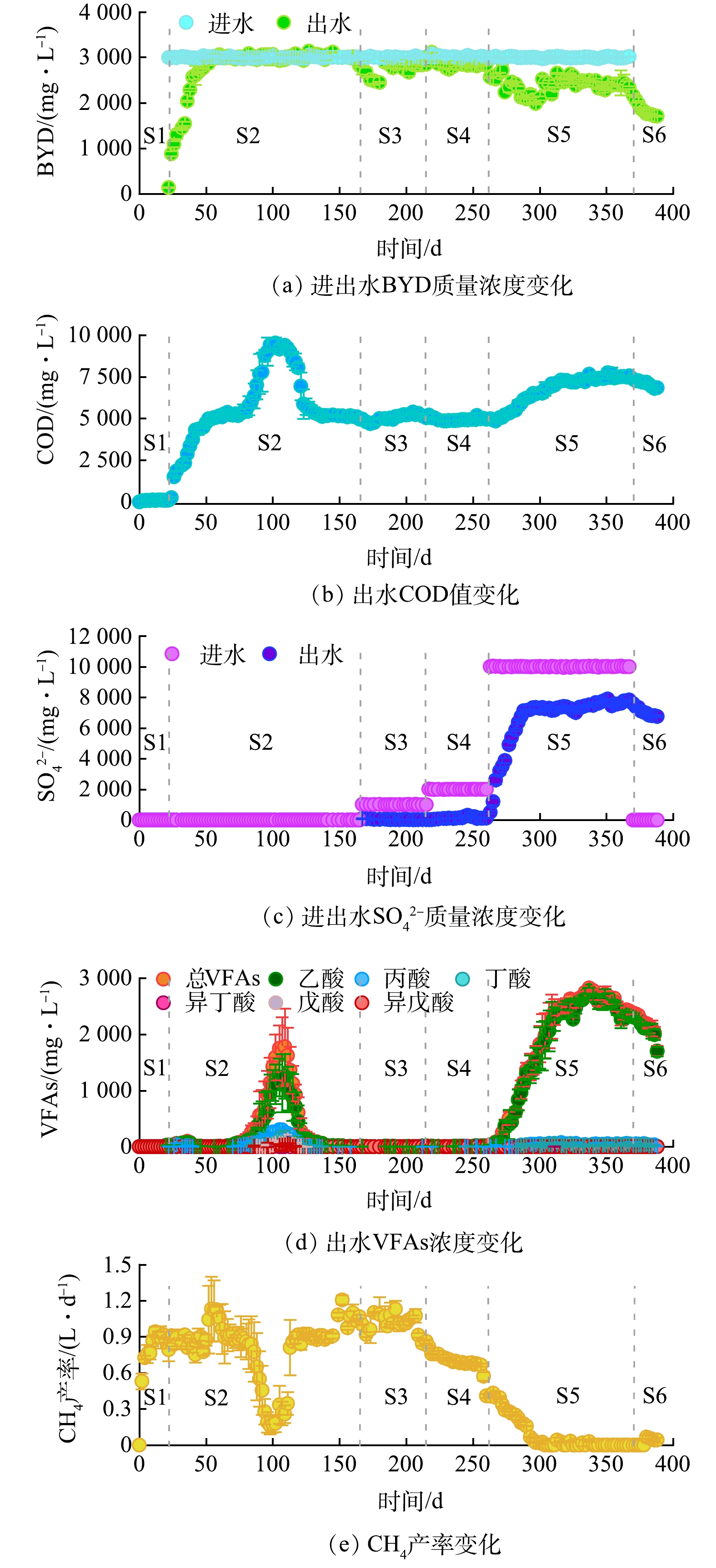
-
六价铬(Cr(Ⅵ))是一种对环境有持久性危害的重金属离子,主要来源于工业生产,可引起肺癌、导致皮肤敏感、造成基因缺陷等[1]。在众多的Cr(Ⅵ)处理方法中,光催化法是一种对环境友好的技术,可将剧毒的Cr(Ⅵ)还原成低毒性三价铬(Cr(Ⅲ))。硫化锌(ZnS)作为重要的直接宽带隙过渡金属硫化物半导体材料,具有光生载流子产生效率高,导带位置相对更负等优点,被广泛应用在光催化还原Cr(Ⅵ)[2-3]。但是,ZnS本身也存在诸多缺点,如带宽太大(块体约为3.7 eV),使其只能吸收占太阳光只有4%左右的紫外光[4]。此外,目前,用于光催化的ZnS粒径较大,导致其比表面积小以及活性位点不足,从而影响了其光催化的效果[5-6]。因此,改善ZnS的光催化能力对于去除Cr(Ⅵ)具有重要的意义。
掺杂可以在材料的晶格内部形成空位等缺陷能级,调节光催化剂的禁带宽度,促进光吸收,是提高光催化剂性能的有效方法[7]。Mn作为过渡金属元素,其自身能级4T1→6A1的跃迁能够在可见光下产生光电子,且具有多种氧化态,不仅可将吸收边带拓展至可见光范围,还能够促进光生载流子的有效分离[8-9]。此外,MnS具有与ZnS相似的晶体结构以及相近的晶格常数,使得Mn更容易掺杂进入ZnS晶格[10]。对于ZnS粒径较大产生的负面效应,则可将材料制备成纳米颗粒。当材料的粒径逐渐减小时:一方面,其比表面积增大,有利于材料吸附水体中的污染物;另一方面,材料表面暴露的原子开始增加,尤其对于量子点材料(3个维度的尺寸均在纳米级别),其表面暴露的原子比例很大,因此,量子点材料表面存在大量的悬挂键和缺陷。有研究[11]表明,缺陷的存在有利于促进光催化反应:缺陷的存在可以为光催化提供更多的活性位点;缺陷能够提高光生电子空穴对的分离效率,有利于光催化反应;表面缺陷会在靠近价带顶的位置形成浅能级,降低禁带宽度,导致光催化剂吸收边带红移[12]。因此,制备锰掺杂硫化锌量子点(ZnS∶Mn QDs)有望改善ZnS的光催化性能。
目前,虽然有大量光催化剂用于还原Cr(Ⅵ),但所用光源大部分为高耗能的汞灯与氙灯(120 W以上),这阻碍了他们在实际工程中的应用。LED灯相对于汞灯和氙灯,具有低能耗和高寿命的优点,故其更有实际应用价值。尽管有部分研究[13]使用LED灯激发ZnS光催化降解有机污染物,但使用蓝光LED灯激发ZnS光催化还原Cr(Ⅵ)的研究却鲜见报道。鉴于此,本研究采用共沉淀法制备了锰掺杂硫化锌量子点(ZnS∶Mn QDs),与纯ZnS相比,ZnS∶Mn QDs具有更窄的禁带宽度,吸收边带延伸至可见光区域,可在450 nm的LED蓝光灯下光催化还原Cr(Ⅵ)并且吸附产物Cr(Ⅲ),从而彻底去除溶液中的铬离子。分别考察了不同因素对光催化的影响,并对光催化机理进行了探讨。本研究可为利用低能耗LED灯光催化还原Cr(Ⅵ)提供参考。
全文HTML
-
七水硫酸锌(ZnSO4·7H2O),四水氯化锰(MnCl4·4H2O),九水硫化钠(Na2S·9H2O),重铬酸钾(K2Cr2O7),盐酸,氢氧化钠。所有使用的化学试剂纯度皆为分析纯,实验中用水为超纯水。
LED灯光源的规格为10 W、450 nm,购于深圳奇异果光电有限公司;透射电子显微镜(TEM,Talos F200X,FEI公司);X射线衍射仪(XRD,D/Max-2400,日本理学公司);紫外-可见分光光度计(UV-vis,UV-2450,日本岛津公司);稳态/瞬态荧光光谱仪(FLS1000型,英国爱丁堡公司);原子吸收光谱仪(AAS,Z-2000型,日本日立公司);紫外-可见分光光度计(TU-1900型,北京普析通用仪器有限公司)。
-
本研究采用共沉淀法制备ZnS∶Mn QDs。分别称取7.189 g ZnSO4·7H2O, x mg MnCl4·4H2O于150 mL圆底烧瓶中(x=0、19.79、59.37、98.95、158.32、217.69、296.85),加入80 mL超纯水并将圆底烧瓶置于磁力搅拌器中。在氮气氛围下,缓慢搅拌上述溶液20 min。称取6.004 5 g Na(SO4)2·9H2O并超声溶解在20 mL超纯水中。在氮气氛围下,将该溶液缓慢滴加到含Zn2+和Mn2+的混合液中,继续搅拌1 h。反应结束后,将白色沉淀物离心分离,水洗后离心(重复3遍),然后置于真空干燥箱60 ℃下干燥24 h。干燥后所得固体粉末呈略微粉红色,且Mn掺杂量越高,该颜色越明显。所得材料记为ZnS∶Mn(x%)(x%为n(Mn2+)/n(Zn2+)的百分数)。当加入的MnCl4·4H2O分别为0、19.79、59.37、98.95、158.32、217.69和296.85 mg时,材料记为ZnS∶Mn(0%)、ZnS∶Mn(1%)、ZnS∶Mn(3%)、ZnS∶Mn(5%)、ZnS∶Mn(8%)、ZnS∶Mn(11%)和ZnS∶Mn(15%)。
所得材料采用TEM、XRD、UV-vis和荧光光谱仪分别表征催化剂的形貌、晶体结构和光学特性。
-
先将1 000 mg·L−1的Cr(Ⅵ)储备液稀释至目标浓度。一定量的ZnS∶Mn QDs光催化剂加入50 mL去离子水并超声5 min。取50 mL Cr(Ⅵ)稀释液加入ZnS∶Mn QDs悬浮液,并用1 mol·L−1盐酸或氢氧化钠调节至一定pH。预先在黑暗条件下搅拌30 min使吸附达到平衡。打开LED灯的开关,调节LED灯距离液面上方5 cm,并在特定的时间取样,取样液经过0.22 μm滤膜过滤后置于5 mL离心管待测。总铬浓度采用火焰原子吸收光谱仪测定,Cr(Ⅵ)浓度采用二苯卡巴肼(DPC)方法测定[14],Cr(Ⅲ)浓度则为总铬浓度与Cr(Ⅵ)浓度之差。采用一级动力学方程(式(1))描述光催化还原Cr(Ⅵ)的过程。
式中:Ct为t时Cr(Ⅵ)的浓度,mg L−1;C0为Cr(Ⅵ)的初始浓度,mg L−1;k为反应动力学的速率,min−1;t为反应时间,min。
1.1. 实验试剂与仪器
1.2. ZnS∶Mn QDs的制备和表征
1.3. ZnS∶Mn QDs蓝光LED灯光催化还原Cr(Ⅵ)
-
1) TEM分析。采用TEM对所获得的ZnS∶Mn QDs的尺寸进行分析。如图1所示,ZnS∶Mn颗粒大小分布均匀,粒径在10 nm以下,这表明合成的催化剂属于量子点材料。图1(b)中箭头所指处为较完整的单个颗粒,可以观察到明显的晶格条纹。
2)晶体结构分析。ZnS∶Mn QDs的XRD表征结果如图2所示。所有样品的XRD数据均与PDF卡片05-0566吻合,说明合成的催化剂属于立方型闪锌矿结构(即β-ZnS),图2中所示的3个衍射峰分别归属于立方型闪锌矿的(111)、(220)、(311)晶面。纯ZnS样品和其他Mn掺杂样品所展示的峰型和位置一致,即Mn掺杂没有改变ZnS的晶体结构,也未导致晶格膨胀,说明ZnS对Mn具有较高的容纳性。Mn掺杂的样品在其他位置未出现杂峰,这表明合成的ZnS∶Mn QDs物相单一,纯度较高。此外,所有样品的衍射峰宽度比较大,这可能与材料结晶度较差和粒径较小有关。相对于高结晶度的催化剂,结晶度差的催化剂可能拥有更多的表面缺陷,从而可以改变材料的光催化性能[15]。
结合XRD图谱及Debye-Scherrer(德拜-谢乐)公式(式(2)),估算了所合成催化剂的晶粒大小。
式中:D为晶粒垂直于晶面方向的平均厚度,nm;K为Scherrer常数,K=0.9;λ为铜靶上激发的X射线波长,为0.154 2 nm;β为衍射峰半峰宽(弧度制,rad);θ为衍射角(弧度制,rad)。最终计算所得的ZnS∶Mn(0%)~ZnS∶Mn(15%)晶粒大小依次为2.8、2.7、2.7、3.1、2.7、2.6和2.5 nm,可见Mn掺杂浓度在0%~15%内对晶粒大小的影响并不大。结合SEM中对粒径的分析结果,可知在ZnS∶Mn QDs之间存在团聚。
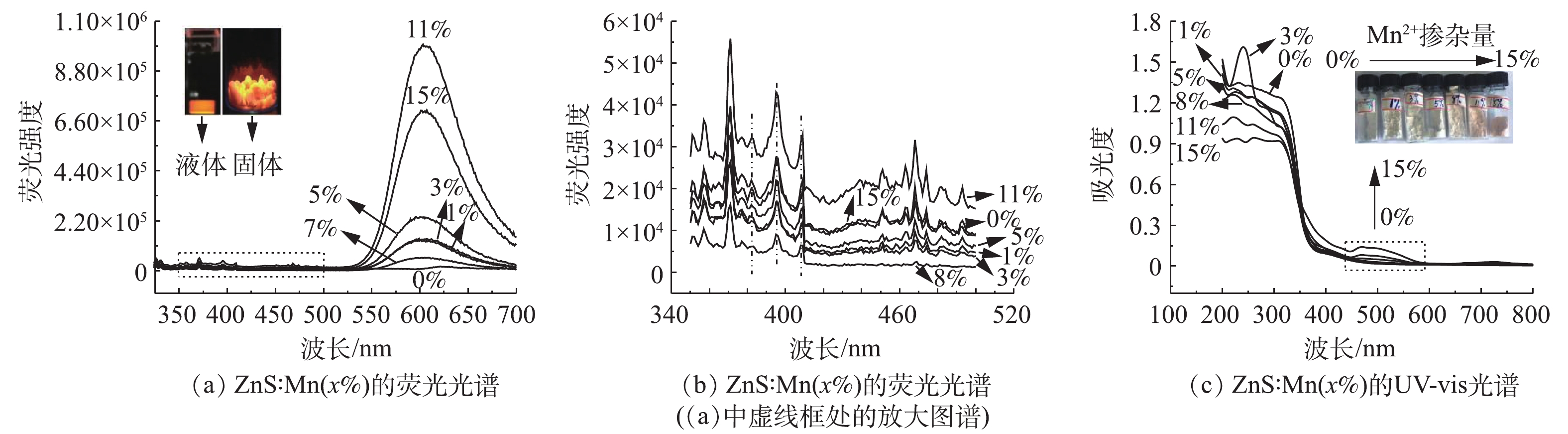
3)光学特性。图3为ZnS∶Mn(3%)的荧光光谱和UV-vis吸收光谱。如图3(a)的荧光光谱所示,在325 nm激发光下,催化剂的荧光发射峰位于598 nm处,为橘黄色荧光。这是由于ZnS∶Mn QDs在吸收激发光后产生空穴,进而被Mn2+捕获,电子和空穴是在Mn2+复合后导致Mn2+的能级4T1→6A1跃迁的结果,这说明Mn2+成功掺杂进入ZnS的晶格。为进一步获得催化剂表面结构信息,继续研究了ZnS∶Mn(3%)在350~500 nm下的光致发光谱(图3(b))。该区域的发射峰对应各种缺陷态的发光。382 nm发射峰与间隙锌有关,而395 nm处可归属于表面硫原子的空轨道[16],对于在409 nm出现的宽峰,一般认为是由于载流子在硫空位和价带之间重新结合导致的[16]。
Mn掺杂也导致ZnS的UV-vis发生了变化。如图3(c)所示,对于纯ZnS而言,其吸收边带为375 nm左右,但随着掺杂Mn2+后,其吸收边带移至了可见光区域,随着Mn离子掺杂浓度的升高,在500 nm处出现一个吸收峰,这是由于Mn2+自身能级跃迁所引起的[17],这表明Mn成功掺杂进ZnS中。因此,Mn掺杂ZnS后催化剂有2段带隙,一段是ZnS自身的带隙,该段带隙位于紫外光区域;另一段则是Mn引入后形成的杂质能级带隙,可以延伸至可见光区域。这显示Mn掺杂可以有效改善ZnS的光学性能,导致比ZnS更窄的禁带宽度,实现在可见光波段的吸收。此外,随着Mn2+掺杂浓度的提高,ZnS的外观颜色也从白色逐渐向粉红色转变。
-
Mn掺杂不仅可以从光学性能上影响催化反应,还可以影响光催化反应的吸附阶段。先前的研究表明,金属阳离子掺杂催化剂后能够调节催化剂表面电荷分布,导致等电点发生明显变化[18-20],从而影响催化剂对污染物的吸附。在催化剂浓度为0.5 g·L−1、Cr(Ⅵ)浓度为25 mg·L−1、温度为25 ℃、不调节pH的反应条件下,考察了Mn掺杂水平对光催化的影响。如图4(a)所示,随着Mn掺杂量增加,材料对Cr(Ⅵ)的吸附量逐渐增加。但当掺杂量为15%时,Cr(Ⅵ)吸附量反而略有下降,这可能是因为过量的Mn掺杂占据了原有的吸附位点所引起的。总体来看,所制备的ZnS∶Mn QDs对Cr(Ⅵ)具有优异的吸附性能。在光催化阶段,随着Mn掺杂含量增加,Cr(Ⅵ)还原效果的总体趋势为先增加后降低(图4(b))。这表明低浓度的Mn掺杂可以作为光生电子或空穴的浅势捕获阱,从而提高光生电子空穴对的分离效率。但过量的Mn掺杂可能导致其成为电子空穴对的复合中心,从而引起催化效率下降。尽管ZnS∶Mn(11%)拥有最好的还原效果,但由于ZnS∶Mn(3%)的还原效率与ZnS∶Mn(11%)非常接近且考虑到原料用量的成本问题,可选用ZnS∶Mn(3%)作为后续研究对象。光催化还原Cr(Ⅵ)的动力学如图4(c)所示,通过计算得到ZnS∶Mn(3%)对Cr(Ⅵ)还原速率为0.163 min−1,接近纯ZnS对Cr(Ⅵ)还原速率(0.089 min−1)的2倍。
我们对反应过程中溶液中剩余的Cr(Ⅲ)含量进行监测,所用催化剂为ZnS∶Mn(3%)。如图4(d)所示,对于Cr(Ⅵ)初始浓度为25 mg·L−1的溶液,反应过程中剩余的Cr(Ⅲ)含量较少,且反应结束后Cr(Ⅲ)能够基本吸附完全。因此,对于低浓度Cr(Ⅵ)溶液,可以认为Cr(Ⅵ)被还原的同时,其产物又被催化剂原位吸附。随着Cr(Ⅵ)浓度的升高,溶液中剩余的Cr(Ⅲ)含量逐渐增加。当Cr(Ⅵ)初始浓度为100 mg·L−1时,反应结束后,仍然有少量Cr(Ⅲ)存在于溶液中,这可能是由于吸附位点不足所致。
-
由于pH可以影响材料表面带电情况以及反应物的空间构型,因此pH是影响光催化的重要因素之一。当溶液pH<4时ZnS则会出现溶解现象[21],实验中将溶液初始pH控制在5~9。在催化剂浓度为0.5 g·L−1、Cr(Ⅵ)浓度为25 mg·L−1、温度为25 ℃的反应条件下,pH对光催化的影响如图5所示。随着pH的升高,光催化速率降低,偏酸性条件有利于光催化。这一现象可从ZnS的表面零电荷点以及pH对Cr(Ⅵ)存在形式的影响进行解释。ZnS的表面零电荷点在7.0~7.5[22]。当pH<7时催化剂表面被质子化,呈现正电荷,当5<pH<7时,Cr(Ⅵ)以
HCrO−4 和Cr2O2−7 阴离子的形式存在[23],因此,偏酸性条件催化剂有利于吸附Cr(Ⅵ),反应速率最高。此外,由能斯特方程(式(3))可知,溶液中H+的浓度越大,则电极电势越大,Cr(Ⅵ)越容易被光催化还原。当pH=7时,ZnS表面处于电中性,而Cr(Ⅵ)主要以Cr2O2−7 阴离子的形式存在,催化剂对Cr(Ⅵ)的吸附降低,反应速率因此下降;当pH=9时,催化剂表面带负电荷,Cr(Ⅵ)以Cr2O2−7 阴离子的形式存在,催化剂和Cr(Ⅵ)处于静电排斥状态,此时催化效率最低;不调节pH时(pH=5.8),Cr(Ⅵ)光催化还原速率接近pH=5下的速率,为简化操作,实际应用中可不调节pH。式中:E0为标准氧化还原电位,V;R为摩尔气体常数,为8.314 J·(K·mol)−1;T为热力学温度,K;n为转移的电子数量,mol;F为法拉第常数,为96 485 C·mol−1。
-
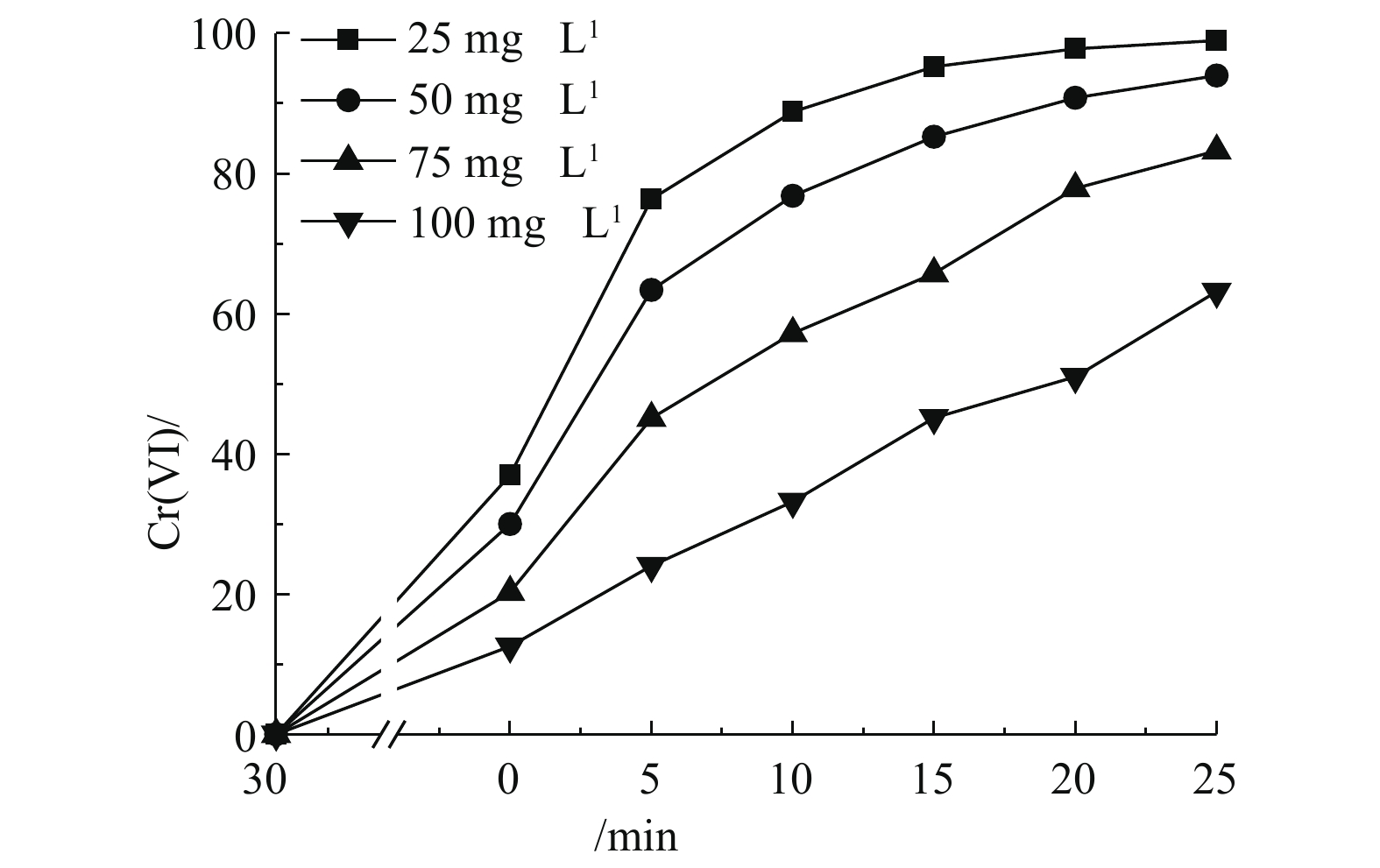
在催化剂浓度为0.5 g·L−1、温度为25 ℃、不调节pH的反应条件下,考察了Cr(Ⅵ)浓度对光催化的影响。如图6所示,随着Cr(Ⅵ)浓度的增加,其光催化还原速率降低。该现象可以从以下3个方面解释:当催化剂用量一定时,表面的吸附位点数量基本保持不变,而随着反应物浓度增加,吸附位点数量显得相对不足,成为了催化反应的限速因素;同时,Cr(Ⅵ)对450 nm处的光有吸收,当Cr(Ⅵ)浓度升高后,其对该处光线的吸收增强,导致了更少的光用于激发催化剂产生光生电子还原Cr(Ⅵ);此外,当Cr(Ⅵ)浓度升高时,溶液中的
CrO2−4 将转化成Cr2O2−7 二聚体,由于Cr2O2−7 二聚体比CrO2−4 具有更大的空间位阻,催化剂对Cr2O2−7 具有更小的吸附量,导致光催化效率有所下降。 -
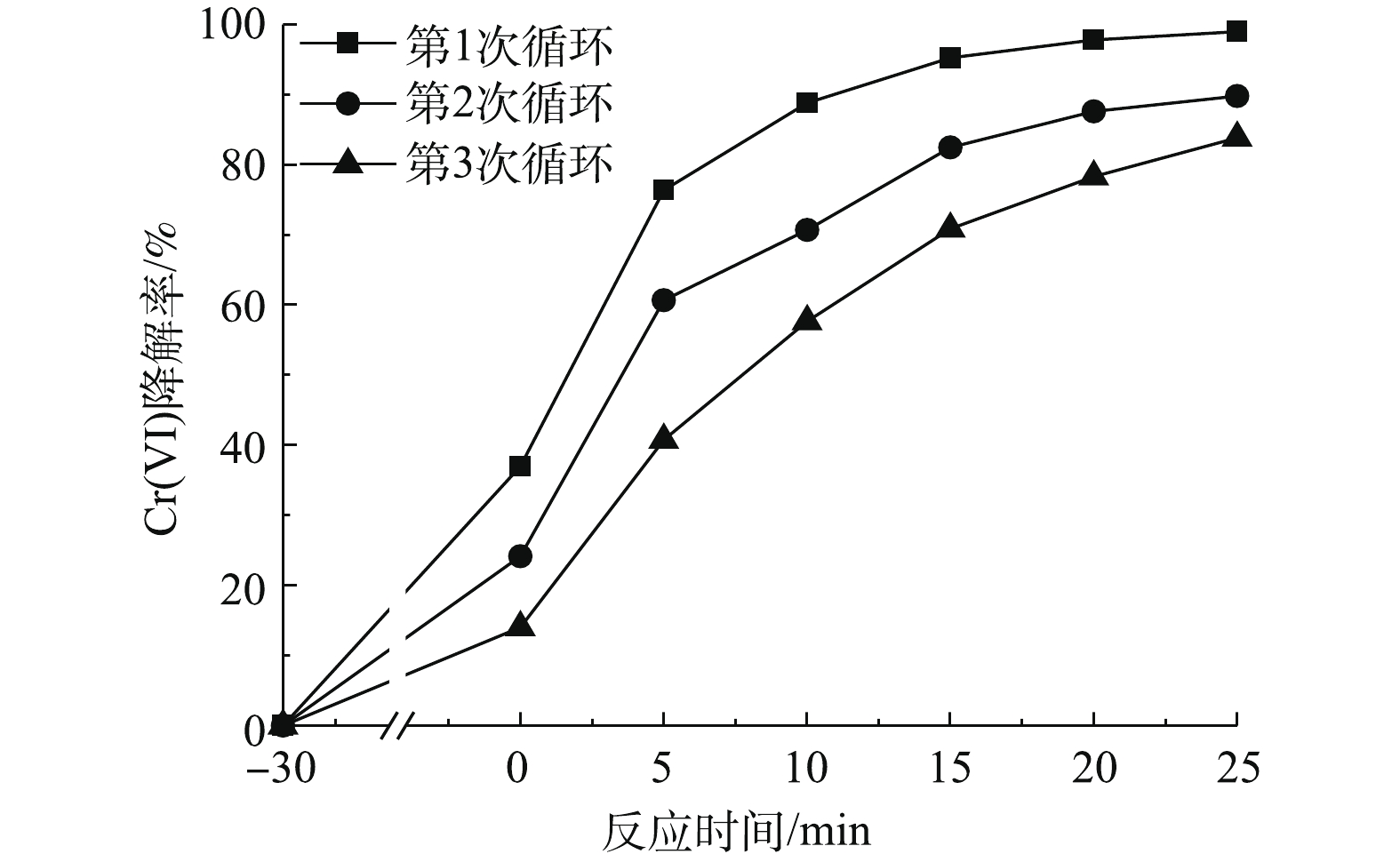
在催化剂浓度为0.5 g·L−1、Cr(Ⅵ)浓度为25 mg·L−1、温度为25 ℃、不调节pH的反应条件下,考察了催化剂的可循环利用性能。将多组光催化后的材料离心去除上清液,真空干燥后再进行光催化实验。如图7所示,随着循环次数的增加,催化剂对Cr(Ⅵ)的还原速率逐渐下降。这是由于在光催化后Cr(Ⅲ)被吸附在催化剂上,导致了活性位点的减少,进而导致反应速率降低。在经过3次循环后,光催化率下降了约15%,在光催化25 min后Cr(Ⅵ)最终去除率为84%。
-
我们对比了不同光催化剂的实验条件对Cr(Ⅵ)还原的影响(表1),包括光源类型、光源功率、光照波长、催化剂浓度、pH、Cr(Ⅵ)浓度、还原时间以及是否能够同时吸附生成的Cr(Ⅲ)。在对比的材料中包括氮化碳、钛化碳、二氧化钛、MOFs及金属硫化物,光源主要涉及氙灯、卤素灯和LED灯。从反应时间来判断,尽管存在部分材料有更高效的Cr(Ⅵ)还原效率,但存在其所用光源功率过高、催化剂浓度更高或Cr(Ⅵ)浓度更低等缺点。例如,虽然PANI/MT具有最高的催化效率,但是与ZnS∶Mn量子点相比,PANI/MT所用催化剂浓度更高,Cr(Ⅵ)浓度更低,且辐射波长在紫外光区域。BUC-21/Cd0.5Zn0.5S催化剂能够在可见光区域高效还原Cr(Ⅵ),且所用光源功率只有5 W,能够节省大量电能,但含有对环境有害的金属元素Cd,这阻碍了其在实际工程的应用。对于催化剂能否吸附还原产物Cr(Ⅲ),具有双面效应:不能吸附Cr(Ⅲ)的催化剂,在光催化结束后能够释放更多活性位点,有利于循环利用,但溶液中的Cr(Ⅲ)需要进一步处理;能够同时吸附还原产物Cr(Ⅲ)的催化剂,往往具有更差的循环性能,这取决于催化剂是否拥有足够的活性位点。综上所述,利用ZnS∶Mn QDs还原Cr(Ⅵ)具有低能耗、环境友好、高效等优点。
-
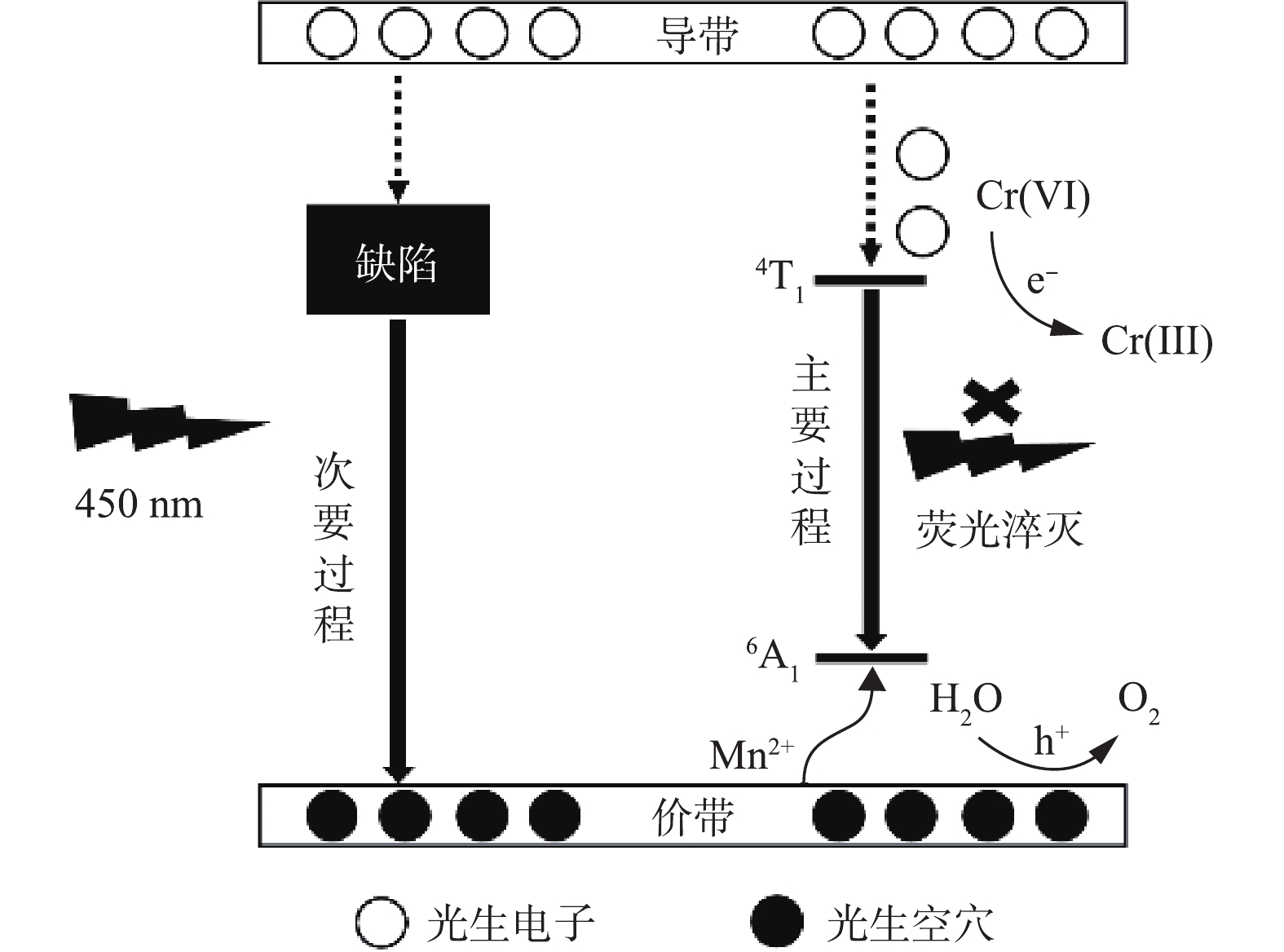
采用365 nm的激发光研究了在光催化过程中荧光强度的变化。如图8所示,在365 nm光照下,ZnS∶Mn QDs发出强烈的橙黄色荧光,这是由于在吸收激发光后,产生的空穴被Mn2+捕获,电子和空穴在Mn2+复合后导致Mn2+的4T1→6A1跃迁。但在加入Cr(Ⅵ)后,荧光被淬灭。淬灭的原因是:一方面,由于Cr(Ⅵ)的UV-vis光谱与365 nm的激发光有重叠,导致了内吸作用,荧光强度下降[33];另一方面,则是光生电子或空穴在复合过程中被捕获,导致他们无法复合发出荧光。由于Cr(Ⅵ)为最高价态,难以捕获光生空穴进行氧化,但可以被光生电子捕获进行还原。随着光催化反应,荧光逐渐恢复,这说明先前加入的Cr(Ⅵ)已经转化为低价态铬离子。由于低价态铬离子无法捕获光生电子再次被还原,因此,光生电子空穴对可以再次复合,荧光逐渐恢复。同时我们注意到,恢复的荧光红移到粉红色,这可能与Cr(Ⅲ)的吸附有关[34-35]。
根据PL光谱表征,ZnS∶Mn QDs主要存在2个区域的荧光峰,即350~500 nm的缺陷态荧光和598 nm处的杂质Mn发光,后者的荧光强度远远高于前者。因此,在450 nm蓝光激发下,主要为Mn2+的跃迁发光。如图9所示,当催化剂被辐射后,导带上产生光生电子,价带则产生相应数量的光生空穴。原本在Mn处用于复合发光的电子被Cr(Ⅵ)捕获,导致Cr(Ⅵ)被还原。根据先前对ZnS基催化剂的研究[36-37]可知,在不添加牺牲剂的条件下,价带上余留的空穴参与水的氧化。相对于纯ZnS,Mn掺杂ZnS具有更高的催化效率,这与UV-vis表征中Mn改善了ZnS的光吸收能力是一致的。因此,ZnS可通过Mn离子掺杂改善光吸收能力来调节其催化性能。
2.1. ZnS∶Mn QDs表征结果
2.2. Mn掺杂水平对光催化的影响
2.3. 溶液pH对光催化的影响
2.4. Cr(Ⅵ)浓度对光催化的影响
2.5. 催化剂的循环利用性能
2.6. 与其他光催化剂的对比
2.7. 光催化机理探究
-
1)锰掺杂硫化锌量子点催化剂能够在低能耗的450 nm蓝光LED灯下光催化还原Cr(Ⅵ),ZnS∶Mn QDs粒径在10 nm以下,Mn的掺杂没有改变ZnS的晶体结构。相对于纯ZnS,ZnS∶Mn QDs展宽了光吸收边带,能够提高光催化还原Cr(Ⅵ)的能力。
2)综合考虑成本、反应速率和操作性,确定了最佳Mn掺杂量为3%,适宜的pH为5.8。对于25 mg·L−1 Cr(Ⅵ),反应25 min后Cr(Ⅵ)被去除了99%。与多数光催化剂还原Cr(Ⅵ)不同,ZnS∶Mn QDs能够吸附还原后的产物Cr(Ⅲ),实现一步去除水中铬离子。
3)光生电子空穴对在杂质Mn处复合产生荧光,Cr(Ⅵ)可以捕获光生电子导致荧光淬灭,同时Cr(Ⅵ)被还原,而价带上余留的空穴参与水的氧化。



 DownLoad:
DownLoad: