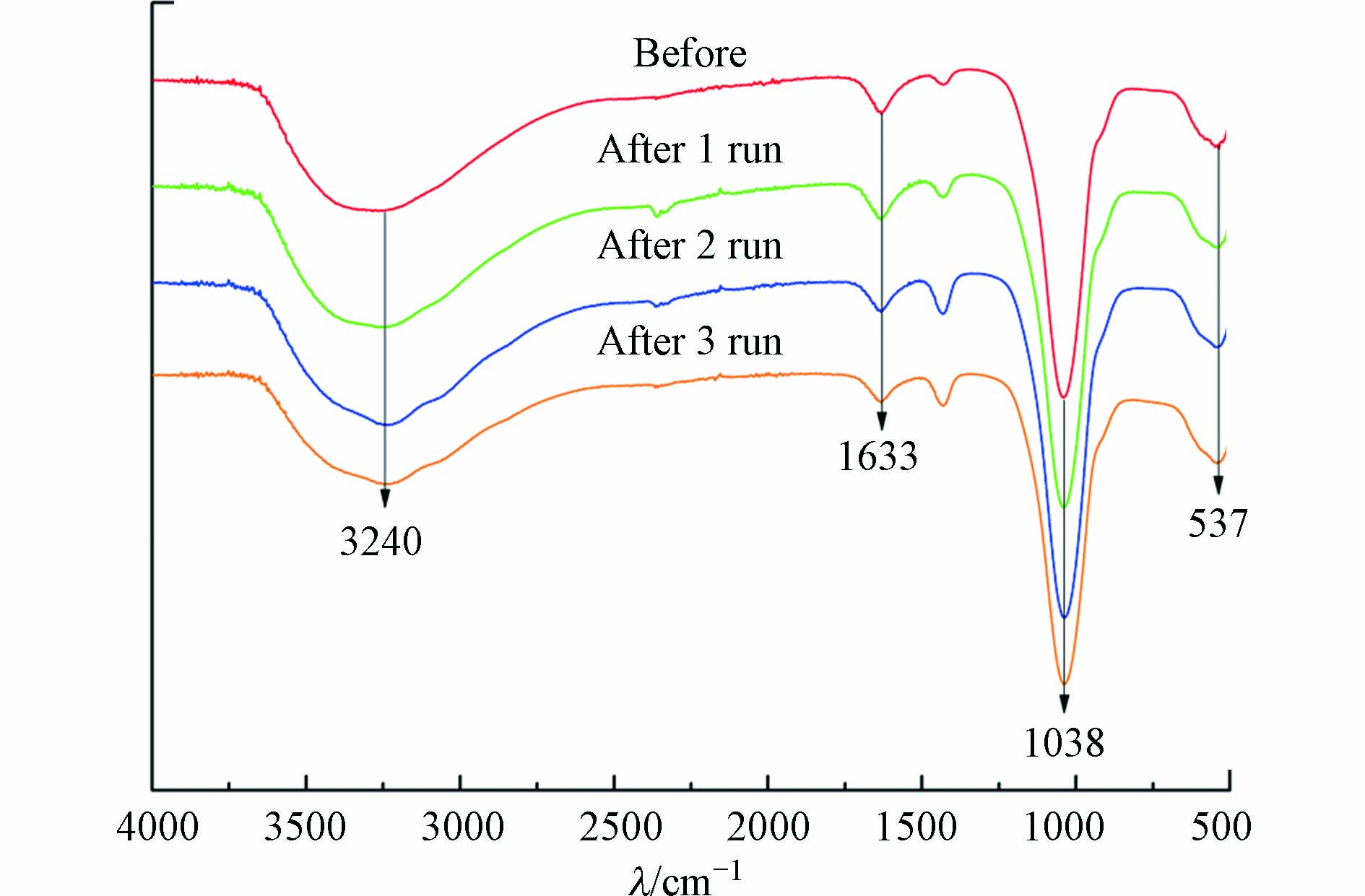
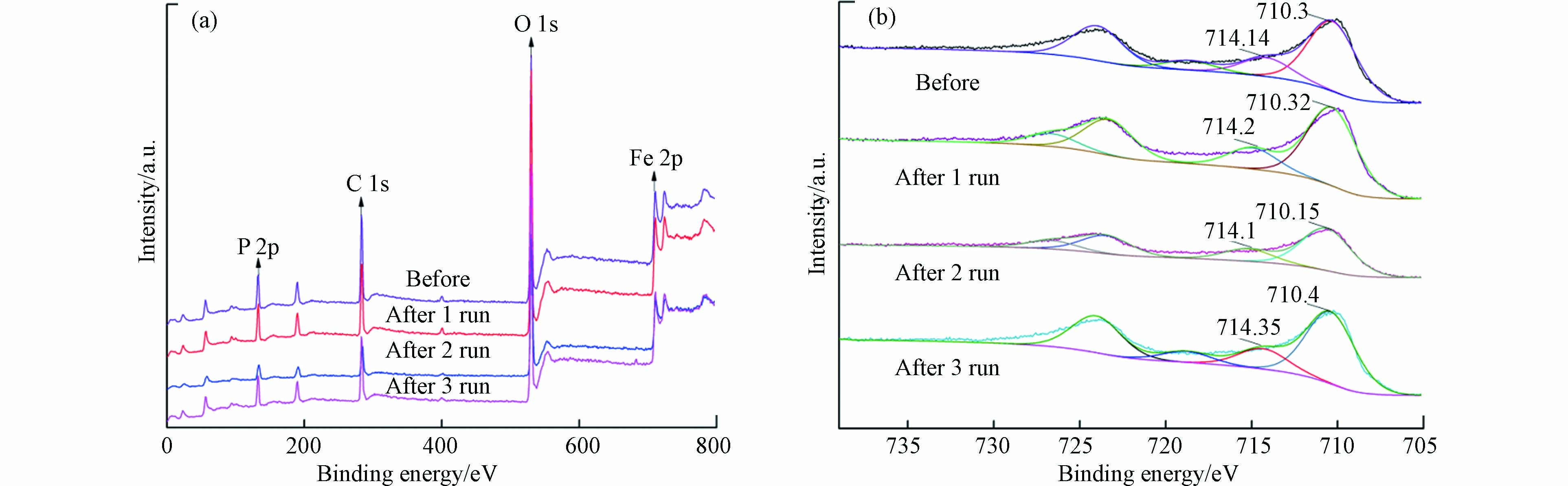
为考察循环使用过程中磷酸铁结构的重现性,对循环使用前后进行了对比,其红外光谱和XPS全光谱见图6和图7。由图6可知,磷酸铁傅里叶红外光谱在波数537、1038、1633、3240 cm−1处有较强的吸收峰,分别对应Fe—O的不对称伸缩振动[33]、P—O—P的反对称伸缩振动、O—H的弯曲振动和结晶水有关的振动[34]。磷酸铁的官能团在多次循环后均未发生明显改变,说明催化反应结束后磷酸铁具有较高的结构重现性。由图7(a)可知, XPS全光谱表明磷酸铁表面主要由Fe、O、P、C等元素组成,该结果与XRF元素分析结果明显不符,后者显示磷酸铁主要由Fe、P、O等3种元素组成,且三者的物质的量之比为1:1:4。由此可以推断,XPS全光谱中显示的C元素可能来自测试过程中加入的碳粉(作为样品基底)或吸附自空气中的CO2。图7(b)是磷酸铁Fe 2P高分辨谱图,710.3 eV和714.14 eV处的峰表明其表面有大量的FeⅢ[35],且Fe—O的结合能在循环前后未发生较大变化,说明磷酸铁在循环使用后具有较高的化学重现性。
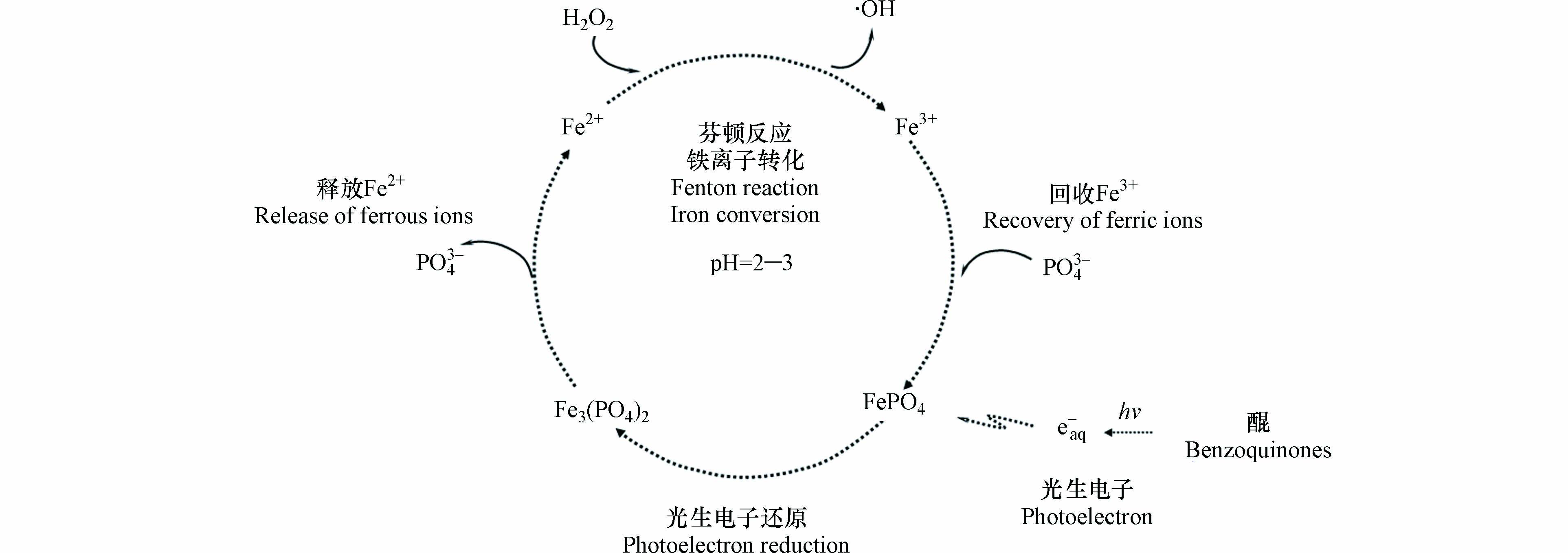
综合以上讨论,可见光下苯醌类化合物诱导磷酸铁芬顿反应的整个过程分为磷酸铁还原、亚铁离子释放、均相芬顿反应、三价铁离子再沉淀等步骤,具体机制如图8所示。
首先,是磷酸铁还原。可见光照射下苯醌类化合物产生光生水合电子,其还原作用将稳定、溶解度低的磷酸铁转化为不稳定、溶解度高的磷酸亚铁,该步骤是整个催化过程的关键步骤(式5—8)。
其次,是亚铁离子释放。溶解度高的磷酸亚铁在酸性条件下释放亚铁离子,导致溶液中亚铁离子浓度增加,该步骤是整个催化过程的决定步骤(式3)。
然后,是均相芬顿反应。亚铁离子与溶液中的过氧化氢发生芬顿反应(式4),体系生成的羟基自由基氧化降解水中的有机污染物橙Ⅱ和苯醌类化合物(式12—15);伴随着苯醌类化合物的降解,其光生水合电子浓度不足,导致亚铁离子释放受限;同时,溶液中的芬顿反应可以将亚铁离子快速转化为铁离子(式4),其结果是亚铁离子浓度出现峰值后急剧下降、铁离子浓度快速上升。
最后,是三价铁离子再沉淀。铁离子与磷酸根发生沉淀反应重新生成磷酸铁(式 9),铁离子浓度达到峰值后快速下降并达标排放,在将释放的磷酸根回收的同时,避免了铁离子的二次污染。
综上,利用可见光下苯醌类化合物产生的光生水合电子,可以调控磷酸铁的还原和亚铁离子的释放,进而引发芬顿反应降解有机污染物,不仅能够开发利用含苯醌类废水“以废治废”的水处理方法,而且能够为废旧磷酸铁锂电池回收副产物磷酸铁的高值化开发提供理论依据。
-
(1)构建了可见光下苯醌类化合物诱导磷酸铁芬顿反应体系,该体系能极大提高橙Ⅱ的脱色和矿化效率。当体系分别添加2-氯-1,4-苯醌、苯醌和对二甲基醌,反应60 min时,橙Ⅱ脱色率分别是99.5%、98.1%和77.7%;反应180 min时,橙Ⅱ矿化率分别为78.8%、77.6%和52.4%。
(2)可见光下苯醌类化合物诱导磷酸铁芬顿反应体系中,磷酸铁是间接的铁源和直接的铁汇。当体系分别添加2-氯-1,4-苯醌、苯醌和对二甲基醌,亚铁离子峰值(对应时间)从基础实验的0.86 mg·L−1 (120 min)分别提高到2.06 mg·L−1 (40 min)、1.61 mg·L−1 (40 min)和1.04 mg·L−1 (120 min);反应结束时,总铁离子的浓度会大幅降低至0.2 mg·L−1左右,可以有效避免铁离子的二次污染。
(3)可见光下苯醌诱导磷酸铁芬顿反应循环降解橙Ⅱ过程中,磷酸铁能够作为铁离子稳定的源和汇,其铁源和铁汇过程具有较高的重现性。循环反应起始,溶液中总铁离子浓度在0.2—0.6 mg·L−1之间;3次循环反应结束时,溶液中总铁离子浓度重新降至0.2—0.4 mg·L−1之间。通过对比分析磷酸铁的FT-IR和XPS图谱,发现其在循环使用后具有较高的结构和化学重现性。
可见光下苯醌类化合物诱导磷酸铁芬顿反应的铁离子源汇机制
Iron-ion source and sink mechanism for Fenton reaction based on iron phosphate induced by benzoquinones under visible light
-
摘要: 构建了可见光下苯醌类化合物诱导磷酸铁产生芬顿反应的体系,分析了苯醌类化合物对磷酸铁释放亚铁离子的影响,比较了苯醌类化合物诱导磷酸铁芬顿反应降解橙Ⅱ的效率,研究了橙Ⅱ降解过程中铁离子和亚铁离子之间的转化以及羟基自由基浓度的变化,讨论了可见光下苯醌类化合物诱导磷酸铁芬顿反应铁离子的源汇机制。结果表明,可见光下苯醌类化合物诱导的磷酸铁是芬顿反应铁离子的间接铁源和直接铁汇。当可见光-磷酸铁-过氧化氢体系分别添加2-氯-1,4-苯醌、苯醌和对二甲基醌,溶液中亚铁离子浓度峰值(对应时间)从基础实验的0.86 mg·L−1 (120 min)分别提高到2.06 mg·L−1 (40 min)、1.61 mg·L−1 (40 min)和1.04 mg·L−1 (120 min);铁离子引发的芬顿反应能极大提高橙Ⅱ的脱色率和矿化率:反应60 min时,橙Ⅱ脱色率分别是99.5%、98.1%和77.7%;反应180 min时,橙Ⅱ矿化率分别为78.8%、77.6%和52.4%;反应结束时,总铁离子的浓度会大幅降低至0.2 mg·L−1左右,能避免铁离子的二次污染。另外,可见光下苯醌诱导磷酸铁芬顿反应循环降解橙Ⅱ的结果表明,铁离子释放和回收过程具有较高的重现性;FT-IR和XPS图谱表明,磷酸铁在循环使用后具有较高的结构和化学重现性。本研究开发了磷酸铁用于有机废水处理高值化利用的途径,为废旧磷酸铁锂电池的资源化利用提供理论依据。Abstract: A new Fenton system was constructed based on iron phosphate induced by benzoquinones (2-Chloro-1,4-benzoquinone, 1,4-Benzoquinone or 2,5-Dimethylquinone) under visible light. The effects of benzoquinones on ferrous ion release from iron phosphate and degradation efficiency of Orange Ⅱ through Fenton process were analyzed in detail. During Orange Ⅱ degradation, the transformation between iron and ferrous ions and the change concentration of hydroxyl radical were detected. The iron-ion source and sink mechanism for Fenton reaction was further investigated. The results showed that iron phosphate was the indirect source and direct sink of iron ions induced by benzoquinones under visible light. When 2-Chloro-1,4-benzoquinone, 1,4-Benzoquinone and 2,5-Dimethylquinone were added to the visible light-iron phosphate-hydrogen peroxide system, the peak values of ferrous ion concentration (corresponding time) was increased from 0.86 mg·L−1 (120 min) to 2.06 mg·L−1 (40 min), 1.61 mg·L−1 (40 min) and 1.04 mg·L−1 (120 min), respectively. Then the decolorization and mineralization rates of Orange Ⅱ could be greatly enhanced through Fenton process using iron ion as catalyst. After 60 min treatment, the decolorization rates of Orange Ⅱ were increased to 99.5%, 98.1% and 77.7%, respectively; after 180 min treatment, the mineralization rates of Orange Ⅱwere up to 78.8%, 77.6% and 52.4%, respectively. In addition, the concentration of total iron ions was decreased to about 0.2 mg·L−1 after treatment, therefor the secondary contamination of iron ions could be avoided. Furthermore, the iron release and recovery process has high reproducibility. Importantly, Fourier transform infrared and X-ray photoelectron spectroscopy showed that iron phosphate had high structural and chemical reproducibility after reaction. This study developed a way of high-value utilization of iron phosphate for organic wastewater treatment, which provided a theoretical support for comprehensive utilization of waste lithium iron phosphate batteries.
-
Key words:
- iron phosphate /
- light-assisted Fenton reaction /
- benzoquinones /
- Orange Ⅱ /
- hydroxyl radical
-
图 1 苯醌类化合物对可见光-磷酸铁-过氧化氢体系中橙Ⅱ脱色(a)及铁离子(b)和亚铁离子浓度(c)的影响([Benzoquinones]=0.2 mmol∙L−1, [Orange Ⅱ]=0.2 mmol∙L−1, pH=3.0, T=30 ℃, FePO4=1 g·L−1, LED灯白光=10 W, H2O2=10 mmol·L−1)
Figure 1. Effects of benzoquinones on Orange Ⅱ decolorization (a) and ferric (b) and ferrous ions concentrations (c) in visible light-iron phosphate-hydrogen peroxide system
-
[1] FORTE F, PIETRANTONIO M, PUCCIARMATI S, et al. Lithium iron phosphate batteries recycling: An assessment of current status [J]. Critical Reviews in Environmental Science and Technology, 2021, 51(19): 2232-2259. doi: 10.1080/10643389.2020.1776053 [2] DELACOURT C, POIZOT P, TARASCON J M, et al. The existence of a temperature-driven solid solution in LixFePO4 for 0 ≤ x ≤ 1 [J]. Nature Materials, 2005, 4(3): 254-260. doi: 10.1038/nmat1335 [3] HARPER G, SOMMERVILLE R, KENDRICK E, et al. Recycling lithium-ion batteries from electric vehicles [J]. Nature, 2019, 575(7781): 75-86. doi: 10.1038/s41586-019-1682-5 [4] TIAN X H, ZHOU Y K, TU X F, et al. Well-dispersed LiFePO4 nanoparticles anchored on a three-dimensional graphene aerogel as high-performance positive electrode materials for lithium-ion batteries [J]. Journal of Power Sources, 2017, 340: 40-50. doi: 10.1016/j.jpowsour.2016.11.049 [5] FREITAS M B J G, CELANTE V G, PIETRE M K. Electrochemical recovery of cobalt and copper from spent Li-ion batteries as multilayer deposits [J]. Journal of Power Sources, 2010, 195(10): 3309-3315. doi: 10.1016/j.jpowsour.2009.11.131 [6] HOREH N B, MOUSAVI S M, SHOJAOSADATI S A. Bioleaching of valuable metals from spent lithium-ion mobile phone batteries using Aspergillus niger [J]. Journal of Power Sources, 2016, 320: 257-266. doi: 10.1016/j.jpowsour.2016.04.104 [7] LI L, LU J, REN Y, et al. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries [J]. Journal of Power Sources, 2012, 218: 21-27. doi: 10.1016/j.jpowsour.2012.06.068 [8] ZENG X L, LI J H, SINGH N. Recycling of spent lithium-ion battery: A critical review [J]. Critical Reviews in Environmental Science and Technology, 2014, 44(10): 1129-1165. doi: 10.1080/10643389.2013.763578 [9] WANG W, WU Y F. An overview of recycling and treatment of spent LiFePO4 batteries in China [J]. Resources, Conservation and Recycling, 2017, 127: 233-243. doi: 10.1016/j.resconrec.2017.08.019 [10] LAW J C F, LEUNG K S Y. Redox mediators and irradiation improve Fenton degradation of acesulfame [J]. Chemosphere, 2019, 217: 374-382. doi: 10.1016/j.chemosphere.2018.11.032 [11] DI Y W, LIU L, MA H C, et al. Bi doping into Ti/Co3O4 NWs (nanowires) for improved photoelectrochemical decolorization of dyeing wastewater (reactive brilliant blue KN-R) [J]. Journal of Materials Science:Materials in Electronics, 2020, 31(12): 9504-9513. doi: 10.1007/s10854-020-03492-7 [12] KHAJONE V B, BHAGAT P R. Synthesis of polymer-supported Brønsted acid-functionalized Zn-porphyrin complex, knotted with benzimidazolium moiety for photodegradation of azo dyes under visible-light irradiation [J]. Research on Chemical Intermediates, 2020, 46(1): 783-802. doi: 10.1007/s11164-019-03990-2 [13] ZHAO Y P, HU J Y. Photo-Fenton degradation of 17β-estradiol in presence of α-FeOOHR and H2O2 [J]. Applied Catalysis B:Environmental, 2008, 78(3/4): 250-258. [14] 林爱秋, 程和发. 芬顿及光芬顿法降解氟喹诺酮类抗生素研究进展 [J]. 环境化学, 2021, 40(5): 1305-1318. doi: 10.7524/j.issn.0254-6108.2021011401 LIN A Q, CHENG H F. Recent development in the degradation of fluoroquinolones by Fenton and photo-Fenton processes [J]. Environmental Chemistry, 2021, 40(5): 1305-1318(in Chinese). doi: 10.7524/j.issn.0254-6108.2021011401
[15] 苗笑增, 戴慧旺, 陈建新, 等. 草酸根对α-FeOOH多相UV-Fenton催化能力的增效实验 [J]. 环境科学, 2018, 39(3): 1202-1211. MIAO X Z, DAI H W, CHEN J X, et al. Experiment to enhance catalytic activity of α-FeOOH in heterogeneous UV-Fenton system by addition of oxalate [J]. Environmental Science, 2018, 39(3): 1202-1211(in Chinese).
[16] 邓曹林, 王京刚, 王颖, 等. 石墨烯改性Al-MCM-41介孔分子筛负载铁芬顿催化剂降解苯酚 [J]. 环境化学, 2015, 34(6): 1185-1192. doi: 10.7524/j.issn.0254-6108.2015.06.2014110301 DENG C L, WANG J G, WANG Y, et al. Fenton catalytic degradation of phenol by using iron-loaded graphene modified mesoporous Al-MCM-41 catalyst [J]. Environmental Chemistry, 2015, 34(6): 1185-1192(in Chinese). doi: 10.7524/j.issn.0254-6108.2015.06.2014110301
[17] 付军, 余艳鸽, 赵昱东, 等. 模拟日光-非均相Fenton光催化降解喹啉 [J]. 环境化学, 2017, 36(5): 1072-1082. doi: 10.7524/j.issn.0254-6108.2017.05.2016090706 FU J, YU Y G, ZHAO Y D, et al. Simulated sunlight-heterogeneous Fenton degradation of quinoline in wastewater [J]. Environmental Chemistry, 2017, 36(5): 1072-1082(in Chinese). doi: 10.7524/j.issn.0254-6108.2017.05.2016090706
[18] 姜自立, 李献众, 刘治庆, 等. 苯醌对聚合硅酸铁多相UV-Fenton体系的增效机制 [J]. 中国环境科学, 2020, 40(7): 2943-2951. doi: 10.3969/j.issn.1000-6923.2020.07.018 JIANG Z L, LI X Z, LIU Z Q, et al. The enhanced mechanism of benzoquinone(BQ) on poly-silicate-ferric(PSF) in heterogeneous UV-Fenton system [J]. China Environmental Science, 2020, 40(7): 2943-2951(in Chinese). doi: 10.3969/j.issn.1000-6923.2020.07.018
[19] 苏晓轩, 徐国鹏, 李献众, 等. PSF多相UV-Fenton体系原儿茶酸与龙胆酸的增效对比 [J]. 中国环境科学, 2021, 41(4): 1624-1633. doi: 10.3969/j.issn.1000-6923.2021.04.015 SU X X, XU G P, LI X Z, et al. Comparison of the enhanced effect of protocatechuic acid and gentisic acid on the heterogeneous UV-Fenton system with Poly-Silicate-Ferric(PSF) as catalyst [J]. China Environmental Science, 2021, 41(4): 1624-1633(in Chinese). doi: 10.3969/j.issn.1000-6923.2021.04.015
[20] QIN Y X, SONG F H, AI Z H, et al. Protocatechuic acid promoted alachlor degradation in Fe(Ⅲ)/H2O2 Fenton system [J]. Environmental Science & Technology, 2015, 49(13): 7948-7956. [21] HOSOKAWA S, SHUKUYA K, SOGABE K, et al. Novel absorbance peak of gentisic acid following the oxidation reaction [J]. PLoS One, 2020, 15(4): e0232263. doi: 10.1371/journal.pone.0232263 [22] ABEDI F, RAZAVI B M, HOSSEINZADEH H. A review on gentisic acid as a plant derived phenolic acid and metabolite of aspirin: Comprehensive pharmacology, toxicology, and some pharmaceutical aspects [J]. Phytotherapy Research, 2020, 34(4): 729-741. doi: 10.1002/ptr.6573 [23] 贺俊梅, 孔令娜, 梁倩, 等. 苯醌类与苯醌亚胺类在日光/Fenton体系中的光敏性 [J]. 中国环境科学, 2016, 36(9): 2638-2644. doi: 10.3969/j.issn.1000-6923.2016.09.013 HE J M, KONG L N, LIANG Q, et al. Photosensitivity of benzoquinones and benzoquinoneimines in the sunlight/Fenton system [J]. China Environmental Science, 2016, 36(9): 2638-2644(in Chinese). doi: 10.3969/j.issn.1000-6923.2016.09.013
[24] 袁文辉, 徐志峰. 混合电镀污泥中铬铁的选择性分离工艺 [J]. 有色金属(冶炼部分), 2016(9): 55-58. YUAN W H, XU Z F. Selective separation technology of chromium and ironfrom mixed electroplating sludge [J]. Nonferrous Metals (Extractive Metallurgy), 2016(9): 55-58(in Chinese).
[25] HALL R D, CHIGNELL C F. Steady-state near-infrared detection of singlet molecular oxygen: A Stern-Volmer quenching experiment with sodium azide [J]. Photochemistry and Photobiology, 1987, 45(4): 459-464. doi: 10.1111/j.1751-1097.1987.tb05403.x [26] CLENNAN E L, PACE A. Advances in singlet oxygen chemistry [J]. Tetrahedron, 2005, 61(28): 6665-6691. doi: 10.1016/j.tet.2005.04.017 [27] WU K Q, YI D X, ZHAO J C, et al. Photo-Fenton degradation of a dye under visible light irradiation [J]. Journal of Molecular Catalysis A:Chemical, 1999, 144(1): 77-84. doi: 10.1016/S1381-1169(98)00354-9 [28] 陈建新. 铁柱撑膨润土催化UV-Fenton降解染料的性能及其机理研究[D]. 杭州: 浙江大学, 2007: 120. CHEN J X. Study on the catalytic properties of Fe-pillared bentonite and mechanism of UV-Fenton degradation of dye[D]. Hangzhou: Zhejiang University, 2007:120(in Chinese).
[29] 王安静. 苯醌类化合物的降解特性[D]. 大连: 大连海事大学, 2018:56. WANG A J. The degradation characteristics of benzoquinones compounds[D]. Dalian, China: Dalian Maritime University, 2018:56(in Chinese).
[30] 王彦广, 吕萍, 张殊佳. 有机化学[M]. 2版. 北京: 化学工业出版社, 2009:396. WANG Y G, LU P, ZHANG S J, et al. Organic chemistry [M]. Beijing: Chemical Industry Press, 2009:396(in Chinese).
[31] GAO J, LIU Y T, XIA X N, et al. Mechanisms for photo assisted Fenton of synthesized pyrrhotite at neutral pH [J]. Applied Surface Science, 2019, 463: 863-871. doi: 10.1016/j.apsusc.2018.09.007 [32] ZENG L Y, GONG J Y, DAN J F, et al. Novel visible light enhanced Pyrite-Fenton system toward ultrarapid oxidation of p-nitrophenol: Catalytic activity, characterization and mechanism [J]. Chemosphere, 2019, 228: 232-240. doi: 10.1016/j.chemosphere.2019.04.103 [33] LI J F, LI J G, LIU X Y, et al. Effect of silicon content on preparation and coagulation performance of poly-silicic-metal coagulants derived from coal gangue for coking wastewater treatment [J]. Separation and Purification Technology, 2018, 202: 149-156. doi: 10.1016/j.seppur.2018.03.055 [34] FU Y, YU S L, HAN C W. Morphology and coagulation performance during preparation of poly-silicic-ferric (PSF) coagulant [J]. Chemical Engineering Journal, 2009, 149(1/2/3): 1-10. [35] HUAI Y Y, PLACKOWSKI C, PENG Y J. The effect of gold coupling on the surface properties of pyrite in the presence of ferric ions [J]. Applied Surface Science, 2019, 488: 277-283. doi: 10.1016/j.apsusc.2019.05.236 -






 下载:
下载: