-
垃圾渗沥液是一种成分复杂且难处理的高浓度有机废水[1],2021年我国生活垃圾清运量达到2.67×108 t,相对于2015年的1.91×108 t增加了约39.8%。目前,卫生填埋是我国城市生活垃圾的主要处理方式之一[2],与垃圾焚烧和堆肥处理相比,卫生填埋成本较低且工艺简单[3],但生活垃圾经过填埋处理后会发生一系列物理化学和生物化学的反应而产生垃圾渗沥液[4],据计算,每吨生活垃圾在填埋周期内约产生0.1 t垃圾渗沥液[5]。垃圾渗沥液污染物复杂且浓度超高、水质水量变化大[6]、处理难度高[2],如果处理不好,就会导致垃圾渗沥液渗入到土壤、地下水、河流中,从而会影响整个生态系统自身的修复和发展[7]。常用的垃圾渗沥液处理技术主要包括好氧生物处理、厌氧生物处理的生物法和用活性炭吸附[8]、离子交换[9]、膜渗析[10]等物理化学法。单独的生物处理方法无法使其处理达标,并且反应的周期较长;发达城市常用的反渗透处理工艺虽能将垃圾渗沥液处理达标但其耗费极大且会产生二次污染[11-14],因此,寻找高效且低成本的处理垃圾渗沥液的方法迫在眉睫。
近年来,微纳米气泡(MB)在水处理领域取得了巨大进步,其表现的特性已经超出了人们对普通气泡的认知[15]。微纳米气泡一般指直径小于100 μm气泡的总称。其中,直径大于1 μm 的为微气泡,直径小于1 μm的气泡为纳米气泡[16]。微米气泡直径其比表面积大,水中停留时间较长,传质效率高、界面电位高。与微米气泡相比,纳米气泡水中停留时间更长,其传质效率更高,界面点位更高,生物效果显著[17]。由于微纳米气泡具有很强的滞留性,同时内压较大,其高溶解能力可为水体提供高含量的溶解氧[18],微纳米气泡用于处理受污染的水体,可降低水体中的有机污染物[19],同时抑制水体中厌氧菌的有机质分解过程,降低水体中氮、磷的含量[20]。微纳米气泡气液界面带负电荷[21],可与特定的污染物反应,此外微纳米气泡破裂时产生的自由基和振动波可以促进污染物的去除[22]。故在水污染处理领域中,微纳米气泡被发现后得到了广泛的关注和应用[23]。
垃圾渗沥液成分复杂,水质不稳定,采用常规生物处理法处理效果不理想。针对于此,高级氧化技术可应用于为有效降解垃圾渗沥液。芬顿和类芬顿技术得到了广泛应用,其原理是利用·OH的强氧化性,从有机物中夺取氢原子使其分解为CO2和水等无机物或小分子有机物[24]。近年来,以SO4−·的高级氧化技术由于其较高的降解能力、优于·OH的存在寿命和对污染物的高度适应性成为目前的研究热点。过硫酸盐(PS)活化技术常用于降解水环境中的有机污染物[25],过硫酸盐在光、热、声、过渡金属离子的条件下会分解过硫酸根生成硫酸根自由基,其具有较高的氧化还原电位(E0=2.5~3.1 eV),具有强氧化能力,其主要通过电子传递和提取氢将有机污染物氧化为无机物或小分子物质,甚至直接分解成CO2和H2O[26]。目前常见的过硫酸盐活化法有热活化、过渡金属催化活化、紫外光活化等[27],有研究表明,在波长小于270 nm的紫外光照射下过硫酸盐[28],过硫酸盐中的过硫酸根会分解生成硫酸根自由基,从而有效降解有机污染物[29]。本研究利用微纳米气泡的特性将其与紫外灯(UV)活化过硫酸盐技术相结合,考察了反应时间、初始pH、过硫酸钾(PS)投加量、紫外灯功率、微纳米气泡(MB)进气量等因素对垃圾渗沥液中COD去除效果的影响。
-
冰乙酸(CH3COOH)购自重庆川东化工集团,正己酸(C6H12O2)(AR,99.0%)、氨水(NH3·H2O)(AR,28%)购自上海麦克林生化科技有限公司,冰乙酸(CH3COOH)(AR,99.5%)购自重庆川东化工集团,正丁酸(CH3CH2CH2COOH)(AR,99.0%)、过硫酸钾(K2S2O8)(AR,99.5%)购自国药集团化学试剂有限公司,5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)(RG,98%)购自阿达玛斯试剂有限公司,实验用水为超纯水。
-
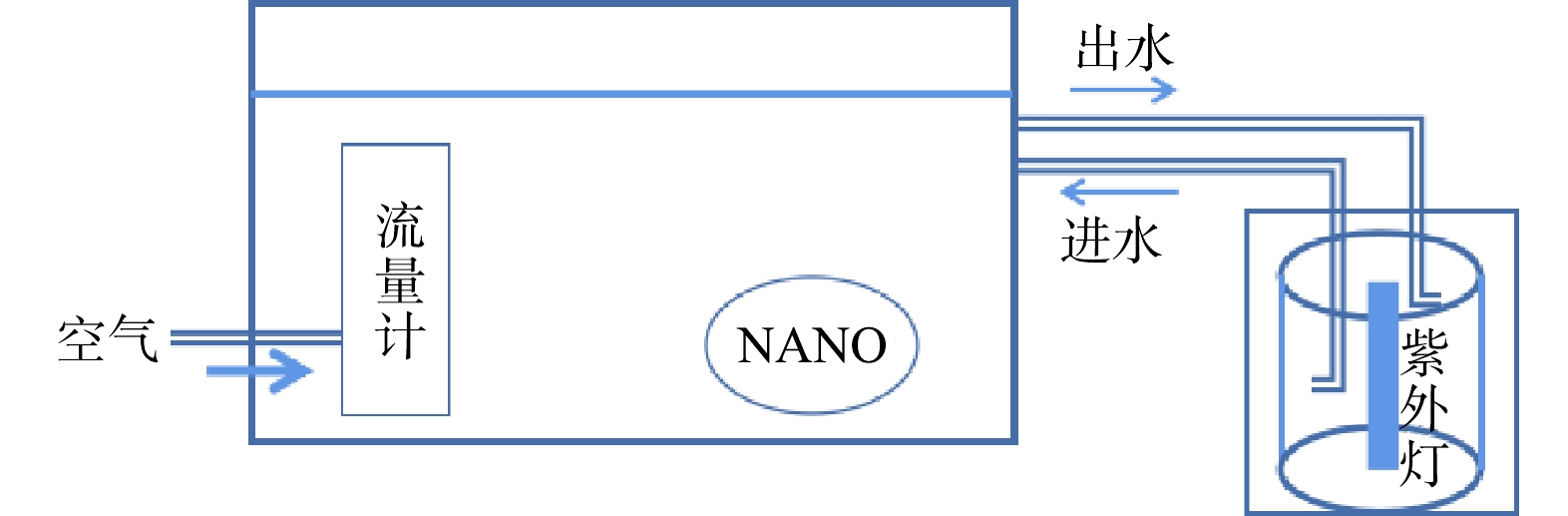
实验装置和仪器见图1,采用体积为1.0 L的烧杯作为反应容器,实验中所用的实验和分析仪器包括:微纳米气泡发生器(LF-1500,上海行恒科技公司),加热消解器(DRB200,美国哈希),紫外分光度计(DR 6000),总有机碳分析仪(TOC-LCPH,日本岛津),连续流动分析仪(荷兰 SKALAR San++),顺磁共振波谱仪(EMXnano,日本岛津)。
-
称取一定量的过硫酸钾加入到由1.74 mmol·L−1冰乙酸、1.08 mmol·L−1正丁酸、2.56 mmol·L−1正己酸和1.94 mmol·L−1氨水溶液组成的1 000 mL模拟垃圾渗沥液中,调节溶液pH,将废水经过微纳米气泡发生器产生微纳米气泡,调节压力阀和进气量至溶液呈乳白色,同时在烧杯中放入254 nm紫外灯并开启紫外灯,每隔30 min取样于紫外分光度计、总有机碳分析仪和连续流动分析仪测试其COD、TOC和氨氮。TOC和氨氮的去除率率按照式(1)、式(2)和式(3)进行计算。
式中:RC为COD去除率,%;C0为初始COD, mg·L−1;Ct为反应t时刻的COD,mg·L−1;RT为TOC去除率,%;T0为其初始TOC,mg·L−1;Tt为t时刻的TOC,mg·L−1;RN为氨氮去除率,%;N0为初始氨氮浓度,mg·L−1;Nt为反应t时刻的氨氮浓度,mg·L−1。
-
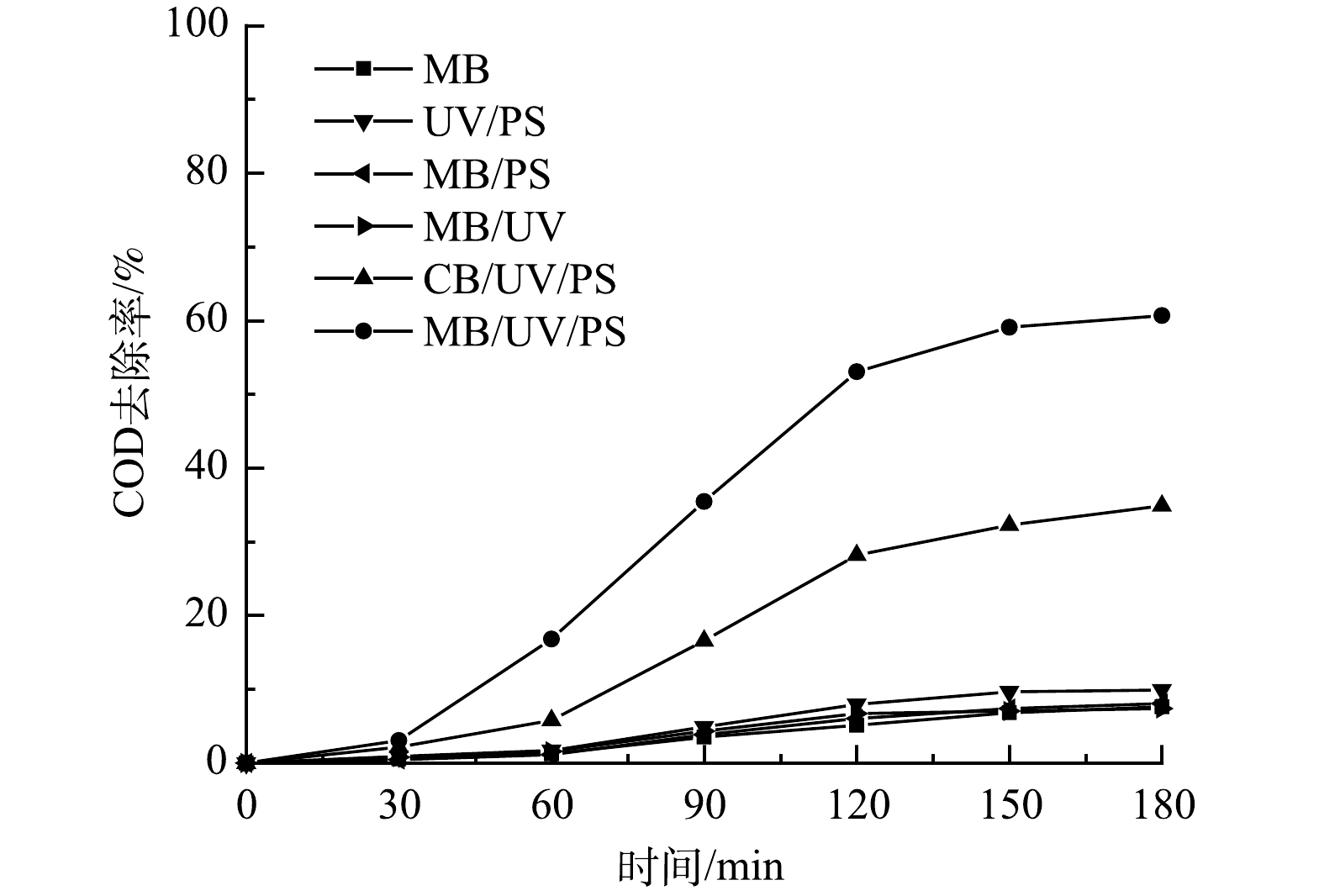
图2为MB进气量为30 mL·min−1,在不同条件下废水COD的去除效果。由图2可以看出,仅有微纳米气泡的条件下,反应180 min后废水COD去除率为7.61%。在MB/PS体系中,PS投加量为4 g·L−1时,180 min后COD去除率为8.07%。在UV/PS体系中,当UV的功率为10W,PS投加量为4 g·L−1时,180 min后垃圾渗沥液的COD去除率为9.89%。在MB/UV体系中,反应180 min后COD去除率为7.39%。在常规气泡(CB)的条件下,CB/UV/PS体系对COD的去除效果有一定的提升,经180 min常规气泡曝气反应后,COD去除率为34.89%,在MB/UV/PS体系中,当紫外灯功率为10 W、过硫酸盐投加量为4 g·L−1时,COD去除率达到了60.68%。由此可见,MB/UV/PS体系对垃圾渗沥液COD有良好的去除效果。这是由于在紫外灯活化过硫酸盐条件下,导入微纳米气泡后,传质加快[17],同时提高了羟基自由基和硫酸根自由基产生的浓度,因此,在该条件下COD的去除率显著提升。
-
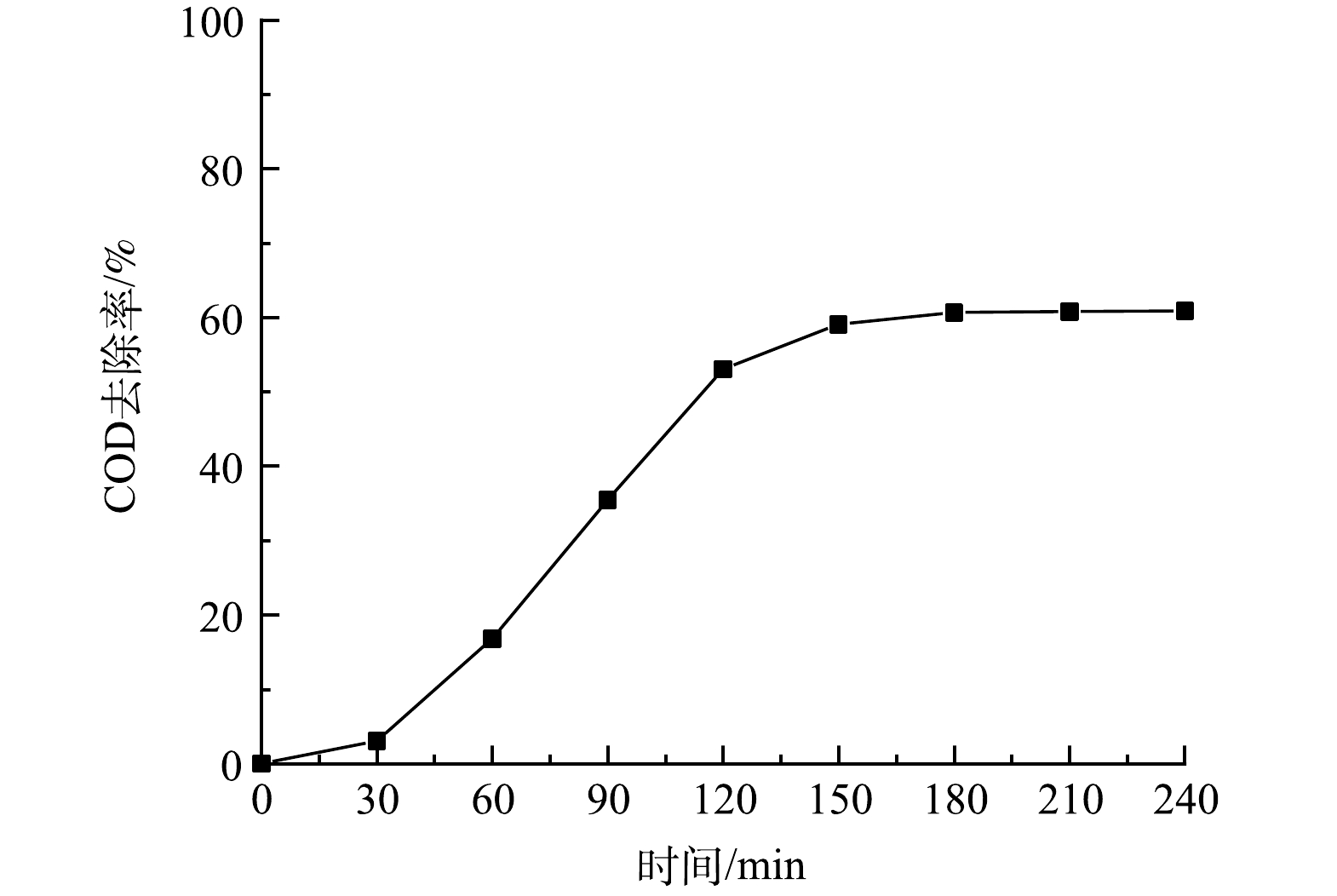
在MB/UV/PS体系中,当溶液pH=6.0,过硫酸盐投加量为4 g·L−1,MB进气量为30 mL·min−1,紫外灯功率为10 W时,COD去除率随反应时间的变化如图3所示。过硫酸盐在微纳米气泡和紫外灯的协同作用下产生硫酸根自由基,过硫酸盐中的过氧键会在氧化还原反应中断裂生成过一硫酸根,过一硫酸根在微纳米气泡和紫外灯的活化下产生羟基自由基和硫酸根自由基,而硫酸根自由基和羟基自由基会通过电子传递和提取氢将垃圾渗沥液中的有机污染物氧化为无机物或小分子物质,随着反应的进行,COD去除率稳步上升,反应180 min时可达到60.68%。
-
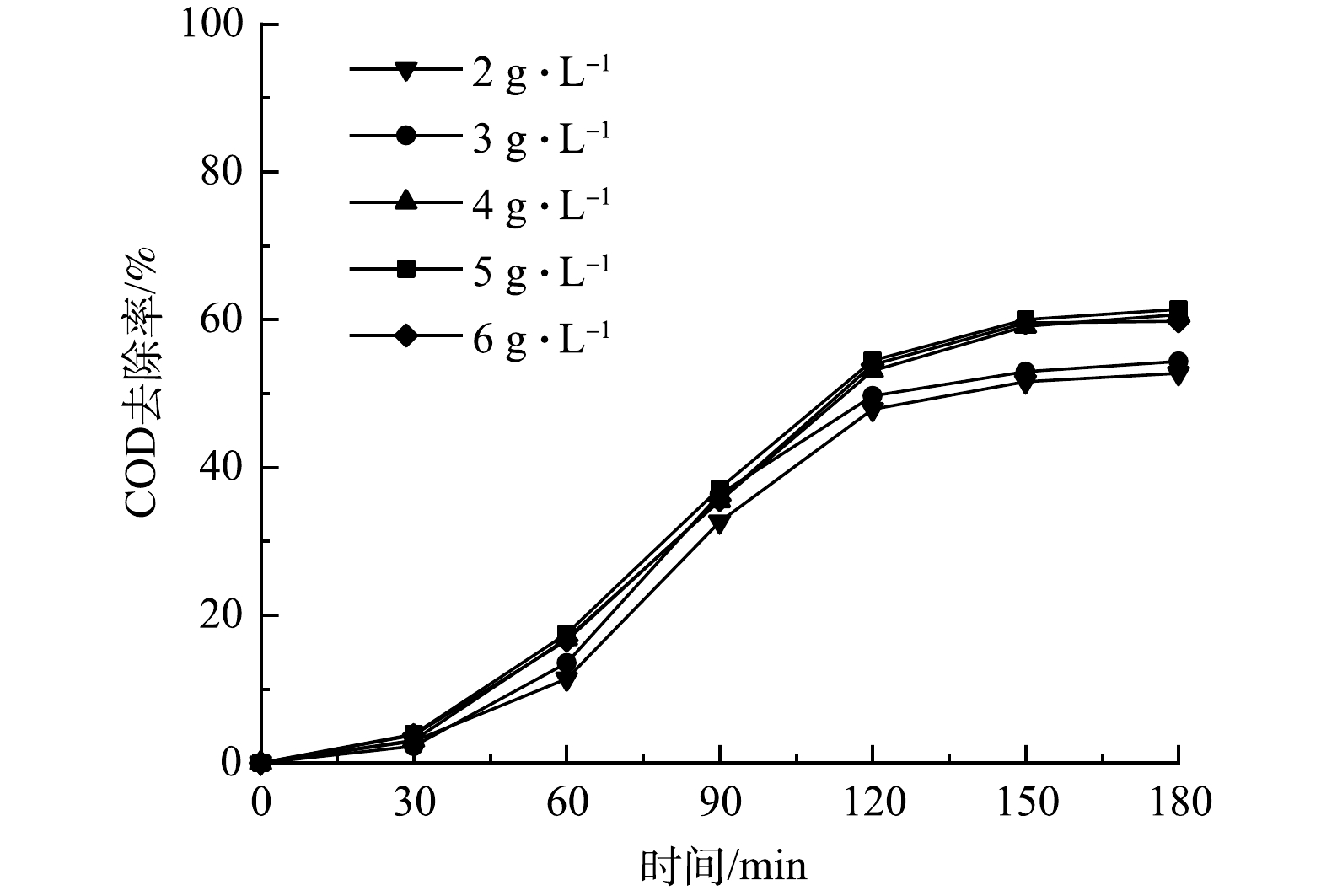
图4反映了PS投加量对垃圾渗沥液COD去除效果的影响。由图4可以看出,随着PS投加量的增加,COD的去除率有所增高。当PS投加量为2、3、4、5、6 g·L−1时,经过3 h的反应,COD的去除率分别为52.73%、54.32%、60.68%、60.57%、59.77%,PS投加量超过4 g·L−1时,COD去除率没有随着PS继续投加而继续增加。这说明溶液中的过硫酸盐已达到饱和,4 g·L−1为PS的最佳投放量。
-
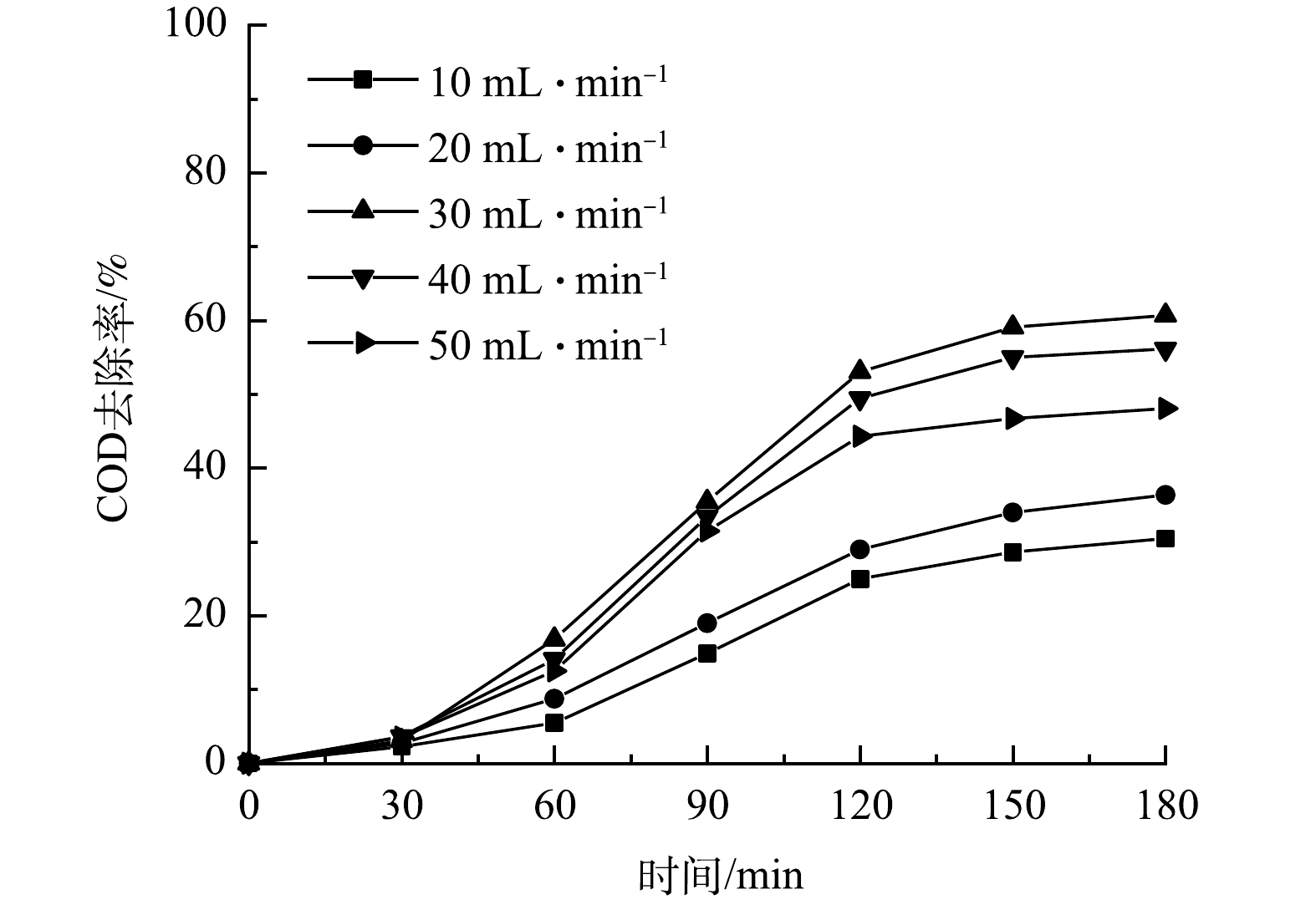
进气量决定微纳米气泡的产生量[17]。图5为MB进气量对COD去除率的影响。可见在MB/UV/PS体系中,MB进气量对模拟废水中COD的去除率随MB的进气量的增加呈先升高后降低的变化趋势。当MB进气量由 10 mL·min−1 增至30 mL·min−1时,COD去除率逐渐升高,并在 30 mL·min−1时达到最高,为60.68%。而随着 MB进气量由30 mL·min−1增加到 60 mL·min−1时,COD去除率降至48.07%。这说明微纳米气泡的最佳进气量为30 mL·min−1。
-
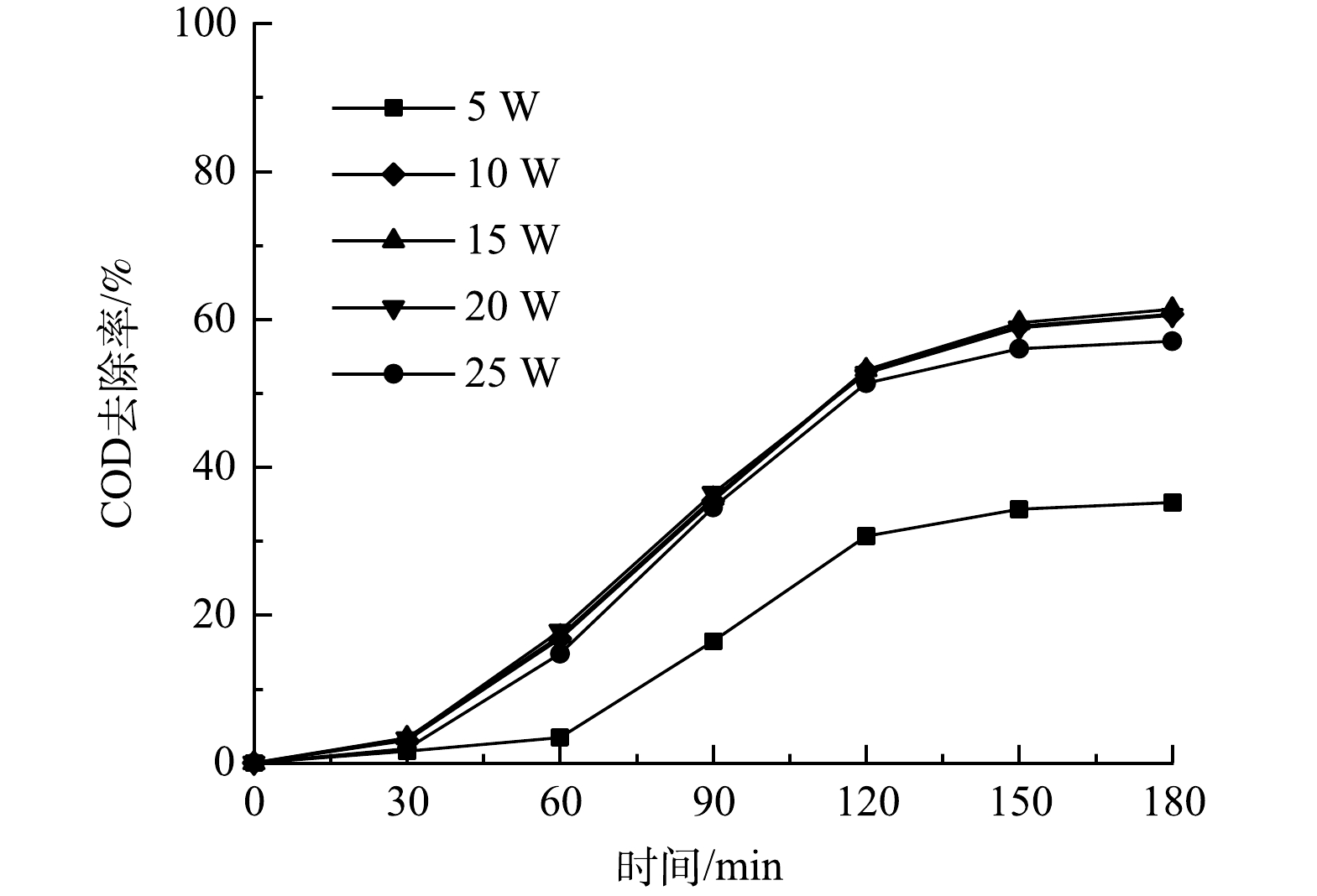
图6为紫外灯(UV)的功率对COD去除率的影响。由图6可见,随着紫外灯功率的不断提升,COD去除率不断增加。当紫外灯为5 W时,COD去除率为35.23%;在紫外灯功率为15 W时去除率达到61.36%。这说明随着紫外灯功率的增加,会产生活性自由基,进而去除有机污染物,但随着紫外灯功率的进一步提升,溶液的COD去除率不再增高。
-
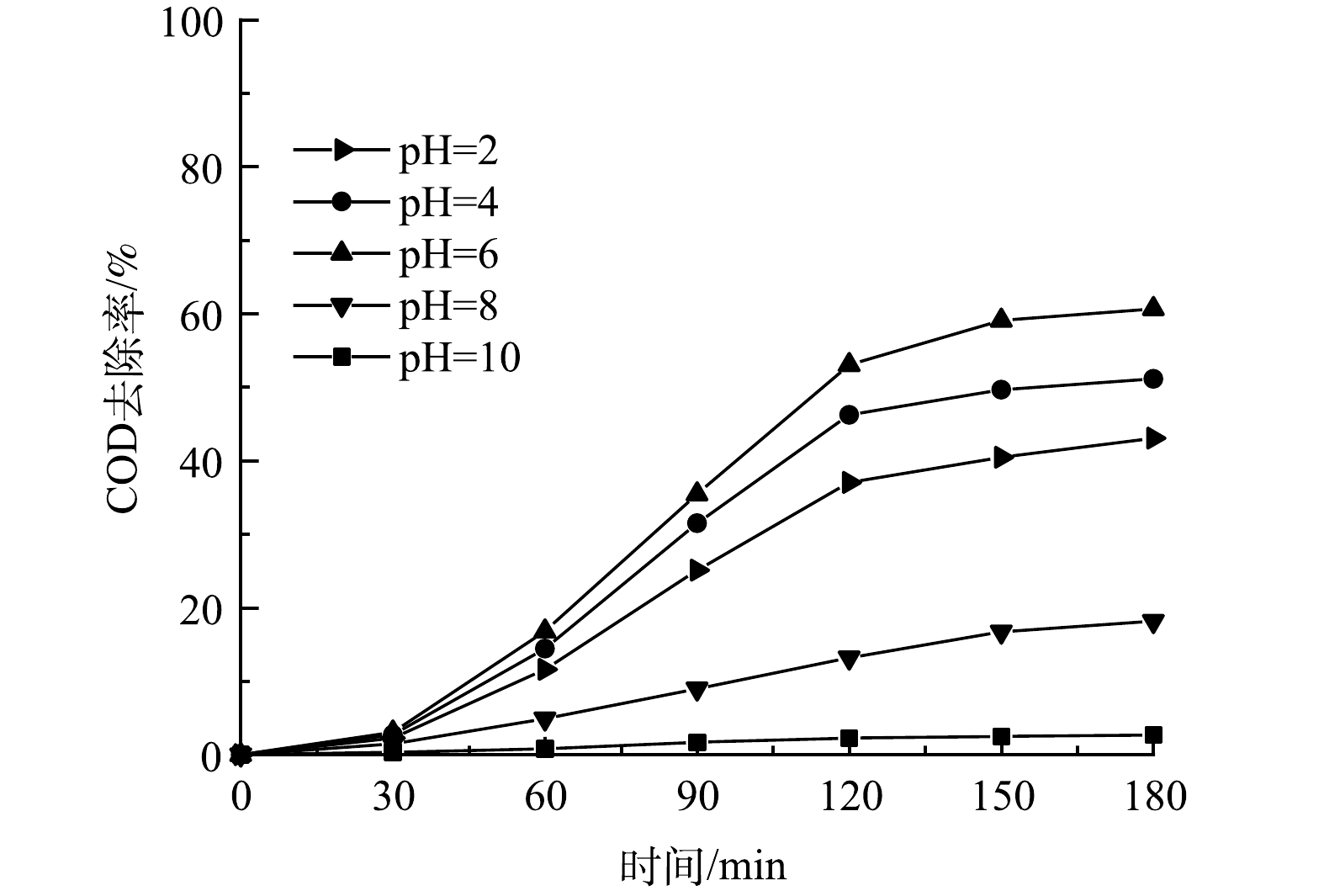
图7为模拟垃圾渗沥液初始pH对后续反应的COD去除效果的影响。可见,在MB/UV/PS体系中,当溶液pH=2时,COD去除率为43.07%;随着pH的增加,COD去除率增加,在pH=6时去除率达到最高,为60.68%。当在pH在碱性条件下COD去除率大幅度降低,当溶液pH=10时,去除率仅仅只有2.73%。这说明废水中的有机酸在碱性条件下生成的盐会抑制污染物的降解。
-
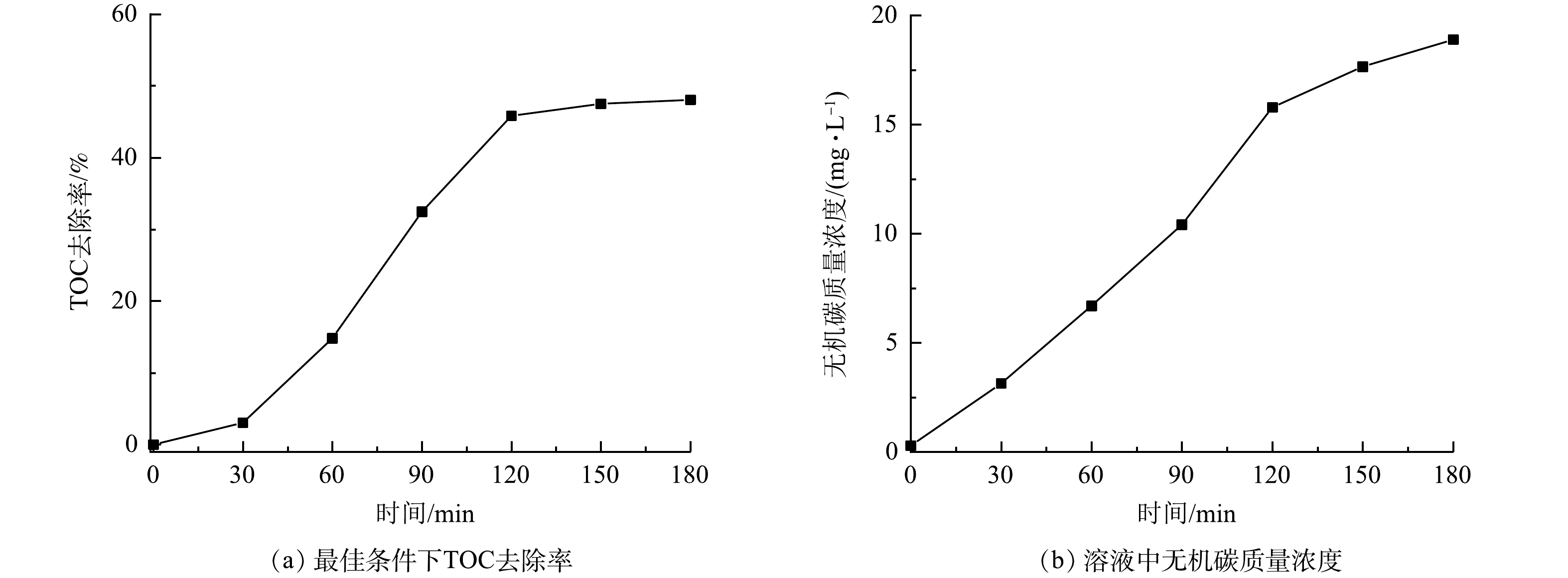
由图8(a)可见,当过硫酸盐投加量为4 g·L−1、微纳米气泡进气量为30 mL·min−1、紫外灯功率为10 W、初始pH为6时,随着反应的进行,溶液中TOC的去除率迅速升高。反应3 h 后,TOC去除率为48.05%。由图8(b)可以看出,在反应前溶液中无机碳质量浓度为0.29 mg·L−1,在180 min时反应中无机碳质量浓度为18.89 mg·L−1。反应中无机碳质量浓度随着反应时间的增加而增加,这说明反应中有机物被分解转化为 CO2 和 H2O。
-
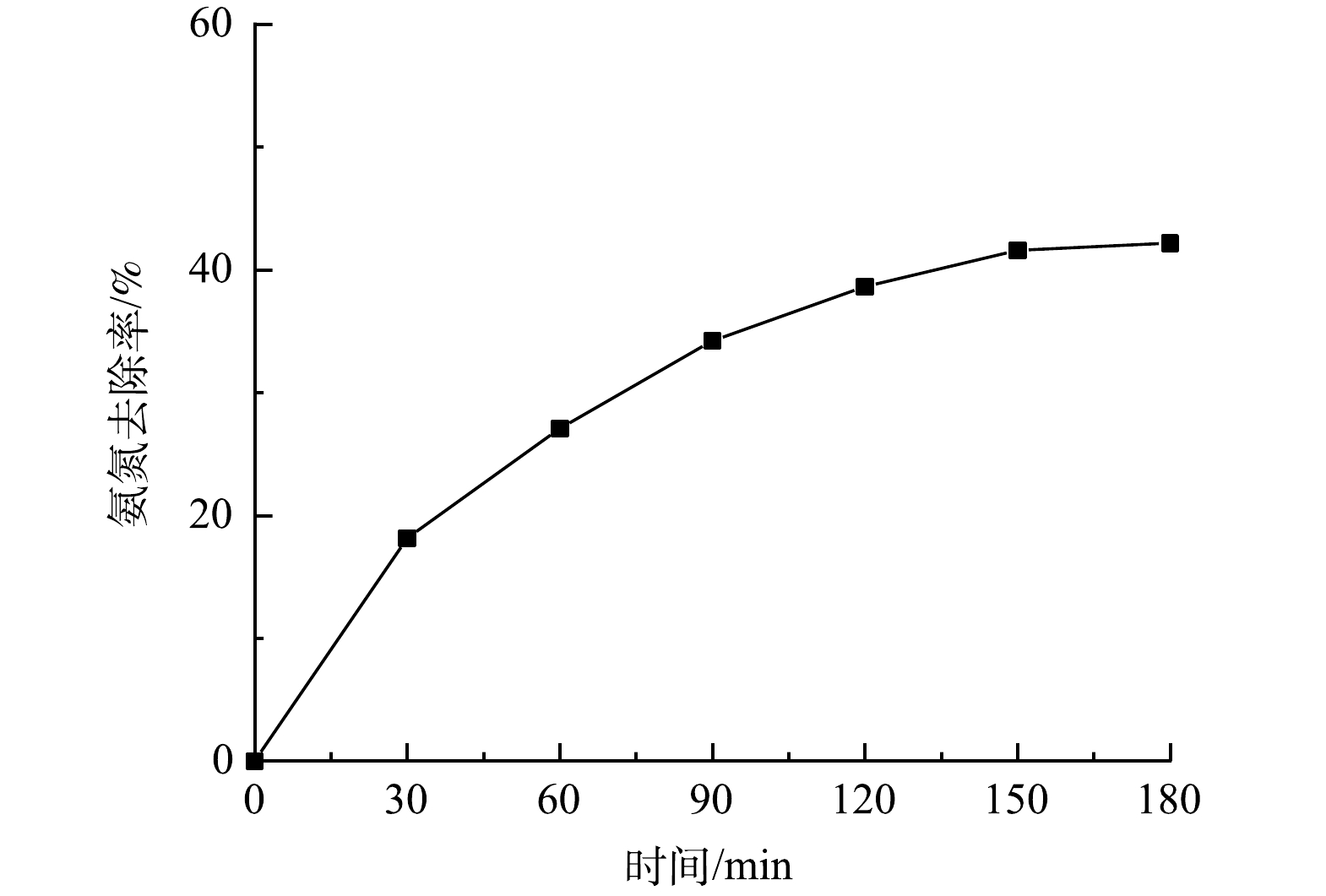
图9反映了当PS为4 g·L−1、MB进气量为30 mL·min−1、UV功率为10 W、初始pH为6时氨氮去除率的变化。可见,当反应时间为30 min时,溶液中氨氮去除率为18.2%;随着反应时间的进行,溶液中氨氮的去除率迅速增高,反应为180 min时,氨氮去除率为42.1%。
-
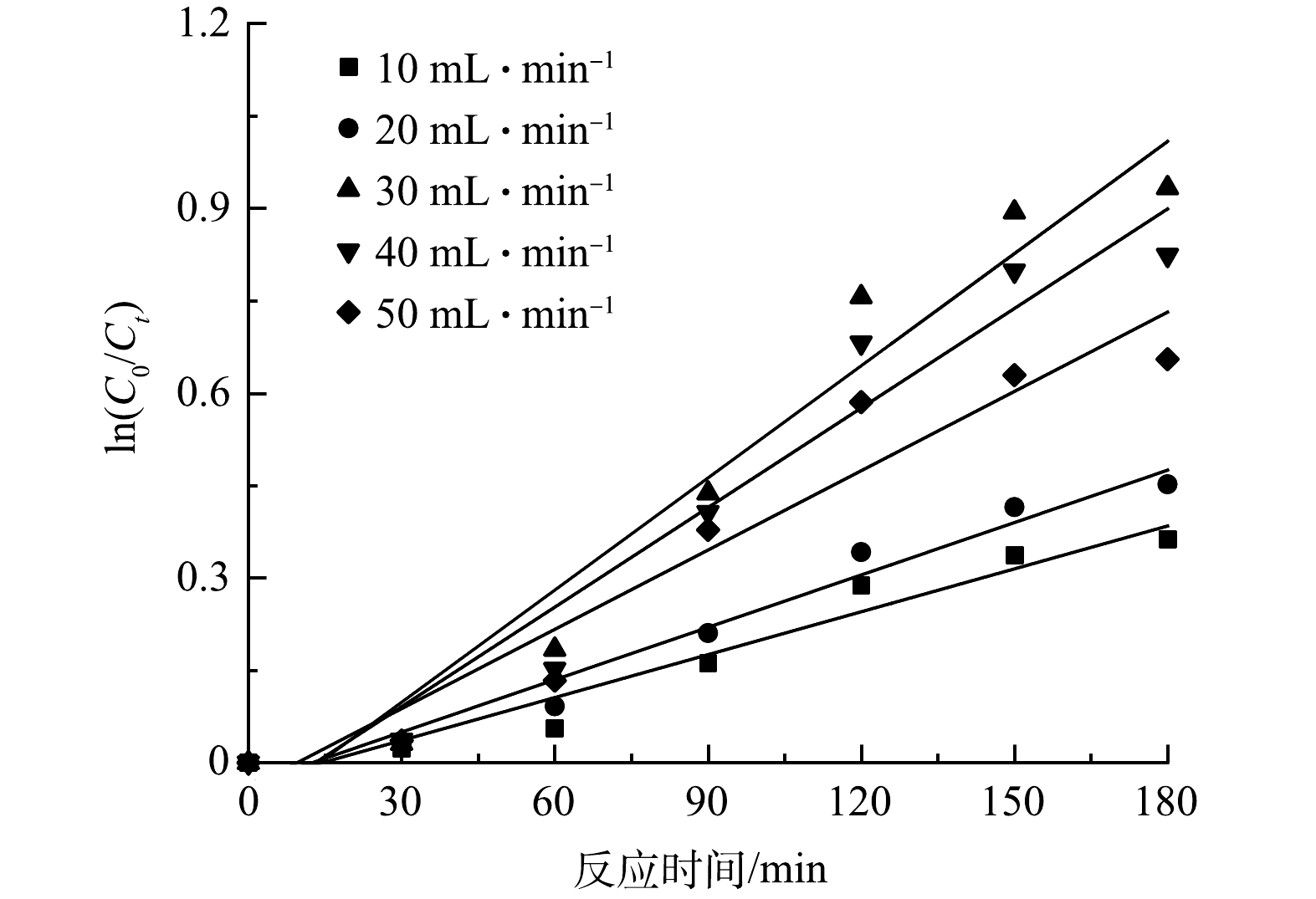
由图10可见,In(C0/Ct)与t基本呈线性关系。这表明微纳米气泡(MB)进气量对垃圾渗沥液的降解过程可用一级反应动力学方程来描述In(C0/Ct)=kt来描述,结果见表1。当MB进气量由10 mL·min−1上升到50 mL·min−1时,K先从0.002 32 min−1上升到0.006 07 min−1,然后下降至0.004 29 min−1,在MB进气量为30 mL·min−1时,半衰期t1/2为114.2 min。这主要是因为产生的微纳米气泡可以促进反应从而有效去除溶液中的有机物,当MB进气量为30 mL·min−1时,反应器中微纳米气泡含量最多,导致在溶液中会产生更多的活性自由基。
-
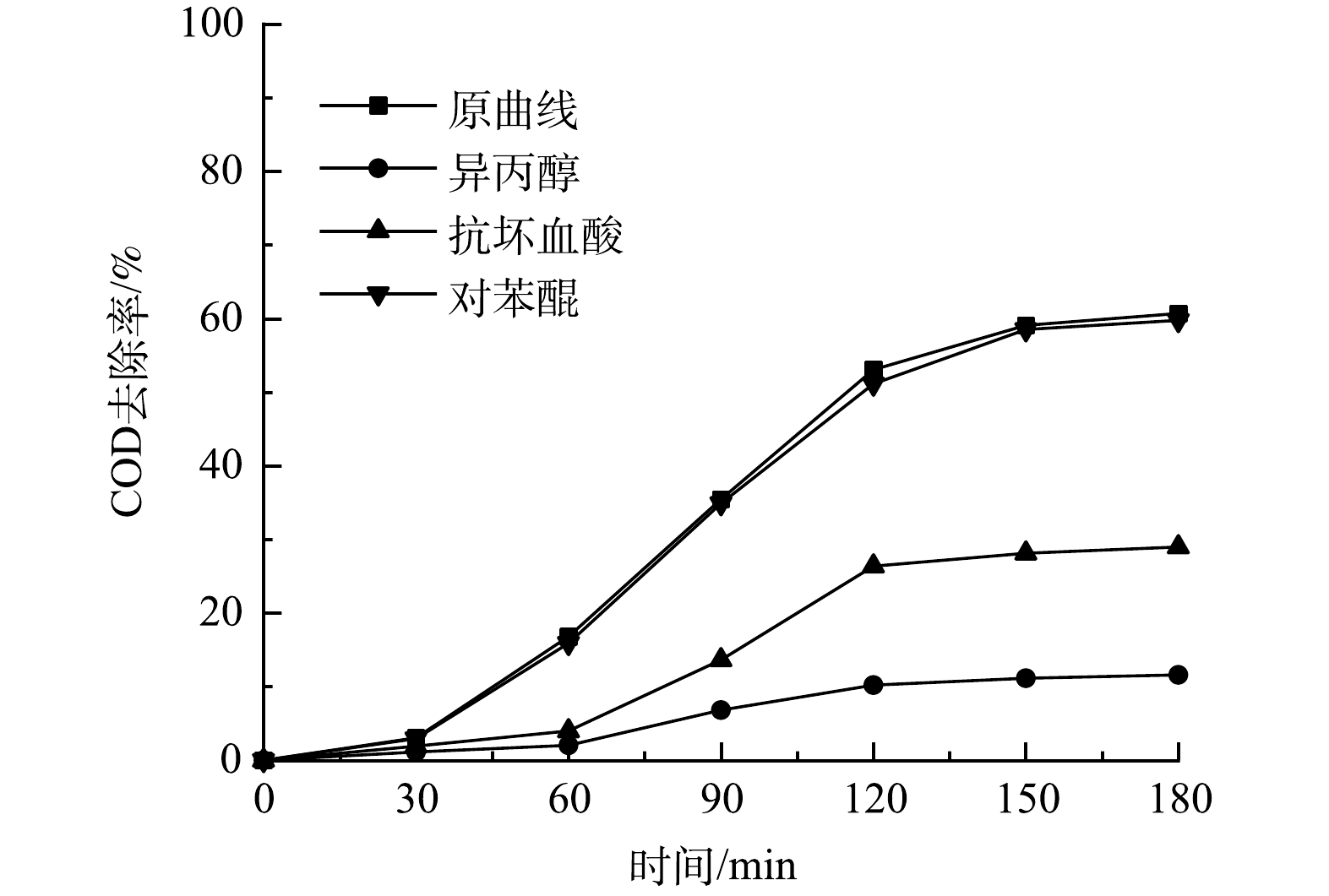
1)自由基淬灭实验。NASSERI等[30]的研究表明,异丙醇(IPA)可淬灭羟基自由基,抗坏血酸(AA)淬灭硫酸根自由基,对苯醌(BQ)可淬灭超氧自由基。图11为加入不同淬灭剂后溶液中COD去除率的变化情况。可见,加入异丙醇(IPA)后溶液COD去除率由60.68%下降为11.59%。这说明在UV/MB/PS体系中生成了羟基自由基。加入了抗坏血酸后去除率由60.68%下降为28.98%。这说明反应中生成了硫酸根自由基。加入了对苯醌后,COD去除率几乎无变化。这证明该体系中没有超氧自由基的生成。
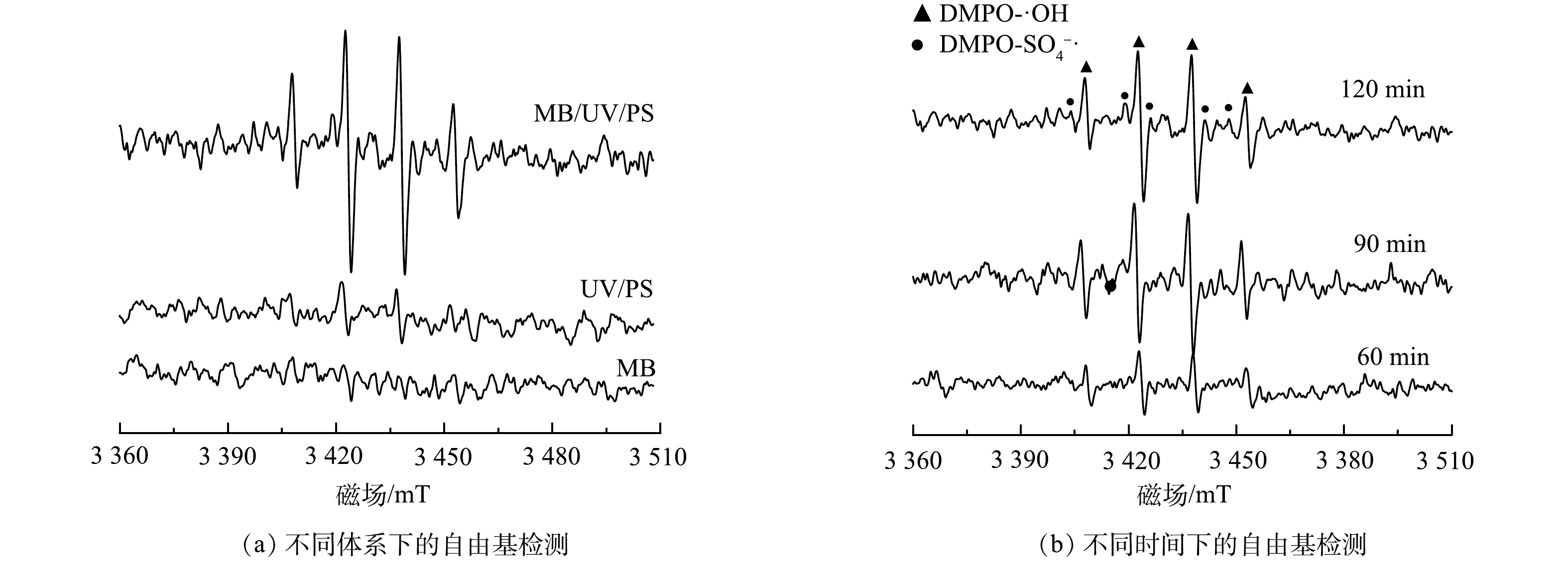
2)不同处理体系中的自由基表征。本研究采用EPR技术检测了3个反应体系中的活性物质。由图12(a)可以看出,在单独MB体系中没有活性自由基产生,而在UV/PS体系中检测出中有少量的羟基自由基和硫酸根自由基。这是由于过硫酸根在低于270 nm的紫外灯照射下分解生成硫酸根自由基,过硫酸根中的过氧键在氧化还原反应中断裂生成过一硫酸根,过一硫酸根在低于270 nm的紫外灯紫外灯照射下分解生羟基自由基和硫酸根自由基(式(4)~式(5))。在MB/UV/PS体系中可以检测出有大量的羟基自由基和硫酸自由基。这说明导入微纳米气泡使反应中的羟基自由基和硫酸根自由基浓度增加,促使反应中的污染物分解。图12(b)为在MB/UV/PS体系中分别在60、90 和120 min时的自由基检测结果。可见,溶液中检测出了羟基自由基和硫酸根自由基,在120 min时自由基浓度最大。这说明溶液中的羟基自由基和硫酸根自由基浓度随着反应时间的延长而增加。
-
1)自由基淬灭实验结果结合EPR表征结果表明,羟基自由基和硫酸根自由基是降解过程中的主要活性物质。
2)单独采用微纳米气泡对模拟垃圾渗沥液中COD的去除率仅为7.61%,使用紫外灯活化过硫酸钾对COD的去除率为9.89%,微纳米气泡活化过硫酸钾对COD的去除率为8.07%,而微纳米气泡联合紫外灯活化过硫酸钾对COD的去除率为60.68%,这表明微纳米气泡和紫外灯活化过硫酸盐体系有明显协同作用。
3)当反应时间为 180 min、PS投加量为4 g·L−1、pH=6、MB 进气量为 30 mL·min−1时,COD的去除率可达60.68%,TOC去除率为48.05%,氨氮去除率为42.1%。
微纳米气泡/UV/K2S2O8协同处理模拟垃圾渗沥液
Treatment of simulated landfill leachate by micro-nano bubble /UV/K2S2O8
-
摘要: 以垃圾渗沥液中的主要污染物冰乙酸、正丁酸、正己酸和氨组成的混合物为处理对象,采用微纳米气泡联合紫外灯活化过硫酸盐法处理模拟垃圾渗沥液,考察了反应时间、过硫酸钾投加量、pH、紫外灯功率、微纳米气泡进气量对污染物去除效果的影响。结果表明:微纳米气泡对紫外灯活化过硫酸盐法有显著的协同作用。当反应时间为180 min、过硫酸钾投加量为4 g·L−1、pH=6、紫外灯功率为10 W、微纳米气泡进气量为30 mL·min−1时,污染物的去除效果最佳,COD去除率为60.68%,TOC去除率为48.05%,氨氮去除率为42.1%。自由基淬灭实验结合EPR表征结果表明,羟基自由基和硫酸根自由基为降解过程中主要的活性物质。溶液中无机碳含量增加,说明废水中有机物分解为二氧化碳和水。Abstract: In this study, the main pollutants in landfill leachate, including glacial acetic acid, n-butyric acid, n-hexanoic acid and ammonia, were treated by micro-nano bubbles combined with UV lamp activated persulfate method. The effects of different reaction time, potassium persulfate dosage, pH, UV lamp power and gas flow of micro and nano bubbles on the removal rate of landfill leachate were studied. The results showed that micro-nano bubbles had a significant synergistic effect on UV lamp activated persulfate method. When the reaction time was 180 min, the dosage of potassium persulfate was 4 g·L−1, pH=6, uv lamp power was 10 W, and the gas flow of micro/nano bubbles was 30 mL·min−1, the removal rates of COD, TOC and ammonia nitrogen were 60.68%, 48.05% and 42.1%, respectively. The results of free radical quenching and EPR showed that the simulated landfill leachate was degraded by the attack of hydroxyl radical and sulfate radical. Total organic carbon analyzer (TOC) analysis measured the increase of inorganic carbon in the solution, indicating the decomposition of organic matter into carbon dioxide and water in wastewater.
-
Key words:
- micro-nano bubbles /
- ultraviolet lamp /
- persulfate /
- landfill leachate
-
垃圾渗沥液是一种成分复杂且难处理的高浓度有机废水[1],2021年我国生活垃圾清运量达到2.67×108 t,相对于2015年的1.91×108 t增加了约39.8%。目前,卫生填埋是我国城市生活垃圾的主要处理方式之一[2],与垃圾焚烧和堆肥处理相比,卫生填埋成本较低且工艺简单[3],但生活垃圾经过填埋处理后会发生一系列物理化学和生物化学的反应而产生垃圾渗沥液[4],据计算,每吨生活垃圾在填埋周期内约产生0.1 t垃圾渗沥液[5]。垃圾渗沥液污染物复杂且浓度超高、水质水量变化大[6]、处理难度高[2],如果处理不好,就会导致垃圾渗沥液渗入到土壤、地下水、河流中,从而会影响整个生态系统自身的修复和发展[7]。常用的垃圾渗沥液处理技术主要包括好氧生物处理、厌氧生物处理的生物法和用活性炭吸附[8]、离子交换[9]、膜渗析[10]等物理化学法。单独的生物处理方法无法使其处理达标,并且反应的周期较长;发达城市常用的反渗透处理工艺虽能将垃圾渗沥液处理达标但其耗费极大且会产生二次污染[11-14],因此,寻找高效且低成本的处理垃圾渗沥液的方法迫在眉睫。
近年来,微纳米气泡(MB)在水处理领域取得了巨大进步,其表现的特性已经超出了人们对普通气泡的认知[15]。微纳米气泡一般指直径小于100 μm气泡的总称。其中,直径大于1 μm 的为微气泡,直径小于1 μm的气泡为纳米气泡[16]。微米气泡直径其比表面积大,水中停留时间较长,传质效率高、界面电位高。与微米气泡相比,纳米气泡水中停留时间更长,其传质效率更高,界面点位更高,生物效果显著[17]。由于微纳米气泡具有很强的滞留性,同时内压较大,其高溶解能力可为水体提供高含量的溶解氧[18],微纳米气泡用于处理受污染的水体,可降低水体中的有机污染物[19],同时抑制水体中厌氧菌的有机质分解过程,降低水体中氮、磷的含量[20]。微纳米气泡气液界面带负电荷[21],可与特定的污染物反应,此外微纳米气泡破裂时产生的自由基和振动波可以促进污染物的去除[22]。故在水污染处理领域中,微纳米气泡被发现后得到了广泛的关注和应用[23]。
垃圾渗沥液成分复杂,水质不稳定,采用常规生物处理法处理效果不理想。针对于此,高级氧化技术可应用于为有效降解垃圾渗沥液。芬顿和类芬顿技术得到了广泛应用,其原理是利用·OH的强氧化性,从有机物中夺取氢原子使其分解为CO2和水等无机物或小分子有机物[24]。近年来,以SO4−·的高级氧化技术由于其较高的降解能力、优于·OH的存在寿命和对污染物的高度适应性成为目前的研究热点。过硫酸盐(PS)活化技术常用于降解水环境中的有机污染物[25],过硫酸盐在光、热、声、过渡金属离子的条件下会分解过硫酸根生成硫酸根自由基,其具有较高的氧化还原电位(E0=2.5~3.1 eV),具有强氧化能力,其主要通过电子传递和提取氢将有机污染物氧化为无机物或小分子物质,甚至直接分解成CO2和H2O[26]。目前常见的过硫酸盐活化法有热活化、过渡金属催化活化、紫外光活化等[27],有研究表明,在波长小于270 nm的紫外光照射下过硫酸盐[28],过硫酸盐中的过硫酸根会分解生成硫酸根自由基,从而有效降解有机污染物[29]。本研究利用微纳米气泡的特性将其与紫外灯(UV)活化过硫酸盐技术相结合,考察了反应时间、初始pH、过硫酸钾(PS)投加量、紫外灯功率、微纳米气泡(MB)进气量等因素对垃圾渗沥液中COD去除效果的影响。
1. 材料与方法
1.1 实验药品
冰乙酸(CH3COOH)购自重庆川东化工集团,正己酸(C6H12O2)(AR,99.0%)、氨水(NH3·H2O)(AR,28%)购自上海麦克林生化科技有限公司,冰乙酸(CH3COOH)(AR,99.5%)购自重庆川东化工集团,正丁酸(CH3CH2CH2COOH)(AR,99.0%)、过硫酸钾(K2S2O8)(AR,99.5%)购自国药集团化学试剂有限公司,5,5-二甲基-1-吡咯啉-N-氧化物(DMPO)(RG,98%)购自阿达玛斯试剂有限公司,实验用水为超纯水。
1.2 实验装置和仪器
实验装置和仪器见图1,采用体积为1.0 L的烧杯作为反应容器,实验中所用的实验和分析仪器包括:微纳米气泡发生器(LF-1500,上海行恒科技公司),加热消解器(DRB200,美国哈希),紫外分光度计(DR 6000),总有机碳分析仪(TOC-LCPH,日本岛津),连续流动分析仪(荷兰 SKALAR San++),顺磁共振波谱仪(EMXnano,日本岛津)。
1.3 实验方法
称取一定量的过硫酸钾加入到由1.74 mmol·L−1冰乙酸、1.08 mmol·L−1正丁酸、2.56 mmol·L−1正己酸和1.94 mmol·L−1氨水溶液组成的1 000 mL模拟垃圾渗沥液中,调节溶液pH,将废水经过微纳米气泡发生器产生微纳米气泡,调节压力阀和进气量至溶液呈乳白色,同时在烧杯中放入254 nm紫外灯并开启紫外灯,每隔30 min取样于紫外分光度计、总有机碳分析仪和连续流动分析仪测试其COD、TOC和氨氮。TOC和氨氮的去除率率按照式(1)、式(2)和式(3)进行计算。
RC=C0−CtC0×100% (1) RT=T0−TtT0×100% (2) RN=N0−NtN0×100% (3) 式中:RC为COD去除率,%;C0为初始COD, mg·L−1;Ct为反应t时刻的COD,mg·L−1;RT为TOC去除率,%;T0为其初始TOC,mg·L−1;Tt为t时刻的TOC,mg·L−1;RN为氨氮去除率,%;N0为初始氨氮浓度,mg·L−1;Nt为反应t时刻的氨氮浓度,mg·L−1。
2. 结果与讨论
2.1 不同条件下垃圾渗沥液中COD的去除效果
图2为MB进气量为30 mL·min−1,在不同条件下废水COD的去除效果。由图2可以看出,仅有微纳米气泡的条件下,反应180 min后废水COD去除率为7.61%。在MB/PS体系中,PS投加量为4 g·L−1时,180 min后COD去除率为8.07%。在UV/PS体系中,当UV的功率为10W,PS投加量为4 g·L−1时,180 min后垃圾渗沥液的COD去除率为9.89%。在MB/UV体系中,反应180 min后COD去除率为7.39%。在常规气泡(CB)的条件下,CB/UV/PS体系对COD的去除效果有一定的提升,经180 min常规气泡曝气反应后,COD去除率为34.89%,在MB/UV/PS体系中,当紫外灯功率为10 W、过硫酸盐投加量为4 g·L−1时,COD去除率达到了60.68%。由此可见,MB/UV/PS体系对垃圾渗沥液COD有良好的去除效果。这是由于在紫外灯活化过硫酸盐条件下,导入微纳米气泡后,传质加快[17],同时提高了羟基自由基和硫酸根自由基产生的浓度,因此,在该条件下COD的去除率显著提升。
2.2 反应时间对COD去除效果的影响
在MB/UV/PS体系中,当溶液pH=6.0,过硫酸盐投加量为4 g·L−1,MB进气量为30 mL·min−1,紫外灯功率为10 W时,COD去除率随反应时间的变化如图3所示。过硫酸盐在微纳米气泡和紫外灯的协同作用下产生硫酸根自由基,过硫酸盐中的过氧键会在氧化还原反应中断裂生成过一硫酸根,过一硫酸根在微纳米气泡和紫外灯的活化下产生羟基自由基和硫酸根自由基,而硫酸根自由基和羟基自由基会通过电子传递和提取氢将垃圾渗沥液中的有机污染物氧化为无机物或小分子物质,随着反应的进行,COD去除率稳步上升,反应180 min时可达到60.68%。
2.3 PS投加量对COD去除效果的影响
图4反映了PS投加量对垃圾渗沥液COD去除效果的影响。由图4可以看出,随着PS投加量的增加,COD的去除率有所增高。当PS投加量为2、3、4、5、6 g·L−1时,经过3 h的反应,COD的去除率分别为52.73%、54.32%、60.68%、60.57%、59.77%,PS投加量超过4 g·L−1时,COD去除率没有随着PS继续投加而继续增加。这说明溶液中的过硫酸盐已达到饱和,4 g·L−1为PS的最佳投放量。
2.4 MB进气量对COD去除效果的影响
进气量决定微纳米气泡的产生量[17]。图5为MB进气量对COD去除率的影响。可见在MB/UV/PS体系中,MB进气量对模拟废水中COD的去除率随MB的进气量的增加呈先升高后降低的变化趋势。当MB进气量由 10 mL·min−1 增至30 mL·min−1时,COD去除率逐渐升高,并在 30 mL·min−1时达到最高,为60.68%。而随着 MB进气量由30 mL·min−1增加到 60 mL·min−1时,COD去除率降至48.07%。这说明微纳米气泡的最佳进气量为30 mL·min−1。
2.5 紫外灯功率对COD去除效果的影响
图6为紫外灯(UV)的功率对COD去除率的影响。由图6可见,随着紫外灯功率的不断提升,COD去除率不断增加。当紫外灯为5 W时,COD去除率为35.23%;在紫外灯功率为15 W时去除率达到61.36%。这说明随着紫外灯功率的增加,会产生活性自由基,进而去除有机污染物,但随着紫外灯功率的进一步提升,溶液的COD去除率不再增高。
2.6 初始pH对COD去除效果的影响
图7为模拟垃圾渗沥液初始pH对后续反应的COD去除效果的影响。可见,在MB/UV/PS体系中,当溶液pH=2时,COD去除率为43.07%;随着pH的增加,COD去除率增加,在pH=6时去除率达到最高,为60.68%。当在pH在碱性条件下COD去除率大幅度降低,当溶液pH=10时,去除率仅仅只有2.73%。这说明废水中的有机酸在碱性条件下生成的盐会抑制污染物的降解。
2.7 最佳条件下TOC去除率
由图8(a)可见,当过硫酸盐投加量为4 g·L−1、微纳米气泡进气量为30 mL·min−1、紫外灯功率为10 W、初始pH为6时,随着反应的进行,溶液中TOC的去除率迅速升高。反应3 h 后,TOC去除率为48.05%。由图8(b)可以看出,在反应前溶液中无机碳质量浓度为0.29 mg·L−1,在180 min时反应中无机碳质量浓度为18.89 mg·L−1。反应中无机碳质量浓度随着反应时间的增加而增加,这说明反应中有机物被分解转化为 CO2 和 H2O。
2.8 最佳条件下氨氮去除率
图9反映了当PS为4 g·L−1、MB进气量为30 mL·min−1、UV功率为10 W、初始pH为6时氨氮去除率的变化。可见,当反应时间为30 min时,溶液中氨氮去除率为18.2%;随着反应时间的进行,溶液中氨氮的去除率迅速增高,反应为180 min时,氨氮去除率为42.1%。
2.9 反应中的一级反应动力学
由图10可见,In(C0/Ct)与t基本呈线性关系。这表明微纳米气泡(MB)进气量对垃圾渗沥液的降解过程可用一级反应动力学方程来描述In(C0/Ct)=kt来描述,结果见表1。当MB进气量由10 mL·min−1上升到50 mL·min−1时,K先从0.002 32 min−1上升到0.006 07 min−1,然后下降至0.004 29 min−1,在MB进气量为30 mL·min−1时,半衰期t1/2为114.2 min。这主要是因为产生的微纳米气泡可以促进反应从而有效去除溶液中的有机物,当MB进气量为30 mL·min−1时,反应器中微纳米气泡含量最多,导致在溶液中会产生更多的活性自由基。
表 1 进气量对COD去除效果和反应速率的影响Table 1. Effect of gas flow on COD removal effect and reaction rateMB进气量/(mL·min−1) K/min−1 t1/2/min R2 10 0.002 32 298.770 336 0.943 1 20 0.002 84 244.065 908 0.964 1 30 0.006 07 114.192 287 0.945 58 40 0.005 39 128.598 735 0.939 7 50 0.004 29 161.572 769 0.924 2 2.10 反应中的活性自由基分析
1)自由基淬灭实验。NASSERI等[30]的研究表明,异丙醇(IPA)可淬灭羟基自由基,抗坏血酸(AA)淬灭硫酸根自由基,对苯醌(BQ)可淬灭超氧自由基。图11为加入不同淬灭剂后溶液中COD去除率的变化情况。可见,加入异丙醇(IPA)后溶液COD去除率由60.68%下降为11.59%。这说明在UV/MB/PS体系中生成了羟基自由基。加入了抗坏血酸后去除率由60.68%下降为28.98%。这说明反应中生成了硫酸根自由基。加入了对苯醌后,COD去除率几乎无变化。这证明该体系中没有超氧自由基的生成。
2)不同处理体系中的自由基表征。本研究采用EPR技术检测了3个反应体系中的活性物质。由图12(a)可以看出,在单独MB体系中没有活性自由基产生,而在UV/PS体系中检测出中有少量的羟基自由基和硫酸根自由基。这是由于过硫酸根在低于270 nm的紫外灯照射下分解生成硫酸根自由基,过硫酸根中的过氧键在氧化还原反应中断裂生成过一硫酸根,过一硫酸根在低于270 nm的紫外灯紫外灯照射下分解生羟基自由基和硫酸根自由基(式(4)~式(5))。在MB/UV/PS体系中可以检测出有大量的羟基自由基和硫酸自由基。这说明导入微纳米气泡使反应中的羟基自由基和硫酸根自由基浓度增加,促使反应中的污染物分解。图12(b)为在MB/UV/PS体系中分别在60、90 和120 min时的自由基检测结果。可见,溶液中检测出了羟基自由基和硫酸根自由基,在120 min时自由基浓度最大。这说明溶液中的羟基自由基和硫酸根自由基浓度随着反应时间的延长而增加。
S2O2−8hv→2SO−4⋅ (4) HSO−5hv→SO−4⋅+OH⋅ (5) 3. 结论
1)自由基淬灭实验结果结合EPR表征结果表明,羟基自由基和硫酸根自由基是降解过程中的主要活性物质。
2)单独采用微纳米气泡对模拟垃圾渗沥液中COD的去除率仅为7.61%,使用紫外灯活化过硫酸钾对COD的去除率为9.89%,微纳米气泡活化过硫酸钾对COD的去除率为8.07%,而微纳米气泡联合紫外灯活化过硫酸钾对COD的去除率为60.68%,这表明微纳米气泡和紫外灯活化过硫酸盐体系有明显协同作用。
3)当反应时间为 180 min、PS投加量为4 g·L−1、pH=6、MB 进气量为 30 mL·min−1时,COD的去除率可达60.68%,TOC去除率为48.05%,氨氮去除率为42.1%。
-
表 1 进气量对COD去除效果和反应速率的影响
Table 1. Effect of gas flow on COD removal effect and reaction rate
MB进气量/(mL·min−1) K/min−1 t1/2/min R2 10 0.002 32 298.770 336 0.943 1 20 0.002 84 244.065 908 0.964 1 30 0.006 07 114.192 287 0.945 58 40 0.005 39 128.598 735 0.939 7 50 0.004 29 161.572 769 0.924 2 -
[1] YUAN Y C, LIU J D, et al. Ozone direct oxidation pretreatment and catalytic oxidation post-treatment coupled with ABMBR for landfill leachate treatment[J]. Science of the Total Environment, 2021, 794(1-4): 148557. [2] LI R W, LI L, ZHANG Z M, et al. Limiting factors of heavy metals removal during anaerobic biological pretreatment of municipal solid waste landfill leachate[J]. Journal of Hazardous Materials, 2021, 416: 126081. doi: 10.1016/j.jhazmat.2021.126081 [3] DAIANA S, HELOISE Q, ROSANGELA B, et al. Presence of endocrine disrupting chemicals in sanitary landfill leachate, its treatment and degradation by Fenton based processes: A review[J]. Process Safety and Environmental Protection, 2019, 131(C): 255-267. [4] CHEN G Y, WU G Y, LI N, et al. Landfill leachate treatment by persulphate related advanced oxidation technologies[J]. Journal of Hazardous Materials, 2021, 418(4): 126355. [5] GAO M, LI S Q, ZOU H J, et al. Aged landfill leachate enhances anaerobic digestion of waste activated sludge[J]. Journal of Environmental Management, 2021, 293(2/3): 112853. [6] LOPEZ A, PAGANO M, VOLPE A, et al. Fenton's pre-treatment of mature landfill leachate[J]. Chemosphere, 2004, 54(7): 1005-1010. doi: 10.1016/j.chemosphere.2003.09.015 [7] SILVA L, ALVESl V M, DANTAS E, et al. Chemical safety assessment of transformation products of landfill leachate formed during the Fenton process[J]. Journal of Hazardous Materials, 2021, 419(80): 126438. [8] ZHOU Y R, HUANG K Y, JIAO X Y, et al. Anaerobic co-digestion of organic fractions of municipal solid waste: Synergy study of methane production and microbial community[J]. Biomass and Bioenergy, 2021, 151: 106137. doi: 10.1016/j.biombioe.2021.106137 [9] RESHADI M, HASANI S S, NAZARIPOUR M, et al. The evolving trends of landfill leachate treatment research over the past 45 years.[J]. Environmental Science and Pollution Research International, 2021, 28(47): 1-19. [10] ATE H, ARGUN M E. Advanced oxidation of landfill leachate: Removal of micropollutants and identification of by-products[J]. Journal of Hazardous Materials, 2021, 413(5): 125326. [11] JIANG N, HUANG L, HUANG M H, et al. Electricity generation and pollutants removal of landfill leachate by osmotic microbial fuel cells with different forward osmosis membranes[J]. Sustainable Environment Research, 2021, 31(1): 22. doi: 10.1186/s42834-021-00095-7 [12] SHI L, CHEN H X, MENG H L, et al. How environmental policy impacts technology adoption: A case of landfill leachate[J]. Journal of Cleaner Production, 2021, 310: 127484. doi: 10.1016/j.jclepro.2021.127484 [13] LEBRON Y, MOREIRA V R, BRASIL Y L, et al. A survey on experiences in leachate treatment: Common practices, differences worldwide and future perspectives[J]. Journal of Environmental Management, 2021, 288: 112475. doi: 10.1016/j.jenvman.2021.112475 [14] GHANBARI F, KHATEBASREH M, MAHDAVIANPOUR M, et al. Evaluation of peroxymonosulfate/O3/UV process on a real polluted water with landfill leachate: Feasibility and comparative study[J]. Korean Journal of Chemical Engineering, 2021, 38(7): 1416-1424. doi: 10.1007/s11814-021-0782-8 [15] 柳伟. 我国垃圾渗滤液处理现状探究[J]. 生物化工, 2021, 7(6): 3. doi: 10.3969/j.issn.2096-0387.2021.06.043 [16] 吴娜, 薛晓莉, 张志立, 等. 微纳米气泡技术在环保领域的应用研究进展[J]. 现代农业科技, 2020(17): 154-155+160. doi: 10.3969/j.issn.1007-5739.2020.17.096 [17] 刘畅, 唐玉朝, 王品之. 微纳米气泡在治理水体污染方面的应用研究[J]. 安徽建筑大学学报, 2020, 28(03): 6. [18] WU Y F, LIN H, YIN W Z, et al. Water quality and microbial community changes in an urban river after micro-nano bubble technology in situ treatment[J]. Water, 2019, 11(1): 66. doi: 10.3390/w11010066 [19] HUAMING, HE, LIANG. Research on the feasibility of spraying micro/nano bubble ozonated water for airborne disease prevention[J]. Ozone:Science & Engineering, 2015, 37(1): 78-84. [20] SHARIFUZZAMAN, M. D, YANG H N, Park S M. Park, et al. Performance comparison of micro-nano bubble, electro-oxidation and ozone pre-treatment in reducing fluoride from industrial wastewater[J]. Engineering in Agriculture, Environment and Food, 2017, 10(3): S1881836617300216. [21] 李恒震, 胡黎明, 辛鸿博. 微纳米气泡技术应用于污染地下水原位修复研究[J]. 岩土工程学报, 2015, 37(S2): 115-120. doi: 10.11779/CJGE2015S2023 [22] 吕宙, 从善畅, 程婷, 等. 微纳米气泡曝气技术在生活污水处理中的应用研究[J]. 广州化工, 2014, 42(7): 4. doi: 10.3969/j.issn.1001-9677.2014.07.046 [23] DAYARATHNE H, CHOI J, JANG A. Enhancement of cleaning-in-place (CIP) of a reverse osmosis desalination process with air micro-nano bubbles[J]. Desalination, 2017, 422: 1-4. doi: 10.1016/j.desal.2017.08.002 [24] 杨世迎, 杨鑫, 王萍, 等. 过硫酸盐高级氧化技术的活化方法研究进展[J]. 现代化工, 2009, 29(4): 13-19. doi: 10.3321/j.issn:0253-4320.2009.04.004 [25] LUO Y T, SU R K, YAO H S, et al. Degradation of trimethoprim by sulfate radical-based advanced oxidation processes: kinetics, mechanisms, and effects of natural water matrices.[J]. Environmental Science and Pollution Research International, 2021, 28(44): 62572-62582. doi: 10.1007/s11356-021-15146-0 [26] LU Y T, SHEN Y L, ZHANG S F, et al. Enhancement of removal of VOCs and odors from wood by microwave-activated persulfate.[J]. ACS Omega, 2021, 6(8): 5945-5952. doi: 10.1021/acsomega.1c00126 [27] GUO J Y, GAO Q F, YANG S Q, et al. Degradation of pyrene in contaminated water and soil by Fe2+-activated persulfate oxidation: Performance, kinetics, and background electrolytes (Cl-, HCO3- and humic acid) effects.[J]. Process Safety and Environmental Protection, 2021, 146: 686-693. doi: 10.1016/j.psep.2020.12.003 [28] 张国珍, 王宏伟, 李晓燕, 等. 紫外活化过硫酸盐降解水中阿莫西林的特性[J]. 中国给水排水, 2021, 37(19): 53-59. doi: 10.19853/j.zgjsps.1000-4602.2021.19.009 [29] 徐朋飞, 郭怡秦, 王光辉, 等. 紫外活化过硫酸盐对甲基橙脱色处理实验研究[J]. 环境工程, 2017, 35(11): 5. doi: 10.13205/j.hjgc.201711013 [30] NASSERI S, MAHVI A H, SEYEDSALEHI M, et al. Degradation kinetics of tetracycline in aqueous solutions using peroxydisulfate activated by ultrasound irradiation: Effect of radical scavenger and water matrix[J]. Journal of Molecular Liquids, 2017: 704-714. -




 下载:
下载: