-
河流上覆水中的重金属可以通过沉淀、吸附、络合等作用,在河床表层底泥中富集[1-2]。当水体条件发生改变时,底泥中的重金属会通过氧化还原、溶解、解吸等作用,从河床表层底泥中释放,造成上覆水体的污染[3-4]。国内外普遍使用疏浚治理河湖底泥,但是疏浚工程会产生大量含有重金属的疏浚底泥,疏浚底泥含水率高、热值低,不适合传统焚烧方法处理[5-6]。近年来,稳定化技术被用于重金属废水、污染土壤等治理工作,通过加入药剂使沉积物中重金属发生物理化学反应,从而降低重金属的溶解性和迁移性,以达到良好的稳定化效果[7]。传统的稳定化药剂采用水泥、磷灰石等化学药剂,但存在处理后土壤板结、增容等缺点[8]。因此,本研究拟采用壳聚糖、膨润土、生物炭等天然材料,开发处理效果好、价廉易得的重金属稳定剂。
壳聚糖 (CTS) 是第二大天然线性化合物,具有无毒、无害、生物可降解性以及能通过自身丰富的基团络合重金属等特性,是一种良好的吸附重金属的材料[9]。宋俊颖等[10]利用CTS处理重金属污染土壤,当CTS投加量为7%时,铜离子的稳定化率达到92.36%。YAN等[11]利用CTS处理Cr和Hg复合型重金属污染的土壤,7 d后,土壤中有效态重金属的含量降低明显且残渣态含量升高。我国膨润土矿产资源丰富,价格低廉,具有较大的表面积、良好的吸附性、离子交换性和黏结性等优势,在底泥重金属稳定化技术中广泛应用[12]。杨秀敏等[13]通过等温吸附实验,研究了钠基膨润土对Cu2+、Zn2+、Cd2+的吸附情况,发现钠基膨润土 (NaBent) 对3种金属具有良好的吸附能力,能够降低土壤中有效型重金属的含量。这2种材料在我国产量大且易得,因此,可以使用CTS对NaBent进行改性,得到一种处理底泥重金属能力更高的复合型稳定剂。
本研究采用壳聚糖改性钠基膨润土稳定剂 (NaBent-CTS) 对底泥中的Cu2+、Zn2+、Cd2+进行单一和复合的重金属稳定化实验,通过改变稳定剂投加量、底泥pH和底泥液固比寻求稳定重金属的最佳工况点;通过毒性特征沥滤方法 (TCLP) 进行重金属浸取,以重金属稳定化率作为处理效果的重要指标,探究实验条件的改变对重金属稳定化效果的影响以及重金属之间存在的竞争吸附关系,旨在为温瑞塘河底泥重金属稳定化处理提供相关的研究基础。
-
实验疏浚底泥取自温州市温瑞塘河,使用环保绞吸式挖泥船采集底泥样品。将采集到的样品灌入洁净的聚乙烯桶中,密封后运回实验室自然风干,研磨,过100目筛,分析其各理化指标。疏浚底泥含水率为55.43%,溶解性有机碳 (DOC) 质量分数为265.63 mg·kg−1,pH为7.68,总磷质量分数为1.22 g·kg−1,氨氮质量分数为30.57 mg·kg−1,重金属Cu、Zn、Cd的质量分数分别为188.62、386.89和161.28 mg·kg−1。对疏浚底泥采用TCLP法进行重金属浸取,浸取后重金属Cu2+、Zn2+和Cd2+的质量浓度分别为0.793、0.960 和1.421 mg·L−1。
由测试结果可知,疏浚底泥中的Zn、Cu和Cd的含量均超出《围填海工程填充物质成分限值》 (GB 30736-2014) 的要求,因此将Zn、Cu和Cd3种重金属作为研究对象。
以未受重金属污染的温瑞塘河底泥为母质,分别添加锌、铜和镉的标准储备液进行实验底泥的配制。保持实验底泥含水率为50%左右,灌入洁净的聚乙烯桶中,在密封、室温的条件下放置14周后,室内自然风干,研磨,过100目筛备用。Cu2+、Zn2+和Cd2+实验底泥重金属浸取液质量浓度分别为1.598、1.714和1.701 mg·L−1。
-
本实验以无毒无害、价廉易得为标准,选取CTS和NaBent作为稳定药剂的制作材料,实验药剂信息如表1所示。
-
取6 g CTS (90%+) 溶于150 mL的5%醋酸溶液中,使用折叶式搅拌器将其缓慢充分溶解。向壳聚糖溶液中,缓慢加入30 g NaBent充分浸润3 h,在46 ℃恒温水浴锅中,连续搅拌4 h成糊状,加入一定量的氢氧化钠溶液,调节pH至9,缓慢搅拌10 min,沉淀壳聚糖2 h,用蒸馏水冲洗沉淀至pH为7~8,在转速为3 500 r·min−1的条件下离心分离15 min,取下层沉淀,放入烘箱在85 ℃下烘干,研磨,过100目筛,制得壳聚糖负载率为9.22%的NaBent-CTS。
-
称取风干过筛的底泥样品60 g,保持底泥pH为7,底泥液固比为1.5∶1,以稳定剂投加量 (稳定剂与干底泥的质量之比) 为1%、3%、5%、7%、10%进行单一重金属和复合重金属稳定化实验;保持稳定剂投加量为5%,底泥液固比为1.5∶1,以底泥pH为5、6、7、8、9进行单一重金属和复合重金属稳定化实验。保持稳定剂投加量为5%,底泥pH为7,以底泥液固比 (液体体积与干底泥质量之比,单位为mL∶g) 为1∶1、1.3∶1、1.5∶1、1.7∶1、2∶1进行单一重金属和复合重金属稳定化实验,每个样品充分混匀8 h,室温下密封放置7 d,进行稳定化处理,稳定后的底泥放置在实验室,自然风干,研磨,过100目筛,每组实验均设置3个平行,均以未经处理的底泥作为对照。
稳定化后的底泥采用TCLP法和我国固体废物标准浸取程序 (水平振荡法,HVM法) 进行重金属的浸取[14]。由于各实验底泥pH均大于5,因此选用2号浸取剂 (将5.7 mL冰醋酸溶入去离子水中,定容至1 L,保持溶液pH为2.88±0.05) 。称取12 g实验底泥,置于500 mL锥形振荡瓶中,按照液固比=20∶1加入浸取剂,在25 ℃条件下,恒温水浴水平往复振荡20 h,用稀硝酸淋洗抽滤器,用0.45 μm的滤膜过滤收集浸取液,4 ℃下密封保存,待测。稳定化率计算方法见式 (1) 。
式中:η为重金属的稳定化率;c0为加稳定剂前底泥样品的重金属浸取液质量浓度;c1为加稳定剂后底泥样品中重金属浸取液质量浓度。
-
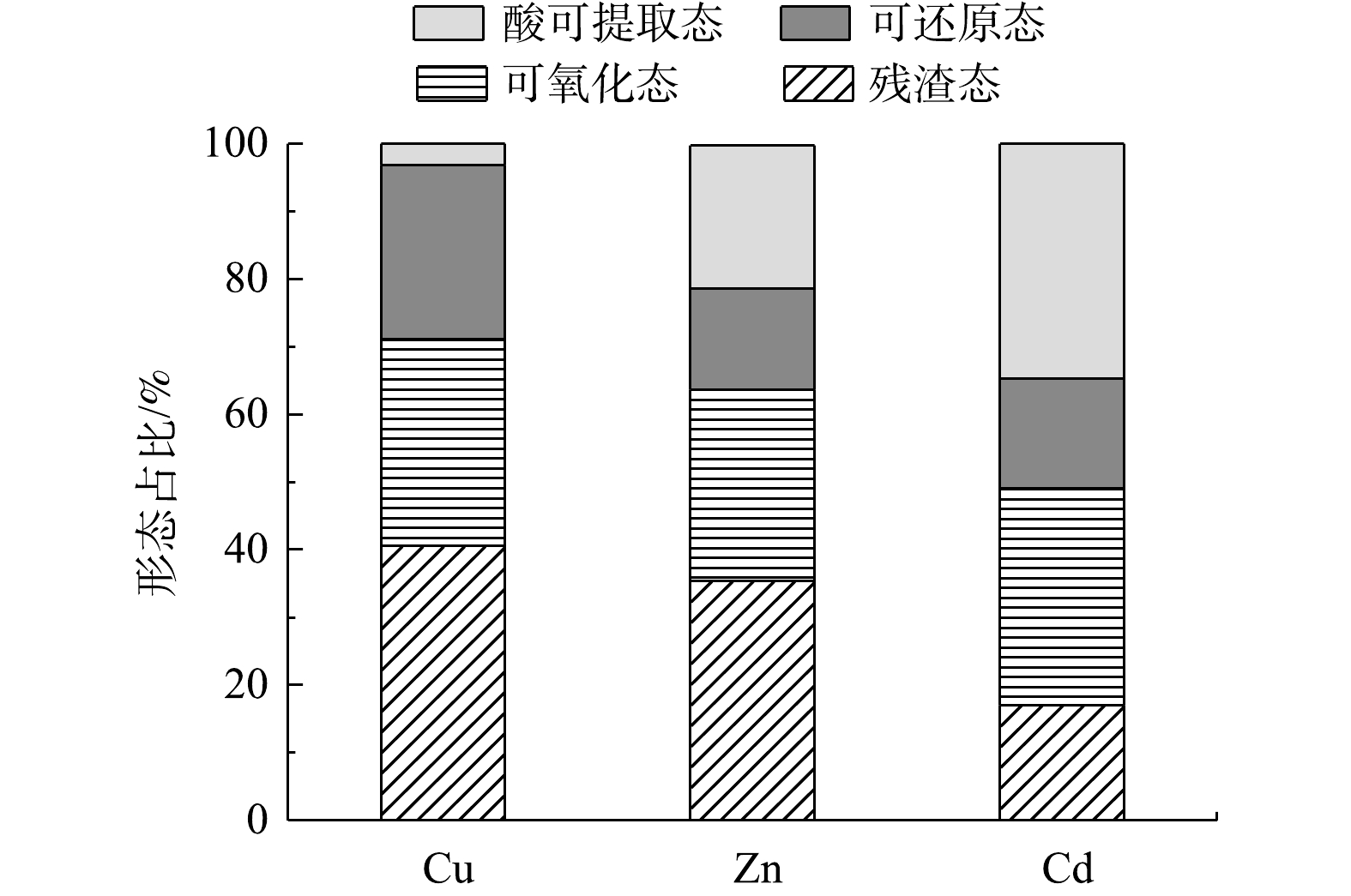
针对稳定化14 d后和未经处理的疏浚底泥,采用BCR连续提取法对其中的重金属进行连续提取。测定不同阶段提取的重金属质量分数,计算疏浚底泥中酸可提取态、可氧化态、可还原态和残渣态的重金属占比,稳定性由大到小为残渣态、可还原态、可氧化态、酸可提取态。
-
使用XRD、SEM、FT-IR、XPS、BET表征手段,观察NaBent-CTS微观结构及形貌特征,分析其晶相组成、晶面取向和基团结构等表面特性。
-
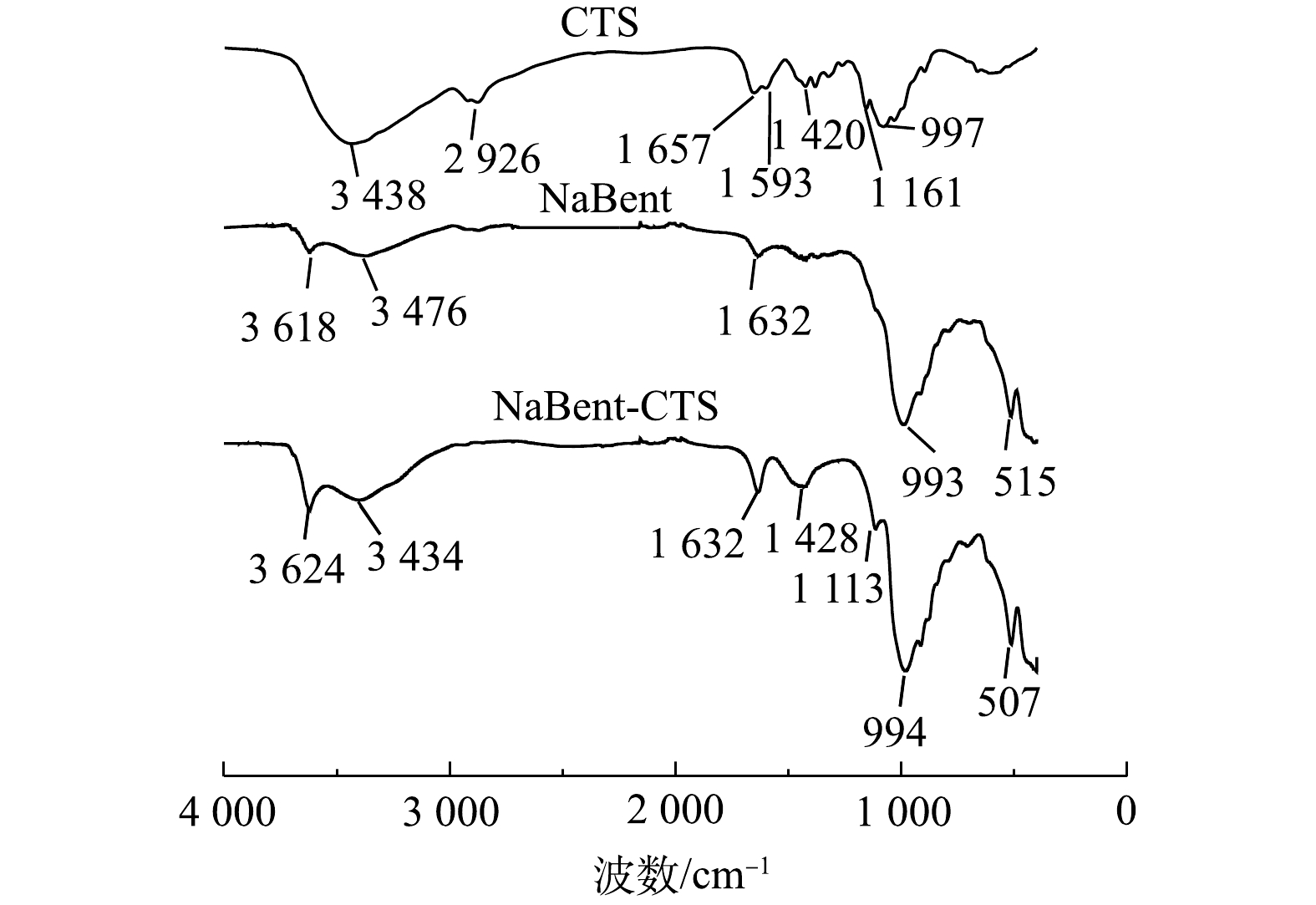
1) FT-IR分析。图1为CTS、NaBent和NaBent-CTS的红外光谱。在CTS红外光谱中,3 438 cm−1处的吸收峰为氨基N—H和羟基O—H的伸缩振动吸收峰,2 926 cm−1处的吸收峰为C—H伸缩振动吸收峰,1 657 cm−1处的吸收峰为酰胺Ⅰ谱带吸收峰,1 593 cm−1处的吸收峰为酰胺Ⅱ谱带吸收峰,1 420 cm−1处的吸收峰为羟基O—H面内弯曲振动吸收峰,1 161 cm−1处为伯羟基O—H的吸收峰,1 072 cm−1处为仲羟基O—H的吸收峰[15]。在NaBent红外光谱中,3 618 cm−1处为NaBent层间Si—Al—OH中羟基O—H伸缩振动峰,3 476 cm−1处为层间水分子的O—H羟基伸缩振动峰,1 632 cm−1处为NaBent层间水分子O—H弯曲振动峰,990 cm−1处为Si—O—Si不对称伸缩振动峰,515 cm−1处为Si—O—Al弯曲振动峰[16]。由NaBent-CTS与CTS和NaBent的红外光谱比较结果可知:3 624 cm−1处的吸收峰显著增强,峰面积变大,说明壳聚糖进入钠基膨润土层间,使层间的O—H羟基基团增多;1 428 cm−1处羟基弯曲振动吸收峰增强,在1 113 cm−1处出现羟基弯曲振动吸收峰,说明壳聚糖成功负载在钠基膨润土上;3 434 cm−1处为钠基膨润土层间水分子O—H羟基伸缩振动峰与壳聚糖中氨基N—H弯曲振动峰的合并峰;507 cm−1处Si—O—Al吸收峰面积和强度增大,表明在Si—O—Al处发生了化学吸附,1 657 cm−1与1 593 cm−1处的酰胺谱带吸收峰消失,因此,壳聚糖上的酰胺与Si—O—Al之间可能发生了化学吸附;994 cm−1处为Si—O—Si与羟基O—H振动峰的合并峰。
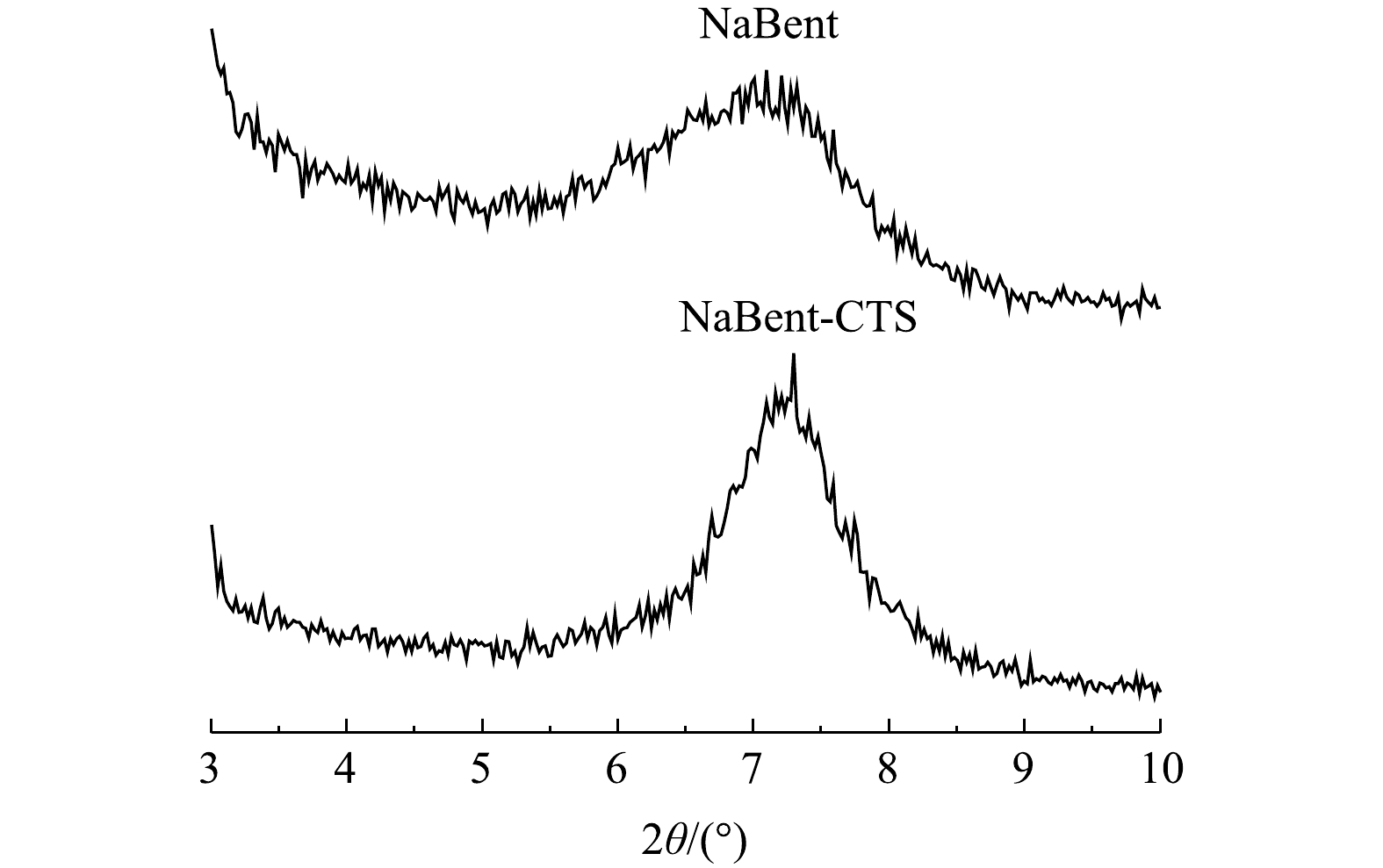
2) XRD分析。由图2可知,NaBent与NaBent-CTS衍射峰首峰的位置θ分别为3.58°和3.567 5°。层间距可根据Bragg方程[17]计算得出。计算方法见式 (2) 。
式中:d为层间距;θ为入射线与反射晶面之间的夹角;λ为波长,Cu靶Ka射线 (λ=0.154 06 nm) ;n为反射级数,n=1。
由式 (2) 可知,NaBent的层间距为1.233 6 nm,NaBent-CTS的层间距为1.237 9 nm,NaBent层间距在负载CTS前后未发生明显改变,由红外光谱分析结果可知,存在部分CTS进入NaBent层间。
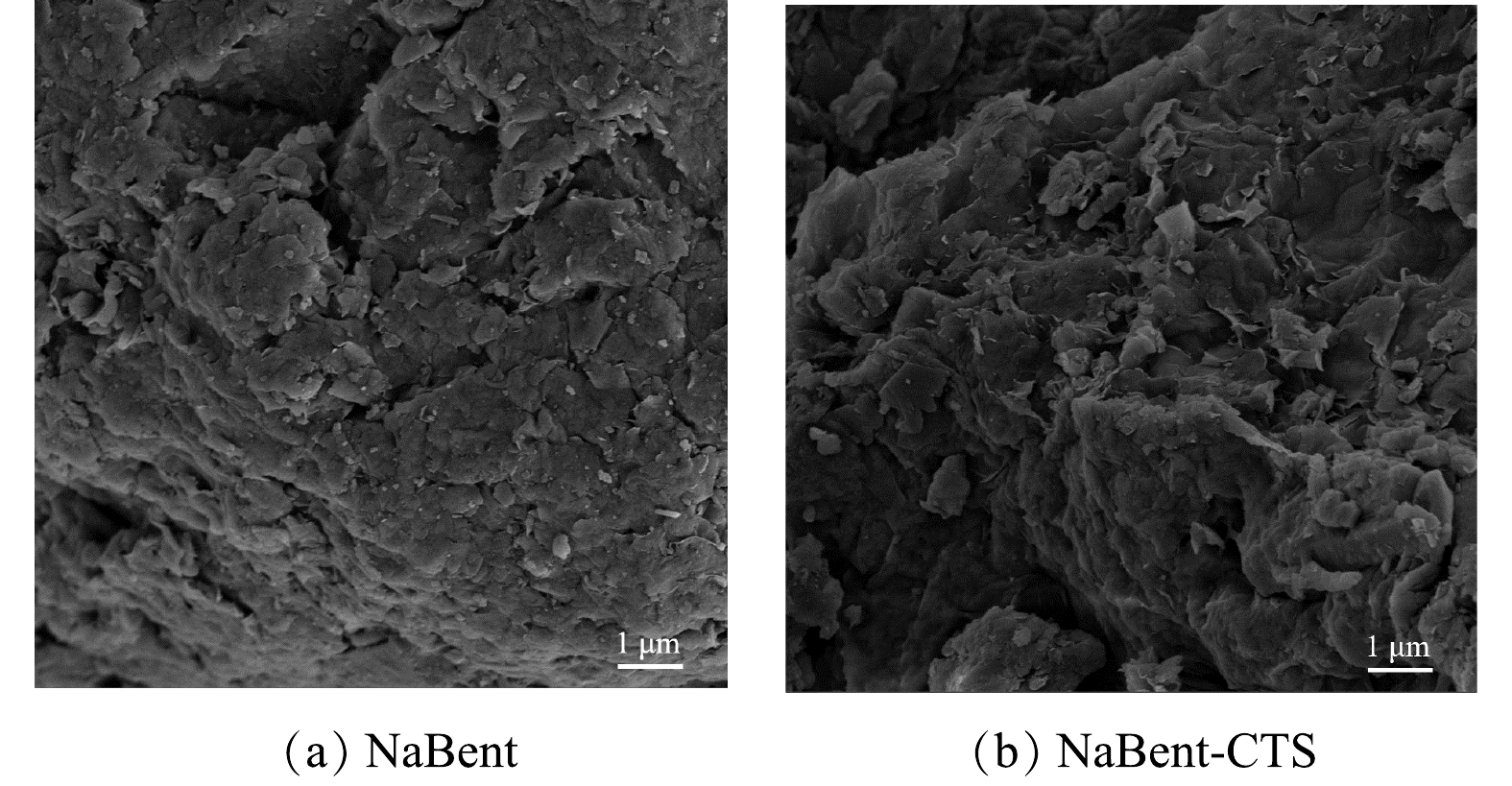
3) SEM与BET分析。由图3可知,NaBent的外貌发生了明显的变化,NaBent颗粒表面结构较平整,NaBent-CTS颗粒表面更加粗糙。经BET分析,NaBent与NaBent-CTS的比表面积分别为21.036 m2·g−1和14.609 m2·g−1,NaBent改性后比表面积减少,这是因为CTS负载在NaBent表面,堵塞了孔隙,导致比表面积降低[18]。
-
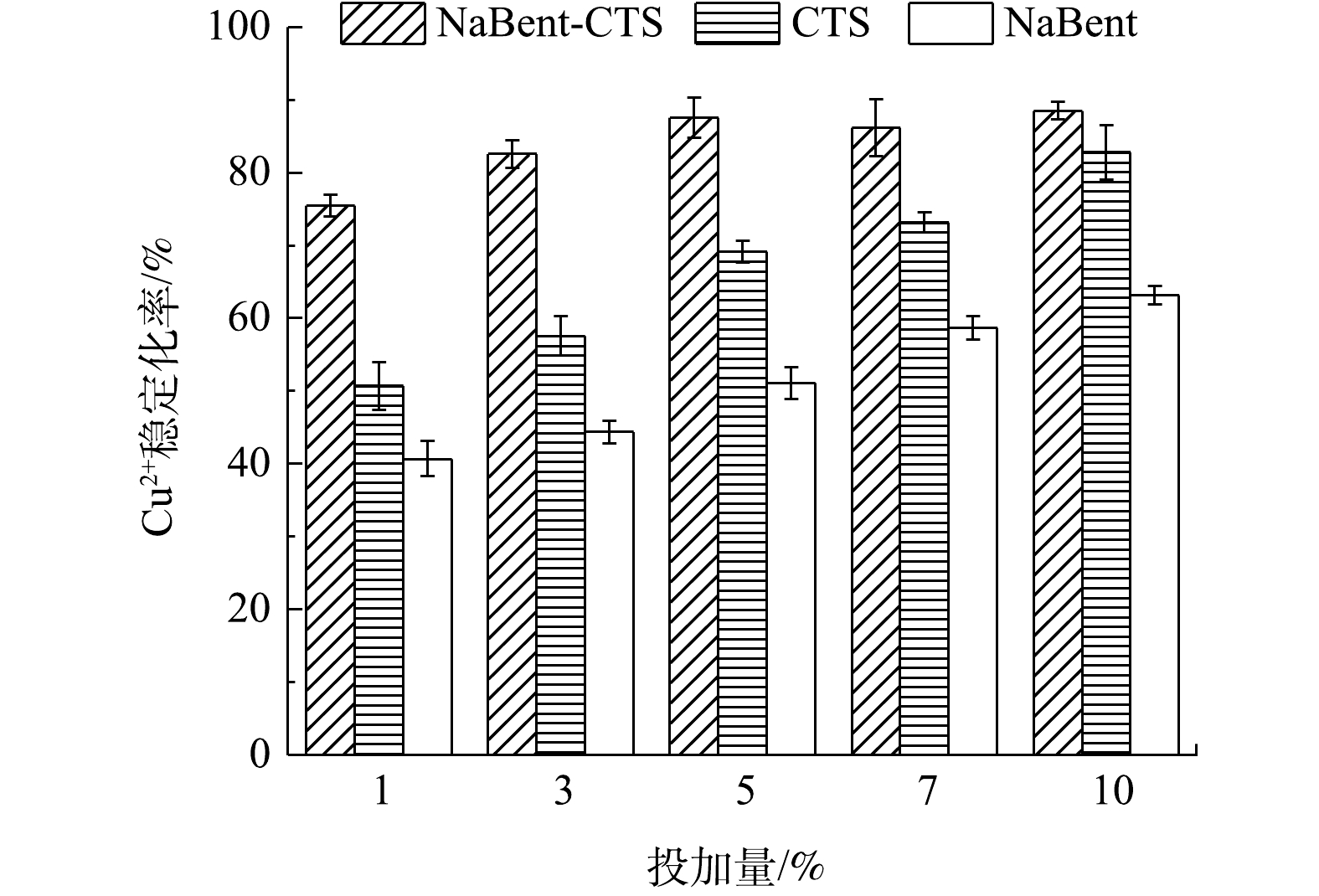
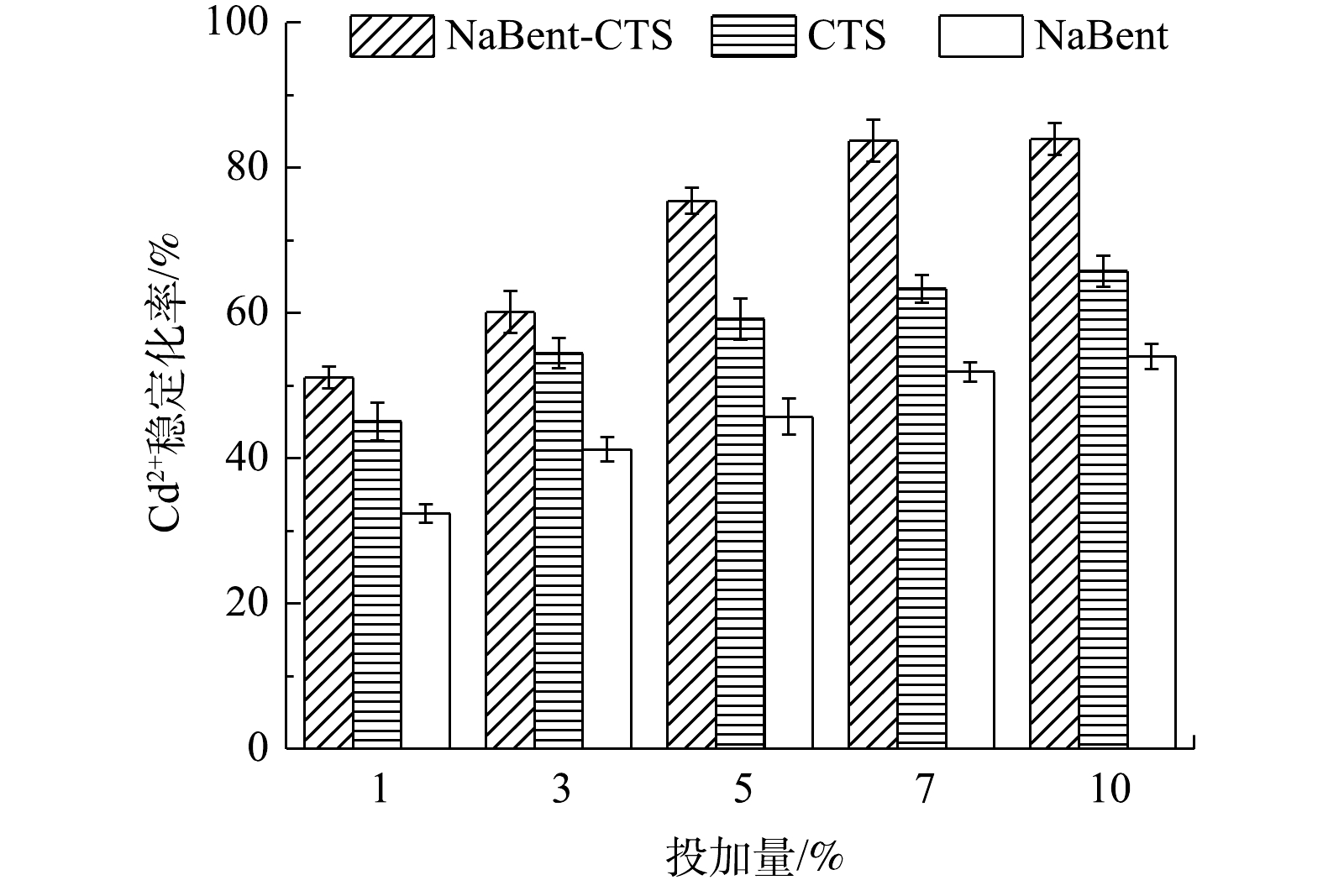
1) 复合前后稳定效果的比较。由图4~图6可知,在pH为7、液固比为1.5:1时,随着3种重金属稳定剂投加量的递增,Cu2+、Zn2+和Cd2+的稳定化率也逐渐递增,达到一定投加量后,NaBent-CTS对Cu2+、Zn2+和Cd2+的稳定化率趋于稳定。对比3种稳定剂效果,NaBent-CTS对Cu2+、Zn2+和Cd2+的稳定效果最佳,且在较低的投加量下可达到较好的稳定效果。投加量为5%时,Cu2+的稳定化率达到稳定,浸取液质量浓度由1.714 mg·L−1降至0.213 mg·L−1,稳定化率为87.56%;投加量为7%时,Zn2+的稳定化率达到稳定,浸取液质量浓度由1.598 mg·L−1降至0.226 mg·L−1,稳定化率为85.85%;投加量为7%时,Cd2+的稳定化率达到稳定,浸取液质量浓度由1.701 mg·L−1降至0.277 mg·L−1,稳定化率为83.71%。与NaBent-CTS相比,CTS稳定重金属效果较差,NaBent稳定效果最差,均在投加量为10%时,稳定化率达到最大。
由此可知,CTS改性NaBent后,NaBent-CTS稳定重金属的能力得到提升,并且在较低投加量的情况下达到较好的稳定效果。虽然NaBent改性后比表面积有一定程度的降低,但NaBent中的CTS中含有大量的羟基和氨基,这2类基团对重金属有极强的螯合能力,通过CTS表面的内扩散作用,重金属离子更易进入NaBent中,与Na+、Al3+等金属离子发生离子交换作用,使NaBent表现出较高的吸附性能[19]。
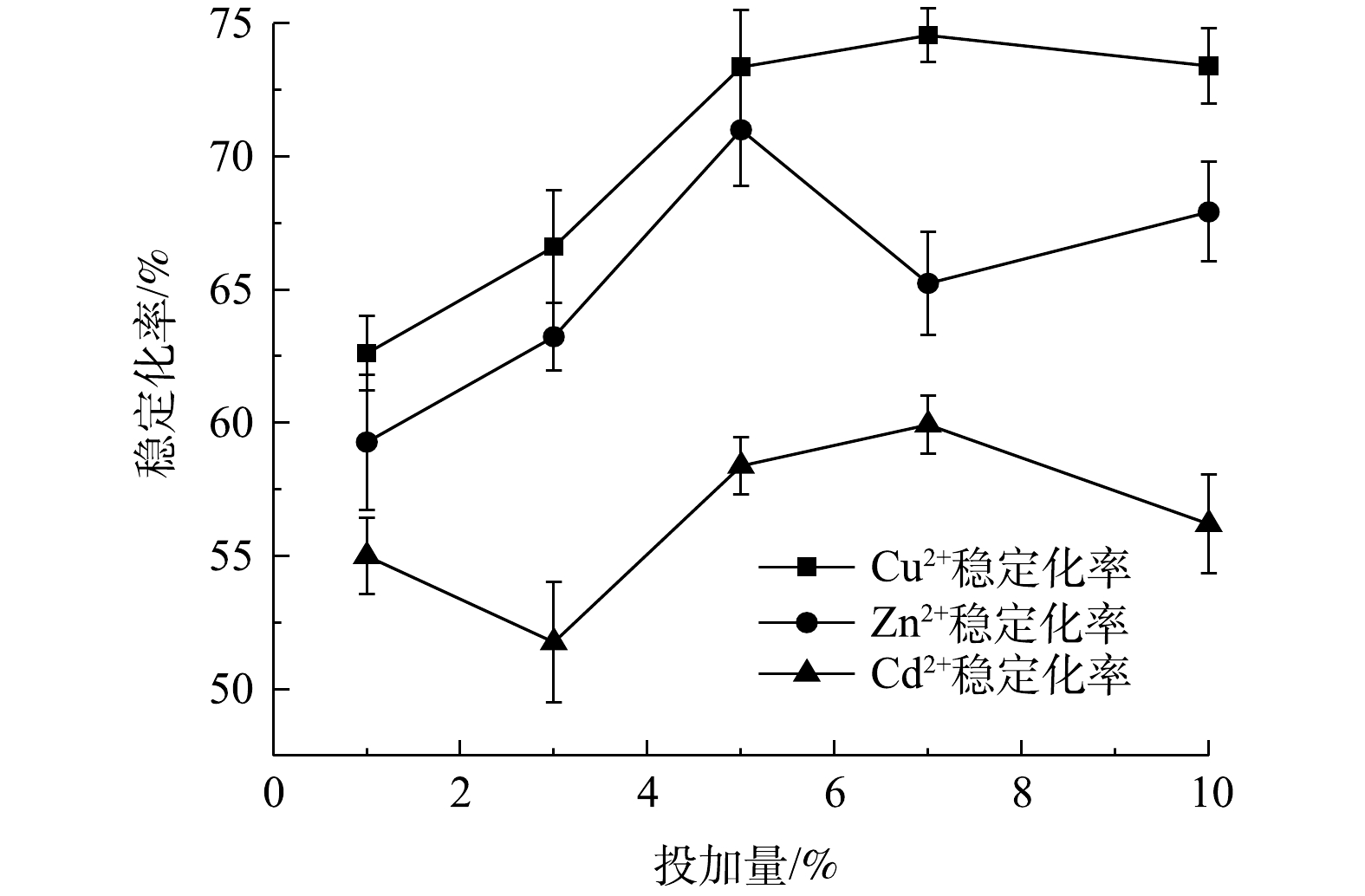
2) NaBent-CTS投加量对单一重金属稳定化率的影响。图7表明了在pH为7与实验底泥液固比为1.5∶1时,稳定剂投加量的变化对Cu2+、Zn2+和Cd2+稳定化率的影响。随着稳定剂投加量的增加,Cu2+、Zn2+和Cd2+的稳定化率也随之升高,达到一定程度后稳定化率基本保持稳定。Cu2+、Zn2+和Cd2+的稳定化率分别在药剂投加量为5%、7%和7%时达到稳定,稳定化率为87.56%、85.85%和83.71%。
NaBent-CTS中存在氨基官能团与羟基官能团,具有与重金属离子形成配位键的能力,从而螯合重金属,并且稳定剂中含有众多Na+、Al3+离子,可通过离子交换作用来吸附重金属。随着稳定剂投加量的增大,能够提供的配位键的数量与吸附比表面积不断增多,能够吸附更多的重金属离子,使3种重金属离子的稳定化率不断提高;稳定剂投加量继续增大,稳定剂颗粒之间相互黏结,比表面积减少,导致稳定化率增幅变小。
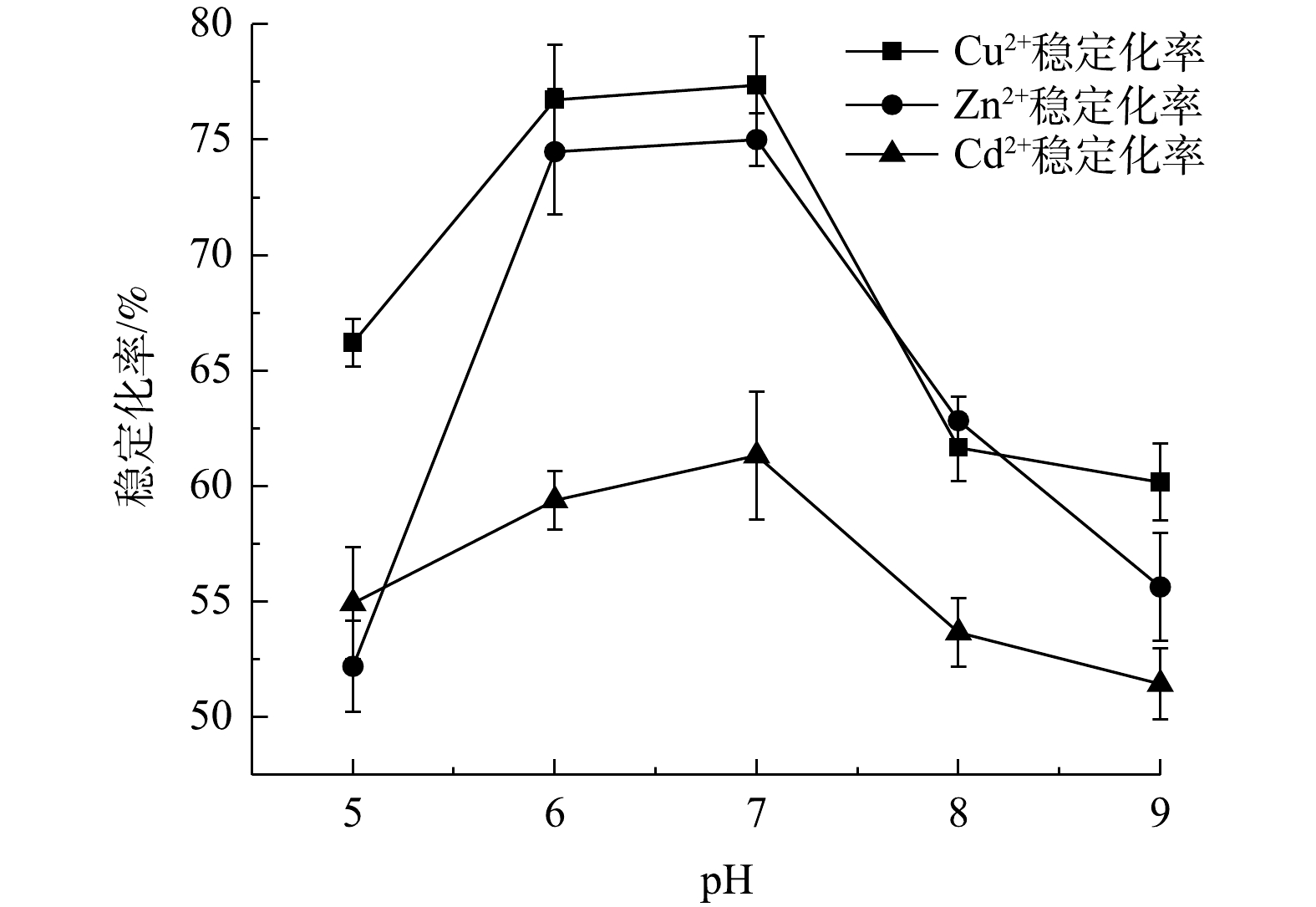
3) 底泥pH对单一重金属稳定化率的影响。图8表明了在稳定剂投加量为5%与实验底泥液固比为1.5:1时,底泥pH对Cu2+、Zn2+和Cd2+稳定化率的影响。3种重金属稳定化率均随pH的上升呈先升高后降低的趋势,Cu2+、Zn2+和Cd2+稳定化率分别在pH为7、6和7时达到稳定,Cu2+浸取液质量浓度由1.714 mg·L−1降至0.283 mg·L−1,Zn2+浸取液质量浓度由1.598 mg·L−1降至0.346 mg·L−1,Cd2+浸取液质量浓度由1.701 mg·L−1降至0.433 mg·L−1,稳定化率分别为83.47%、78.35%和74.57%。出现上述现象的原因如下:当pH小于7时,H+的质量浓度较高,占据了稳定剂的吸附位,与重金属离子形成竞争吸附关系,导致重金属稳定化率较低;当pH大于7时,部分OH−会与重金属离子形成沉淀,难以被稳定剂吸附,经过TCLP浸取实验,氢氧化物沉淀溶于酸性浸取剂中,导致稳定化率下降。
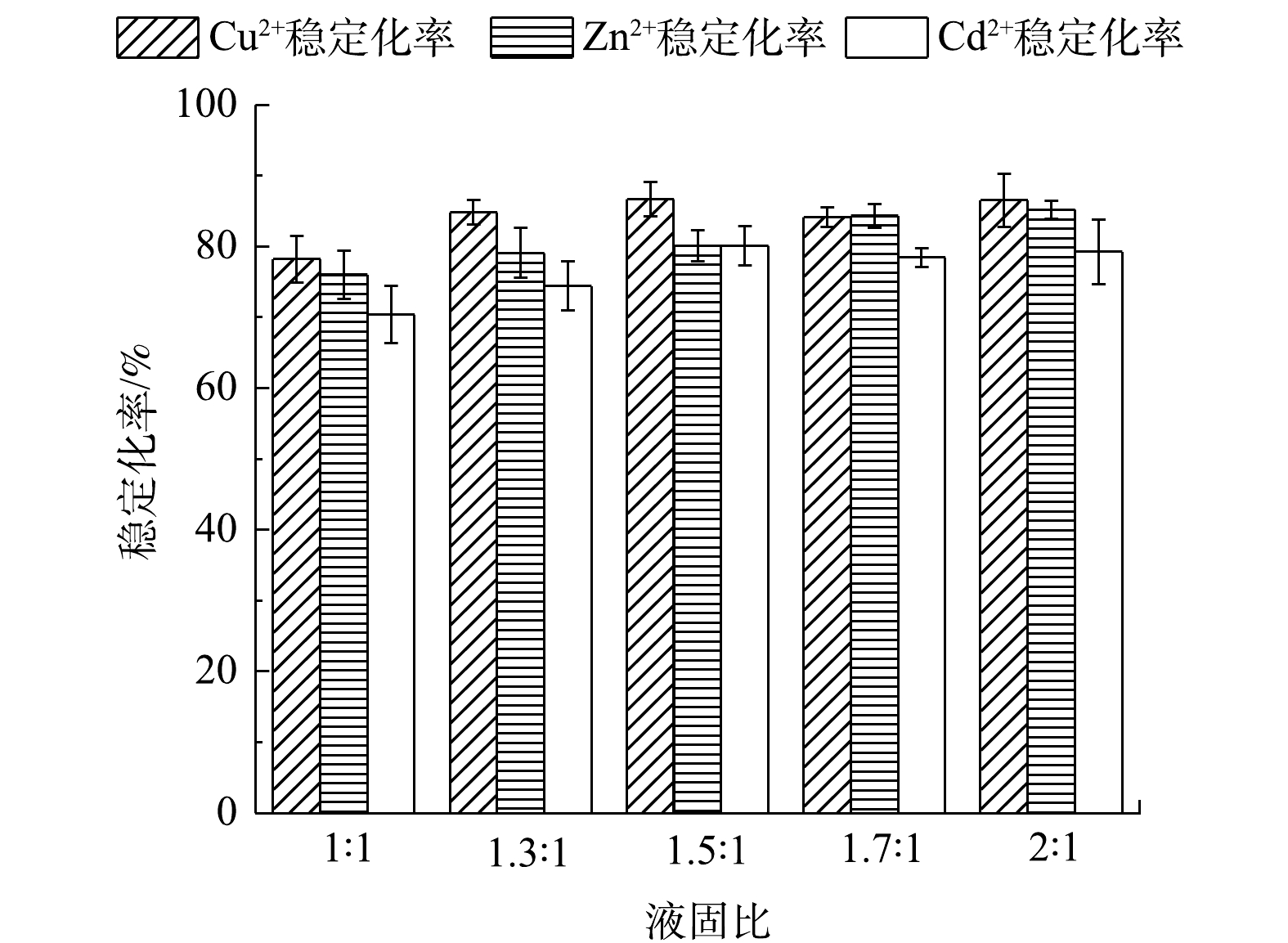
4) 底泥液固比对单一重金属稳定化率的影响。图9表明了在pH为7与稳定剂投加量为5%时,实验底泥液固比的变化对Cu2+、Zn2+和Cd2+稳定化率的影响。液固比对3种重金属稳定化率影响不明显,Cu2+、Zn2+和Cd2+稳定化率分别在液固比为1.3∶1、1.7∶1和1.5∶1时达到稳定,Cu2+浸取液质量浓度由1.714 mg·L−1降至0.260 mg·L−1,Zn2+浸取液质量浓度由1.598 mg·L−1降至0.251 mg·L−1,Cd2+浸取液质量浓度由1.701 mg·L−1降至0.338 mg·L−1,稳定化率分别为84.82%、84.32%和80.13%。出现上述趋势的原因如下,在液固比较小的条件下,溶剂中的重金属质量浓度与底泥孔隙水中的重金属质量浓度在较短的时间内达到平衡,抑制了底泥孔隙水中的重金属向溶剂中扩散的趋势[20]。随着液固比逐渐升高,溶剂与底泥孔隙水中的重金属质量浓度需要在较长的时间内达到平衡,使得扩散作用能够在较长时间内持续进行,释放到溶剂中的重金属也增多,使稳定化率增加。并且含水率不同的实验底泥在7 d稳定化期中内部成分的变化也不同,会间接影响底泥中矿物颗粒与胶体颗粒之间的相互作用,从而改变实验底泥中重金属的存在形态和活性[21]。
-
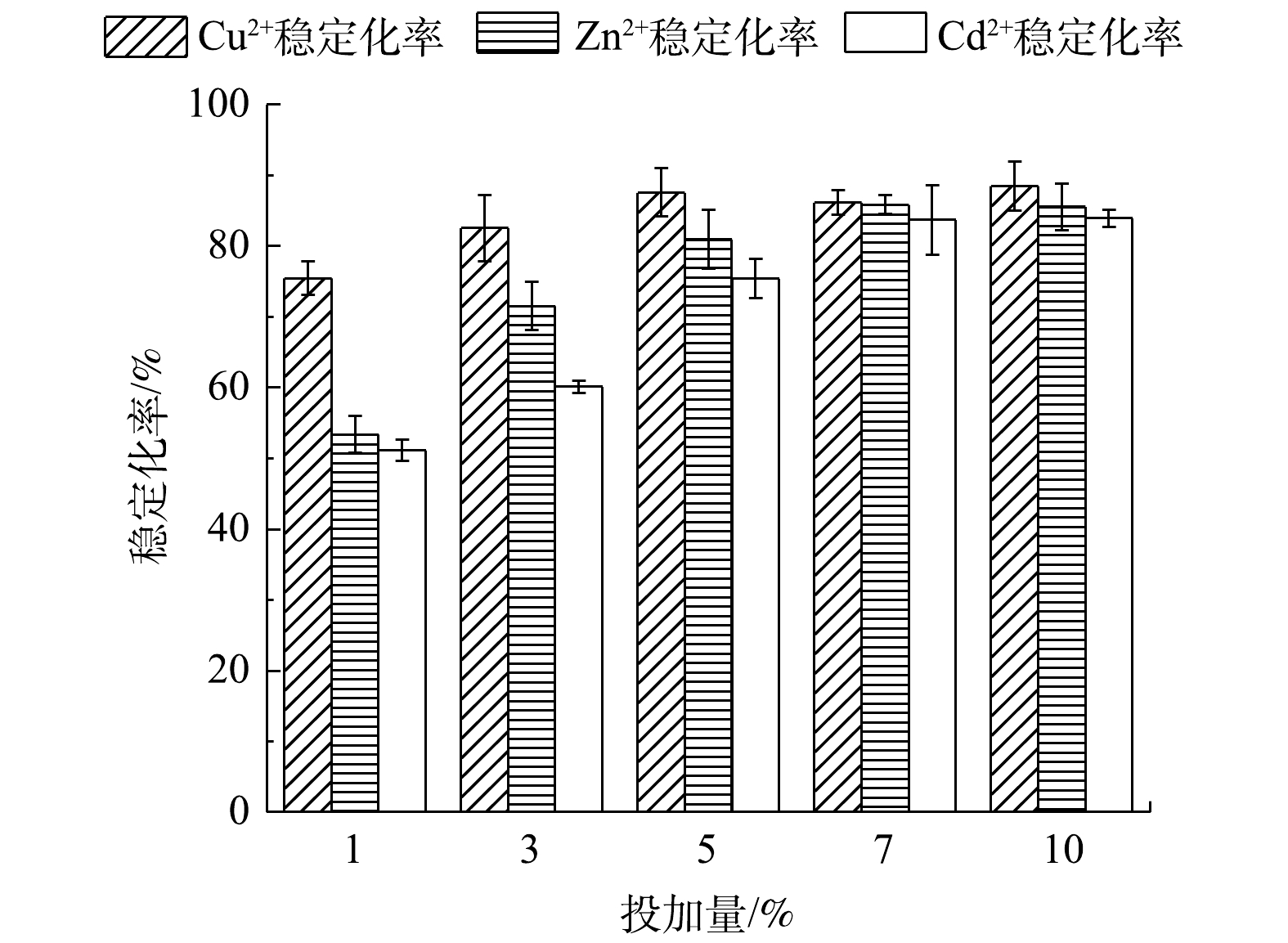
由图10可知,在pH为7与疏浚底泥液固比为1.5∶1时,稳定剂投加量对复合重金属的稳定化率存在较大的影响。随着稳定剂投加量的不断增大,Cu2+、Zn2+和Cd2+稳定化率也逐渐升高。在稳定剂投加量为5%时,稳定化率达到了最佳值,Cu2+浸取液质量浓度由0.793 mg·L−1降至0.211 mg·L−1,Zn2+浸取液质量浓度由0.960 mg·L−1降至0.278 mg·L−1,Cd2+浸取液质量浓度由1.421 mg·L−1降至0.591 mg·L−1,稳定化率分别为73.36%、71.00%、58.38%。
在稳定剂投加量超过5%时,Zn2+和Cd2+稳定化率呈现下降的趋势。这可能是稳定剂颗粒之间相互黏结,比表面积减少,导致重金属离子之间竞争吸附作用增强。而竞争力较弱的Zn2+和Cd2+脱离吸附位点,导致稳定化率下降。
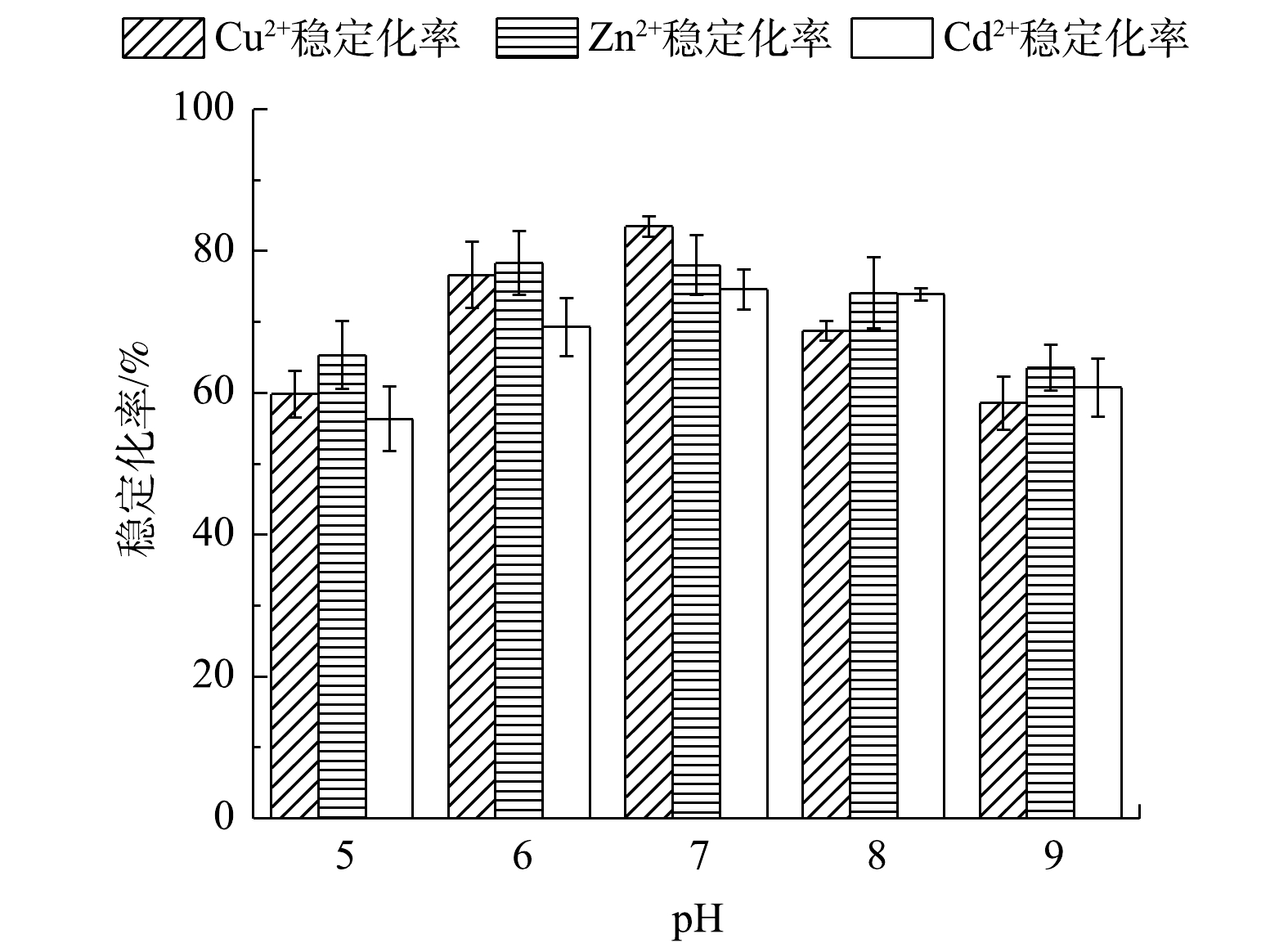
由图11可知,在稳定剂投加量为5%与疏浚底泥液固比为1.5:1时,底泥pH的变化对复合重金属稳定化率的影响非常明显。在pH为7时,复合重金属稳定化率达到了最大值,Cu2+浸取液质量浓度由0.793 mg·L−1降至0.180 mg·L−1,Zn2+浸取液质量浓度由0.960 mg·L−1降至0.239 mg·L−1,Cd2+浸取液质量浓度由1.421 mg·L−1降至0.549 mg·L−1,稳定化率分别为77.24%、75.03%、61.33%。
由图12可知,在pH为7与稳定剂投加量为5%时,随着底泥液固比的增大,Cu2+、Zn2+和Cd2+的稳定化率逐渐上升。在液固比为1.5:1时逐渐稳定,Cu2+浸取液质量浓度由0.793 mg·L−1降至0.180 mg·L−1,Zn2+浸取液质量浓度由0.960 mg·L−1降至0.239 mg·L−1,Cd2+浸取液质量浓度由1.421 mg·L−1降至0.592 mg·L−1,稳定化率分别为77.26%、75.11%、58.32%。
Cd2+的稳定化率随着底泥液固比增大出现降低的趋势。原因可能是,随着液固比的增大,含水率升高,释放到溶剂中的重金属也增多,但稳定剂表面的吸附位点数量一定,使竞争能力较差的Cd2+脱离吸附位点,导致稳定化率下降。
综上所述,在稳定剂投加量为5%、底泥pH为7、底泥液固比为1.5:1时,NaBent-CTS对复合重金属的稳定化率最好,稳定化率分别达到75.95%、73.71%和59.00%。3种重金属离子之间的竞争吸附关系为Cu2+>Zn2+>Cd2+。
-
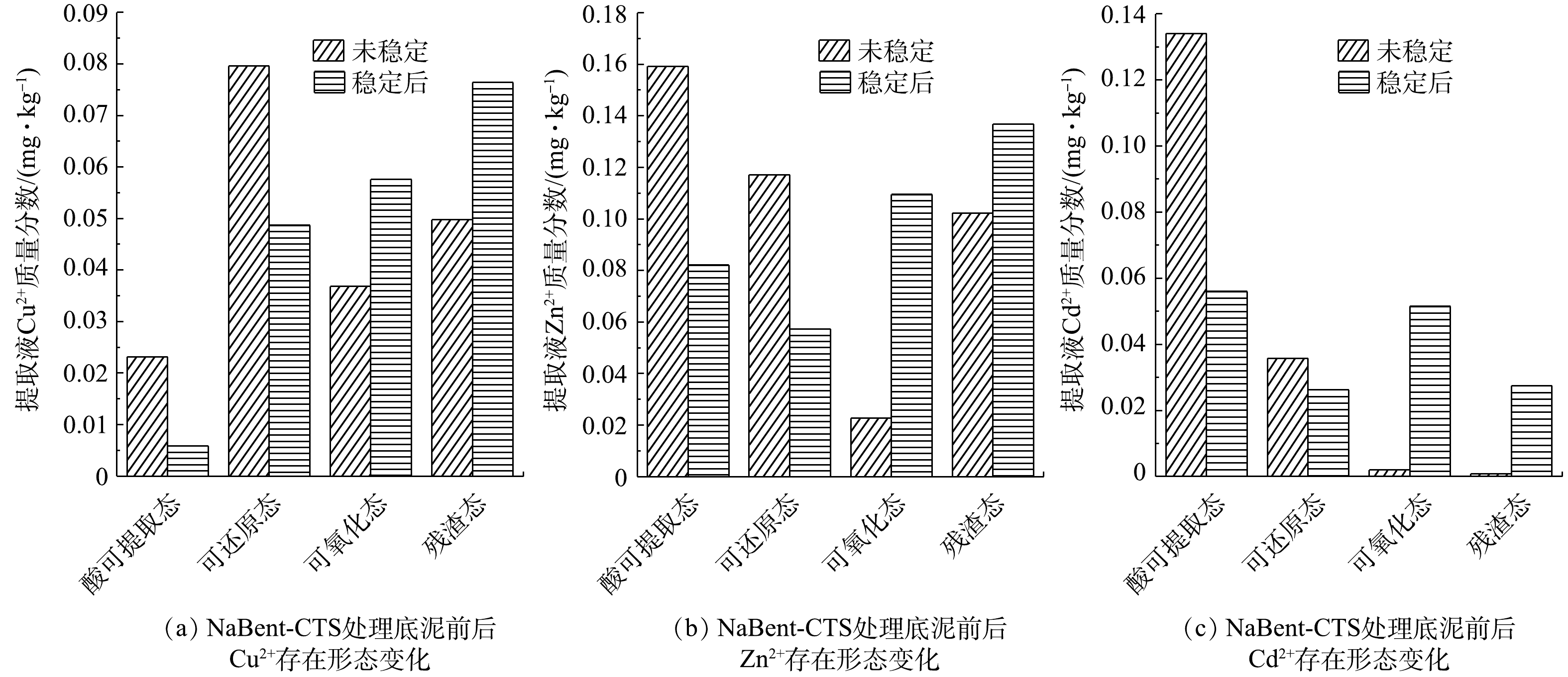
如图13、图14所示,在稳定化处理底泥前,Cu2+、Zn2+、Cd2+的存在形态以酸可提取态和可还原态这两种不稳定形态占比较大,可氧化态和残渣态这两种稳定形态占比较小。经NaBent-CTS稳定化处理14 d后,可氧化态和残渣态这两种形态占比显著提高,表明NaBent-CTS具有良好的稳定重金属的效果。
重金属离子与NaBent-CTS结合后,与稳定剂中的羟基和氨基发生螯合配位作用,并且稳定剂中含有Na+、Al3+等可交换离子,将以酸可提取态和可还原态存在的重金属变成了可氧化态与残渣态的存在形态,从而降低底泥中重金属污染生态环境的风险。
-
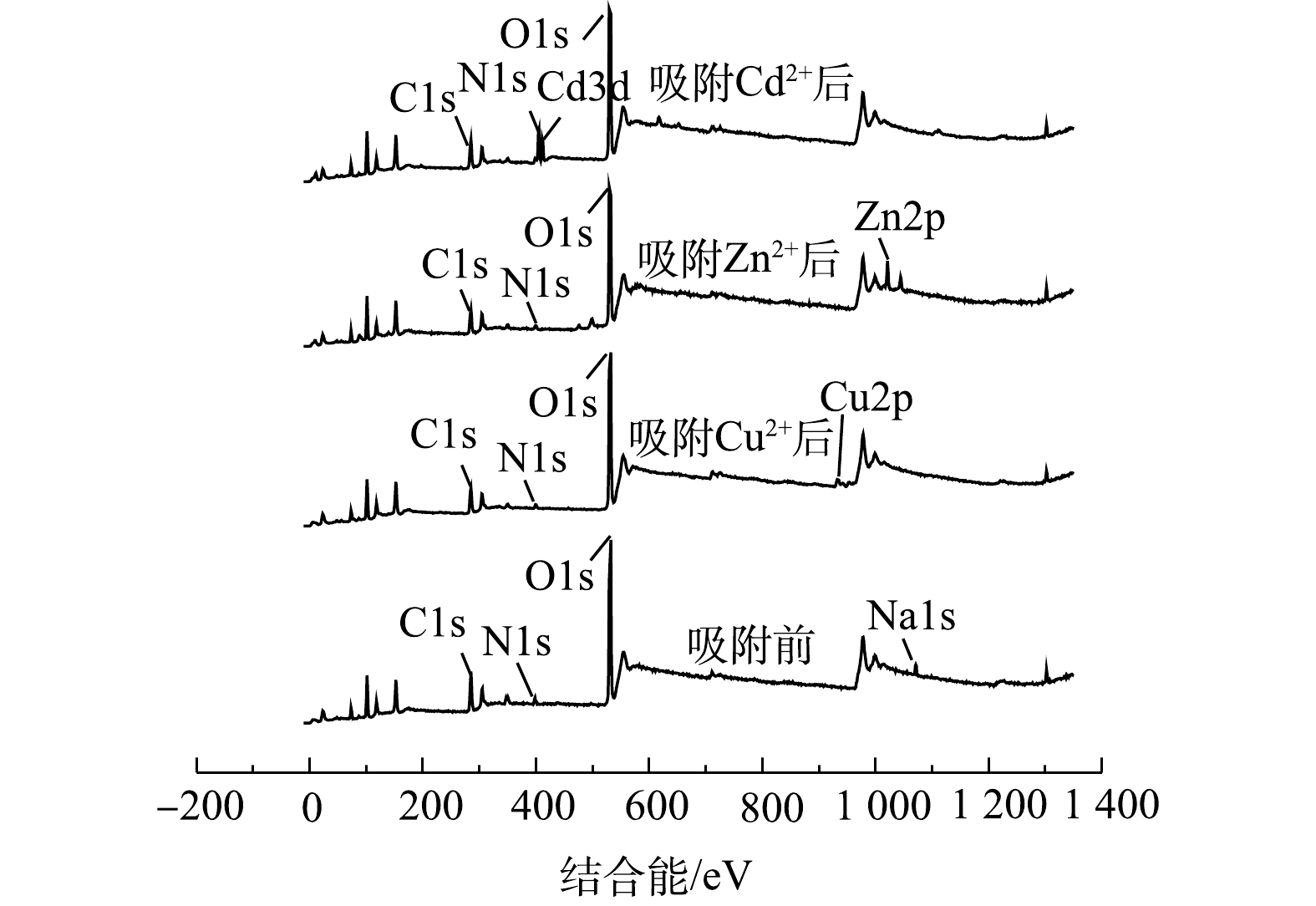
图15为NaBent-CTS在室温条件下,稳定化处理相同质量浓度Cu2+、Zn2+、Cd2+溶液后的XPS全谱图。可以看出,稳定化处理Cu2+、Zn2+和Cd2+后,XPS图谱中出现Cu2p、Zn2p和Cd3d的轨道峰,充分证明Cu2+、Zn2+和Cd2+已吸附在NaBent-CTS上。NaBent-CTS稳定化处理重金属后,XPS图谱中的Na1s谱峰几乎消失,说明稳定化过程中Cu2+、Zn2+、Cd2+与Na+发生离子交换反应,导致稳定剂中Na+含量骤减。
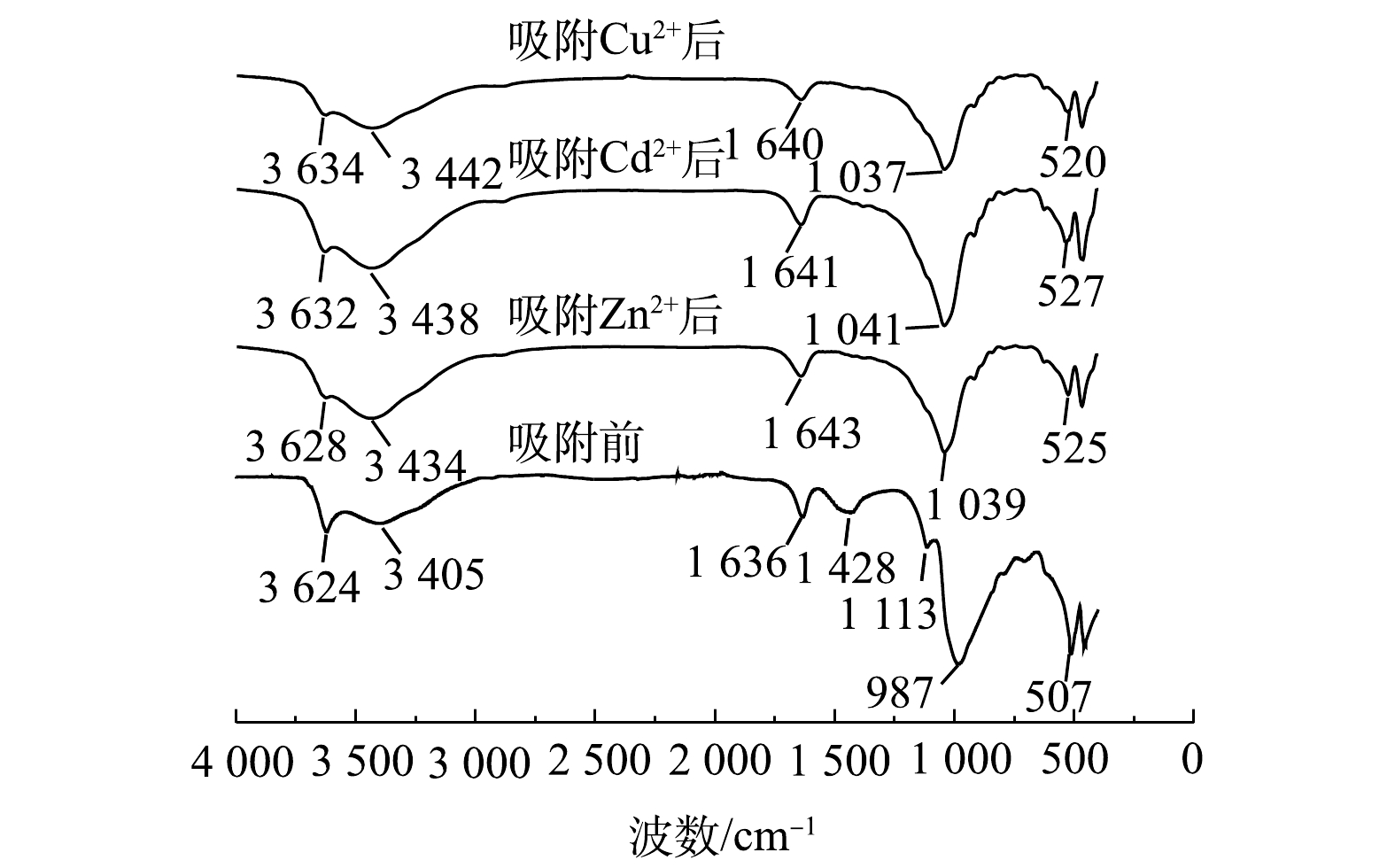
图16为NaBent-CTS在室温条件下,稳定化处理相同质量浓度Cu2+、Zn2+、Cd2+溶液后的FT-IR光谱图。可以看出,NaBent-CTS稳定Cu2+、Zn2+、Cd2+后没有新的峰出现,3 624、3 405、1 636、1 428、1 113、987 cm−1处的羟基与氨基特征峰发生偏移并且峰强度降低,这是由于重金属与基团之间发生了螯合反应;507 cm−1处Si—O—Al特征峰出现波数偏移与强度降低,这是由于重金属与稳定剂中Al3+离子发生了离子交换反应。稳定化处理Cu2+、Zn2+和Cd2+后,特征峰削弱强度不同,说明NaBent-CTS对Cu2+、Zn2+和Cd2+之间出现选择性吸附。由特征峰削弱强度可知,NaBent-CTS对重金属稳定能力由强到弱为Cu2+>Zn2+>Cd2+,这符合稳定剂处理复合重金属污染底泥的实验结果。
综上所述,NaBent-CTS稳定重金属过程中存在螯合反应与离子交换反应,重金属离子的螯合配位可能是由CTS中的氨基和羟基、Si—Al—OH和层间水分子O—H的互相作用,这样生成的螯合物可能是高交联的结构,稳定性极强。
-
1) 由NaBent-CTS表面特性分析结果可知,NaBent和CTS之间存在化学吸附,大量CTS吸附在NaBent表面,稳定剂表面粗糙但比表面积降低。
2) 经过CTS改性后的NaBent稳定重金属的能力显著提高,在投加量为5%时,可达到较好的稳定效果。在投加量为5%、pH为7、液固比为1.3∶1时,NaBent-CTS对Cu2+重金属污染底泥的处理效果最好。在投加量为7%、pH为6、液固比为1.7∶1时,NaBent-CTS对Zn2+重金属污染底泥的处理效果最好。在投加量为7%、pH为7、液固比为1.5∶1时,NaBent-CTS对Cd2+重金属污染底泥的处理效果最好。
3) NaBent-CTS投加量为5%、pH为7、液固比为1.5∶1时,NaBent-CTS对复合重金属污染底泥的重金属稳定化效果最好,Cu2+、Zn2+、Cd2+稳定化率分别达到75.95%、73.71%和59.00%;NaBent-CTS稳定化处理复合重金属污染底泥时,Cu2+、Zn2+、Cd2+之间存在竞争吸附作用,竞争力由强到弱为Cu2+>Zn2+>Cd2+。
4) 采用BCR法分析稳定化处理14 d后底泥中Cu2+、Zn2+、Cd2+的存在形态,可以看出,在NaBent-CTS处理后,底泥中Cu2+、Zn2+、Cd2+的存在形态更加稳定,可氧化态与残渣态比例大幅上升。
壳聚糖改性钠基膨润土稳定剂对重金属污染底泥的处理
Treatment of heavy metal polluted sediment with chitosan modified Na-bentonite stabilizer
-
摘要: 为有效处理重金属污染的疏浚底泥并验证重金属稳定剂的稳定性,采用重金属稳定化的方法,以Cu2+、Zn2+、Cd2+单一和复合重金属污染的疏浚底泥为研究对象,以壳聚糖改性后的钠基膨润土为稳定剂,在不同稳定剂投加量、pH和液固比的条件下,对污染底泥进行稳定化处理,并采用毒性特征沥滤方法 (TCLP) 和BCR连续提取法对稳定化效果进行分析与评价。结果表明:经过壳聚糖改性后的钠基膨润土稳定重金属的能力显著提高,在投加量为5%时,对Cu2+、Zn2+、Cd2+单一重金属污染底泥便可达到较高的稳定化率;聚糖改性钠基膨润土稳定剂 (NaBent-CTS) 处理Cu2+、Zn2+、Cd2+单一重金属污染底泥的最佳投加量为5%~7%,pH为6~7,液固比为1.3:1~1.7:1;NaBent-CTS处理Cu2+、Zn2+、Cd2+复合重金属污染底泥的最佳投加量为5%、pH为7、液固比为1.5:1,稳定化率分别达到75.95%、73.71%和59.00%;NaBent-CTS处理复合重金属污染底泥时重金属存在竞争吸附,由强到弱排列顺序为Cu2+>Zn2+>Cd2+;经NaBent-CTS稳定化处理14 d后,可氧化态和残渣态占比显著提高,稳定性能佳。NaBent-CTS对Cu2+、Zn2+、Cd2+单一和复合重金属污染底泥具有较好的稳定效果。该研究结果可为重金属污染疏浚底泥的稳定化处理提供相关参考。Abstract: In order to effectively treat the dredged sediment polluted by heavy metals and verify the stability of heavy metal stabilizer, a type of heavy metal stabilization method with the stabilizer of the Na-bentonite modified by chitosan was adopted, the dredged sediment polluted by single and complex heavy metals Cu2+, Zn2+ and Cd2+ was taken as the research object to perform the stabilizing treatment under the conditions of different stabilizer dosage, pH and liquid-solid ratio. Toxicity characteristic leaching method (TCLP) and BCR continuous extraction method were used to analyze and evaluate the stabilization effect. The results showed that the ability of Na-bentonite modified by chitosan to stabilize heavy metals increased significantly. When the dosage of sodium bentonite modified by chitosan was 5%, the sediment contaminated by single heavy metal of Cu2+, Zn2+ or Cd2+ could reach a high stabilization rate. The optimal dosage of Na-bentonite stabilizer (NaBent-CTS) treating contaminated sediments by Cu2+, Zn2+ and Cd2+ alone was 5%~7%, pH was 6~7 and the liquid-to-solid ratio was 1.3:1~1.7:1. The optimal dosage of NaBent-CTS treating contaminated sediment by Cu2+, Zn2+ and Cd2+ composite heavy metals was 5%, pH was 7 and liquid-to-solid ratio was 1.5:1, and the stabilization rates were 75.95%, 73.71% and 59.00%, respectively. The competitive adsorption of heavy metals occurred in NaBent-CTS treatment process, and the corresponding competitive order from strong to weak was Cu2+>Zn2+>Cd2+. After 14 days of NaBent-CTS stabilization treatment, the proportions of oxidizable state and residue state for heavy metals increased significantly, and heavy metal stability was good. NaBent-CTS has good stabilization effects on sediment polluted by single and complex heavy metals of Cu2+, Zn2+ and Cd2+. The results can provide a relevant reference for the stabilization treatment of dredged sediment polluted by heavy metals.
-
Key words:
- chitosan /
- Na-bentonite /
- stabilizer /
- heavy metals /
- sediment
-
如今,人类赖以生存的水环境的污染变得日益严重,病原体在水中能够快速的传播,严重影响环境卫生和公众健康。全世界每年约有1 700万人死于环境中的致病菌引起的传染病,而且这个数字仍在增加[1]。因此,寻找高效、环保的方法来灭活水体中的病原体,已经成为了保护环境和人类健康的迫切任务。光催化是一种将太阳光能转化为化学能的环保方法,在多个领域都有广泛的应用前景[2]。近年来,光催化技术已经被广泛的应用于降解污染物[3]、灭活细菌、处理空气污染物[4]等环境净化方面。其中,光催化灭菌技术是一种绿色、高效的灭菌方法,可以杀死水体中大多数的病原微生物[5]。
氧化铟(In2O3)是p区金属氧化物中唯一对可见光有响应的N型半导体,所以关于In2O3的研究较早[6],因为其具有导电性高、催化活性较强和自由载流子迁移率高等优点,在材料领域,环境领域备受大家的关注[7]。有研究[8-9]表明,有机污染物的光催化分解、太阳能电池和气体感应等。由于In2O3独特的三维构造,立方体结构的In2O3被认为是环境中非常稳定的材料之一[10],立体三维In2O3的六个方形面在各种角度和方向上有足够大的光吸收表面,因此使它适合吸收和转换太阳能。In2O3纳米材料的制备方法虽然很简单,但是其可见光响应较差,带隙能较大导致对光能的利用率很低,只能利用太阳能的少部分波段,这样就对其实际应用产生了很大的限制[11]。
三元金属硫化物(CdIn2S4、ZnIn2S4和CuInS2)具有很强的可见光吸收能力和足够的负导带电位[12],其应用于各种光催化领域中,包括将二氧化碳通过光催化转化为碳氢化合物燃料[13]、制氢[14]、Cr(Ⅵ)光还原[15]和有机物降解[15-16]等。其中硫铟镉(Cdln2S4)因其带隙能较小和光催化性能优异的特点备受关注[17],但存在着易发生光腐蚀的缺点,限制了它的应用[18]。
黑磷(BP)作为一种新型的单元素二维(2D)材料,具有类似于石墨烯的二维片状结构,自2014年以来,引起了大家的研究兴趣[19]。作为一种不含金属的半导体,其在储存太阳能[20]、 降解污染物[21]、和光电器件中有着出色的应用[22]。在光催化方面,由于其具有带隙较窄、强大的可见光和近红外光吸收能力、低毒性和生物相容性等突出的优点,在光催化领域受到极大关注[23],这些特性使BP可以作为一种具有潜力的催化剂,用于水分离和其他光氧化反应[24]。LEE等将BP与TiO2成功复合为纳米催化剂,并应用于降解罗丹明B(RhB)中,这是关于BP光催化性能研究最早的报告[25]。但是,BP还存在着一定的缺点,其应用成本高,单一导体的光催化效率低,所以不适合作为主体材料[26-27]。
光催化材料对有机污染和菌污染处理是一种要求严格的水净化过程,因此,考虑到更大程度地利用太阳能,构筑具有良好光能利用率的异质结构是进一步提高In2O3 纳米材料光催化性能的有效方法。综上所述,本研究利用水热法制备了三元复合材料Cdln2S4/In2O3/BP,考察了其对RhB的降解性能以及E.coli的灭菌性能,并探究了不同掺杂比例的Cdln2S4对光催化性能的影响。同时基于自由基淬灭实验推测出了RhB的降解过程以及E.coli的灭活过程中可能发生的反应机制。本研究可为开发新型高效光催化剂和环保灭菌方法提供参考。
1. 实验部分
1.1 实验试剂
主要试剂包括氯化铟(InCl3),氢氧化钠(NaOH),硫酸铟(In2(SO4)3),LB肉汤,LB营养琼脂,硫酸钡(BaSO4),黑磷晶体(BP),乙二醇((CH2OH)2),氯化镉(CdCl2),硫脲(CS(NH2)2)。无水乙醇(C2H6O)。
1.2 In2O3的制备
称取 InCl3 0.221 g(1 mmol)置入烧杯中,加入10 mL蒸馏水,室温下搅拌至澄清透明,此时缓慢加入NaOH颗粒0.2 g(5 mmol),待搅拌均匀后量取5 mL乙二醇加入烧杯中,搅拌30 min后将反应液加入到聚四氟乙烯内衬的不锈钢反应釜中密封。在 180 ℃下持续反应16 h,自然冷却至室温。过滤后得到白色固体粉末产品,依次用无水乙醇、蒸馏水洗涤几次,60 ℃真空干燥12 h,将干燥后的产物用马弗炉在 500 ℃煅烧3 h后取出,研磨后得到In2O3。
1.3 CdIn2S4的制备
将含水氯化镉(CdCl2),硫酸铟(In2(SO4)3),硫脲(CS(NH2)2)按物质的量配比为1∶1∶4加入到50 mL蒸馏水中搅拌30 min,然后将溶液转移至反应釜内,置于烘箱内,180 ℃恒温反应13 h后,将反应釜自然冷却至室温,弃去上清液,将产物依次用蒸馏水和无水乙醇洗涤数次, 于80 ℃真空干燥24 h, 得到粉末,最后将其放入马弗炉中升温400 ℃反应1 h后降至室温,从而得到CdIn2S4颗粒。
1.4 Cdln2S4/In2O3/BP复合催化剂的制备
将含水氯化镉(CdCl2),硫酸铟(In2(SO4)3),硫脲(CS(NH2)2)按物质的量配比为1∶1∶4加入到45 mL蒸馏水中,通过物质的量计算预计得到的CdIn2S4的质量,然后加入与CdIn2S4质量相应倍数(X=50、20、10)的In2O3。将相应质量比的BP(2%复合催化剂)置于5 mLN-甲基吡咯烷酮(NMP)溶液中,超声30 min,超声结束后倒入上述混合液当中,搅拌300 min,转移至反应釜内,然后将反应釜置于180 ℃的烘箱内,恒温反应13 h后,将反应釜自然冷却至室温,弃去上清液,将产物依次用蒸馏水和无水乙醇洗涤数次,于80 ℃烘箱内干燥13 h,得到粉末,最后将其放入马弗炉中升温400 ℃,加热1 h后取出,等待降至室温,研磨,得到催化剂(2% Cdln2S4/In2O3/BP、5% Cdln2S4/In2O3/BP、10% Cdln2S4/In2O3/BP)。
1.5 样品光催化活性测试
向烧杯中加入50 mL RhB (20 mg·L−1)溶液与25 mg催化剂,实验前在黑暗条件下超声10 min,搅拌20 min,使催化剂均匀分布以建立吸附−脱附平衡,随后进行光照实验。以500W氙灯作为光源,装上滤光片(420 nm),光照开始后每20 min取2.5 mL上层清液,用滤膜(d=45 μm)过滤,利用紫外-可见光分光光度计测试清液在540 nm的吸光度,根据式(1)计算RhB的去除率。
R=(C0−Ct)/C0×100% (1) 式中:R为RhB的去除率,%;C0为初始吸光度;Ct为t时刻清液的吸光度,t为反应时间,min。
1.6 自由基淬灭实验
为了确定在光催化降解体系中参与反应的活性物种,进行了自由基淬灭实验。实验中加入异丙醇(1 mmol·L−1 IPA)、乙二胺四乙酸二钠(1 mmol·L−1DTA-2Na)和抗坏血酸(1 mmol·L−1 AC)等活性物种清除剂,选择性地淬灭体系中产生的羟基自由基(·OH)、空穴(h+ ),超氧自由基(·O2−)。实验步骤和试剂用量与1.5节一致。
1.7 灭菌实验
在进行光催化灭活细菌实验之前,将所需要的仪器以及试剂放入安全柜中,打开紫外灯,设定30 min,通风15 min。取原菌液(1×108 CFU·mL−1)4 µL,加入到3.6 mL已灭菌的氯化钠稀释液中依次稀释,取4 mL 1×107 CFU·mL−1加入到36 mL催化剂溶液中,然后取0 min 样品之后放入光催化系统中。然后开始开灯进行光催化灭活细菌实验。在不同的时间间隔(30、60、90、120 min)进行取样,收集样品用灭菌的氯化钠(NaCl)水溶液连续稀释到1×104 CFU·mL−1。然后将1 mL稀释的样品立即涂抹在营养琼脂平板上,并在 37 ℃恒温培养12 h,以确定存活细胞的数量。使用平板计数法,计算出每个光催化剂的灭菌率并绘制成图。
η=(A0−At)/A0×100% (2) 式中:η为灭菌率,%;A0为未光照菌落数;At为t时刻光照后菌落数,t为反应时间,min。
2. 结果与讨论
2.1 XRD分析
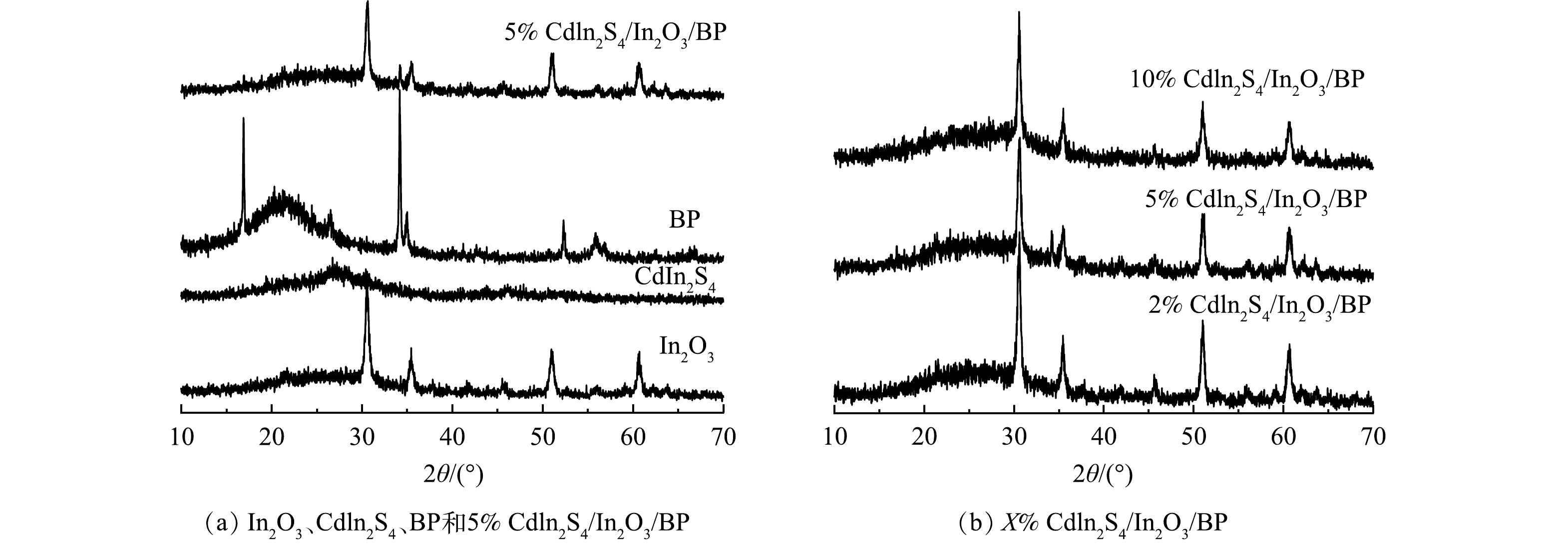
图1为纯In2O3、Cdln2S4、BP以及Cdln2S4/In2O3/BP复合材料的XRD图谱。由图1(a)可见,纯品Cdln2S4的平面2θ在27.2 °和47.4 °分别对应的是(311)和(440)晶面,与标准卡片JCPDS# 27-0060相符合[28]。纯品In2O3在30.46°、37.58°、50.92°、60.9°位置出现的特征峰与卡片库中JCPDS#71-2195号卡片中(222)、(400)、(440)、(622)晶面相对应[41]。纯BP在2θ值为16.9 °、34.2 °、52.3 °、54.6 °出现了特征峰,对应着(020)、(040)、(060)、(042)晶面与标准卡片库中JCPDS#73-1358号卡片一致[29]。制备的5% Cdln2S4/In2O3/BP复合材料与纯品BP、In2O3有着相似的峰形,与纯品In2O3的峰位置一致。此外,分别在34.2 °、27.2 °处出现了特征峰,对应着BP的(040)与Cdln2S4(311)晶面[30-31]。由图1(b)可见,在5% Cdln2S4/In2O3/BP中,在34.2°出现了BP的特征峰。而2% Cdln2S4/In2O3/BP没有出现特征峰的原因可能是BP的掺杂量较少。3种复合材料的特征峰出现位置一致,并且没有出现其他物质的特征衍射峰,证明所制备催化剂的纯度较高。
2.2 FT-IR分析
图2(a)为纯品Cdln2S4、In2O3、BP 和5% Cdln2S4/In2O3/BP 的FT-IR 谱图。可见,所制备的所有样品在1 390 cm−1和1 640 cm−1处均有吸收峰,归因于样品所吸附水分子的弯曲振动峰[32];在3 440 cm−1附近出现的宽吸收带对应的是O—H键的振动[33]。纯品In2O3在540、565和600 cm−1处出现了3个尖锐的吸收峰,与文献中In2O3的出峰位置一致[34],可归因为In—O键的声子振动,同时也说明了In2O3以立方体的形式存在[35]。纯品Cdln2S4在1 100 cm−1附近出现了1个较宽的吸收带,对应的是In-S键的伸缩振动峰[36]。纯品BP在1 006 cm−1和1 160 cm−1 处出现了特征峰,与文献中的出峰位置一致[37]。5%Cdln2S4/In2O3/BP复合材料具备上述3种单品材料的特征峰,均有In2O3、Cdln2S4、BP 3种典型的振动模式。图2(b)为3种不同掺杂比例的Cdln2S4所制备催化剂的红外FT-IR 谱图。由图2(b)可以发现,3种样品出现特征峰的位置相同,此外,883 cm−1和2 970 cm−1处的特征峰峰值,随着Cdln2S4的掺杂量的增加而逐渐增大。
2.3 SEM分析
图3为纯品CdIn2S4、In2O3以及5% CdIn2S4/In2O3和5% CdIn2S4/In2O3/BP的SEM微观结构表征图。由图3可以看出,纯品CdIn2S4整体呈现出直径约为1~2 µm的纳米花球状结构,且可以看到花球是由多个类似的纳米片组合而成的,其表面可以看到很多的空隙。这种结构可以增大材料的表面积,有利于和其他催化剂的复合[38](图3(a))。由图3(b) 可以看出纯品In2O3为40~80 nm的立方体结构。由图3(c)可以看出,CdIn2S4的纳米花球附着在由In2O3立方体结构组成的平面上,同时,花球上的空隙被In2O3颗粒填满。由图3(d)可以清楚看到CdIn2S4/In2O3附着在黑磷基底上,复合催化剂表现出了CdIn2S4和In2O3共同的优点,结构均匀且纳米片更小。结合图3 (a)~(d)综合分析可以得出结论: 5% CdIn2S4/In2O3/BP改变了3种单品的形貌,催化剂比表面积较大,导致其与反应物分子接触面积就更大,从而可提高复合材料的光催化活性。
2.4 TEM分析
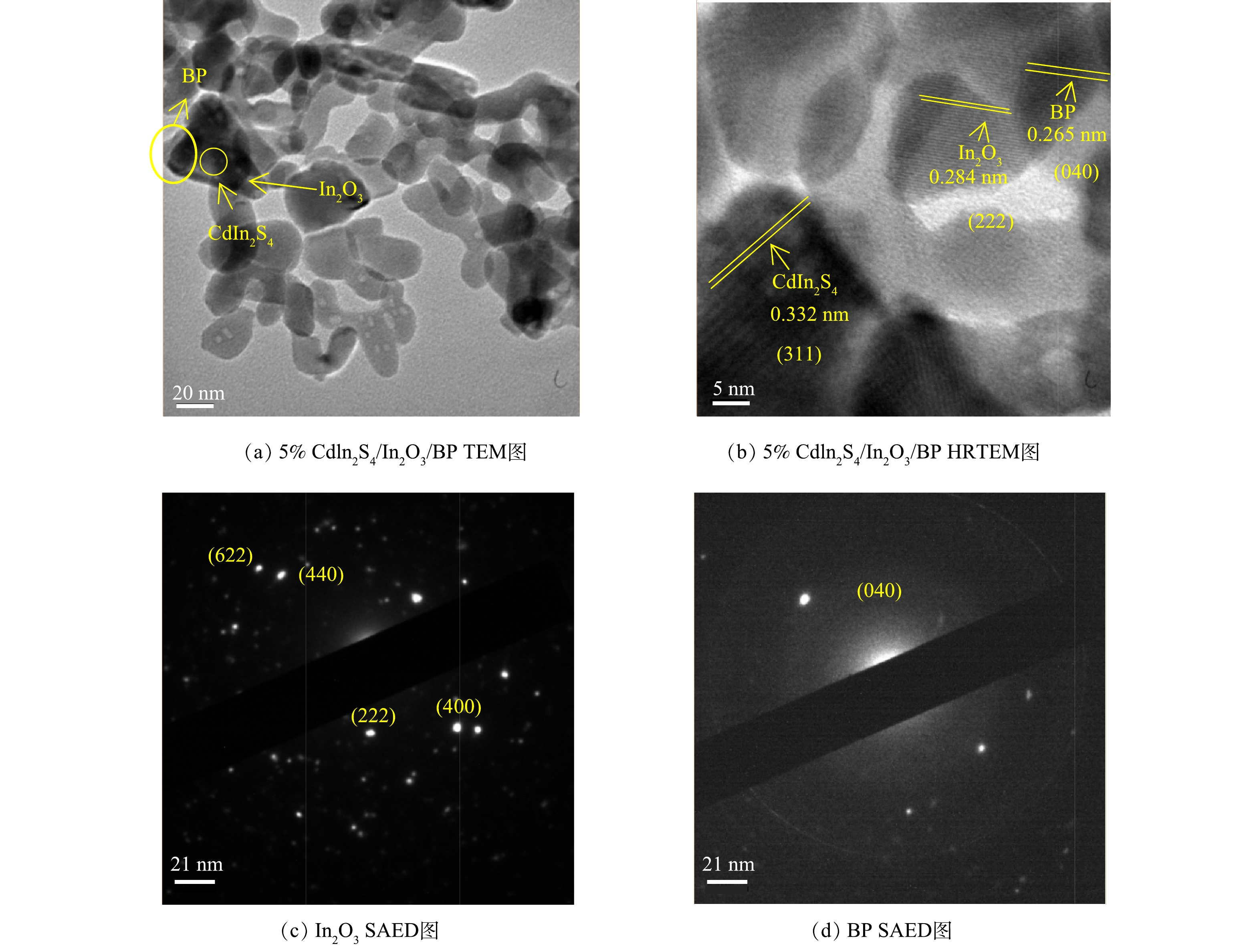
由图4(a)中可以观察到3种物质。图像中黑色的阴影部分为黑磷,球状的CdIn2S4大量附着在In2O3上,复合后的CdIn2S4/In2O3一部分沉积在黑磷上。这说明该复合催化剂Z型异质结构建成功。由图4(b)可以清楚的观察到催化剂表面条纹清晰的晶格,晶格条纹间距为0.284 nm对应的是In2O3的(222)晶面[27],晶格条纹间距为0.332 nm对应为CdIn2S4的(311)晶面[28],晶格条纹间距为0.265 nm对应BP的(040)晶面。这与文献中的研究结果一致[39]。由图4(c)中可见In2O3的立方体结构[28]。这与SEM和FT-IR的表征结果一致。由图4(d)可见,SAED测得的晶面与标准卡片JCPDS#73-1358一致[40]。
2.5 XPS分析
为了获取复合材料表面各元素的化学形态,对5% CdIn2S4/In2O3/BP以及5% CdIn2S4/In2O3复合材料进行了XPS分析,结果如图5所示。图5(a)可以看出,2种复合材料的全谱中主要存在着Cd、In、S、O、C、P6种元素,没有其他元素存在,说明2种复合材料纯度较高。在284.65 eV出现的C1s峰,归因于催化剂表面吸附了CO2[41]。与5% CdIn2S4/In2O3相比,5% CdIn2S4/In2O3/BP在133.7 eV处出现了新的衍射峰,经过数据库和文献查证是P元素的峰[42],证明三元复合催化剂中有BP的存在。图5(b)中上曲线结合能位于168.9 eV处的衍射峰对应着S2p1/2,下曲线在167.6 eV处和169 eV处的衍射峰对应着S2p3/2和S2p1/2,表明S以S2-形式存在[43]。由图5(c)可见,电子结合能于444.2 eV和451.8 eV处的2个对称峰对应于In3d2/5和In3d2/3,结合能差值为7.6 eV。这说明In离子以In3+形式存在,与文献中一致[44]。图5(c)中2种复合材料的衍射峰位置基本一致[35]。图5(d)为O1s谱图,分别在529.6 eV和532.1 eV处出现了衍射峰,529.6 eV处的峰为化学吸附水产生的衍射峰,532.1 eV处的峰归因于O原子和In原子的晶格振动。此结果说明形成In-O键,表明了存在正二价的O[45]。图5(e)中的两种复合材料在结合能位于405 eV和406.1 eV处的峰对应于Cd3d5/2,根据文献和数据库查证,说明Cd以Cd2+形式存在于CdIn2S4化合物中[38]。与5% CdIn2S4/In2O3对比,5% CdIn2S4/In2O3/BP的S2p、In3d、Cd3d的结合能均发生了偏移,说明S、In、Cd元素的化学环境发生了变化[46]。结合能的变化进一步证实了Z型异质结构的存在,而且结合能发生的偏移也有助于电子的快速输运[18]。
2.6 DRS分析
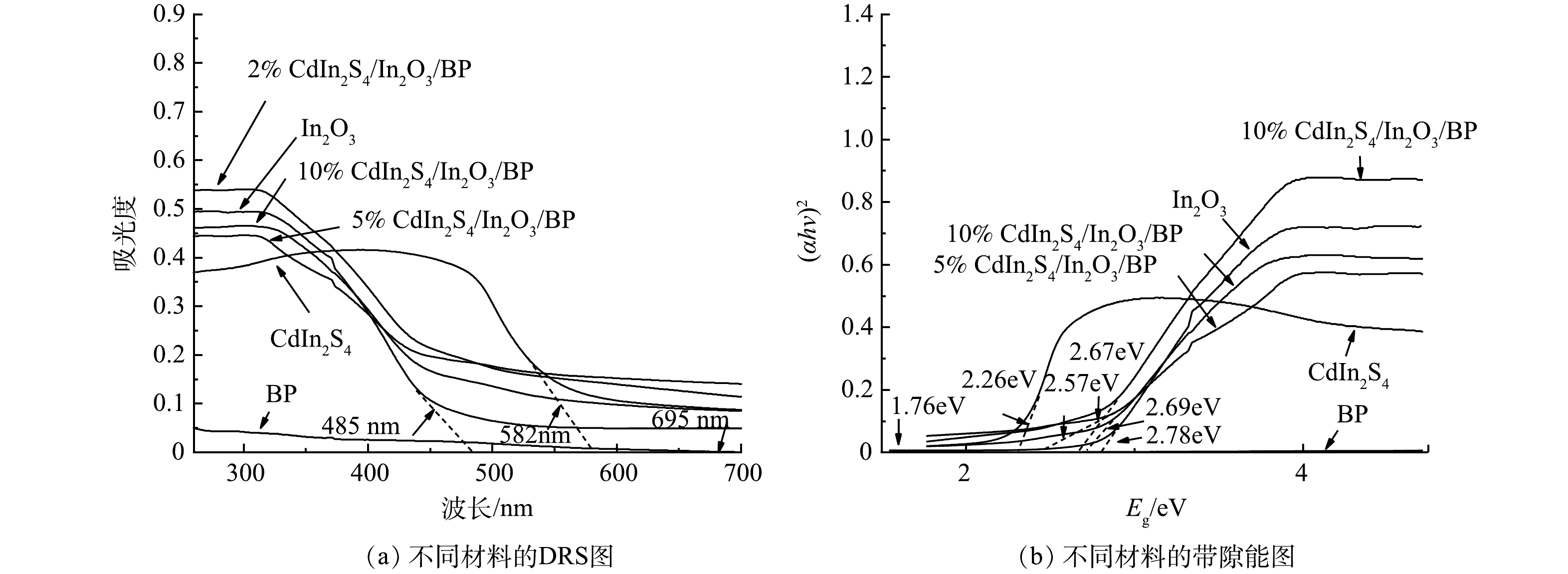
利用紫外-可见漫反射光谱法(DRS)测得纯品Cdln2S4、In2O3、BP以及Cdln2S4/In2O3/BP的光吸收性能。图6(a)中纯In2O3的吸收带边在485 nm左右,Cdln2S4的最大吸收带边在582 nm左右,而BP的最大吸附边缘在695 nm左右。说明BP对可见光有良好的吸收,这可能是其本身内部带隙较窄的原因[47]。将3种物质复合以后,Cdln2S4/In2O3/BP的吸收边长分别达到了512、542、502 nm。3种复合材料均发生了不同程度的红移现象,且复合材料对可见光的吸附能力均优于纯品In2O3。这说明Cdln2S4和BP的引入成功拓宽了In2O3的光吸收范围。由图6(a)中可以看到,Cdln2S4掺杂比例在5%时达到了最大的吸收带边,当Cdln2S4掺杂比例大于5%时,复合材料的吸收带边变小,说明5%Cdln2S4/In2O3/BP的光吸收性能最佳。以测得的DRS数据为基础,根据Kubelka–Munk(式(3))求得复合材料的带隙能。
(αhv)n=k(hv−Eg) (3) 式中:α是吸收系数,k是常数,hv是光子能量,Eg是带隙吸收能,n是常数(直接半导体n=2,间接半导体n=1/2,对于Cdln2S4/In2O3/BP复合材料,n=2)。由图6 (b)可见,纯品Cdln2S4、In2O3、BP的带隙能值分别是2.26、2.78、1.76 eV,与之前的文献相符合。2%Cdln2S4/In2O3/BP为2.67 eV,5%Cdln2S4/In2O3/BP为2.57 eV,10% Cdln2S4/In2O3/BP为2.69 eV。
为进一步探讨光催化灭菌的反应机理,用Mulliken电负性理论并根据式(4)分别对Cdln2S4、In2O3和BP 的价带和导带位置进行计算。
EVB=X−Ee+0.5Eg (4) ECB=EVB−Eg (5) 式中:X是半导体的电负性(组成原子绝对电负性的几何平均值),X(Cdln2S4)=4.84,X(In2O3)=5.28,X(BP)=5.62; Ee是以氢为基准自由电子能(Ee=4.5eV)半导体的带隙能;EVB、ECB分别是价带和导带的边缘电位。通过计算得到Cdln2S4的价带和导带电位分别为+1.47 eV和−0.79 eV,In2O3的价带和导带电位分别为+2.17 eV和−0.61 eV,BP的价带和导带电位分别为+2.0 eV和+0.24 eV。
2.7 罗丹明B的光降解性能
如图7所示,在纯品CdIn2S4、In2O3、BP的存在下对罗丹明B的降解率分别为61.3% 、57%、54.7%,说明单品的催化剂有着一定的光催化活性。而复合之后的催化剂在氙灯照射下对于罗丹明B的脱色有显著提升,证明复合材料的光催化活性有所提升。当掺杂了不同比例的CdIn2S4时,所制得的复合材料与纯品催化剂相比,对于染料溶液的降解率都有提高,2% Cdln2S4/In2O3/BP、5% Cdln2S4/In2O3/BP、10% Cdln2S4/In2O3/BP对罗丹明B的降解率分别达到了87.9%、94.7%、 81%。其中,对罗丹明B降解率最高的是5% Cdln2S4/In2O3/BP,与纯品CdIn2S4、In2O3、BP相比,降解率分别提高了33.4%、37.7%、40%。光降解实验结果与DRS表征数据一致:5% Cdln2S4/In2O3/BP光催化剂的带隙能值最小,光催化性能最好。
2.8 活性物种分析
为了进一步研究光生载流子及活性基团在光催化降解实验中的作用,所以进行了自由基淬灭实验,结果如图8所示。当IPA加入后,对罗丹明B的降解率略有下降,由94.7%下降到了90.2%;而在添加EDTA-2Na后,复合材料对罗丹明B的降解率为84.3%;在反应体系中加入AC后,降解率为40.3%。这说明·OH、h+、·O2−这3种活性物质都参与了光催化降解罗丹明B的过程,其中·O2−是参与反应主要的活性物质。根据上述结果可知,5% Cdln2S4/In2O3/BP在光催化降解反应体系中,可生成·OH、·O2−、h+等活性物种,其中·O2−在反应过程中起主导作用,在降解反应中贡献排序为·O2− >h+>·OH。
2.9 光催化灭菌性能
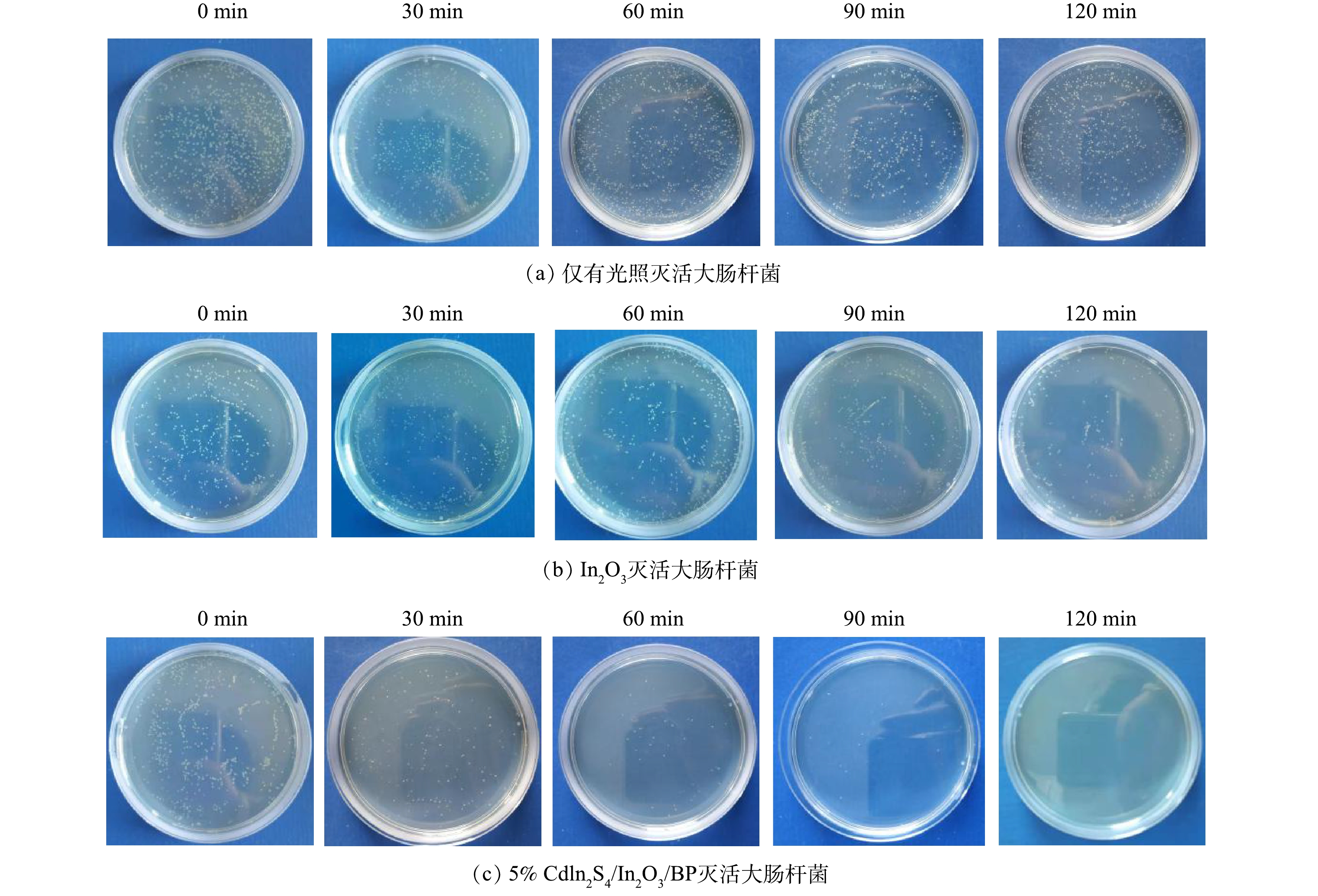
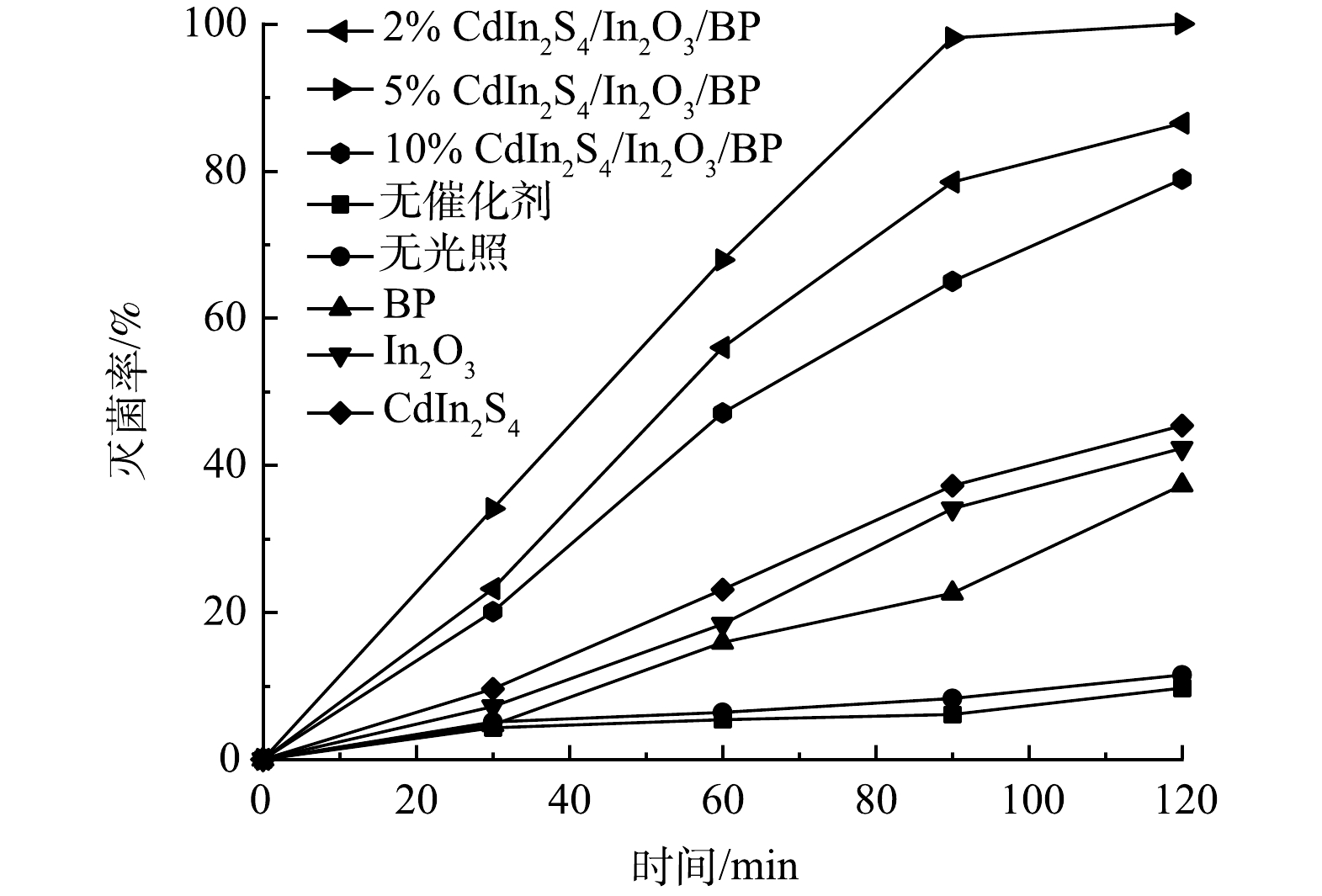
为了考察所制备光催化剂的灭菌活性,通过光催化灭活E.coli实验分别对纯品Cdln2S4、In2O3、BP以及X% Cdln2S4/In2O3/BP的灭菌能力作出分析。将相同浓度的E.coli放入相同浓度(0.5 g·L−1)的抗菌剂中并在氙灯下照射120 min,采用稀释平板法计数评价合成光催化材料的灭菌效果,具体实验结果见图9和图10。
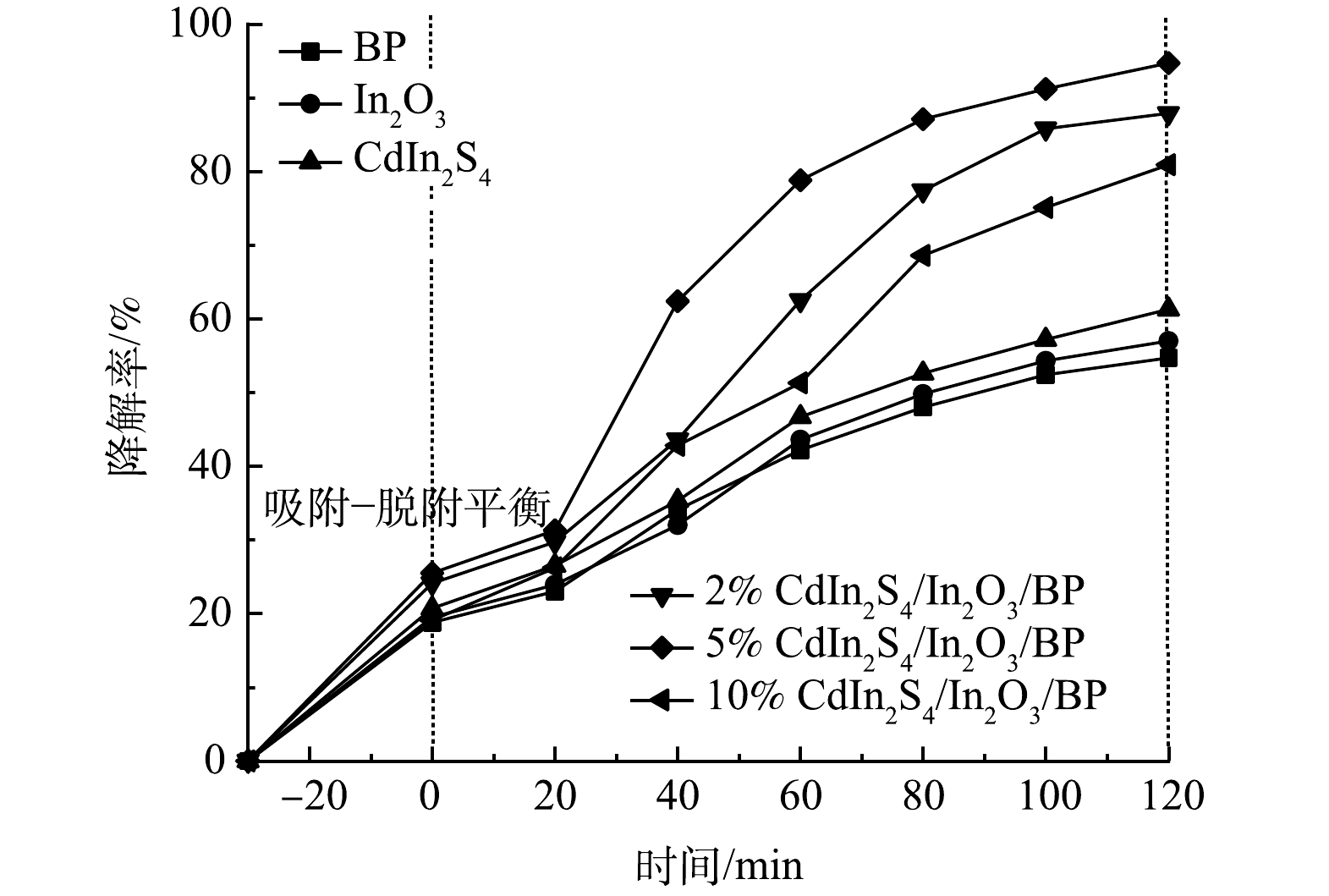
如图10所示,无光照有催化剂和有光照无催化剂的对照组对E.coli的灭菌率较低,反应120 min后,分别达到了11.5%和9.7%。这表明仅有光照射或仅有催化剂的灭菌效果均较差。光照120 min后,纯品Cdln2S4、In2O3和BP对大肠杆菌的灭菌率分别是45.4%、42.3%、37.3%,而掺杂不同比例Cdln2S4的Cdln2S4/In2O3/BP复合材料在光照条件下,对E.coli有更高的灭菌率,且灭菌率随着光照时间的增加而逐渐提高。复合催化剂与纯品催化剂相比,灭菌率均有很大提升。结合表征数据,这可能归因于Cdln2S4与In2O3和BP复合后,提高了催化剂的光催化灭菌性能。由图10可以看到,5% Cdln2S4/In2O3/BP具有最佳的灭菌能力,光照120 min后灭菌率达到了100%,相较于纯品In2O3提高了57.7%。但随着Cdln2S4掺杂量的进一步增加,光催化灭菌效果反而下降,所以5%的Cdln2S4为最佳掺杂比例。这与之前的表征实验和光催化降解罗丹明B的实验结果一致。
2.10 反应机理分析
根据上述实验分析的结果并结合以往报道的文献,推测了Cdln2S4/In2O3/BP复合材料降解罗丹明B以及灭活E.coli的反应机理图(图11)。从2.6得出了图中导带和价带的位置,推测CdIn2S4、In2O3、BP之间存在着Ⅱ型异质结或Z型异质结两种相互作用机制。如果为Ⅱ型异质结构,In2O3和BP价带上的h+倾向于迁移到CdIn2S4价带上,CdIn2S4价带位置(1.47 eV)要低于OH−(1.99 eV)或者H2O(2.68 eV),这使得CdIn2S4价带上积聚的h+无法与吸附在表面的OH−或者H2O分子氧化成·OH,但根据自由基捕获实验结果证明复合催化剂在光降解体系中可以产生·OH,所以为Ⅱ型异质结构的假设是错误的。因此,推测Cdln2S4/In2O3/BP复合材料的结构为双重Z型异质结,在可见光的照射下,Cdln2S4/In2O3/BP受到激发,In2O3和BP导带上的e−会迅速的向Cdln2S4价带迁移与Cdln2S4价带上产生的光诱导空穴重合,这一过程抑制CdIn2S4、In2O3、BP光生载流子的重组。停留在Cdln2S4导带上的e−具有更强的还原能力,因为Cdln2S4导带的位置(−0.79 eV)比O2(−0.046 eV)更负,所以在可见光的激发下,Cdln2S4导带上的e−会转移到催化剂的表面上,并与溶液中的O2发生反应,生成·O2−。停留在In2O3和BP价带上的h+处于低能级,其具有很强的氧化能力,In2O3和BP的价带位置分别为2.17 eV和2.0 eV,要高于OH−(1.99 eV),可以与OH−发生反应生成·OH,这一假设结果与淬灭实验结果一致,因此,Cdln2S4/In2O3/BP为双重Z型异质结构。生成的活性氧物种(·OH、·O2−)可以加快对RhB的降解,也可以氧化E.coli中的辅酶,从而抑制辅酶A在E.coli中的作用,降低了E.coli内蛋白质与糖类的正常代谢,从而导致E.coli死亡[48]。
3. 结论
1)将In2O3、Cdln2S4和BP复合得到复合材料Cdln2S4/In2O3/BP,通过表征可知Cdln2S4/In2O3/BP能够被可见光激发,光催化性能得到了增强,制备的纳米花球状的Cdln2S4和立方体In2O3成功结合并附着在黑磷基底上,CdIn2S4、In2O3、BP形成了双重Z型异质结结构。
2) 5% Cdln2S4/In2O3/BP光催化剂对RhB的降解率最高,达到了94.7%,与纯品Cdln2S4、In2O3、BP相比,降解率分别提高了33.4%、37.7%、40%;5% Cdln2S4/In2O3/BP光催化剂对E.coli的灭菌率也达到最高,为100%。
3) CdIn2S4/In2O3/BP复合催化剂在可见光激发下生成的e−与溶液中的氧分子发生反应,得到的·O2−为降解实验和灭菌实验中起主要作用的活性物质。
-
表 1 实验药剂信息
Table 1. Information of experimental agents
序号 名称 种类 化学式 纯度 稳定机理 厂家 1 CTS 有机 (C6H11NO4)N 化学纯 羟基、氨基等高分子基团与重金属离子螯合配位 Adamas 2 NaBent 无机 Nax(H2O)4(Al2-xMg0.83) (Si4O10) (OH)2 分析纯 Na+、Al2+、Mg2+等离子与重金属离子发生离子交换反应 Adamas -
[1] PENG J, SONG Y, YUAN P, et al. The remediation of heavy metals contaminated sediment[J]. Journal of Hazardous Materials, 2009, 161(2/3): 633-640. [2] SUNDARAY S K, NAYAK B B, LIN S, et al. Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments: A case study: Mahanadi basin, India[J]. Journal of Hazardous Materials, 2011, 186(2/3): 1837-1846. [3] WANG Y, YANG L, KONG L, et al. Spatial distribution, ecological risk assessment and source identification for heavy metals in surface sediments from Dongping Lake, Shandong, East China[J]. Catena, 2015, 125: 200-205. doi: 10.1016/j.catena.2014.10.023 [4] CHIANG Y W, SANTOS R M, GHYSELBRECHT K, et al. Strategic selection of an optimal sorbent mixture for in-situ remediation of heavy metal contaminated sediments: framework and case study[J]. Journal of Environmental Management, 2012, 105: 1-11. [5] SONG Z, GAO H, ZHANG W, et al. Influence of flocculation conditioning on environmental risk of heavy metals in dredged sediment[J]. Journal of Environmental Management, 2021, 297: 113-313. [6] AKCIL A, ERUST C, OZDEMIROGLU S, et al. A review of approaches and techniques used in aquatic contaminated sediments: Metal removal and stabilization by chemical and biotechnological processes[J]. Journal of Cleaner Production, 2015, 86: 24-36. doi: 10.1016/j.jclepro.2014.08.009 [7] BAO J, WANG L, XIAO M. Changes in speciation and leaching behaviors of heavy metals in dredged sediment solidified/stabilized with various materials[J]. Environmental Science and Pollution Research, 2016, 23(9): 8294-8301. doi: 10.1007/s11356-016-6184-5 [8] 王锋, 张顺力, 王宏杰, 等. 河道污染底泥重金属稳定化药剂研究进展[J]. 净水技术, 2020, 39(7): 92-100. doi: 10.15890/j.cnki.jsjs.2020.07.016 [9] JOTHIRAMALINGAM R, LO S L, PHANTHI L A. Chitosan-type bioadditive-modified electronic industry waste sludge for heavy metal stabilization with assistance of microwave heating[J]. Industrial & Engineering Chemistry Research, 2010, 49(6): 2557-2561. [10] 宋俊颖, 何绪文, 黄占斌. 壳聚糖及其衍生物对土壤重金属的稳定化效应[J]. 化工进展, 2019, 38(9): 4308-4319. doi: 10.16085/j.issn.1000-6613.2018-2296 [11] YAN H, LIN G. Usage of chitosan on the complexation of heavy metal contents and vertical distribution of Hg(II) and Cr(VI) in different textural artificially contaminated soils[J]. Environmental Earth Sciences, 2015, 73(5): 2483-2488. doi: 10.1007/s12665-014-3599-5 [12] 姚乐. 改性膨润土吸附处理含砷废水实验研究[J]. 科学技术与工程, 2010(16): 4093-4095. doi: 10.3969/j.issn.1671-1815.2010.16.070 [13] 杨秀敏, 胡振琪, 李宁, 等. 钠基膨润土对重金属离子Cu2+、Zn2+、Cd2+的吸附实验[J]. 煤炭学报, 2009, 34(6): 819-822. doi: 10.3321/j.issn:0253-9993.2009.06.021 [14] 张海军, 于颖, 倪余文, 等. 采用巯基捕收剂稳定化处理垃圾焚烧飞灰中的重金属[J]. 环境科学, 2007, 28(8): 1899-1904. doi: 10.3321/j.issn:0250-3301.2007.08.044 [15] YANG K, WANG G, LIU F, et al. Removal of multiple heavy metal ions using a macromolecule chelating flocculant xanthated chitosan[J]. Water Science and Technology, 2019, 79(12): 2289-2297. doi: 10.2166/wst.2019.230 [16] 杨秀敏, 钟子楠, 潘宇, 等. 重金属离子在钠基膨润土中的吸附特征与机理[J]. 环境工程学报, 2013, 7(7): 2775-2780. [17] SAVITRI E, BUDHYANTORO A. The effect of ratio chitosan-bentonite and processing time on the characterization of chitosan-bentonite composite[J]. Materials Science and Engineering, 2017, 223(1): 161-172. [18] 黄春桃, 刘国光, 姚琨, 等. 壳聚糖-膨润土复合材料的研究进展[J]. 广东农业科学, 2012, 39(8): 161-164. doi: 10.3969/j.issn.1004-874X.2012.08.050 [19] KUMARARAJA P, MANIAIAH K M, DATTA S C, et al. Chitosan-g-poly (acrylic acid)-bentonite composite: A potential immobilizing agent of heavy metals in soil[J]. Cellulose, 2018, 25(7): 3985-3999. doi: 10.1007/s10570-018-1828-x [20] 苗远, 张超, 李本高. 利用螯合剂脱除生化剩余污泥重金属研究[J]. 工业用水与废水, 2018, 49(6): 84-88. doi: 10.3969/j.issn.1009-2455.2018.06.021 [21] 蒋玉广, 袁珊珊, 杨伟, 等. ES稳定重金属污染底泥效果[J]. 环境工程学报, 2015, 9(9): 4376-4384. doi: 10.12030/j.cjee.20150945 -




 下载:
下载: