-
水资源是人类赖以生存的自然资源,是生态系统中必不可少的重要因素,良好的水环境对于维护人体健康和生态安全至关重要。近年来随着经济的飞速发展,采矿、电镀、冶金等工业产生的重金属(类金属)废水不断流入水体,造成了严重的环境污染,其中砷污染尤为突出[1-3]。砷(As)是自然界中广泛存在的一种类金属,也是对植物与人体健康危害最大的一种类金属元素[4-5]。进入水体的砷,不易降解,具有较大的隐蔽性和持久性,可经由食物链通过生物积累或生物放大进入生物体,对人体健康和生态环境造成严重的危害[6-7]。鉴于砷的高危害性,砷被美国环境保护署(EPA)和世界卫生组织 (WHO)列为强致癌物质[8]。当前,废水中砷的处理技术主要有化学沉淀法、氧化法、离子交换法、吸附法等,其中吸附法因其成本低廉、效果稳定、操作简单等优点被广泛应用[9-11]。因此,开发具有良好吸附性能的新型绿色材料对于水体中砷的去除具有重要意义[12]。
废水中的砷元素以As(III)和As(Ⅴ) 2种价态为主,而As(III)的毒性是As(Ⅴ)的60多倍,且更难去除[13]。纳米铁(nZVI)因其环境友好,比表面积大等优点已被广泛用于重金属砷的去除。其中,对含砷废水中As(III)的同步氧化吸附受到越来越多研究者的关注[14-15]。然而,表面能高且自带磁性的特性也使得纳米铁容易产生团聚,导致其吸附及还原能力的降低,限制了其在含砷废水中的应用[16]。因此,需要在纳米铁的制备中添加载体以减少其团聚。生物碳是一种多孔、富含表面官能团的黑色碳质材料,对水体中重金属离子有较好的吸附性[17]。有研究表明,纳米铁负载在生物炭上后,能有效减少其团聚效应,且生物炭自身的良好的导电能力,增强了改性后纳米铁的电子迁移能力,提高了其反应活性[18]。
传统纳米铁的制备多采用硼氢化钠作化学还原剂,但硼氢化钠属于易制爆的危险化学品,有毒且成本较高[19]。近年来,具有成本低廉、环境友好等特点的生物炭负载纳米铁绿色制备方法备受关注[20-21]。该方法利用茶叶、葡萄籽等植物提取液代替传统的硼氢化钠来还原制备纳米铁。据文献报道,葡萄籽提取液中富含原花青素等多酚类物质,其作为一种生物活性还原剂,能将亚铁还原成纳米铁[22-25]。本研究选取了风车草(Cyperus alternifolius)为原料制备了生物炭载体,以葡萄籽提取液为还原剂,通过液相还原法制备了风车草生物炭负载纳米铁(CBC-nZVI),探究了CBC-nZVI对废水中As(III)的去除效应。以期为绿色纳米铁制备技术应用于重金属砷的废水处理提供可行性参考。
-
风车草(采自湖南先导洋湖再生水有限公司);葡萄籽;砷标准溶液、镉标准溶液(均为优级纯,国药集团化学试剂有限公司);亚砷酸钠(NaAsO2)、氢氧化钠(NaOH)、盐酸(HCl)、硫酸亚铁(FeSO4·7H2O)、乙醇(CH3CH2OH)(均为分析纯,国药集团化学试剂有限公司) 。
-
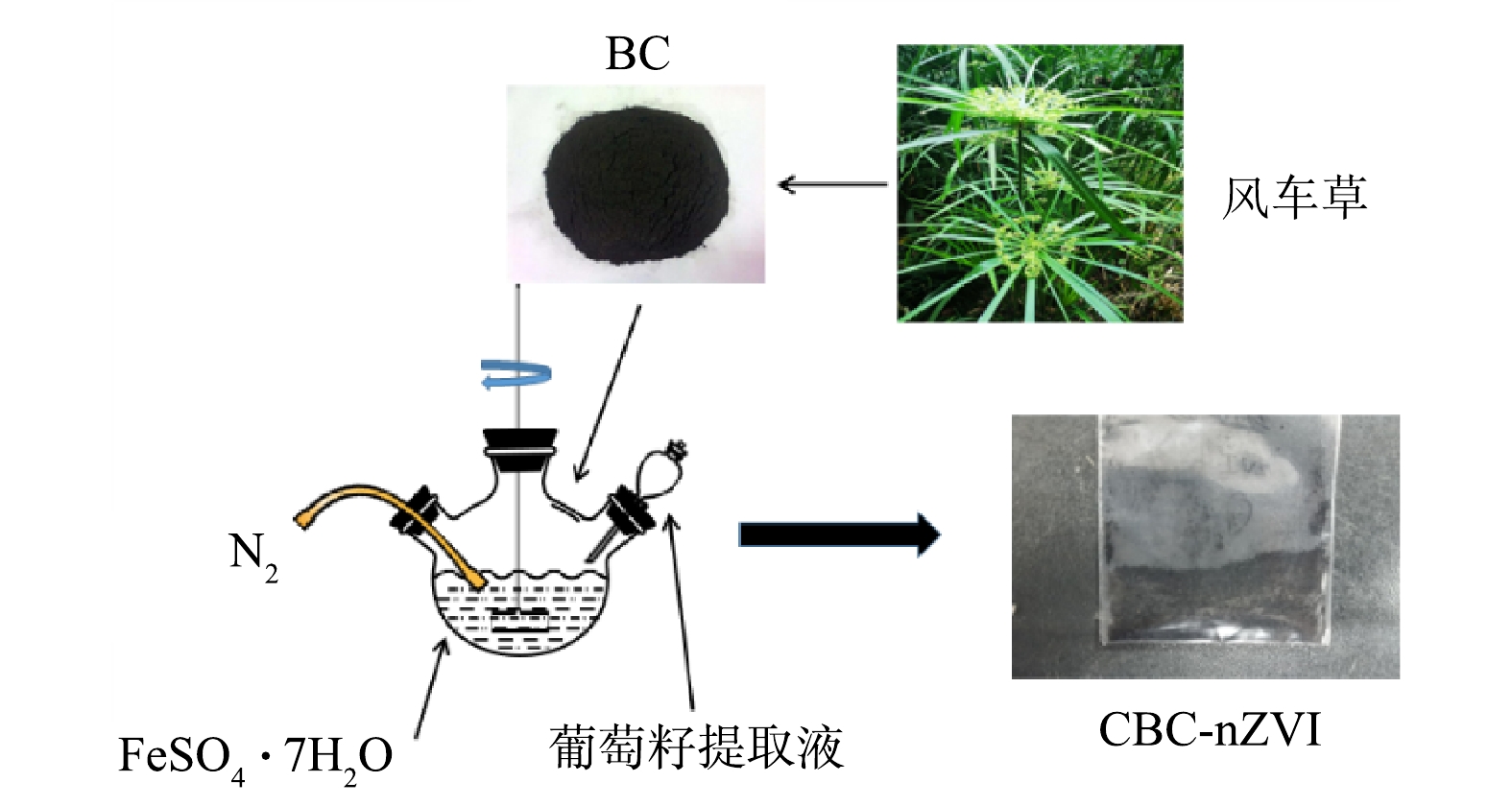
将洗净干燥后的风车草剪至5 cm左右,用粉碎机研磨成粉状后放到大坩埚中,在300 ℃下用马弗炉恒温煅烧120 min,烧制结束取出坩埚并冷却,将烧制的生物炭研磨过100目筛备用。用分析天平称取5 g葡萄籽,用粉碎机研磨成粉状后倒入到含200 mL超纯水的烧杯中,用密封膜密封于48 ℃的条件下超声加热30 min,经滤膜过滤后得到葡萄籽提取液备用。
生物炭负载纳米铁(CBC-nZVI)的制备。用分析天平称取12.41 g的FeSO4·7H2O放入三口烧瓶中,溶于25 mL超纯水和25 mL乙醇的混合溶液中,接通氮气并开启机械搅拌。待FeSO4·7H2O完全溶解后加入5 g的风车草生物炭粉末,继续搅拌30 min后,用蠕动泵缓慢滴加葡萄籽提取液,在滴加结束后继续搅拌60 min,经真空抽滤后用超纯水和无水乙醇各冲洗3次,放入60 ℃真空干燥箱中烘干,制得CBC-nZVI材料。制备过程如图1。
-
采用透射电镜(Talos F200X,Thermo Fisher,美国)分析样品微观形貌;采用能谱仪(SmartEDX,EDAX Inc,美国)分析样品元素分布;采用傅里叶变换红外光谱仪(Nicolet iS20 ,Thermo Fisher,美国)分析样品官能团;采用全自动比表面及孔隙度分析仪(ASAP 2460,Micromeritics,美国)测定样品孔体积及比表面积;使用X 射线光电子能谱仪(Thermo Escalab 250Xi,Thermo Fisher,美国)测定样品的元素组成。
-
取150 mL质量浓度为150 mg·L−1的As(Ⅲ)溶液于锥形瓶中,并投加一定质量的CBC和CBC-nZVI于转速为120 r·min−1的摇床中振荡。在设定时间内取样并通过定性滤纸过滤再分析测定。设定反应时间分别为10、15、20、30、40、60、80、120 min;CBC和CBC-nZVI投加量分别为0.05、0.1、0.2、0.3、0.5、0.8 g;溶液初始pH分别为2.0、3.0、4.0、5.0、6.0、7.0、8.0;溶液温度分别为25、45、65 ℃。采用原子荧光分光光度计测定滤液中As(Ⅲ)的剩余质量浓度,并通过式(1)计算其剩余吸附量。每组实验设计3个平行。
式中:q为CBC-nZVI对As(Ⅲ)的吸附量,mg·g−1;C0和C分别为吸附前后As(Ⅲ)的质量浓度,mg·L−1;V为溶液体积,L;m为吸附剂质量,g。
-
投加CBC和CBC-nZVI各0.2 g于150 mL不同初始质量浓度(20、50、100、150、200、300、400、500 mg·L−1)的As(Ⅲ)溶液中,调节溶液初始pH为5.0,在溶液温度为25 ℃、转速为120 r·min−1的摇床中振荡120 min。通过测定锥形瓶中吸附质的剩余质量浓度,计算出平衡吸附量。采用Freundlich模型(式(2))和Langmuir模型(式(3))对实验数据进行拟合。
式中:Ce为平衡时吸附质质量浓度,mg·L−1 ;qe为平衡吸附容量,mg·g−1 ;qm为最大吸附容量,mg·g−1 ;KF为Freundlich常数;KL为Langmuir常数;n为经验常数。
-
投加CBC和CBC-nZVI各0.2 g于150 mL质量浓度为150 mg·L−1的As(Ⅲ)溶液中,调节溶液初始pH为5.0,在温度为25 ℃、转速为120 r·min−1的摇床中振荡120 min。通过测定锥形瓶中吸附质的剩余质量浓度,计算出平衡吸附量。采用准一级动力学模型(式(4))、准二级动力学模型(式(5))对所得实验数据进行拟合。
式中:t为吸附时间,min;qe和qt分别为平衡时和t时刻的吸附量,mg·g−1;k1为准一级动力学速率常数,min−1; k2为准二级动力学速率常数,g·(mg·min)-1。
-
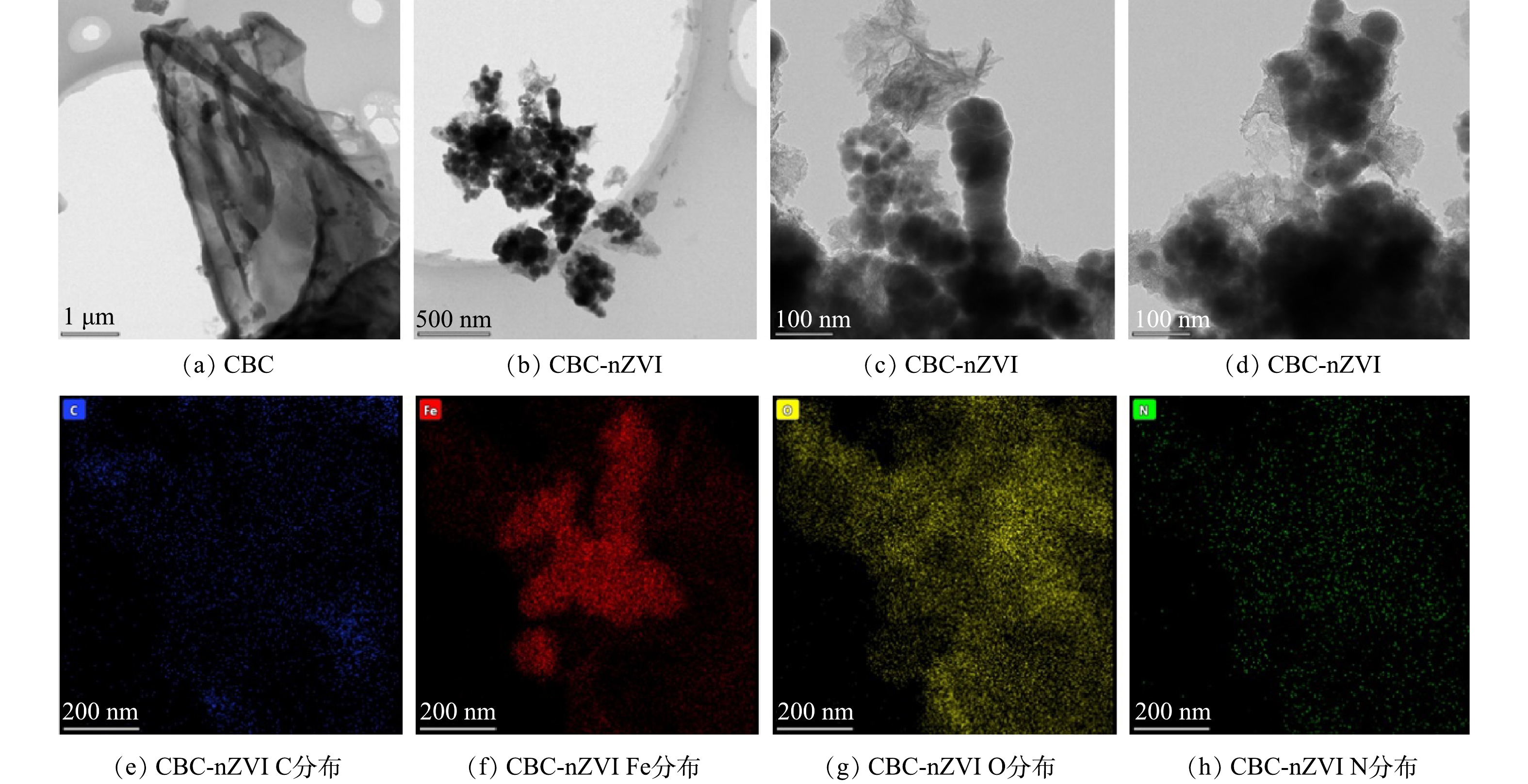
1)扫描透射电镜(STEM)和EDS分析。图2(a)为CBC的扫描透射电镜图。由图2(a)可见,CBC主要呈薄片状堆叠在一起,宽度为微米级,大小不均。图2(b)~(d) 为CBC-nZVI的透射电镜图。在图中可以观察到纳米铁颗粒灰色边缘和黑色中心的对比,即CBC-nZVI表面具有明显的核壳结构,其外部包裹了由一层薄薄的致密金属中心组成的壳层,这是由无定形Fe2O3、FeOOH等铁的各种氧化物组成的[26-27]。同时可以明显地看出,生物炭的存在加强了纳米铁颗粒的分散性,有效防止了纳米铁颗粒的过度团聚。由图2(e)~(h)可见,亮点表明元素C、Fe、O和N的存在,同时Fe元素的映射形状意味着nZVI非均匀地分散在CBC-nZVI的表面上。构成CBC-nZVI复合材料的粒子呈非均匀纳米尺寸。
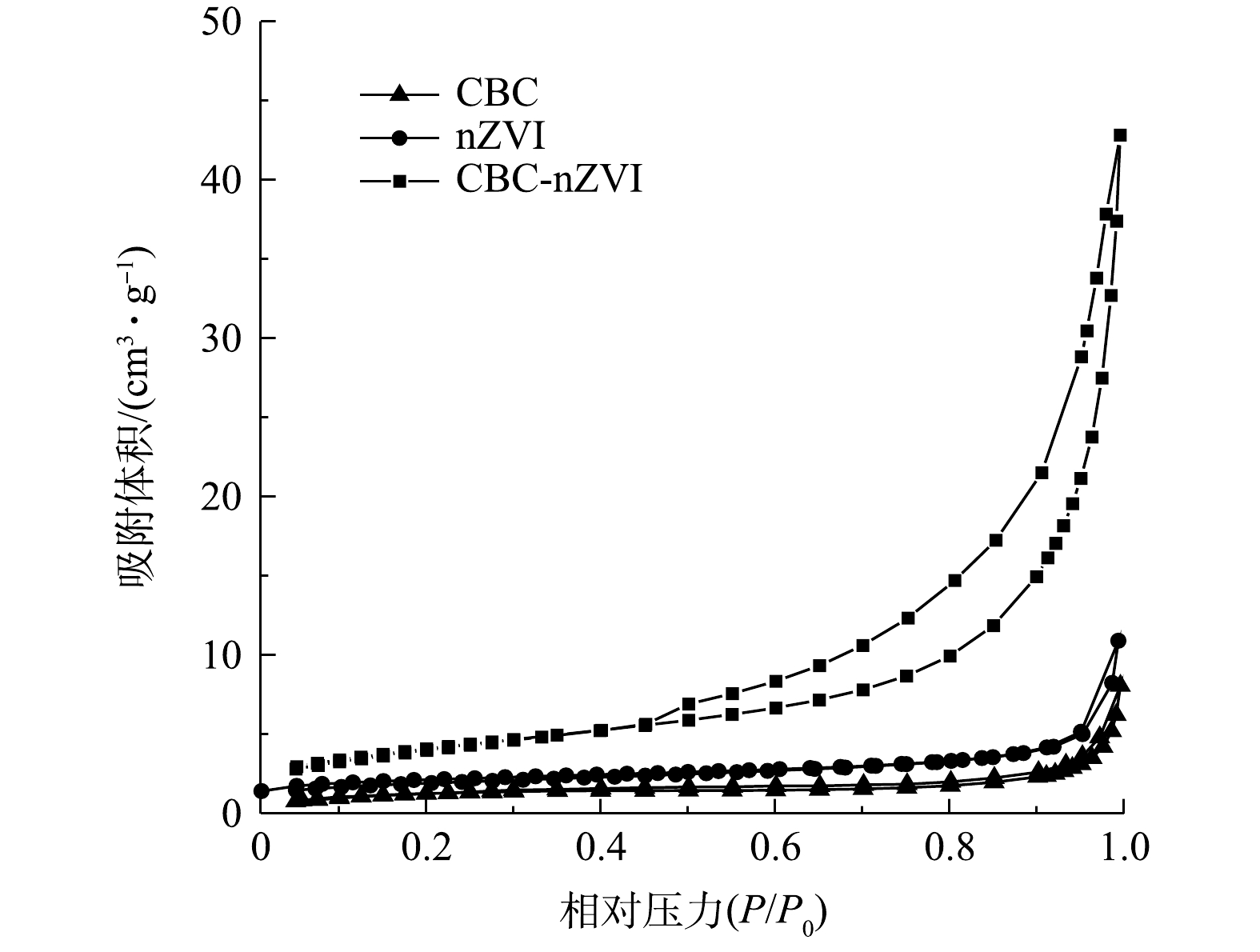
2) BET比表面积与孔体积分析。CBC、nZVI及CBC-nZVI的BET比表面积和孔体积分析结果见图3,X轴的相对压力可以分为低压(0~0.1)、中压(0.3~0.8)和高压(0.9~1.0)3段。CBC和CBC-nZVI在低压区吸附量较低,而在中压区吸附曲线发生了明显的上升,形成了回滞环,呈现出典型的IV型等温吸脱附曲线。这表明材料内部存在着介孔结构。与CBC和nZVI相比,CBC-nZVI的回滞环更加明显,由此可知,CBC-nZVI的比表面积和孔体积大于CBC和nZVI。由表1可见,CBC-nZVI的比表面积和孔体积分别为14.744 m2·g−1和0.066 cm3·g−1,其比表面积为CBC的2.9倍和nZVI的1.9倍。更大的比表面积和孔体积能够为CBC-nZVI提供更多的吸附位点,从而增强CBC-nZVI对重金属离子去除效果[28-29]。
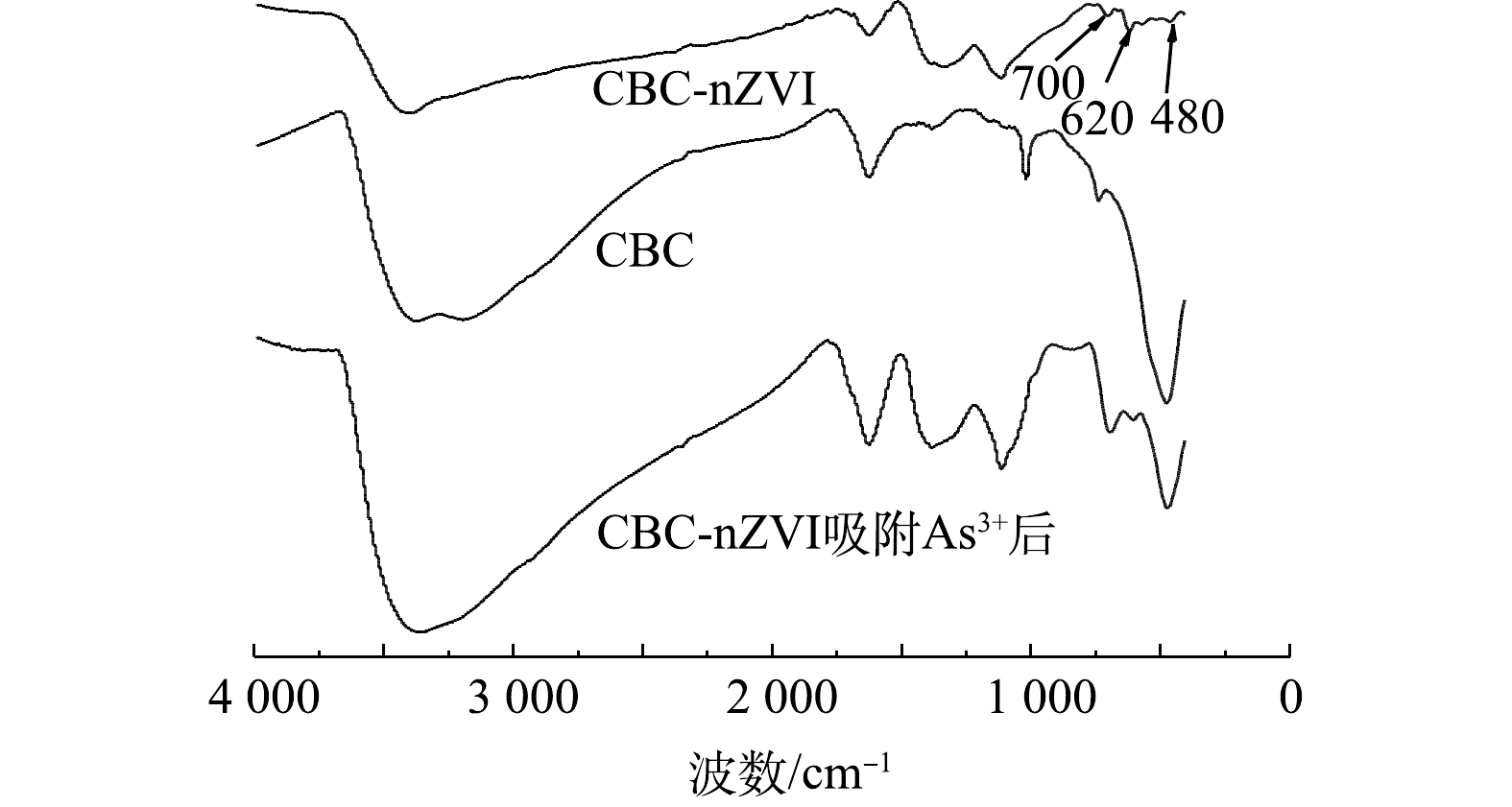
3)傅里叶变换红外光谱(FTIR)分析。图4为CBC和CBC-nZVI红外吸收光谱图(FTIR)。其中3 400、3 200 cm−1附近的宽峰为—OH的伸缩振动,2 970、2 930 cm−1附近的峰主要是烷烃的C—H的伸缩振动峰,1 625 cm−1出现的峰归因于O—H的面内弯曲振动峰,1 380、1 340 cm−1出现的峰为CO32-的C=O的伸缩振动峰[30],1 120 cm−1附近峰主要是C—O的伸缩振动峰。700、620 cm−1为氢氧化(亚)铁的Fe—O—H的弯曲振动峰,580、480 cm−1为氧化(亚)铁的Fe—O振动峰[31],表明CBC-nZVI表面生成了铁的氧化物。CBC表面存在的羟基在与nZVI的合成过程中,能够促进与Fe2+的结合,因此,有利于nZVI的负载。
-
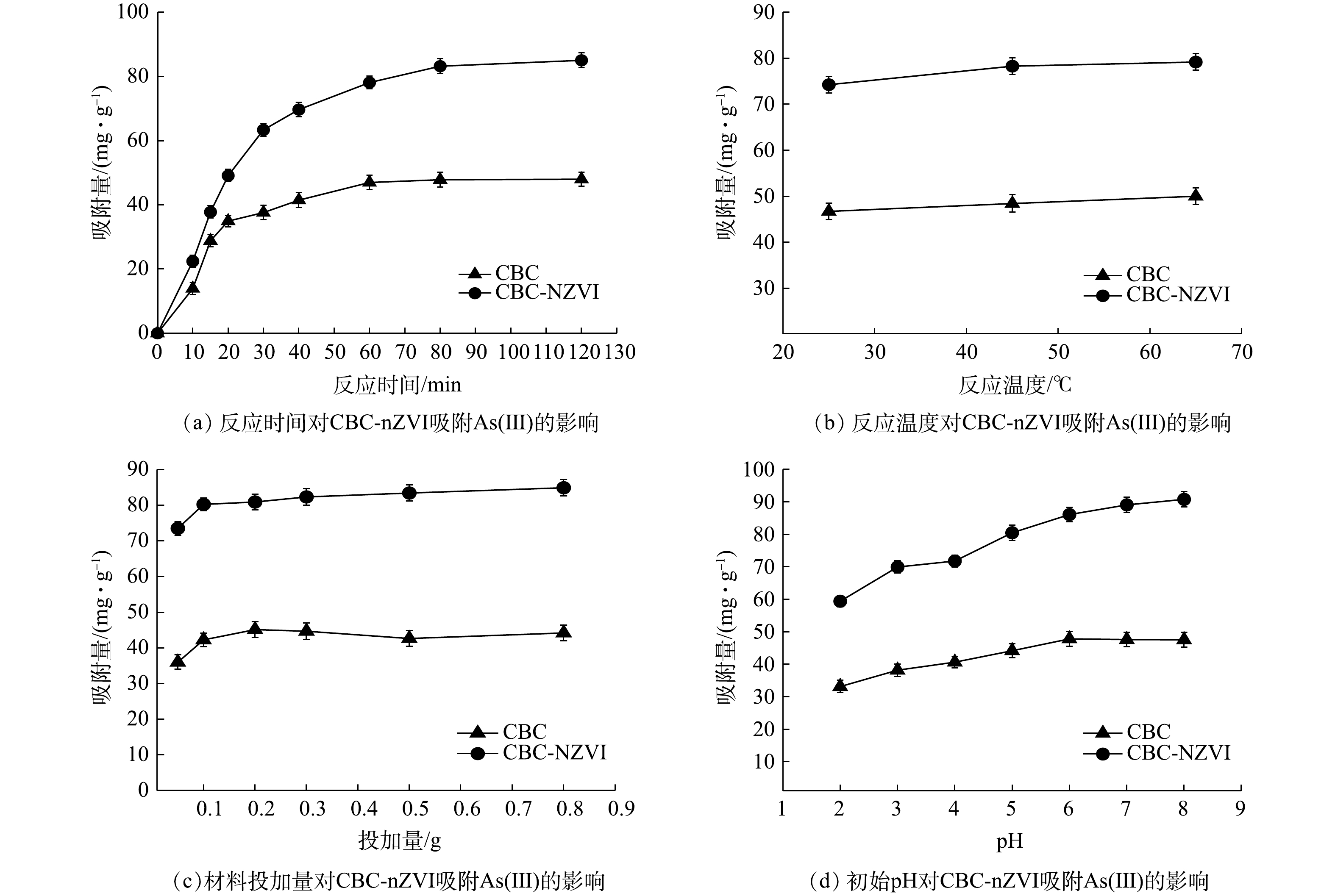
1)反应时间的影响。在25 ℃,自然pH,As(Ⅲ)初始质量质量浓度为150 mg·L−1的条件下,反应时间对CBC-nZVI吸附As(Ⅲ)的影响结果见图5(a)。反应初期As(Ⅲ)迅速抢占材料表面的活性吸附位点,60 min内As(Ⅲ)的去除量显著增加。随着反应时间的延长,活性位点越来越少,As(Ⅲ)去除量增长的也逐渐缓慢,之后继续延长吸附反应时间,As(Ⅲ)的去除量变化趋于稳定,在120 min后达到吸附平衡。
2)溶液温度的影响。在自然pH,反应时间为120 min,As(Ⅲ)初始质量浓度为150 mg·L−1的条件下,研究溶液温度对CBC-nZVI吸附As(Ⅲ)的影响。由图5(b)可知, CBC-nZVI对As(Ⅲ)的吸附量随着溶液温度的升高略有上升,但变化幅度在5%之内。因此,可认为温度对As(Ⅲ)的吸附没有明显影响。吸附量的略微上升可能是随着温度的增高,As(Ⅲ)在水溶液中的运动加剧﹐在迁移运动过程加大了和纳米铁的接触概率,从而有利于As(Ⅲ)的去除。
3) CBC-nZVI投加量的影响。在25 ℃、自然pH,As(Ⅲ)初始质量质量浓度为150 mg·L−1,反应时间为120 min的条件下,吸附剂投加量对CBC-nZVI吸附As(Ⅲ)的影响结果见图5(c)。随着投加量不断增加,CBC-nZVI对As(Ⅲ)的吸附量有小幅上升,在适当的投加范围内,投加量越大,可为As(Ⅲ)提供更多的吸附位点,As(Ⅲ)的吸附总量也随之增大。但随着投加量增加至0.2 g后,As(Ⅲ)的吸附量趋于稳定。这是因为As(Ⅲ)的总量是一定的,此时CBC-nZVI对As(Ⅲ)的吸附量也趋于饱和。当CBC-nZVI的投加量过高时,反而容易导致材料之间因过度拥挤而产生团聚,减小反应的接触面积[32]。
4)溶液初始pH的影响。在25 ℃,As(Ⅲ)初始质量浓度为150 mg·L−1,反应时间为120 min,投加量为0.2 g的条件下,溶液初始pH对CBC-nZVI吸附As(Ⅲ)的影响结果见图5(d)。在pH为2.0~6.0时,CBC-nZVI对As(Ⅲ)的吸附量随着pH的增大而显著提高;当pH为6.0~8.0时,CBC-nZVI对As(Ⅲ)的吸附量随着pH的增大逐渐趋于平稳。有研究表明,当pH<9.2时,As(Ⅲ) 主要以H3AsO3 的形式存在,当pH>9.2时,As(Ⅲ)主要以H2AsO3−的形式存在[33]。低pH环境下,CBC-nZVI对As(Ⅲ)的去除量不高,这是因为强酸环境造成了纳米零价铁的部分溶解,减少了材料表面的反应活性位点[34]。随着pH的增加,有利于Fe2+的氧化,进而生成铁(氢)氧化物,这些氧化物与水中的As(Ⅲ)和As(Ⅴ)发生共沉淀反应,将砷固定于CBC-nZVI实现去除[35-36]。
根据单因素研究结果,在反应时间为120 min、反应温度为45 ℃、投加量为0.2 g、pH=8的条件下进行吸附实验,得出最佳单因素条件下,CBC-nZVI对As(III)的吸附去除量为99.12 mg∙g−1。
-
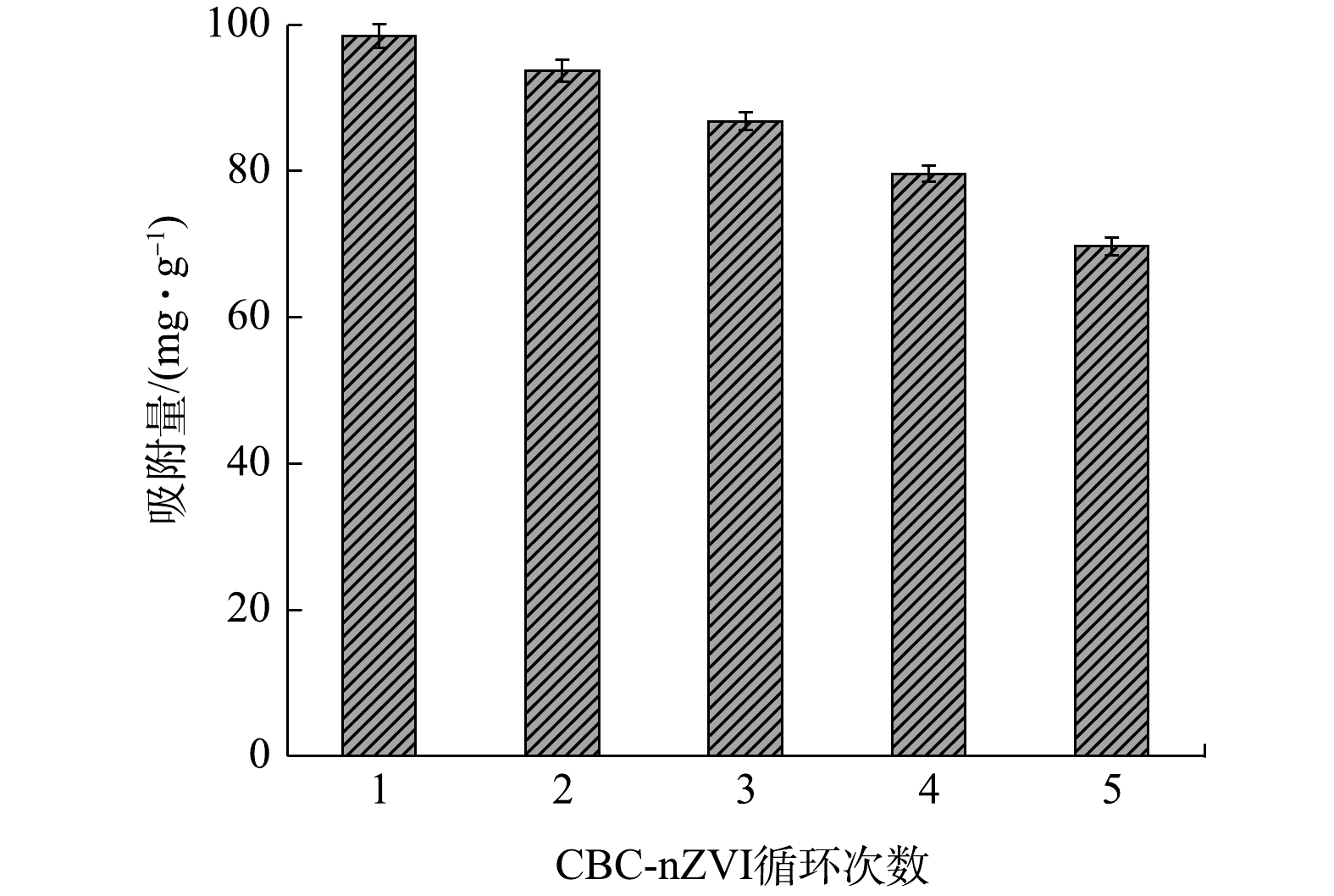
将已达吸附平衡的CBC-nZVI用0.l mol∙L−1 NaOH溶液解析后进行了重复实验,考察CBC-nZVI的稳定性和循环使用可行性。如图6所示,循环使用后的CBC-nZVI对As(Ⅲ)的吸附量呈下降趋势,循环使用5次降低了29.2%,但仍保有69.7 mg∙g−1的吸附量,说明CBC-nZVI具有较好的稳定性和重复利用性能。
-
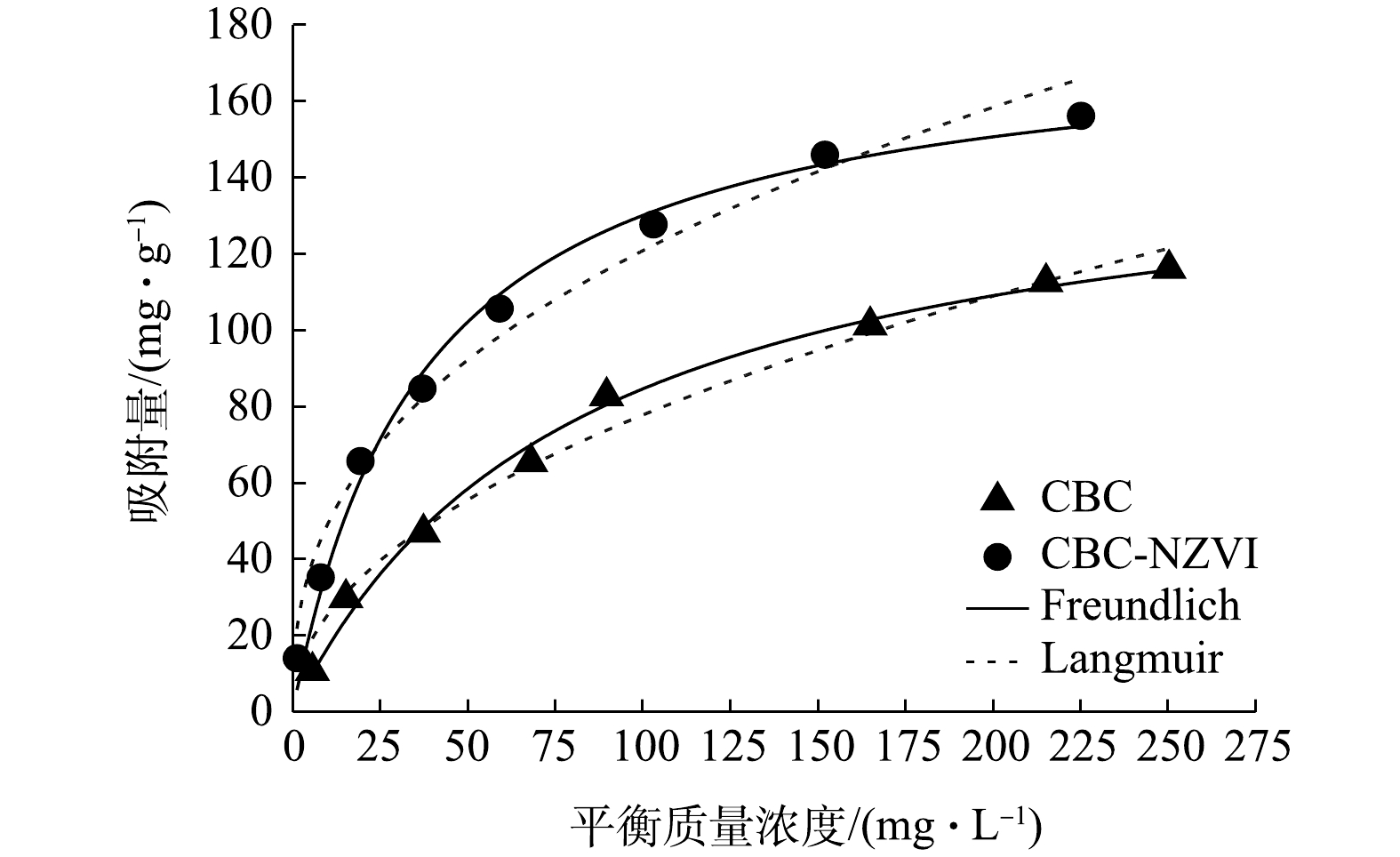
1)等温吸附属性分析。通过Freundlich和Langmuir等温吸附模型来拟合As(Ⅲ)吸附曲线,拟合结果见图7,模型的参数值见表2。由拟合结果可知,CBC和CBC-nZVI使用Langmuir等温吸附模型拟合的R2分别为0.993和0.990,更接近1。因此,用Langmuir等温吸附模型描述CBC和CBC-nZVI对As(Ⅲ)的吸附行为更为准确,表明As(Ⅲ)的吸附是发生在均匀表面的单层吸附[37]。在Langmuir等温吸附模型中,qm为CBC和CBC-nZVI的理论最大吸附量,qm越大,表明材料的吸附能力越强;在Freundlich等温吸附模型中KF是吸附容量指标,1/n表示吸附强度,KF和n越大,表明其吸附容量越大,吸附强度越强[38]。与CBC相比,CBC-nZVI对As(Ⅲ)的吸附常数KF和n分别增大了144.5%和24.9%,由此可知,改性后的CBC-NZV拥有更大的吸附容量和吸附强度,从而增大了其对As(Ⅲ)的吸附能力。这与qm的拟合结果相符。
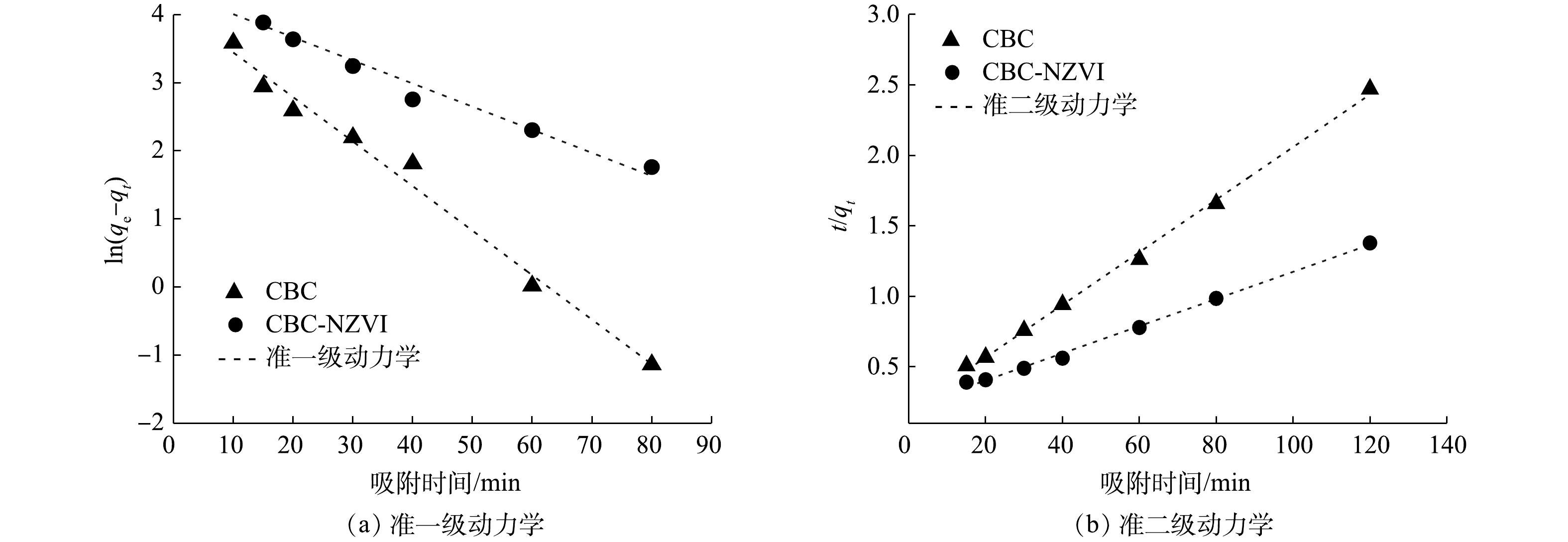
2)吸附动力学属性分析。通过准一级动力学和准二级动力学模型来拟合As(Ⅲ)的吸附曲线,拟合结果见图8,模型的参数值见表3。由拟合结果可知,CBC和CBC-nZVI使用准二级动力学模型拟合的R2分别0.998和0.996,更接近1。因此,用准二级动力学模型描述CBC和CBC-nZVI对As(Ⅲ)的吸附行为更为准确,表明CBC和CBC-nZVI对As(Ⅲ)的吸附过程主要受化学吸附的控制。此外,CBC和CBC-nZVI用准一级动力学模型拟合的R2值皆都大于0.97,表明两种材料对As(Ⅲ)的吸附还存在着非主导的物理吸附过程,这与材料本身的性质有关[39]。
-
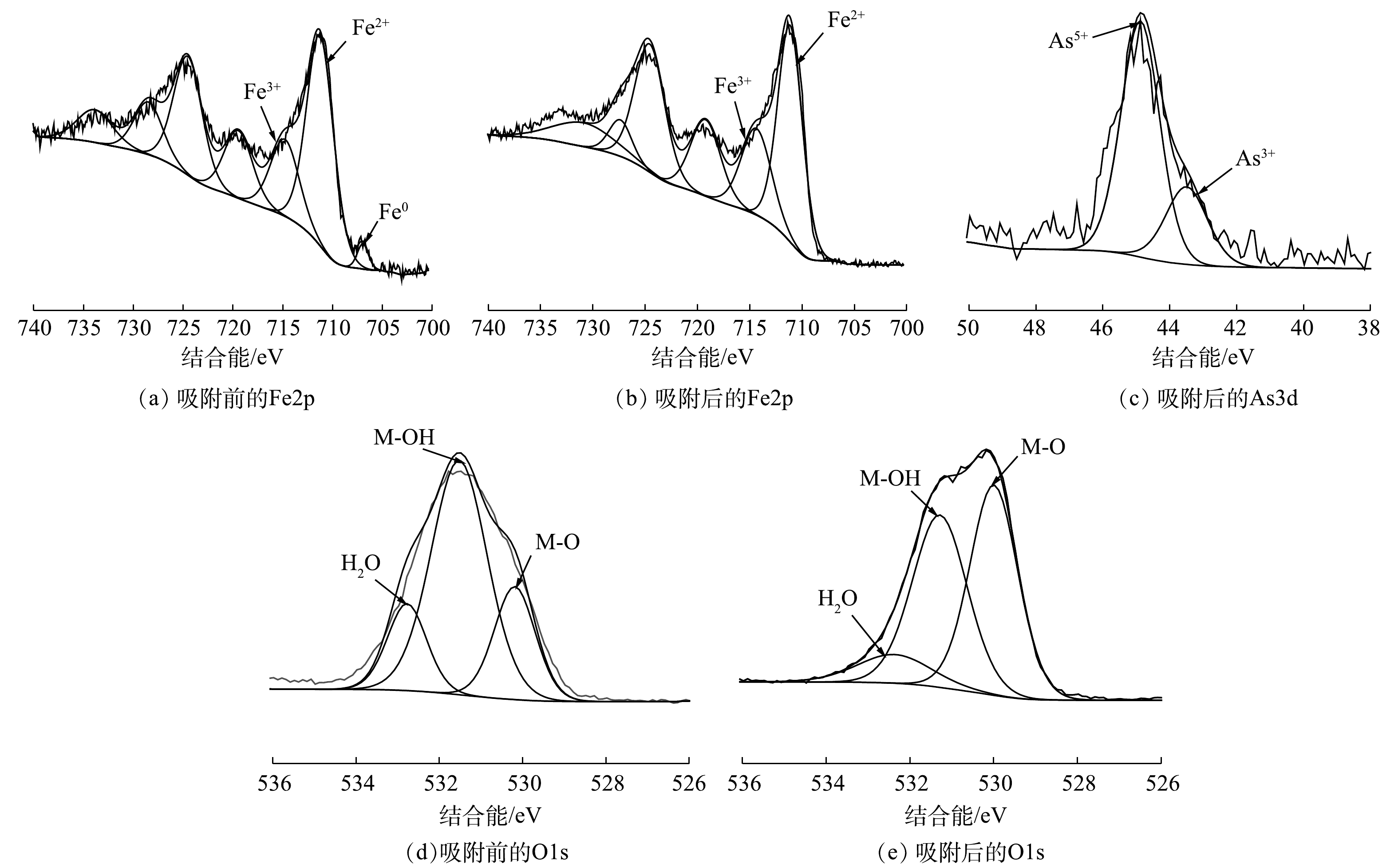
为了进一步分析CBC-nZVI去除As(Ⅲ)的机理,利用FTIR和XPS光谱分析反应前后CBC-nZVI表面的官能团和元素价态变化。由图4可知,反应后CBC-nZVI的主要官能团并未消失。在3 400 cm−1左右处的-OH衍射峰型增强,这可能是由于CBC-nZVI与As(Ⅲ)反应后, CBC-nZVI上的活性位点被As(Ⅲ)所取代,导致CBC-nZVI的表面成分发生了变化[40]。
由图9(a)和图9(b)的Fe2p分峰图可以看出,吸附反应前的CBC-nZVI中在707.18、710.8、715.18 eV处观察到3个明显的特征峰。这表明CBC-nZVI中Fe表现为Fe0、Fe2+、Fe3+共存。吸附反应后Fe3+的相对含量增加,而Fe0的特征峰消失[41-42],这表明在反应过程中Fe0和Fe2+逐渐氧化为Fe3+。由图9(c)的As3d分峰图可以看出,反应后CBC-nZVI全扫描图谱中出现了43.96 eV和45.44 eV2个峰,这是As(Ⅲ)和As(Ⅴ)的特征峰[43],可见砷已经被吸附到CBC-nZVI上。
反应初始溶液中只存在As(Ⅲ),但反应后样品同时出现了As(Ⅲ)和As(Ⅴ)特征峰,说明部分As(Ⅲ)被氧化为As(Ⅴ),可知CBC-nZVI溶液中As(Ⅲ)的去除不是单一的吸附,还包括氧化还原反应。YAN[44]等用高分辨率XPS (HR-XPS)对纳米铁进行多线分析表明,As(Ⅴ)主要分布在纳米铁氧化物表面,As(Ⅲ)主要分布在纳米铁氧化物壳层,说明As(Ⅲ)的氧化有氧化物壳层的贡献作用。由CBC-nZVI的扫描透射电镜图和傅里叶红外光谱图已知,CBC-nZVI的核壳外部有铁的氧化物生成,结合已有研究[45-46]结果推测As(Ⅲ)氧化为As(Ⅴ)的机理为:纳米铁与水和空气的反应过程中被腐蚀,这些Fe0的腐蚀产物将As(Ⅲ)氧化为As(Ⅴ),并对二者进行吸附。RAMOS等[27]和MANNING等[47]的研究已证明某些纳米铁腐蚀过程中产生的部分铁氧化物能够将As(Ⅲ)氧化为As(Ⅴ)。同时,纳米铁的有氧腐蚀能产生活性自由基·OH作用于As(Ⅲ)的氧化[48],反应过程如式(6)和式(7)所示。
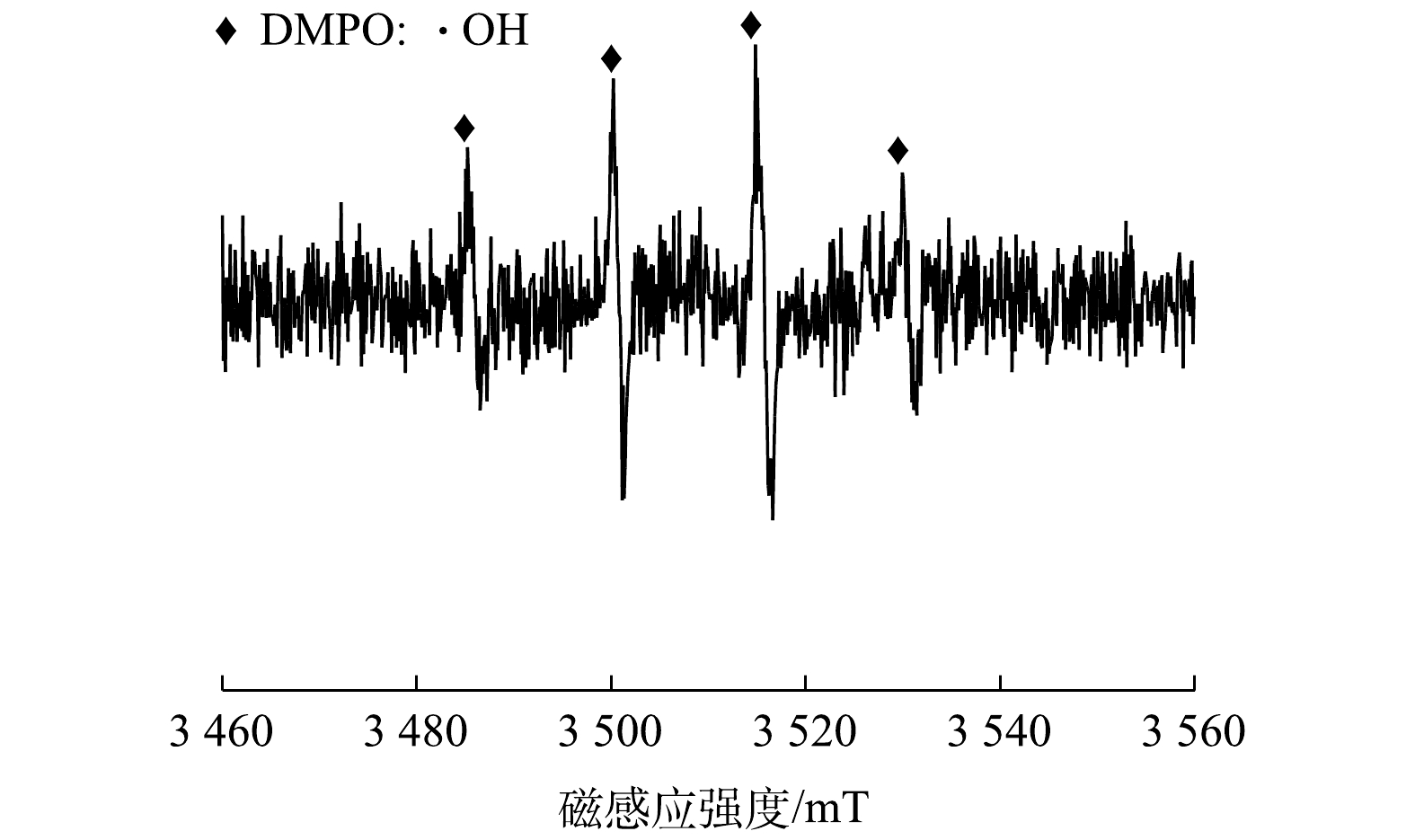
为了进一步确认CBC-nZVI去除As(Ⅲ)的反应过程中是否生成了自由基·OH,本文利用电子自旋共振(ESR)对·OH进行了定性测定。测定结果如图10所示。在CBC-nZVI吸附去除As(Ⅲ)的反应体系中检测到了峰高比为1∶2∶2∶1 的·OH特征峰,证明了CBC-nZVI与水发生有氧腐蚀催化产生了自由基·OH,从而将As(Ⅲ)氧化为As(Ⅴ)。反应过程中,As(Ⅲ)大部分被氧化为毒性较低的As(Ⅴ),被吸附于CBC-nZVI上实现去除。此外,大量研究[33-34,49]已证明CBC-nZVI有氧腐蚀产生的Fe2+和Fe3+会发生复杂的聚合反应,进而生成铁(氢)氧化物,这些铁(氢)氧化物在形成过程中与As(Ⅲ)、As(Ⅴ)发生共沉淀,将砷固定在CBC-nZVI上实现去除。由图9(d)和图9(e)的O1s分峰图可知,CBC-nZVI吸附反应前在530.1、531.3、532.3 eV处观察到3个明显的特征峰,分别归属于金属氧化物(M-O)、金属羟基基团(M-OH)和CBC-nZVI表面吸附的结合水(H2O)[50]。而在吸附反应后的CBC-nZVI中M-OH基团的峰面积下降,M-O基团的峰面积增加。这归因于CBC-nZVI表面上的金属羟基基团均参与对溶液中As(III)和As(V)的吸附,砷与其羟基配位形成内层络合物降低了M-OH基团的含量,共沉淀过程中产生的铁(氢)氧化物增加了M-O基团的含量[51]。总体而言,CBC-nZVI对As(Ⅲ)的去除包括吸附、氧化还原和共沉淀3个过程,探明CBC-nZVI去除As(Ⅲ)的具体步骤和详细机理仍需通过进一步研究。
-
1)通过TEM、BET、XPS、FTIR等表征手段对CBC-nZVI进行分析,结果表明铁元素已被负载于生物炭上,原子百分比为22.79%。生物炭的存在有效地防止了纳米铁颗粒的过度聚集,改性后的CBC-nZVI具有更大的比表面积并提供了更多的有效反应位点。2)随着吸附时间的延长、溶液温度的升高、CBC-nZVI投加量的增加和溶液初始pH的增大,CBC-nZVI对As(Ⅲ)的吸附量不断上升。再生实验表明,CBC-nZVI具有较好的稳定性和循环利用性。3) CBC-nZVI对As(Ⅲ)吸附过程更符合Langmuir模型和准二级吸附动力学模型,表明CBC-nZVI对As(Ⅲ)的吸附是单层吸附,以化学吸附为主。4) CBC-nZVI吸附去除As(Ⅲ)的反应体系中产生了·OH自由基,反应过程中,As(Ⅲ)大部分被氧化为毒性较低的As(Ⅴ),通过吸附、氧化还原和共沉淀实现As(Ⅲ)的最终去除。
葡萄籽提取液还原制备生物炭负载纳米铁对水中As(Ⅲ)的去除性能及机理
Removal performance and mechanism of As(Ⅲ) from water by reduction preparation of biochar supported nano-iron from grape seed extract
-
摘要: 利用植物提取液绿色合成的纳米铁,具有绿色环保、成本低廉等优点。本文采用葡萄籽提取液作为还原剂和稳定剂,风车草生物炭为载体,制备了生物炭负载纳米铁(CBC-nZVI),用于去除废水中的As(Ⅲ)。结果表明,纳米铁(nZVI)成功负载于生物炭表面,具有较大的比表面积和孔体积;随着反应时间的延长,溶液温度的升高,CBC-nZVI投加量的增加和溶液初始pH的增大,CBC-nZVI对As(Ⅲ)的吸附量不断增大;Langmuir等温吸附模型能更准确地描述CBC-nZVI对As(Ⅲ)吸附行为,CBC-nZVI对As(Ⅲ)去除过程符合准二级动力学模型,表明CBC-nZVI对As(Ⅲ)的吸附是单层吸附,以化学吸附为主。ESR表征结果表明CBC-nZVI在有氧反应体系中生成了·OH,反应过程中,As(Ⅲ)大部分被氧化为毒性较低的As(Ⅴ),通过吸附、氧化还原和共沉淀实现As(Ⅲ)的最终去除。Abstract: The green synthesis of iron nanoparticles using plant extracts has the advantages of green environment protection and low cost. In this study, biochar-loaded nano-iron (CBC-nZVI) was prepared for As(Ⅲ) removal from wastewater using grape seed extract as the stabilizer and reducing agent, and Cyperus alternifolius based-biochar as the carrier. The results showed that the nano-iron (nZVI) was successfully loaded on the surface of biochar, and had a large specific surface area and pore volume. The adsorption of As(Ⅲ) by CBC-nZVI increased continuously with the increase of reaction time, solution temperature, dosage and initial pH of the solution. Langmuir isothermal adsorption model could more accurately describe the adsorption behavior of CBC-nZVI on As(Ⅲ) than other ones. The removal of As(Ⅲ) by CBC-nZVI was in accordance with the quasi-secondary kinetic model, indicating that above adsorption process was a monolayer adsorption and was dominated by chemisorption. The characterization of ESR showed that CBC-nZVI produced ·OH in the aerobic reaction system. During the reaction, most of As(Ⅲ) was oxidized to As(V) with lower toxicity, and the final removal of As(Ⅲ) was achieved by adsorption, redox and co-precipitation.
-
Key words:
- biochar /
- nano-iron /
- grape seed extract /
- remove /
- As(Ⅲ)
-
随着电子产品迭代速度的持续加快,电子垃圾的产生量不断攀升。2019年全球共产生5.36×107 t电子垃圾,相比2014年增长了21.0%[1]。拆解是电子垃圾常用的回收处理方法之一,但在拆解过程中,大量有毒有害的重金属常未被有效回收,因而造成严重的土壤污染风险,从而给人群健康带来潜在威胁[2-3]。 因此,电子垃圾拆解区土壤重金属污染分布特征受到了众多学者的广泛研究。梁啸[4]的研究表明,电子垃圾拆解场周边农田土壤中重金属Cd和Cu质量分数均超过国家土壤环境质量标准限值[5];SHI等[6]的研究表明,温岭市电子垃圾拆解场周边水稻田土壤在2006—2016年重金属Cd、Cu、Ni和Zn的质量分数分别增加了0.110、11.8、1.01和6.82 mg·kg−1,这表明电子垃圾拆解会导致严重的重金属污染;张璐瑶等[7]发现,浙江某电子垃圾拆解区内农用地重金属Cd质量分数平均值是土壤背景值的6.3~10.0倍,且部分农作物Cd质量分数超过食品安全限值,这说明电子垃圾拆解不仅会造成土壤重金属污染,而且会严重威胁农产品质量安全。以上研究侧重于电子垃圾拆解区农田土壤重金属质量分数分布特征,但是,针对电子垃圾拆解区不同用地类型土壤重金属的空间分布特征及风险,报道较少。
本研究以广东省汕头市潮阳区贵屿镇的拆解区为研究对象,对拆解地以及周边菜地、稻田和荒地等不同深度土壤样品进行采集,研究Cu、Zn、Cd和Pb等重金属质量分数与形态的空间分布特征,并采用地累积指数法和潜在生态风险指数法分别评价不同用地类型土壤重金属潜在的生态风险,以期为土壤重金属污染防治与修复实践提供理论依据。
1. 材料与方法
1.1 研究区域概况
贵屿镇是国内三大电子垃圾拆解基地之一,20世纪90年代开始涌现大量手工拆解回收电子垃圾的家庭式小作坊,导致大量含重金属污水直接排放到环境中,从而造成土壤重金属污染[8-9]。有研究[10-11]表明,电子垃圾拆解使该区域土壤重金属污染情况进一步恶化,重金属镍、锌、镉和砷的质量分数分别为背景值的141%、198%、206%和181%,并发现有14.3%的稻米样品Pb超标。
1.2 样品采集与分析
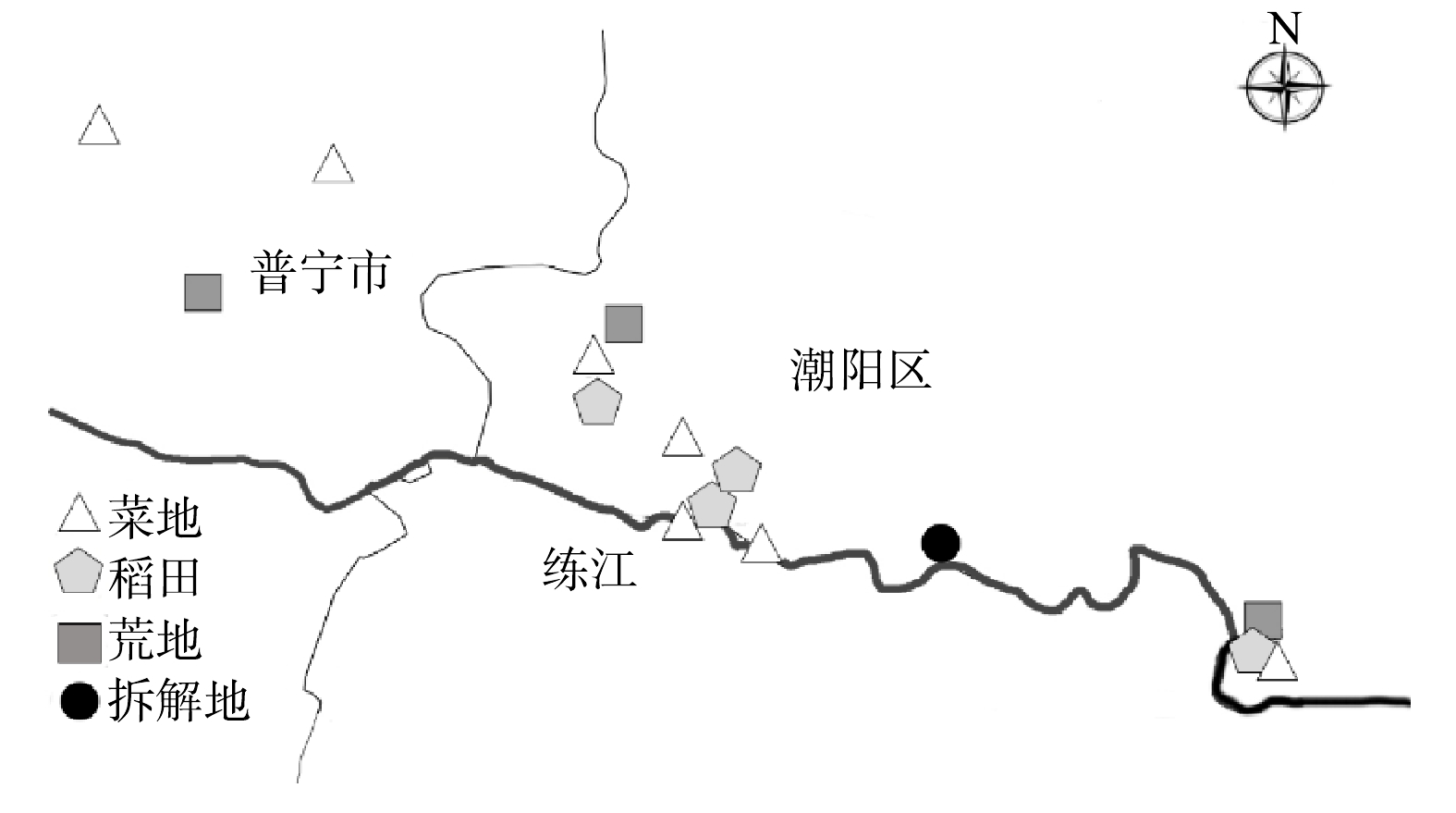
1)样品采集及前处理。以广东省汕头市潮阳区贵屿镇拆解地及周围的菜地、稻田和荒地等不同用地类型土壤为研究对象,共设置15个采样区(图1)。采用对角线布点法在每个采样区布置3个采样点,每个采样点按地表下0~10、10~20、20~40、40~60、60~80、80~100 cm依次钻取6个土层。同时,每层采样点取3个平行样,置于干净通风处晾干,去除石子、杂草、残枝等杂物,用研钵研磨后过100目筛,筛下土样于干燥处密封保存备用[12]。研究区域共采集270份土壤样品,包括菜地土壤样品126份、荒地土壤样品54份、稻田土壤样品72份、拆解地土壤样品18份,总共测定总样品数为4 050个。
2)重金属质量分数测定。取0.1 g土壤样品放入坩埚中,滴入适量去离子水,加入10 mL盐酸,将坩埚置于通风橱内的电热板上120 ℃左右加盖加热。当坩埚内盐酸剩余3 mL左右时,取下稍冷,分别加入5 mL硝酸、5 mL氢氟酸、3 mL高氯酸,中温加盖加热,60 min后开盖飞硅,时常摇动坩埚,冒大量白烟时盖上盖子使坩埚内壁黑色物质消解,待黑色物质被完全消解后,开盖使白烟冒尽。若经过以上步骤坩埚内呈黄色溶液,则可加入1~2 mL的硝酸,若有黑色的物质残余则加入1~2 mL的高氯酸。用水冲洗坩埚盖和内壁,将溶液转移至50 mL容量瓶中,冷却后定容摇匀[13]。重金属质量分数采用石墨炉原子吸收法测定[14-15]。
3)重金属形态测定。采用BCR连续提取法[16]分析重金属形态。酸提取态:准确称取1.00 g样品于50 mL聚丙烯塑料离心管中,加入20 mL 0.1 mol·L−1醋酸溶液,室温下振荡16 h,使用离心机(TD5A,卢湘仪)在3 000 r·min−1下离心,将上清液移出稀释至50 mL,用原子吸收分光光度计(Z2000,日立)测量并计算重金属质量分数。可还原态:加入20 mL 0.1 mol·L−1 NH2OH·HCl(pH=2),室温下振荡16 h,离心、稀释后用原子吸收分光光度计测量并计算重金属质量分数。可氧化态:加入30% H2O2溶液,室温下振荡1 h,85 ℃水浴提取2 h,冷却后加入3 mL 1 mol·L−1 NH4Ac溶液(pH=2),室温下振荡16 h,离心、稀释后用原子吸收分光光度计测量并计算重金属质量分数。残渣态:HNO3-HF-HClO4湿法消解,赶酸至近干,将上清液移出稀释至50 mL,用原子吸收分光光度计测量并计算重金属质量分数。
1.3 评价方法
1)地累积指数法。在评价过程中除了考虑人为污染因素、环境地球化学背景值外,还考虑到自然成岩作用引起背景值变动的因素[17]。地累积指数(Igeo)的计算方法见式(1)。
Igeo=log2[ωi,s/(K·ωi,n)] (1) 式中:
ωi,s i K K ωi,n i 表 1 土壤重金属背景值及毒性系数Table 1. Background value and toxicity coefficient of soil heavy metals重金属元素 背景值/(mg·kg-1) 毒性系数 Cu 11.5 5 Zn 63.3 1 Cd 0.106 30 Pb 43.3 5 表 2 地累积指数分级标准Table 2. Criteria for index of geo-accumulation地累积指数Igeo 分级 污染程度 Igeo≤0 0 无污染 0<Igeo≤1 1 轻度~中等污染 1<Igeo≤2 2 中等污染 2<Igeo≤3 3 中等~强污染 3<Igeo≤4 4 强污染 4<Igeo≤5 5 强污染~极严重污染 5<Igeo≤10 6 极严重污染 2)潜在生态风险指数法。根据重金属性质及其在环境中行为特点,将重金属质量分数、生态效应、环境效应和毒理学联系在一起进行评价[20-21]。某一区域土壤中第i种重金属的潜在生态风险系数(Ei)和土壤中多种重金属的潜在生态风险指数(RI)的计算方法见式(2)和式(3)。
Ei=Ti⋅ωi,sωi,n (2) RI=∑Ei=∑Ti⋅ωi,sωi,n (3) 式中:Ti为元素
i ωi,s i ωi,n i 表 3 潜在生态风险系数及潜在生态风险指数分级标准Table 3. Criteria for potential ecological risk coefficients and potential ecological risk indices潜在生态风险系数Ei 潜在生态风险指数RI 污染程度 Ei<40 RI<150 轻度生态危害 40≤Ei<80 150≤RI<300 中等生态危害 80≤Ei<160 300≤RI<600 强度生态危害 160≤Ei<320 600≤RI<1 200 很强生态危害 320≤Ei 1 200≤RI 极强生态危害 2. 结果与讨论
2.1 重金属质量分数分布特征
根据不同用地类型土壤重金属质量分数的分布特征可知(图2),菜地、稻田和荒地土壤的Cd超标尤为严重,达到《农用地土壤污染风险管控标准》(GB15618-2018)[24]标准限值的1.07~4.00倍。值得注意的是,拆解地土壤4种重金属质量分数均远远超过标准限值,第6层土壤中Cd的质量分数高达28.3 mg·kg−1(图2(d)),相当于环境标准限值的94.2倍,背景值的267倍。同样,于敏等[25]在对贵屿镇拆解地100 cm深处土壤的研究中,发现重金属质量分数相当于土壤背景值的2~200倍。
菜地、稻田和荒地土壤的重金属质量分数随土层深度的增加呈现下降趋势,但拆解地土壤第5和6层的重金属质量分数显著高于1~4层,同时存在严重的Cu、Zn、Cd和Pb污染(图2(d))。菜地、稻田和荒地土壤的重金属质量分数变化趋势与柴艳芳[26]和李科等[27]的研究结果类似,但拆解地土壤重金属沿剖面变化特征与以上研究者调研结果存在显著差异,这说明该拆解地在60~100 cm处土壤重金属污染的影响因素较为复杂。
2.2 重金属形态分布特征
从重金属形态分布特征来看(图3),菜地、稻田和荒地土壤的酸提取态重金属中,比例最高的重金属为Cu(平均21.4%),可还原态重金属占比最高的是Pb,高达38.3%。有研究表明[28],可还原态重金属会与土壤中铁锰氧化物结合,化学键的还原会将重金属离子释放,这说明可还原态可作为土壤Pb的活性组分之一,潜在危害不容忽视[29]。值得注意的是,菜地和稻田的土壤酸提取态Cd占比高于荒地,这可能是因为菜地和稻田土壤中作物的根系分泌物、微生物代谢活动影响了重金属在土壤中的赋存形态,故导致重金属向利于植物吸收富集的酸提取态转化[30-31]。此外,随着土壤深度的增加,重金属Zn形态变化是4种重金属中最为明显的,其中第6层土壤的酸提取态Zn相较于第1层平均下降了48.0%,而残渣态Zn平均上升了48.8%。
拆解地土壤中重金属形态分布与其它类型用地存在明显不同。其中,第5和6层土壤的酸提取态Cd占比极高,分别高达55.3%和79.8%,而酸提取态Cu占比仅为0.52%和3.66%(图3(d))。同样,林娜娜等[32]报道了广东清远某电子垃圾拆解区土壤重金属形态的分布特征,酸提取态Cd占比达60%~70%。拆解地土壤剖面第5和6层重金属污染较为严重,这很有可能是重金属长期地向下迁移所造成的[33-35]。
2.3 地累积指数法评价结果
菜地、稻田和荒地土壤重金属的污染指数,总体呈现随深度增加而降低的变化趋势(表4)。重金属Cu和Zn的地累积指数集中在0~1,整体为轻微污染,Pb是地累积指数均值最低的重金属,仅在0~20 cm土壤出现指数>0的情况。同样,李定龙等[36]的研究表明,台州某拆解地周围稻田重金属Cu的地累积指数为0~1。值得注意的是,稻田0~20 cm处土壤Cd污染程度远比菜地和荒地严重,这是因为,稻田Cd地累积指数均值为2.51,相对于菜地和荒地分别高出21.3%和14.9%。
表 4 不同用地类型土壤中重金属污染地累积指数评价(Igeo)Table 4. Geo-accumulation index (Igeo) of heavy metals in different land-use types用地类型 土层序号 Cu Zn Cd Pb Igeo均值 污染等级 Igeo均值 污染等级 Igeo均值 污染等级 Igeo均值 污染等级 菜地 1 0.93 1 0.42 1 2.17 3 −0.78 0 2 0.59 1 0.33 1 1.97 2 −0.54 0 3 −0.04 0 0.02 1 2.00 3 −1.53 0 4 −0.07 0 0.08 1 1.82 2 −1.36 0 5 −0.15 0 0.07 1 1.60 2 −1.21 0 6 0.19 1 0.11 1 1.56 2 −1.48 0 稻田 1 1.54 2 0.49 1 2.55 3 −0.27 0 2 1.25 2 0.39 1 2.47 3 −0.35 0 3 0.21 1 0.00 1 2.03 3 −1.31 0 4 −0.32 0 −0.05 0 2.31 3 −1.95 0 5 −0.75 0 −0.05 0 1.95 2 −2.38 0 6 −0.58 0 −0.28 0 1.71 2 −2.06 0 荒地 1 1.82 2 0.17 1 2.18 3 −0.12 0 2 0.22 1 0.26 1 2.19 3 0.16 1 3 −0.50 0 0.22 1 1.88 2 −0.80 0 4 −0.25 0 0.25 1 1.68 2 −0.63 0 5 −0.53 0 −0.28 0 1.61 2 −1.89 0 6 −0.53 0 −0.41 0 1.42 2 −1.64 0 拆解地 1 2.80 3 1.00 2 3.22 4 0.68 1 2 3.84 4 1.44 2 3.83 4 1.73 2 3 0.64 1 0.18 1 1.80 2 −1.56 0 4 0.97 1 0.24 1 1.92 2 −2.00 0 5 4.83 5 2.06 3 6.70 6 3.82 4 6 6.64 6 2.64 3 7.47 6 4.36 5 一般情况下,电子垃圾拆解通过酸化和水洗等方式来分解其中有用的物质,其它无法拆解的部分填埋或焚烧,电子垃圾残存的重金属很有可能会渗入土壤中[37]。本研究中,拆解场地土壤不同重金属的污染指数,存在明显差别。第1和2层土壤Cu和Cd为强污染(指数均值3~4),第3和4层土壤污染程度相较于其它层次的土壤更低,甚至重金属Pb处在“无污染”级别,但第5和6层重金属污染程度尤为严重,其中Cd污染指数分别达6.70和7.47,均处于最高的“极严重污染”等级。结合本研究结果与前人研究[7, 38]可知,拆解场地0~20 cm土壤的污染可能是源自电子垃圾填埋、焚烧和废水渗入,且第2层土壤重金属累积量最大。至于其它3种用地类型的土壤,同样遭受了来自拆解地的不同重金属污染[6],其中Cd的累积量最大且垂直迁移能力最强。
2.4 潜在生态风险指数法评价结果
从各重金属潜在生态风险系数(Ei)可以看出(图4),菜地、稻田和荒地各层土壤中Cu、Zn和Pb重金属污染风险系数均<40,均表现为轻度生态危害风险级别。各用地类型土壤Cd的风险系数均>80(图4(c)),其中,稻田Cd的风险系数比菜地和荒地分别高出25.1%和38.6%;李依微等[39]的研究表明,该拆解区内水体Cd污染严重,故使用了含Cd灌溉水可能是导致稻田Cd的风险系数较高的原因。值得注意的是,拆解地重金属Cu、Zn、Cd和Pb的潜在生态风险系数平均值分别为191、4.36、2 340和50.2,其中,第5和6层土壤Cu和Cd的风险系数非常高,尤其是Cd的风险系数在这2层土壤分别达到4 667和7 994(图4(d)),表现为最高的“极强生态危害”等级。
各用地类型综合生态风险指数(RI)表明(图5),4种用地类型土壤中重金属潜在生态风险指数均值分别为229(菜地)、238(稻田)、165(荒地)和2 587(拆解地),其中,菜地、稻田和荒地土壤风险级别为中等污染。值得注意的是,高生态风险指数的拆解地中第5和6层土壤重金属生态风险指数分别高达4 993和8 906,均达到“极强生态危害”等级。同样,梁啸[4]和尹芳华等[40]通过对拆解地及周边农田的调研发现,拆解地重金属生态风险处于极强等级,农田生态风险则为中等以上级别。
根据陈江等[41]的报道,土壤重金属污染分担率可采用重金属潜在生态风险系数与风险指数的比值来计算,从而反映各重金属对潜在生态风险指数的贡献比率。本研究中,Cu、Zn和Pb在4种用地类型各层土壤中的污染分担率为0.01%~11.8%,而Cd在菜地、稻田、荒地和拆解地土壤中的风险指数贡献率分别高达92.6%、94.2%、91.5%和90.5%。4种用地类型土壤中,稻田Cd污染分担率是最高的。同样,ISLAM等[42]通过该评价方法计算拆解地土壤重金属Cd对潜在生态风险指数分担率达77.7%。
3. 结论
1)拆解地土壤Cu、Zn、Cd和Pb的质量分数均较大程度超过《农用地土壤污染风险管控标准》的标准限值,且60~100 cm处土壤的重金属质量分数明显高于0~60 cm层。4种用地类型中各层土壤Cd的质量分数均超过标准限值。
2)菜地和稻田土壤酸提取态Cd占比高于荒地,拆解地中第5和6层土壤酸提取态Cd占比>50%。各用地类型土壤中Zn形态随深度变化显著,随深度的增加,由酸提取态和可还原态向可氧化态和残渣态转化。
3)不同用地类型土壤中重金属潜在的生态风险排序为:拆解地>稻田>菜地>荒地。拆解地土壤重金属潜在生态风险极高,菜地、稻田和荒地均为中等风险。重金属Cd对各用地类型土壤重金属污染组成的贡献最大,土壤累积量最大且垂直迁移能力最强,在稻田土壤Cd元素防控修复工作中尤需关注。
-
表 1 CBC/nZVI/CBC-nZVI的比表面积和孔体积
Table 1. Specific surface area and pore volume of CBC/nZVI/CBC-nZVI
材料名称 比表面积/(m2·g−1) 孔体积/(cm3·g−1) CBC 4.939 0.012 nZVI 7.816 0.007 CBC-nZVI 14.744 0.066 表 2 CBC和CBC-nZVI吸附As(Ⅲ)吸附等温线模型参数
Table 2. Adsorption isotherms fitting parameters of As(Ⅲ) adsorption onto CBC and CBC-nZVI
材料 Freundlich模型 Langmuir模型 R2 kF n R2 KL qm CBC 0.979 8.305 2.05 0.993 0.012 153.27 CBC-nZVI 0.977 20.035 2.56 0.990 0.026 179.03 表 3 CBC和CBC-nZVI吸附As(Ⅲ)吸附动力学模型参数
Table 3. Fitting parameters of As(Ⅲ) adsorption kinetics onto CBC and CBC-nZVI
材料 准一级动力学模型 准二级动力学模型 R2 K1 qe R2 K2 qe CBC 0.986 −0.034 48.56 0.998 0.018 53.59 CBC-nZVI 0.975 −0.065 86.41 0.996 0.009 103.73 -
[1] JAIN C K, SINGH R D. Technological options for the removal of arsenic with special reference to South East Asia[J]. Journal of Environmental Management, 2012, 107: 1-18. [2] 严群, 桂勇刚, 周娜娜, 等. 混凝沉淀法处理含砷选矿废水[J]. 环境工程学报, 2014, 8(9): 3683-3688. [3] 陆俏利, 瞿广飞, 吴斌, 等. 矿区含砷尾矿及废渣稳定化研究[J]. 环境工程学报, 2016, 10(5): 2587-2594. doi: 10.12030/j.cjee.201412257 [4] 徐方男. 新型铁锰氧化物对水体中砷镉吸附性能及机理研究[D]. 杭州: 浙江大学, 2020. [5] 郭凌, 卜玉山, 张曼, 等. 煤基腐殖酸对外源砷胁迫下玉米生长及生理性状的影响[J]. 环境工程学报, 2014, 8(2): 758-766. [6] AREDES S, KLEIN, PAWLIK M. The removal of arsenic from water using natural iron oxide minerals[J]. Journal of Cleaner Production, 2013, 60: 71-76. doi: 10.1016/j.jclepro.2012.10.035 [7] 冷迎祥, 刘菲, 王文娟, 等. 小分子有机酸对纳米铁稳定砷的影响[J]. 环境工程学报, 2017, 11(5): 3195-3203. doi: 10.12030/j.cjee.201606010 [8] WANG M, CHEN Z, SONG W, et al. A review on cadmium exposure in the population and intervention strategies against cadmium toxicity[J]. Bulletin of Environmental Contamination and Toxicology, 2021, 106(1): 65-74. doi: 10.1007/s00128-020-03088-1 [9] WU J, ZHANG H, HE P J, et al. Cr(VI) removal from aqueous solution by dried activated sludge biomass[J]. Journal of Hazardous Materials, 2010, 176(1/2/3): 697-703. [10] BASHA C A, SELVI S J, RAMASAMY E, et al. Removal of arsenic and sulphate from the copper smelting industrial effluent[J]. Chemical Engineering Journal, 2008, 141(1/2/3): 89-98. [11] LUONG V T, KURZ E E C, HELLRIEGEL U, et al. Iron-based subsurface arsenic removal technologies by aeration: A review of the current state and future prospects[J]. Water Research, 2018, 133: 110-122. doi: 10.1016/j.watres.2018.01.007 [12] 乔洪涛, 乔永生, 秦瑞红, 等. 微波酸改性生物炭的制备及其对Cd2+的吸附性能研究[J]. 化工新型材料, 2020, 48(4): 212-216. [13] 蒋国民, 王云燕, 柴立元, 等. 高铁酸钾处理含砷废水[J]. 过程工程学报, 2009, 9(6): 1109-1114. doi: 10.3321/j.issn:1009-606X.2009.06.013 [14] AINIWAER M, ZHANG T, ZHANG N, et al. Synergistic removal of As (III) and Cd (II) by sepiolite-modified nanoscale zero-valent iron and a related mechanistic study[J]. Journal of Environmental Management, 2022, 319: 115658. doi: 10.1016/j.jenvman.2022.115658 [15] SHU H Y, CHANG M C, CHEN C C, et al. Using resin supported nano zero-valent iron particles for decoloration of Acid Blue 113 azo dye solution[J]. Journal of Hazardous Materials, 2010, 184(1/2/3): 499-505. [16] GUAN X, SUN Y, QIN H, et al. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: the development in zero-valent iron technology in the last two decades (1994-2014)[J]. Water Research, 2015, 75: 224-248. doi: 10.1016/j.watres.2015.02.034 [17] 黄菲, 闫梦, 常建宁, 等. 不同菌糠生物炭对水体中Cu2+、Cd2+的吸附性能[J]. 环境化学, 2020, 39(4): 1116-1128. doi: 10.7524/j.issn.0254-6108.2019091604 [18] 曾涛涛, 农海杜, 沙海超, 等. 污泥基生物炭负载纳米零价铁去除Cr(VI)的性能与机制[J]. 复合材料学报, 2022, 40: 1-13. [19] 刘勇, 黄超, 翁秀兰, 等. 绿色合成纳米铁去除水中铬离子[J]. 环境工程学报, 2016, 10(8): 4118-4124. doi: 10.12030/j.cjee.201601146 [20] 金晓英, 杨露, 林强, 等. 绿色合成纳米铁镍去除水中Cr(Ⅵ)的动力学及机理[J]. 环境科学学报, 2022, 42(10): 284-292. [21] SAIF S, TAHIR A, CHEN Y. Green synthesis of iron nanoparticles and their environmental applications and implications[J]. Nanomaterials, 2016, 6(11): 209. doi: 10.3390/nano6110209 [22] MITTAL A K, CHISTI Y, BANERJEE U C. Synthesis of metallic nanoparticles using plant extracts[J]. Biotechnology Advances, 2013, 31(2): 346-356. doi: 10.1016/j.biotechadv.2013.01.003 [23] HOAG G E, COLLINS J B, HOLCOMB J L, et al. Degradation of bromothymol blue by ‘greener’nano-scale zero-valent iron synthesized using tea polyphenols[J]. Journal of Materials Chemistry, 2009, 19(45): 8671-8677. doi: 10.1039/b909148c [24] 曾慎亮, 翁秀兰, 童玉贵, 等. 绿色合成纳米铁同时去除水体中的Pb(Ⅱ)和Cd(Ⅱ)[J]. 环境科学学报, 2015, 35(11): 3538-3544. doi: 10.13671/j.hjkxxb.2015.0008 [25] 李赛. 绿色合成纳米零价铁与膨胀珍珠岩负载纳米零价铁降解混合染料的研究[D]. 太原: 太原理工大学, 2019. [26] MU Y, JIA F, AI Z, et al. Iron oxide shell mediated environmental remediation properties of nano zero-valent iron[J]. Environmental Science:Nano, 2017, 4(1): 27-45. doi: 10.1039/C6EN00398B [27] RAMOS M A V, YAN W, LI X, et al. Simultaneous oxidation and reduction of arsenic by zero-valent iron nanoparticles: understanding the significance of the core−shell structure[J]. Journal of Physical Chemistry C, 2009, 113(33): 14591-14594. doi: 10.1021/jp9051837 [28] XIAO J, GAO B, YUE Q, et al. Removal of trihalomethanes from reclaimed-water by original and modified nanoscale zero-valent iron: characterization, kinetics and mechanism[J]. Chemical Engineering Journal, 2015, 262: 1226-1236. doi: 10.1016/j.cej.2014.10.080 [29] 盛杰, 傅浩洋, 王伟, 等. 纳米零价铁的表征及改性研究进展[J]. 环境化学, 2020, 39(11): 2959-2978. doi: 10.7524/j.issn.0254-6108.2020070803 [30] ÖZÇIMEN D, ERSOY-MERIÇBOYU A. Characterization of biochar and bio-oil samples obtained from carbonization of various biomass materials[J]. Renewable Energy, 2010, 35(6): 1319-1324. doi: 10.1016/j.renene.2009.11.042 [31] MANDAL S, PU S, HE L, et al. Biochar induced modification of graphene oxide & nZVI and its impact on immobilization of toxic copper in soil[J]. Environmental Pollution, 2020, 259: 113851. doi: 10.1016/j.envpol.2019.113851 [32] 李强, 杜玉成, 李杨, 等. 硅藻土基纳米结构AlOOH-MnO2复合氧化物沉积制备及其对As(Ⅴ)吸附性能[J]. 中国粉体技术, 2019, 25(4): 61-69. [33] KANEL S R, MANNING B, CHARLET L, et al. Removal of arsenic (III) from groundwater by nanoscale zero-valent iron[J]. Environmental Science & Technology, 2005, 39(5): 1291-1298. [34] 夏雪芬, 滑熠龙, 黄潇月, 等. 纳米零价铁对水中砷和硒去除的比较研究[J]. 化学学报, 2017, 75(6): 594. [35] SU C, PULS R W. Arsenate and arsenite removal by zerovalent iron: Kinetics, redox transformation, and implications for in situ groundwater remediation[J]. Environmental Science & Technology, 2001, 35(7): 1487-1492. [36] 杜琼. 树脂基纳米零价铁氧化—吸附同步去除水体As(Ⅲ)的特性研究[D]. 南京: 南京大学, 2014. [37] LI T, LIU Y, PENG Q, et al. Removal of lead (II) from aqueous solution with ethylenediamine-modified yeast biomass coated with magnetic chitosan microparticles: Kinetic and equilibrium modeling[J]. Chemical Engineering Journal, 2013, 214: 189-197. doi: 10.1016/j.cej.2012.10.055 [38] 曹玮. 磁性生物炭去除废水中Pb2+、Cd2+的效果及机制初探[D]. 长沙: 中南林业科技大学, 2016. [39] 苏文龙, 成应向, 陈韬, 等. 芬顿污泥制备磁性吸附剂去除水中Sb(Ⅴ)[J]. 环境工程学报, 2022, 16(7): 2165-2177. doi: 10.12030/j.cjee.202202014 [40] XU H, GAO M, HU X, et al. A novel preparation of S-nZVI and its high efficient removal of Cr(VI) in aqueous solution[J]. Journal of Hazardous Materials, 2021, 416: 125924. doi: 10.1016/j.jhazmat.2021.125924 [41] 郭可心, 田佳一, 孙煜璨, 等. 磁性污泥基生物炭对Pb2+的吸附性能[J]. 环境工程学报, 2022, 16(5): 1416-1428. doi: 10.12030/j.cjee.202201003 [42] AHMED W, MEHMOOD S, NÚÑEZ-DELGADO A, et al. Utilization of Citrullus lanatus L. seeds to synthesize a novel MnFe2O4-biochar adsorbent for the removal of U(VI) from wastewater: Insights and comparison between modified and raw biochar[J]. Science of the Total Environment, 2021, 771: 144955. doi: 10.1016/j.scitotenv.2021.144955 [43] 樊建新, 秦亮, 段婷, 等. Fe3O4改性生物质炭对As的吸附特征研究[J]. 重庆交通大学学报(自然科学版), 2021, 40(10): 111-118. [44] YAN W, RAMOS M A V, KOEL B E, et al. As (III) sequestration by iron nanoparticles: Study of solid-phase redox transformations with X-ray photoelectron spectroscopy[J]. Journal of Physical Chemistry C, 2012, 116(9): 5303-5311. doi: 10.1021/jp208600n [45] WANG P, FU F, LIU T. A review of the new multifunctional nano zero-valent iron composites for wastewater treatment: Emergence, preparation, optimization and mechanism[J]. Chemosphere, 2021, 285: 131435. doi: 10.1016/j.chemosphere.2021.131435 [46] O’CARROLL D, SLEEP B, KROL M, et al. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation[J]. Advances in Water Resources, 2013, 51: 104-122. doi: 10.1016/j.advwatres.2012.02.005 [47] MANNING B A, HUNT M L, AMRHEIN C, et al. Arsenic (III) and arsenic (V) reactions with zerovalent iron corrosion products[J]. Environmental Science & Technology, 2002, 36(24): 5455-5461. [48] LIU K, LI F, CUI J, et al. Simultaneous removal of Cd(II) and As(III) by graphene-like biochar-supported zero-valent iron from irrigation waters under aerobic conditions: synergistic effects and mechanisms[J]. Journal of Hazardous Materials, 2020, 395: 122623. doi: 10.1016/j.jhazmat.2020.122623 [49] 李美蓉, 唐晨柳, 张伟贤, 等. 纳米零价铁去除水体中砷的效能与机理[J]. 化学进展, 2022, 34(4): 846-856. [50] LI Z, DENG S, YU G, et al. As(V) and As(III) removal from water by a Ce–Ti oxide adsorbent: Behavior and mechanism[J]. Chemical Engineering Journal, 2010, 161(1/2): 106-113. [51] 周世民. 铁基纳米复合材料的制备及对砷吸附性能研究[D]. 天津: 天津大学, 2016. -





 下载:
下载: