-
作为水生生态系统的主要养分[1],磷(P)是引起湖泊富营养化的关键营养元素,可导致藻类过度繁殖和水质恶化[2],其触发水体富营养化的阈值浓度可低至10 μg·L−1[3]。全球约有30%~50%的水源由于淡水中含有过量溶解磷而面临富营养化问题[4]。调节总磷浓度、减少磷源输入是解决富营养化的重要途径。
传统污水除磷方法主要有生物处理、化学沉淀、离子交换和吸附[5]。其中,吸附具有易于操作、去除效率高、污泥产生少、成本低等特点,被认为是除磷的最佳选择[6]。除了使用沸石、活性炭和生物炭等吸附剂除磷,近年来,由于凹凸棒土易获得且体量大,比传统的吸附剂更环保、更具成本效益[7]。凹凸棒土(Si8O20Mg5 (OH) 2 (H2O) 4·4H2O)是一种水合Mg-Al硅酸盐矿物,具有固有通道和纳米结晶度的纤维层状结构。与其他材料相比,凹凸棒土由于具有高度多孔的微结构、阳离子交换能力强和活性表面官能团多等优势,故可将其作为潜在的除磷吸附剂应用于大规模废水治理[8]。
近年来,相关学者已经探索了对天然凹凸棒土进行改性以克服其局限性并提高吸附磷酸盐能力的方法。稀土具有较活泼的化学性质,能与周期表中许多元素反应,生成络合物,其与无机配体形成络合物的稳定性顺序由大到小为PO4>CO3>F>SO4>SCN>NO3 >Cl[9]。稀土吸附剂具有可选择性、吸附效果好、吸附容量高、易于再生等特点,且能替代贵金属,在含磷废水处理中应用前景广阔,因此,开展稀土在废水除磷方面的研究具有一定意义[10]。在过去的20 a中,由于镧(La)系吸附剂对磷酸盐具有亲和力强、吸附效果好的优点,被广泛用于废水除磷。但使用除La以外的其他稀土元素去除磷酸盐却鲜有报道,且目前大多数研究集中在对高浓度磷的去除,而对低浓度磷去除研究较少。
为此,本研究以凹凸棒土(ATP)为原料,分别使用钕(Nd)、铈(Ce)、钇(Y)、镧(La)、镨(Pr)对凹凸棒土的表面进行了改性,旨在制备出吸附效果好、选择性吸附强的环境友好型高效吸附除磷剂,以期为低浓度含磷废水的处理提供参考。
-
实验所用凹凸棒土取自江苏省盱眙县中材凹凸棒石粘土有限公司,所用药品皆为分析纯(AR):盐酸、氢氧化钠、硝酸钇、硝酸钕、硝酸镨、硝酸镧、硝酸铈、磷酸二氢钾、表面活性剂十八烷基三甲基溴化铵等;实验中所有溶液均由去离子水配制。
-
将破碎研磨后的凹凸棒土过350目筛后,置于马弗炉内400 ℃焙烧1 h 后,自然冷却,备用。称取经过热处理的凹凸棒土置于250 mL锥形瓶中,再加入50 mL浓度为0.4 mol·L−1的硝酸钕,用保鲜膜密封后置于恒温振荡箱中,在40 ℃、200 r·min−1下反应24 h后,加入20 mL浓度为 50 mmol·L−1的十八烷基三甲基溴化铵溶液,继续反应24 h后,再置于105 ℃烘干,所得固体材料即为钕改性凹凸棒土(Nd-ATP)。铈改性凹凸棒土(Ce-ATP)、钇改性凹凸棒土(Y-ATP)、镧改性凹凸棒土(La-ATP)、镨改性凹凸棒土(Pr-ATP)制备方法与钕改性凹凸棒土相同。
-
1)投加量的影响。分别称量0.01~0.05 g的稀土改性凹凸棒土吸附剂(Nd-ATP、Ce-ATP、Y-ATP、La-ATP、Pr-ATP)加入到100 mL质量浓度为1 mg·L−1的TP溶液中,调节pH为3,在25 ℃,300 r·min−1下反应24 h,吸附完成后,测定TP的质量浓度并计算其去除率。
2)初始pH的影响。分别称量0.04 g的稀土改性凹凸棒土吸附剂加入到100 mL质量浓度为1 mg·L−1的TP溶液中,调节pH为3、5、7、9、11,在25 ℃、300 r·min−1下反应24 h,吸附完成后,测定TP的质量浓度并计算其去除率。
3)共存阴离子的影响。分别称量0.04 g的稀土改性凹凸棒土吸附剂加入到100 mL浓度为1 mg·L−1的TP溶液中,按1 mg·L−1投加
HCO−3 、SO2−4 、Cl−以及混合离子溶液,在25 ℃、300 r·min−1下反应24 h,吸附完成后,测定TP的质量浓度并计算其去除率。4)吸附动力学。分别称量0.04 g的稀土改性凹凸棒土吸附剂加入100 mL质量浓度为1 mg·L−1的TP溶液中,调节pH为3,在25 ℃、300 r·min−1下反应,在0~360 min内测定TP的质量浓度,并计算其吸附量。采用准一级动力学(式(1))和准二级动力学模型(式(2))描述稀土改性凹凸棒土吸附剂对TP的吸附过程。
式中:qe为平衡吸附量,mg·g−1; qt为t时刻的吸附量,mg·g−1;t为吸附时间,min; k1为准一级动力学速率常数,min−1;k2为准二级动力学速率常数,g·(mg·min)−1。
5)吸附等温线。分别称量0.05 g的稀土改性凹凸棒土吸附剂加入100 mL浓度为5、10、20、30、40、50 mg·L−1的TP溶液中,调节pH为3,在25 ℃、300 r·min−1下反应24 h,并计算TP的吸附量。采用Langmuir(式(3))和Freundlich模型(式(4))对吸附过程进行拟合。
式中:Qm为最大吸附容量,mg·g−1;Qe为平衡吸附量,mg·g−1;b为Langmuir模型常数,L·mg−1;n为Freundlich模型常数;Kf为Freundlich模型常数,mg·g−1。
-
1) SEM表征分析。稀土改性前后凹凸棒土SEM照片如图1所示。图1(a)中天然凹凸棒土呈板条状或纤维状,通过棒状单晶的紧密排列,形成晶体束然后聚集在一起[11];表面呈现出带有棒束的倒塌层状结构,且具有较为均匀的孔隙,为负载稀土提供了良好环境。改性后凹凸棒土(图1(b)~(f))凹凸棒土单晶排列更加独立和分散,表面孔隙增大。其原因可能是:高温改性致使白云石和碳酸钙等杂质被去除,原有分子之间的键合力减弱,孔道疏通[12],在表面活性剂十八烷基三甲基溴化铵的作用下,晶体束的团聚现象弱化。
2) XRD表征分析。稀土改性前后凹凸棒土的XRD图谱如图2所示。由图2可见,天然凹凸棒土中含有凹凸棒石、白云石和石英,经过高温焙烧后,凹凸棒石、白云石、石英的强度虽均有不同程度地减弱[13],但改性后凹凸棒土XRD谱图的基线基本不变,峰的强度、数量保持相对不变。这说明,在一定温度下凹凸棒土具有热稳定性[14]。凹凸棒土经稀土改性后,在29.1°处有稀土氧化物的衍射峰存在,表明稀土已经成功负载于凹凸棒土表面[15]。
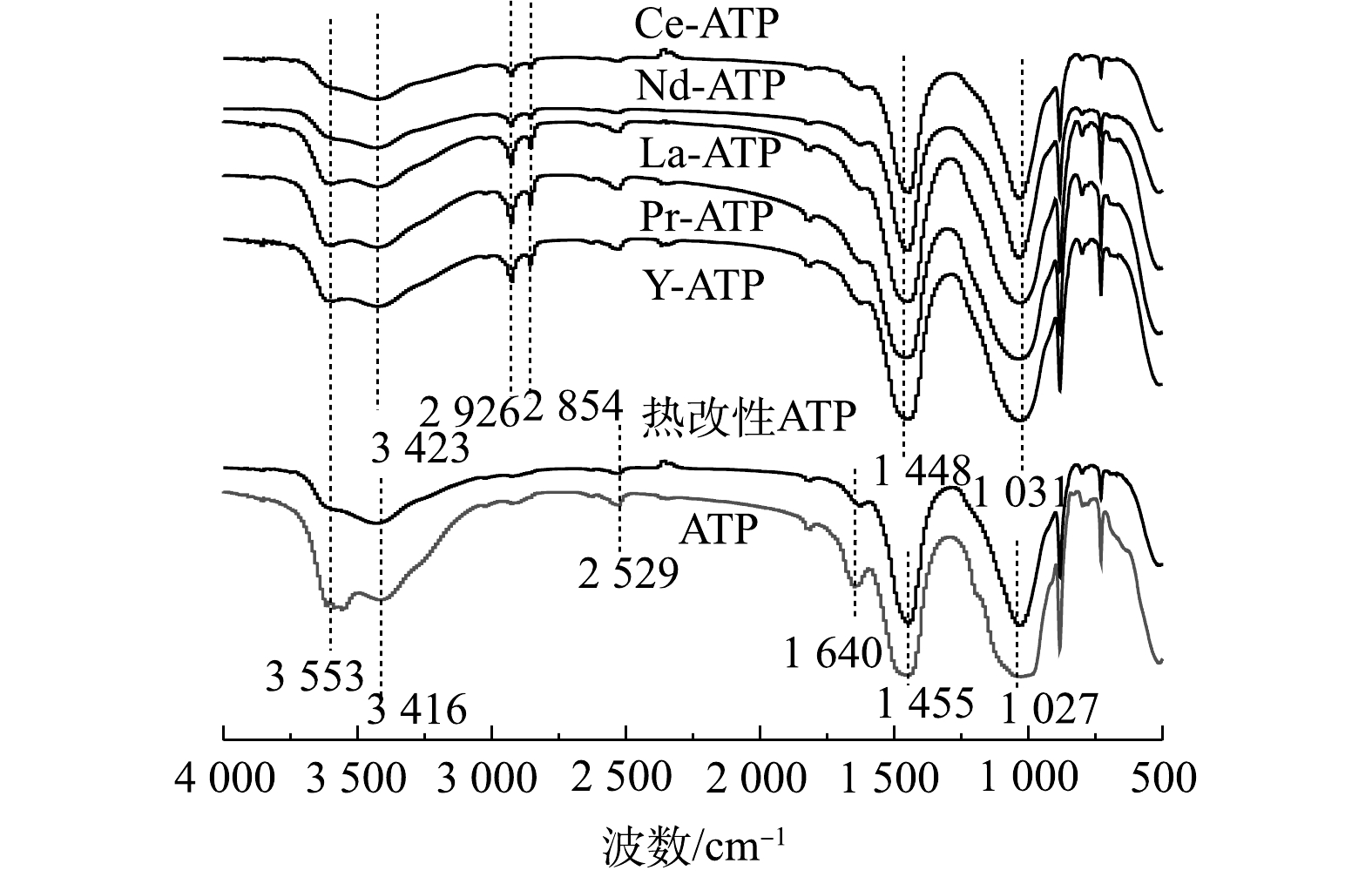
3) FT-IR。稀土改性前后凹凸棒土FT-IR图谱如图3所示。由图3可见:凹凸棒土中四面体和八面体结构的内部—OH拉伸振动峰为3 553 cm−1;3 416 cm −1是结构水中将Mg和Al连接在四面体和八面体之间的羟基官能团的拉伸振动带;Si—O3—C的反对称拉伸振动峰组对应于1 027 cm−1。对比热改性前后凹凸棒土的红外光谱变化可发现,原1 640 cm−1和2 529 cm−1处归属于凹凸棒土吸附水和沸石水的振动峰消失。其原因可能是:高温焙烧后的凹凸棒土表面吸附水、沸石水依次脱羟基,但仍保留结构水和部分结晶水[16]。如图3所示,稀土改性凹凸棒土后,凹凸棒土骨架的振动峰略有偏移,均向波数高的方向移动且凹凸棒土的—OH振动峰均有不同程度上的降低;改性后的凹凸棒土红外光谱中出现了几处新峰,2 854 cm−1 和2 926 cm−1处为—NH2的振动吸收峰,这表明经过表面活性剂十八烷基三甲基溴化铵改性后,已有氨基被修饰到凹凸棒土表面。
4) XRF表征分析。稀土改性前后凹凸棒土XRF数据见表1。由表1可知,与天然凹凸棒土相比,稀土改性后的凹凸棒土中钙、镁、铝、铁等含量均有不同程度的减少,这应该是稀土元素通过与上述离子发生交换反应从而成功负载到凹凸棒土表面[17];Ce-ATP表面负载Ce氧化物含量为3.78%,高于其他几种改性凹凸棒土稀土氧化物占比,其原因可能是Ce元素与钙、镁、铝等离子交换的能力更强。
-
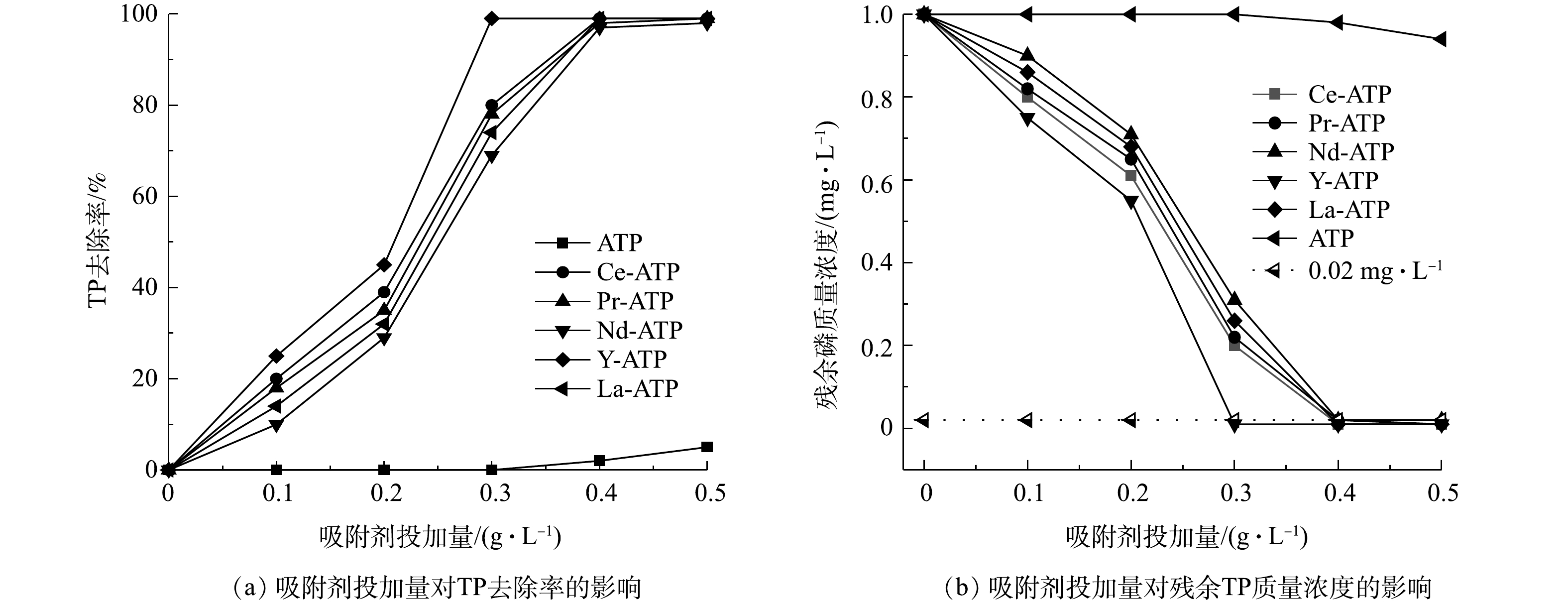
由图4可知,随着投加量逐渐增加,5种稀土改性后的凹凸棒土对TP的去除率均呈现先增大后不变的趋势。一般而言,当投加量过小时,溶液中的磷酸根离子远多于改性凹凸棒土所能提供的吸附位点,因此TP去除率较低;当投加量持续增加时,吸附剂提供的吸附位点也随之增加,溶液中剩余的磷酸根离子可附着在吸附剂的表面,因此,TP去除率不断增加。从实验结果看,当吸附剂用量为 0.3 g·L−1时,5种稀土改性凹凸棒土对TP的去除率均达到了70%,其中Y-ATP的去除率显著高于其余 4 种吸附剂,去除率可达98%。当吸附剂用量增加至 0.4 g·L−1时,5种稀土改性凹凸棒土对TP的去除率均达到了 98% 以上。此时,溶液残余TP质量浓度由0.9 mg·L−1急剧下降到0.02 mg·L−1,达到地表 Ⅰ 类水标准。当投加量继续增加,TP去除率和溶液残余TP质量浓度均不再发生变化。当天然凹凸棒土投加量由0.10 g·L−1增加至0.50 g·L−1时,TP去除率最高为6%,与稀土改性后的凹凸棒土TP去除率相差近17倍,这说明使用稀土改性凹凸棒土去除TP是高效可行的。
-
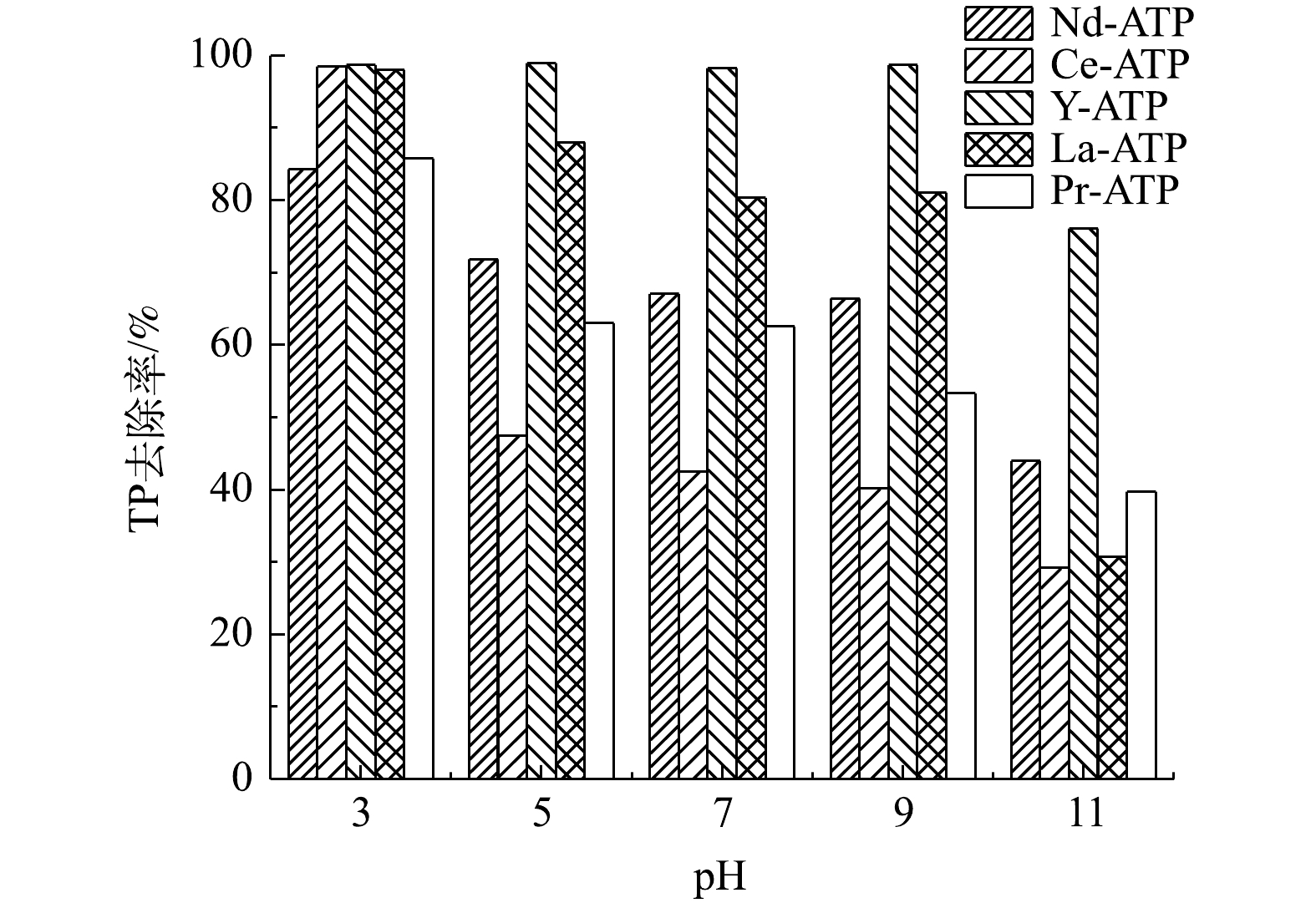
由图5可知,当pH为3时,吸附剂除磷效果最好,其中Ce-ATP、Y-ATP和La-ATP对TP去除率可达98%,Nd-ATP和Pr-ATP效果略差,约为84%。除Y-ATP外,其他几种吸附剂的TP去除率均随pH的增加有不同程度的降低。当pH为3~7时,磷酸盐以
H2PO−4 为主要形式存在于水体中[18],而随着pH的增加,磷酸盐逐渐转化为HPO2−4 和PO3−4 。稀土和磷酸根之间可能的化学反应如式(5)~式(7)所示(以La为例)。随着pH的升高,吸附剂表面所带的负电荷与磷酸根离子之间的静电斥力增加,表面的羟基离子也会与磷酸根离子发生竞争[19],稀土氧化物与磷酸根离子反应形成的络合物减少,从而导致TP的去除率降低。当pH>9时,5种吸附剂的TP去除率骤降,这与稀土在高pH的溶液中发生溶解有关。当pH为3~9时,Y-ATP的去除率较稳定,均可达到98%,表明其受pH的影响较小,可应用于不同pH的水体环境中。
-
由表2可知,
SO2−4 和Cl−对磷酸盐的吸附影响几乎可以忽略,HCO−3 和混合离子对于Nd-ATP、Ce-ATP、Pr-ATP这3种吸附剂除磷效果影响较大,Nd-ATP、Pr-ATP去除率下降近35%,Ce-ATP去除率下降近20%。这可能是由于HCO−3 会与磷酸根离子竞争吸附位点,致使磷酸根离子可用吸附位点减少,稀土氧化物与磷酸根离子反应形成的络合物减少,从而导致TP去除率降低。几种阴离子对磷酸盐吸附效果影响由大到小为混合离子>HCO−3 >SO2−4 >Cl−。 -
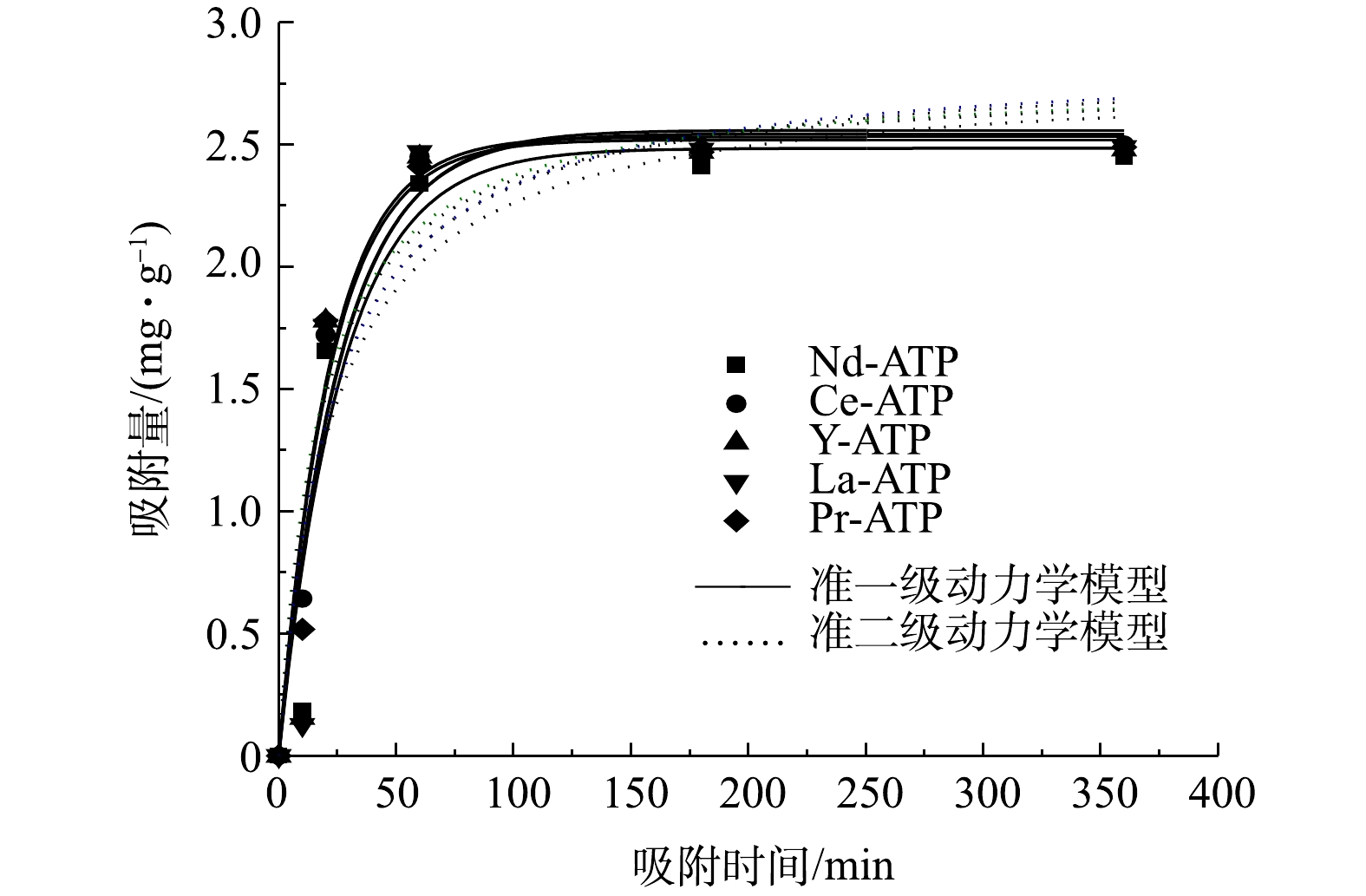
稀土改性凹凸棒土吸附除磷准一级和准二级模型拟合曲线如图6所示。由图6可知,随着吸附时间的增加,5 种稀土改性凹凸棒土对TP的吸附量不断增加直至逐渐趋于稳定。吸附剂除磷初始阶段吸附速率较快,在20 min时达到平衡吸附容量的60%~77%,在40 min内达到平衡吸附容量的92%~97%,绝大部分磷酸盐在前60 min内被吸附,大约在100 min时吸附达到平衡。这可能是因为,经过稀土改性后的凹凸棒土表面存在大量的有效活性位点,磷酸根离子与这些有效活性位点快速结合,随着吸附位点被逐渐占据,反应速率逐渐减慢直至达到平衡[20]。由表3可见,与准一级模型相比,准二级模型对磷酸盐的吸附数据具有更好的适用性(可决系数R2值更高,均大于0.92),表明该过程主要以化学吸附为主。
-
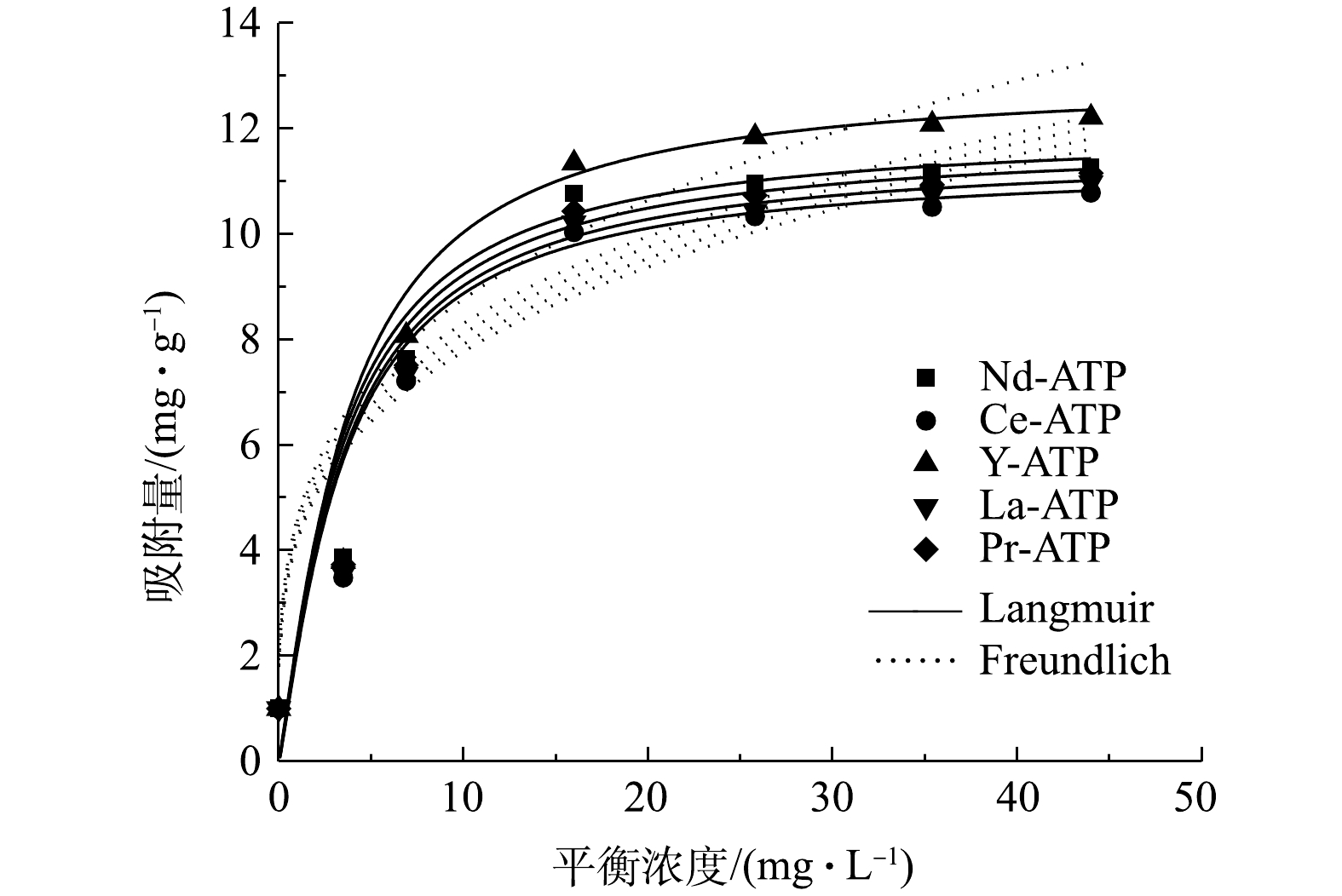
稀土改性凹凸棒土吸附除磷Langmuir和Freundlich模型拟合如图7所示,模型常数和相关系数见表4。由图7可知,在TP浓度较低的情况下,吸附量均随TP平衡浓度的升高而增大,当TP达到一定浓度后,吸附量的上升趋势逐渐趋于平稳。由表4可见,相对Freundlich模型,Langmuir模型的方程可决系数R2(R2>0.99)更高。表明稀土改性凹凸棒土对TP的吸附过程是一种以单分子层化学吸附为主[17]。溶液中磷酸根离子浓度与吸附剂表面液膜的浓度差增加,有利于磷酸根离子向吸附剂表面迁移,最终达到单位质量吸附剂的最大吸附量[19]。由Langmuir 模型可知,Nd-ATP、Ce-ATP、Y-ATP、La-ATP和Pr-ATP 的最大吸附量分别为11.95、11.32、12.94、11.53和 11.76 mg·g−1。
-
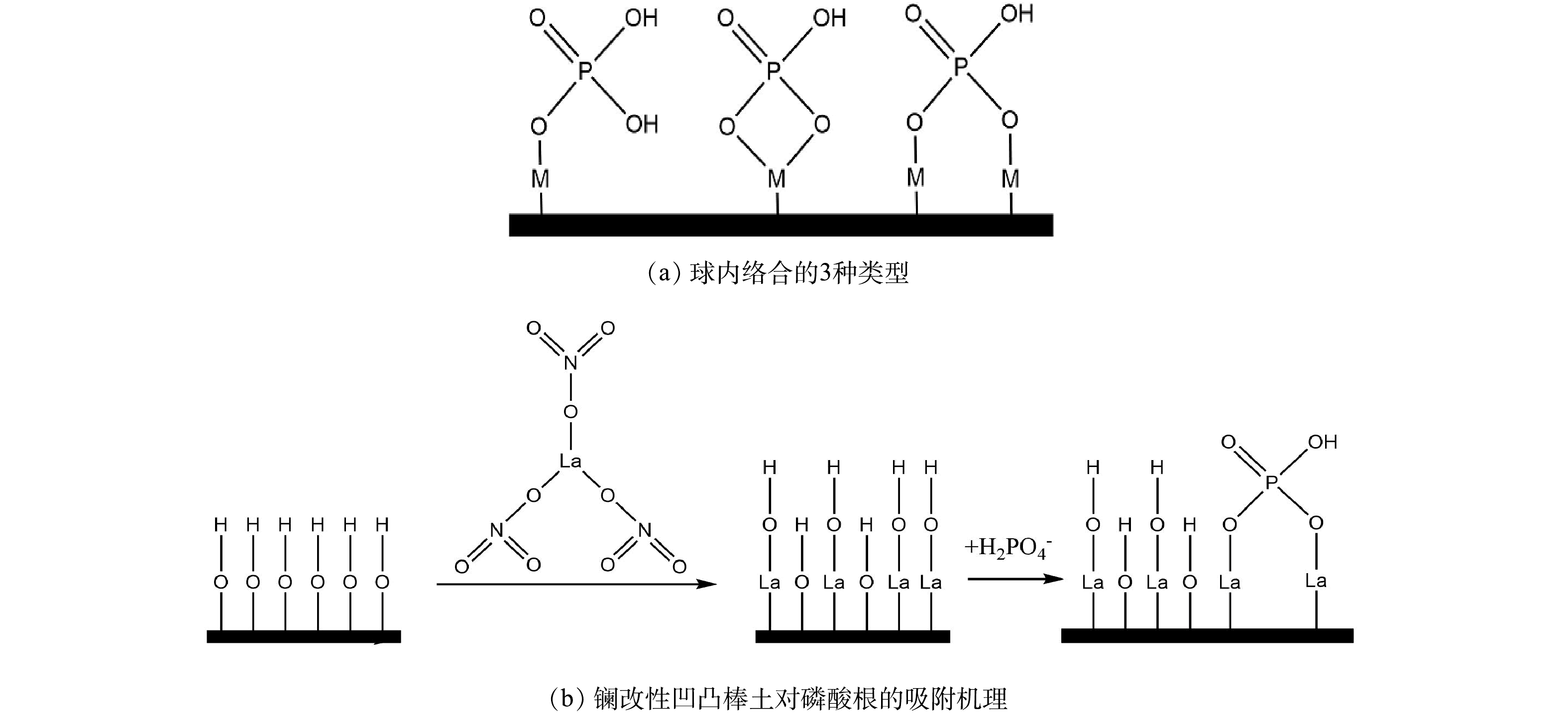
结合已有文献报道以及本研究所得实验结果,可以进一步推测稀土改性凹凸棒土吸附除磷机理。实现对磷酸根离子选择性吸附的3种方法为氢键、形状互补和球内络合,稀土改性凹凸棒土对磷酸根离子的选择性吸附更符合球内络合(图8)。图8(a)阐明了3种类型的球内络合,磷酸根离子可以在金属颗粒表面形成单核单齿(1个金属原子和1个氧来自磷酸根离子)、单核双齿(1个金属原子和2个氧来自磷酸根离子)和双核双齿(2个金属原子和2个氧来自磷酸根离子)络合物[21]。
经表面活性剂十八烷基三甲基溴化铵改性后,阳离子与凹凸棒土纤维晶间的水合阳离子发生了离子交换,晶体内部结构打开,间距加大,提高了凹凸棒土对磷酸根的吸附能力。由XRD和XRF的表征结果可知,经5种稀土元素改性的凹凸棒土均形成相同晶型的稀土氧化物(吸附活性成分),Nd、Ce、La、Pr同属于镧系元素。有研究[22]表明,La和Ce具有相同的除磷机制。由此推测,5种稀土改性凹凸棒土的除磷机理可能相似。以La为例,由图8(b)可知,La以凹凸棒土为载体,负载在凹凸棒土棒晶表面。在含磷废水中,La的表面被羟基覆盖,La改性凹凸棒土表面的基团主要以La-OH形式存在,总体上氢氧化镧表面带正电荷。在配体交换过程中,La表面羟基被另一种配体磷酸根取代,配体与La原子之间形成共价化学键,磷酸盐在La颗粒表面形成双核双齿络合物,从而形成磷酸镧沉淀,继而达到除磷目的。
-
1)稀土改性凹凸棒土(Nd-ATP、Ce-ATP、Y-ATP、La-ATP和Pr-ATP)吸附除磷效率随pH值的增加而降低,Y-ATP在pH 3~9时保持相对稳定的TP去除率;共存
HCO−3 在很大程度上抑制了稀土改性凹凸棒土对TP的吸附。2) 当初始TP浓度为5~50 mg·L−1时,Langmuir吸附模型能更好地描述稀土改性凹凸棒土吸附除磷过程,Y-ATP对TP 的吸附容量最大,为12.94 mg·g−1。当初始TP浓度为1 mg·L−1,准二级动力学模型能更好地描述稀土改性凹凸棒土吸附除磷过程,吸附过程主要以单分子层化学吸附为主。
3) XRD、SEM、FT-IR、和XRF表征结合除磷结果表明,稀土改性凹凸棒土吸附除磷主要遵循球内络合机理,吸附剂表面羟基起关键作用。
4)稀土改性凹凸棒土吸附剂可应用于低浓度含磷废水处理,具有除磷效率高、吸附容量大和选择性吸附等优点。
稀土改性凹凸棒土对低浓度磷的吸附性能
Performance of rare earth modified attapulgite on the adsorption of low concentration phosphorus wastewater
-
摘要: 为提高凹凸棒土对污水中低浓度磷的吸附能力,制备了稀土改性凹凸棒土(Nd-ATP、Ce-ATP、Y-ATP、La-ATP、Pr-ATP),且将其用于低浓度含磷(TP=1 mg·L−1)废水的吸附处理,比较了5种吸附剂的除磷性能、吸附动力学和等温线模型,以及改性前后的结构组成和表面基团变化。结果表明:当pH为3时,改性凹凸棒土除磷效果最好,TP去除率为84%~98%;共存
HCO−3 对吸附剂除磷有较强的抑制作用,SO2−4 和Cl−对TP吸附的影响几乎可以忽略;准二级动力学模型和Langmuir等温线模型能较好描述该吸附除磷过程。其中,pH、投加量、共存阴离子对Y-ATP除磷效果影响最小;Y-ATP对TP的吸附容量最大,为12.94 mg·g−1。稀土改性凹凸棒土吸附剂除磷主要遵循球内络合机理,其中吸附剂表面的羟基基团起关键作用。Abstract: In order to improve the adsorption capacity of attapulgite towards low concentration phosphorus in sewage, rare earth modified attapulgite (Nd-ATP, Ce-ATP, Y-ATP, La-ATP, Pr-ATP neodymium, cerium, yttrium, lanthanum, praseodymium) absorbents were prepared to treat low-concentration phosphorus-containing (TP=1 mg·L−1) wastewater. The phosphorus removal performance, adsorption kinetics and isotherm models of five adsorbents were compared, as well as the changes in the structure, composition and surface groups before and after modification. The results showed that at pH 3 the modified attapulgite had the best phosphorus removal effect, and the TP removal rate was 84%~98%; the coexistingHCO−3 had a strong inhibitory effect on the phosphorus removal, whileSO2−4 and Cl− had almost negligible effects. The quasi-second-order kinetic model and Langmuir isotherm model could better describe the process of phosphorus removal process than other models. Among them, pH, dosage, and coexisting anions had the least influence on the phosphorus removal effect of Y-ATP yttrium modified attapulgite; Y-ATP yttrium modified attapulgite had the largest adsorption capacity for phosphorus TP, which was 12.94 mg·g−1. The phosphorus removal of rare earth modified attapulgite adsorbent mainly followed the intra-sphere complex mechanism, of which the surface hydroxyl groups on the adsorbent played a key role. -
表 1 稀土改性前后凹凸棒土主要化学组成
Table 1. Main chemical composition of attapulgite before and after rare earth modification
样品 化学组成/% ATP Nd-ATP Ce-ATP Y-ATP La-ATP Pr-ATP SiO2 47.26 45.16 44.04 44.93 45.14 45.29 CaO 15.02 14.84 14.18 15.52 14.84 14.94 MgO 13.04 13.04 13.873 12.87 12.89 13.17 Al2O3 11.06 10.75 10.316 10.54 10.71 10.73 Fe2O3 8.987 8.37 7.255 8.533 8.438 8.228 K2O 2.6 2.271 1.808 2.369 2.371 2.249 TiO2 1.45 1.35 1.09 1.37 1.34 1.34 P2O5 0.204 0.194 0.134 0.182 0.191 0.209 MnO 0.109 0.096 7 0.077 3 0.091 6 0.091 2 0.094 5 稀土氧化物 — 2.93 3.78 2.308 3.01 2.73 表 2 共存阴离子对TP去除率的影响
Table 2. The effect of coexisting anions on TP removal rate
共存阴离子 TP去除率% Nd-ATP Ce-ATP Y-ATP La-ATP Pr-ATP HCO−3 53.92 71.15 95.46 90.6 59.22 SO2−4 96.26 94.91 96.71 94.91 93.1 Cl- 96.74 98.09 98.09 98.09 97.64 PO2−4 95.91 95.91 97.67 97.23 97.67 混合离子 57.51 81.29 97.29 97.13 57.51 表 3 稀土改性凹凸棒土吸附除磷动力学拟合参数
Table 3. Kinetic parameters of adsorption and removal of phosphorus by rare earth modified attapulgite
吸附剂 准一级动力学方程 准二级动力学方程 Qe/ (mg·g−1) K1/ min−1 R2 Qe/ (mg·g−1) K2/ (g·(mg·h)−1) R2 Nd-ATP 2.543 0.041 0.885 2.829 0.053 0.933 8 Ce-ATP 2.541 0.050 0.888 2.799 0.066 0.933 7 Y-ATP 2.600 0.038 0.874 2.910 0.047 0.924 9 La-ATP 2.604 0.041 0.873 2.904 0.051 0.924 1 Pr-ATP 2.558 0.046 0.876 2.829 0.059 0.927 5 表 4 稀土改性凹凸棒土吸附除磷等温线拟合参数
Table 4. Fitting parameters of P adsorption isotherm by rare earth modified attapulgite
吸附剂 Langmuir模型 Freundlich模型 Qm/(mg·g−1) b R2 Kf n R2 Nd-ATP 11.95 0.261 0.991 4.550 0.261 0.875 Ce-ATP 11.32 0.271 0.995 4.164 0.271 0.884 Y-ATP 12.94 0.278 0.996 4.602 0.280 0.885 La-ATP 11.53 0.270 0.995 4.240 0.271 0.887 Pr-ATP 11.76 0.266 0.994 4.398 0.266 0.885 -
[1] WANG S, JIN X, ZHAO H, et al. Phosphorus fractions and its release in the sediments from the shallow lakes in the middle and lower reaches of Yangtze River area in China[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2006, 273: 109-116. [2] YIN H, REN C, LI W. Introducing hydrate aluminum into porous thermally-treated calcium-rich attapulgite to enhance its phosphorus sorption capacity for sediment internal loading management[J]. Chemical Engineering Journal, 2018, 348: 704-712. doi: 10.1016/j.cej.2018.05.065 [3] REITZEL K, ANDERSEN F O, EGEMOSE S, et al. Phosphate adsorption by lanthanum modified bentonite clay in fresh and brackish water[J]. Water Research, 2013, 47(8): 2787-2796. doi: 10.1016/j.watres.2013.02.051 [4] YIN H, HAN M, TANG W. Phosphorus sorption and supply from eutrophic lake sediment amended with thermally-treated calcium-rich attapulgite and a safety evaluation[J]. Chemical Engineering Journal, 2016, 285: 671-678. doi: 10.1016/j.cej.2015.10.038 [5] WANG H, WANG X, MA J, et al. Removal of cadmium (II) from aqueous solution: A comparative study of raw attapulgite clay and a reusable waste-struvite/attapulgite obtained from nutrient-rich wastewater[J]. Journal of Hazardous Materials, 2017, 329: 66-76. doi: 10.1016/j.jhazmat.2017.01.025 [6] HONG S H, NDINGWAN A M, YOO S C, et al. Use of calcined sepiolite in removing phosphate from water and returning phosphate to soil as phosphorus fertilizer[J]. Journal of Environmental Management, 2020, 270: 110817. doi: 10.1016/j.jenvman.2020.110817 [7] HUANG R, LIN Q, ZHONG Q, et al. Removal of Cd(II) and Pb(II) from aqueous solution by modified attapulgite clay[J]. Arabian Journal of Chemistry, 2020, 13(4): 4994-5008. doi: 10.1016/j.arabjc.2020.01.022 [8] LI X, ZHANG X, WANG X, et al. Phytoremediation of multi-metal contaminated mine tailings with Solanum nigrum L. and biochar/attapulgite amendments[J]. Ecotoxicology and Environmental Safety, 2019, 180: 517-525. doi: 10.1016/j.ecoenv.2019.05.033 [9] 邓春玲. 稀土吸附剂废水深度脱磷[D]. 昆明: 昆明理工大学, 2002. [10] 李彬. 稀土吸附剂微污染水深度除磷研究[D]. 昆明: 昆明理工大学, 2005. [11] YIN H, YAN X, GU X. Evaluation of thermally-modified calcium-rich attapulgite as a low-cost substrate for rapid phosphorus removal in constructed wetlands[J]. Water Research, 2017, 115: 329-338. doi: 10.1016/j.watres.2017.03.014 [12] XU C, QI J, YANG W, et al. Immobilization of heavy metals in vegetable-growing soils using nano zero-valent iron modified attapulgite clay[J]. Science of the Total Environment, 2019, 686: 476-483. doi: 10.1016/j.scitotenv.2019.05.330 [13] WANG Q, WEN J, HU X, et al. Immobilization of Cr(VI) contaminated soil using green-tea impregnated attapulgite[J]. Journal of Cleaner Production, 2021, 278: 123967. doi: 10.1016/j.jclepro.2020.123967 [14] BOUDRICHE L, CALVET R, CHAMAYOU A, et al. Influence of different wet milling on the properties of an attapulgite clay, contribution of inverse gas chromatography[J]. Powder Technology, 2021, 378: 29-39. doi: 10.1016/j.powtec.2020.09.045 [15] YIN H, YANG P, KONG M, et al. Use of lanthanum/aluminum co-modified granulated attapulgite clay as a novel phosphorus (P) sorbent to immobilize P and stabilize surface sediment in shallow eutrophic lakes[J]. Chemical Engineering Journal, 2020, 385: 123395. doi: 10.1016/j.cej.2019.123395 [16] SUN P, ZHANG W, ZOU B, et al. Preparation of EDTA-modified magnetic attapulgite chitosan gel bead adsorbent for the removal of Cu(II), Pb(II), and Ni(II)[J]. International Journal of Biological Macromolecules, 2021, 182: 1138-1149. doi: 10.1016/j.ijbiomac.2021.04.132 [17] 苗琛琛, 毛林强, 陶德晶, 等. 镧改性凹凸棒土的制备及其对水中磷酸盐的吸附[J]. 环境工程学报, 2016, 10(12): 7070-7074. [18] YIN H, KONG M. Simultaneous removal of ammonium and phosphate from eutrophic waters using natural calcium-rich attapulgite-based versatile adsorbent[J]. Desalination, 2014, 351: 128-137. doi: 10.1016/j.desal.2014.07.029 [19] 刘晨, 张美一, 潘纲. 超薄水滑石纳米片除磷效果与机理[J]. 环境工程学报, 2018, 12(9): 2446-2456. [20] 杨树润, 张世熔, 冯灿, 等. 4种镧改性海藻粉末对养殖废水中磷的去除[J]. 环境工程学报, 2019, 13(10): 2357-2368. [21] WU B, WAN J, ZHANG Y, et al. Selective Phosphate Removal from Water and Wastewater using Sorption: Process Fundamentals and Removal Mechanisms[J]. Environmental Science & Technology, 2020, 54(1): 50-66. [22] 崔有为, 李杰, 杜兆富. 镧、铈改性沸石对污水除磷性能和机制的比较研究[J]. 中国稀土学报, 2016, 34(4): 461-467. -











 下载:
下载: