-
1,4-丁炔二醇(1,4-butynediol, BYD)是一种重要的化工原料,主要用于合成1,4-丁二醇(1,4-butanediol, BDO),进而生产四氢呋喃、聚四亚甲基乙二醇醚(PTMEG)、聚对苯二甲酸丁二醇酯(PBT)和聚丁二酸丁二醇酯(PBS)等重要化工产品[1-3]。目前,我国已经是世界上最大的BDO生产国[4],BDO生产首先利用乙炔和甲醛经铜铋催化合成BYD,BYD再经过精馏提浓,提浓后BYD需要通过阴阳离子树脂脱除含有的铜离子、二氧化硅和醋酸根离子等杂质,进而再催化加氢生成BDO[5]。其中,阴阳离子树脂再生产生的脱离子废液含有高浓度BYD残留[6],废水化学需氧量(chemical oxygen demand, COD)可达到6 000~20 000 mg·L−1,是BDO生产过程产生的主要高浓度有机废水,由于部分企业使用5%的硫酸进行阳离子树脂再生,从而导致该废水中硫酸盐含量也较高(6 000~10 000 mg·L−1)。BYD脱离子废液与生活污水、冲洗废水和BDO精馏废水等低浓浓度有机废水混合后即为BDO生产废水,BYD脱离子废液水量占BDO生产废水的比例为50%~70%。 BYD脱离子废液的高效低耗处理是BDO生产废水处理的关键。
厌氧生物处理技术因为具有能耗低、可回收甲烷气和污泥产量少等优势,广泛应用于高浓度有机废水的预处理[7]。其利用水解产酸菌、互养产氢产乙酸菌和产甲烷菌的协作实现有机物的厌氧甲烷转化[8]。当废水中含有硫酸根离子时,硫酸盐还原菌(sulfate reducing bacteria,SRB)也会参与厌氧代谢过程,在低浓度硫酸盐含量条件下,SRB可以促进难降解有机物的降解和乙酸产生,进而促进甲烷代谢[9];当硫酸盐含量过高时,硫酸盐还原产生的过多硫化氢可以抑制产甲烷古菌和SRB,进而抑制厌氧有机物代谢[10]。考虑到BYD是BDO生产脱离子废液的主要COD贡献者,阐明其在不同厌氧处理条件下的生物降解效果,对于脱离子废液及其他高含BYD废水的处理工艺设计具有重要指导意义。
目前关于BYD可生化性的研究较少,GOTVAJN[11]和TISLER等[12]利用快速生物降解实验方法评估了BYD的好氧可生化性,发现在60 d的培养周期内BYD浓度基本没有降低,认为BYD是一种不易生物降解有机物。陈庆磊等[13]利用批次实验评估了BDO生产废水的厌氧处理效果,发现COD去除率约为56%,没有研究BYD的去除率。且BYD分子中含有内炔烃超共轭结构,化学性质十分稳定[11],明确BYD厌氧可生化性对于指导工程实践具有积极意义。
因此,本研究联合使用批次和连续实验方法评估了BYD在厌氧生化处理过程中的生物降解效果,同时测定了COD、BYD、硫酸盐浓度变化及微生物群落的演替情况,研究结果可为含BYD工业废水的处理提供指导。
-
实验所用BYD购自上海麦克林生化科技股份有限公司(纯度98%),为白色斜方结晶,用纯水配成50 000 mg·L−1储备液,4 ℃保存。厌氧生化实验的外加微量元素营养液组成为[14]:MgCl2 (30 mg·L−1)、NiCl2 (10 mg·L−1)、CoCl2 (10 mg·L−1)、FeCl2 (40 mg·L−1)、CaCl2 (20 mg·L−1)、ZnSO4 (20 mg·L−1)、MnSO4 (20 mg·L−1)。CaCl2等常规化学试剂均为分析纯,购自天津市科密欧化学试剂有限公司。
本实验所用厌氧颗粒污泥为安徽宿州某酒精厂污水处理站的厌氧颗粒污泥,外形为规则球形,颗粒直径为0.5~4.0 mm;活性污泥采自河北石家庄市某市政污水处理厂缺氧池污泥,絮状,沉降性能优良。
-
厌氧批次实验在AMPTS® II自动甲烷潜力测试系统(BPC Instruments AB)中进行。为探究BYD是否可在纯厌氧产甲烷体系降解、复配活性污泥和SO42−还原是否可促进BYD厌氧降解,厌氧批次实验一共设置了3组实验,每组2个平行,同时设置没有厌氧污泥接种的空白组。实验组污泥浓度均控制在15 000 mg·L−1,pH=7.1,具体物料组成见表1,硫酸盐组控制BYD/SO42− =0.299 (对应COD/SO42− 的质量比为0.5)。各组实验均加入1 mL微量元素溶液,反应总体积均为400 mL,利用恒温水浴控制反应温度稳定在37 ℃。
-
厌氧连续实验在2套平行中温有效容积为6.3 L的UASB反应器进行,种泥为30%活性污泥与70%厌氧颗粒污泥复配污泥。进水水质模拟内蒙古某BDO生产企业的实际BYD脱离子废液水质配置(表2),进水COD由葡萄糖和BYD组成,氮源为氨氮,磷源为磷酸二氢钠,碱度由碳酸氢钠贡献,微量元素(Fe、Mn、Zn、Co)添加参考KONG等[10],进水pH调至7.2±0.2,进水基质桶水温由低温水浴控制在4 ℃。反应器各阶段具体运行参数见表2。
-
厌氧发酵气体首先通过二氧化碳吸收瓶(含有3 M的氢氧化钠),之后利用湿式气体流量计记录每日产气量。污水样品经0.22 μm滤膜过滤后,滤液用于各指标测试,其中COD和SO42−测定参照水质测定标准[15]。针对硫酸盐还原体系,滤液首先经过曝气去除溶液中的硫化物,通过硫化物测定试纸(陆恒生物)不变蓝判断硫化物被完全去除,之后再过滤测定COD。采用GC-FID (岛津GC2010 plus)测定BYD浓度,选用SH-Stabilwax-DA柱(30 m×0.25 mm×0.25 μm) 作为气相色谱柱,温度程序如下:100 ℃保留1 min,以20 ℃·min−1升温至120 ℃,保留4 min,之后以20 ℃·min−1升温至220 ℃,保留5 min。进样口和检测器温度分别为240 ℃和260 ℃。氦气作载气,初始压力为400 kPa。采用外标法测定BYD含量,每次测试新配置标准曲线。采用内标法测定挥发性脂肪酸(VFAs)含量,取1mL滤液加入1-丁醇内标(2 000 mg·L−1)后,使用GC-FID (岛津GC2010 plus)测定VFAs浓度,选用Restek Stabilwax-DA柱(30 m×0.53 mm×0.1 μm) 作为气相色谱柱,温度程序如下:70 ℃保留1 min,以20 ℃·min−1升至150 ℃,之后以4 ℃·min−1升至 160 ℃,再以20 ℃·min−1升至210 ℃,保留2 min。进样口和检测器温度分别为240 ℃和260 ℃。氦气作载气,初始压力为167.3 kPa。
-
针对UASB反应器,在每个运行阶段结束时分别从2个平行反应器中收集厌氧污泥样品,使用FastDNATM SPIN Kit (MP Biomedicals, Solon, USA)试剂盒提取DNA,使用NanoDrop分光光度计(ND-2000, NanoDrop Technologies, USA)测定DNA浓度和纯度。提取后DNA使用515F (GTGCCAGCMGCCGCGG)和907R (CCGTCAATTCMTTTRAGTTT)引物扩增细菌16S rRNA基因[16],PCR扩增产物送至上海美吉生物医药科技有限公司进行NovaSeq PE250测序。测序数据存储于NCBI SRA (链接号:PRJNA1108964)。测序数据质控、OUT (operational taxonomic units)聚类、细菌菌属注释和主坐标分析(PCoA)通过美吉生物云平台(https://cloud.majorbio.com/)完成。
-
如图1(a)所示,空白组中BYD不会因为水解、挥发等原因而浓度下降。在单一厌氧颗粒污泥接种的实验组,BYD降解缓慢,31 d实验结束时去除率仅为40.72%,对应的降解速率为6.6 mg·(L·d)−1 (图1(b)),且COD去除与BYD降解表现出较高的一致性(相关系数R2=0.908,P<0.05)。KONG等[17]报道活性污泥与厌氧颗粒污泥的复配可以提高N-N-二甲基甲酰胺的厌氧产甲烷效果,发现N-N-二甲基甲酰胺首先被活性污泥中兼性厌氧水解微生物转化为二甲胺和一甲胺等中间体,再被产甲烷古菌直接利用,从而促进了N-N-二甲基甲酰胺的完全厌氧降解。厌氧颗粒污泥与活性污泥复配有可能也会促进BYD的厌氧降解,因此开展了2种污泥复配的BYD降解实验,结果如图1(c)所示。复配活性污泥后,BYD的降解速率加快,达到9.9 mg·(L·d)−1,在31 d实验结束时BYD的去除率达到了65.71%,较单一厌氧颗粒污泥实验组提升了25.01%。但是需要指出,实验结束时污泥复配体系的COD去除率仅为29.43%,与单一颗粒污泥体系(33.02%)基本一致。将实验结束时残留BYD浓度转化为COD理论值为281.2 mg·L−1,小于COD实测值(658.5 mg·L−1),表明虽然活性污泥接种带入的BYD兼性厌氧水解菌可以将BYD转化为未知有机中间产物,使得其母体物质浓度降低速度加快,但生成的中间产物仍难以被产甲烷古菌降解。除了产甲烷古菌,在硫酸盐含量较高的厌氧体系中,SRB可以利用乳酸、丙酸和醇类等多种有机物作为电子供体[18],SO42−为电子受体,将有机物降解并将SO42−还原成硫化氢[19]。
考虑到BYD分子中含有羟基,有可能作为SRB的电子供体而被降解。因此,在复配污泥作为种泥的条件下进一步加入硫酸盐,探究硫酸盐还原是否可以加速BYD的厌氧降解。结果如图1 (d)所示,加入硫酸盐后,复配污泥中BYD降解速率进一步提升至11.2 mg·(L·d)−1,在31 d实验结束后去除率达到79.46%,比单一厌氧颗粒污泥和复配污泥实验组的BYD去除率分别提高了38.74%和13.75%。同时,COD的最终去除率达到52.03%,比单一厌氧颗粒污泥和复配污泥实验组分别提高了19.01%和22.60%。混合液SO42−的质量浓度变化与BYD和COD的质量浓度变化都表现出显著的正相关关系(P<0.05) (图1 (d))。实验开始后(0~6 d),SO42−质量浓度即快速降低(图1 (d)),表明反应起始硫酸盐还原作用即占据主导,这可能与本研究中采用的低COD/SO42− (0.5)有关。HAO等[20]报道COD/SO42−低于1.5时,SRB会获得生长优势,厌氧代谢以硫酸盐还原为主。在反应结束时,SO42−去除率为57.32%,对应的单位硫酸盐去除所需的COD比例为0.5,低于与WU等之前报道的乙醇和醋酸盐合成废水单位硫酸盐去除所需的COD比例(0.6)[21]。将实验结束时混合液残留BYD的质量浓度转化为理论COD值为171.6 mg·L−1,同样小于实测COD值441 mg·L−1,说明外加硫酸盐虽然提高了BYD的降解,但是体系中仍有部分BYD的厌氧转化产物难以被SRB利用。
-
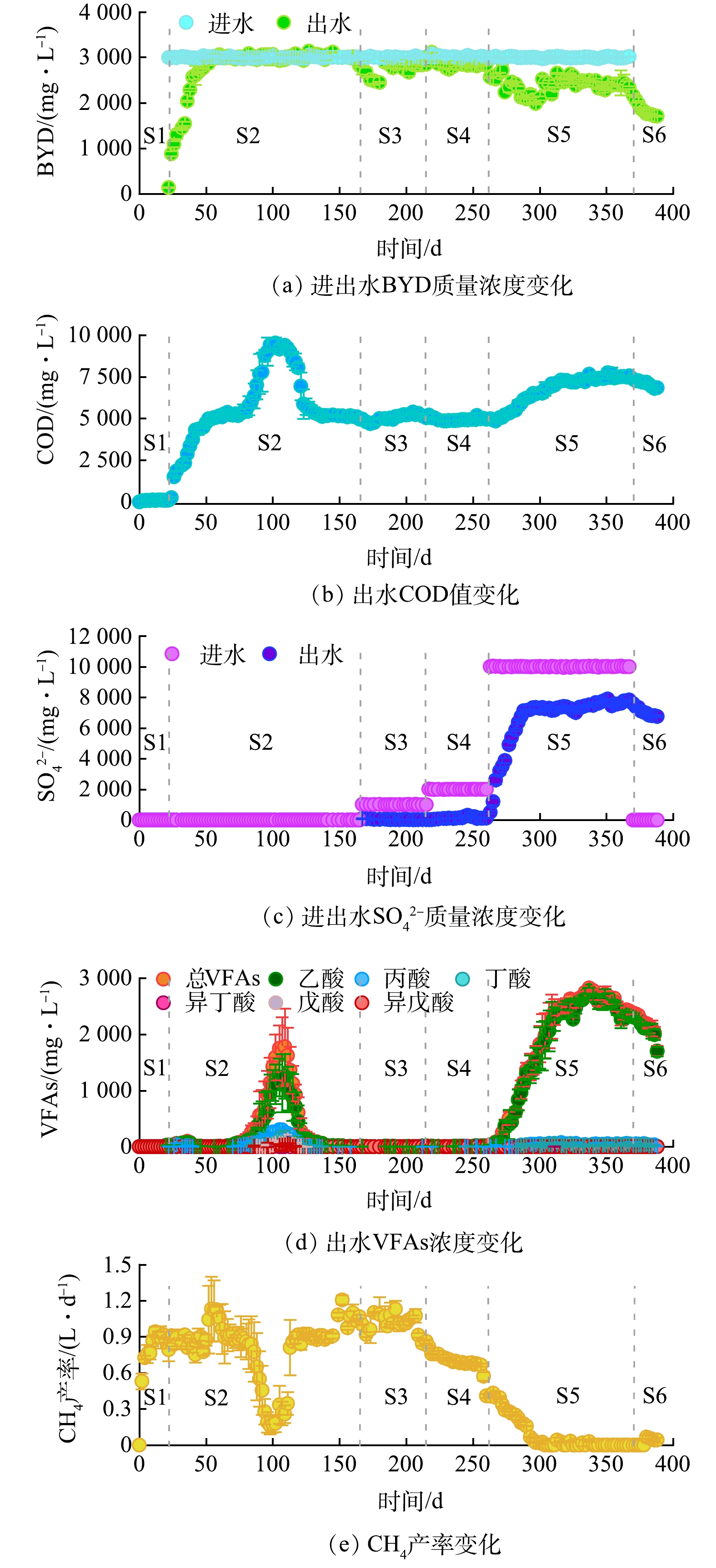
1)反应器运行效果。短期批次实验可以初步评估目标污染物的厌氧降解性能,而长期连续实验可以进一步解析厌氧微生物群落经过长期驯化后对目标污染物的生物降解效果(这与实际废水处理系统运行状况更相似),2种方法的结合可以更全面地解析目标污染物的厌氧降解性能[7]。本研究中,批次实验结果表明BYD在厌氧产甲烷体系中降解较慢,厌氧颗粒污泥复配活性污泥和外加硫酸盐均可以加速BYD的厌氧降解。为此,进一步参考实际BDO生产脱离子废液水质(COD为6 000~20 000 mg·L−1,SO42−含量为6 000~10 000 mg·L−1),建立了2套平行的UASB反应器评估了长期运行情况下BYD的厌氧降解效果,结果如图2所示。UASB反应器一共运行了388 d,根据进水水质变化分为S1~S6 6个阶段(表2)。
UASB反应器以葡萄糖为唯一碳源启动,运行20 d之后出水COD值低于120 mg·L−1,VFAs低于30 mg·L−1 (图2(b)和图2(d)) (S1阶段),证明厌氧污泥活性优良。在第21天,反应器进水加入2 980 mg·L−1的BYD (S2阶段),此后反应器出水COD值和BYD质量浓度逐渐上升,在52 d分别稳定在约5 000 mg·L−1和2 965 mg·L−1,对应的COD和BYD去除率仅约50.33%和0.50% (图2(a)~(b)),且在此过程中CH4产率未出现下降(图2 (e)),VFAs未累积(图2(d))。在第83天,反应器出水VFAs和COD值急剧上升,在105 d VFAs达到了1 780 mg·L−1,主要由乙酸组成(图2(d)),同时出水COD也上升到了9 365 mg·L−1 (图2(b)),COD去除率下降至6.37%,UASB出现了VFAs累积现象。考虑到BYD分子中含有—C≡C—和—OH等结构,易于与金属离子络合[22],当其质量浓度过高时(如本文的2 980 mg·L−1)可络合带走过多铁、钴、镍等微量金属元素,导致可用于产甲烷古菌维持正常生命活动的微量金属元素含量降低,从而导致厌氧产甲烷微生物失活。随后在111 d将进水中微量元素质量浓度翻倍,反应器出水VFAs和COD值随即快速下降,系统产气恢复(图2 (e))。之后继续对UASB反应器进行了42 d的连续监测,系统再未出现抑制情况,但BYD去除率也没有进一步提升,表明即使经过长期驯化厌氧产甲烷微生物代谢体系中BYD降解速率仍较低。
此后,向反应器引入硫酸盐共运行了3个阶段(S3~S5),通过提高进水硫酸盐质量浓度使各阶段COD/SO42−分别为10、5和1。在S3阶段(167~215 d),进水COD/SO42− =10的情况下,UASB反应器CH4产率从0.89 L·d−1提升至1.07 L·d−1,表明少量硫酸盐加入提高了厌氧甲烷产率。同时,该阶段COD去除率为50.56%,SO42−去除率为97.15%,出水BYD质量浓度先下降后上升,最终稳定在2 847 mg·L−1,对应去除率为3.92% (图2 (a)、(b))。
在S4阶段(216~260 d),将COD/SO42−降低至5,CH4产率从1.07 L·d−1迅速降低至0.41 L·d−1,而VFAs未发生累积(图2(d)),BYD去除率略提升至4.83%,COD去除率为51.02%,SO42−去除率93.81%。上述结果说明此阶段厌氧生化系统仍然能保持正常运行,但硫酸盐还原作用已经显著增强,开始与产甲烷代谢共同承担对乙酸的降解。
在S5阶段(263~369 d),进一步将进水COD/SO42−下调到1,出水BYD质量浓度明显降低,去除率从S4阶段的4.83%提高到21.92% (图2(a))。但同时,厌氧生物系统迅速受到抑制,COD去除率从51.02%下降至20.31%,SO42−去除率从93.81%下降至24.48%,并且VFAs (主要为乙酸)快速累积至2 824 mg·L−1 (图2 (d)),这可能是由于在COD/SO42− =1的条件下,硫酸盐还原已经成为主导的厌氧有机物降解途径[23],但是进水SO42−质量浓度过高,导致产生的硫化氢质量浓度超过了SRB和产甲烷古菌的耐受阈值,从而抑制了乙酸的进一步转化。O'FLAHERTY等[10]曾报道游离硫化氢对厌氧微生物的抑制阈值为38~185 mg·L−1,而本研究S5阶段UASB反应器内理论游离硫化氢质量浓度已经超过214.7 mg·L−1 (约占总硫化物8.82%)[19],厌氧微生物活性可被完全抑制。在第370天采取停止进水的方式试图恢复厌氧系统活性(S6阶段),在18 d的恢复期内,反应器内BYD、COD、SO42−和VFAs均有下降趋势,但下降趋势较慢。
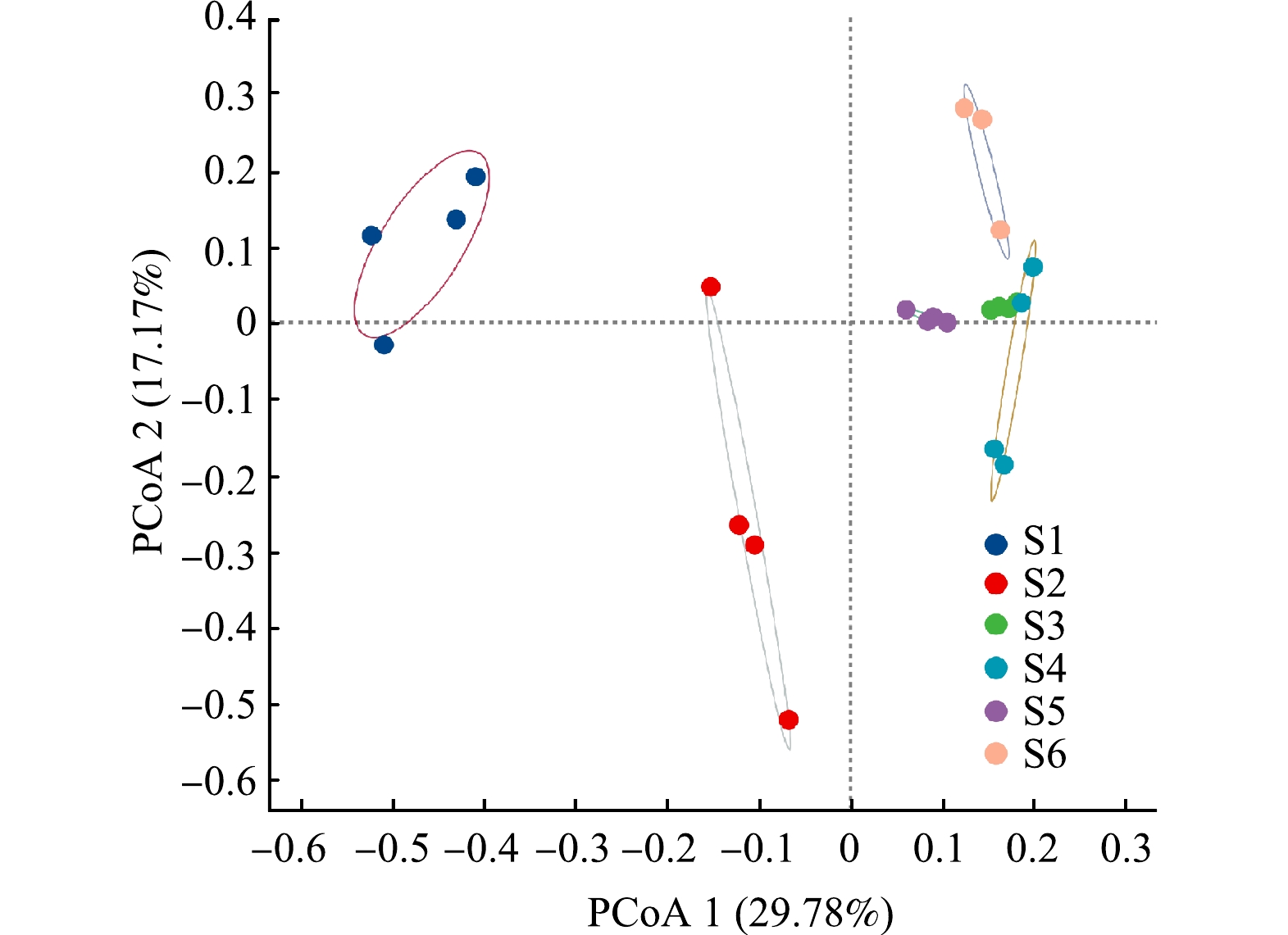
2)微生物群落演替。利用高通量测序技术分析了UASB连续实验过程中厌氧污泥细菌群落的演替情况。如图3所示,BYD的投加(S2)显著改变了污泥中厌氧微生物群落结构,硫酸盐的投加(S3~S6)进一步显著改变了UASB厌氧系统微生物群落的结构,而在S3~S6阶段COD/SO42−的改变及停止进水过程中细菌群落结构相对稳定。
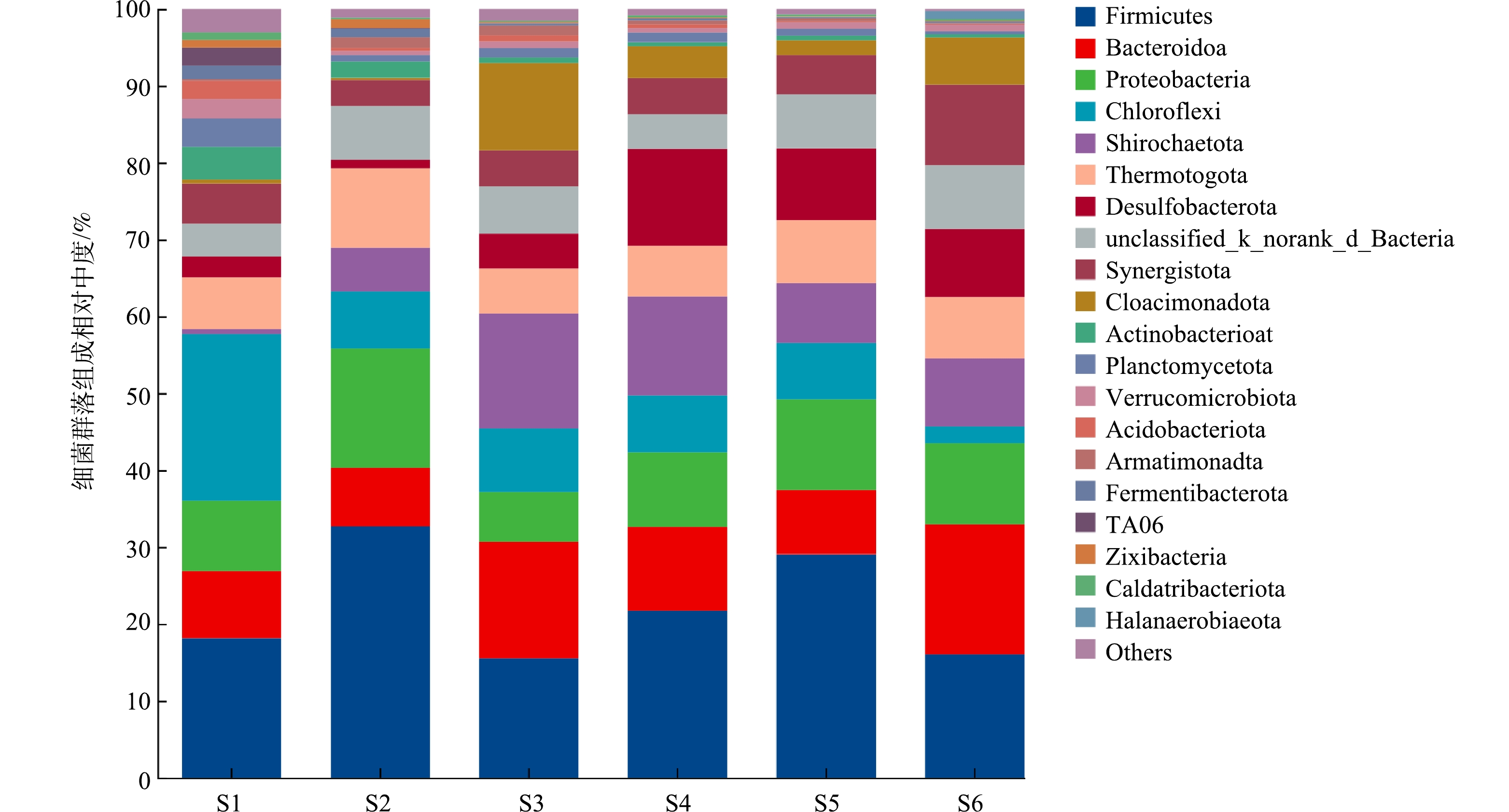
门水平下细菌群落变化如图4所示,种泥(S1阶段)细菌以绿弯菌门(Chloroflexi)、厚壁菌门(Firmicutes)、变形菌门(Proteobacteria)、拟杆菌门(Bacteroidota)、热孢菌门(Thermotogae)、互养菌门(Synergistota)和螺旋体门(Spirochaetota)为主,其中部分Proteobacteria和Spirochaetota细菌具有硫酸盐还原功能[24]。投加BYD后(S2阶段),Firmicutes、Proteobacteria和Spirochaetota相对丰度分别从18.13%、9.17%和0.71%增加到32.68%、15.53%和5.67%,而Chloroflexi门丰度从21.63%减少至7.44%。进水增加硫酸盐后,在S3阶段脱硫杆菌门(Desulfobacterota)、Spirochaetota和Cloacimonadota门细菌相对丰度分别由1.09%、5.67%和0.27%增加至4.53%、14.98%和11.35%,其中Cloacimonadota门细菌通常存在于厌氧发酵系统中,宏基因组组装基因组分析揭示其是一种潜在的乙酸产生菌[25],而多种Desulfobacterota门细菌(如Syntrophobacter、Desulfoglaeba和Desulfovibrio)同时是丙酸氧化产乙酸菌和SRB,在高COD/SO42−条件下可获得更大的生存优势,成为产甲烷古菌的主要互养微生物[20, 26-28]。随着COD/SO42−降低至5(S4阶段),厌氧系统硫酸盐还原作用被进一步加强(图2 (e)),Desulfobacterota和Proteobacteria相对丰度分别从4.53%和6.45%上升到12.64%和9.75%,而Cloacimonadota和Spirochaetota相对丰度略有降低。在S5与S6阶段,Firmicutes与Chloroflexi相对丰度分别从29.08%和7.33%减少至16.04%和2.18%,Bacteroidota和Synergistota相对丰度分别从8.30%和5.07%提升至16.88%和10.49%,而Desulfobacterota和Proteobacteria丰度基本不变(图4)。
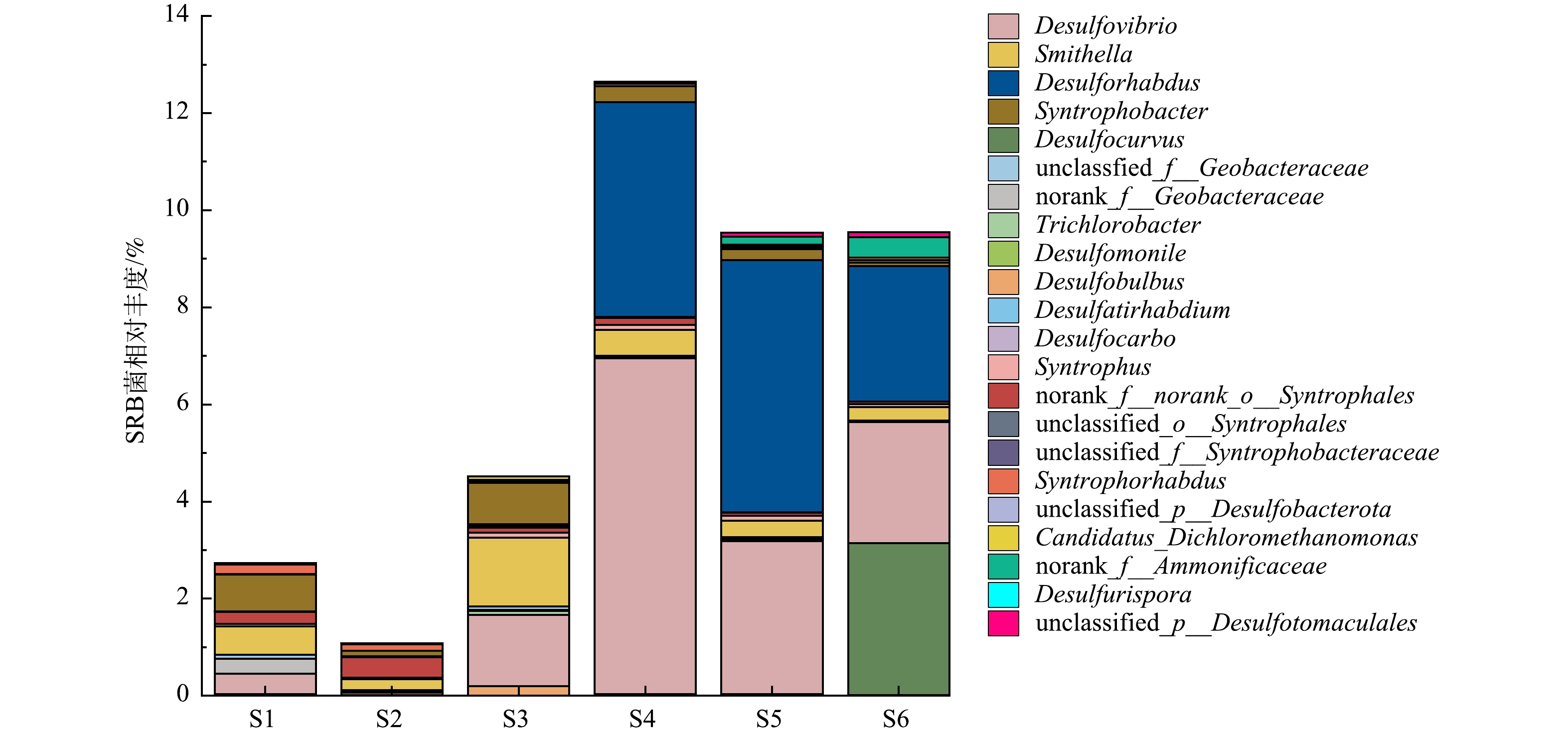
考虑到硫酸盐还原作用可以增强BYD的厌氧降解,进一步在属水平上分析了实验过程中UASB内SRB的变化(图5)。本研究共检出属于5个门64个属的SRB,其中丰度较高的22个SRB在种泥中的总丰度为2.76%,优势SRB菌为互营杆菌属(Syntrophobacter) (0.76%)、史密斯氏菌属(Smithella) (0.60%)和脱硫弧菌属(Desulfovibrio) (0.41%)。S2阶段引入BYD后,厌氧污泥中SRB种类未变,但总丰度明显减少(1.17%) (图5)。进入S3阶段(进水COD/SO42− =10),Desulfovibrio、Syntrophobacter和Smithella快速生长,相对丰度分别从0.05%、0.11%和0.22%增加至1.46%、0.86%和1.42%。这3个菌属均属于Desulfobacterota[28],其中Syntrophobacter与Smithella为互养产乙酸菌,其可利用硫酸盐作为电子受体氧化丙酸等多种有机物,生成乙酸和二氧化碳[29],从而为产甲烷古菌提供更多的甲烷前驱物,促进甲烷代谢。而Desulfovibrio为不完全氧化型SRB[30],在COD/SO42−≥10的条件下,同样可利用硫酸盐作为电子受体氧化乳酸、丙酸等有机物生成乙酸,从而提高了乙酸产量,促进甲烷代谢[21, 29]。
上述3个菌属的富集可能是S3阶段观测到甲烷产率提升的原因(图2(e))。在S4阶段COD/SO42−进一步降低到5后,SRB总丰度增加至12.6%,Desulforhabdus和Desulfovibrio取代Syntrophobacter与Smithella成为优势SRB。其中,Desulforhabdus丰度由S3阶段的0.04%显著提升至4.42%,其是一种完全氧化型SRB,可利用硫酸盐作为电子受体完全氧化乙酸等有机物生成二氧化碳和硫化物[29, 31-32],进而减少了可用于甲烷代谢的乙酸量。因此,完全氧化型SRB与产甲烷古菌对乙酸的竞争应该是S4阶段UASB反应器甲烷产率明显下降(图2(e))的主要原因。
进入S5阶段,虽然SRB总丰度有所降低,但是Desulforhabdus的相对丰度进一步增加至5.19%,说明在低COD/SO42−条件下完全氧化型SRB会获得生存优势。Desulforhabdus的进一步富集,一方面会使更多的乙酸用于硫酸盐还原,从而进一步减少甲烷产生;另一方面,在高硫酸盐存在下,完全氧化型硫酸盐还原产生的过多硫化氢会同时抑制产甲烷古菌和SRB(包括Desulforhabdus)[10],这就导致了在S5阶段基本无甲烷产生(图2(e)),VFAs (特别是乙酸)显著累积(图2(d)),同时硫酸盐去除有限(图2(c))。需要指出,尽管S5阶段硫酸盐还原作用被显著抑制,BYD的降解仍得到显著提升(图2(a)),同时BYD的降解效率提升与Desulforhabdus丰度增加有一定相关性(图2(a)和图5),说明完全氧化型SRB (Desulforhabdus)可能是BYD的潜在降解菌。MOTTERAN等[33]曾报道Desulforhabdus参与了直链烷基苯磺酸盐这类难降解有机物的厌氧降解,且硫酸盐浓度会影响其降解效果。除Desulforhabdus外,部分水解产酸菌(如Caldicoprobacter, Anaerofustis, Lachnoclostridium, Thiobacillus)和互养产酸菌(Thermovirga)的丰度变化与BYD的降解效果同样表现出显著正相关性(Spearman相关,P<0.05)。后续研究需结合纯菌筛选和降解实验以进一步确认BYD的厌氧降解菌。
在S6阶段,另一种不完全氧化型SRB (Desulfocurvus)[34-35]丰度显著增加(图5)。COLIN等[36]同样在含高浓度乙酸盐的河口沉积物(厌氧环境)中检测到高丰度的Desulfocurvus。这可能是因为虽然Desulfocurvus主要利用硫酸盐作为电子受体氧化乳酸或丙酮酸等有机物生成乙酸,但是在H2含量高的厌氧环境其可以利用乙酸作为碳源生长[34],而S6阶段高浓度乙酸残留(图2 (d))和相对高H2含量(Desulfovibrio、Syntrophobacter和Smithella等SRB的代谢产氢)的环境有利于其增殖。
-
本研究表明对于含BYD的废水采用厌氧UASB处理时,厌氧接种污泥最好采用复配污泥;对于含有BYD同时COD和SO42−含量较高的废水(例如BDO生产过程产生的脱离子废液),厌氧UASB工艺可考虑利用硫酸盐还原提升废水的有机质去除效果,但是需要考虑游离硫化氢抑制问题:高强度硫酸盐还原,会产生大量硫化物,同时生成大量游离硫化氢,其会进入细胞,对产甲烷古菌和SRB产生抑制[23, 37],从而导致厌氧有机物去除效率下降(图2)。为了防止游离硫化氢的抑制,一方面可以通过稀释,降低进水硫酸盐质量浓度,比如HU等[23]研究发现在COD/SO42− =1的情况下,降低进水SO42−质量浓度至3 000 mg·L−1,可以保证80%的SO42−被还原去除;另一方面李健等[38]针对UASB反应器,设计出水循环系统和硫化物吹脱塔,即厌氧出水通过泵打入吹脱塔,吹脱塔中布置曝气装置,利用空气曝气将废水中硫化物氧化为硫单质从而降低废水中游离硫化氢含量,吹脱后液体通过泵打回到进水端,与进水混合后进入厌氧塔,从而缓解塔内游离硫化氢的抑制。利用上述措施,李健等[38]实现了高有机硫、高COD制药废水的稳定厌氧处理。最后,高质量浓度BYD本身可以络合金属离子,硫酸盐还原产生的硫化物也可以沉淀金属离子,因此通过硫酸盐还原处理高含BYD废水时,需要注意补加更多的微量元素。
-
1)单纯的厌氧产甲烷体系中BYD的降解较慢,活性污泥和厌氧颗粒污泥复配及添加SO42−均可以提升厌氧生化对BYD的降解速率。
2)高质量浓度BYD会络合过多铁、钴、镍等微量金属元素,导致产甲烷古菌被抑制,厌氧系统VFAs显著累积,通过补加微量元素的方式可以解除抑制。
3)随着进水COD/SO42−降低,硫酸盐还原逐渐替代甲烷代谢成为主要的厌氧代谢途径,同时BYD的厌氧降解率也逐渐升高;在进水COD/SO42−为1时BYD的降解率达到21.92%,完全氧化型硫酸盐还原菌Desulforhabdus成为优势菌属,但此时因为游离硫化氢大量产生,同时抑制了产甲烷古菌和硫酸盐还原菌,使得厌氧体系乙酸大量累积,即使停止进水厌氧系统在短时间内也很难恢复。
4)后续研究需要进一步考察不同质量浓度BYD对厌氧微生物群落的影响,并设计实验评估低COD/SO42−情况下,进水硫酸盐质量浓度对硫酸盐还原降解BYD的影响。此外,还需考察非生物转化途径(如吸附作用)对BYD在厌氧生化系统去除的贡献。
1,4-丁炔二醇的厌氧降解性能评估
Evaluation of the anaerobic biodegradability of 1,4-butynediol
-
摘要: 该研究首先利用批次实验评估了1,4-丁炔二醇(1,4-butynediol,BYD)在三种厌氧生化条件下的降解效果,发现在单纯厌氧颗粒污泥体系中BYD的降解较慢,厌氧颗粒污泥与活性污泥复配及添加SO42−都可以提高BYD降解速率。之后以模拟BYD废水为处理对象,以厌氧颗粒污泥与活性污泥复配污泥作为种泥,研究了UASB反应器长期连续运行条件下BYD在厌氧产甲烷体系和硫酸盐还原体系下的降解情况。发现在厌氧产甲烷体系中BYD去除效果不佳,并且高浓度BYD (2 980 mg·L−1)会络合微量金属元素,导致产甲烷古菌活性降低,长期运行会导致挥发性脂肪酸累积,通过补加微量元素可以恢复产甲烷菌活性。通过增加SO42−浓度降低进水COD/SO42−可以促进硫酸盐还原成为主导厌氧代谢途径,在进水COD/SO42− (质量比)为1,硫酸盐浓度为10 000 mg·L−1时,BYD的去除率可提高至21.92%,硫酸盐还原菌Desulfovibrio和Desulforhabdus成为优势菌属,但此时高浓度游离硫化氢同时抑制了产甲烷古菌和硫酸盐还原菌,使得厌氧体系挥发性脂肪酸再度大量累积,即使停止进水,硫化氢的抑制在短时间内难以恢复。本研究表明BYD为一种厌氧难生物降解有机物,硫酸盐还原作用可以促进其降解,研究结果可为含BYD废水的处理工艺设计提供指导。Abstract: This study evaluated the degradation efficiency of 1,4-butynediol (BYD) under three types of anaerobic biochemical conditions through batch experiments. The results showed that direct BYD degradation was relatively slow by anaerobic granular sludge with BYD as a solo carbon source, whereas the combination of activated sludge and anaerobic granular sludge, as well as sulfate (SO42−) augmentation, could enhance the degradation of BYD. Thereafter, the simulated BYD wastewater was taken as treatment object, anaerobic granular sludge combined with activated sludge was taken as seed sludge, The long-term running of UASB reactors was performed. The BYD degradation was studied under anaerobic methanogenic metabolism condition and sulfate reduction condition, respectively. The result showed that the removal of BYD under anaerobic methanogenic metabolism condition was not good, and the complexation reaction could occur between BYD with high concentration (2 980 mg·L−1) and trace metal elements, which led to the decrease of the methanogenic archaea activity and accumulation of volatile fatty acids after long-term running.The methanogenic archaea activity could be recovered through supplementary addition of trace metal elements. The increase of SO42− concentration and decrease of COD/SO42− ratio in influent could result in sulfate reduction dominating the anaerobic metabolic pathway. At the influent COD/SO42− ratio of 1 (m/m, SO42− concentration of 10 000 mg·L−1), the degradation of BYD increased to 21.92%, and sulfate reducing bacteria Desulfovibrio and Desulforhabdus became the dominant genera in UASB. At this time, the free hydrogen sulfide with high concentration inhibited the methanogenic archaea and sulfate reduction bacteria, causing the high accumulation of volatile fatty acid in anaerobic system occurred again. Even though the influent was stop, the inhibiting results by free hydrogen sulfide was difficult to recover in a short period of time. This study indicated that sulfate reduction can promote the anaerobic biodegradation of BYD, which could provide the guidance for the design of treatment processes for BYD containing wastewater.
-
岩溶水是岩溶系统的重要组成部分,其化学组分受地质、水文地质和人类活动等多种因素的控制,并在径流过程中发生复杂的水化学作用,最终表现出独特的水化学组分特征[1-2]。岩溶水化学系统的变量与控制因素错综复杂,因此如何利用水化学组分特征识别水化学信息、分析各类水文地球化学作用及研究水化学组分的时空变化规律并最终实现对水化学演化过程的重新构建就成为了一个难题[3-4]。国内外对岩溶区水化学的研究主要集中在水化学组分和演化等方面[5-7],主要通过定性分析结合定量模拟的方法来完成水化学分析,例如采用水化学类型分析、离子组合比分析和多元统计分析等方法定性分析水化学各组分的来源和控制因素,采用水文地球化学模拟方法定量模拟分析水化学演化[8-11]。
我国是岩溶地貌最为典型的地区之一,总分布面积可达344×104 km2[12],以滇、黔、桂为主体的西南岩溶区分布最为广泛,东北、内蒙、华北和华东地区也有分布,受气候、地层岩性和地质构造等因素的影响,我国南北方岩溶及岩溶水存在较大的差异。以北方岩溶区为例,大量学者研究表明北方岩溶水水化学特征主要受碳酸盐岩和石膏的风化溶解[13-18]、去白云化的控制[13-15,17-18],同时也受到诸如上覆孔隙水混入[18]、煤系地层伴生硫化物矿物氧化[15-16]、人类工程活动[17]等其他因素的影响,且随着径流途径延伸和深度增加呈现一定的规律变化。而对于南方岩溶区,岩溶水水化学特征则是主要受到碳酸盐岩和石膏的风化溶解[19-25]、阳离子交替吸附作用的控制[19,21],同时也受到诸如硅酸盐矿物溶解[21,25]、孔隙水[24]、采煤活动和农业活动[21-25]等其他因素的制约,大部分地区具有明显的空间分异性[20,22-25]。
本文以滇东高原牛栏江流域寻甸县岩溶区地下水为研究对象,利用水化学数据,探讨地下水化学特征、演化进程及成因。研究区属牛栏江-滇池补水工程补给区,本研究对区域的水化学特征、水质保护和滇池生态恢复具有重要意义。
1. 材料与方法(Materials and methods)
1.1 研究区概况
研究区位于云南省寻甸县境内,南距昆明市区90 km,地理坐标为东经102°41′—103°33′,北纬25°20′—26°01′。研究区属于高原季风气候,多年平均气温为14.5 ℃,多年平均降水量为1020.9 mm。降水多集中在5—10月,占全年降水量约80%。
研究区处于滇东高原盆谷亚区南部,属于云贵高原第二级阶梯[26],地势西北高,呈向东南倾斜阶梯状,中部地势略高,东西两侧低中山之间分布于大小不等的山间槽谷[27],东南侧受牛栏江水系的河流强烈切割,河谷深切。主体山势呈NE-SW向,连绵起伏,峰谷相间。研究区碳酸盐岩地层广泛分布,构造强烈,岩溶地貌发育,岩溶面积约占寻甸县辖区面积的45.6%。
研究区内地下水类型主要是以岩溶水和裂隙水为主,孔隙水零星分布。岩溶含水层可以分为纯质碳酸盐岩含水层和碳酸盐岩夹碎屑岩含水层。纯质碳酸盐岩含水层包括有二叠系茅口组(P1m)和栖霞组(P1q),石炭系上统、中统(C3和C2)、下统摆佐组(C1b)和大塘组上司段(C1ds),以及寒武系龙王庙组(∈1l),岩石裂隙多被网脉状结晶方解石或白云石充填;碳酸盐岩夹碎屑岩含水层包括有三叠系永宁镇组(T1y)、泥盆系宰格组(D3zg)、志留系关底组(S3g)和寒武系双龙潭组(∈2s),可见断续方解石和白云石细脉穿插。碎屑岩含水层包括有三叠系飞仙关组(T1f)、二叠系梁山组(P1l)、石炭系大塘组万寿山段(C1dw)和泥盆系海口组(D2h),多呈条带状分布,岩性以页岩、砂岩和泥岩为主,富水性中—弱,常构成区域内相对隔水层。区内地下水主要补给来源于大气降雨,岩溶地下水直接通过岩溶洼地、漏斗、漏水洞和部分溶蚀裂隙等渗入或注入补给。受地层岩性、地质构造和地形地貌等控制,地下水从中部分水岭部位向两侧径流,并以南侧牛栏江作为排泄基准面整体呈现从北向南的径流方向,与岩层走向和构造线延展方向大体相似,并多在岩层交界面附近以下降泉形式排泄。区内各含水层分布及特征分别见表1和图1。
表 1 区内含水层分布及特征Table 1. Characteristics and distribution of aquifer in study area含水层Aquifer 代号Code 主要岩性特征Main lithologic characteristics 水文地质特征Hydrogeological characteristics 富水性Water abundance 纯质碳酸盐岩含水层 P1m、P1q 灰岩、白云岩等 区内大面积分布,层厚约237—645m,为区内主要的岩溶含水层,岩溶管道极其发育,入渗条件好 强 C3、C2、C1b、C1ds、∈1l 呈NE向条带状分布于研究区中部,岩溶发育,为区内岩溶水的主要排泄层位,地下水动态随季节变化较大 碳酸盐岩夹碎屑岩含水层 T1y、D3zg、S3g、∈2s 碳酸盐岩(灰岩、白云岩)夹碎屑岩(泥岩、页岩等) 呈NE向条带状分布于研究区中部,溶蚀较发育,介质以溶蚀裂隙为主,地下水动态随季节变化较大 中—强 碎屑岩含水层 T1f、D2h 砂岩、页岩、泥岩 呈条带状分布于研究区东侧,富水性、透水性都较弱,水位埋深约100m 中 P1l、C1dw 页岩、砂岩、煤线 呈条带状分布于研究区东侧,水量贫乏,透水性弱,常构成区域相对隔水层 弱 玄武岩含水层 P2β 玄武岩 大面积分布于研究区内,裂隙发育,地下水动态随季节变化大 中—强 1.2 研究方法
采用现场测试和样品室内测试相结合的研究方法,选取具有代表性水样24组,其中包括岩溶泉16组、基岩裂隙水8组。采样时间为2017年8月,采样点位见图1。
pH值采用Multi340i 便携式水质多参数分析仪现场测定,室内分析主要阳离子(Na++K+、Ca2+、Mg2+)和阴离子(
HCO−3 SO2−4 HCO−3 SO2−4 2. 结果与讨论 (Results and discussion)
2.1 水化学组分及特征
对区内24组水样进行分析,水化学离子分析数据和离子概要统计见表2。由表2可知,pH值区间为7.56—9.18,平均为7.56,呈弱碱性,标准偏差较小;TDS与TH分别为22.70—458.40 mg·L−1和9.1—440.1 mg·L-1,平均含量分别为254.03 mg·L−1和220.88 mg·L−1,东西两区含量相差较大,标准偏差较大,主要源于东西两区地层岩性的不同。区内地下水水样的主要阳离子和阴离子的相对丰度分别为Ca2+>Mg2+>Na++K+和
HCO−3 SO2−4 表 2 水文地球化学组分统计表(mg·L−1)Table 2. Statistical table of hydrogeochemical composition分区Region 编号No. pH Na++K+ Ca2+ Mg2+ Cl− SO2−4 HCO−3 游离CO2 Free carbon dioxide 侵蚀性CO2 Corrosive carbon dioxide TH TDS 取样地层Sampling stratum 东区 SY01 7.79 14.44 50.60 33.00 2.83 8.61 342.20 2.07 2.13 262.20 280.60 D3zg SY02 7.27 6.09 51.57 34.18 4.16 12.30 321.90 15.51 1.06 269.50 269.30 C1ds SY03 7.36 4.86 53.35 27.75 5.01 16.40 285.10 8.27 2.13 247.40 249.90 C1ds SY04 7.78 5.38 50.03 71.70 4.26 32.30 478.00 5.17 0.00 420.00 402.70 S3g SY05 7.39 0.19 64.77 60.60 4.68 26.00 460.70 23.78 4.26 411.10 386.60 D3zg SY06 7.50 17.92 21.86 34.82 11.59 5.96 278.60 14.99 0.00 197.90 221.40 D3zg SY07 7.63 140.74 18.54 10.07 2.65 18.60 452.30 2.59 0.00 87.70 416.80 C1ds SY08 7.64 4.66 69.95 5.89 1.62 6.02 244.50 6.20 0.00 198.90 210.40 C1ds SY09 7.54 9.00 80.88 47.88 1.99 22.10 478.90 10.86 0.00 399.00 401.30 C1b SY10 7.34 18.49 100.55 35.20 4.16 27.10 490.30 18.10 0.00 396.00 430.60 C1ds SY11 7.34 1.95 77.32 34.08 2.75 76.70 309.40 13.44 0.00 333.30 347.50 ∈2s SY12 7.70 70.84 7.04 39.78 1.79 40.60 354.40 7.24 0.00 181.30 337.20 S3g SY13 7.89 1.51 101.28 45.49 3.45 107.80 397.80 6.51 0.00 440.10 458.40 S3g SY14 7.58 4.41 70.84 46.16 1.96 31.40 415.80 9.82 112.87 366.90 362.60 S3g SY15 7.16 7.83 96.43 20.87 6.39 48.00 347.20 26.37 10.65 326.70 353.10 P1m SY16 7.20 1.00 92.46 19.45 5.46 35.60 327.20 21.20 10.65 310.90 317.50 P1m 西区 SY17 7.21 2.93 4.78 2.50 1.37 2.35 29.50 3.62 10.65 22.20 28.70 P2β SY18 6.90 5.76 10.44 2.06 1.68 1.08 53.20 9.82 17.04 9.10 47.60 P2β SY19 6.88 4.52 6.56 1.33 1.25 0.82 35.40 7.76 15.97 21.90 32.20 P2β SY20 7.33 3.28 5.18 1.96 1.37 1.91 29.50 2.07 7.45 21.00 28.50 P2β SY21 8.85 12.44 4.70 0.93 1.43 1.79 47.30 0.00 2.13 15.60 68.20 P2β 西区 SY22 7.65 31.92 95.53 16.52 1.42 2.26 453.20 3.10 5.32 306.60 374.20 P2β SY23 7.32 2.63 10.93 4.03 1.35 1.61 56.10 7.76 14.91 43.90 48.60 P2β SY24 9.18 4.71 2.43 1.42 1.51 1.05 23.10 0.00 12.78 11.90 22.70 P2β 最大值 9.18 140.74 101.28 71.70 11.59 107.80 490.30 26.37 112.87 440.10 458.40 最小值 6.88 0.19 2.43 0.93 1.25 0.82 23.10 0.00 0.00 9.10 22.70 平均值 7.56 15.73 47.83 24.90 3.17 22.02 279.65 9.43 9.58 220.88 254.03 标准偏差 0.52 30.44 36.62 20.75 2.36 26.34 171.28 7.37 22.77 155.06 153.82 变异系数 0.07 1.94 0.77 0.83 0.74 1.20 0.61 0.78 2.38 0.70 0.61 根据Piper图解[28](图2)分析,
HCO−3 SO2−4 HCO−3 HCO−3 HCO−3 HCO−3 2.2 地下水补给来源
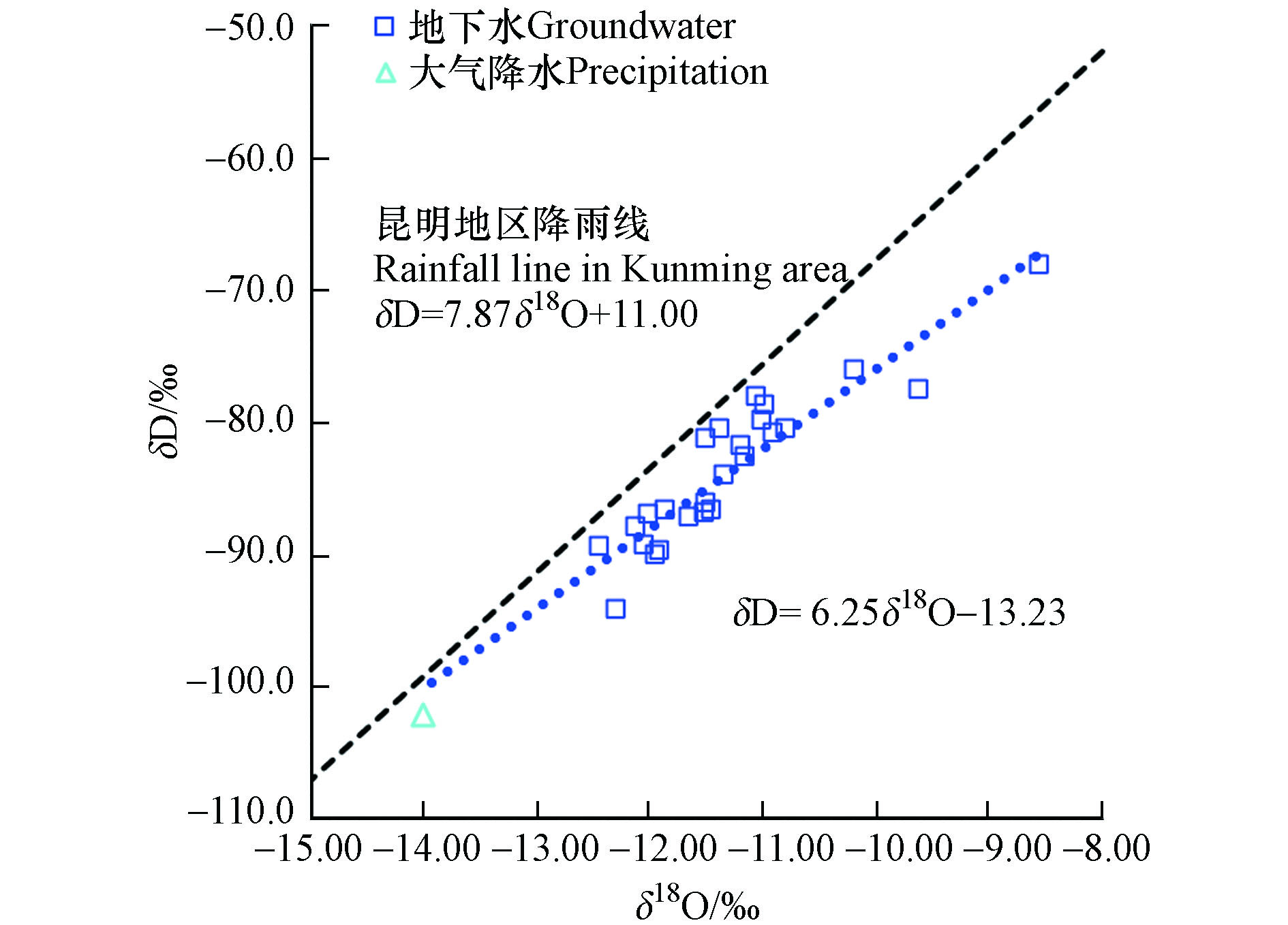
利用研究区地下水中δD和δ18O值绘制氢氧同位素特征关系图(图3),δ18O分布于−8.54‰—−14.00‰,均值为−11.39‰,δD分布于−68.20‰—−102.00‰,均值为−84.40‰,用最小二乘法得出拟合线方程为δD=6.25δ18O−13.23,r=0.8852,δD-δ18O关系方程斜率为6.25,小于昆明地区大气降雨线δD=7.87δ18O+11.00[29]的斜率7.87,有研究表明该地区受温度、雨量、高程、纬度等效应影响,使得降雨过程中δD与δ18O发生同位素分馏,使研究区δD与δ18O关系线斜率小于昆明地区大气降雨线[30]。区内各水样点位的δD与δ18O值间有较好的线性相关性,且略低于昆明地区大气降雨线,表明研究区内地下水的主要补给来源为大气降水,并受到一定程度的蒸发作用影响。
2.3 水化学形成作用与控制因素
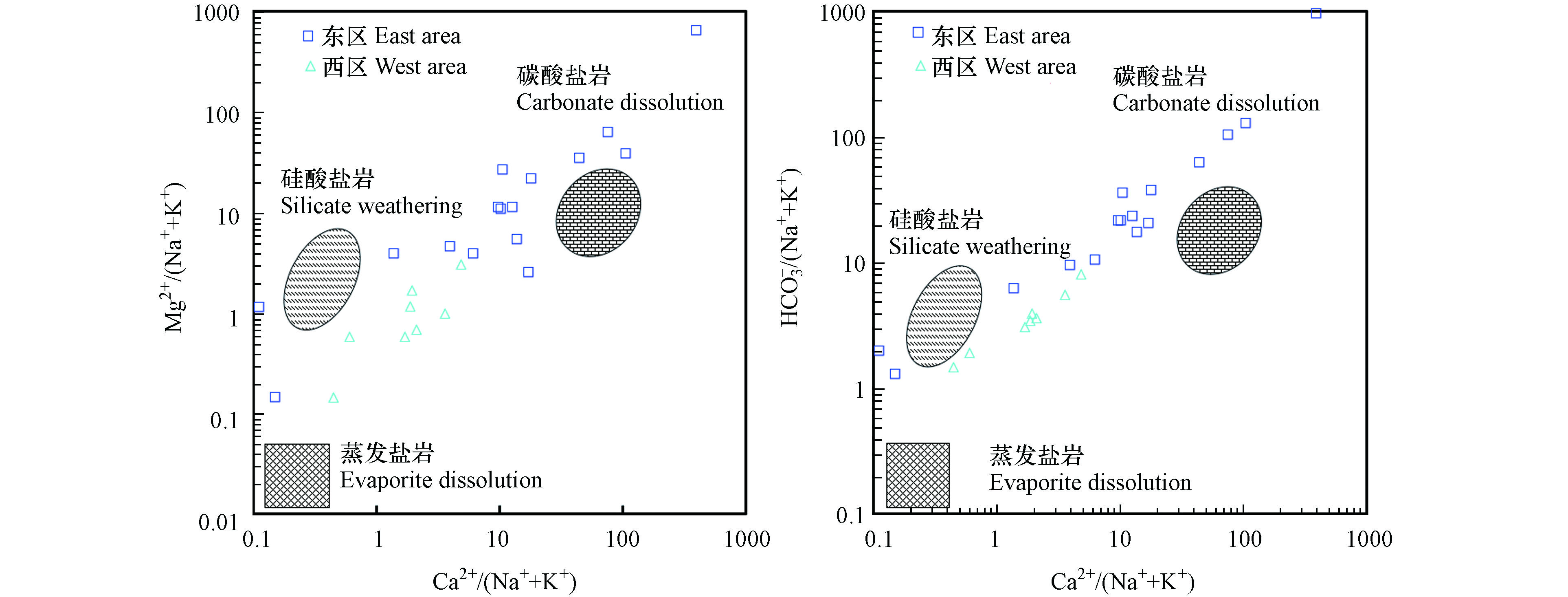
利用Gibbs图[32-33]指示水化学组分的形成作用,并结合Ca2+/(Na++K+)与Mg2+/(Na++K+)离子摩尔浓度比值关系图、Ca2+/(Na++K+)与
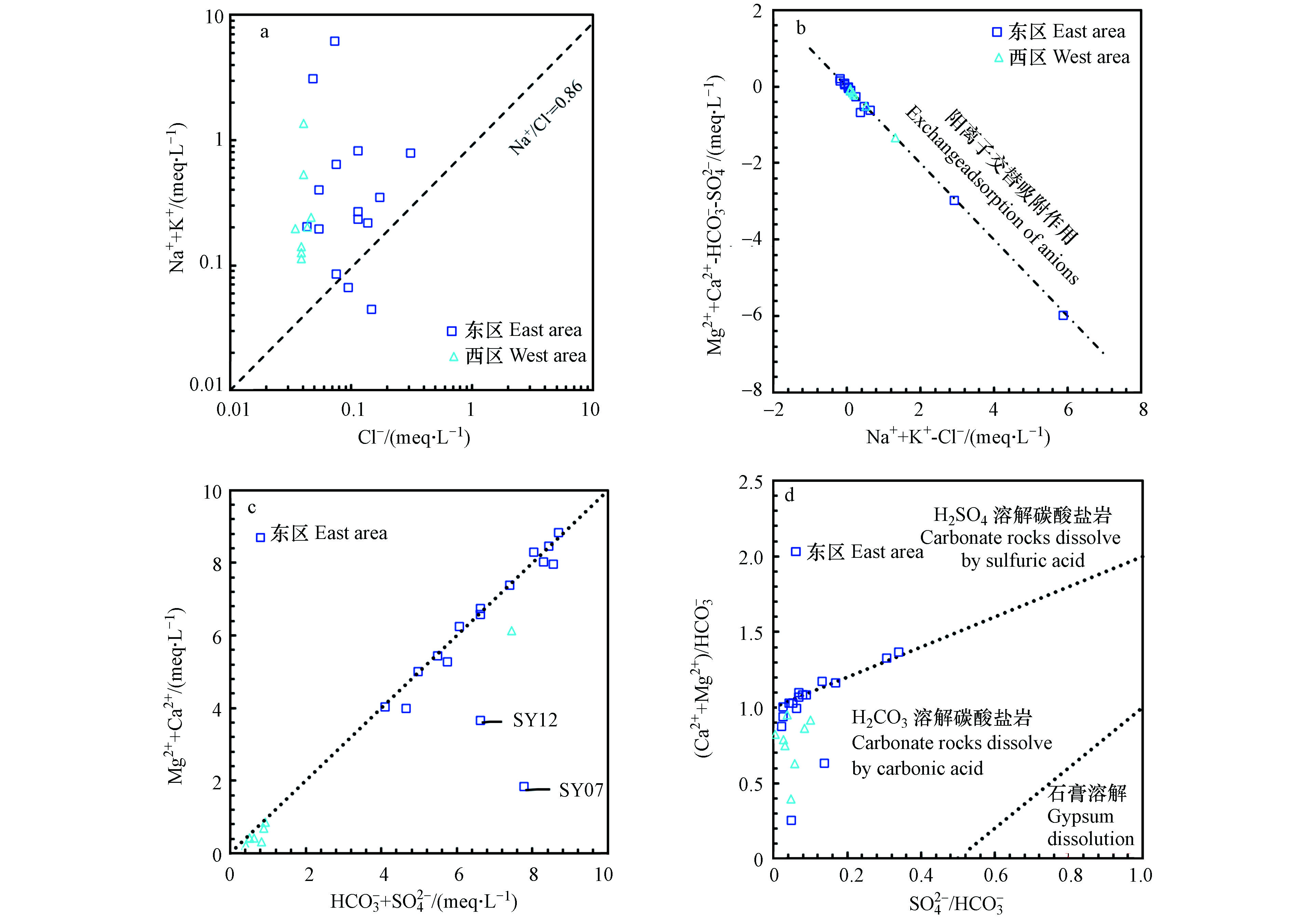
HCO−3 HCO−3 HCO−3 SY07与SY12两个水样的Na++K+离子的毫克当量比例分别可达86.62%和89.56%,SY07位于纯质碳酸盐岩地层(C1ds),与处于同地层相近的SY08水样点水化学组分相差较大,说明二者在径流途径与循环时间上有差异;SY07在泉口部位沉积了大量的泉华,表明发生脱碳酸作用,HCO3-与Ca2+、Mg2+离子形成碳酸盐沉淀,造成Ca2+、Mg2+离子浓度降低[35],同时该水样点附近有居民区,可能受到人类活动影响;SY12位于碳酸盐岩夹碎屑岩地层(S3g),Ca2+离子浓度较低,Na++K+离子浓度较高,说明岩溶水在径流途中可能发生阳离子交替吸附反应,Ca2+离子与围岩中的Na++K+发生了交换,同时该水样点附近有居民区,可能受到人类活动影响。
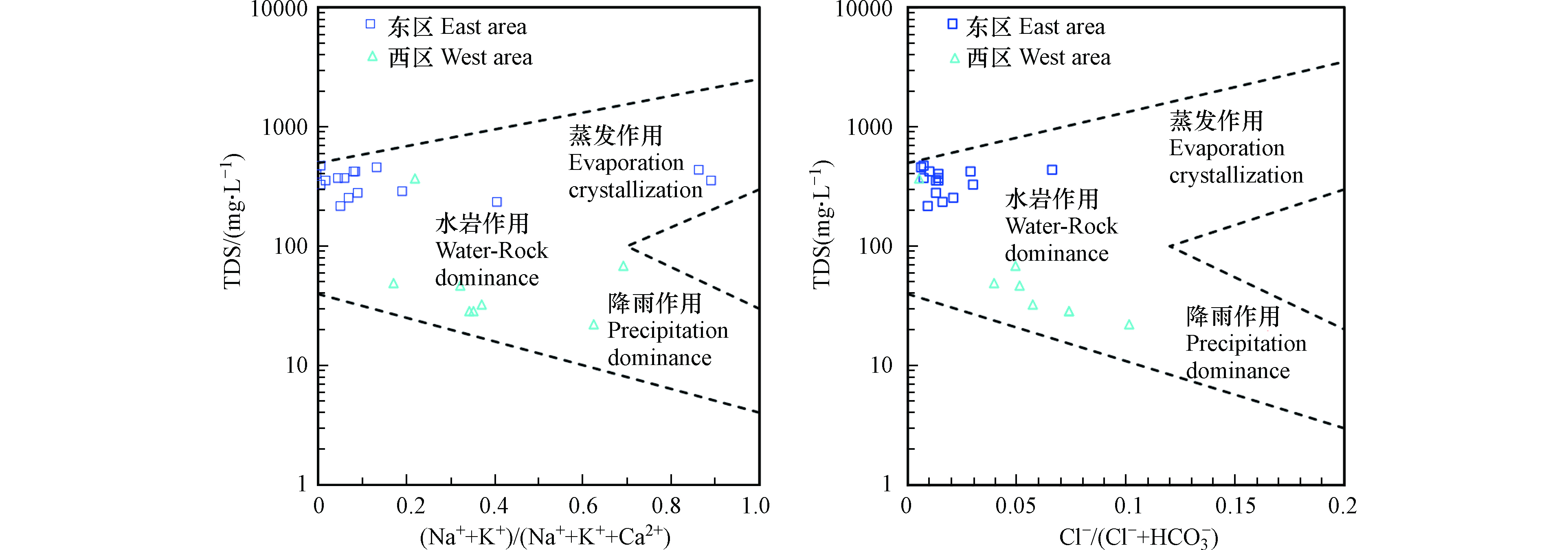
西区水样(8组)的Cl−/(Cl−+
HCO−3 HCO−3 区内地下水中K++Na+的空间变异性较大,东西两区的变异系数分别为188.88%和116.85%,浓度范围分别为0.19—140.74 mg·L−1和2.63— 31.92 mg·L−1。Cl−浓度范围为1.25—11.59 mg·L−1,较低的含量可以认为其主要来源为大气降水,多数水化学样的Na++K+的浓度高于Cl−,且有91.67%的地下水水样分布在雨水线(Na+/Cl−=0.86[36])的上方(图6a),表明其主要来源大气降雨外还有其他来源,盐岩与硅铝酸盐矿物的风化溶解和阳离子交替吸附作用也可能引起Na++K+浓度高于Cl−。通常可用[Ca2++Mg2+−
HCO−3 SO2−4 HCO−3 SO2−4 Pearson相关系数可以表示变量间的线性关系[38]。表3列出了区内水化学组分之间的Pearson相关系数,采用SPSS 19 for Windows系统软件计算。TDS是反映地下水中化学组分多寡的综合指标,与Ca2+、Mg2+、
HCO−3 SO2−4 CaCO3+CO2+H2O=Ca2++2
HCO−3 CaMg(CO3)2+2CO2+2H2O=Ca2++Mg2++4
HCO−3 Ca2+与
SO2−4 HCO−3 SO2−4 HCO−3 SO2−4 SO2−4 HCO−3 SO2−4 HCO−3 HCO−3 HCO−3 表 3 水化学离子Pearson相关系数Table 3. Pearson correlation coefficient of physiochemical parametersNa++K+ Ca2+ Mg2+ Cl− SO2−4 HCO−3 TH TDS pH Na++K+ 1 Ca2+ −0.208 1 Mg2+ −0.076 0.509* 1 Cl− −0.061 0.267 0.396 1 SO2−4 −0.029 0.583** 0.531** 0.184 1 HCO−3 0.298 0.739** 0.792** 0.341 0.486* 1 TH −0.162 0.877** 0.859** 0.380 0.642** 0.882** 1 TDS 0.297 0.775** 0.770** 0.327 0.646** 0.978** 0.891** 1 pH 0.084 −0.190 −0.051 −0.180 −0.040 −0.103 −0.131 −0.086 1 注:*P<0.05;**P<0.01. 3. 结论(Conclusion)
(1)研究区水化学类型主要是以HCO3-Ca·Mg型和HCO3-Mg·Ca为主,水岩作用控制区内的水化学类型。HCO3-和Ca2+是区内主要阴阳离子,分别占89.45%和46.42%,反映了碳酸盐岩溶解对区内水化学特征的控制作用,部分受硅酸盐岩溶解影响。
(2)δD与δ18O关系显示,研究区内地下水主要受大气降水补给。区内地下水化学形成过程受地层岩性影响较为显著,东、西两区地下水水化学特征差异较大。东区主要为碳酸盐岩地层,受水岩作用控制,岩溶发育;西区则是以玄武岩地层为主,溶解性能较差,受大气降水作用的影响更加显著。
(3)Mg2+、Ca2+、
HCO−3 SO2−4 (4)上述结果为对牛栏江-滇池补水工程区的水化学特征、水质保护和滇池生态恢复提供了参考依据,但后续研究工作仍需结合其他方法进一步开展。
-
表 1 厌氧批次实验设计
Table 1. Design of anaerobic batch experiments
mg·L−1 实验组别 接种污泥 BYD 碱度(以CaCO3计) NH4Cl KH2PO4 K2HPO4 SO42− 颗粒污泥组 厌氧颗粒污泥 500 1 000 140 30 30 — 污泥复配组 厌氧颗粒污泥与活性污泥复配a 500 1 000 140 30 30 — 外加硫酸盐组 厌氧颗粒污泥与活性污泥复配a 500 1 000 140 30 30 1 670 注:厌氧颗粒污泥与活性污泥浓度比为2:1。 表 2 厌氧UASB反应器不同阶段的运行参数
Table 2. Operating parameters of anaerobic UASB reactors at different stages
阶段 HRT(d) 有机负荷(以COD计) COD/SO42−(质量比) BYD(mg·L−1) 葡萄糖(mg·L−1) TCOD(mg·L−1) SO42−(mg·L−1) S1 10 0.5 — — 4 686 5 000 — S2 10 1 — 2 980 4 686 10 000 — S3 10 1 10 2 980 4 686 10 000 1 000 S4 10 1 5 2 980 4 686 10 000 2 000 S5 10 1 1 2 980 4 686 10 000 10 000 S6 — — — — — — — -
[1] 崔小明. 1, 4-丁二醇生产技术及国内外市场分析[J]. 精细石油化工进展, 2012, 13(10): 32-38. doi: 10.3969/j.issn.1009-8348.2012.10.009 [2] 柳赛锋, 彭文才, 代斌. 不同介孔载体负载Cu催化合成1, 4-丁炔二醇[J]. 石河子大学学报(自然科学版), 2018, 36(3): 358-362. [3] 王凤阳. 雷尼镍催化1, 4-丁炔二醇选择加氢与工艺优化 [D]. 大连: 大连理工大学, 2021. [4] 张萍. 浅谈1, 4-丁二醇生产工艺及其技术进展[J]. 化工管理, 2016(26): 238-238. doi: 10.3969/j.issn.1008-4800.2016.26.202 [5] 张静, 左童久, 陆江银. 分子筛镍基催化剂对1, 4-丁炔二醇加氢制1, 4-丁烯二醇催化性能研究[J]. 石油炼制与化工, 2021, 52(9): 25-30. [6] WEISSERMEL K, ARPE H J. Industrielle Organische Chemie: Bedeutende Vor-und Zwischenprodukte [M]. VCH-Verlag-Ges, 1998. [7] 余博, 田哲, 池勇志, 等. 土霉素对剩余污泥中温厌氧消化的短期和长期影响[J]. 环境工程学报, 2016, 10(4): 2009-2015. [8] 徐威. 环境微生物学 [M]. 北京: 中国建材工业出版社, 2017: 112-115. [9] 夏涛, 陈立伟, 蔡天明, 等. 硫酸盐还原菌促进厌氧消化中丙酸转化的研究[J]. 环境科学与技术, 2009, 32(5): 40-43. doi: 10.3969/j.issn.1003-6504.2009.05.010 [10] O'FLAHERTY V, COLOHAN S, MULKERRINS D, et al. Effect of sulphate addition on volatile fatty acid and ethanol degradation in an anaerobic hybrid reactor. II: microbial interactions and toxic effects[J]. Bioresource Technology, 1999, 68(2): 109-120. doi: 10.1016/S0960-8524(98)00146-1 [11] GOTVAJN A G, ZAGORC-KONCAN J. Comparison of biodegradability assessment tests for chemical substances in water[J]. Water Science & Technology, 1996, 33(6): 207-212. [12] TISLER T Z-K J. Aquatic toxicity of selected chemicals as a basic criterion for environmental classification[J]. Archives of Industrial Hygiene and Toxicology, 2003, 54(3): 207-213. [13] 陈庆磊, 张书良. 1, 4-丁二醇生产废水处理工艺的研究[J]. 河南化工, 2015, 32(4): 26-27. [14] KONG Z, LI L, LI Y Y. Long-term performance of UASB in treating N, N-dimethylformamide-containing wastewater with a rapid start-up by inoculating mixed sludge[J]. Science of the Total Environment, 2019, 648: 1141-1150. doi: 10.1016/j.scitotenv.2018.08.161 [15] 国家环境保护总局. 水和废水监测分析方法[M]. 4版. 北京: 中国环境科学出版社, 2002. [16] WANG Z, CHEN Z, KOWALCHUK G A, et al. Succession of the resident soil microbial community in response to periodic inoculations[J]. Applied and Environmental Microbiology, 2021, 87(9): e00046. [17] KONG Z, LI L, KURIHARA R, et al. Anaerobic treatment of N, N-dimethylformamide-containing wastewater by co-culturing two sources of inoculum[J]. Water Research, 2018, 139: 228-239. doi: 10.1016/j.watres.2018.03.078 [18] 成建国. 高效硫酸盐还原菌群的筛选、固定化及其在玉米浆脱硫中的应用 [D]. 武汉: 华中农业大学, 2023. [19] 张亚丽. SRB去除凉果废水中高浓度有机物与硫酸盐的作用机制 [D]. 广州: 广州大学, 2023. [20] HAO T W, XIANG P Y, MACKEY H R, et al. A review of biological sulfate conversions in wastewater treatment[J]. Water Research, 2014, 65: 1-21. doi: 10.1016/j.watres.2014.06.043 [21] WU J, NIU Q, LI L, et al. A gradual change between methanogenesis and sulfidogenesis during a long-term UASB treatment of sulfate-rich chemical wastewater[J]. Science of the Total Environment, 2018, 636: 168-176. doi: 10.1016/j.scitotenv.2018.04.172 [22] 刘少友, 唐文华, 邹勇, 等. 镍-磷-碳-氧化学镀层的制备、表征及1, 4-丁炔二醇在体系中的作用[J]. 材料保护, 2006, 39(9): 10-14. doi: 10.3969/j.issn.1001-1560.2006.09.004 [23] HU Y, JING Z, SUDO Y, et al. Effect of influent COD/SO42− ratios on UASB treatment of a synthetic sulfate-containing wastewater[J]. Chemosphere, 2015, 130: 24-33. doi: 10.1016/j.chemosphere.2015.02.019 [24] LOPEZ-CORTES A, FARDEAU M L, FAUQUE G, et al. Reclassification of the sulfate- and nitrate-reducing bacterium Desulfovibrio vulgaris subsp. oxamicus as Desulfovibrio oxamicus sp. nov. , comb. nov[J]. International Journal of Systematic and Evolutionary Microbiology, 2006, 56(7): 1495-1499. [25] JOHNSON L A, HUG L A. Cloacimonadota metabolisms include adaptations in engineered environments that are reflected in the evolutionary history of the phylum[J]. Environmental Microbiology Reports, 2022, 14(4): 520-529. doi: 10.1111/1758-2229.13061 [26] HARMSEN H J, VAN KUIJK B L, PLUGGE C M, et al. Syntrophobacter fumaroxidans sp. nov. , a syntrophic propionate-degrading sulfate-reducing bacterium[J]. International Journal of Systematic and Evolutionary Microbiology, 1998, 48(4): 1383-1387. [27] WALLRABENSTEIN C, HAUSCHILD E, SCHINK B. Syntrophobacter pfennigii sp. nov. , new syntrophically propionate-oxidizing anaerobe growing in pure culture with propionate and sulfate[J]. Archives of Microbiology, 1995, 164: 346-352. [28] YU R Q, BARKAY T. Microbial mercury transformations: Molecules, functions and organisms[J]. Advances in Applied Microbiology, 2022, 118: 31-90. [29] CHEN S, LIU X, DONG X. Syntrophobacter sulfatireducens sp. nov. , a novel syntrophic, propionate-oxidizing bacterium isolated from UASB reactors[J]. International Journal of Systematic and Evolutionary Microbiology, 2005, 55(3): 1319-1324. [30] 周爱娟, 强海峰, 谭慧杰, 等. 一种不完全氧化型硫酸盐还原菌定向筛选和富集的方法: ZL202310440504.4[P]. 2023-04-23. [31] KUEVER J, RAINEY F A, WIDDEL F. Desulforhabdus [M]. Bergey's Manual of Systematics of Archaea and Bacteria. 2015: 1-3. [32] WU J, LIU Q, FENG B, et al. Temperature effects on the methanogenesis enhancement and sulfidogenesis suppression in the UASB treatment of sulfate-rich methanol wastewater[J]. International Biodeterioration & Biodegradation, 2019, 142: 182-190. [33] MOTTERAN F, NADAI B M, BRAGA J K, et al. Metabolic routes involved in the removal of linear alkylbenzene sulfonate (LAS) employing linear alcohol ethoxylated and ethanol as co-substrates in enlarged scale fluidized bed reactor[J]. Science of the Total Environment, 2018, 640-641: 1411-1423. doi: 10.1016/j.scitotenv.2018.05.375 [34] HAMDI O, BEN HANIA W, POSTEC A, et al. Isolation and characterization of Desulfocurvus thunnarius sp. nov. , a sulfate-reducing bacterium isolated from an anaerobic sequencing batch reactor treating cooking wastewater[J]. International Journal of Systematic and Evolutionary Microbiology, 2013, 63(11): 4237-4242. [35] KLOUCHE N, BASSO O, LASCOURREGES J-F, et al. Desulfocurvus vexinensis gen. nov. , sp. nov. , a sulfate-reducing bacterium isolated from a deep subsurface aquifer[J]. International Journal of Systematic and Evolutionary Microbiology, 2009, 59(12): 3100-3104. [36] COLIN Y, GOÑI-URRIZA M, CAUMETTE P, et al. Combination of high throughput cultivation and dsrA sequencing for assessment of sulfate-reducing bacteria diversity in sediments[J]. FEMS Microbiology Ecology, 2013, 83(1): 26-37. doi: 10.1111/j.1574-6941.2012.01452.x [37] REIS M, ALMEIDA J, LEMOS P, et al. Effect of hydrogen sulfide on growth of sulfate reducing bacteria[J]. Biotechnology and Bioengineering, 1992, 40(5): 593-600. doi: 10.1002/bit.260400506 [38] 李健, 王文菊, 东志强, 等. 高机硫和硫酸盐含量高COD制药废水处理工程[J]. 水处理技术, 2018, 44(11): 133-135. -






 DownLoad:
DownLoad: