-
据报道,全球每年释放到环境中的金属镉有3.9×104 t [1,2]。镉作为毒性最强的重金属之一,具有生物累积性和不可生物降解性,可引起癌症、骨变性、肺功能障碍和高血压[3]。因此,找到一个高效、彻底除去废水中Cd2+的方法势在必行。
硫酸盐还原菌(sulfate reducing bacteria,SRB)作为一种绿色环保的去除Cd2+的有效方法,近年来已受到广泛关注[4]。SRB通过将硫酸盐还原为硫化物,硫化物可与重金属离子形成不溶性沉淀,最终达到去除废水中Cd2+的目的[5-7]。董净等[8,9]通过从土壤中分离纯化出耐镉性较好的SRB,对40 mg·L−1 Cd2+的去除率可达到75 %。SRB虽然对废水中Cd2+具有较好去除效果,但存在固液相分离困难、高浓度金属毒性等问题,使其受到限制[10]。
为了解决以上问题,近年来许多研究者通过将SRB固定在合适的载体上[11-14],增加其比表面积,提高物质表面的运输速率,同时保护微生物的酶活性,使得重金属对微生物的毒性减弱,最终提高SRB的生物稳定性且易于分离[12-16]。海藻酸钠是一种从藻类中提取的天然多糖,由于其对微生物的毒性低且价格低廉,所以经常用于SRB的固定化技术中。此外,为进一步提高SRB对Cd2+的去除率,近年来多采用零价铁纳米粒子(Fe0)修饰SRB,并用于原位控制受污染的地下水[15]。PEDRO等[16]使用零价铁纳米粒子(Fe0)与SRB复合后,其对Cd2+的去除率可达到99.8 %。ZHANG等[17]使用聚乙烯醇,海藻酸钠固定SRB和Fe0处理49 mg·L−1的Cd2+,去除率可达到99 %。通过以上研究可发现,SRB与固定化材料的结合在处理低浓度的Cd2+时均具有较好的去除能力。然而,近年来工业废水中Cd2+含量越来越高,很难实现完全去除废水中高浓度的Cd2+。此外,传统的Fe0对Cd2+具有较好的处理能力,但其表面易产生聚集行为且易氧化,从而降低其使用寿命。
基于以上研究成果,为寻求一种高效去除高浓度含Cd2+废水的方法,本研究选用惰性金属镍与Fe0偶联形成的双金属纳米粒子参与反应,因其具有良好的腐蚀稳定性,可增加Fe0催化反应活性并有效降低Fe0的氧化,镍铁双金属纳米粒子通常与其他金属偶联以增加催化反应活性并有效降低铁的氧化[18],所以,本研究构建了以海藻酸钠为载体固定镍-铁和SRB活性生物微球(SSNF),探讨了SSNF对Cd2+的吸附去除性能,考察了镍-铁添加量、pH、Cd2+初始浓度和吸附时间对SSFN吸附Cd2+能力的影响,以期为处理高浓度Cd2+污染废水提供参考。
-
实验所用菌株为实验室筛选得到的SRB,使用改良的Postgate C培养基(0.5 g·L−1 KH2PO4,1.0 g·L−1 NH4Cl,1.0 g·L−1 NaSO4,0.1 g·L−1 CaCl2·2H2O,2.0 g·L−1 MgSO4·7H2O,2.0 g·L−1 DL-乳酸钠,1.0 g·L−1 酵母提取物,1.0 g·L−1 Resazurin,0.5 g·L−1 FeSO4·7H2O,0.1 g·L−1 巯基乙酸钠,0.1 g·L−1 维生素C,pH为7.8,121 ℃灭菌15 min)以10 %的接种量接入菌株,在100 mL厌氧瓶中于37 ℃恒温培养箱中对SRB进行培养。当菌液中产生大量黑色沉淀物后即可进行后续实验。
-
在氮气气氛下,在含有0.2 mol·L−1的FeSO4·7H2O溶液的三口烧瓶中逐滴加入0.4 mol·L−1的NaBH4溶液,室温条件下,使用磁力搅拌器混合均匀。反应过程中亚铁可被还原为Fe0(式(1)),从而合成得到Fe0纳米粒子[18]。使用去离子水冲洗3次Fe0纳米颗粒,将湿Fe0纳米颗粒与0.03 mol·L−1的六水硫酸镍反应,制备得到镍铁双金属纳米颗粒(式(2))。用乙醇和去离子水分别冲洗纳米粒子清除多余的SO42−和Na+离子,再真空冷冻干燥24 h。
-
1)活性生物微球(SSNF)的制备:取2.0 g海藻酸钠加入70 mL去离子水中加热搅拌至溶解,高压灭菌后冷却至室温,加入30 mL的菌液(菌数量约为4.18
×104 个·mL−1),分别加入0.1、0.3、0.5、0.7、0.9 g镍铁双金属纳米粒子,不断搅拌,用针筒逐滴加入到含有2 %CaCl2的溶液中,形成1~2 cm的圆球,在37 ℃恒温培养箱中硬化10 h后,用去离子水多次清洗小球,4 ℃保存备用。2)海藻酸钠+硫酸盐还原菌微球(SS)的制备:取2.0 g海藻酸钠加入70 mL去离子水中加热搅拌至溶解,高压灭菌后冷却至室温,加入30 mL的菌液,不断搅拌,用针筒逐滴加入到含有2%CaCl2的溶液中,形成1~2 cm的圆球,在37 ℃恒温培养箱中硬化10 h后,用去离子水多次清洗珠子,4 ℃保存备用。
3)海藻酸钠微球(S)的制备:取2.0 g海藻酸钠加入100 mL去离子水中加热搅拌至溶解,高压灭菌后冷却至室温,用针筒逐滴加入到含有2%CaCl2的溶液中,形成1~2 cm的球形,硬化10 h后,用去离子水多次清洗珠子,4 ℃保存备用。
-
将制备的SSNF、SS、S微球分别取5.0 g加入到50 mL,含300 mg·L−1 Cd2+的合成液(0.5 g·L−1 KH2PO4,1.0 g·L−1 NH4Cl,1.0 g·L−1 NaSO4,0.1 g·L−1 CaCl2·2H2O,2.0 g·L−1 MgSO4·7H2O,2.0 g·L−1 DL-乳酸钠,0.5 g·L−1 FeSO4·7H2O,0.1 g·L−1 巯基乙酸钠,0.1 g·L−1 维生素C)厌氧瓶中,恒温振荡(37 ℃,180 r·min−1),分别在第1、2、3、4、5、6、7 d取样;溶液通过0.45 μm滤膜过滤,测定反应前后溶液中Cd2+和SO42−浓度变化。计算吸附量。实验均设置3组平行。
通过电感耦合等离子体发射光谱仪(ICP-5000)测定反应前后溶液中Cd2+浓度变化;通过电感耦合原子发射光谱仪(ICPS-7500)测定反应前后溶液中S元素的变化。Cd2+浓度和S元素前的测定需将溶液通过0.45 μm滤膜过滤后进行测定。使用流式细胞仪(BD Accuri C6 Plus,Flow Cytometer)测定菌液中菌的数量。使用溴化钾压片法对反应前后的SSNF进行傅里叶红外光谱(Spectrum Two )分析表征;扫描电镜(JSM-IT500)对反应前后的SSNF,镍-铁和SRB的表面的形貌进行分析;采用X射线衍射图谱(D8 Advance, Bruker, Karlsruhe)分析SSNF反应前后体系的变化。实验样品需预先用去离子水冲洗,真空冷冻干燥后进行傅里叶红外光谱、扫描电镜和X射线衍射图谱分析。
-
分别取0.1、0.3、0.5、0.7、0.9 g 镍-铁制备活性生物微球,然后分别取5.0 g小球分别加入到50 mL含有不同质量梯度的Cd2+(100、200、300、400、500 mg·L−1)合成液的厌氧瓶中,通过使用HCl或NaOH调节pH至4、5、6、7、8、9,恒温振荡(37 ℃,180 r·min−1),并分别在第1、2、3、4、5、6、7 天时取样,溶液过0.45 μm滤膜过滤,使用电感耦合等离子体发射光谱仪测定反应前后 Cd2+浓度。计算吸附量。实验均设置3组平行。
-
通过3个实验周期测定活性生物微球重复使用的可行性。测试均在最佳条件下进行。活性生物微球在pH为7、50 mL Cd2+(300 mg·L−1)合成液的厌氧瓶中,恒温振荡(37 ℃,180 r·min−1) 5 d后,微球经过滤分离后,用无菌生理盐水振荡洗涤1 h,再放入富集培养基中激活12 h后进行下一周期实验。如此重复3次,并计算重复使用后活性生物微球对Cd2+和SO42−的去除率。实验均设置3组平行。
-
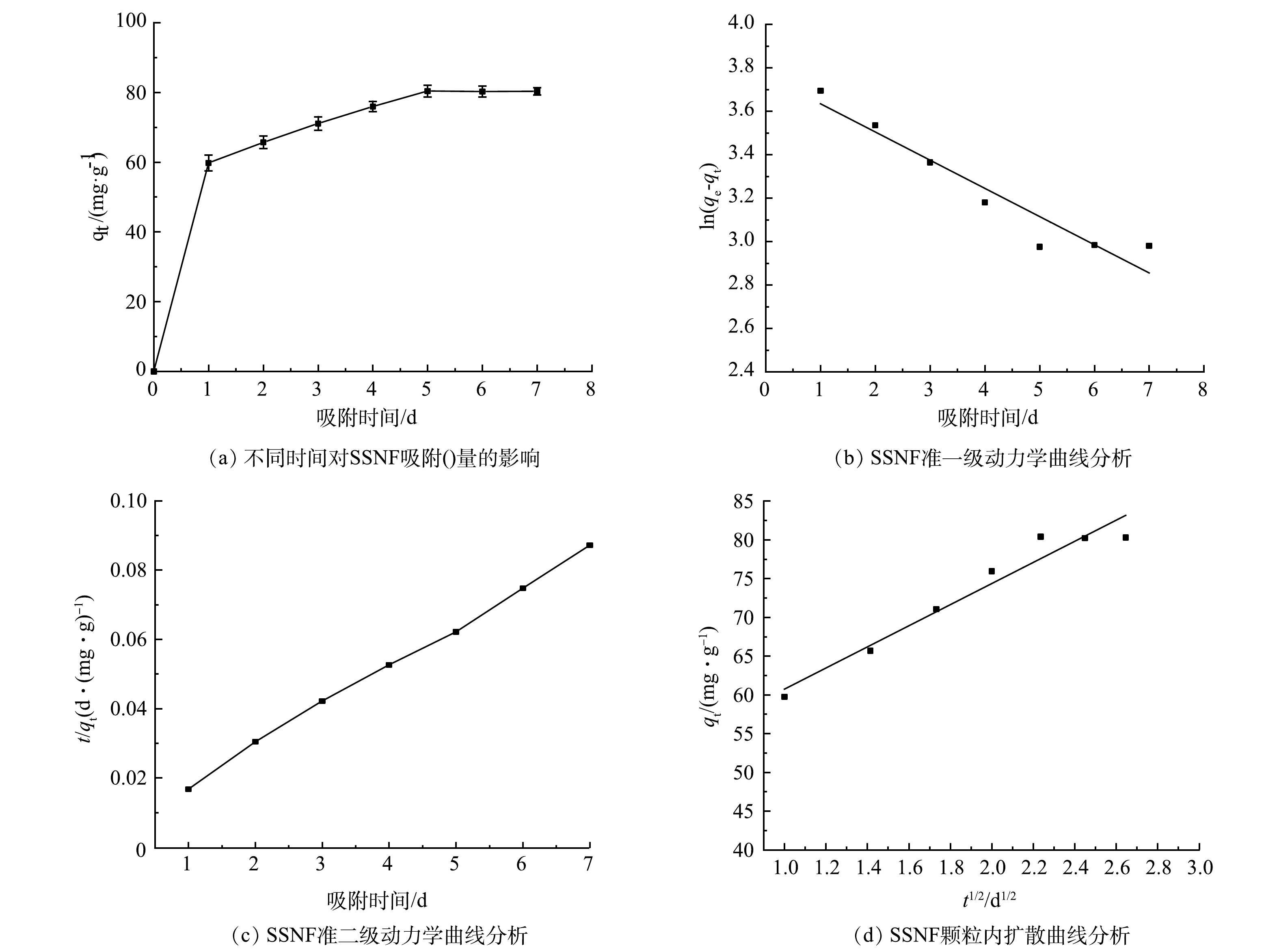
SSNF对Cd2+的吸附动力学分析:取5.0 g SSNF微球(镍-铁加入量为0.3 g)加入到pH为7,50 mL的300 mg·L−1 Cd2+合成液的厌氧瓶中,37 ℃下恒温振荡(180 r·min−1),分别在第1、2、3、4、5、6、7 天取样。溶液通过0.45 μm滤膜过滤,通过电感耦合等离子体发射光谱仪测定反应前后Cd2+浓度,分析其动力学特征。采用准一级动力学方程(式(3))和准二级动力学方程(式(4))分析实验数据,再进一步使用颗粒内扩散模型方程(式(5))分析吸附机理[19]。
式中:qt为某时刻的吸附量,mg·g−1;qe为平衡时的吸附量,mg·g−1;t为吸附时间,d;k1为准一级动力学模型速率常数,d−1;k2为准二级动力学模型速率常数,g·(mg·d)−1;kp为颗粒扩散速率,mg·(g·d1/2) -1;C为厚度、边界层的常数,mg·g−1。
-
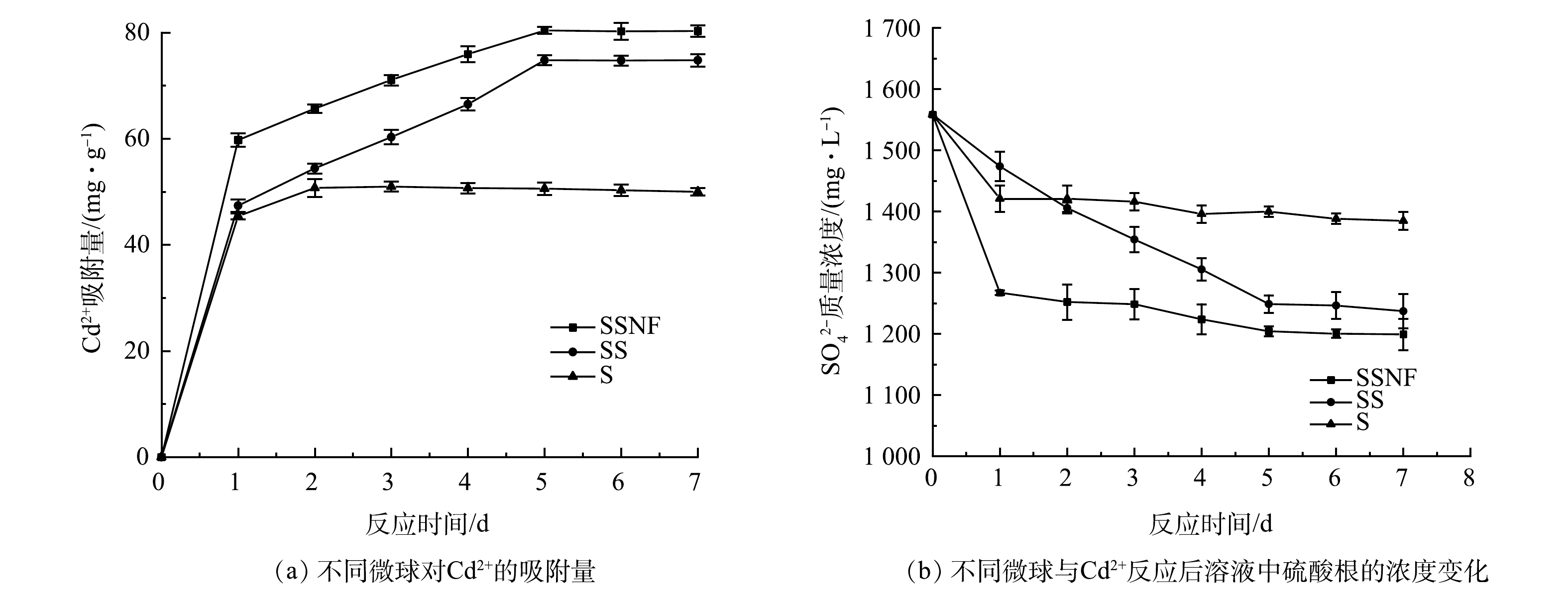
不同微球对Cd2+的吸附量随时间变化如图1(a)所示。在不同微球间存在显著差异。其中SSNF对Cd2+的最大吸附量达到80.40 mg·g−1 ,高于SS(74.79 mg·g−1)和S(50.99 mg·g−1)。但当初始Cd2+含量为400 mg·g−1时,SSNF对Cd2+的最大吸附量达到103.86 mg·g−1 ,高于SS(90.6138 mg·g−1),具有显著差异。这是由于SRB可以将硫酸盐还原成硫化氢,硫化氢与Cd2+形成CdS沉淀[8]。反应过程如式(6)和式(7)所示。
式中,CH2O是有机物,M是重金属离子。镍-铁具有促进SRB与重金属反应的作用。Fe0在厌氧条件下很容易在水中氧化生成H2(式(8)),从而提供电子促进SRB还原硫酸盐((式(9)和式(10))。当Ni0与Fe0偶联时,Ni0可作为原电池的负极,从而加速电子转移[20]。式(11) 是基于式(8)和式(10)得到的。镍-铁对SRB具有协同促进作用,使得SSNF相较于SS和S处理废水中的Cd2+更具优势。
硫酸根浓度可作为SRB活性的参考指标。由图1(b)可见,硫酸根浓度随时间的变化呈先下降后稳定的趋势。这是由于SRB对Cd2+的吸附起主要作用,金属的快速吸附不仅取决于细菌细胞表面的物理吸附和镍-铁与Cd2+的化学反应,海藻酸钠对Cd2+也具有一定的吸附能力。以上结果表明,SSNF对Cd2+具有很好的去除能力,因此,选择SSNF进行后续实验。
-
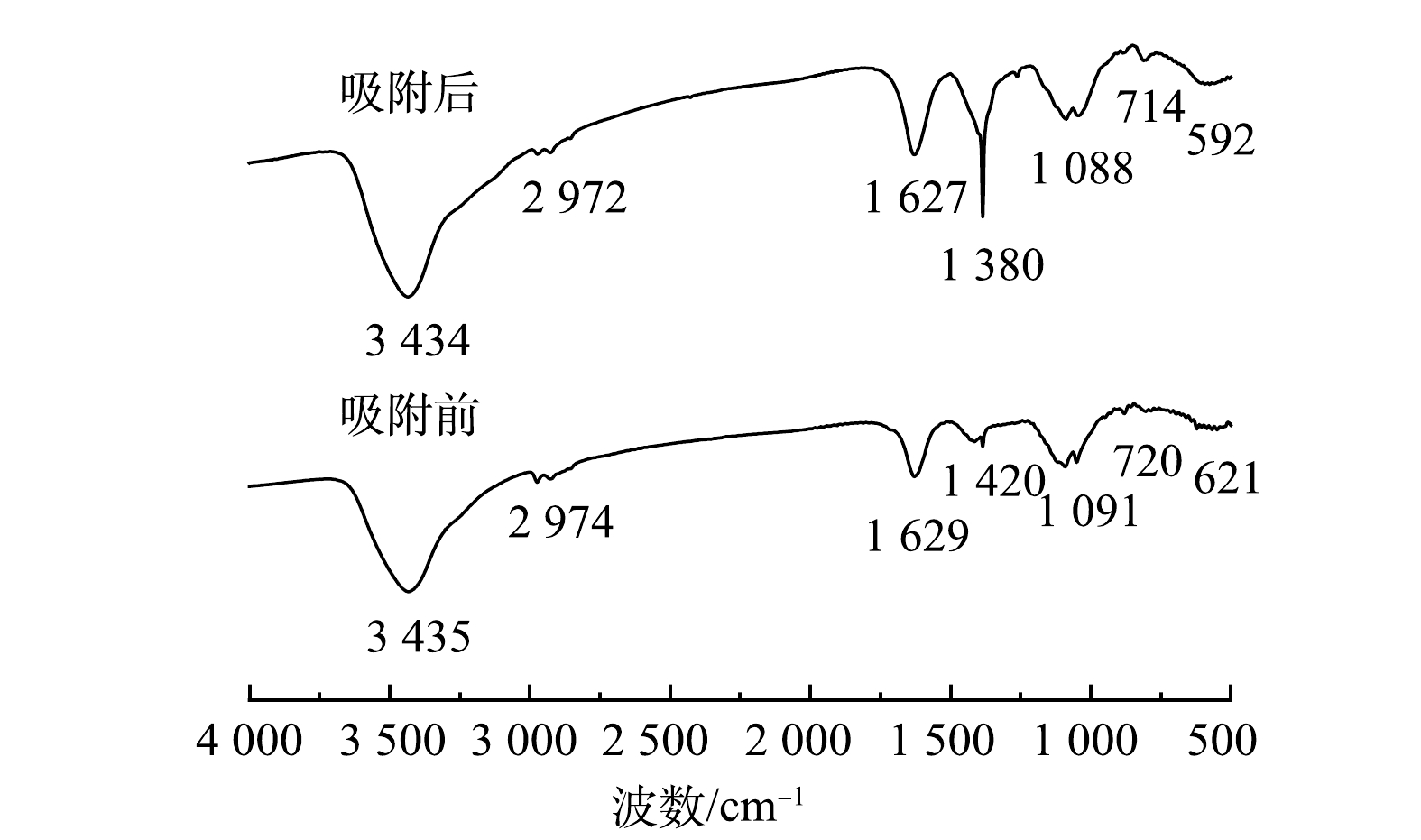
1) FTIR表征分析。图2为SSNF与Cd2+反应前后的FTIR光谱图。在3 434~3 435 cm−1处的特征峰为蛋白质的-NH基团和葡萄糖的羟基基团 [21]。在2 972~2 974 cm−1和621~592 cm−1处观察到是CH2的不对称伸缩振动,与蛋白质中的烷基和海藻酸钠的脂肪酸或CH2基团相对应[22]。1 629~1 627 cm−1处的峰可能是蛋白质或胺的C=O伸缩振动。在1 420~1 380 cm−1附近的强峰表明羧基的存在,其弯曲振动与C=O基团一致。1 088~1 091 cm−1处的峰是多糖的C—O伸缩振动。结果表明,SSNF的特征峰伸缩位于1 420、1 629和3 435 cm−1附近,当SSNF处理Cd2+溶液后,FTIR光谱图中相应的峰发生了强度变化或位移。红外光谱从1 380 cm−1到1 420 cm−1有明显的峰强度变化,表明羧基上的C=O参与了SSNF与Cd2+的反应。峰从1 627 cm−1到1 629 cm−1表明海藻酸钠或SRB中蛋白质或胺基参与了Cd2+的反应。位于3 434 cm−1的峰强度增强,表明海藻酸钠或SRB中羟基和胺基参与了Cd2+的反应[23]。因此,可以合理地假设海藻酸钠和SRB中羧基、羟基、胺基是与Cd2+结合的主要官能团。
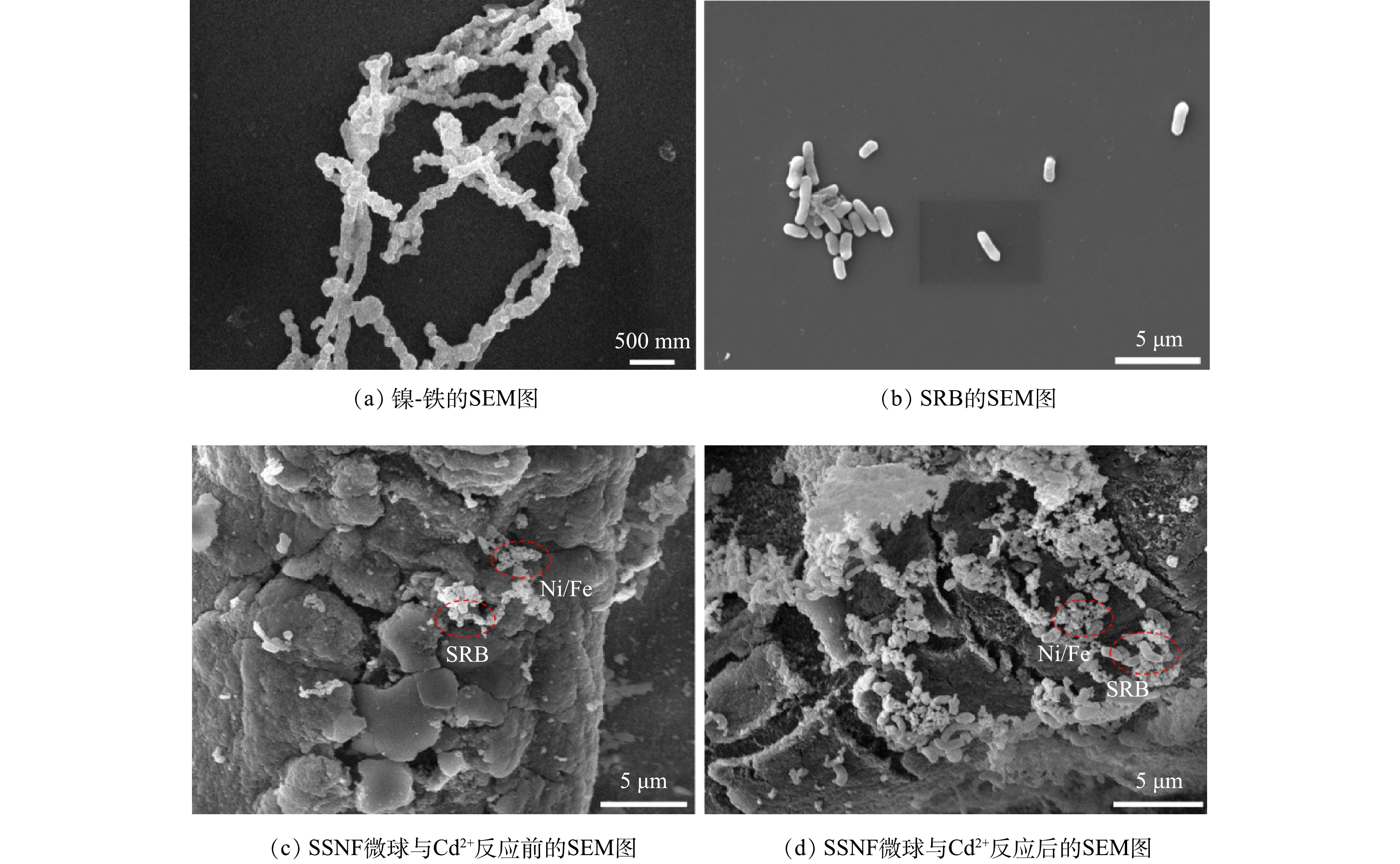
2) SEM分析。由图3(a)可见,因镍-铁具有磁性,故呈串珠状结构[24],尺寸为80~100 nm。由图3(b)可见,SRB呈棒状结构,尺寸为2~3 μm。对比图3(c)和图3(d)可以看出,在与Cd2+反应前,SSNF表面粗糙,具有较大的比表面积,且SRB含量较少;SSNF与Cd2+反应后表面孔隙减少且产生大量的不规则沉淀,镍-铁周围的SRB含量显著增多。由此可推测,沉淀物是通过镍-铁与SRB的氧化还原作用产生的CdS、Fe3O4和Fe2O3[25]。海藻酸钠可以保护SRB的活性,降低Cd2+对其产生的毒性;同时,镍-铁可以制造适宜SRB生长的厌氧环境,使其可以在SSNF表面大量生长,从而实现SSNF对高浓度Cd2+的去除。
3) XRD分析。图4中JCPDS 37-0474为镍-铁的标准卡片,JCPDS 10-0454为CdS的标准卡片。由图4可见,反应前的样品有1个最明显的特征峰在44.74°,属于镍-铁的(110)衍射晶面。反应后在27.31°、44°和81.09°有3个明显的特征峰,分别属于CdS的(111)、(226)和(422)衍射晶面。这表明SRB可将溶液中的SO42−还原为S2−,使溶液中Cd2+转化为CdS沉淀,且在反应后镍-铁的特征峰峰高变低。这说明反应后样品中镍-铁的量降低,推测可能产生Fe3O4或Fe2O3[18]。以上结果表明,SSNF处理含Cd2+废水发生了氧化还原和共沉淀,溶液中Cd2+最终以CdS沉淀的形式被去除。
-
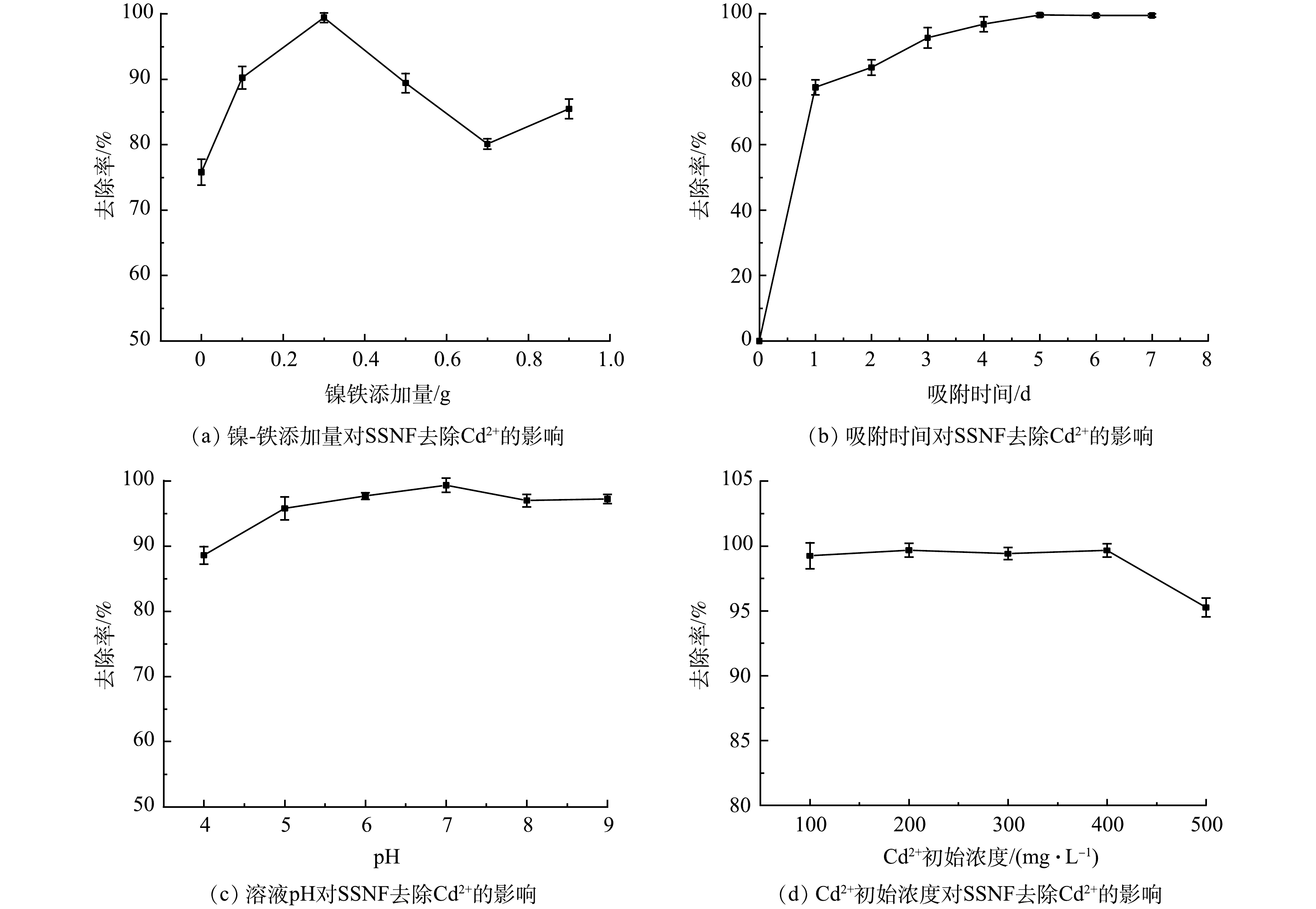
1)镍-铁添加量对SSNF去除Cd2+能力的影响。在Cd2+初始质量浓度为300 mg·L−1、pH为7、反应时间为5 d时的反应条件下,通过添加不同质量的镍-铁制备SSNF微球,考察了其对Cd2+去除能力的影响。由图5(a)可见,SSNF对Cd2+的去除率随镍-铁添加量的增加呈先增加后减少的趋势。当镍-铁添加量由0增加到0.3 g时,SSNF对Cd2+的去除率由75.79%增加到99.41%;当继续增加镍-铁时,SSNF对Cd2+的去除率却呈下降的趋势。由此可确定SSNF中镍-铁的最佳添加量为0.3 g。其原因可能是:当镍-铁添加量过少时,其对SRB处理Cd2+的协同促进作用较小,SRB生长密度过大,导致SRB对营养物质的竞争,从而阻碍微生物生长,降低SSNF对Cd2+的去除效果;此外,由于镍-铁具有磁性,会产生团聚现象[25],所以当镍-铁的添加量过多时,会包裹在SRB的表面,减少了SRB与硫酸盐的接触面积,影响微生物的正常生长,从而降低了SSNF对Cd2+的去除率。
2)反应时间对SSNF去除Cd2+的影响。在Cd2+初始质量浓度为300 mg·L−1、pH为7、SSNF微球中镍-铁添加量为0.3 g时,SSNF对Cd2+的去除率随时间变化如图5(b)所示。可以看出,SSNF对Cd2+的去除率呈先增加,然后趋于平衡的变化特征。其吸附过程分为2个阶段:0~1 d为快速去除阶段,去除率达到77.53%;2~5 d为慢速去除阶段,在第5 d基本达到平衡,去除率达到99.98 %。0~1 d时,由于镍-铁具有促进SRB还原硫酸盐的作用, SSNF微球具有足够的反应位点,使其达到快速去除Cd2+的效果;1~5 d时,SRB进入对数生长期和稳定期,由于重金属的毒性作用使得部分菌死亡,而有研究证明,死菌对Cd2+的去除能力显著低于活菌[8],导致SSNF对Cd2+的去除率增长缓慢;5~7 d时,SRB进入衰亡期,镍-铁由于在反应过程中发生氧化反应导致其还原能力下降[18],所以SSNF对Cd2+的去除率基本趋于平衡。因此,SSNF与Cd2+反应的最佳平衡时间为5 d。
3) pH对SSNF去除Cd2+的影响。在Cd2+初始质量浓度为300 mg·L−1、反应时间为5 d时、镍-铁添加量为0.3 g时,不同pH对SSNF去除Cd2+的影响结果见图5(c)。可见,随pH的增加,SSNF对Cd2+的去除率呈先增大后减小的趋势。当pH为4~7时,去除率由88.59%增大到99.31%;当pH为7~9时,去除率由99.31%减小到97.21%。由此可知,当pH为7时,SSNF对Cd2+的去除效果最好。这可能与溶液中的离子与相应的官能团的静电结合作用有关。在较高pH条件下,SSNF对Cd2+的去除能力高于较低pH条件下,因为在较低pH条件,溶液中H+含量较高,其会与Cd2+竞争SSNF的反应位点,同时H+会与SSNF表面的-OH基团结合形成-OH2+,从而使SSNF表面官能团带正电,从而减少SSNF与Cd2+的反应位点[26]。当溶液pH为9时,Cd(OH)2的浓度商大于溶度积常数,说明溶液中存在Cd(OH)2沉淀,所以此时的去除率相较于pH为8时有所提升。在较高pH条件下,SSNF表面的-OH被电离成带负电的氧(−O–),其可排斥能够吸附Cd2+的SSNF上的带负电的基团 [18],从而降低SSNF对Cd2+的去除能力。
4) Cd2+初始浓度对SSNF去除Cd2+的影响。在SSNF中镍-铁添加量为0.3 g、反应时间为5 d、pH为7时,考察了不同的Cd2+初始浓度对SSNF去除能力的影响。如图5(d)所示,随着Cd2+浓度的增加,其去除率呈先平稳后下降的趋势。当Cd2+质量浓度为100~400 mg·L−1时,SSNF对其的去除率几乎达到100%,其最大吸附量达到103.86 mg·g−1;当Cd2+质量浓度为500 mg·L−1时,SSNF对其的去除率下降至95.25%。当Cd2+浓度较低时,SSNF具有足够的反应位点,且容易与Cd2+结合[27],此外,镍-铁可制造SRB适宜生长的厌氧环境,从而形成较高的Cd2+去除率。当Cd2+浓度过高时,SSNF的反应位点逐渐饱和[28],且高浓度的Cd2+会对SRB产生毒性作用,干扰核酸和酶活性位点,SRB的生长受到抑制,导致SSNF对Cd2+的去除率下降[29]。
-
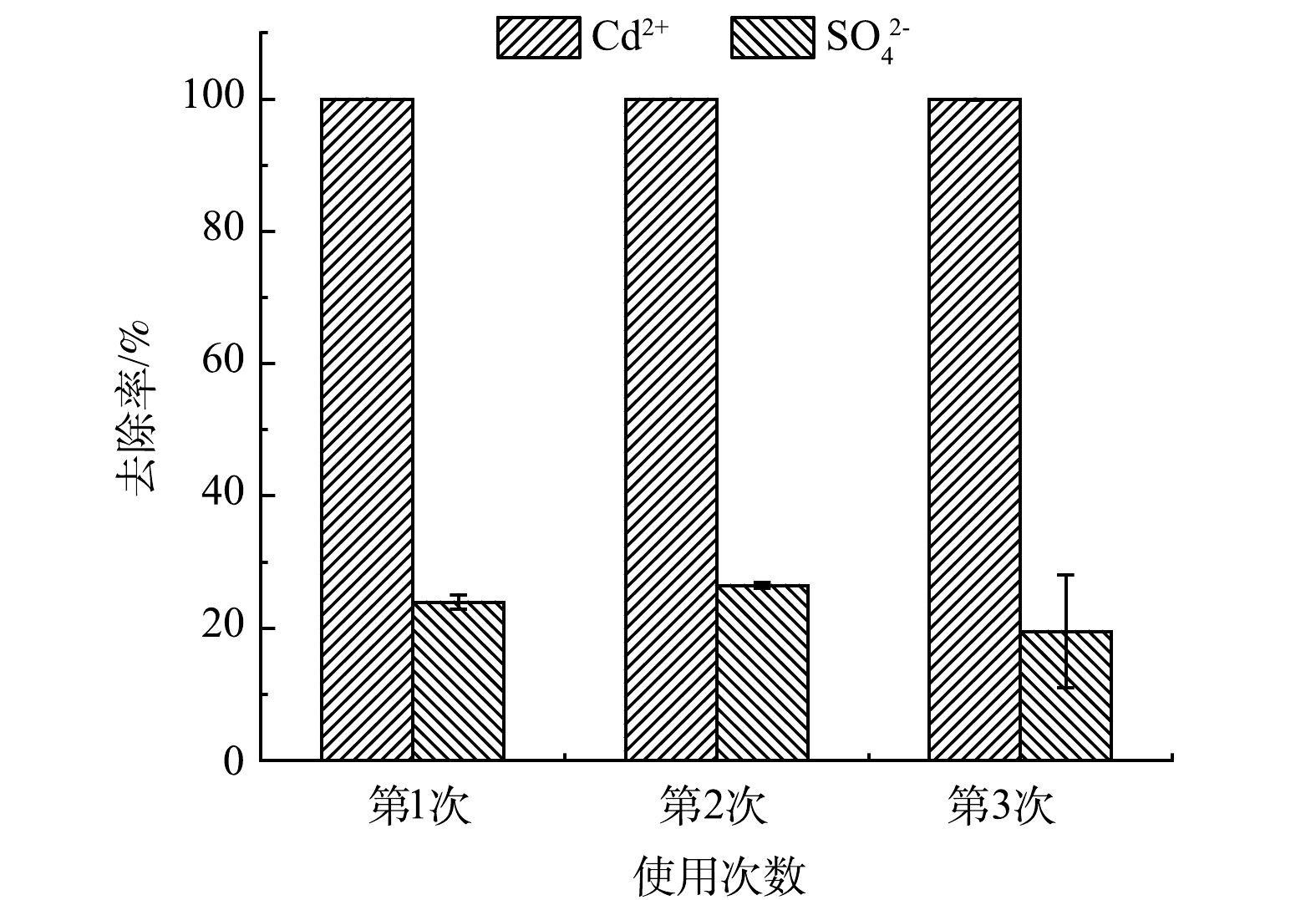
活性生物微球经过3次循环再利用后,对Cd2+的去除率和SO42−的消耗情况如图6所示。SSNF对Cd2+始终保持较高的去除率,而SO42−的消耗较最初仅降低了4.4 %。在每个周期结束后,使用硫酸盐还原菌培养基激活SRB,SSNF在下1个周期仍能对Cd2+保持较高的去除能力。这说明,活性微球可以保护SRB,从而降低Cd2+对其产生的毒性作用,同时,不易被氧化的镍-铁仍可促进SRB与Cd2+发生还原反应,从而保持较高的去除能力。在第1个周期后,微球没有发生断裂;但在后2个周期中,微球发生部分断裂,可能造成微珠内的物质泄露,从而降低活性微球对Cd2+和SO42−的去除率。同时,3个周期实验中释放的Ni质量浓度均小于1 mg·L−1。由此可见,SSNF具有良好的循环再利用性能。
-
由图7(b)、图7 (c)和表1可以看出,SSNF对Cd2+的吸附更加符合准二级动力学。其准一级动力学模型和准二级动力学模型的R2分别为0.914和0.998,均大于0.9,但准二级动力学方程所得的理论平衡吸附量qe(88.50 mg·g−1)相比于准一级动力学方程所得的理论平衡吸附量qe(43.14 mg·g−1)更加接近实验所得吸附量(80.40 mg·g−1),说明SSNF对Cd2+的吸附主要受化学吸附控制,而物理吸附作用为辅[19]。颗粒内扩散模型的拟合曲线未通过原点,说明吸附过程不符合颗粒内扩散模型,其吸附过程受其他吸附阶段的共同控制。这可能是因为吸附过程是通过离子交换或共价电子对形成的共价力即化学吸附起主要作用。SRB还原硫酸盐产生的硫化物与重金属形成的金属硫化物沉淀是发生在SSNF外部,所以重金属离子不易进入SSNF内部[24]。所以,SSNF对Cd2+的吸附过程为:第1步是硫酸盐扩散,被SSNF中的SRB还原生成硫化物;第2步是硫化物与Cd2+形成金属硫化物,沉淀到SSNF表面。
-
1) SSNF对废水中高浓度的Cd2+具有较好的去除能力,镍-铁对SRB具有协同促进作用。SSNF吸附Cd2+的最佳条件为:镍-铁添加量为0.3 g,pH为7,反应时间5 d,Cd2+初始质量浓度为400 mg·L−1.此时Cd2+的去除率达到100%,吸附量为103.86 mg·g−1。
2) SSNF与Cd2+反应前后物质组成的变化说明:反应过程中存在离子交换作用,羧基、羟基、胺基是与Cd2+结合的主要官能团,溶液中的Cd2+转化为CdS沉淀的形式被去除。
3) SSNF吸附Cd2+符合准二级动力学模型,以化学吸附作用为主,物理吸附为辅;离子交换或共价电子可能是SSNF去除Cd2+的主要机制。
海藻酸钠固定硫酸盐还原菌、镍-铁纳米粒子生物微球对污水中Cd2+的去除
Removal of Cd2+ in sewage by sodium alginate immobilized sulfate-reducing bacteria and nickel-iron nanoparticle biological microspheres
-
摘要: 为寻求一种高效去除高浓度含Cd2+废水的方法,本研究以海藻酸钠为载体固定硫酸盐还原菌(SRB)和镍铁双金属纳米粒子,制备了高效去除Cd2+的活性生物微球(SSNF),并通过对比不同的固定化材料来探讨其对废水中高浓度Cd2+的去除效果。通过改变镍-铁添加量,pH,反应时间,初始Cd2+浓度探讨了SSNF去除Cd2+的能力,并结合吸附动力学探究了SSNF对Cd2+的去除过程及相关机制。结果表明,当镍-铁添加量为0.3 g、pH为7、反应时间5 d、Cd2+初始浓度为400 mg·L−1时,SSNF去除率可达到100 %,吸附量为103.86 mg·g−1。镍-铁对 SRB去除Cd2+具有明显的协同促进作用,且符合准二级动力学模型,以化学吸附作用为主,物理吸附为辅。离子交换或共价电子作用可能是SSNF吸附Cd2+的主要机制。利用无菌生理盐水对活性生物微球进行洗涤,重复使用3次后,微球仍能保持较高的去除能力。该研究结果可为解决含有高浓度Cd2+污染的废水问题提供技术参考。Abstract: In order to find an efficient method to remove high-concentration Cd2+ contained in wastewater, sodium alginate was used as a carrier to immobilize sulfate-reducing bacteria (SRB) and nickel-iron bimetallic nanoparticles, and a type of active biological microspheres(SSNF) that can efficiently remove Cd2+ was prepared. Through the comparison of different immobilized materials, its effect on high-concentration Cd2+ removal from wastewater was explored. The Cd2+ removal ability by SSNF was discussed with the variations of the amount of ferronickel, pH, reaction time, and initial Cd2+ concentration, and the adsorption kinetics was combined to study the Cd2+ removal process and related mechanisms by SSNF. The results showed that when ferronickel addition was 0.3 g, pH=7, reaction time was 5 d, and Cd2+ initial concentration was 400 mg·L−1, Cd2+ removal rate by SSNF reached 100 % with the adsorption capacity of 103.86 mg·g−1. Ferronickel has an obvious synergistic promotion effect on Cd2+ removal by SRB, and it conforms to the quasi-second-order kinetic model, and was dominated by chemical adsorption with physical adsorption as supplement. Ion exchange or covalent electron interaction may be the main mechanism for Cd2+ adsorption onto SSNF. The research results can provide a technical reference for solving the problem of high concentration-Cd2+ wastewater treatment.
-
据报道,全球每年释放到环境中的金属镉有3.9×104 t [1,2]。镉作为毒性最强的重金属之一,具有生物累积性和不可生物降解性,可引起癌症、骨变性、肺功能障碍和高血压[3]。因此,找到一个高效、彻底除去废水中Cd2+的方法势在必行。
硫酸盐还原菌(sulfate reducing bacteria,SRB)作为一种绿色环保的去除Cd2+的有效方法,近年来已受到广泛关注[4]。SRB通过将硫酸盐还原为硫化物,硫化物可与重金属离子形成不溶性沉淀,最终达到去除废水中Cd2+的目的[5-7]。董净等[8,9]通过从土壤中分离纯化出耐镉性较好的SRB,对40 mg·L−1 Cd2+的去除率可达到75 %。SRB虽然对废水中Cd2+具有较好去除效果,但存在固液相分离困难、高浓度金属毒性等问题,使其受到限制[10]。
为了解决以上问题,近年来许多研究者通过将SRB固定在合适的载体上[11-14],增加其比表面积,提高物质表面的运输速率,同时保护微生物的酶活性,使得重金属对微生物的毒性减弱,最终提高SRB的生物稳定性且易于分离[12-16]。海藻酸钠是一种从藻类中提取的天然多糖,由于其对微生物的毒性低且价格低廉,所以经常用于SRB的固定化技术中。此外,为进一步提高SRB对Cd2+的去除率,近年来多采用零价铁纳米粒子(Fe0)修饰SRB,并用于原位控制受污染的地下水[15]。PEDRO等[16]使用零价铁纳米粒子(Fe0)与SRB复合后,其对Cd2+的去除率可达到99.8 %。ZHANG等[17]使用聚乙烯醇,海藻酸钠固定SRB和Fe0处理49 mg·L−1的Cd2+,去除率可达到99 %。通过以上研究可发现,SRB与固定化材料的结合在处理低浓度的Cd2+时均具有较好的去除能力。然而,近年来工业废水中Cd2+含量越来越高,很难实现完全去除废水中高浓度的Cd2+。此外,传统的Fe0对Cd2+具有较好的处理能力,但其表面易产生聚集行为且易氧化,从而降低其使用寿命。
基于以上研究成果,为寻求一种高效去除高浓度含Cd2+废水的方法,本研究选用惰性金属镍与Fe0偶联形成的双金属纳米粒子参与反应,因其具有良好的腐蚀稳定性,可增加Fe0催化反应活性并有效降低Fe0的氧化,镍铁双金属纳米粒子通常与其他金属偶联以增加催化反应活性并有效降低铁的氧化[18],所以,本研究构建了以海藻酸钠为载体固定镍-铁和SRB活性生物微球(SSNF),探讨了SSNF对Cd2+的吸附去除性能,考察了镍-铁添加量、pH、Cd2+初始浓度和吸附时间对SSFN吸附Cd2+能力的影响,以期为处理高浓度Cd2+污染废水提供参考。
1. 材料和方法
1.1 硫酸盐还原菌的培养
实验所用菌株为实验室筛选得到的SRB,使用改良的Postgate C培养基(0.5 g·L−1 KH2PO4,1.0 g·L−1 NH4Cl,1.0 g·L−1 NaSO4,0.1 g·L−1 CaCl2·2H2O,2.0 g·L−1 MgSO4·7H2O,2.0 g·L−1 DL-乳酸钠,1.0 g·L−1 酵母提取物,1.0 g·L−1 Resazurin,0.5 g·L−1 FeSO4·7H2O,0.1 g·L−1 巯基乙酸钠,0.1 g·L−1 维生素C,pH为7.8,121 ℃灭菌15 min)以10 %的接种量接入菌株,在100 mL厌氧瓶中于37 ℃恒温培养箱中对SRB进行培养。当菌液中产生大量黑色沉淀物后即可进行后续实验。
1.2 镍-铁的制备
在氮气气氛下,在含有0.2 mol·L−1的FeSO4·7H2O溶液的三口烧瓶中逐滴加入0.4 mol·L−1的NaBH4溶液,室温条件下,使用磁力搅拌器混合均匀。反应过程中亚铁可被还原为Fe0(式(1)),从而合成得到Fe0纳米粒子[18]。使用去离子水冲洗3次Fe0纳米颗粒,将湿Fe0纳米颗粒与0.03 mol·L−1的六水硫酸镍反应,制备得到镍铁双金属纳米颗粒(式(2))。用乙醇和去离子水分别冲洗纳米粒子清除多余的SO42−和Na+离子,再真空冷冻干燥24 h。
Fe(H2O)2+6+2BH−4→Fe0↓+2B(OH)3+7H2↑ (1) Fe0+Ni2+→Fe2++Ni0 (2) 1.3 SSNF的制备
1)活性生物微球(SSNF)的制备:取2.0 g海藻酸钠加入70 mL去离子水中加热搅拌至溶解,高压灭菌后冷却至室温,加入30 mL的菌液(菌数量约为4.18
×104 2)海藻酸钠+硫酸盐还原菌微球(SS)的制备:取2.0 g海藻酸钠加入70 mL去离子水中加热搅拌至溶解,高压灭菌后冷却至室温,加入30 mL的菌液,不断搅拌,用针筒逐滴加入到含有2%CaCl2的溶液中,形成1~2 cm的圆球,在37 ℃恒温培养箱中硬化10 h后,用去离子水多次清洗珠子,4 ℃保存备用。
3)海藻酸钠微球(S)的制备:取2.0 g海藻酸钠加入100 mL去离子水中加热搅拌至溶解,高压灭菌后冷却至室温,用针筒逐滴加入到含有2%CaCl2的溶液中,形成1~2 cm的球形,硬化10 h后,用去离子水多次清洗珠子,4 ℃保存备用。
1.4 SSNF对Cd2+的吸附性能与表征
将制备的SSNF、SS、S微球分别取5.0 g加入到50 mL,含300 mg·L−1 Cd2+的合成液(0.5 g·L−1 KH2PO4,1.0 g·L−1 NH4Cl,1.0 g·L−1 NaSO4,0.1 g·L−1 CaCl2·2H2O,2.0 g·L−1 MgSO4·7H2O,2.0 g·L−1 DL-乳酸钠,0.5 g·L−1 FeSO4·7H2O,0.1 g·L−1 巯基乙酸钠,0.1 g·L−1 维生素C)厌氧瓶中,恒温振荡(37 ℃,180 r·min−1),分别在第1、2、3、4、5、6、7 d取样;溶液通过0.45 μm滤膜过滤,测定反应前后溶液中Cd2+和SO42−浓度变化。计算吸附量。实验均设置3组平行。
通过电感耦合等离子体发射光谱仪(ICP-5000)测定反应前后溶液中Cd2+浓度变化;通过电感耦合原子发射光谱仪(ICPS-7500)测定反应前后溶液中S元素的变化。Cd2+浓度和S元素前的测定需将溶液通过0.45 μm滤膜过滤后进行测定。使用流式细胞仪(BD Accuri C6 Plus,Flow Cytometer)测定菌液中菌的数量。使用溴化钾压片法对反应前后的SSNF进行傅里叶红外光谱(Spectrum Two )分析表征;扫描电镜(JSM-IT500)对反应前后的SSNF,镍-铁和SRB的表面的形貌进行分析;采用X射线衍射图谱(D8 Advance, Bruker, Karlsruhe)分析SSNF反应前后体系的变化。实验样品需预先用去离子水冲洗,真空冷冻干燥后进行傅里叶红外光谱、扫描电镜和X射线衍射图谱分析。
1.5 不同因素对SSNF去除效果的影响
分别取0.1、0.3、0.5、0.7、0.9 g 镍-铁制备活性生物微球,然后分别取5.0 g小球分别加入到50 mL含有不同质量梯度的Cd2+(100、200、300、400、500 mg·L−1)合成液的厌氧瓶中,通过使用HCl或NaOH调节pH至4、5、6、7、8、9,恒温振荡(37 ℃,180 r·min−1),并分别在第1、2、3、4、5、6、7 天时取样,溶液过0.45 μm滤膜过滤,使用电感耦合等离子体发射光谱仪测定反应前后 Cd2+浓度。计算吸附量。实验均设置3组平行。
1.6 SSNF的再利用实验
通过3个实验周期测定活性生物微球重复使用的可行性。测试均在最佳条件下进行。活性生物微球在pH为7、50 mL Cd2+(300 mg·L−1)合成液的厌氧瓶中,恒温振荡(37 ℃,180 r·min−1) 5 d后,微球经过滤分离后,用无菌生理盐水振荡洗涤1 h,再放入富集培养基中激活12 h后进行下一周期实验。如此重复3次,并计算重复使用后活性生物微球对Cd2+和SO42−的去除率。实验均设置3组平行。
1.7 吸附动力学
SSNF对Cd2+的吸附动力学分析:取5.0 g SSNF微球(镍-铁加入量为0.3 g)加入到pH为7,50 mL的300 mg·L−1 Cd2+合成液的厌氧瓶中,37 ℃下恒温振荡(180 r·min−1),分别在第1、2、3、4、5、6、7 天取样。溶液通过0.45 μm滤膜过滤,通过电感耦合等离子体发射光谱仪测定反应前后Cd2+浓度,分析其动力学特征。采用准一级动力学方程(式(3))和准二级动力学方程(式(4))分析实验数据,再进一步使用颗粒内扩散模型方程(式(5))分析吸附机理[19]。
ln(qe−qt)=lnqe−k12.303t (3) tqt=1k2q2e+1qet (4) qt=kpt0.5+C (5) 式中:qt为某时刻的吸附量,mg·g−1;qe为平衡时的吸附量,mg·g−1;t为吸附时间,d;k1为准一级动力学模型速率常数,d−1;k2为准二级动力学模型速率常数,g·(mg·d)−1;kp为颗粒扩散速率,mg·(g·d1/2) -1;C为厚度、边界层的常数,mg·g−1。
2. 结果与讨论
2.1 不同微球对Cd2+的吸附性能
不同微球对Cd2+的吸附量随时间变化如图1(a)所示。在不同微球间存在显著差异。其中SSNF对Cd2+的最大吸附量达到80.40 mg·g−1 ,高于SS(74.79 mg·g−1)和S(50.99 mg·g−1)。但当初始Cd2+含量为400 mg·g−1时,SSNF对Cd2+的最大吸附量达到103.86 mg·g−1 ,高于SS(90.6138 mg·g−1),具有显著差异。这是由于SRB可以将硫酸盐还原成硫化氢,硫化氢与Cd2+形成CdS沉淀[8]。反应过程如式(6)和式(7)所示。
2CH2O+SO2−4+2H+=H2S+CO2+2H2O (6) H2S+M=MS(s)+2H+ (7) 式中,CH2O是有机物,M是重金属离子。镍-铁具有促进SRB与重金属反应的作用。Fe0在厌氧条件下很容易在水中氧化生成H2(式(8)),从而提供电子促进SRB还原硫酸盐((式(9)和式(10))。当Ni0与Fe0偶联时,Ni0可作为原电池的负极,从而加速电子转移[20]。式(11) 是基于式(8)和式(10)得到的。镍-铁对SRB具有协同促进作用,使得SSNF相较于SS和S处理废水中的Cd2+更具优势。
Fe0+2H2O→Fe2++H2+2OH− (8) Fe0→Fe2++2e− (9) SO2−4+4H2+H+→HS−+4H2O (10) 4Fe0+SO2−4+9H+→HS−+4Fe2++4H2O (11) 硫酸根浓度可作为SRB活性的参考指标。由图1(b)可见,硫酸根浓度随时间的变化呈先下降后稳定的趋势。这是由于SRB对Cd2+的吸附起主要作用,金属的快速吸附不仅取决于细菌细胞表面的物理吸附和镍-铁与Cd2+的化学反应,海藻酸钠对Cd2+也具有一定的吸附能力。以上结果表明,SSNF对Cd2+具有很好的去除能力,因此,选择SSNF进行后续实验。
2.2 SSNF的结构表征
1) FTIR表征分析。图2为SSNF与Cd2+反应前后的FTIR光谱图。在3 434~3 435 cm−1处的特征峰为蛋白质的-NH基团和葡萄糖的羟基基团 [21]。在2 972~2 974 cm−1和621~592 cm−1处观察到是CH2的不对称伸缩振动,与蛋白质中的烷基和海藻酸钠的脂肪酸或CH2基团相对应[22]。1 629~1 627 cm−1处的峰可能是蛋白质或胺的C=O伸缩振动。在1 420~1 380 cm−1附近的强峰表明羧基的存在,其弯曲振动与C=O基团一致。1 088~1 091 cm−1处的峰是多糖的C—O伸缩振动。结果表明,SSNF的特征峰伸缩位于1 420、1 629和3 435 cm−1附近,当SSNF处理Cd2+溶液后,FTIR光谱图中相应的峰发生了强度变化或位移。红外光谱从1 380 cm−1到1 420 cm−1有明显的峰强度变化,表明羧基上的C=O参与了SSNF与Cd2+的反应。峰从1 627 cm−1到1 629 cm−1表明海藻酸钠或SRB中蛋白质或胺基参与了Cd2+的反应。位于3 434 cm−1的峰强度增强,表明海藻酸钠或SRB中羟基和胺基参与了Cd2+的反应[23]。因此,可以合理地假设海藻酸钠和SRB中羧基、羟基、胺基是与Cd2+结合的主要官能团。
2) SEM分析。由图3(a)可见,因镍-铁具有磁性,故呈串珠状结构[24],尺寸为80~100 nm。由图3(b)可见,SRB呈棒状结构,尺寸为2~3 μm。对比图3(c)和图3(d)可以看出,在与Cd2+反应前,SSNF表面粗糙,具有较大的比表面积,且SRB含量较少;SSNF与Cd2+反应后表面孔隙减少且产生大量的不规则沉淀,镍-铁周围的SRB含量显著增多。由此可推测,沉淀物是通过镍-铁与SRB的氧化还原作用产生的CdS、Fe3O4和Fe2O3[25]。海藻酸钠可以保护SRB的活性,降低Cd2+对其产生的毒性;同时,镍-铁可以制造适宜SRB生长的厌氧环境,使其可以在SSNF表面大量生长,从而实现SSNF对高浓度Cd2+的去除。
3) XRD分析。图4中JCPDS 37-0474为镍-铁的标准卡片,JCPDS 10-0454为CdS的标准卡片。由图4可见,反应前的样品有1个最明显的特征峰在44.74°,属于镍-铁的(110)衍射晶面。反应后在27.31°、44°和81.09°有3个明显的特征峰,分别属于CdS的(111)、(226)和(422)衍射晶面。这表明SRB可将溶液中的SO42−还原为S2−,使溶液中Cd2+转化为CdS沉淀,且在反应后镍-铁的特征峰峰高变低。这说明反应后样品中镍-铁的量降低,推测可能产生Fe3O4或Fe2O3[18]。以上结果表明,SSNF处理含Cd2+废水发生了氧化还原和共沉淀,溶液中Cd2+最终以CdS沉淀的形式被去除。
2.3 活性生物微球的影响因素分析
1)镍-铁添加量对SSNF去除Cd2+能力的影响。在Cd2+初始质量浓度为300 mg·L−1、pH为7、反应时间为5 d时的反应条件下,通过添加不同质量的镍-铁制备SSNF微球,考察了其对Cd2+去除能力的影响。由图5(a)可见,SSNF对Cd2+的去除率随镍-铁添加量的增加呈先增加后减少的趋势。当镍-铁添加量由0增加到0.3 g时,SSNF对Cd2+的去除率由75.79%增加到99.41%;当继续增加镍-铁时,SSNF对Cd2+的去除率却呈下降的趋势。由此可确定SSNF中镍-铁的最佳添加量为0.3 g。其原因可能是:当镍-铁添加量过少时,其对SRB处理Cd2+的协同促进作用较小,SRB生长密度过大,导致SRB对营养物质的竞争,从而阻碍微生物生长,降低SSNF对Cd2+的去除效果;此外,由于镍-铁具有磁性,会产生团聚现象[25],所以当镍-铁的添加量过多时,会包裹在SRB的表面,减少了SRB与硫酸盐的接触面积,影响微生物的正常生长,从而降低了SSNF对Cd2+的去除率。
2)反应时间对SSNF去除Cd2+的影响。在Cd2+初始质量浓度为300 mg·L−1、pH为7、SSNF微球中镍-铁添加量为0.3 g时,SSNF对Cd2+的去除率随时间变化如图5(b)所示。可以看出,SSNF对Cd2+的去除率呈先增加,然后趋于平衡的变化特征。其吸附过程分为2个阶段:0~1 d为快速去除阶段,去除率达到77.53%;2~5 d为慢速去除阶段,在第5 d基本达到平衡,去除率达到99.98 %。0~1 d时,由于镍-铁具有促进SRB还原硫酸盐的作用, SSNF微球具有足够的反应位点,使其达到快速去除Cd2+的效果;1~5 d时,SRB进入对数生长期和稳定期,由于重金属的毒性作用使得部分菌死亡,而有研究证明,死菌对Cd2+的去除能力显著低于活菌[8],导致SSNF对Cd2+的去除率增长缓慢;5~7 d时,SRB进入衰亡期,镍-铁由于在反应过程中发生氧化反应导致其还原能力下降[18],所以SSNF对Cd2+的去除率基本趋于平衡。因此,SSNF与Cd2+反应的最佳平衡时间为5 d。
3) pH对SSNF去除Cd2+的影响。在Cd2+初始质量浓度为300 mg·L−1、反应时间为5 d时、镍-铁添加量为0.3 g时,不同pH对SSNF去除Cd2+的影响结果见图5(c)。可见,随pH的增加,SSNF对Cd2+的去除率呈先增大后减小的趋势。当pH为4~7时,去除率由88.59%增大到99.31%;当pH为7~9时,去除率由99.31%减小到97.21%。由此可知,当pH为7时,SSNF对Cd2+的去除效果最好。这可能与溶液中的离子与相应的官能团的静电结合作用有关。在较高pH条件下,SSNF对Cd2+的去除能力高于较低pH条件下,因为在较低pH条件,溶液中H+含量较高,其会与Cd2+竞争SSNF的反应位点,同时H+会与SSNF表面的-OH基团结合形成-OH2+,从而使SSNF表面官能团带正电,从而减少SSNF与Cd2+的反应位点[26]。当溶液pH为9时,Cd(OH)2的浓度商大于溶度积常数,说明溶液中存在Cd(OH)2沉淀,所以此时的去除率相较于pH为8时有所提升。在较高pH条件下,SSNF表面的-OH被电离成带负电的氧(−O–),其可排斥能够吸附Cd2+的SSNF上的带负电的基团 [18],从而降低SSNF对Cd2+的去除能力。
4) Cd2+初始浓度对SSNF去除Cd2+的影响。在SSNF中镍-铁添加量为0.3 g、反应时间为5 d、pH为7时,考察了不同的Cd2+初始浓度对SSNF去除能力的影响。如图5(d)所示,随着Cd2+浓度的增加,其去除率呈先平稳后下降的趋势。当Cd2+质量浓度为100~400 mg·L−1时,SSNF对其的去除率几乎达到100%,其最大吸附量达到103.86 mg·g−1;当Cd2+质量浓度为500 mg·L−1时,SSNF对其的去除率下降至95.25%。当Cd2+浓度较低时,SSNF具有足够的反应位点,且容易与Cd2+结合[27],此外,镍-铁可制造SRB适宜生长的厌氧环境,从而形成较高的Cd2+去除率。当Cd2+浓度过高时,SSNF的反应位点逐渐饱和[28],且高浓度的Cd2+会对SRB产生毒性作用,干扰核酸和酶活性位点,SRB的生长受到抑制,导致SSNF对Cd2+的去除率下降[29]。
2.4 SSNF的再利用
活性生物微球经过3次循环再利用后,对Cd2+的去除率和SO42−的消耗情况如图6所示。SSNF对Cd2+始终保持较高的去除率,而SO42−的消耗较最初仅降低了4.4 %。在每个周期结束后,使用硫酸盐还原菌培养基激活SRB,SSNF在下1个周期仍能对Cd2+保持较高的去除能力。这说明,活性微球可以保护SRB,从而降低Cd2+对其产生的毒性作用,同时,不易被氧化的镍-铁仍可促进SRB与Cd2+发生还原反应,从而保持较高的去除能力。在第1个周期后,微球没有发生断裂;但在后2个周期中,微球发生部分断裂,可能造成微珠内的物质泄露,从而降低活性微球对Cd2+和SO42−的去除率。同时,3个周期实验中释放的Ni质量浓度均小于1 mg·L−1。由此可见,SSNF具有良好的循环再利用性能。
2.5 吸附动力学
由图7(b)、图7 (c)和表1可以看出,SSNF对Cd2+的吸附更加符合准二级动力学。其准一级动力学模型和准二级动力学模型的R2分别为0.914和0.998,均大于0.9,但准二级动力学方程所得的理论平衡吸附量qe(88.50 mg·g−1)相比于准一级动力学方程所得的理论平衡吸附量qe(43.14 mg·g−1)更加接近实验所得吸附量(80.40 mg·g−1),说明SSNF对Cd2+的吸附主要受化学吸附控制,而物理吸附作用为辅[19]。颗粒内扩散模型的拟合曲线未通过原点,说明吸附过程不符合颗粒内扩散模型,其吸附过程受其他吸附阶段的共同控制。这可能是因为吸附过程是通过离子交换或共价电子对形成的共价力即化学吸附起主要作用。SRB还原硫酸盐产生的硫化物与重金属形成的金属硫化物沉淀是发生在SSNF外部,所以重金属离子不易进入SSNF内部[24]。所以,SSNF对Cd2+的吸附过程为:第1步是硫酸盐扩散,被SSNF中的SRB还原生成硫化物;第2步是硫化物与Cd2+形成金属硫化物,沉淀到SSNF表面。
表 1 SSNF对Cd2+的动力学模型参数Table 1. Kinetic model parameters of SSNF to Cd2+准一级动力学方程 准二级动力学方程 颗粒内扩散模型 qe/(mg·g−1) K1/d−1 R2 qe/(mg·g−1) K2/(g·(mg·d)−1) R2 C/(mg·g−1) Kp/(mg·(g·d1/2)−1) R2 43.1421 0.129 9 0.914 88.50 0.0113 0.998 47.106 13.626 0.949 3. 结论
1) SSNF对废水中高浓度的Cd2+具有较好的去除能力,镍-铁对SRB具有协同促进作用。SSNF吸附Cd2+的最佳条件为:镍-铁添加量为0.3 g,pH为7,反应时间5 d,Cd2+初始质量浓度为400 mg·L−1.此时Cd2+的去除率达到100%,吸附量为103.86 mg·g−1。
2) SSNF与Cd2+反应前后物质组成的变化说明:反应过程中存在离子交换作用,羧基、羟基、胺基是与Cd2+结合的主要官能团,溶液中的Cd2+转化为CdS沉淀的形式被去除。
3) SSNF吸附Cd2+符合准二级动力学模型,以化学吸附作用为主,物理吸附为辅;离子交换或共价电子可能是SSNF去除Cd2+的主要机制。
-
表 1 SSNF对Cd2+的动力学模型参数
Table 1. Kinetic model parameters of SSNF to Cd2+
准一级动力学方程 准二级动力学方程 颗粒内扩散模型 qe/(mg·g−1) K1/d−1 R2 qe/(mg·g−1) K2/(g·(mg·d)−1) R2 C/(mg·g−1) Kp/(mg·(g·d1/2)−1) R2 43.1421 0.129 9 0.914 88.50 0.0113 0.998 47.106 13.626 0.949 -
[1] ZHOU Q, CHEN Y, YANG M. Enhanced bioremediation of heavy metal from effluent by sulfate-reducing bacteria with copper-iron bimetallic particles support[J]. Bioresour Technology, 2013, 136(5): 413-417. [2] 朱晓丽, 寇志健, 王军强. 生物炭固定化硫酸盐还原菌对Cd2+吸附及作用机制分析[J]. 环境科学学报, 2021, 41(7): 2682-2690. [3] KUMAR R, CHAWLA J, KAUR I. Removal of cadmium ion from wastewater by carbon-based nanosorbents: a review[J]. Journal of Water and Health, 2015, 13(1): 18-33. doi: 10.2166/wh.2014.024 [4] 苗雅慧, 祁诗月, 陈吉. 硫酸盐还原菌在酸性矿山废水处理中的应用[J]. 应用化工, 2021, 50(11): 3074-3078. doi: 10.3969/j.issn.1671-3206.2021.11.034 [5] 徐师, 张大超, 吴梦. 硫酸盐还原菌在处理酸性矿山废水中的应用[J]. 有色金属科学与工程, 2018, 9(1): 92-97. [6] 孟琛, 杨宏, 王少伦. 硫酸盐还原菌包埋固定化及微生物群落分析[J]. 环境工程学报, 2019, 13(8): 1995-2003. [7] 朱煜. 硫酸盐还原菌对重金属污染土壤的处理研究[J]. 环境污染与防治, 2021, 43(8): 952-955. [8] 董净, 代群威, 赵玉连. 硫酸盐还原菌的分纯及对Cd2+钝化研究[J]. 环境科学与技术, 2019, 42(5): 34-40. [9] 姚琪, 黄建洪, 杨磊. 硫酸盐生物还原过程中涉硫组分代谢特性[J]. 环境工程学报, 2018, 12(10): 2783-2790. [10] RAMRAKHIANI L, GHOSH S, MAJUMDAR S. Surface modification of naturally available biomass for enhancement of heavy metal removal efficiency, upscaling prospects, and management aspects of spent biosorbents: A review[J]. Appllied Biochemistry& Biotechnology, 2016, 180(1): 41-78. [11] WANG J, CHEN C. Biosorbents for heavy metals removal and their future[J]. Biotechnology Advances, 2009, 27(2): 195-226. doi: 10.1016/j.biotechadv.2008.11.002 [12] 狄军贞, 王明昕, 赵微. 麦饭石固定化SRB污泥颗粒处理模拟煤矿酸性废水的适应性[J]. 环境工程学报, 2017, 11(7): 3985-3990. [13] 张颖, 李致格, 张磊. 纳米ZrO2-SRB颗粒对铬和氟污染地下水修复的动态实验[J]. 环境工程学报, 2020, 14(9): 2548-2559. [14] 童辉; 乔江涛; 周继梅. 硫酸盐还原菌介导针铁矿表面硫的转化及镉固定脱毒效应[J]. 生态环境学报, 2021, 30(5): 1069-1075. [15] LI X, DAI L, ZHANG C, et al. Enhanced biological stabilization of heavy metals in sediment using immobilized sulfate reducing bacteria beads with inner cohesive nutrient[J]. Journal of Hazardous Materials, 2017, 324: 340-347. doi: 10.1016/j.jhazmat.2016.10.067 [16] 张雅琳, 胡学伟, 夏丽娟. 甘蔗渣为缓释碳源负载SRB处理模拟矿山淋滤水[J]. 环境工程学报, 2016, 10(5): 2355-2360. [17] ZHANG M, WANG H, HAN X. Preparation of metal-resistant immobilized sulfate reducing bacteria beads for acid mine drainage treatment[J]. Chemosphere, 2016, 154: 215-223. doi: 10.1016/j.chemosphere.2016.03.103 [18] WU M, YAN X, LIU K, et al. Application of activated biomaterial in the rapid start-up and stable operation of biological processes for removal cadmium from effluent[J]. Water, Air, & Soil Pollution, 2017, 228(1). [19] WANG N, XU X, LI H, et al. High performance and prospective application of xanthate-modified thiourea chitosan sponge-combined pseudomonas putida and talaromyces amestolkiae biomass for Pb(II) removal from wastewater[J]. Bioresource Technology, 2017, 233: 58-66. doi: 10.1016/j.biortech.2017.02.069 [20] LI Y, LI X, HAN D. New insights into the role of Ni loading on the surface structure and the reactivity of nZVI toward tetrabromo- and tetrachlorobisphenol[J]. Chemical Engineering Journal, 2017, 311: 173-182. doi: 10.1016/j.cej.2016.11.084 [21] HE S, RUAN B, ZHENG Y, et al. Immobilization of chlorine dioxide modified cells for uranium absorption[J]. Environmental Radioactivity, 2014, 137: 46-51. doi: 10.1016/j.jenvrad.2014.06.016 [22] BAI J, YANG X, DU R, et al. Biosorption mechanisms involved in immobilization of soil Pb by bacillus subtilis DBM in a multi-metal-contaminated soil[J]. Journal of Environmental Sciences, 2014, 26(10): 2056-2064. doi: 10.1016/j.jes.2014.07.015 [23] CARPIO I E, MACHADO-SANTELLI G, SAKAnov S K, et al. Copper removal using a heavy-metal resistant microbial consortium in a fixed-bed reactor[J]. Water Research, 2014, 62: 156-166. doi: 10.1016/j.watres.2014.05.043 [24] GOPI KIRAN M, PAKSHIRAJAN K, DAS G. Heavy metal removal from aqueous solution using sodium alginate immobilized sulfate reducing bacteria: Mechanism and process optimization[J]. Journal of Environmental Management, 2018, 218: 486-496. [25] LIANG S, GUO F, DU S, et al. Synthesis of sargassum char-supported Ni-Fe nanoparticles and its application in tar cracking during biomass pyrolysis[J]. Fuel, 2020, 275(C): 117923. [26] YAN G, VIRARAGHAVAN T. Heavy-metal removal from aqueous solution by fungus mucor rouxii[J]. Water Research, 2003, 37(18): 4486-4496. doi: 10.1016/S0043-1354(03)00409-3 [27] LI X, LAN S M, ZHU Z P, et al. The bioenergetics mechanisms and applications of sulfate-reducing bacteria in remediation of pollutants in drainage: A review[J]. Ecotoxicology & Environment Safety, 2018, 158: 162-170. [28] GREENLEE L F, TORREY J D, AMARO R L, et al. Kinetics of zero valent iron nanoparticle oxidation in oxygenated water[J]. Environmental Science & Technology, 2012, 46(3): 12913-12920. [29] 文晓凤, 杜春艳, 袁瀚宇. 改性磁性纳米颗粒固定内生菌Bacillus nealsonii吸附废水中Cd2+的特性研究[J]. 环境科学学报, 2016, 36(12): 4376-4383. -







 下载:
下载: