-
我国每年玉米秸秆产量巨大,实现秸秆的资源化利用,不仅可以有效解决秸秆资源结构性过剩问题,还能推动农业生产向绿色、高效、可持续发展的道路迈进。而秸秆生物乙醇,是近年来蓬勃兴起的战略性新兴产业[1-2];作为一种替代的新型可再生绿色能源,具有良好的经济效益和环境效益[3-4]。以秸秆废弃物为代表的木质纤维素类生物质具有产量大、分布广、可再生、价格低等特点,是生物燃料乙醇产业规模化发展的重要原料来源[5]。
秸秆中木质纤维素的复杂结构限制了其高效利用,需对原料先进行预处理后再进行糖化发酵产乙醇[6-8]。吴亚林[9]采用微波辅助小分子有机酸对玉米秸秆进行预处理,发现预处理可以去除木质素并有效提高酶水解的效果。LI等[10]研究了NaOH-纤维素酶联合预处理对玉米秸秆降解速率和还原糖产量的影响,提到该预处理可以有效增强玉米秸秆厌氧消化性能。不同的预处理方法各有利弊,其中生物法预处理凭借环境友好,成本低,副产物少等优势脱颖而出;然而从自然界分离得到的野生菌种产酶能力较弱、稳定性差,将其用于生物预处理效率较低、难以适应工业化生产的要求[11];针对生物法预处理存在的问题,有研究者通过诱变手段对菌种遗传物质进行改造,选育符合要求的高产菌株以强化生物预处理。目前常用的菌株诱变方式主要分为物理诱变和化学诱变两大类[12],物理诱变技术中最常用的就是紫外诱变,该方法操作简单,速度快,对设备要求较低,主要通过破坏DNA的连接,使其形成胸腺嘧啶二聚体,阻碍碱基间的正常配对,从而引起突变或死亡;如刘庆玉等[13]采用紫外诱变筛选获得高效木质素降解菌株,木质素降解率较出发菌株提高了13.6%。化学诱变剂主要有烷化剂、叠氮化物及碱基类似物等,其中甲基磺酸乙酯 (EMS) 是一种高效稳定的烷化剂,可通过烷基化反应诱发嘌呤和嘧啶之间的转换突变,广泛应用于微生物及植物作物的诱变选育中,具有较高的突变频率。ALI等[14]采用不同浓度的EMS对野生菌株lsl-3进行诱变,得到突变菌株EMS-cys2的乙酰木聚糖酯酶活性较野生菌株有明显增强。
以上研究表明,紫外及甲基磺酸乙酯等诱变手段对于提高微生物酶活性具有一定作用,但目前大多研究集中在不同诱变剂的单一诱变,对于木质素降解菌株的复合诱变选育研究较少,刘登辉[15]采用复合诱变手段选育纤维素酶高产菌株,结果表明复合诱变具有协同效应,较单一诱变效果更好。ETHIRAJ等[16]研究发现UV-EMS复合诱变获得的突变菌株蛋白酶活性 (3 437.6 EU·mL−1) 高于单独的UV诱变 (3 234.9 EU·mL−1) 和单独的EMS诱变菌株酶活性 (2 797 EU·mL−1) ,复合诱变有利于正向突变的累积从而提高诱变效率。因此,本研究以自然筛选出的木质素降解菌株——皮特不动杆菌Acinetobacter pittii FM1为出发菌株,采用紫外线和甲基磺酸乙酯 (UV-EMS) 复合诱变手段进行菌株的诱变选育,并添加突变菌株对秸秆进行预处理及发酵产乙醇试验研究,以期为秸秆的生物法预处理发展提供参考。
-
实验用玉米秸秆取自河北省石家庄市周边农田,风干玉米秸秆粉碎过20目筛后于室温下密封保存。测定其纤维素、半纤维素及木质素含量分别为42.14%±0.40% 、31.02%±0.42% 、14.85%±0.35% 。菌株为Acinetobacter pittii FM1,从环境土壤中筛选保存。实验中所用培养基及其配制如表1所示。
-
1) UV诱变。取出发菌株FM1的单孢子悬浮液10 mL,置于直径为9 mm带转子的无菌培养皿中,并在磁力搅拌下用功率20 W的紫外灯在固定距离30 cm处进行不同时间的照射,暗处理10 min后置于4 ℃冰箱内1.5 h降低回复突变。取不同照射时间的处理液稀释后涂布在LB培养基上,28 ℃恒温避光培养24 h后进行菌落计数,并计算致死率,绘制紫外诱变致死曲线。
2) EMS诱变。吸取2 mL单孢子悬浮液于灭菌的10 mL离心管中,然后立即加入2 mL一定浓度的EMS溶液 (用0.1 mol·L−1的PBS缓冲液配制,0.22 μm滤膜过滤除菌) ,摇晃使其充分混合,设置未加EMS的空白对照组。诱变处理结束后加入等体积的2% Na2S2O3终止反应[17],适当稀释后涂布培养同实验方法1) 中所述。
3) EMS诱变条件优化。采用控制单一变量法分别设置不同的EMS诱变剂体积分数、诱变时间、诱变温度及菌悬液浓度,开展单因素试验,根据单因素试验的结果设计L9 (43) 的正交试验,对诱变条件进一步优化。正交试验设计如表2所示。
4) 复合诱变。根据实验所得最佳条件,对菌株进行UV-EMS的复合诱变。将获得的突变菌株点接于苯胺蓝固体培养基上,根据褪色圈直径D及菌落直径d比值大小进行初筛;之后接种于木质素降解复筛培养基中于30 ℃,120 r·min−1下培养,每隔4 h取样1次并参照诸葛荣夏[18]和GANESH等[19]所述方法测定木质素过氧化物 (Lip) 酶活、锰过氧化物 (Mnp) 酶活,采用紫外分光光度计 (UV-5100,上海元析仪器有限公司) 在280 nm波长处测定吸光度,计算木质素磺酸钠含量及木质素降解率。将筛选获得的菌株多次传代培养,保证突变菌株的遗传稳定性。
5) 秸秆预处理。取5 g粉碎玉米秸秆加入250 mL的锥形瓶中,按照料液比1∶10加入无机盐培养液,接种菌液体积分数为10%,并在30 ℃,120 r·min−1条件下恒温培养。每隔12 h取样1次,5 000 r·min−1离心10 min去上清液保留残渣,置于烘箱中105 ℃烘干至恒重,采用全自动纤维素分析仪 (ANKOM 2000i,北京中科华星科贸有限公司) 测定秸秆组分含量,并参照李红亚[20]所述方法计算木质素降解率变化。
6) 同步糖化发酵产乙醇。同步糖化发酵工艺 (SSF) ,即酶解糖化和发酵在同一反应容器中同时进行[21]。参照郭栋豪[22]所述工艺条件进行试验,采用3, 5-二硝基水杨酸比色法 (DNS法) 测定上清液中还原糖含量 (以葡萄糖计) [23],采用重铬酸钾-DNS氧化比色法测定乙醇含量[24-25]。
-
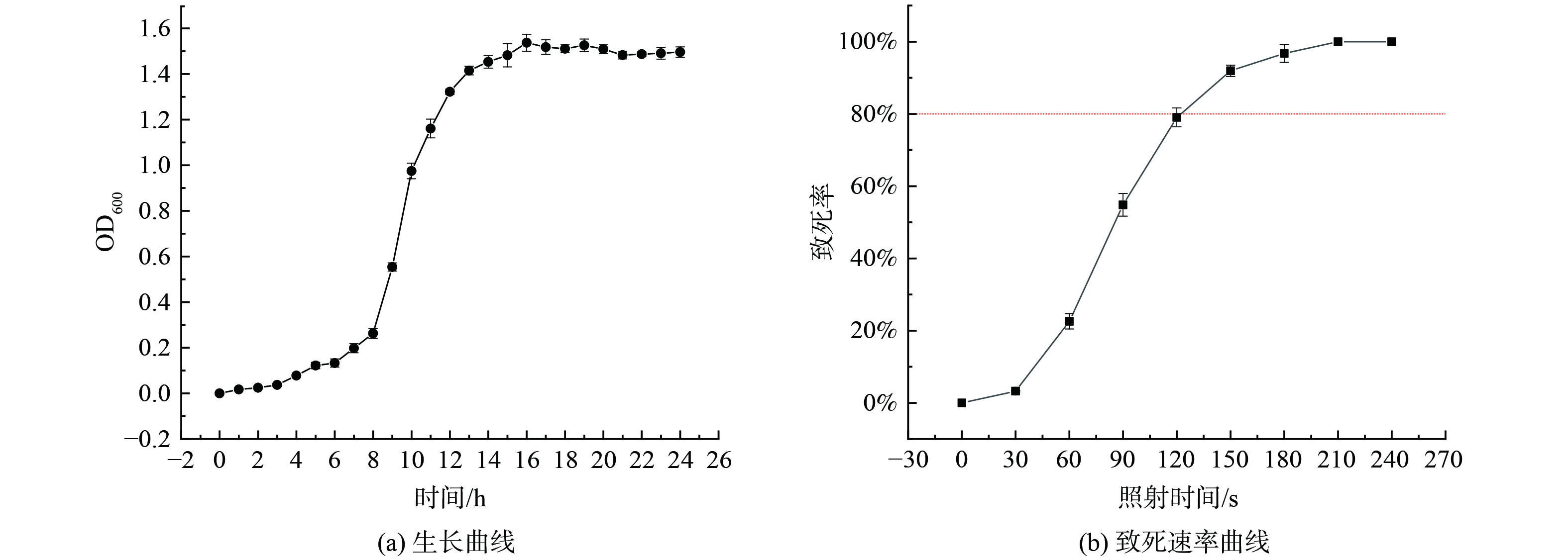
绘制菌株生长曲线如图1(a)所示,取培养8~12 h的对数期生长菌株进行紫外诱变,从图1(b)可得当照射时间为120 s时,紫外诱变致死率达到79.03%;在照射时间210 s时,致死率达到100%。有研究发现,在诱变致死率80%左右时正突变率较高[26],因此选取120 s作为后续紫外诱变照射时间。
-
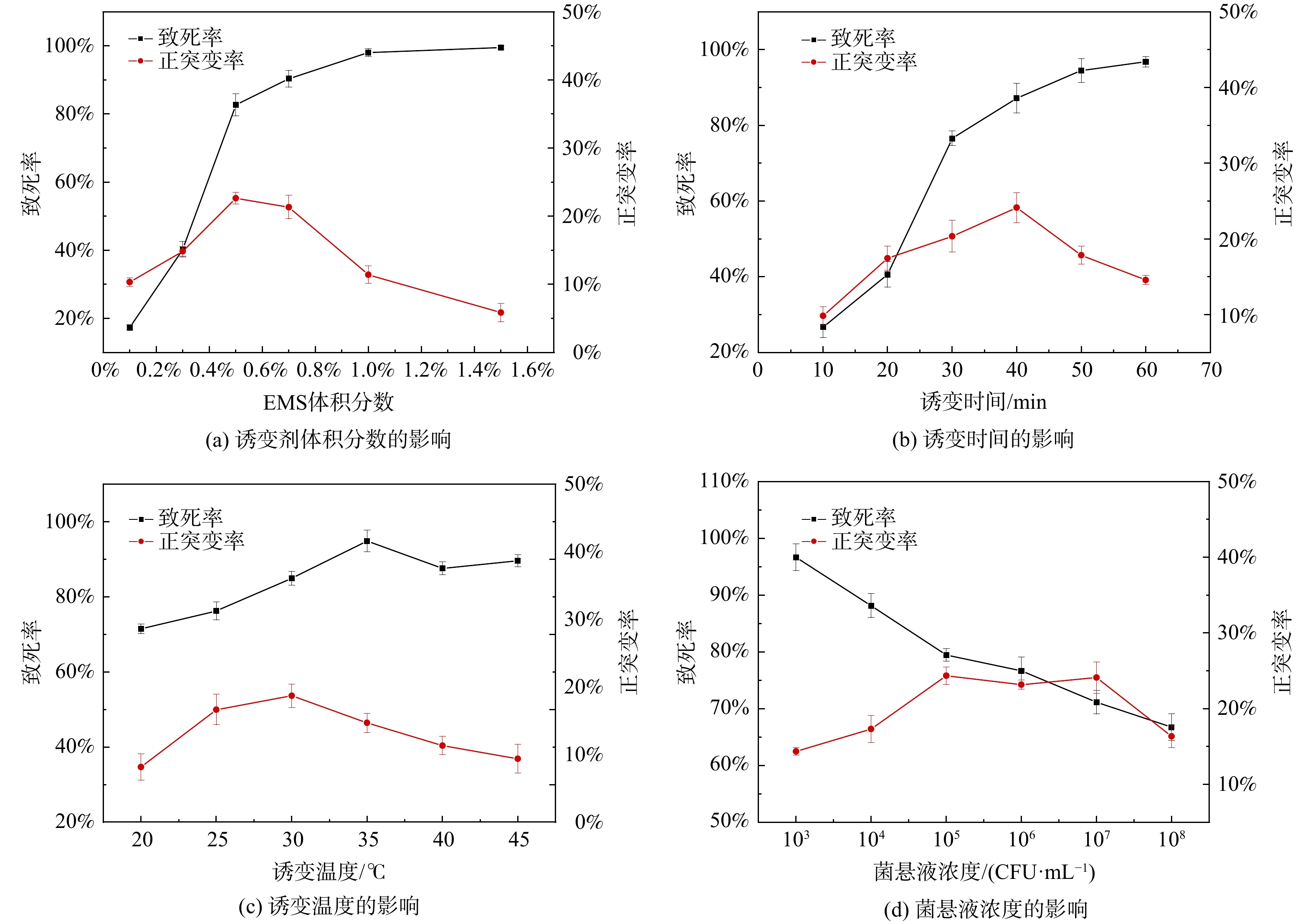
1) 单因素试验结果。不同因素对诱变致死率和正突变率的影响情况如图2所示。由图2(a)、(b)可知,诱变致死率随着EMS体积分数和诱变时间的增加总体呈上升趋势;当EMS体积分数为0.5%时,诱变致死率达到82.7%,此时的正突变率最高,为22.65%;之后随着诱变剂体积分数的提高,致死率缓慢提高,正突变率随之降低;表明EMS诱变剂对菌体的杀伤力具有剂量依赖性,且随EMS体积分数的增加而增强[27];当诱变时间在20~30 min时致死率变化最快,当诱变时间为40 min时,致死率达到87.2%,此时正突变率最高为24.13%。由图2(c)可知,诱变温度对诱变致死率的影响相对较小,致死率基本都在70%~95%之间;当温度大于35 ℃时,致死率明显下降,可能由于温度升高在加快诱变反应速度的同时促进了诱变剂的水解,一定范围内低温更有利于保持化学性质的稳定性。由图2(d)可知,随着菌悬液浓度增大,致死率总体呈下降趋势,原因可能是一定浓度的EMS诱变剂只能对相应的一定数量的菌体起作用;在菌悬液浓度为105~107 CFU·mL−1时,正突变率变化不大,浓度为105 CFU·mL−1时,致死率为74.95%,正突变率为24.35%。根据上述单因素试验结果确定正交试验中因素的种类和水平,进一步研究多因素交互作用并对实验条件进行优化。
2) 正交试验结果。正交试验结果如表3所示。由表3可知,在影响致死率的4个因素中,影响程度分别为,诱变剂体积分数>菌悬液浓度>诱变时间>诱变温度;通过单因素试验的结果可以发现,当诱变致死率在80%左右时,菌株的正突变的概率较高,因此选择本试验中诱变的最佳条件组合为A2B1C2D3,即为,诱变剂体积分数为0.5%、诱变时间为30 min、诱变温度为28 ℃、菌悬液浓度为107 CFU·mL−1,此时诱变致死率为81.23%。以最佳诱变条件进行结果验证性试验,得到菌株致死率为80.53%,正突变率为22.86%,进一步确定了正交试验结果的可靠性。
-
紫外诱变获得突变菌株共39株,其中正向突变菌株7株,表明紫外诱变对菌株遗传性状的影响具有随机性[28];其中测量得到突变菌株UV6的D/d值最大为3.14,是出发菌株FM1的1.24倍。因此选取菌株UV6进行后续EMS诱变处理。
1) 菌株初筛。复合诱变共获得诱变菌株58株,通过苯胺蓝平板初筛,获得了13株正向突变菌株,命名为UEM1~UEM13。测定菌株的褪色圈及菌落直径结果如表4所示。
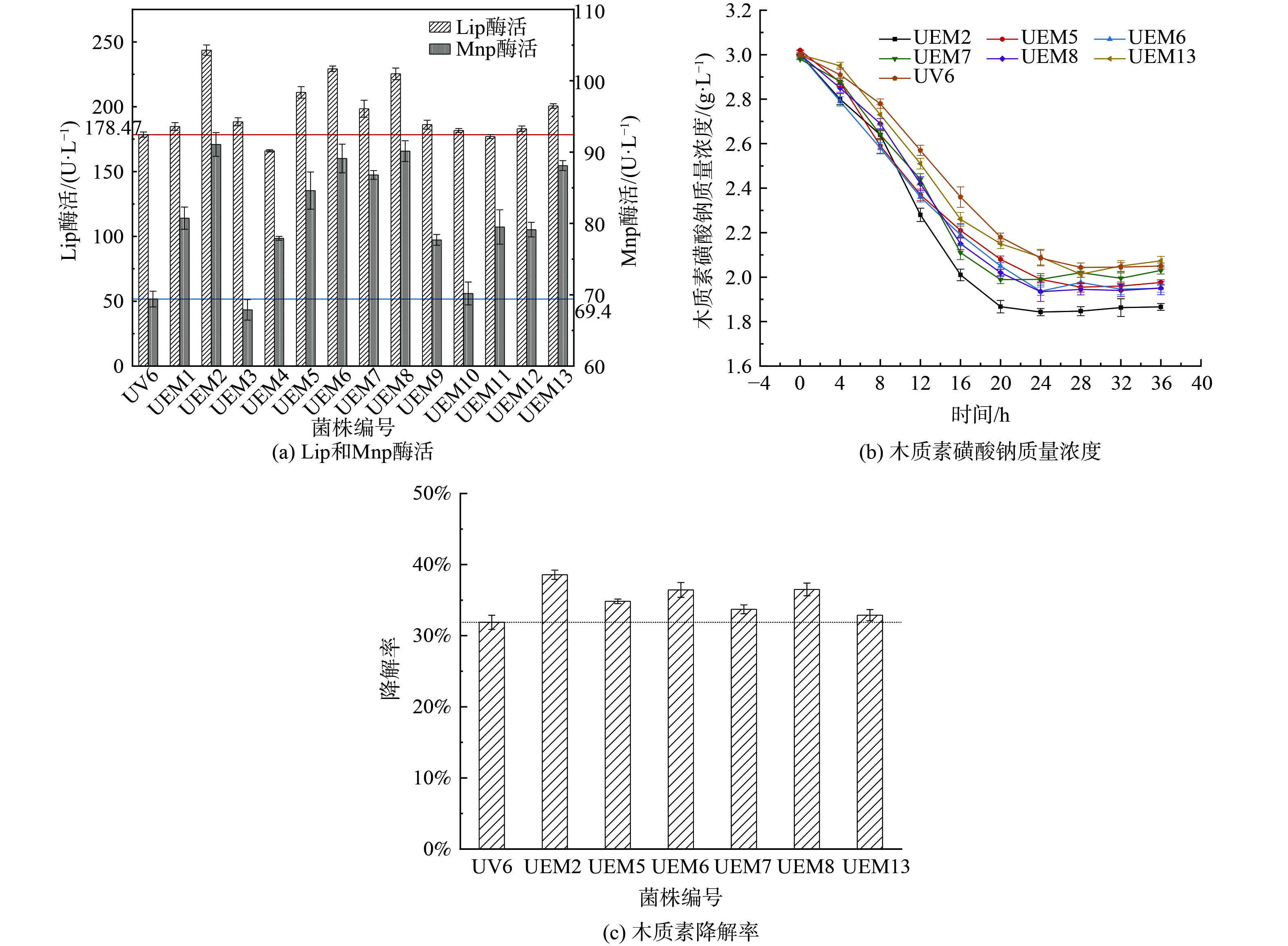
2) 菌株复筛。将上述13株突变菌株分别进行划线培养,接种至木质素降解复筛培养基中,每隔4 h取样1次,测定木质素降解率;在培养24 h后测定相应的木质素降解酶活力,结果如图3所示。木质素生物降解是一个多酶促反应过程,其中LiP和MnP酶是主要的木质素降解酶类,通过催化木质素中的芳香环及连接键发生断裂形成小分子片段,进而在一系列酶的协同作用下进入三羧酸循环,因此LiP和MnP酶活性的高低直接影响着木质素的生物降解。如图3(a)所示,通过对13株正向突变菌株木质素降解酶活的测定,发现Mnp酶活相对于Lip酶活较低,说明筛选菌株在木质素的降解过程中Mnp起主要作用;其中突变菌株UEM2菌株的酶活性最高,Lip (243.6 U·L−1) 和Mnp酶活性 (94.32 U·L−1) 相对于UV6菌株分别提高了36.4%和35.85%,相对于FM1菌株分别提高了58.8%和51.7%,说明复合诱变能够有效提高微生物菌种的产酶性能,且效果优于单一诱变。这与KHALID等[29]的研究结果一致。根据酶活的大小挑选出UEM2、UEM5、UEM6、UEM7、UEM8和UEM13这6株优势菌株,测定280 nm波长处的吸光度并计算不同菌株对木质素的降解能力,结果如图3(b)所示,反映的木质素的降解能力的强弱与酶活的大小基本保持一致;6株菌株在前20 h内均有较高的木质素降解能力,木质素磺酸钠质量浓度迅速下降,说明在该时间段可能是微生物对数生长期,生长活动旺盛,产酶能力较强。在培养20 h之后测定的木质素磺酸钠质量浓度缓慢下降后趋于平缓或出现反弹上升的趋势,由于280 nm波长实际上是苯环中m-位置的吸收波长[30],因此出现该现象的原因可能是当进入衰亡期,一方面生长代谢缓慢,微生物对木质素的降解能力变弱;另一方面可能菌体自溶产生的次级代谢产物中含有苯环结构的物质,从而显示出木质素磺酸钠质量浓度出现平缓或者反弹升高的现象,该结果与陈跃辉[31]实验结果类似。通过对比36 h内不同菌株木质素降解率的最高值,如图3(c)所示,可以发现UEM2突变菌株的木质素降解率达到38.57%,比UV6提高了20.98%。这也证明了物理化学复合诱变方式具有协同效应,比单一诱变效果更好[32]。因此根据初筛和复筛结果,选取UEM2菌株为最佳突变菌株。
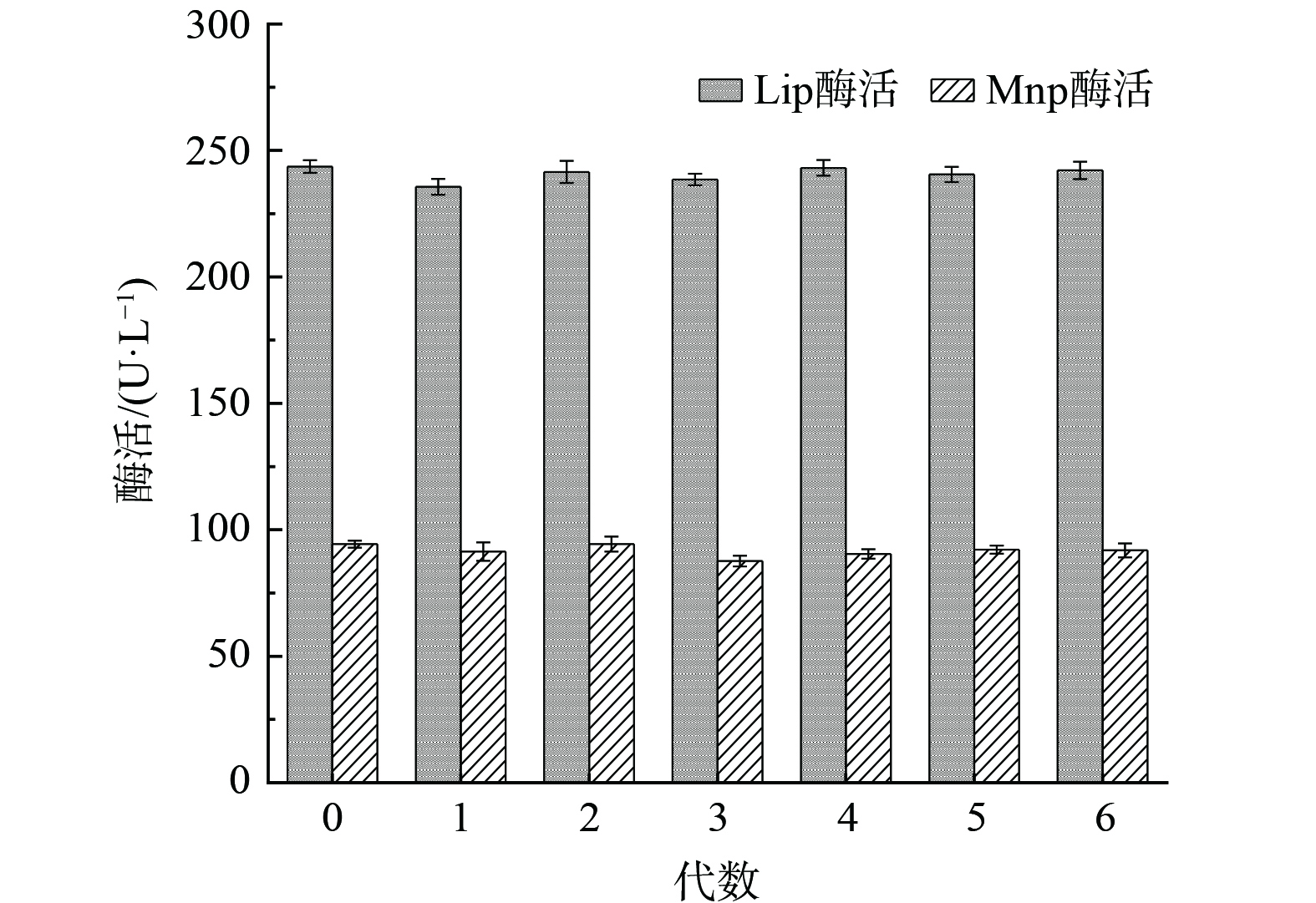
3) 遗传稳定性。由于回复突变情况的存在,突变菌株在连续多次传代培养时,可能会容易出现菌种生物量降低,酶活性减弱,菌种衰退及遗传标记丢失等问题[33]。因此对突变菌株UEM2进行连续多次的传代培养,观察形态学变化,并通过测定其Lip和Mnp酶活,验证其遗传稳定性,其结果见图4。由图4可知,对UEM2菌株同样条件下连续传代6次,随着传代次数的增加,突变菌株UEM2的各代菌株酶活性波动幅度不大,与第0代菌株保持持平或略低于0代,第6代菌株的Lip和Mnp活性分别为242.1和91.88 U·L−1,表明了突变菌株UEM2具备产酶能力强且遗传稳定性好等优良特性,这与XIA等[34]和WANG等[35]的研究结果一致。因此,选取UEM2菌株作为后续预处理试验菌株。
-
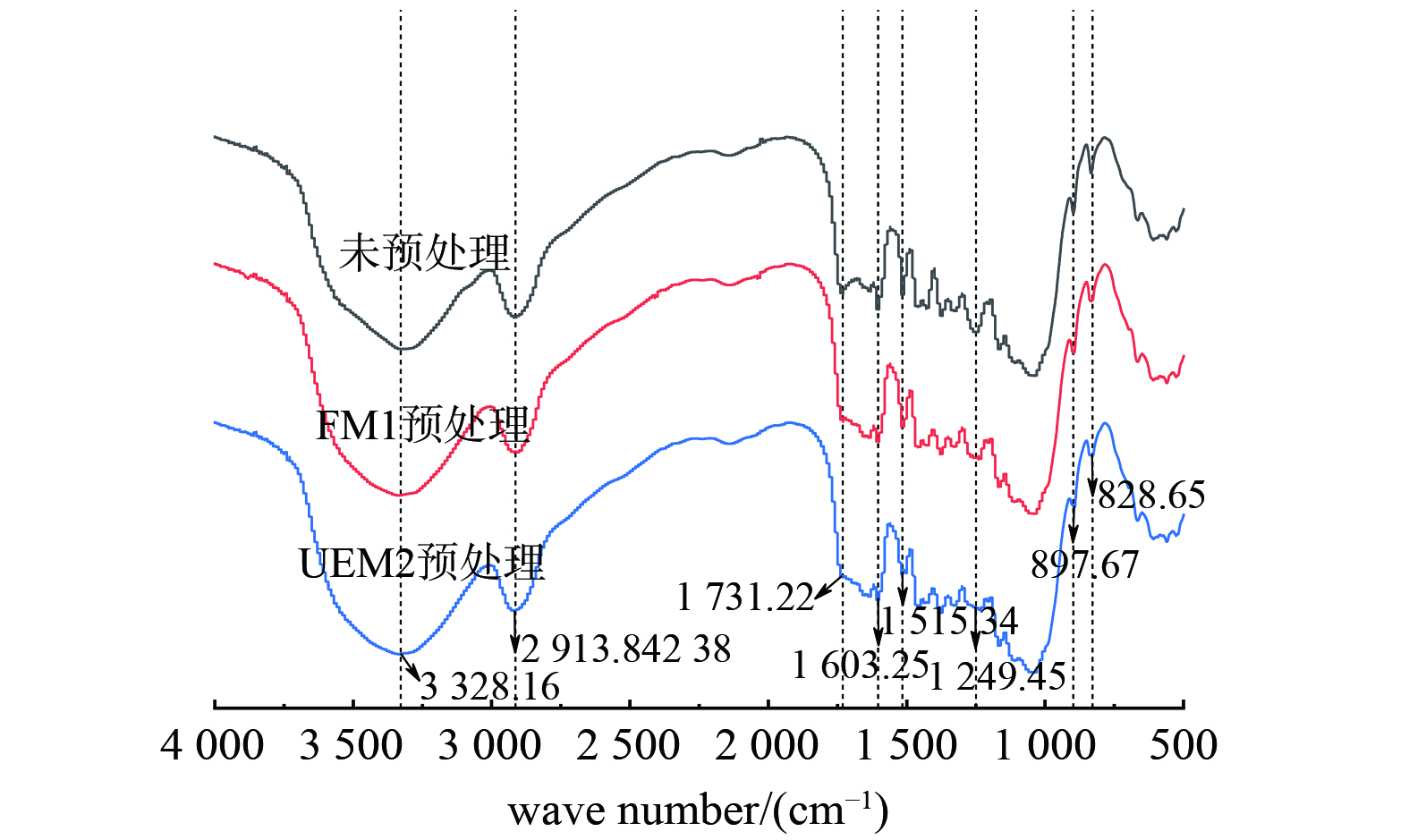
1) 秸秆残渣FTIR分析。分别对未预处理,添加FM1预处理,添加UEM2预处理之后的秸秆残渣进行FTIR分析,得到红外光谱如图5所示。由图5可知,在3 328.16、2 913.97、1 731.22、1 603.25、1 515.34、1 249.45、897.67及828.65 cm−1附近的典型特征峰,分别是由于秸秆中纤维素、半纤维素及木质素在甲基和亚甲基中CH生长振动及-OH的拉伸、乙酰基或羰基的 C=O 伸展、木质素的苯环C=C伸展和芳香骨架振动等所致[36]。通过观察对比发现,添加UEM2预处理组苯环特征吸收峰 (1 603、1 515 和1 249 cm−1) 附近的峰值相较于FM1预处理组和未预处理组明显减弱,说明该组秸秆木质素组分含量显著降低,这与伦晓中等[37]研究结果相似。在1 731 cm−1处的峰归因于半纤维素的乙酰基或木质素的阿魏酸和对香豆酸羧基的酯键,该峰在FM1光谱中下降,而在UEM2光谱中几乎消失,也说明秸秆中木质素得到了有效去除,且UEM2比FM1组去除效果更好。
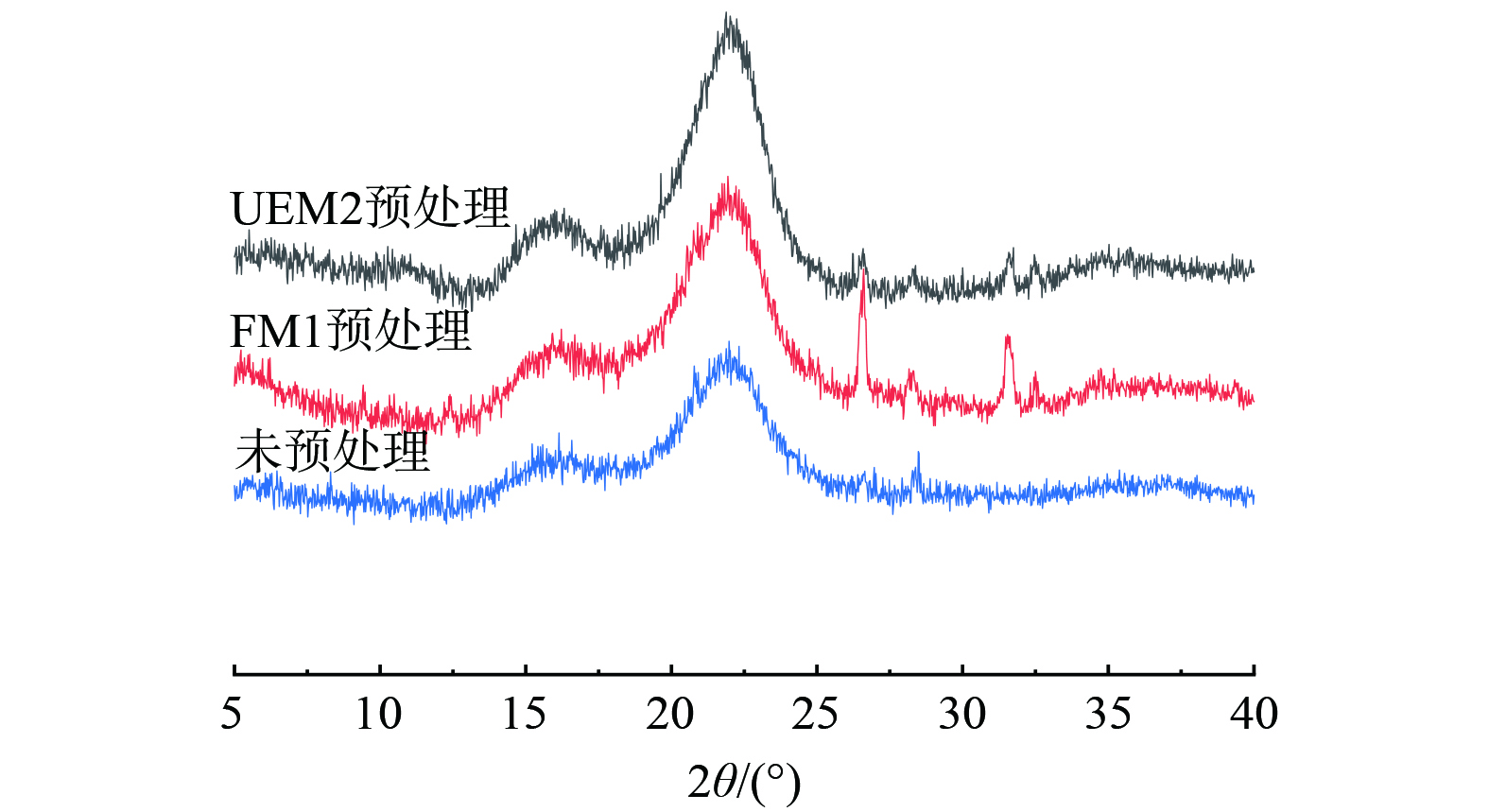
2) 秸秆残渣XRD分析。对预处理前后的秸秆残渣进行XRD分析,可用于研究秸秆纤维素结晶状态变化[38],结果如图6所示。由图6可知,衍射图2θ=22.02和16.1附近2个主要峰位,分别对应于纤维素I型结晶面 (002) 和结晶面 (101) ,使用Segal 方法计算结晶指数CrI,得到未预处理组、FM1组、UEM2组的CrI分别为41.68%、38.87%和32.49%。与前2组相比,UEM2预处理组的结晶指数有所下降,说明在秸秆预处理过程中破坏了纤维素的结晶结构,使得结晶度降低;另一方面也可能是预处理对半纤维素和木质素的去除,导致了纤维素结晶度的变化。通常情况下,对于秸秆的预处理方法大多是通过降低木质素和半纤维素组分对纤维素的包裹和覆盖率,提高纤维素酶对纤维素的可及面积从而提高酶解效率;而实际上纤维素本身的结晶度对于纤维素酶水解效率也存在一定的影响,纤维素酶的敏感性及作用效果与秸秆中纤维素的结晶度和聚合度直接相关。因此从XRD分析结果得出UEM2预处理组相比于其他两组结晶度最低,也说明了添加UEM2菌株对秸秆的预处理效果最好。
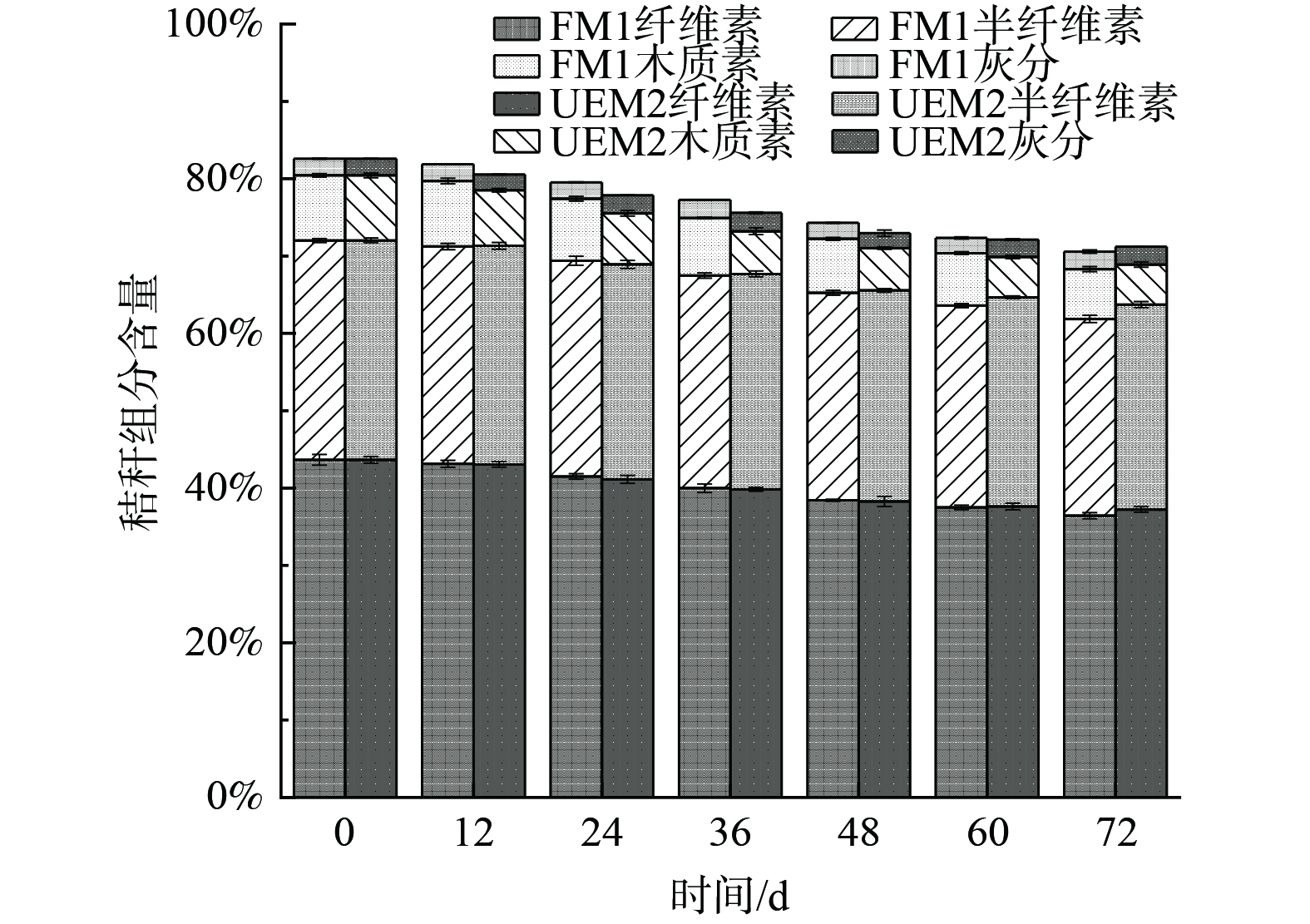
3) 秸秆预处理前后组分分析。定时取样测定预处理过程中秸秆组分含量的变化,结果如图7所示。由图7可知,FM1组木质素含量由8.46%下降至6.45%,秸秆木质素降解率为23.76%;而相同时间内UEM2组木质素含量下降至5.17%,降解率达到38.86%。木质素的降解率在一定程度上反映着玉米秸秆的预处理效果,木质素降解率越高,意味着有越多的被包裹着的纤维素暴露出来,使得纤维素酶的可及表面积增大,从而获得更好的预处理效果。在木质素降解的过程中,秸秆半纤维素的含量基本没有变化,而两组纤维素的含量均有下降趋势,降解木质素的同时部分纤维素也被降解,其中FM1组纤维素含量由43.65%下降至36.43%,损失率为16.5%;UEM2组纤维素含量由43.65%下降至37.23%,损失率为14.7%,略低于FM1组。综上,无论从木质素降解率还是纤维素损失率角度进行对比,UV-EMS复合诱变获得的高酶活突变菌株对于秸秆的预处理均具有明显优势。
-
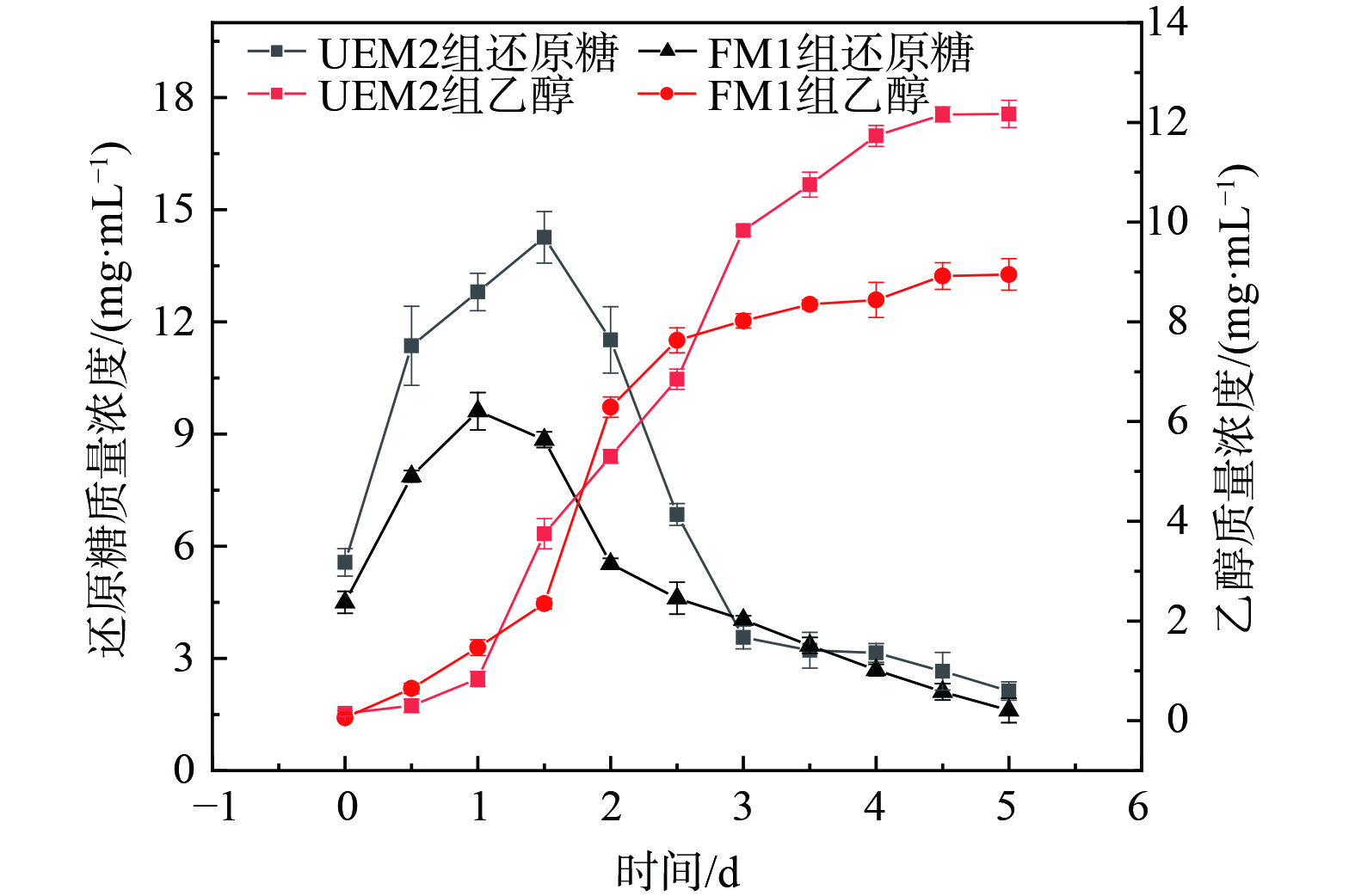
乙醇产量是秸秆生物预处理效果的最直观反映,将2种不同菌株预处理的秸秆进行SSF糖化发酵产乙醇试验,测定5 d内的还原糖 (以葡萄糖计) 和乙醇质量浓度变化,结果如图8所示。由图8可知,反应初期测得的还原糖质量浓度 (以葡萄糖计) 迅速增长,而乙醇质量浓度变化较小;可能由于初期酿酒酵母的活性较低,发酵速率小于糖化速率;随着反应的进行,在反应的第2~3 d,发酵液中溶解氧含量降低从而使酵母的代谢活动主要处于厌氧乙醇发酵,发酵液中微生物通过糖酵解作用将酶解生成的葡萄糖转化为丙酮酸,进一步脱羧形成乙醛,乙醛最终被还原成乙醇[39]。这个过程中积累的葡萄糖被迅速消耗,UEM2组还原糖质量浓度由最大积累值14.26 mg·mL−1下降至3.56 mg·mL−1,FM1组还原糖质量浓度由最大积累值9.61 mg·mL−1下降至4.03 mg·mL−1,之后消耗殆尽;乙醇质量浓度随之增加,在第5d UEM2组乙醇质量浓度达到12.17 mg·mL−1,是FM1组乙醇质量浓度 (8.95 mg·mL−1) 的1.36倍;通过乙醇质量浓度的对比,发现UEM2组相对于FM1组处理具有显著优势,这与孙哲等[40]对微藻进行诱变产乙醇的结果一致,同时也进一步验证了诱变处理的可行性。葡萄糖是发酵生成乙醇的主要前体物,通过预处理破坏木质纤维素的结晶结构,增大秸秆纤维素与纤维素酶的接触面积,得以实现葡萄糖的积累及乙醇产量的提升。因此该实验结果说明UV-EMS复合诱变强化了原始菌株的预处理效果,提高了秸秆生物乙醇的转化效率,对推进秸秆的高效资源化利用及秸秆乙醇产业的发展具有一定意义。
-
1) UV-EMS复合诱变具有协同作用,效果优于单一诱变,可有效提高菌株产酶能力。和单一诱变菌株UV6、野生菌株FM1相比,复合诱变菌株UEM2的LiP (243.6 U·L−1) 和MnP (94.32 U·L−1) 酶活性最高。
2) UEM2的预处理效果显著优于FM1,秸秆木质素降解率达到38.86%,比FM1组提高了63.55%。
3) UEM2预处理组乙醇质量浓度 (12.17 mg·mL−1) 达到FM1组 (8.95 mg·mL−1) 的1.36倍,进一步验证了UV-EMS复合诱变对生物法预处理的强化作用,在此基础上能够更好的实现秸秆的减量化处理及高效资源化利用。
UV-EMS复合诱变强化生物预处理秸秆产乙醇
Enhanced biological pretreatment of straw for ethanol production by UV-EMS composite mutagenesis
-
摘要: 玉米秸秆中木质纤维素结构复杂,采用安全、绿色的生物法对秸秆进行预处理,可以有效提高其能源转化效率进而实现秸秆的减量化及高效资源化利用。针对目前生物预处理法中存在的野生菌株产酶能力较弱、酶活性低的问题,在优化条件下对野生木质素降解菌株FM1进行紫外线照射 (UV) 与甲基磺酸乙酯 (EMS) 复合诱变,并将筛选获得的突变菌株UEM2应用于秸秆生物乙醇制备的预处理过程中,通过秸秆木质素降解率及乙醇产量等指标来对比探究诱变对秸秆预处理的影响。结果表明,UV-EMS复合诱变使菌株木质素降解酶活性得到有效提高,对秸秆的生物法预处理起到了一定的强化作用,且效果优于单一诱变;UEM2预处理后木质素降解率达到38.86%,比FM1处理组提高了63.55%;通过同步糖化发酵(SSF),测定UEM2组生物乙醇质量浓度达到12.17 mg·mL−1,是FM1组的1.36倍,进一步验证了诱变对预处理的强化效果。该研究结果可为秸秆生物法预处理的应用和发展提供理论参考。Abstract: The structure of lignocellulose in corn straw is complex. Using safe and green biological methods to pre-treat straw can effectively improve its energy conversion efficiency and achieve the reduction and efficient resource utilization of straw. In response to the problems of weak enzyme production ability and low enzyme activity of wild strains in current biological pretreatment methods, this study conducted a combination of ultraviolet radiation (UV) and ethyl methanesulfonate (EMS) mutagenesis on the wild lignin degrading strain FM1 under optimized conditions, and applied the selected mutant strain UEM2 to the pretreatment process of straw bioethanol preparation. The effects of mutagenesis on the pretreatment effect of straw were compared and explored through indicators such as straw lignin degradation rate and ethanol yield. The results showed that UV-EMS composite mutagenesis effectively increased the activity of lignin degrading enzymes of the strain, and played a certain strengthening role in the biological pretreatment of straw, and the effect was better than single mutagenesis. After pretreatment with UEM2, the lignin degradation rate reached 38.86%, which was 63.55% higher than that of the FM1 treatment group. By simultaneous saccharification fermentation (SSF), the bioethanol yield in the UEM2 group was determined to reach 12.17 mg·mL−1, which was 1.36 times higher than that of the FM1 group, further verifying the strengthening effect of mutagenesis on pretreatment. The research results can provide theoretical reference for the application and development of straw biological pretreatment.
-
表 1 培养基种类及配制
Table 1. Types of media and preparation methods
序号 培养基种类 培养基配方 1 LB液体 (固体) 培养基 蛋白胨10 g·L−1,酵母浸粉5 g·L−1,NaCl 10 g·L−1, (琼脂16 g·L−1) 2 初筛培养基 苯胺蓝0.1 g·L−1溶于LB培养基 3 复筛培养基 木质素磺酸钠3 g·L−1,(NH4)2SO4 2 g·L−1,K2HPO4 1 g·L−1,KH2PO4 1 g·L−1,MgSO4 0.2 g·L−1,CaCl2 0.1 g·L−1,FeSO4 0.05 g·L−1,MnSO4 0.02 g·L−1 4 产酶发酵培养基 蛋白胨5 g·L−1,葡萄糖4 g·L−1,K2HPO4 2 g·L−1,KH2PO4 1 g·L−1,CaCl2 1 g·L−1 注:培养基均调节pH=7,并于121 ℃灭菌20 min。 表 2 正交试验设计
Table 2. Orthogonal experimental design
水平 因素 A诱变剂体积分数/% B诱变时间/min C诱变温度/ ℃ D菌悬液浓度/ (CFU·mL−1) 1 0.4 30 25 105 2 0.5 35 28 106 3 0.6 40 30 107 表 3 正交试验结果
Table 3. Orthogonal test results
序号 因素 致死率/% A B C D 实验1 1 1 1 1 83.34 实验2 1 2 2 2 76.69 实验3 1 3 3 3 77.08 实验4 2 1 2 3 81.23 实验5 2 2 3 1 93.38 实验6 2 3 1 2 92.48 实验7 3 1 3 2 89.25 实验8 3 2 1 3 90.16 实验9 3 3 2 1 96.39 均值1 79.037 84.707 88.660 91.033 — 均值2 89.130 86.743 84.867 86.140 — 均值3 91.930 88.647 86.570 82.923 — 极差 12.893 3.940 3.793 8.110 — 表 4 复筛菌株褪色圈及菌落直径
Table 4. The diameters of the fading ring and colony of the re-screened strains
菌株编号 褪色圈直径D/cm 菌落直径d/cm UEM1 4.27 1.35 UEM2 5.26 1.23 UEM3 3.58 1.08 UEM4 4.11 1.26 UEM5 5.12 1.45 UEM6 5.39 1.33 UEM7 4.4 1.28 UEM8 4.79 1.16 UEM9 4.16 1.09 UEM10 4.22 1.29 UEM11 3.73 1.12 UEM12 3.98 1.25 UEM13 4.66 1.31 -
[1] SADEGHINEZHAD E, KAZI S N, BADARUDIN A, et al. Sustainability and environmental impact of ethanol as a biofuel[J]. Reviews in Chemical Engineering, 2014, 30(1): 51-72. [2] 王梦, 田晓俊, 陈必强, 等. 生物燃料乙醇产业未来发展的新模式[J]. 中国工程科学, 2020, 22(2): 47-54. [3] ANTAR M, LYU D M, NAZARI M, et al. Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization[J]. Renewable and Sustainable Energy Reviews, 2021, 139: 110691. doi: 10.1016/j.rser.2020.110691 [4] SARAVANAN A, YAASHIKAA P R, SENTHIL K P, et al. Techno-economic and environmental sustainability prospects on biochemical conversion of agricultural and algal biomass to biofuels[J]. Journal of Cleaner Production, 2023, 414: 137749. doi: 10.1016/j.jclepro.2023.137749 [5] 王凯军, 石川, 刘越. 有机固废厌氧发酵产物的转化制备与应用进展[J]. 环境工程学报, 2021, 15(6): 1840-1861. doi: 10.12030/j.cjee.202007004 [6] RAUD M, KIKAS T, SIPPULA O, et al. Potentials and challenges in lignocellulosic biofuel production technology[J]. Renewable and Sustainable Energy Reviews, 2019, 111: 44-56. doi: 10.1016/j.rser.2019.05.020 [7] 姚晓琰, 王润娟, 吕学斌, 等. 碱液预处理玉米秸秆的条件优化及添加剂的选择[J]. 环境工程学报, 2014, 8(7): 3011-3017. [8] WANG S Z, SUN X X, YUAN Q P. Strategies for enhancing microbial tolerance to inhibitors for biofuel production: A review[J]. Bioresource Technology, 2018, 258: 302-309. doi: 10.1016/j.biortech.2018.03.064 [9] 吴亚林. 玉米秸秆制备燃料乙醇的预处理和酶解工艺研究[D]. 扬州: 扬州大学, 2019. [10] LI X X, YAN X H, YE M Y, et al. Enhancement of anaerobic digestion performance of corn straw via combined sodium hydroxide-cellulase pretreatment[J]. Biochemical Engineering Journal, 2022, 187: 108652. doi: 10.1016/j.bej.2022.108652 [11] TALEBNIA F, KARAKASHEV D, ANGELIDAKI I. Production of bioethanol from wheat straw: An overview on pretreatment, hydrolysis and fermentation[J]. Bioresource Technology, 2010, 101(13): 4744-4753. doi: 10.1016/j.biortech.2009.11.080 [12] THURAKIT T, PATHOM A W, PUMAS C, et al. High-efficiency production of biomass and biofuel under two-stage cultivation of a stable microalga Botryococcus braunii mutant generated by ethyl methanesulfonate-induced mutation[J]. Renewable Energy, 2022, 198: 176-188. doi: 10.1016/j.renene.2022.08.029 [13] 刘庆玉, 姚影, 张敏, 等. 紫外诱变筛选高效木质素降解菌株的研究[J]. 可再生能源, 2010, 28(4): 58-61. doi: 10.3969/j.issn.1671-5292.2010.04.014 [14] ALI S, MAHMOOD S. Mutagenesis of a thermophilic Alkalibacillus flavidus for enhanced production of an extracellular acetyl xylan esterase in semi-solid culture of linseed meal[J]. Waste and Biomass Valorization, 2020, 11(7): 3327-3335. doi: 10.1007/s12649-019-00665-2 [15] 刘登辉. 高产纤维素酶菌株的选育研究[D]. 武汉: 武汉工程大学, 2022. [16] ETHIRAJ S, GOPINATH S, RAVI V, et al. Enhancement of serrapeptase hyper producing mutant by combined chemical and uv mutagenesis and its potential for fibrinolytic activity[J]. Journal of Pure and Applied Microbiology, 2020, 14(2): 1295-1303. doi: 10.22207/JPAM.14.2.25 [17] ZHANG Y, HE M L, ZOU S M, et al. Breeding of high biomass and lipid producing Desmodesmus sp. by Ethylmethane sulfonate-induced mutation[J]. Bioresource Technology, 2016, 207: 268-275. doi: 10.1016/j.biortech.2016.01.120 [18] 诸葛荣夏. 腐浆中木质纤维降解菌的筛选及复合菌系构建[D]. 南京: 南京林业大学, 2020. [19] GANESH K A, SEKARAN G, KRISHNAMOORTHY S. Solid state fermentation of Achras zapota lignocellulose by Phanerochaete chrysosporium[J]. Bioresource Technology, 2006, 97(13): 1521-1528. doi: 10.1016/j.biortech.2005.06.015 [20] 李红亚, 李术娜, 王树香, 等. 产芽孢木质素降解菌MN-8的筛选及其对木质素的降解[J]. 中国农业科学, 2014, 47(2): 324-333. doi: 10.3864/j.issn.0578-1752.2014.02.012 [21] DAHNUM D, TASUM S O, TRIWAHYUNI E, et al. Comparison of SHF and SSF processes using enzyme and dry yeast for optimization of bioethanol production from empty fruit bunch[J]. Energy Procedia, 2015, 68: 107-116. doi: 10.1016/j.egypro.2015.03.238 [22] 郭栋豪. 60Co-γ辐照预处理玉米秸秆制备乙醇的研究[D]. 长沙: 湖南大学, 2018. [23] PHWAN C K, CHEW K W, SEBAYANG A H, et al. Effects of acids pre-treatment on the microbial fermentation process for bioethanol production from microalgae[J]. Biotechnology for Biofuels, 2019, 12: 191. doi: 10.1186/s13068-019-1533-5 [24] MADADI M, ZHAO K L, WANG Y M, et al. Modified lignocellulose and rich starch for complete saccharification to maximize bioethanol in distinct polyploidy potato straw[J]. Carbohydrate Polymers, 2021, 265: 118070. doi: 10.1016/j.carbpol.2021.118070 [25] 何川, 章登政, 张俊, 等. 重铬酸钾-DNS比色法测定发酵液中乙醇含量[J]. 生命科学研究, 2013, 17(1): 1-4, 10. doi: 10.3969/j.issn.1007-7847.2013.01.001 [26] 马东林. 优良玫瑰糖多孢菌的选育及在黄酒应用上促进发酵机理解析[D]. 无锡: 江南大学, 2022. [27] SHAFIQUE S, BAJWA R, SHAFIQUE S. Molecular characterisation of UV and chemically induced mutants of Trichoderma reesei FCBP-364[J]. Natural Product Research, 2010, 24(15): 1438-1448. doi: 10.1080/14786410903132399 [28] 杨珠英. 紫外及甲基磺酸乙酯诱变选育竹红菌甲素高产菌株的研究[D]. 苏州: 苏州大学, 2013. [29] KHALID N, ASGHER M, QAMAR S A. Evolving trend of Boletus versicolor IBL-04 by chemical mutagenesis to overproduce laccase: Process optimization, 3-step purification, and characterization[J]. Industrial Crops and Products, 2020, 155: 112771. doi: 10.1016/j.indcrop.2020.112771 [30] 郁红艳. 农业废物堆肥化中木质素的降解及其微生物特性研究[D]. 长沙: 湖南大学, 2007. [31] 陈跃辉. 三国吴简腐蚀斑微生物的分离鉴定及其木质素降解性能研究[D]. 长沙: 中南大学, 2009. [32] 胡悦, 李汉文, 喻晨, 等. LiCl-ARTP复合诱变选育高产碱性蛋白酶菌株及其发酵条件优化[J]. 中国酿造, 2021, 40(2): 59-65. doi: 10.11882/j.issn.0254-5071.2021.02.012 [33] KUN R S, GOMES A C S, HILDEN K S, et al. Developments and opportunities in fungal strain engineering for the production of novel enzymes and enzyme cocktails for plant biomass degradation[J]. Biotechnology Advances, 2019, 37(6): 107361. doi: 10.1016/j.biotechadv.2019.02.017 [34] XIA X K, ZHANG Y E, LEI S J, et al. Identification and iterative combinatorial mutagenesis of a new naringinase-producing strain, Aspergillus tubingensis MN589840[J]. Letters in Applied Microbiology, 2021, 72(2): 141-148. doi: 10.1111/lam.13379 [35] WANG J L, CHEN P Z, LI S P, et al. Mutagenesis of high-efficiency heterotrophic nitrifying-aerobic denitrifying bacterium Rhodococcus sp. strain CPZ 24[J]. Bioresource Technology, 2022, 361: 127692. doi: 10.1016/j.biortech.2022.127692 [36] MOTAUNG T E, MOKHOTHU T H. The influence of supermasscolloider on the morphology of sugarcane bagasse and bagasse cellulose[J]. Fibers and Polymers, 2016, 17(3): 343-348. doi: 10.1007/s12221-016-5430-2 [37] 伦晓中, 寇巍, 赵勇, 等. 膨化预处理玉米秸秆的还原糖酶解工艺研究[J]. 环境工程学报, 2013, 7(1): 317-322. [38] 侯进菊. 稻草秸秆酶解糖化残渣基吸附剂的制备及其强化纤维素乙醇生产的机制研究[D]. 上海: 华东师范大学, 2022. [39] 冯磊, 王宁, 寇巍, 等. 底物浓度对玉米秸秆乙醇发酵及残渣甲烷发酵的影响[J]. 环境工程学报, 2019, 13(1): 186-194. doi: 10.12030/j.cjee.201807058 [40] 孙哲, 孙昕, 李鹏飞, 等. 基于提高乙醇产率的常压室温等离子体微藻诱变育种[J]. 中国环境科学, 2021, 41(8): 3733-3739. doi: 10.3969/j.issn.1000-6923.2021.08.030 -




 下载:
下载: