-
氟喹诺酮类抗生素(fluoroquinolones,FQs)是指具有4-氧代喹啉-6-氟-3-羧基结构的一类药物总称。FQs具有抗菌谱广、杀菌能力强、耐药发生率低、体内分布广、组织浓度高、半衰期长、价格低廉等特点,被广泛应用于水产品、畜禽类及人类疾病预防和治疗过程中,是当前应用最广泛的抗生素类药物之一[1-2]。近年来,随着FQs使用量的不断增加,在地表水[3-4]、饮用水[5-6]、污水处理厂[7-11]以及河流沉积物[12-13]中检出FQs的报道越来越多。因FQs可诱导产生耐药性菌株,破坏微生态平衡,故其在环境中的残留及潜在风险受到国内外学者的普遍关注[14-15]。
随着养殖业的迅猛发展,为追求利益最大化,养殖业开始出现养殖密度过高、喂食量过度、有效隔离空间不足、流行病愈加频繁等问题。为预防及治疗流行病和促进畜禽、鱼及贝类的生长,养殖人员将FQs添入饲料中,以致养殖业出现抗生素应用不合理、消耗过量的现状[16]。使用后的FQs一般只有20%~30%被利用和吸收,其余FQs以母体化合物或代谢物形式排出体外[17],造成养殖场周边及流域环境的FQs污染[18-19]。因此,养殖废水成为FQs进入环境的重要来源之一。目前,虽然国内外已有很多学者对环境[3-11]、饲料[20-21]、食品[22-23]、水产品[24-25]等领域中的FQs开展了相关研究,但这些研究所关注的FQs类的化合物相对较少,很难满足环境中FQs的检测需求。本研究针对养殖废水中可能存在的17种FQs,通过优化前处理和仪器分析条件,实现了对养殖废水中17种FQs的同时检测,为养殖废水中FQs的污染特征研究提供了一种快速、准确的测定方法。此外,还应用本研究所建立的方法对广州某水产养殖场养殖废水中17种FQs的残留量进行了检测。
-
标准贮备液:17种FQs(氟罗沙星、氧氟沙星、培氟沙星、依诺沙星、诺氟沙星、环丙沙星、恩诺沙星、达氟沙星、马波沙星、洛美沙星、奥比沙星、二氟沙星、沙拉沙星、加替沙星、莫西沙星、氟甲喹和那氟沙星),1种替代物(诺氟沙星-d5)及3种进样内标(环丙沙星-d8、13C3-氟甲喹和恩诺沙星-d5)的贮备液均购自天津阿尔塔公司,溶剂为甲醇,质量浓度均为100 mg·L−1,在−20 ℃下避光保存,使用时用甲醇将混标稀释成不同浓度的标准工作液。
实验试剂和材料:甲醇(中国上海,霍尼韦尔贸易有限公司),甲酸(中国北京,迪马科技有限公司);盐酸(中国衡阳,凯信化工试剂有限公司),氨水(中国天津,光复精细化工研究所)。Oasis HLB 固相萃取柱(6 mL,500 mg,美国,Waters公司);CORTECS® C18柱(4.6 mm×100 mm,2.7 µm,美国,waters公司);0.45 μm玻璃纤维膜、2 mL注射器、0.22 μm聚四氟乙烯滤膜(中国广州,国凌仪器有限公司)。
-
Fotector plus(PFCs)高通量全自动固相萃取仪(中国厦门,睿科集团股份有限公司);Agilent 1260 Infinity快速液相色谱(美国,安捷伦科技有限公司)- AB 4000 Qtrap三重四级杆串联质谱(美国,AB SCIEX公司);MGS-2200氮吹浓缩仪(日本,EYELA公司);SCQ-1000C超声波清洗仪(中国上海,声彦超声波仪器有限公司)。
-
量取1 000 mL的水样,用0.45 μm 的聚四氟乙烯滤膜除去水中悬浮颗粒物,加入5%甲醇溶液(体积比),用盐酸调节pH至2.0,并加入20 ng替代物(诺氟沙星-d5),以8-12 mL·min−1的流速匀速通过活化后的HLB固相萃取柱。HLB柱依次用6 mL甲醇、6 mL水及6 mL 盐酸水溶液(pH=2)淋洗活化。样品富集完成后,用6 mL 5%甲醇溶液(体积比)淋洗小柱,干燥25 min,再用9 mL 0.1%甲酸甲醇以1 mL·min−1 的速度洗脱小柱,洗脱液收集于试管中。洗脱液经浓缩装置浓缩至尽干,加入10 ng进样内标(恩诺沙星-d5、环丙沙星-d8和13C3-氟甲喹),最后用0.1%甲酸−5 mmol·L−1甲酸铵水溶液和甲醇混合溶液(体积比为1∶1)定容至1.0 mL,涡旋混匀,过0.22 μm聚四氟乙烯滤膜,待测。
-
色谱柱:Waters CORTECS® C18柱(4.6 mm×100 mm,2.7 µm),流动相为0.1%甲酸−5 mmol·L−1甲酸铵水溶液(A相)和甲醇(B相),流速为0.5 mL·min−1,柱温为40 ℃,进样体积为5 μL。正离子模式下梯度洗脱程序:0~1 min为20%B;1~11 min为20%-40%B;11~14 min为40%-95% B;14~17 min为95%B;17.0~17.1 min为95%-20%B,每个梯度完成后平衡2.9 min。
离子源为电喷雾电离源,气帘气压力为0.17 MPa,雾化气压力为0.41 MPa,辅助气压力为0.41 MPa,正离子模式下离子源电压为5 500 V,去溶剂温度为550 ℃,检测方式为多反应离子扫描模式。
-
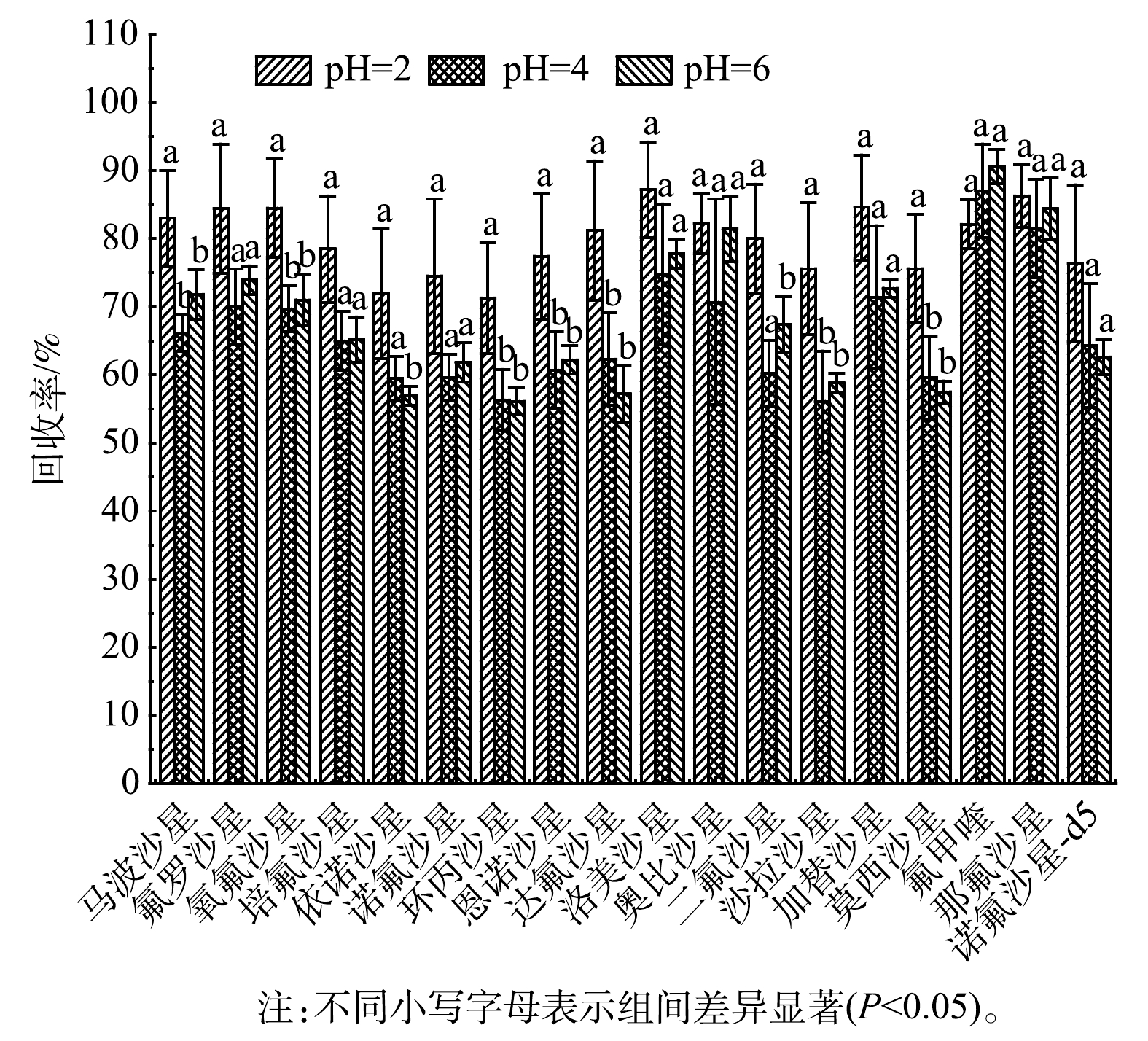
1)样品pH的优化。pH可以改变目标物/吸附剂的离子化或质子化程度,从而影响回收效果。为了考察不同pH对17种FQs的回收效果,本研究采集9份1 000 mL水样,将3份pH调至2.0,3份pH调至4.0,3份pH调至6.0,加标浓度均为20 ng·L−1。如图1所示,当样品的pH调节至2.0时,17种FQs的平均回收率为71.3%-87.2%,RSD为3.6%~11.5%;但当样品的pH调节至4.0和6.0时,依诺沙星、环丙沙星、沙拉沙星及莫西沙星回收率明显下降,均低于60%,RSD为1.3%~11.5%。这可能是因为氟喹诺酮类抗生素的酸度系数(pKa)普遍在4.0~7.0,当样品的pH小于其酸度系数(pKa)2个单位以上时,99%的目标化合物呈中性,有效增强目标化合物在吸附剂上的保留,从而提高回收率。因此,本研究最终选择将水样pH调节至2.0进行富集。
2)固相萃取柱的选择。目前国内外报道的常用于水样中抗生素测定的固相萃取小柱主要有C18柱和HLB柱。为了考察HLB柱和C18柱对17种FQs的萃取效率,本研究做了对比实验。如图2 所示,HLB柱对17种FQs的平均回收率为71.3%~87.2%,显著高于C18柱。这可能与各萃取柱萃取原理有关。有研究表明[26],C18柱是按照疏水非极性作用机理进行萃取,较为适合lgKow≥2.5的非极性到中等极性的化合物的萃取。而FQs为亲水性化合物,其lgKow≤2.0,因此,不适宜用C18柱萃取。HLB柱的填料为亲水亲酯型聚合物,比较适合FQs的保留。因此,本研究最终选用HLB柱对环境水样中17种FQs进行富集。
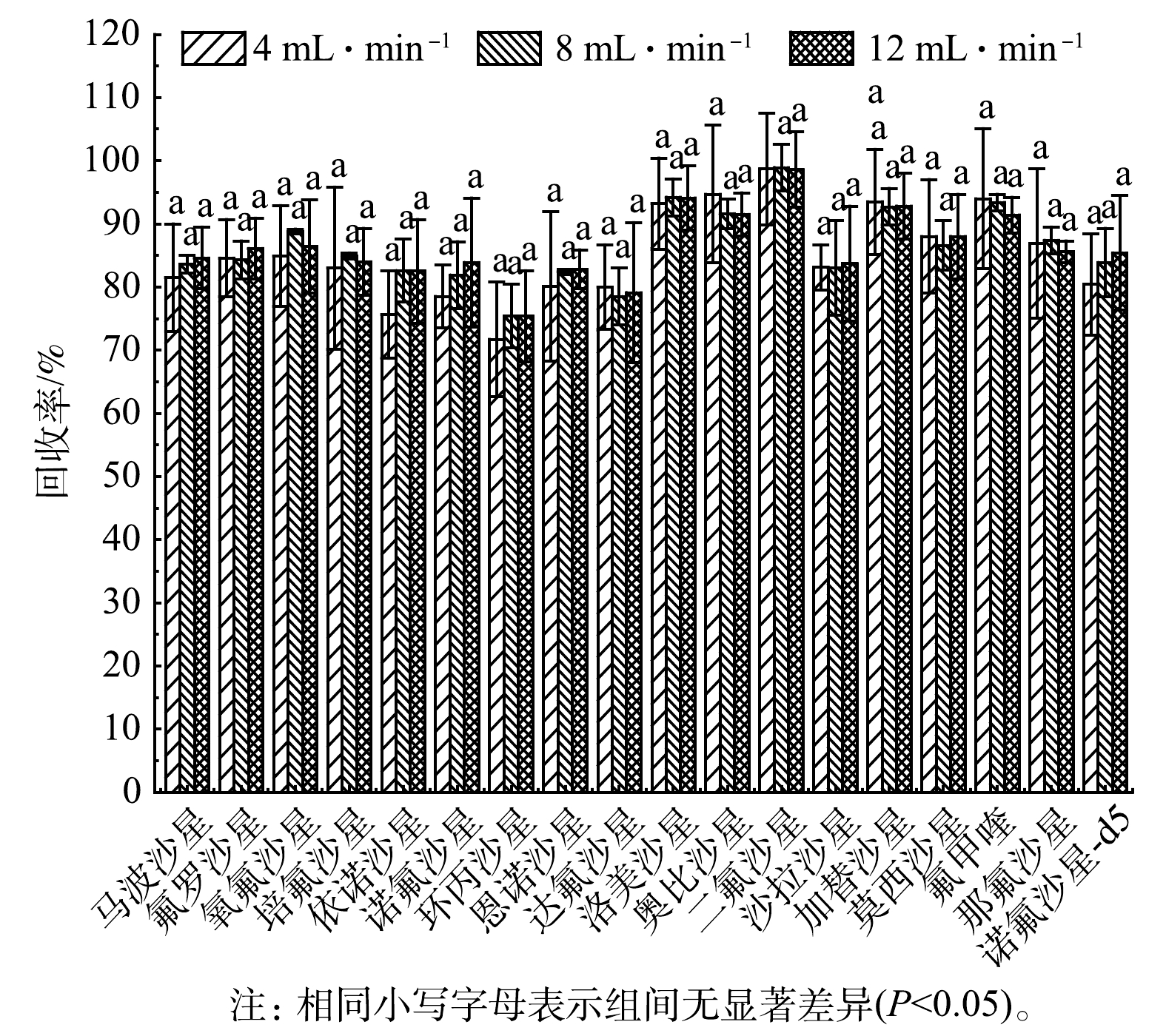
3)上样速度的优化。上样速度是SPE条件优化的一个重要参数。一般来说,上样速度越慢,回收率越高,但样品的预处理时间相对较长;而上样速度过快,固相萃取小柱对目标化合物的吸附往往不充分,容易造成回收率偏低。为筛选出高效、快捷的前处理条件,本研究比较了不同上样速度对17种FQs萃取回收效果的影响。如图3所示,当上样速度为4、8和12 mL·min−1时,对17种FQs平均回收率基本没有影响,说明目标化合物很容易吸附在固相萃取小柱上。在节约时间的同时,考虑到当样品中FQs浓度较高时,上样速度过快可能导致目标物富集效果变差,故本研究最终选择的上样流速为8~12 mL·min−1。
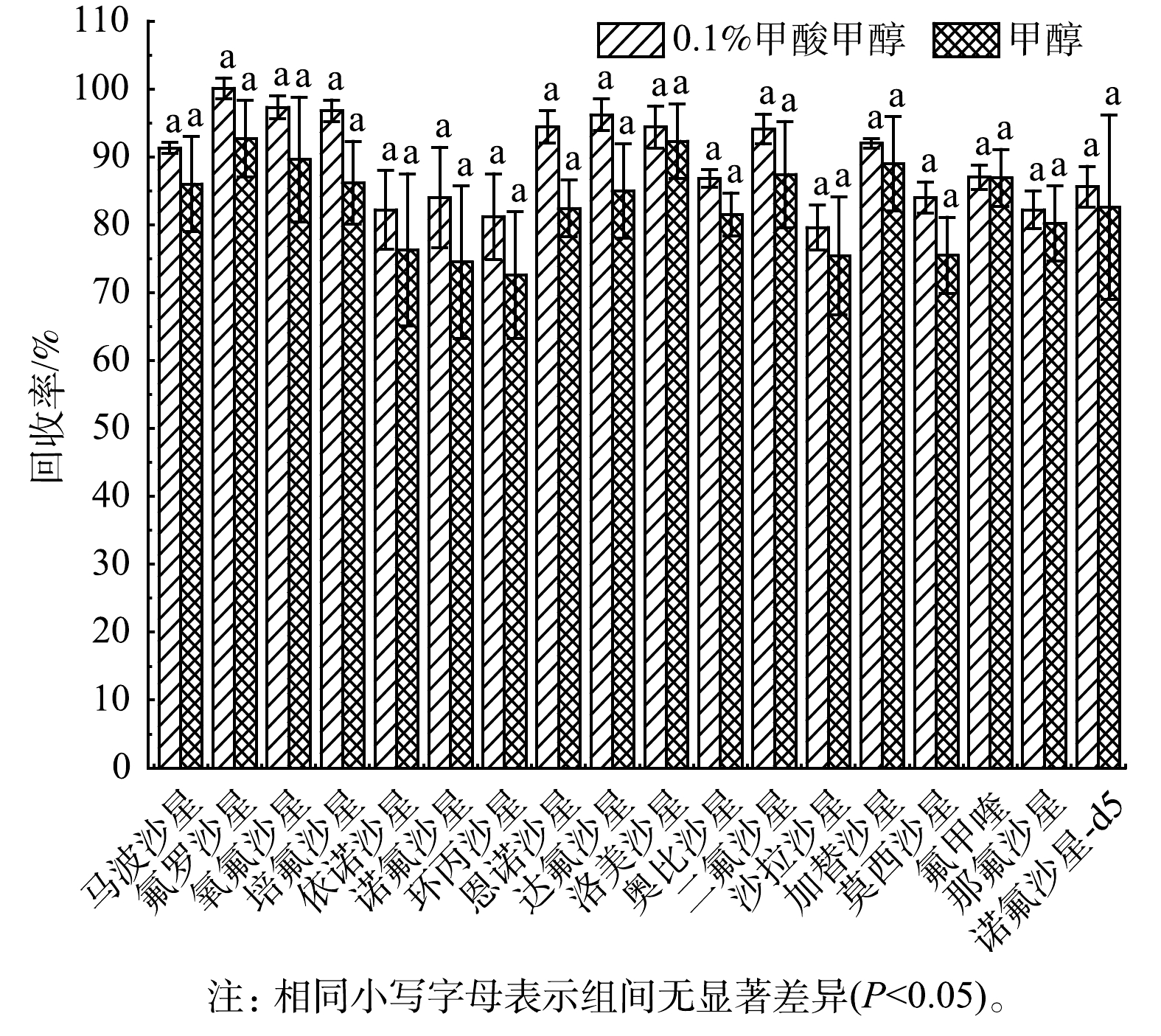
4)洗脱溶剂的选择。为了考察不同洗脱溶剂对17种FQs的洗脱效果,本研究对比了0.1%甲酸甲醇和纯甲醇2种洗脱溶剂。如图4所示。其中用0.1%甲酸甲醇溶液洗脱的平均回收率为79.6%~100%,相对偏差为0.7%~7.4%;而用甲醇洗脱的平均回收率为72.6%~92.7%,相对偏差为3.2%~13.6%。虽然0.1%甲酸甲醇略优于甲醇,但2种洗脱溶剂对17种FQs的回收率均大于70%,因此,上述2种洗脱溶剂均可作为17种FQs的洗脱溶剂。
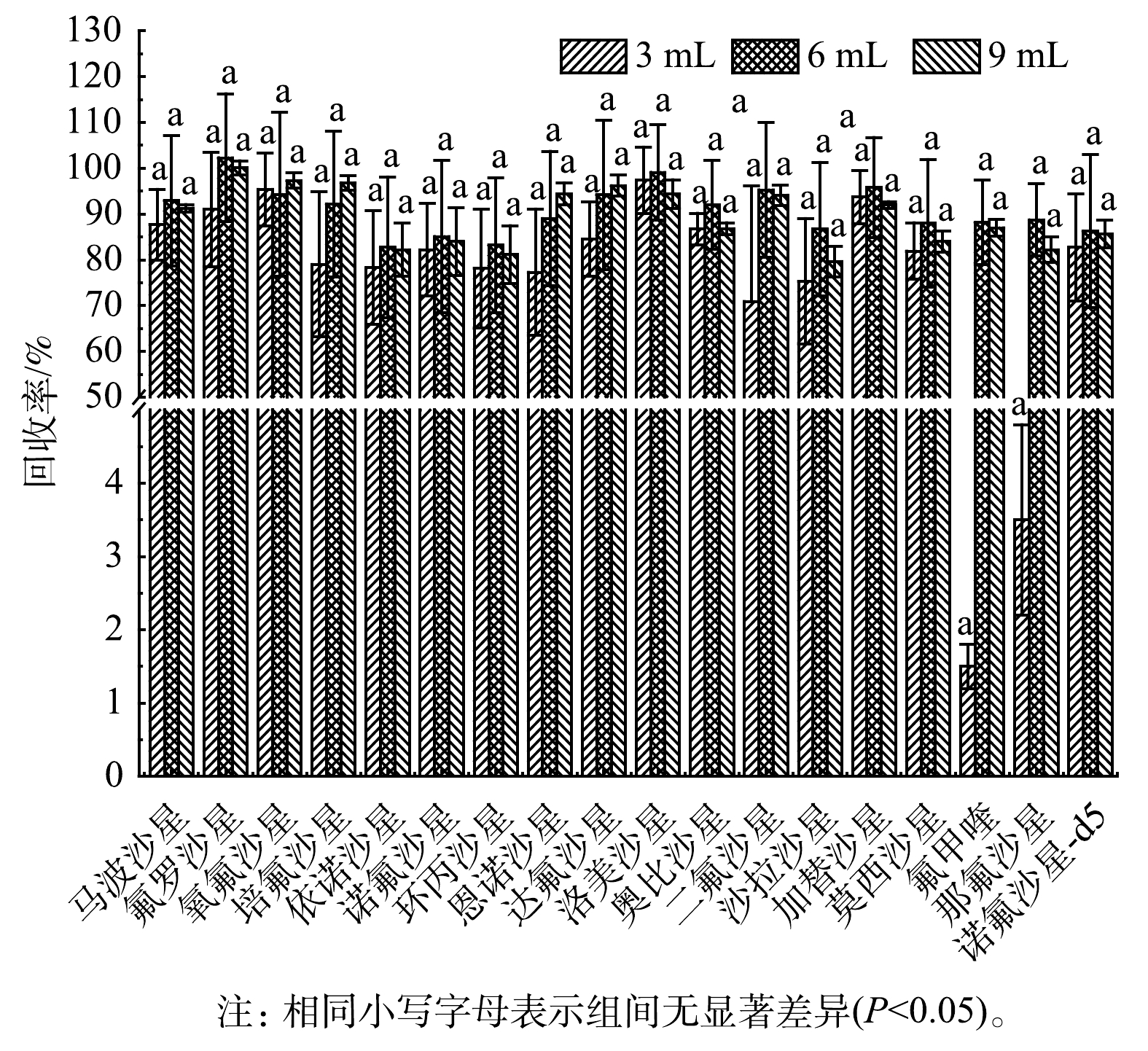
5)洗脱溶剂用量优化。为获取最佳的洗脱溶剂体积,在选定0.1%甲酸甲醇溶液作为洗脱溶剂后,将9份1 000 mL水样(加标质量浓度为20 ng·L−1)平均分成3组,分别对比了3、6和9 mL洗脱体积对17种FQs的回收效果。如图5所示,当洗脱量为3 mL时,那氟沙星和氟甲喹的平均回收率很低,均小于10%,其他目标化合物的平均回收率均在70%以上;当洗脱量为6 mL和9mL时,大部分目标已经被洗脱,且各目标化合物的平均回收率均在80%以上。考虑到养殖废水基质较为复杂,洗脱量较少可导致部分目标化合物未能完全洗脱,回收效果变差,故本研究选取9 mL作为最佳洗脱量。
-
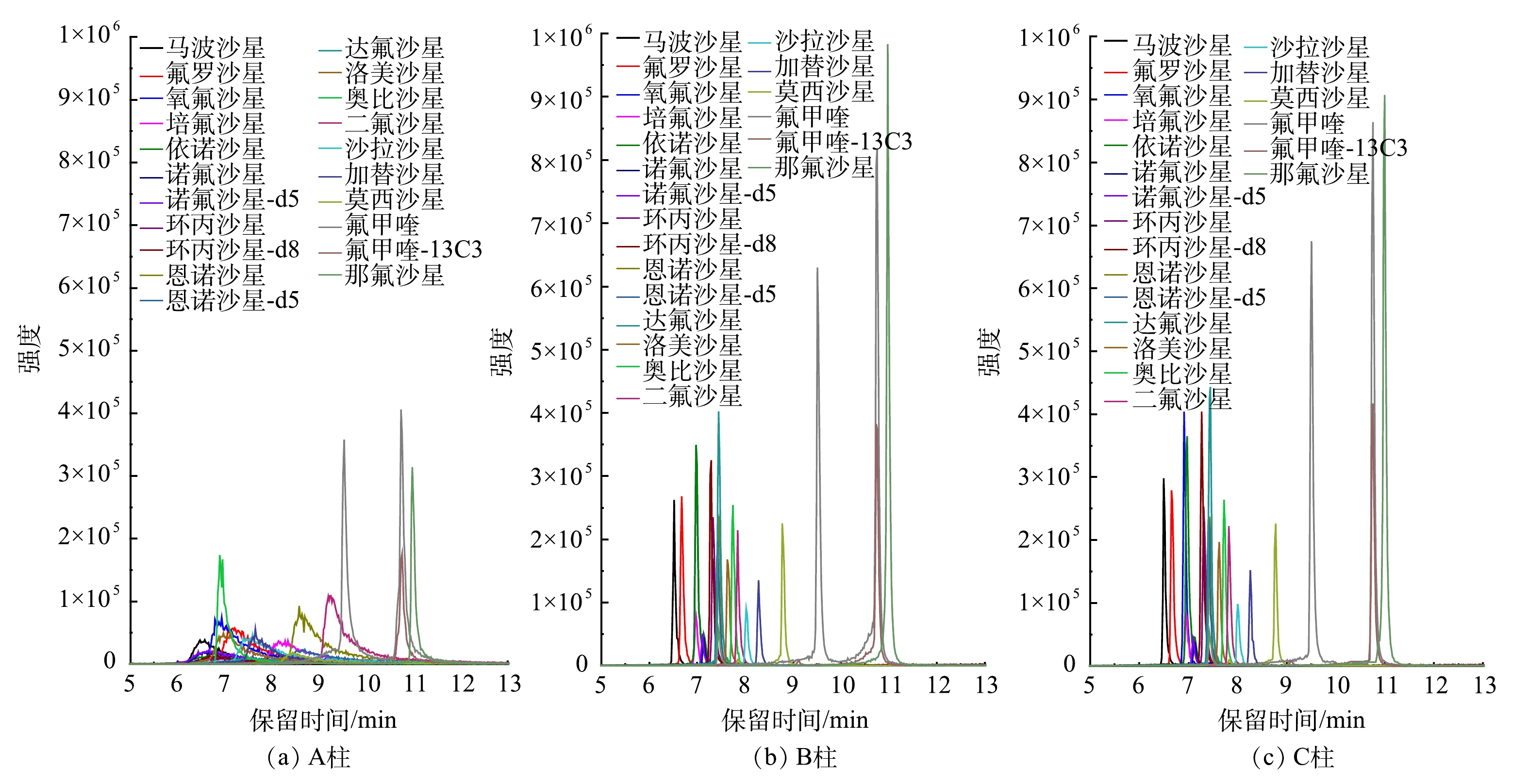
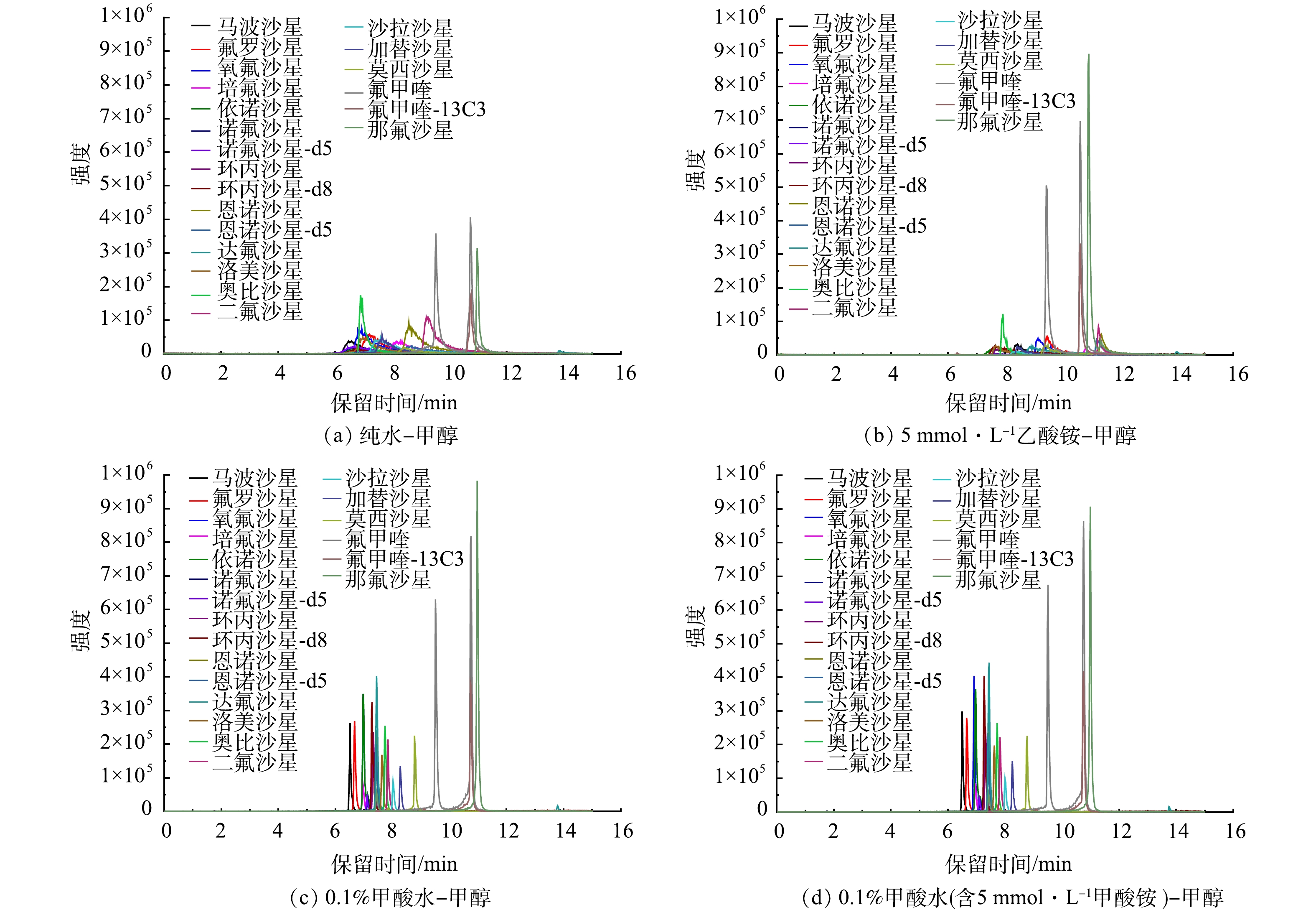
1)色谱柱的选择。FQs多为两性物质,具有一定的水溶性,不同的色谱柱对其分离影响较大。本研究考察了多种常用的C18反相色谱柱对17种FQs的分离效果,如Kinetex-C18®(100 mm×3.0 mm×2.6 μm)(A柱)、XTEERRA-C18® MS C18 (100 mm×2.1 mm×3.5 μm)(B柱)和CORTECS® C18(100 mm×4.6 mm×2.7 μm)(C柱)。如图6所示(进样浓度为20 μg·L−1),使用A柱分离17 种FQs时,出现峰形拖尾现象;使用B柱分离FQs时,莫西沙星和那氟沙星等目标物的色谱峰展宽严重;而使用C柱分离17 种FQs时,不仅分离效果好,峰形对称,而且响应强度也相对较高。因此,本研究选择C柱对17种FQs进行分离。
2)流动相组分优化。确定色谱柱后,为更好的分离各种目标物并获取满意的峰型,本研究对流动相组分进行了优化。首先,比较了不同有机相(甲醇和乙腈)对目标化合物的分离效果。结果表明,使用甲醇时,基线相对较低,并且对目标化合物具有更好的分离效果,因此,确定甲醇为有机相。因在流动相中加入适当的有机酸或缓冲盐可增强目标物的离子化效率,增强分析方法的灵敏度,所以在确定有机相为甲醇后,又比较了不同有机酸、缓冲盐对灵敏度的影响,包括5 mmol·L−1乙酸铵、0.1%甲酸水及0.1%甲酸−5 mmol·L−1甲酸铵等。如图7所示,0.1%甲酸−5 mmol·L−1甲酸铵和甲醇作为流动相时,峰型最好,灵敏度最高,信号强度最强。
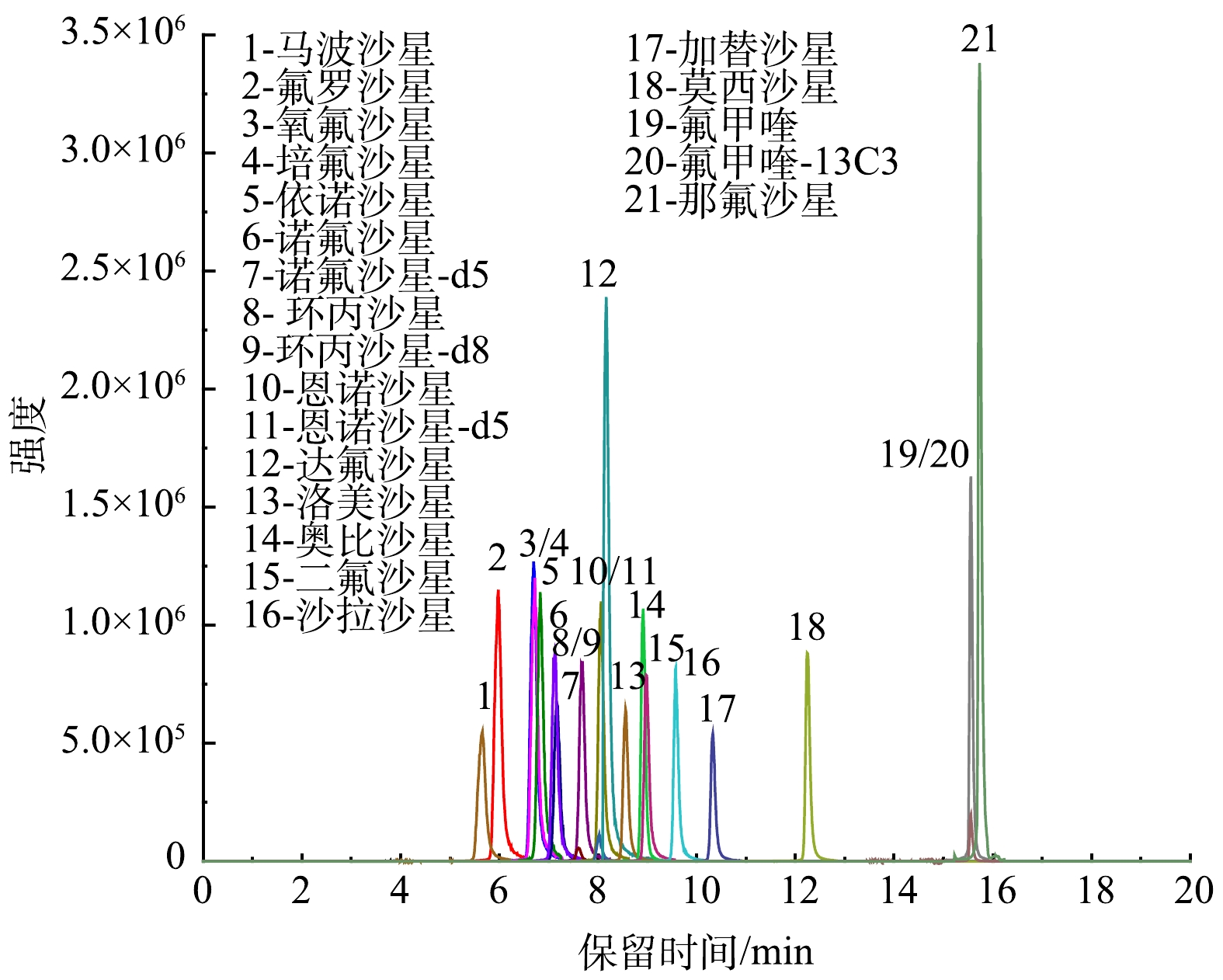
3)洗脱梯度优化。经反复优化洗脱梯度程序后,最终选择1.4.1节所述的洗脱梯度。在选定的洗脱梯度下,所有组分均在20 min以内出峰,且分离效果最为佳,具体色谱图见图8(进样浓度为100 μg·L−1)。
-
配制质量浓度为1.0 mg·L−1的FQs、内标化合物和替代物的混合标准溶液,在正离子模式下,用直接注射进样方式扫描并优化母离子/子离子特征离子对,及相应的锥孔电压、碰撞电压等其他参数,利用获取的全部特征离子及离子间的丰度比进行定性分析,以丰度最高的特征离子响应与浓度的关系进行定量分析,质谱优化参数结果如表1所示。
-
1)方法的线性关系、检出限和定量限。将17种FQs的200 μg·L−1 标准溶液按梯度稀释,梯度浓度分别为0.5、1、2、5、10、 20、50、100 μg·L−1。用已优化好的色谱条件和质谱条件进行测定,内标法定量。以17种FQs的质量浓度为横坐标,定量离子的峰面积为纵坐标,绘制标准曲线。结果表明,17种FQs在0.5-100 μg·L−1内线性关系良好,R2>0.99。根据环境监测分析方法标准制修订技术导则[27],对大约检出限为3-5倍浓度的样品进行7次平行测定,计算测定结果的标准偏差(SD)。其方法检出限(LOD)为3.143倍的SD,定量限(LOQ) 为4倍的 LOD。根据计算结果,LOD为0.08-0.3 ng·L−1,LOQ为0.32-1.2 ng·L−1。
2)加标实验结果。用超纯水进行空白基质加标回收实验,加标质量浓度分别设置为10 ng·L−1和90 ng·L−1,每个浓度水平做6个重复样品,同时设置6个空白样品。按第1.4节中的步骤对样品进行处理,以考察方法的总体回收率、重现性是否达到检测分析要求。如表2所示,当添加量为10 ng·L−1时,17种FQs的平均回收率为58.6%~104.2%,RSD为2.9%~19.3%;当添加量为90 ng·L−1时,平均回收率为65.3%~91.0 %,RSD为2.1%~18.6%。此外,本研究还以养殖废水为基质,设置了6个基质加标实验,加标质量浓度为20 ng·L−1。结果如表3所示,17种FQs的平均回收率为47.8%~118.7%,RSD为3.0%~18.6%。本方法的空白和基质加标回收结果与美国 EPA1694 [28]相当,但与其他几种FQs相比,莫西沙星、达氟沙星和依诺沙星的RSD值相对偏高。这可能是由于现有的富集和净化手段未能有效去除水样的背景干扰,在运用正离子扫描模式时,这些背景会增强或减弱目标化合物的电离结果,进而影响仪器测定结果的准确性,造成方法重现性较差,RSD偏高。
-
为验证本研究建立的检测分析方法,按照《污水监测技术规范》(HJ91.1-2019)[29]中的样品采集规定,采集了广州某水产养殖场的养殖废水并进行了测定。样品采集后,立即用盐酸调节pH至2.0±0.5,4 ℃以下避光运送至实验室。测定结果表明:采集的养殖废水共检测出9种FQs,其中莫西沙星为2.34 ng·L−1,氟甲喹为0.96 ng·L−1,马波沙星为0.24 ng·L−1,氧氟沙星为9.36 ng·L−1,恩诺沙星为1.13 ng·L−1,达氟沙星为5.96 ng·L−1,培氟沙星为2.20 ng·L−1,环丙沙星为0.53 ng·L−1,依诺沙星为3.48 ng·L−1。
-
1)通过优化固相萃取条件、色谱分离条件、质谱检测参数,建立了一种快速、准确测定养殖废水中17种FQs的固相萃取-液相色谱-三重四极杆串联质谱法。该方法通过特征碎片离子和色谱保留时间定性,通过特征准分子离子定量,方法检出限为0.08~0.3 ng·L−1;空白加标平均回收率为58.6%~104.2%,RSD为2.1%~19.3%(n=6);基质加标平均回收率为47.8%~118.7%,RSD小于20%(n=6),满足养殖废水中17种FQs残留分析的要求。
2)所采集的广州某养殖场的养殖废水样品中,含有多种不同含量的氟喹诺酮类抗生素,其中氧氟沙星检出浓度最高(9.36 ng·L−1),达氟沙星次之(5.96 ng·L−1)。
固相萃取-液相色谱-三重四极杆串联质谱测定养殖废水中17种氟喹诺酮类抗生素
Determination of 17 fluoroquinolones antibiotics in aquaculture wastewater using LC-MS/MS coupled with solid phase extraction
-
摘要: 采用固相萃取-液相色谱-三重四极杆串联质谱(SPE-LC-MS/MS)技术,建立了养殖废水中17种氟喹诺酮类抗生素(FQs)的测定方法。水样在采用固相萃取法富集前,先用 0.45 μm 的聚四氟乙烯滤膜过滤,而后加入5%甲醇(体积比),用盐酸溶液将水样pH调节至2.0±0.5,经固相萃取柱富集,最后用9 mL 0.1%甲酸甲醇洗脱。以C18柱为分离柱,0.1%甲酸−5 mmol·L−1 甲酸铵水溶液和甲醇为流动相,采用液相色谱-三重四极杆串联质谱多反应监测离子模式(MRM)对目标化合物进行了检测和分析。在优化实验条件下,17种FQs的线性范围为0.50 -100 μg·L−1时,目标化合物峰面积与内标物质峰面积之比与质量浓度的线性关系良好(R2>0.99),方法检出限为0.08-0.3 ng·L−1。在加标量为0.01 μg·L−1和0.09 μg·L−1时,空白加标的平均回收率为58.6%-104.2% 和65.3%-91.0%,相对标准偏差(RSD)在2.1%-19.3%(n=6)。以养殖废水为基质,17种FQs的加标回收率在47.8%-118.7%,RSD小于20%(n=6)。应用该方法测定了广州某水产养殖场的养殖废水。结果表明,氧氟沙星检出浓度最高(9.36 ng·L−1),达氟沙星次之(5.96 ng·L−1)。该方法快速、准确,可适用于养殖废水中17种FQs的测定。Abstract: A method was developed for the determination of 17 target fluoroquinolones antibiotics (FQs) in aquaculture wastewater by liquid chromatography/tandem triple quadruple bar mass spectrometry (LC-MS/MS), coupled with solid phase extraction (SPE). Before solid phase extraction, samples were filtered by 0.45 μm polytetrafluoroethylene filter membrane, and then 5% methanol (V:V) was added in filtrate and the pH was adjusted to 2.0 with HCl solution. The analytes were efficiently extracted by SPE column and eluted by 9 mL 0.1% formic acid methanol. The separation was performed on a reverse-phase Cl8 column using a mobile phase consisting of 0.1% formic acid-5mmol·L−1 ammonium formate aqueous solution and methanol. All analytes were quantified by LC-MS /MS under multi-reaction monitoring (MRM) mode. Under the optimal conditions, the calibration curves of all 17 targets were linear in the range of 0.5-100μg·L−1 with the correlation coefficients more than 0. 99. The limits of detection (MDL) were 0.08~0.3 ng·L−1.The average recovery rates were in the ranges of 58.6%~104.2% and 58.0%~107.8% and relative standard deviations (RSDs) ranged from 2.1% to 19.3% (n=6) with two spiked levels of 0.01 and 0.09 μg·L−1, respectively. The average recoveries of 17 FQs in the aquaculture wastewater samples were 47.8%~118.7% with RSDs<20% (n=6). A sample of aquaculture wastewater collected from an aquaculture farm in Guangzhou was detected with this method. The results showed that the ofloxacin had the highest detection concentration (9.36 ng·L−1.) in the sample, followed by danofloxacin (5.96 ng·L−1). This method was simple, rapid and accurate, and could be used to detect 17 FQs residues in aquaculture wastewater.
-
氟喹诺酮类抗生素(fluoroquinolones,FQs)是指具有4-氧代喹啉-6-氟-3-羧基结构的一类药物总称。FQs具有抗菌谱广、杀菌能力强、耐药发生率低、体内分布广、组织浓度高、半衰期长、价格低廉等特点,被广泛应用于水产品、畜禽类及人类疾病预防和治疗过程中,是当前应用最广泛的抗生素类药物之一[1-2]。近年来,随着FQs使用量的不断增加,在地表水[3-4]、饮用水[5-6]、污水处理厂[7-11]以及河流沉积物[12-13]中检出FQs的报道越来越多。因FQs可诱导产生耐药性菌株,破坏微生态平衡,故其在环境中的残留及潜在风险受到国内外学者的普遍关注[14-15]。
随着养殖业的迅猛发展,为追求利益最大化,养殖业开始出现养殖密度过高、喂食量过度、有效隔离空间不足、流行病愈加频繁等问题。为预防及治疗流行病和促进畜禽、鱼及贝类的生长,养殖人员将FQs添入饲料中,以致养殖业出现抗生素应用不合理、消耗过量的现状[16]。使用后的FQs一般只有20%~30%被利用和吸收,其余FQs以母体化合物或代谢物形式排出体外[17],造成养殖场周边及流域环境的FQs污染[18-19]。因此,养殖废水成为FQs进入环境的重要来源之一。目前,虽然国内外已有很多学者对环境[3-11]、饲料[20-21]、食品[22-23]、水产品[24-25]等领域中的FQs开展了相关研究,但这些研究所关注的FQs类的化合物相对较少,很难满足环境中FQs的检测需求。本研究针对养殖废水中可能存在的17种FQs,通过优化前处理和仪器分析条件,实现了对养殖废水中17种FQs的同时检测,为养殖废水中FQs的污染特征研究提供了一种快速、准确的测定方法。此外,还应用本研究所建立的方法对广州某水产养殖场养殖废水中17种FQs的残留量进行了检测。
1. 材料与方法
1.1 试剂与材料
标准贮备液:17种FQs(氟罗沙星、氧氟沙星、培氟沙星、依诺沙星、诺氟沙星、环丙沙星、恩诺沙星、达氟沙星、马波沙星、洛美沙星、奥比沙星、二氟沙星、沙拉沙星、加替沙星、莫西沙星、氟甲喹和那氟沙星),1种替代物(诺氟沙星-d5)及3种进样内标(环丙沙星-d8、13C3-氟甲喹和恩诺沙星-d5)的贮备液均购自天津阿尔塔公司,溶剂为甲醇,质量浓度均为100 mg·L−1,在−20 ℃下避光保存,使用时用甲醇将混标稀释成不同浓度的标准工作液。
实验试剂和材料:甲醇(中国上海,霍尼韦尔贸易有限公司),甲酸(中国北京,迪马科技有限公司);盐酸(中国衡阳,凯信化工试剂有限公司),氨水(中国天津,光复精细化工研究所)。Oasis HLB 固相萃取柱(6 mL,500 mg,美国,Waters公司);CORTECS® C18柱(4.6 mm×100 mm,2.7 µm,美国,waters公司);0.45 μm玻璃纤维膜、2 mL注射器、0.22 μm聚四氟乙烯滤膜(中国广州,国凌仪器有限公司)。
1.2 主要仪器
Fotector plus(PFCs)高通量全自动固相萃取仪(中国厦门,睿科集团股份有限公司);Agilent 1260 Infinity快速液相色谱(美国,安捷伦科技有限公司)- AB 4000 Qtrap三重四级杆串联质谱(美国,AB SCIEX公司);MGS-2200氮吹浓缩仪(日本,EYELA公司);SCQ-1000C超声波清洗仪(中国上海,声彦超声波仪器有限公司)。
1.3 样品前处理
量取1 000 mL的水样,用0.45 μm 的聚四氟乙烯滤膜除去水中悬浮颗粒物,加入5%甲醇溶液(体积比),用盐酸调节pH至2.0,并加入20 ng替代物(诺氟沙星-d5),以8-12 mL·min−1的流速匀速通过活化后的HLB固相萃取柱。HLB柱依次用6 mL甲醇、6 mL水及6 mL 盐酸水溶液(pH=2)淋洗活化。样品富集完成后,用6 mL 5%甲醇溶液(体积比)淋洗小柱,干燥25 min,再用9 mL 0.1%甲酸甲醇以1 mL·min−1 的速度洗脱小柱,洗脱液收集于试管中。洗脱液经浓缩装置浓缩至尽干,加入10 ng进样内标(恩诺沙星-d5、环丙沙星-d8和13C3-氟甲喹),最后用0.1%甲酸−5 mmol·L−1甲酸铵水溶液和甲醇混合溶液(体积比为1∶1)定容至1.0 mL,涡旋混匀,过0.22 μm聚四氟乙烯滤膜,待测。
1.4 仪器分析条件
色谱柱:Waters CORTECS® C18柱(4.6 mm×100 mm,2.7 µm),流动相为0.1%甲酸−5 mmol·L−1甲酸铵水溶液(A相)和甲醇(B相),流速为0.5 mL·min−1,柱温为40 ℃,进样体积为5 μL。正离子模式下梯度洗脱程序:0~1 min为20%B;1~11 min为20%-40%B;11~14 min为40%-95% B;14~17 min为95%B;17.0~17.1 min为95%-20%B,每个梯度完成后平衡2.9 min。
离子源为电喷雾电离源,气帘气压力为0.17 MPa,雾化气压力为0.41 MPa,辅助气压力为0.41 MPa,正离子模式下离子源电压为5 500 V,去溶剂温度为550 ℃,检测方式为多反应离子扫描模式。
2. 结果与讨论
2.1 前处理条件的选择和优化
1)样品pH的优化。pH可以改变目标物/吸附剂的离子化或质子化程度,从而影响回收效果。为了考察不同pH对17种FQs的回收效果,本研究采集9份1 000 mL水样,将3份pH调至2.0,3份pH调至4.0,3份pH调至6.0,加标浓度均为20 ng·L−1。如图1所示,当样品的pH调节至2.0时,17种FQs的平均回收率为71.3%-87.2%,RSD为3.6%~11.5%;但当样品的pH调节至4.0和6.0时,依诺沙星、环丙沙星、沙拉沙星及莫西沙星回收率明显下降,均低于60%,RSD为1.3%~11.5%。这可能是因为氟喹诺酮类抗生素的酸度系数(pKa)普遍在4.0~7.0,当样品的pH小于其酸度系数(pKa)2个单位以上时,99%的目标化合物呈中性,有效增强目标化合物在吸附剂上的保留,从而提高回收率。因此,本研究最终选择将水样pH调节至2.0进行富集。
2)固相萃取柱的选择。目前国内外报道的常用于水样中抗生素测定的固相萃取小柱主要有C18柱和HLB柱。为了考察HLB柱和C18柱对17种FQs的萃取效率,本研究做了对比实验。如图2 所示,HLB柱对17种FQs的平均回收率为71.3%~87.2%,显著高于C18柱。这可能与各萃取柱萃取原理有关。有研究表明[26],C18柱是按照疏水非极性作用机理进行萃取,较为适合lgKow≥2.5的非极性到中等极性的化合物的萃取。而FQs为亲水性化合物,其lgKow≤2.0,因此,不适宜用C18柱萃取。HLB柱的填料为亲水亲酯型聚合物,比较适合FQs的保留。因此,本研究最终选用HLB柱对环境水样中17种FQs进行富集。
3)上样速度的优化。上样速度是SPE条件优化的一个重要参数。一般来说,上样速度越慢,回收率越高,但样品的预处理时间相对较长;而上样速度过快,固相萃取小柱对目标化合物的吸附往往不充分,容易造成回收率偏低。为筛选出高效、快捷的前处理条件,本研究比较了不同上样速度对17种FQs萃取回收效果的影响。如图3所示,当上样速度为4、8和12 mL·min−1时,对17种FQs平均回收率基本没有影响,说明目标化合物很容易吸附在固相萃取小柱上。在节约时间的同时,考虑到当样品中FQs浓度较高时,上样速度过快可能导致目标物富集效果变差,故本研究最终选择的上样流速为8~12 mL·min−1。
4)洗脱溶剂的选择。为了考察不同洗脱溶剂对17种FQs的洗脱效果,本研究对比了0.1%甲酸甲醇和纯甲醇2种洗脱溶剂。如图4所示。其中用0.1%甲酸甲醇溶液洗脱的平均回收率为79.6%~100%,相对偏差为0.7%~7.4%;而用甲醇洗脱的平均回收率为72.6%~92.7%,相对偏差为3.2%~13.6%。虽然0.1%甲酸甲醇略优于甲醇,但2种洗脱溶剂对17种FQs的回收率均大于70%,因此,上述2种洗脱溶剂均可作为17种FQs的洗脱溶剂。
5)洗脱溶剂用量优化。为获取最佳的洗脱溶剂体积,在选定0.1%甲酸甲醇溶液作为洗脱溶剂后,将9份1 000 mL水样(加标质量浓度为20 ng·L−1)平均分成3组,分别对比了3、6和9 mL洗脱体积对17种FQs的回收效果。如图5所示,当洗脱量为3 mL时,那氟沙星和氟甲喹的平均回收率很低,均小于10%,其他目标化合物的平均回收率均在70%以上;当洗脱量为6 mL和9mL时,大部分目标已经被洗脱,且各目标化合物的平均回收率均在80%以上。考虑到养殖废水基质较为复杂,洗脱量较少可导致部分目标化合物未能完全洗脱,回收效果变差,故本研究选取9 mL作为最佳洗脱量。
2.2 色谱条件的选择和优化
1)色谱柱的选择。FQs多为两性物质,具有一定的水溶性,不同的色谱柱对其分离影响较大。本研究考察了多种常用的C18反相色谱柱对17种FQs的分离效果,如Kinetex-C18®(100 mm×3.0 mm×2.6 μm)(A柱)、XTEERRA-C18® MS C18 (100 mm×2.1 mm×3.5 μm)(B柱)和CORTECS® C18(100 mm×4.6 mm×2.7 μm)(C柱)。如图6所示(进样浓度为20 μg·L−1),使用A柱分离17 种FQs时,出现峰形拖尾现象;使用B柱分离FQs时,莫西沙星和那氟沙星等目标物的色谱峰展宽严重;而使用C柱分离17 种FQs时,不仅分离效果好,峰形对称,而且响应强度也相对较高。因此,本研究选择C柱对17种FQs进行分离。
2)流动相组分优化。确定色谱柱后,为更好的分离各种目标物并获取满意的峰型,本研究对流动相组分进行了优化。首先,比较了不同有机相(甲醇和乙腈)对目标化合物的分离效果。结果表明,使用甲醇时,基线相对较低,并且对目标化合物具有更好的分离效果,因此,确定甲醇为有机相。因在流动相中加入适当的有机酸或缓冲盐可增强目标物的离子化效率,增强分析方法的灵敏度,所以在确定有机相为甲醇后,又比较了不同有机酸、缓冲盐对灵敏度的影响,包括5 mmol·L−1乙酸铵、0.1%甲酸水及0.1%甲酸−5 mmol·L−1甲酸铵等。如图7所示,0.1%甲酸−5 mmol·L−1甲酸铵和甲醇作为流动相时,峰型最好,灵敏度最高,信号强度最强。
3)洗脱梯度优化。经反复优化洗脱梯度程序后,最终选择1.4.1节所述的洗脱梯度。在选定的洗脱梯度下,所有组分均在20 min以内出峰,且分离效果最为佳,具体色谱图见图8(进样浓度为100 μg·L−1)。
2.3 质谱条件的选择和优化
配制质量浓度为1.0 mg·L−1的FQs、内标化合物和替代物的混合标准溶液,在正离子模式下,用直接注射进样方式扫描并优化母离子/子离子特征离子对,及相应的锥孔电压、碰撞电压等其他参数,利用获取的全部特征离子及离子间的丰度比进行定性分析,以丰度最高的特征离子响应与浓度的关系进行定量分析,质谱优化参数结果如表1所示。
表 1 目标化合物、替代物及内标物的多离子反应监测条件Table 1. The operating parameters of the target compounds, substitutes and internal standard化合物 质荷比 (m/z) 电压/V 定量内标 母离子 定量离子 定性离子 锥孔电压 碰撞电压 氟甲喹 262.1 202 244.1 56 27/45 13C3-氟甲喹 诺氟沙星 320.2 276.2 233.1 101 25/35 环丙沙星d8 依诺沙星 321.1 303.1 232.1 61 29/49 环丙沙星d8 环丙沙星 332.1 314.1 288.1 81 29/27 环丙沙星d8 培氟沙星 334.2 316 290.1 76 29/27 环丙沙星d8 洛美沙星 352.2 308.2 265.1 91 25/33 恩诺沙星-d5 达氟沙星 358.2 340.2 82.1 76 33/73 恩诺沙星-d5 恩诺沙星 360.2 316.1 245.1 76 27/37 恩诺沙星-d5 那氟沙星 361.2 343.2 283.1 85 35/50 13C3-氟甲喹 氧氟沙星 362.2 318.2 261.1 76 27/39 恩诺沙星-d5 马波沙星 363.1 72.1 320.1 80 46/23 环丙沙星d8 氟罗沙星 370.1 326.1 269.1 76 27/37 恩诺沙星-d5 加替沙星 376.2 332.2 261 81 27/41 恩诺沙星-d5 沙拉沙星 386.1 342.1 299.1 106 27/39 恩诺沙星-d5 奥比沙星 396 352 295.2 80 24/32 恩诺沙星-d5 二氟沙星 400.2 356.2 299.1 81 29/39 恩诺沙星-d5 莫西沙星 402.2 384.2 358.2 76 31/29 环丙沙星d8 诺氟沙星-d5 325.2 281.2 238.1 86 25/35 环丙沙星d8 恩诺沙星-d5 365.2 321.2 347.2 81 29/31 — 环丙沙星-d8 340.2 322.1 296.1 91 31/27 — 13C3-氟甲喹 265.1 247.1 205.1 46 25/45 — 注:表中“/”前后是母离子/定量离子和母离子/定性离子优化后的碰撞电压 2.4 方法性能
1)方法的线性关系、检出限和定量限。将17种FQs的200 μg·L−1 标准溶液按梯度稀释,梯度浓度分别为0.5、1、2、5、10、 20、50、100 μg·L−1。用已优化好的色谱条件和质谱条件进行测定,内标法定量。以17种FQs的质量浓度为横坐标,定量离子的峰面积为纵坐标,绘制标准曲线。结果表明,17种FQs在0.5-100 μg·L−1内线性关系良好,R2>0.99。根据环境监测分析方法标准制修订技术导则[27],对大约检出限为3-5倍浓度的样品进行7次平行测定,计算测定结果的标准偏差(SD)。其方法检出限(LOD)为3.143倍的SD,定量限(LOQ) 为4倍的 LOD。根据计算结果,LOD为0.08-0.3 ng·L−1,LOQ为0.32-1.2 ng·L−1。
2)加标实验结果。用超纯水进行空白基质加标回收实验,加标质量浓度分别设置为10 ng·L−1和90 ng·L−1,每个浓度水平做6个重复样品,同时设置6个空白样品。按第1.4节中的步骤对样品进行处理,以考察方法的总体回收率、重现性是否达到检测分析要求。如表2所示,当添加量为10 ng·L−1时,17种FQs的平均回收率为58.6%~104.2%,RSD为2.9%~19.3%;当添加量为90 ng·L−1时,平均回收率为65.3%~91.0 %,RSD为2.1%~18.6%。此外,本研究还以养殖废水为基质,设置了6个基质加标实验,加标质量浓度为20 ng·L−1。结果如表3所示,17种FQs的平均回收率为47.8%~118.7%,RSD为3.0%~18.6%。本方法的空白和基质加标回收结果与美国 EPA1694 [28]相当,但与其他几种FQs相比,莫西沙星、达氟沙星和依诺沙星的RSD值相对偏高。这可能是由于现有的富集和净化手段未能有效去除水样的背景干扰,在运用正离子扫描模式时,这些背景会增强或减弱目标化合物的电离结果,进而影响仪器测定结果的准确性,造成方法重现性较差,RSD偏高。
表 2 17 种FQs化合物的纯水加标回收率(n = 6)及相对标准偏差Table 2. Recovery rate (n = 6) and RSD of 17 fluoroquinolones antibiotics in pure water化合物 添加质量浓度/(ng·L−1) 平均回收率/% RSD/% 化合物 添加质量浓度/(ng·L−1) 平均回收率/% RSD/% 莫西沙星 10 65.7 8.6 恩诺沙星 10 80.1 14 90 73.5 4.7 90 78.3 2.8 二氟沙星 10 63.9 7.8 达氟沙星 10 80.6 19.3 90 69.1 2.1 90 78.9 2.6 奥比沙星 10 95.6 7.1 洛美沙星 10 96.2 3 90 91 6.8 90 88.8 3.8 沙拉沙星 10 58.6 7.1 培氟沙星 10 89.2 12.6 90 65.3 4.8 90 89 4.7 加替沙星 10 85.4 2.9 环丙沙星 10 79.7 9.5 90 84 4.1 90 81.5 2.5 氟罗沙星 10 94.4 7.2 依诺沙星 10 77 9.2 90 90.3 10.8 90 78.5 18.6 马波沙星 10 93.4 3.7 诺氟沙星 10 83.1 4.3 90 87.8 7.8 90 75.9 6.4 氧氟沙星 10 104.2 6.8 氟甲喹 10 86.2 11.4 90 90.4 5.3 90 78 5.8 那氟沙星 10 76.6 12.1 90 78.7 7.3 表 3 17 种FQs化合物的养殖废水加标回收率(n = 6)及相对标准偏差Table 3. Recovery rate (n = 6) and RSD of 17 fluoroquinolones antibiotics in aquaculture wastewater化合物 添加质量浓度/(ng·L−1) 平均回收率/% RSD/% 化合物 添加质量浓度/(ng·L−1) 平均回收率/% RSD/% 莫西沙星 20 85.7 18.6 恩诺沙星 20 66.4 11.3 二氟沙星 20 47.8 11.6 达氟沙星 20 70.8 12.3 奥比沙星 20 59.7 12.6 洛美沙星 20 62.8 10.2 沙拉沙星 20 50.1 14.6 培氟沙星 20 118.7 11.2 加替沙星 20 56.8 13.4 环丙沙星 20 82.7 8.9 氟罗沙星 20 75 8.4 依诺沙星 20 94.4 11.2 马波沙星 20 95.7 12 诺氟沙星 20 54.1 10 氧氟沙星 20 83.4 11 氟甲喹 20 104.8 3 那氟沙星 20 100.4 3.2 2.5 实际样品测定
为验证本研究建立的检测分析方法,按照《污水监测技术规范》(HJ91.1-2019)[29]中的样品采集规定,采集了广州某水产养殖场的养殖废水并进行了测定。样品采集后,立即用盐酸调节pH至2.0±0.5,4 ℃以下避光运送至实验室。测定结果表明:采集的养殖废水共检测出9种FQs,其中莫西沙星为2.34 ng·L−1,氟甲喹为0.96 ng·L−1,马波沙星为0.24 ng·L−1,氧氟沙星为9.36 ng·L−1,恩诺沙星为1.13 ng·L−1,达氟沙星为5.96 ng·L−1,培氟沙星为2.20 ng·L−1,环丙沙星为0.53 ng·L−1,依诺沙星为3.48 ng·L−1。
3. 结论
1)通过优化固相萃取条件、色谱分离条件、质谱检测参数,建立了一种快速、准确测定养殖废水中17种FQs的固相萃取-液相色谱-三重四极杆串联质谱法。该方法通过特征碎片离子和色谱保留时间定性,通过特征准分子离子定量,方法检出限为0.08~0.3 ng·L−1;空白加标平均回收率为58.6%~104.2%,RSD为2.1%~19.3%(n=6);基质加标平均回收率为47.8%~118.7%,RSD小于20%(n=6),满足养殖废水中17种FQs残留分析的要求。
2)所采集的广州某养殖场的养殖废水样品中,含有多种不同含量的氟喹诺酮类抗生素,其中氧氟沙星检出浓度最高(9.36 ng·L−1),达氟沙星次之(5.96 ng·L−1)。
-
表 1 目标化合物、替代物及内标物的多离子反应监测条件
Table 1. The operating parameters of the target compounds, substitutes and internal standard
化合物 质荷比 (m/z) 电压/V 定量内标 母离子 定量离子 定性离子 锥孔电压 碰撞电压 氟甲喹 262.1 202 244.1 56 27/45 13C3-氟甲喹 诺氟沙星 320.2 276.2 233.1 101 25/35 环丙沙星d8 依诺沙星 321.1 303.1 232.1 61 29/49 环丙沙星d8 环丙沙星 332.1 314.1 288.1 81 29/27 环丙沙星d8 培氟沙星 334.2 316 290.1 76 29/27 环丙沙星d8 洛美沙星 352.2 308.2 265.1 91 25/33 恩诺沙星-d5 达氟沙星 358.2 340.2 82.1 76 33/73 恩诺沙星-d5 恩诺沙星 360.2 316.1 245.1 76 27/37 恩诺沙星-d5 那氟沙星 361.2 343.2 283.1 85 35/50 13C3-氟甲喹 氧氟沙星 362.2 318.2 261.1 76 27/39 恩诺沙星-d5 马波沙星 363.1 72.1 320.1 80 46/23 环丙沙星d8 氟罗沙星 370.1 326.1 269.1 76 27/37 恩诺沙星-d5 加替沙星 376.2 332.2 261 81 27/41 恩诺沙星-d5 沙拉沙星 386.1 342.1 299.1 106 27/39 恩诺沙星-d5 奥比沙星 396 352 295.2 80 24/32 恩诺沙星-d5 二氟沙星 400.2 356.2 299.1 81 29/39 恩诺沙星-d5 莫西沙星 402.2 384.2 358.2 76 31/29 环丙沙星d8 诺氟沙星-d5 325.2 281.2 238.1 86 25/35 环丙沙星d8 恩诺沙星-d5 365.2 321.2 347.2 81 29/31 — 环丙沙星-d8 340.2 322.1 296.1 91 31/27 — 13C3-氟甲喹 265.1 247.1 205.1 46 25/45 — 注:表中“/”前后是母离子/定量离子和母离子/定性离子优化后的碰撞电压 表 2 17 种FQs化合物的纯水加标回收率(n = 6)及相对标准偏差
Table 2. Recovery rate (n = 6) and RSD of 17 fluoroquinolones antibiotics in pure water
化合物 添加质量浓度/(ng·L−1) 平均回收率/% RSD/% 化合物 添加质量浓度/(ng·L−1) 平均回收率/% RSD/% 莫西沙星 10 65.7 8.6 恩诺沙星 10 80.1 14 90 73.5 4.7 90 78.3 2.8 二氟沙星 10 63.9 7.8 达氟沙星 10 80.6 19.3 90 69.1 2.1 90 78.9 2.6 奥比沙星 10 95.6 7.1 洛美沙星 10 96.2 3 90 91 6.8 90 88.8 3.8 沙拉沙星 10 58.6 7.1 培氟沙星 10 89.2 12.6 90 65.3 4.8 90 89 4.7 加替沙星 10 85.4 2.9 环丙沙星 10 79.7 9.5 90 84 4.1 90 81.5 2.5 氟罗沙星 10 94.4 7.2 依诺沙星 10 77 9.2 90 90.3 10.8 90 78.5 18.6 马波沙星 10 93.4 3.7 诺氟沙星 10 83.1 4.3 90 87.8 7.8 90 75.9 6.4 氧氟沙星 10 104.2 6.8 氟甲喹 10 86.2 11.4 90 90.4 5.3 90 78 5.8 那氟沙星 10 76.6 12.1 90 78.7 7.3 表 3 17 种FQs化合物的养殖废水加标回收率(n = 6)及相对标准偏差
Table 3. Recovery rate (n = 6) and RSD of 17 fluoroquinolones antibiotics in aquaculture wastewater
化合物 添加质量浓度/(ng·L−1) 平均回收率/% RSD/% 化合物 添加质量浓度/(ng·L−1) 平均回收率/% RSD/% 莫西沙星 20 85.7 18.6 恩诺沙星 20 66.4 11.3 二氟沙星 20 47.8 11.6 达氟沙星 20 70.8 12.3 奥比沙星 20 59.7 12.6 洛美沙星 20 62.8 10.2 沙拉沙星 20 50.1 14.6 培氟沙星 20 118.7 11.2 加替沙星 20 56.8 13.4 环丙沙星 20 82.7 8.9 氟罗沙星 20 75 8.4 依诺沙星 20 94.4 11.2 马波沙星 20 95.7 12 诺氟沙星 20 54.1 10 氧氟沙星 20 83.4 11 氟甲喹 20 104.8 3 那氟沙星 20 100.4 3.2 -
[1] 沈睿智, 曾天映, 张海惠, 等. 氟喹诺酮-3-N-酰胺类衍生物的合成与抗肿瘤活性[J]. 化学通报, 2020, 83(8): 730-734. [2] 王岩. 氟喹诺酮类药物左氧氟沙星联合黄连素治疗细菌性痢疾的效果分析[J]. 中国医药指南, 2020, 18(2): 153-154. [3] HERRERA-HERRERA A V, HERNNDEZ-BORGES J, BORGES-MIQUEL T M, et al. Dispersive liquid-liquid microextraction combined with ultra-high performance liquid chromatographyfor the simultaneous determination of 25 sulfonamide and quinolone antibiotics in water samples[J]. Journal of Pharmaceutical and Biomedical Analysis, 2013, 75: 130-137. doi: 10.1016/j.jpba.2012.11.026 [4] HERRERA-HERRERA A V, HERN NDEZ-BORGES J, BORGES-MIQUEL T M, et al, Dispersive liquid-liquid microextraction combined with nonaqueous capillary electrophoresis for the determination of fluoroquinolone antibiotics in waters[J]. Electrophoresis, 2010, 31(20): 3457-3465. [5] RODRIGUEZ E, NAVARRO-VILLOSLADA F, BENITO-PENA E, et al. Multiresidue determination of ultratrace levels of fluoroquinolone antimicrobials in drinking and aquaculture water samples by automated online molecularly imprinted solid phase extraction and liquid chromatography[J]. Analytical Chemistry, 2011, 83(6): 2046-2055. doi: 10.1021/ac102839n [6] GROS M, RODRGUEZ-MOZAZ, BARCEL D. Fast and comprehensive multi-residue analysis of a broad range of human and veterinary pharmaceuticals and some of their metabolites in surface and treated waters by ultra-high-performance liquid chromatography coupled to quadrupole-linear ion trap tandem mass spectrometry[J]. Journal of Chromatography A, 2012, 1248: 104-121. doi: 10.1016/j.chroma.2012.05.084 [7] ZHOU J L, MASKAOUI K, LUFADEJU A. Optimization of antibiotic analysis in water by solid-phase extraction and high performance liquid chromatography-mass spectrometry/mass spectrometry[J]. Analytica Chimica Acta, 2012, 731: 32-39. doi: 10.1016/j.aca.2012.04.021 [8] DORIVAL-GARC N, ZAFRA-G MEZ A, CANTARERO S, et al. Simultaneous determination of 13 quinolone antibiotic derivatives in wastewater samples using solid-phase extraction and ultra performance liquid chromatography-tandem mass spectrometry[J]. Microchemical Journal, 2013, 106: 323-333. doi: 10.1016/j.microc.2012.09.002 [9] PRIETO A, SCHRADER S, BAUERC, et al. Synthesis of a molecularly imprinted polymer and its application for microextraction by packed sorbent for the determination of fluoroquinolone related compounds in water[J]. Analytica Chimica Acta, 2011, 685(2): 146-152. doi: 10.1016/j.aca.2010.11.038 [10] HUANG X J, QIU N N, YUAN D X, et al. Preparation of a mixed stir bar for sorptive extraction based on monolithic material for the extraction of quinolones from wastewater[J]. Journal of Chromatography A. 2010, 1217(16): 2667-2673. [11] RUSU A, HANCU G, V LGYI G, et al. Separation and determination of quinolone antibacterials by capillary electrophoresis[J]. Journal of Chromatographic Science, 2014, 52(8): 919-925. doi: 10.1093/chromsci/bmt107 [12] MONTESDEOCA-ESPONDA S, SOSA-FERRERA Z, SANTANA-RODR GUEZ J J. Combination of microwave-assisted micellar extraction with liquid chromatography tandem mass spectrometry for the determination of fluoroquinolone antibiotics in coastal marine sediments and sewage sludges samples[J]. Biomedical Chromatography, 2012, 26(1): 33-40. doi: 10.1002/bmc.1621 [13] DORIVAL-GARC A N, ZAFRA-G MEZ A, CAMINO-S NCHEZ F J, et al. Analysis of quinolone antibiotic derivatives in sewage sludge samples by liquid chromatography-tandem mass spectrometry: Comparison of the efficiency of three extraction techniques[J]. Talanta, 2013, 106: 104-118. doi: 10.1016/j.talanta.2012.11.080 [14] 张运尚, 杜加茹, 王伟东, 等. 氟喹诺酮类药物残留限量及检测技术研究进展[J]. 中国畜牧杂志, 2021, 57(4): 39-44. [15] 柴丽月, 柳海, 梁芹芹, 等. 宁波市水产品中氟喹诺酮类药物残留现状分析及对策[J]. 检验检疫刊, 2020, 30(1): 25-27. [16] 杨硕. 畜禽养殖废水的抗生素污染现状及检测方法[J]. 农业与技术, 2020, 40(21): 107-108. [17] HARTMANN A, ALDER A C, KOLLER T, et al. Identification of fluoroquinolone antibiotics as the main source of human genotoxicity in native hospital wastewater[J]. Environmental Toxicology and Chemistry, 1998, 17(3): 377-382. doi: 10.1002/etc.5620170305 [18] MATSUI Y, OZU T, INOUE T, et al. Occurrence of a veterinary antibiotic in streams in a small catchment area with livestock farms[J]. Desalination, 2008, 226(1-3): 215-221. doi: 10.1016/j.desal.2007.01.243 [19] CAMPAGNOLOA E R, JOHNSOn K R, KARPATIA A, et al. Antimicrobial residues in animal waste and water resources proximal to large-scale swine and poultry feeding operations[J]. Science of the Total Environment, 2002, 299(1/2/3): 89-95. [20] 郝燕娟, 李汉敏, 叶卓霖, 等. 饲料中喹诺酮酸酯药物的测定液相色谱—串联质谱法[J]. 广东饲料, 2018, 27(11): 41-45. doi: 10.3969/j.issn.1005-8613.2018.11.011 [21] 周鑫, 张建雄, 李继丰, 等. 超高效液相色谱串联质谱法测定饲料中7种氟喹诺酮类药物[J]. 养殖与饲料, 2018(1): 4-6. doi: 10.3969/j.issn.1671-427X.2018.01.002 [22] 李妍, 闫蕊, 王孝研, 等. 动物源性食品中氟喹诺酮类抗生素残留检测方法的研究进展[J]. 食品安全质量检测学报, 2019, 10(10): 2918-2928. doi: 10.3969/j.issn.2095-0381.2019.10.015 [23] 张宏博, 王洋, 王燕, 等. 肉制品中喹诺酮残留检测[J]. 食品安全导刊, 2018(31): 54-59. doi: 10.3969/j.issn.1674-0270.2018.31.019 [24] 魏丹, 国明, 张菊. 加速溶剂萃取-磁固相萃取-高效液相色谱-串联质谱法测定水产品中10种氟喹诺酮类药物残留[J]. 色谱, 2020, 38(12): 1413-1422. [25] 钱卓真, 朱世超, 魏博娟, 等. 高效液相色谱—串联质谱法测定水产品中19种喹诺酮类药物残留量[J]. 中国渔业质量与标准, 2012(3): 68-76. [26] 陈小华. 固相萃取技术与应用 第2版[M]. 北京: 科学出版社. 2019. [27] 中华人民共和国生态环境部. 环境监测分析方法标准制订技术导则: HJ 168-2020[S]. 北京: 中国环境出版社, 2020. [28] Englert B . Method 1694: Pharmaceuticals and Personal Care Products in Water, Soil, Sediment, and Biosolids by HPLC/MS/MS. 2007. [29] 中华人民共和国生态环境部. 污水监测技术规范: HJ 91.1-2019[S]. 北京: 中国环境科学出版社, 2020. -




 下载:
下载: