-
二十世纪初,人们选用氯消毒作为保障饮用水安全的最后一道工艺,至二十一世纪,已经发展出氯胺消毒、二氧化氯消毒、紫外线消毒、臭氧消毒、光催化消毒、水力空化消毒、高锰酸钾消毒等多种消毒方式。在消毒过程中,消毒剂会不可避免的与水中的部分有机或无机物发生反应,生成一类对人体健康有害的物质,即消毒副产物(disinfection byproducts,DBPs)。自从Rook于1976年首次从消毒后的水中检测出三氯甲烷以来,已有超过600余种消毒副产物被人类识别和发现,主要包括:三卤甲烷、卤乙酸、卤代酮、卤代醛等[1-2]。近年来,有研究表明,在原水中有无机阴离子如溴离子或碘离子存在的情况下,消毒后的水中不仅会生成氯代消毒副产物(chlorinated disinfection byproducts,Cl-DBPs),还会有溴代消毒副产物(brominated disinfection byproducts,Br-DBPs)和碘代消毒副产物(iodinated disinfection byproducts,I-DBPs)的生成[3-4]。在我国,大部分沿海地区的水源是潮汐河流,靠近入海口,经常会受到咸潮入侵的影响,而海水中溴离子和碘离子的质量浓度是一般淡水的3 000多倍和十几倍,这将使得这些地区的水源水中有存在较高质量浓度溴离子和碘离子的风险,进而导致消毒过程中更容易生成Br-DBPs和I-DBPs。此外,在我国北方地区的一些城镇生活饮用水中的碘含量可以超过100 µg·L−1[5-6],2019年全国生活饮用水水碘含量调查报告表明[7],河北、天津、河南、山东等地区水碘含量较高,其中河北最高可达1 113.7 µg·L−1,天津最高可达427.0 µg·L−1,河南最高可达489.0 µg·L−1,山东最高可达901.0 µg·L−1。水体中较高的碘含量,不仅会增加人类患甲状腺疾病的风险,而且还会增加消毒过程中I-DBPs的生成。有研究表明,Br-DBPs和I-DBPs较Cl-DBPs具有更强的细胞毒性和遗传毒性[8-9],而包括中国、美国、以色列、澳大利亚、土耳其等世界各地的饮用水厂的出水中均发现有Br-DBPs和I-DBPs的存在[10-11],控制消毒过程中Br-DBPs和I-DBPs的生成已成为目前饮用水安全研究方面的热点和重点。
由于氯消毒会产生较多的三卤甲烷、卤乙酸等受到严格管控的含碳消毒副产物(carbonaceous disinfection byproducts, C-DBPs),因此,不少饮用水厂转而采用氯胺作为消毒剂。氯胺与消毒副产物前体物的反应活性远小于氯,能够有效减少消毒后三卤甲烷和卤乙酸的生成,且氯胺在管网中形成余氯的持续时间长,能够有效抑制管网中残留细菌的再繁殖。但氯胺消毒会导致一些毒性更强的含氮消毒副产物(nitrogenous disinfection byproducts,N-DBPs)如卤代乙腈、卤代乙酰胺、卤代硝基甲烷、亚硝胺类等生成的增加[12-13]。近年来,国内外学者对于N-DBPs的关注日渐增加,但多集中于氯代N-DBPs,如氯乙腈、氯代硝基甲烷、氯代乙酰胺等的生成特性、生成机理和生物毒性等方面。而当原水中存在高质量浓度的溴离子或碘离子时,氯胺消毒后的水中生成的N-DBPs也会由氯代向溴代和碘代转变,且溴代和碘代N-DBPs的毒性更是远高于氯代N-DBPs。
目前对于DBPs的定性和定量分析主要采用气相色谱、液相色谱结合质谱等方法实现。其中气相色谱与气相色谱质谱联用技术主要针对挥发性和半挥发性的DBPs进行定性和定量分析[14-15],而针对难挥发性或高极性的DBPs,特别是一些溴代和碘代DBPs,气相色谱与气相色谱质谱联用技术难以实现检测和分析。近年来,ZHANG等[16]和DING等[17]使用超高效液相色谱/电喷雾电离-三重四极杆质谱(ultra performance liquid chromatography/electrospray ionization-triple quadruple mass spectrometry,UPLC-ESI-tqMS)可实现对饮用水中极性Br-DBPs和I-DBPs的快速选择性检测。该方法基于质谱中的前体离子扫描(precursor ion scan,PIS)模式,在该模式下,可以检测出产生特定碎片离子的所有母离子分子。例如,可以通过将子离子的质荷比(m/z)设置为79/81,并扫描产生该子离子碎片的所有母离子,进而筛选出水样中的所有Br-DBPs,最后,通过分析同位素的丰度比以及结合其他子离子碎片信息,可以确定这些Br-DBPs的分子结构[18]。同样,通过将子离子的质荷比(m/z)设置为127,可以快速选择性地检测出I-DBPs[19]。
本研究以中国南方某沿海城市的饮用水厂和污水厂出水为研究对象,通过投加溴离子和碘离子来模拟咸潮入侵,考察这2类水体在氯胺消毒过程中溴代和碘代DBPs的生成情况,并采用UPLC/ESI-tqMS的PIS模式,快速选择性地检测和识别出氯胺消毒过程中生成的主要极性卤代DBPs。在此基础上,对2类水体中卤代DBPs的生成种类和数量进行对比。整个研究对于加深溴代和碘代DBPs的认识,保障人们群众的饮水安全具有重要意义。
-
选取福建省漳州市招商局经济技术开发区污水处理厂和自来水厂的出厂水作为研究对象。其中,污水处理厂采用常规二级处理+混凝沉淀+紫外消毒工艺,处理后的水可以达到污水一级A排放标准,并排放入周边海域;自来水厂采用常规处理工艺(混凝-沉淀-过滤-消毒),原水来自当地主要饮用水源——九龙江。采集的水样运回实验室后,经0.45 μm滤膜过滤并于4 ℃下冷藏避光保存。
-
无水硫酸钠(分析纯)购自Aladdin,甲基叔丁基醚(methyl tert-butyl ether,MTBE,色谱纯)、乙腈(色谱纯)均购买自美国Tedia公司,氯化钠(优级纯)、次氯酸钠(分析纯)、溴化钠(分析纯)、硫代硫酸钠(分析纯)均购自西陇科学,碘化钾(分析纯)购买自汕头市达濠精细化学品有限公司,硫酸(优级纯)、氢氧化钠(分析纯)、盐酸(分析纯)、氯化铵(分析纯)均购自国药集团化学试剂有限公司。
-
一氯胺溶液由NaClO与NH4Cl溶液(保持二者质量比为4 mg·L−1 Cl2:1 mg·L−1 NH4+-N)于冰水浴下暗处混合反应30 min制得,需现用现配。制得的溶液采用N,N-二乙基-1,4-苯二胺分光光度法测定其一氯胺含量(以氯计)。
准确量取20 mL 1.1中采集的实际水样于带聚四氟乙烯瓶垫的圆底离心管中。通过加入一定量的溴化钠(500 mg·L−1 Br—)或碘化钾(100 mg·L−1 I—)溶液,使得水样中溴离子质量浓度达到了5 mg·L−1,碘离子质量浓度达到了0.5 mg·L−1,来模拟咸潮入侵的影响。然后以C(TOC):C(Cl2)= 1:5加入氯胺进行消毒,将水样放置于恒温培养箱内25 °C避光反应72 h。反应完毕后,取出水样,用100 mg·L−1的Na2S2O3溶液终止反应。
-
采用液液萃取。具体方法为:取消毒完毕后的水样,加入盐酸(1 mol·L−1)将pH调节至2.0,加入5 mL的MTBE作为萃取剂,再加入8 g氯化钠以促进分层,涡旋振荡1.5 min,静置5 min分层,将上层有机相吸出,加入3 g无水硫酸钠(使用前需经马弗炉400 °C灼烧并用MTBE洗涤)过滤至吹脱管中,氮吹至0.5 mL,与10 mL乙腈混合,氮吹至0.5 mL,移入棕色进样小瓶中,分析前采用超纯水定容至1 mL,经0.22 μm PTFE滤膜过滤后上机检测。
-
所用仪器为Agilent 1290 Infinity LC-6490 MS/MS超高效液相色谱-串联四极杆质谱联用仪,液相色谱柱为Proshell 120 EC-C18(100 mm×2.1 mm×2.7 µm,Agilent)。流动相由超纯水和乙腈组成,流速为0.25 mL·min−1,进样量为10 µL。流动相的梯度洗脱程序为:0~12 min从10%乙腈线性变化到90%,并保持3 min,在1 min内恢复到10%乙腈,16~18 min保持在10%,以在下一次进样之前重新平衡色谱柱。MS/MS检测中,ESI源采用负离子模式,MS参数如下:干燥气(氮气)温度160 °C;干燥气流速,16 L·min−1;鞘气(氮气)温度,350 °C;鞘气流速,11 L·min−1;毛细管电压,3 000 V。采用PIS模式,设置Q1为全扫描状态,Q3为选择离子监测状态,将Q3设置为允许m/z 79/81或m/z 127的特征碎片通过,能够检测到水样中几乎所有可以被ESI电离的溴代DBPs和碘代DBPs。串联UPLC系统,对ESI-tqMS PIS模式检测到的溴代DBPs和碘代DBPs进行分离,确定保留时间(retention time,RT)后,进行子离子扫描,获得分子离子的碎片信息。由于C-Br的键能强于C-I键的键能,并且C-Br的键长比C-I的键长短,因此I-DBPs的最佳碰撞能要小于Br-DBPs,根据ZHANG等人[16-18]的经验,本研究选择30 eV作为I-DBPs的最佳碰撞能,选择40 eV作为Br-DBPs的碰撞能,在此最佳碰撞能下,能够最大程度地减少“假”分子离子的干扰,如Cl2CHC—在碰撞能量超过15 eV 时,倾向于解离为Cl2CH—和Cl—,其对I-DBPs的干扰就可以排除。
-
自来水厂出水和污水厂出水中的总有机碳(total organic carbon, TOC)、氨氮、特征紫外吸光度(specific ultraviolet absorbance, SUVA)、pH、溴离子、碘离子等水质指标如表1所示。
TOC是以碳的含量来表示水样中有机物总量的综合性指标,SUVA是水样在254 nm处的吸光度与溶解性有机碳(dissloved organic carbon, DOC)的比值,可以反映水样中含碳碳双键、苯环等不饱和键有机物的含量。有研究表明,水样的TOC和SUVA往往和DBPs的生成量呈显著正相关关系。由表1可知,污水厂出水的TOC和SUVA 分别为自来水厂出水的1.96倍和2.15倍,由此可以推测,污水厂出水经氯胺消毒后生成的DBPs的总量会多于自来水厂出水。氨氮作为水中氮的主要存在形态,在氯胺消毒过程中,对N-DBPs的生成具有重要影响。由表1可知,污水厂出水中的氨氮为0.63 mg·L−1,远远超过自来水厂出水,因此,可以推测,污水厂出水经氯胺消毒后N-DBPs的生成量会远远超过自来水厂出水。溴和碘离子广泛存在于各种水体中,其作为DBPs的主要无机前体物,对DBPs的生成同样具有重要的影响。而在本研究选取的2个水样中,仅在污水厂出水中检测出81 µg·L−1的溴离子。虽然在一般情况下,水体中都不会存在较高质量浓度的溴离子和碘离子,但考虑到沿海地区的水源时刻面临咸潮入侵的风险,以本研究选取的九龙江为例,在咸潮入侵期间,九龙江原水中的溴离子和碘离子质量浓度最高可分别达到6 mg·L−1和0.2 mg·L−1。因此,有必要考察极端情况下(咸潮入侵导致水源中存在较高质量浓度的溴离子和碘离子)氯胺消毒过程中DBPs的生成情况。
-
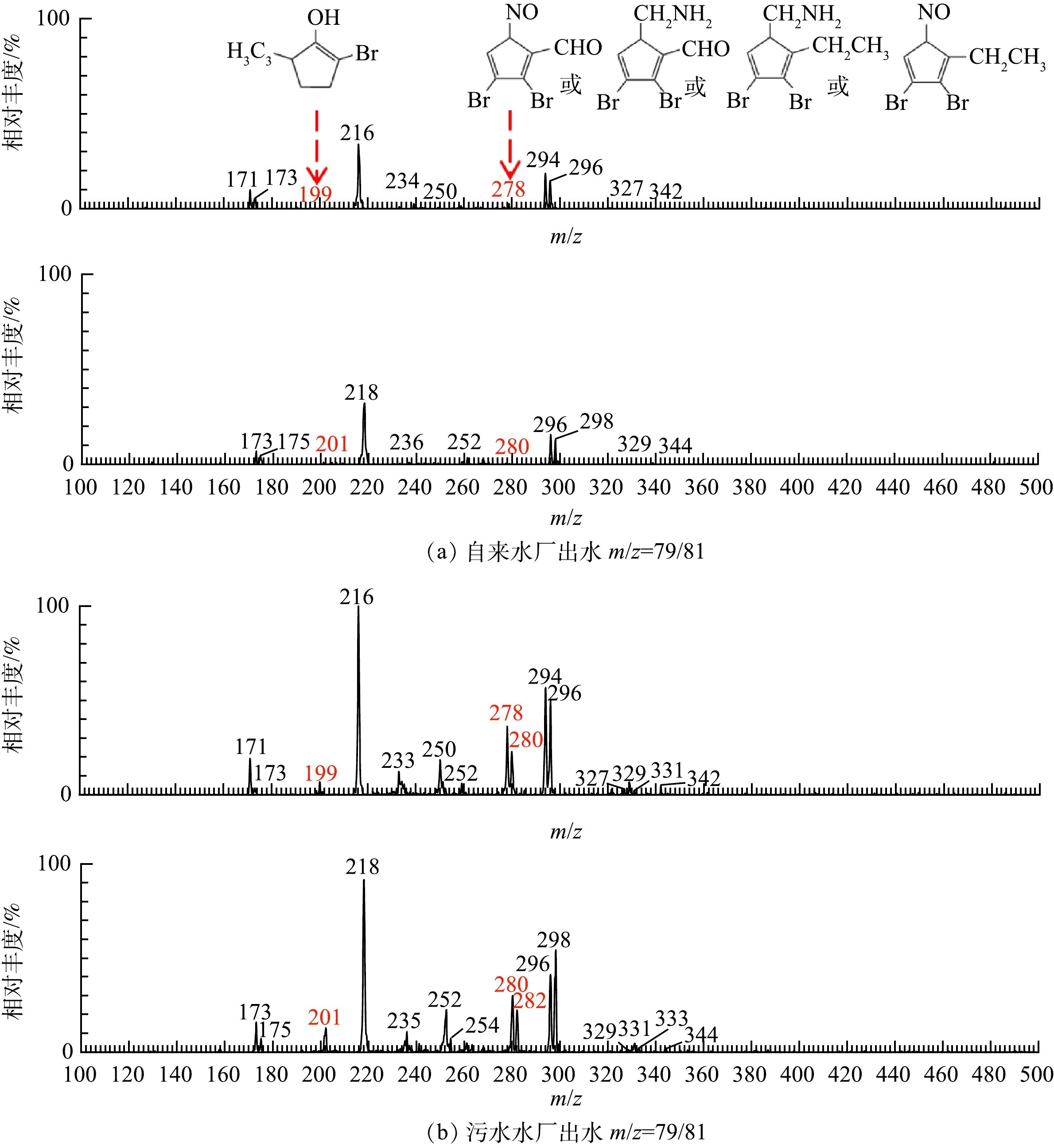
本研究采用UPLC/ESI-tqMS对氯胺消毒后的水样进行检测,首先设置PIS m/z 79/81进行扫描,检测所有的极性Br-DBPs,再根据天然同位素丰度比进一步推断物质中氯、溴原子个数。通过串联UPLC系统,对ESI-tqMS PIS模式检测到的极性Br-DBPs进行分离,确定每个母离子的保留时间后,调整仪器模式为子离子模式,进行子离子扫描,将Q1设置为选择离子监测状态,Q3设置为扫描状态,获得子离子的碎片信息,推断物质结构。水样的ESI-tqMS PIS m/z 79/81扫描质谱图如图1所示。
根据图1可知,m/z 171/173/175、m/z 171/173、m/z 216/218、m/z 233/235/237、m/z 250/252/254、m/z 294/296/298、m/z 327/329/331/333分别对应二溴乙酸(脱羧)、4-溴苯酚、2-溴-4-硝基苯酚、溴-氯-二甲基苯酚或3-溴-5-氯-4-羟基苯甲醛、2-溴-6-氯-4-硝基苯酚、2,6-二溴-4-硝基苯酚和2,4,6-三溴苯酚[18-20]。在本研究中,对检测发现的未知母离子m/z 199/201、m/z 278/280/282进行子离子扫描,得到分子离子的碎片信息,推断其分子结构。
1)离子簇m/z 199/201的分析鉴定。离子簇m/z 199/201有2个同位素峰,峰高比例接近1:1,说明该物质中含有一个溴原子。观测到一个分子质量为40 Da (119-79=40)的碎片丢失,根据Beynon表,该物质可能含有C2H2N、C3H4。由于该物质的出峰时间为6.272 min,说明分子中可能含有苯环或者是环状结构,在子离子扫描质谱图中看到一个m/z 62的子离子碎片,最多包含5个碳原子,说明该物质含有的环状结构很可能是环戊烯。由于该物质的分子质量为200 Da,减去1个溴,再减去环戊烯,剩余的57可能包含C2H2N(40)或C3H4(40)和OH(17)。此外,根据氮律,由C、H、O、N、P和卤素组成的有机化合物,若含有奇数个氮,相对分子质量一定是奇数,若含有偶数个氮或不含氮,相对分子质量一定是偶数,该物质的分子质量为200 Da,因此,该物质不含氮。综上所述,推断该物质的分子式可能为C8H9OBr,满足不饱和度计算公式,推断合理。该物质的可能结构为1-丙二烯-3-溴-2-羟基-环戊烯及其同分异构体。
2)离子簇m/z 278/280/282的分析鉴定。m/z 278/280/282有3个同位素峰,说明该物质中含有2个溴原子或1个氯原子和1个溴原子。3个同位素峰的峰高比例接近1:2:1,说明该化合物分子中含有2个溴原子。在子离子碎片图中观测到一个分子质量为30 Da (280-250=30)的碎片丢失,根据Beynon表,该碎片的分子式可能为NO、CH4N、H2N2、CH2O、C2H6。在子离子碎片图中观测到一个分子质量为58 Da (280-222=58)的碎片丢失,该碎片可能由28+30组成,根据Beynon表,分子质量为28 Da的分子式可能是N2、CO、CH2N或者C2H4。根据氮律与分子组成原则,分子质量为58 Da的碎片的分子式组成可能为NO和CO、NO和C2H4、CH4N和CO、CH4N和C2H4。由于该物质的分子质量为279 Da,减去2个溴(158),再减去58,剩余63的部分最多含有5个碳原子,此外该物质的出峰时间为6.942 min,说明分子中可能含有环状结构,因此,剩余部分很可能是环戊二烯。综上所述,该物质的分子式可能为C6H3O2NBr2、C7H7ONBr2或C8H11NBr2,满足不饱和度计算公式。该物质的可能结构为2,3-二溴-5-硝基-环戊二烯-1-醛、2,3-二溴-1-乙基-5-硝基-环戊二烯、2,3-二溴-5-甲氨基-环戊二烯-1-醛、2,3-二溴-1,-乙基-5-甲氨基-环戊二烯及其同分异构体。
-
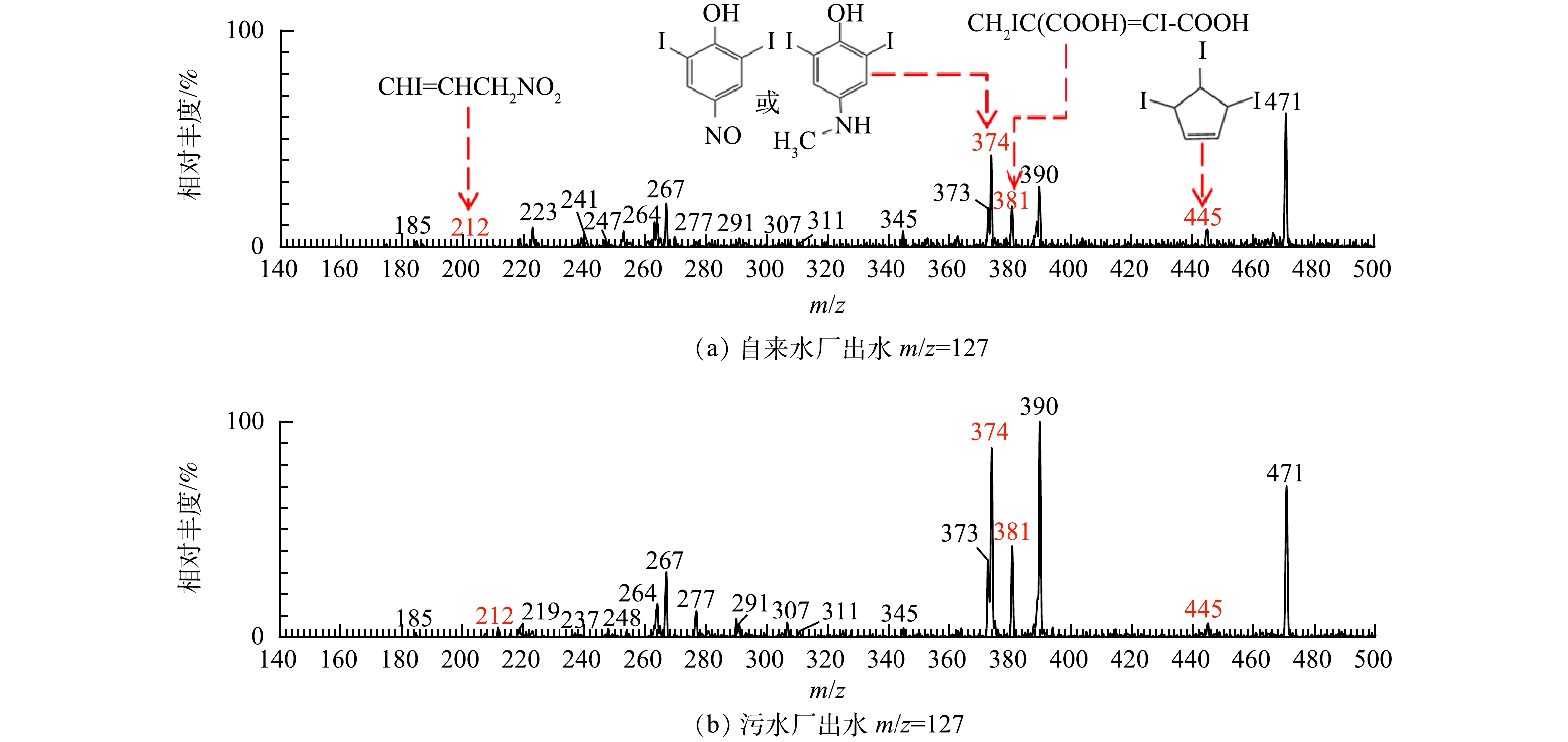
极性I-DBPs的检测是通过设置模式PIS m/z 127来实现,其他步骤与极性Br-DBPs的检测方法一致。水样的ESI-tqMS PIS m/z 127扫描质谱图如图2所示。根据图2可知,m/z 185、m/z 241、m/z 247、m/z 267、m/z 277、m/z 291、m/z 307、m/z 311、m/z 373、m/z 390、m/z 471的物质结构在前人的研究中已提出[17,19-20],分别是一碘乙酸、一碘丁烯二酸、4-羟基-3-碘苯甲醛、二碘乙酸(脱羧)、3-碘-4-羟基-5-甲基-苯甲酸、3-甲酰基-4-羟基-5-碘苯甲酸、4-羟基-5-碘-1,3-苯二甲酸、二碘乙酸或2-碘-3-甲基-1-萘酸、4-羟基-3,5-二碘-苯甲醛、2,6-二碘-4-硝基苯酚、2,4,6-三碘苯酚。本研究中,检测发现未知母离子m/z 212、m/z 374、m/z 381、m/z 445,对4个未知母离子进行子离子扫描,得到分子离子的碎片信息,推断物质结构。
1)离子簇m/z 212的分析鉴定。离子簇m/z 212没有其他同位素峰,说明该物质中不含有溴原子和氯原子,该物质的出峰时间为1.147 min,说明分子中不含有苯环。在子离子扫描质谱图中看到一个m/z 127的子离子碎片,该物质应该仅含有一个碘,否则不符合分子组成原则。在子离子扫描质谱图中看到一个m/z 62的子离子碎片,根据Beynon表,该碎片的分子式可能为CH2O3、CH4NO3、CH6N2O或C2H6O2,由于该物质的分子质量是213 Da,根据氮律可以知道,分子组成中一定含有奇数个氮原子,因此,推断m/z 62的碎片分子式为CH4NO3。213减去62(CH4NO3)与127(I)后(213-62-127=24),剩余部分最多为2个碳原子,则物质分子式可能为C3H4O2NI,满足不饱和度计算公式,推断合理。该物质的可能结构为CHI=CHCH2NO2及其同分异构体。
2) 离子簇m/z 374的分析鉴定。离子簇m/z 374没有其他同位素峰,说明该物质中不含有溴原子和氯原子。在子离子碎片图中观测到一个分子质量为127 Da (374-247=127)的碎片丢失和一个分子质量为254 Da (374-120=254)的碎片丢失,说明该物质结构中有2个I。观测到一个分子质量为30 Da (374-344=30)的碎片, 根据Beynon表,该碎片的分子式可能为NO、H2N2、CH2O、CH4N或C2H6。观测到一个分子质量为92 Da (219-127=92)的碎片丢失, 根据Beynon表,该碎片的分子式可能为C2H4O4、C2H6NO3、C2H8N2O2、C3H8O3、C6H6N、C6H4O或C7H8,由于该物质的出峰时间为6.272 min,说明分子中可能含有苯环,因此,该碎片的分子式可能为C6H6N、C6H4O或C7H8。由于该物质的分子质量为375 Da,根据氮律,该物质分子组成中一定含有奇数个氮原子,因此,推断该物质的分子式可能为C6H7N3I2、C8H11NI2、C6H3O2NI2或C7H7ONI2,其中C6H7N3I2与C8H11NI2不符合分子组成原则,则该物质分子式为C6H3O2NI2或C7H7ONI2,满足不饱和度计算公式,推断合理。该物质的可能结构为2,6-二碘-4-亚硝基苯酚或2,6-二碘-4-甲氨基苯酚及其同分异构体。
3)离子簇m/z 381的分析鉴定。离子簇m/z 381没有其他同位素峰,说明该物质中没有溴原子和氯原子。该物质的出峰时间为1.022 min,说明分子中不含有苯环。在子离子碎片图中观测到一个分子质量为142 Da (381-239=142)的碎片丢失和一个分子质量为284 Da (381-97=284)的碎片丢失,说明该物质结构中有2个碘甲基(CH2I)。观测到一个分子质量为120 Da (381-261=120)的碎片丢失,该碎片可能为2个乙酸(CH3COOH),说明该物质的结构中应该有2个羧基,且很有可能有一个C=C。综上,推测该物质的分子式为C5H4I2O4,符合氮律且满足不饱和度计算公式。该分子结构可能为CH2IC(COOH)=CI-COOH及其同分异构体。
4)离子簇m/z 445的分析鉴定。离子簇m/z 445没有其他同位素峰,说明该物质中没有溴原子和氯原子。在子离子碎片图中观测到一个分子质量为127 Da (445-318=127)的碎片,一个分子质量为254 Da (445-191=254)的碎片丢失和一个分子质量为381 Da (445-64=381)的碎片丢失,说明该物质结构中有3个I。观测到一个分子质量为64 Da (191-127=64)的碎片丢失, 根据Beynon表,该碎片的分子式可能为C5H4。该物质的分子质量为446 Da,因此,推断该物质的分子式为C5H5I3。该物质的出峰时间为6.383 min,说明分子中可能含有环状结构。该物质的分子式为C5H5I3,不可能含有苯环。综上所述,推测该物质的结构可能为三碘代环戊烯,满足不饱和度计算公式,推断合理。
-
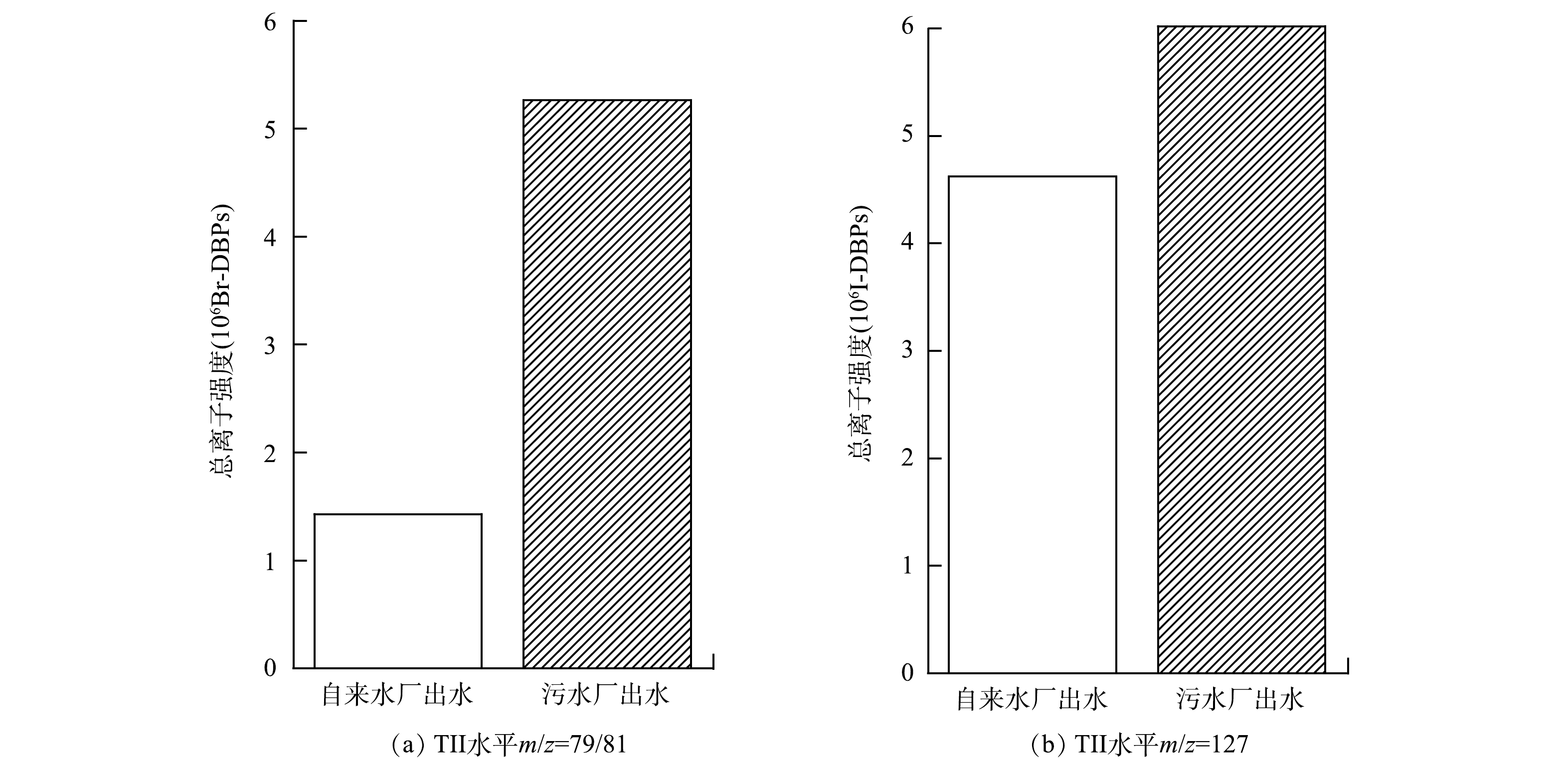
总离子流强度(total ion intensity,TII)为质谱在1次扫描中检测到的所有离子峰的离子流强度之和[17,21]。在本研究中,通过将子离子的m/z设置为 79/81,并将母离子质谱图中m/z在 90~500(m/z> 500的离子峰的离子流强度较低,不计算在内)内的所有离子峰的离子流强度相加,可指示水样中极性Br-DBPs的总生成量。通过将子离子的m/z设置为127,并将母离子质谱图中m/z在130~500内的所有离子峰的离子强度相加,可指示水样中极性I-DBPs的总生成量。图3反映了自来水厂与污水厂出水经氯胺消毒后生成的Br-DBPs与I-DBPs 的总离子强度。由图3可以看出,污水厂出水经氯胺消毒后,Br-DBPs和I-DBPs的TII分别为5265 251.58和6022 404.88,是自来水厂出水的3.68倍和1.30倍。污水厂出水经氯胺消毒后Br-DBPs与I-DBPs 的总生成量均大于自来水厂出水,这主要是因为污水厂出水的TOC(3.00 mg·L−1)大于自来水厂出水(1.53 mg·L−1),在相同条件下,TOC越高的水样,在氯胺消毒过程中生成DBPs的量越高。
表2总结了污水厂与自来水厂出水经氯胺消毒后检测到的所有极性卤代DBPs。从表2中可以看出,污水厂出水经氯胺消毒后生成的极性Br-DBPs的种类显著多于自来水厂出水。其中,m/z 171/173、m/z 199/201、m/z 233/235/237、m/z 250/252/254、m/z 278/280/282、m/z 327/329/331/333等物质在自来水厂出水中均未检测到。此外,污水厂出水中溴代N-DBPs(包括m/z 216/218、m/z 250/252/254、m/z 278/280/282、m/z 294/296/298)的离子强度较自来水厂出水水样高,而N-DBPs通常比C-DBPs的毒性更大。投加碘离子后经氯胺消毒的自来水厂出水水样与污水厂出水水样产生的极性I-DBPs种类基本一致,但污水厂出水水样中I-DBPs的离子强度更高,特别是碘代N-DBPs。导致污水厂出水水样中卤代N-DBPs含量更高的可能原因是污水厂出水中的氨氮较高。此外,污水厂出水中含苯环的芳香族卤代DBPs的含量较高,这可能是由于污水厂出水的SUVA较大,DBPs前体物中苯环或共轭双键等不饱和有机物较自来水厂出水多。
-
1)在有高质量浓度溴离子存在情况下,自来水厂和污水厂出水经氯胺消毒后,均检测到了大量的极性Br-DBPs生成。其中,m/z 199/201和m/z 278/280/282的Br-DBPs是在本研究中首次被检测出,通过对子离子碎片的扫描和分析,推断出其分别为1-丙二烯-3-溴-2-羟基-环戊烯和2,3-二溴-5-硝基-环戊二烯-1-醛及其同分异构体。
2)在有高质量浓度碘离子存在情况下,自来水厂和污水厂出水经氯胺消毒后,均检测到了大量的极性I-DBPs生成。其中,m/z 212、m/z 374、m/z 381和m/z 445等I-DBPs是在本研究中首次被检测出,通过对它们子离子碎片的扫描和分析,推断出它们的分子结构分别为1-碘-3-硝基-丙烯、2,6-二碘-4-亚硝基苯酚、1-碘-2-碘甲基-丁烯二酸和三碘代环戊烯及其同分异构体。
3)污水厂出水经氯胺消毒后生成的Br-DBPs和I-DBPs的TII分别为5265 251.58和602 2404.88,是自来水厂出水的3.68倍和1.30倍,且污水厂出水中部分溴代和碘代N-DBPs的离子强度远高于自来水厂出水。
经氯胺消毒的高溴碘水体中极性卤代消毒副产物的生成及鉴定
Formation and identification of polar halogenated disinfection byproducts during chloramine disinfection of high bromine and iodine water
-
摘要: 本研究以中国南方某沿海地区易受海水入侵影响的自来水厂和污水处理厂出水为研究对象,通过建立基于液液萃取与超高效液相色谱/电喷雾电离-三重四级杆质谱的分析方法,快速选择性地检测这类含高质量浓度溴离子、碘离子的水体在氯胺消毒过程中生成的极性卤代消毒副产物。结果表明,通过设置子离子m/z 79/81和m/z 127,对母离子进行扫描,在氯胺消毒后的2种水样中检测出了10种极性溴代消毒副产物和19种极性碘代消毒副产物。通过对同位素丰度比和子离子碎片的分析,首次提出了2种溴代消毒副产物和4种碘代消毒副产物的分子结构。此外,对比自来水厂和污水厂出水的总离子强度可以发现,污水厂出水中生成的极性卤代消毒副产物,特别是卤代含氮消毒副产物的种类和数量显著高于自来水厂出水。
-
关键词:
- 极性卤代消毒副产物 /
- 超高效液相色谱/电喷雾电离-三重四极杆质谱 /
- 氯胺消毒 /
- 前体离子扫描
Abstract: This study takes the effluents from a drinking water treatment plant and a wastewater treatment plant in a coastal area in southern China which is vulnerable to seawater intrusion as the research object. A detection method based on liquid-liquid extraction combined with ultra performance liquid chromatography/electrospray ionization-triple quadrupole mass spectrometry was established, which can rapidly and selectively detect polar halogenated disinfection byproducts from chloramine disinfection of water containing high concentration of bromide and iodide ions. The results showed that 10 kinds of polar brominated disinfection byproducts and 19 kinds of polar iodinated disinfection byproducts were detected in two water samples by setting precursor ion scans of m/z 79/81 and m/z 127. Based on the analysis of isotopic abundances ratio and particular fragment ions, the molecular structures of 2 brominated disinfection byproducts and 4 iodinated disinfection byproducts were firstly proposed. In addition, the species and the quantities of polar halogenated disinfection byproducts generated from the effluent of wastewater treatment plant were higher than those from the drinking water treatment plant by comparing the total ion intensity, especially for halogenated nitrogenous disinfection byproducts. -
二十世纪初,人们选用氯消毒作为保障饮用水安全的最后一道工艺,至二十一世纪,已经发展出氯胺消毒、二氧化氯消毒、紫外线消毒、臭氧消毒、光催化消毒、水力空化消毒、高锰酸钾消毒等多种消毒方式。在消毒过程中,消毒剂会不可避免的与水中的部分有机或无机物发生反应,生成一类对人体健康有害的物质,即消毒副产物(disinfection byproducts,DBPs)。自从Rook于1976年首次从消毒后的水中检测出三氯甲烷以来,已有超过600余种消毒副产物被人类识别和发现,主要包括:三卤甲烷、卤乙酸、卤代酮、卤代醛等[1-2]。近年来,有研究表明,在原水中有无机阴离子如溴离子或碘离子存在的情况下,消毒后的水中不仅会生成氯代消毒副产物(chlorinated disinfection byproducts,Cl-DBPs),还会有溴代消毒副产物(brominated disinfection byproducts,Br-DBPs)和碘代消毒副产物(iodinated disinfection byproducts,I-DBPs)的生成[3-4]。在我国,大部分沿海地区的水源是潮汐河流,靠近入海口,经常会受到咸潮入侵的影响,而海水中溴离子和碘离子的质量浓度是一般淡水的3 000多倍和十几倍,这将使得这些地区的水源水中有存在较高质量浓度溴离子和碘离子的风险,进而导致消毒过程中更容易生成Br-DBPs和I-DBPs。此外,在我国北方地区的一些城镇生活饮用水中的碘含量可以超过100 µg·L−1[5-6],2019年全国生活饮用水水碘含量调查报告表明[7],河北、天津、河南、山东等地区水碘含量较高,其中河北最高可达1 113.7 µg·L−1,天津最高可达427.0 µg·L−1,河南最高可达489.0 µg·L−1,山东最高可达901.0 µg·L−1。水体中较高的碘含量,不仅会增加人类患甲状腺疾病的风险,而且还会增加消毒过程中I-DBPs的生成。有研究表明,Br-DBPs和I-DBPs较Cl-DBPs具有更强的细胞毒性和遗传毒性[8-9],而包括中国、美国、以色列、澳大利亚、土耳其等世界各地的饮用水厂的出水中均发现有Br-DBPs和I-DBPs的存在[10-11],控制消毒过程中Br-DBPs和I-DBPs的生成已成为目前饮用水安全研究方面的热点和重点。
由于氯消毒会产生较多的三卤甲烷、卤乙酸等受到严格管控的含碳消毒副产物(carbonaceous disinfection byproducts, C-DBPs),因此,不少饮用水厂转而采用氯胺作为消毒剂。氯胺与消毒副产物前体物的反应活性远小于氯,能够有效减少消毒后三卤甲烷和卤乙酸的生成,且氯胺在管网中形成余氯的持续时间长,能够有效抑制管网中残留细菌的再繁殖。但氯胺消毒会导致一些毒性更强的含氮消毒副产物(nitrogenous disinfection byproducts,N-DBPs)如卤代乙腈、卤代乙酰胺、卤代硝基甲烷、亚硝胺类等生成的增加[12-13]。近年来,国内外学者对于N-DBPs的关注日渐增加,但多集中于氯代N-DBPs,如氯乙腈、氯代硝基甲烷、氯代乙酰胺等的生成特性、生成机理和生物毒性等方面。而当原水中存在高质量浓度的溴离子或碘离子时,氯胺消毒后的水中生成的N-DBPs也会由氯代向溴代和碘代转变,且溴代和碘代N-DBPs的毒性更是远高于氯代N-DBPs。
目前对于DBPs的定性和定量分析主要采用气相色谱、液相色谱结合质谱等方法实现。其中气相色谱与气相色谱质谱联用技术主要针对挥发性和半挥发性的DBPs进行定性和定量分析[14-15],而针对难挥发性或高极性的DBPs,特别是一些溴代和碘代DBPs,气相色谱与气相色谱质谱联用技术难以实现检测和分析。近年来,ZHANG等[16]和DING等[17]使用超高效液相色谱/电喷雾电离-三重四极杆质谱(ultra performance liquid chromatography/electrospray ionization-triple quadruple mass spectrometry,UPLC-ESI-tqMS)可实现对饮用水中极性Br-DBPs和I-DBPs的快速选择性检测。该方法基于质谱中的前体离子扫描(precursor ion scan,PIS)模式,在该模式下,可以检测出产生特定碎片离子的所有母离子分子。例如,可以通过将子离子的质荷比(m/z)设置为79/81,并扫描产生该子离子碎片的所有母离子,进而筛选出水样中的所有Br-DBPs,最后,通过分析同位素的丰度比以及结合其他子离子碎片信息,可以确定这些Br-DBPs的分子结构[18]。同样,通过将子离子的质荷比(m/z)设置为127,可以快速选择性地检测出I-DBPs[19]。
本研究以中国南方某沿海城市的饮用水厂和污水厂出水为研究对象,通过投加溴离子和碘离子来模拟咸潮入侵,考察这2类水体在氯胺消毒过程中溴代和碘代DBPs的生成情况,并采用UPLC/ESI-tqMS的PIS模式,快速选择性地检测和识别出氯胺消毒过程中生成的主要极性卤代DBPs。在此基础上,对2类水体中卤代DBPs的生成种类和数量进行对比。整个研究对于加深溴代和碘代DBPs的认识,保障人们群众的饮水安全具有重要意义。
1. 材料与方法
1.1 水样采集
选取福建省漳州市招商局经济技术开发区污水处理厂和自来水厂的出厂水作为研究对象。其中,污水处理厂采用常规二级处理+混凝沉淀+紫外消毒工艺,处理后的水可以达到污水一级A排放标准,并排放入周边海域;自来水厂采用常规处理工艺(混凝-沉淀-过滤-消毒),原水来自当地主要饮用水源——九龙江。采集的水样运回实验室后,经0.45 μm滤膜过滤并于4 ℃下冷藏避光保存。
1.2 药品与试剂
无水硫酸钠(分析纯)购自Aladdin,甲基叔丁基醚(methyl tert-butyl ether,MTBE,色谱纯)、乙腈(色谱纯)均购买自美国Tedia公司,氯化钠(优级纯)、次氯酸钠(分析纯)、溴化钠(分析纯)、硫代硫酸钠(分析纯)均购自西陇科学,碘化钾(分析纯)购买自汕头市达濠精细化学品有限公司,硫酸(优级纯)、氢氧化钠(分析纯)、盐酸(分析纯)、氯化铵(分析纯)均购自国药集团化学试剂有限公司。
1.3 氯胺消毒实验
一氯胺溶液由NaClO与NH4Cl溶液(保持二者质量比为4 mg·L−1 Cl2:1 mg·L−1 NH4+-N)于冰水浴下暗处混合反应30 min制得,需现用现配。制得的溶液采用N,N-二乙基-1,4-苯二胺分光光度法测定其一氯胺含量(以氯计)。
准确量取20 mL 1.1中采集的实际水样于带聚四氟乙烯瓶垫的圆底离心管中。通过加入一定量的溴化钠(500 mg·L−1 Br—)或碘化钾(100 mg·L−1 I—)溶液,使得水样中溴离子质量浓度达到了5 mg·L−1,碘离子质量浓度达到了0.5 mg·L−1,来模拟咸潮入侵的影响。然后以C(TOC):C(Cl2)= 1:5加入氯胺进行消毒,将水样放置于恒温培养箱内25 °C避光反应72 h。反应完毕后,取出水样,用100 mg·L−1的Na2S2O3溶液终止反应。
1.4 水样预处理方法
采用液液萃取。具体方法为:取消毒完毕后的水样,加入盐酸(1 mol·L−1)将pH调节至2.0,加入5 mL的MTBE作为萃取剂,再加入8 g氯化钠以促进分层,涡旋振荡1.5 min,静置5 min分层,将上层有机相吸出,加入3 g无水硫酸钠(使用前需经马弗炉400 °C灼烧并用MTBE洗涤)过滤至吹脱管中,氮吹至0.5 mL,与10 mL乙腈混合,氮吹至0.5 mL,移入棕色进样小瓶中,分析前采用超纯水定容至1 mL,经0.22 μm PTFE滤膜过滤后上机检测。
1.5 UPLC-ESI-tqMS分析测试条件
所用仪器为Agilent 1290 Infinity LC-6490 MS/MS超高效液相色谱-串联四极杆质谱联用仪,液相色谱柱为Proshell 120 EC-C18(100 mm×2.1 mm×2.7 µm,Agilent)。流动相由超纯水和乙腈组成,流速为0.25 mL·min−1,进样量为10 µL。流动相的梯度洗脱程序为:0~12 min从10%乙腈线性变化到90%,并保持3 min,在1 min内恢复到10%乙腈,16~18 min保持在10%,以在下一次进样之前重新平衡色谱柱。MS/MS检测中,ESI源采用负离子模式,MS参数如下:干燥气(氮气)温度160 °C;干燥气流速,16 L·min−1;鞘气(氮气)温度,350 °C;鞘气流速,11 L·min−1;毛细管电压,3 000 V。采用PIS模式,设置Q1为全扫描状态,Q3为选择离子监测状态,将Q3设置为允许m/z 79/81或m/z 127的特征碎片通过,能够检测到水样中几乎所有可以被ESI电离的溴代DBPs和碘代DBPs。串联UPLC系统,对ESI-tqMS PIS模式检测到的溴代DBPs和碘代DBPs进行分离,确定保留时间(retention time,RT)后,进行子离子扫描,获得分子离子的碎片信息。由于C-Br的键能强于C-I键的键能,并且C-Br的键长比C-I的键长短,因此I-DBPs的最佳碰撞能要小于Br-DBPs,根据ZHANG等人[16-18]的经验,本研究选择30 eV作为I-DBPs的最佳碰撞能,选择40 eV作为Br-DBPs的碰撞能,在此最佳碰撞能下,能够最大程度地减少“假”分子离子的干扰,如Cl2CHC—在碰撞能量超过15 eV 时,倾向于解离为Cl2CH—和Cl—,其对I-DBPs的干扰就可以排除。
2. 结果与讨论
2.1 水质指标分析
自来水厂出水和污水厂出水中的总有机碳(total organic carbon, TOC)、氨氮、特征紫外吸光度(specific ultraviolet absorbance, SUVA)、pH、溴离子、碘离子等水质指标如表1所示。
表 1 各水样水质指标Table 1. Water quality indicators of each water sample样品 TOC/(mg·L−1) 氨氮/(mg·L−1) SUVA pH 溴离子/(µg·L−1) 碘离子/(µg·L−1) 自来水厂出水 1.53 0.04 1.65 7.10 / / 污水厂出水 3.00 0.63 3.54 7.36 81 / TOC是以碳的含量来表示水样中有机物总量的综合性指标,SUVA是水样在254 nm处的吸光度与溶解性有机碳(dissloved organic carbon, DOC)的比值,可以反映水样中含碳碳双键、苯环等不饱和键有机物的含量。有研究表明,水样的TOC和SUVA往往和DBPs的生成量呈显著正相关关系。由表1可知,污水厂出水的TOC和SUVA 分别为自来水厂出水的1.96倍和2.15倍,由此可以推测,污水厂出水经氯胺消毒后生成的DBPs的总量会多于自来水厂出水。氨氮作为水中氮的主要存在形态,在氯胺消毒过程中,对N-DBPs的生成具有重要影响。由表1可知,污水厂出水中的氨氮为0.63 mg·L−1,远远超过自来水厂出水,因此,可以推测,污水厂出水经氯胺消毒后N-DBPs的生成量会远远超过自来水厂出水。溴和碘离子广泛存在于各种水体中,其作为DBPs的主要无机前体物,对DBPs的生成同样具有重要的影响。而在本研究选取的2个水样中,仅在污水厂出水中检测出81 µg·L−1的溴离子。虽然在一般情况下,水体中都不会存在较高质量浓度的溴离子和碘离子,但考虑到沿海地区的水源时刻面临咸潮入侵的风险,以本研究选取的九龙江为例,在咸潮入侵期间,九龙江原水中的溴离子和碘离子质量浓度最高可分别达到6 mg·L−1和0.2 mg·L−1。因此,有必要考察极端情况下(咸潮入侵导致水源中存在较高质量浓度的溴离子和碘离子)氯胺消毒过程中DBPs的生成情况。
2.2 氯胺消毒过程中生成的极性Br-DBPs的检测与识别
本研究采用UPLC/ESI-tqMS对氯胺消毒后的水样进行检测,首先设置PIS m/z 79/81进行扫描,检测所有的极性Br-DBPs,再根据天然同位素丰度比进一步推断物质中氯、溴原子个数。通过串联UPLC系统,对ESI-tqMS PIS模式检测到的极性Br-DBPs进行分离,确定每个母离子的保留时间后,调整仪器模式为子离子模式,进行子离子扫描,将Q1设置为选择离子监测状态,Q3设置为扫描状态,获得子离子的碎片信息,推断物质结构。水样的ESI-tqMS PIS m/z 79/81扫描质谱图如图1所示。
根据图1可知,m/z 171/173/175、m/z 171/173、m/z 216/218、m/z 233/235/237、m/z 250/252/254、m/z 294/296/298、m/z 327/329/331/333分别对应二溴乙酸(脱羧)、4-溴苯酚、2-溴-4-硝基苯酚、溴-氯-二甲基苯酚或3-溴-5-氯-4-羟基苯甲醛、2-溴-6-氯-4-硝基苯酚、2,6-二溴-4-硝基苯酚和2,4,6-三溴苯酚[18-20]。在本研究中,对检测发现的未知母离子m/z 199/201、m/z 278/280/282进行子离子扫描,得到分子离子的碎片信息,推断其分子结构。
1)离子簇m/z 199/201的分析鉴定。离子簇m/z 199/201有2个同位素峰,峰高比例接近1:1,说明该物质中含有一个溴原子。观测到一个分子质量为40 Da (119-79=40)的碎片丢失,根据Beynon表,该物质可能含有C2H2N、C3H4。由于该物质的出峰时间为6.272 min,说明分子中可能含有苯环或者是环状结构,在子离子扫描质谱图中看到一个m/z 62的子离子碎片,最多包含5个碳原子,说明该物质含有的环状结构很可能是环戊烯。由于该物质的分子质量为200 Da,减去1个溴,再减去环戊烯,剩余的57可能包含C2H2N(40)或C3H4(40)和OH(17)。此外,根据氮律,由C、H、O、N、P和卤素组成的有机化合物,若含有奇数个氮,相对分子质量一定是奇数,若含有偶数个氮或不含氮,相对分子质量一定是偶数,该物质的分子质量为200 Da,因此,该物质不含氮。综上所述,推断该物质的分子式可能为C8H9OBr,满足不饱和度计算公式,推断合理。该物质的可能结构为1-丙二烯-3-溴-2-羟基-环戊烯及其同分异构体。
2)离子簇m/z 278/280/282的分析鉴定。m/z 278/280/282有3个同位素峰,说明该物质中含有2个溴原子或1个氯原子和1个溴原子。3个同位素峰的峰高比例接近1:2:1,说明该化合物分子中含有2个溴原子。在子离子碎片图中观测到一个分子质量为30 Da (280-250=30)的碎片丢失,根据Beynon表,该碎片的分子式可能为NO、CH4N、H2N2、CH2O、C2H6。在子离子碎片图中观测到一个分子质量为58 Da (280-222=58)的碎片丢失,该碎片可能由28+30组成,根据Beynon表,分子质量为28 Da的分子式可能是N2、CO、CH2N或者C2H4。根据氮律与分子组成原则,分子质量为58 Da的碎片的分子式组成可能为NO和CO、NO和C2H4、CH4N和CO、CH4N和C2H4。由于该物质的分子质量为279 Da,减去2个溴(158),再减去58,剩余63的部分最多含有5个碳原子,此外该物质的出峰时间为6.942 min,说明分子中可能含有环状结构,因此,剩余部分很可能是环戊二烯。综上所述,该物质的分子式可能为C6H3O2NBr2、C7H7ONBr2或C8H11NBr2,满足不饱和度计算公式。该物质的可能结构为2,3-二溴-5-硝基-环戊二烯-1-醛、2,3-二溴-1-乙基-5-硝基-环戊二烯、2,3-二溴-5-甲氨基-环戊二烯-1-醛、2,3-二溴-1,-乙基-5-甲氨基-环戊二烯及其同分异构体。
2.3 氯胺消毒过程中生成的极性I-DBPs的检测与识别
极性I-DBPs的检测是通过设置模式PIS m/z 127来实现,其他步骤与极性Br-DBPs的检测方法一致。水样的ESI-tqMS PIS m/z 127扫描质谱图如图2所示。根据图2可知,m/z 185、m/z 241、m/z 247、m/z 267、m/z 277、m/z 291、m/z 307、m/z 311、m/z 373、m/z 390、m/z 471的物质结构在前人的研究中已提出[17,19-20],分别是一碘乙酸、一碘丁烯二酸、4-羟基-3-碘苯甲醛、二碘乙酸(脱羧)、3-碘-4-羟基-5-甲基-苯甲酸、3-甲酰基-4-羟基-5-碘苯甲酸、4-羟基-5-碘-1,3-苯二甲酸、二碘乙酸或2-碘-3-甲基-1-萘酸、4-羟基-3,5-二碘-苯甲醛、2,6-二碘-4-硝基苯酚、2,4,6-三碘苯酚。本研究中,检测发现未知母离子m/z 212、m/z 374、m/z 381、m/z 445,对4个未知母离子进行子离子扫描,得到分子离子的碎片信息,推断物质结构。
1)离子簇m/z 212的分析鉴定。离子簇m/z 212没有其他同位素峰,说明该物质中不含有溴原子和氯原子,该物质的出峰时间为1.147 min,说明分子中不含有苯环。在子离子扫描质谱图中看到一个m/z 127的子离子碎片,该物质应该仅含有一个碘,否则不符合分子组成原则。在子离子扫描质谱图中看到一个m/z 62的子离子碎片,根据Beynon表,该碎片的分子式可能为CH2O3、CH4NO3、CH6N2O或C2H6O2,由于该物质的分子质量是213 Da,根据氮律可以知道,分子组成中一定含有奇数个氮原子,因此,推断m/z 62的碎片分子式为CH4NO3。213减去62(CH4NO3)与127(I)后(213-62-127=24),剩余部分最多为2个碳原子,则物质分子式可能为C3H4O2NI,满足不饱和度计算公式,推断合理。该物质的可能结构为CHI=CHCH2NO2及其同分异构体。
2) 离子簇m/z 374的分析鉴定。离子簇m/z 374没有其他同位素峰,说明该物质中不含有溴原子和氯原子。在子离子碎片图中观测到一个分子质量为127 Da (374-247=127)的碎片丢失和一个分子质量为254 Da (374-120=254)的碎片丢失,说明该物质结构中有2个I。观测到一个分子质量为30 Da (374-344=30)的碎片, 根据Beynon表,该碎片的分子式可能为NO、H2N2、CH2O、CH4N或C2H6。观测到一个分子质量为92 Da (219-127=92)的碎片丢失, 根据Beynon表,该碎片的分子式可能为C2H4O4、C2H6NO3、C2H8N2O2、C3H8O3、C6H6N、C6H4O或C7H8,由于该物质的出峰时间为6.272 min,说明分子中可能含有苯环,因此,该碎片的分子式可能为C6H6N、C6H4O或C7H8。由于该物质的分子质量为375 Da,根据氮律,该物质分子组成中一定含有奇数个氮原子,因此,推断该物质的分子式可能为C6H7N3I2、C8H11NI2、C6H3O2NI2或C7H7ONI2,其中C6H7N3I2与C8H11NI2不符合分子组成原则,则该物质分子式为C6H3O2NI2或C7H7ONI2,满足不饱和度计算公式,推断合理。该物质的可能结构为2,6-二碘-4-亚硝基苯酚或2,6-二碘-4-甲氨基苯酚及其同分异构体。
3)离子簇m/z 381的分析鉴定。离子簇m/z 381没有其他同位素峰,说明该物质中没有溴原子和氯原子。该物质的出峰时间为1.022 min,说明分子中不含有苯环。在子离子碎片图中观测到一个分子质量为142 Da (381-239=142)的碎片丢失和一个分子质量为284 Da (381-97=284)的碎片丢失,说明该物质结构中有2个碘甲基(CH2I)。观测到一个分子质量为120 Da (381-261=120)的碎片丢失,该碎片可能为2个乙酸(CH3COOH),说明该物质的结构中应该有2个羧基,且很有可能有一个C=C。综上,推测该物质的分子式为C5H4I2O4,符合氮律且满足不饱和度计算公式。该分子结构可能为CH2IC(COOH)=CI-COOH及其同分异构体。
4)离子簇m/z 445的分析鉴定。离子簇m/z 445没有其他同位素峰,说明该物质中没有溴原子和氯原子。在子离子碎片图中观测到一个分子质量为127 Da (445-318=127)的碎片,一个分子质量为254 Da (445-191=254)的碎片丢失和一个分子质量为381 Da (445-64=381)的碎片丢失,说明该物质结构中有3个I。观测到一个分子质量为64 Da (191-127=64)的碎片丢失, 根据Beynon表,该碎片的分子式可能为C5H4。该物质的分子质量为446 Da,因此,推断该物质的分子式为C5H5I3。该物质的出峰时间为6.383 min,说明分子中可能含有环状结构。该物质的分子式为C5H5I3,不可能含有苯环。综上所述,推测该物质的结构可能为三碘代环戊烯,满足不饱和度计算公式,推断合理。
2.4 自来水厂与污水厂出水经氯胺消毒后生成的极性卤代DBPs种类和数量的比较
总离子流强度(total ion intensity,TII)为质谱在1次扫描中检测到的所有离子峰的离子流强度之和[17,21]。在本研究中,通过将子离子的m/z设置为 79/81,并将母离子质谱图中m/z在 90~500(m/z> 500的离子峰的离子流强度较低,不计算在内)内的所有离子峰的离子流强度相加,可指示水样中极性Br-DBPs的总生成量。通过将子离子的m/z设置为127,并将母离子质谱图中m/z在130~500内的所有离子峰的离子强度相加,可指示水样中极性I-DBPs的总生成量。图3反映了自来水厂与污水厂出水经氯胺消毒后生成的Br-DBPs与I-DBPs 的总离子强度。由图3可以看出,污水厂出水经氯胺消毒后,Br-DBPs和I-DBPs的TII分别为5265 251.58和6022 404.88,是自来水厂出水的3.68倍和1.30倍。污水厂出水经氯胺消毒后Br-DBPs与I-DBPs 的总生成量均大于自来水厂出水,这主要是因为污水厂出水的TOC(3.00 mg·L−1)大于自来水厂出水(1.53 mg·L−1),在相同条件下,TOC越高的水样,在氯胺消毒过程中生成DBPs的量越高。
表2总结了污水厂与自来水厂出水经氯胺消毒后检测到的所有极性卤代DBPs。从表2中可以看出,污水厂出水经氯胺消毒后生成的极性Br-DBPs的种类显著多于自来水厂出水。其中,m/z 171/173、m/z 199/201、m/z 233/235/237、m/z 250/252/254、m/z 278/280/282、m/z 327/329/331/333等物质在自来水厂出水中均未检测到。此外,污水厂出水中溴代N-DBPs(包括m/z 216/218、m/z 250/252/254、m/z 278/280/282、m/z 294/296/298)的离子强度较自来水厂出水水样高,而N-DBPs通常比C-DBPs的毒性更大。投加碘离子后经氯胺消毒的自来水厂出水水样与污水厂出水水样产生的极性I-DBPs种类基本一致,但污水厂出水水样中I-DBPs的离子强度更高,特别是碘代N-DBPs。导致污水厂出水水样中卤代N-DBPs含量更高的可能原因是污水厂出水中的氨氮较高。此外,污水厂出水中含苯环的芳香族卤代DBPs的含量较高,这可能是由于污水厂出水的SUVA较大,DBPs前体物中苯环或共轭双键等不饱和有机物较自来水厂出水多。
表 2 PIS模式检测到的极性卤代DBPsTable 2. Summary of polar halogenated DBPs detected by the PIS method保留时间/min m/z 分子式 自来水厂(离子强度) 污水厂(离子强度) 1.138 171/173/175 Br2CH‒(二溴乙酸脱羧) 32 027.30 29 648.04 7.121 171/173 C6H5BrO — 177 050.17 7.329 199/201 C8H9OBr — 26 709.16 6.658 216/218 C6H4BrNO3 59 143.04 294 934.40 3.287 233/235/237 C8H8ClBrO/ C7H4BrClO2 — 23 850.18 4.756 250/252/254 C6H3ClBrNO3 — 20 814.88 6.937 278/280/282 C6H3O2NBr2/C7H7ONBr2/C8H11NBr2 — 31 756.26 5.045 294/296/298 C7H7Br2NO2/ C6H3Br2NO3 12 335.46 61 958.09 9.920 327/329/331/333 C6H3Br3O — 31 623.82 1.045 185 ICH2COOH 10 471.12 2 206.56 1.045 212 C3H4O2NI 10 354.86 37 467.30 4.773 241 HOOCCI=CHCOOH 4 813.60 / 6.248 247 C7H5IO2 19 194.20 11 113.92 1.041 263/265 BrICHCOOH 9 653.30 12 649.32 1.222 267 I2CH‒(碘乙酸脱羧) 86 903.52 175 636.39 1.080 277 C8H7IO3 44 945.46 93 248.87 4.757 291 C8H5IO4 2 252.32 7 910.82 1.230 307 C8H5IO5 1 107.56 979.00 1.222 311 I2CHCOOH 3 467.16 7 815.92 4.573 373 C7H4I2O2 5 0451.95 96 620.75 6.272 374 C6H3O2NI2/C7H7ONI2 29 565.46 87 359.02 1.026 381 C5H4I2O4 56 681.59 94 595.47 6.106 390 C6H3I2NO3 16 543.80 53 547.95 6.380 445 C5H5I3 70 950.00 21 901.90 11.252 471 C6H3I3O 296 380.22 340 645.97 3. 结论
1)在有高质量浓度溴离子存在情况下,自来水厂和污水厂出水经氯胺消毒后,均检测到了大量的极性Br-DBPs生成。其中,m/z 199/201和m/z 278/280/282的Br-DBPs是在本研究中首次被检测出,通过对子离子碎片的扫描和分析,推断出其分别为1-丙二烯-3-溴-2-羟基-环戊烯和2,3-二溴-5-硝基-环戊二烯-1-醛及其同分异构体。
2)在有高质量浓度碘离子存在情况下,自来水厂和污水厂出水经氯胺消毒后,均检测到了大量的极性I-DBPs生成。其中,m/z 212、m/z 374、m/z 381和m/z 445等I-DBPs是在本研究中首次被检测出,通过对它们子离子碎片的扫描和分析,推断出它们的分子结构分别为1-碘-3-硝基-丙烯、2,6-二碘-4-亚硝基苯酚、1-碘-2-碘甲基-丁烯二酸和三碘代环戊烯及其同分异构体。
3)污水厂出水经氯胺消毒后生成的Br-DBPs和I-DBPs的TII分别为5265 251.58和602 2404.88,是自来水厂出水的3.68倍和1.30倍,且污水厂出水中部分溴代和碘代N-DBPs的离子强度远高于自来水厂出水。
-
表 1 各水样水质指标
Table 1. Water quality indicators of each water sample
样品 TOC/(mg·L−1) 氨氮/(mg·L−1) SUVA pH 溴离子/(µg·L−1) 碘离子/(µg·L−1) 自来水厂出水 1.53 0.04 1.65 7.10 / / 污水厂出水 3.00 0.63 3.54 7.36 81 / 表 2 PIS模式检测到的极性卤代DBPs
Table 2. Summary of polar halogenated DBPs detected by the PIS method
保留时间/min m/z 分子式 自来水厂(离子强度) 污水厂(离子强度) 1.138 171/173/175 Br2CH‒(二溴乙酸脱羧) 32 027.30 29 648.04 7.121 171/173 C6H5BrO — 177 050.17 7.329 199/201 C8H9OBr — 26 709.16 6.658 216/218 C6H4BrNO3 59 143.04 294 934.40 3.287 233/235/237 C8H8ClBrO/ C7H4BrClO2 — 23 850.18 4.756 250/252/254 C6H3ClBrNO3 — 20 814.88 6.937 278/280/282 C6H3O2NBr2/C7H7ONBr2/C8H11NBr2 — 31 756.26 5.045 294/296/298 C7H7Br2NO2/ C6H3Br2NO3 12 335.46 61 958.09 9.920 327/329/331/333 C6H3Br3O — 31 623.82 1.045 185 ICH2COOH 10 471.12 2 206.56 1.045 212 C3H4O2NI 10 354.86 37 467.30 4.773 241 HOOCCI=CHCOOH 4 813.60 / 6.248 247 C7H5IO2 19 194.20 11 113.92 1.041 263/265 BrICHCOOH 9 653.30 12 649.32 1.222 267 I2CH‒(碘乙酸脱羧) 86 903.52 175 636.39 1.080 277 C8H7IO3 44 945.46 93 248.87 4.757 291 C8H5IO4 2 252.32 7 910.82 1.230 307 C8H5IO5 1 107.56 979.00 1.222 311 I2CHCOOH 3 467.16 7 815.92 4.573 373 C7H4I2O2 5 0451.95 96 620.75 6.272 374 C6H3O2NI2/C7H7ONI2 29 565.46 87 359.02 1.026 381 C5H4I2O4 56 681.59 94 595.47 6.106 390 C6H3I2NO3 16 543.80 53 547.95 6.380 445 C5H5I3 70 950.00 21 901.90 11.252 471 C6H3I3O 296 380.22 340 645.97 -
[1] HUANG H, ZHU H H, GAN W H, et al. Occurrence of nitrogenous and carbonaceous disinfection byproducts in drinking water distributed in Shenzhen, China[J]. Chemosphere, 2017, 188: 257-264. doi: 10.1016/j.chemosphere.2017.08.172 [2] DU P H, ZHAO H, CAO H B, et al. Transformation of halobenzoquinones with the presence of amino acids in water: Products, pathways and toxicity[J]. Water Research, 2017, 122: 299-307. doi: 10.1016/j.watres.2017.06.007 [3] XIA Y, LIN Y L, XU B, et al. Iodinated trihalomethane formation during chloramination of iodate-containing waters in the presence of zero valent iron[J]. Water Research, 2017, 124: 219-226. doi: 10.1016/j.watres.2017.07.059 [4] ZHANG H F, YANG M. Characterization of brominated disinfection byproducts formed during chloramination of fulvic acid in the presence of bromide[J]. Science of the Total Environment, 2018, 627: 118-124. doi: 10.1016/j.scitotenv.2018.01.215 [5] WANG N, ZHANG G, XIONG R, et al. Synchronous moderate oxidation and adsorption on the surface of gama-MnO2 for efficient iodide removal from water[J]. Environmental Science & Technology, 2022, 56(13): 9417-9427. [6] YANG W, YU S C, CHEN C, et al. Stopping the supply of iodized salt alone is not enough to make iodine nutrition suitable for children in higher water iodine areas: A cross-sectional study in northern China[J]. Ecotoxicology and Environmental Safety, 2020, 188: 109930. doi: 10.1016/j.ecoenv.2019.109930 [7] 中国疾病预防控制中心. 全国生活饮用水水碘含量调查报告[R]. 2019 [8] LI X F, MITCH W A. Drinking water disinfection byproducts (DBPs) and human health effects: Multidisciplinary challenges and opportunities[J]. Environmental Science & Technology, 2018, 52(4): 1681-1689. [9] WAGNER E D, PLEWA M J. CHO cell cytotoxicity and genotoxicity analyses of disinfection byproducts: An updated review[J]. Journal of Environmental Sciences, 2017, 58: 64-76. doi: 10.1016/j.jes.2017.04.021 [10] WANG J, HAO Z N, SHI F, et al. Characterization of brominated disinfection byproducts formed during the chlorination of aquaculture seawater[J]. Environmental Science & Technology, 2018, 52(10): 5662-5670. [11] LUEK J L, SCHMITT K P, MOUSER P J, et al. Halogenated organic compounds identified in hydraulic fracturing wastewaters using ultrahigh resolution mass spectrometry[J]. Environmental Science & Technology, 2017, 51(10): 5337-5385. [12] HU J, SONG H, ADDISON J W, et al. Halonitromethane formation potentials in drinking waters[J]. Water Research, 2010, 44(1): 105-114. doi: 10.1016/j.watres.2009.09.006 [13] JEONG C H, POSTIGO C, RICHARDSON S D, et al. Occurrence and comparative toxicity of haloacetaldehyde disinfection byproducts in drinking water[J]. Environmental Science & Technology, 2015, 49(23): 13749-13759. [14] ZHANG B B, XU G J, LI L, et al. Facile fabrication of MIL-96 as coating fiber for solid-phase microextraction of trihalomethanes and halonitromethanes in water samples[J]. Chemical Engineering Journal, 2018, 350: 240-247. doi: 10.1016/j.cej.2018.05.180 [15] RHYS A A, CARTER D S, LIEW N W, et al. Simultaneous analysis of haloacetonitriles, haloacetamides and halonitromethanes in chlorinated waters by gas chromatography-mass spectrometry[J]. Chemosphere, 2018, 220: 314-323. [16] ZHANG X R, TALLEY J W, BOGGESS B, et al. Fast selective detection of polar brominated disinfection byproducts in drinking water using precursor ion scans[J]. Environmental Science & Technology, 2008, 42(17): 6598-6603. [17] DING G Y, ZHANG X R. A picture of polar iodinated disinfection byproducts in drinking water by (UPLC/ESI-tqMS)[J]. Environmental Science & Technology, 2009, 43(24): 9287-9293. [18] ZHANG D, WU Y, ZHANG X R, et al. Identification, formation and control of polar brominated disinfection byproducts during cooking with edible salt, organic matter and simulated tap water[J]. Water Research, 2020, 172: 115526. doi: 10.1016/j.watres.2020.115526 [19] PAN Y, ZHANG X R, LI Y. Identification, toxicity and control of iodinated disinfection byproducts in cooking with simulated chlor(am)inated tap water and iodized table salt[J]. Water Research, 2016, 88: 60-68. doi: 10.1016/j.watres.2015.10.002 [20] HAN J R, ZHANG X R, LIU J Q, et al. Characterization of halogenated DBPs and identification of new DBPs trihalomethanols in chlorine dioxide treated drinking water with multiple extractions[J]. Journal of Environmental Sciences, 2017, 58: 83-92. doi: 10.1016/j.jes.2017.04.026 [21] YANG M T, ZHANG X R, LIANG Q H, et al. Application of (LC/MS/MS) precursor ion scan for evaluating the occurrence, formation and control of polar halogenated DBPs in disinfected waters: A review[J]. Water Research, 2019, 158: 322-337. doi: 10.1016/j.watres.2019.04.033 -




 DownLoad:
DownLoad: