-
2004—2020年,我国城市生活垃圾焚烧处理量从4.5×106 t增长到1.46×108 t[1],焚烧发电已成为我国城市生活垃圾处理的主要方式。生活垃圾焚烧处理具有减量化和能源利用等优点[2],但同时也会产生二次污染物。飞灰是垃圾焚烧过程中产生的一种含有重金属、二恶英和大量可溶性氯盐的固体废物[3],对人体和环境有害,被全球列为危险废物[4]。飞灰产生量与垃圾焚烧炉的类型有关,我国炉排炉和流化床垃圾焚烧炉的飞灰产生量分别占垃圾焚烧量的3%~5%和10%~20%[5]。随着我国垃圾焚烧量的增加,飞灰的产生量也在逐年增加。
目前,常用的飞灰处理方式有2类。固化/稳定化是指通过技术手段使飞灰中的重金属具有化学惰性或被物理包裹,减少其向环境中迁移[6-7]。其中,水泥固化技术操作简单、成熟,但能耗和占地较大且固化后的产物只能填埋处置[7]。化学稳定化技术可以降低飞灰的浸出毒性并且有长期的稳定性[8],但化学稳定剂的价格相对较高,某些螯合剂还会导致飞灰填埋后产生的渗滤液中NH4+-N等物质的质量浓度升高,增加渗滤液的处理难度[9]。热处理(如熔融、烧结等)[10]技术可将飞灰中大部分有毒的有机化合物分解,并把重金属固定在无定形相中,以降低其浸出率[11]。但该方法能耗高,并且会使飞灰中一些熔点较低的金属氯化物挥发,产生毒性更大的二次飞灰。分离浸出是通过浸取剂(水、酸、碱或生物浸取剂等)分离飞灰中的重金属和盐分等污染物,或分离有价值的元素回收利用,分离后的飞灰被填埋或进行水泥窑协同处置[12-16]。我国新颁布的《生活垃圾焚烧飞灰污染控制技术规范(试行)》(HJ 1134-2020)[17]中规定,应通过预处理控制飞灰处理产物中的可溶性氯质量分数(应不超过2%,以不高于1%为宜)。因此,采用水洗预处理脱除飞灰中的可溶性氯已成为必不可少的步骤。
目前,飞灰水洗方式和工艺已有较多研究[18-20],也有了一些工程应用,但关于水洗液性质和处理的研究较少。飞灰水洗液中含有大量的氯盐(氯化钾、氯化钠和氯化钙等)和一些重金属离子。高盐废水的处理方式主要有超滤、纳滤、反渗透、电渗析[21-23]等。其中,电渗析技术具有浓缩倍率高、消耗药剂少等优点,已被广泛应用[24]。电渗析在飞灰处理方面的研究主要是针对飞灰悬浊液中的重金属去除,该方法对飞灰中Cd、Cu和Zn的去除率可达80%以上,但对Pb的去除率只有12%[25-26]。而且,在电渗析过程中会产生大量的氯气,经过电渗析处理后飞灰的浸出毒性仍然较高[27]。电渗析对垃圾填埋场渗滤液中盐的浓缩效果较好,通过5级电渗析可使渗滤液的TDS浓缩48倍,水的回用率为62.5%,且随着电渗析的时间增长,阴离子膜的离子交换容量会降低,但膜污染并不严重[28]。本研究采用循环式电渗析装置探索飞灰水洗液中盐的浓缩,通过比较电导率变化、盐质量浓度的浓缩倍数、淡液回用率和能耗等因素获得最佳的电渗析浓缩工况;依据K+//Cl−—H2O 三元水盐体系相图,通过蒸发结晶的方式回收电渗析浓缩液中的氯化钾工业盐和混盐,以期为电渗析浓缩飞灰水洗液并回收盐的工业应用提供参考。
-
实验所用飞灰取自某炉排炉生活垃圾焚烧发电厂(处理规模为1 500 t·d−1);烟气处理工艺为,选择性非催化还原脱硝+半干法脱酸+活性炭吸附+袋式除尘。飞灰中主要元素Na、S、Cl、K、Ca的质量分数分别为4.04%、2.10%、21.32%、5.44%、39.89%;重金属Cd、Cr、Cu、Fe、Mn、Ni、Pb、Sb、Zn的质量分数分别为(183.3±4.0)、(25.4±2.6)、(514±15)、(3 961±146)、(142±7)、(8.3±1.9)、(1 233±22)、(35.4±3.6)、(5 361±155) mg·kg−1。本实验所用碳酸钾(K2CO3)、硫酸钠(Na2SO4)、盐酸试剂均为分析纯。
-
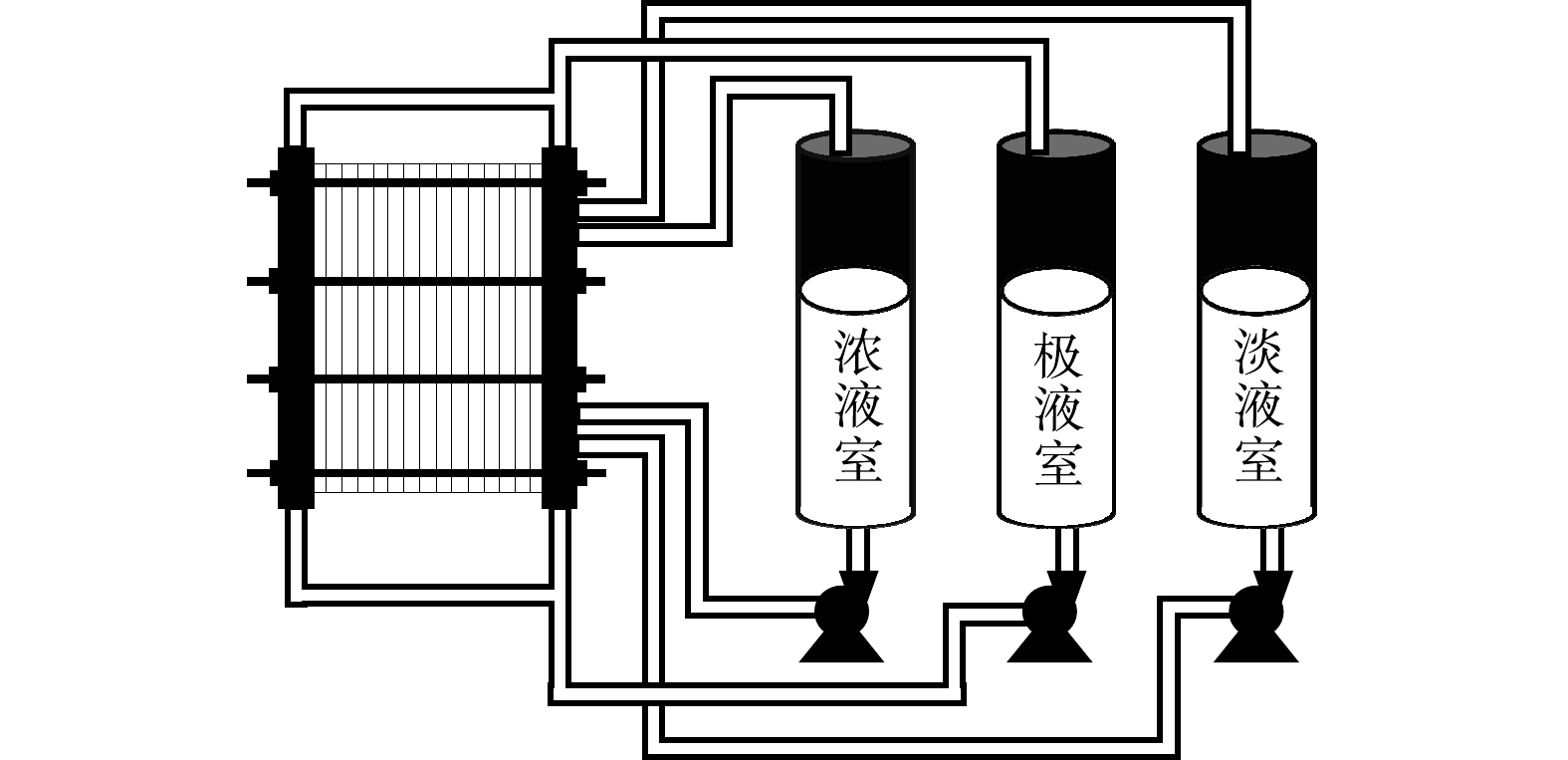
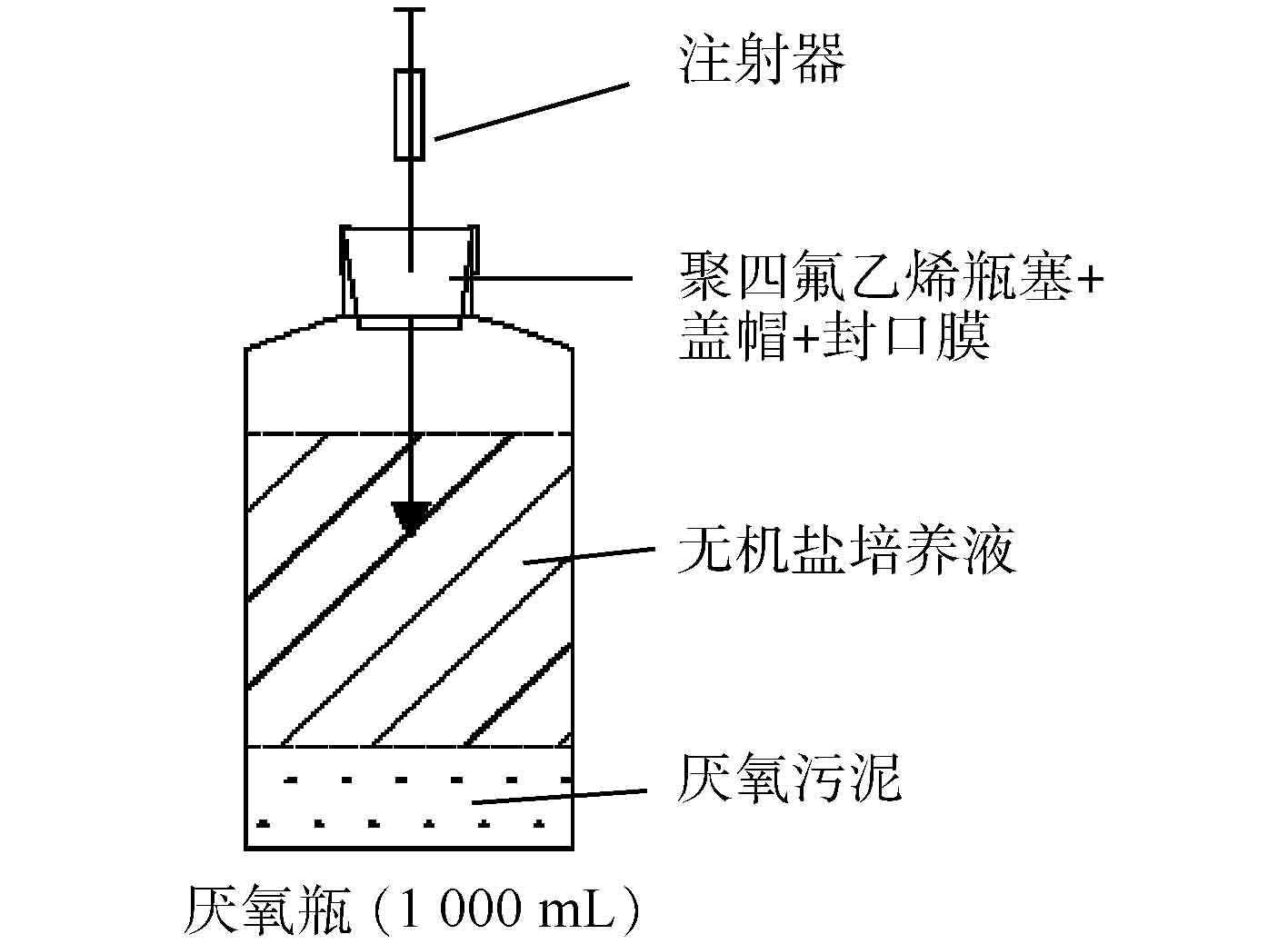
循环式电渗析装置如图1所示。该装置有极液室、淡液室和浓液室3个储液罐;共有10对膜,单张膜有效面积为180 cm2;电极材料为钛涂钌铱;用整流器控制电渗析电压,采用恒压(11 V)模式。
-
1)飞灰水洗液制备。分别称取5.0 g飞灰置于8个100 mL塑料瓶中,以2、4、6、8、10、12、14、16 L·kg−1的液固比向塑料瓶中分别加入蒸馏水。混合后将塑料瓶放入翻转振荡器中,25 ℃、60 r·min−1、振荡30 min。水洗完成后,用0.45 μm滤膜过滤,得到飞灰水洗液。分析上述水洗液中氯质量浓度和氯的脱除率后,选取4 L·kg−1的液固比制备电渗析浓缩用的飞灰水洗液。每次称取50.0 g飞灰,加入200 mL蒸馏水,按上述条件翻转振荡水洗,共制备10 L飞灰水洗液。
2)飞灰水洗液预处理。测定水洗液中的Ca2+质量浓度,计算沉淀所有Ca2+所需的CO32‒量,根据计算结果向水洗液中加入过量的K2CO3,待沉淀完全后过滤。
3)飞灰水洗液电渗析浓缩。将脱钙后的飞灰水洗液加入循环式电渗析装置的淡液室和浓液室中,对比淡液室∶浓液室=2∶1(V/V)1次电渗析浓缩和淡液室∶浓液室=1∶1(V/V)2次电渗析浓缩(从第1次电渗析后的浓液取2 L,均分用作第2次电渗析淡液室和浓液室中的液体)2种工况(分别称为工况1和工况2)的浓缩效果。电渗析循环流量为40 L·h−1,用质量浓度为4%的硫酸钠溶液作为极液,并用电导率仪实时监控淡液室和浓液室中液体(简称淡液和浓液)电导率变化情况,每分钟记录1次数据。
4)回收工业盐。将2次电渗析后的浓缩液蒸发结晶,产物近似看作由氯化钠和氯化钾组成的混盐,根据30 ℃时Na+、K+//Cl−—H2O 三元水盐体系相图计算得到加水量,该加水量可使混盐中氯化钠完全溶解,而氯化钾部分溶解。加水振荡混匀后离心,得到较纯的氯化钾和混有氯化钾与氯化钠的溶液。
-
飞灰的主要元素组成用高性能微区X射线荧光光谱仪(M4 Tornado,Bruker,德国)测定;飞灰中可溶性氯的质量分数根据《危险废物鉴别标准 浸出毒性鉴别》(GB 5085.3-2007)[29]附录F中提供的方法测定;水洗液中氯的质量浓度用离子色谱仪(ISC-600,Thermo Fisher,美国)测定;水洗液中金属离子的质量浓度用电感耦合等离子体发射光谱仪(ICP-OES 5110,Agilent,美国)测定。
-
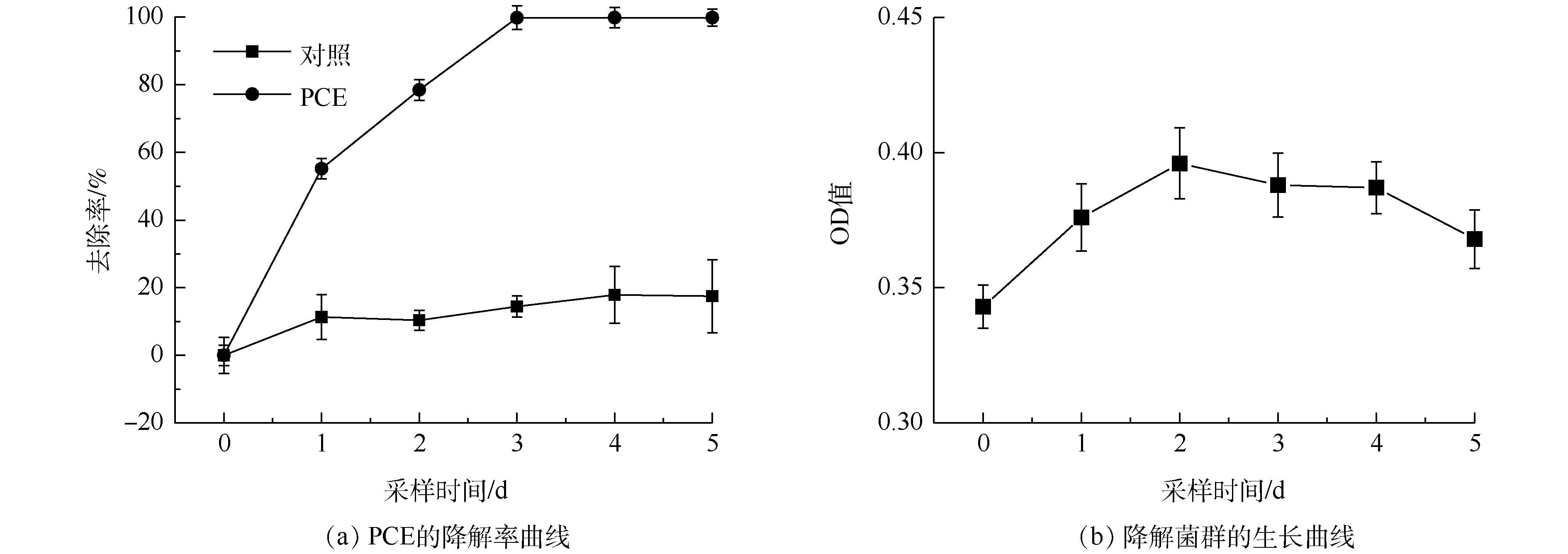
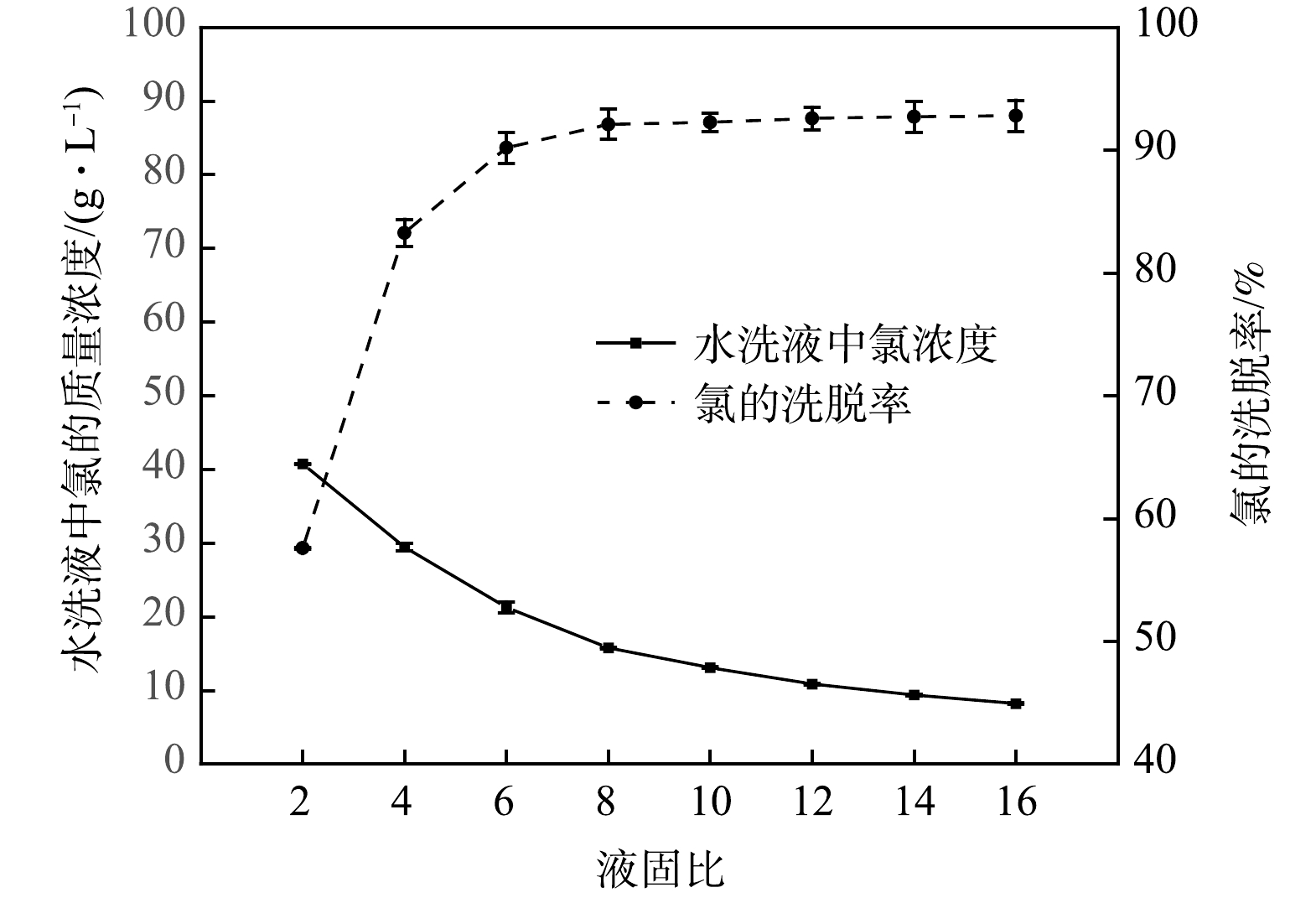
飞灰中可溶性氯质量分数为14.1%。由于飞灰中的氯绝大部分为水溶性氯,因此,液固比对飞灰中氯的洗脱影响很大[30]。图2为不同液固比水洗后,飞灰水洗液中氯的质量浓度和氯的洗脱率。可以看出,随着水洗液固比增大,氯的洗脱率逐渐增大,但水洗液中氯的质量浓度逐渐降低,这与已有研究[19, 31-32]的水洗结果相似。
虽然增大液固比可以提高氯的洗脱率,但会增大用水量,且会使水洗液中氯的质量浓度降低,这会增加后续飞灰水洗液处理的负荷。氯的洗脱率与水洗液固比不是正比关系,液固比大于6 L·kg−1时,氯的洗脱率上升幅度降低,所以用4 L·kg−1的液固比水洗飞灰2次比8 L·kg−1水洗飞灰1次的氯洗脱率更高。因此,工业应用中更倾向于采用低液固比、多级水洗的方式来去除飞灰中可溶性氯,第二级或更多级水洗的洗脱液可以回用于前一级水洗。本实验中,4 L·kg−1液固比水洗可去除飞灰中83.25%的可溶性氯,第二级4 L·kg−1液固比水洗后飞灰中可溶性氯的质量分数为0.817%,在《生活垃圾焚烧飞灰污染控制技术规范(试行)》(HJ 1134-2020)[17]中规定的限值之下,可以填埋、与水泥窑协同处置或作其他利用,而第二级水洗液可以回用于第一级水洗。1次水洗溶液中氯的质量浓度为30.43 g·L−1,已经处于较高的质量浓度。因此,本实验采用4 L·kg−1液固比水洗,第一级飞灰水洗液用作电渗析浓缩,典型元素质量浓度如表1所示。飞灰水洗液中Ca2+质量浓度为9.71 g·L−1,1 L飞灰水洗液中加入33.6 g K2CO3,脱除其中的Ca2+。脱钙后水洗液中的Ca2+质量浓度为0.1 mg·L−1,达到电渗析处理(Ca2+<40 mg·L−1)的要求。
-
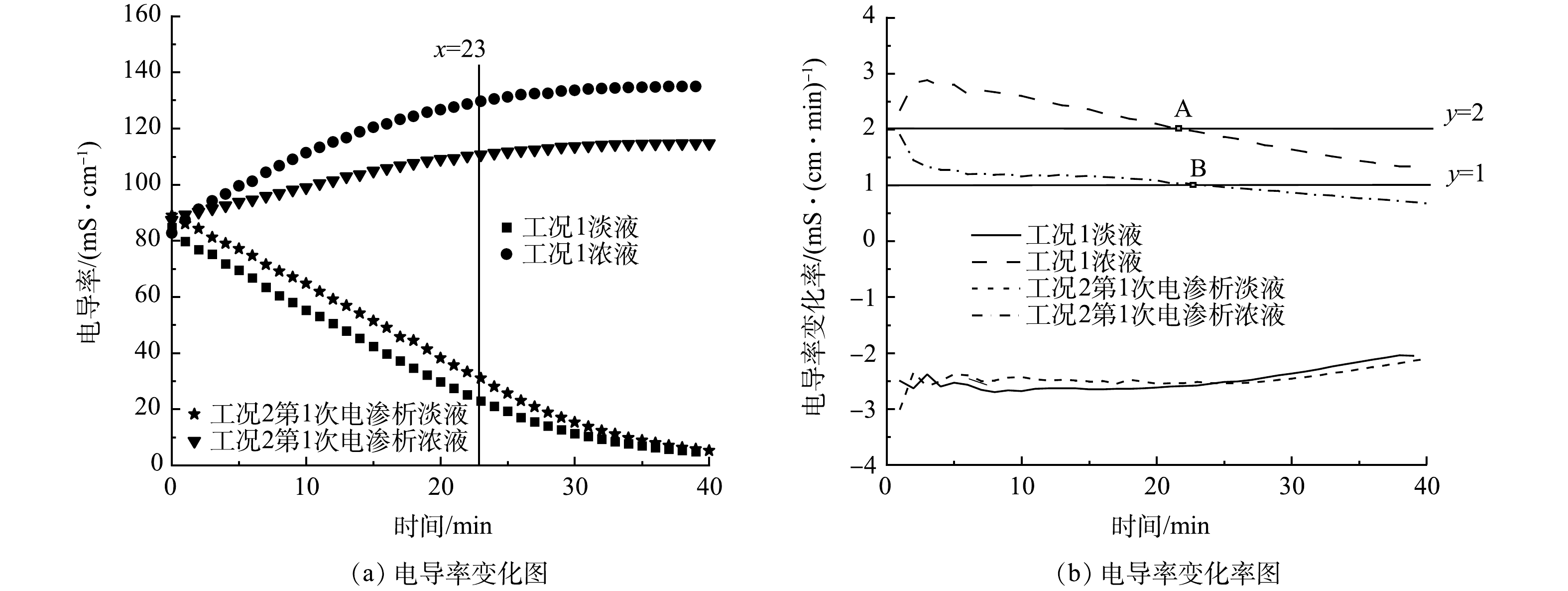
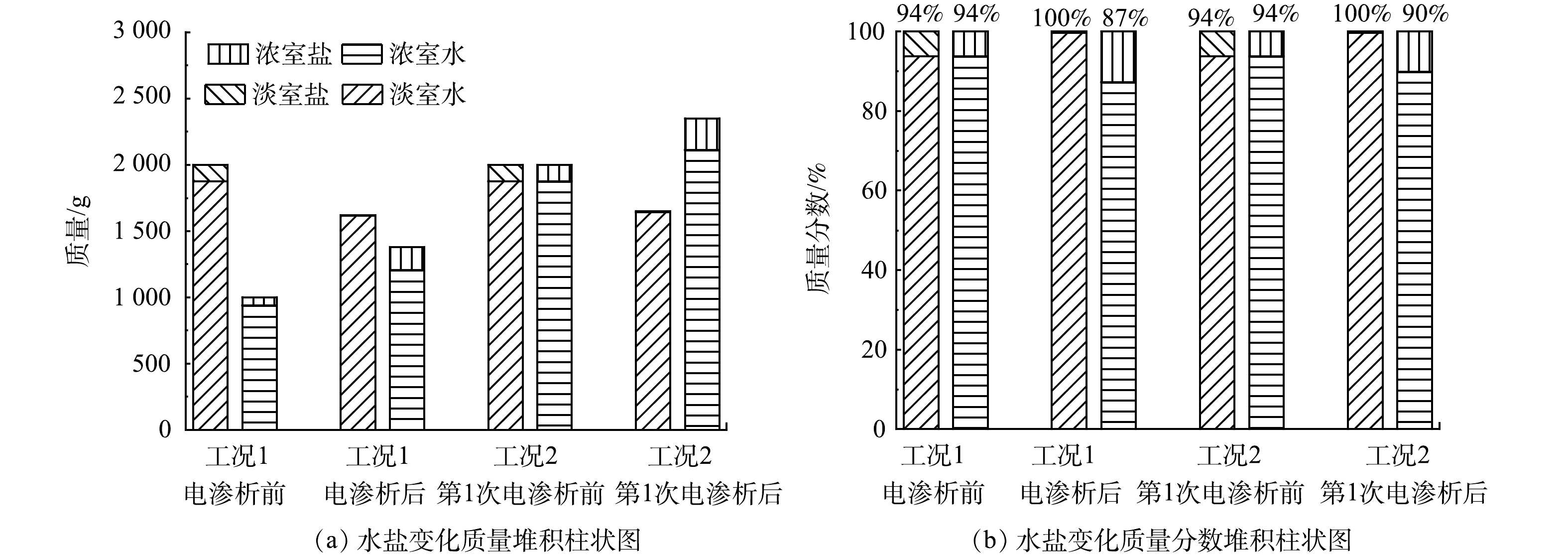
1)电渗析淡液室与浓液室加液体积比对飞灰水洗液盐浓缩的影响。图3(a)为2种加液工况下电渗析过程中淡液与浓液的电导率变化图。随着电渗析时间延长,2种工况下淡液的电导率都可以降到较低的状态(5 mS·cm−1),该盐度下的液体可回用作飞灰的第一级水洗,节约水洗用水量。而2种工况下浓液的电导率从23 min开始,升高均变缓慢。这是因为,当浓液和淡液中盐的质量浓度差超过一定程度后,会导致电渗析膜两侧的渗透压增大,除了电驱动溶液中离子的迁移外,水的迁移量也会增大[24, 33]。溶液中水和盐的迁移情况如图4(b)所示。由于水的移动,电渗析后工况1的浓液电导率小于工况2浓液电导率的1.5倍(没有水分迁移的理想情况下的倍数)。
图3(b)为根据式(1)计算得到的2种工况下电导率的变化率曲线,由于工况1与工况2第1次电渗析的淡液室加液体积相同,所以,2种工况下淡液电导率的变化率曲线呈现相同的变化规律。而工况2第1次电渗析的浓液室加液体积是工况1的2倍,因此,后者电导率的变化率一直是前者的2倍左右。工况1液电导率的变化率等于2时(图3(b)中A点),电渗析进行到第22 min,这说明该点之后水的移动导致浓缩效果不佳;该点处的淡液电导率为25.1 mS·cm−1,该盐度下的液体也可回用于飞灰的第一级水洗。同理,图3(b)中的B点(电导率的变化率等于1)也为工况2第1次电渗析的最佳结束点,该点处(第23 min)的淡液电导率为31 mS·cm−1。
由图4可看出,电渗析后淡液中盐的占比近似为0。工况1比工况2第1次电渗析的浓缩效果好,但前者水的迁移量也比后者的高。这是因为,随着电渗析进行,前者浓液的电导率升高较快,因此,淡液与浓液中盐的质量浓度差比后者的大,所以水的迁移量也更大。表2为这2种工况进行前后的物料质量浓度表,由表中浓缩倍数可以看出,工况2的浓缩效果比工况1的浓缩效果提高了12.3%。但从回用水的角度来看,工况2相当于将每L飞灰水洗液中的盐转移到0.38 L浓液中,得到0.62 L淡液可回用;而工况1相当于将每L飞灰水洗液中的盐转移到0.46 L浓液中,得到0.54 L淡液可回用。所以,工况2的浓缩效果优于工况1的浓缩效果。
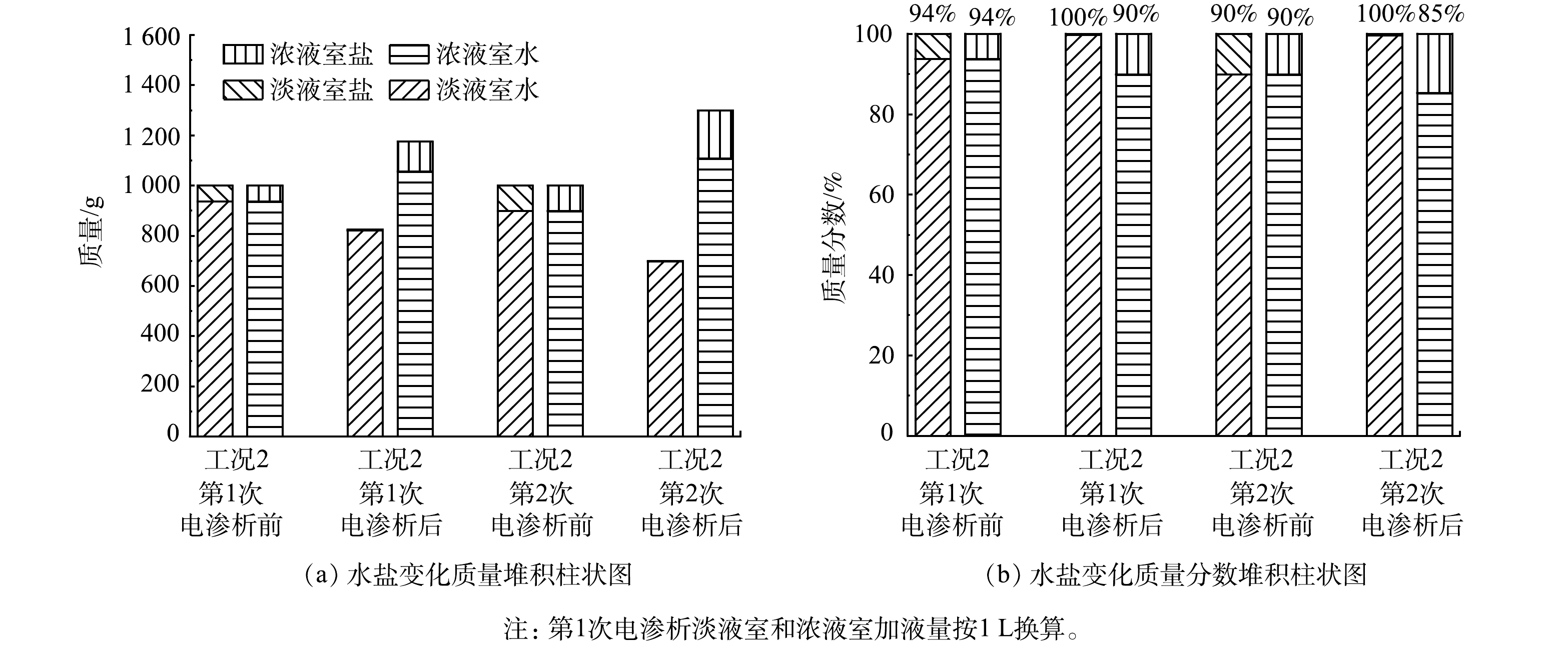
2)电渗析进液盐质量浓度对飞灰水洗液盐浓缩的影响。图5为工况2的电导率及其时间变化率图。可以看出,当电渗析淡液与浓液室中液体体积比相同而盐质量浓度不同时,初始质量浓度高的淡液盐分向浓液中转移的速率越快,而浓液中盐分增长速率相似。这是因为,电渗析进料液体中盐质量浓度越大,液体的电阻越小,在相同电压下,每张电渗析膜上的电流密度也越大,所以淡液中盐的转移速率越快。因此,提高飞灰水洗液中的盐质量浓度可以加快电渗析对其浓缩的速率,之后,可减小水洗的液固比,或回流水洗液的方法来提高水洗液中的盐质量浓度。
由图6和表2可以看出,第2次电渗析后淡液的体积减少量比第1次电渗析的多,且相对于第1次电渗析而言,第2次仅将含盐量提高了5%。这说明,进料液中盐质量浓度越高,越难浓缩。
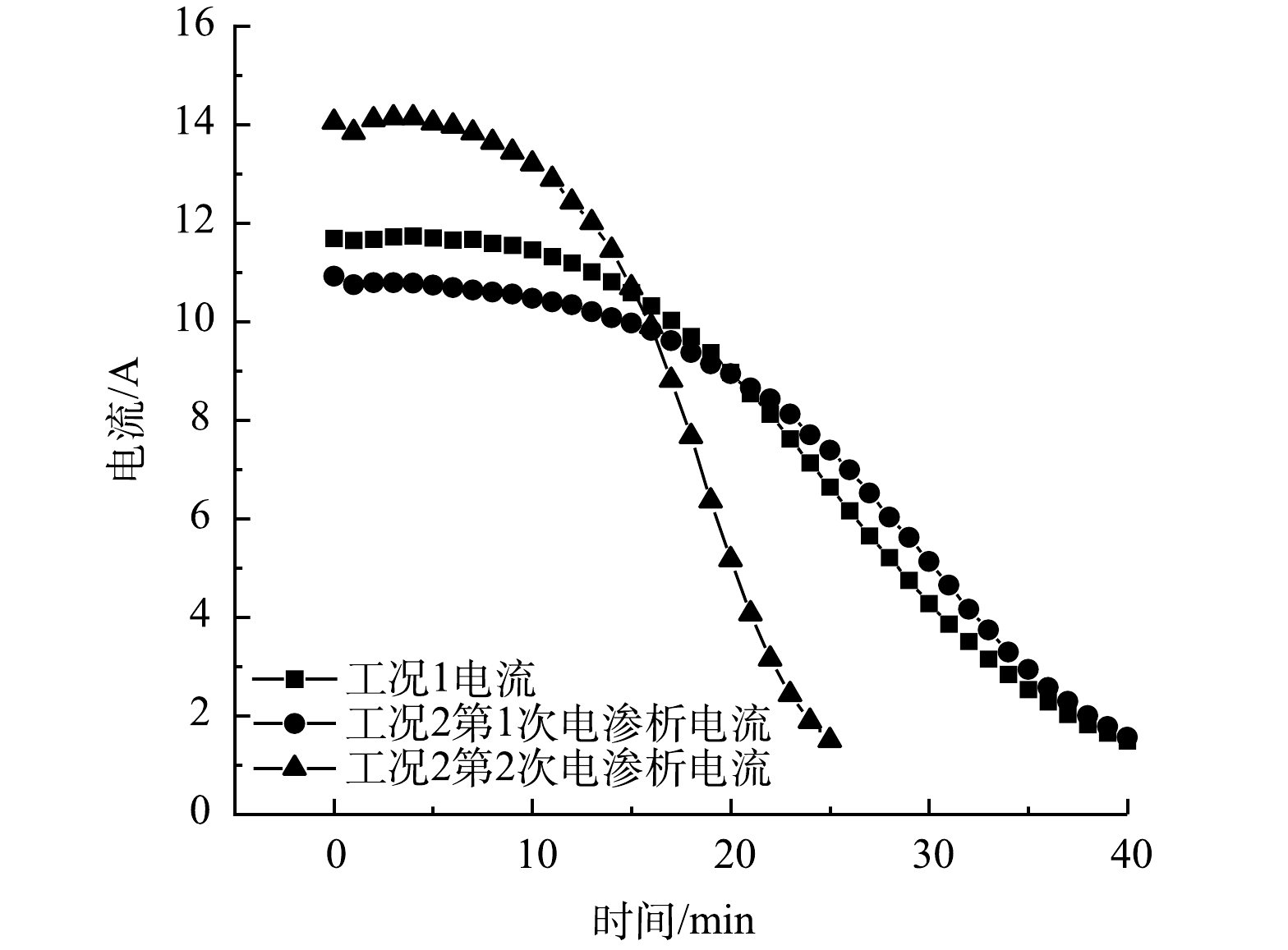
3)电渗析能耗分析。图7为2种工况下电流随时间的变化图。其中,工况1和工况2第1次电渗析的电流曲线变化趋势相同,而工况2第2次电渗析的电流明显高于另外2种情况,且下降速度较快。采用实时电压
× 电流随时间的积分电量总和进行能耗分析,计算得到工况1电渗析的电耗为208.8 kJ,淡液/浓液循环泵2台,每台功率为0.045 kW,极液循环泵1台,功率为0.015 kW,整流器功率按90%计,处理水量为3 L,则该工况的总能耗为482.4 kJ。处理1 t飞灰水洗液需要的电量为160.8 MJ,其中电渗析的能耗占总量的48%。工况2第1次电渗析的电耗为 205.2 kJ,第2次电渗析的电耗为198.0 kJ,处理水量为4 L,泵和整流器的功率与工况1相同,则该工况的总能耗为885.6 kJ。处理1 t飞灰水洗液需要的电量为221.4 MJ,其中电渗析的能耗占总量的46%。本实验用电渗析处理飞灰水洗液所需要的能耗比其他工业上处理高盐废水所需的能耗(6.552 MJ·t−1 [24]; 28.44 MJ·t−1 [34])高。这是因为,飞灰水洗液的含盐量较高、实验终止时,淡液电导率降得太低以及本装置的膜对数和膜的有效面积较小导致的。后续可考虑将电渗析的终止点设置在上述最佳的电渗析点处,可节约能耗并获得所需的浓缩效果。由能耗结果可以看出,工况2处理1 t飞灰水洗液所需的能耗是工况1的1.37倍。结合上述浓缩效果来看,虽然工况2的浓缩效果和水的回收率都比工况1略高,但工况2的能耗较大,以该能耗来提升浓缩效果和水的回收率并不经济。因此,工况1更适合用来浓缩飞灰水洗液。
-
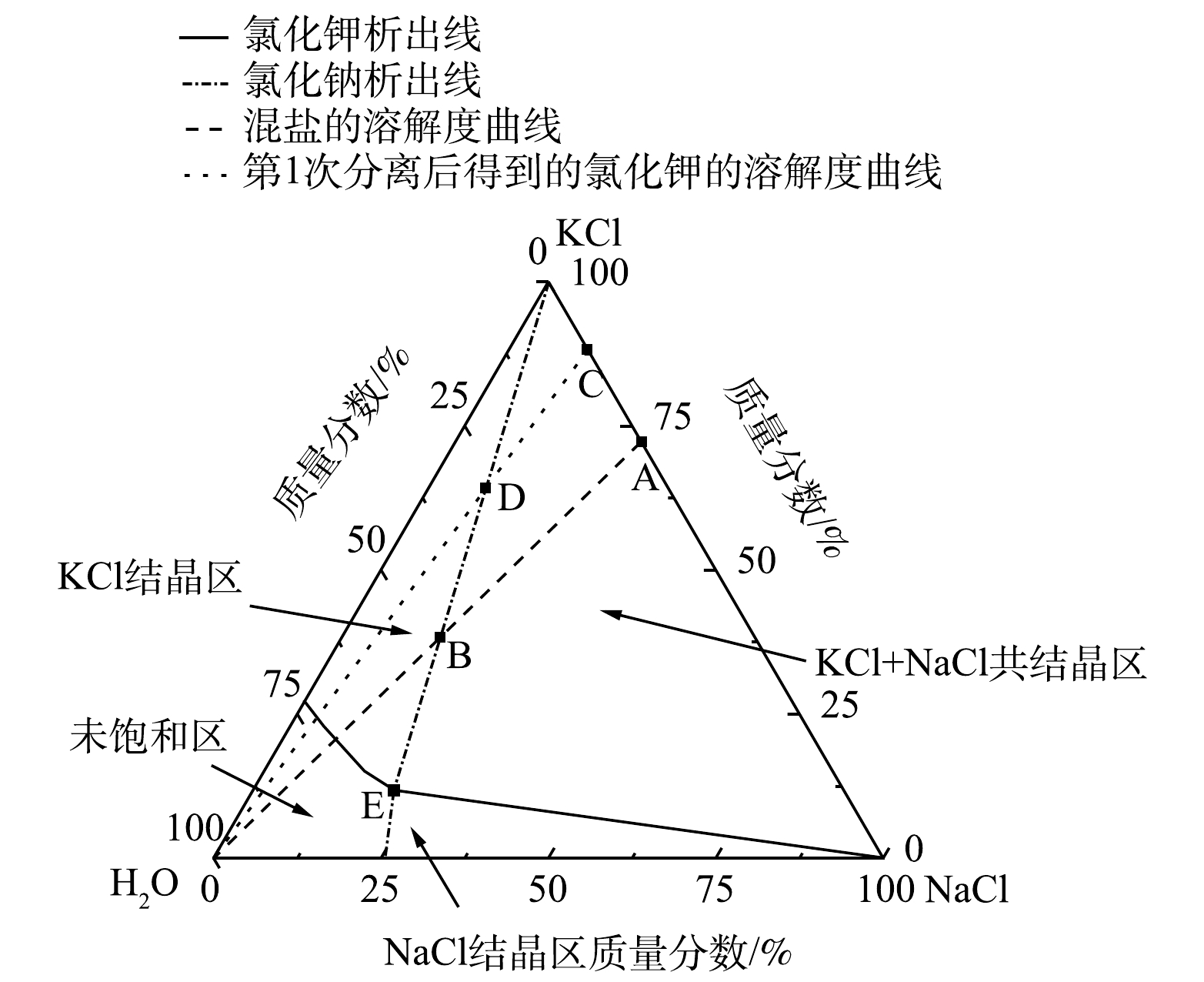
工业盐回收过程中各类盐的组成点以及30 ℃时Na+、K+//Cl−—H2O 三元水盐体系相图如图8所示。其中,混盐的组成为A点(KCl为72.15%、NaCl为27.85%),B点为混盐中氯化钠完全溶解而氯化钾最少溶解的点,离心分离后得到的氯化钾组成为C点(KCl为88.04%、NaCl为11.94%)。对该点处的氯化钾再次进行提纯,可得到纯度为92.2%的工业氯化钾,其中Ba、Mn、Zn的质量分数分别为(0.15±0.03)、(0.07±0.05)、(0.98±0.12) mg·kg−1,As、Cd、Cr、Cu、Ni、Pb、Sb等重金属未检测到,其他杂质如钙镁离子总质量分数为0.05 mg·kg−1、氯化钠质量分数为2.17%,符合《工业氯化钾》(GBT 7118-2008)[35]中的1级标准。由于二恶英类化合物的KOW在104.31~107.27之间[36],属于疏水性有机物,主要被飞灰中的活性炭所吸附,且在飞灰中属于痕量级的存在[37-38],在制备飞灰水洗液时已将活性炭颗粒截留在固相残渣中。因此,本研究未考虑回收的工业氯化钾中二恶英的分析和去除。分离得到的滤液组成都为点E(KCl为11.85%、NaCl为21%),可蒸发结晶后作为融雪剂。
飞灰水洗液中钾的质量浓度为10.1 g·L−1,除钙时1 L飞灰水洗液中加入33.6 g K2CO3,即1 L飞灰水洗液中加入了18.96 g钾,减掉淡液中的钾和实验过程中损失的钾,可将飞灰中洗出的68%的钾回收到工业氯化钾中,19.9%回收到融雪剂中。淡液回用于飞灰的水洗,则可进一步回收飞灰中剩余的盐。
-
1)采用4 L·kg−1液固比2次水洗飞灰,可将飞灰中可溶性氯质量分数降至0.817%,该质量分数已达到《生活垃圾焚烧飞灰污染控制技术规范(试行)》(HJ 1134-2020)中规定,水洗后的飞灰可填埋或水泥窑协同处置。
2)采用循环式电渗析装置浓缩飞灰水洗液。加液量淡液室∶浓液室=2∶1(V/V)1次电渗析浓缩比加液量淡液室∶浓液室=1∶1(V/V)2次电渗析浓缩更适合用于飞灰水洗液的电渗析浓缩。飞灰水洗液中的盐质量浓度越高,电渗析对其浓缩的速度越快,可通过减小水洗的液固比来提高水洗液中的盐质量浓度,以提高电渗析的浓缩速率。
3)根据30 ℃时Na+、K+//Cl−—H2O 三元水盐体系相图,对电渗析浓缩液中的混盐进行2次分离提纯,可得到纯度为92.2%的工业氯化钾,该产物符合《工业氯化钾》(GBT 7118-2008)中的1级标准,分离后的滤液蒸发结晶后可作为融雪剂。飞灰中洗出的钾68%回收为工业氯化钾,19.9%回收到融雪剂中。
利用电渗析浓缩飞灰水洗液并回收工业盐
Concentration of fly ash eluate by electrodialysis and recovery of industrial salt
-
摘要: 为回收飞灰中含有的大量可溶性盐,并利于飞灰后续填埋、水泥窑协同处置或建材利用,采用不同的液固比水洗飞灰并利用循环式电渗析装置浓缩飞灰水洗液,依据Na+、K+//Cl−—H2O 三元水盐体系相图通过蒸发结晶回收浓缩液中的盐。结果表明,以4 L·kg−1的液固比二级水洗飞灰后,可将飞灰中的可溶性氯质量分数降到《生活垃圾焚烧飞灰污染控制技术规范(试行)》(HJ 1134-2020)规定的限值之下。将飞灰一级水洗液加入电渗析装置的淡液室和浓液室,对比淡液室∶浓液室=2∶1 (V/V) 1次电渗析浓缩和淡液室∶浓液室=1∶1 (V/V) 2次电渗析浓缩2种工况可知,后者的浓缩倍数和淡液回用率(2.28倍、62%)比前者(2.03倍、54%)略高,但后者能耗是前者的1.37倍。因此,1次电渗析更适合用于飞灰水洗液的浓缩提盐。提高飞灰水洗液中盐质量浓度,可以提高电渗析浓缩速率。浓缩液蒸发结晶可得到纯度为92.2%的工业氯化钾和融雪剂,并可将飞灰中洗出的68%的钾回收为工业氯化钾。本研究结果可为电渗析浓缩飞灰水洗液并回收盐的工业应用提供参考。Abstract: In order to recover the large amount of soluble salt contained in fly ash and benefit to the subsequent landfilling, co-disposal in cement kiln or utilization as building materials, the fly ash was washed with different liquid to solid (L/S) ratios, and the eluate was concentrated by a circulating electrodialysis device. Then the salt was recovered from the concentrated solution by evaporative crystallization according to the Na+, K+//Cl−—H2O phase diagram. It was found that the mass fraction of soluble chlorine in the fly ash can be reduced to below the limit value of 《Technical specification for pollution control of fly-ash from municipal solid waste incineration》(HJ 1134-2020) when two-stage water washing with a L/S ratio of 4 L·kg−1 was adopted in each stage. The fly ash eluate from first stage was added into the dilute and concentration chamber in the electrodialysis device. The two working conditions were compared, i.e., one-stage electrodialysis with dosage of the fly ash eluate in dilute chamber to concentration chamber = 2:1(V/V), and two-stage of electrodialysis with dosage in dilute chamber to concentration chamber = 1:1(V/V). The results showed that the concentration ratio and the rate of diluted water reuse of the latter (2.28 times and 62%) were slightly higher than those of the former (2.03 times and 54%), but the energy consumption of the latter was 1.37 times that of the former, so one-stage electrodialysis was more suitable for the concentration of fly ash eluate. The speed of concentration by electrodialysis can be increased by increasing the mass concentration of salt in the fly ash eluate. The industrial grade potassium chloride with purity of 92.2% and deicing agent can be obtained by evaporative crystallization, in which 68% of the potassium washed from the fly ash can be recovered as industrial grade potassium chloride. The results of this study can provide a reference for the industrial application of concentration of fly ash eluate by electrodialysis and recovery salt from the concentrate.
-
Key words:
- fly ash /
- water-washing /
- chloride /
- electrodialysis /
- industrial salt /
- potassium chloride
-
随着工农业的迅速发展,氯代烃类有机物作为生产原料和溶剂被人类广泛地应用在工业生产、农药、干洗和医疗等行业[1],造成了一系列环境问题。氯代烃类在环境中随着雨水、径流等渗滤到土壤中[2],被土壤吸附一部分后渗漏到地下含水层,导致地下水污染[3],其中地下水中四氯乙烯、三氯乙烯及四氯化碳等污染尤为普遍[4-6]。本研究中选取一种典型氯代烃PCE作为研究对象,该物质是在常温下易挥发、非易燃的重质非水溶相液体(dense non-aqueous phase liquids,DNAPLs),化学性质稳定,且有很强的生物毒性和潜在的生物累积性,并具有刺激性、致敏性、致突变性、致畸性、致癌性等特性,已被较多国家列为优先控制污染物[7]。一旦PCE通过各种途径进入地下环境,会下渗到地下水深层,严重污染地下水资源,对生态环境和人体健康产生极大的危害[8]。1999年北京市地下水有机污染调查结果表明,北京地区有2处氯代烃污染区域面积超过10 km2,主要污染物为TCE和PCE,最高浓度分别为487.6 μg∙L−1和63.74 μg∙L−1,其中PCE的检出率较高[9]。为了保护生态环境及促进可持续发展,地下水中氯代烃污染问题亟待解决,地下水水质保护和污染修复刻不容缓[10]。
考虑到微生物修复技术具有易于原位修复、处理成本低、无二次污染且可以实现无害化等优点,故进一步研究微生物群落对PCE的降解非常必要[11]。近年来,国内外研究人员对氯代烃的生物降解进行了大量探索和研究[12-19]。在厌氧条件下,氯代烯烃可以作为某些细菌的终端电子受体,通过厌氧微生物作用发生还原脱氯,生成次级产物,最终达到无害化的修复目的,而加入一些有机质进行共代谢可以提高其降解速率[12-14]。有研究[15]发现,将厌氧细菌Y51株和好氧性混合株P. pseudocalcaligenes KF707-D3株综合运用可以将难生物降解的PCE完全分解。实际上,单一的厌氧菌或好氧菌很难彻底快速降解PCE[16]。迄今为止,仅发现Dehalococcoides mccartyi属的菌株能使PCE完全脱氯[17],且该属菌株对碳源要求较高,一般需要醋酸盐作为碳源[18],生长条件较为苛刻。然而,多种常见微生物如脱硫单胞菌,硫磺菌等可以协同合作将PCE还原脱氯[19]。
目前,对微生物修复氯代烃的研究主要集中在单一好氧菌株以及厌氧菌株的运用。因此,有必要研究地下水环境中微生物群落降解氯代烃的特性,从而进一步探索提高氯代烃生物降解速率的可行方法。尽管有很多学者研究某类共代谢基质条件下微生物菌株是否能够成功脱氯,但很少有学者针对微生物群落在不同共代谢基质条件下的脱氯能力开展相关研究[20]。因此,根据现阶段氯代烃修复的研究经验,本研究选取一种典型氯代烃PCE,旨在评估模拟地下水环境下降解菌群的共代谢脱氯能力。本研究采用振荡培养法[21]来筛选并鉴定出PCE优势降解菌群,对环境因素开展实验并对其进行了条件优化,探索了不同共代谢基质条件下PCE降解的规律,建立了反应动力学模型,比较了不同共代谢基质条件下微生物的脱氯能力。
1. 材料与方法
1.1 实验原料
四氯乙烯(C2Cl4)和甲醇(CH3OH)为色谱纯,酵母浸膏和维生素B12为生物试剂,乙醇(CH3CH2OH)、三水合磷酸氢二钾(K2HPO4∙3H2O)、七水合硫酸亚铁(FeSO4∙7H2O)、一水合磷酸二氢钠(NaH2PO4∙H2O)、氯化铵(NH4Cl)、七水合硫酸镁(MgSO4∙7H2O)、七水合硫酸锰(MnSO4∙7H2O)、七水合硫酸锌(ZnSO4∙7H2O)、乳酸钠(dl-C3H5O3Na)、葡萄糖(C6H12O6)、六水合氯化钴(CoCl2∙6H2O)、一水合次氮基三乙酸三钠(N(CH2CO2Na)3∙H2O)均为分析纯。
1.2 实验装置
实验过程中使用的仪器设备:恒温振荡箱(MQL-621R,上海旻泉仪器有限公司)、厌氧培养箱(YQX-Ⅱ型,上海新苗医疗器械制造有限公司)、立式压力蒸汽灭菌锅(YM30型,上海三申医疗器械有限公司)、紫外可见分光光度计(TU-1810,北京普析通用仪器有限责任公司)、氮吹仪器(ANPEL DC12,上海安谱实验科技股份有限公司)、气相色谱仪(Agilent7820A,安捷伦科技有限公司)、Illumina Mi Seq平台(微基生物科技(上海)有限公司)。驯化实验采用厌氧瓶密封操作,实验装置见图1,可定期使用注射器采样。
1.3 实验方法
PCE降解菌群的驯化筛选。以南京某污水处理厂的厌氧活性污泥为菌种来源,采用图1的实验装置对厌氧活性污泥进行驯化培养,整个实验操作过程在厌氧培养箱中完成。实验配制无机盐培养基来模拟地下水[22],其组成成分为:K2HPO4∙3H2O 1.05 g∙L−1、NaH2PO4∙H2O 0.25 g∙L−1、NH4Cl 0.49 g∙L−1、N(CH2CO2Na)3∙H2O 0.03 g∙L−1、dl-C3H5O3Na 0.112 g∙L−1、MgSO4∙7H2O 0.05 g∙L−1、FeSO4∙7H2O 3 mg∙L−1、MnSO4∙7H2O 0.74 mg∙L−1、ZnSO4∙7H2O 0.74 mg∙L−1、CoCl2∙6H2O 0.25 mg∙L−1、维生素B12 0.05 mg∙L−1[23]。PCE降解菌群的驯化采用梯度驯化法[24],按PCE浓度由低到高进行,先将200 mL活性污泥接种到700 mL除氧灭菌后的无机盐培养基中(除非特殊说明,pH均在6.80~7.25),随后采用聚四氟乙烯瓶塞和铝卷曲盖密封血清瓶,并采用无菌注射器加入PCE溶液。PCE溶液浓度初次设定为0.5 mg∙L−1,然后放置于30 ℃、转速为30 r∙min−1的恒温振荡培养箱中进行培养驯化7 d,之后更新培养基并将浓度依次递增为1、2、3、4、5 mg∙L−1[22],每个阶段均培养7 d。
PCE降解特性研究。将100 mL无机盐培养基置于血清瓶中,所有培养基在使用前均利用氮气吹扫30 min,然后用高压灭菌锅在121 ℃下灭菌30 min,最后放入厌氧培养箱中冷却备用。将经过梯度驯化后获得的菌液按5%投加量接入含有100 mL无机盐培养基的血清瓶中,随后采用特氟隆表面橡胶隔膜(美国安捷伦公司)和铝卷曲盖密封血清瓶,PCE初始投加量设定为1 mg∙L−1,随后置于30 ℃、30 r∙min−1条件下恒温振荡器中培养。实验过程中每隔24 h采样1次,测定培养基中残留PCE的浓度以及吸光度,分析驯化所得菌群的PCE降解特性及菌群生长曲线。
环境因素对微生物群落降解PCE的影响。在保持与降解特性实验相应条件不变的前提下,分别研究不同温度(10、20、30 ℃)、不同pH(6、7、8)、不同PCE初始浓度(0.5、1、1.5、2、2.5 mg∙L−1)等条件对菌群降解PCE的影响。在相同的实验条件下,不接种菌液作对照,每组实验重复做3个平行样,取平均值。每隔24 h采样1次,测定培养基中残留PCE的浓度,采样结束后使用硅胶填缝密封盖上留下的针孔以防止PCE挥发。
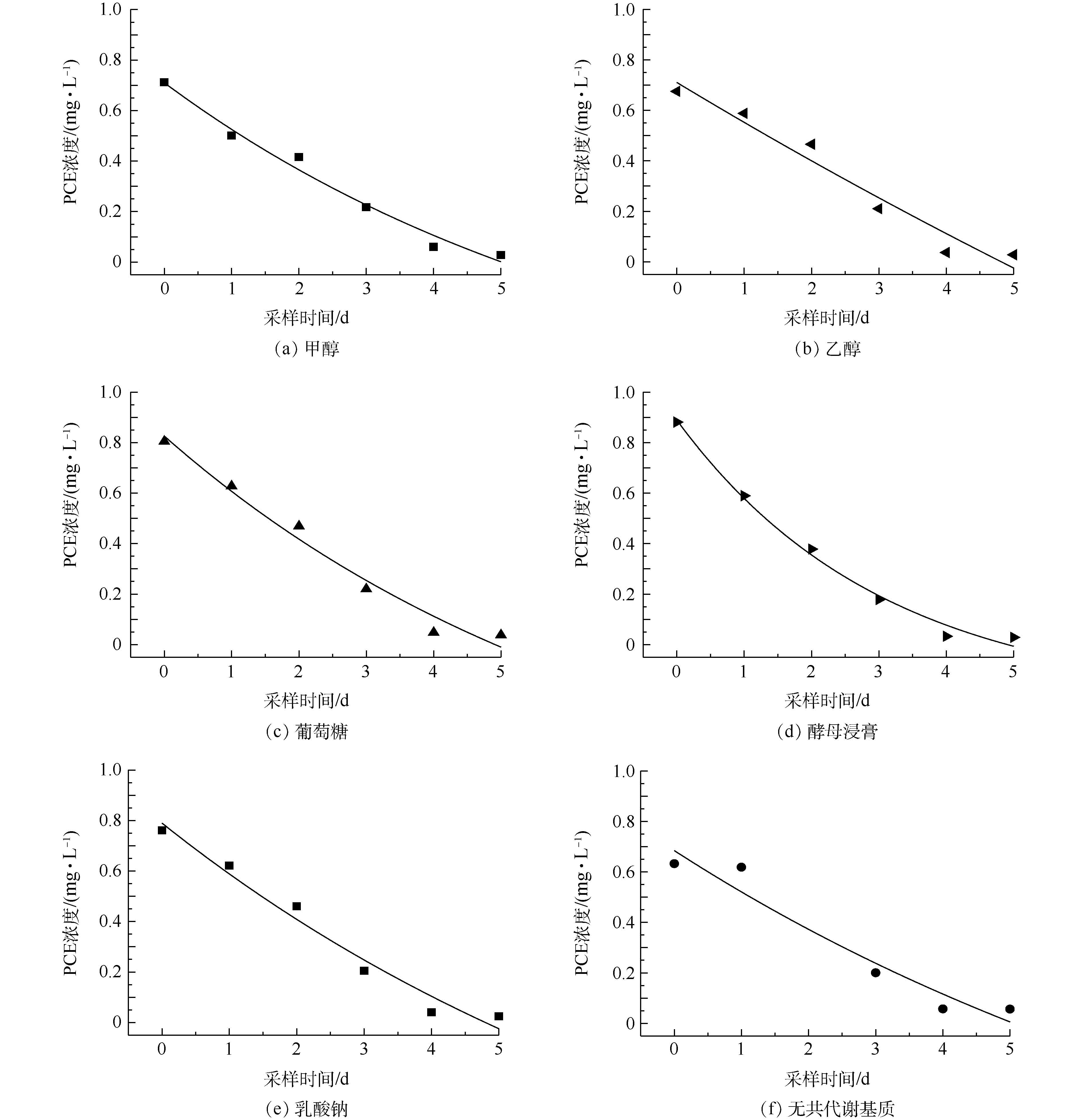
不同共代谢基质对微生物群落降解PCE的影响。为了探索各种共代谢基质对降解菌群性能的影响,将培养所得菌液按5%接种量加入100 mL的无机盐培养液,PCE初始投加量设定为1 mg∙L−1,再分别加入甲醇、乙醇、葡萄糖、酵母浸膏、乳酸钠[25]。其中,葡萄糖以及酵母浸膏浓度为1 g∙L−1、甲醇浓度为0.6 g∙L−1、乙醇浓度为20 mg∙L−1、乳酸钠浓度为0.77 g∙L-1[26]。在30 ℃、30 r∙min−1条件下振荡培养,每24 h采样检测,并利用实验结果建立动力学模型。
1.4 分析方法
实验中PCE浓度采用顶空气相色谱仪[27](Agilent 7820A,美国Agilent公司)及DB-624色谱柱测量。
实验中菌群浓度采用分光光度法[25]测定,取适量实验样品放入1 cm的比色皿中,利用紫外分光光度计测量其在600 nm处的吸光度(A600)。
实验中PCE降解菌群群落结构分析采用16S rDNA[28]来分析,按照柱式细菌基因组抽提试剂盒的使用操作说明,对混合培养体的菌液中的DNA进行提取[29],将纯化后的样品利用Illumina Mi Seq平台进行高通量测序。
2. 结果与讨论
2.1 菌群对PCE降解特性研究
驯化期后PCE降解曲线及菌群生长曲线见图2。由图2(a)可知,PCE能得到高效降解并且去除率已明显高于90%。由图2(b)可知:降解初期(0~2 d),菌群在PCE存在条件下能以较快速度生长;随着采样时间的推移,中期(2~4 d)达到静止期,OD值缓慢下降;后期(4~5 d),由于PCE含量不足,菌群活性被抑制,OD值下降较快。筛选得到的菌群能够高效降解PCE,可以进行菌种鉴定以及环境因素影响实验。
2.2 PCE降解菌群的结构组成
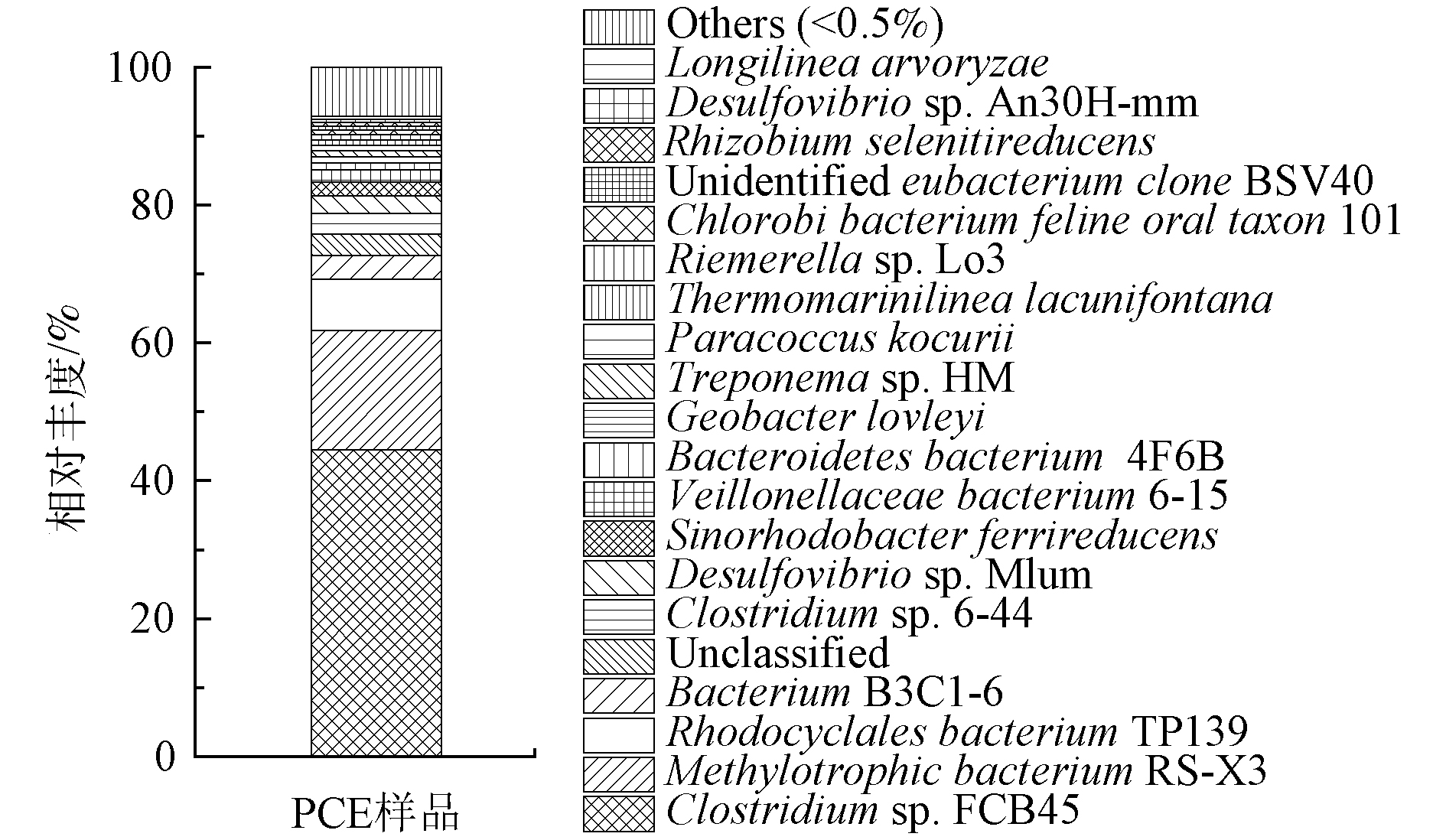
种水平下的样本群落结构组成分布见图3,菌种鉴定结果表明PCE的降解菌群群落结构复杂多样。该菌群中共有16个高于0.50%的种,分别为Clostridium sp. FCB45、Methylotrophic bacterium RS-X3、Rhodocyclales bacterium TP139、Bacterium B3C1-6、Clostridium sp. 6-44、Desulfovibrio sp. Mlhm、Sinorhodobacter ferrireducens、Veillonellaceae bacterium 6-15、Bacteroidetes bacterium 4F6B、Geobacter lovleyi、Treponema sp. HM、Paracoccus kocurii、Thermomarinilinea lacunifontana、Riemerella sp. Lo3、Chlorobi bacterium feline oral taxon 101及Rhizobium selenitireducens。其中,梭状芽孢杆菌(Clostridium sp. FCB45)含量最高,占总量的44.49%,成为优势菌种。这一结果证实了DAVID[12]在微生态研究中推测Clostridium属这种活性较高的梭状菌可以成为降解PCE的潜在微生物的结论。然而,因为这是由普通污水厂的厌氧污泥驯化而来的,故与LOFFLER等[30]在被PCE长期污染的沉积物中所观察到的Desulfuromonas和Dehalococcoides是PCE的优势降解菌有所不同。Methylotrophic bacterium RS-X3含量为17.34%,作为甲基营养菌不仅可以在氯化甲烷上生长[31],并且其被证实含有脱氯酶基因[32],因此,可推测在本研究中Methylotrophic bacterium RS-X3可以促进PCE的生物降解。而占总量7.39%的Rhodocyclales bacterium TP139作为能够利用多种有机化合物生长的多功能细菌的代表[33],首次在PCE降解菌群中被发现并检测到。
有研究表明,脱硫弧菌属(Desulfovibrio sp. Mlhm)可以参与几种氯代污染物的脱氯和硫酸盐还原,包括1, 2-二氯乙烷[34]、1-三氯乙烷[35]和氯仿[36],Treponema属(Treponema sp. HM)能够通过添加醋酸盐将五氯苯酚脱氯成氢气、二氧化碳和甲酸盐[37],Bacteroides属(Bacteroidetes bacterium 4F6B)可降解甲基叔丁基醚[38],Sinorhodobacter ferrireducens能利用肌醇、L-丙氨酸、4-羟基苯甲酸和L-脯氨酸作为碳源[39],Thermomarinilinea属(Thermomarinilinea lacunifontana)能在多种有机物存在时生长(包括明胶、甲壳素、谷氨酸等)[40]。这些菌株也在该菌群中被检测到,其含量分别为2.61%、0.85%、0.97%、1.99%和0.80%,其中Desulfovibrio、Treponema属为潜在PCE降解菌。
2.3 环境因素对降解的影响
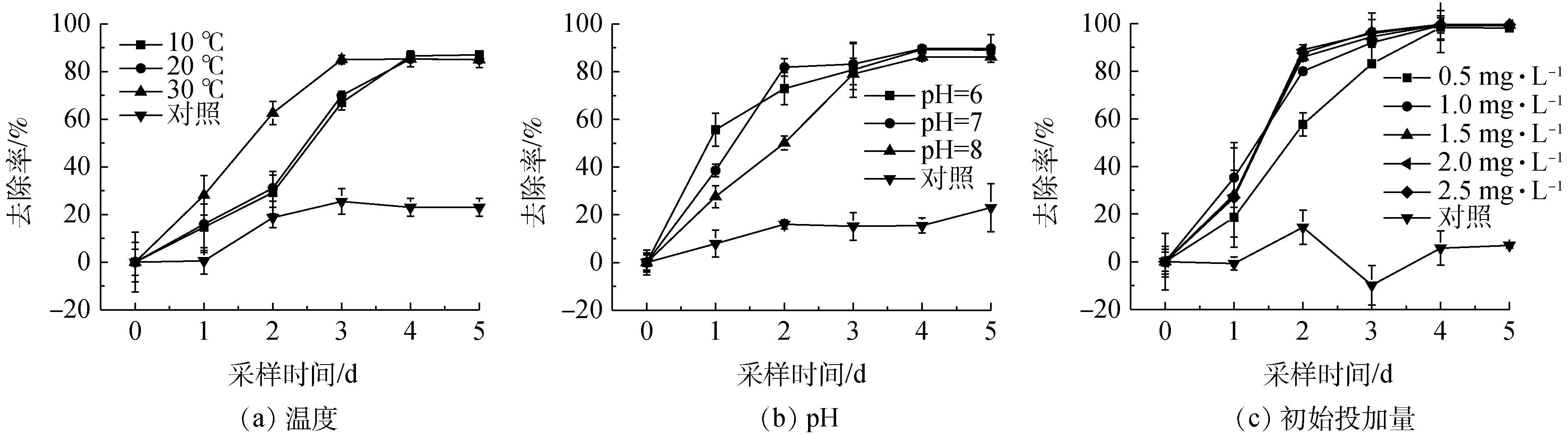
环境因素对PCE降解效果的影响见图4。由图4(a)可知温度对PCE降解率的影响不显著。数据显示3个温度梯度下去除率均高于80%,表明该菌群整体代谢活性较高,具有较宽的温度生态幅,适应不同温度的环境能力较强。在实验初期(0~3 d),菌群在10 ℃以及 20 ℃下对PCE的降解速率相比30 ℃较慢;在后期(4 d后),菌群在10 ℃和20 ℃时对PCE的降解率与其在30 ℃下的降解率相当。ZHANG等[41]也得出TCE去除率随温度的升高(从20 ℃上升到30 ℃)而增加的类似结论。SHARMA等[42]认为,低温下微生物需要更多的时间来矿化疏水性物质。因此,生物反应与化学反应都会减慢速率,而较高的温度会导致生物降解增加。一般地下水的平均温度约为15 ℃(我国西北地区冬季地下水水温仍处于10 ℃左右)[43-45],可以达到该菌群生长的适宜温度范围。
由图4(b)可知:菌群在降解PCE的过程中受非极端pH的影响不显著。在初始pH 6~8时,PCE的降解速率随着初始pH的变化呈现出一定的波动性,但在总体上体系中的微生物群落均能够实现PCE的有效降解。初始pH 7.0为PCE降解的最适值,因为弱酸以及弱碱性的条件不会对降解菌群活性产生抑制,所以在非极端pH的地下水环境中,此菌群也可以发挥作用。
由图4(c)可知:菌群在降解PCE的过程中受PCE初始浓度的影响不显著,但其降解速率存在一定波动性。在此PCE初始投加范围内,该菌群活性没有受到明显抑制,此浓度下的PCE毒性没有破坏菌群体内的酶、核酸以及DNA等的结构,所以菌群能正常进行新陈代谢,但当PCE初始浓度过低时,与酶结合的底物浓度偏低,会使微生物的活性降低,从而导致PCE降解速率下降[46]。
2.4 不同共代谢基质对降解的影响
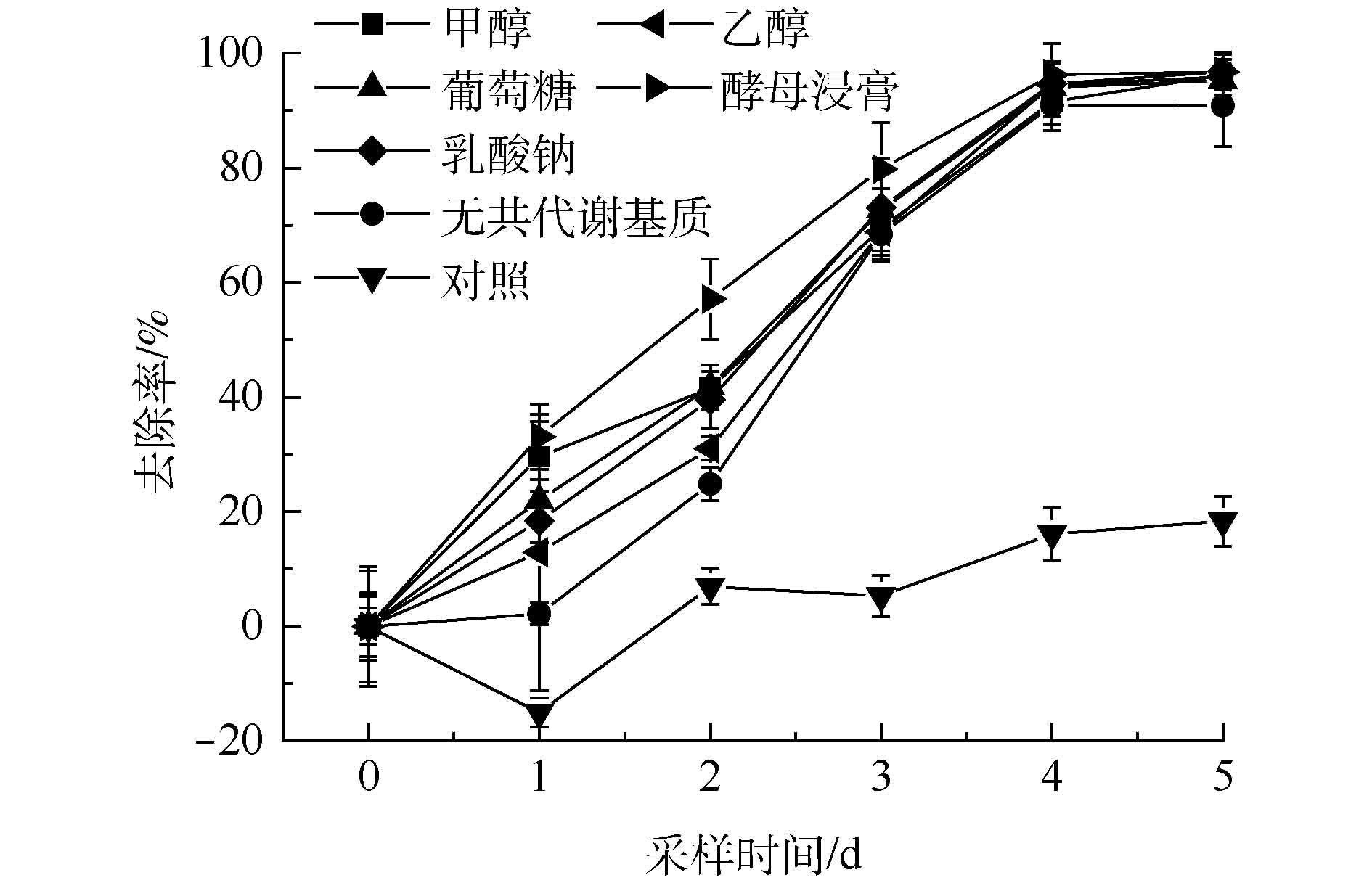
不同共代谢基质对PCE降解效果的影响见图5,其中,酵母浸膏作为共代谢基质时降解效果最好。为了比较不同共代谢基质对微生物脱氯能力的影响,需要建立动力学模型进行比较。一般情况下,反应的动力学方程见式(1),对应一级动力学模型[47]见式(2)。
dc/dt=−kcp(1) c=c0e−k(t−t0)(2) 式中:c为PCE浓度,mg·L−1;p为反应级数;k为反应速率常数,d−1;t为反应时间,d;c0为初始PCE浓度,mg·L−1;t0为初始反应时间,d。
PCE反应动力学模型见图6,根据一级反应动力学拟合方程得到的相关参数见表1。由图6和表1可知,共代谢基质强化的微生物降解过程全部符合一级反应动力学,R2均在0.928以上,除无共代谢对照组只有0.884外,整体拟合效果较好。根据表1进一步可得出,添加了酵母浸膏后实验的半衰期比不添加共代谢基质时减少了0.838 d,并且降解反应速率常数由0.327 d−1降至0.098 d−1,其他共代谢基质(除乙醇外)添加后降解反应速率常数均有提高。上述结果表明,这些共代谢基质的添加均提高了菌群的降解速率。添加不同共代谢基质后的PCE降解反应速率常数有以下关系:酵母浸膏>葡萄糖≈甲醇>乳酸钠>无共代谢基质>乙醇。以酵母浸膏为共代谢基质时,降解反应速率常数最大为0.327 d−1,半衰期为1.571 d,大于其他共代谢基质,所以在此实验条件下,酵母浸膏为最佳的共代谢基质。这可能是因为酵母浸膏含有丰富的维生素、各种氨基酸及核酸降解物等,可以作为促进微生物生长的氮素营养源[22]。乙醇作为共代谢基质时四氯乙烯的降解反应速率常数最小,还小于没有共代谢条件下的降解速率,这可能是因为乙醇对该菌群产生了活性抑制。乙醇因为其特殊的理化性质,会对简单节杆菌中的细胞色素酶等产生抑制作用,使降解菌的降解效率降低[48],乙醇还会改变乳酸杆菌细胞膜的通透性从而影响菌体的代谢活力[49]。因此,可推断同为革兰氏阳性[50]菌的Clostridium sp. FCB45(优势菌种)会出现相似的活性抑制,从而影响PCE降解速率。
表 1 不同共代谢基质对应的PCE的反应动力学模型方程及相关参数Table 1. Reaction kinetics equations and parameters of PCE degradation using different co-substrates共代谢基质 动力学模型 R2 反应速率常数k/d−1 半衰期t1/2/d 甲醇 c=1.39e−t7.02−0.682 
0.972 0.142 1.665 乙醇 c=4.38e−t27.23−3.67 
0.928 0.037 2.296 葡萄糖 c=1.62e−t6.875−0.792 
0.963 0.145 2.095 酵母浸膏 c=1.11e−t3.055−0.223 
0.988 0.327 1.571 乳酸钠 c=1.895e−t8.906−1.106 
0.955 0.112 2.161 无共代谢基质 c=1.746e−t10.155−1.061 
0.884 0.098 2.409 3. 结论
1)利用梯度驯化法获得可将PCE高效降解的菌群,此菌群可以在PCE长期选择压力下生存繁殖。菌种鉴定结果显示:在种水平上,梭状芽孢杆菌Clostridium sp. FCB45含量最高,成为群落中的优势菌种。该群落多样性高,组成复杂,群落稳定性高,易于管理。
2)当温度为30 ℃、pH为中性、PCE初始浓度为1 mg∙L−1和共代谢基质为酵母浸膏时,PCE降解效率最高可达96.75%。此群落可以在不同环境条件下(pH、温度等)表现出较高的降解率,该微生物群落有较宽的生态幅,在非极端环境条件下该群落均能发挥作用。
3)添加共代谢基质强化的微生物实验结果表明,反应模型均符合一级反应动力学,拟合度良好,降解反应速率常数由大到小顺序依次是:酵母浸膏>葡萄糖≈甲醇>乳酸钠>无共代谢基质>乙醇。该菌群在利用酵母浸膏作为共代谢基质时,降解速率常数最大可达0.327 d−1。本研究中酵母浸膏最大程度地提高了四氯乙烯的降解速率。
-
图 8 30 ℃时Na+、K+//Cl-—H2O 三元水盐体系相图[39]及回收工业盐过程中各类盐的组成点
Figure 8. Phase diagram of Na+、K+//Cl-—H2O ternary water salt system at 30 ℃ and composition points of various salts in the process of industrial salt recovery
表 1 液固比为4 L·kg−1的飞灰水洗液中各元素的质量浓度
Table 1. Mass concentrations of elements in the fly ash eluates by washing at a liquid to solid ratio of 4 L·kg−1
Cr/(mg·L−1) Cu/(mg·L−1) K/(g·L−1) Mg/(mg·L−1) Na/(g·L−1) Pb/(mg·L−1) Ba/(mg·L−1) Ca/(g·L−1) Cl/(g·L−1) 0.55±0.01 0.05±0 10.10±0.12 0.05±0 6.48±0.21 17.30±1.67 4.51±0.10 9.71±0.03 30.40±0.32 表 2 电渗析前后物料质量浓度
Table 2. Mass concentration of material before ang after electrodialysis
考察项目 液体量/L 含盐量/(g·L−1) 钾/(g·L−1) 钠/(g·L−1) 预处理后飞灰水洗液 — 62.7 ± 0.9 24.4 ± 0.3 5.68 ± 0.18 淡液(工况1) 1.62 3.62 ± 0.66 0.94 ± 0.03 0.62 ± 0.03 浓液(工况1) 1.38 127 ± 1(*2.03倍) 48.0 ± 0.2(*1.97倍) 15.5 ± 0.1(*2.73倍) 1次电渗析淡液(工况2) 1.65 3.44 ± 0.39 0.96 ± 0.02 0.64 ± 0.06 1次电渗析浓液(工况2) 2.35 101 ± 1(*1.62倍) 34.2 ± 0.4(*1.4倍) 9.20 ± 0.08(*1.17倍) 2次电渗析淡液(工况2) 0.7 3.96 ± 0.22 1.12 ± 0.01 0.71 ± 0.01 2次电渗析浓液(工况2) 1.3 143 ± 1(*2.28倍) 52.4 ± 0.4(*2.14倍) 15.2 ± 0.1(*2.68倍) 注:*为浓缩倍数;浓缩倍数=浓液中该物质的质量浓度/预处理后飞灰水洗液中该物质的质量浓度。 -
[1] 国家统计局. 中国统计年鉴[M]. 北京: 中国统计出版社, 2004-2020. [2] LECKNER B. Process aspects in combustion and gasification Waste-to-Energy (WtE) units[J]. Waste Management, 2015, 37: 13-25. doi: 10.1016/j.wasman.2014.04.019 [3] QUINA M J, BORDADO J C, QUINTA-FERREIRA R M. Treatment and use of air pollution control residues from MSW incineration: An overview[J]. Waste Management, 2008, 28(11): 2097-2121. doi: 10.1016/j.wasman.2007.08.030 [4] JAMBHULKAR H P, SHAIKH S M S, KUMAR M S. Fly ash toxicity, emerging issues and possible implications for its exploitation in agriculture; Indian scenario: A review[J]. Chemosphere, 2018, 213: 333-344. doi: 10.1016/j.chemosphere.2018.09.045 [5] NIE Y. Development and prospects of municipal solid waste (MSW) incineration in China[J]. Frontiers of Environmental Science & Engineering in China, 2008, 2(1): 1-7. [6] FAN C C, WANG B M, ZHANG T T. Review on cement stabilization/solidification of municipal solid waste incineration fly ash[J]. Advances in Materials Science and Engineering, 2018, 2018: 1-7. [7] WANG X N, GAO M, WANG M L, et al. Removal of heavy metals in municipal solid waste incineration fly ash using lactic acid fermentation broth[J]. Environmental Science and Pollution Research, 2021, 28: 62716-62725. doi: 10.1007/s11356-021-14948-6 [8] 何品晶, 吴长淋, 章骅, 等. 生活垃圾焚烧飞灰及其稳定化产物的长期浸出行为[J]. 环境化学, 2008, 27(6): 786-790. doi: 10.3321/j.issn:0254-6108.2008.06.018 [9] 章骅, 曾佳玮, 吕凡, 等. 飞灰螯合剂中挥发性污染物的释放[J]. 中国环境科学, 2019, 39(12): 5182-5190. doi: 10.19674/j.cnki.issn1000-6923.2019.0602 [10] LI R D, LI Y L, YANG T H, et al. A new integrated evaluation method of heavy metals pollution control during melting and sintering of MSWI fly ash[J]. Journal of Hazardous Materials, 2015, 289: 197-203. doi: 10.1016/j.jhazmat.2015.02.055 [11] XUE Y, LIU X M. Detoxification, solidification and recycling of municipal solid waste incineration fly ash: A review[J]. Chemical Engineering Journal, 2021, 420: 130349. doi: 10.1016/j.cej.2021.130349 [12] MEER I, NAZIR R. Removal techniques for heavy metals from fly ash[J]. Journal of Material Cycles and Waste Management, 2018, 20(2): 703-722. doi: 10.1007/s10163-017-0651-z [13] JIANG Y H, XI B D, LI X J, et al. Effect of water-extraction on characteristics of melting and solidification of fly ash from municipal solid waste incinerator[J]. Journal of Hazardous Materials, 2009, 161(2-3): 871-877. doi: 10.1016/j.jhazmat.2008.04.033 [14] WANG X N, WANG M L, ZOU D Z, et al. Comparative study on inorganic Cl removal of municipal solid waste fly ash using different types and concentrations of organic acids[J]. Chemosphere, 2020, 261: 127754. doi: 10.1016/j.chemosphere.2020.127754 [15] KANG D, SON J, YOO Y, et al. Heavy-metal reduction and solidification in municipal solid waste incineration (MSWI) fly ash using water, NaOH, KOH, and NH4OH in combination with CO2 uptake procedure[J]. Chemical Engineering Journal, 2020, 380: 122534. doi: 10.1016/j.cej.2019.122534 [16] YANG J, WANG Q H, LUO Q S, et al. Biosorption behavior of heavy metals in bioleaching process of MSWI fly ash by Aspergillus niger[J]. Biochemical Engineering Journal, 2009, 46(3): 294-299. doi: 10.1016/j.bej.2009.05.022 [17] 中华人民共和国生态环境部. 生活垃圾焚烧飞灰污染控制技术规范: HJ 1134-2020[S]. 北京: 中国环境出版集团, 2020. [18] CHEN X, BI Y, ZHANG H, et al. Chlorides removal and control through water-washing process on MSWI fly ash[J]. Procedia Environmental Sciences, 2016, 31: 560-566. doi: 10.1016/j.proenv.2016.02.086 [19] 白晶晶, 张增强, 闫大海, 等. 水洗对焚烧飞灰中氯及重金属元素的脱除研究[J]. 环境工程, 2012, 30(2): 104-108. doi: 10.13205/j.hjgc.2012.02.032 [20] CHIANG K Y, HU Y H. Water washing effects on metals emission reduction during municipal solid waste incinerator (MSWI) fly ash melting process[J]. Waste Management, 2010, 30(5): 831-838. doi: 10.1016/j.wasman.2009.12.009 [21] FERIA-DíAZ J J, CORREA-MAHECHA F, LóPEZ-MéNDEZ M C, et al. Recent desalination technologies by hybridization and integration with reverse osmosis: A review[J]. Water, 2021, 13(10): 1369. doi: 10.3390/w13101369 [22] ZHANG Y, GHYSELBRECHT K, MEESSCHAERT B, et al. Electrodialysis on RO concentrate to improve water recovery in wastewater reclamation[J]. Journal of Membrane Science, 2011, 378(1-2): 101-110. doi: 10.1016/j.memsci.2010.10.036 [23] MIR N, BICER Y. Integration of electrodialysis with renewable energy sources for sustainable freshwater production: A review[J]. Journal of Environmental Management, 2021, 289: 112496. doi: 10.1016/j.jenvman.2021.112496 [24] 施小林, 王大新, 楼书怿, 等. 电渗析技术在水泥厂废水零排放处理中的应用[J]. 环境工程, 2021, 39(7): 179-184. doi: 10.13205/j.hjgc.202107025 [25] ZHAN X Y, GUNVOR M K. Electrodialytic remediation of municipal solid waste incineration fly ash as pre-treatment before geopolymerisation with coal fly ash[J]. Journal of Hazardous Materials, 2021, 412: 125220. doi: 10.1016/j.jhazmat.2021.125220 [26] KIRKELUND G M, JENSEN P E, VILLUMSEN A, et al. Test of electrodialytic upgrading of MSWI APC residue in pilot scale: focus on reduced metal and salt leaching[J]. Journal of Applied Electrochemistry, 2010, 40: 1049-1060. doi: 10.1007/s10800-009-0059-0 [27] 魏国侠, 刘汉桥, 武振华, 等. 电渗析法分离医疗垃圾焚烧飞灰浸出液中重金属[J]. 过程工程学报, 2014, 14(5): 776-781. [28] SCHOEMAN J J, STEYN A, MAKGAE M. Evaluation of electrodialysis for the treatment of an industrial solid waste leachate[J]. Desalination, 2005, 186(1-3): 273-289. doi: 10.1016/j.desal.2005.04.061 [29] 中华人民共和国国家环境保护总局, 国家质量监督检验检疫总局. 危险废物鉴别标准 浸出毒性鉴别: GB 5085.3-2007[S]. 北京: 中国环境科学出版社, 2007. [30] YANG R B, LIAO W P, WU P H. Basic characteristics of leachate produced by various washing processes for MSWI ashes in Taiwan[J]. Journal of Environmental Management, 2012, 104: 67-76. [31] 罗智宇, 张云月, 张清, 等. 垃圾焚烧飞灰水洗去氯的实验研究[J]. 环境卫生工程, 2008, 3: 16-18,22. doi: 10.3969/j.issn.1005-8206.2008.03.006 [32] CHIMENOS J M, FERNANDEZ A I, CERVANTES A, et al. Optimizing the APC residue washing process to minimize the release of chloride and heavy metals[J]. Waste Management, 2005, 25(7): 686-693. doi: 10.1016/j.wasman.2004.12.014 [33] ZHAO D D, LEE L Y, ONG S L, et al. Electrodialysis reversal for industrial reverse osmosis brine treatment[J]. Separation and Purification Technology, 2019, 213: 339-347. doi: 10.1016/j.seppur.2018.12.056 [34] 陈晓丹, 李正浩, 张淦, 等. 均相膜电渗析在电镀废水零排放中的应用//中国环境科学学会[J]. 2017年中国环境科学学会科学与技术年会论文集(第二卷). 北京, 2017: 1369-1372. [35] 中华人民共和国国家质量监督检验检疫总局, 中国国家标准化管理委员会. 工业氯化钾: GBT 7118-2008[S]. 北京: 中国环境科学出版社, 2008. [36] 黄俊, 余刚, 张彭义. 单苯环氯取代指数法预测二恶英类化合物PCDFs的正辛醇水分配系数[J]. 环境科学研究, 2002, 15(2): 1-5. doi: 10.3321/j.issn:1001-6929.2002.02.001 [37] HUANG Y, TAKAOKA M, TAKEDA N, et al. Polychlorinated biphenyls removal from weathered municipal solid waste incineration fly ash by collector-assisted column flotation[J]. Journal of Hazardous Materials, 2003, 100(1-3): 259-270. doi: 10.1016/S0304-3894(03)00113-4 [38] 王旭, 陆胜勇, 陈志良, 等. 生活垃圾焚烧飞灰水洗液中氯离子的去除研究[J]. 环境科学学报, 2017, 37(6): 2218-2222. doi: 10.13671/j.hjkxxb.2016.0412 [39] 牛自得, 程芳琴. 水盐体系相图及其应用[J]. 天津:天津大学出版社, 2002: 234-239. -




 下载:
下载: