-
自然界中的铬主要以Cr(III)和Cr(VI)的形式存在[1-2],Cr(VI)化合物不能自然降解,其毒性是Cr(III)的100多倍[3]。水体中的Cr(VI)会导致水生生物死亡,有较高的致癌性和致畸性[4]。铬被广泛用于多种行业中,包括电镀、制革、金属加工、冶金、玻璃制造、木材防腐剂和陶瓷等,在生产过程都会产生大量含铬废水。我国《污水综合排放标准》(GB/T 8979-1996)中将Cr(VI)列为第一类污染物,其最高允许排放质量浓度为0.5 mg·L−1[5]。
目前,针对废水中Cr(VI)常见处理方法有化学沉淀法、离子交换法、生物法和吸附法等[6-7]。与其他几种处理方法相比,吸附法设备简单、方便操作、环境友好,成本较低[8]。对于吸附法来说,吸附剂的选择尤为重要。常用Cr(VI)的吸附剂主要有活性炭、分子筛、生物炭和粉煤灰等[9]。在这些吸附剂中,生物炭是一种高度芳香化难降解的固态物质,其表面含有羧基、酚羟基、羰基等官能团,具有较大的比表面积和孔隙度,且其种类丰富,成本低廉,还可以实现固体废物再利用,因此近年来被广泛应用。一般来说,生物炭吸附Cr(VI)的机理主要包括物理吸附、静电作用、离子交换作用以及与生物炭上的矿物质或官能团(羧基、羟基、酚醇等)作用生成沉淀或络合物等[10]。然而,天然生物质材料碳化后对Cr(VI)的吸附能力较差,为了提高其应用价值及适用范围,需对生物炭进行改性。常见的改性方法包括热解前原料改性,热解后生物炭成品改性;从改性原理可分为化学改性和物理改性等[11]。
化学改性是一种有效调控生物炭理化性质的方法,可以通过活化剂刻蚀炭颗粒的内部结构,发生一系列的交联缩聚反应形成丰富的孔结构,是提高生物炭性能的有效途径。常用的活化剂有氯化锌、磷酸和氢氧化钾等[12]。其中,磷酸是三元中强酸,具有脱水和氧化性,进入原料内部后可以与原料中的无机物生成磷酸盐,经酸洗后能够去除磷酸盐从而达到扩孔的目的,从而增加材料的孔结构和比表面积。此外,磷酸活化温度一般在550 ℃以下,能耗低,实验操作危险性较KOH更低。
慈竹在我国西南地区广泛生长,生长速度快,产量大。选择干枯衰老的慈竹作为生物质原料可以充分实现废弃物的再利用。磷酸活化法与其他的材料改性方法相比,过程简单,成本低,且不会引入有毒有害元素,在提高除铬能力的同时,也保证了水环境安全,可以避免对水体造成二次污染。因此,本研究选用慈竹作为生物质原料,经磷酸活化后热解得到生物炭,考察了其对废水中的Cr(VI)的吸附性能及吸附机理。与已经被报道的铬吸附剂相比,本研究制备的吸附剂无论从去除效果、经济成本、安全性、稳定性还是再生性等方面考虑,均具有较强的竞争力和较好的应用前景。
-
重铬酸钾(K2Cr2O7)购自阿拉丁试剂(上海)有限公司;磷酸(H3PO4,85%)、盐酸(HCl,36%~38%)、氢氧化钠(NaOH)、乙醇(C2H6O)、硝酸(HNO3)等试剂购自国药化工试剂有限公司。所有试剂均为分析纯级,无需进一步纯化即可直接使用。
-
1)吸附剂的制备。将慈竹用超纯水冲洗以除去表面杂质,于110 ℃下烘干至恒重。将烘干后的慈竹用研磨机磨至50目以下,用H3PO4作活化剂,固液比(活化剂体积:生物质质量)为2 mL·g−1,搅拌均匀后活化24 h。随后在N2氛围下,将活化后的材料550 ℃热解1.5 h。热解完成后用1 mol·L−1的HCl冲洗以除去灰分等杂质[13],之后再用超纯水洗至中性,烘干待用。为了进行对比,制备了未经磷酸活化的慈竹生物炭,处理步骤同上所述。未活化的慈竹生物炭和经磷酸活化后制得的慈竹生物炭分别命名为SAB和ASAB。
2)吸附剂的表征。SAB和ASAB的比表面积使用ASAP 2020分析仪(Micromeritics, USA)进行分析,并使用Brunauer–Emmet–Teller(BET)模型对相关参数进行计算;采用X射线衍射仪(XRD, D8 Advance,Bruker,Germany)对晶体结构进行测定;利用扫描电镜(SEM,ZEISS SIGMA, Germany)和能谱分析(EDX)对吸附剂的表面形貌进行观察,并分析元素组成;利用傅里叶变换红外光谱(FTIR,Thermo iS10,USA)对官能团进行分析。利用X射线光电子能谱(XPS, Thermo Scientific K-Alpha)测定吸附剂表面的元素价态,C1s位于284.6 eV处的峰值被用作空白来校正光谱值。
3)吸附Cr(VI)实验。通过批量吸附实验评价慈竹生物炭对Cr(VI)的吸附性能。先将2.829 g重铬酸钾(K2Cr2O7)溶于1 000 mL的超纯水中,配制成质量浓度为1 000 mg·L−1的Cr(VI)标准溶液,密封待用。除非另有说明,否则所有的实验中吸附剂与溶液的固液比均为1 g·L−1;混合溶液在25 ℃恒温摇床(ZHWY 334, Zhicheng,中国)下以150 r·min−1振荡24 h。反应结束后,用0.45 μm的PTFE针式过滤器将固液分离,用紫外-可见光分光光度计测定溶液中的Cr(VI)质量浓度。所有的实验数据都经过3次重复实验,最终的结果为平均值。
探究了pH(1~8),反应时间(5、10、20、40、80、160、320、640和1 280 min)、溶液中Cr(VI)的质量浓度(50、100、200、300、400、500和600 mg·L−1)、反应温度(25、35和45 ℃)和共存阴离子(Cl−、NO3−、SO42−和H2PO4−)对Cr(VI)吸附效果的影响。在吸附-脱附实验中,选用1 mol·L−1的NaOH作为脱附剂,固液比为1 g·L−1,时间为8 h。Cr(VI)的去除率和吸附量分别根据式(1)和式(2)进行计算。用吸附等温线模型(Langmuir(式(3))和Freundlich模型(式(4))来拟合ASAB吸附Cr(VI)的过程。并利用准一级动力学方程(式(5))和准二级动力学方程(式(6))对此吸附过程进行了动力学研究。
式中:
η 为去除率,%;C0为Cr(VI)的初始质量浓度,mg·L−1;Ce为平衡质量浓度,mg·L-1; V为溶液体积,L;m为吸附剂质量,g;qe为吸附量,mg·g-1。qmax为最大吸附量,mg·g−1;KL为Langmuir模型的平衡常数;1n 为异质性因子。qt为反应t时间时的吸附量,mg·g−1;k1为准一阶模型的速率常数,min−1;k2为准二阶模型的速率常数,g·(mg·min)−1;t为反应时间,min。 -
如图1(a)~(b)所示,SAB和ASAB的氮气吸脱附曲线均出现了滞后环,表明2种吸附剂均存在介孔结构。结合表1可以看出,SAB中主要以微孔结构为主,而经磷酸活化后的ASAB则以介孔结构为主。ASAB的比表面积较SAB增大了514.02 m2·g−1。这可能是因为在活化过程中,磷酸与原材料中无机物生成磷酸盐,从而使原料膨胀,增大了碳微晶的距离[5]。制备完成的生物炭通过使用HCl洗涤可以除去过量的磷酸盐,这样可以得到较发达的孔结构,大大增加吸附剂表面的活性位点[14],有助于Cr(VI)在ASAB上的吸附。
SEM表征结果(图2(a)~(b)) 表明,ASAB的内部结构疏松,孔洞丰富。这与磷酸活化有关,生物质中大部分有机挥发物经磷酸活化后发生演化,导致生物炭表面发生破裂。此外,在焙烧过程中,磷酸的蒸发会在材料内部形成空腔结构。吸附Cr后的ASAB形貌没有发生明显的变化,但通过mapping图(图2(d)~(f))可以得出,ASAB表明均匀分布着Cr元素,证实Cr被吸附到生物炭上。
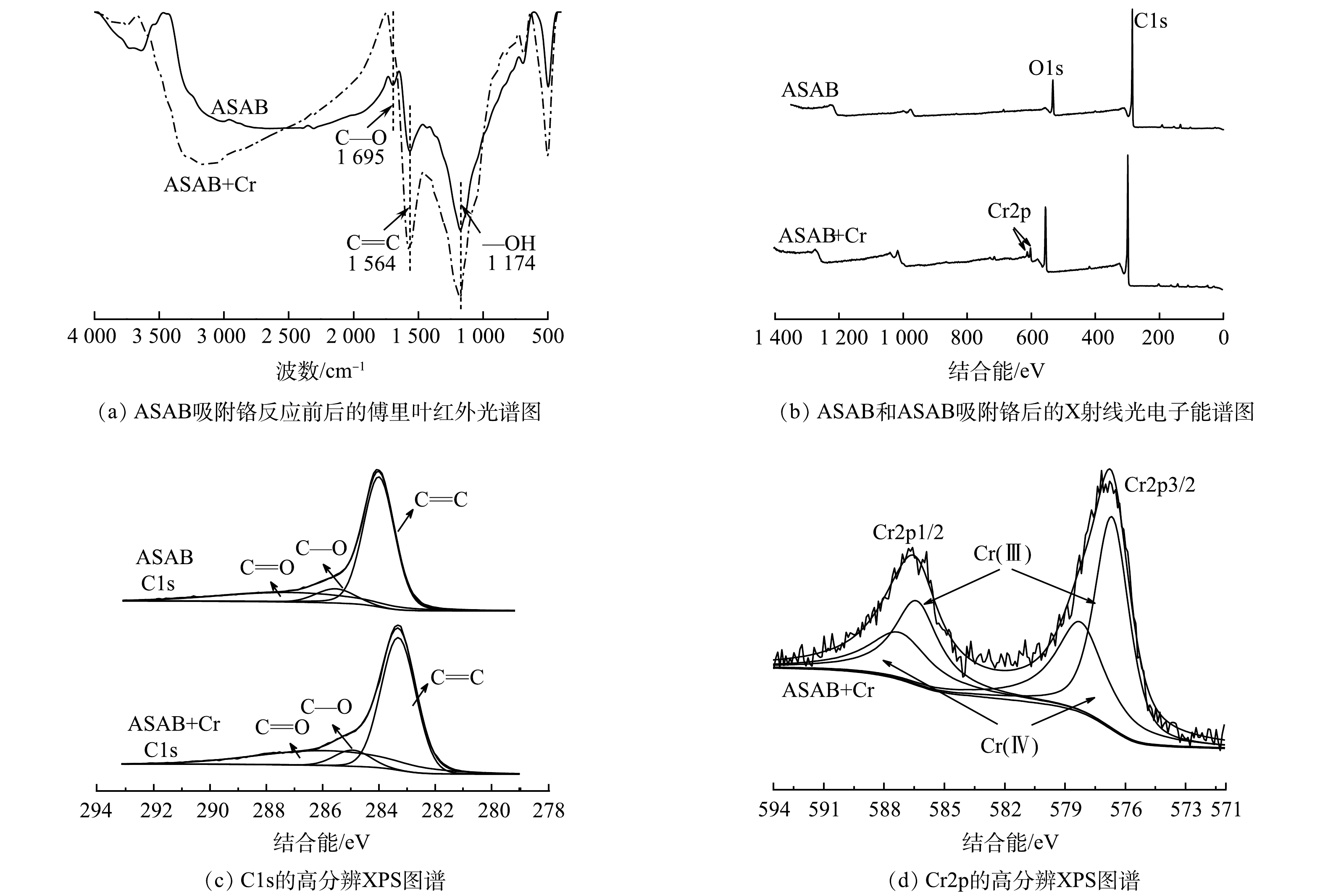
由图3(a)可以看出,在3 500~3 200 cm−1内均出现1个较宽的吸收峰,代表着羟基—OH的伸缩振动峰。在1 564 cm−1处观察到的吸收谱带归属于生物炭中的C=C。吸附Cr(VI)后的ASAB在1 695 cm−1处的C—O消失,说明其参与了吸附反应。
使用X射线光电子能谱对ASAB和ASAB+Cr(VI)进行了表面分析(图3(b)~(d))。图3(c)是C1s的高分辨XPS图谱,其中,C1s能拟合成C—C,C—O(酚、醇或醚),和C=O(羰基)3种基团。吸附Cr之后,ASAB上C—O官能团的峰面积从8.9%降低到7.8%,而C=O增加,这是因为在吸附过程中,C—O官能团被Cr(VI)氧化。由图3(d)可见,Cr(III)和Cr(VI)物种同时存在于吸附剂表面。位于576.7 eV和586.4 eV结合能处的峰对应的是Cr(III),在578.2 eV和587.2 eV处的低强度峰对应的是Cr(VI)。在Cr2p1/2轨道中,Cr(III)和Cr(VI)的占比分别为57.7%和42.3%;在Cr2p3/2轨道中,Cr(III)和Cr(VI)的占比分别为56.3%和43.7%。因此,与ASAB结合的Cr主要以Cr(III)形式存在,这说明绝大多数的Cr(VI)在与ASAB作用后被还原为Cr(III)。
-
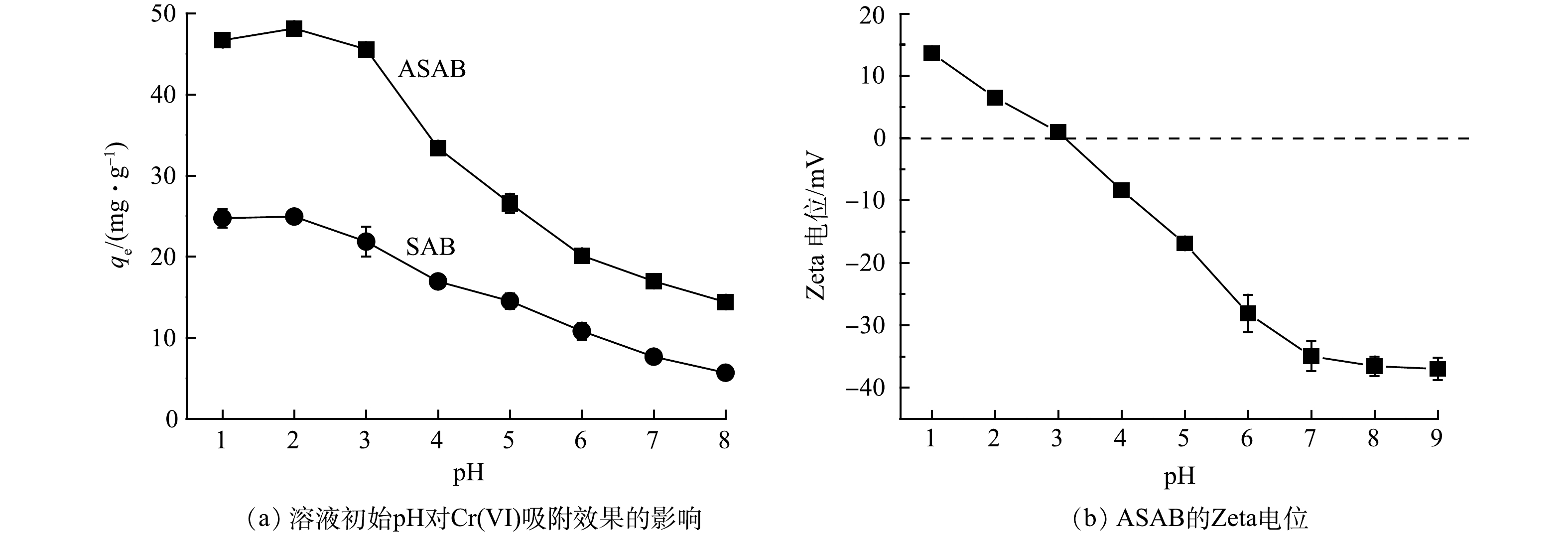
1)溶液初始pH对吸附效果的影响。溶液的初始pH一般会影响吸附剂的表面电荷,进而影响其对Cr(VI)的吸附效果。由图4(a)中可以看出,ASAB对Cr(VI)的去除效果整体要好于SAB。酸性条件更有利于Cr(VI)的吸附,当溶液的初始pH=2时,ASAB对Cr(VI)的吸附量是48.1 mg·g−1,去除率可以达到96.2%。随着pH的增加,吸附效果逐渐变差,这主要是因为当pH较低时,溶液中存在大量的质子,有助于将Cr(VI)还原为Cr(III),导致Cr(VI)的含量降低。随着pH的增加,溶液中OH−逐渐增加,从而和阴离子CrO42−形成竞争吸附,导致Cr(VI)去除率降低。此外,吸附过程还涉及到静电引力作用,通过Zeta电位测试得出ASAB的表面零电荷点(pHPZC=3.1)(图4(b)),因此,当溶液的pH<pHPZC时,吸附剂的表面被质子化而呈现正电性,从而与带负电的铬离子发生静电引力作用,促进吸附剂对铬的吸附[15],当溶液的pH>pHPZC时,Cr(VI)的吸附会受到静电斥力的影响。综上所述,在低pH条件下ASAB对Cr(VI)的去除效果更好。当pH分别为2和3时,ASAB对Cr(VI)的吸附量仅相差2.6 mg·g−1,而对于实际电镀废水,pH更接近于3,因此,后续实验均在pH=3的条件下进行。
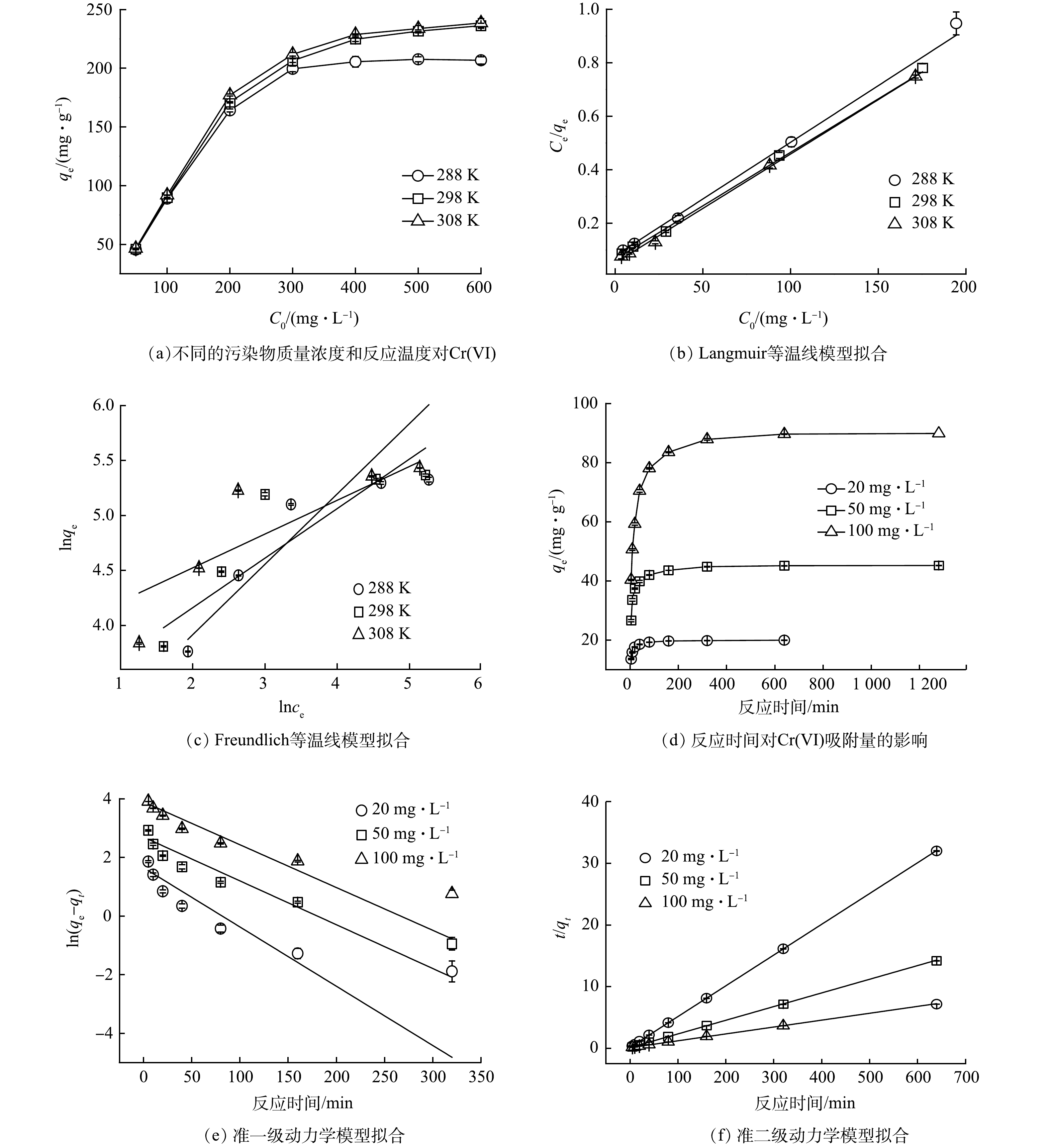
2)Cr(VI)的初始质量浓度和吸附等温线。保持其他的反应条件一致,当溶液中Cr(VI)的初始质量浓度不同时,所对应的吸附量也不相同。如图4(a)所示,随着Cr(VI)初始质量浓度的增加,ASAB对Cr(VI)的吸附量也逐渐增加。这是因为污染物的质量浓度越高,溶液与ASAB表面的质量浓度差越大,传质速率提高,导致与活性位点的碰撞概率增高,吸附量也随之增加,直至达到吸附平衡。
由图5(a)可以看出,当溶液中Cr(VI)的初始质量浓度增加到600 mg·L−1时,在不同的温度下,吸附反应均达到平衡状态。在室温25 ℃时,ASAB对Cr(VI)的最大吸附量为236.2 mg·g−1。拟合结果如图5(b)~(c)所示,模型参数如表2所示。通过Langmuir模型拟合出的参数R2均高于Freundlich模型,且由Langmuir模型拟合出的最大吸附量与实际测量值接近。由此可见,Langmuir模型更符合ASAB对Cr(VI)的吸附过程,说明ASAB对Cr(VI)的吸附是单层吸附,分子之间没有相互作用[16]。
3)反应时间和吸附动力学。由图5(d)可以看出,吸附过程大致分为2个阶段,在0~80 min内,吸附量迅速增加,随后吸附变缓慢,吸附量增加不明显,直至达到吸附平衡状态。这是因为在反应初始阶段,ASAB的吸附位点很多,随着反应的进行,吸附位点变少,导致反应速率也变慢,最终达到饱和状态。
吸附动力学模型能够反映吸附剂的吸附速率。由表3可以看出,由准二级动力学模型拟合出的R2都能达到0.999,且由准二级动力学模型拟合出的理论吸附量与实际吸附量更为接近。因此,准二级动力学模型更适合于描述ASAB对Cr(VI)的吸附过程,说明吸附速率与吸附剂上未被占据的吸附位点的平方成正比,化学吸附占主导作用[16]。
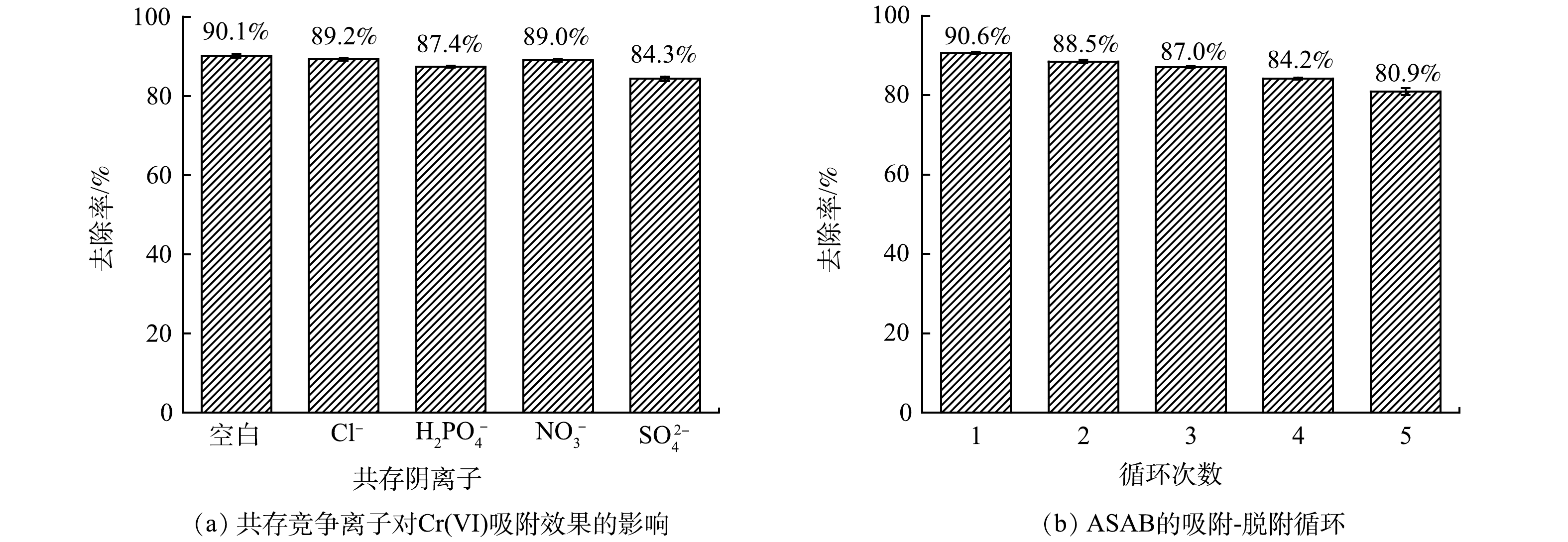
4)阴离子的竞争吸附行为。在实际铬废水中,还可能含有NO3−、H2PO4−、SO42−、Cl−等常见的阴离子,其会和带负电的Cr(VI)竞争吸附位点,从而抑制吸附剂对Cr(VI)的吸附。本文对NO3−(KNO3)、H2PO4−(KH2PO4)、SO42−(K2SO4)、Cl−(KCl)这几种阴离子和Cr(VI)在二元共存条件下的竞争吸附进行了研究,每组实验中,Cr(VI)和竞争离子的质量浓度均为50 mg·L-1。结果如图6(a)所示。当溶液中没有竞争离子时,ASAB对Cr(VI)的去除率是90.1%,而在Cl−、NO3−、H2PO4−、SO42−这4种竞争离子存在条件下,Cr(VI)的去除率分别为89.2%、89.0%、87.4%、84.3%。可以看出,Cl−、NO3−和H2PO4−的存在对Cr(VI)的去除影响较小。这可能是因为3者是单价阴离子,价态低于铬离子,故其竞争能力较弱[5]。然而,SO42−对Cr(VI)的去除影响较大,这可能是因为它是多价态阴离子,与Cr(VI)的竞争能力更强一些。
5)吸附剂的吸附-脱附。吸附剂的重复利用性是衡量其是否能得到广泛应用的重要指标,因为吸附剂的使用次数越多,经济成本越低。采用1 mol·L−1的NaOH作为脱附剂,对吸附Cr(VI)后的ASAB进行洗脱研究。如图6(b)所示,经过5次吸附-脱附后,ASAB仍能去除溶液中80.9%的Cr(VI)。在碱性条件下OH−很多,这使得ASAB表面带负电,从而可以与被吸附的Cr(VI)产生静电相斥作用而把Cr(VI)重新释放到溶液中。在吸附-脱附循环过程中,Cr(VI)的去除率随循环次数的增加而降低,可能是因为解吸剂的应用削弱了吸附剂上某些活性位点的活性。综上所述,ASAB具有良好的解吸再生能力。
6)经济成本分析。吸附剂的经济成本是其是否能广泛应用于实际工程的重要考虑因素。生物炭是在有限的氧气条件下对生物质进行热处理而产生的一种碳材料,原材料非常丰富,经济成本低,而且还可以实现废物再利用。本研究中选用的吸附剂是用慈竹制备的生物炭,来源广泛,价格低廉,而且制备过程简便且易实现。通过与同类型的吸附剂进行比较,由表4可以看出,本研究中制备的ASAB对铬的去除率高,吸附量可以达到236.2 mg·g−1,高于XU等[17]等制备的活性炭球的最大吸附量(230.2 mg·g−1),因此,ASAB具有较大的竞争力,但成本却远低于活性炭球。因此,本研究中制备的ASAB具有潜在的应用价值。
-
ASAB对溶液中Cr(VI)的吸附主要涉及到以下3个过程:第一,经磷酸活化后,生物炭的比表面积增大,从而使吸附污染物的活性位点数量大大增加,此外也增加了吸附剂表面的含氧官能团;第二,当溶液pH低于ASAB的Zeta电位时,ASAB表面会被质子化带正电,从而与带负电的Cr(VI)之间产生静电引力作用来实现对铬的去除;第三,XPS表征结果表明,吸附Cr后ASAB表面的C=O基团数量增加,说明部分C—O被氧化成了C=O,而50%~60%的Cr以Cr(III)的形式存在,则说明发生了氧化还原反应,部分Cr(VI)被还原成Cr(III)。综上所述,ASAB对Cr(VI)的吸附包括静电吸引和氧化还原作用。
-
1)本研究以慈竹为生物质原料,用磷酸对其进行活化,经热解后制得ASAB。在pH为3时,ASAB对Cr(VI)的吸附量最高,为236.2 mg·g−1。
2)等温线和动力学研究分别表明,ASAB对Cr(VI)的吸附是单层吸附,分子之间没有相互作用,且化学吸附占主导作用。整个吸附过程涉及静电吸引作用和氧化还原作用等。
3)溶液中的常见阴离子对Cr(VI)的竞争程度依次为Cl−<NO3−<H2PO4−<SO42−。
4)采用1 mol·L-1的NaOH做脱附剂,经过5次吸附-脱附循环后,ASAB对Cr(VI)的去除率依然可达80.9%,表明吸附剂具有良好的再生性能。
慈竹生物炭对水溶液中Cr(VI)的吸附性能
Adsorption performance of sinocalamus affinis biochar towards Cr(VI) in aqueous solution
-
摘要: 以慈竹(sinocalamus affinis, SA)为原料,用磷酸对其进行活化,后经热解得到活化生物炭(activated sinocalamus affinis biochar, ASAB),用来吸附水溶液中的Cr(VI)。当溶液的初始pH为3时,Cr(VI)的初始质量浓度为20 mg·L−1,吸附剂投加量为1g·L−1时,Cr(VI)去除率高达99.8%,剩余溶液中Cr(VI)的质量浓度低于废水排放标准(0.5 mg·L−1)。保持其他条件不变,改变Cr(VI)初始浓度,吸附剂的最大吸附容量可达236.2 mg·g−1。以上结果均说明ASAB对废水中的Cr(VI)具有良好的吸附效果。采用SEM、BET、FTIR、XPS等表征方法对活化前、后的慈竹生物炭的化学结构和物理组成进行了表征。ASAB的比表面积是844.45 m2·g−1,约为SAB(sinocalamus affinis biochar)的2.6倍,较高的比表面积可以提供更多的活性位点。本研究中,ASAB的除铬的机制包括静电作用和氧化还原作用。经过5个吸附-脱附循环后,ASAB对Cr(VI)的吸附效率依然可以达到80.9%。以上结果表明,作为1种高效的Cr(VI)吸附剂,ASAB可以用于处理废水中的Cr(VI)。Abstract: In this study, sinocalamus affinis (SA) was used as raw material to prepare biochar (ASAB) through activation with phosphoric acid and pyrolysis. The ASAB was used to adsorb Cr(VI) from aqueous solution. At the initial solution pH of 3, the initial Cr(VI) mass concentration of 20 mg·L−1 and the adsorbent dosage of 1 g·L−1, the removal rate of Cr(VI) was as high as 99.8%, and the remaining mass concentration of Cr(VI) in the solution was lower than the wastewater discharge standard (0.5 mg·L−1). When only the initial concentration of Cr(VI) changed and other conditions maintained stable, the maximum adsorption capacity of the ASAB could reach 236.2 mg·g−1. All of these results indicated that the ASAB had a good adsorption effect of Cr(VI) in wastewater. The chemical structure and physical composition of the adsorbent were characterized by SEM, BET, FTIR, and XPS. The specific surface area of the ASAB was 844.45 m2·g−1, which was about 1.6 times higher than that of the SAB(sinocalamus affinis biochar), and the higher specific surface area could provide more active sites. The mechanism of chromium removal by the ASAB included electrostatic and redox actions. After five adsorption-desorption cycles, the adsorption efficiency of the ASAB towards Cr(VI) could still maintain 80.9%. In conclusion, the ASAB is an efficient adsorbent and can be used to treat Cr(VI) in wastewater.
-
Key words:
- sinocalamus affinis biochar /
- Cr(VI) /
- adsorption performance /
- adsorption mechanism /
- kinetic study
-
铅是有毒的环境污染物,被世界卫生组织列为十大危害公共卫生安全的化学物质之一. 环境铅污染主要来源于工业生产和应用. 尽管从20世纪末开始,全世界各个国家先后禁止使用含铅汽油以及含铅钢管和硬聚氯乙烯(PCV)塑料管材,但是由于社会工业的快速发展,铅的使用量仍然逐年增加. 根据2021年3月Statista研究部门的数据统计,全球铅消费量从2004年至2020年由7297万吨增加至11545万吨[1],增长了58.2%,其主要应用领域是铅蓄电池生产,是铅总消费量的80%左右[2]. 铅蓄电池的生产及回收过程使得铅以蒸气和烟尘形式逸散在空气中,并通过呼吸摄入方式进入人体肺部,进一步进入血液循环系统. 当血液中铅含量超过正常范围(400 mg·L−1)[3],可引起铅中毒.
铅中毒已被认为是重大的公共健康问题,尤其在发展中国家. 铅可以随着血液循环转移至身体全身的各个组织器官[4-5],引起神经系统、造血系统、肾脏系统、心血管系统、生殖系统以及骨骼系统等病变[6]. 大脑的中枢神经系统是铅毒性最敏感的器官,与神经退行性疾病(比如阿尔兹海默症)的发生密切相关[7]. 铅暴露对儿童的大脑发育系统具有更显著的毒性效应. 大量研究表明,儿童铅中毒可引起大脑发育障碍、认知能力和智商降低等症状,且这种毒性效应具有持久性甚至永久性[8-9]. 铅对生殖系统具有毒性损伤作用,对男性常见的危害包括降低精子运动性能、减少精子数量、损伤染色体,导致不育、前列腺功能异常和血清睾酮变化等,对女性的危害包括患不孕症、流产、胎膜早破、妊娠高血压和早产等[10]. 此外,孕期铅暴露可直接影响胎儿的正常发育,可能会改变大脑中髓鞘形成的时间,对儿童后期的学习或其他认知功能产生长期的有害影响[11-12]. 综上,铅中毒可严重危害人体健康.
驱铅治疗可以缓解铅中毒的损伤效应. 依地酸二钠钙(CaNa2EDTA)是目前常用的驱铅治疗药物,其原理是利用EDTA与铅离子发生络合反应,形成稳定的水溶性依地酸铅,从而通过肾脏系统随尿液排出[13]. 但是目前临床上铅中毒患者在治疗过程中铅的脱除效率较低,血液中仅部分铅通过尿液排出[14]. 此外,该方法存在血液中铅(血铅)浓度回升的现象,即在治疗初期血铅水平降低,但是一段时间后血铅浓度回升,导致治疗效果较差. 影响铅中毒患者治疗效果的因素来自多方面,可能包括用药因素和个体的生理状态等,具体的影响因素目前还有待研究.
本文以使用CaNa2EDTA进行驱铅治疗的铅中毒患者作为研究对象,分别在用药前、用药后24 h、用药后72 h对全血、血浆以及尿液中的铅含量进行检测,同时检测尿液的pH值,分析铅中毒患者在治疗过程中,血铅、血浆铅和尿铅的变化趋势以及他们的铅脱除率,进一步分析不同体系中铅脱除率与尿液pH值的相关性,进而探究驱铅治疗效果的影响因素.
1. 材料与方法(Materials and methods)
1.1 铅中毒患者的治疗方案及信息统计
本试验共收集24名来中国广东惠州市的铅中毒患者。患者在惠州市职业病防治院接受驱铅治疗,治疗方法为静脉注射CaNa2EDTA,同时口服补充维生素C,其中有3名患者接受了两次驱铅治疗. 分别在患者用药前24 h、用药后24 h、用药后72 h收集血液和尿液样品,共收集了81份. 以上实验由惠州市职业病防治院的伦理委员会批准,并且征得所有患者的同意. 铅中毒患者的信息见表1.
表 1 铅中毒患者的统计数据Table 1. Demographic data of the participants年龄Age 身体质量指数/(kg·m−2)Body mass index 男Male 女Female 40.2 ± 7.2 22.4 ± 2.3 19 (79.2%) 5 (20.8%) 1.2 实验仪器和试剂
实验仪器:离心机(安徽中科中佳科学仪器有限公司);电感耦合等离子体质谱(ICP-MS 8800;安捷伦科技有限公司);石墨烯微波消解仪(JRY-X350;湖南金蓉园仪器设备有限公司);pH测量仪(SevenCompact;梅特勒托利多);超低温冰箱(赛默飞世尔科技公司).
试剂:65%浓硝酸(EMPARTA;德国Merck);30%过氧化氢(优级纯;国药集团化学试剂有限公司);铅标准溶液(Multi-element calibration standard 2A;美国安捷伦科技有限公司);内标溶液(ICP-MS Internal Std Mix;安捷伦科技有限公司).
1.3 样品前处理方法
血液样品:使用肝素钠抗凝管采集铅中毒患者的血液样品(2 mL),常温放置(25 ℃左右),6 h内进行以下前处理:取0.5 mL全血至1.5 mL离心管,用于全血中总铅含量检测,保存于-80 ℃冰箱;对剩余全血样品(1.5 mL)进行离心处理(500 r·min−1,10 min),转移上层血浆至新的1.5 mL离心管中,再次离心(3000 r·min−1,10 min),将上层血浆转移至新的1.5 mL离心管,保存于-80 ℃冰箱,用于血浆中铅含量的检测.
尿液样品:将收集的尿液样品转移至15 mL离心管中,直接冻存于-80 ℃冰箱,用于尿液中铅含量的检测和尿液pH值检测.
1.4 铅含量测定
全血和血浆样本:取200 μL样品,加200 μL浓硝酸消解和60 μL 30%的过氧化氢,95 ℃加热2 h,冷却后使用去离子水定容至10 mL,使用滤网(0.22 μm)进行过滤处理,最后使用ICP-MS测定铅浓度.
尿液样本:使用含2%硝酸的水溶液直接将尿液样品稀释10倍,再使用滤网(0.22 μm)进行过滤处理,最后使用ICP-MS测定铅浓度.
样品检测过程中采用方法空白、空白加标、平行测试来控制测试质量,该方法测定铅浓度的回收率为111.5% ± 1.4%.
1.5 尿液pH值检测
pH校准:使用3种pH标准缓冲溶液(pH4.01、pH7.00、pH9.21)依次进行pH校准,并保存校准结果. 校正不同标准缓冲溶液之间,使用去离子水冲洗电极,并用无尘纸吸取电极表面残留的水分.
尿液样品的pH测定:使用去离子水冲洗电极,使用无尘纸吸取电极表面残留的水分,将电极放入样品中,按读数键开始测量,在读数稳定后,记录数据;随后进行下一个样品的测定. 按照此方法测定所有尿液样品的pH值.
1.6 数据分析和绘图
使用Excel(Microsoft Office 2016)进行数据处理和分析,使用Graphpad软件(GraphPad Prism 8)进行图表绘制,具有显著性差异结果的标准是P < 0.05.
2. 结果与讨论(Results and discussion)
2.1 铅浓度的变化趋势
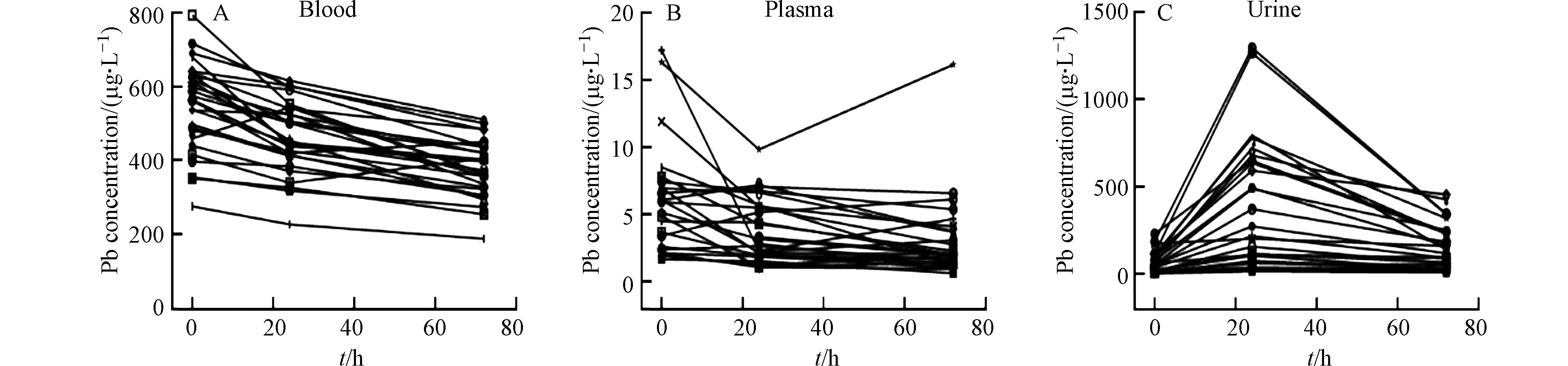
铅中毒患者血铅含量较高,通过使用驱铅药物,可以促进血铅通过肾脏系统随尿液排出体外,从而降低人体血液系统中的铅含量. 本次铅中毒患者在治疗过程中,血铅浓度呈现明显的下降趋势(图1A). 铅中毒患者的血铅含量分布范围广,在用药前其浓度范围为276.00—727.29 μg·L−1;用药后24 h和72 h,血铅浓度范围分别为227.20—615.20 μg·L−1和188.00—510.40 μg·L−1. 从用药前至用药后24 h,血铅浓度显著降低(P < 0.01);从用药后24 h至用药后72 h,血铅浓度也显著下降(P < 0.001). 大部分病人的血浆铅浓度也具有明显的连续降低趋势,而少部分病人的血浆铅浓度先降低后升高(图1B). 在用药前、用药后24 h和用药后72 h,血浆铅的浓度范围分别为1.76—17.18 μg·L−1、1.10—9.84 μg·L−1和0.67—16.13 μg·L−1. 在驱铅治疗的不同阶段血浆铅的变化趋势不具备统一性,这可能与不同病人具有个体差异有关. 此外,血浆铅的浓度是血铅的1.2%左右,说明在血液系统中仅有少部分铅存在于血浆中,而大部分铅赋存于血细胞中,这与之前相关的研究结果是一致的[15]. 几乎所有铅中毒患者的尿铅浓度,在治疗过程中均呈现先升高后降低的趋势(图1C). 在用药前、用药后24 h和用药后72 h,尿铅的浓度范围分别为2.92—230.04 μg·L−1、17.68—1294.26 μg·L−1和10.13—454.91 μg·L−1. 从用药前至用药后24 h,尿铅浓度显著升高(P < 0.001),从用药后24 h至用药后72 h,尿铅浓度显著下降(P < 0.01),说明在用药后24 h,通过尿液排出体外的铅含量比在用药后72 h高. 这可能是因为在用药前期,CaNa2EDTA的浓度较高,其通过络合反应结合的铅的含量较高. CaNa2EDTA是乙二胺四酸(EDTA)的二钠钙盐,可以与多种金属结合生成稳定的可溶络合物. 上述结果显示使用CaNa2EDTA治疗铅中毒患者,血铅和血浆铅的浓度均逐渐降低,同时尿铅含量增加.
2.2 铅脱除率对比
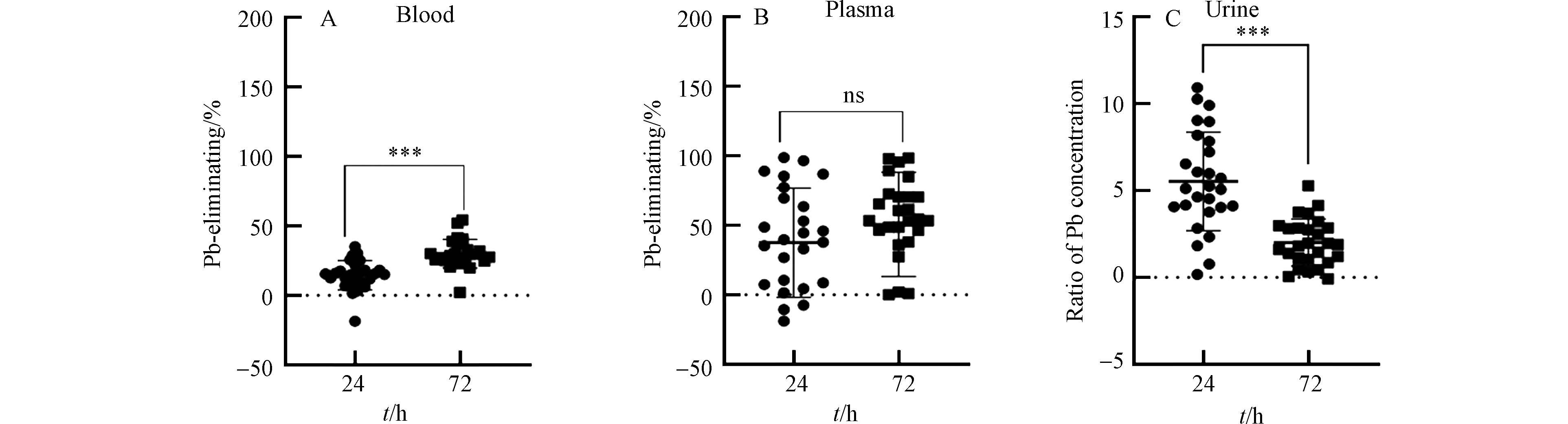
尽管用药具有一定的驱铅效果,但是不同病人的铅脱除率参差不齐,且在不同治疗阶段的铅脱除率不同. 本文定义铅脱除率为相对于用药前的铅浓度降低的百分比,定义铅排出比例为用药后尿铅浓度与用药前尿铅浓度的比值. 在用药后24 h和72 h,血铅中的脱除率分别为(15.92 ± 8.30)%和(30.02 ± 10.32)%,且具有显著差异(图2A). 在用药后24 h和72 h,血浆铅中的脱除率分别为(37.51 ± 39.27)%和(50.62 ± 37.36)%(图2B). 在用药后24 h和72 h,铅的排出比例分别为5.52 ± 2.82和2.01 ± 1.34,且具有显著差异(图2C). 上述结果显示,铅中毒患者治疗过程中,血液系统中铅的整体脱除效果较差,最高的平均脱除率只有50.62%. 铅脱除率可以直接反应铅中毒患者的治疗效果. 上述结果说明,铅中毒患者的驱铅治疗效果较差. 这可能是因为大部分铅中毒患者属于慢性铅中毒,超过90%铅赋存于骨骼系统或其他软组织中[16]. 慢性铅中毒患者在使用驱铅药物后,尽管血铅可以随尿液排出体外,但体内其他部位(骨骼、软组织)的铅可能又转移至血液系统中,从而导致血铅的脱除率较低. 因此,慢性铅中毒患者的驱铅治疗更具挑战性.
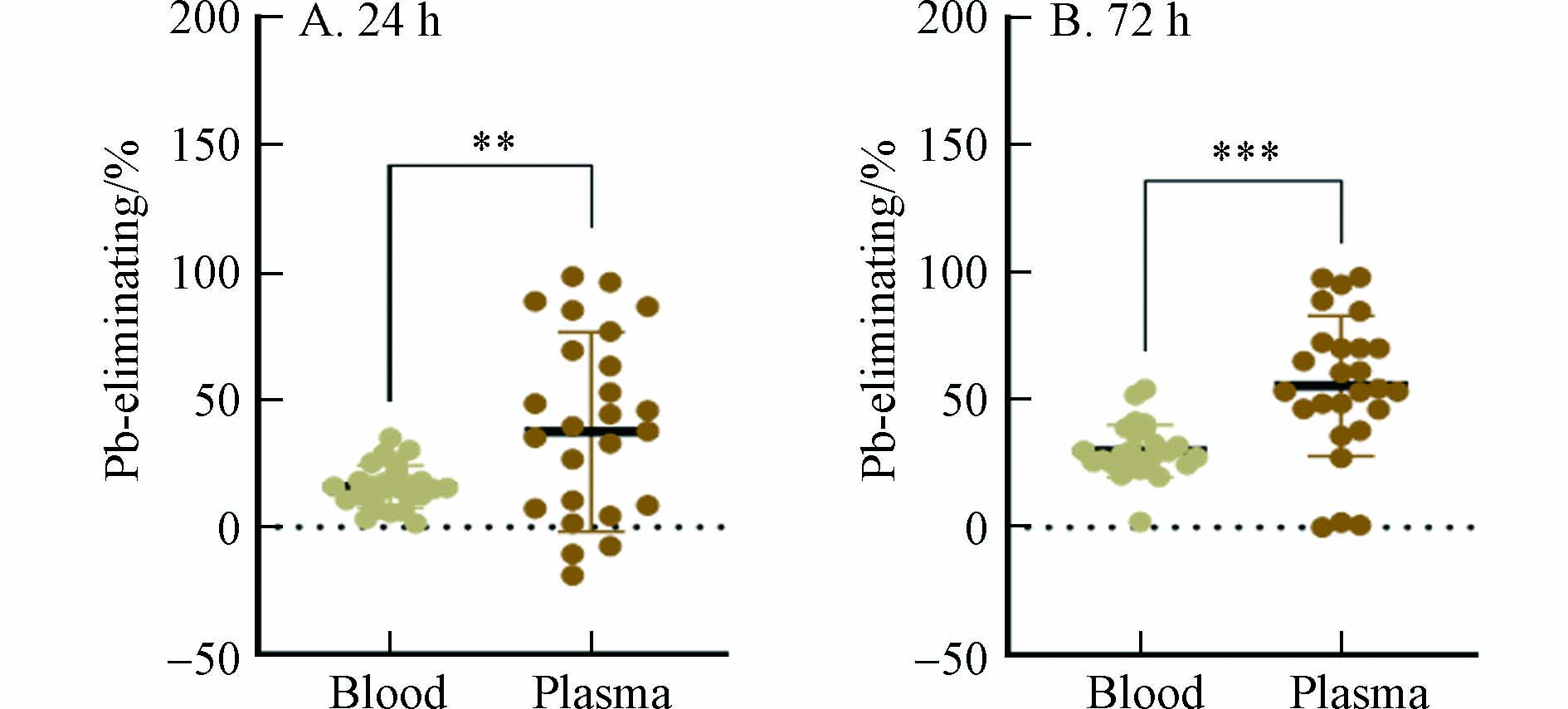
进一步研究发现,血铅和血浆铅的脱除率具有显著差异. 在用药后24 h,血浆铅的脱除率显著高于血铅,同样在用药后72 h,也存在类似的差别(图3). 说明血浆铅与EDTA的络合效率高于血细胞中铅. 血细胞中的铅主要与红细胞中的血红蛋白结合. 尽管EDTA与铅的络合物(结合常数为18.0)比血红蛋白与铅的络合物(结合常数为4.08)稳定[17],但是由于红细胞膜的空间隔离作用,EDTA较难与红细胞中的铅接触发生反应,而更易与血浆铅发生络合,因此,使得血浆铅的脱除率较高.
2.3 血浆铅与铅中毒患者的治疗效果相关
进一步研究显示,尿铅与血浆铅的相关性较强,而与血铅的相关性较弱(图4). 在用药后72 h,血浆铅和尿铅的皮尔逊相关系数高达0.88(P < 0.0001). 对比不同治疗阶段,用药后24 h和72 h的血浆铅与尿铅的相关性比用药前的强,这说明用药治疗促使血浆铅与尿铅的关联性增强. 用药后,EDTA主要与血浆铅发生络合反应,致使血浆铅优先通过尿液排出,因此血浆铅与尿铅的相关性增强. 已有研究显示,血铅和血浆铅之间以及血铅和尿铅之间均呈对数关系[18],这也说明血浆铅与尿铅具有线性相关性. 尽管血浆铅含量较低,但是其流动性和健康危害更大,可以穿过血脑屏障进入大脑组织,也可能穿过胎盘屏障进入胎儿体内. 研究表明,在母体血铅水平升高的情况下,发育中的胎儿可能因母体血浆铅升高而暴露于铅的风险高于全血铅水平所预测的风险[19]. 上述结果说明血浆铅可能是尿铅的直接来源,血浆铅的含量及变化是影响铅中毒患者治疗效果的重要因素.
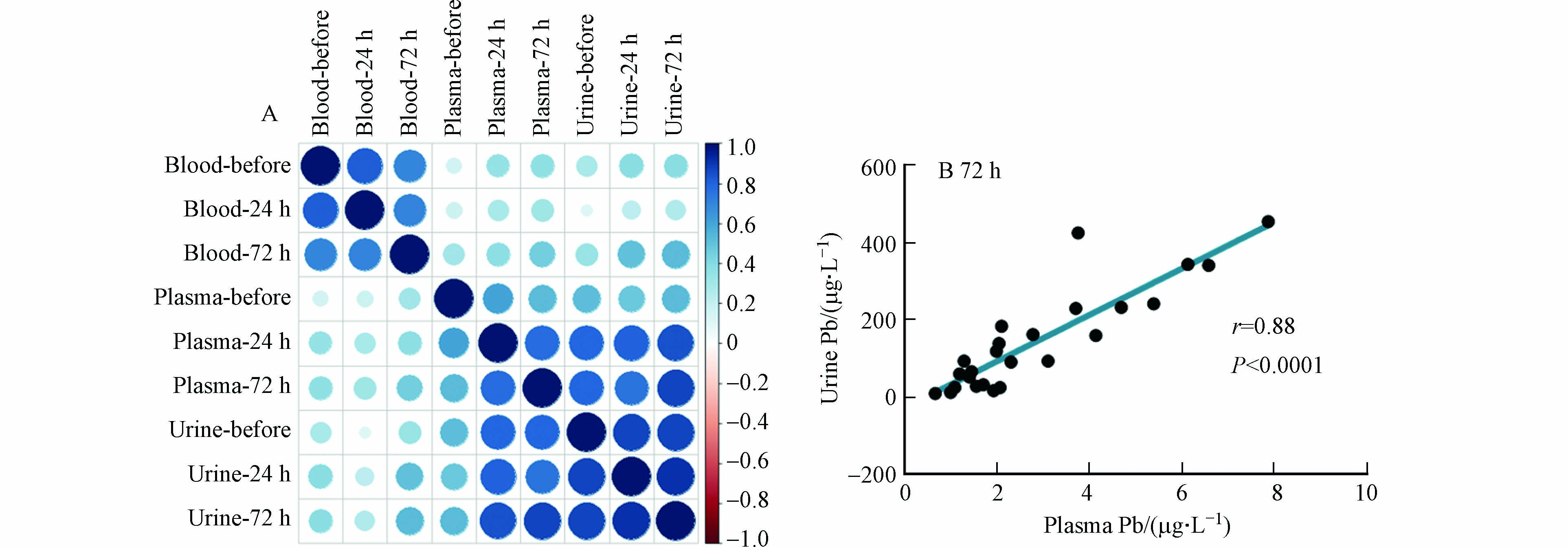 图 4 铅中毒患者在驱铅治疗过程不同样品中铅浓度的相关性分析Figure 4. The correlation of Pb concentration in different samples during medical treatmentA . 不同样品中铅浓度的皮尔逊相关系数热图,B. 用药后72 h血浆铅与尿铅的相关性图A. Heatmap for Pearson correlations of Pb in different samples, B. The correlation of plasma Pb and urine Pb at 72 h after medication
图 4 铅中毒患者在驱铅治疗过程不同样品中铅浓度的相关性分析Figure 4. The correlation of Pb concentration in different samples during medical treatmentA . 不同样品中铅浓度的皮尔逊相关系数热图,B. 用药后72 h血浆铅与尿铅的相关性图A. Heatmap for Pearson correlations of Pb in different samples, B. The correlation of plasma Pb and urine Pb at 72 h after medication血浆铅可以与其他组织器官直接进行物质交换,通过环境暴露从肺部或肠道吸收的铅,首先被吸收进入血浆,进一步可能被血细胞吸收,可能直接从血浆转移至骨骼、肝脏、脾脏或大脑,也可能进入肾脏,通过尿液排出体外. 同时,对于长期铅暴露人群,铅可能从骨骼或其他组织转移至血浆,成为血浆铅[20]. 血浆铅就如一个交换池,血浆铅的易变性也直接影响了铅中毒患者的治疗效果,比如急性铅暴露人群的血浆铅在较短时间内迅速升高,而后逐渐转移至骨骼或其他组织器官,因此对于急性暴露人群的治疗,越快接受治疗,其铅脱除效率可能越好. 铅在血浆和血细胞中的分配平衡可以显著影响血浆铅的含量及其变化. 由于血铅和血浆铅呈现对数关系,因此,当血铅含量较低时,血浆铅随血铅的升高而增加;当血铅含量较高时,血浆铅随着血铅的升高而迅速增加[18],这是因为血细胞中铅的结合接近或处于饱和状态,增加的血铅几乎全部分配于血浆中. 由此,可以推断当血铅含量超过血细胞的铅饱和状态时,铅中毒患者的治疗效果较好.
2.4 尿液pH值可能与铅中毒患者的治疗效果相关
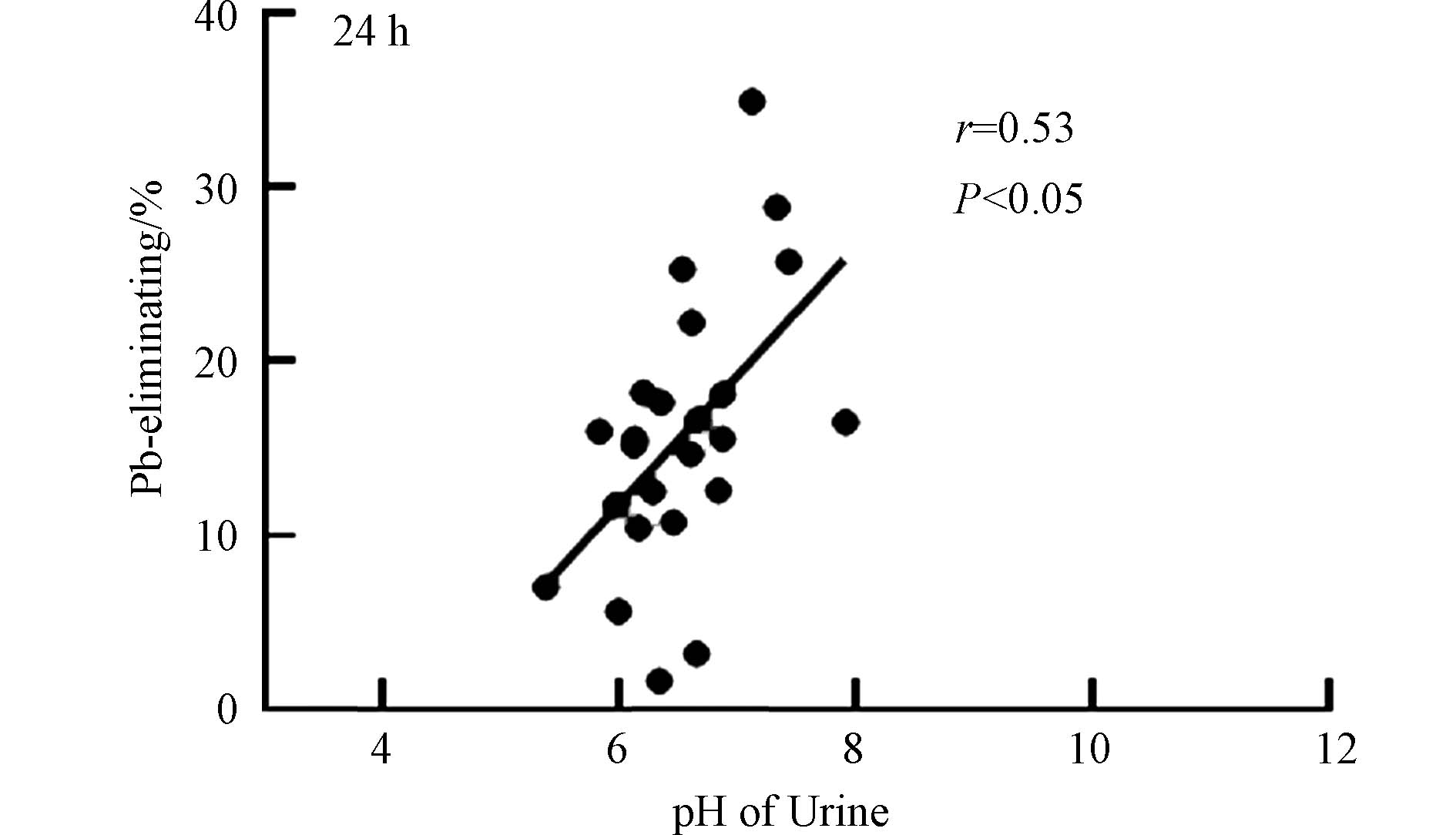
溶液pH是影响EDTA与Pb络合的重要参数. 尿液pH值也是重要的生物化学指标,反映肾脏调节体液酸碱平衡的能力,其变化与多种因素有关,包括每日摄入的食物酸碱性、糖尿病、肾炎等. 实验进一步测定了尿液的pH值,探究了尿液pH与铅中毒患者治疗效果的关系. 研究结果显示,尿液pH值与血铅的脱除率具有关联性,用药后24 h,血铅脱除率与pH值具有显著正相关(r = 0.53,P < 0.05)(图5),说明尿液pH增加可以促进血液中的铅排出体外. 已有较多研究工作探究了土壤pH对重金属的吸附以及生物利用性的影响,一般来说,随着pH值的增加,铅在土壤中的吸附量均呈现明显的上升趋势[21]. 在溶液的酸碱度呈碱性时,铅离子形成氢氧化铅沉淀[22]. 但目前关于人体体液pH值与铅含量变化的研究较少,pH值是影响人体生物化学反应的重要参数,也是评估人体健康的重要指标,因此pH值对铅脱除的影响值得更多的关注和研究.
驱铅治疗的效果与多种因素有关[23-24]. 血铅可以与其他组织器官发生铅交换. 当体内生理状态发生改变时,比如怀孕、骨骼中酸碱环境变化等,铅可能从骨骼中释放,转移至血液系统或其他软组织器官中[25]. 此外,CaNa2EDTA与铅的络合反应与多种生理因素有关,比如血液的酸碱度、血脂含量、其他二价金属含量等. 当血液中酸碱度发生变化,EDTA与铅的络合反应效率会发生变化;当其他二价金属含量较高时,也会与EDTA发生络合反应,从而降低了铅脱除率.
3. 结论(Conclusion)
铅是广泛分布于环境中的有毒重金属,长期铅暴露可导致铅中毒. 本文对铅中毒患者治疗过程中的血铅、血浆铅、尿铅以及尿液pH值进行测定,分析铅浓度的变化趋势以及铅的脱除效率,进一步探究了铅中毒患者治疗效果的影响因素. 本次铅中毒患者在治疗过程中(用药前、用药后24 h、用药后72 h),血铅浓度呈现明显的下降趋势;大部分病人的血浆铅浓度也具有明显的连续降低趋势,而少部分血浆铅浓度先降低后升高;尿铅浓度呈现先升高后降低的趋势. 血液中铅的脱除率整体较低,不超过50%,血浆铅的脱除率显著高于血铅的脱除率. 血浆铅和尿液酸碱度是是影响铅中毒病人驱铅治疗效果的重要因素. 研究发现,尿铅与血浆铅的相关性较强,而与血铅的相关性较弱. 在用药后72 h,血浆铅和尿铅的皮尔逊相关系数高达0.88(P < 0.0001). 尿液pH值与血铅的脱除率具有关联性. 在用药后24 h,血铅脱除率与pH值具有显著正相关(r = 0.53,P < 0.05). 本研究可为提高铅中毒病人的驱铅治疗效果提供科学依据.
-
表 1 SAB和ASAB的孔结构参数
Table 1. Pore structure parameters of SAB and ASAB
吸附剂 全孔面积/(m2·g−1) 微孔面积/(m2·g−1) 介孔面积/(m2·g−1) 孔容/(cm3·g−1) 孔径/nm SAB 330.43 263.38 67.05 0.03 2.89 ASAB 844.45 116.22 728.23 1.68 10.36 表 2 Langmuir和Freundlich模型参数
Table 2. Langmuir and Freundlich model parameters
温度/ ℃ 实际吸附量/(mg·g−1) Langmuir模型 Freundlich模型 KL/(L·mg−1) 理论吸附量/(mg·g−1) R2 KF/(mg(1-n)·Ln·g−1) n R2 15 205.5 0.054 236.4 0.996 13.9 1.564 0.838 25 224.7 0.061 250.0 0.991 26.1 2.221 0.865 35 228.8 0.078 246.3 0.999 49.9 3.257 0.776 表 3 准一级和准二级动力学模型参数
Table 3. Pseudo first and pseudo second order kinetic model parameters
温度/ ℃ 实际吸附量/(mg·g−1) Langmuir模型 Freundlich模型 KL/(L·mg−1) 理论吸附量/(mg·g−1) R2 KF/(mg(1-n)·Ln·g−1) n R2 15 205.5 0.054 236.4 0.996 13.9 1.564 0.838 25 224.7 0.061 250.0 0.991 26.1 2.221 0.865 35 228.8 0.078 246.3 0.999 49.9 3.257 0.776 -
[1] 翟付杰, 张超, 宋刚福, 等. 木棉生物炭对水体中Cr(Ⅵ)的吸附特性和机制研究[J]. 环境科学学报, 2021, 41(5): 1891-1900. [2] 狄军贞, 杨瑞琪, 董艳荣, 等. 改性褐煤对酸性矿山废水中Cr(Ⅵ)的吸附性能研究[J]. 工业水处理, 2022, 42(5): 103-109. [3] NIU J R, JIA X X, ZHAO Y Q, el al. Adsorbing low concentrations of Cr(VI) onto CeO2, @ ZSM-5 and the study of adsorption kinetics, isotherms and thermodynamics[J]. Water Science & Technology, 2018, 77(9): 2327-2340. [4] 袁孝康, 周江敏, 陶月良, 等. 蒙脱石/氧化石墨烯对Cr(Ⅵ)的吸附[J]. 水处理技术, 2022, 48(2): 108-128. [5] JIA X X, ZHANG Y Q, HE Z, et al. Mesopore-rich badam-shell biochar for efficient adsorption of Cr(VI) from aqueous solution[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105634. doi: 10.1016/j.jece.2021.105634 [6] LIANG S, SHI S Q, ZHANG H H, et al. One-pot solvothermal synthesis of magnetic biochar from waste biomass: Formation mechanism and efficient adsorption of Cr(VI) in an aqueous solution[J]. Science of the Total Environment, 2019, 695: 133886. doi: 10.1016/j.scitotenv.2019.133886 [7] JIA K, JI Y, HE X H, et al. One-step fabrication of dual functional Tb3+ coordinated polymeric micro/nano-structures for Cr(VI) adsorption and detection[J]. Journal of Hazardous Materials, 2022, 423: 127166. doi: 10.1016/j.jhazmat.2021.127166 [8] 史航, 李兵, 郭建忠. 功能化枝状复合吸附材料的制备及吸附Cr(Ⅵ)的性能[J]. 浙江农林大学学报, 2022, 39(2): 396-404. [9] 张航, 马蓉, 弓亮, 等. 硅藻基Cr(Ⅵ)表面离子印迹吸附材料的制备及其对Cr(Ⅵ)的吸附性能[J]. 材料导报, 2022, 36(8): 1-6. [10] 陈燕勤, 王雪莉, 宋世方. 核桃壳生物炭对水体中Cr(Ⅵ)的吸附性能研究[J]. 新疆农业大学学报. 2022, 45(4): 317-322. [11] 徐冰冰, 江琳琳, 张建华, 等. 改性夏威夷壳炭对废水中Cr(Ⅵ)的吸附行为研究[J]. 金属矿山, 2021(9): 192-198. [12] 崔艳红, 孙鹏, 汪颖军, 等. 乙醇辅助氨基改性NCR-MCM-48分子筛的制备及其对Cr(Ⅵ)的吸附性能[J]. 硅酸盐通报, 2021, 40(6): 2118-2128. doi: 10.16552/j.cnki.issn1001-1625.20210513.002 [13] ZHAO N, ZHAO C F, LV Y Z, et al. Adsorption and coadsorption mechanisms of Cr(VI) and organic contaminants on H3PO4 treated biochar[J]. Chemosphere, 2017, 186: 422-429. doi: 10.1016/j.chemosphere.2017.08.016 [14] DING P J, NIU J R, CHANG F Q, et al. NiCo2O4 hollow microsphere–mediated ultrafast peroxymonosulfate activation for dye degradation[J]. Chinese Chemical Letters, 2021, 32: 2495-2498. doi: 10.1016/j.cclet.2020.12.063 [15] MIAO S Y, GUO J R, DENG Z M, et al. Adsorption and reduction of Cr(VI) in water by iron-based metal-organic frameworks (Fe-MOFs) composite electrospun nanofibrous membranes[J]. Journal of Cleaner Production, 2022, 370: 133566. doi: 10.1016/j.jclepro.2022.133566 [16] LI Y L, CHEN X, LIU L, et al. Characteristics and adsorption of Cr(VI) of biochar pyrolyzed from landfill leachate sludge[J]. Journal of Analytical and Applied Pyrolysis, 2022, 162: 105449. doi: 10.1016/j.jaap.2022.105449 [17] XU H J, LIU Y X, LIANG H X, et al. Adsorption of Cr(VI) from aqueous solutions using novel activated carbon spheres derived from glucose and sodium dodecylbenzene sulfonate[J]. Science of the Total Environment, 2020, 759(67): 143457. [18] ZENG H Y, ZENG H H, ZHANG H, et al. Efficient adsorption of Cr (VI) from aqueous environments by phosphoric acid activated eucalyptus biochar[J]. Journal of Cleaner Production, 2020, 286: 124964. [19] BAUTISTA-TOLEDO M I, RIVERA-UTRILLA J, R OCAMPO-PEREZ, et al. Cooperative adsorption of bisphenol-A and chromium(III) ions from water on activated carbons prepared from olive-mill waste[J]. Carbon, 2014, 73: 338-350. doi: 10.1016/j.carbon.2014.02.073 [20] LIU N, ZHANG Y T, XU C, et al. Removal mechanisms of aqueous Cr(VI) using apple wood biochar: A spectroscopic study[J]. Journal of Hazardous Materials, 2019, 384: 121371. [21] JIANG B, GONG Y F, GAO J N, et al. The reduction of Cr(VI) to Cr(III) mediated by environmentally relevant carboxylic acids: State-of-the-art and perspectives[J]. Journal of Hazardous Materials, 2019, 365: 205-226. doi: 10.1016/j.jhazmat.2018.10.070 -




 DownLoad:
DownLoad: