-
双酚A(bisphenol A, BPA)是一种被列入内分泌干扰物(endocrine disrupting chemicals, EDCs)名单的重要工业原料,在塑料、阻燃、涂料等产品中广泛使用并最终进入自然环境,因此,BPA在河流、湖泊、海洋等地表水体和地下水中被频繁检出[1]。目前,污水处理厂常规的物理和生物处理法对BPA的去除效果均有限。因此,需开发高效降解BPA的技术,以解决水体BPA的污染问题[2]。
过一硫酸盐(PMS)在被激发后可释放出超氧自由基(O2·−)、羟基自由基(·OH)、硫酸根自由基(SO4·−)等多种强氧化性自由基,这些自由基降解污染物的能力强且反应速度快,因此,基于PMS的高级氧化技术已引起广泛关注[3-7]。与均相反应相比,基于半导体催化剂的非均相反应具有稳定、激发效率高的优势,更适用于多种水质[8],且半导体催化剂中可变价的过渡金属离子会产生氧化还原电对,通过金属离子与PMS间发生电子转移的方式来促进PMS的激发,产生SO4·−或O2·−进而实现对污染物的降解[9]。目前已有大量研究采用半导体催化剂激发PMS[10-11],也有研究利用半导体催化剂的良好光吸收效率,将半导体光催化与PMS的催化氧化耦合联用,通过多种途径激发PMS[11];同时,PMS的加入会促进半导体催化剂光生电荷的转移和分离[8]。因此,太阳光/半导体催化剂/PMS联用的高级氧化技术极具前景和可行性[12-14]。目前的大量相关研究主要是利用氧化电势较高的SO4·−或·OH实现对污染物的降解[10-11];而O2·−具有相对低的氧化电势,同时具有更强的对污染物的选择性,但基于O2·−的PMS催化氧化研究尚处于初级阶段[15-16]。
层状双氢氧化物(layered double hydroxides, LDHs),是一类易于制备的阴离子粘土或水滑石类材料,具备特殊的层状结构和较大的比表面积。LDHs是由构成板层的金属阳离子和层间阴离子构成,其阳离子包括二价和三价离子,且当三价离子所占摩尔比在0.25~0.33时,LDHs结构稳定且结晶度高[17-18]。LDHs的二维层状结构可以在一定程度上诱导电子的转移,抑制电子-空穴对的复合[19];LDHs中存在大量活性位点,可以充分接触并激发PMS;当阳离子中含过渡金属元素时,LDHs会具有更强的电子转移能力和催化能力[20]。目前已有研究者将LDHs作为复合催化剂的一部分,运用光催化与过硫酸盐高级氧化耦合的技术,实现对污染物的降解[9];但半导体与LDHs复合的催化剂的合成常需多步反应完成,而一步水热反应合成的LDHs则具有合成简单高效的优势。
本研究选取了Cu、Mn、Fe 3种元素作为LDHs的金属阳离子,通过水热法合成了Cu/Mn-Fe LDHs,确定了Cu、Mn、Fe的最佳摩尔比,进行多种表征并测试了其催化性能;探究了催化剂投加量和PMS浓度对BPA降解效果的影响并得到降解BPA的最佳反应参数;在不同反应条件下测试了BPA的降解效率,比较了LDHs光催化、PMS催化氧化及二者耦合联用条件下BPA的降解效果;探究了水质指标(包括溶液pH,常见阴离子的种类及浓度)对BPA降解效果的影响;通过活性成分掩蔽实验及电子顺磁共振(ESR)测试进一步验证了反应中的活性成分的种类及不同的活性成分对BPA降解贡献率的大小。
-
过一硫酸盐(2KHSO5 · KHSO4 · K2SO4,PMS)、碳酸氢钾(KHCO3)、磷酸二氢钾(KH2PO4)、氯化钾(KCl)、无水碳酸钠(Na2CO3)、二水合乙二胺四乙酸钠(EDTA·2Na)、对苯醌(C6H4O2,DBQ)、双酚A(BPA)购于阿拉丁试剂(上海)有限公司;九水合硝酸铁(Fe(NO3)3·9H2O)、四水合硝酸锰(Mn(NO3)2·4H2O)、三水合硝酸铜(Cu(NO3)2·3H2O)、无水硫酸钾(K2SO4)、无水硝酸钾(KNO3)、叔丁醇(2-甲基-2-丙醇,C4H10O, TBA)、L-组氨酸(C6H9N3O2)购于上海麦克林生化科技有限公司;氢氧化钠(NaOH)、氢氧化钾(KOH)、甲醇(CH3OH,MeOH)、硫酸(H2SO4)购于国药集团。除甲醇为色谱纯,所有试剂均为分析纯。实验中的用水均为超纯水。
-
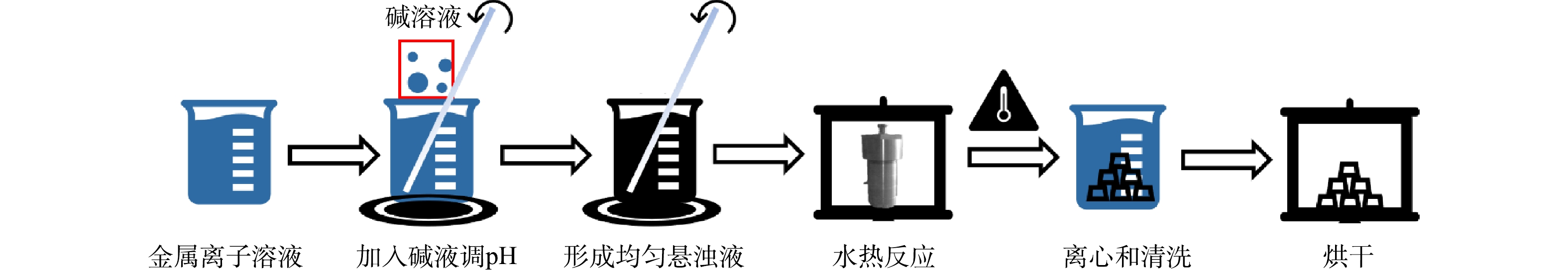
如图1所示,本实验采用水热法制备LDHs。精确称取1 mmol硝酸铁,并根据Cu、Mn、Fe的比例,称取一定质量的硝酸铜和硝酸锰,将三者溶解于50 mL水并置于100 mL的聚四氟乙烯反应釜中,搅拌30 min后,保持高速搅拌并缓慢滴加2 mol·L−1的氢氧化钠和0.6 mol·L−1的碳酸钠溶液至溶液pH至9.1,继续搅拌2 h,将反应釜置于80 ℃下加热24 h,自然冷却到室温,将产物以5 000 r·min−1的速度离心,并用超纯水将所得黑色固体清洗至pH呈中性,最后置于60 ℃下烘干。
-
通过45 kV和200 mA 的X射线衍射光谱(XRD,Rigaku Smartlab))对LDHs的晶体结构进行表征;由以KBr为背景的ALPHA光谱仪(Bruker)的傅里叶变换红外光谱(FT-IR)采集LDHs的表面官能团信息;催化剂的形貌特征由配有能量色散X射线谱仪(EDS)的扫描电子显微镜(SEM,EM-30 PLUS,COXEM)和加速电压为200 kV的透射电子显微镜(TEM,G2 F20,FEI)获取;催化剂的比表面积由ASAP2020氮吸附装置开展Bruauer-Emmett-Teller(BET)测得;使用以BaSO4作为参考的UV 3600 Plus分光光度计进行紫外可见漫反射光谱(UV-vis DRS)的检测;LDHs的元素组成由以Al Ka射线为激发源的EscaLab Xi +光谱仪X射线光电子能谱(XPS)测得;使用电子自旋共振(ESR)光谱仪(E500,Bruke)测得反应体系中的自由基和其他活性成分。
-
称取一定量的催化剂加入到浓度为20 mg·L−1的BPA溶液中,并缓慢地搅拌;待吸附解吸平衡后加入0.2~0.8 mmol·L−1的PMS溶液并打开模拟太阳光照射的氙灯光源(光强为60 mA·cm−2),反应过程中在特定时间取样,加入等量甲醇进行淬灭并使用0.45 μm滤膜过滤样品,使用高效液相色谱(HPLC, Agilent)检测样品,HPLC使用C18色谱柱(250 mm×4.6 mm×5 μm)和紫外检测器(280 nm),在流速为1 mL·min−1、甲醇相与水相比例为70∶30的条件下,BPA的出峰时间位于2.26 min,利用积分所得峰面积确定BPA的浓度。通过液相色谱-质谱联用(LC-MS (Q-Exactive), Agilent)确定反应的降解产物。
-
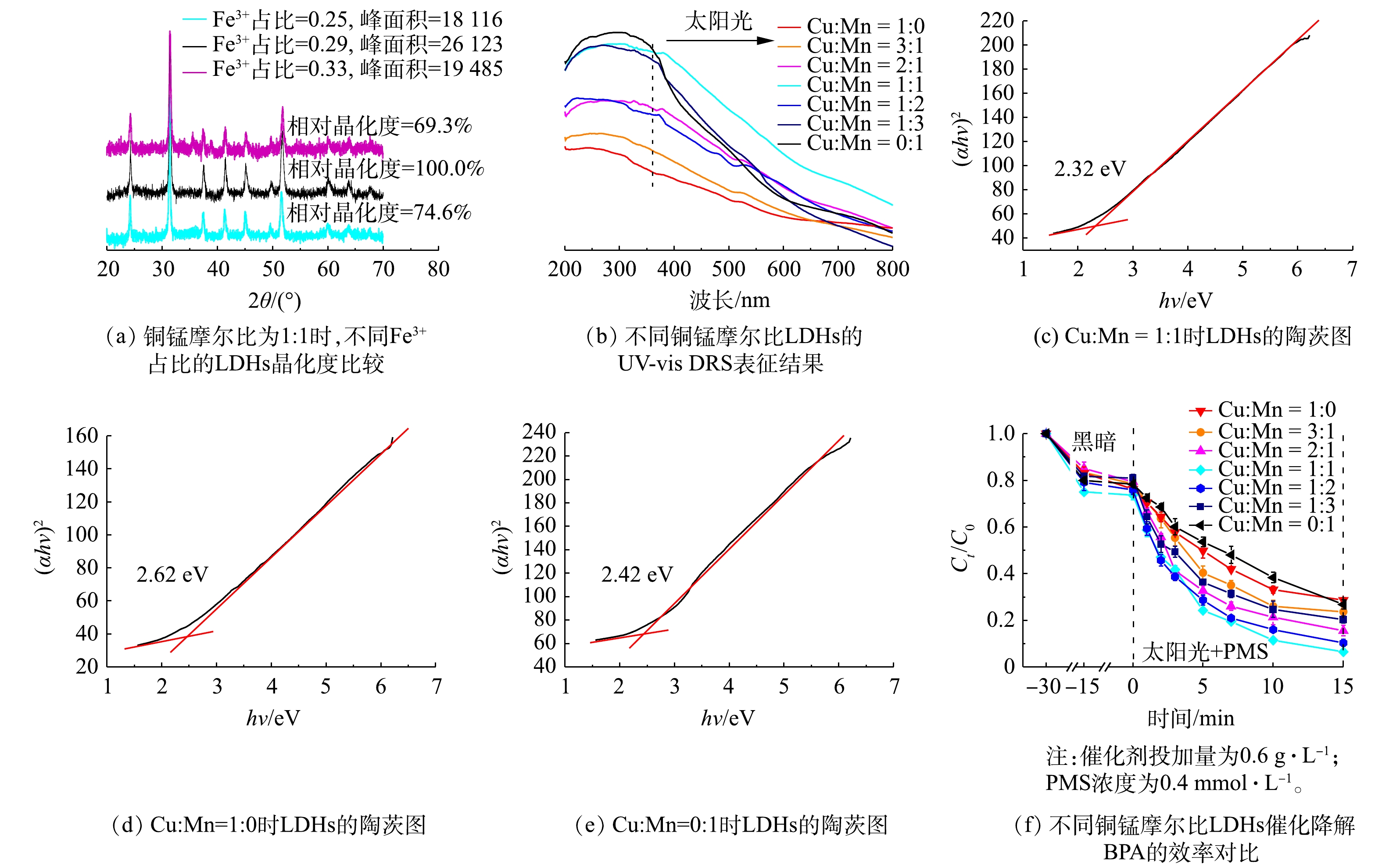
LDHs的三价离子在摩尔占比为0.25~0.33时具有稳定的晶体结构[17]。为确定三价离子的最佳比例,在Cu∶Mn(摩尔比) = 1∶1的条件下,通过XRD表征对不同Fe3+占比的LDHs结晶度进行了测试,结果如图2(a)所示。当Fe元素占比为0.29时,LDHs的XRD响应峰强度最高且半峰宽最小,说明此LDHs结晶度最高;而当Fe3+占比为0.25和0.33时,LDHs的相对晶化度仅为Fe3+占比为0.29的LDHs的69.3%和74.6%。因此,Fe3+的最佳摩尔比例为0.29。
在铁离子摩尔占比为0.29的条件下,为确定最佳铜锰摩尔比,使用了不同摩尔比(1∶0、3∶1、2∶1、1∶1、1∶2、1∶3、0∶1)的Cu、Mn元素合成Cu/Mn-Fe LDHs,并应用UV-vis DRS对太阳光的吸收效率进行了检测,结果如图2(b)所示。当Cu∶Mn(摩尔比)为0∶1时,Mn-Fe LDHs具有很强的紫外波段(200~400 nm)光的吸收能力,但几乎不吸收可见波段(400~800 nm)光;而Cu∶Mn(摩尔比)为 1∶0的Cu-Fe LDHs在紫外、可见波段均表现出较弱的光吸收能力,且光吸收能力随波长增大而减弱。随着Cu、Mn的同时加入,LDHs紫外波段具有更强吸收能力,而Cu元素占比的提高会使LDHs的光吸收曲线更加平滑。铜锰摩尔比为1∶1时,所得的Cu/Mn-Fe LDHs具有最强的太阳光(360~800 nm)吸收能力;而其他铜锰摩尔比的LDHs则在各波段的吸收能力均明显弱于摩尔比为1∶1的LDHs。因此,当铜锰摩尔比为1∶1时,太阳光利用效率最高。如图2(c)所示,其禁带宽度为2.32 eV,能够有效利用波长小于534.5 nm的光,相比于图2(d)和图2(e)中的Cu-Fe LDHs(2.62 eV)和Mn-Fe LDHs(2.42 eV)有所降低。此外,我们也对不同铜锰摩尔比的Cu/Mn-Fe LDHs光催化活化PMS降解BPA的性能进行了考察,结果如图2(f)所示。随着铜锰摩尔比的降低,BPA的去除率会呈现出先升高后降低的趋势,当铜锰摩尔比达到1∶1时(此时Cu∶Mn∶Fe = 1.2∶1.2∶1),催化剂的催化效果最佳。对20 mg·L−1的BPA溶液,0.6 g·L−1的铜锰摩尔比为1:1的Cu/Mn-Fe LDHs(简称1∶1 CMF)可吸附去除26.3%的BPA;加入PMS并进行光照后,BPA的去除速率为0.177 min−1,经过15 min的降解反应后,BPA去除率可达到93.5%。因此,综合考虑光吸收效率及催化能力,将1∶1 CMF作为催化降解BPA能力最强的LDHs,并对其进行表征测试。
-
1) XRD分析。通过XRD对所制备的LDHs的组成及纯度信息进行了分析,结果如图3所示。在2θ 为24.2°、31.4°、37.6°、41.5°、45.1°和51.8°处观察到衍射峰,分别对应于(012)、(104)、(110)、(113)、(202)、(018)晶面[21];相比于Cu-Fe LDHs和Mn-Fe LDHs,1∶1 CMF的(002)、(104)、(110)峰宽小,强度高且几乎无杂峰,因此,具有更高的结晶度和纯度。Mn-Fe LDHs在21.1°和54.0°出现了衍射峰,对应MnOOH标准图谱(JCPDS 012-0733)的(110)和(221)晶面;而Cu-Fe LDHs在14.7°、17.4°和57.9°的衍射峰,分别对应于Cu(OH)2CO3标准图谱(JCPDS 004-0309)的(020)、(120)和(002)晶面。此外,Cu-Fe LDHs及Mn-Fe LDHs的衍射峰较宽,说明其结晶度低,其中Cu-Fe LDHs的峰形宽且杂峰多,这与Cu元素的姜-泰勒效应(Jahn-Teller effect)有关。
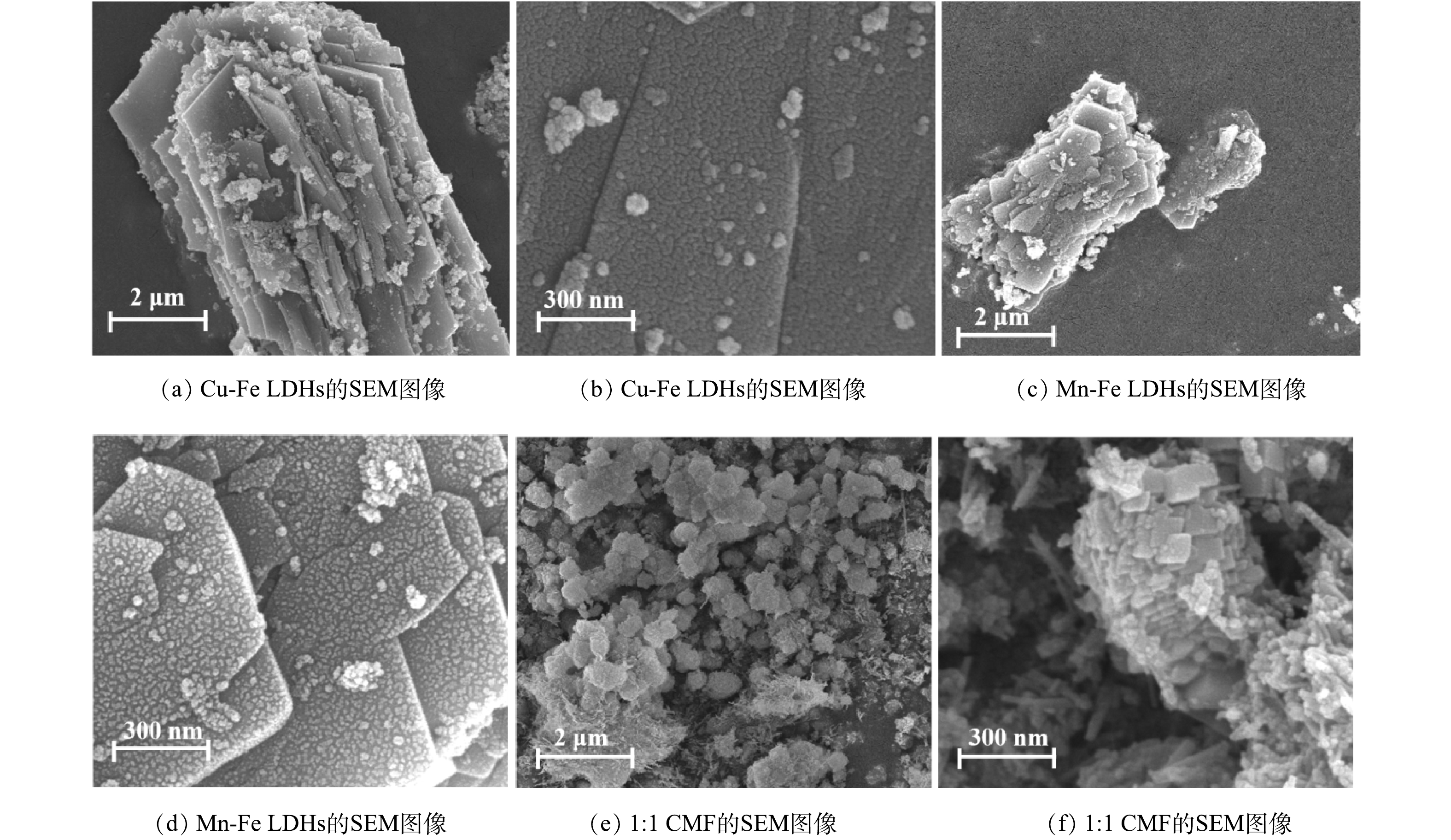
2) SEM和TEM分析。如图4(a)和图4(c)所示,Cu-Fe LDHs和Mn-Fe LDHs具有明显的二维层状堆积结构,其中Cu-Fe LDHs堆积更加密集,且片状堆积过程中呈现出一定的卷曲趋势;如图4(b)和图4(d)所示,在Cu-Fe LDHs和Mn-Fe LDHs层状结构表面具有一定的杂质晶粒,这也与XRD表征的结果相同。而当Cu、Mn元素同时作为LDHs的二价元素时,1∶1 CMF仍具有LDHs特有的层状与片状结构,但呈现出如图4(e)所示的絮状与片状共存的状态。相比于Cu-Fe LDHs和Mn-Fe LDHs,如图4(f)所示,1∶1 CMF晶体的单片面积减小,同时堆积更加紧密。1∶1 CMF呈现出小面积薄片的二维堆积结构,堆积层数更多,因此,也增大了LDHs的比表面积。BET测试结果表明,1∶1 CMF的BET比表面积可达264.60 m²·g−1,约为普通LDHs的2.2倍[18],因此,1∶1 CMF具有大量的催化活性点位和较强的吸附性能。
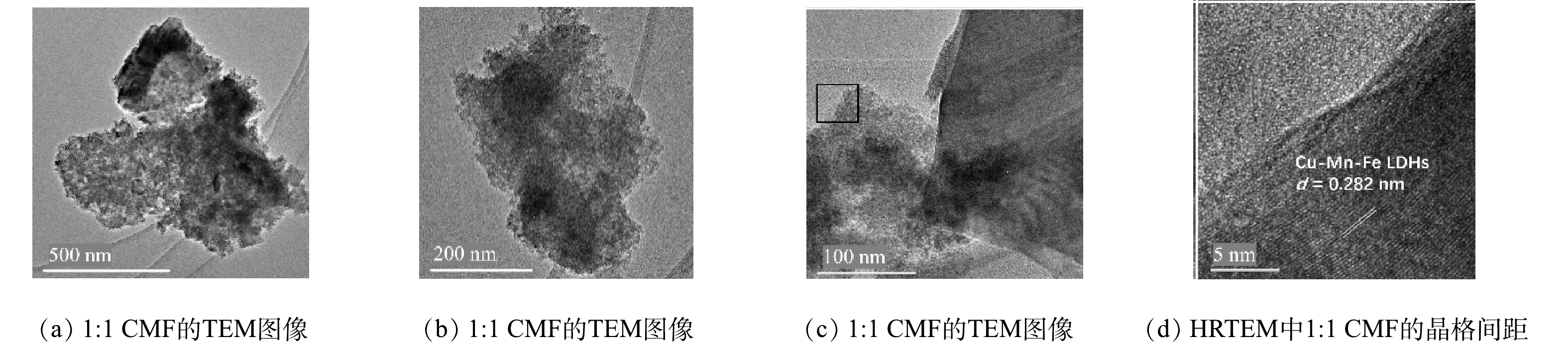
TEM分析结果如图5(a)~(c)所示。1∶1 CMF呈现出规则的片状结构,整体堆积较为均匀,在堆叠密集区域呈现出更深的颜色,因此,1∶1 CMF可堆叠多层。1∶1 CMF的晶格如图5(d)所示,其0.282 nm的晶格间距与XRD的(104)晶面相对应[21]。
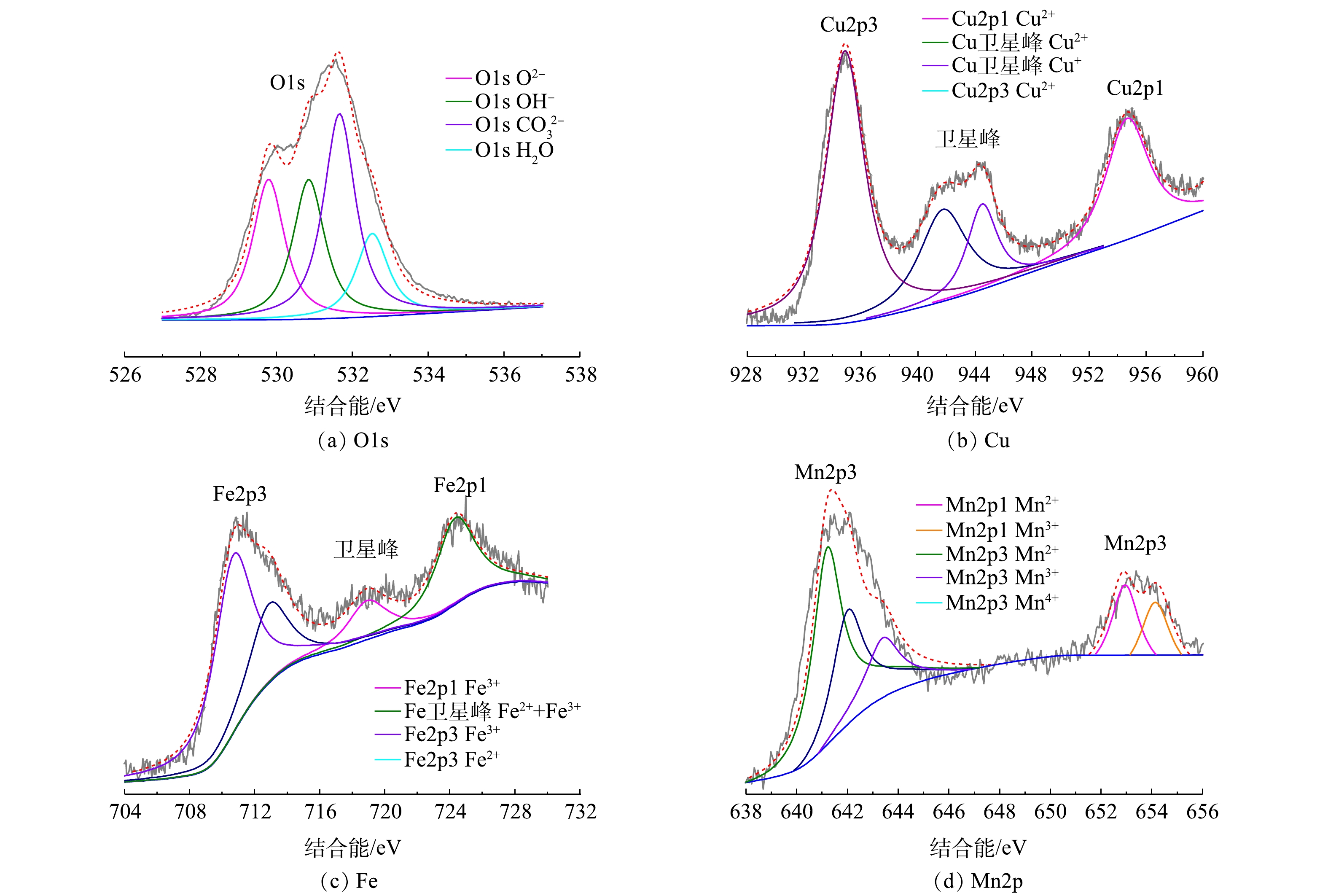
3) XPS分析。1∶1 CMF的XPS分析结果如图6所示。由图6(a)可以看出,O1s可以分为位于529.80、530.90、531.70和532.50 eV的4个峰,其中位于529.80 eV的峰代表了晶格中氧离子(O2−)与金属元素(Cu2+、Fe3+、Mn2+)的结合键,可被认定为M—O或M—O—M[22-23];位于530.90 eV的峰属于表面羟基基团[22];位于531.70 eV的峰代表了层间碳酸根基团[24];而位于532.50 eV的峰是由层间水分子引起的[22, 24]。由图6(b)可知,Cu2p谱图可分为4处峰,934.90 eV和954.70 eV分别代表2p3/2和2p1/2,在962.90 eV处存在卫星峰,证明Cu主要以二价形式存在[24-25];而在两峰之间位于941.80 eV和944.50 eV的两峰之间存在卫星峰,这说明了催化剂表面同时存在Cu+和Cu2+[26]。由图6(c)可知,Fe2p谱图可以分为位于711.50 eV和724.50 eV的2p1/2和2p3/2 主峰:其中2p3/2峰可分为代表Fe2+和Fe3+,加之在Fe2p3/2与Fe2p1/2之间的718.90 eV处存在卫星峰,证明了表面Fe2+与Fe3+同时存在[27],而2p1/2位于724.50 eV,证明Fe主要以三价形式存在[26]。由图6(d)可知,Mn2p谱图可以分为分别代表2p3/2和2p1/2的位于641.90 eV和653.40 eV的2个主峰,其中2p3/2处可进一步分为3个峰,根据结合能由小到大排列分别为641.20、642.10和643.50 eV,依次代表Mn2+、Mn3+和Mn4+[28],证明Mn是以多种价态混合物的形式存在。且从峰强可以看出,Mn的主要存在形式为Mn2+,而Mn3+和Mn4+的含量很低[29]。由此可见,1∶1 CMF是以碳酸根作为层间阴离子,Cu、Mn、Fe的主要存在形式分别是Cu(Ⅱ)、Mn(Ⅱ)和Fe(Ⅲ)。
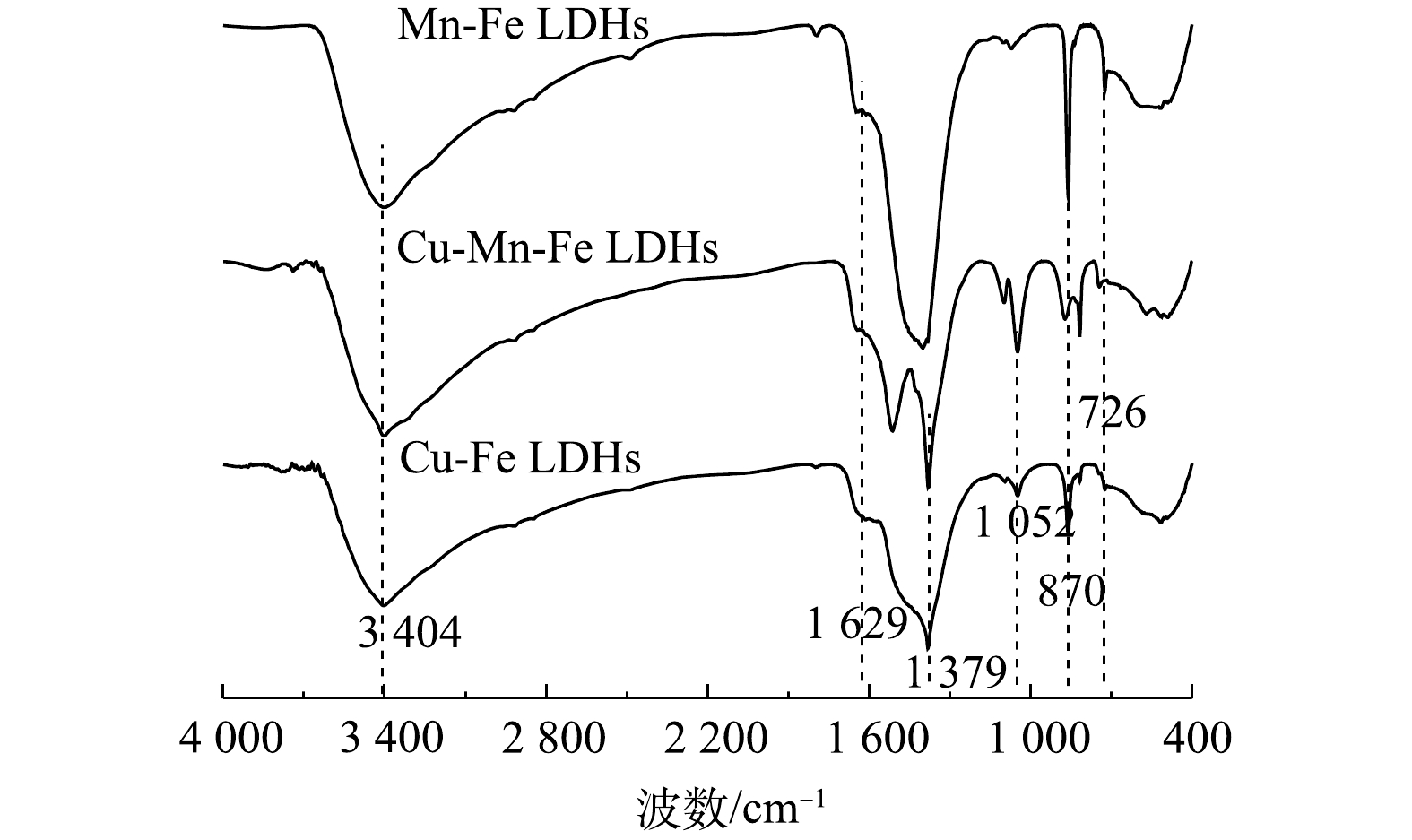
4) FT-IR分析。Mn-Fe LDHs、Cu-Fe LDHs和1∶1 CMF的FT-IR表征结果如图7所示。位于3 404 cm−1处的强宽峰源于羟基(—OH)基团的伸缩振动,这主要来自于水滑石表面的羟基或水分子中的羟基[30];1 629 cm−1处的吸收峰表明了LDHs层间水分子的存在[31];位于1 379 cm−1的峰源于C=O的反对称伸缩振动峰[32],而870 cm−1处的峰是由C—O伸缩振动产生,加上在1∶1 CMF的1 514 cm−1处产生了吸收强度较高的C—O不对称分裂峰,证明合成的LDHs为碳酸根型LDHs。位于1 052 cm−1处的峰对应于金属原子与氧原子基团间的弯曲振动(M—O—M);而450~700 cm−1的宽峰则是由金属与羟基间发生的晶格振动(M—O或M—OH)而产生[17, 32]。因此,以上结果证明所合成的1∶1 CMF具有碳酸根型LDHs的典型特征。
-
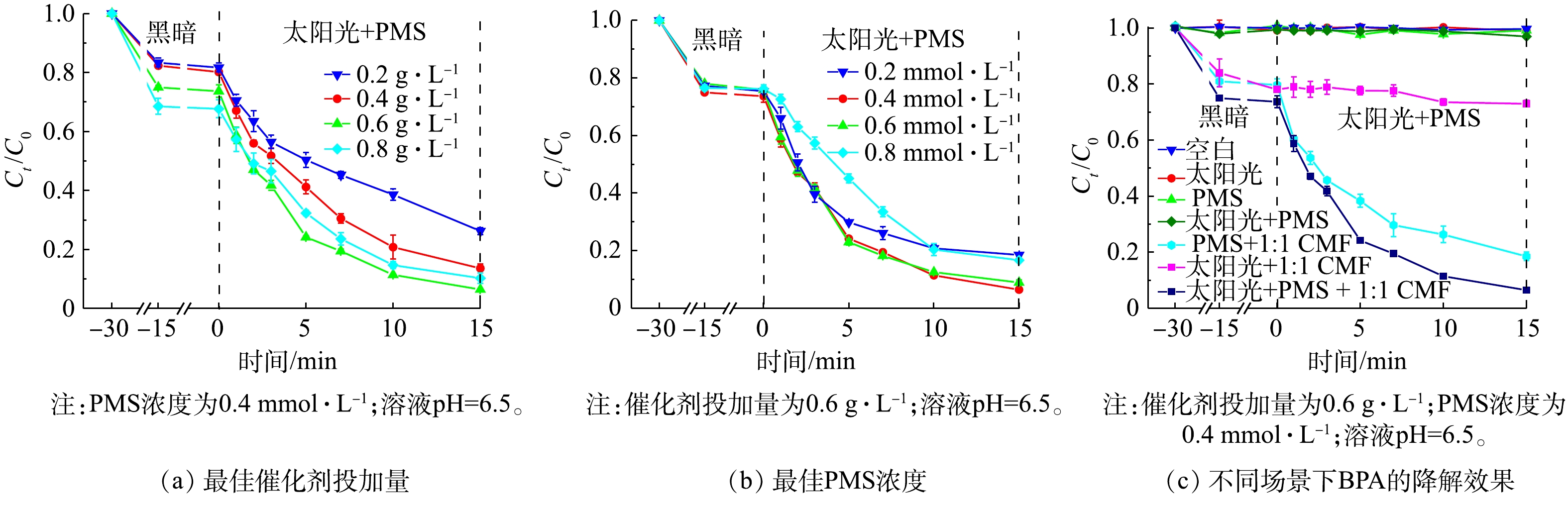
确定了太阳光/1∶1 CMF/PMS体系降解BPA的最佳反应条件,包括最佳的催化剂投加量和PMS浓度,并研究了在最佳反应条件下不同体系中的BPA去除效果,结果如图8所示。
1)催化剂投加量的优化。如图8(a)所示,随着催化剂质量浓度由0.2 g·L−1增加到0.6 g·L−1,吸附去除BPA的比例逐渐提高,BPA的总去除率在催化剂质量浓度为0.6 g·L−1时达到93.5%,且降解速率也由0.079 min−1提高至0.177 min−1。这是由于随着催化剂质量浓度的提高,反应体系吸收光子的能力提高,带来了更多激发PMS的活性点位[33]。但随着催化剂质量浓度由0.6 g·L−1提高到0.8 g·L−1时,反应速率降至0.137 min−1。这可能是由于过量的催化剂本身发生团聚效应,减少了催化剂的活性点位,从而降低了催化效果[34];或是过量的催化剂使得溶液对光的透射减弱,使得催化剂的光利用效率有所降低[9]。因此,最佳催化剂的投加量选为0.6 g·L−1。
2) PMS投加量的优化。如图8(b)所示,当PMS浓度由0.2 mmol·L−1提高到0.4 mmol·L−1时,反应速率由0.118 min−1提高至0.177 min−1,降解效果达到最佳。这是由于适量浓度的PMS有助于产生更多的活性自由基,包括SO4·−、·OH、O2·−等;而进一步提高PMS浓度至0.8 mmol·L−1时,降解速率降至0.110 min−1,降解率也有大幅度下降。如式(1)~式(3)所示,过量的PMS会与生成的强活性自由基(SO4·−、·OH、O2·−等),生成氧化电势仅为1.1 V的SO5·−自由基,导致降解速率的降低[35]。因此,本研究确认最佳PMS的浓度为0.4 mmol·L−1。
3)不同反应场景下BPA的降解效率。图8(c)反映了不同反应条件下BPA的去除效果。BPA几乎不能被模拟太阳光降解[36],虽然1∶1 CMF可以吸收太阳光的能量,但仅产生少量光生空穴,因此,在太阳光/1∶1 CMF的反应体系中,BPA去除速率仅为0.004 min−1;在单独向BPA溶液中投加PMS的情况下,由于PMS稳定不会自激发[37],无法产生活性成分降解BPA;而由于模拟太阳光对PMS的激发能力有限[15],光/PMS的反应体系中几乎不会产生强氧化性自由基,降解BPA的效果几乎可以忽略不计。当向溶液中加入1∶1 CMF及PMS时,LDHs中的大量活性点位会激发PMS产生多种活性自由基,BPA的去除速率达0.112 min−1,去除率可在15 min时达到81.5%;在1∶1 CMF/PMS体系中加以太阳光照,则会进一步提高其催化活性,最终可达到0.177 min−1的反应速率,并在15 min达到93.5%的去除率。这说明将光催化与PMS催化耦合联用会大幅提高BPA的降解速率并达到较高的去除率。
-
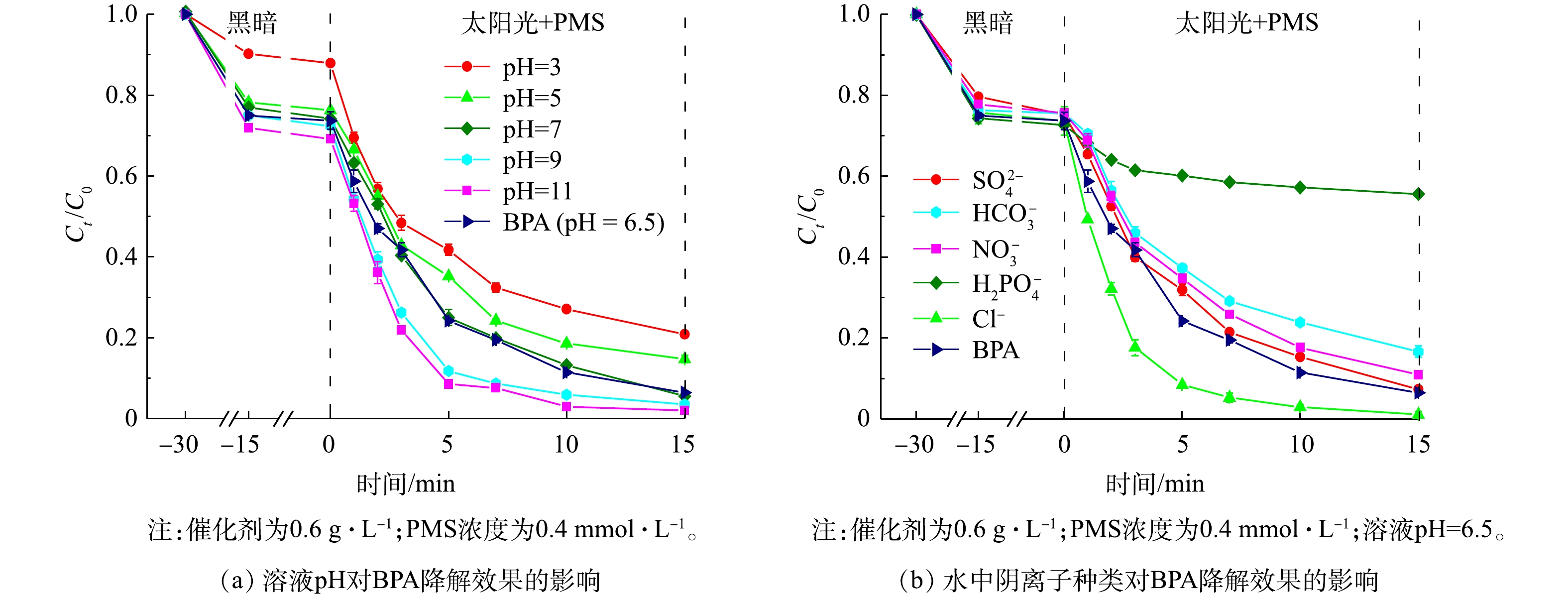
1)溶液pH的影响。我们研究了水质指标对太阳光/1∶1 CMF/ PMS体系降解BPA效果的影响。溶液pH对BPA降解效果的影响如图9(a)所示。太阳光/1∶1 CMF/ PMS体系在较宽的初始溶液pH范围内均表现出良好的光催化活性,且在较宽的pH范围内保留较好的降解性能,即使是在溶液pH为3时也可在15 min达到79.3%的降解率,此时的降解速率为0.114 min−1。随着溶液pH的增大,BPA的降解效果逐渐变好,在溶液pH为11时可达到0.279 min−1的速率,并达到99.5%以上的去除率。PMS在激发产生O2·−时会生成H+,碱性条件可以通过清除H+促进PMS的激发[38-39],因此,碱性条件会提高反应体系降解BPA的能力;而酸性条件会降低反应体系的降解能力。这一现象的原因有3点:首先,当pH较低时,PMS的主要存在形式为难被激发的H2SO5 (pKa = 9.4) [40];其次,在酸性条件下,LDHs组分易产生浸出,不利于LDHs对BPA的吸附[9],例如溶液pH=3的条件下,吸附解吸平衡后LDHs仅吸附了12.1%的BPA,仅为pH=7时的一半,不利于反应的进行;最后,溶液中存在的大量H+会清除体系中产生的多种活性自由基,包括·OH、SO4·−、O2·−,如式(4)~式(6)所示[41]。因此,在pH=3时,BPA的降解速率会下降至0.114 min−1。
2)溶液中阴离子的影响。溶液中阴离子的种类对降解效果的影响如图9(b)所示。当离子浓度为1 mmol·L−1时,SO42−对降解BPA的反应几乎无影响;Cl−会促进降解反应的进行,使反应速率由0.177 min−1提高到0.318 min−1;而H2PO4−、HCO3−和NO3−均会抑制降解反应的进行,分别使反应速率由0.177 min−1降至0.023、0.113和0.139 min−1。水中氯离子的存在会使得·OH和SO4·−氧化Cl−形成Cl·, 其生成途径如式(7)~式(9)所示[42],而Cl·对BPA具有更快的响应和氧化速度[43],因此,氯离子的存在会促进BPA降解。
如式(10)~式(11)所示,H2PO4−会掩蔽·OH和SO4·−等自由基产生H2PO4·,且H2PO4−还会与催化剂的表面活性位点结合,从根本上抑制了PMS的活化和自由基的产生[44]。同样,HCO3−[45]和NO3−[46]会与SO4·−、·OH等强氧化性自由基反应,释放出氧化能力更弱的CO3·−和NO3·自由基(式(12)~式(15)),从而降低了反应速率。
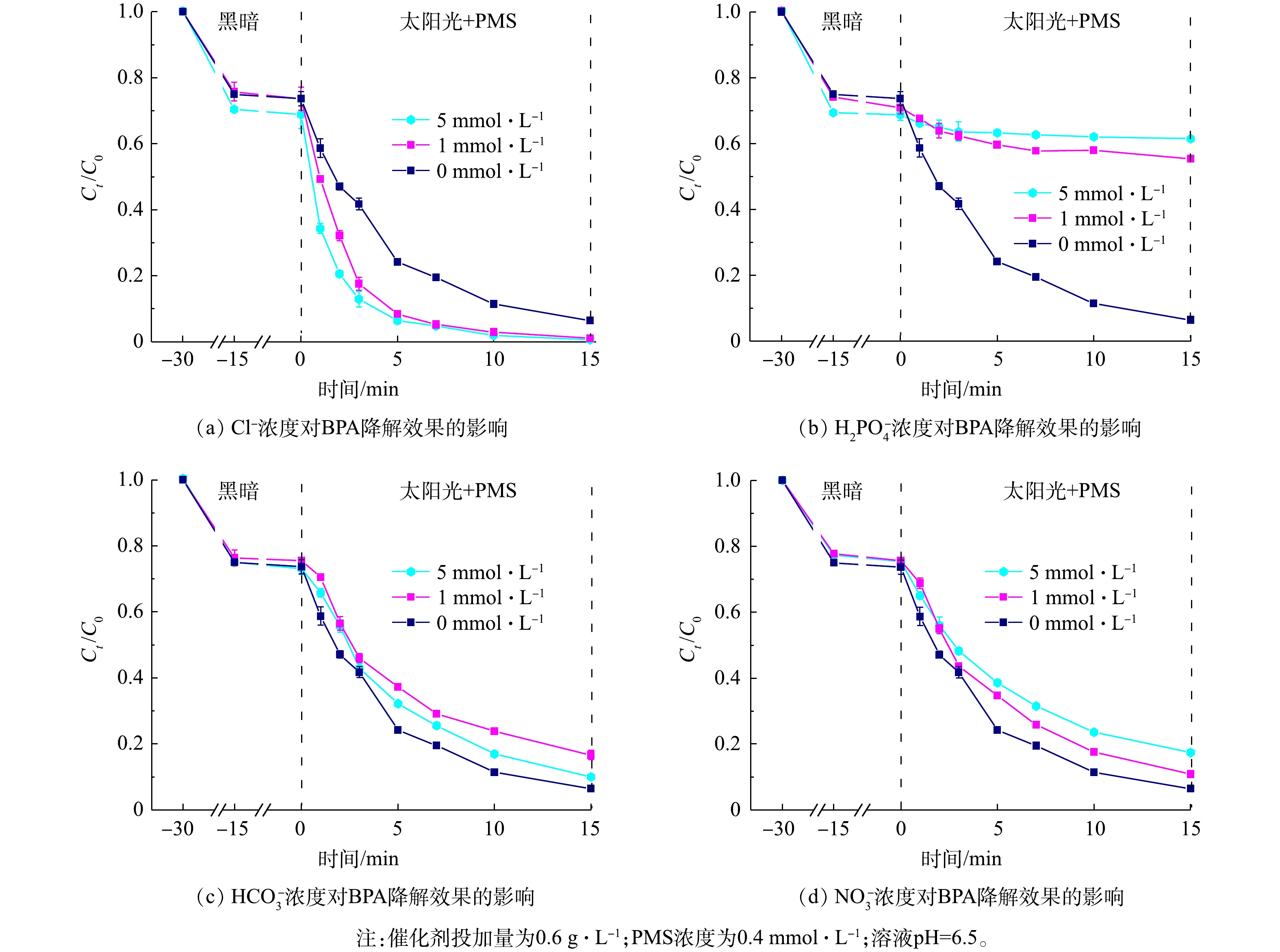
进一步研究了上述4种离子对降解效果的影响,结果如图10所示。随着硝酸根、磷酸二氢根离子、氯离子浓度由1 mmol·L−1增大到5 mmol·L−1,其对BPA降解效果的抑制或促进作用会更为显著。但当碳酸氢根的离子浓度提高到5 mmol·L−1时,其抑制作用反而会有所降低。这是由于,当碳酸氢根离子浓度提高时,会导致pH有所升高,促进了PMS的活化;另一方面,因PMS分子具有不对称性,易受到具有亲核性的HCO3−、CO32−等分子的攻击,从而激发产生SO4·−[47]。
-
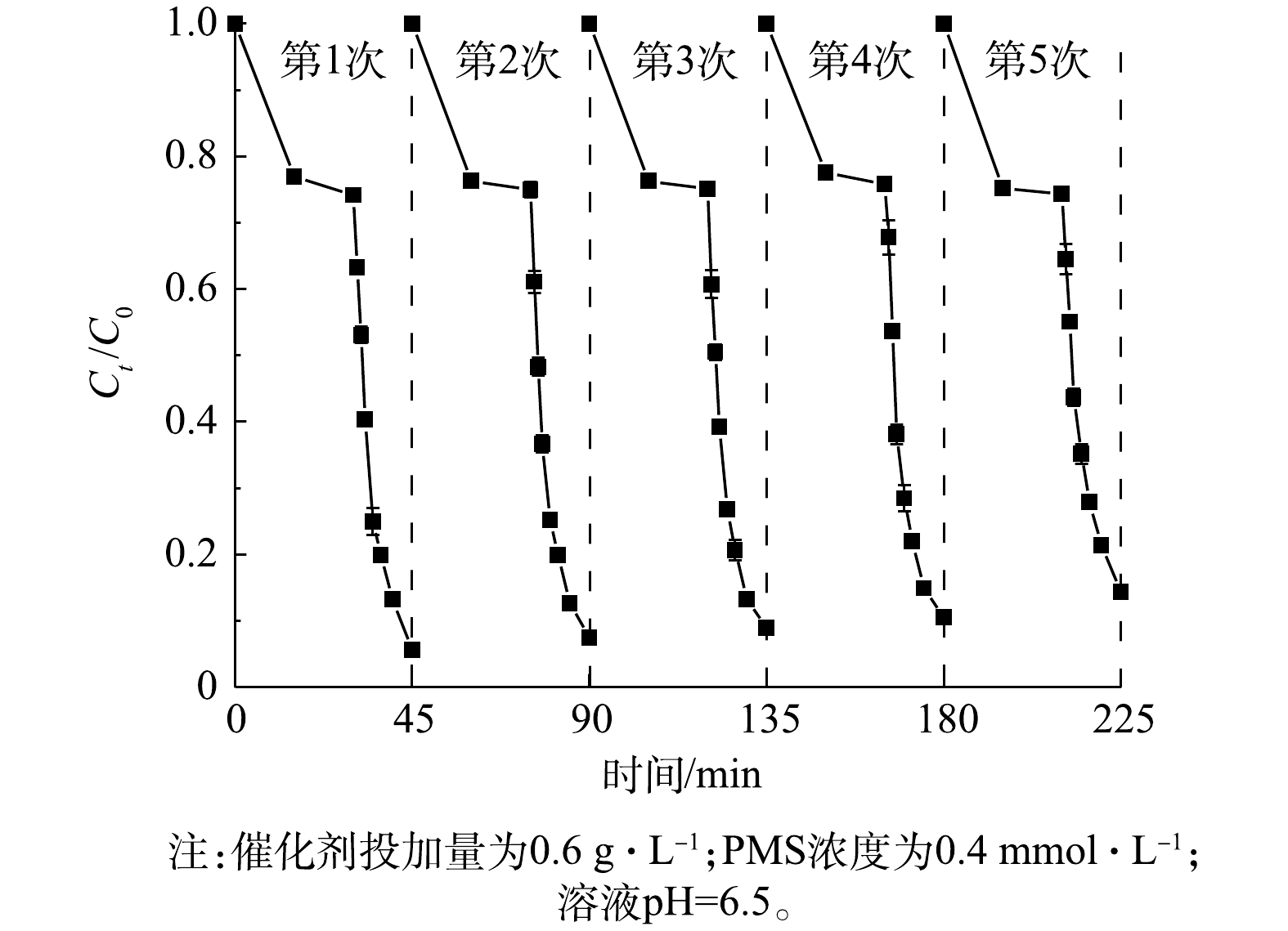
通过连续5轮的降解实验,证实了1∶1 CMF催化剂具有良好的稳定性。如图11所示,在4次循环实验之内,LDHs的催化性能几乎没有减弱;当进行第5轮循环反应时,BPA的去除率在反应15 min后仍可达到85.7%,相较于第1轮反应仅降低了7.8%,这也说明1∶1 CMF具有较好的稳定性及可重复利用性。通过ICP-MS对反应后溶液中的过渡金属元素进行了检测,Cu元素的质量浓度为340.08 μg·L−1,Mn元素的质量浓度为33.02 μg·L−1,Fe元素的质量浓度为160.20 μg·L−1,均小于饮用水水质标准的要求(Cu、Mn、Fe的限量标准分别是1、0.1和0.3 mg·L−1)。由此可见,该催化剂的应用较为安全。
-
1)反应体系中活性成分的生成和贡献。为确定太阳光/1∶1 CMF/PMS中的作用活性基对降解BPA的贡献率,通过掩蔽实验确定了各活性成分在BPA降解反应中的贡献率,并通过ESR对该体系中存在的活性成分进行验证,并据此判断反应中的主要作用活性成分。
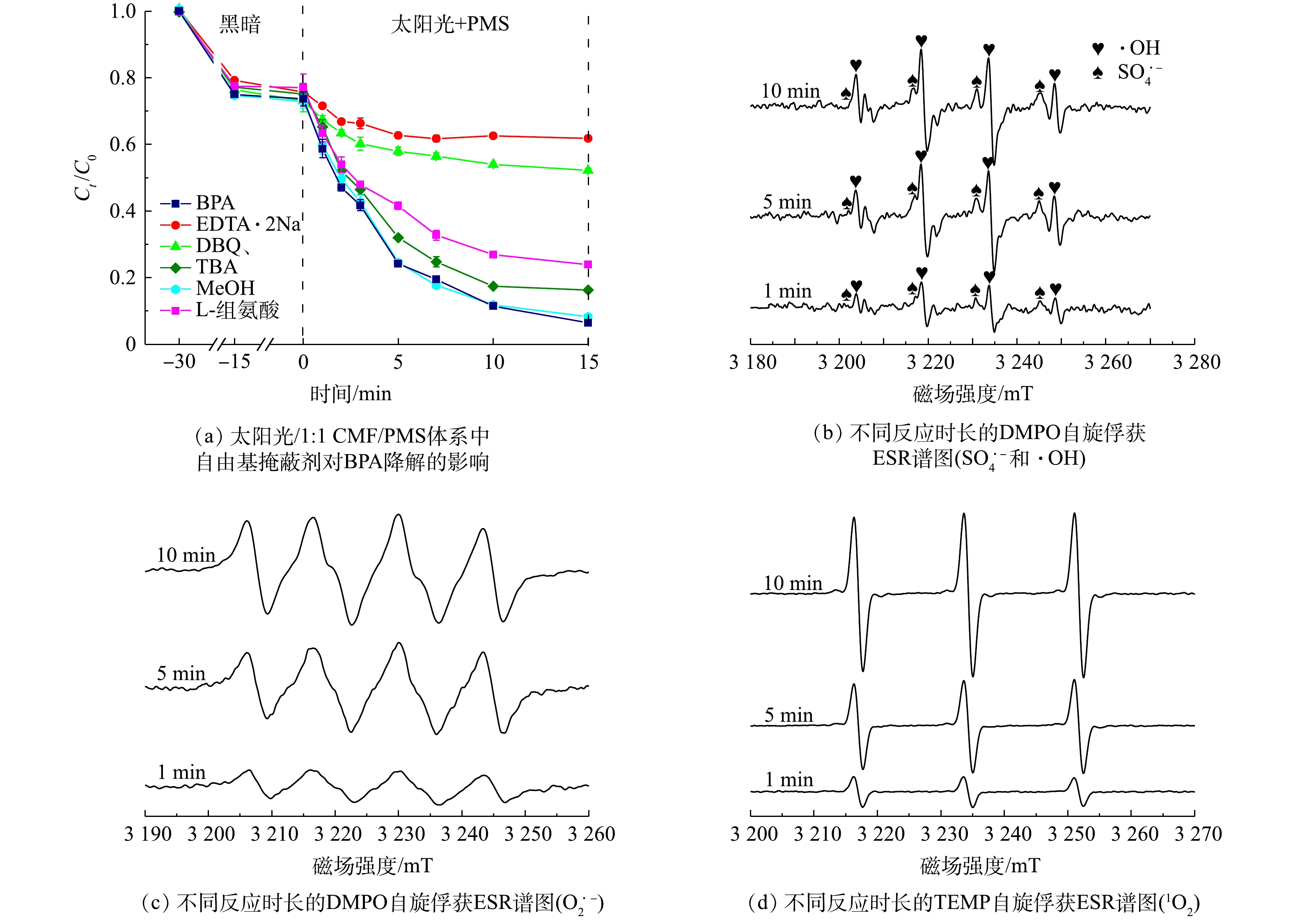
在掩蔽实验中,EDTA-2Na是h+的掩蔽剂,叔丁醇是常用的·OH掩蔽剂,甲醇是SO4·−的掩蔽剂,对苯醌是O2·−的掩蔽剂,L-组氨酸是1O2的掩蔽剂。为确认太阳光/1∶1 CMF/PMS体系中的反应活性成分,分别使用这5种掩蔽剂进行掩蔽实验,结果如图12(a)所示。反应活性成分对BPA降解的作用从大到小的顺序为h+>O2·−>1O2>·OH>SO4·−,其中h+和O2·−在反应中起到了最重要的作用。这与已有研究结果相似[48]:当h+被掩蔽时,15 min时BPA的降解率由67.3%降低至13.9%;而当O2·−被掩蔽时,15 min时BPA的降解率降低至21%,这也说明了h+和O2·−是降解BPA的主要反应活性成分;当·OH、1O2在被掩蔽时,反应15 min时BPA降解率分别降低了9.9%和17.5%,说明他们在反应体系中具有一定的贡献率;而SO4·−的掩蔽对降解反应几乎无影响,因此,可视其为非主要活性自由基。
为了确认反应中起作用的主要自由基种类,通过使用5,5-dimethylpyrroline-oxide (DMPO)作为捕获剂,使用ESR检测了SO4·−、·OH和O2·−;使用TEMP作为捕获剂检测了1O2,结果如图12(b)~(d)所示。图12(b)表明,在光照条件下加入PMS和催化剂,该反应体系中会同时产生·OH和SO4·−,但SO4·−含量较低且不会随时间增加而增加,而代表着·OH的1∶2∶2∶1峰的峰强逐渐升高;如图12(c)和图12(d)所示,随着时间的延长,代表着O2·−和1O2的DMPO-O2·−、TEMP-1O2的响应强度会逐渐增加[16, 38],说明了O2·−和1O2在体系中可大量产生。因此,ESR测试证明,O2·−,·OH和1O2在太阳光/1∶1 CMF/PMS体系中可大量产生且随时间的延长而增多,故其是反应中发挥作用的自由基,这与掩蔽实验的结果一致。相对地,SO4·−的产生较少且含量稳定,说明了SO4·−在反应体系中不是主要的反应活性成分。
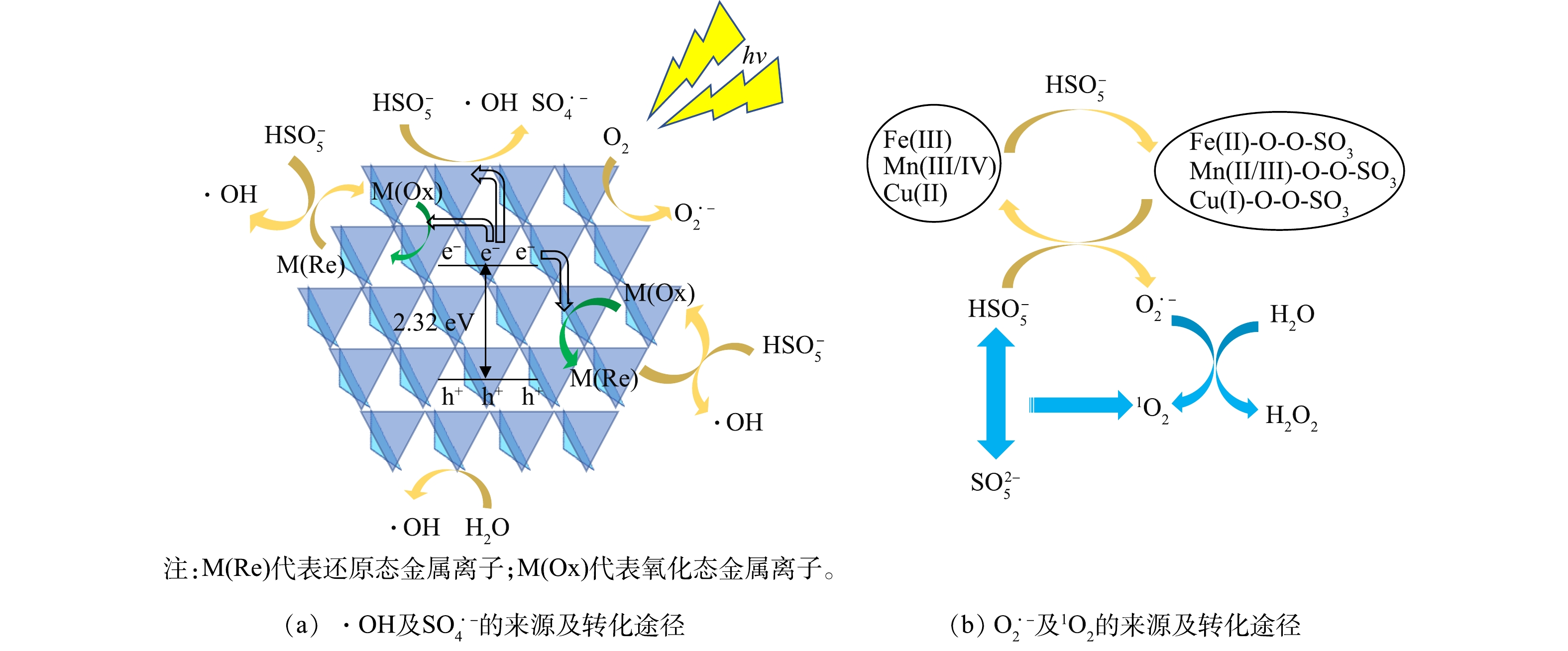
2)反应机制推测。由掩蔽实验和ESR的结果可知,该体系中的主要活性成分为h+和O2·−,降解BPA的过程中,活性成分的贡献率由高到低依次是h+、O2·−、·OH、1O2、SO4·−。如图13(a)所示,在光照过程中,1∶1 CMF会吸收光子后并发生电子的定向迁移,进而产生自由电子e−和空穴h+,但如果不加入PMS仅进行1∶1 CMF的光催化,仅有少量光生h+产生,故反应速率较慢。体系中同时存在1∶1 CMF与PMS时,如式(16)所示,PMS将获得1∶1 CMF的e−被激发,产生SO4·−和·OH,促进1∶1 CMF中h+的产生[38, 49-50];且由于1∶1 CMF中存在多组氧化还原电对(Fe3+和Fe2+,Mn4+和Mn3+,Mn3+和Mn2+,Cu2+和Cu+)(式(17)~(19)),他们会与HSO5−发生电子转移反应从而激发PMS产生·OH[9]。在碱性条件下,PMS激发后的主要产物SO4·−会很快转化为·OH,因此, 在该反应体系中几乎不存在SO4·−[51]。如图13(b)所示,在反应过程除溶解氧吸收电子产生的O2·−外[38],如式(20)~式(27)所示,过渡金属离子通过与HSO5−进行络合反应形成M-SO5中间体,并通过与HSO5−分子的进一步反应产生O2·−[37-38],这也是反应体系中O2·−的主要来源。如式(28)~(29)所示,1O2可源于HSO5−与SO52−分子间的直接作用,或通过O2·−与水的反应产生[16, 38]。最终,这些活性成分会氧化降解BPA。
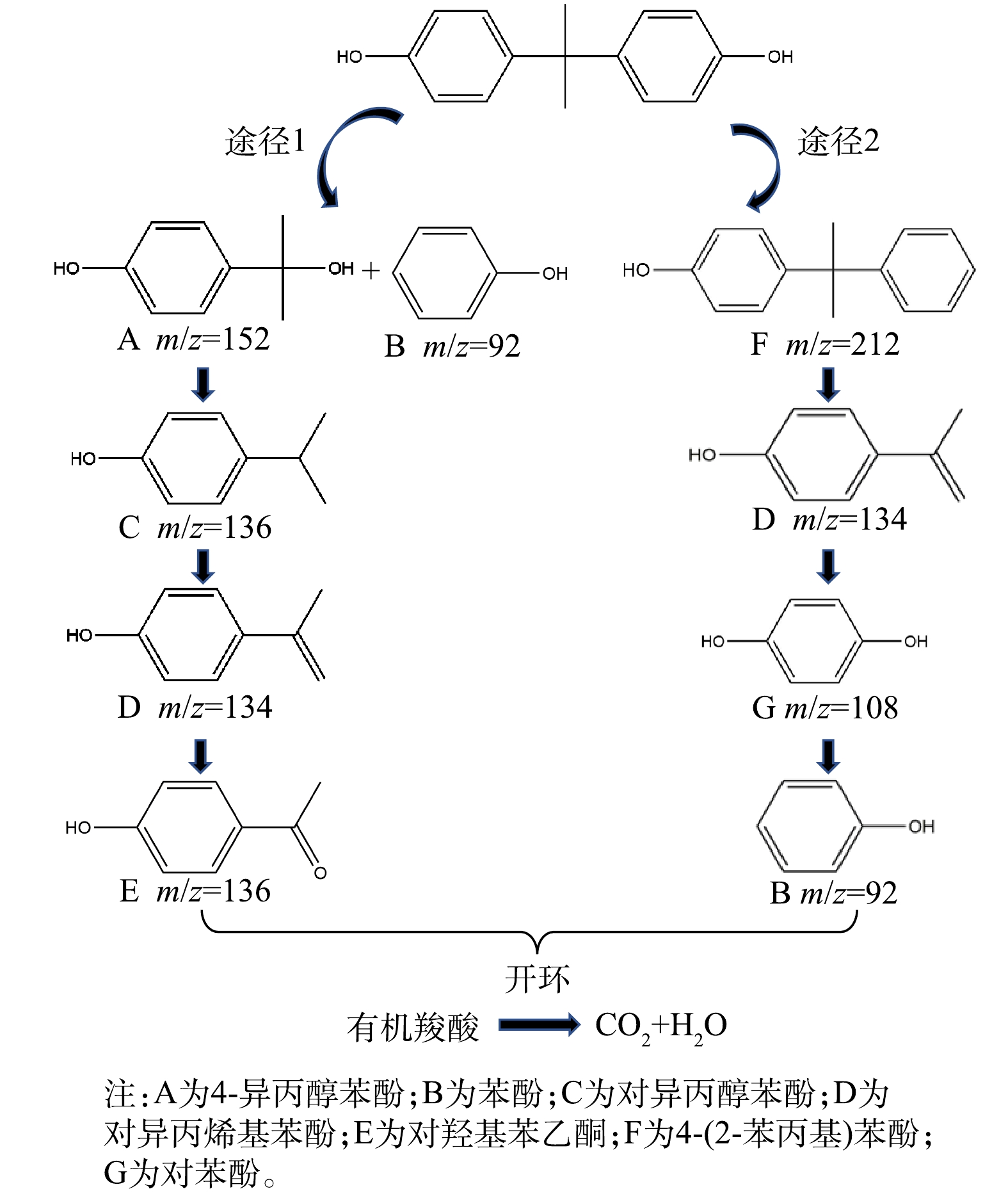
3)反应中的降解产物及途径。为确定BPA降解产物及其降解途径,使用LC-MS检测了反应产物,结果如图14所示。BPA的降解途径主要有以下2条:首先,反应活性成分攻击异丙基与苯环的C—C键,进而生成4-异丙醇苯酚(A)和苯酚(B),前者发生脱羟基反应产生对异丙醇苯酚(C),然后通过脱氢反应和光促羰基化反应,生成对异丙烯基苯酚(D)和对羟基苯乙酮(E);其次,反应活性成分攻击C—O键,产生4-(2-苯丙基)苯酚(F),然后攻击富电子基团产生对异丙烯基苯酚(D),D进一步发生氧化作用产生对苯酚(G),并借由反应活性成分攻击C—O键产生苯酚(B)。最后,所有中间产物将进一步被氧化为乙酸、草酸等小分子物质,最终被矿化为CO2和H2O。
-
1)采用水热法合成了CuMnFe LDHs,当Fe3+摩尔占比为0.29,Cu、Mn摩尔比为1∶1时,LDHs具有最佳的太阳光利用效率及最强的催化能力。
2) 1∶1 CMF具有良好的晶格结构和絮状外观的层状结构,且单层的薄片面积小于Cu-Fe LDHs或Mn-Fe LDHs,具有较大的比表面积和吸附能力;1∶1 CMF中金属元素存在多组氧化还原电对,使1∶1 CMF有更强的电子转移能力,因此,具有较强的催化活性。
3)在太阳光/1∶1 CMF/PMS反应体系中,在PMS最佳浓度为0.4 mmol·L−1和催化剂最佳投加量为0.6 g·L−1时, BPA的去除率在15 min内达到93.5%,该降解反应的过程符合一级反应动力学。
4)在酸性条件下,H2PO4−、NO3−和HCO3−会抑制太阳光/1∶1 CMF/PMS体系对BPA的降解,而碱性条件和Cl−会增强其降解BPA的能力。
5)反应体系中起主要作用的自由基为O2·−,活性成分的作用强弱依次为h+>O2·−>1O2>·OH>SO4·−。h+由1∶1 CMF受光照产生,而PMS会促进h+的产生;O2·−则主要来源于LDHs中过渡金属离子与PMS分子间的两级转化。
6) BPA的降解途径共有2条:途径一的降解中间产物依次为双酚A、4-异丙醇苯酚和苯酚、对异丙醇苯酚、对异丙烯基苯酚、对羟基苯乙酮;途径二的降解中间产物依次为4-(2-苯丙基)苯酚、对异丙烯基苯酚、对苯酚、苯酚。最后,所有中间产物进一步被氧化为乙酸、草酸等小分子物质,最终被矿化为CO2和H2O。
太阳光/CuMnFe LDHs催化剂/过一硫酸盐体系降解双酚A
Degradation of bisphenol A using CuMnFe LDHs catalyst and peroxymonosulfate under solar light
-
摘要: 利用水热法合成了具有高催化活性的CuMnFe三元金属氢氧化物(LDHs)催化剂,确定了最佳的Cu、Mn、Fe摩尔比,通过XRD、XPS、UV-vis DRS、FT-IR、SEM、TEM、BET等方法对催化剂进行了表征,并探究了催化剂投加量、PMS浓度、溶液pH、阴离子的种类和浓度对体系BPA去除的影响,进而通过LC-MS探究了BPA的降解途径与产物。结果表明:在Fe离子摩尔占比为0.29时,LDHs的结晶度最高,且在Cu与Mn摩尔比为1∶1时,1∶1 Cu/Mn-Fe LDHs具有最强的光利用效率和催化活性。在模拟太阳光照射下,在催化剂用量为0.6 g·L−1和PMS投加量为0.4 mmol·L−1时,15 min内对20 mg·L−1的BPA的去除率可达到93.5%。1∶1 Cu/Mn-Fe LDHs具有良好的稳定性和重复利用性,在初始溶液pH 3~11内可保持良好的催化性能,但其催化性能会在pH为 3~5的酸性条件下有所降低,在pH 为3时BPA的去除率仍达79.3%;溶液中的Cl−会促进体系对BPA的降解反应,而H2PO4−、
NO−3 、HCO−3 会抑制体系对BPA的降解。此外,还分析了水中阴离子浓度对该体系降解BPA的影响,并探究了不同活性成分对降解相对贡献的大小。结果表明,超氧自由基(O2·−)和空穴(h+)是本反应体系中最主要的活性成分,PMS掠夺自由电子从而大幅促进了h+的产生,而LDHs的过渡金属元素会与PMS形成稳定中间体,通过掠夺其他PMS的电子进而激发产生O2·−。Abstract: The CuMnFe layered trimetallic hydroxides (LDHs) catalyst with high catalytic activity was synthesized by the hydrothermal method, and the optimal molar ratio of Cu, Mn and Fe was determined. XRD, XPS, UV-vis DRS, FT-IR, SEM, TEM and BET were used to characterize the LDHs, and the effects of catalyst dosage, PMS concentration, initial pH, the type and concentration of anions on the degradation of bisphenol A were studied. LC-MS was also used to identify the degradation products and pathways. The results showed that the highest crystallinity of LDHs occurred when the molar ratio of Fe was 0.29, and the strongest light utilization efficiency and catalytic activity occurred when the molar ratio of Cu∶Mn was 1∶1. Under the simulated solar light irradiation, the removal rate for 20mg.L−1 BPA was 93.5% after 15 min oxidation at the optimized 0.6 g·L−1 1∶1 Cu/Mn-Fe LDHs (1∶1 CMF) and 0.4 mmol·L−1 PMS. 1∶1 Cu/Mn-Fe LDHs had a good stability and reusability. Within pH 3~11 of raw solution, LDHs maintained a good catalysis performance, which would decrease at acidic conditions of pH 3~5, and the removal rate of BPA was 79.3%. Cl− accelerated the degradation of BPA, while H2PO4−, NO3− and HCO3− inhibited the reaction. In addition, the effects of anions concentrations on BPA degradation by this system was analyzed, and the relative contributions of different active species were also discussed. Superoxide radicals (O2·−) and holes (h+) were the most important active components in this reaction system. PMS withdrew free electrons and greatly promoted the production of h+, while the transition metal elements of LDHs could interact with PMS to generate stable intermediates, which withdrew the electron from other PMS to generate O2·−. -
-
[1] YANG Y, OK Y S, KIM K H, et al. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review[J]. Science of the Total Environment, 2017, 596-597: 303-320. doi: 10.1016/j.scitotenv.2017.04.102 [2] LV S W, LIU J M, LI C Y, et al. Two novel MOFs@COFs hybrid-based photocatalytic platforms coupling with sulfate radical-involved advanced oxidation processes for enhanced degradation of bisphenol A[J]. Chemosphere, 2020, 243: 125378. doi: 10.1016/j.chemosphere.2019.125378 [3] GHANBARI F, MORADI M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review[J]. Chemical Engineering Journal, 2017, 310: 41-62. doi: 10.1016/j.cej.2016.10.064 [4] HUANG Y, NENGZI L C, ZHANG X, et al. Catalytic degradation of ciprofloxacin by magnetic CuS/Fe2O3/Mn2O3 nanocomposite activated peroxymonosulfate: Influence factors, degradation pathways and reaction mechanism[J]. Chemical Engineering Journal, 2020, 388: 124274. doi: 10.1016/j.cej.2020.124274 [5] GUO R, WANG Y, LI J, et al. Sulfamethoxazole degradation by visible light assisted peroxymonosulfate process based on nanohybrid manganese dioxide incorporating ferric oxide[J]. Applied Catalysis B:Environmental, 2020, 278: 119297. doi: 10.1016/j.apcatb.2020.119297 [6] GUO R, NENGZI L C, CHEN Y, et al. Construction of high-efficient visible photoelectrocatalytic system for carbamazepine degradation: Kinetics, degradation pathway and mechanism[J]. Chinese Chemical Letters, 2020, 31(10): 2661-2667. doi: 10.1016/j.cclet.2020.03.068 [7] XU H, JIANG N, WANG D, et al. Improving PMS oxidation of organic pollutants by single cobalt atom catalyst through hybrid radical and non-radical pathways[J]. Applied Catalysis B: Environmental, 2020, 263: 118350. [8] DIKDIM J M D, GONG Y, NOUMI G B, et al. Peroxymonosulfate improved photocatalytic degradation of atrazine by activated carbon/graphitic carbon nitride composite under visible light irradiation[J]. Chemosphere, 2019, 217: 833-842. doi: 10.1016/j.chemosphere.2018.10.177 [9] ZHANG H, NENGZI L C, WANG Z, et al. Construction of Bi2O3/CuNiFe LDHs composite and its enhanced photocatalytic degradation of lomefloxacin with persulfate under simulated sunlight[J]. Journal of Hazardous Materials, 2020, 383: 121236. doi: 10.1016/j.jhazmat.2019.121236 [10] AHMED M M, BRIENZA M, GOETZ V, et al. Solar photo-Fenton using peroxymonosulfate for organic micropollutants removal from domestic wastewater: Comparison with heterogeneous TiO2 photocatalysis[J]. Chemosphere, 2014, 117: 256-261. doi: 10.1016/j.chemosphere.2014.07.046 [11] CHEN X, WANG W, XIAO H, et al. Accelerated TiO2 photocatalytic degradation of acid orange 7 under visible light mediated by peroxymonosulfate[J]. Chemical Engineering Journal, 2012, 193-194: 290-295. doi: 10.1016/j.cej.2012.04.033 [12] ZHANG H, NENGZI L C, LI X, et al. Construction of CuBi2O4/MnO2 composite as Z-scheme photoactivator of peroxymonosulfate for degradation of antibiotics[J]. Chemical Engineering Journal, 2020, 386: 124011. doi: 10.1016/j.cej.2020.124011 [13] LI B, NENGZI L C, GUO R, et al. Novel synthesis of Z-scheme α-Bi2O3/g-C3N4 composite photocatalyst and its enhanced visible light photocatalytic performance: Influence of calcination temperature[J]. Chinese Chemical Letters, 2020, 31(10): 2705-2711. doi: 10.1016/j.cclet.2020.04.026 [14] CUI Y, NENGZI L C, GOU J, et al. Fabrication of dual Z-scheme MIL-53(Fe)/α-Bi2O3/g-C3N4 ternary composite with enhanced visible light photocatalytic performance[J]. Separation and Purification Technology, 2020, 232: 115959. doi: 10.1016/j.seppur.2019.115959 [15] LIM J, HOFFMANN M R. Peroxymonosulfate (PMS) activation on cobalt-doped TiO2 nanotubes: degradation of organics under dark and solar light irradiation conditions[J]. Environmental Science-Nano, 2020, 7(5): 1602-1611. doi: 10.1039/D0EN00131G [16] HUANG J, ZHANG H. Mn-based catalysts for sulfate radical-based advanced oxidation processes: A review[J]. Environmental International, 2019, 133: 105141. doi: 10.1016/j.envint.2019.105141 [17] WEN T, WU X, TAN X, et al. One-pot synthesis of water-swellable Mg-Al layered double hydroxides and graphene oxide nanocomposites for efficient removal of As(V) from aqueous solutions[J]. ACS Applied Material Interfaces, 2013, 5(8): 3304-3311. doi: 10.1021/am4003556 [18] GUO R, ZHU Y, CHENG X, et al. Efficient degradation of lomefloxacin by Co-Cu-LDH activating peroxymonosulfate process: Optimization, dynamics, degradation pathway and mechanism[J]. Journal of Hazardous Materials, 2020, 399: 122966. doi: 10.1016/j.jhazmat.2020.122966 [19] ZHANG G, ZHANG X, MENG Y, et al. Layered double hydroxides-based photocatalysts and visible-light driven photodegradation of organic pollutants: A review[J]. Chemical Engineering Journal, 2020, 392: 123684. doi: 10.1016/j.cej.2019.123684 [20] TANG S, WANG Z, YUAN D, et al. Enhanced photocatalytic performance of BiVO4 for degradation of methylene blue under LED visible light irradiation assisted by peroxymonosulfate[J]. International Journal of Electrochemical Science, 2020, 15(3): 2470-2480. [21] OTGONJARGAL E, KIM Y S, PARK S M, et al. Mn-Fe layered double hydroxides for adsorption of As(III) and As(V)[J]. Separation Science and Technology, 2012, 47(14/15): 2192-2198. [22] LI S, GUO Y, XIAO M, et al. Enhanced arsenate removal from aqueous solution by Mn-doped MgAl-layered double hydroxides[J]. Environmental Science and Pollution Research International, 2019, 26(12): 12014-12024. doi: 10.1007/s11356-019-04667-4 [23] CHEN Y, HONG H, CAI J, et al. Highly Efficient CO2 to CO transformation over Cu-Based catalyst derived from a CuMgAl‐layered double hydroxide (LDH)[J]. ChemCatChem, 2020, 13(2): 656-663. [24] WAGNER C D, ZATKO D A, RAYMOND R H. Use of the oxygen KLL auger lines in identification of surface chemical-states by electron-spectroscopy for chemical-analysis[J]. Analytical Chemistry, 1980, 52(9): 1445-1451. doi: 10.1021/ac50059a017 [25] WU L, ZHOU X, WAN G, et al. Novel hierarchical CuNiAl LDH nanotubes with excellent peroxidase-like activity for wide-range detection of glucose[J]. Dalton Transactions, 2021, 50(1): 95-102. doi: 10.1039/D0DT03288C [26] WANG S, ZHU J, ZHANG S, et al. The catalytic degradation of nitrobenzene by the Cu-Co-Fe-LDH through activated oxygen under ambient conditions[J]. Dalton Transactions, 2020, 49(13): 3999-4011. doi: 10.1039/C9DT03794B [27] OU B, WANG J, WU Y, et al. Efficient removal of Cr (VI) by magnetic and recyclable calcined CoFe-LDH/g-C3N4 via the synergy of adsorption and photocatalysis under visible light[J]. Chemical Engineering Journal, 2020, 380: 122600. doi: 10.1016/j.cej.2019.122600 [28] UMEZAWA Y, REILLEY C N. Effect of argon ion-bombardment on metal-complexes and oxides studied by X-ray photoelectron-spectroscopy[J]. Analytical Chemistry, 1978, 50(9): 1290-1295. doi: 10.1021/ac50031a025 [29] WU X, FENG Y, LIU X, et al. Redox & acidity optimizing of LDHs-based CoMnAl mixed oxides for enhancing NH3-SCR performance[J]. Applied Surface Science, 2019, 495: 143513. doi: 10.1016/j.apsusc.2019.07.255 [30] LU H, ZHU Z, ZHANG H, et al. Simultaneous removal of arsenate and antimonate in simulated and practical water samples by adsorption onto Zn/Fe layered double hydroxide[J]. Chemical Engineering Journal, 2015, 276: 365-375. doi: 10.1016/j.cej.2015.04.095 [31] XIE L, ZHONG Y, XIANG R, et al. Sono-assisted preparation of Fe(II)-Al(III) layered double hydroxides and their application for removing uranium (VI)[J]. Chemical Engineering Journal, 2017, 328: 574-584. doi: 10.1016/j.cej.2017.07.051 [32] EL GAINI L, LAKRAIMI M, SEBBAR E, et al. Removal of indigo carmine dye from water to Mg-Al-CO3-calcined layered double hydroxides[J]. Journal of Hazardous Materials, 2009, 161(2/3): 627-632. [33] ABDELHALEEM A, CHU W. Monuron photodegradation using peroxymonosulfate activated by non-metal-doped TiO2 under visible LED and the modeling via a parallel-serial kinetic approach[J]. Chemical Engineering Journal, 2018, 338: 411-421. doi: 10.1016/j.cej.2018.01.036 [34] 李立, 吴丽颖, 董正玉, 等. 高晶度Mn-Fe LDH催化剂活化过一硫酸盐降解偶氮染料[J]. 环境科学, 2020, 41(6): 10. [35] WU Y, FANG Z, SHI Y, et al. Activation of peroxymonosulfate by BiOCl@Fe3O4 catalyst for the degradation of atenolol: Kinetics, parameters, products and mechanism[J]. Chemosphere, 2019, 216: 248-257. doi: 10.1016/j.chemosphere.2018.10.012 [36] ZHAN M J, YANG X, XIAN Q M, et al. Photosensitized degradation of bisphenol A involving reactive oxygen species in the presence of humic substances[J]. Chemosphere, 2006, 63(3): 378-386. doi: 10.1016/j.chemosphere.2005.08.046 [37] ALI J, WENLI L, SHAHZAD A, et al. Regulating the redox centers of Fe through the enrichment of Mo moiety for persulfate activation: A new strategy to achieve maximum persulfate utilization efficiency[J]. Water Research, 2020, 181: 115862. doi: 10.1016/j.watres.2020.115862 [38] SHAHZAD A, ALI J, IFTHIKAR J, et al. Non-radical PMS activation by the nanohybrid material with periodic confinement of reduced graphene oxide (rGO) and Cu hydroxides[J]. Journal of Hazardous Materials, 2020, 392: 122316. doi: 10.1016/j.jhazmat.2020.122316 [39] ZHU S, LI X, KANG J, et al. Persulfate activation on crystallographic manganese oxides: Mechanism of singlet oxygen evolution for nonradical selective degradation of aqueous contaminants[J]. Environmental Science and Technology, 2019, 53(1): 307-315. doi: 10.1021/acs.est.8b04669 [40] GONG Y, ZHAO X, ZHANG H, et al. MOF-derived nitrogen doped carbon modified g-C3N4 heterostructure composite with enhanced photocatalytic activity for bisphenol A degradation with peroxymonosulfate under visible light irradiation[J]. Applied Catalysis B:Environmental, 2018, 233: 35-45. doi: 10.1016/j.apcatb.2018.03.077 [41] YANG L, XU L, BAI X, et al. Enhanced visible-light activation of persulfate by Ti3+ self-doped TiO2/graphene nanocomposite for the rapid and efficient degradation of micropollutants in water[J]. Journal of Hazardous Materials, 2019, 365: 107-117. doi: 10.1016/j.jhazmat.2018.10.090 [42] XU Y, LIN Z, ZHANG H. Mineralization of sucralose by UV-based advanced oxidation processes: UV/PDS versus UV/H2O2[J]. Chemical Engineering Journal, 2016, 285: 392-401. doi: 10.1016/j.cej.2015.09.091 [43] LEI Y, CHENG S, LUO N, et al. Rate constants and mechanisms of the reactions of Cl· and Cl2·− with trace organic contaminants[J]. Environmental Science and Technology, 2019, 53(19): 11170-11182. doi: 10.1021/acs.est.9b02462 [44] YAN J, LI J, PENG J, et al. Efficient degradation of sulfamethoxazole by the CuO@Al2O3 (EPC) coupled PMS system: Optimization, degradation pathways and toxicity evaluation[J]. Chemical Engineering Journal, 2019, 359: 1097-1110. doi: 10.1016/j.cej.2018.11.074 [45] CHEN H, XU Y, ZHU K, et al. Understanding oxygen-deficient La2CuO4-δ perovskite activated peroxymonosulfate for bisphenol A degradation: The role of localized electron within oxygen vacancy[J]. Applied Catalysis B:Environmental, 2021, 284: 119732. doi: 10.1016/j.apcatb.2020.119732 [46] JAAFARZADEH N, GHANBARI F, AHMADI M. Catalytic degradation of 2, 4-dichlorophenoxyacetic acid (2, 4-D) by nano-Fe2O3 activated peroxymonosulfate: Influential factors and mechanism determination[J]. Chemosphere, 2017, 169: 568-576. doi: 10.1016/j.chemosphere.2016.11.038 [47] YANG S, WANG P, YANG X, et al. Degradation efficiencies of azo dye acid orange 7 by the interaction of heat, UV and anions with common oxidants: Persulfate, peroxymonosulfate and hydrogen peroxide[J]. Journal of Hazardous Materials, 2010, 179(1/2/3): 552-558. doi: 10.1016/j.jhazmat.2010.03.039 [48] CHEN P, ZHANG Q, SHEN L, et al. Insights into the synergetic mechanism of a combined vis-RGO/TiO2/peroxodisulfate system for the degradation of PPCPs: Kinetics, environmental factors and products[J]. Chemosphere, 2019, 216: 341-351. doi: 10.1016/j.chemosphere.2018.10.096 [49] XIA D, HE H, LIU H, et al. Persulfate-mediated catalytic and photocatalytic bacterial inactivation by magnetic natural ilmenite[J]. Applied Catalysis B:Environmental, 2018, 238: 70-81. doi: 10.1016/j.apcatb.2018.07.003 [50] GOLSHAN M, KAKAVANDI B, AHMADI M, et al. Photocatalytic activation of peroxymonosulfate by TiO2 anchored on cupper ferrite (TiO2@CuFe2O4) into 2, 4-D degradation: Process feasibility, mechanism and pathway[J]. Journal of Hazardous Materials, 2018, 359: 325-337. doi: 10.1016/j.jhazmat.2018.06.069 [51] TAKDASTAN A, KAKAVANDI B, AZIZI M, et al. Efficient activation of peroxymonosulfate by using ferroferric oxide supported on carbon/UV/US system: A new approach into catalytic degradation of bisphenol A[J]. Chemical Engineering Journal, 2018, 331: 729-743. doi: 10.1016/j.cej.2017.09.021 -







 下载:
下载: