-
随着我国餐饮、养殖、制药、食品和皮革等产业的发展,产生了大量的高氨氮废水,如果这些废水排入河流湖泊和地下水,会导致水体富营养化[1-2]。对于此类高氨氮废水,传统的硝化反硝化技术需要大量曝气,在碳氮比较低的情况下还需额外补充大量碳源,产生大量的经济成本。此外,传统的硝化反硝化技术脱氮效率较低,需要较长的水力停留时间(hydraulic retention time, HRT)和较大的占地面积,还会产生大量剩余污泥[3-4]。因此,亟需突破传统脱氮技术运行能耗高、脱氮效率低、占地面积大、剩余污泥产量多等技术瓶颈,开发一种节能、高效、省地和产泥量少的快速脱氮处理技术。厌氧氨氧化(anammox)是迄今最高效节能的脱氮方式[5-6],可以在不加碳源的条件下实现自养高负荷脱氮,污泥产量低。然而,厌氧氨氧化菌(anAOB)的自养性和生长缓慢增加了污水处理工艺的启动期[7]。因此,加快anAOB的生长速率,实现anammox工艺的快速启动及高效脱氮,对于anammox的推广应用及污水处理运行节能减排具有重要的现实意义。
目前文献中报道的anAOB的倍增周期为10~20 d,anAOB的缓慢生长导致反应器启动时间较长[8]。全球第一座大规模anammox污水处理厂于2002年在鹿特丹建成并投入运行,启动耗时3.5 a,比预期的2 a要长[9]。anAOB的缓慢生长速度和底物的抑制作用以及控制复杂等使得anammox工艺实际应用受到限制[10]。以前的研究通常在反应器中接种厌氧污泥[11]、硝化污泥[12]、反硝化污泥[13],甚至anammox絮凝污泥[14],但由于群落中anammox丰度较低,这些策略通常必须在低进水负荷下启动,导致启动时间更长。显然,尽管anammox工艺已经研究了20多年,但启动周期长仍是其实际应用的主要障碍[15-16]。
Anammox颗粒污泥相对于絮体污泥具有较强的环境适应能力和脱氮性能[17-18],将其作为接种污泥将有助于anammox的启动。此外,贾方旭等[13]关于anAOB与其他细菌之间的协同竞争关系的研究表明,anAOB与硝化菌和反硝化菌在不同条件下存在竞争和协同关系。然而,利用anAOB与其他菌群之间的协同作用加速anammox启动的研究则相对较少,同时硝化菌和反硝化菌相对于anAOB更容易获得。因此,本研究的重点是接种少量成熟anammox颗粒污泥到含有硝化污泥和反硝化污泥的反应器,以验证接种少量anammox颗粒污泥,利用菌群协同机制是否可实现anammox工艺快速启动,并通过氮素转化和氮平衡的计算分析了anammox反应器的脱氮性能,同时采用高通量技术分析了anammox反应器中微生物群落丰度的变化规律。
-
采用人工配制的模拟废水作为进水,模拟废水的NH3-N、
NO−2 -N和无机碳源使用NH4Cl、NaNO2、NaHCO3按需配置,其他组分包括27.2 mg·L−1 KH2PO4、135.92 mg·L−1 CaCl2·2H2O、300 mg·L−1 MgSO4·7H2O、570 mg·L−1KCl以及1 mL·L−1微量元素I和微量元素II。微量元素I包括5 g·L−1 EDTA和5 g·L−1 FeSO4·7H2O;微量元素II包括15 g·L−1 EDTA、0.014 g·L−1 H3BO4、0.845 g·L−1 MnCl2·4H2O、0.25 g·L−1 CuSO4·5H2O、0.43 g·L−1 ZnSO4·7H2O、0.19 g·L−1 NiCl2·6H2O、0.16 g·L−1 NaSeO4·10H2O、0.22 g·L−1 NaMoO4·2H2O、0.24 g·L−1 CoCl2·6H2O[19]。接种污泥取自实验室培养的anammox种泥、反硝化污泥和硝化污泥,其悬浮固体质量浓度为(mixed liquor suspended solids, MLSS)分别为8 553、6 834和6 532 mg·L−1,挥发性悬浮固体质量浓度(mixed liquid volatile suspended solids, MLVSS)分别为7 095、5 331和5 265 mg·L−1。 -
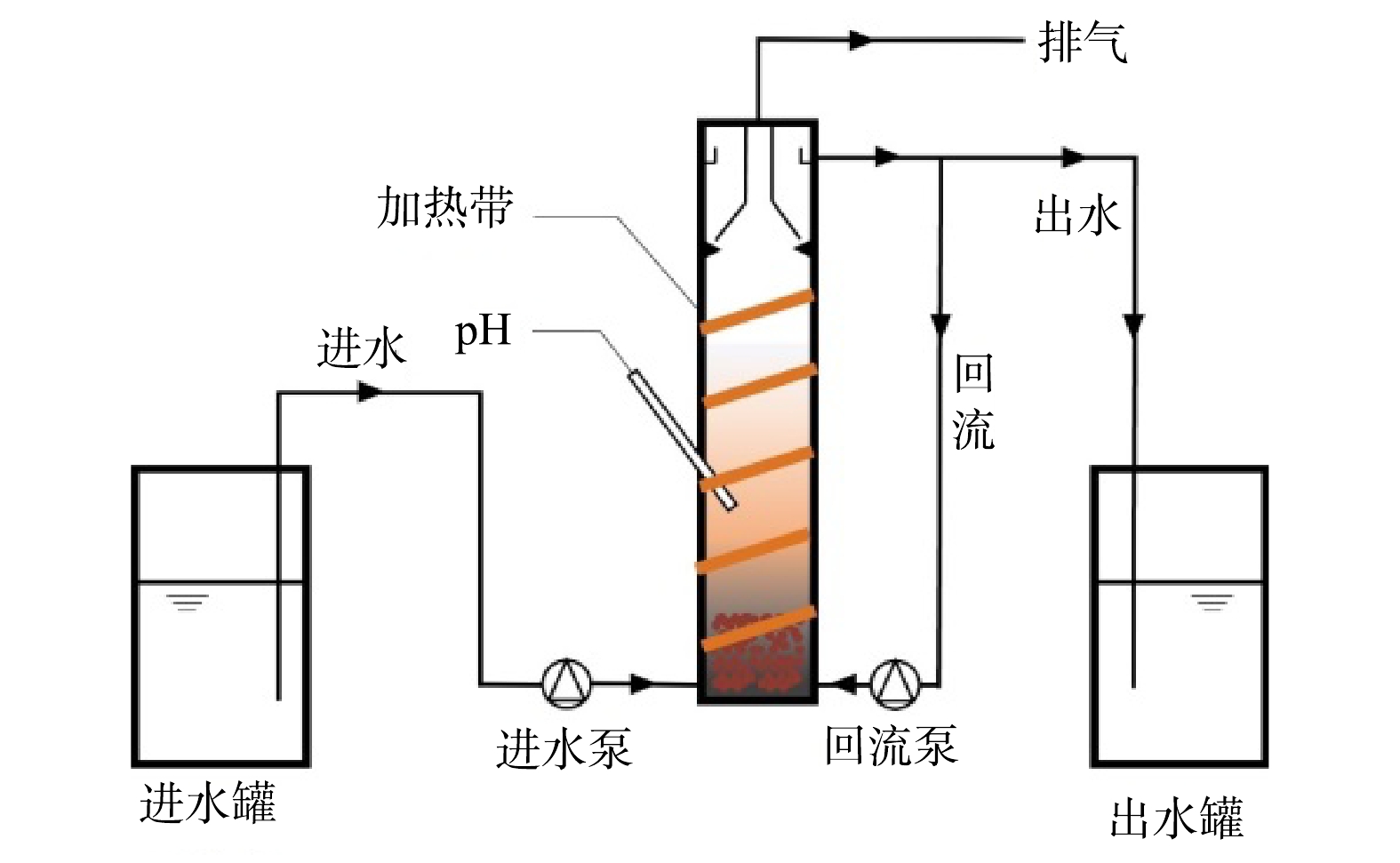
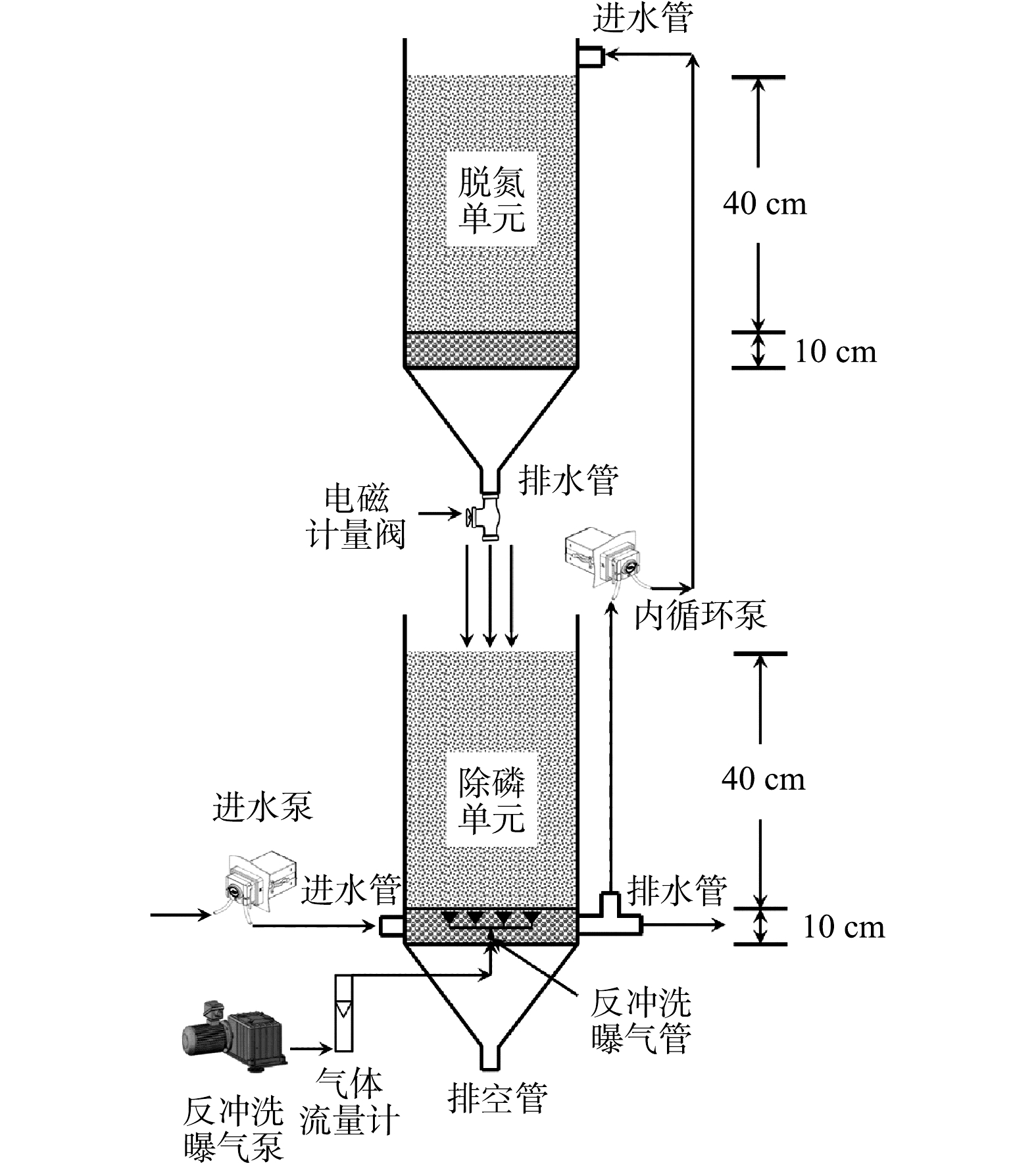
本研究所用的实验装置如图1所示。实验所用的上流式厌氧污泥床反应器(up-flow anaerobic sludge bed,UASB),反应器由圆柱形有机玻璃制成,内径为7 cm,高为30 cm,工作体积为1 L。反应器内混合液温度通过外置加热带控制,由底部进水,上部溢流出水,底部设置有回流管,部分出水可通过回流管进入反应器内,可通过调整回流液的流量调整反应器回流比(reflux ration, R)。
-
本研究设置3组实验:A组只接种 500 mg·L−1的anammox污泥,作为空白组;B组接种500 mg·L−1的anammox污泥和5 000 mg·L−1的反硝化污泥;C组接种500 mg·L−1的anammox污泥和5 000 mg·L−1的硝化污泥(污泥质量浓度均以MLSS计)。在整个实验期间,控制3组反应器温度为(35±1) ℃,为了消除pH对反应器性能的影响,通过投加30 g·L−1碳酸氢钠溶液调节系统pH至7.5~8.0。实验分为3个阶段:阶段I(1~54 d),主要考察底物质量浓度对反应器脱氮效果的影响;阶段II(55~96 d),主要考察在高负荷条件下,调整HRT和回流比对反应器脱氮效果的影响;阶段III(97~116 d),通过降低进水负荷考察其对反应器脱氮性能恢复的影响。不同阶段的运行参数见表1。每天取进出水水样并检测水质变化。
-
MLSS、MLVSS、NH3-N、
NO−2 -N和NO−3 -N用国标法测定[20];COD使用快速测定仪(连华5B-3C,连华科技)测定;DO使用便携式溶解氧仪(HACH-HQ30,哈希水质分析仪器(上海)有限公司)测定和pH使用pH计(FE20K,梅特勒-托利多国际贸易(上海)有限公司)测定;反应器中的游离氨(FA)和游离亚硝酸(FNA)的质量浓度分别按照式(1)和式(2)进行计算[21]。式中:
CFA 为游离氨质量浓度,mg·L−1;CFNA 为游离亚硝酸质量浓度,mg·L−1;CNH3−N 为氨氮质量浓度,mg·L−1;CNO−2−N 为亚硝酸盐质量浓度,mg·L−1;T为温度,℃。 -
取3组反应器中不同阶段混合液各 50 mL,8 000 r·min−1下离心去除上清液,剩余固体用于微生物分析。依据 E.Z.N.A.®soil DNA kit 对样品进行 DNA 抽提,并使用凝胶电泳和NanoDrop2000检测提取DNA的质量和纯度;使用338F(5’-ACTCCTACGGGAGGCAGCAG-3’) 和806R(5’-GACTACHVGGGTWTCTAAT-3’) 对16S rRNA基因V3~V4可变区进行PCR扩增。PCR扩增程序为:首先在95 ℃预变性3 min,然后在95 ℃变性30 s,在55 ℃退火30 s,在72 ℃延伸30 s,并执行27个循环,然后在72 ℃稳定延伸10 min,最后在4 ℃进行保存。每个样本3个重复。将同一样本的PCR扩增产物混合后使用2%琼脂糖凝胶回收,利用AxyPrep DNA Gel Extraction Kit 进行回收产物纯化,2%琼脂糖凝胶电泳检测,并用Quantus™ Fluorometer 对回收产物进行检测定量。最后通过Miseq PE300平台进行测序。
-
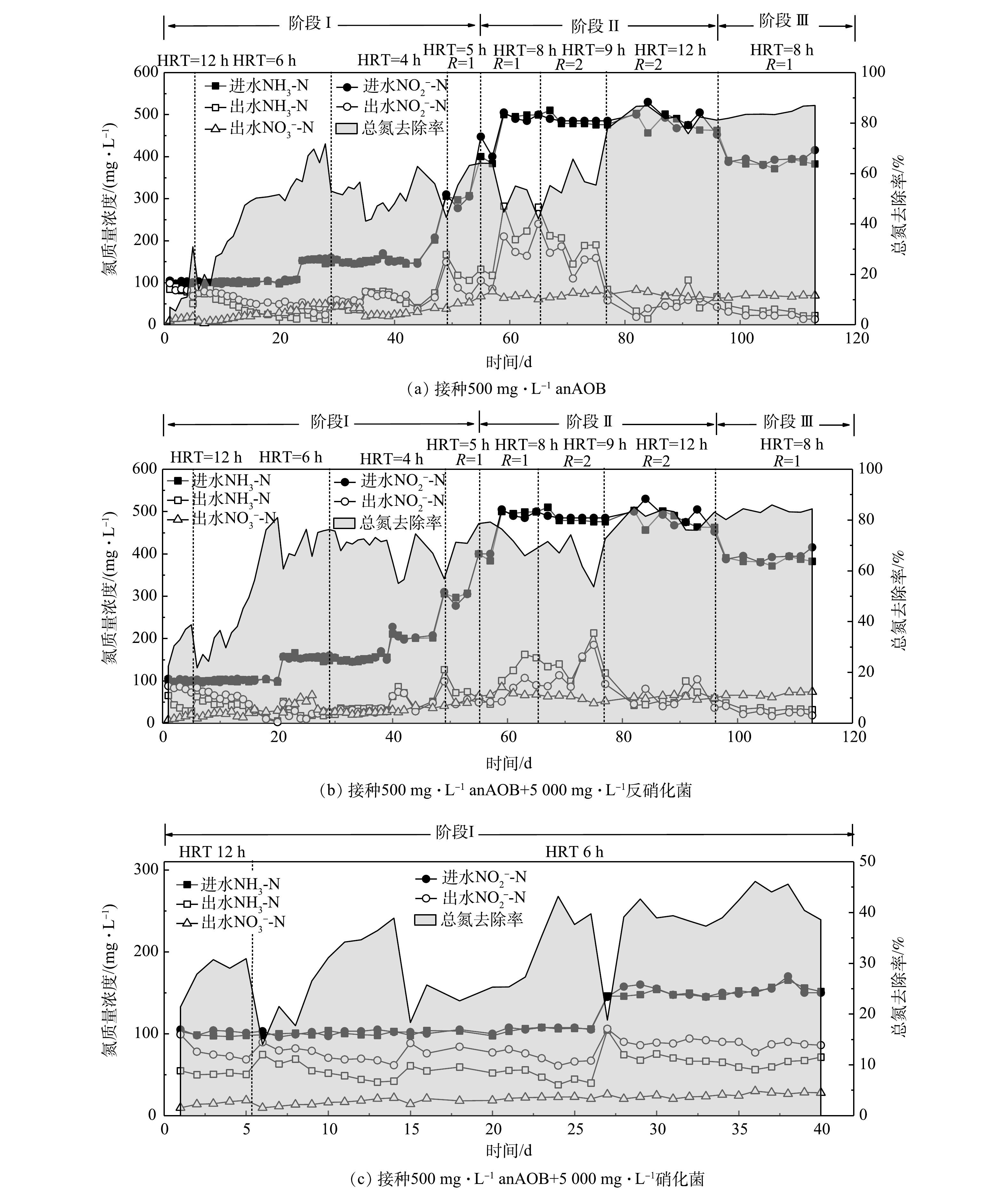
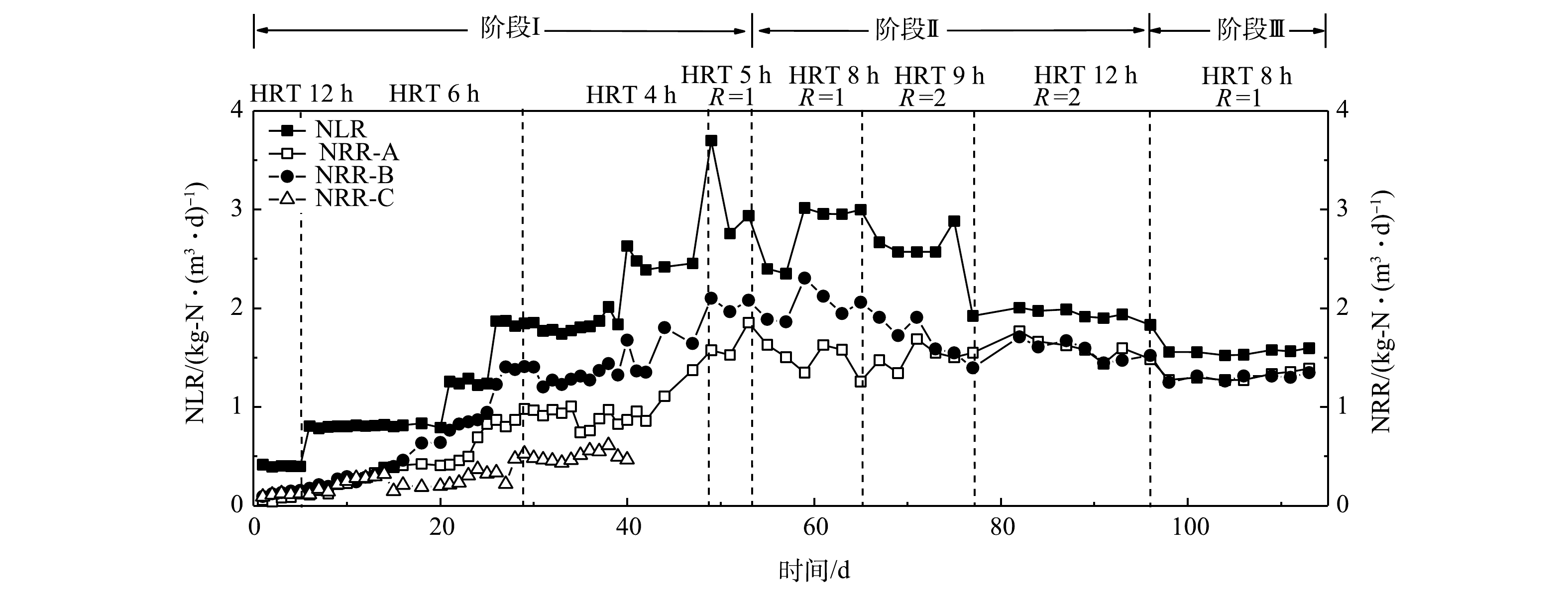
3个反应器的进出水氮素变化如图2所示。在第I阶段,在进水负荷相同的条件下,A组和B组的TN去除率随时间延长逐渐升高,在进水总氮质量浓度由200 mg·L−1提高至600 mg·L−1的过程中,2组的TN去除率整体呈现出逐渐升高的趋势。其中,B组的TN去除率最高,平均去除率可达70%左右;A组的TN平均去除率约为60%。这说明反硝化菌的存在有利于促进系统TN的去除。图3反映了3组反应器在脱氮过程中的总氮负荷率和总氮去除效率(NRR)的变化。在第I阶段(0~54 d),在相同的NLR下,B组表现出最高的总氮去除效率,启动30 d后,B组的NRR达到了1.41 kg·(m3·d)−1,相对于A组(0.97 kg·(m3·d)−1)提高了31.2%。这同样说明反硝化菌的存在有利于TN的去除。XU等 [22]关于anammox污泥中anAOB和其他菌之间关系的研究结果也表明,反硝化菌Denitratisoma 的存在可提高anAOB活性。由此可推测,通过接种反硝化菌和anAOB可提高anAOB的活性。相对于A组和B组,C组的TN去除率最低(图2),仅有40%左右,且在后期没有升高的趋势,C组的NRR仅有0.48 kg·(m3·d)−1,低于空白组A组(50.5%)。这可能是由于硝化菌与anAOB存在底物竞争,不利于anAOB的富集增长,这与贾方旭等[8]的研究结果相似。因此,C组运行40 d后停止运行,第II和III阶段主要对比A组和B组的脱氮效果。
在第II阶段(55~96 d),升高进水NH3-N和
NO−2 -N质量浓度至500 mg·L−1,A组和B组的出水NH3-N和NO−2 -N波动较大。将HRT从8 h延长到9 h,同时升高回流比R为2,然而出水水质依然有波动,B组较A组波动较小;延长HRT为12 h后,出水水质有所好转,但90 d后依然有上升趋势。这充分证明,当进水总氮质量浓度升至1 000 mg·L−1后,导致系统脱氮效果变差,也说明过高的进水负荷抑制anAOB活性。李媛[23]也做了类似的研究,当进水总氮质量浓度由200 mg·L−1升高到600 mg·L−1、HRT由8 h缩短到6 h时,NRR迅速下降,NRR由1.2 kg·(m3·d)−1下降到1.03 kg·(m3·d)−1。在第III阶段(97~116 d),为了恢复系统的脱氮性能,提高系统的稳定性,分别降低进水NH3-N和
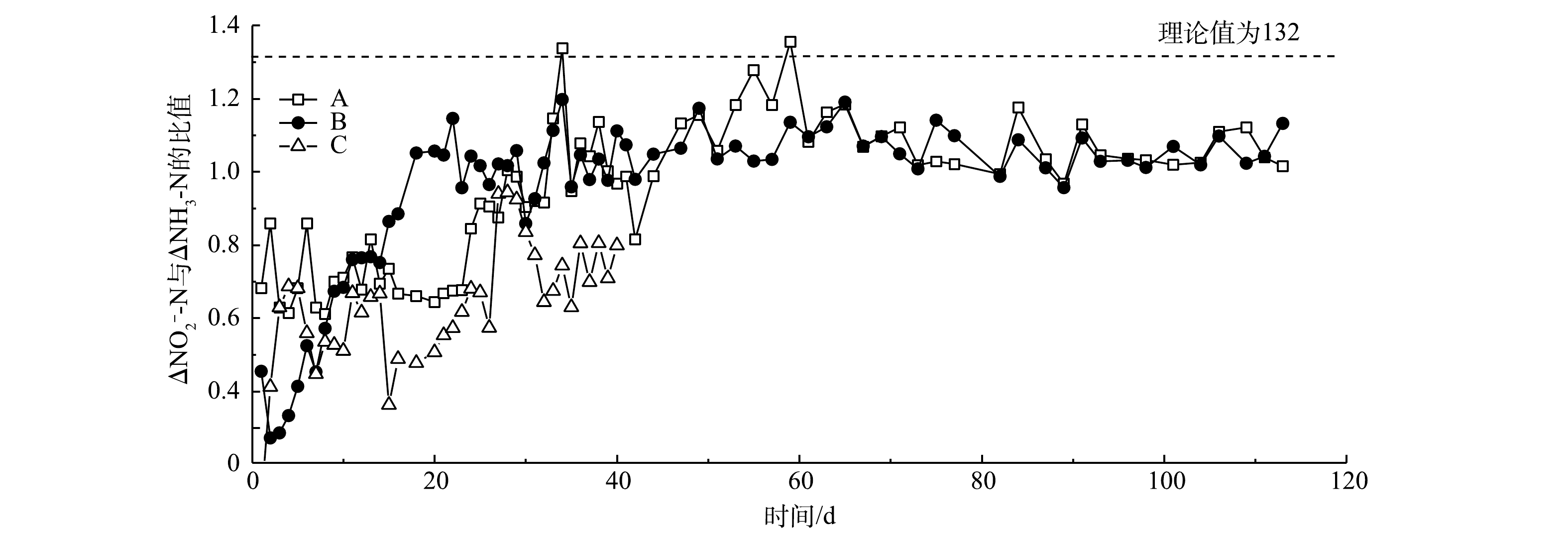
NO−2 -N质量浓度至400 mg·L−1,系统出水基本稳定,A组和B组的TN去除率稳定在80%以上,NRR维持在1.3 kg·(m3·d)−1左右。这说明通过降低进水负荷能够缓解对anAOB的抑制,有助于系统脱氮性能的恢复。图4反映了3组反应器脱氮过程中
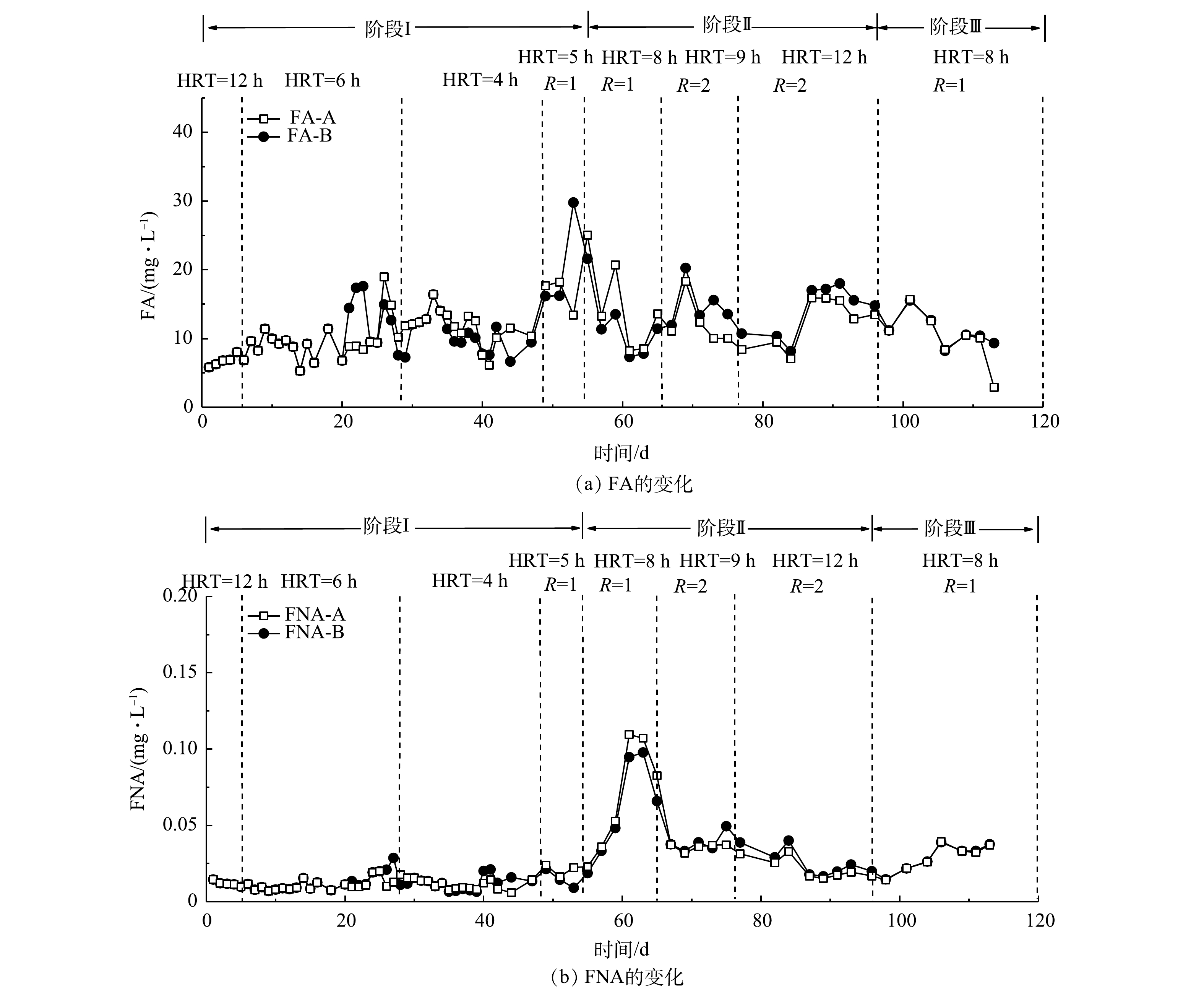
NO−2 -N去除量(△NO−2 -N)与NH3-N去除量(△NH3-N)的比值变化。A、B和C 3组反应器中NO−2 -N去除量与NH3-N去除量比例开始呈上升趋势,后续趋于稳定,基本保持在1.1左右。由厌氧氨氧化反应方程[24-25]可知,在厌氧氨氧化稳定运行时,NO−2 -N去除量与NH3-N去除量的理论比值为1.32。本研究的NO2−-N去除量与NH3-N去除量的比值与理论值差异较小。其原因为:一是由于进水中含有一定量的溶解氧,会发生亚硝化和硝化作用,导致少量NH3-N转化为NO−2 -N或者NO−3 -N;二是菌群在富集过程中可能会释放氧化剂[26]。有研究表明,在低浓度厌氧氨氧化菌培养条件下,系统由于菌群浓度降低会产生氧化剂(超氧化物或羟基自由基),导致中NH3-N的消耗量增高[27]。B组的NO−2 -N去除量与NH3-N去除量比例在20 d后基本保持稳定,而A组在30 d后才趋于稳定,C组持续表现出较大波动。这充分说明反硝化菌的存在有利于anammox的启动,使anammox在最短的时间内在反应器内起主导作用,而硝化菌与anAOB存在底物竞争,从而导致C组NO−2 -N去除量与NH3-N去除量比例持续处于较低水平,并且有较大波动,对于anammox的启动具有抑制作用。游离氨(FA)和游离亚硝酸(FNA)的质量浓度对于anammox过程具有很大的影响[20]。为了评估FNA和FA对anammox过程的影响,本研究计算了A组和B组anammox过程的FA和FNA,探究高负荷进水条件下anammox受抑制的机理。图5反映了A组和B组脱氮过程中FA和FNA的变化。A组和B组在整个anammox脱氮过程中FA的质量浓度低于30 mg·L−1。已有研究[22]表明,控制FA的质量浓度低于50 mg·L−1对于anammox没有抑制作用。而在第II阶段,FNA的质量浓度已经达到0.05 mg·L−1以上,甚至超过了0.1 mg·L−1。anammox过程FNA质量浓度超过0.05 mg·L−1对于anAOB具有明显的抑制作用[19, 28]。这充分说明,当进水
NO−2 -N质量浓度达到500 mg·L−1时,会导致FNA的质量浓度升高,当FNA的质量浓度超过0.05 mg·L−1时,会抑制anAOB的活性。通过降低进水NO−2 -N浓度,可使FNA低于0.05 mg·L−1,进而使系统逐渐恢复。 -
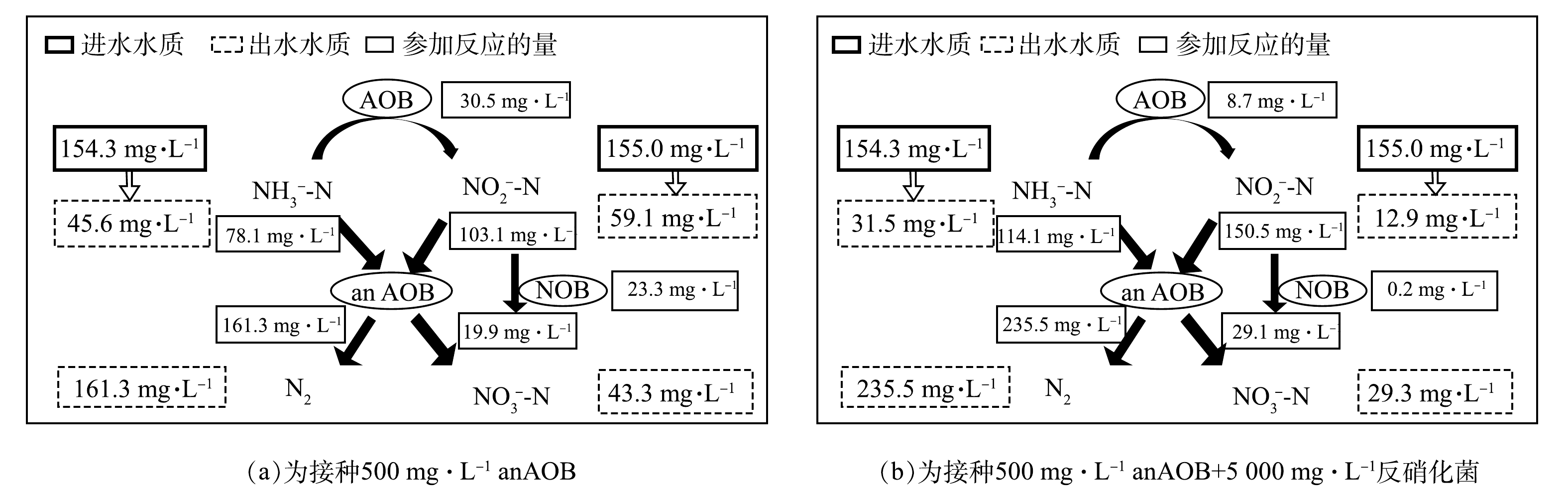
为了进一部分解析A组和B组2种启动方式对anammox启动的差异以及氮素转化的规律,通过对启动30 d后A组和B组的进出水氮素指标核算系统的氮素转化,结果如图6所示。在进水NH3-N为154.3 mg·L−1,在
NO−2 -N为155.0 mg·L−1的条件下,A组的总氮去除率达到52.2%,B组的总氮去除率达到76.2%,2组的总氮去除均是通过anammox实现的,B组比A组提高了24%。这充分说明,反硝化菌的存在有利于提高anAOB的活性。此结果与已有的研究结果一致[20]。此外,A组有30.5 mg·L−1的NH3-N被氨氧化菌氧化成NO−2 -N,同时有23.3 mg·L−1的NO−2 -N被亚硝酸盐氧化菌氧化成了NO−3 -N。而B组中有仅有8.7 mg·L−1的NH3-N被氨氧化菌氧化成NO−2 -N,0.2 mg·L−1的NO−2 -N被亚硝酸盐氧化菌氧化成了NO−3 -N,硝化反应的占比远低于anammox。该结果说明,B组可为anAOB提供较好的启动环境,更大程度的避免硝化菌等其他杂菌的影响,使anAOB最短的时间在反应器内起主导作用,实现快速启动。 -
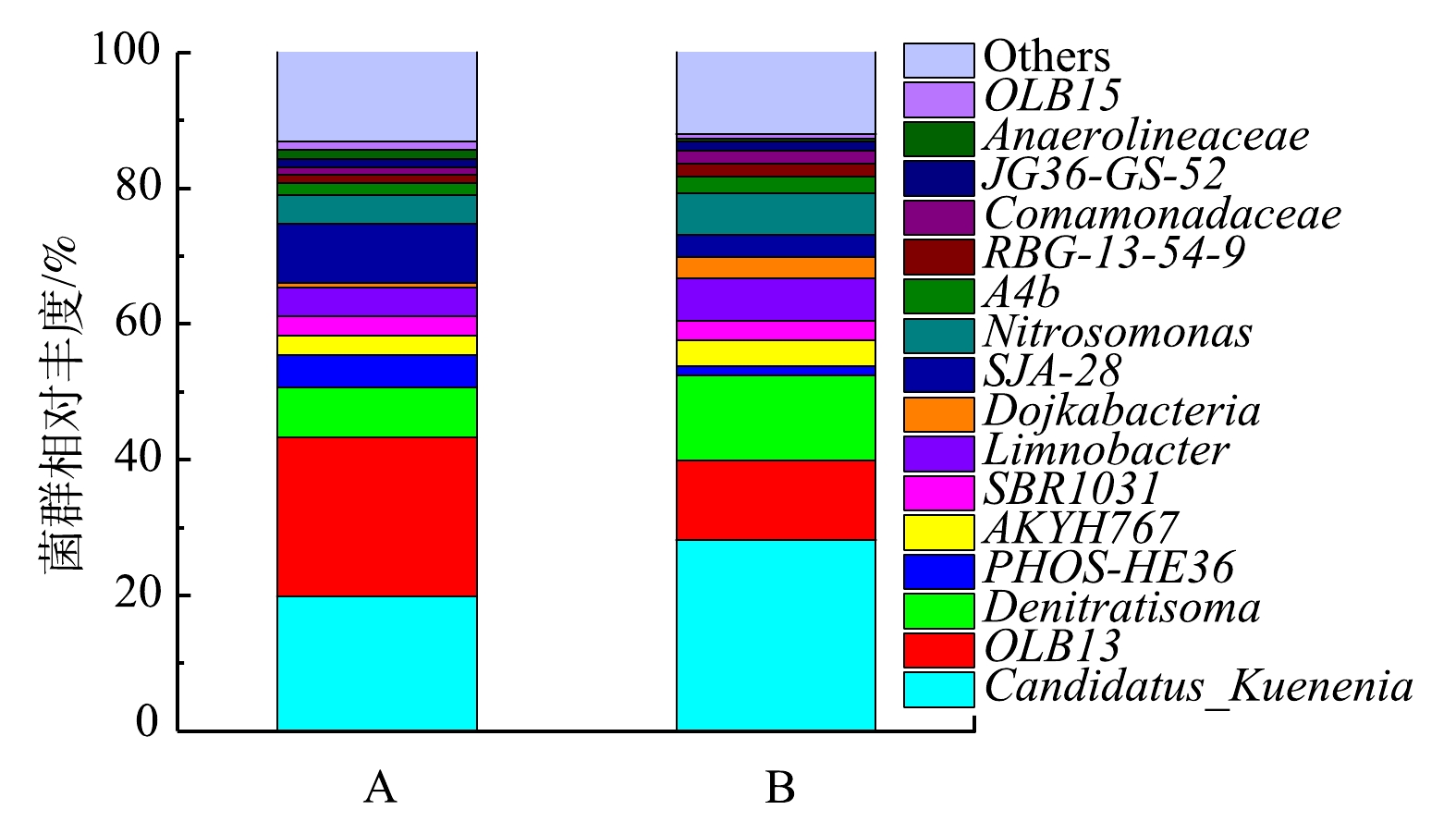
反应器anammox的启动过程是anAOB富集的过程。为了进一部分解析A组和B组接种方式对anAOB菌群丰度的影响,将A组和B组启动30 d后,取样进行高通量测序。细菌种群在属水平上的相对丰度如图7所示。2组反应器中主要的菌属为Candidatus Kuenenia, OLB13和Denitratisoma。其中Candidatus Kuenenia是anAOB,在A组中的丰度占比为19.8%,在B组中的丰度占比为28.1%,相对于A组提高了41%。这说明B组的接种方式有利于anAOB菌群丰度的提升,从而促进anAOB的活性。这也充分印证了在第2.1节中的推测。Denitratisoma是常见的反硝化菌属[29],在A组和B组中的丰度占比分别为7.3%和12.6%。ZHANG等[30]的研究表明,Candidatus Kuenenia 的相对丰度与Denitratisoma 的相对丰度趋势具有一定的正相关性,说明 Candidatus Kuenenia与Denitratisoma 有很强的正相互作用,侧面验证了Denitratisoma的存在有利于体现Candidatus Kuenenia的活性。OLB13属于厌氧菌,是厌氧消化的核心微生物种群之一[31-32]。已有研究[12]表明,脱氮反应系统中OLB13和Denitratisoma的存在对于Candidatus Kuenenia 的活性表达具有积极作用。因此,通过反硝化菌和anAOB的同时接种可以为anAOB提供一种较好的增殖环境,从而有利于提高anAOB丰度,促进反应器anammox脱氮效率的提升。
-
1)向反应器中投加5 000 mg·L−1反硝化污泥(以MLSS计)和500 mg·L−1 anammox污泥(以MLSS计)进行接种,可以实现anammox的快速启动。启动30 d以后,总氮去除负荷可达1.41 kg·(m3·d)−1以上。
2)进水
NO−2 -N质量浓度达到500 mg·L−1时,会导致FNA质量浓度超过0.05 mg·L−1,从而严重影响anAOB的活性,进而导致反应器出水水质波动;通过降低进水NO−2 -N质量浓度可以使系统恢复。3)本研究所用的脱氮系统中,主导anammox的anAOB为Candaditue Kuenenia,与单独接种anammox污泥相比,接种反硝化污泥和anammox污泥可使Candaditue Kuenenia的丰度提高40.0%,从而使反应器脱氮效率提高了31.2%。
低接种量条件下实现厌氧氨氧化快速启动的策略
Strategy on fast start-up of anaerobic ammonia oxidation under low inoculation conditions
-
摘要: 厌氧氨氧化(Anaerobic ammonia oxidation, anammox)是目前最高效节能的脱氮方式,可以在不加碳源的条件下实现自养高负荷脱氮。然而,厌氧氨氧化菌(Anaerobic ammonia oxidizing bacterial, anAOB)因其生长缓慢,会导致污水处理工艺的启动周期较长。因此,为缩短anammox的启动周期,设置了3种不同污泥接种方式(A:接种500 mg·L−1 anAOB; B:接种500 mg·L−1 anAOB+5 000 mg·L−1反硝化菌;C:接种500 mg·L−1 anAOB+5 000 mg·L−1硝化菌)开展anammox启动实验,分析了底物浓度对脱氮效果的影响以及不同接种条件下微生物群落的差异。结果表明,采用向反应器投加500 mg·L−1 anAOB+5 000 mg·L−1反硝化菌的接种方式,可以实现anammox的快速启动;启动30 d后,总氮去除效率(nitrogen removal rate, NRR)可达1.41 kg·(m3·d)−1以上。微生物群落分析结果表明,反应器中主要存在的anAOB为Candaditue Kuenenia;与单独接种anammox污泥相比,接种反硝化污泥和anammox污泥会使Candaditue Kuenenia的相对丰度提高了40.0%,从而使脱氮效率提高了31.2%。底物浓度对anammox过程的影响结果表明,进水
NO−2 -N质量浓度达到500 mg·L−1时会导致游离亚硝酸(FNA)浓度升高,而当FNA质量超过0.05 mg·L−1时,会严重影响anAOB活性,导致反应器出水水质波动;通过降低进水NO−2 -N浓度,可以使系统恢复。以上研究结果说明,通过接种反硝化菌和anAOB的方式可实现anammox的快速启动,加速实现anammox工艺在污水处理中大规模应用。Abstract: Anaerobic ammonia oxidation (anammox) was by far the most efficient and energy-saving method of nitrogen removal, which achieved autotrophic and high-load nitrogen removal without adding carbon sources. However, the slow growth characteristic of anaerobic ammonia oxidizing bacteria (anAOB) prolonged the start-up period of wastewater treatment process. Therefore, in order to shorten the start-up cycle of anammox, three sludge inoculation methods (A inoculation with 500 mg·L−1 anAOB; B inoculation with 500 mg·L−1 anAOB+5 000 mg·L−1 denitrifying bacteria; C inoculation with 500 mg·L−1 anAOB+5 000 mg·L−1 nitrifying bacteria) were set up to conduct experiment of anammox. The effect of substrate concentration on the nitrogen removal and the difference of microbial community under different inoculation conditions were analyzed. The results indicated that the method of inoculating low-concentration anammox sludge (500 mg·L−1) and denitrification sludge (5 000 mg·L−1) achieved a rapid start of anammox, and the nitrogen removal rate (NRR) exceeded 1.41 kg·(m3·d)−1 after 30 days. The result of microbial analysis showed that the main anammox bacteria in the reactor was Candidatus Kuenenia. Comparing with the inoculation with anammox sludge alone, the abundance of Candaditue Kuenenia in the inoculation with denitrification sludge and anammox sludge increased by 40.0%, thereby the nitrogen removal efficiency increased by 31.2%. The result of the effect of substrate load showed that when the concentration ofNO−2 -N reached 500 mg·L−1, the concentration of free nitrous acid (FNA) increased, FNA concentration exceeding 0.05 mg·L−1 would seriously affect the activity of anAOB and caused the unstable quality of effluent. The system could be restored by reducing the nitrite nitrogen concentration of influent. These results indicated the rapid start of anammox could be achieved by inoculating denitrifying bacteria and anAOB, which will accelerate the large-scale application of the anammox process in sewage treatment.-
Key words:
- anaerobic ammonia oxidation (anammox) /
- fast start-up /
- inoculation /
- nitrogen removal /
- microorganism
-
提高人工快速渗滤(constructed rapid infiltration,CRI)系统的脱氮除磷性能是确保该工艺高效处理城镇生活污水的关键[1-2]。在诸多研究中,强化CRI系统中基于亚硝化的全程自养脱氮(completely autotrophic nitrogen removal over nitrite,CANON)作用被认为是提高该工艺脱氮效果的重要途径,此技术随之用于城镇生活污水处理[3]。截至目前,有学者相继开展了常温和低
NH+4 NH4+ 作为另一种备受关注的生物脱氮除磷新技术,反硝化除磷(denitrifying phosphorus removal,DPR)工艺可消耗有机碳源,并可发挥“一碳两用”的功能,使除磷和反硝化在缺氧环境下同时完成[9]。有研究结果初步证实,反硝化除磷耦合ANAMMOX作用的生物同步脱氮除磷工艺具备高效处理市政污水的潜力[10-11],如能在CRI系统中实现CANON作用与DPR作用的耦合,则CANON型CRI工艺的上述缺陷便有可能得到弥补。
本研究尝试构建了基于同步短程硝化、ANAMMOX、反硝化和反硝化除磷(simultaneous partial nitrification,ANAMMOX,denitrification and denitrifying phosphorus removal,SNADPR)作用的复合式人工快速渗滤(hybrid constructed rapid infiltration,H-CRI)系统,考察和探究了该系统的运行效能及微生物特性,而后对其中氮磷元素的归趋进行了解析。期望通过此研究,可探寻有效措施以弥补CANON型CRI工艺在处理城镇生活污水时的缺陷,提高其脱氮除磷性能及其稳定性,进而推动新型生物同步脱氮除磷工艺的研发及应用。
1. 材料与方法
1.1 实验装置
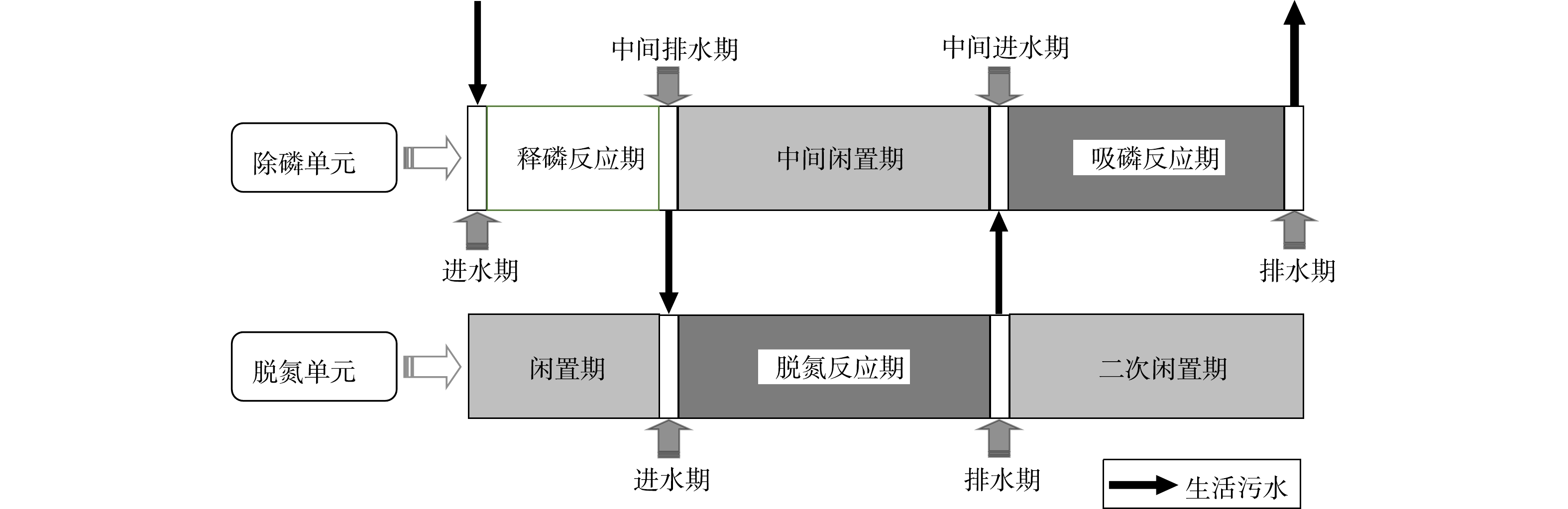
SNADPR型H-CRI装置构型见图1。
前期实验分别构建了CANON型CRI系统和DPR型CRI系统[12-13]。其中:CANON型CRI系统由PVC管制成,表面积约314 cm2(φ=20 cm),其填料层厚度为50 cm且孔隙率约为37%:底部为5 cm厚砾石承托层,粒径为1~3 cm;上部为45 cm厚沸石层,粒径为5~10 mm。装置顶部和底部分别设有进水管和出水管,出水管附有计量阀控制装置的排水速率(vd)。前期研究中,当vd为0.5 L·min−1时,系统中的CANON作用得以强化,其TN和
NH+4 NO−3 NO−x SNADPR型H-CRI系统由CANON型CRI装置(标记为脱氮单元)和DPR型CRI装置(标记为除磷单元)耦合而成(图1),即两单元上下堆叠,并设置3台泵控制其运行。其中,1台为进水泵,与除磷单元的进水管相连;1台为内循环泵,分别与除磷单元的排水管和脱氮单元的进水管相连,可将前者出水泵入后者;1台为反冲洗曝气泵,与除磷单元的反冲洗曝气管相连。此外,本研究另设置1套CANON型CRI系统作为对照组,用于和SNADPR型H-CRI系统进行对比。
1.2 运行方式
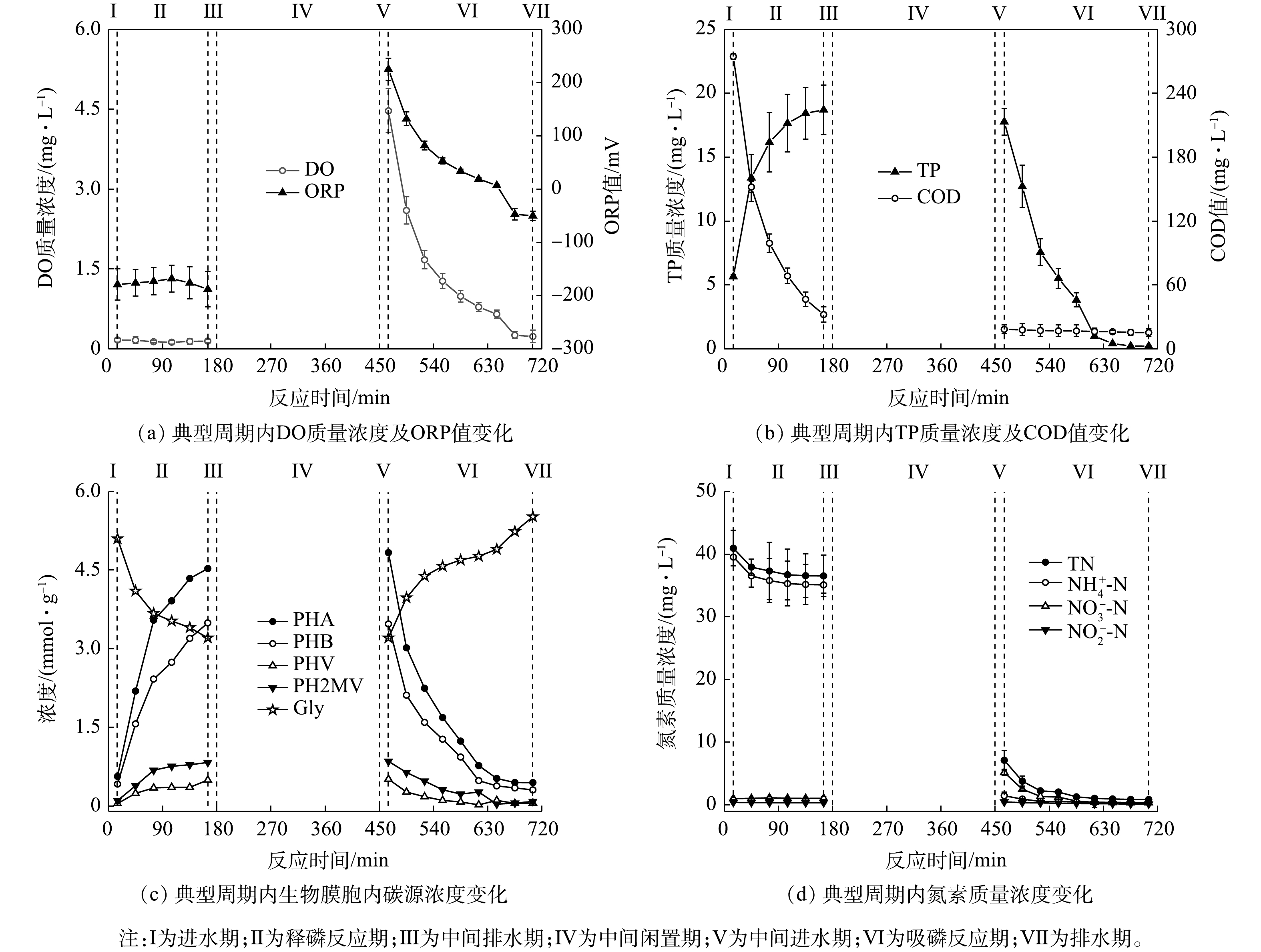
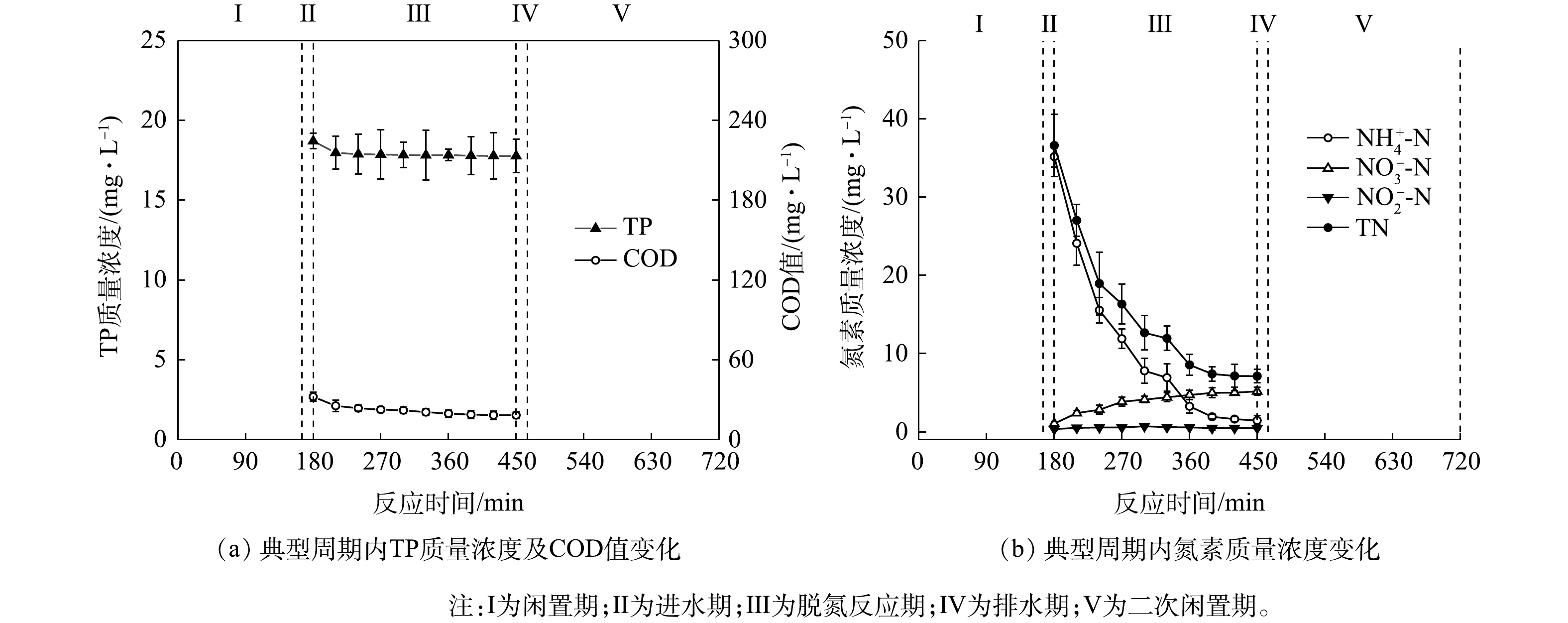
考虑到好氧氨氧化菌(AOB)、厌氧氨氧化菌(AnAOB)、反硝化菌和反硝化聚磷菌(DPAOs)等功能微生物各自的生理生化特性,需对SNADPR型H-CRI系统进行空间或时间上的分割与交替,才能确保上述功能微生物的高效协同。为此,将该系统按照内循环潮汐流模式连续运行150个周期(图2),每个周期时长为12 h。运行方式为:将5 L进水泵入除磷单元中,使其进入释磷反应期,期间脱氮单元处于闲置期;随后,内循环泵将污水自除磷单元泵入脱氮单元中,使后者进入脱氮反应期,期间除磷单元处于中间闲置期;待脱氮单元的脱氮反应期结束后,此单元中的污水通过排水管以0.5 L·min−1的排水速率跌入除磷单元中,则除磷单元进入吸磷反应期,而脱氮单元则进入二次闲置期;最后,除磷单元将处理后的污水排出系统。按照时间顺序,上述典型周期内除磷单元的运行过程依次标记为进水期(时长为15 min)、释磷反应期(时长为150 min)、中间排水期(时长为15 min)、中间闲置期(时长为270 min)、中间进水期(时长为15 min)、吸磷反应期(时长为240 min)和排水期(时长为15 min)7个阶段;脱氮单元的运行过程依次为闲置期(时长为165 min)、进水期(时长为15 min)、脱氮反应期(时长为270 min)、排水期(时长为15 min)和二次闲置期(时长为255 min)5个阶段。H-CRI系统的污水处理量为10 L·d−1,水力负荷(HLR)为0.18 m3·(m2·d)−1。
对照组在实验阶段以潮汐流模式亦连续运行了150个周期,每个周期时长为12 h,包括进水期(时长为15 min)、淹水期(时长为270 min)、排水期(时长为15 min,vd=0.5 L·min−1)和闲置期(时长为420 min)4个阶段。该系统的污水处理量为10 L·d−1,HLR为0.32 m3·(m2·d)−1。
1.3 反冲洗操作
每周对H-CRI系统中的除磷单元进行反冲洗,以去除其中老化的生物膜,维持DPAOs活性并确保填料层的孔隙率。采用气-水联合反冲洗方式对此单元进行反冲洗:先对其单独气洗4 min,而后气-水联合反冲洗5 min,最后漂洗9 min。此过程中水洗和气洗的强度分别为6 L·(m2·s)−1和12 L·(m2·s)−1。清洗液和脱落的生物膜通过该单元的排空管进行收集。
1.4 进水水质
实验用水为安徽农业大学园区内生活污水,将其初沉后的上清液作为2组装置进水,其耗氧有机物(以COD计)、TN、
NH+4 NO−3 NO−2 1.5 分析方法
1)水样采集及分析方法。每天采集装置进出水水样;当各系统稳定运行时,在其典型周期内实时采集填料层中的水样。水样中COD、TN、
NH+4 NO−3 NO−2 2)填料样品采集。在实验阶段末采集2组实验装置中的填料样品,样品编号分别标记S1(取自对照组)、S2(取自H-CRI系统的脱氮单元)和S3(取自H-CRI系统的除磷单元)。
3)脱氮除磷性能测定。对于S1和S2,其亚硝酸化活性(PPNA)、硝酸化活性(PNA)、反硝化活性(PDA)、短程反硝化活性(PBDA)及厌氧氨氧化比活性(SAA)分别参照文献[15-16]中方法进行测定;对于S3,其生物膜中PHA、PHB、PHV、PH2MV和糖原(Gly)含量的测定参照文献[17],DPAOs占PAOs比例(即DPAOs/PAOs)的测定参照文献[18]。填料样品中全氮和全磷含量的测定方法均参照文献[19]。
4)基于16S rDNA的Illumina平台高通量测序。获取上述填料样品表面的生物膜,而后将其送至上海美吉生物科技医药公司进行高通量分析测序。测序分析后,根据Barcode序列区分各个样本的数据,进行嵌合体过滤,得到可用于后续分析的有效数据,即Clean reads。为了研究样品的物种组成多样性,对所有样品的Clean reads进行聚类,以97%的一致性(identity)将序列聚类成OTUs (operational taxonomic units),然后对OTUs的代表序列进行物种注释。
1.6 数据处理
采用SPSS 22.0对试验数据进行统计分析;采用one-way ANOVA进行方差分析;采用Origin 2018作图,图中相关数据为平均值±标准差;文中污染物去除(转化)率(量)、累积率(量)等的计算方法均参照文献[20];实验装置中氮磷去除途径的解析方法参考文献[21]。
2. 结果与讨论
2.1 运行性能
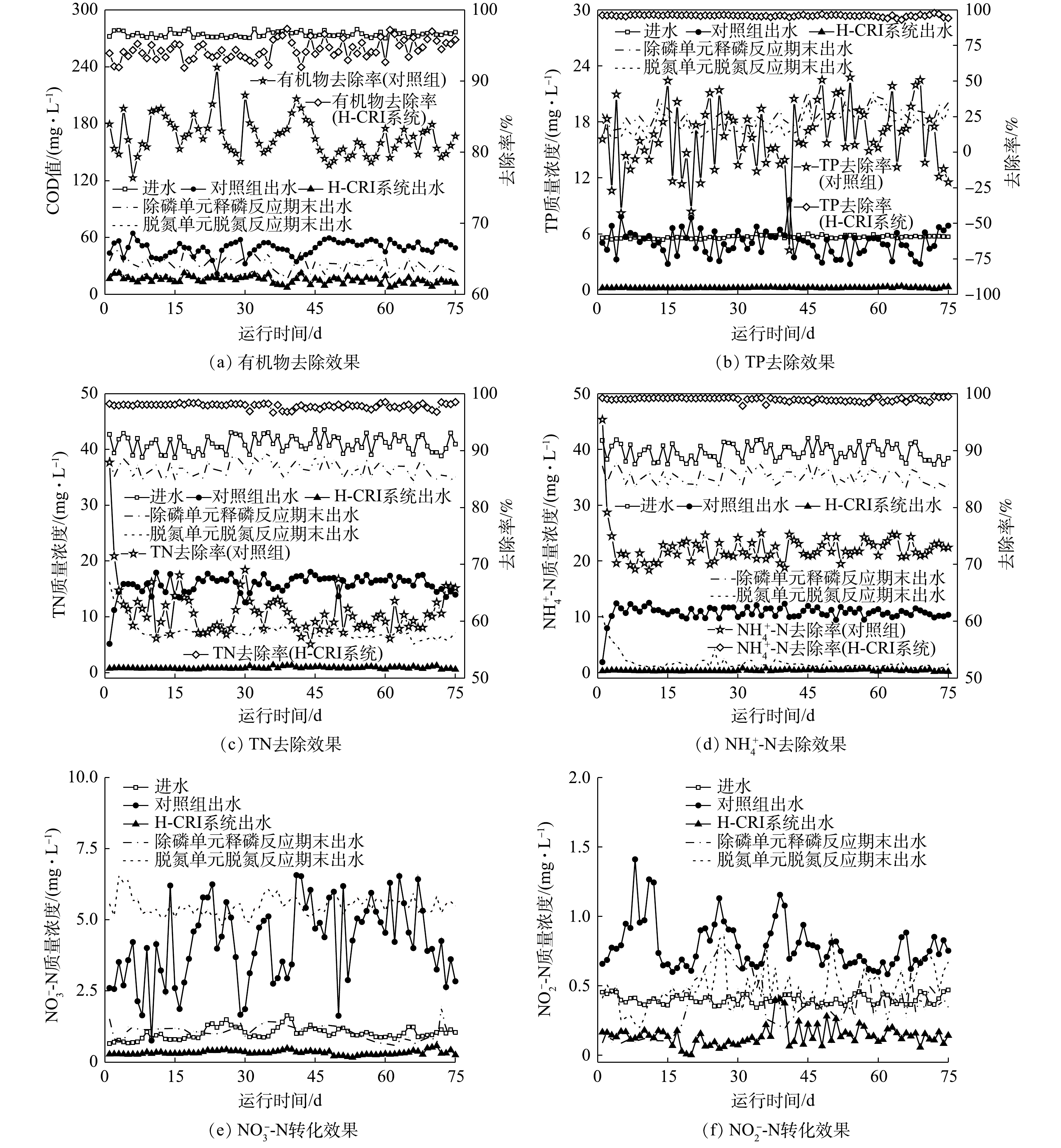
由图3可知,作为前期已成功启动的CANON型CRI系统,对照组具有良好的有机物去除性能[η≈(82.10±0.12)%],其出水的COD值<50 mg·L−1。有研究[4]表明,由于生物膜结构及其内部微环境的复杂性,某些CANON工艺中仍存在相当数量的异养型微生物,其可获得高效的有机物去除效果。然而,进水中的有机物却使对照组的脱氮性能出现下降,其TN和
NH+4 在稳定运行期间,SNADPR型H-CRI系统对进水中有机物、TN、
NH+4 NH+4 NO−3 NO−2 NH+4 NH+4 NO−3 NOx NO−3 NH+4 NO−3 上述结果表明,相较于对照组,H-CRI系统具备更佳的有机物及氮磷去除性能,可实现对生活污水的高效处理。对于该耦合装置,内循环潮汐流运行模式的设置可使其中的脱氮单元和除磷单元高效协作,且2单元中CANON作用和DPR作用的强度及稳定性均可得到充分保障。
2.2 微生物群落组成
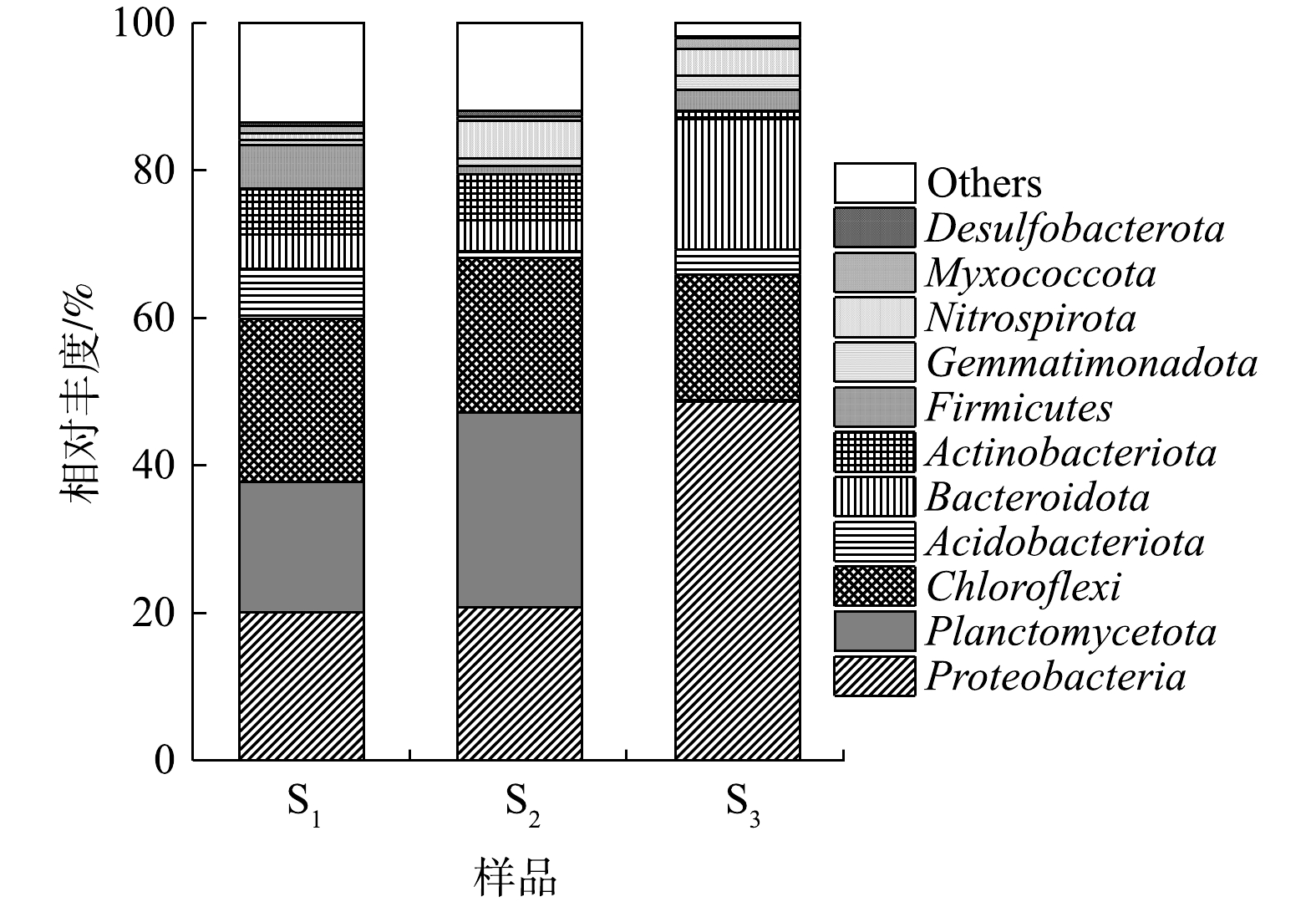
由图6可知,变形菌门(Proteobacteria)、浮霉菌门(Planctomycetota)和绿弯菌门(Chloroflexi)是S1和S2中相对丰度较高的3个菌门。其中,Proteobacteria在2组样本中的占比均>20%;Planctomycetota在S1中的相对丰度为17.66%,但其在S2中的含量却可达26.40%;此外,S1中硝化螺旋菌门(Nitrospirota)的相对丰度较S2(5.16%)显著下降至0.85%,但其中酸杆菌门(Acidobacteriota)的含量却增至6.72%,高于S2(0.91%)。有研究者指出,多数AOB(如Nitrosococcus、Nitrosomonas等)属Proteobacteria;AnAOB共包括5个属9个菌种,均属于Planctomycetota;亚硝酸盐氧化菌(NOB)属于Nitrospirota;反硝化菌则主要存在于Proteobacteria;另外,Acidobacteriota中的部分功能菌也具备还原
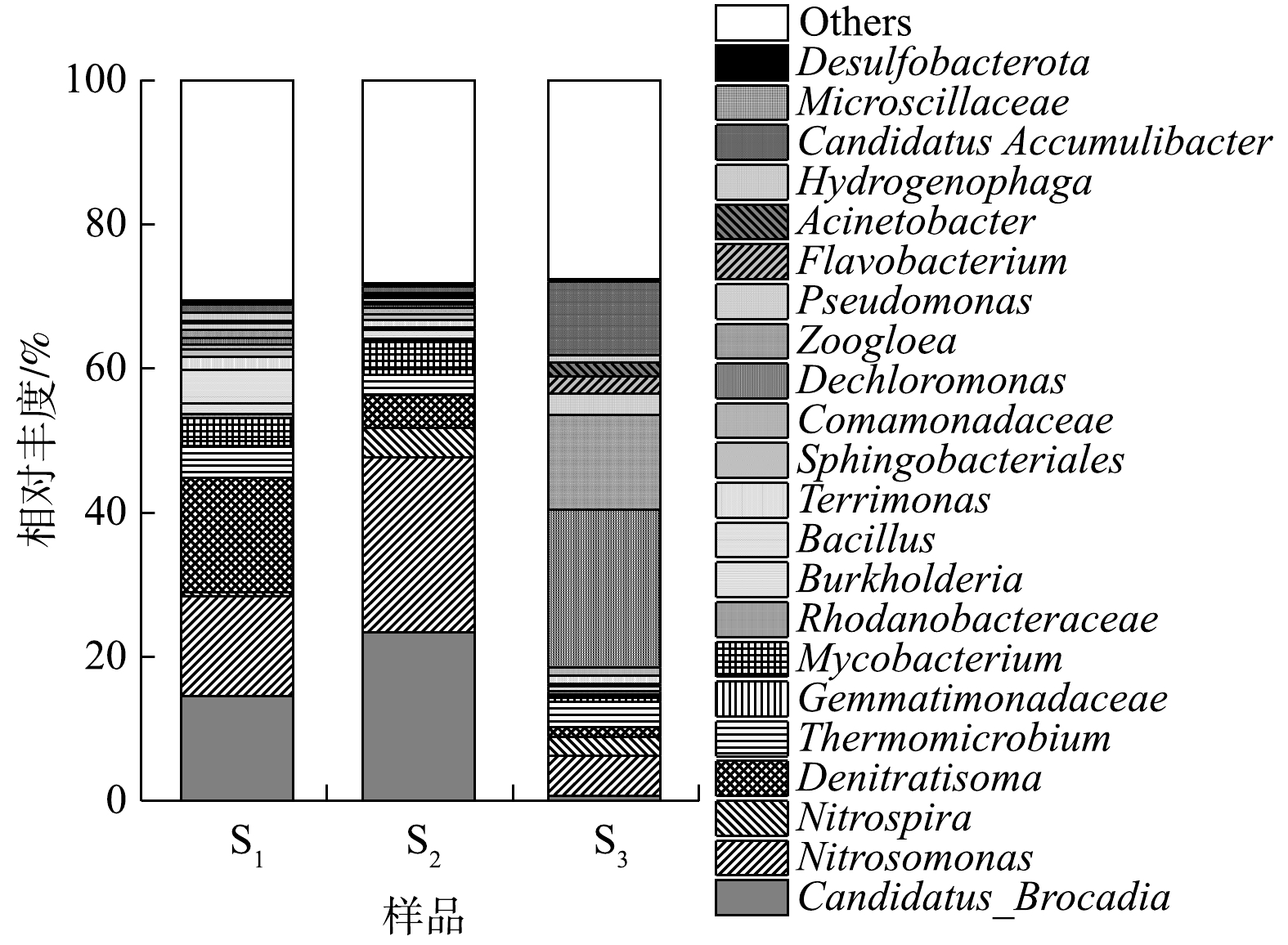
NO−x 由图7可知,S1和S2中的优势菌属均包括Candidatus Brocadia和Nitrosomonas,2种菌属与CANON反应相关[24]。其中,Candidatus Brocadia是上述样本中检出的唯一AnAOB,此结果与HU等[26]和GONZALEZ等[27]的研究结论相符。Nitrosomonas是各样本中唯一检出的AOB,相较于Nitrospira与Nitrospina,该菌属被证实更易在CANON系统中生长[28]。与图6相呼应,Candidatus Brocadia和Nitrosomonas在S1中的相对丰度(14.56%和13.80%)低于S2(23.38%和24.25%);硝化螺旋菌属(Nitrospira)在S1中的含量也明显低于S2。然而,属红环菌科(Rhodocyclaceae)的反硝化菌属——Denitratisoma此时却是S1中的优势菌属,其相对丰度可达15.89%,这表明,对照组的反硝化性能较H-CRI系统的脱氮单元有所增强。上述结果进一步证实:对照组和H-CRI系统的脱氮单元中均存在CANON反应体系;对照组由于受到进水中较高浓度有机物的影响,导致其中的异养菌(包括反硝化菌)滋生,随之影响了AOB与AnAOB的丰度及活性,进而影响了CANON作用的强度。而对于H-CRI系统的脱氮单元,由于除磷单元与之耦合后可缓解有机物对其微生物群落结构的冲击,则脱氮单元中CANON作用的强度及稳定性得到保障。S3中的第1大优势菌属为Dechloromonas(21.83%)。在侧流EBPR工艺中,Dechloromonas是常见的优势微生物,其具备缺氧吸磷的能力[29]。此结果表明H-CRI系统的除磷单元具备较理想的反硝化除磷性能。Zoogloea(13.11%)是S3中的第2大优势菌属,其能够合成胞内碳源进行内源反硝化作用[30],此样品中较高丰度的Zoogloea预示着H-CRI系统的除磷单元还具有一定的内源反硝化性能。值得注意的是,S3中还存在一定含量的Candidatus Accumulibacter (10.23%)和Pseudomonas (3.01%),此2种微生物均可进行反硝化除磷作用[31-32],则两者均对除磷单元的反硝化除磷效果有促进作用。有研究表明,除了
NO−3 2.3 功能微生物活性
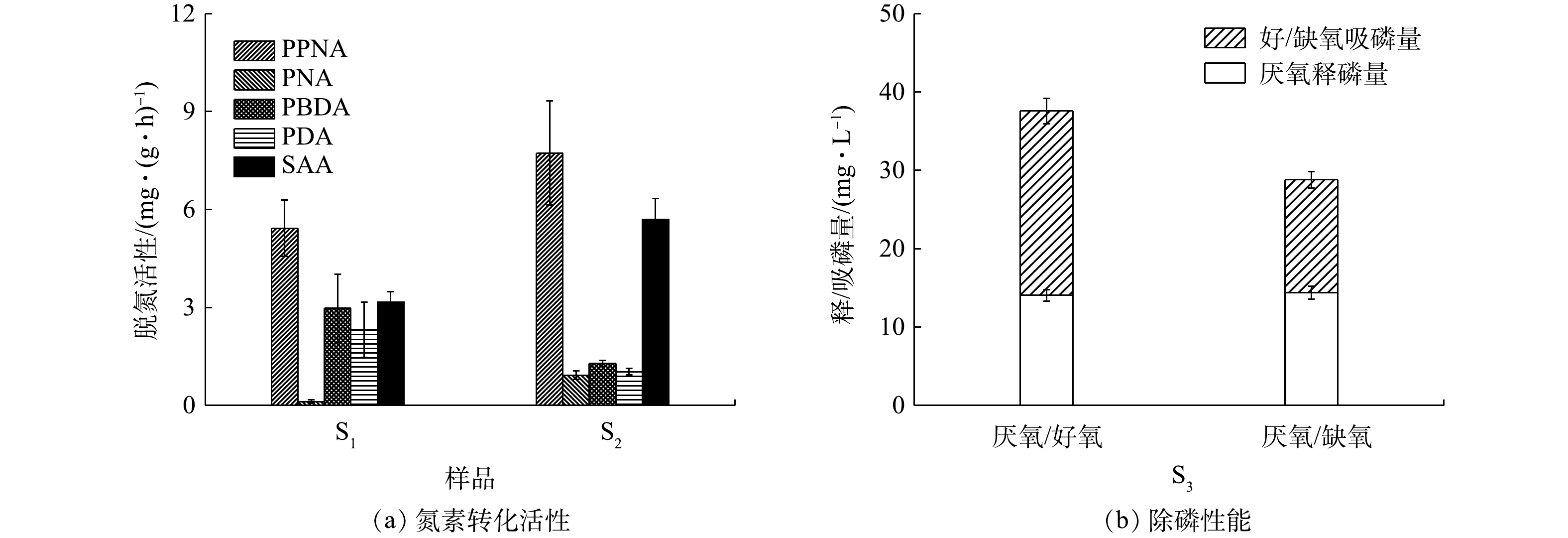
由图8可知,对照组的PPNA和SAA分别为(5.43±0.86) mg·(g·h)−1和(3.16±0.32) mg·(g·h)−1,而其PBDA和PDA则分别为(2.98±1.04) mg·(g·h)−1和(2.32±0.85) mg·(g·h)−1。与同类型研究[4]相比,此系统中CANON作用的强度偏低,但其反硝化性能却有较大幅度的提升。相较于对照组,H-CRI系统中脱氮单元的PPNA和SAA分别增至(7.73±1.60) mg·(g·h)−1和(5.70±0.64) mg·(g·h)−1,但该单元的PBDA和PDA有所下降,分别稳定在(1.29±0.08) mg·(g·h)−1和(1.03±0.10) mg·(g·h)−1。据此判断,脱氮单元中的CANON作用此时具备较高的强度,其反硝化性能也得到一定程度的强化。进水C/N会影响CANON系统中各功能微生物之间的竞争和协同关系[24]:当进水中有机物浓度适量时,各类脱氮功能微生物可共存并互相促进,即系统中的AOB和AnAOB协作完成CANON反应,反硝化菌则以有机碳源为电子供体,将体系中剩余的
NO−2 NO−3 上述结果表明,与对照组相比,内循环潮汐流运行模式下的H-CRI系统凭借其2个子单元的高效耦合分别为AOB、AnAOB、异养反硝化菌和PAOs(包括DPAOs)提供了其各自适宜的生态位,确保了此4类功能微生物对底物的合理竞争,进而实现了对生活污水的高效处理。其中:除磷单元中富集的PAOs(包括DPAOs)可在释磷反应期内利用有机物合成胞内碳源,因而削弱乃至消除了有机物对脱氮单元运行性能的影响,确保了其中CANON作用的稳定;除磷单元中的PAOs在吸磷反应期通过消耗内碳源实现了对脱氮单元出水中TP的超量吸收;脱氮单元出水中的
NOx− 2.4 氮磷去除途径解析
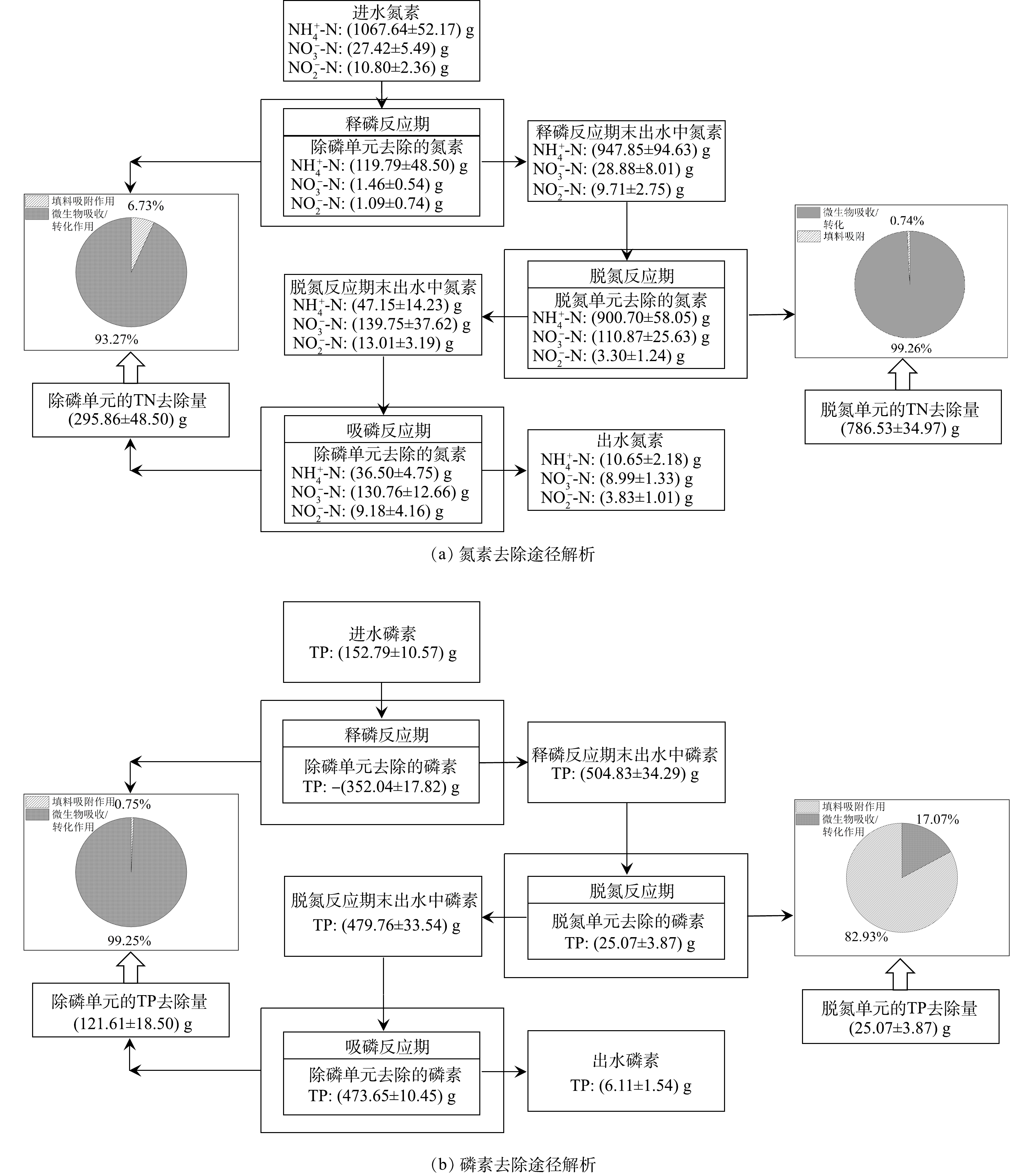
由图9可知,H-CRI系统在实验阶段的TN去除量为(1 082.39±27.82) g。其中,除磷单元的
NH+4 NO−3 NO−2 NH+4 NO−3 NO−2 综上所述,内循环潮汐流运行模式可将DPR型CRI装置与CANON型CRI装置成功耦合为SNADPR型H-CRI系统,此工艺在处理生活污水时可摆脱低
NH4+ 3. 结论
1)对于由CANON型CRI装置和DPR型CRI装置耦合而成的H-CRI系统,当其按照内循环潮汐流模式连续运行时,有助于其中SNADPR作用的发生和强化。
2)当HLR为0.18 m3·(m2·d)−1时,SNADPR型H-CRI系统可实现对生活污水中有机物及氮磷的高效同步去除,其对有机物、TN、
NH+4 3)脱氮单元中的CANON作用是SANDPR型H-CRI系统脱氮的主要途径,而系统中磷素的去除主要依赖于除磷单元中PAOs的过量吸磷作用,两者去除的氮磷量分别占H-CRI系统脱氮除磷总量的(72.13±6.12)%和(82.29±5.58)%。
-
表 1 厌氧氨氧化反应器不同阶段的运行参数
Table 1. Operating parameters of different stages of anammox reactor
阶段 运行时间/d 进水NH3-N质量浓度/(mg·L−1) 进水 NO−2 R HRTa/h NLR b/(kg·(m3·d)−1) I 1~6 100 100 0 12 0.8 7~28 100 100 0 6 1.6 29~48 150 150 0 4 1.8 49~54 300 300 1 5 2.88 II 55~65 500 500 1 8 3 66~76 500 500 2 9 2.67 77~96 500 500 2 12 2 III 97~116 400 400 1 8 2.4 注:a HRT为水力停留时间;b NLR为总氮负荷。 -
[1] 赵志瑞, 马斌, 张树军, 等. 高氨氮废水与城市生活污水短程硝化系统菌群比较[J]. 环境科学, 2013, 34(4): 1448-1456. [2] 闫家望. 高氨氮废水处理技术及研究现状[J]. 中国资源综合利用, 2018, 36(3): 99-101. doi: 10.3969/j.issn.1008-9500.2018.03.035 [3] 陈重军, 王建芳, 张海芹, 等. 厌氧氨氧化污水处理工艺及其实际应用研究进展[J]. 生态环境学报, 2014, 23(3): 521-527. doi: 10.3969/j.issn.1674-5906.2014.03.023 [4] 徐峥勇. 基于亚硝化、厌氧氨氧化与反硝化的脱氮耦合工艺及其控制策略研究[D]. 长沙: 湖南大学, 2011. [5] LI X, LU M Y, HUANG Y, et al. Influence of seasonal temperature change on autotrophic nitrogen removal for mature landfill leachate treatment with high-ammonia by partial nitrification-Anammox process[J]. Journal of Environmental Sciences, 2021, 102: 291-300. doi: 10.1016/j.jes.2020.09.031 [6] CHEN G, LI J, DENG H, et al. Study on Anaerobic ammonium oxidation (Anammox) sludge immobilized in different gel carriers and its nitrogen removal performance[J]. Journal of Residuals Science & Technology, 2015, 12: S47-S54. [7] WANG J X, LIANG J D, SUN L, et al. Granule-based immobilization and activity enhancement of anammox biomass via PVA/CS and PVA/CS/Fe gel beads[J]. Bioresource Technology, 2020, 309: 123448. doi: 10.1016/j.biortech.2020.123448 [8] 贾方旭, 彭永臻, 杨庆. 厌氧氨氧化菌与其他细菌之间的协同竞争关系[J]. 环境科学学报, 2014, 34(6): 1351-1361. [9] VAN DER STAR W R L, ABMA W R, BLOMMERS D, et al. Startup of reactors for anoxic ammonium oxidation: Experiences from the first full-scale anammox reactor in Rotterdam[J]. Water Research, 2007, 41(18): 4149-4163. doi: 10.1016/j.watres.2007.03.044 [10] HU B L, ZHENG P, TANG C J, et al. Identification and quantification of anammox bacteria in eight nitrogen removal reactors[J]. Water Research, 2010, 44(17): 5014-5020. doi: 10.1016/j.watres.2010.07.021 [11] BI Z, QIAO S, ZHOU J T, et al. Fast start-up of Anammox process with appropriate ferrous iron concentration[J]. Bioresource Technology, 2014, 170: 506-512. doi: 10.1016/j.biortech.2014.07.106 [12] WANG T, ZHANG H M, YANG F L, et al. Start-up of the anammox process from the conventional activated sludge in a membrane bioreactor[J]. Bioresource Technology, 2009, 100: 2501-2506. doi: 10.1016/j.biortech.2008.12.011 [13] CHEN H, HU H Y, CHEN Q Q, et al. Successful start-up of the anammox process: Influence of the seeding strategy on performance and granule properties[J]. Bioresource Technology, 2016, 211: 594-602. doi: 10.1016/j.biortech.2016.03.139 [14] 王晓霞. ASBR反应器内厌氧氨氧化的快速启动及其脱氮性能研究[D]. 青岛: 青岛大学, 2012. [15] PHAN T N, TRUONG T T V, Ha N B, et al. High rate nitrogen removal by ANAMMOX internal circulation reactor (IC) for old landfill leachate treatment[J]. Bioresource Technology, 2017, 234: 281-288. doi: 10.1016/j.biortech.2017.02.117 [16] WANG S H, Guo J B, LIAN J, et al. Rapid start-up of the anammox process by denitrifying granular sludge and the mechanism of the anammox electron transport chain[J]. Biochemical Engineering Journal, 2016, 115: 101-107. doi: 10.1016/j.bej.2016.09.001 [17] 王朝朝, 冀颖, 闫立娜, 等. 厌氧氨氧化颗粒污泥UASB反应器的快速启动[J]. 中国给水排水, 2019, 35(11): 15-20. [18] 许冬冬, 康达, 郭磊艳, 等. 厌氧氨氧化颗粒污泥研究进展[J]. 微生物学通报, 2019, 46(8): 1988-1997. [19] ZHANG Y L, HE S L, NIU Q G, et al. Characterization of three types of inhibition and their recovery processes in an anammox UASB reactor[J]. Biochemical Engineering Journal, 2016, 109: 212-221. doi: 10.1016/j.bej.2016.01.022 [20] 国家环境保护总局. 水和废水监测分析方法[M]. 4版. 北京: 中国环境科学出版社, 2002. [21] PARK, S, BAE, W. Modeling kinetics of ammonium oxidation and nitrite oxidation under simultaneous inhibition by free ammonia and free nitrous acid[J]. Process Biochemistry, 2009, 44(6): 631-640. doi: 10.1016/j.procbio.2009.02.002 [22] XU L Z J, ZHANG Q, FU J J, et al. Deciphering the microbial community and functional genes response of anammox sludge to sulfide stress[J]. Bioresource Technology, 2020, 302: 122885. doi: 10.1016/j.biortech.2020.122885 [23] 李媛. 厌氧氨氧化工艺启动和运行特性及其受抑机理研究[D]. 无锡: 江南大学, 2014. [24] STROUS M, KUENEN J G, JETTEN M S M. Key physiol-ogy of anaerobic ammonium oxidation[J]. Applied and Environmental Microbiology, 1999, 65(7): 3248-3250. doi: 10.1128/AEM.65.7.3248-3250.1999 [25] STROUS M, HEIJNEN J J, KUENEN J G, et al. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms[J]. Applied Microbiology and Biotechnology, 1998, 50(5): 589-596. doi: 10.1007/s002530051340 [26] HYOKWAN B, MINKYU C, CHANGSOO L, et al. Enrichment of ANAMMOX bacteria from conventional activated sludge entrapped in poly(vinyl alcohol)/sodium alginate gel[J]. Chemical Engineering Journal, 2015, 281: 531-540. doi: 10.1016/j.cej.2015.06.111 [27] ZHANG Z, LIU S. Insight into the overconsumption of ammonium by anammox consortia under anaerobic conditions[J]. Journal of Apply Microbiology, 2014, 117(6): 1830-1838. doi: 10.1111/jam.12649 [28] NIU Q G, HE S L, ZHANG Y L, et al. Process stability and the recovery control associated with inhibition factors in a UASB-anammox reactor with a long-term operation[J]. Bioresource Technology, 2016: 132-141. [29] FAHRBACH M, KUEVER J, MEINKE R, et al. Denitratisoma oestradiolicum gen. nov., sp. nov., a 17b-oestradiol-degrading, denitrifying betaproteobacterium[J]. International Journal of Systematic and Evolutionary Microbiology, 2006, 56: 1547-1552. doi: 10.1099/ijs.0.63672-0 [30] ZHANG Q, WU J, YE Y Y, et al. Microbial and genetic responses of anammox process to the successive exposure of different antibiotics[J]. Chemical Engineering Journal, 2020: 127576. [31] ZHOU S, ZHANG Z, SUN Z L, et al. Responses of simultaneous anammox and denitrification (SAD) process to nitrogen loading variation: Start-up, performance, sludge morphology and microbial community dynamics[J]. Science of the Total Environment, 2021, 795: 148911. doi: 10.1016/j.scitotenv.2021.148911 [32] XIA Y, WANG Y, WANG Y, et al. Cellular adhesiveness and cellulolytic capacity in Anaerolineae revealed by omics-based genome interpretation[J]. Biotechnology Biofuels, 2016, 9: 111. doi: 10.1186/s13068-016-0524-z -









 下载:
下载: