-
垃圾焚烧厂产生的NOx主要来自垃圾中N元素(为了与其他文献保持一致,后简称“燃料N”)的转化[1]。目前国内多地已大幅收严垃圾焚烧厂的NOx排放限值,但超量投加脱硝药剂会造成氨逃逸的增加[2-3],而选择性催化还原(selective catalytic reduction,SCR)脱硝工艺的成本又很高,因此,亟需研究垃圾焚烧生成NOx的影响因素,以及在垃圾分类背景下的源头防控机理,并进一步开发低成本、低风险的NOx减排技术。
尽管20世纪90年代已有学者提出流化床焚烧炉中燃料N可能的转化路径[4-5],但国内外有关垃圾焚烧厂NOx源头防控的研究仍然有限。一方面,此类研究局限于模拟垃圾的小试实验,内容包括模拟燃料中H/N、O/N等元素比与燃料N转化率之间的关系,考察温度、升温速率对燃料N转化动力学的影响[6-7],以及风量、温度等因素对中间产物HCN、NH3的影响[8-9];另一方面,此类局限于对各反应阶段影响因素的研究尚不成系统。燃料N的转化包含3个反应阶段。在热解阶段,燃料中蛋白质、环二肽、吡啶等含氮杂环化合物需要较高的热解温度[10],而热解温度在700 ℃以上时有利于缩短反应时间[7]、提高热解产物中HCN/NH3的比例[9];在氧化阶段,氧气和水均可作氧化剂[6, 11]将NH3和HCN氧化为NO和少量的N2O,增加反应供氧量会拉动前一阶段NH3和HCN的生成,从而促进NO生成[8, 12-14];在第3阶段,已生成的NO会在还原气体氛围或焦炭表面还原为N2 [15-17],且焦炭表面的还原效率更高,故燃料固定碳含量越高则其N转化率越低。总体而言,尚未系统地探明垃圾焚烧实际工程中燃料N转化的全过程机理,不足以指导实际工程中的NOx防控。因此,多数焚烧厂对NOx的源头防控仅以调控风量和氧含量为主,未能结合垃圾的成分特征提出更优的解决方案。
本研究基于现有垃圾焚烧过程中燃料N转化的3个阶段特征,利用多家垃圾焚烧厂的垃圾成分特征、运行工况及焚烧生成NOx的质量浓度(简称“NOx生成浓度”)的成对数据,以期探明垃圾焚烧实际工程中燃料N转化的全过程机制,将转化路径图从定性推进到半定量,分析了垃圾分类背景下分离厨余垃圾对NO x 源头防控的作用,为垃圾焚烧厂实现低成本、低风险的NOx减排提供参考。
-
本研究收集了国内近百家垃圾焚烧厂的环境影响评价报告和竣工环保验收报告,获取相关工程的垃圾组分、元素含量、NOx生成浓度等数据。但受限于业内普遍不重视NOx的生成浓度,未在竣工环保验收中监测NOx生成浓度,而垃圾焚烧厂投入运行联入监管网络后很难再通过停用脱硝装置的方式监测NOx生成浓度,因此,仅获取到分别位于华北、华中、华南和西南地区的8家垃圾焚烧厂14台焚烧炉的燃料特性及NOx生成浓度和实时工况。
-
结合文献中分析过的燃料N转化影响因素,将8家焚烧厂14台焚烧炉的实时工况概化为炉温(T)、过剩空气系数(EA)等2个参数,将燃料特性概化为含水率(M)、挥发分含量(V)、固定碳含量(FC)、固定碳占可燃分的百分比(R)、N元素含量(N)、H元素与N元素的质量比(H/N)、O元素与N元素的质量比(O/N)等7个参数,将NOx生成浓度与理论生成浓度的比值换算为燃料N转化率(η)。以上10个参数及其数值见表1。
为识别各参数对燃料N转化率(η)的影响强弱,将表1中各参数与燃料N转化率(η)做相关分析。因各参数分布未知,且多呈现多峰偏态分布,故仅计算Spearman秩相关系数[18]。与燃料N转化率(η)较为相关的参数为固定碳含量(FC)、N元素含量(N)、H元素与N元素的质量比(H/N)、O元素与N元素的质量比(O/N)等4个,相应的Spearman秩相关系数超过±0.5。这4个参数中,燃料N转化率(η)与固定碳含量(FC)在 p > 0.1时相关性不显著,在 p ≤ 0.1时,与后3个参数显著相关。
-
1)工况参数的影响。图1反映出垃圾焚烧实际工况中的燃料N转化率与工况参数(炉温T、过剩空气系数EA)间的相关性不显著,Spearman秩相关系数的显著性水平见表2。
首先,N转化率与炉温(T)的相关性不显著。这是因为垃圾焚烧实际工程中垃圾燃料和烟气的停留时间均大于文献[19]所提出的最短停留时间(分别为10 min[7]和800 ℃以上停留1.7 s),可认为垃圾燃料中的挥发分N和固定碳N都会通过热解过程迁移到气相中,从而削弱了与炉温的相关性。
其次,N转化率与过剩空气系数(EA)的相关性不显著。这是因为表1中数据之间的组间方差和组内方差相互干扰所致。以焚烧厂B、G、H为例,焚烧厂G垃圾中固定碳含量是其它焚烧厂的1.5~2.0倍,H/N、O/N分别是其他焚烧厂的0.17~0.41倍和0.17~0.51倍,从而导致焚烧厂G的燃料N转化率约为其他焚烧厂的0.5倍。实际上就单个焚烧厂而言,垃圾燃料及焚烧炉特性的差异不大,燃料N转化率较为接近。此外,尽管理论上供氧量的减少有利于抑制NO的生成[12],但为了保障热值不均匀的垃圾燃料稳定燃烧,很难依靠动态调节过剩空气系数的方式来精准抑制NOx源头生成。
2)垃圾特性的影响。图1表明垃圾焚烧实际工况中的燃料N转化率与固定碳含量(FC)之间具有显著相关性,而与挥发分含量(V)之间无显著相关性。这是因为气相中燃料N的热解、氧化和还原主要由温度、氧浓度和气体的混合程度所决定,与挥发分含量相关性较小,但固定碳含量会直接影响气固相的接触面积和碳活性位点的数量,进而影响NOx的还原效率[17]。
此外,燃料N转化率与含水率(M)之间的相关性不显著。这主要是因为垃圾含水率普遍处于较高水平,导致其对燃料N转化率的影响已近阈值。表1中,垃圾含水率为42.06% ~ 55.32%,每吨垃圾焚烧大约生成3 817 m3干烟气(标准状态)[1],则焚烧炉内生成湿烟气的含水率约为12.03% ~ 15.28%。水分子在高温条件下生成的大量H自由基与HCN发生加氢反应生成NH3[20],并与焦炭发生水煤气反应生成CO和H2[17],可增强烟气的还原性[21]。然而,当含水率大于10%时,HCN和焦炭的浓度不足以继续支持上述反应,从而导致还原作用的效果不再明显[11]。
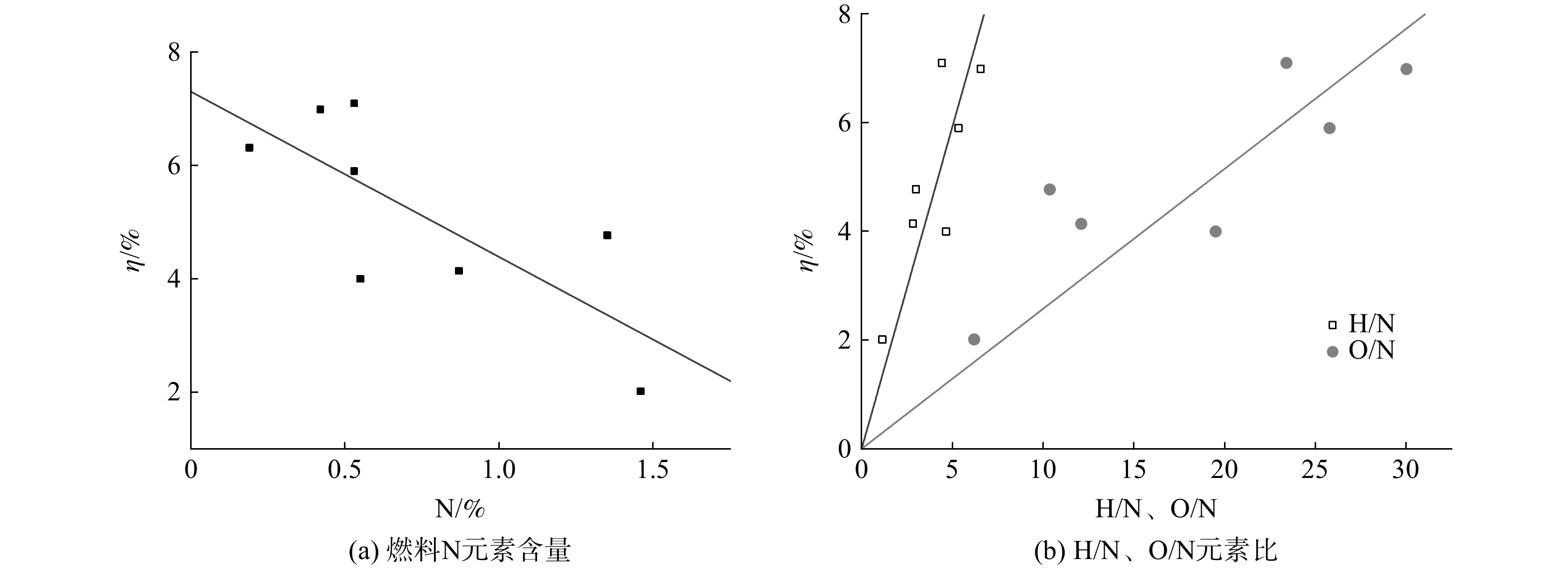
3)垃圾中元素含量的影响。图1表明,垃圾焚烧实际工况中的燃料N转化率与N元素含量、H/N、O/N之间的相关系数依次为−0.84、0.74、0.82,呈显著相关性。进一步的线性回归分析结果表明,燃料N转化率随N元素含量增加而降低(如图2(a)所示),而随H/N、O/N增加而升高(如图2(b)所示)。这与CHYANG等[12]研究结论相似。导致上述结果的原因为:燃料N热解过程中,更高的N元素含量导致热解产物中N2增加[12],从而降低燃料N转化率;而H元素是中间产物HCN和NH3的必需元素,提高H元素浓度有利于增大HCN和NH3的产率[22]、降低N2产率,从而增大燃料N转化率;HCN和NH3转化为NO x 的过程离不开O元素的参与[6, 23],提高O元素浓度同样有利于增大燃料N转化率。
-
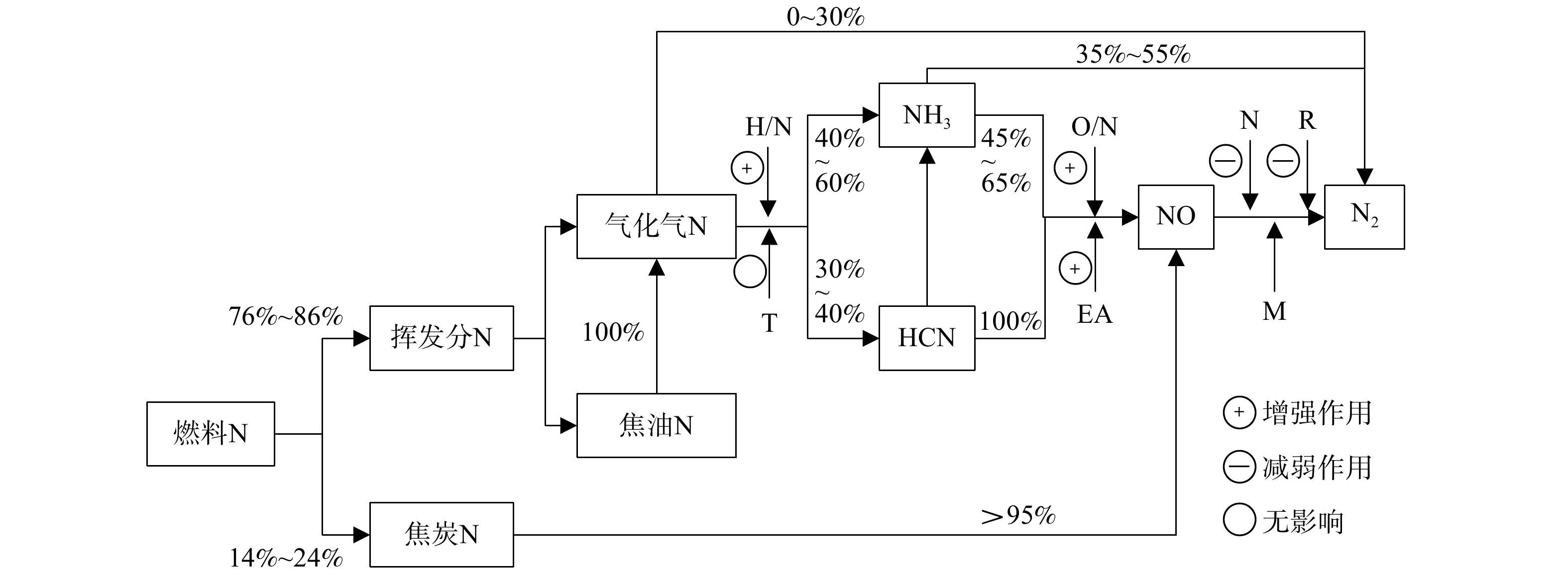
综合上述关于垃圾焚烧实际工况中燃料N转化的影响因素分析,根据生活垃圾的燃料特性,在已有的燃料N定性转化路径的基础上,确定了燃料N的定量转化路径,结果如图3所示。
1)燃料N的热解。生活垃圾可燃分中挥发分和固定碳含量分别为76%~86%和14%~24%(见表1)。挥发分N热解产物一般为气化气态的N(简称“气化N”)和焦油态的N(简称“焦油N”),但在垃圾焚烧厂的高温高湿条件下,焦油N可完全热解为气化N,故焦油N转化为气化N的比例为100%。
气化N进一步热解时,除了大部分转化为HCN和NH3,也会有0~30%直接转化为N2[11, 24]。气化N转化为N2的比例取决于垃圾的元素成分:N元素含量越高,或H/N、O/N元素比越小,则气化N转化为N2的比例越大。生活垃圾中的厨余垃圾蛋白质含量高,会生成较多杂环状的吡咯氮和吡啶氮,更易转化为HCN[25-26]。然而,在湿烟气中水分子的作用下,只有30%~40%燃料N会转化为HCN,40%~60%燃料N会热解生成NH3 [9, 27]。
2) NO的生成。HCN氧化的主要产物为NO(见表3中的反应1和2),而NH3氧化的主要产物为NO和N2(见表3中反应3和4)。反应4在850℃条件下的反应速率约为反应3的390倍,但反应4的焓变约为反应3的1.5倍[28],所以,反应3和4竞争的强弱主要受到催化剂(焦炭)的影响。参考煤燃烧中反应3和4的比例[4-5],确定NH3氧化为NO和N2的比例分别为45%~65%和35%~55%。此外,因为焦炭中的N(简称“焦炭N”)与焦炭会被同时氧化并迁移到烟气中,而生活垃圾焚烧的炉渣热灼减率<5%,所以,焦炭N的转化率>95%。
3) NO的还原。氧化生成的NO还会被还原为N2,主要还原剂包括HCN的氧化产物CNO、未参与氧化的NH3、以CO和CH4为代表的气化气、焦炭,主要反应见表3中的反应5~反应9 [4-5]。反应6为均相反应,其他反应均在固体催化剂的作用条件下发生,反应的催化剂主要为床料和焦炭。已有研究表明,约有86%~98%的NO会再次被还原为N2[4-5, 17, 29],即最终剩余的NO仅为氧化生成NO的2%~14%。这一阶段是影响燃料N转化率的关键步骤,SNCR脱硝和SCR脱硝实际上是通过外源还原剂来增强这一还原过程。
-
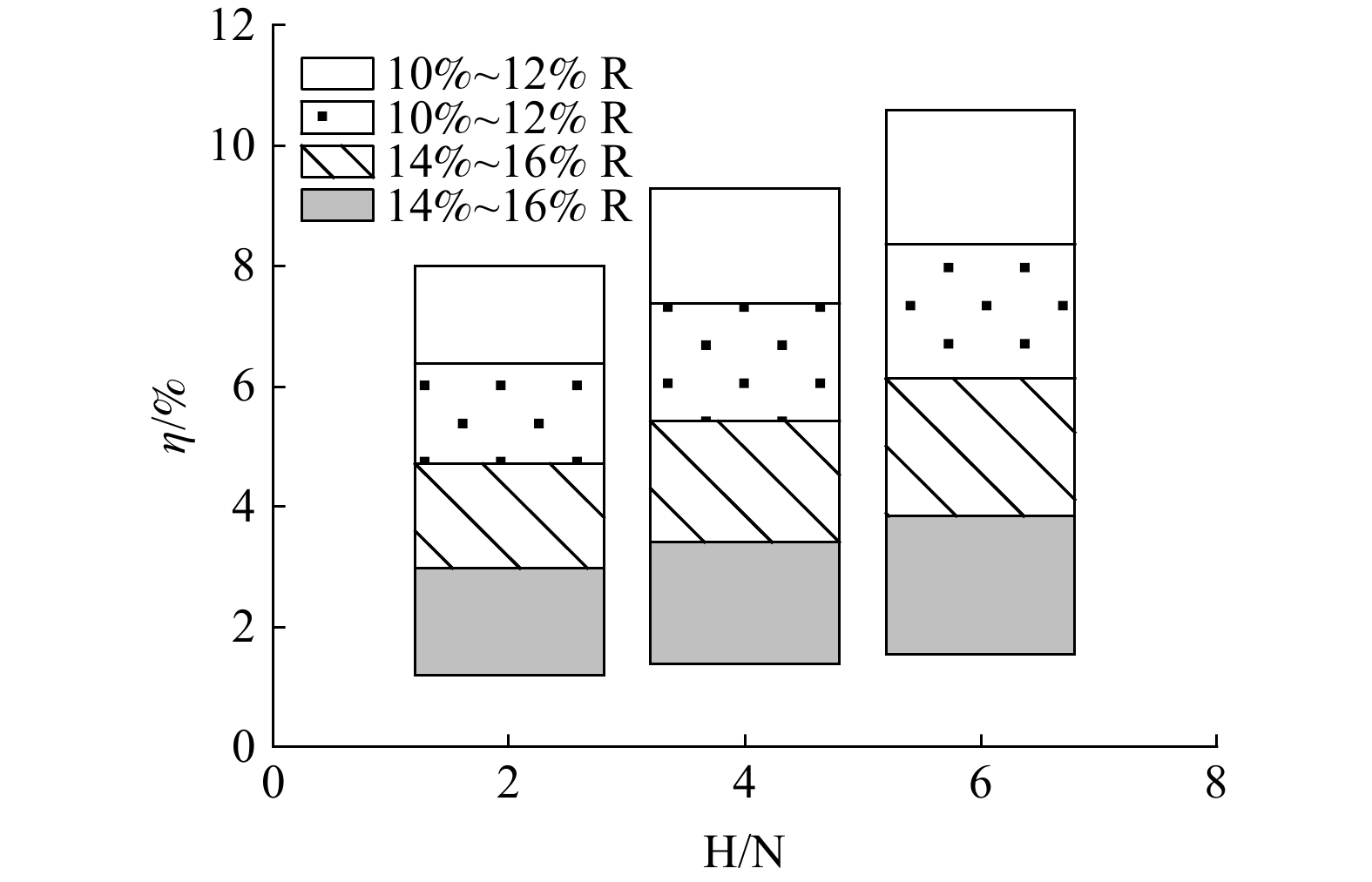
根据图1和图3,可找出在垃圾焚烧厂中对燃料N转化率具有显著影响的独立参数,分别为H/N和R。其中,H/N作为燃料中元素含量对燃料N转化率影响的代表,R作为燃料中固定碳含量对燃料N转化率影响的代表。H/N主要影响气化N热解产物N2、HCN和NH3之间的比例,R主要影响NO的还原效率。将H/N分为低、中、高3个等级,取值为1~7,各等级对应的热解产物以典型值作为代表,如表4所示。当H/N不在此区间内可按照最接近的等级计算N2、HCN和NH3的分配比例。
结合前文得出的数据可知,R为10%~18%时,NOx还原效率均匀分布于86%~98%。故将R分为4个等级,分别为10%~12%、12%~14%、14%~16%、16%~18%,对应NOx还原效率为86%~89%、89%~92%、92%~95%、95%~98%。当R低于10%或高于18%时,可分别按最低和最高还原效率计算。在NOx的一次生成过程中,固定碳中N元素转化率高于挥发分中的N元素转化率,固定碳中N元素的增加有利于NOx生成;然而,固定碳对NOx的还原作用又会阻碍NOx
的生成,故整体上呈现出2种相反作用的叠加效果。将2种因素控制下的燃料N转化率划分为图4所示的12个区间。整体而言,燃料N的转化率随H/N升高而升高,随R的升高而降低。但在每一区间内,燃料N转化率随R的升高而升高。将本研究获得的8家垃圾焚烧厂的相关数据(H/N和R)代入图4,可得只有焚烧厂E和F中燃料N转化率不在预测区间内,准确率达到75%,故具有一定可信性。 -
一般来说,送入垃圾焚烧厂的可燃物主要包括5种:厨余垃圾、纸类、木竹类、纺织类、橡塑类。其中,厨余垃圾的干基N元素含量约为3%,纺织类中N元素含量约为2.3%,其他成分中N元素含量小于1%[30-31]。厨余垃圾中因含有较多糖类和脂肪,N元素含量较高,故可认为热解产物只有HCN和NH3。根据文献[10, 25],蛋白质热解产物中HCN/NH3为2.2~9.5,估算热解产物中HCN约为80%,NH3约为20%。纺织物中的N元素主要以聚酰胺的形态存在,H/N约为0.8,热解产物分为40% N2、35% HCN和25% NH3[19]。因其他组分N元素含量低,且无较多杂环氮,采取表4中高H/N等级,热解产物中60% NH3和40% HCN。由此可估算厨余垃圾、纺织类和其他组分中燃料N的转化比例分别为91%、65%和73%。对于厨余垃圾占比40%以上的生活垃圾来说,通过垃圾分类,可将富含N元素的厨余垃圾从生活垃圾中分离而避免被送入垃圾焚烧厂,有利于垃圾焚烧中的NO x 源头防控。
-
表5为我国4座城市的生活垃圾组分含量表。根据垃圾中各组分含量,可确定垃圾中固定碳和挥发分的含量[32]及N元素的含量[31],进而可确定在不同垃圾组分中,NOx的还原效率所在图4中所处的区间,进而可得到NOx的预测浓度。将4座城市中的生活垃圾分出不同比例的厨余垃圾,并分析燃料N转化率和NOx生成浓度的影响,结果见图5。
如图5所示,不进行垃圾分类时,一线城市NOx生成浓度为300~350 mg∙m−3,二线城市为200~300 mg∙m−3。将厨余垃圾全部分出时,生成的NOx降低比例为22%~55%。以目前的最低排放标准(80 mg∙m−3)为超低排放限值,以150 mg∙m−3为将来的趋势标准(福建省和海南省)为例,假设SNCR脱硝效率为50%,可得NOx生成浓度分别应不高于160 mg∙m−3和300 mg∙m−3。由在一线城市中,当厨余垃圾分出的比例大于80%时,仅通过SNCR脱硝即可满足低于150 mg∙m−3的要求。尽管要达到超低排放要求(80 mg∙m−3)还需增加其他脱硝措施,但分出厨余垃圾仍可降低脱硝剂的用量,降低氨逃逸风险。在二线城市中,将厨余垃圾全部分出时,仅通过SNCR脱硝即可达到超低排放要求。
厨余垃圾的剔除,一方面会降低R,从而导致NO x 还原效率降低;另一方面会降低N元素含量,从而导致NO x 一次转化的综合转化率降低。这两个方面结果对燃料N转化的作用是相反的。随着厨余垃圾剔除比例的提高,焚烧过程中剩余垃圾的NO x 生成浓度整体呈先略微升高再加速下降的趋势。这是由于随着厨余垃圾不同程度地被剔除,R的降低使得NO x 还原效果减弱,导致燃料N的转化率不降反增。然而,N元素含量的微弱下降不足以支撑NO x 生成浓度的下降。R的进一步降低对NO x 还原过程的削弱效果减弱,特别是当R降至低于10%时,其不再对NO x 的还原效率造成削弱,表现为NO x 生成浓度呈加速下降趋势。图5中部分节点出现燃料N转化率上升,主要是受NO x 还原效率降低的影响。随着厨余垃圾剔除比例的提高,燃料N转化率的变化范围越来越小,甚至变为恒值,这是因为很低的R已很难再影响NO x 的还原效率。
-
1)在正常运行的生活垃圾焚烧厂中,焚烧炉膛温度、烟气停留时间、垃圾含水率这3个影响因素与燃料N转化率之间不存在显著的相关性。H/N、O/N能增强燃料N的热解和氧化过程,与燃料N转化率呈现正相关性;垃圾中N元素含量、固定碳含量能增强NOx的还原效率,与燃料N转化率呈现负相关性。
2)燃料特性对燃料N转化率其决定性作用,将H/N分为3个等级、固定碳占可燃分的百分比(R)分为4个等级,建立燃料N转化率与这2个参数之间的关系,可通过这2个因素预测燃料N转化率可能的取值范围,准确率达到75%。
3)通过垃圾分类可减少入炉垃圾中的厨余垃圾占比,能有效降低垃圾焚烧中NOx的
生成浓度。将一线城市生活垃圾中的厨余垃圾剔除80%以上,仅通过SNCR脱硝即可使垃圾焚烧的NOx排放浓度不超过150 mg∙m−3;将二线城市生活垃圾中的厨余垃圾全部剔除后,仅通过SNCR脱硝亦可使垃圾焚烧的NOx排放浓度达到超低排放要求,即不超过80 mg∙m−3。
垃圾分类背景下厨余垃圾剔除比例对生活垃圾焚烧厂NOx排放
Influencing of kitchen waste removal ratio on NOx emission of domestic waste incineration plant in the era of waste classification
-
摘要: 收集了生活垃圾焚烧厂关停SNCR时燃料的N排放数据以及与之对应的工况和燃料特性数据,并分析各影响因素对燃料N转化过程中不同环节的影响机理。结果表明,在工业化生产中,燃料N转化率与炉温、垃圾含水率之间不存在显著联系,而随N元素含量和固定碳含量的增加而降低,随过剩空气系数、H/N、O/N的增加而升高。据此提出了符合实际生产条件的燃料N转化路径图。该路径图表明,燃料特性对燃料N转化率和NOx生成浓度起到了决定性作用,并进一步量化了H/N和固定碳含量这2个参数与燃料N转化率之间的关系。提出通过将厨余垃圾从生活垃圾分出的方法来减少NOx生成,并以国内典型城市为例,研究了分出不同比例厨余垃圾的情况下,燃料N转化率和NOx生成浓度的变化趋势。本研究表明通过分出厨余垃圾的方法,能够降低垃圾焚烧厂的脱硝成本及氨逃逸风险,可为进一步控制生活垃圾焚烧过程中的NOx排放提供参考。Abstract: To analyze the factors in different process of fuel-N conversion, fuel-N emission data and corresponding operating conditions and fuel characteristics data of the waste incineration plant were collected when SNCR is closed. The results showed that no significant correlation between the fuel-N conversion efficiency and furnace temperature and moisture content in industrial production. The fuel-N conversion efficiency decreased with the nitrogen content and fixed carbon content, yet increased with the excess air ratio, H/N and O/N weight ratio. The fuel-N conversion pathway under the conditions of industrial production was proposed. The fuel characteristics played a crucial role in the fuel-N conversion efficiency and NOx production. The relationship between the fuel-N conversion and H/N weight ratio and char content was further quantified. Separating kitchen waste from municipal solid waste (MSW) was proposed as an effective method to reduce NOx emission, and the evolving trends of fuel-N conversion efficiency and NOx emission were estimated under the different scenarios of kitchen waste separation rates in the representative cities in China. The estimation indicated that the cost of denitration and the risk of ammonia escape in waste incineration plants could be reduced by separating kitchen waste from MSW.
-
Key words:
- waste incineration /
- fuel-N conversion /
- source control /
- waste classification /
- kitchen waste
-
卡马西平(carbamazepine,CBZ)是一种常见且重要的精神类药物,在临床上常用于控制癫痫的发作、三叉神经痛和舌咽神经痛,并且可以预防和治疗狂躁症和抑郁症,每年消耗量达1 214 t[1-2]。同时,CBZ也是水环境和城镇污水中典型的药物和个人护理用品(pharmaceutical and personal care products,PPCPs)类污染物。与众多PPCPs类污染物类似,CBZ具有难降解特性,其半衰期长达(990±10) d[3]。因此,CBZ随污水排放后,将在环境中持续存在,其在地表水和土壤中的残留质量浓度可高达830 μg·L−1[4-5]和50 μg·kg−1[3]。同时,CBZ还具有较强的生物累积性,在ng·L−1级别就会产生神经毒性、胚胎发育影响、器官损伤等显著的生态毒性效应,其在食物链的富集作用对水生生态系统和人体健康产生深远影响[6]。对于CBZ的处理技术,在传统的污水处理厂中,CBZ的去除率通常低于10%[7-8]。高级氧化技术(advanced oxidation processes,AOPs),包括Fenton氧化、超声氧化、臭氧氧化和紫外线/过硫酸盐氧化等,可原位生成强氧化剂(·OH、1O2、·O2−、O3、HOCl和SO4·−)[9-11],以实现CBZ的快速氧化降解。然而,能量和不稳定试剂的大量投入、二次污染物生成仍是AOPs面临的关键问题。因此,研发高效、低耗的CBZ降解技术,从受污染水体中有效去除CBZ等PPCPs类污染物、缓解其环境生态风险具有重要意义。
有研究表明,光电催化(photoelectrocatalysis,PEC)技术已被证明是应对能源危机和环境污染的一种重要AOPs方法[12]。PEC系统耦合了光催化过程和电化学过程,能有效解决电催化过程能耗高、光催化过程效能低的问题,提高有机污染物的降解效果。例如,WANG等[13]的研究证明,以TiO2为光阳极的PEC系统对CBZ的降解率为(73.1±1.7)%,高于光催化(photocatalysis,PC)和电催化(electrocatalysis,EC)工艺的总和。然而,以CBZ为代表的PPCPs污染物,其在污水中质量浓度为仅为ng·L−1~μg·L−1[14],要实现CBZ的高效降解往往需要投加更多的电能。近年来,光催化燃料电池(photocatalytic fuel cell,PFC)结合了光催化和传统燃料电池的原理,可在无需外加电能情况下,驱动有机污染物的光电催化降解过程,并可同步产生电能[15],其有望为水中PPCPs类污染物的高效、低耗去除提供新的解决方案。
对于PFC系统,研究表明通过改进阳极材料增加光电流是有限的,难以实现系统性能的大幅度提升,加之电子从光阳极迁移到阴极的由于动力学缓慢等原因无法快速消耗。阴极过程已成为整个PFC系统的限制性因素[16]。目前,PFC过程通常采用2种功能性阴极,一是催化氧气4电子还原反应的普通阴极,如ZHANG等[17]构建光催化燃料电池,在模拟太阳光照射下,CN-WO3/W阳极在1.2 V(相对于Ag/AgCl)下,以有机污染物为燃料,显示出6.1 mA·cm−2的稳态光电流密度,且对PFOA的降解率高达95%。二是进行2电子还原反应的H2O2电合成阴极,该阴极可以利用光阳极产生的电子在阴极表面选择性合成H2O2,通过类芬顿过程和光阳极作用,促进体系中ROS的生成和CBZ等水中微量有机污染物的降解[18-19]。然而,值得注意的是,在PFC系统中,阴阳极通过光电流密切相关,阴极的选择直接影响了系统产电和阳极光催化性能。O2/H2O2的标准电极电势为0.695 V vs SHE仅为O2/H2O的50%,因而从热力学角度来看,采用H2O2电合成阴极对于电能回收、光阳极空穴-电子有效分离和空穴有效利用是不利的。
因此,本研究主要以PFC系统高效低耗降解CBZ为目标,研究不同阴极对PFC系统产电、CBZ去除效能的影响,选择效能最优的阴极开展影响因素分析实验、并通过淬灭实验和中间产物分析,探究PFC系统对CBZ的降解机制,为PFC系统低耗高效去除水中的CBZ提供数据参考。
1. 材料与方法
1.1 阳极与阴极的制备
1)TiO2/g-C3N4/CQDs(TCNC)阳极的制备。本研究主要采用可见光相应的TCNC光阳极开展研究,具体制备步骤如下[20]:将方阻为7 Ω的FTO导电玻璃放入1 : 1 : 1的异丙醇:丙酮:超纯水混合液中,超声60 min后取出清洗;将40 mL去离子水与40 mL浓盐酸进行混合搅拌5 min,然后,加入1.35 mL钛酸四丁酯,再搅拌5 min,混合液放入Teflon高压反应釜中;然后把FTO导电面朝上放入反应釜中,烘箱中150 ℃反应5 h,随后取出并清洗干净;称取2.0 g二氰二胺放入坩埚当中,然后将负载了TiO2的导电玻璃导电面朝下放入坩埚当中;将坩埚放入马弗炉中550 ℃反应3 h,随后取出并清洗干净即得TiO2/g-C3N4阳极;在50 mL (1 mol·L−1)葡萄糖中加入50 mL (1mol·L−1)的NaOH,超声处理2 h得到棕色溶液,用盐酸调至中性即得CQDs水溶液;将TiO2/g-C3N4浸泡在CQDs溶液中并放入烘箱中60 ℃干燥10 h,即可得到TCNC阳极。
2)类芬顿空气扩散阴极(GF)的制备。称取45 mg铁锰铜三元催化剂(铁锰铜质量比为3 : 1 : 8)[21]和90 mg石墨再加入10 mL塑料离心管中,然后量取100 μL去离子水,800 μL 5%全氟磺酸溶液,400 μL异丙醇,漩涡振荡5 min。将混合物快速涂于防水透气碳布(单面涂敷了PTFE防水透气涂层)的碳纤维裸露面,室温干燥24 h,即获得类芬顿催化剂与石墨比例为1:2的GF阴极。此外,石墨电极(PG)为类芬顿催化剂与石墨比例为0:2的空气扩散阴极。
3)铂碳(Pt/C)空气扩散阴极的制备。称取15 mg 47.5% Pt催化剂和45 mg碳黑(XC-72R,美国卡波特有限公司)于塑料瓶中,加入50 μL去离子水振荡60 s,加400 μL 5% Nafion溶液,200 μL异丙醇,振荡摇匀,均匀涂在防水碳布的碳纤维裸露面,即获得载Pt量为0.5 mg·cm−2的Pt/C阴极。
1.2 CBZ的去除实验
以负载TCNC的导电玻璃为光阳极,选择功能性阴极,制备单室反应器,阴阳极的有效面积为7 cm2,反应器有效体积为14 mL,采用可见光LED白灯作为激发光源,光功率密度为80 mW·cm−2,CBZ初始质量浓度为1 mg·L−1,PFC运行时间为300 min。对比分析不同功能性阴极、不同条件反应对CBZ的去除效果。
1.3 分析和计算方法
1)材料表征。通过场发射扫描电镜(SU8010型,HITACHI 日立)观察空气阴极扩散层的表面形貌。通过自动比表面积和孔径分析仪(ASAP,
2460 3.01, Micromeritics USA)测量催化剂负载在阴极形成的孔径和比表面积。2)溶出实验。通过总有机碳分析仪(TOC-L,岛津)测定PFC系统运行过程中,溶出的的TOC浓度,通过电感耦合等离子体质谱仪(Aglient-
7800 ,安捷伦)测定溶出的金属离子浓度。3)光电性能测试。采用电化学工作站(DH7000型,江苏东华分析仪器有限公司)对光电化学性能进行了测试,测试项目包括线性扫描伏安扫描、功率密度曲线和电化学阻抗谱。
4)自由基分析。使用电子顺磁共振光谱仪(JES FA200,日本电子JEOL)对测试样品中的自由基进行分析,采用DMPO分别在水溶液和甲醇溶液中捕获·OH和·O2− [22];通过投加自由基淬灭剂[23]研究CBZ降解过程中·OH、·O2−和h+的贡献。
5) CBZ浓度和中间产物的测定。采用高效液相色谱法(Alliance e2695,沃特世科技有限公司)测定CBZ的浓度,色谱柱为C18色谱柱(4.6 mm×250 mm,5 μm,瑞典Akzo Nobel公司),流动相为60甲醇 : 40水(0.1%乙酸) (V : V),流速为1.0 mL·min−1,紫外检测器波长为280 nm,柱温35 ℃。中间产物采用超高效液相色谱-四极杆-静电场轨道阱高分辨质谱(UPLC-Q-Orbitrap HRMS)进行检测分析[24]。
2. 结果与讨论
2.1 DP、PC、PFC体系对CBZ的去除影响
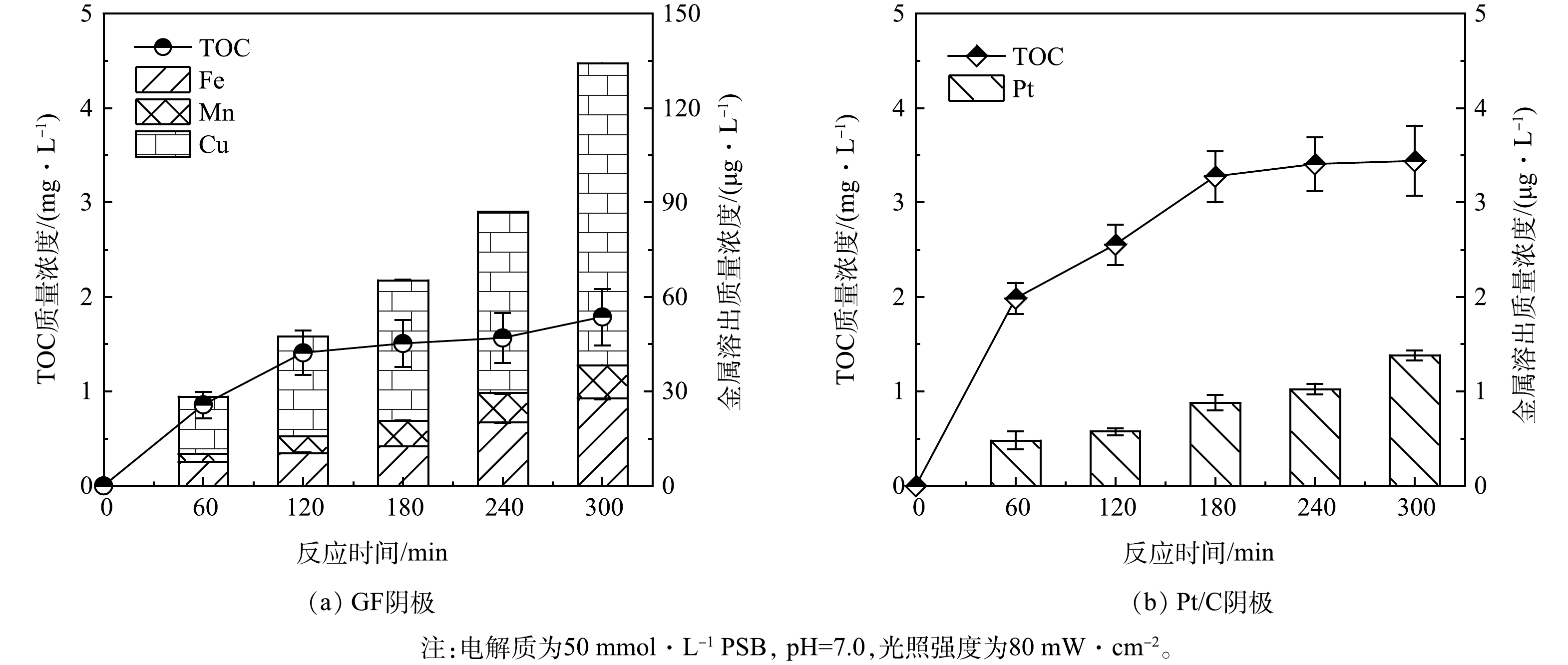
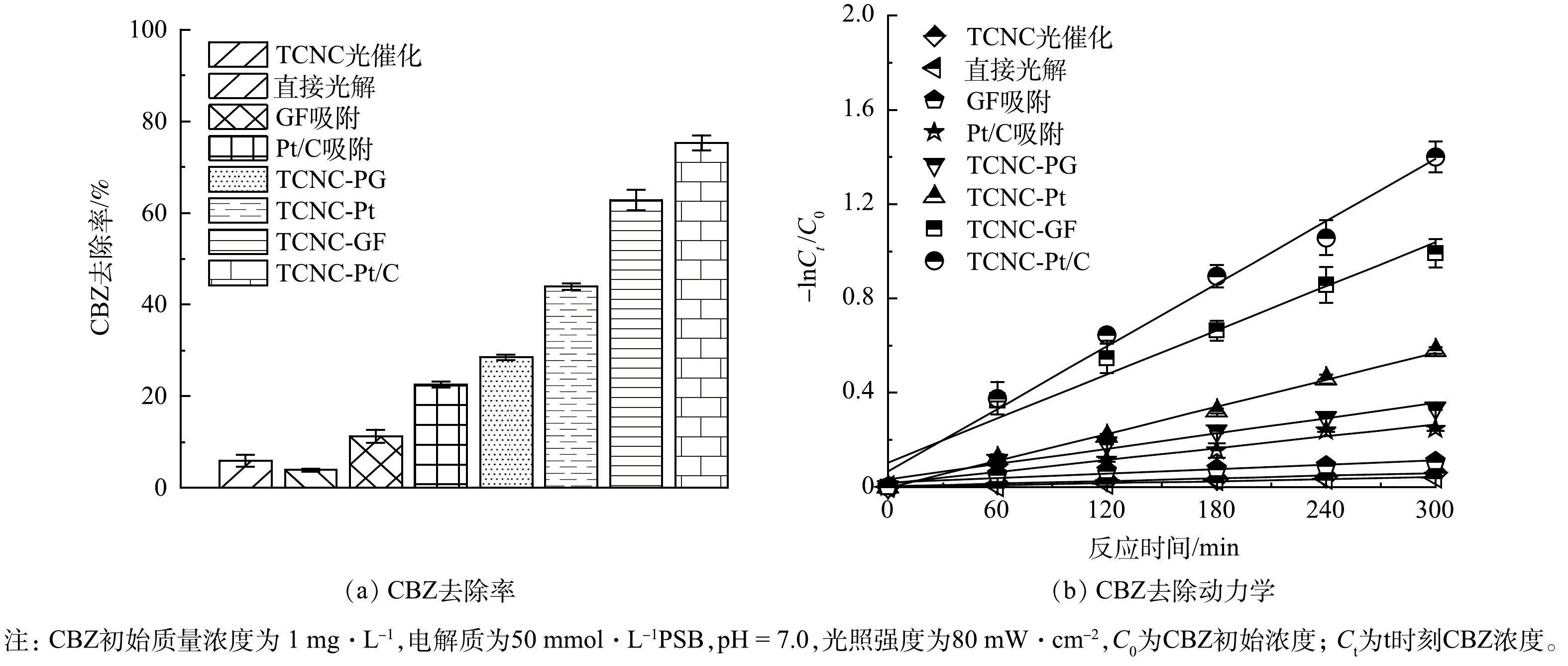
在PFC系统中,有机物、H2O和OH−是电子主要来源。空穴氧化和光激发过程产生的电子可在阴阳极电势差驱动下,在PFC回路中定向移动,可有效抑制光催化剂中载流子复合,提高光催化效率和CBZ去除效率。如图1(a)所示,对于卡马西平CBZ直接光解和TCNC光催化过程,CBZ去除率较低,仅为(3.9±0.3)%和(5.8±1.3)%。值得关注的是,在PFC系统中,阴极材料会直接影响CBZ的去除效率。为了提升CBZ的降解效率,我们制备并比较了类芬顿空气扩散电极(GF电极)和Pt/C空气扩散电极两种功能性阴极。首先,该两种阴极对CBZ都具有一定的吸附效果。从阴极表面的SEM图(图2)中可以看出,GF阴极和Pt/C阴极表面都均匀负载了催化剂,且Pt/C阴极表面的粗糙程度大于GF阴极。同时,Pt/C阴极具有较小的孔径、更大的比表面积和微孔体积(表1),因此Pt/C表现为更高的吸附去除效果。实验结果表明,Pt/C阴极和GF阴极对CBZ的吸附去除效率分别为(22.5±0.8)%和(11.3±1.4)%。
表 1 GF阴极和Pt/C阴极的比表面积和孔径分析Table 1. Specific surface area and pore analysis of GF and Pt/C cathodes阴极 BET面积/(m2·g−1) 孔径/nm 微孔体积/(cm3·g−1) GF阴极 9.897 6 11.730 7 0.053 539 Pt/C阴极 11.763 6 10.791 9 0.069 410 对于光电催化过程,当以铂(Pt)电极作为阴极构建PFC系统后,光阳极生成的光生电子传递给阴极,在阴极上发生O2+4H++4e− → H2O反应,CBZ去除率显著提升至(43.9±0.7)%。而当采用Pt/C空气扩散电极代替Pt电极,由于其优越的氧气扩散性能[25],强化了阴极表面电子转移,CBZ去除率可进一步提升至(75.2±1.6)%。然而,当采用石墨(PG)空气扩散阴极,由于其主要催化的是氧气2电子还原产H2O2的过程[26],系统对光生电子的驱动力下降,CBZ去除率仅为(28.5±0.6)%。虽然通过在阴极材料中引入类芬顿催化剂制备GF阴极,可活化H2O2并强化ROS生成,使CBZ去除率提升至(62.8±2.2)%,但其去除反应动力学常数(Kobs)仅为Pt/C的70.6%(图1(b))。结果表明在PFC系统中,相对于TCNC - GF系统利用类芬顿过程强化ROS生成,TCNC - Pt/C系统则是通过提升阴极的电子转移能力,强化光电流和载流子分离能力,对CBZ的强化去除更为有利。因此,TCNC - Pt/C系统对CBZ具有更高的去除效能。此外,为了佐证电极材料在反应过程中的稳定性,我们同时开展了TOC和金属溶出实验,如图3所示,PFC系统运行300 min后,GF阴极和Pt/C阴极的TOC溶出质量浓度分别为(1.8±0.3) mg·L−1和(3.4±0.4) mg·L−1,而GF阴极的金属溶出质量浓度为134.3 μg·L−1,远大于Pt/C阴极的金属溶出质量浓度(1.4 μg·L−1),因此,从材料稳定性方面考虑,本研究选择Pt/C阴极构建PFC体系。
2.2 PFC体系的电池性能分析
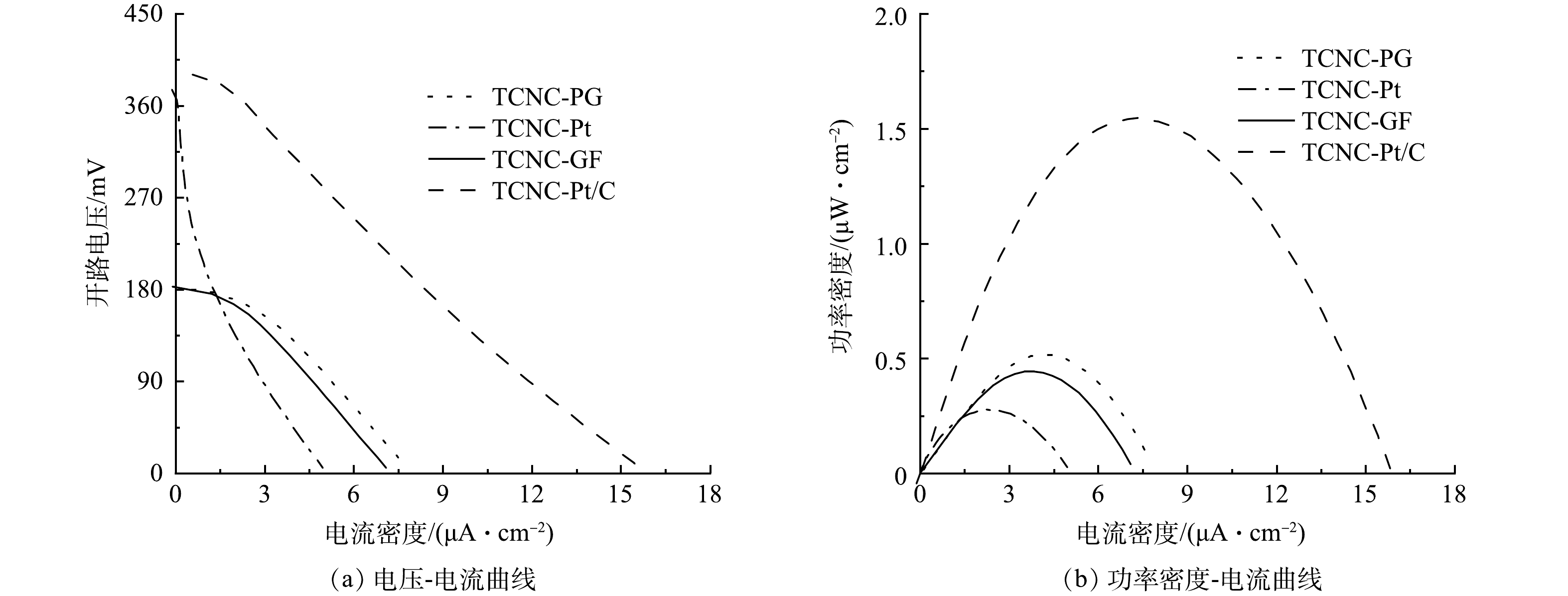
对于PFC系统,处理污染物去除效率外,产电效能也是衡量系统性能的关键指标,主要包括开路电压(Voc)、短路光电流密度(Jsc)、最大功率密度(Pmax)等关键参数[27]。如图4(a)所示,PG阴极和GF阴极的Voc分别为185.6 mV和187.5 mV,而Pt阴极和Pt/C阴极的Voc为375.5 mV和401.2 mV。Voc主要反映PFC阳极和阴极之间的电势差,主要取决于阴极反应。PG和GF阴极主要进行氧气的2电子还原反应,E0 = 0.68 V,而对于Pt和Pt/C阴极则主要为氧气4电子还原反应,E0 = 1.23 V。理论上,Pt和Pt/C阴极的电势约为PG和GF阴极的2倍,则Pt和Pt/C阴极的Voc也是PG和GF阴极的2倍左右。Jsc可以间接反映体系中的由阳极传递至阴极的电子数量,主要取决于阴阳极电势差以及PFC的内阻,阳极空穴的有效消耗以及阴极电子的迅速转化,可以有效提高体系的Jsc以及Pmax[27],本研究中,Pt/C阴极由于较高的阴极电势以及空气扩散阴极的优势,表现出较高的Jsc (15.9 μA·cm−2),约为PG和GF阴极的2倍,为Pt阴极的3倍。表明,Pt/C阴极可显著增大光电流,从而提升CBZ的去除效果。相似地,TCNC - Pt/C系统的Pmax达1.5 μW·cm−2,分别是TCNC - Pt、TCNC - PG和TCNC - GF系统的5、3.75和3倍。因此,Pt/C阴极有利于发电。
2.3 PFC体系的光电化学特性分析
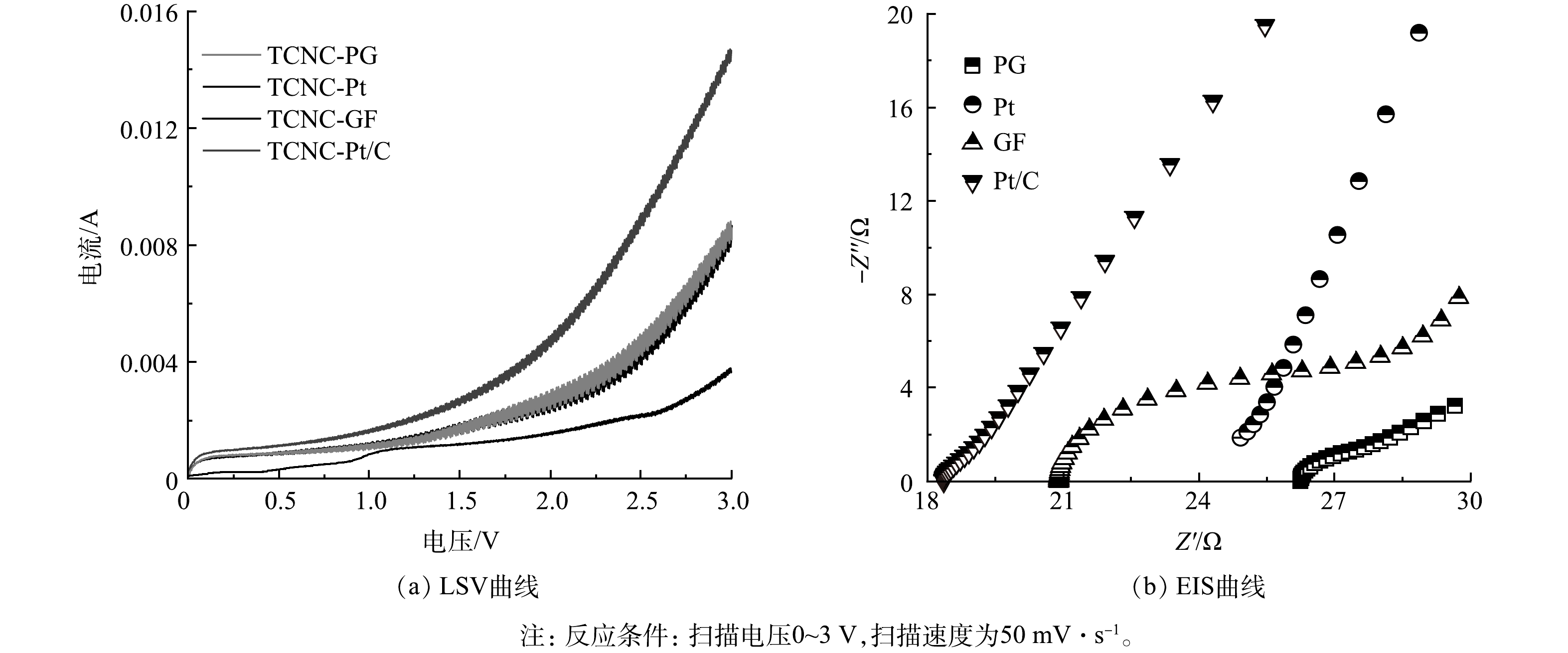
进一步对不同PFC体系的光电化学特性开展研究,各功能性阴极的线性伏安扫描结果表明(图5(a)),阴极表面的电子转移能力为Pt/C > PG > GF > Pt,由于采用相同的光阳极,各系统的电荷转移能力与阴极表面的电子转移能力相一致,Pt/C表现出较高的光电流,与J-V曲线结果一致,证明Pt/C阴极具有优异的电子转移能力和良好的氧气还原活性[28]。此外,GF与PG相比,电流没有明显增加,说明PG阴极添加铁锰铜金属催化剂后,并不能提升系统的电荷转移能力,TCNC - GF对于CBZ去除效能的提升主要是由于PFC体系中的ROS产量的增加。同时,电化学阻抗图谱分析结果(图5(b))表明,Pt的圆弧最大,其Rct达170 Ω,而Pt/C的Rct仅为0.5 Ω,远低于PG(6.4 Ω)和GF(8.0 Ω)阴极,Pt/C阴极与其他3种功能性阴极相比的低电阻,解释了其在PFC中的较高功率输出。因此,本研究后续选择Pt/C作为阴极,研究PFC系统的影响因素及CBZ降解机制分析。
2.4 PFC系统对CBZ去除过程的影响因素分析
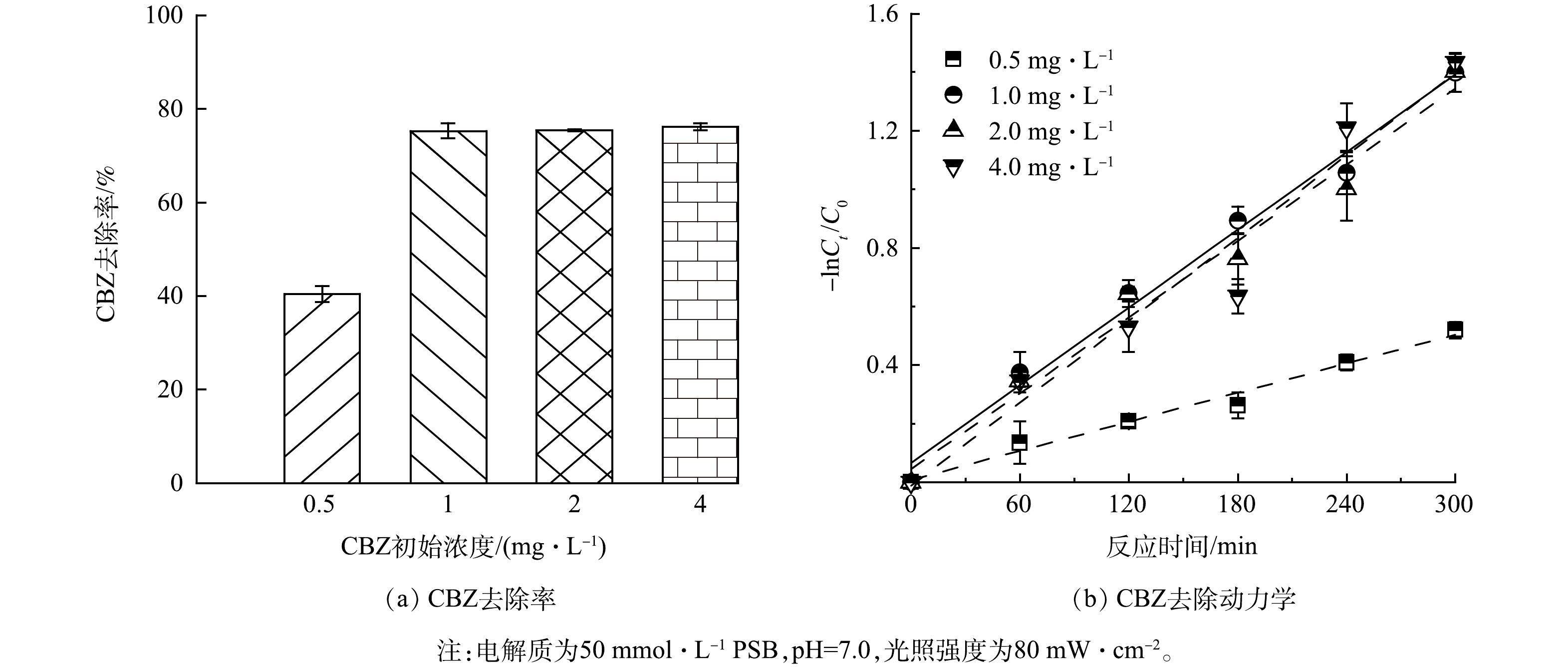
1) CBZ初始质量浓度对PFC体系去除CBZ效果的影响。底物初始质量浓度对TCNC-Pt/C系统的影响如图6所示,当CBZ初始质量浓度为0.5、1、2和4 mg·L−1时,反应300 min后对应的CBZ去除率分别为(40.5±1.7)%、(75.3±1.6)%、(75.4±0.2)%和(76.2±0.8)%,Kobs分别为0.001 7、0.004 4、0.004 3和0.004 7 min−1。随着CBZ初始质量浓度由0.5 mg·L−1增加至1 mg·L−1,CBZ的去除率提升约86%,但当CBZ初始质量浓度大于1 mg·L−1,CBZ去除效果无明显变化。上述结果表明,在PFC系统中,该CBZ浓度范围对其去除率的影响较低,推测由于本研究采用的光电催化体系中可以产生充足的ROS,使CBZ与阳极活性位点或者ROS碰撞的概率趋于稳定。
2)电解液种类对PFC体系去除CBZ效果的影响。PFC系统中电解液的主要作用是传导离子,降低体系的欧姆内阻,但同时电解液的种类也会影响PFC去除CBZ的效能。如图7所示,当电解液分别为50 mmol·L−1的NaNO3、NaCl、Na2SO4和PBS时,反应300 min后CBZ去除率分别为54.9%、83.8%、63.0%和75.3%。上诉CBZ去除率的差异可以通过体系中不同ROS的形成来解释[29]。当电解质为NaCl时,CBZ去除量最高,反应速率达0.005 8 min−1,推测其原因是Cl−能在PFC体系中能生成活性氯自由基 (Cl2·−和HOCl) [30],从而促进CBZ的去除。当电解质为Na2SO4时,体系中生成·SO4−和·OH,虽然可提升CBZ的去除,但也可能增加电子-空穴对复合的可能性[31],从而使CBZ的降解效率下降。此外,电解液携带电荷的数量可直接影响PFC的产电效能并对CBZ的去除产生影响,在相同的摩尔浓度下,电解液的电导率为PBS > Na2SO4 > NaNO3,因此,PBS携带电荷能力更强,因此,系统产生的光电流更高[32],强化了光电子和空穴的分离,CBZ去除效率较高。
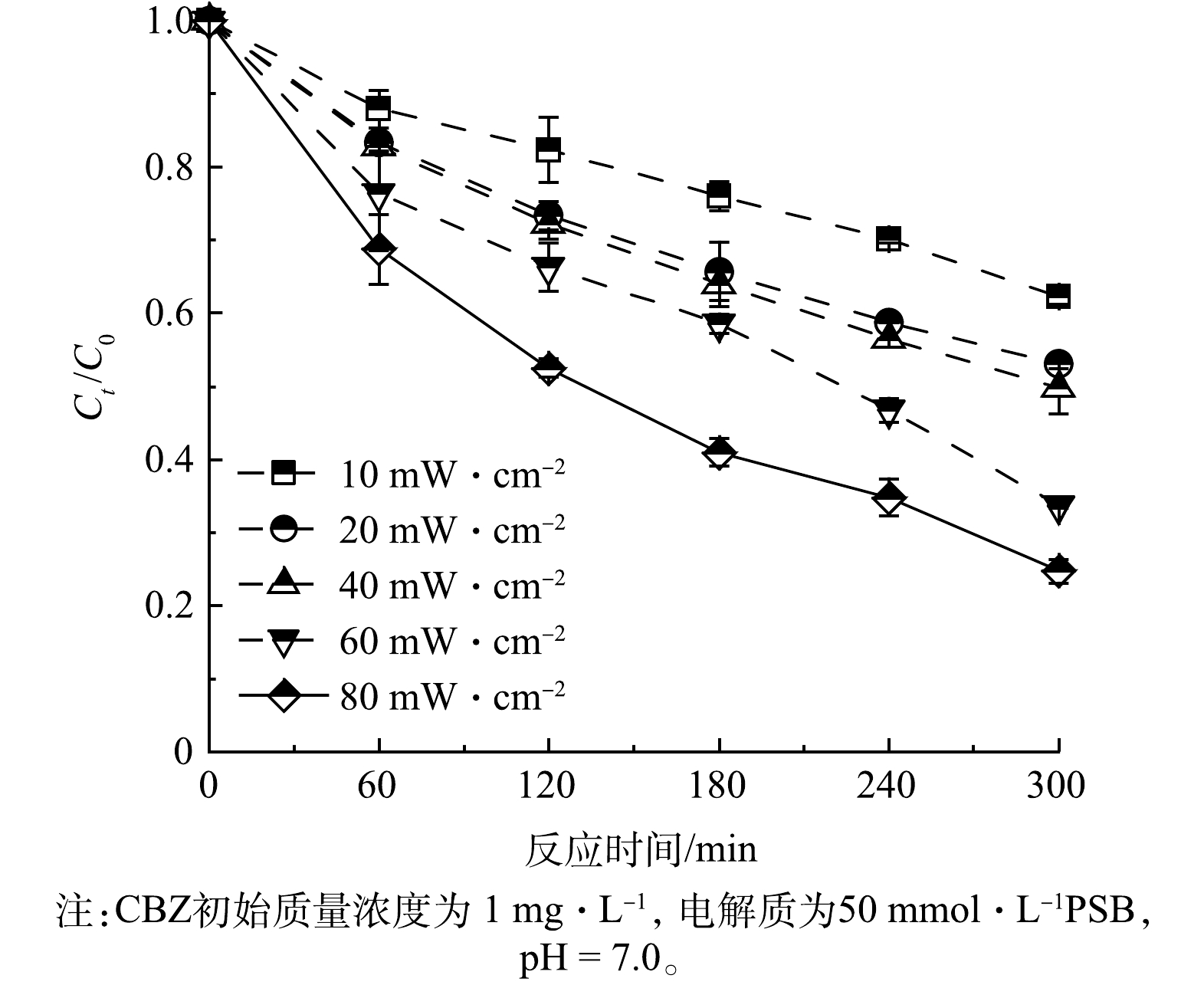
3)光照强度对PFC体系去除CBZ效果的影响。当光照强度为10、20、40、60和80 mW·cm−2时,PFC运行结果如图8所示,随着光照强度的逐渐增大,CBZ的去除率也在不断增加。产生这种结果的原因是由于光电催化反应是一种界面反应,随着光照强度的增大,照射到阳极催化剂表面的光子数量就会增多,激发得到的光电流密度也随之增大,同时促进了光生电子对的分离,激发出更多的光生空穴,进而产生更多的ROS促进CBZ的去除[33]。此外,光照强度的增大也会使PFC体系整体的温度上升,加速CBZ和ROS的布朗运动,增加两者的碰撞、反应概率,从而加速了CBZ的去除。
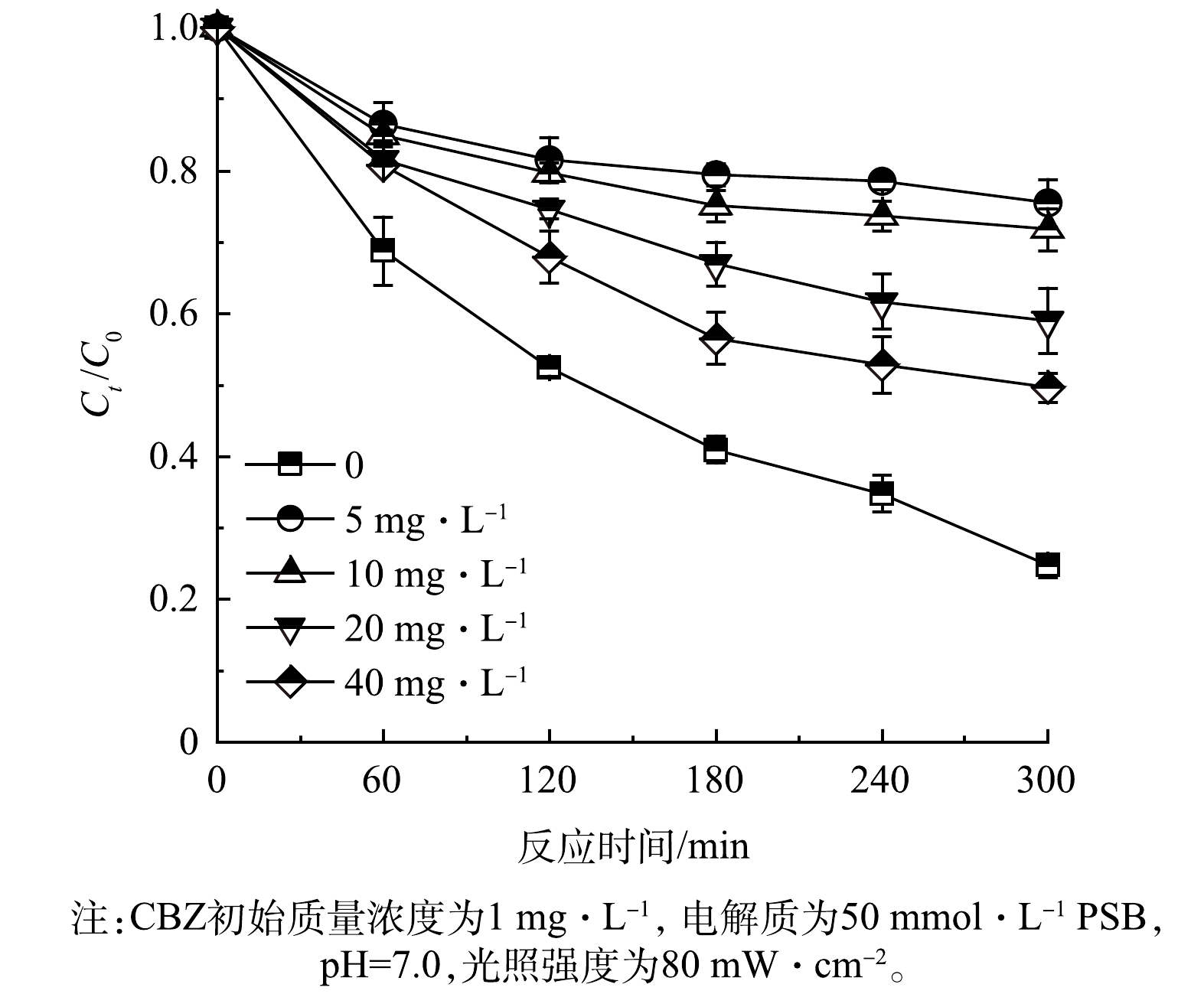
4)腐殖酸的初始质量浓度对PFC体系去除CBZ效果的影响。为了模拟自然水体中天然有机物存在条件下,PFC系统对CBZ的去除效果,本文通过添加不同质量浓度(0、5、10、20和40 mg·L−1)的腐殖酸(HA),考察其对TCNC-Pt/C系统去除CBZ的影响。实验结果如图9所示,在上述HA浓度下,CBZ去除率分别为75.3%、24.6%、28.2%、41.0%和50.4%。整体上,腐殖酸作为大分子有机质会和CBZ竞争活性位点和ROS,因此HA的存在对CBZ的去除起抑制作用[34];然而,随着腐殖酸质量浓度的增加,CBZ的去除效率有所提升,这主要归因于HA是水生环境中常见的光敏有机物,容易被激发产生活性中间体,如·O2−和·OH,从而促进有机污染物的光降解过程[35]。
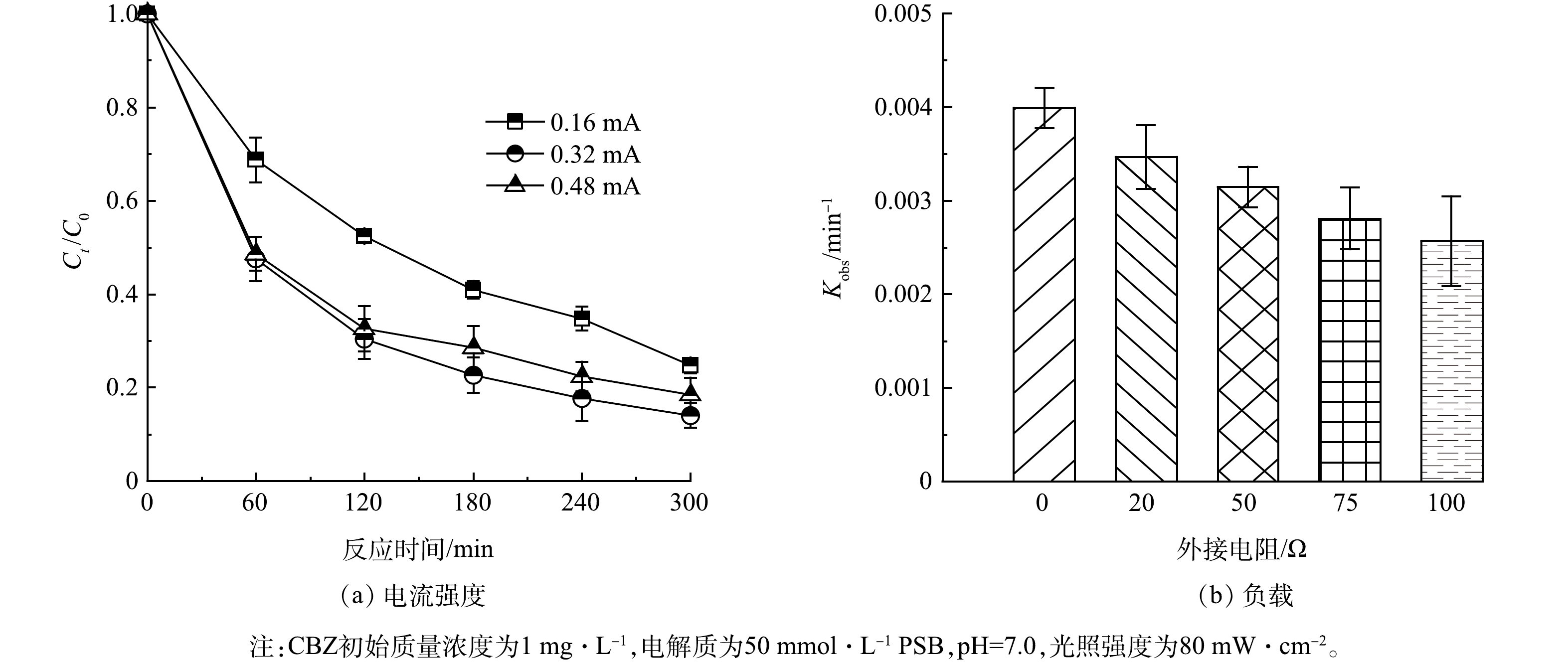
5)电流强度和负载对CBZ去除效果的影响。在可见光激发下,TCNC-Pt/C系统可产生0.16 mA的电流,当强制将体系电流提升一倍时,CBZ的去除效率升至85.9%(图10(a)),因为随着体系电流的增加,会加速阳极的电子向阴极转移,进一步抑制光生空穴-电子复合,从而促进CBZ的去除[36]。但当电流提升2倍时,CBZ去除效率仅为81.9%,CBZ去除率出现降低的情况,这可能由于过高偏置电位下空间电荷层的重新分配导致光生载流子的减少[37]。此外,负载也会对PFC系统造成影响,实验结果如图10(b)所示,当负载电阻由0 增加到100 Ω时,CBZ去除速率由0.004 0 min−1降到0.002 6 min−1,上述结果表明PFC的CBZ去除效能依赖于阳极和阴极之间的电荷转移,并受到体系负载的影响。
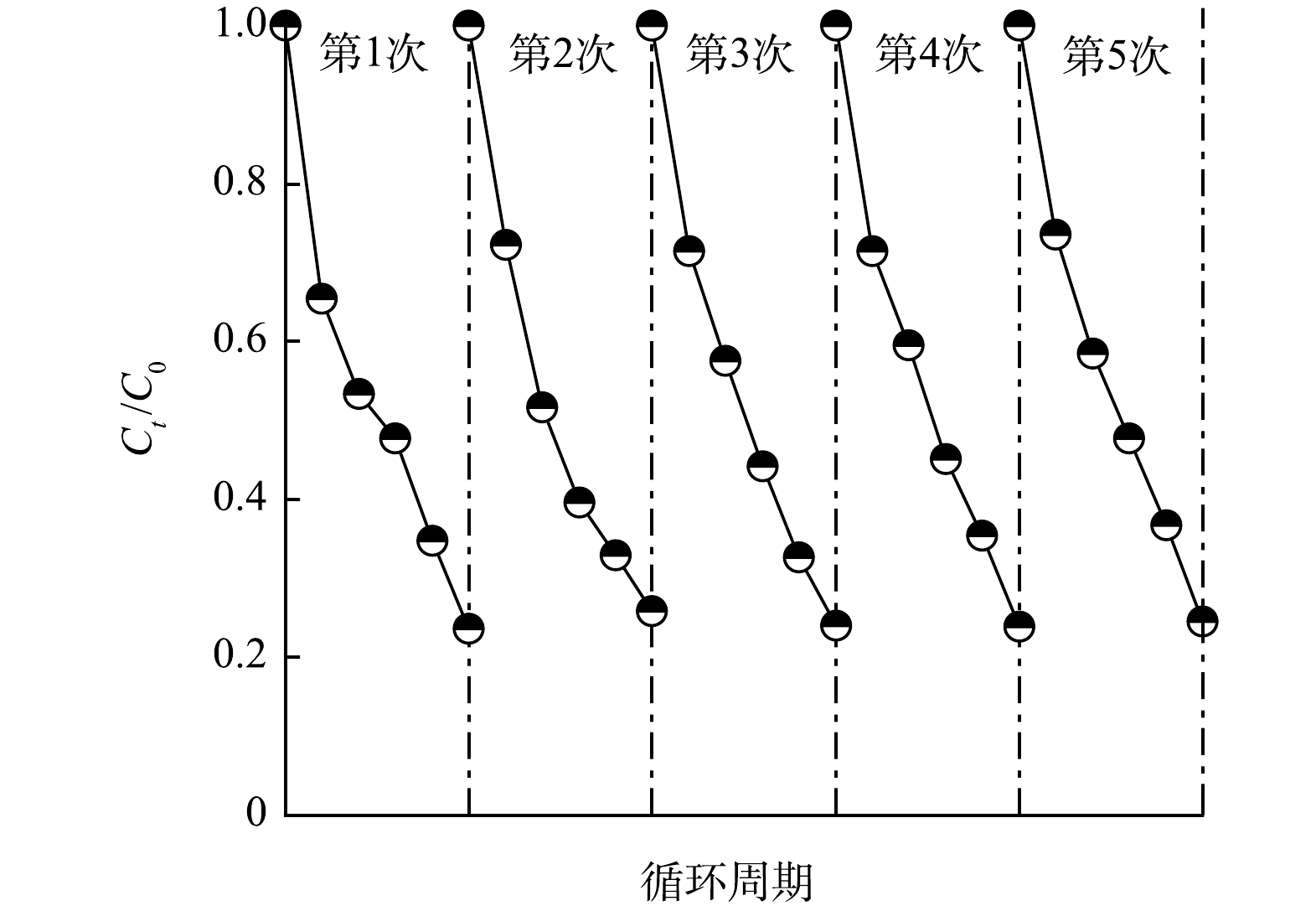
6)TCNC - Pt/C系统循环稳定性实验。为了评估TCNC - Pt/C系统的实际应用前景,对该系统进行循环稳定性测试,结果如图11所示。经过5次循环周期,TCNC - Pt/C系统对于CBZ的去除效率从第1次的76.4%降到第5次的75.5%,仍然具有良好的去除效果。
2.5 活性氧物种的鉴定
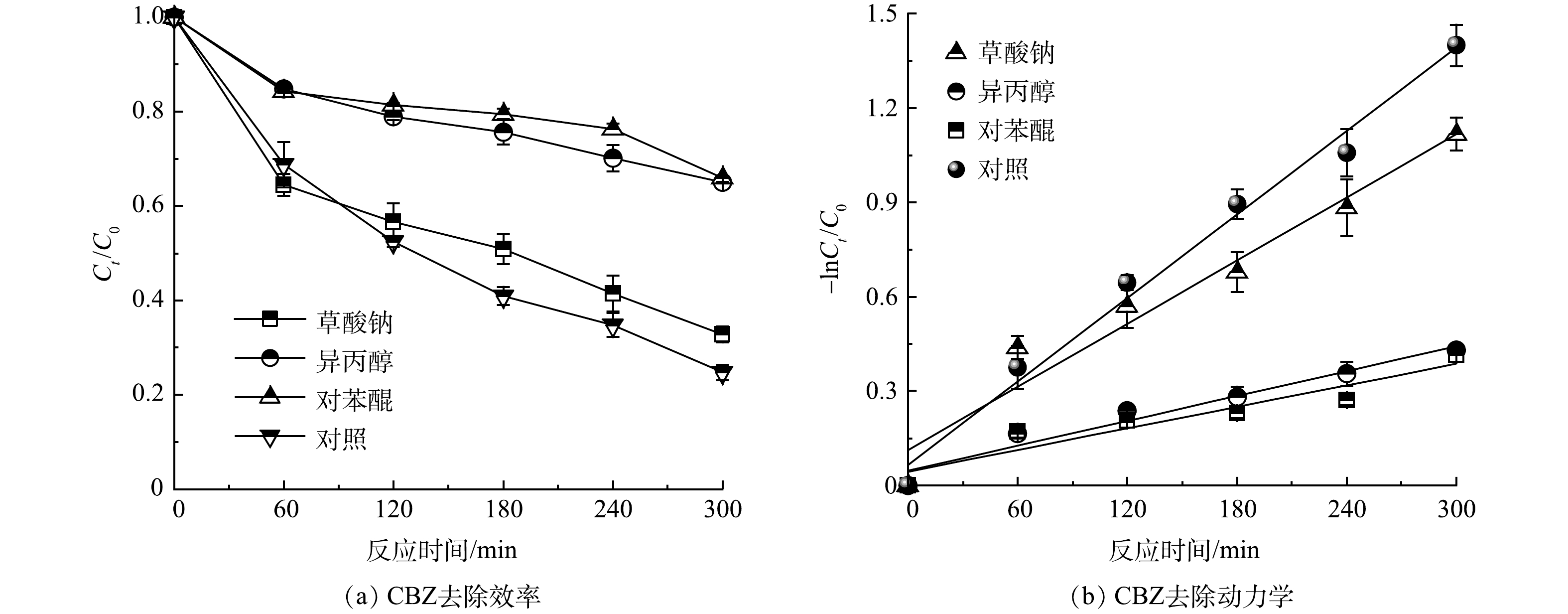
如图12所示,加入10 mmol·L−1的异丙醇、对苯醌和草酸钠分别对·OH、·O2−和h+进行淬灭时,CBZ的去除效率由75.3%分别下降到35%、34%和67.3%,Kobs从0.004 4 min−1(R2 = 0.985)分别下降至0.001 3 min−1(R2 = 0.959)、0.001 1 min−1(R2 = 0.873)和0.003 3 min−1(R2 = 0.951)。因此·O2−、·OH和h+对于CBZ降解的贡献度分别为:54.8%、53.5%和10.6%。这是因为TCNC阳极产生的·O2−和·OH起主要作用,与SU[19]结果一致。
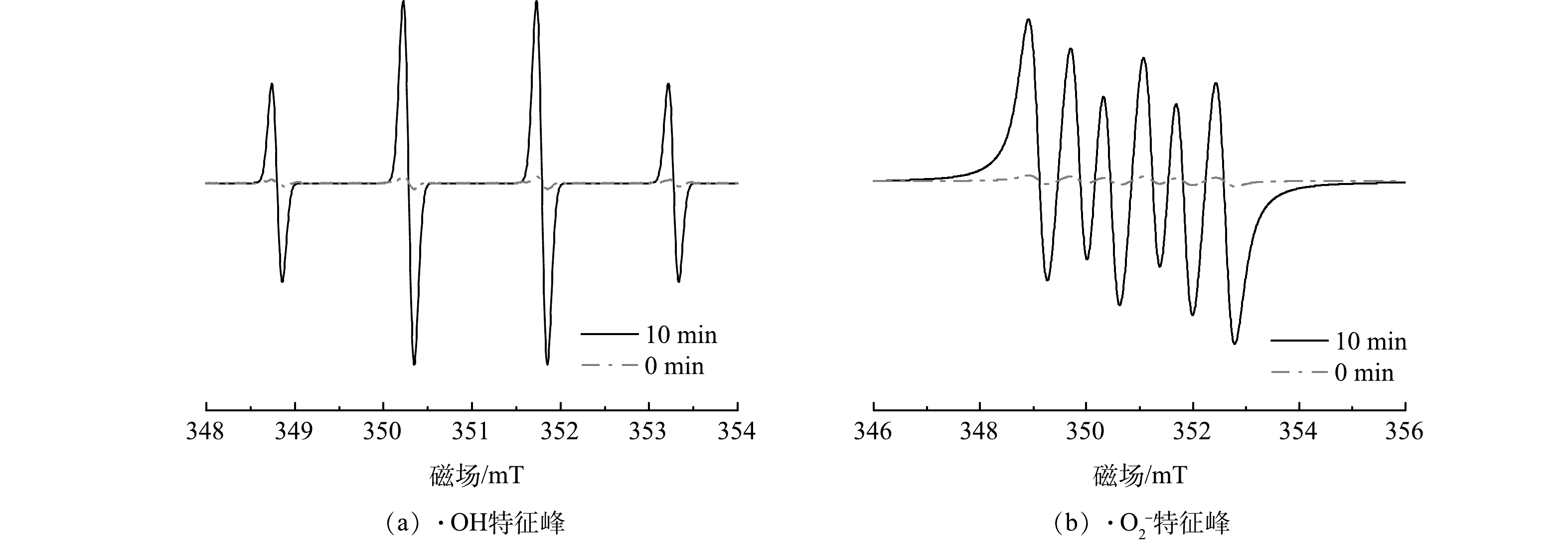
为了验证TCNC - Pt/C系统在降解CBZ过程中产生的·O2−和·OH,利用电子顺磁共振光谱仪(EPR)检测反应10 min前后的ROS产生情况。在图13中可以清晰地观察到峰强比例为1 : 2 : 2 : 1的DMPO - ·OH特征峰和1 : 1 : 1 : 1的DMPO - ·O2− 特征峰,证明TCNC-Pt/C系统中存在羟基自由基和超氧自由基。
2.6 CBZ降解机理研究
利用UPLC-Q-Orbitrap HRMS检测CBZ在降解过程中的中间产物(P1-P20),其可能得降解路径如图14所示。CBZ在·O2−和·OH作用下,可发生脱氢、羟基化和酰胺基裂解等反应,形成六种化合物(P1-P6)。例如CBZ可被ROS攻击而断键生成P1或P2,或者CBZ的杂环羟基化形成P5和P6两种同分异构体。P1逐步氧化成P7 → P12,再被氧化成P18。主要鉴定的中间体是P3(10,11- 环氧卡马西平),它是CBZ降解过程中最常检测到的中间产物[38]。P3可以通过环收缩形成P9,而后进行酰胺基丢失过程形成P20。同时,P3也可以水解形成P4,或者通过杂环开环过程转化为P9,然后通过P9的亲电芳香取代的分子内环化得到P13。P13进一步发生乙酰基的裂解以形成P16,或者P13的醛基被氧化以形成P17。P16和P17都可以通过醛基和胺/羧基的裂解转化为P18。最后CBZ被降解成H2O、CO[39-40]等小分子物质。
2.7 PFC技术优势与展望
对于CBZ的常规处理技术,如物理吸附法,145 μg·L−1CBZ去除效率可达43% [41],然而该方法只能转移水中的CBZ,无法实现其转化和降解;若采用生物处理技术,在10 d内仅能使1 mg·L−1CBZ下降30%左右[42]。对于高级氧化法,常规类芬顿法需要投加3.4 g·L−1的H2O2和1.0 g·L−1Fe3O4才能实现CBZ的去除[43]。在提供1.8 mA·cm−2电流密度的条件下,利用电芬顿技术能去除水中66%的CBZ(12 mg·L−1)[44]。而在本研究中,采用Pt/C作为功能性阴极的PFC系统,在无需投入额外电能和大量药剂的情况下,仅需提供电解质即可实现75.3%的CBZ去除效率,在电能消耗、药剂投加和二次污染产生等方面具有优势。
然而,本研究提供的TCNC - Pt/C系统,在CBZ去除过程中仅能产生0.16 mA的电流,功率密度仅为1.5 μW·cm−2。考虑到高电流密度对CBZ去除的促进作用,因此可进一步针对光阳极和阴极材料、反应器结构、运行参数等方面进行优化,以提升PFC系统的电流密度、电能产率以及CBZ去除效率。其次,PFC对实际废水中CBZ等PPCPs类污染物的去除效率和关键性影响因素也需进一步深入研究,以实现PFC在污水处理和生态修复中的推广应用。
3. 结论
1)选择Pt/C为功能性阴极的PFC系统,反应300 min后,1.0 mg·L−1的CBZ去除效率达75.3%,优于其他系统和功能性阴极。
2)通过功率密度曲线、LSV和EIS分析,证实了以TCNC为阳极,Pt/C为阴极,具有良好的光电催化性能和电能回收潜力,最大功率密度为1.5 μW·cm−2。
3)获得TCNC - Pt/C系统的最佳操作条件为50 mmol·L−1的PBS电解质和80 mW·cm−2的光照强度。在TCNC - Pt/C系统中,腐殖酸会与CBZ竞争ROS,降低CBZ的去除效能,但同时腐殖酸的光敏特性可能对去除CBZ有一定的促进作用。强制提升电流强度在一定程度上可以促进CBZ的去除,但作用并不明显;且负载会降低CBZ的去除效率。重复批次实验表明TCNC - Pt/C系统具有良好的稳定性,可以实现CBZ的持续去除。
4) TCNC - Pt/C体系在降解CBZ的过程中起主要作用的ROS是·O2−和·OH,贡献率分别达54.8%和53.5%。ROS主要通过脱氢、羟基化和酰胺基裂解等反应对CBZ进行降解,明确了CBZ的主要降解路径。
-
表 1 燃料N转化率及其可能的影响因素
Table 1. fuel-N conversion and possible influencing factors
焚烧厂编号 工况 垃圾特性参数 燃料N转化率η/% T/℃ EA M/% V/% FC/% R/% N/% H/N O/N A 1 049.0 3.09 50.99 42.09 6.52 13.42 0.19 6.77 37.03 6.31 B 975.8 1.96 50.21 39.21 6.55 14.32 0.55 4.65 19.51 3.48 B 1 006.0 2.49 50.21 39.21 6.55 14.32 0.55 4.65 19.51 4.16 B 1 036.9 2.45 50.21 39.21 6.55 14.32 0.55 4.65 19.51 4.35 C 986.2 2.59 52.1 41.49 6.49 13.53 0.42 6.57 30.02 6.98 D 1 116.6 1.25 54.03 40.84 7.19 14.97 0.53 5.36 25.79 6.14 D 1 087.7 1.25 54.03 40.84 7.19 14.97 0.53 5.36 25.79 5.66 E 1 015.0 1.48 54.07 33.71 6.64 16.47 0.53 4.43 23.42 7.09 F 1 016.8 1.22 55.32 43.90 6.65 13.15 0.87 2.84 12.1 4.14 G 972.6 1.67 55.03 39.21 10.50 21.12 1.46 1.16 6.21 1.87 G 1 005.2 1.72 55.03 39.21 10.50 21.12 1.46 1.16 6.21 1.94 G 996.9 1.93 55.03 39.21 10.50 21.12 1.46 1.16 6.21 2.22 H 1 040.0 1.58 42.06 35.80 6.68 15.72 1.35 3.01 10.37 5.01 H 972.0 1.51 42.06 35.80 6.68 15.72 1.35 3.01 10.37 4.53 注:垃圾特性参数分别为含水率(M)、挥发分含量(V)、固定碳含量(FC)、固定碳占可燃分的百分比(R)、N元素含量(N)、H元素与N元素的质量比(H/N)、O元素与N元素的质量比(O/N)。 表 2 10个参数Spearman秩相关系数的显著性水平
Table 2. 10 Correlation significance analysis of variables
T EA M V FC R N H/N O/N η T 0.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 EA 0.39 0.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 M 0.98 0.21 0.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 V 0.21 0.77 0.20 0.00 1.00 0.30 1.00 1.00 1.00 1.00 FC 0.83 0.04 0.07 0.42 0.00 0.02 0.18 0.20 0.25 1.00 R 0.29 0.39 0.41 0.01 0.00 0.00 0.36 0.30 0.30 1.00 N 0.07 0.50 0.44 0.12 0.00 0.01 0.00 0.00 0.00 0.01 H/N 0.09 0.26 0.13 0.11 0.01 0.01 0.00 0.00 0.00 0.09 O/N 0.06 0.52 0.43 0.08 0.01 0.01 0.00 0.00 0.00 0.01 η 0.08 0.95 0.28 0.74 0.05 0.19 0.00 0.00 0.00 0.00 序号 反应方程式 反应速率方程 动力学参数 1 HCN+O2→CNO R1=k1CO2CHCN k1 = 2.14×105exp(−10000T) 2 CNO + 0.5O2→NO + CO R2=k1CO2CHCN×(11+k5CNO) — 3 NH3+1.25O2→NO + 1.5H2O R3=k3CO2CNH3 k3 = 5.07×1014exp(−38160T) 4 2NH3+1.5O2→N2+1.5H2O R4=k4CNH3CO2ka+CO2 k4 = 2.89×106exp(−10000T)ka=0.054 5 CNO + NO→N2+CO+0.5O2 R5=k1CO2CHCN×(k51+k5CNO) k5 = 1.02×109exp(−25460T) 6 NO+NH3+0.25O2→N2+1.5H2O R6=k6C0.5NOC0.5NH3C0.5O20.5 k6 = 1.1×1012exp(−27680T) 7 NO + CO→0.5N2+CO2 R7=k7×(kbCNO(kcCCO+kd)kbCNO+kcCCO+kd) k7=1.952×1010exp(−19000T)kb=0.186,kc=0.00786,kd=0.00253 8 NO + C→0.5N2+CO R8=k8CNONcharπd2char k8 = 5.85×107exp(−12000T) 9 4NO+CH4→2N2+2H2O+CO2 R9=k9CCH4CNO k9 = 2.7×106exp(−9466T) 表 4 H/N元素比与热解产物对应表
Table 4. Correspondence table of H/N weight ratio and pyrolysis product
% 表 5 典型城市中垃圾各组分的质量分数
Table 5. The composition of municipal solid waste in several typical cities
% 城市 厨余垃圾 纸类 橡塑类 纺织类 木竹类 一线城市A1 61.11 9.46 19.95 2.80 1.48 一线城市A2 52.42 8.95 16.77 8.43 1.98 二线城市B1 52.41 9.17 17.06 3.38 2.47 二线城市B2 64.48 6.71 10.12 1.22 0.05 -
[1] LU J W, ZHANG S, HAI J, et al. Status and perspectives of municipal solid waste incineration in China: A comparison with developed regions[J]. Waste Management, 2017, 69: 170-186. doi: 10.1016/j.wasman.2017.04.014 [2] XU C, HONG J, CHEN J, et al. Is biomass energy really clean? An environmental life-cycle perspective on biomass-based electricity generation in China[J]. Journal of Cleaner Production, 2016, 133: 767-776. doi: 10.1016/j.jclepro.2016.05.181 [3] 方熙娟. SNCR-SCR脱硝技术在500t/d垃圾焚烧炉的应用研究 [D]. 北京: 清华大学, 2015. [4] JENSEN A, JOHNSSON J E, ANDRIES J, et al. Formation and reduction of NOx in pressurized fluidized bed combustion of coal[J]. Fuel, 1995, 74(11): 1555-1569. doi: 10.1016/0016-2361(95)00155-X [5] DESROCHES-DUCARNE E, DOLIGNIER J C, MARTY E, et al. Modelling of gaseous pollutants emissions in circulating fluidized bed combustion of municipal refuse[J]. Fuel, 1998, 77(13): 1399-1410. doi: 10.1016/S0016-2361(98)00060-X [6] LIU X, LUO Z, YU C, et al. Conversion mechanism of fuel-N during pyrolysis of biomass wastes[J]. Fuel, 2019, 246: 42-50. doi: 10.1016/j.fuel.2019.02.042 [7] RIAZA J, MASON P, JONES J M, et al. High temperature volatile yield and nitrogen partitioning during pyrolysis of coal and biomass fuels[J]. Fuel, 2019, 248: 215-220. doi: 10.1016/j.fuel.2019.03.075 [8] SUKSANKRAISORN K, PATUMSAWAD S, VALLIKUL P, et al. Co-combustion of municipal solid waste and Thai lignite in a fluidized bed[J]. Energy Conversion and Management, 2004, 45(6): 947-962. doi: 10.1016/S0196-8904(03)00187-0 [9] HANSSON K M, SAMUELSSON J, TULLIN C, et al. Formation of HNCO, HCN, and NH3 from the pyrolysis of bark and nitrogen-containing model compounds[J]. Combustion and Flame, 2004, 137(3): 265-277. doi: 10.1016/j.combustflame.2004.01.005 [10] HANSSON K M, SAMUELSSON J, AMAND L E, et al. The temperature's influence on the selectivity between HNCO and HCN from pyrolysis of 2, 5-diketopiperazine and 2-pyridone[J]. Fuel, 2003, 82(18): 2163-2172. doi: 10.1016/S0016-2361(03)00206-0 [11] YAMAMOTO T, KUWAHARA T, NAKASO K, et al. Kinetic study of fuel NO formation from pyrrole type nitrogen[J]. Fuel, 2012, 93: 213-220. doi: 10.1016/j.fuel.2011.09.032 [12] CHYANG C S, WU K T, LIN C S. Emission of nitrogen oxides in a vortexing fluidized bed combustor[J]. Fuel, 2007, 86(1): 234-243. [13] CHAIKLANGMUANG S, JONES J M, POURKASHANIAN M, et al. Conversion of volatile-nitrogen and char-nitrogen to NO during combustion[J]. Fuel, 2002, 81(18): 2363-2369. doi: 10.1016/S0016-2361(02)00175-8 [14] ROGAUME T, JABOUILLE F, TORERO J L. Effect of excess air on grate combustion of solid wastes and on gaseous products[J]. International Journal of Thermal Sciences, 2008, 48(1): 165-173. [15] GLARBORG P. Fuel nitrogen conversion in solid fuel fired systems[J]. Progress in Energy and Combustion Science, 2003, 29(2): 89-113. doi: 10.1016/S0360-1285(02)00031-X [16] ROGAUME T, KOULIDIATI J, RICHARD F, et al. A model of the chemical pathways leading to NOx formation during combustion ofmixtures of cellulosic and plastic materials[J]. International Journal of Thermal Sciences, 2006, 45(4): 359-366. doi: 10.1016/j.ijthermalsci.2005.07.005 [17] DU H L, ZHANG M, ZHANG Y, et al. Characteristics of NO reduction by char layer in fixed-bed coal combustion[J]. Energy Sources, Part A:Recovery, Utilization, and Environmental Effects, 2017, 39(10): 963-970. doi: 10.1080/15567036.2016.1249810 [18] 张利田, 卜庆杰, 杨桂华, 等. 环境科学领域学术论文中常用数理统计方法的正确使用问题[J]. 环境科学学报, 2007,27(1): 171-173. doi: 10.3321/j.issn:0253-2468.2007.01.030 [19] LEICHTNAM J N. The behaviour of fuel-nitrogen during fast pyrolysis of polyamide at high temperature[J]. Journal of Analytical and Applied Pyrolysis, 2000, 55(2): 255-268. doi: 10.1016/S0165-2370(00)00075-9 [20] 詹昊, 张晓鸿, 阴秀丽, 等. 生物质热化学转化过程含N污染物形成研究[J]. 化学进展, 2016, 28(12): 1880-1890. doi: 10.7536/PC160438 [21] ZHANG G, ZHU C, GE Y, et al. Fluidized bed combustion in steam-rich atmospheres for high-nitrogen fuel: Nitrogen distribution in char and volatile and their contributions to NOx[J]. Fuel, 2016, 186: 204-214. doi: 10.1016/j.fuel.2016.08.071 [22] CHEN G, YANG R, CHENG Z, et al. Nitric oxide formation during corn straw/sewage sludge co-pyrolysis/gasification[J]. Journal of Cleaner Production, 2018, 197: 97-105. doi: 10.1016/j.jclepro.2018.06.073 [23] ZHANG H, ZHANG X, SHAO J, et al. Effect of temperature on the product characteristics and fuel-nitrogen evolution during chromium-tanned solid wastes pyrolysis polygeneration[J]. Journal of Cleaner Production, 2020, 254: 120020. doi: 10.1016/j.jclepro.2020.120020 [24] CHEN H, WANG Y, XU G, et al. Fuel-N evolution during the pyrolysis of industrial biomass wastes with high nitrogen content[J]. Energies, 2012, 5(12): 5418-5438. doi: 10.3390/en5125418 [25] HANSSONA K M, MANDA L E A, HABERMANNB A, et al. Pyrolysis of poly-L-leucine under combustion-like conditions[J]. Fuel, 2003, 82: 653-660. doi: 10.1016/S0016-2361(02)00357-5 [26] LU G, LIU H, ZHANG Q, et al. Nitrogen conversion during the homogeneous and heterogeneous stages of sludge steam gasification: Synergistic effects of Fenton's reagent and CaO conditioner[J]. Fuel, 2019, 241: 1109-1116. doi: 10.1016/j.fuel.2018.12.109 [27] ZHAN H, ZHUANG X, SONG Y, et al. Formation and regulatory mechanisms of N-containing gaseous pollutants during stage-pyrolysis of agricultural biowastes[J]. Journal of Cleaner Production, 2019, 236: 117706. doi: 10.1016/j.jclepro.2019.117706 [28] 孙洪春, 曲振平. 选择性催化氧化含氨废气为氮气的研究进展[J]. 科学通报, 2020, 65(26): 2835-2865. [29] LI H, HAN J, ZHANG N, et al. Effects of high-temperature char layer and pyrolysis gas on NOx reduction in a typical decoupling combustion coal-fired stove[J]. Journal of Thermal Science, 2019, 28(1): 40-50. doi: 10.1007/s11630-018-1022-3 [30] LIU H, MA X, LI L, et al. The catalytic pyrolysis of food waste by microwave heating[J]. Bioresource Technology, 2014, 166: 45-50. doi: 10.1016/j.biortech.2014.05.020 [31] 许崇涛, 曹阳, 武桐, 等. 城市生活垃圾焚烧过程中NOx的生成与控制研究进展[J]. 工业锅炉, 2014(4): 1-6. doi: 10.3969/j.issn.1004-8774.2014.04.001 [32] 刘富强, 朱兆华. 普通工业垃圾燃烧特性实验研究[J]. 环境科学与技术, 2008, 31(8): 113-116. doi: 10.3969/j.issn.1003-6504.2008.08.031 -






 下载:
下载: