-
由于锂离子电池具有安全环保,电池容量大,输出功率大,自放电小等诸多优点,被广泛用于便携式电子产品,航空航天,医疗器械等领域[1~2]。据报道,2022年中国锂电池正极材料和负极材料产量分别有1.85×106 t和1.4×106 t,而2022年锂电池回收量仅有4.15×105 t,只有15%不到的锂电池得到有效回收,因锂电池使用寿命只有1~3年,如果回收率不变,到2025年可能会有2.835×106 t废电池不能得到有效回收。这些废旧锂电池中含有钴、镍等有毒重金属,直接丢弃会对土壤、河流等造成严重污染,而锂离子电池中锂、镍、钴、锰等金属的含量远高于一般矿物,具有极高的资源性和价值,随着科技不断发展和资源的不断开发,对资源的可循环利用和环境保护的要求被日益重视[3],因此废旧锂离子电池的回收显得尤为重要并已经迫在眉睫[4-5]。
溶剂萃取法[6]因其具有高提取率、选择分离性高、流程简单和操作连续化等优点,已经成为处理废旧锂电池中金属离子分离回收的主要方法。目前废旧三元锂电池中有价金属提取主要是在无机酸 (硫酸、盐酸等)体系中进行,因此,萃取分离的研究重点主要在硫酸、盐酸等无机酸体系中开展。如:代梦雅等[7]对电极材料使用硫酸浸出,浸出液经过P204除锰,在pH=5.2条件下,分别以0.5 mol·L−1 P507和0.6 mol·L−1 Cyanex272为萃取剂,经过两级错流萃取,钴萃取率分别为98.21%和99.44%, 镍共萃取率分别为24.42%和4.26%, 锂共萃取率分别为15.84%和5.11%;鲁桃等[8]在硫酸体系中使用Cyanex272,初始pH为4,萃取剂体积分数为50%,皂化率75%左右,O/A=1∶1,振荡时间为2 min,钴的一级萃取率为98.38%,而镍的损失率为2.28%。但在无机酸体系提取过程中易产生Cl2、SO2等有害气体、设备易腐蚀、铜、铝等杂质浸出率高等缺点,近年来,更多研究选用酸性较为温和、更为环保、杂质浸出率低的有机酸对正极材料中有价金属进行浸出被越来越多的研究者所关注[9]。如孟飞[10]使用柠檬酸对废旧三元电池浸出,镍钴锂锰的浸出率均大于90%,而铜、铝的浸出率均小于30%;高桂兰[11]对正极材料在柠檬酸浓度1.2 mol·L−1、硫代硫酸钠浓度0.3 mol·L−1、固液比20 g·L−1、浸出温度70 ℃、浸出时间30 min,钴的浸出率可达96%左右,锂的浸出率可达99%左右;但目前,关于在有机酸体系萃取分离废旧三元锂电池中的有价金属的文献较少。
本研究选用的是有机酸中较为便宜的D, L-苹果酸浸出废旧锂电池正极材料,经过前期一定的探索实验探究有机酸体系各种萃取剂对有价金属元素的萃取率;对浸出液选用一种或几种萃取剂来对其中的锰和钴进行分步萃取,研究不同影响因素下萃取剂对苹果酸浸出液中金属元素的萃取效果,为有机酸体系高效萃取锰、钴提供一定的参考。
-
本实验采用模拟的废旧三元电池正极材料LiNi0.6Co0.2Mn0.2O2的苹果酸浸出液。浸出液Ⅰ的成分为:Mn 2.26 g·L−1、Ni 5.7 g·L−1、Co 2.5 g·L−1、Li 1.24 g·L−1,苹果酸质量浓度为100 g·L−1。浸出液Ⅱ的成分为:Mn 4.51 g·L−1,苹果酸质量浓度为100 g·L−1。浸出液Ⅲ的成分为:Co 5.31 g·L−1,苹果酸质量浓度为100 g·L−1。浸出液IV的成分为:Co 2.50 g·L−1、Ni 4.94 g·L−1、Li 1.28 g·L−1,苹果酸质量浓度为100 g·L−1。萃取剂P204和萃取剂Cyanex272由重庆康普化学工业股份有限公司生产、萃取剂溶剂为煤油 (航空煤油)、NaOH为天津市风船化学试剂科技有限公司生产,苹果酸为湖县利乐生物科技实业有限公司生产。
-
实验时,首先使用30%NaOH溶液对浸出液pH值进行调节,然后按设定相比将浸出液与有机相混于分液漏斗中,在震荡器上震荡一段时间,取出分液漏斗静置分相,随后对负载有机相使用极低质量浓度的被萃金属苹果酸溶液进行洗涤,静置分相并分离,随后用一定质量浓度苹果酸溶液与洗涤后的负载有机相进行反萃处理,静置分相并分离得到反萃液。方程式如式(1)~式(3)所示,其中Men+代表被萃金属离子,Mn+代表其它金属离子,HR表示萃取剂。
式(1)是萃取剂将浸出液中的金属元素萃取到有机相中,式(2)是通过低质量浓度的苹果酸溶液将其它金属离子洗涤到溶液中,式(3)是使用低质量浓度苹果酸溶液将负载有机相中的金属离子反萃到苹果酸溶液中。
-
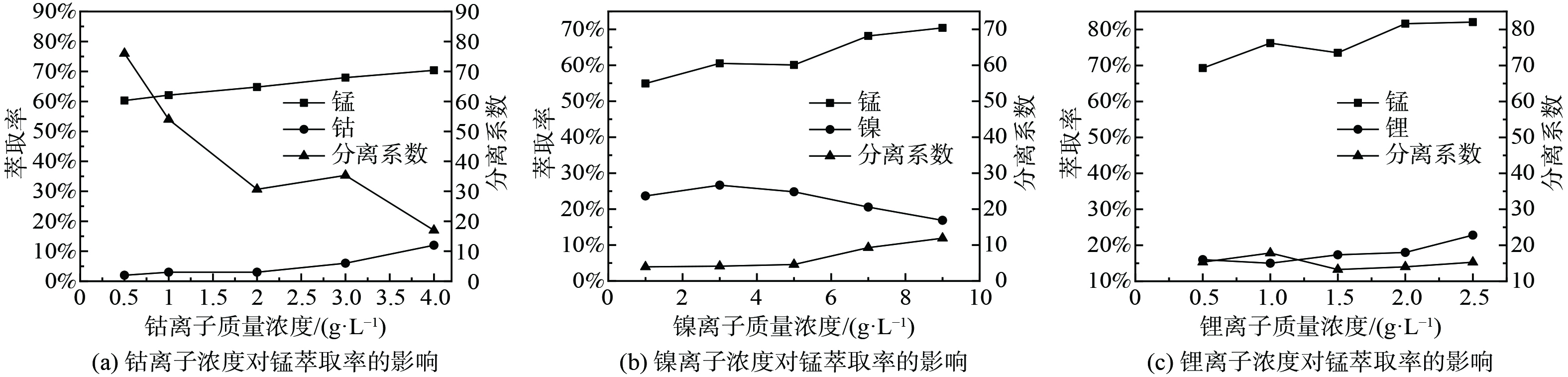
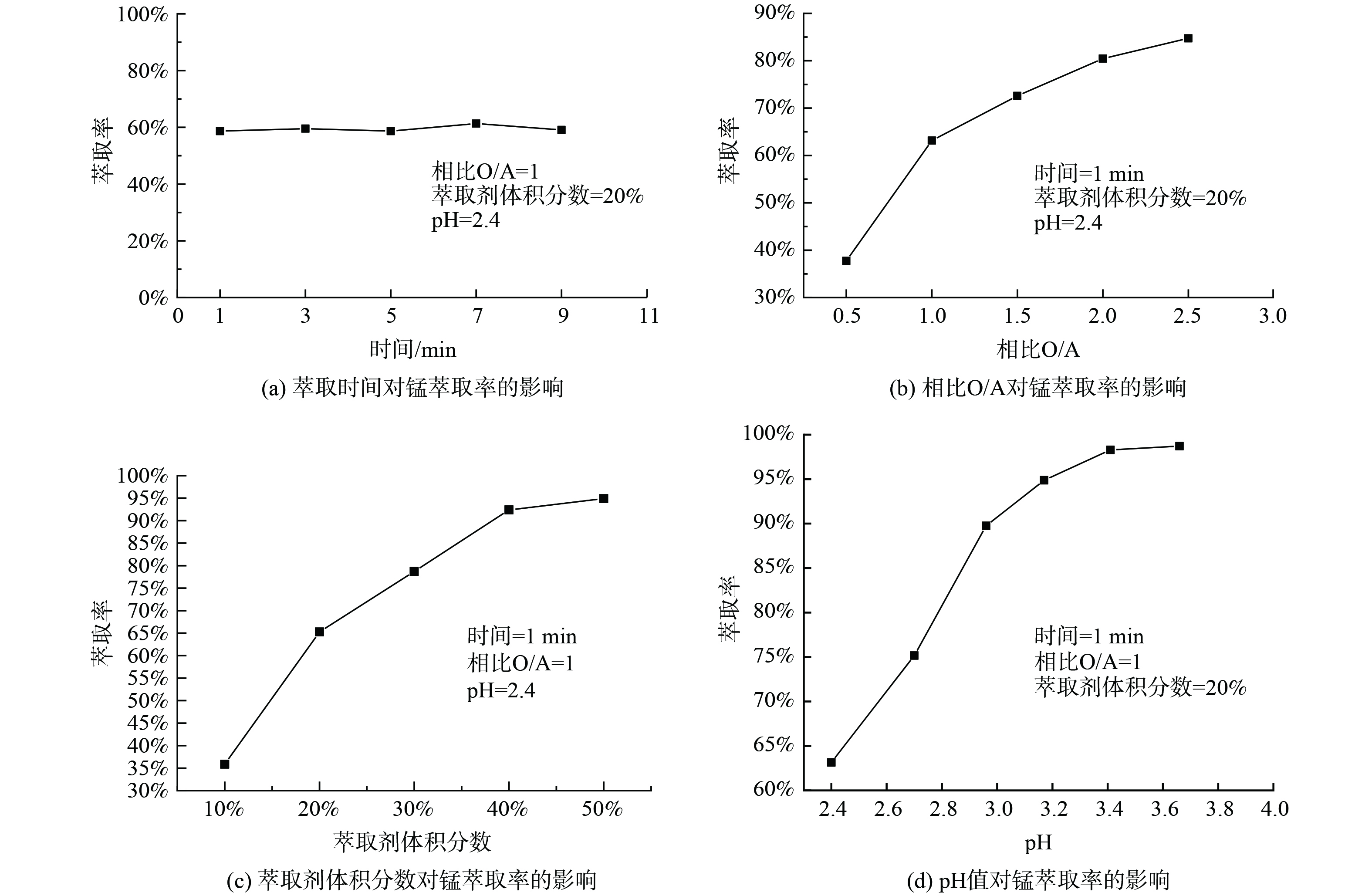
1)萃取条件对P204萃取Mn2+的影响。取浸出液Ⅱ20 mL,进行温度为25 ℃,萃取时间为1~9 min,相比O/A为1∶2至5∶2,萃取剂体积分数为10%~50%,浸出液pH为2.40~3.70系列条件下的萃取实验,实验结果如图1所示。如图1(a)所示,随着时间的增加,萃取率有轻微变化,但起伏不大,说明反应进行较快。当萃取时间为1 min时,反应已达到平衡,萃取率在60.00%左右;如图1(b)所示,随着相比O/A增加,锰的萃取率先迅速增加后趋于稳定,这是因为在溶液中其他离子质量浓度确定的情况下,锰的萃取率取决于萃取剂P204量的多少,增大相比O/A就等于增加萃取剂的量,当相比O/A较小时,萃取剂P204基本与锰完全反应,因溶液中锰的含量是一定的,所以最终锰的萃取率是趋于稳定的。考虑到原料利用率,相比过大导致体系粘度增加,影响分相效率及分相速率等问题,选择最佳相比O/A为1∶1,其萃取率为63.15%。由图1(c)可知,当萃取剂体积分数分别为10%、20%、30%时,萃取率分别为35.87%、65.25%、78.70%,随着萃取剂体积分数的增加,锰的萃取率也迅速增加,最后趋于平衡,这是因为随着P204含量的增加,萃取剂与锰的接触几率增加,形成萃合物的几率增加,从而萃取率增加,因锰的含量是固定的,所以最后萃取率趋于平衡。考虑到成本及萃取率增加速率,选择最佳萃取剂体积分数为20%。由图1(d)可知,随着初始pH的增加,萃取率迅速增加,然后趋于平缓,这是因为在萃取反应过程中金属离子会与萃取剂反应产生H+离子,pH值的增加有利于反应向生成萃合物的方向进行,当pH=2.96时,萃取率为89.75%,再继续增加pH,萃取率增加相对较小,因此选择最佳pH为2.96。取浸出液Ⅰ在最佳实验条件下进行三级逆流萃取实验,锰的萃取率可达到99.99%,镍、钴、锂的共萃率分别为12.11%、9.20%、3.23%。
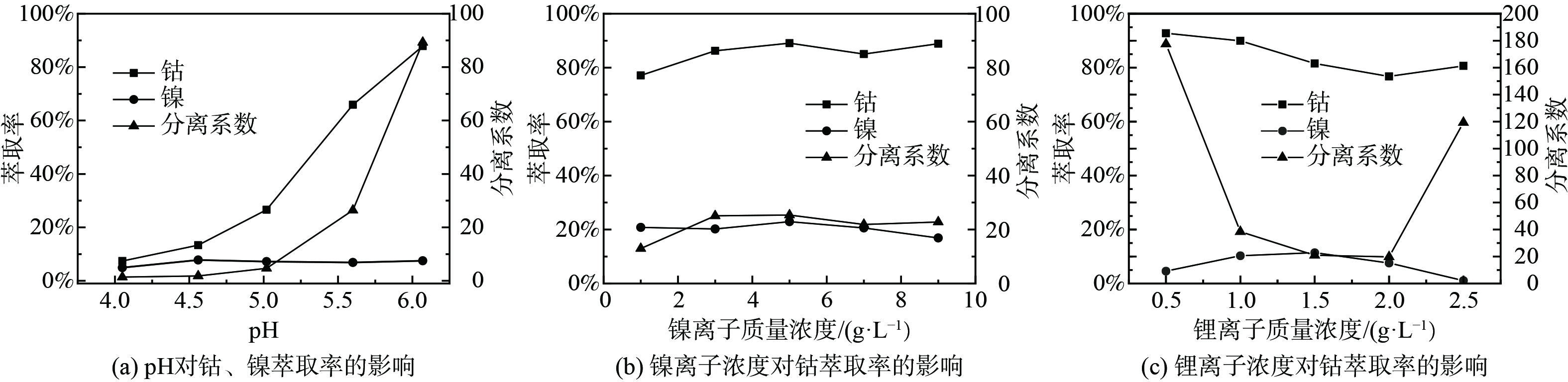
2)其他金属离子对P204萃取Mn2+的影响。取浸出液Ⅱ20 ml,分别加入一定量碳酸钴、碳酸镍、碳酸锂,进行温度为 25 ℃,萃取时间为1 min,相比O/A=1∶1,萃取剂体积分数为20%的萃取实验,实验结果如图2所示。随着钴离子质量浓度的增加,锰离子萃取率在缓慢增加。由于添加碳酸钴导致初始pH发生变化,初始pH变为2.44~2.65,在无钴离子存在的情况下,P204对锰的萃取率从63%左右升至73%左右,而添加钴离子后,锰的萃取率从60.30%增加到70.40%,说明锰离子萃取率的增加主要是由于添加碳酸钴导致的初始pH值变化引起的。由于钴的萃取率在在逐渐增加,从而使锰、钴的分离系数在不断降低。由图2(b)可知,随着镍离子质量浓度的增加,锰离子的萃取率不断增加。由于加入了碳酸镍,导致初始pH值变为2.48~2.78,在无镍离子存在的情况下,锰的萃取率从64.00%左右升至78.00%左右,而添加镍离子后,锰的萃取率从54.93%升到70.40%,说明锰离子萃取率的增加主要是由于添加碳酸镍导致的pH值变化引起的。而镍离子的萃取率先增加后降低,这是因为P204对镍的萃取容量达到了最大值,继续增加镍离子质量浓度,萃取率降低。由图2(c)可知,随着锂离子质量浓度的增加,锰离子的萃取率不断增加,由于加入了碳酸锂,初始pH值变为2.70~3.28,在无锂离子存在的情况下,P204对锰的萃取率从75.00%左右上升到95.00%左右,添加锂离子后,萃取率由69.28%上升至82.06%,说明锰离子萃取率的增加主要是由于添加碳酸锂导致的pH值变化引起的。由图2的(a)、(b)、(c)可知,3种离子与锰的分离系数均较差,为了保证纯度,需要在后续对负载有机相进行水洗以除去其中大部分的其他金属离子。
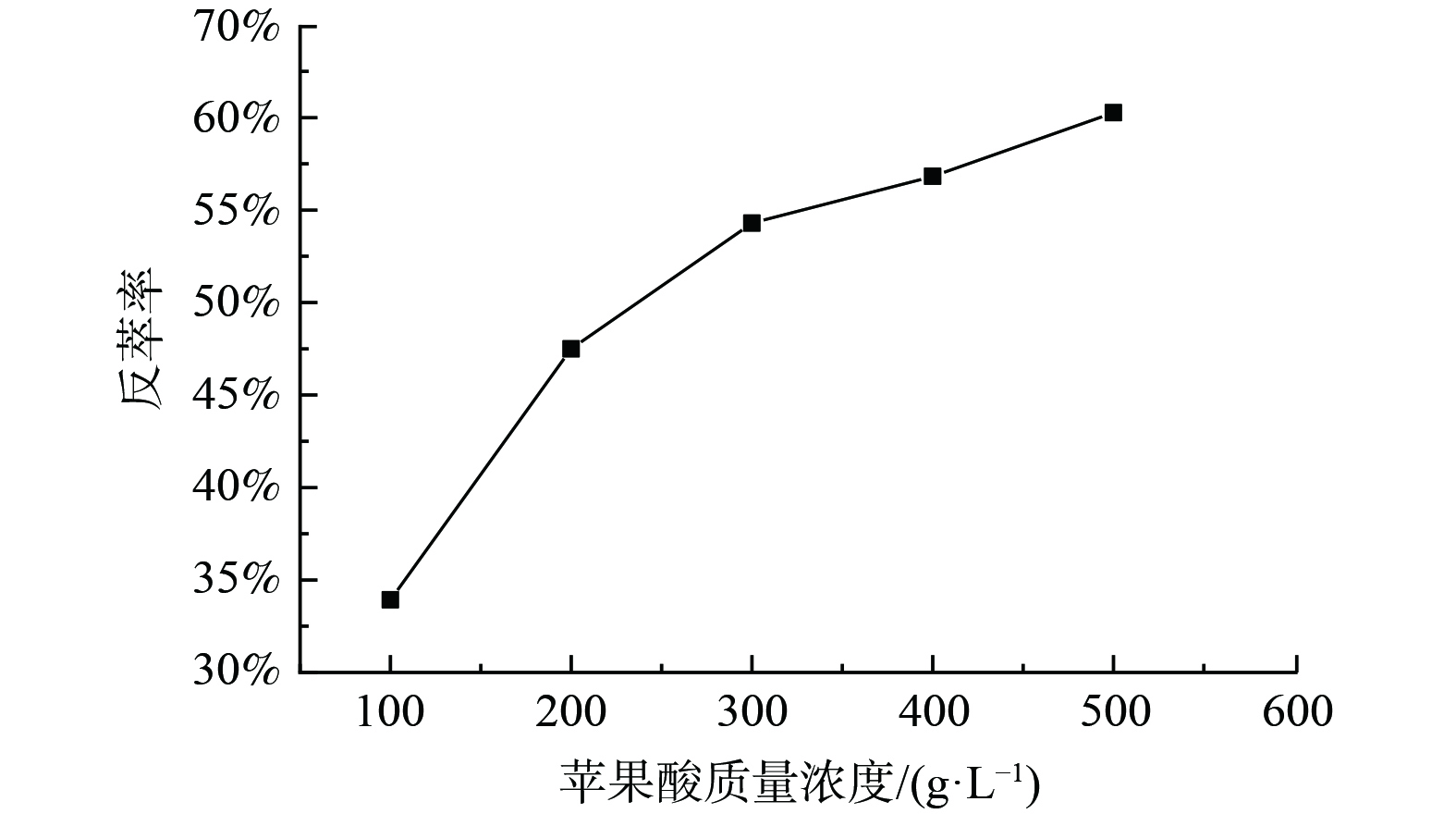
3) P204负载有机相反萃锰的研究。为了抑制有机相中锰被洗出,使用含1 g·L−1锰的100 g·L−1苹果酸溶液以相比O/A=1∶1对浸出液Ⅰ使用P204进行三级逆流萃取后的负载锰有机相洗涤,洗涤前有机相中Mn为2.255 g·L−1,Ni为0.69 g·L−1,Co为0.23g·L−1,洗涤后有机相中Mn为2.51 g·L−1,Ni为0.04 g·L−1,Co仅含0.03 g·L−1,锂离子质量浓度太低未检测到,表明洗涤效果较良好。对洗涤后的负载有机相进行反萃,固定条件为:反萃介质为苹果酸,相比O/A=1∶1、反萃时间20 min、温度25 ℃,实验结果如图3。由图3可知,在此反萃条件下,Mn的单级反萃率可达55.00%左右。在同样反萃条件下对Mn负载有机相进行三级逆流反萃,反萃率可达88.80%,由此可见,苹果酸溶液能将大部分的锰反萃。
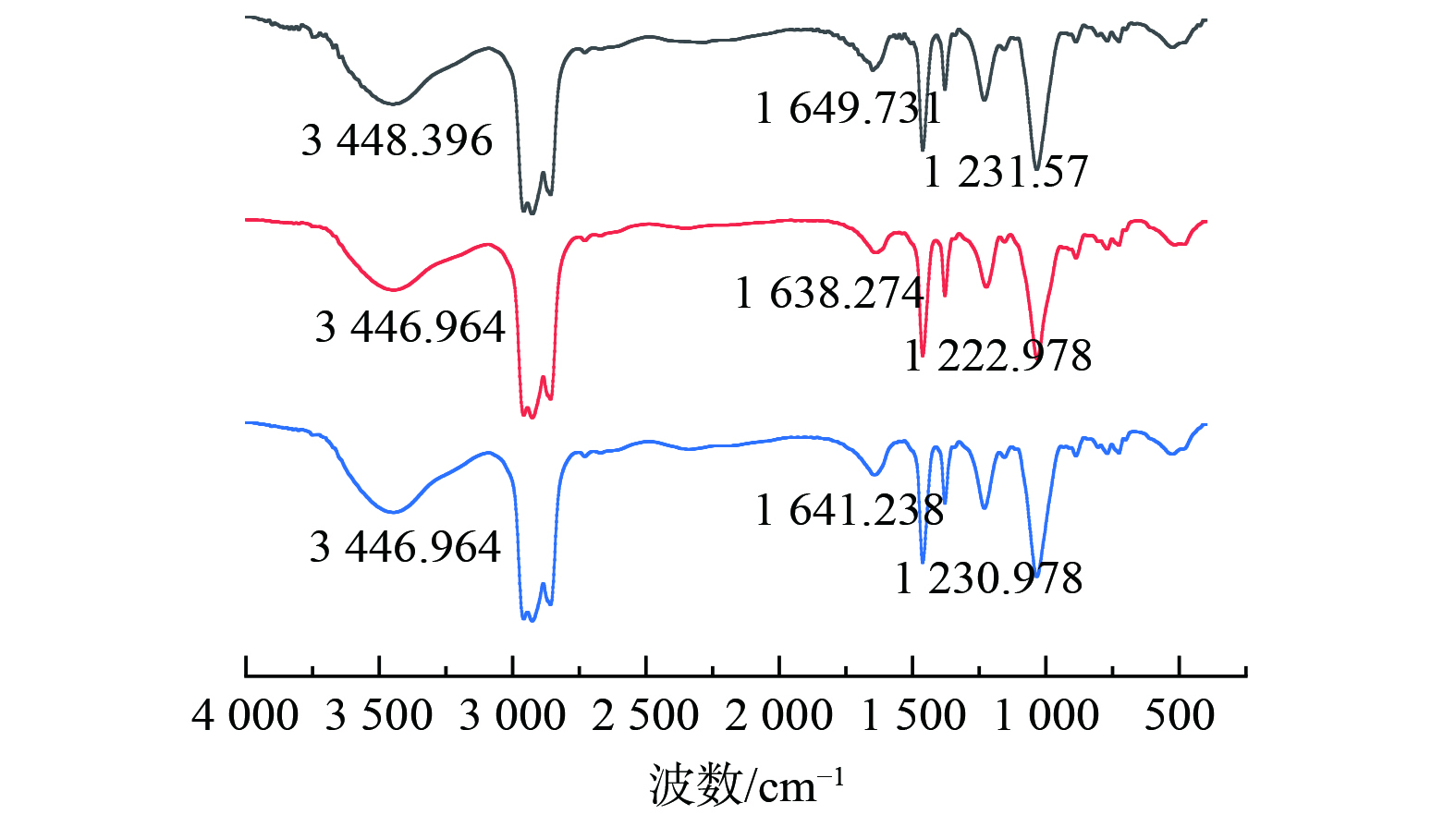
4)红外光谱测试分析P204萃取锰对P204有机相萃取前后及反萃后进行了红外光谱分析,如图由图4可知,在3 448.396 cm−1出现二聚体分子间氢键伸缩振动峰,在1 649.731 cm−1出现二聚体的O-H面内弯曲振动峰。在萃取后,3 448.396 cm−1振动峰强度降低但是仍能观察到二聚体峰,说明分子间形成的-OH键中的H被锰取代,但是P204过量,未能完全消除二聚体的影响。而1 649.731 cm−1的峰向低波数1 638.274移动,且峰强度有所降低,这是因为P-O→Mn的生成的同时使氢键电子云降低。在反萃后,振动峰强度有一定恢复且向高波数偏移至1 641.238 cm−1,这是因为反萃后锰被氢离子置换出去,但是仍未置换完全。在1 231.570 cm−1处出现P=O的伸缩振动峰,在萃取后,由高波数的1 231.570 cm−1位移到1 222.978 cm−1说明P=O键与Mn离子发生配位,波数变化是由于P=O键与Mn形成P=O→Mn配位键,使P=O双键的电子云密度降低,键的强度减弱,从而导致其震动频率下降。同时因为Mn与P=O的配位,使P204的对称性发生变化,P=O键的偶极矩发生变化,导致峰强度减弱[12-14]。即P204萃取锰的反应的实质是Mn与P-O-H中的氢发生置换反应,且与P=O形成配位键进而被萃取。
-
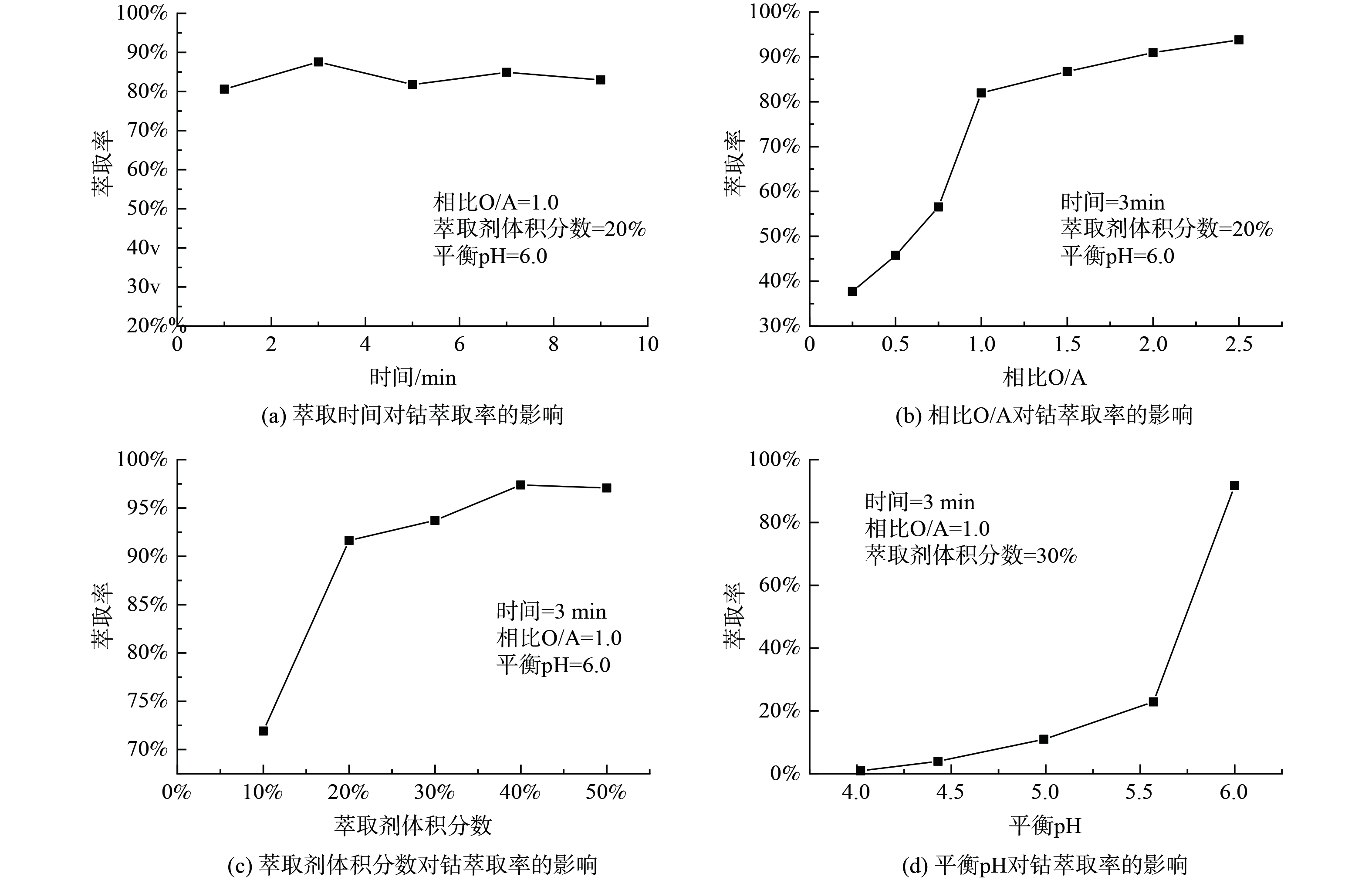
1)萃取条件对Cyanex272萃取Co2+的影响。取浸出液Ⅲ 20ml,进行温度为25 ℃,萃取时间为1~9 min,相比O/A为1∶4至5∶2,萃取剂体积分数为10%~50%,平衡pH为4~6的萃取实验,实验结果如图5所示。如图5(a)所示,整个反应进行较快,萃取率整体变化不大。当萃取时间为3min时,萃取率为87.56%,达到最大值,因此,3 min为最佳萃取时间。由图5(b)可知,随着相比O/A的不断增加,萃取率也在不断增加。因溶液中钴离子的含量是一定的,相比O/A的不断增加,最终钴的萃取率时趋于稳定的。考虑到成本,原料利用率,以及相比过大时导致影响分相效率及分相速率低,选择最佳相比O/A为1∶1,钴的最佳萃取率为81.97%。由图5(c)可知,随着萃取剂体积分数的不断增加,萃取率也是在不断增加的。这是因为Cyanex272的增加,萃取剂与钴的接触几率也在不断增加,形成萃合物的几率增加,最终萃取率增加。当萃取剂体积分数分别10%、20%、30%,萃取率分别为71.92%、91.65%、93.72%,因萃取剂体积分数为20%时,萃取率上升幅度较大,所以最佳萃取剂体积分数为20%。由图5(d)可知,随着pH的增加,萃取率不断增加,当pH为5~6时,萃取率有较大幅度增加。当平衡pH=6,钴的萃取率为91.75%。随着萃取反应的进行,溶液的pH在不断降低,增加水相的平衡pH有利于萃取反应发生,进而增大钴的萃取率,最终选择最佳平衡pH为6。取浸出液IV在最佳实验条件下进行三级逆流萃取钴实验,钴的萃取率为94.01%,锂、镍的共萃率分别为11.21%和0.02%。
2)其他金属离子对Cyanex272萃取Co2+的影响。取浸出液Ⅲ20ml,分别加入一定量碳酸镍、碳酸锂,并重新定容,进行温度为25 ℃,萃取时间为3min,相比O/A=1∶1,萃取剂体积分数为20%,平衡pH=6的萃取实验,实验结果如图6所示。因Cyanex272对钴、镍均具有良好的萃取效果,所以测试在添加一定量镍离子后,在萃取剂体积分数为20%,相比O/A=1∶1,萃取时间3 min,镍离子质量浓度为10.4 g·L−1条件下,不同平衡pH下Cyanex272对镍、钴的萃取效果,结果如图6(a)所示,随着平衡pH的增加,钴离子的萃取率在不断增加,镍离子的萃取率基本保持不变。当pH=6时,钴的萃取率为89.62%,镍的萃取率未超过10%,说明不同pH下,镍离子对Cyanex272萃钴过程基本无影响。由图6(b)可知,随着镍离子质量浓度的增加,钴离子萃取率先增后趋于平衡,与未添加镍离子时相比,钴的的萃取率有一定的下降,即镍离子对Cyanex272萃钴有抑制作用,但是随着镍离子质量浓度的增加,这种抑制作用在不断降低。镍离子萃取率基本维持在20.00%左右,当镍离子质量浓度为3 g·L−1时,钴的萃取率为86.24%,此时镍钴的分离系数为20.2,两者较易进行分离。由图6(c)可知,随着锂离子质量浓度增加,钴离子萃取率整体呈下降趋势,说明锂离子对钴离子的萃取有一定抑制作用,且这种抑制作用在不断增强。
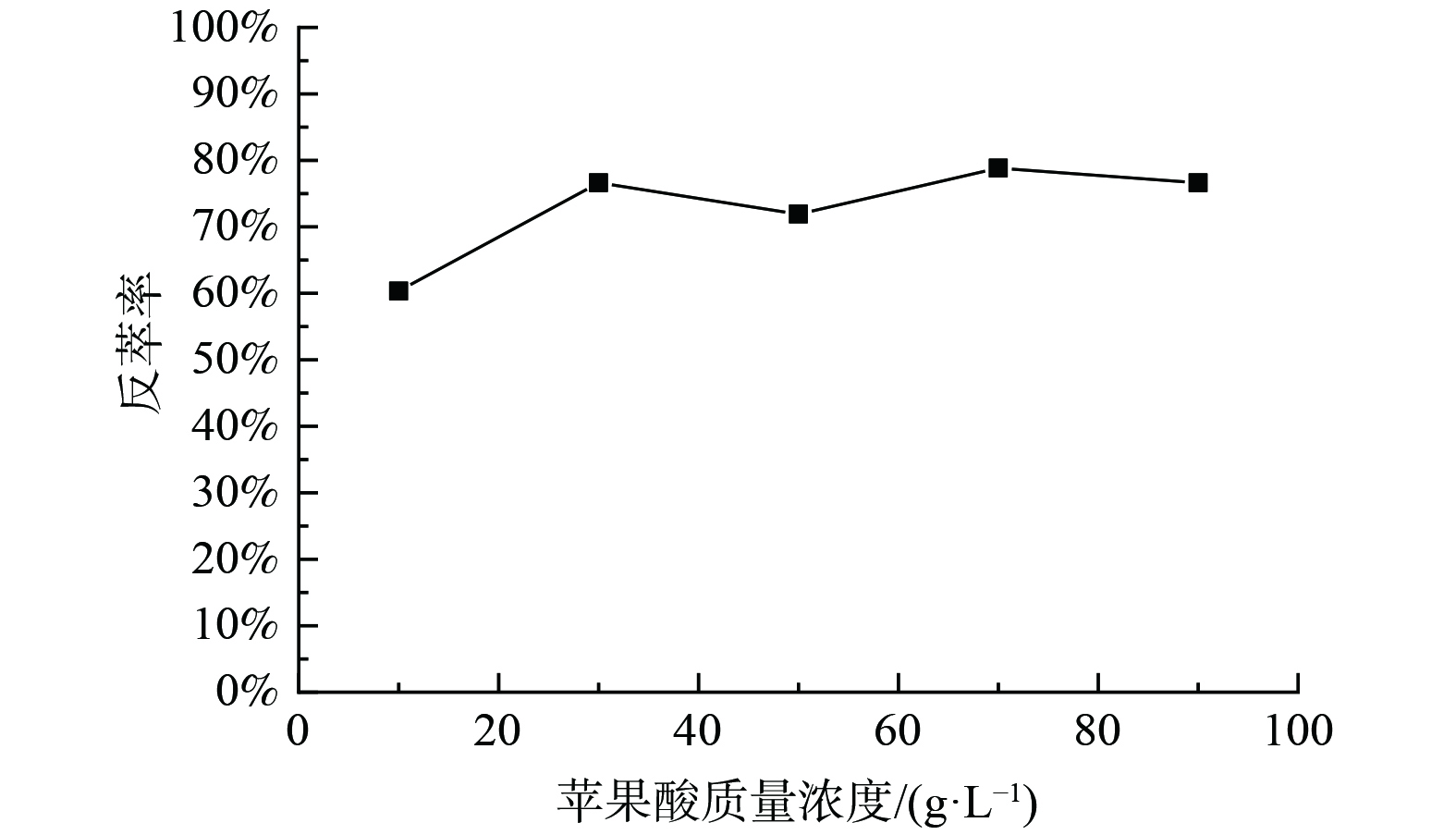
3) Cyanex272负载有机相反萃钴的研究。为了抑制有机相中的钴被洗出,使用含1 g·L−1钴的10 g·L−1苹果酸溶液以相比O/A=1∶1对浸出液IV使用Cyanex272进行三级逆流萃取后的负载钴有机相洗涤,洗涤前有机相中Co 2.31 g·L−1,Ni 0.01 g·L−1,Li 0.14 g·L−1,洗涤后有机相中Co为2.81 g·L−1,镍未检测出,锂仅含5.47 mg·L−1,洗涤效果良好。对洗涤后的负载有机相进行反萃,固定条件为:反萃介质为苹果酸、相比O/A=1∶1、反萃时间10min、温度25 ℃。实验结果如图7。由图7可知,当反萃介质苹果酸质量浓度为30 g·L−1时,Co的单级反萃率可达76.61%。在同样条件下进行三级逆流反萃Co后,Co反萃率为99.98%。
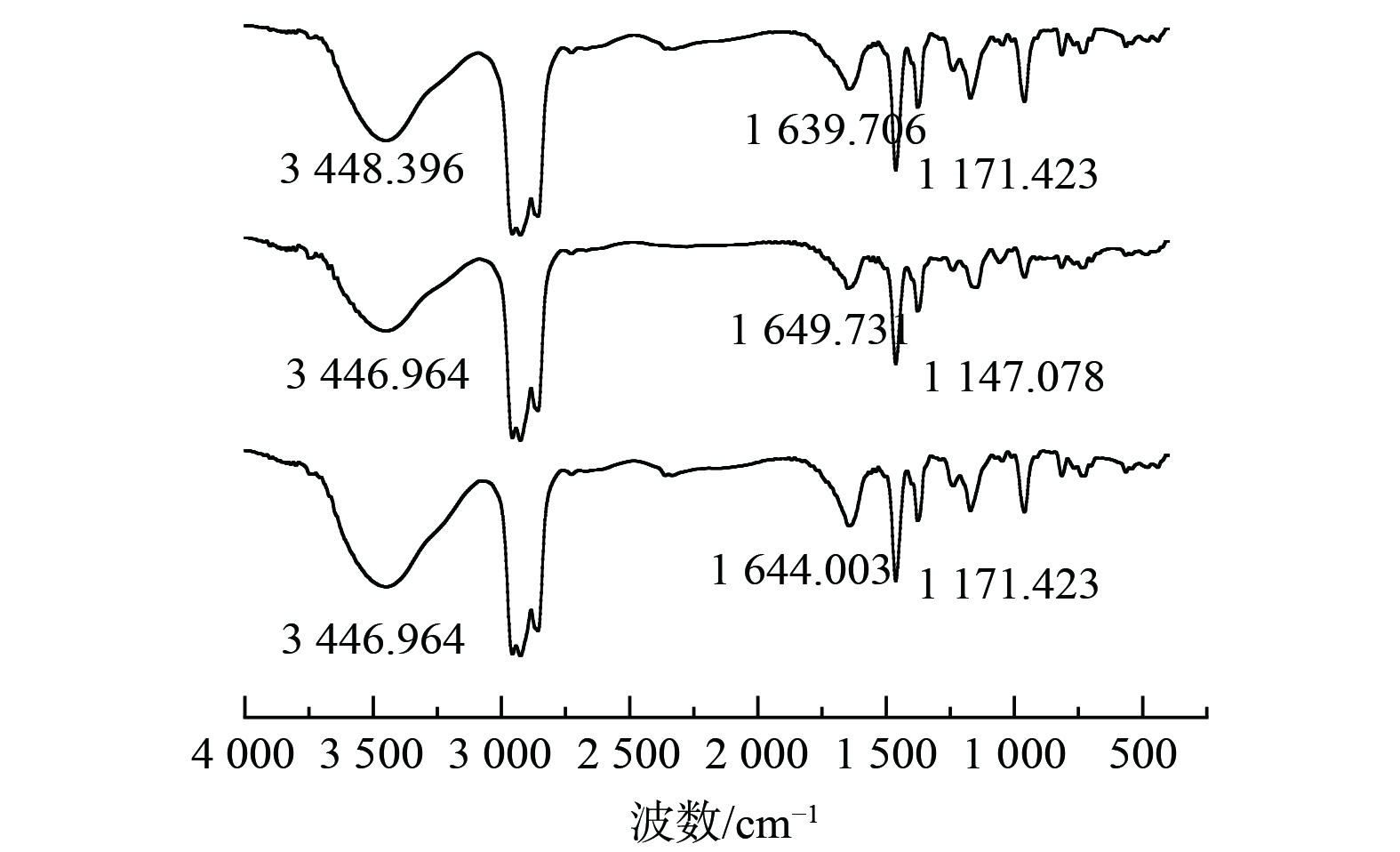
4)红外光谱测试分析Cyanex272萃取钴对Cyanex272有机相萃取前后及反萃后进行了红外光谱分析, 如图8所示。在3 448.396 cm−1附近和1 639.706 cm−1附近出现了二聚体分子间氢键伸缩振动峰, 和二聚体的O-H面内弯曲振动峰,在1 171.423 cm−1附近出现了P=O的伸缩振动峰。萃取后,位于3 448.396 cm−1的吸收峰的强度变低和位于1 639.706cm−1的峰向高波位1 649.731 cm−1移动,且峰强度有所降低,在反萃后有所恢复,说明-OH中的氢与锰发生了置换反应,而在萃取后,由高波位的1 171.423 cm−1的P=O的伸缩振动峰位移到低波位的1 147.078 cm−1,且峰强度变弱,这说明P=O键与钴离子发生配位反应,使P=O的电子云密度降低,减弱了P=O键的强度,使其振动频率减弱。因为P=O与Co的配位,使得Cyanex272的对称性改变,P=O键的偶极矩发生变化,导致峰强度减弱。即Cyanex272萃取钴的反应的实质是钴与P-O-H中的氢发生置换反应,且与P=O形成配位键进而被萃取。
-
1)在苹果酸体系中, P204做萃取剂通过三级逆流萃取可使锰的萃取率达99.99%,其中除萃取时间外,相比O/A、萃取剂体积分数、pH这3个因素对P204萃锰的萃取率有显著影响;通过三级逆流反萃,锰的反萃率可达88.88%。
2)在苹果酸体系中,Cyanex272做萃取剂通过三级逆流萃取可使钴的萃取率达94.01%,其中平衡pH对Cyanex272萃取钴的萃取率影响最为显著,其次分别是相比O/A和萃取剂体积分数。通过三级逆流萃取,钴的反萃率可达99.98%。
3)根据红外光谱结果表明,P204萃锰、Cyanex272萃钴的原理为金属离子与P-O-H中的氢发生置换反应,且与P=O形成配位键进而被萃取。
废旧锂电池正极材料浸出液中锰、钴的萃取分离
Extraction and separation of manganese and cobalt from the leaching solution of cathode materials of waste lithium batteries
-
摘要: 针对废旧锂电池中正极材料中有价金属未得到有效回收的情况,对废旧锂电池正极材料用苹果酸浸出后的浸出液进行分步萃取,以回收其中有价金属。研究了萃取时间、相比O/A、萃取剂体积分数等因素对各金属元素萃取率的影响。结果表明:在最佳萃取条件下,用P204 经过三级逆流萃取后,锰的萃取率达到99.99%,镍、钴、锂的共萃率分别为12.11%、9.20%、3.23%。负载锰的有机相用含1 g·L−1锰的苹果酸溶液洗脱共萃取的镍、钴、锂,然后用300 g·L−1的苹果酸三级逆流反萃锰,锰的反萃率达到了88.80%;用Cyanex272为萃取剂通过三级逆流萃取,钴的萃取率达到了94.01%,锂、镍的共萃率分别为11.21%和0.02%,负载钴的有机相用含1 g·L−1钴的苹果酸溶液洗脱共萃取的锂,随后用30 g·L−1的苹果酸对负载钴的有机相进行三级逆流反萃,钴的反萃率达到了99.98%;提出了先用P204从废旧锂电正极材料苹果酸浸出液中萃取分离Mn,而后再用 Cyanex272萃取分离Co的工艺流程,可实现苹果酸浸出液中Mn和Co的分离。本研究结果可为有机酸体系高效萃取锰、钴提供参考。Abstract: In response to the situation that the valuable metals in the cathode materials of waste lithium batteries were not effectively recovered, fractional extraction of malic acid lechate was conducted to recover the valuable metals therein. The effects of extraction time, ratio O/A, and extractant volume fraction on the extraction rate of each metal element were investigated. The results showed that, after three-stage countercurrent extraction using P204 under the optimal extraction conditions, 99.99% manganese was obtained, while the co-extraction of nickel, cobalt and lithium was 12.11%, 9.20%, 3.23%, respectively. The co-extracted nickel, cobalt, lithium of manganese loaded organic phase were eluted with malic acid solution containing 1 g·L−1 manganese, and then manganese was stripping with three-stage countercurrent stripping of 300 g·L−1 malic acid, and the manganese stripping rate reached 88.80%. The extraction rate of cobalt reached 94.01% by three-stage countercurrent extraction using Cyanex272, 11.21% and 0.02% for lithium and nickel, respectively. The co-extracted lithium of cobalt loaded organic phase were eluted with malic acid solution containing 1 g·L−1 cobalt, and then cobalt was stripping by three-stage countercurrent with 30 g·L−1 malic acid, achieving 99.98% for cobalt. The process flowsheet was proposed first extracting and separating Mn from the malic acid leachate with P204 and then extracting and separating Co with Cyanex272, and the separation of Mn and Co from the malic acid leachate can be achieved. The results of this study can provide a reference for the efficient extraction of manganese and cobalt by organic acid systems.
-
Key words:
- malic acid leachate /
- solvent extraction /
- P204 /
- Cyanex272
-
由于锂离子电池具有安全环保,电池容量大,输出功率大,自放电小等诸多优点,被广泛用于便携式电子产品,航空航天,医疗器械等领域[1~2]。据报道,2022年中国锂电池正极材料和负极材料产量分别有1.85×106 t和1.4×106 t,而2022年锂电池回收量仅有4.15×105 t,只有15%不到的锂电池得到有效回收,因锂电池使用寿命只有1~3年,如果回收率不变,到2025年可能会有2.835×106 t废电池不能得到有效回收。这些废旧锂电池中含有钴、镍等有毒重金属,直接丢弃会对土壤、河流等造成严重污染,而锂离子电池中锂、镍、钴、锰等金属的含量远高于一般矿物,具有极高的资源性和价值,随着科技不断发展和资源的不断开发,对资源的可循环利用和环境保护的要求被日益重视[3],因此废旧锂离子电池的回收显得尤为重要并已经迫在眉睫[4-5]。
溶剂萃取法[6]因其具有高提取率、选择分离性高、流程简单和操作连续化等优点,已经成为处理废旧锂电池中金属离子分离回收的主要方法。目前废旧三元锂电池中有价金属提取主要是在无机酸 (硫酸、盐酸等)体系中进行,因此,萃取分离的研究重点主要在硫酸、盐酸等无机酸体系中开展。如:代梦雅等[7]对电极材料使用硫酸浸出,浸出液经过P204除锰,在pH=5.2条件下,分别以0.5 mol·L−1 P507和0.6 mol·L−1 Cyanex272为萃取剂,经过两级错流萃取,钴萃取率分别为98.21%和99.44%, 镍共萃取率分别为24.42%和4.26%, 锂共萃取率分别为15.84%和5.11%;鲁桃等[8]在硫酸体系中使用Cyanex272,初始pH为4,萃取剂体积分数为50%,皂化率75%左右,O/A=1∶1,振荡时间为2 min,钴的一级萃取率为98.38%,而镍的损失率为2.28%。但在无机酸体系提取过程中易产生Cl2、SO2等有害气体、设备易腐蚀、铜、铝等杂质浸出率高等缺点,近年来,更多研究选用酸性较为温和、更为环保、杂质浸出率低的有机酸对正极材料中有价金属进行浸出被越来越多的研究者所关注[9]。如孟飞[10]使用柠檬酸对废旧三元电池浸出,镍钴锂锰的浸出率均大于90%,而铜、铝的浸出率均小于30%;高桂兰[11]对正极材料在柠檬酸浓度1.2 mol·L−1、硫代硫酸钠浓度0.3 mol·L−1、固液比20 g·L−1、浸出温度70 ℃、浸出时间30 min,钴的浸出率可达96%左右,锂的浸出率可达99%左右;但目前,关于在有机酸体系萃取分离废旧三元锂电池中的有价金属的文献较少。
本研究选用的是有机酸中较为便宜的D, L-苹果酸浸出废旧锂电池正极材料,经过前期一定的探索实验探究有机酸体系各种萃取剂对有价金属元素的萃取率;对浸出液选用一种或几种萃取剂来对其中的锰和钴进行分步萃取,研究不同影响因素下萃取剂对苹果酸浸出液中金属元素的萃取效果,为有机酸体系高效萃取锰、钴提供一定的参考。
1. 材料与方法
1.1 实验原料
本实验采用模拟的废旧三元电池正极材料LiNi0.6Co0.2Mn0.2O2的苹果酸浸出液。浸出液Ⅰ的成分为:Mn 2.26 g·L−1、Ni 5.7 g·L−1、Co 2.5 g·L−1、Li 1.24 g·L−1,苹果酸质量浓度为100 g·L−1。浸出液Ⅱ的成分为:Mn 4.51 g·L−1,苹果酸质量浓度为100 g·L−1。浸出液Ⅲ的成分为:Co 5.31 g·L−1,苹果酸质量浓度为100 g·L−1。浸出液IV的成分为:Co 2.50 g·L−1、Ni 4.94 g·L−1、Li 1.28 g·L−1,苹果酸质量浓度为100 g·L−1。萃取剂P204和萃取剂Cyanex272由重庆康普化学工业股份有限公司生产、萃取剂溶剂为煤油 (航空煤油)、NaOH为天津市风船化学试剂科技有限公司生产,苹果酸为湖县利乐生物科技实业有限公司生产。
1.2 实验方案
实验时,首先使用30%NaOH溶液对浸出液pH值进行调节,然后按设定相比将浸出液与有机相混于分液漏斗中,在震荡器上震荡一段时间,取出分液漏斗静置分相,随后对负载有机相使用极低质量浓度的被萃金属苹果酸溶液进行洗涤,静置分相并分离,随后用一定质量浓度苹果酸溶液与洗涤后的负载有机相进行反萃处理,静置分相并分离得到反萃液。方程式如式(1)~式(3)所示,其中Men+代表被萃金属离子,Mn+代表其它金属离子,HR表示萃取剂。
stringUtils.convertMath(!{formula.content}) (1) stringUtils.convertMath(!{formula.content}) (2) stringUtils.convertMath(!{formula.content}) (3) 式(1)是萃取剂将浸出液中的金属元素萃取到有机相中,式(2)是通过低质量浓度的苹果酸溶液将其它金属离子洗涤到溶液中,式(3)是使用低质量浓度苹果酸溶液将负载有机相中的金属离子反萃到苹果酸溶液中。
2. 结果与讨论
2.1 P204萃取Mn2+的研究
1)萃取条件对P204萃取Mn2+的影响。取浸出液Ⅱ20 mL,进行温度为25 ℃,萃取时间为1~9 min,相比O/A为1∶2至5∶2,萃取剂体积分数为10%~50%,浸出液pH为2.40~3.70系列条件下的萃取实验,实验结果如图1所示。如图1(a)所示,随着时间的增加,萃取率有轻微变化,但起伏不大,说明反应进行较快。当萃取时间为1 min时,反应已达到平衡,萃取率在60.00%左右;如图1(b)所示,随着相比O/A增加,锰的萃取率先迅速增加后趋于稳定,这是因为在溶液中其他离子质量浓度确定的情况下,锰的萃取率取决于萃取剂P204量的多少,增大相比O/A就等于增加萃取剂的量,当相比O/A较小时,萃取剂P204基本与锰完全反应,因溶液中锰的含量是一定的,所以最终锰的萃取率是趋于稳定的。考虑到原料利用率,相比过大导致体系粘度增加,影响分相效率及分相速率等问题,选择最佳相比O/A为1∶1,其萃取率为63.15%。由图1(c)可知,当萃取剂体积分数分别为10%、20%、30%时,萃取率分别为35.87%、65.25%、78.70%,随着萃取剂体积分数的增加,锰的萃取率也迅速增加,最后趋于平衡,这是因为随着P204含量的增加,萃取剂与锰的接触几率增加,形成萃合物的几率增加,从而萃取率增加,因锰的含量是固定的,所以最后萃取率趋于平衡。考虑到成本及萃取率增加速率,选择最佳萃取剂体积分数为20%。由图1(d)可知,随着初始pH的增加,萃取率迅速增加,然后趋于平缓,这是因为在萃取反应过程中金属离子会与萃取剂反应产生H+离子,pH值的增加有利于反应向生成萃合物的方向进行,当pH=2.96时,萃取率为89.75%,再继续增加pH,萃取率增加相对较小,因此选择最佳pH为2.96。取浸出液Ⅰ在最佳实验条件下进行三级逆流萃取实验,锰的萃取率可达到99.99%,镍、钴、锂的共萃率分别为12.11%、9.20%、3.23%。
2)其他金属离子对P204萃取Mn2+的影响。取浸出液Ⅱ20 ml,分别加入一定量碳酸钴、碳酸镍、碳酸锂,进行温度为 25 ℃,萃取时间为1 min,相比O/A=1∶1,萃取剂体积分数为20%的萃取实验,实验结果如图2所示。随着钴离子质量浓度的增加,锰离子萃取率在缓慢增加。由于添加碳酸钴导致初始pH发生变化,初始pH变为2.44~2.65,在无钴离子存在的情况下,P204对锰的萃取率从63%左右升至73%左右,而添加钴离子后,锰的萃取率从60.30%增加到70.40%,说明锰离子萃取率的增加主要是由于添加碳酸钴导致的初始pH值变化引起的。由于钴的萃取率在在逐渐增加,从而使锰、钴的分离系数在不断降低。由图2(b)可知,随着镍离子质量浓度的增加,锰离子的萃取率不断增加。由于加入了碳酸镍,导致初始pH值变为2.48~2.78,在无镍离子存在的情况下,锰的萃取率从64.00%左右升至78.00%左右,而添加镍离子后,锰的萃取率从54.93%升到70.40%,说明锰离子萃取率的增加主要是由于添加碳酸镍导致的pH值变化引起的。而镍离子的萃取率先增加后降低,这是因为P204对镍的萃取容量达到了最大值,继续增加镍离子质量浓度,萃取率降低。由图2(c)可知,随着锂离子质量浓度的增加,锰离子的萃取率不断增加,由于加入了碳酸锂,初始pH值变为2.70~3.28,在无锂离子存在的情况下,P204对锰的萃取率从75.00%左右上升到95.00%左右,添加锂离子后,萃取率由69.28%上升至82.06%,说明锰离子萃取率的增加主要是由于添加碳酸锂导致的pH值变化引起的。由图2的(a)、(b)、(c)可知,3种离子与锰的分离系数均较差,为了保证纯度,需要在后续对负载有机相进行水洗以除去其中大部分的其他金属离子。
3) P204负载有机相反萃锰的研究。为了抑制有机相中锰被洗出,使用含1 g·L−1锰的100 g·L−1苹果酸溶液以相比O/A=1∶1对浸出液Ⅰ使用P204进行三级逆流萃取后的负载锰有机相洗涤,洗涤前有机相中Mn为2.255 g·L−1,Ni为0.69 g·L−1,Co为0.23g·L−1,洗涤后有机相中Mn为2.51 g·L−1,Ni为0.04 g·L−1,Co仅含0.03 g·L−1,锂离子质量浓度太低未检测到,表明洗涤效果较良好。对洗涤后的负载有机相进行反萃,固定条件为:反萃介质为苹果酸,相比O/A=1∶1、反萃时间20 min、温度25 ℃,实验结果如图3。由图3可知,在此反萃条件下,Mn的单级反萃率可达55.00%左右。在同样反萃条件下对Mn负载有机相进行三级逆流反萃,反萃率可达88.80%,由此可见,苹果酸溶液能将大部分的锰反萃。
4)红外光谱测试分析P204萃取锰对P204有机相萃取前后及反萃后进行了红外光谱分析,如图由图4可知,在3 448.396 cm−1出现二聚体分子间氢键伸缩振动峰,在1 649.731 cm−1出现二聚体的O-H面内弯曲振动峰。在萃取后,3 448.396 cm−1振动峰强度降低但是仍能观察到二聚体峰,说明分子间形成的-OH键中的H被锰取代,但是P204过量,未能完全消除二聚体的影响。而1 649.731 cm−1的峰向低波数1 638.274移动,且峰强度有所降低,这是因为P-O→Mn的生成的同时使氢键电子云降低。在反萃后,振动峰强度有一定恢复且向高波数偏移至1 641.238 cm−1,这是因为反萃后锰被氢离子置换出去,但是仍未置换完全。在1 231.570 cm−1处出现P=O的伸缩振动峰,在萃取后,由高波数的1 231.570 cm−1位移到1 222.978 cm−1说明P=O键与Mn离子发生配位,波数变化是由于P=O键与Mn形成P=O→Mn配位键,使P=O双键的电子云密度降低,键的强度减弱,从而导致其震动频率下降。同时因为Mn与P=O的配位,使P204的对称性发生变化,P=O键的偶极矩发生变化,导致峰强度减弱[12-14]。即P204萃取锰的反应的实质是Mn与P-O-H中的氢发生置换反应,且与P=O形成配位键进而被萃取。
2.2 Cyanex272萃取钴的研究
1)萃取条件对Cyanex272萃取Co2+的影响。取浸出液Ⅲ 20ml,进行温度为25 ℃,萃取时间为1~9 min,相比O/A为1∶4至5∶2,萃取剂体积分数为10%~50%,平衡pH为4~6的萃取实验,实验结果如图5所示。如图5(a)所示,整个反应进行较快,萃取率整体变化不大。当萃取时间为3min时,萃取率为87.56%,达到最大值,因此,3 min为最佳萃取时间。由图5(b)可知,随着相比O/A的不断增加,萃取率也在不断增加。因溶液中钴离子的含量是一定的,相比O/A的不断增加,最终钴的萃取率时趋于稳定的。考虑到成本,原料利用率,以及相比过大时导致影响分相效率及分相速率低,选择最佳相比O/A为1∶1,钴的最佳萃取率为81.97%。由图5(c)可知,随着萃取剂体积分数的不断增加,萃取率也是在不断增加的。这是因为Cyanex272的增加,萃取剂与钴的接触几率也在不断增加,形成萃合物的几率增加,最终萃取率增加。当萃取剂体积分数分别10%、20%、30%,萃取率分别为71.92%、91.65%、93.72%,因萃取剂体积分数为20%时,萃取率上升幅度较大,所以最佳萃取剂体积分数为20%。由图5(d)可知,随着pH的增加,萃取率不断增加,当pH为5~6时,萃取率有较大幅度增加。当平衡pH=6,钴的萃取率为91.75%。随着萃取反应的进行,溶液的pH在不断降低,增加水相的平衡pH有利于萃取反应发生,进而增大钴的萃取率,最终选择最佳平衡pH为6。取浸出液IV在最佳实验条件下进行三级逆流萃取钴实验,钴的萃取率为94.01%,锂、镍的共萃率分别为11.21%和0.02%。
2)其他金属离子对Cyanex272萃取Co2+的影响。取浸出液Ⅲ20ml,分别加入一定量碳酸镍、碳酸锂,并重新定容,进行温度为25 ℃,萃取时间为3min,相比O/A=1∶1,萃取剂体积分数为20%,平衡pH=6的萃取实验,实验结果如图6所示。因Cyanex272对钴、镍均具有良好的萃取效果,所以测试在添加一定量镍离子后,在萃取剂体积分数为20%,相比O/A=1∶1,萃取时间3 min,镍离子质量浓度为10.4 g·L−1条件下,不同平衡pH下Cyanex272对镍、钴的萃取效果,结果如图6(a)所示,随着平衡pH的增加,钴离子的萃取率在不断增加,镍离子的萃取率基本保持不变。当pH=6时,钴的萃取率为89.62%,镍的萃取率未超过10%,说明不同pH下,镍离子对Cyanex272萃钴过程基本无影响。由图6(b)可知,随着镍离子质量浓度的增加,钴离子萃取率先增后趋于平衡,与未添加镍离子时相比,钴的的萃取率有一定的下降,即镍离子对Cyanex272萃钴有抑制作用,但是随着镍离子质量浓度的增加,这种抑制作用在不断降低。镍离子萃取率基本维持在20.00%左右,当镍离子质量浓度为3 g·L−1时,钴的萃取率为86.24%,此时镍钴的分离系数为20.2,两者较易进行分离。由图6(c)可知,随着锂离子质量浓度增加,钴离子萃取率整体呈下降趋势,说明锂离子对钴离子的萃取有一定抑制作用,且这种抑制作用在不断增强。
3) Cyanex272负载有机相反萃钴的研究。为了抑制有机相中的钴被洗出,使用含1 g·L−1钴的10 g·L−1苹果酸溶液以相比O/A=1∶1对浸出液IV使用Cyanex272进行三级逆流萃取后的负载钴有机相洗涤,洗涤前有机相中Co 2.31 g·L−1,Ni 0.01 g·L−1,Li 0.14 g·L−1,洗涤后有机相中Co为2.81 g·L−1,镍未检测出,锂仅含5.47 mg·L−1,洗涤效果良好。对洗涤后的负载有机相进行反萃,固定条件为:反萃介质为苹果酸、相比O/A=1∶1、反萃时间10min、温度25 ℃。实验结果如图7。由图7可知,当反萃介质苹果酸质量浓度为30 g·L−1时,Co的单级反萃率可达76.61%。在同样条件下进行三级逆流反萃Co后,Co反萃率为99.98%。
4)红外光谱测试分析Cyanex272萃取钴对Cyanex272有机相萃取前后及反萃后进行了红外光谱分析, 如图8所示。在3 448.396 cm−1附近和1 639.706 cm−1附近出现了二聚体分子间氢键伸缩振动峰, 和二聚体的O-H面内弯曲振动峰,在1 171.423 cm−1附近出现了P=O的伸缩振动峰。萃取后,位于3 448.396 cm−1的吸收峰的强度变低和位于1 639.706cm−1的峰向高波位1 649.731 cm−1移动,且峰强度有所降低,在反萃后有所恢复,说明-OH中的氢与锰发生了置换反应,而在萃取后,由高波位的1 171.423 cm−1的P=O的伸缩振动峰位移到低波位的1 147.078 cm−1,且峰强度变弱,这说明P=O键与钴离子发生配位反应,使P=O的电子云密度降低,减弱了P=O键的强度,使其振动频率减弱。因为P=O与Co的配位,使得Cyanex272的对称性改变,P=O键的偶极矩发生变化,导致峰强度减弱。即Cyanex272萃取钴的反应的实质是钴与P-O-H中的氢发生置换反应,且与P=O形成配位键进而被萃取。
3. 结论
1)在苹果酸体系中, P204做萃取剂通过三级逆流萃取可使锰的萃取率达99.99%,其中除萃取时间外,相比O/A、萃取剂体积分数、pH这3个因素对P204萃锰的萃取率有显著影响;通过三级逆流反萃,锰的反萃率可达88.88%。
2)在苹果酸体系中,Cyanex272做萃取剂通过三级逆流萃取可使钴的萃取率达94.01%,其中平衡pH对Cyanex272萃取钴的萃取率影响最为显著,其次分别是相比O/A和萃取剂体积分数。通过三级逆流萃取,钴的反萃率可达99.98%。
3)根据红外光谱结果表明,P204萃锰、Cyanex272萃钴的原理为金属离子与P-O-H中的氢发生置换反应,且与P=O形成配位键进而被萃取。
-
-
[1] TIRATH R, KUPPAM C, NARESH A K, et al. Recycling of cathode material from spent lithium-ion batteries: Challenges and future perspectives[J]. Journal of Hazardous Materials, 2022, 429: 128312. doi: 10.1016/j.jhazmat.2022.128312 [2] JIN S, MU D Y, LU Z A, et al. A comprehensive review on the recycling of spent lithium-ion batteries: Urgent status and technology advances[J]. Journal of Cleaner Production, 2022, 340: 130535. doi: 10.1016/j.jclepro.2022.130535 [3] 荆俊杰, 谢吉民. 微量元素锰污染对人体的危害[J]. 广东微量元素科学, 2008, 15(2): 6-9. doi: 10.3969/j.issn.1006-446X.2008.02.002 [4] MESHRAM P, PANDEY B D, MANKHAND T R. Hydrometallurgical processing of spent lithium ion batteries (LIBs)in the presence of a reducing agent with emphasis on kinetics of leaching[J]. Chemical Engineering Journal, 2015, 281: 418-427. doi: 10.1016/j.cej.2015.06.071 [5] MESHRAM P, PANDEY B D, MANKHAND T R. Recovery of valuable metals from cathodic active material of spent lithium ion batteries: Leaching and kinetic aspects[J]. Waste Management, 2015, 45(11): 306-313. [6] 范丹丹. 抗坏血酸浸出报废镍钴锰三元电池中有价金属的研究[D]. 上海: 上海第二工业大学, 2019: 4-8. [7] 代梦雅, 张亚茹, 张可, 等. 用溶剂萃取—沉淀法从废锂离子电池正极材料中回收钴镍锂[J]. 湿法冶金, 2019, 38(4): 276-282. doi: 10.13355/j.cnki.sfyj.2019.04.005 [8] 鲁桃, 程洁红. 溶剂萃取分离废锂离子电池中的钴[J]. 江苏理工学院学报, 2019, 25(6): 22-28. doi: 10.3969/j.issn.1674-8522.2019.06.004 [9] 徐平, 陈钦, 张西华, 等. 废锂离子电池中锂提取技术研究进展[J]. 过程工程学报, 2019, 19(5): 853-864. doi: 10.12034/j.issn.1009-606X.219221 [10] 孟飞. 废弃三元锂离子电池金属元素选择性浸出及分离特性研究[D]. 重庆: 重庆大学, 2020: 87-100. [11] 高桂兰. 有机酸还原性体系浸出回收废弃锂离子电池正极材料的研究[D]. 上海: 上海大学, 2019: 81-82. [12] 吴诗婷. P204-添加剂改性萃取体系萃取钕、钆的研究[D]. 南昌: 南昌航空大学, 2018: 47-52. [13] 李剑虹, 张兴. P204-HCl-HAc体系萃取La的机理分析与萃取平衡常数[J]. 稀有金属与硬质金, 2010, 38(2): 11-13. [14] 徐志高, 王力军, 吴延科, 等. DIBK-P204体系萃取分离锆和铪的机理[J]. 中国有色金属学报, 2013, 23(7): 2061-2068. doi: 10.19476/j.ysxb.1004.0609.2013.07.039 -




 下载:
下载: