-
砷(As)是一种广泛分布在自然环境中的有毒类金属元素,被列为I类致癌物[1]。自然水体中砷的浓度较低(1—5 μg·L−1),主要以无机砷砷酸盐(AsⅤ)和亚砷酸盐(AsⅢ)的形态存在[2-3]。砷的毒性不仅由总量决定,更取决于其形态[2];相对于无机砷,有机砷如一甲基砷酸(MMA)、二甲基砷酸(DMA)、三甲基砷酸(TMA)、砷甜菜碱(AsB)、砷胆碱(AsC)、砷糖(arsenosugar)和砷脂(arsenolipid)等[4-6]的毒性要小得多。目前砷糖(arsenosugar)和砷脂(arsenolipid)的毒性尚未充分阐明,但已有报道,部分砷脂对人体细胞具有一定毒性[7]。微藻是一类广泛分布在陆地和海洋中的自养生物,种类和数量繁多,在地球生态系统中具有重要地位。砷对微藻有较强的毒性作用,但微藻在与砷长期共存的过程中,进化出了多种砷解毒机制,主要有细胞表面的吸附,细胞吸收后对砷形态的转化,包括AsⅢ的氧化、AsⅤ的还原、AsⅢ的甲基化或甲基化后挥发、胞内巯基物质对砷的络合、合成砷糖和砷脂等[3, 8]。不同条件下微藻对砷的解毒机制明显不同,然而造成这些差异的原因尚不清楚。
砷被微藻吸收后,可通过多种解毒途径进行形态转化并与不同细胞组分结合,根据其溶解性大体可分为水溶态、脂溶态和残渣态等3种组分[9]。水溶态砷指胞内亲水性砷化物,一般以无机砷为主,也存在少量MMA、DMA、AsB、AsC、砷糖等[10];脂溶态砷指胞内含砷脂质,包括含砷脂肪酸、含砷烃、含砷长链醇、砷糖磷脂等[11];经过水和有机类试剂连续萃取后剩余的部分是残渣态砷,这类砷与细胞中不溶性物质相结合,主要以未知结构的阳离子形式存在[12]。
以往的研究多用单一提取法[13-14]提取砷形态(提取剂为水或稀硝酸等),这种方法操作简单,然而对于一些结构复杂的砷化物,若只用一种提取剂,无法将其完全分离,提取率较低。例如,Miyashita等[15]研究表明,刚毛藻Cladophora glomerata和莱茵衣藻Chlamydomonas reinhardtii具有较多的脂溶态砷化物,仅用水提取这些藻类的效率很低(C. glomerata为16%,C. reinhardtii为32%)。连续分级提取法[10, 16]即根据砷与细胞不同组分的结合特性,选择水和有机溶剂等多种提取剂,将样品中不同砷化物依序分离出来,其提取效率更高,且利于鉴定更多种类的砷形态。
C. reinhardtii是一种广泛存在于水体环境中的模式藻类,具有很强的砷富集、吸收能力[17-18],然而不同砷浓度处理下莱茵衣藻的代谢规律与胞内各组分砷的分配尚不清楚。因此,本文采用不同AsⅤ浓度处理莱茵衣藻,测定其砷富集、吸收和培养基中含量;采用连续分级提取法,分离定量胞内水溶、脂溶和残渣态砷,并运用高效液相色谱-电感耦合等离子体质谱联用技术(HPLC-ICP-MS)分析培养基中砷的形态,探究莱茵衣藻对砷酸盐的富集、分配和形态转化,进一步揭示微藻砷解毒机制。
-
C. reinhardtii购于美国明尼苏达大学衣藻资源中心。采用TAP(tris-acetate-phosphate)培养基(pH 7.0),121 ℃灭菌30 min;培养温度为(25±2 )℃;光照强度为2000 lux,并维持光暗比(12 h∶12 h);且在藻种传代扩增及培养过程中均保证无菌操作。
-
AsⅤ处理浓度:5、10、20、50、100 μg·L−1,并设置空白对照两组(有藻无砷和有砷无藻)。每个处理设置3个平行。将生长至对数期的莱茵衣藻接入新鲜的TAP培养基,藻细胞初始OD680=0.10,整个接种转移过程均在无菌超净工作台中进行,每天使用SpectraMax i3X多功能酶标仪(Molescular Devices)对其OD680进行测定。
-
培养7 d后离心收集上清液和藻样(7291 r·min−1,2 min),并将藻样分为两部分,一部分用0.1 mol·L−1磷酸盐缓冲液(PBS,pH=7.0)和去离子水清洗3遍洗去藻细胞表面吸附的砷,用于测定莱茵衣藻对砷的吸收量;另一部分藻样用于测定莱茵衣藻对砷的富集量。将藻样冷冻干燥后称取0.05 g至消煮管内,加入2 mL硝酸(UP级),浸没过夜。使用石墨消解仪(海能SH230)在(120±2)℃下电热消解至溶液澄清透明,开盖赶酸至管内剩下0.5 mL的液体,静置冷却后将消煮液转移至10 mL容量瓶,用去离子水定容。同时以不加藻样的相同体系作为试剂空白。最后使用0.45 μm的水系滤器过滤,待测。采用ICP-MS(NexION2000)测定藻样和培养基中砷含量。本文使用动态反应池(DRC)技术,通过让砷离子与氧反应形成在m/z为91时可测量的75As16O+离子的方式减少氯离子的干扰,使结果更加准确。
-
莱茵衣藻胞内的砷分为水溶态、脂溶态和残渣态,采用连续分级提取,方法如下。
水溶态砷[19]:准确称取加砷处理的经冷冻干燥后的藻样0.10 g,加入2 mL水为萃取剂,用超声波清洗仪超声10 min(100 W,40 Hz),7291 r·min−1离心2 min。重复3次。将离心后的上清液合并,使用0.45 μm的水系滤器过滤。过滤后样品(水溶态)放置−60 ℃低温冰箱保存,待测。
脂溶态砷[16]:向提取过水溶态的藻样干燥后加入萃取剂二氯甲烷/甲醇(2:1,V/V)混合溶液6 mL,充分震荡并超声10 min。随后7291 r·min−1离心2 min收集上清液,过滤后样品(脂溶态)经旋转蒸发后浓缩至微量并放置−60 ℃低温冰箱保存,待测。
残渣态砷[6]:在经过水溶态和脂溶态连续提取后,剩下的即为残渣态。将藻样放入通风橱待有机试剂挥发完全后再放置−60 ℃低温冰箱保存,待测。
不同组分砷含量的测定方法同1.2.2节。将收集的上清液经0.22 μm的水系滤器过滤后采用HPLC-ICP-MS测定培养基中砷形态,同时配置不同浓度的混合砷标准溶液(AsⅢ、DMA、MMA、AsⅤ)作标准曲线。具体仪器条件见表1。
-
砷含量的测定以115In为内标,以砷的标准曲线进行定量;采用消解标准物质羊栖菜(NMIJ CRM 7405-a)和鱼肝(DOLT-5)对本试验测定结果的可靠性进行评估,标准物质的消解与样品同时进行,并设置3个平行。测定的两标准物质的总砷回收率为91.3%—95.7%,数据精密度较好,说明分析方法不存在明显的系统误差。此外,仪器的检测限可达0.02 μg·L−1,内标回收率99.24%—111.66%,相对标准偏差(RSD)<5%。此外,本文采用连续分级提取法对羊栖菜和鱼肝的胞内砷形态进行提取并测定,以各组分砷含量相加之和占总砷含量的百分比作为提取率,两种标准物质的提取率为92.84%—93.72%,说明此方法可靠。
以不加莱茵衣藻、加入5—100 μg·L−1 AsⅤ处理的TAP培养基为空白对照,放置于同等试验条件下7 d后,用HPLC-ICP-MS检测培养基中的砷形态,结果发现AsⅢ含量均低于检出限,说明整个培养期内AsⅤ形态稳定。
-
试验数据采用Origin 96进行处理、作图,并采用SPSS 20.0进行差异显著性分析(P<0.05)。试验数据为平均值±标准偏差(n=3)。
-
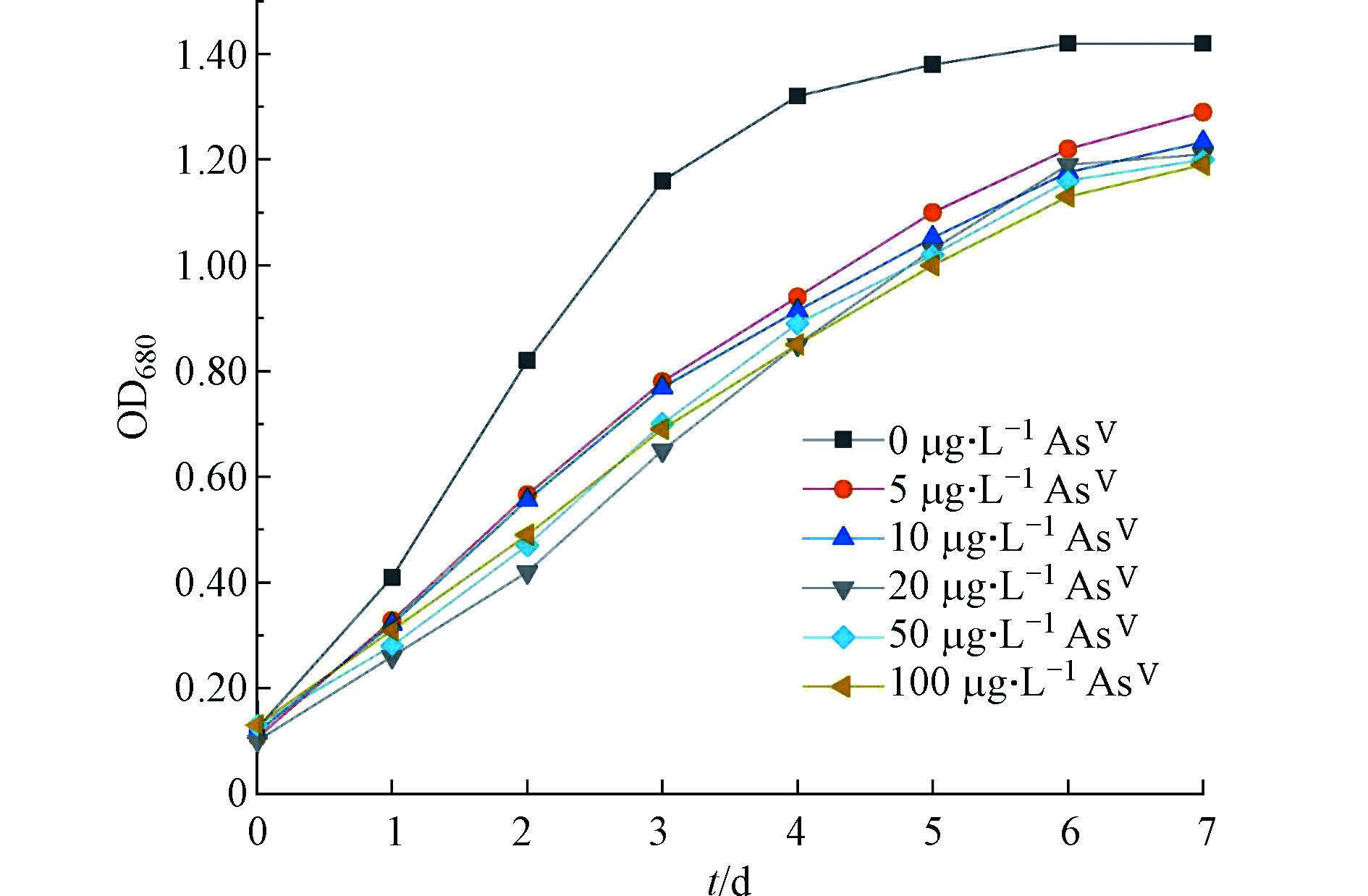
对照组(不加AsⅤ)莱茵衣藻在第4 天进入生长稳定期,而加砷处理的莱茵衣藻随着时间的增加缓慢生长,在第7 天趋于平稳(图1)。相对于对照组,AsⅤ的加入对莱茵衣藻的生长有显著抑制,但最终的生长OD680值维持在1.19—1.29,各处理组之间无显著差异。说明本研究采用的莱茵衣藻对AsⅤ比较敏感.
-
随着AsⅤ处理浓度的增加,C. reinhardtii对砷的富集和吸收量显著增加(表2),这与Yin等[17]的结果一致。而莱茵衣藻对砷的吸附量较少(0.92—1.83 μg·g−1),且处理间无明显差异。由此可知,不同AsⅤ浓度处理下C. reinhardtii均以对砷的吸收为主,其吸收量占富集量的比例为81.92%—96.79%。李崇华等[20]用20 μg·L−1的AsⅤ处理莱茵衣藻6 h,发现其对砷的富集、吸收和吸附量随着AsⅤ暴露时间的增加呈现先增加后减少的趋势,在4 h时对砷的富集量达到最大值((90.83±0.49 )μg·g−1),高于本文相同浓度处理7 d后的砷富集量13.77 μg·g−1(表2),说明莱茵衣藻短期内可富集吸收大量的砷,但最终将大部分砷外排来减小砷的毒性。
-
由图2可知,莱茵衣藻胞内主要以脂溶态砷为主,残渣态和水溶态含量较少。这与Miyashita等[15]的结果保持一致。研究表明,除了莱茵衣藻,还有许多藻类及鱼类也存在脂溶态砷[21-24]。有研究表示,砷脂可能在生物细胞膜化学中起作用,细胞膜中脂质动态排列的微小变化可能影响细胞信号传导等膜功能[25],对人体健康有潜在的风险[11]。
随着AsⅤ浓度的增加,莱茵衣藻脂溶态砷含量呈增加的趋势,由3.61 μg·g−1增加至22.19 μg·g−1,然而脂溶态砷占总砷的比例先减少后增加(最高可达72.2%)(图3);水溶态砷的含量随着AsⅤ浓度的增加而显著增加,由0.41 μg·g−1增加至4.31 μg·g−1,而水溶态砷占总砷的比例虽先增加后减少,但整体成上升的趋势(图3)。Glabonjat等[16]研究表示随着AsⅤ浓度的增加,盐藻Dunaliella tertiolecta产生的水溶态砷的含量及所占比例显著增加,与本文的结果一致;而盐藻砷脂的含量虽显著增加,但占总砷的比例维持不变。Xue等[10]对施加AsⅤ处理的念珠藻Nostoc sp. PCC 7120进行连续分级提取砷形态,发现念珠藻产生了AsⅤ、DMA、甘油砷糖和磷酸砷糖,还生成了两种砷脂,且随着AsⅢ浓度的增加,DMA及两种砷糖的含量逐渐减少;说明随着砷浓度的增加,莱茵衣藻的解毒途径可能发生了改变,将毒性较强的水溶态无机砷转化为毒性较弱的脂溶态砷。
-
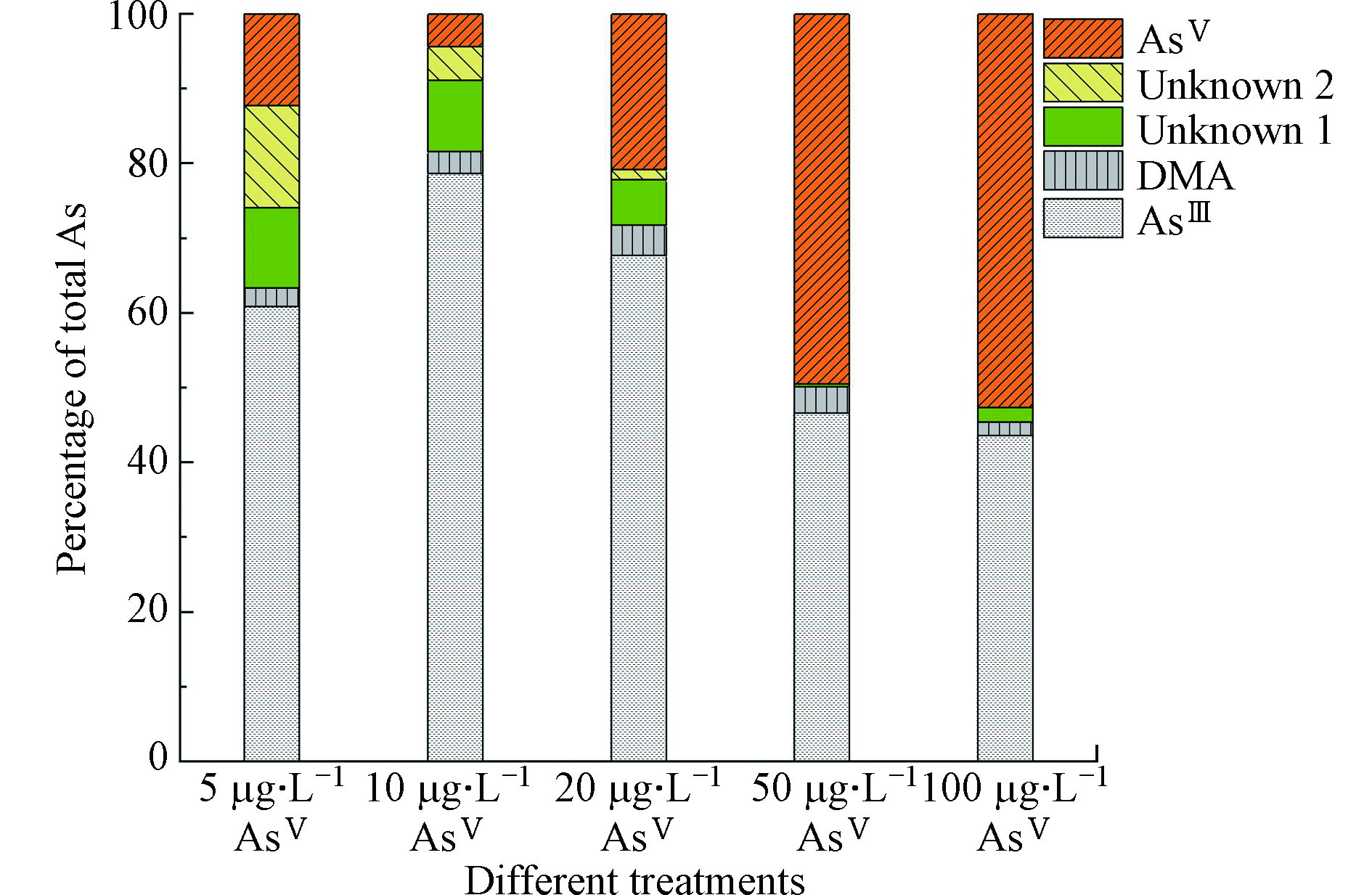
随着AsⅤ浓度的逐渐增加,在培养7 d后,培养基中的砷含量为(1.62±0.11)、(4.02±0.37)、(12.50±0.26)、(42.01±0.95)、(73.70±6.22) μg·L−1,分别占施加总砷含量的23.65%、37.37%、52.74%、77.94%、77.54%,说明随着砷浓度的增加,莱茵衣藻对砷的富集逐渐减少。培养基中砷形态的含量及占比如表3、图3、4所示。在5—20 μg·L−1 AsⅤ处理下,培养基中的砷主要以AsⅢ为主(60.89%—78.66%)且其含量随砷浓度的增加而增加,含有少量的DMA和AsⅤ,未检测到MMA,但检测到两种未知物质,这与Miyashita等[26]的研究一致;由此可知莱茵衣藻能介导砷形态的转化,将AsⅤ还原为AsⅢ随后进行甲基化,生成了未知砷化物并外排至培养基。根据已有研究[27-28],未知形态1、2可能分别是磷酸砷糖和磺酸砷糖。Murray等[29]发现将C. vulgaris暴露于AsⅤ 7 d后不仅生成了AsⅢ、DMA,还产生了磷酸砷糖、磺酸砷糖和甘油砷糖。50—100 μg·L−1 AsⅤ处理下,培养基中AsⅤ的含量及所占比例增加(49.50%—52.63%),说明随着砷浓度的增加,莱茵衣藻抑制了AsⅤ的吸收来减轻毒性;此外,培养基中AsⅢ含量其次(43.57%—46.60%),也含有较少的DMA,相较于低浓度砷处理,磷酸砷糖含量虽有显著增加,但所占比例明显减少且未检测到磺酸砷糖。Miyashita等[26]发现具有代表性的脂溶态砷化物--磷脂酰砷糖的基本结构中包含甘油砷糖和磷酸砷糖,因此认为这些含氧砷糖可能是砷脂合成的前体。Xue等[30-31]发现Synechocystis sp. PCC 6803在亚砷酸盐的胁迫下生成了DMA、磷酸砷糖、磺酸砷糖和两种砷糖磷脂,并阐明了砷糖生物合成途径的前两个步骤。藻细胞内砷组分分配的变化说明,随着砷胁迫的增加,除AsⅤ还原外排外,莱茵衣藻的解毒机制由以AsⅢ甲基化生成DMA和砷糖等过程为主,转变为以减少吸收并生成更多的脂溶态砷等途径为主,藻细胞减少对AsⅤ的吸收,DMA、磷酸砷糖和磺酸砷糖等含有甲基砷的化合物可能作为前体,进一步合成脂溶态砷化物。
-
在不同浓度AsⅤ处理下,莱茵衣藻生长受到一定抑制,对砷的富集以吸收为主;胞内脂溶态砷占主要部分,水溶态和残渣态砷的含量较少,且随着砷浓度的增加,脂溶态砷占总砷的比例先减少后增加,而水溶态砷所占比例先增加后减少,说明砷的解毒途径存在差异。结合培养基中砷形态变化可推断,在砷酸盐胁迫下,莱茵衣藻主要将AsⅤ还原为AsⅢ,并大量外排;同时,AsⅢ甲基化为DMA,可能合成砷糖并外排。随着AsⅤ浓度的增加,藻细胞减少对AsⅤ的吸收,甲基化程度加大,合成更多的脂溶态砷。因此,深入解析脂溶态砷的生物合成过程、研究微藻对砷的分配和形态转化,有助于揭示微藻对砷的解毒机制,进一步明确微藻在砷生物地球化学循环中的作用。
莱茵衣藻对砷酸盐的富集分配和形态转化
Accumulation, distribution and transformation of arsenate by Chlamydomonas reinhardtii
-
摘要: 已知微藻可将吸收的无机砷转化为不同形态砷,但其在细胞不同组分(水溶态、脂溶态、残渣态)中的分配规律还不清楚。本文采用不同浓度(5—100 μg·L−1)砷酸盐(AsⅤ)处理莱茵衣藻,7 d后测定莱茵衣藻生长情况和砷富集吸收及培养基中砷含量,并采用连续分级提取法测定藻细胞内水溶态、脂溶态和残渣态砷的含量。结果表明,不同浓度AsⅤ处理下,莱茵衣藻均以对砷的吸收为主,胞内脂溶态砷含量较多,水溶态和残渣态含量较少;随着砷浓度的增加,脂溶态砷占总砷的比例先减少后增加,而水溶态砷所占比例先增加后减少。培养基中的砷形态以AsⅢ和AsⅤ为主,含有少量DMA,同时检测出两种可能为砷糖的未知化合物。在低砷胁迫下,莱茵衣藻将AsⅤ还原为AsⅢ,通过甲基化等过程转化为DMA和砷糖等形态外排;随着砷处理浓度的增加,除上述途径外,莱茵衣藻还通过减少As(V)吸收和生成更多的脂溶态砷等途径进行解毒。Abstract: It is known that microalgae can transform absorbed inorganic arsenic (As) into different species. However, As distribution in various cellular components (water-soluble, lipid-soluble, and residual) is not clear. In this paper, the growth of Chlamydomonas reinhardtii, As accumulation and absorption by Chlamydomonas reinhardtii, and As concentration and speciation in the medium were determined after 7 days that Chlamydomonas reinhardtii was exposured to different concentrations of arsenate (AsⅤ) (5—100 μg·L−1). The sequential extraction method was used to determine the contents of water-soluble, lipid-soluble, and residual As in the cell. The results showed thatthe absorption dominated the As accumulation of C. reinhardtii upon the As exposure. The content of lipid-soluble As was higher than that of water-soluble and residual As in the cell. With the increase of As concentration, the proportion of lipid-soluble As in the total intracellular decreased first and then increased, and water-soluble As increased first and then decreased. Arsenite (AsⅢ) and AsⅤ were the major As species in the culture medium, which also contained a small amount of DMA and two unknown As compounds, possibly arsenosugars. Therefore, under low As stress, AsⅤ was reduced to AsⅢ, followed by methylation to DMA and arsenosugars, and efflux occurred in C. reinhardtii cells. With the increase of As concentration, in addition to the above processes, C. reinhardtii detoxified As through decrease of AsⅤ absorption and generation of more lipid-soluble As.
-
Key words:
- Chlamydomonas reinhardtii /
- AsⅤ /
- sequential extraction /
- speciation transformation
-
砷(As)是一种广泛分布在自然环境中的有毒类金属元素,被列为I类致癌物[1]。自然水体中砷的浓度较低(1—5 μg·L−1),主要以无机砷砷酸盐(AsⅤ)和亚砷酸盐(AsⅢ)的形态存在[2-3]。砷的毒性不仅由总量决定,更取决于其形态[2];相对于无机砷,有机砷如一甲基砷酸(MMA)、二甲基砷酸(DMA)、三甲基砷酸(TMA)、砷甜菜碱(AsB)、砷胆碱(AsC)、砷糖(arsenosugar)和砷脂(arsenolipid)等[4-6]的毒性要小得多。目前砷糖(arsenosugar)和砷脂(arsenolipid)的毒性尚未充分阐明,但已有报道,部分砷脂对人体细胞具有一定毒性[7]。微藻是一类广泛分布在陆地和海洋中的自养生物,种类和数量繁多,在地球生态系统中具有重要地位。砷对微藻有较强的毒性作用,但微藻在与砷长期共存的过程中,进化出了多种砷解毒机制,主要有细胞表面的吸附,细胞吸收后对砷形态的转化,包括AsⅢ的氧化、AsⅤ的还原、AsⅢ的甲基化或甲基化后挥发、胞内巯基物质对砷的络合、合成砷糖和砷脂等[3, 8]。不同条件下微藻对砷的解毒机制明显不同,然而造成这些差异的原因尚不清楚。
砷被微藻吸收后,可通过多种解毒途径进行形态转化并与不同细胞组分结合,根据其溶解性大体可分为水溶态、脂溶态和残渣态等3种组分[9]。水溶态砷指胞内亲水性砷化物,一般以无机砷为主,也存在少量MMA、DMA、AsB、AsC、砷糖等[10];脂溶态砷指胞内含砷脂质,包括含砷脂肪酸、含砷烃、含砷长链醇、砷糖磷脂等[11];经过水和有机类试剂连续萃取后剩余的部分是残渣态砷,这类砷与细胞中不溶性物质相结合,主要以未知结构的阳离子形式存在[12]。
以往的研究多用单一提取法[13-14]提取砷形态(提取剂为水或稀硝酸等),这种方法操作简单,然而对于一些结构复杂的砷化物,若只用一种提取剂,无法将其完全分离,提取率较低。例如,Miyashita等[15]研究表明,刚毛藻Cladophora glomerata和莱茵衣藻Chlamydomonas reinhardtii具有较多的脂溶态砷化物,仅用水提取这些藻类的效率很低(C. glomerata为16%,C. reinhardtii为32%)。连续分级提取法[10, 16]即根据砷与细胞不同组分的结合特性,选择水和有机溶剂等多种提取剂,将样品中不同砷化物依序分离出来,其提取效率更高,且利于鉴定更多种类的砷形态。
C. reinhardtii是一种广泛存在于水体环境中的模式藻类,具有很强的砷富集、吸收能力[17-18],然而不同砷浓度处理下莱茵衣藻的代谢规律与胞内各组分砷的分配尚不清楚。因此,本文采用不同AsⅤ浓度处理莱茵衣藻,测定其砷富集、吸收和培养基中含量;采用连续分级提取法,分离定量胞内水溶、脂溶和残渣态砷,并运用高效液相色谱-电感耦合等离子体质谱联用技术(HPLC-ICP-MS)分析培养基中砷的形态,探究莱茵衣藻对砷酸盐的富集、分配和形态转化,进一步揭示微藻砷解毒机制。
1. 材料与方法 (Materials and methods)
1.1 试验材料与培养条件
C. reinhardtii购于美国明尼苏达大学衣藻资源中心。采用TAP(tris-acetate-phosphate)培养基(pH 7.0),121 ℃灭菌30 min;培养温度为(25±2 )℃;光照强度为2000 lux,并维持光暗比(12 h∶12 h);且在藻种传代扩增及培养过程中均保证无菌操作。
1.2 试验方法
1.2.1 不同AsⅤ浓度处理莱茵衣藻
AsⅤ处理浓度:5、10、20、50、100 μg·L−1,并设置空白对照两组(有藻无砷和有砷无藻)。每个处理设置3个平行。将生长至对数期的莱茵衣藻接入新鲜的TAP培养基,藻细胞初始OD680=0.10,整个接种转移过程均在无菌超净工作台中进行,每天使用SpectraMax i3X多功能酶标仪(Molescular Devices)对其OD680进行测定。
1.2.2 藻样砷总量的测定
培养7 d后离心收集上清液和藻样(7291 r·min−1,2 min),并将藻样分为两部分,一部分用0.1 mol·L−1磷酸盐缓冲液(PBS,pH=7.0)和去离子水清洗3遍洗去藻细胞表面吸附的砷,用于测定莱茵衣藻对砷的吸收量;另一部分藻样用于测定莱茵衣藻对砷的富集量。将藻样冷冻干燥后称取0.05 g至消煮管内,加入2 mL硝酸(UP级),浸没过夜。使用石墨消解仪(海能SH230)在(120±2)℃下电热消解至溶液澄清透明,开盖赶酸至管内剩下0.5 mL的液体,静置冷却后将消煮液转移至10 mL容量瓶,用去离子水定容。同时以不加藻样的相同体系作为试剂空白。最后使用0.45 μm的水系滤器过滤,待测。采用ICP-MS(NexION2000)测定藻样和培养基中砷含量。本文使用动态反应池(DRC)技术,通过让砷离子与氧反应形成在m/z为91时可测量的75As16O+离子的方式减少氯离子的干扰,使结果更加准确。
1.2.3 藻样不同组分砷的提取测定
莱茵衣藻胞内的砷分为水溶态、脂溶态和残渣态,采用连续分级提取,方法如下。
水溶态砷[19]:准确称取加砷处理的经冷冻干燥后的藻样0.10 g,加入2 mL水为萃取剂,用超声波清洗仪超声10 min(100 W,40 Hz),7291 r·min−1离心2 min。重复3次。将离心后的上清液合并,使用0.45 μm的水系滤器过滤。过滤后样品(水溶态)放置−60 ℃低温冰箱保存,待测。
脂溶态砷[16]:向提取过水溶态的藻样干燥后加入萃取剂二氯甲烷/甲醇(2:1,V/V)混合溶液6 mL,充分震荡并超声10 min。随后7291 r·min−1离心2 min收集上清液,过滤后样品(脂溶态)经旋转蒸发后浓缩至微量并放置−60 ℃低温冰箱保存,待测。
残渣态砷[6]:在经过水溶态和脂溶态连续提取后,剩下的即为残渣态。将藻样放入通风橱待有机试剂挥发完全后再放置−60 ℃低温冰箱保存,待测。
不同组分砷含量的测定方法同1.2.2节。将收集的上清液经0.22 μm的水系滤器过滤后采用HPLC-ICP-MS测定培养基中砷形态,同时配置不同浓度的混合砷标准溶液(AsⅢ、DMA、MMA、AsⅤ)作标准曲线。具体仪器条件见表1。
表 1 砷形态分析的HPLC-ICP-MS仪器条件Table 1. Experimental conditions for As speciation by HPLC-ICP-MS高效液相色谱HPLC PerkinElmer 电感耦合等离子体质谱ICP-MS NexION 2000 色谱柱Chromatographic column Hamilton PRP-X100阴离子交换色谱分析柱Anion exchange chromatography column(250 mm×4.1 mm, 10 μm) 等度分离流动相Equally separated mobile phase 17.5 mmol·L−1 (NH4)2HPO4, pH 5.8 进样体积Injection volume 50 μL 流速Flow velocity 1.5 mL·min−1 运行时间Run time 8 min 1.3 质量控制
砷含量的测定以115In为内标,以砷的标准曲线进行定量;采用消解标准物质羊栖菜(NMIJ CRM 7405-a)和鱼肝(DOLT-5)对本试验测定结果的可靠性进行评估,标准物质的消解与样品同时进行,并设置3个平行。测定的两标准物质的总砷回收率为91.3%—95.7%,数据精密度较好,说明分析方法不存在明显的系统误差。此外,仪器的检测限可达0.02 μg·L−1,内标回收率99.24%—111.66%,相对标准偏差(RSD)<5%。此外,本文采用连续分级提取法对羊栖菜和鱼肝的胞内砷形态进行提取并测定,以各组分砷含量相加之和占总砷含量的百分比作为提取率,两种标准物质的提取率为92.84%—93.72%,说明此方法可靠。
以不加莱茵衣藻、加入5—100 μg·L−1 AsⅤ处理的TAP培养基为空白对照,放置于同等试验条件下7 d后,用HPLC-ICP-MS检测培养基中的砷形态,结果发现AsⅢ含量均低于检出限,说明整个培养期内AsⅤ形态稳定。
1.4 数据分析
试验数据采用Origin 96进行处理、作图,并采用SPSS 20.0进行差异显著性分析(P<0.05)。试验数据为平均值±标准偏差(n=3)。
2. 结果与讨论 (Results and discussion)
2.1 不同AsⅤ浓度处理下莱茵衣藻的生长
对照组(不加AsⅤ)莱茵衣藻在第4 天进入生长稳定期,而加砷处理的莱茵衣藻随着时间的增加缓慢生长,在第7 天趋于平稳(图1)。相对于对照组,AsⅤ的加入对莱茵衣藻的生长有显著抑制,但最终的生长OD680值维持在1.19—1.29,各处理组之间无显著差异。说明本研究采用的莱茵衣藻对AsⅤ比较敏感.
2.2 不同AsⅤ浓度处理下莱茵衣藻对砷的富集和吸收
随着AsⅤ处理浓度的增加,C. reinhardtii对砷的富集和吸收量显著增加(表2),这与Yin等[17]的结果一致。而莱茵衣藻对砷的吸附量较少(0.92—1.83 μg·g−1),且处理间无明显差异。由此可知,不同AsⅤ浓度处理下C. reinhardtii均以对砷的吸收为主,其吸收量占富集量的比例为81.92%—96.79%。李崇华等[20]用20 μg·L−1的AsⅤ处理莱茵衣藻6 h,发现其对砷的富集、吸收和吸附量随着AsⅤ暴露时间的增加呈现先增加后减少的趋势,在4 h时对砷的富集量达到最大值((90.83±0.49 )μg·g−1),高于本文相同浓度处理7 d后的砷富集量13.77 μg·g−1(表2),说明莱茵衣藻短期内可富集吸收大量的砷,但最终将大部分砷外排来减小砷的毒性。
表 2 不同AsⅤ浓度处理下莱茵衣藻富集、吸收的砷含量 (μg·g−1 DW)Table 2. Arsenic accumulation and absorption by C. reindardtii exposed to different AsⅤ concentrations (μg·g−1 DW)AsⅤ处理/(μg·L−1)AsⅤ treatment 富集Accumulation 吸收Absorption 5 6.25±0.53e 5.12±0.12e 10 10.93±0.48d 9.83±0.24d 20 13.77±0.62c 12.65±0.21c 50 16.43±0.58b 15.38±0.18b 100 28.62±0.38a 27.70±0.25a 注:不同小写字母表示不同AsⅤ浓度处理下莱茵衣藻的砷富集和吸收含量的显著性差异(P<0.05). Note: Different letters are significant difference at P<0.05 among As accumulation and absorption by C. reindardtii exposed to AsⅤ under different concentrations. 2.3 不同AsⅤ浓度处理下莱茵衣藻胞内不同组分砷含量
由图2可知,莱茵衣藻胞内主要以脂溶态砷为主,残渣态和水溶态含量较少。这与Miyashita等[15]的结果保持一致。研究表明,除了莱茵衣藻,还有许多藻类及鱼类也存在脂溶态砷[21-24]。有研究表示,砷脂可能在生物细胞膜化学中起作用,细胞膜中脂质动态排列的微小变化可能影响细胞信号传导等膜功能[25],对人体健康有潜在的风险[11]。
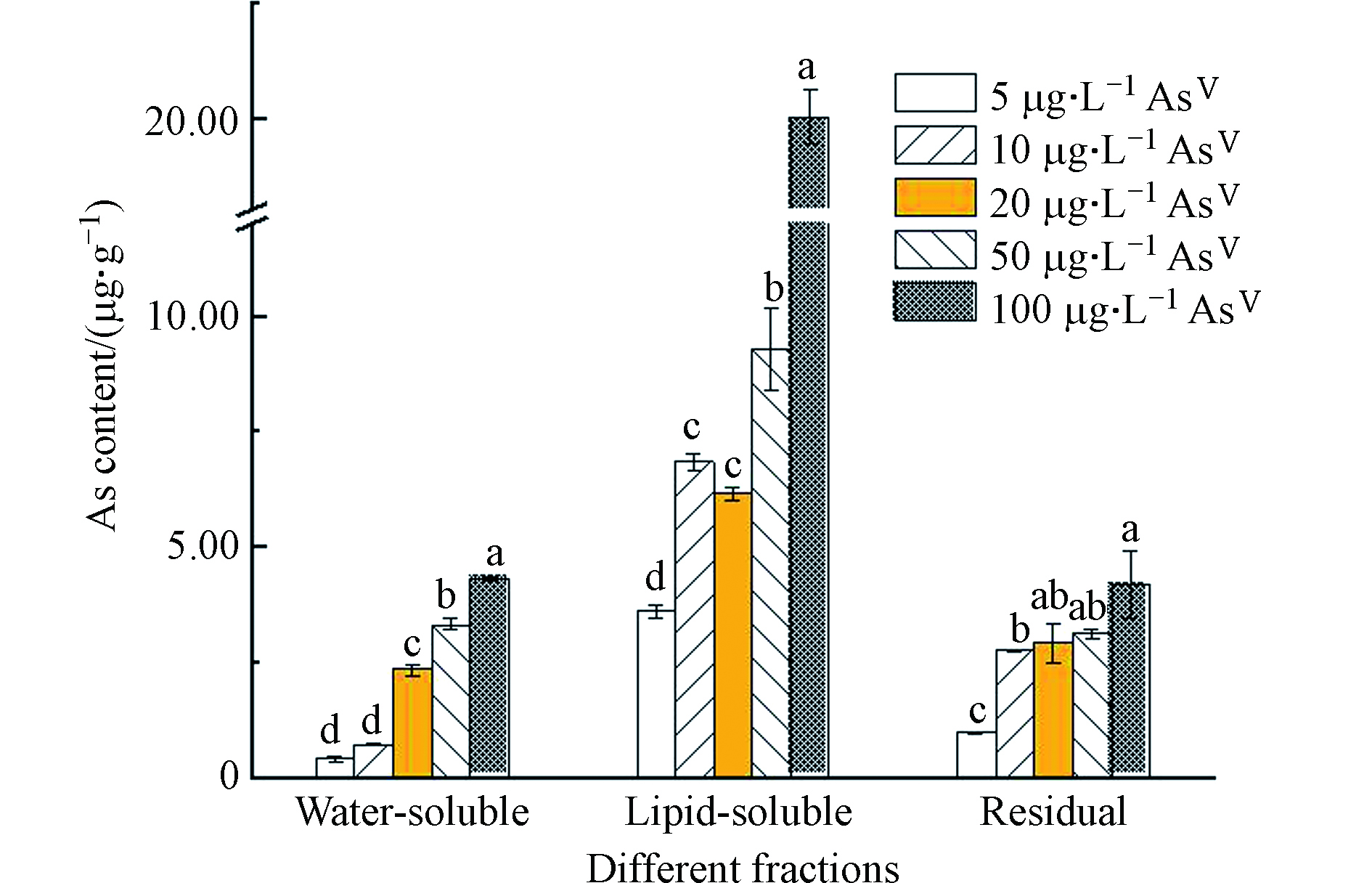 图 2 不同AsⅤ浓度处理下莱茵衣藻不同组分的砷含量Figure 2. Arsenic contents of different components in C. reinhardtii exposed to different AsⅤ concentrations注:不同小写字母表示不同AsⅤ浓度处理下莱茵衣藻各组分砷含量的显著性差异(P<0.05)Note: Different letters are significant difference at P<0.05 among As contents of different components by C. reinhardtii exposed to AsⅤ under different concentrations
图 2 不同AsⅤ浓度处理下莱茵衣藻不同组分的砷含量Figure 2. Arsenic contents of different components in C. reinhardtii exposed to different AsⅤ concentrations注:不同小写字母表示不同AsⅤ浓度处理下莱茵衣藻各组分砷含量的显著性差异(P<0.05)Note: Different letters are significant difference at P<0.05 among As contents of different components by C. reinhardtii exposed to AsⅤ under different concentrations随着AsⅤ浓度的增加,莱茵衣藻脂溶态砷含量呈增加的趋势,由3.61 μg·g−1增加至22.19 μg·g−1,然而脂溶态砷占总砷的比例先减少后增加(最高可达72.2%)(图3);水溶态砷的含量随着AsⅤ浓度的增加而显著增加,由0.41 μg·g−1增加至4.31 μg·g−1,而水溶态砷占总砷的比例虽先增加后减少,但整体成上升的趋势(图3)。Glabonjat等[16]研究表示随着AsⅤ浓度的增加,盐藻Dunaliella tertiolecta产生的水溶态砷的含量及所占比例显著增加,与本文的结果一致;而盐藻砷脂的含量虽显著增加,但占总砷的比例维持不变。Xue等[10]对施加AsⅤ处理的念珠藻Nostoc sp. PCC 7120进行连续分级提取砷形态,发现念珠藻产生了AsⅤ、DMA、甘油砷糖和磷酸砷糖,还生成了两种砷脂,且随着AsⅢ浓度的增加,DMA及两种砷糖的含量逐渐减少;说明随着砷浓度的增加,莱茵衣藻的解毒途径可能发生了改变,将毒性较强的水溶态无机砷转化为毒性较弱的脂溶态砷。
2.4 不同AsⅤ浓度处理下莱茵衣藻培养基中的砷含量及形态
随着AsⅤ浓度的逐渐增加,在培养7 d后,培养基中的砷含量为(1.62±0.11)、(4.02±0.37)、(12.50±0.26)、(42.01±0.95)、(73.70±6.22) μg·L−1,分别占施加总砷含量的23.65%、37.37%、52.74%、77.94%、77.54%,说明随着砷浓度的增加,莱茵衣藻对砷的富集逐渐减少。培养基中砷形态的含量及占比如表3、图3、4所示。在5—20 μg·L−1 AsⅤ处理下,培养基中的砷主要以AsⅢ为主(60.89%—78.66%)且其含量随砷浓度的增加而增加,含有少量的DMA和AsⅤ,未检测到MMA,但检测到两种未知物质,这与Miyashita等[26]的研究一致;由此可知莱茵衣藻能介导砷形态的转化,将AsⅤ还原为AsⅢ随后进行甲基化,生成了未知砷化物并外排至培养基。根据已有研究[27-28],未知形态1、2可能分别是磷酸砷糖和磺酸砷糖。Murray等[29]发现将C. vulgaris暴露于AsⅤ 7 d后不仅生成了AsⅢ、DMA,还产生了磷酸砷糖、磺酸砷糖和甘油砷糖。50—100 μg·L−1 AsⅤ处理下,培养基中AsⅤ的含量及所占比例增加(49.50%—52.63%),说明随着砷浓度的增加,莱茵衣藻抑制了AsⅤ的吸收来减轻毒性;此外,培养基中AsⅢ含量其次(43.57%—46.60%),也含有较少的DMA,相较于低浓度砷处理,磷酸砷糖含量虽有显著增加,但所占比例明显减少且未检测到磺酸砷糖。Miyashita等[26]发现具有代表性的脂溶态砷化物--磷脂酰砷糖的基本结构中包含甘油砷糖和磷酸砷糖,因此认为这些含氧砷糖可能是砷脂合成的前体。Xue等[30-31]发现Synechocystis sp. PCC 6803在亚砷酸盐的胁迫下生成了DMA、磷酸砷糖、磺酸砷糖和两种砷糖磷脂,并阐明了砷糖生物合成途径的前两个步骤。藻细胞内砷组分分配的变化说明,随着砷胁迫的增加,除AsⅤ还原外排外,莱茵衣藻的解毒机制由以AsⅢ甲基化生成DMA和砷糖等过程为主,转变为以减少吸收并生成更多的脂溶态砷等途径为主,藻细胞减少对AsⅤ的吸收,DMA、磷酸砷糖和磺酸砷糖等含有甲基砷的化合物可能作为前体,进一步合成脂溶态砷化物。
表 3 不同AsⅤ浓度处理下莱茵衣藻培养基中各砷形态的浓度Table 3. Concentrations of As species in the medium of C. reinhardtii exposed to different AsⅤ concentrations各砷形态的浓度 / (μg·L−1)As species concentrations AsⅤ处理 / (μg·L−1)AsⅤ treatment AsⅢ DMA Unknown 1 AsⅤ Unknown 2 5 0.72±0.08d 0.03±0.00b 0.13±0.02d 0.15±0.03c 0.16±0.00a 10 2.37±0.33d 0.09±0.01b 0.29±0.03cd 0.13±0.05c 0.14±0.02b 20 6.22±0.29c 0.37±0.09b 0.56±0.08c 1.91±0.10c 0.12±0.00b 50 15.79±0.05b 1.21±0.12a 1.12±0.01b 16.77±0.88b — 100 34.92±1.80a 1.45±0.25a 1.60±0.10a 42.18±1.93a — 注:不同小写字母表示不同AsⅤ浓度处理下莱茵衣藻培养基中各砷形态的浓度的显著性差异(P<0.05). Note: Different letters are significant difference at P<0.05 among the concentrations of As species in medium by C. reinhardtii exposed to AsⅤ under different concentrations. 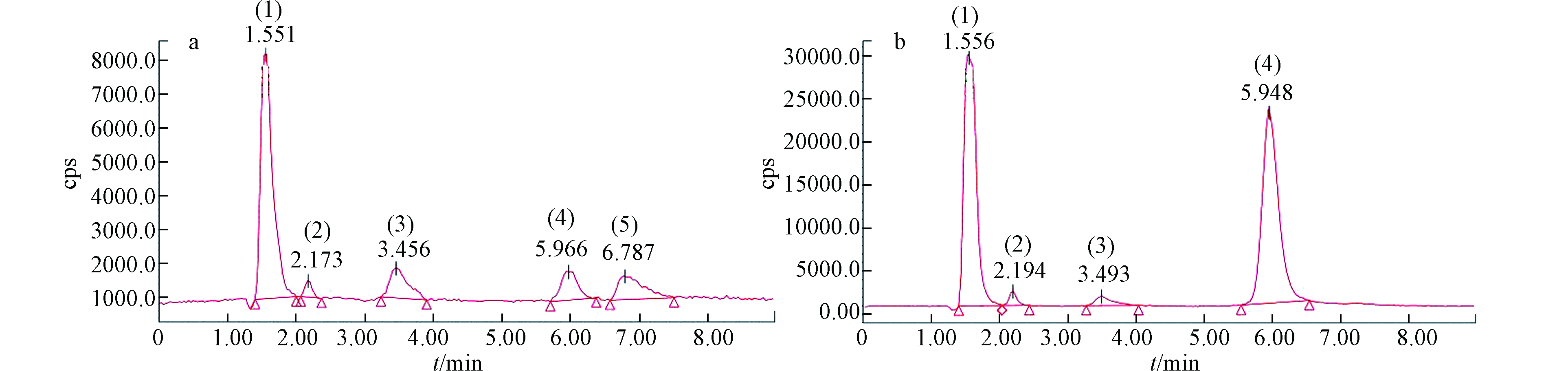 图 4 不同AsⅤ浓度处理下莱茵衣藻培养基中砷形态HPLC-ICP-MS谱图Figure 4. HPLC-ICP-MS spectra of As species in the medium of C. reinhardtii exposed to different As(Ⅴ) concentrations (5, 100 μg·L−1)a: 5 μg·L−1 AsⅤ, b: 100 μg·L-1 AsⅤ(稀释10倍)注:各砷形态分别用不同数字表示:(1)AsⅢ;(2)DMA;(3)未知形态1;(4)AsⅤ;(5)未知形态2a: 5 μg·L−1AsⅤ, b: 100 μg·L−1 AsⅤ (diluted 10 times) Note: As species are indicated by numbers: (1) AsⅢ; (2) DMA; (3) Unknown 1; (4) AsⅤ; (5) Unknown 2
图 4 不同AsⅤ浓度处理下莱茵衣藻培养基中砷形态HPLC-ICP-MS谱图Figure 4. HPLC-ICP-MS spectra of As species in the medium of C. reinhardtii exposed to different As(Ⅴ) concentrations (5, 100 μg·L−1)a: 5 μg·L−1 AsⅤ, b: 100 μg·L-1 AsⅤ(稀释10倍)注:各砷形态分别用不同数字表示:(1)AsⅢ;(2)DMA;(3)未知形态1;(4)AsⅤ;(5)未知形态2a: 5 μg·L−1AsⅤ, b: 100 μg·L−1 AsⅤ (diluted 10 times) Note: As species are indicated by numbers: (1) AsⅢ; (2) DMA; (3) Unknown 1; (4) AsⅤ; (5) Unknown 23. 结论 (Conclusion)
在不同浓度AsⅤ处理下,莱茵衣藻生长受到一定抑制,对砷的富集以吸收为主;胞内脂溶态砷占主要部分,水溶态和残渣态砷的含量较少,且随着砷浓度的增加,脂溶态砷占总砷的比例先减少后增加,而水溶态砷所占比例先增加后减少,说明砷的解毒途径存在差异。结合培养基中砷形态变化可推断,在砷酸盐胁迫下,莱茵衣藻主要将AsⅤ还原为AsⅢ,并大量外排;同时,AsⅢ甲基化为DMA,可能合成砷糖并外排。随着AsⅤ浓度的增加,藻细胞减少对AsⅤ的吸收,甲基化程度加大,合成更多的脂溶态砷。因此,深入解析脂溶态砷的生物合成过程、研究微藻对砷的分配和形态转化,有助于揭示微藻对砷的解毒机制,进一步明确微藻在砷生物地球化学循环中的作用。
-
表 1 砷形态分析的HPLC-ICP-MS仪器条件
Table 1. Experimental conditions for As speciation by HPLC-ICP-MS
高效液相色谱HPLC PerkinElmer 电感耦合等离子体质谱ICP-MS NexION 2000 色谱柱Chromatographic column Hamilton PRP-X100阴离子交换色谱分析柱Anion exchange chromatography column(250 mm×4.1 mm, 10 μm) 等度分离流动相Equally separated mobile phase 17.5 mmol·L−1 (NH4)2HPO4, pH 5.8 进样体积Injection volume 50 μL 流速Flow velocity 1.5 mL·min−1 运行时间Run time 8 min 表 2 不同AsⅤ浓度处理下莱茵衣藻富集、吸收的砷含量 (μg·g−1 DW)
Table 2. Arsenic accumulation and absorption by C. reindardtii exposed to different AsⅤ concentrations (μg·g−1 DW)
AsⅤ处理/(μg·L−1)AsⅤ treatment 富集Accumulation 吸收Absorption 5 6.25±0.53e 5.12±0.12e 10 10.93±0.48d 9.83±0.24d 20 13.77±0.62c 12.65±0.21c 50 16.43±0.58b 15.38±0.18b 100 28.62±0.38a 27.70±0.25a 注:不同小写字母表示不同AsⅤ浓度处理下莱茵衣藻的砷富集和吸收含量的显著性差异(P<0.05). Note: Different letters are significant difference at P<0.05 among As accumulation and absorption by C. reindardtii exposed to AsⅤ under different concentrations. 表 3 不同AsⅤ浓度处理下莱茵衣藻培养基中各砷形态的浓度
Table 3. Concentrations of As species in the medium of C. reinhardtii exposed to different AsⅤ concentrations
各砷形态的浓度 / (μg·L−1)As species concentrations AsⅤ处理 / (μg·L−1)AsⅤ treatment AsⅢ DMA Unknown 1 AsⅤ Unknown 2 5 0.72±0.08d 0.03±0.00b 0.13±0.02d 0.15±0.03c 0.16±0.00a 10 2.37±0.33d 0.09±0.01b 0.29±0.03cd 0.13±0.05c 0.14±0.02b 20 6.22±0.29c 0.37±0.09b 0.56±0.08c 1.91±0.10c 0.12±0.00b 50 15.79±0.05b 1.21±0.12a 1.12±0.01b 16.77±0.88b — 100 34.92±1.80a 1.45±0.25a 1.60±0.10a 42.18±1.93a — 注:不同小写字母表示不同AsⅤ浓度处理下莱茵衣藻培养基中各砷形态的浓度的显著性差异(P<0.05). Note: Different letters are significant difference at P<0.05 among the concentrations of As species in medium by C. reinhardtii exposed to AsⅤ under different concentrations. -
[1] OREMLAND R S, STOLZ J F. The ecology of arsenic [J]. Science, 2003, 300(5621): 939-944. doi: 10.1126/science.1081903 [2] 苑春刚, LE X C. 砷形态分析 [J]. 化学进展, 2009, 21(2): 467-473. YUAN C G, LE X C. Arsenic speciation analysis [J]. Progress in Chemistry, 2009, 21(2): 467-473(in Chinese).
[3] LIU C, LUO C, GAO Y, et al. Arsenic contamination and potential health risk implications at an abandoned tungsten mine, southern China [J]. Environmental Pollution, 2010, 158(3): 820-826. doi: 10.1016/j.envpol.2009.09.029 [4] LUVONGA C, RIMMER C A, YU L L, et al. Organoarsenicals in seafood: Occurrence, dietary exposure, toxicity, and risk assessment considerations-a review [J]. Journal of Agricultural and Food Chemistry, 2020, 68(4): 943-960. doi: 10.1021/acs.jafc.9b07532 [5] WANG Y, WANG S, XU P, et al. Review of arsenic speciation, toxicity and metabolism in microalgae [J]. Reviews in Environmental Science & Biotechnology, 2015, 14(3): 427-451. [6] PETURSDOTTIR A H, FLETCHER K, GUNNLAUGSDOTTIR H, et al. Environmental effects on arsenosugars and arsenolipids in Ectocarpus (Phaeophyta) [J]. Environmental Chemistry, 2016, 13(1): 21-33. doi: 10.1071/EN14229 [7] MEYER S, SCHULZ J, JEIBMANN A, et al. Arsenic-containing hydrocarbons are toxic in the in vivo model Drosophila melanogaster [J]. Metallomics, 2014, 6(11): 2010-2014. doi: 10.1039/C4MT00249K [8] QIN J, LEHR C R, YUAN C, et al. Biotransformation of arsenic by a Yellowstone thermoacidophilic eukaryotic alga [J]. Proceedings of the National Academy of Sciences, 2009, 106(13): 5213-5217. doi: 10.1073/pnas.0900238106 [9] FOSTER S, THOMSON D, MAHER W. Uptake and metabolism of arsenate by anexic cultures of the microalgae Dunaliella tertiolecta and Phaeodactylum tricornutum [J]. Marine Chemistry, 2008, 108(3): 172-183. [10] XUE X M, YAN Y, XIONG C, et al. Arsenic biotransformation by a cyanobacterium Nostoc sp. PCC 7120 [J]. Environmental Pollution, 2017, 228(3): 111-117. [11] GLABONJAT R A, RABER G, JENSEN K B, et al. Quantification of arsenolipids in the certified reference material NMIJ 7405-a (Hijiki) using HPLC/mass spectrometry after chemical derivatization [J]. Analytical Chemistry, 2014, 86(20): 10282-10287. doi: 10.1021/ac502488f [12] NAVRATILOVA J, RABER G, FISHER S J, et al. Arsenic cycling in marine systems: Degradation of arsenosugars to arsenate in decomposing algae, and preliminary evidence for the formation of recalcitrant arsenic [J]. Environmental Chemistry, 2011, 8(1): 44-51. doi: 10.1071/EN10107 [13] PELL A, KOKKINIS G, MALEA P, et al. LC-ICP-MS analysis of arsenic compounds in dominant seaweeds from the Thermaikos Gulf (Northern Aegean Sea, Greece) [J]. Chemosphere, 2013, 93(9): 2187-2194. doi: 10.1016/j.chemosphere.2013.08.003 [14] JIA Y Y, WANG L, MA L, et al. Speciation analysis of six arsenic species in marketed shellfish: extraction optimization and health risk assessment [J]. Food Chemistry, 2018, 244: 311-316. doi: 10.1016/j.foodchem.2017.10.064 [15] MIYASHITA S, SHIMOYA M, KAMIDATE Y, et al. Rapid determination of arsenic species in freshwater organisms from the arsenic-rich Hayakawa River in Japan using HPLC-ICP-MS [J]. Chemosphere, 2009, 75(8): 1065-1073. doi: 10.1016/j.chemosphere.2009.01.029 [16] GLABONJAT R A, EHGARTNER J, DUNCAN E G, et al. Arsenolipid biosynthesis by the unicellular alga Dunaliella tertiolecta is influenced by As/P ratio in culture experiments [J]. Metallomics, 2017, 10(1): 145-153. [17] YIN X, WANG L, DUAN G, et al. Characterization of arsenate transformation and identification of arsenate reductase in a green alga Chlamydomonas reinhardtii [J]. Journal of Environmental Sciences, 2011(7): 134-141. [18] WANG N X, LI Y, DENG X H, et al. Toxicity and bioaccumulation kinetics of arsenate in two freshwater green algae under different phosphate regimes [J]. Water Research, 2013, 47(7): 2497-2506. doi: 10.1016/j.watres.2013.02.034 [19] MIYASHITA S, FUJIWARA S, TSUZUKI M, et al. Cyanobacteria produce arsenosugars [J]. Environmental Chemistry, 2012, 9(5): 474-484. doi: 10.1071/EN12061 [20] 李崇华, 赵方慈, 喻琪盛, 等. 胞外聚合物对莱茵衣藻砷富集和形态转化的影响 [J]. 湖南农业大学学报(自然科学版), 2019, 45(4): 384-390. LI C H, ZHAO F C, YU Q S, et al. Effects of extracellular polymeric substances on the accumulation and transformation of arsenic by Chlamydomonas reinhardtii [J]. Journal of Hunan Agricultural University (Natural Sciences), 2019, 45(4): 384-390(in Chinese).
[21] SARA G S, RABER G, RAML R, et al. Arsenosugar phospholipids and arsenic hydrocarbons in two species of brown macroalgae [J]. Environmental Chemistry, 2012, 9(9): 63-66. [22] RAAB A, NEWCOMBE C, PITTON D, et al. Comprehensive analysis of lipophilic arsenic species in a brown alga (Saccharina latissima) [J]. Analytical Chemistry, 2013, 85(5): 2817-2824. doi: 10.1021/ac303340t [23] ARROYO-ABAD U, PFEIFER M, MOTHES S, et al. Determination of moderately polar arsenolipids and mercury speciation in freshwater fish of the River Elbe (Saxony, Germany) [J]. Environmental Pollution, 2015, 208: 458-466. [24] TALESHI M S, RABER G, EDMONDS J S, et al. Arsenolipids in oil from blue whiting Micromesistius poutassou-evidence for arsenic-containing esters [J]. Scientific Reports, 2015, 4(1): 7492-7498. doi: 10.1038/srep07492 [25] SELE V, SLOTH J J, LUNDEBYE A K, et al. Arsenolipids in marine oils and fats: A review of occurrence, chemistry and future research needs [J]. Food Chemistry, 2012, 133(3): 618-630. doi: 10.1016/j.foodchem.2012.02.004 [26] MIYASHITA S, FUJIWARA S, TSUZUKI M, et al. Rapid biotransformation of arsenate into oxo-arsenosugars by a freshwater unicellular green alga, Chlamydomonas reinhardtii [J]. Bioscience Biotechnology and Biochemistry, 2011, 75(3): 522-530. doi: 10.1271/bbb.100751 [27] XUE X M, YE J, RABER G, et al. Arsenic methyltransferase is involved in arsenosugar biosynthesis by providing DMA [J]. Environmental science technology, 2017, 51(3): 1224-1230. doi: 10.1021/acs.est.6b04952 [28] MADSEN A D, GOESSLER W, PEDERSEN S N, et al. Characterization of an algal extract by HPLC-ICP-MS and LC-electrospray MS for use in arsenosugar speciation studies [J]. Journal of Analytical Atomic Spectrometry, 2000, 15(6): 657-662. doi: 10.1039/b001418o [29] MURRAY L A, RAAB A, MARR I L, et al. Biotransformation of arsenate to arsenosugars by Chlorella vulgaris [J]. Applied Organometallic Chemistry, 2003, 17: 669-674. doi: 10.1002/aoc.498 [30] XUE X M, YE J, RABER G, et al. Identification of steps in the pathway of arsenosugar biosynthesis [J]. Environmental Science Technology, 2019, 53: 634-641. doi: 10.1021/acs.est.8b04389 [31] XUE X M, RABER G, FOSTER S, et al. Biosynthesis of arsenolipids by the cyanobacterium Synechocystis sp. PCC 6803 [J]. Environmental Chemistry, 2014, 11(5): 506-513. doi: 10.1071/EN14069 -




 DownLoad:
DownLoad: