-
我国能源供应以煤炭为主[1-2],且燃煤发电是煤炭消耗的较为清洁的利用方式,煤炭消耗中电煤比例达50%以上[3-4]。随着排放限值的渐趋严格,燃煤电厂排放的颗粒物、SO2、NOx等常规污染物减排幅度显著,尤其是超低排放实施以来,常规污染物控制技术水平得到大幅提升,排放指标远优于发达国家[5-8]。
值得注意的是,目前燃煤电厂对颗粒物的排放仅考核可过滤颗粒物(filterable particulate matter, FPM),并未涉及可凝结颗粒物(condensable particulate matter,CPM),而CPM的冷凝核一般都小于1 μm,也属于PM2.5[9],经烟囱排到大气环境后会迅速冷凝成固态或液态颗粒物,因此,根据美国环保署(EPA)规定,CPM和FPM均属于固定源排放的一次颗粒物。已有研究[10-11]发现,燃煤电厂排放的PM2.5中CPM占比达50%以上,且PM2.5通常还会富集各种重金属、有机物等,对环境及人类健康危害性极大[12-13],因此,CPM的排放也应引起足够的重视。
另外,燃煤烟气排放的Hg、SO3、有机物等也尚未得到广泛关注。这些污染物虽然排放浓度不高,但相关研究表明:燃煤电厂排放的Hg浓度在几至十几μg·m−3,主要以难以脱除的元素汞(Hg0)为主[14-17];燃煤电厂排放的SO3浓度在几至几十mg·m−3,配以低-低温电除尘器的超低排放机组SO3排放浓度普遍较低[18-22];燃煤电厂排放的有机物浓度多为0~20 mg·m−3,且有机物的种类与其总浓度没有明显关系[23-28]。因此,这些非常规污染物的排放及危害需要深入研究。
目前,针对燃煤电厂污染物减排的研究,多是针对常规污染物的超低排放,偶有涉及非常规污染物的报道,也大多仅讨论了1~2种非常规污染物,系统性不强。本研究针对某典型的超低排放机组,开展了不同工况条件下CPM、Hg、SO3、多环芳烃(PAHs)等非常规污染物的现场实测,对各类非常规污染物的梯级脱除特性开展了系统性研究,以期为后续燃煤电厂非常规污染物的排放控制提供参考。
全文HTML
-
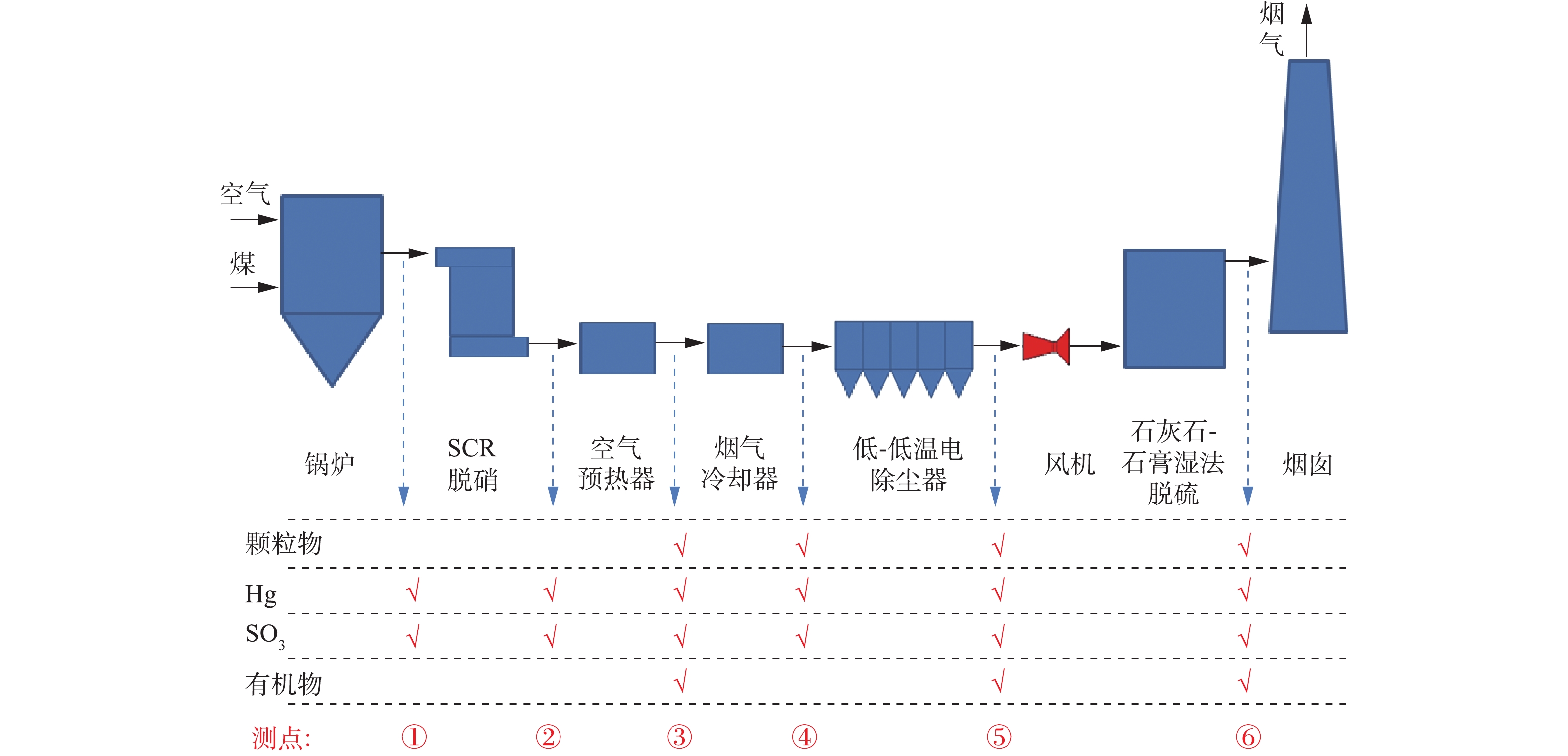
以某1 000 MW燃煤电厂超低排放机组为研究对象,使用锅炉为超临界参数变压运行螺旋管直流炉,采用一次中间再热、四角切圆燃烧方式,煤粉燃烧器为四角布置、切向燃烧、摆动式燃烧器。机组配套SCR脱硝装置、烟气冷却器、低-低温电除尘器、石灰石-石膏湿法脱硫装置。SCR脱硝布置了3层催化剂,并预留第4层催化剂位置;烟气冷却器采用H型金属翅片管式换热器,设计出口烟气温度为(90±5) ℃;低-低温电除尘器为3室5电场电除尘器,设计除尘效率为99.94%;湿法脱硫为石灰石-石膏湿法脱硫,设计脱硫效率>97%,设计出口烟气温度50 ℃。实验期间机组负荷稳定(变化范围±5%),分别在满负荷、75%负荷条件下开展测试实验。机组的设计煤种、校核煤种和实验实烧煤种如表1所示,且保证实验期间煤质来源稳定,以煤种收到基硫分、灰分和挥发分为例,实验期间各成分波动分别在12%、10%、7%以内。分别测定不同工况条件下各测点烟气中颗粒物(FPM和CPM)、Hg、SO3和PAHs浓度,并分析其污染物脱除及排放特性。
-
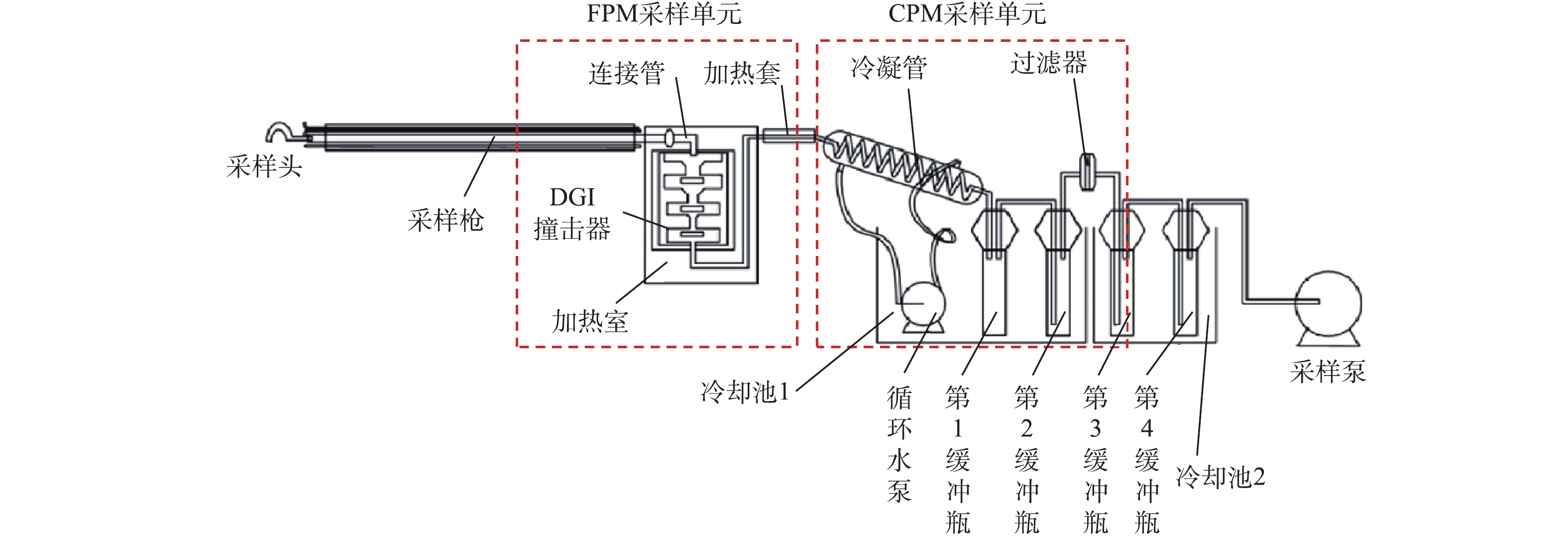
燃煤电厂烟气治理技术路线及测点布置如图1所示。借鉴国内外先进的测试标准及方法[29-36],对烟气中FPM和CPM进行同步测量,颗粒物采样系统如图2所示。加热枪伸入烟道中以等速采集烟气,采集的烟气首先进入FPM采集单元,通过DGI多级撞击器对不同粒径颗粒进行分级,并在末级撞击器出口处布置滤膜(0.3 μm颗粒捕集效率>99%),撞击器及滤膜分别用于收集>10 μm、2.5~10 μm、1~2.5 μm和≤1 μm的FPM,且撞击器外置电加热装置(约120 ℃),防止CPM的干扰,FPM采集单元的测试方法符合标准ISO 23210: 2009的相关规定;脱除FPM后的高温烟气进入后级CPM采集单元,分别通过冷凝管(内径4 mm、总长约200 mm)、第1缓冲瓶、第2缓冲瓶和过滤器,对CPM进行高效收集,冷却池的水温控制在30 ℃以下,从而最大限度地提高CPM捕集效率。待采样结束后,立即用N2对系统进行吹脱,以去除溶解的SO2等污染物,CPM采样单元的测试方法符合标准EPA Method 202的相关规定。FPM质量直接通过对采集的颗粒物样品称质量获得;CPM通过润洗液对冷凝管、缓冲瓶及相关连接件清洗获得,且为了研究CPM的组分,先采用去离子水获得无机组分,再采用丙酮溶液获得有机组分。整个系统为等速采样,结合采样体积,计算获得烟气中各类颗粒物的质量浓度。
-
在测点③~测点⑥ 4个位置分别测定烟气中FPM和CPM,并计算颗粒物的合计值。为提高数据的有效性,每个测点至少重复测定3次,计算平均值,并开展相应的空白实验,以判定测试数据是否失真。低-低温电除尘器为3室5电场电除尘器,因此,其进、出口烟道分别为3支分烟道,测点④、⑤位于分烟道上,通过加权平均的方式计算颗粒物的加权平均值,计算方法如式(1)所示。
式中:C1~C3分别为3支分烟道测得的颗粒物浓度,mg·m−3;Q1~Q3分别为3支分烟道测得的烟气量,m3·h−1。
测试并计算得到不同负荷条件下FPM和CPM质量浓度,结果如表2所示。FPM按空气动力学粒径不同分为PM1、PM2.5、PM10和总FPM,CPM分为有机组分和无机组分,FPM和CPM合计为总颗粒物。
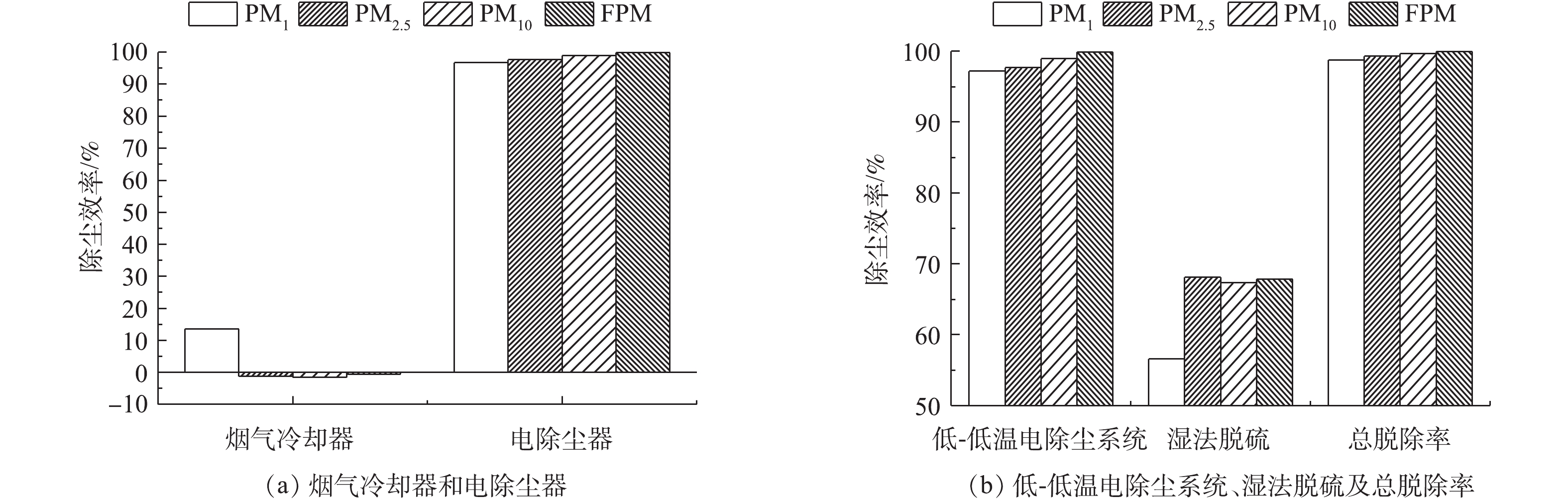
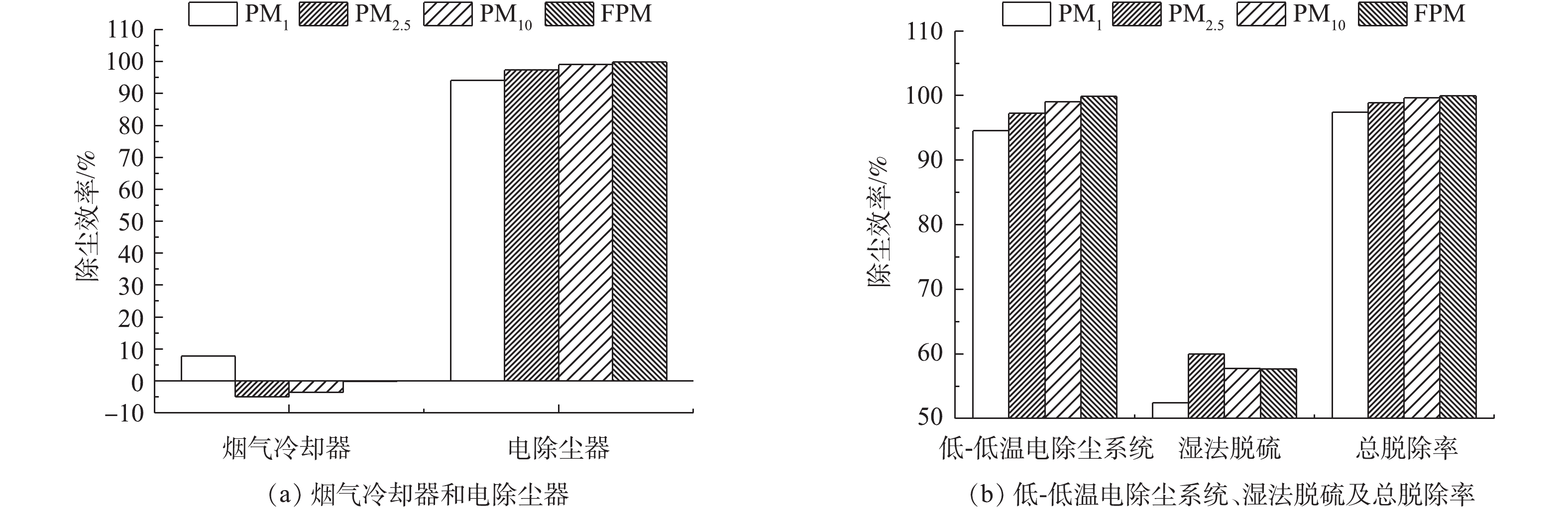
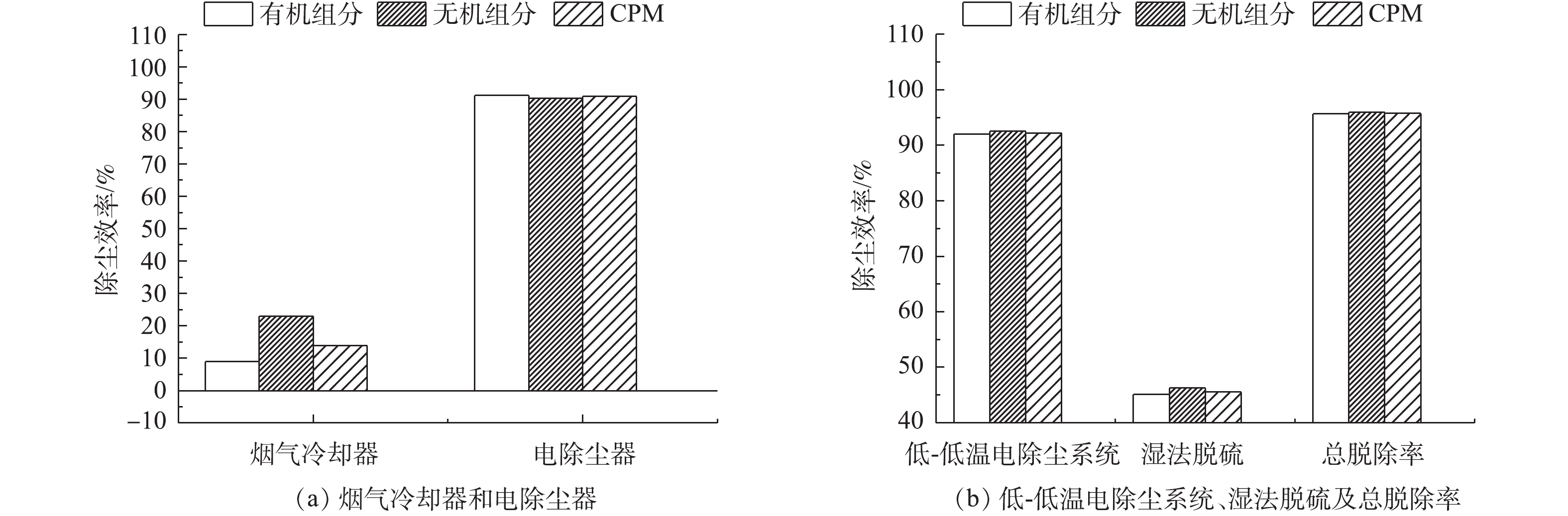
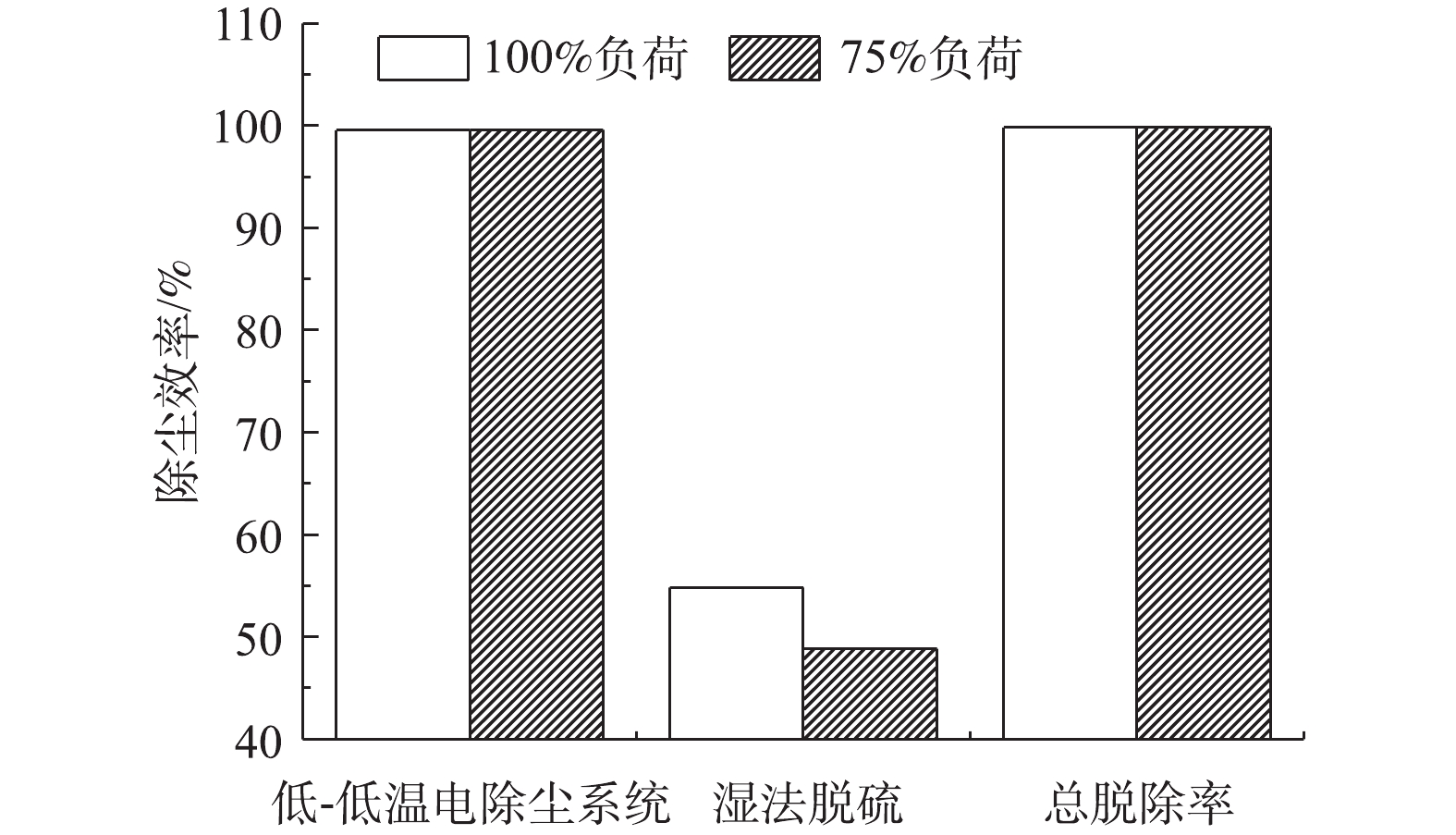
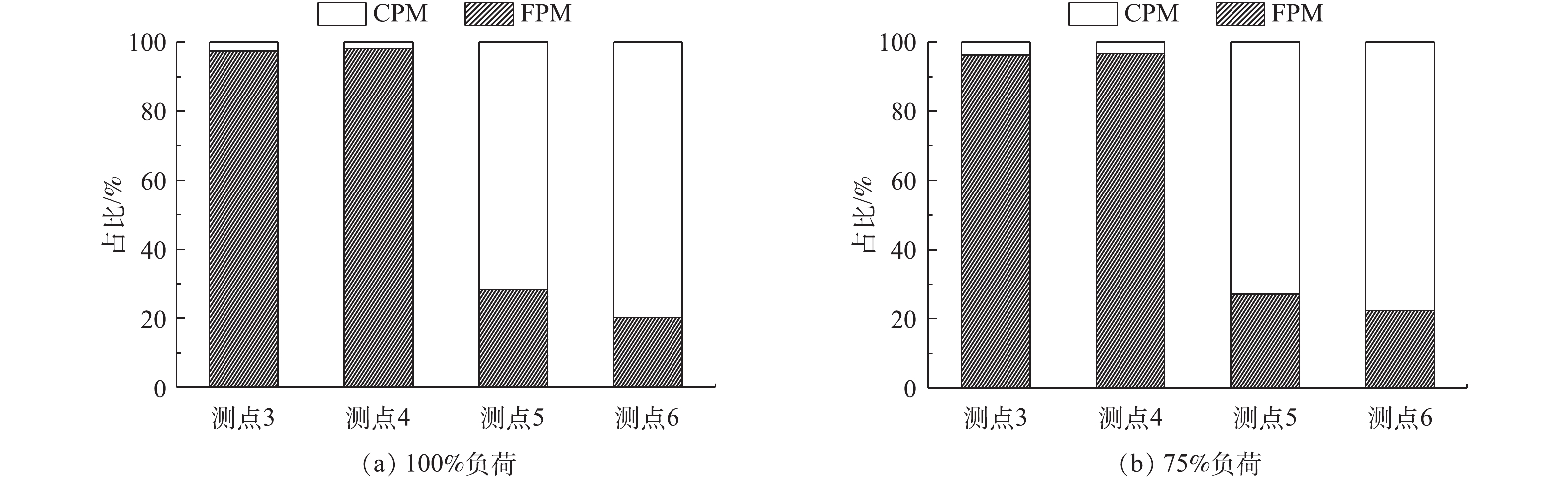
计算不同负荷条件下各污染物脱除设备对FPM、CPM及总颗粒物的脱除率,结果分别如图3~图7所示。从颗粒物的生成来看,FPM的浓度明显高过CPM一个数量级,且负荷不同,其颗粒物浓度也有差异,75%负荷时FPM和CPM浓度均略高于100%负荷,考虑是因为低负荷时,燃料的燃烧效率不及高负荷时高,因此,在相当原料消耗的前提下,FPM和CPM中均含更多的未燃尽成分,质量浓度略高。经过低-低温电除尘系统(烟气冷却器+电除尘器)后,烟气中CPM质量浓度明显高于FPM,这是因为系统对2类颗粒物的脱除率不同,100%、75%负荷时低-低温电除尘系统对FPM脱除率分别为99.87%、99.89%,对CPM脱除率明显低于FPM,分别为87.15%、92.20%,且负荷降低,电除尘系统对2类颗粒物的脱除率均有明显提升。这是因为负荷降低,烟气流速下降,对应的电除尘器比集尘面积增加,变相提高了电除尘性能,这与文献报道的规律[37]一致。烟气冷却器将烟气温度从121、122 ℃降至93、90 ℃,降温后,CPM的有机组分和无机组分均有不同程度的降低,FPM质量浓度增加。这是因为随着温度的降低,部分高露点的CPM会冷凝吸附到FPM上,从而增加了FPM浓度;FPM中PM1浓度未增反减,可能是因为CPM冷凝吸附到FPM表面后,改变了颗粒物表面性质,促进了小粒径颗粒间、小颗粒与大颗粒间的团聚长大所致[38-39]。湿法脱硫对2类颗粒物脱除率有限,100%、75%负荷时,湿法脱硫对FPM脱除率分别为67.85%、57.69%,对CPM脱除率明显低于FPM,分别为49.65%、45.55%,且负荷降低,湿法脱硫对2类颗粒物的脱除率均有明显降低。这是因为湿法脱硫对颗粒物的捕集主要依靠石膏液滴的惯性捕集及除雾器的惯性脱除[40],负荷降低后烟气流速降低,不利于湿法脱硫对2类颗粒物的捕集。100%、75%负荷时,整个系统对FPM脱除率分别为99.96%、99.95%,对CPM脱除率分别为93.53%、95.75%。经计算,100%、75%负荷时低-低温电除尘系统对总颗粒物脱除率分别为99.56%、99.59%,湿法脱硫对总颗粒物脱除率分别为54.82%、48.83%,整个污染物控制系统对颗粒物的总脱除率均约为99.80%。不同负荷下CPM和FPM的浓度分布比例如图8所示。烟气中颗粒物初始以FPM为主,占比分别为98%、96%。经过除尘后发生反转,以CPM为主,占比分别为72%、73%,最终排放的颗粒物中,FPM分别为3.6、4.4 mg·m−3,均满足超低排放要求,但此时CPM却达14.2、15.3 mg·m−3,总颗粒物高达17.8、19.7 mg·m−3,CPM的浓度远超FPM,因此,CPM的排放也应引起足够的重视。
2.1. 颗粒物测试方法
2.2. 颗粒物测试结果及分析
-
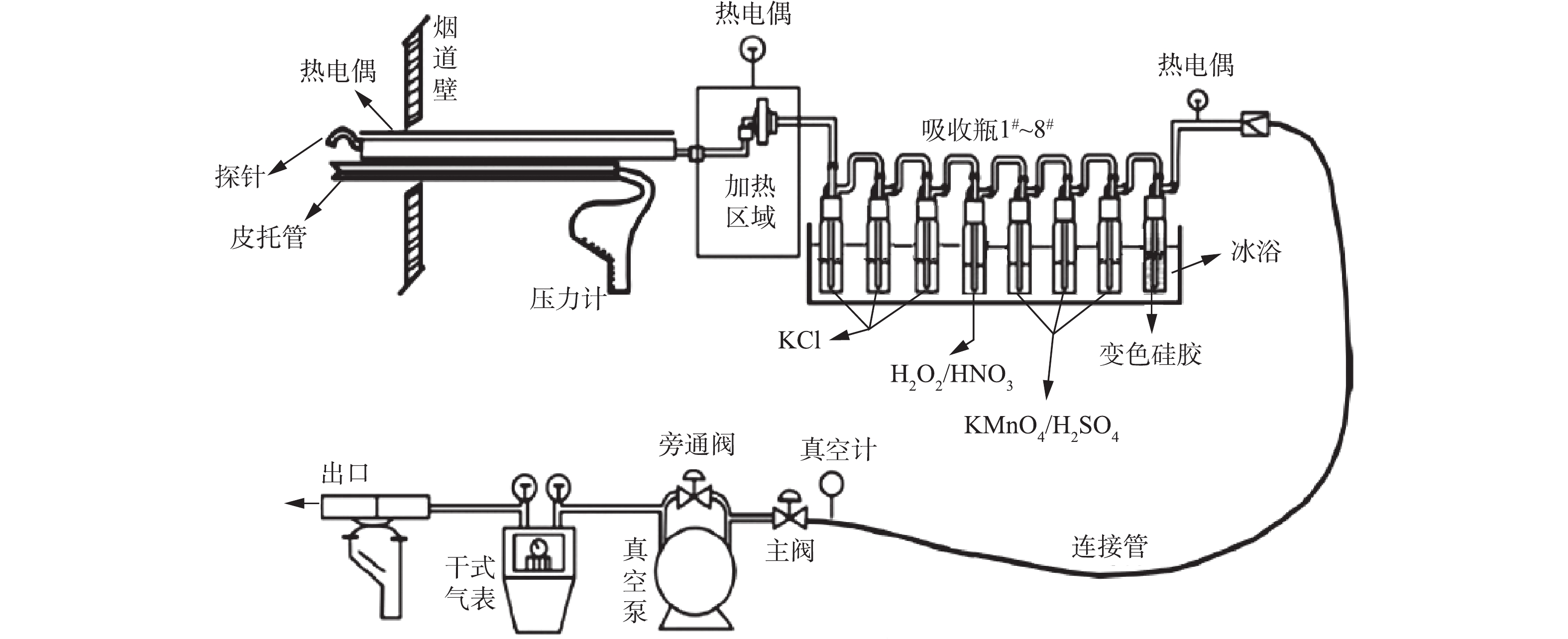
燃煤烟气中的Hg有元素Hg(Hg0)、二价Hg(Hg2+)和颗粒Hg(Hgp) 3种赋存形态,借鉴国内外先进的测试标准及方法[41-43],对烟气中Hg采样采用安大略法(OHM)进行测试,采样系统如图9所示。采样石英玻璃管及过滤系统均配置电加热,温度控制在120 ℃,防止水蒸气凝结造成烟气中Hg的沿壁损失。采样时对吸收瓶进行冷浴处理,保证烟气Hg的充分吸收。过滤系统用于采集飞灰,并测定飞灰颗粒中的Hg(Hgp),测定仪器采用高频塞曼直接测汞仪RA-915F。吸收瓶箱装有8个吸收瓶,其中,前3个氯化钾溶液吸收瓶用于吸收烟气中的Hg2+;后面氧化氢/硝酸溶液和高锰酸钾/硫酸溶液用于吸收烟气中的Hg0。溶液中各个价态的Hg采用RA-915W冷原子吸收汞分析仪进行测定。最末级吸收瓶装有硅胶,以对烟气进行干燥,避免对泵的不良影响。整个系统为等速采样,结合采样体积,计算获得烟气中各价态Hg的质量浓度。为验证测试数据的准确性,根据物料的质量守恒原则,对于整个电厂系统的Hg平衡进行核算[44],即Hg的输入量与输出量应该是相等的。Hg的输入主要是煤,输出包括烟气、渣、电除尘器收集的飞灰、脱硫石膏、脱硫废水等。液体样品中Hg含量采用RA-915W冷原子吸收汞分析仪进行测定,固体样品采用采用高频塞曼直接测汞仪RA-915F,并配以固体样品热解附件PYRO915#493,能通过高温热解样品直接测量样品中的汞浓度。
-
在满负荷条件下,测得各测点不同价态Hg的浓度,测试结果如表3所示。经计算,各设备对不同价态Hg的脱除率如图10所示。SCR脱硝、空预器、烟气冷却器、电除尘器、湿法脱硫对总Hg均有脱除效果,其脱除率分别为0.85%、0.43%、4.29%、64.66%、28.05%,电除尘器的脱Hg效率最高,主要是因为有相当一部分Hg是以Hgp形态存在,电除尘器在高效除尘的同时也脱除了几乎100%的Hgp。经计算,低-低温电除尘系统(烟气冷却器+电除尘器)对总Hg的脱除率为64.81%,整个烟气治理系统的脱Hg效率达75.5%,Hgp全部被脱除,剩余的是难以脱除的Hg0、Hg2+,脱除率分别为63.01%、64.29%,排放浓度分别为5.4、0.5 μg·m−3。在各个价态的Hg中,Hg0是最难被脱除的。虽然SCR脱硝对总Hg脱除效果不明显,但可有效促进Hg0向Hg2+、Hgp的迁移转化。经计算,其Hg0转化效率达44.52%,跟以往的报道数据[43-45]大致相当,主要与SCR催化剂的成分、烟气中卤族元素含量等有关。烟气冷却器对Hg0脱除率达28.21%,明显高于空预器。可能是因为烟气冷却器将烟气温度降至酸露点以下,此时气态的硫酸冷凝成硫酸雾,被飞灰吸附后,与飞灰中的碱性物质中和,避免了SO3与Hg争夺吸附点位[46]。湿法脱硫对Hg2+脱除率较高,达82.76%。这是因为Hg2+易溶于水,但对Hg0脱除能力有限,且浆液中的Hg2+可能与
SO2−3 、Fe2+、Mn2+等2价离子发生反应[47],被还原为Hg0,造成浓度不降反升。对于烟气中较难脱除的Hg0、Hg2+,若需进一步减排,除了进一步强化现有超低排放设备的协同脱除能力,如SCR脱硝的Hg0氧化性能等,还需配置额外的脱Hg系统,如活性炭或改性活性炭喷射脱Hg技术。为了验证测试数据的准确性,根据物料的质量守恒原则,对整个电厂系统的Hg平衡进行了核算。根据该机组的实际日耗煤量及炉渣、粉煤灰、石膏、废水产量及烟气量,计算得到各输出单元的分布系数,计算方法如式(2)所示。
式中:
ηi 为各输出单元的分布系数;Fiout 为某个输出单元的日输出量,μg·d−1;Fin 为整个系统的日输入量,μg·d−1。整个系统的平衡系数计算方法如式(3)所示。
式中:
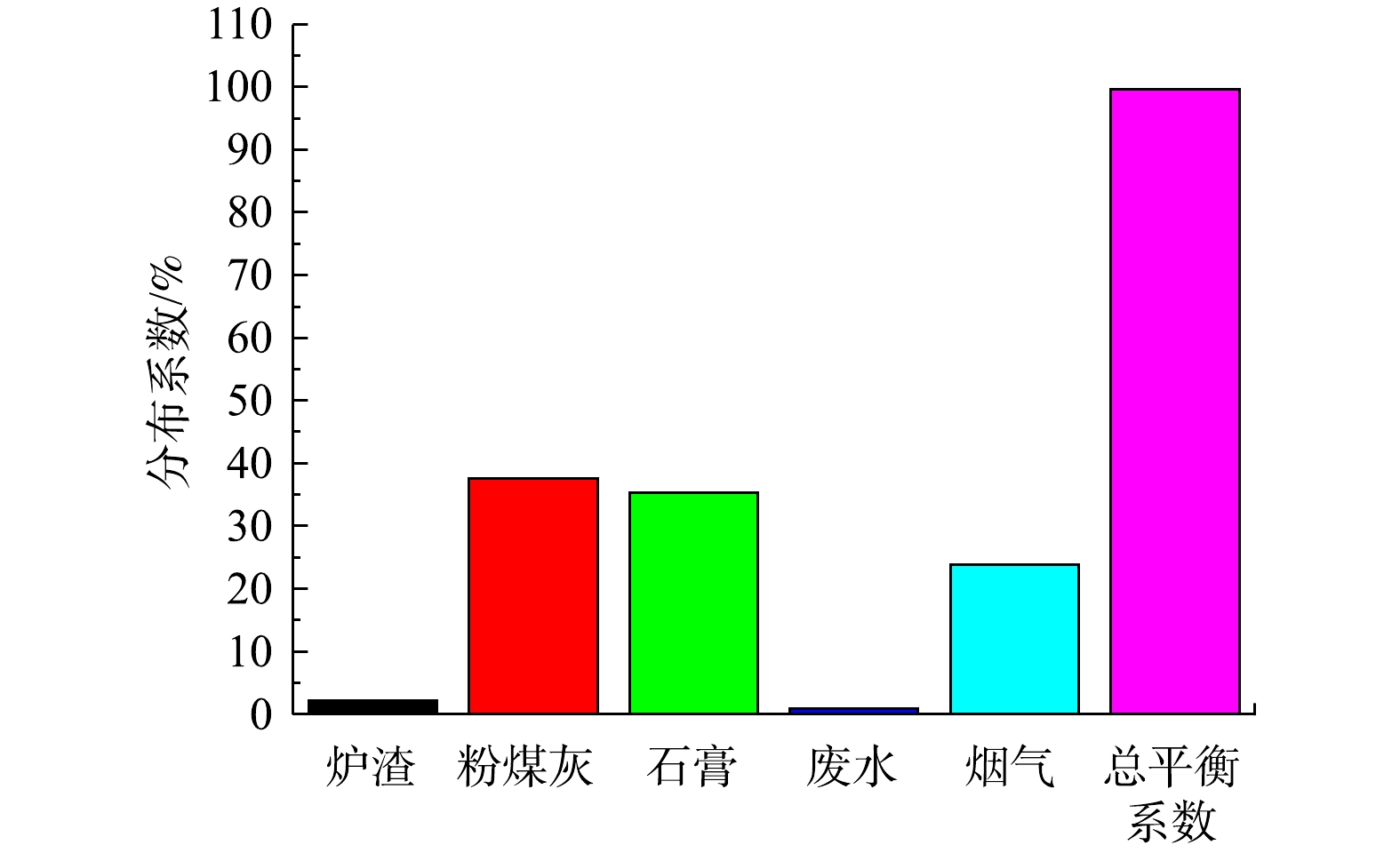
η 为各输出单元的分布系数;Fout 为整个系统的日输出量,即n∑iFiout ,μg·d−1。计算结果如图11所示,总平衡系数达99.7%,测试结果可信度较高。在各输出单元中,最终通过烟气排入大气环境中的Hg占比为23.8%,可见其仍有进一步脱Hg的潜力和必要。固/液样品中粉煤灰、石膏中Hg分布系数最高,分别为37.6%、35.3%。
3.1. Hg测试方法
3.2. Hg测试结果及分析
-
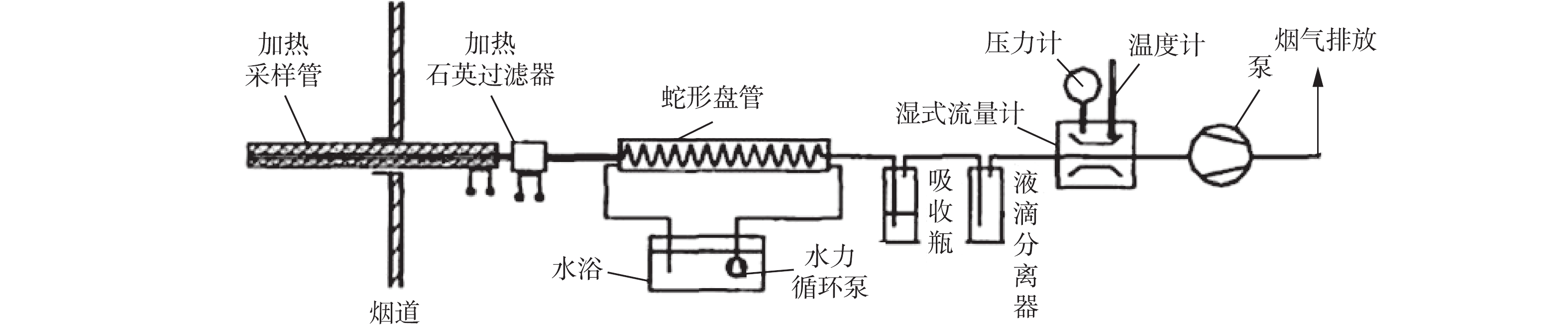
参照国标GB/T 21508-2008规定的控制冷凝法对烟气中SO3进行测定,采样系统如图12所示。采样管内壁为石英玻璃管,采样管全程伴热,加热温度控制在180 ℃,以防止SO3在壁面冷凝损失。过滤石英棉用于过滤FPM,以防止FPM中硫酸根对最终SO3测定结果的影响。蛇形盘管布置在65 ℃的恒温水浴中,将SO3冷凝成硫酸雾滴,并通过惯性捕集下来。采样结束后,用去离子水清洗蛇形盘管,硫酸根离子的测定采用哈希DR 6000紫外-可见分光光度计。整个系统为等速采样,结合采样体积,计算获得烟气中SO3的质量浓度。
-
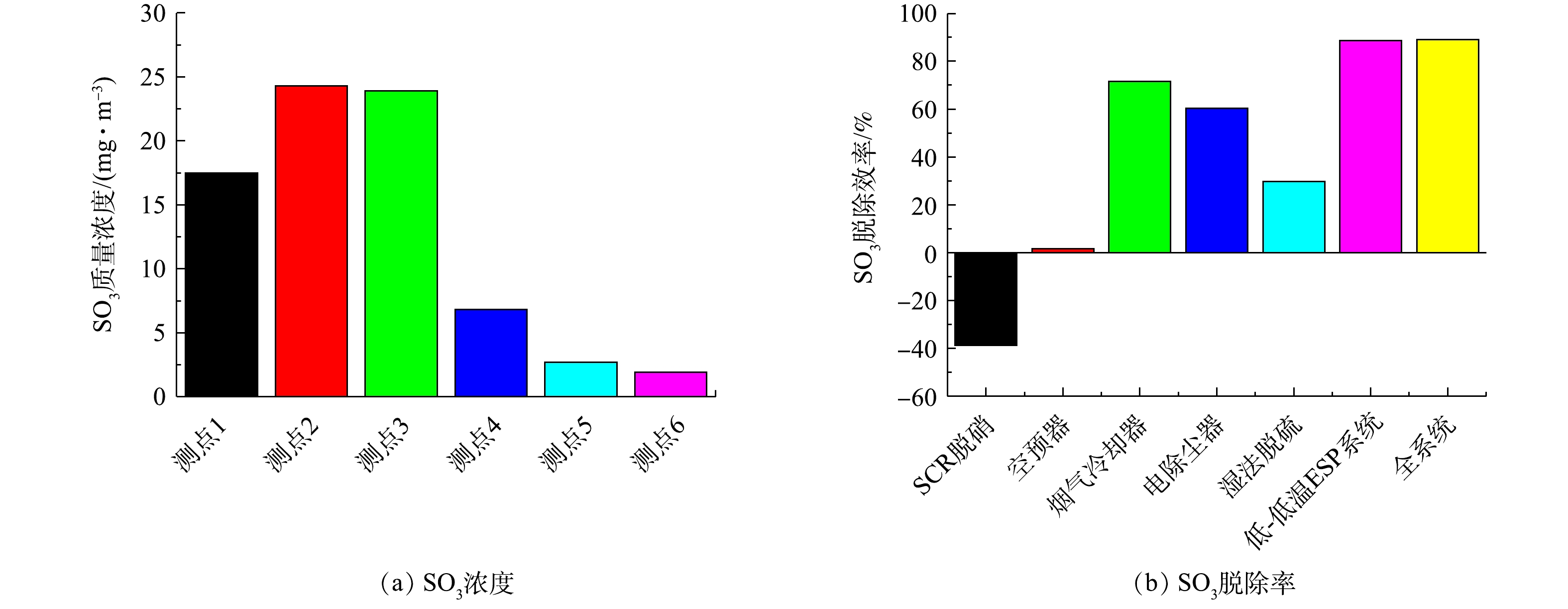
在满负荷条件下各测点测得烟气中SO3的浓度,并计算各污染物脱除设备的SO3脱除率,结果如图13所示。燃煤烟气中SO3除了来自炉膛燃烧过程中煤的硫分氧化形成,另外还会在SCR脱硝过程中,催化剂将部分SO2氧化成了SO3,因此,实测得到SCR脱硝前后SO3浓度分别为17.5、24.3 mg·m−3,增加了6.8 mg·m−3。实验期间,SCR脱硝前SO2浓度约1 g·m−3,经计算,在SCR脱硝过程中,催化剂将SO2氧化成SO3的转化率约为0.7%。烟气冷却器将烟气温度从121 ℃降至93 ℃,降至酸露点以下,此时气态的硫酸冷凝成硫酸雾,并被飞灰吸附后与飞灰中的碱性物质中和,因此,烟气中SO3浓度大幅降低,烟气冷却器对SO3脱除率为71.55%。与电除尘器配合使用,低-低温电除尘系统(烟气冷却器+电除尘器)可脱除88.7%的SO3,是各污染物脱除设备中脱除率最高的。湿法脱硫主要用于脱除SO2,此时SO3是以硫酸气溶胶颗粒的形式存在,粒径很小,脱硫浆液与硫酸气溶胶颗粒之间的传质作用主要依靠惯性碰撞、布朗扩散等作用实现,因此,湿法脱硫对SO3的脱除率并不高,仅为29.63%。整个系统(含SCR脱硝)对SO3脱除率为89.14%,最终SO3排放浓度为1.9 mg·m−3。
4.1. SO3测试方法
4.2. SO3测试结果及分析
-
PAHs是一类具有2个及以上苯环的有机污染物,危害性大,且不宜分解。美国环保署(EPA)规定优先控制16种PAHs,分别有1个2环(萘)、5个3环(苊、二氢苊、芴、菲、蒽)、4个4环(荧蒽、芘、苯并(a)蒽、屈)、4个5环(苯并(b)萤蒽、苯并(k)萤蒽、苯并(a)芘、二苯并(a,h)蒽)和2个6环(茚并(1,2,3-cd)芘、苯并(g,h,i)苝)PAHs。参照行标HJ 646-2013的相关规定,对16种PAHs进行测定,采样系统如图14所示。滤筒保温箱内置玻纤滤筒,用于收集烟气中固相PAHs,气相吸附柱内置XAD-2树脂,用于收集气相PAHs。最终使用色谱质谱联用仪(GC-MS)定量分析PAHs浓度。分别在测点③、⑤、⑥开展测试,整个系统为等速采样,结合采样体积,计算获得烟气中PAHs的质量浓度。
-
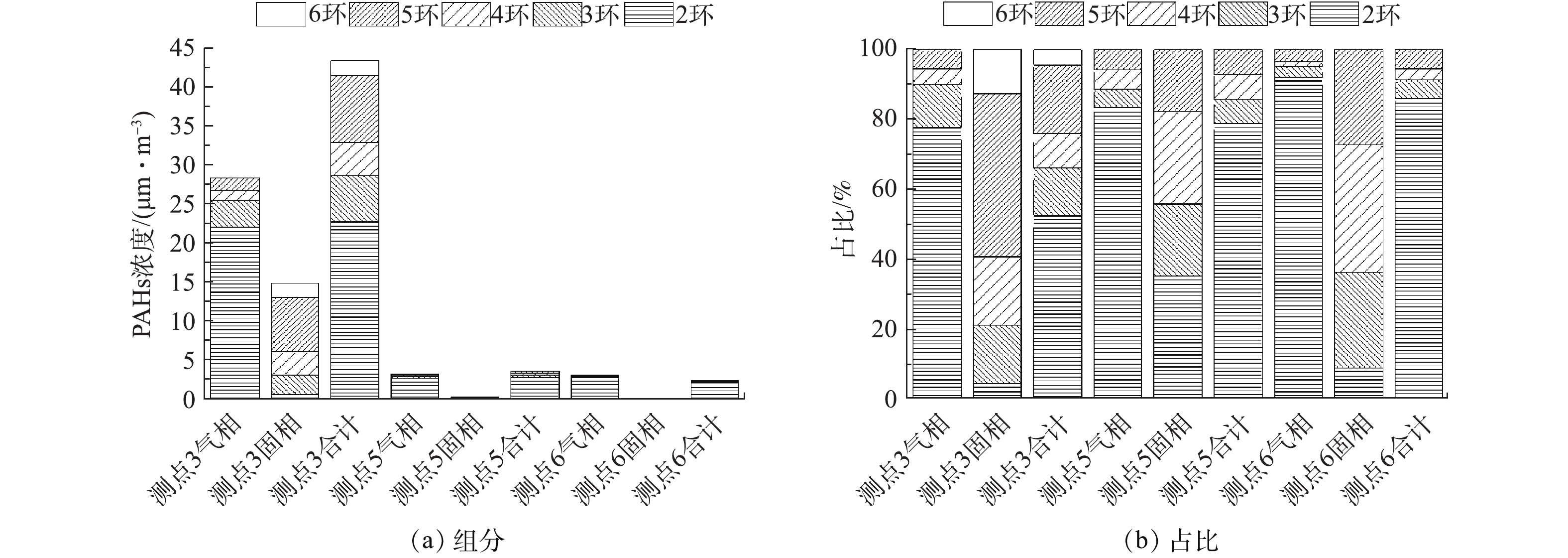
在满负荷条件下,在测点③、⑤、⑥测得烟气中PAHs的浓度,结果如表4所示。烟气中气相PAHs浓度明显高于固相,气相、固相PAHs浓度分别为28.49、15.02 μg·m−3。值得注意的是,PAHs沸点、熔点较高,在室温下通常以固相或液相存在,因此,表2中CPM的有机组分明显高过无机组分。测点⑤、⑥测得烟气中气相PAHs的浓度也明显高于固相,这是因为低-低温电除尘系统、湿法脱硫对FPM具有显著的脱除效果。最终PAHs排放为2.5 μg·m−3,其中,2环、3环、4环、5环、6环的浓度分别为2.15、0.13、0.08、0.14、0 μg·m−3。
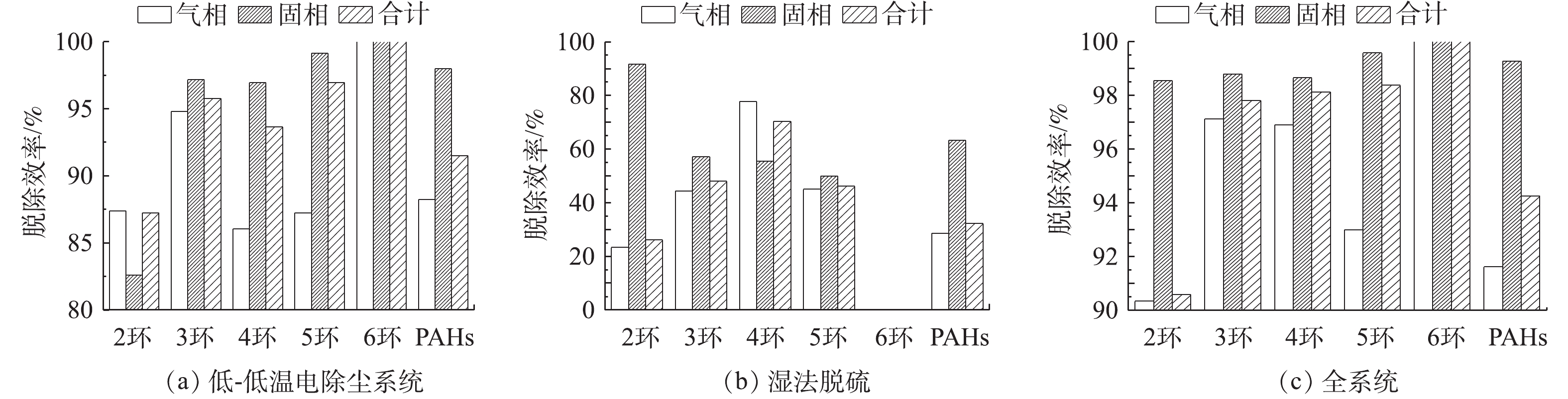
不同测点处PAHs组分有明显差异,各组分及其比例如图15所示。初始PAHs中以2环和5环为主,其中,气相以2环为主,固相以5环为主;经过低-低温电除尘系统后,气相中2环比例进一步提高,固相中5环比例减少,2、3、4环比例增加,总的PAHs中2环比例增加;经过湿法脱硫后,主要以2环PAHs为主。低-低温电除尘系统对PAHs脱除率如图16(a)所示,低-低温电除尘系统可脱除91.52%的PAHs,其中,气相脱除率明显低于固相,分别为88.24%、98%;6环PAHs脱除率达100%,2环PAHs脱除率相对最低,为87.25%。有研究[48]表明,燃煤电厂电除尘器对PAHs脱除率在90%左右,与本研究结果相当。湿法脱硫对PAHs脱除率如图16(b)所示,湿法脱硫可脱除32.25%的PAHs。气相脱除率明显低于固相,分别为28.66%、63.33%;随着环数的增加,脱除率逐渐提高,2环固相PAHs脱除率最高,达91.57%。全系统对PAHs的脱除率如图16(c)所示,PAHs脱除率可达94.25%,气相、固相脱除率分别为91.61%、99.27%;随着环数的增加,脱除率逐渐提高,且固相的脱除率明显高于气相,2环~6环PAHs脱除率在90.58%~100%,其中,固相在98.55%~100%,气相在90.33%~100%。
5.1. PAHs测试方法
5.2. PAHs测试结果及分析
-
1)通过FPM和CPM一体化采样系统测定满负荷和75%负荷条件下颗粒物的梯级脱除特性,发现FPM的生成量明显高过CPM一个数量级,但CPM的排放浓度却远超FPM,因此,CPM的排放也应引起足够的重视。低负荷时存在燃烧不完全,FPM和CPM质量浓度均略高些;负荷降低,有利于提高电除尘性能,但不利于湿法脱硫对颗粒物的捕集。
2)通过安大略法(OHM)测定满负荷条件下各个价态Hg的梯级脱除特性,发现在各个价态的Hg中,Hg0是最难被脱除的,Hgp全部被脱除,Hg0、Hg2+排放浓度分别为5.4、0.5 μg·m−3。
3)通过控制冷凝法测定满负荷条件下SO3的梯级脱除特性,发现SCR脱硝对SO3脱除呈负贡献;低-低温电除尘系统可脱除88.7%的SO3,是各污染物脱除设备中脱除率最高的;湿法脱硫对SO3的脱除率并不高,仅为29.63%;整个系统(含SCR脱硝)对SO3脱除率为89.14%。
4)采用HJ 646-2013规定的测试方法在满负荷条件下对16种PAHs进行测定,发现烟气中气相、固相PAHs浓度分别为28.49、15.02 μg·m−3,其中,气相以2环为主,固相以5环为主;全系统对PAHs脱除率达94.25%,随着环数增加,脱除率逐渐提高,且固相的脱除率明显高于气相。











 下载:
下载: