-
据《全国土壤污染调查公报 (2014年) 》[1]显示,我国目前受重金属污染的耕地面积约2.0×107 hm2,其中砷 (As) 是我国耕地、林地、草地的主要污染之一,超标率达到2.7%,重度污染点位占比为0.1%[2]。过度暴露于As污染环境中可导致皮炎、膀胱癌、神经毒性高血压等疾病[3-4]。美国有毒物质和疾病登记署 (TSDR) 基于重金属污染频率、毒性及潜在人体暴露可能性,评估得出As、Pb、Hg、Cd为4种最需控制的重金属[5]。固化/稳定化技术主要通过修复材料改变重金属形态,降低重金属可移动性及毒性,具有经济、快速的特点[6],生物炭作为一种稳定化材料对As具有一定的固定作用,因其来源广泛、价格低廉、环境友好等特点,在As修复研究中受到了极大关注[7]。然而,相比于其他重金属 (如Pb、Cd、Cr) ,生物炭对As稳定化效果更易受到环境影响。由于生物炭并未降低土壤中As总量,主要改变了As的形态,因此需要关注生物炭对As修复效果的长期稳定性。
通常认为,生物炭是一种稳定的炭材料,但目前越来越多证据表明生物炭施入土壤后会发生老化作用,其稳定性远低于预期[7-9]。KUZYAKOV等[10]研究表明,生物炭中超过80%的脂类和磷脂在3.5年内被分解,7%的稠合芳烃丢失。生物炭施入土壤2年后,其碳损失量高达11%~27%[9]。老化作用不仅使生物炭材料内不稳定碳发生矿化分解[7],降低碳总量,对碳形态、生物炭基本性质也会产生影响。例如,老化作用可能会导致生物炭中溶解性有机碳 (DOC) 质量分数增加,生物炭比表面积、表面官能团种类及数量、Zeta电位、灰分含量等发生变化。
生物炭材料老化是否会对生物炭钝化重金属效果产生影响?近几年一些学者开展了相关方面的研究。SU等[11]在对生物炭进行氧化老化的研究中发现,氧化老化会降低生物炭中K、Ca、Na等元素及C-O、O-C=O官能团数量,导致生物炭与Cd间共沉淀及π-π作用减弱;而ZHANG等[12]对生物炭进行了180 d高温和冻融老化后,发现老化后生物炭-OH、C=O官能团增强,进一步增强了其与Hg(II)的络合作用;黄晓雅等[13]通过30次干湿循环老化实验,发现老化作用提高了铈锰改性生物炭对红壤中As的稳定化效果,其原因可能是老化作用使生物炭表面酸性基团增强、pH下降、零点电荷增加,进而增强了生物炭对As静电吸附作用。但KIM等[14]发现,经25次干湿循环和冻融循环老化后,生物炭释放的DOC浓度分别是未老化生物炭的3.7、8.9倍,同时As的迁移性显著增强。老化条件下,生物炭与重金属间作用机制对重金属钝化效果的影响可能不同,然而,目前关于老化后生物炭对As稳定化效果和迁移行为影响仍不明确。实验室模拟老化实验可通过模拟自然环境中温度、水分等因素变化对生物炭的碳总量、形态[15]和基本理化性质[16]产生的影响,探究老化作用对生物炭与重金属间的吸附、钝化行为的影响。
本研究以As为主要研究对象,13C标记的生物炭为原料,通过吸附实验,制备As吸附饱和的13C标记生物炭,将其进行2种实验室模拟老化实验,拟通过分析老化前后生物炭的基本性质、碳总量、碳形态与生物炭钝化As的稳定性、As形态变化,探究老化作用对生物炭钝化As效果的影响,以期为评估生物炭钝化As的长期修复提供参考。
-
1) 13C标记生物炭的制备。采用脉冲标记法获取具有13C标记功能的玉米秸秆[17],通常随着制备温度提高,生物炭比表面积、孔隙率增大[18],碳稳定增强,产量降低,有利于生物炭对重金属的吸附;但过高的热解温度会导致生物炭孔道结构坍塌,从而降低吸附性能[19],兼顾生物炭稳定性、产量与重金属吸附效果,热解温度通常为500~700 ℃[20]。故本研究将13C标记的玉米秸秆放入马弗炉中在550 ℃下,热解3 h,得到13C标记生物炭,研磨过100目筛后备用。13C丰度 (δ) 测定表明,经过3次脉冲标记,生物炭δ13C由-13.28‰提高至27.81‰,13C已成功负载于生物炭中。
2) 饱和吸附As生物炭的制备。根据吸附动力学和吸附热力学实验,确定生物炭对As的吸附平衡时间为24 h,最大饱和吸附量为47.37 mg·kg−1,本实验需要获得饱和吸附As的生物炭,作为老化实验材料,因此将As2O3溶于水配置浓度为2 mmol·L−1的亚砷酸溶液,准确称取0.15 g 13C标记生物炭于50 mL聚乙烯离心管中,加入40 mL亚砷酸溶液,以0.01 M的NaNO3溶液作为电解质,调节溶液pH为5 (用0.01 M NaOH或HNO3调节) ,25 ℃、250 r·min−1条件下振荡24 h,离心后,过0.22 μm滤膜,收集滤上物35 ℃下烘干,得到As饱和吸附的生物炭,并根据以上方法批量制备,共得饱和吸附As生物炭20 g,过100目筛后收集备用,记为BC。
-
1) 生物炭老化实验。对饱和吸附As的生物炭 (BC) 分别进行为期60 d冻融老化 (freeze-thaw aging) 和自然老化 (spontaneous aging) ,分别记为BCFTC、BCSPON:冻融老化:将生物炭置于密封不透光容器中保持最大持水率的40%,每天在-79 ℃下保持19 h,25 ℃下保持5 h进行循环培养,共培养60 d;自然老化:将生物炭置于密封不透光容器中保持最大持水率40%,置于实验室桌面在室温下进行老化,共培养60 d。老化全过程中所有老化样品均在黑暗中培养,并设置3组平行。
2) 老化作用对生物炭基本性质影响。在老化第0、20、40、60 d进行非破坏性取样,通过pH计 (pHS-3C,中国上海雷磁科学仪器股份有限公司) 测定生物炭pH (生物炭与水比为1∶200) ;通过马尔文电位粒度仪 (Zetasizer Nano ZSP,英国马尔文仪器有限公司) 对生物炭Zeta电位进行测定。本实验通过比表面积分析仪 (BET,V-sorb 2800,北京金埃谱科技有限公司) 测定老化前后生物炭比表面积、孔体积;通过傅里叶变换红外光谱仪 (Nicolet IS 5,美国赛默飞世尔科技有限公司) 定性分析老化前后生物炭表面官能团。
3) 老化作用对生物炭的碳总量、碳形态的影响。通过老化前后生物炭中13C丰度测定,定量分析生物炭中碳总量变化。采用同位素质谱仪 (Isoprime-100,艾力蒙塔贸易 (上海) 有限公司) 对生物炭的13C/12C进行测定,计算生物炭13C丰度,计算公式见式(1)。
式中:δ13C为生物炭13C丰度,‰;R样为被测样品的13C与12C的稳定同位素丰度比值;RPDB为标准物质的13C与12C的稳定同位素丰度比值,为常数0.011 237 2。
通过高温煅烧法测定老化前后生物炭灰分质量分数[21];通过元素分析仪 (Elementar Vario EL,德国Elementar公司) 测定老化前后生物炭中C、H、N、S元素质量分数,O元素质量分数通过差减法计算而得,计算公式见式(2)。
式中:ωO为生物炭中O元素质量分数,%;ωC为生物炭中C元素质量分数,%;ωN为生物炭中N元素质量分数,%;ωH为生物炭中H元素质量分数,%;ωS为生物炭中S元素质量分数,%;ω灰分为生物炭中灰分质量分数,%。
在老化第0、20、40、60 d取样,采用TOC分析仪 (Muti N/C 3100 TOC,德国耶拿分析仪器股份公司) 测定生物炭DOC质量分数 (生物炭与水比为1∶100) 。
4) 老化作用对生物炭钝化As稳定性影响。在老化第0、20、40、60 d取样,通过美国环境保护署毒性特征浸出程序 (Method 1311: Toxicity characteristic leaching procedure,TCLP) [22]评估不同老化处理生物炭中As的浸出毒性 (TCLP-As) [23-24]。通过X射线光电子能谱仪 (XPS,EscaL-ab 250Xi,赛默飞世尔科技 (中国) 有限公司) 测定老化前后As价态变化;采用Tessier连续浸提法分析老化前后As (Tessier-As) 形态变化[25],并参照KE等[26]方法,根据提取难度将固定在生物炭中的As的形态划分为“易生物利用态” (可交换态) 、“潜在生物利用态” (碳酸盐结合态) 和“非生物利用态” (铁锰氧化态、有机结合态和残渣态) 对老化前后生物炭形态进行分析。通过原子荧光光度计 (AFS-8220,北京吉天仪器有限公司) 测定As浓度。同时,所有处理均设3组平行,取3次实验的平均值,所有平行样的分析误差均小于6%,加标回收率为96.0%~109.3%,符合《水质汞、砷、硒、铋和锑的测定 原子荧光法》 (HJ 694-2014) 标准[27-28]。
-
1) 老化作用对生物炭表观结构的影响。生物炭老化前后的比表面积和孔体积参数见表1。BC的比表面积和孔体积分别为1.786 m2·g−1和0.012 cm3·g−1,经60 d冻融老化和自然老化后,相较于BC,BCFTC和BCSPON比表面积和孔体积增幅分别为205.8%、137.5%和275.0%、250.0%。这可能是由于老化作用促进了生物炭石墨层膨胀、破裂及重组[16]。此外,温度、水分等因素也可能加速生物炭孔隙中无定形碳的溶解,导致原本被覆盖或堵塞的孔隙暴露[29],因此60 d老化作用增加了生物炭比表面积和孔体积。
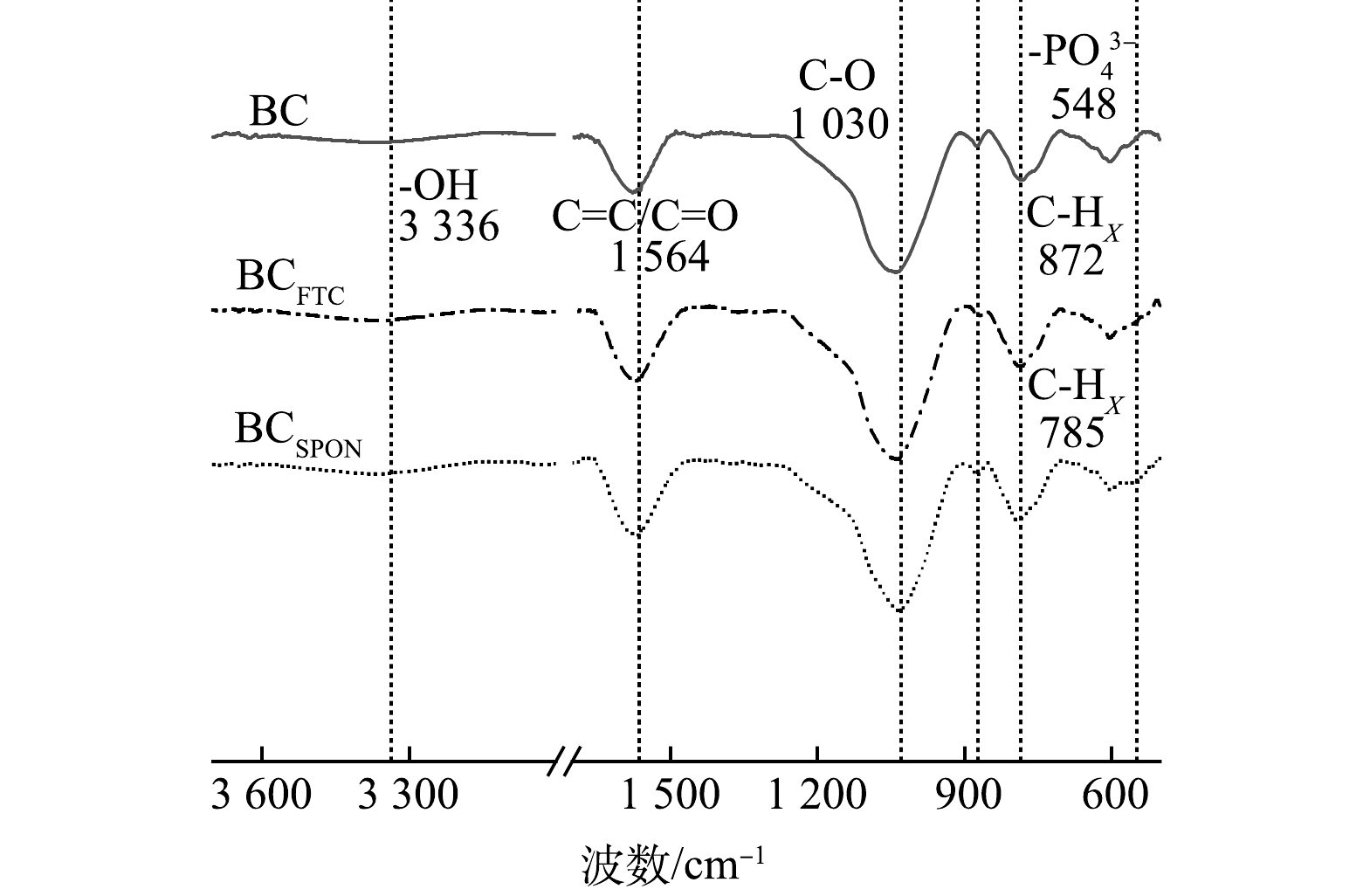
2) 老化作用对生物炭表面官能团影响。为探究老化作用对生物炭表面官能团种类及数量影响,对老化前后生物炭进行傅里叶红外光谱 (FTIR) 表征分析见图1。由图1可知,60 d冻融老化和自然老化后,生物炭表面的脂肪族官能团-OH (3336 cm−1) 、C=C/C=O (1564 cm−1) [21]、C-O (1030 cm−1) [14]、-CHX (872 cm−1和785 cm−1) [21]的吸收峰振幅小幅增强。这与XU等[30]和ZHANG等[12]报道一致,其主要原因可能是老化过程中生物炭发生脱氢脱氧反应或生物炭表面发生羟基化,增加了含氧官能团数量,可为生物炭吸附As提供更多吸附位点。此外,BCSPON在548 cm−1处出现了-PO43−的新峰,表明自然老化过程中生物炭表面可能发生破裂,有利于生物炭内部P等物质的溶出[23],因而新增-PO43−特征峰。
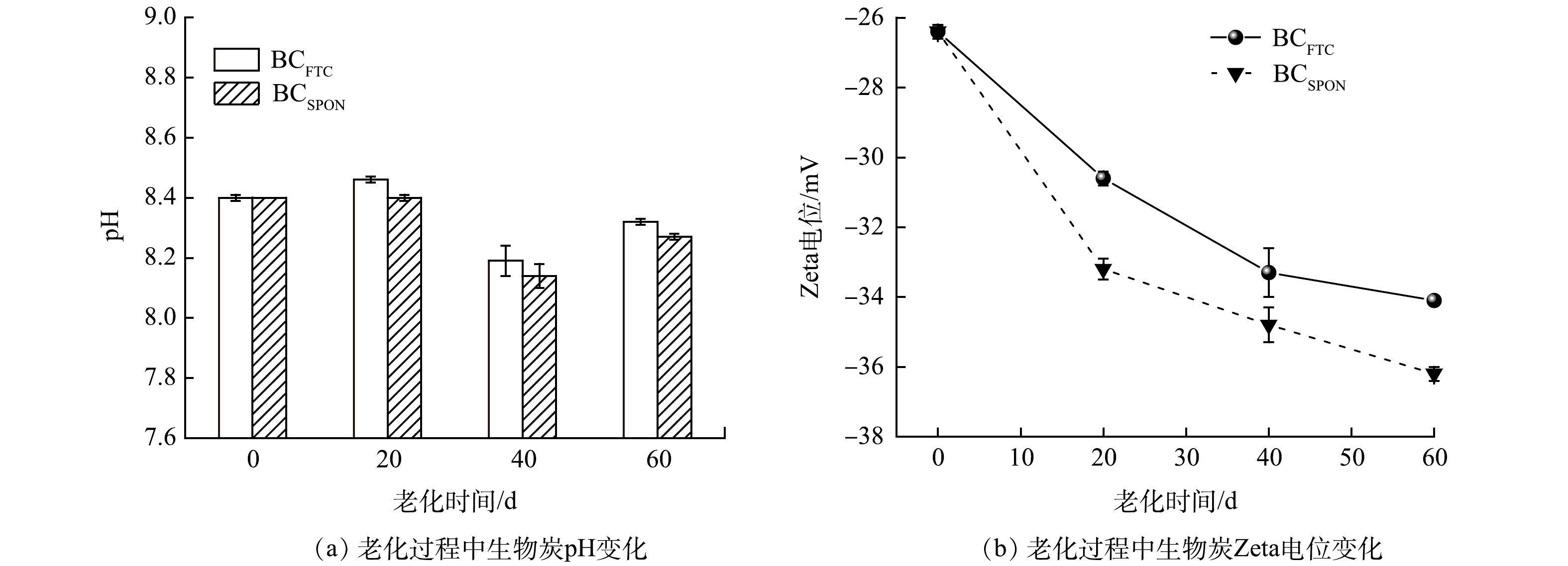
3) 老化作用对生物炭pH和Zeta电位影响。老化过程中BCFTC、BCSPON的pH变化如图2(a)所示。2种老化作用初期生物炭pH均呈现升高趋势,其原因为老化作用促进生物炭内部碱性物质释放增加了pH;在老化第40 d时,2种老化生物炭的pH均较20 d时降低,可能由于氧化作用增加了生物炭表面酸性官能团数量[24-25],或老化过程中生物炭吸收CO2生成碳酸盐[31]。但相较BC,老化60 d后,BCFTC、BCSPON的pH变化并不明显。
图2(b)为2种老化生物炭Zeta电位的随老化时间的变化。老化过程中生物炭Zeta负电位随老化时间增加而增强,可能由2方面原因造成:首先,老化作用增加了生物炭-OH数量,因而生物炭中可去质子化基团数量增加,表面负电荷相应增加;其次,在生物炭的碳骨架中,当O、N原子与C、H原子结合时,电子会向电负性更强的O、N原子转移,增加生物炭表面负电荷量,由表2可知,老化作用增加了生物炭(O+N)/C比值,故生物炭表面负电荷增加[32],Zeta负电位增强。由于生物炭与As表面均呈现电负性,老化过程中生物炭电负性的增强可能导致生物炭与As间静电斥力增强,增加As释放的风险。
-
1) 老化作用对生物炭碳总量的影响。稳定同位素示踪技术具有高度的专一性和灵敏度,可以通过测量同位素丰度比值来精确定量生物炭中碳总量[33],60 d老化前后生物炭δ13C测定结果如表2所示,结果表明,60 d老化作用后,δ13C由28.81‰ (BC) 分别降低至27.73‰ (BCFTC) 、27.13‰ (BCSPON) ,这可能归因于老化作用促进生物炭中部分不稳定碳以CO2形式释放。由于老化作用后仍有97.7%~99.71%的碳赋存于生物炭中,表明老化作用对生物炭碳总量影响较小。
生物炭老化前后元素组成、灰分质量分数、原子比值测定分析如表2所示。60 d老化后,BCFTC和BCSPON中O元素质量分数由21.48% (BC) 分别增加至22.36%和25.29%,主要归因于氧化作用提高了生物炭表面含氧官能团数量,与FTIR分析结果一致。老化后,BCFTC和BCSPON的灰分质量分数分别降低了1.82%和2.87%,这可能是由于在氧化和浸出过程中去除了部分可溶性固体和碱性盐[26]。生物炭H/C、O/C和(N+O)/C值常被用于评估生物炭的亲水性、芳香性和极性[34],2种老化作用均提高了生物炭的H/C、O/C和(N+O)/C值,这与SORRENTI等[35]和刘文慧等[36]研究结果一致,表明老化作用降低了生物炭的亲水性和芳香性程度,增强了生物炭极性,其中极性的增强可能对重金属的钝化产生影响[37]。
2) 老化作用对生物炭碳形态的影响。老化过程中生物炭DOC质量分数测定如图3所示,冻融老化20 d后DOC质量分数达到最大值,由1.27 mg·g−1增加至4.14 mg·g−1,自然老化40 d后DOC质量分数达到最大值,由1.27 mg·g−1增加至4.75 mg·g−1;老化60 d后,BCFTC和BCSPON中DOC质量分数分别为3.82、4.4 mg·g−1,分别是老化前的3.01、3.46倍。老化作用促进生物炭中无定形碳以DOC形式释放,由于DOC表面含大量含氧官能团且迁移性较强,可通过络合作用固定As,并作为载体促进DOC-As共迁移[38],因而DOC质量分数的增加可能增加As释放风险。老化作用下,生物炭中DOC质量分数的增加可能与本研究中制备生物炭所使用的生物质有关。本研究以玉米秸秆为生物质制备生物炭,玉米秸秆中纤维素质量分数较高,因而制备而成的生物炭中无定形碳所占比重较大[39]。老化作用下,受到温度、水分等因素的影响,生物炭石墨层可能发生放热膨胀,孔隙中无定形碳可能以溶解性炭黑、苯环、DOC等形式释放[40],故生物炭中DOC质量分数增加。
-
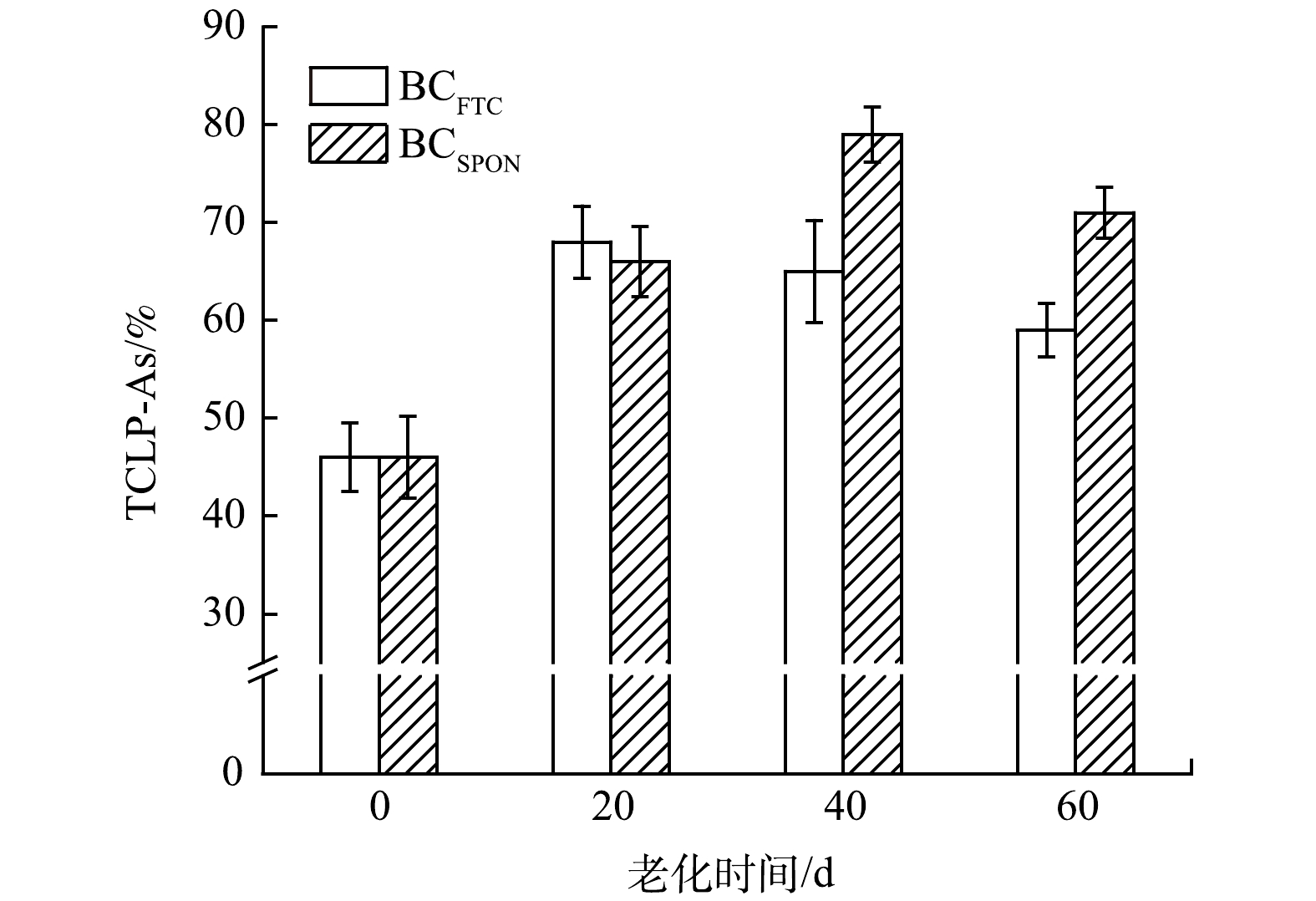
1) 老化过程中生物炭钝化As的稳定性分析。由于生物炭通过孔道扩散、静电作用、络合作用、共沉淀等机制固定As[16],故老化作用下生物炭基本性质、碳总量、碳形态的变化可能会对其钝化重金属稳定性产生影响。老化作用对生物炭中TCLP-As影响如图4所示。老化前TCLP-As为46%,BCFTC在老化20 d后TCLP-As达到最大值为68%,老化60 d后TCLP-As降低至59%,老化60 d较老化前增幅为28.3%;BCSPON在老化40 d后TCLP-As达到最大值为79%,老化60 d后TCLP-As降低至71%,老化60 d较老化前增幅为54.3%。2种老化作用后,TCLP-As浸出率均明显提高,这可能与生物炭中DOC质量分数、Zeta电位、pH、含氧官能团种类及数量等因素有关。
不同老化作用下TCLP-As与DOC质量分数、Zeta电位、pH相关性分析如表3所示。冻融老化、自然老化生物炭的TCLP-As均与DOC质量分数呈显著正相关,与Zeta电位、pH间负相关性并不显著,这表明老化作用导致的生物炭中DOC质量分数的增加是促进As活化的主要驱动因子。在生物炭固定As应用中,灰分可在生物炭表面提供带正电的活性位点,通过静电作用与共沉淀作用固定As[41],灰分中K+、Ca2+、Mg2+等离子可通过离子交换作用增强生物炭对As的钝化[42],本研究中,2种老化作用下,生物炭中灰分质量分数均有所降低,共沉淀作用可能降低,这对TCLP-As浸出率提高也具有重要影响。
对比2种老化方式,自然老化作用下TCLP-As浸出率高于冻融老化,这可能由于与冻融老化相比,自然老化下生物炭中DOC质量分数和Zeta负电位均较高,生物炭与As的静电斥力增强,灰分质量分数较低,As与灰分中重金属共沉淀减弱,均不利于As的固定,此外,自然老化提高了PO43−的溶出,PO43−与As会竞争生物炭表面的吸附点位,从而促进As从生物炭表面解吸,导致生物炭已吸附As的活化[43];另一方面,老化作用增加了生物炭比表面积、孔体积,为As提高更多吸附位点,也增强了As在生物炭中孔道扩散作用[13],可部分抵消DOC、PO43−、Zeta电位对As的活化作用。其中,冻融老化对生物炭比表面积、孔体积的增加更为明显,故老化后TCLP-As浸出率表现为:自然老化>冻融老化。
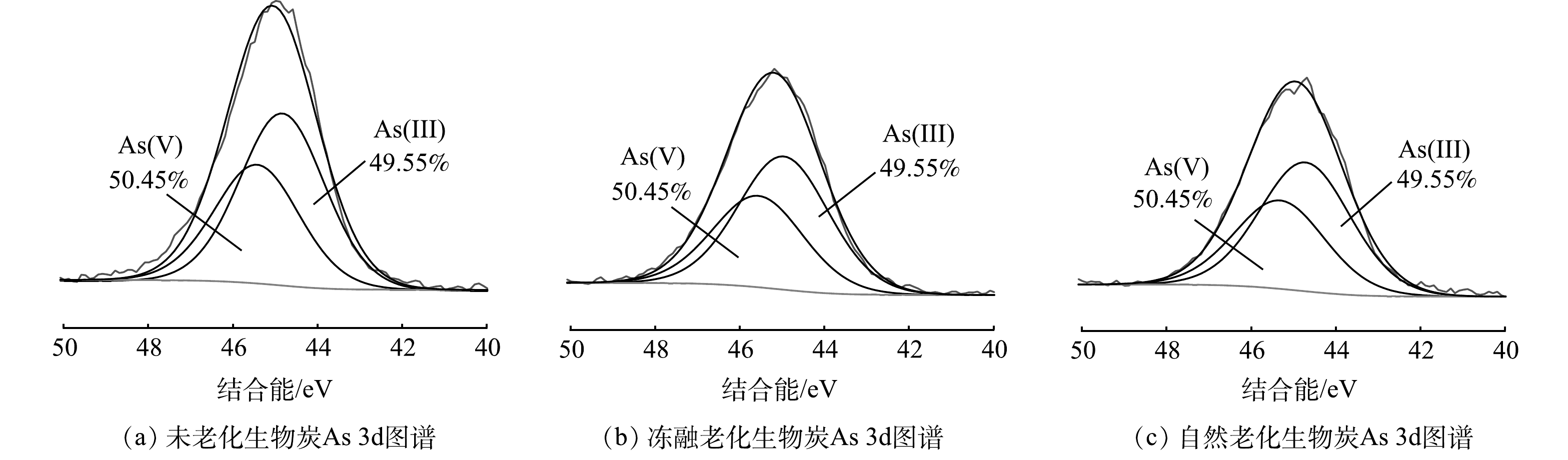
2) 老化作用对As形态的影响。通过XPS As 3d精细图谱表征,分析老化前后As的价态的变化,表征结果如图5所示。60 d的冻融老化和自然老化并未对生物炭表面吸附的As的价态产生明显影响,可能归因于As价态转化主要依赖于微生物作用[44]。然而,本实验中使用的灭菌后的生物炭,且实验环境并不适合微生物生长,故老化作用对生物炭吸附As的价态基本不产生影响。
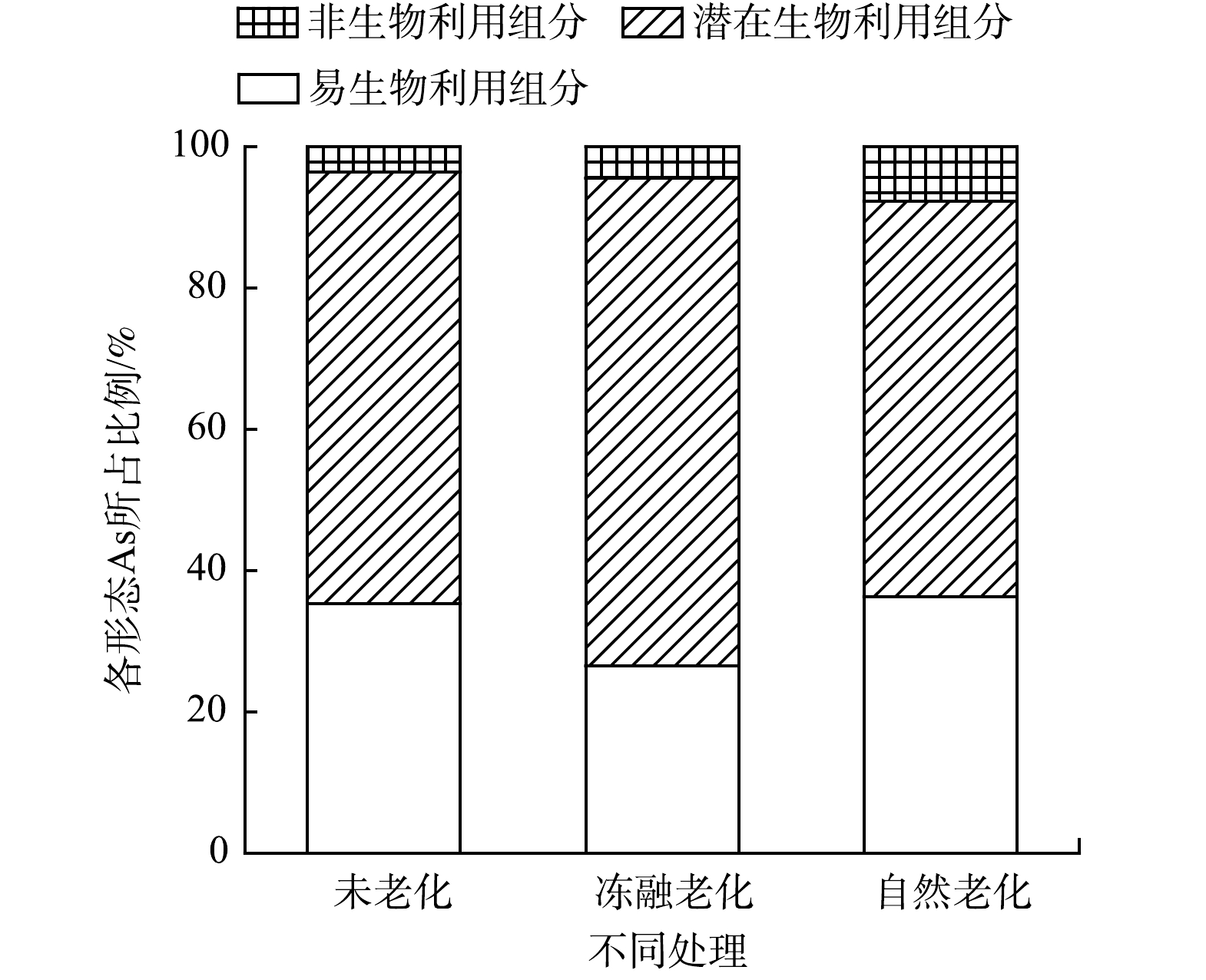
As的毒性与其赋存形态密切相关。基于Tessier形态提取法的As形态分析结果如图6所示。2种老化前后As各形态所占比例均表现为:潜在生物利用态>易生物利用态>非生物利用态。相较于BC,BCFTC中As易生物利用态所占比例降低8.8%、潜在生物利用态所占比例升高7.9%、非生物利用态所占比例升高0.9%,表明冻融老化促进As由易生物利用态向潜在生物利用态转化,这可能归因于冻融老化提高了生物炭对As的物理吸附;BCSPON中As易生物利用态所占比例增加1%、潜在生物利用态所占比例降低5.1%、非生物利用态所占比例升高4.1%,表明自然老化促进As由潜在生物利用态向非生物利用态和易生物利用态转化,这可能由于老化过程中pH的降低导致As由碳酸盐结合态向铁锰氧化态发生转化,易生物利用态的提高可能是DOC质量分数和PO43−数量的提升,导致As与DOC络合或被P竞争生物炭表面的吸附点位而活化。
从As形态分布可以看出 (图6) ,BC、BCFTC、BCSPON中As的易生物利用态分别占35.3%、26.5%和35.3%,潜在生物利用部分分别占61.1%、69%、61.1%,明显高于非生物利用部分,表明老化与未老化生物炭上吸附的As均存在较大环境风险,老化作用对生物炭中As形态影响并不明显。
-
1) 60 d的冻融老化和自然老化明显提高了生物炭中TCLP-As浸出率,生物炭中DOC质量分数的增加是降低生物炭钝化As效率的主要因素;此外,Zeta负电位增强、灰分质量分数降低、PO43−数量增加等对降低生物炭钝化As效率也具有一定的影响作用。
2) 在生物炭钝化As应用中,可通过优化生物炭制备条件、生物炭改性等方法,减少DOC-As复合物对As活化和协同迁移的影响[16]、降低生物炭与As间静电斥力,增强生物炭对As钝化的稳定性。
3) 本研究主要探究了温度变化引起的老化作用对生物炭钝化As的影响,但自然条件下,土壤理化性质、气候条件、其他污染物竞争吸附等,也可能影响生物炭在真实环境中对As钝化稳定性,未来可进一步研究在田间条件下长期老化对生物炭钝化As稳定性的影响。
基于模拟老化和同位素技术的生物炭砷钝化效率评估
Assessment of biochar immobilization on arsenic based on simulated aging and isotope technology
-
摘要: 为探究生物炭钝化砷长期效果,运用实验室模拟老化和稳定同位素技术,对饱和吸附砷 (As) 的13C标记生物炭分别进行60 d冻融老化和自然老化实验,通过老化前后生物炭材料、碳总量、碳形态、TCLP提取态As (TCLP-As) 、As形态变化,评估生物炭材料老化对钝化砷稳定性的影响。结果表明,老化作用未明显改变As的价态 (As3+,As5+) ,但降低了生物炭对As的钝化效果,TCLP-As由老化前的46%分别增加至60% (冻融老化) 和63.6% (自然老化) 。这主要归因于老化作用促进生物炭中部分不稳定碳转化为溶解性有机碳 (DOC) ,而DOC具有较强迁移性,其丰富的含氧官能团可以与As络合,形成DOC-As团聚体而发生共迁移;此外,老化后生物炭Zeta负电位、PO43−数量的增加,灰分质量分数、pH的降低,导致生物炭与As间静电斥力增强,离子交换、沉淀作用减弱,促进了As的解吸释放,故生物炭对As的钝化效果降低。对比2种老化,自然老化后,生物炭与As之间静电斥力、As与PO43−竞争生物炭表面吸附点位更强,沉淀作用减弱更为明显;而冻融老化后生物炭比表面积相对较大,可提供更多吸附点位,故自然老化后生物炭钝化As稳定性低于冻融老化。本研究结果可为生物炭钝化砷的应用提供参考。Abstract: Simulated aging and stable isotope technology was conducted in laboratory to investigate the long-term immobilization of biochar on arsenic (As). 13C-labeled biochar with As-saturated adsorption was obtained through adsorption experiment, and then the freeze-thaw aging and spontaneous aging experiments were carried out for 60 days. The carbon stability of biochar was evaluated by the change of biochar material, total carbon content and morphology of biochar, immobilization of As by TCLP extraction and Tessier sequential extraction before and after the aging. The results showed that the As valence state (As3+, As5+) was not significantly affected by aging effect. However, the TCLP-As was increased from 46% (unaged) to 60% (freeze-thaw aging) and 63.6% (spontaneous aging) respectively. This was mainly due to the transformation of unstable carbon in biochar into dissolved organic carbon (DOC) after aging, which had strong mobility and rich oxygen-containing functional groups. The DOC was easy complex with As and co-migration as DOC-As complex. In addition, the increased Zeta negative potential and the concentration of PO43− and the decreased ash mass fraction and pH of biochar enhanced electrostatic repulsion between biochar and As, and then reduced the ion exchange and precipitation between biochar and As. Compared with freeze-thaw aging and spontaneous aging, the freeze-thaw aging was more conducive to pore adsorption, while for spontaneous aging, the electrostatic repulsion and competitive adsorption between biochar and As were stronger, and obviously weakening of precipitation of As and PO43−. Therefore, the stability of biochar immobilization of As after spontaneous aging was lower than that of freeze-thaw aging. This study could provide a good reference for the application of biochar passivation of arsenic.
-
Key words:
- freeze-thaw aging /
- spontaneous aging /
- As morphology /
- 13C stable isotope /
- dissolved organic carbon
-
表 1 老化前后生物炭比表面积与孔体积分析
Table 1. Analysis of specific surface area and pore volume of original and aged biochar
生物炭类型 比表面积/(m2·g−1) 比表面积增幅/% 孔体积/ (cm3·g−1) 孔体积增幅/% BC 1.786 — 0.012 — BCFTC 5.462 205.8 0.045 275.0 BCSPON 4.241 137.5 0.042 250.0 表 2 生物炭老化前后δ13C、元素组成、灰分质量分数和元素摩尔比
Table 2. The δ13C, elemental composition, ash Mass fraction and molar ratio of elements of original and aged biochar
生物炭类型 δ13C/‰ 元素质量分数/% ω (灰分) /% 原子比 N C H S O H/C O/C (O+N)/C BC 27.81 1.81 54.76 1.74 0.17 21.48 20.04 0.29 0.38 0.32 BCFTC 27.73 1.85 55.60 1.82 0.15 22.36 18.22 0.30 0.39 0.33 BCSPON 27.13 1.78 54.11 1.72 0.13 25.29 17.17 0.35 0.38 0.38 表 3 不同老化过程中As浸出量与生物炭Zeta电位、DOC质量分数、pH相关性分析
Table 3. Correlation analysis between As leaching ratios with biochar Zeta potential, DOC mass fraction, pH during different aging processes
老化方式 TCLP-As与Zeta电位相关性 TCLP-As与DOC质量分数相关性 TCLP-As与pH相关性 冻融老化 −0.452 0.956* −0.177 自然老化 −0.932 0.963* −0.805 注:*表示在0.05水平 (双侧) 上显著相关。 -
[1] 环境保护部, 国土资源部. 全国土壤污染状况调查公报[EB/OL]. [2014-04-17]. https://www.mee.gov.cn/gkml/sthjbgw/qt/201404/W020140417558995804588.pdf. [2] ZENG S, MA J, YANG Y, et al. Spatial assessment of farmland soil pollution and its potential human health risks in China[J]. Science of the Total Environment, 2019, 687: 642-653. doi: 10.1016/j.scitotenv.2019.05.291 [3] 罗海艳. 铁锰改性生物炭对土壤镉砷形态及水稻积累镉砷的影响[D]. 湖南农业大学, 2019. [4] PODGORSKI J, BERG M. Global threat of arsenic in groundwater[J]. Science, 2020, 368(6493): 845-850. doi: 10.1126/science.aba1510 [5] GONG H, ZHAO L, RUI X, et al. A review of pristine and modified biochar immobilizing typical heavy metals in soil: Applications and challenges[J]. Journal of Hazardous Materials, 2022: 128668. [6] AHMED W, MEHMOOD S, NÚÑEZ-DELGADO A, et al. Adsorption of arsenic(III) from aqueous solution by a novel phosphorus-modified biochar obtained from Taraxacum mongolicum Hand-Mazz: Adsorption behavior and mechanistic analysis[J]. Journal of Environmental Management, 2021, 292: 112764. doi: 10.1016/j.jenvman.2021.112764 [7] HAN L, SUN K, YANG Y, et al. Biochar’s stability and effect on the content, composition and turnover of soil organic carbon[J]. Geoderma, 2020, 364: 114184. doi: 10.1016/j.geoderma.2020.114184 [8] KNICKER H, HILSCHER A, De la ROSA J M, et al. Modification of biomarkers in pyrogenic organic matter during the initial phase of charcoal biodegradation in soils[J]. Geoderma, 2013, 197: 43-50. [9] ROMBOLA A G, FABBRI D, MEREDITH W, et al. Molecular characterization of the thermally labile fraction of biochar by hydropyrolysis and pyrolysis-GC/MS[J]. Journal of Analytical and Applied Pyrolysis, 2016, 121: 230-239. doi: 10.1016/j.jaap.2016.08.003 [10] KUZYAKOV Y, BOGOMOLOVA I, GLASER B. Biochar stability in soil: decomposition during eight years and transformation as assessed by compound-specific 14C analysis[J]. Soil Biology and Biochemistry, 2014, 70: 229-236. doi: 10.1016/j.soilbio.2013.12.021 [11] SU Y, WEN Y, YANG W, et al. The mechanism transformation of ramie biochar’s cadmium adsorption by aging[J]. Bioresource Technology, 2021, 330: 124947. doi: 10.1016/j.biortech.2021.124947 [12] ZHANG S, YANG X, JU M, et al. Mercury adsorption to aged biochar and its management in China[J]. Environmental Science and Pollution Research, 2019, 26: 4867-4877. doi: 10.1007/s11356-018-3945-3 [13] 黄晓雅, 李莲芳, 朱昌雄, 等. 干湿交替对铈锰改性生物炭固定红壤As的影响[J]. 环境科学, 2021, 42(21): 5997-6005. doi: 10.13227/j.hjkx.202105007 [14] KIM H, KIM J, KIM T, et al. Interaction of biochar stability and abiotic aging: Influences of pyrolysis reaction medium and temperature[J]. Chemical Engineering Journal, 2021, 411: 128441. doi: 10.1016/j.cej.2021.128441 [15] JIANG S, DAI G, LIU Z, et al. Field-scale fluorescence fingerprints of biochar-derived dissolved organic matter (DOM) provide an effective way to trace biochar migration and the downward co-migration of Pb, Cu and As in soil[J]. Chemosphere, 2022, 301: 134738. doi: 10.1016/j.chemosphere.2022.134738 [16] WANG L, O CONNOR D, RINKLEBE J, et al. Biochar aging: mechanisms, physicochemical changes, assessment, and implications for field applications[J]. Environmental Science & Technology, 2020, 54(23): 14797-14814. [17] LIANG Y, LI X, YANG F, et al. Tracing the synergistic migration of biochar and heavy metals based on 13C isotope signature technique: Effect of ionic strength and flow rate[J]. Science of the Total Environment, 2023, 859: 160229. doi: 10.1016/j.scitotenv.2022.160229 [18] SIDDIQ O M, TAWABINI B S, SOUPIOS P, et al. Removal of arsenic from contaminated groundwater using biochar: a technical review[J]. International Journal of Environmental Science and Technology, 2022: 1-14. [19] LENG L, HUANG H. An overview of the effect of pyrolysis process parameters on biochar stability[J]. Bioresource Technology, 2018, 270: 627-642. doi: 10.1016/j.biortech.2018.09.030 [20] MCBEATH A V, WURSTER C M, BIRD M I. Influence of feedstock properties and pyrolysis conditions on biochar carbon stability as determined by hydrogen pyrolysis[J]. Biomass and Bioenergy, 2015, 73: 155-173. doi: 10.1016/j.biombioe.2014.12.022 [21] CHANG R, SOHI S P, JING F, et al. A comparative study on biochar properties and Cd adsorption behavior under effects of ageing processes of leaching, acidification and oxidation[J]. Environmental Pollution, 2019, 254: 113123. doi: 10.1016/j.envpol.2019.113123 [22] United States Environmental Protection Agency: Washington DC. Method 1311: Toxicity characteristic leaching procedure[S]. 1992: 1-35. [23] 陈昱, 梁媛, 郑章琪, 等. 老化作用对水稻秸秆生物炭吸附Cd(Ⅱ)能力的影响[J]. 环境化学, 2016, 35(11): 2337-2343. doi: 10.7524/j.issn.0254-6108.2016.11.2016031601 [24] HUFF M D, LEE J W. Biochar-surface oxygenation with hydrogen peroxide[J]. Journal of Environmental Management, 2016, 165: 17-21. [25] HALE S, HANLEY K, LEHMANN J, et al. Effects of chemical, biological, and physical aging as well as soil addition on the sorption of pyrene to activated carbon and biochar[J]. Environmental Science & Technology, 2011, 45(24): 10445-10453. [26] KE Y, ZHANG F, ZHANG Z, et al. Effect of combined aging treatment on biochar adsorption and speciation distribution for Cd(II)[J]. Science of the Total Environment, 2023: 161593. [27] 环境保护部. 水质汞、砷、硒、铋和锑的测定 原子荧光法: HJ 694-2014[S]. 北京: 中国环境科学出版社, 2014. [28] 刘丹丹, 刘菲, 缪德仁. 土壤重金属连续提取方法的优化[J]. 现代地质, 2015, 29(2): 390-396. doi: 10.3969/j.issn.1000-8527.2015.02.024 [29] CHEN J, WANG P, DING L, et al. The comparison study of multiple biochar stability assessment methods[J]. Journal of Analytical and Applied Pyrolysis, 2021, 156: 105070. doi: 10.1016/j.jaap.2021.105070 [30] XU Z, XU X, TSANG D C, et al. Contrasting impacts of pre-and post-application aging of biochar on the immobilization of Cd in contaminated soils[J]. Environmental Pollution, 2018, 242: 1362-1370. doi: 10.1016/j.envpol.2018.08.012 [31] XU X, KAN Y, ZHAO L, et al. Chemical transformation of CO2 during its capture by waste biomass derived biochars[J]. Environmental Pollution, 2016, 213: 533-540. doi: 10.1016/j.envpol.2016.03.013 [32] TAN Z, YUAN S, HONG M, et al. Mechanism of negative surface charge formation on biochar and its effect on the fixation of soil Cd[J]. Journal of Hazardous Materials, 2020, 384: 121370. doi: 10.1016/j.jhazmat.2019.121370 [33] NIER A O. Determination of isotopic masses and abundances by mass spectrometry[J]. Science, 1955, 121(3152): 737-744. doi: 10.1126/science.121.3152.737 [34] TANG W, JING F, LAURENT Z B L G, et al. High-temperature and freeze-thaw aged biochar impacts on sulfonamide sorption and mobility in soil[J]. Chemosphere, 2021, 276: 130106. doi: 10.1016/j.chemosphere.2021.130106 [35] SORRENTI G, MASIELLO C A, DUGAN B, et al. Biochar physico-chemical properties as affected by environmental exposure[J]. Science of the Total Environment, 2016, 563: 237-246. [36] 刘文慧, 王昱璇, 陈丹丹, 等. 老化作用对生物炭理化特性的影响[J]. 工程热物理学报, 2021, 42(6): 1575-1582. [37] XU D, ZHAO Y, SUN K, et al. Cadmium adsorption on plant-and manure-derived biochar and biochar-amended sandy soils: impact of bulk and surface properties[J]. Chemosphere, 2014, 111: 320-326. doi: 10.1016/j.chemosphere.2014.04.043 [38] ZHANG L, ZHU D, WANG H, et al. Humic acid‐mediated transport of tetracycline and pyrene in saturated porous media[J]. Environmental Toxicology and Chemistry, 2012, 31(3): 534-541. doi: 10.1002/etc.1726 [39] XU Z, WAN Z, SUN Y, et al. Electroactive Fe-biochar for redox-related remediation of arsenic and chromium: Distinct redox nature with varying iron/carbon speciation[J]. Journal of Hazardous Materials, 2022, 430: 128479. doi: 10.1016/j.jhazmat.2022.128479 [40] LIAN F, XING B. Black carbon (biochar) in water/soil environments: molecular structure, sorption, stability, and potential risk[J]. Environmental Science & Technology, 2017, 51(23): 13517-13532. [41] BENIS K Z, DAMUCHALI A M, SOLTAN J, et al. Treatment of aqueous arsenic–A review of biochar modification methods[J]. Science of the Total Environment, 2020, 739: 139750. doi: 10.1016/j.scitotenv.2020.139750 [42] CUI X, FANG S, YAO Y, et al. Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar[J]. Science of the Total Environment, 2016, 562: 517-525. doi: 10.1016/j.scitotenv.2016.03.248 [43] KIM H, KIM S, JEON E, et al. Effect of dissolved organic carbon from sludge, Rice straw and spent coffee ground biochar on the mobility of arsenic in soil[J]. Science of the Total Environment, 2018, 636: 1241-1248. doi: 10.1016/j.scitotenv.2018.04.406 [44] 张林. 微生物介导下砷和锑迁移规律及机制的研究[D]. 西安建筑科技大学, 2018. 期刊类型引用(2)
1. 黄旭敏. 膜生物反应技术在环境工程污水处理中的应用. 造纸装备及材料. 2024(01): 125-127 .  百度学术
百度学术
2. 单明娥,李辉. 膜生物反应污水处理技术对水质优化的影响研究. 能源与环保. 2024(10): 159-164+170 .  百度学术
百度学术
其他类型引用(0)
-






 下载:
下载: