-
砷是一种有毒的类金属元素[1],在地下水中主要以As(Ⅲ)和As(Ⅴ)的络阴离子形式存在,其中As(Ⅲ)对人体的危害远高于As(Ⅴ)[2],地方性的高砷地下水砷中毒问题已在环境地质方面成为当今国际社会所面临的最棘手的问题之一[3]。高砷地下水在世界内分布广泛,遍及全球70 多个国家,包括孟加拉国、印度、中国、墨西哥、匈牙利、越南等[4]。长期饮用砷浓度高于10 μg·L−1地下水的症状通常包括皮肤病(如色素沉着,皮肤角质化过度和皮肤癌等)、心血管疾病、神经系统疾病、肝癌、肾癌和前列腺癌等[5]。在中国北部,尤其是内蒙古、山西、新疆有很多高砷地下水区,寻找替代水资源或有效治理高砷水源面临巨大挑战。
地球成因的高砷水被普遍认为是水-岩相互作用的结果[6];沉积物中可交换态砷在水交替缓慢的条件下,经过长期的水-岩相互作用极易进入地下水中形成高砷地下水[7]。Mukherjee 等的研究表明富砷矿物与全新世冲积沉积物的第四纪沉积物有关[8]。高存荣等认为沉积环境和沉积物性质对地下水中砷的成因与迁移有重要影响[9]。Zhang等发现地下水水位波动和停滞时间是影响地下水中砷的迁移与分布的重要因素[10]。余倩等认为磷酸盐与砷的竞争吸附对地下水中砷的迁移转化有重要影响[11]。因此,查明含水层水文地球化学特征对砷运移的影响可以为揭示高砷地下水成因机理与地下水砷污染的防控提供科学依据。
我国高砷地下水主要分布在干旱内陆盆地及河流三角洲[12]。尽管奎屯河流域是中国首个大规模地方性砷中毒病区[13],但与松嫩平原、大同盆地等区域相比,奎屯河流域高砷地下水地球化学的研究程度仍不够深入,人们对地下水中的高砷成因及砷运移机制还不甚了解。因此,本研究主要目的是:(1)阐述研究区含水层水文地球化学特征;(2)评估地下水中As富集的成因和程度;(3)评价控制砷运移的主要地球化学因子。
全文HTML
-
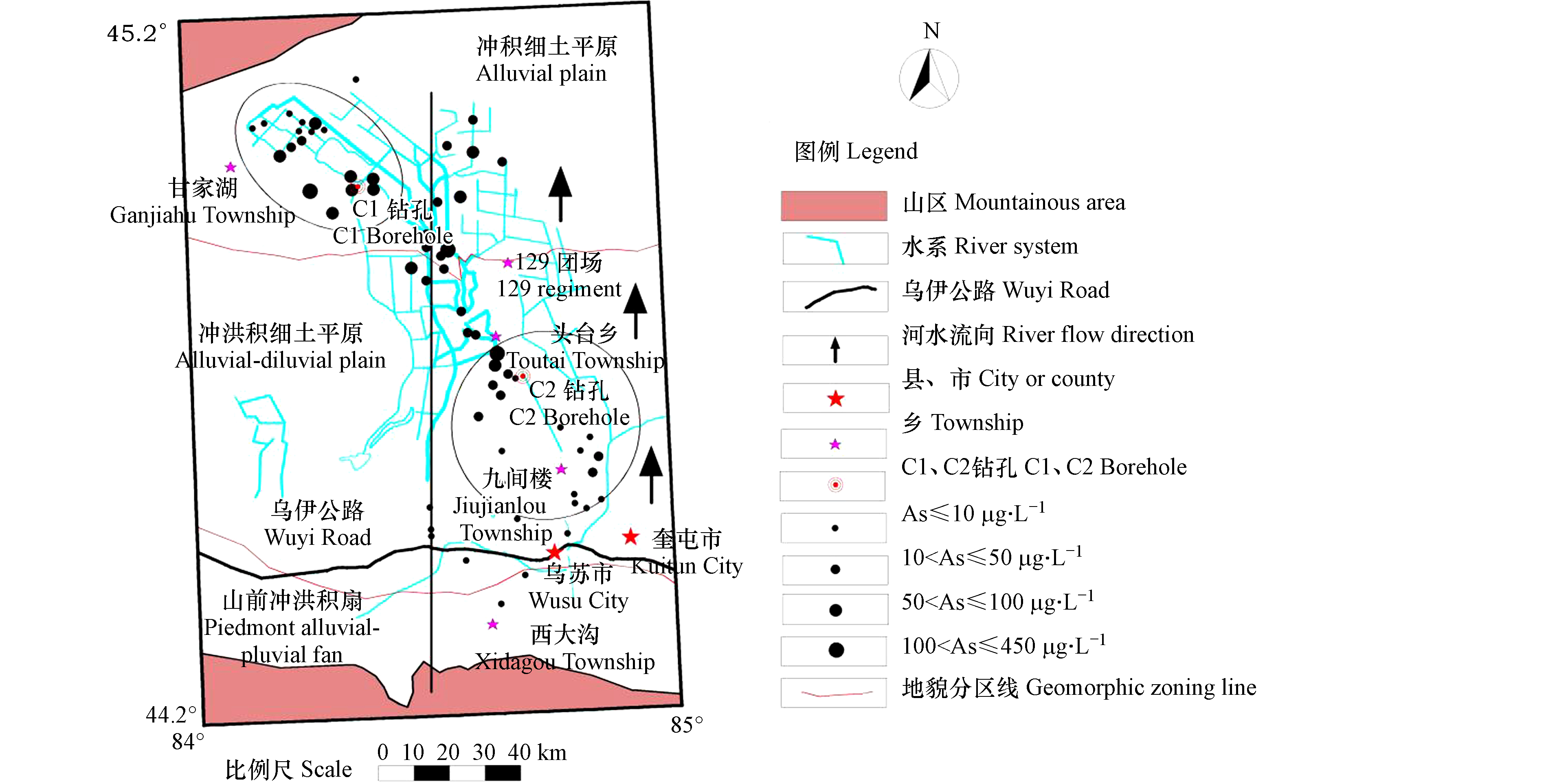
奎屯河流域(图1)在新疆乌鲁木齐西220 km,准噶尔盆地边缘。流域东与巴音沟河流域交界,西与托托河流域相交,南与天山分水岭相邻,北与扎伊尔山分水岭连壤。流域内有奎屯河、古尔图河和四棵树河,均源自博罗科努山、依连哈比尔尕山,流入准噶尔盆地及山间盆地的低洼带[14]。奎屯河流域地处欧亚大陆腹地,位于气候干旱的北温带,冬寒夏热,蒸发量大降水少,温差大且空气干燥。年均气温在7 ℃左右,年均降水量150—170 mm,年均蒸发量1710—1930 mm,蒸发量是降水量的10—12.9倍。研究区地下水径流补给均来自山区融雪水和降水,其中山区融雪水补给量占总补给量的25%—35%。
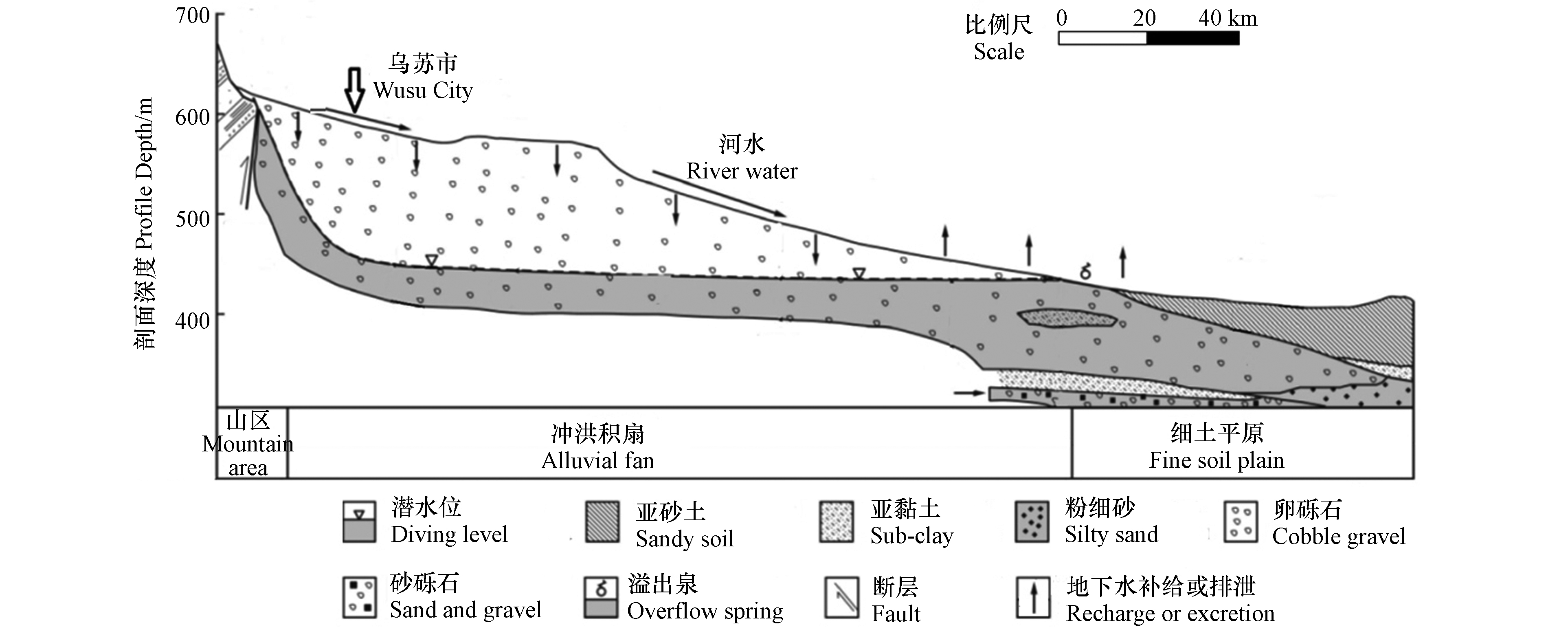
从古近纪起,受喜马拉雅运动的强烈影响,山地与盆地间断块式的升降运动增强,使中生代地层断裂和褶皱。随着山前拗陷西迁北移,到新近纪时形成以乌苏-奎屯为沉积中心,再次接受新的堆积,此期,在四棵树河东面主要呈现坳褶,在四棵树河西面主要呈现块断陷落[15]。进入第四纪,近期构造运动仍很强烈,地壳的变化以垂直升降运动为主,水平运动次之。山前冲洪积扇(图2)构成含水层的岩性为卵砾石,随着地面倾斜方向其颗粒由粗变细;在冲洪积细土平原上部,含水层的岩性以细砂为主;冲洪积细土平原下部,潜水含水层岩性为粉细砂、细砂,局部有砂砾层。研究区下游的冲积细土平原区,含水层岩性为多层结构,由上至下依次为亚砂土、卵砾石、粉细砂、砂砾石,局部有亚黏土弱透水层。
-
2017年6月和2019年8月在奎屯河流域分别采集45组、16组地下水水样,所取水样均来自当地已有机井,采样点分布见图1。现场采集样品时,采用多参数便携水质分析仪DDB-303A、JPBJ-608溶解氧分析仪、PHB-4等仪器测试地下水温度、pH、电导率、溶解氧、Eh和碱度等,并记录水井类型、井深、水位埋深等信息;水样采集前要先用稳定后的地下水原液润洗采样瓶,然后把地下水水样用0.45 μm的滤膜过滤后,再装入聚乙烯采样瓶中密封,在低温状态下保存并及时送检。水样的采集、保存及送样过程严格按照《地下水环境监测技术规范(HL/T164—2004)》的要求执行。样品的测试由新疆地矿局第二水文地质大队化验室完成,测试项目包括:
HPO2−4 、pH、NO−2 、Eh、K+、F−、Na+、NO−3 、Ca2+、Mg2+、HCO−3 、CO2−3 、Cl−、SO2−4 、TDS、NH+4 、As、TFe、Mn、SiO2、H2SiO3、COD、总硬度。运用阴阳离子平衡法进行计算可知,所有水样数据的电荷平衡绝对误差均小于5%,为可靠数据。 -
2019年8月在奎屯河流域冲积细土平原的石桥乡梧桐村开凿1个90 m钻孔C1,在冲洪积细土平原头台乡三泉居民点开凿了1个45 m钻孔C2(图1)。在C1、C2钻孔中分别采集沉积物样品30组、14组。每3 m采集1 组沉积物样品,如果岩性突变,则加大采样密度。取样时,用刻刀剥去泥皮,取沉积物岩心,放入干净的保鲜袋中密封,再低温保存送往实验室。
沉积物样品取回实验室后,将样品置于无风阴凉处,使其进行自然干燥,然后研磨至200目以下,将样品进行标记后取10 g样品于中国科学院新疆生态与地理研究所生态与环境分析测试中心进行沉积物化学组分检测,测试项目包括:As、Fe、Mn、Ca、Mg、Cu。土壤标准物质GBW07454(GSS-25)的测定值与标准值吻合,样品加标回收率在93.4%—103.6%范围内,符合控制范围要求。
1.1. 研究区概况
1.2. 地下水水样采集与分析
1.3. 沉积物样品采集与测试
-
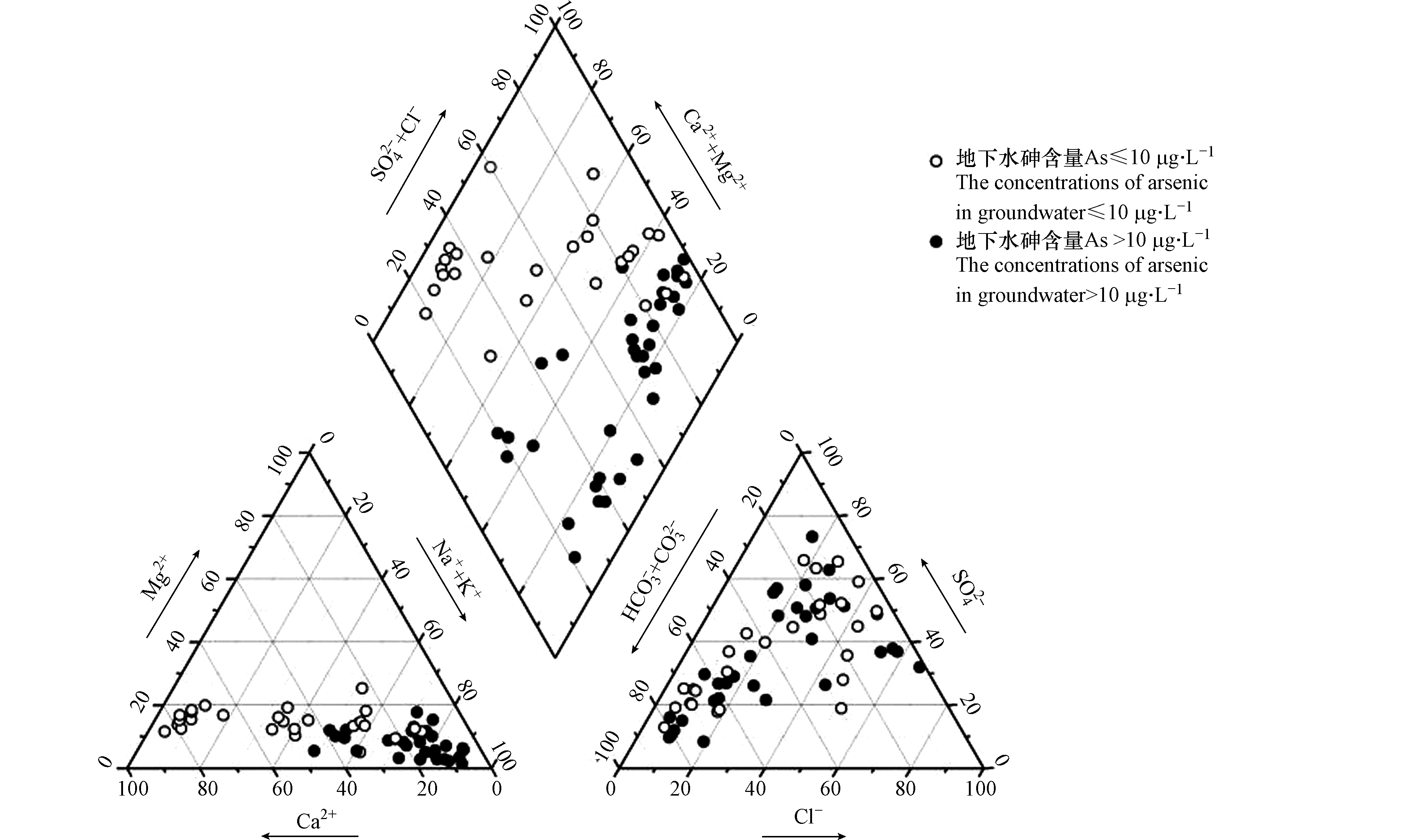
研究区61组地下水样品的水化学组分信息见表1,井深为18—500 m。首先把地下水水样的水化学参数数据绘制于Piper三线图中,然后以水样中As含量是否超过国家标准来进行区分(图3);按舒卡列夫分类方法对地下水水化学类型分类[16-17]。高砷地下水的优势阳离子为Na+,阴离子以Cl·HCO3型、SO4·HCO3型为主,水化学类型主要为SO4·HCO3-Na型和Cl·HCO3-Na型。
由表1可知,高砷地下水pH值为7.7—9.0之间,呈现出碱性环境;
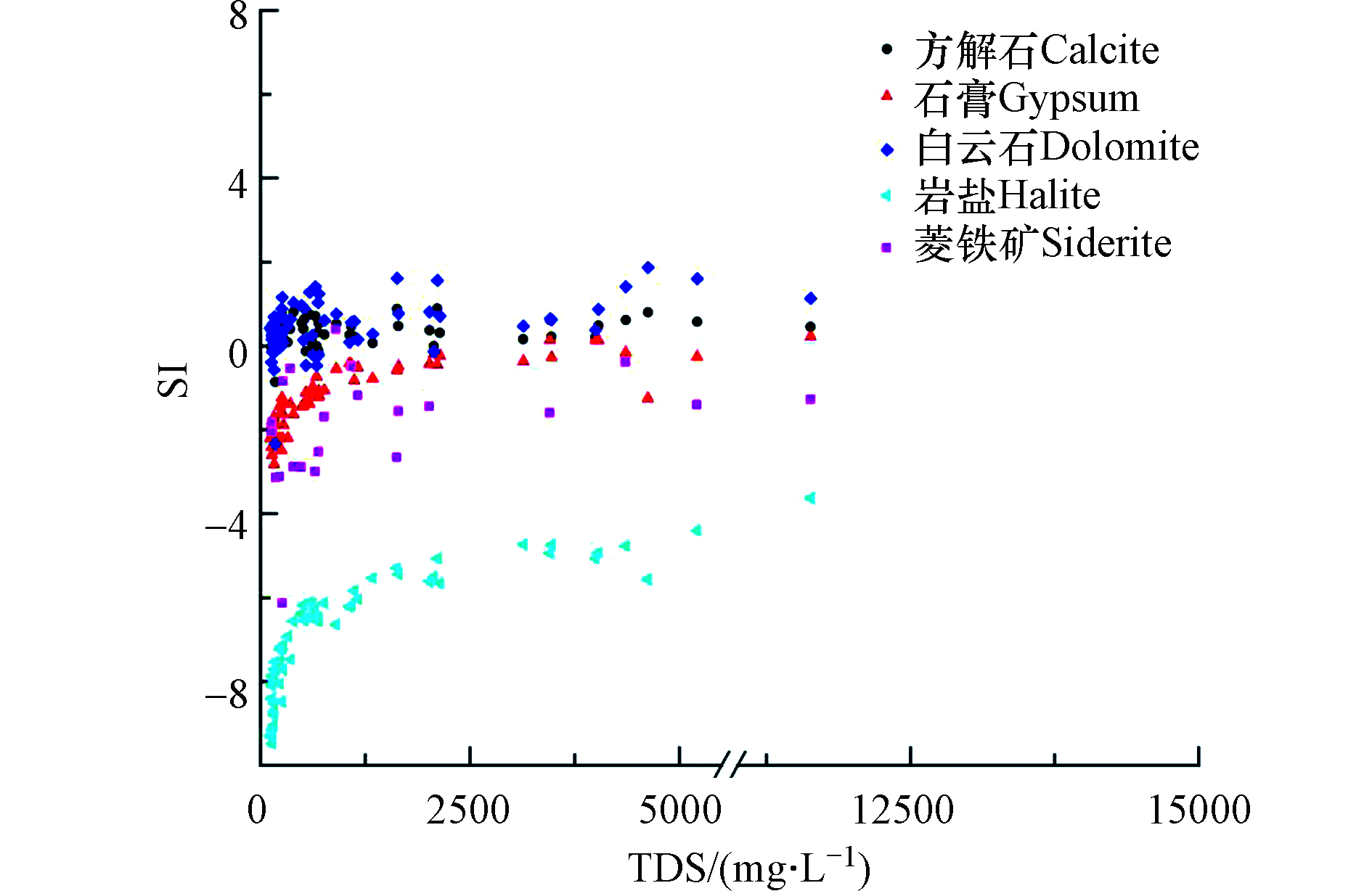
HCO−3 的浓度为78.18—572.06 mg·L−1,平均值为143.52 mg·L−1;PO3−4 的浓度为ND(未检出)—1.43 mg·L−1,检出率为68.9%,平均值为0.25 mg·L−1;高砷地下水Eh主要为−216— −3 mV,属还原环境。Fe和Mn的浓度分别为ND—0.62 mg·L−1、ND—2.41 mg·L−1,有22份检测出Fe,检出率为36.1 %,平均值为0.11 mg·L−1,28份检测出Mn,检出率为 45.9%,平均值0.49 mg·L−1。NO−3 的浓度为ND—40.25 mg·L−1,检出率为75.4%,平均值为4.34 mg·L−1;NO−2 的浓度为 ND—0.75 mg·L−1,检出率为24.6%,平均值为0.18 mg·L−1;NH+4 的浓度为ND—25.20 mg·L−1,检出率为95.1%,平均值为1.18 mg·L−1。运用PHREEQC软件计算出地下水中矿物饱和指数(SI)。如图4所示,在大部分地下水中方解石和白云石均处于过饱和状态,这表明
HCO−3 浓度的升高不仅受含水层中碳酸盐溶解的控制[18],而且可能受到沉积物和地下水中有机物氧化的影响[19]。地下水中岩盐全部处于不饱和状态;此外,在大多数地下水水样中,菱铁矿及蒸发岩(石膏)的SI值为负值处于不饱和状态。在富砷含水层中,菱铁矿的沉淀是一种重要的水文地球化学过程。 -
在奎屯河流域所采集的61 组地下水水样中(表1),As浓度为 ND—444.40 μg·L−1,其中变异系数为1.69,属极强变异,表明研究区地下水中As含量在空间分布上的变化极大。研究区采样点As浓度最高达444.40 μg·L−1(该水样取自车排子镇哈拉苏村,井深500 m,含水层岩性为粉细砂),这可能是由于研究区内古地理环境和封闭的构造条件长期以来形成了有利于As富集的还原环境。由图1可以看出,地下水水样As含量未超标的区域集中分布在山前冲洪积扇(南起乌苏市西大沟镇北至乌伊公路一带)和冲积细土平原(甘家湖一带);其中,冲洪积扇地下水砷含量未超标,是由于该区域地形较陡(图2),地下水水力坡度较大、地下水的滞留时间较短。冲积细土平原地下水砷含量未超标的均为浅层地下水,可能是由于浅层地下水与地表水交换频繁的原因[20]。
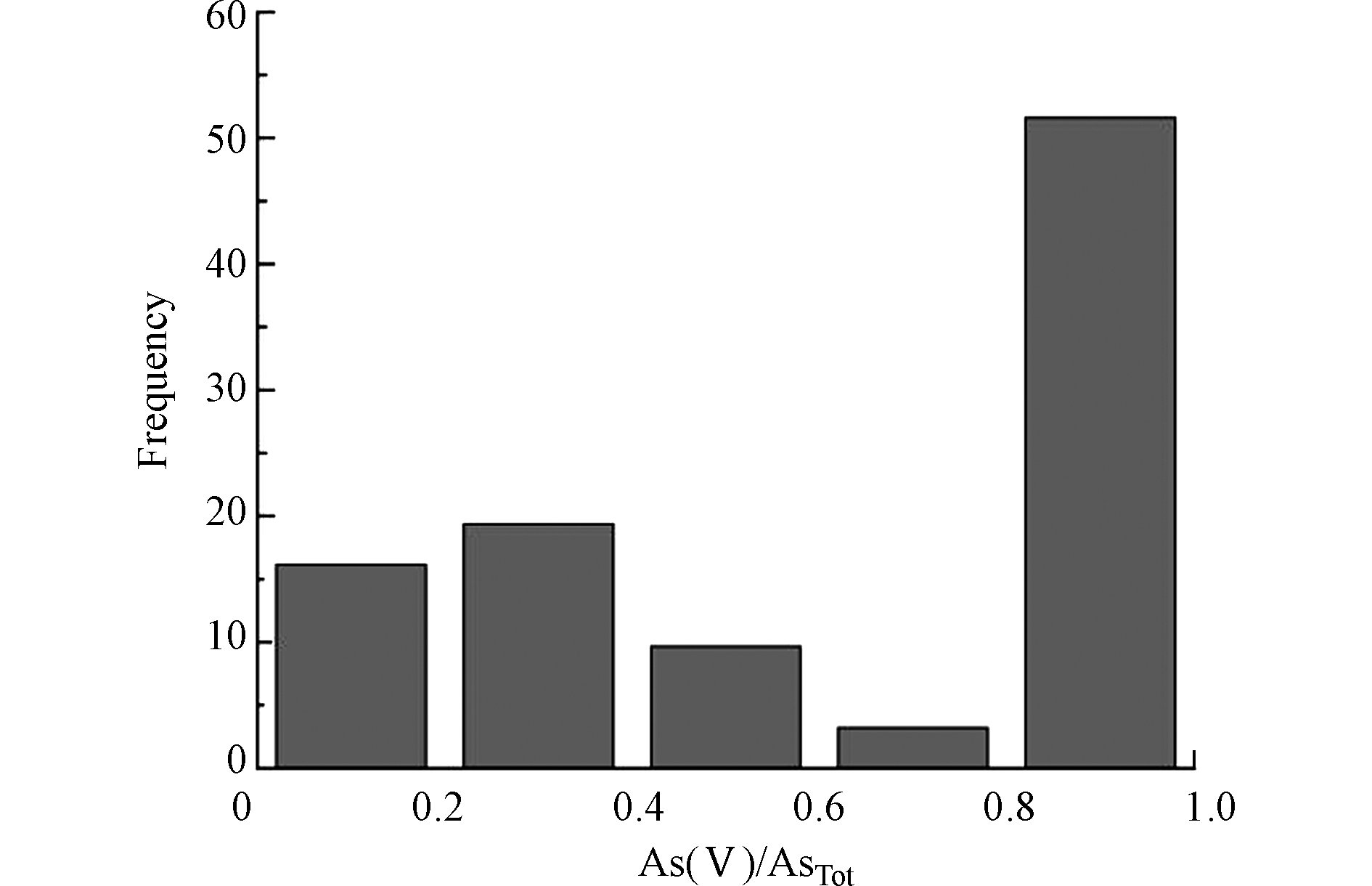
地下水中As(Ⅴ)浓度在ND—434.94 μg·L−1的范围内,平均值和中值分别为49.5 μg·L−1、33.7 μg·L−1。在分析的样品中,无机As(Ⅲ)占总溶解性砷的 22.7%,55%的As(Ⅴ)与总砷之比大于60%(图5),As(Ⅴ)是地下水中As的主要存在形式,是由于高pH下铁氧化物对As(Ⅴ)的吸附亲和力明显小于As(Ⅲ),表明该研究区地下水中砷毒性相对较轻。
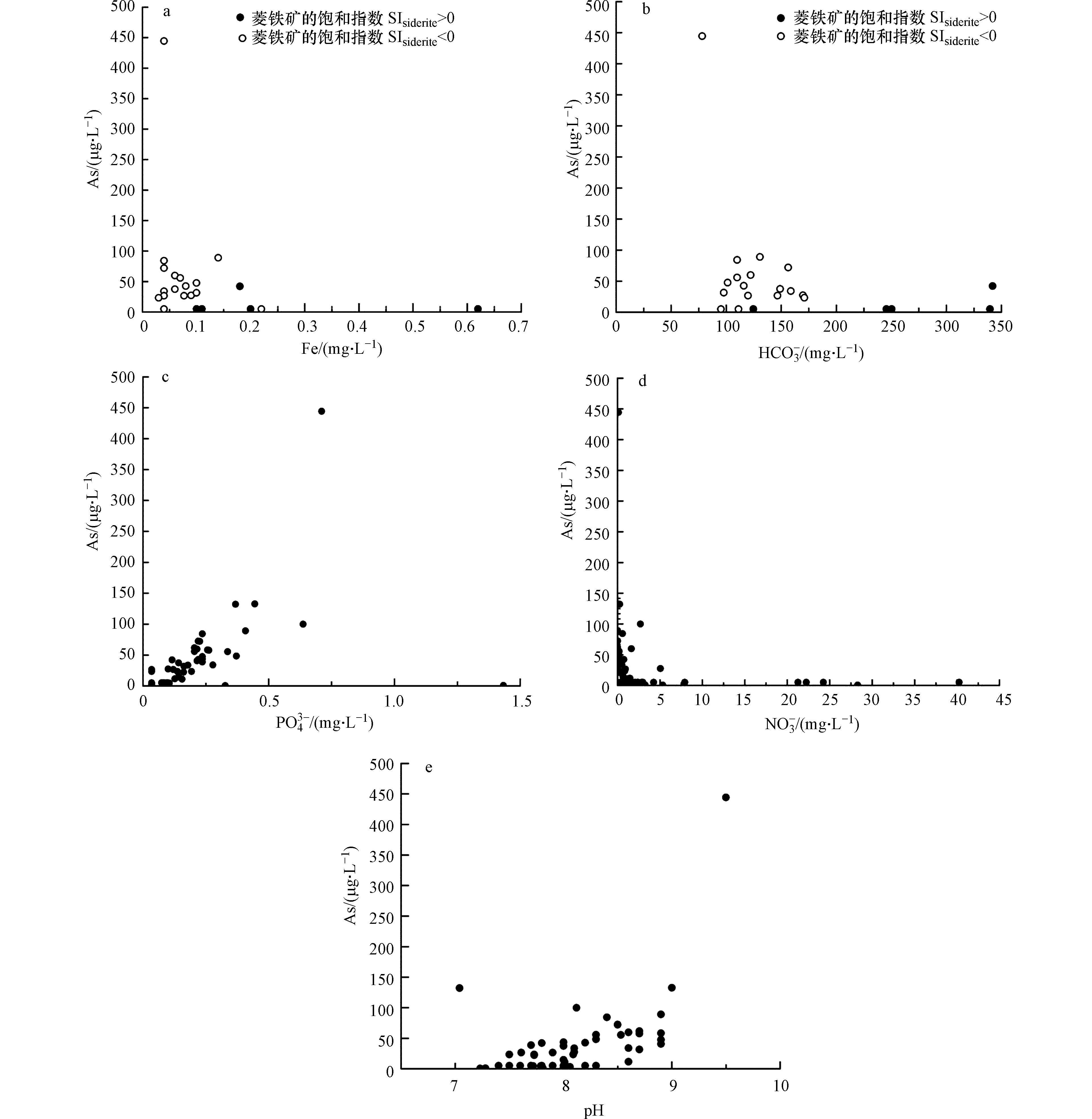
研究区地下水中As与Fe的关系如图6a所示,由图6a可以看出随着Fe浓度的升高As浓度呈下降的趋势,该地区地下水中Fe的浓度并不高。当SIsiderite(菱铁矿的饱和指数)>0时,菱铁矿的沉淀降低了铁的含量,导致As与Fe呈较低的负相关关系(r=−0.16);在SIsiderite<0时,As与Fe呈现负相关关系(r=−0.21),可能是由于Fe的氢氧化物还原溶解产生的Fe2+会再次被残留矿物吸附[21]。研究区地下水中As和
HCO−3 的关系见图6b,当SIsiderite>0时,As与HCO−3 呈正相关关系(r = 0.51),可能是因为地下水中HCO−3 的含量较高,可与As之间呈现竞争吸附作用,从而促进As的解吸附;而在SIsiderite<0的情况下,As与HCO−3 具有负相关性(r=−0.43),可能是由于地下水中HCO3−与As的硫化物反应生成稳定的As-CO2−3 复合物,从而导致地下水中As浓度增大[22]。因为磷酸根(PO3−4 )与砷酸根(AsO3−4 )均能专性吸附在矿物表面,所以两种离子会在金属氧化物表面产生竞争吸附作用[23]。地下水中As和PO3−4 的关系如图6c所示,PO3−4 与As之间呈现显著正相关关系(r=0.47),表明PO3−4 的竞争吸附是地下水中As富集的主要原因。研究区地下水中As和NO−3 的关系见图6d,NO−3 与As相关性不显著(r=−0.20)。地下水中As浓度随着NO−3 浓度的上升而逐渐下降,NO−3 浓度越高时,铁锰氧化物越稳定,含砷铁锰氧化物就不会发生还原反应将砷释放到地下水中[24],因此部分区域高浓度NO−3 所指示的氧化环境可以在一定程度上制约As的释放。地下水中As与pH的关系见图6e,pH与As之间呈现显著正相关关系(r=0.53),当pH值范围在4—9之间时,随着pH的增大会使胶体及黏土矿物带更多的负电荷,降低其对以阴离子形式存在的As的吸附能力,导致地下水中As的浓度增大[25]。 -
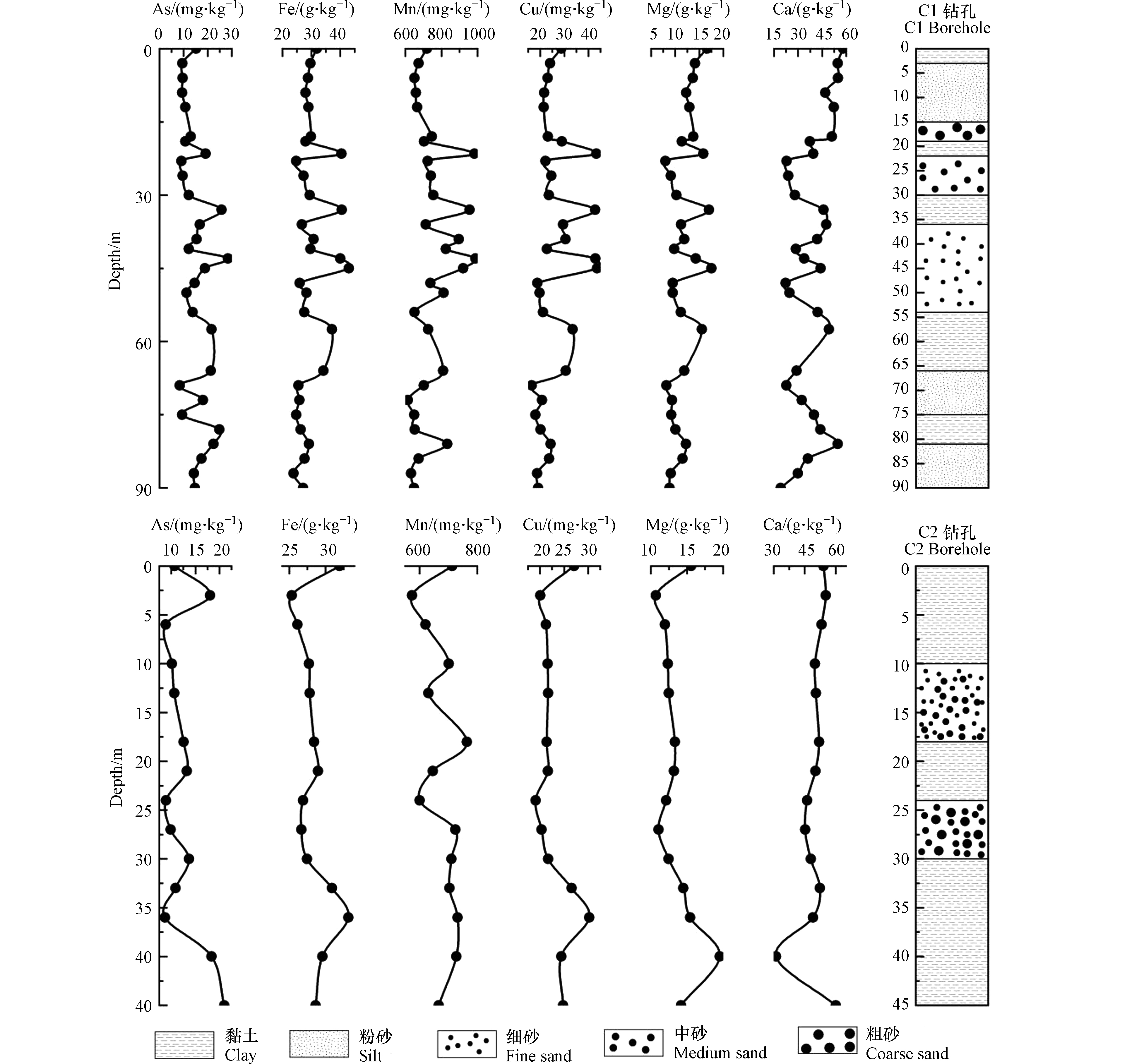
对研究区2个钻孔中所取的44组沉积物样品的化学组分进行了分析(图7),由图7可以看出,C1钻孔中沉积物As含量为8.36—28.41 mg·kg−1,平均值和中值分别为15.26 mg·kg−1、14.43 mg·kg−1。C2钻孔中沉积物As含量为8.69—21.01 mg·kg−1,平均值和中值分别为 12.54 mg·kg−1、10.78 mg·kg−1。两处钻孔中As含量均高于典型的现代松散型沉积物(5—10 mg·kg−1)[6]。C1钻孔中,在地下3 m以内岩性为黏土,颜色为暗黄色;在地下3—81 m之间岩性以粉细砂为主,颜色以黄褐(棕)色为主;地表以下81—90 m之间岩性以粉砂为主,沉积物颜色多以黄色为主。C1钻孔中地下43 m处的黄褐色细砂中As含量最高为28.41 mg·kg−1。C2钻孔中,在地下10 m以内为黏土,颜色多为黄色;10—18 m处岩性为中细砂,18—24 m处岩性为黏土,颜色为黑色,24—30 m处岩性为中粗砂,颜色为黑褐色。30—45 m处岩性为黏土,颜色以黄色为主。C2钻孔中沉积物在地下45 m处As含量最高为21.01 mg·kg−1。
C1、C2钻孔在接近地表3 m内的黏土层中As含量较高,C1钻孔在地表测出As含量为15.18 mg·kg−1,C2钻孔在地下3 m处As含量为18.07 mg·kg−1,这可能是对化肥及含砷农药等长期使用所造成的[26]。由沉积物中岩性与砷含量随深度的变化趋势可知黏土层中As含量较高,砂层中As含量较低,这表明沉积物中As含量与岩性关系密切。仅在C1钻孔中39—43 m、66—72 m处的深色细砂层出现砷含量极高的情况,这可能是由于该砂层有机质含量相对较高,导致沉积物较容易富集砷[27]。
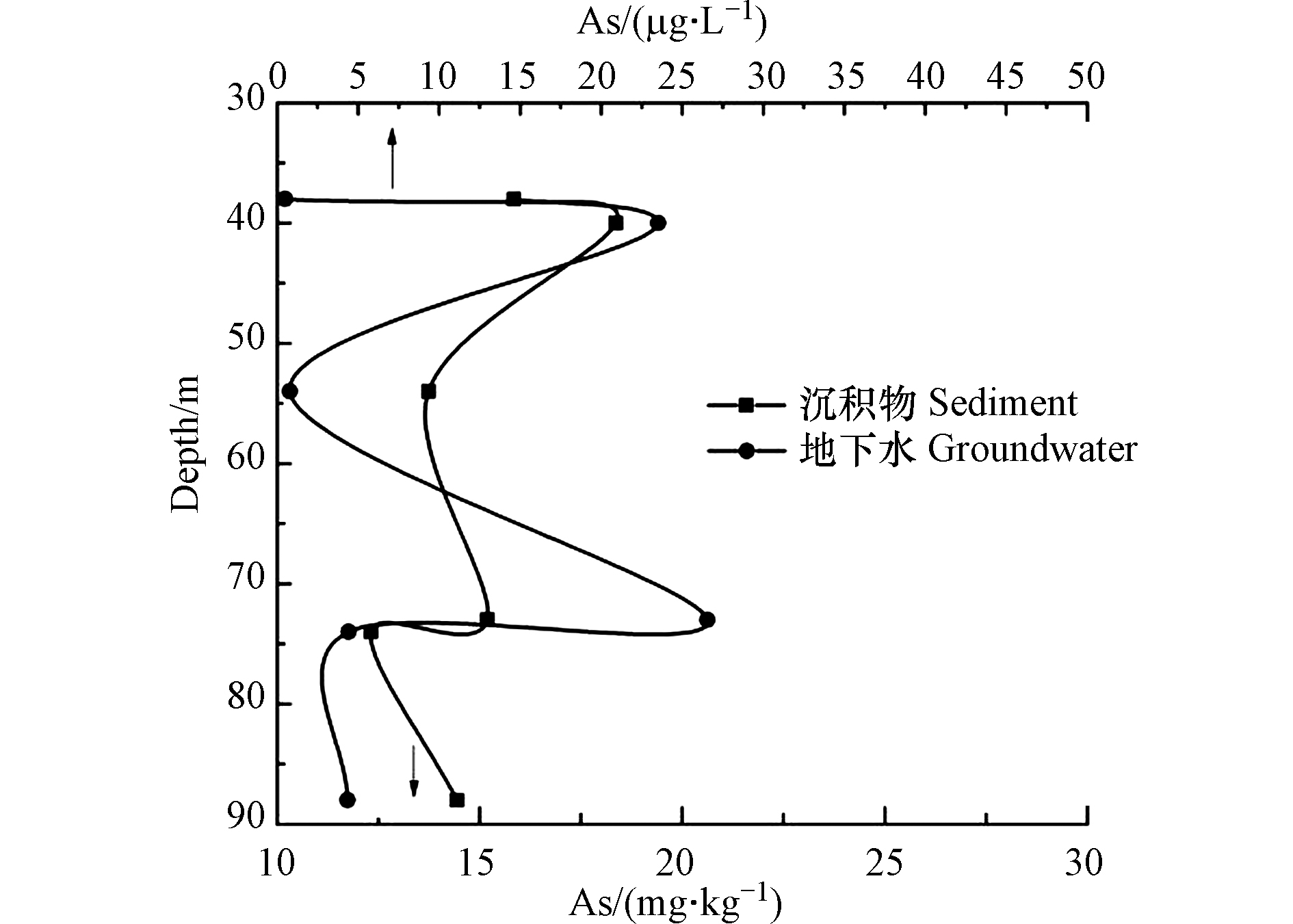
在2个钻孔周围圈出临近地下水取样点(图1),将相同深度的地下水、沉积物样品中As含量进行对比,地下水和沉积物中As随深度的变化规律见图8,沉积物与相应深度地下水中As含量间呈现正相关关系(r = 0.58),表明沉积物中的As含量是影响地下水中As浓度的一个因素。C1钻孔中随深度的增加沉积物各元素含量之间具有相关性,在As含量相对较低的砂层中(包括两处As含量较高的细砂层),对应的Fe、Mn、Cu、Mg、Ca的含量也较低(较高)。
-
运用SPSS软件对C1钻孔沉积物中各元素进行相关性分析,如表2所示,可以发现As与Fe、Mn含量之间均呈现显著正相关,表明砷更倾向于在Fe、Mn含量高的沉积物中富集。平原区的沉积物颗粒较细,地下水水力坡度小,流速慢,水-岩相互作用时间长,导致氧化剂短缺,沉积物中Fe、Mn氧化物发生还原反应将表面上吸附的As释放到地下水中[28]。因此,在富含Fe、Mn矿物的黏土层中As含量较高。
C2钻孔中As与Fe、Mn含量在强还原环境下呈现负相关,如表3所示,可能是微生物利用有机碳作为电子供体将Fe、Mn氧化物还原溶解生成Fe2+、Mn2+,游离态的Fe2+、Mn2+被铁的氢氧化物重新吸附所致[29]。
-
研究区高砷地下水的化学特征和砷的区域分布表明,地下水中砷的迁移主要是在还原条件下发生的,对高砷地下水集中分布区域的沉积地层和岩性的调查表明,在沉积物(< 3 m)中广泛存在以黏土为主的地层,这种地质环境将限制大气中氧气向含水层的扩散,这可能是在受砷影响的含水层中观察到还原条件的主要原因。阴离子的竞争吸附可能是促进As迁移的重要因素,如重碳酸根、磷酸根与砷酸根在铁氧化物上竞争吸附;Fe/Mn的氢氧化物的还原溶解可能是促进As迁移的另一个因素。地下水中砷的直接来源被认为是沉积物中含砷矿物的还原溶解,如沉积物中含砷铁锰氧化物发生还原反应时会将砷释放到地下水中。研究区地下水中As(Ⅴ)的溶解态砷所占比例很高,As(Ⅴ)的吸附能力比As(Ⅲ)强[30],大量的As(Ⅴ)会降低地下水中砷在空间上的迁移能力。此外,高砷区域地下水流动缓慢,这将限制对含水层的冲刷、溶质(As)的运移。地下水流动缓慢的地区对上述各种水文地球化学过程释放出的少量As非常敏感。
2.1. 地下水化学
2.1.1. 地下水水化学特征
2.1.2. 地下水砷的空间分布
2.2. 沉积物的地球化学特征
2.2.1. 沉积物的岩性特征及化学组成
2.2.2. 沉积物中Fe、Mn含量对砷的影响
2.3. 控制砷运移的因素
-
本文在奎屯河流域地下水及沉积物地球化学特征研究的基础上,分析了含水系统砷运移影响的因素。结论如下:
(1)奎屯河流域地下水水样中As浓度在未检出—444.40 μg·L−1的范围内,超标率为 56%;高砷地下水pH值主要在 7.7—9.0 的范围内,呈现出碱性环境。高砷地下水Eh主要在−216— −3 mV之间,主要存在还原环境中。
(2)研究区内沉积物As含量在 8.36—28.41 mg·kg−1之间,黏土层As含量较高,砂层中As含量较低,这表明沉积物中As含量与岩性关系密切。沉积物中As与Fe、Mn含量之间通常呈现显著正相关,表明砷更倾向于在Fe、Mn含量高的沉积物中富集,但在强还原环境下,会由于铁的氢氧化物吸附解析态砷导致As与Fe、Mn含量呈现负相关。
(3)地下水中As的运移不只受Fe、Mn的氢氧化物的还原溶解的影响,而且还受到了
PO3−4 和HCO−3 竞争吸附的控制。As(Ⅴ)是地下水中As的主要存在形式,大量的As(Ⅴ)会降低地下水中砷在空间上的迁移能力。









 下载:
下载: