-
人工湿地是一种集生态和景观性于一体的高效低耗的废水生态处理技术。随着人工湿地越来越广泛的应用于处理垃圾渗滤液、畜禽养殖废水厌氧消化液和食品废水等高氮低碳废水,有机碳源的匮乏已成为制约人工湿地高效脱氮的瓶颈,特别是针对总氮(TN)的高效去除[1-2]。长期以来,反硝化作用被人们一直认为是将活性氮转化为氮气(N2)的唯一途径,然而在实际运行的湿地中,针对高氮低碳废水的传统硝化和反硝化脱氮能力被限制在一定水平,且由于传统厌氧反硝化过程需要大量碳源,往往需要外加碳源以补充其不足,从而导致其处理废水成本较高[1, 3]。
近年来日益成熟的厌氧氨氧化处理工艺为解决此问题提供了新的途径。厌氧氨氧化(anaerobic ammonia oxidation, anammox)过程是指在厌氧条件下分别以氨盐(NH4+)和亚硝酸盐(NO2−)为电子供体和电子受体的高效生物脱氮过程,该过程无需碳源,且伴随硝酸盐(NO3−)的生成[4-5]。随着分子生物学技术的发展,荧光定量PCR和高通量测序已被用于人工湿地中厌氧氨氧化菌的研究[5-7]。厌氧氨氧化过程已经被证明可以成为人工湿地中主要的脱氮过程,发挥高效的脱氮作用[1, 6, 8]。同时有研究表明,在人工湿地中厌氧氨氧化菌呈现出种群多样性[6, 8];C/N比、植物等环境因子和反硝化作用等脱氮过程对厌氧氨氧化菌的群落、丰度有着重要的影响[5, 9-10]。
目前虽然有学者对不同C/N条件下厌氧氨氧化菌在人工湿地的存在特征有少量研究,但在不同C/N和湿地植物多重耦合作用下针对处理高氮低碳废水的人工湿地中厌氧氨氧化菌的存在特征却尚乏报道。基于此,本文通过不同C/N比和植物配置,构建4组垂直流人工湿地处理高氮低碳废水,考察各湿地中氮污染物的去除差异;利用荧光定量PCR和高通量测序技术,对比研究各湿地的微生物群落、主要功能微生物及厌氧氨氧化菌的存在特征,进一步探讨C/N和湿地植物多重耦合作用下对湿地中厌氧氨氧化菌的影响特征。
-
4组垂直流人工湿地反应器均采用3 mm厚的有机玻璃进行构建,反应器尺寸均为20 cm × 17.0 cm × 40 cm。湿地填料(砾石)取自处理高氮低碳废水的、运行3年后闲置1年的潜流人工湿地中试系统,每个人工湿地的填料层高度为30 cm,砾石粒径分布为5—22 mm,孔隙率为0.38,湿地床高为35 cm。反应器进水方式均为自下而上,进水口位于反应器底部。
-
根据进水C/N比和湿地植物配置将4个人工湿地分别命名为T1、T2、T3、T4,具体为(1)T1:无碳源(C/N = 0),无湿地植物;(2)T2:无碳源(C/N = 0),湿地植物为鸢尾;(3)T3:有碳源(C/N = 0.5),无湿地植物;(4)T4:有碳源(C/N = 0.5),湿地植物为鸢尾。4个湿地反应器均采用间歇进水,周期为3 h,包括进水阶段0.5 h和反应阶段2.5 h,水力负荷均为2 cm·d−1。根据猪场养殖污水厌氧消化液的高氮低碳特性,本试验用水为人工模拟配制,其实际进水的NH4+-N(NH4Cl)、NO2−-N(NaNO2)、NO3−-N(KNO3)和TN的质量浓度分别为(282.58±16.58)、(395.98±24.31)、(88.03±19.26)、(779.33±53.38) mg·L−1;葡萄糖为有机碳源,COD浓度按需配制。各湿地的水力停留时间均为4.1 d。通过高纯氮气(纯度>99.9%)对湿地配水吹脱0.5 h控制湿地进水溶解氧DO<1 mg·L−1。进水pH值控制在7.5—8.5。各湿地的运行环境温度为(25±2) ℃。各湿地开始运行之前,试运行40 d以恢复湿地微生物的活性,之后开始正式运行,运行时间为90 d。
-
正式运行期间,各湿地进出水样品采集周期均为每2 天采集1次。各湿地运行结束(第90 天)后,进行填料样品采集,从每个湿地中的不同层(0—10、10—20、20—30 cm)采集填料样品,混合均匀后将其保存于–20 ℃待DNA的提取和后续分子生物学的分析。
-
水质指标NH4+-N、NO2−-N、NO3−-N和TN的测定参照《水和废水监测分析方法》(第四版)[11]。溶解氧和pH测定采用Muti 3620 IDS水质分析仪。
-
将采集到的湿地填料经摇床重复振荡洗脱3次至生理盐水中,收集洗脱液并经0.2 μm的水系滤膜抽滤,洗脱的微生物即存在于滤膜上,遂将滤膜剪碎,以用于DNA的提取。湿地填料中DNA提取采用FastDNATM Spin Kit for Soil DNA提取试剂盒(MP Biomedicals, USA),并用Nano Drop 2000UV-Vis Spectrophotometer(Thermo Fisher Scientific, USA)测定DNA浓度。采用引物Amx368f/Amx820r扩增厌氧氨氧化菌16S rRNA基因片段。采用1%凝胶电泳检测扩增产物质量和特异性。PCR扩增产物外送用于厌氧氨氧化菌16S rRNA质粒标准品的制备(美吉生物,上海),载体为pMD 18-T。目标基因定量引物序列及退火温度如表1所示。
-
荧光定量PCR:厌氧氨氧化菌16S rRNA基因定量采用TransStart® Top Green qPCR SuperMix(SuperMix)试剂盒(全式金,北京)进行荧光定量PCR(qPCR)反应(LightCycler480Ⅱ, Roche, Germany)。用引物Amx368f/Amx820r定量厌氧氨氧化菌16S rRNA的拷贝数,20 µL反应体系为:DNA模板2 µL,引物各0.4 µL,SuperMix 10 µL,ddH2O 7.2 µL,反应程序为:94 ℃ 30 s,94 ℃ 5 s,56 ℃ 15 s,72 ℃ 10 s,40个循环。每个样品做3次平行,最终结果扩增效率在90%—110%之间,可决系数R2 > 0.99,溶解曲线为单一峰。
高通量测序:提取的填料样品DNA送生工生物工程(上海)公司开展Miseq高通量测序。细菌16sRNA的V3-V4区域的PCR扩增引物为341F(CCTACGGGNGGCWGCAG)和805R(GACTACHVGGGTATCTAATCC)。
-
利用和Origin 8.0和SPSS软件,进行数据分析和绘图。并采用单因素方差(One-way ANOVA)分析多组数据间的差异性,其显著水平设为P<0.05。图中相关数据为平均值±标准差。
-
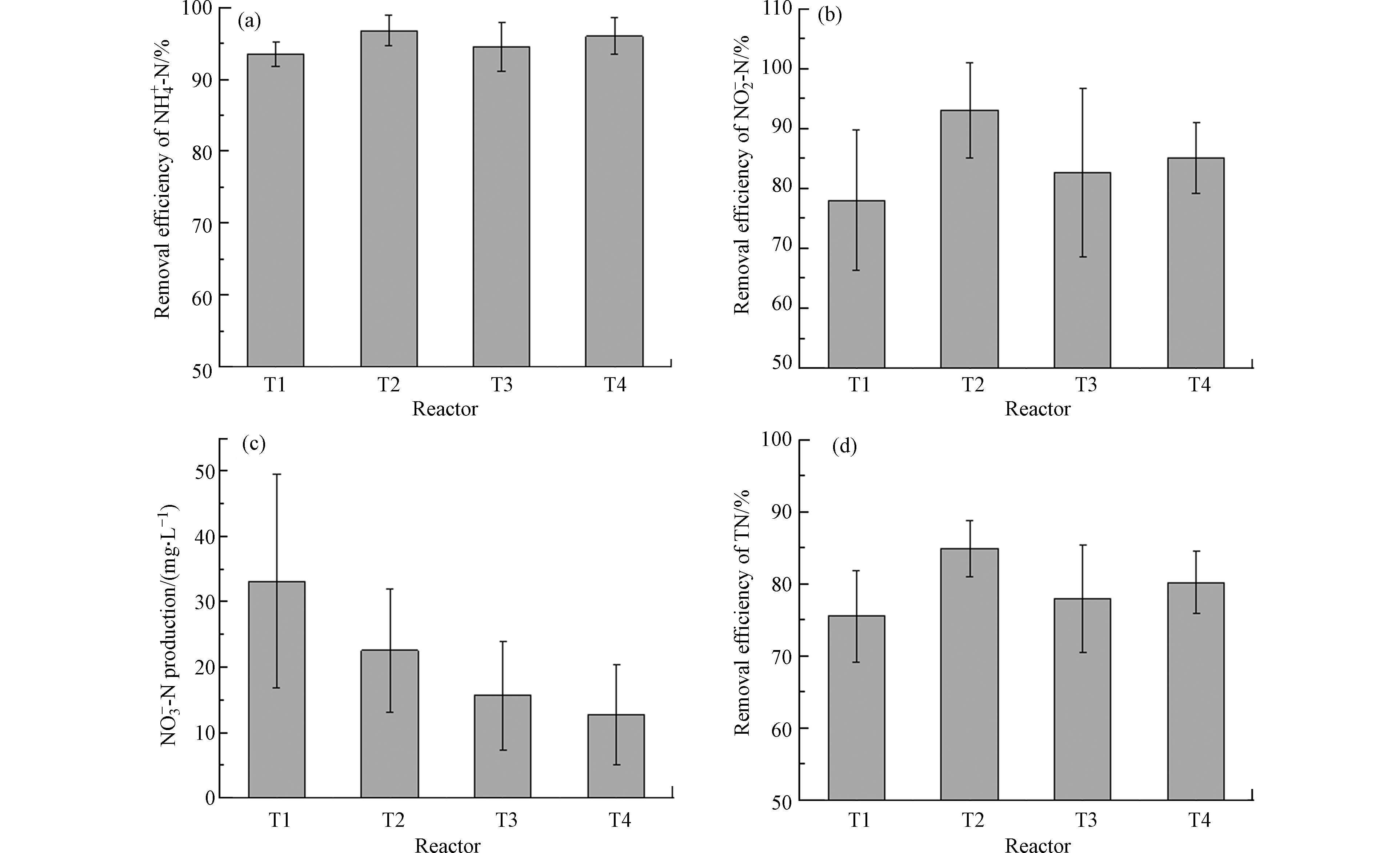
经90 d的运行,4个人工湿地对NH4+-N、NO2−-N、NO3−-N和TN的去除效果如图1所示。T1、T2、T3和T4对NH4+-N的去除率分别为(93.52±1.72)%、(96.74±2.16)%、(94.50±3.35)%和(95.97±2.52)%,去除效果均较好,无显著性差异(P>0.05),其中T2的去除效果最优。此外T2对NO2−-N的去除率亦最高,为(93.06±7.91)%,T1湿地去除率最低,仅为(77.96±11.72)%,T1和T2对NO2−-N的去除存在显著性差异(P<0.05)。T1、T2、T3和T4中NO3−-N均有不同程度的生成,呈现T4<T3<T2<T1,这表明有植物的湿地NO3−-N生成量均低于无植物湿地,有碳源湿地均低于无碳源湿地,这与Lin等[13]研究一致。TN作为考察人工湿地脱氮性能的重要指标,T1、T2、T3和T4的TN去除率分别为(75.48±6.41)%、(84.90±3.96)%、(77.94±7.43)%和(80.16±4.32)%,且T2与T1、T3、T4对TN的去除均存在显著性差异(P<0.05)。T1和T2中C/N为0,T3和T4中C/N(=0.5)亦较低,但各湿地仍发挥出较高的TN去除效果且伴随NO3−-N生成,而反硝化脱氮过程需要足够的碳源(C/N=2—5)作为电子供体将硝酸盐转化为氮气[14],厌氧氨氧化过程是将NH4+-N和NO2−-N转化为氮气,同时生成NO3−-N,此过程无需碳源,因此猜测厌氧氨氧化过程是各湿地中氮污染物去除的主要途径。
T1、T2、T3和T4的TN去除效果呈现出T1<T3<T4<T2,表明有植物无碳源湿地的脱氮效果最佳;有碳源湿地的脱氮效果优于无碳源无植物湿地;且有植物湿地的脱氮效果优于无植物湿地。Lin等[13]发现添加碳源后,种植植物湿地去除NO3−-N的能力明显高于无植物湿地。夏艳阳等[10]发现,有碳源有植物系统对NH4+-N和TN的去除效果优于有碳源无植物系统的去除效果,与本研究一致。此外,添加的碳源有助于反硝化过程,去除一部分由厌氧氨氧化过程产生的NO3−-N,提高TN的去除效果[10]。
-
采集到的湿地填料样品提取DNA后,针对厌氧氨氧化菌16S rRNA基因进行PCR扩增并经1%凝胶电泳特异性检测,初步检出各湿地中存在厌氧氨氧化菌,之后通过qPCR定量填料样品中的厌氧氨氧化菌16S rRNA基因拷贝数。Anammox bacterial 16S rRNA是参与厌氧氨氧化过程中NH4+-N和NO2−-N转化的关键基因,厌氧氨氧化菌的基因丰度分布特征如图2所示。T1、T2、T3和T4湿地的厌氧氨氧化菌基因丰度分别为2.20 × 108、2.88 × 108、2.34 × 108、2.42 × 108 copies·g−1。相较于T1、T3和T4中的厌氧氨氧化菌基因丰度略微提高,但增长不显著,而T2的厌氧氨氧化菌基因丰度增长幅度最大,这表明有植物无碳源的湿地系统可能更有利于厌氧氨氧化菌的生长富集,可能是因为植物根际提供的好氧-缺氧-厌氧微环境促进了厌氧氨氧化菌的生长,且植物根际会分泌一部分碳源,在反硝化菌的作用下利用碳源将硝酸盐还原为亚硝酸盐为厌氧氨氧化菌提供底物[10]。
-
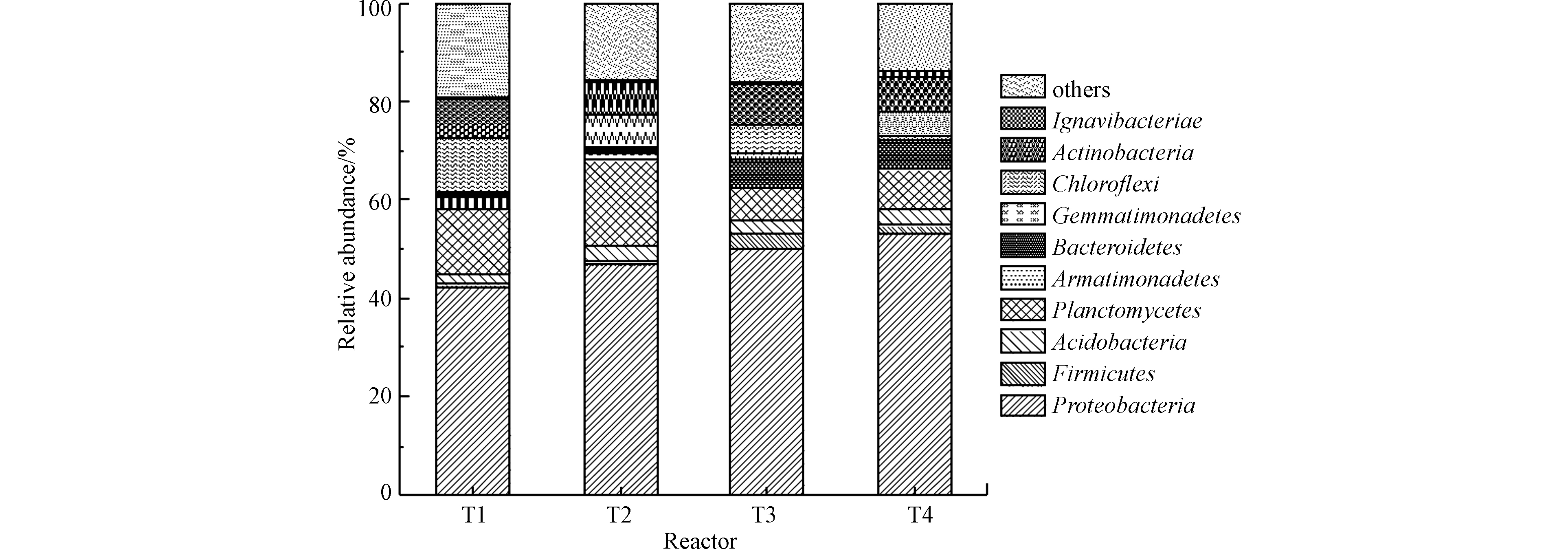
基于反应器90 d的运行,利用高通量测序技术对4组人工湿地的微生物群落结构进行分析,如图3所示,门相对丰度大于1%。由图3可知,变形菌门(Proteobacteria)、浮霉菌门(Planctomycetes)、绿弯菌门(Chloroflexi)、放线菌门(Actinobacteria)和拟杆菌门(Bacterodidetes)等在各人工湿地中相对丰度较高,且是人工湿地和厌氧氨氧化反应器的优势菌种[4-6, 9]。
变形菌门中包含许多参与硝化-反硝化脱氮过程和有机物生物降解转化的菌属[15],相较于其他菌门其在各湿地中的相对丰度均最高(>40%)。厌氧氨氧化菌属于浮霉菌门,因此其占比可反映出各湿地的厌氧氨氧化性能。T1、T2、T3和T4湿地的浮霉菌门的相对丰度存在显著差异,分别为13.38%、17.48%、6.54%、8.14%,表明植物对浮霉菌门的富集发挥促进作用,而有机物表现出抑制作用,猜测原因可能是有机物对厌氧氨氧化菌生长的抑制[4]。绿弯菌门是厌氧氨氧化系统常见的细菌,可产生生物膜结构,在缺氧条件下清除厌氧氨氧化菌可产生的代谢物且有助于厌氧氨氧化菌形成颗粒态[16]。绿弯菌门相对丰度呈现出T1>T2>T3>T4。装甲菌门(Armatimonadetes)也是厌氧氨氧化系统中常见的细菌,其可能会导致氨氮的过度消耗,导致厌氧氨氧化细菌可利用底物不足[17]。T3(2.95%)和T4(1.71%)中的厚壁菌门(Firmicutes)相对丰度高于T1(0.69%)和T2(0.66%),这是因为厚壁菌门具有反硝化作用,而T3和T4中外加的碳源为其提供了足够的底物[4]。放线菌门属于革兰氏阳性细菌,大部分是异养细菌,对有机物有一定的降解能力,其相对丰度呈现T3>T1>T4>T2,表明适当的有机物和植物对其生长富集有强化作用。植物和碳源显著影响湿地系统内部微生物群落结构与多样性,因此湿地植物、碳源与微生物之间存在相互影响、相互作用关系[18-20]。从整体来看,4组垂直流湿地中的微生物群落均较为丰富,但微生物群落结构存在显著性差异。
基于4组垂直流人工湿地中的微生物群落结构发生了显著性变化,对各湿地中参与脱氮过程的主要功能微生物进行分析,探究各湿地内部主要功能微生物的存在特征及差异。
-
硝化-反硝化过程被认为是人工湿地中重要的微生物脱氮途径,其主要参与者为硝化细菌(氨氧化细菌、亚硝化细菌)和异养反硝化细菌,基于高通量测序结果对各湿地中硝化细菌和反硝化细菌进行筛选分析,共检测出8种反硝化菌属和6种硝化菌属,见表2。
Flavobacterium属是具有代表性的反硝化菌属[6, 21],其在T1、T2、T3和T4中相对丰度均最高,且显著高于其他反硝化菌属,是各湿地反硝化菌中的优势菌属。除Flavobacterium属外,Thermomonas属是常见的兼性异养反硝化细菌,Pseudomonas属是需氧自养菌[6]。Thermomonas属在T2、T3和T4中相对丰度显著高于其他反硝化菌属,而T1中Pseudomonas属占比最高。T1、T2、T3和T4中的反硝化菌相对丰度分别为2.48%、5.83%、8.63%、10.17%,呈现出T4>T3>T2>T1,表明有机物是反硝化菌生长富集的重要限制因子,且植物对反硝化菌的富集有积极作用[10]。Ye等[22]研究发现植物根系分泌物可以加速反硝化细菌的生长,同时能一定程度上提高微生物丰富度和生物多样性。此外,植物的根际可为反硝化菌提供一部分碳源,强化其生长富集。硝化菌属包含氨氧化菌属和亚硝酸盐氧化菌属。如表2所示,其中Nitrosomonas和Nitrosovibrio是将NH4+-N转化为NO2−-N的氨氧化菌[6, 23],Nitrospira、Nitrosococcus、Nitrolancea和Nitrobacter是将NO2−-N转化为NO3−-N的亚硝酸盐氧化菌[24-25]。相较于反硝化菌属,各湿地中的硝化菌属相对丰度明显较低。T4中的硝化菌属相对丰度最高(0.89%),而T1、T2、T3中硝化菌属占比无明显差别(0.44%、0.56%、0.42%),表明有植物有碳源的湿地系统更有利于硝化细菌的富集生长。Nitrosomonas和Nitrobacter均是T1和T2中的硝化菌中的优势菌属,而Nitrosomonas是T3和T4中的优势菌属,且湿地中的Nitrosomonas和Nitrobacter属相对丰度具有较大差异,这表明碳源的添加可显著改变湿地系统的硝化菌属结构。从整体来看,植物和碳源对4组人工湿地中硝化菌属和反硝化菌属的群落结构及占比具有显著影响,可见植物和碳源是硝化反硝化菌群落演替的重要因子。
-
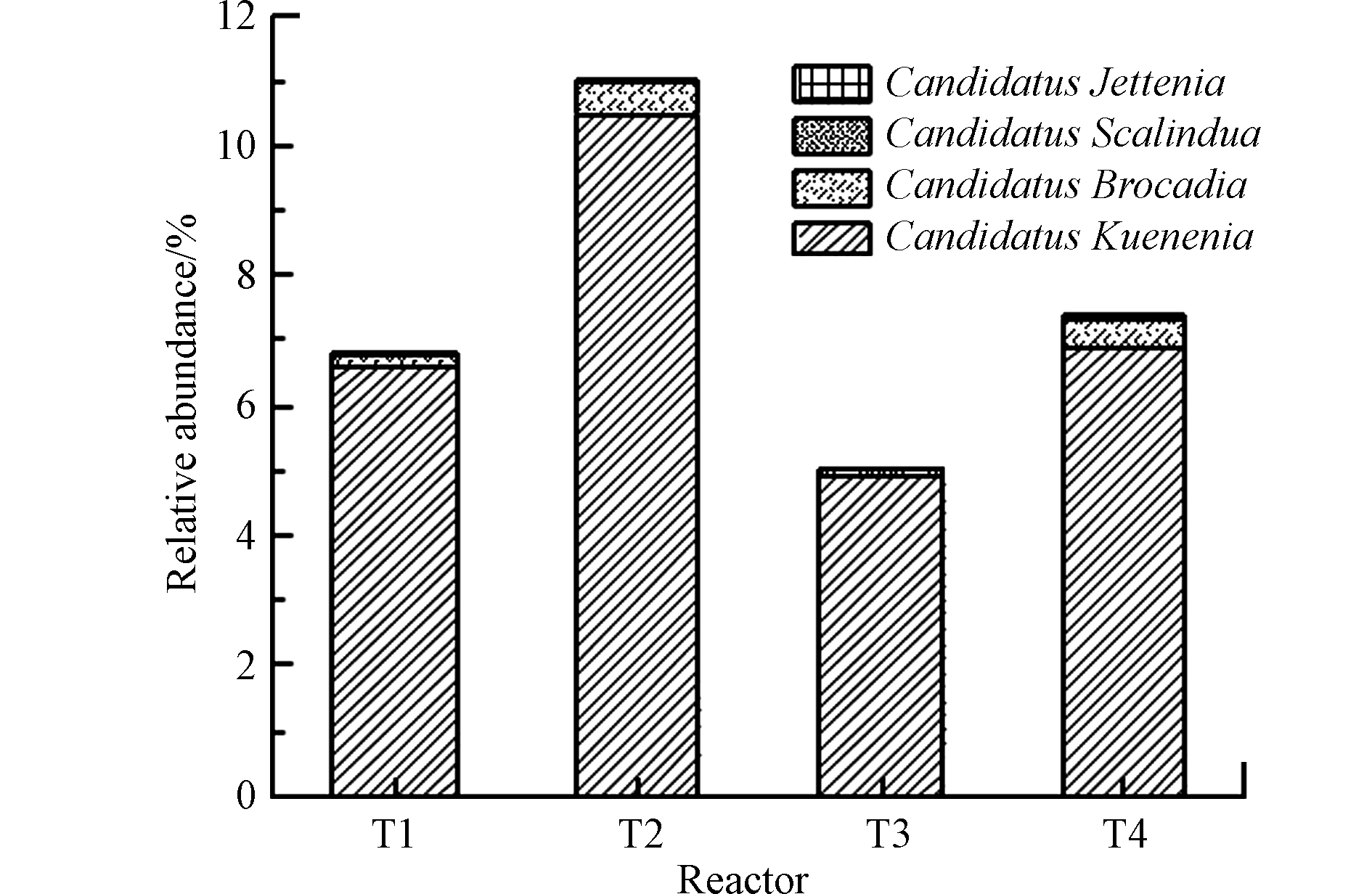
厌氧氨氧化过程作为一种新型微生物脱氮过程,其主要参与者是厌氧氨氧化菌[5],为考察在有无植物和碳源情况下厌氧氨氧化菌群落的存在特征,通过高通量测序共检出4种厌氧氨氧化菌属,如图4所示。在4组湿地系统中均检出Candidatus Kuenenia、Candidatus Brocadia、Candidatus Scalindua和Candidatus Jettenia属。Candidatus Kuenenia属在各湿地中相对丰度最高,分别为6.61%、10.47%、4.91%和6.92%,且均占厌氧氨氧化菌属的比例很高(>90%),说明其是4组湿地系统中厌氧氨氧化菌属的重要组成,发挥积极的脱氮作用。此外,T2(0.51%)和T4(0.43%)中的Candidatus Scalindua属相对丰度显著高于T1(0.19%)和T3(0.13%)。而Candidatus Brocadia和Candidatus Jettenia属相对丰度在各湿地之间无明显差异。Candidatus Kuenenia属更适宜在低氮负荷下生长,而Candidatus Brocadia属更适宜在高氮负荷下富集[7],这与本研究的结果相反。这可能是因为本研究中湿地进水中的亚硝酸盐底物相对充足,而Candidatus Kuenenia属相较Candidatus Brocadia属对亚硝酸盐底物有更高的亲和力[26]。van der Star等[7]研究表明厌氧氨氧化菌属群落的组成受氮负荷、C/N比、溶解氧浓度等因素的影响较大。T1、T2、T3和T4中的厌氧氨氧化菌属相对丰度分别为6.83%、11.03%、5.06%和7.38%,表明厌氧氨氧化菌属是各湿地系统中的优势脱氮菌,呈现出T2>T4>T1>T3。厌氧氨氧化菌属相对丰度在各湿地之间存在显著性差异(P<0.05)。Wang等[9]研究表明,相较于反硝化过程,C/N比低于2时更有利于厌氧氨氧化过程的进行。在高C/N比下,NO2可能主要用于反硝化过程,而非厌氧氨氧化过程[14]。本研究中,较低的C/N比和较高的氮污染物浓度可为厌氧氨氧化菌的生长提供适宜的生境,但碳源的添加和植物的配置仍会对厌氧氨氧化菌的富集生长产生一定的影响。以上结果表明,有植物无碳源的湿地系统更加有利于厌氧氨氧化菌的生长繁殖,而碳源的添加一定程度上会对起抑制作用,但植物在一定程度上可缓解碳源对厌氧氨氧化菌生长的抑制。
-
(1)各人工湿地系统对NH4+-N、NO2−-N和TN均有较好且稳定的去除效果。其中有植物无碳源湿地系统的脱氮效果最佳,TN平均去除率可达84.90%;有碳源湿地系统的脱氮效果优于无碳源无植物湿地系统;且有植物湿地的脱氮效果优于无植物湿地。各人工湿地系统的主要脱氮途径为厌氧氨氧化过程,且在不同程度上存在厌氧氨氧化与反硝化的协同耦合脱氮。
(2)各湿地中的微生物群落均较为丰富,其中优势菌门为变形菌门、浮霉菌门、绿弯菌门、放线菌门和拟杆菌门。但由于C/N和植物配置差异,微生物群落结构存在显著性差异。
(3)各湿地系统中均检出4种厌氧氨氧化菌属,其中Candidatus Kuenenia属在各湿地中占比最高(>90%),且其在无碳源有植物湿地中显著高于其他3组湿地。受C/N和植物配置差异影响,各湿地中的厌氧氨氧化菌属结构之间存在一定差异。相较于其他三组湿地,有植物无碳源湿地更有利于厌氧氨氧化菌的生长富集。
处理高氮低碳废水的垂直流人工湿地中厌氧氨氧化菌分布特征
Distribution characteristics of anammox bacteria in vertical flow constructed wetlands for treating high-nitrogen wastewater with low carbon
-
摘要: 为探明C/N和湿地植物对湿地中厌氧氨氧化菌的影响特征,基于不同C/N比和植物配置,构建4组垂直流人工湿地处理高氮低碳废水,研究其对氮污染物的去除特征差异,并利用荧光定量PCR技术和高通量测序技术对湿地中的主要功能微生物及厌氧氨氧化菌的分布特征进行分析。结果表明,在进水TN浓度为779.33 mg·L−1时,各湿地的TN平均去除率分别为75.48%、84.90%、77.94%和80.16%。整体来看,无碳源有植物湿地的脱氮效果最佳;有碳源湿地的脱氮效果优于无碳源无植物湿地;且有植物湿地的脱氮效果优于无植物湿地。各湿地中微生物群落结构在门上平上存在显著差异,且厌氧氨氧化菌属结构存在显著差异。 各湿地中均检出4种厌氧氨氧化菌属,其中Candidatus Kuenenia属在各湿地中占比最高(> 90%)。无碳源有植物湿地中厌氧氨氧化菌属相对丰度最高,且有植物湿地均高于无植物湿地,表明有植物无碳源的湿地系统更加有利于厌氧氨氧化的生长繁殖,而碳源的添加会对其起抑制作用,但植物在一定程度上可缓解碳源对厌氧氨氧化菌生长的抑制。各人工湿地系统的主要脱氮途径为厌氧氨氧化过程,且在不同程度上存在厌氧氨氧化与反硝化的协同耦合脱氮。Abstract: Based on different C/N ratio and plant configurations, four sets of vertical flow constructed wetlands (CWs) were constructed to treat high-nitrogen wastewater with low-carbon. The differences in characteristics of nitrogen pollutants treatment were studied, and the distribution characteristics of the main functional microorganisms and anaerobic ammonia oxidation (anammox) bacteria were analyzed. The results showed that when the influent TN concentration was 779.33 mg·L−1, the average removal efficiencies of TN in each CWs were 75.48%, 84.90%, 77.94% and 80.16%, respectively. By comparison, the CW treatment with plants and no carbon source showed the best nitrogen removal performance, and the CW treatment with carbon sources attained better nitrogen treatment performance than CW treatment with no carbon sources and no plants. Meantime, the CWs treatment with plants nitrogen treatment performance was better in than CWs without plants. At phylum level, there were significant differences in the microbial community structure in each CWs. Besides, there were significant differences in the structure of anammox bacteria among CWs. Four anammox bacteria genera were detected in each CWs, in which Candidatus Kuenenia accounted for the highest proportion (> 90%). The relative abundance of anammox bacteria genera in CW with plants and no carbon sources was the highest, and CWs with plants are higher than CWS without plants, indicating that CW system with plants and no carbon sources are more conducive to the growth and reproduction of anammox bacteria. The addition of carbon source inhibited the growth and reproduction of anammox bacteria, but plants could alleviate the inhibition of carbon source on the growth of anammox bacteria to a certain extent. The main nitrogen removal pathway of each CWs system was anammox process. And to varying degrees, there was synergistic coupling of anammox process and denitrification process for nitrogen removal.
-
重金属污染场地,指长期进行矿产冶炼、电镀加工、不锈钢生产、仪表机械制造等产业的重金属企业厂区,因没有采取严格规范的环保措施[1],产生废气沉降、废液灌溉和废弃物堆积[2]等,从而直接或间接污染土壤的工业场地,直接或间接造成的被重金属元素污染土壤的工矿业场地。重金属污染具有隐蔽性强、潜伏期长、污染后果严重等特点[3]。据2014年发布的《全国污染土壤调查状况公报》[4](简称公报),我国土壤的总超标率达到了16.1%。其中,工矿业废弃地的土壤环境问题突出,比较典型且污染严重的地块类型有3种:重污染企业用地的招标点位占调查总数的36.3%;工业废弃地的超标点位占调查总数的34.9%;工业园区的超标点位占调查总数的29.4%。土壤污染主要由无机污染物造成。该公报列举了铜、汞、镍、镉、铬、砷、铅、锌8种重金属元素作为无机污染物,并对其进行了详细说明。
随着我国“退二进三”的城市化发展及产业结构升级,城市的工业污染场地引起人们关注,其中重金属是主要污染源,影响周边居民健康,制约土地资源的二次利用[5]。近年来,我国针对重金属污染场地的污染修复技术发展日渐成熟,基于已有的技术研究,董家麟[6]以国内外大量文献综合分析各项技术的优缺点及应用范围;宋立杰等[7]介绍且分析了各项修复技术的实施方法;晁文彪[8]通过研究专利技术对重金属污染修复技术的发展趋势作出了展望。但目前大多数研究聚焦于重金属污染土壤的整体技术发展现状,单独对场地修复技术进行研究的综述类文献较少。本研究对重金属污染场地修复技术专利(简称专利)进行了计量分析,探究各项技术的发展趋势。
1. 数据来源与分析方法
本研究是基于国家知识产权局专利检索及分析网站进行的检索,设计检索式以关键词“重金属”和“土壤”为主,排除了农、矿、垃圾、肥、污泥等几个关键词,此外,在中国知识资源综合数据库(简称中国知网)以同一个检索式进行二次检索,以专利公开日为日期标准,截取时间段为2002年1月1日—2019年5月24日的专利,筛选排除了农田土壤修复、土壤重金属检测方法、土壤重金属来源分析及风险评估方法等专利,得到关于重金属污染场地修复技术的公开专利1 556项。梳理筛选得到的专利,将专利的名称、公开日、授权日、公开号、研究机构、发明人、技术类型、目标重金属、应用效果、优点等录入Excel并利用筛选功能、查找功能进行公开年份(分析年度变化均以此为标准)、目标重金属、技术类型的统计;利用Origin软件制图。文中2019年的专利数量为2019年1月1日—2019年5月24日的专利数量总和,采用文献计量学方法系统总结了重金属污染场地修复技术的现状、特点及发展趋势。
2. 数据分析
2.1 专利总量的统计与分析
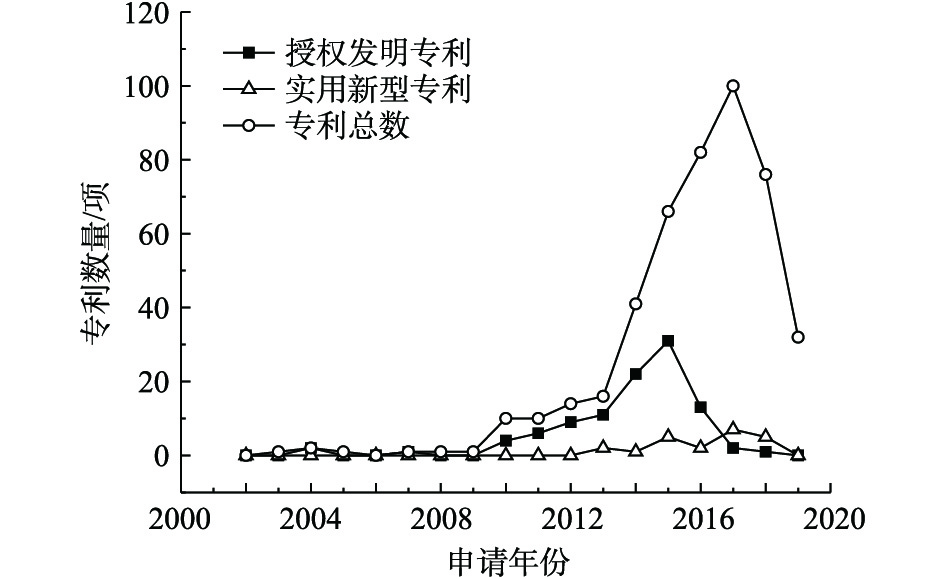
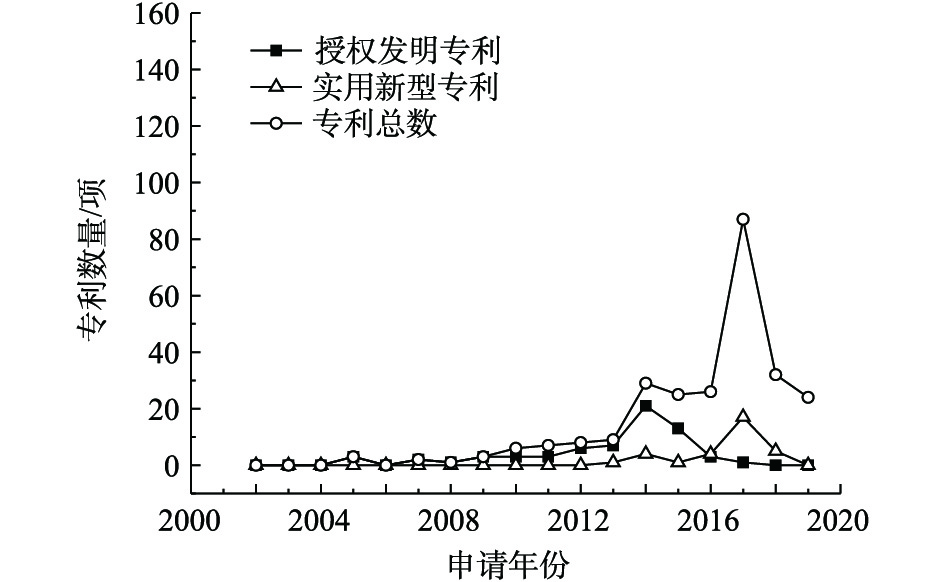
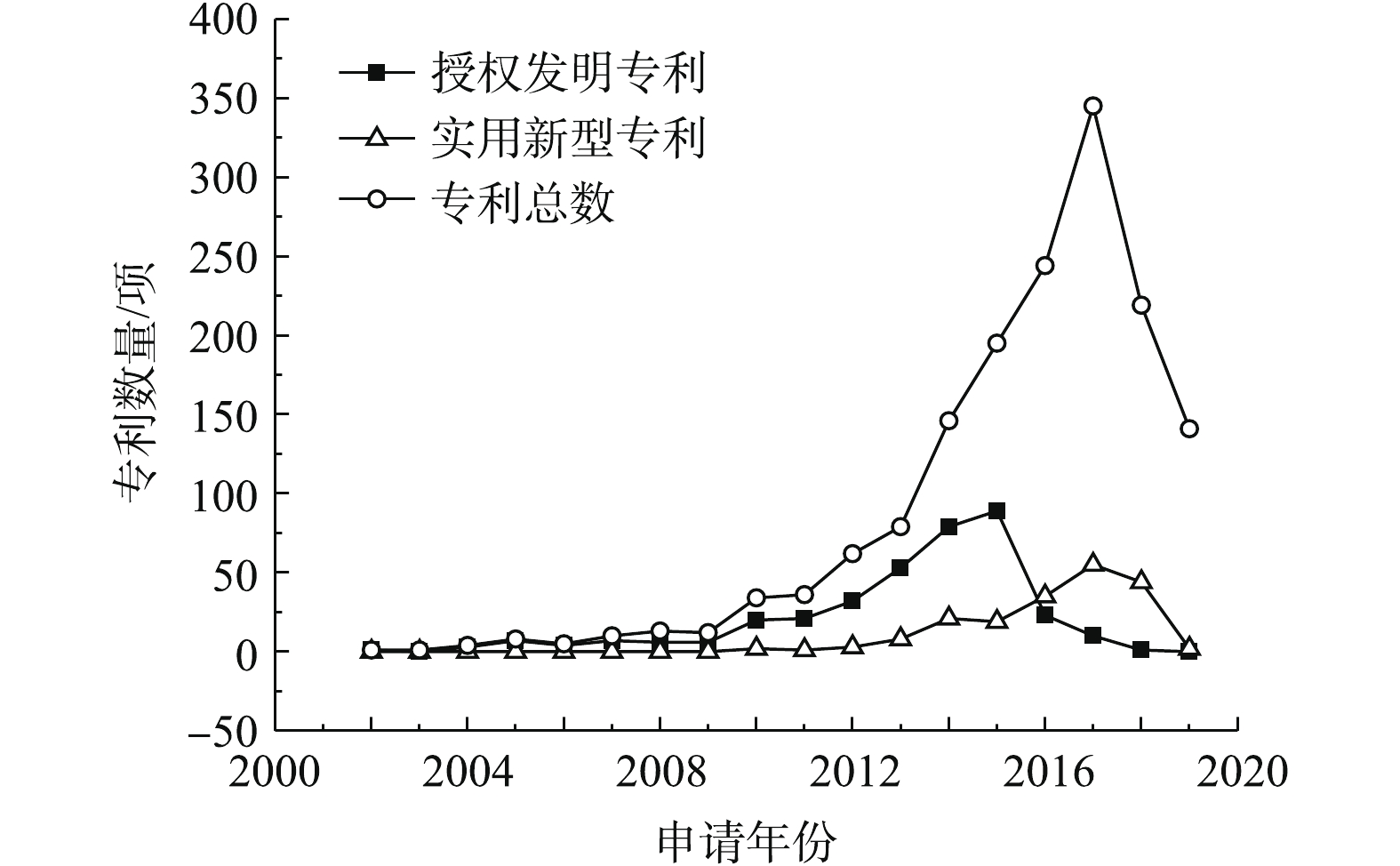
1)专利总量年度变化。2002—2019年公开的专利总数、授权发明专利数量及实用新型专利数量年度变化趋势见图1。公开专利的研究进程可以分为4个阶段。第1阶段为2002—2009年,该阶段公开的专利数量增长几乎停滞;第2阶段为2009—2013年,国内研究处于起步阶段,平均增长率约为16项·a−1;2013年进入了重金属污染场地修复的第3阶段,公开的专利数量快速增长,平均增长率为67项·a−1,尤其是2016—2017年,公开的专利数量增长了101项;第4阶段是2017年至今,公开的专利数量稍有回落,趋于稳定,2018年公开的专利数量为219项,2019年1—5月公开的专利数量为141项。第3阶段的公开专利数量增长可能因为2012年国家将土壤修复列入《“十二五”国家战略性新兴产业发展规划》(国发[2012]28号)的“先进环保产业发展路线图”,江苏省、广东省、重庆市、湖南省等地普遍关停了多家企业,开展了工矿企业搬迁原址场地土壤的修复[9],激发了土壤修复产业的活力。
在此期间,授权专利数量总体呈增长趋势,实用新型专利在2014年开始发展,专利数量占专利总数的比例逐渐增大,2014年占比为13.70%,2016年占比为14.64%,2017年占比为15.23%。这说明修复治理重金属污染场地的各项技术研究成熟后,研发具有自主知识产权、符合我国国情的重金属污染场地修复装置和设备也引起了人们重视。
2)专利总量区域分布和机构分布。申请公开专利数量占据全国前10名的区域见表1。由表1可知,江苏省、湖南省、广东省、北京市是主要的专利申请地区,被公开的申请专利数量分别为225、155、153和147项。这4个省份公开专利数量比较多,其主要原因为:第一,可能是因为当地科研机构多、科研氛围浓厚。全国范围内申请重金属污染场地修复技术专利的机构总数为611个,其中江苏省、湖南省、广东省、北京市的机构数量分别为71、58、72和64个,4个省份的机构数量总和近乎占据了全国研究机构总量的一半;第二,可能是当地的工业结构和产业需求促进了重金属污染土壤修复技术的发展。2011年,廖晓勇等[5]提出,我国达到工业规模级别的重金属冶炼企业及重金属压延加工企业数量众多,其中这类企业在江苏省的数量最多,浙江省、广东省、湖南省名列前茅,在产业结构调整和城市化发展的推动下,重金属污染场地的修复成了这些省份必须解决的问题,因此,修复技术的研究发展也相应较快。
表 1 区域专利数量前10名和区域机构数量前10名Table 1. Top 10 regions in patent numbers and top 10 regions in organizations区域 专利数量/项 区域 机构数量/个 江苏省 225 广东省 72 湖南省 155 江苏省 71 广东省 153 北京市 64 北京市 147 湖南省 58 上海市 92 上海市 38 浙江省 91 山东省 35 湖北省 86 湖北省 33 四川省 75 安徽省 32 山东省 66 浙江省 31 安徽省 57 四川省 29 3)公开专利的重金属研究对象及其适用技术分析。常见的重金属研究对象为镉、铅、铜、锌、铬、砷、汞、镍,其中研究镉、铬、铜、铅、锌等5种重金属的公开专利尤其多,用于处理镉、铅、铜、锌、铬、砷、汞、镍为主的污染场地的公开专利分别为324、257、186、163、140、91、77和58项,关于其余重金属污染场地修复技术的公开专利均不足50项,这说明以上列举的几种重金属的处理技术研究比较成熟。其中,铜、铬、锌、镍、锰等几种重金属的技术研究均是从生物修复技术起步,再扩展研究其他技术的应用。固化/稳定化技术在各类重金属污染场地的技术占比为31.48%~54.55%,可以普遍应用于各种重金属污染场地,适用性广;生物修复技术则常用于镉、铅、铜、锌、锰等几种重金属污染场地,技术占比为25.77%~41.18%,这主要取决于可筛选应用于不同重金属污染场地的生物资源丰富度;联合修复技术比较适用于铬污染场地,技术占比达到24.29%,对于一些其他重金属污染,如钴、锶、锑、铯、硒,这几种金属的联合修复技术研究也在2014年和2017年有所发展;淋洗修复技术常用于修复镉、铅、铜、锌、镍、砷、汞等重金属污染场地,技术占比均为10%~15%;物理修复技术在镍、锰、铬、铜、汞几种重金属污染场地应用得比较多,技术占比为9.09%~12.14%,主要是吸附修复技术和电动修复技术发展得比较好,热脱附修复技术主要应用在汞污染场地和砷污染场地中,磁分离修复技术则主要应用于铬污染场地、铜污染场地和铅污染场地。
2.2 各项技术的统计与分析
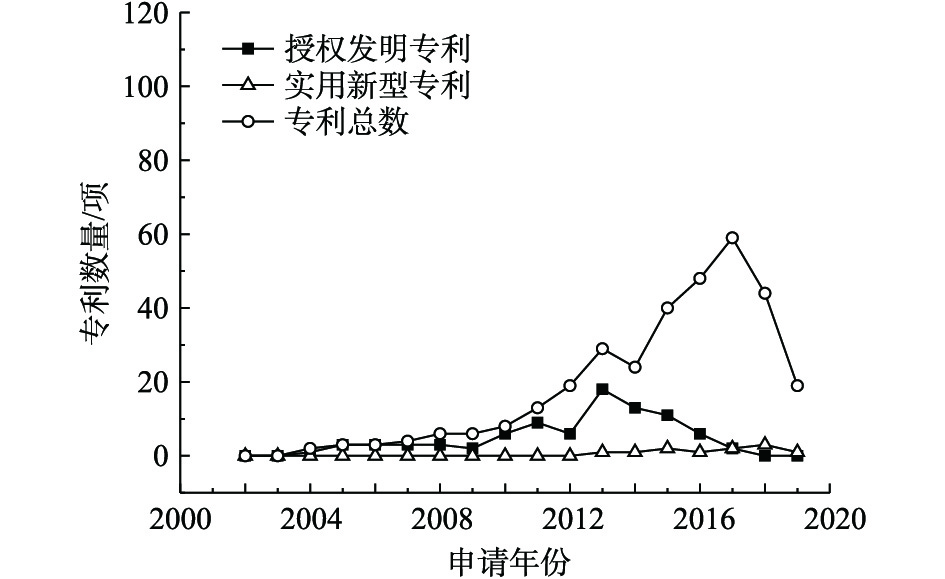
固化/稳定化技术修复重金属污染场地的公开专利总数、授权发明专利数量和实用新型专利数量统计结果见图2。该项技术在2010年才开始发展,整体呈现上升趋势,2013—2017年发展最为迅猛,近2年发展趋势有所回落。迄今为止,2015年固化/稳定化技术的授权发明专利数量最多,实用新型专利变化一直波动不大。固化/稳定化技术是一种高效、快速治理重金属污染场地的技术,该技术主要通过调节土壤pH、离子交换、螯合作用、络合作用、吸附作用等方式改变重金属离子的赋存形态,降低其生物有效性和生态毒性,从而达到固化/稳定化重金属的目的。近几年,固化稳定化技术的主要发展趋势是研发绿色环保固化/稳定化药剂、降低二次污染的风险。以往大量研究选用水泥、石灰等作为固化/稳定化修复药剂的主要成分进行修复,易出现土壤板结或过度石灰化的现象,既覆盖了重金属污染土壤的表面,影响治理效果,也不利于污染土壤的二次利用;部分研究选用化学药剂作为固化/稳定化修复药剂,易造成土壤盐碱化或者酸化、碱化土壤。因此,天然材料如秸秆、贝壳粉、木质素等材料的应用得到了重视。此外,国内重视以废治废、变废为宝的理念,对钢渣、废弃石膏、电石渣等进行利用,但由于易造成二次污染,材料逐渐由钢渣转变成具有生物可降解性的废料,如糖醛渣、农林废弃物、城市污水厂的污泥,降低成本和环境风险。
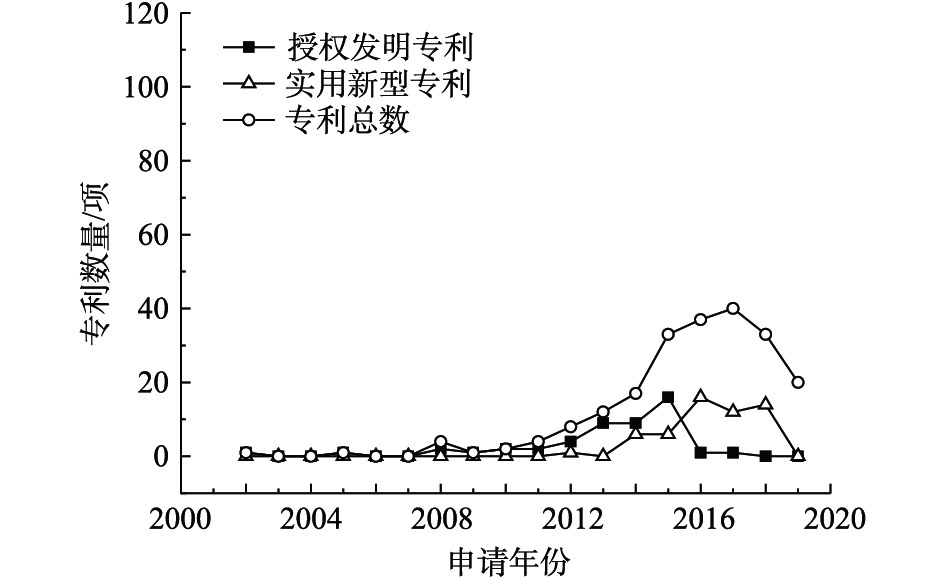
利用生物修复技术修复重金属污染场地的公开专利总数、授权发明专利数量和实用新型专利数量统计结果见图3。自2005年起,该项技术的专利总数基本呈现上升趋势,授权发明专利的数量在2013年后呈递减趋势,实用新型专利发展缓慢。生物修复技术是指利用自然界的生物资源,如通过微生物菌株、超累积植物和蚯蚓等吸收或移除土壤中的重金属,从而对重金属污染场地进行修复。该技术主要包括植物修复技术、动物修复技术、微生物修复技术、生物联合修复技术。目前,研究者偏向于发展动物和微生物辅助超累积植物吸收重金属为主的生物联合修复技术。近几年,研究重点主要包括3个方面:一是如何提高植物对重金属的吸收效率,常用方法包括施加生物炭基肥[10-12]、微生物复合菌剂[13-15]或动物[16-17]等;二是如何保证种子在重金属污染土壤中的存活率,现有的解决办法包括生态修复床[18-19]、人工包埋种子[20]、增加纤维丝隔离层[21-22]等;三是如何筛选生物资源,植物修复技术一般采用生物量大、生长迅速、重金属超累积容量大的植物,微生物修复技术则一般采用对重金属耐受性较高的菌株,例如芽孢杆菌和霉菌。
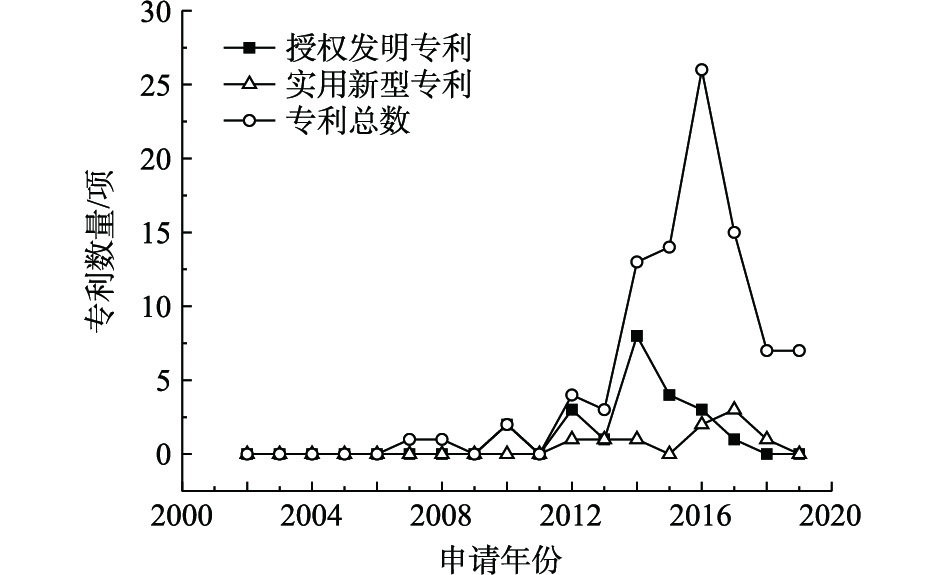
淋洗技术修复重金属污染场地的公开专利数量、授权发明专利数量和实用新型专利数量统计结果见图4。淋洗修复技术的公开专利在2011年开始发展,专利总数基本呈现上升趋势,2017年专利总数达到44项。淋洗修复技术主要是异位修复,须依赖装置设备,提高修复效果,降低淋洗剂用量[23-27]。其核心在于淋洗剂的选用和实际场地的实施,这2个要素决定了淋洗技术能否在重金属污染场地修复领域中广泛被应用。淋洗常选用酸溶液作为淋洗剂,但EDDS、聚环氧琥珀酸、柠檬酸、苹果酸等可生物降解的酸溶液价格昂贵,因此,常配合水、硝酸或盐酸、氯化铁水溶液、吐温80等其他淋洗剂使用,从而降低环境风险和药剂成本;场地实施一般利用振荡进行淋滤,为加快淋洗速度,郭红岩等[28]和郭军康等[29]开始研究通过微波/超声波等促进淋滤过程,目前,郭军康等[30]已同步设计了超声波强化萃取装置,但尚未有应用实例。
物理修复技术是指利用阻隔、吸附、电迁移、电泳、电渗析、热脱附、磁场效应等物理原理对重金属污染场地进行修复的技术,主要包括电动修复技术、吸附修复技术、阻隔修复技术、磁分离修复技术和热脱附修复技术。
电动修复技术的公开专利数量、授权发明专利数量和实用新型专利数量统计结果见图5。2017年之前电动修复技术的公开专利数量总体呈上升趋势,2017年达到最大值27项;授权发明专利数量有所波动,2017年前实用新型专利的数量呈持续上升的趋势。目前,电动修复技术的研究热点主要是电动装置的创新,既包括电极、电解液等的材料创新,也包括电动装置本身的结构组成创新。
吸附修复技术的公开专利数量、授权发明专利数量和实用新型专利数量统计结果见图6。2014年授权发明专利达到峰值,2015年实用新型专利开始发展。目前,研究比较成熟的有海藻酸钠制备吸附板和吸附微球[31-33]、农业废弃物制备生物炭[34-36]等,技术重点主要是对吸附材料进行改性提高其吸附效率,增大吸附容量;常用的原材料一般是黏土矿物,如凹凸棒土、膨润土、沸石、二元水滑石等,也有有机高分子材料,例如树脂、凝胶球等。
其他物理修复技术还包括磁分离修复技术、热脱附修复技术和阻隔修复技术等。磁分离修复技术只能应用于磁性重金属,2014年后,每年的公开专利数量维持在4~5项,2018年、2019年的公开专利总数均为6项;热脱附修复技术从2014年开始发展,整体呈缓慢的上升趋势,主要适用于含汞、砷的重金属污染场地,应用范围狭窄、耗能较高,研究重点是不同类型的热脱附装置[37-39],2014年开始发展,整体呈缓慢上升趋势;阻隔修复技术的公开专利数量保持波动。
联合修复技术的公开专利数量、授权发明专利数量和实用新型专利数量统计结果见图7,该项技术从2013年开始发展,公开专利数量总体呈现上升趋势,2017年,达到最大值为87项,主要集中为研究原位修复重金属污染的阻隔装置[40-42];授权发明专利的数量在2014年较多;实用新型专利则在2017年达到峰值。
联合修复技术指联合1种或2种以上技术进行重金属污染场地的修复治理,通常是以固化/稳定化技术、生物修复技术和淋洗修复技术为主,物理修复技术为辅进行。电动修复技术[43-46]、吸附修复技术[47-49]和热脱附修复技术[50-53]均常与淋洗修复技术联用,通过吸附等方式富集淋出液中的重金属后,淋出液可以进行回用,此外,其也常与生物技术联用,加快植物吸收重金属的速率;磁分离技术逐渐受到重视,该项技术配合固化/稳定化技术应用于重金属污染场地,可有效移除场地土壤中的重金属[54-55],配合淋洗技术使用则可以快速吸附淋出液的重金属,使得淋出液可以回用[56-59]。
3. 结论
1)近几年,研究者更加重视相关装置的发展,直接反映为实用新型专利的数量占比显著提高。
2)国内重金属污染场地修复技术的公开专利发展可分为4个阶段,2013—2017年发展迅猛,平均增长率为66项·a−1,近2年发展渐缓。其中,江苏省、北京市、广东省、湖南省的科研机构众多,公开专利总数也比较多。
3)综合适用对象范围、研究机构数量、公开专利数量及年份变化趋势等因素进行评估,可以看出,目前,发展较好的重金属污染场地修复技术依次为固化/稳定化技术、淋洗修复技术、物理修复技术,以废治废、降低成本仍然是研究热点。
-
表 1 目标基因定量引物序列及退火温度
Table 1. Primer sequences and annealing temperature for target genes of q-PCR
目标基因Target gene 引物Primers 引物序列 (5′-3′)Primer sequences 退火温度/℃Annealing temperature 引物长度/bp Primer length 参考文献Reference Anammox 16S rRNA Amx368f TTCGCAATGCCCGAAAGG 56 470 [12] Amx820r AAAACCCCTCTACTAGTGCCC 表 2 硝化-反硝化相关菌属存在特征
Table 2. Characteristics of nitrification-denitrification bacteria related genus
菌属 Bacterial genera 相对丰度/% Relative abundance T1 T2 T3 T4 反硝化相关菌属 Flavobacterium 2.01 4.06 7.01 8.06 Thermomonas 0.10 1.47 1.17 1.79 Enterobacter 0.00 0.00 0.00 0.00 Pseudomonas 0.16 0.17 0.17 0.20 Hydrogenophaga 0.07 0.02 0.07 0.02 Aeromonas 0.00 0.01 0.00 0.01 Janthinobacterium 0.02 0.01 0.01 0.02 Acidovorax 0.12 0.09 0.19 0.06 总计 2.48 5.83 8.63 10.17 硝化相关菌属 Nitrosomonas 0.12 0.13 0.23 0.70 Nitrosovibrio 0.00 0.01 0.01 0.01 Nitrospira 0.04 0.03 0.05 0.05 Nitrosococcus 0.03 0.03 0.01 0.01 Nitrolancea 0.08 0.08 0.04 0.03 Nitrobacter 0.17 0.28 0.07 0.08 总计 0.44 0.56 0.42 0.89 -
[1] WANG Z, HUANG M L, QI R, et al. Enhancing nitrogen removal via the complete autotrophic nitrogen removal over nitrite process in a modified single-stage tidal flow constructed wetland [J]. Ecological Engineering, 2017, 103: 170-179. doi: 10.1016/j.ecoleng.2017.04.005 [2] WU S B, KUSCHK P, BRIX H, et al. Development of constructed wetlands in performance intensifications for wastewater treatment: A nitrogen and organic matter targeted review [J]. Water Research, 2014, 57: 40-55. doi: 10.1016/j.watres.2014.03.020 [3] DĄBROWSKI W, KAROLINCZAK B, MALINOWSKI P. Application of SS-VF bed for the treatment of high concentrated reject water from autothermal termophilic aerobic sewage sludge digestion [J]. Journal of Ecological Engineering, 2018, 19(4): 103-110. doi: 10.12911/22998993/89663 [4] 秦榕, 宋佳宇, 齐碧薇, 等. 厌氧氨氧化反应器菌群动态演替分析[J]. 环境科学与技术, 2020, 43(增刊1): 23-28. QIN R, SONG J Y, QI B W, et al. Dynamic succession analysis of bacteria in anaerobic ammonia oxidation reactor for refining wastewater treatment[J]. Environmental Science & Technology, 2020, 43(Sup 1): 23-28(in Chinese).
[5] 吕露遥, 杨永哲, 张雷, 等. 多级垂直潮汐流人工湿地厌氧氨氧化脱氮研究 [J]. 水处理技术, 2019, 45(10): 114-120. LV L Y, YANG Y Z, ZHANG L, et al. Study on nitrogen removal by anaerobic ammonium oxidation in multi-stage vertical tidal flow constructed wetlands [J]. Technology of Water Treatment, 2019, 45(10): 114-120(in Chinese).
[6] CHEN D Y, GU X S, ZHU W Y, et al. Denitrification- and anammox-dominant simultaneous nitrification, anammox and denitrification (SNAD) process in subsurface flow constructed wetlands [J]. Bioresource Technology, 2019, 271: 298-305. doi: 10.1016/j.biortech.2018.09.123 [7] VAN DER STAR W R, MICLEA A I, VAN DONGEN U G, et al. The membrane bioreactor: A novel tool to grow anammox bacteria as free cells [J]. Biotechnology and Bioengineering, 2008, 101(2): 286-294. doi: 10.1002/bit.21891 [8] YUAN C B, ZHAO F C, ZHAO X H, et al. Woodchips as sustained-release carbon source to enhance the nitrogen transformation of low C/N wastewater in a baffle subsurface flow constructed wetland [J]. Chemical Engineering Journal, 2020, 392: 124840. doi: 10.1016/j.cej.2020.124840 [9] WANG X J, YANG R L, GUO Y, et al. Investigation of COD and COD/N ratio for the dominance of anammox pathway for nitrogen removal via isotope labelling technique and the relevant bacteria [J]. Journal of Hazardous Materials, 2019, 366: 606-614. doi: 10.1016/j.jhazmat.2018.12.036 [10] 夏艳阳, 崔理华, 黄小龙. 污水碳源对复合垂直流-水平流人工湿地脱氮效果的影响 [J]. 环境工程学报, 2017, 11(1): 638-644. doi: 10.12030/j.cjee.201509223 XIA Y Y, CUI L H, HUANG X L. Effect of internal carbon source supplement on nitrogen removal in integrated vertical-flow and horizontal-flow constructed wetland [J]. Chinese Journal of Environmental Engineering, 2017, 11(1): 638-644(in Chinese). doi: 10.12030/j.cjee.201509223
[11] 国家环境保护总局. 水和废水监测分析方法[M]. 第4版. 北京: 中国环境出版社, 2002: 258-282. China Environmental Protection Administration. Methods for monitoring and analysis of water and wastewater[M]. 4th ed. Beijing: China Environmental Science Press, 2002: 258-282 (in Chinese) .
[12] SCHMID M C, MAAS B, DAPENA A, et al. Biomarkers for in situ detection of anaerobic ammonium-oxidizing (anammox) bacteria [J]. Applied and Environmental Microbiology, 2005, 71(4): 1677-1684. doi: 10.1128/AEM.71.4.1677-1684.2005 [13] LIN Y F, JING S R, WANG T W, et al. Effects of macrophytes and external carbon sources on nitrate removal from groundwater in constructed wetlands [J]. Environmental Pollution, 2002, 119(3): 413-420. doi: 10.1016/S0269-7491(01)00299-8 [14] COBAN O, KUSCHK P, KAPPELMEYER U, et al. Nitrogen transforming community in a horizontal subsurface-flow constructed wetland [J]. Water Research, 2015, 74: 203-212. doi: 10.1016/j.watres.2015.02.018 [15] 吕纯剑, 高红杰, 宋永会, 等. 潮汐流-潜流组合人工湿地微生物群落多样性研究 [J]. 环境科学学报, 2018, 38(6): 2140-2149. LÜ C J, GAO H J, SONG Y H, et al. Microbial community diversity in the combined tide flow-subsurface flow constructed wetland [J]. Acta Scientiae Circumstantiae, 2018, 38(6): 2140-2149(in Chinese).
[16] LI X R, DU B, FU H X, et al. The bacterial diversity in an anaerobic ammonium-oxidizing (anammox) reactor community [J]. Systematic and Applied Microbiology, 2009, 32(4): 278-289. doi: 10.1016/j.syapm.2009.03.002 [17] ZHANG Z, LIU S. Insight into the overconsumption of ammonium by anammox consortia under anaerobic conditions [J]. Journal of Applied Microbiology, 2014, 117(6): 1830-1838. doi: 10.1111/jam.12649 [18] 张燕, 刘雪兰, 王月明, 等. 中国规模化畜禽养殖污水处理中人工湿地的研究进展 [J]. 环境科学与技术, 2016, 39(1): 87-92. ZHANG Y, LIU X L, WANG Y M, et al. Research progress of constructed wetland treating intensive livestock and poultry wastewater in China [J]. Environmental Science & Technology, 2016, 39(1): 87-92(in Chinese).
[19] 廖新俤, 骆世明. 人工湿地对猪场废水有机物处理效果的研究 [J]. 应用生态学报, 2002, 13(1): 113-117. doi: 10.3321/j.issn:1001-9332.2002.01.025 LIAO X D, LUO S M. Treatment effect of constructed wetlands on organic matter in wastewater from pig farm [J]. Chinese Journal of Applied Ecology, 2002, 13(1): 113-117(in Chinese). doi: 10.3321/j.issn:1001-9332.2002.01.025
[20] 陈永华, 吴晓芙, 陈明利, 等. 人工湿地污水处理系统中植物套种模式根际微生物多样性研究 [J]. 环境科学, 2011, 32(8): 2397-2402. CHEN Y H, WU X F, CHEN M L, et al. Analysis of microorganism species diversity in plant intercropping models in a wetland system constructed for treatment of municipal sewage [J]. Environmental Science, 2011, 32(8): 2397-2402(in Chinese).
[21] PICHINOTY F, BIGLIARDI-ROUVIER J, MANDEL M, et al. The isolation and properties of a denitrifying bacterium of the genus Flavobacterium [J]. Antonie Van Leeuwenhoek, 1976, 42(3): 349-354. doi: 10.1007/BF00394134 [22] YE J, ZHANG P Y, SONG Y H, et al. Influence of operational mode, temperature, and planting on the performances of tidal flow constructed wetland [J]. Desalination and Water Treatment, 2016, 57(17): 8007-8014. doi: 10.1080/19443994.2015.1055310 [23] ZHANG P F, PENG Y K, LU J L, et al. Microbial communities and functional genes of nitrogen cycling in an electrolysis augmented constructed wetland treating wastewater treatment plant effluent [J]. Chemosphere, 2018, 211: 25-33. doi: 10.1016/j.chemosphere.2018.07.067 [24] SHEN J P, ZHANG L M, DI H J, et al. A review of ammonia-oxidizing bacteria and archaea in Chinese soils [J]. Frontiers in Microbiology, 2012, 3: 296. [25] ZENG L P, TAO R, TAM N F Y, et al. Differences in bacterial N, P, and COD removal in pilot-scale constructed wetlands with varying flow types [J]. Bioresource Technology, 2020, 318: 124061. doi: 10.1016/j.biortech.2020.124061 [26] ZHU G B, WANG S Y, FENG X J, et al. Anammox bacterial abundance, biodiversity and activity in a constructed wetland [J]. Environmental Science & Technology, 2011, 45(23): 9951-9958. -






 下载:
下载: