-
六溴环十二烷(HBCD)是一种高含溴量的添加型阻燃剂,其产量仅次于多溴联苯醚(decaBDE)和四溴双酚A(TBBPA),被广泛用于建筑、纺织材料、电子设备、塑料制品等产品中[1]。基于其广泛污染及具有富集性、远距离迁移性、生物毒性等特点,HBCD作为持久性有机污染物,于2013年被列入斯德哥尔摩公约受控名单[2]。
HBCD具有手性中心,理论而言共含有16种同分异构体,常见的有α-HBCD、β-HBCD和γ-HBCD,以及其相应的(+)和(−)对映体。不同立体构型的HBCD在环境中的富集、代谢和毒性行为均存在差异[3]。一般而言,在土壤[4]和水体[5]等非生物介质中γ-HBCD含量较高,在生物介质中α-HBCD则占主导。尽管β-HBCD在环境介质中的检出浓度一般不是最高的,但有研究表明,其累积能力和毒性作用不容忽视。对斜生栅藻[6]和玉米[7]的研究发现,β-HBCD在其体内的累积动力学速率和最高吸收量均高于α-和γ-HBCD。奥斯卡鱼肠对β-和γ-HBCD的吸收速率也高于对α-HBCD的吸收[8]。在生物毒性方面,Palace等[9]发现,与α-HBCD相比,β和γ-HBCD会显著增高鱼体内脱碘酶的活性,从而降低鱼对碘的吸收能力。对人肝细胞L02和人肝癌细胞HepG2的研究表明β-HBCD的毒性影响高于γ-和α-HBCD[10]。可见,在特定环境下β-HBCD的富集能力及生物毒性高于其他异构体。同时,在生物体内亦有研究表明,γ-HBCD会发生向β-HBCD的异构体转化,导致β-构型浓度增加[11],加剧其环境生态风险。因此,在研究HBCD的环境行为及影响时,对β-HBCD的考察需要引起重视。而目前关于β-HBCD的毒性研究较为匮乏,亟待开展更多工作。
有关HBCD植物毒性方面的考察相对较少,植物是生态系统中的第一营养级,植物对污染物的吸收及其迁移转化、环境影响和归趋均有重要作用,研究HBCD对植物的毒性作用具有深远意义。Zhang等[12]研究了商品HBCD混合物对拟南芥的基因表达和蛋白功能的毒性作用,发现机体能量产生-转化和氨基酸转运-代谢方面均受到不同程度的负面影响。Huang等[13]在体外研究α-和γ-HBCD对玉米细胞色素P450(CYP)酶的影响表明,(−)/(+)γ-HBCD会诱导CYP酶活性增加,α-HBCD则表现为抑制,且(−)α-HBCD对CYP酶活性的抑制高于(+)α-HBCD。本团队前期研究发现HBCD可诱导玉米体内羟基自由基产生,造成一定程度的DNA损伤,其中β-HBCD的毒性高于γ-HBCD[7];在对映体水平上也分别考察了α-和γ-HBCD对玉米的生长、形态改变、抗氧化酶和DNA损伤方面的对映体选择性影响[14-15]。然而遗憾的是上述研究均未开展对β-HBCD对映体的相关探讨,不同光旋纯活性的β-HBCD是否会对植物产生选择性毒性还需要进一步判断。
本研究选择玉米为受试植物,采用不同浓度梯度的β-HBCD及其对映体对玉米开展水培暴露研究,通过玉米体内抗氧化物质活性及含量变化,从抗氧化酶系统和非酶抗氧化系统两方面说明机体对β-HBCD选择性毒性的响应,结合细胞内典型活性氧(ROS)物质超氧阴离子(
O⋅−2 )水平和植物根系活力,判断暴露后玉米体内ROS失衡对其生长代谢的对映体选择性影响机制,为全面评价HBCD的环境生态风险提供重要信息。 -
主要试剂:β-HBCD标准品(50 μg·mL−1)购于AccuStandard公司。流动相甲醇为色谱纯,其余试剂均为分析纯。
主要仪器:高效液相色谱(HPLC,岛津LC-20AD)、紫外-可见分光光度计(岛津UV-2550)、高速冷冻离心机(Multifuge X1R,Thermo公司)、恒温培养箱(A1000,Conviron公司)、多功能酶标仪(SPARK,德国TECAN公司)、电子天平(精度±0.0001g,上海双杰有限公司)。
-
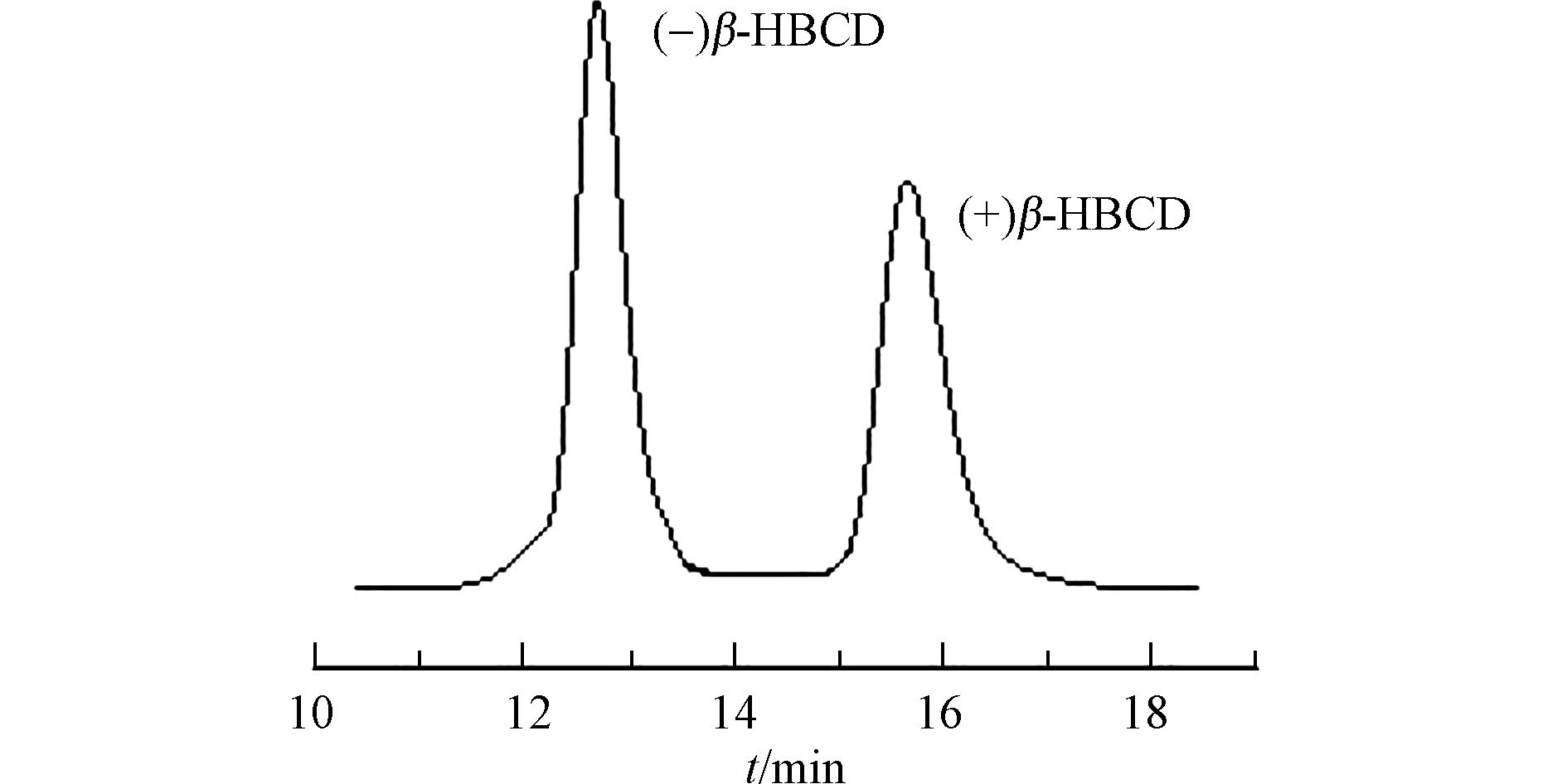
选择β-PM手性柱(200 mm×4 mm,5 μm,德国MN公司),采用高效液相色谱法(HPLC)优化得到最佳色谱分离条件:流动相为95%甲醇:5%水,流速0.4 mL·min−1,柱温25 ℃,检测器波长220 nm。在最佳分离条件下制备单一对映体,根据紫外检测器出峰信号和保留时间手动收集相应的流出液,并采用外标法进行定量。根据Zhang等[16]的研究,流出检测器的对映体依次为(−)β-HBCD、(+)β-HBCD对映体。分离后的对映体纯度均在98%以上,最终获得的(+)和(−)β-HBCD浓度分别为1.81 μg·mL−1和1.63 μg·mL−1。分离谱图如图1所示。
-
选取均匀且饱满的玉米种子(购于中国农业科学研究院),去离子水清洗后,用10%H2O2的消毒溶液浸泡10 min,用去离子清洗干净后,将玉米种子均匀平铺在覆盖有湿滤纸的托盘上,置于(27±0.5)℃的恒温培养箱中于暗处发芽,保持相对湿度在60%—80%。配置不同浓度梯度(0.5、1.0、2.0、3.0、5.0 μg·L−1)的(+)β-HBCD、(rac)β-HBCD和(−)β-HBCD暴露液。挑选培养4 d后长势均一的玉米幼苗转移至盛有150 mL HBCD溶液的黑色玻璃水培罐中进行暴露,每盆放置5棵幼苗,每24 h更换1次暴露液,更换暴露液前检测溶液中对映体外消旋化的比率(< 9%),确定对映体转化的影响可忽略不计。恒温培养箱每天设置14 h光照时间,相对湿度为60%—80%,每天改变玉米幼苗的位置以减少光照和温度的空间差异。暴露3 d后进行毒性指标测定。实验中,每个处理设置3个平行,每个实验重复3次,设置去离子水和甲醇空白对照,两组对照结果一致,说明溶剂甲醇未对植物生长产生影响。
-
将玉米幼苗用去离子水清洗并用滤纸吸干,分别称取0.5 g玉米根部与地上部,置于研钵中,在冰浴下加入6 mL磷酸缓冲液(0.05 mol·L−1、pH =7.8)研磨成匀浆,于4 ℃,5000 r·min−1离心15 min,提取上清液,即得到样品粗酶液。
采用Bradford的方法[17],以100 μg·mL−1牛血清蛋白为标准溶液,0.005%的考马斯亮蓝G-250溶液为染料,在595 nm下绘制标准曲线,检测样品中的蛋白水平。超氧化物歧化酶(SOD)的测定参照Beauchamp等[18]的方法并略作改动。采用氮蓝四唑(NBT)光还原法进行检测,将粗酶液与显色液(50 mmol·L−1磷酸缓冲液(pH =7.8)、130 mmol·L−1甲硫氨酸、750 μmol·L−1氮蓝四唑、100 μmol·L−1 EDTA-Na2及20 μmol·L−1核黄素)混匀,将1支对照管避光放置作为空白,其它于4000 lx光照下反应15—30 min,在560 nm处测定各管吸光值。抗坏血酸过氧化物酶(APX)的测定参照Nakano[19]的方法,0.5 mL上清液混于1 mL反应体系(包含50 mmol·L−1磷酸钾,0.5 mmol·L−1抗坏血酸,0.1 mmol·L−1过氧化氢以及0.1 mmol·L−1 EDTA),在290 nm处测定各管吸光值以确定抗坏血酸活性。
-
称取幼苗叶片0.10 g,剪成1—2 mm碎片,置于5 mL 2%的盐酸甲醇溶液中,于37 ℃恒温箱中放置3 h,直到叶片用肉眼观察已完全变白,取出过滤获得上清液,于530 nm下测花青素的含量,由于可溶性糖和叶绿素的干扰,需要在620 nm下测可溶性糖和650 nm下叶绿素的光密度值。计算公式为:
其中,ε为花青素的摩尔消光系数值为4.62×106;V为所取上清液体积(mL);m为所取样品质量(g);nmol·g−1为每克样品中的花青素含量。
-
植物样品的提取和粗酶液的提取方法一致,获得的上清液采用NBT法[20]检测。取0.5 mL样品、0.5 mL 65 mol·L−1的磷酸缓冲液和1.5 mL 1.0 mol·L−1的盐酸羟胺混匀,于25 ℃保温1 h,加入2 mL 17 mmol·L−1对氨基苯磺酸和2 mL 7 mmol·L−11-萘胺,摇匀后在25 ℃保温20 min,在波长530 nm下测吸光度。
-
根系活力采用TTC法[21]并略作改动。在0.1 mL 1%的2,3,5-三苯基氯化四氮唑溶液中加入少许Na2S2O4粉末,无水甲醇定容至10 mL,即得到三苯甲月替(TTF)标准溶液,以空白作参比,在485 nm下测定吸光度值绘制标准曲线。称取0.3 g根样品于小烧杯中,加入5 mL 1% TTC和5 mL 0.1 mol·L−1磷酸缓冲液(pH =7.0)摇匀,使溶液完全浸没根,37 ℃无光保温2 h,加入2 mL 1 mol·L−1硫酸停止反应。取出根,用滤纸吸干反应液并切段置于10 mL甲醇中,于40 ℃恒温箱中放置,使根尖切段完全变白为止(4—6 h),在485 nm下测定吸光度值,通过标准曲线计算出四氮唑还原量。
-
所有数据均为3次重复实验所得结果,实验数据以平均值±标准偏差(Mean±SD)来表示。采用Origin 8.0对数据进行回归分析,SPSS 17.0对数据进行显著性检验。
-
SOD是生物体抗氧化系统的第一道防线,可催化
O⋅−2 自由基发生歧化反应,生成氧气(O2)和过氧化氢(H2O2),APX以抗坏血酸(ASA)为电子供体,将H2O2转化为H2O[22]。它们在植物体内可清除过量的活性氧,使植物在一定程度上减缓伤害。随着β-HBCD暴露浓度的增加,玉米体内SOD和APX活性(除(−)β-HBCD处理外)均呈现显著的先增加后减少的趋势(图2),表明β-HBCD诱发了玉米机体的氧化应激效应。图2a显示玉米根部SOD活性在(+)、(rac)和(−)β-HBCD最低暴露浓度下(0.5 μg·L−1)即显著增加到最大值(P < 0.05),分别为对照组的1.85倍、1.71倍和1.63倍,但三者之间未呈现出显著性差异,可能是因为低浓度的污染物对玉米SOD活性的影响较小;在最高浓度5.0 μg·L−1时,SOD活性降到最低,但仍比对照组增加了58.7%、56.0%和26.8%,表明β-HBCD对玉米产生了氧化胁迫,且不同立体构型产生的毒性为(+)β-HBCD > (rac)β-HBCD > (−)β-HBCD。在(+)和(rac)β-HBCD暴露下,玉米地上部SOD活性分别在3.0 μg·L−1与2.0 μg·L−1时达到最大(图2b),较对照组分别增加了15.6%和9.5%,表明根部受到的影响更大。(−)β-HBCD处理的地上部SOD活性与对照组相比无显著差异,可见(−)β-HBCD对玉米的影响较小。
β-HBCD及其对映体均诱导了玉米根部和地上部APX活性增加(图2c和d)。玉米根部在暴露浓度为1.0 μg·L−1时APX活性最高,(+)、(rac)和(−)β-HBCD处理分别是对照组的5.26倍、4.91倍和3.90倍,随后与浓度呈现负相关关系。地上部分,APX活性在(+)和(rac)β-HBCD浓度为3.0 μg·L−1时达到最大值,较对照组分别增加了114.6%和97.6%,随后逐渐降低;(−)β-HBCD处理的玉米地上部APX活性在低浓度下无显著差异,仅在最大暴露浓度(5.0 μg·L−1)时显著增加到对照组的1.45倍。可见,APX活性变化结果与SOD类似,均表现出了(+)β-HBCD的影响最大,其次是(rac)β-HBCD,(−)β-HBCD影响最小。Fang等[23]在甲霜灵对映体对烟草幼苗的影响研究中发现:R-对映体比S-对映体对SOD活性的促进作用更高,对幼苗的影响更大,说明同种物质不同立体构型对生物体产生的毒性作用亦是存在差异的。
外源污染物会影响植物体内的活性氧(ROS)平衡,ROS的大量生成,可以激发植物自身的抗氧化防御系统,显著诱导酶活性增加以消除过量的ROS[24]。本研究中β-HBCD诱导了玉米体内ROS的产生,使细胞抗氧化酶SOD和APX活性增加以清除玉米体内的
O⋅−2 与H2O2。随着胁迫的加深,SOD和APX活性逐渐降低,可能是由于植物机体内其他抗氧化机制也被诱发,而使得SOD和APX活性所受的影响变小,但其活性并未降到对照组以下,说明SOD和APX仍对β-HBCD有响应。由于根部先于地上部接触暴露液,且有研究表明,在培养玉米的过程中,地上部对β-HBCD的积累主要来源于根部吸收和茎向传输,且总富集量低于根部[25],因此使得根部SOD和APX活性在较低浓度下即产生应激响应,且变化量均高于地上部的最大变化量。与α-HBCD[14]和γ-HBCD[15]的玉米毒性研究对比,在异构体水平上,对SOD酶活性的影响为β-HBCD>α-HBCD>γ-HBCD;在对映体水平上,α-、β-和γ-HBCD均分别表现出(+)体>(rac)体>(−)体,且对于毒性较强的(+)构型,又呈现(+)α-HBCD>(+)β-HBCD>(+)γ-HBCD的顺序,(−)构型则为(−)β-HBCD>(−)α-HBCD>(−)γ-HBCD。因此,在生物体内不同异构体和对映体可能表现出不同的效应程度。同时,数据结果显示玉米体内APX活性的变化程度明显高于SOD活性的变化,可能是β-HBCD诱发了sod基因表达量上升[26],从而减轻了对SOD活性的影响压力,也可能是由于抗氧化酶APX比SOD更加敏感导致。 -
植物在受到氧化胁迫时,为保护自身生理发育及新陈代谢的正常进行,形成了一套复杂完善的抗氧化保护系统,包括抗氧化酶系统和非酶系统,花青素是非酶系统中一种重要的抗氧化剂,属于生物类黄酮物质,主要存在于植物叶片中,和酚类化合物的作用机理相似,花青素可通过酚羟基与自由基反应生成较稳定的半醌式自由基,从而终止自由基链式反应[27]。
随着β-HBCD暴露浓度的增加,玉米地上部花青素含量与抗氧化酶类似,亦呈现先增加后减少的趋势(图3)。1.0 μg·L−1的(+)、(rac)和(−)β-HBCD处理下玉米叶片中花青素含量最高,分别达到对照组的3.55倍、3.22倍和2.28倍;在最高浓度5.0 μg·L−1时,花青素含量最低,(+)、(rac)和(−)β-HBCD处理组分别比对照组增加了77.6%、94.2%和43.9%。研究表明,花青素具有清除自由基、促进激活抗氧化酶系统的能力,是植物抗氧化的基础[27],本研究中植物体内抗氧化酶系统和非酶系统随β-HBCD暴露的变化趋势一致,说明其在植物防御外源氧化胁迫过程中同样起到非常重要的作用。不同对映体处理组相比,与抗氧化酶系统的SOD和APX活性检测结果一致,均表现出对玉米的毒性作用大小顺序为(+)β-HBCD > (rac)β-HBCD > (−)β-HBCD。
-
β-HBCD的暴露诱导了玉米体内SOD、APX酶活性和花青素含量的增加,那么这种应激响应是否对机体内部的ROS进行了有效的控制呢?本研究进一步考察了玉米组织中的
O⋅−2 水平,以衡量植物对逆境抗性的防御程度。结果显示(图4),玉米体内的
O⋅−2 含量随暴露浓度的增加呈现先增后降趋势,且不同对映体之间存在显著差异(P < 0.05)。玉米根部O⋅−2 含量在最低暴露浓度(0.5 μg·L−1)的(+)和(−)β-HBCD、1.0 μg·L−1的(rac)β-HBCD处理下达到最大值,分别是对照组的2.70、2.14、2.39倍;在最大暴露浓度5.0 μg·L−1时,(rac)和(−)β-HBCD处理组玉米根部O⋅−2 水平与对照组无显著差异,(+)β-HBCD处理组仍高于对照组,(+)构型的β-HBCD对机体影响更大;在玉米地上部中,(+)β-HBCD处理的O⋅−2 含量在2.0 μg·L−1时最高,达到空白对照组的2.02倍,且不同浓度间无显著差异,(rac)和(−) β-HBCD处理组的地上部O⋅−2 水平分别在1.0 μg·L−1和0.5 μg·L−1时达到最高,分别是空白对照组的1.86倍和1.71倍,随后显著下降。在外界胁迫下,生物体内更多的分子氧经过单电子还原反应转变为
O⋅−2 ,进而衍生出羟基自由基等一系列的ROS物质[28],破坏植物细胞膜及蛋白、核酸等生物大分子。本研究发现β-HBCD能显著诱导玉米体内的O⋅−2 水平,进而机体抗氧化系统被激活,高效清除过量的ROS,然而仅高浓度的(rac)和(−)β-HBCD处理组的植物根部O⋅−2 含量恢复到对照组水平,相比而言,(+)β-HBCD胁迫下的玉米体内O⋅−2 增加得最快、水平最高、恢复得也最慢,表明(+)β-HBCD对玉米的毒性作用更强。这与Zhang等[16]关于β-HBCD对肝细胞毒性作用的结果(+)β-HBCD毒性更强一致。 -
根系是植物获得营养和水分,维持稳定较大生物量的关键部位,根系活力的高低直接影响作物的正常生长并间接干扰叶片的光合效率[29-30]。本研究中玉米根系是直接接触暴露液的部位,根部
O⋅−2 的变化程度比地上部更显著,为了明确植物中O⋅−2 的失衡是否会影响其健康状态,本研究进一步开展了植物根系活力的检测。由图5可以看出,经(+)和(rac)β-HBCD胁迫后,幼苗根系活力在0.5 μg·L−1时与空白对照组无显著差异,在1.0 μg·L−1时显著下降,于最大浓度(5.0 μg·L−1)时降到最低,分别较对照组减少了65.1%和39.7%;(−)β-HBCD胁迫下,幼苗根系活力仅在最大浓度胁迫下,较空白对照组显著下降了28.5%,其余处理均与空白无显著差异。显然,尽管植物机体存在庞大的抗氧化保护系统,但β-HBCD及其对映体仍降低了玉米幼苗的根系活力,影响了其健康状态,且不同对映体对幼苗根系活力的抑制作用为(+)β-HBCD > (rac)β-HBCD > (−)β-HBCD。植物根系活力反映根系整体的代谢强度,包括根系呼吸、氧化、还原和合成能力等[31],植物处于逆境生理环境时,其呼吸代谢关键酶表达量会发生改变,进而影响植物的呼吸过程[30]。本研究中β-HBCD很可能是通过诱导ROS的变化影响了玉米根部代谢关键酶的表达或活性,从而导致其代谢强度的改变,根系活力发生变化。这与Zhang等[32]关于碱环境胁迫下大米机体ROS累积导致根系损伤的作用结果一致。不同的β-HBCD对映体对幼苗根系活力的抑制作用存在显著差异,可能与其空间立体构型有关。有文献报道,不同立体构型的HBCD与细胞内代谢相关酶的结合位点具有差异性,其中(−)β-HBCD能够选择性进入玉米代谢相关酶-细胞色素氧化酶(CYP)和谷胱甘肽转移酶(GST)亚型酶CYP71C3v2、GST31的活性位点并与其键合,进而被转化成不同的代谢产物,从而降低了其在植物体内的浓度[25],这可能是(−)β-构型产生的毒性效应较小的主要原因。
-
手性化合物β-HBCD对玉米的生长代谢具有显著的对映体选择性影响,毒性大小为(+)β-HBCD>(rac)β-HBCD>(−)β-HBCD,主要源于植物抗氧化酶系统和非酶抗氧化系统未能有效控制玉米体内
O⋅−2 水平,ROS自由基平衡被打破,进而幼苗根系活力显著下降,这是导致植物生长代谢受阻的重要影响因素。本研究从植物角度出发,在对映体水平上评价了β-HBCD对植物生长代谢的影响,为评价HBCD的环境行为和毒理提供重要数据,对综合评估其环境生态风险具有重要意义。
β-六溴环十二烷对玉米生长代谢的对映体选择性影响
Enantioselective effects of β-HBCD on the growth metabolism of maize
-
摘要: 为了探讨不同光旋纯活性的β-HBCD对植物生长代谢的对映体选择性影响,本研究采用高效液相色谱法(HPLC)优化分离并制备得到β-六溴环十二烷(β-HBCD)单一对映体((+)β-HBCD和(−)β-HBCD),开展了不同浓度梯度的(+)、(rac)和(−)β-HBCD溶液对玉米幼苗的水培暴露实验。随着β-HBCD暴露浓度的增加,玉米体内超氧化物歧化酶(SOD)和抗坏血酸过氧化物酶(APX)活性(除(−)β-HBCD处理外)、以及花青素含量均呈现显著的先增加后减少的趋势,表明β-HBCD诱发了玉米机体的抗氧化酶系统和非酶系统的应激反应,其中(+)β-HBCD诱导SOD、APX活性和花青素水平增加量最大,在玉米根部分别达到空白对照组的1.85、5.26、3.55倍,(−)β-HBCD处理增加量最小,分别达到空白对照组的1.63、3.90、2.28倍;玉米体内的活性氧(ROS)平衡被破坏,超氧阴离子(
O⋅−2 )水平被显著诱导,诱导量为(+)β-HBCD > (rac)β-HBCD > (−)β-HBCD;幼苗根系活力受到显著的抑制,(+)、(rac)和(−)β-HBCD处理组的抑制量均在最高暴露浓度时达到最大,分别较空白对照组减少了65.1%、39.7%和28.5%,推测O⋅−2 水平失衡,根系活力下降,是导致植物生长代谢受阻的重要影响因素。综上所有证据表明β-HBCD对玉米的生长代谢产生了对映体选择性影响,毒性顺序为(+)β-HBCD > (rac)β-HBCD > (−)β-HBCD。本研究对综合评价HBCD的环境行为和生态风险具有重要意义。Abstract: In order to explore the enantioselective effects of β-HBCD with different optical activity on plant growth metabolism, the optimized separation and preparation of (+) β-HBCD and (−) β-HBCD were performed by high performance liquid chromatography (HPLC). Hydroponic exposure experiments of maize seedlings were conducted at solutions of (+), (rac) and (−) β-HBCDs with different concentrations. The activities of superoxide dismutase (SOD) and ascorbate peroxidase (APX) (except for (−) β-HBCD treatments) and anthocyanin content in maize increased significantly at first and then decreased with the increasing exposure concentrations of β-HBCD, indicating that β-HBCD induced stress response of antioxidant enzyme system and non-enzymatic system in maize. The strongest induced effects on SOD, APX activity and anthocyanin level were found in (+) β-HBCD treatments, which reached 1.85, 5.26 and 3.55 times higher than blank control group in maize roots, respectively. The induced effects of (−) β-HBCD treatments were the smallest, reaching 1.63, 3.90 and 2.28 times higher than blank control group, respectively. The ROS balance in maize was destroyed, andO⋅−2 level was significantly induced in the order of (+) β-HBCD > (rac) β-HBCD > (−) β-HBCD. The root activity of seedlings was significantly inhibited, and the maximum inhibition reached at the highest exposure concentration treatment, which decreased by 65.1%, 39.7% and 28.5%, respectively, compared with blank control group for (+), (rac) and (−) β-HBCD treatments. It is speculated that the imbalance ofO⋅−2 level and the decrease of root activity are the important factors that affect the growth and metabolism of plants. All the results demonstrated the significant enantioselective effects of β-HBCD on maize growth and metabolism with the toxicity order of (+) β-HBCD > (rac) β-HBCD > (−) β-HBCD. This study is of great significance for comprehensive assessment of the environmental behavior and ecological risk of HBCD.-
Key words:
- β-hexabromocyclododecanes /
- enantioselectivity /
- antioxidant enzyme /
- superoxide anion /
- root activity
-
六溴环十二烷(HBCD)是一种高含溴量的添加型阻燃剂,其产量仅次于多溴联苯醚(decaBDE)和四溴双酚A(TBBPA),被广泛用于建筑、纺织材料、电子设备、塑料制品等产品中[1]。基于其广泛污染及具有富集性、远距离迁移性、生物毒性等特点,HBCD作为持久性有机污染物,于2013年被列入斯德哥尔摩公约受控名单[2]。
HBCD具有手性中心,理论而言共含有16种同分异构体,常见的有α-HBCD、β-HBCD和γ-HBCD,以及其相应的(+)和(−)对映体。不同立体构型的HBCD在环境中的富集、代谢和毒性行为均存在差异[3]。一般而言,在土壤[4]和水体[5]等非生物介质中γ-HBCD含量较高,在生物介质中α-HBCD则占主导。尽管β-HBCD在环境介质中的检出浓度一般不是最高的,但有研究表明,其累积能力和毒性作用不容忽视。对斜生栅藻[6]和玉米[7]的研究发现,β-HBCD在其体内的累积动力学速率和最高吸收量均高于α-和γ-HBCD。奥斯卡鱼肠对β-和γ-HBCD的吸收速率也高于对α-HBCD的吸收[8]。在生物毒性方面,Palace等[9]发现,与α-HBCD相比,β和γ-HBCD会显著增高鱼体内脱碘酶的活性,从而降低鱼对碘的吸收能力。对人肝细胞L02和人肝癌细胞HepG2的研究表明β-HBCD的毒性影响高于γ-和α-HBCD[10]。可见,在特定环境下β-HBCD的富集能力及生物毒性高于其他异构体。同时,在生物体内亦有研究表明,γ-HBCD会发生向β-HBCD的异构体转化,导致β-构型浓度增加[11],加剧其环境生态风险。因此,在研究HBCD的环境行为及影响时,对β-HBCD的考察需要引起重视。而目前关于β-HBCD的毒性研究较为匮乏,亟待开展更多工作。
有关HBCD植物毒性方面的考察相对较少,植物是生态系统中的第一营养级,植物对污染物的吸收及其迁移转化、环境影响和归趋均有重要作用,研究HBCD对植物的毒性作用具有深远意义。Zhang等[12]研究了商品HBCD混合物对拟南芥的基因表达和蛋白功能的毒性作用,发现机体能量产生-转化和氨基酸转运-代谢方面均受到不同程度的负面影响。Huang等[13]在体外研究α-和γ-HBCD对玉米细胞色素P450(CYP)酶的影响表明,(−)/(+)γ-HBCD会诱导CYP酶活性增加,α-HBCD则表现为抑制,且(−)α-HBCD对CYP酶活性的抑制高于(+)α-HBCD。本团队前期研究发现HBCD可诱导玉米体内羟基自由基产生,造成一定程度的DNA损伤,其中β-HBCD的毒性高于γ-HBCD[7];在对映体水平上也分别考察了α-和γ-HBCD对玉米的生长、形态改变、抗氧化酶和DNA损伤方面的对映体选择性影响[14-15]。然而遗憾的是上述研究均未开展对β-HBCD对映体的相关探讨,不同光旋纯活性的β-HBCD是否会对植物产生选择性毒性还需要进一步判断。
本研究选择玉米为受试植物,采用不同浓度梯度的β-HBCD及其对映体对玉米开展水培暴露研究,通过玉米体内抗氧化物质活性及含量变化,从抗氧化酶系统和非酶抗氧化系统两方面说明机体对β-HBCD选择性毒性的响应,结合细胞内典型活性氧(ROS)物质超氧阴离子(
O⋅−2 1. 实验部分 (Experimental section)
1.1 试剂与仪器
主要试剂:β-HBCD标准品(50 μg·mL−1)购于AccuStandard公司。流动相甲醇为色谱纯,其余试剂均为分析纯。
主要仪器:高效液相色谱(HPLC,岛津LC-20AD)、紫外-可见分光光度计(岛津UV-2550)、高速冷冻离心机(Multifuge X1R,Thermo公司)、恒温培养箱(A1000,Conviron公司)、多功能酶标仪(SPARK,德国TECAN公司)、电子天平(精度±0.0001g,上海双杰有限公司)。
1.2 对映体的分离与制备
选择β-PM手性柱(200 mm×4 mm,5 μm,德国MN公司),采用高效液相色谱法(HPLC)优化得到最佳色谱分离条件:流动相为95%甲醇:5%水,流速0.4 mL·min−1,柱温25 ℃,检测器波长220 nm。在最佳分离条件下制备单一对映体,根据紫外检测器出峰信号和保留时间手动收集相应的流出液,并采用外标法进行定量。根据Zhang等[16]的研究,流出检测器的对映体依次为(−)β-HBCD、(+)β-HBCD对映体。分离后的对映体纯度均在98%以上,最终获得的(+)和(−)β-HBCD浓度分别为1.81 μg·mL−1和1.63 μg·mL−1。分离谱图如图1所示。
1.3 植物的暴露与培养
选取均匀且饱满的玉米种子(购于中国农业科学研究院),去离子水清洗后,用10%H2O2的消毒溶液浸泡10 min,用去离子清洗干净后,将玉米种子均匀平铺在覆盖有湿滤纸的托盘上,置于(27±0.5)℃的恒温培养箱中于暗处发芽,保持相对湿度在60%—80%。配置不同浓度梯度(0.5、1.0、2.0、3.0、5.0 μg·L−1)的(+)β-HBCD、(rac)β-HBCD和(−)β-HBCD暴露液。挑选培养4 d后长势均一的玉米幼苗转移至盛有150 mL HBCD溶液的黑色玻璃水培罐中进行暴露,每盆放置5棵幼苗,每24 h更换1次暴露液,更换暴露液前检测溶液中对映体外消旋化的比率(< 9%),确定对映体转化的影响可忽略不计。恒温培养箱每天设置14 h光照时间,相对湿度为60%—80%,每天改变玉米幼苗的位置以减少光照和温度的空间差异。暴露3 d后进行毒性指标测定。实验中,每个处理设置3个平行,每个实验重复3次,设置去离子水和甲醇空白对照,两组对照结果一致,说明溶剂甲醇未对植物生长产生影响。
1.4 酶提取与测定
将玉米幼苗用去离子水清洗并用滤纸吸干,分别称取0.5 g玉米根部与地上部,置于研钵中,在冰浴下加入6 mL磷酸缓冲液(0.05 mol·L−1、pH =7.8)研磨成匀浆,于4 ℃,5000 r·min−1离心15 min,提取上清液,即得到样品粗酶液。
采用Bradford的方法[17],以100 μg·mL−1牛血清蛋白为标准溶液,0.005%的考马斯亮蓝G-250溶液为染料,在595 nm下绘制标准曲线,检测样品中的蛋白水平。超氧化物歧化酶(SOD)的测定参照Beauchamp等[18]的方法并略作改动。采用氮蓝四唑(NBT)光还原法进行检测,将粗酶液与显色液(50 mmol·L−1磷酸缓冲液(pH =7.8)、130 mmol·L−1甲硫氨酸、750 μmol·L−1氮蓝四唑、100 μmol·L−1 EDTA-Na2及20 μmol·L−1核黄素)混匀,将1支对照管避光放置作为空白,其它于4000 lx光照下反应15—30 min,在560 nm处测定各管吸光值。抗坏血酸过氧化物酶(APX)的测定参照Nakano[19]的方法,0.5 mL上清液混于1 mL反应体系(包含50 mmol·L−1磷酸钾,0.5 mmol·L−1抗坏血酸,0.1 mmol·L−1过氧化氢以及0.1 mmol·L−1 EDTA),在290 nm处测定各管吸光值以确定抗坏血酸活性。
1.5 花青素的测定
称取幼苗叶片0.10 g,剪成1—2 mm碎片,置于5 mL 2%的盐酸甲醇溶液中,于37 ℃恒温箱中放置3 h,直到叶片用肉眼观察已完全变白,取出过滤获得上清液,于530 nm下测花青素的含量,由于可溶性糖和叶绿素的干扰,需要在620 nm下测可溶性糖和650 nm下叶绿素的光密度值。计算公式为:
OD花青素=(OD530−OD620)−0.1×(OD650−OD620) (1) 花青素含量(nmol⋅g−1)=[(OD花青素/ε)×(V/m)×106] (2) 其中,ε为花青素的摩尔消光系数值为4.62×106;V为所取上清液体积(mL);m为所取样品质量(g);nmol·g−1为每克样品中的花青素含量。
1.6 超氧阴离子的检测
植物样品的提取和粗酶液的提取方法一致,获得的上清液采用NBT法[20]检测。取0.5 mL样品、0.5 mL 65 mol·L−1的磷酸缓冲液和1.5 mL 1.0 mol·L−1的盐酸羟胺混匀,于25 ℃保温1 h,加入2 mL 17 mmol·L−1对氨基苯磺酸和2 mL 7 mmol·L−11-萘胺,摇匀后在25 ℃保温20 min,在波长530 nm下测吸光度。
1.7 根系活力检测
根系活力采用TTC法[21]并略作改动。在0.1 mL 1%的2,3,5-三苯基氯化四氮唑溶液中加入少许Na2S2O4粉末,无水甲醇定容至10 mL,即得到三苯甲月替(TTF)标准溶液,以空白作参比,在485 nm下测定吸光度值绘制标准曲线。称取0.3 g根样品于小烧杯中,加入5 mL 1% TTC和5 mL 0.1 mol·L−1磷酸缓冲液(pH =7.0)摇匀,使溶液完全浸没根,37 ℃无光保温2 h,加入2 mL 1 mol·L−1硫酸停止反应。取出根,用滤纸吸干反应液并切段置于10 mL甲醇中,于40 ℃恒温箱中放置,使根尖切段完全变白为止(4—6 h),在485 nm下测定吸光度值,通过标准曲线计算出四氮唑还原量。
1.8 数据分析
所有数据均为3次重复实验所得结果,实验数据以平均值±标准偏差(Mean±SD)来表示。采用Origin 8.0对数据进行回归分析,SPSS 17.0对数据进行显著性检验。
2. 结果与讨论 (Results and discussion)
2.1 β-HBCD对玉米抗氧化酶活性的影响
SOD是生物体抗氧化系统的第一道防线,可催化
O⋅−2 随着β-HBCD暴露浓度的增加,玉米体内SOD和APX活性(除(−)β-HBCD处理外)均呈现显著的先增加后减少的趋势(图2),表明β-HBCD诱发了玉米机体的氧化应激效应。图2a显示玉米根部SOD活性在(+)、(rac)和(−)β-HBCD最低暴露浓度下(0.5 μg·L−1)即显著增加到最大值(P < 0.05),分别为对照组的1.85倍、1.71倍和1.63倍,但三者之间未呈现出显著性差异,可能是因为低浓度的污染物对玉米SOD活性的影响较小;在最高浓度5.0 μg·L−1时,SOD活性降到最低,但仍比对照组增加了58.7%、56.0%和26.8%,表明β-HBCD对玉米产生了氧化胁迫,且不同立体构型产生的毒性为(+)β-HBCD > (rac)β-HBCD > (−)β-HBCD。在(+)和(rac)β-HBCD暴露下,玉米地上部SOD活性分别在3.0 μg·L−1与2.0 μg·L−1时达到最大(图2b),较对照组分别增加了15.6%和9.5%,表明根部受到的影响更大。(−)β-HBCD处理的地上部SOD活性与对照组相比无显著差异,可见(−)β-HBCD对玉米的影响较小。
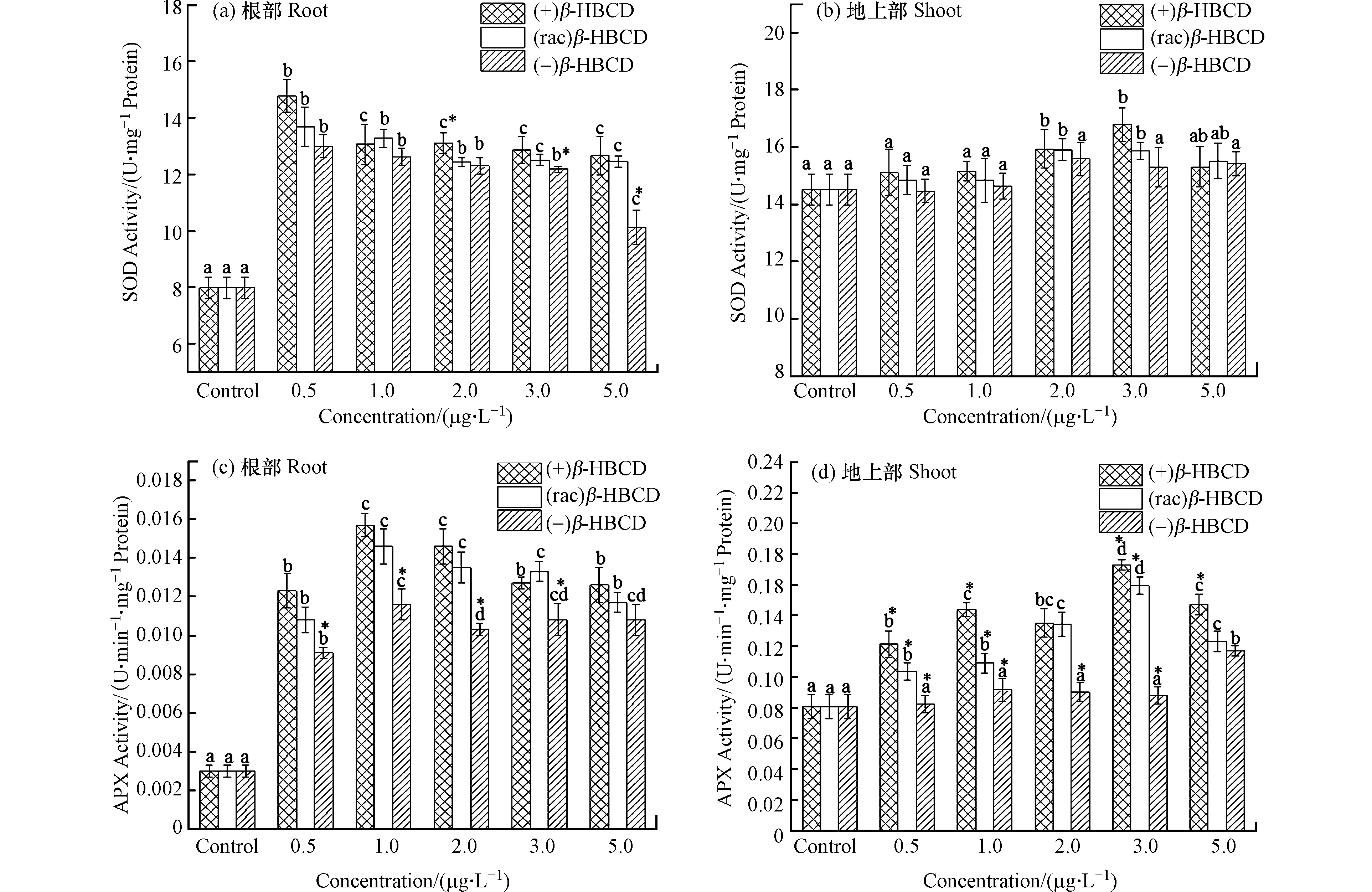 图 2 β-HBCD对玉米体内SOD和APX活性的影响Figure 2. Effects of β-HBCD on SOD and APX activity in maize(不同字母表示不同浓度之间存在显著差异(P < 0.05),* 表示不同对映体之间存在显著差异(P < 0.05))(Different letters represent statistically significant difference between different concentrations at P << 0.05,while * represents a significant difference between the enantiomers at P < 0.05)
图 2 β-HBCD对玉米体内SOD和APX活性的影响Figure 2. Effects of β-HBCD on SOD and APX activity in maize(不同字母表示不同浓度之间存在显著差异(P < 0.05),* 表示不同对映体之间存在显著差异(P < 0.05))(Different letters represent statistically significant difference between different concentrations at P << 0.05,while * represents a significant difference between the enantiomers at P < 0.05)β-HBCD及其对映体均诱导了玉米根部和地上部APX活性增加(图2c和d)。玉米根部在暴露浓度为1.0 μg·L−1时APX活性最高,(+)、(rac)和(−)β-HBCD处理分别是对照组的5.26倍、4.91倍和3.90倍,随后与浓度呈现负相关关系。地上部分,APX活性在(+)和(rac)β-HBCD浓度为3.0 μg·L−1时达到最大值,较对照组分别增加了114.6%和97.6%,随后逐渐降低;(−)β-HBCD处理的玉米地上部APX活性在低浓度下无显著差异,仅在最大暴露浓度(5.0 μg·L−1)时显著增加到对照组的1.45倍。可见,APX活性变化结果与SOD类似,均表现出了(+)β-HBCD的影响最大,其次是(rac)β-HBCD,(−)β-HBCD影响最小。Fang等[23]在甲霜灵对映体对烟草幼苗的影响研究中发现:R-对映体比S-对映体对SOD活性的促进作用更高,对幼苗的影响更大,说明同种物质不同立体构型对生物体产生的毒性作用亦是存在差异的。
外源污染物会影响植物体内的活性氧(ROS)平衡,ROS的大量生成,可以激发植物自身的抗氧化防御系统,显著诱导酶活性增加以消除过量的ROS[24]。本研究中β-HBCD诱导了玉米体内ROS的产生,使细胞抗氧化酶SOD和APX活性增加以清除玉米体内的
O⋅−2 2.2 β-HBCD对玉米体内花青素含量的影响
植物在受到氧化胁迫时,为保护自身生理发育及新陈代谢的正常进行,形成了一套复杂完善的抗氧化保护系统,包括抗氧化酶系统和非酶系统,花青素是非酶系统中一种重要的抗氧化剂,属于生物类黄酮物质,主要存在于植物叶片中,和酚类化合物的作用机理相似,花青素可通过酚羟基与自由基反应生成较稳定的半醌式自由基,从而终止自由基链式反应[27]。
随着β-HBCD暴露浓度的增加,玉米地上部花青素含量与抗氧化酶类似,亦呈现先增加后减少的趋势(图3)。1.0 μg·L−1的(+)、(rac)和(−)β-HBCD处理下玉米叶片中花青素含量最高,分别达到对照组的3.55倍、3.22倍和2.28倍;在最高浓度5.0 μg·L−1时,花青素含量最低,(+)、(rac)和(−)β-HBCD处理组分别比对照组增加了77.6%、94.2%和43.9%。研究表明,花青素具有清除自由基、促进激活抗氧化酶系统的能力,是植物抗氧化的基础[27],本研究中植物体内抗氧化酶系统和非酶系统随β-HBCD暴露的变化趋势一致,说明其在植物防御外源氧化胁迫过程中同样起到非常重要的作用。不同对映体处理组相比,与抗氧化酶系统的SOD和APX活性检测结果一致,均表现出对玉米的毒性作用大小顺序为(+)β-HBCD > (rac)β-HBCD > (−)β-HBCD。
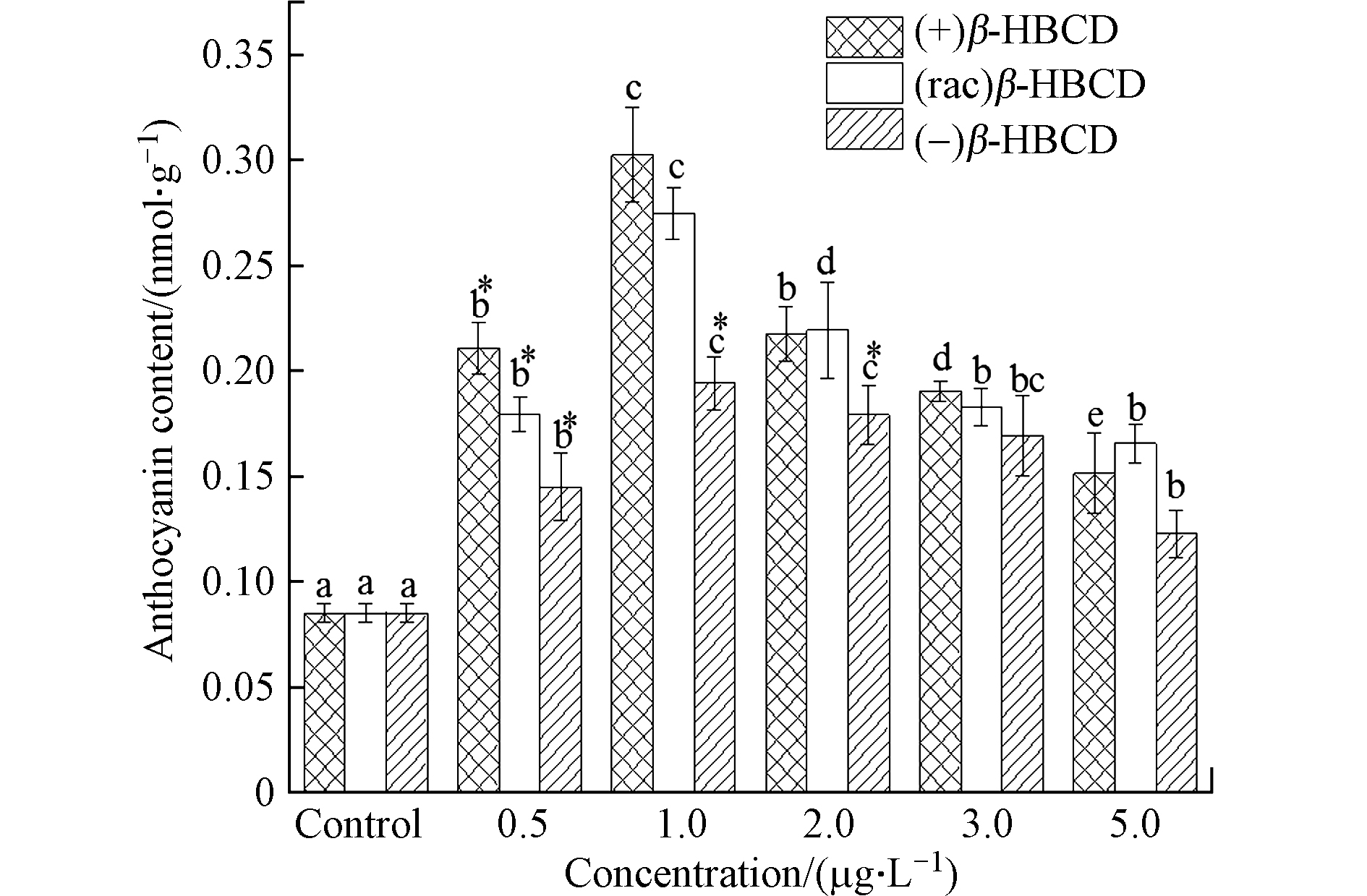 图 3 β-HBCD对花青素含量的影响Figure 3. Effect of β-HBCD on anthocyanidin content in maize(不同字母表示不同浓度之间存在显著差异(P < 0.05),* 表示不同对映体之间存在显著差异(P < 0.05))(Different letters represent statistically significant difference between different concentrations at P < 0.05,while * represents a significant difference between the enantiomers at P < 0.05)
图 3 β-HBCD对花青素含量的影响Figure 3. Effect of β-HBCD on anthocyanidin content in maize(不同字母表示不同浓度之间存在显著差异(P < 0.05),* 表示不同对映体之间存在显著差异(P < 0.05))(Different letters represent statistically significant difference between different concentrations at P < 0.05,while * represents a significant difference between the enantiomers at P < 0.05)2.3 β-HBCD对玉米超氧阴离子含量的影响
β-HBCD的暴露诱导了玉米体内SOD、APX酶活性和花青素含量的增加,那么这种应激响应是否对机体内部的ROS进行了有效的控制呢?本研究进一步考察了玉米组织中的
O⋅−2 结果显示(图4),玉米体内的
O⋅−2 O⋅−2 O⋅−2 O⋅−2 O⋅−2 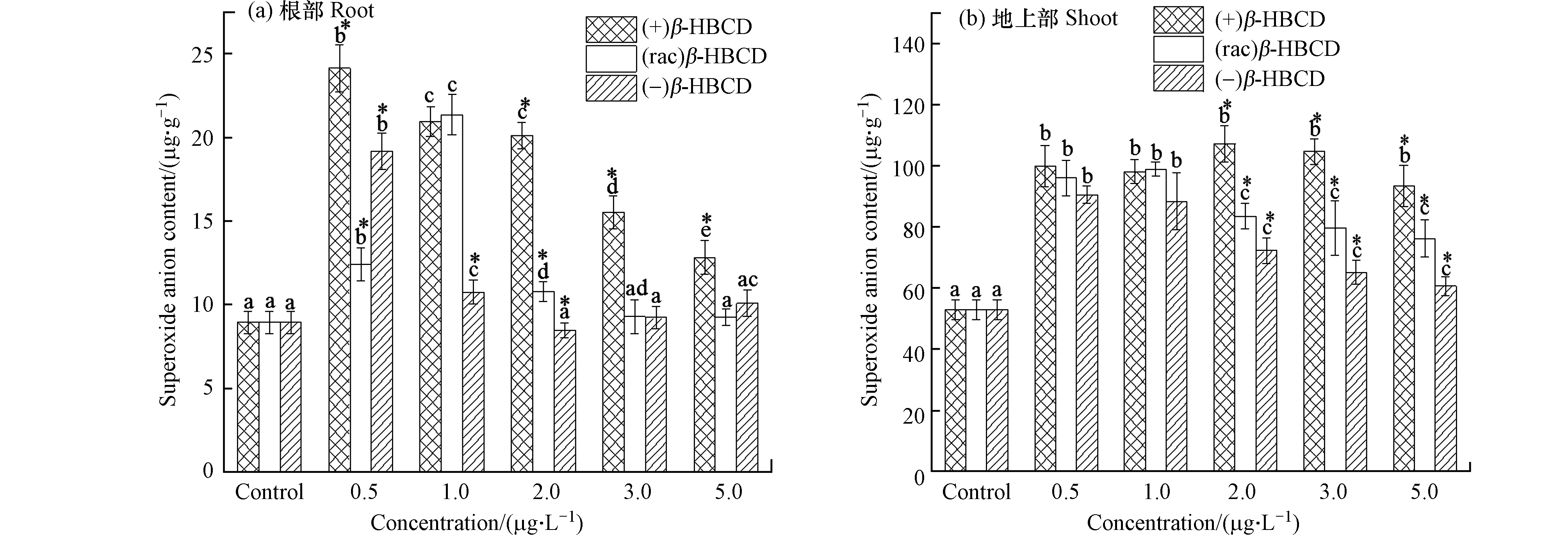 图 4 β-HBCD对映体对玉米体内
图 4 β-HBCD对映体对玉米体内O⋅−2 Figure 4. Effects of β-HBCD enantiomers onO⋅−2 (不同字母表示不同浓度之间存在显著差异(P < 0.05),* 表示不同对映体之间存在显著差异(P < 0.05))(Different letters represent statistically significant difference between different concentrations at P < 0.05,while * represents a significant difference between the enantiomers at P < 0.05)在外界胁迫下,生物体内更多的分子氧经过单电子还原反应转变为
O⋅−2 O⋅−2 O⋅−2 O⋅−2 2.4 β-HBCD对玉米根系活力的影响
根系是植物获得营养和水分,维持稳定较大生物量的关键部位,根系活力的高低直接影响作物的正常生长并间接干扰叶片的光合效率[29-30]。本研究中玉米根系是直接接触暴露液的部位,根部
O⋅−2 O⋅−2 由图5可以看出,经(+)和(rac)β-HBCD胁迫后,幼苗根系活力在0.5 μg·L−1时与空白对照组无显著差异,在1.0 μg·L−1时显著下降,于最大浓度(5.0 μg·L−1)时降到最低,分别较对照组减少了65.1%和39.7%;(−)β-HBCD胁迫下,幼苗根系活力仅在最大浓度胁迫下,较空白对照组显著下降了28.5%,其余处理均与空白无显著差异。显然,尽管植物机体存在庞大的抗氧化保护系统,但β-HBCD及其对映体仍降低了玉米幼苗的根系活力,影响了其健康状态,且不同对映体对幼苗根系活力的抑制作用为(+)β-HBCD > (rac)β-HBCD > (−)β-HBCD。植物根系活力反映根系整体的代谢强度,包括根系呼吸、氧化、还原和合成能力等[31],植物处于逆境生理环境时,其呼吸代谢关键酶表达量会发生改变,进而影响植物的呼吸过程[30]。本研究中β-HBCD很可能是通过诱导ROS的变化影响了玉米根部代谢关键酶的表达或活性,从而导致其代谢强度的改变,根系活力发生变化。这与Zhang等[32]关于碱环境胁迫下大米机体ROS累积导致根系损伤的作用结果一致。不同的β-HBCD对映体对幼苗根系活力的抑制作用存在显著差异,可能与其空间立体构型有关。有文献报道,不同立体构型的HBCD与细胞内代谢相关酶的结合位点具有差异性,其中(−)β-HBCD能够选择性进入玉米代谢相关酶-细胞色素氧化酶(CYP)和谷胱甘肽转移酶(GST)亚型酶CYP71C3v2、GST31的活性位点并与其键合,进而被转化成不同的代谢产物,从而降低了其在植物体内的浓度[25],这可能是(−)β-构型产生的毒性效应较小的主要原因。
 图 5 β-HBCD对玉米根系活力的影响Figure 5. Effects of β-HBCD on root activity in maize(不同字母表示不同浓度之间存在显著差异(P < 0.05),* 表示不同对映体之间存在显著差异(P < 0.05))(Different letters represent statistically significant difference between different concentrations at P < 0.05,while * represents a significant difference between the enantiomers at P < 0.05)
图 5 β-HBCD对玉米根系活力的影响Figure 5. Effects of β-HBCD on root activity in maize(不同字母表示不同浓度之间存在显著差异(P < 0.05),* 表示不同对映体之间存在显著差异(P < 0.05))(Different letters represent statistically significant difference between different concentrations at P < 0.05,while * represents a significant difference between the enantiomers at P < 0.05)3. 结论 (Conclusion)
手性化合物β-HBCD对玉米的生长代谢具有显著的对映体选择性影响,毒性大小为(+)β-HBCD>(rac)β-HBCD>(−)β-HBCD,主要源于植物抗氧化酶系统和非酶抗氧化系统未能有效控制玉米体内
O⋅−2 -
[1] ZHANG Y Q, LI Q F, LU Y L, et al. Hexabromocyclododecanes (HBCDDs) in surface soils from coastal cities in north China: Correlation between diastereoisomer profiles and industrial activities [J]. Chemosphere, 2016, 148: 504-510. doi: 10.1016/j.chemosphere.2016.01.051 [2] SINDIKU O, BABAYEMI O, OSIBANJO O, et al. Polybrominated diphenyl ethers listed as Stockholm Convention POPs, other brominated flame retardants and heavy metals in e-waste polymers in Nigeria [J]. Environmental Science and Pollution Research, 2015, 22(19): 14489-14501. doi: 10.1007/s11356-014-3266-0 [3] ZHANG Y W, SUN H W, LIU F, et al. Hexabromocyclododecanes in limnic and marine organisms and terrestrial plants from Tianjin, China: Diastereomer- and enantiomer-specific profiles, biomagnification, and human exposure [J]. Chemosphere, 2013, 93(8): 1561-1568. doi: 10.1016/j.chemosphere.2013.08.004 [4] HUANG H L, WANG D, WAN W N, et al. Hexabromocyclododecanes in soils and plants from a plastic waste treatment area in North China: Occurrence, diastereomer- and enantiomer-specific profiles, and metabolization [J]. Environmental Science and Pollution Research, 2017, 24(27): 21625-21635. doi: 10.1007/s11356-017-9792-9 [5] XIANG N, CHEN L, MENG X Z, et al. Occurrence of hexabromocyclododecane (HBCD) in sewage sludge from Shanghai: Implications for source and environmental burden [J]. Chemosphere, 2015, 118: 207-212. doi: 10.1016/j.chemosphere.2014.08.058 [6] ZHANG Y W, SUN H W, ZHU H K, et al. Accumulation of hexabromocyclododecane diastereomers and enantiomers in two microalgae, Spirulina subsalsa and Scenedesmus obliquus [J]. Ecotoxicology and Environmental Safety, 2014, 104: 136-142. doi: 10.1016/j.ecoenv.2014.02.027 [7] WU T, WANG S, HUANG H L, et al. Diastereomer-specific uptake, translocation, and toxicity of hexabromocyclododecane diastereoisomers to maize [J]. Journal of Agricultural and Food Chemistry, 2012, 60(34): 8528-8534. doi: 10.1021/jf302682p [8] LUO X J, RUAN W, ZENG Y H, et al. Trophic dynamics of hexabromocyclododecane diastereomers and enantiomers in fish in a laboratory feeding study [J]. Environmental Toxicology and Chemistry, 2013, 32(11): 2565-2570. [9] PALACE V, PARK B, PLESKACH K, et al. Altered thyroxine metabolism in rainbow trout (Oncorhynchus mykiss) exposed to hexabromocyclododecane (HBCD) [J]. Chemosphere, 2010, 80(2): 165-169. doi: 10.1016/j.chemosphere.2010.03.016 [10] HUANG X M, CHEN C, SHANG Y, et al. In vitro study on the biotransformation and cytotoxicity of three hexabromocyclododecane diastereoisomers in liver cells [J]. Chemosphere, 2016, 161: 251-258. doi: 10.1016/j.chemosphere.2016.07.001 [11] SZABO D T, DILIBERTO J J, HAKK H, et al. Toxicokinetics of the flame retardant hexabromocyclododecane gamma: Effect of dose, timing, route, repeated exposure, and metabolism[J]. Toxicological Sciences, 2010 , 117: 282–293. [12] ZHANG X, HERGER A G, REN Z et al. Resistance effect of flavonols and toxicology analysis of hexabromocyclododecane based on soil-microbe-plant system [J]. Chemosphere, 2020, 257: 127248. doi: 10.1016/j.chemosphere.2020.127248 [13] HUANG H L, WANG D, WEN B, et al. Roles of maize cytochrome P450 (CYP) enzymes in stereo-selective metabolism of hexabromocyclododecanes (HBCDs) as evidenced by in vitro degradation, biological response and in silico studies [J]. Science of the Total Environment, 2019, 656: 364-372. doi: 10.1016/j.scitotenv.2018.11.351 [14] 武彤, 田柳, 崔建升, 等. 六溴环十二烷对映体对玉米的生理和基因损伤研究 [J]. 环境科学学报, 2018, 38(12): 4864-4872. WU T, TIAN L, CUI J S, et al. Physiological and genetic damage effects of hexabromocyclododecane enantiomers on maize [J]. Acta Scientiae Circumstantiae, 2018, 38(12): 4864-4872(in Chinese).
[15] 崔建升, 刘颖, 武彤, 等. γ-六溴环十二烷对映体对玉米的氧化损伤 [J]. 环境化学, 2016, 35(9): 1762-1768. doi: 10.7524/j.issn.0254-6108.2016.09.2016012606 CUI J S, LIU Y, WU T, et al. Oxidative damage of γ-hexabromocyclododecane enantiomers to maize [J]. Environmental Chemistry, 2016, 35(9): 1762-1768(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.09.2016012606
[16] ZHANG X L, YANG F X, CHAO X, et al. Cytotoxicity evaluation of three pairs of hexabromocyclododecane (HBCD) enantiomers on Hep G2 cell [J]. Toxicology in Vitro, 2008, 22(6): 1520-1527. doi: 10.1016/j.tiv.2008.05.006 [17] BRADFORD M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dyebinding [J]. Analytical Biochemistry, 1976, 72(1/2): 248-254. [18] BEAUCHAMP C, FRIDOVICH I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels [J]. Analytical Biochemistry, 1971, 44(1): 276-287. doi: 10.1016/0003-2697(71)90370-8 [19] NAKANO, Y, ASADA, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts [J]. Plant and Cell Physiology, 1981, 22(5): 867-880. [20] JABS T, DIETRICH R A, DANGL J L. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide [J]. Science (New York, N. Y. ), 1996, 273(5283): 1853-1856. doi: 10.1126/science.273.5283.1853 [21] YAMAUCHI T, WATANABE K, FUKAZAWA A, et al. Ethylene and reactive oxygen species are involved in root aerenchyma formation and adaptation of wheat seedlings to oxygen-deficient conditions [J]. Journal of Experimental Botany, 2014, 65(1): 261-273. doi: 10.1093/jxb/ert371 [22] 刘冬峰. 砂梨对高温胁迫的响应及耐热机理研究[D]. 杭州: 浙江大学, 2014: 1-131. LIU D F. Studies on the resoponse of sand pear to high-temperature and high-tolerance mechanism[D], Hangzhou: Zhejiang University, 2014: 1-131 (in Chinese).
[23] FANG S, TAO Y, ZHANG Y Z, et al. Effects of metalaxyl enantiomers stress on root activity and leaf antioxidant enzyme activities in tobacco seedlings [J]. Chirality, 2018, 30(4): 469-474. doi: 10.1002/chir.22810 [24] LIU X L, ZHANG S Z, SHAN X Q, et al. Combined toxicity of cadmium and arsenate to wheat seedlings and plant uptake and antioxidative enzyme responses to cadmium and arsenate co-contamination [J]. Ecotoxicology and Environmental Safety, 2007, 68(2): 305-313. doi: 10.1016/j.ecoenv.2006.11.001 [25] HUANG H L, ZHANG S Z, LV J T, et al. Experimental and theoretical evidence for diastereomer- and enantiomer-specific accumulation and biotransformation of HBCD in maize roots [J]. Environmental Science & Technology, 2016, 50(22): 12205-12213. [26] HONG H Z, LV D M, LIU W X, et al. Toxicity and bioaccumulation of three hexabromocyclododecane diastereoisomers in the marine copepod Tigriopus japonicas [J]. Aquatic Toxicology, 2017, 188: 1-9. doi: 10.1016/j.aquatox.2017.04.010 [27] 蔡珊, 黄亚梅, 张易华, 等. 花青素生理活性及其抗氧化机制 [J]. 陕西农业科学, 2018, 64(12): 40-43. doi: 10.3969/j.issn.0488-5368.2018.12.011 CAI S, HUANG Y M, ZHANG Y H, et al. Physiological activity and antioxidant mechanism of anthocyanin [J]. Shaanxi Journal of Agricultural Sciences, 2018, 64(12): 40-43(in Chinese). doi: 10.3969/j.issn.0488-5368.2018.12.011
[28] BANERJEE B D, SETH V, AHMED R S. Pesticide-induced oxidative stress: perspectives and trends [J]. Reviews on Environmental Health, 2001, 16(1): 1-40. doi: 10.1515/REVEH.2001.16.1.1 [29] XU C M, CHEN L P, CHEN S, et al. Effects of rhizosphere oxygen concentration on root physiological characteristics and anatomical structure at the tillering stage of rice [J]. Annals of Applied Biology, 2020, 177(1): 61-73. doi: 10.1111/aab.12589 [30] 李志霞, 秦嗣军, 吕德国, 等. 植物根系呼吸代谢及影响根系呼吸的环境因子研究进展 [J]. 植物生理学报, 2011, 47(10): 957-966. LI Z X, QIN S J, LV D G, et al. Research progress in root respiratorymetabolism of plant and the environmental influencing factors [J]. Plant Physiology Journal, 2011, 47(10): 957-966(in Chinese).
[31] CSERESNYÉS I, RAJKAI K, TAKÁCS T. Indirect monitoring of root activity in soybean cultivars under contrasting moisture regimes by measuring electrical capacitance [J]. Acta Physiologiae Plant, 2016, 38(5): 1-21. doi: 10.1007/s11738-016-2149-z [32] ZHANG H, LIU X L, ZHANG R X, et al. Root damage under alkaline stress is associated with reactive oxygen species accumulation in rice (Oryza sativa L. ) [J]. Frontiers in Plant Science, 2017, 8: 1580. doi: 10.3389/fpls.2017.01580 -











 下载:
下载:








































