-
罗丹明B(RhB)又称玫瑰红B,水溶液呈碱性,性质稳定,具有致畸、致癌和诱变性,广泛应用于有色玻璃,纺织、涂料等行业。由于其难以被生物降解,若富含RhB的废水未经有效处理直接排放,将会对人类身体健康和环境造成潜在危害[1-2]。近年来,研究者们越发关注过硫酸盐(PS)高级氧化技术,这是因为PS被活化后能产生强氧化性的
SO−4⋅ ,可以无选择性地降解水中的有机污染物,具有反应高效,无污染等特点[3]。截至目前,已知活化PS的方式有:热活化、紫外活化、电活化、过渡金属活化和炭活化等[3-4]。生物炭是生物质残渣(植物、果壳、木屑等)限氧热解产生的一种炭材料[5],与活性炭结构相似,利用其活化PS,不仅成本更低,且自身的吸附性能可以协同去除水中污染物[6]。而作为一种重要的农业废弃物资源,目前以动物粪便为原料制备生物炭的研究较少,随着我国规模化养殖业迅速发展,畜禽粪便的不合理排放已经成为农业面源污染的主要来源,需要迫切的解决其带来的环境污染问题[7-8]。
因此,本研究以RhB为目标污染物,选用猪粪为原料制备生物炭,利用“硫酸/氢氧化钾+超声波+高温活化”进行改性处理,对材料进行表征分析,探寻其活化PS去除RhB的最优体系及影响因素,以期获得一种低成本的废水处理方法,达到以废治废的目的。
-
主要试剂与用品:聚偏氟乙烯(PVDF)微孔膜,浓硫酸,氢氧化钠,过硫酸钾(PS),罗丹明B,均为分析纯购自阿拉丁试剂(上海)有限公司;猪粪采自广州市某养猪场;实验室用水为二次去离子水。
主要仪器:扫描电镜(SEM,蔡司Sigma 300);比表面积分析仪(micromeritics,ASAP 2460);傅里叶红外光谱仪(FTIR,德国布鲁克AVANCE NEO);X射线衍射光谱(XRD,日本理学SmartLab);紫外-可见分光光度计(Ultra-3660,北京普源精电科技有限公司);数控超声波清洗器(KQ-500DE,昆山超声仪器有限公司);水浴恒温振荡器(THZ-82A,常州澳华仪器有限公司)。
-
生物炭制备:猪粪采自广州市某养猪场,风干72 h,并拣出石粒、树枝等杂质,捣碎后过60目筛,装于坩埚中盖好,放入马弗炉中,选择在限氧条件下以10 ℃·min−1的升温梯度加热至700 ℃恒温120 min,热解产物冷却至室温再研磨过100目筛,用去离子水清洗,滤后于烘箱中105 ℃烘干,装入棕色广口瓶中,即制得猪粪生物炭,标记为:BC。
生物炭改性:对文献[9]方法做出优化。取制备好的BC于锥形瓶中,每5 g生物炭分别加入200 mL体积分数5%的氢氧化钾或硫酸溶液,25 ℃下,在水浴恒温振荡器中以120 r·min−1的转速改性24 h,后在功率为200 W的超声波下改性30 min,调节溶液pH至中性,结束后经离心机固液分离,用去离子水和无水乙醇反复清洗后倒出上清液,于160 ℃活化24 h,即得到改性后的猪粪制生物炭。氢氧化钾和硫酸改性后的生物炭分别标记为:KBC和SBC。
-
采用扫描电子显微镜(SEM,含EDS能谱分析)表征材料的表面形貌并分析元素的分布情况;比表面积分析仪(BET)对材料表面特性进行分析;傅里叶红外光谱仪(FTIR)分析材料表面官能团;X射线衍射仪(XRD)分析材料晶体结构。
-
探究3种生物炭BC、KBC、SBC对RhB的吸附特性。取200 mL初始浓度10 mg·L−1的RhB于250 mL锥形瓶中,加入0.05 g生物炭,调初始pH值至7,在160 r·min−1的频率下振荡14 h。在设定时间(5、10、20、30、60、90、120、240、360、480、600、720、840 min)取上清液经0.22 μm滤膜过滤,测定RhB的质量浓度。根据式1计算吸附容量,根据式(2、3、4)计算一级,二级吸附动力学和颗粒扩散模型的拟合参数
式中,
Qe (mg·g−1),Qt(mg·g−1),C0 (mg·L−1),Ce (mg·L−1),V(L),W(g),di,k1 [g·(mg·h)−1],k2 [g·(mg·h)−1],kid[mg·g−1·h−1/2]分别为平衡时的吸附容量,时间t(min)时的吸附容量,溶液的初始浓度,溶液的平衡浓度,溶液体积,生物炭投加量,边界层厚度,一级速率常数,二级速率常数和粒子扩散速率常数。 -
取200 mL初始浓度10 mg·L−1的RhB于250 mL锥形瓶中,加入一定量生物炭和PS,调节初始pH,在160 r·min−1的频率下反应120 min,分别在设定时间(5、10、20、30、60、90、120 min)取上清液用0.22 μm滤膜过滤,测定RhB的吸光度,计算质量浓度和去除率(式5)。在生物炭重复利用实验中,同时做多组平行以保证使用后的生物炭足量,将反应终点溶液经0.45 μm抽滤再经冷冻干燥即可得到使用后的生物炭,避光封闭保存以重复使用。
式中,R是RhB去除率(%),C为t时刻RhB的浓度(mg·L−1),C0为RhB的初始浓度(mg·L−1)。
-
用紫外-可见分光光度计(Ultra-3660,北京普源精电科技有限公司RIGOL)在RhB可见光最大吸收波长552 nm处测定其吸光度。
-
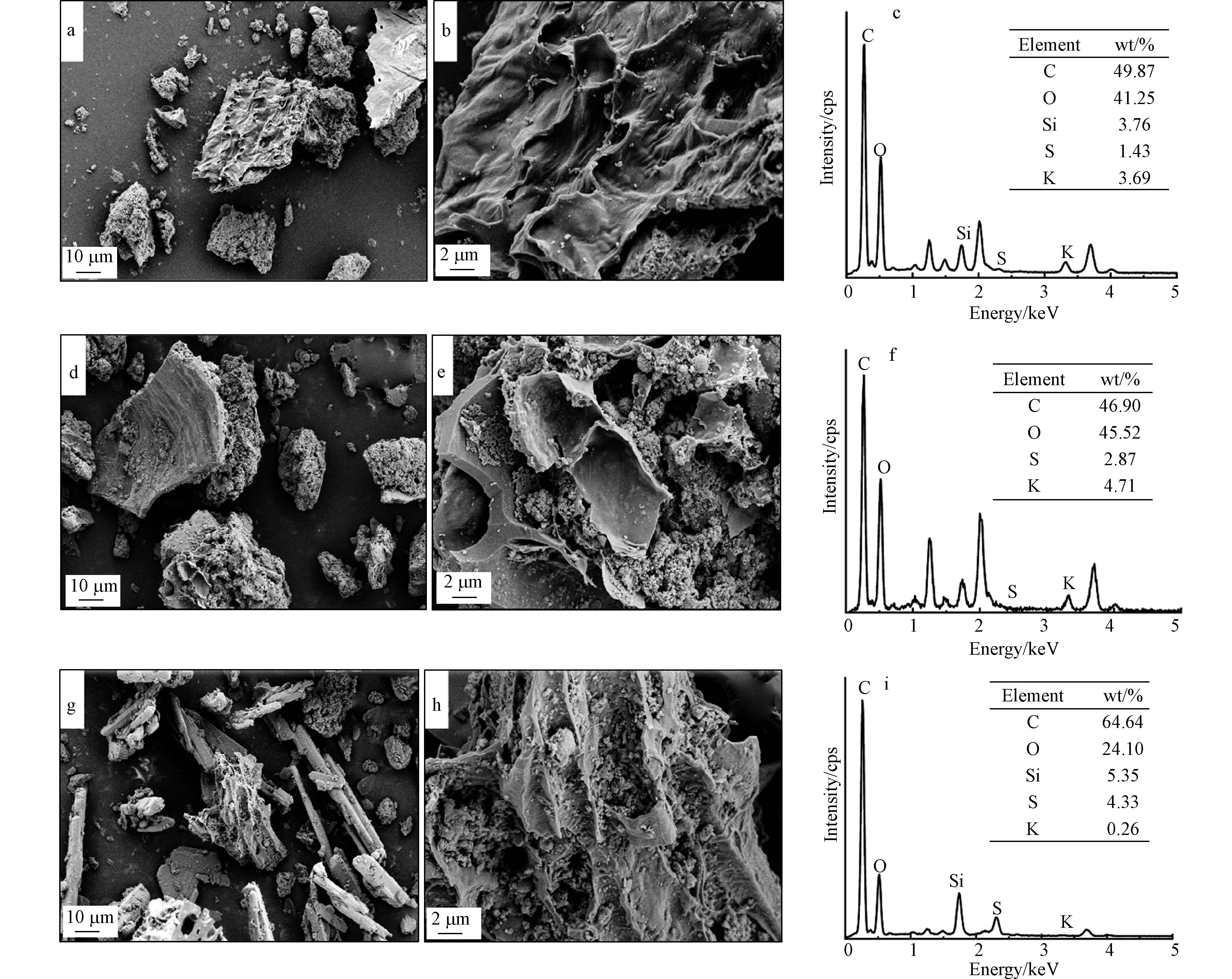
由图1可见,3种生物炭表面呈现凹凸不平状态,均为不规则多孔结构,这是因为有机质在高温热解(700 ℃)中生成CO2和H2O等气体,有利于形成孔隙[10];其中,KBC和SBC的多孔结构更明显,这是因为强酸强碱具有氧化性,对生物炭表面侵蚀在一定程度上具有造孔作用,原本堵塞孔道的灰分也会被清洗。通过EDS能谱结果表明,猪粪制生物炭C和O的含量较高,有一定量的Si,经改性后,KBC和SBC中元素K和S的含量明显增加。
-
BET(比表面积分析)是衡量多孔材料的重要参数,结果如表1所示,KBC和SBC的比表面积较改性前分别提高了4.2倍和6倍,总孔体积提高了1.9倍和3.7倍,微孔孔容提高了4.2倍和2.4倍,3种生物炭均属于介孔材料。据文献[9]报道,以猪粪为原料,在700 ℃制备生物炭,用“硫酸+超声波”改性90 min后,生物炭比表面积为46.250 m2·g−1,这是因为硫酸的氧化性和超声波的空化作用协同有助于疏通生物炭堵塞的孔隙,产生空洞结构,但是,比表面积远小于本文制备的SBC(106.611 m2·g−1)。结合EDS分析,这或许是因为本实验生物炭更长时间(24 h)浸润硫酸溶液后再经活化(160 ℃)能更好的掺杂硫元素,这种杂原子的修饰与碳原子会形成局部电势密度差,产生表面缺陷,形成大比表面积,可以为PS提供更多的反应活性位点[11]。
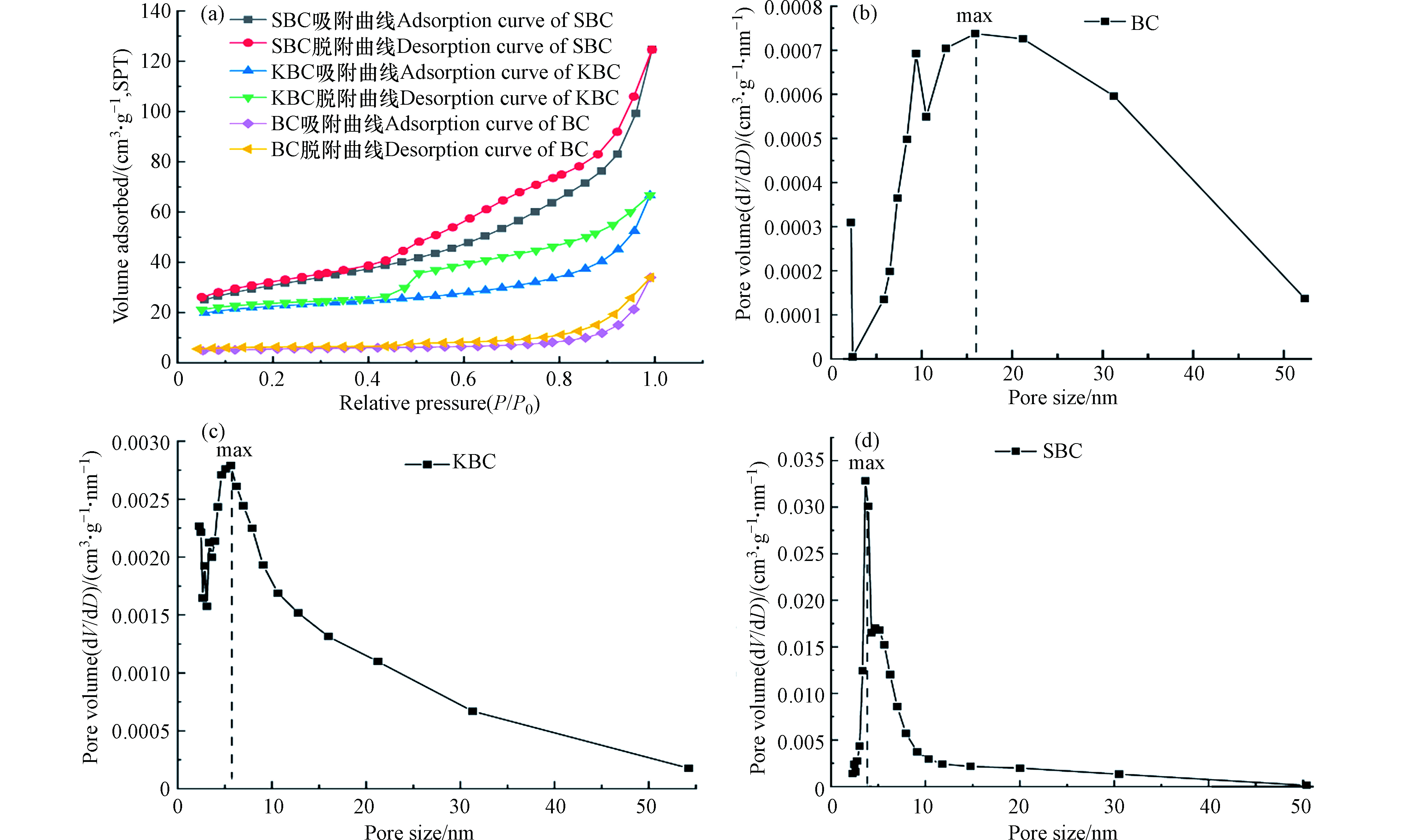
图2为3种生物炭的氮气吸附-脱附和孔径分布曲线。结果表明,3种生物炭的吸附-脱附等温线均不重合且脱附曲线均位于吸附曲线上方,产生吸附滞后,存在H2型滞后环,这种滞后现象与孔的形状及其大小有关。3种生物炭在吸附压力较高区间(P/P0>0.8)气体吸附线急剧上升,N2吸附量速率明显加快,发生中孔毛细凝聚,表现出Ⅵ型N2吸附-脱附等温线[12],KBC和SBC的滞后环较宽,说明生物炭孔大小分布更宽且含有更多的介孔[13]。BC,KBC和SBC的孔径分别在10—20 nm,5—10 nm和2.5—8 nm间较为密集,最可几孔径分别为:15.898 nm、5.605 nm和4.658 nm,均大于RhB的分子尺寸(1.59 nm×1.18 nm×0.56 nm)[14],这样利于RhB的吸附和扩散。
-
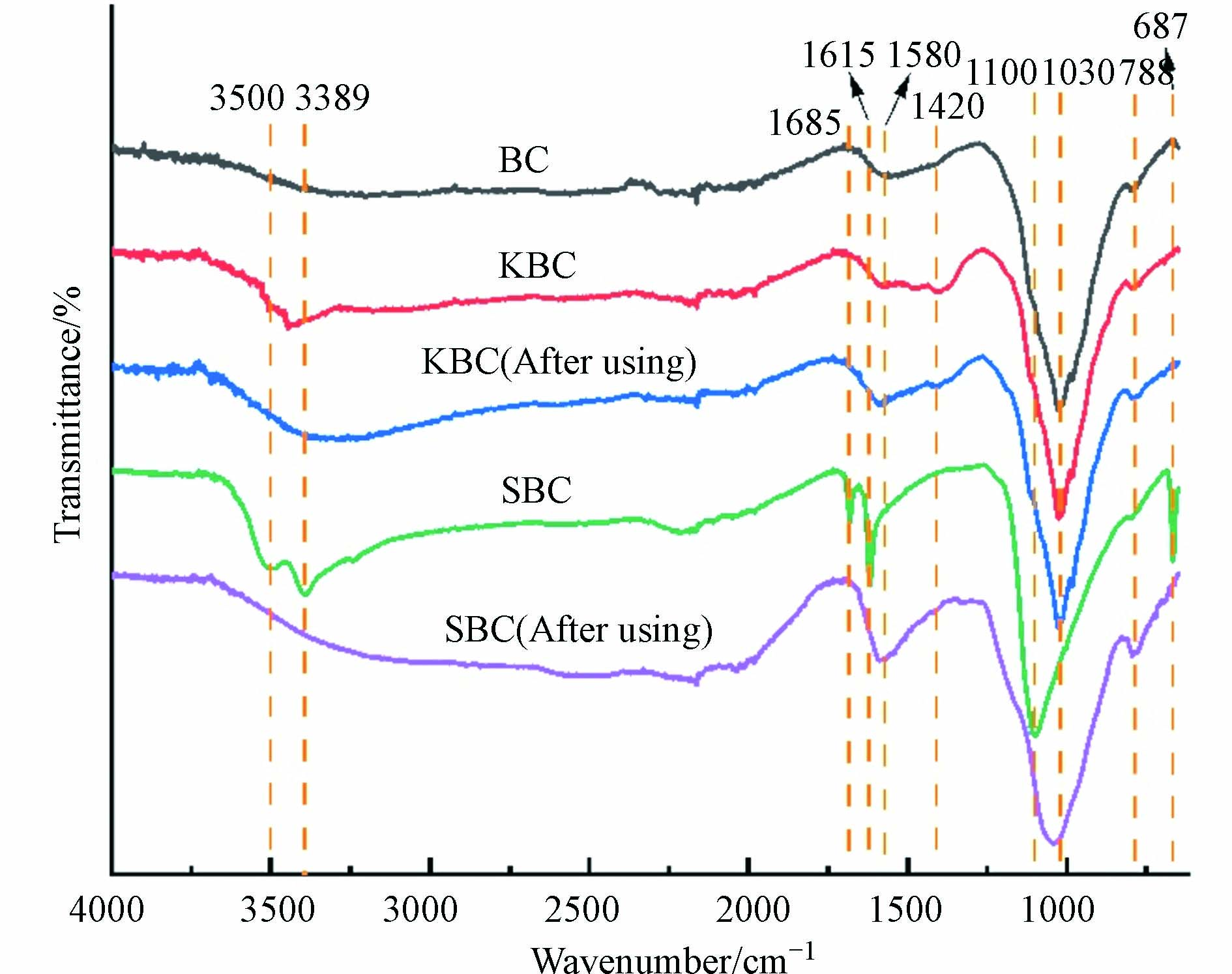
图3是生物炭活化PS降解RhB前后的FTIR曲线,主要的特征峰为:3750—3300 cm−1处吸收峰是醇酚羟基(—OH)振动[15],1700—1600 cm−1处为C=O或C=C振动[16-17];1420 cm−1振动归结于脂肪族的C—H键[18];1100—1030 cm−1处有很强的伸缩振动峰,归结于C—O键或Si—O[19-20];810—750 cm−1处的伸缩振动归结于芳香族的C—H键[21];687 cm−1处存在S—H振动[13]。
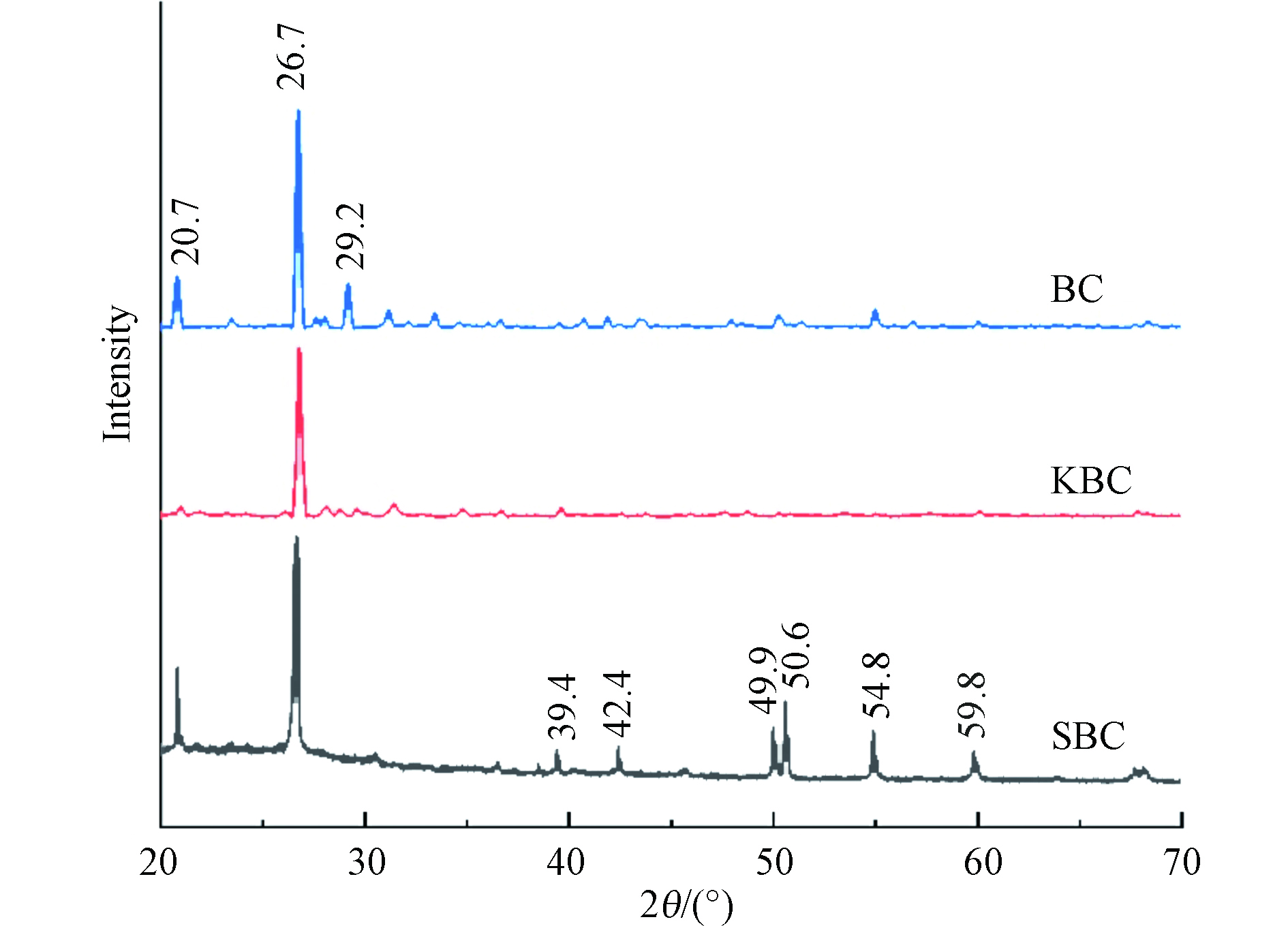
综合EDS和FTIR分析,猪粪制生物炭具有芳香性,含有硅酸盐;改性后含有丰富的C—C、C—O、C=O键以及更多的官能团;SBC比KBC具有更多的官能团种类和数量,活化PS后SBC和KBC的含氧官能团(—OH)的伸缩振动峰强度均明显减弱,并且SBC的官能团S-H的伸缩振动峰也消失,说明这些官能团参与到了活化PS降解RhB的过程。图4为材料的XRD图像,为了确定材料的晶型结构,通过Jade 6.5进行物相检索,猪粪制生物炭(BC)表面主要存在SiO2(PDF卡片号46—1045)和CaCO3(PDF卡片号41—1475),改性后生物炭的这些衍射峰强度明显减弱,说明SiO2和CaCO3可能在改性后消失,而SBC伴随着新的衍射峰出现,这些归因于CaSO4(PDF卡片号37—0184),可能是因为H2SO4改性与CaCO3反应所致。
-
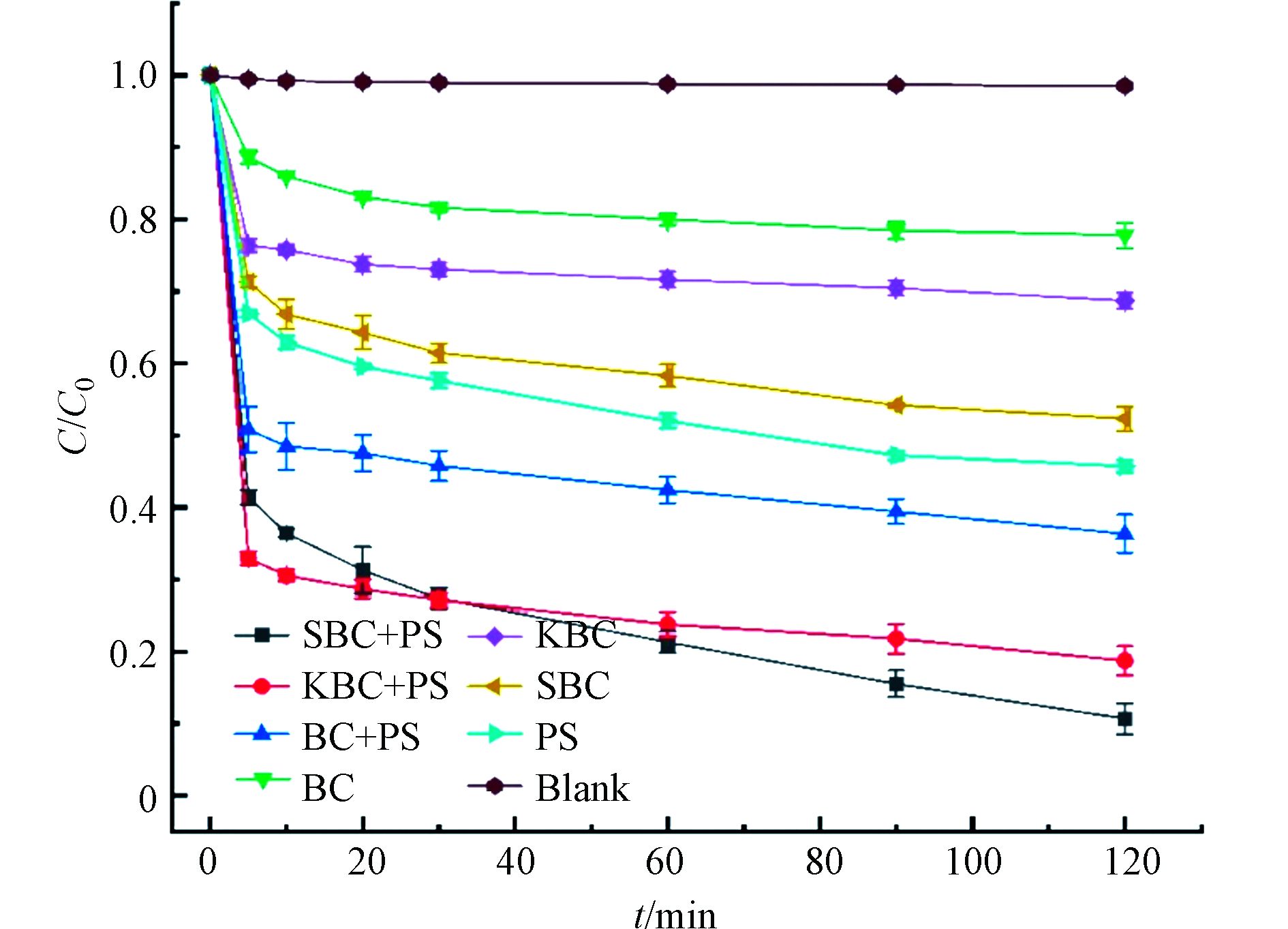
为了考察生物炭催化PS去除RhB的性能,本文探究了相同反应条件下不同体系对RhB的去除效果,结果如图5所示。体系的去除效果由低到高依次为:Blank(1.5%)、BC(22.22%)、KBC(31.30%)、SBC(47.66%)、PS(54.26%)、BC+PS(63.63%)、KBC+PS(81.25%)、SBC+PS(89.30%)。由结果分析,空白对照组RhB的去除率是1.5%,这是因为实验室光源条件下RhB被少量光解;单独的生物炭对RhB有吸附性,SBC的吸附效果最好;单一的PS可以氧化降解RhB,这是因为PS会水解产生少量的
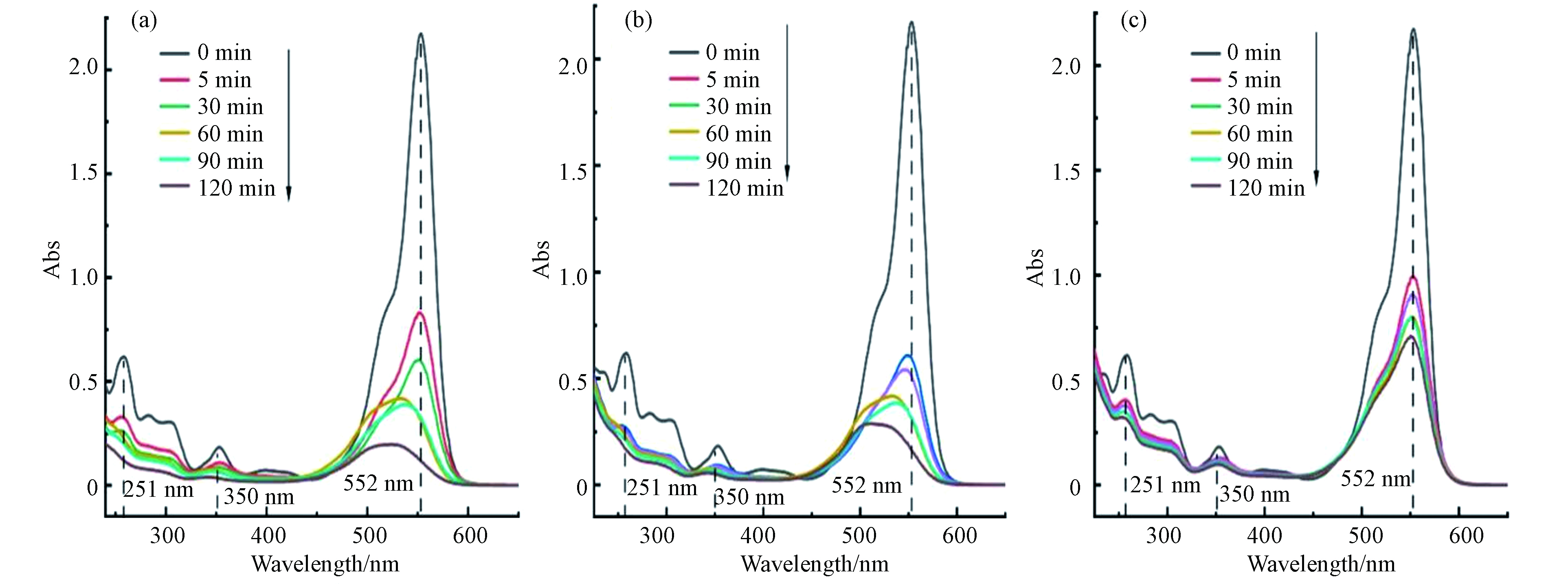
SO−4⋅ [22](式6);“生物炭/过硫酸盐”体系能明显提高对RhB的去除率,因为生物炭的表面官能团可以有效活化PS产生SO−4⋅ [23](式7),体系“SBC+PS”的去除效果最好,结合BET和FTIR分析,这是由于SBC具有更大的比表面积和更丰富的官能团。为了探究RhB的降解变化,不同反应时间的紫外可见吸收光谱见图6。从0 min的谱图可以看到,RhB的主要特征峰位于251、350、552 nm处。其中,250—350 nm的吸收峰归结于芳香环,350 nm处的吸收峰为共轭双键[24],峰强随着反应时间的进行而明显减弱,这说明芳香环和共轭双键被破坏,552 nm的特征峰表现出相同的趋势,而且波长发生蓝移现象,说明RhB发生了脱乙基化反应并伴随着新物质的产生[25]。因此,全波长扫描表明,随着反应的进行,RhB的脱色团,取代基和分子骨架不同程度的被破坏,这说明“生物炭/过硫酸盐”体系中RhB的去除存在吸附和降解2种途径。
-
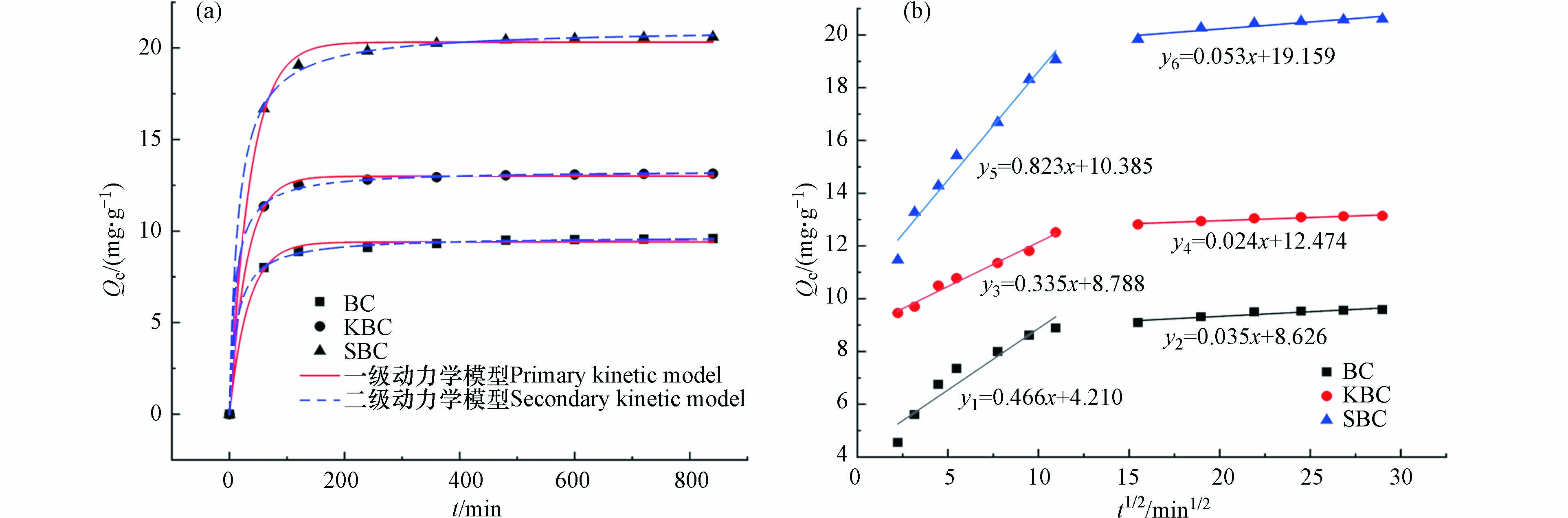
生物炭吸附在RhB的去除中会产生积极影响,按照1.4实验方案,采用一级和二级动力学模型对吸附实验结果进行拟合,结果如表2和图7(a)所示。从结果分析,3种生物炭(BC、KBC、SBC)吸附RhB过程的二级动力学理论平衡吸附量与吸附饱和时的实验值(qe1=9.584 mg·g−1、qe2=13.138 mg·g−1、qe3=20.605 mg·g−1)更吻合,相关系数拟合度较高,说明符合二级动力学模型,是一种化学吸附过程[26]。
为了进一步解释扩散机理,利用颗粒扩散模型对实验结果进行拟合,结果如表3和图7(b)。3种生物炭对RhB的吸附呈二级线性关系,吸附量均随t1/2的增大而增大,k1明显大于k2,这说明第一阶段RhB通过边界层迅速聚集到生物炭表面,外表面吸附达到饱和后染料分子会进入到孔内部,第二阶段拟合线斜率趋于水平,扩散阻力增大,吸附逐渐达到饱和,且拟合曲线不过原点,说明内扩散过程不是唯一限速步骤,生物炭吸附RhB的过程受表面吸附和颗粒内扩散共同控制[27]。
-
由不同体系对RhB的去除结果比较后发现,体系“SBC+PS”效果最为显著,所以需要进一步探究不同因素对该体系脱色RhB的影响,结果如图8。首先,溶液初始pH是影响过硫酸盐体系降解污染物的重要参数,因此,考察了pH(3—11)范围内对RhB的去除影响。结果如图8(a)所示,pH从3增加到11时,RhB的去除率依次降低(95.88%、91.89%、89.30%,、83.91%、63.19%),说明酸性条件更有利于反应进行。这是因为酸性环境有更多的H+能高效活化
S2O2−8 产生更多的SO−4⋅ (式8—9),而在碱性条件下,S2O2−8 会转化为·OH,其氧化性和半衰期都小于SO−4 ·(式10—11),所以RhB的去除率随着pH的升高而降低,这与王艳等[28]利用Zn/BC活化PS降解金橙Ⅱ的研究结果一致。另一方面,生物炭在酸性条件下更有利于吸附RhB。当溶液pH较低时,生物炭表面官能团质子化,RhB以阳离子形式存在,这样有利于生物炭和RhB进行离子交换进而提高去除率;而溶液pH较高时,RhB以两性离子存在,形成大分子的二聚体,不利于被生物炭吸附[29]。生物炭和PS投加量对去除RhB的影响如图8(b、c)所示。生物炭投加量从250 mg·L−1增加到1000 mg·L−1时,RhB的去除率逐渐增加(89.30%、93.37%、97.96%、98.79%),这是因为随着生物炭投加量的增加吸附效能逐渐增强,可以为PS提供活化位点产生更多的
SO−4⋅ 。同时,随着PS投加量的增加,RhB的去除率会表现出相同趋势(73.02%、89.30%、92.51%、93.75%),这是因为随着PS的增加提高了SO−4⋅ 的产生速率。但需要注意的是,当投加量从1.5 mmol·L−1增加到3.5 mmol·L−1时,RhB的去除效率并未显著提升,这是因为SBC表面活性位点被占用,也可能是因为过量的SO−4⋅ 会相互消耗(式12),从而降低了脱色效果[30]。 -
对于体系“SBC+PS”,
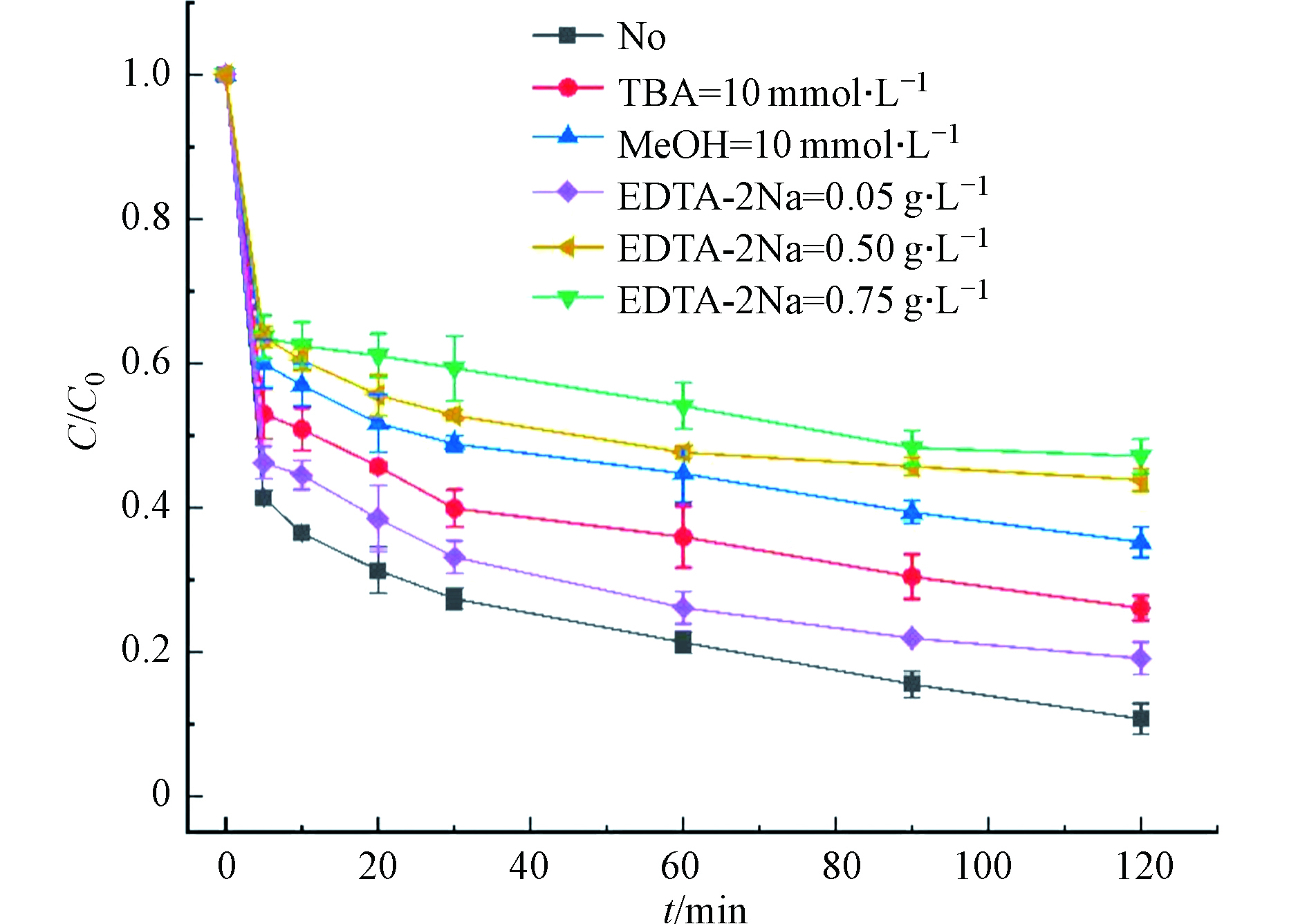
SO−4⋅ 和·OH都可能是降解RhB的原因,为了确定作用机制,往往加入醇类以淬灭自由基。甲醇(MeOH)对SO−4⋅ 和·OH的反应速率常数相差不多,分别是1.1×107 mol·(L·S)−1和9.7×109 mol·(L·S)−1;叔丁醇(TBA)与·OH的反应速率比SO−4⋅ 要快得多,分别为6×108 mol·(L·S)−1和8.4×105 mol·(L·S)−1 [31],结果如图9所示。由于叔丁醇和甲醇的加入,RhB的去除率由89.30%分别下降到64.80%和73.93%,这说明两种淬灭剂均能表现出对RhB降解的抑制作用,SO−4⋅ 和·OH共同参与了反应,其中·OH是主导自由基,SO−4⋅ 起辅助作用。而一些研究发现,活化PS降解有机物的过程不局限于自由基机制,可能同时存在非自由基降解有机污染物,吴瑶[24]以水稻秸秆生物炭为催化剂活化过硫酸盐降解苯胺和RhB时推测,生物炭在活化PS的同时,表面会形成更高活性的空穴以降解污染物。EDTA-2Na往往作为活性物种空穴和光生电子的捕获剂(式13—17)[32-33],所以在反应体系中加入了不同浓度的EDTA-2Na后发现,随着投加量的增加会显著抑制RhB的降解,当EDTA-2Na浓度为0.75 g·L−1时,RhB的去除率保持在52.82%,与单独SBC的吸附去除率(47.66%)相近,但是这种非自由基机制还仅仅是实验推测,有待进一步的探究和论证.
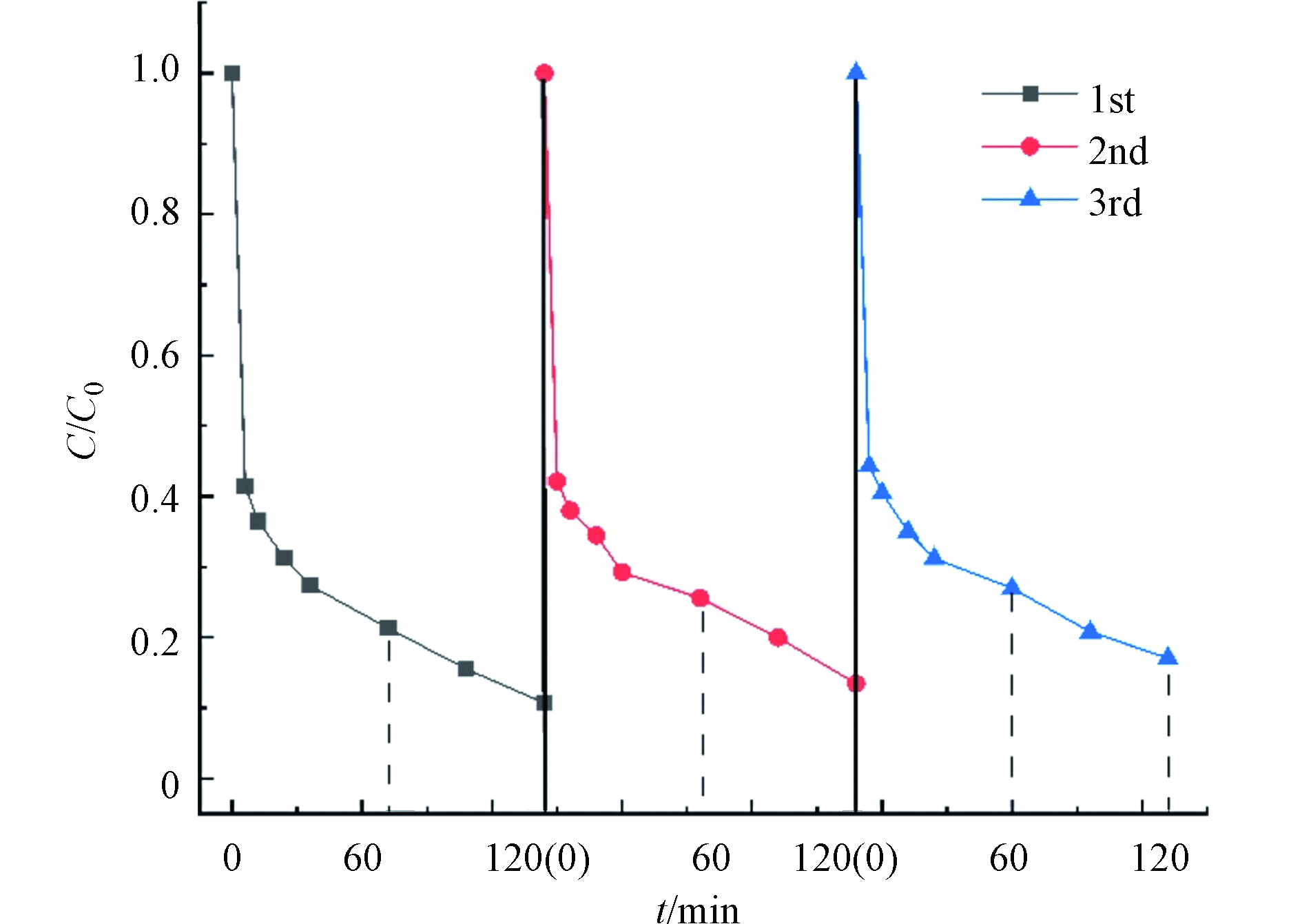
催化剂的重复利用性能是衡量材料的重要指标,因此考察了SBC重复使用3次后RhB的去除情况,结果如图10。SBC在重复使用过程中保持了较高的活性,RhB的去除率分别为:89.30%,86.56%,82.93%,随着多次使用之后,生物炭的吸附和活化性能会逐渐降低,但总体而言SBC可以作为活化PS的一种有效催化剂。
-
(1)结合SEM,BET,FTIR,XRD分析,猪粪制生物炭属于介孔材料,有芳香性,改性后具有大比表面积,总孔体积和丰富的官能团;生物炭含氧官能团可以有效参与活化PS。
(2)3种生物炭吸附RhB的过程受表面吸附和颗粒内扩散共同控制,更符合二级动力学,是一种化学吸附;“SBC+PS”体系脱色RhB的效果最好,结合UV-vis分析表明去除RhB的过程存在吸附和降解两种途径,RhB的结构会受到一定程度的破坏。
(3)体系“SBC+PS”脱色RhB的过程受初始pH,生物炭和PS投加量的影响。总体而言,酸性条件更有利于反应进行,生物炭和PS投加量越多,RhB的降解率越高。
(4)体系“SBC+PS”中去除RhB可能同时存在自由基和非自由基两种机制。·OH是体系中的主导自由基,
SO−4⋅ 起辅助作用;基于RhB的降解被引入的EDTA-2Na(一种空穴捕获剂)显著抑制的实验结果,说明可能存在非自由基降解机制。SBC具有良好的重复利用性能,可以作为活化PS的一种有效催化剂。
改性猪粪制生物炭活化过硫酸盐(PS)去除罗丹明B
Removal of Rhodamine B by modified pig manure made biochar-activated persulfate(PS)
-
摘要: 为了探究生物炭活化过硫酸盐(PS)去除罗丹明B(RhB)的效能,实验以猪粪为原料,采用限氧热解法(700 ℃)制备生物炭并进行酸碱改性,通过扫描电镜(SEM),比表面积分析仪(BET),傅里叶红外光谱仪(FTIR)和X射线衍射仪(XRD)对材料进行了表征分析,同时探究了生物炭吸附RhB的机理以及作为催化剂活化PS脱色RhB的最佳体系,影响因素和反应机制。结果表明,改性后生物炭的比表面积,总孔体积显著增加,含有丰富的含氧官能团;生物炭吸附RhB的过程受表面吸附和颗粒内扩散共同控制,更符合二级动力学,是一种化学吸附过程;体系“SBC(硫酸改性生物炭)+PS”脱色RhB的效果最优,酸性条件更有利于反应进行,RhB去除率随着生物炭和PS投加量的增大而增大; SBC重复利用效能良好,可以作为活化PS的有效催化剂,体系“SBC+PS”中可能同时存在自由基和非自由基两种机制协同降解RhB。综上,本研究致力于探索一种低成本的废水处理方案,以期达到以废治废的目的。Abstract: In order to investigate the effectiveness of biochar activated persulfate (PS) for Rhodamine B (RhB) removal, biochar was prepared by oxygen-limited pyrolysis (700 ℃) using pig manure as raw material and modified with acid-base. The paper also investigated the mechanism of RhB adsorption on biochar and the optimal system for the activation of PS decolorization of RhB as a catalyst, the influencing factors and the reaction mechanism. The results showed that, The specific surface area and total pore volume of the modified biochar increased significantly and contained abundant oxygen-containing functional groups; The adsorption of RhB by biochar was controlled by both surface adsorption and intraparticle diffusion, which was more in line with secondary kinetics and was a chemisorption process; The "SBC (Sulfuric acid-modified biochar)+PS" system has the best effect on RhB decolorization, the acidic condition is more favorable to the reaction, and the removal rate of RhB increases with the increase of biochar and PS dosage; SBC has good reuse efficiency and can be used as an effective catalyst for activation of PS, and there may be both free radicals and non-free radicals in the "SBC+PS" system. In conclusion, this study is dedicated to explore a low-cost solution for wastewater treatment in order to achieve the goal of treating waste with waste.
-
Key words:
- biochar from pig manure /
- persulfate /
- Rhodamine B /
- modified
-
罗丹明B(RhB)又称玫瑰红B,水溶液呈碱性,性质稳定,具有致畸、致癌和诱变性,广泛应用于有色玻璃,纺织、涂料等行业。由于其难以被生物降解,若富含RhB的废水未经有效处理直接排放,将会对人类身体健康和环境造成潜在危害[1-2]。近年来,研究者们越发关注过硫酸盐(PS)高级氧化技术,这是因为PS被活化后能产生强氧化性的
SO−4⋅ 生物炭是生物质残渣(植物、果壳、木屑等)限氧热解产生的一种炭材料[5],与活性炭结构相似,利用其活化PS,不仅成本更低,且自身的吸附性能可以协同去除水中污染物[6]。而作为一种重要的农业废弃物资源,目前以动物粪便为原料制备生物炭的研究较少,随着我国规模化养殖业迅速发展,畜禽粪便的不合理排放已经成为农业面源污染的主要来源,需要迫切的解决其带来的环境污染问题[7-8]。
因此,本研究以RhB为目标污染物,选用猪粪为原料制备生物炭,利用“硫酸/氢氧化钾+超声波+高温活化”进行改性处理,对材料进行表征分析,探寻其活化PS去除RhB的最优体系及影响因素,以期获得一种低成本的废水处理方法,达到以废治废的目的。
1. 材料与方法(Materials and methods)
1.1 试剂与仪器
主要试剂与用品:聚偏氟乙烯(PVDF)微孔膜,浓硫酸,氢氧化钠,过硫酸钾(PS),罗丹明B,均为分析纯购自阿拉丁试剂(上海)有限公司;猪粪采自广州市某养猪场;实验室用水为二次去离子水。
主要仪器:扫描电镜(SEM,蔡司Sigma 300);比表面积分析仪(micromeritics,ASAP 2460);傅里叶红外光谱仪(FTIR,德国布鲁克AVANCE NEO);X射线衍射光谱(XRD,日本理学SmartLab);紫外-可见分光光度计(Ultra-3660,北京普源精电科技有限公司);数控超声波清洗器(KQ-500DE,昆山超声仪器有限公司);水浴恒温振荡器(THZ-82A,常州澳华仪器有限公司)。
1.2 生物炭制备及改性
生物炭制备:猪粪采自广州市某养猪场,风干72 h,并拣出石粒、树枝等杂质,捣碎后过60目筛,装于坩埚中盖好,放入马弗炉中,选择在限氧条件下以10 ℃·min−1的升温梯度加热至700 ℃恒温120 min,热解产物冷却至室温再研磨过100目筛,用去离子水清洗,滤后于烘箱中105 ℃烘干,装入棕色广口瓶中,即制得猪粪生物炭,标记为:BC。
生物炭改性:对文献[9]方法做出优化。取制备好的BC于锥形瓶中,每5 g生物炭分别加入200 mL体积分数5%的氢氧化钾或硫酸溶液,25 ℃下,在水浴恒温振荡器中以120 r·min−1的转速改性24 h,后在功率为200 W的超声波下改性30 min,调节溶液pH至中性,结束后经离心机固液分离,用去离子水和无水乙醇反复清洗后倒出上清液,于160 ℃活化24 h,即得到改性后的猪粪制生物炭。氢氧化钾和硫酸改性后的生物炭分别标记为:KBC和SBC。
1.3 材料表征
采用扫描电子显微镜(SEM,含EDS能谱分析)表征材料的表面形貌并分析元素的分布情况;比表面积分析仪(BET)对材料表面特性进行分析;傅里叶红外光谱仪(FTIR)分析材料表面官能团;X射线衍射仪(XRD)分析材料晶体结构。
1.4 吸附实验
探究3种生物炭BC、KBC、SBC对RhB的吸附特性。取200 mL初始浓度10 mg·L−1的RhB于250 mL锥形瓶中,加入0.05 g生物炭,调初始pH值至7,在160 r·min−1的频率下振荡14 h。在设定时间(5、10、20、30、60、90、120、240、360、480、600、720、840 min)取上清液经0.22 μm滤膜过滤,测定RhB的质量浓度。根据式1计算吸附容量,根据式(2、3、4)计算一级,二级吸附动力学和颗粒扩散模型的拟合参数
Qe=(C0−Ce)×VW (1) ln(Qe−Qt)=lnQe−k1t (2) tQt=1k2×Q2e+tQe (3) Qt=kid×t1/2+di (4) 式中,
Qe C0 Ce 1.5 降解实验
取200 mL初始浓度10 mg·L−1的RhB于250 mL锥形瓶中,加入一定量生物炭和PS,调节初始pH,在160 r·min−1的频率下反应120 min,分别在设定时间(5、10、20、30、60、90、120 min)取上清液用0.22 μm滤膜过滤,测定RhB的吸光度,计算质量浓度和去除率(式5)。在生物炭重复利用实验中,同时做多组平行以保证使用后的生物炭足量,将反应终点溶液经0.45 μm抽滤再经冷冻干燥即可得到使用后的生物炭,避光封闭保存以重复使用。
R=(1−C/C0)×100% (5) 式中,R是RhB去除率(%),C为t时刻RhB的浓度(mg·L−1),C0为RhB的初始浓度(mg·L−1)。
1.6 测试方法
用紫外-可见分光光度计(Ultra-3660,北京普源精电科技有限公司RIGOL)在RhB可见光最大吸收波长552 nm处测定其吸光度。
2. 结果与讨论(Results and discussion)
2.1 生物炭的表征分析
2.1.1 生物炭SEM分析
由图1可见,3种生物炭表面呈现凹凸不平状态,均为不规则多孔结构,这是因为有机质在高温热解(700 ℃)中生成CO2和H2O等气体,有利于形成孔隙[10];其中,KBC和SBC的多孔结构更明显,这是因为强酸强碱具有氧化性,对生物炭表面侵蚀在一定程度上具有造孔作用,原本堵塞孔道的灰分也会被清洗。通过EDS能谱结果表明,猪粪制生物炭C和O的含量较高,有一定量的Si,经改性后,KBC和SBC中元素K和S的含量明显增加。
2.1.2 生物炭BET分析
BET(比表面积分析)是衡量多孔材料的重要参数,结果如表1所示,KBC和SBC的比表面积较改性前分别提高了4.2倍和6倍,总孔体积提高了1.9倍和3.7倍,微孔孔容提高了4.2倍和2.4倍,3种生物炭均属于介孔材料。据文献[9]报道,以猪粪为原料,在700 ℃制备生物炭,用“硫酸+超声波”改性90 min后,生物炭比表面积为46.250 m2·g−1,这是因为硫酸的氧化性和超声波的空化作用协同有助于疏通生物炭堵塞的孔隙,产生空洞结构,但是,比表面积远小于本文制备的SBC(106.611 m2·g−1)。结合EDS分析,这或许是因为本实验生物炭更长时间(24 h)浸润硫酸溶液后再经活化(160 ℃)能更好的掺杂硫元素,这种杂原子的修饰与碳原子会形成局部电势密度差,产生表面缺陷,形成大比表面积,可以为PS提供更多的反应活性位点[11]。
表 1 生物炭的表面特征测试结果Table 1. Test results of surface characteristics of biochar比表面积/(m2·g−1)Specific surface area 孔容/( cm3·g−1)Pore volume 微孔孔容/(cm3·g−1)Micropore volume 平均孔径/nmAverage pore diameter BC 17.756 0.052 0.005 11.824 KBC 73.965 0.103 0.022 5.576 SBC 106.611 0.193 0.013 7.236 图2为3种生物炭的氮气吸附-脱附和孔径分布曲线。结果表明,3种生物炭的吸附-脱附等温线均不重合且脱附曲线均位于吸附曲线上方,产生吸附滞后,存在H2型滞后环,这种滞后现象与孔的形状及其大小有关。3种生物炭在吸附压力较高区间(P/P0>0.8)气体吸附线急剧上升,N2吸附量速率明显加快,发生中孔毛细凝聚,表现出Ⅵ型N2吸附-脱附等温线[12],KBC和SBC的滞后环较宽,说明生物炭孔大小分布更宽且含有更多的介孔[13]。BC,KBC和SBC的孔径分别在10—20 nm,5—10 nm和2.5—8 nm间较为密集,最可几孔径分别为:15.898 nm、5.605 nm和4.658 nm,均大于RhB的分子尺寸(1.59 nm×1.18 nm×0.56 nm)[14],这样利于RhB的吸附和扩散。
2.1.3 生物炭FTIR和XRD分析
图3是生物炭活化PS降解RhB前后的FTIR曲线,主要的特征峰为:3750—3300 cm−1处吸收峰是醇酚羟基(—OH)振动[15],1700—1600 cm−1处为C=O或C=C振动[16-17];1420 cm−1振动归结于脂肪族的C—H键[18];1100—1030 cm−1处有很强的伸缩振动峰,归结于C—O键或Si—O[19-20];810—750 cm−1处的伸缩振动归结于芳香族的C—H键[21];687 cm−1处存在S—H振动[13]。
综合EDS和FTIR分析,猪粪制生物炭具有芳香性,含有硅酸盐;改性后含有丰富的C—C、C—O、C=O键以及更多的官能团;SBC比KBC具有更多的官能团种类和数量,活化PS后SBC和KBC的含氧官能团(—OH)的伸缩振动峰强度均明显减弱,并且SBC的官能团S-H的伸缩振动峰也消失,说明这些官能团参与到了活化PS降解RhB的过程。图4为材料的XRD图像,为了确定材料的晶型结构,通过Jade 6.5进行物相检索,猪粪制生物炭(BC)表面主要存在SiO2(PDF卡片号46—1045)和CaCO3(PDF卡片号41—1475),改性后生物炭的这些衍射峰强度明显减弱,说明SiO2和CaCO3可能在改性后消失,而SBC伴随着新的衍射峰出现,这些归因于CaSO4(PDF卡片号37—0184),可能是因为H2SO4改性与CaCO3反应所致。
2.1.4 不同体系对RhB去除的影响
为了考察生物炭催化PS去除RhB的性能,本文探究了相同反应条件下不同体系对RhB的去除效果,结果如图5所示。体系的去除效果由低到高依次为:Blank(1.5%)、BC(22.22%)、KBC(31.30%)、SBC(47.66%)、PS(54.26%)、BC+PS(63.63%)、KBC+PS(81.25%)、SBC+PS(89.30%)。由结果分析,空白对照组RhB的去除率是1.5%,这是因为实验室光源条件下RhB被少量光解;单独的生物炭对RhB有吸附性,SBC的吸附效果最好;单一的PS可以氧化降解RhB,这是因为PS会水解产生少量的
SO−4⋅ SO−4⋅ 为了探究RhB的降解变化,不同反应时间的紫外可见吸收光谱见图6。从0 min的谱图可以看到,RhB的主要特征峰位于251、350、552 nm处。其中,250—350 nm的吸收峰归结于芳香环,350 nm处的吸收峰为共轭双键[24],峰强随着反应时间的进行而明显减弱,这说明芳香环和共轭双键被破坏,552 nm的特征峰表现出相同的趋势,而且波长发生蓝移现象,说明RhB发生了脱乙基化反应并伴随着新物质的产生[25]。因此,全波长扫描表明,随着反应的进行,RhB的脱色团,取代基和分子骨架不同程度的被破坏,这说明“生物炭/过硫酸盐”体系中RhB的去除存在吸附和降解2种途径。
2S2O2−8+2H2O⟶3SO2−4+SO−4⋅+O−2⋅+4H+ (6) Carbon surface−OH+S2O2−8⟶Carbon surface−O·+SO−4⋅+HSO−4 (7) 2.2 生物炭对RhB的吸附动力学
生物炭吸附在RhB的去除中会产生积极影响,按照1.4实验方案,采用一级和二级动力学模型对吸附实验结果进行拟合,结果如表2和图7(a)所示。从结果分析,3种生物炭(BC、KBC、SBC)吸附RhB过程的二级动力学理论平衡吸附量与吸附饱和时的实验值(qe1=9.584 mg·g−1、qe2=13.138 mg·g−1、qe3=20.605 mg·g−1)更吻合,相关系数拟合度较高,说明符合二级动力学模型,是一种化学吸附过程[26]。
表 2 生物炭吸附RhB的一级、二级动力学模型拟合参数Table 2. Primary and secondary kinetic model fitting parameters for RhB adsorption on biochar一级动力学模型Primary kinetic model 二级动力学模型Secondary kinetic model qe1/(mg·g−1) k1/[g·(mg·h−1)] R21 qe2/(mg·g−1) k2/[g·(mg·h−1)] R22 BC 9.007 0.091 0.939 9.437 0.015 0.990 KBC 12.254 0.230 0.919 12.702 0.031 0.969 SBC 19.111 0.117 0.894 20.042 0.009 0.968 为了进一步解释扩散机理,利用颗粒扩散模型对实验结果进行拟合,结果如表3和图7(b)。3种生物炭对RhB的吸附呈二级线性关系,吸附量均随t1/2的增大而增大,k1明显大于k2,这说明第一阶段RhB通过边界层迅速聚集到生物炭表面,外表面吸附达到饱和后染料分子会进入到孔内部,第二阶段拟合线斜率趋于水平,扩散阻力增大,吸附逐渐达到饱和,且拟合曲线不过原点,说明内扩散过程不是唯一限速步骤,生物炭吸附RhB的过程受表面吸附和颗粒内扩散共同控制[27]。
表 3 生物炭吸附RhB的颗粒扩散模型拟合参数Table 3. Fitting parameters of particle diffusion model for RhB adsorption by biochar颗粒扩散模型Particle diffusion model k1/[mg·g−1·h−1/2] c1 R2 k2/[mg·g−1·h−1/2] c2 R2 BC 0.466 4.210 0.916 0.035 8.626 0.877 KBC 0.335 8.788 0.983 0.024 12.474 0.935 SBC 0.823 10.385 0.975 0.053 19.159 0.867 2.3 实验条件对RhB脱色的影响
由不同体系对RhB的去除结果比较后发现,体系“SBC+PS”效果最为显著,所以需要进一步探究不同因素对该体系脱色RhB的影响,结果如图8。首先,溶液初始pH是影响过硫酸盐体系降解污染物的重要参数,因此,考察了pH(3—11)范围内对RhB的去除影响。结果如图8(a)所示,pH从3增加到11时,RhB的去除率依次降低(95.88%、91.89%、89.30%,、83.91%、63.19%),说明酸性条件更有利于反应进行。这是因为酸性环境有更多的H+能高效活化
S2O2−8 SO−4⋅ S2O2−8 SO−4 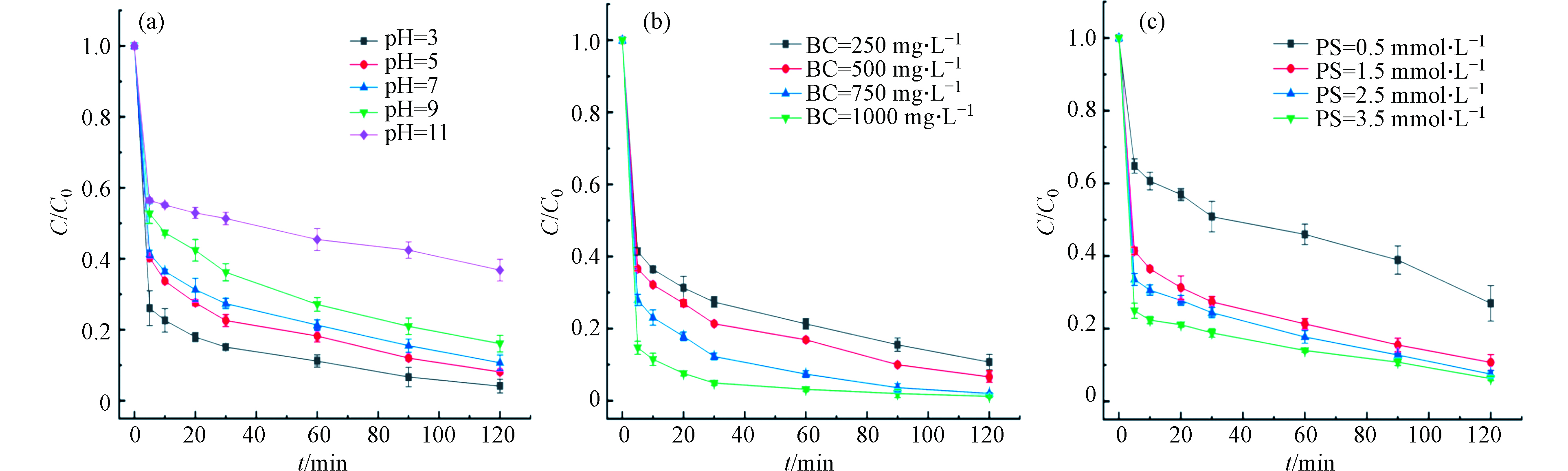 图 8 “SBC+PS”体系降解RhB的影响因素Figure 8. Factors influencing the degradation of RhB by“SBC+PS”system:(a)初始pH,(b)生物炭投加量,(c)PS投加量(反应条件:C0=10 mg·L−1,V=0.2 L,pH=7,BC=250 mg·L−1,PS=1.5 mmol·L−1)(a)Initial pH,(b)Biochar dosage,(c)PS dosage (Reaction conditions:C0=10 mg·L−1,V=0.2 L,pH=7,BC=250 mg·L−1,PS=1.5 mmol··L−1)
图 8 “SBC+PS”体系降解RhB的影响因素Figure 8. Factors influencing the degradation of RhB by“SBC+PS”system:(a)初始pH,(b)生物炭投加量,(c)PS投加量(反应条件:C0=10 mg·L−1,V=0.2 L,pH=7,BC=250 mg·L−1,PS=1.5 mmol·L−1)(a)Initial pH,(b)Biochar dosage,(c)PS dosage (Reaction conditions:C0=10 mg·L−1,V=0.2 L,pH=7,BC=250 mg·L−1,PS=1.5 mmol··L−1)生物炭和PS投加量对去除RhB的影响如图8(b、c)所示。生物炭投加量从250 mg·L−1增加到1000 mg·L−1时,RhB的去除率逐渐增加(89.30%、93.37%、97.96%、98.79%),这是因为随着生物炭投加量的增加吸附效能逐渐增强,可以为PS提供活化位点产生更多的
SO−4⋅ SO−4⋅ SO−4⋅ S2O2−8+H+⟶HS2O2−8 (8) HS2O2−8⟶SO−4⋅+HSO−4 (9) S2O2−8+OH−⟶SO2−4+·OH (10) SO−4·+H2O⟶HSO−4+·OH (11) SO−4⋅+SO−4⋅⟶2SO2−4 (12) 2.4 降解作用机制及材料的重复使用性能
对于体系“SBC+PS”,
SO−4⋅ SO−4⋅ SO−4⋅ SO−4⋅ SO−4⋅ 而一些研究发现,活化PS降解有机物的过程不局限于自由基机制,可能同时存在非自由基降解有机污染物,吴瑶[24]以水稻秸秆生物炭为催化剂活化过硫酸盐降解苯胺和RhB时推测,生物炭在活化PS的同时,表面会形成更高活性的空穴以降解污染物。EDTA-2Na往往作为活性物种空穴和光生电子的捕获剂(式13—17)[32-33],所以在反应体系中加入了不同浓度的EDTA-2Na后发现,随着投加量的增加会显著抑制RhB的降解,当EDTA-2Na浓度为0.75 g·L−1时,RhB的去除率保持在52.82%,与单独SBC的吸附去除率(47.66%)相近,但是这种非自由基机制还仅仅是实验推测,有待进一步的探究和论证.
催化剂的重复利用性能是衡量材料的重要指标,因此考察了SBC重复使用3次后RhB的去除情况,结果如图10。SBC在重复使用过程中保持了较高的活性,RhB的去除率分别为:89.30%,86.56%,82.93%,随着多次使用之后,生物炭的吸附和活化性能会逐渐降低,但总体而言SBC可以作为活化PS的一种有效催化剂。
R-CH2-COO−+hvB+⟶R-CH2-COO·+H2O⟶R-H+CO2+CH2O+H+ (13) CH2O+H2Ohv⟶HCOOH+H2 (14) HCOOH+hvB+⟶HCOO·+H+ (15) HCOO·⟶CO2+H++e−cB (16) 2H++2e−cB⟶H2 (17) 3. 结论(Conclusion)
(1)结合SEM,BET,FTIR,XRD分析,猪粪制生物炭属于介孔材料,有芳香性,改性后具有大比表面积,总孔体积和丰富的官能团;生物炭含氧官能团可以有效参与活化PS。
(2)3种生物炭吸附RhB的过程受表面吸附和颗粒内扩散共同控制,更符合二级动力学,是一种化学吸附;“SBC+PS”体系脱色RhB的效果最好,结合UV-vis分析表明去除RhB的过程存在吸附和降解两种途径,RhB的结构会受到一定程度的破坏。
(3)体系“SBC+PS”脱色RhB的过程受初始pH,生物炭和PS投加量的影响。总体而言,酸性条件更有利于反应进行,生物炭和PS投加量越多,RhB的降解率越高。
(4)体系“SBC+PS”中去除RhB可能同时存在自由基和非自由基两种机制。·OH是体系中的主导自由基,
SO−4⋅ -
表 1 生物炭的表面特征测试结果
Table 1. Test results of surface characteristics of biochar
比表面积/(m2·g−1)Specific surface area 孔容/( cm3·g−1)Pore volume 微孔孔容/(cm3·g−1)Micropore volume 平均孔径/nmAverage pore diameter BC 17.756 0.052 0.005 11.824 KBC 73.965 0.103 0.022 5.576 SBC 106.611 0.193 0.013 7.236 表 2 生物炭吸附RhB的一级、二级动力学模型拟合参数
Table 2. Primary and secondary kinetic model fitting parameters for RhB adsorption on biochar
一级动力学模型Primary kinetic model 二级动力学模型Secondary kinetic model qe1/(mg·g−1) k1/[g·(mg·h−1)] R21 qe2/(mg·g−1) k2/[g·(mg·h−1)] R22 BC 9.007 0.091 0.939 9.437 0.015 0.990 KBC 12.254 0.230 0.919 12.702 0.031 0.969 SBC 19.111 0.117 0.894 20.042 0.009 0.968 表 3 生物炭吸附RhB的颗粒扩散模型拟合参数
Table 3. Fitting parameters of particle diffusion model for RhB adsorption by biochar
颗粒扩散模型Particle diffusion model k1/[mg·g−1·h−1/2] c1 R2 k2/[mg·g−1·h−1/2] c2 R2 BC 0.466 4.210 0.916 0.035 8.626 0.877 KBC 0.335 8.788 0.983 0.024 12.474 0.935 SBC 0.823 10.385 0.975 0.053 19.159 0.867 -
[1] 刘琦, 陈嘉磊, 张启灵, 等. 稻壳灰吸附剂对罗丹明B的吸附性能研究 [J]. 化学研究与应用, 2019, 31(8): 1482-1491. doi: 10.3969/j.issn.1004-1656.2019.08.013 LIU Q, CHEN J L, ZHANG Q L, et al. Studies on the adsorption of Rhodamine B by rice husk ash adsorbent [J]. Chemical Research and Application, 2019, 31(8): 1482-1491(in Chinese). doi: 10.3969/j.issn.1004-1656.2019.08.013
[2] RICHARDSON S D, WILLSON C S, RUSCH K A. Use of rhodamine water tracer in the marshland upwelling system [J]. Ground Water, 2004, 42(5): 678-688. doi: 10.1111/j.1745-6584.2004.tb02722.x [3] 王行梁, 李晓良, 王子, 等. 硫酸根自由基高级氧化技术的发展及应用[J]. 现代化工, 2020, 40(增刊1): 49-53. WANG X L, LI X L, WANG Z, et al. Development and application of advanced oxidation technology based on sulfate radical[J]. Modern Chemical Industry, 2020, 40(Sup 1): 49-53(in Chinese).
[4] 王肖磊, 吴根华, 方国东, 等. 过渡金属活化过硫酸盐在环境修复领域的研究进展 [J]. 生态与农村环境学报, 2021, 37(2): 145-154. WANG X L, WU G H, FANG G D, et al. Transition metal activated persulfate for environmental remediation: A review [J]. Journal of Ecology and Rural Environment, 2021, 37(2): 145-154(in Chinese).
[5] AZARGOHAR R, DALAI A K. Biochar as a precursor of activated carbon [J]. Applied Biochemistry and Biotechnology, 1996, 131(1/2/3): 762-773. [6] FANG G D, LIU C, GAO J, et al. Manipulation of persistent free radicals in biochar to activate persulfate for contaminant degradation [J]. Environmental Science & Technology, 2015, 49(9): 5645-5653. [7] 孙良媛, 刘涛, 张乐. 中国规模化畜禽养殖的现状及其对生态环境的影响 [J]. 华南农业大学学报(社会科学版), 2016, 15(2): 23-30. SUN L Y, LIU T, ZHANG L. The pollution of scale livestock and poultry breeding and its influence on eco-environment [J]. Journal of South China Agricultural University (Social Science Edition), 2016, 15(2): 23-30(in Chinese).
[8] 武淑霞, 刘宏斌, 黄宏坤, 等. 我国畜禽养殖粪污产生量及其资源化分析 [J]. 中国工程科学, 2018, 20(5): 103-111. doi: 10.15302/J-SSCAE-2018.05.016 WU S X, LIU H B, HUANG H K, et al. Analysis on the amount and utilization of manure in livestock and poultry breeding in China [J]. China Engineering Science, 2018, 20(5): 103-111(in Chinese). doi: 10.15302/J-SSCAE-2018.05.016
[9] 陈佼, 张建强, 陆一新, 等. 改性猪粪生物炭对水中Cr(Ⅵ)的吸附性能 [J]. 水处理技术, 2017, 43(4): 31-35,41. CHEN J, ZHANG J Q, LU Y X, et al. Adsorption properties of Cr(Ⅵ) in aquatic solutions by modified pig manure biochar [J]. Technology of Water Treatment, 2017, 43(4): 31-35,41(in Chinese).
[10] LIU X Q, DING H S, WANG Y Y, et al. Pyrolytic temperature dependent and ash catalyzed formation of sludge char with ultra-high adsorption to 1-naphthol [J]. Environmental Science & Technology, 2016, 50(5): 2602-2609. [11] WANG H Z, GUO W Q, LIU B H, et al. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism [J]. Water Research, 2019, 160: 405-414. doi: 10.1016/j.watres.2019.05.059 [12] LIANG C J, LIN Y T, SHIN W H. Persulfate regeneration of trichloroethylene spent activated carbon [J]. Journal of Hazardous Materials, 2009, 168(1): 187-192. doi: 10.1016/j.jhazmat.2009.02.006 [13] 桑倩倩, 王芳君, 赵元添, 等. 铁硫改性生物炭去除水中的磷 [J]. 环境科学, 2021, 42(5): 2313-2323. SANG Q Q, WANG F J, ZHAO Y T, et al. Removal of phosphorus from water by iron-sulfur modified biochar [J]. Environmental Science, 2021, 42(5): 2313-2323(in Chinese).
[14] 李艳春, 张鹏会, 张强, 等. 4种生物炭对阳离子染料吸附性能 [J]. 环境科学与技术, 2020, 43(7): 101-110. LI Y C, ZHANG P H, ZHANG Q, et al. Adsorption of cationic dye in aqueous solution by four biochars [J]. Environmental Science & Technology, 2020, 43(7): 101-110(in Chinese).
[15] SANTAMARINA J C, KLEIN K A, WANG Y H, et al. Specific surface: Determination and relevance [J]. Canadian Geotechnical Journal, 2002, 39(1): 233-241. doi: 10.1139/t01-077 [16] ZHANG S Q, YANG X, LIU L, et al. Adsorption behavior of selective recognition functionalized biochar to Cd(Ⅱ) in wastewater [J]. Materials (Basel, Switzerland), 2018, 11(2): E299. doi: 10.3390/ma11020299 [17] 任洁青, 王朝旭, 张峰, 等. 改性稻壳生物炭对水中Cd2+的吸附性能研究 [J]. 生态与农村环境学报, 2021, 37(1): 73-79. REN J Q, WANG C X, ZHANG F, et al. Adsorption of Cd2+ from aqueous solution by modified rice husk-derived biochars [J]. Journal of Ecology and Rural Environment, 2021, 37(1): 73-79(in Chinese).
[18] 崔志文, 任艳芳, 王伟, 等. 碱和磁复合改性小麦秸秆生物炭对水体中镉的吸附特性及机制 [J]. 环境科学, 2020, 41(7): 3315-3325. CUI Z W, REN Y F, WANG W, et al. Adsorption characteristics and mechanism of cadmium in water by alkali and magnetic composite modified wheat straw biochar [J]. Environmental Science, 2020, 41(7): 3315-3325(in Chinese).
[19] 罗元, 谢坤, 冯弋洋, 等. 镧改性核桃壳生物炭制备及吸附水体磷酸盐性能 [J]. 化工进展, 2021, 40(2): 1121-1129. LUO Y, XIE K, FENG Y Y, et al. Preparation of lanthanum modified walnut shell biochar and adsorption of phosphate from aqueous solutions [J]. Chemical Industry and Engineering Progress, 2021, 40(2): 1121-1129(in Chinese).
[20] 桂向阳, 刘晨, 许吉宏, 等. 畜禽粪便生物炭的二维红外光谱分析 [J]. 光谱学与光谱分析, 2020, 40(11): 3606-3612. GUI X Y, LIU C, XU J H, et al. Two-dimensional perturbation correlation infrared spectroscopy analysis of animal manure biochar [J]. Spectroscopy and Spectral Analysis, 2020, 40(11): 3606-3612(in Chinese).
[21] 许利娜, 杨小华, 丁海阳, 等. 生物基N, S双掺杂荧光碳点的制备及其应用 [J]. 高校化学工程学报, 2019, 33(4): 951-956. doi: 10.3969/j.issn.1003-9015.2019.04.022 XU L N, YANG X H, DING H Y, et al. Synthesis and application of bio-based N, S co-doped fluorescent carbon dots [J]. Journal of Chemical Engineering of Chinese Universities, 2019, 33(4): 951-956(in Chinese). doi: 10.3969/j.issn.1003-9015.2019.04.022
[22] GU W, LI X D, XING M C, et al. Removal of phosphate from water by amine-functionalized copper ferrite chelated with La(III) [J]. Science of the Total Environment, 2018, 619/620: 42-48. doi: 10.1016/j.scitotenv.2017.11.098 [23] 姚淑华, 马锡春, 李士凤. 秸秆生物炭活化过硫酸盐氧化降解苯酚 [J]. 中国环境科学, 2018, 38(11): 4166-4172. doi: 10.3969/j.issn.1000-6923.2018.11.023 YAO S H, MA X C, LI S F. Straw biochar activated persulfate oxidation and degradation of phenol [J]. China Environmental Science, 2018, 38(11): 4166-4172(in Chinese). doi: 10.3969/j.issn.1000-6923.2018.11.023
[24] 吴瑶. 水稻秸秆生物炭协同过硫酸钠降解水中苯胺和罗丹明B的效果与机制研究[D]. 南京: 南京农业大学, 2018. WU Y. Rapid degradation of aniline and rhodamine B in aqueous solution by persulfate combined with rice straw biochar and their mechanisms[D]. Nanjing: Nanjing Agricultural University, 2018(in Chinese).
[25] CHEN L Q, ZHANG Z H, WANG Y J, et al. Photocatalytic properties and electrochemical characteristic of a novel biomimetic oxygenase enzyme photocatalyst iron(II) tetrahydroxymethyl Tetra(1, 4-dithiin) porphyrazine for the degradation of organic pollutants [J]. Journal of Molecular Catalysis A:Chemical, 2013, 372: 114-120. doi: 10.1016/j.molcata.2013.02.013 [26] 刘冬冬, 李金铭, 赵博骏, 等. 秸秆水热炭与热裂解炭结构表征及铅吸附机制研究 [J]. 农业机械学报, 2020, 51(12): 304-314. doi: 10.6041/j.issn.1000-1298.2020.12.033 LIU D D, LI J M, ZHAO B J, et al. Pb2+ absorption mechanism and structure characterization of hydrochar and pyrochar from straw [J]. Transactions of the Chinese Society for Agricultural Machinery, 2020, 51(12): 304-314(in Chinese). doi: 10.6041/j.issn.1000-1298.2020.12.033
[27] 施超, 冯景伟, 彭书传, 等. 活性炭纤维对水中罗丹明B的吸附性能 [J]. 环境化学, 2013, 32(3): 394-401. SHI C, FENG J W, PENG S C, et al. Adsorption propreties of Rhodamine B by activater carbon fiber in aqueous solutions [J]. Environmental Chemistry, 2013, 32(3): 394-401(in Chinese).
[28] 王艳, 杨硕, 张米雪, 等. ZnFe/BC活化过硫酸盐降解金橙Ⅱ [J]. 环境化学, 2018, 37(12): 2630-2637. doi: 10.7524/j.issn.0254-6108.2018042504 WANG Y, YANG S, ZHANG M X, et al. Degradation of Orange Ⅱ by ZnFe/BC catalyzed persulfate [J]. Environmental Chemistry, 2018, 37(12): 2630-2637(in Chinese). doi: 10.7524/j.issn.0254-6108.2018042504
[29] 王章鸿, 郭海艳, 沈飞, 等. 蚯蚓粪便制备生物炭及其对罗丹明B吸附的研究 [J]. 环境科学学报, 2015, 35(10): 3170-3177. WANG Z H, GUO H Y, SHEN F, et al. Production of biochar by vermicompost carbonization and its adsorption to Rhodamine-B [J]. Acta Scientiae Circumstantiae, 2015, 35(10): 3170-3177(in Chinese).
[30] 李飞跃, 桂向阳, 刘晨, 等. 改性生物炭催化过硫酸盐脱色金橙Ⅱ [J]. 环境污染与防治, 2018, 40(11): 1207-1213. LI F Y, GUI X Y, LIU C, et al. Decoloration of dye acid orange 7 by modified biochar catalyzed persulfate [J]. Environmental Pollution & Control, 2018, 40(11): 1207-1213(in Chinese).
[31] YAN J C, LEI M, ZHU L H, et al. Degradation of sulfamonomethoxine with Fe3O4 magnetic nanoparticles as heterogeneous activator of persulfate [J]. Journal of Hazardous Materials, 2011, 186(2/3): 1398-1404. [32] VELO-GALA I, LÓPEZ-PEÑALVER J J, SÁNCHEZ-POLO M, et al. Role of activated carbon surface chemistry in its photocatalytic activity and the generation of oxidant radicals under UV or solar radiation [J]. Applied Catalysis B:Environmental, 2017, 207: 412-423. doi: 10.1016/j.apcatb.2017.02.028 [33] HU X Y, ZHOU K F, CHEN B Y, et al. Graphene/TiO2/ZSM-5 composites synthesized by mixture design were used for photocatalytic degradation of oxytetracycline under visible light: Mechanism and biotoxicity [J]. Applied Surface Science, 2016, 362: 329-334. doi: 10.1016/j.apsusc.2015.10.192 -










 下载:
下载: