-
砷(As)是一种有毒的类重金属元素,普遍存在于土壤、淡水和海洋生态系统中。含砷化合物的毒性和生物有效性由其形态所决定,对于大多数生物体,亚砷酸盐(As(Ⅲ))的毒性大于砷酸盐(As(Ⅴ)),而无机砷的毒性大于五价甲基砷(包括一甲基砷(Monomethyl arsenic,MMAs)、二甲基砷(Dimethyl arsenic,DMAs)和三甲基砷氧化物(Trimethyl arsenic oxide,TMAsO))、砷胆碱和砷甜菜碱[1]。硅藻是一类重要的水生光合自养微藻,对全球初级生产力贡献约20%,在海洋和淡水生态系统的金属生物地球化学循环中发挥重要作用[2-3]。已有研究发现6种海洋硅藻具有砷转化能力,在胞内将As(Ⅴ)转化为As(Ⅲ),随后生成MMAs和DMAs,其中布氏双尾藻(Ditylum brightwellii)的细胞体积相对较大,表现出较高的砷甲基化效率[4]。淡水硅藻中也存在砷抗性和砷转化功能物种,淡水水华硅藻链状弯壳藻(Achnanthidium minutissimum)能在100 μmol·L−1 As(Ⅴ)和10 μmol·L−1 As(Ⅲ)条件下生长,在河流生物膜中为主要的砷耐受类群[5-6]。A. minutissimum在含1 μmol·L−1 As(Ⅴ)的培养液中能将As(Ⅴ)进行胞内还原后转化为二甲基砷和其他有机砷产物(约占总砷10%),并将胞内As(Ⅲ)排出胞外(约占50%)[6]。然而,不同淡水硅藻对无机砷底物的转化能力和砷抗性差异的研究较为缺乏。
水体中溶解态硅酸盐是硅藻类浮游植物体生长所必需的营养成分[7]。硅藻通过特定的膜蛋白(硅酸盐转运蛋白SITs)能对胞外硅酸盐进行特异性识别和跨膜转运,利用硅合成硅质细胞壁合成和维持细胞代谢[8]。硅酸盐代谢与硅藻多种生理过程关系紧密,包括呼吸和光合作用。硅酸盐的补充能增加硅藻光合作用速率和中心代谢三羧酸循环酶的丰度,并有助于其细胞分裂[9]。在重金属胁迫下(如Cd),硅转运蛋白转录活性显著下调,可能降低溶解性硅酸盐的吸收从而减少细胞壁的生物合成[10]。硅酸盐有效性对海洋硅藻重金属敏感性产生影响,在硅酸盐缺乏的条件下硅藻的Cd、Cu和Pb的耐受性明显降低,而硅酸盐补充后硅藻能通过高表达细胞膜和液泡金属转运蛋白和更高的抗氧化活性,呈现更强的重金属抗性[11-12]。因此,硅酸盐与硅藻重金属抗性关系密切,但硅酸盐有效性对淡水硅藻砷转化的影响及其作用机制尚不清楚。
菱形藻属(Nitzschia)是常见的淡水硅藻属,其繁殖速度快、分布广、易获取且具有重金属抗性等特点,有应用于水体环境中重金属修复的潜力[13]。本研究选取菱形藻属中的针状菱形藻(Nitzschia acicularis)和谷皮菱形藻(Nitzschia palea)为研究对象,探究不同淡水硅藻对无机砷As(Ⅴ)和As(Ⅲ)的转化能力和As(Ⅲ)抗性,以及硅酸盐对硅藻砷转化的影响和此过程中相关基因的转录表达,为探究淡水硅藻参与砷生物地球化学循环及其影响机制提供参考。
-
实验采用的针状菱形藻(Nitzschia acicularis) (FACHB-2868)、谷皮菱形藻(Nitzschia palea) (FACHB-2263)购买自中国科学院武汉水生生物研究所淡水藻种库。采用CSI培养基进行培养[6],调节pH值为(7.00±0.02),在121 ℃下进行高压灭菌。
在无菌条件下将针状菱形藻(Nitzschia acicularis)和谷皮菱形藻(Nitzschia palea)转接至CSI培养基中,瓶口用透气封口膜封口后放入光照培养箱内培养。在温度(25±1) ℃、光强强度3 000 lux、光暗循环为12 h:12 h的光照培养箱内进行培养,每天定时摇瓶3~4次,以保证藻细胞所利用矿质营养的均匀度。
-
1) 硅藻对As(Ⅲ)和As(Ⅴ)的转化实验。将生长对数期的2种藻细胞分别接种于CSI培养基中,使得培养起始细胞密度(OD680)为0.04。As(Ⅲ)和As(Ⅴ)浓度分别设置为15和30 μmol·L−1,每组设置3个平行,分别在第0、3、5、7、10天取400 μL藻液,经0.22 μm滤膜过滤,得到上清液,利用NexION 300X高效液相色谱-电感耦合等离子体-质谱仪(HPLC-ICP-MS,PerkinElmer,USA)测定砷形态。
2) As(Ⅲ)影响下硅藻生长曲线和叶绿素含量。将生长对数期的2种藻细胞分别接种于CSI培养基中,使得培养起始细胞密度(OD680)为0.04。As(Ⅲ)和As(Ⅴ)浓度分别设置为15和30 μmol·L−1,每组设置3个平行,分别在第0、3、5、7、10天取400 μL藻液,经0.22 μm滤膜过滤,得到上清液,利用NexION 300X高效液相色谱-电感耦合等离子体-质谱仪(HPLC-ICP-MS,PerkinElmer,USA)测定砷形态。
在培养第10天时,用无水乙醇法测定所有处理中硅藻的叶绿素含量[14]。取1 mL藻液在12 000 r·min−1转速下离心5 min,弃去上清液后,用50 μL蒸馏水重悬藻液,然后加入0.95 mL无水乙醇并在75 ℃下水浴加热5 min,待冷却后在波长665 nm、649 nm和750 nm下测定吸光度,并通过式 (1)~(3) 计算叶绿素质量浓度。
式中:Chla为叶绿素a的质量浓度,Chlb为叶绿素b的质量浓度,G为总叶绿素质量浓度, 单位均为mg·L−1。
3) As(Ⅲ)胁迫下硅藻扫描电镜图(SEM)。利用SEM对0和15 μmol·L−1 As(Ⅲ)浓度处理下培养14 d后的2种硅藻进行表面形貌观察。取40 mL藻液在4 000 g下离心15 min,然后用PBS(0.01 mol·L−1)冲洗3次,再将藻细胞样品在2.5%的戊二醛中固定24 h,接着用0.1 mol·L−1磷酸盐缓冲液洗涤4次后,样品经不同浓度酒精(30、50、70、90、100%)脱水和叔丁醇浸泡置换,冷冻干燥和喷金镀膜,最后用SEM(S-3000 N,Hitachi)进行测试。
4) 硅酸盐对硅藻砷转化的影响实验。将生长于CSI培养基中(含0.1 g·L−1硅酸钠Na2SiO3·9H2O)的硅藻作为空白对照,另设置As(Ⅲ)(15 μmol·L−1)和As(Ⅲ)处理下额外添加0.05、0.15和0.25 g·L−1硅酸钠,共5个处理,每个处理3个平行。培养6 d后取上清液过膜后用HPLC-ICP-MS测定砷形态。
5) RNA提取方法。根据硅藻细胞在生长初期基因表达活性较高,取上述培养2 d后的针状菱形藻的藻液50 mL,在室温下以4 500 r·min−1离心5 min后收获细胞,弃上清液并重新悬浮在1 mL的DEPC水中溶解沉淀,接着转移至已灭菌的2 mL离心管内,将样品在室温下4 500 r·min−1离心5 min,弃去上清液,收集的藻细胞放置于液氮中。对藻细胞进行研磨后转移至装有1 mL的Trizol试剂液的离心管中,再向其中加入0.2 mL的氯仿(CHCl3),在4 ℃条件下12 000 r·min−1离心15 min,取上层水相,再加入2/3水相体积的异丙醇,摇匀后室温沉淀10 min,在相同条件下离心5 min,弃上清。用0.5 mL提前冷却的体积分数为75%的乙醇(DEPC水和无水乙醇的体积比为1:3)洗涤沉淀,并离心5 min,弃上清,再重复一次该步骤。于超净台风干乙醇后加入适量(10~40 μL)DEPC水,在65 ℃下助融5 min,然后RNA样品置于–80 ℃冰箱备用。
6) 反转录荧光定量PCR(RT-qPCR)。用PrimeScrip RT reagent Kit with gDNA Eraser (Takara,Japan)对RNA进行反转录,然后以反转录后的cDNA样品为模板,对β-actin、砷甲基转移酶基因(arsM)、线粒体核糖体RNA 12S基因(12S)和硅酸盐转运基因(sit)进行相对定量。实时荧光定量PCR反应体系为20 μL,包括10 μL SYBR Green Master Mix (GenStar,China)、目的基因上下游引物各0.5 μL(10μmol·L−1)、1 μL DNA模板、8 μL RNase-free Water。β-actin、12S和sit基因引物和qPCR反应条件参考文献[10](表1)。对于arsM基因,以Nitzschia属的砷甲基转移功能基因作为参考序列,基于序列保守区用NCBI Primer Blast进行引物设计。荧光定量PCR扩增反应使用CFX 384 Real-Time PCR Detection System (Bio-Rad Laboratories, USA)进行测定。以DEPC处理后的水为模板做负对照,PCR反应特异性用PCR产物溶解曲线确定。
-
硅藻特定基因的相对表达量用2−ΔΔCt方法计算,根据4个生物学重复计算平均值和标准误差。对于处理间差异的显著性,采用SPSS 26软件进行方差分析。采用Origin 2022b完成结果绘图。
-
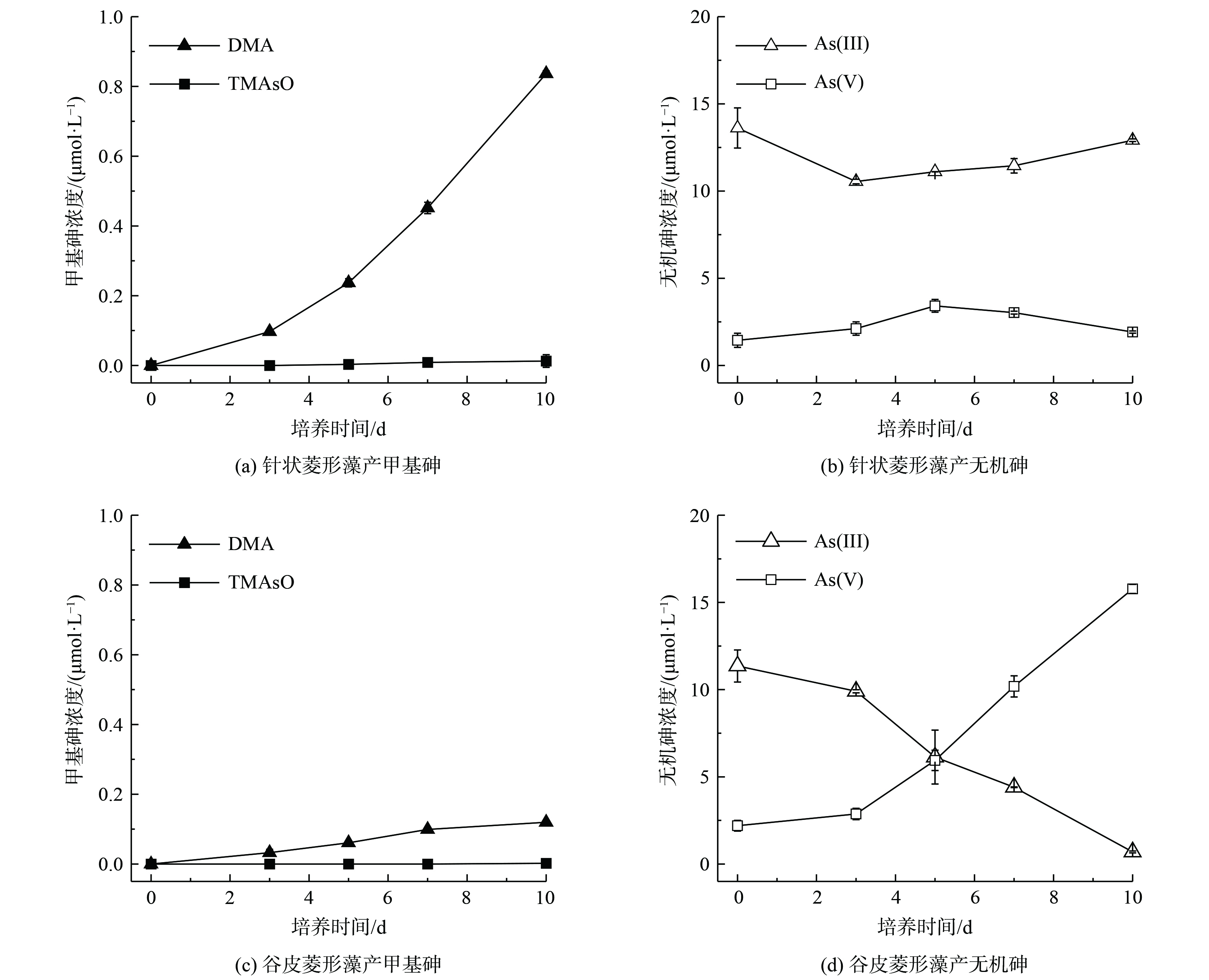
针状菱形藻和谷皮菱形藻分别暴露于15 μmol·L−1 As(Ⅲ)时,在10 d培养期内甲基砷浓度随时间增加而增加,产物形态主要为DMA。硅藻生长在前10 d内活性较高,细胞的砷代谢较为活跃,这表明2种硅藻在生长增殖过程中都有砷甲基化作用。第10天时,针状菱形藻中DMA浓度达到0.84 μmol·L−1,TMAsO浓度达到0.013 μmol·L−1,转化率分别为5.54%和0.17%(图1(a)),同时有明显的As(Ⅴ)生成(1.92 μmol·L−1),转化率为3.2%(图1(b))。对于谷皮菱形藻,10 d培养后生成的DMA浓度为0.12 μmol·L−1,转化率为0.8%,但未检测到TMAsO(图1(c)),并发现有大量As(Ⅴ)生成(15.8 μmol·L−1),转化率为90.1%(图1(d))。以上结果表明,针状菱形藻和谷皮菱形藻在As(Ⅲ)暴露下都具有As(Ⅲ)砷甲基化能力,将As(Ⅲ)转化为毒性较低的甲基砷。QIN在2009年首次鉴定了藻类红藻(Cyanidioschyzon sp. isolate 5508)中的亚砷酸甲基转移功能基因CmarsM和验证了其酶学特性。CmarsM能在体外将As(Ⅲ)转化为一甲基亚砷酸,而后生成二甲基亚砷酸和三甲基砷气体,CmarsM基因的异源表达能赋予砷敏感型大肠杆菌细胞对As(Ⅲ)的抗性,使得砷甲基化反应具有解毒的生理意义[15]。此后,还有其他藻类被发现具有As(Ⅲ)甲基化功能,如蛋白核小球藻和莱茵衣藻等[16]。本研究中2种藻砷甲基化反应主要生成DMA,皆未检测到一甲基砷,可能是由于一甲基亚砷酸中间产物在胞内的连续甲基化,而未能外排至胞外溶液中。针状菱形藻的甲基化能力高于谷皮菱形藻,显示出不同淡水硅藻物种的砷甲基化效率存在差异。除了砷甲基化,暴露于As(Ⅲ)的2种藻在培养期内明显发生了As(Ⅲ)氧化反应。QIN也发现红藻能氧化As(Ⅲ)为As(Ⅴ),发生于细胞质区室外,可能与其胞外酶有关,类似于碳酸酐酶和磷酸酶对胞外无机碳和磷酸盐的摄取等胞外酶驱动的反应[15]。由于光合自养型硅藻不能利用砷氧化产生能量,藻的砷氧化作用是潜在的As(Ⅲ)胁迫下的一种生存策略。谷皮菱形藻的砷甲基化能力虽然较弱,但具有较强的砷氧化能力,表现出不同硅藻采取不同的方式缓解As(Ⅲ)胁迫。由于微藻介导的砷氧化反应产生毒性和移动性较小的五价砷,较强砷氧化能力的微藻具有应用于砷污染水体修复的潜力[17]。
暴露于30 μmol·L−1 As(Ⅴ)下的针状菱形藻和谷皮菱形藻在10 d培养期内产生甲基砷,并在溶液中随时间增加而累积。结果如图2所示,10 d后针状菱形藻产生的DMA浓度为0.26 μmol·L−1,转化率为0.85%(图2(a)),谷皮菱形藻中DMA浓度为0.07 μmol·L−1,转化率为0.24%(图2(c))。这表明针状菱形藻对As(Ⅴ)的砷甲基化作用较强于谷皮菱形藻,与2种藻As(Ⅲ)砷甲基化差异结果一致。淡水硅藻A. minutissimum能甲基化As(Ⅴ)为DMA,即在淡水水体中硅藻能通过胞内还原后甲基化,生成毒性较低的甲基砷产物[5]。同时,在针状菱形藻培养液中还检测出明显的As(Ⅲ)(0.32 μmol·L−1)(图2(b)),但在谷皮菱形藻中未检出(图2(d)),这与谷皮菱形藻较弱的砷甲基化能力相对应。这些结果表明硅藻吸收As(Ⅴ)后可通过2个途径进行解毒:一是将As(Ⅴ)在胞内还原为As(Ⅲ),然后对As(Ⅲ)进行甲基化生成DMA;二是将胞内还原产生的As(Ⅲ)外排至胞外从而降低胞内砷浓度[15]。微藻胞内砷还原是砷抗性代谢中重要的一步,将绿藻Chlamydomonas reinhardtii砷还原酶CrACR2s表达于砷还原功能缺失的大肠杆菌中,能恢复其对As(Ⅴ)的抗性[18]。真核生物中发现的砷酸盐还原酶为双特异性磷酸酶中的一员,来源于酵母Saccharomyces cerevisiae的Acr2蛋白酶的作用机制类似于细菌中的arsC,利用谷氧还蛋白和谷胱甘肽作为电子供体还原As(Ⅴ)[19]。微藻中的谷胱甘肽还会与As(Ⅲ)形成As-GSH复合物,使砷保持还原态进行连续甲基化反应[20],或是转移储存于液泡中减少砷的毒性[21]。另外,微藻对水溶液中As(Ⅴ)的还原作用,减少As(Ⅴ)有效性,降低As(Ⅴ)和磷酸盐的竞争性转运,有利于微藻对水溶液中的磷酸盐的吸收利用。
-
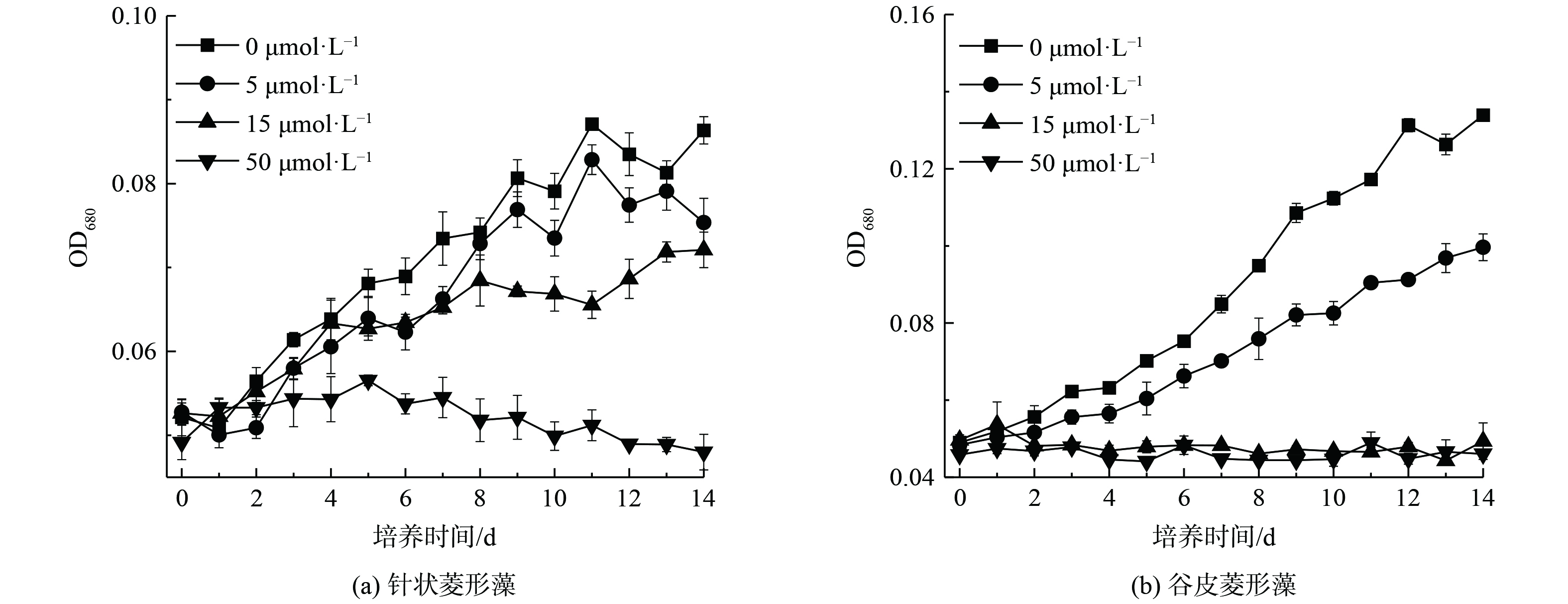
针状菱形藻和谷皮菱形藻暴露于不同浓度As(Ⅲ)下的OD值变化如图3所示,14 d培养过程中无砷处理的2种硅藻生物量随着时间增加而增加。当As(Ⅲ)存在时,生物量随As(Ⅲ)浓度增加而减少,并在As(Ⅲ)存在条件下生长至14 d时处于稳定期后期。5和15 μmol·L−1 As(Ⅲ)对针状菱形藻生长有抑制作用,使培养14 d后的OD分别下降了12.7%和16.5%,且针状菱形藻不能在50 μmol·L−1 As(Ⅲ)条件下生长(图3(a))。相对无砷处理,谷皮菱形藻在5 μmol·L−1 As(Ⅲ)存在时14 d后的生物量减少13.4%,15和50 μmol·L−1 As(Ⅲ)明显抑制其生长(图3(b))。类似的As(Ⅲ)对硅藻生长的抑制作用也发现于淡水水华硅藻链状弯壳藻(Achnanthidium minutissimum)中,A. minutissimum能在10 μmol·L−1 As(Ⅲ)条件下生长,但100 μmol·L−1 As(Ⅲ)完全抑制其生长。本研究中针状菱形藻比谷皮菱形藻有较强的砷抗性,显示出不同硅藻的砷抗性存在差异,可能与其采用的砷解毒机制不同有关[6]。
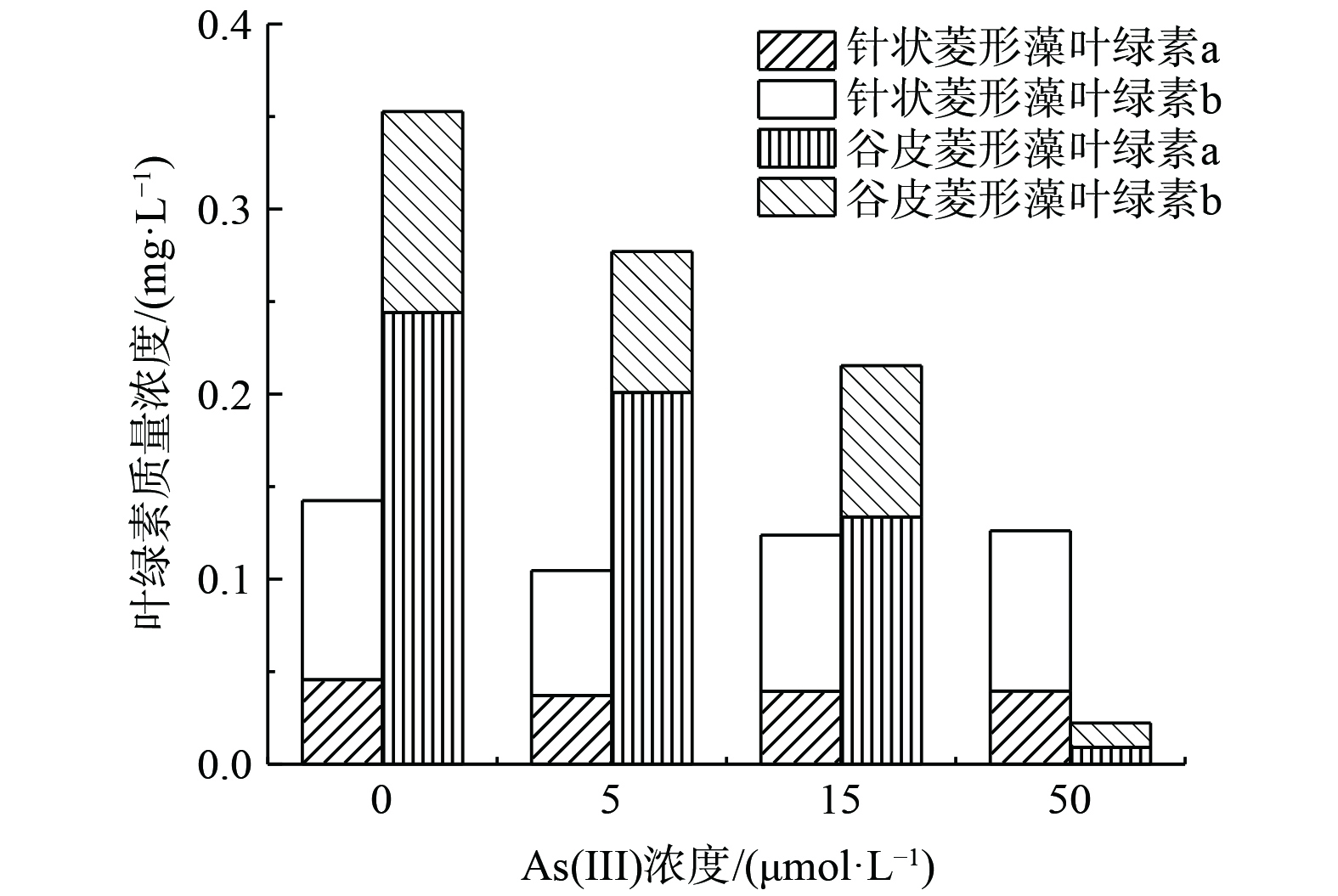
如图4所示,在第10天硅藻细胞生长至稳定期时测定各处理的叶绿素含量,发现不同浓度As(Ⅲ)胁迫对硅藻的叶绿素a和b含量也产生了负面影响。与对照组相比,5、15和50 μmol·L−1 As(Ⅲ)使针状菱形藻叶绿素a和b分别降低了13.4%~18.6%和10.5%~30.2%。对于谷皮菱形藻,叶绿素含量随着As(Ⅲ)浓度增加明显下降,5、15和50 μmol·L−1 As(Ⅲ)相较于对照使叶绿素a和b分别下降了17.7%~96.3%和24.7%~88.0%。已有研究表明,As(Ⅲ)存在会减少光合蓝细菌Nostoc muscorum的叶绿素含量,可能是由于As(Ⅲ)减少了合色素合成前体、增加叶绿素降解酶,或取代叶绿素四吡咯环中心的镁原子从而降低了捕光色素蛋白复合物的效率[22]。另外,砷暴露还会干扰生物体呼吸电子传递链,影响还原力NADPH和能量ATP的形成,降低1,5—二磷酸核酮糖羧化酶/加氧酶(RuBisCo)活性和固碳效率,从而降低其生长速率[22]。对应于As(Ⅲ)胁迫下硅藻生物量减少,谷皮菱形藻的叶绿素含量降低较针状菱形藻更为明显,表现出硅藻间砷敏感度的差异。
-
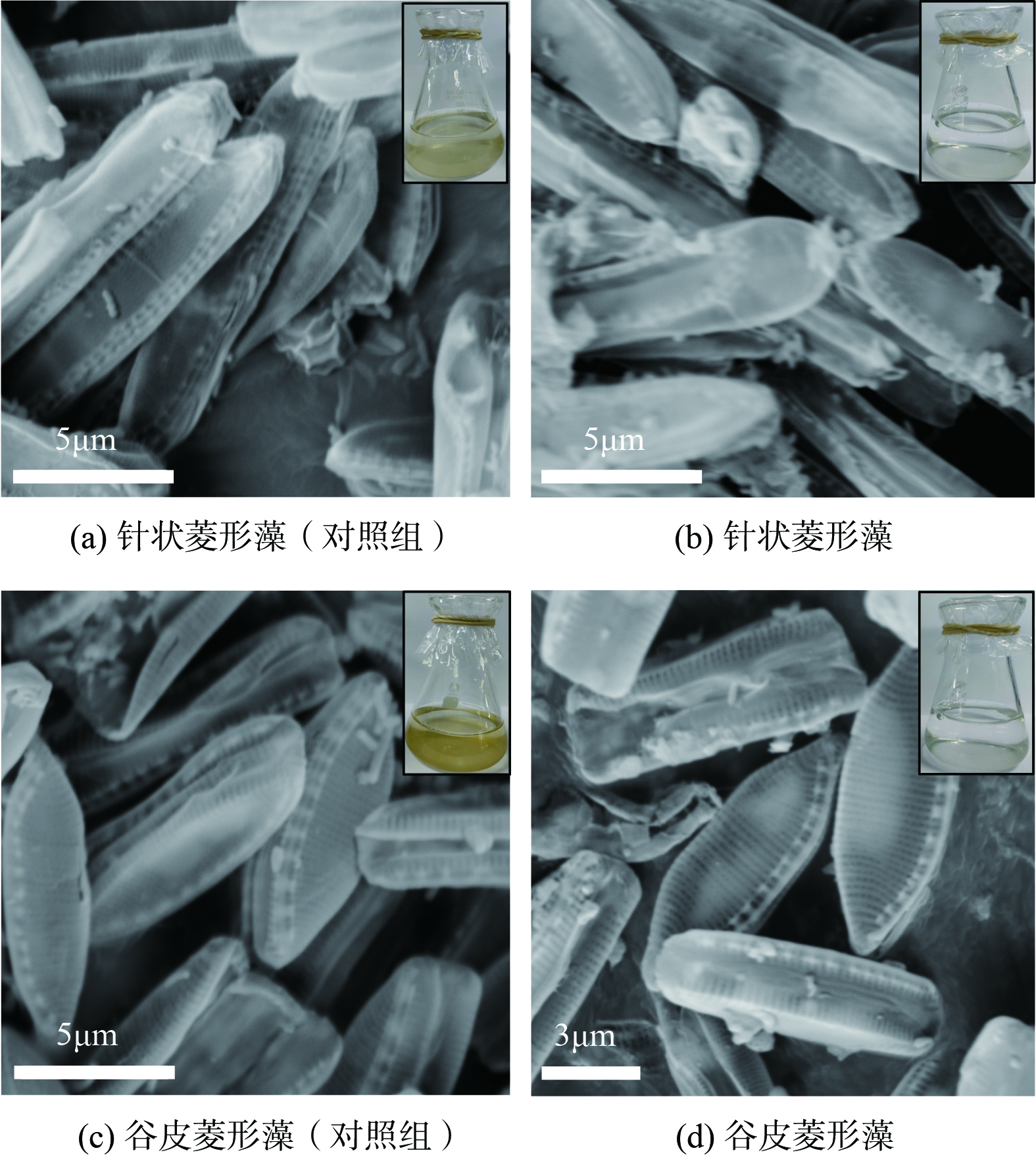
通过SEM表征有无15 μmol·L−1 As(Ⅲ)添加下2种硅藻的表面形貌,结果显示相较于无砷培养中完整的硅藻细胞,有砷处理下针状菱形藻表面出现更多絮状物,细胞壁有所破损,壳孔的完整度降低,展现出砷的毒性导致的硅藻表面损伤(图5(a)、(b))。砷存在时谷皮菱形藻除了出现更多的表面絮状物外,壳面有所变形(图5(c)、(d))。这与文献报道的As(Ⅲ)胁迫会引起舟形藻(Navicula sp.)硅藻细胞表面破损[23]是一致的。
-
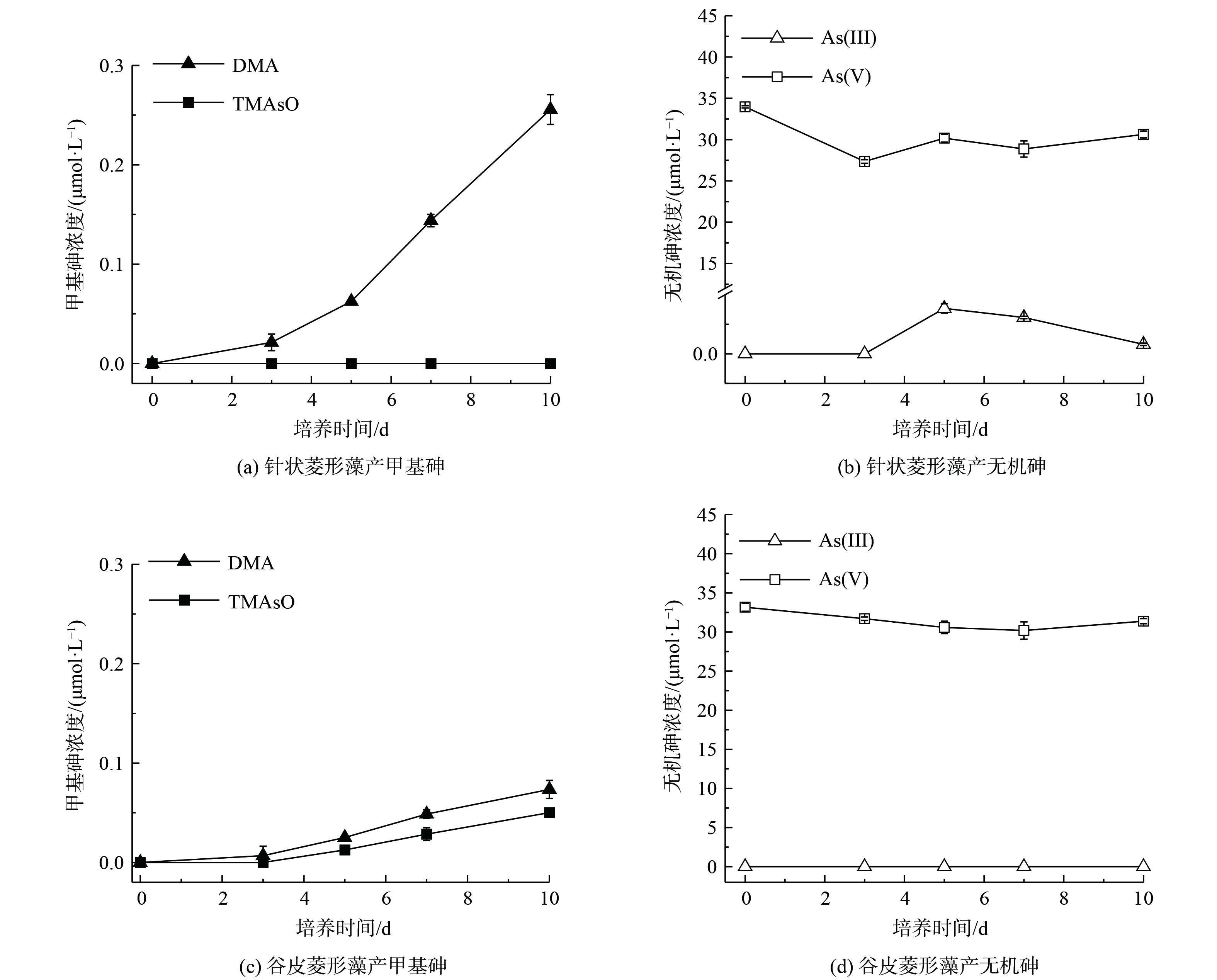
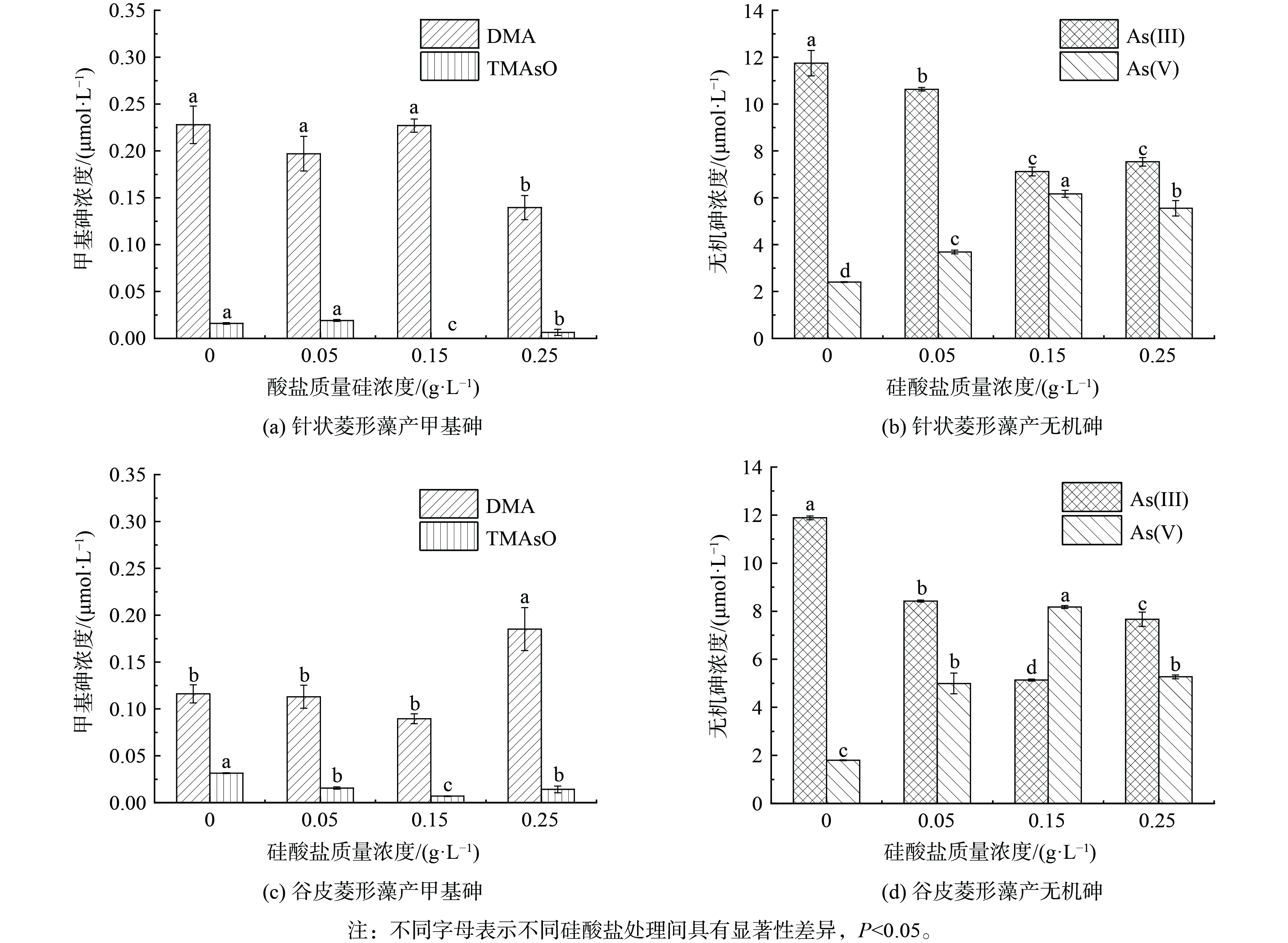
15 μmol·L−1 As(Ⅲ)处理中针状菱形藻培养6 d后,硅藻处于生长期中期,此时转化产生的DMA浓度为0.23 μmol·L−1,TMAsO浓度为0.016 μmol·L−1,砷氧化产生As(Ⅴ)的浓度达2.4 μmol·L−1 (图6(a))。额外添加0.05和0.15 g·L−1硅酸盐对主要产物DMA生成没有明显影响,而0.25 g·L−1硅酸盐处理下DMA产生量显著下降,高浓度硅酸盐添加也显著降低了TMAsO生成。同时,添加硅酸盐显著增加了As(Ⅴ),特别是0.15 g·L−1硅酸盐处理下As(Ⅴ)浓度为6.2 μmol·L−1,相比无额外添加提高了2.6倍,该处理中相应的As(Ⅲ)浓度显著下降(图6(b))于谷皮菱形藻,As(Ⅲ)处理下培养6 d后DMA、TMAsO和As(Ⅴ)浓度分别为0.12、0.03和1.8 μmol·L−1 (图6(c))。0.05和0.15 g·L−1硅酸盐对DMA生成没有明显影响,而0.25 g·L−1硅酸盐处理下DMA显著增加。与针状菱形藻类似,额外添加硅酸盐显著增加了谷皮菱形藻As(Ⅴ)的浓度,在质量浓度为0.15 g·L−1时,As(Ⅴ)达到最高(8.2 μmol·L−1)。相比无额外添加提高4.5倍(图6(d))的来说,硅酸盐添加会对硅藻砷甲基化产生负面影响,特别是对于砷甲基化能力较强的针状菱形藻,同时显著增强了2种硅藻的砷氧化能力。由于亚砷酸和硅酸盐具有相似的结构,其能共享相同的硅转运蛋白进行吸收,如水稻细胞的硅转运蛋白同时介导了As(Ⅲ)和Si的吸收[24]。亚砷酸和硅酸盐在硅藻中的吸收也可能共享SIT硅转运蛋白[21],因此硅酸盐添加通过与As(Ⅲ)竞争吸收,从而减少细胞对砷的转运和接下来的胞内甲基化反应。此外,高浓度硅酸盐促进了砷氧化,As(Ⅲ)作为甲基化底物其有效性的降低也会导致砷甲基化效率降低。硅酸盐作为自然水体中常见的含氧阴离子,其质量浓度范围为0.45~14 mg·L−1 Si。本研究硅藻生长的硅酸盐背景质量浓度为0.1 g·L−1 Na2SiO3·9H2O(即9.9 mg·L−1 Si),与自然条件下硅藻的生长条件接近,并在0.05、0.15和0.25 g·L−1硅酸钠处理中0.15 g·L−1硅酸钠添加后砷氧化率最高,因此建议该浓度为硅藻调控水体砷氧化时的参考浓度。
-
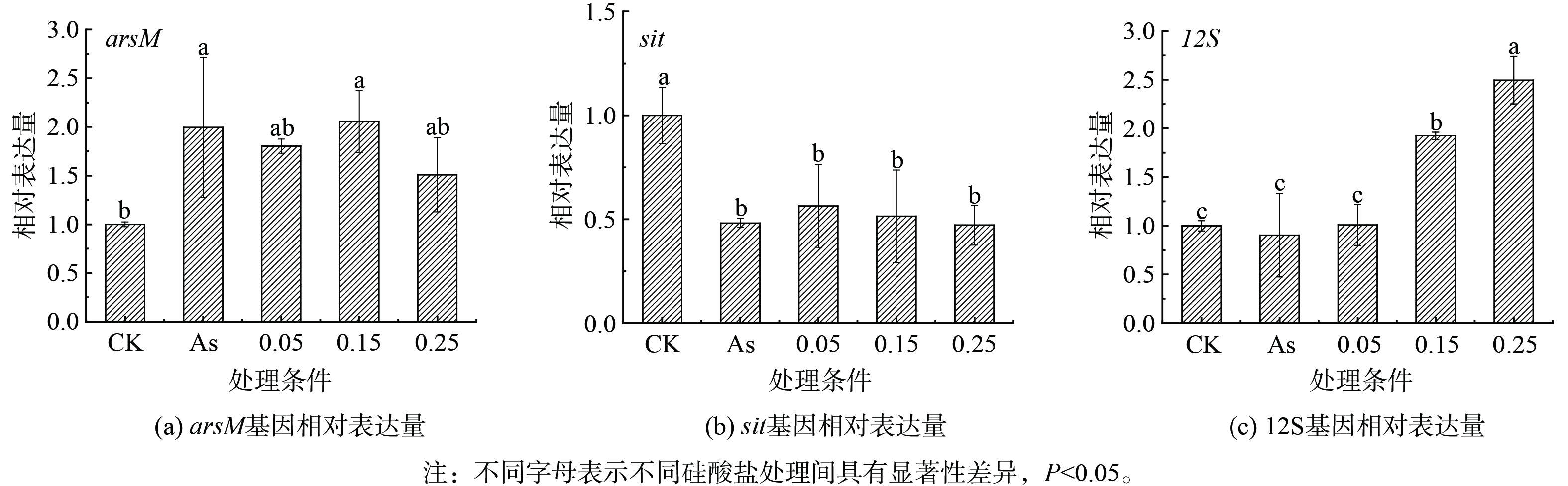
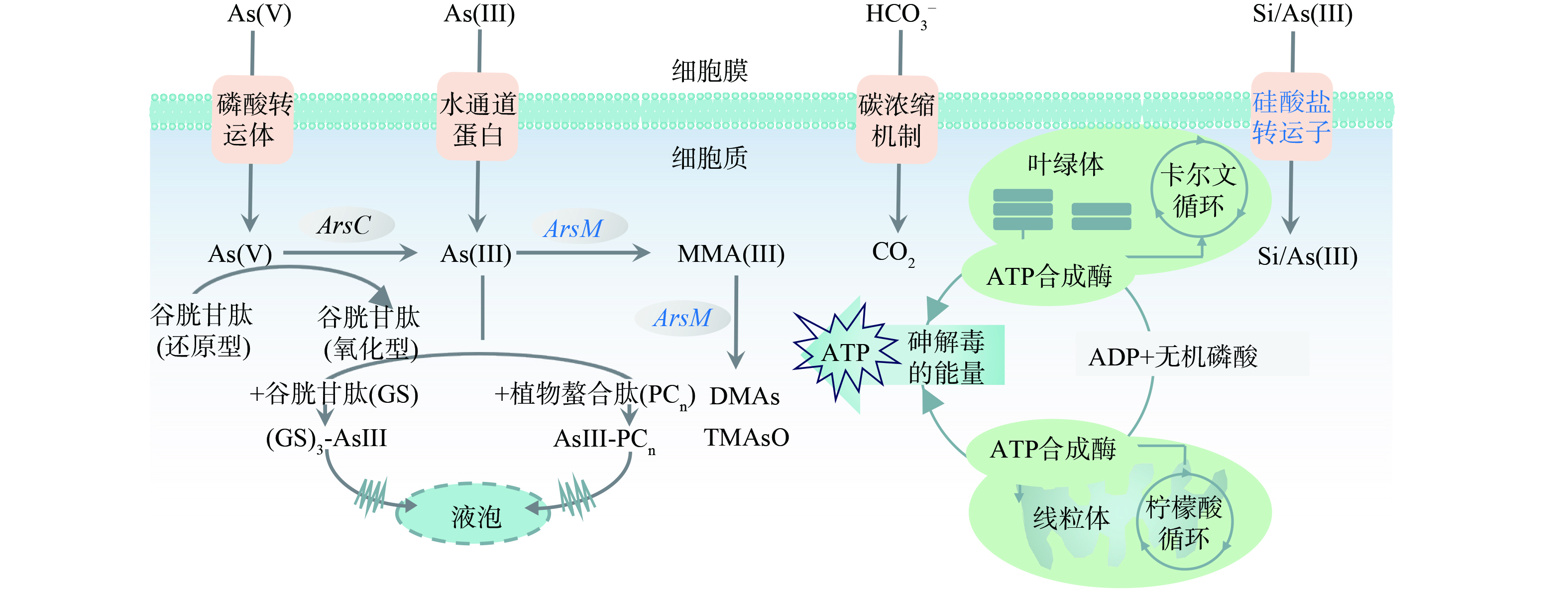
不同处理下针状菱形藻的砷甲基转移酶基因(arsM)、硅酸盐转运基因(sit)和线粒体核糖体RNA 12S基因(12S)相对表达量结果表明,相对无砷处理,15 μmol·L−1 As(Ⅲ)存在时藻细胞显著上调arsM基因表达,并显著下调了sit基因(图7(a)和7(b))。这些结果表明硅藻在As(Ⅲ)暴露下通过显著上调arsM基因转录表达驱动砷甲基化,证明arsM介导的砷甲基化反应是硅藻应对砷胁迫的抗性机制之一。As(Ⅲ)暴露后sit基因下调表明在转录水平上砷对硅藻硅酸盐利用产生抑制作用,已有研究也发现谷皮菱形藻响应于重金属胁迫时(例如Cd),会显著下调sit基因[10]。因为As(Ⅲ)和硅酸盐可能共用SIT硅转运蛋白转运[21],sit基因下调降低As(Ⅲ)吸收,能缓解细胞的砷暴露程度。此外,高浓度硅酸盐条件下砷氧化显著增加,As(Ⅲ)有效性的降低也会导致砷甲基化效率降低。额外添加硅酸盐对arsM表达没有明显影响,与硅酸盐影响下针状菱形藻培养液中的甲基砷浓度变化相对应。已有研究表明,硅酸盐添加会引起硅藻sit基因或水稻植物细胞硅转运蛋白基因的下调,从而降低细胞对砷的吸收转运[25-26]。在本研究中,硅酸盐对砷胁迫下sit基因表达没有明显影响,表明硅转运蛋白活性不是硅酸盐影响硅/砷吸收代谢的主要因素。As(Ⅲ)存在对12S基因表达无明显影响,硅酸盐添加后显著上调了12S基因表达,并在质量浓度为0.25 g·L−1时达到最高(图7(c)),这说明硅酸盐添加可提高硅藻线粒体数量,以维持砷胁迫下细胞的ATP产生[27]。综上所述,硅藻应对As(Ⅲ)胁迫提高砷甲基化活性,降低硅/砷转运活性,有利于胞内砷解毒和减少As(Ⅲ)吸收,硅酸盐增加不会直接影响砷甲基化活性,但能促进As(Ⅲ)氧化为As(Ⅴ)降低砷毒性,并且提高呼吸产能活性,从而有助于强化硅藻对砷的解毒作用(图8)。
-
1)淡水硅藻针状菱形藻和谷皮菱形藻能对As(Ⅲ)进行甲基化和氧化,同时对As(Ⅴ)能进行还原后甲基化,且主要甲基化产物形态为二甲基砷。将毒性较强的三价无机砷转化为低毒的甲基砷,或氧化为低毒的五价砷,成为硅藻主要的砷解毒机制。
2)针状菱形藻对As(Ⅲ)的砷甲基化能力比谷皮菱形藻强,而谷皮菱形藻的As(Ⅲ)氧化作用比针状菱形藻强。不同硅藻的主要砷转化途径和主要产物形态不同,表现出硅藻抵御As(Ⅲ)胁迫的生存策略存在差异。
3)暴露于As(Ⅲ)的针状菱形藻上调砷甲基转移功能基因arsM转录活性,并显著下调了硅酸盐转运基因sit转录,表明硅藻通过表达相关功能基因驱动砷甲基化反应,和减少硅/砷转运活性,能够帮助硅藻胞内砷解毒和减少As(Ⅲ)吸收,体现出硅藻在As(Ⅲ)胁迫下转录水平的代谢响应特征。
4)硅酸盐的添加对针状菱形藻砷甲基转移酶基因表达活性没有显著影响,但上调线粒体核糖体RNA 12S基因转录活性,并促进了As(Ⅲ)向As(Ⅴ)转化,表明硅藻在砷胁迫下提高细胞的呼吸产能活性,同时通过促进砷氧化降低砷毒性,有助于强化硅藻对砷的抗性。
淡水硅藻的砷甲基化和砷氧化代谢机制
Metabolic mechanisms of arsenic methylation and oxidation in freshwater diatoms
-
摘要: 硅藻砷甲基化和氧化过程在淡水生态系统砷生物地球化学循环中发挥重要作用,并且硅藻的重金属抗性与营养元素硅酸盐有效性紧密相关。然而,不同硅藻对无机砷的转化能力和砷抗性差异,硅酸盐对硅藻砷转化的影响机制尚不清楚。选取2种淡水硅藻,即针状菱形藻(Nitzschia acicularis)和谷皮菱形藻(Nitzschia palea),作为研究对象,探究其砷甲基化和氧化作用,以及硅酸盐影响下砷转化和此过程中的相关基因转录活性。结果表明,硅藻暴露于As(Ⅲ)的10 d培养期内主要产生二甲基砷,针状菱形藻的二甲基砷转化率为5.54%,高于谷皮菱形藻的转化率(0.80%)。谷皮菱形藻的As(Ⅲ)氧化作用比针状菱形藻强,前者和后者的氧化率分别为90.1%和3.2%。2种硅藻砷甲基化和氧化能力的差异表明不同硅藻As(Ⅲ)胁迫下的主要砷抗性策略不同。针状菱形藻在As(Ⅲ)胁迫下显著上调砷甲基转移功能基因(arsM)表达,并显著下调硅酸盐转运基因(sit)表达。这表明硅藻驱动胞内砷甲基化反应和减少硅/砷转运活性,从而有助于胞内砷解毒和减少As(Ⅲ)吸收。硅酸盐添加对针状菱形藻的arsM基因表达没有明显影响,但上调了线粒体核糖体RNA 12S基因表达,同时促进As(Ⅲ)向As(Ⅴ)转化,这表明硅酸盐可促进硅藻砷氧化和呼吸产能活性,有助于增加硅藻对砷的抗性。本研究证实了硅藻间砷甲基化和氧化能力差异,并且硅酸盐是影响其砷转化的重要因素,可为硅藻应用于砷污染水体修复提供参考。Abstract: Arsenic (As) methylation and oxidation processes in freshwater diatoms play an important role in the biogeochemical cycle of As in freshwater ecosystems, and the heavy metal resistance of diatoms is closely related to the availability of nutrient silicates. However, the transformation ability and As resistance of different diatoms to inorganic arsenic vary, and the mechanism of the silicate effect on the As transformation in diatoms are still unclear. In this study, two freshwater diatoms (Nitzschia acicularis and Nitzschia palea) were selected to explore their As methylation and oxidation, as well as the influence of silicate on the As transformation and the transcriptional activity of related genes during this process. The results showed that diatoms exposed to As(III) mainly produced dimethylarsenic (DMAs) during the 10-day culture period mainly produced dimethyl-arsenic, and the conversion rate of DMAs in the Nitzschia acicularis was 5.54%, higher than that in the Nitzschia palea (0.8%). The oxidation of As(III) was stronger in Nitzschia acicularis than in Nitzschia palea with the oxidation rate of 90.1% and 3.2%, respectively. The differences of As methylation and oxidation capacities bwtween the two diatoms indicated that the main arsenic resistance strategies under As(III) stress were different. With exposing to As(III), the expression of arsenic methyl transfer functional gene (arsM) was significantly up-regulated and the expression of silicate transport gene (sit) was significantly down-regulated. This suggested that diatoms drived intracellular arsenic methylation and reduced Si/As transport activity, thereby contributing to intracellular arsenic detoxification and reducing As(III) absorption.The addition of silicate had insignificant effect on transcriptional activity of arsM in Nitzschia acicularis, but up-regulated the express of mitochondrial ribosomal RNA 12S gene, and promoted the conversion of As(III) to As(V). These results indicated that silicate could promote As oxidation and respiratory activity of diatoms, and contribute to the increase of arsenic resistance of diatoms. This study confirmed the differences in As methylation and oxidation capacity among diatoms, and that silicate was an important factor affecting As transformation, wihch can provide theoretical support for the application of diatoms in the remediation of As-contaminated water.
-
砷(As)是一种有毒的类重金属元素,普遍存在于土壤、淡水和海洋生态系统中。含砷化合物的毒性和生物有效性由其形态所决定,对于大多数生物体,亚砷酸盐(As(Ⅲ))的毒性大于砷酸盐(As(Ⅴ)),而无机砷的毒性大于五价甲基砷(包括一甲基砷(Monomethyl arsenic,MMAs)、二甲基砷(Dimethyl arsenic,DMAs)和三甲基砷氧化物(Trimethyl arsenic oxide,TMAsO))、砷胆碱和砷甜菜碱[1]。硅藻是一类重要的水生光合自养微藻,对全球初级生产力贡献约20%,在海洋和淡水生态系统的金属生物地球化学循环中发挥重要作用[2-3]。已有研究发现6种海洋硅藻具有砷转化能力,在胞内将As(Ⅴ)转化为As(Ⅲ),随后生成MMAs和DMAs,其中布氏双尾藻(Ditylum brightwellii)的细胞体积相对较大,表现出较高的砷甲基化效率[4]。淡水硅藻中也存在砷抗性和砷转化功能物种,淡水水华硅藻链状弯壳藻(Achnanthidium minutissimum)能在100 μmol·L−1 As(Ⅴ)和10 μmol·L−1 As(Ⅲ)条件下生长,在河流生物膜中为主要的砷耐受类群[5-6]。A. minutissimum在含1 μmol·L−1 As(Ⅴ)的培养液中能将As(Ⅴ)进行胞内还原后转化为二甲基砷和其他有机砷产物(约占总砷10%),并将胞内As(Ⅲ)排出胞外(约占50%)[6]。然而,不同淡水硅藻对无机砷底物的转化能力和砷抗性差异的研究较为缺乏。
水体中溶解态硅酸盐是硅藻类浮游植物体生长所必需的营养成分[7]。硅藻通过特定的膜蛋白(硅酸盐转运蛋白SITs)能对胞外硅酸盐进行特异性识别和跨膜转运,利用硅合成硅质细胞壁合成和维持细胞代谢[8]。硅酸盐代谢与硅藻多种生理过程关系紧密,包括呼吸和光合作用。硅酸盐的补充能增加硅藻光合作用速率和中心代谢三羧酸循环酶的丰度,并有助于其细胞分裂[9]。在重金属胁迫下(如Cd),硅转运蛋白转录活性显著下调,可能降低溶解性硅酸盐的吸收从而减少细胞壁的生物合成[10]。硅酸盐有效性对海洋硅藻重金属敏感性产生影响,在硅酸盐缺乏的条件下硅藻的Cd、Cu和Pb的耐受性明显降低,而硅酸盐补充后硅藻能通过高表达细胞膜和液泡金属转运蛋白和更高的抗氧化活性,呈现更强的重金属抗性[11-12]。因此,硅酸盐与硅藻重金属抗性关系密切,但硅酸盐有效性对淡水硅藻砷转化的影响及其作用机制尚不清楚。
菱形藻属(Nitzschia)是常见的淡水硅藻属,其繁殖速度快、分布广、易获取且具有重金属抗性等特点,有应用于水体环境中重金属修复的潜力[13]。本研究选取菱形藻属中的针状菱形藻(Nitzschia acicularis)和谷皮菱形藻(Nitzschia palea)为研究对象,探究不同淡水硅藻对无机砷As(Ⅴ)和As(Ⅲ)的转化能力和As(Ⅲ)抗性,以及硅酸盐对硅藻砷转化的影响和此过程中相关基因的转录表达,为探究淡水硅藻参与砷生物地球化学循环及其影响机制提供参考。
1. 材料与方法
1.1 材料及培养条件
实验采用的针状菱形藻(Nitzschia acicularis) (FACHB-2868)、谷皮菱形藻(Nitzschia palea) (FACHB-2263)购买自中国科学院武汉水生生物研究所淡水藻种库。采用CSI培养基进行培养[6],调节pH值为(7.00±0.02),在121 ℃下进行高压灭菌。
在无菌条件下将针状菱形藻(Nitzschia acicularis)和谷皮菱形藻(Nitzschia palea)转接至CSI培养基中,瓶口用透气封口膜封口后放入光照培养箱内培养。在温度(25±1) ℃、光强强度3 000 lux、光暗循环为12 h:12 h的光照培养箱内进行培养,每天定时摇瓶3~4次,以保证藻细胞所利用矿质营养的均匀度。
1.2 实验方法
1) 硅藻对As(Ⅲ)和As(Ⅴ)的转化实验。将生长对数期的2种藻细胞分别接种于CSI培养基中,使得培养起始细胞密度(OD680)为0.04。As(Ⅲ)和As(Ⅴ)浓度分别设置为15和30 μmol·L−1,每组设置3个平行,分别在第0、3、5、7、10天取400 μL藻液,经0.22 μm滤膜过滤,得到上清液,利用NexION 300X高效液相色谱-电感耦合等离子体-质谱仪(HPLC-ICP-MS,PerkinElmer,USA)测定砷形态。
2) As(Ⅲ)影响下硅藻生长曲线和叶绿素含量。将生长对数期的2种藻细胞分别接种于CSI培养基中,使得培养起始细胞密度(OD680)为0.04。As(Ⅲ)和As(Ⅴ)浓度分别设置为15和30 μmol·L−1,每组设置3个平行,分别在第0、3、5、7、10天取400 μL藻液,经0.22 μm滤膜过滤,得到上清液,利用NexION 300X高效液相色谱-电感耦合等离子体-质谱仪(HPLC-ICP-MS,PerkinElmer,USA)测定砷形态。
在培养第10天时,用无水乙醇法测定所有处理中硅藻的叶绿素含量[14]。取1 mL藻液在12 000 r·min−1转速下离心5 min,弃去上清液后,用50 μL蒸馏水重悬藻液,然后加入0.95 mL无水乙醇并在75 ℃下水浴加热5 min,待冷却后在波长665 nm、649 nm和750 nm下测定吸光度,并通过式 (1)~(3) 计算叶绿素质量浓度。
Chla=13.7(OD665−OD750)−5.76(OD649−OD750) (1) Chlb=25.8(OD649−OD750)−7.6(OD665−OD750) (2) G=Chla+Chlb=6.10(OD665−OD750)+20.04(OD649−OD750) (3) 式中:Chla为叶绿素a的质量浓度,Chlb为叶绿素b的质量浓度,G为总叶绿素质量浓度, 单位均为mg·L−1。
3) As(Ⅲ)胁迫下硅藻扫描电镜图(SEM)。利用SEM对0和15 μmol·L−1 As(Ⅲ)浓度处理下培养14 d后的2种硅藻进行表面形貌观察。取40 mL藻液在4 000 g下离心15 min,然后用PBS(0.01 mol·L−1)冲洗3次,再将藻细胞样品在2.5%的戊二醛中固定24 h,接着用0.1 mol·L−1磷酸盐缓冲液洗涤4次后,样品经不同浓度酒精(30、50、70、90、100%)脱水和叔丁醇浸泡置换,冷冻干燥和喷金镀膜,最后用SEM(S-3000 N,Hitachi)进行测试。
4) 硅酸盐对硅藻砷转化的影响实验。将生长于CSI培养基中(含0.1 g·L−1硅酸钠Na2SiO3·9H2O)的硅藻作为空白对照,另设置As(Ⅲ)(15 μmol·L−1)和As(Ⅲ)处理下额外添加0.05、0.15和0.25 g·L−1硅酸钠,共5个处理,每个处理3个平行。培养6 d后取上清液过膜后用HPLC-ICP-MS测定砷形态。
5) RNA提取方法。根据硅藻细胞在生长初期基因表达活性较高,取上述培养2 d后的针状菱形藻的藻液50 mL,在室温下以4 500 r·min−1离心5 min后收获细胞,弃上清液并重新悬浮在1 mL的DEPC水中溶解沉淀,接着转移至已灭菌的2 mL离心管内,将样品在室温下4 500 r·min−1离心5 min,弃去上清液,收集的藻细胞放置于液氮中。对藻细胞进行研磨后转移至装有1 mL的Trizol试剂液的离心管中,再向其中加入0.2 mL的氯仿(CHCl3),在4 ℃条件下12 000 r·min−1离心15 min,取上层水相,再加入2/3水相体积的异丙醇,摇匀后室温沉淀10 min,在相同条件下离心5 min,弃上清。用0.5 mL提前冷却的体积分数为75%的乙醇(DEPC水和无水乙醇的体积比为1:3)洗涤沉淀,并离心5 min,弃上清,再重复一次该步骤。于超净台风干乙醇后加入适量(10~40 μL)DEPC水,在65 ℃下助融5 min,然后RNA样品置于–80 ℃冰箱备用。
6) 反转录荧光定量PCR(RT-qPCR)。用PrimeScrip RT reagent Kit with gDNA Eraser (Takara,Japan)对RNA进行反转录,然后以反转录后的cDNA样品为模板,对β-actin、砷甲基转移酶基因(arsM)、线粒体核糖体RNA 12S基因(12S)和硅酸盐转运基因(sit)进行相对定量。实时荧光定量PCR反应体系为20 μL,包括10 μL SYBR Green Master Mix (GenStar,China)、目的基因上下游引物各0.5 μL(10μmol·L−1)、1 μL DNA模板、8 μL RNase-free Water。β-actin、12S和sit基因引物和qPCR反应条件参考文献[10](表1)。对于arsM基因,以Nitzschia属的砷甲基转移功能基因作为参考序列,基于序列保守区用NCBI Primer Blast进行引物设计。荧光定量PCR扩增反应使用CFX 384 Real-Time PCR Detection System (Bio-Rad Laboratories, USA)进行测定。以DEPC处理后的水为模板做负对照,PCR反应特异性用PCR产物溶解曲线确定。
表 1 实时荧光定量PCR引物序列及反应条件Table 1. Primers and reaction conditions for real-time quantitative PCR目的基因 引物名称 引物序列 扩增条件 β-actin act-F CTTGGCTGGTCGTGACTTGA 95 ℃预变性 2 min; 95 ℃ 10 s,60 ℃ 20 s, 40个循环。 act-R ACCATCGGGAAGCTCGAAAG arsM arsM-F GATGGGCTGGTCGTTGGCAT arsM-R TGGGAGCAAGGCAAAAGGCA 12S 12S-F AGGATGCAAGTGTTATCCGGA 12S-R CAATATCTACGCATTTCACCGCT sit sit-F ATCAACGCTACCACCTGCAT sit-R GAGCCTCGTGGGTAACAACA 1.3 数据分析方法
硅藻特定基因的相对表达量用2−ΔΔCt方法计算,根据4个生物学重复计算平均值和标准误差。对于处理间差异的显著性,采用SPSS 26软件进行方差分析。采用Origin 2022b完成结果绘图。
2. 结果与讨论
2.1 硅藻驱动As(Ⅲ)和As(Ⅴ)转化
针状菱形藻和谷皮菱形藻分别暴露于15 μmol·L−1 As(Ⅲ)时,在10 d培养期内甲基砷浓度随时间增加而增加,产物形态主要为DMA。硅藻生长在前10 d内活性较高,细胞的砷代谢较为活跃,这表明2种硅藻在生长增殖过程中都有砷甲基化作用。第10天时,针状菱形藻中DMA浓度达到0.84 μmol·L−1,TMAsO浓度达到0.013 μmol·L−1,转化率分别为5.54%和0.17%(图1(a)),同时有明显的As(Ⅴ)生成(1.92 μmol·L−1),转化率为3.2%(图1(b))。对于谷皮菱形藻,10 d培养后生成的DMA浓度为0.12 μmol·L−1,转化率为0.8%,但未检测到TMAsO(图1(c)),并发现有大量As(Ⅴ)生成(15.8 μmol·L−1),转化率为90.1%(图1(d))。以上结果表明,针状菱形藻和谷皮菱形藻在As(Ⅲ)暴露下都具有As(Ⅲ)砷甲基化能力,将As(Ⅲ)转化为毒性较低的甲基砷。QIN在2009年首次鉴定了藻类红藻(Cyanidioschyzon sp. isolate 5508)中的亚砷酸甲基转移功能基因CmarsM和验证了其酶学特性。CmarsM能在体外将As(Ⅲ)转化为一甲基亚砷酸,而后生成二甲基亚砷酸和三甲基砷气体,CmarsM基因的异源表达能赋予砷敏感型大肠杆菌细胞对As(Ⅲ)的抗性,使得砷甲基化反应具有解毒的生理意义[15]。此后,还有其他藻类被发现具有As(Ⅲ)甲基化功能,如蛋白核小球藻和莱茵衣藻等[16]。本研究中2种藻砷甲基化反应主要生成DMA,皆未检测到一甲基砷,可能是由于一甲基亚砷酸中间产物在胞内的连续甲基化,而未能外排至胞外溶液中。针状菱形藻的甲基化能力高于谷皮菱形藻,显示出不同淡水硅藻物种的砷甲基化效率存在差异。除了砷甲基化,暴露于As(Ⅲ)的2种藻在培养期内明显发生了As(Ⅲ)氧化反应。QIN也发现红藻能氧化As(Ⅲ)为As(Ⅴ),发生于细胞质区室外,可能与其胞外酶有关,类似于碳酸酐酶和磷酸酶对胞外无机碳和磷酸盐的摄取等胞外酶驱动的反应[15]。由于光合自养型硅藻不能利用砷氧化产生能量,藻的砷氧化作用是潜在的As(Ⅲ)胁迫下的一种生存策略。谷皮菱形藻的砷甲基化能力虽然较弱,但具有较强的砷氧化能力,表现出不同硅藻采取不同的方式缓解As(Ⅲ)胁迫。由于微藻介导的砷氧化反应产生毒性和移动性较小的五价砷,较强砷氧化能力的微藻具有应用于砷污染水体修复的潜力[17]。
暴露于30 μmol·L−1 As(Ⅴ)下的针状菱形藻和谷皮菱形藻在10 d培养期内产生甲基砷,并在溶液中随时间增加而累积。结果如图2所示,10 d后针状菱形藻产生的DMA浓度为0.26 μmol·L−1,转化率为0.85%(图2(a)),谷皮菱形藻中DMA浓度为0.07 μmol·L−1,转化率为0.24%(图2(c))。这表明针状菱形藻对As(Ⅴ)的砷甲基化作用较强于谷皮菱形藻,与2种藻As(Ⅲ)砷甲基化差异结果一致。淡水硅藻A. minutissimum能甲基化As(Ⅴ)为DMA,即在淡水水体中硅藻能通过胞内还原后甲基化,生成毒性较低的甲基砷产物[5]。同时,在针状菱形藻培养液中还检测出明显的As(Ⅲ)(0.32 μmol·L−1)(图2(b)),但在谷皮菱形藻中未检出(图2(d)),这与谷皮菱形藻较弱的砷甲基化能力相对应。这些结果表明硅藻吸收As(Ⅴ)后可通过2个途径进行解毒:一是将As(Ⅴ)在胞内还原为As(Ⅲ),然后对As(Ⅲ)进行甲基化生成DMA;二是将胞内还原产生的As(Ⅲ)外排至胞外从而降低胞内砷浓度[15]。微藻胞内砷还原是砷抗性代谢中重要的一步,将绿藻Chlamydomonas reinhardtii砷还原酶CrACR2s表达于砷还原功能缺失的大肠杆菌中,能恢复其对As(Ⅴ)的抗性[18]。真核生物中发现的砷酸盐还原酶为双特异性磷酸酶中的一员,来源于酵母Saccharomyces cerevisiae的Acr2蛋白酶的作用机制类似于细菌中的arsC,利用谷氧还蛋白和谷胱甘肽作为电子供体还原As(Ⅴ)[19]。微藻中的谷胱甘肽还会与As(Ⅲ)形成As-GSH复合物,使砷保持还原态进行连续甲基化反应[20],或是转移储存于液泡中减少砷的毒性[21]。另外,微藻对水溶液中As(Ⅴ)的还原作用,减少As(Ⅴ)有效性,降低As(Ⅴ)和磷酸盐的竞争性转运,有利于微藻对水溶液中的磷酸盐的吸收利用。
2.2 不同As(Ⅲ)浓度下硅藻生长曲线和叶绿素含量
针状菱形藻和谷皮菱形藻暴露于不同浓度As(Ⅲ)下的OD值变化如图3所示,14 d培养过程中无砷处理的2种硅藻生物量随着时间增加而增加。当As(Ⅲ)存在时,生物量随As(Ⅲ)浓度增加而减少,并在As(Ⅲ)存在条件下生长至14 d时处于稳定期后期。5和15 μmol·L−1 As(Ⅲ)对针状菱形藻生长有抑制作用,使培养14 d后的OD分别下降了12.7%和16.5%,且针状菱形藻不能在50 μmol·L−1 As(Ⅲ)条件下生长(图3(a))。相对无砷处理,谷皮菱形藻在5 μmol·L−1 As(Ⅲ)存在时14 d后的生物量减少13.4%,15和50 μmol·L−1 As(Ⅲ)明显抑制其生长(图3(b))。类似的As(Ⅲ)对硅藻生长的抑制作用也发现于淡水水华硅藻链状弯壳藻(Achnanthidium minutissimum)中,A. minutissimum能在10 μmol·L−1 As(Ⅲ)条件下生长,但100 μmol·L−1 As(Ⅲ)完全抑制其生长。本研究中针状菱形藻比谷皮菱形藻有较强的砷抗性,显示出不同硅藻的砷抗性存在差异,可能与其采用的砷解毒机制不同有关[6]。
如图4所示,在第10天硅藻细胞生长至稳定期时测定各处理的叶绿素含量,发现不同浓度As(Ⅲ)胁迫对硅藻的叶绿素a和b含量也产生了负面影响。与对照组相比,5、15和50 μmol·L−1 As(Ⅲ)使针状菱形藻叶绿素a和b分别降低了13.4%~18.6%和10.5%~30.2%。对于谷皮菱形藻,叶绿素含量随着As(Ⅲ)浓度增加明显下降,5、15和50 μmol·L−1 As(Ⅲ)相较于对照使叶绿素a和b分别下降了17.7%~96.3%和24.7%~88.0%。已有研究表明,As(Ⅲ)存在会减少光合蓝细菌Nostoc muscorum的叶绿素含量,可能是由于As(Ⅲ)减少了合色素合成前体、增加叶绿素降解酶,或取代叶绿素四吡咯环中心的镁原子从而降低了捕光色素蛋白复合物的效率[22]。另外,砷暴露还会干扰生物体呼吸电子传递链,影响还原力NADPH和能量ATP的形成,降低1,5—二磷酸核酮糖羧化酶/加氧酶(RuBisCo)活性和固碳效率,从而降低其生长速率[22]。对应于As(Ⅲ)胁迫下硅藻生物量减少,谷皮菱形藻的叶绿素含量降低较针状菱形藻更为明显,表现出硅藻间砷敏感度的差异。
2.3 As(Ⅲ)胁迫下硅藻扫描电镜图(SEM)
通过SEM表征有无15 μmol·L−1 As(Ⅲ)添加下2种硅藻的表面形貌,结果显示相较于无砷培养中完整的硅藻细胞,有砷处理下针状菱形藻表面出现更多絮状物,细胞壁有所破损,壳孔的完整度降低,展现出砷的毒性导致的硅藻表面损伤(图5(a)、(b))。砷存在时谷皮菱形藻除了出现更多的表面絮状物外,壳面有所变形(图5(c)、(d))。这与文献报道的As(Ⅲ)胁迫会引起舟形藻(Navicula sp.)硅藻细胞表面破损[23]是一致的。
2.4 硅酸盐对硅藻As(Ⅲ)转化的影响
15 μmol·L−1 As(Ⅲ)处理中针状菱形藻培养6 d后,硅藻处于生长期中期,此时转化产生的DMA浓度为0.23 μmol·L−1,TMAsO浓度为0.016 μmol·L−1,砷氧化产生As(Ⅴ)的浓度达2.4 μmol·L−1 (图6(a))。额外添加0.05和0.15 g·L−1硅酸盐对主要产物DMA生成没有明显影响,而0.25 g·L−1硅酸盐处理下DMA产生量显著下降,高浓度硅酸盐添加也显著降低了TMAsO生成。同时,添加硅酸盐显著增加了As(Ⅴ),特别是0.15 g·L−1硅酸盐处理下As(Ⅴ)浓度为6.2 μmol·L−1,相比无额外添加提高了2.6倍,该处理中相应的As(Ⅲ)浓度显著下降(图6(b))于谷皮菱形藻,As(Ⅲ)处理下培养6 d后DMA、TMAsO和As(Ⅴ)浓度分别为0.12、0.03和1.8 μmol·L−1 (图6(c))。0.05和0.15 g·L−1硅酸盐对DMA生成没有明显影响,而0.25 g·L−1硅酸盐处理下DMA显著增加。与针状菱形藻类似,额外添加硅酸盐显著增加了谷皮菱形藻As(Ⅴ)的浓度,在质量浓度为0.15 g·L−1时,As(Ⅴ)达到最高(8.2 μmol·L−1)。相比无额外添加提高4.5倍(图6(d))的来说,硅酸盐添加会对硅藻砷甲基化产生负面影响,特别是对于砷甲基化能力较强的针状菱形藻,同时显著增强了2种硅藻的砷氧化能力。由于亚砷酸和硅酸盐具有相似的结构,其能共享相同的硅转运蛋白进行吸收,如水稻细胞的硅转运蛋白同时介导了As(Ⅲ)和Si的吸收[24]。亚砷酸和硅酸盐在硅藻中的吸收也可能共享SIT硅转运蛋白[21],因此硅酸盐添加通过与As(Ⅲ)竞争吸收,从而减少细胞对砷的转运和接下来的胞内甲基化反应。此外,高浓度硅酸盐促进了砷氧化,As(Ⅲ)作为甲基化底物其有效性的降低也会导致砷甲基化效率降低。硅酸盐作为自然水体中常见的含氧阴离子,其质量浓度范围为0.45~14 mg·L−1 Si。本研究硅藻生长的硅酸盐背景质量浓度为0.1 g·L−1 Na2SiO3·9H2O(即9.9 mg·L−1 Si),与自然条件下硅藻的生长条件接近,并在0.05、0.15和0.25 g·L−1硅酸钠处理中0.15 g·L−1硅酸钠添加后砷氧化率最高,因此建议该浓度为硅藻调控水体砷氧化时的参考浓度。
2.5 硅酸盐对硅藻砷甲基化和硅代谢相关基因转录表达的影响
不同处理下针状菱形藻的砷甲基转移酶基因(arsM)、硅酸盐转运基因(sit)和线粒体核糖体RNA 12S基因(12S)相对表达量结果表明,相对无砷处理,15 μmol·L−1 As(Ⅲ)存在时藻细胞显著上调arsM基因表达,并显著下调了sit基因(图7(a)和7(b))。这些结果表明硅藻在As(Ⅲ)暴露下通过显著上调arsM基因转录表达驱动砷甲基化,证明arsM介导的砷甲基化反应是硅藻应对砷胁迫的抗性机制之一。As(Ⅲ)暴露后sit基因下调表明在转录水平上砷对硅藻硅酸盐利用产生抑制作用,已有研究也发现谷皮菱形藻响应于重金属胁迫时(例如Cd),会显著下调sit基因[10]。因为As(Ⅲ)和硅酸盐可能共用SIT硅转运蛋白转运[21],sit基因下调降低As(Ⅲ)吸收,能缓解细胞的砷暴露程度。此外,高浓度硅酸盐条件下砷氧化显著增加,As(Ⅲ)有效性的降低也会导致砷甲基化效率降低。额外添加硅酸盐对arsM表达没有明显影响,与硅酸盐影响下针状菱形藻培养液中的甲基砷浓度变化相对应。已有研究表明,硅酸盐添加会引起硅藻sit基因或水稻植物细胞硅转运蛋白基因的下调,从而降低细胞对砷的吸收转运[25-26]。在本研究中,硅酸盐对砷胁迫下sit基因表达没有明显影响,表明硅转运蛋白活性不是硅酸盐影响硅/砷吸收代谢的主要因素。As(Ⅲ)存在对12S基因表达无明显影响,硅酸盐添加后显著上调了12S基因表达,并在质量浓度为0.25 g·L−1时达到最高(图7(c)),这说明硅酸盐添加可提高硅藻线粒体数量,以维持砷胁迫下细胞的ATP产生[27]。综上所述,硅藻应对As(Ⅲ)胁迫提高砷甲基化活性,降低硅/砷转运活性,有利于胞内砷解毒和减少As(Ⅲ)吸收,硅酸盐增加不会直接影响砷甲基化活性,但能促进As(Ⅲ)氧化为As(Ⅴ)降低砷毒性,并且提高呼吸产能活性,从而有助于强化硅藻对砷的解毒作用(图8)。
3. 结论
1)淡水硅藻针状菱形藻和谷皮菱形藻能对As(Ⅲ)进行甲基化和氧化,同时对As(Ⅴ)能进行还原后甲基化,且主要甲基化产物形态为二甲基砷。将毒性较强的三价无机砷转化为低毒的甲基砷,或氧化为低毒的五价砷,成为硅藻主要的砷解毒机制。
2)针状菱形藻对As(Ⅲ)的砷甲基化能力比谷皮菱形藻强,而谷皮菱形藻的As(Ⅲ)氧化作用比针状菱形藻强。不同硅藻的主要砷转化途径和主要产物形态不同,表现出硅藻抵御As(Ⅲ)胁迫的生存策略存在差异。
3)暴露于As(Ⅲ)的针状菱形藻上调砷甲基转移功能基因arsM转录活性,并显著下调了硅酸盐转运基因sit转录,表明硅藻通过表达相关功能基因驱动砷甲基化反应,和减少硅/砷转运活性,能够帮助硅藻胞内砷解毒和减少As(Ⅲ)吸收,体现出硅藻在As(Ⅲ)胁迫下转录水平的代谢响应特征。
4)硅酸盐的添加对针状菱形藻砷甲基转移酶基因表达活性没有显著影响,但上调线粒体核糖体RNA 12S基因转录活性,并促进了As(Ⅲ)向As(Ⅴ)转化,表明硅藻在砷胁迫下提高细胞的呼吸产能活性,同时通过促进砷氧化降低砷毒性,有助于强化硅藻对砷的抗性。
-
表 1 实时荧光定量PCR引物序列及反应条件
Table 1. Primers and reaction conditions for real-time quantitative PCR
目的基因 引物名称 引物序列 扩增条件 β-actin act-F CTTGGCTGGTCGTGACTTGA 95 ℃预变性 2 min; 95 ℃ 10 s,60 ℃ 20 s, 40个循环。 act-R ACCATCGGGAAGCTCGAAAG arsM arsM-F GATGGGCTGGTCGTTGGCAT arsM-R TGGGAGCAAGGCAAAAGGCA 12S 12S-F AGGATGCAAGTGTTATCCGGA 12S-R CAATATCTACGCATTTCACCGCT sit sit-F ATCAACGCTACCACCTGCAT sit-R GAGCCTCGTGGGTAACAACA -
[1] NIEGEL C, MATYSIK F-M. Analytical methods for the determination of arsenosugars—A review of recent trends and developments[J]. Analytica Chimica Acta, 2010, 657(2): 83-99. doi: 10.1016/j.aca.2009.10.041 [2] ROSENWASSER S, GRAFF VAN CREVELD S, SCHATZ D, et al. Mapping the diatom redox-sensitive proteome provides insight into response to nitrogen stress in the marine environment[J]. Proceedings of the National Academy of Sciences, 2014, 111(7): 2740-2745. doi: 10.1073/pnas.1319773111 [3] 丁腾达, 倪婉敏, 张建英. 硅藻重金属污染生态学研究进展[J]. 应用生态学报, 2012, 23(3): 857-866. doi: 10.13287/j.1001-9332.2012.0117 [4] PAPRY R I, ISHII K, MAMUN M A A, et al. Arsenic biotransformation potential of six marine diatom species: effect of temperature and salinity[J]. Scientific Reports, 2019, 9(1): 10226. doi: 10.1038/s41598-019-46551-8 [5] BARRAL-FRAGA L, MORIN S, ROVIRA M D M, et al. Short-term arsenic exposure reduces diatom cell size in biofilm communities[J]. Environmental Science and Pollution Research, 2016, 23(5): 4257-4270. doi: 10.1007/s11356-015-4894-8 [6] HASEGAWA H, PAPRY R I, IKEDA E, et al. Freshwater phytoplankton: Biotransformation of inorganic arsenic to methylarsenic and organoarsenic[J]. Scientific Reports, 2019, 9(1): 12074. doi: 10.1038/s41598-019-48477-7 [7] PAN Y, SUBBA RAO D V, MANN K H, et al. Effects of silicate limitation on production of domoic acid, a neurotoxin, by the diatom Pseudo-nitzschia multiseries. I. Batch culture studies[J]. Marine Ecology Progress Series, 1996, 131(1-3): 225-233. [8] SAPRIEL G, QUINET M, HEIJDE M, et al. Genome-wide transcriptome analyses of Silicon metabolism in phaeodactylum tricornutum reveal the multilevel regulation of Silicic acid transporters[J]. PLOS ONE, 2009, 4(10): e7458. doi: 10.1371/journal.pone.0007458 [9] MACHADO M, VAZ M G M V, BROMKE M A, et al. Metabolic stability of freshwater Nitzschia palea strains under silicon stress associated with triacylglycerol accumulation[J]. Algal Research, 2021, 60: 102554. doi: 10.1016/j.algal.2021.102554 [10] KIM TIAM S, LAVOIE I, DOOSE C, et al. Morphological, physiological and molecular responses of Nitzschia palea under cadmium stress[J]. Ecotoxicology, 2018, 27(6): 675-688. doi: 10.1007/s10646-018-1945-1 [11] ZHOU B, MA J, CHEN F, et al. Mechanisms underlying silicon-dependent metal tolerance in the marine diatom Phaeodactylum tricornutum[J]. Environmental Pollution, 2020, 262: 114331. doi: 10.1016/j.envpol.2020.114331 [12] MA J, ZHOU B, DUAN D, et al. Silicon limitation reduced the adsorption of cadmium in marine diatoms[J]. Aquatic Toxicology, 2018, 202: 136-144. doi: 10.1016/j.aquatox.2018.07.011 [13] SANTOS J, ALMEIDAl S F, FIGUEIRA E. Cadmium chelation by frustulins: a novel metal tolerance mechanism in Nitzschia palea (Kutzing) W. Smith[J]. Ecotoxicology, 2013, 22(1): 166-73. doi: 10.1007/s10646-012-1013-1 [14] WINTERMANS J F G M, DE MOTS A. Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol[J]. Biochimica et Biophysica Acta (BBA) - Biophysics including Photosynthesis, 1965, 109(2): 448-453. doi: 10.1016/0926-6585(65)90170-6 [15] QIN J, LEHR C R, YUAN C, et al. Biotransformation of arsenic by a Yellowstone thermoacidophilic eukaryotic alga[J]. Proceedings of the National Academy of Sciences, 2009, 106(13): 5213-5217. doi: 10.1073/pnas.0900238106 [16] YE J, RENSING C, ROSEN B P, et al. Arsenic biomethylation by photosynthetic organisms[J]. Trends in Plant Science, 2012, 17(3): 155-162. doi: 10.1016/j.tplants.2011.12.003 [17] HUSSAIN M M, WANG J, BIBI I, et al. Arsenic speciation and biotransformation pathways in the aquatic ecosystem: The significance of algae[J]. Journal of Hazardous Materials, 2021, 403. [18] YIN X, WANG L, DUAN G, et al. Characterization of arsenate transformation and identification of arsenate reductase in a green alga Chlamydomonas reinhardtii[J]. Journal of Environmental Sciences, 2011, 23(7): 1186-1193. doi: 10.1016/S1001-0742(10)60492-5 [19] BOBROWICZ P, WYSOCKI R, OWSIANIK G, et al. Isolation of three contiguous genes, ACR1, ACR2 andACR3, involved in resistance to Arsenic compounds in the yeast Saccharomyces cerevisiae[J]. Yeast, 1997, 13(9): 819-828. doi: 10.1002/(SICI)1097-0061(199707)13:9<819::AID-YEA142>3.0.CO;2-Y [20] 王培培, 陈松灿, 朱永官, 等. 微生物砷甲基化及挥发研究进展[J]. 农业环境科学学报, 2018, 37(07): 1377-1385. doi: 10.11654/jaes.2018-0542 [21] XU D, SCHAUM C-E, LI B, et al. Acclimation and adaptation to elevated pCO2 increase arsenic resilience in marine diatoms[J]. The ISME Journal, 2021, 15(6): 1599-1613. doi: 10.1038/s41396-020-00873-y [22] PATEL A, TIWARI S, PRASAD S M. Toxicity assessment of arsenate and arsenite on growth, chlorophyll a fluorescence and antioxidant machinery in Nostoc muscorum[J]. Ecotoxicology and Environmental Safety, 2018, 157: 369-379. doi: 10.1016/j.ecoenv.2018.03.056 [23] ZHANG J, NI Y, DING T, et al. The role of humic acid in the toxicity of arsenite to the diatom Navicula sp[J]. Environmental Science and Pollution Research, 2014, 21(6): 4366-75. doi: 10.1007/s11356-013-2413-3 [24] MA J F, YAMAJI N, MITANI N, et al. Transporters of arsenite in rice and their role in arsenic accumulation in rice grain[J]. Proceedings of the National Academy of Sciences, 2008, 105(29): 9931-9935. doi: 10.1073/pnas.0802361105 [25] ZHANG S, GENG L, FAN L, et al. Spraying silicon to decrease inorganic arsenic accumulation in rice grain from arsenic-contaminated paddy soil[J]. Science of the Total Environment, 2020, 704. [26] SHRESTHA ROSHAN P, HILDEBRAND M. Evidence for a regulatory role of diatom Silicon transporters in cellular Silicon responses[J]. Eukaryotic Cell, 2015, 14(1): 29-40. doi: 10.1128/EC.00209-14 [27] KIM TIAM S, FEUITET-MAZEL A, DELMAS F, et al. Development of q-PCR approaches to assess water quality: effects of cadmium on gene expression of the diatom Eolimna minima[J]. Water Research, 2012, 46(4): 934-42. doi: 10.1016/j.watres.2011.11.005 期刊类型引用(1)
1. 刘雯清,黄中情,何发涛,钟彪,宋梦萍,曾绍宇,阮俊杰,杨常亮,陈晨. 不同暴露条件下克氏原螯虾组织中砷的积累与转化. 环境化学. 2024(02): 497-505 .  百度学术
百度学术
其他类型引用(1)
-






 DownLoad:
DownLoad: