-
氨选择性催化还原 (NH3 selective catalytic reduction,NH3-SCR) 脱除氮氧化物 (NOx) 技术具有效率高、运行稳定等优点,已广泛应用于各类大型工业连续排放源。NH3-SCR技术关键为催化剂,其应用成本通常占60%[1]。V2O5-WO3(MoO3)/TiO2为最常见的商业催化剂,其温段 (300~400 ℃) 催化活性较高且抗硫抗水性较好[2]。然而,由于脱硝装置布设在除尘和脱硫设备前,SO2可被其催化剂氧化成SO3,进而与还原剂NH3生成(NH4)2SO4和NH4HSO4,附着于催化剂表面,导致催化活性降低[3];同时,烟气中较多的粉尘会对SCR反应器造成冲刷与磨损。为避免上述问题,推荐采用尾部低尘低温 (<250 ℃) 布置方式,将SCR反应器安装于脱硫装置和布袋除尘器之后。目前,我国有100余家大型垃圾焚烧发电厂采用低温SCR技术,其运行温度通常为180~220 ℃。然而,较低的温度使NH3转化率偏低,显著增加氨逃逸[4],因此研发能高效稳定脱硝且减少氨逃逸的低温SCR催化剂成为NH3-SCR领域研究热点。
锰 (Mn) 有多种价态,具有很强的氧化还原活性。Mn基催化剂在低温NH3-SCR催化剂领域应用较多[5]。同时,Cr2O3催化剂表现出良好脱硝活性[6],采用浸渍法对V2O5-WO3/TiO2进行Cr负载改性,可使催化剂的低温脱硝性能和抗硫性有较大提升[7]。负载Cr可增加催化剂表面酸性位点,从而增强催化剂表面氧吸附能力及对NH3的催化氧化能力[8]。此外,Mo负载可提升MnOx催化剂的脱硝性能,有利于Mn3+的形成,并增加烟气中NH3的吸附[9]。亚单层MoO3增加表面强酸位点数量,催化NH3氧化并增加其N2选择性[10]。添加Mo可提高CeO2-TiO2的脱硝效率,增加催化剂抗硫抗水性[11]。因此,本研究尝试将Mn基催化剂负载改性元素铬 (Cr) 和钼 (Mo) ,制备高效且具有降低氨逃逸功能的焚烧烟气低温脱硝制剂。
由于现有Mn基催化剂多使用含Mn的分析纯试剂制备金属氧化物复合物,或以碳纳米管和分子筛等为载体制备负载型Mn氧化物[12-16],使得催化剂生产成本较高。本课题组拟以天然锰矿粉 (manganese ore powder, MOP) 为基质材料制备相关催化剂,从而保证成本足够低廉,且制造的催化剂可以粉体状态直接注入烟气并一次性使用。
本研究拟通过浸渍法制备负载Cr和Mo的MOP催化剂,以天然锰矿粉作为对比,全面考察负载改性MOP催化剂在NH3存在和不存在2种氛围下的脱硝活性,以及NO存在和不存在2种氛围下的脱氨活性,并通过NH3全自动程序升温化学吸附仪及质谱联用 (NH3-TPD-MS) 、X-射线衍射 (XRD) 、场发射扫描电子显微镜 (FE-SEM) —能量色散X射线光谱仪 (EDS) 和X射线光电子能谱 (XPS) 表征分析,观测催化剂酸性位点、形貌、晶型结构及表面元素分布、价态变化,探索Cr和Mo负载改性对MOP脱硝和脱氨性能的影响机理,以期为廉价高效的低温脱硝催化剂制备提供参考。
-
天然锰矿粉 (球磨后过200目筛) 购买于湖南某厂家。经表征分析,锰矿粉中Mn的质量分数为42.22% (Zetium X射线荧光光谱仪,荷兰马尔文帕纳科公司) ,比表面积为14.97 m2∙g−1,总孔容4.16×10−2 cm3∙g−1,平均孔径3.905 nm (QuadraSorb SI4物理吸附仪,美国康塔仪器公司) ,锰矿粉粒径范围为0.028~399.05 μm,面积权重平均粒径为1.54 μm,粒径为10~200 μm的颗粒体积占总体积的64.9% (Mastersizer2000激光衍射粒度分析仪,英国马尔文仪器有限公司) 。
Cr和Mo负载所使用的Cr(NO3)3·9H2O和(NH4)6Mo7O24·4H2O,以及气流中NH3质量浓度测定用的铵离子标准溶液、酒石酸钾钠和纳氏试剂 (NaOH、HgCl2和KI) ,全部为分析纯试剂。实验用压缩空气 (纯度99.999%) 、NO标准气 (999.3mg∙L−1,N2为平衡气) 和NH3标准气 (999.5 mg∙L−1,N2为平衡气) 均购买于大连光明特种气体有限公司。
-
1) 锰矿粉基催化剂的制备。天然锰矿粉在负载改性前进行了加热处理,在105 ℃下烘干12 h,再放入马弗炉中400 ℃中焙烧5 h,冷却研磨混匀。负载2%和5%Cr锰矿粉 (2%Cr-MOP和5%Cr-MOP) 的制备方法为:分别称取7.70 g和15.39 g Cr(NO3)3·9H2O溶解于50 mL去离子水中,各加入20 g锰矿粉,搅拌后静置4 h,在85 ℃烘干12 h,再放入马弗炉于400 ℃焙烧5 h,冷却研磨混匀。负载质量分数分别为2%和5%Mo锰矿粉 (2%Mo-MOP和5%Mo-MOP) 的制备方法与Cr负载锰矿粉基本相同,分别称取1.84 g和3.68 g (NH4)6Mo7O24·4H2O溶解于50 mL去离子水中,再分别加入20 g锰矿粉,烘干、焙烧并研磨混匀。文中涉及到的负载数据均为质量分数。
2) 实验装置及过程。NOx和NH3催化脱除实验装置由配气系统、反应系统及分析监测系统构成。配气系统配送的模拟烟气中,NH3、NO和压缩空气的调配根据实验方案,通过流量控制器和流量计调节。在脱硝性能测试中,在NH3存在(NO+NH3+O2+N2)氛围下,气流流速为2.5 L∙min−1,NO、NH3质量浓度和O2体积分数分别为280~300 mg∙m−3、280~300 mg∙m−3和7%~8%;在NH3不存在(NO+O2+N2)氛围下,气流流速为1 L∙min−1,NO质量浓度和O2体积分数分别为370~390 mg∙m−3和12%~13%。在脱氨性能测试中,在NO存在(NO+NH3+O2+N2)氛围下,气流流速和NO、NH3质量浓度及O2体积分数同脱硝性能测试中NH3存在氛围保持一致;在NO不存在 (NH3+O2+N2) 氛围下,气流流速为2.5 L∙min−1,NH3质量浓度和O2体积分数分别为380~400 mg∙m−3和12%~13%。
反应系统为管式炉固定床立式反应器 (QSH-VGP-1200T,上海全硕电炉有限公司) ,炉内放置石英反应管 (外径30 mm) 。分别称取10 g直径为2 mm的玻璃珠、10 g直径为1 mm的玻璃珠和5 g石英砂,按次序平铺于石英管底部,再加入均匀混合的5 g催化剂与15 g石英砂,最后再称取10 g直径为1 mm玻璃珠置于催化剂上层。
在分析监测系统中,反应装置进口、出口气流中NO、NO2质量浓度、O2体积分数采用烟气分析仪 (崂应3016,青岛崂应海纳光电环保集团有限公司) 在线监测,NH3质量浓度采用稀硫酸溶液吸收-纳氏试剂分光光度法检测。鉴于N2O通常在400 ℃以上才会有明显生成[17],故本研究没有进行分析。在反应期间,反应装置出口气流分成3路。第1路气流与烟气分析仪相接,采样速率为1 L∙min−1;第2路气流连接串联的吸收管 (体积为100 mL) 2只,每只装有50 mL 0.01 mol∙L−1稀硫酸溶液,同时采用气体采样泵 (流速为1 L∙min−1) 抽气;第3路气流经稀硫酸溶液脱NH3后引入通风橱放空。脱硝和脱氨实验采用固定升温和降温程序,通过温控器实现反应床层温度的精确控制,温度分别设定为150 ℃、200 ℃、250 ℃、300 ℃、350 ℃和400 ℃,达到预设温度5 min后开始采样。采样持续时间30 min。当采样结束后,将2只吸收管中的稀酸溶液合并,定容至100 mL。根据《环境空气和废气氨的测定纳氏试剂分光光度法》 (HJ 533-2009) 测定NH4+离子浓度,计算气流中NH3质量浓度。
3) 计算方法。NO转化率Rn、NH3转化率Ra (脱氨效率) 、NO2生成率YNO2、无NO存在条件下脱氨反应的NO生成率YNO和脱硝效率Rd计算公式见式 (1)~(5) 。
式中:[NO]in和[NH3]in分别为反应器进口气流中NO的平均质量浓度和NH3测定质量浓度;[NO]out、[NO2]out和[NH3]out分别为反应器出口气流中NO、NO2的平均质量浓度和NH3测定质量浓度。以上质量浓度单位均为mg∙m−3。
4) 催化剂表征方法。催化剂表面NH3吸附通过NH3-TPD-MS (AutoChem II,美国Micromeritics公司及GSD350 OmniStar,德国Pfeiffer VacuumGmbH公司) 测定。在催化剂XRD (X'pert Pro MPD,荷兰Nalytical公司) 表征分析中,压片法制样后采用Cu Kα辐射,10~90° (2θ) 扫描,扫描步长为0.033(°)∙s−1。催化剂结构和形貌分析采用FE-SEM-EDS (JEOL JSM-7800F,日本电子株式会社) ,加速电压0.01~30 kV。催化剂表面元素含量和价态分布通过XPS (ESCALA Xi+,赛默飞世尔科技公司) 测试,X射线源Al Kα,能谱扫描范围0~1350 eV,宽幅扫描间距1 eV,窄幅扫描间距0.1 eV。
-
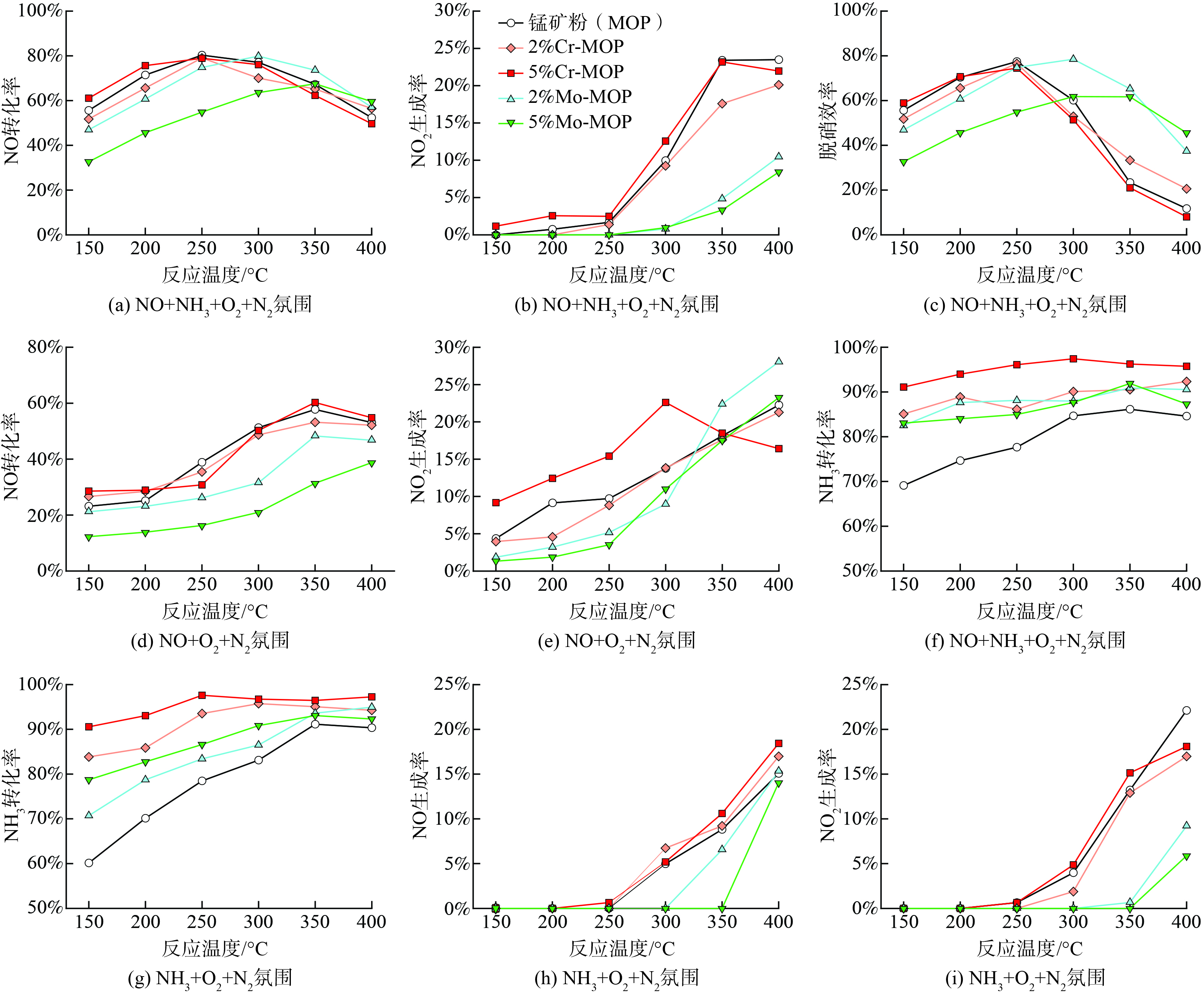
1) 负载Cr锰矿粉的脱硝效率。在(NO+NH3+O2+N2)氛围下,Cr负载几乎未对MOP的催化脱硝产生影响。图1 (a)~(c) 表明,原锰矿粉、2%Cr-MOP和5%Cr-MOP有相似的NO转化率、NO2生成率和脱硝效率。当反应温度由150 ℃升至250 ℃,3种催化剂的脱硝效率持续增加。在250 ℃时,原锰矿粉、2%Cr-MOP和5%Cr-MOP的脱硝效率达到峰值,分别为77.4%、76.5%和74.5%。在(NO+O2+N2 )(无NH3) 氛围下,负载Cr对NO转化率没有明显影响。如图1 (d) 所示,3种催化剂的NO转化率在150~350 ℃时,随温度增加而持续上升,400 ℃时表现出降低趋势。在150~300 ℃下,由于5%Cr-MOP的NO2生成率明显高于原锰矿粉和2%Cr-MOP (图1 (e) ) ,故低温负载5%Cr具有更强的NO催化氧化生成NO2能力。
2) 负载Cr锰矿粉的脱氨效率。在(NO+NH3+O2+N2)氛围下,Cr负载大幅提升了MOP的NH3转化率。图1 (f) 表明,当温度为150 ℃时,原锰矿粉的脱氨效率 (NH3转化率) 为69.1%,负载2%和5%Cr后分别提高到85.1%和91.1%。当温度由150 ℃升至300 ℃,3种催化剂的NH3转化率均随温度增加,均表现出升高趋势。当温度为300 ℃时,5%Cr-MOP的脱氨效率达到峰值 (97.4%) 。鉴于5%Cr负载MOP的NH3转化率明显高于2%负载条件,故增加Cr负载量可提高MOP的脱氨效率。
在(NH3+O2+N2) (无NO) 氛围下,Cr负载也显著增加MOP的脱氨效率,尤其在150~250 ℃低温条件下。图1 (g) 表明,当温度为150 ℃时,原锰矿粉的NH3转化率为60.1%,负载2%和5%Cr后分别提高到83.8%和90.6%。当温度为250~400 ℃时,2%Cr-MOP和5%Cr-MOP的脱氨效率一直维持在93%以上。此外,Cr负载对MOP催化NH3氧化生成NO和NO2没有明显影响 (图1 (h) 和图1 (i) ) 。当温度为150~250 ℃时,几乎无NO和NO2生成,当温度从250 ℃增至400 ℃,NO和NO2生成量持续增加。这说明负载Cr在提升MOP催化剂 NH3转化率同时并未导致NH3的过度氧化。
3) 负载Mo锰矿粉的脱硝效率。在(NO+NH3+O2+N2)氛围下,当温度为150~250 ℃,与原锰矿粉相比,5%Mo负载导致NO转化率和脱硝效率明显降低,2%Mo负载表现为轻微下降;但当温度为350~400 ℃时,2%Mo-MOP和5%Mo-MOP的脱硝效率大幅增加 (图1 (a) 和图1 (c) ) ,均比原锰矿粉高25%以上。较高的脱硝效率源于NO2生成率的显著减少 (图1 (b) ) 。在350~400 ℃的温度条件下,原锰矿粉的NO2生成率较2%Mo-MOP和5%Mo-MOP高1~6倍,这说明Mo负载对NH3和NO氧化生成NO2有很强的抑制作用。
在(NO+O2+N2 )(无NH3) 氛围下,当温度为150~400 ℃时,5%Mo负载明显降低MOP的NO转化率 (图1 (d) ) ,但当温度为150~200 ℃时,2%Mo-MOP的NO转化率与原锰矿粉接近。同时,当温度为150~300 ℃时,2%Mo-MOP和5%Mo-MOP的NO2生成率均明显低于原锰矿粉 (图1 (e) ) 。综上所述,2%Mo负载在不显著影响NO低温 (150~200 ℃) 催化转化效率的同时,会抑制NO氧化为NO2。
4) 负载Mo锰矿粉的脱氨效率。在(NO+NH3+O2+N2)氛围下,Mo负载明显增加了MOP的脱氨效率,但幅度小于Cr负载。如图1 (f) 所示,当温度为150 ℃时,Mo负载NH3转化率的提升幅度较其他温度更大,与原锰矿粉相比,2%Mo-MOP和5%Mo-MOP的脱氨效率分别提高了10.6%和18.6%。然而,随温度增加 (150~300 ℃) ,Mo负载引起的NH3转化率增幅逐渐减小,2%Mo-MOP和5%Mo-MOP的脱氨效率没有明显差别。这意味着当Mo负载量达到2%以后,继续增加负载量可能不会再显著促进MOP脱氨效率。
在(NH3+O2+N2) (无NO) 氛围下,Mo负载也明显提升了MOP基催化剂的脱氨效率。同样,提升幅度亦低于Cr负载 (图1 (g) ) 。基本上,随Mo负载量增加,MOP的NH3转化率也上升。值得注意的是,Mo负载显著抑制了MOP催化NH3氧化生成NO和NO2 (图1 (h) 和图1 (i) ) 。当温度在300 ℃以下时,添加2%Mo-MOP和5%Mo-MOP后并未检测到NO和NO2的生成;同时,温度350 ℃时NO2仍极少量生成,说明Mo负载可抑制NH3氧化为NO和NO2。
-
1) NH3的吸附特性。催化剂表面酸性位点的变化通过NH3-TPD分析 (图2) 。鉴于TCD热导检测器不能严格区分脱附物种 (图2 (a) ) ,实验同时选择了m/z=16通道的质谱进行联合检测以相互印证 (图2 (b) ) 。测试结果表明,3种催化剂均在中温段有最强脱附峰。此时,与MOP相比,5%Cr和5%Mo负载的NH3吸附能力有较大提升,Mo负载尤其显著。5%Mo改性MOP不仅中温段脱附峰向高温方向移动,而且低温段峰值也较MOP和5%Cr-MOP更大。这说明Mo负载不仅同时增加表面弱酸和中强酸性位点数量,而且使中强酸性位点酸性增强,总体促进NH3吸附能力 (图2 (b) ) 。
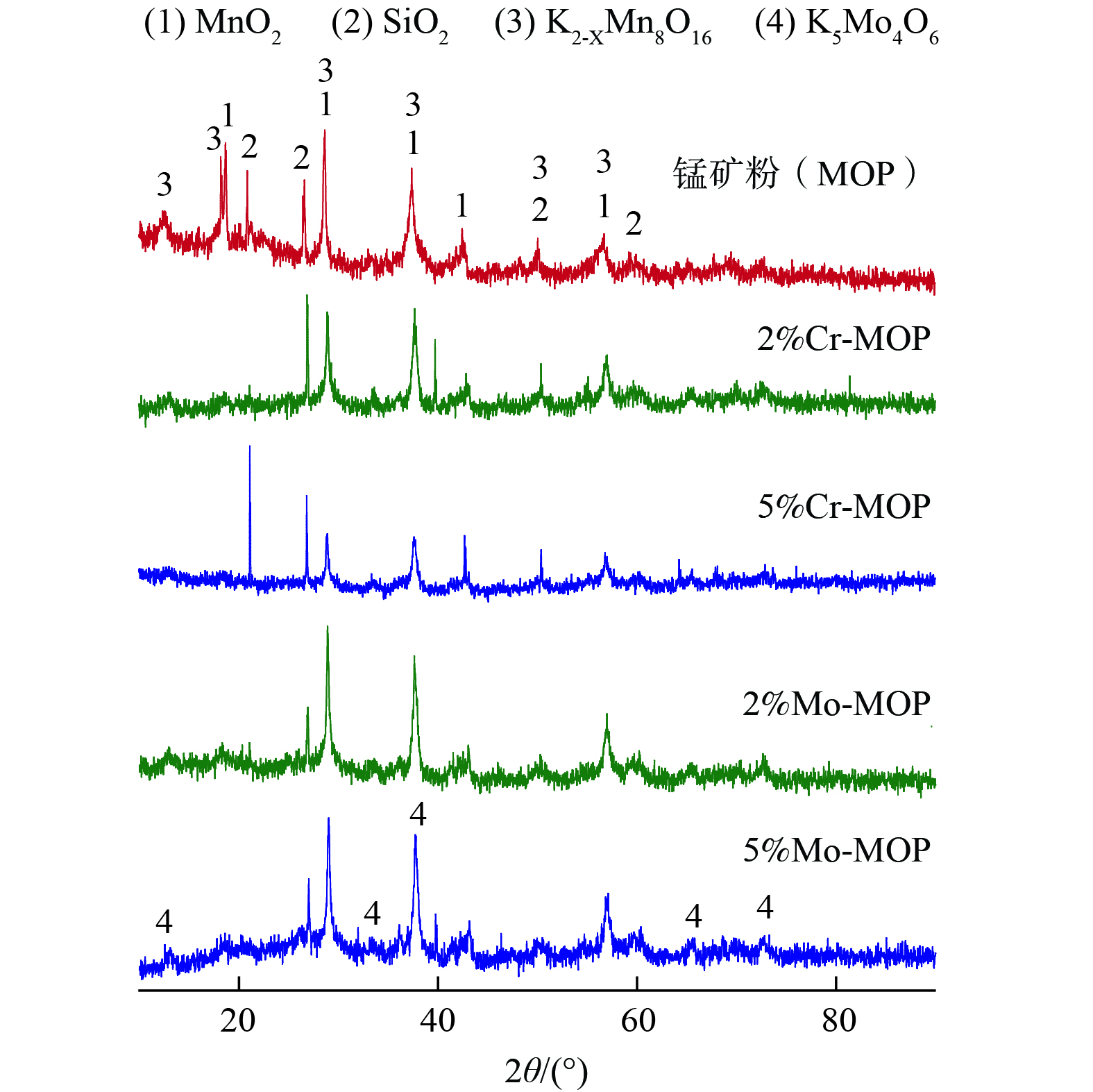
2) FE-SEM及表面EDS分析。FE-SEM图片表明,负载改性锰矿粉表面为不规则的块状形貌 (图3 (a)~(d) ) 。EDS测试表明,5%Cr和5%Mo改性MOP以Mn、O、K、Al、Si 和Fe元素为主 (图3 (e) ) 。由Mapping分析结果发现,负载的Cr和Mo与MOP中的Mn和O元素分布基本一致,这说明负载的Cr和Mo主要分布在MOP表面。对比可见,随Cr和Mo负载量的增加,Cr和Mo点密度明显增加,但Mn点密度没有改变,说明Cr和Mo的负载未破坏MOP表面结构。
3) XRD分析。图4表明,原锰矿粉与负载改性锰矿粉基催化剂中均鉴定出MnO2、SiO2和K2-xMn8O16特征峰,但Cr负载MOP中没有观测到CrOx特征峰,这说明催化剂制备中使用的Cr(NO3)3经加热处理后没有转化成晶型CrOx,而是以无定形态存在。此外,Cr负载明显降低MnO2和K2-xMn8O16的特征峰强度,而MnO2和K2-xMn8O16中的Mn均为Mn4+。鉴于Mn4+是MOP具有良好脱硝活性的主要原因之一,因此Cr负载没有提升其负载改性MOP的催化脱硝效率。5%Mo-MOP中观测到了K5Mo4O6特征峰 (图4) ,说明以(NH4)6Mo7O24·4H2O形式加入的Mo加热处理后在MOP表面与K反应转化生成了钾钼盐结晶,这会占据表面活性位点,导致脱硝活性的下降,与5%Mo-MOP低温脱硝活性显著下降的实验现象一致。2%Mo-MOP中没有观测到K5Mo4O6特征峰,故较低的Mo负载量对NO转化率影响轻微。
4) XPS分析。对原锰矿粉、5%Cr-MOP和5%Mo-MOP表面的Mn (Mn 2p) 进行XPS分析。如图5 (a) 所示,原锰矿粉中Mn的电子结合能主要位于642.07 eV (Mn3+) 和 643.51 eV (Mn4+) 处,这说明Mn主要以MnO2和Mn2O3形式存在。负载Cr后峰值分别位于642.07eV (Mn3+) 和643.67eV (Mn4+) 处 (图5 (b) ) ,与原锰矿粉几乎相同,这说明Cr与Mn之间没有明显的相互作用。负载Mo后峰值分别位于641.52eV (Mn3+) 和643.49eV (Mn4+) 处 (图5 (c) ) ,与原锰矿粉相比,特征峰出现展宽现象且向低结合能方向偏移,说明Mo与Mn之间产生明显相互作用。负载Mo使Mn价层电子得失能力增强,而金属活性离子价态频繁变换极易形成配合物,有利于NH3吸附及迅速活化,通过E-R机理促使吸附态NH3形成-NH2中间体后再与NO反应,从而提高N2选择性[18],这可能是Mo负载提升MOP脱氨效率的重要原因之一。有研究表明,低温时MOP基催化剂E-R和L-H机理同时存在。对于L-H机理,NH3和NO均吸附于催化剂表面,NO生成NO2、硝酸盐或亚硝酸盐反应中间体后与配位态NH3发生氧化还原反应[19]。由此推测,在NO+O2+N2 (无NH3) 氛围下,低温段5%Cr负载NO2生成率高的原因与L-H机理更相关。
MnOx脱硝活性从强到弱排序依次为MnO2> Mn5O8> Mn2O3> Mn3O4> MnO[20],Mn4+占比越高,催化剂的脱硝性能越好[21]。将图5 (a)~(c) 中Mn3+和Mn4+分峰分别积分并计算峰面积,进一步计算Mn4+/(Mn3++Mn4+)比值。结果表明,原锰矿粉Mn4+的相对含量为44.7%,负载Cr和Mo后分别降至39.0%和20.6%。Mo负载降幅更大,可能导致NO向NO2的转化大幅减少并显著降低脱硝效率。
进一步对原锰矿粉、5%Cr-MOP和5%Mo-MOP表面的O (O 1s) 进行XPS分析。如图5 (d)~(f) 所示,结合能为532.56 eV处的特征峰归属于表面化学吸附氧 (Oα:O22-/O−) ,结合能为529.90 eV处为晶格氧 (Oβ:O2-) 。其中Oα为活性氧,具有很强的化学反应活性,因而催化剂表面Oα占比越高,其脱硝性能越好。将Oα和Oβ分峰分别积分计算峰面积,再计算Oα/(Oα+Oβ)值。结果表明,原锰矿粉Oα相对含量为0.806,而Cr和Mo负载MOP分别为0.649和0.698。这意味着Cr和Mo均可能导致催化剂脱硝活性下降。然而,在脱硝实验中,Cr改性没有观测到脱硝效率的明显变化,仅5%Mo负载的抑制作用表现明显。
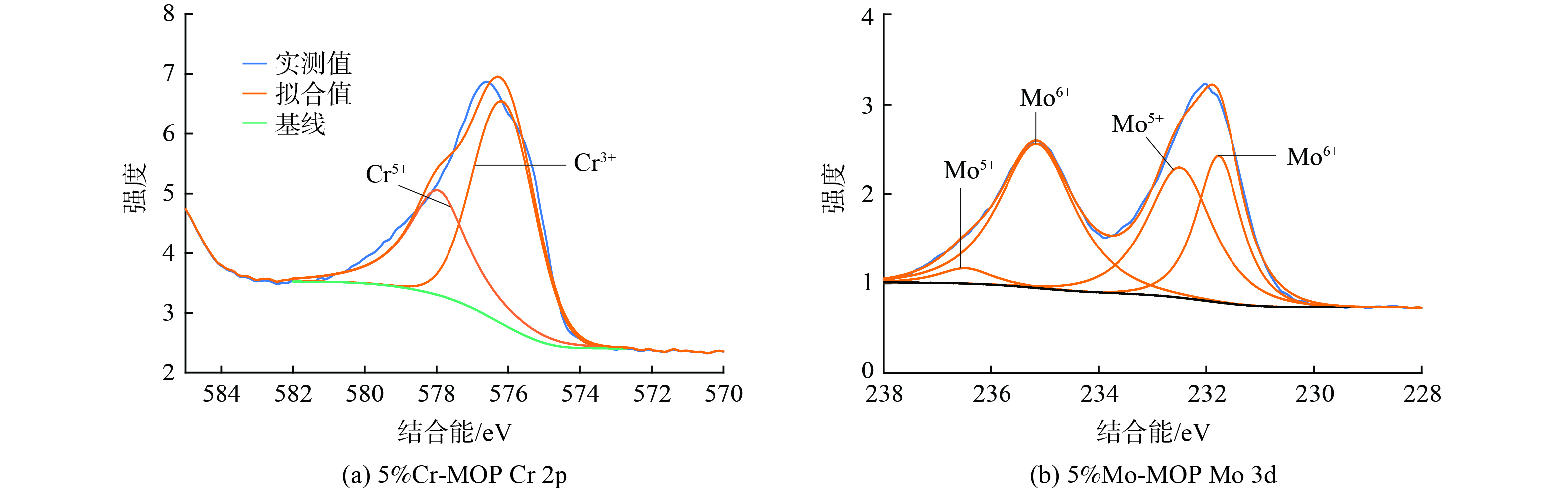
图6 (a) 显示了5%Cr-MOP表面的Cr (Cr 2p) XPS分析结果。Cr的电子结合能主要位于576.14eV (Cr3+) 和577.95eV (Cr5+) 处,这说明Cr主要以Cr3+和Cr5+形式存在。将Cr3+和Cr5+分峰分别积分计算出各自的峰面积,计算出Cr5+/Cr3+为0.47。XRD测试结果也表明,Cr主要以无定形Cr2O3形式物理附着于催化剂表面。Cr2O3为两性偏酸性化合物,有利于Cr负载MOP NH3吸附及脱氨活性的提升。
图6 (b) 为5%Mo-MOP表面的Mo (Mo 3d) XPS分析结果。通过分峰拟合获得4个电子结合能特征峰,其中232.49 eV和236.44eV归属于Mo5+,231.77eV和235.15eV归属于Mo6+。将各峰分别积分并计算出峰面积,得到Mo5+/Mo6+ = 0.53,说明Mo6+的相对含量比Mo5+高约1倍。Mo6+有更强的NH3吸附能力[10]。这与Mo改性促进MOP催化脱氨性能和NH3-TPD-MS测试表现一致。此外,Mo6+和钾能够形成多种形式的钾钼盐KxMoyOz[22],因此在XRD中可观察到K5Mo4O6特征峰。
-
1) 天然锰矿粉具有较高的催化脱硝和脱氨活性。Cr负载进一步大幅提升其NH3转化率,但对脱硝效率没有显著影响。负载的Cr主要以无定形Cr2O3物理附着于MOP表面,Cr与Mn之间没有明显的相互作用。2) Mo负载明显提升了MOP的NH3催化转化率,但提升幅度低于Cr负载。Mo负载增加表面酸性位点数量,促进NH3吸附能力。负载的Mo与MOP表面K反应生成钾钼盐结晶,占据了MOP表面的活性位点,并导致MOP表面Mn4+和Oα的相对含量降低。尽管如此,2%Mo负载仅对MOP在150~250 ℃时的脱硝效率有轻微抑制,但350~400 ℃却表现出显著促进作用。3) 负载Cr或负载少量Mo可大幅提升锰矿粉基催化剂的脱氨活性,同时对脱硝效率产生不值得关注的影响。价格低廉的MOP经Cr和Mo改性后将具有良好的脱氨性能,在大型垃圾焚烧设施使用时可将粉末态Cr和Mo负载MOP一次性喷入烟气,在减少NOx排放的同时降低非选择性催化还原 (SNCR) 脱硝引起的氨逃逸。
负载Cr和Mo锰矿粉基催化剂的脱硝脱氨性能
Denitration and deamination performance of manganese ore powder-based catalysts with Cr and Mo loading
-
摘要: 氨选择性催化还原 (NH3-SCR) 技术在焚烧烟气NOx减排控制中广泛应用。然而,目前广泛商业化使用的SCR催化剂价格昂贵且低温活性差。以价格低廉的天然锰矿粉 (MOP) 为载体,采用浸渍法制备了Cr和Mo负载改性MOP,在温度范围150~400 ℃,对比研究了MOP和负载改性MOP的脱硝和脱氨催化效率,并基于NH3-TPD-MS、FE-SEM-EDS、XRD和XPS对所制备的催化剂进行表征。结果表明:MOP具有较高的催化脱硝脱氨活性;Cr负载可大幅提升MOP的氨转化率,且对脱硝效率没有明显影响;Cr主要以无定形Cr2O3形式物理附着于MOP表面,Cr与Mn之间没有明显的相互作用。同时,Mo负载对MOP的氨催化转化也有明显促进作用,2%Mo负载仅轻微减少了MOP在150~250 ℃时的脱硝效率。在5%Mo改性MOP表面,酸性位点数量和NH3吸附能力显著提高,但Mo与K反应生成钾钼盐结晶,导致Mn4+和Oα的相对含量降低。该研究结果可为低温低尘尾部布设SCR的同时脱硝脱氨提供参考。Abstract: NH3-SCR technology is extensively used in control of NOx emission from incineration flue gas. However, in recent decades the widely adopted commercial SCR catalysts were expensive and their activity at lower temperature was decreased. In the experiment, the cheap natural manganese ore powder (MOP) was selected as the carrier, and by impregnation method the modified MOP with Cr and Mo was prepared, respectively. In the temperature range of 150~400 ℃, NO and NH3 conversion efficiency between MOP and modified MOP was compared, and based on NH3-TPD-MS, FE-SEM-EDS, XRD and XPS analysis the prepared catalysts were characterized. The results demonstrated that MOP had high catalytic de-NOx and ammonia removal activity. Cr loading on MOP significantly enhanced NH3 conversion performance, and had no significant effects on the denitration efficiency. Cr was mainly physically adsorbed on the surface of MOP as amorphous Cr2O3. There was no evident interactions occurred between Cr and Mn. Meanwhile, Mo loading also remarkably promoted catalytic deamination activity of MOP, and the denitration efficiency over MOP doped with 2% Mo was only slightly declined at 150~250 ℃. On the surface of 5% Mo-modified MOP, acid sites and NH3 adsorption capacity was notably increased, but crystal K5Mo4O6 was formed due to the reaction between Mo and K, leading to the lowered relative content of Mn4+ and adsorbed oxygen (Oα). In summary, the experiment results provided the important references for simultaneous denitration and deamination of SCR located at the downstream under the conditions of low dust and temperature.
-
Key words:
- manganese ore powder /
- SCR at lower temperature /
- denitration /
- deamination
-
-
[1] 孔杨, 张泽凯, 李浙飞, 等. 近十年来(2011-2021)钒基NH3-SCR催化剂的研究进展[J]. 工业催化, 2022, 30(12): 16-33. [2] 李泽清, 陈红萍, 魏永林. 低温钒基脱硝催化剂的研究进展[J]. 广州化工, 2022, 50(7): 15-18,35. [3] 张延兵. Mn-基低温脱硝催化剂制备、性能及在聚苯硫醚滤料上的应用[D]. 福州: 福州大学, 2017. [4] 盛雨佳. 锰基复合氧化物低温脱硝及氨氧化研究[D]. 合肥: 合肥工业大学, 2021. [5] JO SH, SHIN BK, SHIN MC, et al. Dispersion and valence state of MnO2/Ce(1- x )Zr x O2-TiO2 for low temperature NH3-SCR[J]. Catalysis Communications, 2014, 57: 134-137. doi: 10.1016/j.catcom.2014.08.014 [6] 鲁文质, 赵秀阁, 王辉, 等. NO的催化氧化[J]. 催化学报, 2000, 21(5): 423-427. [7] 李春晓, 李坚, 梁文俊, 等. Cr负载V2O5-WO3/TiO2催化剂的低温NH3-SCR脱硝活性[J]. 高等学校化学学报, 2019, 40(7): 1447-1455. [8] YANG R, HUANG H F, CHEN Y J, et al. Performance of Cr-doped vanadia/titania catalysts for low-temperature selective catalytic reduction of NO x with NH3[J]. Chinese Journal of Catalysis, 2015, 36(8): 1256-1262. doi: 10.1016/S1872-2067(15)60884-1 [9] YANG G, ZHAO HT, LUO X, et al. Promotion effect and mechanism of the addition of Mo on the enhanced low temperature SCR of NO x by NH3 over MnO x /γ-Al2O3 catalysts[J]. Applied Catalysis B:Environmental, 2019, 245: 743-752. doi: 10.1016/j.apcatb.2018.12.080 [10] 杜月瑶. 负载型二维钼基催化剂脱硝性能及抗碱金属中毒性能研究[D]. 厦门: 华侨大学, 2021. [11] LI LL, LI PX, TAN W, et al. Enhanced low-temperature NH3-SCR performance of CeTiO x catalyst via surface Mo modification[J]. Chinese Journal of Catalysis, 2020, 41(2): 364-374. doi: 10.1016/S1872-2067(19)63437-6 [12] LI J, CHANG H, MA L, et al. Low-temperature selective catalytic reduction of NO x with NH3 over metal oxide and zeolite catalysts-A review[J]. Catalysis Today, 2011, 175: 147-156. doi: 10.1016/j.cattod.2011.03.034 [13] 吴大旺, 张秋林, 林涛, 等. Fe对Mn/CeO2-TiO2催化剂低温NH3选择性催化还原NO的影响[J]. 无机材料学报, 2012, 27(5): 495-500. [14] SU Y, FAN B, WANG L, et al. MnO x supported on carbon nanotubes by different methods for the SCR of NO with NH3[J]. Catalysis Today, 2013, 201: 115-121. doi: 10.1016/j.cattod.2012.04.063 [15] MOUSAVI SM, NIAEI A, GÓMEZ MJI, et al. Characterization and activity of alkaline earth metals loaded CeO2-MO x (M=Mn, Fe) mixed oxides in catalytic reduction of NO[J]. Materials Chemistry and Physics, 2014, 143: 921-928. doi: 10.1016/j.matchemphys.2013.09.017 [16] 黄增斌, 李翠清, 王振, 等. 不同分子筛负载锰铈催化剂的低温NH3-SCR脱硝性能[J]. 燃料化学学报, 2016, 44(11): 1388-1393. [17] ZHU M H, LAI J-K, WACHS IE. Formation of N2O greenhouse gas during SCR of NO with NH3 by supported vanadium oxide catalysts[J]. Applied Catalysis B:Environmental, 2018, 224: 836-840. doi: 10.1016/j.apcatb.2017.11.029 [18] ZHENG HL, SONG WY, ZHOU Y, et al. Mechanistic study of selective catalytic reduction of NO x with NH3 over Mn-TiO2: a combination of experimental and DFT study[J]. Journal of Physical Chemistry C, 2017, 121(36): 19859-19871. doi: 10.1021/acs.jpcc.7b06715 [19] QI G, YANG RT. Low-temperature selective catalytic reduction of NO with NH3 over iron and manganese oxides supported on titania[J]. Applied Catalysis B Environmental, 2003, 44(3): 217-225. doi: 10.1016/S0926-3373(03)00100-0 [20] KAPTEIJN F, SINGOREDJO L, ANDREINI A, et al. Activity and selectivity of pure manganese oxides in the selective catalytic reduction of nitric oxide with ammonia[J]. Applied Catalysis B:Environmental, 1994, 3: 173-189. doi: 10.1016/0926-3373(93)E0034-9 [21] 张先龙, 胡晓芮, 刘仕雯, 等. 锰基累托石低温NH3-SCR催化剂的制备方法[J]. 环境化学, 2022, 41(3): 1043-1051. [22] 姜明, 卞国柱, 姜伟, 等. 几种氧化物载体上钾相作用物种的结构形式[J]. 化学物理学报, 1993, 6(4): 370-376. -




 下载:
下载: