-
挥发性有机化合物(VOCs)作为PM2.5和臭氧的重要前驱体,已经对大气环境质量和人体健康造成直接和间接的危害[1-2],VOCs废气治理成为了近年来的环境热点话题之一。在VOCs废气的众多处理技术中,催化燃烧技术因处理效率高、能耗低、二次污染小而在工业上应用广泛[3]。为达到VOCs催化燃烧所需温度,工业上多采用电加热使VOCs废气达到起燃温度。由于电加热是采用热传导的加热方式,因而对大气量的VOCs废气加热时能耗巨大。另外,VOCs催化燃烧时,持续的高温环境会使催化剂活性组分烧结而影响VOCs降解效果[4]。微波加热应用于VOCs催化燃烧是一种新技术,它利用电磁波的选择性仅对催化剂进行加热,因而能耗低且催化剂受热均匀、快速[5]。而且,微波对催化剂活性组分的热点效应有利于引发VOCs的催化燃烧,同时微波的偶极极化作用还可降低VOCs的反应阈能而促进其氧化降解[6]。卜龙利等[7]、姚泽等[8]利用吸波型催化剂证实了微波催化燃烧VOCs效果优于电加热催化燃烧。
微波催化燃烧技术的关键是高效而稳定的催化剂。过渡金属氧化物催化剂种类丰富、经济性好、不易中毒,但其对于某些难降解有机物的催化活性差、低温活性以及热稳定性有待提高[9]。贵金属催化剂具有催化活性高、高温下稳定性好和适用范围广的优点,但也存在资源稀少和价格高昂的问题[10]。目前,市面上较为常见的是铜锰铈三元金属氧化物催化剂。胡旭睿[11]研究证实,铜锰铈氧化物催化剂对芳香烃类、醇类、酮类等有机物具有良好的氧化性能,但其矿化效果不佳且对芳香烃的降解效果低于醇、酮类。贵金属Pt、Pd等在低温时对芳烃类和3个碳以上的直链烷烃活化能力强[12]。已有研究证实,将贵金属与过渡金属复合可以增强催化剂活性。SHI等[13]通过还原法和离子交换法合成了Pt /Ce-USY催化剂,其对1,2-二氯乙烷的催化活性高于Pt/USY催化剂,Pt与CeO2之间的相互作用抑制了碳物质的沉积,从而增强了催化剂的耐久性。LEE等[14]将贵金属金、钯沉积在CeO2表面,证实适量Au的加入使得Pd/CeO2催化剂活性增强。CHEN等[15]合成了Pd/Fe3O4催化剂用于去除CO,当沉积适量Fe3O4时,CO氧化的起燃温度明显降低。因此,本研究在铜锰铈三元催化剂基础上复合微量贵金属Pt,以期提高催化剂对芳烃类VOCs的活化能力,进而提高总VOCs的去除效果。
本研究选取油墨印刷VOCs废气中含量最多的2种物质甲苯和乙酸乙酯作为目标污染物,以蜂窝状堇青石为载体制备Pt复合铜锰铈(CMC)负载型催化剂(Pt-CMC/堇青石),重点考察Pt复合前后催化剂对模拟VOCs废气催化燃烧效果的差异,以探究低含量贵金属添加对铜锰铈氧化物催化剂催化活性的影响程度。
-
蜂窝状正方体堇青石(7目,边长150 mm);硝酸铜、硝酸锰(50 wt%)、硝酸铈、氯铂酸,分析纯;硅溶胶,分析纯。超声波清洗器(KQ3200,昆山市超声仪器有限公司);电热鼓风干燥箱(101-3AB,天津泰斯特仪器有限公司); 箱式电阻炉(SX-4-10,天津泰斯特仪器有限公司);单模腔微波装置(ZDM-2,南京汇研微波系统工程有限公司);气相色谱仪(GC/FID,6890 N,美国安捷伦科技公司);泵吸式VOC检测仪(XS-2000-VOC,希思智能科技);扫描电子显微镜(JSM-6510LV型,日本电子);比表面积及孔径分析仪(V-sorb2800P型,北京金埃谱公司);X射线衍射仪(X’Pert型,荷兰帕纳科)。
-
本研究采用等体积浸渍法进行负载型催化剂的制备,即载体吸水率测试基础上配置适量体积的浸渍液、然后浸渍液被载体完全吸收的一种催化剂制备方法。首先,将正方体堇青石切割成圆柱型载体(D×L=27×150 mm),称重并测其吸水率,根据吸水率确定助剂硅溶胶的用量;其次,按3∶3∶1的质量负载比例分别称取铜(2.5%(质量分数))、锰、铈的金属盐,称取一定量的硅溶胶以及少量氯铂酸(Pt的质量分数0.01%),加入去离子水超声震荡30 min配制完全溶解的浸渍液;最后,将堇青石载体置于浸渍液中将浸渍液完全吸收,样品静置风干后放入烘箱中80 ℃过夜烘干,电阻炉内500 ℃煅烧4 h,自然冷却后即可制得Pt-CMC/堇青石催化剂。不加入氯铂酸、仅加入氯铂酸和其余步骤均相同情况下,可分别制得CMC/堇青石、Pt /堇青石催化剂。
利用扫描电子显微镜观察催化剂表面形貌及活性组分分布状况,借助BET测试仪分析催化剂的比表面积及孔径孔体积,使用X射线衍射仪测试活性组分的晶体结构及晶粒大小。
-
实验装置如图1所示,整个装置分为配气、催化燃烧和尾气净化3部分。在配气系统中,空气依次通过变色硅胶柱和活性炭柱去除水分和有机物,其流量通过气体流量计控制;实验时采用微量注射泵将液态甲苯和乙酸乙酯(按一定比例混合)以恒定速度连续均匀地注入三口烧瓶中,三口烧瓶被电加热套加热而将有机物气化,气化的甲苯和乙酸乙酯被空气带出并在混合瓶内混合均匀。实验开始前催化剂置于石英管内,石英管填装催化剂的长度为360 mm,锥形收口的长度是20 mm,上下两端开口外径分别为32 mm和9 mm,壁厚2 mm;石英管固定在单模腔微波装置上形成固定床反应器,实验开始后微波通过单模腔持续辐照在固定床上,催化剂吸波升温达到VOCs催化燃烧温度;石英管下端连接混合瓶,通入的VOCs气体在固定床上发生催化燃烧反应,床中插入的热电偶探针与温度显示仪连接以实时监测床层温度变化,石英管上下分别设进、出气采样口。催化燃烧后的VOCs尾气分别通过缓冲瓶和装有乙醇和碱液的尾气吸收瓶,净化后的废气经通风橱排空。
本研究利用CMC/堇青石、Pt-CMC/堇青石和Pt /堇青石催化剂在处理气量0.12 m3·h−1、气时空速(gas hourly space velocity,GHSV)1 500 h−1条件下,考察了不同微波功率(30、40、50、60、70 W)和不同VOCs浓度(1 000、1 500、2 000 mg·m−3)下甲苯和乙酸乙酯的催化燃烧效率,分析微波催化燃烧甲苯反应动力学及Pt复合影响,比较3种催化剂的催化活性及Pt复合对总VOCs去除效率的提升作用,探究Pt-CMC/堇青石催化剂对甲苯、乙酸乙酯和总VOCs催化活性的稳定性。
-
本研究主要利用气相色谱仪对甲苯和乙酸乙酯的进气和出气质量浓度进行定量分析,气相色谱检测条件为[16]:柱箱初始温度100 ℃,以20 ℃·min−1的速率升温至180 ℃,保持3 min,加热器温度为190 ℃,检测器温度300 ℃;设定分流比为50∶1;尾气吹脱用氮气,流量30 mL·min−1。
使用手持泵吸式VOC检测仪对反应器进气和出气的总VOCs浓度进行检测,从而分析反应过程中总VOCs的催化燃烧效率。文中数据为2次平行实验的平均值,以此消除实验偶然性误差的影响。
-
1) BET。根据表1数据可知,与堇青石载体相比,CMC/堇青石与Pt-CMC/堇青石2种催化剂的比表面积有不同程度的增加,这表明催化剂的吸附能力也相应增强。与堇青石载体相比,CMC/堇青石催化剂总孔容相对减小,平均孔径有所增大。推测其原因是,活性组分负载后,载体表面的部分孔隙被活性组分填充和覆盖,载体结构的原有孔道被拓宽。Pt-CMC/堇青石催化剂活性晶粒间相互积聚生成了包括微孔和介孔在内的新孔道,催化剂的比表面积及孔体积都明显增大,这有利于污染物分子在催化剂表面的吸附与活化。因此,Pt的复合在一定程度上增强了催化剂的吸附活化性能。
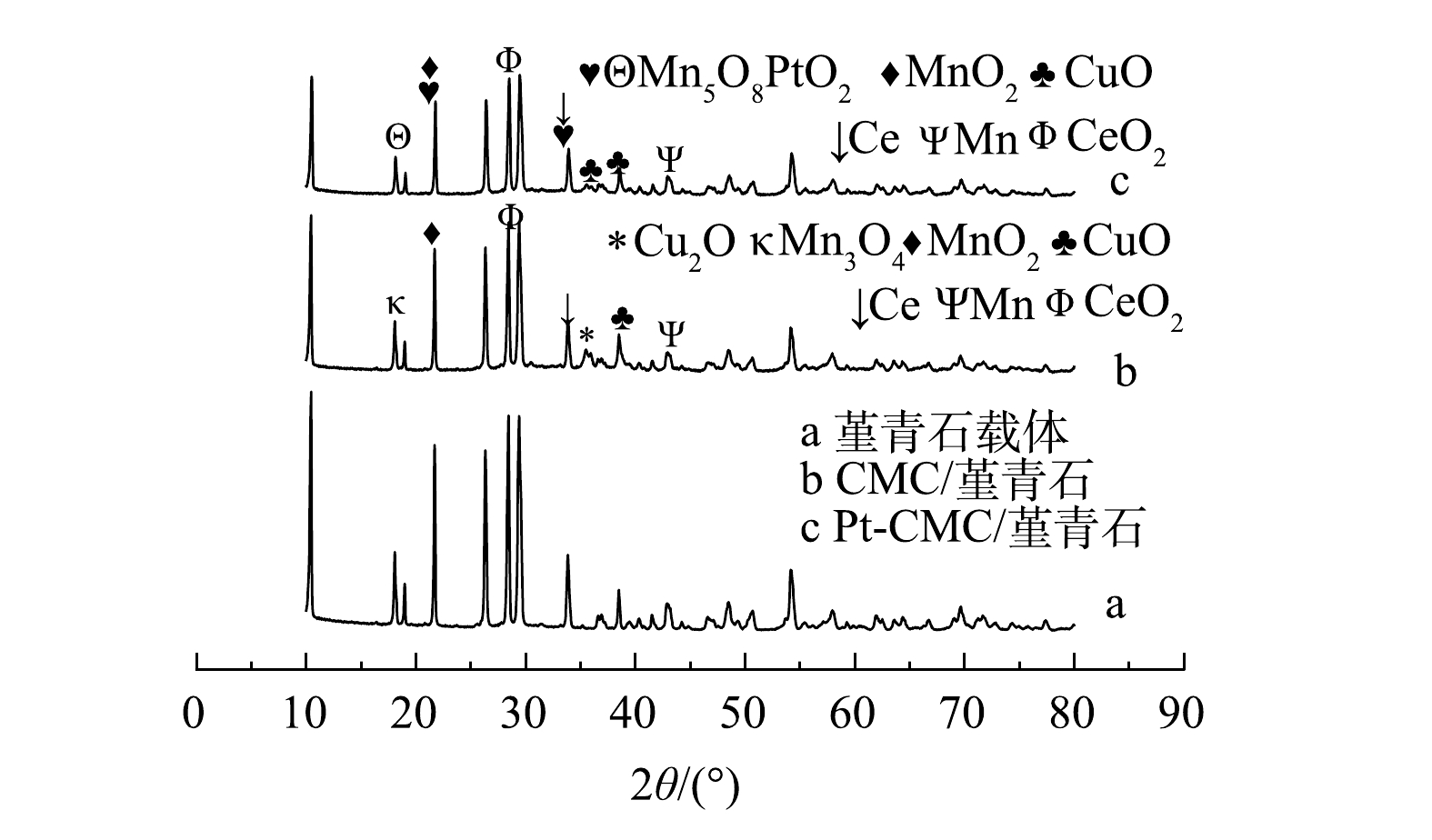
2) XRD。如图2所示,2 θ在10°~40°之间存在着较为密集的堇青石特征衍射峰。对比XRD谱图可以发现,活性组分的负载和制备时的高温煅烧不会改变堇青石自身的晶体结构;与堇青石载体相比,CMC/堇青石和Pt-CMC/堇青石催化剂的特征衍射峰强度减弱,活性晶粒的平均尺寸有不同程度的减小。活性组分负载后堇青石特征峰峰强减弱的原因可能是活性组分一定程度上覆盖和屏蔽了堇青石表面的特征峰[17];贵金属Pt复合后,金属氧化物等活性组分晶体特征峰的峰高有微弱增大,这可能是不同尖晶石活性组分共存所致。6个处于17.5°、21.9°、28.7°、34.1°、36.7°、38.3°位置较为明显的特征峰,均归属于铜锰铈及其金属氧化物,这说明在贵金属Pt复合前后,铜锰铈金属氧化物都是微波催化燃烧VOCs的主要活性组分。在堇青石载体、CMC/堇青石催化剂和Pt-CMC/堇青石催化剂上,3种催化剂的晶粒平均尺寸分别为50.7、13.9和14.5 nm。Pt复合后,催化剂晶粒尺寸变化较小,而比表面积却从0.66 m2·g−1增大到2.95 m2·g−1,由此可以说明贵金属Pt的复合使活性颗粒的分散性增大[18]。
结合特征峰组分分析可知,少量贵金属Pt的添加改变了催化剂上铜和锰的晶相。贵金属Pt复合前,锰以+4价的MnO2和中间价态的Mn3O4形式存在;贵金属Pt复合后,部分金属锰的价态升高,以Mn5O8形式存在,且Cu2O的晶体在掺杂后未检测到,检测出以+4价存在的PtO2。有文献报道,Cu、Mn金属离子价态升高,可以增强催化剂的活性[19]。
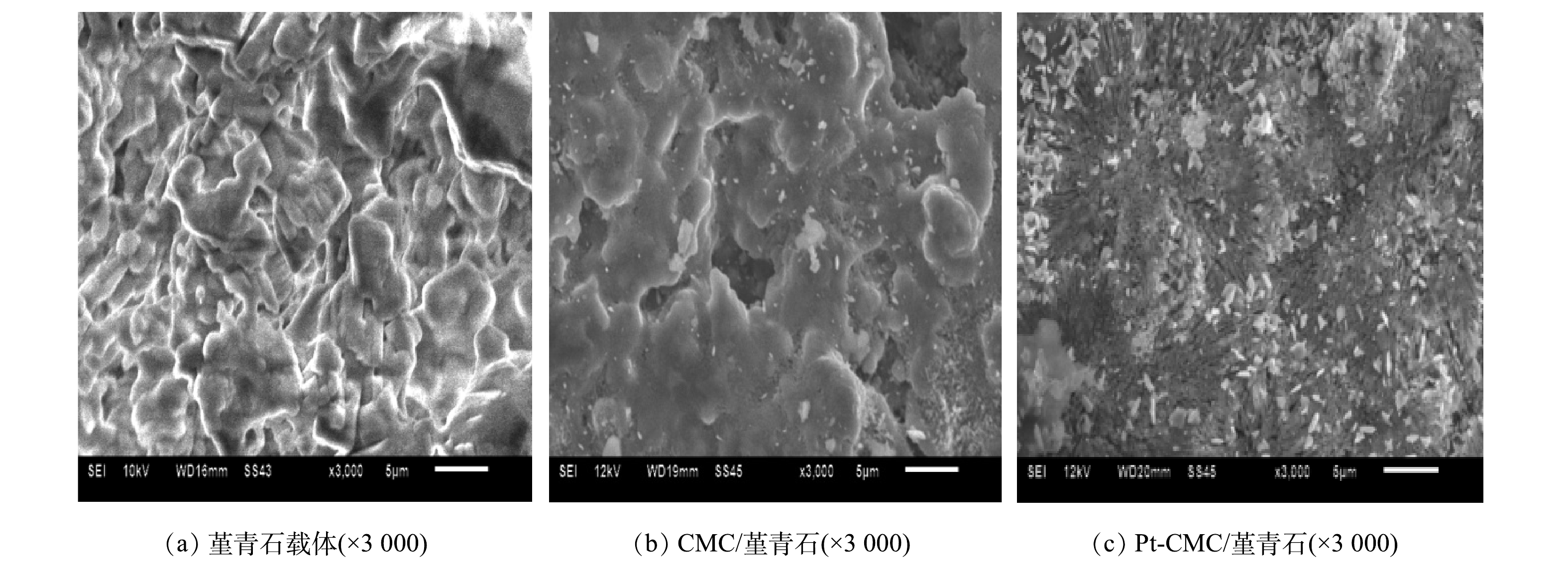
3) SEM。图3给出堇青石载体和CMC/堇青石、Pt-CMC/堇青石催化剂的表面形貌。可以看出,堇青石载体呈明显的层状结构,表面孔隙较多且分布均匀,有利于活性组分的负载。CMC/堇青石催化剂表面存在着较多微米尺寸的活性颗粒,不均匀地分布在载体表面;部分活性组分经高温煅烧后团聚成大块与堇青石结构连结在一起,填充载体原有孔隙的同时也产生了新的孔隙。Pt-CMC/堇青石催化剂表面的活性颗粒在载体表面上分布更均匀、分散度更高。对比Pt复合前后催化剂的微观形貌,可以看出,Pt的复合改变了活性颗粒的分散性,活性物质的再分散可能导致催化剂的吸附性改变。
-
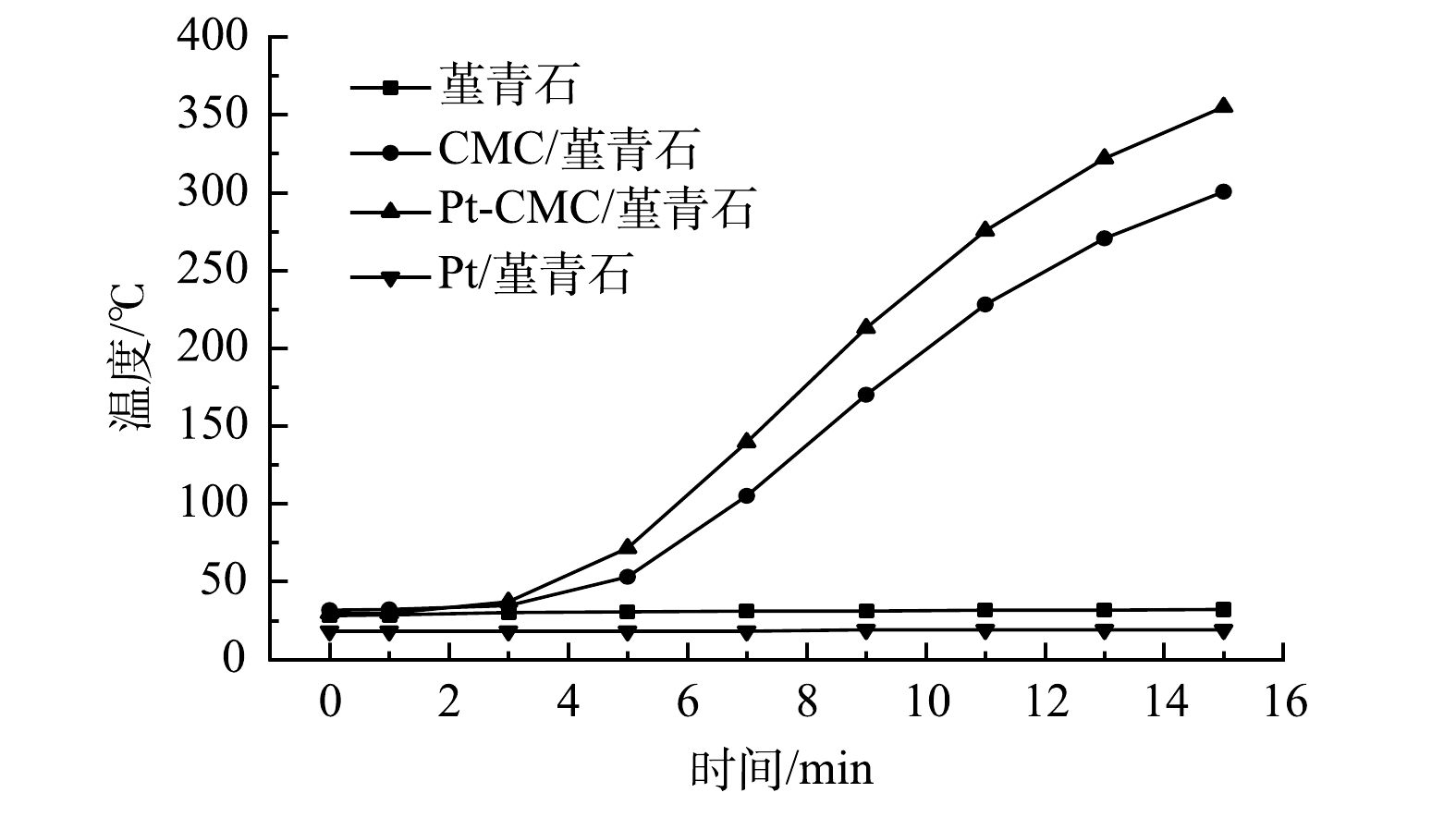
1)吸波升温曲线。图4为堇青石载体、CMC/堇青石、Pt-CMC/堇青石和Pt/堇青石在200 W微波功率辐照下的吸波升温曲线。由图4可见,堇青石载体温度几乎不变,这表明其吸波性能很差;而Pt/堇青石催化剂在15 min内温度也仅升高了3 ℃,其原因是贵金属具有高热稳定性,且吸波性能差。CMC/堇青石和Pt-CMC/堇青石催化剂升温效果明显好于前2种,微波辐照15 min时分别升温至300和355 ℃,说明铜锰铈及其金属氧化物具有良好的吸波性能,是2种催化剂吸波升温的主要原因;Pt复合催化剂升温速率更快,这可能是Pt的复合提高了催化剂上活性晶粒的分散性,降低了金属对微波的反射,以及颗粒尺寸变大增强了微波辐照时的局部热点效应,从而使得催化剂的吸波升温性能提高[20-21]。催化剂的快速升温有助于微波热点效应和高温活性区的出现,从而有助于甲苯和乙酸乙酯的快速氧化与彻底降解。
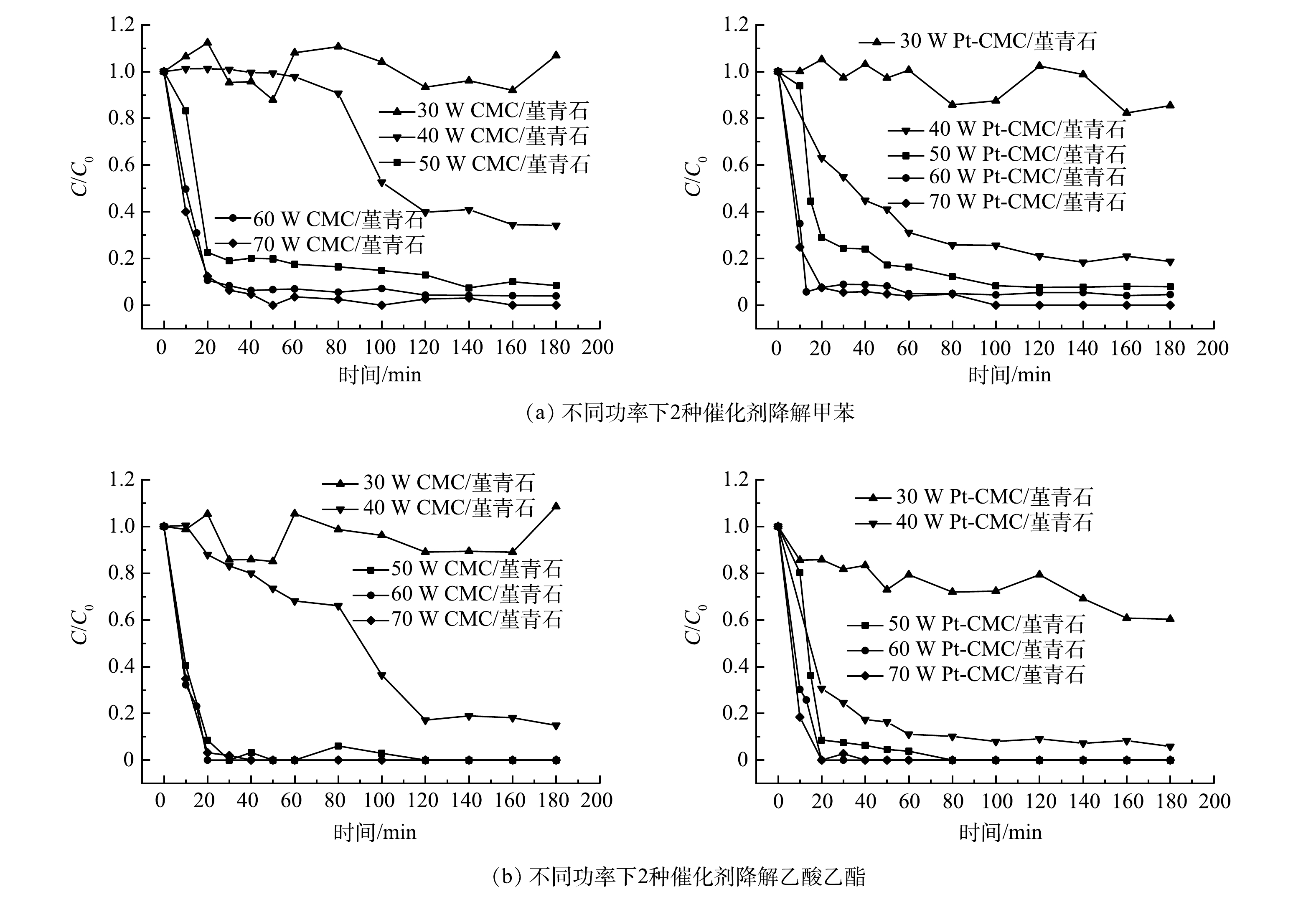
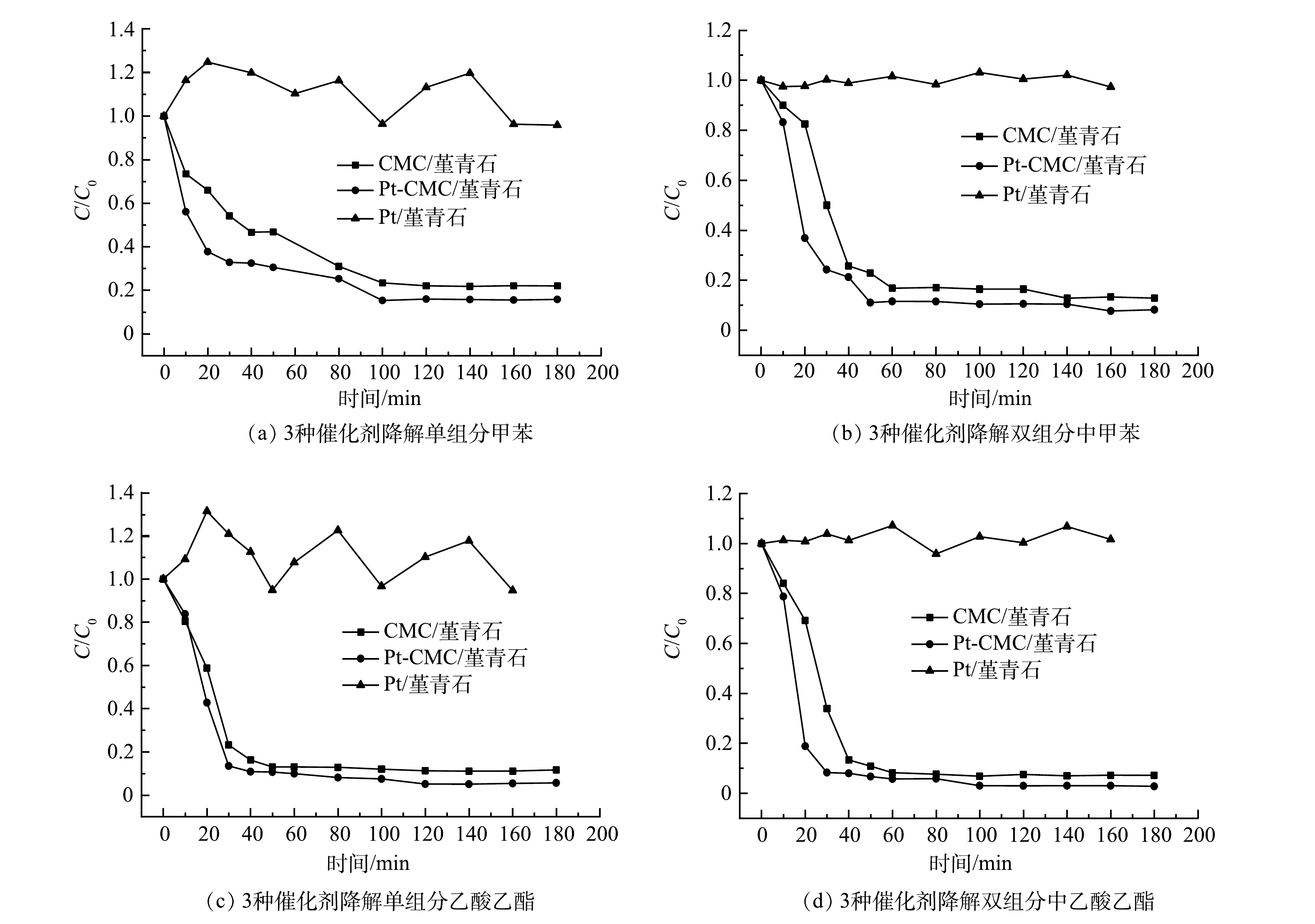
2)催化剂活性。在甲苯和乙酸乙酯的进气浓度分别为1 000和500 mg·m−3,进气量为0.12 m3·h−1,微波功率分别选取30、40、50、60、70 W的条件下,开展CMC/堇青石和Pt-CMC/堇青石催化剂对甲苯和乙酸乙酯双组分VOCs废气的催化燃烧性能试验,结果如图5所示。图5中纵坐标C/C0为反应实时浓度与进气浓度比值(图6同),可直观表示降解效率并使数据间具有可比性。
由图5可见,微波功率30 w时,2种催化剂对甲苯和乙酸乙酯的降解呈不稳定态势,其原因是床层温度偏低(CMC/堇青石床层温度低于66 ℃),催化剂对污染物的去除以吸附-脱附为主。随着微波功率增大,甲苯和乙酸乙酯的降解效率也随之升高,但2种催化剂对甲苯和乙酸乙酯降解效率的差异却由大变小:40 W时,催化剂对甲苯和乙酸乙酯降解效率的差异最大;70 W时,这种差异最小,此时固定床温度高,污染物在催化剂表面完全燃烧,温度的作用掩盖了Pt的复合作用。
同一微波功率下,降解甲苯与乙酸乙酯时, Pt-CMC/堇青石催化剂表现出更高的VOCs催化活性。40 W微波功率下,Pt-CMC/堇青石对甲苯和乙酸乙酯的降解效率分别为82%和93%,而CMC/堇青石降解甲苯和乙酸乙酯的效率分别为66%和82%。推测其原因是,Pt-CMC/堇青石的比表面积相比CMC/堇青石大幅增加,从而有利于甲苯与乙酸乙酯的吸附与活化;同时,活性组分更好的分散性使得Pt-CMC/堇青石升温更快,而高温则有利于VOCs的催化燃烧。如果要使CMC/堇青石催化剂对甲苯和乙酸乙酯的降解效率与Pt-CMC/堇青石相同,则须提高微波功率至47 W。由此可见,Pt的复合提高了催化剂的低温催化活性,有效降低了VOCs的催化燃烧成本。
贺丽娜等[16]证明了甲苯的微波催化燃烧为假一级反应。因此,依据一级反应动力学方程ln(C0/C)=kt,对CMC/堇青石和Pt-CMC/堇青石3种催化剂在微波功率分别为50、60、70 W时降解双组分VOCs废气中甲苯的反应动力学进行线性拟合,相应方程与参数如表2所示。结果表明,CMC/堇青石和Pt-CMC/堇青石微波催化燃烧甲苯的反应符合假一级反应动力学,同时R2值随微波功率的增加而增大,此时一级动力学拟合程度越高,证明高温下的催化燃烧反应为一级反应。反应速度常数k值对比发现,微波功率越大,k值越大,催化反应速度越快;微波功率60 W时,Pt-CMC/堇青石降解甲苯的k值为0.129,明显高于CMC/堇青石的0.111 9。可见,Pt的复合增强了甲苯的吸附活化作用而加快了反应速率,进而提高CMC活性组分对甲苯的微波催化燃烧效率。
3)催化剂比较。图6为微波功率40 W、进气量0.12 m3·h−1,甲苯和乙酸乙酯浓度各为1 000 mg·m−3条件下,CMC/堇青石、Pt-CMC/堇青石和Pt/堇青石催化剂降解单组分甲苯、乙酸乙酯及二者混合的效率曲线。无论是单、双组分甲苯和乙酸乙酯的催化燃烧降解,均是Pt-CMC/堇青石的活性最高,CMC/堇青石次之,而Pt/堇青石则未表现出催化活性。对于Pt/堇青石而言,其在微波辐照下几乎不升温,从而无法为VOCs的催化燃烧提供所需的温度条件,因此甲苯和乙酸乙酯几乎不降解。无论是甲苯或乙酸乙酯的单组分降解,还是双组分废气降解,乙酸乙酯的降解效率均高于甲苯。以Pt-CMC/堇青石为例,单组分甲苯和乙酸乙酯的降解效率分别为84%和95%(此时床层温度相近)。乙酸乙酯比甲苯更易于降解的原因可能为:一是乙酸乙酯等含氧有机物比甲苯更易被催化剂吸附活化;二是乙酸乙酯上的C-O键比甲苯的C-H键键能低而更易断裂[22-23]。
比较分析图6中甲苯和乙酸乙酯的降解效率发现:对于单组分甲苯和乙酸乙酯的降解,Pt-CMC/堇青石较CMC/堇青石有约7%和6%的提升;对于甲苯与乙酸乙酯的双组分降解,Pt-CMC/堇青石较CMC/堇青石有约5%和4%的提升。由此可见,Pt复合对甲苯降解效率的提升大于乙酸乙酯。分析认为,CMC/堇青石对于一些C-H键较弱、吸附性好的含氧有机物如乙酸乙酯、丙酮等具有良好的活化性能,但催化氧化C-H键较强的芳香类物质的能力则稍弱,而贵金属Pt活化C-H键的能力很强,弥补了铜锰铈金属氧化物活化苯环能力的不足[24]。此外,CMC/堇青石和Pt-CMC/堇青石对甲苯和乙酸乙酯双组分废气的降解效率明显高于各自单组分,其原因是双组分VOCs废气的初始浓度更高,催化燃烧时放出更多热量使得床层温度更高,从而有利于VOCs的氧化降解。
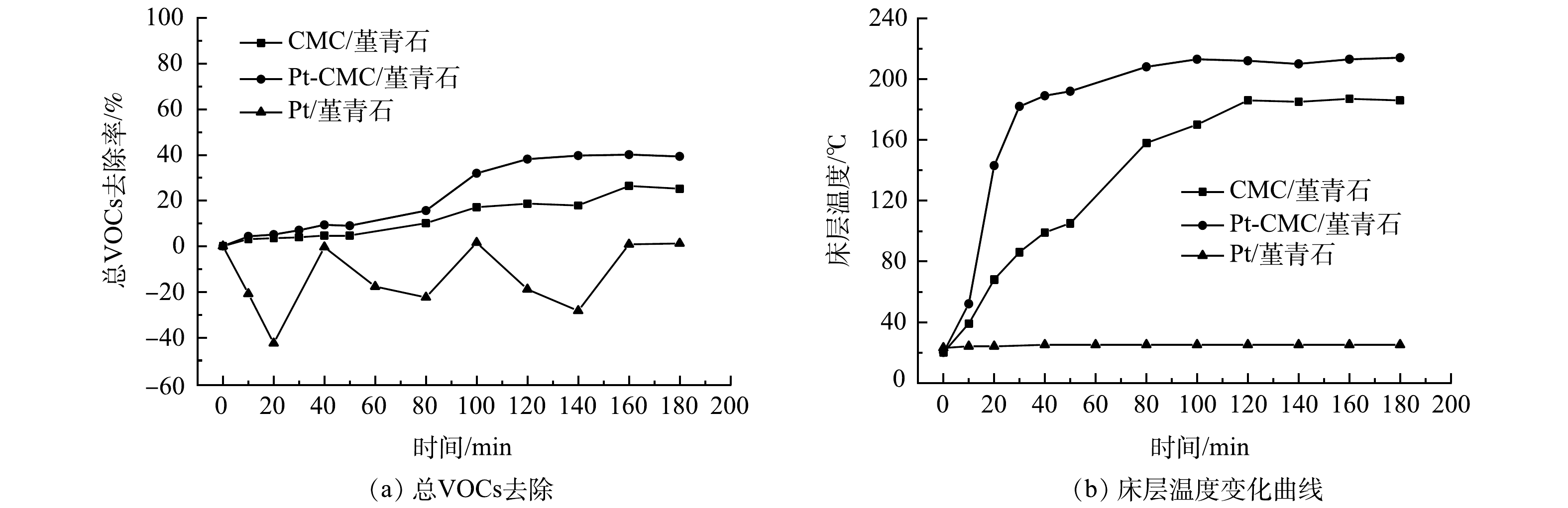
4)总VOCs去除。微波功率40 W时,对催化剂催化燃烧1 000 mg·m−3甲苯的总VOCs去除率进行测试,其结果和床层温度变化如图7所示。由图7(a)可见,Pt/堇青石的总VOCs去除率为负值且波动幅度大,CMC/堇青石和Pt-CMC/堇青石的总VOCs去除率分别为25%和40%,Pt的复合将总VOCs的去除效率提高了15%。如图7(b)所示,Pt/堇青石的不吸波使其床层温度为常温,催化燃烧反应未发生,甲苯在催化剂表面进行吸附-脱附动态平衡过程,因而其总VOCs去除率波动大且多为负值;Pt-CMC/堇青石床层升温速率快于CMC/堇青石,稳定后的床层温度高出CMC/堇青石床层30 ℃,这是总VOCs去除效率提高的主要原因。分析认为,Pt的复合在提升催化剂吸波升温能力的同时,其对甲苯C-H键的活化能力也有助于提高总VOCs的去除效率。随着微波功率的增加,床层温度急剧升高,总VOCs去除率也随之升高,催化剂对总VOCs去除效率的差别也随之减小,Pt复合的影响被床层温度影响掩盖而体现不出。因此,Pt的复合提高了催化剂的低温催化活性,有助于低温下VOCs的催化氧化。
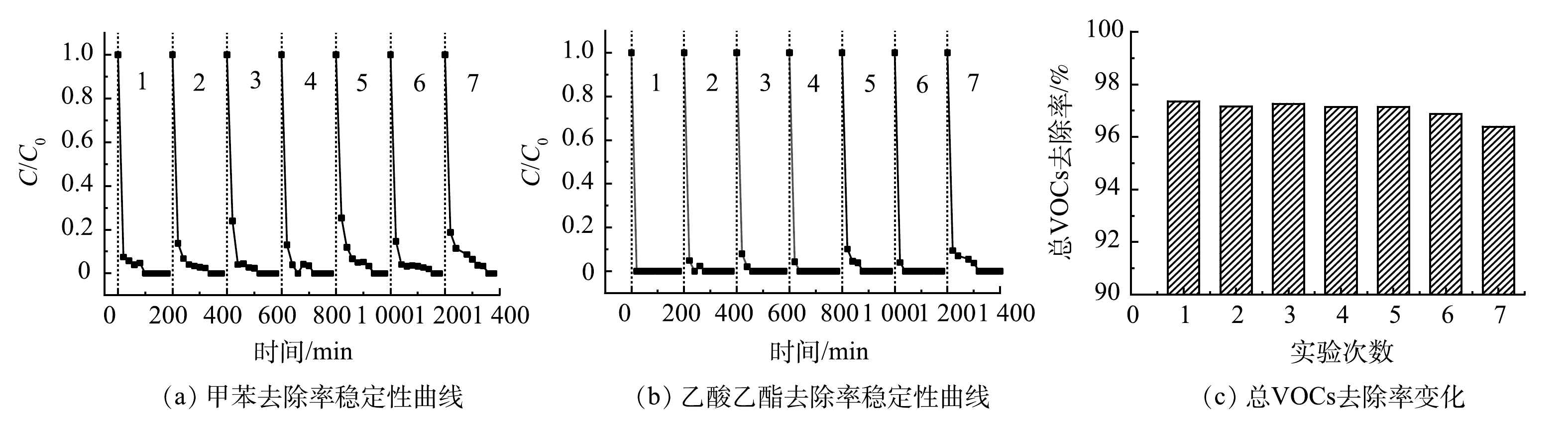
5)稳定性实验。根据图5的实验结果可知,微波功率70 W时Pt-CMC/堇青石催化剂对甲苯和乙酸乙酯的催化效果最好,同时总VOCs的去除率也最高。因此,在微波功率70 W、进气量0.12 m3·h−1、双组分气体中甲苯和乙酸乙酯初始浓度各为1 000 mg·m−3和500 mg·m−3条件下对Pt-CMC/堇青石催化剂进行了连续7次(每次实验3 h)的稳定性测试,结果如图8所示。
如图8(a)、8(b)所示,连续稳定性试验中,甲苯和乙酸乙酯被完全去除,乙酸乙酯的去除效率较甲苯更稳定,催化剂展示出良好的催化活性和稳定性。图8(c)的总VOCs去除率从97.5%略微降至96.5%,表明Pt-CMC/堇青石对双组分VOCs废气具有高的矿化效率与稳定性。微波功率70 W的稳定性实验中,催化剂床层温度320 ℃,高于40 W时的210 ℃,可见,床层温度的升高是提升总VOCs去除率的有效手段之一。连续的高温稳定性实验,会引起催化剂孔隙结构的微变和活性颗粒的团聚,进而引起双组分VOCs(特别是甲苯)去除效率的波动;然而,Pt复合增强了催化剂表面活性颗粒的分散性,增大了催化剂的比表面积与孔容,从而有效提高了催化剂活性,保证了催化剂性能的稳定。
-
1)等体积浸渍法制备的Pt-CMC/堇青石催化剂比CMC/堇青石催化剂具有更大的活性颗粒尺寸,Pt的再分散作用使得催化剂表面活性组分分布更加均匀;Pt的复合提高了催化剂的孔隙率,进而有利于VOCs分子在催化剂表面的吸附与活化;Pt复合后的尖晶石活性组分峰有微弱增大,Cu、Mn价态升高而使催化剂活性增强。
2)复合Pt后,催化剂的吸波升温能力有一定的增强,这与吸波活性组分分布均匀、散热能力增强有关。反应动力学分析表明,Pt的复合加快了催化燃烧反应速率,高温下甲苯的催化燃烧反应为一级反应;复合Pt后的催化剂其低温催化活性明显提高,Pt-CMC/堇青石催化剂表面总VOCs去除效率比CMC/堇青石提高15%。
3)对于Pt-CMC/堇青石和CMC/堇青石催化剂而言,乙酸乙酯的降解效率高于甲苯,可认为含氧的乙酸乙酯比甲苯更易被催化剂吸附活化,C-O键比甲苯的C-H键键能低而更易断裂;Pt复合对甲苯降解效率的提高大于乙酸乙酯,其原因是Pt对苯环中C-H键的活化能力强而有助于苯环的氧化与开环。
Pt-CuMnCeOx/堇青石微波催化燃烧VOCs性能
Application of Pt-CuMnCeOx/Cordierite catalyst in microwave catalytic combustion of VOCs
-
摘要: 微波催化燃烧是一项新的VOCs处理技术,提升VOCs处理效率的关键在于开发催化活性更高、性能更稳定的催化剂。将贵金属Pt与铜锰铈金属氧化物复合制备了Pt-CuMnCeOx/堇青石催化剂,SEM、BET和XRD表征基础上测试催化剂对双组分VOCs-甲苯和乙酸乙酯的催化活性与稳定性。研究表明,Pt的复合提高了催化剂表面活性颗粒的分散性,增大了催化剂的比表面积和孔体积,升高了铜锰价态而提高了催化剂的低温催化活性。微波功率40 W时,Pt-CuMnCeOx/堇青石催化剂的甲苯和乙酸乙酯的降解效率高出CuMnCeOx/堇青石16%和11%;微波功率70 W时,Pt-CuMnCeOx/堇青石催化剂可完全去除甲苯和乙酸乙酯,总VOCs去除率达97%左右且活性保持稳定。本研究结果可为微波催化燃烧VOCs技术提供参考。
-
关键词:
- 微波 /
- CuMnCeOx/堇青石 /
- Pt复合 /
- 催化燃烧 /
- VOCs
Abstract: Microwave catalytic combustion is an emerging technology for VOCs treatment, and preparation of the catalyst with higher catalytic activity and stability is the key to highly efficient VOCs removal. Noble metal Pt was composited with copper-manganese-cerium oxides and loaded on the surface of honeycomb cordierite carrier to prepare a Pt-CuMnCeOx/Cordierite catalyst by incipient-wetness impregnation method. Based on SEM, BET and XRD characteristics, the catalytic activity and stability of three catalysts in microwave catalytic combustion of bi-component VOCs toluene and ethyl acetate were tested in this work. The experimental results showed that Pt composition improved the dispersion of active particles on the catalyst surface, increased specific surface area and pore volume of the catalyst and lifted valence states of copper and manganese to enhance the low temperature catalytic activity of the catalyst greatly. The removal efficiencies of toluene and ethyl acetate on the surface of Pt-CuMnCeOx/Cordierite catalyst were higher 16% and 11% than CuMnCeOx/Cordierite catalyst under 40 W of microwave power. Toluene and ethyl acetate were degraded completely by Pt-CuMnCeOx/Cordierite catalyst under 70 W of microwave power, and the removal rate of total VOCs reached 97% simultaneously. Pt-CuMnCeOx/cordierite catalyst exhibited a high catalytic activity and an excellent stability during consecutive seven times test. This study can provide a reference for microwave catalytic combustion of VOCs technology.-
Key words:
- microwave /
- CuMnCeOx/Cordierite /
- Pt composition /
- catalytic combustion /
- VOCs
-
挥发性有机化合物(VOCs)作为PM2.5和臭氧的重要前驱体,已经对大气环境质量和人体健康造成直接和间接的危害[1-2],VOCs废气治理成为了近年来的环境热点话题之一。在VOCs废气的众多处理技术中,催化燃烧技术因处理效率高、能耗低、二次污染小而在工业上应用广泛[3]。为达到VOCs催化燃烧所需温度,工业上多采用电加热使VOCs废气达到起燃温度。由于电加热是采用热传导的加热方式,因而对大气量的VOCs废气加热时能耗巨大。另外,VOCs催化燃烧时,持续的高温环境会使催化剂活性组分烧结而影响VOCs降解效果[4]。微波加热应用于VOCs催化燃烧是一种新技术,它利用电磁波的选择性仅对催化剂进行加热,因而能耗低且催化剂受热均匀、快速[5]。而且,微波对催化剂活性组分的热点效应有利于引发VOCs的催化燃烧,同时微波的偶极极化作用还可降低VOCs的反应阈能而促进其氧化降解[6]。卜龙利等[7]、姚泽等[8]利用吸波型催化剂证实了微波催化燃烧VOCs效果优于电加热催化燃烧。
微波催化燃烧技术的关键是高效而稳定的催化剂。过渡金属氧化物催化剂种类丰富、经济性好、不易中毒,但其对于某些难降解有机物的催化活性差、低温活性以及热稳定性有待提高[9]。贵金属催化剂具有催化活性高、高温下稳定性好和适用范围广的优点,但也存在资源稀少和价格高昂的问题[10]。目前,市面上较为常见的是铜锰铈三元金属氧化物催化剂。胡旭睿[11]研究证实,铜锰铈氧化物催化剂对芳香烃类、醇类、酮类等有机物具有良好的氧化性能,但其矿化效果不佳且对芳香烃的降解效果低于醇、酮类。贵金属Pt、Pd等在低温时对芳烃类和3个碳以上的直链烷烃活化能力强[12]。已有研究证实,将贵金属与过渡金属复合可以增强催化剂活性。SHI等[13]通过还原法和离子交换法合成了Pt /Ce-USY催化剂,其对1,2-二氯乙烷的催化活性高于Pt/USY催化剂,Pt与CeO2之间的相互作用抑制了碳物质的沉积,从而增强了催化剂的耐久性。LEE等[14]将贵金属金、钯沉积在CeO2表面,证实适量Au的加入使得Pd/CeO2催化剂活性增强。CHEN等[15]合成了Pd/Fe3O4催化剂用于去除CO,当沉积适量Fe3O4时,CO氧化的起燃温度明显降低。因此,本研究在铜锰铈三元催化剂基础上复合微量贵金属Pt,以期提高催化剂对芳烃类VOCs的活化能力,进而提高总VOCs的去除效果。
本研究选取油墨印刷VOCs废气中含量最多的2种物质甲苯和乙酸乙酯作为目标污染物,以蜂窝状堇青石为载体制备Pt复合铜锰铈(CMC)负载型催化剂(Pt-CMC/堇青石),重点考察Pt复合前后催化剂对模拟VOCs废气催化燃烧效果的差异,以探究低含量贵金属添加对铜锰铈氧化物催化剂催化活性的影响程度。
1. 材料与方法
1.1 实验原料与仪器
蜂窝状正方体堇青石(7目,边长150 mm);硝酸铜、硝酸锰(50 wt%)、硝酸铈、氯铂酸,分析纯;硅溶胶,分析纯。超声波清洗器(KQ3200,昆山市超声仪器有限公司);电热鼓风干燥箱(101-3AB,天津泰斯特仪器有限公司); 箱式电阻炉(SX-4-10,天津泰斯特仪器有限公司);单模腔微波装置(ZDM-2,南京汇研微波系统工程有限公司);气相色谱仪(GC/FID,6890 N,美国安捷伦科技公司);泵吸式VOC检测仪(XS-2000-VOC,希思智能科技);扫描电子显微镜(JSM-6510LV型,日本电子);比表面积及孔径分析仪(V-sorb2800P型,北京金埃谱公司);X射线衍射仪(X’Pert型,荷兰帕纳科)。
1.2 催化剂制备与表征
本研究采用等体积浸渍法进行负载型催化剂的制备,即载体吸水率测试基础上配置适量体积的浸渍液、然后浸渍液被载体完全吸收的一种催化剂制备方法。首先,将正方体堇青石切割成圆柱型载体(D×L=27×150 mm),称重并测其吸水率,根据吸水率确定助剂硅溶胶的用量;其次,按3∶3∶1的质量负载比例分别称取铜(2.5%(质量分数))、锰、铈的金属盐,称取一定量的硅溶胶以及少量氯铂酸(Pt的质量分数0.01%),加入去离子水超声震荡30 min配制完全溶解的浸渍液;最后,将堇青石载体置于浸渍液中将浸渍液完全吸收,样品静置风干后放入烘箱中80 ℃过夜烘干,电阻炉内500 ℃煅烧4 h,自然冷却后即可制得Pt-CMC/堇青石催化剂。不加入氯铂酸、仅加入氯铂酸和其余步骤均相同情况下,可分别制得CMC/堇青石、Pt /堇青石催化剂。
利用扫描电子显微镜观察催化剂表面形貌及活性组分分布状况,借助BET测试仪分析催化剂的比表面积及孔径孔体积,使用X射线衍射仪测试活性组分的晶体结构及晶粒大小。
1.3 实验装置与方法
实验装置如图1所示,整个装置分为配气、催化燃烧和尾气净化3部分。在配气系统中,空气依次通过变色硅胶柱和活性炭柱去除水分和有机物,其流量通过气体流量计控制;实验时采用微量注射泵将液态甲苯和乙酸乙酯(按一定比例混合)以恒定速度连续均匀地注入三口烧瓶中,三口烧瓶被电加热套加热而将有机物气化,气化的甲苯和乙酸乙酯被空气带出并在混合瓶内混合均匀。实验开始前催化剂置于石英管内,石英管填装催化剂的长度为360 mm,锥形收口的长度是20 mm,上下两端开口外径分别为32 mm和9 mm,壁厚2 mm;石英管固定在单模腔微波装置上形成固定床反应器,实验开始后微波通过单模腔持续辐照在固定床上,催化剂吸波升温达到VOCs催化燃烧温度;石英管下端连接混合瓶,通入的VOCs气体在固定床上发生催化燃烧反应,床中插入的热电偶探针与温度显示仪连接以实时监测床层温度变化,石英管上下分别设进、出气采样口。催化燃烧后的VOCs尾气分别通过缓冲瓶和装有乙醇和碱液的尾气吸收瓶,净化后的废气经通风橱排空。
本研究利用CMC/堇青石、Pt-CMC/堇青石和Pt /堇青石催化剂在处理气量0.12 m3·h−1、气时空速(gas hourly space velocity,GHSV)1 500 h−1条件下,考察了不同微波功率(30、40、50、60、70 W)和不同VOCs浓度(1 000、1 500、2 000 mg·m−3)下甲苯和乙酸乙酯的催化燃烧效率,分析微波催化燃烧甲苯反应动力学及Pt复合影响,比较3种催化剂的催化活性及Pt复合对总VOCs去除效率的提升作用,探究Pt-CMC/堇青石催化剂对甲苯、乙酸乙酯和总VOCs催化活性的稳定性。
1.4 分析方法
本研究主要利用气相色谱仪对甲苯和乙酸乙酯的进气和出气质量浓度进行定量分析,气相色谱检测条件为[16]:柱箱初始温度100 ℃,以20 ℃·min−1的速率升温至180 ℃,保持3 min,加热器温度为190 ℃,检测器温度300 ℃;设定分流比为50∶1;尾气吹脱用氮气,流量30 mL·min−1。
使用手持泵吸式VOC检测仪对反应器进气和出气的总VOCs浓度进行检测,从而分析反应过程中总VOCs的催化燃烧效率。文中数据为2次平行实验的平均值,以此消除实验偶然性误差的影响。
2. 结果与讨论
2.1 催化剂表征
1) BET。根据表1数据可知,与堇青石载体相比,CMC/堇青石与Pt-CMC/堇青石2种催化剂的比表面积有不同程度的增加,这表明催化剂的吸附能力也相应增强。与堇青石载体相比,CMC/堇青石催化剂总孔容相对减小,平均孔径有所增大。推测其原因是,活性组分负载后,载体表面的部分孔隙被活性组分填充和覆盖,载体结构的原有孔道被拓宽。Pt-CMC/堇青石催化剂活性晶粒间相互积聚生成了包括微孔和介孔在内的新孔道,催化剂的比表面积及孔体积都明显增大,这有利于污染物分子在催化剂表面的吸附与活化。因此,Pt的复合在一定程度上增强了催化剂的吸附活化性能。
表 1 堇青石载体和2种催化剂的比表面积、孔体积及孔径数据表Table 1. Data table of specific surface area, pore volume and pore diameter of Cordierite and two catalysts供试样品 比表面积/(m2·g−1) 微孔面积/(m2·g−1) 孔体积/(cm3·g−1) 总孔容/(cm3·g−1) 平均孔径/nm 堇青石 0.11 ND ND 0.30 36.79 CMC/堇青石 0.66 ND ND 0.09 41.23 Pt-CMC/堇青石 2.95 1.11 0.000 47 0.38 36.85 备注:ND为未检出。 2) XRD。如图2所示,2 θ在10°~40°之间存在着较为密集的堇青石特征衍射峰。对比XRD谱图可以发现,活性组分的负载和制备时的高温煅烧不会改变堇青石自身的晶体结构;与堇青石载体相比,CMC/堇青石和Pt-CMC/堇青石催化剂的特征衍射峰强度减弱,活性晶粒的平均尺寸有不同程度的减小。活性组分负载后堇青石特征峰峰强减弱的原因可能是活性组分一定程度上覆盖和屏蔽了堇青石表面的特征峰[17];贵金属Pt复合后,金属氧化物等活性组分晶体特征峰的峰高有微弱增大,这可能是不同尖晶石活性组分共存所致。6个处于17.5°、21.9°、28.7°、34.1°、36.7°、38.3°位置较为明显的特征峰,均归属于铜锰铈及其金属氧化物,这说明在贵金属Pt复合前后,铜锰铈金属氧化物都是微波催化燃烧VOCs的主要活性组分。在堇青石载体、CMC/堇青石催化剂和Pt-CMC/堇青石催化剂上,3种催化剂的晶粒平均尺寸分别为50.7、13.9和14.5 nm。Pt复合后,催化剂晶粒尺寸变化较小,而比表面积却从0.66 m2·g−1增大到2.95 m2·g−1,由此可以说明贵金属Pt的复合使活性颗粒的分散性增大[18]。
结合特征峰组分分析可知,少量贵金属Pt的添加改变了催化剂上铜和锰的晶相。贵金属Pt复合前,锰以+4价的MnO2和中间价态的Mn3O4形式存在;贵金属Pt复合后,部分金属锰的价态升高,以Mn5O8形式存在,且Cu2O的晶体在掺杂后未检测到,检测出以+4价存在的PtO2。有文献报道,Cu、Mn金属离子价态升高,可以增强催化剂的活性[19]。
3) SEM。图3给出堇青石载体和CMC/堇青石、Pt-CMC/堇青石催化剂的表面形貌。可以看出,堇青石载体呈明显的层状结构,表面孔隙较多且分布均匀,有利于活性组分的负载。CMC/堇青石催化剂表面存在着较多微米尺寸的活性颗粒,不均匀地分布在载体表面;部分活性组分经高温煅烧后团聚成大块与堇青石结构连结在一起,填充载体原有孔隙的同时也产生了新的孔隙。Pt-CMC/堇青石催化剂表面的活性颗粒在载体表面上分布更均匀、分散度更高。对比Pt复合前后催化剂的微观形貌,可以看出,Pt的复合改变了活性颗粒的分散性,活性物质的再分散可能导致催化剂的吸附性改变。
2.2 催化剂性能实验
1)吸波升温曲线。图4为堇青石载体、CMC/堇青石、Pt-CMC/堇青石和Pt/堇青石在200 W微波功率辐照下的吸波升温曲线。由图4可见,堇青石载体温度几乎不变,这表明其吸波性能很差;而Pt/堇青石催化剂在15 min内温度也仅升高了3 ℃,其原因是贵金属具有高热稳定性,且吸波性能差。CMC/堇青石和Pt-CMC/堇青石催化剂升温效果明显好于前2种,微波辐照15 min时分别升温至300和355 ℃,说明铜锰铈及其金属氧化物具有良好的吸波性能,是2种催化剂吸波升温的主要原因;Pt复合催化剂升温速率更快,这可能是Pt的复合提高了催化剂上活性晶粒的分散性,降低了金属对微波的反射,以及颗粒尺寸变大增强了微波辐照时的局部热点效应,从而使得催化剂的吸波升温性能提高[20-21]。催化剂的快速升温有助于微波热点效应和高温活性区的出现,从而有助于甲苯和乙酸乙酯的快速氧化与彻底降解。
2)催化剂活性。在甲苯和乙酸乙酯的进气浓度分别为1 000和500 mg·m−3,进气量为0.12 m3·h−1,微波功率分别选取30、40、50、60、70 W的条件下,开展CMC/堇青石和Pt-CMC/堇青石催化剂对甲苯和乙酸乙酯双组分VOCs废气的催化燃烧性能试验,结果如图5所示。图5中纵坐标C/C0为反应实时浓度与进气浓度比值(图6同),可直观表示降解效率并使数据间具有可比性。
由图5可见,微波功率30 w时,2种催化剂对甲苯和乙酸乙酯的降解呈不稳定态势,其原因是床层温度偏低(CMC/堇青石床层温度低于66 ℃),催化剂对污染物的去除以吸附-脱附为主。随着微波功率增大,甲苯和乙酸乙酯的降解效率也随之升高,但2种催化剂对甲苯和乙酸乙酯降解效率的差异却由大变小:40 W时,催化剂对甲苯和乙酸乙酯降解效率的差异最大;70 W时,这种差异最小,此时固定床温度高,污染物在催化剂表面完全燃烧,温度的作用掩盖了Pt的复合作用。
同一微波功率下,降解甲苯与乙酸乙酯时, Pt-CMC/堇青石催化剂表现出更高的VOCs催化活性。40 W微波功率下,Pt-CMC/堇青石对甲苯和乙酸乙酯的降解效率分别为82%和93%,而CMC/堇青石降解甲苯和乙酸乙酯的效率分别为66%和82%。推测其原因是,Pt-CMC/堇青石的比表面积相比CMC/堇青石大幅增加,从而有利于甲苯与乙酸乙酯的吸附与活化;同时,活性组分更好的分散性使得Pt-CMC/堇青石升温更快,而高温则有利于VOCs的催化燃烧。如果要使CMC/堇青石催化剂对甲苯和乙酸乙酯的降解效率与Pt-CMC/堇青石相同,则须提高微波功率至47 W。由此可见,Pt的复合提高了催化剂的低温催化活性,有效降低了VOCs的催化燃烧成本。
贺丽娜等[16]证明了甲苯的微波催化燃烧为假一级反应。因此,依据一级反应动力学方程ln(C0/C)=kt,对CMC/堇青石和Pt-CMC/堇青石3种催化剂在微波功率分别为50、60、70 W时降解双组分VOCs废气中甲苯的反应动力学进行线性拟合,相应方程与参数如表2所示。结果表明,CMC/堇青石和Pt-CMC/堇青石微波催化燃烧甲苯的反应符合假一级反应动力学,同时R2值随微波功率的增加而增大,此时一级动力学拟合程度越高,证明高温下的催化燃烧反应为一级反应。反应速度常数k值对比发现,微波功率越大,k值越大,催化反应速度越快;微波功率60 W时,Pt-CMC/堇青石降解甲苯的k值为0.129,明显高于CMC/堇青石的0.111 9。可见,Pt的复合增强了甲苯的吸附活化作用而加快了反应速率,进而提高CMC活性组分对甲苯的微波催化燃烧效率。
表 2 微波催化燃烧甲苯反应动力学Table 2. Reaction kinetics of microwave catalytic combustion of toluene功率条件 催化剂 ln(C0/C)=kt k R2 50 W Pt-CMC/堇青石 y=0.0594x−0.0204 0.0594 0.9828 60 W CMC/堇青石 y=0.1119x−0.1398 0.1119 0.9553 60 W Pt-CMC/堇青石 y=0.129x−0.0797 0.129 0.9887 70 W CMC/堇青石 y=0.1045x−0.0428 0.1045 0.995 70 W CMC/堇青石 y=0.1299x+0.031 0.1299 0.9983 3)催化剂比较。图6为微波功率40 W、进气量0.12 m3·h−1,甲苯和乙酸乙酯浓度各为1 000 mg·m−3条件下,CMC/堇青石、Pt-CMC/堇青石和Pt/堇青石催化剂降解单组分甲苯、乙酸乙酯及二者混合的效率曲线。无论是单、双组分甲苯和乙酸乙酯的催化燃烧降解,均是Pt-CMC/堇青石的活性最高,CMC/堇青石次之,而Pt/堇青石则未表现出催化活性。对于Pt/堇青石而言,其在微波辐照下几乎不升温,从而无法为VOCs的催化燃烧提供所需的温度条件,因此甲苯和乙酸乙酯几乎不降解。无论是甲苯或乙酸乙酯的单组分降解,还是双组分废气降解,乙酸乙酯的降解效率均高于甲苯。以Pt-CMC/堇青石为例,单组分甲苯和乙酸乙酯的降解效率分别为84%和95%(此时床层温度相近)。乙酸乙酯比甲苯更易于降解的原因可能为:一是乙酸乙酯等含氧有机物比甲苯更易被催化剂吸附活化;二是乙酸乙酯上的C-O键比甲苯的C-H键键能低而更易断裂[22-23]。
比较分析图6中甲苯和乙酸乙酯的降解效率发现:对于单组分甲苯和乙酸乙酯的降解,Pt-CMC/堇青石较CMC/堇青石有约7%和6%的提升;对于甲苯与乙酸乙酯的双组分降解,Pt-CMC/堇青石较CMC/堇青石有约5%和4%的提升。由此可见,Pt复合对甲苯降解效率的提升大于乙酸乙酯。分析认为,CMC/堇青石对于一些C-H键较弱、吸附性好的含氧有机物如乙酸乙酯、丙酮等具有良好的活化性能,但催化氧化C-H键较强的芳香类物质的能力则稍弱,而贵金属Pt活化C-H键的能力很强,弥补了铜锰铈金属氧化物活化苯环能力的不足[24]。此外,CMC/堇青石和Pt-CMC/堇青石对甲苯和乙酸乙酯双组分废气的降解效率明显高于各自单组分,其原因是双组分VOCs废气的初始浓度更高,催化燃烧时放出更多热量使得床层温度更高,从而有利于VOCs的氧化降解。
4)总VOCs去除。微波功率40 W时,对催化剂催化燃烧1 000 mg·m−3甲苯的总VOCs去除率进行测试,其结果和床层温度变化如图7所示。由图7(a)可见,Pt/堇青石的总VOCs去除率为负值且波动幅度大,CMC/堇青石和Pt-CMC/堇青石的总VOCs去除率分别为25%和40%,Pt的复合将总VOCs的去除效率提高了15%。如图7(b)所示,Pt/堇青石的不吸波使其床层温度为常温,催化燃烧反应未发生,甲苯在催化剂表面进行吸附-脱附动态平衡过程,因而其总VOCs去除率波动大且多为负值;Pt-CMC/堇青石床层升温速率快于CMC/堇青石,稳定后的床层温度高出CMC/堇青石床层30 ℃,这是总VOCs去除效率提高的主要原因。分析认为,Pt的复合在提升催化剂吸波升温能力的同时,其对甲苯C-H键的活化能力也有助于提高总VOCs的去除效率。随着微波功率的增加,床层温度急剧升高,总VOCs去除率也随之升高,催化剂对总VOCs去除效率的差别也随之减小,Pt复合的影响被床层温度影响掩盖而体现不出。因此,Pt的复合提高了催化剂的低温催化活性,有助于低温下VOCs的催化氧化。
5)稳定性实验。根据图5的实验结果可知,微波功率70 W时Pt-CMC/堇青石催化剂对甲苯和乙酸乙酯的催化效果最好,同时总VOCs的去除率也最高。因此,在微波功率70 W、进气量0.12 m3·h−1、双组分气体中甲苯和乙酸乙酯初始浓度各为1 000 mg·m−3和500 mg·m−3条件下对Pt-CMC/堇青石催化剂进行了连续7次(每次实验3 h)的稳定性测试,结果如图8所示。
如图8(a)、8(b)所示,连续稳定性试验中,甲苯和乙酸乙酯被完全去除,乙酸乙酯的去除效率较甲苯更稳定,催化剂展示出良好的催化活性和稳定性。图8(c)的总VOCs去除率从97.5%略微降至96.5%,表明Pt-CMC/堇青石对双组分VOCs废气具有高的矿化效率与稳定性。微波功率70 W的稳定性实验中,催化剂床层温度320 ℃,高于40 W时的210 ℃,可见,床层温度的升高是提升总VOCs去除率的有效手段之一。连续的高温稳定性实验,会引起催化剂孔隙结构的微变和活性颗粒的团聚,进而引起双组分VOCs(特别是甲苯)去除效率的波动;然而,Pt复合增强了催化剂表面活性颗粒的分散性,增大了催化剂的比表面积与孔容,从而有效提高了催化剂活性,保证了催化剂性能的稳定。
3. 结论
1)等体积浸渍法制备的Pt-CMC/堇青石催化剂比CMC/堇青石催化剂具有更大的活性颗粒尺寸,Pt的再分散作用使得催化剂表面活性组分分布更加均匀;Pt的复合提高了催化剂的孔隙率,进而有利于VOCs分子在催化剂表面的吸附与活化;Pt复合后的尖晶石活性组分峰有微弱增大,Cu、Mn价态升高而使催化剂活性增强。
2)复合Pt后,催化剂的吸波升温能力有一定的增强,这与吸波活性组分分布均匀、散热能力增强有关。反应动力学分析表明,Pt的复合加快了催化燃烧反应速率,高温下甲苯的催化燃烧反应为一级反应;复合Pt后的催化剂其低温催化活性明显提高,Pt-CMC/堇青石催化剂表面总VOCs去除效率比CMC/堇青石提高15%。
3)对于Pt-CMC/堇青石和CMC/堇青石催化剂而言,乙酸乙酯的降解效率高于甲苯,可认为含氧的乙酸乙酯比甲苯更易被催化剂吸附活化,C-O键比甲苯的C-H键键能低而更易断裂;Pt复合对甲苯降解效率的提高大于乙酸乙酯,其原因是Pt对苯环中C-H键的活化能力强而有助于苯环的氧化与开环。
-
表 1 堇青石载体和2种催化剂的比表面积、孔体积及孔径数据表
Table 1. Data table of specific surface area, pore volume and pore diameter of Cordierite and two catalysts
供试样品 比表面积/(m2·g−1) 微孔面积/(m2·g−1) 孔体积/(cm3·g−1) 总孔容/(cm3·g−1) 平均孔径/nm 堇青石 0.11 ND ND 0.30 36.79 CMC/堇青石 0.66 ND ND 0.09 41.23 Pt-CMC/堇青石 2.95 1.11 0.000 47 0.38 36.85 备注:ND为未检出。 表 2 微波催化燃烧甲苯反应动力学
Table 2. Reaction kinetics of microwave catalytic combustion of toluene
功率条件 催化剂 ln(C0/C)=kt k R2 50 W Pt-CMC/堇青石 y=0.0594x−0.0204 0.0594 0.9828 60 W CMC/堇青石 y=0.1119x−0.1398 0.1119 0.9553 60 W Pt-CMC/堇青石 y=0.129x−0.0797 0.129 0.9887 70 W CMC/堇青石 y=0.1045x−0.0428 0.1045 0.995 70 W CMC/堇青石 y=0.1299x+0.031 0.1299 0.9983 -
[1] 张新民, 薛志钢, 孙新章, 等. 中国大气挥发性有机物控制现状及对策研究[J]. 环境科学与管理, 2014, 39(1): 16-19. doi: 10.3969/j.issn.1673-1212.2014.01.004 [2] 杨栋. 长治市主城区环境空气臭氧污染较重时期VOCs污染特征分析[J]. 山西化工, 2020, 40(2): 120-122. [3] ZHU A M, ZHOU Y, WANG Y, et al. Catalytic combustion of VOCs on Pt/CuMnCe and Pt/CeY honeycomb monolithic catalysts[J]. J Rare Earth, 2018, 36(12): 1272-1277. doi: 10.1016/j.jre.2018.03.032 [4] 张钰彩, 卜龙利, 王晓晖, 等. 微波加热下苯的催化氧化性能研究[J]. 环境科学, 2012, 33(8): 2759-2765. [5] 蔡春芳. 微波加热技术在生物质能源领域的应用研究进展[J]. 精细与专用化学品, 2018, 26(7): 49-52. [6] 周德良, 刘洁. 微波加热及其量子特性[J]. 黑龙江八一农垦大学学报, 2019, 31(1): 74-77. doi: 10.3969/j.issn.1002-2090.2019.01.013 [7] 卜龙利, 张钰彩, 王晓晖, 等. 微波辅助催化氧化苯高性能催化剂实验研究[J]. 燃料化学学报, 2012, 40(7): 878-885. doi: 10.3969/j.issn.0253-2409.2012.07.018 [8] 姚泽. 微波加热α-MnO2催化去除甲苯和臭氧的研究[D]. 哈尔滨: 哈尔滨工业大学, 2016. [9] 徐少娟, 张洪祥, 林建翔, 等. VOCs催化燃烧催化剂的研究进展[J]. 广东化工, 2020, 47(22): 96-97. doi: 10.3969/j.issn.1007-1865.2020.22.040 [10] 曾俊淋, 刘霄龙, 王健, 等. 贵金属催化剂对VOCs催化氧化的研究进展[J]. 环境工程, 2015, 33(11): 72-77. [11] 胡旭睿. 微波催化燃烧 VOCs 催化剂制备及性能研究[D]. 石家庄: 河北科技大学, 2016. [12] 左满宏, 吕宏安. 催化燃烧与催化剂材料在VOCs治理方面研究进展[J]. 天津化工, 2007(4): 8-10. doi: 10.3969/j.issn.1008-1267.2007.04.003 [13] SHI Y J, LI Z M, WANG J L, et al. Synergistic effect of Pt/Ce and USY zeolite in Pt-based catalysts with high activity for VOCs degradation[J]. Applied Catalysis B:Environmental, 2021: 286. [14] LEE D S, CHEN Y W. The mutual promotional effect of Au–Pd/CeO2 bimetallic catalysts on destruction of toluene[J]. Journal of Taiwan Institute of Chemical Engineers, 2013, 44(1): 40-44. doi: 10.1016/j.jtice.2012.08.002 [15] CHEN S T, SI R, TAYLOR E, et al. Synthesis of Pd/Fe3O4 hybrid nanocatalysts with controllable interface and enhanced catalytic activities for CO oxidation[J]. Journal of Physical Chemistry C, 2012, 116(23): 12969-12976. [16] 贺利娜, 卜龙利, 都琳, 等. 微波催化燃烧气态甲苯特性及床层温度分布[J]. 中国环境科学, 2019, 39(8): 3242-3248. doi: 10.3969/j.issn.1000-6923.2019.08.014 [17] 刘艳春, 王兆春, 曾令可, 等. 堇青石蜂窝陶瓷的表面改性[J]. 分析测试学报, 2014, 33(9): 1044-1049. doi: 10.3969/j.issn.1004-4957.2014.09.010 [18] 余鸿敏. Cu-Mn-Ce复合氧化物催化剂掺杂改性和热稳定性研究[D]. 杭州: 浙江工业大学. 2011. [19] 刘超, 李彦秋, 柳璐, 等. VOCs催化燃烧的锰铈催化剂研究进展[J]. 辽宁化工, 2020, 49(8): 968-970. doi: 10.3969/j.issn.1004-0935.2020.08.020 [20] 刘海楠. 二氧化钛复合型催化剂制备及其微波辅助催化氧化甲苯性能试验研究[D]. 西安: 西安建筑科技大学, 2013. [21] BO L L, ZHANG Y B, QUAN X, et al. Microwave assisted catalytic oxidation of p-nitrophenol in aqueous solution using carbon-supported copper catalyst[J]. Journal of Hazardous Materials, 2008, 153(3): 1201-1206. doi: 10.1016/j.jhazmat.2007.09.082 [22] ADITI R G, JEANETTE S R, FIGUEIREDO J L, et al. Manganese oxide OMS-2 as an effective catalyst for total oxidation of ethyl acetate[J]. Applied Catalysis B:Environmental, 2007, 72(1-2): 129-135. doi: 10.1016/j.apcatb.2006.10.017 [23] GOTZ V, MURTAZA Z, LANNY D S. Ignition in alkane oxidation on nobel metal catalysts[J]. Catal Today, 1999, 47(1): 219-228. [24] 卢晗锋. 低温催化燃烧VOCs的复合氧化物催化剂活性、稳定性及整体化研究[D]. 杭州: 浙江工业大学, 2010. -





 下载:
下载: