-
水力压裂技术是低渗透油藏及非常规油气开发的主要手段[1-4]。压裂过程中产生的压裂返排液含有油、酚类、甲醛、胍胶等多种有害物质[5],组分复杂,乳化程度高,稳定性强,处理难度大[6],若不能经有效处理而排放或回注地层,会对环境造成严重污染和破坏[7-9]。研究人员多采用物理法、化学法、生物法的联合工艺来处理压裂返排液[10-12],传统的处理方法普遍存在处理工艺流程长、设备多等问题,且所加入的化学药剂也容易造成二次污染。
相较于传统处理方法,电化学方法具有环保、反应条件适中、降解效率高等特点,已成为新兴的研究方向。樊玉新等[13]研究了电絮凝预处理+电化学氧化工艺处理压裂返排液,处理后耗氧有机污染物(以COD计)的浓度降至80 mg·L−1以下。孟宣宇等[14]考察了各操作条件对电絮凝处理效果的影响,确定最佳条件为反应时间60 min,电流密度10.0 mA·cm−2,极板间距3 cm,pH=7。王啸等[15]采用絮凝预处理+电絮凝氧化组合法处理海上压裂返排废水,处理后污水COD去除率达到85.9%。吴磊等[16]通过电絮凝电化学氧化-臭氧氧化耦合处理技术去除压裂返排液中的总有机碳(TOC),在不分室和分室情况下TOC去除率分别为80%和95%。以上研究对于电化学处理压裂返排液具有重要意义,但多集中在工艺参数的优化[17-20]和处理效率的提升方面,对电化学处理油田压裂返排液的机理研究则较少。
本研究采用电絮凝和电化学氧化方法处理压裂返排液,采用响应面法对电化学处理过程进行拟合,并分析相关因素对COD去除效果的影响;通过探究COD去除效果与反应时间的关系研究电化学法处理压裂返排液的反应动力学;通过Al-Ferron 逐时络合比色法和自由基淬灭实验,分析了电化学处理过程中COD的去除机理;研究了电化学反应前后极板表面的变化以及影响极板钝化的因素,以期为电化学技术处理油田压裂返排液的工业化应用提供参考。
-
实验采用的水样为新疆油田压裂返排液。对压裂返排液进行测定和分析,其pH为7.57,总硬度为1 544 mg·L−1,碱度为4 880 mg·L−1,浊度为123.2 NTU,COD为1 520 mg·L−1,表明其硬度高、有机物含量高。压裂返排液中颗粒的Zeta电位为-35.2~-40.9 mV,表明水中颗粒物状态稳定不易沉降。随着自然沉降时间的增加,压裂返排液中的颗粒粒径变化小,说明压裂返排液中的胶体颗粒物处于非常稳定的状态。实验数据均为3次测量取平均值。
-
实验所用主要试剂:铬黑T指示剂、甲基橙指示剂、酚酞指示剂、异丙醇、对苯醌、Ferron 试剂、邻菲罗啉、无水醋酸钠、盐酸羟胺、盐酸和铝标液等,上述药品均为分析纯。
实验所用主要仪器:MS305D直流电源(东莞迈豪电子科技有限公司);DR1010 COD快速测定仪(美国哈希公司);WZB-175浊度计(上海仪电科学仪器有限公司);PHS-3C pH计(上海雷磁公司);Tescan MIRA 3场发射扫描电子显微镜(捷克泰斯肯公司);气质联用450GC-320MS(varian公司) ;SALD-2300激光粒度衍射仪(日本岛津制作所);UV-1600紫外可见分光光度计(上海美谱达仪器有限公司)。
-
如图1所示,实验采用的电化学反应器为定制的矩形反应器,长、宽、高度分别为6 、4.7 、12.5 cm,有效容积为250 mL,其材质为有机玻璃,反应器内有固定电极板的凹槽,凹槽间隔为5 mm,本实验中将极板间距固定为40 mm。电极板固定在凹槽内,通过导线与直流电源相连。实验所用到的极板为铝极板、Ti/RuO2-IrO2极板和石墨极板,其规格一致,长、宽、厚度分别为5、10、0.1 cm。
-
取压裂返排液于电化学反应器中进行电絮凝和电化学氧化实验,设置电流密度为10 mA·cm−2,极板间距为 40 mm,搅拌速度为 500 r·min−1,电絮凝所用阳极极板为铝板,阴极极板为石墨;电化学氧化所用阳极极板为Ti/RuO2-IrO2,阴极极板为石墨,电化学处理完成后取上层液测量COD。
电化学反应动力学实验:取压裂返排液进行电絮凝和电化学氧化处理,将电解时间设置为20~240 min,处理完成后,取上层液测量COD,取3次测量的平均值,采用反应动力学模型拟合电化学反应后COD随电解时间的变化。
Al含量及分布实验:利用铝标准溶液和Ferron络合显色剂反应绘制标准曲线,拟合得到标准曲线方程。取反应后溶液与 Ferron 络合显色剂反应,根据标准曲线求出总铝含量AlT。采用Al-Ferron 逐时络合比色法测定溶液中铝的形态分布,取反应后溶液与 Ferron 络合显色剂反应,根据标准曲线及不同时间段吸光度的变化来确定铝各形态的含量,铝的形态主要分为Ala(单体形态),Alb(聚合铝形态)和Alc(溶胶或凝胶聚合物形态)[21],AlT = Ala+Alb+Alc。
活性物质分析实验:取压裂返排液进行电絮凝和电化学氧化处理,分别加入异丙醇和对苯醌,异丙醇为羟基自由基(OH·)的特征淬灭剂[22-26],对苯醌为超氧负离子(O2·−)的特征淬灭剂,这2种自由基均可快速实现有机污染物的降解甚至矿化。通过向压裂返排液中加入异丙醇和对苯醌,设置异丙醇和对苯醌浓度梯度为1~4 mmol·L−1,进行电化学处理,处理完成后,取上层液测定COD,取3次测量的平均值,通过比较处理后的COD分析对应电化学反应过程中的活性物质。
有机物成分分析:取100 mL水样用硫酸调节pH<2,使用石油醚作为萃取剂与其在分液漏斗中混合,摇晃均匀后,静置30 min,待水相和有机相完全分层后将水相从下层取出,有机相从上层取出,剩余水样继续用石油醚萃取,总共萃取3次,将3次所得的有机相合并进行气相色谱质谱联用(GC-MS)分析。
电极板表面分析实验:取处理过压裂返排液的电絮凝和电化学氧化的阴阳极板,通过扫描电镜(SEM)对其表面形貌进行表征,并通过X射线能谱分析(EDS)对其组成成分进行分析。
-
1)响应面分析:根据响应面法设计原理,采用Box-bohken模型对电絮凝法处理压裂返排液的主要性能指标进行3因素3 水平试验设计,如表1所示,以电流密度(A)、极板间距(B)、电解时间(C)为考察因素(自变量),以+1、0、−1 分别代表自变量的高、中、低3 因素水平,对自变量进行编码。
2)反应动力学分析。采用反应动力学模型对电化学处理后水的COD变化进行拟合,零级反应动力学方程如式(1)所示,一级反应动力学方程如式(2)所示。
式中:cCOD为当前时间水中的COD,mg·L−1,k0为反应速率,mg·(L·min)−1,t为反应时间,min,A0为常数。
式中:lncCOD为当前时间水中的COD的对数,k1为反应速率,min−1,t为反应时间,min,A1为常数。
-
1)电絮凝法处理的响应面分析。电絮凝法处理压裂返排液的响应面结果如表2所示。通过Design-Expert 12对表2中的数据进行多元回归拟合,得到电絮凝处理压裂返排液的电流密度、极板间距和电解时间与COD去除率之间的二次多项式回归方程(表2下注1),对该回归方程进行方差分析,结果如表3所示。
由表3的方差分析结果可知:TP 模型的F 值为6.95,P值为0.023( P值小于0.05 视为模型显著),表明所得的模型达到显著水平,即该模型在被研究的整个回归区域内拟合较好。多元相关系数R2=0.926 0,说明相关性好。由响应面分析得电絮凝处理最优实验条件:电流密度为8.78 mA·cm−2,极板间距为3.95 cm,电解时间为46.29 min,在此条件下的COD最大去除率可达88.2%。
各因素对COD去除率影响的响应面曲线与等高线如图2所示。由图2(a)可知,电流密度和极板间距对COD去除率的影响均不显著。由图2(b)可以看出,电解时间的曲面更加陡峭,而电流密度的影响较小,说明电解时间对COD去除率的影响程度更加显著。由图2(c)可见,极板间距和电解时间对COD去除率的影响均十分显著,两者交互作用较好,电解时间的P值(P=0.010 3)小于极板间距(P=0.015 5),表明相比于极板间距电解时间更占优势。
2)电化学氧化法处理的响应面分析,电化学氧化法处理压裂返排液的响应面结果如表4所示。利用软件对表4实验数据进行多元回归拟合,得到电化学氧化处理压裂返排液的电流密度、极板间距和电解时间与COD去除率之间的二次多项式回归方程(表2下注2),对该回归方程进行方差分析结果如表5所示。
由表5中的方差分析结果可知,TP 模型的F值为11.88,P值为0.007 0,表明所得的模型达到显著水平,即该模型在被研究的整个回归区域内拟合较好。多元相关系数R2=0.955 3,说明相关性好。综上所述,回归方程对电化学氧化去除压裂返排液的COD可提供一个合适的模型。由响应面分析结果可得到电化学氧化处理最优实验条件:电流密度为8.66 mA·cm−2,极板间距为2.81 cm,电解时间为43.21 min,最大COD去除率可达100.0%。
各因素影响COD去除率的响应面曲线与等高线如图3所示。由图3(a)可知,相比于极板间距,电流密度的曲面更加陡峭,说明电流密度对COD去除率的影响程度更不显著。图3(b)中电流密度的曲面更加陡峭,而电解时间的影响较小,说明相比于电解时间,电流密度对COD去除率的影响程度更加显著,对于电化学氧化处理而言,电流密度越大,有机物氧化的效率越高,处理效果越显著。图3(c)中极板间距和电解时间对COD去除率的影响均十分显著,两者交互作用较好,电解时间的P值(P=0.003 9)小于极板间距(P=0.257 7),表明相比于极板间距电解时间更占优势。
-
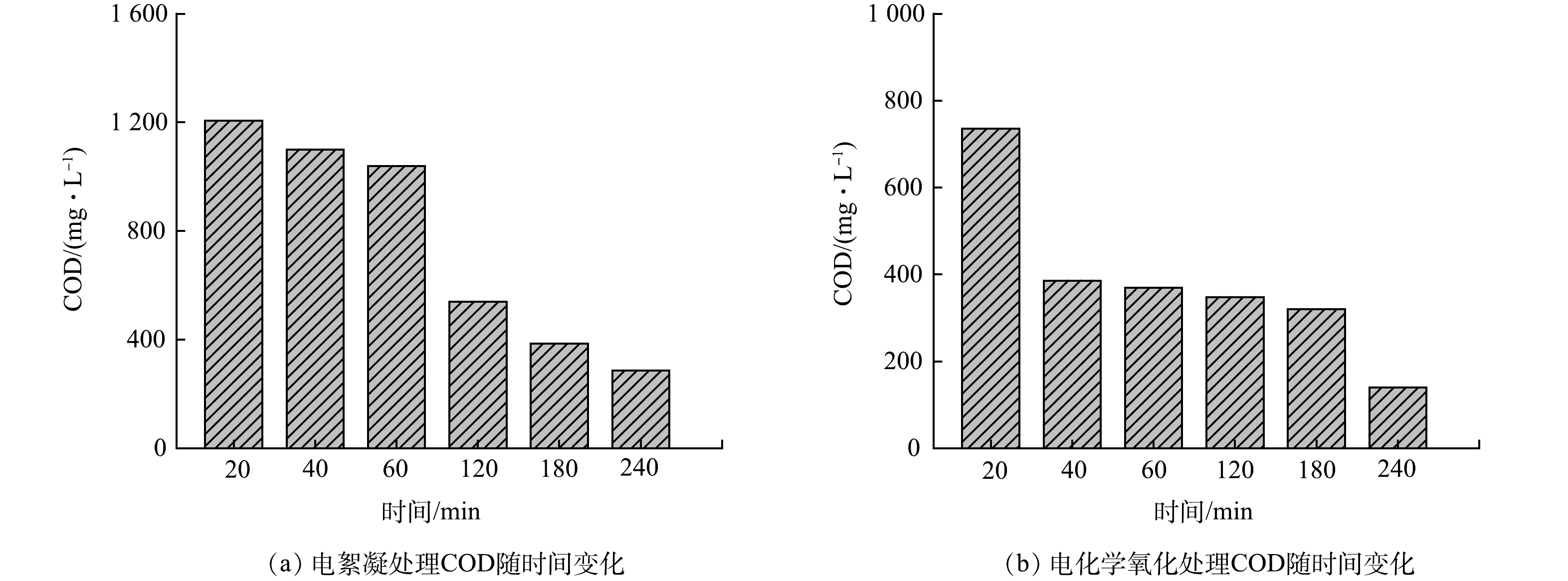
由图4(a)可知,随处理时间增加,COD去除率从19.1%增大至80.1%,表明增加电絮凝时间有利于COD的去除。这是由于延长电絮凝时间使更多的铝离子在水中形成的氢氧化物和多羟基配合物絮体,这些絮体在同向絮凝和异向絮凝的共同作用下,由小的絮凝体叠加形成大的絮凝集团,通过网捕、吸附架桥和电性中和等的协同作用[27]去除水中的油、悬浮物等有机杂质,使水的COD降低。
由图4(b)可知,随处理时间增长,COD去除率从50.6%增大至90.6%,增加电化学氧化时间有利于COD的去除。这是由于增长电化学氧化时间将使更多的污染物直接在阳极表面通过电子传递被氧化,或被电解产生的活性物质间接氧化[28],进而导致水的COD降低。在相同的实验参数条件下,电化学氧化法去除COD的效果优于电絮凝法。
通过比较各级动力学模型的特征,采用零级动力学模型对电絮凝处理压裂返排液COD随时间的变化进行分析,如表6所示,在电絮凝处理压裂返排液的0~4 h,其反应速率常数为4.49 mg·(L·min)−1,零级动力学方程式为cCOD =−4.49t+1253.6,该拟合方程的相关系数为0.935 8,拟合相关性显著。根据零级反应动力学模型的特征,反应速率在反应过程中保持恒定,与残余COD无关。
通过比较各级动力学模型的特征,采用一级动力学拟合模型对电化学氧化处理压裂返排液COD随时间的变化进行分析,如表6所示,在电化学氧化处理压裂返排液的0~4 h,其反应速率常数为0.005 4 min−1,一级动力学方程式为lncCOD = −0.005 4t+6.433,该拟合方程的相关系数为0.773 7。根据一级反应动力学模型的特征,反应速率随着残余COD降低而降低,因此,电絮凝时间可设置为1~2 h,并及时更新反应器中的压裂返排液,这样可使反应器中的压裂返排液保持高COD,进而使COD去除速率维持在较高水平。
-
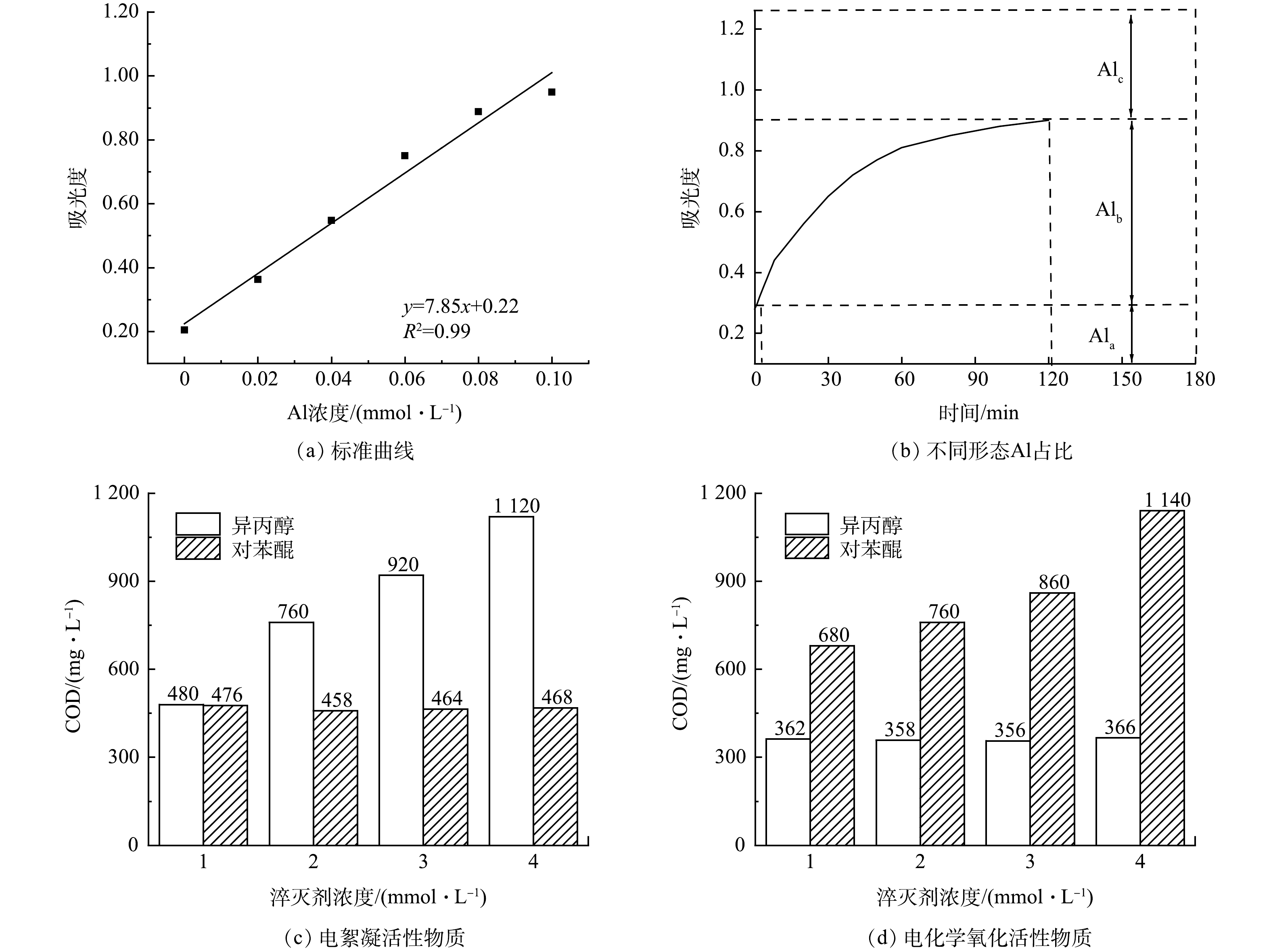
1)电化学法处理的活性物质分析。采用铝标准溶液配制不同浓度的铝溶液,加入等量Ferron络合显色剂,定容后用紫外可见分光光度计测定370 nm处吸光度,绘制的标准曲线如图5(a)所示。
根据总铝含量的测定方法,在370 nm下测得经电絮凝处理后水的总吸光度为1.256,根据标准曲线算得总铝含量AlT为0.132 mmol·L−1。按照Al-Ferron 逐时络合比色法,由一定时间内的吸光度变化可得,经电絮凝处理过后水中Ala、Alb、Alc吸光度占比分别为23.6%、48.0%、28.4%。如图5(b)所示,通过公式计算可得Ala为0.296 mmol·L−1,Alb为0.603 mmol·L−1,Alc为0.357 mmol·L−1。由Al的不同形态分布可知,Alb占比接近总铝含量的50%,Alb主要由聚十三铝(AlO4Al12(OH)24(H2O)127+,简称Al13)构成,Al13具有很强的电中和能力和优异的絮凝作用,为经典的Keggin 结构,其结构中心为Al—O四面体,外围是12个八面体配位的Al(OH)2+,在中性pH条件下,结构形态较稳定,不易继续水解,表现出一定的稳态,Al13有很强的正电荷和吸附聚集能力,因而具有很强的电中和与粘附架桥能力,对于去除水中的有机物具有关键作用。
除了铝的聚合体,特定种类的自由基对于去除水中有机物也有一定的作用。由图5(c)可知,随着水中异丙醇浓度的增大,电絮凝处理后水的COD由480 mg·L−1增至1 120 mg·L−1,COD去除率由67.8%降至24.8%,表明加入的异丙醇对水中的OH·起到淬灭作用,在异丙醇醇分子中,由于OH·的影响,使得α-氢较活泼,容易被氧化和脱氢[29],OH·与异丙醇主要发生夺氢反应,而且主要是夺取α-氢[30]。电絮凝作用而产生的OH·,来自于电极在电场作用下对水的氧化分解,或是氢氧根离子的直接氧化(式(3)~(4))[31]。OH·的减少使反应速率大大降低,抑制污染物的降解,COD的去除率降低,因此,OH·在电絮凝处理压裂返排液的过程中对COD的去除起到重要作用。而随着水中对苯醌浓度的增大,经电絮凝处理后水的COD基本不变,COD去除率保持在68%~70%,表明O2·−在该过程中未发挥明显作用。
由图5(d)可见,随着水中异丙醇的浓度增大,经电化学氧化处理后水的COD基本不变,COD去除率保持在75%~76%。此结果表明OH·在该过程中未发挥作用。随着加入对苯醌的浓度增大,经电化学氧化处理后水的COD从680 mg·L−1增至1 420 mg·L−1,COD去除率由54.4%下降至23.5%,表明加入的对苯醌对水中的O2·−起到特征淬灭作用,O2·−是含有未配对电子的高活性物质,可通过电子传递而与水中有机物发生氧化还原反应[32],使有机物降解为小分子物质,O2·−的淬灭使有机污染物的降解过程受阻,反应速率大大降低,COD的去除率降低。因此,O2·−是电化学氧化处理压裂返排液过程中对于COD的去除起到主要作用的活性物质。
2)电化学法处理前后有机物分析。由表7可知,经GC-MS分析鉴定,电絮凝处理1 h后压裂返排液中的大部分有机物被去除,剩余有机物主要为C10H16O2和C22H13NO4。经过电化学氧化处理1 h后,水中的长链有机物大部分被氧化为短链有机物,其中含 Si和含 I的有机物均被去除。Si存在于C9H20O2Si中,鉴定其为环己基甲基二甲氧基硅烷,在电絮凝过程中OH·使C—Si和C—C键断裂,生成3-氯己烷和正己醇。I存在于C14H29I、C18H37I和C20H41I中,经GC-MS鉴定其分别1-碘十四烷、1-碘十八烷和1-碘-2-辛基十二烷,在电化学氧化中O2·−断裂C—I键使其变为短链小分子物质,如氯己烷和正己醇等。
选取2,4-二叔丁基苯酚和环己基甲基二甲氧基硅烷作为代表性有机物分析电絮凝和电化学氧化过程中有机物的降解途径。在电絮凝过程中,OH·首先攻击苯环上的C—C键,使之断裂后生成微溶于水的苯酚和叔丁醇[33],苯酚经铝聚合物的吸附聚集作用被絮凝去除,叔丁醇被OH·进一步氧化为CO2和H2O。在电化学氧化过程中,O2·−使C—Si键断裂,生成环己醇、丙三醇和二氧化硅[34],O2·−的强氧化作用使初级产物进一步氧化为二氧化碳和水。
-
1)电絮凝处理后极板表面形貌及组成分析。如图6(a)所示,由扫描电镜(SEM)图可以看出,电絮凝阳极铝板表面有较多坑洞。这些坑洞是由于铝失电子变成铝离子进入水中导致。此外,存在不规则块状晶体附着在铝极板表面(图6(b))。结合X射线能谱分析(EDS)(表8)可知,由于压裂返排液的总硬度较高,这些块状晶体可能是水中的钙离子结合水中的碳酸根所形成的碳酸钙。电絮凝阴极石墨极板在微观上为片层状结构(图6(c)),其表面覆盖着絮体和晶体颗粒(图6(d)),由EDS分析(表8)检测到阴极表面主要元素为C、O、Ca、Na和Mg,石墨极板上的絮体可能是带正电的有机物在电场作用下吸附到极板表面,同时水中的钙离子和镁离子结合水中的氢氧根和碳酸根生成的不溶盐结晶在极板表面。
2)电化学氧化处理后极板表面形貌及组成分析。如图7(a)所示,电化学氧化的阳极Ti/RuO2-IrO2极板表面存在着细小的颗粒,在20 000倍条件下观察这些细小颗粒形状不规则(图7(b)),结合EDS分析结果(表9)可知,这些细小颗粒应为压裂返排液中的有机污染物经电化学氧化后,裂解为小分子有机物附着在Ti/RuO2-IrO2极板上。电化学氧化阴极石墨极板上覆盖着致密的条状垢(图7(c)),在50 000倍条件下观察,这些条状垢中也有块状晶体(图7(d)),结合EDS分析(表9)可知,这些致密的条状垢主要是有机污染物,而块状晶体则是钙镁碳酸盐。
-
1)电絮凝和电化学氧化能有效去除油田压裂返排液的COD,电絮凝和电化学氧化处理去除COD的二次响应面回归模型相关性显著,实验可信度、精确度和精密度均在合理范围内,在最佳实验条件下,COD去除率分别可达88.2%和100.0%。
2)电絮凝和电化学氧化处理压裂返排液去除COD的动力学分别表现为零级和一级反应动力学的特征,其反应速率常数分别为4.49 mg·(L·min)−1和0.005 4 min−1。
3)对于处理压裂返排液的2种不同的电化学技术,电絮凝法中铝聚合物的絮凝作用和OH·的氧化分解作用可协同去除水中的有机污染物;电化学氧化法主要依靠强氧化性的O2·−,O2·−可将绝大部分长链有机物降解为小分子有机物和无机物。
4)在电絮凝反应过程中,压裂返排液中的钙离子结合水中的碳酸根所形成的碳酸钙附着在铝极板表面,阴极石墨极板上存在有机物絮体、钙镁碳酸盐;电化学氧化过程中,阳极Ti/RuO2-IrO2极板表面存在着小分子有机物,阴极石墨极板上覆盖着致密的条状有机污染物以及钙镁碳酸盐。
油田压裂返排液的电化学处理
Electrochemical treatment of oilfield fracturing flowback fluid
-
摘要: 为探究电絮凝和电化学氧化法处理油田压裂返排液的机理,采用响应面法拟合了反应过程,考察了电化学反应动力学、活性物质以及电极板的形貌和成分的变化。结果表明,电絮凝和电化学氧化法的响应面模型相关性显著,精确度和可信度均在合理范围内,在最优实验条件下其对应的COD去除率分别可达88.2%和100.0%;压裂返排液经电絮凝和电化学氧化处理后去除COD的动力学分别适用于零级和一级动力学模型,反应速率常数分别为4.49 mg·(L·min)−1和0.005 4 min−1;电絮凝和电化学氧化处理压裂返排液起主要作用的活性物质分别是OH·和O2·−;电絮凝反应后,阳极和阴极表面分别附有碳酸钙和絮体有机物,电化学氧化反应后,阳极和阴极表面分别覆盖着致密的有机污染物和钙镁碳酸盐。Abstract: To investigate the mechanism of electrocoagulation and electrochemical oxidation methods treating oilfield fracturing backflow fluid, response surface methodology was used to fit the reaction process, and the electrochemical reaction kinetics, active substances, and changes in the morphology and composition of electrode plates were studied. The results showed that the correlation between the response surface models of electrocoagulation and electrochemical oxidation methods was significant, and the accuracy and reliability were in a reasonable range. Under the optimal experimental conditions, their COD removal rates could reach 88.16% and 100.00%, respectively. The COD removal kinetics for fracturing flowback fluid after electrocoagulation and electrochemical oxidation treatment were applicable to the zero order and first order kinetic models, respectively, and the reaction rate constant were 4.49 mg·L−1·min−1 and 0.005 4 min−1 , respectively. The active substances that played a major role in the treatment of fracturing backflow fluid by electrocoagulation and electrochemical oxidation were OH· and O2·− , respectively. After the electrochemical reaction, the electrocoagulation anode and cathode were coated with calcium carbonate and flocculent organic matter on the surface, respectively, while the electrochemical oxidation anode and cathode surfaces were covered with dense organic pollutants and calcium magnesium carbonate, respectively.
-
水力压裂技术是低渗透油藏及非常规油气开发的主要手段[1-4]。压裂过程中产生的压裂返排液含有油、酚类、甲醛、胍胶等多种有害物质[5],组分复杂,乳化程度高,稳定性强,处理难度大[6],若不能经有效处理而排放或回注地层,会对环境造成严重污染和破坏[7-9]。研究人员多采用物理法、化学法、生物法的联合工艺来处理压裂返排液[10-12],传统的处理方法普遍存在处理工艺流程长、设备多等问题,且所加入的化学药剂也容易造成二次污染。
相较于传统处理方法,电化学方法具有环保、反应条件适中、降解效率高等特点,已成为新兴的研究方向。樊玉新等[13]研究了电絮凝预处理+电化学氧化工艺处理压裂返排液,处理后耗氧有机污染物(以COD计)的浓度降至80 mg·L−1以下。孟宣宇等[14]考察了各操作条件对电絮凝处理效果的影响,确定最佳条件为反应时间60 min,电流密度10.0 mA·cm−2,极板间距3 cm,pH=7。王啸等[15]采用絮凝预处理+电絮凝氧化组合法处理海上压裂返排废水,处理后污水COD去除率达到85.9%。吴磊等[16]通过电絮凝电化学氧化-臭氧氧化耦合处理技术去除压裂返排液中的总有机碳(TOC),在不分室和分室情况下TOC去除率分别为80%和95%。以上研究对于电化学处理压裂返排液具有重要意义,但多集中在工艺参数的优化[17-20]和处理效率的提升方面,对电化学处理油田压裂返排液的机理研究则较少。
本研究采用电絮凝和电化学氧化方法处理压裂返排液,采用响应面法对电化学处理过程进行拟合,并分析相关因素对COD去除效果的影响;通过探究COD去除效果与反应时间的关系研究电化学法处理压裂返排液的反应动力学;通过Al-Ferron 逐时络合比色法和自由基淬灭实验,分析了电化学处理过程中COD的去除机理;研究了电化学反应前后极板表面的变化以及影响极板钝化的因素,以期为电化学技术处理油田压裂返排液的工业化应用提供参考。
1. 材料与方法
1.1 实验所用压裂返排液水质
实验采用的水样为新疆油田压裂返排液。对压裂返排液进行测定和分析,其pH为7.57,总硬度为1 544 mg·L−1,碱度为4 880 mg·L−1,浊度为123.2 NTU,COD为1 520 mg·L−1,表明其硬度高、有机物含量高。压裂返排液中颗粒的Zeta电位为-35.2~-40.9 mV,表明水中颗粒物状态稳定不易沉降。随着自然沉降时间的增加,压裂返排液中的颗粒粒径变化小,说明压裂返排液中的胶体颗粒物处于非常稳定的状态。实验数据均为3次测量取平均值。
1.2 试剂和仪器
实验所用主要试剂:铬黑T指示剂、甲基橙指示剂、酚酞指示剂、异丙醇、对苯醌、Ferron 试剂、邻菲罗啉、无水醋酸钠、盐酸羟胺、盐酸和铝标液等,上述药品均为分析纯。
实验所用主要仪器:MS305D直流电源(东莞迈豪电子科技有限公司);DR1010 COD快速测定仪(美国哈希公司);WZB-175浊度计(上海仪电科学仪器有限公司);PHS-3C pH计(上海雷磁公司);Tescan MIRA 3场发射扫描电子显微镜(捷克泰斯肯公司);气质联用450GC-320MS(varian公司) ;SALD-2300激光粒度衍射仪(日本岛津制作所);UV-1600紫外可见分光光度计(上海美谱达仪器有限公司)。
1.3 实验装置
如图1所示,实验采用的电化学反应器为定制的矩形反应器,长、宽、高度分别为6 、4.7 、12.5 cm,有效容积为250 mL,其材质为有机玻璃,反应器内有固定电极板的凹槽,凹槽间隔为5 mm,本实验中将极板间距固定为40 mm。电极板固定在凹槽内,通过导线与直流电源相连。实验所用到的极板为铝极板、Ti/RuO2-IrO2极板和石墨极板,其规格一致,长、宽、厚度分别为5、10、0.1 cm。
1.4 实验方法
取压裂返排液于电化学反应器中进行电絮凝和电化学氧化实验,设置电流密度为10 mA·cm−2,极板间距为 40 mm,搅拌速度为 500 r·min−1,电絮凝所用阳极极板为铝板,阴极极板为石墨;电化学氧化所用阳极极板为Ti/RuO2-IrO2,阴极极板为石墨,电化学处理完成后取上层液测量COD。
电化学反应动力学实验:取压裂返排液进行电絮凝和电化学氧化处理,将电解时间设置为20~240 min,处理完成后,取上层液测量COD,取3次测量的平均值,采用反应动力学模型拟合电化学反应后COD随电解时间的变化。
Al含量及分布实验:利用铝标准溶液和Ferron络合显色剂反应绘制标准曲线,拟合得到标准曲线方程。取反应后溶液与 Ferron 络合显色剂反应,根据标准曲线求出总铝含量AlT。采用Al-Ferron 逐时络合比色法测定溶液中铝的形态分布,取反应后溶液与 Ferron 络合显色剂反应,根据标准曲线及不同时间段吸光度的变化来确定铝各形态的含量,铝的形态主要分为Ala(单体形态),Alb(聚合铝形态)和Alc(溶胶或凝胶聚合物形态)[21],AlT = Ala+Alb+Alc。
活性物质分析实验:取压裂返排液进行电絮凝和电化学氧化处理,分别加入异丙醇和对苯醌,异丙醇为羟基自由基(OH·)的特征淬灭剂[22-26],对苯醌为超氧负离子(O2·−)的特征淬灭剂,这2种自由基均可快速实现有机污染物的降解甚至矿化。通过向压裂返排液中加入异丙醇和对苯醌,设置异丙醇和对苯醌浓度梯度为1~4 mmol·L−1,进行电化学处理,处理完成后,取上层液测定COD,取3次测量的平均值,通过比较处理后的COD分析对应电化学反应过程中的活性物质。
有机物成分分析:取100 mL水样用硫酸调节pH<2,使用石油醚作为萃取剂与其在分液漏斗中混合,摇晃均匀后,静置30 min,待水相和有机相完全分层后将水相从下层取出,有机相从上层取出,剩余水样继续用石油醚萃取,总共萃取3次,将3次所得的有机相合并进行气相色谱质谱联用(GC-MS)分析。
电极板表面分析实验:取处理过压裂返排液的电絮凝和电化学氧化的阴阳极板,通过扫描电镜(SEM)对其表面形貌进行表征,并通过X射线能谱分析(EDS)对其组成成分进行分析。
1.5 分析方法
1)响应面分析:根据响应面法设计原理,采用Box-bohken模型对电絮凝法处理压裂返排液的主要性能指标进行3因素3 水平试验设计,如表1所示,以电流密度(A)、极板间距(B)、电解时间(C)为考察因素(自变量),以+1、0、−1 分别代表自变量的高、中、低3 因素水平,对自变量进行编码。
表 1 响应面实验设计因素水平和编码Table 1. Factor level and code for response surface experimental design变量 因素 电流密度(A)/(mA·cm−2) 极板间距(B)/cm 电解时间(C)/min −1 1 2 20 0 5.5 3 40 1 10 4 60 2)反应动力学分析。采用反应动力学模型对电化学处理后水的COD变化进行拟合,零级反应动力学方程如式(1)所示,一级反应动力学方程如式(2)所示。
stringUtils.convertMath(!{formula.content}) (1) 式中:cCOD为当前时间水中的COD,mg·L−1,k0为反应速率,mg·(L·min)−1,t为反应时间,min,A0为常数。
stringUtils.convertMath(!{formula.content}) (2) 式中:lncCOD为当前时间水中的COD的对数,k1为反应速率,min−1,t为反应时间,min,A1为常数。
2. 结果与讨论
2.1 电化学法处理压裂返排液的响应面分析
1)电絮凝法处理的响应面分析。电絮凝法处理压裂返排液的响应面结果如表2所示。通过Design-Expert 12对表2中的数据进行多元回归拟合,得到电絮凝处理压裂返排液的电流密度、极板间距和电解时间与COD去除率之间的二次多项式回归方程(表2下注1),对该回归方程进行方差分析,结果如表3所示。
表 2 电絮凝处理的响应面结果Table 2. Response surface results of electrocoagulation treatment序号 X1 X2 X3 COD去除率/% 1 −1 −1 0 63.2 2 1 −1 0 75.6 3 −1 1 0 59.7 4 1 1 0 70.3 5 −1 0 −1 52.0 6 1 0 −1 69.5 7 −1 0 1 67.4 8 1 0 1 74.7 9 0 −1 −1 58.2 10 0 1 −1 43.8 11 0 −1 1 75.0 12 0 1 1 54.7 13 0 0 0 69.8 14 0 0 0 69.2 15 0 0 0 70.3 注1:Y1 = 0.697 7+0.059 7A − 0.054 4B+0.060 4C − 0.004 5AB − 0.025 5AC − 0.014 8BC+0.027A2 − 0.052 7B2 − 0.065 7C2。 表 3 电絮凝回归方程的方差分析Table 3. Analysis of variance for the regression equation of electrocoagulation来源 平方和 自由度 均方和 F P 模型 0.114 0 9 0.012 7 6.95 0.023 A 0.028 6 1 0.028 6 15.66 0.010 8 B 0.023 7 1 0.023 7 12.97 0.015 5 C 0.029 2 1 0.029 2 15.99 0.010 3 AB 0.000 1 1 0.000 1 0.044 4 0.841 4 AC 0.002 6 1 0.002 6 1.43 0.285 9 BC 0.009 0 1 0.009 0 0.477 3 0.520 4 A2 0.002 7 1 0.002 7 1.48 0.277 9 B2 0.010 3 1 0.010 3 5.63 0.063 8 C2 0.015 9 1 0.015 9 8.74 0.031 6 残差 0.009 1 5 0.001 8 失拟项 0.009 1 3 0.003 0 纯误差 0.000 1 2 0 由表3的方差分析结果可知:TP 模型的F 值为6.95,P值为0.023( P值小于0.05 视为模型显著),表明所得的模型达到显著水平,即该模型在被研究的整个回归区域内拟合较好。多元相关系数R2=0.926 0,说明相关性好。由响应面分析得电絮凝处理最优实验条件:电流密度为8.78 mA·cm−2,极板间距为3.95 cm,电解时间为46.29 min,在此条件下的COD最大去除率可达88.2%。
各因素对COD去除率影响的响应面曲线与等高线如图2所示。由图2(a)可知,电流密度和极板间距对COD去除率的影响均不显著。由图2(b)可以看出,电解时间的曲面更加陡峭,而电流密度的影响较小,说明电解时间对COD去除率的影响程度更加显著。由图2(c)可见,极板间距和电解时间对COD去除率的影响均十分显著,两者交互作用较好,电解时间的P值(P=0.010 3)小于极板间距(P=0.015 5),表明相比于极板间距电解时间更占优势。
2)电化学氧化法处理的响应面分析,电化学氧化法处理压裂返排液的响应面结果如表4所示。利用软件对表4实验数据进行多元回归拟合,得到电化学氧化处理压裂返排液的电流密度、极板间距和电解时间与COD去除率之间的二次多项式回归方程(表2下注2),对该回归方程进行方差分析结果如表5所示。
表 4 电化学氧化处理的响应面结果Table 4. Response surface results of electrochemical oxidation treatment序号 X1 X2 X3 COD去除率/% 1 −1 −1 0 62.0 2 1 −1 0 97.9 3 −1 1 0 62.4 4 1 1 0 94.4 5 −1 0 −1 59.4 6 1 0 −1 81.8 7 −1 0 1 78.9 8 1 0 1 96.1 9 0 −1 −1 62.9 10 0 1 −1 67.0 11 0 −1 1 100.0 12 0 1 1 78.3 13 0 0 0 100.0 14 0 0 0 100.0 15 0 0 0 95.9 注2:Y2=0.985 3+0.134 4A − 0.025 9B+0.102 8C − 0.009 7AB − 0.013 0AC − 0.064 5BC -0.087 3A2 − 0.107 3B2 − 0.108 5C2。 表 5 电化学氧化回归方程的方差分析Table 5. Analysis of variance for the regression equation of electrochemical oxidation来源 平方和 自由度 均方和 F P 模型 0.351 1 9 0.039 0 11.88 0.007 0 A 0.144 5 1 0.144 5 43.98 0.001 2 B 0.005 4 1 0.005 4 1.63 0.257 7 C 0.084 5 1 0.084 5 25.71 0.003 9 AB 0.000 4 1 0.000 4 0.115 8 0.747 5 AC 0.000 7 1 0.000 7 0.205 8 0.669 1 BC 0.016 6 1 0.016 6 5.07 0.074 2 A2 0.028 1 1 0.028 1 8.56 0.032 8 B2 0.042 5 1 0.042 5 12.94 0.015 6 C2 0.043 5 1 0.043 5 13.24 0.014 9 残差 0.016 4 5 0.003 3 失拟项 0.015 3 3 0.005 1 纯误差 0.001 1 2 0.000 6 由表5中的方差分析结果可知,TP 模型的F值为11.88,P值为0.007 0,表明所得的模型达到显著水平,即该模型在被研究的整个回归区域内拟合较好。多元相关系数R2=0.955 3,说明相关性好。综上所述,回归方程对电化学氧化去除压裂返排液的COD可提供一个合适的模型。由响应面分析结果可得到电化学氧化处理最优实验条件:电流密度为8.66 mA·cm−2,极板间距为2.81 cm,电解时间为43.21 min,最大COD去除率可达100.0%。
各因素影响COD去除率的响应面曲线与等高线如图3所示。由图3(a)可知,相比于极板间距,电流密度的曲面更加陡峭,说明电流密度对COD去除率的影响程度更不显著。图3(b)中电流密度的曲面更加陡峭,而电解时间的影响较小,说明相比于电解时间,电流密度对COD去除率的影响程度更加显著,对于电化学氧化处理而言,电流密度越大,有机物氧化的效率越高,处理效果越显著。图3(c)中极板间距和电解时间对COD去除率的影响均十分显著,两者交互作用较好,电解时间的P值(P=0.003 9)小于极板间距(P=0.257 7),表明相比于极板间距电解时间更占优势。
2.2 电化学法处理压裂返排液的反应动力学
由图4(a)可知,随处理时间增加,COD去除率从19.1%增大至80.1%,表明增加电絮凝时间有利于COD的去除。这是由于延长电絮凝时间使更多的铝离子在水中形成的氢氧化物和多羟基配合物絮体,这些絮体在同向絮凝和异向絮凝的共同作用下,由小的絮凝体叠加形成大的絮凝集团,通过网捕、吸附架桥和电性中和等的协同作用[27]去除水中的油、悬浮物等有机杂质,使水的COD降低。
由图4(b)可知,随处理时间增长,COD去除率从50.6%增大至90.6%,增加电化学氧化时间有利于COD的去除。这是由于增长电化学氧化时间将使更多的污染物直接在阳极表面通过电子传递被氧化,或被电解产生的活性物质间接氧化[28],进而导致水的COD降低。在相同的实验参数条件下,电化学氧化法去除COD的效果优于电絮凝法。
通过比较各级动力学模型的特征,采用零级动力学模型对电絮凝处理压裂返排液COD随时间的变化进行分析,如表6所示,在电絮凝处理压裂返排液的0~4 h,其反应速率常数为4.49 mg·(L·min)−1,零级动力学方程式为cCOD =−4.49t+1253.6,该拟合方程的相关系数为0.935 8,拟合相关性显著。根据零级反应动力学模型的特征,反应速率在反应过程中保持恒定,与残余COD无关。
表 6 电化学处理压裂返排液的反应速率常数和动力学方程式Table 6. Reaction rate constant and kinetic equation of treating fracturing flowback fluid by electric flocculation and electrochemical oxidation处理方法 反应速率常数k 动力学方程式 R2 电絮凝 4.49 mg·(L·min)−1 cCOD = −4.49t+1253.6 0.935 8 电化学氧化 0.005 4 min−1 ln cCOD = −0.0054t+6.433 0.773 7 通过比较各级动力学模型的特征,采用一级动力学拟合模型对电化学氧化处理压裂返排液COD随时间的变化进行分析,如表6所示,在电化学氧化处理压裂返排液的0~4 h,其反应速率常数为0.005 4 min−1,一级动力学方程式为lncCOD = −0.005 4t+6.433,该拟合方程的相关系数为0.773 7。根据一级反应动力学模型的特征,反应速率随着残余COD降低而降低,因此,电絮凝时间可设置为1~2 h,并及时更新反应器中的压裂返排液,这样可使反应器中的压裂返排液保持高COD,进而使COD去除速率维持在较高水平。
2.3 电化学法处理压裂返排液的机理分析
1)电化学法处理的活性物质分析。采用铝标准溶液配制不同浓度的铝溶液,加入等量Ferron络合显色剂,定容后用紫外可见分光光度计测定370 nm处吸光度,绘制的标准曲线如图5(a)所示。
根据总铝含量的测定方法,在370 nm下测得经电絮凝处理后水的总吸光度为1.256,根据标准曲线算得总铝含量AlT为0.132 mmol·L−1。按照Al-Ferron 逐时络合比色法,由一定时间内的吸光度变化可得,经电絮凝处理过后水中Ala、Alb、Alc吸光度占比分别为23.6%、48.0%、28.4%。如图5(b)所示,通过公式计算可得Ala为0.296 mmol·L−1,Alb为0.603 mmol·L−1,Alc为0.357 mmol·L−1。由Al的不同形态分布可知,Alb占比接近总铝含量的50%,Alb主要由聚十三铝(AlO4Al12(OH)24(H2O)127+,简称Al13)构成,Al13具有很强的电中和能力和优异的絮凝作用,为经典的Keggin 结构,其结构中心为Al—O四面体,外围是12个八面体配位的Al(OH)2+,在中性pH条件下,结构形态较稳定,不易继续水解,表现出一定的稳态,Al13有很强的正电荷和吸附聚集能力,因而具有很强的电中和与粘附架桥能力,对于去除水中的有机物具有关键作用。
除了铝的聚合体,特定种类的自由基对于去除水中有机物也有一定的作用。由图5(c)可知,随着水中异丙醇浓度的增大,电絮凝处理后水的COD由480 mg·L−1增至1 120 mg·L−1,COD去除率由67.8%降至24.8%,表明加入的异丙醇对水中的OH·起到淬灭作用,在异丙醇醇分子中,由于OH·的影响,使得α-氢较活泼,容易被氧化和脱氢[29],OH·与异丙醇主要发生夺氢反应,而且主要是夺取α-氢[30]。电絮凝作用而产生的OH·,来自于电极在电场作用下对水的氧化分解,或是氢氧根离子的直接氧化(式(3)~(4))[31]。OH·的减少使反应速率大大降低,抑制污染物的降解,COD的去除率降低,因此,OH·在电絮凝处理压裂返排液的过程中对COD的去除起到重要作用。而随着水中对苯醌浓度的增大,经电絮凝处理后水的COD基本不变,COD去除率保持在68%~70%,表明O2·−在该过程中未发挥明显作用。
stringUtils.convertMath(!{formula.content}) (3) stringUtils.convertMath(!{formula.content}) (4) 由图5(d)可见,随着水中异丙醇的浓度增大,经电化学氧化处理后水的COD基本不变,COD去除率保持在75%~76%。此结果表明OH·在该过程中未发挥作用。随着加入对苯醌的浓度增大,经电化学氧化处理后水的COD从680 mg·L−1增至1 420 mg·L−1,COD去除率由54.4%下降至23.5%,表明加入的对苯醌对水中的O2·−起到特征淬灭作用,O2·−是含有未配对电子的高活性物质,可通过电子传递而与水中有机物发生氧化还原反应[32],使有机物降解为小分子物质,O2·−的淬灭使有机污染物的降解过程受阻,反应速率大大降低,COD的去除率降低。因此,O2·−是电化学氧化处理压裂返排液过程中对于COD的去除起到主要作用的活性物质。
2)电化学法处理前后有机物分析。由表7可知,经GC-MS分析鉴定,电絮凝处理1 h后压裂返排液中的大部分有机物被去除,剩余有机物主要为C10H16O2和C22H13NO4。经过电化学氧化处理1 h后,水中的长链有机物大部分被氧化为短链有机物,其中含 Si和含 I的有机物均被去除。Si存在于C9H20O2Si中,鉴定其为环己基甲基二甲氧基硅烷,在电絮凝过程中OH·使C—Si和C—C键断裂,生成3-氯己烷和正己醇。I存在于C14H29I、C18H37I和C20H41I中,经GC-MS鉴定其分别1-碘十四烷、1-碘十八烷和1-碘-2-辛基十二烷,在电化学氧化中O2·−断裂C—I键使其变为短链小分子物质,如氯己烷和正己醇等。
表 7 电化学法处理前后有机物成分变化Table 7. Changes in organic matter composition before and after electrochemical treatment水样 主要成分 压裂返排液 C9H20O2Si、C13H14OS3、C14H22O、C14H29I、C15H32、C16H34、C17H36、C18H37I、C19H40C20H41I、C26H54、C29H60、C32H66 电絮凝处理后 C10H16O2、C22H13NO4 电化学氧化处理后 C5H10N6、C6H14O、C6H13Cl、C8H7F3O3S2、C9H14O2、C12H12F6O2、C22H13NO4 选取2,4-二叔丁基苯酚和环己基甲基二甲氧基硅烷作为代表性有机物分析电絮凝和电化学氧化过程中有机物的降解途径。在电絮凝过程中,OH·首先攻击苯环上的C—C键,使之断裂后生成微溶于水的苯酚和叔丁醇[33],苯酚经铝聚合物的吸附聚集作用被絮凝去除,叔丁醇被OH·进一步氧化为CO2和H2O。在电化学氧化过程中,O2·−使C—Si键断裂,生成环己醇、丙三醇和二氧化硅[34],O2·−的强氧化作用使初级产物进一步氧化为二氧化碳和水。
2.4 电化学法处理压裂返排液的电极板表面形貌及组成分析
1)电絮凝处理后极板表面形貌及组成分析。如图6(a)所示,由扫描电镜(SEM)图可以看出,电絮凝阳极铝板表面有较多坑洞。这些坑洞是由于铝失电子变成铝离子进入水中导致。此外,存在不规则块状晶体附着在铝极板表面(图6(b))。结合X射线能谱分析(EDS)(表8)可知,由于压裂返排液的总硬度较高,这些块状晶体可能是水中的钙离子结合水中的碳酸根所形成的碳酸钙。电絮凝阴极石墨极板在微观上为片层状结构(图6(c)),其表面覆盖着絮体和晶体颗粒(图6(d)),由EDS分析(表8)检测到阴极表面主要元素为C、O、Ca、Na和Mg,石墨极板上的絮体可能是带正电的有机物在电场作用下吸附到极板表面,同时水中的钙离子和镁离子结合水中的氢氧根和碳酸根生成的不溶盐结晶在极板表面。
表 8 电絮凝阳极极板的EDS分析Table 8. EDS analysis of electroflocculation anode plate电絮凝阳极极板 电絮凝阴极极板 元素 质量比/% 原子百分比/% 元素 质量比/% 原子百分比/% C 49.75 66.25 O 49.04 60.55 Al 39.59 23.47 C 8.63 14.18 O 10.11 10.11 Ca 26.55 13.08 Ca 0.16 1.06 Na 8.62 7.41 Fe 0.08 0.11 Mg 2.92 2.37 2)电化学氧化处理后极板表面形貌及组成分析。如图7(a)所示,电化学氧化的阳极Ti/RuO2-IrO2极板表面存在着细小的颗粒,在20 000倍条件下观察这些细小颗粒形状不规则(图7(b)),结合EDS分析结果(表9)可知,这些细小颗粒应为压裂返排液中的有机污染物经电化学氧化后,裂解为小分子有机物附着在Ti/RuO2-IrO2极板上。电化学氧化阴极石墨极板上覆盖着致密的条状垢(图7(c)),在50 000倍条件下观察,这些条状垢中也有块状晶体(图7(d)),结合EDS分析(表9)可知,这些致密的条状垢主要是有机污染物,而块状晶体则是钙镁碳酸盐。
表 9 电化学氧化阳极极板的EDS分析Table 9. EDS analysis of electrochemical oxidation anode plate电化学氧化阳极极板 电化学氧化阴极极板 元素 质量比/% 原子百分比/% 元素 质量比/% 原子百分比/% O 21.90 40.97 O 46.45 56.93 Ti 54.33 33.96 C 14.64 23.91 C 6.93 17.27 Ca 28.64 14.01 Ru 12.06 3.57 Mg 2.81 2.26 S 1.51 1.41 Na 1.95 1.66 3. 结论
1)电絮凝和电化学氧化能有效去除油田压裂返排液的COD,电絮凝和电化学氧化处理去除COD的二次响应面回归模型相关性显著,实验可信度、精确度和精密度均在合理范围内,在最佳实验条件下,COD去除率分别可达88.2%和100.0%。
2)电絮凝和电化学氧化处理压裂返排液去除COD的动力学分别表现为零级和一级反应动力学的特征,其反应速率常数分别为4.49 mg·(L·min)−1和0.005 4 min−1。
3)对于处理压裂返排液的2种不同的电化学技术,电絮凝法中铝聚合物的絮凝作用和OH·的氧化分解作用可协同去除水中的有机污染物;电化学氧化法主要依靠强氧化性的O2·−,O2·−可将绝大部分长链有机物降解为小分子有机物和无机物。
4)在电絮凝反应过程中,压裂返排液中的钙离子结合水中的碳酸根所形成的碳酸钙附着在铝极板表面,阴极石墨极板上存在有机物絮体、钙镁碳酸盐;电化学氧化过程中,阳极Ti/RuO2-IrO2极板表面存在着小分子有机物,阴极石墨极板上覆盖着致密的条状有机污染物以及钙镁碳酸盐。
-
表 1 响应面实验设计因素水平和编码
Table 1. Factor level and code for response surface experimental design
变量 因素 电流密度(A)/(mA·cm−2) 极板间距(B)/cm 电解时间(C)/min −1 1 2 20 0 5.5 3 40 1 10 4 60 表 2 电絮凝处理的响应面结果
Table 2. Response surface results of electrocoagulation treatment
序号 X1 X2 X3 COD去除率/% 1 −1 −1 0 63.2 2 1 −1 0 75.6 3 −1 1 0 59.7 4 1 1 0 70.3 5 −1 0 −1 52.0 6 1 0 −1 69.5 7 −1 0 1 67.4 8 1 0 1 74.7 9 0 −1 −1 58.2 10 0 1 −1 43.8 11 0 −1 1 75.0 12 0 1 1 54.7 13 0 0 0 69.8 14 0 0 0 69.2 15 0 0 0 70.3 注1:Y1 = 0.697 7+0.059 7A − 0.054 4B+0.060 4C − 0.004 5AB − 0.025 5AC − 0.014 8BC+0.027A2 − 0.052 7B2 − 0.065 7C2。 表 3 电絮凝回归方程的方差分析
Table 3. Analysis of variance for the regression equation of electrocoagulation
来源 平方和 自由度 均方和 F P 模型 0.114 0 9 0.012 7 6.95 0.023 A 0.028 6 1 0.028 6 15.66 0.010 8 B 0.023 7 1 0.023 7 12.97 0.015 5 C 0.029 2 1 0.029 2 15.99 0.010 3 AB 0.000 1 1 0.000 1 0.044 4 0.841 4 AC 0.002 6 1 0.002 6 1.43 0.285 9 BC 0.009 0 1 0.009 0 0.477 3 0.520 4 A2 0.002 7 1 0.002 7 1.48 0.277 9 B2 0.010 3 1 0.010 3 5.63 0.063 8 C2 0.015 9 1 0.015 9 8.74 0.031 6 残差 0.009 1 5 0.001 8 失拟项 0.009 1 3 0.003 0 纯误差 0.000 1 2 0 表 4 电化学氧化处理的响应面结果
Table 4. Response surface results of electrochemical oxidation treatment
序号 X1 X2 X3 COD去除率/% 1 −1 −1 0 62.0 2 1 −1 0 97.9 3 −1 1 0 62.4 4 1 1 0 94.4 5 −1 0 −1 59.4 6 1 0 −1 81.8 7 −1 0 1 78.9 8 1 0 1 96.1 9 0 −1 −1 62.9 10 0 1 −1 67.0 11 0 −1 1 100.0 12 0 1 1 78.3 13 0 0 0 100.0 14 0 0 0 100.0 15 0 0 0 95.9 注2:Y2=0.985 3+0.134 4A − 0.025 9B+0.102 8C − 0.009 7AB − 0.013 0AC − 0.064 5BC -0.087 3A2 − 0.107 3B2 − 0.108 5C2。 表 5 电化学氧化回归方程的方差分析
Table 5. Analysis of variance for the regression equation of electrochemical oxidation
来源 平方和 自由度 均方和 F P 模型 0.351 1 9 0.039 0 11.88 0.007 0 A 0.144 5 1 0.144 5 43.98 0.001 2 B 0.005 4 1 0.005 4 1.63 0.257 7 C 0.084 5 1 0.084 5 25.71 0.003 9 AB 0.000 4 1 0.000 4 0.115 8 0.747 5 AC 0.000 7 1 0.000 7 0.205 8 0.669 1 BC 0.016 6 1 0.016 6 5.07 0.074 2 A2 0.028 1 1 0.028 1 8.56 0.032 8 B2 0.042 5 1 0.042 5 12.94 0.015 6 C2 0.043 5 1 0.043 5 13.24 0.014 9 残差 0.016 4 5 0.003 3 失拟项 0.015 3 3 0.005 1 纯误差 0.001 1 2 0.000 6 表 6 电化学处理压裂返排液的反应速率常数和动力学方程式
Table 6. Reaction rate constant and kinetic equation of treating fracturing flowback fluid by electric flocculation and electrochemical oxidation
处理方法 反应速率常数k 动力学方程式 R2 电絮凝 4.49 mg·(L·min)−1 cCOD = −4.49t+1253.6 0.935 8 电化学氧化 0.005 4 min−1 ln cCOD = −0.0054t+6.433 0.773 7 表 7 电化学法处理前后有机物成分变化
Table 7. Changes in organic matter composition before and after electrochemical treatment
水样 主要成分 压裂返排液 C9H20O2Si、C13H14OS3、C14H22O、C14H29I、C15H32、C16H34、C17H36、C18H37I、C19H40C20H41I、C26H54、C29H60、C32H66 电絮凝处理后 C10H16O2、C22H13NO4 电化学氧化处理后 C5H10N6、C6H14O、C6H13Cl、C8H7F3O3S2、C9H14O2、C12H12F6O2、C22H13NO4 表 8 电絮凝阳极极板的EDS分析
Table 8. EDS analysis of electroflocculation anode plate
电絮凝阳极极板 电絮凝阴极极板 元素 质量比/% 原子百分比/% 元素 质量比/% 原子百分比/% C 49.75 66.25 O 49.04 60.55 Al 39.59 23.47 C 8.63 14.18 O 10.11 10.11 Ca 26.55 13.08 Ca 0.16 1.06 Na 8.62 7.41 Fe 0.08 0.11 Mg 2.92 2.37 表 9 电化学氧化阳极极板的EDS分析
Table 9. EDS analysis of electrochemical oxidation anode plate
电化学氧化阳极极板 电化学氧化阴极极板 元素 质量比/% 原子百分比/% 元素 质量比/% 原子百分比/% O 21.90 40.97 O 46.45 56.93 Ti 54.33 33.96 C 14.64 23.91 C 6.93 17.27 Ca 28.64 14.01 Ru 12.06 3.57 Mg 2.81 2.26 S 1.51 1.41 Na 1.95 1.66 -
[1] 严志虎, 戴彩丽, 赵明伟, 等. 压裂返排液处理技术研究与应用进展[J]. 油田化学, 2015, 32(3): 444-445. [2] KHAIR E, ZHANG S, MA S, et al. Performance and application of new anionic D3F-AS05 viscoelastic fracturing fluid[J]. Journal of Petroleum Science and Engineering, 2011, 78(1): 131-138. doi: 10.1016/j.petrol.2011.05.011 [3] GEETANJALI C, KEKA O, CH. V. A novel and cleaner bio-polymer Gum Karaya-based Silica nano-composite fracturing fuid for high-temperature application[J]. Journal of Petroleum Exploration and Production Technology (2021) 11: 3785–3795. [4] HAO H, HUANG X, GAO C, et al. Application of an integrated system of coagulation and electro-dialysis for treatment of wastewater produced by fracturing[J]. Desalination and Water Treatment, 2015, 55(8): 2034-2043. doi: 10.1080/19443994.2014.930700 [5] 薛承瑾. 页岩气压裂技术现状及发展建议[J]. 石油钻探技术, 2011, 39(3): 24-29. [6] LIU D, FAN M, YAO L, et al. A new fracturing fluid with combination of single phase microemulsion and gelable polymer system[J]. Journal of Petroleum Science and Engineering, 2010, 73(3/4): 267-271. [7] 李兰, 杨旭, 杨德敏. 油气田压裂返排液治理技术研究现状[J]. 环境工程, 2011, 29(4): 54-56,70. [8] 叶春松, 郭京骁, 周为, 等. 页岩气压裂返排液处理技术的研究进展[J]. 化工环保, 2015, 35(1): 21-26. [9] 杨博丽, 张勉, 徐迎新. 电絮凝处理胍胶压裂返排废水实验研究[J]. 水处理技术, 2019, 45(1): 38-39. [10] 王顺武, 赵晓非, 李子旺, 等. 油田压裂返排液处理技术研究进展[J]. 化工环保, 2016, 36(5): 494. [11] 李健, 赵立志, 刘军, 等. 压裂返排废液达标排放的实验研究[J]. 油气田环境保护, 2002, 12(3): 26-28. [12] 杨志刚, 魏彦林, 吕雷, 等. 页岩气压裂返排废水回用处理技术研究与应用[J]. 天然气工业, 2015, 35(5): 131-137. [13] 樊玉新, 张海兵, 黄伟强, 等. 电化学工艺处理油田聚合物型压裂返排液[J]. 工业水处理, 2022, 42(10): 139-145. [14] 孟宣宇, 朱营莉, 林雯杰, 等. 页岩气压裂返排液电絮凝处理技术研究[J]. 工业水处理, 2017, 37(11): 58-61. [15] 王啸, 冉玉莹, 刘长亮, 等. 海上油田压裂返排废水COD处理实验研究[J]. 应用化工, 2023, 52(5): 1329-1332. [16] 吴磊, 谷梅霞, 杨阳, 等. 页岩气田压裂返排液电化学臭氧耦合处理技术实验研究[J]. 辽宁化工, 2023, 52(5): 761-766. [17] 赵忠山. 压裂返排液对原油乳化液介电特性的影响研究[J]. 油气田地面工程, 2017, 36(6): 51-53. [18] 林啸, 姚媛元, 陈果. 胍胶压裂返排废水残渣净化处理技术[J]. 石油钻采工艺, 2016, 38(5): 689-692. [19] 刘宇程, 吴东海, 袁建梅, 等. 膜蒸馏处理页岩气井压裂返排废水[J]. 环境工程学报, 2017, 1(1): 48-54. [20] 蒋继辉, 冀忠伦, 任小荣, 等. 聚合硅酸铝铁絮凝剂处理油井压裂废水[J]. 化工环保, 2013, 3(94): 363-366. [21] 张梦迪, 张维, 姚继明. 靛蓝废水无机盐环境下铝极板溶解与电化学行为分析[J]. 精细化工, 2023, 40(5): 1124-1126. [22] MONTEAGUDO J, DURAN A, SAN M, et al. Roles of different intermediate active species in the mine-ralization reactions of phenolic pollutants under a UV-A/C photo-Fenton process[J]. Applied Catalysis B-Environmental, 2011, 106: 242-249. [23] HWANG S, HHLING S, KO S. Fenton-like degradation of MTBE: Effect if iron counter anion and radical scavenger[J]. Chemosphere, 2010, 78: 563-568. doi: 10.1016/j.chemosphere.2009.11.005 [24] XU L, WANG J. Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-Like heterogeneous catalyst for degradation of 4-Chlorphenol[J]. Environmental Science & Technology, 2012, 46: 10145-10153. [25] LI G, WONG K, ZHANG X, et al. Deradation of Acid Orange 7 using magnetic AgBr under visible light: The roles of oxidizing species[J]. Chemosphere, 2009, 76(9): 1185-1191. doi: 10.1016/j.chemosphere.2009.06.027 [26] ZHANG X, SUN D, LI G, et al. Investigation of the roles of active oxygen species in photodegradation of azo dye AO7 in TiO2 photocatalysis illuminated by microwave electrodeless lamp[J]. Journal of Photochemistry and Photobiology A-Chemistry, 2008, 199(2-3): 311-315. doi: 10.1016/j.jphotochem.2008.06.009 [27] FERNANDES A, PACHECO M, CIRIACO L, et al. Review on the electrochemical processes for the treatment of sanitary landfill leachates: present and future[J]. Applied Catalysis B: Environmental. 2015, 176-177: 183-200. [28] NICOT J, SCANLON B, REEDY R, et al. Source and fate of hydraulic fracturing water in the Barnett Shale: A historical perspective[J]. Environmental Science & Technology, 2014, 48(4): 2464-2471. [29] XUE X, HANNA K, DESPAS C, et al. Effect of chelating agent on the oxidation rate of PCP in the magnetite/ H2O2 system at neutral pH[J]. Journal of Molecular Catalyst A:Chemical, 2009, 311: 29-35. doi: 10.1016/j.molcata.2009.06.016 [30] BUXTON G, GREENSTOCK C, HELMAN W, et al. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O-) in aqueous solution[J]. Journal of Physical and Chemical Reference Data, 1988, 17: 513-886. doi: 10.1063/1.555805 [31] 陈建孟, 潘伟伟, 刘臣亮. 电化学体系中羟基自由基产生机理与检测的研究进展[J]. 浙江工业大学学报, 2008, 36(4): 416-421. [32] 吴飞鹏, 蔡继业, 马淑媛, 等. 壳聚糖对超氧自由基的清除作用[J]. 高分子材料科学与工程, 2008, 24(8): 124-125. [33] ISTVAN I, ZSUZSANNA L, ANDRAS D, et al. Investigation of the photodecomposition of phenol in near-UVirradiatedaqueous TiO2 suspensions. I: Effect of charge-trappingspecies on the degradation kinetics[J]. Applied Catalysis A:General, 1999, 180: 25-33. doi: 10.1016/S0926-860X(98)00355-X [34] ANDREW B, THOMAS N, WILLEM H, et al. Rapid reaction of superoxide with insulin-tyrosyl radicals to generate a hydroperoxide with subsequent glutathione addition[J]. Free Radical Biology and Medicine, 2014, 70: 86-95. doi: 10.1016/j.freeradbiomed.2014.02.006 -





 下载:
下载: