-
大气颗粒物尤其是细颗粒物,由于其构成成分复杂,能够长距离传播并且会随人类呼吸进入体内,因此其被认为是重要的环境风险物质[1]。大量的流行病学与环境毒理学研究表明,人类呼吸系统疾病的发病率与大气细颗粒物的暴露之间呈显著的正相关关系[2-4]。针对大气颗粒物的人体健康损害机理研究是近年来的热门研究问题,目前大量的研究结果表明ROS(reactive oxygen species,ROS)的过度累积导致机体的氧化应激效应是大气污染物危害人体健康的重要的原因之一。
ROS是指含有氧、且具有较高氧化反应活性的一类物质的总称,常见的ROS种类包括超氧自由基(O2•−)、羟基自由基(HO•)、单线态氧(1O2)、过氧羟基自由基(HO2•)和过氧化氢(H2O2)等[5]。ROS也被称为氧化潜能或氧化还原活性,研究表明ROS的过量积累会导致人体氧化应激反应。氧化应激是指人体内氧化还原反应失衡,更倾向于氧化作用,从而对机体造成损害,直至产生病理反应的过程[6]。该过程类似于金属物质的氧化作用,同理,当ROS过量时,也会导致我们身体“生锈”。
大气ROS主要分为颗粒物诱导作用生成的颗粒相ROS和气相ROS,颗粒物诱导机体产生ROS的途径主要有两种:(1)直接氧化:氧化物质会伴随颗粒物一同沉积在呼吸系统内,造成细胞氧化应激;(2)间接氧化:颗粒物中的某些化学组分刺激细胞产生过量的ROS,或颗粒物中的某些化学组分与产生ROS的特定生物化学物质催化相互作用[7-9]。
研究表明,大气颗粒物中的化学组分可与呼吸道上皮细胞相互作用产生ROS[10],进而损害人体细胞[7, 11],进一步还会造成呼吸道炎症和其他呼吸系统疾病[12-14]。此外大气颗粒物在肺细胞内可产生ROS,并随后诱发氧化应激和气道炎症[8, 10]。在人呼吸道上皮衬里液中,空气中的污染物可以与机体细胞发生作用,如臭氧和羟基自由基与表面活性剂和抗氧化剂(抗坏血酸、谷胱甘肽等)发生反应,生成二次氧化剂,与细颗粒物(包括醌类、铁和铜离子等)中的氧化还原活性组分可以触发和维持产生ROS的循环反应[15]。
气相ROS主要是通过光化学作用和多相反应产生的,如羟基自由基,超氧自由基、单线态氧以及环氧化物都源于臭氧的光解和伴随的自由基反应[16-17]。现有的研究成果表明,颗粒相ROS比气相ROS 对人体健康的危害更大,因为大多数气相ROS具有较高的水溶性和分子扩散性,更容易被皮肤的粘液涂层吸收[18],这不同于颗粒相ROS会伴随颗粒物进入人体内并持续造成健康损害。气相ROS更主要的是会影响大气光化学作用并且会与部分大气污染物发生反应,起到减少大气污染的作用[19],如羟基自由基在大气自清洁中起关键作用,可与大多数空气污染物发生反应,将它们转化为可通过湿沉积有效去除的水溶性物质[20]。因此,羟基自由基也经常被描述为“大气的清洁剂”。
-
目前已经有多种ROS检测方法,根据检测方案的种类可以将其分为细胞内检测和细胞外检测两种。细胞内检测主要是通过将小鼠肺泡的巨噬细胞暴露于PM环境中并检测其生成的ROS[21-22]。而相较于细胞内检测方案,细胞外检测方法由于其简单、便捷,目前已成为了多数研究ROS的首选方案。本文主要简述几种常见的细胞外ROS分析方法。
-
细胞衬里液法是模拟抗坏血酸,谷胱甘肽等细胞中的抗氧化剂,它们会在在细胞衬里液中与大气颗粒物发生氧化还原反应,其消耗速率即可反映颗粒物的ROS水平[23-25]。抗坏血酸和谷胱甘肽随时间的消耗量可用高效液相色谱法和紫外-可见吸光光谱检测,其消耗速率即可量化ROS的浓度水平[26]。目前关于抗坏血酸和谷胱甘肽的方法基本上都在细胞衬里液里进行。
-
2’,7’-二氢二氯荧光素(DCFH)本身不发荧光,需要辣根过氧化物酶作为催化剂与颗粒物中的ROS发生氧化反应后生成荧光物质(DCF)[27]。DCF在激发波长485 nm,发射波长535 nm下可检测到,ROS的浓度与DCF的生成量呈正比[28]。用不同浓度的H2O2与DCFH反应结果作为标准浓度最终得到ROS的含量。二氯荧光素的方法虽然简单,但由于荧光物质容易分解,所以背景信号相对较高[29-32]。
-
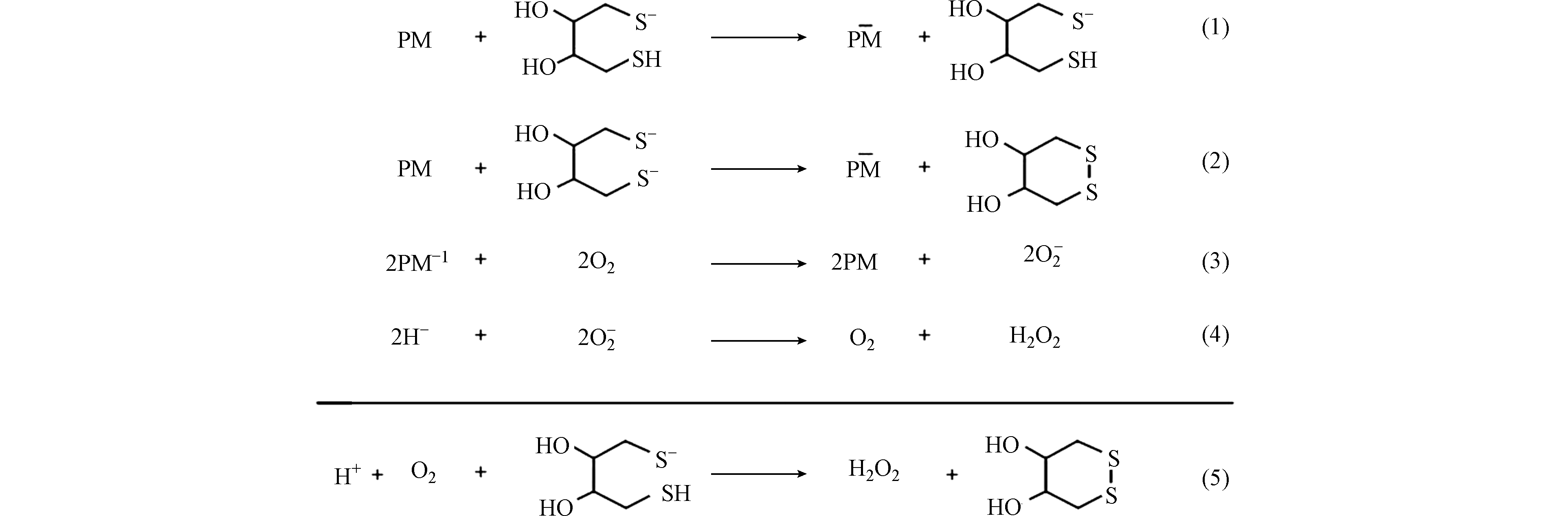
二硫苏糖醇(DTT)是一种还原剂,可以与颗粒物中的ROS物质发生氧化还原反应如图1所示。它模拟细胞还原剂如还原型辅酶Ⅱ(NADPH)的氧化, 通过DTT的消耗速率评估大气PM的氧化潜能[14]。DTT本身不吸光,与5,5’-二硫代双-(2-硝基苯甲酸)(DTNB)反应后变成黄色的2-硝基-5-硫基苯甲酸,该物质在412 nm处有吸收峰,一般用紫外-可见吸光光谱仪检测。ROS与DTT反应后会使DTT的含量下降,再通过DTT与DTNB的显色反应检测剩余的DTT含量,DTT的消耗速率即可用来量化颗粒物中ROS浓度水平。该方法是目前最常用的ROS检测方案
-
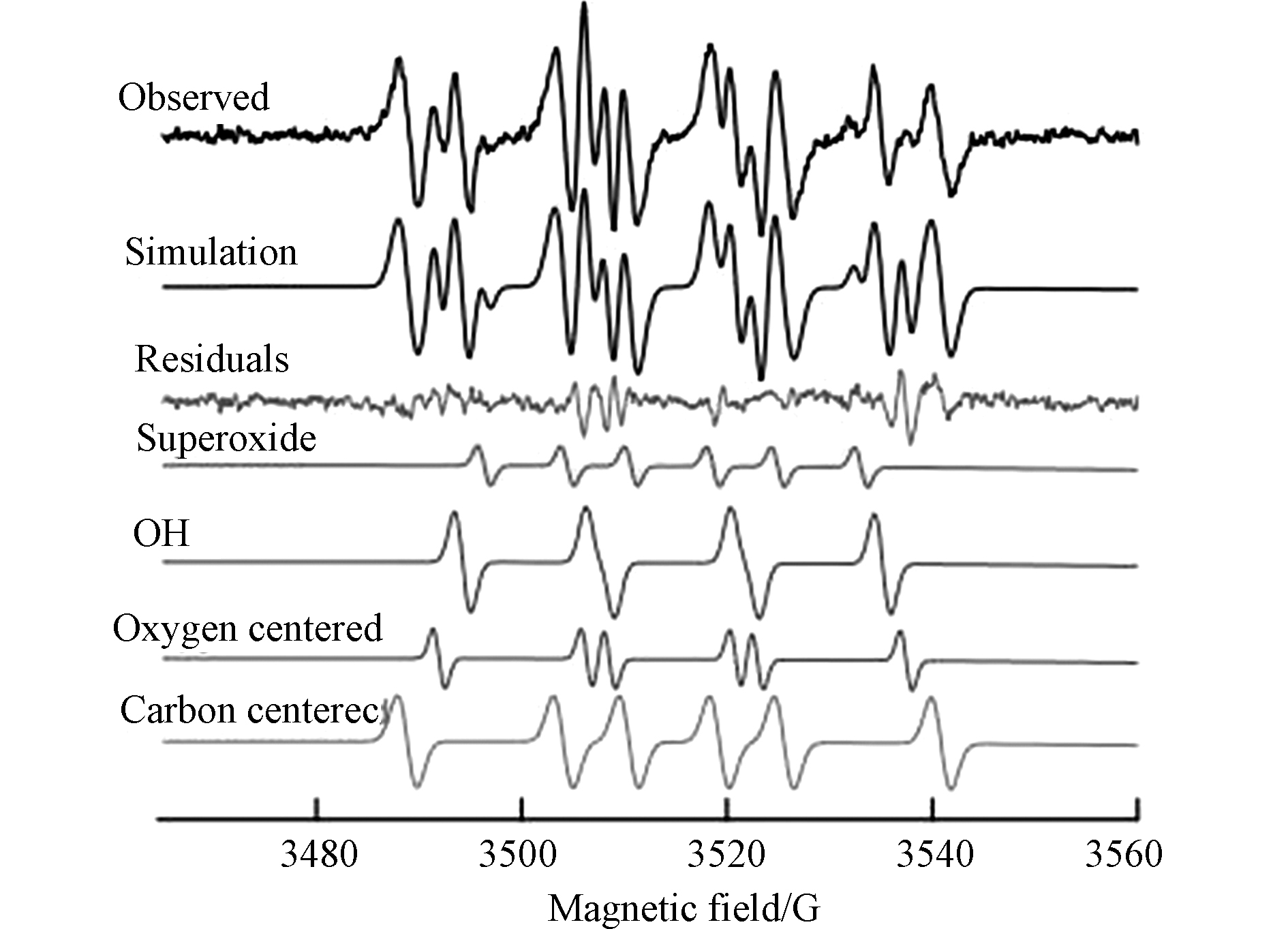
电子顺磁共振波谱法是通过检测样品中单电子的数量而确定自由基的存在,由于ROS自由基的寿命较短,在检测的过程中通常需要使用自由基捕获剂。O2•−、HO•等可以用5-叔丁基羰基-5-甲基-1-吡咯-N-氧化物(BMPO)或5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)捕获[33],而1O2可以用4-羟基-2,2,6,6-四甲基哌啶(TEMP)捕获[34]。通过对比样品EPR谱图的特征峰高度和位置即可确定ROS自由基的种类,自由基的浓度可以用自旋量(spin)表示。图2为BMPO捕获的大气颗粒物中自由基的EPR谱图[35]。
-
气相ROS具有较高的水溶性和分子扩散性,其在大气环境中的存在时间往往较短,传统的离线检测方案往往是对样品长时间保存后进行检测,并不适用于气相ROS的检测。近年来已经有少数研究人员开发了基于空气样品液化器(PILS)和DCFH分析结合的ROS在线检测系统,如Gali 等[36]使用PILS在线采集气溶胶并对样品进行实时荧光探针检测。其他的一些研究也都采取了类似的原理以期得到实际大气环境中ROS的浓度水平,这些研究结果普遍表明离线ROS检测会低估气溶胶的氧化活性[37-40],但目前仍缺乏规范的气相ROS检测方案。
-
决定大气ROS生成机制的主要因素包括水溶性颗粒物的粒径特征和主要成分,目前大多数研究成果表明,细颗粒物是诱导ROS生成的重要成分[41-42],而颗粒物中的水溶性有机物及金属离子则是诱导ROS生成的主要组分,此外近年来也有一些研究提出生物气溶胶对大气ROS具有一定的贡献[43-44]。
粒径分布研究如Hung[28]对中国台北市大气颗粒物及汽车尾气中不同粒径颗粒物中ROS的浓度进行了研究,发现人行道上的颗粒物活性较高,相较于大粒径颗粒物,细颗粒物的氧化潜能更高。Cho[14]等以美国洛杉矶不同地区的大气颗粒物为研究对象,对颗粒物的化学组成和氧化潜能进行了全面研究。结果表明,超细颗粒物的氧化还原活性最高,其诱导消耗细胞内谷胱甘肽的作用最强,这与诱导氧化应激有关。此外,Chirizzi、Prasanna等[43-46]的研究也都表明了细颗粒物是诱导ROS生成的主要物质。Arangio等[35]采用EPR法对德国美因茨不同粒径大气颗粒物中ROS的种类及浓度进行了分析,发现超细颗粒物生成ROS的种类最多,包括羟基自由基、超氧自由基、以碳为中心的自由基以及以氧为中心的自由基,而随着粒径的增大,羟基自由基的生成量占比会逐渐增加,这种现象可能是由于随着颗粒物粒径增加金属离子的含量会增加,金属离子与过氧化物的芬顿反应是产生羟基自由基的重要机制。
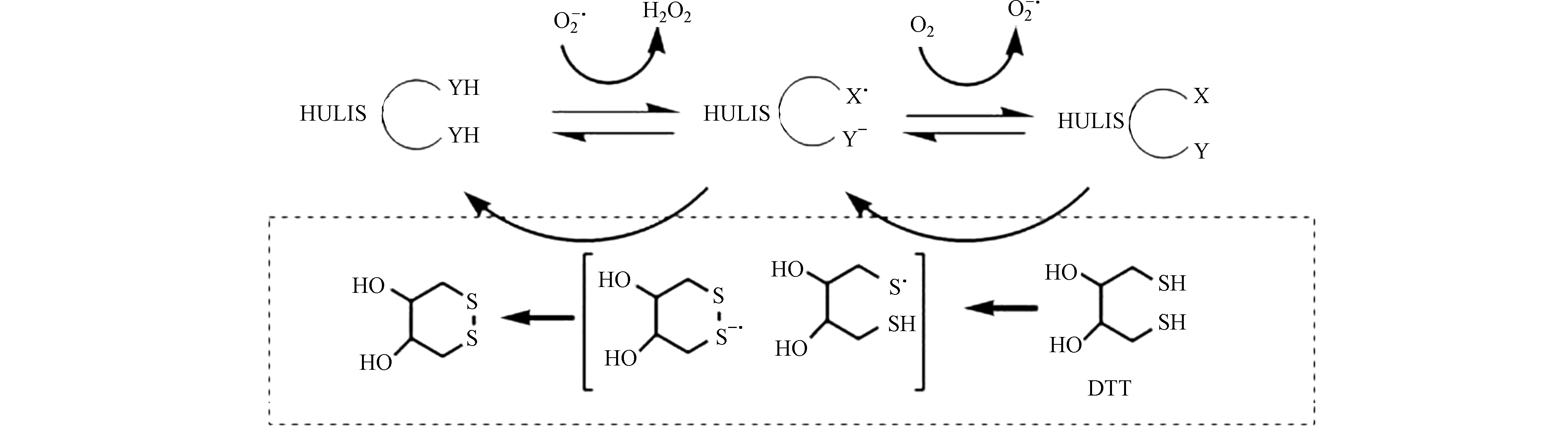
随着对大气颗粒物ROS的深入研究,水溶性及水不溶性有机物变为了ROS的研究重点,例如Lin等[47]以我国长三角地区大气中腐殖质类物质(HULIS)的氧化潜能为研究对象,发现了HULIS是大气颗粒中水溶性有机组分中产生ROS的主要成分。如图3所示为HULIS诱导ROS生成并造成DTT的消耗,HULIS作为可以作为电子的载体,以氧化还原反应中间体的形式催化ROS的生成。Verma等[48]研究了美国亚特兰大水溶性和不溶性组分及其疏水/亲水组分的ROS水平,结果表明甲醇提取物的DTT活性明显比水溶性组分更高,大气颗粒物中水溶性有机碳和水不溶性有机碳的含量影响了甲醇和水提取物的DTT活性。Verma等[49]研究了美国有机气溶胶的氧化潜能,结果表明生物质燃烧和氧化性更强的有机气溶胶的水溶性颗粒物的DTT活性相关性更强。有机气溶胶贡献了约60%的DTT活性,金属离子则可能影响了其他DTT活性。对于水不溶性有机物,其生成ROS的机制目前仍有待探究,部分研究认为大分子的多环芳烃或醌类物与细胞氧化剂或金属离子结合可能是水不溶性有机物生成ROS的重要机制[47-49]。
金属离子则被认为是大气ROS的另一主要诱导成分,这包括了金属离子的直接诱导及金属离子与其他有机物共同作用诱导ROS的生成。
Cheung等[50]研究交通气溶胶发现可溶性铁与颗粒物ROS活性密切相关,此外一些其他的金属物质如钙、锌和磷等都与DTT活性密切相关。See等[51]采用二氯荧光素法对大气颗粒物中ROS与金属离子的相关性进行了研究,具体研究对象包括交通车辆排放、餐饮及燃料燃烧。研究发现水溶性金属可能对大气ROS有重要影响。Verma等[52]研究发现金属离子(V、Ni和Cd)及水溶性有机碳与ROS有显著的相关性。此外,他们还发现一次颗粒物和二次颗粒物都具有较高的氧化还原活性。
Yu等[53]针对颗粒物中不同组分的拮抗作用及协同作用在ROS生成中的影响进行了研究。结果表明在DTT消耗中,铁与醌的相互作用是加和性的,其在羟基生成中具有强的协同作用。铜在DTT消耗和羟基生成过程中都表现出与醌类化合物的拮抗作用。锰在DTT氧化过程中与醌类化合物具有协同作用,但在羟基生成过程中起拮抗作用。Xiong等[54]发现铁本身不会对DTT活性造成影响,但在与其他醌类物质共同存在的条件下会生成羟基自由基。Lyu等[55]研究了上海市不同污染天气条件下的大气ROS,结果发现在雾霾天气,颗粒物的DTT活性是正常天气样品的2.4倍。通过相关性分析研究表明,铜和锰等金属离子贡献了约55%±13%的DTT活性,而醌类化合物仅占8%±3%。
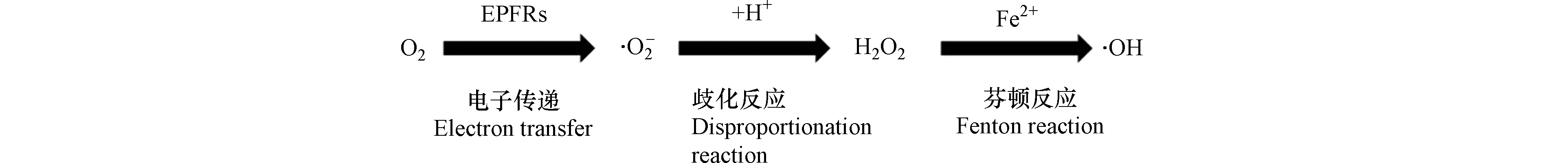
此外,在近年研究中,一种新型的环境风险物质环境持久性自由基(environmental persistent free radicals,EPFRs)也被发现可以同氧气作用生成ROS。EPFRs广泛存在于大气及土壤环境中,它不同于羟基自由基等短寿命自由基,其半衰期可以长达数月甚至1年,因此在进入人体后会持续对人体健康造成危害[56-60]。已经有研究证明,EPFRs可以造成心血管和呼吸功能障碍,以及肺部炎症甚至肺癌,而造成这些症状的原因则被认为是ROS与氧气作用生成的ROS[61-65],如图4所示为EPFRs 生成 ROS 的作用机制,首先 EPFRs 将电子转移给氧分子生成超氧阴离子,然后超氧阴离子发生气化反应生成过氧化氢,最后反应体系内发生芬顿反应并生成羟基自由基[66-67]。由于 EPFRs 具有长寿命的特征,因此当大气颗粒物进入人体后,该反应体系可能会持续作用生成ROS并对人体造成健康危害。
-
大气ROS的来源较为复杂,如表1所示为国内外不同地区ROS来源特征研究的对比,虽然不同的地区具有一定的差异性,但整体的研究结果表明人为排放如汽车尾气,燃料燃烧和二次硫酸盐等是导致大气中ROS生成的主要来源[68-70]。
国内关于颗粒物ROS的研究如Liu等[71]研究了天津市PM2.5中ROS的来源。他们利用正向矩阵因子模型(PMF)和多元线性回归(MLR)法对颗粒物的DTT活性进行了来源解析,结果表明冬季特别是采暖期煤炭燃烧对DTT的贡献较高。而交通来源则在夏季贡献最高,此外二次源在夏季也有一定贡献,工业源则对春季贡献较大。Ma等[72]研究了北京市大气颗粒物中HULIS产生ROS的来源,PMF解析的结果表明煤炭燃烧、生物质燃烧、交通源、其他燃烧及二次气溶胶为主要来源,其中DTT活性的70%以上由燃烧源贡献。Wang等[73]研究了杭州市PM2.5产生ROS的日变化规律,结果表明二次无机气溶胶与相应的DTT活性有较好的相关性,并表现出显著的日变化,白天较夜间具有更好地相关性,这表明白天的ROS的生成受到了二次光化学作用影响。Yu等[74]研究了北京大气PM2.5中ROS的来源特征,结果表明ROS主要来源依次为车辆排放>二次硫酸盐和工业排放> 燃煤>粉尘。Brehmer等[75]研究了四川绵阳农村地区的大气ROS,研究表明与ROS相关性最好的为二次硫酸盐有关的元素(As,Mo,Zn)。Wang等[76]运用DTT检测法研究了西安市PM2.5中ROS的主要来源并发现,煤炭燃烧、二次硫酸盐和机动车排是ROS主要来源,其浓度水平为冬季>春季>夏季>秋季。Huang等[40]采用在线ROS检测方法发现,北京市冬季的ROS浓度明显大于春季,这与冬季的大规模供暖有关。Gali等[36]采用在线ROS分析方案对香港主要道路及城市地区ROS浓度水平进行了分析并发现道路中心ROS浓度水平要显著高于住宅地区,这表明交通排放是影响ROS的重要因素。
国外关于ROS来源的研究如Verma等[77]采用了PMF和化学质量平衡(CMB)法对美国佐治亚州的3个不同城市大气颗粒物的氧化潜能进行了研究,两种研究方法的分析结果基本一直,但CMB的分析结果中汽车尾气的贡献比PMF的高,其研究结果表明在全年的不同季节,交通排放的贡献是一致的,而在夏季和冬季,二次氧化过程和燃烧则占主导地位。此外,在秋夏两个气候较为干旱的季节,粉尘有一定的贡献。Bates等[78]采用DTT法测定水溶性PM2.5的ROS,对美国亚特兰大ROS水平进行了研究,研究采用了CMB法和线性回归分析法对ROS来源进行了评估,发现燃烧源对DTT总活性的贡献最大。此外,该研究还对DTT活性的历史水平进行了估算,用于对1998—2009年期间的流行病学分析,通过将流行病学数据与颗粒物氧化应激水平进行相关性分析,结果发现相较于PM2.5,ROS水平与心力衰竭、哮喘等心血管疾病就诊率的相关性更高。Weber等[79]研究了法国大气颗粒物氧化潜能的来源分配,该研究采用PMF方法分析颗粒物及ROS来源。结果发现生物质燃烧和交通排放源的是氧化活性的主要来源。King等[38]对美国亚特兰大城市及农村地区5—7月的ROS浓度水平同时进行了在线和离线方法进行了分析,在线和离线分析的结果都表明城市地区的ROS浓度要高于农村地区,他们认为这与城市大量的交通排放有关。Wang等[39]采用在线分析法对美国纽约夏季进行了连续7 d的ROS分析,结果表明工作日ROS浓度水平要高于周末,这与交通排放有关,而白天则要高于夜晚,这表明白天强烈的光化学作用会生成ROS。
-
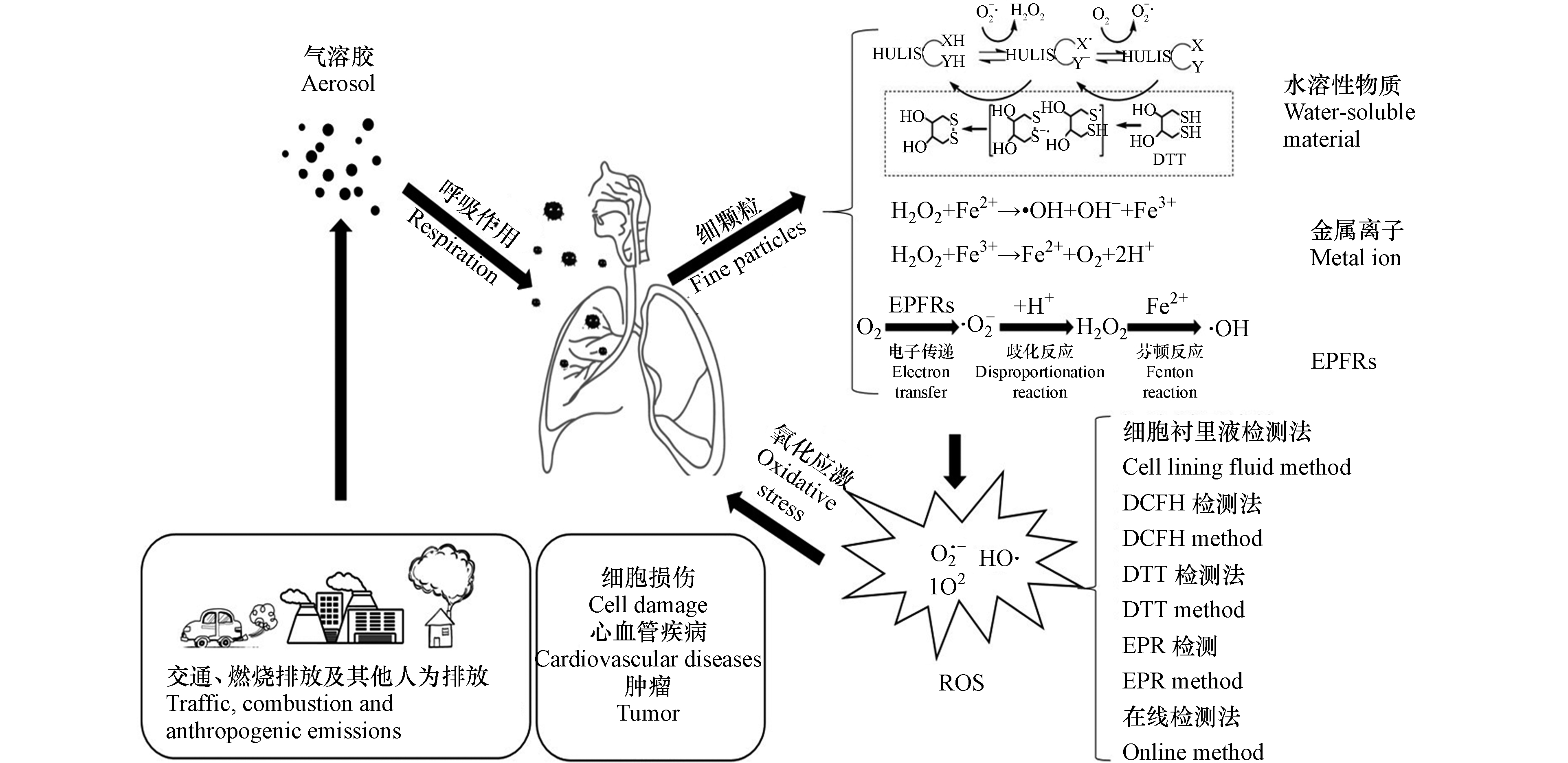
氧化潜能作为评估大气颗粒物健康风险的重要指标,研究大气ROS的污染特性具有十分重要的价值,现有的研究成果已经对大气ROS的污染特征有了初步的掌握,图5对本文的主要内容进行了概括总结。在未来的研究中确定其具体的来源特征并探究气溶胶化学组分与氧化潜能的关系是该领域研究的热点问题。目前研究者们普遍认为ROS的主要来源为金属离子的氧化还原过程,也有部分研究者认为有机组分作为中间体诱发的氧化还原反应可能是大气中ROS的重要来源,因此在未来的ROS研究中可以将以下几个方面作为研究重点。
(1)现有的研究成果已经表明大气颗粒物可以通过产生ROS危害人体健康,但鉴于大气颗粒物组成的复杂性,不同成分对ROS的生成贡献及生成机理都有一定的差异性。因此在未来的研究中可以通过实验室模拟实际大气颗粒物中的不同组分并探究其生成ROS的能力,以确定诱导大气ROS的主导成分。
(2)EPFRs是近年来的研究热点,它作为一种可以持续产生ROS的环境风险物质,目前对其具体的生成机制及物理化学特性的研究还不够全面,在未来的研究中需要证明其与其他体系反应生成ROS的具体机制。
(3)气相ROS的相关研究表明,目前常用的离线ROS检测方案可能无法准确检测到大气ROS的实际浓度水平,因此对气相ROS的研究应是一项研究重点。
(4)现有大气污染的评价及防控指标主要是依据大气污染物的浓度,其最终目的仍是降低大气污染的健康风险。ROS作为评价大气污染物健康风险的重要指标,亟需根据大气污染物的氧化潜能建立相应的评价体系,以期更好的降低大气污染物的健康风险。
大气活性氧物质污染特征、机制及其来源研究进展
Advances in research on the pollution characteristics, mechanisms and sources of active reactive oxygen species in the atmosphere
-
摘要: 活性氧(reactive oxygen species,ROS)是指含有氧、且具有较高氧化反应活性的一类物质的总称,因其会由大气颗粒物中的不同组分诱导生成并危害人体健康而广受关注。本文主要概述了大气ROS的国内外研究进展,总结了大气ROS的检测方案,并详细介绍了细胞衬里液法、二硫苏糖醇检测法等几种常用的胞外ROS检测方案。大气细颗粒物是大气ROS的主要诱导组分,具体的成分为水溶性有机物和金属离子,环境持久性自由基也可能是潜在的ROS诱导物质。ROS的来源研究表明燃烧过程和交通排放等人为来源是大气ROS的主要来源。在未来的研究中可以以大气ROS的生成机制为研究重点,通过实验室模拟等具体实验手段全面研究ROS的污染特征,为ROS污染评价体系的制定提供数据支撑。Abstract: Reactive Oxygen Species (ROS) refers to the general term for a class of substances that contain oxygen and have high oxidation reaction activity. They are widely concerned because they are induced by different components in atmospheric particulates and endanger human health. This article mainly summarizes the research progress of atmospheric reactive oxygen species at home and abroad, summarizes the detection schemes of atmospheric reactive oxygen species, and introduces in detail several common extracellular ROS detection schemes such as cell lining liquid method and dithiothreitol detection method. Atmospheric fine particulate matter is the main inducing component of atmospheric ROS, and the specific components are water-soluble organic matter and transition metals. Persistent free radicals in the environment may also be potential ROS inducing substances. Studies on the sources of ROS have shown that man-made sources such as combustion processes and traffic emissions are the main sources of atmospheric ROS. In the future research, we can focus on the generation mechanism of atmospheric ROS, and comprehensively study the pollution characteristics of ROS through specific experimental methods such as laboratory simulation, so as to provide data support for the ROS pollution evaluation system.
-
Key words:
- atmosphere /
- ROS /
- pollution characteristics
-

-
表 1 国内外ROS来源研究情况
Table 1. Research on the sources of ROS in China and foreign countries
地区
Region分析模型
Analysis modelROS主要来源
Main source of ROS国内
China天津 PMF、MLR 交通源、燃煤 北京 PMF、在线分析 煤炭/生物质燃烧、交通排放 绵阳 富集因子法 生物质燃烧、二次硫酸盐 西安 PMF、MLR 煤炭燃烧、二次硫酸盐 杭州 富集因子法 二次过程 国外
Foreign country美国 PMF、CMB、在线分析 交通源、燃烧 法国 PMF 生物质燃烧、交通源 -
[1] BAUMGARTNER J, SCHAUER J J, EZZATI M, et al. Indoor air pollution and blood pressure in adult women living in rural China [J]. Environmental Health Perspectives, 2011, 119(10): 1390-1395. doi: 10.1289/ehp.1003371 [2] CHAN C K, YAO X. Air pollution in mega cities in China [J]. Atmospheric Environment, 2008, 42(1): 1-42. doi: 10.1016/j.atmosenv.2007.09.003 [3] BRUNEKREEF B, FORSBERG B. Epidemiological evidence of effects of coarse airborne particles on health [J]. The European Respiratory Journal, 2005, 26(2): 309-318. doi: 10.1183/09031936.05.00001805 [4] MAUDERLY J L, CHOW J C. Health effects of organic aerosols [J]. Inhalation Toxicology, 2008, 20(3): 257-288. doi: 10.1080/08958370701866008 [5] LU C, SONG G Q, LIN J M. Reactive oxygen species and their chemiluminescence-detection methods [J]. TrAC Trends in Analytical Chemistry, 2006, 25(10): 985-995. doi: 10.1016/j.trac.2006.07.007 [6] MATÉS J M, SEGURA J M, PÉREZ-GÓMEZ C, et al. Antioxidant enzymatic activities in human blood cells after an allergic reaction to pollen or house dust mite [J]. Blood Cells Molecules and Diseases, 1999, 25(2): 103-109. doi: 10.1006/bcmd.1999.0234 [7] LI N, SIOUTAS C, CHO A, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage [J]. Environmental Health Perspectives, 2003, 111(4): 455-460. doi: 10.1289/ehp.6000 [8] AYRES J G, BORM P, CASSEE F R, et al. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential—A workshop report and consensus statement [J]. Inhalation Toxicology, 2008, 20(1): 75-99. doi: 10.1080/08958370701665517 [9] SQUADRITO G L, CUETO R, DELLINGER B, et al. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter [J]. Free Radical Biology and Medicine, 2001, 31(9): 1132-1138. doi: 10.1016/S0891-5849(01)00703-1 [10] KELLY F J, FUSSELL J C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter [J]. Atmospheric Environment, 2012, 60: 504-526. doi: 10.1016/j.atmosenv.2012.06.039 [11] DONALDSON K, BROWN D, CLOUTER A, et al. The pulmonary toxicology of ultrafine particles [J]. Journal of Aerosol Medicine, 2002, 15(2): 213-220. doi: 10.1089/089426802320282338 [12] NEL A. Air pollution-related illness: Effects of particles [J]. Science, 2005, 308(5723): 804-806. doi: 10.1126/science.1108752 [13] POPE C A, DOCKERY D W. Health effects of fine particulate air pollution: Lines that connect [J]. Journal of the Air and Waste Management Association, 2006, 56(6): 709-742. doi: 10.1080/10473289.2006.10464485 [14] CHO A K, SIOUTAS C, MIGUEL A H, et al. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin [J]. Environmental Research, 2005, 99(1): 40-47. doi: 10.1016/j.envres.2005.01.003 [15] LAKEY P, BERKEMEIER T, TONG H, et al. Chemical exposure-response relationship between air pollutants and reactive oxygen species in the human respiratory tract [J]. Scientific Reports, 2016, 6: 32916. doi: 10.1038/srep32916 [16] SHIRAIWA M, SOSEDOVA Y, ROUVIÈRE A, et al. The role of long-lived reactive oxygen intermediates in the reaction of ozone with aerosol particles [J]. Nature Chemistry, 2011, 3(4): 291-295. doi: 10.1038/nchem.988 [17] ZIEMANN P J, ATKINSON R. ChemInform abstract: Kinetics, products, and mechanisms of secondary organic aerosol formation [J]. ChemInform, 2012, 43(48): 6582-6605. [18] CHEN X, HOPKE P K, CARTER W P L. Secondary organic aerosol from ozonolysis of biogenic volatile organic compounds: Chamber studies of particle and reactive oxygen species formation [J]. Environmental Science & Technology, 2011, 45(1): 276-282. [19] COOPER O R, PARRISH D D, STOHL A, et al, et al. Revisiting the evidence of increasing springtime ozone mixing ratios in the free troposphere over western North America [J]. Geophysical Research Letters, 2015, 42(20): 8719-8728. doi: 10.1002/2015GL065311 [20] PÖSCHL U, SHIRAIWA M. Multiphase chemistry at the atmosphere–biosphere interface influencing climate and public health in the anthropocene [J]. Chemical Reviews, 2015, 115(10): 4440-4475. doi: 10.1021/cr500487s [21] TUET W Y, FOK S, VERMA V, et al. Dose-dependent intracellular reactive oxygen and nitrogen species (ROS/RNS) production from particulate matter exposure: Comparison to oxidative potential and chemical composition [J]. Atmospheric Environment, 2016, 144: 335-344. doi: 10.1016/j.atmosenv.2016.09.005 [22] TUET W Y, CHEN Y L, FOK S, et al. Inflammatory responses to secondary organic aerosols (SOA) generated from biogenic and anthropogenic precursors [J]. Atmospheric Chemistry and Physics, 2017, 17(18): 11423-11440. doi: 10.5194/acp-17-11423-2017 [23] GODRI K J, DUGGAN S T, FULLER G W, et al. Particulate matter oxidative potential from waste transfer station activity [J]. Environmental Health Perspectives, 2010, 118(4): 493-498. doi: 10.1289/ehp.0901303 [24] GODRI K J, HARRISON R M, EVANS T, et al. Increased oxidative burden associated with traffic component of ambient particulate matter at roadside and urban background schools sites in London [J]. Plos One, 2011, 6(7): e21961. doi: 10.1371/journal.pone.0021961 [25] CALAS A, UZU G, KELLY F J, et al. Comparison between five acellular oxidative potential measurement assays performed with detailed chemistry on PM10 samples from the city of Chamonix (France) [J]. Atmospheric Chemistry and Physics, 2018, 18(11): 7863-7875. doi: 10.5194/acp-18-7863-2018 [26] VISENTIN M, PAGNONI A, SARTI E, et al. Urban PM2.5 oxidative potential: Importance of chemical species and comparison of two spectrophotometric cell-free assays [J]. Environmental Pollution, 2016, 219: 72-79. doi: 10.1016/j.envpol.2016.09.047 [27] MONTESINOS V N, SLEIMAN M, COHN S, et al. Detection and quantification of reactive oxygen species (ROS) in indoor air [J]. Talanta, 2015, 138: 20-27. doi: 10.1016/j.talanta.2015.02.015 [28] HUNG H F, WANG C S. Experimental determination of reactive oxygen species in Taipei aerosols [J]. Journal of Aerosol Science, 2001, 32(10): 1201-1211. doi: 10.1016/S0021-8502(01)00051-9 [29] LIN V S, GRANDBOIS M, MCNEILL K. Fluorescent molecular probes for detection of one-electron oxidants photochemically generated by dissolved organic matter [J]. Environmental Science & Technology, 2017, 51(16): 9033-9041. [30] MURPHY K R, STEDMON C A, GRAEBER D, et al. Fluorescence spectroscopy and multi-way techniques. PARAFAC [J]. Analytical Methods, 2013, 5(23): 6557. doi: 10.1039/c3ay41160e [31] LIU Q Y, BAUMGARTNER J, ZHANG Y X, et al. Oxidative potential and inflammatory impacts of source apportioned ambient air pollution in Beijing [J]. Environmental Science & Technology, 2014, 48(21): 12920-12929. [32] PATEL A, RASTOGI N. Oxidative potential of ambient fine aerosol over a semi-urban site in the Indo-Gangetic Plain [J]. Atmospheric Environment, 2018, 175: 127-134. doi: 10.1016/j.atmosenv.2017.12.004 [33] KHACHATRYAN L, VEJERANO E, LOMNICKI S, et al. Environmentally persistent free radicals (EPFRs). 1. Generation of reactive oxygen species in aqueous solutions [J]. Environmental Science & Technology, 2011, 45(19): 8559-8566. [34] LIANG P, ZHANG C, DUAN X, et al. N-doped graphene from metal-organic frameworks for catalytic oxidation of p-hydroxylbenzoic acid: N-functionality and mechanism [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(3): 2693-2701. [35] ARANGIO A M, TONG H J, SOCORRO J, et al. Quantification of environmentally persistent free radicals and reactive oxygen species in atmospheric aerosol particles [J]. Atmospheric Chemistry and Physics, 2016, 16(20): 13105-13119. doi: 10.5194/acp-16-13105-2016 [36] GALI N K, STEVANOVIC S, BRIMBLECOMBE P, et al. The diurnal characteristics of PM-bound ROS and its influencing factors at urban ambient and roadside environments [J]. Atmospheric Research, 2020, 244: 105039. doi: 10.1016/j.atmosres.2020.105039 [37] FULLER S J, WRAGG F P H, NUTTER J, et al. Comparison of on-line and off-line methods to quantify reactive oxygen species (ROS) in atmospheric aerosols [J]. Atmospheric Environment, 2014, 92: 97-103. doi: 10.1016/j.atmosenv.2014.04.006 [38] KING L E, WEBER R J. Development and testing of an online method to measure ambient fine particulate reactive oxygen species (ROS) based on the 2', 7'-dichlorofluorescin (DCFH) assay [J]. Atmospheric Measurement Techniques, 2013, 6(7): 1647-1658. doi: 10.5194/amt-6-1647-2013 [39] WANG Y G, HOPKE P K, SUN L P, et al. Laboratory and field testing of an automated atmospheric particle-bound reactive oxygen species sampling-analysis system [J]. Journal of Toxicology, 2011, 2011: 419476. [40] HUANG W, ZHANG Y X, ZHANG Y, et al. Development of an automated sampling-analysis system for simultaneous measurement of reactive oxygen species (ROS) in gas and particle phases: GAC-ROS [J]. Atmospheric Environment, 2016, 134: 18-26. doi: 10.1016/j.atmosenv.2016.03.038 [41] JANSSEN N A H, YANG A, STRAK M, et al. Oxidative potential of particulate matter collected at sites with different source characteristics [J]. Science of the Total Environment, 2014, 472: 572-581. doi: 10.1016/j.scitotenv.2013.11.099 [42] PIETROGRANDE M C, DALPIAZ C, DELL'ANNA R, et al. Chemical composition and oxidative potential of atmospheric coarse particles at an industrial and urban background site in the alpine region of northern Italy [J]. Atmospheric Environment, 2018, 191: 340-350. doi: 10.1016/j.atmosenv.2018.08.022 [43] SAMAKE A, UZU G, MARTINS J M F, et al. The unexpected role of bioaerosols in the Oxidative Potential of PM [J]. Scientific Reports, 2017, 7(1): 1-10. doi: 10.1038/s41598-016-0028-x [44] JIN L, XIE J W, WONG C K C, et al. Contributions of city-specific fine particulate matter (PM2.5) to differential in vitro oxidative stress and toxicity implications between Beijing and Guangzhou of China [J]. Environmental Science & Technology, 2019, 53(5): 2881-2891. [45] CHIRIZZI D, CESARI D, GUASCITO M R, et al. Influence of Saharan dust outbreaks and carbon content on oxidative potential of water-soluble fractions of PM2.5 and PM10 [J]. Atmospheric Environment, 2017, 163: 1-8. doi: 10.1016/j.atmosenv.2017.05.021 [46] VENKATACHARI P, HOPKE P K, BRUNE W H, et al. Characterization of wintertime reactive oxygen species concentrations in Flushing, New York [J]. Aerosol Science and Technology, 2007, 41(2): 97-111. doi: 10.1080/02786820601116004 [47] LIN P, YU J Z. Generation of reactive oxygen species mediated by humic-like substances in atmospheric aerosols [J]. Environmental Science & Technology, 2011, 45(24): 10362-10368. [48] VERMA V, FANG T, XU L, et al. Organic aerosols associated with the generation of reactive oxygen species (ROS) by water-soluble PM2.5 [J]. Environmental Science & Technology, 2015, 49(7): 4646-4656. [49] VERMA V, RICO-MARTINEZ R, KOTRA N, et al. Contribution of water-soluble and insoluble components and their hydrophobic/hydrophilic subfractions to the reactive oxygen species-generating potential of fine ambient aerosols [J]. Environmental Science & Technology, 2012, 46(20): 11384-11392. [50] CHEUNG K L, NTZIACHRISTOS L, TZAMKIOZIS T, et al. Emissions of particulate trace elements, metals and organic species from gasoline, diesel, and biodiesel passenger vehicles and their relation to oxidative potential [J]. Aerosol Science and Technology, 2010, 44(7): 500-513. doi: 10.1080/02786821003758294 [51] SEE S W, WANG Y H, BALASUBRAMANIAN R. Contrasting reactive oxygen species and transition metal concentrations in combustion aerosols [J]. Environmental Research, 2007, 103(3): 317-324. doi: 10.1016/j.envres.2006.08.012 [52] VERMA V, NING Z, CHO A K, et al. Redox activity of urban quasi-ultrafine particles from primary and secondary sources [J]. Atmospheric Environment, 2009, 43(40): 6360-6368. doi: 10.1016/j.atmosenv.2009.09.019 [53] YU H R, WEI J L, CHENG Y L, et al. Synergistic and antagonistic interactions among the particulate matter components in generating reactive oxygen species based on the dithiothreitol assay [J]. Environmental Science & Technology, 2018, 52(4): 2261-2270. [54] XIONG Q S, YU H R, WANG R R, et al. Rethinking dithiothreitol-based particulate matter oxidative potential: Measuring dithiothreitol consumption versus reactive oxygen species generation [J]. Environmental Science & Technology, 2017, 51(11): 6507-6514. [55] LYU Y, GUO H B, CHENG T T, et al. Particle size distributions of oxidative potential of lung-deposited particles: Assessing contributions from quinones and water-soluble metals [J]. Environmental Science & Technology, 2018, 52(11): 6592-6600. [56] KHACHATRYAN L, DELLINGER B. Environmentally persistent free radicals (EPFRs)-2. are free hydroxyl radicals generated in aqueous solutions? [J]. Environmental Science & Technology, 2011, 45(21): 9232-9239. [57] DELLINGER B, LOMNICKI S, KHACHATRYAN L, et al. Formation and stabilization of persistent free radicals [J]. Proceedings of the Combustion Institute, 2007, 31(1): 521-528. doi: 10.1016/j.proci.2006.07.172 [58] CHEN Q C, WANG M M, WANG Y Q, et al. Rapid determination of environmentally persistent free radicals (EPFRs) in atmospheric particles with a quartz sheet-based approach using electron paramagnetic resonance (EPR) spectroscopy [J]. Atmospheric Environment, 2018, 184: 140-145. doi: 10.1016/j.atmosenv.2018.04.046 [59] YANG L L, LIU G R, ZHENG M H, et al. Highly elevated levels and particle-size distributions of environmentally persistent free radicals in haze-associated atmosphere [J]. Environmental Science & Technology, 2017, 51(14): 7936-7944. [60] WANG P, PAN B, LI H, et al. The overlooked occurrence of environmentally persistent free radicals in an area with low-rank coal burning, Xuanwei, China [J]. Environmental Science & Technology, 2018, 52(3): 1054-1061. [61] SHALTOUT A A, BOMAN J, SHEHADEH Z F, et al. Spectroscopic investigation of PM2.5 collected at industrial, residential and traffic sites in Taif, Saudi Arabia [J]. Journal of Aerosol Science, 2015, 79: 97-108. doi: 10.1016/j.jaerosci.2014.09.004 [62] DELA CRUZ A L N, COOK R L, LOMNICKI S M, et al. Effect of low temperature thermal treatment on soils contaminated with pentachlorophenol and environmentally persistent free radicals [J]. Environmental Science & Technology, 2012, 46(11): 5971-5978. [63] OYANA T J, LOMNICKI S M, GUO C Q, et al. A scalable field study protocol and rationale for passive ambient air sampling: A spatial phytosampling for leaf data collection [J]. Environmental Science & Technology, 2017, 51(18): 10663-10673. [64] LIAO S H, PAN B, LI H, et al. Detecting free radicals in biochars and determining their ability to inhibit the germination and growth of corn, wheat and rice seedlings [J]. Environmental Science & Technology, 2014, 48(15): 8581-8587. [65] CHEN Q C, WANG M M, SUN H Y, et al. Enhanced health risks from exposure to environmentally persistent free radicals and the oxidative stress of PM2.5 from Asian dust storms in Erenhot, Zhangbei and Jinan, China [J]. Environment International, 2018, 121: 260-268. doi: 10.1016/j.envint.2018.09.012 [66] MARTIN-NETO L, ROSELL R, SPOSITO G. Correlation of spectroscopic indicators of humification with mean annual rainfall along a temperate grassland climosequence [J]. Geoderma, 1998, 81(3/4): 305-311. [67] ALDERMAN S L, FARQUAR G R, POLIAKOFF E D, et al. An infrared and X-ray spectroscopic study of the reactions of 2-chlorophenol, 1, 2-dichlorobenzene, and chlorobenzene with model CuO/silica fly ash surfaces [J]. Environmental Science & Technology, 2005, 39(19): 7396-7401. [68] HU S, POLIDORI A, ARHAMI M, et al. Redox activity and chemical speciation of size fractioned PM in the communities of the Los Angeles-Long Beach harbor [J]. Atmospheric Chemistry and Physics, 2008, 8(21): 6439-6451. doi: 10.5194/acp-8-6439-2008 [69] VERMA V, POLIDORI A, SCHAUER J J, et al. Physicochemical and toxicological profiles of particulate matter in los angeles during the October 2007 southern California wildfires [J]. Environmental Science & Technology, 2009, 43(3): 954-960. [70] ALAM M S, DELGADO-SABORIT J M, STARK C, et al. Using atmospheric measurements of PAH and quinone compounds at roadside and urban background sites to assess sources and reactivity [J]. Atmospheric Environment, 2013, 77: 24-35. doi: 10.1016/j.atmosenv.2013.04.068 [71] LIU W J, XU Y S, LIU W X, et al. Oxidative potential of ambient PM2.5 in the coastal cities of the Bohai Sea, Northern China: Seasonal variation and source apportionment [J]. Environmental Pollution, 2018, 236: 514-528. doi: 10.1016/j.envpol.2018.01.116 [72] MA Y Q, CHENG Y B, QIU X H, et al. Sources and oxidative potential of water-soluble humic-like substances (HULISWS) in fine particulate matter (PM2.5) in Beijing [J]. Atmospheric Chemistry and Physics, 2018, 18(8): 5607-5617. doi: 10.5194/acp-18-5607-2018 [73] WANG J P, LIN X, LU L P, et al. Temporal variation of oxidative potential of water soluble components of ambient PM2.5 measured by dithiothreitol (DTT) assay [J]. Science of the Total Environment, 2019, 649: 969-978. doi: 10.1016/j.scitotenv.2018.08.375 [74] YU S Y, LIU W J, XU Y S, et al. Characteristics and oxidative potential of atmospheric PM2.5 in Beijing: Source apportionment and seasonal variation [J]. Science of the Total Environment, 2019, 650: 277-287. doi: 10.1016/j.scitotenv.2018.09.021 [75] BREHMER C, LAI A, CLARK S, et al. The oxidative potential of personal and household PM2.5 in a rural setting in southwestern China [J]. Environmental Science & Technology, 2019, 53(5): 2788-2798. [76] WANG Y Q, WANG M M, LI S P, et al. Study on the oxidation potential of the water-soluble components of ambient PM2.5 over Xi’an, China: Pollution levels, source apportionment and transport pathways [J]. Environment International, 2020, 136: 105515. doi: 10.1016/j.envint.2020.105515 [77] VERMA V, FANG T, GUO H, et al. Reactive oxygen species associated with water-soluble PM2.5 in the southeastern United States: Spatiotemporal trends and source apportionment [J]. Atmospheric Chemistry and Physics, 2014, 14(23): 12915-12930. doi: 10.5194/acp-14-12915-2014 [78] BATES J T, WEBER R J, ABRAMS J, et al. Reactive oxygen species generation linked to sources of atmospheric particulate matter and cardiorespiratory effects [J]. Environmental Science & Technology, 2015, 49(22): 13605-13612. [79] WEBER S, UZU G, CALAS A, et al. An apportionment method for the oxidative potential of atmospheric particulate matter sources: Application to a one-year study in Chamonix, France [J]. Atmospheric Chemistry and Physics, 2018, 18(13): 9617-9629. doi: 10.5194/acp-18-9617-2018 -



 下载:
下载: