-
左旋葡聚糖(LG)即1,6-脱水-β-D-葡萄糖,是一种由纤维素热分解产生的水溶性无水糖[1]。一直以来, 左旋葡聚糖(LG)被当做生物质燃烧的有机分子标记物受到大量关注[2]。左旋葡聚糖(LG)来源广泛,当燃烧温度高于300 ℃时,纤维素和半纤维素的热裂解会生成LG[2-8],在淀粉和糖类的热变过程中也会生成LG[9],同时木质纤维素和泥炭木加热后生成的产物中也有LG存在[10]。LG分布广泛[11-15],在大气中具有明显的区域分布特征,整体表现为农村高于城市[11-13, 16-25]、高于森林海洋等偏远地区[14-15, 26-37]。在对LG研究不断深入的过程中,可采用多种分析检测技术,有气相色谱-质谱法(GC-MS)[38]、热解吸气相色谱质谱法(TD-GC-MS)[39]、高效液相色谱(HPLC)与质谱相结合法[40]、离子色谱法(IC)[41]、离子色谱-质谱-电喷雾电离法(IC-MS-ESI)[42]法等方法。近几十年,很多研究者已对LG的来源、分布、检测方法等方面进行了综述,例如Bhattarai等 [43]对LG的测量分析技术,排放特性(包括形成,稳定性,比率)和空间变化做了很好的总结,Janoszka等[44]和Schkolnik等[45]对LG测定方法也做了很好的总结。
LG作为生物质燃烧过程的独特示踪剂,一度被认为是在大气中具有高稳定性、高丰度的生物质燃烧产物[43]。然而,近年的研究却表明,LG在大气条件下也会发生不同程度的光化学降解,同时也在不同相态的实验室模拟实验得到该结论,甚至外场的同位素观察也证实了LG在大气条件下的不稳定性[41, 44]。但是,目前并没有研究者从不同相态的角度来对LG的光化学降解进行总结。本文总结了实验室模拟中液相[30,46-52]、气相[53-55]和非均相[56-57]下自由基、离子、温度、湿度等因素对LG的光化学的影响,以及大气中LG的光化学影响因素和LG排放进入大气后的光化学反应机制,并对减缓LG光化学降解的研究进行了展望。
-
基于大气中存在云滴水相体系,也存在雾滴的水非均相体系,实验室模拟了这类大气条件下LG的光化学稳定性[43]。
-
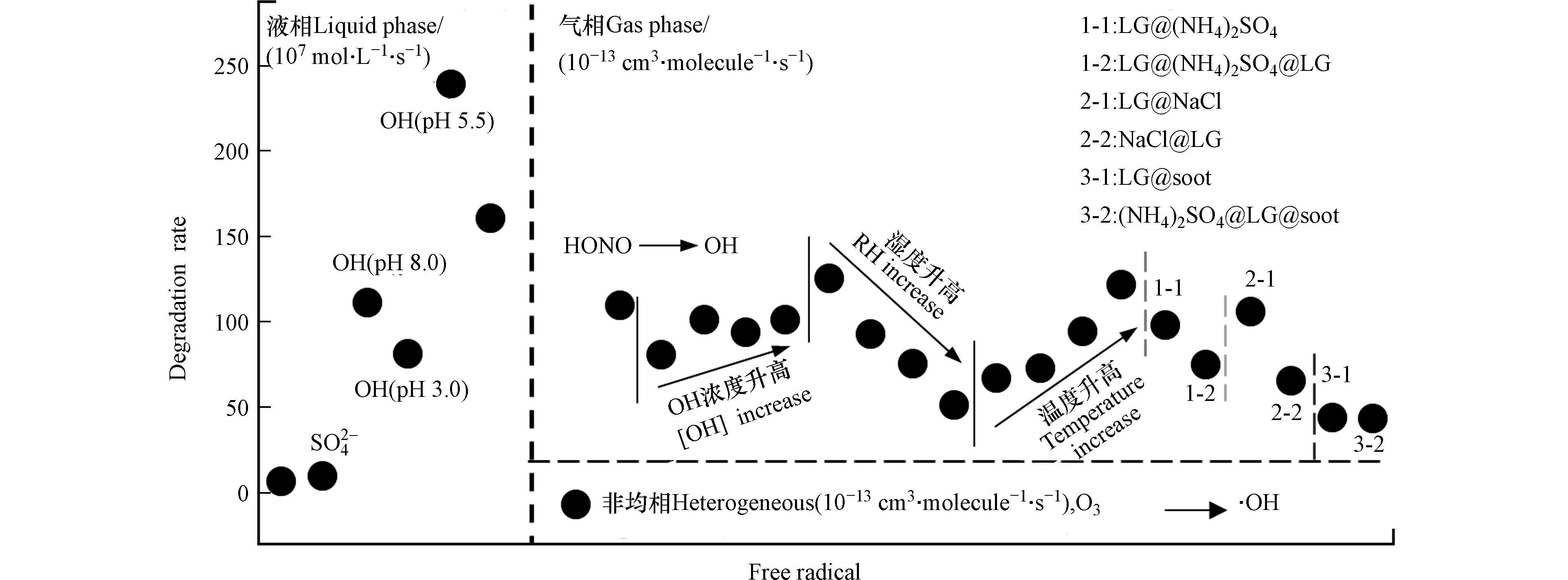
对于液相体系中的光氧化反应,Zhao等[50]和Teraji等[52]利用H2O2产生的羟基自由基(•OH)促进LG发生降解。一方面Zhao[50]等测定了室温下LG与羟基自由基反应的光降解速率常数为(1.08±0.16)×109 mol·L−1·s−1,并指出水相光氧化导致的LG损失显著。另一方面在Teraji等[52]的文章中提到,因为羟基自由基是水环境中的一种有效氧化剂,LG的寿命由它们与羟基自由基的相对反应性控制。LG与羟基自由基之间的双分子速率常数和羟基自由基的稳态浓度决定了反应性,因此在20 ℃的条件下,pH=3.0时LG的光降解速率为(0.79±0.11)×109 mol·L−1·s−1;pH=5.5时,LG的光降解速率为(2.4±0.28)×109 mol·L−1·s−1;pH=8.0时,LG的光降解速率为(1.6±0.08)×109 mol·L−1·s−1由此可知,在羟基自由基的催化降解实验中,随着pH值的升高,LG的光降解速率呈现先升高后降低的趋势,在pH 5.5时,其降解速率最高。Jing等[58]首次利用量子化学方法研究LG的降解,发现298 K时,LG与羟基自由基反应的速率常数为2.21×10−13 mol·L−1·s−1,大气寿命为26 d([•OH]=2.0×106 mol·L−1),其中羟基自由基的催化降解为主要反应,而且LG的大气反应机理随温度而变化。研究发现温度对LG的降解也有一定的影响。在液相反应条件下,本课题组黄通等[59]分别在5 ℃、15 ℃、28 ℃的液相环境中进行了光氧化实验,实验结果得出在5 ℃的温度下LG的降解速率为4.0×10−2 min−1,15℃时降解速率为9.7×10−2 min−1,28 ℃时LG的降解速率增至28.9×10−2 min−1。随着温度的升高,LG的降解速率加快。Hoffmann等[30]研究发现,LG在白天很容易被羟基自由基氧化,夏季平均降解约为7.2 ng·m−3·h−1,冬季约为4.7 ng·m−3·h−1。对于大气中硝基自由基
(NO−3) 和硫酸自由基(SO2−4) (式1),Hoffmann等发现其光解速率常数分别为(1.6 ± 0.2) ×107 mol·L−1·s−1和(5.2 ± 0.9)×107 mol·L−1·s−1,较羟基自由基慢很多[30]。研究还发现,LG在高相对湿度条件下的氧化速度更快。除了这几种自由基,大气中还存在Criegee中间体[52]。Shinichi[51]等研究了Criegee中间体与LG在水中的反应,证明了在周围气溶胶和土壤中发现的糖与气-水界面处的氯自由基具有很高的反应性。Criegee中间体在很大程度上与糖反应,而不是水分子反应。此研究说明Criegee中间体产生的氯自由基同样可以导致LG的光氧化降解(式2)。
此外,本课题组黄通等[59]研究也发现无机离子
(SO2−4、NO−3、NO−2) 对LG的降解有一定的影响。低浓度的SO2−4 的存在会消耗部分的羟基自由基,从而减弱对LG的氧化作用。通过液相光氧化实验结果可以看出,未添加无机离子之前,LG的降解速率为28.9×10−2 min−1,加入一定量的SO2−4 和NO−3 后,LG的降解速率逐渐变慢。当其浓度大于0.1 mmol·L−1时,对LG降解的抑制作用减弱。当NO−2 浓度为1 mmol·L−1时,LG的降解速率降为0.5×10−2 min−1。Holmes等[25]研究了Fe3+ 光氧化体系中(式3—5)LG的聚合反应,LG在这个体系中形成糖苷键聚合生成吡喃糖低聚物,Fe3+ 加入后,促进了LG光解。此外,本课题组黄通等[59]研究也发现无机离子(SO2−4、NO−3、NO−2) 对LG的降解有一定的影响。低浓度的SO2−4 的存在会消耗部分的羟基自由基,从而减弱对LG的氧化作用。通过液相光氧化实验得出的数据可以看出,未添加无机离子之前,LG的降解速率为28.9×10-2 min−1,加入一定量的SO2−4 和NO−2 后,LG的降解速率逐渐变慢。当其浓度大于0.1 mmol·L−1后,对LG降解的抑制作用减弱。当NO−2 浓度为1 mmol·L−1时,LG的降解速率降为0.5×10−2 min−1。Holmes等[25]研究了Fe3+ 光氧化体系中(式3—5)LG的聚合反应,LG在这个体系中形成糖苷键聚合生成吡喃糖低聚物,Fe3+ 加入后,促进了LG光解。以上研究结果表明,自由基能促进液相体系中LG的光化学降解,其中作用最大的为羟基自由基,此外硝基自由基、硫酸自由基、Criegee中间体产生的氯自由基等自由基也促进LG的光化学降解。在自由基影响光化学降解的过程中,环境(如温度和相对湿度)、无机离子(如
SO2−4,NO−3,NO−2 )对LG的降解有一定的影响。对于雾滴相的光化学反应[30],实验室采用流动管实验,以硫酸铵和LG形成颗粒物,同时保持一定湿度,通
O3 进行光解,发现这种颗粒物水相中的LG也具有较高的反应活性(表1),其中O3 经过254 nm紫外光照射光解产生羟基自由基参与LG的光化学降解(式6—8)。Hennigan等[53]的研究表明,在1×106 molecule·cm−3羟基自由基的暴露条件下,LG的大气寿命为0.7—2.2 d。Lai等[54]利用流动反应器整合羟基的对照实验,实验假设了12h羟基自由基的平均典型浓度为1.5×106 molecule·cm−3,研究不同的环境条件及混合状态下的,发现其大气寿命为1.2—3.9 d。此外,他们还发现在1.5×106 molecule·cm−3羟基自由基中,LG的大气寿命为(1.7±0.2)d。Bai等[58]指出在2×106 molecule·cm−3羟基自由基中,LG的大气寿命为26 d。以上研究表明在雾滴相中,羟基自由基浓度同样影响光化学降解。Bryan等[48-49]模拟了云水中潜在的二次示踪剂,提出假设,除羟基自由基反应外,酸催化的低聚反应也可能是大气水性气溶胶中LG的重要去除途径。大气含水气溶胶的天然酸有助于通过酸催化的低聚反应去除游离LG。Abdilla等[46]利用Bronsted酸(硫酸和乙酸)催化LG降解,其降解速率远远慢于自由基的催化作用。Hoffmann等[30]以建模的方式探究了LG的大气稳定性,结果表明LG在大气中的稳定性类似于与大气中醇和多元醇,并且其浓度可以通过大气处理而显著降低。
通过以上的比较可知,LG的大气寿命既受到羟基自由基等各种自由基和无机离子浓度的影响,又受到一些环境因素(如反应温度、压力等)的影响[54]。
-
LG的降解不仅存在于大气的液相体系中,在气相和非均相体系中也会发生。对于气相中LG的光化学稳定性,受到湿度、温度、自由基等因素的影响,其中较高相对湿度(RH)下[30]自由基氧化途径和较长时间空气质量的老化都会对LG的氧化反应产生影响[54](式9)。Lai等[54]研究发现,LG的降解能力随湿度的增加而降低,随温度的上升而增加,以及随着羟基自由基浓度的增加而逐渐上升并趋于平稳。Pratap等[55]在烟雾室中研究LG在-8—10 ℃温度范围内的变化,在10 ℃左右时LG降解速率显著衰减。Jonathan等在[60]假设白天平均OH浓度为2×106 molecule·cm−3(约1 ×10−7 µg·mL−1)的条件下得出在干燥条件和RH=40%时,LG的大气寿命分别约为27 d和13 d。
LG具有较高的排放因子和大气气溶胶浓度,即使在不同的环境条件下被氧化降解,它仍然是生物质燃烧的理想标志物。此外,当受体与排放源的距离较近时,LG也可以作为良好的示踪剂。Sang等[57]进行了实验室模拟对照实验,指出在
O3 的光解反应中,促进了羟基自由基的产生,并且和O(1 D)与水的后续反应有直接的关联。Hennigan等[53]采用烟雾箱,模拟了LG的光化学稳定性,以亚硝酸光化学产生的羟基自由基来实现其光降解,在这种条件下LG的光解速率为(1.1±0.5)×10−11 cm3·molecule−1·s−1。Hennigan等[61]假设OH浓度恒定为1×106 molecule·cm−3,LG和K+内部混合后发现,每次实验中LG与K+ 比值均显著下降,与在燃烧室中测量的数据相比,它在实验模拟的光氧化阶段结束时,平均下降了80%。这个实验结果表明,LG光降解能力受无机离子的影响也非常明显。此外,Vikram等[55]在木烟雾室中对LG实验表明,LG的表观化学寿命除了与温度和有机气溶胶浓度及蒸气壁损失率有关外,化学结构(如碳数,环状部分和含氧的官能团)也在挥发反应的重要性中起主导作用[56] 。Sean [56]对纯赤藓糖醇和LG的非均相氧化实验中发现,赤藓糖醇的颗粒质量下降与母体物质浓度下降的比率是LG的约3倍,这表明化学结构(例如碳数,环状部分和含氧的官能团)在反应中起主导作用。其中,具有两个环状部分的LG可以进行两个裂解反应而不会解离成两个独立的分子,因此蒸气压不会像赤藓糖醇那样急剧增加。因此,相对于氧化的质量损失率较低,这表明具有环结构和较高分子量的化合物可能有助于延长有机气溶胶的寿命(式10)。Sang等[55]在水溶液和混合溶液(NH4)2 SO−4 -LG颗粒暴露在OH中这两种情况下,对样品进行同位素分析。LG浓度随着OH暴露的增加呈下降趋势。对于80%以上的LG转化,化学老化同位素计算的灵敏度将最高。以上研究结表明在气相和非均相反应中,LG光化学降解受到自由基浓度、无机离子、化学结构的影响。

-
化学示踪剂的稳定性对其适用性具有重要影响,即使是理想条件下的示踪剂也会存在不稳定现象。前人的研究表明,LG在大气中可以稳定存在10 d左右,且不会发生光化学降解[43, 52]。然而,最近有相关研究指出,LG在大气中有显著的化学反应,并借助模拟和模型研究讨论了大气中LG随时间变化(即稳定性)的问题[63]。LG的不稳定性不仅得到了实验室模拟的证实,而且外场观测的同位素数据(δ13 C)也说明大气中LG的确存在光化学降解,大约50%的LG在大气传输中发生了降解[62]。因此,使用LG作为惰性生物质燃烧分子标记可能会大大低估生物质燃烧对样品组成的影响,而LG的碳同位素比测量可用于纠正这种偏差[62]。
大气中情况更为复杂,Saarnio和本课题组均发现不仅仅存在上述的氧化体系,还存在TiO2光化学体系[5, 64]。不同的光化学体系反应速率不同(图1),因此贡献也可能有所不同,实际大气中应该是这些体系综合贡献。
理解LG的降解取决于几个外部因素,包括pH,RH,温度和生物活性,还需要正确理解动力学同位素效应,结合气团的后向轨迹理解主要排放途径的贡献。此外,使用受控(封闭系统)以及周围环境(开放系统),可以使用更准确的衰减常数和传输时间(即老化)的测量来计算排放源的原始左旋葡聚糖浓度[43, 65]。值得注意的是,LG及其异构体M和G都存在于大气中,LG浓度取决于生物质燃烧类型和燃烧条件[26],还可能受到环境的影响。LG作为单一的生物质燃烧标志物,可能因为降解而影响其源解析。但是,LG与其异构体之间的比例可以弥补这个问题。同样的,异构体之间一般是可以转化的[41, 66],研究发现LG通过手性立体化学在大气中异构化为M和G,在模拟光-芬顿条件下进行氧化降解。LG的异构化在实验室模拟和LG / (G + M)比中的观察表明,同分异构体的比例的变化会受到排放的各种类型的生物量变化影响[64, 66]。
-
本文综述了LG在液相、气相和非均相等模拟条件中的光化学降解,总结了影响LG降解的几类因素。虽然LG在大气中分布广泛,常被用作生物质燃烧的标记物,但是已有研究表明其在大气中并不是稳定存在的,进入大气后会发生一系列光化学反应。首先,在液相、气相和非均相的体系中羟基自由基、Bronsted酸和Criegee中间体等都会促进LG发生降解,其中羟基自由基的催化降解作用最明显。其次,随着pH值的升高LG的降解速率呈现先升后降的趋势。大气中的无机离子
(SO2−4、NO−3、NO−2) 也会对LG的降解产生影响。随着SO2−4 和NO−3 浓度的增加,LG的降解速率呈现一种先减后增的趋势;但随着NO−2 浓度增加,NO−2 对LG降解抑制作用增强。除此之外,温湿度也可以影响LG的降解,温度的升高会加快LG的降解,但湿度对LG降解的影响与温度相反,湿度增加会抑制它的降解。尽管LG的在大气中光化学研究已经有大量报道,但是仍然存在不足。首先,虽然LG和它的同分异构体M和G在大气中有较高的浓度,但是将它作为针对生物质燃烧的单一标志物可能会由于降解而影响其源解析,近年来已有研究发现LG与它的同分异构体之间的比例可以弥补这个问题,但还需要更加深入研究。其次,由于目前分析和检测技术所存在的不足使LG原位、在线鉴定受到影响,需要不断探索更加精确的仪器和更加准确的方法对LG进行研究。最后为了LG的研究更加精确,需要发现更多影响LG光化学降解的因素。总之,这些方面都需要研究者们进行不断的探索。
大气颗粒物中左旋葡聚糖光化学的研究进展
Photochemistry of levoglucosan in atmospheric aerosols: A review
-
摘要: 大量的有机污染物通过生物质燃烧排到大气中,从而影响着空气质量、气候变化以及人类健康等。左旋葡聚糖长期以来被视作生物质燃烧的标志物,在大气化学的源解析中具有重要的意义。本文综述了左旋葡聚糖的光化学稳定性,全面讨论了在实验室模拟研究中左旋葡聚糖在液相、非均相和气相等模拟条件中发生的光化学氧化反应。此外,还结合本实验室的工作总结了左旋葡聚糖的光化学机制,对比了它在不同相态中的降解速率。Abstract: A large number of organic pollutants are discharged into the atmosphere through biomass burning, thereby affecting air quality, climate change, and human health. Levoglucosan has widely been regarded as a marker of biomass burning and has important significance in the source analysis of atmospheric chemistry. This article reviews the photochemical transformation of levoglucosan and comprehensively discusses the photochemical oxidation of levoglucosan in the liquid phase, heterogeneous phase, and gas phase in the laboratory simulation. In addition, combined with the work of our laboratory, we summarized the photochemical mechanism of levoglucosan and compared its degradation rate in different phases.
-
Key words:
- levoglucosan /
- atmospheric chemistry /
- mechanism /
- biomass burning
-
表 1 不同相态中左旋葡聚糖光化学反应参数
Table 1. The parameters of photochemical reaction of levoglucosan in different phase
反应类型Reaction type 自由基Free radical 反应物降解速率Reactant degradation rate 大气寿命Atmosphere lifetime 参考文献Ref 液相酸催化 H+ (0.599±0.009) mol·L−1·min−1 [46] 无 (0.023±0.008) mol·L−1·min−1 模型模拟 •OH (2.4±0.3)×109 mol·L−1·min−1 [30] NO3 (1.6±0.2)×107 mol·L−1·min−1 SO2−4 (5.2±0.9)×107 mol·L−1·min−1 液相芬顿 •OH (1.28±0.45)×109 mol·L−1·min−1 [48-49] 液相 •OH (1.08±0.16)×109 mol·L−1·min−1 [50] 液相 •OH(pH 3.0—8.0) 7.9×108—2.4×109 mol·L−1·min−1 [52] 液相 SO2−4 28.9×10−2 min−1 [30] SO2−4 21.9×10−2 min−1 SO2−4 25.79×10−2 min−1 SO2−4 26.5×10−2 min−1 液相 NO−3 28.9×10−2 min−1 [62] NO−3 17.9×10−2 min−1 NO−3 13.9×10−2 min−1 NO−3 20.2×10−2 min−1 液相 NO−2 28.9×10−2 min−1 [30] NO−2 17.3×10−2 min−1 NO−2 9.6×10−2 min−1 NO−2 0.5×10−2 min−1 非均相 O3 (3.09±0.18)×10−13 cm3·molecule−1·s−1 [54] 气相 •OH (1.1±0.5)×10−11 cm3 ·molecule−1·s−1 0.7—2.2 d [54] 气相 •OH (4.04±0.29)×10−12—(12.5±0.17)×10−12 cm3·molecule−1·s−1 1.2—3.9 d [54] -
[1] SUCIU L G, MASIELLO C A, GRIFFIN R J. Anhydrosugars as tracers in the earth system [J]. Biogeochemistry, 2019, 146(3): 209-256. doi: 10.1007/s10533-019-00622-0 [2] SIMONEIT B R T, SCHAUER J J, NOLTE C G, et al. Levoglucosan, a tracer for cellulose in biomass burning and atmospheric particles [J]. Atmospheric Environment, 1999, 33(2): 173-182. doi: 10.1016/S1352-2310(98)00145-9 [3] FABBRI D, TORRI C, SIMONEIT B R T, et al. Levoglucosan and other cellulose and lignin markers in emissions from burning of Miocene lignites [J]. Atmospheric Environment, 2009, 43(14): 2286-2295. doi: 10.1016/j.atmosenv.2009.01.030 [4] FRASER M P, LAKSHMANAN K. Using levoglucosan as a molecular marker for the long-range transport of biomass combustion aerosols [J]. Environmental Science & Technology, 2000, 34(21): 4560-4564. [5] SAARNIO K, TEINILÄ K, AURELA M, et al. High-performance anion-exchange chromatography-mass spectrometry method for determination of levoglucosan, mannosan, and galactosan in atmospheric fine particulate matter [J]. Analytical and Bioanalytical Chemistry, 2010, 398(5): 2253-2264. doi: 10.1007/s00216-010-4151-4 [6] SAARNIO K, TEINILÄ K, SAARIKOSKI S, et al. Online determination of levoglucosan in ambient aerosols with particle-into-liquid sampler–high-performance anion-exchange chromatography–mass spectrometry (PILS–HPAEC–MS) [J]. Atmospheric Measurement Techniques, 2013, 6(10): 2839-2849. doi: 10.5194/amt-6-2839-2013 [7] SIMONEIT B R T. Biomass burning—a review of organic tracers for smoke from incomplete combustion [J]. Applied Geochemistry, 2002, 17(3): 129-162. doi: 10.1016/S0883-2927(01)00061-0 [8] ZHANG X L, YANG W H, BLASIAK W. Thermal decomposition mechanism of levoglucosan during cellulose pyrolysis [J]. Journal of Analytical and Applied Pyrolysis, 2012, 96: 110-119. doi: 10.1016/j.jaap.2012.03.012 [9] LAKSHMANAN C M, HOELSCHER H E. Production of levoglucosan by pyrolysis of carbohydrates pyrolysis in hot inert gas stream [J]. Starch - Stä rke, 1970, 22(8): 261-264. [10] GALLETTI G C, BOCCHINI P. Pyrolysis/gas chromatography/mass spectrometry of lignocellulose [J]. Rapid Communications in Mass Spectrometry, 1995, 9(9): 815-826. doi: 10.1002/rcm.1290090920 [11] CASEIRO A, OLIVEIRA C. Variations in wood burning organic marker concentrations in the atmospheres of four European cities [J]. Journal of Environmental Monitoring:JEM, 2012, 14(8): 2261-2269. doi: 10.1039/c2em10849f [12] MAENHAUT W, VERMEYLEN R, CLAEYS M, et al. Assessment of the contribution from wood burning to the PM10 aerosol in Flanders, Belgium [J]. Science of the Total Environment, 2012, 437: 226-236. doi: 10.1016/j.scitotenv.2012.08.015 [13] KŘŮMAL K, MIKUŠKA P, VOJTĚŠEK M, et al. Seasonal variations of monosaccharide anhydrides in PM1 and PM2.5 aerosol in urban areas [J]. Atmospheric Environment, 2010, 44(39): 5148-5155. doi: 10.1016/j.atmosenv.2010.08.057 [14] WANG X Y, THAI P K, MALLET M, et al. Emissions of selected semivolatile organic chemicals from forest and savannah fires [J]. Environmental Science & Technology, 2017, 51(3): 1293-1302. [15] de OLIVEIRA ALVES N, BRITO J, CAUMO S, et al. Biomass burning in the Amazon region: Aerosol source apportionment and associated health risk assessment [J]. Atmospheric Environment, 2015, 120: 277-285. doi: 10.1016/j.atmosenv.2015.08.059 [16] XU J S, XU H H, XIAO H, et al. Aerosol composition and sources during high and low pollution periods in Ningbo, China [J]. Atmospheric Research, 2016, 178/179: 559-569. doi: 10.1016/j.atmosres.2016.05.006 [17] 朱红霞, 陶雪梅, 王超, 等. 北京及周边6个城市大气PM2.5中左旋葡聚糖及其异构体的时空分布特征 [J]. 环境科学, 2020, 41(4): 1544-1549. ZHU H X, TAO X M, WANG C, et al. Spatial and temporal distribution characteristics of levoglucosan and its isomers in PM2.5 in Beijing and six surrounding cities [J]. Environmental Science, 2020, 41(4): 1544-1549(in Chinese).
[18] MA S X, WANG Z Z, BI X H, et al. Composition and source of saccharides in aerosols in Guangzhou, China [J]. Chinese Science Bulletin, 2009, 54(23): 4500-4506. [19] WANG G H, KAWAMURA K, LEE S C, et al. Molecular, seasonal, and spatial distributions of organic aerosols from fourteen Chinese cities [J]. Environmental Science & Technology, 2006, 40(15): 4619-4625. [20] ZHAO Z Z, CAO J J, ZHANG T, et al. Stable carbon isotopes and levoglucosan for PM2.5 elemental carbon source apportionments in the largest city of Northwest China [J]. Atmospheric Environment, 2018, 185: 253-261. doi: 10.1016/j.atmosenv.2018.05.008 [21] FU P Q, KAWAMURA K, PAVULURI C M, et al. Molecular characterization of urban organic aerosol in tropical India: Contributions of primary emissions and secondary photooxidation [J]. Atmospheric Chemistry and Physics, 2010, 10(6): 2663-2689. doi: 10.5194/acp-10-2663-2010 [22] SEVIMOGLU O, ROGGE W F. Organic compound concentrations of size-segregated PM10 during sugarcane burning and growing seasons at a rural and an urban site in Florida, USA [J]. Aerosol and Air Quality Research, 2015, 15(5): 1720-1736. doi: 10.4209/aaqr.2015.02.0069 [23] NIRMALKAR J, DESHMUKH D K, DEB M K, et al. Mass loading and episodic variation of molecular markers in PM2.5 aerosols over a rural area in eastern central India [J]. Atmospheric Environment, 2015, 117: 41-50. doi: 10.1016/j.atmosenv.2015.07.003 [24] CAO F, ZHANG S C, KAWAMURA K, et al. Chemical characteristics of dicarboxylic acids and related organic compounds in PM2.5 during biomass-burning and non-biomass-burning seasons at a rural site of Northeast China [J]. Environmental Pollution, 2017, 231: 654-662. doi: 10.1016/j.envpol.2017.08.045 [25] WAN X, KANG S C, LI Q L, et al. Organic molecular tracers in the atmospheric aerosols from Lumbini, Nepal, in the northern Indo-Gangetic Plain: Influence of biomass burning [J]. Atmospheric Chemistry and Physics, 2017, 17(14): 8867-8885. doi: 10.5194/acp-17-8867-2017 [26] SCHKOLNIK G, FALKOVICH A H, RUDICH Y, et al. New analytical method for the determination of levoglucosan, polyhydroxy compounds, and 2-methylerythritol and its application to smoke and rainwater samples [J]. Environmental Science & Technology, 2005, 39(8): 2744-2752. [27] PIO C A, LEGRAND M, ALVES C A, et al. Chemical composition of atmospheric aerosols during the 2003 summer intense forest fire period [J]. Atmospheric Environment, 2008, 42(32): 7530-7543. doi: 10.1016/j.atmosenv.2008.05.032 [28] HU Q H, XIE Z Q, WANG X M, et al. Levoglucosan indicates high levels of biomass burning aerosols over oceans from the Arctic to Antarctic [J]. Scientific Reports, 2013, 3: 3119. doi: 10.1038/srep03119 [29] FU P Q, KAWAMURA K. Diurnal variations of polar organic tracers in summer forest aerosols: A case study of a Quercus and Picea mixed forest in Hokkaido, Japan [J]. Geochemical Journal, 2011, 45(4): 297-308. doi: 10.2343/geochemj.1.0123 [30] HOFFMANN D, TILGNER A, IINUMA Y, et al. Atmospheric stability of levoglucosan: A detailed laboratory and modeling study [J]. Environmental Science & Technology, 2010, 44(2): 694-699. [31] KAWAMURA K, ISHIMURA Y, YAMAZAKI K. Four years' observations of terrestrial lipid class compounds in marine aerosols from the western North Pacific [J]. Global Biogeochemical Cycles, 2003, 17(1): 3-1. [32] FU P Q, KAWAMURA K, BARRIE L A. Photochemical and other sources of organic compounds in the Canadian high Arctic aerosol pollution during winter-spring [J]. Environmental Science & Technology, 2009, 43(2): 286-292. [33] von SCHNEIDEMESSER E, SCHAUER J J, HAGLER G S W, et al. Concentrations and sources of carbonaceous aerosol in the atmosphere of Summit, Greenland [J]. Atmospheric Environment, 2009, 43(27): 4155-4162. doi: 10.1016/j.atmosenv.2009.05.043 [34] HARA K, OSADA K, YABUKI M, et al. Haze episodes at Syowa Station, coastal Antarctica: Where did they come from? [J]. Journal of Geophysical Research:Atmospheres, 2010, 115(D14): D14205. doi: 10.1029/2009JD012582 [35] ZANGRANDO R, BARBARO E, VECCHIATO M, et al. Levoglucosan and phenols in Antarctic marine, coastal and plateau aerosols [J]. Science of the Total Environment, 2016, 544: 606-616. doi: 10.1016/j.scitotenv.2015.11.166 [36] SANG X F, ZHANG Z S, CHAN C, et al. Source categories and contribution of biomass smoke to organic aerosol over the southeastern Tibetan Plateau [J]. Atmospheric Environment, 2013, 78: 113-123. doi: 10.1016/j.atmosenv.2012.12.012 [37] CONG Z, KANG S, KAWAMURA K, et al. Carbonaceous aerosols on the south edge of the Tibetan Plateau: Concentrations, seasonality and sources [J]. Atmospheric Chemistry and Physics, 2015, 15(3): 1573-1584. doi: 10.5194/acp-15-1573-2015 [38] PASHYNSKA V, VERMEYLEN R, VAS G, et al. Development of a gas chromatographic/ion trap mass spectrometric method for the determination of levoglucosan and saccharidic compounds in atmospheric aerosols. Application to urban aerosols [J]. Journal of Mass Spectrometry, 2002, 37(12): 1249-1257. doi: 10.1002/jms.391 [39] SHEESLEY R J, MIERITZ M, DEMINTER J T, et al. Development of an in situ derivatization technique for rapid analysis of levoglucosan and polar compounds in atmospheric organic aerosol [J]. Atmospheric Environment, 2015, 123: 251-255. doi: 10.1016/j.atmosenv.2015.10.047 [40] YTTRI K E, DYE C, SLØRDAL L H, et al. Quantification of monosaccharide anhydrides by liquid chromatography combined with mass spectrometry: Application to aerosol samples from an urban and a suburban site influenced by small-scale wood burning [J]. Journal of the Air & Waste Management Association, 2005, 55(8): 1169-1177. [41] ENGLING G, CARRICO C M, KREIDENWEIS S M, et al. Determination of levoglucosan in biomass combustion aerosol by high-performance anion-exchange chromatography with pulsed amperometric detection [J]. Atmospheric Environment, 2006, 40: 299-311. doi: 10.1016/j.atmosenv.2005.12.069 [42] KIRCHGEORG T, SCHÜPBACH S, KEHRWALD N, et al. Method for the determination of specific molecular markers of biomass burning in lake sediments [J]. Organic Geochemistry, 2014, 71: 1-6. doi: 10.1016/j.orggeochem.2014.02.014 [43] BHATTARAI H, SAIKAWA E, WAN X, et al. Levoglucosan as a tracer of biomass burning: Recent progress and perspectives [J]. Atmospheric Research, 2019, 220: 20-33. doi: 10.1016/j.atmosres.2019.01.004 [44] JANOSZKA K, CZAPLICKA M. Methods for the determination of levoglucosan and other sugar anhydrides as biomass burning tracers in environmental samples - A review [J]. Journal of Separation Science, 2019, 42(1): 319-329. doi: 10.1002/jssc.201800650 [45] SCHKOLNIK G, RUDICH Y. Detection and quantification of levoglucosan in atmospheric aerosols: A review [J]. Analytical and Bioanalytical Chemistry, 2006, 385(1): 26-33. doi: 10.1007/s00216-005-0168-5 [46] ABDILLA R M, RASRENDRA C B, HEERES H J. Kinetic studies on the conversion of levoglucosan to glucose in water using brønsted acids as the catalysts [J]. Industrial & Engineering Chemistry Research, 2018, 57(9): 3204-3214. [47] HOSOYA T, KAWAMOTO H, SAKA S. Different pyrolytic pathways of levoglucosan in vapor- and liquid/solid-phases [J]. Journal of Analytical and Applied Pyrolysis, 2008, 83(1): 64-70. doi: 10.1016/j.jaap.2008.06.008 [48] HOLMES B J, PETRUCCI G A. Water-soluble oligomer formation from acid-catalyzed reactions of levoglucosan in proxies of atmospheric aqueous aerosols [J]. Environmental Science & Technology, 2006, 40(16): 4983-4989. [49] HOLMES B J, PETRUCCI G A. Oligomerization of levoglucosan by Fenton chemistry in proxies of biomass burning aerosols [J]. Journal of Atmospheric Chemistry, 2007, 58(2): 151-166. doi: 10.1007/s10874-007-9084-8 [50] ZHAO R, MUNGALL E L, LEE A K Y, et al. Aqueous-phase photooxidation of levoglucosan–a mechanistic study using aerosol time-of-flight chemical ionization mass spectrometry (Aerosol ToF-CIMS) [J]. Atmospheric Chemistry and Physics, 2014, 14(18): 9695-9706. doi: 10.5194/acp-14-9695-2014 [51] ENAMI S, HOFFMANN M R, COLUSSI A J. Criegee intermediates react with levoglucosan on water [J]. The Journal of Physical Chemistry Letters, 2017, 8(16): 3888-3894. doi: 10.1021/acs.jpclett.7b01665 [52] TERAJI T, ARAKAKI T. Bimolecular rate constants between levoglucosan and hydroxyl radical: Effects of pH and temperature [J]. Chemistry Letters, 2010, 39(8): 900-901. doi: 10.1246/cl.2010.900 [53] HENNIGAN C J, SULLIVAN A P, COLLETT JR J L, et al. Levoglucosan stability in biomass burning particles exposed to hydroxyl radicals [J]. Geophysical Research Letters, 2010, 37(9): L09806. [54] LAI C Y, LIU Y C, MA J Z, et al. Degradation kinetics of levoglucosan initiated by hydroxyl radical under different environmental conditions [J]. Atmospheric Environment, 2014, 91: 32-39. doi: 10.1016/j.atmosenv.2014.03.054 [55] PRATAP V, BIAN Q J, KIRAN S A, et al. Investigation of levoglucosan decay in wood smoke smog-chamber experiments: The importance of aerosol loading, temperature, and vapor wall losses in interpreting results [J]. Atmospheric Environment, 2019, 199: 224-232. doi: 10.1016/j.atmosenv.2018.11.020 [56] KESSLER S H, SMITH J D, CHE D L, et al. Chemical sinks of organic aerosol: Kinetics and products of the heterogeneous oxidation of erythritol and levoglucosan [J]. Environmental Science & Technology, 2010, 44(18): 7005-7010. [57] SANG X F, GENSCH I, KAMMER B, et al. Chemical stability of levoglucosan: An isotopic perspective [J]. Geophysical Research Letters, 2016, 43(10): 5419-5424. doi: 10.1002/2016GL069179 [58] BAI J, SUN X M, ZHANG C X, et al. The OH-initiated atmospheric reaction mechanism and kinetics for levoglucosan emitted in biomass burning [J]. Chemosphere, 2013, 93(9): 2004-2010. doi: 10.1016/j.chemosphere.2013.07.021 [59] 黄通, 杨池, 张春燕, 等. 无机离子对左旋葡聚糖光降解的影响 [J]. 环境化学, 2021, 40(2): 624-631. HUANG T, YANG C, ZHANG C Y, et al. Effect of inorganic ions on photodegradation of Levoglucosan [J]. Environmental Chemistry, 2021, 40(2): 624-631(in Chinese).
[60] SLADE J H, KNOPF D A. Multiphase OH oxidation kinetics of organic aerosol: The role of particle phase state and relative humidity [J]. Geophysical Research Letters, 2014, 41(14): 5297-5306. doi: 10.1002/2014GL060582 [61] HENNIGAN C J, MIRACOLO M A, ENGELHART G J, et al. Chemical and physical transformations of organic aerosol from the photo-oxidation of open biomass burning emissions in an environmental chamber [J]. Atmospheric Chemistry and Physics, 2011, 11(15): 7669-7686. doi: 10.5194/acp-11-7669-2011 [62] GENSCH I, SANG-ARLT X F, LAUMER W, et al. Using δ13C of levoglucosan as a chemical clock [J]. Environmental Science & Technology, 2018, 52(19): 11094-11101. [63] FAVEZ O, EL HADDAD I, PIOT C, et al. Inter-comparison of source apportionment models for the estimation of wood burning aerosols during wintertime in an Alpine City (Grenoble, France) [J]. Atmospheric Chemistry and Physics, 2010, 10(12): 5295-5314. doi: 10.5194/acp-10-5295-2010 [64] YANG C, ZHANG C Y, LUO X S, et al. Isomerization and degradation of levoglucosan via the photo-Fenton process: Insights from aqueous-phase experiments and atmospheric particulate matter [J]. Environmental Science & Technology, 2020, 54(19): 11789-11797. [65] STYLER S A, DONALDSON D J. Heterogeneous photochemistry of oxalic acid on Mauritanian sand and Icelandic volcanic ash [J]. Environmental Science & Technology, 2012, 46(16): 8756-8763. [66] XU C Y, LIN X M, YIN S S, et al. Enantioselectivity in biotransformation and bioaccumulation processes of typical chiral contaminants [J]. Environmental Pollution, 2018, 243: 1274-1286. doi: 10.1016/j.envpol.2018.09.095 -




 下载:
下载:





























