-
磷是生命活动中必不可少的重要营养物质,但水体磷浓度超标,会导致藻类过度生长,从而大量消耗水中的溶解氧,影响水质[1],引起水体富营养化,是农业生产中环境影响较大的问题[2]。根据2020年中国生态环境状况公报数据显示,我国近30%地表水呈现富营养化状态。磷为不可再生资源,从废水中分离与回收含磷化合物,不仅能解决水体富营养化问题,保护生态环境,还能有效回收利用磷资源,具有很大的经济价值和社会效应,也是一项迫在眉睫的艰巨任务[3]。
近年来,多种解决水体磷污染的方法取得重要的进展。例如化学沉淀法、生物法、吸附法、离子交换法、结晶法以及电渗析等。其中,吸附法具有简单、高效的优势受到人们的普遍关注,合成经济有效的吸附剂,在水体磷污染治理领域应用潜力很大[4]。对固体载体材料进行固载化,使其具备吸附磷酸盐的特征又可循环利用,具有重要的研究意义。以沸石[5]、碳材料[6]、二氧化硅[7]、生物炭[8]、纤维素等生物质[9]作为载体制备除磷材料的研究有了显著的进展,但它们在应用上也存在一些不足。例如,天然矿石如沸石材料热稳定性不高;多孔材料孔内修饰困难,而且水相目标分子难以进入孔径之中限制其应用;碳材料如石墨烯价格昂贵;此外,粉末载体材料回收不便,易导致二次污染。因此,制备高效稳定、绿色环保可循环利用的新型磷酸盐吸附材料吸附和回收水体磷酸盐具有重要研究价值。
腈纶纤维,作为一种成熟的合成纤维,通常应用于衣织物和室内装饰纺织品的加工[10]。具有柔软蓬松、强度高、弹性好、耐热和耐光[11]等特点,其表面含有的丰富的氰基,可以通过化学改性的方法使其转化为酰胺、羧基、偕肟胺等基团[12],是理想的载体材料,并广泛应用于固载有机小分子催化有机反应[13],吸附重金属离子[14]或有机污染物[15]。本课题组已制备了许多不同结构和性质的功能性纤维,如载铁离子负载胺功能化纤维[16]净化水体磷酸盐,研究发现,腈纶纤维改性的铁离子负载胺功能化纤维,有利于提高对磷酸盐的吸附能力(24 mg·g−1 P),是一种环保、高效的磷酸盐吸附剂,且可重复使用5次以上[16]。因此,通过化学修饰引入新的官能团,制备功能化纤维具有较高的可行性,对提高废水中磷酸盐的去除和回收具有重要的研究意义。
作为先前功能化纤维的合成及其对水体磷酸盐去除方面工作[16-17]的延伸。本文以腈纶纤维为载体,将与水体磷具有交换作用的阴离子修饰到纤维表层,通过改变卤代烃烷基链的长度调控纤维表面亲疏水性,制备系列极性可调的季铵盐功能化纤维(图1),通过红外光谱、扫描电镜、X-射线粉末衍射等技术进行表征,以证明纤维的成功修饰。系统研究了不同极性修饰得季铵盐功能化纤维对水体磷的净化与回收,探究了 pH、吸附时间、磷酸盐初始浓度等对磷酸盐的影响,旨在为水体磷污染防控及纺织纤维资源化利用提供新思路。
-
主要试剂:腈纶纤维、N, N-二甲基-1,3丙二胺、正溴丁烷、正溴己烷、正溴辛烷、无水乙醇、硫酸、盐酸、氢氧化钠、2,4-二硝基酚指示剂、抗坏血酸、钼酸胺,所用的试剂均为分析纯,实验用水均为去离子水。
主要仪器:SZCL-2数显智能控温磁力搅拌器、DZTW型电子调温电热套、SHB-Ⅲ 循环式多用真空泵、84-1磁力搅拌器、DHG-9070A型鼓风干燥箱、FE20-型pH计(METTLER TOLEDO)、上海722G可见分光光度计。
-
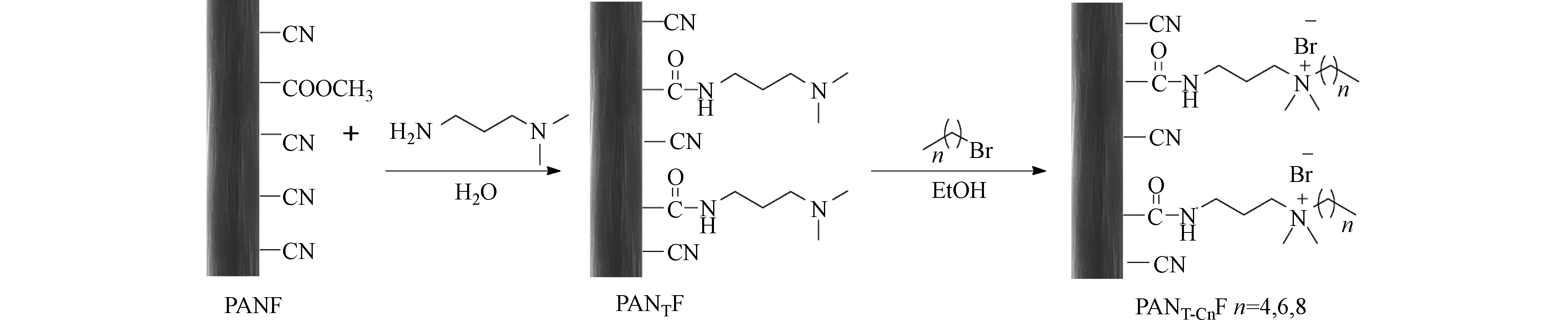
腈纶纤维放在60 °C的烘箱中烘干,然后称取干燥的腈纶纤维(3.0 g)放入250 mL圆底烧瓶中,量取N, N-二甲基-1,3丙二胺(45 mL)和去离子水(30 mL)加入烧瓶。电磁搅拌加热回流5 h后,取出纤维,用去离子水(60 °C)反复冲洗直至滤液呈中性,放入烘箱中(60 °C)烘干(12 h),得到胺化纤维PANTF。
将干燥的PANTF(0.5 g)和相对于纤维表层叔胺5倍含量(5.45 mmol)的相应卤化物(正溴丁烷、正溴己烷、正溴辛烷)浸入圆底烧瓶(100 mL)中,倒入无水乙醇(20 mL)。电磁搅拌加热回流5 h后取出纤维,用无水乙醇和去离子水(60 °C)反复冲洗,放入烘箱(60 °C)烘干(12 h),得到季铵化纤维PANT-C4F、PANT-C6F、PANT-C8F。通过改变电磁搅拌加热回流时间(0.5、3、5 h),进一步制备了不同增重的正溴丁烷修饰的季铵盐功能化纤维PANT-C4F,得到3种不同修饰程度的季铵化纤维PANT-C4F。
-
本论文通过纤维的增重(1)和官能度(2)来表示季铵化功能纤维的修饰程度,计算公式如下:
式中,m1表示胺化纤维PANTF的质量,m2表示季铵化功能纤维的质量,M为修饰前后增加的分子量。
-
磷酸盐溶液的pH分别使用0.1 mg·L−1 NaOH和0.1 mg·L−1 HCl调节,吸附实验设置3个重复取平均值,通过分光光度计在700 nm波长下分析其磷酸盐浓度[18]。
-
本文主要针对工业尾水等高浓度含磷废水,含磷废水经过一级反应处理后P浓度约为30 mg·L−1 ,再经过二级中和反应会降低至10 mg·L−1左右。因此,将磷酸盐初始浓度设定为20 mg·L−1 P[19],能够更好地模拟含磷工业尾水的处理。分别取10 mg干燥的季铵化纤维PANT-C4F、PANT-C6F、PANT-C8F,各浸入10 mL的20 mg·L−1 P 的KH2PO4溶液中,电磁搅拌2 h,取出纤维,可见分光光度计在波长700 nm处测试剩余溶液中磷酸盐浓度。纤维对磷酸盐吸附量Q(mg·g−1 P)根据式(3)进行计算:
式中,C1表示吸附后溶液的磷浓度(mg·L−1 P),C2表示吸附前溶液的磷浓度(mg·L−1 P),V表示所用磷酸盐溶液体积(mL),m表示吸附时所用功能纤维的质量(mg)。
-
取10 mg干燥的季铵化纤维PANT-C4F置于20 mL的KH2PO4溶液(C0=20 mg·L−1 P)中,用电磁搅拌10 min,取出纤维用去离子水冲洗后,浸入20 mL洗脱液溶液(NaCl,0.5 mol·L−1)中,室温搅拌30 min,重复进行5次,按1.5.1节的步骤处理并测定。纤维对磷酸盐的吸附率R(%)和解吸率D(%)分别根据式(4)和(5)进行计算:
式中,Ce表示吸附后溶液的磷浓度(mg·L−1 P),C0表示吸附前溶液的磷浓度(mg·L−1 P),Cd表示解吸后溶液的磷浓度(mg·L−1 P)。
-
取20 mg·L−1 P的KH2PO4溶液20 mL,将初始pH调至3—9。各加入10 mg干燥的功能化纤维PANT-C4F,电磁搅拌1 h,按1.5.1节的步骤处理并测定。
-
将20 mg·L−1 P的KH2PO4溶液,调节至pH 7。取20 mL各加入10 mg干燥的PANT-C4F,在温度293 K下,控制电磁搅拌的时间(0—20 min),按1.5.1节的步骤处理并测定。分别用准一级动力学方程(6)和准二级动力学(7)进行拟合,公式如下:
式中,qt和qe分别为t时刻和平衡时间对磷酸盐的吸附量,k1和k2为速率常数。
-
配制不同浓度的KH2PO4溶液(4—30 mg·L−1 P),调节至pH 7,分别取20 mL不同浓度的KH2PO4溶液,各加入10 mg干燥的PANT-C4F,电磁搅拌10 min,按1.5.1节的步骤处理并测定。分别用Langmuir(8)和Freundlich(9)模型进行拟合,公式如下:
式中,qmax为理论最大吸附容量,k1为Langmuir常数,n为非均质因子,kf为Freundlich常数。
-
功能纤维及其增重与官能度如表1所示。正溴丁烷修饰的季铵盐功能化纤维PANT-C4F,随着反应时间的延长,增重与官能度也随之增大,反应5 h增重为35.4%,官能度高达1.91 mmol·g−1。此外,正溴己烷和正溴辛烷修饰的季铵盐功能化纤维PANT-C6F和PANT-C8F,官能度分别为1.31 mmol·g−1和1.42 mmol·g−1。以上结果也说明随着卤代烃链长增加,其与叔胺功能化纤维的反应活性降低。
-
纤维的傅里叶变换红外光谱(FTIR)如图2所示,从PANF的光谱中(图2a)可以看到,在2245 cm−1和1734 cm−1处由于C≡N和C=O的拉伸振动引起的两个峰。胺化和季铵化后(图2b-d),在1650 cm−1处出现了一个新的吸收峰,是由于N—C=O拉伸振动引起的,表明功能组分通过酰胺键被成功接枝到PANF上。
-
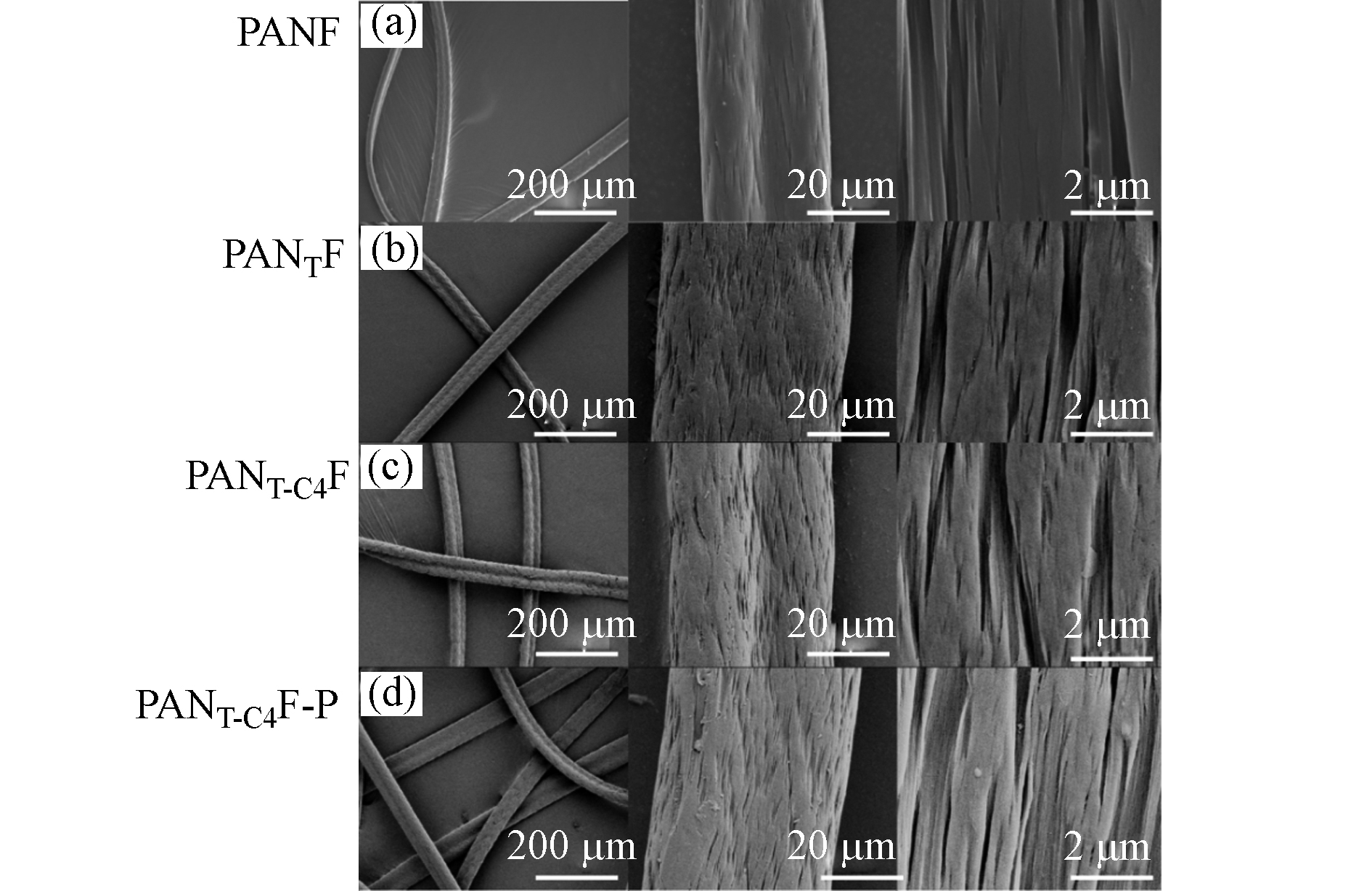
通过扫描电子显微镜(SEM)对纤维表面形貌进行表征(图3)。纤维在200倍的低放大率的图像中(图3a-d)都是连续的,呈现完整的结构。在2000倍放大率下,纤维表面仍然呈线形分布。PANTF和PANT-C4F(图3b-c) 由于发生化学接枝过程导致纤维溶胀[20],在20000倍的高倍镜下,由于增加和水中污染物的接触,可以看出纤维表面有一些裂纹。吸附磷酸盐后的PANT-C4F-P(图3d),与PANT-C4F(图3c)相比,没有明显的损坏,其结构仍呈具有完整性。
-
用X射线粉末衍射(XRD)对纤维的内部晶体结构进行了表征(图4)。由于PANF的六角形晶格(100)表面折射[21],PANF的XRD图像在2θ=17o处出现了1个特征峰(图4a)。经过改性后,纤维的XRD图谱与初始PANF一致,峰值强度略有下降(图4b-d)。研究结果表明,尽管进行了多步接枝反应,纤维的内部晶体结构仍保持良好,说明纤维载体的稳定性。
-
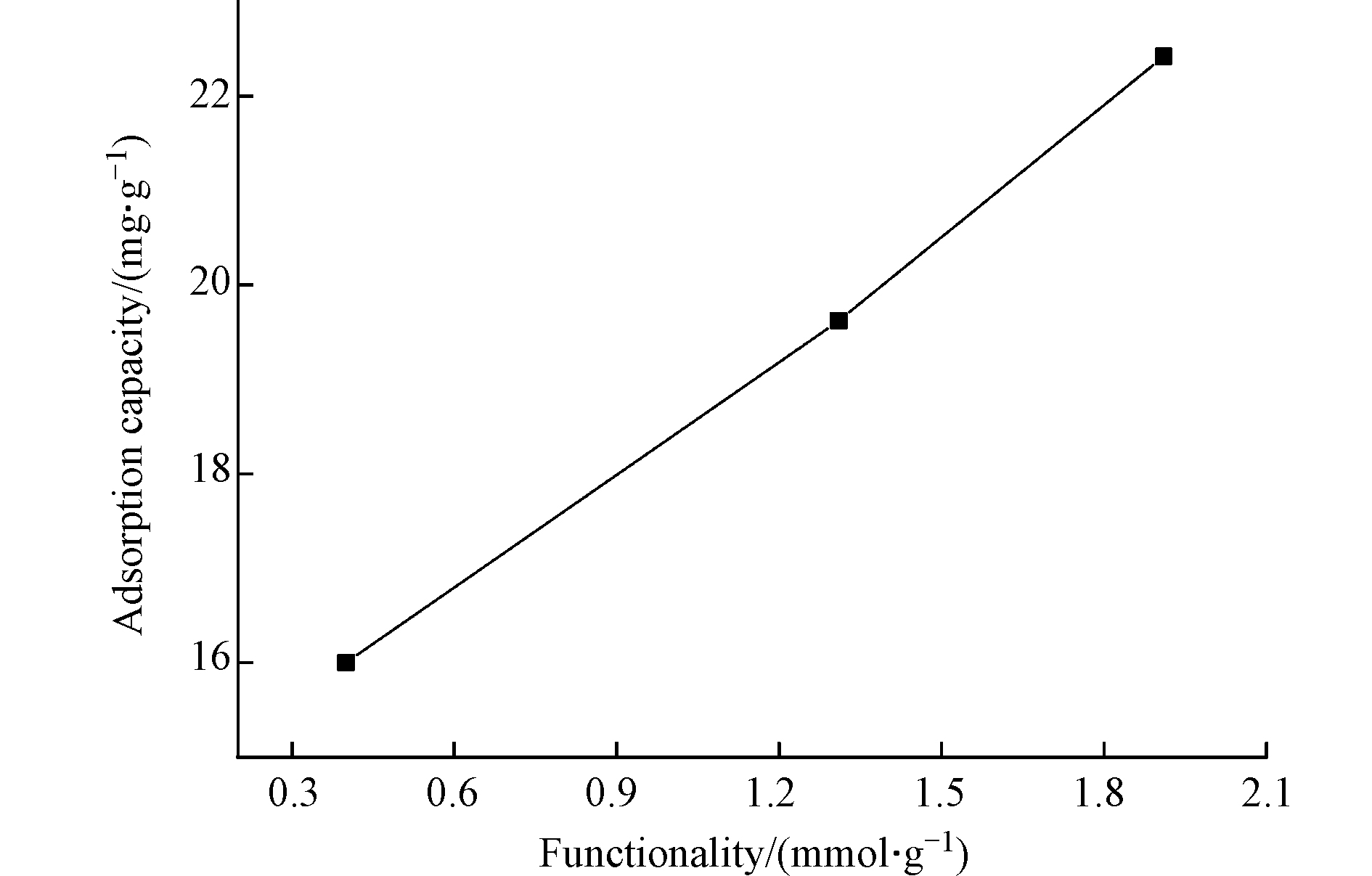
选取接枝正溴己烷、正溴辛烷的功能化纤维PANT-C6F、PANT-C8F和官能度近似的PANT-C4F(1.26 mmol·g−1)比较功能化纤维对水体磷的吸附能力,其结果如表2所示,PANT-C4F对磷的吸附性能最好。随着接枝链长的增加,季铵盐功能化纤维对磷的去除能力逐渐降低,这是因为烷烃的链长越长其疏水性越强,所以正溴丁烷修饰的功能化纤维(PANT-C4F)亲水性较其他两种卤代烃好,因此更易于和亲水性的磷酸根离子发生交换,由此本文选择PANT-C4F作为研究的最佳纤维。进一步研究不同官能度的PANT-C4F对磷酸盐的吸附性能,如图5所示,官能度为1.91 mmol·g−1的PANT-C4F对磷的吸附能力最强,说明接枝的正溴丁烷越多,可供离子交换的位点就越多,吸附量就越大。因此,选择官能度为1.91 mmol·g−1左右的PANT-C4F进行后续的吸附实验。
一种良好的固载型磷吸附材料需具备较好的可重复使用能力,因此,研究了吸附磷前后季铵化功能纤维的元素含量变化以证明该吸附材料的循环使用性能。表3列出了PANF、PANTF、PANT-C4F、PANT-C4F-P(吸附磷酸盐)、PANT-C4F-1(循环1次)的元素分析结果。由于N, N-二甲基-1,3丙二胺中C含量低于PANF,而H含量高于PANF,所以与PANF相比,PANTF的C含量显著下降,H含量增加,同时,与N, N-二甲基-1,3丙二胺的反应释放氨气,PANTF的N含量也降低。在季铵化过程中,伴随着Br离子的引入,与PANTF相比,PANT-C4F的含量C、N和H含量均呈下降趋势。此外PANT-C4F-P的C、N、H含量全部由于发生磷酸盐的交换而增加。从表3可以看出,PANT-C4F-1的C、N、H含量与PANT-C4F相似,说明PANT-C4F具有良好循环使用能力和稳定性能。
为进一步证实该功能化纤维的可重复使用性,进行循环性能测试。在浓度为20 mg·L−1 P的20 mL KH2PO4溶液中吸附磷酸盐,吸附达到饱和后,反复冲洗PANT-C4F表面附着的磷酸盐,并用NaCl溶液(0.5 mol·L−1)对PANT-C4F进行解吸附。实验表明,PANT-C4F对磷酸盐的第一次解吸率高达99.4%,并且在五次循环后解吸率仍在85%以上(图6),具有较高解吸效率。值得注意的是,在第一次吸附解吸过程中,会有一定的Br−释放,但浓度在0.004 μg·L−1左右,远小于污染风险,第二次解吸主要通过Cl−,其大量存于水体中,不会造成环境风险。结果证明了其具有优秀的循环使用能力,对磷资源的回收利用与水体磷污染防控具有重要意义。
-
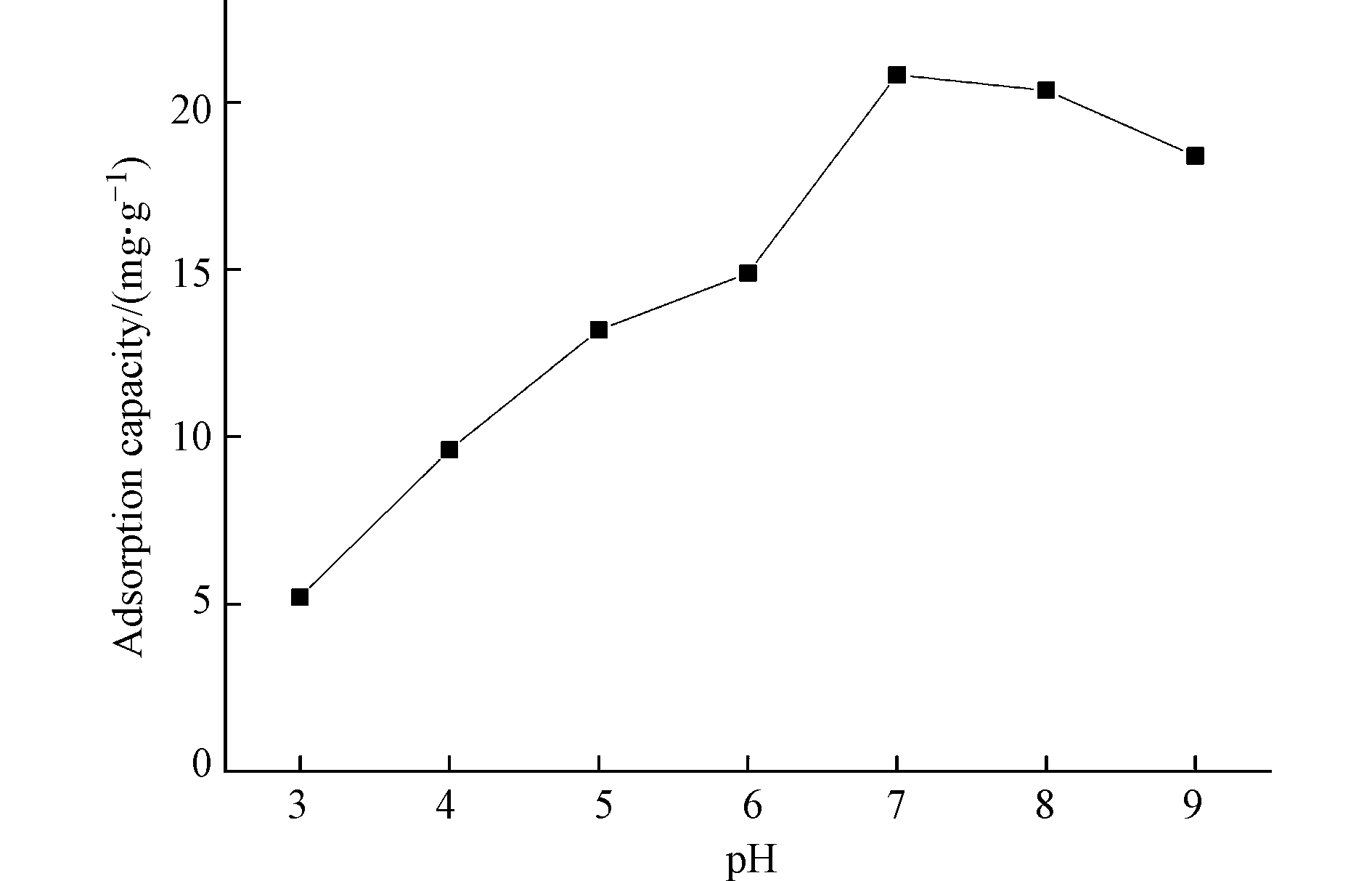
PANT-C4F(官能度为1.26 mmol·g−1)在不同pH值下对磷的平衡吸附量的影响如图7所示,在强酸条件(pH<4)下对磷的吸附能力较低。
此条件下磷酸盐多以磷酸分子形式存在,难以与Br−交换,因此导致吸附量较低;随着pH的增大,吸附量增大,在pH 7左右,功能化纤维对磷酸盐的吸附量达到最大。这是因为随着pH的增大,磷酸盐多以H2PO4−/HPO42-/PO43-形式存在,水中H2PO4−/HPO42-/PO43-越多越容易与功能化纤维上的Br−进行交换;当pH值大于7时,由于较多的OH−存在,与磷酸根离子之间发生静电排斥,纤维对磷的吸附能力略微下降。综上所述,功能纤维在碱性条件下的吸附性能大于酸性,在中性附近吸附性能最好,与生活污水的典型pH值范围6.5—8.5之间[22]相符,证明季铵化功能纤维具有较高的实际应用价值。
-
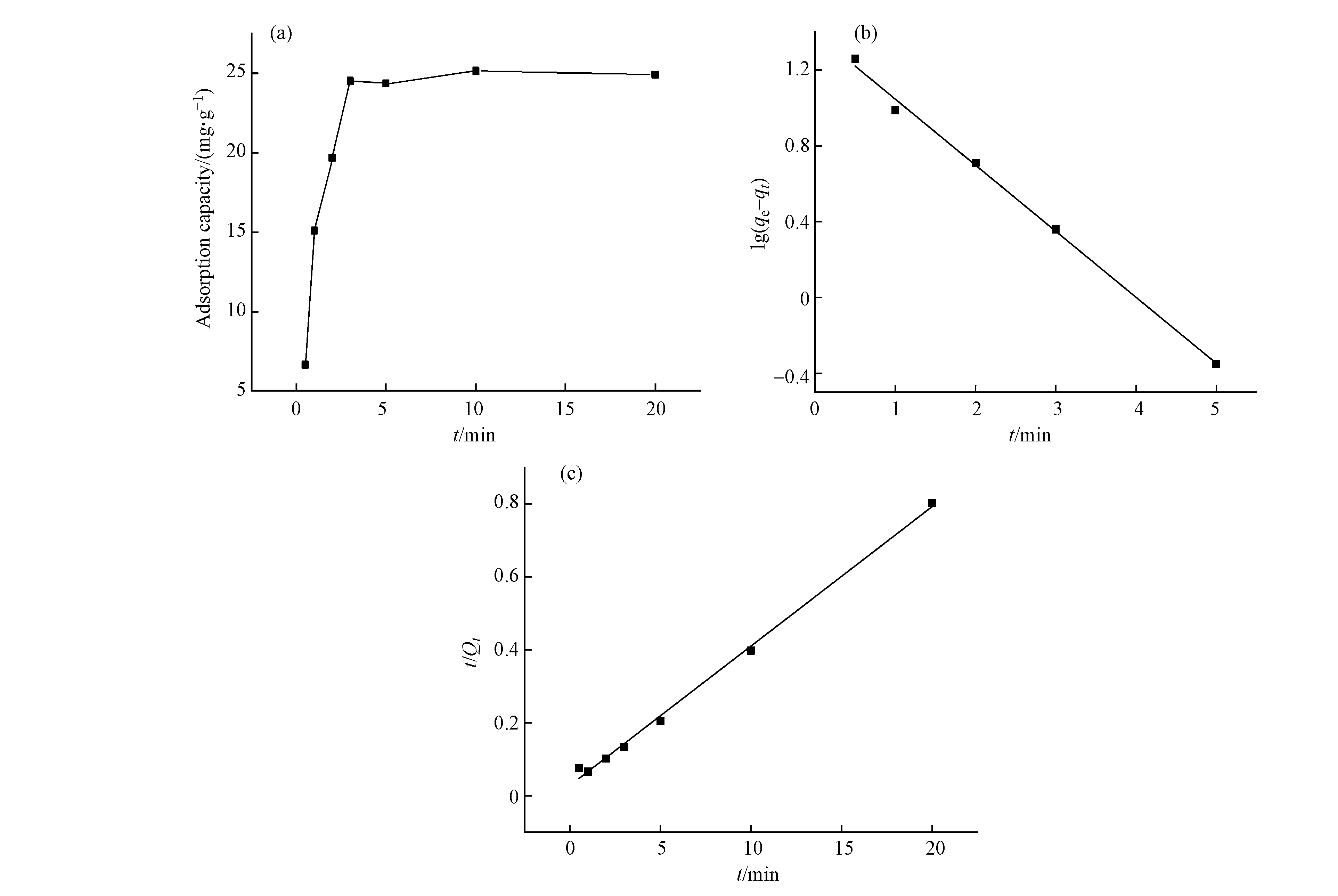
在最优pH条件下,用官能度为1.91 mmol·g−1的PANT-C4F测试吸附时间对吸附量的影响,结果如图8a所示。季铵化功能纤维对磷的吸附在1 min左右达到半饱和,在3 min左右趋向平衡,最高吸附量可达25 mg·g−1 P,较镁铁层状双金属氢氧化物[23]、CaO-生物炭复合材料[24]和其他除磷材料展现出优秀的吸附效率。采用准一级动力学模型和准二级动力学模型进行了拟合(图8b-c和表4),准二级动力学模型(R2=0.9967)比准一级动力学模型(R2=0.9956)更能描述PANT-C4F吸附磷酸盐的动力学数据,说明PANT-C4F对磷酸盐的吸附主要以化学吸附为主[25]。
-
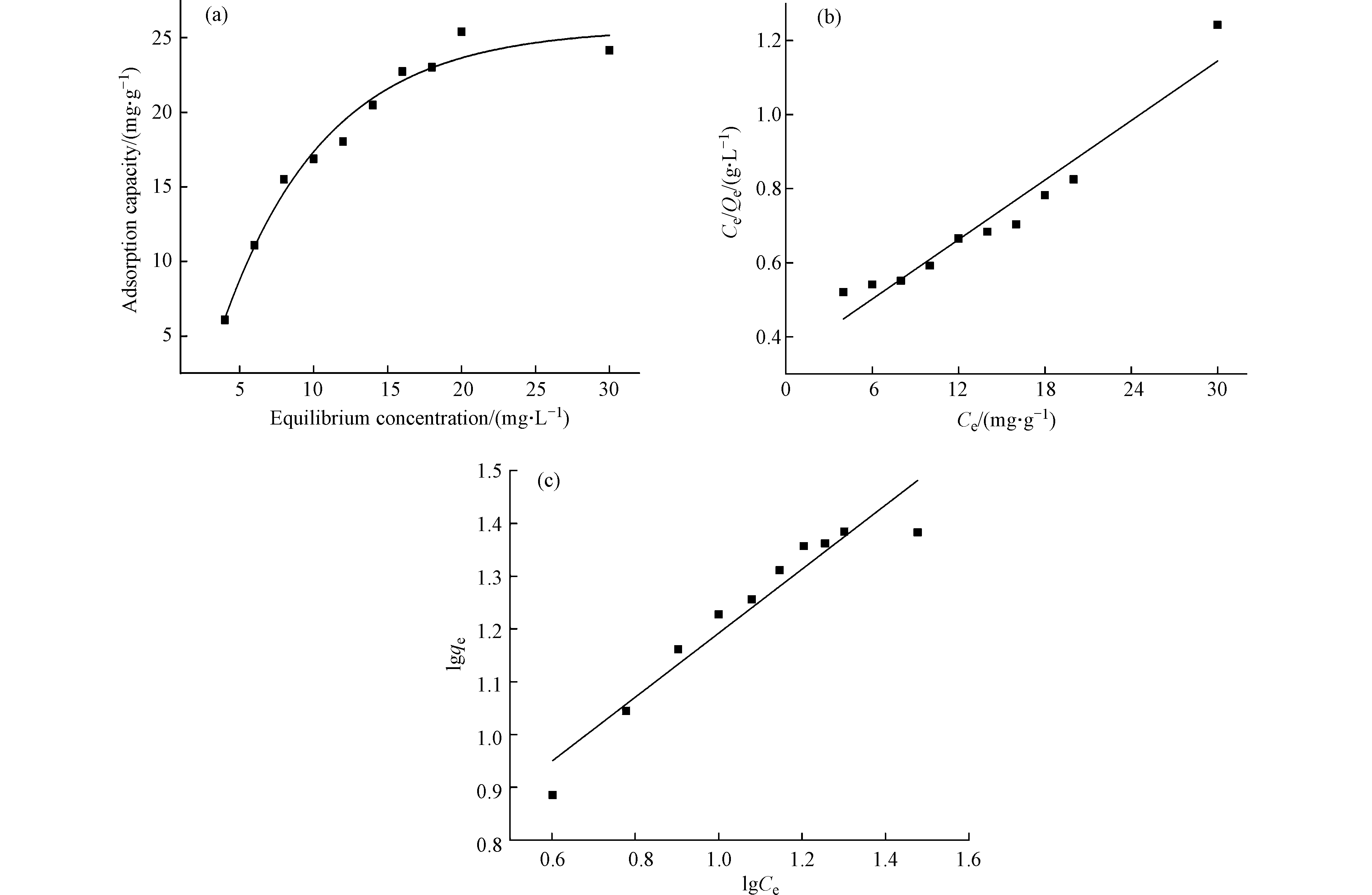
用修饰程度为1.91 mmol·g−1的PANT-C4F研究其在不同初始磷酸盐浓度溶液中的吸附性能,结果如图9所示。功能化纤维对磷酸盐的吸附随着磷初始浓度的增加而增加,直到达到吸附平衡。因为磷浓度较低时,纤维上有大量易于接触的吸附位点可供吸附。然而,随浓度的增加,到达了平衡,纤维上没有更多的位点可用。本实验纤维最大吸附量是25 mg·g−1 P,与天然沸石[26]、壳糖复合材料[27]等部分除磷材料相比存在较大优势。采用Langmuir模型和Freundlich模型对改性材料的吸附等温线进行拟合(图9b-c和表4),Langmuir模型拟合(R2=0.9273)更优于Freundlich(R2=0.9113),说明改性纤维材料对磷酸盐的吸附是均匀的单分子层吸附[28]。
-
吸附动力学和吸附等温线结果表明,PANT-C4F对磷酸盐的吸附主要是通过单分子层化学吸附。由此,提出一种可能的PANT-C4F对磷酸盐协同吸附机理,原理图如图10所示。
吸附机制归因于功能化纤维表层溴离子和磷酸盐离子之间的离子交换。为了验证PANT-C4F的离子交换机理,测试了纤维的能谱 (图11)。PANF和PANTF主要包含C、N和O(图11a-b)。对于季铵化的PANT-C4F, 出现了新的峰Br(图11c)。特别是在吸附磷酸盐后,在能谱中形成了新的P峰(图11d),而Br的峰强度明显降低,表明PANT-C4F通过Br离子交换成功吸附磷酸盐。
-
季铵盐功能化纤维在净化回收水体磷酸盐领域具有广阔应用前景,以衣物纺织常见的腈纶纤维为原材料,制备的新型极性可调功能化纤维,不仅能为水体磷酸盐的净化回收,纤维固废的减量化、无害化和资源化提供新途径,同时,回收的水体磷作为液体肥料的主要原料,还可用于农作物生长,实现废水中磷资源的循环利用,减轻水体磷污染。本研究设计合成了系列极性可调的季铵化功能纤维并对其磷的吸附性能进行了研究,通过对纤维进行表征,以证实接枝和应用的成功。吸附动力学和吸附等温学实验表明PANT-C4F在3 min实现最大吸附能力(25 mg·g−1 P),准二级动力学模型和Langmuir模型能够更好的描述改性纤维材料的吸附动力学和吸附等温线,表明PANT-C4F对磷酸盐的吸附主要是单分子层化学吸附,吸附机理为溴和磷酸盐之间的离子交换。利用0.5 mol·L−1 NaCl溶液对季铵化功能纤维进行解吸附测试,解吸率达99.4%,可重复循环5次以上,证明该纤维具有循环能力,能够净化回收水体磷。由此说明,PANT-C4F对治理磷污染的废水具有很大的应用优势,是一种高效净化与回收水体磷酸盐的吸附材料。
极性可调功能化纤维的构建及其对废水磷酸盐的去除
The construction of polarity regulable functionalized fibers and its removal of phosphate in wastewater
-
摘要: 本文利用腈纶纤维(PANF)便于回收、易于修饰、价格低廉等优点,通过不同链长卤代烃的季铵化反应,构建系列极性可调的功能化腈纶纤维,通过红外光谱、扫描电镜、X-射线衍射等技术进行表征,并研究纤维表层极性调控对水体磷酸盐的吸附性能的影响。结果表明,正溴丁烷修饰的季铵化纤维(PANT-C4F)对磷的吸附能力(25 mg·g−1 P)优于正溴己烷和正溴辛烷修饰的季铵盐功能化纤维(PANT-C6F、PANT-C8F)。改性纤维材料的吸附动力学更符合准二级动力学模型,吸附等温线采用Langmuir模型拟合更优,说明功能化纤维对磷的去除主要为单分子层化学吸附。PANT-C4F在pH 7左右时,对磷的吸附效果最好,且3 min达到平衡,因此具有较高的吸附效率。此外,PANT-C4F吸附的磷酸盐可以在NaCl溶液中解吸附,至少可以循环5次以上,实现功能化纤维的循环利用和磷的有效回收。研究表明,PANT-C4F是一种高效的水体磷酸盐吸附材料,具有较高的实际应用价值。Abstract: This study utilized the characteristic of polyacrylonitrile fiber (PANF) which can be easily recycled and modified with low price, we constructed a series of polarity regulable functional polyacrylonitrile fibers by the quaternary ammonium reaction with halogenated hydrocarbons of different chain lengths, which was characterized by FTIR, SEM and XRS, the adsorption ability effect of polar regulation on water phosphate was also be investigated. The results showed that phosphorus adsorption capacity (25 mg·g−1 P) of n-brombutane modified quaternary ammonium fibers (PANT-C4F) was better than quaternary ammonium fibers modified with n-bromhexane and n-bromocane (PANT-C6F、PANT-C8F). The second-order plot for modified polyacrylonitrile fiber materials are more suitable to describe the adsorption kinetics, and the Langmuir model are better to fit the adsorption isotherms, which indicate the phosphorus adsorption by functional fibers is mainly occurred through single molecular layer chemisorption. When the pH was about 7, the functional fibers had the best adsorption effect on phosphorus, and reached the balance of suction in 3 min which proved it has high adsorption efficiency. In addition, those phosphate adsorbed by PANT-C4F can be desorbed in NaCl solution to realize the recycling of functionalized fibers and the effective recovery of phosphorus at least 5 times, the results showed the PANT-C4F is a kind of high efficient material for phosphorus adsorption in wastewater with high practical application value.
-
Key words:
- polyacrylonitrile fibers /
- polarity /
- quaternization /
- adsorption /
- phosphate
-
寻求和利用可再生绿色清洁能源替代化石燃料,是解决能源与环境危机的重要途径。微生物燃料电池(microbial fuel cell, MFC)作为一种集污水处理与生物产电于一体的新型技术,以产电细菌为主体,可将化学能转化为电能,同时去除水体中的污染物[1-2]。电极材料是影响MFC性能的关键因素之一,也是MFC产电微生物的附着载体和生长场所[3]。因此,找到一种可供微生物大量附着和生长的载体,同时具有良好导电性能的材料至关重要。
MFC电极多采用碳质材料,拥有良好的生物相容性、导电性和化学稳定性[4]。碳质材料一般包括石墨烯、碳毡、碳布、生物炭等。其中石墨烯电极机械强度较好,但其材料表面相对光滑,不利于微生物附着,因而导致胞外电子传递效率低[5-6];碳毡电极柔韧性良好,但其在MFC运行时,由于材质较厚,生物膜会妨碍底物由外向内的扩散,影响对污染物的降解效率;碳布电极表面粗糙但机械强度较差,不适于投入大规模的实际工程应用中[7]。相比于传统电极材料,生物炭材料具有来源广泛、成本低廉、电化学性能较好、比表面积高和孔隙结构多等优点。2018年CHEN等[8]大麻槿秸秆通过简单的碳化处理制成MFC阳极,其电流密度达到了32.5 A·m−2,是对照组石墨棒电极的3倍,由此可见生物炭作为MFC电极材料是具有一定优势的。
据2020年中国统计年鉴统计,我国核桃栽培面积为5.54×1010 m²,约1.3×109株[9]。每年有大量的废弃核桃壳产生,如何有效处理这些固体废物,实现减量化和资源化是环境领域的研究热点。采用高温裂解法制备生物炭,再通过化学活化,可使其表面结构相对于碳基材料的平面结构更为粗糙,更有效的提升活性表面积[10-13]。常见的生物炭化学活化剂包括ZnCl2、HPO4、KOH等,其中ZnCl2活化制备的活性炭具有产率高、过渡孔发达、价廉易得等优点[14],JIANG等通过ZnCl2活化甘蔗渣发现,锌离子浓度越高,比表面积越大[15]。
目前,以改性核桃壳作为电极材料的研究鲜有报道。因此,本研究主要以改性核桃壳作为生物炭基电极材料,通过不同温度的碳化、不同浓度的ZnCl2活化、不同比例的材料复合制成微生物燃料电池电极,通过表征分析,考察不同制备方法制备出的材料的性能差异,分析其在MFC中产电性能的差异,以及最佳条件MFC去除污染物的能力,为微生物燃料电池的发展方向提供参考。
1. 材料和方法
1.1 电极材料的制备
将市场上购买的核桃取果皮后粉碎,过40目分子筛后,置于石英舟中,再将其放入管式炉(OTF-1200 X),真空400 ℃炭化90 min后,得到黑色产物。称取一定量的黑色产物与氯化锌固体按质量比分别为5:1、5:3、5:5,置于烧杯中加去离子水刚好完全淹没,搅拌后,再将其置于105 ℃烘箱中烘干24 h。将烘干好的黑色产物放于管式炉中央,分别在400、600、800 ℃温度条件下真空煅烧2 h,反应结束后在真空保护下冷却至室温。煅烧好的样品先用10% HCl溶液洗涤,然后用去离子水洗涤,直至中性,最后将其置于105 ℃烘箱中烘干24 h,得到核桃壳碳化产物。
制备好的生物炭样品与聚苯胺和热熔胶按5:1:4和5:1:5质量比进行混合,然后将混合材料置于刚玉舟模具中压实,再放入200 ℃管式炉进行真空热熔,热熔30 min后,自然冷却至室温取出。制备后的电极材料样品尺寸为2 cm×3 cm×0.5(±0.05) cm。
1.2 表征与测试
实验采用扫描电镜(捷克TESCAN MIRA LMS),通过磨成粉末过0.4 mm筛网制样,对生物炭基电极材料表面形貌进行表征;采用拉曼光谱(激光器波长532 nm,扫描范围50~4 000 cm−1)分析电极材料的石墨化程度;采用孔隙及比表面积分析仪(康塔4000 e,脱气温度120 ℃)分析电极材料的比表面积、孔体积和孔径;采用电化学工作站(CHI 660 e),通过LSV、EIS测试,分析电极材料的导电性能的差异;采用HACH高量程(20~1 500 mg·L−1)消解法测定COD;采用国标纳氏试剂比色法测定氨氮;采用电流电压数据采集器(KEYSIGHT 34972A)测定MFC产电性能、采集电流电压及功率密度。
1.3 MFC的构建
实验采用空气阴极单室MFC反应器,由有机玻璃制成,内径为10 cm,高度为14 cm,设置溢流堰用于出水,底部设置0.4 cm有机玻璃管用于进水。反应器阴阳极用尼龙螺栓固定,有效容积为377 mL。配制模拟废水(实验所用的去离子水为灭菌除氧后的水)用于MFC产电性能分析,每隔24 h进出水150 mL,其组成为1.356 g·L−1 C4H4Na2O4,0.15 g·L−1 (NH4)2SO4,0.253 5 g·L−1 KH2PO4,0.125 g·L−1 MgSO4·7H2O,0.125 g·L−1 NaCl,0.002 5 g·L−1 FeSO4·7H2O,0.002 g·L−1 MnSO4·H2O,1 mL·L−1 微量元素。其中微量元素包括1.5 g·L−1 FeC13·6H2O,0.02 g·L−1 CuC12·2H2O,0.18 g·L−1 KI,0.12 g·L−1 MnCl2·4H2O,0.01 g·L−1 ZnC12,0.06 g·L−1 Na2MoO4·2H2O,0.15 g·L−1 CoC12·6H2O,0.15 g·L−1 H3BO3,0.06 g·L−1 Na2MoO4·2H2O。
1.4 脱氮微生物接种
实验所用接种微生物为本研究组前期实验筛选出的异养硝化-好氧反硝化菌[16]。通过扩大培养,鉴定其异养硝化与好氧反硝化性能后接种至MFC反应器。具体过程如下:将菌种接种至LB液体培养基,在(30±2) ℃培养箱中培养24 h后,取菌种培养液与去离子水以1:9比例混合,用10 mL离心管离心去除上清液后加适量水摇匀,倒入好氧反硝化培养基,恒温振荡培养(160 min−1,30 ℃),每12 h测1次硝氮浓度和OD600;再取培养液置于异养硝化培养基,厌氧箱中恒温培养(30 ℃),每12 h测1次氨氮浓度和OD600。实验所用LB液体培养基含有3.0 g·L−1 牛肉膏、5.0 g·L−1 NaCl、10.0 g·L−1 蛋白胨(pH=7.0);好氧反硝化培养基(100 mL)含有1.356 g·L−1 C4H4 Na2O4、0.064 1 g·L−1 KNO3、0.253 5 g·L−1 KH2PO4、0.125 g·L−1 MgSO4·7H2O、0.125 g·L−1 NaCl、0.002 5 g·L−1 FeSO4·7H2O、0.002 g·L−1 MnSO4·H2O;异养硝化培养基(100 mL)含有1.356 g·L−1 C4H4Na2O4、0.253 5 g·L−1 KH2PO4、0.125 g·L−1 MgSO4·7H2O、0.125 g·L−1 NaCl、0.002 5 g·L−1 FeSO4·7H2O、0.002 g·L−1 MnSO4·H2O、0.15 g·L−1 (NH4)2SO4。
1.5 MFC启动
将目标菌株菌悬液接种至MFC反应器中,接种比例10%,上下电极间距4 cm。MFC置于恒温气候箱中,温度和湿度分别控制30 ℃、50%。进水pH为7.0±0.1,连接1 kΩ的外电阻,每5 min对MFC输出电流电压进行实时监控。在MFC电压达到稳定输出时,测量分析极化曲线和功率密度曲线(文中功率密度和电流密度以反应器有效容积为参比)。具体方法为:依次将外电阻由2 000 Ω调到100 Ω,每30 s记录1次MFC外阻的电流电压值,其中每更换一次电阻需等待3 min让电压值稳定。
2. 结果与讨论
2.1 生物炭/氯化锌质量比对电极材料性能的影响
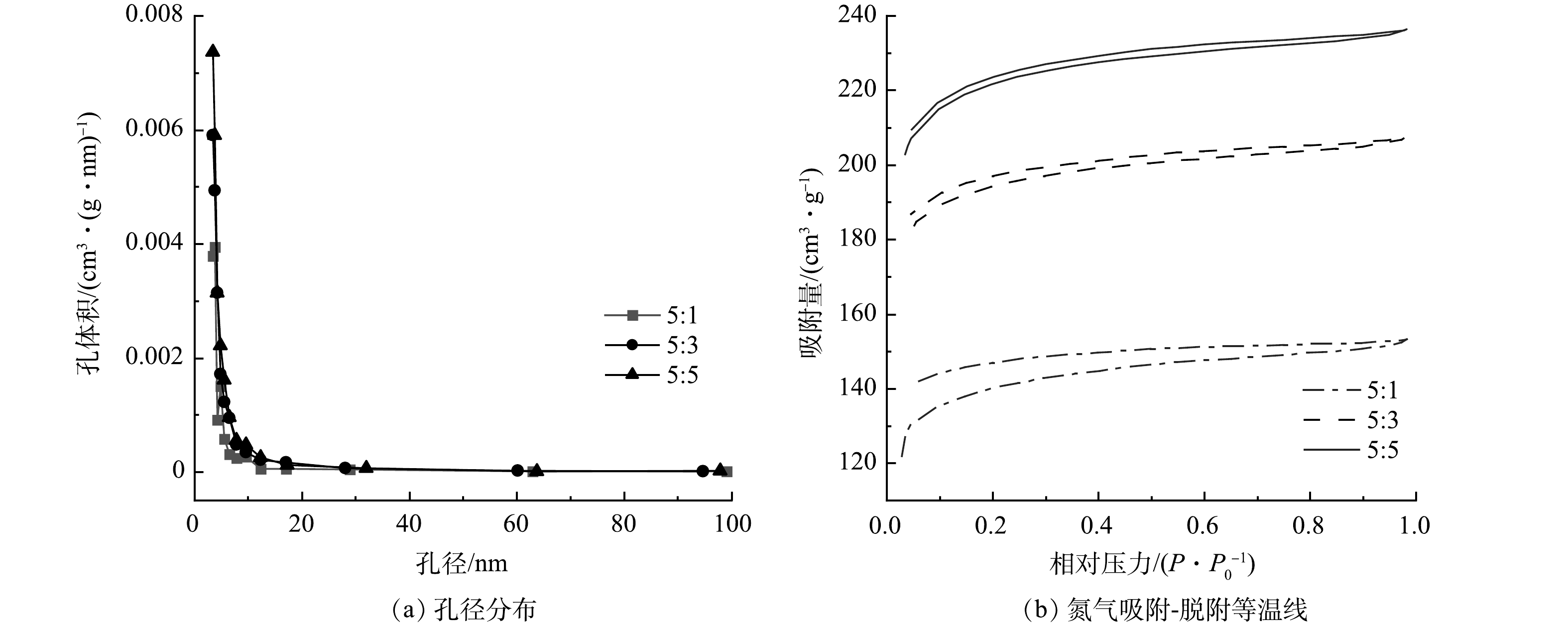
为阐明ZnCl2用量对生物炭材料孔隙结构的影响,实验采用生物炭/氯化锌质量比分别为5:1、5:3、5:5的电极材料,在600 ℃煅烧后,通过比表面积和孔径分布来评估核桃壳生物炭的比表面积及相应孔径分布。由图1可知,ZnCl2活化后的生物炭孔结构的孔径主要集中在3.5 nm附近,在相对压力为0.1~1.0内出现较显著的滞后环,按照国际纯化学和应用化学联合会的定义,核桃壳生物炭是典型的Ⅰ型和Ⅱ型特性[17],说明核桃壳生物炭的结构属于尺寸较小的介孔结构。由表1可知,随着ZnCl2质量比的不断增加,改性核桃壳生物炭的比表面积由590 m2·g−1增加到883 m2·g−1,孔容由0.009 cm3·g−1逐渐增加到0.017 cm3·g−1。这说明随着ZnCl2用量的增加,活化后的核桃壳生物炭的比表面积也越大,可为微生物的生长提供更多的场所[18],做成MFC电极后其微生物负载量可得到提升,从而促进MFC的产电。
表 1 BET测量时获得的比表面积、孔径和孔容Table 1. Specific surface area, pore size and pore volume determined by BET measurement生物炭/氯化锌质量比 比表面积/(m2·g−1) 孔径/nm 孔容/(cm3·g−1) 5:1 590 3.818 0.009 5:3 657 3.424 0.015 5:5 883 3.421 0.017 2.2 煅烧温度对电极材料性能的影响
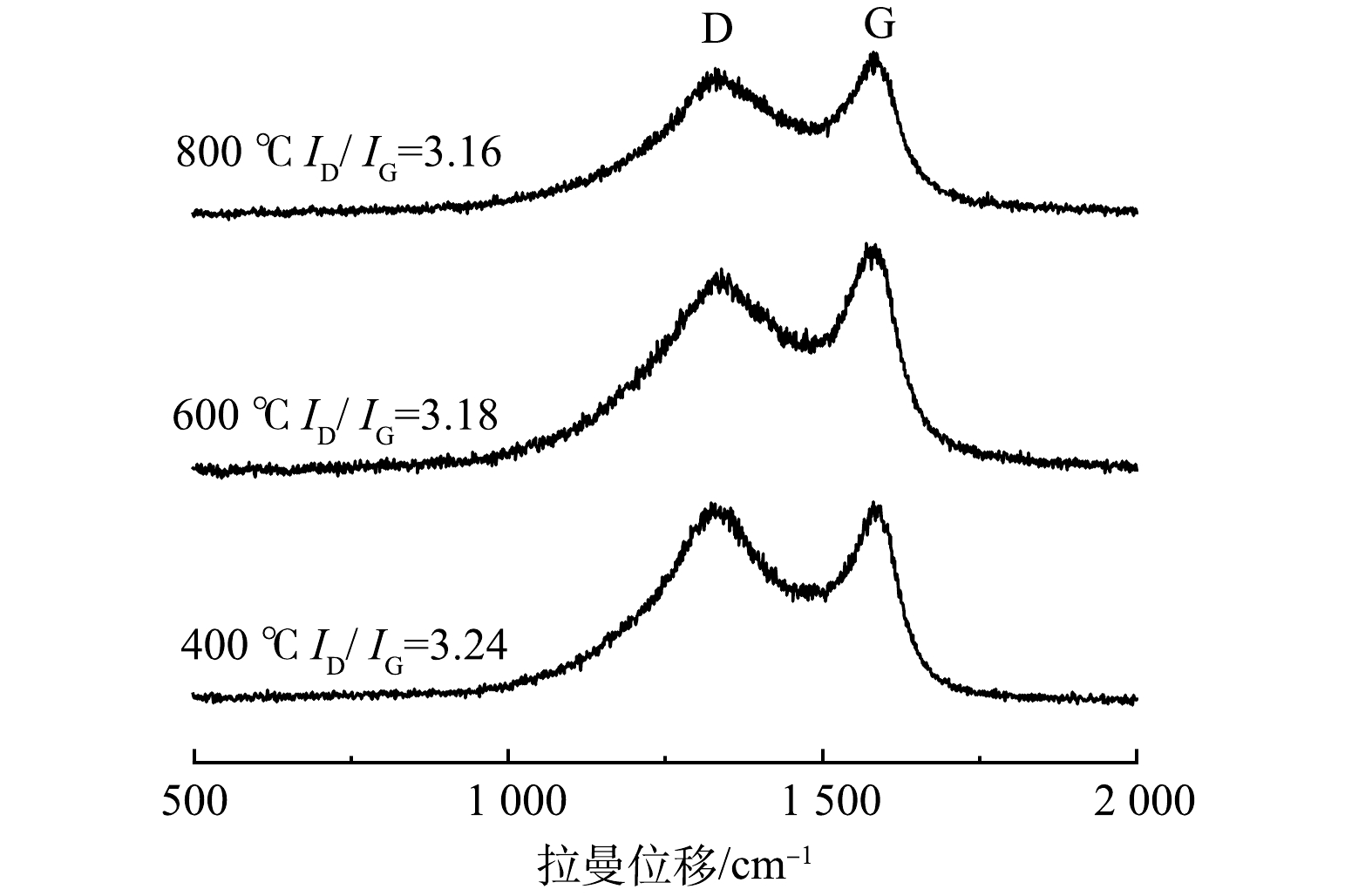
为阐明热处理温度对生物炭材料分子结构的影响规律,控制生物炭/氯化锌质量比为5:5,分别在400、600、800 ℃热处理条件下,对制备的生物炭进行拉曼光谱分析,实验结果如图2所示。由图2可见,核桃壳生物炭在1 316 cm−1和1 586 cm−1处有2个显著的拉曼峰,分别为炭材料的特征D峰和G峰[19-20]。其中D峰主要是芳香环之间的C—C结构,为环数大于6环的芳香环结构,是由炭材料缺陷引起;G峰与炭材料的C=C键Sp2杂化有关。D峰与G峰的强度比(ID/IG)可以在一定程度反映材料的缺陷程度,ID/IG值越高,代表材料的无序率越高;ID/IG值越低,说明材料的石墨化程度越高,导电性能越好[21]。根据拉曼光谱图Gauss拟合曲线方法可以得到ID/IG。由图2可以看出,随着热解温度的增加,ID/IG比值变小。这说明材料的石墨化程度增加,导电性能越好,制作的MFC电极性能越好。
2.3 生物炭/聚苯胺/热熔胶复合比例对电极材料性能的影响
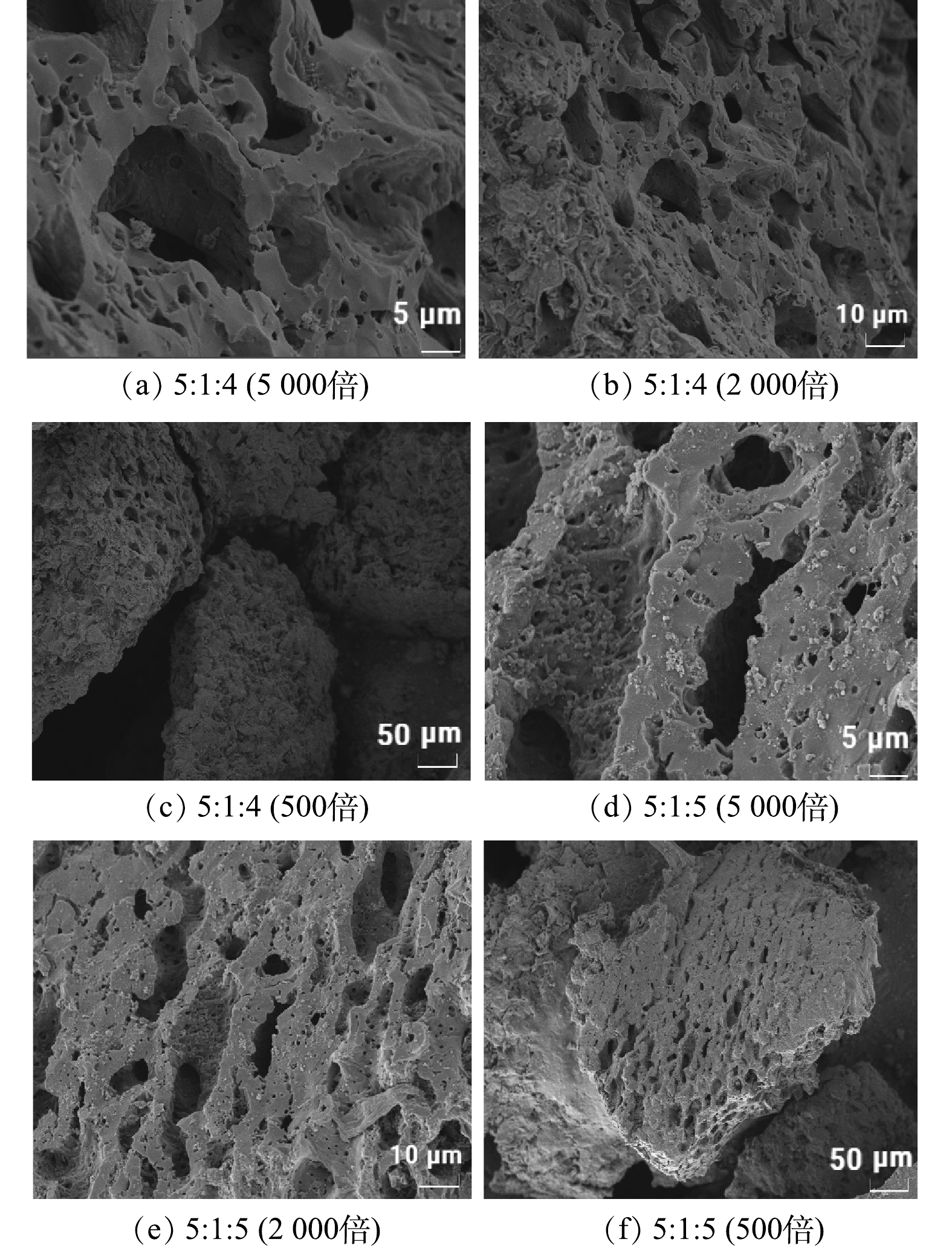
聚苯胺与热熔胶比例也是考察MFC电极制作过程的因素之一。图3为在真空煅烧温度为600 ℃、生物炭与氯化锌活化质量比为5:3的条件下,生物炭/聚苯胺/热熔胶比例分别为5:1:4和5:1:5所制成的MFC复合电极的扫描电镜图。
由于当生物炭/聚苯胺/热熔胶的比例为5:1:1和5:1:2时,经过煅烧的生物炭电极几乎不成型,依旧保持粉末状态;当生物炭/聚苯胺/热熔胶添加比例为5:1:3时,经过煅烧的生物炭电极机械强度低,易碎,因此这些复合比例均不能到达作为MFC电极材料的要求,故实验选用的生物炭/聚苯胺/热熔胶比例为5:1:4和5:1:5。由图3可以看出,随着热熔胶中聚乙烯粉末和导电态聚苯胺的加入,其对生物炭的表面起到了修饰作用,但未对生物炭的多孔结构产生明显的影响。
2.4 生物炭/聚苯胺/热熔胶复合电极电化学表征
图4反映了电解池三电极体系中生物炭/聚苯胺/热熔胶复合电极的电化学性能。以1 cm2的铂片作为辅助电极,以Ag/AgCl作为参比电极,将制备得到BPP 5:1:4和BPP 5:1:5的复合电极分别连接在电极夹上作为工作电极。所有的电化学性能测试均是在1 mmol·L−1 铁氰化钾混合溶液(0.1 mol·L−1 KCl)中完成。
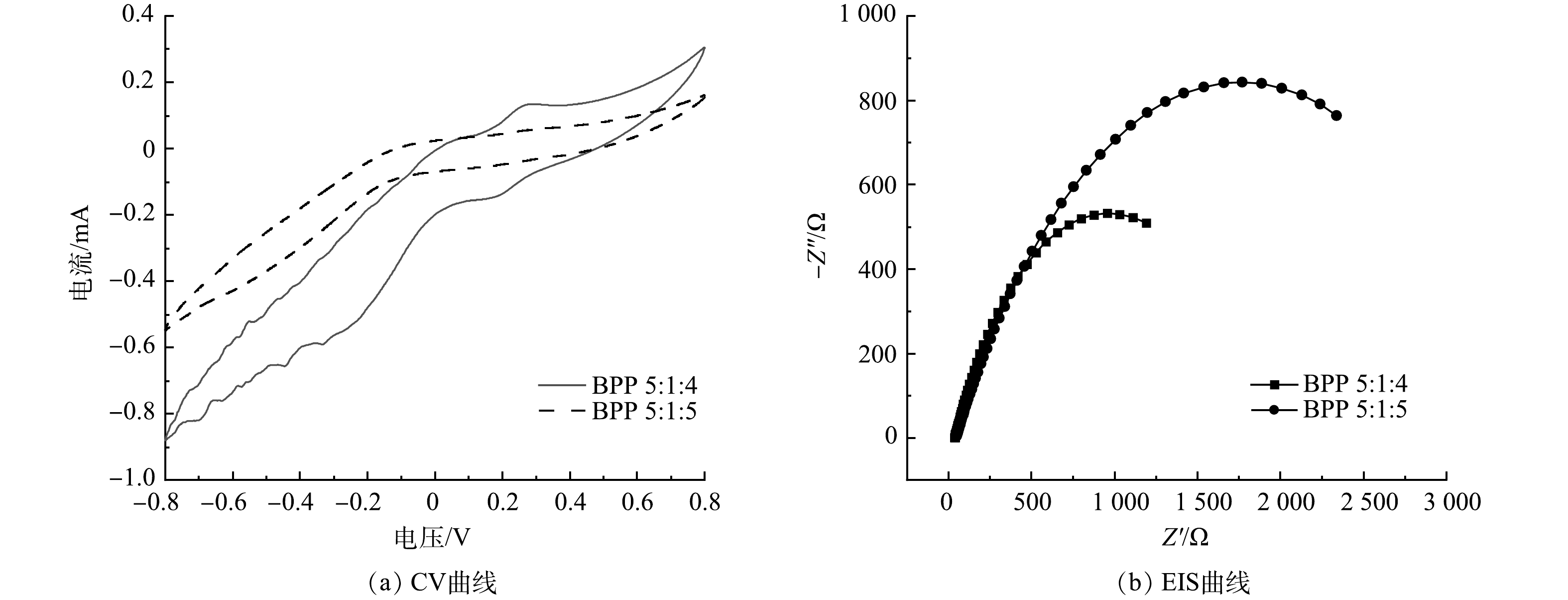
如图4(a)所示,BPP 5:1:4材料在-0.8~0.8 V内的电流为0.305~-0.879 9 mA,且CV曲线有2对微弱的氧化还原峰;而BPP 5:1:5材料的电流为0.154~-0.546 mA,CV曲线没有氧化还原峰。材料氧化还原峰越多越明显,材料的电子传递能力越好[22],因此,BPP 5:1:4材料比BPP 5:1:5材料具有更良好的电子传递能力,更易促进氧化还原反应。
交流阻抗(EIS)曲线如图4(b)所示,其中,正弦信号频率为0.01~105 Hz,交流振幅为0.006。MFC中EIS的表征大多用于分析欧姆内阻和扩散内阻,由于低频区对扩散内阻的表征存在较大偏差,所以在数据拟合过程中未将低频区部分纳入拟合范围[23]。本次拟合使用软件Zview2,等效电路模型中RΩ为欧姆内阻,Rct为电荷转移内阻,电荷转移内阻与一个双电层电容并联,但因弥散效应的存在,该电容偏离理想双电层电容器,因而在本次拟合电路中使用常相位角原件代替传统双电层电容器。根据拟合结果,BPP 5: 1: 4欧姆内阻为37.17 Ω,电荷转移内阻为1 854 Ω,BPP 5:1:5欧姆内阻为46.54 Ω,电荷转移内阻为343 Ω。总的来看,Rct均大于RΩ,说明MFC系统内组主要受Rct控制。就Rct而言,BPP 5:1:4小于BPP 5:1:5,Rct主要反映电活性微生物与电极之间电子传递过程的内阻[24],Rct越小,其电子传递速率越快,因此,BPP 5:1:4生物电化学活性优于BPP 5:1:5。
2.5 菌株硝化反硝化能力的分析
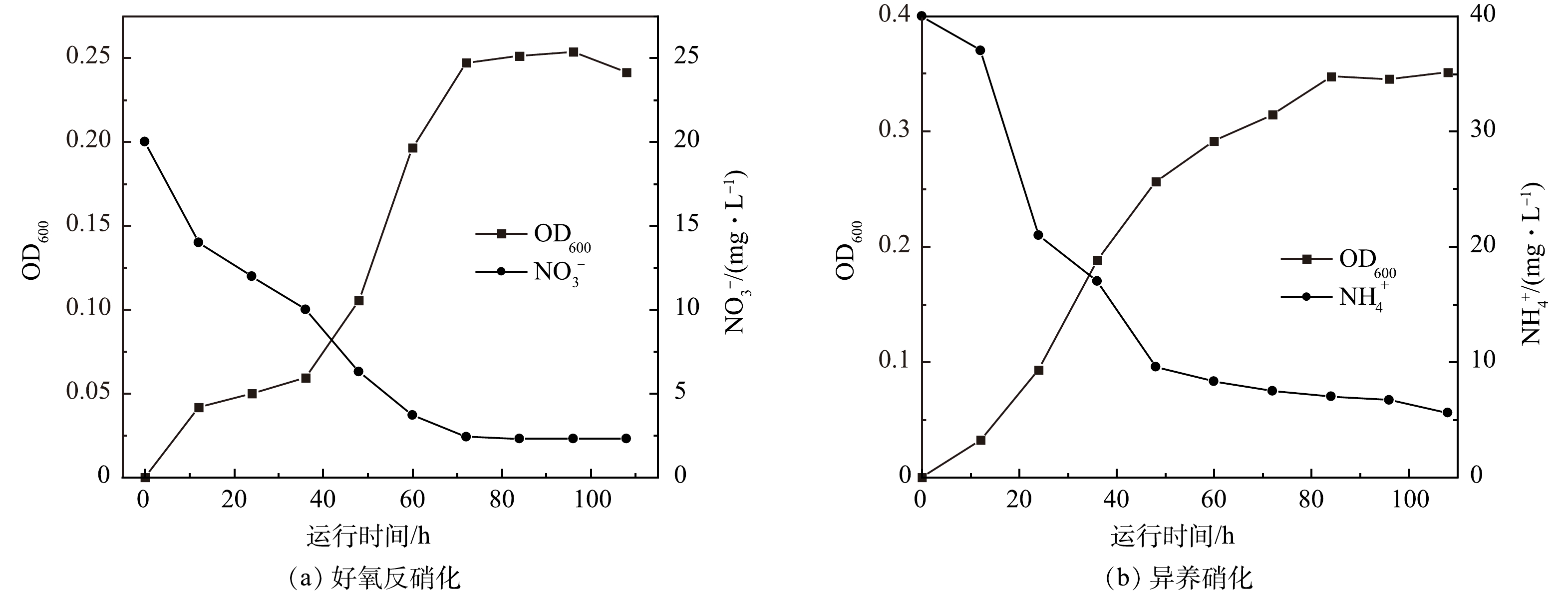
本研究组前期筛选出的异养硝化-好氧反硝化菌[16],通过扩大培养后,接种到异养硝化与好氧反硝化培养基中。由图5可见,在好氧反硝化、异养硝化培养基中菌株不断进行自我繁殖,并消耗培养基中的硝酸根与氨氮。这说明实验接种的微生物具有好氧反硝化与异养硝化能力,后续实验将采用此细菌做为MFC的产电菌。
2.6 改性核桃壳生物炭电极MFC产电性能
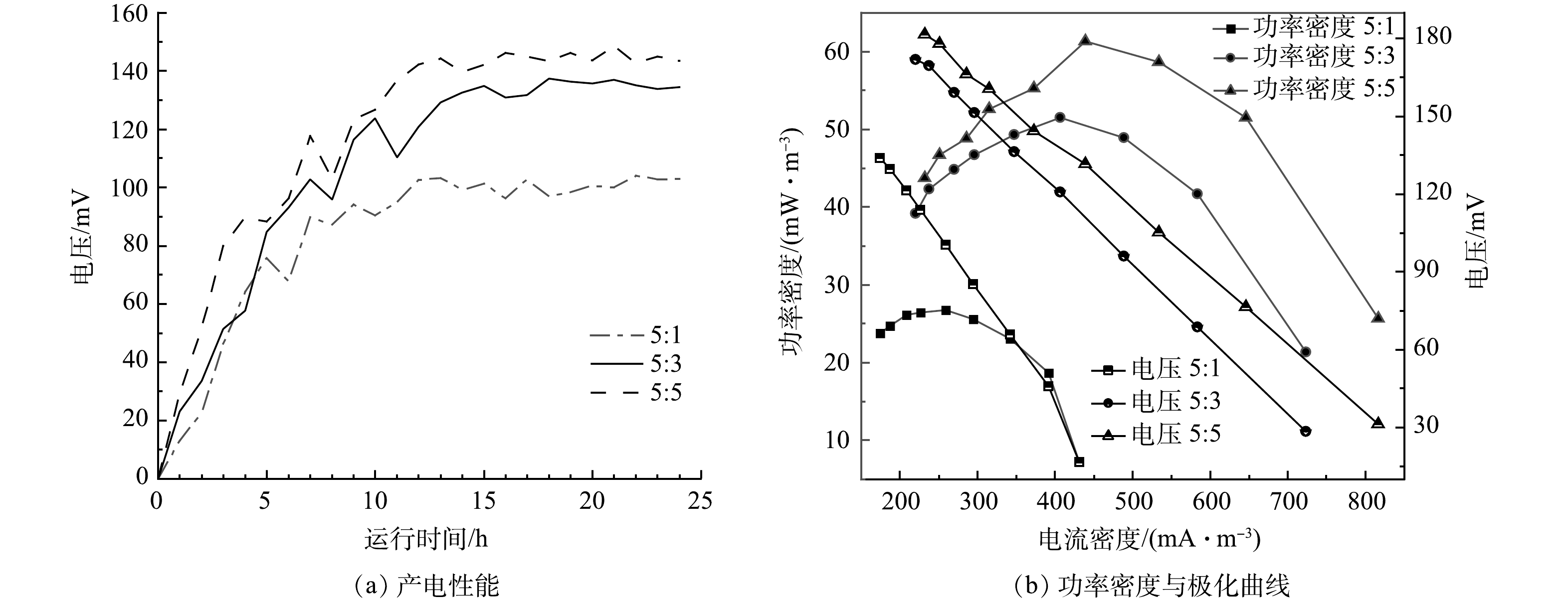
实验构建单室MFC反应器,将脱氮菌株接种至MFC反应器中,连接不同条件下制备的改性核桃壳基生物炭电极材料,每5 min对MFC输出电流电压进行实时监控,其产电性能、功率密度与极化曲线如图6所示。
在真空煅烧温度为600 ℃,BPP为5:1:4的条件下,考察了不同浓度氯化锌对产电性能的影响,结果如图6(a)和图6(b)所示。当生物炭/氯化锌质量比为5:1时,MFC电极最大输出电压为0.103 V,随外电阻由大到小变化,反应器极化曲线电压由133 mV降至16 mV,最大体积功率为26 mW·m−3,电流密度为259 mW·m−3;当生物炭/氯化锌质量比为5:3时,最大输出电压为0.137 V,随外电阻由大到小变化,反应器极化曲线电压由171 mV降至28 mV,最大体积功率为51 mW·m−3,电流密度为406 mA·m−3;当生物炭/氯化锌质量比为5:5时,MFC电极最大输出电压为0.148 V,随外电阻由大到小变化,反应器极化曲线电压由181 mV降低至31 mV,最大功率密度为61 mW·m−3,电流密度为438 mA·m−3。根据极化曲线斜率可以得出MFC电极电阻,斜率越小MFC的内阻越大[25]。随着ZnCl2质量比的增加,MFC的产电能力增加,内阻逐渐减小,电极材料的产电性能越好。当生物炭/氯化锌质量比从5:3提升到5:5,MFC的产电性能提升不大,可能原因是ZnCl2对生物炭的造孔能力几乎达到饱和[26]。
在BPP为5:1:4,生物炭/氯化锌比为5:3条件下,考察了煅烧温度对电极性能的影响,结果如图7(a)和图7(b)所示。在400 ℃煅烧条件下,MFC电极最大输出电压为0.096 V,随外电阻由大到小变化,反应器极化曲线电压由119 mV降低至17 mV,最大功率密度为22 mW·m−3,电流密度为238 mA·m−3;在600 ℃煅烧条件下,最大输出电压为0.137 V,随外电阻由大到小变化,反应器极化曲线电压由171 mV降低至28 mV,最大体积功率为51 mW·m−3,电流密度为406 mA·m−3;在800 ℃煅烧条件下,MFC电极最大输出电压为0.143 V,随外电阻由大到小变化,反应器极化曲线电压由181 mV降低至29 mV,最大功率密度约为57 mW·m−3,此时的电流密度为436 mA·m−3。随着煅烧温度的增加,MFC的产电能力增加,内阻逐渐减小,电极材料的产电性能越好。结合图2可知,这是由于材料石墨化程度的增加,导致MFC的内阻减小。由图7可见,在600 ℃和800 ℃条件下,制备的电极性能相差不大。由此可见,当煅烧温度达到一定程度,MFC的产电性能提升不大,可能的原因是温度的增加破坏了部分生物炭的微孔和大孔,虽然生物炭石墨化程度增加,但是微生物的负载量减少[27]。
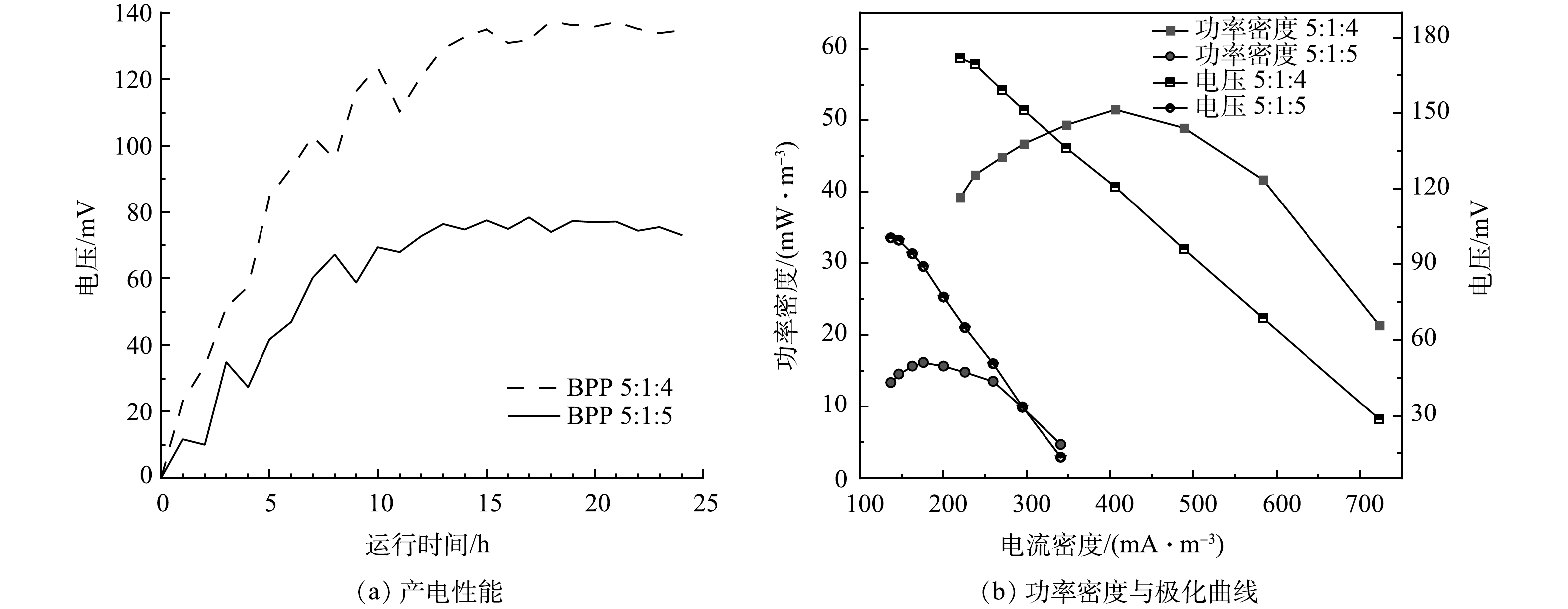
在生物炭/氯化锌比为5:3,真空煅烧温度为600 ℃条件下,考察了不同材料复合情况对电极产电性能的影响,结果如图8(a)和图8(f)所示。当生物炭/聚苯胺/热熔胶复合比例为5:1:4时,最大输出电压为0.137 V,随外电阻由大到小变化,反应器极化曲线电压由171 mV降至28 mV,最大体积功率为51 mW·m−3,电流密度为406 mA·m−3;当生物炭/聚苯胺/热熔胶复合比例为5:1:5时,最大输出电压为0.077 V,随外电阻由大到小变化,反应器极化曲线电压由100 mV降至13mV,最大体积功率为16 mW·m−3,电流密度为176 mA·m−3。由此可见,生物炭的含量对复合材料有显著影响。结合图3可知,在保证材料成型的前提下,生物炭含量越高,MFC的内阻越小,材料的产电性能越好[28]。
2.7 改性核桃壳生物炭MFC对污染物的去除性能
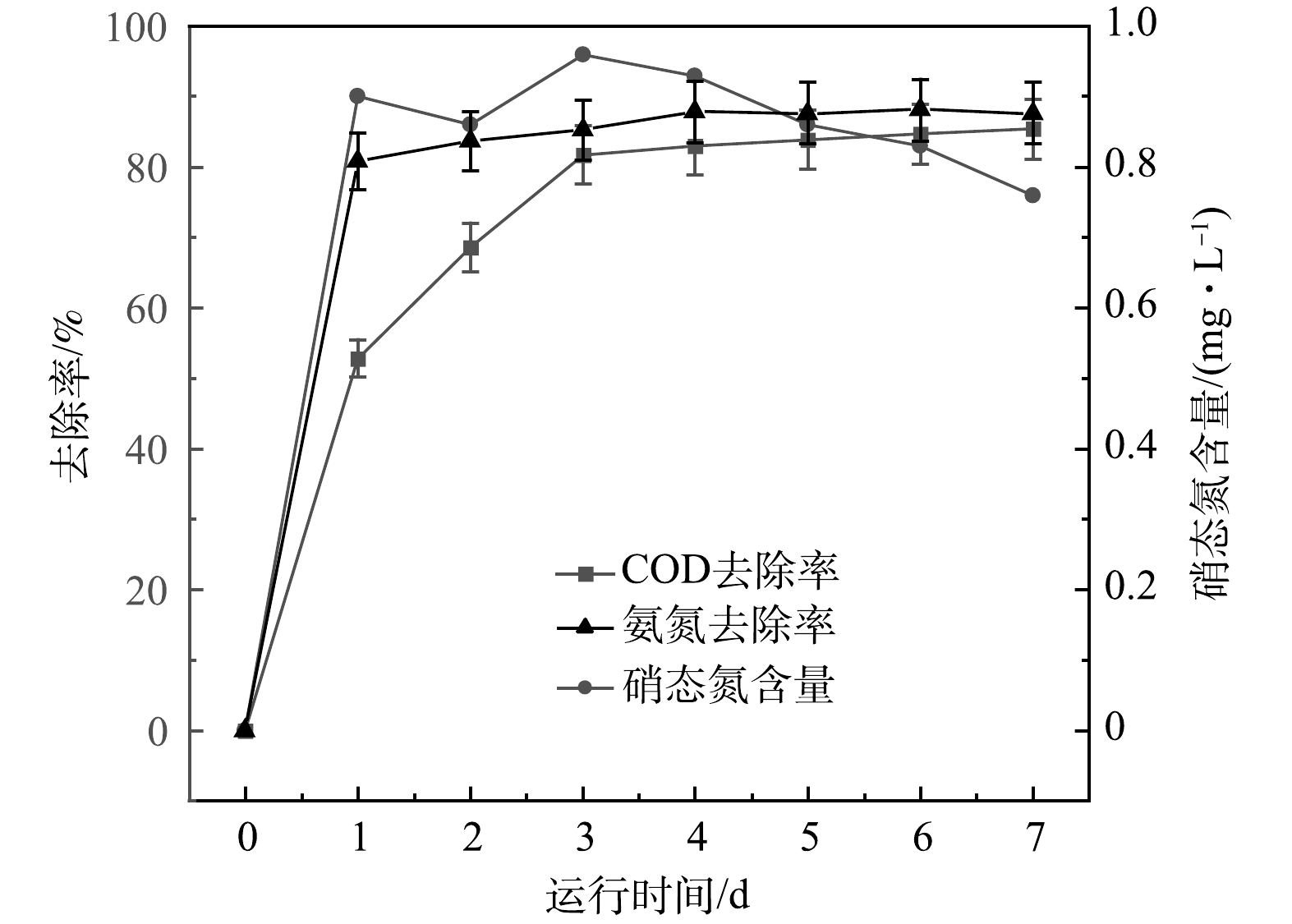
根据以上结果,确认电极制备的最佳条件为BPP 5:1:4、煅烧温度600 ℃、生物炭/氯化锌比5:3时,即节约了生产成本,又达到最大产电量的90%,为减少对环境的污染,选用此种方法制备的电极材料用来探讨改性核桃壳生物炭电极材料用于MFC反应器降解污染物的长期效果,结果如图9所示。可以看出,随着时间的推移,MFC中出水COD由685 mg·L−1降至100 mg·L−1。第1天时,出水COD大幅下降,这说明微生物在反应初期消耗废水中大量有机物用于增殖。随着微生物增殖所需能量减少,有机物需求也逐渐减少,最终COD稳定去除率为85%。出水氨氮质量浓度由38 mg·L−1降低至4.5 mg·L−1,在第4天达到稳定,改性核桃壳生物炭MFC对氨氮的去除率最终达到88%。水体中的硝氮在7 d内先上升后下降,可以看出硝化菌株产生的硝态氮会被好氧反硝化菌株利用,MFC具有较好的脱硝态氮能力。
3. 结论
1) MFC电极的最佳制备条件:活化时生物炭/氯化锌质量比5:3,真空煅烧温度为600 ℃,生物炭/聚苯胺/热熔胶复合电极比例为5:1:4,应用于MFC中最大的体积功率密度可达51 mW·m−3,对模拟废水中COD和氨氮的去除率分别为85%和88%。
2)相较于传统生物炭电极在水体中易碎,通过复合聚苯胺与热熔胶来制作生物炭电极可以在模拟废水中稳定运行。
3)用核桃壳生物炭通过简单的过程制备的MFC电极,为成本低廉,绿色清洁,操作简单的MFC发展方向提供了新的选择。
-
表 1 纤维的增重和官能团
Table 1. The weight gain and functionality of the fibers
纤维Fiber 增重/% Weight 官能度/(mmol·g−1) Functionality PANTF 28.9 2.18 PANT-C4F-a 5.9 0.41 PANT-C4F-b 20.8 1.26 PANT-C4F-c 35.4 1.91 PANT-C6F 27.7 1.31 PANT-C8F 37.8 1.42 注:PANT-C4F(a-c)分别为0.5、3、5 h时间下,制备的不同增重正溴丁烷修饰的季铵盐功能化纤维. 表 2 功能化纤维对磷的吸附性能比较
Table 2. Comparison of quaternary ammonium fibers
季铵化功能纤维Quaternary ammonium functional fibers m/mg C1/(mg·L−1 P) C2/(mg·L−1 P) Q/(mg·g−1 P) PANT-C4F 11.0 5.30 20 13.36 PANT-C6F 11.0 6.15 20 12.59 PANT-C8F 11.0 6.97 20 11.85 表 3 元素分析数据
Table 3. Elemental analysis data of different fibers
条目Entry 纤维类型Sample C/% N/% H/% 1 PANF 66.25±0.12 25.49±0.07 5.12±0.02 2 PANTF 59.68±0.01 22.02±0.03 6.85±0.07 3 PANT-C4F 53.87±0.30 18.25±0.01 6.58±0.07 4 PANT-C4F-P 53.51±0.07 17.77±0.12 6.50±0.01 5 PANT-C4F-1 53.39±0.01 17.50±0.08 6.56±0.01 表 4 改性腈纶纤维吸附磷的动力学及等温线拟合方程参数
Table 4. Kinetic and isotherm parameters for phosphorus adsorption onto modified polyacrylonitrile fibers
模型Model 参数 R2 准一级 k1=0.8020 qe=24.7634 mg·g−1 P 0.9956 准二级 k2=0.0519 qe=26.1712 mg·g−1 P 0.9967 Langmuir k=0.0784 qmax=37.3552 mg·g−1 P 0.9273 Freundlich kf=3.8498 n=1.6486 0.9113 -
[1] 柳儒, 方利平, 李季. 磁性绿锈吸附固定厌氧水体磷酸盐及其影响因素 [J]. 环境科学与技术, 2019, 42(12): 53-60. LIU R, FANG L P, LI J. Phosphate sequestration by magnetic green rusts in anarobic conditions and its implication for eutrophication control [J]. Environmental Science & Technology, 2019, 42(12): 53-60(in Chinese).
[2] 卫凯平, 武慧君, 黄莉, 等. 农业生产系统氮磷环境影响分析: 以安徽省为例 [J]. 农业环境科学学报, 2018, 37(8): 1802-1810. doi: 10.11654/jaes.2018-0053 WEI K P, WU H J, HUANG L, et al. Analysis of environmental impact derived from nitrogen and phosphorus in agricultural production systems: A case study of Anhui Province [J]. Journal of Agro-Environment Science, 2018, 37(8): 1802-1810(in Chinese). doi: 10.11654/jaes.2018-0053
[3] HUANG W Y, ZHANG Y M, LI D. Adsorptive removal of phosphate from water using mesoporous materials: A review [J]. Journal of Environmental Management, 2017, 193: 470-482. [4] 王方嘉, 徐武松, 郑文杰, 等. 改性秸秆去除水体污染物的研究进展 [J]. 化学与生物工程, 2020, 37(9): 13-16,26. doi: 10.3969/j.issn.1672-5425.2020.09.03 WANG F J, XU W S, ZHENG W J, et al. Research progress in removal of water pollutant by modified straw [J]. Chemistry & Bioengineering, 2020, 37(9): 13-16,26(in Chinese). doi: 10.3969/j.issn.1672-5425.2020.09.03
[5] 吴小龙, 林建伟, 张宏华, 等. 物理扰动对锆改性沸石改良底泥磷吸附和移动的影响 [J]. 环境化学, 2019, 38(5): 1119-1127. WU X L, LIN J W, ZHANG H H, et al. Effect of physical disturbance on phosphorus sorption and immobilization onto/in zirconium-modified zeolite-amended sediments [J]. Environmental Chemistry, 2019, 38(5): 1119-1127(in Chinese).
[6] HARIJAN D K L, CHANDRA V. Akaganeite nanorods decorated graphene oxide sheets for removal and recovery of aqueous phosphate [J]. Journal of Water Process Engineering, 2017, 19: 120-125. doi: 10.1016/j.jwpe.2017.07.019 [7] CHEN L, LI Y Z, SUN Y B, et al. La(OH)3 loaded magnetic mesoporous nanospheres with highly efficient phosphate removal properties and superior pH stability [J]. Chemical Engineering Journal, 2019, 360: 342-348. doi: 10.1016/j.cej.2018.11.234 [8] 罗元, 谢坤, 张克强, 等. 生物炭及其金属改性材料脱除水体磷酸盐研究进展 [J]. 环境化学, 2020, 39(8): 2175-2186. doi: 10.7524/j.issn.0254-6108.2019052701 LUO Y, XIE K, ZHANG K Q, et al. Research progress on removal of phosphate from aqueous solution by biochar and its metal modified materials [J]. Environmental Chemistry, 2020, 39(8): 2175-2186(in Chinese). doi: 10.7524/j.issn.0254-6108.2019052701
[9] URE D, AWADA A, FROWLEY N, et al. Greenhouse tomato plant roots/carboxymethyl cellulose method for the efficient removal and recovery of inorganic phosphate from agricultural wastewater [J]. Journal of Environmental Management, 2019, 233: 258-263. [10] 李甫, 董永春, 程博闻, 等. 改性聚丙烯腈纤维与金属离子的配位反应及其应用进展 [J]. 纺织学报, 2017, 38(6): 143-150. LI F, DONG Y C, CHENG B W, et al. Recent progress in coordination of modified polyacrylonitrile fiber with metal ions and applications [J]. Journal of Textile Research, 2017, 38(6): 143-150(in Chinese).
[11] 张超, 李梦星, 周清, 等. 聚丙烯腈螯合纤维的研究进展 [J]. 化工新型材料, 2015, 43(11): 239-241. ZHANG C, LI M X, ZHOU Q, et al. Progress in polyacrylonitrile chelated fiber [J]. New Chemical Materials, 2015, 43(11): 239-241(in Chinese).
[12] LI G W, XIAO J, ZHANG W Q. ChemInform abstract: Knoevenagel condensation catalyzed by a tertiary-amine functionalized polyacrylonitrile fiber [J]. Green Chemistry, 2011, 13(7): 1828-1836. [13] SHI X L, SUN B, CHEN Y J, et al. Tuning anion species and chain length of ligands grafted on the fiber for an efficient polymer-supported Ni(II) complex catalyst in one-pot multicomponent A3-coupling [J]. Journal of Catalysis, 2019, 372: 321-329. doi: 10.1016/j.jcat.2019.03.020 [14] 王力, 陈兆文, 苗雷, 等. 聚丙烯腈基改性功能纤维选择性吸附饮用水中Cu2+研究 [J]. 合成纤维工业, 2019, 42(5): 51-53. doi: 10.3969/j.issn.1001-0041.2019.05.011 WANG L, CHEN Z W, MIAO L, et al. Selective adsorption of polyacrylonitrile modified functional fiber for Cu2+ in drinking water [J]. China Synthetic Fiber Industry, 2019, 42(5): 51-53(in Chinese). doi: 10.3969/j.issn.1001-0041.2019.05.011
[15] WANG B, CHEN P Y, ZHAO R X, et al. Carbon-dot modified polyacrylonitrile fibers: Recyclable materials capable of selectively and reversibly adsorbing small-sized anionic dyes [J]. Chemical Engineering Journal, 2020, 391: 123484. doi: 10.1016/j.cej.2019.123484 [16] XU W S, ZHENG W J, WANG F J, et al. Using iron ion-loaded aminated polyacrylonitrile fiber to efficiently remove wastewater phosphate [J]. Chemical Engineering Journal, 2021, 403: 126349. doi: 10.1016/j.cej.2020.126349 [17] XU G, XU W S, TIAN S, et al. Enhanced phosphate removal from wastewater by recyclable fiber supported quaternary ammonium salts: Highlighting the role of surface polarity [J]. Chemical Engineering Journal, 2021, 416: 127889. doi: 10.1016/j.cej.2020.127889 [18] LI J, LIB, HUANG H M, et al. Removal of phosphate from aqueous solution by dolomite-modified biochar derived from urban dewatered sewage sludge [J]. Science of the Total Environment, 2019, 687: 460-469. doi: 10.1016/j.scitotenv.2019.05.400 [19] 魏凯. 磷肥工业废水处理及回用 [J]. 安徽化工, 2020, 46(2): 101-102,11. doi: 10.3969/j.issn.1008-553X.2020.02.027 WEI K. Phosphate fertilizer industrial wastewater treatment and reuse [J]. Anhui Chemical Industry, 2020, 46(2): 101-102,11(in Chinese). doi: 10.3969/j.issn.1008-553X.2020.02.027
[20] NATARAJ S K, KIM B H, DELA CRUZ M, et al. Free standing thin webs of porous carbon nanofibers of polyacrylonitrile containing iron-oxide by electrospinning [J]. Materials Letters, 2009, 63(2): 218-220. doi: 10.1016/j.matlet.2008.09.060 [21] GAO R J, XU G, ZHENG L S, et al. A highly selective and sensitive reusable colorimetric sensor for Ag+ based on thiadiazole-functionalized polyacrylonitrile fiber [J]. Journal of Materials Chemistry C, 2016, 4(25): 5996-6006. doi: 10.1039/C6TC00621C [22] JIANG J X, KIM D I, DORJI P, et al. Phosphorus removal mechanisms from domestic wastewater by membrane capacitive deionization and system optimization for enhanced phosphate removal [J]. Process Safety and Environmental Protection, 2019, 126: 44-52. doi: 10.1016/j.psep.2019.04.005 [23] 吴俊麟, 林建伟, 詹艳慧, 等. 镁铁层状双金属氢氧化物对磷酸盐的吸附作用及对内源磷释放的控制效果及机制 [J]. 环境科学, 2020, 41(1): 273-283. WU J L, LIN J W, ZHAN Y H, et al. Adsorption of phosphate on Mg/Fe layered double hydroxides(Mg/Fe-LDH)and use of Mg/Fe-LDH as an amendment for controlling phosphorus release from sediments [J]. Environmental Science, 2020, 41(1): 273-283(in Chinese).
[24] LIU X N, SHEN F, QI X H. Adsorption recovery of phosphate from aqueous solution by CaO-biochar composites prepared from eggshell and rice straw [J]. Science of the Total Environment, 2019, 666: 694-702. doi: 10.1016/j.scitotenv.2019.02.227 [25] ZHOU K, WU B R, SU L H, et al. Enhanced phosphate removal using nanostructured hydrated ferric-zirconium binary oxide confined in a polymeric anion exchanger [J]. Chemical Engineering Journal, 2018, 345: 640-647. doi: 10.1016/j.cej.2018.01.091 [26] ANDRÉS E, ARAYA F, VERA I, et al. Phosphate removal using zeolite in treatment wetlands under different oxidation-reduction potentials [J]. Ecological Engineering, 2018, 117: 18-27. doi: 10.1016/j.ecoleng.2018.03.008 [27] ZHANG B, CHEN N, FENG C P, et al. Adsorption for phosphate by crosslinked/non-crosslinked-chitosan-Fe(III) complex sorbents: Characteristic and mechanism [J]. Chemical Engineering Journal, 2018, 353: 361-372. doi: 10.1016/j.cej.2018.07.092 [28] WANG Y, XIE X M, CHEN X L, et al. Biochar-loaded Ce3+-enriched ultra-fine ceria nanoparticles for phosphate adsorption [J]. Journal of Hazardous Materials, 2020, 396: 122626. doi: 10.1016/j.jhazmat.2020.122626 -






 下载:
下载: