-
制药废水具有高盐度、高色度、高化学需氧量(chemical oxygen demand,COD)等特点,属于难降解有机工业废水之一。经过生化处理后,仍存在溶解性有机物(dissolved organic matter,DOM)浓度较高、毒性较大等问题,难以达到排放标准和回用水要求[1]。生化处理后,废水中DOM由腐殖质类、多糖类、蛋白类、脂质类和小分子的氨基酸等组成[2],含有未完全降解的小分子有机物,以及长链烷烃、芳香烃等大分子质量有机物,废水多呈现淡黄色[3]。DOM排入水体后将对水环境造成不利影响,并产生一定的生态风险。因此,须对制药废水进行深度处理,以消减DOM物质排放量,降低生物毒性,减少水环境生态风险。
废水深度处理工艺主要包括高级氧化法(advanced oxidation processes,AOPs)、膜分离法、吸附法和生物法等[4-6]。AOPs由于其氧化能力强、清洁、无二次污染得到广泛应用。AOPs可在水中生成羟基自由基(HO·),HO·具有高氧化活性和无选择性特征,能够氧化大多数有机污染物,因此得到广泛关注[7]。传统臭氧化技术存在HO·产生效率差、臭氧利用率低、气液传质速率慢等问题,使得难降解有机物矿化效率有限,制约了其实际应用[8]。微气泡(microbubble,MB)通常是指直径小于50 μm的微小气泡,有研究表明,微气泡臭氧传质速率是普通气泡的1.3~1.5倍,且能够有效加速臭氧分解产生HO·,进而提高臭氧化能力[9]。同时,有研究[10-13]表明,微气泡表面存在电荷聚集效应,在液相收缩过程中表面电荷密度和ζ电位急剧升高,并在破裂时可产生HO·,产生类催化效应。在微气泡催化臭氧化中,臭氧微气泡类催化效应与催化剂催化效应协同,可显著提高HO·氧化能力,使得臭氧利用率和有机物矿化率显著提高[9, 14-15],并可提高废水的可生化性,降低其生物毒性[16]。LIU等[17]采用微气泡臭氧化技术处理阿特拉津废水,发现臭氧微气泡通过臭氧分解以及微气泡破裂可提高阿特拉津降解率。ZHANG等[18]以活性炭为催化剂催化微气泡臭氧化处理酸性大红3R废水,可将TOC去除率由传统气泡(coarse bubble,CB)的36.2%提升至91.2%。可见,微气泡臭氧化技术在废水深度处理领域具有良好的应用前景。
非均相催化臭氧化通过将臭氧转化为HO·来提高氧化能力和污染物去除效率[19-20]。选用活性金属离子制备具有水滑石结构的层状双羟基复合金属氢氧化物(layered double hydroxides,LDHs),往往具有优异的催化性能,但其应用于催化臭氧化的研究甚少。HASSANI等[21]采用镍基层状氢氧化物纳米材料催化臭氧化处理模拟染料废水,其对COD的去除率可由传统臭氧化的30%提高到72%。FU等[22]采用钴铁层状双氢氧化物催化臭氧化降解甲苯磺酸溶液,TOC的去除率可达85%。
本研究将微气泡与催化臭氧化技术相结合,采用镁铝层状双氢氧化物催化剂(Mg-Al-LDHs,MAL)催化微气泡臭氧化实际制药废水,考察了普通气泡臭氧化(CB/O3)、普通气泡催化臭氧化(MAL/CB/O3)、微气泡臭氧化(MB/O3)和微气泡催化臭氧化(MAL/MB/O3)体系处理实际制药废水的效能及有机物降解特征,分析了废水中亲水性酸(hydrophilic acid,HIA)、亲水性碱(hydrophilic base,HIB)、亲水性中性(hydrophilic neutral,HIN)、疏水性酸(hydrophobic acid,HOA)、疏水性碱(hydrophobic base,HOB)、疏水性中性(hydrophobic neutral,HON)DOM组分的变化,以期为制药废水深度处理提供参考。
-
本研究所用实际制药废水取自石家庄某化学合成制药企业生化处理出水,其水质指标为:510~600 mg·L−1COD、55~65 mg·L−1BOD5、140~160 mg·L−1SS、30~40 mg·L−1 NH4+-N、895±5 mg·L−1 Cl−、(3825±20) mg·L−1 SO42−、(195±5) mg·L−1 CO32−、(38±2) mg·L−1 PO43−、UV254为4.0~5.0、pH为6.8~7.8。
-
将0.03 mol·L−1 Mg(NO3)2·6H2O与0.01 mol·L−1 Al(NO3)3·9H2O溶于100 mL去离子水中,获得金属盐溶液;将0.75 mol·L−1 NaHCO3溶于500 mL去离子水中,获得碱性溶液;在60℃水浴加热搅拌下将金属盐溶液滴入碱性溶液中,滴加过程中用2 mol·L−1 NaOH调节pH,使pH保持在8.5;将悬浊液在60 ℃水浴加热21 h,抽滤、洗涤至与去离子水pH相同,将沉淀物在78 ℃下干燥12 h,获得镁铝层状双氢氧化物催化剂。采用SEM、TEM和XRD对催化剂形貌和层状结构进行表征。
-
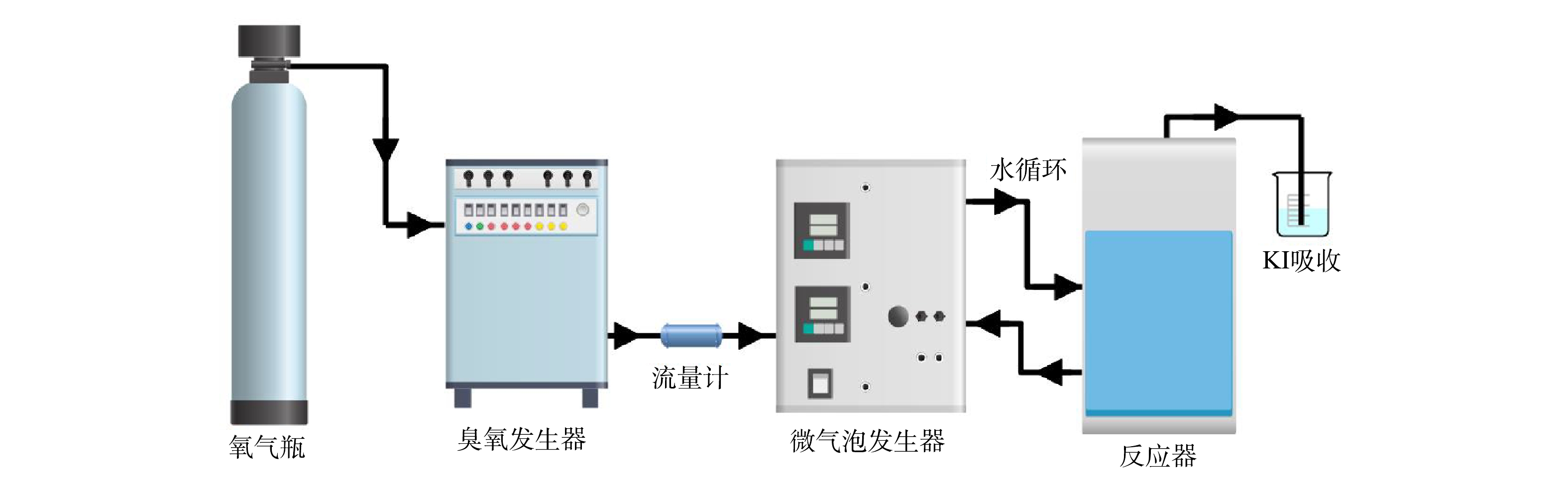
本研究实验装置示意如图1所示。主要包括氧气源臭氧发生器(OZ-10G,广州大环)、微气泡发生器(北京晟峰恒泰科技有限公司)、有机玻璃反应器(有效容积15 L)。在MB/O3处理中,臭氧发生器产生臭氧气体,通过气体流量计控制臭氧气体流量。臭氧气体与反应器中循环废水混合后,进入微气泡发生器产生臭氧微气泡,由底部进入反应器。在CB/O3处理中,臭氧气体通过反应器底部曝气头产生臭氧普通气泡。处理后尾气由反应器顶部排气口进入尾气吸收瓶中,通过碘化钾溶液吸收。
-
本研究采用批次实验方法,分别通过CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3体系深度处理实际制药废水,处理时间均为90 min。处理过程中,控制臭氧气体流量为0.5 L·min−1(此流量下产生微气泡平均直径小于50 μm),同时控制臭氧气体浓度,使得臭氧投加总量与处理废水初始COD总量之比约为0.6,催化剂用量为0.5 g·L−1。处理过程中通过测定COD、BOD5、UV254、三维荧光(3D-EEM)光谱、可生化性、生物毒性,评估处理效能。此外,对各体系处理前后废水DOM组分中COD、UV254和3D-EEM进行测定,分析处理过程中DOM不同组分的变化特征。
-
COD采用重铬酸钾微波消解法测定[23];UV254采用分光光度计(UV-5100,METASH)进行测定;BOD5采用生物化学需氧量测定仪(LH-BOD601,连华科技)测定;生物毒性采用Mithras LB 940多功能分析仪测定[24]。采用荧光光谱仪(FluoroMax-4,日本HORIBA)对样品进行3D-EEM分析。采用比表面测定仪(D/MAX-2500,日本Rigaku)按照BET氮吸附法对MAL比表面积进行测定。
废水中DOM组分采用树脂分级法分离收集,树脂采用(Supelite)XAD-8大孔径树脂、Dowex Marathon MSC(H)阳离子交换树脂、Duolite A-7阴离子交换树脂,使用前采用有机溶剂索氏提取进行预处理,去除树脂中可溶性有机物,然后使用不同浓度的HCl和NaOH溶液活化树脂[25-26]。废水中DOM通过处理活化后离子交换树脂分离,将DOM分为HIA、HIB、HIN、HOA、HOB、HON组分[27],而后对各组分COD、UV254和3D-EEM进行测定分析。
-
MAL/MB/O3体系协同效应值根据式(1)进行计算[28]。
式中:R为协同效应值;ka为MB/O3/LDHs体系COD去除准一级速率常数,min−1;kb为MB/N2、CB/O3以及MAL吸附过程中COD去除准一级速率常数之和,min−1。
-
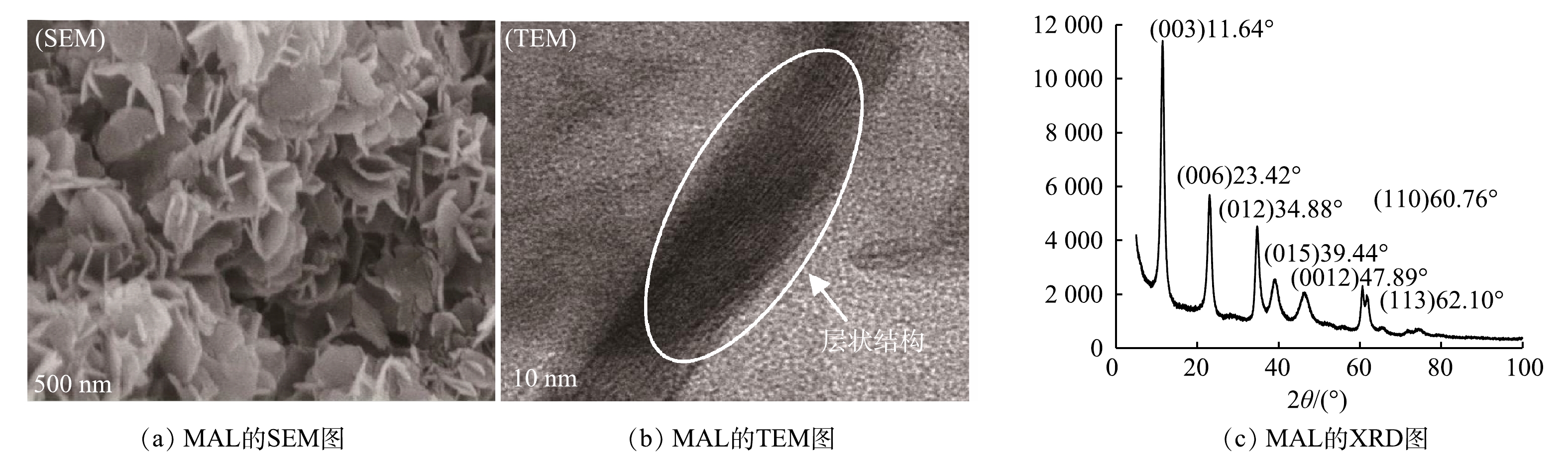
MAL呈粉末状,比表面积为60 m2·g−1。由图2(a)和图2(b)可见,MAL形貌呈现片状结构,其内部具有明显的层状结构。由图2(c)可见,在2θ为11.64°、23.42°、34.88°、60.76°处有尖锐衍射峰,对应(003)、(006)、(012)和(110)晶面,符合层状双氢氧化物的典型特征[29],其与镁铝层状双氢氧化物标准物的衍射峰基本匹配。这表明镁铝层状双氢氧化物成功制备。
-
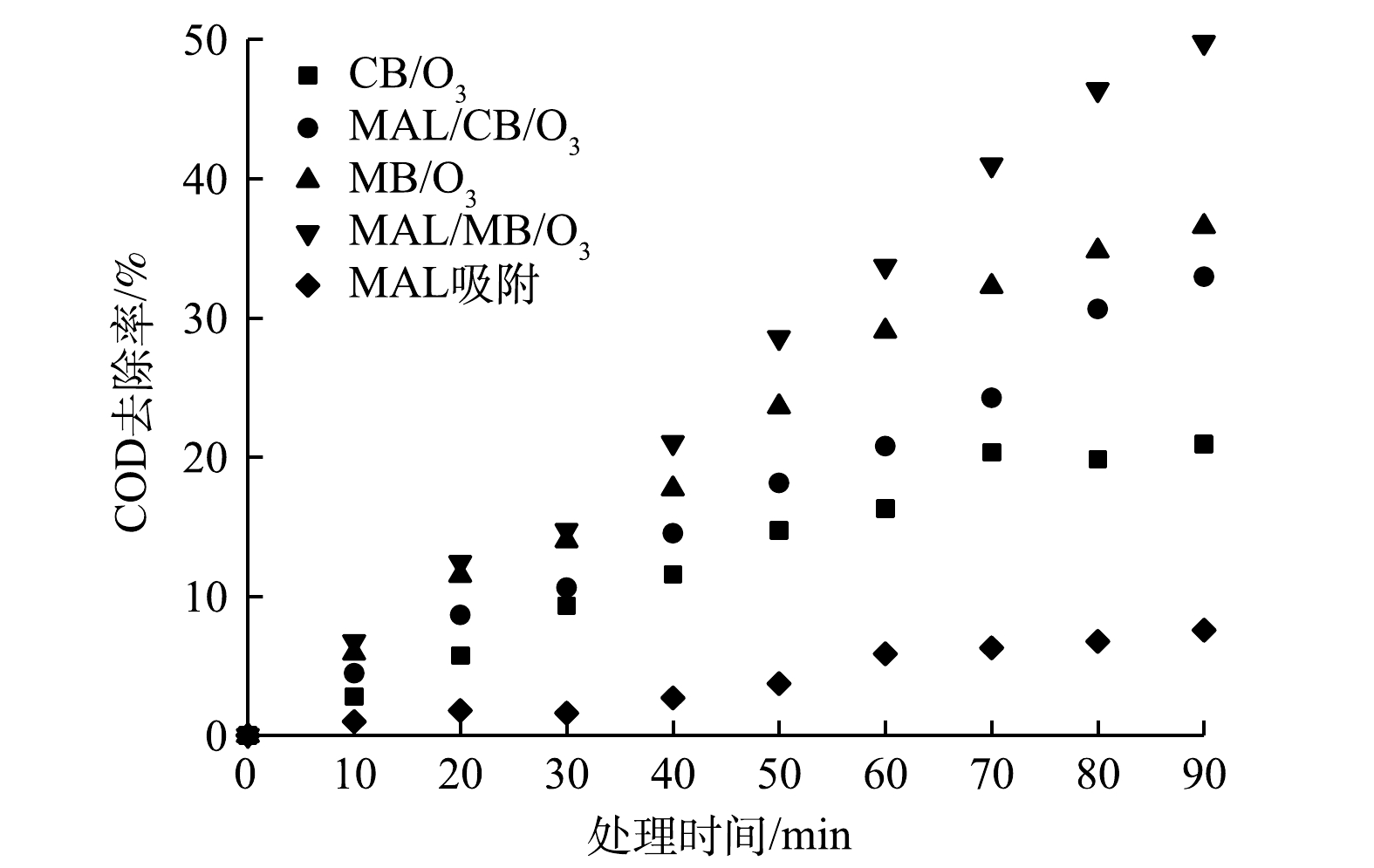
1) COD整体去除。在CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3体系及MAL催化剂吸附处理实际制药废水过程中,整体COD去除率随时间变化如图3所示。其中,MAL吸附对COD去除率不足7%。可以看到,在CB/O3体系中,COD去除率在处理70 min后达到20.93%,而后几乎保持不变;在MAL/CB/O3、MB/O3和MAL/MB/O3体系中,COD去除率在整个处理过程中呈现不断上升的趋势,处理90 min后COD去除率分别达到32.96%、36.57%和49.79%。微气泡臭氧化可增强对COD的去除性能,MB/O3体系比CB/O3体系对COD去除率提高了15.64%。MAL/MB/O3体系比MB/O3体系对COD去除率提高了13.22%。以上结果表明,MAL催化通过镁铝双金属可提高催化体系电子传递速率,且层状结构可提供更多的催化反应活性位点,可加速有机物氧化降解进程,进一步提高COD氧化去除效率。因此,微气泡臭氧化和MAL催化对整体COD去除均有明显促进作用。计算得出CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3体系中COD去除量与臭氧消耗量的比值R,分别为0.38、0.60、0.62和0.83,这表明MAL/MB/O3体系单位臭氧消耗量去除COD更多,臭氧反应效率更高。
为进一步分析微气泡臭氧化和MAL催化作用对COD去除是否存在协同效应,分别测定和计算MAL催化剂吸附、氮气微气泡(MB/N2)、CB/O3以及MAL/MB/O3处理过程去除COD反应速率常数,其值分别为8.693×10−4、2.189×10−3、2.903×10−3和7.293×10−3 min−1。在此基础上,根据式(1)计算MAL/MB/O3协同效应R值,为1.223,表明微气泡臭氧化与MAL催化作用对COD去除存在一定程度的协同效应[30]。
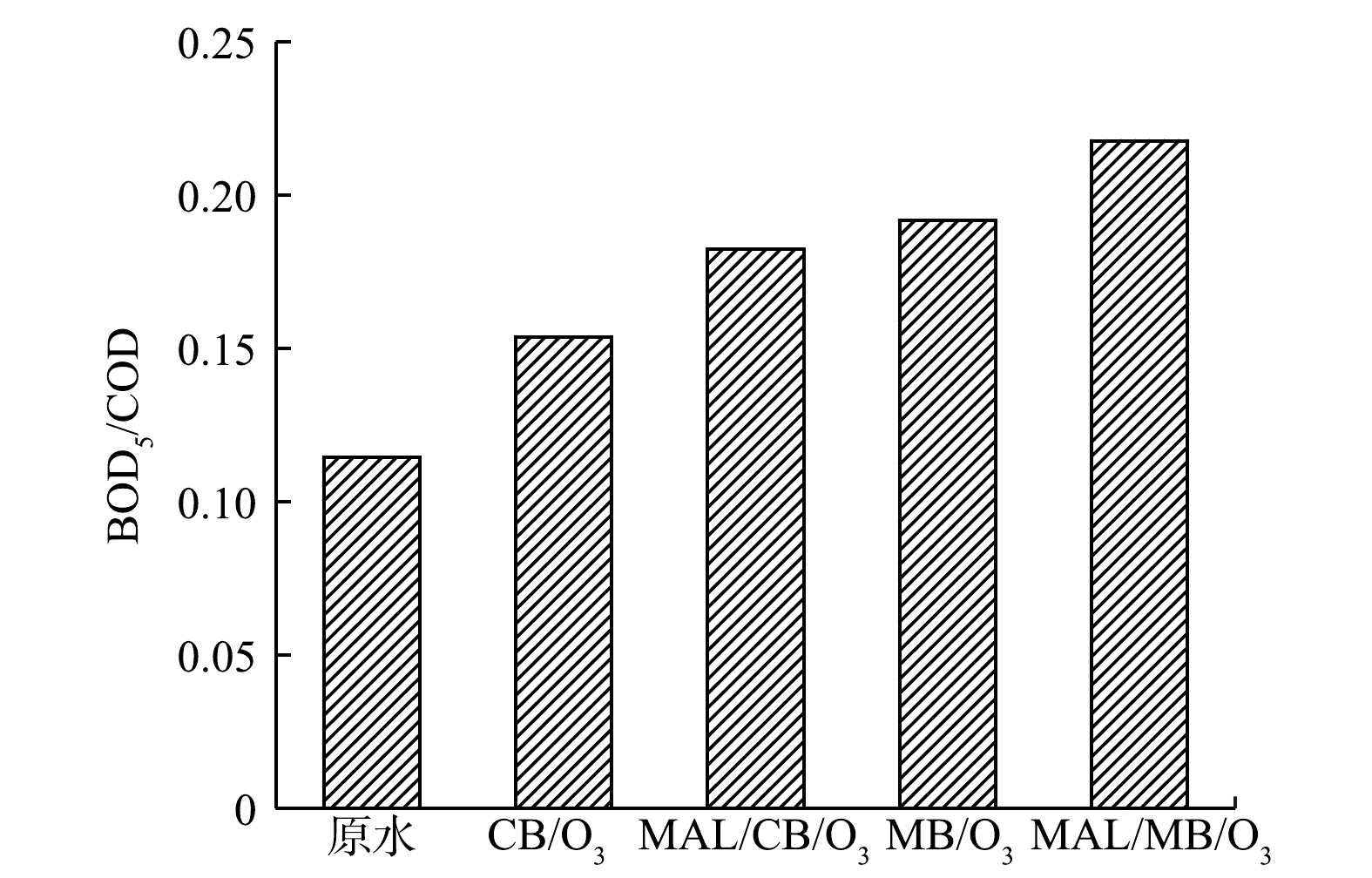
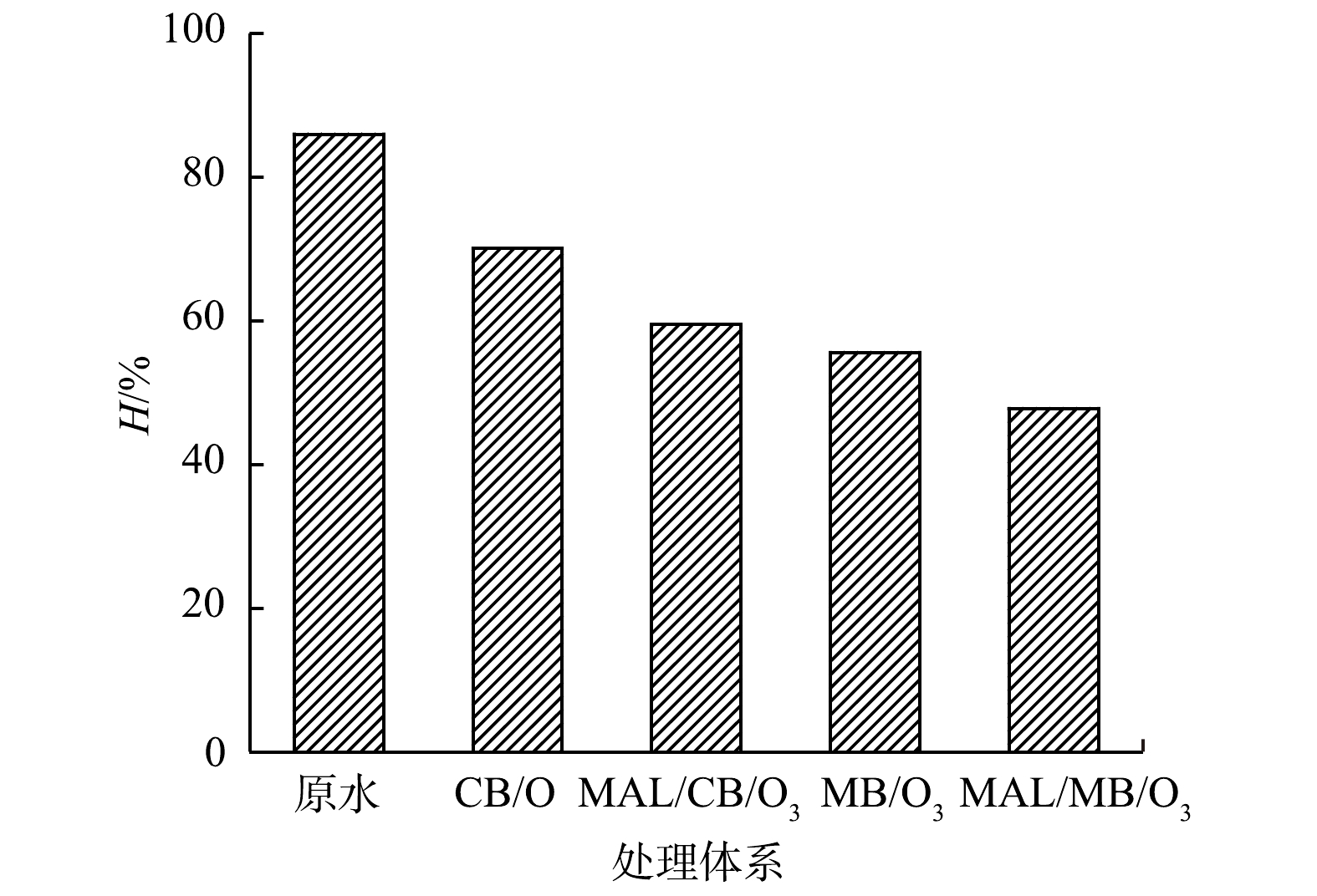
2)可生化性与生物毒性变化。CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3体系深度处理实际制药废水前后,BOD5/COD值的变化如图4所示,发光细菌抑制率H的变化如图5所示。可以看到,处理前废水BOD5/COD值为0.114,可生化性较差,且发光细菌抑制率H为86.0%,属于高毒水平。各体系处理90 min后,废水BOD5/COD分别提高至0.153、0.182、0.191和0.217,废水可生化性得到一定改善;同时,发光细菌抑制率H分别降低至70.0%、59.5%、55.6%和47.8%,其中MAL/MB/O3处理后可降低至中毒水平[31]。可见,有机物深度降解和去除可改善废水可生化性和降低生物毒性,而MAL/MB/O3体系作用更为明显。
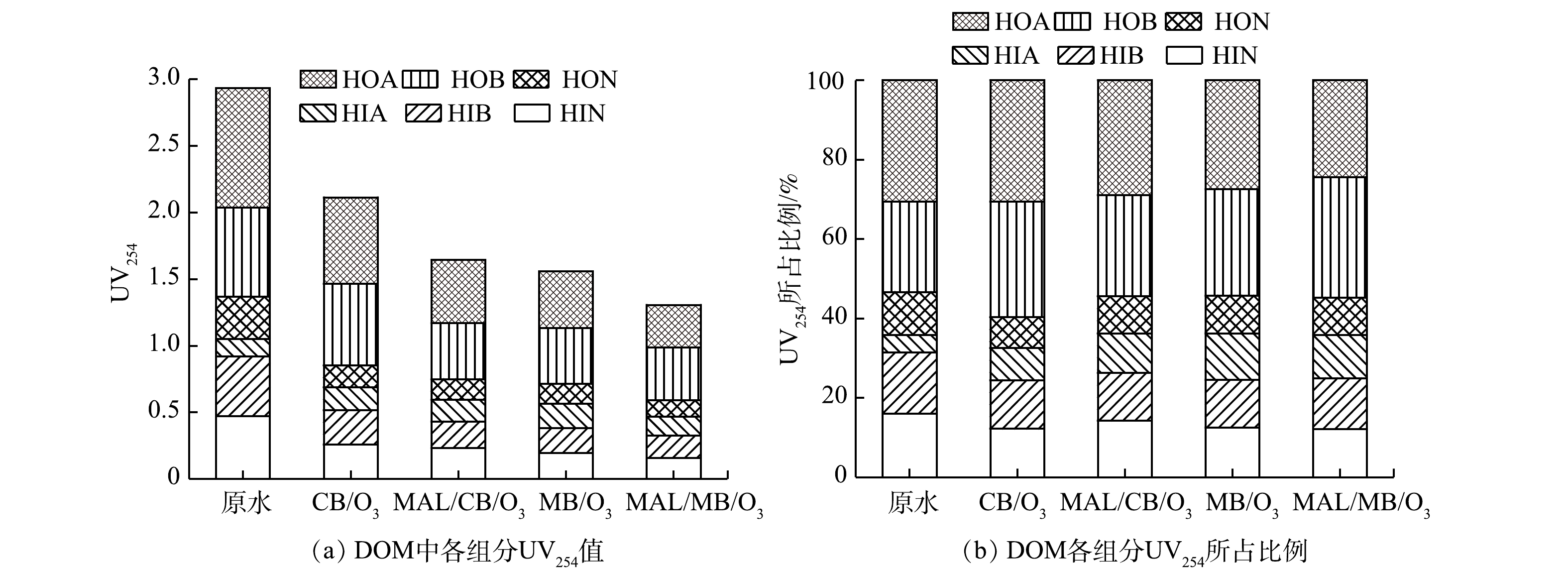
3) DOM组分浓度变化。在COD整体去除基础上,分析了CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3处理制药废水前后,废水中DOM各组分COD浓度变化以及不同体系处理前后DOM各组分COD浓度及其所占比例的变化,结果如图6所示。由图6(a)可以看到,原水DOM疏水性组分浓度整体高于亲水性组分,其中HOA组分浓度最高,HIA组分浓度最低。处理90 min后,CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3对疏水性组分去除率比值为0.54∶0.76∶0.85∶1,对亲水性组分去除率比值为0.28∶0.69∶0.68∶1,疏水性组分去除效率均高于亲水性组分,且MB/O3对疏水性、亲水性组分去除明显优于CB/O3,CB/O3对亲水性组分去除明显低于疏水性组分。产生上述结果的原因可能是,臭氧直接氧化可去除疏水性组分,而亲水性组分去除主要依赖HO·氧化。MAL/MB/O3同时具有强化臭氧传质、提高臭氧直接氧化能力以及催化HO·氧化和微气泡强化HO·氧化作用[28],因此对DOM的去除效率最高,可达到52.51%,其中疏水性组分整体去除率56.67%,亲水性组分整体去除率46.93%。
值得注意的是,HIA浓度在CB/O3、MAL/CB/O3和MB/O3处理中分别增加了27.27%、9.09%和44.37%。其原因可能是其他组分被氧化转化为HIA,而臭氧直接氧化和HO·氧化对<C5脂肪酸降解效果较差[32-33],从而导致HIA的积累。
由图6(b)可以看出,不同体系处理制药废水前后DOM各组分COD所占该体系整体COD的比例。对于疏水性组分,HOA所占比例明显下降,HOB所占比例保持稳定,HON所占比例有所上升;对于亲水性组分,HIA所占比例明显升高,HIB所占比例也有所升高,而HIN所占比例下降明显。可见,HOA和HIN组分相对更容易降解或转化,而HIA降解最为困难。
-
1) DOM组分UV254变化。CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3体系处理实际制药废水前后DOM中各组分UV254值,以及4种体系处理实际制药废水前后DOM中各组分UV254值所占该体系UV254值的比例如图7(a)和图7(b)所示。由图7(a)可以看到,原水DOM疏水性组分UV254值整体高于亲水性组分,原水疏水性组分的UV254值与原水疏水性组分的COD的比值为亲水性组分的1.34倍,因而疏水性组分不饱和度更强。对疏水性组分而言,HOA、HOB和HON组分UV254值与该组分COD之比的比值为1∶0.88∶1.37。由此可以推测,HOA中C5~C9脂肪酸和芳香环酸共存,不饱和度居中;HOB中以芳香环胺为主,不饱和度最强;HON中以长链(>C9)脂肪酸为主,不饱和度较低[32]。对亲水性组分而言,HIN主要为<C5脂肪酰胺和多官能团醇类,HIA主要为<C5脂肪酸和羟基酸等,HIB主要为<C9脂肪胺和吡啶的两性蛋白质,HIN、HIA和HIB组分UV254值与该组分COD之比的比值为1∶1.04∶0.79,可见HIN和HIA不饱和度相当且较强,HIB不饱和度相对较弱。
各体系处理90 min后,CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3对疏水性组分UV254去除率比值为0.44∶0.80∶0.85∶1,对亲水性组分UV254去除率比值为0.62∶0.78∶0.84∶1。可见, CB/O3/LDHs、MB/O3和MB/O3/LDHs体系中亲、疏水性组分UV254去除率基本一致。其原因是:由于存在较强HO·氧化作用,因而对芳香环结构和不饱和键均具有较强破坏作用;而CB/O3对亲水性组分去除率较低,对UV254去除率相对较高,表明臭氧直接氧化可破坏亲水性组分的不饱和结构,但完全矿化去除较为困难。
不同体系处理制药废水前后DOM各组分UV254所占该体系UV254的比例如图7(b)所示。各体系处理过程中,疏水性组分中,HOA所占比例明显下降,HOB所占比例有所升高,HON所占比例略有下降;亲水性组分中,HIA所占比例明显升高,HIB和HIN所占比例均有所下降。DOM各组分的UV254所占比例变化与COD变化有关,如HOA、HIA、HIN组分UV254与COD所占比例变化一致;同时也与组分不饱和度有关,如HOB不饱和度最强,虽COD所占比例不变,但UV254所占比例升高,而HON和HIB不饱和度较弱,虽COD所占比例升高,但UV254所占比例下降。
值得注意的是,HIA组分UV254值在处理后均出现升高现象,包括经过MAL/MB/O3处理后,HIA浓度降低的同时,其UV254值也有所升高。同时,原水以及经CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3体系处理后的HIA中COD比值为1∶1.27∶1.09∶1.44∶0.95,UV254比值为1∶1.32∶1.26∶1.40∶1.09,表明除HIA组分COD升高外,HIA组分不饱和度有所增强也是UV254值升高的原因之一。由于处理中存在其他组分向HIA组分转化过程,转化产生的HIA组分可能存留较多原有组分未被完全氧化的不饱和结构,因此,增强了处理后HIA组分不饱和度。
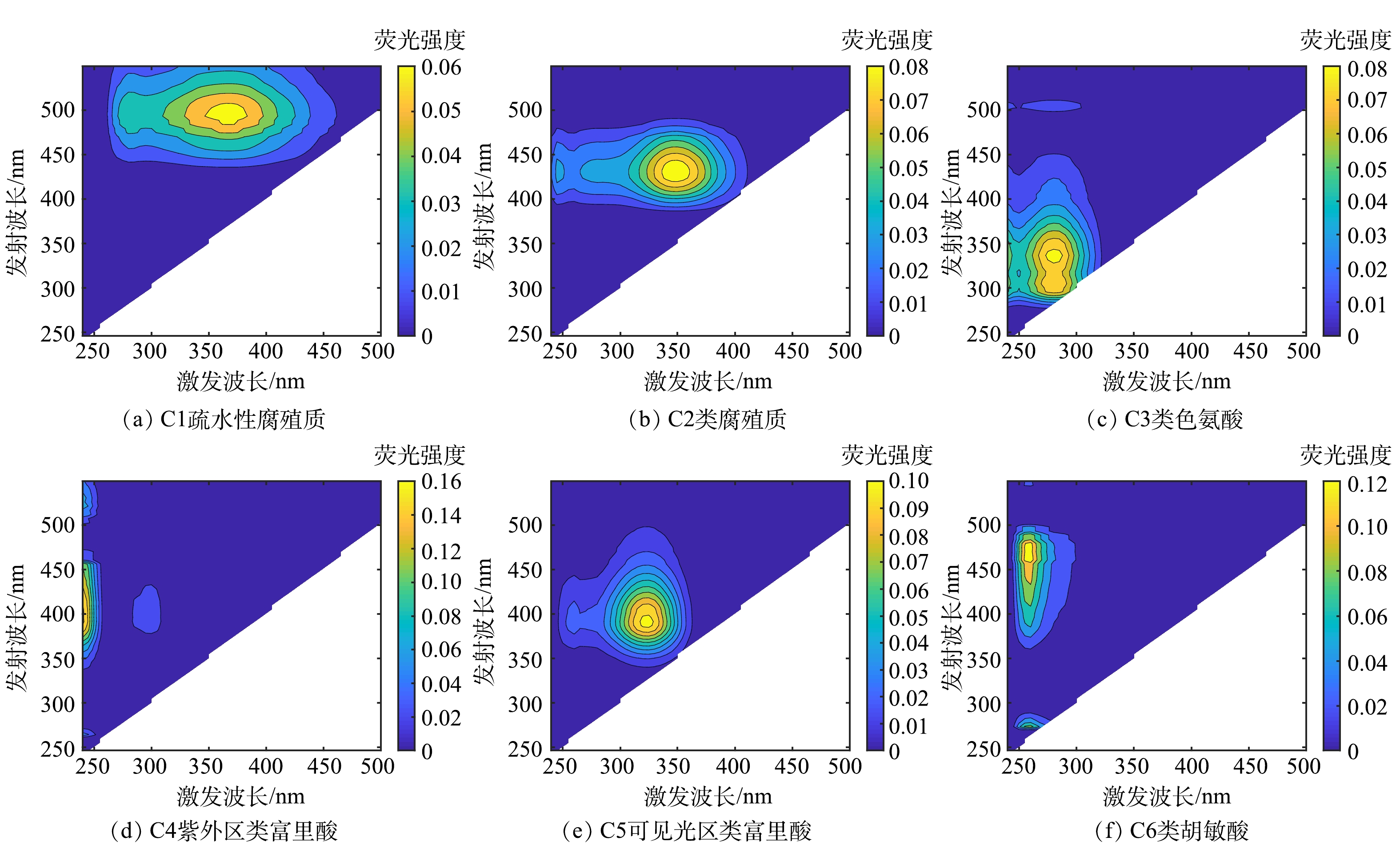
2) DOM组分3D-EEM图谱分析。由于实际制药废水中存在较多复杂有机物,其3D-EEM光谱通常是多个荧光峰重叠的结果,因此,采用平行因子分析法将复合的荧光峰分解为几个独立的荧光峰成分,进而进行主成分分析。CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3体系处理实际制药废水前后,废水DOM组分3D-EEM光谱经平行因子分析法解析,可得到6类物质模型(图8)。C1(Ex/Em为375 nm/500 nm)为疏水性腐殖质;C2(Ex/Em为350 nm/430 nm)为类腐殖质;C3(Ex/Em为280 nm/340 nm)为类色氨酸;C4(Ex/Em为240 nm/410 nm)为紫外区类富里酸;C5(Ex/Em为325 nm/385 nm)为可见光区类富里酸;C6(Ex/Em为250 nm/470 nm)为类胡敏酸[34-38]。原水中HOA组分包含C1、C2和C5,以C2居多;HOB组分包含C2 、C4、C5和C6,以C5居多;HON组分包含C4和C6,以C4居多;HIA组分包含C3和C5,以C3居多;HIB组分包含C2和C5,以C2居多;HIN组分包含C2、C4和C5,以C5居多。
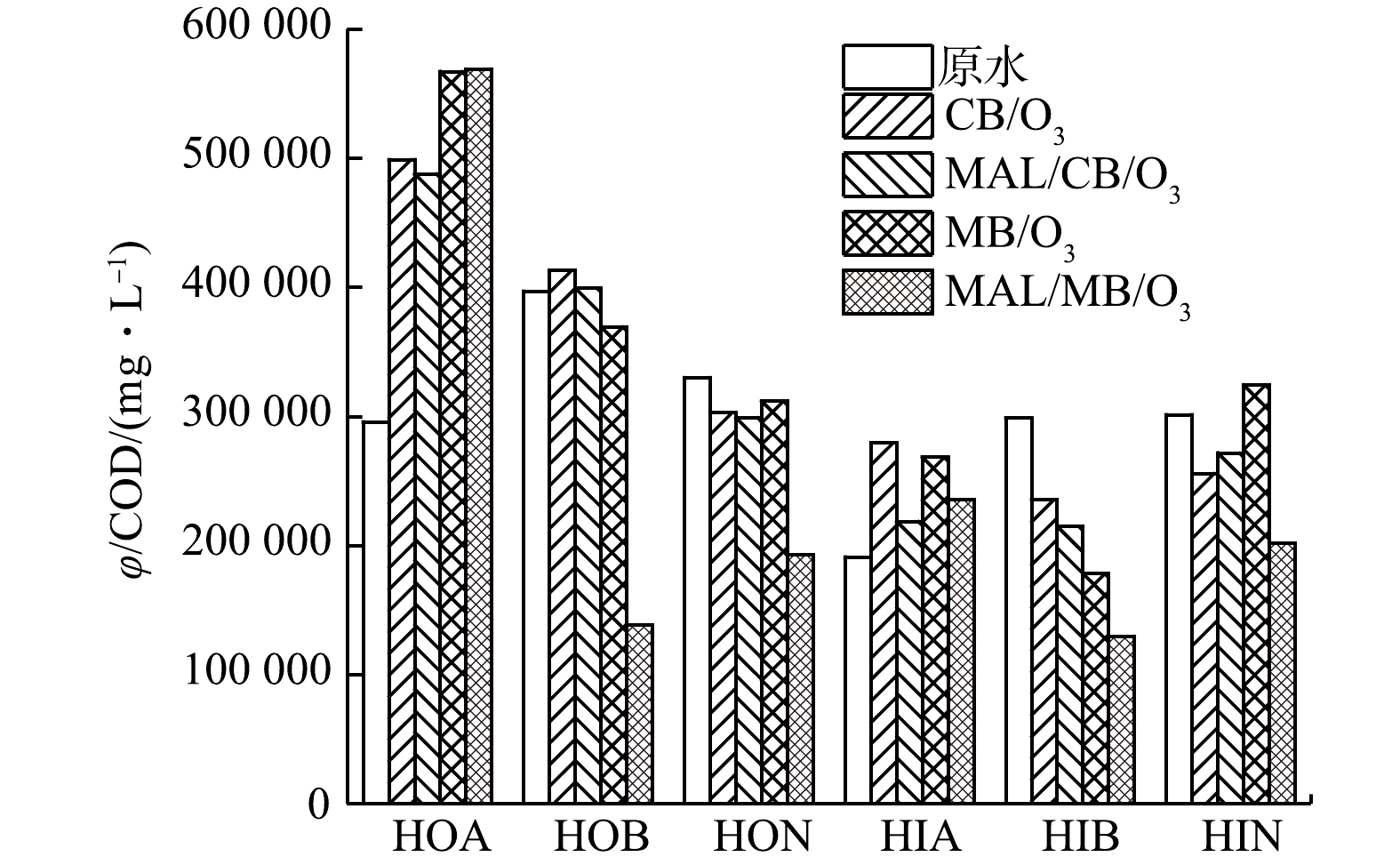
经CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3处理后,废水中DOM各组分中C1~C6荧光强度显著变化(变化率>±30%),结果如图9所示。整体而言,C1~C6荧光强度在HOB、HON、HIB和HIN中呈现下降趋势,在HOA和HIA中呈现增加趋势,表明DOM官能团在氧化过程中整体向酸性官能团转化。此外,C1、C2荧光强度明显下降,C4~C6荧光强度明显增强,表明类腐殖质在氧化中存在向类富里酸和类胡敏酸转化的趋势。HIA组分中主要物质C3荧光强度下降,而其他物质荧光强度增加,也证实了其他组分、特别是疏水性组分向HIA的转化。MAL/MB/O3体系中C1~C6荧光强度下降最显著,表明其对于各物质荧光结构的破坏最显著。
计算处理前后DOM各组分荧光区域积分体积φ值[33]与COD的比值,结果如图10所示。物质荧光强度与电子共轭度直接相关,而电子共轭度受到取代基团的供/吸电子能力的影响。原水中HOA和HIA组分φ与COD比值相对较低,这可能是由于其分子中存在大量羧酸吸电子基团,从而降低了芳香环结构的电子共轭度;而处理后φ与COD比值显著提高,则可能是由于氧化过程中羧酸基团相对芳香环结构被破坏更为显著,从而减小了对芳香环结构电子共轭度的影响。其他组分处理前后φ与COD比值呈现波动趋势。其原因是各组分中C1~C6存在的转化过程造成了各组分荧光强度的变化,但整体仍以下降为主。除HIA外,MAL/MB/O3处理后各组分φ与COD比值大幅下降,表明其对相应组分中荧光基团结构的氧化破坏效应最强。
-
1) MAL/MB/O3处理制药废水性能明显优于CB/O3、MAL/CB/O3和MB/O3。处理90 min后,整体COD去除率可达49.79%,COD去除量与臭氧消耗量的比值为0.83,BOD5/COD值由0.114提高至0.217,发光细菌抑制率由86.0%下降至47.8%。
2)制药废水DOM中疏水性组分氧化去除率高于亲水性组分。臭氧直接氧化可去除疏水性组分,而亲水性组分去除主要依赖HO·氧化。MAL/MB/O3对DOM去除率最高,可达到52.51%,其中疏水性组分整体去除率为56.67%,亲水性组分整体去除率为46.93%。
3)废水DOM在氧化处理中存在官能团向酸性基团转化、类腐殖质向类富里酸和类胡敏酸转化、其他组分向HIA组分转化趋势。MAL/MB/O3强氧化能力对于DOM组分不饱和结构和荧光结构的破坏作用最为显著。
微气泡催化臭氧化深度处理制药废水的效果及DOM组分特性的变化
Performance of advanced treatment of pharmaceutical wastewater by microbubble catalytic ozonation and component variation characteristics of dissolved organic matter
-
摘要: 采用镁铝层状双氢氧化物(MAL)微气泡催化臭氧化(MAL/MB/O3)体系深度处理实际制药废水,考察了该体系对溶解性有机物(DOM)的深度去除性能及DOM组分的变化特性,并与普通气泡臭氧化(CB/O3)、普通气泡催化臭氧化(MAL/CB/O3)、微气泡臭氧化(MB/O3)工艺进行了比较。结果表明,MAL/MB/O3处理性能优于CB/O3、MAL/CB/O3和MB/O3,在臭氧投加总量与处理废水初始COD值之比为0.6、气流量0.5 L·min−1、催化剂投加量0.5 mg·L−1的条件下,整体COD去除率可达49.79%,COD去除量与臭氧消耗量的比值为0.83,废水的可生化性得到了一定改善,生物毒性显著下降。废水DOM中疏水性组分氧化去除率高于亲水性组分。臭氧直接氧化可去除疏水性组分,而亲水性组分去除主要依赖HO·氧化。MAL/MB/O3对DOM的去除效率最高,可达到52.51%,其中疏水性组分整体去除率56.67%,亲水性组分整体去除率46.93%。废水DOM在氧化处理中存在官能团向酸性基团转化、类腐殖质向类富里酸和类胡敏酸转化、其他组分向亲水性酸(HIA)组分转化的趋势。MAL/MB/O3强氧化能力对于DOM组分不饱和结构和荧光结构的破坏作用最为显著。以上研究结果可为制药废水深度处理提供参考。Abstract: The catalytic microbubble ozonation with magnesium-aluminum layered double hydroxides (MAL) as catalyst (MAL/MB/O3) was used to perform advanced treatment of actual pharmaceutical wastewater. In comparison with common bubble ozonation (CB/O3), catalytic common bubble ozonation (MAL/CB/O3) and microbubble ozonation (MB/O3), the performance of advanced treatment of pharmaceutical wastewater by microbubble catalytic ozonation and component variation characteristics of dissolved organic matter (DOM) were studied. The results showed that the overall treatment performance of MAL/MB/O3 was better than that of CB/O3, MAL/CB/O3 and MB/O3. When the ratio of total ozone dosage to total initial COD of treated wastewater was 0.6, gas flow was 0.5 L·min−1 and catalyst dosage was 0.5 mg·L−1, the total COD removal efficiency could reach 49.79%, and the ratio of COD removal to ozone consumption was 0.83 in MAL/MB/O3. The biodegradability of MAL/MB/O3 treated wastewater was improved and the biological toxicity decreased significantly. The oxidation removal efficiency of hydrophobic components in wastewater DOM was higher than that of hydrophilic components. The hydrophobic components could be removed by ozone direct oxidation, while the hydrophilic components removal mainly depended on HO· oxidation. MAL/MB/O3 had the highest DOM removal efficiency of 52.51%, including 56.67% removal of hydrophobic components and 46.93% removal of hydrophilic components. During the oxidation treatment of wastewater DOM, some conversion tendencies occurred, such as functional groups to acidic groups, humic-like substances to fulvic acids-like and humic acids-like, and other components to hydrophilic acid (HIA) components. The strong oxidation ability of MAL/MB/O3 had the most significant destructive effect on the unsaturated structure and fluorescent structure of DOM components. This study can provide a theoretical and technical reference for advanced treatment of pharmaceutical wastewater.
-
制药废水具有高盐度、高色度、高化学需氧量(chemical oxygen demand,COD)等特点,属于难降解有机工业废水之一。经过生化处理后,仍存在溶解性有机物(dissolved organic matter,DOM)浓度较高、毒性较大等问题,难以达到排放标准和回用水要求[1]。生化处理后,废水中DOM由腐殖质类、多糖类、蛋白类、脂质类和小分子的氨基酸等组成[2],含有未完全降解的小分子有机物,以及长链烷烃、芳香烃等大分子质量有机物,废水多呈现淡黄色[3]。DOM排入水体后将对水环境造成不利影响,并产生一定的生态风险。因此,须对制药废水进行深度处理,以消减DOM物质排放量,降低生物毒性,减少水环境生态风险。
废水深度处理工艺主要包括高级氧化法(advanced oxidation processes,AOPs)、膜分离法、吸附法和生物法等[4-6]。AOPs由于其氧化能力强、清洁、无二次污染得到广泛应用。AOPs可在水中生成羟基自由基(HO·),HO·具有高氧化活性和无选择性特征,能够氧化大多数有机污染物,因此得到广泛关注[7]。传统臭氧化技术存在HO·产生效率差、臭氧利用率低、气液传质速率慢等问题,使得难降解有机物矿化效率有限,制约了其实际应用[8]。微气泡(microbubble,MB)通常是指直径小于50 μm的微小气泡,有研究表明,微气泡臭氧传质速率是普通气泡的1.3~1.5倍,且能够有效加速臭氧分解产生HO·,进而提高臭氧化能力[9]。同时,有研究[10-13]表明,微气泡表面存在电荷聚集效应,在液相收缩过程中表面电荷密度和ζ电位急剧升高,并在破裂时可产生HO·,产生类催化效应。在微气泡催化臭氧化中,臭氧微气泡类催化效应与催化剂催化效应协同,可显著提高HO·氧化能力,使得臭氧利用率和有机物矿化率显著提高[9, 14-15],并可提高废水的可生化性,降低其生物毒性[16]。LIU等[17]采用微气泡臭氧化技术处理阿特拉津废水,发现臭氧微气泡通过臭氧分解以及微气泡破裂可提高阿特拉津降解率。ZHANG等[18]以活性炭为催化剂催化微气泡臭氧化处理酸性大红3R废水,可将TOC去除率由传统气泡(coarse bubble,CB)的36.2%提升至91.2%。可见,微气泡臭氧化技术在废水深度处理领域具有良好的应用前景。
非均相催化臭氧化通过将臭氧转化为HO·来提高氧化能力和污染物去除效率[19-20]。选用活性金属离子制备具有水滑石结构的层状双羟基复合金属氢氧化物(layered double hydroxides,LDHs),往往具有优异的催化性能,但其应用于催化臭氧化的研究甚少。HASSANI等[21]采用镍基层状氢氧化物纳米材料催化臭氧化处理模拟染料废水,其对COD的去除率可由传统臭氧化的30%提高到72%。FU等[22]采用钴铁层状双氢氧化物催化臭氧化降解甲苯磺酸溶液,TOC的去除率可达85%。
本研究将微气泡与催化臭氧化技术相结合,采用镁铝层状双氢氧化物催化剂(Mg-Al-LDHs,MAL)催化微气泡臭氧化实际制药废水,考察了普通气泡臭氧化(CB/O3)、普通气泡催化臭氧化(MAL/CB/O3)、微气泡臭氧化(MB/O3)和微气泡催化臭氧化(MAL/MB/O3)体系处理实际制药废水的效能及有机物降解特征,分析了废水中亲水性酸(hydrophilic acid,HIA)、亲水性碱(hydrophilic base,HIB)、亲水性中性(hydrophilic neutral,HIN)、疏水性酸(hydrophobic acid,HOA)、疏水性碱(hydrophobic base,HOB)、疏水性中性(hydrophobic neutral,HON)DOM组分的变化,以期为制药废水深度处理提供参考。
1. 材料与方法
1.1 实验用水
本研究所用实际制药废水取自石家庄某化学合成制药企业生化处理出水,其水质指标为:510~600 mg·L−1COD、55~65 mg·L−1BOD5、140~160 mg·L−1SS、30~40 mg·L−1 NH4+-N、895±5 mg·L−1 Cl−、(3825±20) mg·L−1 SO42−、(195±5) mg·L−1 CO32−、(38±2) mg·L−1 PO43−、UV254为4.0~5.0、pH为6.8~7.8。
1.2 镁铝层状双氢氧化物催化剂(MAL)的制备和表征
将0.03 mol·L−1 Mg(NO3)2·6H2O与0.01 mol·L−1 Al(NO3)3·9H2O溶于100 mL去离子水中,获得金属盐溶液;将0.75 mol·L−1 NaHCO3溶于500 mL去离子水中,获得碱性溶液;在60℃水浴加热搅拌下将金属盐溶液滴入碱性溶液中,滴加过程中用2 mol·L−1 NaOH调节pH,使pH保持在8.5;将悬浊液在60 ℃水浴加热21 h,抽滤、洗涤至与去离子水pH相同,将沉淀物在78 ℃下干燥12 h,获得镁铝层状双氢氧化物催化剂。采用SEM、TEM和XRD对催化剂形貌和层状结构进行表征。
1.3 实验装置
本研究实验装置示意如图1所示。主要包括氧气源臭氧发生器(OZ-10G,广州大环)、微气泡发生器(北京晟峰恒泰科技有限公司)、有机玻璃反应器(有效容积15 L)。在MB/O3处理中,臭氧发生器产生臭氧气体,通过气体流量计控制臭氧气体流量。臭氧气体与反应器中循环废水混合后,进入微气泡发生器产生臭氧微气泡,由底部进入反应器。在CB/O3处理中,臭氧气体通过反应器底部曝气头产生臭氧普通气泡。处理后尾气由反应器顶部排气口进入尾气吸收瓶中,通过碘化钾溶液吸收。
1.4 实验方法
本研究采用批次实验方法,分别通过CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3体系深度处理实际制药废水,处理时间均为90 min。处理过程中,控制臭氧气体流量为0.5 L·min−1(此流量下产生微气泡平均直径小于50 μm),同时控制臭氧气体浓度,使得臭氧投加总量与处理废水初始COD总量之比约为0.6,催化剂用量为0.5 g·L−1。处理过程中通过测定COD、BOD5、UV254、三维荧光(3D-EEM)光谱、可生化性、生物毒性,评估处理效能。此外,对各体系处理前后废水DOM组分中COD、UV254和3D-EEM进行测定,分析处理过程中DOM不同组分的变化特征。
1.5 分析方法
COD采用重铬酸钾微波消解法测定[23];UV254采用分光光度计(UV-5100,METASH)进行测定;BOD5采用生物化学需氧量测定仪(LH-BOD601,连华科技)测定;生物毒性采用Mithras LB 940多功能分析仪测定[24]。采用荧光光谱仪(FluoroMax-4,日本HORIBA)对样品进行3D-EEM分析。采用比表面测定仪(D/MAX-2500,日本Rigaku)按照BET氮吸附法对MAL比表面积进行测定。
废水中DOM组分采用树脂分级法分离收集,树脂采用(Supelite)XAD-8大孔径树脂、Dowex Marathon MSC(H)阳离子交换树脂、Duolite A-7阴离子交换树脂,使用前采用有机溶剂索氏提取进行预处理,去除树脂中可溶性有机物,然后使用不同浓度的HCl和NaOH溶液活化树脂[25-26]。废水中DOM通过处理活化后离子交换树脂分离,将DOM分为HIA、HIB、HIN、HOA、HOB、HON组分[27],而后对各组分COD、UV254和3D-EEM进行测定分析。
1.6 协同效应计算
MAL/MB/O3体系协同效应值根据式(1)进行计算[28]。
R=kakb (1) 式中:R为协同效应值;ka为MB/O3/LDHs体系COD去除准一级速率常数,min−1;kb为MB/N2、CB/O3以及MAL吸附过程中COD去除准一级速率常数之和,min−1。
2. 结果与讨论
2.1 MAL催化剂表征
MAL呈粉末状,比表面积为60 m2·g−1。由图2(a)和图2(b)可见,MAL形貌呈现片状结构,其内部具有明显的层状结构。由图2(c)可见,在2θ为11.64°、23.42°、34.88°、60.76°处有尖锐衍射峰,对应(003)、(006)、(012)和(110)晶面,符合层状双氢氧化物的典型特征[29],其与镁铝层状双氢氧化物标准物的衍射峰基本匹配。这表明镁铝层状双氢氧化物成功制备。
2.2 对有机物去除的效能
1) COD整体去除。在CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3体系及MAL催化剂吸附处理实际制药废水过程中,整体COD去除率随时间变化如图3所示。其中,MAL吸附对COD去除率不足7%。可以看到,在CB/O3体系中,COD去除率在处理70 min后达到20.93%,而后几乎保持不变;在MAL/CB/O3、MB/O3和MAL/MB/O3体系中,COD去除率在整个处理过程中呈现不断上升的趋势,处理90 min后COD去除率分别达到32.96%、36.57%和49.79%。微气泡臭氧化可增强对COD的去除性能,MB/O3体系比CB/O3体系对COD去除率提高了15.64%。MAL/MB/O3体系比MB/O3体系对COD去除率提高了13.22%。以上结果表明,MAL催化通过镁铝双金属可提高催化体系电子传递速率,且层状结构可提供更多的催化反应活性位点,可加速有机物氧化降解进程,进一步提高COD氧化去除效率。因此,微气泡臭氧化和MAL催化对整体COD去除均有明显促进作用。计算得出CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3体系中COD去除量与臭氧消耗量的比值R,分别为0.38、0.60、0.62和0.83,这表明MAL/MB/O3体系单位臭氧消耗量去除COD更多,臭氧反应效率更高。
为进一步分析微气泡臭氧化和MAL催化作用对COD去除是否存在协同效应,分别测定和计算MAL催化剂吸附、氮气微气泡(MB/N2)、CB/O3以及MAL/MB/O3处理过程去除COD反应速率常数,其值分别为8.693×10−4、2.189×10−3、2.903×10−3和7.293×10−3 min−1。在此基础上,根据式(1)计算MAL/MB/O3协同效应R值,为1.223,表明微气泡臭氧化与MAL催化作用对COD去除存在一定程度的协同效应[30]。
2)可生化性与生物毒性变化。CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3体系深度处理实际制药废水前后,BOD5/COD值的变化如图4所示,发光细菌抑制率H的变化如图5所示。可以看到,处理前废水BOD5/COD值为0.114,可生化性较差,且发光细菌抑制率H为86.0%,属于高毒水平。各体系处理90 min后,废水BOD5/COD分别提高至0.153、0.182、0.191和0.217,废水可生化性得到一定改善;同时,发光细菌抑制率H分别降低至70.0%、59.5%、55.6%和47.8%,其中MAL/MB/O3处理后可降低至中毒水平[31]。可见,有机物深度降解和去除可改善废水可生化性和降低生物毒性,而MAL/MB/O3体系作用更为明显。
3) DOM组分浓度变化。在COD整体去除基础上,分析了CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3处理制药废水前后,废水中DOM各组分COD浓度变化以及不同体系处理前后DOM各组分COD浓度及其所占比例的变化,结果如图6所示。由图6(a)可以看到,原水DOM疏水性组分浓度整体高于亲水性组分,其中HOA组分浓度最高,HIA组分浓度最低。处理90 min后,CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3对疏水性组分去除率比值为0.54∶0.76∶0.85∶1,对亲水性组分去除率比值为0.28∶0.69∶0.68∶1,疏水性组分去除效率均高于亲水性组分,且MB/O3对疏水性、亲水性组分去除明显优于CB/O3,CB/O3对亲水性组分去除明显低于疏水性组分。产生上述结果的原因可能是,臭氧直接氧化可去除疏水性组分,而亲水性组分去除主要依赖HO·氧化。MAL/MB/O3同时具有强化臭氧传质、提高臭氧直接氧化能力以及催化HO·氧化和微气泡强化HO·氧化作用[28],因此对DOM的去除效率最高,可达到52.51%,其中疏水性组分整体去除率56.67%,亲水性组分整体去除率46.93%。
值得注意的是,HIA浓度在CB/O3、MAL/CB/O3和MB/O3处理中分别增加了27.27%、9.09%和44.37%。其原因可能是其他组分被氧化转化为HIA,而臭氧直接氧化和HO·氧化对<C5脂肪酸降解效果较差[32-33],从而导致HIA的积累。
由图6(b)可以看出,不同体系处理制药废水前后DOM各组分COD所占该体系整体COD的比例。对于疏水性组分,HOA所占比例明显下降,HOB所占比例保持稳定,HON所占比例有所上升;对于亲水性组分,HIA所占比例明显升高,HIB所占比例也有所升高,而HIN所占比例下降明显。可见,HOA和HIN组分相对更容易降解或转化,而HIA降解最为困难。
2.3 DOM组分的变化
1) DOM组分UV254变化。CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3体系处理实际制药废水前后DOM中各组分UV254值,以及4种体系处理实际制药废水前后DOM中各组分UV254值所占该体系UV254值的比例如图7(a)和图7(b)所示。由图7(a)可以看到,原水DOM疏水性组分UV254值整体高于亲水性组分,原水疏水性组分的UV254值与原水疏水性组分的COD的比值为亲水性组分的1.34倍,因而疏水性组分不饱和度更强。对疏水性组分而言,HOA、HOB和HON组分UV254值与该组分COD之比的比值为1∶0.88∶1.37。由此可以推测,HOA中C5~C9脂肪酸和芳香环酸共存,不饱和度居中;HOB中以芳香环胺为主,不饱和度最强;HON中以长链(>C9)脂肪酸为主,不饱和度较低[32]。对亲水性组分而言,HIN主要为<C5脂肪酰胺和多官能团醇类,HIA主要为<C5脂肪酸和羟基酸等,HIB主要为<C9脂肪胺和吡啶的两性蛋白质,HIN、HIA和HIB组分UV254值与该组分COD之比的比值为1∶1.04∶0.79,可见HIN和HIA不饱和度相当且较强,HIB不饱和度相对较弱。
各体系处理90 min后,CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3对疏水性组分UV254去除率比值为0.44∶0.80∶0.85∶1,对亲水性组分UV254去除率比值为0.62∶0.78∶0.84∶1。可见, CB/O3/LDHs、MB/O3和MB/O3/LDHs体系中亲、疏水性组分UV254去除率基本一致。其原因是:由于存在较强HO·氧化作用,因而对芳香环结构和不饱和键均具有较强破坏作用;而CB/O3对亲水性组分去除率较低,对UV254去除率相对较高,表明臭氧直接氧化可破坏亲水性组分的不饱和结构,但完全矿化去除较为困难。
不同体系处理制药废水前后DOM各组分UV254所占该体系UV254的比例如图7(b)所示。各体系处理过程中,疏水性组分中,HOA所占比例明显下降,HOB所占比例有所升高,HON所占比例略有下降;亲水性组分中,HIA所占比例明显升高,HIB和HIN所占比例均有所下降。DOM各组分的UV254所占比例变化与COD变化有关,如HOA、HIA、HIN组分UV254与COD所占比例变化一致;同时也与组分不饱和度有关,如HOB不饱和度最强,虽COD所占比例不变,但UV254所占比例升高,而HON和HIB不饱和度较弱,虽COD所占比例升高,但UV254所占比例下降。
值得注意的是,HIA组分UV254值在处理后均出现升高现象,包括经过MAL/MB/O3处理后,HIA浓度降低的同时,其UV254值也有所升高。同时,原水以及经CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3体系处理后的HIA中COD比值为1∶1.27∶1.09∶1.44∶0.95,UV254比值为1∶1.32∶1.26∶1.40∶1.09,表明除HIA组分COD升高外,HIA组分不饱和度有所增强也是UV254值升高的原因之一。由于处理中存在其他组分向HIA组分转化过程,转化产生的HIA组分可能存留较多原有组分未被完全氧化的不饱和结构,因此,增强了处理后HIA组分不饱和度。
2) DOM组分3D-EEM图谱分析。由于实际制药废水中存在较多复杂有机物,其3D-EEM光谱通常是多个荧光峰重叠的结果,因此,采用平行因子分析法将复合的荧光峰分解为几个独立的荧光峰成分,进而进行主成分分析。CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3体系处理实际制药废水前后,废水DOM组分3D-EEM光谱经平行因子分析法解析,可得到6类物质模型(图8)。C1(Ex/Em为375 nm/500 nm)为疏水性腐殖质;C2(Ex/Em为350 nm/430 nm)为类腐殖质;C3(Ex/Em为280 nm/340 nm)为类色氨酸;C4(Ex/Em为240 nm/410 nm)为紫外区类富里酸;C5(Ex/Em为325 nm/385 nm)为可见光区类富里酸;C6(Ex/Em为250 nm/470 nm)为类胡敏酸[34-38]。原水中HOA组分包含C1、C2和C5,以C2居多;HOB组分包含C2 、C4、C5和C6,以C5居多;HON组分包含C4和C6,以C4居多;HIA组分包含C3和C5,以C3居多;HIB组分包含C2和C5,以C2居多;HIN组分包含C2、C4和C5,以C5居多。
经CB/O3、MAL/CB/O3、MB/O3和MAL/MB/O3处理后,废水中DOM各组分中C1~C6荧光强度显著变化(变化率>±30%),结果如图9所示。整体而言,C1~C6荧光强度在HOB、HON、HIB和HIN中呈现下降趋势,在HOA和HIA中呈现增加趋势,表明DOM官能团在氧化过程中整体向酸性官能团转化。此外,C1、C2荧光强度明显下降,C4~C6荧光强度明显增强,表明类腐殖质在氧化中存在向类富里酸和类胡敏酸转化的趋势。HIA组分中主要物质C3荧光强度下降,而其他物质荧光强度增加,也证实了其他组分、特别是疏水性组分向HIA的转化。MAL/MB/O3体系中C1~C6荧光强度下降最显著,表明其对于各物质荧光结构的破坏最显著。
计算处理前后DOM各组分荧光区域积分体积φ值[33]与COD的比值,结果如图10所示。物质荧光强度与电子共轭度直接相关,而电子共轭度受到取代基团的供/吸电子能力的影响。原水中HOA和HIA组分φ与COD比值相对较低,这可能是由于其分子中存在大量羧酸吸电子基团,从而降低了芳香环结构的电子共轭度;而处理后φ与COD比值显著提高,则可能是由于氧化过程中羧酸基团相对芳香环结构被破坏更为显著,从而减小了对芳香环结构电子共轭度的影响。其他组分处理前后φ与COD比值呈现波动趋势。其原因是各组分中C1~C6存在的转化过程造成了各组分荧光强度的变化,但整体仍以下降为主。除HIA外,MAL/MB/O3处理后各组分φ与COD比值大幅下降,表明其对相应组分中荧光基团结构的氧化破坏效应最强。
3. 结论
1) MAL/MB/O3处理制药废水性能明显优于CB/O3、MAL/CB/O3和MB/O3。处理90 min后,整体COD去除率可达49.79%,COD去除量与臭氧消耗量的比值为0.83,BOD5/COD值由0.114提高至0.217,发光细菌抑制率由86.0%下降至47.8%。
2)制药废水DOM中疏水性组分氧化去除率高于亲水性组分。臭氧直接氧化可去除疏水性组分,而亲水性组分去除主要依赖HO·氧化。MAL/MB/O3对DOM去除率最高,可达到52.51%,其中疏水性组分整体去除率为56.67%,亲水性组分整体去除率为46.93%。
3)废水DOM在氧化处理中存在官能团向酸性基团转化、类腐殖质向类富里酸和类胡敏酸转化、其他组分向HIA组分转化趋势。MAL/MB/O3强氧化能力对于DOM组分不饱和结构和荧光结构的破坏作用最为显著。
-
-
[1] ZHAO F Z, JU F, HUANG K L, et al. Comprehensive insights into the key components of bacterial assemblages in pharmaceutical wastewater treatment plants[J]. Science of the Total Environment, 2019, 651: 2148-2157. doi: 10.1016/j.scitotenv.2018.10.101 [2] 张静, 张守敬, 刘春, 等. 工业废水水质对微气泡臭氧化深度处理影响[J]. 环境科学, 2020, 41(4): 1752-1760. [3] 赵立军, 郭磊, 陈进富, 等. 抗生素废水的GC-MS分析与显色物质的初步确定[J]. 环境工程学报, 2009, 3(10): 1830-1834. [4] FARIAS T, HAJIZADEH S, YE L. Cryogels with high cisplatin adsorption capacity: Towards removal of cytotoxic drugs from wastewater[J]. Separation and Purification Technology, 2020, 235: 116203. doi: 10.1016/j.seppur.2019.116203 [5] ISARI A A, MEHREGAN M, MEHREGAN S, et al. Sono-photocatalytic degradation of tetracycline and pharmaceutical wastewater using WO3 /CNT heterojunction nanocomposite under US and visible light irradiations: A novel hybrid system[J]. Journal of Hazardous Materials, 2020, 390: 122050. doi: 10.1016/j.jhazmat.2020.122050 [6] PRASERTKULSAK S, CHIEMCHAISRI C, CHIEMCHAISRI W, et al. Removals of pharmaceutical compounds at different sludge particle size fractions in membrane bioreactors operated under different solid retention times[J]. Journal of Hazardous Materials, 2019, 368: 124-132. doi: 10.1016/j.jhazmat.2019.01.050 [7] CAI Q Q, WU M Y, LI R, et al. Potential of combined advanced oxidation: Biological process for cost-effective organic matters removal in reverse osmosis concentrate produced from industrial wastewater reclamation: Screening of AOP pre-treatment technologies[J]. Chemical Engineering Journal, 2020, 389: 123419. doi: 10.1016/j.cej.2019.123419 [8] 张静, 杜亚威, 刘晓静, 等. 臭氧微气泡处理酸性大红3R废水特性研究[J]. 环境科学, 2015, 36(2): 584-589. [9] WU C, LI P, XIA S J, et al. The role of interface in microbubble ozonation of aromatic compounds[J]. Chemosphere, 2019, 220: 1067-1074. doi: 10.1016/j.chemosphere.2018.12.174 [10] SWART B, ZHAO Y, KHAKU M, et al. In situ characterisation of size distribution and rise velocity of microbubbles by high-speed photography[J]. Chemical Engineering Science, 2020, 225: 115836. doi: 10.1016/j.ces.2020.115836 [11] FAN W, ZHOU Z, WANG W, et al. Environmentally friendly approach for advanced treatment of municipal secondary effluent by integration of micro-nano bubbles and photocatalysis[J]. Journal of Cleaner Production, 2019, 237(Nov.10): 117821-117828. [12] HU L, XIA Z. Application of ozone micro-nano-bubbles to groundwater remediation[J]. Journal of Hazardous Materials, 2018, 342: 446-453. doi: 10.1016/j.jhazmat.2017.08.030 [13] LIU Y X, ZHOU Y P, WANG T Z, et al. Micro-nano bubble water oxygation: Synergistically improving irrigation water use efficiency, crop yield and quality[J]. Journal of Cleaner Production, 2019, 222: 835-843. doi: 10.1016/j.jclepro.2019.02.208 [14] ABDISA J, PALLAB G. A comparative study on the removal of dimethyl sulfoxide from water using microbubbles and millibubbles of ozone[J]. Journal of Water Process Engineering, 2021, 40: 101937. doi: 10.1016/j.jwpe.2021.101937 [15] JOTHINATHAN L. CAI Q Q. ONG S L. et al. Organics removal in high strength petrochemical wastewater with combined microbubble-catalytic ozonation process[J]. Chemosphere, 2021, 263: 127980. doi: 10.1016/j.chemosphere.2020.127980 [16] DENG S H, LAKSHMI J, CAI Q Q, et al. FeOx@GAC catalyzed microbubble ozonation coupled with biological process for industrial phenolic wastewater treatment: Catalytic performance, biological process screening and microbial characteristics[J]. Water research, 2021, 190: 116687. doi: 10.1016/j.watres.2020.116687 [17] LIU Y, WANG S, SHI L, et al. Enhanced degradation of atrazine by microbubble ozonation[J]. Environmental Science:Water Research and Technology, 2020, 6: 227-233. [18] ZHANG J, HUANG G Q, LIU C, et al. Synergistic effect of microbubbles and activated carbon on the ozonation treatment of synthetic dyeing wastewater[J]. Separation and Purification Technology, 2018, 201: 10-18. doi: 10.1016/j.seppur.2018.02.003 [19] HUANG Y, YANG T, LIANG M, et al. Ni-Fe layered double hydroxides catalized ozonation of synthetic wastewater containing Bisphenol A and municipal secondary effluent[J]. Chemosphere, 2019, 235: 143-152. doi: 10.1016/j.chemosphere.2019.06.162 [20] SHI Y, LI S, WANG L, et al. Compositional characteristics of dissolved organic matter in pharmaceutical wastewater effluent during ozonation[J]. Science of the Total Environment, 2021, 21: 146278. [21] EL HASSANI K, KALNINA D, Turks M, et al. Enhanced degradation of an azo dye by catalytic ozonation over Ni-containing layered double hydroxide nanocatalyst[J]. Separation and Purification Technology, 2019, 210: 764-774. doi: 10.1016/j.seppur.2018.08.074 [22] FU X, HUANG Y, WANG Y, et al. Ozonation catalyzed by CoxFe1 layered double hydroxide for the degradation of P -toluenesulfonic acid[J]. Ozone Science and Engineering, 2020, 5: 1766946. [23] 张志君, 何婷. 重铬酸盐法测定水中化学需氧量[J]. 中国科技信息, 2020(13): 49. [24] 李瑞霞. 利用发光细菌法对制药废水生物急性毒性的研究[D]. 北京: 清华大学, 2013. [25] LEENHEER J A. Comprehensive approach to preparative isolation and fractionation of dissolved organic carbon from natural waters and wastewaters[J]. Environmental Science & Technology, 1981, 15(5): 578-588. [26] ZHANG T, LU J F, MA J, et al. Comparative study of ozonation and synthetic goethite-catalyzed ozonation of individual NOM fractions isolated and fractionated from a filtered river water[J]. Water Research, 2008, 42(6-7): 1563-1570. doi: 10.1016/j.watres.2007.11.005 [27] FU L Y, WU C Y, ZHOU Y X, et al. Ozonation reactivity characteristics of dissolved organic matter in secondary petrochemical wastewater by single ozone, ozone/H2O2, and ozone/catalyst[J]. Chemosphere, 2019, 233: 34-43. doi: 10.1016/j.chemosphere.2019.05.207 [28] GU Z P, CHEN W M, LI Q B, et al. Kinetics study of dinitrodiazophenol industrial wastewater treatment by a microwave-coupled ferrous-activated persulfate process[J]. Chemosphere, 2019, 215: 82-91. doi: 10.1016/j.chemosphere.2018.10.009 [29] TAMBOLI A H, JADHAV A R, CHUNG W, et al. Structurally modified cerium doped hydrotalcite-like precursor as efficient catalysts for hydrogen production from sodium borohydride hydrolysis[J]. Energy, 2015, 93: 955-962. doi: 10.1016/j.energy.2015.09.059 [30] ZHANG H, HE Y L, LAI L D, et al. Catalytic ozonation of bisphenol A in aqueous solution by Fe3O4-MnO2 magnetic composites: Performance, transformation pathways and mechanism[J]. Separation and Purification Technology, 2019, 245: 116449. [31] 那广水, 张月梅, 陈彤, 等. 发光细菌法评价排污口污水中总有机污染物毒性[J]. 中国环境监测, 2010, 26(5): 61-64. doi: 10.3969/j.issn.1002-6002.2010.05.017 [32] SWIETLIK J, DABROWSKA A, RACZYK-STANISLAWIAK U, et al. Reactivity of natural organic matter fractions with chlorine dioxide and ozone[J]. Water Research, 2004, 38(3): 547-558. doi: 10.1016/j.watres.2003.10.034 [33] GUNTEN U V. Ozonation of drinking water: part I. Oxidation kinetics and product formation[J]. Water Research, 2003, 37(7): 1443-1467. doi: 10.1016/S0043-1354(02)00457-8 [34] GUO L, LU M, LI Q, et al. Three-dimensional fluorescence excitation-emission matrix (EEM) spectroscopy with regional integration analysis for assessing waste sludge hydrolysis treated with multi-enzyme and thermophilic bacteria[J]. Bioresource Technology, 2014, 171: 22-28. doi: 10.1016/j.biortech.2014.08.025 [35] COBLE P G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy[J]. Marine Chemistry, 1996, 51(4): 325-346. doi: 10.1016/0304-4203(95)00062-3 [36] CHENG Y Y, WANG S L, HU S B, et al. The fluorescence characteristics of dissolved organic matter(DOM)in the seagrass ecosystem from hainan by fluorescence excitation-emission matrix spectroscopy[J]. Spectroscopy & Spectral Analysis, 2015, 35(1): 141-145. [37] LI W, XU Z, WU Q, et al. Characterization of fluorescent-dissolved organic matter and identification of specific fluorophores in textile effluents[J]. Environmental Science & Pollution Research International, 2015, 22(6): 4183. [38] COBLE P G. Marine optical biogeochemistry: The chemistry of ocean color[J]. Cheminform, 2007, 38(2): 402-418. -




 下载:
下载: