-
挥发性有机物 (volatile organic compounds,VOCs) 是PM2.5和O3的重要前体物,因此,成为影响大气环境的主要污染物及防控防治的重点[1-2]。焦化行业是重要的VOCs排放源,其排放呈现点位多、成分复杂、浓度不一的特征,且往往混杂多种类型污染物,治理较为困难。以焦化废水VOCs排放为代表,其来源覆盖焦化生产所有用水环节,通过无组织形式逸散的VOCs废气浓度低、含湿量高、成分复杂,往往存在含硫的硫醇、硫醚等小分子VOCs组分[3]。针对焦化行业复杂的VOCs排放体系,吸附浓缩+后处理的组合工艺逐渐成为主流技术之一。在常见吸附剂中,分子筛具备比表面积大、孔道结构均一、孔容高、热稳定性好等优点,已成为现阶段工业VOCs吸附浓缩处理领域的研究热点[4-7]。
目前,有关分子筛吸附VOCs的报道集中于合成方法、吸附机理和控制吸附过程的关键因素等方面。现有研究表明,分子筛孔道尺寸与VOCs分子大小接近时具有较好的吸附性能[8],Y型分子筛的孔隙结构适合苯系物的吸附净化[9],但Y型分子筛硅铝比低,亲水性强,在废气湿度较高时水分子具有竞争吸附作用,对有机物的吸附能力较低[10-12]。而常规高硅分子筛 (如USY分子筛) 抗水性能强,但由于孔道尺寸大,吸附脱除小分子含硫VOCs效率不高。因此,从工业适用性出发,含湿条件下分子筛对小分子含硫VOCs (如硫醇、硫醚等) 的吸附性能仍有待提高。
对分子筛进行离子交换改性后,通过金属离子与含硫VOCs中S原子的相互作用,可提高吸附材料对含硫VOCs的吸附性能。改性分子筛常见应用于石油化工生产的燃料油和重烃液相中含硫化合物的脱除[13-15]。交换离子可通过π络合吸附或S-M机理作用吸附硫化物分子[16-18],从而对含硫化合物进行脱除。而对分子筛在管道天然气吸附脱硫的应用研究表明,不同交换离子对分子筛脱硫性能的影响不同,部分离子交换分子筛能显著提升气相有机硫的脱除性能[19-22]。但目前对于分子筛吸附脱除气相含硫VOCs的研究相对较少,且水分对于气相含硫VOCs吸附的影响尚无报道。而实际上焦化VOCs废气往往含有一定湿度,且组分复杂,主要含有苯、甲苯、二甲苯等苯系物和硫醇、硫醚等含硫有机物。
基于此,本研究选取不同金属离子 (Ag、Cu、Mg、Zn、Ce、Ca等) ,采用液相离子交换法对NaY和USY-3分子筛进行改性,并以二甲基硫醚 (DMS) 和对二甲苯 (PX) 分别作为小分子含硫VOCs和普通VOCs模型物,考察改性分子筛在对含硫VOCs的吸脱附性能及循环再生性能,并研究多组份VOCs的竞争吸附,以探究其在含湿条件下吸附含硫VOCs的应用可能性。
-
1) 材料。沸石分子筛包括NaY分子筛 (卓然环保科技(大连)有限公司) 和USY-3分子筛 (卓然环保科技(大连)有限公司) 2种。其他材料有高纯N2、高纯O2、二甲基硫醚 (DMS) 、对二甲苯 (PX) 。
2) 试剂。氢氧化钠 (NaOH,98%,AR) 和硝酸铜 (Cu(NO3)2,99.99%) 购于上海麦克林生化科技有限公司。四基氢氧化铵 (C12H29NO,50% in H2O) 、硝酸银 (AgNO3,99.8%,AR) 、乙酸镁 (C4H6O4Mg,99.99%) 、六水合硝酸锌 (Zn(NO3)2∙6H2O,99%,AR) 、六水合硝酸铈 (Ce(NO3)3∙6H2O,99.95%) 、二水合硝酸钙 (Ca(NO3)2∙2H2O,99%,AR) 均购于阿拉丁试剂 (上海) 有限公司。
-
采用液相离子交换法对分子筛进行改性,制备离子交换分子筛。以硝酸铜溶液处理NaY分子筛为例:取一定量分子筛,加入0.5 mol·L−1硝酸铜溶液中 (每克分子筛加20 mL溶液) ,在80 ℃水浴条件下持续搅拌交换24 h。然后用去离子水洗涤、过滤,并在110 ℃下干燥12 h。干燥产物在500 ℃煅烧4 h获得样品,所得材料记为CuY。同理,Ag、Mg、Zn、Ce、Ca交换样品依次记为AgY、MgY、ZnY、CeY、CaY;USY-3分子筛交换样品记为xUSY-3 (x为交换离子) 。
-
采用X射线衍射仪 (X'Pert PRO MPD,PANalytical) 对分子筛的晶体结构进行表征,分子筛的孔径分布信息采用物理吸附仪 (Autosorb iQ,Quanta Chrome) 进行表征。采用X射线荧光光谱仪 (ZSX 100e,Rigaku) 、电感耦合等离子体发射光谱仪 (Optima 5300DV,PerkinElmer) 对分子筛Si/Al比进行分析测定。采用X射线光电子能谱仪 (ESCAlab 250,Thermo) 对分子筛元素价态进行分析。采用扫描电镜 (S-4800 Hitachi,Japan) 和透射电镜 (Tecnai G2 F20,FEI) 对分子筛的微观形貌进行分析。
-
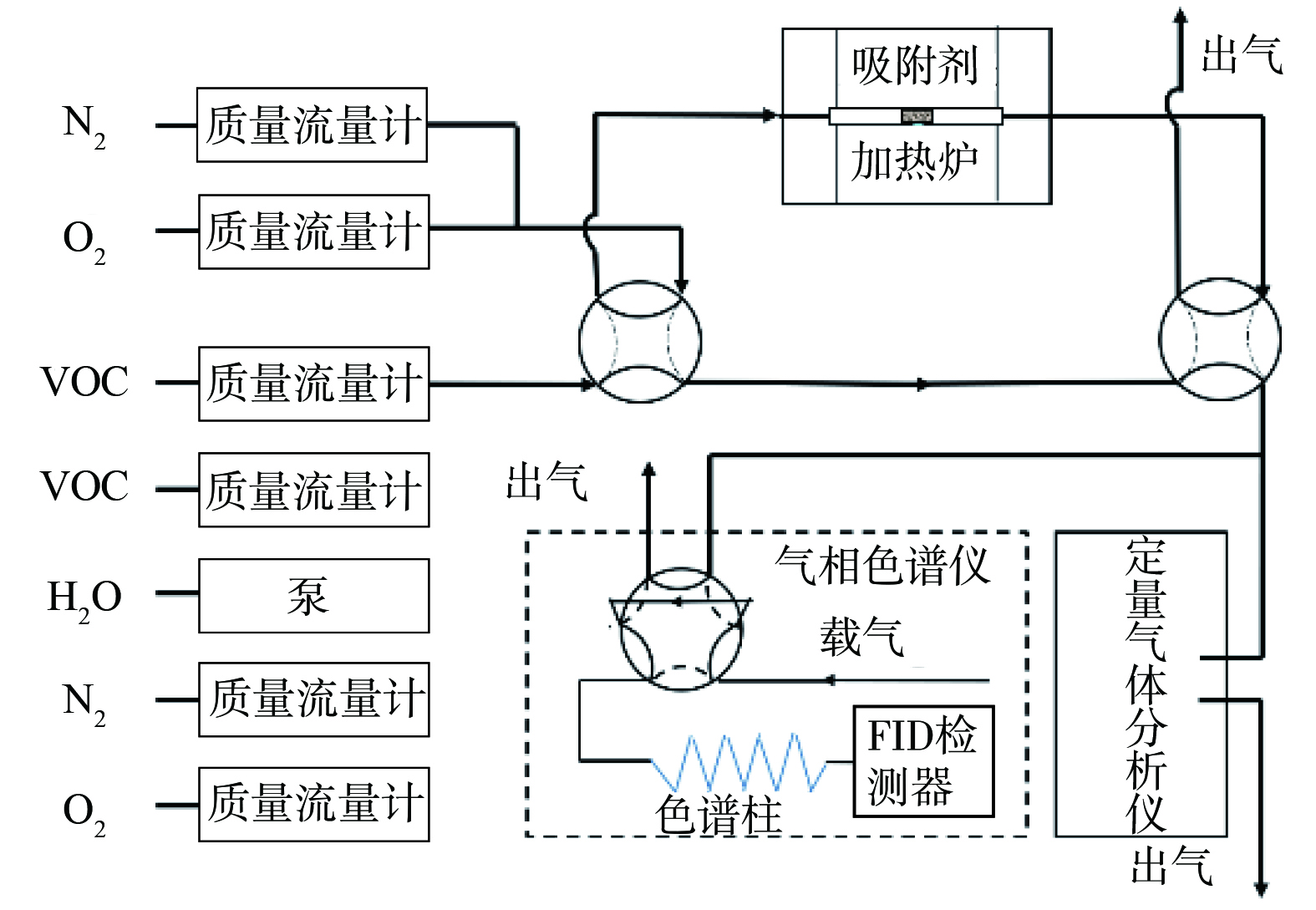
1) 分子筛吸附性能测定实验方法。采用固定床—气相色谱评价装置测定分子筛吸附性能,实验装置如图1所示。
吸附过程中将100 mg吸附剂 (40-60目) 装入石英反应管 (内径4 mm) 中,在300 ℃N2气氛下预处理1 h,以去除水和其他吸附杂质。吸附实验在35 ℃下进行,N2、O2、VOCs为钢瓶气,H2O采用注射泵和加热炉发生,并通过N2吹入气体管路,采用质量流量计对各路气体进行精确控制和调节 (控制精度满足设定值的±0.35%) ,气体组成 (体积分数) 为:0.005% VOC+20% O2+N2 (平衡气) ,保持通入气体总流量为100 mL·min−1,体系中需通入水蒸气时,体积分数为1.5%。用气相色谱仪 (GC7890A,Agilent) 和定量气体分析仪 (Hiden QGA,北京英格海德分析技术有限公司) 在线检测出口VOCs质量浓度,获得穿透吸附曲线。吸附剂的VOCs吸附容量计算如下:
式中:q为分子筛的吸附量,mg·g−1;F为气体流速,mL·min−1;m为吸附剂用量,g;t为吸附时间,min;C0为入口VOCs质量浓度,mg·m−3;Ct为吸附t min后出口VOCs质量浓度,mg·m−3;ts为Ct达到某一特定值所需的时间,min。特定地,当Ct降至C0的10%时,定义此时达到穿透吸附;当Ct稳定且等于C0时,定义此时达到饱和吸附。
2) 分子筛VOCs脱附及分子筛循环再生性能实验。分子筛样品用量取5~10 mg,分别在N2气氛和N2+O2气氛下 (气体总流量均为50 mL·min−1,需要通入O2时,设定其体积分数为20%) ,对VOCs吸附饱和的吸附剂进行程序升温脱附 (30~800 ℃,升温速率为10 ℃·min−1) ,获得TG曲线,以TG一阶导绘制样品的DTG曲线,评价吸附剂脱附性能。此外,在固定床反应器中对VOCs吸附饱和的分子筛进行程序升温脱附 (0~600 ℃,升温速率10 ℃·min−1) ,通过质谱在线检测分析吸附剂热脱附产物。同时,为探究吸附剂的循环再生吸附性能,对VOCs吸附饱和吸附剂在300 ℃空气吹扫下热再生,对吸附剂进行多次吸附再生循环实验。通过气相色谱在线检测计算每次再生吸附量,以评价分子筛吸附的稳定性。
-
1) 孔结构形貌表征。取50~100 mg待测粉末样品置于脱气管中,在300 ℃下真空脱气3 h,然后在-186 ℃ (87 K) 条件下进行Ar吸附/脱附测试。用Brunauer-Emmett-Teller (BET) 方法由吸附等温线计算样品总比表面积 (Vtotal) 。通过t-plot法计算样品的微孔表面积 (Smicro) 和微孔体积 (Vmicro) ,Stotal减去Smicro是介孔表面积 (Smeso) 。样品的总孔体积 (Vpore) 和介孔体积 (Vmeso) 通过脱附等温线使用Barrett-Joyner-Halenda (BJH) 方法测定。测试结果如表1所示。经离子交换后,所有改性NaY、USY-3分子筛的Si/Al均未发生明显变化,但总比表面积、孔体积均减小,分子筛微孔面积及体积明显降低,尤其是AgY分子 (微孔面积806.1 m2·g−1降至550.8 m2·g−1) 和AgUSY-3 (微孔面积由731.6 m2·g−1降至611.7 m2·g−1) 。这说明离子交换改性在一定程度上对微孔结构造成破坏。
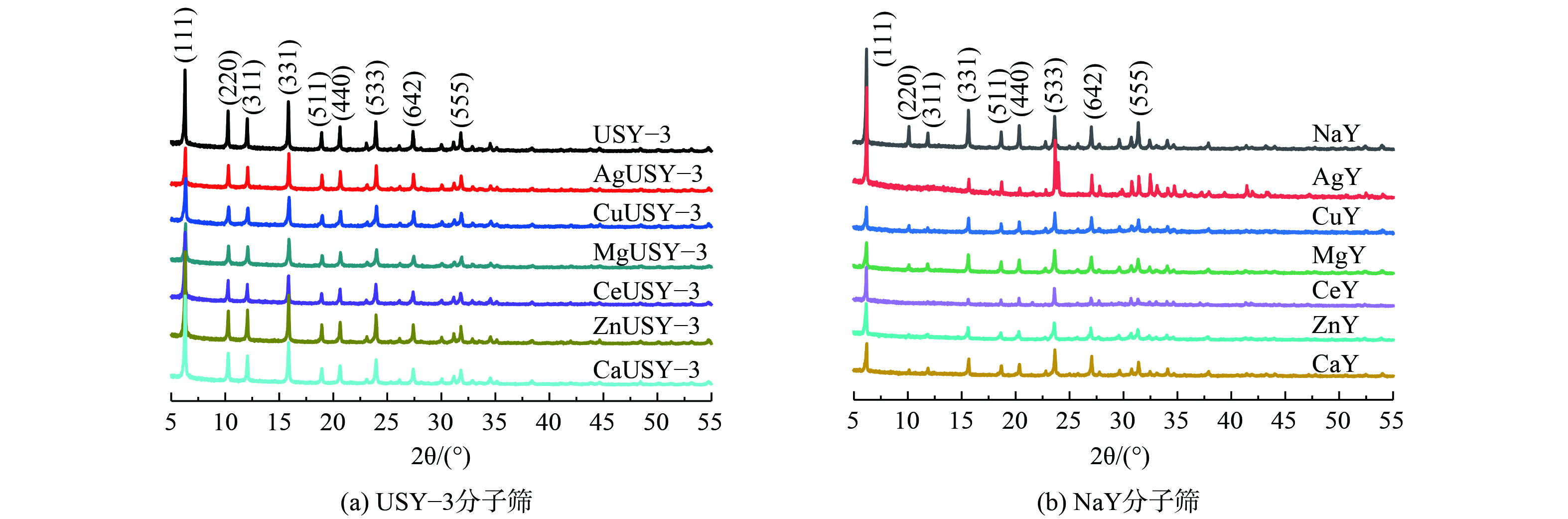
2) XRD表征。离子交换改性前后分子筛的XRD结果如图2所示。USY-3及NaY分子筛特征峰对应的 (111) 、 (220) 、 (311) 、 (331) 、 (533) 、 (642) 等特征晶面,均可归属于FAU型分子筛的典型晶体衍射峰[23-25]。不同离子交换后,USY-3分子筛 (图2(a)) 特征峰的强度变化较小,这说明分子筛的结晶度及晶体结构稳定性基本未发生太大变化。除AgY分子筛外,不同离子交换的NaY分子筛特征峰强度均有稍微下降 (图2(b)) 。这说明离子交换在一定程度上降低了结晶度,这可能是由于NaY分子筛离子交换容量大于USY-3分子筛,其离子交换程度大,对晶体结构影响更大,但其晶体结构仍保持稳定。
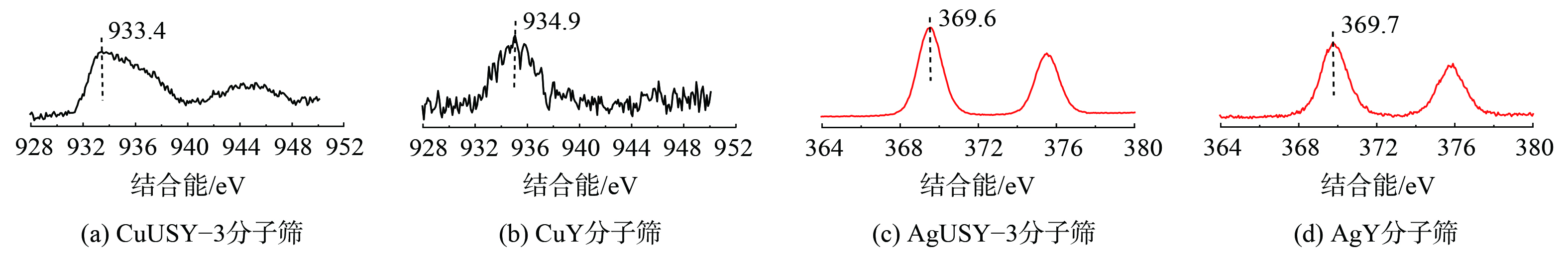
3) XPS表征。Cu、Ag离子交换的分子筛XPS分析如图3所示。由Cu 2p图可知,CuUSY-3及CuY在结合能933.4 eV和934.9 eV的峰为Cu 2p3/2的特征峰,对应表现出二价Cu2+的特性。特别地,分子筛中一价Cu+的XPS峰结合能为932~933 eV,无任何震动,配位的Cu2+则为933~936 eV[26]。由Ag 3d图可知,在AgUSY-3及AgY结合能为369.6 eV和369.7 eV处检测出Ag 3d特征峰[27]。这表明Ag元素均以一价Ag+离子存在于离子交换分子筛中。
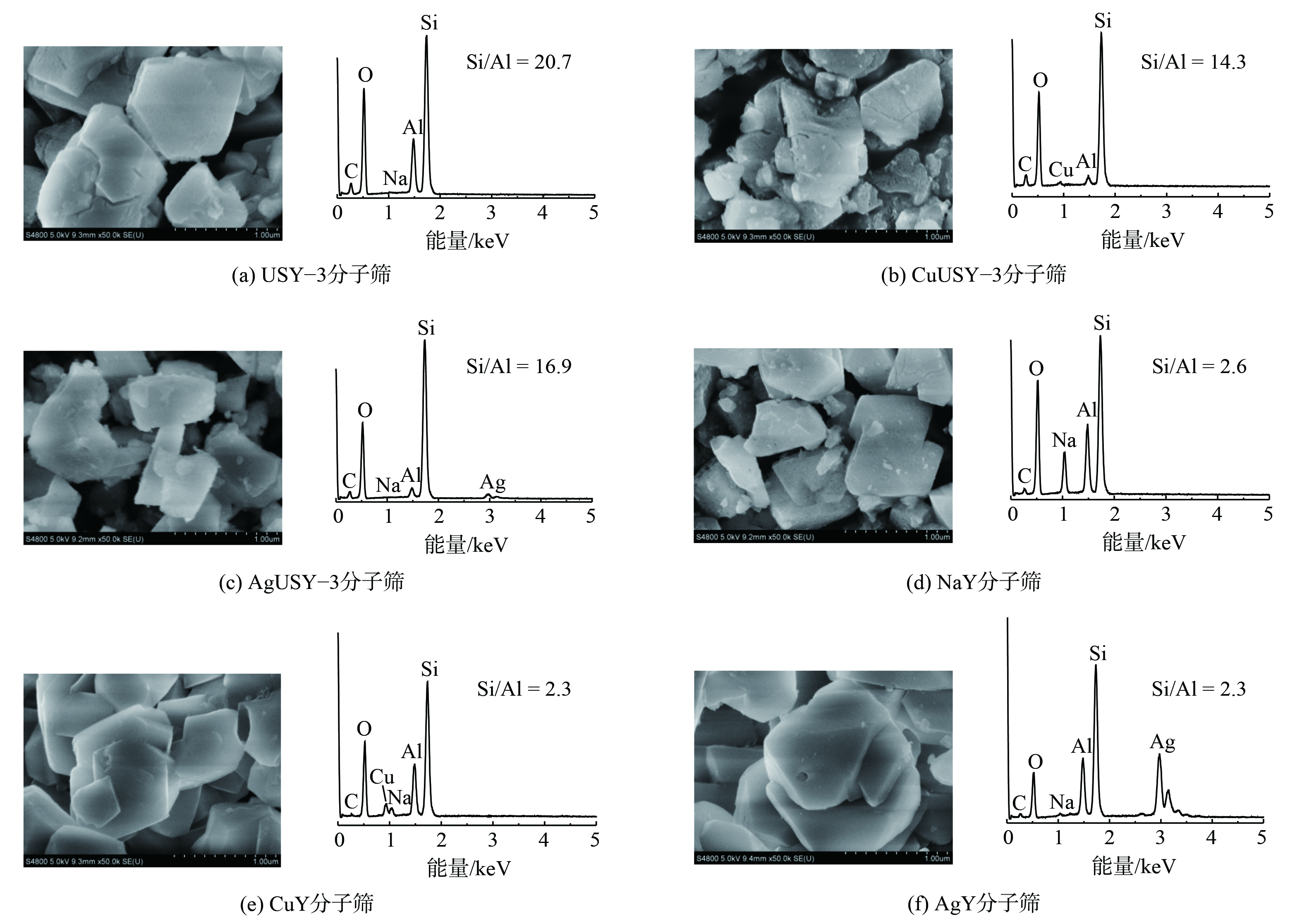
4) 微观形貌表征。离子交换分子筛的SEM图及EDS剖面如图4所示。改性前后USY-3和NaY分子筛均呈规则正八面体形状,其晶体结构完整,这与XRD表征结果一致。离子交换后分子筛EDS剖面 (图4(b)、(c)、(e)、(f)) 出现了Cu、Ag的能量峰,且CuY、AgY分子筛信号较CuUSY-3、AgUSY-3强,说明NaY分子筛的离子交换量更大。
5) 离子交换分子筛的组成表征。如表2所示,经过离子交换改性,Cu2+和Ag+均被引入USY-3和NaY分子筛骨架中,但NaY比USY-3分子筛的离子交换度高,这说明NaY具有更高的离子交换容量。此外,与Cu2+相比,更多的Ag+离子交换到分子筛上。如AgY分子筛中Ag+离子摩尔含量达8.89%,离子交换度为87.8%,CuY分子筛中Cu2+离子摩尔含量和离子交换度分别为2.69%及51.9%。
式中:IE Ag为Ag离子交换度,%;X Ag为Ag的摩尔分数,%;XAl为Al的摩尔分数,IECu为Cu离子交换度,%;X Cu为Cu的摩尔分数,%。
-
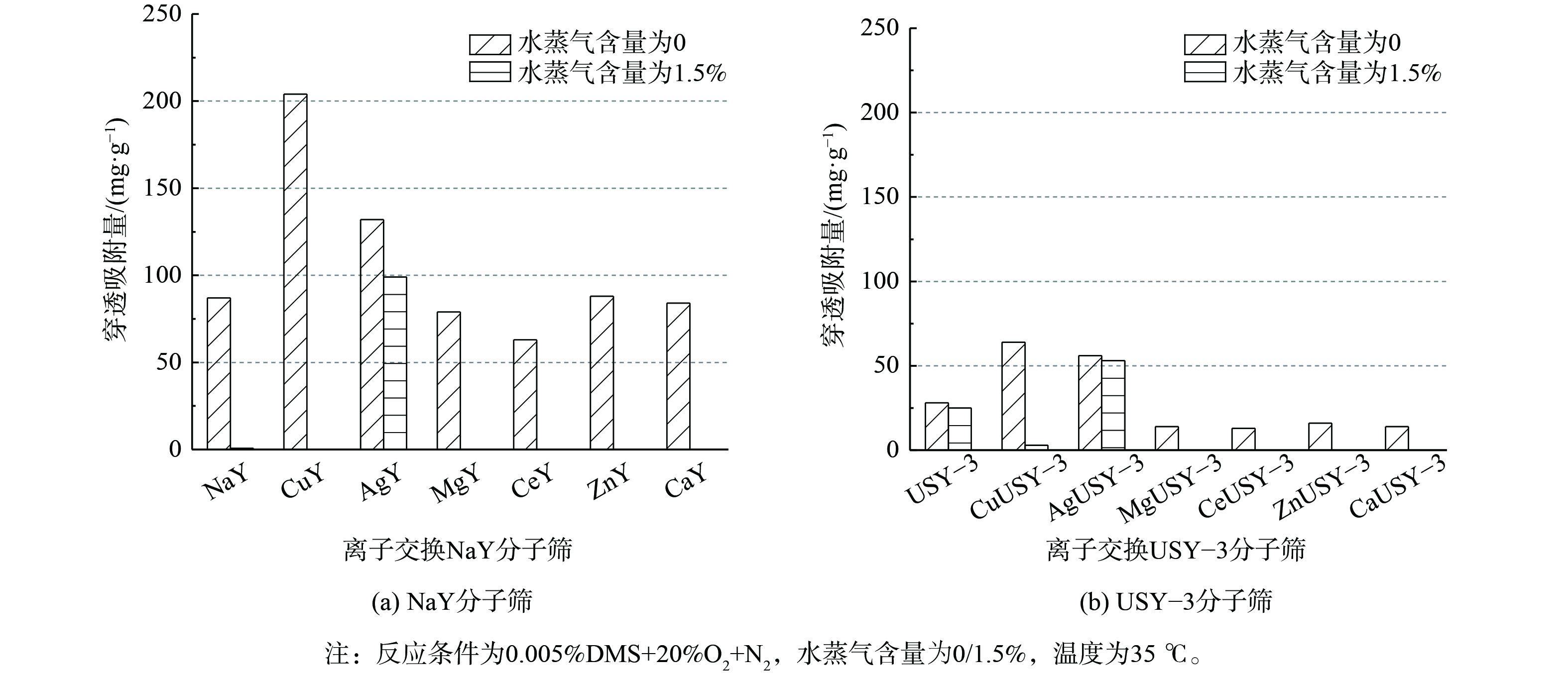
固定吸附温度35 ℃,分子筛用量100 mg,通入总流量为100 mL·min−1的混合气 (体积分数) :0.005% VOC (0.005% DMS)+20% O2+N2 (平衡气) 。通过固定床吸附实验得出Y型分子筛的DMS穿透吸附量如图5所示。在无水蒸气存在时,Y型分子筛DMS吸附能力比较结果为NaY>USY-3分子筛。Cu、Ag离子交换改性后,NaY及USY-3分子筛的DMS穿透吸附量大幅提升,其中Cu-Y及CuUSY-3分子筛的DMS穿透吸附量均提高了约130% (CuY穿透吸附量为203 mg·g−1,CuUSY-3穿透吸附量为64 mg·g−1) 。其他离子交换分子筛的吸附性能均下降,这可能与分子筛改性后微孔面积下降有关 (表1) 。Cu、Ag离子交换分子筛的微孔面积降低,但DMS吸附性能提高。这说明Cu、Ag离子可与DMS进行配位络合,将对DMS的物理吸附转变为化学吸附,结合更加牢固,从而提升吸附性能。CuUSY-3、AgUSY-3分子筛的DMS穿透吸附量 (64 mg·g−1、56 mg·g−1) 较CuY、AgY分子筛 (203 mg·g−1、132 mg·g−1) 更低。结合分子筛组成结果分析认为,这是由于NaY较USY-3分子筛离子交换容量高,Cu、Ag离子占比高,可为DMS提供更多吸附位点。
当体系中通入1.5%的水蒸气时,CuUSY-3及CuY分子筛的DMS吸附量几乎为0,而AgY、AgUSY-3分子筛可保持较高的DMS吸附性能 (AgY的穿透吸附量为99 mg·g−1,AgUSY-3的穿透吸附量为53 mg·g−1) 。这说明Ag离子交换可提高分子筛的DMS吸附性能,同时削弱水的竞争吸附影响。这与前人研究报道的结果一致,Ag离子的存在可避免H2O与分子筛的相互作用,抑制H2O的竞争吸附效应[19]。
-
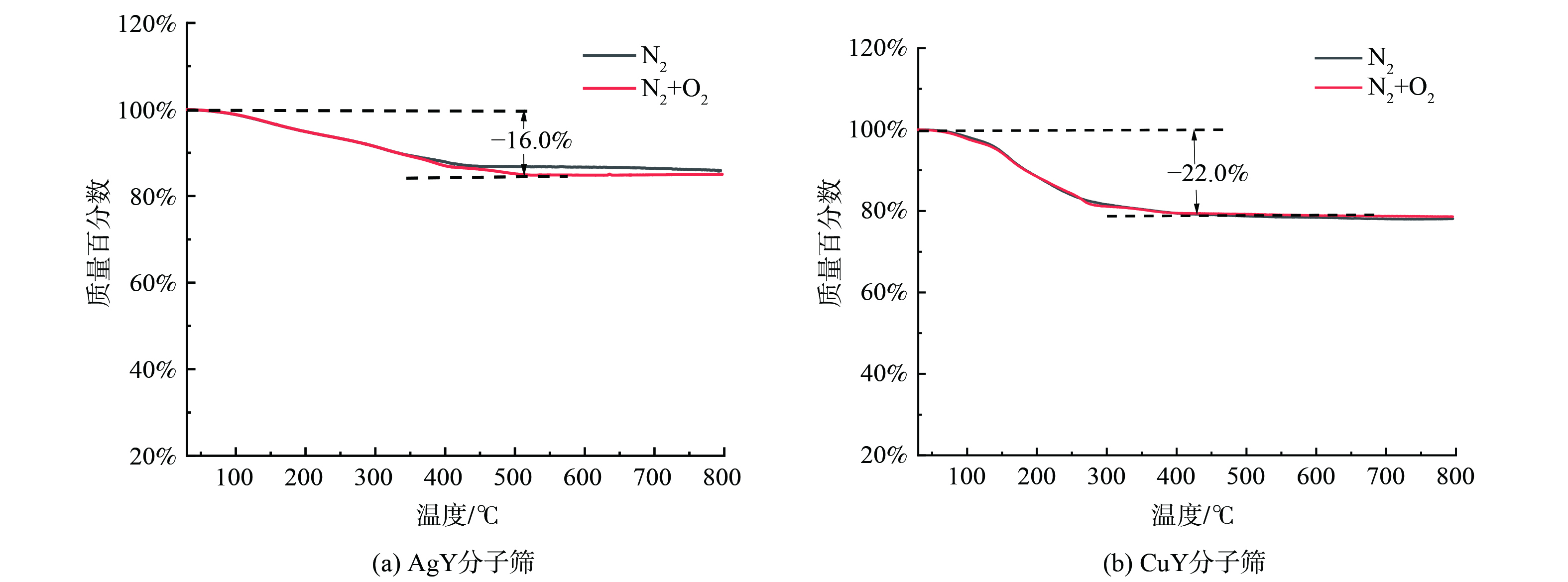
AgY、CuY分子筛不同脱附气氛下的脱附TG曲线如图6所示。其中,30~500 ℃内的失重归因于吸附在分子筛中DMS的解吸。由图6(a)可知,当AgY分子筛吸附饱和后,在N2、N2+O2两种气氛下脱附失重速率几乎相同,但N2脱附较N2+O2脱附失重略早达到平衡,分子筛失重百分比分别为N2脱附的13.5%及N2+O2脱附的16.0%,这基本对应吸附DMS在分子筛上的质量占比 (11.7%)。对比图6(b),CuY分子筛不同气氛脱附失重曲线几乎保持一致,失重速率及最终失重百分比基本相同,保持在约22.0%,对应吸附DMS在分子筛上的质量占比 (17.6%)。此外,CuY分子筛较AgY分子筛脱附曲线斜率更大,且更早达到平衡,这表明DMS在CuY分子筛中更易脱附。
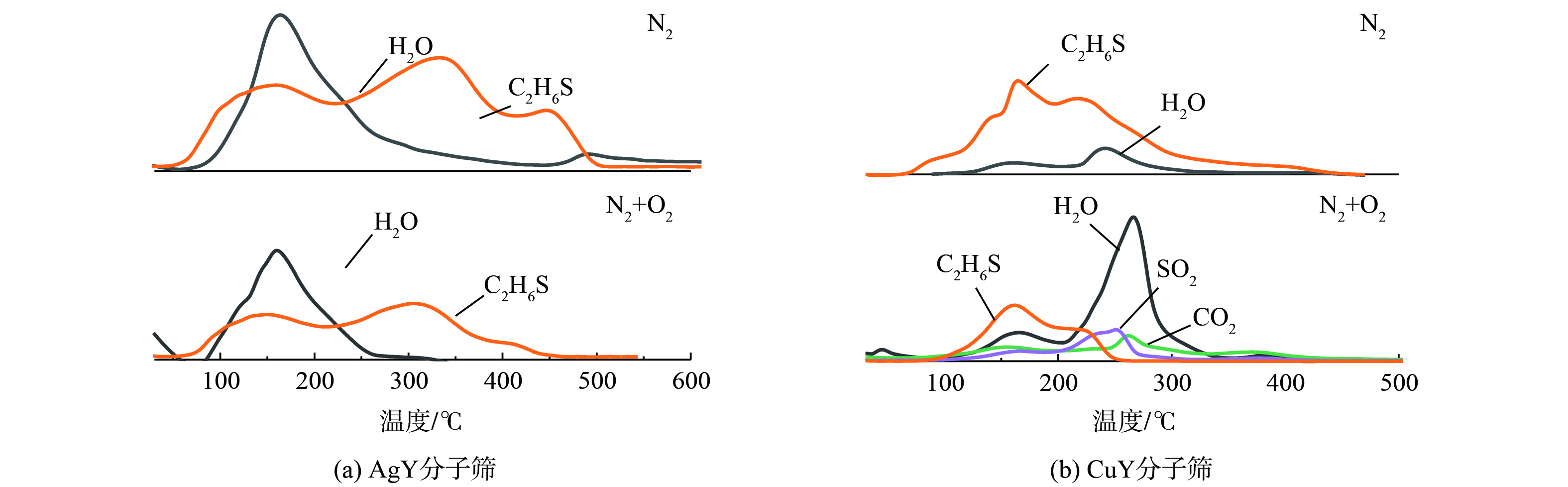
离子交换分子筛脱附产物结果如图7所示。在N2及N2+O2脱附气氛下 (图7(a)) ,AgY分子筛的DMS脱附曲线均在150、350 °C左右有两个明显脱附峰,说明在这两个温度范围内吸附的DMS脱附速率最快。温度继续升高,曲线逐渐下降趋于平缓,DMS的解吸在450 ℃后才逐渐完成,与TG曲线结果一致。此外,体系中还检测到部分H2O的信号 (约200 ℃) ,说明程序升温过程中有H2O从分子筛中解吸 (可能与低硅分子筛亲水,且配气中不可避免的含有微量H2O有关) 。应特别注意,在N2+O2气氛下,AgY分子筛在TPD过程中峰强度较弱,这可能是由于DMS分解为H2S和C2H4[28],但由于浓度较低,没有检测到明显信号。CuY分子筛脱附时 (图7(b)) ,DMS均只有一个主要的脱附峰 (200 °C左右) ,且相对AgY分子筛而言,DMS解吸信号下降得更早更快,在400 ℃后逐渐解吸完全。这说明DMS在CuY分子筛上解吸更加容易。此外,在N2与N2+O2的气氛下,脱附产物会存在差别。在N2脱附条件下,脱附产物中仅有DMS和H2O,而在N2+O2气氛下同时还检测出了CO2及SO2的信号。这说明通入O2后,吸附的DMS在脱附过程中发生反应,产生CO2及SO2等产物。
-
以DMS为模型物,对吸附饱和的AgY及CuY分子筛进行循环动态吸脱附实验。脱附在300 ℃下通入N2+O2的气氛下进行吹扫热再生。如图8所示,经5次循环再生后,CuY分子筛环DMS穿透吸附性能可稳定维持在97%以上,具备良好的热稳定性和再生性能。与CuY分子筛相比,AgY分子筛对DMS络合吸附作用更强,吸附分子更难从AgY分子筛中脱除,所以热脱附并不彻底。AgY分子筛 (133 mg·g−1) 再生一次穿透吸附量降低21% (105 mg·g−1) ,可能归因于活性Ag物种形貌分布在热再生过程中产生了某些变化,但经5次循环再生后,穿透吸附性能又逐渐恢复至约90% (122 mg·g−1) 。这说明AgY分子筛可恢复并保持稳定吸附。
-
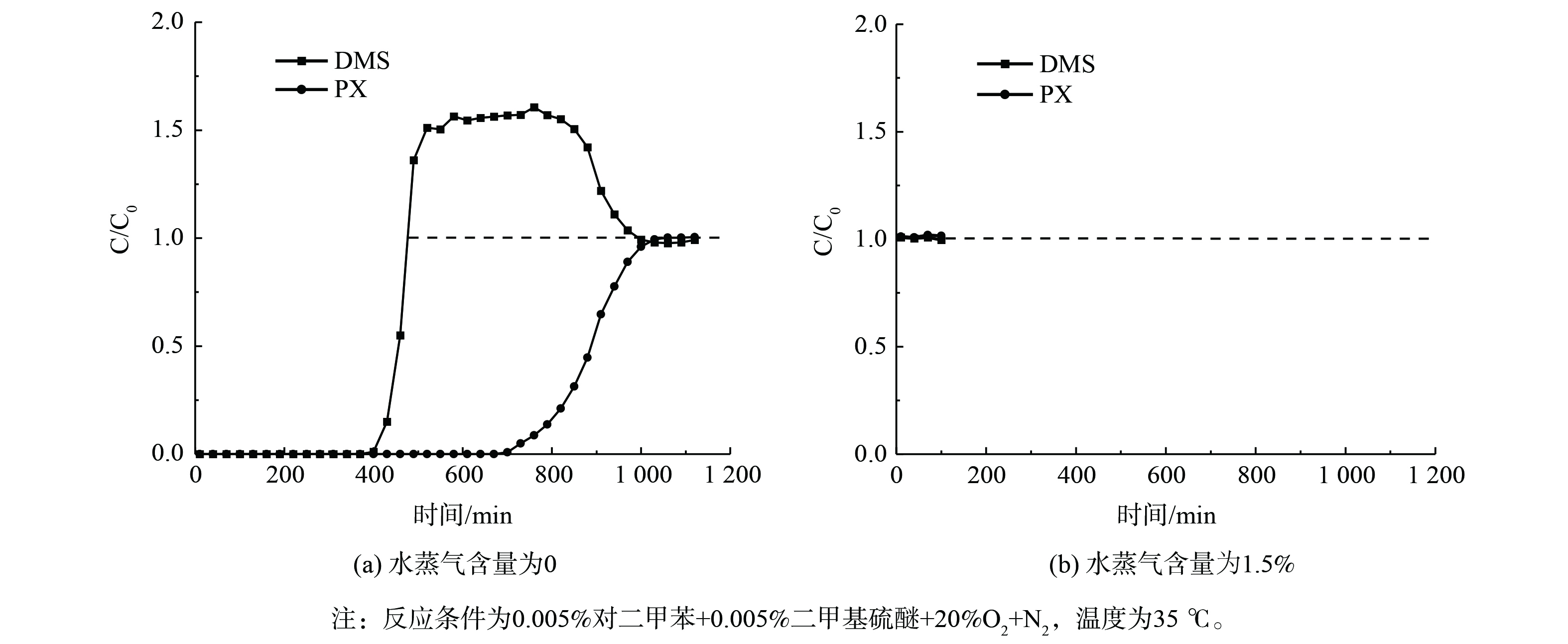
NaY分子筛对双组分 (体积分数) VOCs (0.005% PX+0.005% DMS) 的吸附穿透如图9所示。无水蒸气存在时下,PX穿透慢 (约730 min) ,DMS穿透快 (约430 min) ,且吸附470 min后DMS的C/C0>1.0,直至PX吸附饱和才逐渐趋于平衡。这说明NaY分子筛对PX吸附能力远优于DMS,PX对分子筛吸附位点的竞争力更强,且穿透后部分吸附的DMS逐渐被PX取代。这主要归因于与较DMS相比,PX的分子大小更接近分子筛孔道尺寸,故更易被吸附。而当存在1.5%水蒸气时,NaY分子筛对PX和DMS吸附能力均为0,吸附曲线均迅速穿透。这说明H2O在分子筛上的竞争吸附能力最强,可优先VOCs占据吸附位点。
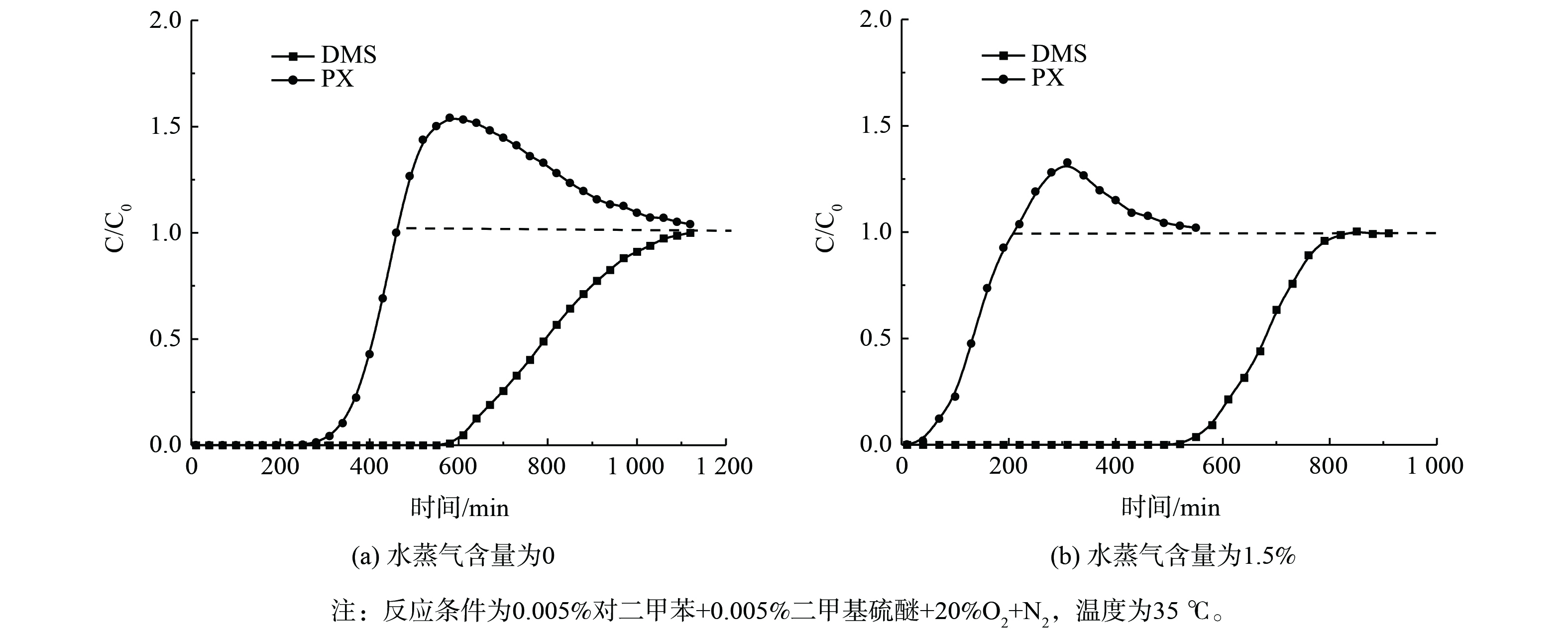
AgY分子筛对PX、DMS的混合体系的竞争吸附穿透曲线如图10所示。无水蒸气存在时,PX比DMS提前300 min穿透,在吸附460 min后PX的C/C0>1.0,直至DMS吸附饱和才趋于平衡。这说明混合气氛下,AgY分子筛具有优异的DMS吸附性能,DMS比PX具有竞争吸附优势,可优先占据吸附位点。而当1.5% 水蒸气存在时,由于DMS及H2O的竞争吸附,PX的附性能更差,穿透时间缩短 (约145 min) 。而DMS穿透时间依然可维持在600 min以后,吸附性能受H2O影响较小。这可能是由于DMS小分子在微孔AgY分子筛内部快速扩散,与Ag离子络合形成Ag—S键,从而被优先吸附抑制H2O的竞争吸附[19]。
AgY分子筛对VOCs (PX、DMS) 的竞争吸附穿透吸附量总结如表3所示。无水蒸气存在时,NaY分子筛仅能有效吸附PX,对DMS的吸附量相对较低。AgY分子筛总VOCs吸附量略低,但能同时吸附两种VOCs,且吸附量均高于80 mg·g−1。而当1.5% 水蒸气存在时,NaY分子筛对VOCs的吸附能力为0,而AgY分子筛对VOCs总吸附量保持在96 mg·g−1,且对DMS具有绝对的竞争吸附优势。结果表明,Ag离子交换改性可提高NaY分子筛的抗水性,将其使用范围拓宽至含湿条件,同时可提高对DMS等小分子硫化物的吸附选择性。
-
1) Cu及Ag离子交换分子筛的DMS吸附量显著提升。无水蒸气存在时,CuY分子筛的DMS穿透吸附量最高 (203 mg·g−1) ,穿透吸附量提升130%。1.5% 水蒸气存在时,只有Ag离子能抑制H2O的竞争吸附,AgY分子筛的DMS穿透吸附量最高 (99 mg·g−1) 。两种分子筛多次循环再生后吸附容量无明显下降。
2) 分子筛DMS吸附性能的提升主要受Cu、Ag离子交换容量的影响,Cu、Ag离子交换分子筛的微孔面积降低,物理吸附性能减弱。然而,Cu、Ag离子可与DMS进行配位络合,化学吸附增强,从而提升吸附性能。NaY较USY-3分子筛具有更高的离子交换容量,可为DMS提供更多吸附位点,故其改性后吸附性能更优。
3) AgY分子筛可同时吸附PX、DMS两种VOCs,且具有较好地抗水性和循环再生性能,可用于含湿条件下VOCs中小分子硫化物的吸附净化。
离子交换法改性的NaY分子筛对吸附含硫VOCs的性能提升
Improvement of the adsorption performance of sulfur-containing VOCs by NaY zeolite modified by ion exchange method
-
摘要: 为解决分子筛在含湿条件下对小分子含硫VOCs吸附性能差的问题,并实现高效捕集,采用液相离子交换法选取不同金属离子 (Ag、Cu、Mg、Zn、Ce、Ca等) 对NaY和USY-3分子筛进行改性,以二甲基硫醚 (DMS) 作为模型物,考察2种改性分子筛对高湿度含硫VOCs脱除效果,进而优选性能优异的改性分子筛,再通过多组分VOCs竞争吸附实验来模拟实际应用效果。结果表明,NaY和USY-3分子筛改性后均可保持结构稳定,NaY型分子筛较USY-3分子筛具有更高的离子交换容量,改性后吸附性能提升更明显,CuY、AgY分子筛的DMS吸附性能优异 (CuY的穿透吸附量为203 mg·g−1,AgY的穿透吸附量为132 mg·g−1) 。然而,仅有Ag离子交换可提高NaY分子筛的抗水性,在1.5%水蒸气存在时,DMS穿透吸附量最高可达99 mg·g−1,且5次循环再生后吸附容量仍在90%。在对二甲苯 (PX) 存在时,分子筛对2种VOCs的吸附量均高于80 mg·g−1,且DMS具有绝对的竞争吸附优势。本研究表明AgY分子筛具有优异的抗水性和吸附选择性,可为其在含湿条件下对VOCs中小分子硫化物的吸附净化应用提供参考。
-
关键词:
- 挥发性有机物(VOCs) /
- Y型分子筛 /
- 吸脱附 /
- 离子交换
Abstract: In order to solve the problem of poor adsorption performance of zeolites for small sulfur-containing VOCs under wet conditions and achieve efficient capture , different metal ions (Ag, Cu, Mg, Zn, Ce, Ca, etc.) were selected to modify NaY and USY-3 zeolites by liquid phase ion exchange method. The sulfur-containing VOCs adsorption performance of modified zeolites under aqueous conditions was investigated by using Dimethyl sulfide (DMS) as a model. Furthermore, the ion-modified molecular sieves with excellent performance were used in multi-component VOCs competitive adsorption experiments to test their performance under actual industrial conditions. The results showed that both NaY and USY-3 molecular sieves could maintain structural stability after modification. Compared with USY-3 zeolites , NaY zeolites had higher ion exchange capacity, and the adsorption performance was improved significantly after ion exchange modification. CuY and AgY molecular sieves had excellent DMS adsorption performance ( the penetration adsorption of CuY was 203 mg·g -1 , and that of AgY was 132 mg·g -1 ). However, only Ag ion exchange improved the water-resistance of NaY molecular sieves. The maximum DMS penetration adsorption capacity of AgY zeolites was 99 mg·g -1 in the presence of 1.5% water vapor, and the adsorption capacity decreased by no more than 10% after 5 cycles of regeneration. In the presence of para-xylene (PX), the adsorption capacity of PX and DMS was higher than 80 mg·g -1 , and DMS had an absolute competitive adsorption advantage. The experimental data showed that AgY molecular sieves had excellent water resistance and adsorption selectivity, and could provide reference to for its application in the adsorption and purification of small molecular sulfides in VOCs under wet conditions.-
Key words:
- volatile organic compounds(VOCs) /
- Y zeolite /
- adsorption and desorption /
- ion-exchanged
-
表 1 离子交换改性前后分子筛结构参数
Table 1. Textural parameters for the zeolite before and after ion exchange modification
分子筛样品种类 Stotal/(m2·g−1) Smicro/(m2·g−1) Smeso/(m2·g−1) Vpore/(cm3·g−1) Vmicro/(cm3·g−1) Vmeso/(cm3·g−1) Si/Al比 XRF ICP NaY 856.6 806.1 50.5 0.41 0.32 0.09 3.4 2.7 CuY 851.7 802.8 48.9 0.36 0.27 0.09 3.4 2.7 AgY 603.4 550.8 52.6 0.31 0.20 0.11 3.7 2.7 MgY 754.3 703.2 51.1 0.37 0.27 0.10 3.5 2.8 ZnY 719.9 674.2 45.7 0.33 0.25 0.08 3.4 2.8 CaY 745.8 702.4 43.4 0.37 0.29 0.08 3.1 2.7 CeY 718.2 669.6 48.6 0.35 0.27 0.08 3.3 2.7 USY-3 879.3 731.6 147.7 0.61 0.34 0.27 25.5 20.5 CuUSY-3 807.8 652.8 155.9 0.52 0.24 0.28 25.4 20.0 AgUSY-3 767.4 611.7 155.7 0.51 0.24 0.27 26.2 20.8 MgUSY-3 678.8 494.6 183.9 0.51 0.18 0.33 25.8 20.6 ZnUSY-3 809.2 660.7 148.5 0.51 0.24 0.27 24.9 20.6 CaUSY-3 794.1 643.4 150.7 0.52 0.24 0.28 25.4 20.0 CeUSY-3 766.2 599.6 166.6 0.57 0.23 0.34 25.7 21.2 表 2 离子交换改性前后分子筛结构组成
Table 2. Structual composition of molecular sieves before and after ion-exchanged modification
分子筛样品种类 化学组成 离子交换量/% 离子交换度/% USY-3 Na0.004Al0.116Si2.404O7.476 — — CuUSY-3 Na0.053Cu0.036Al0.175Si2.498O7.239 0.36 41.4 AgUSY-3 Na0.001Ag0.105Al0.159Si2.690O7.045 1.05 66.1 NaY Na0.652Al0.825Si2.175O6.348 — — CuY Na0.293Cu0.269Al1.035Si2.375O6.028 2.69 51.9 AgY Na0.070Ag0.889Al1.013Si2.366O5.661 8.89 87.8 表 3 离子交换改性分子筛对PX和DMS的穿透吸附量
Table 3. Penetration adsorption capacity of PX and DMS on ion-exchange modified molecular sieves
分子筛 水蒸气含量 VOCs穿透吸附量/(mg·g−1) DMS穿透吸附量/(mg·g−1) PX穿透吸附量/(mg·g−1) NaY 0 241 59 182 AgY 0 169 88 81 NaY 1.5% 0 0 0 AgY 1.5% 96 81 15 -
[1] 荀志萌, 李照海, 何娇, 等. 大风量低浓度VOCs气体二次吸附浓缩净化技术开发[J]. 环境工程学报, 2016, 10(1): 283-288. doi: 10.12030/j.cjee.20160146 [2] 党小庆, 王琪, 曹利, 等. 吸附法净化工业VOCs的研究进展[J]. 环境工程学报, 2021, 15(11): 3479-3492. doi: 10.12030/j.cjee.202011052 [3] 李晓宁. 煤化工企业污水处理站异味治理工艺方案设计 [D]. 济南: 山东大学, 2018. [4] 刘星园, 张永锋, 肖凯, 等. 分子筛材料在VOCs吸附中的研究进展[J]. 化工进展, 2021, 41(5): 2504-2510. doi: 10.16085/j.issn.1000-6613.2021-0958 [5] LI X, ZHANG L, YANG Z, et al. Adsorption materials for volatile organic compounds (VOCs) and the key factors for VOCs adsorption process: A review[J]. Separation and Purification Technology, 2020, 235: 116213. doi: 10.1016/j.seppur.2019.116213 [6] 李照海, 羌宁, 刘涛, 等. 活性炭和沸石分子筛处理非稳定排放VOCs气体的性能比较[J]. 环境工程学报, 2017, 11(5): 2933-2939. doi: 10.12030/j.cjee.201611026 [7] 梁欣欣, 卜龙利, 刘嘉栋, 等. 分子筛负载型吸附剂对典型VOCs的吸附行为特性[J]. 环境工程学报, 2016, 10(6): 3152-3160. doi: 10.12030/j.cjee.201501108 [8] SHEN X, DU X, YANG D, et al. Influence of physical structures and chemical modification on VOCs adsorption characteristics of molecular sieves[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106729. doi: 10.1016/j.jece.2021.106729 [9] 李智, 王建英, 王勇, 等. NaY沸石分子筛在VOCs处理中的应用[J]. 环境工程学报, 2020, 14(8): 2211-2221. doi: 10.12030/j.cjee.201910071 [10] 吴琼, 栾志强, 崔振, 等. Y型蜂窝分子筛疏水改性及对二甲苯吸附性能研究[J]. 应用化工, 2021, 50(06): 1495-1498. doi: 10.3969/j.issn.1671-3206.2021.06.009 [11] 周瑛, 卢晗锋, 王稚真, 等. VOCs和水在Y型分子筛表面的竞争吸附[J]. 环境工程学报, 2012, 6(5): 1653-1657. [12] 姚露露, 周燕芳, 郭珊珊, 等. Y型与ZSM-5型分子筛吸附VOCs性能的对比[J]. 环境工程学报, 2022, 16(1): 182-189. doi: 10.12030/j.cjee.202104061 [13] 赵亚伟, 沈本贤, 孙辉, 等. 过渡金属改性Y型分子筛吸附脱除低碳烃中二甲基二硫醚[J]. 化工进展, 2017, 36(6): 2190-2196. doi: 10.16085/j.issn.1000-6613.2017.06.032 [14] 周广林, 蒋晓阳, 周红军. 吸附法脱除液化石油气中的二甲基二硫醚[J]. 现代化工, 2014, 34(1): 72-74. doi: 10.16606/j.cnki.issn0253-4320.2014.01.027 [15] 吕梦颖, 李芹, 王晓胜, 等. 改性ZSM-5分子筛吸附脱除甲基叔丁基醚中的二甲基二硫醚[J]. 天然气化工(C1化学与化工), 2018, 43(3): 15-19. [16] SONG H, CUI X H, SONG H L, et al. Characteristic and adsorption desulfurization performance of Ag–Ce bimetal ion-exchanged Y zeolite[J]. Industrial & Engineering Chemistry Research, 2014, 53(37): 14552-14557. [17] RUI J, LIU F, WANG R, et al. Adsorptive desulfurization of model gasoline by using different Zn sources exchanged NaY zeolites[J]. Molecules, 2017, 22(2): 305. doi: 10.3390/molecules22020305 [18] 陈晓陆, 张朋, 高爽, 等. 改性Y型分子筛对硫化物吸附脱除规律的研究进展[J]. 山东化工, 2018, 47(18): 45-47. doi: 10.3969/j.issn.1008-021X.2018.18.020 [19] SATOKAWA S, KOBAYASHI Y, FUJIKI H. Adsorptive removal of dimethylsulfide and t-butylmercaptan from pipeline natural gas fuel on Ag zeolites under ambient conditions[J]. Applied Catalysis B Environmental, 2005, 56(1-2): 51-56. doi: 10.1016/j.apcatb.2004.06.022 [20] DEZHI Y, HUAN H, XUAN M, et al. Adsorption–desorption behavior and mechanism of dimethyl disulfide in liquid hydrocarbon streams on modified Y zeolites[J]. Applied Catalysis B:Environmental, 2014, 148-149: 377-386. doi: 10.1016/j.apcatb.2013.11.027 [21] LIDAN L, JIE Z, CHONGPIN H, et al. Adsorptive separation of dimethyl disulfide from liquefied petroleum gas by different zeolites and selectivity study via FT-IR[J]. Separation and Purification Technology, 2014, 125: 247-255. doi: 10.1016/j.seppur.2014.02.002 [22] CHEN X, SHEN B, SUN H, et al. Ion-exchange modified zeolites X for selective adsorption desulfurization from Claus tail gas: Experimental and computational investigations[J]. Microporous and Mesoporous Materials, 2018, 261: 227-236. doi: 10.1016/j.micromeso.2017.11.014 [23] DEHGHANI M, TADJARODI A. Formation and characterization of zeolite Y-platinum nanoparticles by rapid method of ultrasonic irradiation and investigation of its electrochemical property[J]. Journal of Nanostructures, 2020, 10(3): 486-496. [24] MENG B, REN S, LI Z, et al. Intra-crystalline mesoporous zeolite [Al, Zr]-Y for catalytic cracking[J]. ACS Applied Nano Materials, 2020, 3(9): 9293-9302. doi: 10.1021/acsanm.0c01925 [25] MENG F, DING Y, MENG W, et al. Modification of molecular sieves USY and their application in the alkylation reaction of benzene with cyclohexene[J]. ChemistrySelect, 2020, 5(29): 8935-8941. doi: 10.1002/slct.202002320 [26] KAREN M G-R, BETHZAELY F-R, FéLIX R R, et al. A hierarchical porous carbon – Mn+ FAU (Mn+=Ni2+ or Cu2+) adsorbent: Synthesis, characterization and adsorption of salicylic acid from water[J]. Microporous and Mesoporous Materials, 2014, 200: 225-234. doi: 10.1016/j.micromeso.2014.08.055 [27] SONG H, CUI X H, SONG H L, et al. Characteristic and adsorption desulfurization performance of Ag-Ce bimetal ion-exchanged Y zeolite[J]. Industrial & Engineering Chemistry Research, 2015, 53(37): 14552-14557. [28] LEE D, KO E Y, LEE H C, et al. Adsorptive removal of tetrahydrothiophene (THT) and tert-butylmercaptan (TBM) using Na-Y and AgNa-Y zeolites for fuel cell applications[J]. Applied Catalysis A General, 2008, 334(1/2): 129-136. -




 下载:
下载: