-
钴白合金是铜钴矿深加工过程中的副产物,由于钴的存在,使得该合金具有良好的硬度及耐热性[1]。钴白合金的成分基本为钴、铜、铁,其他元素的含量极低[2]。我国可利用的钴矿石资源较少,大部分钴矿石依赖进口[3]。世界上最主要的钴资源是刚果(金)和赞比亚的铜钴矿,一般含钴品位为0.1%~0.5%,高品味的可达到2%~3%。但是,其副产物钴白合金中钴的含量可达10%左右;此外,在钴白合金中,还含有大量的铜、铁等元素,使其具有较高的回收价值[1-5]。
目前,钴白合金的回收处理工艺主要有火法、湿法和微生物浸出等[6]。火法处理的常规工艺为造渣熔炼-浸出工艺[7]。该工艺先通过向钴白合金中掺入碳酸钙等配料,之后再在高温下焙烧,以实现钴、铜与其他杂质金属的分离,最终通过硫酸酸浸得到钴和铜的浸出液。但是,火法处理的能耗较高,操作也相对复杂,而且对有价金属的回收不彻底[8-9]。湿法处理主要有常压氧化酸浸法[10]、加压氧化酸浸法[11]、机械活化-酸浸法[12]、电化学溶解法[13]。相比于火法处理,湿法处理能耗低,但是对于处理设备的要求较高,同时也会产生一定的环境污染。有研究结果表明,使用微生物浸出钴白合金可实现钴、铜的高效回收[14]。胡国宏等[15]使用A.f菌(氧化亚铁硫杆菌)进行钴白合金的浸出,钴和铜的浸出率分别可以达到了99.5%和99.0%,而且浸出率高、成本低。
本研究通过消解分析钴白合金中各种金属的含量,初步估计其资源化利用的价值;并通过梯度实验探究接触浸出和非接触浸出的最佳固液比,以选出最佳工艺的最佳处理条件;最终,通过接触浸出和非接触浸出实验结果的对比分析,探究这2种方法对钴白合金中钴和铜的浸出机理。
全文HTML
-
供试钴白合金来源于河南某有色冶炼厂。盐酸(HCl)、硝酸(HNO3)、高氯酸 (HClO4)、氢氟酸(HF)、硫磺(S)、黄铁矿(FeS2)、硫酸铵((NH4)2SO4)、磷酸二氢钾 (KH2PO4)、无水氯化钙 (CaCl2)、七水合硫酸镁(MgSO4·7H2O)、醋酸(HOAc)、盐酸羟胺(NH2OH·HCl)、双氧水(H2O2)、醋酸铵(NH4OAc)均为分析纯。
-
电感耦合等离子发射光谱仪(OPTIMA 8300,珀金埃尔默股份有限公司)用于测定溶液中金属元素的浓度;X射线衍射仪(VG MK II,英国VG公司)用于分析固体样品的晶体结构;扫描电子显微镜SEM(Quanta FEG 250,美国FEI公司)用于观察固体样品的微观形貌;电热恒温鼓风干燥箱(DHP-9032,上海一恒科学仪器有限公司);pH计(DELTA320,梅特勒-托利多仪器有限公司);恒温水浴振荡器(THZ-82,金坛市荣华仪器制造有限公司)用于培养混合菌液。
-
将钴白合金置于电热恒温鼓风干燥箱中105 ℃烘干至恒重,粉碎研磨,过100目筛筛分后备用。钴白合金的金属含量测定采用三酸消解法[1, 16],目标金属的赋存形态采用BCR连续萃取法[17-19]。
-
取若干容积为250 mL的锥形瓶,先分别加入85 mL无机盐培养基[20-23](2.0 g·L−1 (NH4)2SO4、1.0 g·L−1 KH2PO4、0.25 g·L−1 CaCl2、0.5 g·L−1 MgSO4·7H2O)、0.8 g硫磺、0.8 g黄铁矿;之后,接入At、Af、Lf菌液[24-25]各5 mL;透气膜封口后,置于恒温水浴振荡器中,在35 ℃条件下,以135 r·min−1振荡,培养至体系pH下降至0.8,便可用于生物淋滤实验。
-
将培养稳定的菌液高速离心后,使菌体和生物酸分离。向pH为0.8的生物酸中直接加入钴白合金样品,并置于恒温水浴振荡器中,在35 ℃、135 r·min−1的条件下反应24 h,此为非接触淋滤实验[26-28]。该浸出过程无细菌参与。设定淋滤的固液比(g∶mL)为1∶100、2∶100、3∶100、4∶100、5∶100、6∶100,每个梯度做3个平行实验。反应结束后,测定上清液中目标金属浓度。
按照上述最优淋滤效果对应的固液比,做非接触循环富集实验。浸出完成后,通过抽滤实现固液分离;向上清液中加入之前分离出的菌体,密封;之后,于恒温水浴震荡器中,培养条为35 ℃、135 r·min−1,培养至体系pH下降至0.8;再进行非接触淋滤实验。如此循环淋滤10次,之后测定每次淋滤后上清液中的目标金属浓度。
-
向pH已达到0.8的菌液中直接加入钴白合金,置于恒温水浴震荡器中,在35 ℃、135 r·min−1条件下培养,隔天取样测钴、铜的浸出浓度,此为接触淋滤[26-28]。在该过程中,生物酸和细菌同时参与浸出。设定淋滤的固液比(g∶mL)为1∶100、2∶100、3∶100、4∶100。每个梯度做3个平行实验。在反应的第1、3、5、7、9 d取上清液测定目标金属浓度。
1.1. 供试材料与试剂
1.2. 实验仪器与检测用途
1.3. 钴白合金的理化性质分析
1.4. 实验所用菌液的培养
1.5. 钴白合金的非接触淋滤实验
1.6. 钴白合金的接触淋滤实验
-
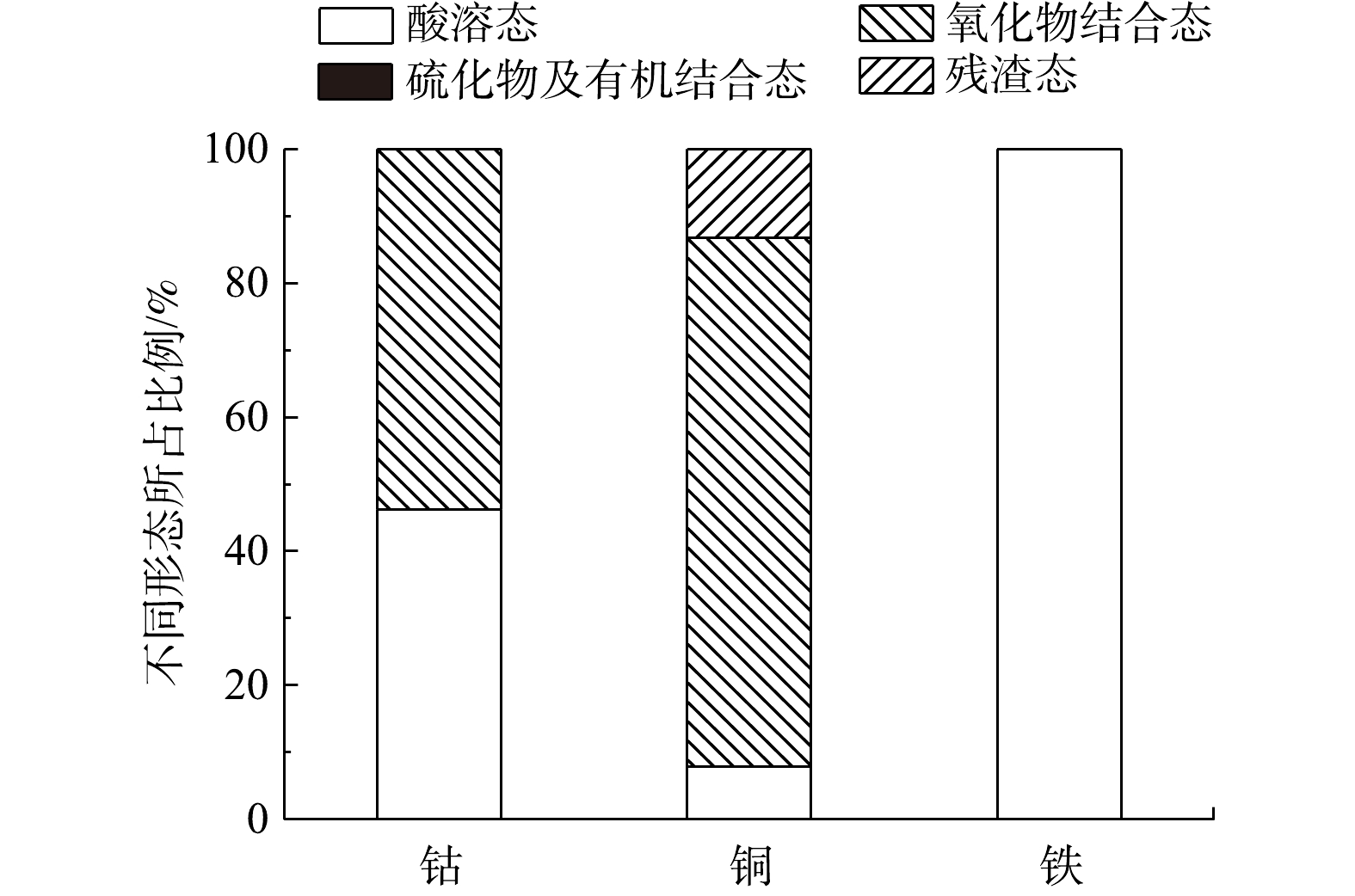
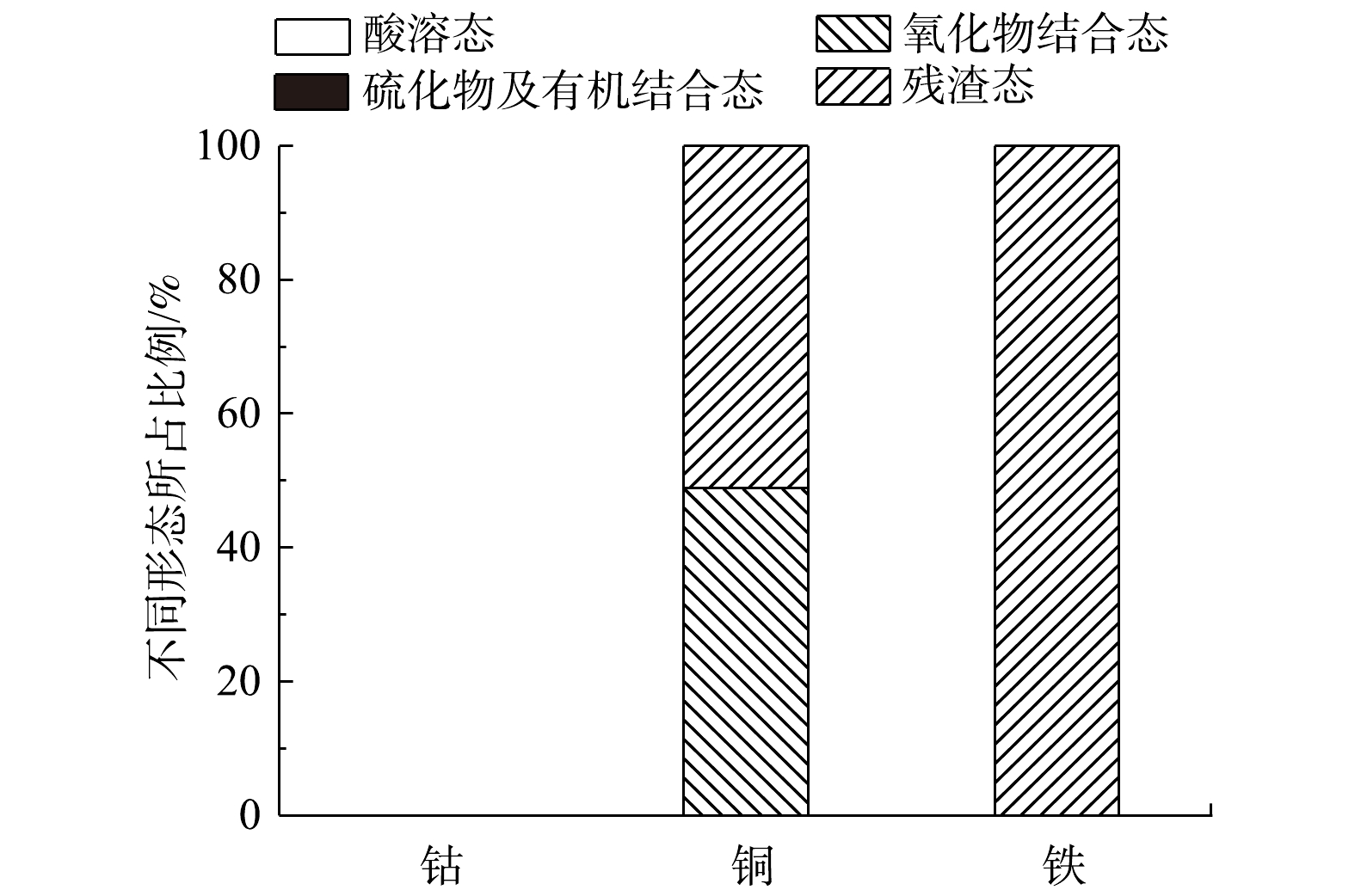
钴白合金样品中的金属种类及含量如表1中所示。其中,钴百合金中含铜量为10.81%、钴为13.66%、铁为20.53%,即有一定的回收价值。但该样品中铁含量过高,在浸出过程中会产生铁钒沉淀,会影响铜、钴的浸出效果。因此,为设计出更加合理的浸出工艺参数,使用BCR连续萃取技术处理钴白合金,以分析其中钴、铜、铁的金属赋存形态[18],所得结果如图1所示。如图1所示,钴白合金中的钴、铜、铁均不存在硫化物及有机结合态。其中,钴的存在形态为酸溶态(46.27%)和氧化物结合态(53.73%);铜的存在形态为酸溶态(7.86%)、氧化物结合态(78.84%)、残渣态(13.3%);铁的存在形态全部为酸溶态。通过BCR实验结果可知,在生物淋滤过程中,样品中的钴通过生物酸中氢离子的作用,基本上可以被完全浸出;铜的浸出除了生物酸的作用外,还需要一定的氧化反应,并可通过菌体的接触实现残渣态铜的浸出。
-
钴白合金样品的XRD谱图如图2所示。从图中可以看出,钴白合金在43°和45°有2个非常明显的峰值。通过与标准图谱卡片PDF#50-0795和PDF#85-1326[29]对比可知,钴白合金中所含的晶体成分主要为钴铁的合金态Co7Fe3和单质铜Cu。
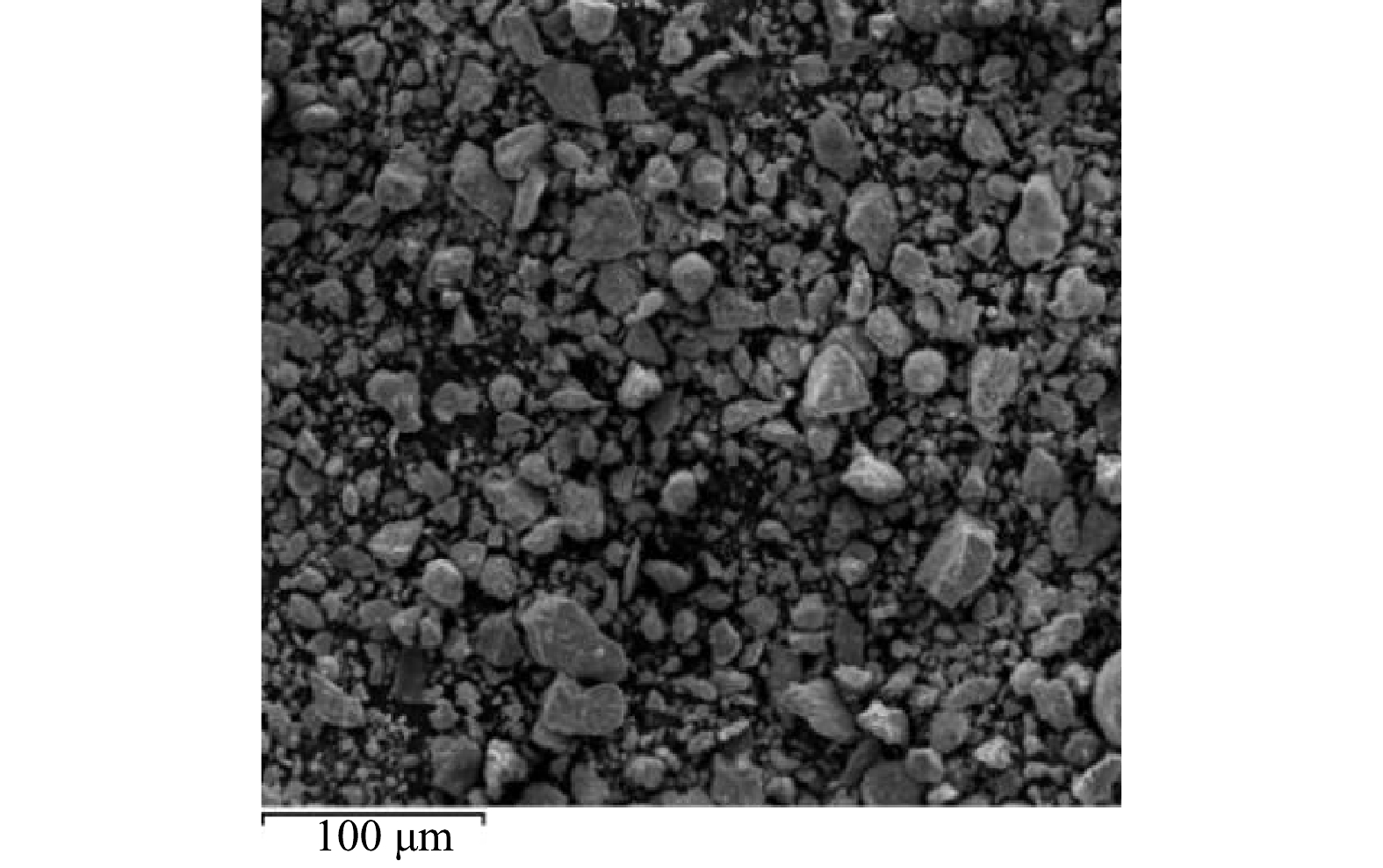
通过SEM能够直接观察钴白合金的形貌特征、颗粒尺寸,由图3可知,钴白合金的形状均为不规则的球体和长方体,粒度较小,分布均匀[30]。
-
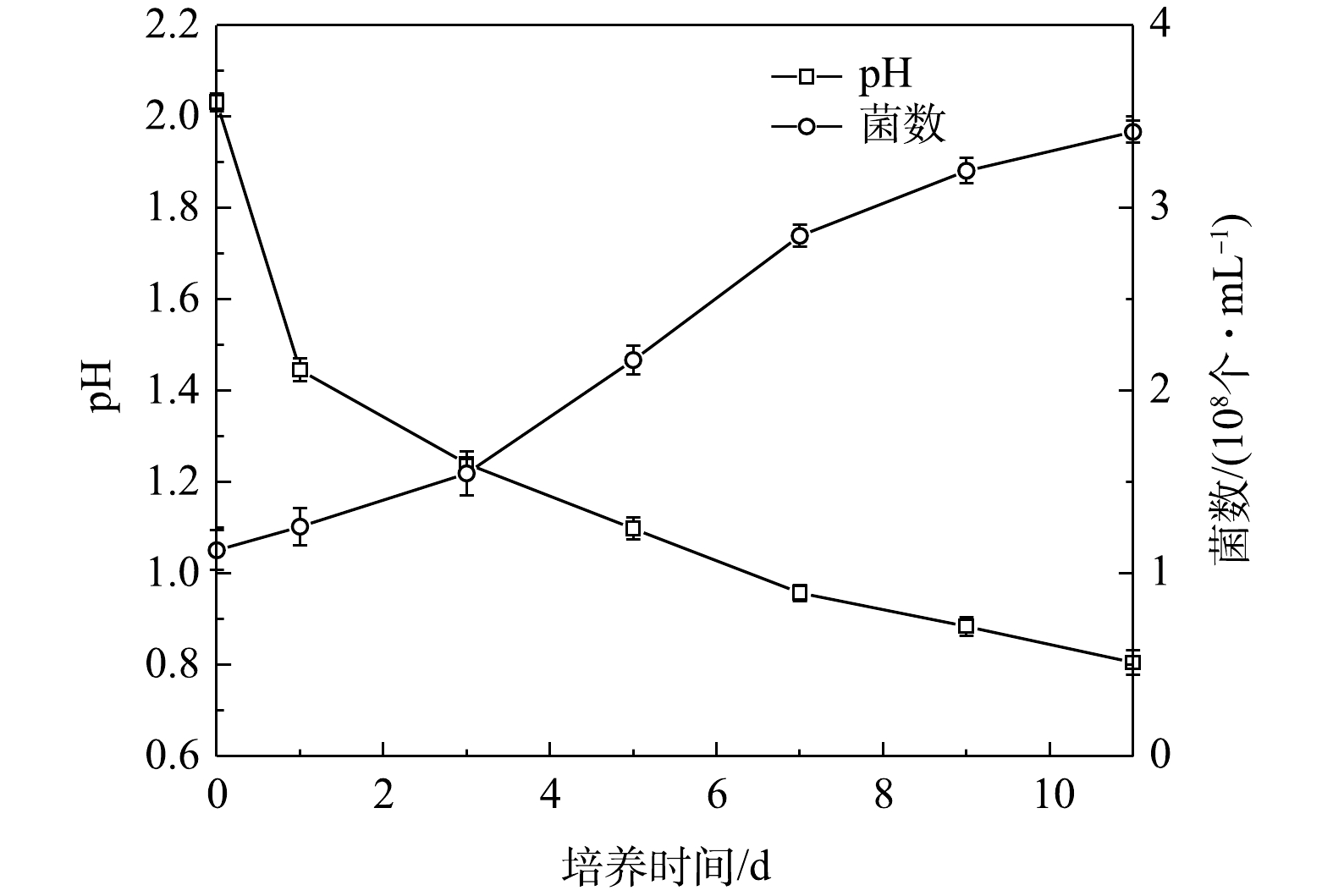
采用At、Af、Lf这3种菌株的混合培养体系,在35 ℃下连续培养,菌体数量在培养至11 d时增长至3.42×108 个·mL−1,菌液的pH从2.0降至0.8(图4)。这是因为,At菌将能源底物中的硫磺转化成了硫酸和供自身生长所需的能量[31]。直接向该菌液中加入钴白合金为接触浸出,该过程具有生物酸和菌体的共同作用;将菌液中的菌体通过高速离心去除,留下pH为0.8的生物酸浸出钴白合金为非接触浸出,该过程无菌体作用。
从图5中可以看出,随着培养时间的延长,菌液中的Fe3+质量浓度不断升高,Fe2+质量浓度极少。这是因为,Af、Lf氧化分解黄铁矿获取能量生长的过程中,伴随着氧化生成了Fe3+;同时,黄铁矿中分解出的硫被At菌转化成硫酸而进入体系中[32-34]。
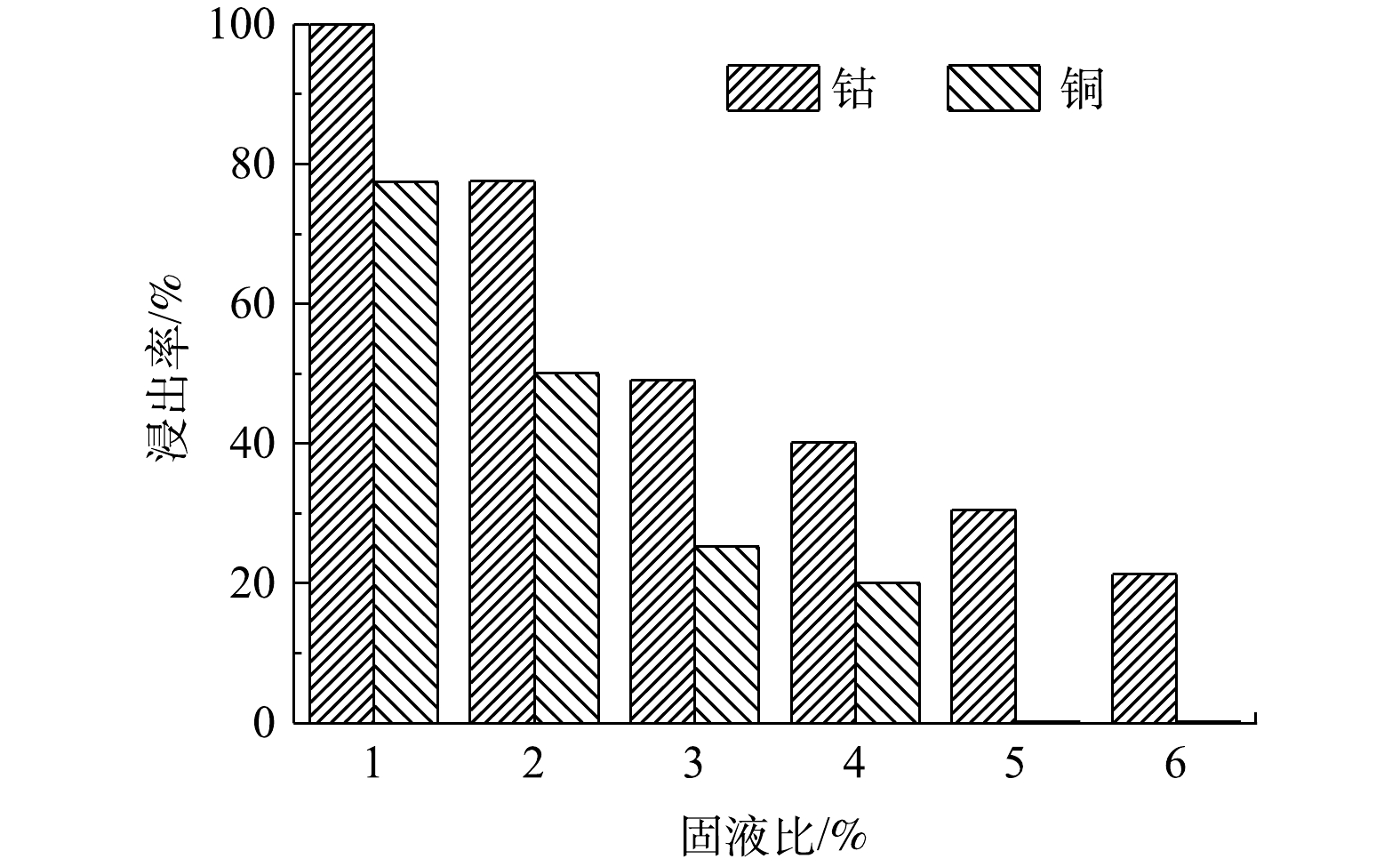
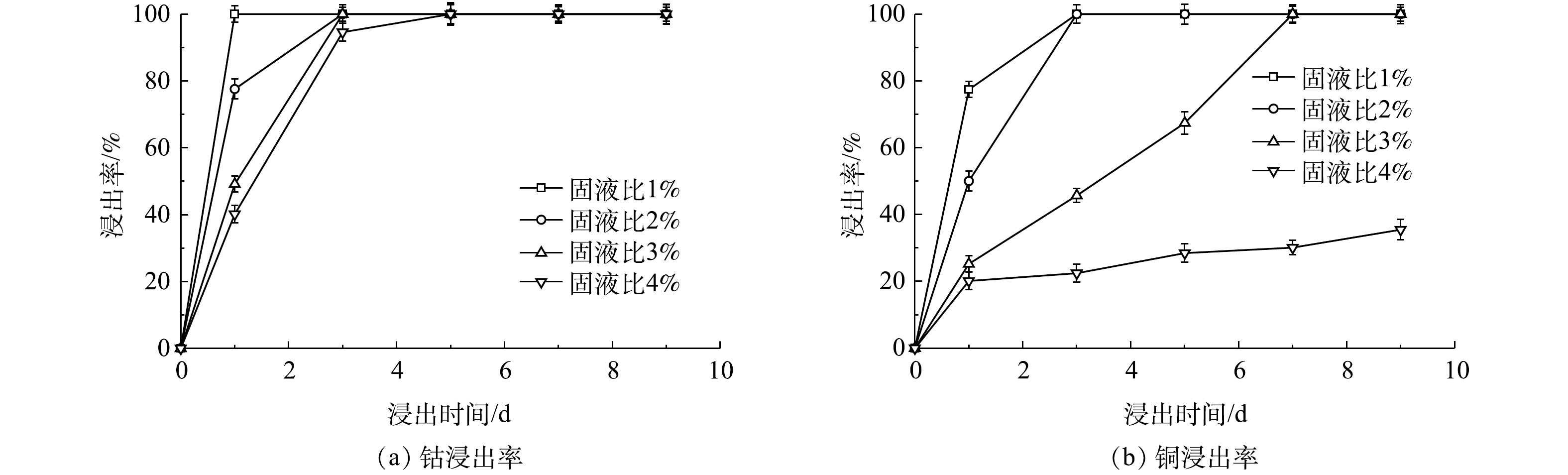
经非接触浸出1 d后,测得不同固液比下目标金属铜和钴的浸出率如图6所示。随着固液比的升高,钴和铜的浸出率均逐渐下降。从非接触浸出钴白合金的实验中可以看出,最优固液比为1%,在pH为0.8的生物酸下,钴白合金中的酸溶态和氧化物结合态的钴能够100%浸出,其浸出液质量浓度为1 356.14 mg·L−1;而铜的浸出率为77.42%,浸出液质量浓度为837.19 mg·L−1。
将1%固液比下的浸出渣多次水洗后,分析浸出渣中铜、钴、铁的赋存形态。由图7可以看出,浸出渣中未检测出钴;残留的铜中含有48.84%的氧化物结合态和51.15%的残渣态;铁被浸出后,又生成了铁钒沉淀进入了残渣态。因此,在浸出过程中,可先将样品中的钴优先浸出到溶液中;将铜留存在渣相中,富集后再次浸出,以实现钴白合金浸出过程中的铜、钴分离。
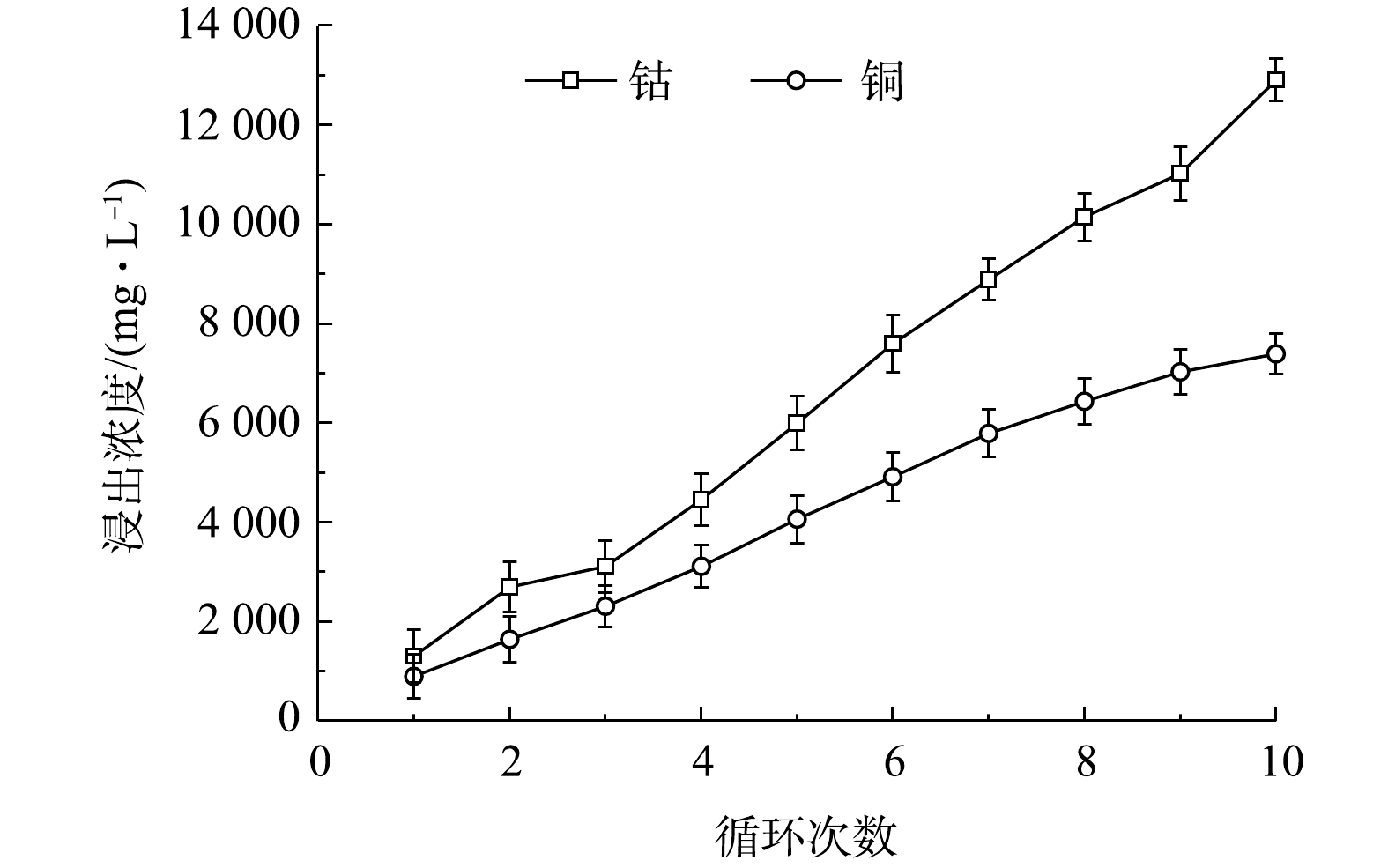
固液比1%下非接触循环第1次到第10次溶液中,铜和钴的质量浓度如图8所示。由图8可知,在循环的过程中,溶液中铜和钴的质量浓度基本呈倍数上升;而且,在实验过程中可以观察到,溶液的颜色有着明显的加深。循环到第10次时,溶液中钴的质量浓度可达12 877.25 mg·L−1,铜的质量浓度可达7 358.67 mg·L−1。
-
不同固液比下,接触浸出钴白合金中钴和铜的浸出率如图9所示。从不同固液比的接触浸出实验中可以看出,钴白合金中钴的浸出效果要明显优于铜的浸出效果。在接触浸出1 d,随着固液比的升高,铜和钴的浸出率逐渐下降。但浸出7 d,在固液比3%下的接触浸出过程中,钴白合金中的钴和铜依然能够完全浸出。
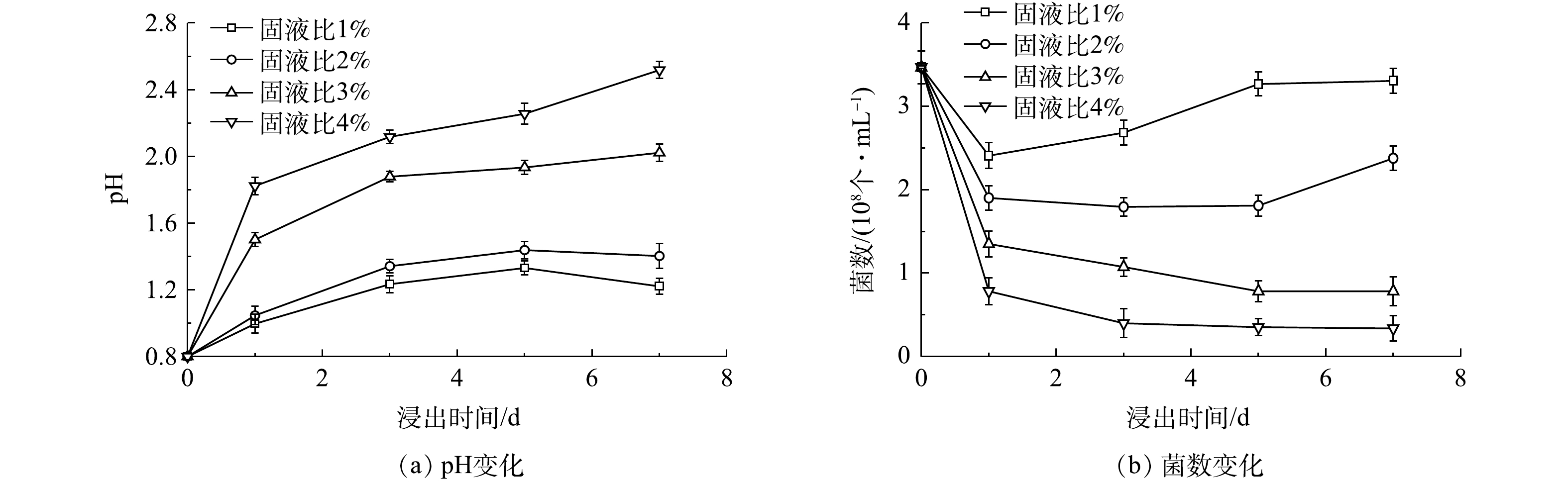
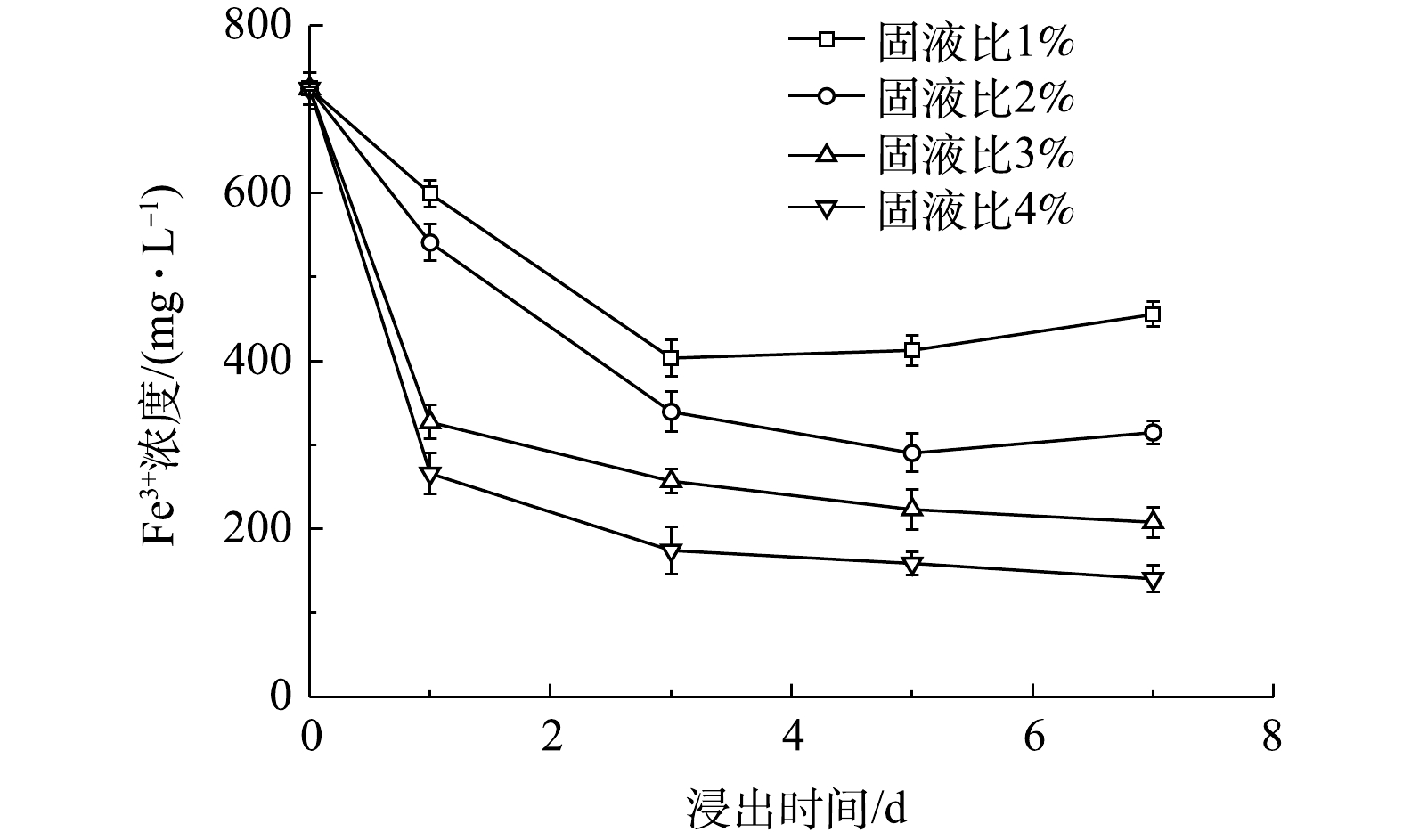
在非接触浸出实验中,在固液比1%下,仅生物酸作用,浸出反应1 d,钴100%浸出,铜浸出77.42%。这一结果与接触浸出实验中,在生物酸和细菌的双重作用下,浸出反应1 d的浸出效果基本一直。由于钴白合金的加入,抑制了菌体的生长,菌体数量降至2.41×108个·mL−1。但随着浸出时间的延长,菌体逐渐适应了周围的浸出环境,菌体逐渐生长,在浸出5 d时生长到了3.26×108个·mL−1,基本与接触浸出前的菌数一致(图10(b)),从而代谢出了新的生物酸(图10(a))。从图11接触浸出过程中Fe3+的浓度变化可以看出,随着浸出时间的延长,在固液比1%下,浸出3 d,Fe3+的质量浓度降到最低403.67 mg·L−1,铜达到100%浸出。3 d后,Fe3+的浓度逐渐上升。这是因为,铜的浸出需要Fe3+的参与;同时,在Af和Lf的作用下,将生成的Fe2+氧化生成Fe3+[35]。因此,提高接触浸出的固液比,并在足够的额浸出时间下,当铜的浸出所消耗Fe3+的速度与细菌氧化Fe2+成Fe3+的速度一致时,即浸出完全。
-
通过对比接触浸出和非接触浸出钴白合金的结果,并结合钴白合金中的金属赋存形态以及钴白合金淋滤前后的XRD图谱变化,可以推测出钴白合金中铜和钴的浸出机理。图12为钴白合金淋滤前后的XRD对比图,可见,淋滤前钴白合金XRD衍射图谱中有较为明显的吸收峰;淋滤后钴白合金XRD衍射图谱中几乎没有吸收峰,这表明钴铁合金和单质铜的结构被破坏[14]。此外,经生物淋滤处理后的钴白合金的硬度有着明显的下降,表面也更加疏松,均表明其结构发生了改变。
在接触浸出1 d后,对不同固液比下的浸出渣中的钴、铜做金属赋存形态(图13)进行了对比分析。由图13(a)可知,随着固液比的升高,钴的浸出率逐渐降低,浸出渣中残余的钴也越来越多。经与原样中钴的对比可以看出,酸溶态的钴被优先浸出到溶液中,而浸出渣中残留的钴均为氧化物结合态。随着浸出时间的延长,体系内的细菌逐渐适应了周围的生存环境,持续代谢出新的生物酸,从而将浸出渣中的钴浸出到溶液中[36]。
同样,由图13(b)可知,随着固液比的升高,铜的浸出率也在逐渐降低。与原样对比,酸溶态的铜也被率先浸出到溶液中,浸出渣中剩余的铜为氧化物结合态和残渣态,该形态的浸出需要Fe3+的参与,最终生成Cu2+和Fe2+进入溶液中[37]。而随着浸出时间的延长,溶液中的Fe2+被细菌又逐渐氧化成Fe3+,Fe3+继续与固相中的铜发生反应,直至Fe3+的生成速率大于其反应的消耗速率时,整个铜的浸出反应达到完全。
2.1. 钴白合金中的金属种类及其形态分析
2.2. 钴白合金的晶体成分及微观形貌分析
2.3. 非接触浸出钴白合金及循环富集
2.4. 接触浸出钴白合金
2.5. 钴白合金中铜、钴的浸出机理
-
1)钴白合金中所含有价金属主要为铜、钴和铁,而且含量均很高(均在10%以上),资源化利用潜力巨大。铜和钴大部分以酸溶态和氧化物结合态存在,适宜于生物淋滤处理。
2)非接触浸出钴白合金的最适固液比为1%,此时钴可100%浸出,铜浸出率为77.42%。非接触循环富集进行10次,最终浸出液中钴的质量浓度可达12 877.25 mg·L−1、铜的质量浓度可达7 358.67 mg·L−1。
3)钴白合金在接触浸出中,随着浸出时间的延长,铜和钴最终均能完全浸出。从浸出渣中钴和铜的赋存形态可以看出,钴浸出仅需生物酸的作用,铜的浸出除了需生物酸作用,还需Fe3+的参与。在菌体的直接作用下,浸出体系内部形成了Fe3+的生成和消耗的循环,以供给铜浸出。



 DownLoad:
DownLoad: