-
近年来,荧光纳米材料因在光催化、生物传感、消毒、离子检测、光电器件和污染物去除等许多领域具有广阔的应用前景而备受关注[1-4]. 荧光纳米材料包括半导体量子点、氮化碳、纳米金刚石、碳纳米管、石墨烯量子点、碳点以及各种复合材料. 含重金属的量子点具有毒性,故环保型荧光纳米材料的制备和应用成为重要的发展趋势.
氮化碳是一种非常有前途的应用材料[5],目前已广泛用于水分解、太阳能传输、污染物去除和检测等领域[6-8],一般通过高温热解工艺由富含氮的前体(如三聚氰胺,氰胺和双氰胺)制备,所得的氮化碳通常具有粒径大、水溶性差、发光性能弱等特点. 近年来,光响应性能增强的氮化碳纳米薄膜和氮化碳量子点的制备和应用受到越来越多研究者的关注[9-10]. Liu等[11]利用有机胺制备光致发光氮化碳量子点,将其作为新型类过氧化物酶,运用比色法检测H2O2和葡萄糖. Zhang等[12]采用水热合成法制备具有光氧化还原催化性能的富碳氮化碳纳米薄膜. 石墨相C3N4纳米片的制备及应用于生物成像、生物传感和离子检测也有相关报道[13-20]. Qiao等[21]报道了硒掺杂石墨氮化碳纳米薄膜的合成方法,并应用于过氧化氢和黄嘌呤比色法检测. Zheng等[22]报道了磁性氮化碳纳米片的简便合成及其在食用油样品中多环芳烃磁性固相萃取中的应用. 研究利用简单的方法合成具有不同形态的氮化碳纳米材料,拓展其应用领域是很有意义的工作.
氯(Cl2)、次氯酸(HClO)和次氯酸根离子(ClO−)具有强氧化性,常用作水的消毒剂. 水中溶解的Cl2、HClO和ClO−的总和定义为游离氯[23]. 游离氯在水中的残留浓度不宜过高,监测其在水中的含量很有必要. 游离氯的测定方法包括碘滴定法[24]、比色法[25]、化学发光法[26]、离子色谱法[27]、液相色谱法[28]、流动注射分析[29]等. 尽管每种方法都有其优点,但仍普遍存在检测灵敏度低、选择性差、使用有毒试剂或应用程序复杂等缺点,开发简便、有效和环保的游离氯测定方法显得很重要.
本研究应用简便的微波辅助溶剂热法,以柠檬酸和三聚氰胺为前驱体制备荧光氮化碳纳米材料(CNNPs),以CNNPs为荧光探针建立对游离氯的定量测定方法,并用于真实水样中游离氯的测定.
-
一水合柠檬酸(CA)、三聚氰胺和油酸均为分析纯,购自上海国药化学试剂有限公司(中国);硫酸奎宁,分析纯,购自阿拉丁工业公司(中国上海);超纯水通过Milli-Q系统(Millipore,Bedford,MA,美国)制备.其他试剂均为分析纯试剂,无需进一步纯化即可使用.
-
利用简单的一步微波辅助溶剂热法制备CNNPs. 将1.5 g CA分别和1.5、0.75、0.5、0.3 g三聚氰胺置于30 mL反应管中,加入10 mL油酸,在1200 r·min−1的磁力搅拌条件下,将混合物置于微波合成器(Anton Paar Monowave 300,奥地利)中在220 ℃下加热15 min,反应过程中压力保持在2.32 MPa. 反应完成后,反应管自然冷却至室温,收集棕色沉淀物. 将沉淀物用正己烷充分洗涤,分散在超纯水中,并以5000 r·min−1离心30 min以除去大颗粒产物. 对应产物分别命名为:CNNPs-1、CNNPs-2、CNNPs-3、CNNPs-5.
-
利用TEM(FEI Tecnai G2 F20)分析CNNPs材料的表面形貌,利用Bruker DAVINCI D8 ADVANCE衍射仪测定X射线衍射(XRD)光谱,利用Thermo ESCALAB 250XI多功能成像电子光谱仪(Thermo Fisher)测定X射线光电子能谱(XPS),利用Magna-IR 750傅里叶变换红外(FT-IR)光谱仪(Nicolet)测定FT-IR光谱,利用UV-2550分光光度计(Shimadzu)测定紫外-可见(UV-Vis)光谱,利用Cary Eclipse PL分光光度计(Varian)测定荧光光谱,使用Vario ELⅢ 元素分析仪(Elementar, Germany)测定元素组成.
-
以硫酸奎宁为参比,通过比较积分荧光强度(PL)计算CNNPs的量子产率. 将硫酸奎宁溶解在0.1 mol·L−1硫酸中,并将获得的CNNPs溶解在超纯水中(η=1.33),所测吸光度保持在0.1以下. CNNPs的量子产率由以下方程式确定:
式中,Q为量子产率,I为积分荧光强度,η为溶剂的折射率,A为吸光度,下标R表示参比样品. 硫酸奎宁在激发波长350 nm下的量子产率为0.577,0.1 mol·L−1 硫酸溶液和超纯水的折射率均为1.33.
-
实验中,CNNPs-5溶液的浓度均为2.5 μg·mL−1. 往CNNPs-5溶液中加入50 μmol·L−1的次氯酸钠,测定溶液在0—60 min内的荧光强度变化. 测定CNNPs-5溶液在添加30 μmol·L−1的次氯酸钠和未添加次氯酸钠时,荧光强度在pH 4—10范围内的变化,分别记为F和F0. 游离氯浓度检测实验中,往CNNPs-5溶液中分别添加0、2、4、6、8、10、15、30、50、60、70、80、90、100 μmol·L−1的次氯酸钠,测定425 nm处的发射荧光强度,根据3倍标准偏差规则(LOD=3Sd/s)计算检测限(LOD).
-
设置两个对照实验,在选择性响应实验中选取16种离子,包括ClO−、Cl−、
ClO−3 、ClO−4 、Br−、BrO−3 、NO−2 、NO−3 、HPO2−4 、PO3−4 、SO2−3 、SO2−4 、Ca2+、Ag+、Cu2+和Pb2+,浓度均为100 μmol·L−1,分别加入到CNNPs-5水溶液中,并记录425 nm处的荧光强度响应. 其中阴离子添加的是其对应的钠盐,Ca2+、Ag+、Cu2+和Pb2+添加的分别是CaCl2、AgNO3、CuCl2和Pb(NO3)2. 在另一个对照干扰实验中,将100 μmol·L−1的ClO−溶液和300 μmol·L−1的上述离子加入CNNPs-5水溶液中,未添加干扰离子的溶液作为对照组,分别记录荧光猝灭响应. -
采集不同位置的2份自来水样品,自来水样品不经处理,添加3种不同浓度的游离氯溶液,运用上述检测方法对所含游离氯浓度进行检测,并计算相对标准偏差.
-
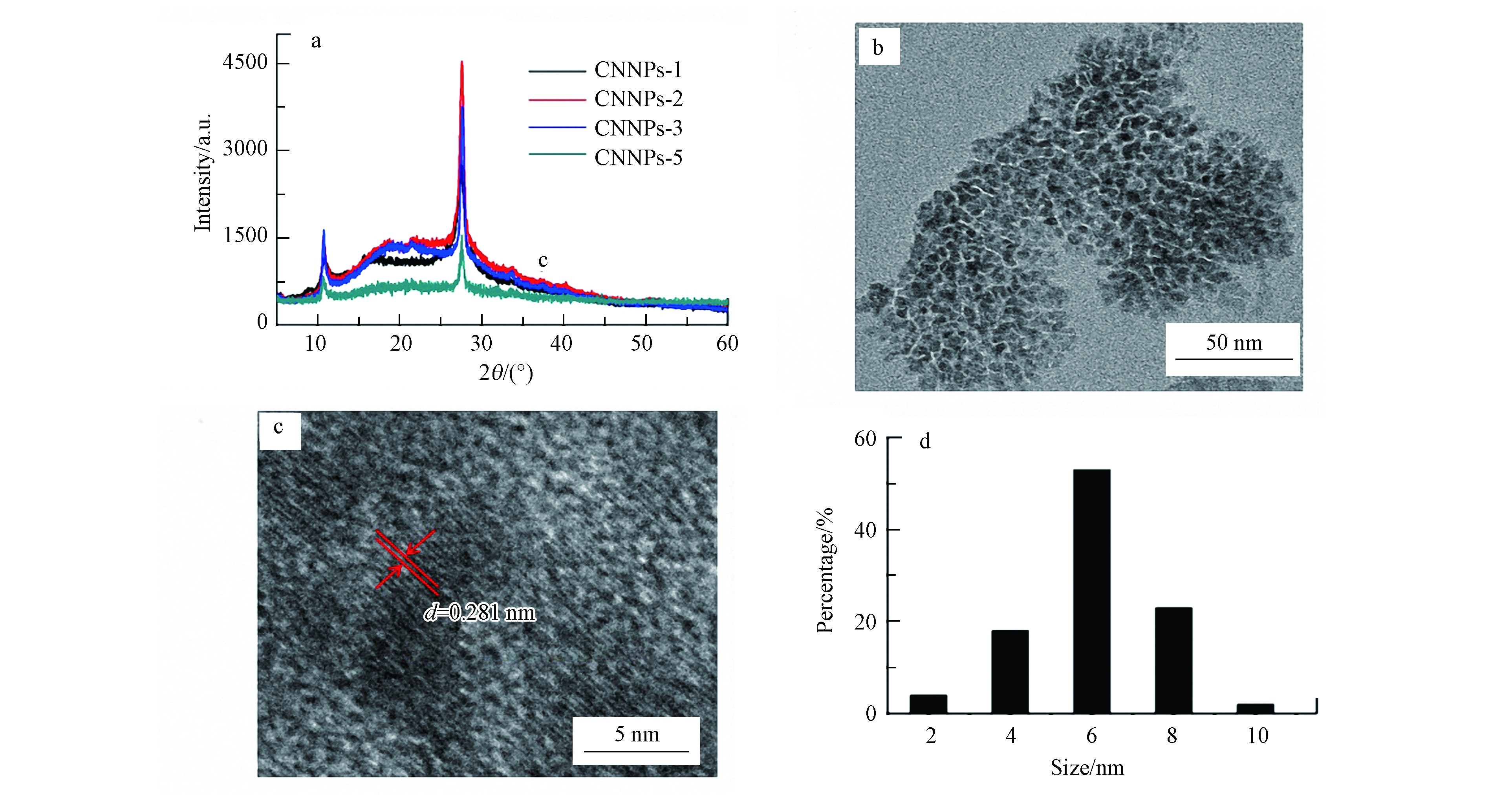
本研究制备的CNNPs样品XRD谱图如图1(a)所示。可以看出,各样品的谱峰均集中在27.5°,与石墨相氮化碳的相关报道一致[5,30],表明成功制备了CNNPs. 柠檬酸的用量越大,所制备的CNNPs水溶性增加,即相对其他3种合成CNNPs材料(CNNPs-1、CNNPs-2、CNNPs-3),CNNPs-5的水溶性最佳,后续选取CNNPs-5作进一步表征和实验. CNNPs-5的微观形貌和粒径分布如图1(b)—(d)所示:CNNPs-5为准球形粒子;具有明显的晶格条纹,测得晶格参数为0.281 nm,与石墨相氮化碳的(002)晶面一致[17];随机测定TEM视野下CNNPs-5材料的50个颗粒粒径大小,结果显示粒径主要分布在4.0—6.0 nm的范围内.
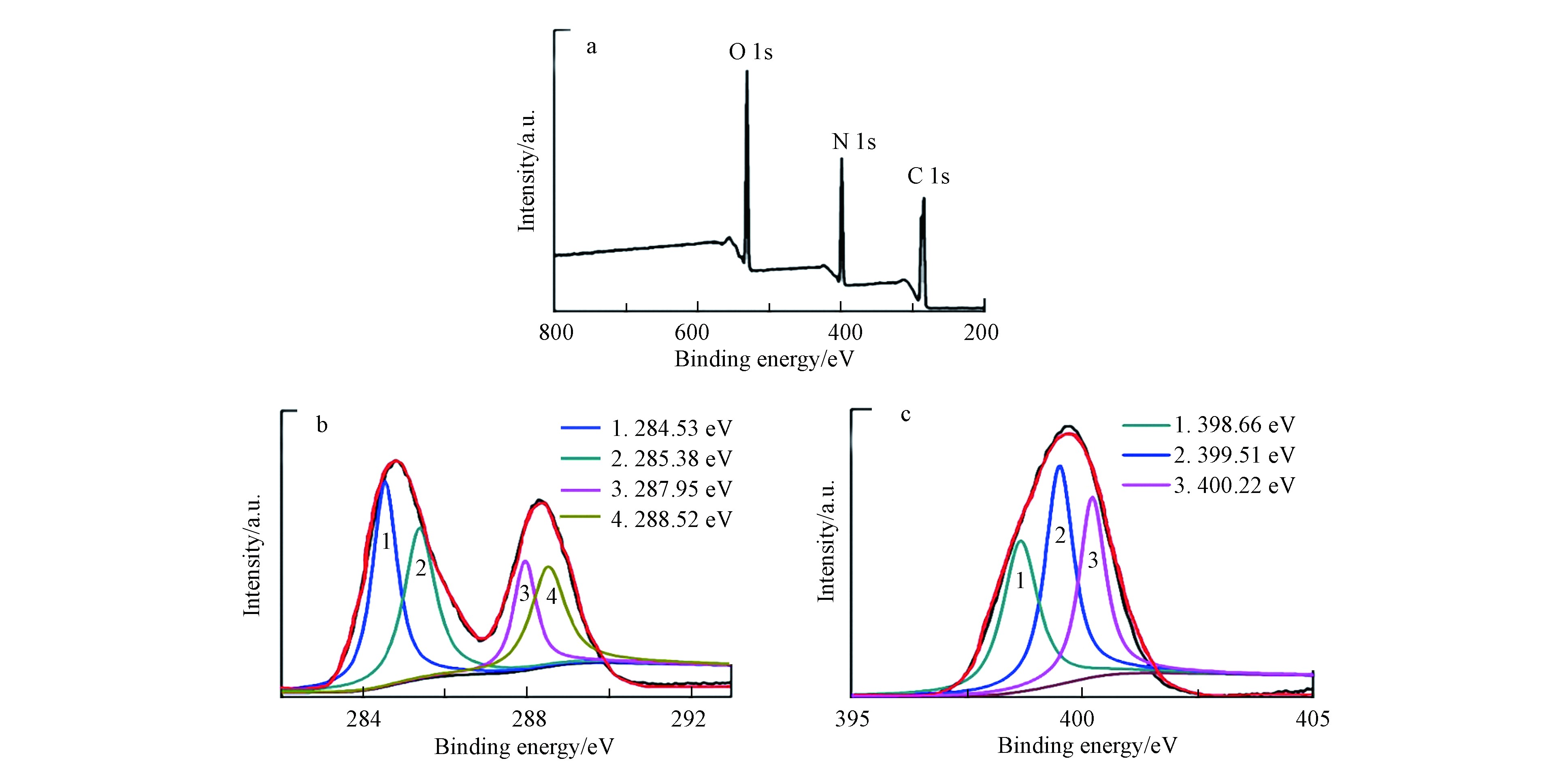
CNNPs-5的XPS谱图(图2a)在285.4、398.4、531.3 eV处有3个峰,可分别归属于C-1s、N-1s和O-1s.C-1s光谱可分解为284.53、285.38、287.95、288.52 eV处的4个峰(图2b),表明存在4种类型的碳键:sp2 C=C或sp3 C—C,C—N或C—O,sp2 C=N,C=O. N-1s光谱的分解表明存在3种类型的氮键:C—N—C(398.66 eV)、N—(C)3(399.51 eV)和C—N—H(400.22 eV)(图2c)[31].
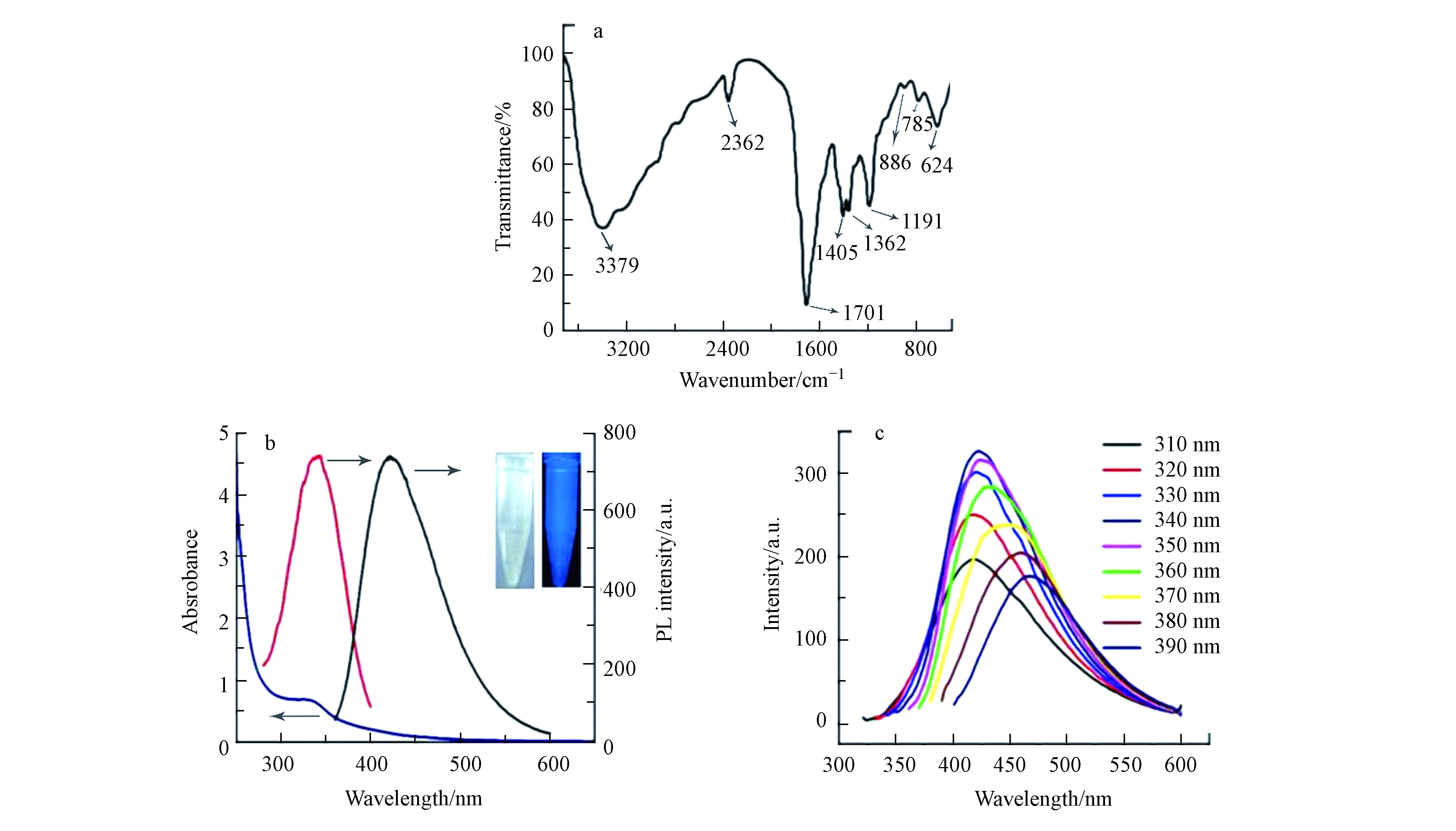
FT-IR光谱(图3a)在1405和1362 cm−1处显示两个特征峰,可归因于芳族C=N伸缩振动. 指纹区域中785 cm−1处的特征峰为s-三嗪环的呼吸振动模式[32],1701 cm−1处的强峰归因于不对称的C=O和C=N的伸缩振动,3379 cm−1处的宽峰归因于N—H和O—H的伸缩振动.
紫外可见光谱(图3b)显示存在一个以335 nm为中心的特征峰,可归因于表面激发态能量的捕获[33],吸收带延伸至550 nm. 激发依赖荧光光谱(图3c)显示,随激发波长在310—350 nm范围内增大,发射波长基本保持不变;随激发波长在350—390 nm范围内增大,发射波长由425 nm红移至460 nm. 随激发波长的增大,荧光强度先增大后减小,在340 nm的激发波长下,425 nm处可观察到最强发射峰,与氮化碳纳米粒子的UV-vis吸收特征相符. 使用硫酸奎宁作为参照,计算得CNNPs-5的量子产率为15.1 %. CNNPs-5在365 nm紫外光下显示出亮蓝色的荧光(图3b,插图).
元素分析结果表明,产物元素组成(质量分数)包含33.11 %的碳,38.46 %的氮,4.15 %的氢和24.28 %的氧. 可以看出,CNNPs-5含有丰富的氧和氮,碳化程度相对较低,含氧和氮的官能团(如羟基和氨基)可能位于石墨氮化碳单元的边缘,这些官能团使产物具有良好的水溶性. Messina等[34]认为富氮碳纳米点的荧光源于壳表面的电子态,可认为本研究所获得的CNNPs-5为功能性氮化碳纳米粒子.
从上述表征结果分析,本研究合成方法具有反应温度较低、操作简便、回收率高、后处理简单等优点,借助微波场的反应时间相对较短,提供了一种合成水溶性氮化碳纳米粒子的简单方法. 合成的材料既具有氮化碳的结构,同时表面丰富的官能团赋予了纳米材料很好的水溶性.
-
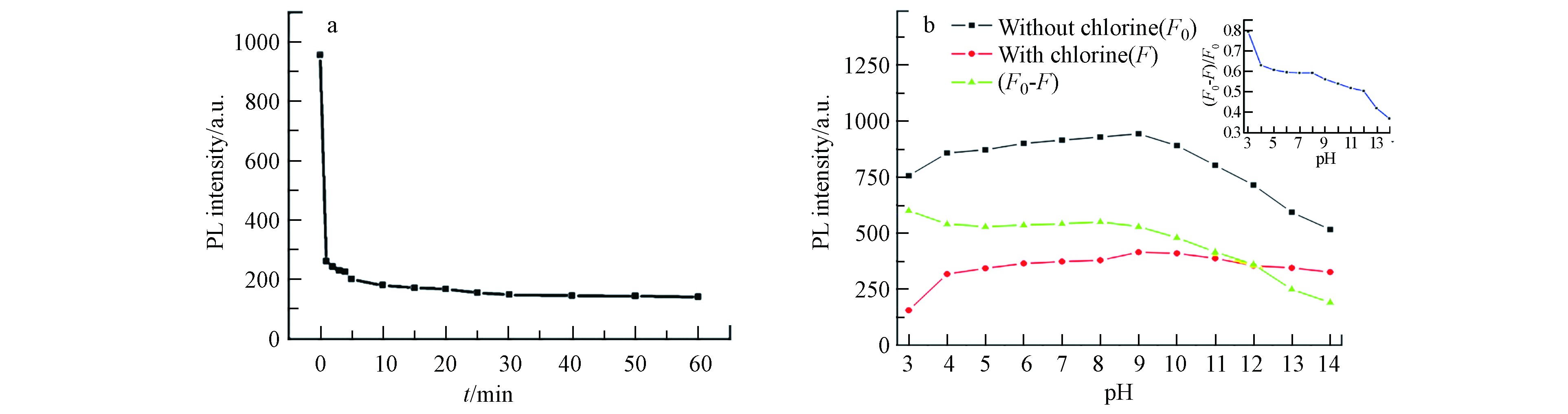
将获得的CNNPs-5用于游离氯的检测,结果如图4a所示,在425 nm处,最初1 min内,50 μmol·L−1次氯酸钠浓度下CNNPs-5的荧光强度猝灭率约为86%. 在接下来的60 min内,荧光强度基本保持稳定. 该结果表明,游离氯对所制备的CNNPs的荧光猝灭非常迅速,可用于溶液中游离氯的快速测定.
图4b显示了次氯酸盐作用下,不同pH值对CNNPs-5荧光响应的影响. 在没有游离氯的情况下,CNNPs-5的荧光强度在4—10的pH范围内基本保持不变,但在较高酸性(pH=3)或较大碱性(pH>10)的情况下明显降低. 在游离氯存在情况下,荧光随pH值变化的趋势与无游离氯时的相似,但在酸性介质中,游离氯对CNNPs-5荧光的猝灭程度比在碱性介质中更为显著. pH值会影响HClO、ClO−和Cl2之间的化学平衡,在酸性至弱碱性溶液(pH=3—9)中,游离氯主要为HClO;在强碱性溶液(pH>9)中,游离氯主要以ClO−的形式存在[35]. HClO的氧化能力比ClO−强,因此氧化可能是荧光猝灭的主要原因. 基于这些实验,该测定方法适用于酸性至弱碱性环境.
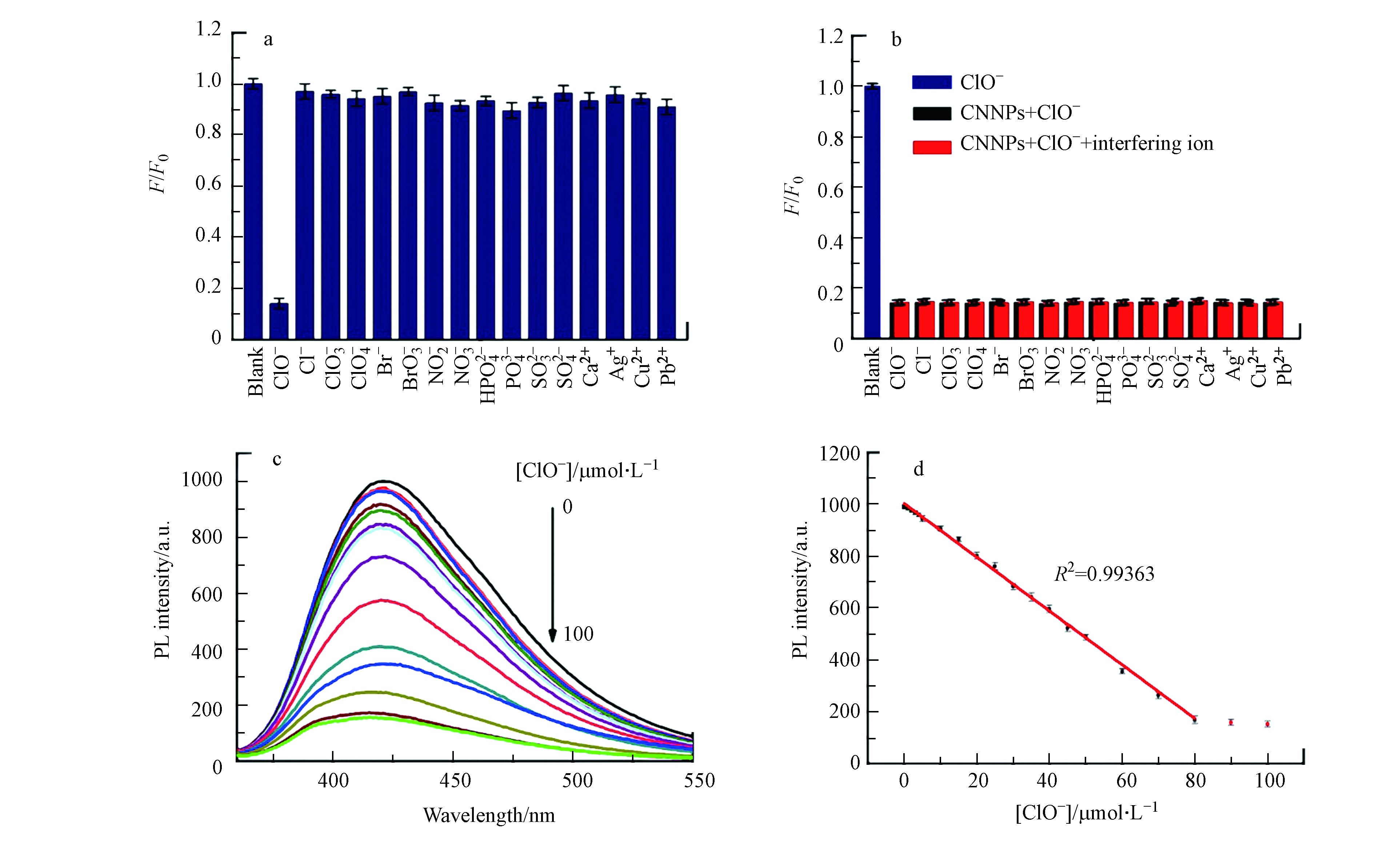
为了评估CNNPs-5测定游离氯方法的选择性,进行了两个对照实验(pH=7.0). 其中选择性响应实验分别添加100 μmol·L−1与生物和环境密切相关的离子,实验结果如图5a所示,在加入ClO−的情况下可观察到明显的荧光猝灭,而其他离子的影响可忽略不计. 在另一个干扰实验中,将ClO−和选择性响应实验离子加入CNNPs-5水溶液的实验结果如图5b(红色条),未添加干扰离子的溶液作为对照组(黑色条,图5b). 实验表明,其他共存离子对ClO−猝灭的影响可以忽略. 这两个对照实验表明,所制备的CNNP-5对ClO−具有高度选择性.
基于以上CNNPs-5的荧光猝灭结果,将不同浓度的ClO−添加到CNNPs-5水溶液中,随着ClO−浓度的增加,荧光强度逐渐降低. 图5c表明,在0—80 μmol·L−1的范围内,荧光强度与ClO−的浓度之间存在良好的线性关系(R2=0.9936). 图5d显示添加各种浓度ClO−后CNNPs-5的荧光发射光谱. 根据3倍标准偏差规则(LOD=3Sd·s−1)计算得检测限(LOD)为0.22 μmol·L−1. 如表1所示,所获得的LOD较利用其他荧光探针的线性范围更宽,或检测限更低[23,36-41]. 表明该分析方法对ClO−离子具有出色的选择性和灵敏度. 不同批次合成的CNNPs-5对ClO−离子的响应一致.
-
自来水样品在未经任何预处理的情况下,掺入不同浓度的ClO−离子,运用上述方法进行分析. 结果如表2所示,从不同位置采集的2份自来水样品中的游离氯浓度分别为1.12、1.09 μmol·L−1,样品的回收率为96.3%—107.5%,相对标准偏差(RSD)小于3.70%(n=3),表明该方法适用于天然水样品的检测.
-
本研究建立了一种简单的利用微波辅助溶剂热法,在适中的反应温度下,利用柠檬酸和三聚氰胺在油酸介质中制备获得荧光氮化碳纳米材料(CNNPs),所制备的CNNPs具有中等的荧光量子产率(15.1%),简单纯化处理可直接用于测定水中游离氯浓度. 该方法简便、快速、低成本且环境友好,测试结果与比色法测试结果一致,可用于检测实际样品中的游离氯.
微波辅助法制备荧光氮化碳纳米材料及其在游离氯检测中的应用
Microwave-assisted preparation of fluorescent carbon nitride nanoparticles and their application in the detection of free chlorine
-
摘要: 在220 ℃下借助微波辅助溶剂热法,利用柠檬酸和三聚氰胺制备荧光氮化碳纳米材料(CNNPs),通过多种分析技术表征了制备的CNNPs。基于游离氯能快速猝灭CNNPs的荧光,将CNNPs做为荧光探针用于游离氯的定量测定,其荧光猝灭速度小于1 min;选择性响应实验和干扰实验表明该测定方法具有较好的灵敏度和选择性,游离氯的线性响应范围为0—80 μmol·L−1,检测限低至0.22 μmol·L−1。该方法适用于实际水样中游离氯的检测。Abstract: Fluorescent carbon nitride nanoparticles (CNNPs) were prepared from citric acid and melamine by a facile microwave-assisted solvothermal method at moderate reaction temperature (220 ℃). The obtained CNNPs were characterized by multiple analytical techniques. Free dissolved chlorine can quench the fluorescence of the CNNPs with excellent sensitivity and selectivity. The quenching speed was rather quick (less than 1 min), selective response experiments and interference experiments showed that this determination method had excellent sensitivity and selectivity. The linear response range of free chlorine was from 0 to 80 μmol·L−1. The limit of detection was as low as 0.22 μmol·L−1. We conclude that this method is applicable to detect free chlorine in real water samples.
-
Key words:
- carbon nitride nanoparticles /
- fluorescent quenching /
- free chlorine /
- melamine /
- citric acid
-
挥发性有机物(volatile organic compounds,VOCs)是城市大气中一种重要的大气污染物。VOCs化学反应活性较高,是产生臭氧的重要前体物质[1-2],并参与雾霾形成[3],故受到越来越多研究者关注。王佳颖等[4]的研究结果表明,北京市夏季大气中O3的生成主要受VOCs控制,特别是受人为源VOCs控制;罗恢泓等[5]发现,上海市中部及东部大气O3的生成亦主要受VOCs控制;韩旸等[6]发现,天津市大气中VOCs的化学反应活性较强,尤其是低碳(C2~C5)的烯烃和烷烃。部分VOCs具有一定的致癌和非致癌毒性作用,如苯、甲苯和1,3-丁二烯等,长期暴露则对人体具有健康风险[7]。现有研究结果表明,工业源是我国 VOCs 的主要排放源[8],因此,加强工业污染控制是大气治理的首要内容[9]。
厦门市位于福建省南部,毗邻台湾海峡。近年来多位学者的研究表明,厦门空气质量呈现明显下降趋势[10]。NIU等[11]研究了厦门市海沧工业区 VOCs 的污染特征;徐慧等[12]研究了厦门冬春季大气VOCs的污染特征及臭氧生成潜势;王坚[13]进行了厦门市VOCs排放特征及光化学反应特征研究。目前,针对厦门市VOCs的相关研究多集中于VOCs污染排放特征及臭氧生成潜势方面,少有针对厦门市具体企业VOCs治理工艺效果及区域性治理效果的评估。空间地理信息系统(geographic information system,GIS)结合区域地理信息,可更有针对性地对各区域VOCs来源及分布等情况进行评估。高爽等[14]借助GIS对常州市区各类大气污染贡献源进行了分析,结果表明工业污染是常州市区最主要的大气污染源,主要污染行业为热电厂和钢铁厂,交通污染次之。黄络萍[15]借助GIS平台对成都市工业源VOCs空间分布特征进行了研究,得出了工业源、溶剂使用源、工艺过程源、化石燃烧源、生物质燃烧源等的相关空间分布及相关地区排放贡献率。
本研究以厦门市8大重点监测行业的企业为代表,通过企业实地调研,实地设备检测等方式,得到VOCs治理设备参数和去除率,进而相关对治理工艺的去除效果进行了评估。同时,运用空间地理信息系统(GIS)将VOCs去除率、排风口VOCs浓度等企业实测参数在地图上进行可视化呈现,据此对厦门市VOCs治理情况进行区域性评价,并与治理工艺的特点相结合进行分析,提出针对不同治理工艺的应用建议,以期为厦门市各重点监测行业的VOCs治理技术选用策略提供参考。
1. 研究对象和方法
1.1 研究对象
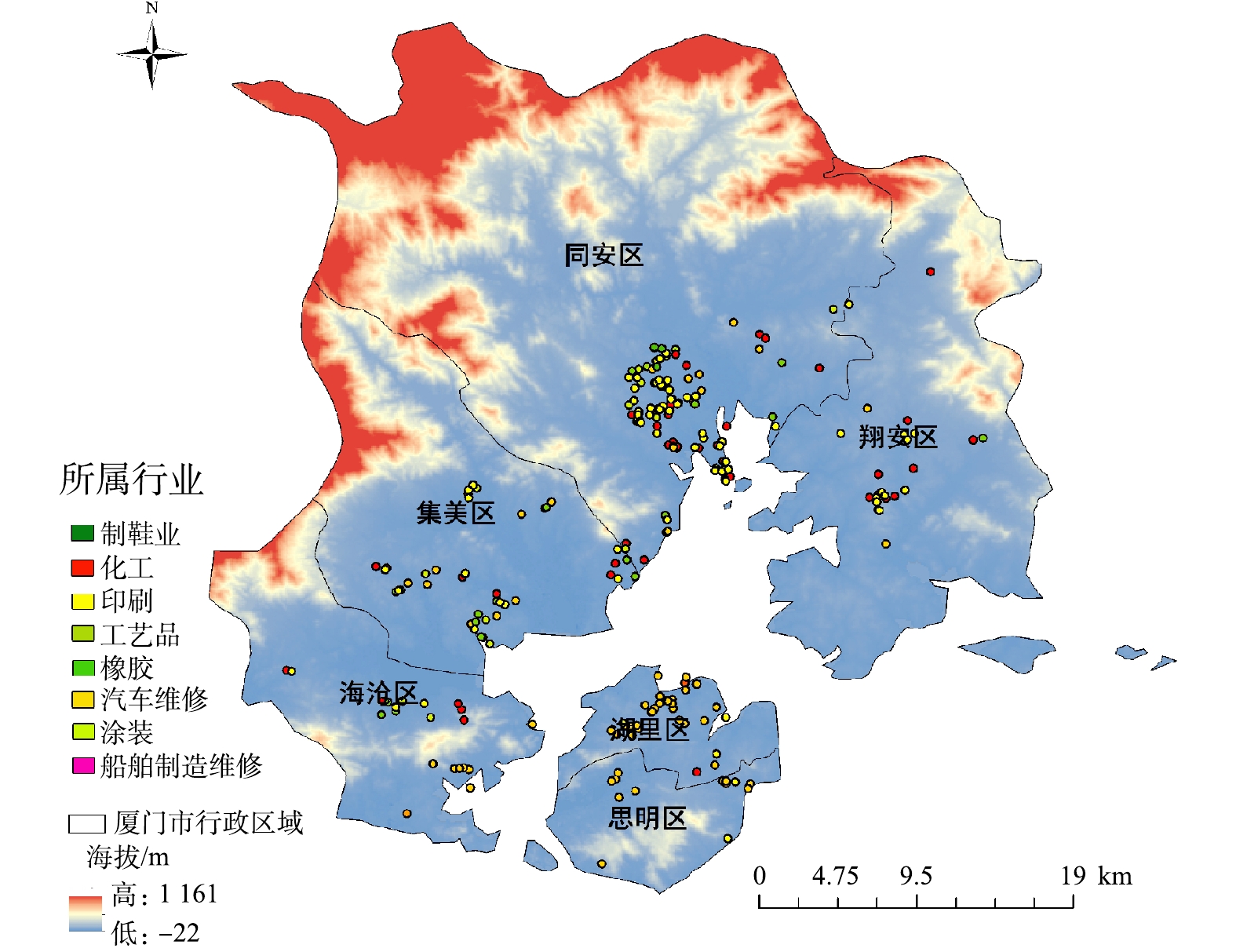
综合考虑所调研行政区各行业占比、企业VOCs排放量、国家行业规范以及各级政府管理文件的具体要求,本研究选取厦门市地区8个VOCs重点监测行业(汽车维修、工业涂装、印刷、橡胶、化工、工艺品、制鞋业、船舶维修)的370 家典型工业企业共计439套设备为研究对象,进行了3个阶段的调查(各行业企业数占比如图1所示)。图1数据表明,汽车维修、印刷、化工、橡胶制造4个行业为此次调查所涉及到的主要行业,相关企业分布在厦门全市6个辖区(思明区、湖里区、集美区、海沧区、同安区、翔安区),涉及区域广、VOCs排放占比高。
1.2 研究方法
1)数据获取方法。本次调查数据来自于厦门市8大行业VOCs调研,通过实地调研对企业相关处理设备参数(活性炭填装量及更换周期、紫外灯管功率及安装数目、催化剂种类等)进行收集;通过实地检测,对企业设备进出口风量、进出口相关物质浓度(VOCs、苯、甲苯、二甲苯)等进行测量和记录。采用HJ 732-2014《固定污染源废气挥发性有机物的采样气袋法》[16]、HJ 734-2014《固定污染源废气挥发性有机物的测定固相吸附-热脱附/气相色谱质谱法》[17]及HJ 584-2010《环境空气苯系物的测定活性炭吸附/二硫化碳解吸-气相色谱法》[18]对废气进行收集与检测,符合国家相关检测方法规定。
2)VOCs治理工艺实际效果评估方法。工业 VOCs 治理工艺的去除率(η)根据式(1)计算得到。
stringUtils.convertMath(!{formula.content}) (1) 式中:C1、C2 分别为治理前、后VOCs浓度,mg·m−3;Q1、Q2分别为治理前、后采样口在分析状态下的气体流量,m3·h−1; 根据式(1)可计算出各治理工艺对VOCs的去除率。将苯系物进出口浓度带入C1、C2,根据式(1)可计算各治理工艺对苯、甲苯、二甲苯等苯系物的去除率。
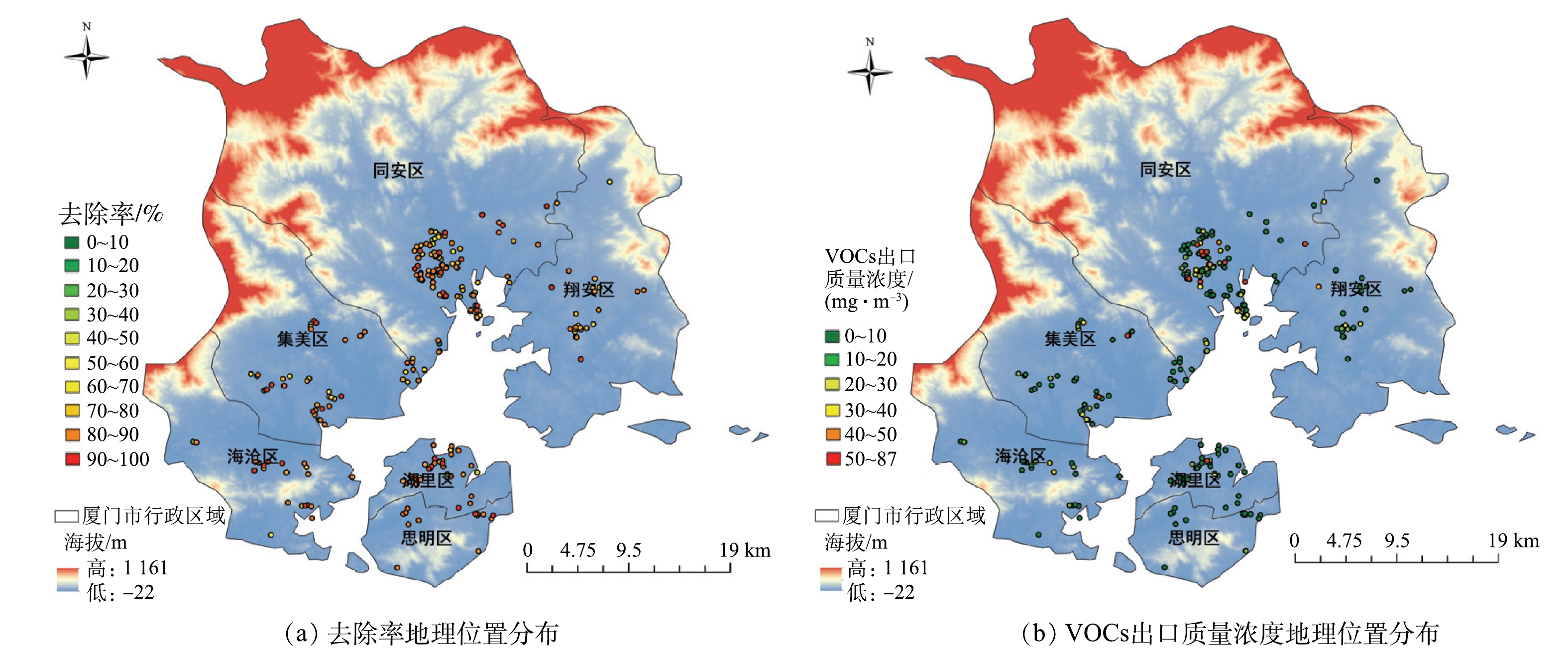
3)基于GIS平台的治理效果评估方法。运用ArcGIS软件将受调查企业标注在厦门市地图上,并按照行业、设备去除率、出口VOCs浓度进行分类,不同梯度采用不同颜色的点进行标注(部分企业存在多套相同工艺设备,需将对应参数取平均值后再进行分类标注),得到相关参数的区域分布图,结合区域图对VOCs的治理效果进行区域性评估。
2. 结果与讨论
2.1 VOCs排放企业分析
根据国民经济行业分类梳理8大行业的细分情况、各子行业排放源及各行业VOCs产生量,结果如表1所示。由表1可见,各种有机化学原料、橡胶、活性剂、黏合剂等原辅材料被广泛使用,印刷、化工及涂装行业大量使用粘合剂、稀释剂等高VOCs产生量的原辅材料,导致这些行业VOCs产生量较大(>1 000 kg·d−1),其中印刷行业VOCs产生量最大,达到5 158.1 kg·d−1,其余行业的VOCs产生量较低。
表 1 行业划分及VOCs产生量情况Table 1. Industry classification and VOCs production行业大类 行业子类 排放源 企业数量/家 平均VOCs产生量/(kg·d−1) 化工 基础化学原料制造 乙烯、丙烯、丙烯腈、丁乙烯、苯乙烯、苯和化学原料药 8 1 651.0 涂料、油墨、颜料及类似产品制造 油墨、油漆、炭黑、染料和印染 10 合成材料制造 合成橡胶、丙烯腈、乙二醇、尼纶、涤纶、合成纤维单体、腈纶、丙纶、维纶和黏胶纤维等 36 专用/其他专用化学品制造 促进剂和黏合剂 5 日用化学品制造 合成洗涤剂香料和活性剂 11 涂装 金属家具涂层 — 3 1 479.9 木质家具涂层 — 6 金属制品业 — 7 机械设备制造 设备和机床防腐、卷材、其他涂料和装配用胶黏剂 6 橡胶 橡胶制品制造 橡胶制品 24 64.6 其他橡胶制品制造 橡胶制品 11 橡胶和塑料制品业 人造革和合成革 13 印刷 装订及印刷相关服务 装订用胶黏剂 15 5 158.1 包装装潢及其他印刷 油墨印刷润版液和稀释剂 85 汽修 汽车喷涂 轿车、汽车(大车)和摩托车修补涂料 111 344.1 工艺品 雕塑工艺品制造 油漆、固化剂和天那水 2 1.3 金属工艺品制造 油漆、固化剂和天那水 2 其他工艺品制造 油漆、固化剂和天那水 7 船舶维修 船舶改装与维修 船舶涂料 2 14.2 制鞋业 橡胶鞋制造 胶黏剂 1 67.5 皮鞋制造 胶黏剂 5 厦门市8大行业企业地理位置分布情况如图2所示。由图2可见,本次调查企业广泛分布于厦门市6个区(海沧区、集美区、同安区、翔安区、湖里区、思明区),其中岛外区域企业数量远多于岛内区域。岛外区域主要以印刷、化工、涂装等高VOCs产生量(>1 000 kg·d−1)行业为主。岛内区域主要以汽车、船舶维修等低VOCs产生量(<1 000 kg·d−1)的服务业行业为主,基本不涉及印刷、化工、涂装等行业,这与厦门市辖区规划和产业布局有关。由于岛内地理位置靠近海域且辖区面积较小,岛内产业主要以旅游、软件、电子以及服务类行业为主,岛外则以大规模的工业企业为主,所以VOCs产生量及排放量也呈现出岛外>岛内的特点。
2.2 各类VOCs废气治理工艺的应用情况
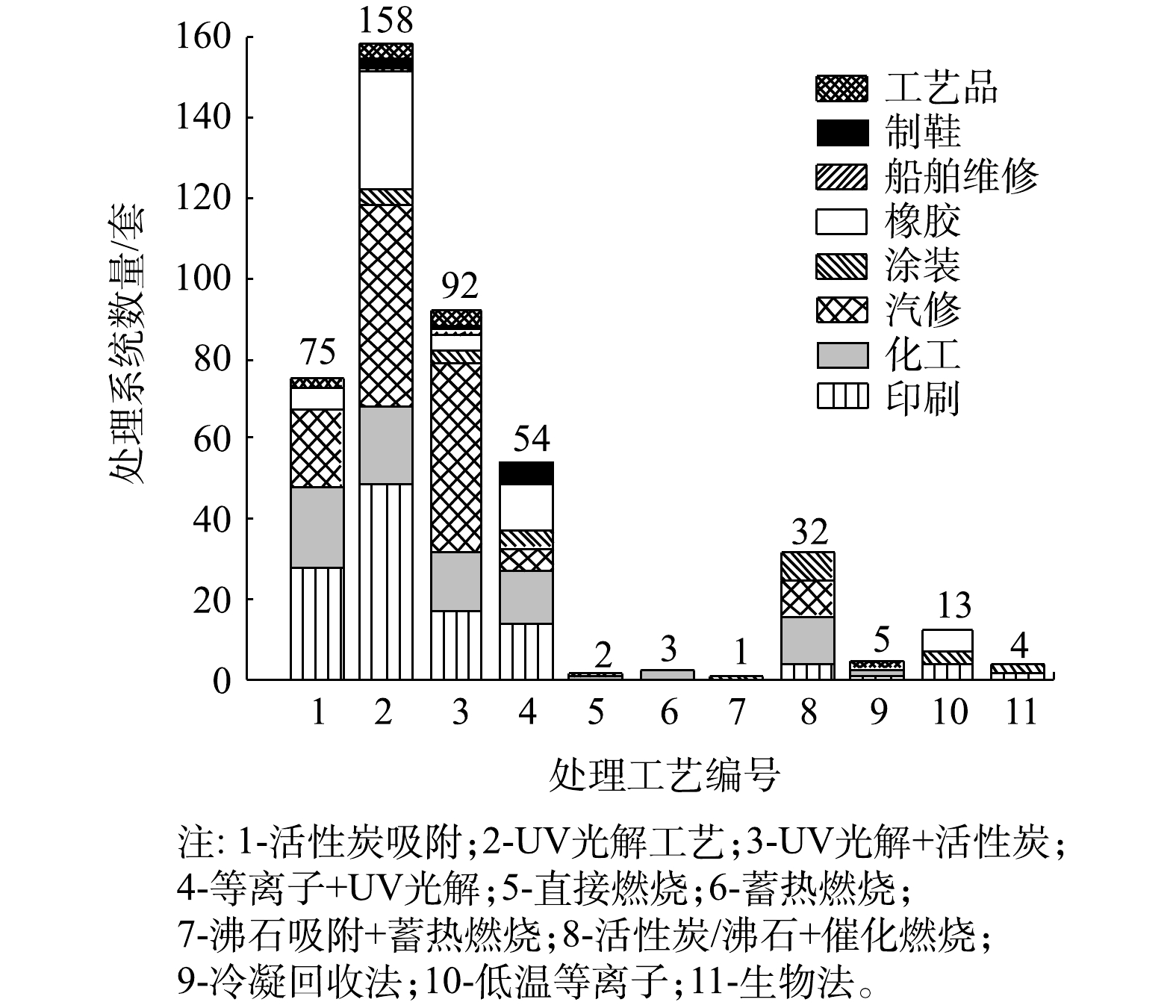
各类VOCs治理工艺在厦门市8大行业中的应用情况如图3所示。由图3可见,从工艺组合上看,多数企业采用单一VOCs治理技术,占调研企业的59.5%,采用组合式VOCs治理技术的企业占被调研企业总数的40.5%。从治理技术的使用分布上看,UV光解(包含UV光解和UV光催化)及其相关组合工艺应用最广,共有304套处理系统,占总数的69.2%,涉及行业主要为汽修、印刷、化工和橡胶;其次为活性炭吸附工艺,共有75套处理系统,占总数的17.1%,其中采用含蜂窝活性炭的处理系统有125套,含颗粒活性炭吸附的处理系统有24套,含纤维活性炭的有18套,涉及行业主要为印刷、化工和汽修;燃烧类工艺(包括直接燃烧、蓄热燃烧、沸石吸附+蓄热燃烧和活性炭/沸石+催化燃烧工艺)在本次调研企业中也有一定比例的应用,共计32套设备,占总数的7.3%,涉及行业主要为化工、汽修和涂装;另有个别企业采用除上述工艺的其他工艺,因数量较少,将其归为其他类工艺进行分析。
2.3 不同治理技术的处理效果及影响因素
2.3.1 对VOCs的去除率
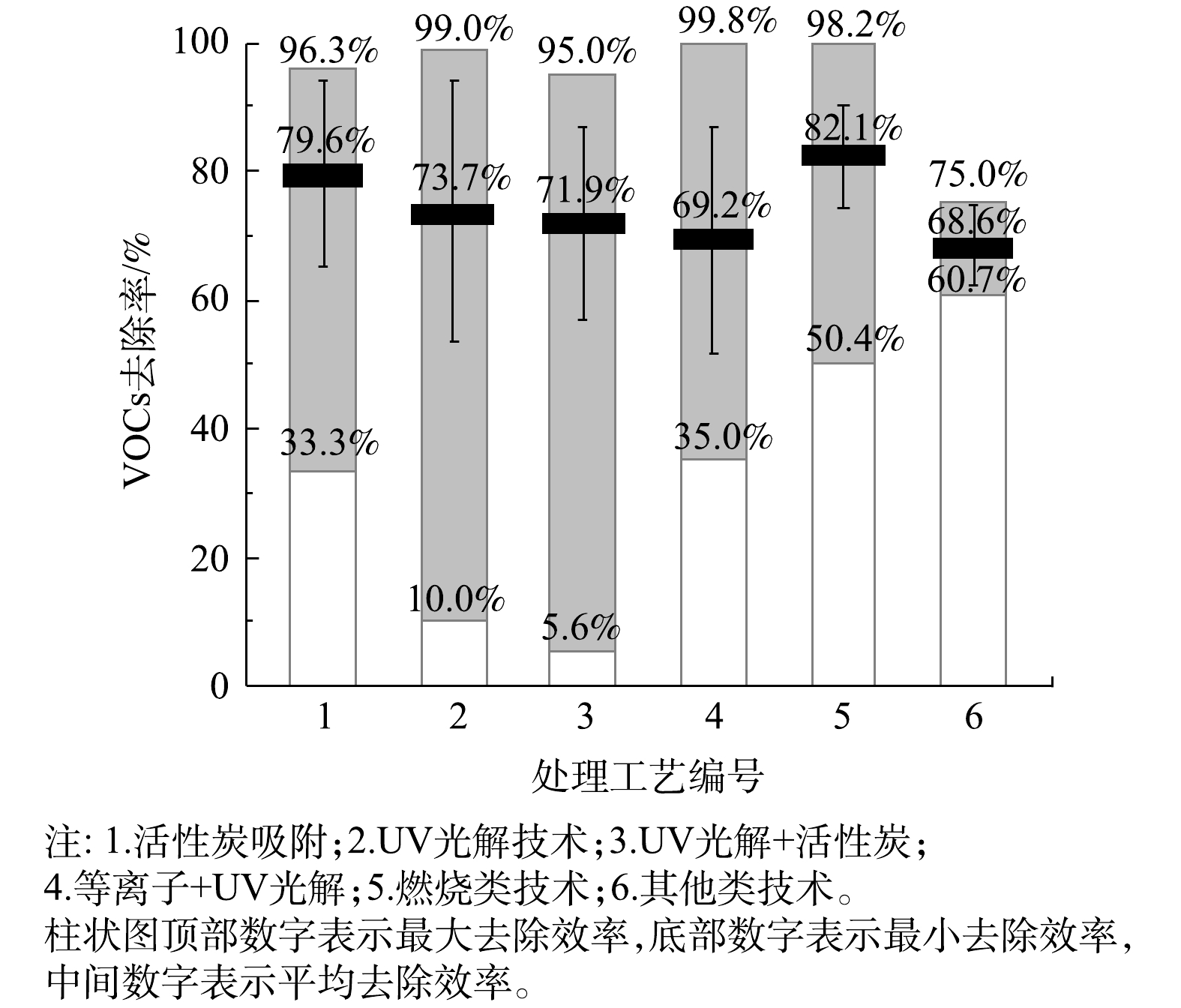
6类处理技术的VOCs去除率如图4所示。在处理的稳定性方面,与活性炭吸附及UV光解有关的工艺VOCs去除率波动较大,去除率的标准偏差也较大,其中“UV光解+活性炭”工艺的标准偏差最大。其可能原因有3点:1)不同行业存在原辅材料、生产工艺和现场环境等方面的差异,故采用同一治理工艺,去除效果也有差异;2)活性炭吸附及其组合工艺存在活性炭相关参数和更换周期差异问题;3)UV光解及相关组合工艺的处理效果受紫外灯管品牌和功率差异、催化剂装填量和方式不同等问题影响。
在平均VOCs去除率方面,6类治理工艺在现场实测条件下的平均去除率为68.6%~82.1%,其中燃烧类工艺的平均去除率最高,其次为活性炭吸附工艺。燃烧类工艺中“活性炭/沸石+催化燃烧”工艺的平均去除率达到80.5%,直接燃烧、蓄热燃烧、“沸石吸附+蓄热燃烧”等工艺虽采用企业较少,但平均去除率也均在90%以上;UV光解及其组合工艺平均去除率较低(均低于75%)。其他类工艺中生物法、低温等离子法、冷凝回收法的平均去除率均在70%左右。
不同处理系统在各个行业中的平均处理效率如表2所示。由表2可知,活性炭吸附工艺对工艺品、印刷、汽修行业的平均去除率较高(均高于80%),治理效果较好,但对橡胶行业的平均去除率仅为63.4%,治理效果较差;UV光解及其组合工艺对汽修行业的平均去除率较高(80%左右),但在印刷、化工、橡胶等行业中平均去除率均较低,治理效果差。燃烧类工艺在各行业处理效果均较好(高于75%),其中在印刷、化工行业处理效果最好,平均处理率均在80%以上。其他类工艺在各行业中发平均去除率为61.2%~74%,处理效果相对较差。
表 2 不同处理工艺在各行业的处理效果Table 2. Treatment efficiency of process in various industries处理工艺 各行业VOCs平均去除率/% 印刷 化工 汽修 涂装 橡胶 船舶维修 制鞋 工艺品 活性炭吸附 83.0 78.0 85.3 — 63.4 — — 92.5 UV光解工艺 69.2 75.3 81.6 72.5 69.2 53.1 83.1 67.4 UV光解+活性炭 60.0 70.9 79.0 77.0 72.0 62.3 52.6 76.5 等离子+UV光解 75.4 70.3 92.9 72.5 53.7 — 57.0 — 燃烧类工艺 92.1 81.4 76.1 77.2 — — — — 其他工艺 61.2 72.4 — 74.0 72.3 — — 63.0 2.3.2 对苯系物的去除效果
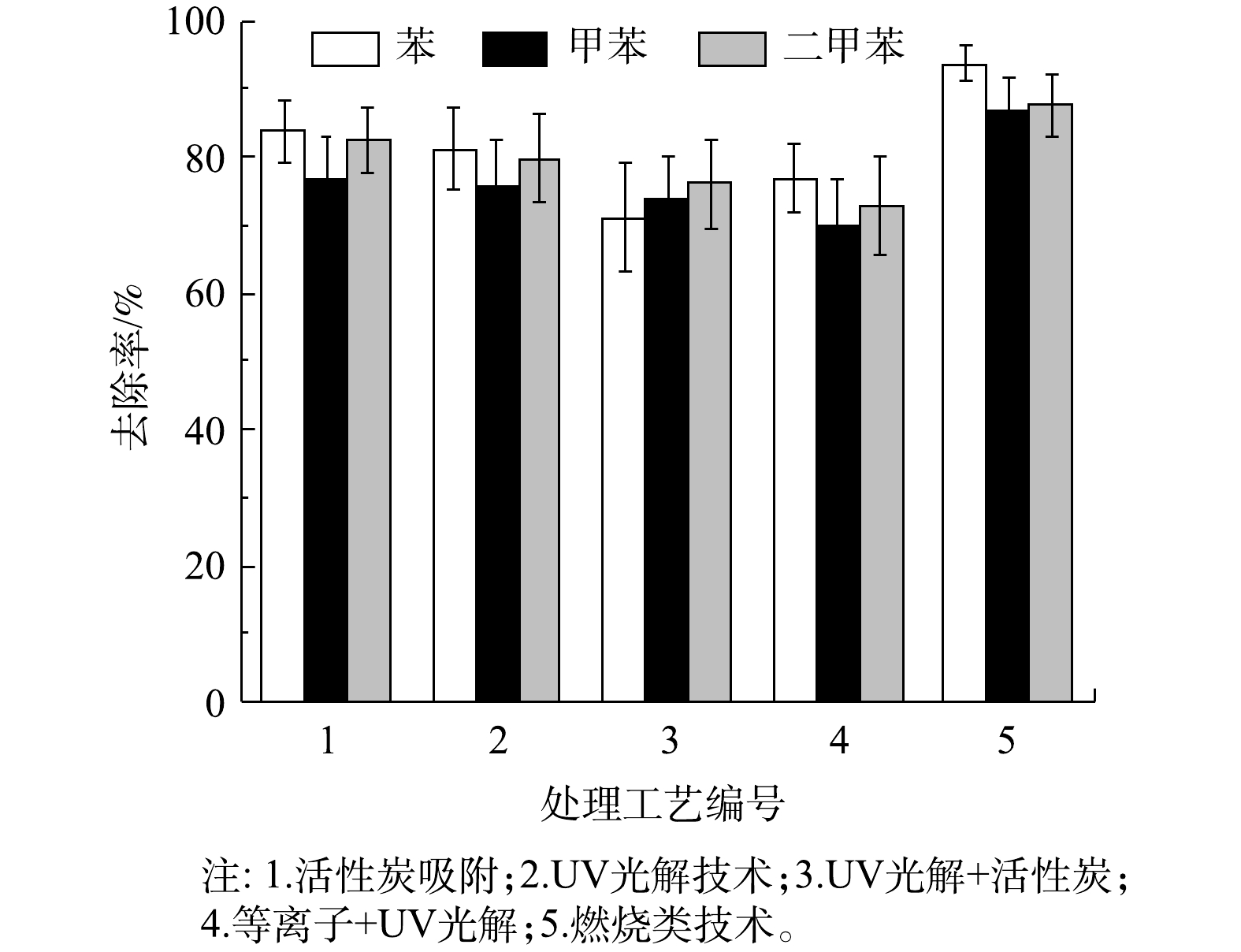
甲苯、二甲苯属于苯的同系物,都是煤焦油分馏或石油的裂解产物[19]。苯系化合物已被世界卫生组织确定为强烈致癌物质[20]。由于本次调查涉及大量汽修以及印刷行业企业,部分涂装行业企业,其中含苯系物的油漆、涂料和防水材料的溶剂或稀释剂被广泛使用,故将苯系物去除率也作为VOCs去除效果评定指标。
5 种处理工艺的苯系物去除率如图5 所示。5种工艺中,燃烧类工艺对苯系物的去除率最高,对苯、甲苯、二甲苯的平均去除率分别达到93.6%、86.9%、87.6%;其次是活性炭吸附工艺,对苯、甲苯、二甲苯的平均去除率分别为83.8%、76.8%、82.5%;“UV光解+活性炭联用”工艺,因为与活性炭工艺联用,去除率有一定的提升,高于UV光解及“等离子+UV光解”工艺,但整体UV光解及其组合工艺对苯系物去除率较低。
2.3.3 对VOCs处理效果的影响因素
1)进口VOCs浓度。各处理工艺在不同进口VOCs 浓度下的去除率如表3所示。由表3可见,进口VOCs浓度对各种治理技术的处理效果有较大影响。各工艺VOCs去除率均随着进口VOCs浓度的升高而增大,且波动范围变小,处理效果更加稳定。经分析,其主要原因为VOCs浓度偏低,影响了吸附反应、化学或光化学反应的速率,在相同反应时间内,VOCs的转化速率下降,从而影响VOCs的去除效率。
表 3 不同进口 VOCs 浓度下各种处理工艺的处理效果Table 3. Treatment efficiency of various processes under different VOCs concentrations at the inlet处理工艺 ρ1下的去除率/% ρ2下的去除率/% ρ3下的去除率/% ηmin ηadv ηmax ηmin ηadv ηmax ηmin ηadv ηmax 活性炭吸附 33.3 77.6 96.3 86 90.5 95 87.3 91.2 95 UV光解 10 72.6 99 70.5 70.2 85.1 80.6 89 95.2 UV光解+活性炭 5.6 71.4 95 63.9 82.5 92.1 89.6 90.4 90.6 等离子+UV光解 35 67.5 99.1 60.9 80.7 99.8 82.9 82.9 82.9 燃烧类工艺 50.4 77.1 95.3 — — — 82.9 91.5 98.2 其他 60.7 68.6 75 — — — — — — 注:ρ1 ~ ρ3 分别表示ρ<100 mg·m−3、100 mg·m−3≤ρ<200 mg·m−3、ρ≥200 mg·m−3;ηmin、ηadv、ηmax分别表示最小去除率、平均去除率、最大去除率。 2)进口风量。各处理工艺在不同进口风量下的VOCs去除率如表4所示。由表4可见,进口风量对UV光解类工艺影响较大,随着风量的增大,这类工艺的VOCs去除率下降,且波动变大;UV光解类工艺在高风量(>20 000 mg·m−3)条件下对VOCs去除率较低。活性炭吸附和燃烧类工艺受风量影响较小,这2类工艺在高风量(>20 000 mg·m−3)条件下,对VOCs仍保持较高的去除率(高于80%)。
表 4 不同进口风量下各种处理工艺的处理效果Table 4. Treatment efficiency of various processes under different air volume of inlet处理工艺 V1下的去除率/% V2下的去除率/% ηmin ηadv ηmax ηmin ηadv ηmax 活性炭吸附 50.4 80.1 95.6 33.3 80.2 96.3 UV光解 29.1 75.4 99 10 71.9 90.2 UV光解+活性炭 33.1 75.4 95 5.6 68.3 91.6 等离子+UV光解 53 74.9 99.8 35 61.2 97 燃烧类工艺 50.4 80.3 90 57.6 81.5 98.2 其他 — — — 60.7 68.6 75 注:V1、V2分别表示V<20 000 m3·h−1、V≥20 000 m3·h−1情况;ηmin、ηadv、ηmax分别表示最小去除率、平均去除率、最大去除率。 3)设计参数选取。对于不同的 VOCs 治理工艺,要发挥其最佳处理效果,必须选取合适的设计参数。本次调研的企业中,大部分处理效率不佳的VOCs处理设备均存在设计参数选取不当的问题。如采用活性炭吸附和其组合工艺的企业中,有58家企业的设备不符合《厦门市生态环境局关于加强挥发性有机物污染防治的通告》[21-23](后简称“通告”)中的相关规定。根据规定中的要求,采用不具备脱附功能的吸附法治理废气时,每10 000 m3·h−1设计风量的吸附剂装填量应不小于1 m3,否则会出现活性炭填装量不足的现象,从而导致部分设备VOCs去除率偏离正常值。又如采用UV光解及其组合工艺的企业中,有93家企业设备使用的紫外灯管在每10 000 m3·h−1风量下总功率低于8 kW,有7家企业设备每10 000 m3·h−1风量停留时间不足1 s,不符合通告[21-23]中“采用光催化氧化法治理废气时,每10 000 m3·h−1设计风量的紫外灯管总功率不得低于8 kW,废气停留时间不得低于1 s”的相关要求。紫外灯管功率与废气停留时间均不足,导致UV光解类工艺大量设备处理效率低于理论值。
4)工艺联用。本次调研企业采用的组合工艺为“UV光解+活性炭工艺”、“等离子+UV光解工艺”、“活性炭/沸石吸附+催化燃烧工艺”和“沸石吸附+蓄热燃烧工艺”。不同治理工艺的联用,可在反应上相互促进,能在提高处理VOCs去除率的同时节省能耗,如燃烧类工艺(催化燃烧、蓄热燃烧)与活性炭、沸石等吸附工艺联用,将大风量、低浓度的有机废气被浓缩成小风量、高浓度的废气后再进行燃烧处理,大大提升了工艺的VOCs去除率、热量的利用效率和对工况条件的适应性。其中,“活性炭/沸石吸附+催化燃烧”工艺利用燃烧余热对吸附饱和的活性炭进行脱附,不仅促进了热量循环,也在一定程度上克服了由于活性炭性质差异、更换周期过长以及填料不足所带来的负面影响。而本次调查中UV光解相关组合工艺去除率低于UV光解工艺,其可能原因有:①UV光解工艺本身存在设计参数选取和治理设施维护问题,导致部分设备VOCs去除率低于正常值;②与活性炭工艺联用中,部分设备活性炭装填量不足或更换周期过长,导致活性炭吸附效果较差,甚至出现脱附现象;③等离子体工艺中,等离子体产生量少而实际有效反应区的风速过大、停留时间过短,无法有效净化VOCs[24]。
5)治理设施维护。不同VOCs 治理技术要达到稳定的处理效果,都需要做好维护工作,但因缺少专业人员维护和节约成本等原因,本次调研的企业中普遍存在日常管理及维护问题。采用活性炭吸附相关工艺的企业中,有57%的企业活性炭更换周期超过3个月,部分企业更换周期超过12个月,存在活性炭更换不及时的现象。活性炭吸附过程同时也是吸附和解离相平衡的过程,当活性炭吸附饱和时,就会出现脱附大于吸附的现象[25]。这样的设备运行时会因为吸附饱和活性炭的脱附现象导致VOCs的去除率降低,甚至出现处理后VOCs浓度高于处理前的现象。部分采用UV光解类工艺的企业紫外灯管损坏维修不及时,设备无法达到设计功率,故导致VOCs去除率偏低。
2.4 区域治理效果评估
各企业治理效果的分布情况如图6所示,从处理效果上看,思明区90%以上企业去除率高于80%,50%左右企业去除率高于90%,治理效果最好;其次为湖里区,该区绝大多数企业去除率高于70%,超过70%企业的去除率在80%以上。可见,岛内2个区整体治理效果较好。岛外4个区域中,海沧区去除率在80%~90%与90%~100%区域上的企业最多,处理效果最好,但有部分企业去除率为50%~70%,治理效果差于岛内2区;翔安区80%左右企业去除率在70%~80%,但是中部及北部少数企业处理效率在40%~60%,处理效果一般。集美区与同安区虽有部分企业去除率高于80%,但整体去除率偏低,大量企业去除率分布在50%~70%,同安区中超过40%企业去除率低于70%,在6个区域内治理效果最差。
从出口VOCs浓度来看,除湖里区某企业出口质量浓度高于50 mg·m−3外,岛内各企业VOCs出口质量浓度均低于20 mg·m−3,出口浓度处在较低水平。在岛外区域,海沧区有2家企业出口质量浓度为20~30 mg·m−3,翔安区有3家企业出口质量浓度为20~40 mg·m−3,集美区中有3家企业出口质量浓度为20~40 mg·m−3、2家企业浓度超过50 mg·m−3,部分企业出口浓度偏高。除这部分企业外,上述3区各企业VOCs的出口质量浓度均低于20 mg·m−3。同安区中,有将近40%左右的企业VOCs出口浓度高于20 mg·m−3,中部地区有大量企业VOCs出口质量浓度高于50 mg·m−3,甚至出现了87 mg·m−3的高质量浓度值。该区整体VOCs出口质量浓度处于较高水平。综上所述,对厦门市域各行政区8大VOCs 重点监测行业的企业整体治理效果排序为:思明区>湖里区>海沧区>翔安区>集美区>同安区。
2.5 VOCs废气治理技术的应用建议
1)在使用活性炭工艺处理VOCs 废气时,应确保活性炭填装量符合通告规定的标准,并在活性炭达到吸附饱和时及时进行更换。
2)在应用UV光解及其组合工艺时,应确保紫外灯管总功率和废气停留时间符合通告中的相应技术规范。当处理苯系物含量较高的VOCs废气时,建议用活性炭吸附工艺进行替代或联用,以确保对苯系物的高效去除。光催化工艺中的催化剂优先考虑蜂窝陶瓷负载的光催化剂。
3)在应用燃烧类工艺处理低浓度废气时,应优先考虑通过吸附方式进行浓缩后再燃烧。直接燃烧能耗相对较高,但净化更为彻底。蓄热燃烧可降低能源消耗。“活性炭/沸石+催化燃烧”工艺可在一定程度上避免因活性炭所导致的问题,对工况适应性较强,但当废气中含有硫、硅和卤素等易使催化剂中毒的物质时,应优先考虑直接燃烧、蓄热燃烧等燃烧类工艺。
4)生物法、冷凝回收法、低温等离子等3种工艺的处理效率较低,但也具有低成本的优点,可视具体情况应用于低VOCs排放企业。
5)由于同安区、集美区、翔安区中印刷、化工等高VOCs产生量企业较多,整体治理效果较差,因此在今后应加强这3个区域的治理,且不建议同安区、翔安区、集美区、海沧区等高VOCs产生量(>1 000 kg·d−1)与高进口风量(>20 000 m3·h−1)的企业使用UV光解及其组合工艺,建议以燃烧类工艺进行替代。
3. 结论
1)本次调查的6类治理工艺中,燃烧类工艺对VOCs处理效果最好,平均去除率为82.1%;活性炭吸附类工艺在工艺品、印刷、汽修行业的治理效果较好,但在橡胶行业治理效果较差;UV光解类工艺VOCs处理效果最差,但这类治理工艺在汽修行业具有较好的治理效果。
2)燃烧类工艺对苯系物去除率最高,对苯、甲苯、二甲苯的平均去除率分别为93.6%、86.9%、87.6%,其次是活性炭吸附工艺,UV光解及其组合工艺对苯系物去除率较低。
3) UV光解及其组合工艺、活性炭吸附工艺在设计参数选取和治理设施维护中存在问题,导致UV光解及其组合工艺的整体去除效果较差。活性炭吸附工艺部分设备去除率低于正常值。在今后的废气治理中,应充分考虑各类影响因素,选择合适的处理技术,并应做好设备日常维护。
4)对厦门市VOCs 8大行业所进行的区域治理效果评估表明,厦门市各区的VOCs治理效果的排序为,思明区>湖里区>海沧区>翔安区>集美区>同安区。
-
图 3 CNNPs-5的IR光谱(a),UV-Vis光谱和光致发光光谱(b),激发依赖发射光谱(c)(b)中插图分别是在日光(左)和365 nm紫外光(右)下拍摄的照片
Figure 3. The IR spectrum (a), UV-Vis and photoluminescence spectrum (b), and excitation-dependent emission spectrum (c) of the CNNPs-5. The two illustrations in (b) were photos taken under daylight (left) and 365 nm ultraviolet light (right).
表 1 不同方法检测ClO−的性能比较
Table 1. Comparison of performance of detecting ClO− by different methods
表 2 自来水样品中游离氯的测定
Table 2. Determination of free chlorine in tap water samples
样品Samples 添加量/(µmol·L−1)Added 总氯/(µmol·L−1)Total found 样品值/(µmol·L−1)Found 回收率/%Recovery 相对标准偏差/%RSD 1 0.1 1.21 1.12 96.3 3.40 0.5 1.62 100.6 3.10 1.0 2.10 97.6 1.61 2 0.1 1.20 1.09 107.5 2.70 0.5 1.61 103.4 3.70 1.0 2.06 96.8 2.05 -
[1] LIM SY, SHEN W, GAO Z. Carbon quantum dots and their applications [J]. Chemical Society Reviews, 2015, 44: 362-381. doi: 10.1039/C4CS00269E [2] 余致汐, 贺南南, 陈欢, 等. 甲壳素复合石墨相氮化碳的制备及光催化杀菌性能 [J]. 环境化学, 2020, 39(5): 1271-1278. doi: 10.7524/j.issn.0254-6108.2019081206 YU Z X, HE N N, CHEN H, et al. Preparation of chitin composite graphite phase carbonitride and its photocatalytic sterilization performance [J]. Environmental Chemistry, 2020, 39(5): 1271-1278(in Chinese). doi: 10.7524/j.issn.0254-6108.2019081206
[3] El-KHATIB A M, YOUSEFN S, GHATASS Z F, et al. Synthesized silver carbon nanotubes and zinc oxide nanoparticles and their ability to remove methylene blue dye [J]. Journal of Nanoneuroscience, 2019, 56: 1-16. [4] YUE L, LI H, SUN Q, et al. Red-Emissive ruthenium-containing carbon dots for bioimaging and photodynamic cancer therapy [J]. ACS Applied Nano Materials, 2020, 3: 869-876. [5] CHAMORRO-POSADA P, DANTE R C, VAZQUEZ-CABO J, et al. Experimental and theoretical investigations on a CVD grown thin film of polymeric carbon nitride and its structure [J]. Diamond and Related Materials, 2021, 111: 108169. doi: 10.1016/j.diamond.2020.108169 [6] LIU J, LIU Y, LIU N, et al. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway [J]. Science, 2015, 347: 970-974. doi: 10.1126/science.aaa3145 [7] ZHU J, XIAO P, LI H, et al. Graphitic carbon nitride: synthesis, properties, and applications in catalysis [J]. ACS Applied Materials & Interfaces, 2014, 6: 16449-16465. [8] 章家立, 李阳, 彭小明, 等. g-C3N4在水环境污染物去除和检测方面的应用研究进展 [J]. 华东交通大学学报, 2019, 36(1): 109-116. ZHANG J L, LI Y, PENG X M, et al. On applications of graphitic carbon nitride in pollutant removal and detection in water environment [J]. Journal of East China Jiaotong University, 2019, 36(1): 109-116(in Chinese).
[9] ZHANG J, CHEN Y, WANG X. Two-dimensional covalent carbon nitride nanosheets: synthesis, functionalization, and applications [J]. Energy & Environmental Science, 2015, 8: 3092-3108. [10] 马贺成, 刘建军, 于迎春, 等. 二维石墨相氮化碳纳米片的制备及其在光催化领域的研究进展 [J]. 应用化学, 2019, 36(3): 259-268. doi: 10.11944/j.issn.1000-0518.2019.03.180241 MA H C, LIU J J, YU Y C, et al. Research progress in preparation and photocatalysis of two-dimensional graphitic carbon nitride nanosheets [J]. Chinese Journal of Applied Chemistry, 2019, 36(3): 259-268(in Chinese). doi: 10.11944/j.issn.1000-0518.2019.03.180241
[11] LIU S, TIAN J, WANG L, et al. A general strategy for the production of photoluminescent carbon nitride dots from organic amines and their application as novel peroxidase-like catalysts for colorimetric detection of H2O2 and glucose [J]. RSC Advances, 2012, 2: 411-413. doi: 10.1039/C1RA00709B [12] ZHANG P, LI X, SHAO C, et al. Hydrothermal synthesis of carbon-rich graphitic carbon nitride nanosheets for photoredox catalysis [J]. Journal of Materials Chemistry A, 2015, 3: 3281-3284. doi: 10.1039/C5TA00202H [13] ASAITHAMBI S, SAKTHIVEL P, KARUPPAIAH M, et al. The bifunctional performance analysis of synthesized Ce doped SnO2/g-C3N4 composites for asymmetric supercapacitor and visible light photocatalytic applications [J]. Journal of Alloys and Compounds, 2021: 158807. [14] LIU Y, WANG Q, LEI J, et al. Anodic electrochemiluminescence of graphitic-phase C3N4 nanosheets for sensitive biosensing [J]. Talanta, 2014, 122: 130-134. doi: 10.1016/j.talanta.2014.01.018 [15] RONG M, LIN L, SONG X, et al. Fluorescence sensing of chromium(VI) and ascorbic acid using graphitic carbon nitride nanosheets as a fluorescent “switch” [J]. Biosensors & Bioelectronics, 2015, 68: 210-217. [16] 陈珠灵, 林敏秀, 宋志平, 等. 基于石墨相氮化碳量子点直接荧光猝灭法检测碘离子的研究 [J]. 光谱学与光谱分析, 2019, 39(7): 2029-2033. CHEN Z L, LIN M X, SONG Z P, et al. Study of direct fluorescencence quenching of graphitic carbon nitride for the detection of iodine ions [J]. Spectroscopy and Spectral Analysis, 2019, 39(7): 2029-2033(in Chinese).
[17] ZHANG H, HUANG Y, HU S, et al. Fluorescent probes for “off-on” sensitive and selective detection of mercury ions and L-cysteine based on graphitic carbon nitride nanosheets [J]. Journal of Materials Chemistry C, 2015, 3: 2093-2100. doi: 10.1039/C4TC02394C [18] ABDOLMOHAMMAD-ZADEH H, RAHIMPOUR E. A novel chemosensor based on graphitic carbon nitride quantum dots and potassium ferricyanidechemiluminescence system for Hg(Ⅱ) ion detection [J]. Sensors and Actuators B: Chemical, 2016, 225: 258-266. doi: 10.1016/j.snb.2015.11.052 [19] HAN J, ZOU HY, GAO MX, et al. A graphitic carbon nitride based fluorescence resonance energy transfer detection of riboflavin [J]. Talanta, 2016, 148: 279-284. doi: 10.1016/j.talanta.2015.10.038 [20] XIE H, DONG J, DUAN J, et al. Magnetic nanoparticles-based immunoassay for aflatoxin B1 using porous g-C3N4 nanosheets as fluorescence probes [J]. Sensors and Actuators B:Chemical, 2019, 278: 147-152. doi: 10.1016/j.snb.2018.09.089 [21] QIAO F, WANG J, AI S, et al. As a new peroxidase mimetics: The synthesis of selenium doped graphitic carbon nitride nanosheets and applications on colorimetric detection of H2O2 and xanthine [J]. Sensors and Actuators B: Chemical, 2015, 216: 418-427. doi: 10.1016/j.snb.2015.04.074 [22] ZHENG H B, DING J, ZHENG S J, et al. Facile synthesis of magnetic carbon nitride nanosheets and its application in magnetic solid phase extraction for polycyclic aromatic hydrocarbons in edible oil samples [J]. Talanta, 2016, 148: 46-53. doi: 10.1016/j.talanta.2015.10.059 [23] DONG Y, LI G, ZHOU N, et al. Graphene quantum dots as a green and facile sensor for free chlorine in drinking water [J]. Analytical Chemistry, 2012, 84: 8378-8382. doi: 10.1021/ac301945z [24] ZHOU M, LI T, ZU M, et al. Membrane-based colorimetric flow-injection system for online free chlorine monitoring in drinking water [J]. Sensors and Actuators B:Chemical, 2021, 327: 128905. doi: 10.1016/j.snb.2020.128905 [25] MA Z, CHEN X, WANG C, et al. A novel ratiometric fluorescence probe for hypochlorite detection and its application in cell imaging [J]. Journal of Molecular Structure, 2020, 1221: 128812. doi: 10.1016/j.molstruc.2020.128812 [26] CLAVER J B, MIRON M C V, CAPITAN-VALLVEY L F. Determination of hypochlorite in water using a chemiluminescent test strip [J]. Analytica Chimica Acta, 2004, 522: 267-273. doi: 10.1016/j.aca.2004.06.051 [27] CHEN P, WEI W Z, YAO S Z. Different valency chlorine species analysis by non-suppressed ion-chromatography with double cell quartz crystal detector [J]. Talanta, 1999, 49: 571-576. doi: 10.1016/S0039-9140(99)00041-7 [28] WATANABE T, IDEHARA T, YOSHIMURA Y, et al. Simultaneous determination of chlorine dioxide and hypochlorite in water by high-performance liquid chromatography [J]. Journal of Chromatography A, 1998, 796: 397-400. doi: 10.1016/S0021-9673(97)01009-1 [29] POBOZY E, PYRZYNSKA K, SZOSTEK B, et al. Flow-injection spectrophotometric determination of free residual chlorine in waters with 3, 3′-dimethylnaphtidine [J]. Microchemical Journal, 1995, 51: 379-86. doi: 10.1006/mchj.1995.1044 [30] ZHOU J, YANG Y, ZHANG C. A low-temperature solid-phase method to synthesize highly fluorescent carbon nitride dots with tunable emission [J]. Chemical Communications, 2013, 49: 8605-8607. doi: 10.1039/c3cc42266f [31] LIN L, RONG M, LU S, et al. A facile synthesis of highly luminescent nitrogen-doped graphene quantum dots for the detection of 2, 4, 6-trinitrophenol in aqueous solution [J]. Nanoscale, 2015, 7: 1872-1878. doi: 10.1039/C4NR06365A [32] CAO X, MA J, LIN Y, et al. A facile microwave-assisted fabrication of fluorescent carbon nitride quantum dots and their application in the detection of mercury ions [J]. Spectrochimica Acta Part A, 2015, 151: 875-880. doi: 10.1016/j.saa.2015.07.034 [33] ANIKUMAR P, WANG X, CAO L, et al. Toward quantitatively fluorescent carbon-based “quantum” dots [J]. Nanoscale, 2011, 3: 2023-2027. doi: 10.1039/c0nr00962h [34] MESSINA F, SCIORTINO L, POPESCU R, et al. Fluorescent nitrogen-rich carbon nanodots with an unexpected β-C3N4 nanocrystallinestructure [J]. Journal of Materials Chemistry C, 2016, 4: 2598-2605. doi: 10.1039/C5TC04096E [35] LIU J, SHANGGUAN M, ZENG X, et al. Phosphorescent iridium (Ⅲ) complex for efficient sensing of hypochlorite and imaging in living cells [J]. Analytical Biochemistry, 2020, 592: 113573. doi: 10.1016/j.ab.2019.113573 [36] SHIRAISHI Y, YAMADA C, TAKAGI S, et al. Fluorometric and colorimetric detection of hypochlorous acid and hypochlorite by a naphthalimide–dicyanoisophorone conjugate [J]. Journal of Photochemistry and Photobiology A:Chemistry, 2021, 406: 112997. doi: 10.1016/j.jphotochem.2020.112997 [37] NING Y, CUI J, LU Y, et al. De novo design and synthesis of a novel colorimetric fluorescent probe based on naphthalenone scaffold for selective detection of hypochlorite and its application in living cells [J]. Sensors and Actuators B:Chemical, 2018, 269: 322-330. [38] WU H, ZHANG W, WU Y, et al. A 7-diethylaminocoumarin-based chemosensor with barbituric acid for hypochlorite and hydrazine [J]. Microchemical Journal, 2020, 159: 105461. doi: 10.1016/j.microc.2020.105461 [39] ZHANG Y M, FANG H, ZHU W, et al. Ratiometric fluorescent sensor based oxazolo-phenazine derivatives for detect hypochlorite via oxidation reaction and its application in environmental samples [J]. Dyes and Pigments, 2020, 172: 107765. doi: 10.1016/j.dyepig.2019.107765 [40] RHA C J, LEE H, KIM C. Development of an azo-naphthol-based probe for detecting hypochlorite (ClO−) via color change in aqueous solution [J]. Inorganic Chemistry Communications, 2020, 121: 108244. doi: 10.1016/j.inoche.2020.108244 [41] WANG H, ZHANG L, GUO X, et al. Comparative study of Cl, N-Cdots and N-Cdots and application for trinitrophenol and ClO− sensor and cell-imaging [J]. Analytica Chimica Acta, 2019, 1091: 76-87. doi: 10.1016/j.aca.2019.09.019 -







 下载:
下载: