-
氰根离子能够与多种金属离子形成络合物,所以被广泛地应用于电镀、冶金等行业中[1]。在镀铜工艺中,由于氰化镀铜操作简便,且所获得的镀层表面光亮、结晶细微、孔隙率低、容易抛光、具有良好的导电性和可焊性,因此,尽管氰化物具有毒性,且会造成环境危害,氰化镀铜仍被广泛应用,因而产生了大量的铜氰废水。氰化物具有很强的毒性,短期内接触氰化物会增加呼气次数、心率且还产生其他神经系统毒害作用。人体长期接触氰化物会导致体重减轻,影响甲状腺,使神经系统退化甚至最终导致死亡。皮肤接触含有氰化物的液体也会引起溃疡和皮肤伤害[2]。因此,对于废水中氰化物的排放各国都制定了严格的标准。
在铜氰废水中,当pH小于9时,氰根(CN−)容易和氢离子(H+)形成剧毒气体氢氰酸(HCN),因此,处理铜氰废水必须在碱性条件下进行[3]。目前处理铜氰废水的主要方法为碱式氯化法,但这种方法可能会产生有毒的氯化氰副产物[4]。此外,也有光催化法[5-6]、物化沉淀法[7]、臭氧法[8]等用于处理铜氰废水。虽然这些方法均可对铜氰具有一定的处理效果,但也存在一些缺陷:光催化法由于光生空穴与光生电子容易复合,导致光催化效率较低;物化沉淀法需要投加大量药剂,成本高、效果差。因此,需要寻找一种绿色高效的处理铜氰废水的方法。
电化学法处理铜氰废水已经被广泛研究[9],通过电化学作用还能将铜氰废水中的铜离子进行回收。过一硫酸盐(KHSO5,PMS)是一种绿色环保的氧化剂,因其具有强氧化性、无二次污染等优点而被应用于水处理领域。过一硫酸盐在光、热、碱、电及过渡金属离子的活化作用下能够产生强氧化性的硫酸根自由基(
SO−4 ·),可用以氧化多种有机物。有研究结果[10-12]表明,铜离子可以活化PMS产生SO−4 ·,进而有效降解抗生素、双酚A等物质。过氧化氢(H2O2)因其清洁廉价无毒害也被广泛应用于水处理领域。H2O2可用于处理含高浓度的氰化物废水[13]。本研究分别采用PMS和H2O2,以强化电化学去除铜氰络合物Cu(CN3)2−的处理效率,并考察了不同试剂浓度和电流密度对Cu(CN3)2−去除效果的影响。
全文HTML
-
实验用水取自某电镀园区实际铜氰废水,废水呈浅黄色,主要成分为铜氰络合物Cu(CN3)2−、氢氧化钠(NaOH)、碳酸钠(Na2CO3)、酒石酸钾钠(C4H4KNaO6·4H2O)。该废水水质情况:pH为11.3,总氰的质量浓度为680.24 mg·L−1,总铜的质量浓度为554.32 mg·L−1,COD为228.00 mg·L−1,电导率为1.6 S·m。
-
主要试剂:过一硫酸钾(KHSO5)、浓硫酸(H2SO4)、氢氧化钠(NaOH)、磷酸(H3PO4)、乙二酸四乙酸二钠(C10H14N2O8Na2·2H2O)、氯胺T(CH3C6H4SO2NClNa·3H2O)、磷酸二氢钾(KH2PO4)、异烟酸(C6H5NO2)、巴比妥酸(C4H4N2O3)、过氧化氢(H2O2, 30%),所有试剂均于国药集团化学试剂有限公司购置,均为分析纯。
主要仪器:DH1765-1 型直流稳压电源、紫外分光光度计(T6 新世纪,北京普析通用仪器有限责任公司)、TAS-990原子吸收分光光度计、磁力搅拌器(德国艾卡仪器设备有限公司)、HACH DRB200 消解仪、DR2800型COD 比色仪、有机玻璃反应器(规格为6 cm×5 cm×5 cm)、RuO2/Ti网状阳极(规格为5 cm×2 cm,孔径为0.2 cm×0.5 cm)、钛阴极(规格为5 cm×2 cm)。
-
为保证实验过程中pH不低于9,在实验开始前使用NaOH和H2SO4将pH调节至12。反应开始前取100 mL水样置于有机玻璃反应器中,将pH调节到12后放置在磁力搅拌器上,将RuO2/Ti网状阳极和钛阴极连接电源插入反应器中电极间距为2 cm,反应器外部用水浴降温。加入一定量的H2O2或PMS到废水中,反应开始,然后在不同反应时间点取样,测定总氰、铜离子和COD,并进行分析。
在检测总氰时,需要先将络合态的氰转化为游离态,需加入磷酸和乙二酸四乙酸二钠,在pH小于2的条件下加热蒸馏,冷凝后通过氢氧化钠溶液吸收。此时络合态的氰全部转化为游离态,之后使用异烟酸-巴比妥酸紫外分光光度计法进行检测,检测波长为610 nm。铜离子使用火焰原子吸收光谱法进行检测,COD采用加热消解比色法测定。
1.1. 实验用水水质
1.2. 试剂与仪器
1.3. 实验方法
-
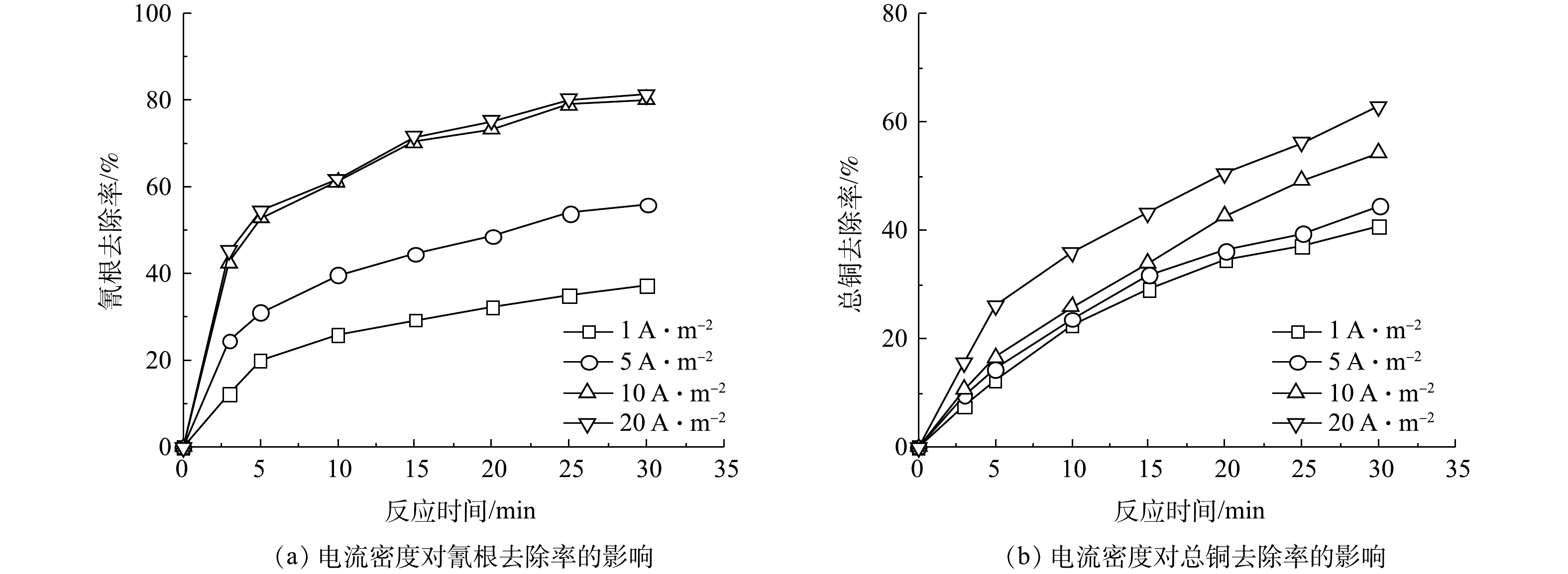
首先研究了氰根的去除过程中电流密度铜离子去除率和氰根去除率的影响。如图1所示,当反应30 min后,在电流密度分别为1、5、10、20 A·m−2时,氰根的去除率分别为37.50%、57.02%、80.29%、81.47%,总铜的去除率分别为40.80%、44.66%、54.41%、63.05%。氰根的去除率随着电流密度的增大而增加,这是由于增加电流密度可以提升体系的电极电位,从而提高氰化物的氧化效率[14]。此外,增加电流密度也会使铜离子更容易吸附到电极表面上(式(1))。
但当电流密度由10 A·m2提高到20 A·m−2时,氰根的去除率仅由80.29%上升到81.47%。这是由于在高电流密度下容易发生二次反应,从而降低对氰化物的氧化效率[15]。由能源消耗的角度来看,去除氰化物的最佳电流密度宜为10 A·m−2。
-
在确认最佳电流密度后,考察了当H2O2投加初始浓度为0.1~0.4 mol·L−1时,对总氰去除率和铜离子吸附的影响。在电氧化反应开始前,加入H2O2确实可以提高氰化物的氧化效率。这是由于H2O2本身在碱性条件下会解离出分子氧,分子氧也可以参与氰化物的氧化,从而提高氰化物的氧化效率[16]。有研究结果表明,H2O2氧化氰化物时,氰化物首先被氧化为低毒性的氰酸盐(式(2)),之后氰酸根经过水解反应被分解为铵根和碳酸根(式(3))[17]。
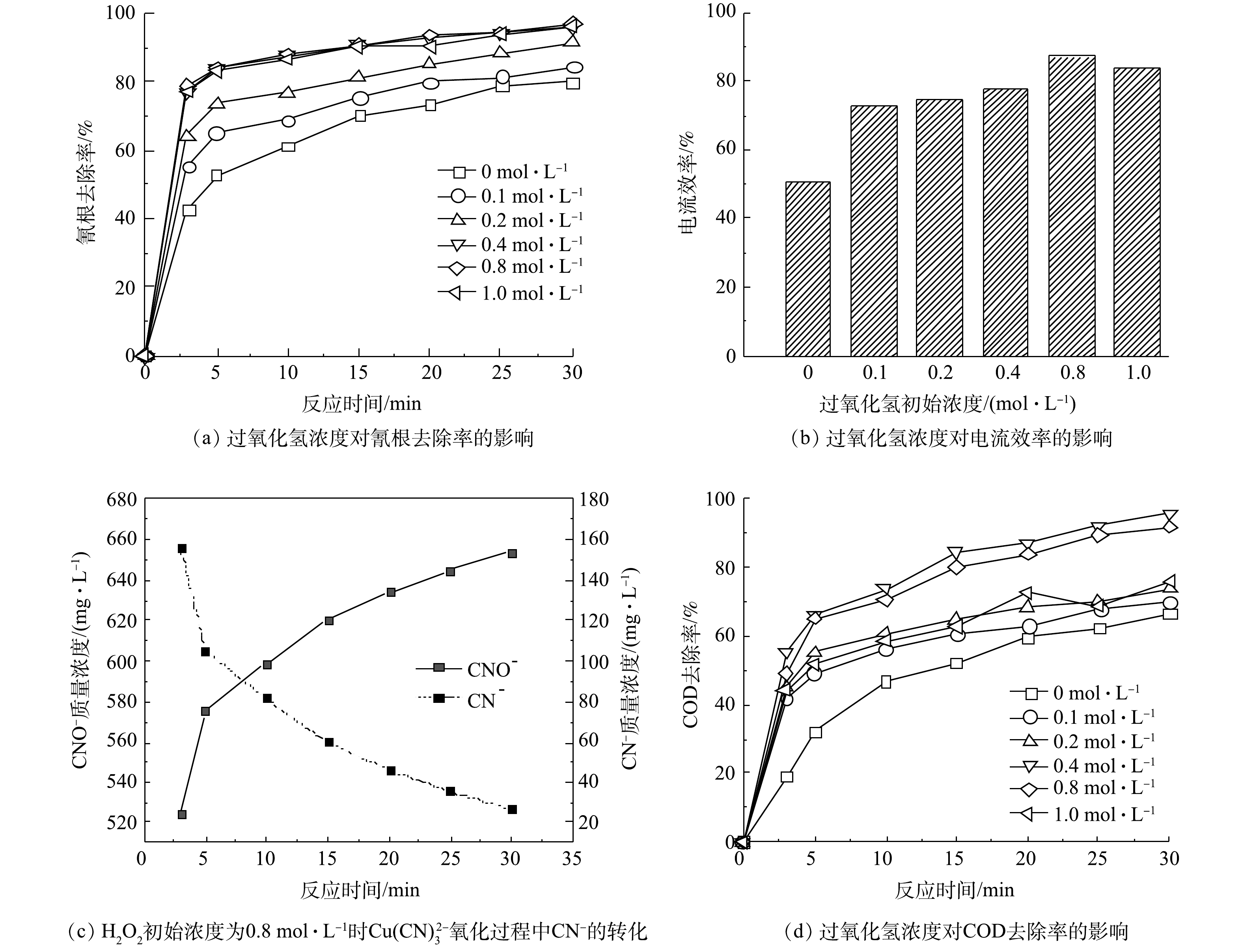
如图2(a)所示,在H2O2初始浓度在0.1~0.4 mol·L−1时,氰根的去除率随着H2O2浓度的增加而升高,30 min时的氰根去除率由84.77%增加至96.12%;在H2O2浓度高于0.4 mol·L−1时氰根的去除效率不再升高;在H2O2浓度为1 mol·L−1时氰根去除率反而呈现下降的趋势。分析其原因为,当H2O2投加浓度过高时,反应开始时初始产生的·OH越多,但其中一部分并未参与到氧化反应,而是发生自身链反应被消耗。电流效率也是随着H2O2初始浓度的增加而增加,当H2O2浓度为0.8 mol·L−1时电流效率最高,为87.30%;当H2O2初始浓度为1 mol·L−1时,由于H2O2浓度过高发生了分解反应,从而导致电流效率下降。检测了CN−的氧化产物CNO−,如图2(c)所示,CNO−随着CN−的减少而增加,且为检测到硝酸根、亚硝酸根、氨氮,说明CNO−为本实验的主要产物。COD的去除率也随着H2O2初始浓度的增加出现了先升高后降低的现象,在H2O2浓度为0.4 mol·L−1时,30 min时耗氧有机物(以COD计)的质量浓度为9.24 mg·L−1,COD去除率为95.94%。因此,去除氰化物的最佳H2O2浓度为0.4 mol·L−1。但H2O2对于低浓度的氰根处理效率极低,在氰化物去除率超过80%后,由于铜离子转化为氧化铜的量增加,氧化铜对PMS的催化效率不如铜离子,导致反应速率明显减慢,故在30 min时不能将氰根完全去除。
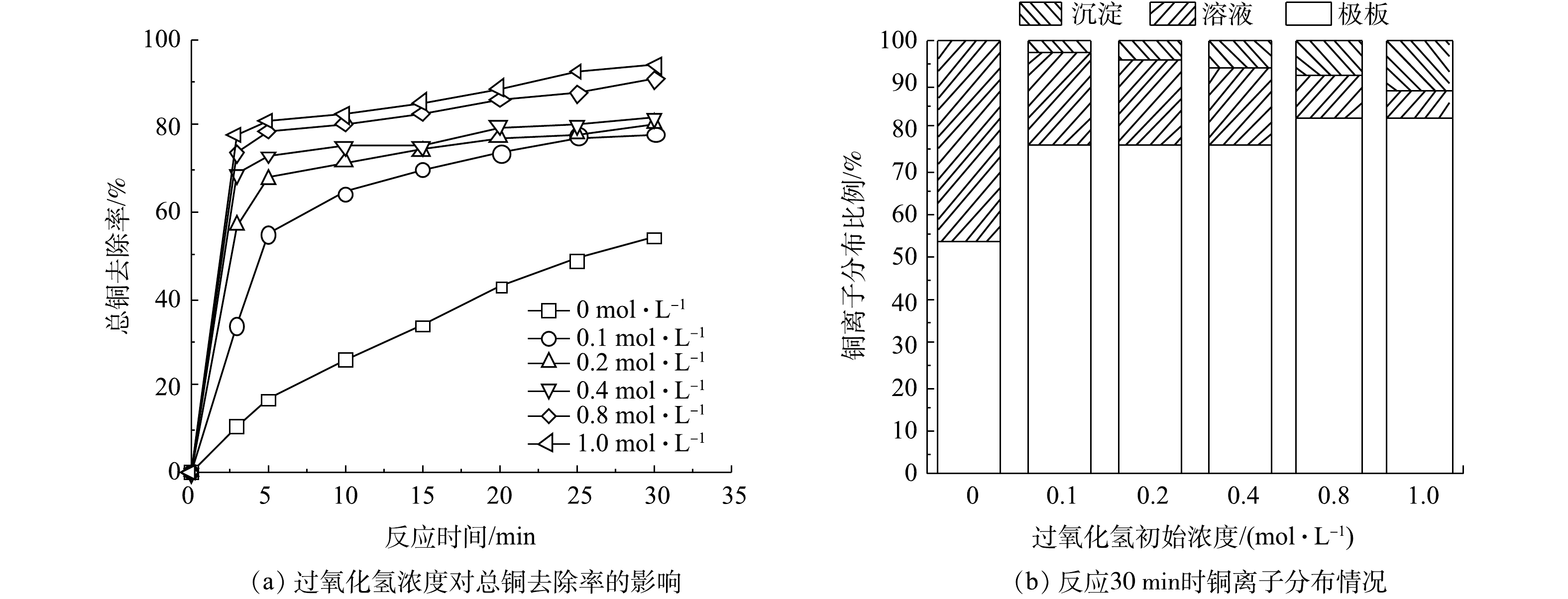
在电氧化反应开始前投入H2O2也可提高铜离子的去除率。如图3(a)所示,铜离子的去除率随着H2O2初始浓度增加而升高,当H2O2浓度为0.4 mol·L−1、反应30 min时,铜离子去除率为81.93%。但去除的铜离子并没有完全被吸附在极板上,一部分铜离子在氧化过程中形成了氢氧化铜和氧化铜沉淀。将反应结束后的极板泡入稀硝酸中,待铜完全溶解后测量了吸附在极板的铜的含量。如图3(b)所示,沉淀物随着H2O2浓度的增加而增加,当H2O2为1 mol·L−1时沉积在极板上的铜占总铜的82.11%,沉淀物质占总铜为11.91%。
-
本研究中考察了不同PMS浓度对铜氰废水处理效果的影响。PMS可以促进电化学体系对于铜氰废水的氧化效果,随着PMS浓度的增加,氰根的去除率也随之升高。某些金属可以作为PMS的活化剂(Fe(0)、Fe(II)、Ag(I)等[18-20])。高浓度的PMS可以促进Cu(CN3)2−的分解,Cu(CN3)2−分解出的Cu2+可以活化PMS产生
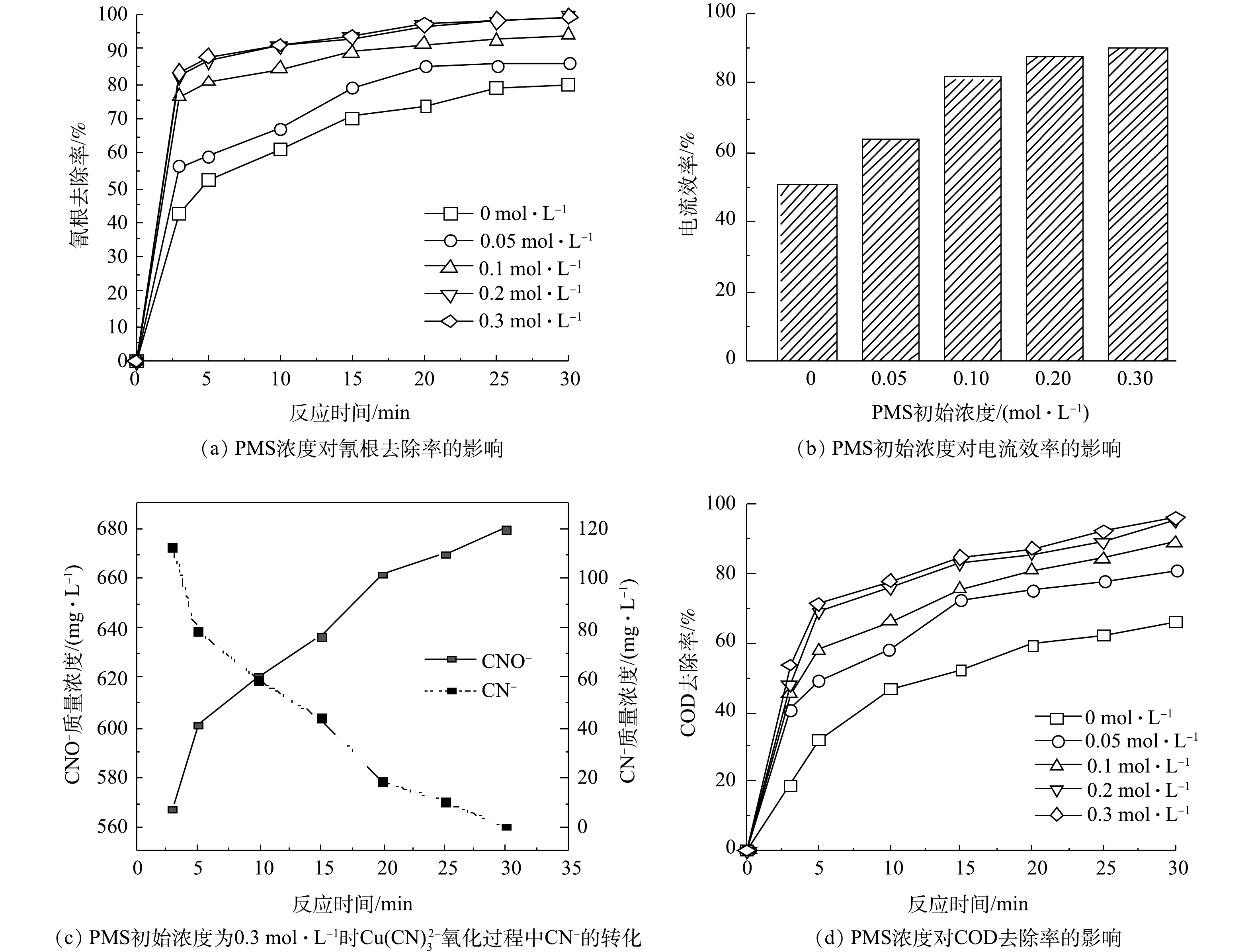
SO−4 ·,SO−4 ·可以高效的氧化CN−,将CN−转化为低毒性的CNO−[21]。一部分铜离子在阳极和溶液中形成的CuO也可以对PMS进行活化,产生更多的SO−4 ·,进而再对CN−进行氧化[22]。PMS的浓度对铜氰废水中氰根的去除率有着重要的影响,结果如图4所示。当不投加PMS时,反应30 min后氰根的去除率为80.29%;当PMS浓度为0.2 mol·L−1时,反应30 min后,氰根去除率约为100%,小于0.3 mg·L−1符合《电镀污染物排放标准》(GB 21900-2008)。当PMS浓度为0.3 mol·L−1时,氰根去除率与PMS浓度为0.2 mol·L−1时的基本相同。这是因为,随着PMS投加量的增加,产生了大量的
SO−4 ·,过多的SO−4 ·发生淬灭反应,降低了SO−4 ·的利用率[23]。电流效率也随着PMS初始浓度的增加而升高,其最高为90.21%。CN−的氧化产物CNO−的检测结果如图4(c)所示。CNO−随着CN−的减少而增加,且为检测到硝酸根、亚硝酸根和氨氮,说明CNO−为本实验的主要产物。COD的去除率也是随着PMS浓度的增加而升高,当PMS浓度为0.2 mol·L−1和0.3 mol·L−1时,COD的去除率差异较小。因此,用于处理铜氰废水的最佳PMS浓度为0.2 mol·L−1。
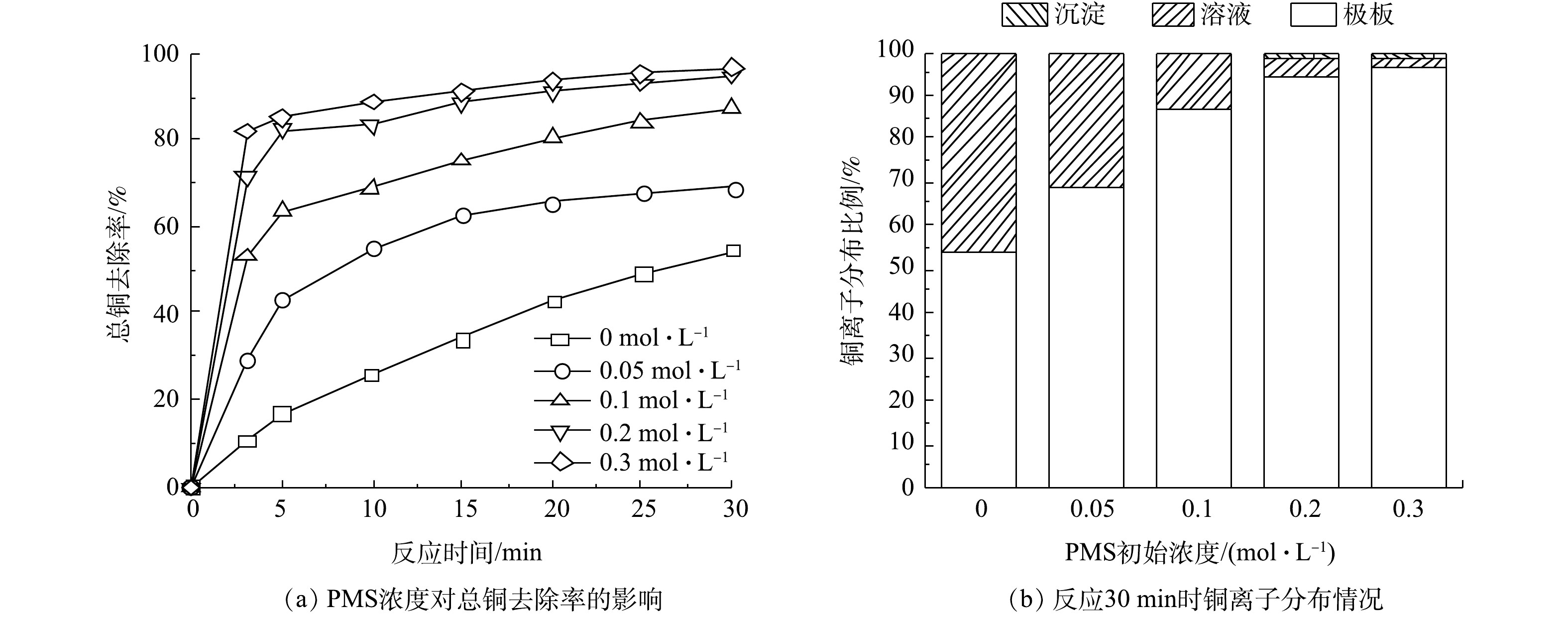
PMS可促进Cu(CN3)2−的分解,使得Cu2+更容易吸附到极板上,从而增加电化学体系对铜的回收效率。如图5所示,总铜的去除率随着PMS浓度的增加而升高,当PMS浓度从0 mol·L−1增加到0.3 mol·L−1时,总铜的去除率由54.41%提高至97.16%,沉积在电极上的铜占总铜的比例由54.41%上升至95.66%。除了在电极上沉积的铜以外,有部分铜形成了氧化铜沉淀,沉淀部分的铜对总铜的占比也随着PMS浓度的升高而升高,当PMS浓度为0.3 mol·L−1时,氧化铜沉淀对总铜的占比为1.50%。
2.1. 电化学处理铜氰废水效果
2.2. H2O2强化电化学处理铜氰废水
2.3. PMS强化电化学处理铜氰废水
-
1) H2O2和PMS均可强化电氧化处理铜氰废水的效果,氰化物的氧化效率和总铜的去除率均随着投药剂量的增加而升高;在投药剂量达到一定量后,会发生副反应,进而降低氰化物的氧化速率。
2) H2O2加强电化学体系对于低浓度的氰化物氧化速率较低,在有限的时间内,不能将氰根完全转化为氰酸根、铵根、碳酸根等物质,无法达到排放标准;在反应过程中,由于氰酸根和水中耗氧有机物(以COD计)的氧化反应会产生出大量气泡,铜离子会被转化为氧化铜和氢氧化铜沉淀,进而影响铜的回收。
3)相比于H2O2,PMS的强化效果更明显。低浓度的氰化物也可以在有限时间内完全处理,由于PMS促进了Cu(CN3)2−的分解,使得Cu2+更容易吸附到极板上,使得在相同时间内沉积在极板表面的铜更多,因而能够回收更多的铜。





 下载:
下载: