-
硝基苯(NB)是一种无色或微黄色的具有苦杏仁味的油状液体,主要应用于制造染料、塑料、农药、炸药和药品,是典型的硝基芳香化合物,具有毒性大、难降解的特点[1-2]。环境中的NB主要来源于化工厂、染料厂、制药厂的废水废气或泄露事故,尤其是苯胺(AN)染料厂排放的废水中含大量NB[3]。NB包括苯环和硝基,硝基吸电子能力强,容易被还原而难于被氧化[4],因此,化学还原成为处理水环境中NB的重要技术方法[5]。纳米零价铁被广泛用于降解硝基苯,但存在易团聚、易氧化、成本高等问题[6-7],因此需要寻求高效廉价的还原剂。
天然硫铁矿主要存在于水体、湖泊、沉积物以及地下水中,是储备最丰富的天然矿物之一[8]。相比其他的还原物质,硫铁矿主要成分为FeS和FeS2,理论上具有较强的还原性能[9-10],且稳定性较好,容易贮存。然而,天然矿物粒径大、比表面积小,实际应用中与污染物接触效率较低,影响其对污染物的降解效果和反应速率[11]。有研究[12]表明,利用球磨法将天然矿物制成微纳级粉末,使矿物表面的悬空未配位键和点缺陷等增多,扩大比面积、增加活性位点和提高反应活性。
还原法降解NB的主要产物通常为AN,仍为有毒有害物质,因此,对其需要进一步处理。高级氧化法可以实现对AN的高效彻底降解[13]。过硫酸盐(PS)具有较强的氧化能力,在环境中的稳定性较好,且通过热、碱、紫外或过渡金属离子等方式活化,可以产生氧化能力更强的硫酸根自由基(
SO−4 ·)和羟基自由基(OH·),对有机污染物具有较强的降解和矿化效果[14-16]。基于上述分析,本文采用湿式球磨法制备微纳级硫铁矿粉末(BMP),并考察BMP单独处理及与PS联合处理对水中NB和AN的去除特性;采用TEM、XRD、FT-IR、XPS等对BMP及其反应后的残渣进行表征,分析反应溶液中的主要离子,阐明BMP/PS对NB的联合降解机制。最后,采用响应面法设计多因素实验寻求最优反应条件,旨在为含NB废水处理或地下水NB污染修复提供新方法。
全文HTML
-
实验材料:天然硫铁矿,购于安阳鑫泽冶金耐材有限公司;过硫酸盐、醋酸钠、冰醋酸、邻啡罗琳试剂、盐酸羟胺、硫酸亚铁铵、甲醇、乙醇、盐酸和氢氧化钠均为分析纯,购于国药化学试剂有限公司;实验室用水为超纯水。
实验仪器:高效液相色谱仪(HPLC,1260II,美国Agilent);X射线衍射仪(XRD,DMAX2500,日本JEOL);高分辨率透射电子显微镜(TEM,JEM-2100F,日本JEOL);X射线光电子能谱(XPS,Axis Ultra DLD,美国Thermo);总有机碳/总氮分析仪(TOC,multi n/c 2100,德国Analytik Jena);立式行星球磨仪(XQM-4,泰安科比钢球);傅里叶变换红外光谱仪(FT-IR,Nicolet iS50,美国赛默飞世尔);高效离子色谱仪(ICS-600,美国赛默飞);紫外-可见光分光光度仪(T6新世纪,北京普析仪器)。
-
采用立式行星式球磨仪制备微纳级BMP粉末。球磨筒有效体积为1 L,磨筒和磨球均为不锈钢材质。挑选晶形较好的天然硫铁矿,人工粉碎至3 mm左右,在厌氧手套箱内平衡2 h,按如下条件装料:15、8、3 mm的大球、中球、小球,个数比为1∶4∶4。磨球与硫铁矿质量比为10∶1,加入一定量乙醇,球磨仪转速400 r·min−1,活化24 h,在超声清洗仪中静置30 min,防止团聚,然后放入真空干燥箱中烘干,存于真空厌氧箱。
采用TEM分析样品的形貌和尺寸大小;采用XPS测试样品的元素变化;采用XRD测试样品的成分和晶体结构变化(铜靶,扫描角度20°~90°,扫描速度为2 (°)·min−1);采用FT-IR测试样品反应前后官能团、化学结构变化。
-
在BMP单独处理实验中,考察BMP投加量和pH对NB降解的影响。室温下,将100 mL浓度为100 mg·L−1的NB水溶液倒入锥形瓶,投加0.5~10 g·L−1的BMP,密封后,放入恒温振荡箱中,以200 r·min−1的转速振荡反应180 min,间隔取样过0.45 μm针式滤膜,采用高效液相色谱仪检测NB和AN的浓度。固定BMP投加量,调节溶液pH为3~11,在恒温振荡箱中振荡反应,分别在0、15、30、60和180 min取样,进一步检测NB和AN浓度。
在BMP/PS联合处理实验中,BMP投加量为5 g·L−1,考察PS投加量对去除NB和AN的影响。将100 mL浓度为100 mg·L−1的NB水溶液倒入锥形瓶,投加5 g·L−1的BMP,密封,放入恒温振荡箱中,以200 r·min−1的转速振荡反应60 min,随即立刻投加1~20 g·L−1的PS,密封,放入恒温振荡箱中,以200 r·min−1的转速继续振荡反应60 min,间隔时间取样,过0.22 μm针式滤膜,采用高效液相色谱仪检测反应溶液中NB和AN的浓度,通过总有机碳总氮测定仪测定TOC的含量,通过紫外分光光度仪检测Fe(Ⅱ)和Fe(Ⅲ)的含量,通过离子色谱仪检测
SO2−4 的含量。根据单因素实验结果,采用Box-Behnken模型设计中心组合实验,选取BMP投加量、还原时间和PS投加量、氧化时间4个因素,以NB去除率和AN浓度作为响应值,进行4因素3水平实验,具体参数设置如表1所示。
1.1. 实验材料与仪器
1.2. BMP制备与表征
1.3. 降解实验
-
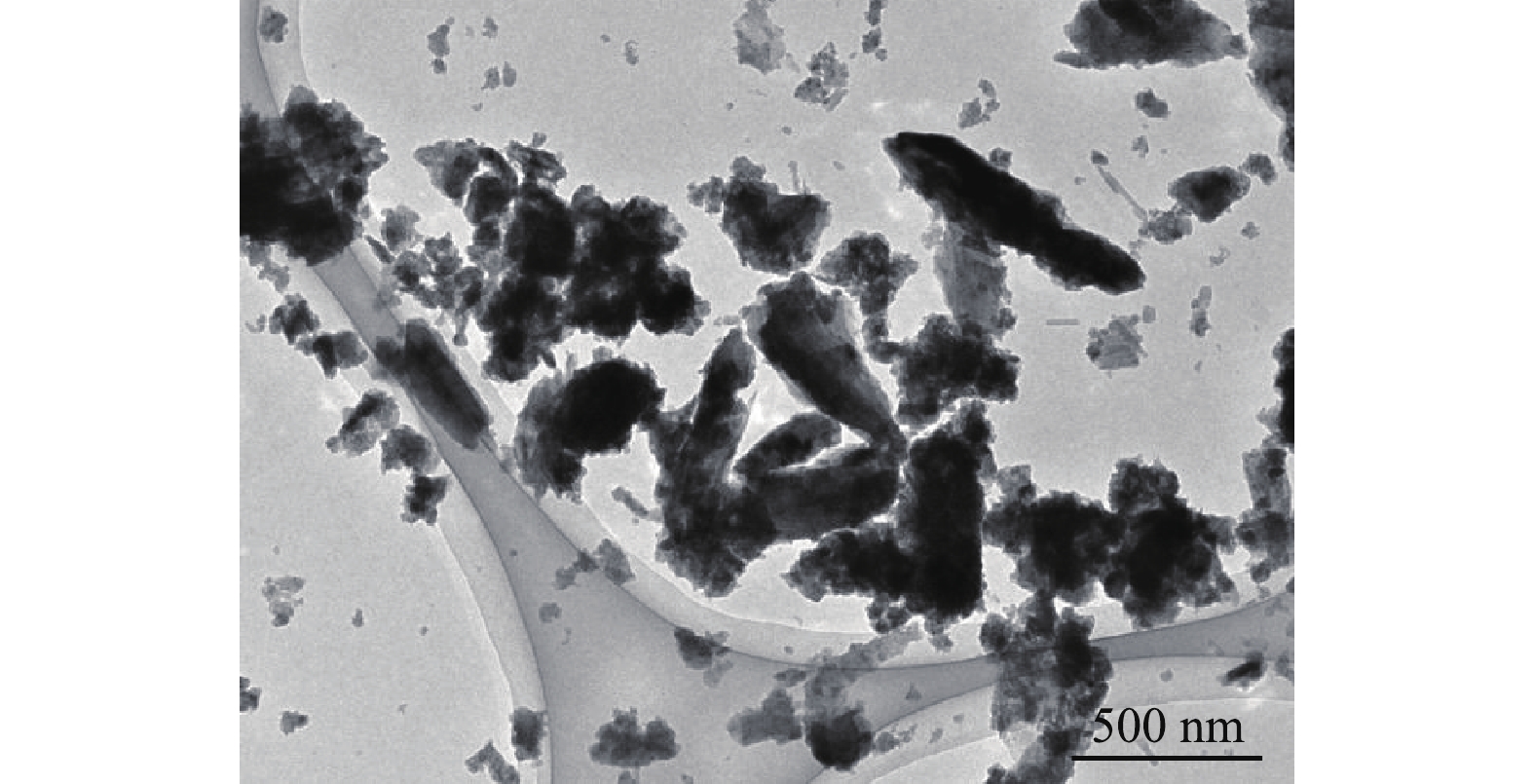
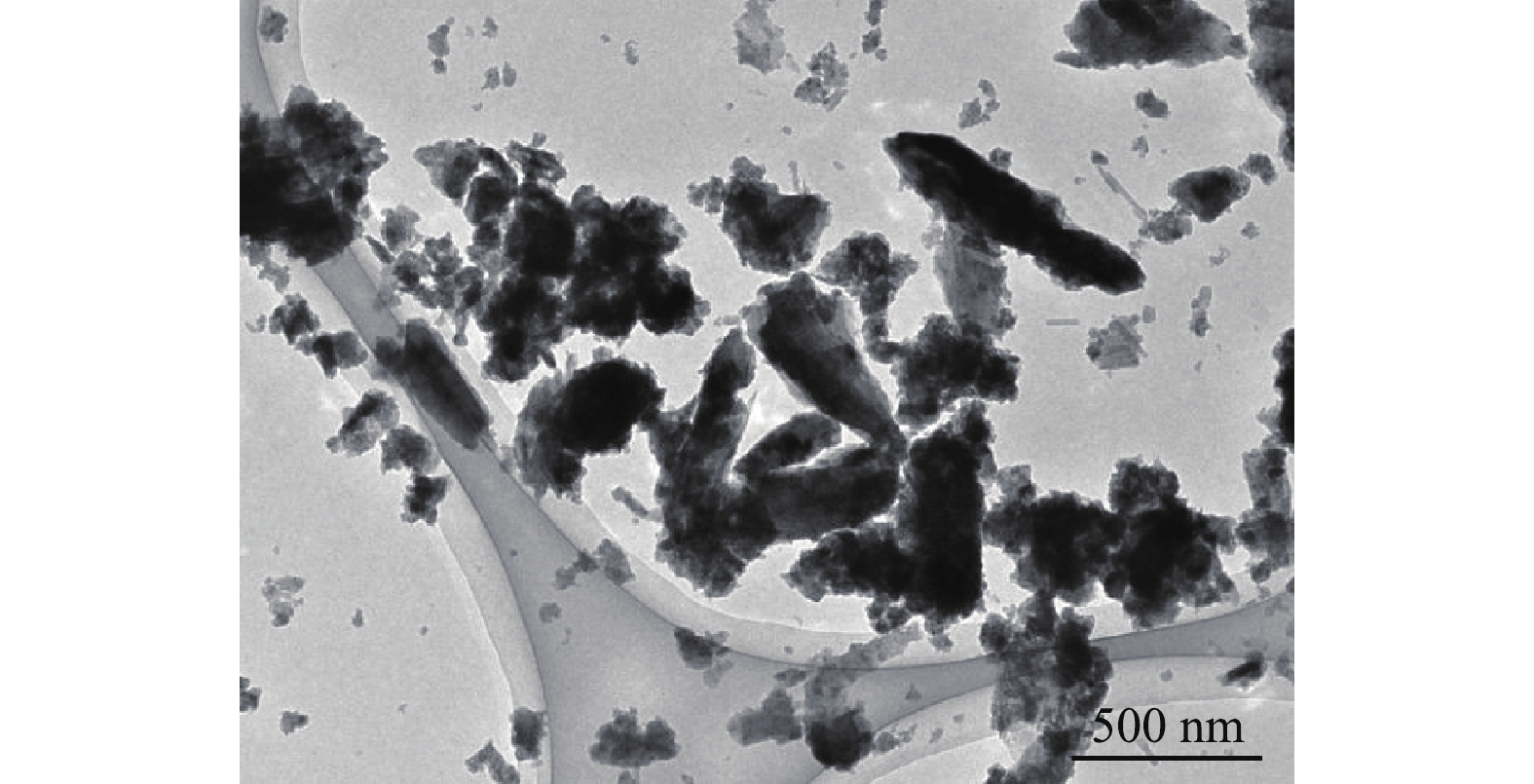
图1为BMP的TEM图,粒径为89~714 nm,均值为217 nm,说明制得的BMP符合微纳米级别的要求。样品内部颜色深,四周颜色浅,这可能是制备过程中表层形成了一层氧化物[17]。样品离散效果好,结构分布均匀,表明晶体结构完整和稳定[18]。
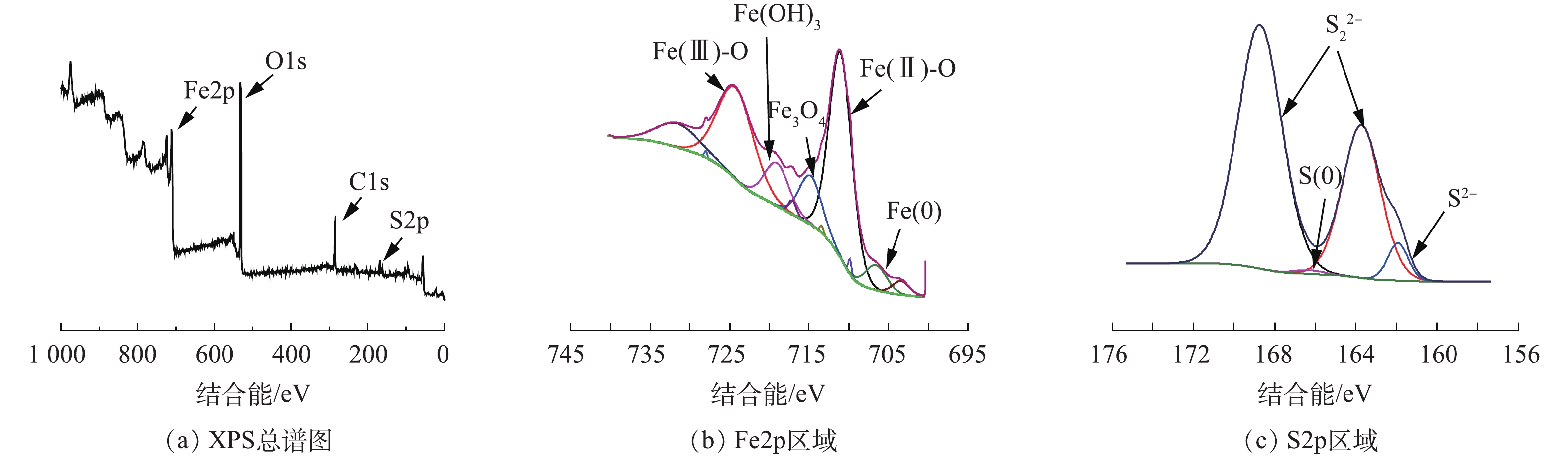
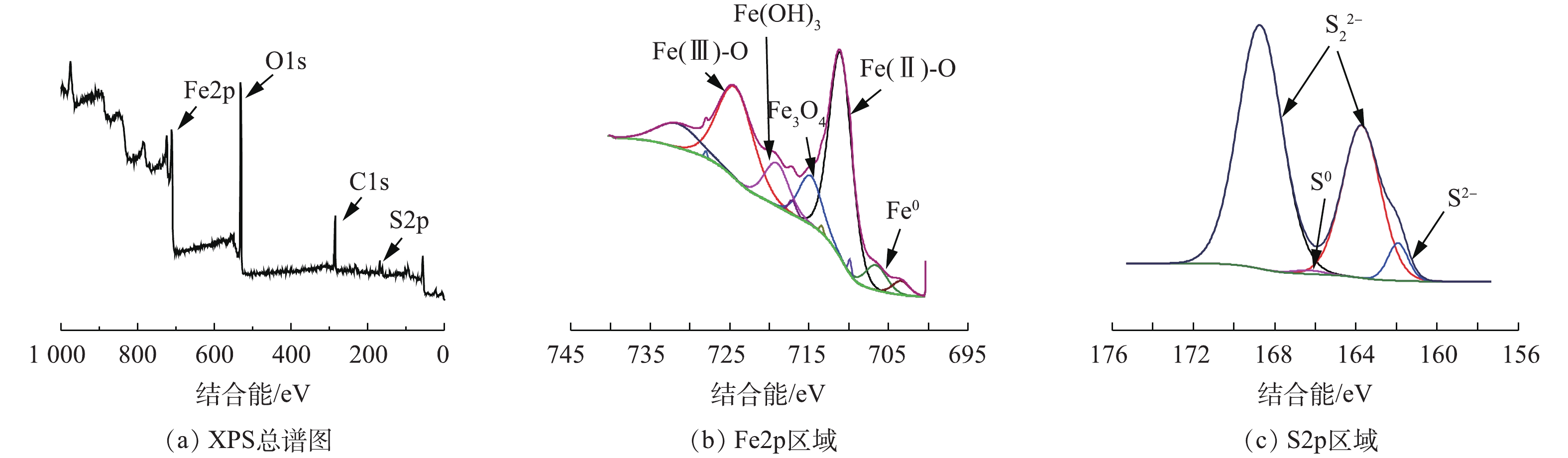
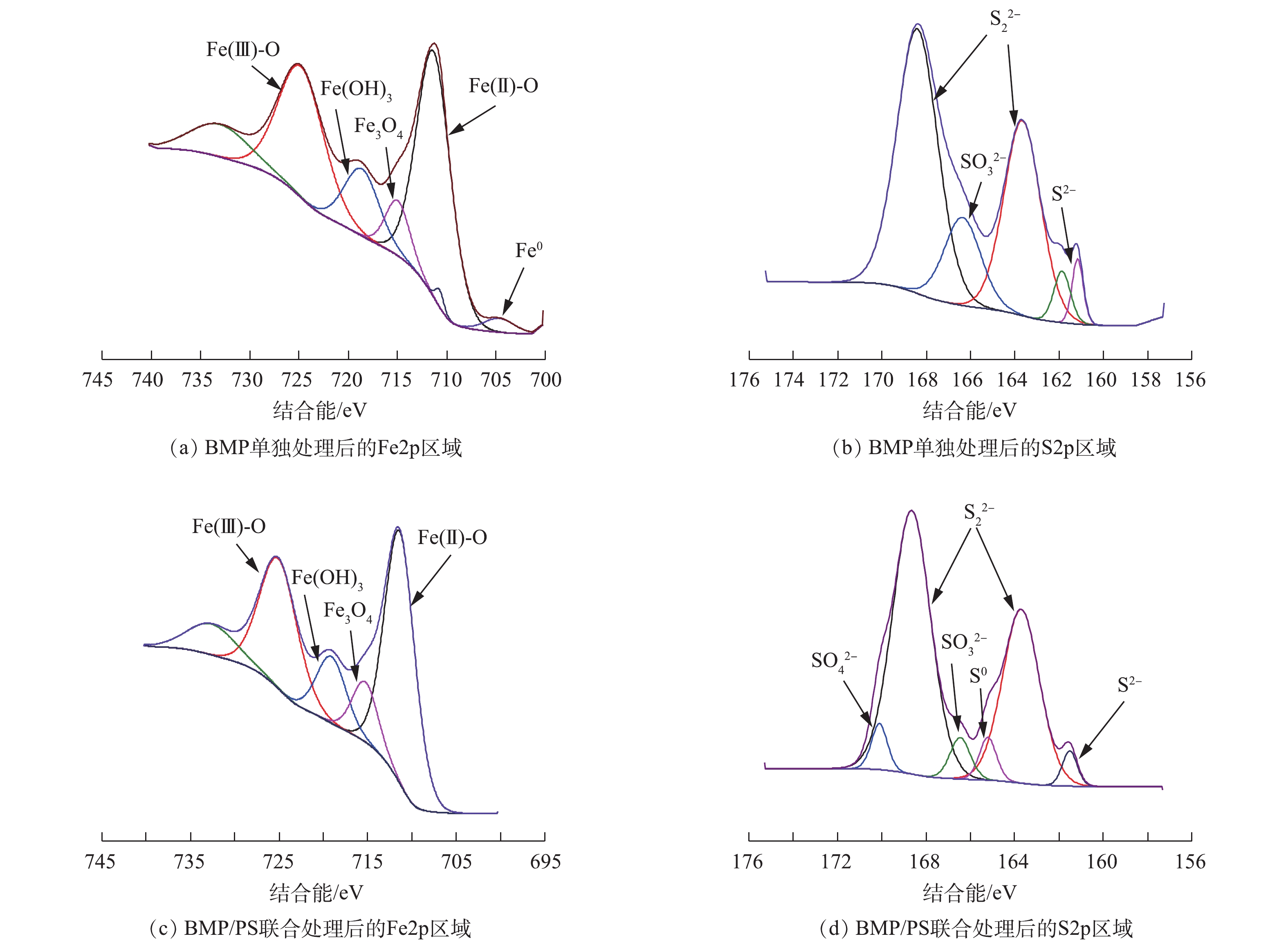
采用XPS对BMP成分进行深入分析,BMP样品的XPS谱图见图2。图2(a)为BMP的XPS总谱图,BMP的元素组成包括C、O、Fe和S。由图2(b)中的Fe2p区域分析结果可知,结合能为711.29 eV和725.03 eV处分别对应Fe(Ⅱ)和Fe(Ⅲ)的衍射峰,含量分别为29.1%和22.41%。Fe0有少量存在[19];S2p区域的窄谱扫描中,结合能为161.98 eV和163.73 eV对应S2−和
S2−2 的衍射峰,含量分别为14.73%和8.32%[20],S0的含量为4.96%。根据XPS表征结果可以推断,BMP主要元素为Fe和S,此外,BMP表面还存在少量Fe0、S0和铁硫氧化物。 -
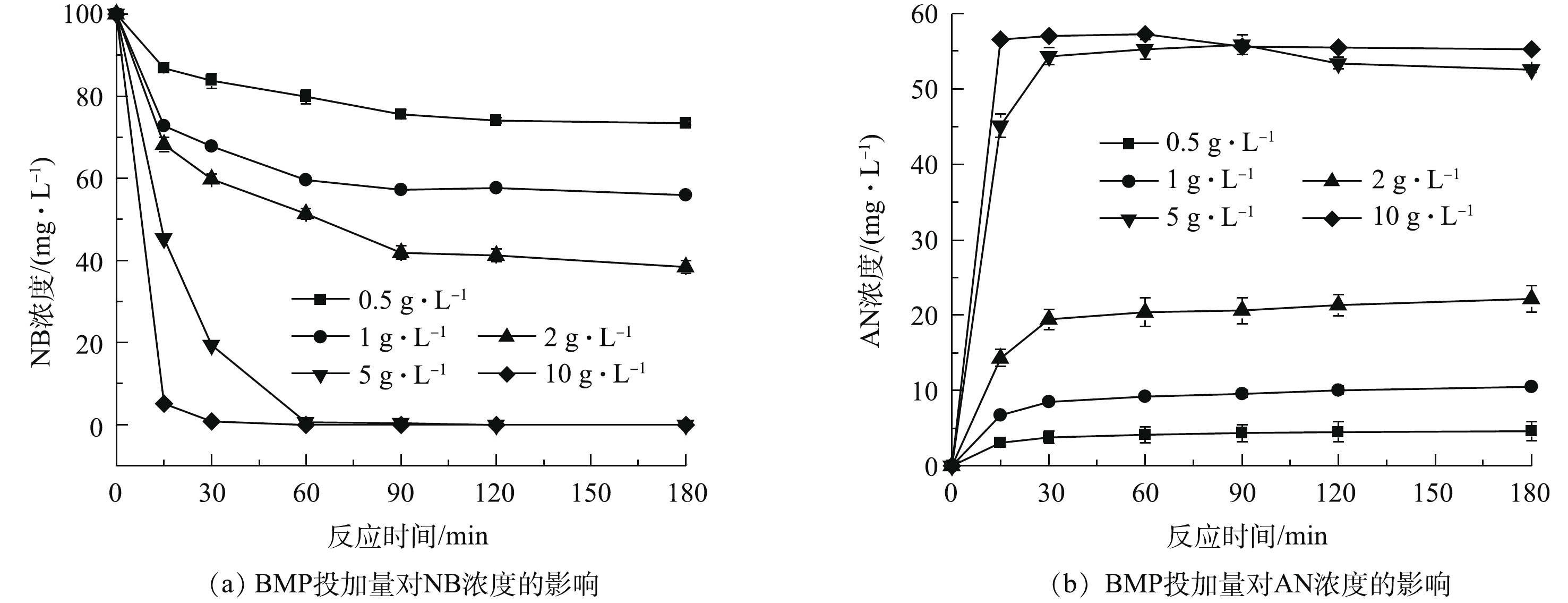
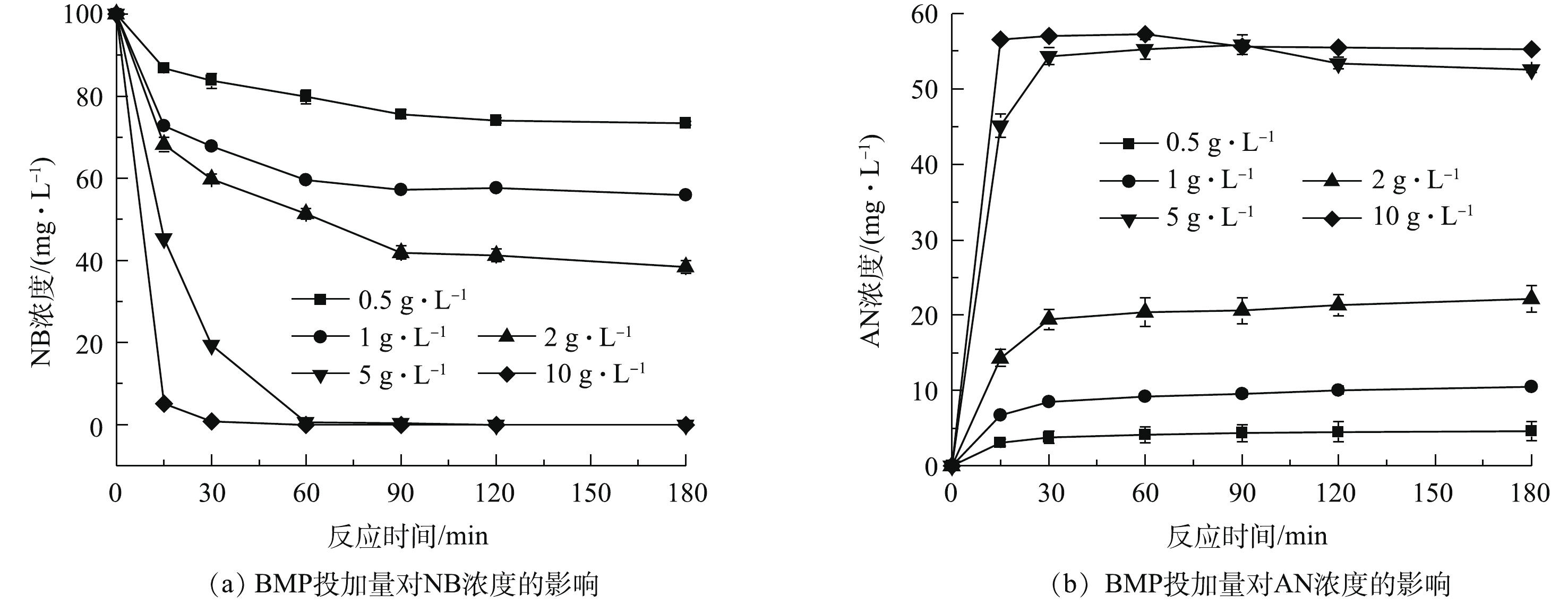
当水溶液体积为100 mL、NB浓度为100 mg·L−1、溶液初始pH=7、BMP投加量为0.5~10 g·L−1、振荡反应时间为180 min时,单独投加BMP对NB和AN浓度的影响结果如图3所示。由图3(a)可知,NB浓度随着BMP投加量的增加而下降,NB浓度在前30 min内迅速降低,随后缓慢下降;当BMP投加量大于5 g·L−1时,NB浓度在15 min内迅速降至0.1 mg·L−1以下,NB浓度的快速下降是由于BMP投加量增加,使得反应活性位点增加,从而迅速降解NB。当BMP投加量为5 g·L−1时,NB在60 min内全部去除,因此,继续增加BMP投加量可加速NB还原,但不会提高NB的去除率。在NB降解的同时,还伴随着AN浓度的迅速增加,由图3(b)可见,当BMP投加量大于5 g·L−1时,AN浓度稳定在55 mg·L−1左右,这表明BMP对NB具有高效还原降解效果,主要产物为AN[21-22]。
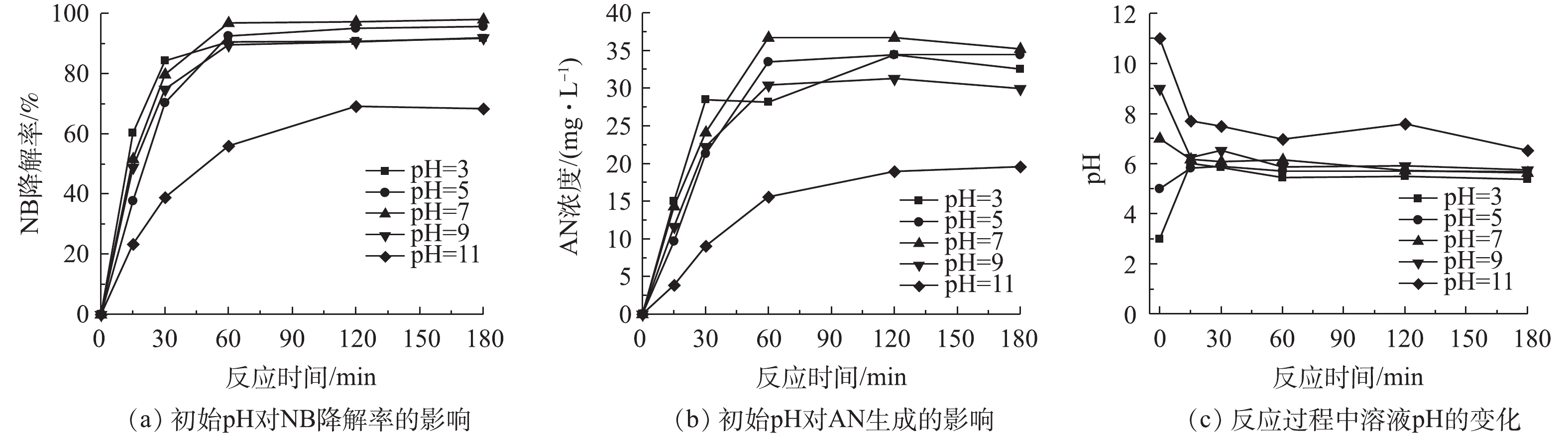
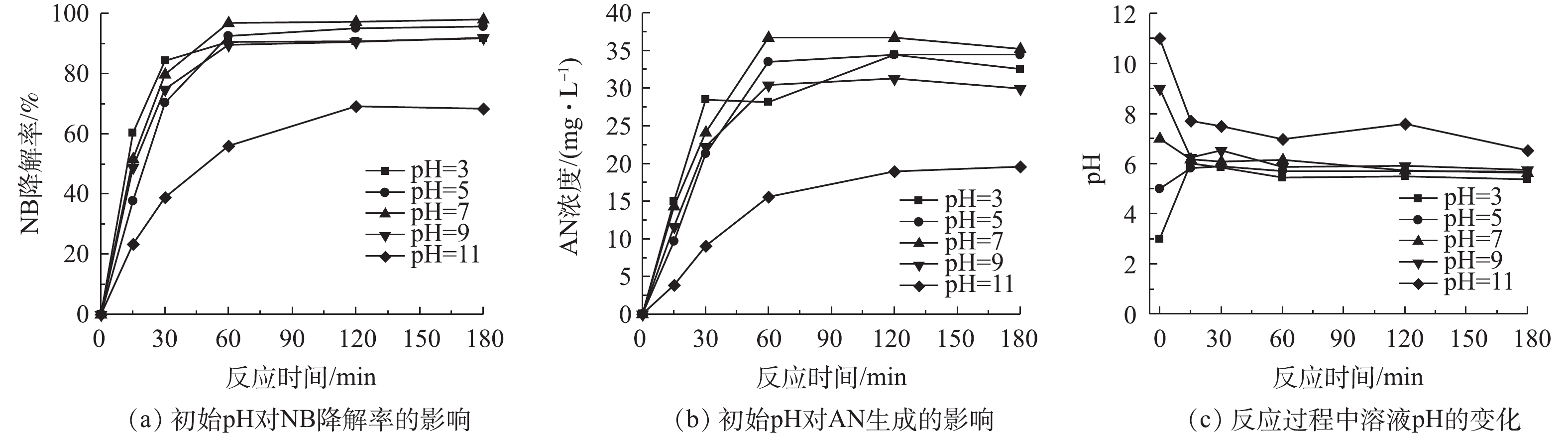
单独投加BMP时,初始pH对NB降解的影响如图4所示。当初始pH为酸性至中性时,对BMP除率NB废水的影响很小,NB去除的反应速率相似,NB去除率在95%以上,中性时NB的去除率达到100%,实验产生的AN浓度在30 mg·L−1左右。在强碱环境中,NB的去除率为60%,AN浓度为20 mg·L−1。原因是在强碱环境下,BMP的活性降低,即NB的降解反应活性下降,相应生成的AN浓度也下降。图4(c)为溶液在反应过程中pH的变化情况。随着反应的进行,在不同初始pH的溶液中,在反应时pH在15 min内迅速变化,然后保持相对稳定的趋势,pH接近6,表明BMP反应体系具有较强的酸碱缓冲性。这是由于在水溶液中FeS2和FeS会与水和溶解氧反应,产生H+和OH−,使反应体系保持一个相对稳定的酸性值,反应[23]如式(2)和式(3)所示。
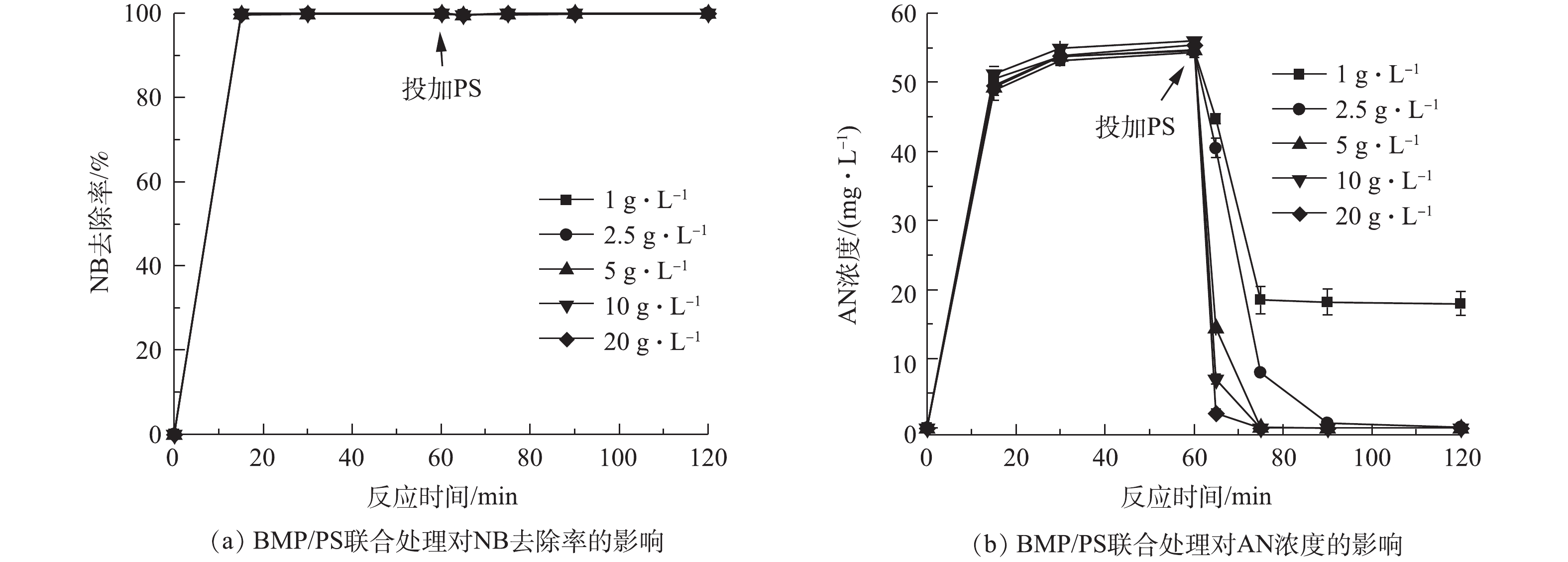
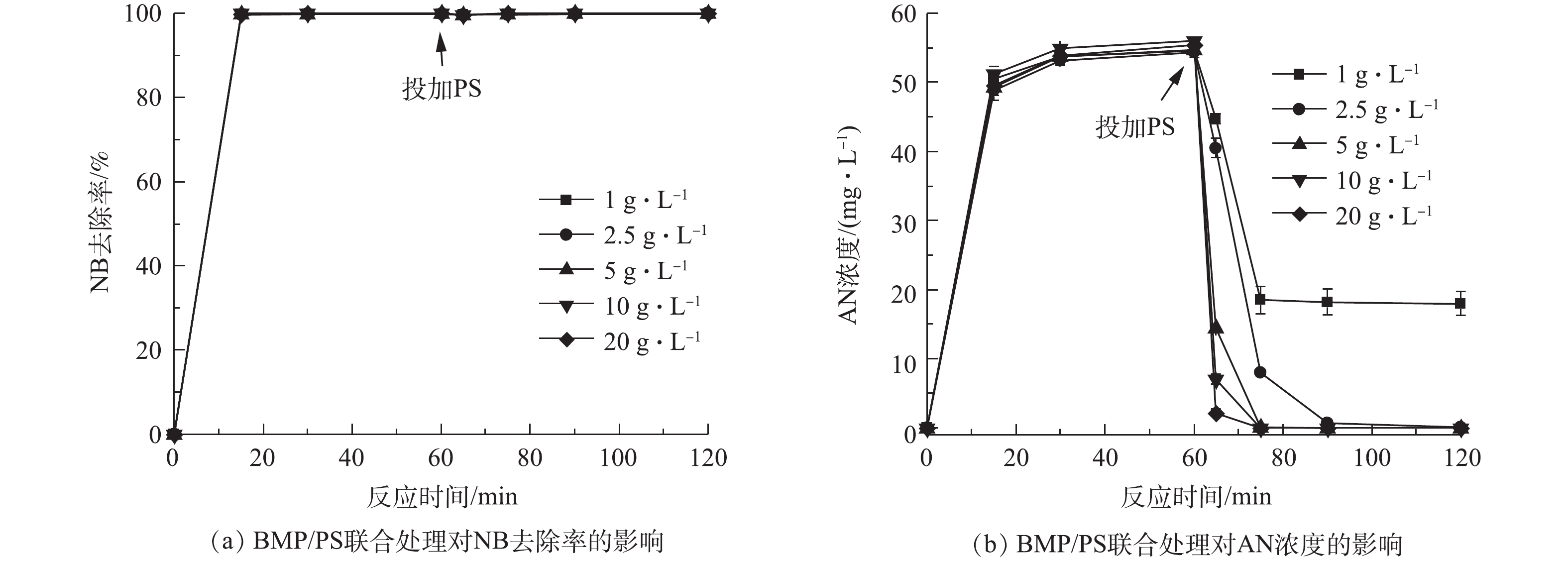
当水溶液体积为100 mL、NB浓度为100 mg·L−1、溶液初始pH=7、BMP投加量固定为5 g·L−1、还原时间为60 min,反应60 min后分别投加1~20 g·L−1的PS,氧化时间为60 min,在0、15、30、60、65、75、90和120 min取样测定。不同PS投加量对AN浓度的影响结果如图5所示。由图5(a)可知,NB的去除率在投加PS前已达到100%,投加PS后,NB去除率保持不变,说明NB完全降解,PS的投加量不再影响NB去除率的变化,这说明在联合处理中,NB主要依赖BMP的还原作用。图5(b)为AN浓度随反应时间的变化。在PS投加前,AN浓度不断上升,这是由于BMP与NB反应产生了AN,AN浓度达到55 mg·L−1,投加PS后,AN浓度迅速下降,且随着PS投加量的增加,下降幅度越大。PS投加量大于2.5 g·L−1时,AN被完全降解。
当BMP投加量为5 g·L−1时,经过60 min后,NB完全降解,AN浓度不断上升,最高达到55 mg·L−1,加入不同投加量的PS后发现,AN浓度急剧下降,降幅随PS投加量的增加而增加;当PS投加量大于2.5 g·L−1时,AN被完全降解。这是由于BMP中的Fe(Ⅱ)活化PS生成具有强氧化性的
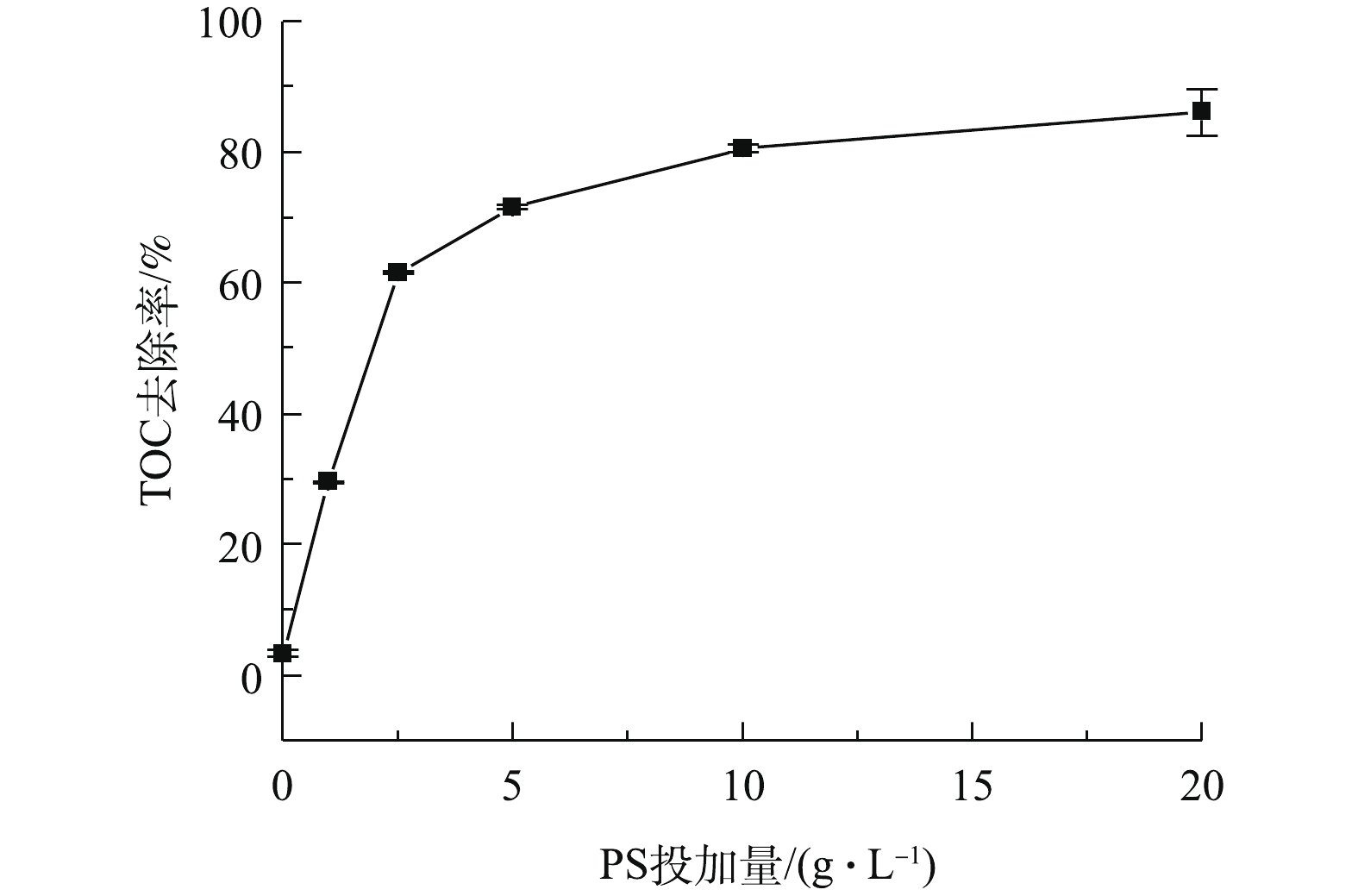
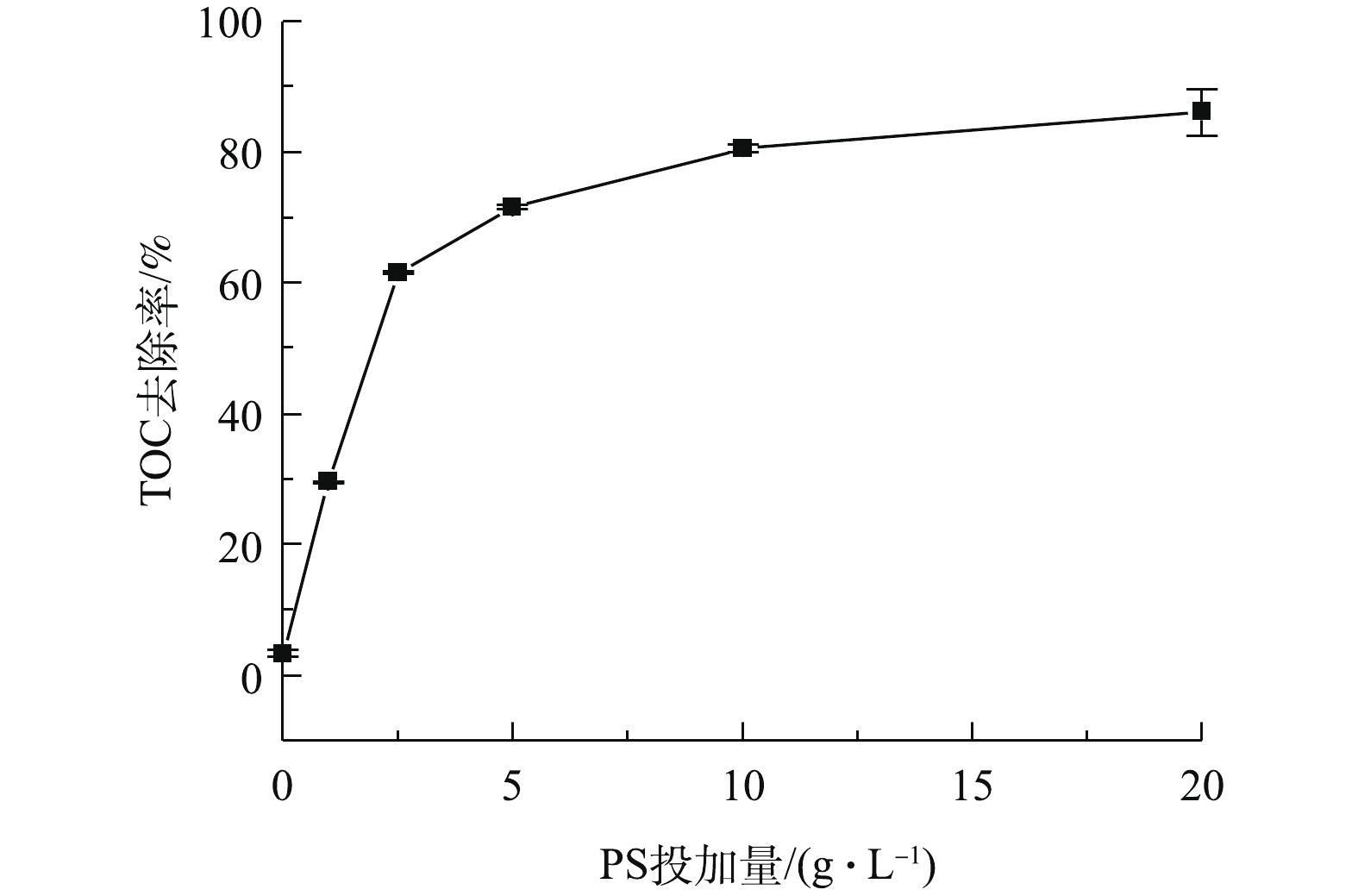
SO−⋅4 和HO·,实现对AN的高效降解[24-25]。反应溶液中TOC去除率的变化如图6所示。在BMP/PS联合处理中,反应120 min后取样检测TOC含量,由图6可知,TOC去除率随着PS投加量的增加而逐渐提高,当PS投加量为1、2.5、5、10和20 g·L−1时,TOC的去除率分别为29.5%、61.6%、71.6%、80.6%和86.1%。这表明联合处理对NB的矿化效果较好,NB降解较为彻底,理论上产物主要为CO2、H2O以及无机酸[26-27]。
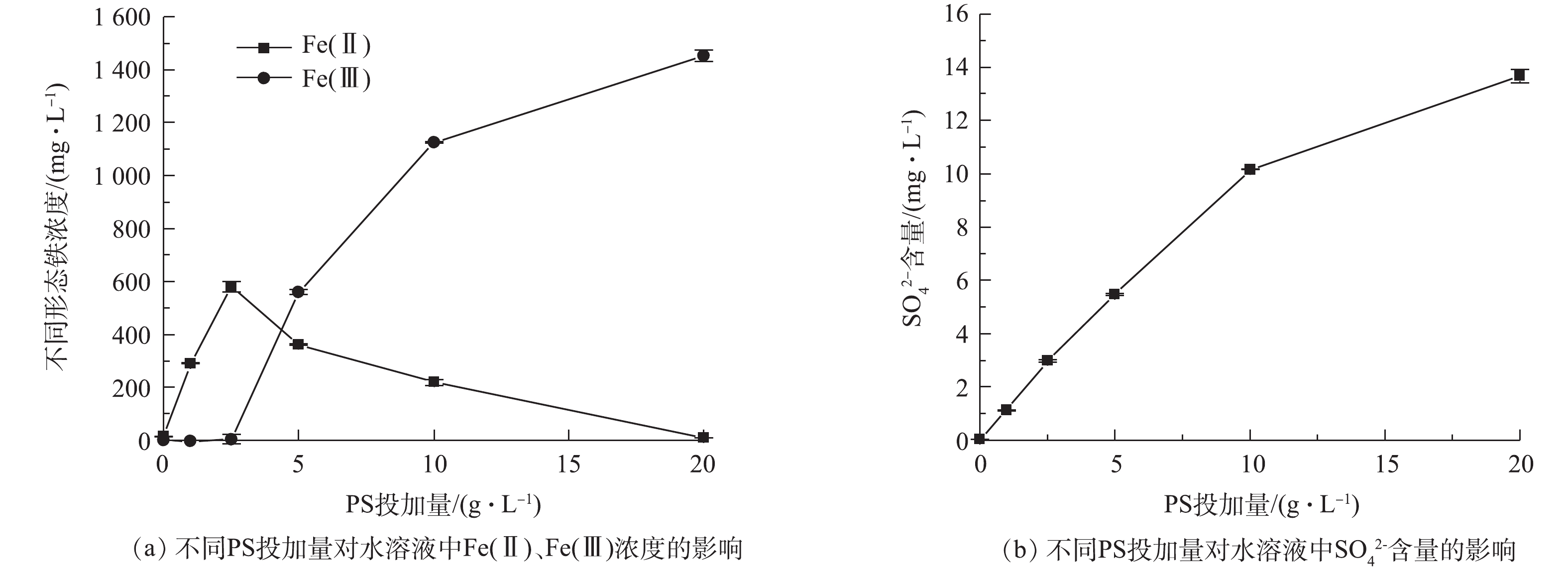
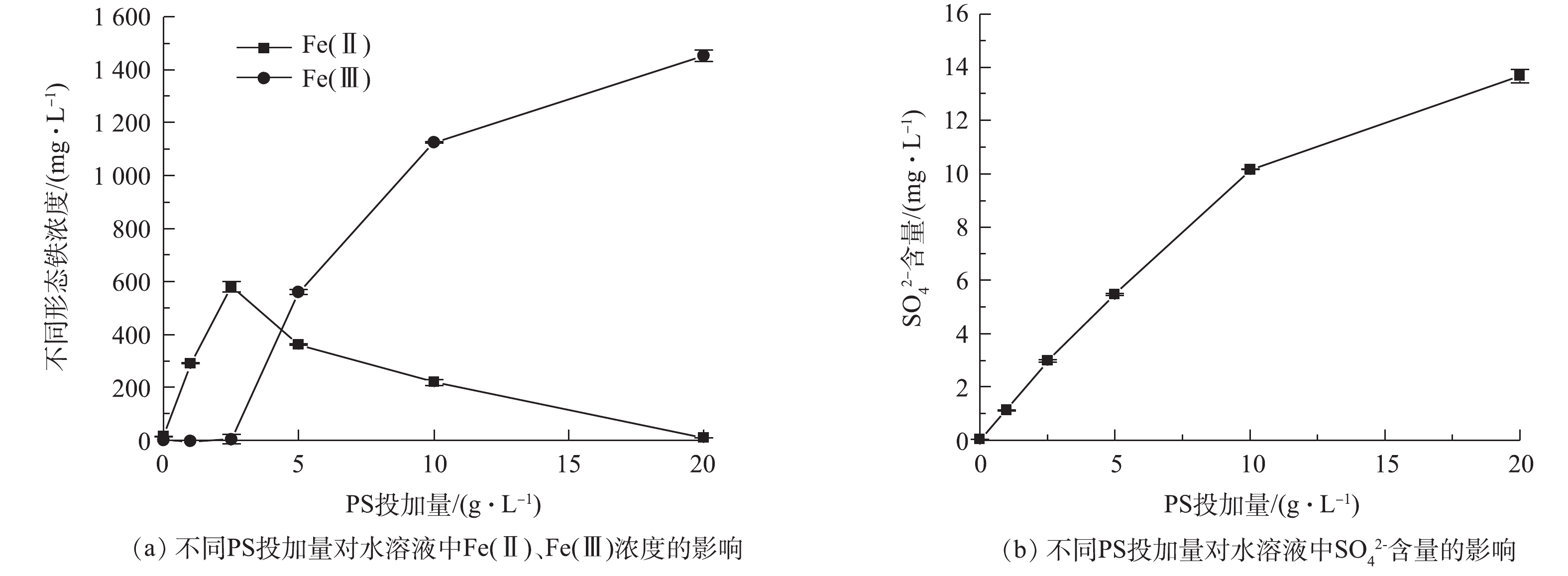
反应溶液中Fe(Ⅱ)、Fe(Ⅲ)以及
SO2−4 含量的变化见图7。由图7(a)可知,当PS投加量低于5 g·L−1时,水溶液中存在Fe(Ⅱ),且随着PS投加量的增加而增加,氧化剂能加速BMP中Fe(Ⅱ)的释放;当PS投加量大于5 g·L−1后,水中Fe(Ⅱ)的含量逐渐降低,同时Fe(Ⅲ)的含量增加。由图7(b)可知,随着PS投加量的增加,SO2−4 含量持续增加。因此,少量PS能促进BMP产生Fe(Ⅱ),但PS含量低不利于AN的降解,随着PS含量增加,当PS含量大于5 g·L−1时,BMP中Fe(Ⅱ)溶出催化PS产生SO⋅−4 、SO2−4 和Fe(Ⅲ)。因此,增加PS的投加量,溶液的中Fe(Ⅱ)浓度下降,Fe(Ⅲ)和SO2−4 浓度逐渐增加。 -
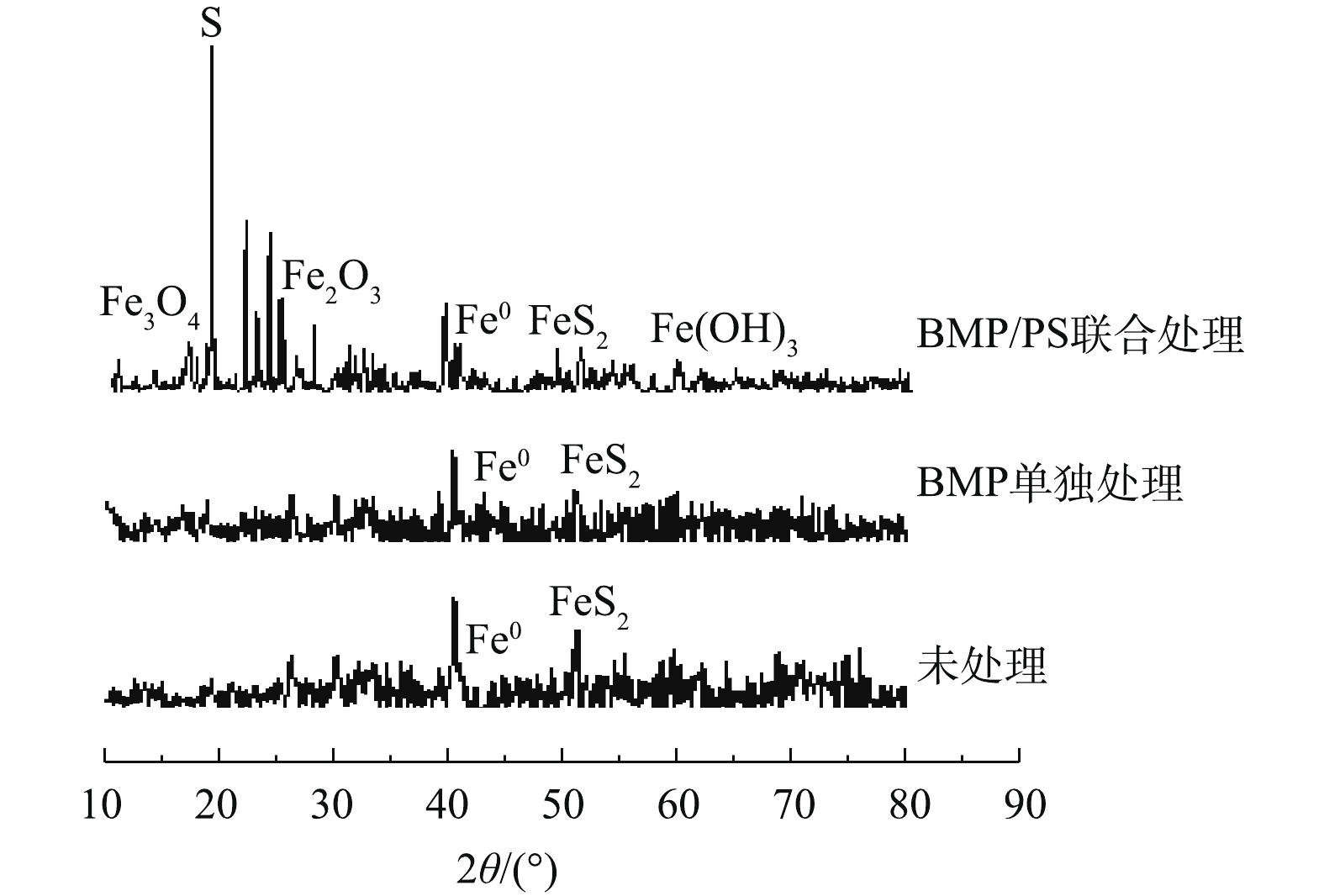
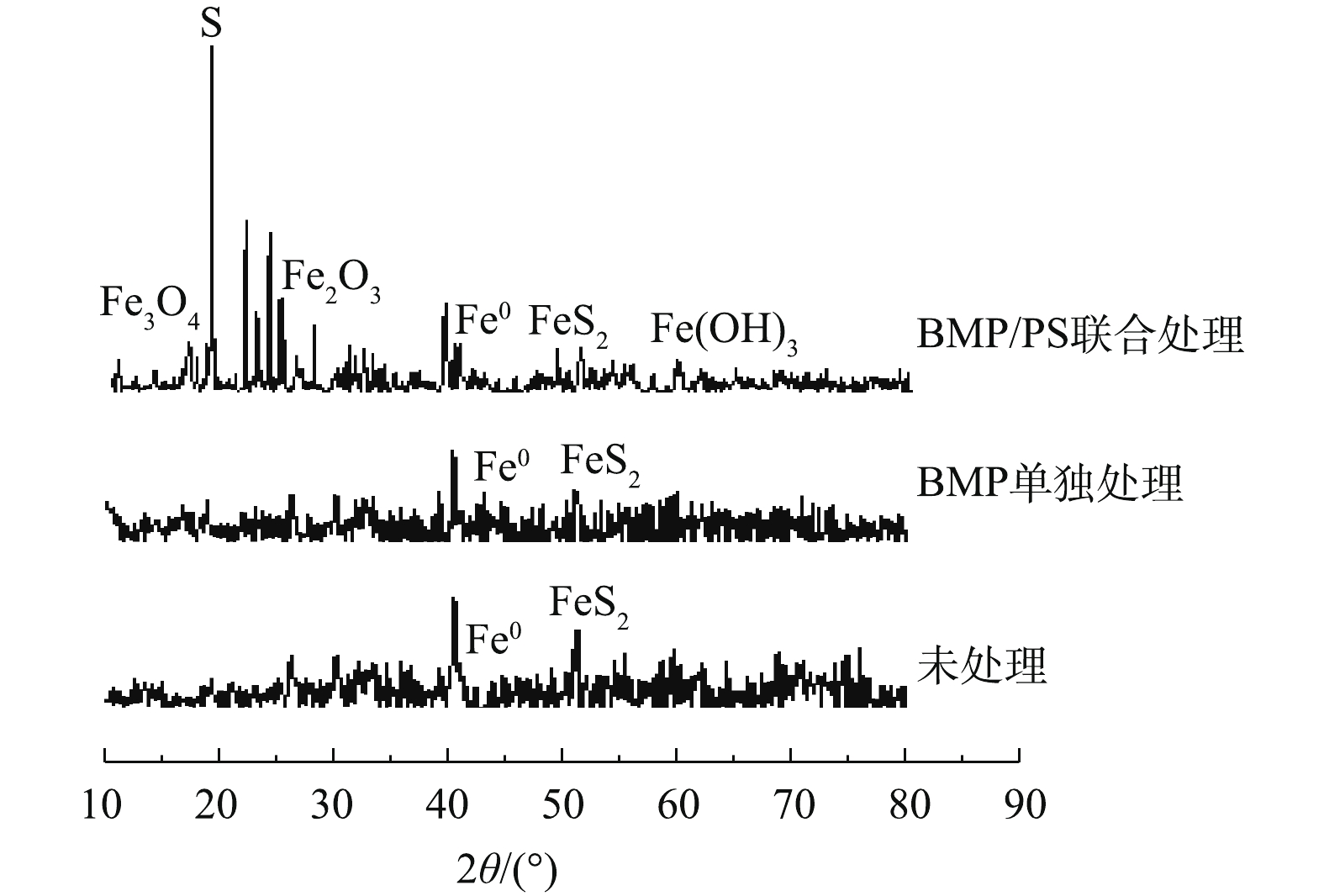
图8为不同处理条件下BMP的XRD图谱表征结果。由图8可知,未处理的BMP样品主要成分为FeS和FeS2,与处理前相比,BMP单独处理后的XRD图谱变化较小,FeS的峰值略有下降。BMP/PS联合处理后的XRD图谱变化较大,主要成分变成单质S,同时产生了部分Fe3O4、Fe2O3和Fe(OH)3,这是由于BMP中FeS被PS氧化生成单质S和Fe(Ⅲ),Fe(Ⅱ)和Fe(Ⅲ)与水中的OH−或O2反应,生成铁氧化物[28-29]。
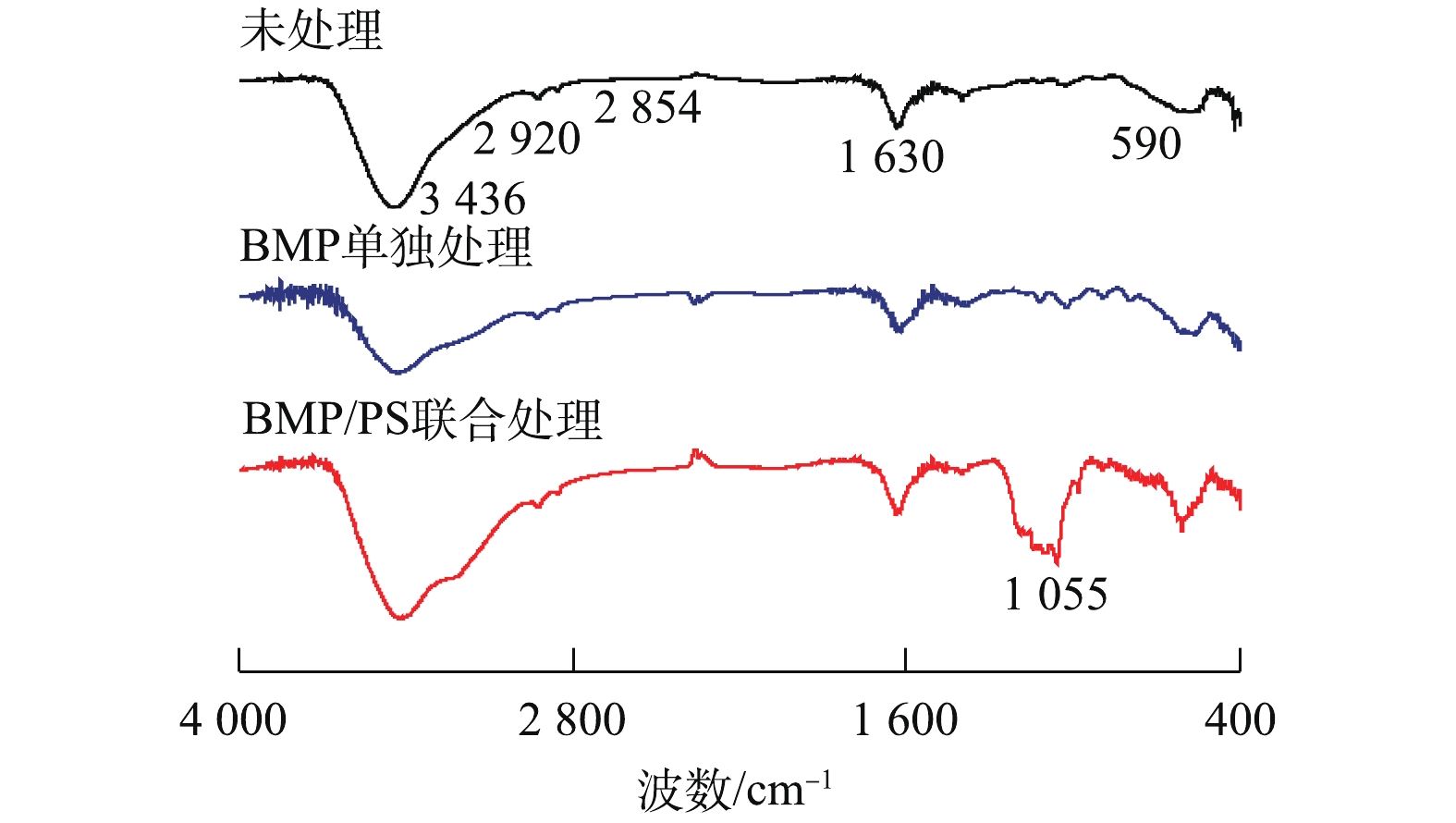
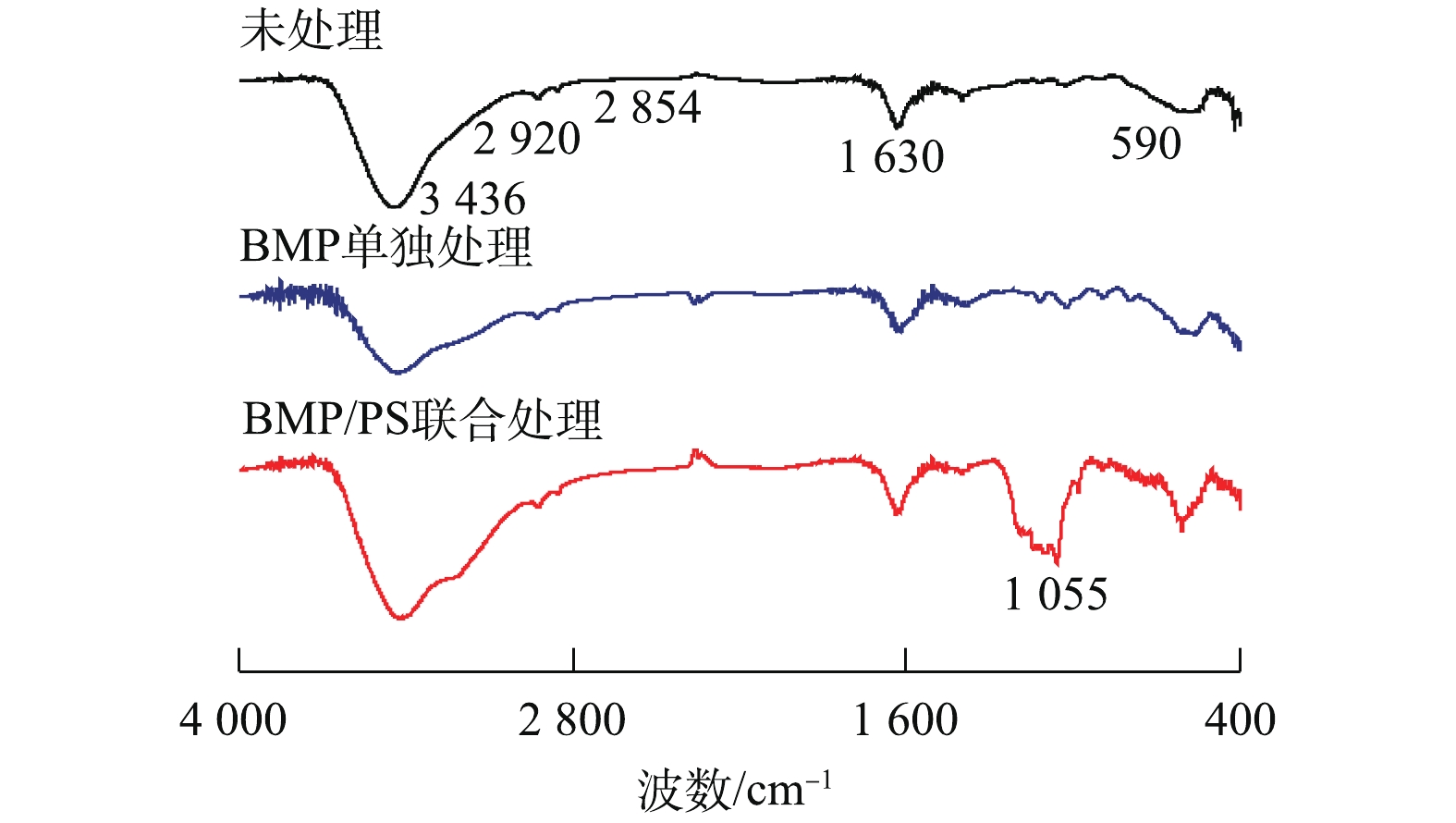
图9为不同处理下BMP的FT-IR图谱。由图9可知,3 436 cm−1处为羟基(—OH)的强拉伸振动吸收峰,2 920 cm−1和2 854 cm−1处为C—H键的振动吸收峰,1 630 cm−1处吸收峰代表羧基(—COOH)的非对称拉伸带;而在指纹区的590 cm−1处的吸收峰为Fe(Ⅱ)—S2−的伸缩振动带[30-31]。与反应前比较发现,BMP单独处理后FT-IR图谱变化较小,BMP/PS联合处理后3 436 cm−1吸收峰振动显著增强,这是由于产生了Fe(OH)3,发生团聚和沉淀[32];1 055 cm−1处出现新的吸收峰,可能是由于
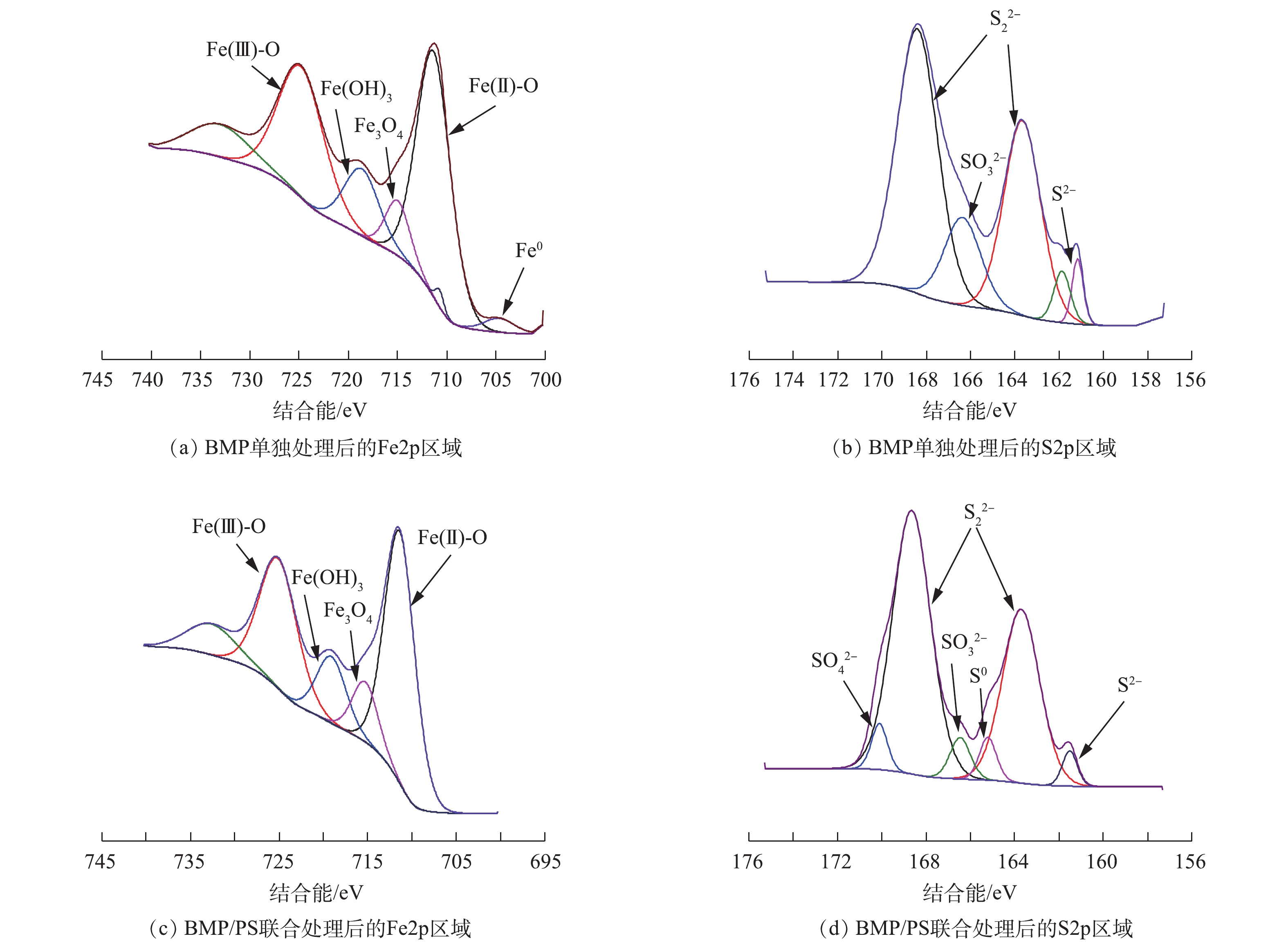
SO2−4 的产生所致[33]。图10为不同处理下BMP的XPS谱图。BMP单独处理后的样品(图10(a)和图10(b)),Fe2p区域内Fe(Ⅱ)-S的衍射峰消失;S2p区域则是微弱的S0衍射峰消失,同时,在光电子结合能为166.48 eV处产生了
SO2−3 的衍射峰。这是由于反应前BMP样品中含有大量FeS和FeS2,反应后BMP经过还原作用消耗了Fe(Ⅱ)和S2−2 ,生成的Fe(Ⅲ)含量由22.41%增加至34.38%,相关反应[34]如式(6)~式(8)所示。BMP/PS联合处理后的样品(图10(c)和图10(d)),Fe2p区域内Fe0衍射峰消失,Fe(OH)3和Fe3O4的衍射峰峰值变大,这表明Fe0与PS接触被氧化成Fe(Ⅱ),Fe(Ⅱ)继续与PS反应生成 Fe(Ⅲ)。Fe(Ⅱ)及Fe(Ⅲ)的含量分别由原来的29.1%和22.41%变为25.56%和28.2%,此外,铁硫氧化物的含量也有所增加[35]。与单独BMP处理比较发现,联合处理后Fe(OH)3和Fe3O4的含量分别由7.86%和5.17%降至6.61%和4.48%,这表明联合处理有效利用了固相铁氧化物,并将其转化为可利用的Fe(Ⅲ)。S2p区域内位于166.58 eV的S0衍射峰重新出现,同时在光电子结合能为170.13 eV处产生了
SO2−4 的衍射峰,这是由于BMP活化PS产生的SO−⋅4 和HO·,其与AN反应后生成SO2−4 和单质S[26],两者占比分别为1.56%和2.08%。综合以上BMP样品变化过程的表征结果,反应前样品含有FeS、FeS2、Fe0和单质硫等。FeS2上的硫的状态是
S2−2 ,S2−2 容易断键从Fe(Ⅱ)获得电子形成稳定的S2−和Fe(Ⅲ);FeS在水溶液中遇H+生成Fe(Ⅱ),S2−经过还原反应后产生了SO2−3 ,消耗了Fe(Ⅱ)-S,进一步加入PS反应后产生了SO−⋅4 、SO2−4 ,同时消耗了样品中的少量Fe0,Fe(Ⅲ)含量进一步增多[36]。BMP降解NB过程中,BMP充当电子供体,NB接受电子被还原。有研究[37]表明,NB还原生成的中间产物主要有亚硝基苯和苯羟胺,最终产物则主要为AN。在本研究中,NB被BMP还原生成大量AN,加入PS后,BMP或Fe(Ⅱ)活化PS产生SO−⋅4 和HO·攻击AN。活化过硫酸盐氧化降解AN的中间产物主要有苯酚、对苯醌、4-氨基苯酚、马来酸等,最终矿化为CO2和H2O[38]。 -
设置水溶液体积为100 mL、NB浓度为100 mg·L−1、BMP的投加量为2.5~7.5 g·L−1、还原时间为15~45 min、PS投加量为2.5~7.5 g·L−1、氧化时间为5~30 min。使用Design Expert 8.05中的Box-Behnken Design设计实验方案,具体实验的设计及实验数据见表2。
采用二次多项式和逐步回归法对实验结果进行拟合,得到如式(9)和式(10)所示的模型方程。
式中:Y1、Y2为响应值,分别代表NB的去除率和AN浓度;A、B、C、D分别代表BMP投加量、还原时间、PS投加量和氧化时间。正项系数表示增加此因素值会提高响应值;反之,负项系数表示增加此因素值会降低响应值。NB去除率的模型中自变量一次项A、B、D,二次项AD、A2和B2显著(P<0.05),表明该模型具有统计学意义,模型P<0.000 1,回归模型极为显著[39-40]。模型的R2为0.949 3,表明独立变量之间的相关性较好,该模型可用于实际预测。AN是在实验中NB还原产生,继而氧化消耗,AN浓度的模型中一次项A和C显著,说明BMP投加量和PS投加量对AN浓度的影响较大,模型P<0.000 1,极为显著。模型的R2为0.928 9,表明独立变量之间的相关性较好,说明该模型可用于实际预测。
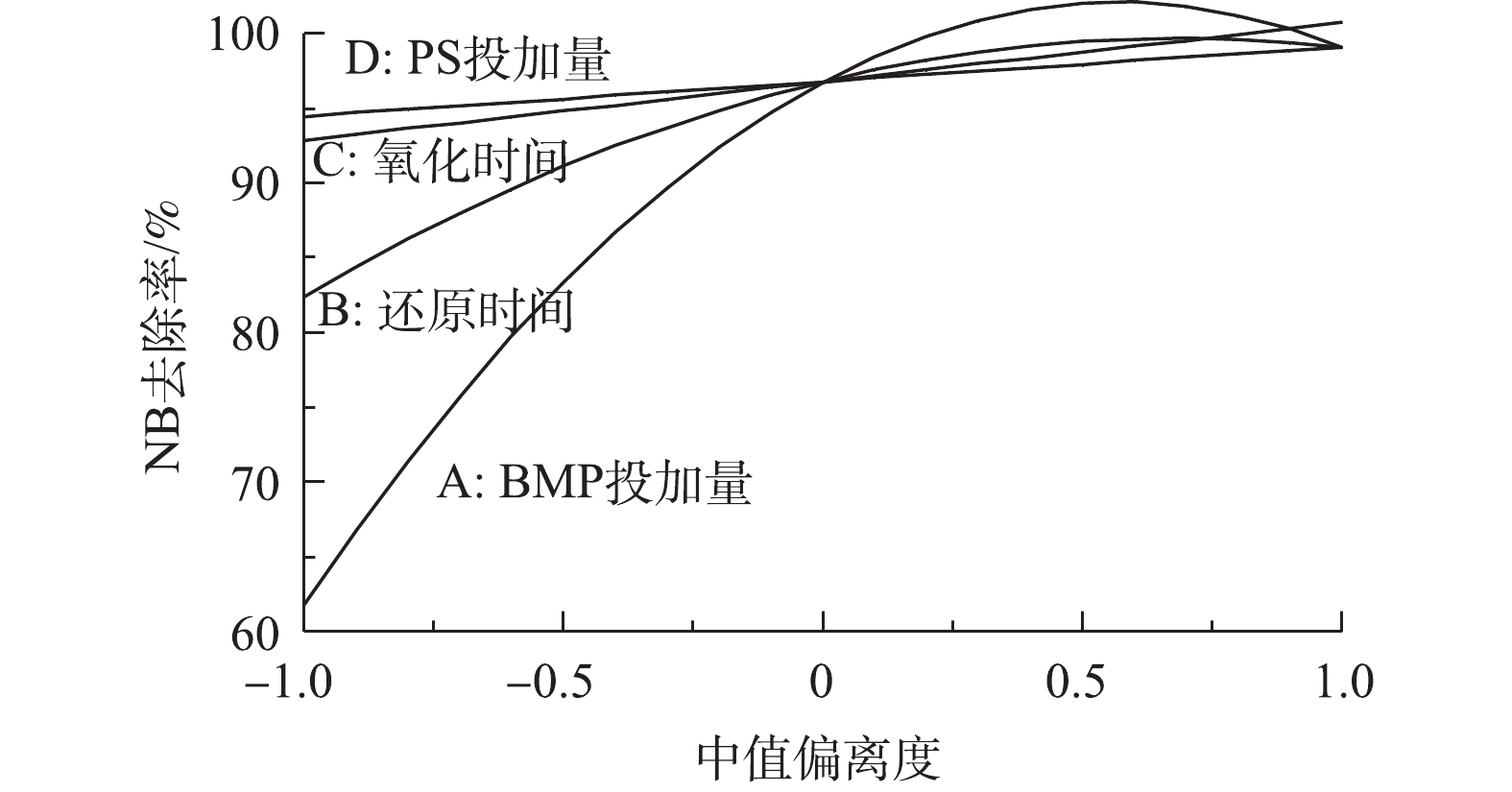
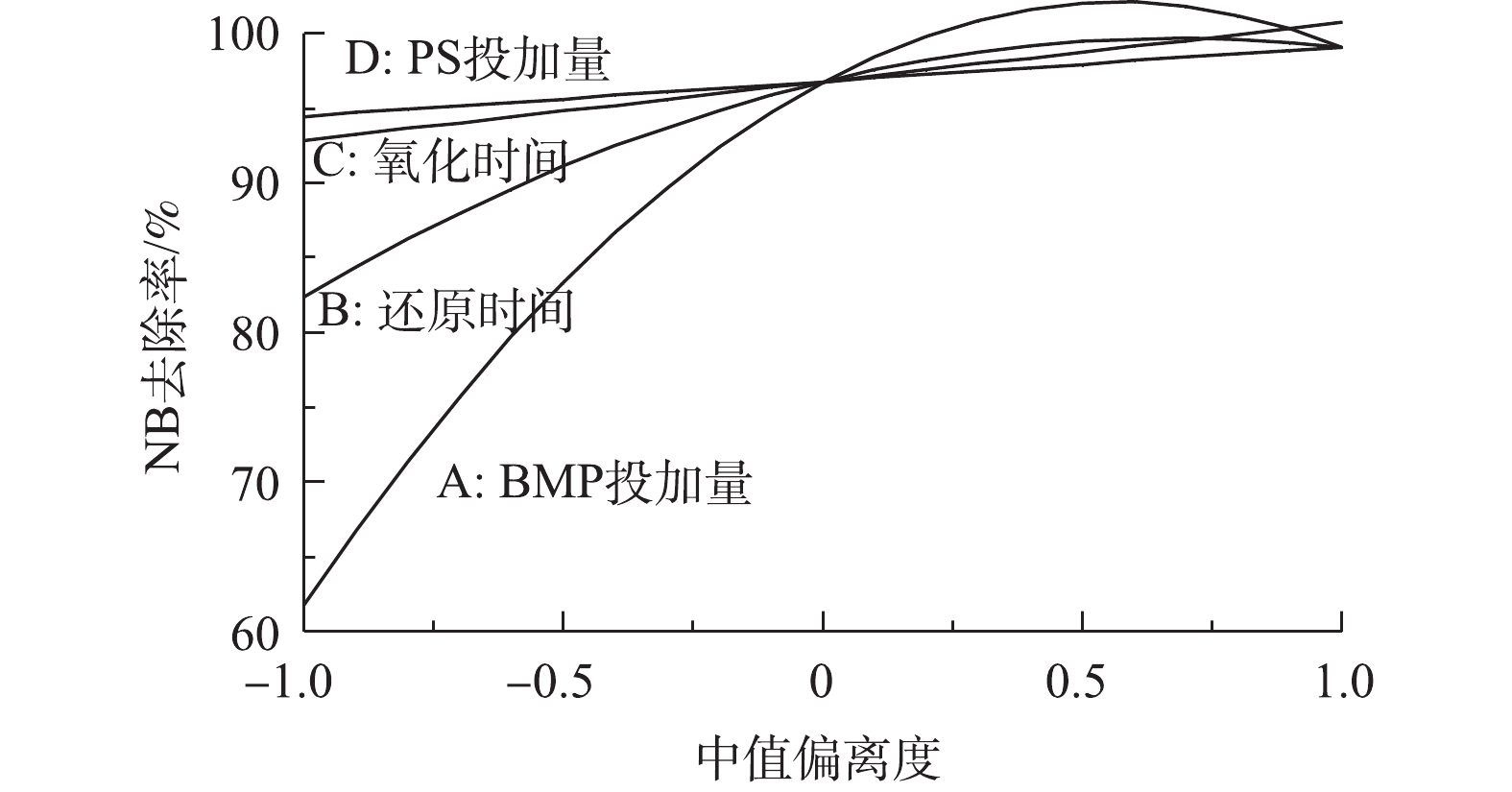
各反应条件对NB去除率的影响程度见图11。BMP投加量对NB去除率的影响程度最大,其次是还原时间,他们均与NB去除率呈二次多项式关系;氧化时间和PS投加量对NB去除率的影响很小。
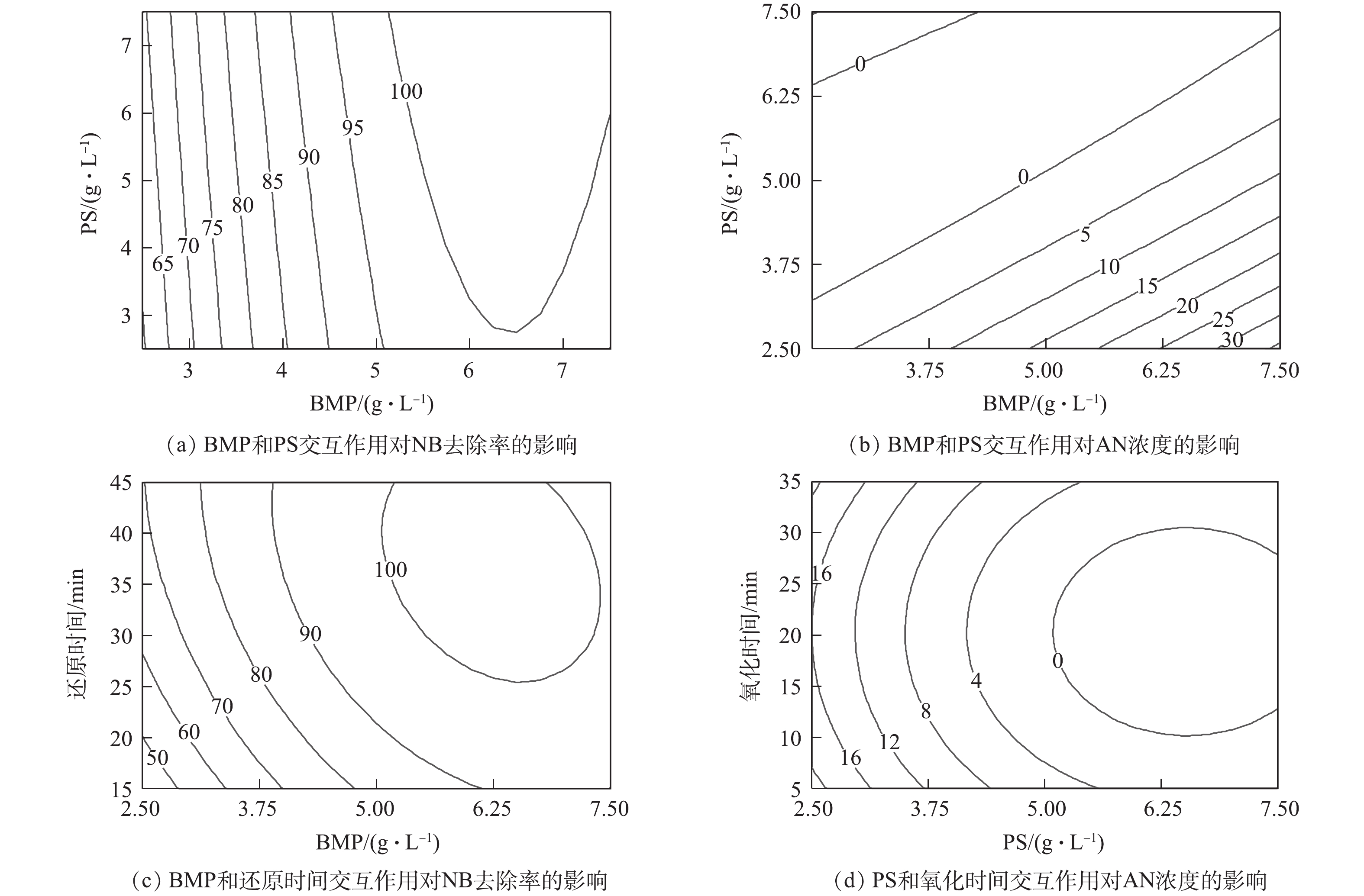
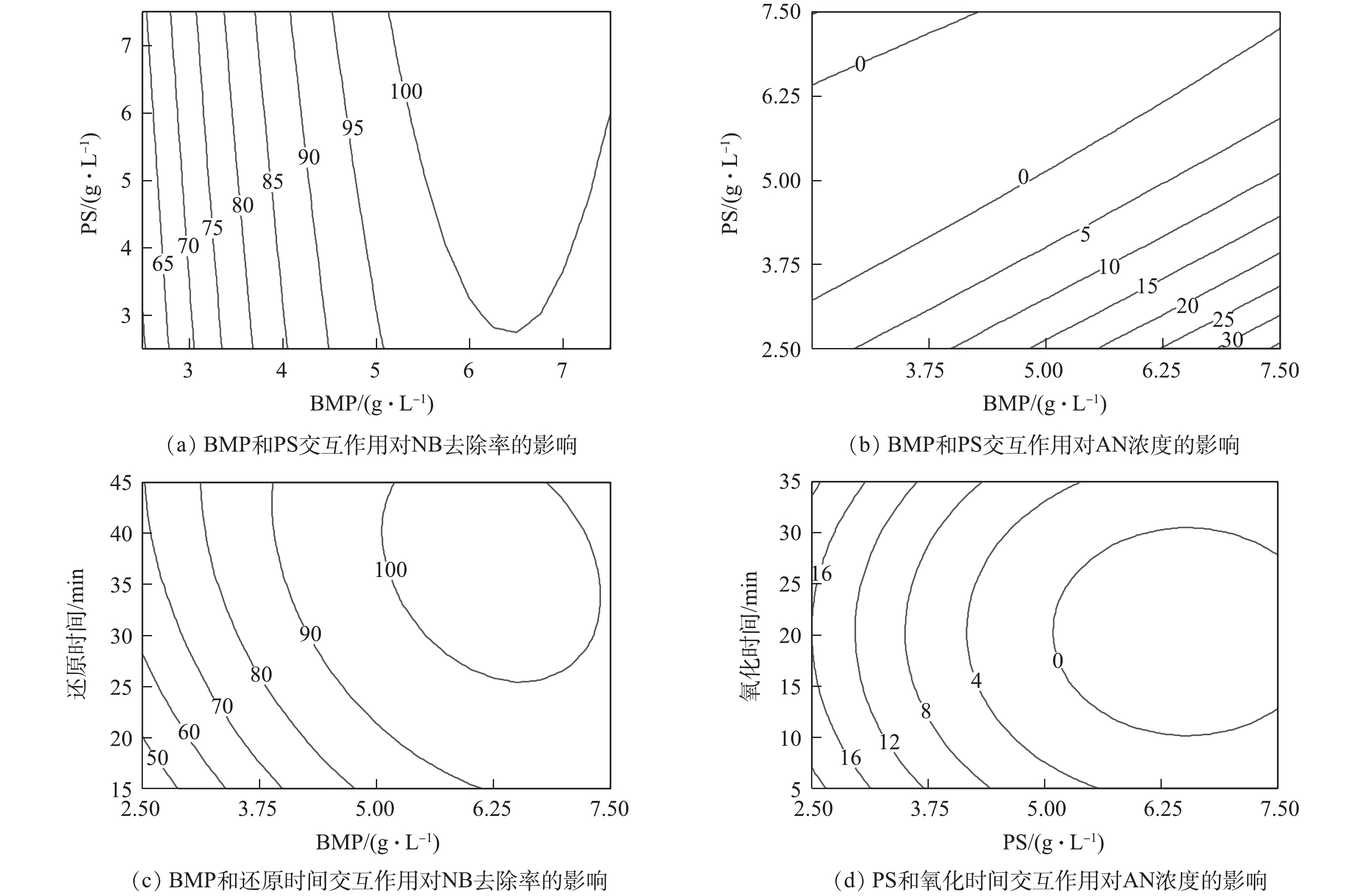
各反应条件间的交互作用对NB去除率和AN浓度的影响结果见图12。其中,图12(a)和图12(b)分别为当还原时间为30 min、氧化时间为17.5 min时,BMP投加量和PS投加量之间的交互作用对NB去除率和AN浓度的影响。由图12(a)可知,BMP投加量对NB去除率起主要作用,且当PS投加量一定时,随着BMP投加量的增加,NB去除率迅速提高;PS投加量对NB去除率的影响很小。由图12(b)可知,BMP和PS投加量分别对AN浓度的影响呈线性关系。当BMP投加量一定时,PS投加量越多,AN浓度越低;当PS投加量一定时,BMP投加量越多,AN浓度越高。PS投加量对AN浓度的影响幅度显著大于BMP投加量。
图12(c)为当PS投加量为5 g·L−1、氧化时间为17.5 min时,BMP和还原时间的交互作用对NB去除率的影响。当BMP投加量较少时,还原时间的变化对NB去除率的影响幅度较大。当还原时间较短时,BMP投加量对NB去除率的影响幅度也比较大。但还原时间的影响幅度显著小于BMP投加量的影响,表明NB去除率对于BMP投加量更为敏感。图12(d)为当BMP投加量为5 g·L−1、还原时间为30 min时,PS投加量和氧化时间的交互作用对AN浓度的影响。为当氧化时间一定时,增加PS投加量显著降低出水中的AN浓度;当PS投加量一定时,随着氧化时间的增加,出水AN浓度先降低后升高,PS投加量对出水AN浓度的影响显著大于氧化时间。
设定优化目标为NB去除率最高和AN浓度最低,通过最优化求解得到最优反应条件为:BMP投加量为7.5 g·L−1、还原时间为45 min、PS投加量为7.5 g·L−1、氧化时间为30 min时,模型预测NB的去除率为99.6%,加入PS后AN浓度为0.028 mg·L−1,和验证实验得出的NB去除率100%、AN浓度降为0 mg·L−1接近,表明拟合方程具有较高的精确度。该条件下NB去除率可达100%,NB还原产生的AN被完全降解。
2.1. BMP表征
2.2. BMP/PS对NB的降解性能
2.3. BMP/PS对NB的降解机理
2.4. 响应面分析
-
1)采用球磨法制备的BMP粒径为89~714 nm,均值为217 nm,离散效果好,分布较均匀。XRD和XPS表征结果表明,BMP主要成分为FeS和FeS2,此外,还含有少量Fe0、单质S和铁硫氧化物。
2)溶液初始pH在酸性和碱性时,对NB和AN的降解效果好。采用BMP单独处理时,NB去除率与BMP投加量呈正相关关系,主要还原产物为AN;采用BMP/PS联合处理时,随着 PS投加量的增加,AN浓度迅速降低,TOC去除率可达80%以上,这表明两者联合处理对NB的降解和矿化效果均较好。
3) XRD、FI-TR、XPS表征结果表明,BMP单独处理时Fe(Ⅱ)和S2−作为活性物质与NB反应,生成
SO2−3 和Fe(Ⅲ)以及降解产物AN;联合处理中BMP中的Fe(Ⅱ)活化PS生成具有强氧化性的SO4−·和HO·,其可与AN反应生成SO2−4 和单质S,Fe(Ⅱ)和Fe(Ⅲ)与水中的OH−或O2反应,生成铁氧化物。4)采用二次多项式和逐步回归法较好地拟合了NB去除率和AN浓度与反应条件之间的关系。BMP投加量对NB去除率的影响最大,其次是还原时间,氧化时间和PS投加量的影响最小。当BMP的投加量为7.5 g·L−1、还原时间为45 min、PS投加量为7.5 g·L−1、氧化时间为30 min 时,NB的去除率为99.6%,AN浓度为0.028 mg·L−1,与验证实验结果相近,这表明该模型有较好的精度和预测能力。











 DownLoad:
DownLoad: