-
目前,针对土壤有机污染物的传统治理技术,通常难以达到去除低环PAHs的修复要求[1-2]。萘 (naphthalene,Nap) 作为一种具有致畸、致突变与致癌的典型低环半挥发性PAHs[3],研究其在土壤介质中的降解规律,并完善其修复治理技术,对于评估低环PAHs的环境风险和提出相应的污染管控措施具有重要的意义。
近年来,过硫酸盐高级氧化技术在污染控制领域受到越来越多的关注[4-6],PS是一种强氧化剂,可以通过热[7-9]、碱[10-11]、过渡金属离子[12-14]、超声[15-17]和微波[18-21]等能量或材料进行活化。研究表明,提升温度可有效增加过硫酸盐的活性,但常规加热存在能耗高、效率低的问题[22]。微波诱导具有加热快速、能耗相对较低、无二次污染等优点[23-24],但缺少机理研究,且大多数人使用市售微波炉,单磁控管能量密度低,很难达到预期活化效果。
工业上绝大多数情况使用多磁控管微波源提高微波功率,然而微波加热的不均匀性在很大程度上限制了微波技术的发展和应用[25]。微波加热不均匀的主要原因是电磁场强度分布不均匀[26]。目前,改善微波加热不均匀性的措施主要是通过改变谐振腔的尺寸及微波馈口数量[27]和相对位置[28],使谐振腔具有尽可能多的振荡模式[29],不同模式叠加后能够获得更加均匀的能量分布状态。本研究拟应用COMSOL Multiphysics[30-31]多物理场仿真软件优化谐振腔内电磁场分布,参照模拟结论,制备微波反应器,并通过MW诱导PS强化降解土壤中萘的实验研究,考察高浓度萘污染土壤的微波诱导修复效果及影响因素,以期为实现该技术的工程应用提供参考。
-
实验所用试剂主要有过硫酸钠 (Na2S2O8) 、萘 (C10H8) 、甲醇 (CH3OH) 、氯化钠 (NaCl) 、石英砂 (SiO2) 、无水乙醇 (CH3CH2OH) 、磷酸 (H3PO4) 均为分析纯、高纯氮气 (N2,99.999%) 。实验所用土壤采自河北科技大学绿地,取表层约20 cm处土壤,去除土壤中根系等杂质后,研磨过60目筛后,在室内经自然风干储存备用。土壤理化性质如表1所示。
-
气相色谱仪 (7820A,安捷伦科技有限公司) 、自动顶空进样器 (7697A,安捷伦科技有限公司) 、气相色谱质谱联用仪 (7890B,安捷伦科技有限公司) 、数显水浴恒温振荡器 (SHA-B,常州荣华仪器制造有限公司) 、电子天平 (FA2004B,上海佑科仪器仪表有限公司) 、红外热成像仪 (DP-22,深圳点扬科技有限公司) 、微波反应器 (山东科宏微波能有限公司) 。
-
1) 矩形谐振腔电磁场模拟。以COMSOL Multiphysics仿真软件构建出微波辐照多物理场仿真模型,通过模拟谐振腔内电磁场分布及土壤样品温度场分布情况,参照模拟结论,制备微波反应器,搭建微波诱导PS强化降解土壤中萘的实验平台。
2) 单因素实验。分别对PS浓度0.2、0.5、0.8、1.0、1.2 mol·L−1,微波辐照时间3、6、10、15、20、30、40、60、80 min,温度40、60、80 ℃,土壤含水率8%、10%、12%、15%这4个影响因子进行单因素实验。为得到稳定可靠的结果,每组实验设置 3 组平行实验,确定各影响因素的最佳取值。
3) 不同方式活化过硫酸盐的效果比较。对比微波诱导与常规电加热实验,探索同功率强度下的微波诱导和电热活化的不同费效关系,探索不同加热方式的能耗特征。同时,从反应动力学角度进行研究,通过反应速率对比,揭示活化能降低规律。
微波诱导。取适量土样置于石英玻璃容器中,加入适量的过硫酸钠溶液,设置微波功率和辐照时间,实验结束后取出污染土样,立即装入22 mL顶空瓶中并加入10 mL饱和NaCl溶液,采用顶空-气相色谱仪测定其中萘含量。常规电热活化。除使用马弗炉设备外,其他与上述步骤相同。
4) 土壤中萘的降解产物分析。实验使用自制微波反应器,在PS浓度1.0 mol·L−1、MW温度80 ℃、土壤含水率15%、MW处理60 min的条件下进行实验,分别在3、6、10、15、20、30、40、60 min 时收集气相样品,采用气相色谱-质谱联用仪 (GC-MS) 进行检测。
-
1) 气相色谱仪 (GC-FID) 检测条件。柱箱初始温度为100 ℃,升温速率8 ℃·min−1升温至100 ℃ (保持1 min) ,升温速率20 ℃·min−1升温至200 ℃ (保持10 min) ;进样口温度220 ℃;检测器温度240 ℃。
2) 气相色谱-质谱联用仪 (GC-MS) 检测条件。柱箱初始温度为35 ℃,升温速率15 ℃·min−1升温至150 ℃ (保持5 min) ,升温速率3 ℃·min−1到280 ℃ (保持2 min) ;进样口温度280 ℃,不分流;电子轰击源 (EI) ;离子原温度230 ℃;离子化能量70 eV。
-
矩形谐振腔的模式数越多,谐振波型数量越多,为了取得较均匀的能量分布,应使矩形谐振腔具有尽可能多的振荡模式[32]。
1) 矩形谐振腔尺寸选择。假设在空间直角坐标系中矩形谐振腔具有无穷多个分离的震荡模式,每一种谐振模式对应一种场分布,对应唯一的谐振频率,矩形谐振腔的谐振频率如式(1)所示,对应在自由空间中的谐振波长如式(2)所示。
式中: a、b、d分别为谐振腔x、y、z方向上的长度;m、n、p 为矩形谐振腔模式系数,分别表示在a、b、d上分布的半驻波波长个数;
C 表示光速,取3×108 m∙s−1。谐振波长
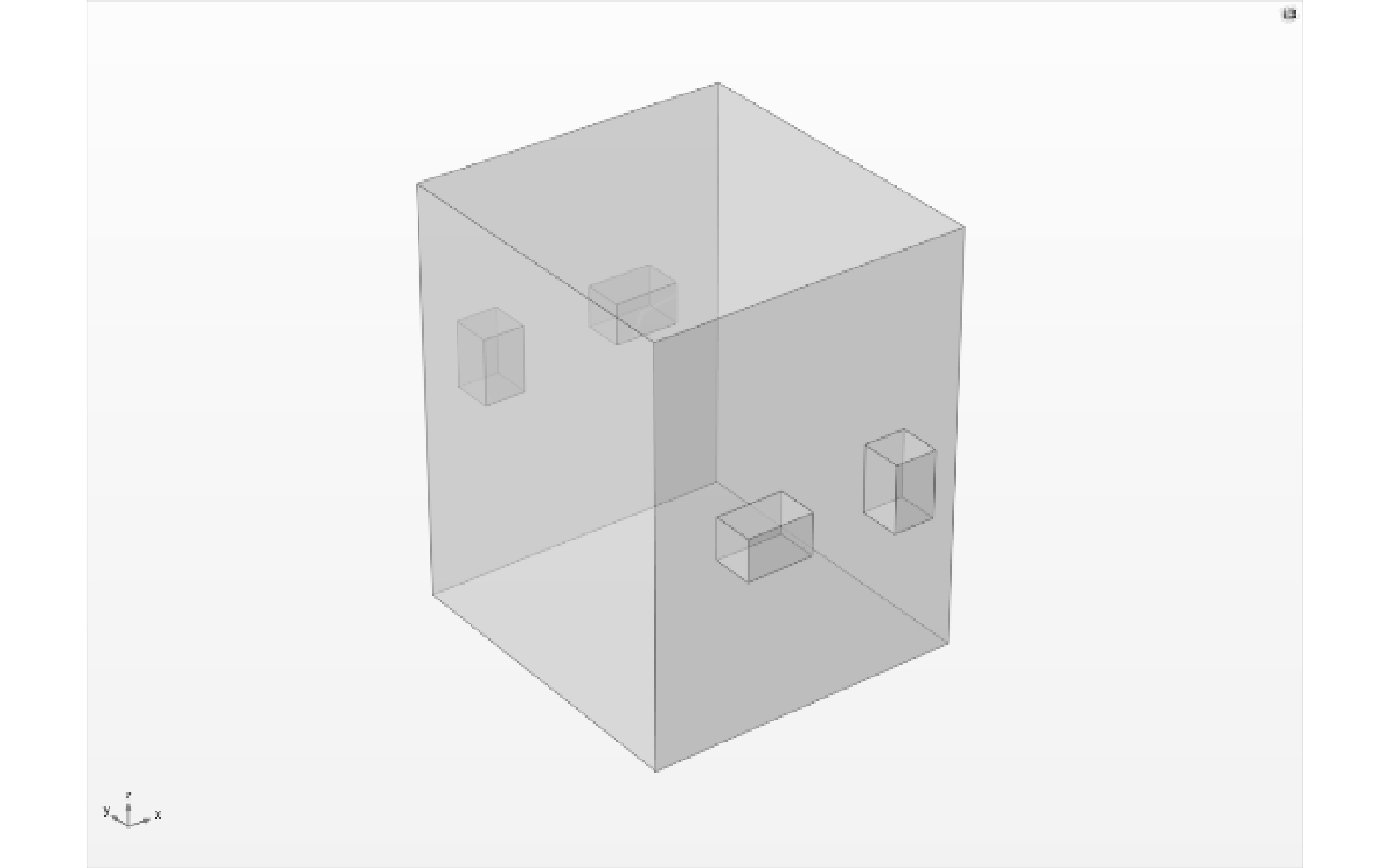
λ 大于工作波长的对应谐振模式应考虑为可存在的谐振模式[33]。根据式(1)、式(2)可求出满足上述限定条件的 m、n、p可能存在的组合数量,在工作频率为2.45 GHz条件下,取a = 400 mm,选取b = 300~400 mm和d = 400~500 mm范围内,借助VC++编写程序代码计算谐振腔模式数,当工作频率为2.45 GHz时,模式最多的腔体尺寸为400 mm×400 mm×480 mm。2) 矩形谐振腔建模。采用COMSOL Multiphysics多物理场仿真软件中RF模块,联合求解有限元边界条件下的麦克斯韦方程组和能量守恒方程,对谐振腔进行了微波场均匀性的仿真研究。谐振腔三维模型如图1所示。
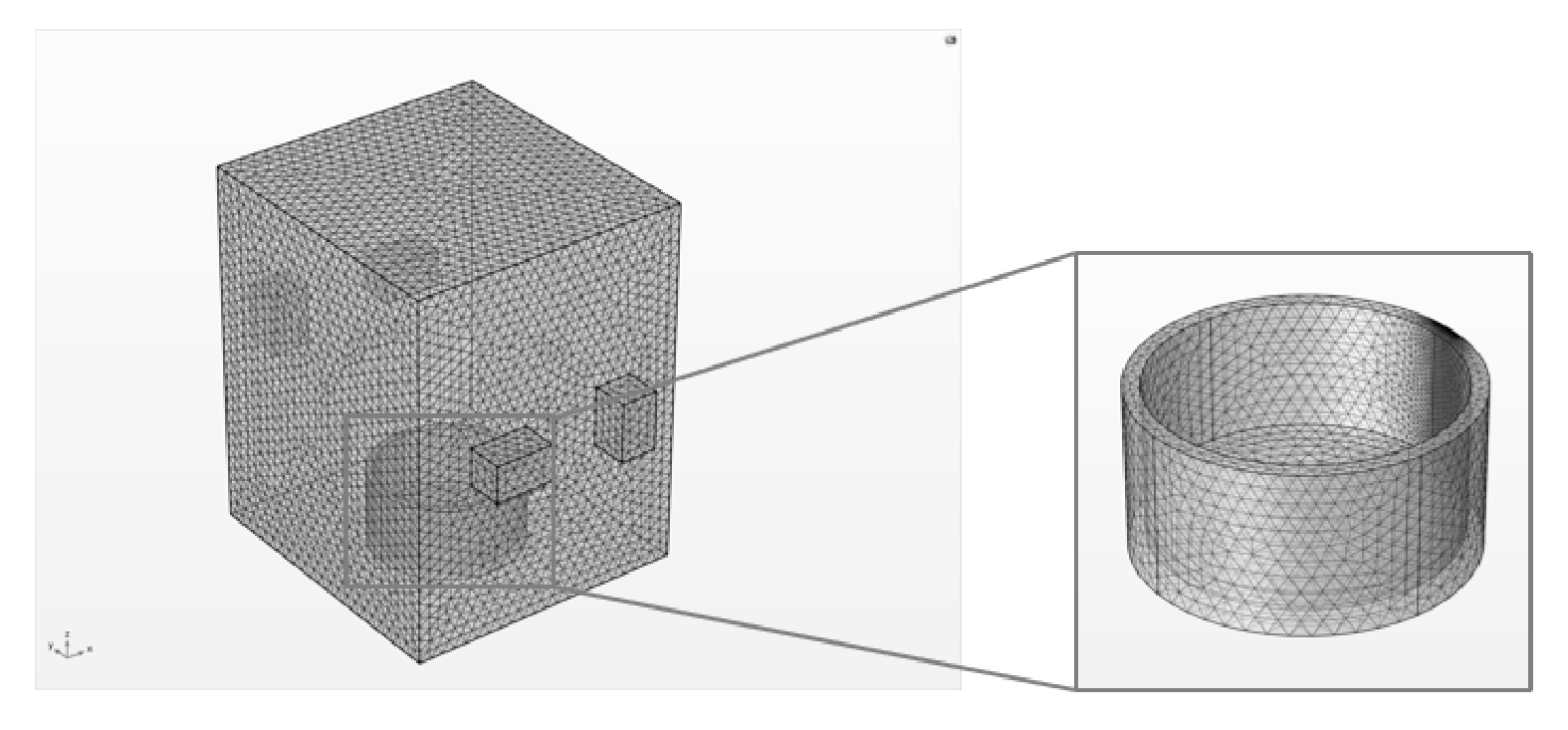
3) 网格划分。利用COMSOL Multiphysics构建整个有限元模型后,设置最大单元为0.024 47 m,最小单元为7.25×10−4 m,最大单元增长率为1.3,曲率因子为0.2,狭窄区域分辨率为1。网格划分如图2所示。
4) 仿真理论。在利用 COMSOL Multiphysics模拟时主要涉及到了麦克斯韦方程和能量守恒方程的联立求解。通过求解一个特殊形式的麦克斯韦方程得到谐振腔内的三维电磁场强度分布,如式(3)所示。
式中:
E 表示电场强度,V∙m−1;μr 表示相对磁导率,j 是虚数单位;σ 是电导率;ω 是角频率;ϵr 是相对介电常数;ϵ0 是真空介电常数,取3×108 m∙s−1 ;k0 为真空中波数。传热场计算可由含非齐次项的偏微分方程式(4)给出。
式中:
ρ 表示密度,kg∙m−3;CP 表示比热容,J∙(kg∙K)−1,;К 为热导率。 -
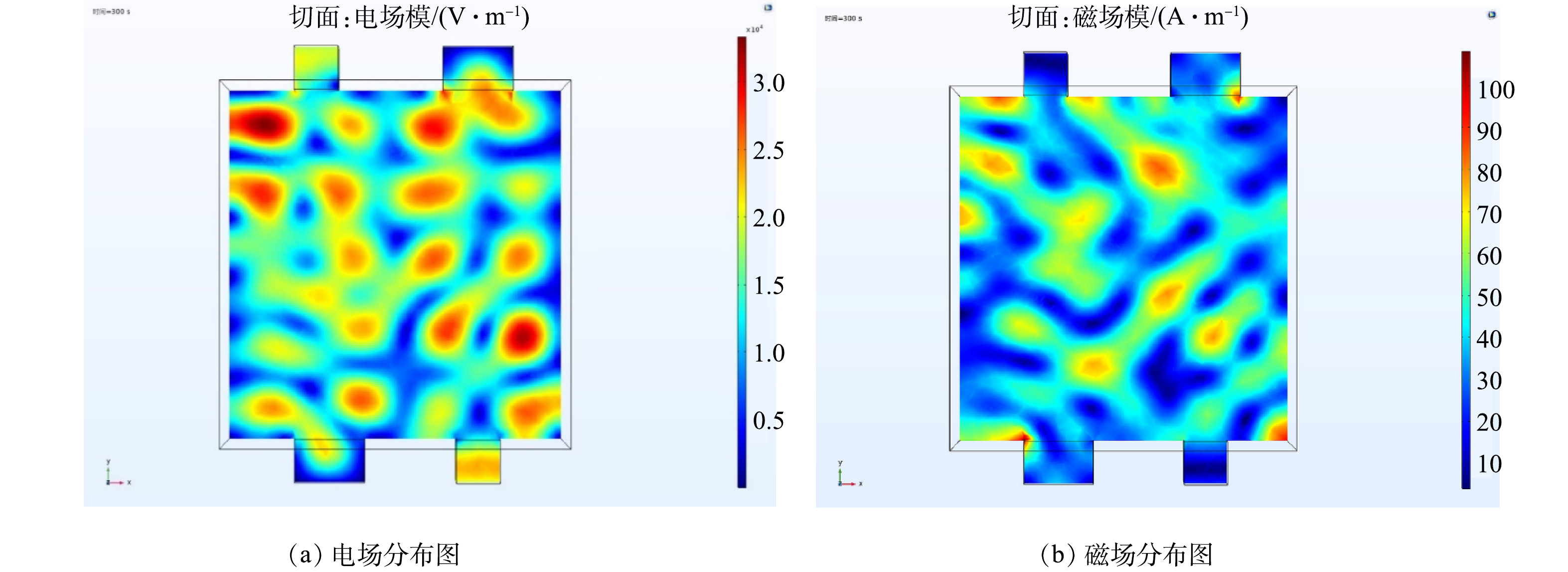
1) 谐振腔内电磁场分布模拟。谐振腔电场平面分布如图3(a)所示。靠近腔体壁的区域电场强度较低,腔体内部区域电场强度较高,这说明谐振腔内部区域的微波功率密度高于靠近腔体壁的区域。并且谐振腔内部电场差值较小,表明腔体内的微波功率密度较一致。这可能是因为靠近腔体壁区域的电场叠加较少,微波源反射到箱体中央与其他磁控管的电场叠加较多,提高了电磁场的均匀性。图3(b)为谐振腔磁场平面分布图,磁场分布情况与电场类似。采用微波加热时,应将样品放在谐振腔的中心位置,以使介质最大程度的吸收能量,提高升温速率。
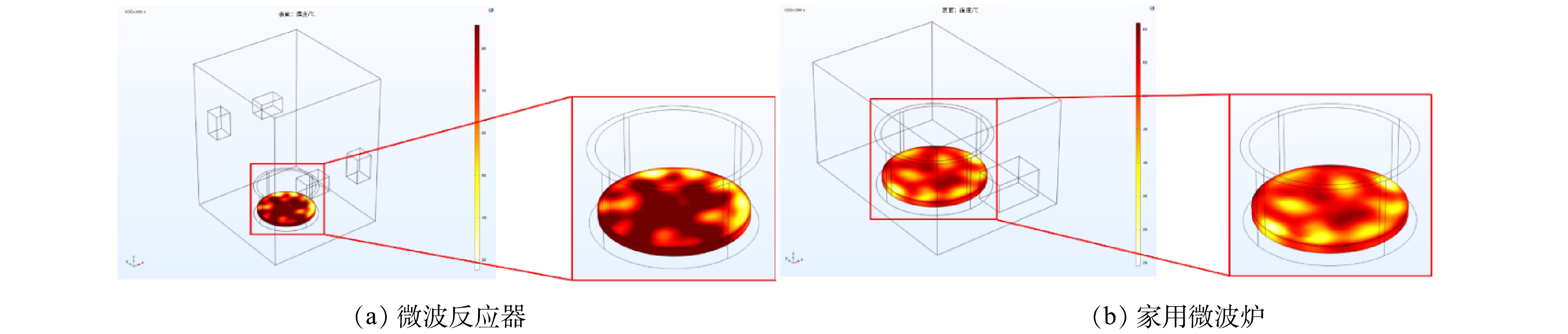
2) 模型加热温度分布模拟。微波反应器中样品温度分布如图4(a)所示。微波反应器中样品温度大部分处于60~80 ℃,温度分布均匀性较好。家用微波炉中样品温度分布如图4(b)所示。大部分样品温度集中在40~60 ℃,且分布均匀性较差。谐振腔负载土壤样品后,会导致电磁场分布改变,进而影响土壤温度分布。增加馈口数量和改变馈口位置可增加电磁场的模式数[34],减少驻波产生,以保证谐振腔内电磁场分布的均匀性。馈口不对称分布时,馈口本身及馈口间反射功率较小,提高了对土壤样品的加热效率及均匀性。
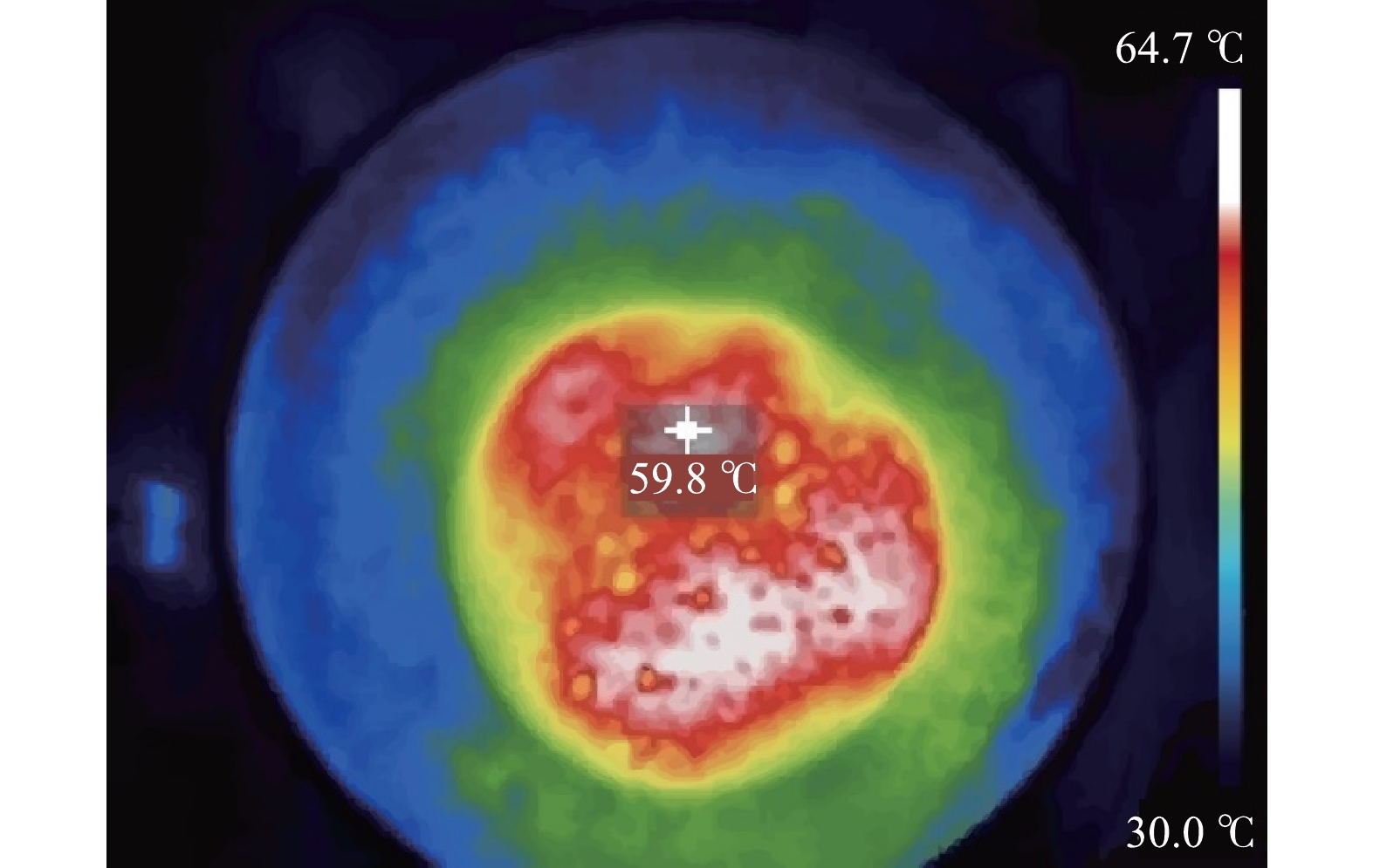
3) 微波反应器加热土壤实验。为了验证上述仿真结果的有效性,将土壤样品放入微波反应器中进行实验,并采用红外热成像仪测定土壤温度分布。结果如图5所示。土壤大部分区域温度在60 ℃左右,最高温度可达64.7 ℃。对比仿真结果显示,实验测得的土壤温度分布与仿真所得到的温度分布基本一致。
-
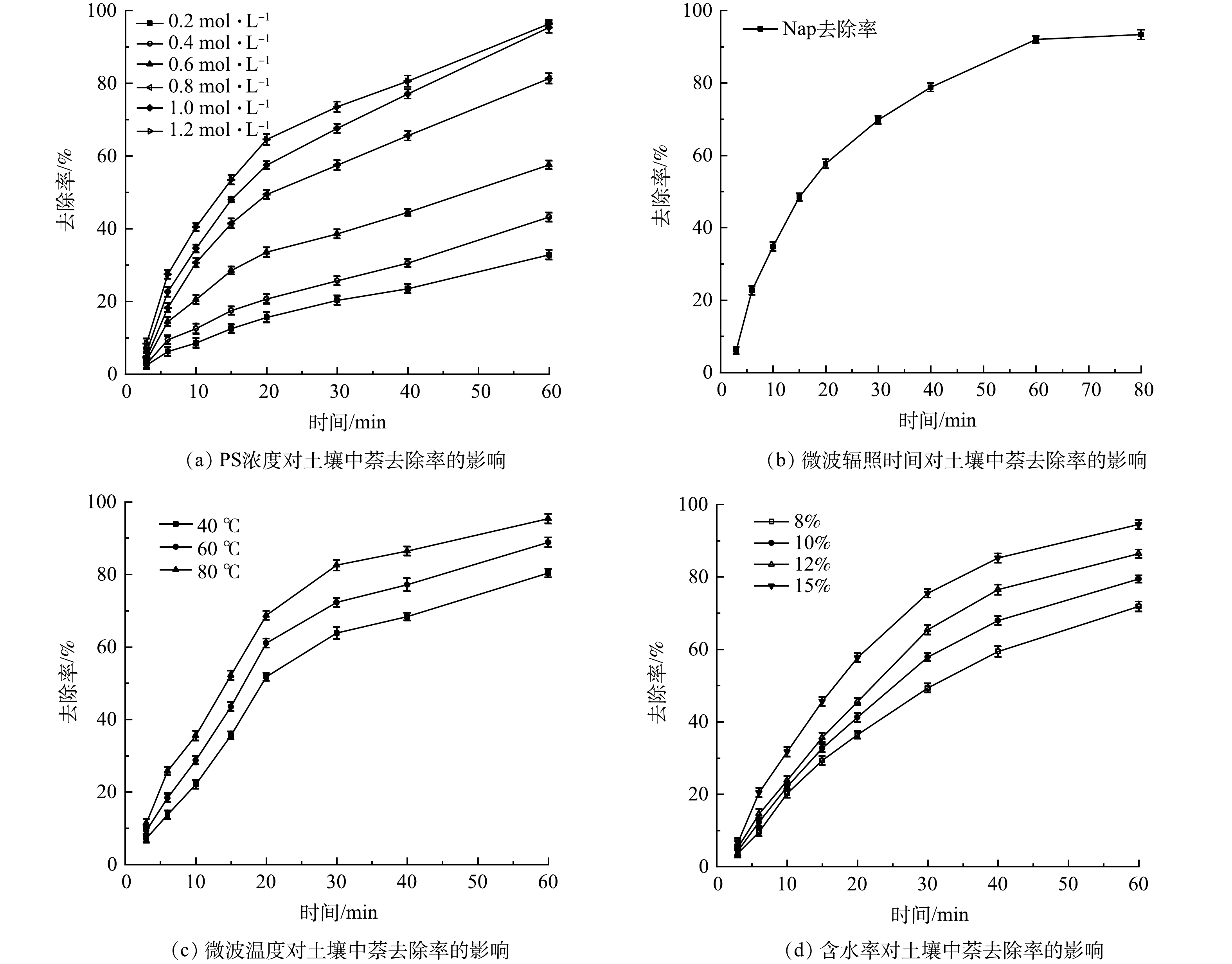
1) PS浓度对萘去除率的影响。PS浓度对萘去除率的影响如图6(a)所示。萘的去除率随PS浓度增加而增加。PS浓度为0.2 mol·L−1时,60 min后约24.2%的萘被去除,而当PS剂量增加到1.2 mol·L−1时,萘的去除率达到了97.5%。随着氧化剂初始浓度的增加,生成的硫酸根自由基水平会更高,从而提高萘的去除率。此外,土壤中的其他可氧化物也会与萘竞争硫酸根自由基[35],高水平的硫酸根自由基对于克服这一问题至关重要。然而,较高的氧化剂剂量可能会产生较高水平的硫酸盐,对土壤造成二次污染。其次,残留的过硫酸盐和硫酸根自由基本身对硫酸根自由基也会产生猝灭反应,反应方程式如式(5)、式(6)所示。
为实现低成本高效益的修复,并将修复过程对地下环境的负面影响降至最低。考虑经济因素及修复效果,选用1.0 mol·L−1的过硫酸盐溶液。
2) 微波辐照时间对萘去除率的影响。微波辐照时间对萘去除率的影响如图6(b)所示。萘的去除率随辐照时间增加先快速升高后趋于平缓。0~30 min可降解70%左右的污染物,30~80 min降解了约23.5%的污染物。通常多环芳烃从土壤固体中的解吸表现出双相行为,具有初始快速阶段和随后的缓慢阶段[36]。因此,容易解吸的萘被过硫酸盐快速氧化,而强螯合萘的氧化受到土壤固体解吸的限制,需要更长的时间来降解。实验时60 min去除率到达92.2%,80 min时去除率为93.5%。综合污染物去除率和能耗因素,选择的微波辐照时间为60 min。
3) 微波温度对萘去除率的影响。微波温度对萘去除率的影响如图6(c)所示。温度从40 ℃提高到80 ℃,60 min时萘的去除率从84.2%增加到95.3%。当温度升高时,一方面体系中的大量的过硫酸盐会更快被活化,可以产生更多的SO4-·;另一方面,温度升高会加速SO4-·与目标污染物分子之间的碰撞,进而促进污染物的降解。温度是影响PS活化过程中的一个重要因素,温度对过硫酸盐氧化降解多环芳烃的速率和效率有重要影响[37]。在标准大气压下,Nap的沸点为217.9 ℃,实验检测反应土壤的表观温度最高达到80 ℃,理论上萘很难从土壤中挥发。在微波诱导下,过硫酸盐及萘分子动能增加,含有碳-碳双键以及苯环的挥发性有机物很容易被氧化活性官能团降解,该结论与HUANG等[38]研究结论相似。
4) 土壤含水率对萘去除率的影响。土壤含水率对萘去除率的影响如图6(d)所示。随着含水率从8%增加到15%,达到80 ℃的时间由12 min缩短到5 min,萘的去除率由71.9%提高到94.3%。由于水的存在,微波优先被含有过硫酸盐溶液的区域吸收[39],使该区域温度快速升高。在微波加热过程中,土壤水分逐渐转化为水蒸气,从而通过蒸馏去除污染物。同时,水蒸气的存在可以增加土壤孔隙度,有利于污染物的传质,土壤湿度的增加会提高污染物的去除效率,这与FALCIGLIA等[40]的观点一致。微波辐射使水极化,导致氢键快速断裂和重新定向[41],引起水分子之间氢键的高频变化,降低其粘度,有利于S2O82-扩散。
-
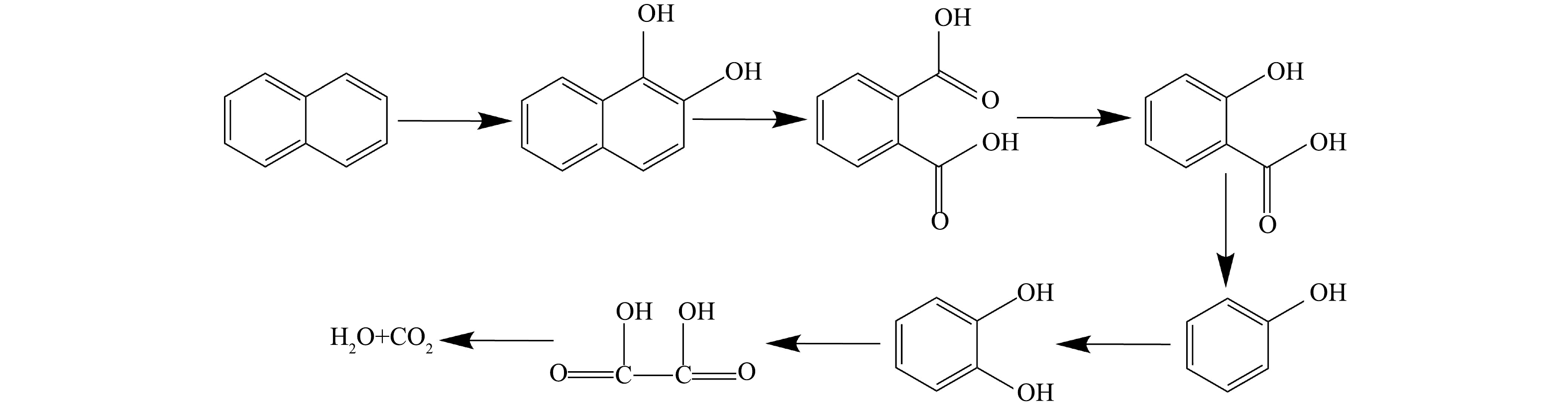
通过GC-MS对MW/PS体系降解萘的产物进行分析,结果如表2所示。萘降解的中间产物主要包括1,2-二羟基萘、邻苯二甲酸、水杨酸、1,2-苯二酚、苯酚。
在反应开始阶段,由于反应的快速进行,大量的SO4−·与水反应生成HO·,由于萘的双环结构没有侧链,与HO·发生加成反应生成1,2-二羟基萘,进而在SO4-·的作用下,苯环开始断裂,生成邻苯二甲酸及水杨酸,这些中间体进一步被SO4-·氧化,生成苯酚,最后苯酚被氧化生成邻二苯酚,进一步开环形成小分子酸,最后分解为H2O及CO2。通过以上产物推测体系可能发生的降解途径如图7所示。
-
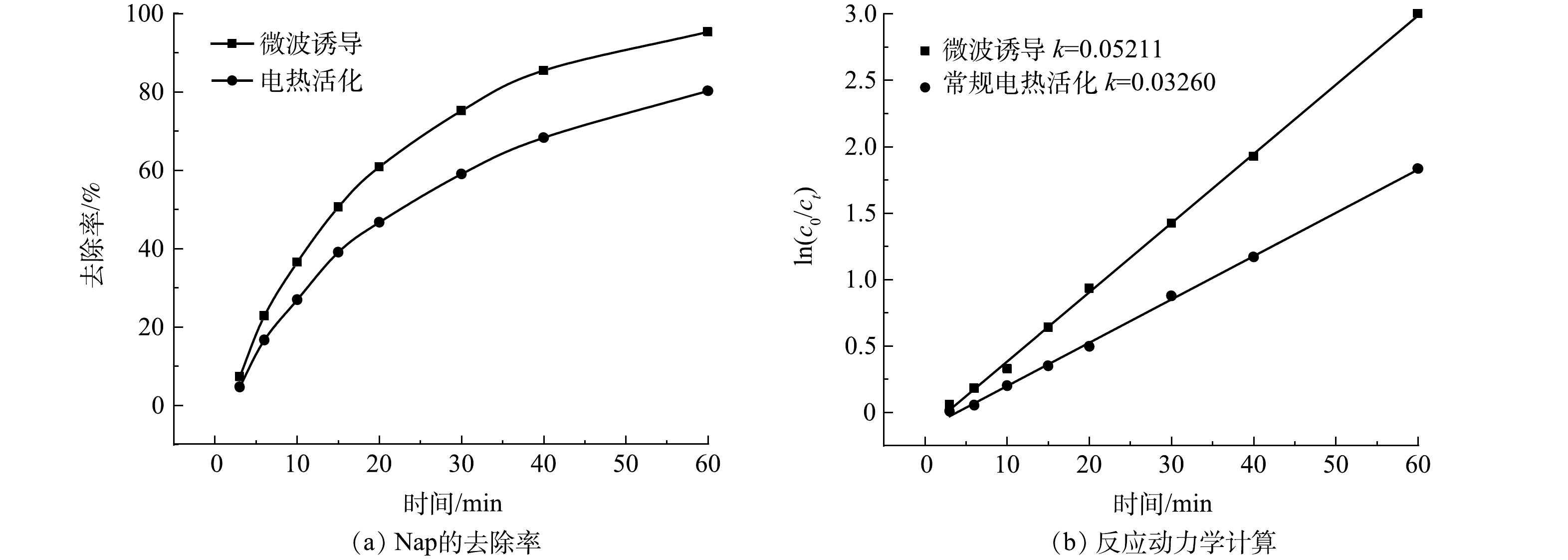
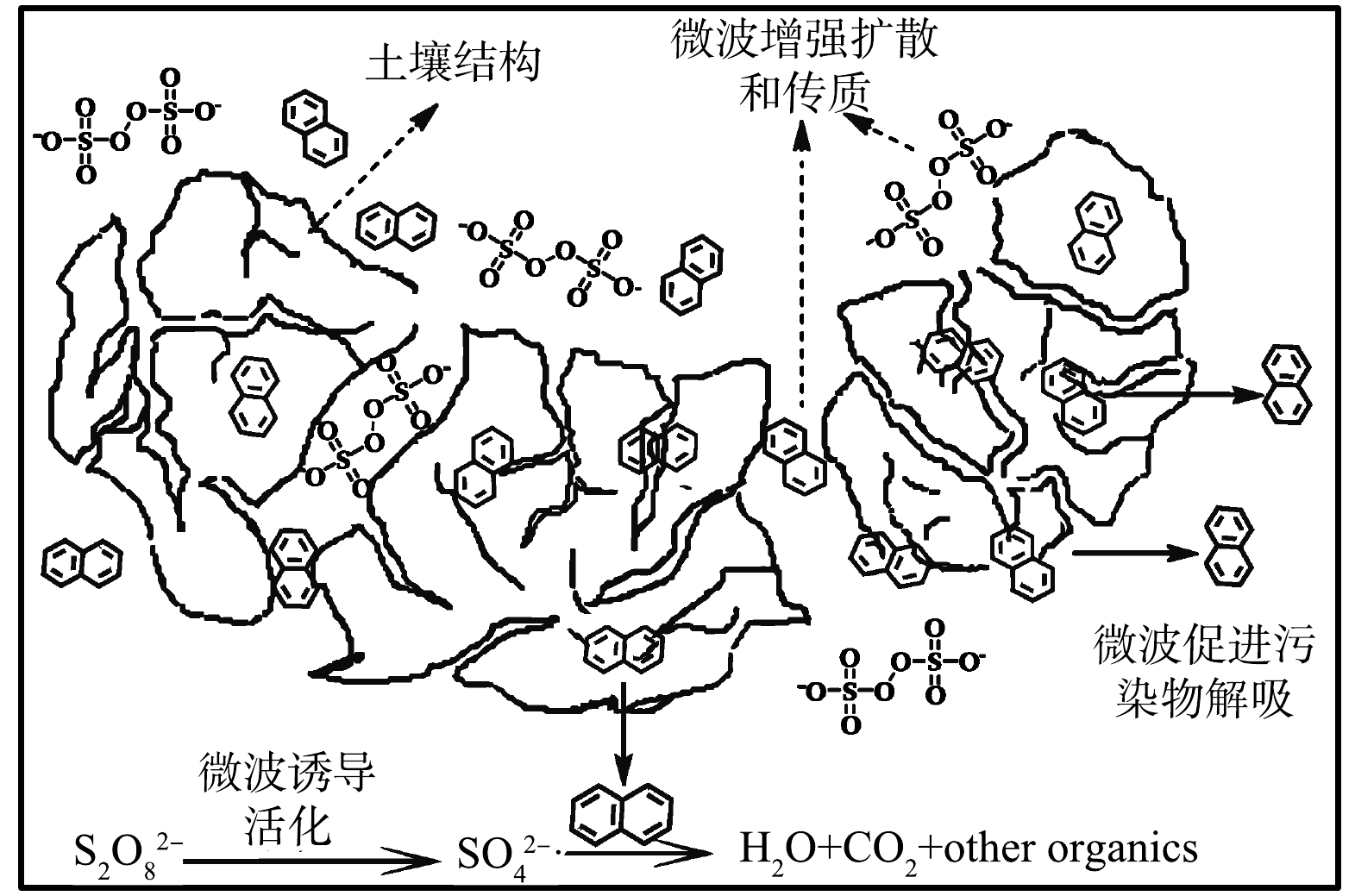
1) 不同活化条件下萘去除效果研究。不同活化条件萘的去除率如图8(a)所示。60 min时微波诱导去除率为94.2%,常规电热活化去除率为80.2%,这可能与微波的高效加热性及强化效应有关。反应动力学计算如图8(b)所示。微波诱导的反应速率常数k为常规电热活化的1.6倍,证明微波除热效应外还具有一定的强化效应。微波除热效应以外,还存在量子层面的晶格震荡及不规律相变等强化效应[42-43]。利用微波诱导过硫酸盐时,偶极分子高频往复运动加热土壤的效应最为显著[44]。其次,在微波交变电场的作用下使得有机质胶体颗粒空隙结构改变,优化传质。同时,被吸附的有机污染物在微波作用下高频震动,更容易发生脱附,从而被氧化去除。微波的特定效应可以加速材料的扩散和传质,这与HINRIKUS等[45]的研究结果相同。
微波诱导过硫酸盐修复萘污染土壤机理如图9所示。土壤修复过程中,微波可使原子配位壳层结构变差,降低原子键级,改变量子间传质、传热环境,降低活化能[46-47],达到高效、节能的效果,以增强土壤中的过硫酸盐修复性能。
2) 污染物去除动力学研究。通过动力学计算的方法对微波的强化效应进行分析,采用伪一级反应动力学计算方法进行验证。通过Arrhenius公式计算反应速率常数与温度的关系,如式(7)所示。
式中:
k 反应速率常数;R 为摩尔气体常数,取8.314 kJ∙(mol·K)−1;A为指前因子;T 为活化温度,K;Ea 为表观活化能,kJ·mol−1。对式(7)取对数可得到式(8)。
根据式(8)的形式,以
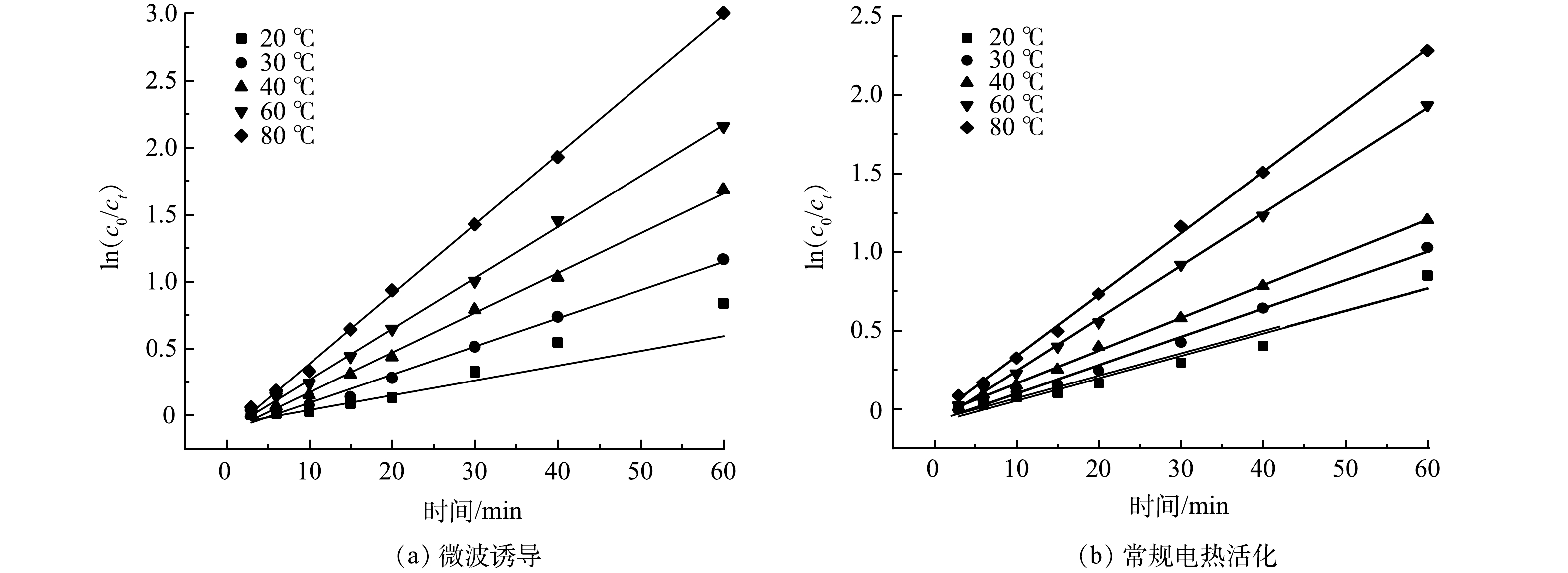
lnk 为纵坐标,1T 为横坐标,可以得到一条斜率为−EaR ,截距为lnA 的直线,据此计算活化能Ea 。微波诱导与电热活化条件下分别选取了20、30、40、60、80 ℃这5个温度梯度,测量不同温度条件反应过程中萘的浓度,根据实验结果计算出各时刻的ln
(c0/ct) ,不同条件下的动力学计算如图10所示。由图10可以看出,ln
(c0/ct) 与时间t的关系可用一次函数表示,且相关性高,可以认为在选取的温度范围内,其反应过程基本符合伪一级动力学模型。不同温度条件下的反应动力学常数如表3、表4所示。通过计算得出,微波诱导条件下,活化能Ea =14.85 kJ·mol−1;常规电加热条件下,活化能Ea =17.63 kJ·mol−1。由动力学分析结果可以看出,微波诱导条件下反应速率常数更大,说明微波诱导条件下,过硫酸盐可以更快速氧化降解土壤中的萘,微波辐射的强化作用促进了过硫酸盐的分解,并且可以增强过硫酸盐在土壤颗粒中的扩散[48],提高污染物去除效率。其次,微波辐射可以降低反应所需的活化能,分析其原因可能是通过改变土壤中有机质极性,使土壤中硫酸根自由基与萘分子碰撞频率加大和减弱化学键的结合强度等方式来实现的。
-
1) 利用COMSOL Multiphysics多物理场仿真软件优化谐振腔内电磁及温度场分布,腔体两侧磁控管不对称分布时电磁场分布最均匀,根据仿真结论优化后的微波反应器具有更好的加热均匀性。
2) 通过单因素实验分析各条件对反应体系的影响,相对较高的PS浓度、MW处理时间、MW辐射温度和土壤含水率可提高萘的降解率,反应在PS浓度1.0 mol·L−1、MW温度80 ℃、土壤含水率15%、MW处理60 min的条件下,萘的去除率最高可达到96.5%。
3) 对MW/PS体系的表观动力学进行研究,MW/PS体系可以快速有效降解土壤中的萘,MW/PS体系反应速率常数是常规电加热的1.6倍,反应速率常数随温度增加而增大,微波可以降低反应所需的活化能。
微波诱导过硫酸盐修复萘污染土壤的模拟优化
Simulation and optimization of naphthalene polluted soil remediation by microwave induced persulfate
-
摘要: 微波 (microwave,MW) 诱导过硫酸盐 (persulfate,PS) 去除土壤中的有机污染物是一种有效的土壤修复手段。以萘为土壤中典型的多环芳烃 (polycyclic aromatic hydrocarbons,PAHs) 有机污染物,利用COMSOL Multiphysics多物理场计算软件模拟,结合电磁耦合理论分析探索了微波电磁辐照过程中土壤有机物消解过程机制,并考察了PS浓度、MW辐照时间、MW温度、土壤含水率等工艺参数对萘去除效果的影响。结果表明,根据仿真优化后,微波反应器具有更好的加热均匀性。在微波谐振腔内,萘的降解过程符合伪一级反应动力学模型,MW诱导PS反应速率常数是常规电加热的1.6倍,这可能是因为极性过硫酸盐分子在微波交变电场的作用下胶体运动趋势增加;同时,多环芳烃有机物在微波场中的土壤传质性能得到优化,降低反应所需的活化能。当PS浓度为1.0 mol·L−1、MW温度为80 ℃、土壤含水率为15%、MW处理时间为60 min时,萘的去除率最高可达到96.5%。本研究结果可为微波诱导过硫酸盐技术在多环芳烃污染土壤修复中的应用提供参考。Abstract: PS (persulfate) induced by MW (microwave) has been proved as an effective strategy for organic polluted soil remediation. In this study, naphthalene was selected as the target soil contaminant, which was a typical kind of PAHs (polycyclic aromatic hydrocarbons). The mechanism of naphthalene removal during microwave electromagnetic irradiation process was explored by COMSOL Multiphysics software simulation together with the theoretical analysis of electromagnetic coupling. Moreover, the effects of PS concentration, MW irradiation time, MW temperature, and soil water content on naphthalene removal performance were investigated. The results indicated that the optimized microwave reactor based on software simulations revealed better heating uniformity. The degradation process of naphthalene conformed to the pseudo-first order kinetic model. Besides, the k value of PS induced by MW reaction were 1.6 times higher than those of conventional electrical heating. These results could be attributed to the polar PS molecules movement under the microwave alternating electric field, thereby optimizing the naphthalene mass transfer process in the soil and reducing the activation energy required for the reaction. The maximum naphthalene removal efficiency could reach 96.5% when the PS concentration, MW irradiation time, MW temperature, and soil water content was 1.0 mol L−1, 80 ℃, 60 min, and 15%, respectively. The results obtained from this study could provide theoretical support for practical applications of PS induced by MW to treat PAHs in soil.
-
Key words:
- microwave induced /
- persulfate /
- naphthalene /
- analogue simulation /
- soil remediation
-
目前,针对土壤有机污染物的传统治理技术,通常难以达到去除低环PAHs的修复要求[1-2]。萘 (naphthalene,Nap) 作为一种具有致畸、致突变与致癌的典型低环半挥发性PAHs[3],研究其在土壤介质中的降解规律,并完善其修复治理技术,对于评估低环PAHs的环境风险和提出相应的污染管控措施具有重要的意义。
近年来,过硫酸盐高级氧化技术在污染控制领域受到越来越多的关注[4-6],PS是一种强氧化剂,可以通过热[7-9]、碱[10-11]、过渡金属离子[12-14]、超声[15-17]和微波[18-21]等能量或材料进行活化。研究表明,提升温度可有效增加过硫酸盐的活性,但常规加热存在能耗高、效率低的问题[22]。微波诱导具有加热快速、能耗相对较低、无二次污染等优点[23-24],但缺少机理研究,且大多数人使用市售微波炉,单磁控管能量密度低,很难达到预期活化效果。
工业上绝大多数情况使用多磁控管微波源提高微波功率,然而微波加热的不均匀性在很大程度上限制了微波技术的发展和应用[25]。微波加热不均匀的主要原因是电磁场强度分布不均匀[26]。目前,改善微波加热不均匀性的措施主要是通过改变谐振腔的尺寸及微波馈口数量[27]和相对位置[28],使谐振腔具有尽可能多的振荡模式[29],不同模式叠加后能够获得更加均匀的能量分布状态。本研究拟应用COMSOL Multiphysics[30-31]多物理场仿真软件优化谐振腔内电磁场分布,参照模拟结论,制备微波反应器,并通过MW诱导PS强化降解土壤中萘的实验研究,考察高浓度萘污染土壤的微波诱导修复效果及影响因素,以期为实现该技术的工程应用提供参考。
1. 材料与方法
1.1 实验原料
实验所用试剂主要有过硫酸钠 (Na2S2O8) 、萘 (C10H8) 、甲醇 (CH3OH) 、氯化钠 (NaCl) 、石英砂 (SiO2) 、无水乙醇 (CH3CH2OH) 、磷酸 (H3PO4) 均为分析纯、高纯氮气 (N2,99.999%) 。实验所用土壤采自河北科技大学绿地,取表层约20 cm处土壤,去除土壤中根系等杂质后,研磨过60目筛后,在室内经自然风干储存备用。土壤理化性质如表1所示。
表 1 土壤样品的基本理化性质Table 1. Basic physical and chemical properties of soil samplespH 水分/% 有机质质量分数/% 阳离子交换量/ (cmol·kg−1) 粒径分布/% 砂粒 粉粒 黏粒 8.36 4.37 3.45 5.43 19.37 71.35 9.28 1.2 实验装置
气相色谱仪 (7820A,安捷伦科技有限公司) 、自动顶空进样器 (7697A,安捷伦科技有限公司) 、气相色谱质谱联用仪 (7890B,安捷伦科技有限公司) 、数显水浴恒温振荡器 (SHA-B,常州荣华仪器制造有限公司) 、电子天平 (FA2004B,上海佑科仪器仪表有限公司) 、红外热成像仪 (DP-22,深圳点扬科技有限公司) 、微波反应器 (山东科宏微波能有限公司) 。
1.3 实验方法
1) 矩形谐振腔电磁场模拟。以COMSOL Multiphysics仿真软件构建出微波辐照多物理场仿真模型,通过模拟谐振腔内电磁场分布及土壤样品温度场分布情况,参照模拟结论,制备微波反应器,搭建微波诱导PS强化降解土壤中萘的实验平台。
2) 单因素实验。分别对PS浓度0.2、0.5、0.8、1.0、1.2 mol·L−1,微波辐照时间3、6、10、15、20、30、40、60、80 min,温度40、60、80 ℃,土壤含水率8%、10%、12%、15%这4个影响因子进行单因素实验。为得到稳定可靠的结果,每组实验设置 3 组平行实验,确定各影响因素的最佳取值。
3) 不同方式活化过硫酸盐的效果比较。对比微波诱导与常规电加热实验,探索同功率强度下的微波诱导和电热活化的不同费效关系,探索不同加热方式的能耗特征。同时,从反应动力学角度进行研究,通过反应速率对比,揭示活化能降低规律。
微波诱导。取适量土样置于石英玻璃容器中,加入适量的过硫酸钠溶液,设置微波功率和辐照时间,实验结束后取出污染土样,立即装入22 mL顶空瓶中并加入10 mL饱和NaCl溶液,采用顶空-气相色谱仪测定其中萘含量。常规电热活化。除使用马弗炉设备外,其他与上述步骤相同。
4) 土壤中萘的降解产物分析。实验使用自制微波反应器,在PS浓度1.0 mol·L−1、MW温度80 ℃、土壤含水率15%、MW处理60 min的条件下进行实验,分别在3、6、10、15、20、30、40、60 min 时收集气相样品,采用气相色谱-质谱联用仪 (GC-MS) 进行检测。
1.4 分析方法
1) 气相色谱仪 (GC-FID) 检测条件。柱箱初始温度为100 ℃,升温速率8 ℃·min−1升温至100 ℃ (保持1 min) ,升温速率20 ℃·min−1升温至200 ℃ (保持10 min) ;进样口温度220 ℃;检测器温度240 ℃。
2) 气相色谱-质谱联用仪 (GC-MS) 检测条件。柱箱初始温度为35 ℃,升温速率15 ℃·min−1升温至150 ℃ (保持5 min) ,升温速率3 ℃·min−1到280 ℃ (保持2 min) ;进样口温度280 ℃,不分流;电子轰击源 (EI) ;离子原温度230 ℃;离子化能量70 eV。
1.5 微波反应器优化设计
矩形谐振腔的模式数越多,谐振波型数量越多,为了取得较均匀的能量分布,应使矩形谐振腔具有尽可能多的振荡模式[32]。
1) 矩形谐振腔尺寸选择。假设在空间直角坐标系中矩形谐振腔具有无穷多个分离的震荡模式,每一种谐振模式对应一种场分布,对应唯一的谐振频率,矩形谐振腔的谐振频率如式(1)所示,对应在自由空间中的谐振波长如式(2)所示。
f=C√(ma)2+(nb)2+(pd)22 (1) λ=2√(ma)2+(nb)2+(pd)2 (2) 式中: a、b、d分别为谐振腔x、y、z方向上的长度;m、n、p 为矩形谐振腔模式系数,分别表示在a、b、d上分布的半驻波波长个数;
C 谐振波长
λ 2) 矩形谐振腔建模。采用COMSOL Multiphysics多物理场仿真软件中RF模块,联合求解有限元边界条件下的麦克斯韦方程组和能量守恒方程,对谐振腔进行了微波场均匀性的仿真研究。谐振腔三维模型如图1所示。
3) 网格划分。利用COMSOL Multiphysics构建整个有限元模型后,设置最大单元为0.024 47 m,最小单元为7.25×10−4 m,最大单元增长率为1.3,曲率因子为0.2,狭窄区域分辨率为1。网格划分如图2所示。
4) 仿真理论。在利用 COMSOL Multiphysics模拟时主要涉及到了麦克斯韦方程和能量守恒方程的联立求解。通过求解一个特殊形式的麦克斯韦方程得到谐振腔内的三维电磁场强度分布,如式(3)所示。
∇×μ−1r(∇×E)−k20(εr−jσωε0)E=0 (3) 式中:
E μr j σ ω ϵr ϵ0 k0 传热场计算可由含非齐次项的偏微分方程式(4)给出。
ρCP∂T∂t=∇⋅(К∇T)+Q (4) 式中:
ρ CP К 2. 结果与讨论
2.1 矩形谐振腔微波场均匀性研究
1) 谐振腔内电磁场分布模拟。谐振腔电场平面分布如图3(a)所示。靠近腔体壁的区域电场强度较低,腔体内部区域电场强度较高,这说明谐振腔内部区域的微波功率密度高于靠近腔体壁的区域。并且谐振腔内部电场差值较小,表明腔体内的微波功率密度较一致。这可能是因为靠近腔体壁区域的电场叠加较少,微波源反射到箱体中央与其他磁控管的电场叠加较多,提高了电磁场的均匀性。图3(b)为谐振腔磁场平面分布图,磁场分布情况与电场类似。采用微波加热时,应将样品放在谐振腔的中心位置,以使介质最大程度的吸收能量,提高升温速率。
2) 模型加热温度分布模拟。微波反应器中样品温度分布如图4(a)所示。微波反应器中样品温度大部分处于60~80 ℃,温度分布均匀性较好。家用微波炉中样品温度分布如图4(b)所示。大部分样品温度集中在40~60 ℃,且分布均匀性较差。谐振腔负载土壤样品后,会导致电磁场分布改变,进而影响土壤温度分布。增加馈口数量和改变馈口位置可增加电磁场的模式数[34],减少驻波产生,以保证谐振腔内电磁场分布的均匀性。馈口不对称分布时,馈口本身及馈口间反射功率较小,提高了对土壤样品的加热效率及均匀性。
3) 微波反应器加热土壤实验。为了验证上述仿真结果的有效性,将土壤样品放入微波反应器中进行实验,并采用红外热成像仪测定土壤温度分布。结果如图5所示。土壤大部分区域温度在60 ℃左右,最高温度可达64.7 ℃。对比仿真结果显示,实验测得的土壤温度分布与仿真所得到的温度分布基本一致。
2.2 单因素结果分析
1) PS浓度对萘去除率的影响。PS浓度对萘去除率的影响如图6(a)所示。萘的去除率随PS浓度增加而增加。PS浓度为0.2 mol·L−1时,60 min后约24.2%的萘被去除,而当PS剂量增加到1.2 mol·L−1时,萘的去除率达到了97.5%。随着氧化剂初始浓度的增加,生成的硫酸根自由基水平会更高,从而提高萘的去除率。此外,土壤中的其他可氧化物也会与萘竞争硫酸根自由基[35],高水平的硫酸根自由基对于克服这一问题至关重要。然而,较高的氧化剂剂量可能会产生较高水平的硫酸盐,对土壤造成二次污染。其次,残留的过硫酸盐和硫酸根自由基本身对硫酸根自由基也会产生猝灭反应,反应方程式如式(5)、式(6)所示。
SO4−⋅+SO4−⋅=S2O82− (5) SO4−⋅+S2O82−=SO42−+S2O8− (6) 为实现低成本高效益的修复,并将修复过程对地下环境的负面影响降至最低。考虑经济因素及修复效果,选用1.0 mol·L−1的过硫酸盐溶液。
2) 微波辐照时间对萘去除率的影响。微波辐照时间对萘去除率的影响如图6(b)所示。萘的去除率随辐照时间增加先快速升高后趋于平缓。0~30 min可降解70%左右的污染物,30~80 min降解了约23.5%的污染物。通常多环芳烃从土壤固体中的解吸表现出双相行为,具有初始快速阶段和随后的缓慢阶段[36]。因此,容易解吸的萘被过硫酸盐快速氧化,而强螯合萘的氧化受到土壤固体解吸的限制,需要更长的时间来降解。实验时60 min去除率到达92.2%,80 min时去除率为93.5%。综合污染物去除率和能耗因素,选择的微波辐照时间为60 min。
3) 微波温度对萘去除率的影响。微波温度对萘去除率的影响如图6(c)所示。温度从40 ℃提高到80 ℃,60 min时萘的去除率从84.2%增加到95.3%。当温度升高时,一方面体系中的大量的过硫酸盐会更快被活化,可以产生更多的SO4-·;另一方面,温度升高会加速SO4-·与目标污染物分子之间的碰撞,进而促进污染物的降解。温度是影响PS活化过程中的一个重要因素,温度对过硫酸盐氧化降解多环芳烃的速率和效率有重要影响[37]。在标准大气压下,Nap的沸点为217.9 ℃,实验检测反应土壤的表观温度最高达到80 ℃,理论上萘很难从土壤中挥发。在微波诱导下,过硫酸盐及萘分子动能增加,含有碳-碳双键以及苯环的挥发性有机物很容易被氧化活性官能团降解,该结论与HUANG等[38]研究结论相似。
4) 土壤含水率对萘去除率的影响。土壤含水率对萘去除率的影响如图6(d)所示。随着含水率从8%增加到15%,达到80 ℃的时间由12 min缩短到5 min,萘的去除率由71.9%提高到94.3%。由于水的存在,微波优先被含有过硫酸盐溶液的区域吸收[39],使该区域温度快速升高。在微波加热过程中,土壤水分逐渐转化为水蒸气,从而通过蒸馏去除污染物。同时,水蒸气的存在可以增加土壤孔隙度,有利于污染物的传质,土壤湿度的增加会提高污染物的去除效率,这与FALCIGLIA等[40]的观点一致。微波辐射使水极化,导致氢键快速断裂和重新定向[41],引起水分子之间氢键的高频变化,降低其粘度,有利于S2O82-扩散。
2.3 萘的降解途径分析
通过GC-MS对MW/PS体系降解萘的产物进行分析,结果如表2所示。萘降解的中间产物主要包括1,2-二羟基萘、邻苯二甲酸、水杨酸、1,2-苯二酚、苯酚。
表 2 萘降解的中间产物Table 2. Intermediate products of naphthalene degradation序号 化合物 分子式 分子量 结构式 1 1,2-Dihydroxynaphthalene C10H8O2 160.17 
2 1,2-Benzenedicarboxylic acid C8H6O4 166.13 
3 Salicylic acid C7H6O4 138.12 
4 Catechol C6H6O2 110.11 
5 phenyl hydroxide C6H6O 94.11 
在反应开始阶段,由于反应的快速进行,大量的SO4−·与水反应生成HO·,由于萘的双环结构没有侧链,与HO·发生加成反应生成1,2-二羟基萘,进而在SO4-·的作用下,苯环开始断裂,生成邻苯二甲酸及水杨酸,这些中间体进一步被SO4-·氧化,生成苯酚,最后苯酚被氧化生成邻二苯酚,进一步开环形成小分子酸,最后分解为H2O及CO2。通过以上产物推测体系可能发生的降解途径如图7所示。
2.4 过硫酸盐活化效果研究及动力学分析
1) 不同活化条件下萘去除效果研究。不同活化条件萘的去除率如图8(a)所示。60 min时微波诱导去除率为94.2%,常规电热活化去除率为80.2%,这可能与微波的高效加热性及强化效应有关。反应动力学计算如图8(b)所示。微波诱导的反应速率常数k为常规电热活化的1.6倍,证明微波除热效应外还具有一定的强化效应。微波除热效应以外,还存在量子层面的晶格震荡及不规律相变等强化效应[42-43]。利用微波诱导过硫酸盐时,偶极分子高频往复运动加热土壤的效应最为显著[44]。其次,在微波交变电场的作用下使得有机质胶体颗粒空隙结构改变,优化传质。同时,被吸附的有机污染物在微波作用下高频震动,更容易发生脱附,从而被氧化去除。微波的特定效应可以加速材料的扩散和传质,这与HINRIKUS等[45]的研究结果相同。
微波诱导过硫酸盐修复萘污染土壤机理如图9所示。土壤修复过程中,微波可使原子配位壳层结构变差,降低原子键级,改变量子间传质、传热环境,降低活化能[46-47],达到高效、节能的效果,以增强土壤中的过硫酸盐修复性能。
2) 污染物去除动力学研究。通过动力学计算的方法对微波的强化效应进行分析,采用伪一级反应动力学计算方法进行验证。通过Arrhenius公式计算反应速率常数与温度的关系,如式(7)所示。
k=Ae−EaRT (7) 式中:
k R T Ea 对式(7)取对数可得到式(8)。
lnk=−EaR⋅1T+lnA (8) 根据式(8)的形式,以
lnk 1T −EaR lnA Ea 微波诱导与电热活化条件下分别选取了20、30、40、60、80 ℃这5个温度梯度,测量不同温度条件反应过程中萘的浓度,根据实验结果计算出各时刻的ln
(c0/ct) 由图10可以看出,ln
(c0/ct) Ea Ea 表 3 微波诱导条件下不同温度的反应动力学计算Table 3. Calculation of reaction kinetics at different temperatures under microwave radiation温度/k 拟合方程式 相关系数R2 反应速率常数 293 y=0.01540x−0.10868 0.989 2 0.015 40 303 y=0.02106x−0.11731 0.992 1 0.021 06 313 y=0.02895x−0.09313 0.997 0 0.028 95 333 y=0.04074x−0.02565 0.996 0 0.040 74 353 y=0.05232x−0.15931 0.997 3 0.052 32 表 4 常规加热条件下不同温度的反应动力学计算Table 4. Calculation of reaction kinetics at different temperatures under conventional heating conditions温度/k 拟合方程式 相关系数R2 反应速率常数 293 y=0.01427x−0.08939 0.963 9 0.014 27 303 y=0.01806x−0.08402 0.993 1 0.018 06 313 y=0.02077x−0.09313 0.998 6 0.020 77 333 y=0.03403x−0.02565 0.995 3 0.034 03 353 y=0.03961x−0.15931 0.997 4 0.039 61 由动力学分析结果可以看出,微波诱导条件下反应速率常数更大,说明微波诱导条件下,过硫酸盐可以更快速氧化降解土壤中的萘,微波辐射的强化作用促进了过硫酸盐的分解,并且可以增强过硫酸盐在土壤颗粒中的扩散[48],提高污染物去除效率。其次,微波辐射可以降低反应所需的活化能,分析其原因可能是通过改变土壤中有机质极性,使土壤中硫酸根自由基与萘分子碰撞频率加大和减弱化学键的结合强度等方式来实现的。
3. 结论
1) 利用COMSOL Multiphysics多物理场仿真软件优化谐振腔内电磁及温度场分布,腔体两侧磁控管不对称分布时电磁场分布最均匀,根据仿真结论优化后的微波反应器具有更好的加热均匀性。
2) 通过单因素实验分析各条件对反应体系的影响,相对较高的PS浓度、MW处理时间、MW辐射温度和土壤含水率可提高萘的降解率,反应在PS浓度1.0 mol·L−1、MW温度80 ℃、土壤含水率15%、MW处理60 min的条件下,萘的去除率最高可达到96.5%。
3) 对MW/PS体系的表观动力学进行研究,MW/PS体系可以快速有效降解土壤中的萘,MW/PS体系反应速率常数是常规电加热的1.6倍,反应速率常数随温度增加而增大,微波可以降低反应所需的活化能。
-
表 1 土壤样品的基本理化性质
Table 1. Basic physical and chemical properties of soil samples
pH 水分/% 有机质质量分数/% 阳离子交换量/ (cmol·kg−1) 粒径分布/% 砂粒 粉粒 黏粒 8.36 4.37 3.45 5.43 19.37 71.35 9.28 表 2 萘降解的中间产物
Table 2. Intermediate products of naphthalene degradation
序号 化合物 分子式 分子量 结构式 1 1,2-Dihydroxynaphthalene C10H8O2 160.17 2 1,2-Benzenedicarboxylic acid C8H6O4 166.13 3 Salicylic acid C7H6O4 138.12 4 Catechol C6H6O2 110.11 5 phenyl hydroxide C6H6O 94.11 表 3 微波诱导条件下不同温度的反应动力学计算
Table 3. Calculation of reaction kinetics at different temperatures under microwave radiation
温度/k 拟合方程式 相关系数R2 反应速率常数 293 y=0.01540x−0.10868 0.989 2 0.015 40 303 y=0.02106x−0.11731 0.992 1 0.021 06 313 y=0.02895x−0.09313 0.997 0 0.028 95 333 y=0.04074x−0.02565 0.996 0 0.040 74 353 y=0.05232x−0.15931 0.997 3 0.052 32 表 4 常规加热条件下不同温度的反应动力学计算
Table 4. Calculation of reaction kinetics at different temperatures under conventional heating conditions
温度/k 拟合方程式 相关系数R2 反应速率常数 293 y=0.01427x−0.08939 0.963 9 0.014 27 303 y=0.01806x−0.08402 0.993 1 0.018 06 313 y=0.02077x−0.09313 0.998 6 0.020 77 333 y=0.03403x−0.02565 0.995 3 0.034 03 353 y=0.03961x−0.15931 0.997 4 0.039 61 -
[1] 周宏仓, 薛鸿斌, 张翠翠, 等. 低环多环芳烃(萘、苊)在活性炭上的热脱附行为[J]. 中国环境科学, 2011, 31(9): 1497-1502. [2] 陈春红, 徐成华, 于天, 等. 低环多环芳烃异位热脱附行为分析[J]. 环境工程, 2022, 40(1): 78-85. [3] GAO W C. Study on Adsorption Mechanism of Naphthalene in Soil by Humic Acid-Based Materials[J]. Journal of Materials, 2022, 6(1): 37-43. [4] 钱林波, 李航宇, 陈梦舫. 高级氧化技术修复苯并[a]芘污染土壤研究进展[J]. 环境化学, 2022, 41(10): 3205-3213. doi: 10.7524/j.issn.0254-6108.2021110203 [5] 高焕方, 龙飞, 曹园城, 等. 新型过硫酸盐活化技术降解有机污染物的研究进展[J]. 环境工程学报, 2015, 9(12): 5659-5664. doi: 10.12030/j.cjee.20151202 [6] 谷得明, 郭昌胜, 冯启言, 等. 基于硫酸根自由基的高级氧化技术及其在环境治理中的应用[J]. 环境化学, 2018, 37(11): 2489-2508. doi: 10.7524/j.issn.0254-6108.2018012102 [7] WANG L, PENG L B, XIE L L, et al. Compatibility of surfactants and thermally activated persulfate for enhanced subsurface remediation[J]. Environmental Science & Technology, 2017, 51(12): 7055-7064. [8] WU H, SUN L, WANG H, et al. Persulfate oxidation for the remediation of petroleum hydrocarbon-contaminated soils[J]. Polish Journal of Environmental Studies, 2016, 25(2): 851-857. doi: 10.15244/pjoes/60857 [9] 栾海彬, 杨立翔, 鲁帅, 等. 热活化过硫酸盐对双酚A的降解及其机理[J]. 环境工程学报, 2016, 10(05): 2459-2464. doi: 10.12030/j.cjee.201412139 [10] ZHAO D, LIAO X Y, YAN X L, et al. Effect and mechanism of persulfate activated by different methods for PAHs removal in soil[J]. Journal of Hazardous Materials, 2013, 254-255: 228-235. doi: 10.1016/j.jhazmat.2013.03.056 [11] LOMINCHAR M A, SANTOS A, MIGUEL E, et al. Remediation of aged diesel contaminated soil by alkaline activated persulfate[J]. Science of the Total Environment, 2018, 622-623: 41-48. doi: 10.1016/j.scitotenv.2017.11.263 [12] HAN D H, WAN J Q, MA Y W, et al. New insights into the role of organic chelating agents in Fe(II) activated persulfate processes[J]. Chemical Engineering Journal, 2015, 269: 425-433. doi: 10.1016/j.cej.2015.01.106 [13] 王继鹏, 虞敏达, 蔡小波, 等. Fe2+活化过硫酸钠原位修复氯苯污染地下水[J]. 环境工程学报, 2016, 10(3): 1275-1280. [14] 高金龙, 马玉琳, 孟庆来, 等. 二价铁活化过硫酸盐降解土壤中十溴联苯醚[J]. 环境工程学报, 2016, 10(12): 7339-7343. doi: 10.12030/j.cjee.201507210 [15] WEI Z S, VILLAMENA F A, WEAVERS L K. Kinetics and mechanism of ultrasonic activation of persulfate: An in situ EPR spin trapping study[J]. Environmental Science & Technology, 2017, 51(6): 3410-3417. [16] PENG L B, WANG L, HU X T, et al. Ultrasound assisted, thermally activated persulfate oxidation of coal tar DNAPLs[J]. Journal of Hazardous Materials, 2016, 318: 497-506. doi: 10.1016/j.jhazmat.2016.07.014 [17] DENG D Y, LIN X T, OU J M, et al. Efficient chemical oxidation of high levels of soil-sorbed phenanthrene by ultrasound induced, thermally activated persulfate[J]. Chemical Engineering Journal, 2015, 265: 176-183. doi: 10.1016/j.cej.2014.12.055 [18] KAN H S, WANG T C, YU J X, et al. Remediation of organophosphorus pesticide polluted soil using persulfate oxidation activated by microwave[J]. Journal of Hazardous Materials, 2021, 401: 1-11. [19] PENG L B, DENG D Y, YE F T. Efficient oxidation of high levels of soil-sorbed phenanthrene by microwave-activated persulfate: implication for in situ subsurface remediation engineering[J]. Journal of Soils and Sediments, 2016, 16(1): 28-37. doi: 10.1007/s11368-015-1176-5 [20] 苗铎. 微波活化过硫酸盐降解土壤中乙基对硫磷/多环芳烃性能及机理[D]. 西北农林科技大学, 2021. [21] 赵琪, 赵哲颖, 谭德俊, 等. 微波活化过硫酸钾深度处理印染废水[J]. 环境工程学报, 2014, 8(05): 1807-1811. [22] WANG F, WU C W, LI Q B. Treatment of refractory organics in strongly alkaline dinitrodiazophenol wastewater with microwave irradiation-activated persulfate[J]. Chemosphere, 2020, 254: 126773-126783. doi: 10.1016/j.chemosphere.2020.126773 [23] QI C, LIU X. Degradation of pentachlorophenol by microwave activated persulfate[J]. Environmental Pollution, 2014, 22: 4670-4679. [24] Chien Y C. Field study of in situ remediation of petroleum hydrocarbon contaminated soil on site using microwave energy[J]. Journal of Hazardous Materials, 2011, 199: 457-461. [25] MOHAMMAD R H, BYUN D Y, PRASHANTA D. Analysis of microwave heating for cylindrical shaped objects[J]. International Journal of Heat and Mass Transfer, 2010, 53(23): 5129-5138. [26] 韩清华, 李仪凡, 赵东林, 等. 多馈源箱式加热器的微波场均匀性仿真分析[J]. 包装与食品机械, 2012, 30(06): 6-9. doi: 10.3969/j.issn.1005-1295.2012.06.002 [27] DOMINGUEZ T E, PLAZA G P, DIAZ Mo A, et al. Optimisation of electric field uniformity in microwave heating systems by means of multi-feeding andgenetic algorithms[J]. International Journal of Materials & Product Technology, 2007, 29(4): 149-162. [28] WANG S M, HU Z C, HAN Y B, et al. Effects of magnetron arrangement and power combination of microwave on drying uniformity of carrot[J]. Drying Technology, 2013, 31(11): 1206-1211. doi: 10.1080/07373937.2013.783590 [29] 王顺民, 胡志超, 韩永斌, 等. 微波干燥均匀性研究进展[J]. 食品科学, 2014, 35(17): 297-300. doi: 10.7506/spkx1002-6630-201417056 [30] CHE W S, MA J. The study on the uniform drying of microwave heating system with multiple waveguides[J]. Applied Mechanics & Materials, 2013, 329: 219-225. [31] 许丹芸, 张亚宁, 朱玲, 等. 基于COMSOL模拟的有机污染土壤ERH修复工艺优化[J]. 环境工程学报, 2021, 15(11): 3642-3650. doi: 10.12030/j.cjee.202101051 [32] 曹盈盈. 材料微波加热的计算机模拟仿真研究[D]. 昆明理工大学, 2011. [33] 赵刚, 刘庆凯, 王筠. 矩形谐振腔微波场的均匀化研究[J]. 科技视界, 2016, 18: 24-28. doi: 10.3969/j.issn.2095-2457.2016.03.015 [34] KUSTUREE J, MUDTAPHA Y, PHADUNGSAK R. Design and analysis of the commercialized drier processing using a combined unsymmetrical double-feed microwave and vacuum system[J]. Chemical Engineering & Processing:Process Intensification, 2010, 49(4): 389-395. [35] CUYPERS C, GROTENHUIS T, JOZIASSE J, et al. Rapid persulfate oxidation predicts PAH bioavailability in soils and sediments[J]. Environmental Science & Technology, 2000, 34(10): 2057-2063. [36] LUO L, LIN S, HUANG H L, et al. Relationships between aging of PAHs and soil properties[J]. Environmental Pollution, 2012, 170(8): 177-182. [37] MIAO D, ZHAO S, ZHU K C, et al. Activation of persulfate and removal of ethyl-parathion from soil: Effect of microwave irradiation[J]. Chemosphere, 2020, 253(C): 1-8. [38] HUANG K C, ZHAO Z Q, GEORGE E H, et al. Degradation of volatile organic compounds with thermally activated persulfate oxidation[J]. Chemosphere, 2005, 61(4): 551-560. doi: 10.1016/j.chemosphere.2005.02.032 [39] CHEN W M, WANG F, HE C, et al. Molecular-level comparison study on microwave irradiation-activated persulfate and hydrogen peroxide processes for the treatment of refractory organics in mature landfill leachate[J]. Journal of Hazardous Materials, 2020, 397(122785): 1-9. [40] FALCIGLIA P P, SCANDURA P, FEDERICO G A V. Modelling of in situ microwave heating of hydrocarbon-polluted soils: Influence of soil properties and operating conditions on electric field variation and temperature profiles[J]. Journal of Geochemical Exploration:Journal of the Association of Exploration Geochemists, 2017, 174: 91-99. doi: 10.1016/j.gexplo.2016.06.005 [41] GALINADA W A, GEORGEES G. Influence of microwave irradiation on the intraparticle diffusion of an insulin variant in reversed-phase liquid chromatography under linear conditions[J]. Journal of Chromatography A, 2007, 1163(1): 157-168. [42] HU Y, HE Y, CHENG H. Microwave-induced degradation of N-nitrosodimethylamine (NDMA) sorbed in zeolites: Effect of mineral surface chemistry and non-thermal effect of microwave[J]. Journal of Cleaner Production, 2018, 174: 1224-1233. doi: 10.1016/j.jclepro.2017.11.014 [43] ZHAI C K, TENG N, PAN B H, et al. Revealing the importance of non-thermal effect to strengthen hydrolysis of cellulose by synchronous cooling assisted microwave driving[J]. Carbohydrate Polymers, 2018, 197: 414-421. doi: 10.1016/j.carbpol.2018.06.031 [44] KOSTAS E T, DANIEL B, ROBINSON J. The application of microwave heating in bioenergy: A review on the microwave pre-treatment and upgrading technologies for biomass[J]. Renewable and Sustainable Energy Reviews, 2017, 77: 12-27. doi: 10.1016/j.rser.2017.03.135 [45] HINRIKU H, JAANUS L, DENIS K, et al. Microwave effect on diffusion: a possible mechanism for non-thermal effect[J]. Electromagnetic biology and medicine, 2015, 34(4): 1-7. [46] COSTA C, SANTOS V H S, ARAUGO P H. et al. Microwave-assisted rapid decomposition of persulfate[J]. European Polymer Journal, 2009, 45(7): 2011-2016. doi: 10.1016/j.eurpolymj.2009.04.006 [47] SAPTARSHI B, MATHAVA K. Microwave-assisted persulfate/peroxymonosulfate process for environmental remediation[J]. Current Opinion in Chemical Engineering, 2022, 36: 1-9. [48] QI C D, LIU X T, ZHAO W, et al. Degradation and dechlorination of pentachlorophenol by microwave-activated persulfate[J]. Environmental science and pollution research international, 2015, 22(6): 4670-4679. doi: 10.1007/s11356-014-3718-6 -




 DownLoad:
DownLoad: