-
当今在污水处理领域中,以能源自给、资源回收为核心的“碳中和”理念逐渐渗透,成为新型污水处理技术的根本要求。厌氧氨氧化工艺(anaerobic ammonium oxidation,anammox,后简写为AMX)作为新型脱氮技术,因其具有能耗低、流程短、污泥产量少等优势已经成为研究热点[1]。部分亚硝化/厌氧氨氧化工艺(partial nitritation/anammox,PN/A)是基于AMX的典型污水脱氮技术,其机理是利用好氧氨氧化菌(AOB)将氨氮部分氧化为亚硝酸盐,而后厌氧氨氧化菌(AnAOB)利用生成的亚硝酸盐和剩余的氨氮生成氮气。AOB和AnAOB的高效协同是PN/A工艺稳定的基本要求。
PN/A工艺已成功应用于城镇污水厂侧流脱氮和高浓度氨氮工业废水处理,但在城镇污水厂主流工艺应用中面临重大挑战,如氨氮浓度较低、废水温度随季节性波动较大,在冬季温度会低至10 ℃ 左右等。有研究[2]表明,AnAOB最佳生长温度为30~40 ℃,温度下降会降低反应器中的AnAOB活性。杨朝晖等[3]研究不同的降温策略对AnAOB活性的影响,发现AnAOB活性与降温幅度之间存在显著负相关关系,即降温幅度越大,AnAOB活性越低。废水温度的波动给PN/A工艺的稳定运行带来挑战,温度冲击对PN/A脱氮性能的影响有待深入研究。
温度下降不仅降低PN/A系统中颗粒污泥活性,还会导致菌群结构以及胞外聚合物(EPS)的变化。温度对微生物活性产生不同程度的影响,导致菌群间的竞争关系变化,易引起原有菌群间的协同关系失衡。有研究表明,经历长期低温培养后,AnAOB的最适温度前移,PN/A颗粒污泥在低温下的脱氮处理效率趋于稳定[4]。LAURENI等[5]通过逐步降低温度适应低温微生物驯化,发现AMX系统可以耐受10~15 ℃ 的温度。但温度冲击对PN/A污泥的脱氮性能以及微生物种群协同的影响尚不清晰。EPS作为微生物的分泌物及颗粒污泥的重要组成部分,在PN/A污泥系统运行过程中环境发生剧烈变化时,其含量和组分就会相应变化[6]。然而,在低温废水处理过程中,PN/A反应器的EPS特性尚未得到充分的研究。近年来群体感应(quorum sensing, QS)作为细菌交流的一种手段受到关注,即利用细菌产生的自诱导信号分子来调控种群密度和生物膜、颗粒污泥的形成。AnAOB的有些生理特性,如SAA、生长速率和EPS分泌都与QS相关[7],进一步了解EPS与QS的相互作用有助于PN/A工艺的实际工程应用。
本研究考察了PN/A颗粒污泥系统在短期温度冲击下的应激效应及恢复能力,系统考察了短期温度冲击以及不同的降温幅度对PN/A颗粒污泥中AOB、AnAOB和NOB活性及菌群间协同效应的影响和脱氮性能恢复的可行性;建立了温度与系统脱氮负荷的变化模型,分析了EPS对温度冲击的响应规律,为PN/A工艺在主流污水处理中的应用及其提高其应对温度波动的脱氮稳定性提供参考。
-
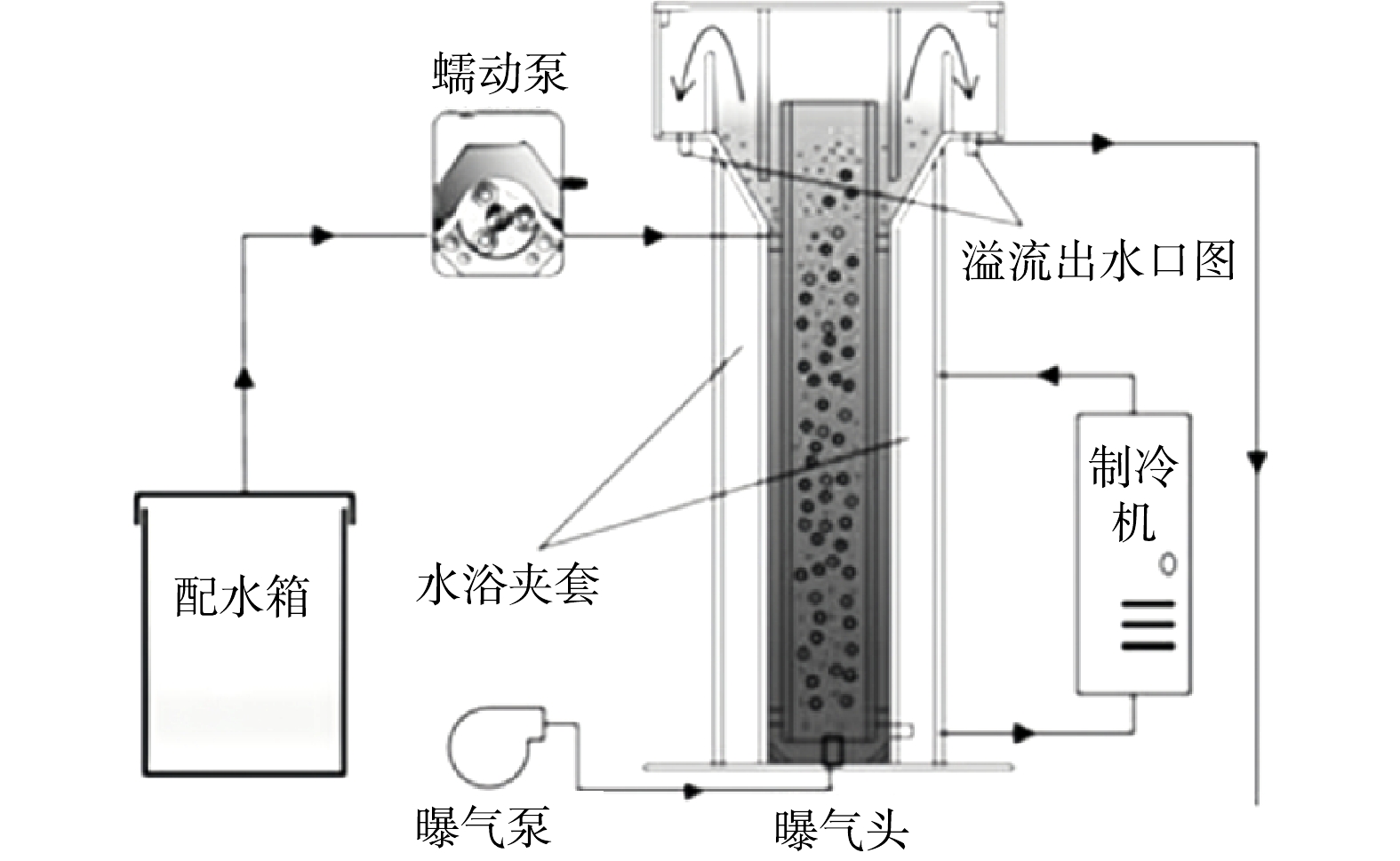
本研究采用圆柱形气提式反应器,有效容积为2 L(图1)。进水和压缩空气均由反应器中心管底部进入,颗粒污泥在气提条件下完全呈流化状态,在顶部沉淀区泥水分离后出水,污泥经内外循环再回到中心管。反应器外壁设有夹套,可通过冷热水浴调控反应温度。
接种污泥为实验室常温下培养的PN/A颗粒污泥,颜色为棕黄色,平均粒径为1.0 mm,反应器污泥质量浓度(MLSS)约为4.5 g·L−1, MLVSS/MLSS为0.88, SVI5值在52 mL·g−1左右,污泥具有良好的沉降性能。
整个实验过程维持进水氨氮质量浓度为100 mg·L−1,不外加有机碳源,水力停留时间(HRT) 为1.5 h,氨氮负荷维持在1.63 kg·(m3·d)−1。具体运行工况如表1所示。实验共分为5个阶段,第Ⅰ阶段在30 ℃ 下稳定运行1~30 d,后续4个阶段以不同幅度(5、10、18和23 ℃ ) 进行降温与恢复,考察温度冲击对PN/A脱氮性能的影响以及温度冲击后PN/A的性能恢复情况。期间pH稳定在7.43~7.74,溶解氧质量浓度维持在0.8~1.4 mg·L−1。
-
NH4+-N、NO3−-N、NO2−-N和TN浓度分别采用纳氏试剂光度法、紫外分光光度法、N-(1-萘基)-乙二胺光度法和过硫酸钾氧化-紫外分光光度法测定。pH和溶解氧(DO)分别采用便携式pH计(METTLER TOLEDO)和HACH HQ30d型溶解氧仪。
通过批次实验测定温度冲击对PN/A污泥氮转化性能的影响以此表征微生物的活性变化,具体操作参考文献[8]。从初始的30 ℃ 分别降温至25、20、12、7 ℃ ,取反应器中各阶段的颗粒污泥混合液10 mL,装入配备有透气硅胶塞的150 mL锥形瓶中,加入90 mL反应器进水(pH=7.6~7.8),并置于机械摇床中,控制转速为160 r·min−1。每隔10 min或20 min,取样测定上清液中亚硝态氮浓度、硝态氮和氨氮,并计算氨素转化速率。
为了更好地理解温度变化对EPS的影响,本研究提取位于核心的紧密结合的TB-EPS、中间层松散结合的LB-EPS和最外层黏液的S-EPS[9],并采用三维荧光光谱(EEM) 法进行分析[10]。颗粒污泥胞外聚合物(EPS) 采用甲醇-NaOH 法[11]提取,蛋白质(PN)和多糖(PS)分别采用 Lowry 法和改进苯酚-硫酸法测定[12]。
-
采用批次实验考察不同运行工况下污泥的脱氮性能,并且结合拟合线性分析,通过测定氮素含量变化分别计算氨氮比降解速率(q(NH4+ -N))、硝态氮比累积速率(q(NO3--N))、亚硝态氮的比累积速率(q(NO2--N))和总氮比降解速率(q(TN))(以 N/MLVSS 计),单位为 mg·(g·h)−1,计算方法参考文献[8]。
-
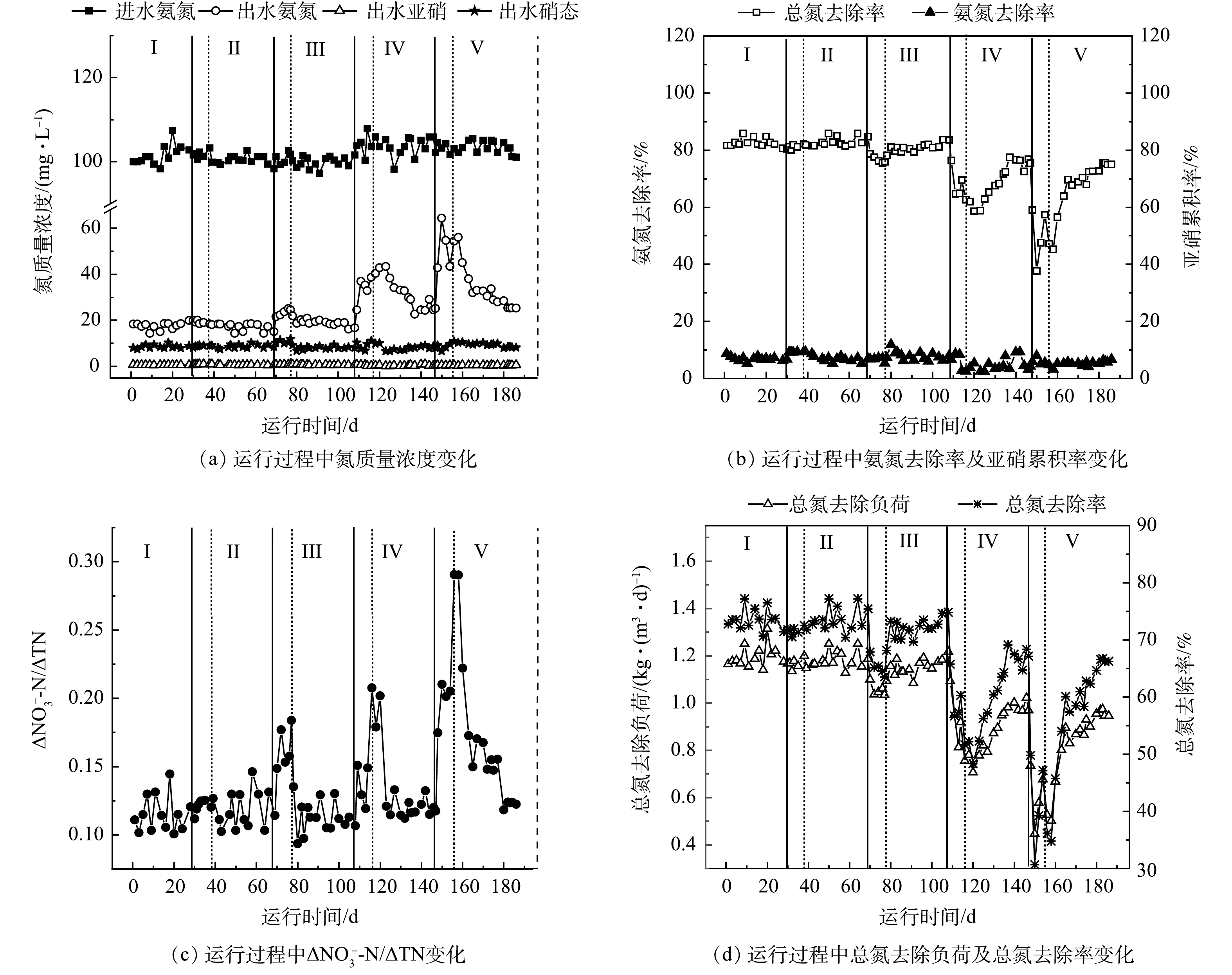
不同温度冲击与恢复过程中反应器运行效能如图2所示。第I阶段(1~30 d) ,将常温培养的PN/A颗粒污泥接种于30 ℃反应器中,系统运行性能稳定,总氮平均去除率维持在73.48%左右。在第II阶段(31~38 d) ,降温至25 ℃ ,系统脱氮性能未出现明显变化。在第39天温度恢复到30 ℃ 运行,总氮去除率维持稳定。如图2(c) 所示,ΔNO3−-N/ΔTN也一直在0.11左右。
第III阶段,系统在第70天由30 ℃ 降温至20 ℃ ,运行7 d后,总氮去除率略有下降,由73.64%降至65.32%,ΔNO3−-N/ΔTN值上升至0.15。而后系统温度恢复至30 ℃ 后再运行30 d,这一阶段总氮平均去除率为72.06%,总氮去除率在2 d内恢复至73.26%。ΔNO3−-N/ΔTN值始终稳定在0.11,恢复到未受过低温冲击的初始水平。
第IV阶段,在第109天,温度由30 ℃ 降温至12 ℃ 运行7 d。期间,出水氨氮去除率由最初的82.52%急剧下降至67.62%。图2(b) 中亚硝累积率有所上升(由6.68%升至8.61%),ΔNO3−-N/ΔTN值升至0.15。而图2(d) 中总氮去除率及总氮去除负荷均有下降,其中总氮去除率下降至58.35%。而后,系统在30 ℃ 条件下恢复运行30 d,总氮去除率缓慢逐渐提升至61.28%左右,与第I阶段的脱氮性能相比下降了16.6%。
第V阶段(148~155 d) 时,温度从30 ℃ 降至7 ℃ ,降幅达23 ℃ ,系统脱氮性能快速下降,7 d内,总氮去除率下降至40.6%,而ΔNO3−-N/ΔTN均值从0.12上升至0.30,总氮去除负荷降至0.67 kg·(m3·d)−1。系统在第156 天恢复至30 ℃ 运行30 d后,最终总氮去除率仅恢复至66.27%,与第I阶段相比,总氮去除率下降21.6%。当经历过7 ℃ 冲击后,再次将系统温度恢复至30 ℃ ,ΔNO3−-N/ΔTN也会下降,最终ΔNO3−-N/ΔTN平均值约为0.15,较第I阶段升高了近38.73%。
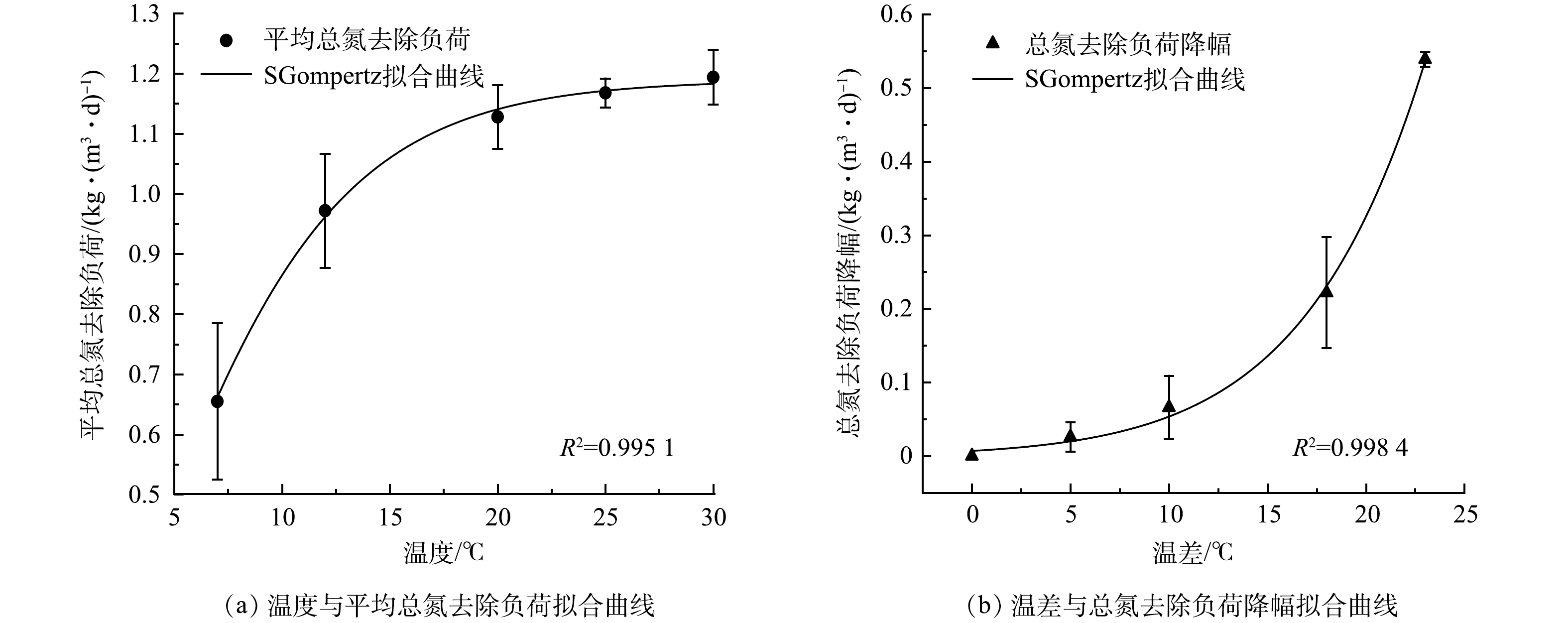
SGompertz模型曾成功用于拟合海藻糖添加条件下单周期内氨素的降解过程[13]。本实验采用该模型拟合平均总氮去除负荷与温度以及总氮去除负荷差值与降温幅度的关系,可决系数R2分别为0.9951和0.9984。如图3所示,本实验数据符合预测值, 7 ℃ 下实验得出平均总氮负荷为0.67 kg·(m3·d)−1,曲线拟合预测值为0.66 kg·(m3·d)−1。预测值与实验值具有很好的相关性,表明该模型可以很好地描述温度冲击与脱氮效能的关系。温度在25~30 ℃ ,总氮去除负荷相对稳定,温度低于20 ℃ ,总氮去除负荷快速下降(图3(a) ) 。温度降幅越大,总氮去除负荷降幅呈指数级增长(图3(b) ) 。采用SGompertz模型对温度与系统总氮去除负荷,以及温度降幅与总氮去除负荷变化具有很好的拟合效果,可以用于指导实际工程运行中系统稳定运行以及应对温度变化提供最佳运行参数。
-
本研究的第I~II阶段,脱氮性能无明显变化,这与钱飞跃等[4]研究结果一致。PN/A颗粒污泥经低温驯化后,最佳温度可由原来的35 ℃ 降至为25 ℃ 。有研究表明,温差2、4、6 ℃的波动对AMX系统脱氮性能不会产生明显影响[14]。HU等[15]的研究表明,AnAOB的生物反应器温度在1 d内从30 ℃ 降至25 ℃ ,脱氮性能无不良影响。
在工程实践中常用温度依赖性(θ)值来表征颗粒污泥的敏感性,其随着温度的降低而增加[16]。通常AnAOB较AOB、NOB对温度更加敏感,在低温条件下AnAOB的活性更易受抑制[17- 18]。LOTTI等[19]的研究也证实AnAOB的θ值在较低温度(10~20 ℃ ) 下增加。20℃ 通常被认为是影响PNA系统脱氮效率的分界线。温度从35 ℃ 降到20 ℃ ,会 导致生物量比活性大幅下降[20],但在P/NA反应器中仍可实现了71.4%的高脱氮效率[21]。这与本研究的结论相似。本研究PN/A颗粒污泥在这一温度范围内变化, AOB和AnAOB都能保持良好的性能和微生物间的协同,得益于PN/A颗粒的特殊结构,AOB位于颗粒的最外层,NOB紧随其后,位于较薄的氧化区, AnAOB位于颗粒内核,这种特殊的传质结构使得AnAOB可以耐受降温的冲击[22]。本研究发现25 ℃ 时总氮去除速率及去除率都较高,分别为6.60 mg·(g·h)−1和77.84%。常温PN/A颗粒污泥可适应低温驯化,导致AnAOB的最适温度前移[4]。
本研究低温冲击后, AnAOB活性受抑制,出现亚硝酸盐未能及时被消耗而在体系内积累。有研究证实,在10 ℃ 下,高浓度AMX系统中,亚硝酸盐积累导致AnAOB活性下降75%[23]。此外,伴随温度的降低,AOB活性被抑制,氧的利用率下降,导致颗粒污泥中氧的渗透深度增加,AnAOB的生存空间减小,使其生长受到进一步的抑制。AINA等研究发现,15 ℃ 时AOB活性下降, AOB层氧气消耗量减少,导致氧气从颗粒外层向内部渗透,抑制AnAOB活性,反而更有利于NOB的生长[24]。在低温下,维持稳定的亚硝化(PN) 是实现PN/A脱氮的关键前提。温度在10 ℃ 到15 ℃ 条件下,AOB、NOB的活性都下降,但NOB的活性往往高于AOB[25]。本研究也证实,在低温7 ℃ 和12 ℃ 时AOB的氨氧化效率下降,NOB对亚硝酸盐和O2的亲和力更高,占据主导地位,导致ΔNO3−-N/ΔTN值升高, 这也增加PN/A颗粒污泥低温运行调控的难度。
在本研究中,当PN/A颗粒温度降低到7 ℃ 后,AnAOB对底物亲和力、酶活性和传质速率等都受到显著抑制。这主要是由于微生物对温度依赖性的不同,微生物种群结构发生变化,脱氮性能及活性在短时间内无法恢复至最佳状态。宋成康等[26]研究发现在温度 20~33 ℃ 下,SBR反应器的厌氧氨氧化性能稳定高效, SAA大于0.32 gN·(gVSS·d)−1,当温度降至 10 ℃ 时,SAA较33 ℃ 时活性下降91%。即使温度恢复微生物活性亦无法恢复至最佳状态,与本研究结果非常相似。
-
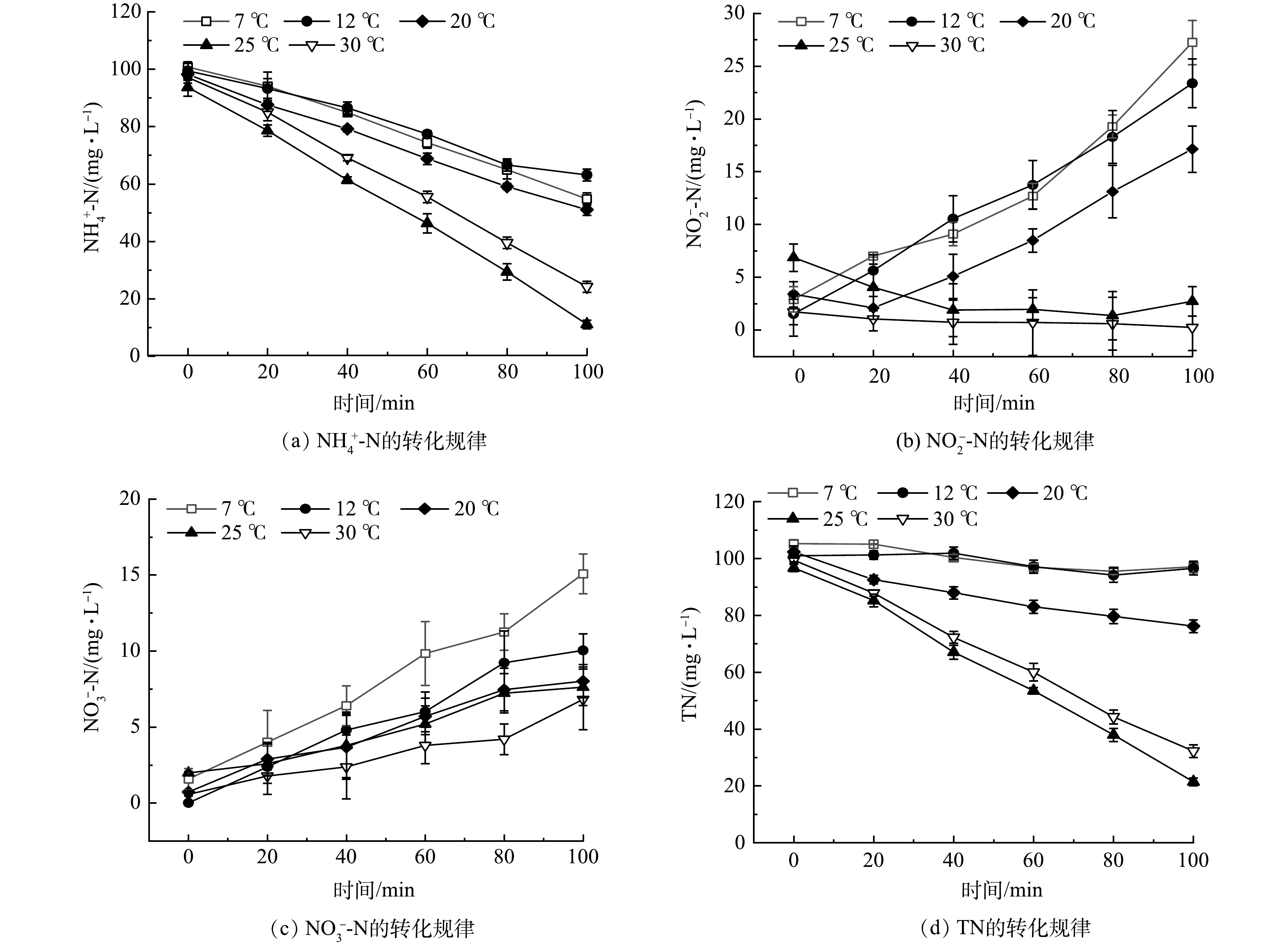
PN/A颗粒污泥中功能微生物对温度变化的响应不完全一致,本研究通过分析不同温度条件下氮元素的转化规律(图4) ,揭示温度与微生物活性的关系。由图4(a)可见,在30 ℃和25 ℃ 时q(NH4+-N) 的最佳降解速率相近,分别为 6.60 mg·(g·h)−1和7.48 mg·(g·h)−1, 25 ℃ 活性较高可能是该颗粒污泥在接种前经过长期低温驯化导致了最适温度出现前移的现象,这与KAWAGOSHI等的观点一致[27]。而7 、12 和20 ℃ 时,q(NH4+-N)有显著下降,均值为3.91 mg·(g·h)−1。
在30 ℃ 和25 ℃ 的批次实验中未出现亚硝酸盐积累,当温度降至12 ℃ 和7 ℃ 时,出现显著的亚硝酸盐积累,且温度越低,亚硝酸盐积累率越高(图4(b)),其质量浓度已达到25.30~28.25 mg·L−1, q(NO3--N)也相应增加,分别达到0.91 mg·(g·h)−1和1.22 mg·(g·h)−1,因此,出水硝酸盐浓度增加(图4(c))。这表明在低温下NOB具有更高的活性,部分亚硝态氮被NOB利用生成硝酸盐,最终导致q(TN)显著下降(图4(d))。在12 ℃和7 ℃时,q(TN)分别为0.40 mg·(g·h)−1和0.74 mg·(g·h)−1,相对于最佳温度为25 ℃ 时,q(TN)下降了94.13%和89.13%。这与温度冲击对PN/A颗粒污泥连续运行的影响具有很好的一致性。批次实验中出现TN下降,亚硝酸积累,证实AnAOB对低温更加敏感。这可能是由于低温使AMX过程的关键酶活性降低及蛋白质合成减缓影响跨膜转运,从而抑制了反应进程,导致亚硝态氮的积累[28]。KUMAR 等[29]也发现,AnAOB的活性在低于 10 ℃ 时会被完全抑制。
综上所述,温度对PN/A颗粒污泥中 AOB、AnAOB以及NOB菌群活性影响不同,各种功能微生物对底物(O2、NO2−-N等)的利用速率有显著差异,当有温度冲击时,功能微生物种群竞争与协同性下降。
-
本研究分析了温度冲击下不同阶段EPS组成和变化规律。如图5所示,TB-EPS 和 LB-EPS中 PS 含量逐渐增加,易于形成三维网状结构,利于PN和PS 的相互协作及细胞间物质转换和能量传递,同时,增加AnAOB和TB-EPS中EPS修饰酶活性,使EPS分层更加趋于稳定。
在实验的低温冲击阶段,PN/A颗粒污泥从30 ℃ 降至20 ℃ 时,EPS总量及3层组分基本无变化;而当 PN/A颗粒污泥中经历30 ℃ 突降至12 ℃ 后,EPS总量较30 ℃ 增加了12.14%, LB层的蛋白质含量增加至原来的5倍;当受到更低的温度冲击(7 ℃) ,EPS总量也增长了原来的50%,TB层的蛋白质大幅增长至268.36 mg·g−1,SB层和LB层的多糖及蛋白质与30 ℃ 及20 ℃ 相比都略有增长。研究实际污水厂污泥时,发现EPS含量与温度存在负相关,冬季时污泥的EPS含量高于夏季,温度下降时,多糖、蛋白质、腐植酸组分均会升高,这主要是由于在低温环境下微生物分泌了更多的EPS来应对环境变化[30]。从本实验结果看,EPS对微生物低温生长起到保护作用,但若EPS过量则会改变颗粒污泥的传质结构,使污泥沉降性变差,最终导致脱氮性能恶化。
AnAOB适应低温时,QS信号分子通过改变细菌细胞膜的结构和冷应激蛋白的含量以及积累抗低温胁迫代谢物,进而影响细菌低温下的代谢调控[31]。低温可能会刺激分泌更多QS的信号分子来调节EPS,霍唐燃等发现在 25 ℃ 条件下酰化高丝氨酸内酯(AHLs,Acyl-homoserine lactones)合成的前体物质 SAM 显著增加,导致AnAOB用于种内通讯的关键信号分子 AHLs 的含量增加[32]。 AHLs在AnAOB菌群的微生物聚集中具有重要作用,AHLs增加了AnAOB菌群中特定氨基酸的含量,从而诱导了PN的产生[33],ZHAO等[34]发现AMX颗粒污泥反应器中含AHLs的上清液对PN和PS的合成分别起到33.5%和48.6%的促进作用。QS与EPS的关系应用为提高PNA工艺脱氮性能提供了新的思路。
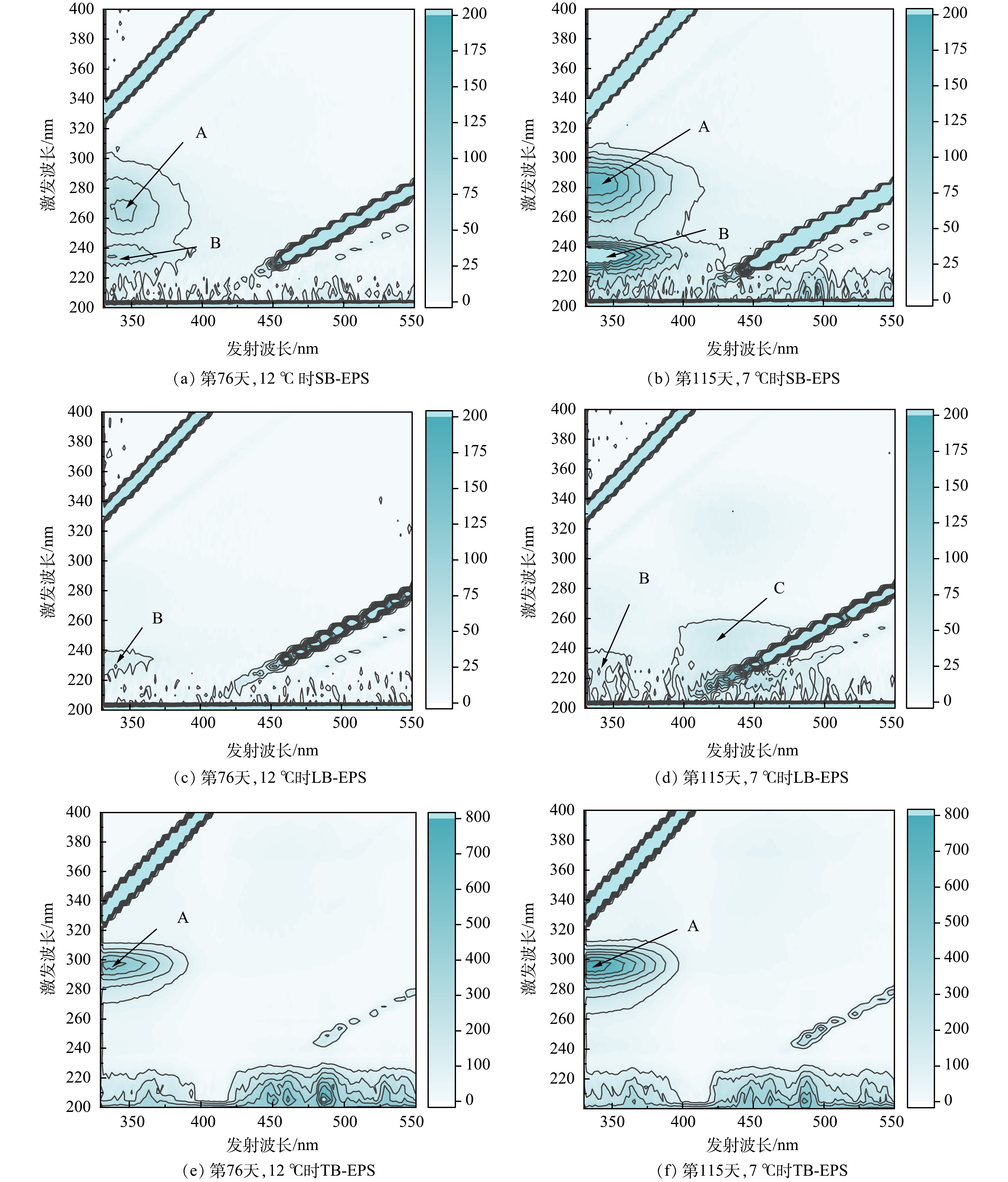
为了进一步了解EPS组分,运用三维荧光光谱(EEM) 探究了12 ℃ 及7 ℃ 污泥样品中的EPS,以检测 EPS 含有的荧光特性物质。不同的荧光峰位置代表不同的物质,强度则表示其相对含量大小。由图6可见,峰A位于激发波长/发射波长(Ex/Em)为290~300 nm/340~350 nm处,为色氨酸类蛋白物质;峰B和峰C分别位于Ex/Em为220~260 nm/400~440 nm和220~350 nm/430~440 nm处,为腐殖酸物质及芳香类蛋白物质。有研究表明,酪氨酸和色氨酸均属于芳香类蛋白,这类蛋白是污泥结构稳定的关键[35]。本研究中色氨酸或腐殖酸物质及芳香类蛋白物质的荧光强度显示出与PN相同的变化趋势。
在LB-EPS和TB-EPS的荧光光谱中仅观察到A峰和B峰,表明蛋白质是主要成分。这与EPS组成分析结果(图6)一致。此外,温度为7 ℃ 时,A峰和B峰的荧光强度明显高于温度为12 ℃ 时,尤其是TB-EPS。这进一步表明,低温刺激可增加EPS的分泌,特别是含有大量蛋白质的TB-EPS,可以丰富内部细胞的营养,从而保护微生物免受环境危害。
-
1) PN/A颗粒污泥在25~30 ℃ 性能最佳,低温冲击会抑制PN/A脱氮性能。12 ℃ 以下的低温冲击后,系统性能难以完全恢复。温度降至7 ℃ ,总氮去除率下降至40.6%,恢复至30 ℃ 运行,最终总氮去除率恢复至66.27%,相对于初始稳定状态,总氮去除率约下降10%。
2) SGompertz模型可有效拟合温度与系统总氮去除负荷,以及温度降幅与总氮去除负荷波动的关系,相关系数R2均在0.995以上,经验证预测值与实验值无明显差异,具有很好的相关性。
3)温度对PN/A颗粒污泥中 AOB、AnAOB以及NOB菌群活性影响不同,AnAOB对低温更加敏感。温度低于15 ℃ 时,AOB和AnAOB种群难以有效协同。
4)在20~30 ℃ 时,PN/A颗粒污泥中EPS总量及TB、LB、SB组分稳定,温度降至 12 ℃ 以下,EPS总量和TB等各组分都大幅增加,在7 ℃ 时 EPS总量较30 ℃ 时增加了50%,TB层的蛋白质增长至268.36 mg·g−1。根据EEM检测发现,低温可刺激TB-EPS分泌更多色氨酸类蛋白物质。
短期低温冲击对PN/A颗粒污泥脱氮效能及微生物性能的影响
Effect of short-term low-temperature shock on the denitrification efficiency of PN/A granular sludge and its microbial performance
-
摘要: 常温部分亚硝化/厌氧氨氧化(partial nitritation/anammox,PN/A)颗粒污泥中不同功能菌群对温度的响应机制不同,在低温条件下易导致脱氮系统失衡。为此,探讨PN/A颗粒污泥系统在温度冲击下的应激效应,包括脱氮性能、微生物活性和EPS对温度冲击的响应,并考察了温度冲击后系统性能恢复的可行性 。结果表明: PN/A颗粒污泥在25~30 ℃时脱氮性能最佳,平均总氮去除率可达到73.48%;低温冲击会抑制PN/A的脱氮性能,温度越低,其对总氮去除率影响越大,12 ℃以下的低温冲击导致平均总氮去除率下降至40.6%,且即使温度回升至30 ℃,平均总氮去除率只能恢复至66.27%。SGompertz模型可有效拟合温度与系统总氮去除负荷以及温度降幅与总氮去除负荷变化的关系,拟合所得可决系数R2均在0.995以上。通过分析温度对微生物活性影响发现,温度对PN/A颗粒污泥中 AOB、AnAOB以及NOB菌群活性影响不同,AnAOB对低温更加敏感。在12 ℃和7 ℃时,总氮比降解速率q(TN) 分别为0.40 mg·(g·h)−1和0.74 mg·(g·h)−1,相对于30 ℃时,q(TN)下降了93.42%和87.83%。在20~30 ℃时,EPS总量和TB、LB、SB组分基本稳定,温度降至 12 ℃以下,EPS总量及各组分均会大幅增加。EEM检测结果表明,低温可刺激TB-EPS分泌更多色氨酸类蛋白质。Abstract: Different functional bacterial groups in normal temperature partial nitritation/anammox (PN/A) granular sludge have different response mechanisms to temperature, which can easily lead to an imbalance of the denitrification system under low temperature conditions. In this study, the stress effects of PN/A granular sludge system under temperature shock were investigated, including the response of denitrification performance, microbial activity and EPS to temperature shock, and the feasibility of system performance recovery after temperature shock was also investigated. The results showed that PN/A granular sludge had the best performed on denitrification at 25~30 °C, with an average total nitrogen removal rate of 73.48%, and low temperature shock could inhibit PN/A denitrification performance, the lower the temperature was, the greater the effect on the total nitrogen removal rate was. The low-temperature shock below 12°C could lead to the decrease of the average TN removal rate to 40.6%, even when the temperature rose back to 30°C, the average TN removal rate only returned to 66.27%. The SGompertz model could effectively fit the relationship between temperature and total nitrogen removal load, as well as the relationship between temperature reduction and total nitrogen removal load varaition, with the determination coefficients R2 above 0.995. The effects of temperature on the activity of AOB, AnAOB and NOB flora in PN/A granular sludge were different, and AnAOB was more sensitive to low temperature. At 12 °C and 7 °C, total nitrogen specific degradation rate (q(TN)) was 0.40 mg·(g·h)−1 and 0.74 mg·(g·h)−1, respectively, which decreased by 93.42% and 93.42% compared with that at 30 °C. The total amount of EPS and TB, LB and SB fractions were essentially stable at 20~30 °C. When the temperature dropped below 12 °C, the total amount of EPS and each EPS fraction increased significantly. According to the EEM analysis, low temperature could stimulate TB-EPS to secrete more tryptophan-like protein substances.
-
表 1 各阶段运行工况说明
Table 1. Operating parameters of the reactor at different operational stages
阶段 运行时间/d 进水NH4+-N浓度/(mg·L−1) 温度/℃ 容积负荷/(kg·(m3·d)−1) I 1~30 100 30 1.63 II 31~38 100 25 1.63 39~69 30 III 70~77 100 20 1.63 78~108 30 IV 109~116 100 12 1.63 117~147 30 V 148~155 100 7 1.63 156~186 30 -
[1] GONG X, WANG B, QIAO X, et al. Performance of the anammox process treating low-strength municipal wastewater under low temperatures: Effect of undulating seasonal temperature variation[J]. Bioresource Technology, 2020, 312: 123590. doi: 10.1016/j.biortech.2020.123590 [2] ISAKA K, DATE Y, KIMURA Y, et al. Nitrogen removal performance using anaerobic ammonium oxidation at low temperatures[J]. FEMS Microbiology Letters, 2008, 282(1): 32-38. doi: 10.1111/j.1574-6968.2008.01095.x [3] 杨朝晖, 徐峥勇, 曾光明, 等. 不同低温驯化策略下的厌氧氨氧化活性[J]. 中国环境科学, 2007, 27(3): 300-305. doi: 10.3321/j.issn:1000-6923.2007.03.003 [4] 刘雨馨, 王建芳, 钱飞跃, 等. 低温下全自养脱氮颗粒污泥适应低基质效能[J]. 环境科学, 2020, 41(9): 4161-4168. doi: 10.13227/j.hjkx.202003036 [5] LAURENI M, FALAS P, ROBIN O, et al. Mainstream partial nitritation and anammox: Long-term process stability and effluent quality at low temperatures[J]. Water Research, 2016, 101: 628-639. doi: 10.1016/j.watres.2016.05.005 [6] TENG Z, SHAO W, ZHANG K, et al. Pb biosorption by leclercia adecarboxylata: Protective and immobilized mechanisms of extracellular polymeric substances[J]. Chemical Engineering Journal, 2019, 375: 122113-122113. doi: 10.1016/j.cej.2019.122113 [7] ZHANG Q, FAN N S, FU J J, et al. Role and application of quorum sensing in anaerobic ammonium oxidation (anammox) process: A review[J]. Critical Reviews in Environmental Science and Technology, 2021, 51(6): 626-648. doi: 10.1080/10643389.2020.1738166 [8] 陈希, 钱飞跃, 王建芳, 等. 环境因子对全自养脱氮颗粒污泥功能菌协同效应的影响[J]. 环境科学, 2018, 39(4): 1756-1762. doi: 10.13227/j.hjkx.201708251 [9] GU C, GAO P, YANG F, et al. Characterization of extracellular polymeric substances in biofilms under long-term exposure to ciprofloxacin antibiotic using fluorescence excitation-emission matrix and parallel factor analysis[J]. Environmental Science & Pollution Research, 2017, 24(15): 13536-13545. [10] MIAO L, WANG S, CAO T, et al. Advanced nitrogen removal from landfill leachate via anammox system based on sequencing biofilm batch reactor (SBBR): Effective protection of biofilm[J]. Bioresource Technology, 2016, 220: 8-16. doi: 10.1016/j.biortech.2016.06.131 [11] 李金璞, 张雯雯, 杨新萍. 活性污泥污水处理系统中胞外多聚物的作用及提取方法[J]. 生态学杂志, 2018, 37(9): 2825-2833. doi: 10.13292/j.1000-4890.201809.001 [12] ADAV S S, LEE D J. Extraction of extracellular polymeric substances from aerobic granule with compact interior structure[J]. Journal of Hazardous Materials, 2008, 154(1/2/3): 1120-1126. doi: 10.1016/j.jhazmat.2007.11.058 [13] 杨振琳, 于德爽, 李津, 等. 海藻糖强化厌氧氨氧化耦合反硝化工艺处理高盐废水的脱氮除碳效能[J]. 环境科学, 2018, 39(10): 4612-4620. doi: 10.13227/j.hjkx.201803218 [14] MENG Y, ZHOU Z, MENG F. Impacts of diel temperature variations on nitrogen removal and metacommunity of anammox biofilm reactors[J]. Water Research, 2019, 160(SEP.1): 1-9. [15] HU Z, LOTTI T, DE KREUK M, et al. Nitrogen removal by a nitritation-anammox bioreactor at low temperature[J]. Applied and Environmental Microbiology, 2013, 79(8): 2807-2812. doi: 10.1128/AEM.03987-12 [16] PARK G, TAKEKAWA M, SODA S, et al. Temperature dependence of nitrogen removal activity by anammox bacteria enriched at low temperatures[J]. Journal of Bioscience and Bioengineering, 2017, 123(4): 505-511. doi: 10.1016/j.jbiosc.2016.11.009 [17] REINO C, SUÁREZ-OJEDA M E, PÉREZ J, et al. Kinetic and microbiological characterization of aerobic granules performing partial nitritation of a low-strength wastewater at 10 ℃[J]. Water Research, 2016, 101: 147-156. doi: 10.1016/j.watres.2016.05.059 [18] ZHOU H, LI X, XU G, et al. Overview of strategies for enhanced treatment of municipal/domestic wastewater at low temperature[J]. Science of the Total Environment, 2018, 643: 225-237. doi: 10.1016/j.scitotenv.2018.06.100 [19] LOTTI T, KLEEREBEZEM R, VAN LOOSDRECHT M C M. Effect of temperature change on anammox activity[J]. Biotechnology & Bioengineering, 2015, 112(1): 98-103. [20] VÁZQUEZ-PADÍN J R, FERNANDEZ I, MORALES N, et al. Autotrophic nitrogen removal at low temperature[J]. Water Science and Technology, 2011, 63(6): 1282-1288. doi: 10.2166/wst.2011.370 [21] JEONG D, LIM H, KIM W. Low-ammonia wastewater treatment by integration of partial nitritation and anaerobic ammonium oxidation (anammox) at 20℃[J]. Journal of Environmental Chemical Engineering, 2021, 9(2): 105122. doi: 10.1016/j.jece.2021.105122 [22] ZHANG L, OKABE S. Ecological niche differentiation among anammox bacteria[J]. Water Research, 2020, 171: 115468. doi: 10.1016/j.watres.2020.115468 [23] LOTTI T, KLEEREBEZEM R, VAN ERP TAALMAN KIP C, et al. Anammox growth on pretreated municipal wastewater[J]. Environmental Science & Technology, 2014, 48(14): 7874-7880. [24] SOLER-JOFRA A, WANG R, KLEEREBEZEM R, et al. Stratification of nitrifier guilds in granular sludge in relation to nitritation[J]. Water Research, 2019, 148: 479-491. doi: 10.1016/j.watres.2018.10.064 [25] YANG Q, PENG Y, LIU X, et al. Nitrogen removal via nitrite from municipal wastewater at low temperatures using real-time control to optimize nitrifying communities[J]. Environmental Science & Technology, 2007, 41(23): 8159-8164. [26] 宋成康, 王亚宜, 韩海成, 等. 温度降低对厌氧氨氧化脱氮效能及污泥胞外聚合物的影响[J]. 中国环境科学, 2016, 36(7): 2006-2013. doi: 10.3969/j.issn.1000-6923.2016.07.015 [27] KAWAGOSHI Y, FUJISAKI K, TOMOSHIGE Y, et al. Temperature effect on nitrogen removal performance and bacterial community in culture of marine anammox bacteria derived from sea-based waste disposal site[J]. Journal of Bioscience and Bioengineering, 2012, 113(4): 515-520. doi: 10.1016/j.jbiosc.2011.11.024 [28] 丁爽. 厌氧氨氧化关键技术及其机理的研究[D]. 杭州: 浙江大学, 2014. [29] KUMAR M, LIN J G. Co-existence of anammox and denitrification for simultaneous nitrogen and carbon removal: Strategies and issues[J]. Journal of Hazardous Materials, 2010, 178(1-3): 1-9. doi: 10.1016/j.jhazmat.2010.01.077 [30] WILÉN B M, LUMLEY D, MATTSSON A, et al. Relationship between floc composition and flocculation and settling properties studied at a full scale activated sludge plant[J]. Water Research, 2008, 42(16): 4404-4418. doi: 10.1016/j.watres.2008.07.033 [31] 郑贝贝. 耐冷菌Bacillus cereus MYB41-22群体感应系统与其温度适应性相关功能研究[D]. 昆明: 昆明理工大学, 2018. [32] 霍唐燃, 潘珏君, 刘思彤. 基于代谢组的厌氧氨氧化菌群对温度的响应机制[J]. 微生物学通报, 2019, 46(8): 1936-1945. doi: 10.13344/j.microbiol.china.190322 [33] TANG X, GUO Y, WU S, et al. Metabolomics uncovers the regulatory pathway of acyl-homoserine lactones based quorum sensing in anammox consortia[J]. Environmental Science & Technology, 2018, 52(4): 2206-2216. [34] ZHAO R, ZHANG H, ZHANG F, et al. Fast start-up anammox process using acyl-homoserine lactones (AHLs) containing supernatant[J]. Journal of Environmental Sciences, 2018, 65: 127-132. doi: 10.1016/j.jes.2017.03.025 [35] ZHU L, ZHOU J, LV M, et al. Specific component comparison of extracellular polymeric substances (EPS) in flocs and granular sludge using EEM and SDS-PAGE[J]. Chemosphere, 2015, 121: 26-32. doi: 10.1016/j.chemosphere.2014.10.053 -




 下载:
下载: