-
氨法烟气脱硫是目前应用较广泛的湿法脱硫技术之一,被应用于煤化工、钢铁、火电和有色金属、造纸等行业[1-2]。尽管氨法烟气脱硫法所需吸收剂氨水的成本高[3-9],但因其副产物硫酸铵((NH4)2SO4) 价值较高,仍被广泛应用于农业和工业领域[10-12]。在该工艺中,因存在结晶率低、晶核形成差[3]现象,会导致氨法脱硫系统出现分离难、运行不稳定等问题,严重制约了技术应用。实现有效的(NH4)2SO4结晶调控过程以获得晶粒大、数量多、近似棱柱体的(NH4)2SO4晶体,对氨法烟气脱硫技术的应用具有现实意义。
影响氨法烟气脱硫中(NH4)2SO4结晶的因素包括操作条件和杂质引入等[13-18]。其中,烟气杂质,包括有机杂质(如油类、酚类)和无机杂质(飞灰、Al3+、Fe3+、Cl−)[19-26],是影响(NH4)2SO4结晶的重要因素。飞灰是燃烧后烟气的重要组成部分,对(NH4)2SO4结晶的影响至关重要。一些学者关注到飞灰固体质量浓度对氨法烟气脱硫中(NH4)2SO4结晶的影响。徐亚琳等[19]和LIU等[27]发现飞灰固体质量浓度存在一个最佳值,当飞灰固体质量浓度过小时,(NH4)2SO4晶体呈多边形块状,而当飞灰固体质量浓度过大时,(NH4)2SO4晶体呈球状。溶液中存在适量飞灰,可防止大量(NH4)2SO4晶核产生。在实际运行过程中,飞灰的性质(尺寸、组成等)受原料、燃烧工况的影响较大[20]。因此,研究飞灰固体质量浓度及性质对(NH4)2SO4结晶的影响机制具有重要意义。
本研究分别从微观和宏观尺度系统研究了飞灰固体质量浓度、飞灰粒径及飞灰含碳量对(NH4)2SO4结晶晶形及粒径分布的影响,通过平均粒径结合均匀性对(NH4)2SO4结晶进行定量比较,并分析飞灰对(NH4)2SO4结晶的影响机制,以期为氨法烟气脱硫中(NH4)2SO4结晶的有效调控提供参考。
-
实验所用(NH4)2SO4试剂为分析纯(≥ 98.0 %,国药集团化学试剂有限公司),实验用水均为去离子水(电导率<0.1 μS·cm−1),配制成质量分数为33%的(NH4)2SO4溶液备用。
实验所用飞灰均由取自山西省长治市的煤样制备,其工业分析和元素分析如表1所示。将煤样置于烘箱中于(105±3)℃烘干3 h,破碎、筛分后按照煤灰成分分析方法(GB/T 1574-2007),将煤样置于850 ℃的马弗炉(Z-CTM500B,徐州泰瑞仪器设备有限公司)内,通过控制燃烧时间为35、30、25、20 min,分别制得含碳量为1.65%、2.56%、4%、15%的未燃尽飞灰样品,用于模拟电厂实际运行中不同工况下产生的飞灰。
-
减压蒸发结晶装置(图1)主要由恒温水浴锅、循环式真空泵、温度和旋转速率控制器、反应瓶、真空压力表等构成。实验过程包括配置实验溶液、开展结晶实验、抽滤浓缩液和干燥滤饼4个步骤。首先配制质量分数为33%的(NH4)2SO4溶液,通过在 (NH4)2SO4溶液中加入不同含量的飞灰,使(NH4)2SO4溶液中飞灰固体质量浓度分别为400、800、1 200、1 600、2 000 mg·L−1。随后取50 mL溶液放入反应瓶中,通过调整三通阀控制实验真空度为83 kPa,待有大量晶体析出时,关闭真空泵,冷却2 h得浓缩液。再经抽滤和无水乙醇多次洗涤后得到滤饼,将其放入培养皿,在105℃的烘箱中干燥1 h得固体晶体,以此研究飞灰固体质量浓度对硫酸铵结晶的影响。然后通过在(NH4)2SO4溶液中加入不同粒径(≤50 µm、50~75 µm、75~120 µm、≥120 µm)的飞灰,在不改变减压蒸发结晶条件的情况下得到晶体,以此研究飞灰粒径对硫酸铵结晶的影响。最后通过在(NH4)2SO4溶液中加入不同含碳量(0%,1.65%,2.56%,4%,15%)的飞灰,在不改变减压蒸发结晶条件的情况下得到晶体,以此研究飞灰含碳量对硫酸铵结晶的影响。
-
1)微观形貌分析。利用扫描电镜能谱仪(SEM-EDS,JMS-IT500HR,日本)研究固体表面的微观形貌。
2)粒径分析。利用标准筛测定(NH4)2SO4晶体的粒径分布,并基于粒径分布结果分析晶体的平均粒径及均匀性,其均匀性计算见式(1)。
式中
,Y 为晶体的均匀性;Mmax、Mmin 为(NH4)2SO4晶体分布的最大质量分数与最小质量分数;Dmax、Dmin 为(NH4)2SO4晶体的最大粒径与最小粒径。3)溶液pH。利用pH计(FE38,美国梅特勒-托利多公司)测定(NH4)2SO4溶液的pH。
4)晶体矿相组成分析。利用X射线晶体衍射仪(XRD,Bruker D2,德国)对晶体进行矿相组成分析。用步进扫描法记录X射线衍射谱图(2θ =10~80°),并参考ICDD粉末衍射数据库,在衍射图中确定样品中的矿相组成。
-
如图2所示,不同飞灰固体质量浓度条件下,(NH4)2SO4晶体的晶形均呈棱柱状,这表明 (NH4)2SO4溶液中的飞灰固体质量浓度对结晶后晶体的晶形影响不大。然而,随着(NH4)2SO4溶液中飞灰固体质量浓度的增加,(NH4)2SO4晶体晶形的规则性会发生变化,当飞灰固体质量浓度由0增至800 mg·L−1时,(NH4)2SO4溶液结晶后晶体的规则性变化不明显;当飞灰固体质量浓度为1 200 mg·L−1时,(NH4)2SO4溶液结晶后晶体的规则性最强;当飞灰固体质量浓度从1 200 mg·L−1继续增至2 000 mg·L−1时,(NH4)2SO4溶液结晶后晶体的规则性会降低。因此,飞灰对(NH4)2SO4晶体规则性的影响存在最佳值,即1 200 mg·L−1。图2表明,飞灰固体质量浓度为1 200 mg·L−1时(NH4)2SO4溶液结晶后的晶体尺寸最大,这说明(NH4)2SO4晶体的粒径同样会受飞灰固体质量浓度的影响。
如图3所示,不同飞灰固体质量浓度下(NH4)2SO4溶液结晶所得晶体的粒径分布规律大致相同,均为粒径在165~247 µm的(NH4)2SO4晶体所占质量分数最大,分别为41%、54.18%、54.73%、65.45%、47.04%和37.39%。随着飞灰固体质量浓度的增加,粒径大于165 µm的(NH4)2SO4晶体所占质量分数先增加后减小,在飞灰固体质量浓度为1 200 mg·L−1时,较大粒径(NH4)2SO4晶体的质量分数最大。这一宏观定量结果与图2中的微观定性分析结果基本一致。
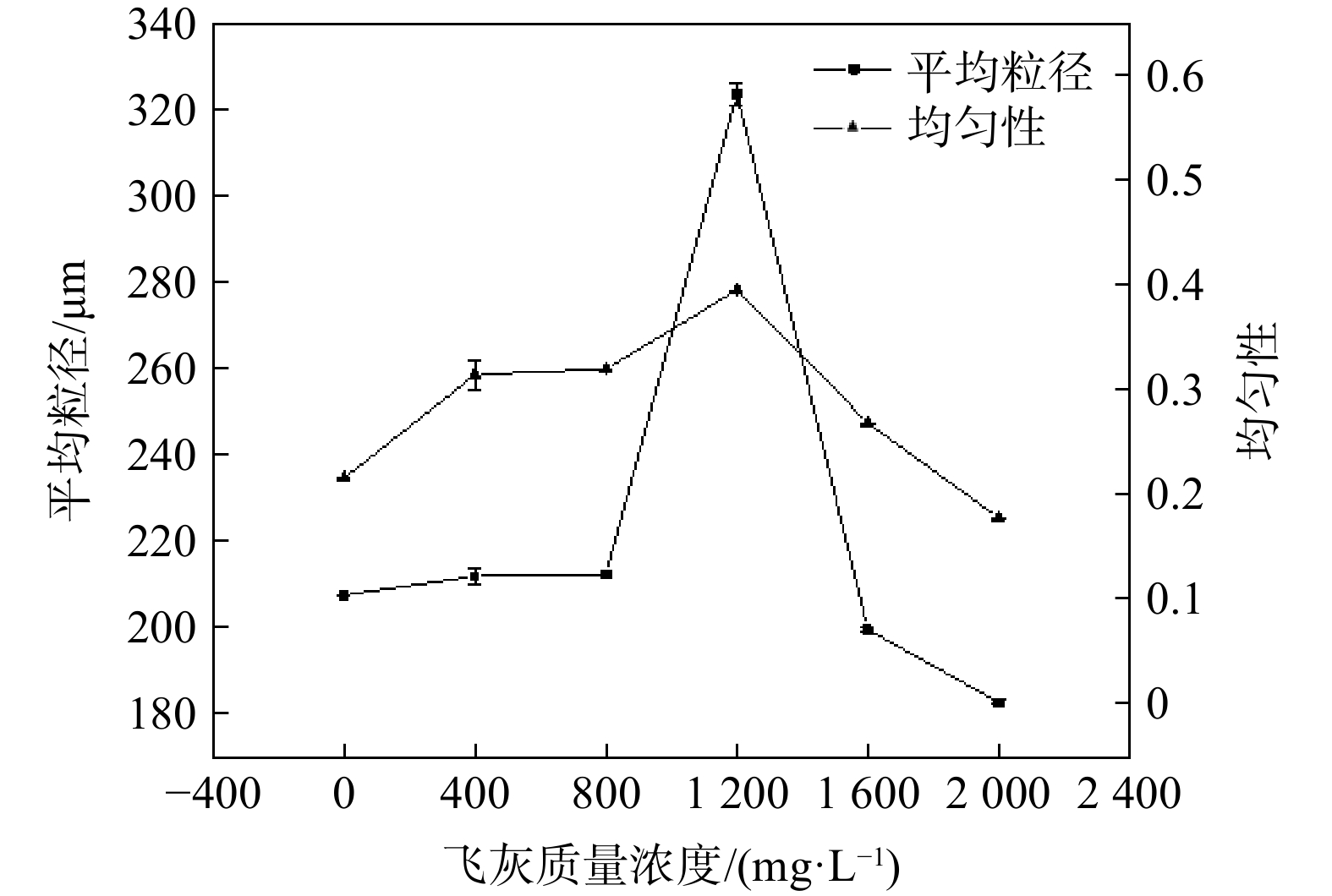
如图4所示,随着(NH4)2SO4溶液中飞灰固体质量浓度的增加,(NH4)2SO4晶体的平均粒径和均匀性亦随之逐渐增加。当飞灰固体质量浓度为1 200 mg·L−1时,(NH4)2SO4晶体的平均粒径增大至约320 µm,均匀性为0.40;随着(NH4)2SO4溶液中飞灰固体质量浓度继续增加,(NH4)2SO4晶体的平均粒径和均匀性呈下降趋势,当飞灰固体质量浓度增至2 000 mg·L−1时,(NH4)2SO4晶体的平均粒径低至180 µm,均匀性低至0.18。这表明适量飞灰的存在(1 200 mg·L−1)有利于(NH4)2SO4晶体平均粒径的提高和均匀性的增加。
-
在实际工业生产中,燃烧前燃料的入炉粒径、燃烧中燃料的燃烧工况和燃烧后烟尘的分离效率都直接影响进入氨法脱硫工序中飞灰的粒径。如图5所示,不同飞灰粒径条件下,(NH4)2SO4晶体的晶形均呈棱柱状,这表明飞灰粒径对(NH4)2SO4结晶后晶体的晶形影响不大。然而,随着(NH4)2SO4溶液中飞灰粒径的增大,(NH4)2SO4晶体晶形的规则性会增加,但其规则性仍不及图2(d)中全粒径飞灰下的硫酸铵晶形。SEM结果亦表明,在不同飞灰粒径下,随着(NH4)2SO4结晶后晶体规则性的变化,(NH4)2SO4晶体的粒径也发生相应变化。
图6表明,对于不同飞灰粒径,(NH4)2SO4溶液结晶后晶体在124~165 µm分布的质量分数最高,分别为36.3 %、35.61 %、36.11 %和40.67 %,这与图3中全粒径飞灰下(NH4)2SO4晶体粒径分布质量分数最高的165~247 µm相比略小,亦表明全粒径飞灰条件下更有利于大粒径(NH4)2SO4晶体的形成。
图7表明,随着飞灰粒径由小于50 μm增至大于120 μm,(NH4)2SO4晶体的平均粒径不断增加。当飞灰粒径为120 μm时,平均粒径最大,约为260 µm,均匀性约为0.495。这与图4中1 200 mg·L−1全粒径飞灰条件下得到的(NH4)2SO4晶体相比,平均粒径仍相对较小,这与图6所示结果一致。
-
在实际工业生产中,受燃烧工况的影响,飞灰中常存在一定量的残碳。若燃烧工况控制良好,飞灰中残碳常低于4%;但若燃烧工况控制不当,飞灰中残碳可能大于10%。图8表明,当飞灰固体质量浓度为1 200 mg·L−1时,不同飞灰含碳量条件下,(NH4)2SO4晶体的晶形均呈现棱柱状。这表明飞灰含碳对(NH4)2SO4结晶后晶体的晶形影响不大。但需要注意的是,飞灰中不含碳或含有高于4%的碳,均有利于(NH4)2SO4晶体规则性的提高。
图9表明,当飞灰固体质量浓度增至2 000 mg·L−1时,不同残碳量条件下(NH4)2SO4晶体均呈不规则的棱柱状,残碳量的增加使得(NH4)2SO4晶体逐渐趋于规则。该结果进一步证实了2.1节中报告的过多飞灰的存在不利于(NH4)2SO4结晶规则的结果,亦说明飞灰固体质量浓度对(NH4)2SO4晶形的影响高于飞灰含碳量。
图10表明,在飞灰固体质量浓度为1 200 mg·L−1时,不同飞灰含碳量条件下(NH4)2SO4溶液结晶所得晶体中粒径分布为65%~70%所对应的粒径均为165~247 µm。粒径≥247 µm的晶体质量分数随飞灰含碳量的增加,先降低后升高。这一宏观粒径随飞灰含碳量的变化规律与图8显示的微观形貌的规则性变化规律一致。
图11表明,随着飞灰含碳量由0增加至1.65 %,(NH4)2SO4晶体的平均粒径由323 µm降低至283 µm,均匀性由0.39增至0.43;当飞灰含碳量由1.65%增至4%时,(NH4)2SO4晶体的平均粒径由283 µm增至293 µm,当飞灰含碳量为15%时,(NH4)2SO4晶体的平均粒径继续增至303 µm,均匀性为0.39。该结果与图10中显示的粒径分布结果一致,亦说明少量碳的存在会增加小粒径(NH4)2SO4晶体生成的可能性。当碳含量较高时,大粒径(NH4)2SO4晶体开始生成,均匀性有所降低。
-
图12表明,随着飞灰固体质量浓度、粒径及含碳量的改变,(NH4)2SO4溶液结晶后产物晶体的衍射峰位置基本一致。这表明飞灰理化性质的变化对(NH4)2SO4晶体的矿相组成影响较小。随着飞灰固体质量浓度由0增至2 000 mg·L−1,(NH4)2SO4溶液结晶后晶体各晶面的匹配生长会发生变化。其中,当飞灰固体质量浓度为1 200 mg·L−1时,(020)面及(040)面与其他晶面的生长更为匹配。在低飞灰固体质量浓度下,(020)面及(040)面的过快生长与高飞灰固体质量浓度下(200)面的生长抑制均不利于规则(NH4)2SO4晶体的生成。这可能与少量飞灰晶核对特定晶面生长的促进和过量飞灰晶核对晶面遮挡抑制晶面生长有关。飞灰粒径的增加可显著促进(NH4)2SO4晶体(020)面与(040)面的生长,但无论何种粒径,均无法实现(020)面、(040)面与其他晶面的合理匹配。这也是2.2节中不同粒径飞灰无法获得较理想(NH4)2SO4晶形的主要原因。随着飞灰含碳量由0增至2.56 %,(NH4)2SO4晶体的(020)面、(040)面、(200)面均受到明显抑制,不利于规则(NH4)2SO4晶形的形成;当飞灰含碳量继续增加时,对(NH4)2SO4晶体(020)面、(040)面、(200)面的抑制作用有所缓解。
通过以上结果,可推断飞灰对(NH4)2SO4结晶的影响机制。如图13所示,(NH4)2SO4结晶主要分为晶核形成和晶核长大两个过程,成核过程又分为均相成核和异相成核,其中异相成核是以溶液中的杂质颗粒为结晶核心。飞灰的存在会增加异相成核的机率,当(NH4)2SO4溶液中含有适量飞灰时,(NH4)2SO4分子以飞灰晶核为核心聚集生长。当溶液中飞灰质量浓度过高时,飞灰晶核的增多会导致附着在每个飞灰晶核表面的(NH4)2SO4减少,进而抑制 (NH4)2SO4晶体的长大。飞灰的含量、粒径及含碳量不仅会影响异相成核过程,还可能通过对特定晶面的促进或抑制影响到(NH4)2SO4晶体的进一步生长,从而影响到(NH4)2SO4晶体的宏观粒径分布。
-
1)适量飞灰对(NH4)2SO4的结晶有促进作用。当(NH4)2SO4溶液中飞灰固体质量浓度为1 200 mg·L−1时,(NH4)2SO4晶体的晶形最规则,此时晶体的平均粒径最大(320 µm),均匀性最高(0.40)。
2)飞灰的粒径、含碳量均会对(NH4)2SO4结晶产生影响,粒径的增大可适当提高(NH4)2SO4晶体的晶形规则性、平均粒径和均匀性,但全粒径飞灰下(NH4)2SO4溶液结晶后的晶体更为理想;飞灰中不含碳或含有高于4 %的碳,均有利于(NH4)2SO4晶体的晶形规则性、平均粒径的提高,但均匀性会略有下降。飞灰固体质量浓度对硫酸铵结晶的影响高于飞灰粒径或飞灰含碳量。
3)飞灰对硫酸铵结晶的影响主要源于飞灰的存在会增加异相成核的机率,可通过对飞灰晶核数量的影响,进一步影响到(NH4)2SO4晶面的生长,从而影响到(NH4)2SO4晶体的粒径和均匀性。
飞灰对氨法烟气脱硫中硫酸铵结晶的影响机制
Effect mechanism of fly ash on crystallization of (NH4)2SO4 in flue gas desulfurization by ammonia process
-
摘要: 燃烧后烟气中飞灰的存在,对氨法烟气脱硫中硫酸铵((NH4)2SO4)结晶的影响至关重要。系统研究了飞灰固体质量浓度、粒径及含碳量对(NH4)2SO4结晶晶形、粒径大小和均匀性的影响,并分析了其影响机制。结果表明,当(NH4)2SO4溶液中飞灰固体质量浓度为1 200 mg·L−1时,(NH4)2SO4晶体的晶形最规则,晶体的平均粒径最大(320 µm),均匀性最高(0.40);飞灰粒径的增大对 (NH4)2SO4晶体的晶形规则性、平均粒径和均匀性的提高均有一定作用,飞灰中不含碳或含有高于4 %的碳,均有利于(NH4)2SO4晶体的晶形规则性、平均粒径的提高,但均匀性会略有下降。飞灰对(NH4)2SO4结晶的影响机制为:飞灰晶核影响(NH4)2SO4晶核的形成和生长,进而影响(NH4)2SO4晶面生长,从而影响(NH4)2SO4晶体的尺寸和均匀性。本研究可为氨法烟气脱硫中(NH4)2SO4结晶的有效调控提供参考。Abstract: The fly ash in the flue gas is very important to the crystallization of (NH4)2SO4 in ammonia flue gas desulfurization. In this paper, the effects of content, particle size and carbon content of fly ash on the crystal shape and particle size distribution of (NH4)2SO4 were studied systematically, and the effect mechanism was analyzed. The results showed that when the content of fly ash in (NH4)2SO4 solution was 1 200 mg·L−1, the crystal shape of (NH4)2SO4 crystals was the most regular, the average particle size of (NH4)2SO4 crystals was the largest (320 µm), and the uniformity was the highest (0.40). The increase of particle size could improve crystal shape, particle size and uniformity of (NH4)2SO4 crystals. When the fly ash contained no carbon or more than 4% carbon content it was helpful for improving crystal shape and particle size of (NH4)2SO4 crystals and was disadvantageous for the uniformity of (NH4)2SO4 crystals. The influence mechanism was that the formation of fly ash nucleus affected the formation and growth of (NH4)2SO4 crystal nucleus, and then affected the growth of (NH4)2SO4 crystal plane, and thus affected the size and uniformity of (NH4)2SO4 crystal.
-
Key words:
- ammonia flue gas desulfurization /
- (NH4)2SO4 /
- fly ash /
- effect mechanism
-
表 1 煤样的工业分析和元素分析
Table 1. Industrial and elemental analysis of coal sample
样品 工业分析 元素分析 Mar Aar Var FCar Cd Hd O d * Nd Sd 煤样 0.65% 8.97% 12.39% 77.99% 53.7% 2.82% 29.86% 1.07% 2.93% 注:*表示O通过差减法计算得出;ar—收到基;d—干燥基;M—水分;A—灰分;V—挥发分;FC—固定碳。 -
[1] 李承泉, 肖婷. 氨法脱硫烟气治理技术[J]. 煤炭加工与综合利用, 2017(2): 59-61. [2] 张全斌, 周琼芳. 中国燃煤烟气脱硫技术回顾与展望[J]. 科技创新与生产力, 2020(5): 27-32. [3] 李露, 黄帮福, 张桂芳, 等. 氨法脱硫副产物硫酸铵蒸发结晶研究与进展[J]. 现代化工, 2020, 40(4): 36-40. [4] 申林艳, 李文彦, 陈军, 等. 氨法烟气脱硫技术及几个问题的探讨[J]. 煤矿现代化, 2005(6): 60-61. doi: 10.3969/j.issn.1009-0797.2005.06.039 [5] 柏顺, 袁泽华. 燃煤排放烟气脱硫技术概述[J]. 化工管理, 2017(13): 77-78. doi: 10.3969/j.issn.1008-4800.2017.13.050 [6] 陈一铭. 燃煤电厂烟气脱硫技术探讨[J]. 低碳世界, 2016(7): 4-5. [7] 崔名双, 周建明, 张鑫, 等. 燃煤工业锅炉烟气脱硫技术及经济性分析[J]. 洁净煤技术, 2019, 25(5): 131-137. [8] 陈欢哲, 何海霞, 万亚萌, 等. 燃煤烟气脱硫技术研究进展[J]. 无机盐工业, 2019, 51(5): 6-11. [9] 董继红, 李占印, 吴俊, 等. 燃煤电厂烟气脱硫技术综述[J]. 化工设计, 2017, 27(6): 3-6. doi: 10.3969/j.issn.1007-6247.2017.06.002 [10] 艾玉春, 张永春, 宁运旺. 氨法脱硫副产品硫酸铵的农业应用前景[J]. 江苏农业科学, 2018, 46(23): 308-313. doi: 10.15889/j.issn.1002-1302.2018.23.077 [11] 周绿山, 明大增, 李志祥, 等. 硫酸铵的应用研究进展[J]. 化学工程师, 2013, 27(5): 60-62. doi: 10.3969/j.issn.1002-1124.2013.05.020 [12] 张云峰, 白健, 高志娟, 等. 硫酸铵在煤粉炉粉煤灰提取氧化铝活化工艺过程中的应用研究[J]. 轻金属, 2018(8): 14-18. [13] HU X T, ZHU T, JIANG X D, et al. A pilot investigation on oxidation of ammonium sulfite by streamer corona plasma[J]. Chemical Engineering Journal, 2007, 139(3): 469-474. [14] WANG L D, WU S Y, LIU S, et al. Cobalt impregnated porous catalyst promoting ammonium sulfate recovery in an ammonia-based desulfurization process[J]. Chemical Engineering Journal, 2018, 331: 416-424. doi: 10.1016/j.cej.2017.08.127 [15] 顾晓婧, 何仁初, 万皓, 等. 氨法脱硫中亚硫酸铵的氧化工艺参数[J]. 上海大学学报(自然科学版), 2015, 21(6): 701-708. [16] JIA Y, ZHONG Q, FAN X. Kinetics of oxidation of total sulfite in the ammonia-based wet flue gas desulfurization process[J]. Chemical Engineering Journal, 2010, 164(1): 132-138. doi: 10.1016/j.cej.2010.08.041 [17] DING H L GAO X, DU Z. Gas-liquid absorption reaction between (NH4)2SO3 solution and SO2 for ammonia-based wet flue gas desulfurization[J]. Applied Energy, 2010, 87(8): 2647-2651. doi: 10.1016/j.apenergy.2010.03.023 [18] JIA Y, YIN L G, XU Y L. A model for performance of sulfite oxidation of ammonia-based flue gas desulfurization system[J]. Atmospheric Pollution Research, 2015, 6(6): 997-1003. doi: 10.1016/j.apr.2015.05.005 [19] LIU X, ZHONG Q, WANG J. Study on ammonium sulfate crystallization in the ammonium desulphurization process in a coal-based power plant in the petrochemical industry[J]. Energy Sources, 2011, 33(21-24): 2027-2035. [20] PANT A, PARSONS M T, BERTRAM A K. Crystallization of aqueous ammonium sulfate particles internally mixed with soot and kaolinite: crystallization relative humidities and nucleation rates[J]. Journal of Physical Chemistry A, 2006, 110(28): 8701-8709. doi: 10.1021/jp060985s [21] 许小静, 张圆圆, 郑鹏艳, 等. 氨法脱硫中杂质对硫酸铵结晶的影响[J]. 煤炭转化, 2020, 43(6): 78-86. [22] RAULS A M, BARTOSCH B K, KIND C M, et al. The influence of impurities on crystallization kinetics – a case study on ammonium sulfate[J]. Journal of Crystal Growth, 2000, 213(1/2): 116-128. [23] OATIS S, IMRE D, MCGRAW R. Heterogeneous nucleation of a common atmospheric aerosol: Ammonium sulfate[J]. Geophysical Research Letters, 2013, 25(24): 4469-4472. [24] 高毅颖, 张懿, 王静康, 等. 不同类型媒晶剂对硫酸铵晶体形态学指标的影响[J]. 化工学报, 2011, 62(12): 3575-3579. doi: 10.3969/j.issn.0438-1157.2011.12.040 [25] BUCHFINK R, SCHMIDT C, ULRICH J. Fe3+ as an example of the effect of trivalent additives on the crystallization of inorganic compounds, here ammonium sulfate[J]. Crystengcomm, 2011, 13(4): 1118-1122. doi: 10.1039/C0CE00107D [26] SHIM K M, KIM J W, KOO K K. Molecular interaction of solvent with crystal surfaces in the crystallization of ammonium sulfate[J]. Journal of Crystal Growth, 2013, 373(15): 64-68. [27] 徐亚琳, 刘新亚, 殷李国, 等. 氨法烟气脱硫副产物硫酸铵蒸发结晶实验研究[J]. 应用化工, 2016, 45(11): 2039-2042. doi: 10.16581/j.cnki.issn1671-3206.2016.11.010 -





 下载:
下载: