-
盐酸四环素(tetracycline hydrochloride, TC)是由4个线性稠合苯环组合而成的氢环并苯环的一类广谱抗生素。TC的大量使用导致水环境中抗生素的积累,潜在地威胁生态系统功能和人类健康,传统水处理方法无法有效去除TC[1-2]。目前水中抗生素的去除方法包括光催化[3]、吸附[4]、生物处理[5]、高级氧化[6]和膜分离[7]等。其中,光催化法可能产生毒性更大的中间产物;高级氧化法和膜分离法处理费用过高;吸附法由于处理方法简单且效果好得到广泛应用[8]。但吸附剂再生困难、费用高,高效吸附剂及其简单再生方法的研发已成为吸附法去除水中四环素的关键。
类水滑石(hydrotalcite-like compounds, LDHs)是由常见的正二价和正三价金属离子组成层状双氢氧化物,结构与粘土的相似,通式为[M2+1–xM3+x(OH)2]x+[(An-)x/nmH2O]x-,其中M2+指的是二价金属阳离子(Mg2+、Cu2+、Mn2+、Zn2+、Ca2+),M3+指的是三价金属阳离子(Al3+、Fe3+、Cr3+),An-指可交换的阴离子(CO2- 3、NO- 3、Cl−和SO2- 4) [9-10],通常可通过共沉淀法[11]和水热法[12]合成。类水滑石能够吸附水中各种无机和有机阴离子及抗生素[13-15]。CHEN等[16]用共沉淀法制备Mg-Al水滑石,结果表明,正滴定法或反滴定法制备的水滑石为孔径较大的H1型介孔材料,正反滴定法制备的水滑石为孔径较小的H3型介孔材料,不同方法制备的水滑石结构不同。OGATA等[17]通过水热法制备的Fe-Mg水滑石对六价铬的吸附量为19.80 mg·g−1;潘国祥等[18]通过共沉淀法制备的Fe-Mg水滑石对六价铬的吸附量为71.12 mg·g−1,制备方法不同的同种水滑石的吸附性能不同。吴永娟等[19]通过煅烧法制备的Mg-Fe类水滑石对甲基橙的吸附量为306.7 mg·g−1; LU等[20]通过共沉淀法制备的Ni-Fe水滑石对甲基橙的吸附量为205.76 mg·g−1,阳离子种类不同的类水滑石的吸附性能也不同。由此可见,水滑石的制备方法对水滑石吸附性能的影响有待深入研究。
此外,含有Zn、Cu、Ni、Cr等元素的水滑石具有光催化活性,曹根庭等[21]采用Zn-Al类水滑石光降解亚甲基蓝,紫外光光照180 min后,降解率达到95%; MAO等[22]采用Cu-Al类水滑石降解罗丹明B和Cr(Ⅵ),紫外光光照30 min,去除率达90.1%;徐敏虹等[23]采用Zn-M-Cr水滑石光催化降解罗丹明B,紫外光光照120 min后,降解率达到90.72%。因此,制备兼具吸附和光催化性能的水滑石,先通过吸附去除水中TC,再通过光催化实现吸附剂的再生,为水中TC的去除开辟新道路。
本研究分别采用共沉淀法和水热法制备了具有光催化活性的Cu-Mg-Al类水滑石(LDHs-C和LDHs-H),通过XRD、SEM、BET、FTIR等方法表征了LDHs-C和LDHs-H的理化性质,通过静态实验研究了LDHs-C和LDHs-H对水中TC的吸附性能,探讨了吸附机理,对吸附饱和的吸附剂的光再生效果进行了评价。
-
三水合硝酸铜(Cu(NO3)2·3H2O)、六水合硝酸镁(Mg(NO3)2·6H2O)、九水合硝酸铝(Al(NO3)3·9H2O)、氢氧化钠(NaOH)、碳酸钠(Na2CO3)均为分析纯、购自国药集团化学试剂有限公司。实验用水为超纯水。
-
共沉淀法制备Cu-Mg-Al类水滑石 (LDHs-C):称取0.02 mol的三水合硝酸铜、0.01 mol的六水合硝酸镁和0.01 mol九水合硝酸铝溶于100 mL去离子水中,制得蓝色液体A;称取2.88 g氢氧化钠和2.544 g碳酸钠溶于100 mL去离子水中并剧烈搅拌使其冷却至室温,制得无色透明溶液B。将A、B两溶液分别置于恒压漏斗中以控制流速(每秒1滴)滴至烧杯,过程中控制pH在9.5~10,滴定完后继续搅拌30 min,得到蓝色胶状沉淀,将其置于60 ℃烘箱中晶化24 h后,用超纯水洗涤至中性,过滤后在60 ℃干燥、研磨得到灰蓝色粉末。
水热法制备Cu-Mg-Al类水滑石(LDHs-H):量取上述A溶液20 mL、B溶液20 mL、加入含有20 mL超纯水的烧杯中搅拌混合均匀,控制pH在9.5~10,转移到内衬聚四氟乙烯的100 mL压力反应釜中,将反应釜放入恒温的电热鼓风干燥箱中,120 ℃反应10 h后取出反应釜,自然冷却至室温,用超纯水离心洗涤至中性,过滤后在60 ℃下干燥过夜,研磨得到灰蓝色粉末。
-
采用XD-3型X射线衍射仪(北京普析通用仪器有限责任公司)测试样品的物相结构,Cu Kα射线,管电流为20.0 mA,管电压为36.0 kV,步进角度为0.02°。采用ASAP-2020型N2物理吸附分析仪(美国Micromeritics公司)测试样品的比表面积和平均孔径,以N2为吸附质,吸附温度为77.0 K,测定前,样品在200.0 ℃下真空脱气2.0 h。采用JSM-6710F扫描电镜(日本JEOL公司)测试样品的表面形貌。采用Perkin-Elmer 550s型傅里叶红外光谱仪(美国珀金埃尔默股份有限公司)用于推断化合物的结构和官能团种类及所处化学环境。
-
吸附实验:分别取50 mg LDHs-C、LDHs-H加入50 mL、20 mg·L−1的盐酸四环素(TC)溶液中,在磁力搅拌下进行吸附实验,反应6 h取样,经离心机分离,取上层清液,于TU-1 810SPC型紫外可见分光光度计中在波长357 nm下测定吸光度,计算得出TC质量浓度。改变初始pH探讨pH对吸附效果的影响。在pH为7,温度为(25±1) ℃条件下进行吸附动力学及热力学实验,分别取50 mg LDHs-C、LDHs-H加入50 mL质量浓度分别为10、20、30、50、100、150 mg·L−1模拟四环素废水中,反应进行至0.5、1、2、4、6、8 h时取上清液测定TC质量浓度。
脱附再生实验:取100 mg吸附饱和后的吸附剂加入含有100 mL蒸馏水的石英管中,在紫外光下照射30 min,脱附完成后,所得样品在烘箱中烘干,再次用于吸附TC,并计算吸附量。多次重复吸附-脱附。记录吸附次数和吸附量。
水滑石对TC的吸附量q、去除率R和再生率p分别按(式(1)~式(3))计算。采用准一级动力学模型(式(4))、准二级动力学模型(式(5))、颗粒内扩散模型(式(6))对吸附动力学进行研究。采用Langmuir(式(7))、Freundlich(式(8))吸附等温模型对实验结果进行拟合。热力学参数∆G、∆H和∆S按(式(9)~式(11))计算。
式中:C0为初始溶液中的TC质量浓度,mg·L−1;Ct为t时刻溶液中的TC质量浓度,mg·L−1;m为吸附剂质量,g;V为溶液体积,L;qn为再生n次后吸附剂的吸附量,mg·g−1;q1为全新吸附剂的吸附量,mg·g−1。qe为平衡吸附量,mg·g−1;qt为t时的吸附量,mg·g−1;t为接触时间,min;k1为准一级吸附速率常数,min−1;k2为准二级吸附速率常数,g·(mg·min)−1;kd为颗粒内扩散常数,mg·g−1·min1/2;C为表征溶质扩散到液相中的常数。Ce是吸附平衡时的溶液质量浓度,mg·L−1;qm为构成单分子层吸附时单位饱和量,mg·g−1;b为Langmuir吸附等温模型常数;KF为系统的常数,与键能有关,可定义为吸附或分配系数,表示TC吸附在吸附剂上,达到单位平衡质量浓度。1/n表示TC在吸附剂上的吸附强度或表面不均匀性。∆G为吸附自由能变,kJ·mol−1;∆H为吸附焓变,kJ·mol−1;∆S为吸附熵变,J·(mol·K)−1;R是理想气体常数, 8.314 J·(mol·K)−1;T为绝对温度,K;Kd分配系数。
-
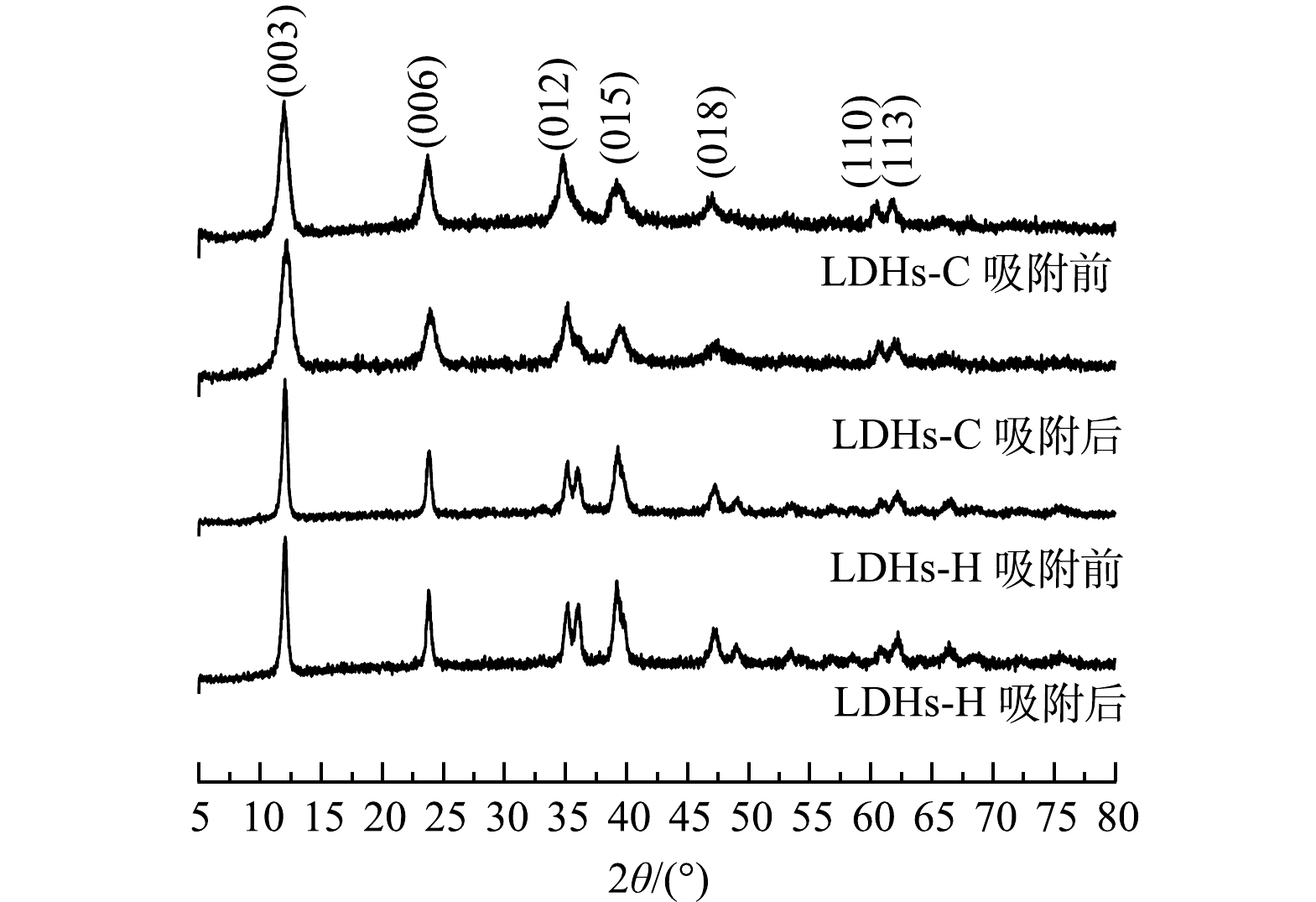
1) XRD分析。图1为LDHs-C和LDHs-H及其吸附前后样品的XRD图谱。由图1可知,LDHs-C具有典型的水滑石特征衍射峰,在2θ为12 °和23.7 °处的衍射峰精细且呈对称结构。这说明样品具有水滑石的层状结构,从而具备阴离子交换和吸附特性。在2θ为34.9 °、39.3 °和46.8 °处的衍射峰表明样品具有六方晶型堆叠对称结构。在2θ为60.0 °和61.5 °处的衍射峰表明样品含有金属氢氧化物Mg(OH)2和Al(OH)2[24]。相较于LDHs-C,LDHs-H (003)、(006)、(012)、(015)和(018)晶面的衍射峰更尖锐,说明LDHs-H的结晶度更高。LDHs-H (012)晶面处的衍射峰出现分叉可能是由于反应温度和时间所引起的同一物质2个不同的晶型形态[25]。吸附前后LDHs-C、LDHs-H衍射峰的峰形没有明显变化,表明吸附剂结构稳定[26]。
表1为LDHs-C和LDHs-H晶胞结构参数。合成的LDHs具有六方晶型结构且晶面间距d由布拉格定律(2dsinθ=nλ)确定,网格参数a (a=2d 110)表示指定同一层的2个金属离子之间的原子间直线距离,晶胞厚度c (c=3d 003)为层与层之间的间距。由表1可知,LDHs-C晶体尺寸较LDHs-H大。
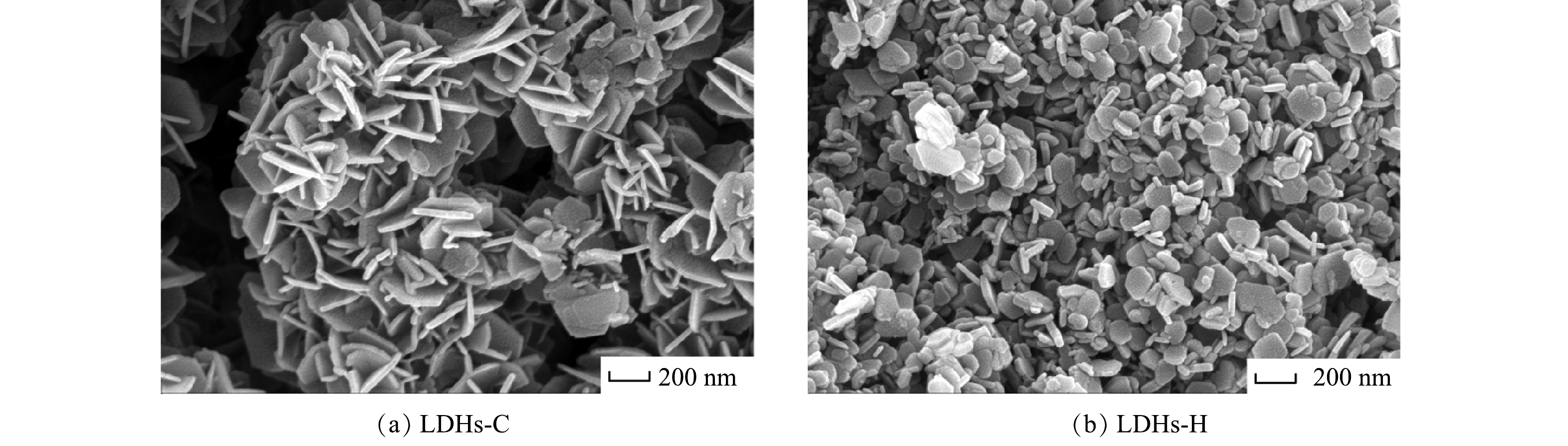
2) SEM分析。由图2可以看出,LDHs-C和LDHs-H均为片状六边形,与XRD结果一致,具有明显的LDHs层状结构[27]。但LDHs-C由尺寸大约为200 nm的片层结构堆叠而成,呈花簇状,片层之间存在大量的空隙;LDHs-H由尺寸大约为100 nm的片层结构聚集而成,结构紧密,空隙较少。
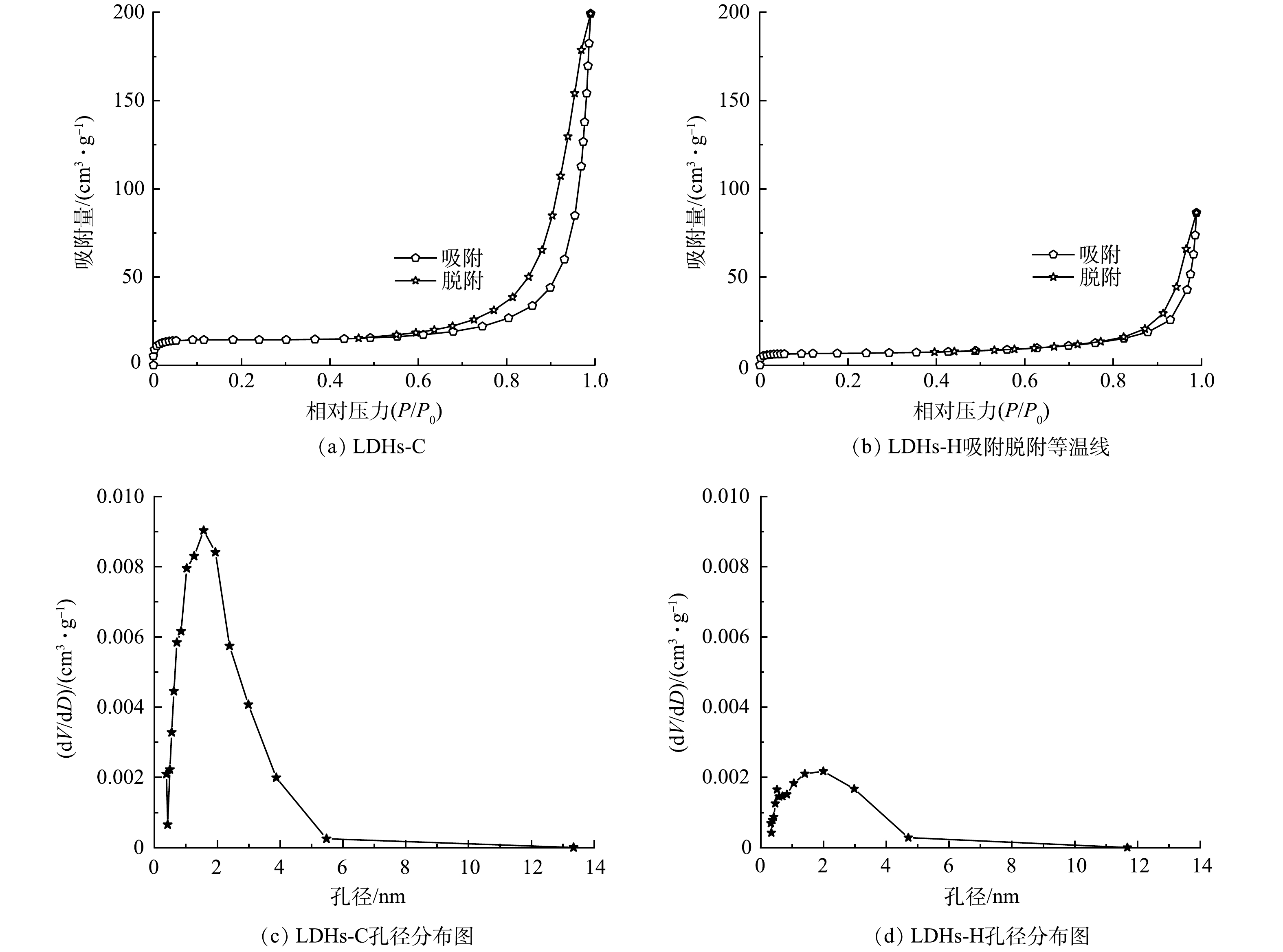
3) BET分析。对LDHs-C、LDHs-H进行 BET 比表面积和孔结构分析,LDHs-C、LDHs-H的 BET 测试相关孔隙参数见表2;N2吸附-解吸等温曲线及孔径分布见图3。由表2可知,LDHs-C的比表面积、孔容、孔径均大于LDHs-H。由SEM、BET分析结果可知,LDHs-C、LDHs-H的晶体大小、形貌结构不同,共沉淀法合成的LDHs-C结晶度差,晶体尺寸大,但片层结构堆叠而成的花簇状结构可防止片间的聚集,改善了分散性,增加了比表面积、孔径和孔容;水热法合成的LDHs-H结晶度好,晶体尺寸小、但片层结构层层堆积造成了片间聚集,减小了比表面积、孔容和孔径。由图3(a)和图3(b)可知,LDHs-C和LDHs-H的N2吸附-解吸等温曲线均为IUPAC分类IV型,吸附和解吸分支之间存在滞回环,均是孔径低于40 nm的介孔材料[27]。回滞环的形状符合IUPAC H3型,进一步说明LDHs-C、LDHs-H为孔径较小的介孔材料,吸附性能好。由孔径分布图3(c)和图3(d)可以发现,LDHs-C孔径均一,孔径分布在2 nm左右,LDHs-H的孔径在1.5~2.5 nm间均有分布。
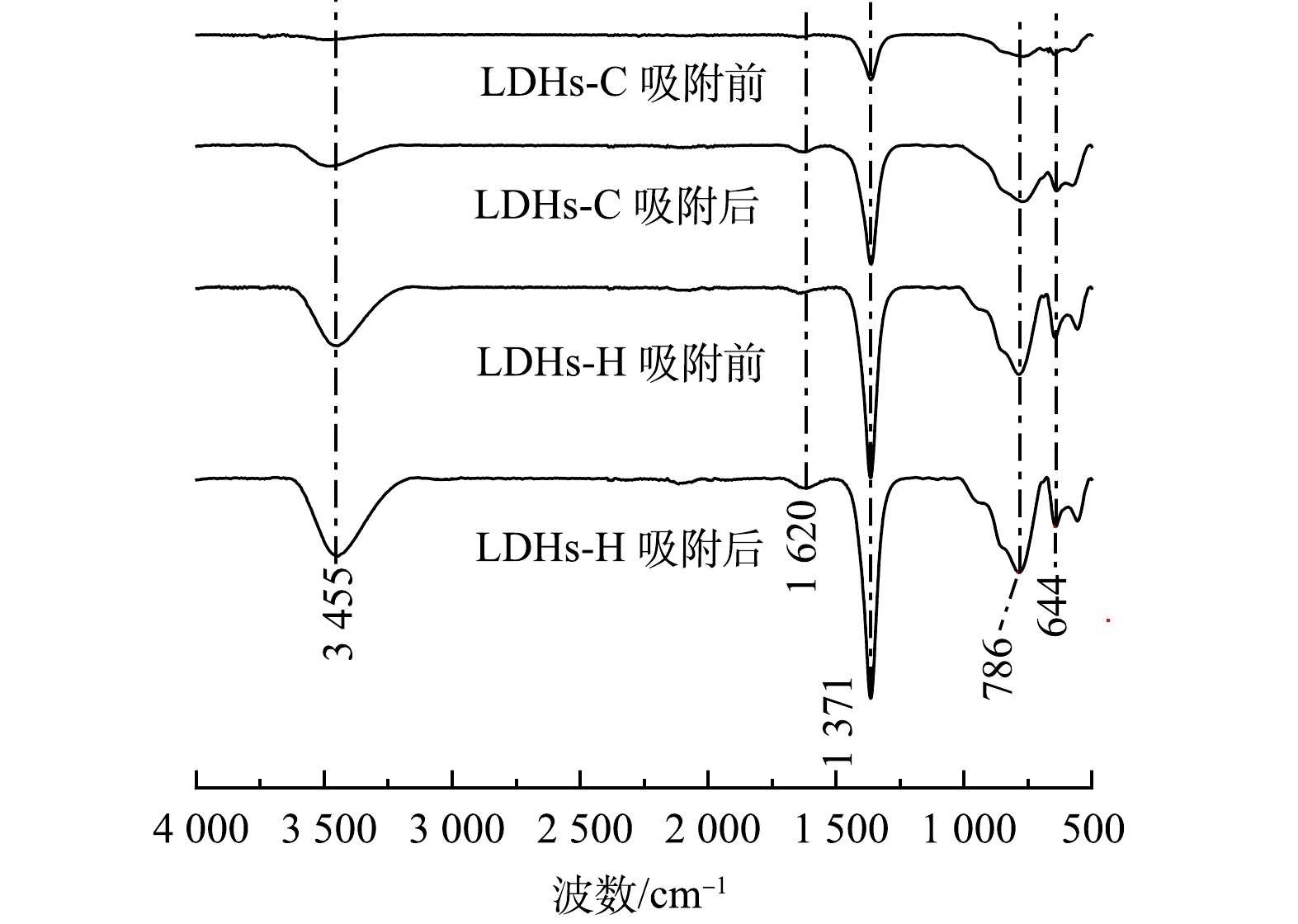
4) FTIR分析。图4为LDHs-C和LDHs-H及其吸附后样品的红外光谱图。LDHs-C和LDHs-H化学结构相同,红外光谱图中,3 455 cm−1处的特征峰宽而强,对应于LDHs各种金属结合的层间羟基和物理吸附的水分子中结构羟基的伸缩振动。以1 620 cm−1为中心的特征峰是由层间水分子结构羟基的振动引起的。以1 371 cm−1为中心的特征峰归因于碳氧键的伸缩振动说明壁间空间中存在碳酸根阴离子。位于786 cm−1和644 cm−1的特征峰对应于金属氢氧化物和氧—金属—氧(M—OH和M—O—M)的振动特性[28]。比较吸附前后的LDHs-C和LDHs-H的红外光谱图,发现吸附后的样品在1 371 cm−1和3 455 cm−1处的谱峰增强,是由于TC分子和LDHs之间相互作用引起。
-
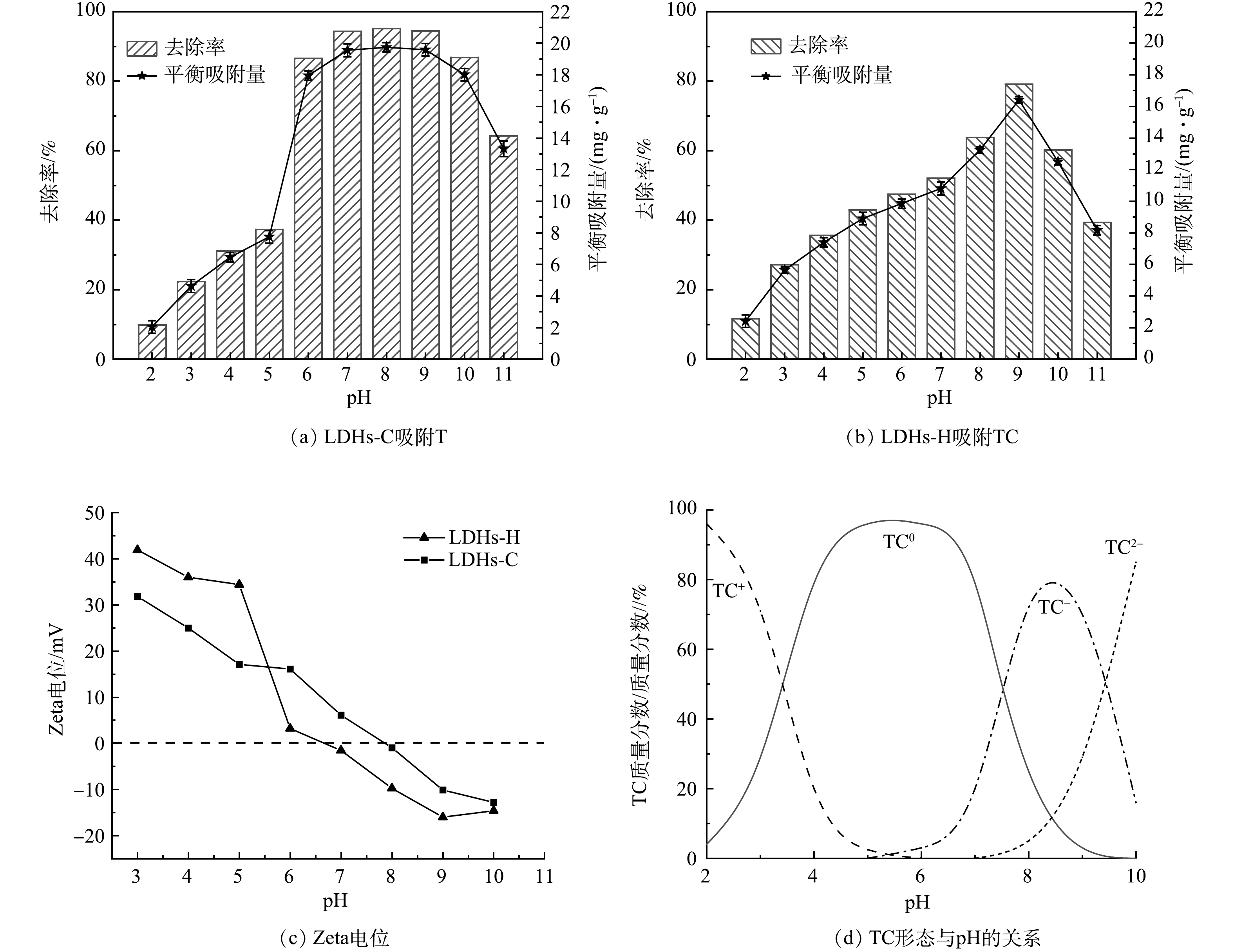
pH不仅影响吸附剂,而且影响TC在水溶液中的存在形态[29]。图5(a)和图5(b)显示了溶液初始pH对LDHs-C和LDHs-H吸附TC的影响。由图5(a)和图5(b)可知,随pH的增加,LDHs-C,LDHs-H对TC的吸附量先增大后减小,LDHs-C在pH为7~9对TC的吸附量较高,LDHs-H在pH为9时对TC的吸附量达到最高。由图5(c)中LDHs-C和LDHs-H 的Zeta电位可知,随着pH的增加,LDHs-C和LDHs-H的Zeta电位由正变负,LDHs-C的等电点为7.9,LDHs-H的等电点为6.6。水溶液TC形态随pH的变化见图5(d)。TC中含有一个共同的碱性核,是一种季弱酸。从图5(d)可知,当pH < 3.3时,四环素分子结构中的二甲氨基被质子化,主要以阳离子(TC+)的形式存在;pH为3.3~7.5时,四环素分子中的酚二酮基失去质子,以两性离子(TC0)的形式存在;当pH>7.5时,以胺化阴离子(TC−)或二价阴离子(TC2-)形式存在[30]。由此可知,在强酸或强碱条件下,吸附剂和吸附质所带电荷相同,静电斥力增加,吸附量下降。
在中性、碱性条件下LDHs-C Zeta电位较LDHs-H高,与TC2-静电斥力小。此外,由XRD、BET、SEM分析可知LDHs-C晶体尺寸较大,呈花簇状结构,比表面积大。因此LDHs-C对TC的吸附效果优于LDHs-H。
-
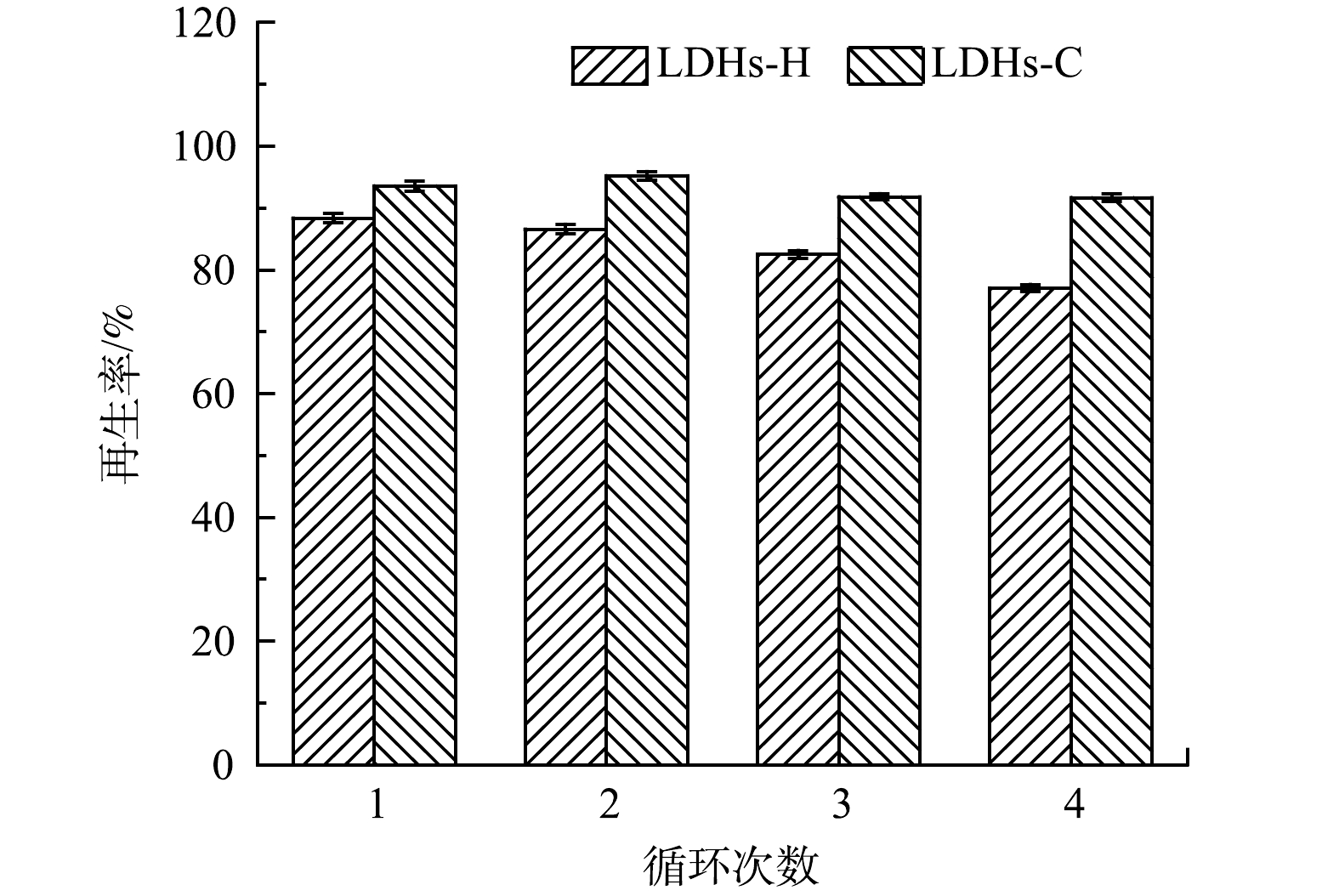
图6为LDHs-C和LDHs-H对TC的脱附再生效果,LDHs-C和LDHs-H具有光催化特性,在紫外光的照射下LDHs被激发,其价带电子跃迁至导带,在价带中生成空穴,即产生光生电荷(e– 、h+)。这些光生电子和空穴可在催化剂内部自由移动,当其移动至LDHs表面时,溶液中溶解O2与电子作用生成 ·O2–,价带上的空穴与H2O 反应可生成 ·OH,在活性物种(e–、h+、·O2–、·OH )的共同作用下,吸附在表面的TC分子发生氧化还原反应,从而实现吸附剂再生[31]。LDHs-H对TC的吸附能力随着循环次数的增加而减少,是由于LDHs-H光催化降解TC不完全,吸附剂未完全再生造成。LDHs-C经过4次吸附解吸循环后仍然保持原有吸附量90%,表明LDHs-C光催化降解TC较完全,在紫外光照射下即可实现脱附再生,吸附剂可以重复利用,因此LDHs-C在TC吸附及水处理领域有着广泛的应用前景。
-
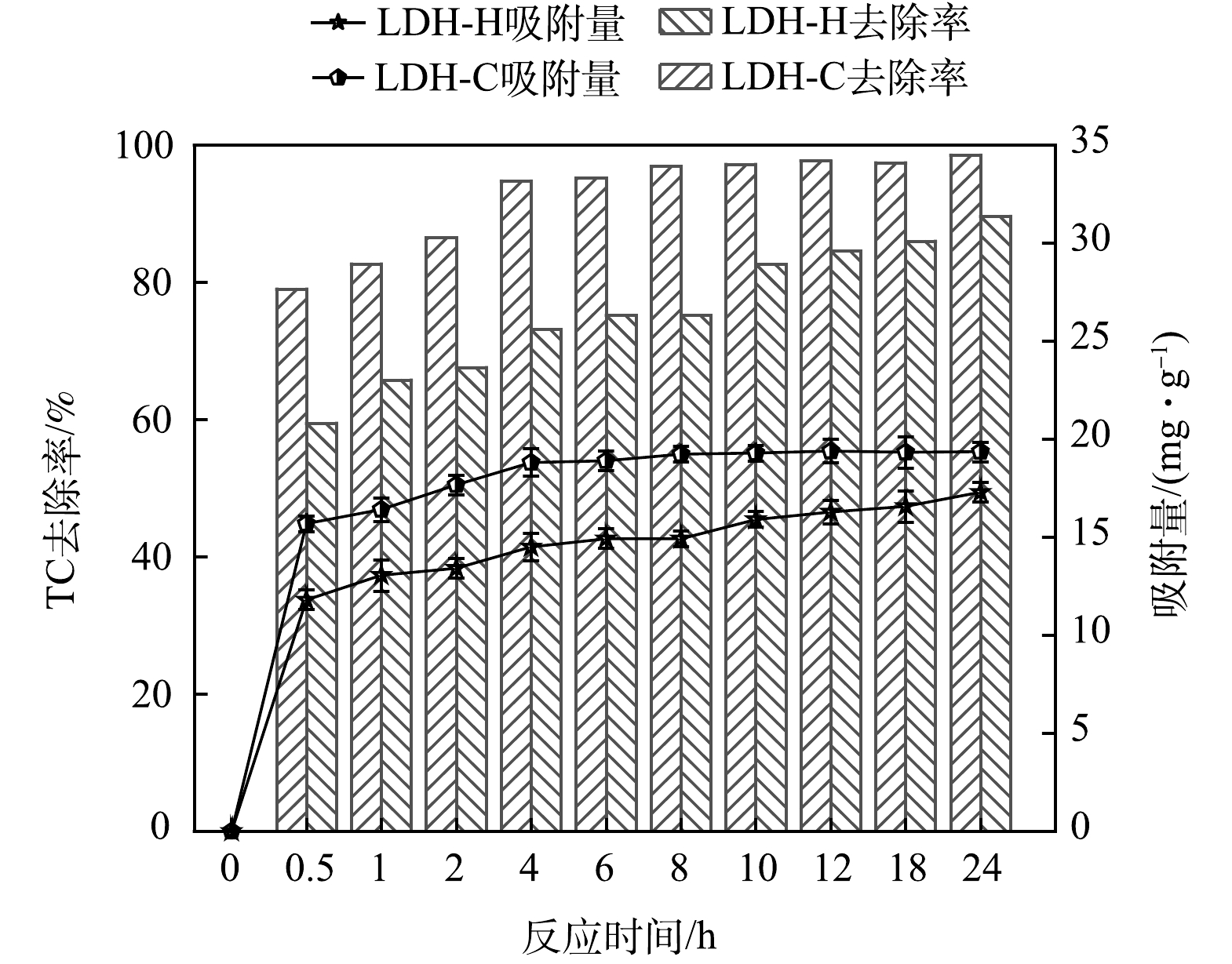
1) 吸附动力学。图7为LDHs-C 和 LDHs-H对TC的吸附动力学。由图7可知,吸附6 h后, 吸附TC达到平衡,LDHs-C和LDHs-H对TC的去除率分别为95.2%和75.2% , LDHs-C对TC的吸附效果优于LDHs-H对TC的吸附效果。LDHs-C和LDHs-H 对TC的平衡吸附量分别为19.24 mg·g−1和14.93 mg·g−1,较有机改性后的凹凸棒土对TC的平衡吸附量(14.39 mg·g−1[32]),矿化垃圾对TC的平衡吸附吸附量(9.68 mg·g−1[33])都高,说明铜镁铝类水滑石对TC的吸附效果良好。
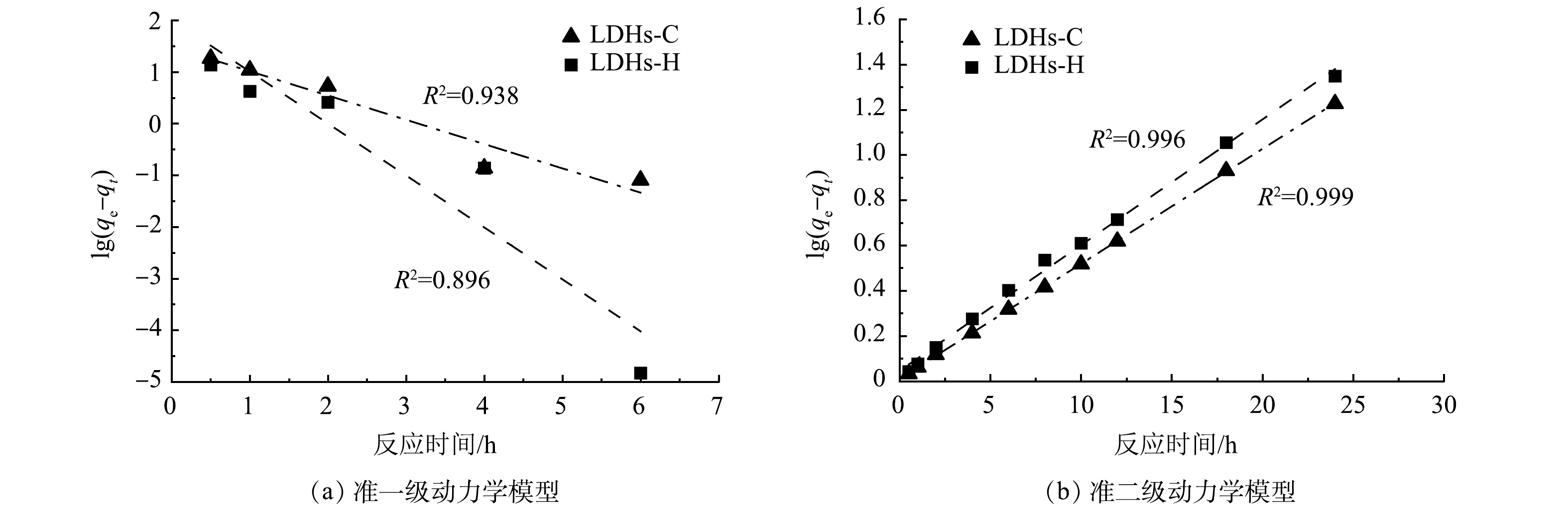
图8为LDHs-C和LDHs-H吸附TC的准一级动力学模型和准二级动力学模型线性拟合,表3为LDHs-C和LDHs-H吸附TC的动力学模型拟合参数。由表3可知,LDHs-C吸附TC的动力学模型相关系数R2均大于0.93,两种模型都可以用来描述该过程。相比较而言,准二级动力学的相关系数( R2=0.99)更高且准二级动力学模型计算的吸附量值与实验值接近。LDHs-H吸附TC亦是如此。准二级动力学模型以化学吸附为基础[34],表明类水滑石吸附TC 的过程是化学吸附过程。XIAO等[35]采用氧化铜吸附四环素,结果表明该过程也符合基于化学吸附的准二级动力学模型。
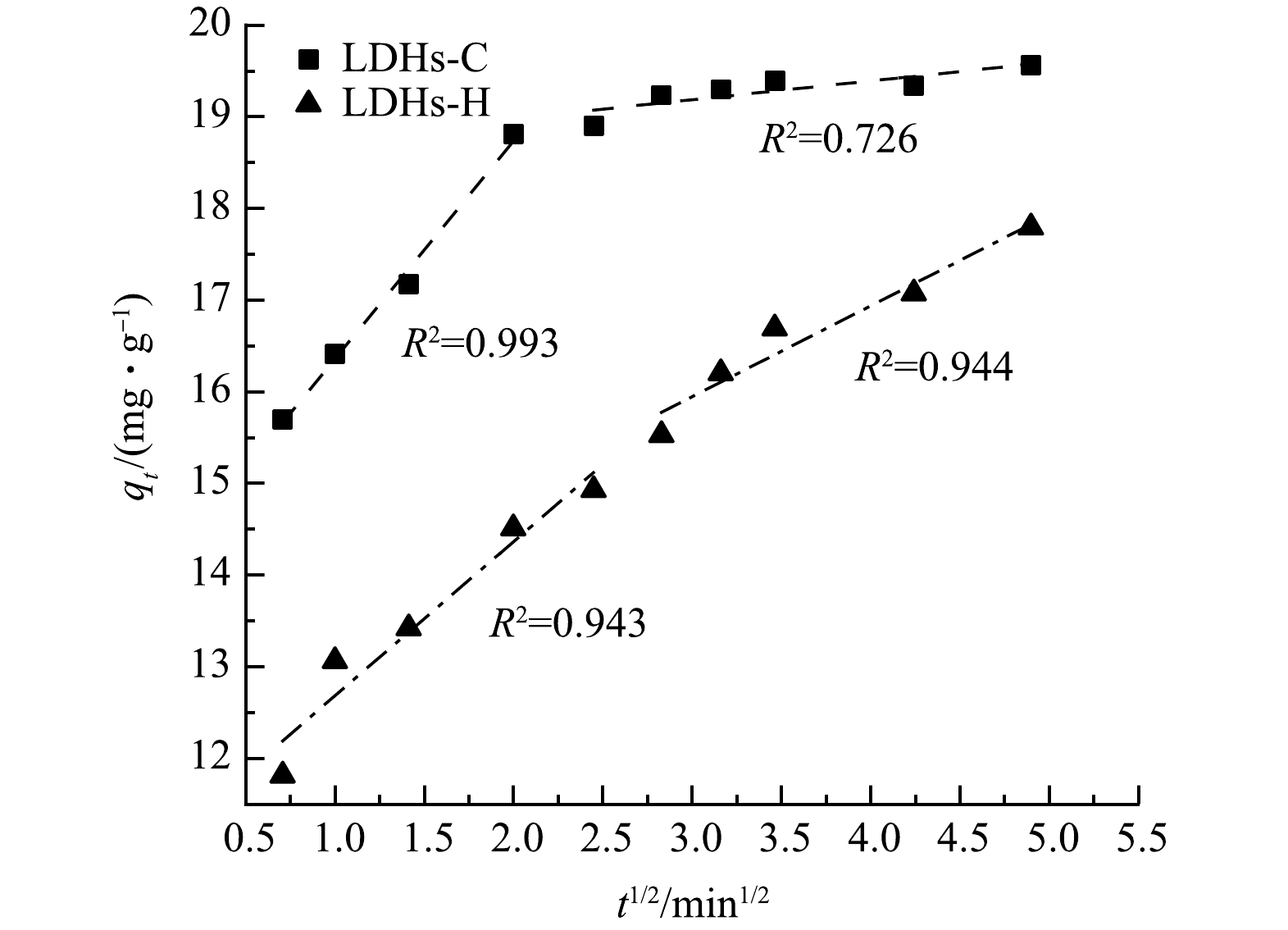
图9为LDHs-C和LDHs-H吸附TC的颗粒内扩散模型拟合,TC在水滑石上的吸附分2个阶段:第1阶段是吸附剂表面吸附;第2阶段是孔道缓慢扩散。表4反映了LDHs-C和LDHs-H吸附TC的颗粒内扩散参数,其中常数C的值不等于0,表明颗粒内扩散不是控制TC在水滑石上的吸附速率的唯一步骤[36]。
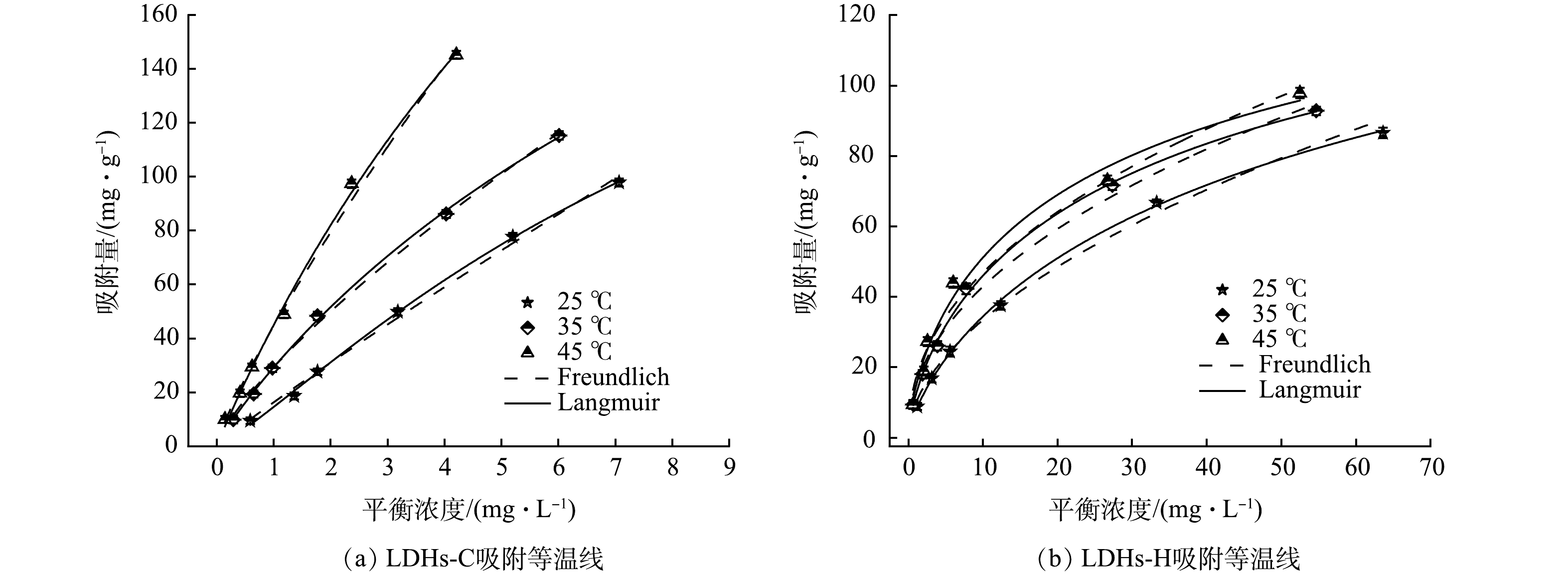
2)吸附等温线。图10显示了LDHs-C和LDHs-H吸附TC吸附等温线,表5为TC在LDHs-C和LDHs-H的吸附等温线模型模型拟合参数。由表5可知,TC在LDHs-C和LDHs-H的吸附等温线符合Langmuir和Freundlich模型,相关系数均大于0.95。LDHs-C吸附TC更符合Langmuir等温吸附模型,表明LDHs-C对TC的吸附过程为单分子层吸附,材料表面的活性位点均匀。Freundlich等温线拟合中,1/n为1.04低于2表示容易吸附。而LDHs-H吸附TC更符合Freundlich吸附等温模型。
3)吸附热力学。LDHs-C和LDHs-H吸附TC的热力学参数如表6所示,不同温度下∆G为负值表示LDHs-C和LDHs-H吸附TC的过程是自发进行的。随着温度升高时∆G绝对值增大,这表明该过程随着温度的升高反应过程的推动力增大。∆H为正值表明该吸附反应过程为吸热过程,升高温度有利于该反应过程的进行。通常物理吸附的∆H变化在小于40 kJ·mol−1的范围内;化学吸附∆H变化在50~80 kJ·mol−1[37]。LDHs-C吸附TC时40 kJ·mol−1<∆H<50 kJ·mol−1,表明LDHs-C吸附TC是物理吸附与化学吸附相结合。LDHs-H 吸附TC时∆H<40 kJ·mol−1,表明LDHs-H吸附TC是物理吸附。
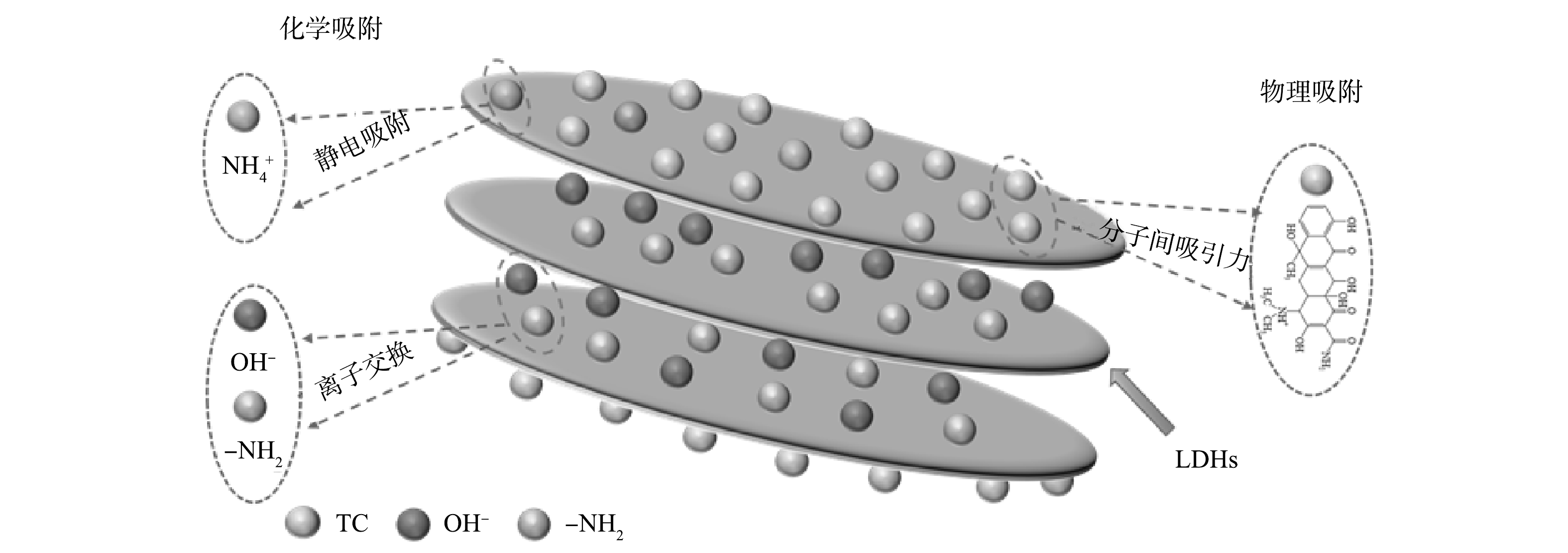
4)机理分析。综上所述,LDHs-C和LDHs-H吸附TC的机理如图11所示。首先水滑石表面具有大量活性位点,TC由液面分子扩散到达水滑石表面与活性位点结合通过范德华力吸附到水滑石表面。其次,在中性或偏碱性条件下,四环素以胺化阴离子(TC−)或二价阴离子(TC2-)形式存在,可与水滑石层间阴离子进行离子交换进入水滑石层间。此外,水滑石还可以通过表面暴露的电子与四环素裸漏的NH+相结合。TC通过物理吸附、离子交换、化学吸附作用吸附到LDHs-C和LDHs-H水滑石表面。
-
1)分别用共沉淀法和水热法制备了Cu-Mg-Al类水滑石,水热法制备的LDHs-H晶体尺寸小、结晶形态好、片层结构聚集,比表面积为23.938 m2·g−1,对TC去除率为75.2%;共沉淀法制备的LDHs-C结晶度差,晶体尺寸大,呈花簇状结构,比表面积为51.245 m2·g−1,在中性条件下荷正电,对TC去除率为95.2%。
2)在pH为7,温度为(25±1) ℃条件下LDHs-C对TC的饱和吸附量可达19.24 mg·g−1,吸附饱和的LDHs-C在紫外线下光照半小时即可实现再生,经4次循环后,LDHs-C对TC的吸附量仍能保持原有的90%以上。
3) LDHs-C对TC的吸附符合准二级吸附动力学模型、Langmuir等温线模型。LDHs-C通过物理吸附、离子交换、化学吸附作用吸附TC。
制备方法对铜镁铝类水滑石吸附性能的影响
Effect of preparation method on adsorption properties of Cu-Mg-Al hydrotalcite-like compounds
-
摘要: 为了考察制备方法对水滑石结晶形态及吸附性能的影响,同时解决吸附剂再生困难且易产生二次污染的问题,分别采用共沉淀法和水热法合成具有光催化作用的Cu-Mg-Al类水滑石,通过X射线衍射(XRD)、扫描电镜(SEM)、傅里叶变换红外光谱(FTIR) 等方法对制备的材料进行了表征,考察了其对盐酸四环素(TC)的吸附性能,吸附饱和的类水滑石采用紫外光照再生。结果表明,共沉淀法合成的LDHs-C吸附性能优于水热法合成的LDHs-H,对TC的去除率分别为95.2%和75.2%。Cu-Mg-Al类水滑石吸附TC的过程均符合准二级动力学方程和Langmuir吸附等温模型。吸附饱和的吸附剂在紫外线照射下30 min即可实现再生,经4次循环后,LDHs-C对TC的吸附量仍能保持原有的90%以上。Abstract: In order to investigate the influence of synthesis methods on the crystal morphology and adsorption performance of hydrotalcite, and solve the problem of difficult regeneration of adsorbent and easily causing secondary pollution, Cu-Mg-Al hydrotalcite-like compounds (LDHs) with photocatalysis were synthesized by the coprecipitation method and the hydrothermal method, respectively, and the prepared materials were characterized by X-ray diffraction (XRD), Scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR) and other methods, the adsorption of tetracycline hydrochloride (TC) was investigated. The saturated hydrotalcite -like compounds were regenerated under UV light. The results show that the adsorption performance of LDHs-C synthesized by the coprecipitation method was better than that of LDHs-H synthesized by the hydrothermal method, their removal rates of TC were 95.2% and 75.2%, respectively. The adsorption process of Cu-Mg-Al hydrotalcite-like compounds conformed to the quasi second order kinetic equation and Langmuir adsorption isotherm model. The saturated adsorbent could be regenerated by 30min UV irradiation. After 4 cycles, the adsorption capacity of TC on LDHs-C could still maintain over 90% of the original.
-
伴随着国内原油品质逐渐降低,氢燃料电池的高速发展,以及人们对环境保护意识的增强等,氢气的需求正在迅速增加。低浓度含氢废气中存在一定比例的氢气,以及N2、CH4、CO2等其他气体,直接作为燃料进行管网燃烧,不仅未能高效利用,还会对环境产生较大污染。因此,最适宜的途径是从现有的含氢废气中高效地回收氢气和其他气体[1-2]。目前,变压吸附(pressure swing adsorption,PSA)工艺已广泛应用于含氢气体处理中,其设备简单,提纯纯度高,易于自动化;吸附剂通常以颗粒状或柱状堆积于固定床吸附塔中,在交变压力下,尺寸较小的吸附剂粒子间相互摩擦严重,随气流逸失或堵塞孔道,而采用较大尺寸的颗粒又增加了分子向内扩散的时间,增大吸附剂的库存量,且导致装置能量利用效率低[3-5]。
沸石分子筛具有比表面积大、孔径均一、可再生等特点,可用于筛分不同尺寸的流体分子[6-8]。其中有效孔径为0.5 nm的5A沸石分子筛被广泛应用于工业含氢气体的变压吸附提纯过程[9-11]。结构化5A分子筛吸附剂是一种具有复合结构的吸附材料,克服了传统分子筛吸附剂的不足[12-13],可避免在交变压力下分子筛粒子间的磨损,提供了适用于高频操作的高表面活性通道,极大地提高了吸附材料的传质速率,有利于开发更紧凑的吸附装备[12-14]。LARA等[15]采用土聚凝胶-热转化(GGTC)结合浸涂法,ERIK等[16]采用水热合成法,均在氧化铝基体表面制备出沸石分子筛涂层,用于气体的分离提纯,但涂层厚度较薄,气体吸附量较低;ASIER等[17]采用浸涂法和水热合成法分别在不锈钢网上制备沸石分子筛涂层,研究结果表明浸涂法具有较大的负载量,但其与基体结合强度较弱;RYAN等[18]采用干喷、湿淬纺丝法制备出沸石分子筛内芯,醋酸纤维素外护套的双层中空纤维管,在循环变压吸附中,产品的纯度可达99.2%,表现出优异的气体分离性能。为提高吸附材料强度、分子筛负载量以及材料在吸附分离时的传质特性,本研究以泡沫镍金属为基体,采用浸渍提拉工艺制备结构化5A分子筛吸附材料,优化了制备工艺参数,并对其性能进行了评价。
1. 材料与方法
1.1 材料与试剂
泡沫镍(厚0.6 mm,孔隙率96%);5A分子筛原粉,平均粒径约3 μm;对比样5A分子筛球,平均粒径1 mm;可溶性淀粉,主要成分为玉米粉;酸性硅溶胶,固含量30%,化学纯;纳米氧化铝溶胶,固含量20%,化学纯;聚乙二醇(PEG),相对分子质量2 000;氢氧化钠,分析纯;乙醇,分析纯;丙酮,分析纯。
1.2 结构化5A分子筛吸附材料制备
1)泡沫镍预处理:将泡沫镍薄片分别在丙酮、无水乙醇、去离子水中超声洗涤15 min,在60 ℃的质量分数为10%的氢氧化钠溶液中碱洗30 s,经60 ℃的热水冲洗30 s,然后放入沸水中处理3 min,吹干。
2)5A分子筛浆液的制备:为获得结合性能良好的分子筛涂层,优选出可溶性淀粉为造孔剂,聚乙二醇为分散剂,硅溶胶和铝溶胶为黏结剂,去离子水作为溶剂,经球磨混合30 min,获得5A分子筛浆液。
3)5A分子筛涂覆:采用浸渍提拉法在泡沫镍薄片上浸涂分子筛浆液,设置提拉速度为20 mm·min−1,浸渍提拉完成后置于自制的全自动旋转仪上,设置旋转速度为5 r·min−1,并在60 ℃温度下缓慢烘干,然后置于马弗炉中焙烧2 h。
1.3 样品的表征方法
采用日本日立公司的S-4800型场发射扫描电子显微镜对样品表面和断面形貌进行观察;采用珀金埃尔默仪器(上海)有限公司的TGA-4000热重分析仪对样品热稳定性进行分析;采用英国牛津仪器公司的D/max2550V型X射线粉末衍射仪对样品进行定性分析;采用美国麦克仪器有限公司的TriStar II 3flex型比表面积及孔径分析仪测定样品的比表面和孔径分布及N2吸附-脱附等温线。
1.4 结合强度测试
采用超声振荡法对结构化5A分子筛进行结合强度测试。样品置于丙酮溶液中,超声波振荡器振荡频率为40 kHz,测试时间为30 min,抽真空烘干后称量。根据超声振荡前后质量差计算质量损失率,见式(1)。
Q=M−NN−X×100% (1) 式中:W为样品质量变化量;Q为样品质量损失率(%);M为样品超声振荡前重量,N为样品超声振荡后的重量,X为基体(泡沫镍)重量。
1.5 穿透曲线测定及吸附容量计算
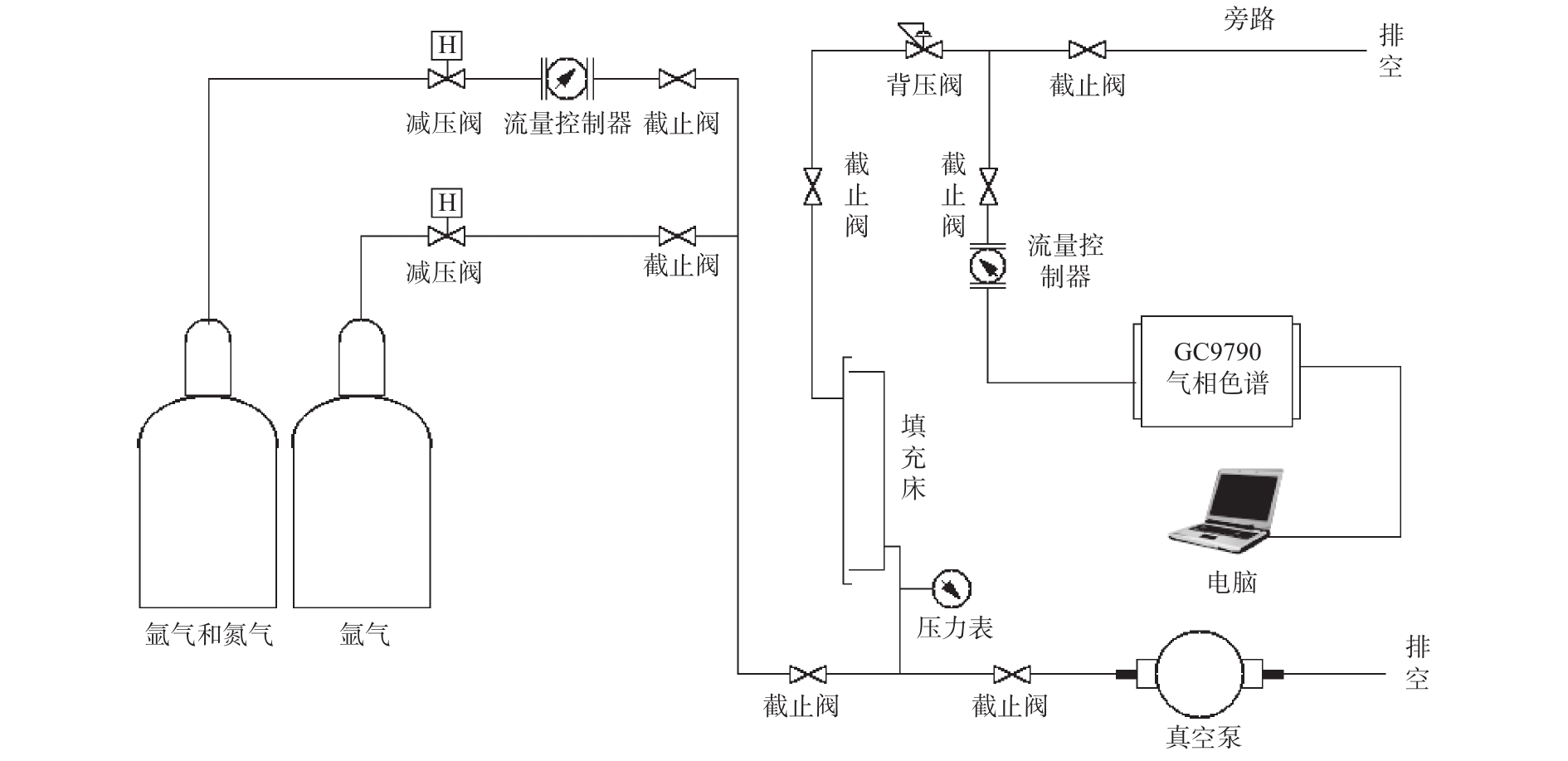
穿透曲线测定实验装置如图1所示,由气源、吸附实验、气体成分分析部分组成。实验中气源部分有2块质量流量控制器,分别控制充压氦气和由体积分数为75%的H2与25%的N2组成的混合气的气体流量,吸附实验是将混合气通入填充床进行吸附,填充床为200 mm×50 mm×10 mm的不锈钢槽,槽内装填若干片结构化的吸附材料或球状吸附剂。气体成分分析是将填充床出口气体通入GC9790气相色谱(填充相TDX01),采用TCD检测器在线测试气体各组分浓度。
根据氮气穿透时间、标气中氮气含量以及尾气中氮气含量,利用如下公式计算样品的N2吸附容量:
q=6.1Pvτ(Co−C)×10−6m (2) 式中:q为吸附材料对氮气的累计吸附容量,mmol·g−1;P为吸附压力,MPa;v为混合气体流过填充床的线速度,cm·s−1;
τ 为临界穿透时间,min;C0、C为标气及尾气中氮气的平均浓度,10−6。实验中以C>1×10−6作为穿透临界时间点;m为填充床中吸附材料填充量,g;常数6.1为填充床参数常量,由实验填充床计算获得。2. 结果与讨论
2.1 结构化5A分子筛的表征
2.1.1 形貌分析
泡沫镍和结构化5A分子筛吸附材料的形貌如图2所示。图2(a)为泡沫镍表面SEM图,可以看出,泡沫镍具有明显的三维孔结构,其特殊的结构为5A分子筛浆液的附着提供支撑,极高的孔隙率有利于提高浆液的附着量。图2(b)和图2(c)分别为结构化5A分子筛吸附材料表面显微照片和SEM图,可以看出,泡沫镍基体镶嵌于分子筛层中,并与镍骨架紧密接合,且吸附材料表面均匀分布着利于气体扩散的细微孔隙。图2(d)和图2(e)分别为结构化5A分子筛断面SEM图和断面局部放大SEM图,可以看出,经焙烧,断面处金属镍骨架包埋在分子筛层中,分子筛颗粒间通过黏结剂桥接作用紧密结合在一起,有利于提高吸附材料整体的机械性能。
2.1.2 热重分析
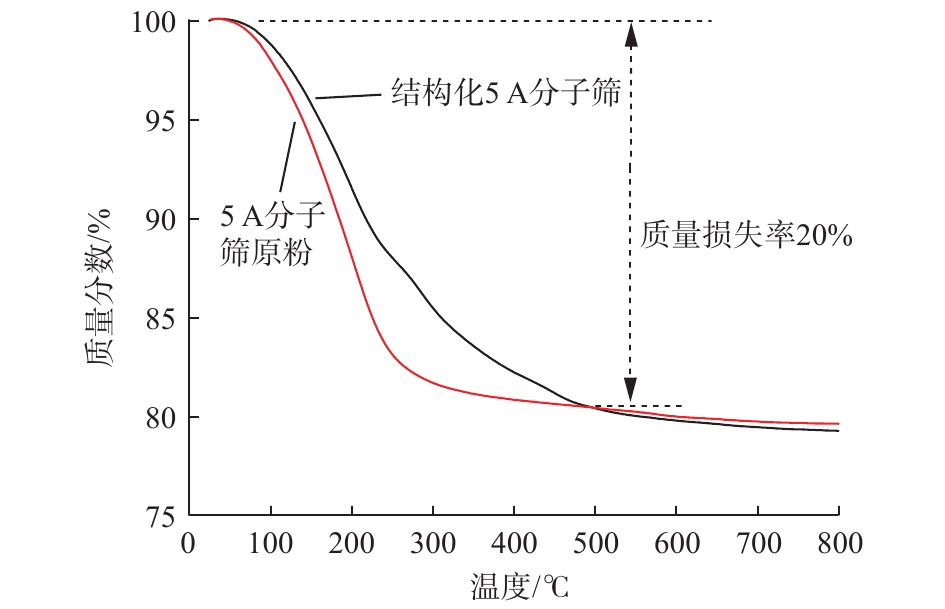
图3为550 ℃焙烧后获得的结构化5A分子筛和5A分子筛原粉的热重分析曲线,可以看出,两者的失重率相当,均在20%左右。100 ℃左右的质量损失,主要是由物理吸附在5A分子筛颗粒表面的吸附水脱除造成的;100~500 ℃质量损失为5A分子筛颗粒上化学吸附的结合水和结构水,以及残余的沸石水的脱除;当温度高于500 ℃时,结构化5A分子筛及其原粉质量均保持不变。由此可见,制备的结构化5A分子筛中无残余的分散剂和造孔剂,且具有良好的热稳定性。
2.2 工艺参数对结构化5A分子筛性能的影响
2.2.1 焙烧温度对结构化5A分子筛性能的影响
图4为不同焙烧温度下结构化5A分子筛XRD图。可以看出,在不同焙烧温度时,特征衍射峰位置基本相同,均没有出现明显不同的衍射峰。焙烧温度在550~650 ℃时,结构化5A分子筛的X射线特征衍射峰位置和相对强度与5A分子筛原粉基本一致;焙烧温度为750 ℃时,某些晶面的长程有序性变差,相对于5A分子筛原粉,特征衍射峰变宽;焙烧温度达到850 ℃时,结构化5A分子筛无尖锐的X射线特征衍射峰,晶型结构完全发生改变,呈无定型态。这说明焙烧后分子筛浆料中的PEG和可溶性淀粉均已被去除,浆液中少量的黏结剂未对5A分子筛原粉的结构产生破坏,并且其衍射峰强度很低。在焙烧温度升高过程中,高温促使分子筛晶粒细化,颗粒结晶度变差,当焙烧温度达到850 ℃时,5A分子筛的孔道坍塌,晶型结构转变成无吸附性能的无定型态。
2.2.2 硅/铝溶胶固含量比对结构化5A分子筛性能的影响
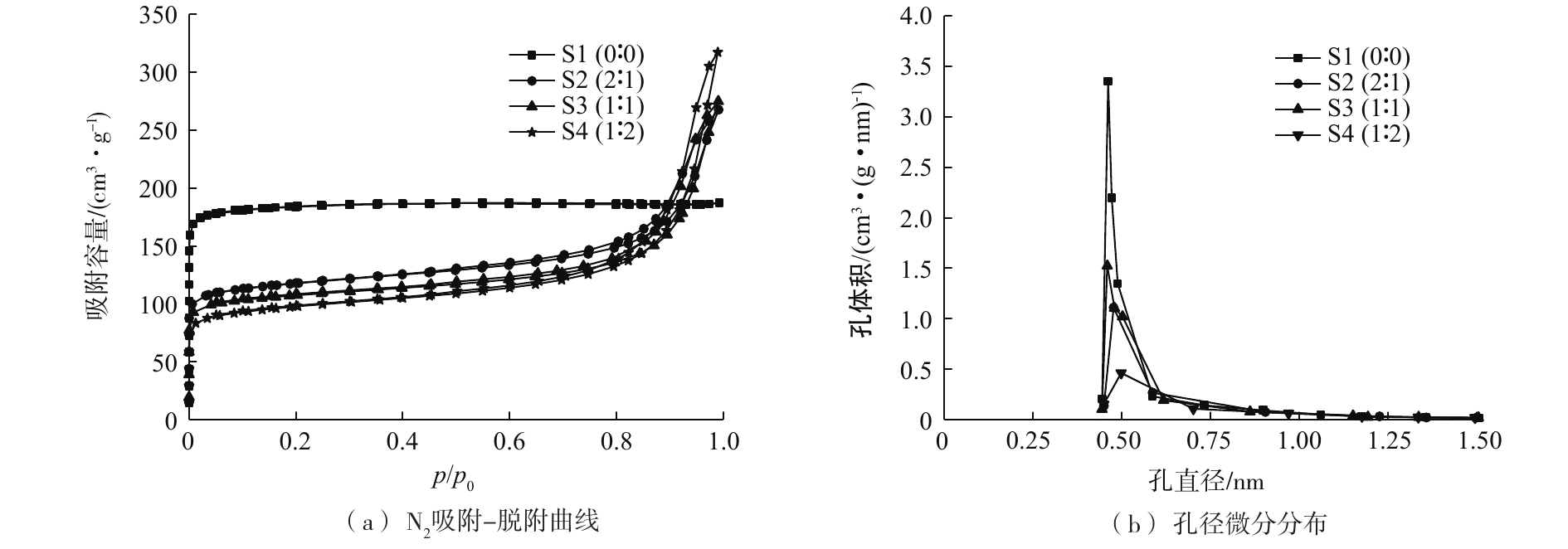
图5为不同硅/铝溶胶固含量比下经550 ℃焙烧后获得的结构化5A分子筛的N2吸附-脱附曲线及孔结构参数,其中S1(0:0))为5A分子筛原粉,S2(2:1)、S3(1:1)和S4(1:2)中w(硅溶胶):w(铝溶胶)分别为2:1、1:1和1:2。从图5(a)可以看出,5A分子筛原粉的吸附-脱附曲线为Ⅰ型。加入硅溶胶和铝溶胶后,结构化5A分子筛吸附材料的吸附-脱附曲线属于Ⅰ+Ⅳ型。这说明5A分子筛原粉为孔道特征规则的微孔,而结构化5A分子筛为微孔和介孔并存的复杂性孔道。从图5(b)可以看出,5A分子筛原粉和结构化5A分子筛的孔径均集中于0.5 nm(5A)位置。随着硅/铝溶胶比值的减小,集中于0.5 nm处的孔的孔体积呈先增大后减小的趋势。当硅/铝溶胶固含量比为1:1时,结构化5A分子筛吸附材料在0.5 nm孔径位置孔体积最大。通过分析认为:当硅/铝溶胶固含量比较大时,分子筛浆液黏度较小,与分子筛骨架同种电性的硅胶粒子,在一定范围内形成硅胶膜包裹分子筛颗粒,阻碍气体分子进入分子筛孔道;当硅/铝溶胶固含量比较小时,酸性的硅溶胶较少,在与弱碱性的铝溶胶混合后,分子筛浆液pH增大,促使溶胶转变为凝胶,导致浆液中5A分子筛原粉未被完全分散开,产生团聚,致使部分分子筛颗粒未完全起到吸附作用;当硅/铝溶胶固含量比为1:1时,分子筛浆液的pH偏弱酸性,浆液中的颗粒物可以均匀地分散在水溶液中,焙烧后分子筛颗粒分布均匀。
2.2.3 分子筛原粉固含量对结构化5A分子筛性能的影响
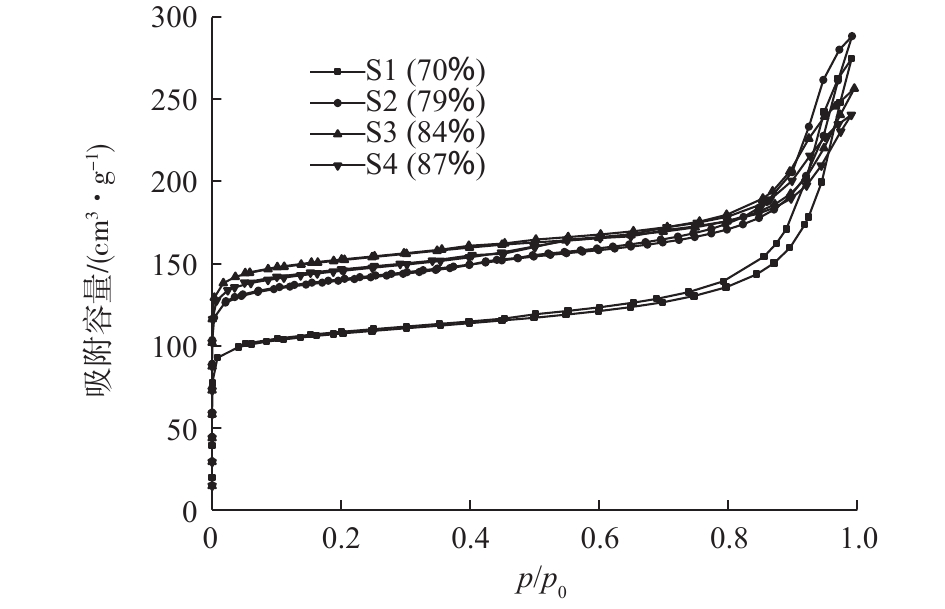
图6为不同5A分子筛原粉固含量下经550 ℃焙烧后获得的结构化材料的N2吸附-脱附曲线。从图6可以看出,样品S1(70%)、S2(79%)、S3(84%)、S4(87%)均为Ⅰ型和Ⅳ型组成的混合型吸附-脱附曲线。在较低相对压力下,吸附量迅速上升,而后出现饱和;在中等相对压力下,吸附曲线的吸附支和脱附支在一定相对压力范围内不重合,分离形成环状;在较高相对压力区域没有表现出明显的饱和,为典型的H1型回滞环。这一现象通常归因于在中孔吸附材料上发生了毛细管冷凝[19-20]。这进一步证实样品为微孔和介孔并存结构,且介孔的孔径分布均匀,均为两端开口的圆筒状孔。另外,随着5A分子筛原粉固含量的增加,单位吸附材料的吸附量增加,其原因可能是分子筛原粉固含量较少时,包裹在分子筛颗粒表面的高温黏结剂在一定程度上会阻碍气体分子进入分子筛孔道,不利于吸附传质;增大5A分子筛原粉固含量,分子筛颗粒表面的黏结剂由包裹状态逐渐转变为部分黏连,气体分子可以顺利地进入分子筛孔道。
表1为不同5A分子筛原粉固含量对结构化吸附材料孔径结构及结合性能的影响。可以看出,随着分子筛原粉固含量的增加,样品的BET比表面(SBET)、微孔比表面积(Smic)及微孔孔容(Vmic)均不断增加,外比表面积(Sext)先增大后保持不变,且当5A分子筛原粉固含量由70%增加到79%时,这一变化尤为明显。这一现象与前面黏结剂包裹分子筛原粉颗粒的分析推断相印证。实验也表明,不断增大5A分子筛原粉固含量,分子筛层脱落率Q增大较为明显,因此,浆料配制时分子筛原粉加入量需兼顾结构化5A分子筛吸附材料的比表面积大及结合强度高的要求。
表 1 5A分子筛原粉固含量对吸附材料孔结构及结合强度的影响Table 1. Effect of 5A molecular sieve content on pore structure and bonding strength of adsorbent样品号 分子筛原粉固含量/% SBET/(m2·g−1) Smic/(m2·g−1) Sext/(m2·g−1) Vmic/(cm3·g−1) Q/% S1 70 414 326 88 0.128 0.54 S1 79 544 437 108 0.170 0.75 S1 84 554 451 103 0.174 3.39 S1 89 569 466 103 0.180 5.42 2.3 结构化5A分子筛吸附材料吸附性能
以N2和H2的混合气为原料,模拟工业含氢废气,分析经550 ℃焙烧后获得的结构化分子筛的N2吸附性能。所采用的实验温度为298 K,柱前压为0.4 MPa,气体流速为1.2 cm·s−1,分别测量5A分子筛球和工艺参数优化后的结构化5A分子筛吸附材料对N2的吸附穿透曲线,结果如图7所示。根据实验穿透曲线,分别计算出吸附材料对N2的工作吸附容量,如表2所示。由图7可以看出,结构化分子筛的穿透曲线形状比分子筛球的更陡峭,且前沿的波幅更短,说明结构化5A分子筛吸附材料具有更小的传质区与传质阻力。产生上述不同的原因是,结构化5A分子筛是以泡沫镍金属为基体,整体厚度小于分子筛球粒径,其气体的传质区间相较于5A分子筛球更短;造孔剂的分解逸散,产生丰富大孔孔道,又为气体分子提供了较大的流动通道。由表2可以看出,在相同测试条件下,由于存在基体等非有效吸附成分,单位质量结构化吸附材料的N2吸附量降低,但有效吸附质(5A分子筛原粉)的吸附量与分子筛球相比,仅降低8.6%,表明结构化工艺对分子筛的吸附量影响较小。
表 2 不同5A分子筛吸附材料结构形式填充床上的N2吸附性能Table 2. Adsorption properties of N2 on packed bed with different 5A molecular sieve adsorbent structure样品 填充柱长/cm 柱前压/MPa 透过时间/min 吸附量/(mmol·g−1) 有效成分吸附量/(mmol·g−1) 5A分子筛球 20 0.4 22 0.81 0.81 结构化5A分子筛 20 0.4 14 0.50 0.74 3. 结论
1)以泡沫镍为基体制备出结构化5A分子筛吸附材料。泡沫镍骨架镶嵌于分子筛层中,并与分子筛层相互封锁,结合强度高,结构化吸附材料涂层脱落率可低于1%。
2)在550~650 ℃焙烧温度下,可保持5A分子筛晶型结构完整,随着温度的升高,5A分子筛结构被破坏。结构化5A分子筛吸附材料随着硅/铝溶胶固含量比的降低,材料吸附性能下降,当硅/铝溶胶固含量比为1:1时,吸附材料微孔孔体积最大。随着5A分子筛原粉固含量的增加,结构化材料吸附性能逐渐提高,但结合强度降低。
3)结构化5A分子筛中微孔和介孔并存,具有较高的单位质量吸附量,且与球状吸附剂相比,结构化吸附材料具有更小的传质区与传质阻力,表现出优异的吸附特性。
-
表 1 LDHs-C和LDHs-H晶胞结构参数
Table 1. The cell parameters of LDHs-C and LDHs-H
吸附剂 D 110/nm a/nm d 003/nm c/nm LDHs-C 0.152 7 0.305 4 0.738 1 2.214 3 LDHs-H 0.152 2 0.304 4 0.734 5 2.203 5 表 2 LDHs-C和LDHs-H的相关孔隙性质参数
Table 2. Porosity parameters of LDHs-C and LDHs-H
吸附剂 全孔面积/(m2·g−1) 孔容/(cm3·g−1) 孔径/nm LDHs-C 51.245 0.305 3.724 LDHs-H 23.938 0.132 3.189 表 3 LDHs-C和LDHs-H吸附TC的动力学模型参数
Table 3. Kinetic model parameters of TC adsorption on LDHs-C and LDHs-H
吸附剂 准一级动力学模型 准二级动力学模型 qe,实验值 qe,计算值 K1 R2 qe,计算值 K2 R2 LDHs-H 14.934 7.492 1.005 0.896 17.995 0.067 0.996 LDHs-C 19.237 4.415 0.471 0.938 19.677 0.247 0.999 表 4 LDHs-C和LDHs-H吸附TC的颗粒内扩散参数
Table 4. Parameters of the intraparticle diffusion model of TC adsorption on LDHs-C and LDHs-H
吸附剂 第1阶段 第2阶段 C Kd1 R2 C Kd1 R2 LDHs-C 13.980 2.376 0.993 18.569 0.205 0.726 LDHs-H 10.999 1.681 0.943 12.972 6.9906 0.944 表 5 LDHs-C和LDHs-H吸附TC的 Langmuir、Freundlich等温线模型参数
Table 5. Langmuir, Freundlich isothermal model parameters of TC adsorption on LDHs-C and LDHs-H
吸附剂 Langmuir模型 Freundlich模型 qm b R2 1/n KF R2 LDHs-C 232.55 0.07 0.984 1.04 13.77 0.957 LDHs-H 68.03 0.12 0.986 0.58 8.44 0.995 表 6 LDHs-C和LDHs-H吸附TC的热力学参数
Table 6. Thermodynamic parameters for TC adsorption on LDHs-C and LDHs-H
吸附剂 T/K ∆G/(kJ·mol−1) ∆H/(kJ·mol−1) ∆S/(J·(K·mol)−1) LDHs-H 298 −21.52 16.98 0.13 308 −22.81 318 −24.10 LDHs-C 298 −23.69 49.28 0.24 308 −26.14 318 −28.59 -
[1] 朱建宇,党清平,杨帆,等. MnFeCu-LDHs活化PMS降解氯四环素的效能及机制[J]. 环境工程学报, 2022, 16(12): 3895-3905. doi: 10.12030/j.cjee.202209108 [2] XU L,ZHANG H,XIONG P,et al. Occurrence,fate,and risk assessment of typical tetracycline antibiotics in the aquatic environment:A review[J]. Science of the Total Environment, 2020, 753: 141975. [3] ZHU Z R,XIA H W,LI H. Boosting photocatalytic degradation efficiency of tetracycline by a visible-light-activated NiMoO4/g-C3N4 heterojunction photocatalyst in the water environment[J]. Solid State Sciences, 2023, 139: 107164. doi: 10.1016/j.solidstatesciences.2023.107164 [4] 张宏,朱振亚,姜英宇,等. 壳聚糖和FeS改性生物炭吸附四环素:吸附机制与位能分布[J]. 环境科学学报, 2020, 40(12): 4306-4317. doi: 10.13671/j.hjkxxb.2020.0202 [5] HU P,SHAO J Y,QIAN G S,et al. Removal of tetracycline by aerobic granular sludge from marine aquaculture wastewater:A molecular dynamics investigation[J]. Bioresource Technology, 2022, 355: 127286. doi: 10.1016/j.biortech.2022.127286 [6] LI Y H,LIN D Y,LI Y F,et al. Nonradical-dominated peroxymonosulfate activation through bimetallic Fe/Mn-loaded hydroxyl-rich biochar for efficient degradation of tetracycline[J]. Nano Research, 2023, 16(1): 155-165. doi: 10.1007/s12274-022-4640-8 [7] XUE Y S,TANG W H,GU H X,et al. Flexible Bi2MoO6/N-doped carbon nanofiber membrane enables tetracycline photocatalysis for environmentally safe growth of vigna radiata[J]. Journal of Alloys and Compounds, 2022, 902: 154-167. [8] 李亚娟,赵传起,洪沛东,等. 磁性还原石墨烯的制备及其对抗生素的吸附性能[J]. 环境工程学报, 2018, 12(1): 15-24. doi: 10.12030/j.cjee.201705157 [9] WANG Z H,ZHANG W,LI C Q,et al. Recent progress of hydrogenation and hydrogenolysis catalysts derived from layered double hydroxides[J]. Catalysts, 2022, 12(11): 1125-1133. [10] LOZANO-LUNAR A,ALVAREZ J I,NAVARRO-BLASCO I,et al. Optimisation of mortar with Mg-Al-Hydrotalcite as sustainable management strategy lead waste[J]. Applied Clay Science, 2021, 212: 336-378. [11] VU V N,PHAM T H T,CHANTHAVONG M,et al. Enhanced photocatalytic degradation of rhodamine-B under led light using CuZnAl Hydrotalcite synthesized by co-precipitation technique[J]. Inorganics, 2022, 10(7): 567-678. [12] MAEGAWA K,ZHANG F,JOHNSON Q,et al. Control of micro- and nanostructures of layered double hydroxides by hydrothermal treatment[J]. Crystal Growth & Design, 2023, 23(4): 2128-2137. [13] GAO F,XU X R,YANG J Y. Removal of p-nitrophenol from simulated sewage using MgCo-3D hydrotalcite nanospheres:Capability and mechanism[J]. Rsc Advances, 2022, 12(41): 27044-27054. doi: 10.1039/D2RA01883G [14] COSANO D,ESQUIVEL D,ROMERO-SALGUERO F J,et al. Efficient removal of nonylphenol isomers from water by use of organo-hydrotalcites[J]. International Journal of Environmental Research and Public Health, 2022, 19(12): 1117-1122. [15] CHEN Y,SHI J,DU Q,et al. Antibiotic removal by agricultural waste biochars with different forms of iron oxide[J]. RSC Advances, 2019, 9(25): 14143-14153. doi: 10.1039/C9RA01271K [16] CHEN R,CHEN T,ZHU C,et al. Effect of coprecipitation method on Mg-Al hydrotalcite properties:application in the synthesis of diethylene glycol di- (methyl carbonate) [J]. Journal of the Iranian Chemical Society, 2020, 17(10): 2507-2513. doi: 10.1007/s13738-020-01945-8 [17] OGATA F,UETA E,KAWASAKI N. Characteristics of a novel adsorbent Fe-Mg-type hydrotalcite and its adsorption capability of As (III) and Cr (VI) from aqueous solution[J]. Journal of Industrial and Engineering Chemistry, 2018, 59: 56-63. doi: 10.1016/j.jiec.2017.10.005 [18] 潘国祥 钱萍萍 曹枫,等. 镁铁水滑石衍生复合氧化物制备、表征与吸附六价铬性能[J]. 稀有金属材料与工程, 2012, 41(s3): 41. [19] 吴永娟,王晓兰,闫俊英. Fe3O4/镁铁类水滑石复合物的制备及吸附性能[J]. 工业水处理, 2019, 39(11): 49-53. doi: 10.11894/iwt.2018-0930 [20] LU Y,JIANG B,FANG L,et al. High performance NiFe layered double hydroxide for methyl orange dye and Cr (VI) adsorption[J]. Chemosphere, 2016, 152: 415-422. doi: 10.1016/j.chemosphere.2016.03.015 [21] 曹根庭,薛继龙,夏盛杰,等. 不同阴离子插层锌铝水滑石对亚甲基蓝的光催化性能[J]. 硅酸盐学报, 2016, 44(5): 726-732. doi: 10.14062/j.issn.0454-5648.2016.05.16 [22] MAO N,JIAO Y. CuAl hydrotalcite formed CuAl-Mixed metal oxides for photocatalytic removal of rhodamine B and Cr (VI) [J]. Chemistryselect, 2018, 3(44): 12676-12681. doi: 10.1002/slct.201801888 [23] 徐敏虹,潘国祥,汪小华,等. Zn-M-Cr三元类水滑石的合成及其光催化性能[J]. 硅酸盐学报, 2017, 45(8): 1175-1182. [24] CHATLA A,ALMANASSRA I W,KOCHKODAN V,et al. Efficient removal of eriochrome black T (EBT) dye and chromium (Cr) by hydrotalcite-derived Mg-Ca-Al mixed metal oxide composite[J]. Catalysts, 2022, 12(10): 1247. doi: 10.3390/catal12101247 [25] 刘定鹏,军秦,吕晴等. 以粉煤灰为原料制备镁铝水滑石[J]. 硅酸盐学报, 2020, 48(08): 1341-1347. doi: 10.14062/j.issn.0454-5648.20190736 [26] XU Z,FAN J,ZHENG S,et al. On the adsorption of tetracycline by calcined magnesium-aluminum hydrotalcites[J]. Journal of Environmental Quality, 2009, 38(3): 1302-1310. doi: 10.2134/jeq2008.0246 [27] ZIYAT H,BENNANI M N,DEHMANI Y,et al. Adsorptive performance of a synthesized Mg-Al Hydrotalcite compound for removal of malachite green:Kinetic,isotherm,thermodynamic,and mechanism study[J]. International Journal of Environmental Analytical Chemistry, 2022, 306: 1-20. [28] HUANG P,LIANG Z,ZHAO Z,et al. Synthesis of hydrotalcite-like compounds with drinking water treatment residuals for phosphorus recovery from wastewater[J]. Journal of Cleaner Production, 2021, 301: 126976. doi: 10.1016/j.jclepro.2021.126976 [29] LIU N,WANG M X,LIU M M,et al. Sorption of tetracycline on organo-montmorillonites[J]. Journal of Hazardous Materials, 2012, 225: 28-35. [30] SHAN R-R,YAN L-G,YANG Y-M,et al. Highly efficient removal of three red dyes by adsorption onto Mg-Al-layered double hydroxide[J]. Journal of Industrial and Engineering Chemistry, 2015, 21: 561-568. doi: 10.1016/j.jiec.2014.03.019 [31] SONG Z Z,GAO H Y,LIAO G Y,et al. A novel slag-based Ce/TiO2@LDH catalyst for visible light driven degradation of tetracycline:performance and mechanism[J]. Journal of Alloys and Compounds, 2022, 901: 163525. doi: 10.1016/j.jallcom.2021.163525 [32] 柴琴琴,呼世斌,刘建伟,等. 有机改性对凹凸棒黏土吸附四环素类抗生素的影响[J]. 中国环境监测, 2018, 34(5): 95-103. doi: 10.19316/j.issn.1002-6002.2018.05.14 [33] 杨伟伟,高晓红,张鑫,等. 四环素在矿化垃圾上的吸附特性及动态过程[J]. 环境化学, 2022, 41(5): 1726-1735. doi: 10.7524/j.issn.0254-6108.2021122404 [34] ZIYAT H,ELMZIOUI S,NACIRI BENNANI M,et al. Kinetic,isotherm,and mechanism investigations of the removal of nitrate and nitrite from water by the synthesized hydrotalcite Mg-Al[J]. Research on Chemical Intermediates, 2021, 47(6): 2605-2627. doi: 10.1007/s11164-021-04414-w [35] XIAO Q,ZHAO Y,LIAO Y. The adsorption performance of tetracycline over copper oxide[J]. Applied Chemical Industry, 2022, 51(11): 3190. [36] OZCAN A,OMEROGLU C,ERDOGAN Y,et al. Modification of bentonite with a cationic surfactant:An adsorption study of textile dye Reactive Blue 19[J]. Journal of Hazardous Materials, 2007, 140(1-2): 173-179. doi: 10.1016/j.jhazmat.2006.06.138 [37] KWIKIMA M M,CHEBUDE Y,MESHESHA B T. Kinetics,adsorption isotherms,thermodynamics,and desorption studies of cadmium removal from aqueous solutions using bamboo sawdust/rice husk biochar[J]. Biomass Conversion and Biorefinery, 2022, 32: 1-13. 期刊类型引用(1)
1. 吴梦豪,姜应和,李大琳. 三种ZnAl-稀有金属LDHs除磷效果对比试验与机理分析. 武汉理工大学学报. 2024(07): 98-106 .  百度学术
百度学术
其他类型引用(4)
-






 下载:
下载: