-
挥发性有机化合物(volatile organic compounds,VOC)是室内最常见的环境污染物之一,而甲醛(HCHO)是其中一种典型污染物[1]。当人体处在甲醛质量浓度大于0.1 mg·m-3环境中时,即可能引发呼吸系统受损,而长期暴露会对人体其他器官造成损害[2]。甲醛已被列为第一类致癌物[3-4]。目前,防治室内甲醛污染的方法有物理法、生物法和化学法,其中物理法包括自然通风和吸附法等,生物法包括植物生态净化和微生物降解等,化学法包括等离子体法、催化氧化技术等[5-7]。物理法成本低、效率高,但不能彻底解决甲醛污染问题,亦可能造成二次污染[8];生物法绿色环保,处理成本低,无二次污染,但存在受环境温/湿度、菌种选择等条件限制,效率低等问题[9];等离子体技术虽受环境影响小,但需施加外部条件,耗能高,安全性差[10-11];催化氧化技术已被证明是一种行之有效的甲醛去除方法,其具体包括热催化、光催化、常温催化、臭氧净化等[12-14]。其中,常温催化氧化技术具有操作简单、耗能低、无二次污染、净化效率高等优点而备受广泛关注,其在实现去除室内低浓度气态甲醛方面有着重要应用价值。
常温催化氧化催化剂可分为两大类:一是贵金属催化剂;二是过渡金属氧化物催化剂。贵金属如Pt、Pd、Au和Ag等,通常以TiO2、Al2O3、SiO2、Co3O4、CeO2等高比表面积为载体制备而成,其活性高、降解性能优越[15-17],但是贵金属资源稀缺、价格昂贵,限制了其广泛范围。过渡金属氧化物因资源丰富、氧化活性高、稳定性良好等优势而具备替代贵金属的潜力,且过渡金属锰氧化物(MnOx)在降解甲醛性能方面表现优异,氧化能力强、CO2选择性高,且晶型、价态、形貌可调,应用前景广泛[18-23]。LU等[24]通过水热法制备了石墨烯-MnO2复合纳米材料,相较于单金属催化剂MnO2,复合催化剂完全催化甲醛所需温度降低了75 ℃,催化性能提高,并且石墨烯增强了Mn3+和Mn4+间的电荷转移,其催化剂表面产生了丰富的氢氧自由基,提升了甲醛催化氧化中间产物-甲酸盐的降解活性。ZHU等[25]通过氧化还原法及铈改性制备了掺杂型MnO2,研究发现随着Ce掺杂量的增加,MnO2结晶度降低,催化剂比表面积增大。当Ce-MnO2中铈锰摩尔比达1∶10时,催化剂常温催化性能稳定,且在100 ℃下可将低浓度甲醛完全氧化为CO2和H2O,这是由于Ce掺杂的MnO2具有更多的表面吸附氧,减小了CeO2纳米团簇与MnO2间的晶界间距,提升了表面氧和晶格氧的氧化还原能力和迁移速率。LI等[26]利用Pechini法制备了MnxCe1-xO2 (x=0.3~0.9) 催化剂,其中Mn0.5Ce0.5O2催化剂活性最佳,且随着质量分数为5% CuOx的掺入,催化剂可进一步降低甲醛100%转化时所需的反应温度。氧化铜的引入增强了氧化物间的协同作用,提高了材料分散性和氧化能力,促进催化材料中氧的释放,进而优化了催化氧化甲醛性能。
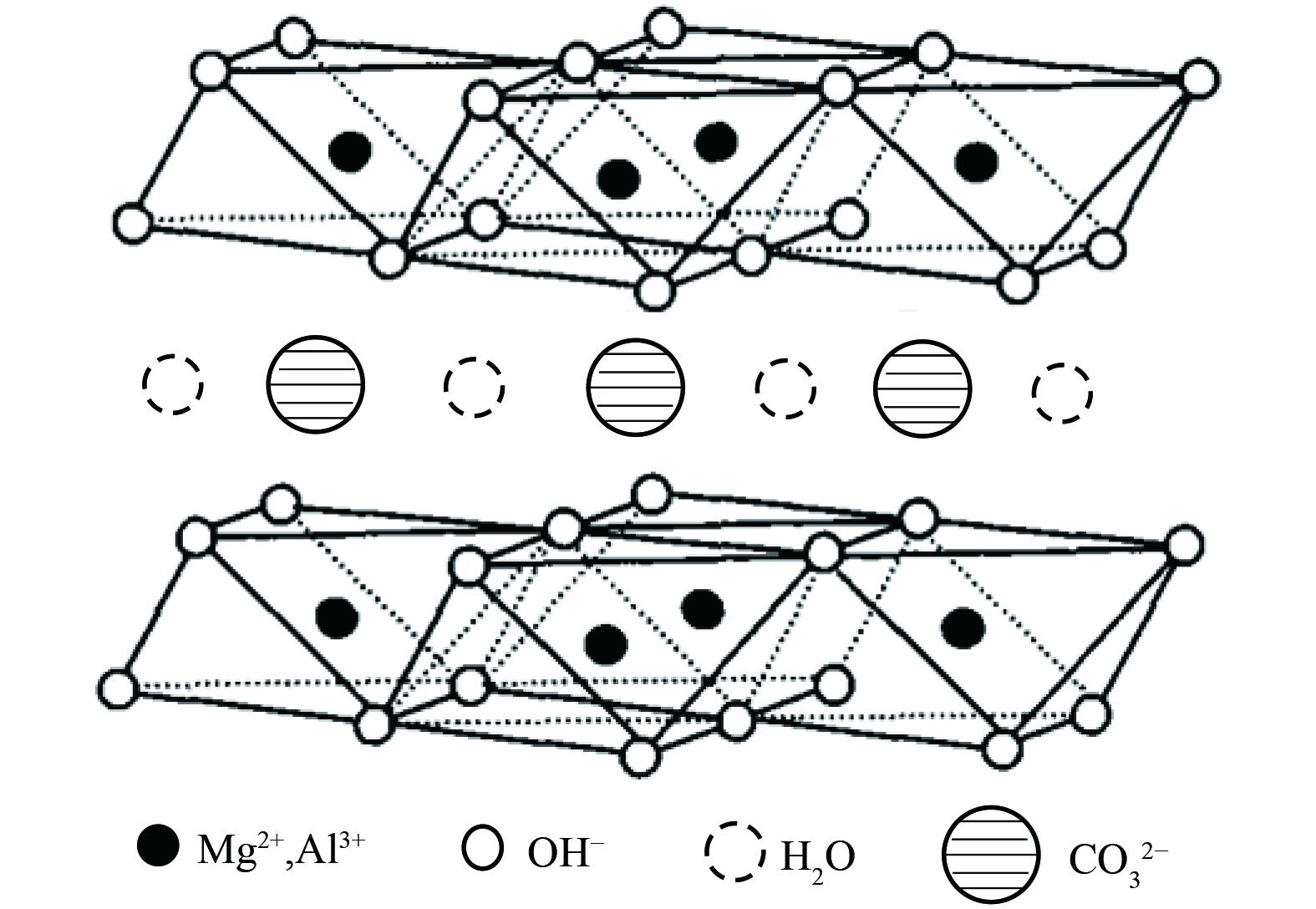
如今催化氧化技术已取得长足进展,但探求高效稳定、抗水蒸气能力强、低成本、常温条件去除低浓度甲醛催化剂仍面临挑战。类水滑石化合物(LDHs)是近年来发展迅速的阴离子型粘土材料[27],其通式表示为[M2+1-xM3+x(OH)2]x+[An-x/n]·mH2O,结构如图1所示。其中,M2+是二价金属阳离子,M3+是三价金属阳离子,An-是层间阴离子,通常为无机阴离子Cl-、CO32-等。因其具有耐酸碱性、热稳定性、吸附性、层间阴离子可交换性及高比表面积等特点,在吸附、催化、阻燃、医药等领域已被广泛应用[28-29]。为此本研究提出采用共沉淀法制备了MnOx/类水滑石催化剂,以类水滑石为载体、片状氧化锰为活性组分,探究载体各组分、煅烧温度、氧化锰负载量等对催化剂常温催化氧化性能的影响,运用XRD、SEM、BET、EPR、XPS等技术表征分析探究催化剂微观结构,考察并分析其常温催化氧化甲醛活性和稳定性,以克服锰基氧化物常温条件下因大量吸附水蒸气而稳定性降低的缺点,研制一种高效稳定的过渡金属氧化物催化剂,为常温催化氧化催化剂的研制提供参考。
-
1) 类水滑石载体的制备。准确称取一定量六水氯化镁和九水合硝酸铝,并将其溶解于100 mL去离子水中,充分搅拌得溶液A;并按照一定比例配制碳酸钠和氢氧化钠的混合溶液B;再将溶液B缓慢滴加到溶液A中,继续搅拌直至pH达10为止,继续搅拌3 h;再将上述溶液离心分离,并用去离子水和无水乙醇各清洗3次,将所得水凝胶在烘箱80 ℃干燥过夜,再置于马弗炉中100 ℃煅烧4 h,根据n(Mg)/n(Al)命名为Mg1Al1、Mg4Al1等类水滑石载体。
2) MnOx/类水滑石负载催化剂的制备。采用共沉淀法制备MnOx/类水滑石催化剂,具体方法如下:准确称量4.0 g Mg1Al1类水滑石载体,将其浸泡于50 mL去离子水中,充分搅拌,并向其中加入一定量硝酸锰溶液,获得悬浊液;再配制0.2 mol·L−1氢氧化钠溶液,并将其缓慢滴加至上述悬浊液中直至pH=10,继续搅拌3 h。再将上述溶液离心分离,并用去离子水和无水乙醇各清洗3次,将得沉淀物在烘箱80 ℃干燥过夜和马弗炉100 ℃、4 h煅烧,即制得负载量为10%的MnOx/Mg4Al1催化剂,其他不同n(Mg)/n(Al)、煅烧温度、MnOx负载量以及类水滑石组分催化剂均采用上述方法制得。
-
实验在50 cm×50 cm×50 cm的密闭玻璃反应器中室温条件下进行。首先在该反应器内采用甲醛溶液挥发方式配制初始低浓度气态甲醛(1.0~1.1 mg·m−3),并将1.0 g的催化剂粉末均匀铺设于一培养皿中(Φ=6 cm),并将其置于反应器底部,并迅速密封该反应器,其反应器内甲醛质量浓度采用甲醛分析仪(PPM-400ST)测定,每12 h连续测定3次,取平均值,连续观察其48 h甲醛质量浓度变化情况,其甲醛去除率按下式计算。
式中:Ct表示每12 h后反应器内甲醛质量浓度,mg·m−3;C0表示反应器内初始甲醛质量浓度,mg·m−3。
催化剂稳定性测试在上述氧化活性测试基础上进行。催化剂经48 h测试后,直接采用注入甲醛溶液方式再次使其在玻璃反应器内挥发至设定的甲醛质量浓度(1.0~1.1 mg·m-3),然后迅速取出甲醛溶液并密封该反应器,并测定其浓度作为第二次活性测试的起始浓度,过程中催化剂保持固定不变,反复操作进行即可。
-
为探究催化剂微观结构,采用X射线衍射仪(XRD, D/teX Ultra 250 detector, Shimadzu XRD-6100, 日本)对催化剂进行XRD分析,检测过程使用Cu-K α辐射(λ=0.154 06 nm),并在40 kV和40 mA下进行;表面形貌采用德国蔡司公司生产的Gemini SEM扫描电镜检测分析,工作电压5.0 kV;比表面积、孔径大小及分布分别基于Brunauer-Emmett-Teller (BET)和Barrett-Joyner-Halenda (BJH)方法(Micromeritics ASAP 2460)在77 K下对催化剂进行N2吸附脱附条件下进行检测;室温下采集了电子顺磁共振图谱(EPR),其X波段微波频率为9.43 GHz,功率为1.5 mW。运用X射线光电子能谱(Thermo ESCALAB 250XI) 分析材料表面的元素组成、化学状态及元素种类, 放射源为Al-Kα (hv=1486.8 eV) ,所得能谱采用C 1s (284.6 eV) 的结合能校准,使用15 kV和300 W的Al-Kα X射线源分析元素化学结合能。
-
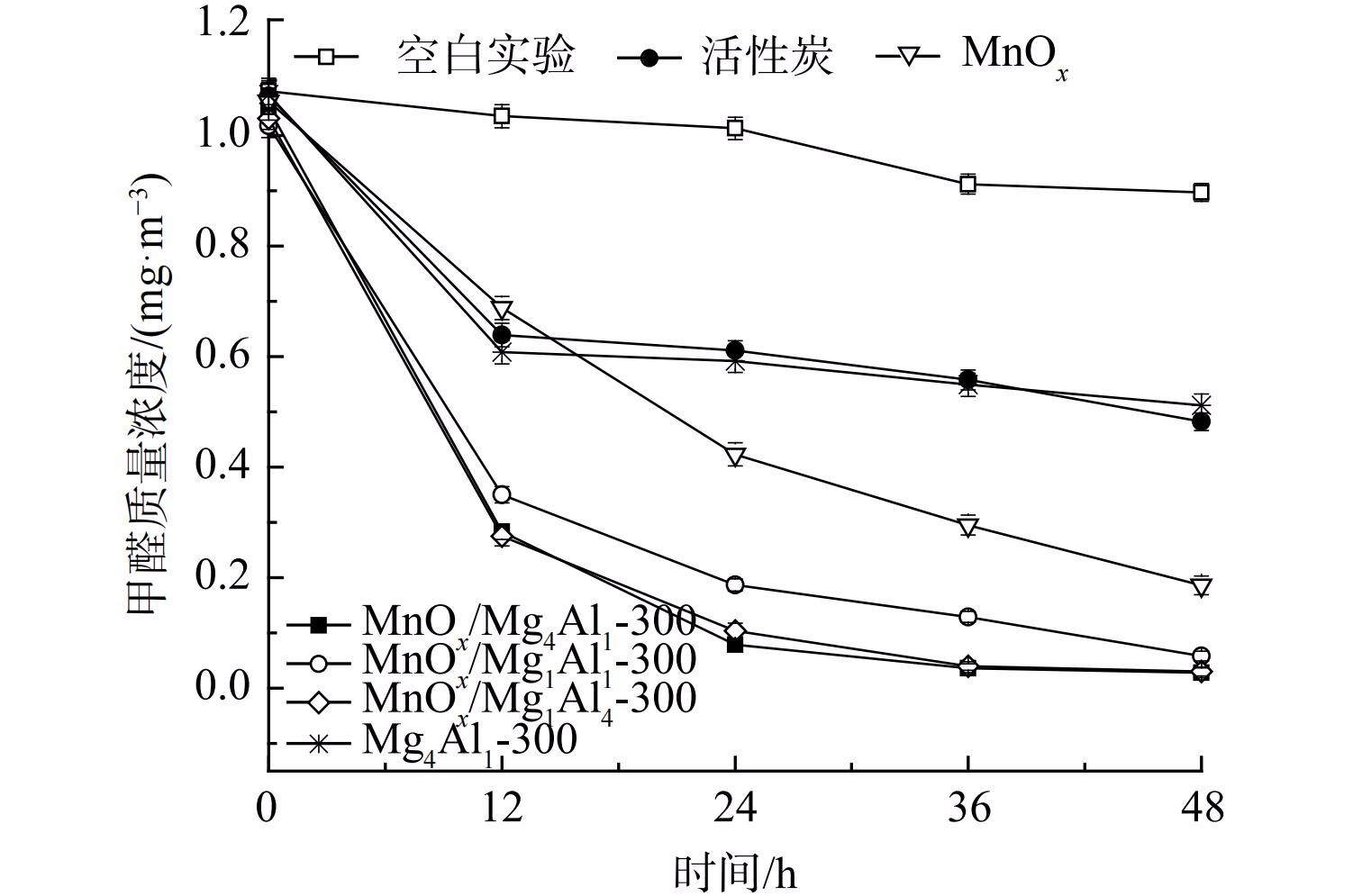
1) n(Mg)/n(Al)对催化剂氧化性能的影响。为对比分析,研究考察了空白反应器以及等量活性炭的吸附性能 (图2) 。空白反应器内甲醛48 h略有下降,下降率仅为16.9%,其可能为吸附及分解等所致。这表明该反应器较为稳定,不存在大量吸附及漏气等情况。等量活性炭因具有较高比表面积而展示出较优异的吸附性能,48 h甲醛吸附率为55.0%。但其氧化活性远低于锰氧化物,这是由于锰氧化物具有更优异的吸附+常温催化性能。载体可对催化剂氧化性能产生显著深刻影响。为此,考察了n(Mg)/n(Al)对MnOx/类水滑石常温催化氧化甲醛性能的影响。在同等测试条件下,纯水滑石Mg4Al1-300性能最差,其48 h甲醛去除率仅为52.3%,仅显示出一定吸附性能。这表明类水滑石Mg4Al1主要作为载体而存在,而纯氧化锰粉末展示出良好的吸附+常温催化氧化性能,其甲醛去除率明显高于比表面积更高的Mg4Al1水滑石(58.2 m2·g−1),表明其可能发生了催化氧化反应。还将MnOx负载至水滑石载体上,其催化剂氧化活性较MnOx显著提升。当n(Mg)∶n(Al)=4∶1、MnOx负载量为40%和煅烧温度为300 ℃时,MnOx/Mg4Al1(比表面积:81.4 m2·g−1)催化剂氧化性能最优,48 h内可将甲醛质量浓度由1.049 mg·m−3降至0.028 mg·m−3,去除率高达97.3%,其前24 h甲醛去除率高达92.5%。而随着时间推移其甲醛去除率逐渐降低,反应受反应物扩散速率控制。相比其他MnOx/MgAl,MnOx/Mg1Al1催化剂性能最差,其比表面积(79.0 m2·g−1)、孔容(0.187 cm3·g−1)和孔径(6.5 nm)亦最低。虽MnOx/Mg1Al4比表面积最高,达156.7 m2·g−1,但其孔径明显低于MnOx/Mg4Al1,其一定程度上限制了甲醛的吸附和反应产物脱附,致使其氧化活性与MnOx/Mg4Al1相当。3种催化剂的氧化活性均高于MnOx(22.6 m2·g−1),这表明其材料的比表面积和孔容孔径是影响催化剂氧化活性的因素之一。
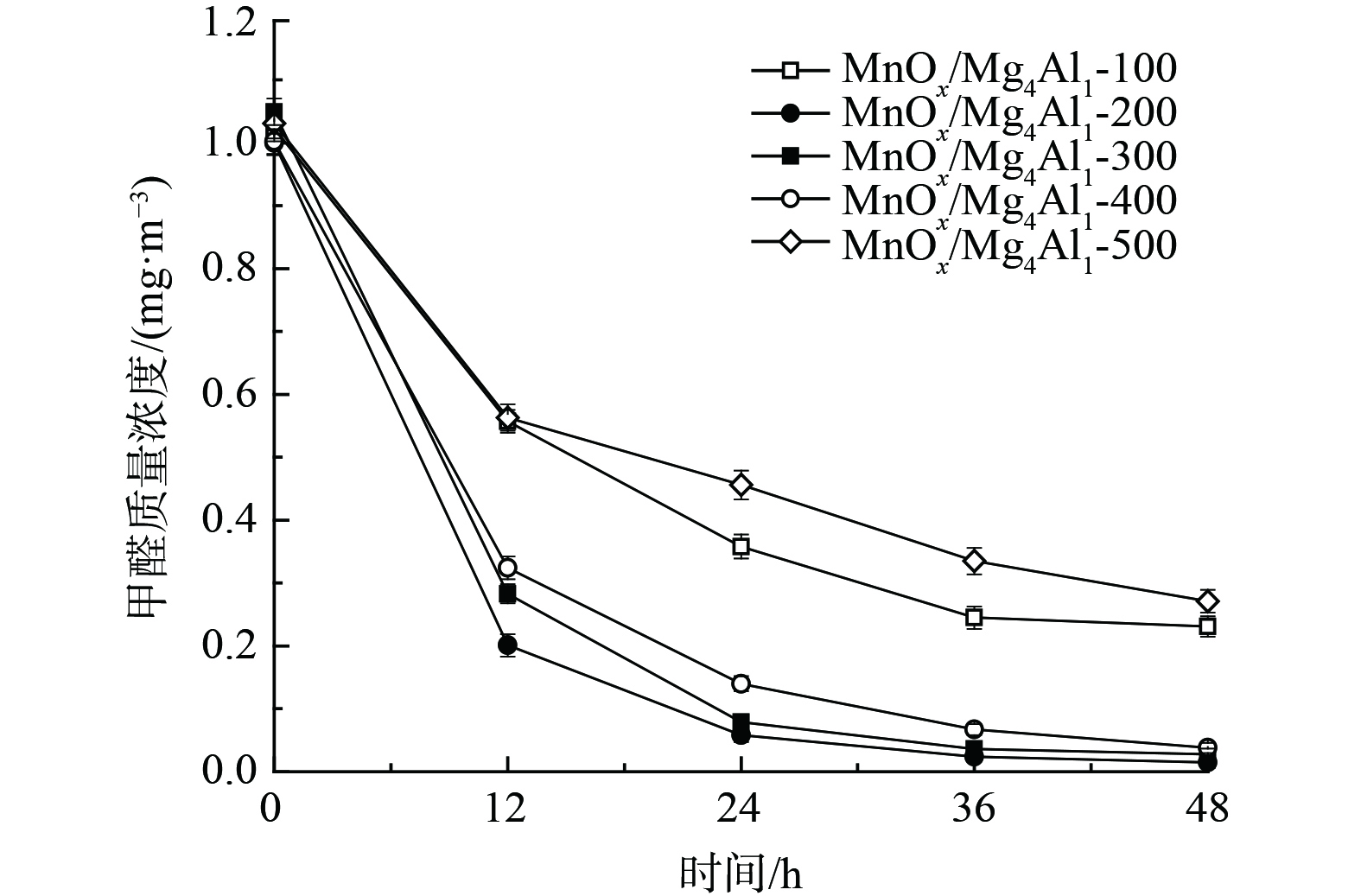
2) 煅烧温度对催化剂氧化性能的影响。图3为不同煅烧温度条件对MnOx/Mg4Al1催化剂氧化性能的影响。随着煅烧温度的提升,其甲醛去除率先升高后持续降低。当煅烧温度为200 ℃时,催化剂表现出最佳的氧化活性,甲醛去除率高达98.5%,其质量浓度由1.000 mg·m−3降至0.015 mg·m−3,明显优于其他同类催化剂。其中,经500 ℃高温煅烧所制催化剂性能最差,48 h甲醛去除率仅为73.7%。这表明其氧化锰晶体及催化剂表面结构可能发生了显著变化,这一结果与XRD和SEM相一致。
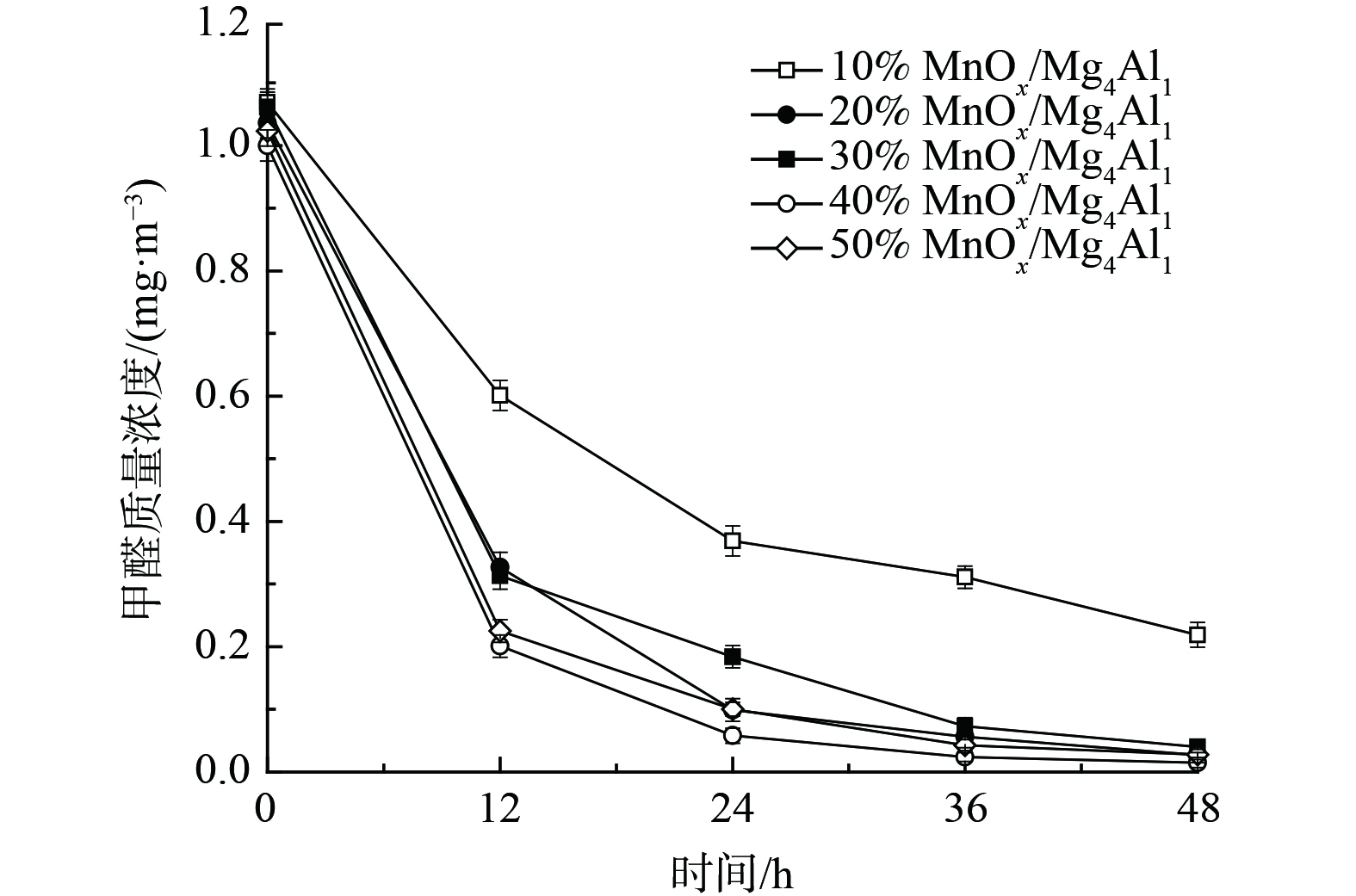
3) MnOx负载量对催化剂氧化性能的影响。为进一步提升催化剂氧化性能、降低成本,考察了MnOx负载量对催化剂常温催化氧化甲醛的性能影响 (图4) 。随着MnOx负载量由40%降至10%,其氧化性能持续降低。当MnOx负载量为10%时,48 h内甲醛去除率为79.5%。而随着MnOx负载量提升至50%时,其氧化性能亦未得到显著提升,反而有所降低,其可能与催化剂比面积下降有关。这表明其MnOx负载量并非越多越佳,存在最佳比例。上述结果表明,MnOx负载量在20%~40%均可接受,其催化剂氧化性能良好,为此选择MnOx负载量为20%进行后续研究。
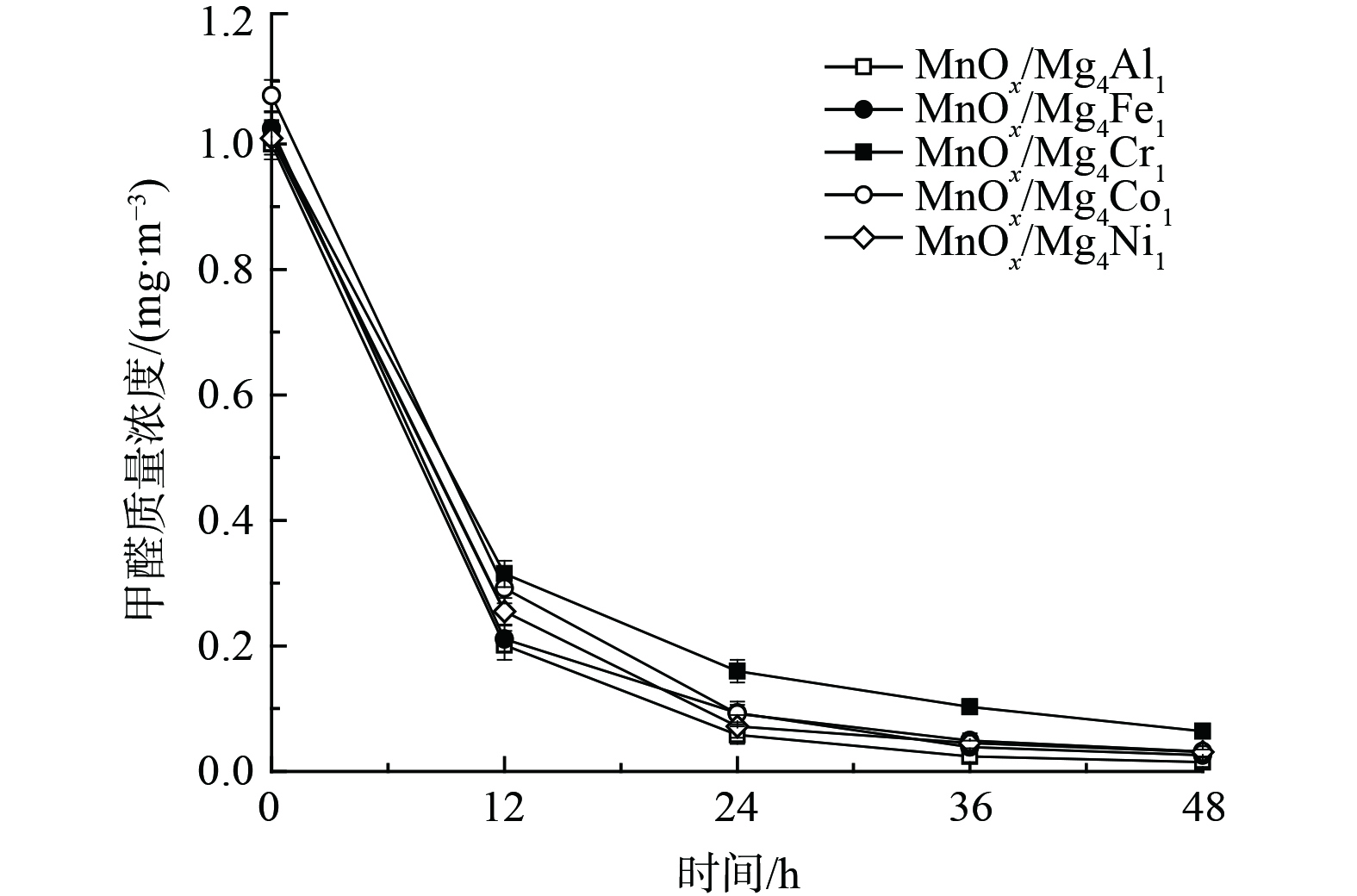
4) 类水滑石组分对催化剂氧化性能的影响。考虑到载体组成成分亦可能会对催化剂降解甲醛能力产生差异,考察了5种组分的类水滑石对催化剂氧化性能的影响 (图5) 。不同组分对催化剂催化效果影响差异较小,48 h内MnOx/Mg4Al1、MnOx/Mg4Fe1、MnOx/Mg4Cr1、MnOx/Co4Al1和MnOx/Ni4Al1催化剂的甲醛转化率依次为98.5%、97.5%、93.7%、97.0%和96.9%。相比之下,MnOx/Mg4Cr1催化剂活性较低,而MnOx/Mg4Al1催化活性最佳。结果表明,不同组分的类水滑石对催化剂的氧化性能影响较小,且对负载有MnOx的MnOx/类水滑石催化剂氧化性能均有显著提升。
5) 催化剂稳定性研究。图6为氧化性能最佳的20% MnOx/Mg4Al1-200催化剂常温催化氧化甲醛的稳定性研究结果。在相同条件下对该催化剂进行了连续3次活性测试,其48 h甲醛去除率依次为97.5%、96.3%和96.9%,催化剂稳定性良好,未见明显的因吸附大量水蒸气而导致的吸附和氧化活性下降的趋势。这表明,催化剂抗水蒸气性能良好,可反复使用,保持着较高的甲醛去除效率,对实际应用有参考价值。
-
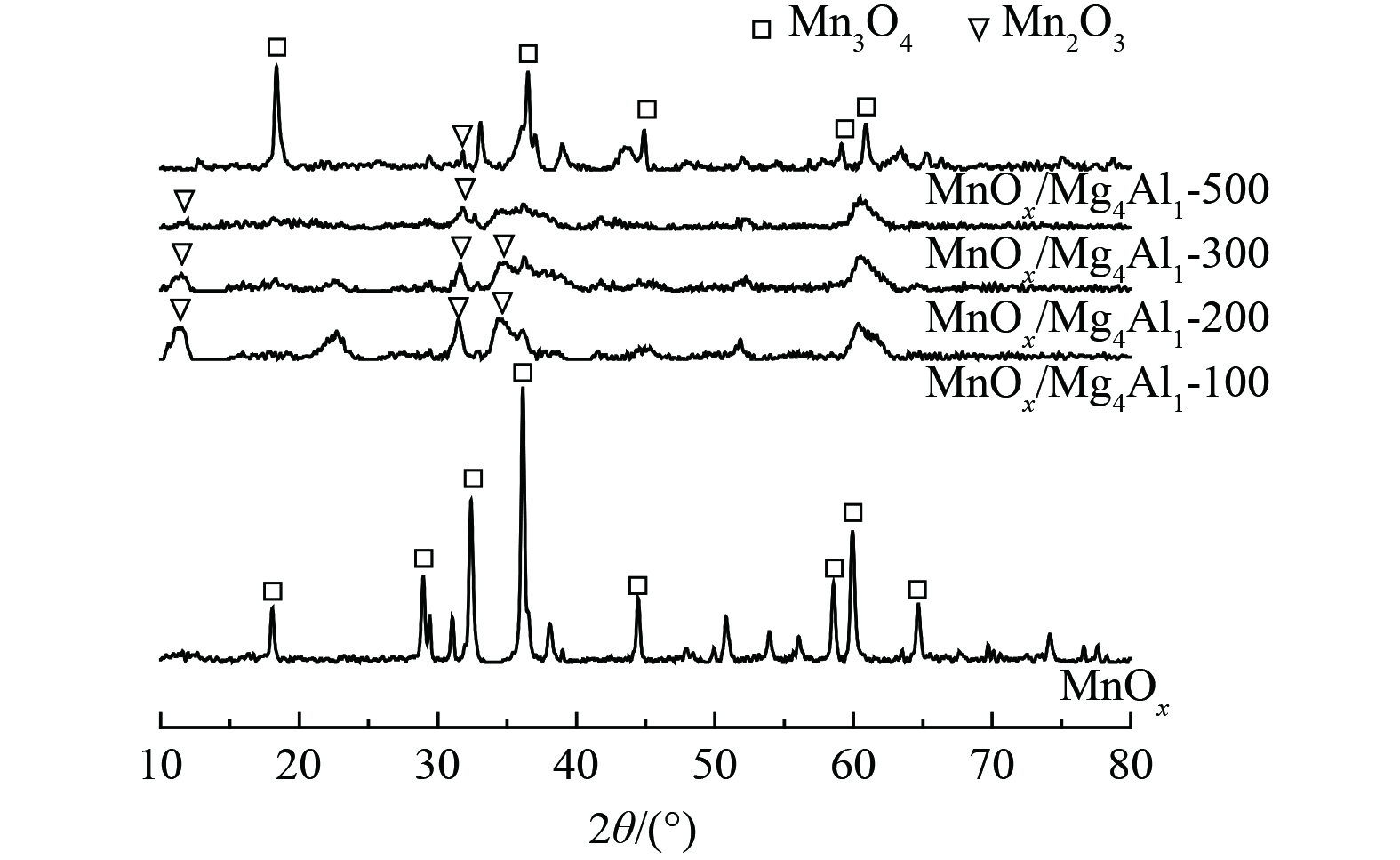
1) 结构形态分析。为探究催化剂结构对催化剂性能的影响,对经过不同温度煅烧后的MnOx/Mg4Al1催化剂及MnOx进行了XRD分析,如图7所示。对于MnOx,所显现的特征峰均为Mn3O4的特征衍射峰(PDF#80-0382),而未发现有其他氧化锰的特征衍射峰。对于MnOx/Mg4Al1催化剂,当煅烧温度为100、200和300 ℃时,MnOx/Mg4Al1催化剂中观察到少量Mn2O3 (PDF#73-1826)和Mn3O4相关晶体衍射峰,衍射峰较宽,晶体粒径较小。这说明晶体高度分散于类水滑石载体上,且衍射峰强度较小,亦表明Mn2O3和Mn3O4部分可能仍以无定形晶体形式存在。在2θ=11.2°、22.4°、34.5°和60.5°处的特征峰与镁铝类水滑石的(003)、(006)、(012)和(110)晶面相匹配[30]。这表明形成了与类水滑石结构相似的片层结构,随着温度升高,催化剂中类水滑石结构的特征峰逐渐消失,可能是由于温度的升高导致层间水损失。而典型的片层结构的峰消失,则说明类水滑石结构在煅烧过程中坍塌。当煅烧温度达到500 ℃时,Mn3O4和Mn2O3衍射峰明显变为尖峰,晶体粒径增大,其不利于催化剂氧化性能的发挥,这一结果与氧化活性结果相一致。
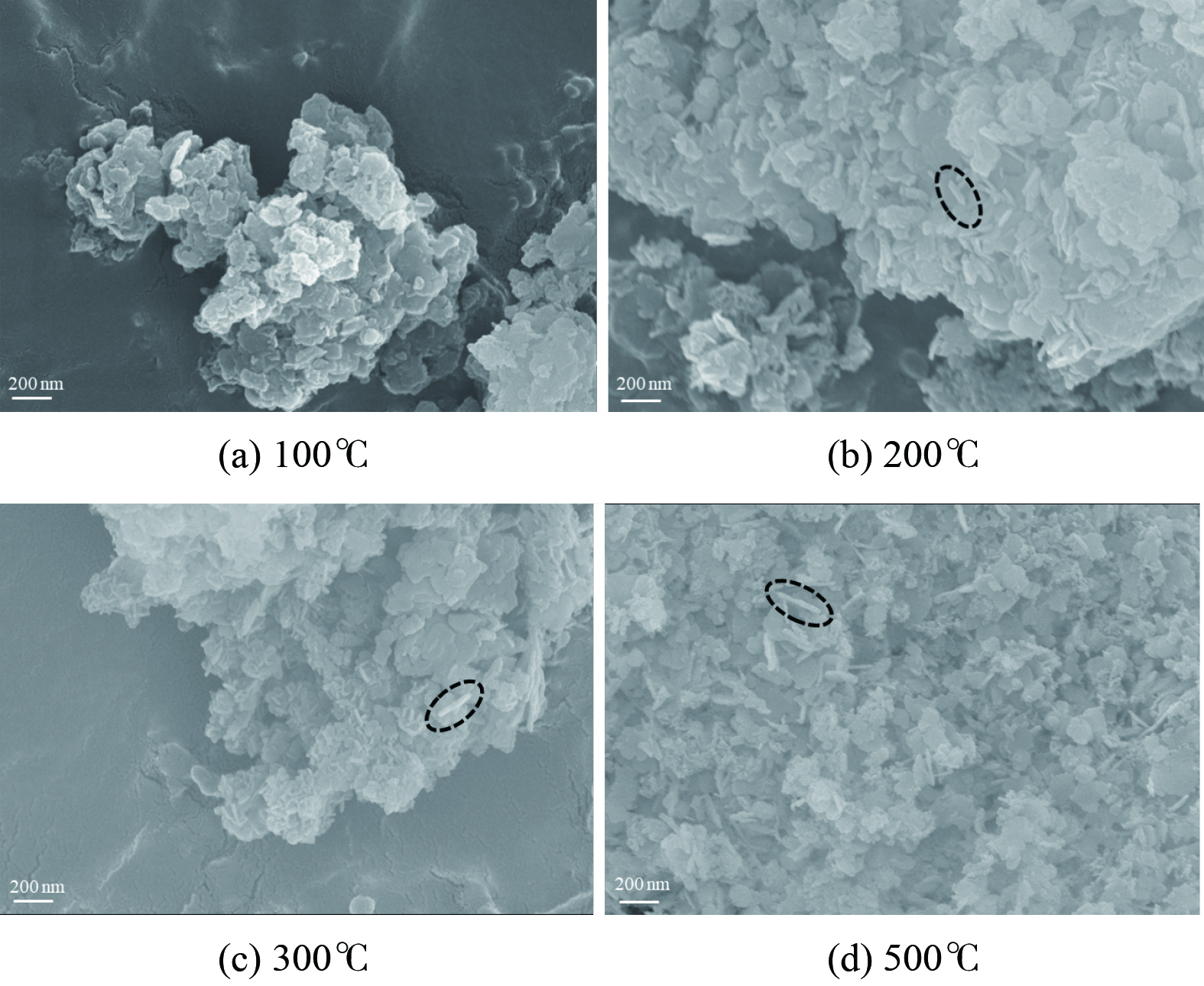
为探究煅烧温度对催化剂的表面结构形态的影响,利用SEM对MnOx/Mg4Al1催化剂进行了表观分析 (图8) 。图8 (a)为经100 ℃煅烧的MnOx/Mg4Al1催化剂SEM图,该催化剂显示为颗粒物块状结构,凹凸不平,这可能是由于催化剂煅烧不充分未能较好的形成片状Mg4Al1所致;当煅烧温度升高至200 ℃和300 ℃时(图8 (b, c)),催化剂表面呈现明显层状结构,即Mg4Al1类水滑石,且表面含有棒状结构物质。随着煅烧温度的提升,其棒状结构物质分布和含量降低。当煅烧温度达到500 ℃时(图8 (d)),催化剂表面结构松散,即Mg4Al1类水滑石片状结构遭到破坏,亦发现大量小片状或棒状结构物质,孔径、孔体积增大,比表面积降低致使催化剂氧化活性降低。这与BET和活性测试结果相一致。
2) 比表面积分析。为探究煅烧温度对MnOx/MgAl催化剂的比表面积和及孔容孔径的影响,对经200 ℃煅烧的MnOx、Mg4Al1及分别经100、200、300和500 ℃煅烧的MnOx/Mg4Al1催化剂进行了BET分析,如表1所示。相较于MnOx,Mg4Al1载体显现出较MnOx更高的比表面积和更低的孔容孔径,其吸附及降解性能应优于MnOx。但结果与事实相反,MnOx展示出更佳的吸附及降解性能。这表明甲醛在MnOx上发生催化降解作用,而Mg4Al1载体仅显现出吸附甲醛性能,无降解作用。研究亦发现,MnOx/类水滑石催化剂比表面积和孔容孔径较MnOx和Mg4Al1显著增大,且比表面积呈现出先增加后降低趋势,其与催化剂氧化活性次序相一致。相较于氧化性能最佳的MnOx/Mg4Al1-200催化剂(78.8 m2·g−1),MnOx/Mg1Al4-300催化剂比表面积更高,达156.7 m2·g−1,但其氧化活性未见显著提升。这表明比表面积是影响催化剂氧化性能的因素之一,但不是唯一决定性因素,其组成亦深刻影响催化剂性能。
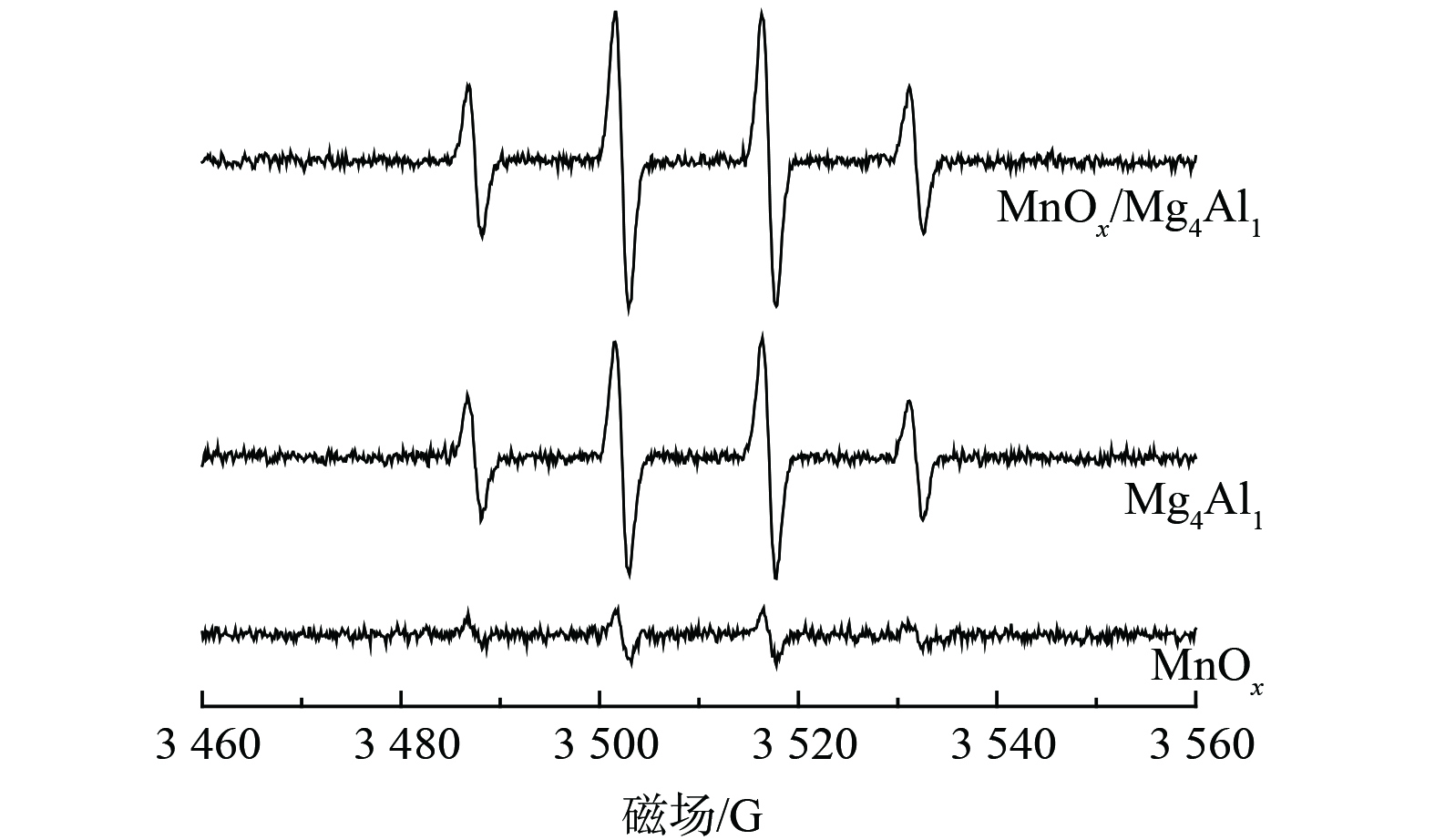
3) EPR分析。为探究催化反应后催化剂表面反应成分组成情况,对MnOx、Mg4Al1、MnOx/Mg4Al1进行了EPR分析 (图9) 。图中显示出4个明显的特征峰,从左到右,其特征峰的比例为1∶2∶2∶1,对应羟基自由基(·OH)的EPR谱图。这表明MnOx/Mg4Al1催化剂中的大量羟基来源于类水滑石载体,而非活性组分MnOx。这些存在于催化剂表面的羟基可有效吸附气态甲醛及中间产物[31],促进催化剂的氧化活性,进而提升催化剂对甲醛的催化降解速率。
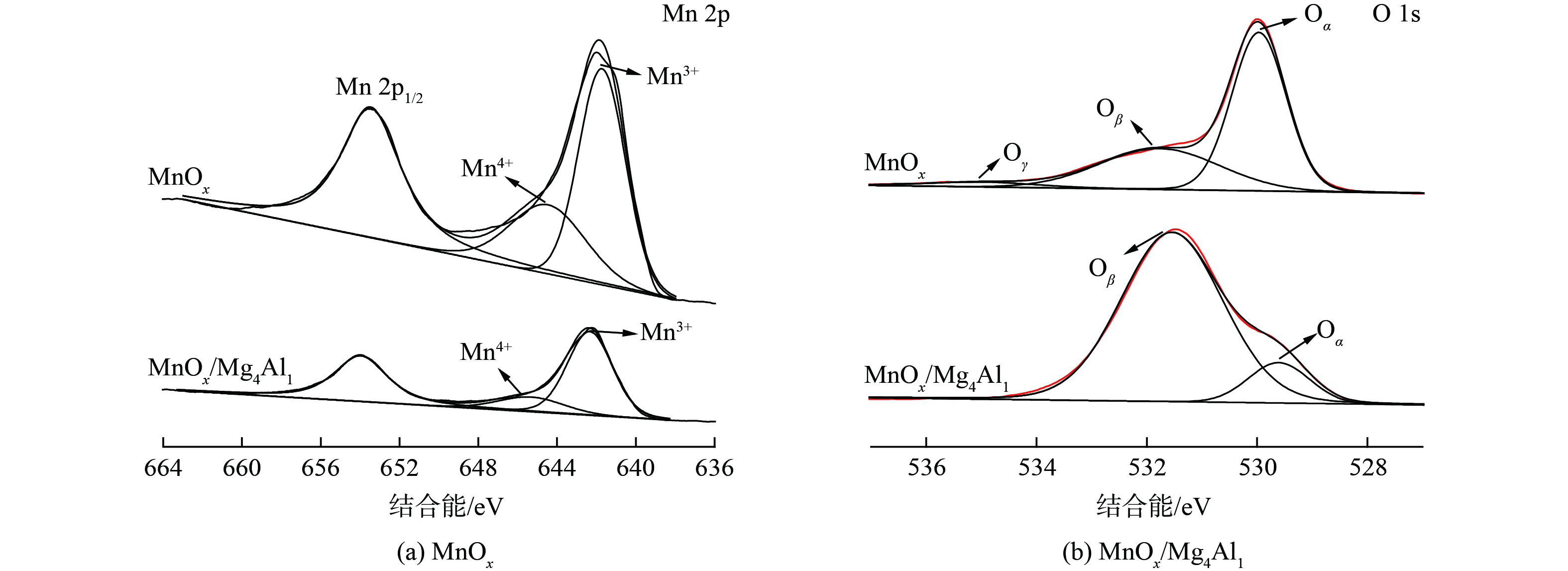
4) XPS分析。为确定催化剂中Mn和O元素的组成及化学结合状态,对MnOx和MnOx/Mg4Al1催化剂进行了XPS分析 (图10) 。图10 (a) Mn 2p 光谱表明,结合能值在653.5 eV和641.9 eV处的特征峰归属为MnO2的Mn 2p1/2和Mn 2p3/2 自旋轨道峰,且Mn 2p3/2反褶积为2个特征峰[32],结合能值为644.5 eV和642 eV处分别归属于Mn4+和Mn3+,且MnOx和MnOx/Mg4Al1催化剂中Mn3+/(Mn3++Mn4+)相对含量比例分别为63.9%和79.8%,可见在MnOx/Mg4Al1催化剂中,Mn3+占比更大。这表明MnOx/Mg4Al1催化剂具有更多氧空位,有利于催化氧化反应的进行。图10 (b) O 1s 光谱表明,结合能值在535.3、531.8和529.6 eV处分别归属于羟基或吸附水(Oγ),表面氧(Oβ)和晶格氧(Oα),而在MnOx/Mg4Al1催化剂中,活性氧的数量和种类发生了显著变化,结合能值在531.5 eV和529.6 eV处分别归属为表面氧(Oβ)和晶格氧(Oα),不存在羟基或吸附水(Oγ)的特征峰[33]。2种催化剂相比,MnOx/Mg4Al1催化剂中的表面氧(Oβ)比例明显上升,其Oβ/(Oα+Oβ)相对含量比例分别为35.7%和88%。由于表面氧(Oβ)在常温催化氧化反应中起着重要作用,且在MnOx/Mg4Al1催化剂中占比更大。这表明MnOx/Mg4Al1催化剂具有较MnOx更高的氧化性能可能是与表面氧(Oβ)的含量高有关。
-
1) 当n(Mg)∶n(Al)为4∶1、煅烧温度200 ℃、MnOx负载量为40%时,催化剂常温催化氧化活性最佳,48 h可将质量浓度为1.000 mg·m−3甲醛降、至0.015 mg·m−3,去除率达98.5%,且展示出良好的催化稳定性。2) 负载型MnOx/类水滑石催化剂催化降解甲醛性能优异,其主要是由于类水滑石载体表面存有大量羟基基团,有利于吸附低浓度甲醛及中间产物,且无明显的与水蒸气竞争吸附而导致的活性下降趋势,同时丰富的表面氧(Oβ)和氧空位、较大的比表面积和较小的颗粒物粒径均利于催化剂氧化性能提升,具有重要的实际应用潜力。
高表面氧MnOx/类水滑石催化剂常温催化低浓度甲醛的性能
Performance of high surface oxygen MnOx/ hydrotalcite-like catalyst for the catalysis of low concentration formaldehyde at ambient temperature
-
摘要: 常温催化氧化技术是去除室内低浓度甲醛(HCHO)的一种有效方法,其中过渡金属氧化物锰基催化剂在催化降解方面性能优异,相较于贵金属催化剂,具有成本低、活性高、稳定性良好、应用前景广等优点。采用共沉淀法制备了类水滑石载体和锰基/类水滑石催化剂,将类水滑石与锰基氧化物相结合用于常温催化氧化,克服了锰基氧化物常温条件下因大量吸附水蒸气而导致稳定性降低的缺点,同时研究考察了载体组成、煅烧温度、氧化锰负载量等对催化剂常温催化氧化性能的影响,并通过XRD、SEM、BET、EPR、XPS等技术对催化剂进行了微观表征。结果表明,当类水滑石中n(Mg)∶n(Al)=4∶1,氧化锰负载量 (质量分数) 为40%,煅烧温度为200 ℃时,所述MnOx/Mg4Al1催化剂性能最佳,其48 h甲醛去除率高达98.5%,甲醛质量浓度由1.000 mg·m-3降至0.015 mg·m−3。类水滑石载体表面存有大量羟基基团,有利于吸附低浓度甲醛及中间产物进而利于催化剂催化氧化性能提升。通过XPS分析可知,相较于MnOx催化剂,MnOx/Mg4Al1催化剂中Oβ/(Oα+Oβ)相对含量比例由35.7%提升至88.0%,表面氧含量(Oβ)大幅提升,其在甲醛常温催化氧化过程中发挥着极其重要作用。本研究结果可为常温催化氧化甲醛提供参考。Abstract: Catalytic oxidation at ambient temperature is an effective method for the removal of low-concentrated formaldehyde (HCHO). The transition metal oxide manganese based catalyst has excellent catalytic degradation performance. Compared with the precious metal catalyst, it has the advantages of low cost, high activity, good stability and wide application prospect. In this study, the hydrotalcite-like and manganese oxides/ hydrotalcite-like catalysts were synthesized by the co-precipitation method. The combination of hydrotalcite-like and manganese oxides for catalytic oxidation of HCHO at ambient temperature overcomed the disadvantage of stability reduction caused by large amount of water vapor adsorption of mangan-based oxides at room temperature. Meanwhile, the effects of carrier composition, calcination temperature and manganese oxides supporting, etc. on the catalytic oxidation at ambient temperature were investigated and characterized by XRD, SEM, BET, EPR and XPS. The results demonstrated that the highest performance of the catalyst was MnOx/Mg4Al1 with n(Mg)∶n(Al)=4∶1, 40 % manganese oxides supporting and calcining at 200 °C. The removal rate of HCHO was up to 98.5% within 48 h, and the concentration was decreased from 1.000 mg·m-3 to 0.015 mg·m-3. The presence of a large number of hydroxyl groups on the surface of the hydrotalcite-like was beneficial to the adsorption of low concentration of HCHO and intermediate by-products, which also improved the oxidation activity of the catalysts. According to XPS analysis, compared to the MnOx catalyst, the Oβ/(Oα+Oβ) ratio of the MnOx/Mg4Al1 was increased from 35.7% to 88.0% and the surface oxygen content (Oβ) also enhanced significantly. The results suggested that the high surface oxygen content played an important role in the catalytic oxidation of HCHO at ambient temperature. This study can provide a reference for the reduction for the catalytic oxidation of HCHO at ambient temperature.
-
表 1 催化剂的比表面积、孔体积和平均孔径
Table 1. Specific surface area, pore volume and average pore diameter of catalysts
样品 比表面积(BET)/(m2·g−1) 孔体积/(cm3·g−1) 平均孔径/nm MnOx-200 22.6 0.222 28.4 Mg4Al1-200 58.2 0.109 5.6 MnOx/Mg4Al1-100 62.0 0.231 13.0 MnOx/Mg4Al1-200 78.8 0.437 14.2 MnOx/Mg4Al1-300 81.4 0.497 19.1 MnOx/Mg4Al1-500 66.3 0.566 16.4 MnOx/Mg1Al1-300 79.0 0.187 6.5 MnOx/Mg1Al4-300 156.7 0.438 8.7 -
[1] HU Q Q, LIU K J, YE J W, et al. Hierarchical Pt/NiCo2O4 nanosheets decorated carbon nanofibers for room-temperature catalytic formaldehyde oxidation[J]. Applied Surface Science, 2023, 623: 157012. doi: 10.1016/j.apsusc.2023.157012 [2] MIAO L, WANG J L, ZHANG, P Y. Review on manganese dioxide for catalytic oxidation of airborne formaldehyde[J]. Applied Surface Science, 2019, 466: 441-453. doi: 10.1016/j.apsusc.2018.10.031 [3] FANG R M, HUANG, X Y, SUN Y J, et al. Excellent stability for catalytic oxidation formaldehyde over defective δ-MnO2 nanoparticles at room temperature[J]. Journal of Environmental Chemical Engineering, 2023, 11(1): 109064. doi: 10.1016/j.jece.2022.109064 [4] 武强, 潘奕君, 裴晶晶. 黑色TiO2/活性炭复合材料的制备及其光催化净化甲醛性能[J]. 环境工程学报, 2023, 17(3): 841-849. [5] YE J, ZHU X, CHENG B, et al. Few-layered graphene-like boron nitride: a highly efficient adsorbent for indoor formaldehyde removal[J]. Environmental Science & Technology Letters, 2017, 4: 20-25. [6] LI Q, ODOOM-WUBAH T, ZHOU Y P, et al. Coral-like CoMnOx as a highly active catalyst for benzene catalytic oxidation[J]. Industrial & Engineering Chemistry Research, 2019, 58(8): 2882-2890. [7] 潘菊霜, 麻春艳, 宋茂勇. 环境水对FeMnO3和NiMnO3室温催化氧化甲醛的影响[J]. 环境科学学报, 2022, 42(6): 346-356. [8] 蒋昕楠, 孔振凯, 王际童, 等. 高锰酸钾改性球形中孔炭的甲醛吸附性能[J]. 环境工程学报, 2018, 12(6): 1676-1 682. [9] 孙婷, 刘凤娟. 室内甲醛污染治理方法研究进展[J]. 环境生态学, 2021, 3(8): 91-95. [10] 雷春生, 朱晓峰. 胺化凹凸棒黏土的制备及甲醛吸附性能研究[J]. 环境工程, 2016, 34(6): 95-100. [11] BOYRAZ E, YALCINKAYA F. Hydrophilic surface-modified PAN nanofibrous membranes for efficient oil-water emulsion separation[J]. Polymers, 2021, 13(2): 197. doi: 10.3390/polym13020197 [12] LI J Y, CUI W, CHEN P, et al. Unraveling the mechanism of binary channel reactions in photocatalytic formaldehyde decomposition for promoted mineralization[J]. Applied Catalysis B:Environmental, 2020, 260: 118130. doi: 10.1016/j.apcatb.2019.118130 [13] BERRICHI A, BACHIR R, BEDRANE S, et al. Heterogeneous bimetallic Au-Co nanoparticles as new efficient catalysts for the three-component coupling reactions of amines, alkynes and CH2Cl2[J]. Research on Chemical Intermediates, 2019, 45(6): 3481-3495. doi: 10.1007/s11164-019-03803-6 [14] 张珍珍, 李鑫恒. 基于催化氧化技术去除甲醛的研究进展[J]. 分子催化, 2019, 33(4): 381-389. doi: 10.16084/j.cnki.issn1001-3555.2019.04.005 [15] 朱杰, 孙月吟, 顾名扬, 等. TiO2负载MnFeOx催化剂的制备及常温催化氧化甲醛性能研究[J]. 功能材料, 2022, 53(4): 4011-4019. [16] CHEN X Y, WANG H H, CHEN M, et al. Co-function mechanism of multiple active sites over Ag/TiO2 for formaldehyde oxidation[J]. Applied Catalysis B:Environmental, 2021, 282: 119543. doi: 10.1016/j.apcatb.2020.119543 [17] TIAN M Z, LIU S J, WANG L L, et al. Complete degradation of gaseous methanol over Pt/FeOx catalysts by normal temperature catalytic ozonation[J]. Environmental Science & Technology, 2020, 54(3): 1938-1945. [18] TIAN Z Y, NGAMOU P H T, VANNIER V, et al. Catalytic oxidation of VOCs over mixed Co-Mn oxides[J]. Applied Catalysis B:Environmental, 2012, 117/118: 125-134. doi: 10.1016/j.apcatb.2012.01.013 [19] YAO X, GAO M X, WEI Z D, et al. Removal of hexanal in cooking fume by combination of storage and plasma-catalytic oxidation on alkali-modified Co-Mn solid solution[J]. Chemosphere, 2019, 220: 738-747. doi: 10.1016/j.chemosphere.2018.12.201 [20] FANG R M, HUANG H B, JI J, et al. Efficient MnOx supported on coconut shell activated carbon for catalytic oxidation of indoor formaldehyde at room temperature[J]. Chemical Engineering Journal, 2018, 334: 2050-2 057. [21] 薛花, 崔家浩, 邢中鹏, 等. MnOx/ACF负载型催化剂常温催化氧化二氯甲烷[J]. 化工环保, 2021, 41(1): 77-82. doi: 10.3969/j.issn.1006-1878.2021.01.013 [22] LI R, SHI, X, HUANG Y, et al. Catalytic oxidation of formaldehyde on ultrathin Co3O4 nanosheets at room temperature: Effect of enhanced active sites exposure on reaction path[J]. Applied Catalysis B:Environmental, 2022, 319: 121902. doi: 10.1016/j.apcatb.2022.121902 [23] 赵海楠, 王健, 徐文青, 等. 锰氧化物催化氧化挥发性有机物(VOCs)研究进展[J]. 环境工程, 2019, 37(10): 157-167. doi: 10.13205/j.hjgc.201910027 [24] LU L, TIAN H, HE J H, et al. Graphene-MnO2 hybrid nanostructure as a new catalyst for formaldehyde oxidation[J]. The Journal of Physical Chemistry C, 2016, 120(41): 23660-23668. doi: 10.1021/acs.jpcc.6b08312 [25] ZHU L, WANG J L, RONG S P, et al. Cerium modified birnessite-type MnO2 for gaseous formaldehyde oxidation at low temperature[J]. Applied Catalysis B:Environmental, 2017, 211: 212-221. doi: 10.1016/j.apcatb.2017.04.025 [26] LI J W, PAN K L, YU S J, et al. Removal of formaldehyde over MnxCe1-xO2 catalysts: thermal catalytic oxidation versus ozone catalytic oxidation[J]. Journal of Environmental Sciences, 2014, 26(12): 2546-2553. doi: 10.1016/j.jes.2014.05.030 [27] SZABÓ V, MESZAROS R, KÓNYA Z, et al. Preparation and characterization of MnIn-layered double hydroxides (LDHs), extension of the synthesis to fabricate MnM (III)-LDHs (M=Al, Sc, Cr, Fe, Ga), and the comparison of their photocatalytic and catalytic activities in the oxidation of hydroquinone[J]. Journal of Molecular Structure, 2022, 1261: 132966. doi: 10.1016/j.molstruc.2022.132966 [28] YAN Q H, HOU X T, LIU G C, et al. Recent advances in layered double hydroxides (LDHs) derived catalysts for selective catalytic reduction of NOx with NH3[J]. Journal of Hazardous Materials, 2020, 400: 123260. doi: 10.1016/j.jhazmat.2020.123260 [29] DANIEL S, THOMAS S. Layered double hydroxides: fundamentals to applications[M]. Cambridge: Woodhead Publishing, 2020: 1-76. [30] LI, D D, YANG G L, LI P L, et al. Promotion of formaldehyde oxidation over Ag catalyst by Fe doped MnOx support at room temperature[J]. Catalysis Today, 2016, 277: 257-265. doi: 10.1016/j.cattod.2016.02.040 [31] YU H R, LIU D X, WANG H Y, et al. Singlet oxygen synergistic surface-adsorbed hydroxyl radicals for phenol degradation in CoP catalytic photo-Fenton[J]. Chinese Journal of Catalysis, 2022, 43(10): 2678-2689. doi: 10.1016/S1872-2067(22)64117-2 [32] WU Z B, JIN R B, WANG H Q, et al. Effect of ceria doping on SO2 resistance of Mn/TiO2 for selective catalytic reduction of NO with NH3 at low temperature[J]. Catalysis Communications, 2009, 10(6): 935-939. doi: 10.1016/j.catcom.2008.12.032 [33] 张先龙, 彭真, 刘鹏等. 基于PPS的锰基催化脱硝-除尘功能一体化滤料的制备及其低温SCR脱硝[J]. 功能材料, 2015, 46(S2): 160-164. -




 下载:
下载: